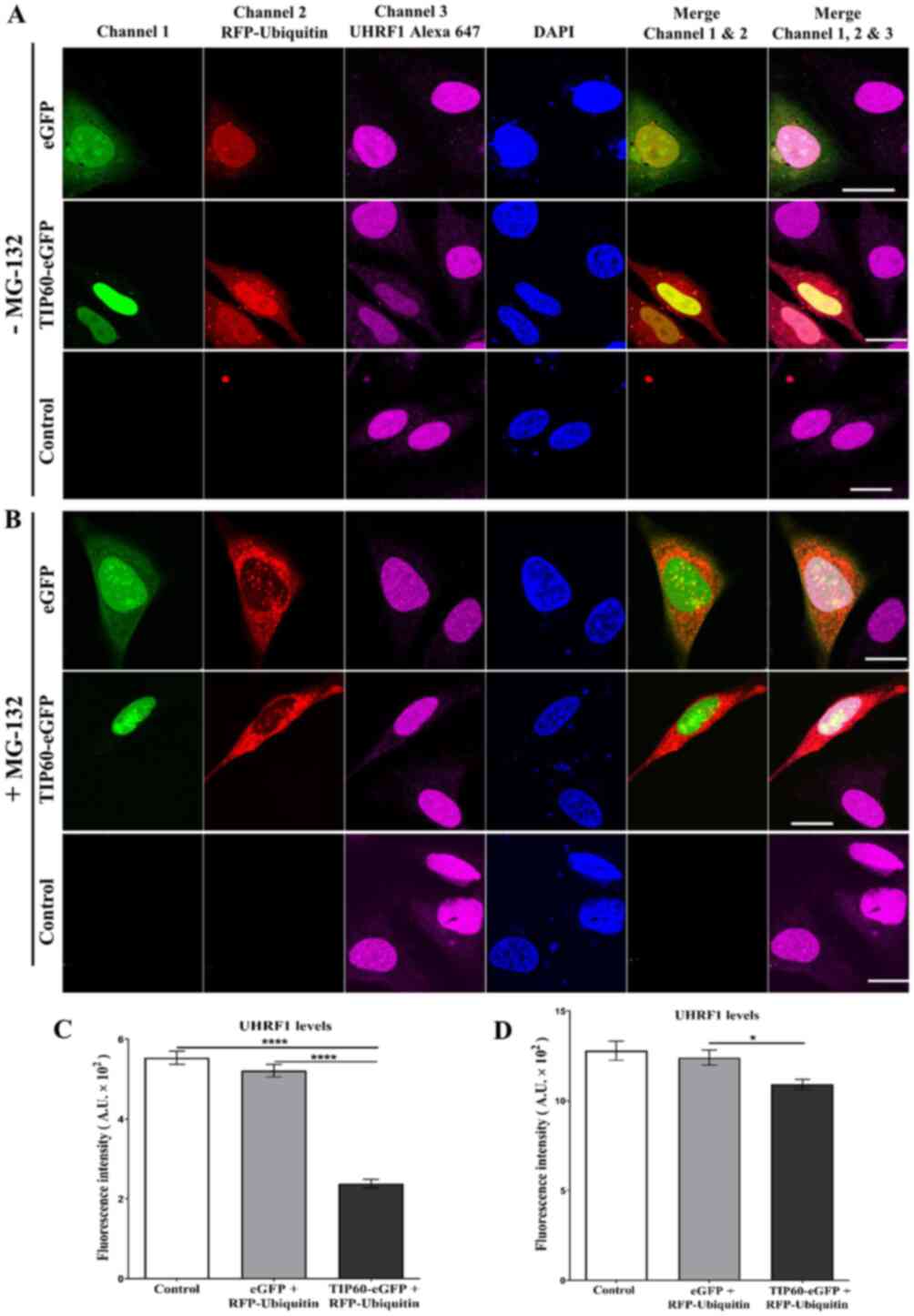
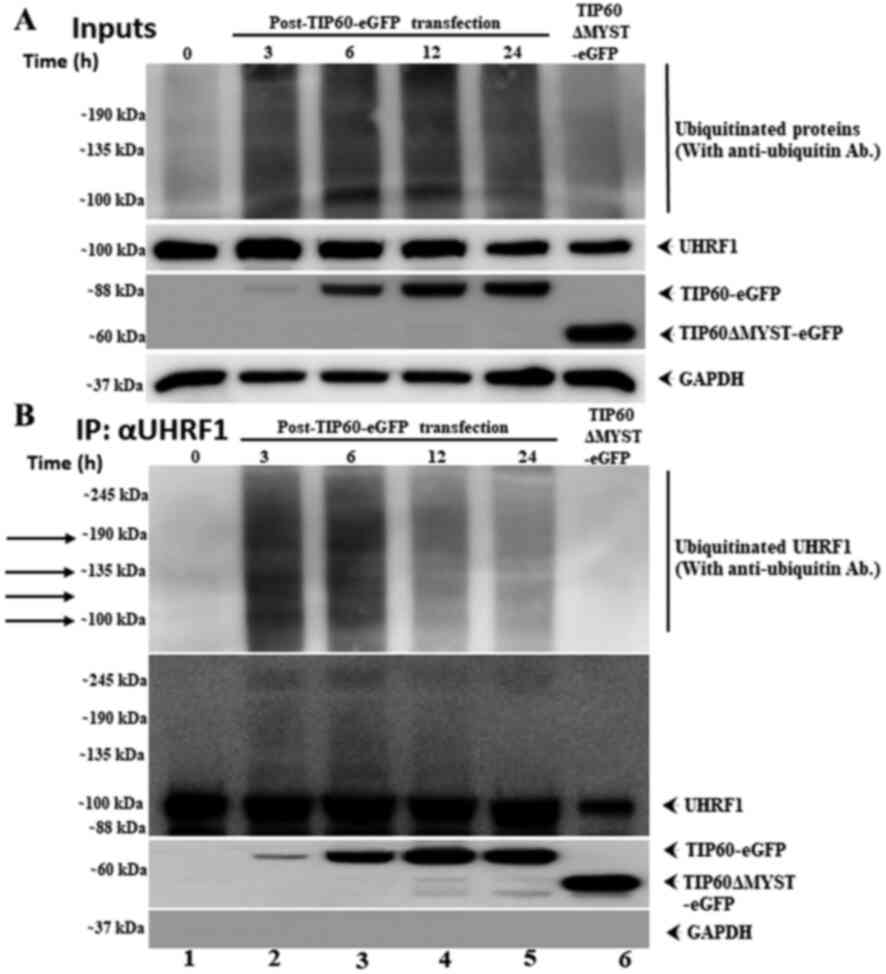
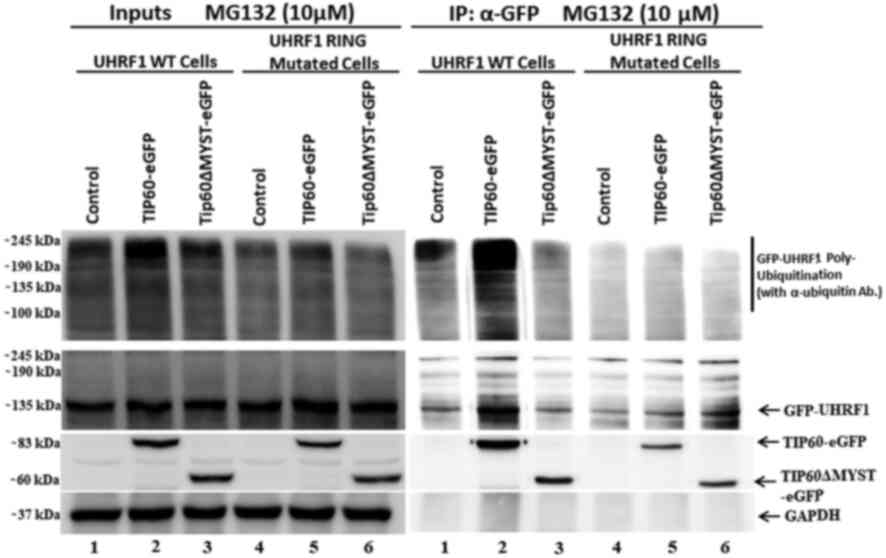
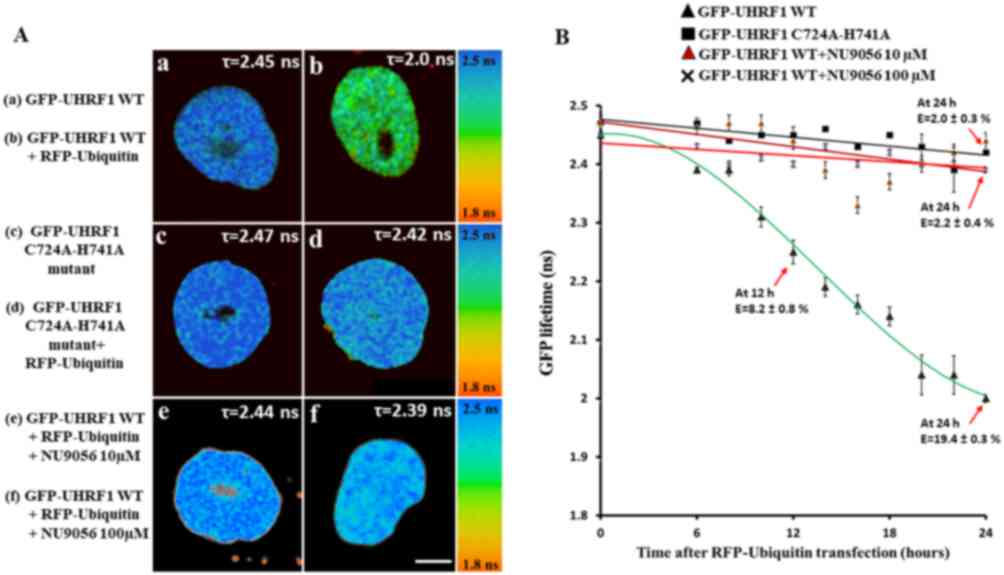
|
1
|
Hopfner R, Mousli M, Jeltsch JM, Voulgaris
A, Lutz Y, Marin C, Bellocq JP, Oudet P and Bronner C: ICBP90, a
novel human CCAAT binding protein, involved in the regulation of
topoisomerase IIalpha expression. Cancer Res. 60:121–128.
2000.PubMed/NCBI
|
|
2
|
Hopfner R, Mousli M, Garnier JM, Redon R,
du Manoir S, Chatton B, Ghyselinck N, Oudet P and Bronner C:
Genomic structure and chromosomal mapping of the gene coding for
ICBP90, a protein involved in the regulation of the topoisomerase
IIalpha gene expression. Gene. 266:15–23. 2001. View Article : Google Scholar
|
|
3
|
Krifa M, Alhosin M, Muller CD, Gies JP,
Chekir-Ghedira L, Ghedira K, Mély Y, Bronner C and Mousli M:
Limoniastrum guyonianum aqueous gall extract induces apoptosis in
human cervical cancer cells involving p16 INK4A re-expression
