Introduction
The multidomain protein, ubiquitin-like, containing
PHD and RING finger domains 1 (UHRF1, also known as ICBP90 in
humans) (1,2), is an important epigenetic integrator
responsible for the faithful transmission of DNA methylation
patterns from parent strands to daughter strands during DNA
replication (1,3-8).
UHRF1 performs this role by recognizing the CpG motifs in
hemi-methylated DNA through its SRA domain (SET and RING-associated
domain) and by recruiting DNA methyltransferase 1 (DNMT1) (6-10).
TTD and PHD domains help UHRF1 to read the histone marks (11-13). The RING domain of UHRF1 has
intrinsic ubiquitin E3 ligase activity by which UHRF1 can
ubiquitinate itself (auto-ubiquitination) (14,15) or other proteins including histones
(16,17). The ubiquitination of H3K23 and
H3K18 by UHRF1 is important for the creation of binding sites for
DNMT1 (7,18-21). The N-terminal UBL domain of UHRF1
binds directly to DNMT1 and increases its enzymatic activity
towards chromatin by controlling H3 ubiquitination (20-22). UHRF1 is also involved in the DNA
damage response (4,23,24) and the regulation of the stability
and function of several other proteins, such as p53, promyelocytic
leukemia protein (PML) and DNMT1 through the collaboration with
other epigenetic partners such as ubiquitin-specific-processing
protease 7 (USP7), histone deacetylase 1 (HDAC1) and Tat
interactive protein, 60 kDa (TIP60) (4,17,25,26).
The authors of the present study first reported the
interaction of UHRF1 with TIP60 in a previous study (27). Indeed, UHRF1 and TIP60 were found
to be in the same macromolecular complex and interact with each
other (17,25,27). TIP60 was originally recognized as
an interacting partner of HIV-1 Tat protein (28). TIP60 (also known as KAT5) belongs
to the MYST family (MOZ, YBF2/SAS3, SAS2 and TIP60) having an
evolutionary conserved domain which harbors histone
acetyltransferase (HAT) activity (29-32). At its N-terminus, TIP60 has a
chromodomain (CRD), while its C-terminus contains the conserved
enzymatic MYST domain (33).
TIP60 reads histone marks (H3K4me2/H3K9me3) through its CRD domain
(34) and translates it through
MYST domain (35). Inside the
MYST domain, there is the catalytic HAT domain which binds to
acetyl coenzyme A and catalyzes acetylation of both histone and
non-histone proteins (36,37).
This acetyltransferase activity is stimulated by a zinc finger
which helps TIP60 to interact with the targeted substrates
(38-40). Through its enzymatic activity,
TIP60 is a central player in many key cellular processes like
chromatin remodeling, DNA damage response, transcription
regulation, genomic integrity, cell cycle and apoptosis (25,36,39,41-43). For instance, it interacts with and
regulates the transcription of nuclear hormone receptors, p53,
c-MYC and NF-κB (39,42,44). Of note, it also regulates p53
activity in an acetylation-dependent (K120 of p53) and -independent
manner (25). The acetylation of
p53 activates p21 and the PUMA pathway, which leads to cell growth
arrest and apoptosis, and thus, ensures tumor suppression (25).
The downregulation of TIP60 inhibits both p21
activation and growth arrest (45). During the M-phase, TIP60 is
essential for chromosomal segregation (46) and cell cycle progression (47-49). Cells lacking TIP60
acetyltransferase activity lose their ability to repair DNA and
ultimately, cell cycle control (41). The heterozygous deletion of the
TIP60 gene (HTATIP) has a lethal effect on embryos (50). In a number of cancer types, TIP60
levels are low as compared to normal cells, supporting its tumor
suppressive role (25,41,45,51-56). In accordance with this role, high
levels of UHRF1 have been shown to interfere with the TIP60-p53
interplay and prevent p53 activation, which leads to tumorigenesis
and/or tumor progression (25).
Therefore, targeting UHRF1 in cancer cells would permit the
'rescue' of p53 levels and would enhance the coordinated dialogue
between p53 and TIP60. In a previous study, the authors
demonstrated that UHRF1 interacts with the MYST domain of TIP60
(57). Moreover, UHRF1, through
its E3 ligase activity, ubiquitinates DNMT1 and thus affects its
expression levels (17,58,59). Although it has already been shown
that TIP60 overexpression downregulates UHRF1 levels in HeLa cells
(57), the mechanisms responsible
for the TIP60-mediated downregulation of UHRF1 in cancer cells
remain elusive. The present study demonstrates that TIP60
interferes with the UHRF1-USP7 association. Following dissociation
from USP7, UHRF1 is auto-ubiquitinated by its RING domain. The
resulting downregulation of UHRF1 activates p73-mediated apoptosis.
Taken together, these observations provide new insight into the
tumor suppressive role of TIP60 by controlling the UHRF1
levels.
Materials and methods
Materials
MG-132
(C26H41N3O5) was
purchased from Selleckchem.com -Bioactive Compounds
Expert (cat. no. S2619). MG-132 was dissolved in pure DMSO
(Sigma-Aldrich; Merck KGaA) and stored at −80°C. Propidium iodide
(PI; cat. no. 130-093-233) was purchased from Miltenyi Biotec GmbH,
while Annexin V-iFluor™ 350 conjugate (cat. no. 20090)
was purchased from AAT Bioquest. The TIP60 inhibitor,
5-(1,2-thiazol-5-yldisulfanyl)-1,2-thiazole (NU9056), was purchased
from Tocris Bioscience (cat. no. 1450644-28-6).
Cells and cell culture
HeLa cells (ATCC, CCL-2, Amp, cervical
adenocarcinoma; human) were grown in Dulbecco's modified Eagle's
medium (DMEM 1X + GlutaMAX™, Pyruvate, Gibco; Thermo Fischer
Scientific, Inc.) which was supplemented with 10% fetal bovine
serum (FBS, cat. no. S1810-500; Dominique Dutscher), in addition to
mixture of penicillin (100 U/ml) and streptomycin (100 U/ml) (cat.
no. 17-602E; Lonza Group, Ltd.), at 37°C with 5% CO2 in
a humidified environment. HeLa cell lines stably expressing either
GFP-UHRF1 WT or GFP-UHRF1 C724A-H741A protein, were constructed
using the pOZ-N plasmid (Addgene) in which the GFP-UHRF1 WT or
GFP-UHRF1 C724A-H741A mutant cDNAs had been subcloned as previously
described (15). Mycoplasma
testing has been performed for the cell lines. Plasmids (TIP60
wild-type and its mutants: ΔHAT and ΔMYST; supplied by EpiGex) were
transfected (at a concentration of 1 µg/2 ml of media, for a
time period of 24 h) into HeLa cells with either jetPEI™
or jetPRIME (2 µl) (cat. no. 101-10N and cat. no. 114-15;
PolyPlus-transfection SA) according to the manufacturer's
protocol.
Plasmid constructs
TIP60 wild-type and mutants (ΔHAT and ΔMYST,
respectively) were cloned into a pEGFP-N1 plasmid (supplied by
EpiGex) to express eGFP-labeled TIP60 proteins in HeLa cells.
RFP-Ubiquitin was purchased from Addgene (cat. no. 11935).
Antibodies
Mouse monoclonal anti-UHRF1 (1:2,000) antibody was
engineered as previously described (1). Other antibodies used included rabbit
polyclonal anti-HAUSP/USP7 (1:5,000; cat. no. ab4080, Abcam), mouse
monoclonal anti-DNMT1 (1:5,000; cat. no. PTG-MAB0079, ProteoGenix),
mouse monoclonal anti-ubiquitin (1:500; cat. no. 05-944,
Sigma-Aldrich; Merck KGaA), mouse monoclonal eGFP (1:1,000; cat.
no. 66,002-1-Ig, Proteintech Group, Inc.; and cat. no. A-11120,
Thermo Fisher Scientific, Inc.), mouse monoclonal anti-GAPDH
(1:5,000; cat. no. MAB374, Merck KGaA), mouse monoclonal anti-GFP
(1:1,000; cat. no. 66002-1-Ig, Proteintech Group, Inc.), mouse
monoclonal anti-p73 (1:500; cat. no. 558785, BD Biosciences),
rabbit polyclonal anti-caspase-3 (1:1,000; cat. no. 9661, Cell
Signaling Technology, Inc.), mouse monoclonal anti-BCL2 (1:1,000;
cat. no. 05-826, Merck KGaA), mouse monoclonal
anti-poly(ADP-ribose) polymerase (PARP; 1:1,000; cat. no.
51-6639GR, BD Biosciences) and rabbit polyclonal anti-BAX (1:1,000;
cat. no. AB2930, Merck KGaA).
Western blot analysis
For western blot analysis, cells were collected at
24 h following transfection by trypsinization. For ubiquitination
experiments, cells were treated with MG-132 (10 µM) 8 h
prior to cell harvesting. Following centrifugation (500 × g for 4
min at 4°C), the medium was discarded, and the cell pellet was
washed with PBS. Cells were lysed with ice cold lysis buffer (10 mM
Tris-HCl pH 7.5, 1 mM EDTA, 150 mM NaCl and 1% NP40 supplemented
with protease inhibitors (cat. no. 1183617000;1 cOmplete mini
EDTA-free protease inhibitor cocktail tablets, Roche Diagnostics
GmbH). Following denaturation at 95°C for 7 min in 4X Laemmli
sample buffer freshly supplemented with β-mercaptoethanol (cat. no.
1610747 Bio-Rad Laboratories, Inc.), 40 µg of the protein
from cell lysates were loaded on 7.5 and 10% SDS-PAGE gels which
after separation were transferred to previously activated
polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life
Sciences, Cytiva). Membranes were blocked by 3% blotting-grade
blocker (Bio-Rad Laboratories, Inc.) in Tris-Buffered Saline, with
Tween®-20, pH 8.0 (TBS-T) (Sigma-Aldrich; Merck KGaA).
Proteins were identified by anti-UHRF1 (1:2,000 dilution in
blocking buffer), anti-ubiquitin (1:500 dilution in blocking
buffer), anti-DNMT1 (1:5,000 dilution in blocking buffer),
anti-USP7 (1:5,000 dilution in blocking buffer), anti-eGFP (1:1,000
dilution in blocking buffer) and anti-GAPDH (1:5,000 dilution in
blocking buffer) primary antibodies, with overnight incubation at
4°C. Membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies, anti-mouse (1:10,000
dilution in blocking buffer; cat. no. W402B; Promega Corporation)
or anti-rabbit (1:10,000 dilution in blocking buffer; cat. no.
