1. Introduction
Nuclear protein-1 (NUPR1; also known as Com-1 or p8)
was initially discovered in 1997 and expressed as a small protein
in the rat and as a novel gene that is activated in the acute phase
of induced pancreatitis and during pancreatic development (1). The molecule, then named p8 was shown
to be related to cell death and gene transcription. During the
following 2 years, the same study group discovered the human
version of p8, which shares a 74% similarity with rat p8 (1,2).
Independently, in the same year, a second group discovered a new
molecule in their search for candidate gene(s) associated with
brain metastasis of breast cancer (3). In metastatic brain tumours, the
expression of one gene was found to be elevated, compared with the
parent breast cancer, and was named candidate of metastasis-1 or
Com-1. It was soon found that these molecules were identical. In
2003, Quirk et al isolated a gene clone from pituitary
derived cells and found it to encode p8 (4). These findings have since triggered
active research into the role of this protein in cancer and other
pathological conditions, such as pancreatitis. NUPR-2 or NUPR1-like
protein was then identified (5),
which appears antagonistic to some degree, to the function of
NUPR1. Together, NUPRs indicate a fascinating area of research. The
present review aimed to summarise the progress in studies on NUPR1,
primarily in cancer and to a limited degree, in other pathological
conditions.
2. Cellular function of NUPR1
There are some excellent reviews available in the
literature which provide detailed knowledge of the molecular and
cellular function of NUPR1 at the beginning of this decade
(6,7). The present review describes the
advancements made in the understanding of NUPR1 in various cancer
types, and some benign disorders, which have taken place since, and
summarizes the key functions of NUPR1in cells, as well as
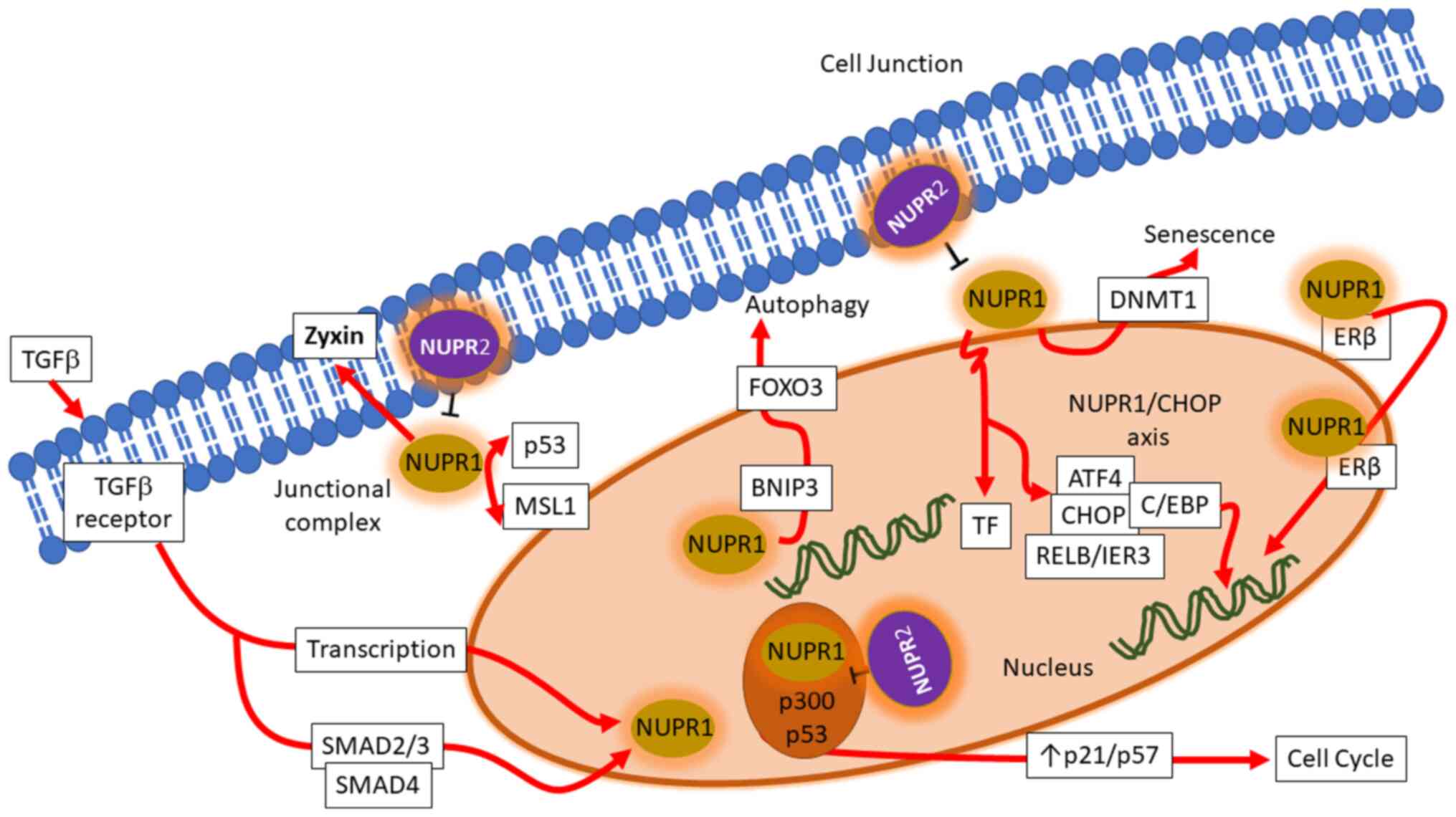
associated genetic interactions, which are illustrated in Fig. 1.
Transcriptional regulations of NUPR1
NUPR1 is a transcription regulator protein with its
gene located on chromosome 16. It is typically expressed in
response to stress signals induced by genotoxic signals and agents.
Transforming growth factor β (TGFβ) is an important regulator of
NUPR1 transcription. TGFβ, upon binding to its receptor, initiates
a canonical cascade, in which phosphorylated small mothers against
decapentaplegic (SMAD)-2/3 proteins form a heteromeric complex with
cofactor SMAD-4 and translocates into the nucleus. By binding on to
the promotor at the 5′-untranslated region (5′-UTR), it elevates
the transcription of NUPR1 through binding, a regulation appearing
at a rapid pace (8).
DNA damage and repair
NUPR1 influences cancer cell resistance to metabolic
stress-induced glucose starvation and hypoxia through the
downstream regulation of Aurora kinase A (AURKA) expression. The
inhibition of AURKA triggers a cytotoxin that can lead to DNA
damage (9). NUPR1 is also
important in γ-irradiation-induced damage and repair (10). It negatively controls DNA repair
following γ-irradiation in the presence of MSL complex subunit 1
(MSL1) by regulating histone acetyltransferase (HAT) activity and
is involved in intercommunication with p53 binding protein (P53BP1)
(10). It has also been shown
that the inhibition of NUPR1 by the organic synthetic molecule,
ZZW-115, sensitizes cells to DNA damage, resulting in the reduction
of SUMOylation of several proteins involved in DNA damage response
by inhibiting the nuclear translocation of NUPR1. This decreases
the SUMOylation-dependent functions of proteins involved in the DNA
damage response (6).
Cell stress and cell death
NUPR1 regulates cellular damage and death in
different forms, depending on the cell context and the types of
stress induced. NUPR1 is involved in D9-tetrahydrocannabinol
(THC)-induced cancer cell death through the downstream targeting of
death inducible telomere repeat-binding factor 3 (TRB3) protein,
transcription factor activating transcription factor 4 (ATF-4) and
the protein C/EBP homologous protein (CHOP) following endoplasmic
reticulum stress (ERS) elevated from the synthesis of ceramide and
collectively provokes apoptosis (11). NUPR1 downregulation in
hepatocellular carcinoma (HCC) cells can enhance cell sensitivity
to sorafenib treatment, which further controls cell growth through
the RELB/IER3 pathway (12). The
previous study by Santofimia-Castaño et al (2018)
demonstrated that cell death observed following the knockdown of
NUPR1 expression could be reversed by incubation with
necrostatin-1, but not by the inhibition of caspase activity
(13). The authors of that study
thus described a model in which inactivation of NUPR1 in pancreatic
cancer cells resulted in ERS that induced a mitochondrial
malfunction, a deficient ATP production and, as consequence, cell
death mediated by a programmed necrosis (13).
Cell growth, autophagy and death
NUPR1 promotes the proliferation of cancer cells by
influencing cell cycle progression. NUPR1 can aid cells to enter
the S phase by bypassing the G0/G1 checkpoint. Escape from the
G0/G1 phase is achieved by an association between NUPR1 and cyclin
inhibitory proteins resulting in downregulation of p21 and p57
(14). The knockdown of NUPR1 is
able to regulate autophagy by interfering with FoxO3, promoting
Bnip3 transcription in the control of autophagy, a stress-dependent
self-defence mechanism that helps cells eliminate the toxic
microenvironment (15). It has
been reported that NUPR1 silencing suppresses autophagic activities
and induces autophagy-mediated apoptosis in multiple myeloma (MM)
cells through the PI3K/AKT/mammalian target of rapamycin (mTOR)
pathway, which exhibits potential as a treatment strategy for MM
(16), and that NUPR1 is a potent
regulator of autolysosomal dynamics and is required for the
progression of certain epithelial cancers as it regulates the late
stages of autolysosome processing through the induction of the
synaptosomal-associated protein (SNAP)-receptor (SNARE) protein
synaptosomal-associated protein, 25 kDa (SNAP25), which forms a
complex with the lysosomal SNARE-associated protein, VAMP8. NUPR1
depletion deregulates autophagic flux and impairs autolysosomal
clearance, inducing massive cytoplasmic vacuolization and premature
senescence in vitro and tumour suppression in vivo
(17).