related to UHRF1 and DNMT1 down-regulation. J Exp Clin Cancer Res.
32:302013. View Article : Google Scholar
|
|
4
|
Ashraf W, Ibrahim A, Alhosin M, Zaayter L,
Ouararhni K, Papin C, Ahmad T, Hamiche A, Mély Y, Bronner C and
Mousli M: The epigenetic integrator UHRF1: On the road to become a
universal biomarker for cancer. Oncotarget. 8:51946–51962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bronner C, Achour M, Arima Y, Chataigneau
T, Saya H and Schini-Kerth VB: The UHRF family: Oncogenes that are
drugable targets for cancer therapy in the near future? Pharmacol
Ther. 115:419–434. 2007. View Article : Google Scholar
|
|
6
|
Bostick M, Kim JK, Estève PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar
|
|
7
|
Bronner C, Alhosin M, Hamiche A and Mousli
M: Coordinated dialogue between UHRF1 and DNMT1 to ensure faithful
inheritance of methylated DNA patterns. Genes (Basel). 10:652019.
View Article : Google Scholar
|
|
8
|
Avvakumov GV, Walker JR, Xue S, Li Y, Duan
S, Bronner C, Arrowsmith CH and Dhe-Paganon S: Structural basis for
recognition of hemi-methylated DNA by the SRA domain of human
UHRF1. Nature. 455:822–825. 2008. View Article : Google Scholar
|
|
9
|
Sharif J, Muto M, Takebayashi S, Suetake
I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T,
Okamura K, et al: The SRA protein Np95 mediates epigenetic
inheritance by recruiting Dnmt1 to methylated DNA. Nature.
450:908–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arita K, Ariyoshi M, Tochio H, Nakamura Y
and Shirakawa M: Recognition of hemi-methylated DNA by the SRA
protein UHRF1 by a base-flipping mechanism. Nature. 455:818–821.
2008. View Article : Google Scholar
|
|
11
|
Nady N, Lemak A, Walker JR, Avvakumov GV,
Kareta MS, Achour M, Xue S, Duan S, Allali-Hassani A, Zuo X, et al:
Recognition of multivalent histone states associated with
heterochromatin by UHRF1 protein. J Biol Chem. 286:24300–24311.
