MicroRNAs (miRNAs/miRs) are endogenous,
19-23-nucleotide-long, non-coding single-stranded RNA molecules
that act as regulators of gene expression by associating with the
multiprotein RNA-induced silencing complex (RISC) (1,2).
RISC silences specific mRNA species by pairing with the
3′-untranslated region (3′-UTR) of the target mRNAs to impact their
expression (3-5). The biogenesis of miRNAs is composed
of multiple steps. First, primary miRNAs are cleaved into stem-loop
precursor structures of ~70 nucleotides, known as precursor-miRNAs
(premiRNAs), by the Drosha enzyme (6,7).
Ultimately, premiRNAs are digested to mature 22-nucleotide-long
miRNAs by the RNase III enzyme Dicer (8).
miR-222, a member of the miR-221/222 family, is
located on the X chromosome p11.3 of the human genome (9). Mature miR-222 sequences have a
hairpin precursor with different arms called the 5' or 3' arm,
which are also known as -5p or -3p, respectively (10,11). Dysregulated miR-222-3p expression
has been reported in various human diseases, such as in cataract
pathogenesis and chordomas (12-15) and appears to be a promising
biomarker for cancer diagnosis and prognosis (16-18).
The development of tumors is a multistep process
that includes continuous proliferation signaling, evading growth
inhibitors and inducing angiogenesis, invasion and metastasis
(19). miRNAs have important
roles in the initiation, development and progression of various
types of cancer, including breast and prostate cancer (20-22). A growing number of studies have
indicated that miR-222-3p has multiple functions in tumorigenesis
(23), cancer cell proliferation
and apoptosis (24), cancer cell
invasion and migration (16,25), therapeutic resistance (26) and the tumor microenvironment
(27,28).
A number of studies have recently manifested the
association of miR-222-3p dysregulation with cancer initiation and
progression (29-31). Despite recent developments in
cancer diagnosis and treatment, there are still numerous problems
associated with the pathogenesis of cancer progression, disease
recurrence and drug resistance. The present review thoroughly
discusses the clinical value of miR-222-3p in cancer and whether
miR-222-3p acts as an oncogene or a cancer suppressor by reviewing
and summarizing studies covering cells, cancer tissues and
biofluids. A comprehensive overview of these findings and the
implications for molecular research are provided.
Increasing studies have suggested that miR-222-3p
expression may be a potential predictor of tumor type, tumor grade
and lymph node metastasis in multiple types of human tumors, such
as prostate cancer, uveal melanoma, papillary thyroid carcinoma and
gastric cancer (32-35). Circulating miR-222-3p, alone or in
combination with other miRNAs in plasma/serum, may act as a
candidate biomarker for the early detection of cancer (12,22,36). Moreover, tumor cell-derived
miR-222-3p serves as a prognostic factor for the survival of
patients with hepatocellular carcinoma and epithelial ovarian
cancer (23,37). The expression pattern of
miR-222-3p has been extensively studied and compared in non-tumor
and tumor tissues of different types of human cancer (Table I).
Aberrant miR-222-3p expression is closely associated
with the clinical characteristics of patients with cancer. For
instance, miR-222-3p was found to be overexpressed in papillary
thyroid carcinoma (PTC) compared with in normal thyroid and benign
cancer tissues (38).
Additionally, Di Fazio et al (39) reported that miR-222-3p could be
used to distinguish patients with typical and atypical lung
carcinoid. Furthermore, miR-222-3p combined with a panel of miRNAs
(miR-7-5p and miR-146b-5p) exhibited high sensitivity and
specificity for identifying different subtypes of PTC (40). This panel of miRNAs could
distinguish non-invasive follicular thyroid neoplasms from
papillary-like nuclear features, follicular adenomas and
infiltrative follicular variants of PTC (40-42). miR-222-3p was reported to be
differentially expressed in some types of cancer, as shown in
Table I. For example, miR-222-3p
expression is decreased in prostate cancer, while it is increased
in other types of cancer, including bladder and breast cancer;
however, miR-222-3p expression in ovarian carcinoma remains
controversial (Table I).
In addition, dysregulated miR-222-3p expression was
found to be closely associated with tumor stage, invasion and
metastasis. In gastric carcinoma, high miR-222-3p expression was
positively associated with advanced clinical stage and lymph node
metastasis (44), and could
predict the survival of patients who were unable to receive
chemotherapy after surgery (45).
Rinnerthaler et al (46)
reported that miR-222-3p expression was associated with the
progesterone receptor, and elevated miR-222-3p expression was
involved in breast cancer development, tumor spread, proliferation
and drug resistance. Moreover, miR-222-3p was found to be highly
associated with the tumor stage and lymph node metastasis in
estrogen receptor α (ERα)-negative patients with endometrial cancer
(EC), and lower miR-222-3p expression was detected in ERα-negative
EC with lower grades (P=0.0145) and earlier stages (I vs. II,
P=0.05; II vs. III, P=0.0043; I vs. III, P=0.0002) (26). By contrast, miR-222-3p
upregulation exhibited a positive association with overall survival
in patients with epithelial ovarian cancer (EOC), and its
expression level was negatively associated with tumor growth in an
EOC mouse model (37).
miR-222-3p serves as a specific biomarker for
various types of tumors in body fluids, such as serum, plasma and
urine (13,47,48) indicating that miR-222-3p has the
potential to be developed into a non-invasive diagnostic biomarker
for tumors. High expression levels of circulating miR-222-3p were
significantly associated with lymph node metastasis (P=0.009) and
clinical stages (P<0.001) in gastric cancer in an analysis of 38
plasma samples (49). Chang et
al (50) revealed that
miR-222-3p was negatively associated with clinical staging and
lymph node metastasis status in plasma samples collected from
patients with oral carcinoma, and miR-222-3p may be a useful
diagnostic biomarker for the differentiation of oral squamous cell
carcinoma and oral leukoplakia plaque. Furthermore, Fredsoe et
al (51) developed a
three-microRNA ratio model (miR-222-3p*/miR-24-3p/miR-30c-5p),
which provided accurate markers in the differential diagnosis of
benign prostatic hyperplasia and prostate cancer (PCa). Several
studies have found that serum miRNAs could be used to predict the
risk of non-muscle invasive bladder cancer, and risk scores were
generated according to the combination of the three miRNA ratios
(miR-29a-3p/miR-222-3-p, miR-150-5-p/miR-331-3p and
miR-409-3-p/miR-433-5-p) (52,53). Similar results were obtained in
PTC (38,54). As aforementioned, dysregulated
miR-222-3p expression has been observed in numerous types of human
cancer, thus providing powerful rationales for its application as a
non-invasive diagnostic biomarker.
As well as having a strong potential in the
diagnosis of cancer, miR-222-3p also serves an important role in
predicting the prognosis of patients with cancer. An increasing
number of studies has demonstrated that dysregulated miR-222-3p
expression can predict the progression and poor prognosis of
patients with cancer, including prostate cancer and papillary
thyroid carcinoma (30,31).
KRAS and BRAF are poor prognostic indicators used
for colorectal cancer (CRC; frequency of mutation in patients:
KRAS, 38-44.9%; BRAF, 4.2-5%); KRAS and BRAF mutations were
positively associated with a poor prognosis in patients with CRC
(57-59). Therefore, more sensitive
prognostic biomarkers are required for CRC. A panel of 16 miRNAs
(including miR-222-3p) associated with improved 5-year DFS time for
stage II and III CRC was identified (57). The signature was identified as an
independent prognostic factor for improved 5-year DFS time by
multivariate analyses (57).