W401B; Promega Corporation), for 1 h at room temperature, to label
the primary antibodies. Signals were detected on an Image Quant LAS
4000 apparatus (GE Healthcare Life Sciences, Cytiva) with
chemiluminescent ECL system (Clarity™ ECL western blotting
substrate; cat. no. 170-5060, Bio-Rad Laboratories, Inc.). Image
Studio Lite (Li-Core Biosciences, Inc.) was used to analyze the
images.
Immunoprecipitation (IP)
For IP, cells were collected and lysed by freeze
shock. Mild sonication was performed in ice-cold PBS freshly
supplemented with protease inhibitors cocktail tablet. Input
controls were established by taking 40 µg of protein from
each lysate. A total of 1,000 to 1,500 µg of protein lysate
were incubated with anti-UHRF1 antibody at 4°C for 3 h or with
anti-USP7 antibody at 4°C overnight. After washing and
equilibration, 60 µl of Dynabeads® protein A
(cat. no. 1002D; Thermo Fischer Scientific, Inc.) were added to the
lysate-antibody mixture and incubated for 1 h at 4°C. The beads
were then collected and washed 3-5 times with ice-cold PBS freshly
supplemented with protease inhibitors tablet. Finally, beads were
resuspended in Laemmli sample buffer. Proteins were denatured by
heating at 95°C for 7 min and examined by western blot
analysis.
UHRF1 auto-ubiquitination assay
HeLa cells stably expressing GFP-UHRF1 WT and
GFP-UHRF1 C724A-H741A mutant proteins were transfected with either
TIP60 WT (1 µg/2 ml of media) or TIP60ΔMYST (1 µg/2
ml of media) mutant using jetPRIME reagent (2 µl), for a
time duration of 24 h. Samples were treated with 10 µM
MG-132, 8 h before harvesting the cells. IP (as described above)
was performed with anti-GFP antibody to immunoprecipitate the
GFP-tagged UHRF1 protein. Samples were resolved by western blot
analysis.
Confocal microscopy
To examine the effect of TIP60 overexpression on the
UHRF1 and DNMT1 levels, HeLa cells were seeded on a cover glass and
transfected with eGFP (1 µg/2 ml of media) or TIP60-eGFP (1
µg/2 ml of media) or TIP60ΔMYST-eGFP (1 µg/2 ml of
media) plasmids using jetPEI™ reagent (2 µl) as
described in the manufacturer's protocol. At 24 h
post-transfection, cells were fixed with 4% paraformaldehyde for 10
min and then permeabilized with 0.2% Triton X-100 for 20 min at
room temperature. Subsequently, blocking was performed with 1% BSA
for 1 h, prior to incubation with a primary antibody against either
UHRF1 or DNMT1 for 3 h at 4°C. After washing three times with PBS,
cells were incubated with secondary antibody labeled with Alexa
Fluor 568 (goat anti-mouse, cat. no. A11031; Invitrogen; Thermo
Fischer Scientific, Inc.) for 60 min at room temperature. The cells
were then washed three times and labeled with DAPI (Invitrogen
Hoechst stain, cat. no. 33258; Thermo Fischer Scientific, Inc.).
Finally, cells were imaged with a confocal Leica TCS SPE microscope
equipped with a 20X air (0.7 NA) immersion lens objective. For
DAPI, Alexa Fluor 568 and eGFP, excitation was performed with a 405
nm laser (25 mW), 561 nm laser (10 mW) and 488 nm laser (25 mW),
respectively. The detection range for the three dyes was 430-480,
570-630 and 500-523 nm, respectively.
To examine the effect of TIP60 overexpression on the
co-localization of UHRF1 and ubiquitin, HeLa cells were
co-transfected with either eGFP (1 µg/2 ml of media) and
RFP-Ubiquitin (1 µg/2 ml of media) or TIP60-eGFP (1
µg/2 ml of media) and RFP-Ubiquitin (1 µg/2 ml of
media) using jetPEI™ reagent (2 µl), for a time duration of
24 h. One group of samples was treated with MG-132 (10 µM) 8
h before cell fixation, to block the proteasomal degradation of
UHRF1. Cells were labeled with anti-UHRF1 as primary antibody for 3
h at 4°C and Alexa Fluor 647-labeled goat anti-mouse, cat. no.
A-21237 (Thermo Fischer Scientific, Inc.) as secondary antibody,
for 1 h at room temperature. DAPI staining was done to visualize
the nucleus. All samples were imaged with a confocal Leica TCS SPE
equipped with an oil immersion objective (HXC PL APO 63X/1.40 OIL
CS). For DAPI, RFP, Alexa Fluor 647 and eGFP, excitation was
performed with a 405 nm laser (25 mW), 561 nm laser (10 mW), 635 nm
laser (18 mW) and 405 nm laser (25 mW), respectively. The detection
range for the four dyes was 430-480, 570-630, 640-702 and 500-523
nm, respectively.
For the UHRF1-USP7 association analysis, HeLa cells
were transfected with either TIP60-eGFP (1 µg) or
TIP60ΔMYST-eGFP (1 µg) using jetPEI™ reagent (2
µl), for a time duration of 24 h. One group of samples was
treated with MG-132 (10 µM) 8 h before cell fixation, to
block the proteasomal degradation of UHRF1 and USP7. Cells were
labeled with anti-UHRF1 (mouse) and anti-USP7 (rabbit) antibodies
overnight at 4°C. The cells were then incubated with secondary
antibody labeled with Alexa Fluor 568 (goat anti-rabbit, cat. no.
A11011; Invitrogen; Thermo Fischer Scientific, Inc.) for USP7 and
Alexa Fluor 647 (goat anti-mouse) for UHRF1. DAPI staining (100
µg/ml in PBS for 20 min at room temperature) was performed
to stain the nuclei. All samples were imaged with a confocal Leica
TCS SPE equipped with an oil immersion objective (HXC PL APO
63X/1.40 OIL CS). For DAPI, Alexa Fluor 568, Alexa Fluor 647 and
eGFP, excitation was performed with a 405 nm laser (25 mW), 561 nm
laser (10 mW), 635 nm laser (18 mW) and 405 nm laser (25 mW),
respectively. The detection range for the four dyes was 430-480,
570-625, 644-707 and 500-531 nm, respectively. All the images were
processed using ImageJ software (1.52p; Wayne Rasband, NIH;
http://imagej.nih.gov/ij).
Fluorescence lifetime imaging microscopy
(FLIM)
HeLa cells stably expressing GFP-UHRF1 WT or
GFP-UHRF1 C724A-H741A protein, were seeded (105 cells
per dish) in a µ-dish (Ibidi) with 35-mm wells. Cells were
transfected with 1 µg RFP-Ubiquitin plasmid using jetPEI™
reagent. Cells were fixed with 4% paraformaldehyde. Following
fixation, cells were imaged with a homemade two-photon excitation
scanning microscope based on an Olympus IX70 inverted microscope
with a 60X 1.2 NA water immersion objective operating in the
descanned fluorescence collection mode as previously described
(60,61). Two-photon excitation at 930 nm was
provided by an Insight DeepSee laser (Spectra Physics, Inc.).
Fluorescence photons were collected using a short-pass filter with
a cut-off wavelength of 680 nm (cat. no. F75-680; Analysentechnik)
and a band-pass filter of 520±17 nm (cat. no. F37-520;
Analysentechnik). The fluorescence was directed to a fibre-coupled
APD (cat. no. SPCM-AQR-14-FC; Perkin Elmer Inc., USA), which was
connected to a time-correlated single photon counting module (cat.
no. SPC830: Becker & Hickl). FLIM data were analyzed using
SPCImage v 7.3 (Becker & Hickel) and the Förster resonance
energy transfer (FRET) efficiency was calculated according to
E=1-(τDA/τD), where τDA is the
lifetime of the donor (GFP) in the presence of acceptor (RFP) and
τD is the lifetime of GFP in the absence of
acceptor.
Apoptosis analysis
Flow cytometry was used to analyze TIP60-induced
apoptosis. HeLa cells were seeded in six-well plates. Cells were
transfected with TIP60-eGFP by using jetPEI™ reagent. TIP60
transfected cells were compared to control cells or cells treated
with jetPEI™ only. Cells were collected after mild trypsinization
and incubated with PI and Annexin V-iFluor™350 conjugate. The
samples were then analyzed with a Guava easyCyte™ flow cytometer
(Merck KGaA). InCyte Software for Guava (Merck KGaA) was used to
analyze the results.
Retrospective TIP60 expression
analysis
To evaluate the differential expression of
TIP60 in normal and cancer cervical tissues retrospectively,
the expression profile of TIP60 was retrieved from GDS3233
(62) at NCBI Gene Expression
Omnibus data base (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS3233).
The expression analysis was done by using Affymetrix U133A
oligonucleotide microarray (Santa Clara, CA) which contains 14500
probes for analysis. This dataset included expression profile of
TIP60 in 24 normal cervical tissues and 28 cervical cancer
tissues which were compared by using an unpaired Student's
t-test.
RNAseq expression analysis
Raw counts RNAseq expression data of cancer samples
were downloaded from the TCGA website (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga),
normalized using DESeq2 (63) and
used to plot the distribution of expression of three genes (UHRF1,
TIP60 and USP7) in each cancer type, and represented as box plots
in Figs. S4, S5 and S9. When
available, the mean expression level of the corresponding non-tumor
tissue was calculated (and shown as a red circle in the
corresponding figures). Co-expression of TIP60 and UHRF1 was
determined by linear regression analysis. Simple linear regression
was used between y-axis and x-axis. The delivered score is the
R2, also known as the linear association, characterizing
the percentage of explained variance. Here caution is advised,
R2 score is indicating the behavior/tendency towards the
association of two genes.
Survival probability analysis
To investigate the association between the
expression of either the TIP60/KAT5, UHRF1 or USP7 gene and the
probability of survival of TCGA cancer patients for whom survival
data were available (meta data available from the TCGA site, as
well as from our custom website http://epimed.univ-grenoble-alpes.fr/database/series),
a two-step bioinformatics analysis was performed: i) Seeking for a
significant association between the expression value and survival
probability, using the Cox model; and ii) when the P-value of the
Cox model was significant (P<0.05), samples were grouped by
quintiles of expression (from the lowest expression <20th
percentile to the highest >80th percentile) and survival
probabilities were compared between the groups with a log-rank
test. Survival plots are only shown for the cancer types in which
an association between the expression of each gene and survival was
found.
Statistical analysis
The results were statistically analyzed using
GraphPad Prism (version 9; GraphPad Software, Inc.) software.
