Introduction
Anti-Müllerian hormone (AMH), a TGFβ family member,
acts by binding to its specific receptor (AMH type II receptor;
AMHRII) that recruits type I receptors (AMHRIs) activin
receptor-like kinase (ALK)2, ALK3 and ALK6. AMHRI phosphorylation
induces SMAD 1/5/8 phosphorylation and their migration into the
nucleus where, through SMAD4, they regulate various genes depending
on the target tissue (1,2). Preclinical in vitro and in
vivo studies and data obtained using clinical samples have
demonstrated that AMHRII and the AMH/AMHRII signaling pathway are
potential therapeutic targets in gynecological tumors (3-9),
particularly in ovarian carcinoma (10).
The AMH/AMHRII signaling cascade can be targeted
using anti-AMHRII antibodies. Among the available anti-AMHRII
antibodies (11) and antibody
fragments (12,13), the monoclonal antibody (MAb) 12G4
and its humanized version (GM-102 or murlentamab) have been
extensively evaluated in preclinical studies (14-17), and murlentamab is now tested in
clinical trials (trial nos. NCT02978755 and NCT03799731). The
mechanism of action of murlentamab involves antibody-dependent
cell-mediated cytotoxicity and antibody-dependent cell
phagocytosis, but no or low apoptosis depending on the model,
suggesting that its efficacy is not directly related to the AMH
signaling pathway (14,15).
To understand why the AMH signaling pathway is not
implicated in the underlying mechanisms of the effects of
murlentamab, the present study aimed to analyze the role of the
three AMHRIs (ALK2, ALK3 and ALK6) in ovarian carcinoma cell lines
and primary carcinoma cells isolated from ascites samples of
patients with ovarian carcinoma. Although the roles of ALK2, ALK3
and ALK6 have been studied in several cell types during development
and in other physiological conditions (18-24), limited data are available on their
roles in cancer. Basal et al (25) have demonstrated that AMHRII, ALK2,
ALK3 and ALK6 are expressed in epithelial ovarian cancer specimens,
but have not assessed their functions.
The results of the present study demonstrated that
ALK2 and ALK3 were the two main AMHRIs implicated in AMH signaling
in four ovarian cancer cell lines, and that their role was
regulated by AMH concentration. Specifically, in the presence of
supraphysiological concentrations of AMH (25 nM recombinant AMH),
ALK3 was recruited, heterodimerized with AMHRII, and induced
apoptotic effects. Conversely, at physiological endogenous AMH
concentration (10 pM), AMH promoted cancer cell viability through
ALK2 recruitment. Therefore, bispecific antibodies (BsAb) against
AMHRII and ALK2, and against AMHRII and ALK3 were designed and
evaluated. The results demonstrated that the anti-AMRII-ALK2 BsAb
12G4-2F9 reduced the growth of COV434-AMHRII tumor cell xenografts
in vivo. Taken together, these results may provide a deeper
understanding of the AMH signaling pathways and may lead to an
innovative therapeutic approach to modulate AMH signaling using
anti-AMHRII/AMHRI BsAbs.
Materials and methods
AMH production and assay
Active recombinant AMH (LR-AMH) (9,26)
was produced in CHO cells (Evitria AG) using proprietary media and
culture conditions according to the WO2014/164891 patent (27) (Fig.
S1). LR-AMH is a full-length protein that is completely
cleaved, thus combining efficiency and stability (26,28). It contains the 24AA leader
sequence of albumin instead of the AMH leader sequence to increase
production and secretion, and the RARR/S furin/kex2 consensus site
instead of the native AMH RAQR/S sequence at position 423-428 to
improve cleavage. AMH was quantified using the Elecsys®
AMH PLUS kit (Roche Diagnostics). For western blot analysis,
recombinant AMH commercialized by Origen (cat. no. TP308397) was
used as the control AMH with an anti-AMH antibody (cat. no.
ab84952; Abcam) that recognizes the C-terminal domain. All
experiments involving LR-AMH were performed in culture medium
containing 1% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) as bovine AMH can signal through human AMHRII
(29). In these experimental
conditions, endogenous AMH concentration in fresh medium ranged
between 5 and 10 pM.
Selection of anti-ALK2 and anti-ALK3
single-chain variable fragments (scFvs)
Anti-ALK2 and anti-ALK3 scFvs were isolated from the
human scFv phage display library Husc I (30,31) by three successive panning rounds
using an ECD-Fc construct (cat. no. 637-AR-100; R&D Systems,
Inc.) for ALK2 and an ECD-Fc (cat. no. 2406-BR-100; R&D
Systems, Inc.) or an ECD-Poly-His (cat. no. 10446-H08H; Sino
Biological, Inc.) construct for ALK3. Two scFv antibodies were
selected for each target according to the following criteria: i)
Their binding to the receptor in ELISA; ii) their variable peptide
sequence to target different epitopes; and iii) the number of
potential glycosylation sites present in their sequences to limit
the need for mutagenesis. All potential glycosylation sites in the
heavy chain variable domain (VH) and light chain variable domain
(VL) of the selected scFv antibodies were suppressed by
site-directed mutagenesis (Genewiz, Inc.): The VH threonine 75
(Kabat numbering) was reversed to the germline lysine, whereas
serine to alanine mutations were performed in the
complementarity-determining region Asn-x-Ser motifs (1, 1 and 4 for
2C1, 2F9 and 3D7, respectively) to avoid binding inhibition due to
N-glycosylation.
BsAb constructs
A generic platform of tetravalent IgG1-like BsAbs
was used (32,33). As described in our previous study
(32), BsAb synthesis requires
the production of one fused heavy chain and two free light chains.
The fused heavy chain contained, from the N- to the C-terminus, the
VH/heavy chain constant domain (CH)1 domain of the anti-AMHRII MAb
12G4 (11) in position 2
(Fig. S2), the VH/CH1 domain of
an anti-AMHRI antibody (anti-ALK2 or ALK3 Mab) in position 1, and a
human IgG1 Fc domain, as well as two free light chains, namely the
light chains of the anti-AMHRII and anti-AMHRI MAbs. The fragment
antigen binding (Fab) sequences from the anti-AMHRII MAb were
inserted in position 2 of each BsAb construct (the furthest away
from the Fc fragment) to ensure specific binding to their target
(32). The anti-ALK2 and
anti-ALK3 Fab sequences were inserted in position 1 of the BsAb
constructs, closer to the Fc fragment. To control the heavy
chain/light chain ratio, two plasmids were constructed. Plasmid I
was a bicistronic construct that contained, from the 5′ to the 3′
end: i) The cDNA encoding the complete sequence of anti-AMHRII MAb
light chain under the control of the cytomegalovirus (CMV)
promoter; ii) an internal ribosome entry site (IRES) sequence from
ECMV (34); and iii) the cDNA
encoding the complete fused heavy chain bearing the VH/CH1 domain
of the anti-AMHRII MAb, the VH/CH1 domain of the anti-ALK2 or
anti-ALK3 MAb and the cDNA encoding the human IgG1 Fc domain.
Plasmid II contained the cDNA encoding the complete sequence of the
anti-AMHRI MAb light chain (anti-ALK2 or anti-ALK3 MAb) under the
control of the CMV promoter. In the control BsAb that targeted only
AMHRII and CD5 (anti-AMHRII-CD5), the cDNA encoding the VH and VL
of the anti-human CD5 MAb 0490 (35) were inserted into plasmids I and II
instead of the anti-AMHRI MAb sequences.
Antibody production
Anti-ALK2 and anti-ALK3 IgG1 and BsAbs were produced
in CHO (Evitria AG) and 293T cells (ATCC® CRL-1573) For
antibody production in 293T cells, the cells were cultured in 150
mm2 dishes to 70% confluence. A 1:1 mixture of 30
µg of plasmids I and II, and 240 µg of the
transfection agent polyethylenimine (PolyScience) was maintained at
room temperature (RT) for 10 min and subsequently added to the
cells for 6 h at 37°C. Following incubation, the transfection
medium was replaced with DMEM-F12 (Gibco; Thermo Fisher Scientific,
Inc.) without FBS. After 5 days at 37°C, the supernatant was
collected by centrifugation at 300 × g for 5 min at 4°C and diluted
(1:1) with 40 mM sodium phosphate buffer, pH 8, filtered through a
0.22-µm filter and purified on a 1-ml protein A column for
24 h at 4°C. The antibodies were eluted with glycine, pH 3, and
immediately stabilized with Tris buffer, pH 9.
Amicon-ultra® filters (MiliporeSigma) with a cut-off of
50 kDa were used for antibody concentration in PBS; 200 ml cell
culture provided ~1 mg of purified antibody.
ELISA assay
The half maximal effective concentration
(EC50) of the antibodies was determined by ELISA.
Maxisorp nunc-immuno® plates (Thermo Fisher Scientific,
Inc.) were coated with 500 ng/ml anti-HIS antibody (clone HIS-1;
cat. no. A7058; Sigma-Aldrich; Merck KGaA) overnight at 4°C to
capture the respective antigens of recombinant human ALK2 (cat. no.
10227-H08B; Sino Biological, Inc.), ALK3 (cat. no. 10446-H08H; Sino
Biological, Inc.) or AMHRII (4749-MR-050; R&D Systems, Inc.)
used at 500 ng/ml. The plates were subsequently washed three times
with PBS/0.01% Tween-20 and incubated with PBS/0.01% Tween-20/2%
BSA (Sigma-Aldrich; Merck KGaA) solution for 2 h at RT for
blocking. Following three washes with PBS/0.01% Tween-20, the
antibodies were added to a final concentration of ranging between
0.08 and 333 nM, and incubated at 37°C for 90 min, followed by
treatment with the anti-human Fc peroxidase secondary antibody
(HRP-conjugated; cat. no. A0170; Sigma-Aldrich; Merck KGaA) for 30
min and the enzyme substrate (3,3′,5,5′-tetramethylbenzidine; cat.
no. 34028; Thermo Fisher Scientific, Inc.). Absorbance was read at
450 nm using a microplate reader after stopping the enzyme reaction
with sulfuric acid.
Cell lines
The human COV434 (sex cord stromal tumor) (36,37) and KGN (granulosa cell tumor)
(38) cell lines were kind gifts
from Dr. Schrier (Department of Clinical Oncology, Leiden
University Medical Center, Leiden, Netherlands) and Dr Yanase
(Kyushu University, Fukuoka, Japan), respectively. The SKOV3 cell
line (high grade serous ovarian cancer) was obtained from ATCC
(ATCC® HTB-77), and the NIH-OVCAR8 cell line (high grade
serous ovarian cancer) was obtained from the Division of Cancer
Treatment and Diagnosis, NCI, Frederick, MD, USA. Cells were
cultured in DMEM:F12 medium without phenol red containing 10%
heat-inactivated FBS. COV434-AMHRII and SKOV3-AMHRII cells were
supplemented with 0.33 mg/ml geneticin (cat. no. ant-gn-1;
InvivoGen). Cells were cultured at 37°C in a humidified atmosphere
with 5% CO2, and the medium was replaced twice per week.
Cells were harvested with 0.5 mg/ml trypsin and 0.2 mg/ml EDTA. All
culture media and supplements were purchased from Thermo Fisher
Scientific, Inc. 293T cells were cultured in DMEM:F12 with phenol
red containing 10% heat-inactivated FBS. Cells were tested for the
absence of Mycoplasma every other week using
MycoAlert® mycoplasma detection kit (cat. nos. LT07-318
and LT07-518; Lonza Group AG). All cell lines were authenticated by
Eurofins Human Cell Line Authentication Services.