2011. View Article : Google Scholar
|
|
12
|
Rajakumara E, Wang Z, Ma H, Hu L, Chen H,
Lin Y, Guo R, Wu F, Li H, Lan F, et al: PHD finger recognition of
unmodified histone H3R2 links UHRF1 to regulation of euchromatic
gene expression. Mol Cell. 43:275–284. 2011. View Article : Google Scholar
|
|
13
|
Hu L, Li Z, Wang P, Lin Y and Xu Y:
Crystal structure of PHD domain of UHRF1 and insights into
recognition of unmodified histone H3 arginine residue 2. Cell Res.
21:1374–1378. 2011. View Article : Google Scholar
|
|
14
|
Jenkins Y, Markovtsov V, Lang W, Sharma P,
Pearsall D, Warner J, Franci C, Huang B, Huang J, Yam GC, et al:
Critical role of the ubiquitin ligase activity of UHRF1, a nuclear
RING finger protein, in tumor cell growth. Mol Biol Cell.
16:5621–5629. 2005. View Article : Google Scholar
|
|
15
|
Ibrahim A, Alhosin M, Papin C, Ouararhni
K, Omran Z, Zamzami MA, Al-Malki AL, Choudhry H, Mély Y, Hamiche A,
et al: Thymoquinone challenges UHRF1 to commit auto-ubiquitination:
A key event for apoptosis induction in cancer cells. Oncotarget.
9:28599–28611. 2018. View Article : Google Scholar
|
|
16
|
Tauber M and Fischle W: Conserved linker
regions and their regulation determine multiple chromatin-binding
modes of UHRF1. Nucleus. 6:123–132. 2015. View Article : Google Scholar
|
|
17
|
Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang
S, Kao HY, Xu Y, Willis J, Markowitz SD, et al: DNMT1 stability is
regulated by proteins coordinating deubiquitination and
acetylation-driven ubiquitination. Sci Signal. 3:ra802010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishiyama A, Yamaguchi L, Sharif J,
Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T,
Ishikawa F, et al: Uhrf1-dependent H3K23 ubiquitylation couples
maintenance DNA methylation and replication. Nature. 502:249–253.
2013. View Article : Google Scholar
|
|
19
|
Qin W, Wolf P, Liu N, Link S, Smets M, La
Mastra F, Forné I, Pichler G, Hörl D, Fellinger K, et al: DNA
methylation requires a DNMT1 ubiquitin interacting motif (UIM) and
histone ubiquitination. Cell Res. 25:911–929. 2015. View Article : Google Scholar
|
|
20
|
Foster BM, Stolz P, Mulholland CB, Montoya
A, Kramer H, Bultmann S and Bartke T: Critical role of the UBL
domain in stimulating the E3 ubiquitin ligase activity of UHRF1
toward chromatin. Mol Cell. 72:739–752.e9. 2018. View Article : Google Scholar
|
|
21
|
Mishima Y, Brueckner L, Takahashi S,
Kawakami T, Otani J, Shinohara A, Takeshita K, Garvilles RG,
Watanabe M, Sakai N, et al: Enhanced processivity of Dnmt1 by
monoubiquitinated histone H3. Genes Cells. 25:22–32. 2020.
View Article : Google Scholar
|
|
22
|
Li T, Wang L, Du Y, Xie S, Yang X, Lian F,
Zhou Z and Qian C: Structural and mechanistic insights into
UHRF1-mediated DNMT1 activation in the maintenance DNA methylation.
Nuclic Acids Res. 46:3218–3231. 2018. View Article : Google Scholar
|
|
23
|
Alhosin M, Omran Z, Zamzami MA, Al-Malki
AL, Choudhry H, Mousli M and Bronner C: Signalling pathways in
UHRF1-dependent regulation of tumor suppressor genes in cancer. J
Exp Clin Cancer Res. 35:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai C, Shi D and Gu W: Negative regulation
of the acetyltransferase TIP60-p53 interplay by UHRF1
(ubiquitin-like with PHD and RING finger domains 1). J Biol Chem.
288:19581–19592. 2013. View Article : Google Scholar :
|
|
26
|
Guan D, Factor D, Liu Y, Wang Z and Kao
HY: The epigenetic regulator UHRF1 promotes ubiquitination-mediated
degradation of the tumor-suppressor protein promyelocytic leukemia
protein. Oncogene. 32:3819–3828. 2013. View Article : Google Scholar :
|
|
27
|
Achour M, Fuhrmann G, Alhosin M, Rondé P,
Chataigneau T, Mousli M, Schini-Kerth VB and Bronner C: UHRF1
recruits the histone acetyltransferase Tip60 and controls its
expression and activity. Biochem Biophys Res Commun. 390:523–528.