Cell senescence
It has been found that in disordered pancreatic
mouse cells, the inhibition of NUPR1 facilitates Kras-induced
cellular senescence through the genomic downregulation of Dnmt1
expression, an enzyme transferring methyl groups onto DNA, which in
turn decreases DNA methylation, crucial for transformation that
helps the induction of Kras-dependent pancreatic cancer (18). NUPR1 can directly regulate the
expression of DNA (cytosine-5)-methyltransferase 1 (Dnmt1) by
interfering with its transcription process by binding on to the
promoter (19). The silencing of
NUPR1 promotes stress-induced senescence upon the enlarging
flattened phenotype of cells provoking spatial pressure (14). As discussed above, NUPR1 is
aberrantly expressed in a subset of cancer cells and predicts low
overall survival rates for patients with lung cancer. NUPR1
depletion deregulates autophagic flux and impairs autolysosomal
clearance, inducing massive cytoplasmic vacuolization and premature
senescence (17).
Endothelial cells
As previously discussed by Cai et al (2016)
in the context of methamphetamine (METH)-induced endothelial
apoptosis, NURP1 functions as a fundamental regulator throughout
the whole process (20).
METH-associated disruptive effects give rise to the formation of
ERS for compensating cell damage in endothelial cells. NUPR1
elevates transcription factor CHOP production in response to ERS,
which then couples to the PUMA promoter through the mediation of
p53, a downstream protein after the nupr1/chop axis. The succeeding
cascades are achieved based on the presentation of NUPR1. The
action of CHOP dissociates anti-apoptotic BCL-2 and upregulates
pro-apoptotic BAX, as well as altering the membrane potential of
the mitochondria. The resulting increased BAX/Bcl-2 ratio drives
the apoptogenic factor cytochrome c importing from
mitochondria into the cell cytosol, which then successfully
triggers endothelial caspase-mediated cell death. A previous study
by Tang et al (2015) also revealed the importance of a
reciprocal association between NUPR1 and ER stress by investing in
Shigella enterotoxin (Shiga) toxin-induced enterocyte
apoptosis (21).
Influence on metabolism
In 2018, Santofimia-Castaño et al examined
NUPR1 depletion cooperating with ERS, inducing pancreatic cancer
cell apoptosis and programmed necrosis by mediating cellular
metabolism. Following NUPR1 knock-down, there was a decrease in ATP
production, resulting in deficient oxygen availability (13). Mitochondrial membrane potential
disruption following Ca2+ uptake from the cytoplasm is
also initiated by a shortage of NUPR1 together with ERS. The
updated mitochondria content triggers the alteration in membrane
permeability resulting in the discharge of cytochrome c,
which leads to cell death (20).
Other research has elucidated a confirmed association between the
deficiency of NUPR1 and bone metabolism, by mediating the receptor
activator of nuclear factor kappa-B ligand or (RANK ligand or
RANKL) and sclerostin, which in turn enhances the proliferation of
osteoblasts and the downregulation of osteoclasts (22). NUPR1 interacts with and activates
SNAP25 to initiate an autolysosomal process that results in
premature senescence and autophagy process (17). NUPR1 is an essential regulator in
protein metabolism and glucose homeostasis (23). It interacts with p300 and Pax2
through which it regulates the transactivation activity of Pax2A
and Pax2B and influences the promoter activities of the glucagon
gene (24). It is also a mediator
of glucose induced growth of beta cells in the pancreas (25). It is also downstream of Zyxin, an
adhesion junctional regulator protein with a controversial role in
cancer (26).
3. NUPR1 in malignant disease
Overall involvement in cancer
Investigations into the role of NUPR1 have increased
since the discovery that it was linked to brain metastasis in
breast cancer (3). By injecting
the human breast cancer cell line, MA-11, into athymic rats, brain
metastases were established. In comparisons between the primary
cancer cells and metastatic breast cancers of the brain using
differential RNA display and protein analysis, NUPR1 was found to
be present in metastatic cells and not in primary cancer cells and
was found to be in aggressive MDA MB-231, but not in MCF-7 cells
which are less aggressive (3).
The authors of that study also demonstrated that there was a rapid
rise in establishing a clinical link between NUPR1 and the
development, progression and clinical outcome in various types of
cancer. The tumorigenic effect of NUPR1 was subsequently
demonstrated in that embryonic fibroblasts from NUPR1+/+
mice, when transformed with ras V12 mutation became tumorigenic and
spread into the peritoneal cavity of mice compared with the same
transformation of NUPR1−/−fibroblasts, which were
non-tumorigenic (27,28). It was surprising to note that
NUPR1+/+ cells with ras V12 mutation transformation grew
at a slower rate than the NUPR1−/−cells with ras V12
mutation transformation, in clear contrast to the in vivo
results in the same study (28).
Another observation made by the same authors was that the
NUPR1-deficient cells grew more rapidly than the
NUPR1+/+ cells (27).
The clear discrepancy and disconnection between in vitro
cell growth and in vivo tumour growth remain
unexplained.
However, these early observations have driven a
marked interest in examining the role of the molecule in individual
human cancers. A summary of some of the key findings from previous
studies is presented in Table
I.
 | Table INUPR1 in clinical cancers. |
Table I
NUPR1 in clinical cancers.
| Cancer type | Methods
applied | Clinical
relevance | (Refs.) |
|---|
| Breast cancer | Northern blot
analysis (n=81) | High levels in
tumours. No correlations with clinical and pathological parameters
nor with uPA (urokinase-type plasminogen activator) and uPAR
(urokinase-type plasminogen activator receptor) | (100) |
| IHC and transcript
analysis (n=120) | Reduced nucleus and
increased cytoplasmic staining in cancer cells. High level is
linked to good prognosis | (32) |
| Genetic analysis of
early stage breast cancer (n=145) | Simultaneous gain
of NUPR1 and ERBB2 (receptor tyrosine-protein kinase erbB2
precursor) gene copy number indicate poorer clinical outcome | (33) |
| Gene transcript by
PCR (n=96) | Stepwise increase
of NUPR1 mRNA from normal, stage 1, 2, 3 and 4. | (36) |
| Pancreatic
cancer | IHC and PCR (38
pancreatic cancer, 5 liver metastasis and 7 metastatic lymph
nodes) | Tumour tissues
stained positive for p8 and normal tissue mostly negative. | (43) |
| IHC (n=44) | Highly positive in
nodal positive tumours and is inversely correlated with the
presence of apoptotic cells. No correlation with survival. | (42) |
| IHC on pancreatic
ductal adenocarcinoma (n=34, TMA) | Level of the NUPR1
expression, together with hypoxia inducible factor 1 subunit α
(HIF1α) are inversely correlated with survival time | (9,44) |
| IHC (n=36) | NUPR1 is linked
with cannibalism of pancreatic cancer which in turn linked to
prognosis | (101) |
| Colorectal
cancer | IHC and
quantitative gene transcript analysis (n=80) | Tumour tissues had
higher levels of NUPR1 transcript than normal tissues. High stage
tumours had less NUPR1. NUPR1 protein was more visible in nucleus
in normal epithelial and in tumour cells stronger staining in the
cytoplasm | (38) |
| NUPR1 transcript
analysis by PCR (n=50) | NUPR1 mRNA was
highly raised in tumour tissues than normal tissues | (39) |
|
Cholangio-carcinoma | IHC (n=10) | NUPR1 mostly
nuclear staining in ductal epithelial cells, increased nuclear
staining in cholangiocarcinoma cells | (60) |
| Prostate
cancer | IHC | Reduced nucleus and
cytoplasmic staining in prostate cancer cells, compared with normal
prostate epithelial cells | (49) |
| Bladder cancer | IHC (n=37) | Mainly cytoplasmic
staining, with invasive cancer cells stained less intensively | (50) |
| Lung cancer | Quantitative gene
transcript analysis | High levels of
NUPR1 mRNA in adenocarcinoma, squamous cell carcinoma and
adenosquamous carcinoma compared with the adjacent normal
tissues | (52) |
| IHC (n=118), NSCLC
(non small cell lung cancer) | High level staining
of NUPR1 in cancer tissues and high staining associated with
shorter survival. | (17) |
| Endometrial
cancer | IHC (n=198) and
gene transcript analysis (n=32) | High levels of
NUPR1 protein and mRNA in deep tumours compared with superficial
tumours | (57) |
| Multiple
myeloma | Geodata analysis
(n=152) | | (58) |
| (MM) | Bone marrow from MM
(N=4) and normal | High levels of
NUPR1 mRNA in MM than in normal | (59) |
| Hepatocellular
carcinoma (HCC) | Gene transcript
analysis (N=23) | A portion of the
tumours (4 out of 23) has high level | (62) |
| IHC and gene array
(n=35 including normal liver, non-tumour and tumour tissues) | Increase in NUPR1
staining in liver cancer (strong in nucleus and also with
cytoplasmic staining). Transcription ratio (tumour to normal) 1.667
(P<0.005) | (63) |
| HCC (n=21),
cirrhotic liver (n=3) and normal liver (n=3) by IHC and qPCR. | HCC with high
levels of nuclear staining of NUPR1 and NUPR1 mRNA than normal
liver tissues | (12) |
| NUPR1 transcript
analysis (n=158) | Significantly
higher in HCC than normal liver tissues and in tumours with high
vascular invasion. Combined expression of NUPR1 and thyroxin
receptors significantly correlated with the survival of the
patients | (66) |
| Thyroid cancer | IHC (n=150) | Most normal and
tumour tissues positive for staining and tumour tissues tended to
be over-expressed. Anaplastic type less intense in staining than
papillary and follicular types. Large tumours and those with lymph
node involvement more intense. | (47) |
| IHC (n=30)
medullary carcinoma | 43.4% regarded as
highly expressed and high degree of staining linked to lymph node
metastasis and recurrence | (48) |
| Glioma | IHC and QPCR
(n=122) | High levels of
NUPR1 mRNA seen in glioma tissues than normal tissues. High levels
staining is associated with shorter survival of the patients. | (68) |
| Osteosarcoma | QPCR (n=58) | Osteosarcomas have
significantly high levels of NUPR1 transcript than non-tumour
tissues. | (67) |
Breast cancer
In breast cancer cells, NUPR1 has been shown to form
a complex with p300 and p53 together with the p21 promoter, to
upregulate the expression of p21, allowing breast cancer cell to
progress through the cell cycle (29). In MCF-7 breast cancer cells, a
HSP9 chaperone protein p23 was shown to result in 7.6 fold
downregulation, although the response to oestradiol was less
prominent (30). It has been
reported that breast cancer cells have less nuclear staining than
normal cells, but more cytoplasmic staining in mammary tumour
tissues (31). A study on gene
transcripts also demonstrated that NUPR1 was reduced in aggressive
tumours and that high levels of NUPR1 transcript were associated
with a longer survival (31). The
finding that NUPR1 levels in oestrogen receptor (ER) and
ERβ-negative tumours has an important bearing in survival led us to
a further discovery that NUPR1 plays an interactive role with ERβ
and that 17-β-oestradiol is able to impact the cellular location of
NUPR1 in breast cancer cells (32). Using genetic analysis to detect
copy numbers of NUPR1 and ERBB2 (her2), Jung et
al (2012) found that a gain of copy numbers of both genes in
early breast cancer was a valuable predictor for patients with
early-stage breast cancer (33).