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. In addition, comparisons between
two groups were analyzed using an unpaired Student's t-test. All
data are presented as the mean ± standard error of the mean (SEM)
of at least three independent experiments. P<0.05 was considered
to indicate a statistically significant difference.
Results
TIP60 overexpression induces the
ubiquitination of UHRF1
The authors have previously demonstrated that TIP60
overexpression downregulates UHRF1 and DNMT1 expression (57). The present study, using confocal
microscopy experiments, confirmed that a significant (P<0.0001)
decrease in UHRF1 and DNMT1 fluorescence intensity was detected in
the TIP60-eGFP WT-transfected cells, while TIP60ΔMYST-eGFP
transfection only marginally affected the UHRF1 and DNMT1
fluorescence intensity (Fig.
S1).
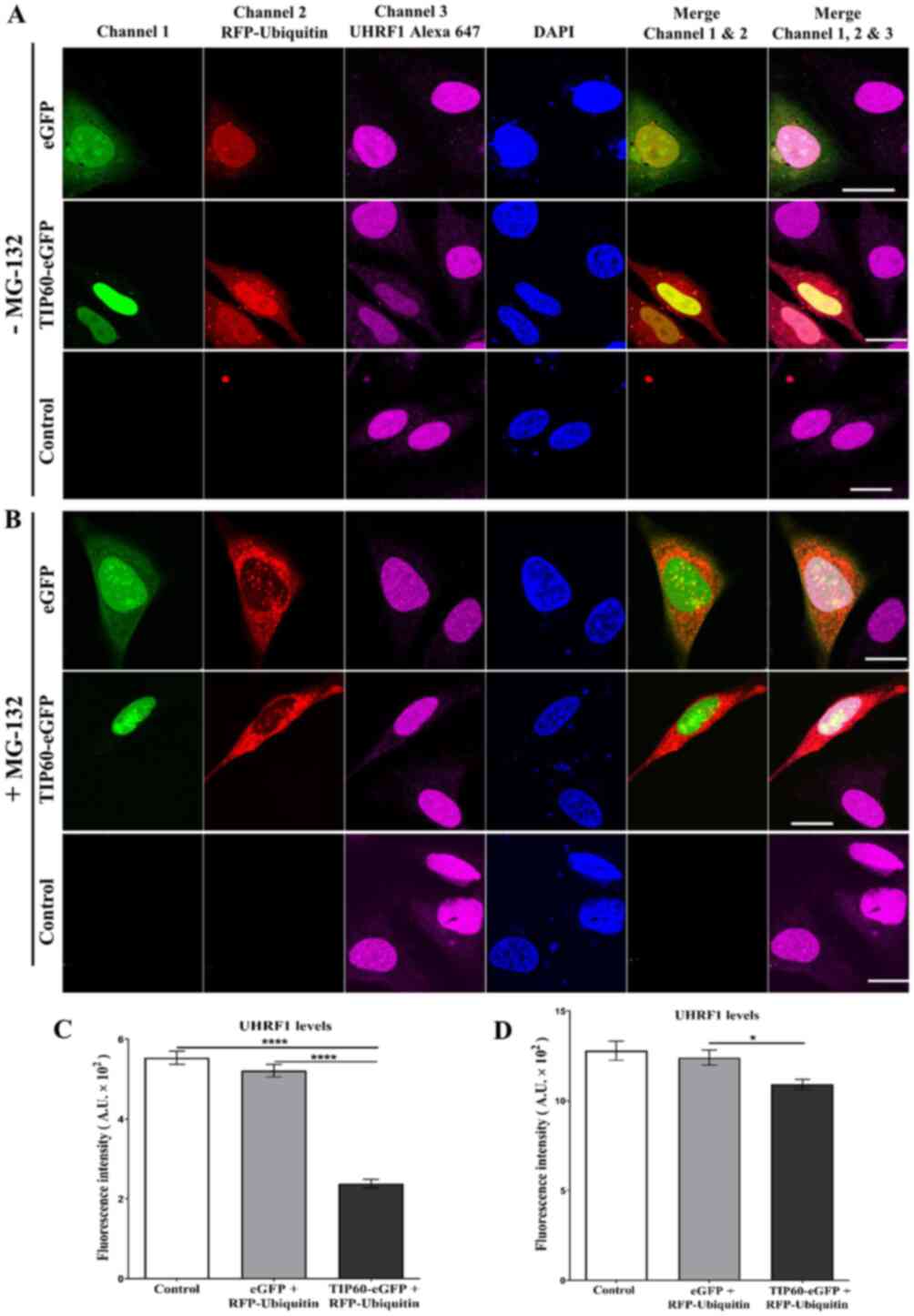
As shown in Fig.
1, HeLa cells were co-transfected with TIP60-eGFP +
RFP-ubiquitin. Untreated HeLa cells and eGFP +
RFP-ubiquitin-co-transfected cells served as the controls.
Endogenous UHRF1 levels were detected using a specific primary
antibody against UHRF1 and Alexa 647-labeled secondary antibody.
TIP60, UHRF1 and ubiquitin were well co-localized in the nucleus
(Fig. 1). A clearly visible
decrease in UHRF1 expression was observed in the TIP60 +
ubiquitin-co-transfected cells as compared with the adjacent
untransfected cells in the same sample or control samples (Fig. 1A). The quantification of the mean
fluorescence intensity of Alexa 647 revealed a significant
(P<0.0001) decrease in the UHRF1 fluorescence intensity (57%) in
the TIP60 + ubiquitin-co-transfected cells (Fig. 1C). The decrease in fluorescence
intensity was comparable with both the control or eGFP +
ubiquitin-transfected cells (6%) (Fig. 1C). UHRF1 expression was restored
in the TIP60 + ubiquitin-co-transfected cells treated with the
proteasomal inhibitor, MG-132 (Fig.
1B). The mean fluorescence intensity of UHRF1 was partially
recovered (Fig. 1D).
Furthermore, western blot analysis was performed to
support the findings of the confocal microscopy experiments
(Fig. S2). HeLa cells were
transfected with either TIP60 WT or TIP60ΔMYST mutant. One group of
samples was treated with MG-132. In the TIP60 WT-transfected sample
(Fig. S2A, -MG-132 group, lane
2), the UHRF1 level significantly decreased as compared with either
the control or TIP60ΔMYST mutant samples (lane 1 and 3,
respectively). Incubation with MG-132 stabilized the UHRF1 levels
in the TIP60 WT overexpressing sample (Fig. S2A, + MG-132 group, lane 2). The
improvement in the expression levels of UHRF1 was comparable to
that of the control and TIP60ΔMYST mutant-transfected samples
(Fig. S2B). In the TIP60
WT-transfected sample, a prominent smear and ubiquitinated protein
bands were observed (indicated with arrows) over UHRF1 following
treatment with MG-132 that the mutant failed to reproduce (Fig. S2A, + MG-132 lanes 2 and 3). Of
note, this observation was observed even with a lower expression of
TIP60 WT compared to the mutant TIP60ΔMYST (Fig. S2A, + MG-132 lane 2).
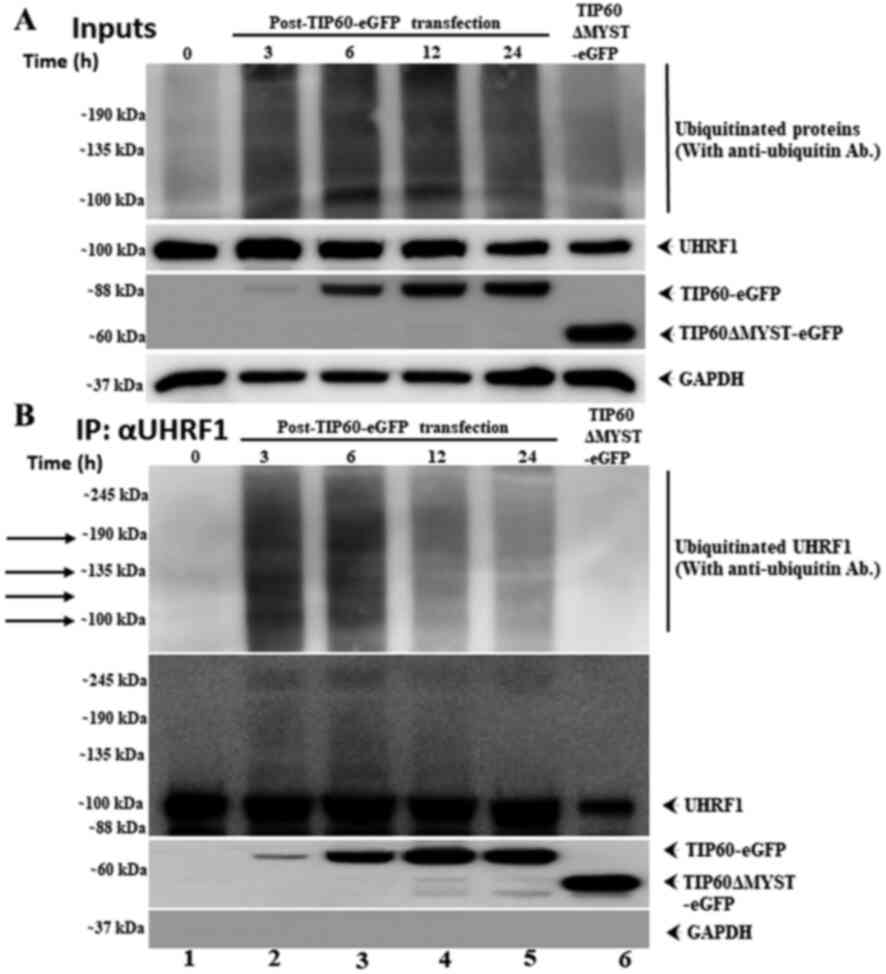
Subsequently, the effects of TIP60 overexpression on
UHRF1 ubiquitination as a function of time (Fig. 2) were examined. HeLa cells were
transfected with either TIP60 WT or TIP60ΔMYST mutant. Cells were
collected at different time intervals following transfection. IP
was performed with an anti-UHRF1 antibody. A prominent
ubiquitination smear was observed with the ubiquitinated UHRF1
bands at 3 and 6 h post-TIP60 WT transfection, suggesting that
TIP60-mediated UHRF1 ubiquitination was an early event (Fig. 2B, IP lanes 2 and 3). However, this
effect was still observed at 12 and 24 h post-TIP60 WT
transfection. In the case of TIP60ΔMYST, no ubiquitination of UHRF1
was observed up to 24 h post-transfection. These results
demonstrated that TIP60 overexpression induced ubiquitination that
did not lead to the degradation of UHRF1, due to proteasome
inhibition by MG-132.