The COV434-AMHRII and SKOV3-AMHRII cell lines were
generated by transfection of the cDNA encoding full-length human
AMHRII as previously described (17). The cDNA encoding full-length human
AMHRII in the pCMV6 plasmid (gifted by Dr Teixeira, Pediatric
Surgical Research Laboratories, Massachusetts General Hospital,
Harvard Medical School, Boston, USA) was first subcloned in the
pcDNA3.1.myc-His vector (Invitrogen; Thermo Fisher Scientific,
Inc.) using the EcoRI and XhoI restriction sites
(enzymes from New England BioLabs, Inc.), and subsequently, using
the EcoRI and SalI sites, in the pIRES1-EGFP vector,
gifted by Dr F Poulat (Institut de Génétique Humaine, CNRS,
Montpellier, France). At 24 h prior to transfection, COV434 cells
were seeded in 150-mm cell culture dishes at 80% confluence. The
AMHRII construct was transfected using the Fugene transfection kit
(Promega Corporation) according to the manufacturer's protocol.
Following a 48-h incubation at 37°C, the transfection medium was
replaced with fresh medium containing 0.5 mg/ml geneticin and was
subsequently changed twice per week for two weeks. The cells were
harvested and sorted using a FACSAria cytometer (Becton-Dickinson
and Company) in 96-well plates. For each cell line, a clone that
strongly expressed AMHRII was selected and designated as
COV434-AMHRII and SKOV3-AMHRII, respectively (Fig. S3).
Primary tumor cells from ascites
Ascites samples from three patients with ovarian
cancer were obtained from the Institut du Cancer de Montpellier
(ICM) according to the French laws and after their informed written
consent. All patient samples and data were retrieved from the ICM
ovarian cancer clinical-biological database that had been approved
by the independent Sud Méditerranée III Ethics Committee (study
reference, 2016.09.06). Patients were chemotherapy-naïve and
waiting for surgical intervention. Freshly obtained ascites were
aliquoted in 50 ml conical centrifuge tubes and centrifuged at 300
× g for 5 min at 4°C. Cell pellets were resuspended in
ammonium-chloride-potassium buffer (pH:7.2) (150 nM
NH4Cl, 10 nM KHCO3 and 0.1 nM
Na2EDTA) to lyse red blood cells on ice for 5 min. The
process was repeated until the lysis was complete. Subsequently,
the cell pellets were plated in 150-mm cell culture dishes with 20
ml DMEM F12-Glutamax (Gibco; Thermo Fisher Scientific, Inc.) and
10% FBS. Cells were subsequently plated in DMEM:F12 with 10% FBS
for 30 min to rapidly eliminate adherent fibroblasts as previously
described (39). Non-adherent
cells were transferred to new dishes with DMEM:F12 and 10% FBS.
Low-passage (between 2 and 6) cells were used for experiments or
frozen in liquid nitrogen.
Small interfering (si)RNA
transfection
siRNA sequences were designed using the Rosetta
algorithm by Sigma-Aldrich; Merck KGaA. A pool of three siRNAs
(siRNA Nano Scale; Sigma-Aldrich; Merck KGaA) was used for each ALK
receptor (ALK2: SIHK0175, SIHK0173, SIHK0174; ALK3: SIHK0170,
SIHK0171, SIHK0172; ALK6: SIHK0175, SIHK0173, SIHK0174). MISSION
esiRNA (cat. no. EHU121781; Sigma-Aldrich; Merck KGaA), a
heterogeneous mixture of siRNA that target human AMH, was used to
silence AMH. COV434-AMHRII and SKOV3-AMHRII cells were plated in
24-well plates at 60-80% confluency. Transfection was performed in
DMEM:F12 medium with 1% FBS using Lipofectamine® RNAiMax
Transfection Reagent diluted in Opti-MEM Medium (cat. nos.
13778-150 and 11058021; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer' s instructions. siRNAs were diluted
to 3 pM, and the siRNA-Lipofectamine (1:1) mixture was added to the
cells for 6 h at 37°C. Cells were washed and cultured in DMEM: F12
with 1% FBS. Experiments with siRNA-transfected cells were
performed at 24 (COV434-AMHRII cells) or 48 (SKOV3-AMHRII cells) h
post-transfection.
Western blot analysis
Cells were washed with PBS and scraped immediately
in RIPA lysis buffer (Santa Cruz Biotechnology, Inc.) with 200 mM
PMSF, 100 mM sodium orthovanadate and a protease inhibitor
cocktail. The protein concentration was determined using the BCA
assay protein quantitation kit (Interchim). Cell extracts were
heated at 95°C for 5 min, separated (50 µg protein/lane) by
10% SDS-PAGE in reducing conditions (5% 2β-mercaptoethanol), and
transferred to PVDF membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked in Tris-buffered saline with 0.1% Tween-20
and 5% non-fat dry milk, and probed with the relevant primary
antibodies at RT for 1 h. Following washing with PBS-Tween 0.01%,
peroxidase-conjugated IgG secondary antibodies were added
(1:10,000) at RT for 1 h. After washing, antibody-antigen
interactions were detected using Immobilon® ECL Ultra
Western HRP Substrate (Merck KGaA). To verify equal loading,
immunoblots were also probed with an anti-GAPDH monoclonal antibody
diluted at 1:1,000 (cat. no. 8884; Cell Signaling Technology,
Inc.).
Reverse transcription-PCR
RNA from 1×106 COV434-AMHRII and
SKOV3-AMHRII cells was extracted using the RNeasy Mini Kit (Qiagen
GmbH) according to the manufacturer's instructions. A total of 1
µg RNA was reverse transcribed using SuperScript III Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. PCR was performed to amplify the cDNA
sequences with specific primers for each ALK receptor using the
Dream Taq Green PCR Master Mix (Thermo Fisher Scientific, Inc.), 10
µM primers and 2.5 µl cDNA with the following
conditions: initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 60 sec, 56.9°C for 90 sec and 72°C for 90 sec,
and final extension at 72°C for 5 min. PCR analysis of Alk receptor
expression was performed using the following primers: ALK2 forward,
5′-TTAAAAGGCGCAACCAAGA-3′ and reverse, 5′-CGTACAACGATCCCATTTCA-3′;
ALK3 forward, 5′-TTTATGGCACCCAAGGAAAG-3′ and reverse,
5′-TGGTATTCAAGGGCACATCA-3′; ALK6 forward,
5′-CTCAGGGAGCGACCTGGGCA-3′ and reverse, 5′-GCGGCCCCAAATGCAGGGAT-3′.
A total of 20 µl of each PCR sample was used for agarose gel
electrophoresis and visualized under UV using G:BOX (Pxi 4;
Syngene).
AMH pathway analysis
OVCAR8, KGN, COV434-AMHRII and SKOV3-AMHRII cells
were cultured in DMEM:F12 with 1% FBS medium overnight and
incubated with LR-AMH (0-25 nM) at 37°C for 6 h. Western blotting
was performed as aforementioned using anti-phosphorylated
(p)SMAD1/5 (cat. no. 9516Cell Signaling Technologies, Inc.),
anti-pAKT (cat. no. 9271; Cell Signaling Technologies, Inc.),
anti-cleaved caspase-3 (cat. no. 9661; Cell Signaling Technologies,
Inc.,), anti-cleaved PARP (cat. no. 9546; Cell Signaling
Technologies, Inc.), anti-GAPDH, anti-ALK2 (cat. no. ab60158;
Abcam) and anti-ALK3 (cat. no. ab38560; Abcam) primary antibodies
diluted at 1:1,000 overnight at 4°C, followed by anti-rabbit and
anti-goat IgG HRP secondary antibodies (1:10,000; cat. nos. AP106P
and AP188P; Sigma-Aldrich; Merck KGaA) at RT for 1 h. Since
previous studies demonstrated that total SMAD1/5, total AKT,
uncleaved caspase-3 and uncleaved PARP were not modified under AMH
treatment, these total proteins were not systematically blotted
(7,40,41).
Clonogenic survival
COV434-AMHRII and SKOV3-AMHRII cells were plated in
24-well plates (50 cells/well) in DMEM:F12 with 1% FBS and cultured
overnight under normal culture conditions (5% CO2,
37°C). LR-AMH (0-25 nM) was subsequently added to the medium for 11
days. For COV434-AMHRII cells, the colonies were fixed in
methanol/acetic acid solution (3:1) at 4°C for 20 min, stained with
10% Giemsa and visible clones were counted manually. For
SKOV3-AMHRII cells, for which direct clone counting was difficult,
the Giemsa-stained clones were bleached with 70% ethanol for 30 min
at RT, and the bleaching solution was analyzed at 595 nm by a plate
reader to obtain a global estimate of the clone number and
size.
Apoptosis assay
Apoptosis initiation was determined using the
Caspase-Glos-3/7 assay (cat. no. G8090; Promega Corporation). Cells
plated in white 96-well plates were incubated with LR-AMH (0-25 nM)
for 6 h at 37°C. Upon addition of the proluminescent caspase-3/7
DEVD-aminoluciferin substrate, caspase-3/7 cleavage of this
substrate releases aminoluciferin that is consumed by luciferase to
produce a luminescent signal, proportional to the caspase-3/7
activity. The luminescent signal was quantified with a PHERASTAR
microplate reader 30 min after substrate addition.
Apoptosis was assessed using the Annexin V-FITC
Apoptosis Detection kit (cat. no. IM3614; Beckman Coulter, Inc.). A
total of ~1×105 COV434-AMHRII and SKOV3-AMHRII
cells/well were seeded in 24-well plates and incubated with or
without 25 nM LR-AMH, or 150 nM staurosporin (positive control) for
24 h. Adherent and detached cells were collected and centrifuged at
200 × g for 5 min at 4°C. Following washing with PBS, cells were
stained with 130 µl of a mixture containing 10 µl
FITC-labeled Annexin V and 20 µl 7AAD in 10 µl
annexin buffer on ice in the dark for 15 min. Following addition of
400 µl annexin buffer, the fluorescence data were acquired
by a Kaluza flow cytometer (Beckman Coulter, Inc.) within 30 min,
and analyzed with the Kaluza Flow Analysis software 2.1 (Beckman
Coulter, Inc.). Flow cytometry data were expressed as the
percentage of FITC-, AAD- or FITC and AAD-positive cells in
5×10= counted cells.
Immunofluorescence
For each experiment, 3×104 OVCAR8, KGN,
COV434-AMHRII and SKOV3-AMHRII cells were cultured on 22-mm square
glass coverslips in 35-mm culture dishes in DMEM:F12 with 10% FBS
overnight at 37°C. Cells were subsequently starved with 1% FBS
medium for 24 h prior to incubation with 25 nM LRMIS for 90 min at
37°C. Cells were then fixed and permeabilized in 3.7%
paraformaldehyde/PBS for 40 min, washed twice with PBS and once
with PBS/0.1% BSA, and blocked with PBS/1%BSA for 1 h at RT.
Subsequently, cells were incubated with P3X63 (42) or 13R4 (30) (1:200; irrelevant antibodies used
as negative controls), the anti-AMHRII 12G4, and anti-ALK2 (cat.
no. AF637), anti-ALK3 (cat. no. AF346) and anti-ALK6 (cat. no.
MAB5051) (all from R&D Systems, Inc.) primary antibodies in the
dark for 1 h. Following three washes with PBS, cells were incubated
with anti-goat (1:1,000; cat. no Ab6881; Abcam) or anti-mouse
(1:1,000; cat. no Ab6785; Abcam) FITC-labeled secondary antibodies
in PBS/0.1% BSA for 1 h, followed by three washes with PBS/0.1%
Tween-20 and three washes with PBS. Coverslips were mounted with
EverBrite™ Hardest Mounting Medium with DAPI (Biotium, Inc.,). For
each cell line, >150 cells were examined to select the
representative fields, and ≥3 representative fields for each sample
were analyzed the following day under a fluorescence Zeiss Axioplan
2 Imaging microscope (Zeiss AG). Receptor (green) and nucleus
(blue) labeling were estimated with ImageJ bundled with 64-bit Java
1.8.0_172 (National Institutes of Health) using the Color Histogram
plugin (http://rsb.info.nih.gov/ij/plugins/color-histogram.html)
to present the data as receptor/nucleus ratios.