2009. View Article : Google Scholar
|
|
28
|
Kamine J, Elangovan B, Subramanian T,
Coleman D and Chinnadurai G: Identification of a cellular protein
that specifically interacts with the essential cysteine region of
the HIV-1 Tat transactivator. Virology. 216:357–366. 1996.
View Article : Google Scholar
|
|
29
|
Yamamoto T and Horikoshi M: Novel
substrate specificity of the histone acetyltransferase activity of
HIV-1-Tat interactive protein Tip60. J Biol Chem. 272:30595–30598.
1997. View Article : Google Scholar
|
|
30
|
Hilfiker A, Hilfiker-Kleiner D, Pannuti A
and Lucchesi JC: mof, a putative acetyl transferase gene related to
the Tip60 and MOZ human genes and to the SAS genes of yeast, is
required for dosage compensation in Drosophila. EMBO J.
16:2054–2060. 1997. View Article : Google Scholar
|
|
31
|
Lee KK and Workman JL: Histone
acetyltransferase complexes: One size doesn't fit all. Nat Rev Mol
Cell Biol. 8:284–295. 2007. View Article : Google Scholar
|
|
32
|
Doyon Y, Selleck W, Lane WS, Tan S and
Côté J: Structural and functional conservation of the NuA4 histone
acetyltransferase complex from yeast to humans. Mol Cell Biol.
24:1884–1896. 2004. View Article : Google Scholar
|
|
33
|
Voss AK and Thomas T: MYST family histone
acetyltransferases take center stage in stem cells and development.
Bioessays. 31:1050–1061. 2009. View Article : Google Scholar
|
|
34
|
Sheikh BN and Akhtar A: The many lives of
KATs-detectors, integrators and modulators of the cellular
environment. Nat Rev Genet. 20:7–23. 2019. View Article : Google Scholar
|
|
35
|
Kim CH, Kim JW, Jang SM, An JH, Seo SB and
Choi KH: The chromodomain-containing histone acetyltransferase
TIP60 acts as a code reader, recognizing the epigenetic codes for
initiating transcription. Biosci Biotechnol Biochem. 79:532–538.
2015. View Article : Google Scholar
|
|
36
|
Squatrito M, Gorrini C and Amati B: Tip60
in DNA damage response and growth control: Many tricks in one HAT.
Trends Cell Biol. 16:433–442. 2006. View Article : Google Scholar
|
|
37
|
Kimura A, Matsubara K and Horikoshi M: A
decade of histone acetylation: Marking eukaryotic chromosomes with
specific codes. J Biochem. 138:647–662. 2005. View Article : Google Scholar
|
|
38
|
Kim MY, Ann EJ, Kim JY, Mo JS, Park JH,
Kim SY, Seo MS and Park HS: Tip60 histone acetyltransferase acts as
a negative regulator of Notch1 signaling by means of acetylation.
Mol Cell Biol. 27:6506–6519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sapountzi V, Logan IR and Robson CN:
Cellular functions of TIP60. Int J Biochem Cell Biol. 38:1496–1509.
2006. View Article : Google Scholar
|
|
40
|
Putnik J, Zhang CD, Archangelo LF, Tizazu
B, Bartels S, Kickstein M, Greif PA and Bohlander SK: The
interaction of ETV6 (TEL) and TIP60 requires a functional histone
acetyltransferase domain in TIP60. Biochim Biophys Acta.
1772:1211–1224. 2007. View Article : Google Scholar
|
|
41
|
Ikura T, Ogryzko VV, Grigoriev M, Groisman
R, Wang J, Horikoshi M, Scully R, Qin J and Nakatani Y: Involvement
of the TIP60 histone acetylase complex in DNA repair and apoptosis.
Cell. 104:463–473. 2000. View Article : Google Scholar
|
|
42
|
Judes G, Rifaï K, Ngollo M, Daures M,
Bignon YJ, Penault-Llorca F and Bernard-Gallon D: A bivalent role
of TIP60 histone acetyl transferase in human cancer. Epigenomics.
7:1351–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Idrissou M, Rifaï K, Daures M,
Penault-Llorca F, Bignon YJ and Bernard-Gallon D: Exciting history
of Tip60 and its companions in carcinogenesis across the
heterochromatin landscapes. OMICS. 22:626–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Frank SR, Parisi T, Taubert S, Fernandez
P, Fuchs M, Chan HM, Livingston DM and Amati B: MYC recruits the
TIP60 histone acetyltransferase complex to chromatin. EMBO Rep.