TIP60 overexpression induces the
auto-ubiquitination of UHRF1
The RING finger domain of UHRF1 has E3 ligase
activity through which it can either ubiquitinate itself
(auto-ubiquitination) (14,15) or other proteins (16,17). Therefore, the present study
investigated whether the downregulation of UHRF1 levels is the
consequence of UHRF1 auto-ubiquitination activity or whether other
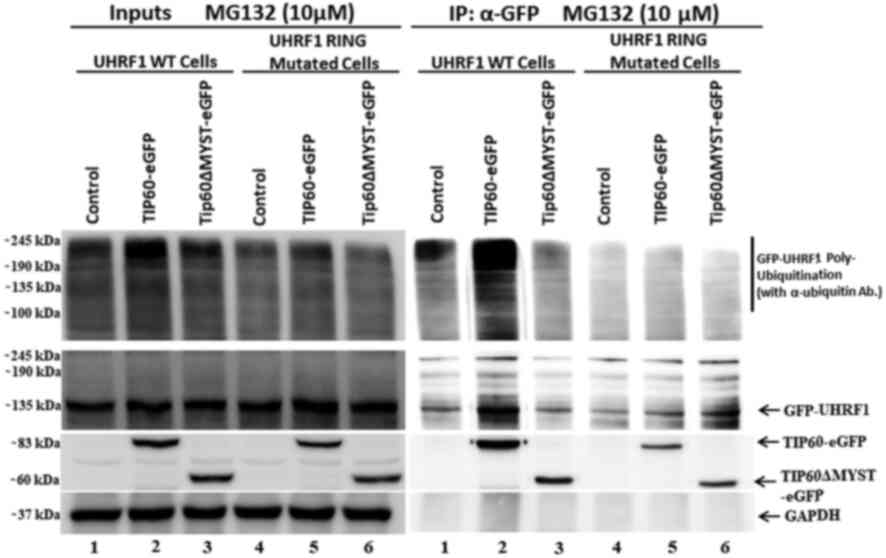
E3 ligases are responsible for this. This experiment was performed
using HeLa cells stably expressing either UHRF1 WT protein or UHRF1
C724A-H741A mutant protein having impaired RING finger domain
activity. Cells were transfected with either TIP60 WT or TIP60ΔMYST
mutant and treated with MG-132. The poly-ubiquitination of UHRF1 WT
was observed when TIP60 WT was overexpressed, as compared with
either the controls or ΔMYST mutant samples (Fig. 3, IP lane 2). Notably, in the case
of UHRF1 C724A-H741A, no ubiquitination smear and bands above the
UHRF1 band were observed (Fig. 3,
IP lanes 4, 5 and 6). This indicated that following TIP60
overexpression, UHRF1 was auto-ubiquitinated via its RING finger.
By contrast, UHRF1 bearing the RING finger domain mutation failed
to be auto-ubiquitinated following TIP60 overexpression.
UHRF1 interacts with ubiquitin
The interaction between UHRF1 and ubiquitin inside
the cell was further confirmed using FRET experiments. FRET between
GFP- and RFP-labeled proteins only occurs when they are <8 nm
apart, a distance relative to intermolecular protein-protein
interactions (60). FRET
efficiency is deduced from the decrease in GFP fluorescence
lifetime measured by Fluorescence Lifetime Imaging Microscopy
(FLIM) (64). FLIM technique
allows to derive and color code the fluorescence lifetime (τ) of
GFP at each pixel of the image (Fig.
4). In comparison to the fluorescence intensity, τ does not
depend on the fluorophore concentration or instrumentation. HeLa
cells expressing either GFP-UHRF1 WT or GFP-UHRF1 C724A-H741A
mutant were used for the experiments.
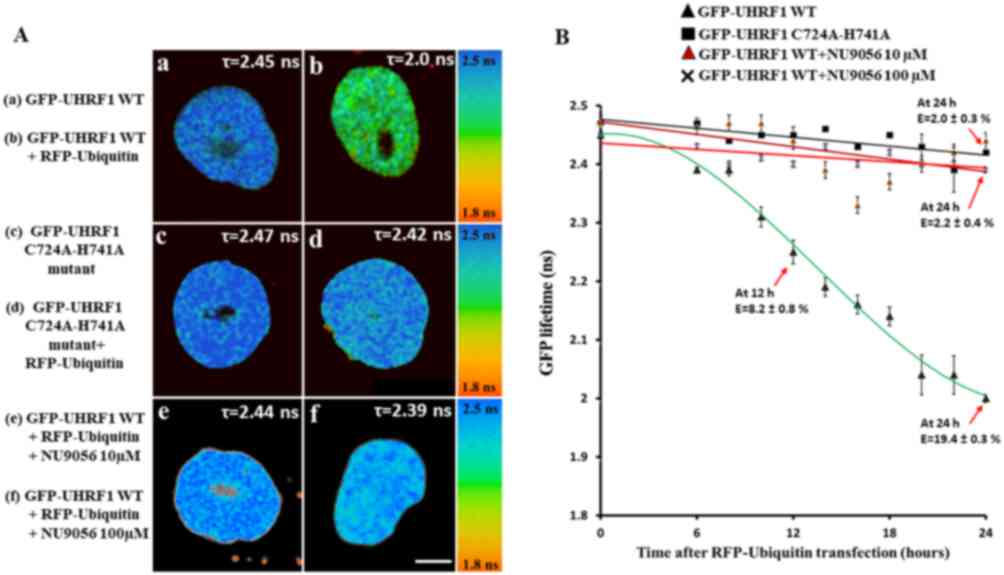 | Figure 4Interaction of UHRF1 and ubiquitin,
as determined by FRET-FLIM. (A) Representative 30x30 µm FLIM
images of HeLa cells stably expressing (a) GFP-UHRF1 WT and (b)
co-transfected with RFP-ubiquitin, HeLa cells (c) stably expressing
GFP-UHRF1 C724A-H741A and (d) co-transfected with RFP-ubiquitin.
The lifetime values are shown by a color code ranging from red (1.8
ns) to blue (2.5 ns). Scale bar, 10 µm. In comparison to
cells (a) expressing only GFP-UHRF1 WT, a marked decrease in the
GFP lifetime and thus, a strong FRET efficiency was observed when
HeLa cells were (b) transfected with RFP-ubiquitin. By contrast, no
substantial difference in lifetime or FRET efficiency was observed
when HeLa cells expressing GFP-UHRF1 C724A-H741A were transfected
with RFP-ubiquitin (compare panels d and c). No difference in
lifetime or FRET efficiency was observed in HeLa cells expressing
GFP-UHRF1 WT in the presence of the TIP60 inhibitor NU9056 (e) 10
µM or (f) 100 µM. FLIM data indicate that UHRF1
interacts with ubiquitin while this interaction is impaired in case
of UHRF1 having a RING finger domain mutation. (B) Change in GFP
lifetime as a function of time. Values are the mean ± SEM from
three independent experiments. The fluorescence lifetimes of
GFP-UHRF1 WT (without and with TIP60 inhibitor NU9056 10 µM,
100 µM) or GFP-UHRF1 C724A-H741A were measured at different
times following transfection with RFP-Ubiquitin. FRET efficiency
was calculated according to E=1-(τDA/τD),
where τDA is the lifetime of the donor (GFP) in the
presence of acceptor (RFP) and τD is the lifetime of GFP
in the absence of acceptor. UHRF1, ubiquitin-like, containing PHD
and RING finger domains 1; TIP60, Tat interactive protein, 60
kDa. |
These cells were co-transfected with RFP-labeled
ubiquitin and fixed at different time points, between 6 and 24 h.
The fluorescence lifetime of GFP-UHRF1 WT used as a control was
2.45±0.01 ns (n=36 cells) (Fig.
4A-a). The lifetime of GFP-UHRF1 was observed to decrease as a
function of time when the GFP-UHRF1 WT cells were transfected with
RFP-ubiquitin (Fig. 4B). A
substantial decrease (8.2% FRET) in lifetime was observed after 12
h of RFP-ubiquitin transfection (2.25±0.02 ns, n=26 cells) and a
further decrease in lifetime was observed after 24 h (2.00±0.01 ns,
n=20 cells) (Fig. 4A-bs). The
corresponding FRET efficiency (E) was 8.2±0.8 and 19.4±0.3% after
12 and 24 h of RFP-ubiquitin transfection, respectively.
Subsequently, the interaction between RFP-labeled ubiquitin and
GFP-labeled UHRF1 having a RING Finger domain mutation, as a
function of time was examined. The lifetime of GFP-UHRF1
C724A-H741A taken as a control was 2.47±0.01 ns (n=28 cells)
(Fig. 4A-c). Notably, no
considerable decrease was observed in the lifetime of GFP-UHRF1
C724A-H741A following RFP-ubiquitin transfection. Following 24 h of
RFP-ubiquitin transfection, the lifetime was 2.42±0.01 ns (n=18
cells) (Fig. 4A-d), which
corresponded to a FRET efficiency of 2.0±0.3%, below the commonly
accepted 5% threshold value for protein-protein interaction
(65). Thus, these data suggest
that mutation in the RING finger domain of UHRF1 can impair its
interaction with the ubiquitin.
Furthermore, the present study examined the effects
of the inhibition of TIP60 acetylation activity on the interaction
between UHRF1 and ubiquitin, by using the specific TIP60 inhibitor,
NU9056. First, the effect of NU9056 at various concentrations
between 1 and 100 µM after 24 h treatment was examined, and
the UHRF1-ubiquitin interaction was analyzed through FLIM, using
HeLa cells expressing GFP-UHRF1 WT protein. The interaction between
UHRF1 and ubiquitin could be still detected in the presence of 1, 3
and 5 µM of NU9056 (FRET efficiency was 12, 10 and 8.8%,
respectively), but was impaired at 10, 30 and 100 µM (FRET
efficiency 5.6, 4.8 and 3.6% respectively) (Fig. S3). Further experiments were
carried out to examine the effects of NU9056 on the UHRF1-ubiquitin
interaction in a time-dependent manner at 10 and 100 µM.
Under these conditions, no considerable decrease in the lifetime of
GFP-UHRF1 was observed at any time intervals in the presence of
TIP60 inhibitor, as shown in Fig.
4A-e and f. Overall, the FLIM data suggest that TIP60 favors
UHRF1-Ubiquitin interaction, while the inhibition of
acetyltransferase activity of TIP60 results in the impairment of
this interaction.