In animal models, NUPR1 together with BMP4, Cyr6, plod2 and
angiopietin2, were shown to be markedly downregulated in
transcription factor E2F knockdown mice and that collectively,
these reductions were thought to contribute to the retardation of
metastasis and presence of circulating cancer cells (34).
A more recent study has revealed that NUPR1 was
essential for tumour repopulating cells in breast cancer cells
(MCF-7) to grow, forming colonies and tumours in vivo
(35). That study demonstrated
that NUPR1 overexpression suppressed nestin and human telomerase
reverse transcriptase (hTERT), both clonogenic markers, via the p53
pathway, leading to the inhibition of repopulation by this small
population of cancer cells and a reduction in tumour growth in
vivo, together revealing a tumour suppressor role for NUPR1 in
breast cancer and indeed ovarian cancer (35). NUPR1 has been found to be present
at high levels in breast cancer cells metastasised to bone,
although not those to the brain, when compared with the parent
cells (36). This is an
interesting finding and that, together with a recent study
demonstrating that NUPR1 may be linked to the osteoclastic
activities of bone (22), may
suggest that NUPR1 plays a pivotal role in bone metastasis from
breast cancer. This possibility is strengthened by findings that
NUPR1 is also an important factor in the growth of bone marrow
mesenchymal cells (37).
Colorectal cancer
Colorectal tumour tissues exhibit high levels of the
NUPR1 transcript (38,39) and more cytoplasmic NUPR1 staining,
compared with normal tissues (38). High stage tumours exhibited a less
obvious presence of NUPR1. The same team demonstrated that the
knockdown of NUPR1 from colorectal cancer cells (RKO and CaCo2)
resulted in less growth, less colony formation and increased
apoptosis, with minimal roles played in cellular migration,
presenting a somewhat contrasting role to the clinical findings
(39,40); Wang et al demonstrated that
the knockdown of NUPR1 reduced invasiveness and that the
overexpression of NUPR1 exerted opposite effects (39). It appears the NUPR1-like protein
(NUPR2 and NUPR1l) plays a contrasting role to NUPR1 in colorectal
cancer cells since the suppression of NUPR1l by miR2277 results in
an increase in cell migration and cell growth (41).
Pancreatic cancer
Early reports of the clinical significance of NUPR1
in pancreatic cancer came from Su et al (42,43), which demonstrated that pancreatic
cancers, particularly metastatic and node-positive tumours
exhibited high levels of NUPR1 protein, although no association
with survival was established. A very compelling investigation
revealed that the staining of NUPR1 protein in pancreatic ductal
adenocarcinoma was inversely associated with the clinical outcome
of patients over a 24-month follow-up period (44). That study also revealed that
NUPR1, together with RelB and IER3, formed a group of markers not
only for predicting patient outcome, but also in the development of
intraductal neoplasia of the pancreas (44). Furthermore, NUPR1, together with
hypoxia-inducible factor α (HIFα) and AURKA participated in the
regulation of pancreatic cancer cell autophagy response to hypoxia
and glucose deprivation (9). The
loss/deletion of NUPR1 would result in the malfunction of the
mitochondria due to ERS, leading to cell death (13). The pancreatic cancer cell line,
colo357, is amongst the most sensitive cell types to a marked
increase in NUPR1 expression in the response to TGFβ1 (8). In pancreatic cancer, NUPR1 is
intimately involved in homotypic cannibalism, or cell-in-cell, in
that there is virtually no NUPR1. The cell-in-cell phenomenon can
be enhanced by TGFβ in PANC1 cells (45). Using an elegant Pdx1-cre;
LSL-KrasG12D; Ink4a/Arffl/fl (KIC) mouse
model, Cano et al (2014) demonstrated that a proficient
NUPR1 expression resulted in the development of murine pancreatic
ductal adenocarcinoma. However, the deletion of the NUPR1 gene in
these mice, although causing substantial perinatal death due to
NUPR1 and Ink4a/Arf inactivation, the surviving mice exhibited a
prolonged survival and less pancreatic cancer-related deaths
(46). Pancreatic cancer cells
derived from NUPR1-proficient KIC mice displayed a high degree of
stemness and anchorage-independent growth compared with
NUPR1-deficient cells (46).
Thyroid cancer
Normal thyroid tissue tends to have a low degree of
NUPR1 staining while thyroid tumours have high levels (47). Papillary and follicular tumours
stain stronger than anaplastic tumours and normal tissues. Node
positive tumours also stain stronger than node-negative tumours
(47). One of the most
interesting observations from the study is the cellular protein
location. In normal follicles, NUPR1 is exclusively displayed as
nuclear staining. The same nuclear staining is observed in
follicular tumours. However, papillary tumours largely stain the
cytoplasmic region, particularly in those large tumour and tumours
with lymph node metastasis (47).
The majority of medullary tumours stain strongly for NUPR1 and high
levels are linked to lymph node metastasis and recurrence (48).
Urological cancers
In a limited study on prostate cancer, nuclear and
cytoplasmic staining were found in normal prostate epithelial cells
and both staining patterns were reduced in prostate cancer cells
(49). The knockdown of NUPR1 in
PC3, DU145 and CAHPV10 prostate cancer cells resulted in an
increase in the invasiveness of the cells in their response to an
invasion inducer, hepatocyte growth factor (HGF) (49). Bladder transitional cells largely
have cytoplasmic staining, with invasive cancer staining at a lower
intensity (50). The knockdown of
NUPR1 from bladder cancer cell lines (RT112 and EJ138) results in
an increase in both cell growth and invasiveness. In multiple
bladder cancer cell lines, CUPR1 is one of the few epigenetically
upregulated non-CpG island genes, together with TIMP1, TNFRSF14,
ITGB4 and downregulated genes including MMP11 and FGF18 (51).
Lung cancer
Non-small cell lung cancer (NSCLC) tissues exhibit
markedly high levels of NUPR1 protein staining compared with
adjacent normal tissues and those with high levels in tumours have
a significantly shorter overall survival (28 months), a marked
difference from those with low levels (80 months) (17). There is otherwise no significant
association with tumour staging, smoking or age. Tumour tissues
(adenocarcinoma, squamous cell carcinoma and mixed type) have
significantly higher levels of NUPR1 transcript than their normal
counterpart tissues (52). The
same group have also shown that multiple human lung cancer cell
lines, namely A549, SKME1, 95-D, NCI-H460, H1299, all highly
express NUPR1 (52). In these
lung cancer cells, knock-down of NUPR1 by siRNA results in the
cells forming less colonies, becoming more apoptotic and forming
less tumours in in vivo models (17,52). It is also a key bystander response
gene in the lung cancer cell line, H1299, when irradiated (53) and a responsive gene that is
downregulated following the knockdown of a mitochondrial protein
c3orf1 (translocase of inner mitochondrial membrane
domain-containing protein 1) from the lung cancer cell line, 95D
(54). In this case, the
reduction in NUPR1 expression was also linked to a change in the
cell cycle and the reduction in cellular migration.
Skin cancers
In K14ΔNLef1/K14L61Rac1 double-transgenic mice, an
exclusive skin tumour type, sebaceous carcinoma-like tumours are
prevalent and are characterised by aggressive growth and
progression (55). A previous
study identified NUPR1 as one of the few responsive genes
contributing to tumour progression as the result of RAC1 knockdown.
In melanoma, the BRAF inhibitor, encorafenib, increased the
expression of ATF4, CHOP and NUPR1 and induced the expression of
PUMA (56).