4:575–580. 2003. View Article : Google Scholar
|
|
45
|
Berns K, Hijmans EM, Mullenders J,
Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M,
Nijkamp W, Weigelt B, et al: A large-scale RNAi screen in human
cells identifies new components of the p53 pathway. Nature.
428:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mo F, Zhuang X, Liu X, Yao PY, Qin B, Su
Z, Zang J, Wang Z, Zhang J, Dou Z, et al: Acetylation of Aurora B
by TIP60 ensures accurate chromosomal segregation. Nat Chem Biol.
12:226–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
DeRan M, Pulvino M, Greene E, Su C and
Zhao J: Transcriptional activation of histone genes requires
NPAT-dependent recruitment of TRRAP-Tip60 complex to histone
promoters during the G1/S phase transition. Mol Cell Biol.
28:435–447. 2008. View Article : Google Scholar
|
|
48
|
Niida H, Katsuno Y, Sengoku M, Shimada M,
Yukawa M, Ikura M, Ikura T, Kohno K, Shima H, Suzuki H, et al:
Essential role of Tip60-dependent recruitment of ribonucleotide
reductase at DNA damage sites in DNA repair during G1 phase. Genes
Dev. 24:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taubert S, Gorrini C, Frank SR, Parisi T,
Fuchs M, Chan HM, Livingston DM and Amati B: E2F-dependent histone
acetylation and recruitment of the Tip60 acetyltransferase complex
to chromatin in late G1. Mol Cell Biol. 24:4546–4556. 2004.
View Article : Google Scholar :
|
|
50
|
Hu Y, Fisher JB, Koprowski S, McAllister
D, Kim MS and Lough J: Homozygous disruption of the Tip60 gene
causes early embryonic lethality. Dev Dyn. 238:2912–2921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sakuraba K, Yasuda T, Sakata M, Kitamura
YH, Shirahata A, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa
G, et al: Down-regulation of Tip60 gene as a potential marker for
the malignancy of colorectal cancer. Anticancer Res. 29:3953–3955.
2009.
|
|
52
|
Sakuraba K, Yokomizo K, Shirahata A, Goto
T, Saito M, Ishibashi K, Kigawa G, Nemoto H and Hibi K: TIP60 as a
potential marker for the malignancy of gastric cancer. Anticancer
Res. 31:77–79. 2011.PubMed/NCBI
|
|
53
|
Gorrini C, Squatrito M, Luise C, Syed N,
Perna D, Wark L, Martinato F, Sardella D, Verrecchia A, Bennett S,
et al: Tip60 is a haplo-insufficient tumour suppressor required for
an oncogene-induced DNA damage response. Nature. 448:1063–1067.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA,
Tran C, Chen C, Chung CH, Huber O, Rose DW, et al: Transcriptional
regulation of a metastasis suppressor gene by Tip60 and
beta-catenin complexes. Nature. 434:921–926. 2005. View Article : Google Scholar
|
|
55
|
Jha S, Vande Pol S, Banerjee NS, Dutta AB,
Chow LT and Dutta A: Destabilization of TIP60 by human
papillomavirus E6 results in attenuation of TIP60-dependent
transcriptional regulation and apoptotic pathway. Mol Cell.
38:700–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brown JA, Bourke E, Eriksson LA and Kerin
MJ: Targeting cancer using KAT inhibitors to mimic lethal
knockouts. Biochem Soc Trans. 44:979–986. 2016. View Article : Google Scholar :
|
|
57
|
Ashraf W, Bronner C, Zaayter L, Ahmad T,
Richert L, Alhosin M, Ibrahim A, Hamiche A, Mely Y and Mousli M:
Interaction of the epigenetic integrator UHRF1 with the MYST domain
of TIP60 inside the cell. J Exp Clin Cancer Res. 36:1882017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang ZM, Rothbart SB, Allison DF, Cai Q,
Harrison JS, Li L, Wang Y, Strahl BD, Wang G and Song J: An
allosteric interaction links USP7 to deubiquitination and chromatin
targeting of UHRF1. Cell Re. 12:1400–1406. 2015. View Article : Google Scholar
|
|
59
|
Cheng J, Yang H, Fang J, Ma L, Gong R,
Wang P, Li Z and Xu Y: Molecular mechanism for USP7-mediated DNMT1
stabilization by acetylation. Nat Commun. 6:70232015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Clamme JP, Azoulay J and Mély Y:
Monitoring of the formation and dissociation of
polyethylenimine/DNA complexes by two photon fluorescence
correlation spectroscopy. Biophys J. 84:1960–1968. 2003. View Article : Google Scholar
|
|
61
|
El Meshri SE, Dujardin D, Godet J, Richert
L, Boudier C, Darlix JL, Didier P, Mély Y and de Rocquigny H: Role
of the nucleocapsid domain in HIV-1 Gag oligomerization and
trafficking to the plasma membrane: A fluorescence lifetime imaging
microscopy investigation. J Mol Biol. 427:1480–1494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M and Murty VV: Identification of copy
number gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar
|
|
63
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Becker W: Advanced time-correlated single
photon counting applications. Springer; Heidelberg: 2015,
View Article : Google Scholar
|
|
65
|
Voss TC, Demarco IA and Day RN:
Quantitaive imaging of protein interactions in the cell nucleus.