In vivo ovarian cancer cell xenograft
assay
All animal experiments were performed in compliance
with the guidelines of the French government and the INSERM
regulations (agreement no. D34-172-27). Athymic nude Hsd female
mice (6-8-week-old; Envigo RMS SARL) were used. At day 0 (D0),
7×106 human COV434-AMHRII cells in 150 µl BD
Matrigel® (ratio 1:1), were subcutaneously grafted into
the right flank. Mice were anesthetized with inhaled isoflurane.
Inhalation anesthesia was delivered by an induction chamber and a
face mask with the following dosing: 4-5% for induction; 1-2% for
maintenance. At D14, when tumor volume reached 80-100
mm3, mice were randomized (n=8-10 mice/group) and
treatments (17 mg/kg BsAbs or saline) were administered by
intraperitoneal injection twice per week for 4 weeks. Tumor
diameters (D) were measured in the 3 dimensions with a caliper once
per week, and tumor volumes were calculated using the formula:
Volume = D1 × D2 × D3/2. Tumor growth inhibition was calculated as
T/C% = (median tumor volume of treated group at day X/median tumor
volume of control group at day X) ×100. When the tumors reached a
volume of 1,500 mm3, mice were sacrificed by exposure to
CO2 without removing animals from their home cage with a
flow rate displacing 20% of the cage volume per minute. The
presumed death after exposure to carbon dioxide was confirmed based
on careful assessment of the animal for unambiguous signs of death,
such as cardiac arrest or fixed, dilated pupils. Sacrifice dates
ranged from D31 (February 27th, 2020; first mouse in the saline
control group) to D62 (March 29th, 2020; last mouse in the 12G4-2F9
BsAb group).
Statistical analysis
Statistical analyses of caspase-3/7 activity and
cell viability/proliferation data were performed using GraphPad
Prism software 7 (GraphPad Software, Inc.) by one-way ANOVA with
Tukey's multiple comparison test. Other statistical analyses were
performed using the STATA 16.0 software (StataCorp LP). A linear
mixed regression model was used to determine the association
between tumor growth and the number of days post-graft. The fixed
part of the model included variables corresponding to the number of
days post-graft and the different groups. Interaction terms were
built into the model. Random intercept and random slope were
included to account for the time effect. The coefficients of the
model were estimated by maximum likelihood. P<0.05 was
considered to indicate a statistically significant difference.
Results
Recombinant A MH induces A MH signaling
in COV434-AMHRII and SKOV3-AMHRII cells
To enable the study of various AMHRIs in AMH/AMHRII
signaling, two ovarian cancer cell lines that stably expressed
AMHRII were established: COV434-AMHRII (17) and SKOV3-AMHRII. AMHRII expression
levels in cell lines derived from ovarian carcinomas and ascites
rapidly and progressively decrease after long-term culture
(9,15), thus limiting experimental
reproducibility. The present study further aimed to determine
whether AMH signaling and its downstream effects were intact in
COV434-AMHRII and SKOV3-AMHRII cells. In both cell lines, SMAD1/5
phosphorylation was induced by increasing concentrations of the
recombinant AMH protein LR-AMH (9,26)
(from 1.6 to 25 nM; Fig. 1A).
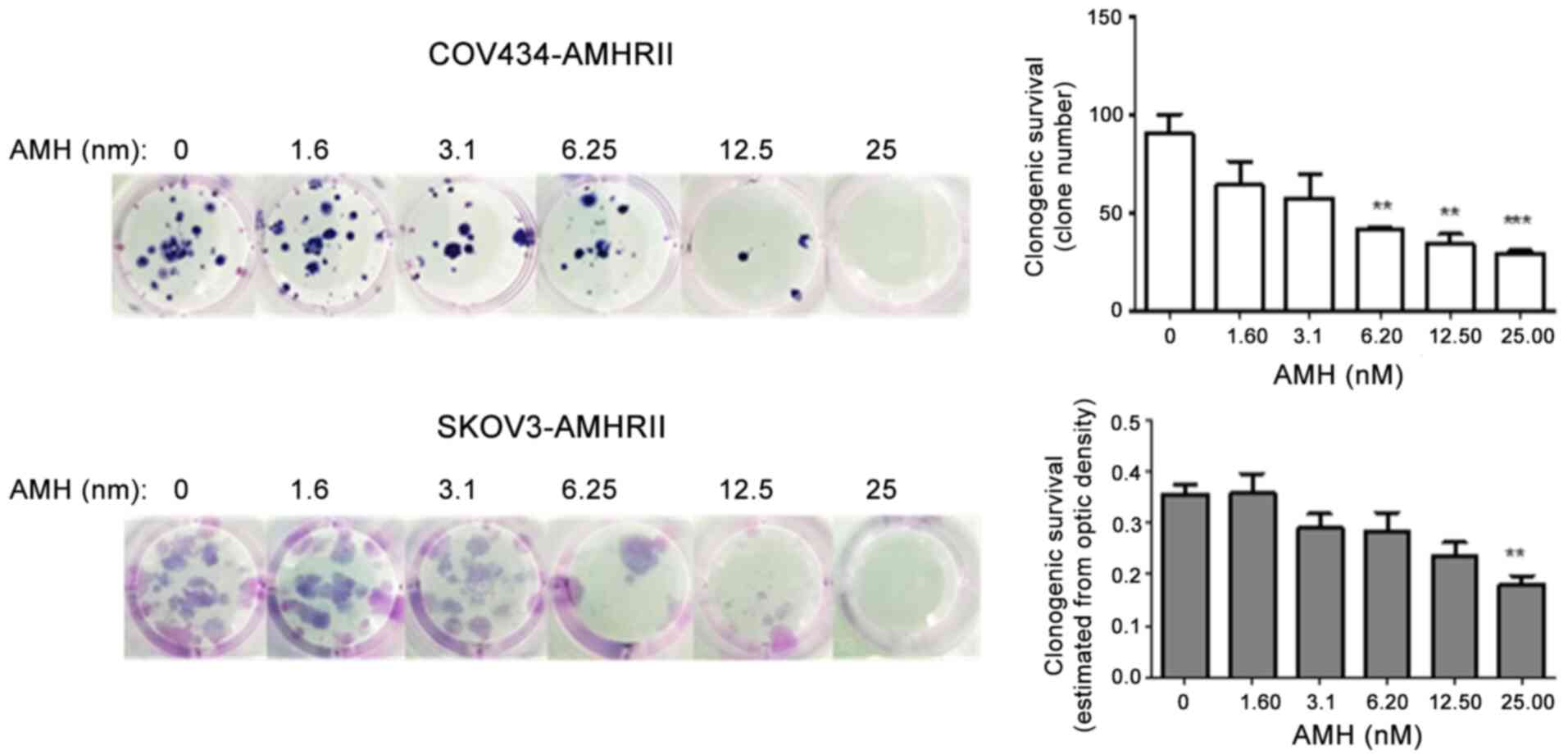
Apoptosis, evaluated by measuring caspase-3/7 activity, was
significantly induced compared with vehicle-treated cells starting
at 12.5 nM LR-AMH in COV434-AMHRII cells and at 6.3 nM LR-AMH in
SKOV3-AMHRII cells (Fig. 1B,
upper panels). Apoptosis induction was further confirmed by western
blot analysis of cleaved caspase-3/7 and cleaved PARP (Fig. 1B, lower panels). In addition, flow
cytometry analysis results demonstrated that incubation with 25 nM
LR-AMH for 24 h increased the apoptotic rate in COV434-AMHRII cells
compared with that in untreated cells (15.3 vs. 5.1% Annexin
V-positive cells and 11.8 vs. 5.6% Annexin V/7AAD-positive cells),
as well as in SKOV3-AMHRII cells compared with that in the
corresponding untreated cells (8.5 vs. 7.4% Annexin V-positive
cells and 13.7 vs. 4.8% Annexin V/7AAD-positive cells) (Figs. 1C and S4). AMH concentrations of 6.2 and 25 nM
reduced the clonogenic ability by >50% in COV434-AMHRII and
SKOV3-AMHRII cells, respectively, compared with that in the
corresponding untreated cells (Fig.
2). These results suggested that COV434-AMHRII and SKOV3-AMHRII
cells may be suitable models to study the roles of ALK2, ALK3 and
ALK6 in AMH signaling.