Similar observations were reported in gastric carcinoma (45) and thyroid cancer (54). Additionally, elevated serum
miR-222-3p expression may significantly predict a poor probability
of 2-year DFS time in patients with glioblastoma (60). Notably, a novel logistic
regression model, comprising five urinary miRNAs (miR-151a-5p,
miR-204-5p, miR-222-3p, miR-23b-3p and miR-331-3p) and serum
prostate-specific antigen (PSA), was established successfully by
Fredsoe et al (32) and
could predict time to biochemical recurrence in 215 patients with
PCa (univariate Cox regression analysis HR, 3.12; P<0.001).
To investigate the prognostic value of miR-222-3p in
the survival of patients with various types of cancer, miR-222-3p
expression in human cancers was analyzed from The Cancer Genome
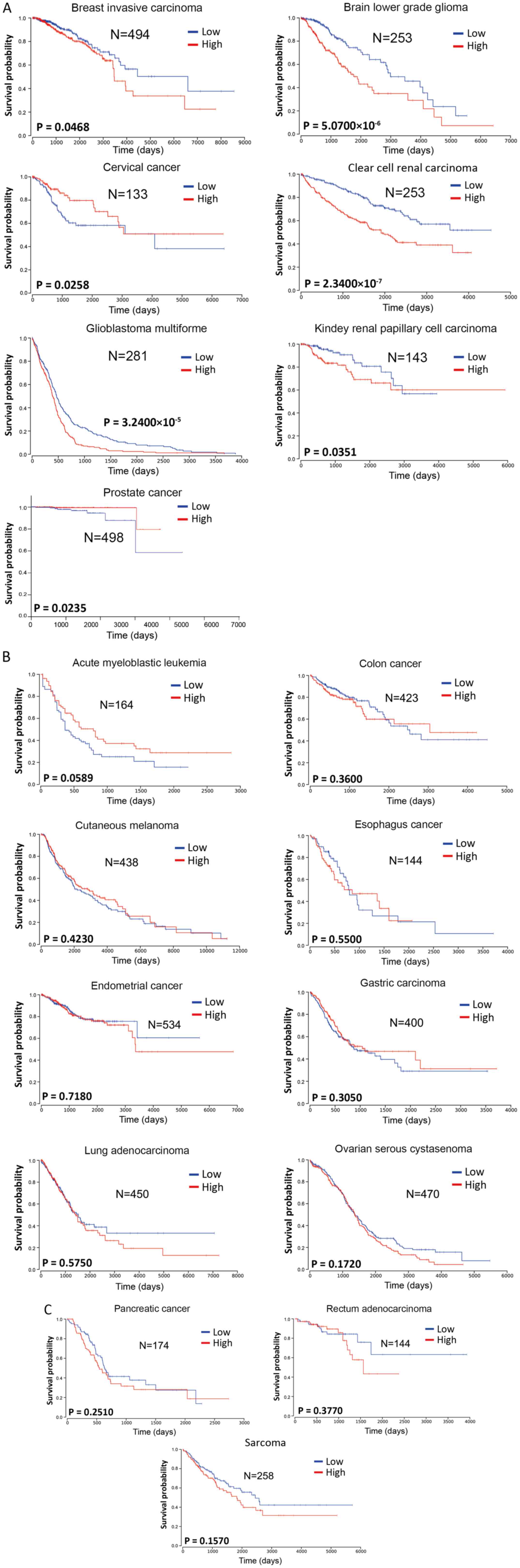
Atlas database (https://tcga-data.nci.nih.gov/tcga/). The Kaplan-Meier
analysis method was used for survival analysis using GraphPad Prism
7.00 (GraphPad Software, Inc.). Log-rank P<0.05 was considered
to indicate a statistically significant difference. Considering the
mid- and late-stage crossovers, the weighted method of Cramer-von
Mises testing was used. The patients were divided into two groups
according to the different expression levels of miR-222-3p in each
tumor type, either lower or higher than the mean value.
miR-222-3p expression was significantly associated
with the survival of patients with breast invasive carcinoma, brain
lower grade glioma, clear cell renal carcinomas, glioblastoma
multiforme or kidney renal papillary cell carcinoma (Fig. 1A), with high miR-222-3p expression
predicting a poorer overall survival compared with low miR-222-3p
expression. In 11 other types of cancer (acute myeloblastic
leukemia, colon cancer, cutaneous melanoma, esophageal cancer, EC,
gastric carcinoma, lung adenocarcinoma, ovarian serous cystadenoma,
pancreatic cancer, rectum adenocarcinoma and sarcoma), the
expression levels of miR-222-3p were not significantly associated
with overall survival (Fig. 1B and
C). Although higher miR-222-3p expression seemed to predict
longer overall survival in acute myeloblastic leukemia, there was
no significant difference between the two groups (P=0.0589;
Fig. 1B). Additionally, higher
miR-222-3p expression in cervical and prostate cancer predicted a
longer overall survival (Fig.
1A). Although these data may be affected by the sample size and
stability of the sequencing method in the miR-222-3p
quantification, the aforementioned data suggest that miR-222-3p
expression may exhibit prognostic value only in certain types of
human cancer.
Therapeutic resistance is a major risk factor for a
poor prognosis in tumor patients who undergo chemo- and
radio-therapy (61,62). A previous study indicated that
miR-222-3p increased raloxifene resistance through suppressing ERα
expression in EC cells (26).
This mechanism is also observed in the resistance to gemcitabine, a
nucleoside analogue with activity against NSCLC, with acquired
gemcitabine resistance being a major obstacle in NSCLC treatment
(63).
Aberrant miR-222-3p expression serves a crucial role
in numerous types of human tumors (64), and it is closely associated with
certain aspects of cancer biology, including tumorigenesis
(65-68). Recently, several reports have
indicated that miR-222-3p exhibited an oncogenic function,
including in CRC (18) and EC
(69). Moreover, miR-222-3p
expression drives cancer stem cell renewal in CRC, making it a
potential target for therapy (70). Helicobacter pylori
infection acts as a trigger in the carcinogenesis of gastric cancer
(71), and increasing studies
(53,72) suggest that H. pylori
affects miRNA expression. Tan et al (53) revealed that miR-222-3p expression
was markedly increased in the cancer group [H. pylori (+)]
compared with in the normal group [H. pylori (-)].
miR-222-3p associated with H. pylori targets
homeodomain-interacting protein kinases 2 (HIPK2) to promote cell
proliferation and invasion, and to inhibit apoptosis in gastric
cancer (72). Functional
experiments demonstrated that miR-222-3p overexpression
significantly enhanced the proliferative activity while inhibiting
the apoptosis of SGC7901 gastric cancer cells, but
miR-222-3p-knockdown exhibited the opposite effects (35). Consistent with this finding, an
in vivo experiment revealed that downregulated miR-222-3p
expression in AN3CA cells inhibited EC tumor growth in a mouse
xenograft model (26). By
contrast, Fu et al (37)
demonstrated that higher miR-222-3p expression was associated with
improved overall survival in patients with EOC, and its level was
negatively associated with tumor growth in vivo. Thus,
miR-222-3p may govern key processes during the development of
various types of cancer.