TIP60 overexpression interferes with the
USP7-UHRF1 association
In order to decipher the origin of the activation of
UHRF1 auto-ubiquitination, an alteration of the protective role of
USP7 was hypothesized. Indeed, USP7 interacts with UHRF1 and
protects it from ubiquitin-mediated proteasomal degradation
(58,66). To assess this hypothesis, HeLa
cells were transfected with either TIP60-eGFP or TIP60ΔMYST-eGFP
mutant. Anti-UHRF1 antibody was used to immunoprecipitate the
endogenous UHRF1 and its associated partner, USP7. The association
between USP7 and UHRF1 was observed in the untreated sample
(control) as USP7 was co-immunoprecipitated with UHRF1 (Fig. 5D). In the TIP60 overexpressed
sample, USP7 was barely detected following co-precipitation with
UHRF1 (Fig. 5D, lane 2). By
contrast, with TIP60ΔMYST-eGFP mutant this association was not
affected (Fig. 5D, lane 3). In a
reciprocal experiment, anti-USP7 antibody was used to
immunoprecipitate endogenous USP7. It was observed that following
TIP60 WT overexpression, reduced levels of endogenous UHRF1 were
co-precipitated as compared with the control and TIP60ΔMYST-eGFP
mutant sample (Fig. 5E, compare
lane 2 to lanes 1 and 3). Taken together, these results suggest
that TIP60 regulates the interaction of UHRF1 with USP7, which
conditions the auto-ubiquitination activity of UHRF1.
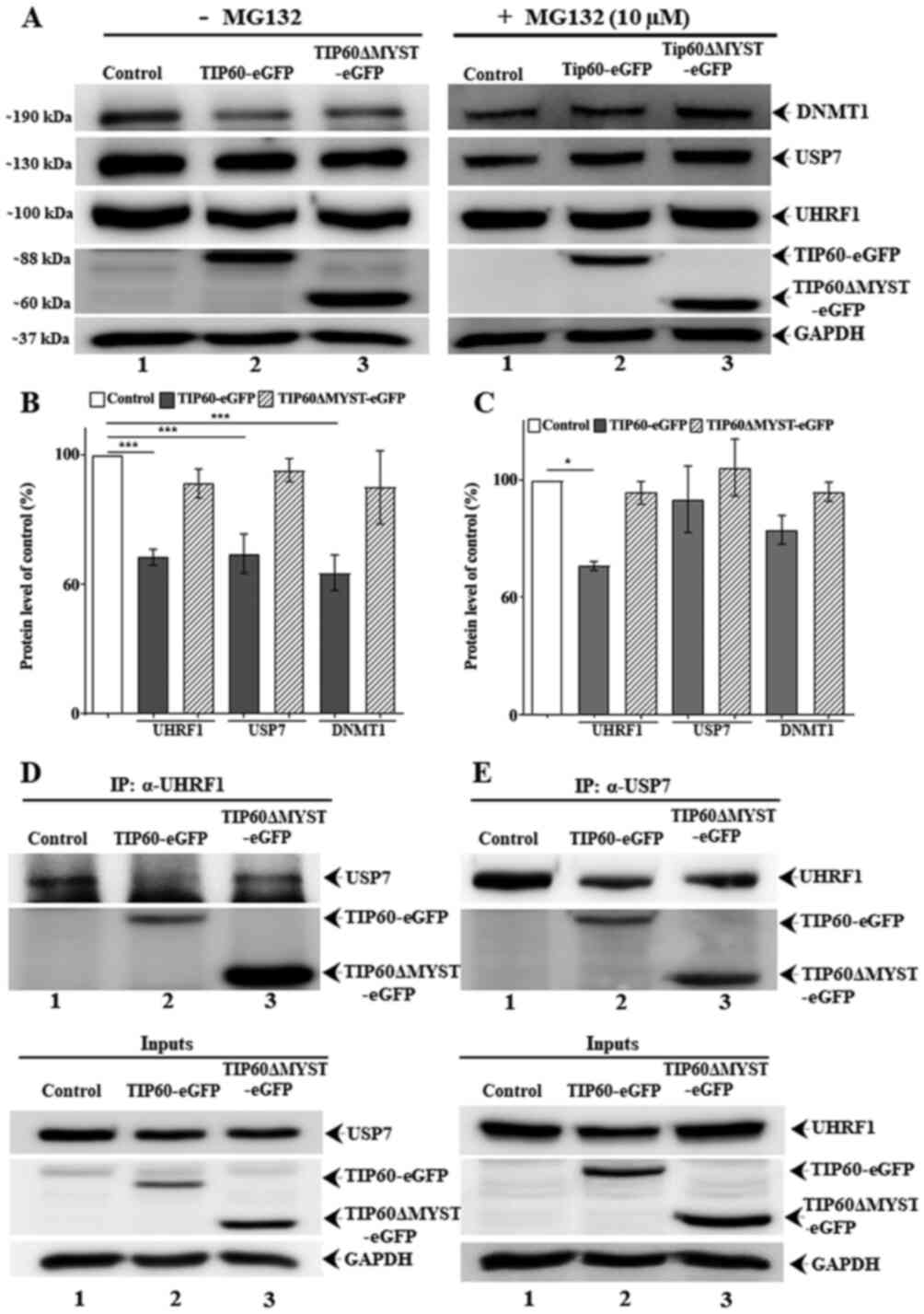 | Figure 5TIP60 interferes with UHRF1-USP7
association and their expression levels. HeLa cells were
transfected with either TIP60-eGFP WT or TIP60ΔMYST-eGFP mutant.
Western blot and immunoprecipitated samples were resolved by
SDS-PAGE and immunoblotted with anti-UHRF1, anti-USP7 and
anti-DNMT1 antibodies. (A) Effect of TIP60 on UHRF1, USP7 and DNMT1
levels with or without MG-132 treatment, respectively. (B and C)
Quantification of the effect of TIP60 on UHRF1, USP7 and DNMT1
levels with or without MG-132 treatment, respectively. Values are
the mean ± SEM from at least three independent experiments which
were analyzed statistically by one-way ANOVA with Tukey's post hoc
test (*P<0.05; ***P<0.001 vs. control
group). (D) Anti-UHRF1 antibody was used to co-immunoprecipitate
UHRF1 and its partner USP7. (E) In a reciprocal experiment,
anti-USP7 antibody was used to co-immunoprecipitate USP7 and UHRF1.
Inputs and IP gels were processed in parallel under similar
conditions. UHRF1, ubiquitin-like, containing PHD and RING finger
domains 1; TIP60, Tat interactive protein, 60 kDa; USP7,
ubiquitin-specific-processing protease 7; DNMT1, DNA
methyltransferase 1. |
TIP60 WT overexpression induced the downregulation
of UHRF1, USP7 and DNMT1 protein expression in comparison to
control or TIP60 ΔMYST mutant (Fig.
5A, left panel). However, treatment with MG-132 (Fig. 5A, right panel) led to a recovery
in the expression levels of these proteins. Quantitative analysis
of the input fractions revealed a significant (P<0.001) decrease
in the UHRF1, USP7 and DNMT1 levels following TIP60 WT
overexpression (Fig. 5B). MG-132
treatment fully restored the USP7 and DNMT1 levels, whereas it only
partially restored the UHRF1 levels (P<0.05) (Fig. 5C). To examine the expression
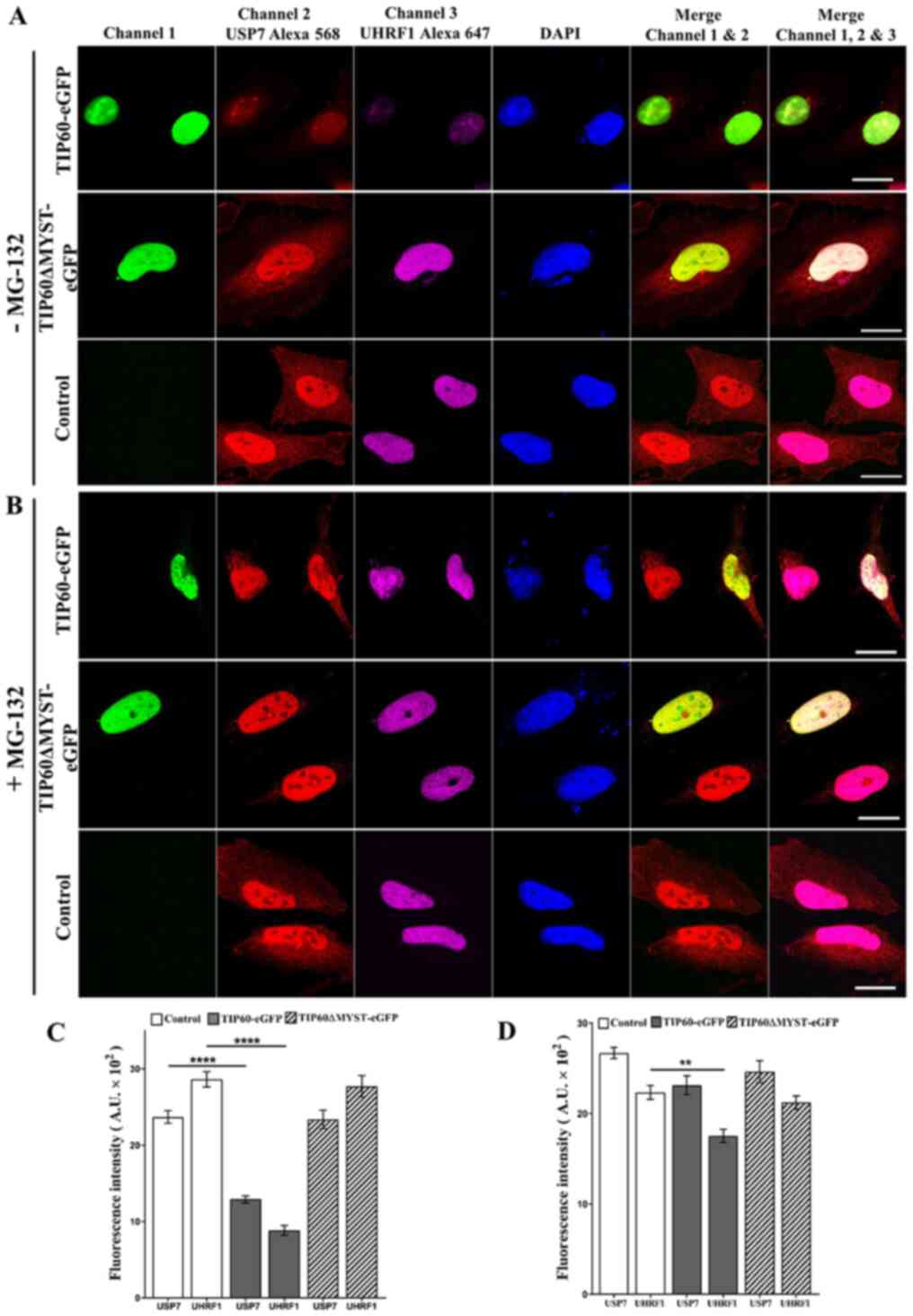
levels of USP7 and UHRF1 inside the cells following TIP60
overexpression, confocal microscopy experiments were performed. The
endogenous levels of UHRF1 and USP7 were examined in the same cells
by labeling with respective antibodies. Based on the mean
fluorescence intensity of the Alexa 568- and Alexa 647-labeled
secondary antibodies, the USP7 and UHRF1 levels were found to be
significantly decreased (P<0.0001) following TIP60
overexpression (Fig. 6A and C).