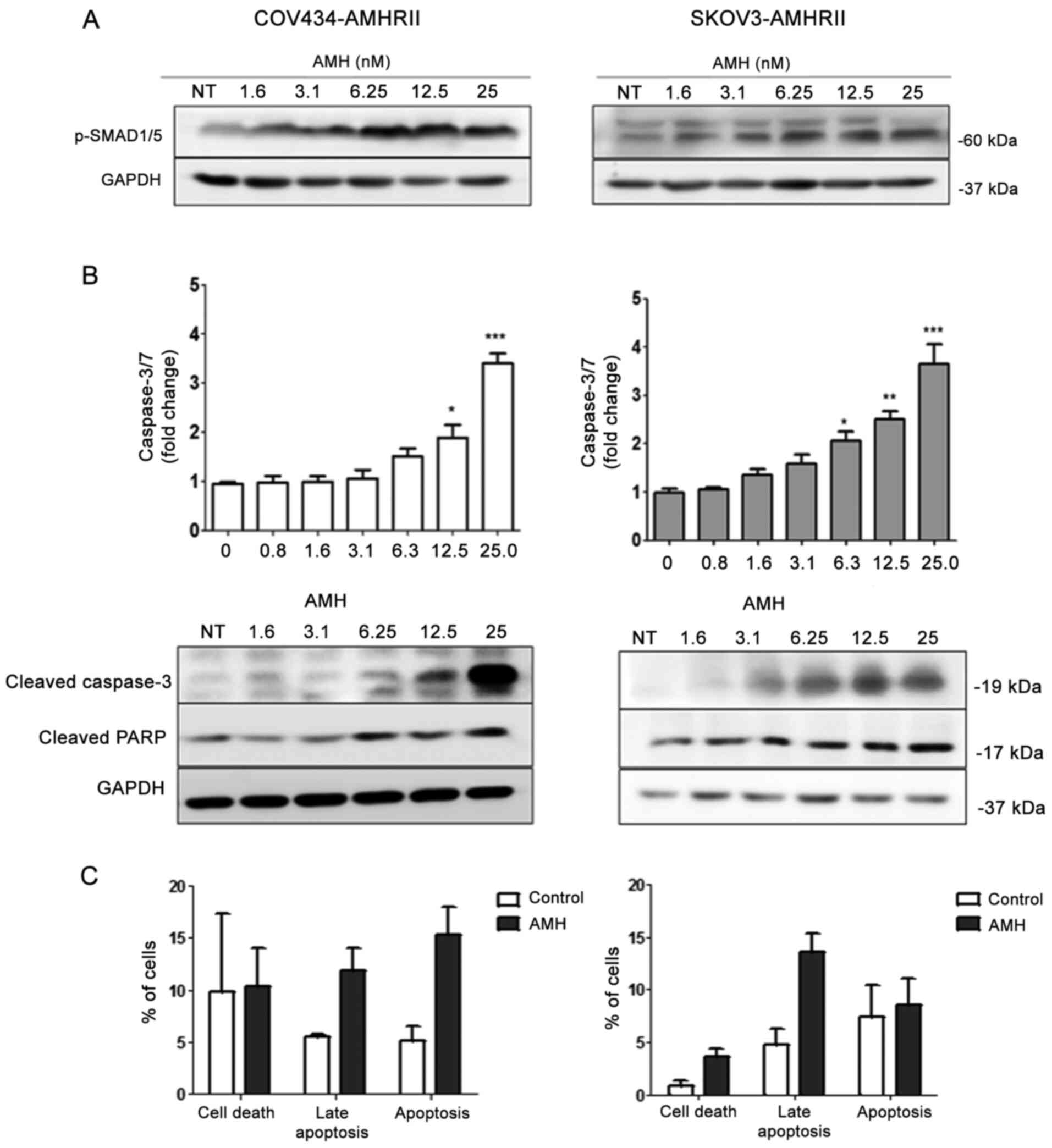 | Figure 1LR-AMH induces AMH signaling in
COV434-AMHRII and SKOV3-AMHRII cells. (A and B) Incubation with
1.6-25 nM LR-AMH for 6 h increased the levels of (A) pSMAD1/5 and
(B) apoptosis (upper panels, caspase-3/7 activity; middle panels,
cleaved caspase-3 and PARP analysis by western blotting) compared
with NT samples. (C) Apoptosis was also analyzed using the Annexin
V-7AAD assay following incubation with 25 nM LR-AMH for 24 h. Data
are presented as the mean ± SEM. COV434-AMHRII, n=3; SKOV3-AMHRII,
n=4. *P<0.05, **P<0.01 and
***P<0.001 vs. control. Apoptosis, Annexin V-positive
cells; late apoptosis, Annexin V/7AAD-positive cells; AMH,
anti-Müllerian hormone; LR-AMH, active recombinant AMH; AMHRII, AMH
type II receptor; PARP, poly(ADP-ribose) polymerase; NT,
non-treated. |
ALK3 mediates AMH-induced increase in the
levels of phosphorylated SMAD1/5 and caspase-3 and PARP activation
in ovarian cancer cells
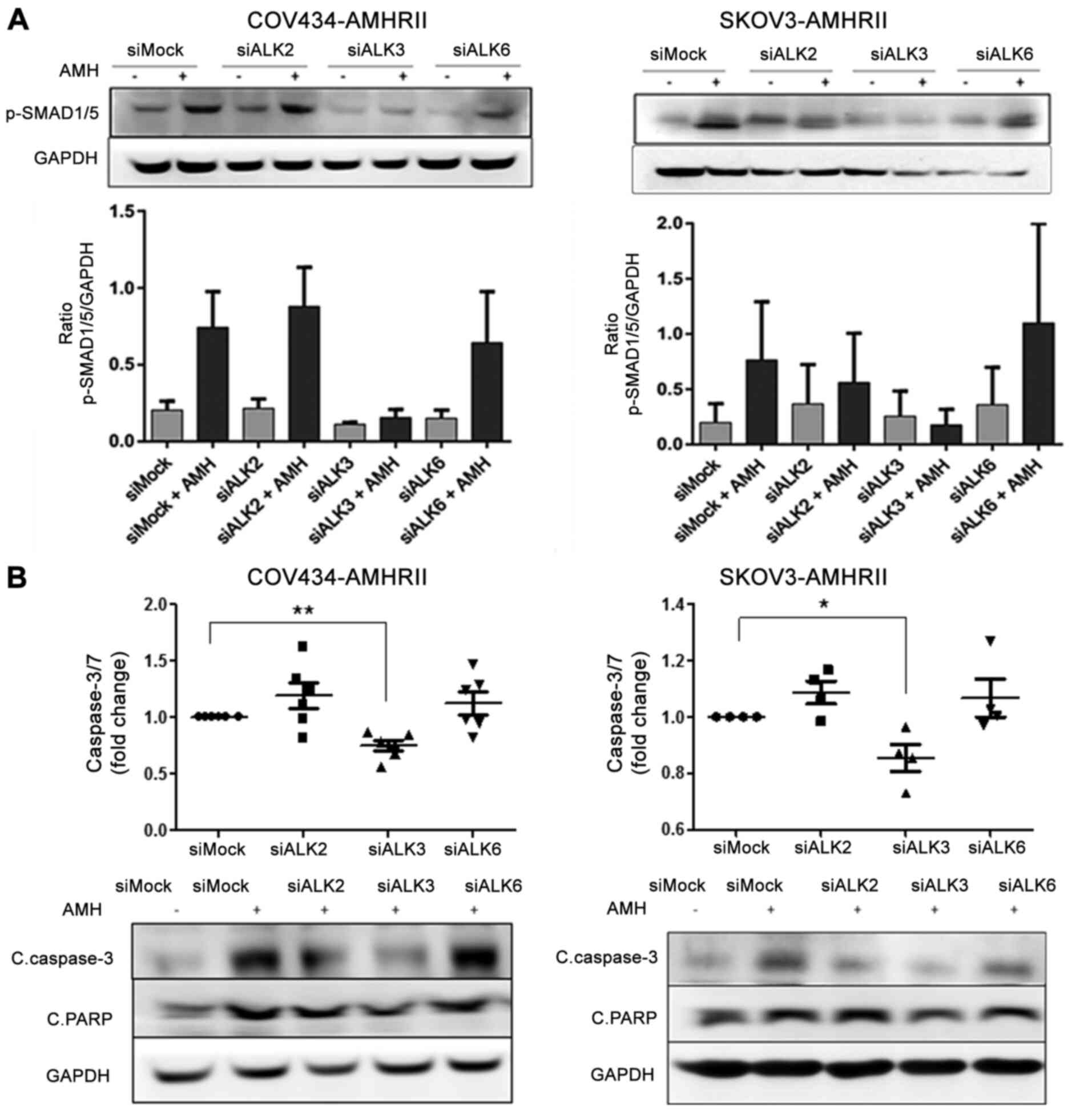
To analyze the involvement of AMHRI in AMH signaling
in ovarian cancer cells, knockdown experiments were performed in
COV434-AMHRII and SKOV3-AMHRII cells by transfecting a mixture of
three siRNAs against each AMHRI. PCR and western blot analyses
revealed that the siRNA mixtures against ALK2 (siAlk2) and
against ALK6 (siAlk6) efficiently inhibited their expression
(Fig. S5). By contrast,
ALK3 silencing (siAlk3) had limited efficiency, particularly
in COV434-AMHRII cells (Fig.
S5). The effects of LR-AMH (at the supraphysiological
concentration of 25 nM for 6 h) on the levels of pSMAD1/5 was
subsequently evaluated in cells transfected with the siRNAs. The
results demonstrated that only siAlk3 transfection suppressed the
levels of pSMAD1/5 in COV434-AMHRII and SKOV3-AMHRII cells compared
with those observed following siMock transfection (Fig. 3A). Similarly, levels of
caspase-3/7 activity and cleavage in COV434-AMHRII and SKOV3-AMHRII
cells were not significantly different in siAlk2- and
siAlk6-transfected cells compared with those in the mock siRNA
control cells (Fig. 3B, top). By
contrast, initiation of apoptosis as measured by caspase-3 cleavage
was reduced by ~25% in siAlk3-transfected COV434-AMHRII and
SKOV3-AMHRII cells compared with those in the corresponding control
cells (Fig. 3B, top). These
results were validated by western blot analysis of cleaved PARP and
caspase-3/7 levels (Fig. 3B,
bottom). Taken together, these findings suggested that AMH
signaling was significantly reduced only by Alk3 silencing (which
was incomplete) in COV434-AMHRII and SKOV3-AMHRII cells,
demonstrating that ALK3 may be the functional AMHRI receptor
implicated in apoptosis of ovarian cancer cells exposed to
supraphysiological AMH concentrations.
AMH concentration regulates the
differential modulation of ALK2 and ALK3 expression, pSMAD1/5,
cleaved caspase-3 and PARP, and pAKT levels
The present study subsequently investigated the
consequences of exposure to AMH on AMHRII, ALK2, ALK3 and ALK6
expression levels in four AMHRII-positive ovarian cancer cell
lines: COV434-AMHRII (sex cord stromal tumor) (36,37), SKOV3-AMHRII (high grade serous
ovarian cancer), OVCAR8 (high grade serous ovarian cancer) and KGN
(granulosa cell tumor) (38).
These cell lines represent different ovarian cancer types and
include AMHRII-transfected and non-transfected cell lines. AMHRII
and ALK2 expression was detected in all four cell lines at
physiological endogenous AMH concentration (basal conditions with
1% FBS corresponding to 10 pM AMH). AMHRII expression was not
modulated by incubation with supraphysiological LR-AMH
concentrations (25 nM for 90 min), whereas ALK2 expression was
decreased by ~30% compared with that under basal conditions, as
estimated by immunofluorescence quantification of receptor/nucleus
labeling (Fig. 4). In all four
cell lines, ALK3 expression was not detected at physiological
endogenous AMH concentrations, but only following exposure to 25 mM
LR-AMH (Fig. 4). ALK6 was not
detectable at either AMH concentration, suggesting that this AMHRI
may not be implicated in the present experimental conditions.
Therefore, the present study further focused on ALK2 and ALK3.
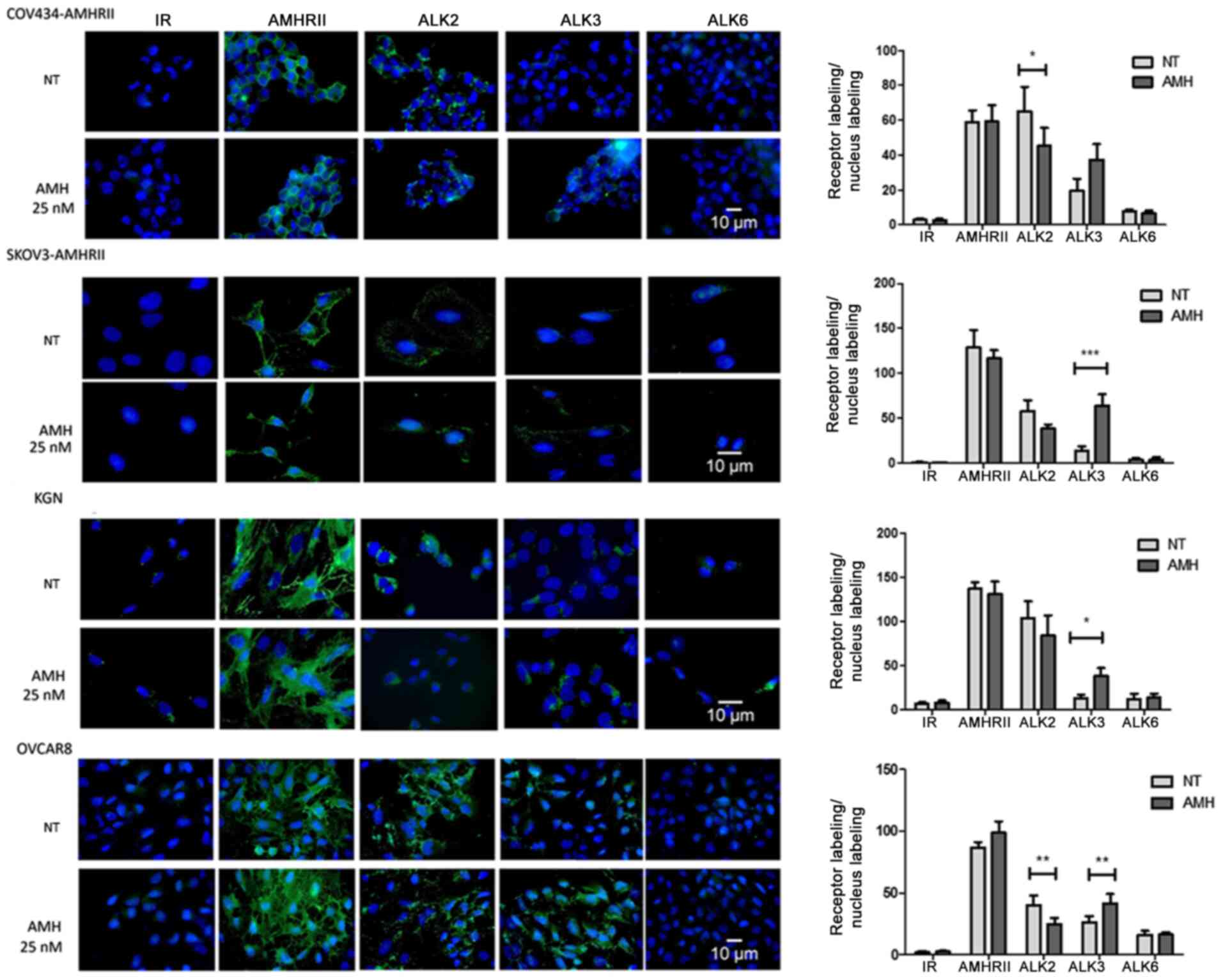 | Figure 4Incubation with recombinant LR-AMH
modulates ALK2 and ALK3 expression in COV434-AMHRII, SKOV3-AMHRII,
OVCAR8 and KGN ovarian cancer cells. Immunofluorescence analysis of
AMHRII, ALK2, ALK3 and ALK6 expression in basal conditions and
after incubation with 25 nM LR-AMH for 90 min. Data are presented
as the ratio of receptor (green) and nucleus (blue) labeling.