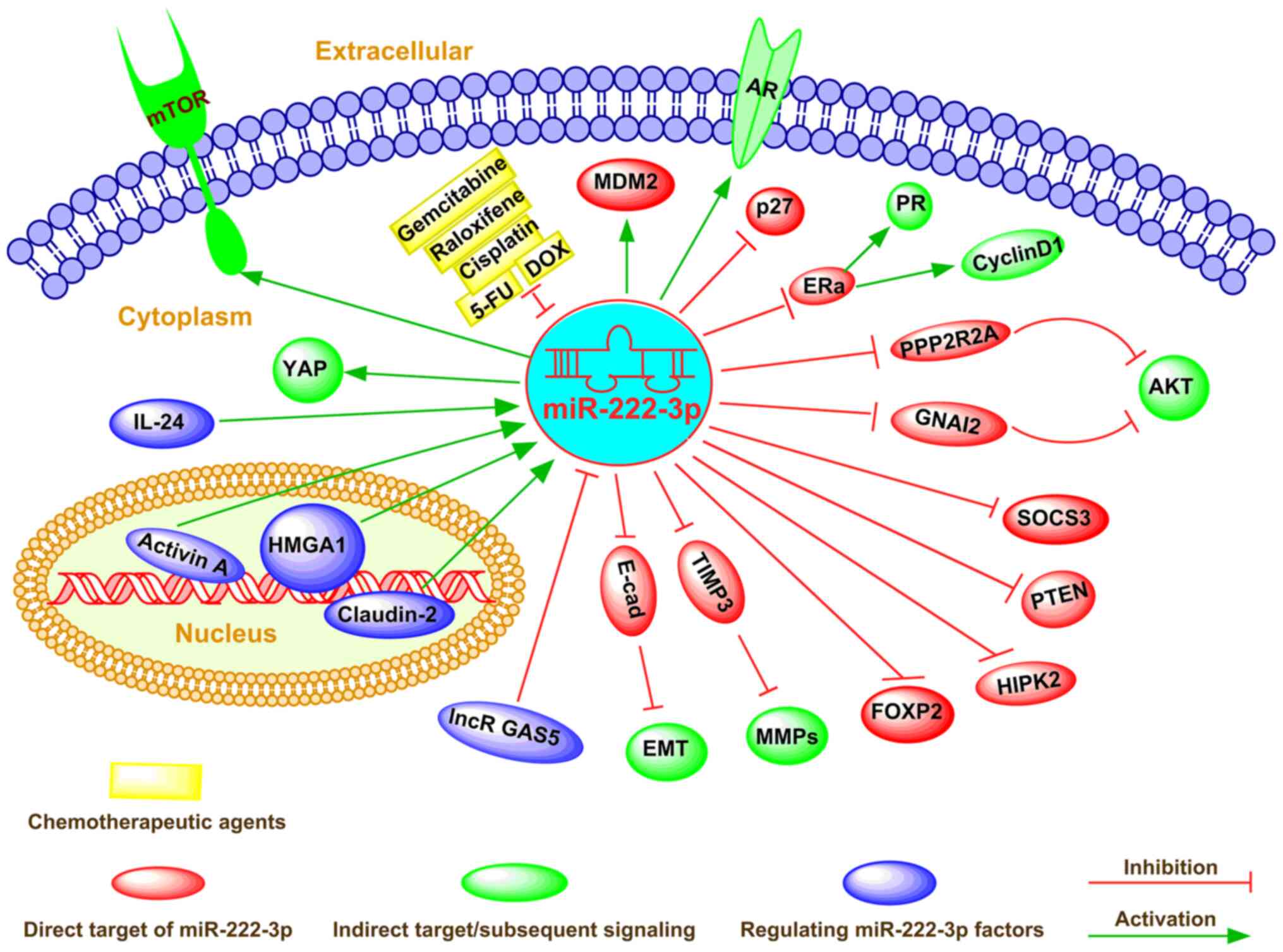
As a cellular regulator, miR-222-3p affects gene
expression via direct binding to complementary sequences in the
3′-UTR of target mRNAs in numerous types of cancer cells (73). miR-222-3p determines the
malignancy of cell proliferation or apoptosis in various types of
cancer, including breast cancer and colorectal carcinoma (74-76). The global regulatory mechanism of
miR-222-3p in determining the fate of cancer cells is shown in
Fig. 2.
In addition, it has been reported that
overexpression of miR-222-3p induces alteration of the cell cycle
(a high ratio of G1 to S phase) and promotes cell
proliferation (26). Conversely,
miR-222-3p-knockdown significantly decreases cell proliferation by
upregulating cyclin D1 (26). The
upregulation of miR-222-3p markedly stimulated cell proliferation
and repressed apoptosis by releasing endogenous IL-24 and
activating the phosphatidylinositol 3 (PI3K)/AKT signaling pathway
in lung cancer (77). Further
investigation indicated that activation of the PI3K/AKT signaling
pathway directly suppressed high mobility group AT-hook 1 (HMGA1)
expression, with the dysregulation of phosphatase and tensin
homology deleted on chromosome ten (PTEN) by miR-222-3p (77,78). By contrast, Coarfa et al
(17) identified a panel of 12
miRNAs (including miR-222-3p) with a proteomic footprint (using
reversed-phase proteomic arrays), and the expression levels of
these miRNAs were markedly decreased in metastatic PCa. This miRNA
panel significantly decreased cell proliferation and targeted key
tumor-associated signaling pathways involving the androgen receptor
axis and the Akt/mTOR signaling pathway (17). Additionally, other studies
revealed that miR-222-3p expression was decreased upon progression
to high-grade PCa (Gleason score 8-10 or PSA level >20 ng/ml;
clinical stage, T3a) (79-81).
A similar result was obtained in a study of PCa by Tong et
al (82). Ottley et al
(80) reported that
cyclin-dependent kinase inhibitor 1B expression was accompanied by
a decrease in miR-93, miR-222-3p and miR-18a expression in activin
A-treated prostate cancer LNCaP cells. Furthermore, a previous
study observed higher expression levels of miR-222-3p in
androgen-independent LNCaP cells than in LNCaP cells, indicating
its growth-promoting role in PCa (83). A small molecule inhibitor of
murine double minute 2 (MDM2), nutlin-3, selectively disrupted the
interaction between MDM2 and p53 (84). Moreover, genome-wide miRNA
expression analysis revealed that the expression levels of
miR-34a-5p, miR-182-5p, miR-203a, miR-222-3p and miR-432-5p were
upregulated following nutlin-3 treatment in a p53-dependent manner
(85). Notably, miR-222-3p
overexpression promoted apoptosis and suppressed proliferation in
neuroblastoma cells (85).
In cancer cells, different types of mutations may
mediate amplification or reduction of gene expression and lead to
altered protein expression patterns (86). Although miR-222-3p is hypothesized
to regulate specific genes, it may not affect some of its predicted
genes (26). The function of
miR-222-3p mainly affects cell fate-associated signaling
pathways.
Aberrant miRNA expression has been reported in
metastatic cancers, which universally display an aggressive
pathophysiology (20). Consistent
with this finding, miR-222-3p has been shown to be essential for
the invasion and metastasis of different types of cancer, including
osteosarcoma, endometrial carcinoma and prostate cancer (25,26,87) (Fig.
2).
The resistance of tumors to various anticancer drugs
is an important factor that increases the invasiveness and
metastasis of tumor cells (88,89), which depend on escaping apoptosis
and increasing drug efflux (24,90). Dysregulated miR-222-3p expression
may contribute to drug resistance by regulating gene expression
(37), the cell cycle and
apoptosis (26,37). Therefore, miR-222-3p is commonly
perceived to be responsive to cancer treatment and has been
emphasized as a new drug target (91) (Fig.
2).
miR-222-3p induces cisplatin resistance in EOC cells
by targeting the 3′-UTR of G protein a inhibiting activity
poly-peptide 2 (37).
Additionally, several studies (26,34,37) revealed that the upregulation of
miR-222-3p expression promoted cell survival in cisplatin-treated
ovarian cancer cells. In addition, miR-222-3p increased raloxifene
resistance via suppressing ERα expression in EC cells (26). Increased miR-222-3p expression
directly targeted the 3'-UTR of claudin-2 mRNA to decrease
apoptosis and induce CRC resistance to 5-fluo-rouracil (92). Transfection of miR-222-3p
inhibitor into doxycycline (DOX)-resistant colon cancer cells
(LoVo/ADR cells) decreased the expression levels of apoptotic
proteins, such as poly (ADP-ribose) polymerase (PARP) and caspase
3, and significantly increased the expression levels of typical
antiapoptotic proteins (BAX, cleaved PARP and cleaved caspase 3)
(24). These results indicate
that miR-222-3p may be a promising therapeutic target for
overcoming chemotherapy resistance in human cancer.