Gynaecological cancers
In endometrial cancer cells, NUPR1, together with
Nidogen 1 was found to be a target gene of the ETV5 transcription
factor which is key to myoendometrial invasion by cancer cells
(57). Eliminating NUPR1 in
endometrial cells minimises cellular migration induced by ETV5
overexpression. NUPR1 was also shown to be highly expressed in the
deep (invasive) endometrial cancers of the patients (57).
Myeloma
Using available GEO databases, Di Martino et
al (2015) identified that the action of NUPR1 was one of the
key mechanisms in myeloma progression and was connected with the
downregulation of 6 genes, including BNIP3, GINS1,
GRAMD3, KIF11, SHCBP1 and SPIN4 and the
upregulation of the 2 genes, ELMOD1 and SLC16A6
(58). Bone marrow from multiple
myeloma patients tends to have higher levels of NUPR1 transcript
than healthy volunteers, although in that study the sample number
was small (59). The silencing
NUPR1 in myeloma cells also resulted in a reduction of cell
proliferation and induction of apoptosis and cell cycle blockage
(59).
Biliary cancers
In cholangiocarcinoma, NUPR1 is predominantly
stained in the nucleus and more so in cancer cells (60). A human HuCCT1 cholangiocarcinoma
cell was found to have a reduced rate of cell growth, and migration
and invasiveness in response to EGF and serum, following NUPR1
knockdown by siRNA (60).
Hepatocellular carcinoma (HCC)
TGFβ1 was able to markedly increase the expression
of NUPR1 in the Hep1 hepatocellular carcinoma cell line (8). In a pathway search study, NUPR1 was
found amongst the top down-regulated genes in non-viral HCCs,
namely in alcohol consumption-related HCC (z ratio-3.0) and
non-alcoholic fatty liver disease related HCC (z ratio-4.5)
(61). HCC tissues tend to have
high levels of NUPR1 protein, mostly in the nucleus, although no
association was observed with TNM staging (12). Lee et al (2015) reported
that NUPR1 was a key transcription regulator which leads to
defective mitochondria-regulating genes in HCC (62). In this process, which is linked to
metabolism of the cancer type, granulin is the key downstream
effector protein of NUPR1. Knockdown of NUPR1 leads to a calcium
signalling-dependent reduction of cellular invasion (62). Of note, the evaluation of NUPR1
mRNA expression in the limited clinical cohort only revealed a
small portion of increase in NUPR1 transcript. NUPR1 can be
activated by hepatitis X protein (HBx) via the HBx-Smad4 pathway,
which results in the reduction of cell death and the induction of
vasculogenic mimicry in HCC (63). The same study demonstrated that
NUPR1 transcript was found to be significantly upregulated in HCC
compared with normal liver tissues by a ratio of 1.667. The
knockdown of NUPR1 resulted in HCCs that were less mobile, had
lower invasiveness and were less tumorigenic in vivo. It
further identified the NUPR1/RELB/IER3/RUNX2 pathway as key in
these events (12). In a
comprehensive search for the transcriptomic and histone
modification profiles during the transition from non-alcoholic
steatohepatitis to HCC, it was found that NUPR1 plays an important
role in this complex network (64). NUPR1 can inhibit lysine
acetyltransferase 8, which in turn influences one of the key
generic alterations of gene patterns for the deacetylation of
histone H4 Lysin 16 during the transition process. Serum from
patients with chronic hepatitis B has been shown to be able to
activate expression of NUPR1 in HCC cells (65). Thyroxin (T3) is a potent inducer
of NUPR1 expression in HCC cell lines, by over 30-fold, an effect
attributable to the transcriptional regulation of thyroxin receptor
protein directly binding and activating the transcriptional
response elements of the NUPR1 promoter (66). Clinically, NUPR1 is positively
associated with thyroxin receptors and the high levels of
expression of both are significantly linked to shorter overall and
disease-free survival (60).
Osteosarcoma
Osteosarcoma tissue has significantly higher levels
of NUPR1 transcript compared with normal tissues (67). Together with miR4443, NUPR1 plays
a regulatory role in a long non-coding RNA, FEZF1-AS1, induced cell
growth, and the migration and invasiveness of osteosarcoma cells.
lncRNA FAL1 and FEZF1-AS1 have been shown to require NUPR1 in their
cancer inducing activities, acting respectively with miR637 and
miR4443 (39,67).
Neurological tumours
In gliomas, the NUPR1 transcript level is
significantly higher than in normal tissues (68). High-grade glioma stains more
strongly than normal brain tissue and low-grade tumours: Again,
high levels of staining are associated with a shorter survival and
also indicates NUPR1 to be an independent prognostic indicator
(68). The study further
confirmed that the knockdown of NUPR1 in multiple glioblastoma
cells resulted in a reduction of cell migration and proliferation,
and cell cycle arrest at the G0/G1 phase (69), events appearing to be coordinated
by intracellular signalling events involving ERK1/2, p38 MAPK and
cleavage of caspase-3 and p27 (68).
Pituitary tumours
There are currently no studies available on human
tumours as yet, at least to the best of our knowledge. NUPR1
expression is generally quiescent in the pituitary gland. However,
an animal study conducted by Mohammad et al (2004)
demonstrated that a parent cell of GH3 somatolactotrope genotype
and a gonadotropic pituitary cell, LbT2 was tumorigenic in nude
mice, whereas when NUPR1 expression was reduced in these cell
lines, it lost its ability to form tumours in vivo (70). It was subsequently established
that at least in LbT2 gonadotropic pituitary cells, NUPR1 allows
the cell to avoid the G0/G1 phase of the cell cycle (14) and that NUPR1 is transcriptively
regulated by activating transcription factor 4 (ATF4), as in other
cell types, such as HeLa cells (71,72). The GCN/ATF4 pathway appears to
involve the amino acid response element (AARE) with the NUPR1
promoter (73).
4. NUPR1 in other conditions
Neurological disorders
NUPR1 is one of the responsive genes in the cerebral
cortex, which is downregulated in response to oestradiol (E2)
induction (74). NUPR1, together
with a few other proteins appears to be a key responsive molecule
in neural cells and tissues following a challenge with
methamphetamine (75). Treatment
with this substance results in an increase in NUPR1 expression,
which is associated with an increase in apoptosis and autophagy in
neural cells in vivo and in cell lines in vitro
(68).
Cardiac hypertrophy
Cardiac hypertrophy is the abnormal enlargement, or
thickening, of the heart muscle, resulting from increases in
cardiomyocyte size. It has been reported that NUPR1 is an important
factor in cardiomyocyte hypertrophy, induced by endothelin and
phenylephrine (76). NUPR1 is
also key to transforming growth factor α (TNFα)-induced
metalloproteinases in heart fibroblasts. These are key contributing
factors in heart failure. It has also been observed that NUPR1 also
partners with some of the muscle specific genes such as p68 (Ddx5)
and MyoD in myoblasts (77).
Liver toxicity
NUPR1 is part of a protection mechanism in CCL4
[Chemokine (C-C motif) ligands 4] induced liver injury, by
coordinating with cytochrome P450 2E1 which converts the chemical
to toxic products (78).
Inflammation
The loss of NUPR1 in mice has been shown to result
in increased death when challenged by lipopolysaccharide (LPS),
together with an increase in TNFα, and in the reactive oxygen
species, myeloperoxidase and hydroperoxide, suggesting that the
loss of protection of stress injuries by NUPR1 (27,79). Conversely, TNFα via NFkB mediates
the expression of NUPR1 (80). It
has also been indicated to be involved in the pathophysiological
process of arthritis, in that osteoarthritic cartilage has higher
levels of NUPR1 and that NUPR1 appears to be a key mediator in
interleukin-1β induced MMP13 expression in chondrocytes (81).
Pancreatitis
It has been shown that LPS is able to rapidly induce
the expression of NUPR1a mRNA, detected by northern blot analysis
in pancreatic acinar cells, in the pancreas, liver and kidneys
(82). On the other hand, NUPR1
has been shown to coordinate with pancreatic associated protein-1
(PAP1) in protecting the pancreas from inflammation inducers
(24). During chronic
pancreatitis, NUPR1 is induced to express and protect pancreatic
acinar cells from becoming apoptotic (83).
Diabetes
The loss of NUPR1 has also been observed with
increases in the beta cell mass of the pancreas, suggesting that it
is involved in glucose metabolism in the body (84). It has also been shown that NUPR1
plays a vital role in the protection of β-cells from apoptosis,
related degradation of insulin storages and subsequent secretion
during inflammatory and obesity-related tissue stress (82).
5. Therapeutic considerations
Therapeutic regulation of NUPR1
Bratland et al reported that vitamin
1,25(OH)2D3 was able to upregulate NUPR1
expression in MCF-7 breast cancer cells and in doing so, reduce
colony formation and provoke cell cycle arrest at the G1 phase,
attributable to the regulation of p21Kip1 (85).