*P<0.05, **P<0.01 and
***P<0.001 n=6. AMH, anti-Müllerian hormone; LR-AMH,
active recombinant AMH; AMHRII, AMH type II receptor; ALK, activin
receptor-like kinase; IR, irrelevant antibody; NT, non-treated. |
To determine the roles of ALK2 and ALK3, their
expression levels as well as the levels pSMAD1/5 were assessed in
basal conditions and following incubation with increasing
concentrations of LR-AMH (1.6-25 nM) for 6 h. In all four cell
lines, ALK2 expression decreased upon incubation with increasing
concentrations of LR-AMH, and was almost undetectable between 6.25
and 12.5 nM LR-AMH (Figs. 5 and
S6). By contrast, ALK3
expression levels increased following LR-AMH exposure compared with
those in the untreated cells. In addition, the levels of pSMAD1/5
and cleaved caspase-3 and PARP increased concomitantly with ALK3
expression (Figs. 5 and S6). To analyze the involvement of
non-canonical pathways in AMH signaling (41,43), pAKT levels were also detected; the
results demonstrated that the levels of pAKT decreased following
incubation with LR-AMH, similar to the ALK2 expression levels
(Figs. 5 and S6).
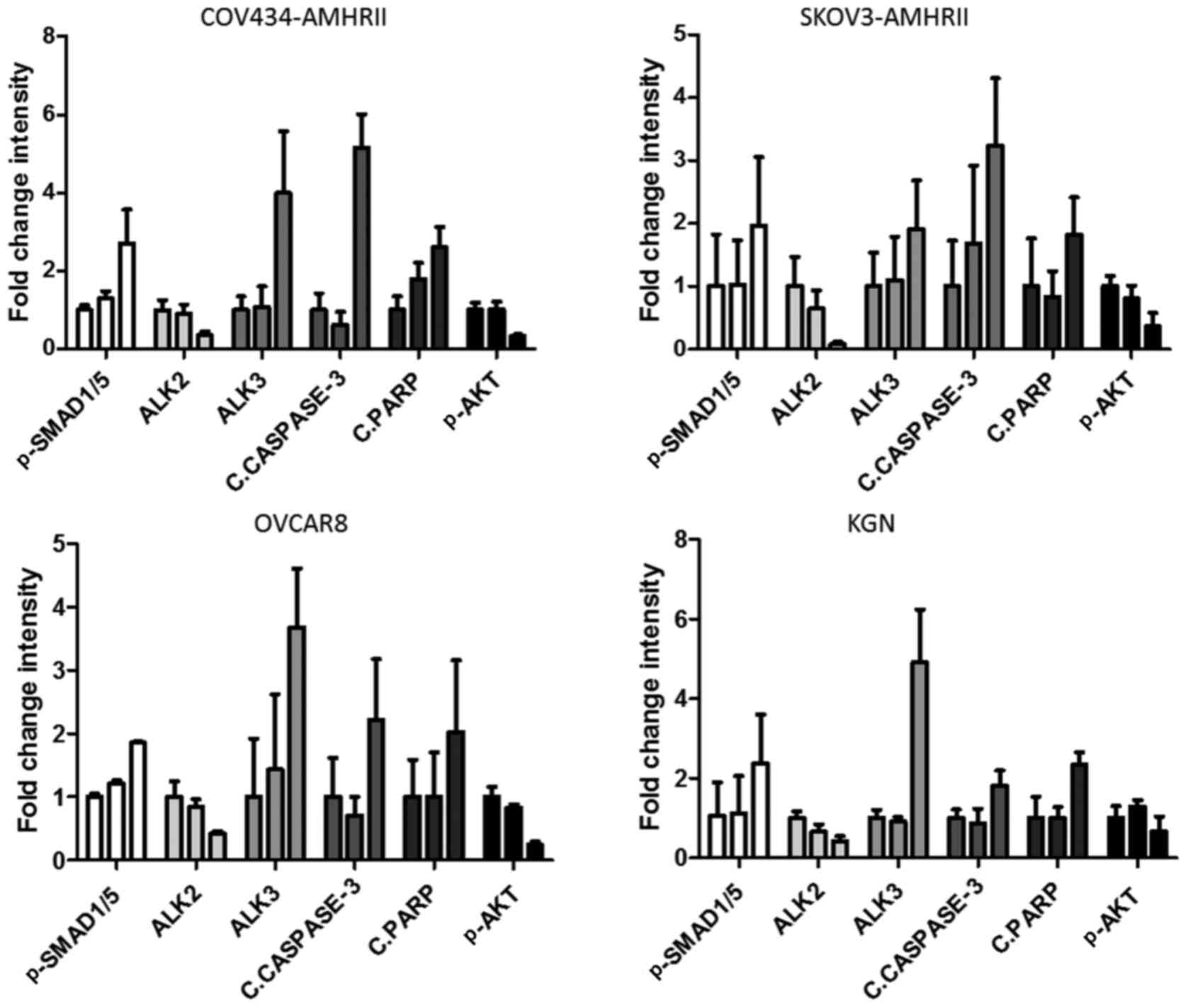 | Figure 5Differential expression of ALK2 and
ALK3 following LR-AMH treatment is associated with the expression
of cell survival and apoptosis markers in COV434-AMHRII,
SKOV3-AMHRII, OVCAR8 and KGN ovarian cancer cells. Quantification
of the western blot analysis of AMH signaling (pSMAD1/5), ALK2 and
ALK3 expression, apoptosis induction (cleaved caspase-3 and PARP)
and pAKT levels following incubation with 1.6 and 25 nM LR-AMH for
6 h. Data are presented as the fold-change relative to control (no
LR-AMH) by bars corresponding from left to right to 0, 1.6 and 25
nM for each protein expression. n>3. Raw data are presented in
Fig. S5. AMH, anti-Müllerian
hormone; LR-AMH, active recombinant AMH; AMHRII, AMH type II
receptor; ALK, activin receptor-like kinase; p, phosphorylated; c,
cleaved; PARP, poly(ADP-ribose) polymerase. |
The same analyses were subsequently repeated in
cancer cells isolated from ascites samples of three patients with
ovarian cancer. and similar results were observed (Figs. 6 and S7) with a certain amount of
variability. The sampled tumors were high-grade papillary serous
carcinomas, of which two harbored wild-type BRCA1 and one presented
with mutated BRCA1. The AMH concentration required to induce
apoptosis, determined by the levels of cleaved caspase-3 and PARP,
appeared to be higher compared with that used in cell lines (50 vs.
25 nM). Notably, despite the enhanced expression levels of
apoptotic markers, pSMAD1/5 levels were paradoxically decreased in
cells incubated with 50 nM recombinant AMH compared with those in
cells treated with 25 nM AMH.
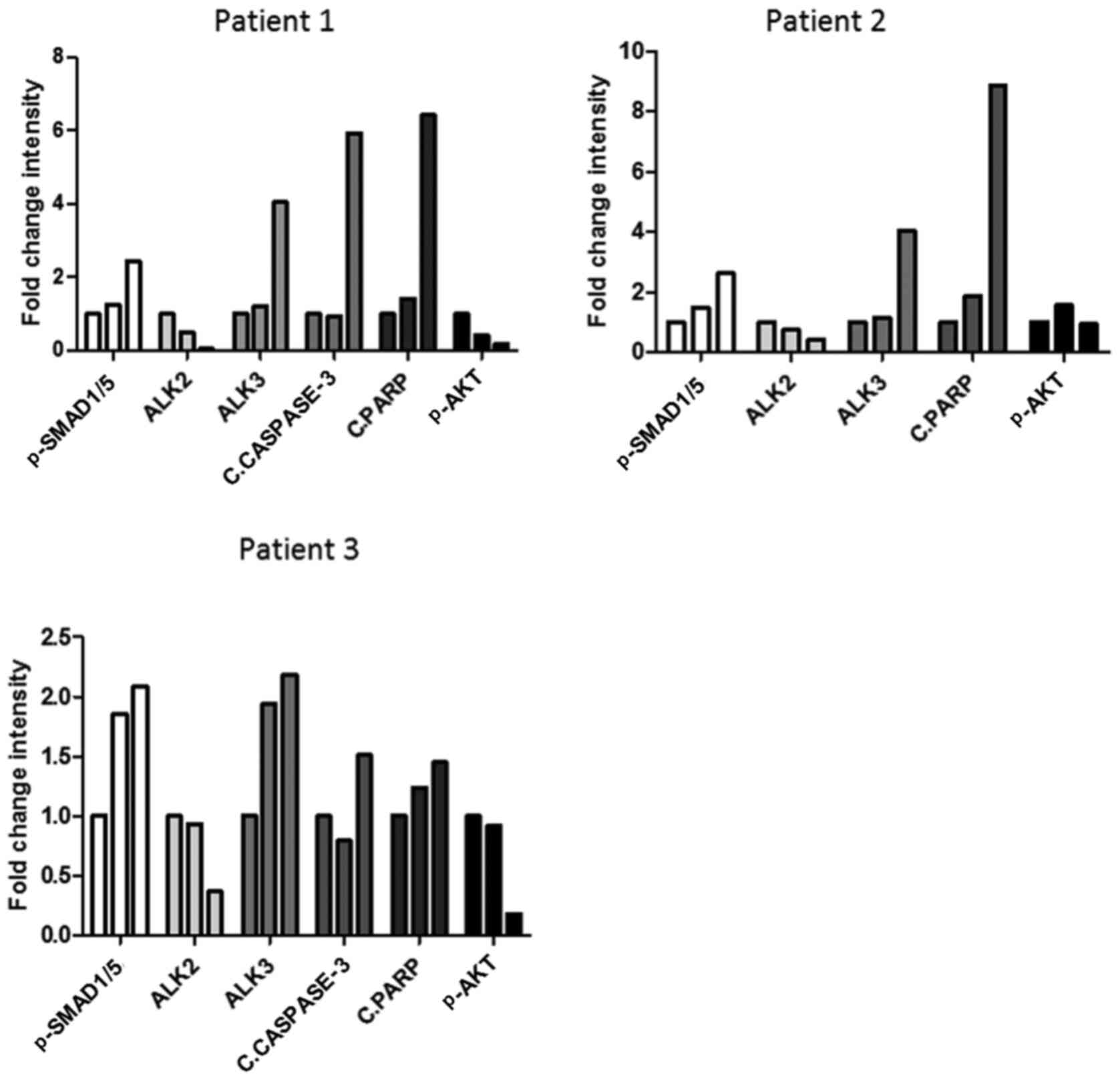 | Figure 6Differential expression of ALK2 and
ALK3 following LR-AMH treatment is associated with the expression
of cell survival and apoptosis markers in primary ovarian cancer
cells. Quantification of the western blot analysis of AMH signaling
(pSMAD1/5), ALK2 and ALK3 expression, apoptosis induction (cleaved
caspase-3 and PARP) and pAKT levels following incubation of cells
isolated from ascites of three patients with ovarian cancer with
1.6 and 25 nM LR-AMH for 6 h. Data are presented as the fold change
relative to control (no LR-AMH) by bars corresponding from left to
right to 0, 1.6 and 25 nM for each protein expression. n=1 for each
patient. Raw data are presented in Fig. S6. AMH, anti-Müllerian hormone;
LR-AMH, active recombinant AMH; AMHRII, AMH type II receptor; ALK,
activin receptor-like kinase; P, phosphorylated; c, cleaved; PARP,
poly(ADP-ribose) polymerase. |
These results suggested that in ovarian carcinoma
cells, supraphysiological AMH concentrations induced ALK3 and
inhibited ALK2 expression, and that this shift may be associated
with proapoptotic signaling.