Biotechniques. 38:413–424. 2005. View Article : Google Scholar :
|
|
66
|
Ma H, Chen H, Guo X, Wang Z, Sowa ME,
Zheng L, Hu S, Zeng P, Guo R, Diao J, et al: M phase
phosphorylation of the epigenetic regulator UHRF1 regulates its
physical association with the deubiquitylase USP7 and stability.
Proc Natl Acad Sci USA. 109:4828–4833. 2012. View Article : Google Scholar :
|
|
67
|
Alhosin M, Abusnina A, Achour M, Sharif T,
Muller C, Peluso J, Chataigneau T, Lugnier C, Schini-Kerth VB,
Bronner C and Fuhrmann G: Induction of apoptosis by thymoquinone in
lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent
pathway which targets the epigenetic integrator UHRF1. Biochem
Pharmacol. 79:1251–1260. 2010. View Article : Google Scholar
|
|
68
|
Achour M, Mousli M, Alhosin M, Ibrahim A,
Peluso J, Muller CD, Schini-Kerth VB, Hamiche A, Dhe-Peganon S and
Bronner C: Epigallocatechin-3-gallate up-regulates tumor suppressor
gene expression via a reactive oxygen species-dependent
down-regulation of UHRF1. Biochem Biophys Res Commun. 430:208–212.
2013. View Article : Google Scholar
|
|
69
|
León-González AJ, Jara-Palacios MJ, Abbas
M, Heredia FJ and Schini-Kerth VB: Role of epigenetic regulation on
the induction of apoptosis in Jurkat leukemia cells by white grape
pomace rich in phenolic compounds. Food Nut. 8:4062–4069. 2017.
|
|
70
|
Sharif T, Alhosin M, Auger C, Minker C,
Kim JH, Etienne-Selloum N, Bories P, Gronemeyer H, Lobstein A,
Bronner C, et al: Aronia melanocarpa juice induces a
redox-sensitive p73-related caspase-3-dependent apoptosis in human
leukemia cells. PLoS One. 7:e325262012. View Article : Google Scholar
|
|
71
|
Kim JK, Estève PO, Jacobsen SE and Pradhan
S: UHRF1 binds G9a and participates in p21 transcriptional
regulation in mammalian cells. Nucleic Acids Res. 37:493–505. 2009.
View Article : Google Scholar :
|
|
72
|
Polepalli S, George SM, Valli Sri Vidya R,
Rodrigues GS, Ramachandra L, Chandrashekar R, DN M, Rao PPN,
Pestell RG and Rao M: Role of UHRF1 in malignancy and its function
as a therapeutic target for molecular docking towards the SRA
domain. Int J Biochem Cell Biol. 114:1055582019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Boukhari A, Alhosin M, Bronner C, Sagini
K, Truchot C, Sick E, Schini-Kerth VB, André P, Mély Y, Mousli M
and Gies JP: CD47 activation-induced UHRF1 over-expression is
associated with silencing of tumor suppressor gene p16INK4A in
glioblastoma cells. Anticancer Res. 35:149–157. 2015.PubMed/NCBI
|
|
74
|
Jeanblanc M, Mousli M, Hopfner R, Bathami
K, Martinet N, Abbady AQ, Siffert JC, Mathieu E, Muller CD and
Bronner C: The retinoblastoma gene and its product are targeted by
ICBP90: A key mechanism in the G1/S transition during the cell
cycle. Oncogene. 24:7337–7345. 2005. View Article : Google Scholar
|
|
75
|
Unoki M, Brunet J and Mousli M: Drug
discovery targeting epigenetic codes: The great potential of UHRF1,
which links DNA methylation and histone modifications, as a drug
target in cancers and toxoplasmosis. Biochem Pharmacol.
78:1279–1288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang F, Yang YZ, Shi CZ, Zhang P, Moyer
MP, Zhang HZ, Zou Y and Qin HL: UHRF1 promotes cell growth and
metastasis through repression of p16(ink4a) in colorectal cancer.