Targeting
Trifluoperazine, a drug used for psychiatric
conditions has been found to interact with NUPR1 and block
NUPR1-dependent tumour growth. Novel derivatives from
trifluoperazine, namely ZZW-115, have been synthesised since with
improved binding and potent effects on pancreatic cancer cells,
providing positive prospects of novel agents in therapeutically
targeting NUPR1 (7,86). Novel compounds that can target
NUPR1 have been recently identified and shown to suppress
pancreatic tumour development in the context of targeting NUPR1
(87). Although it is still too
early to tell, it does indicate that NUPR1 would be a valuable
therapeutic target in tumours closely linked to NUPR1 expression,
pancreatic cancer being an excellent example. A recent study by
Deng et al (2017) found that a STAT3 inhibitor,
fluorofenidone was able to reduce the level of NUPR1 in lung
adenocarcinoma cell lines, reproducibly demonstrated in vivo
and in vitro, in line with reductions in cell growth, colony
formation and tumour growth (88). Specific peptides have been shown
to block the interaction between NUPR1 and one of its key partner
proteins RING1B, indicating a value for targeting the action of
NUPR1 (89). Cationic solid lipid
nanoparticles have been tested as a means with which to deliver
anti-NUPR1 plasmids to HCC cancer cells and have successfully
resulted in reduction of NUPR1 in these cells (90). Moreover, Lan et al (2020)
demonstrated that ZZW-115 sensitized cancer cells to genotoxic
agents by the inhibition of NUPR1 nuclear translocation, which in
turn reduced the SUMOylation-dependent functions of DNA damage
response proteins (6).
Chemoresistance
NUPR1 has been found to be involved in the
resistance of thyroid cancer cells to Lenvatinib therapy (91). Low levels of NUPR1 in fibroblasts
have been shown to result in resistance to adriamycin (79). In breast cancer cells, NUPR1 also
confers resistance to chemo-therapeutic agents, including Taxol and
doxorubicin, by involving the NUPR1-PI3K/Akt-phospho-p21 axis
(29,92). NUPR1-deficient pancreatic cancer
cells are more sensitive to chemotherapeutic drugs, with the
activation of NUPR1 increasing drug resistance to gemcitabine
(46,93) and also becoming more sensitive to
HSP90 inhibitors (94) and mTOR
inhibitors in squamous cell carcinoma of skin (95). Likewise, knockdown of NUPR1 in
liver cancer cells sensitises their response to sorafenib, which
itself induces the expression of NUPR1, resulting in acquired
resistance (12,66). However, the link may be more
complex than just an NUPR1 connection. In a very interesting
preliminary study, a small number of patients with cervical cancers
were tested for changes in a panel of molecules involved in DNA
repair and candidate drug resistance before and after two cycles of
cisplatin treatment (96). NUPR1
was found to be one of the few proteins that was consistently
reduced following treatment; however, this change was not connected
to the clinical and pathological response. This important
observation, the very first in a clinical setting, has raised a
number of important questions; for example, the candidacy of NUPR1
as a drug resistance regulator in the body in vitro for
cisplatin, leads to the question of whether NUPR1 should be
considered together with other key partners. The question is
whether an increase in the reduction of NUPR1 is in fact a signal
for drug resistance in the context of whole body for instance. This
interesting research topic, to be expanded further by the
researchers, would shed important light on such questions. In a
colon cancer cell line (HCT116) spheroid model, NUPR1 was found to
be markedly reduced (activation z ratio-1.387) in a full nutrient
environment but less reduced (z ratio-0.632) in a glucose deprived
environment when cells were treated with irinotecan and chloroquine
(97). Whilst this suggests that
glucose is important in resistance to chemotherapy, it again raises
questions as to the role of NUPR1 in the response to chemotherapy
in different cancer types. In prostate cancer cell lines (DU145 and
PC3) with acquired resistance to docetaxel, single-cell
RNA-sequencing determined differential clusters of sensitive vs.
resistant cells (98). Protein
ubiquitination was the most differentially regulated pathway and
one of the top regulators was identified to be NUPR1. NUPR1 gene
modifications revealed that NUPR1 conferred docetaxel resistance in
both cell lines, indicating that it is a mediator of prostate
cancer drug resistance and hence a target for resistance-reversal
(98).
6. Challenges and future perspectives
NUPR1 is a highly interesting molecule to explore in
the context of cancer, as demonstrated in the evidence gathered
over the past 2 decades. It is involved in multiple aspects of
cancer, from DNA repair, transcription regulation, cell cycle and
death to metabolic activity of cancer cells. It also appears to be
involved in a wide variety of cancer types. Clinically, there are
demonstrable links between NUPR1 and disease progression and
clinical outcome of patients, at least in certain cancer types.
There are also early signs that it has a therapeutic value by
targeting NUPR1 in the treatment of cancer. However, whilst
exciting, a cautious approach is necessary until some of the key
issues are resolved. The central issue is the inconsistencies in
the role of NUPR1 in different cancer types and is a repeatedly
occurring pattern. The following provides some perspectives for the
likely reasons that this is observed.
Discrepancies in clinical and in vitro
findings
Whilst NUPR1 itself has some contrasting roles in
different types of cancer cells, a recently discovered family
member, namely NUPR1-like or NUPR2, may provide some insight.
NUPR1L closely resembles NUPR1 but has contrasting functions to
NUPR1 (5,99). NUPR2 is regulated by p53
responsive elements and its expression is dependent on p53, a
classic tumour suppressor (5).
NUPR2 expression is also induced by p53 inducers, including serum
starvation and oxaliplatin. Furthermore, NUPR1 and NUPR2 mutually
suppress each other by way of the transcription of regulation.
NUPR2 expression downregulates that of NUPR1 and vice versa. NUPR1
has been shown to be able to revert NUPR2 induced cell cycle
arrest, making it a classic agonist-antagonist player in cells
(5). NUPR2 is able to bind the
same partners of NUPR1, including RING1B, at a similar affinity and
most interestingly binds to NUPR1 (87). NUPR2 also appears to play an
inhibitory role in colorectal cancer cells and can be targeted by
miRF2277, which binds to the UTR of the human NUPR2 gene and
down-regulate NUPR2, and in doing so, increase the growth and
migration of cancer cells (41).
Thus, the clinical association between NUPR1 and cancer would
require the consideration of NUPR2, in the same setting, namely in
the same cancer types and the same cohort, in order to establish a
solid link.
Nuclear vs. cytoplasmic
One of the most interesting observations from
published studies is the cellular location of the protein. In
normal follicles of the thyroid gland, NUPR1 exclusively displays
nuclear staining. The same nuclear staining is seen in follicular
tumours. However, papillary tumours largely exhibit cytoplasmic
staining, particularly in large tumours and tumours with lymph node
metastasis (47). In breast
cancer, both cytoplasmic and nuclear staining was observed
(31). These preliminary
observations indicate that cytoplasmic distribution of NUPR1 in
cancer cells may aid the aggressiveness and poor differentiation
phenomenon of cancer cells. Pancreatic tumours also exhibit nuclear
and cytoplasmic staining (42,43). Again, heavy cytoplasmic staining
was seen in NSCLC cancers (17).
A study with a larger cohort enabling us to comprehensively
evaluate the cellular location of NUPR1 would answer these
questions. In addition, further cell-based investigations to
discern the functional discrepancies for cytoplasmic NUPR1 and
nuclear NUPR1 was thought to be necessary. The nuclear vs.
cytoplasmic localisation of NUPR1 remained an enigma until a recent
study by Lan et al (2020), whose elegant study on the effect
of ZZW-115-dependent inhibition of NUPR1 nuclear translocation by
changes in SUMOylation-dependent functions of DNA damage response
proteins (6).
A further point is that one should analyse NUPR1
together with its positive and negative regulators in the same
study. For example, it would be invaluable to co-investigate NUPR1
and its potential partners such as ERBB2 in breast cancer (33). Finally, when considering targeting
NUPR1 as a therapy, it is important to focus on the tumour type(s)
that has/have a demonstrable connection, such as pancreatic cancer,
whereas other cancer types would require additional work for the
relationship to be firmly established. This is an exciting avenue
of research that we anticipate could reveal new targets in cancer
treatment in the future.
Availability of data and materials
Not applicable.
Authors' contributions
WGJ and TAM conceived the design of the study. AXL,
AJS, RH, LY and KF all contributed to the content (literature
search and writing of the text) of the present review. All authors
read and approved of the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank to the assistance
of Dr Gregory Harrison (School of Medicine, Cardiff University) in
formatting the manuscript.
Funding
No funding was received.
References
|
1
|
Mallo GV, Fiedler F, Calvo EL, Ortiz EM,
Vasseur S, Keim V, Morisset J and Iovanna JL: Cloning and
expression of the rat p8 cDNA, a new gene activated in pancreas
during the acute phase of pancreatitis, pancreatic development, and
regeneration, and which promotes cellular growth. J Biol Chem.
272:32360–32369. 1997. View Article : Google Scholar
|
|
2
|
Vasseur S, Vidal Mallo G, Fiedler F,
Bödeker H, Cánepa E, Moreno S and Iovanna JL: Cloning and
expression of the human p8, a nuclear protein with mitogenic
activity. Eur J Biochem. 259:670–675. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ree AH, Tvermyr M, Engebraaten O, Rooman
M, Røsok O, Hovig E, Meza-Zepeda LA, Bruland OS and Fodstad O:
Expression of a novel factor in human breast cancer cells with
metastatic potential. Cancer Res. 59:4675–4680. 1999.PubMed/NCBI
|
|
4
|
Quirk CC, Seachrist DD and Nilson JH:
Embryonic expression of the luteinizing hormone β gene appears to
be coupled to the transient appearance of p8, a high mobility
group-related transcription factor. J Biol Chem. 278:1680–1685.