Anti-AMHRII-ALK2 and anti-AMHRII-ALK3
bispecific antibodies affect AMH/AMHRII signaling
As differential roles of ALK2 and ALK3 in AMH/AMHRII
signaling were observed in ovarian cancer cells, the present study
developed antibodies against these receptors to induce apoptosis in
these cells. To limit side effects due to the ubiquitous expression
of ALK2 and ALK3, bispecific antibodies (BsAbs) against AMHRII and
ALK2, and against AMHRII and ALK3 were developed. The presence of
the antibody fragment against AMHRII (the tumor-specific receptor)
conferred tumor specificity to the BsAbs (Fig. S2). A total of eight anti-ALK2 and
six anti-ALK3 scFv antibodies were isolated from the human scFv
phage display library Husc I (30,31). For each ALK receptor, the two most
efficient binders in ELISA assays were formatted as full human IgG1
and BsAbs (Table I). For all
antibodies and targets, the EC50 of the IgG1 and BsAbs
were comparable. The mean EC50 of the MAb 12G4 was 0.34
nM, which was close to its known KD (0.8 nM) (11,15). To favor BsAb targeting by the
anti-AMHRII arm, affinity maturation of the anti-ALK2 and anti-ALK3
scFv was not performed, providing an advantage to the 12G4 arm.
 | Table IAffinity of the MAbs and BsAbs for
their targets estimated by assessing the EC50 by
ELISA. |
Table I
Affinity of the MAbs and BsAbs for
their targets estimated by assessing the EC50 by
ELISA.
A, MAbs
|
|---|
| Antibody | EC50, nM
|
|---|
| AMHRII | ALK2 | ALK3 |
|---|
| 12G4 | 0.35±1.42 | | |
| 2C1 | | 26.80±1.30 | |
| 2F9 | | 74.56±1.41 | |
| 3D7 | | | 50.03±1.90 |
| 3H6 | | | 2.07±2.13 |
|
B, BsAbs
|
| Antibody | EC50, nM
|
| AMHRII | ALK2 | ALK3 |
|
| 12G4-2C1 | 0.28±1.56 | 29.93±1.26 | |
| 12G4-2F9 | 0.64±1.46 | 85.56±1.36 | |
| 12G4-3D7 | 0.39±1.64 | | 48.45±1.41 |
| 12G4-3H6 | 0.30±1.57 | | 1.14±1.41 |
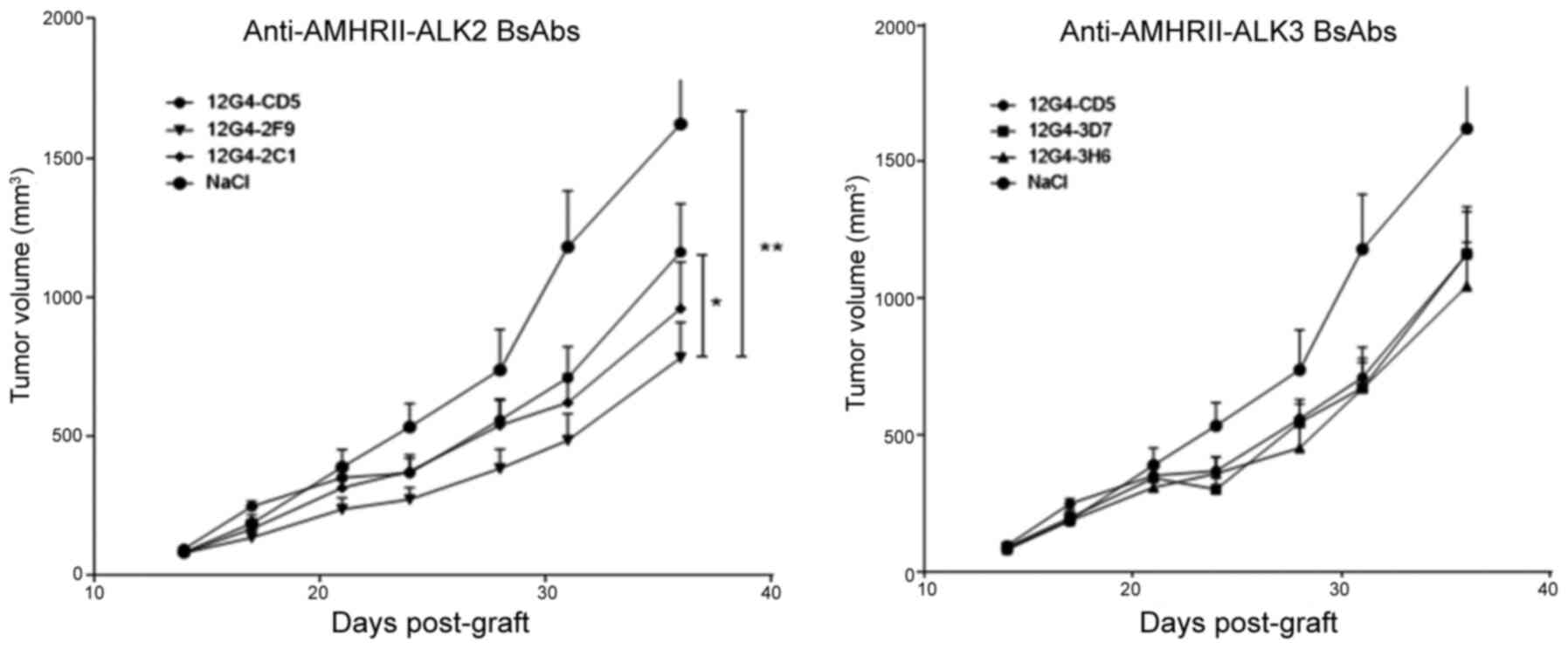
Anti-AMHRII-ALK2 BsAb 12G4-2F9 reduces
the growth of COV434-AMHRII cell xenografts in vivo
To evaluate the in vivo antitumor activity of
the anti-AMHRII-AMHRI BsAbs, mice with established COV434-AMHRII
cell xenografts (8-10 mice/group) were treated with the two
anti-AMHRII-ALK2 BsAbs (12G4-2C1 and 12G4-2F9), the two
anti-AMHRII-ALK3 BsAbs (12G4-3D7 and 12G4-3H6), vehicle control
(NaCl) or a control BsAb against AMHRII and CD5 (12G4-CD5)
(32,35). The 12G4-CD5 BsAb was used as a
control that retained AMHRII recognition, but bound to an
irrelevant antigen (CD5) instead of ALK2 and ALK3. Mice received 17
mg/kg of BsAb (molar equivalent to 10 mg/kg for MAbs) by i.p.
injection twice per week for 4 weeks. Treatment started at D14
after tumor grafting (mean tumor volume, 85 mm3; no
differences among the six groups). On D31, when tumor volumes
reached 1,500 mm3 in three mice of the saline vehicle
group (mean ± SEM, 1180±201 mm3), the mean tumor volume
was lower in the antibody-treated groups, including that in mice
treated with the control BsAb 12G4-CD5, suggesting baseline
efficacy by the anti-AMHRII Fab alone (709±110 mm3). The
tumor volumes in mice treated with the anti-AMHRII-ALK3 BsAbs
12G4-3D7 and 12G4-3H6, or the anti-AMHRII-ALK2 BsAb 12G4-2C1
(671±92.3, 669±111.8 and 620±102 mm3, respectively) were
not significantly different compared those in mice treated with the
control anti-AMHRII BsAb 12G4-CD5 (Fig. 7). By contrast, mice treated with
the AMHRII-ALK2 12G4-2F9 BsAb developed significantly smaller
tumors (484±95 mm3) compared with those treated with the
control BsAb 12G4-CD5. Accordingly, the T/C% values of the 12G4-2C1
and 12G4-2F9 BsAbs were 52 and 41%, respectively. Throughout the
experiment, tumor growth was significantly lower in the
antibody-treated mice compared with that in the vehicle group
(vehicle vs. 12G4-3D7, P=0.018; vehicle vs. 12G4-CD5, 12G4-2C1 or
12G4-3H6, P=0.001) (Fig. 7).
However, only tumor growth in 12G4-2F9-treated mice was
statistically lower compared with that in the 12G4-CD5 group
(P=0.048).
Discussion
The AMH/AMHRII signaling pathway has been studied
during development, but the role of the three AMHRIs ALK2, ALK3 and
ALK6 in the physiological effects of AMH has not been fully
elucidated. Similar to other TGFβ family members, these effects are
dependent on cellular context (44). The predominant AMHRI partner to
AMHRII depends on the cell type, as demonstrated in Sertoli
(18), Leydig (20) and granulosa cells (22), and on the development stage during
Müllerian duct regression (19-21,24). During embryogenesis, the
spatiotemporal patterns of ALK2 and ALK3 expression suggest that
they act sequentially as AMHRIs by high-dose AMH-induced signaling
(42.5 nM in in vitro experiments) (24). ALK2 is implicated at the early
stages of Müllerian duct regression that involve the
epithelial-mesenchymal transition, whereas ALK3 acts later during
apoptosis (24). Orvis et
al (21) have reported that
following AMH binding to the AMHRII expressed on the duct
mesenchyme, ALK2 and ALK3 act redundantly in a complex with AMHRII
to activate the Müllerian duct mesenchyme signals responsible for
the regression of the Müllerian duct mesoepithelium. These
experiments have also suggested that AMH uses ALK3 as its primary
AMHRI to induce regression through apoptosis (21). Thus, the balance between ALK2 and
ALK3 recruitment may differently affect AMHRII-mediated cell fate
during embryogenesis. In cancer, little is known about the
contribution of the various AMHRIs to AMH-AMHRII signaling. Basal
et al (25) analyzed the
expression of AMHRII, ALK2, ALK3 and ALK6 by immunohistochemistry
in 262 epithelial ovarian carcinoma samples; AMHRII was expressed
in 73.4% of the samples, as previously reported (5,11,45,46). In the 235 samples in which all
four receptors could be assessed, 36% expressed
AMHRII/ALK2/ALK3/ALK6, 34% AMHRII/ALK2/ALK3, 18% ALK2/ALK3, and 7%
ALK2/ALK3/ALK6, and these expression profiles were not associated
with the disease stage, overall or disease-free survival (25). In the present study, using two
ovarian cancer cell lines COV434-AMHRII and SKOV3-AMHRII, ALK3 was
demonstrated to be the predominant AMHRI responsible for AMH
signaling, resulting in the induction of apoptosis. In four ovarian
cancer cell lines (COV434-AMHRII, SKOV3-AMHRII, OVCAR8 and KGN),
ALK2 and ALK3 membrane expression was demonstrated to depend on
LR-AMH concentration: ALK2 was expressed at physiological
endogenous AMH concentration (<10 pM), whereas ALK3 was
preferentially expressed at supraphysiological concentrations and
was responsible of apoptosis induction. These results were
validated in tumor cells isolated from ascites samples of three
patients with ovarian carcinoma.