Ann Surg Oncol. 19:2753–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xue B, Zhao J, Feng P, Xing J, Wu H and Li
Y: Epigenetic mechanism and target therapy of UHRF1 protein complex
in malignancies. Onco Targets Ther. 12:549–559. 2019. View Article : Google Scholar :
|
|
78
|
Subbaiah VK, Zhang Y, Rajagopalan D,
Abdullah AN, Yeo-Teh NS, Tomaić V, Banks L, Myers MP, Chow EK and
Jha S: E3 ligase EDD1/UBR5 is utilized by the HPV E6 oncogene to
destabilize tumor suppressor TIP60. Oncogene. 35:2062–2074. 2016.
View Article : Google Scholar
|
|
79
|
Rajagopalan D, Pandey AK, Xiuzhen MC, Lee
KK, Hora S, Zhang Y, Chua BH, Kwok HS, Bhatia SS, Deng LW, et al:
TIP60 represses telomerase expression by inhibiting Sp1 binding to
the TERT promoter. PLoS Pathog. 13:e10066812017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Achour M, Jacq X, Rondé P, Alhosin M,
Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A,
Hughes AD, et al: The interaction of the SRA domain of ICBP90 with
a novel domain of DNMT1 is involved in the regulation of VEGF gene
expression. Oncogene. 27:2187–2197. 2008. View Article : Google Scholar
|
|
81
|
Gao Y, Wang Y, Zhou C, Kong S, Lu J, Wang
H and Yang J: Ubiquitin-specific protease 7 (USP7) is essential for
endometrial stromal cell decidualization in mice. Dev Growth
Differ. 61:176–185. 2019. View Article : Google Scholar
|
|
82
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Felle M, Joppien S, Németh A, Diermeier S,
Thalhammer V, Dobner T, Kremmer E, Kappler R and Längst G: The
USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1
and regulates the stability of UHRF1. Nucleic Acids Res.
39:8355–8365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mistry H, Gibson L, Yun JW, Sarras H,
Tamblyn L and McPherson JP: Interplay between Np95 and Eme1 in the
DNA damage response. Biochem Biophys Res Commun. 375:321–325. 2008.
View Article : Google Scholar
|
|
85
|
Chen H, Ma H, Inuzuka H, Diao J, Lan F,
Shi YG, Wei W and Shi Y: DNA damage regulates UHRF1 stability via
the SCF(β-TrCP) E3 ligase. Mol Cell Biol. 33:1139–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He J, Zhu Q, Wani G, Sharma N, Han C, Qian
J, Pentz K, Wang QE and Wani AA: Ubiquitin-specific protease 7
regulates nucleotide excision repair through deubiquitinating XPC
protein and preventing XPC protein from undergoing ultraviolet
light-induced and VCP/p97 protein-regulated proteolysis. J Biol
Chem. 289:27278–27289. 2014. View Article : Google Scholar :
|
|
87
|
He M, Zhu Z, Shah AA, Zou H, Tao J, Chen Q
and Wan Y: The emerging role of deubiquitinating enzymes in genomic
integrity, diseases, and therapeutics. Cell Biosci. 6:622016.
View Article : Google Scholar :
|
|
88
|
Kwon SK, Saindane M and Baek KH: p53
stability is regulated by diverse deubiquitinating enzymes. Biochim
Biophys Acta Rev Cancer. 1868:404–411. 2017. View Article : Google Scholar
|
|
89
|
Sheng Y, Saridakis V, Sarkari F, Duan S,
Wu T, Arrowsmith CH and Frappier L: Molecular recognition of p53
and MDM2 by USP7/HAUSP. Nat Struct Mol Biol. 13:285–291. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hou H, Sun D and Zhang X: The role of MDM2
amplification and overexpression in therapeutic resistance of
malignant tumors. Cancer Cell Int. 19:2162019. View Article : Google Scholar :
|
|
91
|
Nininahazwe L, Liu B, He C, Zhang H and
Chen ZS: The emerging nature of ubiquitin-specific protease 7
(USP7): A new target in cancer therapy. Drug Discov Today.
26:490–502. 2021. View Article : Google Scholar
|
|
92
|
Bronner C: Control of DNMT1 abundance in
epigenetic inheritance by acetylation, ubiquitylation, and the
histone code. Sci Sign. 4:pe32011.
|
|
93
|
Jang SY, Hong D, Jeong SY and Kim JH:
Shikonin causes apoptosis by up-regulating p73 and down-regulating
ICBP90 in human cancer cells. Biochem Biophys Res Commun.
465:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|