2003. View Article : Google Scholar
|
|
5
|
Lopez MB, Garcia MN, Grasso D, Bintz J,
Molejon MI, Velez G, Lomberk G, Neira JL, Urrutia R and Iovanna J:
Functional characterization of Nupr1L, A Novel p53-regulated
isoform of the high-mobility group (HMG)-related protumoral protein
Nupr1. J Cell Physiol. 230:2936–2950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan W, Santofimia-Castaño P, Swayden M,
Xia Y, Zhou Z, Audebert S, Camoin L, Huang C, Peng L,
Jiménez-Alesanco A, et al: ZZW-115-dependent inhibition of NUPR1
nuclear translocation sensitizes cancer cells to genotoxic agents.
JCI Insight. 5:e1381172020. View Article : Google Scholar
|
|
7
|
Santofimia-Castaño P, Rizzuti B, Xia Y,
Abian O, Peng L, Velázquez-Campoy A, Iovanna JL and Neira JL:
Designing and repurposing drugs to target intrinsically disordered
proteins for cancer treatment: Using NUPR1 as a paradigm. Mol Cell
Oncol. 6:e16126782019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pommier RM, Gout J, Vincent DF, Cano CE,
Kaniewski B, Martel S, Rodriguez J, Fourel G, Valcourt U, Marie JC,
et al: The human NUPR1/P8 gene is transcriptionally activated by
transforming growth factor β via the SMAD signalling pathway.
Biochem J. 445:285–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamidi T, Cano CE, Grasso D, Garcia MN,
Sandi MJ, Calvo EL, Dagorn JC, Lomberk G, Urrutia R, Goruppi S, et
al: Nupr1-aurora kinase A pathway provides protection against
metabolic stress-mediated autophagic-associated cell death. Clin
Cancer Res. 18:5234–5246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gironella M, Malicet C, Cano C, Sandi MJ,
Hamidi T, Tauil RM, Baston M, Valaco P, Moreno S, Lopez F, et al:
p8/nupr1 regulates DNA-repair activity after double-strand gamma
irradiation-induced DNA damage. J Cell Physiol. 221:594–602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carracedo A, Lorente M, Egia A, Blázquez
C, García S, Giroux V, Malicet C, Villuendas R, Gironella M,
González-Feria L, et al: The stress-regulated protein p8 mediates
cannabinoid-induced apoptosis of tumor cells. Cancer Cell.
9:301–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emma MR, Iovanna JL, Bachvarov D, Puleio
R, Loria GR, Augello G, Candido S, Libra M, Gulino A, Cancila V, et
al: NUPR1, a new target in liver cancer: Implication in controlling
cell growth, migration, invasion and sorafenib resistance. Cell
Death Dis. 7:e22692016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santofimia-Castaño P, Lan W, Bintz J,
Gayet O, Carrier A, Lomberk G, Neira JL, González A, Urrutia R,
Soubeyran P and Iovanna J: Inactivation of NUPR1 promotes cell
death by coupling ER-stress responses with necrosis. Sci Rep.
8:169992018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brannon KM, Million Passe CM, White CR,
Bade NA, King MW and Quirk CC: Expression of the high mobility
group A family member p8 is essential to maintaining tumorigenic
potential by promoting cell cycle dysregulation in LbetaT2 cells.
Cancer Lett. 254:146–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong DK, Georgescu SP, Cano C, Aronovitz
MJ, Iovanna JL, Patten RD, Kyriakis JM and Goruppi S: Deficiency of
the transcriptional regulator p8 results in increased autophagy and
apoptosis, and causes impaired heart function. Mol Biol Cell.
21:1335–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li A, Li X, Chen X, Zeng C, Wang Z, Li Z
and Chen J: NUPR1 Silencing induces autophagy-mediated apoptosis in
multiple myeloma cells through the PI3K/AKT/mTOR pathway. DNA Cell
Biol. 39:368–378. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mu Y, Yan X, Li D, Zhao D, Wang L, Wang X,
Gao D, Yang J, Zhang H, Li Y, et al: NUPR1 maintains autolysosomal
efflux by activating SNAP25 transcription in cancer cells.
Autophagy. 14:654–670. 2018. View Article : Google Scholar :
|
|
18
|
Grasso D, Garcia MN, Hamidi T, Cano C,
Calvo E, Lomberk G, Urrutia R and Iovanna JL: Genetic inactivation
of the pancreatitis-inducible gene Nupr1 impairs PanIN formation by
modulating KrasG12D-induced senescence. Cell Death Differ.
21:1633–1641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grasso D, Bintz J, Lomberk G, Molejon MI,
Loncle C, Garcia MN, Lopez MB, Urrutia R and Iovanna JL: Pivotal
role of the chromatin protein Nupr1 in Kras-induced senescence and
transformation. Sci Rep. 5:175492015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai D, Huang E, Luo B, Yang Y, Zhang F,
Liu C, Lin Z, Xie WB and Wang H: Nupr1/Chop signal axis is involved
in mitochondrion-related endothelial cell apoptosis induced by
methamphetamine. Cell Death Dis. 7:e21612016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang B, Li Q, Zhao XH, Wang HG, Li N, Fang
Y, Wang K, Jia YP, Zhu P, Gu J, et al: Shiga toxins induce
autophagic cell death in intestinal epithelial cells via the
endoplasmic reticulum stress pathway. Autophagy. 11:344–354. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiraki M, Xu X, Iovanna JL, Kukita T,
Hirata H, Kamohara A, Kubota Y, Miyamoto H, Mawatari M and Kukita
A: Deficiency of stress-associated gene Nupr1 increases bone volume
by attenuating differentiation of osteoclasts and enhancing
differentiation of osteoblasts. FASEB J. 33:8836–8852. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maida A, Zota A, Sjøberg KA, Schumacher J,
Sijmonsma TP, Pfenninger A, Christensen MM, Gantert T, Fuhrmeister
J, Rothermel U, et al: A liver stress-endocrine nexus promotes
metabolic integrity during dietary protein dilution. J Clin Invest.
126:3263–3278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffmeister A, Ropolo A, Vasseur S, Mallo
GV, Bodeker H, Ritz-Laser B, Dressler GR, Vaccaro MI, Dagorn JC,
Moreno S and Iovanna JL: The HMG-I/Y-related protein p8 binds to
p300 and Pax2 trans-activation domain-interacting protein to
regulate the transactivation activity of the Pax2A and Pax2B
transcription factors on the glucagon gene promoter. J Biol Chem.
277:22314–22319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Päth G, Opel A, Knoll A and Seufert J:
Nuclear protein p8 is associated with glucose-induced pancreatic
beta-cell growth. Diabetes. 53(Suppl 1): S82–S85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong C, Yu J, Li D, Jiang K, Tang Y, Yang
M, Shen H, Fang X, Ding K, Zheng S and Yuan Y: Zyxin as a potential
cancer prognostic marker promotes the proliferation and metastasis
of colorectal cancer cells. J Cell Physiol. Jan 29–2019.Epub ahead
of print.
|
|
27
|
Vasseur S, Hoffmeister A, Garcia S, Bagnis
C, Dagorn JC and Iovanna JL: p8 is critical for tumour development
induced by rasV12 mutated protein and E1A oncogene. EMBO Rep.
3:165–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iovanna JL: Expression of the
stress-associated protein p8 is a requisite for tumor development.
Int J Gastrointest Cancer. 31:89–98. 2002. View Article : Google Scholar
|
|
29
|
Clark DW, Mitra A, Fillmore RA, Jiang WG,
Samant RS, Fodstad O and Shevde LA: NUPR1 interacts with p53,
transcriptionally regulates p21 and rescues breast epithelial cells
from doxorubicin-induced genotoxic stress. Curr Cancer Drug
Targets. 8:421–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simpson NE, Lambert WM, Watkins R,
Giashuddin S, Huang SJ, Oxelmark E, Arju R, Hochman T, Goldberg JD,
Schneider RJ, et al: High levels of Hsp90 cochaperone p23 promote
tumor progression and poor prognosis in breast cancer by increasing
lymph node metastases and drug resistance. Cancer Res.
70:8446–8456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang WG, Watkins G, Douglas-Jones A,
Mokbel K, Mansel RE and Fodstad O: Expression of Com-1/P8 in human
breast cancer and its relevance to clinical outcome and ER status.
Int J Cancer. 117:730–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang WG, Davies G and Fodstad O: Com-1/P8
in oestrogen regulated growth of breast cancer cells, the ER-beta
connection. Biochem Biophys Res Commun. 330:253–262. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung SH, Lee A, Yim SH, Hu HJ, Choe C and
Chung YJ: Simultaneous copy number gains of NUPR1 and ERBB2
predicting poor prognosis in early-stage breast cancer. BMC Cancer.
12:3822012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hollern DP, Honeysett J, Cardiff RD and
Andrechek ER: The E2F transcription factors regulate tumor
development and metastasis in a mouse model of metastatic breast
cancer. Mol Cell Biol. 34:3229–3243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia Q, Zhou W, Yao W, Yang F, Zhang S,
Singh R, Chen J, Chen JJ, Zhang Y, Wei F, et al: Downregulation of
YAP-dependent Nupr1 promotes tumor-repopulating cell growth in soft
matrices. Oncogenesis. 5:e2202016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fish L, Zhang S, Yu JX, Culbertson B, Zhou
AY, Goga A and Goodarzi H: Cancer cells exploit an orphan RNA to
drive meta-static progression. Nat Med. 24:1743–1751. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsunaga K, Fujisawa K, Takami T,
Burganova G, Sasai N, Matsumoto T, Yamamoto N and Sakaida I: NUPR1
acts as a pro-survival factor in human bone marrow-derived
mesenchymal stem cells and is induced by the hypoxia mimetic
reagent deferoxamine. J Clin Biochem Nutr. 64:209–216. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davies ML, Parr C, Sanders AJ, Fodstad O
and Jiang WG: The transcript expression and protein distribution
pattern in human colorectal carcinoma reveal a pivotal role of
COM-1/p8 as a tumour suppressor. Cancer Genomics Proteomics.