The tumor microenvironment serves an important role
in cancer initiation, progression and metastasis (93). The primary components of the tumor
micro-environment are fibroblasts, immune cells, endothelial cells,
extracellular matrix and cytokines (94,95). Among them, immune cells serve
vital roles in enhancing cancer progression by secreting numerous
proinflammatory factors (96).
According to previous studies (97-100), miR-222-3p can modulate the
function of immune cells, such as natural killer (NK) cells and
cancer-associated fibroblasts, and serve an important role in the
tumor microenvironment.
miR-222-3p serves various roles in the initiation,
progression, metastasis and treatment response of cancer, and its
expression can be dysregulated by multiple factors in human cancer
(104-106). For example, the Chinese
medicinal herb andrographolide was reported to inhibit hematoma
tumor growth by altering the miRNA profile (107). Mechanistically, miR-222-3p
expression can be regulated via both transcriptional factors and
epigenetic factor-induced mechanisms in cancer cells (70,108,109) (Fig. 2). Additionally, Ignacio et
al (110) elucidated that
several miRNA (including miR-222-3p) changes caused by ethanol were
reversed by social activity and caused a number of novel epigenetic
mechanisms; for example, prenatal alcohol exposure imposed a
long-lasting effect on neuronal and, ultimately, behavioral
function in adolescents.
miR-222-3p expression was significantly decreased
after activin A treatment in LNCaP cells (83). Recently, miR-222-3p was found to
be positively regulated by HMGA1, an architectural transcription
factor that participates in the biological progression of different
types of human cancer, including uveal melanoma and lung cancer
(33,109). Moreover, HMGA1 overexpression
exacerbated tumor progression by activating miR-222-3p via the
PI3K/Akt/MMP-9 signaling pathway in uveal melanoma (33). Consistent with this finding,
DOX-mediated IL-24 expression markedly decreased HMGA1 mRNA and
protein expression by downregulating miR-222-3p expression, which
resulted in a substantial increase in phosphatase 2A subunit B
expression and a concomitant decrease in phosphorylated
AKTT308/S473 expression (109).
In addition, small interfering (si)RNA-mediated knockdown of HMGA1
significantly decreased AKT T308/S473 protein expression and
markedly decreased cell migration and invasion by targeting
miR-222-3p in lung cancer (109), suggesting that HMGA1 siRNA or
miR-222-3p inhibitor may be used as effective treatments for lung
cancer. Paquet-Fifield et al (70) revealed that activation of
claudin-2 resulted in increased miR-222-3p expression, which
activated the Yes-associated protein and promoted CRC cell
self-renewal. In addition, the long non-coding RNA growth
arrest-specific 5 (GAS5) activated the PTEN/AKT signaling pathway
as a competing endogenous RNA of miR-222-3p in PTC (34). Coincidently, Liu et al
(18) demonstrated that lncRNA
GAS5 dramatically increased PTEN expression by decoying miR-222-3p,
thus inhibiting CRC cell migration and invasion, and promoting cell
autophagy.
Exosomes are membranous extracellular vesicles, with
a diameter of 30-100 nm, that are critical mediators of
intercellular communications (111,112). miRNAs, which may act as
post-transcriptional regulators of gene expression, have also been
identified in exosomes (113).
There is differential expression between cancer and normal exosomes
with specific onco-genic and tumor suppressive miRNAs, which may
provide diagnostic or prognostic potential of circulating exosomal
miRNAs in cancer (114).
Notably, Ostenfeld et al (27) isolated cancer-derived epithelial
cell adhesion molecule-positive-exosomes from the serum and plasma
of patients with CRC, and revealed that increased exosomal
miR-222-3p may be an effective marker in the early stage of CRC.
Higher miR-222-3p expression was also observed in serum-derived
exosomes from patients with EOC compared with in healthy
individuals (97). The
aforementioned studies suggest that miR-222-3p, alone or in
combination with a panel of other miRNAs, may serve as a
non-invasive biomarker in cancer diagnosis. Ryu et al
(105) reported that a panel of
five candidate miRNAs (miR-320e, miR-4454, miR-222-3p, miR-21-5p
and miR-25-3p) predicted poor survival outcomes in patients with
extranodal NK/T-cell lymphoma (ENKTL), indicating the prognostic
value of serum-derived exosomal miRNA profiles in patients with
ENKTL. Circulating exosomal miR-342-5p, miR-222-3p and miR-574-5p
have been reported to be the diagnostic and prognostic markers for
early-stage lung adenocarcinoma (108,115). Jiang et al (31) revealed that significantly
upregulated expression levels of miR-146b-5p and miR-222-3p from
plasma exosomes may be potential biomarkers for lymph node
metastasis in papillary thyroid carcinoma. Via high-throughput
microarrays, the dysregulated miRNAs in paired serum samples from
patients with breast cancer before and after surgery were screened,
and miR-222-3p was identified as an independent prognostic factor
for DFS (HR, 13.19; 95% CI, 1.06-163.59; P=0.045) (56). In addition, increased exosomal
miR-222-3p expression tended to predict a worse prognosis in
patients with NSCLC and exosomal miR-222-3p expression in serum may
be used as a potential prognostic biomarker for predicting
gemcitabine sensitivity in patients with NSCLC (16). Wei et al (16) revealed that gemcitabine-resistant
cells contributed to the development of NSCLC tumor malignancy via
exosome-mediated transfer of miR-222-3p. Moreover, exosome-derived
miR-222-3p enhanced the migration and invasion of
gemcitabine-resistant cells by directly targeting the promoter of
SOCS3 in NSCLC (16). Therefore,
gemcitabine-resistant A549 lung cancer cells could transmit their
malignant phenotype to gemcitabine-sensitive A549 parental cells
via exosome-derived miR-222-3p (16). In addition, HCV infection can
present as an acute manifestation and can cause various
complications, such as chronic hepatitis, liver fibrosis, cirrhosis
and HCC (98,99). Santangelo et al (100) recently revealed that HCV-derived
exosomes suppressed NK cell activity, and this is associated with
high miR-222-3p expression.
In studies of several types of tumors, the
expression levels and function of miR-222-3p exhibited opposite
results (Table I). These findings
may be due to the inconsistent quality of the studies. For example,
the use of miR-222-3p inhibitors and mimics should be further
confirmed in functional verification studies. There are no
high-quality controls for negative and positive products, which
strongly affects the consistency of the data. In future miR-222-3p
studies, the guidelines recommended for miRNA studies should be
applied (116-118). Although the biological functions
and mechanisms of miR-222-3p have been extensively studied
(Fig. 2), further studies on its
clinical application are required. miR-222-3p is widely involved in
the regulation of numerous cellular, physiological and pathological
processes. Additionally, miR-222-3p expression is aberrant during
cancer progression (34,109). Thus, miR-222-3p may be used as a
potential biomarker to predict cancer malignancy. Notably, it may
be possible to develop an innovative strategy to treat various
types of cancer by targeting miR-222-3p. miR-222-3p can directly or
indirectly regulate multiple downstream molecules, which are
involved in multiple tumor signaling pathways, including PI3K/AKT,
PTEN, JAK/STAT, TRPS1/ZEB1 and EMT, between which crosstalks
usually exist, thus constituting a complex signaling network.