As compared with the control samples, decreases in fluorescence of
45 and 60% were observed for USP7 and UHRF1, respectively. By
contrast, the overexpression of the TIP60ΔMYST-eGFP mutant only
marginally affected the fluorescence intensities of USP7 and UHRF1
(Fig. 6A and C). Thus, these data
demonstrate that TIP60 overexpression can downregulate the USP7 and
UHRF1 levels simultaneously. Due to its downregulation, USP7 was
likely unable to protect the UHRF1 degradation via the proteasomal
pathway. As a significant decrease (P<0.0001) was observed in
the USP7 levels following TIP60 overexpression, the USP7 levels
were further examined following treatment with MG-132. Of note, the
expression levels of USP7 were improved in the TIP60 overexpressing
samples following treatment with MG-132 (Fig. 6B and D). The expression levels of
UHRF1 were also improved, although to a lesser extent as compared
with those of USP7, which suggests that once UHRF1 is degraded
through the proteasomal degradation pathway, its levels are not
restored immediately (Fig. 6B and
D).
TIP60 overexpression induces the
activation of p73
Since the experiments indicated that TIP60 regulated
UHRF1 expression by governing its auto-ubiquitination, the
physiological or physiopathological consequences of this regulation
were then investigated. Tumor suppressor protein p73 is important
for genomic stability by responding to a number of stress signals
and is under the control of UHRF1 (67,68). The p73-mediated apoptosis leads to
the activation of the mitochondria-dependent apoptotic pathway
through the transactivation of pro-apoptotic proteins (e.g., BAX)
and the downregulation of pro-survival proteins (e.g., BCL2)
(69,70). Therefore, the present study
examined the effect of the TIP60-mediated UHRF1 downregulation on
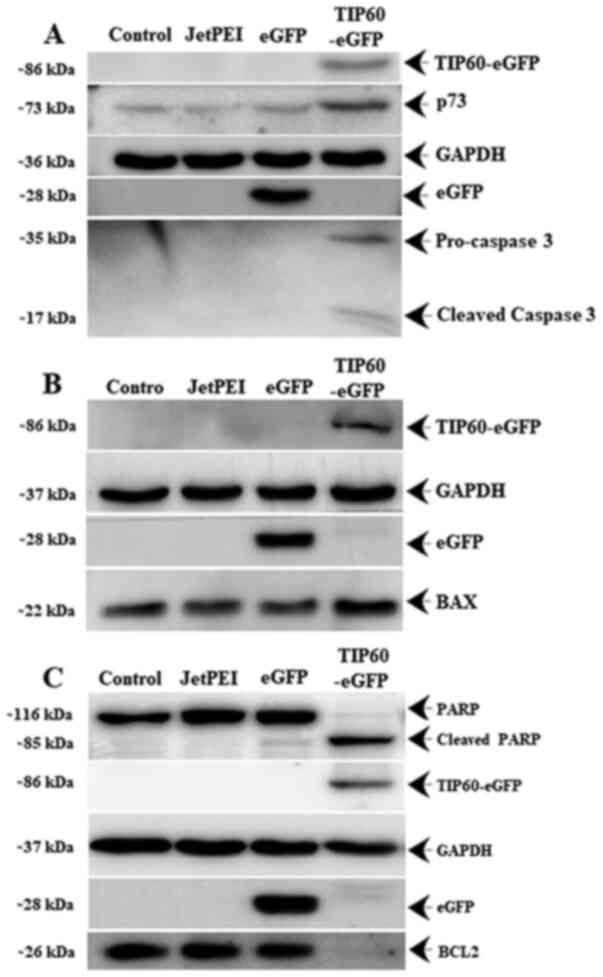
the levels of p73. It was found that the overexpression of TIP60
induced an increase in the expression of p73 (Fig. 7A) and BAX protein (Fig. 7B), whereas it induced a decrease
in the expression of the anti-apoptotic BCL2 protein (Fig. 7C). Furthermore, it was observed
that TIP60 overexpression induced caspase-3 activation from its
precursor pro-caspase-3 (Fig.
7A), which in turn triggered the cleavage of PARP to induce
apoptosis.
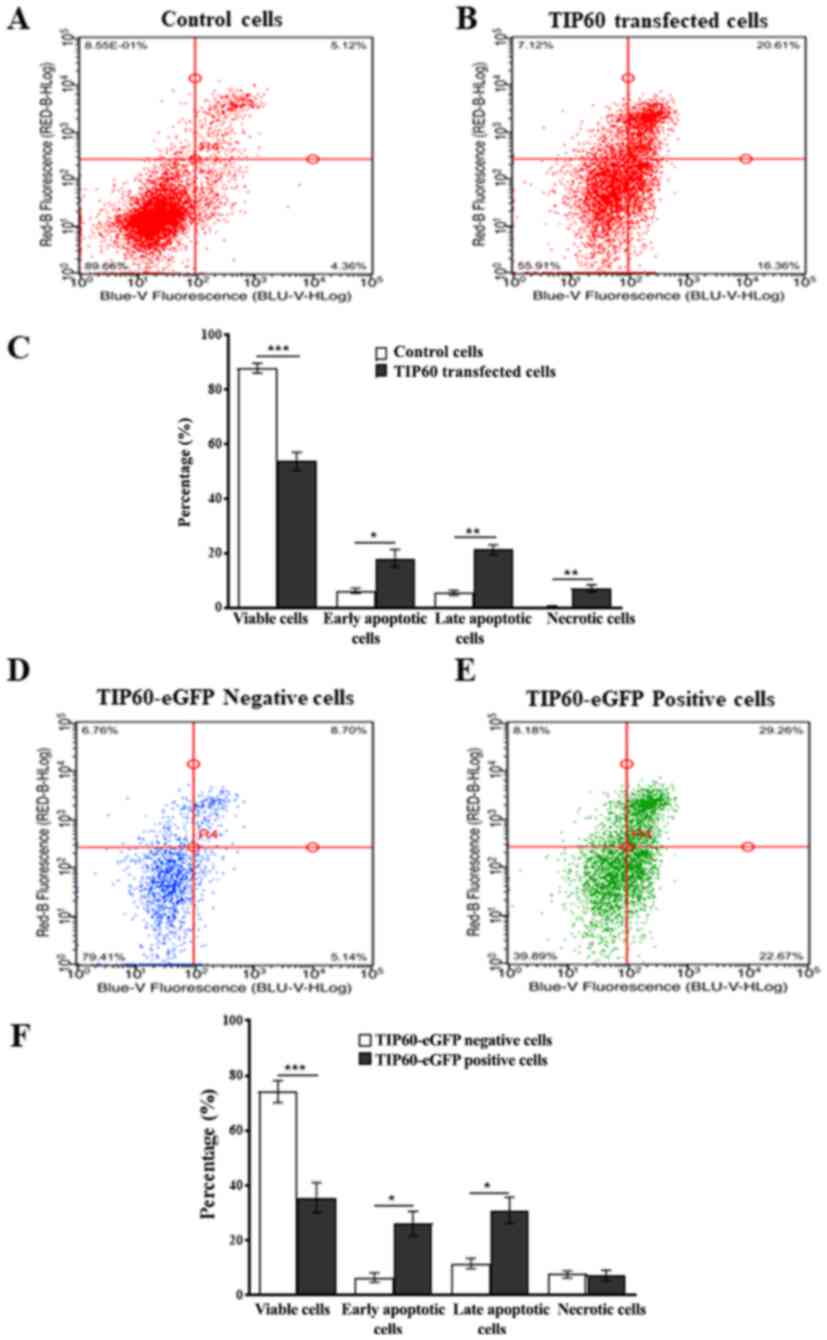
In order to assess the effect of TIP60
overexpression on downstream signaling pathways of p73, flow
cytometric experiments were performed. TIP60-eGFP transfected cells
were analyzed by FACS and compared with cells transfected with the
vector, jetPEI. PI and Annexin V-iFluor™ 350 staining aided the
detection of late and early phases of apoptosis. A significant
(P<0.001) decrease (34%) in cell viability was observed in the
TIP60-transfected cells as compared with the control cells. Along
with the decrease in cell viability following TIP60 overexpression,
an increase of 12% (P<0.05) and 16% (P<0.01) in the number of
early and late apoptotic cells was also observed, respectively
(Fig. 8A-C).
To confirm the aforementioned results, the total
population of TIP60-eGFP-transfected cells was separated into
TIP60-eGFP-positive and TIP60-eGFP-negative cell populations based
on eGFP fluorescence. The average transfection efficiency of
TIP60-eGFP was 61%, so that significant populations of both types
of cells could be obtained. This separation allowed the comparison
of apoptosis induction in TIP60-eGFP-expressing cells and
non-expressing cells, in the same sample. The viability of the
TIP60-eGFP-expressing cells decreased (P<0.001) by 39% as
compared with the cells not expressing TIP60-eGFP (Fig. 8D-F). In the TIP60-eGFP-transfected
cells, there was also a marked increase in the number of early and
late apoptotic cells (Fig. 8E and
F). As UHRF1 exhibits anti-apoptotic properties (5,67),
targeting UHRF1 expression can thus activate apoptotic pathways in
cancer cells. Cumulatively, these data explain the association
between the TIP60-mediated downregulation of the epigenetic
integrator, UHRF1, and the induction of apoptosis in cancer cells
to maintain the cellular and genomic integrity.