7:75–80. 2010.PubMed/NCBI
|
|
39
|
Wang L, Jiang F, Xia X and Zhang B: LncRNA
FAL1 promotes carcinogenesis by regulation of miR-637/NUPR1 pathway
in colorectal cancer. Int J Biochem Cell Biol. 106:46–56. 2019.
View Article : Google Scholar
|
|
40
|
Li X, Martin TA and Jiang WG: COM-1/p8
acts as a tumour growth enhancer in colorectal cancer cell lines.
Anticancer Res. 32:1229–1237. 2012.PubMed/NCBI
|
|
41
|
Gao Q, Lei F, Zeng Q, Gao Z, Niu P,
Junnan, Ning, Li J and Zhang J: Functional passenger-strand miRNAs
in exosomes derived from human colon cancer cells and their
heterogeneous paracrine effects. Int J Biol Sci. 16:1044–1058.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su SB, Motoo Y, Iovanna JL, Berthézène P,
Xie MJ, Mouri H, Ohtsubo K, Matsubara F and Sawabu N:
Overexpression of p8 is inversely correlated with apoptosis in
pancreatic cancer. Clin Cancer Res. 7:1320–1324. 2001.PubMed/NCBI
|
|
43
|
Su SB, Motoo Y, Iovanna JL, Xie MJ, Mouri
H, Ohtsubo K, Yamaguchi Y, Watanabe H, Okai T, Matsubara F and
Sawabu N: Expression of p8 in human pancreatic cancer. Clin Cancer
Res. 7:309–313. 2001.PubMed/NCBI
|
|
44
|
Hamidi T, Algül H, Cano CE, Sandi MJ,
Molejon MI, Riemann M, Calvo EL, Lomberk G, Dagorn JC, Weih F, et
al: Nuclear protein 1 promotes pancreatic cancer development and
protects cells from stress by inhibiting apoptosis. J Clin Invest.
122:2092–2103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cano CE, Hamidi T, Sandi MJ and Iovanna
JL: Nupr1: The Swiss-knife of cancer. J Cell Physiol.
226:1439–1443. 2011. View Article : Google Scholar
|
|
46
|
Cano CE, Hamidi T, Garcia MN, Grasso D,
Loncle C, Garcia S, Calvo E, Lomberk G, Dusetti N, Bartholin L, et
al: Genetic inactivation of Nupr1 acts as a dominant suppressor
event in a two-hit model of pancreatic carcinogenesis. Gut.
63:984–995. 2014. View Article : Google Scholar
|
|
47
|
Ito Y, Yoshida H, Motoo Y, Miyoshi E,
Iovanna JL, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et
al: Expression and cellular localization of p8 protein in thyroid
neoplasms. Cancer Lett. 201:237–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ito Y, Yoshida H, Motoo Y, Iovanna JL,
Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et
al: Expression of p8 protein in medullary thyroid carcinoma.
Anticancer Res. 25:3419–3423. 2005.PubMed/NCBI
|
|
49
|
Jiang WG, Davies G, Martin TA, Kynaston H,
Mason MD and Fodstad O: Com-1/p8 acts as a putative tumour
suppressor in prostate cancer. Int J Mol Med. 18:981–986.
2006.PubMed/NCBI
|
|
50
|
Du P, Ye L, Yang Y and Jiang WG: Candidate
of metastasis 1 regulates in vitro growth and invasion of bladder
cancer cells. Int J Oncol. 42:1249–1256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Veerla S, Panagopoulos I, Jin Y, Lindgren
D and Höglund M: Promoter analysis of epigenetically controlled
genes in bladder cancer. Genes Chromosomes Cancer. 47:368–378.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo X, Wang W, Hu J, Feng K, Pan Y, Zhang
L and Feng Y: Lentivirus-mediated RNAi knockdown of NUPR1 inhibits
human nonsmall cell lung cancer growth in vitro and in vivo. Anat
Rec (Hoboken). 295:2114–2121. 2012. View Article : Google Scholar
|
|
53
|
Ghandhi SA, Ponnaiya B, Panigrahi SK,
Hopkins KM, Cui Q, Hei TK, Amundson SA and Lieberman HB: RAD9
deficiency enhances radiation induced bystander DNA damage and
transcriptomal response. Radiat Oncol Lond Engl. 9:2062014.
View Article : Google Scholar
|
|
54
|
Wu H, Wang W and Xu H: Depletion of
C3orf1/TIMMDC1 inhibits migration and proliferation in 95D lung
carcinoma cells. Int J Mol Sci. 15:20555–20571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Frances D, Sharma N, Pofahl R, Maneck M,
Behrendt K, Reuter K, Krieg T, Klein CA, Haase I and Niemann C: A
role for Rac1 activity in malignant progression of sebaceous skin
tumors. Oncogene. 34:5505–5512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Niessner H, Sinnberg T, Kosnopfel C,
Smalley KSM, Beck D, Praetorius C, Mai M, Beissert S, Kulms D,
Schaller M, et al: BRAF inhibitors amplify the proapoptotic
activity of MEK inhibitors by inducing ER stress in NRAS-mutant
melanoma. Clin Cancer Res. 23:6203–6214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pedrola N, Devis L, Llauradó M, Campoy I,
Martinez-Garcia E, Garcia M, Muinelo-Romay L, Alonso-Alconada L,
Abal M, Alameda F, et al: Nidogen 1 and Nuclear Protein 1: Novel
targets of ETV5 transcription factor involved in endometrial cancer
invasion. Clin Exp Metastasis. 32:467–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Di Martino MT, Guzzi PH, Caracciolo D,
Agnelli L, Neri A, Walker BA, Morgan GJ, Cannataro M, Tassone P and
Tagliaferri P: Integrated analysis of microRNAs, transcription
factors and target genes expression discloses a specific molecular
architecture of hyperdiploid multiple myeloma. Oncotarget.
6:19132–19147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zeng C, Li X, Li A, Yi B, Peng X, Huang X
and Chen J: Knockdown of NUPR1 inhibits the growth of U266 and
RPMI8226 multiple myeloma cell lines via activating PTEN and
caspase activation-dependent apoptosis. Oncol Rep. 40:1487–1494.
2018.PubMed/NCBI
|
|
60
|
Kim KS, Jin DI, Yoon S, Baek SY, Kim BS
and Oh SO: Expression and roles of NUPR1 in cholangiocarcinoma
cells. Anat Cell Biol. 45:17–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Seshachalam VP, Sekar K and Hui KM:
Insights into the etiology-associated gene regulatory networks in
hepatocellular carcinoma from The Cancer Genome Atlas. J
Gastroenterol Hepatol. 33:2037–2047. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee YK, Jee BA, Kwon SM, Yoon YS, Xu WG,
Wang HJ, Wang XW, Thorgeirsson SS, Lee JS, Woo HG and Yoon G:
Identification of a mitochondrial defect gene signature reveals
NUPR1 as a key regulator of liver cancer progression. Hepatology.
62:1174–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bak Y and Shin H: Hepatitis B virus X
promotes hepatocellular carcinoma development via nuclear protein 1
pathway. Biochem Biophys Res Commun. 466:676–681. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
de Conti A, Dreval K, Tryndyak V, Orisakwe
OE, Ross SA, Beland FA and Pogribny IP: Inhibition of the cell
death pathway in nonalcoholic steatohepatitis (NASH)-related
hepatocarcino-genesis is associated with histone H4 lysine 16
deacetylation. Mol Cancer Res. 15:1163–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ji Y, Wang Z, Chen H, Zhang L, Zhuo F and
Yang Q: Serum from chronic hepatitis B patients promotes growth and
proliferation via the IGF-II/IGF-IR/MEK/ERK signaling pathway in
hepato-cellular carcinoma cells. Cell Physiol Biochem. 47:39–53.
2018. View Article : Google Scholar
|
|
66
|
Chen CY, Wu SM, Lin YH, Chi HC, Lin SL,
Yeh CT, Chuang WY and Lin KH: Induction of nuclear protein-1 by
thyroid hormone enhances platelet-derived growth factor A mediated
angiogen-esis in liver cancer. Theranostics. 9:2361–2379. 2019.
View Article : Google Scholar :
|
|
67
|
Zhou C, Xu J, Lin J, Lin R, Chen K, Kong J
and Shui X: Long non-coding RNA FEZF1-AS1 promotes osteosarcoma
progression by regulating miR-4443/NUPR1 axis. Oncol Res.
26:1335–1343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li J, Ren S, Liu Y, Lian Z, Dong B, Yao Y
and Xu Y: Knockdown of NUPR1 inhibits the proliferation of
glioblastoma cells via ERK1/2, p38 MAPK and caspase-3. J
Neurooncol. 132:15–26. 2017. View Article : Google Scholar
|
|
69
|
Li J, Lian ZG, Xu YH, Liu RY, Wei ZQ, Li
T, Lv HT, Zhao YS, Liu YJ, Dong B and Fu X: Downregulation of
nuclear protein-1 induces cell cycle arrest in G0/G1 phase in
glioma cells in vivo and in vitro via P27. Neoplasma. 67:843–850.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mohammad HP, Seachrist DD, Quirk CC and
Nilson JH: Reexpression of p8 contributes to tumorigenic properties
of pituitary cells and appears in a subset of prolactinomas in
transgenic mice that hypersecrete luteinizing hormone. Mol
Endocrinol. 18:2583–2593. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Passe CM, Cooper G and Quirk CC: The
murine p8 gene promoter is activated by activating transcription
factor 4 (ATF4) in the gonadotrope-derived LbetaT2 cell line.