Additionally, miR-222-3p is extensively involved in cancer cell
differentiation, proliferation, apoptosis, invasion, metastasis and
metabolism modulation via targeting gene expression at the
post-transcriptional level. Furthermore, miR-222-3p functions as
either a tumor suppressor or an oncogene in different types of
tumors, indicating its potential as a new target for cancer
treatment. Despite miR-222-3p being extensively involved in cancer
progression, numerous potential target mRNAs of miR-222-3p remain
to be identified, and its functions and mechanisms in tumor
metabolism and tumor immunity require to be further investigated.
Overall, an improved understanding of miR-222-3p and its mechanism
of action may provide research ideas for potentially developing a
novel therapeutic intervention for cancer treatment and an
increased overall survival rate.
It is well known that miRNAs are involved in the
development of cancer and may function as promising biomarkers for
early detection, diagnosis and prognosis. The present review
highlighted the scientific discoveries of miR-222-3p in human
cancer research and outlined the advances and challenges of
miR-222-3p as a diagnostic tool for cancer, as well as providing
biological and clinical insights on this topic. miR-222-3p
functions as an oncogene in some tumors and as a tumor suppressor
in others, suggesting that the function of miR-222-3p is tumor- and
cellular context-dependent. Its biological functions are involved
in the occurrence, progression, metastasis and drug resistance of
cancer, indicating its potential as a new target for cancer
treatment.
Not applicable.
DW, YS and TS wrote the manuscript. PK and YC
consulted relevant literature and completed English revision. TS,
LZ and YD completed the figures and tables. WL and ZT contributed
to conception and design of the framework. WL completed critical
revisions and proofread the manuscript. All authors have checked
all the raw data to ensure its legitimacy and have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by a grant from the National
Natural Science Foundation of China Youth Science Foundation
Project (grant no. 81802571) and the Zhejiang Medical and Health
Science and Technology Project (grant no. 2019RC039).
|
1
|
Orso F, Quirico L, Dettori D, Coppo R,
Virga F, Ferreira LC, Paoletti C, Baruffaldi D, Penna E and Taverna
D: Role of miRNAs in tumor and endothelial cell interactions during
tumor progression. Semin Cancer Biol. 60:214–224. 2020. View Article : Google Scholar
|
|
2
|
Iwakawa HO and Tomari Y: The functions of
MicroRNAs: mRNA decay and translational repression. Trends Cell
Biol. 25:651–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar :
|
|
6
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar
|
|
7
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chendrimada TP, Gregory RI, Kumaraswamy E,
Norman J, Cooch N, Nishikura K and Shiekhattar R: TRBP recruits the
Dicer complex to Ago2 for microRNA processing and gene silencing.
Nature. 436:740–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao JJ, Chu ZB, Hu Y, Lin J, Wang Z,
Jiang M, Chen M, Wang X, Kang Y, Zhou Y, et al: Targeting the
miR-221-222/PUMA/BAK/BAX pathway abrogates dexamethasone resistance
in multiple myeloma. Cancer Res. 75:4384–4397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang KW, Kao SY, Wu YH, Tsai MM, Tu HF,
Liu CJ, Lui MT and Lin SC: Passenger strand miRNA miR-31* regulates
the phenotypes of oral cancer cells by targeting RhoA. Oral Oncol.
49:27–33. 2013. View Article : Google Scholar
|
|
11
|
Ogawa T, Enomoto M, Fujii H, Sekiya Y,
Yoshizato K, Ikeda K and Kawada N: MicroRNA-221/222 upregulation
indicates the activation of stellate cells and the progression of
liver fibrosis. Gut. 61:1600–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasmeen S, Kaur S, Mirza AH, Brodin B,
Pociot F and Kruuse C: miRNA-27a-3p and miRNA-222-3p as novel
modulators of phosphodiesterase 3a (PDE3A) in cerebral
microvascular endothelial cells. Mol Neurobiol. 56:5304–5314. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gulluoglu S, Tuysuz EC, Kuskucu A, Ture U,
Atalay B, Sahin F and Bayrak OF: The potential function of microRNA
in chordomas. Gene. 585:76–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Liu Z, Ma L, Pei C, Qin L, Gao N, Li
J and Yin Y: MiRNAs regulate oxidative stress related genes via
binding to the 3'UTR and TATA-box regions: A new hypothesis for
cataract pathogenesis. BMC Ophthalmol. 17:2–8. 2017. View Article : Google Scholar
|
|
15
|
Verjans R, Peters T, Beaumont FJ, van
Leeuwen R, van Herwaarden T, Verhesen W, Munts C, Bijnen M, Henkens
M, Diez J, et al: MicroRNA-221/222 family counteracts myocardial
fibrosis in pressure overload-induced heart failure. Hypertension.
71:280–288. 2018. View Article : Google Scholar
|
|
16
|
Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng
L, Ding L, Zhang Y, Zhang L, Li N, et al: Exosomes derived from
gemcitabine-resistant cells transfer malignant phenotypic traits
via delivery of miRNA-222-3p. Mol Cancer. 16:132–147. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coarfa C, Fiskus W, Eedunuri VK,
Rajapakshe K, Foley C, Chew SA, Shah SS, Geng C, Shou J, Mohamed
JS, et al: Comprehensive proteomic profiling identifies the
androgen receptor axis and other signaling pathways as targets of
microRNAs suppressed in metastatic prostate cancer. Oncogene.
35:2345–2356. 2016. View Article : Google Scholar
|
|
18
|
Liu L, Wang HJ, Meng T, Lei C, Yang XH,
Wang QS, Jin B and Zhu JF: lncRNA GAS5 inhibits cell migration and
invasion and promotes autophagy by targeting miR-222-3p via the
GAS5/PTEN-signaling pathway in CRC. Mol Ther Nucleic Acids.
17:644–656. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jafri MA, Al-Qahtani MH and Shay JW: Role
of miRNAs in human cancer metastasis: Implications for therapeutic
intervention. Semin Cancer Biol. 44:117–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alves Dos Santos K, Clemente Dos Santos
IC, Santos Silva C, Gomes Ribeiro H, de Farias Domingos I and
Nogueira Silbiger V: Circulating exosomal miRNAs as biomarkers for
the diagnosis and prognosis of Colorectal Cancer. 22:3462020.
|
|
22
|
Fong M, Yan W, Ghassemian M, Wu X, Zhou X,
Cao M, Jiang L, Wang J, Liu X, Zhang J and Wang SJ: Cancer-secreted
miRNAs regulate amino-acid-induced mTORC1 signaling and fibroblast
protein synthesis. EMBO Rep. 22:e512392020.PubMed/NCBI
|
|
23
|
Wang X, Liao X, Huang K, Zeng X, Liu Z,
Zhou X, Yu T, Yang C, Yu L, Wang Q, et al: Clustered microRNAs
hsa-miR-221-3p/hsa-miR-222-3p and their targeted genes might be
prognostic predictors for hepatocellular carcinoma. J Cancer.
10:2520–2533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Deng Z, Chen X, Cai J, Ma T, Zhong
Q, Li R, Li L and Li T: Downregulation of miR-222-3p reverses
doxorubicin-resistance in LoVo cells through upregulating forkhead
box protein P2 (FOXP2) protein. Med Sci Monit. 25:2169–2178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo J, Liu Q, Li Z, Guo H, Bai C and Wang
F: miR-222-3p promotes osteosarcoma cell migration and invasion
through targeting TIMP3. Onco Targets Ther. 11:8643–8653. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu B, Che Q, Qiu H, Bao W, Chen X, Lu W,
Li B and Wan X: Elevated MiR-222-3p promotes proliferation and
invasion of endometrial carcinoma via targeting ERalpha. PLoS One.