Discussion
UHRF1 and TIP60 are within the same epigenetic
complex with other partners, such as DNMT1, USP7, HDAC1,
proliferating cell nuclear antigen (PCNA) and euchromatic
histone-lysine N methyltransferase 2 (EHMT2, also known as G9a)
(17,25,27,66,71). Higher expression levels of UHRF1
have been reported in the majority of cancers (4,72)
and are related to suppression of TSGs expression, tumor invasion,
poor prognosis and resistance towards chemotherapy (4,73-77). In contrast to UHRF1, TIP60
expression is low in cancer cells. TIP60 is considered to play a
tumor suppressor role by maintaining the cellular and genomic
stability (24,41,45,51-56). UHRF1 directly interacts with the
MYST domain of TIP60 (57) and
regulates TIP60 expression and activity (25,27). There is thus a fragile balance
between UHRF1 and TIP60 broken in favor of UHRF1 in cancers. Thus,
it is considered that the role of TIP60 is to maintain UHRF1 at
physiological levels in the UHRF1/DNMT1 macromolecular complex.
The present study performed bioinformatics analysis
to investigate expression of TIP60 (Fig. S4) and UHRF1 (Fig. S5) in various types of cancer
(Table SI) which revealed that
TIP60 expression was mostly downregulated in the majority of
cancers, while on the other hand, UHRF1 expression was upregulated
in the majority of cancers. Further analysis revealed that a higher
TIP60 expression was associated with a better prognosis (Fig. S6) and a higher UHRF1 expression
was associated with a poor prognosis (Fig. S7) of cancer patients. The
investigation of the co-expression of both genes revealed that they
were expressed independently in the majority of cancer types.
However, in kidney renal clear cell carcinoma (KIRC) and brain
lower grade glioma (LGG) cancers, both genes were found to have the
tendency towards an opposite association (Fig. S8). In addition, a higher TIP60
and a lower UHRF1 expression in these two cancer types was
associated with a better prognosis. The distribution of USP7
expression in various cancer types did not seem to change in
correspondence to the non-tumor samples (Fig. S9) with the exception of
glioblastoma multiforme (where USP7 expression was lower) and
kidney chromophobe (where USP7 expression was higher). The
association between USP7 expression and the probability of patient
survival was found only in LGG, where a higher USP7 expression was
associated with a better prognosis (Fig. S10). However, a limitation to the
present study is that more thorough bioinformatics analyses are
required for the validation of the results. Caution is advised,
only mRNA levels were monitored, while protein stability may be
important at the protein final level.
In a previous study by the authors (57), HeLa cells were used to investigate
the interaction between UHRF1 and TIP60. It was reported that UHRF1
interacts with the MYST domain of TIP60, and that TIP60
overexpression leads to the downregulation of UHRF1 and DNMT1
levels (57). The objective of
the present study was to investigate the mechanism behind the
TIP60-mediated downregulation of UHRF1. Therefore, experiments were
performed within the same cell line. Furthermore, the basal level
of TIP60 is low in HeLa cells due to the presence of viral
oncoproteins (HPV E6 and E7). E6 protein leads to the proteasomal
mediated degradation of TIP60 by EDD1 E3 ligase (55,78). Notably, this matches observations
of cervical cancer where these viral proteins induce the
downregulation of TIP60, leading to apoptosis inhibition (79). Additionally, retrospective data
analysis comparing the differential expression of TIP60 gene in a
dataset (GDS3233) of normal cervix vs. cervical cancer samples
(62) revealed that TIP60
expression was significantly (P<0.01) downregulated in cancerous
tissues (Fig. S11). Taken
together, these data validate the relevance of HeLa cells for the
present study, while it would be of interest to observe if whether
the TIP60/UHRF1 pathway is a general mechanism relevant in other
cancer cell lines.
In the present study, using western blot analysis
and confocal microscopy, it was confirmed that TIP60 downregulates
the UHRF1/DNMT1 tandem. Of note, the ΔMYST mutant (lacking
acetyltransferase activity) was unable to affect the expression of
both proteins, indicating that the acetyltransferase activity of
TIP60 is required for downregulating both proteins. It is thus
suggested that TIP60 drives the degradation of UHRF1 and
consequently DNMT1, considering that this latter has been shown to
be under the control of UHRF1 (80). A direct control of TIP60 on DNMT1,
in an USP7-dependent way, may also occur (17).
Ubiquitination is a post-translational modification
which adds single or multiple ubiquitin molecules to proteins
marking them for proteasomal degradation, cellular trafficking,
autophagy, DNA repair, receptor internalization or regulation of
enzymatic activity (81,82). USP7 is a deubiquitinating enzyme
which protects many proteins from ubiquitination including p53,
UHRF1, PTEN, MDM2 and Myc. Its expression levels are high in a
number of cancers. Dysregulation in ubiquitination/deubiquitination
can play a critical role in several diseases, including cancer
(81). USP7 interacts with UHRF1
and protects it from degradation (58,83) while during the M phase, UHRF1 is
degraded as a result of its dissociation from USP7 (66). Zhang et al (58) reported that TIP60 acetylates UHRF1
at K659, which decreases the interaction of USP7-UHRF1. The data of
the present study indicated that the overexpression of TIP60, but
not of its ΔMYST mutant, interfered with the association and
expression levels of USP7 and UHRF1. The dissociation of USP7 from
UHRF1 likely condemns this latter becoming a prey for E3 ligases
that have been reported to ubiquitinate UHRF1 (84,85) or belong to the UHRF1 complex
(15). The role of USP7 as an
oncogene or tumor suppressor gene is still a matter of debate.
Indeed, USP7 protects XPC, a crucial damage recognition factor in
DNA repair, from proteolysis (86,87). USP7 also interacts with the tumor
suppressor gene p53 (88) and
regulates its stability (89).
However, the overexpression of USP7 and MDM2 leads to the
inactivation of p53, resulting in cancer initiation and progression
(90). This appears to be a
result of a protection of the MDM2 E3 ligase which ubiquitinates
p53 by proteosomal degradation. The inhibition of USP7 can
reactivate p53 (90). In the
majority of cancers, the overexpression of USP7 is observed
(91). Consistently, almost all
inhibitors of USP7 lead to cancer cell proliferation arrest which
favors the idea that USP7 rather plays a role of oncogene. Such a
role for USP7 has also been supported by the study of Felle et
al (83) on the colon cancer
cell line, HCT116, in which it was shown that USP7 favors de
novo and maintenance DNA methylation activity of DNMT1. This
suggests that DNA methylation patterns, particularly those of tumor
suppressor silenced genes, are transmitted throughout mitosis. It
is not excluded that this mechanism may also be involved in the
onset of tumorigenesis by the de novo hypermethylation of
the promoters of tumor suppressor genes. Indeed, the downregulation
of UHRF1 via the downregulation of USP7 allows the re-expression of
tumor suppressor genes (15). In
the present study, the TIP60-mediated interference with USP7-UHRF1
association was observed in HeLa cells. However, it would be of
interest to investigate and validate the role of USP7 and its
association with UHRF1 in other cancer cell lines.
In the present study, the ubiquitination of UHRF1
was observed following TIP60 overexpression, which is likely a
consequence of TIP60-mediated UHRF1-USP7 dissociation, as it has
been reported for DNMT1 (17,92). Due to this dissociation, USP7 is
no more able to protect UHRF1 from degradation through the
proteasomal pathway. This hypothesis was confirmed by use of MG-132
that helped recovering initial UHRF1 levels. The RING domain of
UHRF1 has E3 ligase activity through which it can either
ubiquitinate itself or other proteins (15,17). The present study demonstrated that
TIP60 overexpression controlled the auto-ubiquitination of UHRF1,
but not that of UHRF1 C724A-H741A mutant, having impaired RING
domain activity. The data further indicated that the interaction
between UHRF1 and ubiquitin occurred in a time-dependent manner and
that UHRF1 mutant, having impaired E3 ligase activity, was not able
to interact with ubiquitin. It was also found that TIP60 favored
the UHRF1/ubiquitin interaction, while the inhibition of its
acetyltransferase activity impaired this interaction. Subsequently,
UHRF1 was degraded via the proteasome, as treatment with MG132 was
able to recover the initial UHRF1 levels.
A downregulation of UHRF1 induces a recovery in
numerous tumor suppressor genes including RB1, p16INK4A
(CDKN2A), CDH13, SOCS3, BRCA1, CDX2, RUNX3, FOXO4, PPGARG, PML
and p73 (4,24). The present study focused on p73,
as it is known that TIP60 positively regulates apoptosis (41) and that UHRF1 positively regulates
p73 (68). It was found that
TIP60 overexpression induced an enhanced p73 expression. Therefore,
it is suggested that TIP60-mediated apoptosis occurs via the
upregulation of p73. However, it cannot be excluded that p73 is
involved upstream of TIP60, since it has been previously observed
that p73 also negatively regulates UHRF1 (54,93). Furthermore, in agreement with the
present study, it has been observed that p73-mediated apoptosis
involves a caspase-dependent pathway (93).