Endocrine. 30:81–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jin HO, Seo SK, Woo SH, Choe TB, Hong SI,
Kim JI and Park IC: Nuclear protein 1 induced by ATF4 in response
to various stressors acts as a positive regulator on the
transcriptional activation of ATF4. IUBMB Life. 61:1153–1158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Averous J, Lambert-Langlais S, Cherasse Y,
Carraro V, Parry L, B'chir W, Jousse C, Maurin AC, Bruhat A and
Fafournoux P: Amino acid deprivation regulates the stress-inducible
gene p8 via the GCN2/ATF4 pathway. Biochem Biophys Res Commun.
413:24–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sárvári M, Kalló I, Hrabovszky E, Solymosi
N, Tóth K, Likó I, Molnár B, Tihanyi K and Liposits Z: Estradiol
replacement alters expression of genes related to neurotransmission
and immune surveillance in the frontal cortex of middle-aged,
ovariectomized rats. Endocrinology. 151:3847–3862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu X, Huang E, Tai Y, Zhao X, Chen X, Chen
C, Chen R, Liu C, Lin Z, Wang H and Xie WB: Nupr1 modulates
methamphet-amine-induced dopaminergic neuronal apoptosis and
autophagy through CHOP-Trib3-mediated endoplasmic reticulum stress
signaling pathway. Front Mol Neurosci. 10:2032017. View Article : Google Scholar
|
|
76
|
Goruppi S, Patten RD, Force T and Kyriakis
JM: Helix-loop-helix protein p8, a transcriptional regulator
required for cardiomyocyte hypertrophy and cardiac fibroblast
matrix metalloprotease induction. Mol Cell Biol. 27:993–1006. 2007.
View Article : Google Scholar :
|
|
77
|
Sambasivan R, Cheedipudi S, Pasupuleti N,
Saleh A, Pavlath GK and Dhawan J: The small chromatin-binding
protein p8 coordinates the association of anti-proliferative and
pro-myogenic proteins at the myogenin promoter. J Cell Sci.
122:3481–3491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Taïeb D, Malicet C, Garcia S, Rocchi P,
Arnaud C, Dagorn JC, Iovanna JL and Vasseur S: Inactivation of
stress protein p8 increases murine carbon tetrachloride
hepatotoxicity via preserved CYP2E1 activity. Hepatology.
42:176–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vasseur S, Hoffmeister A, Garcia-Montero
A, Mallo GV, Feil R, Kühbandner S, Dagorn JC and Iovanna JL:
p8-deficient fibroblasts grow more rapidly and are more resistant
to adriamycin-induced apoptosis. Oncogene. 21:1685–1694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kallwellis K, Grempler R, Günther S, Päth
G and Walther R: Tumor necrosis factor alpha induces the expression
of the nuclear protein p8 via a novel NF kappaB binding site within
the promoter. Horm Metab Res. 38:570–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yammani RR and Loeser RF: Brief report:
Stress-inducible nuclear protein 1 regulates matrix
metalloproteinase 13 expression in human articular chondrocytes.
Arthritis Rheumatol. 66:1266–1271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang YF, Vaccaro MI, Fiedler F, Calvo EL
and Iovanna JL: Lipopolysaccharides induce p8 mRNA expression in
vivo and in vitro. Biochem Biophys Res Commun. 260:686–690. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Motoo Y, Iovanna JL, Mallo GV, Su SB, Xie
MJ and Sawabu N: P8 expression is induced in acinar cells during
chronic pancreatitis. Dig Dis Sci. 46:1640–1646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hollenbach M, Klöting N, Sommerer I,
Lorenz J, Heindl M, Kern M, Mössner J, Blüher M and Hoffmeister A:
p8 deficiency leads to elevated pancreatic beta cell mass but does
not contribute to insulin resistance in mice fed with high-fat
diet. PLoS One. 13:e02011592018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bratland A, Risberg K, Maelandsmo GM,
Gützkow KB, Olsen OE, Moghaddam A, Wang MY, Hansen CM, Blomhoff HK,
Berg JP, et al: Expression of a novel factor, com1, is regulated by
1,25-dihydroxyvitamin D3 in breast cancer cells. Cancer Res.
60:5578–5583. 2000.PubMed/NCBI
|
|
86
|
Santofimia-Castaño P, Xia Y, Lan W, Zhou
Z, Huang C, Peng L, Soubeyran P, Velázquez-Campoy A, Abián O,
Rizzuti B, et al: Ligand-based design identifies a potent NUPR1
inhibitor exerting anticancer activity via necroptosis. J Clin
Invest. 129:2500–2513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Neira JL, Bintz J, Arruebo M, Rizzuti B,
Bonacci T, Vega S, Lanas A, Velázquez-Campoy A, Iovanna JL and
Abián O: Identification of a drug targeting an intrinsically
disordered protein involved in pancreatic adenocarcinoma. Sci Rep.
7:397322017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Deng ZH, Meng J, Tang J, Hu GY and Tao LJ:
Fluorofenidone Inhibits the Proliferation of Lung Adenocarcinoma
Cells. J Cancer. 8:1917–1926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Santofimia-Castaño P, Rizzuti B, Abián O,
Velázquez-Campoy A, Iovanna JL and Neira JL: Amphipathic helical
peptides hamper protein-protein interactions of the intrinsically
disordered chromatin nuclear protein 1 (NUPR1). Biochim Biophys
Acta Gen Subj. 1862:1283–1295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Botto C, Augello G, Amore E, Emma MR,
Azzolina A, Cavallaro G, Cervello M and Bondì ML: Cationic solid
lipid nanoparticles as non viral vectors for the inhibition of
hepatocellular carcinoma growth by RNA interference. J Biomed
Nanotechnol. 14:1009–1016. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khan HY, Ge J, Nagasaka M, Aboukameel A,
Mpilla G, Muqbil I, Szlaczky M, Chaker M, Baloglu E, Landesman Y,
et al: Targeting XPO1 and PAK4 in 8505C anaplastic thyroid cancer
cells: Putative implications for overcoming lenvatinib therapy
resistance. Int J Mol Sci. 21:2372019. View Article : Google Scholar
|
|
92
|
Vincent AJ, Ren S, Harris LG, Devine DJ,
Samant RS, Fodstad O and Shevde LA: Cytoplasmic translocation of
p21 mediates NUPR1-induced chemoresistance: NUPR1 and p21 in
chemoresistance. FEBS Lett. 586:3429–3434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Palam LR, Gore J, Craven KE, Wilson JL and
Korc M: Integrated stress response is critical for gemcitabine
resistance in pancreatic ductal adenocarcinoma. Cell Death Dis.
6:e19132015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Augello G, Emma MR, Cusimano A, Azzolina
A, Mongiovì S, Puleio R, Cassata G, Gulino A, Belmonte B,
Gramignoli R, et al: Targeting HSP90 with the small molecule
inhibitor AUY922 (luminespib) as a treatment strategy against
hepatocellular carcinoma. Int J Cancer. 144:2613–2624. 2019.
View Article : Google Scholar
|
|
95
|
Yu SL, Lee DC, Baek SW, Cho DY, Choi JG
and Kang J: Identification of mTOR inhibitor-resistant genes in
cutaneous squamous cell carcinoma. Cancer Manag Res. 10:6379–6389.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Real NE, Castro GN, Darío Cuello-Carrión
F, Perinetti C, Röhrich H, Cayado-Gutiérrez N, Guerrero-Gimenez ME
and Ciocca DR: Molecular markers of DNA damage and repair in
cervical cancer patients treated with cisplatin neoadjuvant
chemotherapy: An exploratory study. Cell Stress Chaperones.
22:811–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schroll MM, LaBonia GJ, Ludwig KR and
Hummon AB: Glucose restriction combined with autophagy inhibition
and chemotherapy in HCT 116 spheroids decreases cell clonogenicity
and viability regulated by tumor suppressor genes. J Proteome Res.
16:3009–3018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Schnepp PM, Shelley G, Dai J, Wakim N,
Jiang H, Mizokami A and Keller ET: Single-cell transcriptomics
analysis identifies nuclear protein 1 as a regulator of docetaxel
resistance in prostate cancer cells. Mol Cancer Res. 18:1290–1301.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Urrutia R, Velez G, Lin M, Lomberk G,
Neira JL and Iovanna J: Evidence supporting the existence of a
NUPR1-like family of helix-loop-helix chromatin proteins related
to, yet distinct from, AT hook-containing HMG proteins. J Mol
Model. 20:23572014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ree AH, Pacheco MM, Tvermyr M, Fodstad O
and Brentani MM: Expression of a novel factor, com1, in early tumor
progression of breast cancer. Clin Cancer Res. 6:1778–1783.
2000.PubMed/NCBI
|
|
101
|
Cano CE, Sandí MJ, Hamidi T, Calvo EL,
Turrini O, Bartholin L, Loncle C, Secq V, Garcia S, Lomberk G, et
al: Homotypic cell cannibalism, a cell-death process regulated by
the nuclear protein 1, opposes to metastasis in pancreatic cancer.
EMBO Mol Med. 4:964–979. 2012. View Article : Google Scholar : PubMed/NCBI
|