9:e875632014. View Article : Google Scholar
|
|
27
|
Ostenfeld MS, Jensen SG, Jeppesen DK,
Christensen LL, Thorsen SB, Stenvang J, Hvam ML, Thomsen A,
Mouritzen P, Rasmussen MH, et al: miRNA profiling of circulating
EpCAM(+) extracellular vesicles: Promising biomarkers of colorectal
cancer. J Extracell Vesicles. 5:3402–3417. 2016. View Article : Google Scholar
|
|
28
|
Korabecna M, Koutova L and Tesarova P: The
potential roles of vesicle-enclosed miRNAs in communication between
macrophages and cancer cells in tumor microenvironment. Neoplasma.
64:406–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gasparello J, Papi C, Allegretti M,
Giordani E, Carboni F, Zazza S, Pescarmona E, Romania P, Giacomini
P, Scapoli C, et al: A distinctive microRNA (miRNA) signature in
the blood of colorectal cancer (CRC) patients at surgery. Cancers
(Basel). 12:24102020. View Article : Google Scholar
|
|
30
|
Pudova E, Krasnov G, Nyushko K,
Kobelyatskaya A, Savvateeva M, Poloznikov A, Dolotkazin D, Klimina
K, Guvatova Z, Simanovsky S, et al: miRNAs expression signature
potentially associated with lymphatic dissemination in locally
advanced prostate cancer. BMC Med Genomics. 13(Suppl 8): S1292020.
View Article : Google Scholar
|
|
31
|
Jiang K, Li G, Chen W, Song L, Wei T, Li
Z, Gong R, Lei J, Shi H and Zhu J: Plasma exosomal miR-146b-5p and
miR-222-3p are potential biomarkers for lymph node metastasis in
papillary thyroid carcinomas. Onco Targets Ther. 13:1311–1319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fredsoe J, Rasmussen AKI, Mouritzen P,
Borre M, Orntoft T and Sorensen KD: A five-microRNA model (pCaP)
for predicting prostate cancer aggressiveness using cell-free
urine. Int J Cancer. 145:2558–2567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng Y, Cheng T, Zhao Y and Qu Y: HMGA1
exacerbates tumor progression by activating miR-222 through
PI3K/Akt/MMP-9 signaling pathway in uveal melanoma. Cell Signal.
63:52019. View Article : Google Scholar
|
|
34
|
Zhang XF, Ye Y and Zhao SJ: LncRNA Gas5
acts as a ceRNA to regulate PTEN expression by sponging miR-222-3p
in papillary thyroid carcinoma. Oncotarget. 9:3519–3530. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan X, Tang H, Bi J, Li N and Jia Y:
MicroRNA-222-3p associated with Helicobacter pylori targets HIPK2
to promote cell proliferation, invasion, and inhibits apoptosis in
gastric cancer. J Cell Biochem. 119:5153–5162. 2018. View Article : Google Scholar
|
|
36
|
Ma S, Kong S, Gu X, Xu Y, Tao M, Shen L,
Shen X and Ju S: As a biomarker for gastric cancer, circPTPN22
regulates the progression of gastric cancer through the EMT
pathway. Cancer Cell Int. 21:442021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu X, Li Y, Alvero A, Li J, Wu Q, Xiao Q,
Peng Y, Hu Y, Li X, Yan W, et al: MicroRNA-222-3p/GNAI2/AKT axis
inhibits epithelial ovarian cancer cell growth and associates with
good overall survival. Oncotarget. 7:80633–80654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rosignolo F, Memeo L, Monzani F, Colarossi
C, Pecce V, Verrienti A, Durante C, Grani G, Lamartina L, Forte S,
et al: MicroRNA-based molecular classification of papillary thyroid
carcinoma. Int J Oncol. 50:1767–1777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Fazio P, Montalbano R, Neureiter D,
Alinger B, Schmidt A, Merkel AL, Quint K and Ocker M:
Downregulation of HMGA2 by the pan-deacetylase inhibitor
panobinostat is dependent on hsa-let-7b expression in liver cancer
cell lines. Exp Cell Res. 318:1832–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jahanbani I, Al-Abdallah A, Ali RH,
Al-Brahim N and Mojiminiyi O: Discriminatory miRNAs for the
management of papillary thyroid carcinoma and noninvasive
follicular thyroid neoplasms with papillary-like nuclear features.
Thyroid. 28:319–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Denaro M, Ugolini C, Poma AM, Borrelli N,
Materazzi G, Piaggi P, Chiarugi M, Miccoli P, Vitti P and Basolo F:
Differences in miRNA expression profiles between wild-type and
mutated NIFTPs. Endocr Relat Cancer. 24:543–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Borrelli N, Denaro M, Ugolini C, Poma AM,
Miccoli M, Vitti P, Miccoli P and Basolo F: miRNA expression
profiling of 'noninvasive follicular thyroid neoplasms with
papillary-like nuclear features' compared with adenomas and
infiltrative follicular variants of papillary thyroid carcinomas.
Mod Pathol. 30:39–51. 2017. View Article : Google Scholar
|
|
43
|
de Conti A, Ortega JF, Tryndyak V, Dreval
K, Moreno FS, Rusyn I, Beland FA and Pogribny IP: MicroRNA
deregulation in nonalcoholic steatohepatitis-associated liver
carcinogenesis. Oncotarget. 8:88517–88528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim BH, Hong SW, Kim A, Choi SH and Yoon
SO: Prognostic implications for high expression of oncogenic
microRNAs in advanced gastric carcinoma. J Surg Oncol. 107:505–510.
2013. View Article : Google Scholar
|
|
45
|
Zhang L, Huang Z, Zhang H, Zhu M, Zhu W,
Zhou X and Liu P: Prognostic value of candidate microRNAs in
gastric cancer: A validation study. Cancer Biomark. 18:221–230.
2017. View Article : Google Scholar
|
|
46
|
Rinnerthaler G, Hackl H, Gampenrieder SP,
Hamacher F, Hufnagl C, Hauser-Kronberger C, Zehentmayr F, Fastner
G, Sedlmayer F, Mlineritsch B and Greil R: miR-16-5p is a
stably-expressed house-keeping MicroRNA in breast cancer tissues
from primary tumors and from metastatic sites. Int J Mol Sci.