Based on these differential roles of ALK2 and ALK3
in AMH/AMHRII signaling in ovarian cancer cells, the present study
designed BsAbs against AMHRII and ALK2, and against AMHRII and ALK3
that reduced tumor growth in ovarian cancer. The BsAb strategy used
in the present study allowed us to overcome potential side effects
due to the ubiquitous expression of ALK2 and ALK3 (47), since the anti-AMHRII antibody
fragments conferred tumor specificity.
BsAbs are one of the pillars of the next-generation
antibody drugs (48,49). A limited number of BsAbs are
currently used in the clinic, for instance catumaxomab (CD3-EpCam)
and blinatumomab (CD3-CD19); however, >30 are being evaluated in
clinical trials (50). A variety
of BsAb formats (>100) are available at present, from small
diabodies to complex molecules larger than the conventional IgG
used as therapeutic antibodies (48). These formats differ in size,
arrangement, valency and flexibility, as well as in their
distribution and pharmacokinetic properties, thus offering the
possibility to select the best format for any desired application
(48). BsAbs may be useful for
cancer therapies as they can simultaneously activate and inhibit
various mechanisms of action by directly targeting different
molecules. For instance, they can target two antigens present on
the surface of the same cell (51) or on two different cells, such as a
tumor cell and an immune cell, which is currently the most
developed strategy (52). Among
the BsAbs against two membrane receptors, the RG7992/BFKB8488A
antibody against FGFR1 and KLB, developed as a potential FGF21
mimetic, corresponds to a strategy similar to the one used for the
anti-AMHRII-AKL BsAbs in the present study.
In the BsAbs developed in the present study, the
relative affinity of the anti-AMHRII Fab (mean EC50,
0.34 nM) compared with that of the anti-ALK Fab corresponded to a
1:100 ratio for the MAbs 2C1, 2F9 and 3D7 (EC50 from 26
to 85 nM) and to a 1:10 ratio for the Mab 3H6 (EC50, ~2
nM). These affinity differences and the BsAb design ensured tumor
specificity through AMHRII as the first step of the binding, and
potential signaling through ALK2 or ALK3 binding. Notably, the BsAb
with the highest affinity ratio and thus the lowest anti-ALK
affinity (12G4-2F9) was the most efficient in vivo. In
vivo, all BsAbs used in the present study reduced COV434-AMHRII
tumor growth compared with that observed in the vehicle-treated
group. The BsAb 12G4-2F9 was the most efficient with a T/C% of 41%
(59% growth inhibition) and 68% (32% growth inhibition) compared
with the vehicle and the BsAb targeting only AMHRII (12G4-CD5),
respectively. The stronger antitumor effects of anti-AMHRII-ALK2
BsAbs compared with those of anti-AMHRII-ALK3 BsAbs was in line
with the observation that ALK2 was mainly expressed at
physiological endogenous AMH concentrations, whereas ALK3 was
expressed at supraphysiological AMH concentrations. Accordingly, no
exogenous AMH was added in the in vivo experiments.
Pretreatment with AMH, as performed in our previous study of the
AAV9-LR-AMH strategy (9), may
favor ALK3 expression and increase the efficiency of the
anti-AMHRII-ALK3 BsAbs. Alternatively, as ALK3 is mostly implicated
in apoptosis induction, an anti-ALK3 MAb or an anti-AMHRII-ALK3
BsAb would have to be agonistic to reduce tumor growth. Agonist
antibodies are rarely obtained unless specific and complex
screening strategies are employed. Based on the present results,
potential improvements of the anti-AMHRII-ALK2 BsAb will be
considered, including new anti-ALK2 antibodies and other BsAb
formats to compare them with the 12G4-2F9 BsAb.
In the present study, the effects of the newly
developed BsAbs were compared in vivo, as these experiments
may determine their therapeutic potential. Further detailed
analyses of the effects of these BsAb on signaling will be the
subject of subsequent studies. Even if the potential toxicity of
the anti-AMHRII-ALK2 BsAb developed in the present study cannot be
precisely analyzed in a murine model, the high affinity of the
anti-AMHRII Fab (12G4) should limit it to AMHRII-expressing cells
in the ovaries. From a medical point of view, these concerns are
relatively minor, considering that the ovaries are typically
surgically removed as part of the standard of care of ovarian
cancers.
The original observation of the present study that
ALK2 and ALK3 serve opposing roles in AMH signaling in ovarian
cancer cells may improve the understanding of the involvement of
this hormone in cancer and enable the development of novel
anticancer strategies. The results of the present study constituted
the proof of concept that BsAbs targeting AMHRII-ALK2 may inhibit
tumor growth in ovarian cancer models, and may have enhanced
efficacy compared with antibodies that target AMHRII alone. This is
remarkable considering that these experiments were carried out in
cells that overexpress AMHRII, since this overexpression may have
limited the efficacy of the anti-ALK2 arm and of the AMHRII-ALK2
BsAb as a whole. The design of a potential clinical trial
evaluating the anti-AMHRII-ALK2 BsAb will be based on the results
of the current clinical trials performed in gynecological
(NCT02978755) and colorectal (NCT03799731) cancers with the
anti-AMHRII MAb (GM102, murlentamab). This strategy may offer an
alternative to or complement the use of recombinant AMH to induce
cancer cell apoptosis (9,26).
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AP, MCha and TC conceived the study. MCha, VG,
DPDB, MDC, KD, MChe, BR, MJ, INT, PM and SC performed the
experiments. PEC provided the resources. AP and MCha confirm the
authenticity of all raw data. AP wrote the original draft. AP, TC,
DP, PEC, PM, INT, LG, DPDB, MCha, MDC, VG, MChe, BR, MJ and SC
reviewed and edited the manuscript. MCha designed the figures. AP
supervised the study, was responsible for project administration
and acquired funding. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ascites samples from patients with ovarian cancer
were obtained from the Institut du Cancer de Montpellier (ICM)
according to the French laws and after obtaining informed consent.
All samples were retrieved from the ICM ovarian cancer
clinical-biological database that had been approved by the
independent Sud Méditerranée III Ethics Committee (reference no.
2016.09.06). All experimental protocols using these human samples
were approved by the ICM Comité de Recherche Translationnelle and
were carried out in accordance with the French Guidelines and
Regulations for Human Samples. All animal experiments were
performed in compliance with the guidelines of the French
government and INSERM regulations for experimental animal studies
(agreement no. D34-172-27). All experimental protocols were
approved by the Comité d'éthique en Expérimentation Animale
Languedoc Roussillon.
Patient consent for publication
Not applicable.
Competing interests
Maëva Chauvin, Myriam Chentouf, Pierre Martineau,
André Pèlegrin and Bruno Robert are inventors in: Anti-Müllerian
Inhibiting Substance Type I Receptor Antibodies And Uses Thereof.
EP19306214.8 filed on 2019, September 27. All other authors confirm
that they have no compssseting interests.
Acknowledgments
The authors would like to thank Dr Christel
Larbouret and Dr Muriel Brengues (Immunotargeting and Radiobiology
in Oncology team at IRCM, Montpellier, France) for fruitful
discussions and Dr Adeline Torro (IRCM animal facility manager,
Montpellier, France) for support in in vivo experiments. The
human COV434 and KGN cell lines were kind gifts from Dr Schrier
(Department of Clinical Oncology, Leiden University Medical Center,
Leiden, Netherlands) and Dr Yanase (Kyushu University, Fukuoka,
Japan), respectively. The cDNA encoding full-length human AMHRII in
the pCMV6 plasmid was gifted by Dr Teixeira (Pediatric Surgical
Research Laboratories, Massachusetts General Hospital, Harvard
Medical School, Boston, USA). The pIRES1-EGFP vector was gifted by
Dr F Poulat (Institut de Génétique Humaine, CNRS, Montpellier,
France).
Funding
This study was funded by the French National Research Agency
under the program Investissements d'Avenir Grant Agreement LabEx
MAbImprove (grant no. ANR-10-LABX-53), the Ligue Nationale Contre
le Cancer, INSERM Transfert (Proof of Concept grant no. MISRII-MRI
BsAbs) and the SIte de Recherche Intégrée sur le Cancer (SIRIC)
Montpellier-Cancer (grant no. INCa-DGOS-Inserm 6045).
References
|
1
|
di Clemente N, Jamin SP, Lugovskoy A,
Carmillo P, Ehrenfels C, Picard JY, Whitty A, Josso N, Pepinsky RB
and Cate RL: Processing of anti-mullerian hormone regulates
receptor activation by a mechanism distinct from TGF-beta. Mol
Endocrinol. 24:2193–2206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Josso N and Clemente N: Transduction
pathway of anti-Müllerian hormone, a sex-specific member of the
TGF-beta family. Trends Endocrinol Metab. 14:91–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Masiakos PT, MacLaughlin DT, Maheswaran S,
Teixeira J, Fuller AF Jr, Shah PC, Kehas DJ, Kenneally MK,
Dombkowski DM, Ha TU, et al: Human ovarian cancer, cell lines, and
primary ascites cells express the human Mullerian inhibiting
substance (MIS) type II receptor, bind, and are responsive to MIS.
Clin Cancer Res. 5:3488–3499. 1999.PubMed/NCBI
|
|
4
|
Renaud EJ, MacLaughlin DT, Oliva E, Rueda
BR and Donahoe PK: Endometrial cancer is a receptor-mediated target
for Mullerian Inhibiting Substance. Proc Natl Acad Sci USA.
102:111–116. 2005. View Article : Google Scholar
|
|
5
|
Bakkum-Gamez JN, Aletti G, Lewis KA,
Keeney GL, Thomas BM, Navarro-Teulon I and Cliby WA: Müllerian
inhibiting substance type II receptor (MISIIR): A novel,
tissue-specific target expressed by gynecologic cancers. Gynecol
Oncol. 108:141–148. 2008. View Article : Google Scholar
|
|
6
|
Wei X, Dombkowski D, Meirelles K,
Pieretti-Vanmarcke R, Szotek PP, Chang HL, Preffer FI, Mueller PR,
Teixeira J, MacLaughlin DT, et al: Mullerian inhibiting substance
preferentially inhibits stem/progenitors in human ovarian cancer
cell lines compared with chemotherapeutics. Proc Natl Acad Sci USA.