17:156–167. 2016. View Article : Google Scholar
|
|
47
|
Fredsoe J, Rasmussen AKI, Thomsen AR,
Mouritzen P, Hoyer S, Borre M, Orntoft TF and Sorensen KD:
Diagnostic and prognostic MicroRNA biomarkers for prostate cancer
in cell-free urine. Eur Urol Focus. 4:825–833. 2018. View Article : Google Scholar
|
|
48
|
Fang R, Zhu Y, Hu L, Khadka VS, Ai J, Zou
H, Ju D, Jiang B, Deng Y and Hu X: Plasma MicroRNA pair panels as
novel biomarkers for detection of early stage breast cancer. Front
Physiol. 9:1879–1880. 2018. View Article : Google Scholar
|
|
49
|
Fu Z, Qian F, Yang X, Jiang H, Chen Y and
Liu S: Circulating miR-222 in plasma and its potential diagnostic
and prognostic value in gastric cancer. Med Oncol. 31:164–175.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang YA, Weng SL, Yang SF, Chou CH, Huang
WC, Tu SJ, Chang TH, Huang CN, Jong YJ and Huang HD: A
Three-MicroRNA signature as a potential biomarker for the early
detection of oral cancer. Int J Mol Sci. 19:7582018. View Article : Google Scholar
|
|
51
|
Fredsoe J, Rasmussen AKI, Laursen EB, Cai
Y, Howard KA, Pedersen BG, Borre M, Mouritzen P, Orntoft T and
Sorensen KD: Independent validation of a diagnostic noninvasive
3-MicroRNA ratio model (uCaP) for prostate cancer in cell-free
urine. Clin Chem. 65:540–548. 2019. View Article : Google Scholar
|
|
52
|
Uchino K, Takeshita F, Takahashi RU,
Kosaka N, Fujiwara K, Naruoka H, Sonoke S, Yano J, Sasaki H, Nozawa
S, et al: Therapeutic effects of microRNA-582-5p and -3p on the
inhibition of bladder cancer progression. Mol Ther. 21:610–619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tan X, Tang H, Bi J, Li N and Jia Y:
MicroRNA-222-3p associated with Helicobacter pylori targets HIPK2
to promote cell proliferation, invasion, and inhibits apoptosis in
gastric cancer. J Cell Biochem. 119:5153–5162. 2018. View Article : Google Scholar
|
|
54
|
Rosignolo F, Sponziello M, Giacomelli L,
Russo D, Pecce V, Biffoni M, Bellantone R, Lombardi CP, Lamartina
L, Grani G, et al: Identification of thyroid-associated serum
microRNA profiles and their potential use in thyroid cancer
follow-up. J Endocr Soc. 1:3–13. 2017.PubMed/NCBI
|
|
55
|
Ulivi P, Petracci E, Marisi G, Baglivo S,
Chiari R, Billi M, Canale M, Pasini L, Racanicchi S, Vagheggini A,
et al: Prognostic role of circulating miRNAs in Early-stage
non-small cell lung cancer. J Clin Med. 8:131–142. 2019. View Article : Google Scholar :
|
|
56
|
Wang Y, Yin W, Lin Y, Yin K, Zhou L, Du Y,
Yan T and Lu J: Downregulated circulating microRNAs after surgery:
Potential noninvasive biomarkers for diagnosis and prognosis of
early breast cancer. Cell Death Discov. 4:2–8. 2018. View Article : Google Scholar
|
|
57
|
Kara M, Yumrutas O, Ozcan O, Celik OI,
Bozgeyik E, Bozgeyik I and Tasdemir S: Differential expressions of
cancer-associated genes and their regulatory miRNAs in colorectal
carcinoma. Gene. 567:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Spindler KL, Pallisgaard N, Vogelius I and
Jakobsen A: Quantitative cell-free DNA, KRAS, and BRAF mutations in
plasma from patients with metastatic colorectal cancer during
treatment with cetuximab and irinotecan. Clin Cancer Res.
18:1177–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Domingo E, Camps C, Kaisaki PJ, Parsons
MJ, Mouradov D, Pentony MM, Makino S, Palmieri M, Ward RL, Hawkins
NJ, et al: Mutation burden and other molecular markers of prognosis
in colorectal cancer treated with curative intent: Results from the
QUASAR 2 clinical trial and an Australian community-based series.
Lancet Gastroenterol Hepatol. 3:635–643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao H, Shen J, Hodges TR, Song R, Fuller
GN and Heimberger AB: Serum microRNA profiling in patients with
glioblastoma: A survival analysis. Mol Cancer. 16:59–70. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cooper J and Giancotti FG: Integrin
signaling in cancer: Mechanotransduction, stemness, epithelial
plasticity, and therapeutic resistance. Cancer Cell. 35:347–367.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Friedmann Angeli JP, Krysko DV and Conrad
M: Ferroptosis at the crossroads of cancer-acquired drug resistance
and immune evasion. Nat Rev Cancer. 19:405–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tooker P, Yen WC, Ng SC, Negro-Vilar A and
Hermann TW: Bexarotene (LGD1069, Targretin), a selective retinoid X
receptor agonist, prevents and reverses gemcitabine resistance in
NSCLC cells by modulating gene amplification. Cancer Res.
67:4425–4433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Garofalo M, Romano G, Di Leva G, Nuovo G,
Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al:
EGFR and MET receptor tyrosine kinase-altered microRNA expression
induces tumorigenesis and gefitinib resistance in lung cancers. Nat
Med. 18:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang
Y, Liu Z, Cao X, Chen P, Chen X, et al: A microRNA 221- and
222-mediated feedback loop maintains constitutive activation of
NFκB and STAT3 in colorectal cancer cells. Gastroenterology.
147:847–859.e11. 2014. View Article : Google Scholar
|
|
68
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li Z, Yu Z, Meng X, Zhou S, Xiao S, Li X,
Liu S and Yu P: Long noncoding RNA GAS5 impairs the proliferation
and invasion of endometrial carcinoma induced by high glucose via
targeting miR-222-3p/p27. Am J Transl Res. 11:2413–2421.
2019.PubMed/NCBI
|
|
70
|
Paquet-Fifield S, Koh SL, Cheng L, Beyit
LM, Shembrey C, Molck C, Behrenbruch C, Papin M, Gironella M,
Guelfi S, et al: Tight junction protein Claudin-2 promotes
Self-renewal of human colorectal cancer Stem-like cells. Cancer
Res. 78:2925–2938. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Polk DB and Peek RM Jr: Helicobacter
pylori: Gastric cancer and beyond. Nat Rev Cancer. 10:403–414.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ishiguro H, Kimura M and Takeyama H: Role
of microRNAs in gastric cancer. World J Gastroenterol.
20:5694–5699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma
X, Coppola D and Cheng JQ: MicroRNA-221/222 negatively regulates
estrogen receptor alpha and is associated with tamoxifen resistance
in breast cancer. J Biol Chem. 291:31079–31086. 2016. View Article : Google Scholar
|
|
75
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun K, Wang W, Zeng JJ, Wu CT, Lei ST and
Li GX: MicroRNA-221 inhibits CDKN1C/p57 expression in human
colorectal carcinoma. Acta Pharmacol Sin. 32:375–384. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Y, Ma T, Yang S, Xia M, Xu J, An H,
Yang Y and Li S: High-mobility group A1 proteins enhance the
expression of the oncogenic miR-222 in lung cancer cells. Mol Cell
Biochem. 357:363–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ying SY, Chang DC, Miller JD and Lin SL:
The microRNA: Overview of the RNA gene that modulates gene
functions. Methods Mol Biol. 342:1–18. 2006.PubMed/NCBI
|
|
79
|
Fuse M, Kojima S, Enokida H, Chiyomaru T,
Yoshino H, Nohata N, Kinoshita T, Sakamoto S, Naya Y, Nakagawa M,
et al: Tumor suppressive microRNAs (miR-222 and miR-31) regulate
molecular pathways based on microRNA expression signature in
prostate cancer. J Hum Genet. 57:691–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ottley EC, Nicholson HD and Gold EJ:
Activin A regulates microRNAs and gene expression in LNCaP cells.
Prostate. 76:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tong AW, Fulgham P, Jay C, Chen P, Khalil
I, Liu S, Senzer N, Eklund AC, Han J and Nemunaitis J: MicroRNA
profile analysis of human prostate cancers. Cancer Gene Ther.
16:206–216. 2009. View Article : Google Scholar
|
|
82
|
Ottley EC, Nicholson HD and Gold EJ:
Activin A regulates microRNAs and gene expression in LNCaP cells.