107:18874–18879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anttonen M, Färkkilä A, Tauriala H,
Kauppinen M, Maclaughlin DT, Unkila-Kallio L, Bützow R and
Heikinheimo M: Anti-Müllerian hormone inhibits growth of AMH type
II receptor-positive human ovarian granulosa cell tumor cells by
activating apoptosis. Lab Invest. 91:1605–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meirelles K, Benedict LA, Dombkowski D,
Pepin D, Preffer FI, Teixeira J, Tanwar PS, Young RH, MacLaughlin
DT, Donahoe PK, et al: Human ovarian cancer stem/progenitor cells
are stimulated by doxorubicin but inhibited by Mullerian inhibiting
substance. Proc Natl Acad Sci USA. 109:2358–2363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pépin D, Sosulski A, Zhang L, Wang D,
Vathipadiekal V, Hendren K, Coletti CM, Yu A, Castro CM, Birrer MJ,
et al: AAV9 delivering a modified human Mullerian inhibiting
substance as a gene therapy in patient-derived xenografts of
ovarian cancer. Proc Natl Acad Sci USA. 112:E4418–E4427. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, MacLaughlin DT and Donahoe PK:
Müllerian inhibiting substance/anti-Müllerian hormone: A novel
treatment for gynecologic tumors. Obstet Gynecol Sci. 57:343–357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salhi I, Cambon-Roques S, Lamarre I, Laune
D, Molina F, Pugnière M, Pourquier D, Gutowski M, Picard JY, Xavier
F, et al: The anti-Müllerian hormone type II receptor: Insights
into the binding domains recognized by a monoclonal antibody and
the natural ligand. Biochem J. 379:785–793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan QA, Simmons HH, Robinson MK, Russeva
M, Marasco WA and Adams GP: Development of engineered antibodies
specific for the Müllerian inhibiting substance type II receptor: A
promising candidate for targeted therapy of ovarian cancer. Mol
Cancer Ther. 5:2096–2105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan QA, Robinson MK, Simmons HH, Russeva
M and Adams GP: Isolation of anti-MISIIR scFv molecules from a
phage display library by cell sorter biopanning. Cancer Immunol
Immunother. 57:367–378. 2008. View Article : Google Scholar
|
|
14
|
Bougherara H, Némati F, Nicolas A,
Massonnet G, Pugnière M, Ngô C, Le Frère-Belda MA, Leary A,
Alexandre J, Meseure D, et al: The humanized anti-human AMHRII mAb
3C23K exerts an anti-tumor activity against human ovarian cancer
through tumor-associated macrophages. Oncotarget. 8:99950–99965.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Estupina P, Fontayne A, Barret J-M,
Kersual N, Dubreuil O, Le Blay M, Pichard A, Jarlier M, Pugnière M,
Chauvin M, et al: The anti-tumor efficacy of 3C23K, a
glyco-engineered humanized anti-MISRII antibody, in an ovarian
cancer model is mainly mediated by engagement of immune effector
cells. Oncotarget. 8:37061–37079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gill SE, Zhang Q, Keeney GL, Cliby WA and
Weroha SJ: Investigation of factors affecting the efficacy of
3C23K, a human monoclonal antibody targeting MISIIR. Oncotarget.
8:85214–85223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kersual N, Garambois V, Chardès T, Pouget
JP, Salhi I, Bascoul-Mollevi C, Bibeau F, Busson M, Vié H,
Clémenceau B, et al: The human Müllerian inhibiting substance type
II receptor as immunotherapy target for ovarian cancer. Validation
using the mAb 12G4. MAbs. 6:1314–1326. 2014. View Article : Google Scholar
|
|
18
|
Belville C, Jamin SP, Picard JY, Josso N
and di Clemente N: Role of type I receptors for anti-Müllerian
hormone in the SMAT-1 Sertoli cell line. Oncogene. 24:4984–4992.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons
KM and Donahoe PK: Müllerian inhibiting substance signaling uses a
bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and
induces SMAD6 expression. Mol Endocrinol. 15:946–959.
2001.PubMed/NCBI
|
|
20
|
Josso N, Racine C, di Clemente N, Rey R
and Xavier F: The role of anti-Müllerian hormone in gonadal
development. Mol Cell Endocrinol. 145:3–7. 1998. View Article : Google Scholar
|
|
21
|
Orvis GD, Jamin SP, Kwan KM, Mishina Y,
Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen
A, et al: Functional redundancy of TGF-beta family type I receptors
and receptor-Smads in mediating anti-Mullerian hormone-induced
Mullerian duct regression in the mouse. Biol Reprod. 78:994–1001.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sèdes L, Leclerc A, Moindjie H, Cate RL,
Picard JY, di Clemente N and Jamin SP: Anti-Müllerian hormone
recruits BMPR-IA in immature granulosa cells. PLoS One.
8:e815512013. View Article : Google Scholar
|
|
23
|
Visser JA, Olaso R, Verhoef-Post M, Kramer
P, Themmen AP and Ingraham HA: The serine/threonine transmembrane
receptor ALK2 mediates Müllerian inhibiting substance signaling.
Mol Endocrinol. 15:936–945. 2001.PubMed/NCBI
|
|
24
|
Zhan Y, Fujino A, MacLaughlin DT,
Manganaro TF, Szotek PP, Arango NA, Teixeira J and Donahoe PK:
Müllerian inhibiting substance regulates its receptor/SMAD
signaling and causes mesenchymal transition of the coelomic
epithelial cells early in Müllerian duct regression. Development.
133:2359–2369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basal E, Ayeni T, Zhang Q, Langstraat C,
Donahoe PK, Pepin D, Yin X, Leof E and Cliby W: Patterns of
Müllerian inhibiting substance type II and candidate type I
receptors in epithelial ovarian cancer. Curr Mol Med. 16:222–231.
2016. View Article : Google Scholar
|
|
26
|
Pépin D, Hoang M, Nicolaou F, Hendren K,
Benedict LA, Al-Moujahed A, Sosulski A, Marmalidou A, Vavvas D and
Donahoe PK: An albumin leader sequence coupled with a cleavage site
modification enhances the yield of recombinant C-terminal Mullerian
Inhibiting Substance. Technology (Singap). 1:63–71. 2013.
View Article : Google Scholar
|
|
27
|
Pépin D: Modified mullerian inhibiting
substance (mis) proteins and uses thereof for the treatment of
diseases. WO2014/164891. 2014
|
|
28
|
Wilson CA, di Clemente N, Ehrenfels C,
Pepinsky RB, Josso N, Vigier B and Cate RL: Mullerian inhibiting
substance requires its N-terminal domain for maintenance of
biological activity, a novel finding within the transforming growth
factor-beta superfamily. Mol Endocrinol. 7:247–257. 1993.PubMed/NCBI
|
|
29
|
Cate RL, Mattaliano RJ, Hession C, Tizard
R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, et al:
Isolation of the bovine and human genes for Müllerian inhibiting
substance and expression of the human gene in animal cells. Cell.
45:685–698. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philibert P, Stoessel A, Wang W, Sibler
AP, Bec N, Larroque C, Saven JG, Courtête J, Weiss E and Martineau
P: A focused antibody library for selecting scFvs expressed at high
levels in the cytoplasm. BMC Biotechnol. 7:812007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robin G and Martineau P: Synthetic
customized scFv libraries. Methods Mol Biol. 907:109–122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Golay J, Choblet S, Iwaszkiewicz J,
Cérutti P, Ozil A, Loisel S, Pugnière M, Ubiali G, Zoete V,
Michielin O, et al: Design and validation of a novel generic
platform for the production of tetravalent IgG1-like bispecific
antibodies. J Immunol. 196:3199–3211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kadouche J, Mach JP, Michielin O, Zoete V,
Iwaszkiewicz J, Cerutti M, Choblet S and Golay J: Multispecific
Antibodies. Patent WO2013005194. Filed July 6, 2012; issued January
10, 2013.
|
|
34
|
Duke GM, Hoffman MA and Palmenberg AC:
Sequence and structural elements that contribute to efficient
encephalomyocarditis virus RNA translation. J Virol. 66:1602–1609.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loisel S, André PA, Golay J, Buchegger F,
Kadouche J, Cérutti M, Bologna L, Kosinski M, Viertl D, Delaloye
AB, et al: Antitumour effects of single or combined monoclonal
antibodies directed against membrane antigens expressed by human B
cells leukaemia. Mol Cancer. 10:422011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chan-Penebre E, Armstrong K, Drew A,
Grassian AR, Feldman I, Knutson SK, Kuplast-Barr K, Roche M,
Campbell J, Ho P, et al: Selective killing of SMARCA2- and
SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic
type cells by inhibition of EZH2: In vitro and in vivo preclinical
models. Mol Cancer Ther. 16:850–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang H, Vollmer M, De Geyter M,
Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P,
Holzgreve W and De Geyter C: Characterization of an immortalized
human granulosa cell line (COV434). Mol Hum Reprod. 6:146–153.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I,
Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al: Establishment
and characterization of a steroidogenic human granulosa-like tumor
cell line, KGN, that expresses functional follicle-stimulating
hormone receptor. Endocrinology. 142:437–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O Donnell RL, McCormick A, Mukhopadhyay A,
Woodhouse LC, Moat M, Grundy A, Dixon M, Kaufman A, Soohoo S,
Elattar A, et al: The use of ovarian cancer cells from patients
undergoing surgery to generate primary cultures capable of
undergoing functional analysis. PLoS One. 9:e906042014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chung YJ, Kim HJ, Park SH, Yoon JH, Kim
MR, Nam SW, MacLaughlin DT, Donahoe PK and Kim JH: Transcriptome
analysis reveals that Müllerian inhibiting substance regulates
signaling pathways that contribute to endometrial carcinogenesis.
Int J Oncol. 46:2039–2046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beck TN, Korobeynikov VA, Kudinov AE,
Georgopoulos R, Solanki NR, Andrews-Hoke M, Kistner TM, Pépin D,
Donahoe PK, Nicolas E, et al: Anti-Müllerian hormone signaling
regulates epithelial plasticity and chemoresistance in lung cancer.
Cell Rep. 16:657–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Köhler G, Howe SC and Milstein C: Fusion
between immunoglobulin-secreting and nonsecreting myeloma cell
lines. Eur J Immunol. 6:292–295. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang YE: Non-Smad signaling pathways of
the TGF-β family. Cold Spring Harb Perspect Biol. 9:92017.
View Article : Google Scholar
|
|
44
|
Horbelt D, Denkis A and Knaus P: A
portrait of transforming growth factor β superfamily signalling:
Background matters. Int J Biochem Cell Biol. 44:469–474. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mazumder S, Johnson JM, Swank V, Dvorina
N, Martelli E, Ko J and Tuohy VK: Primary immunoprevention of
epithelial ovarian carcinoma by vaccination against the
extracellular Domain of anti-Müllerian hormone receptor II. Cancer
Prev Res (Phila). 10:612–624. 2017. View Article : Google Scholar
|
|
46
|
Song JY, Chen KY, Kim SY, Kim MR, Ryu KS,
Cha JH, Kang CS, MacLaughlin DT and Kim JH: The expression of
Müllerian inhibiting substance/anti-Müllerian hormone type II
receptor protein and mRNA in benign, borderline and malignant
ovarian neoplasia. Int J Oncol. 34:1583–1591. 2009.PubMed/NCBI
|
|
47
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brinkmann U and Kontermann RE: The making
of bispecific antibodies. MAbs. 9:182–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carter PJ and Lazar GA: Next generation
antibody drugs: Pursuit of the 'high-hanging fruit'. Nat Rev Drug
Discov. 17:197–223. 2018. View Article : Google Scholar
|
|
50
|
Labrijn AF, Janmaat ML, Reichert JM and
Parren PW: Bispecific antibodies: A mechanistic review of the
pipeline. Nat Rev Drug Discov. 18:585–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kontermann RE: Dual targeting strategies
with bispecific antibodies. MAbs. 4:182–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spiess C, Zhai Q and Carter PJ:
Alternative molecular formats and therapeutic applications for
bispecific antibodies. Mol Immunol. 67:95–106. 2015. View Article : Google Scholar : PubMed/NCBI
|