Prostate. 76:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu G, Wu J, Zhou L, Chen B, Sun Z, Zhao F
and Tao Z: Characterization of the small RNA transcriptomes of
androgen dependent and independent prostate cancer cell line by
deep sequencing. PLoS One. 5:e155192010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rihani A, Van Goethem A, Ongenaert M, De
Brouwer S, Volders PJ, Agarwal S, De Preter K, Mestdagh P, Shohet
J, Speleman F, et al: Genome wide expression profiling of p53
regulated miRNAs in neuroblastoma. Sci Rep. 5:9027–9044. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Narrandes S and Xu W: Gene expression
detection assay for cancer clinical use. J Cancer. 9:2249–2265.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu W, Wang X, Wang Y, Dai Y, Xie Y, Ping
Y, Yin B, Yu P, Liu Z, Duan X, et al: SGK1 inhibition-induced
autophagy impairs prostate cancer metastasis by reversing. EMT J
Exp Clin Cancer Res. 37:732018. View Article : Google Scholar
|
|
88
|
Liu W, Wang X, Liu Z, Wang Y, Yin B, Yu P,
Duan X, Liao Z, Chen Y, Liu C, et al: SGK1 inhibition induces
autophagy-dependent apoptosis via the mTOR-Foxo3a pathway. Br J
Cancer. 117:1139–1153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mavrogiannis A, Kokkinopoulou I, Kontos C
and Sideris DJ: Effect of vinca alkaloids on the expression levels
of microRNAs targeting apoptosis-related genes in breast cancer
cell lines. Curr Pharm Biotechnol. 19:1076–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jacob H, Stanisavljevic L, Storli KE,
Hestetun KE, Dahl O and Myklebust MP: Identification of a
sixteen-microRNA signature as prognostic biomarker for stage II and
III colon cancer. Oncotarget. 8:87837–87847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tan HY, Wang N, Lam W, Guo W, Feng Y and
Cheng YC: Targeting tumour microenvironment by tyrosine kinase
inhibitor. Mol Cancer. 17:43–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Vuong L, Kotecha RR, Voss MH and Hakimi
AA: Tumor microenvironment dynamics in clear-cell renal cell
carcinoma. Cancer Discov. 9:1349–1357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vitale I, Manic G, Coussens LM, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhou S, Liu R, Yuan K, Yi T, Zhao X, Huang
C and Wei Y: Proteomics analysis of tumor microenvironment:
Implications of metabolic and oxidative stresses in tumorigenesis.
Mass Spectrom Rev. 32:267–311. 2013. View Article : Google Scholar
|
|
96
|
Ying X, Wu Q, Wu X, Zhu Q and Wang X,
Jiang L, Chen X and Wang X: Epithelial ovarian cancer-secreted
exosomal miR-222-3p induces polarization of tumor-associated
macro-phages. Oncotarget. 7:43076–43087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cabibbo G, Celsa C, Calvaruso V, Petta S,
Cacciola I, Cannavo MR, Madonia S, Rossi M, Magro B, Rini F, et al:
Direct-acting antivirals after successful treatment of early
hepatocellular carcinoma improve survival in HCV-cirrhotic
patients. J Hepatol. 71:265–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Degasperi E, D'Ambrosio R, Iavarone M,
Sangiovanni A, Aghemo A, Soffredini R, Borghi M, Lunghi G, Colombo
M and Lampertico P: Factors associated with increased risk of de
novo or recurrent hepatocellular carcinoma in patients with
cirrhosis treated with direct-acting antivirals for HCV infection.
Clin Gastroenterol Hepatol. 17:1183–1191.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Santangelo L, Bordoni V, Montaldo C,
Cimini E, Zingoni A, Battistelli C, D'Offizi G, Capobianchi MR,
Santoni A, Tripodi M and Agrati C: Hepatitis C virus direct-acting
antivirals therapy impacts on extracellular vesicles microRNAs
content and on their immunomodulating properties. Liver Int.
38:1741–1750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Varchetta S, Mele D, Mantovani S, Oliviero
B, Cremonesi E, Ludovisi S, Michelone G, Alessiani M, Rosati R,
Montorsi M and Mondelli MU: Impaired intrahepatic natural killer
cell cytotoxic function in chronic hepatitis C virus infection.
Hepatology. 56:841–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
van der Meer AJ, Feld JJ, Hofer H, Almasio
PL, Calvaruso V, Fernandez-Rodriguez CM, Aleman S, Ganne-Carrie N,
D'Ambrosio R, Pol S, et al: Risk of cirrhosis-related complications
in patients with advanced fibrosis following hepatitis C virus
eradication. J Hepatol. 66:485–493. 2017. View Article : Google Scholar
|
|
102
|
Fugier E, Marche H, Thélu MA, Macek
Jilková Z, Van Campenhout N, Dufeu-Duchesne T, Leroy V, Zarski JP,
Sturm N, Marche PN and Jouvin-Marche E: Functions of liver natural
killer cells are dependent on the severity of liver inflammation
and fibrosis in chronic hepatitis C. PLoS One. 9:e956142014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tölle A, Jung K, Friedersdorff F, Maxeiner
A, Lein M, Fendler A and Stephan C: The discriminative ability of
Prostate Health Index to detect prostate cancer is enhanced in
combination with miR-222-3p. Cancer Biomark. Dec 15–2020.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ryu K, Lee J, Choi M, Yoon S, Cho J, Ko Y,
Shim J, Kim W, Park C and Kim SJ: Serum-derived exosomal MicroRNA
profiles can predict poor survival outcomes in patients with
extranodal natural Killer/T-cell lymphoma. Cancers (Basel).
12:35482020. View Article : Google Scholar
|
|
105
|
Zhai S, Xu Z, Xie J, Zhang J, Wang X, Peng
C, Li H, Chen H, Shen B and Deng X: Epigenetic silencing of LncRNA
LINC00261 promotes c-myc-mediated aerobic glycolysis by regulating
miR-222-3p/HIPK2/ERK axis and sequestering IGF2BP1. Oncogene.
40:277–291. 2021. View Article : Google Scholar :
|
|
106
|
Lu B, Sheng Y, Zhang J, Zheng Z and Ji L:
The altered microRNA profile in andrographolide-induced inhibition
of hepatoma tumor growth. Gene. 588:124–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gumbiner BM and Kim NG: The Hippo-YAP
signaling pathway and contact inhibition of growth. J Cell Sci.
127:709–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Panneerselvam J, Srivastava A,
Muralidharan R, Wang Q, Zheng W, Zhao L, Chen A, Zhao YD, Munshi A
and Ramesh R: IL-24 modulates the high mobility group (HMG)
A1/miR222/AKT signaling in lung cancer cells. Oncotarget.
7:70247–70263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ignacio C, Mooney SM and Middleton FA:
Effects of acute prenatal exposure to ethanol on microRNA
expression are ameliorated by social enrichment. Front Pediatr.
2:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pant S, Hilton H and Burczynski ME: The
multifaceted exosome: Biogenesis, role in normal and aberrant
cellular function, and frontiers for pharmacological and biomarker
opportunities. Biochem Pharmacol. 83:1484–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Han Z, Li Y, Zhang J, Guo C, Li Q, Zhang
X, Lan Y, Gu W, Xing Z, Liang L, et al: Tumor-derived circulating
exosomal miR-342-5p and miR-574-5p as promising diagnostic
biomarkers for early-stage Lung Adenocarcino. Int J Med Sci.
17:1428–1438. 2020. View Article : Google Scholar :
|
|
115
|
Ortega MM and Bouamar H: Guidelines on
designing MicroRNA sponges: From construction to stable cell line.
Methods Mol Biol. 1509:221–233. 2017. View Article : Google Scholar
|
|
116
|
Wang Z: The guideline of the design and
validation of MiRNA mimics. Methods Mol Biol. 676:211–223. 2011.
View Article : Google Scholar
|
|
117
|
Arroyo J, Gallichotte E and Tewari M:
Systematic design and functional analysis of artificial microRNAs.
Nucleic Acids Res. 42:6064–6077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ganju A, Khan S, Hafeez BB, Behrman SW,
Yallapu MM, Chauhan SC and Jaggi M: miRNA nanotherapeutics for
cancer. Drug Discov Today. 22:424–432. 2017. View Article : Google Scholar :
|
|
119
|
Chen Y, Gao DY and Huang L: In vivo
delivery of miRNAs for cancer therapy: challenges and strategies.
Adv Drug Deliv Rev. 81:128–141. 2015. View Article : Google Scholar
|
|
120
|
Bofill-De Ros X and Gu S: Guidelines for
the optimal design of miRNA-based shRNAs. Methods. 103:157–166.
2016. View Article : Google Scholar : PubMed/NCBI
|