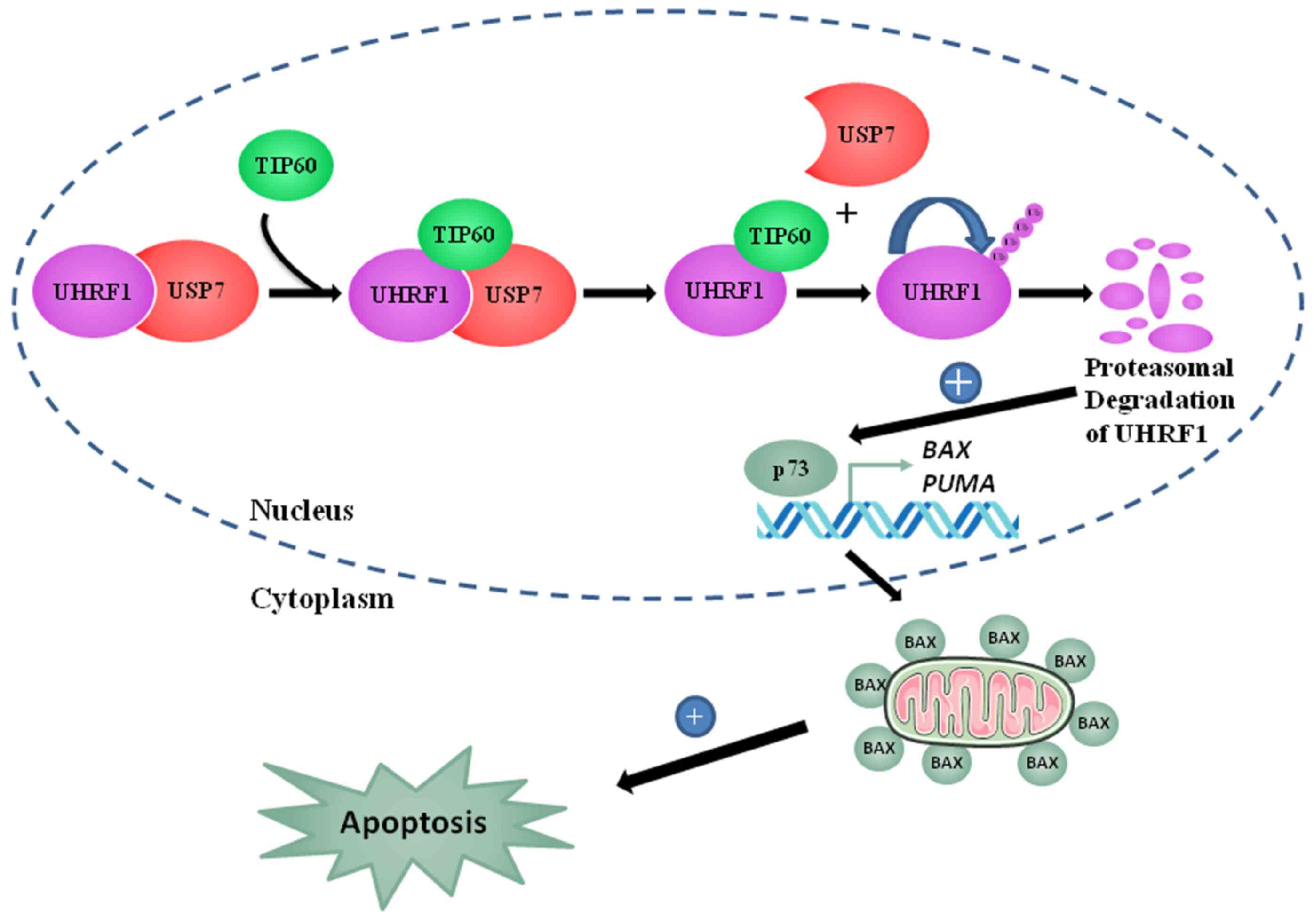
In conclusion, the present study proposes a model
(Fig. 9) depicting the tumor
suppressor role of TIP60 as a tumor suppressor gene. TIP60
upregulation induced apoptosis by the activation of the
p73-mediated downstream signaling pathway. TIP60 overexpression led
to a decrease in BCL2 and an increase in BAX expression, which
activated caspase-3. Caspase-3 activated the cleavage of PARP and
induced apoptosis. Overall, these observations support a tumor
suppressor role of TIP60 through the regulation of the
auto-ubiquitination activity of UHRF1. This interplay directly
governs the expression of TSGs, such as p73, explaining why TIP60
plays a role in apoptosis and cell cycle regulation.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TA conducted all the experiments with the
assistance of WA, AI and LZ under the supervision of MM and CB. MM,
CB, AH and YM were involved in the conception and design of the
study. CDM assisted with the flow cytometry experiments. TA, CB and
MM wrote most of the manuscript and confirm the authenticity of all
raw data with the guidance and assistance of AH and YM. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
The authors would like to thank Mr. Romain
Vauchelles (engineer, Plate-forme d'Imagerie Quantitative-PIQ) and
Dr Ludovic Richert (University of Strasbourg) for providing
assistance with the Confocal and FLIM experiments, respectively.
The authors are also thankful to Dr Sophie Rousseaux (MD, PhD,
INSERM Research Director), Dr Florent Chuffart (PhD, INSERM
Research Engineer) and Dr Ekaterina Flin (University Grenoble Alpes
Research Engineer) (EpiMed core facility; http://epimed.univ-grenoble-alpes.fr) for their
assistance with the bioinformatics analysis. The authors also wish
to thank Dr Nicolas Reynoird (Institute for Advanced Biosciences,
University Grenoble Alpes) for the critical reading of the
manuscript.
Abbreviations:
|
UHRF1
|
ubiquitin-like, containing PHD and
RING finger domains 1
|
|
TIP60
|
Tat interactive protein, 60 kDa
|
|
FLIM
|
fluorescence lifetime imaging
microscopy
|
|
DNMT1
|
DNA methyltransferase 1
|
|
USP7
|
ubiquitin-specific-processing
protease 7
|
|
HDAC1
|
histone deacetylase 1
|
|
IP
|
immunoprecipitation
|
|
WT
|
wild-type
|
|
ΔMYST
|
MYST domain mutant
|
|
FRET
|
Förster Resonance Energy Transfer
|
|
PCNA
|
proliferating cell nuclear
antigen
|
|
EHMT2
|
euchromatic histone-lysine N
methyltransferase 2
|
References
|
1
|
Hopfner R, Mousli M, Jeltsch JM, Voulgaris
A, Lutz Y, Marin C, Bellocq JP, Oudet P and Bronner C: ICBP90, a
novel human CCAAT binding protein, involved in the regulation of
topoisomerase IIalpha expression. Cancer Res. 60:121–128.
2000.PubMed/NCBI
|
|
2
|
Hopfner R, Mousli M, Garnier JM, Redon R,
du Manoir S, Chatton B, Ghyselinck N, Oudet P and Bronner C:
Genomic structure and chromosomal mapping of the gene coding for
ICBP90, a protein involved in the regulation of the topoisomerase
IIalpha gene expression. Gene. 266:15–23. 2001. View Article : Google Scholar
|
|
3
|
Krifa M, Alhosin M, Muller CD, Gies JP,
Chekir-Ghedira L, Ghedira K, Mély Y, Bronner C and Mousli M:
Limoniastrum guyonianum aqueous gall extract induces apoptosis in
human cervical cancer cells involving p16 INK4A re-expression
related to UHRF1 and DNMT1 down-regulation. J Exp Clin Cancer Res.
32:302013. View Article : Google Scholar
|
|
4
|
Ashraf W, Ibrahim A, Alhosin M, Zaayter L,
Ouararhni K, Papin C, Ahmad T, Hamiche A, Mély Y, Bronner C and
Mousli M: The epigenetic integrator UHRF1: On the road to become a
universal biomarker for cancer. Oncotarget. 8:51946–51962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bronner C, Achour M, Arima Y, Chataigneau
T, Saya H and Schini-Kerth VB: The UHRF family: Oncogenes that are
drugable targets for cancer therapy in the near future? Pharmacol
Ther. 115:419–434. 2007. View Article : Google Scholar
|
|
6
|
Bostick M, Kim JK, Estève PO, Clark A,
Pradhan S and Jacobsen SE: UHRF1 plays a role in maintaining DNA
methylation in mammalian cells. Science. 317:1760–1764. 2007.
View Article : Google Scholar
|
|
7
|
Bronner C, Alhosin M, Hamiche A and Mousli
M: Coordinated dialogue between UHRF1 and DNMT1 to ensure faithful
inheritance of methylated DNA patterns. Genes (Basel). 10:652019.
View Article : Google Scholar
|
|
8
|
Avvakumov GV, Walker JR, Xue S, Li Y, Duan
S, Bronner C, Arrowsmith CH and Dhe-Paganon S: Structural basis for
recognition of hemi-methylated DNA by the SRA domain of human
UHRF1. Nature. 455:822–825. 2008. View Article : Google Scholar
|
|
9
|
Sharif J, Muto M, Takebayashi S, Suetake
I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T,
Okamura K, et al: The SRA protein Np95 mediates epigenetic
inheritance by recruiting Dnmt1 to methylated DNA. Nature.
450:908–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arita K, Ariyoshi M, Tochio H, Nakamura Y
and Shirakawa M: Recognition of hemi-methylated DNA by the SRA
protein UHRF1 by a base-flipping mechanism. Nature. 455:818–821.
2008. View Article : Google Scholar
|
|
11
|
Nady N, Lemak A, Walker JR, Avvakumov GV,
Kareta MS, Achour M, Xue S, Duan S, Allali-Hassani A, Zuo X, et al:
Recognition of multivalent histone states associated with
heterochromatin by UHRF1 protein. J Biol Chem. 286:24300–24311.
2011. View Article : Google Scholar
|
|
12
|
Rajakumara E, Wang Z, Ma H, Hu L, Chen H,
Lin Y, Guo R, Wu F, Li H, Lan F, et al: PHD finger recognition of
unmodified histone H3R2 links UHRF1 to regulation of euchromatic
gene expression. Mol Cell. 43:275–284. 2011. View Article : Google Scholar
|
|
13
|
Hu L, Li Z, Wang P, Lin Y and Xu Y:
Crystal structure of PHD domain of UHRF1 and insights into
recognition of unmodified histone H3 arginine residue 2. Cell Res.
21:1374–1378. 2011. View Article : Google Scholar
|
|
14
|
Jenkins Y, Markovtsov V, Lang W, Sharma P,
Pearsall D, Warner J, Franci C, Huang B, Huang J, Yam GC, et al:
Critical role of the ubiquitin ligase activity of UHRF1, a nuclear
RING finger protein, in tumor cell growth. Mol Biol Cell.
16:5621–5629. 2005. View Article : Google Scholar
|
|
15
|
Ibrahim A, Alhosin M, Papin C, Ouararhni
K, Omran Z, Zamzami MA, Al-Malki AL, Choudhry H, Mély Y, Hamiche A,
et al: Thymoquinone challenges UHRF1 to commit auto-ubiquitination:
A key event for apoptosis induction in cancer cells. Oncotarget.
9:28599–28611. 2018. View Article : Google Scholar
|
|
16
|
Tauber M and Fischle W: Conserved linker
regions and their regulation determine multiple chromatin-binding
modes of UHRF1. Nucleus. 6:123–132. 2015. View Article : Google Scholar
|
|
17
|
Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang
S, Kao HY, Xu Y, Willis J, Markowitz SD, et al: DNMT1 stability is
regulated by proteins coordinating deubiquitination and
acetylation-driven ubiquitination. Sci Signal. 3:ra802010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishiyama A, Yamaguchi L, Sharif J,
Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T,
Ishikawa F, et al: Uhrf1-dependent H3K23 ubiquitylation couples
maintenance DNA methylation and replication. Nature. 502:249–253.
2013. View Article : Google Scholar
|
|
19
|
Qin W, Wolf P, Liu N, Link S, Smets M, La
Mastra F, Forné I, Pichler G, Hörl D, Fellinger K, et al: DNA
methylation requires a DNMT1 ubiquitin interacting motif (UIM) and
histone ubiquitination. Cell Res. 25:911–929. 2015. View Article : Google Scholar
|
|
20
|
Foster BM, Stolz P, Mulholland CB, Montoya
A, Kramer H, Bultmann S and Bartke T: Critical role of the UBL
domain in stimulating the E3 ubiquitin ligase activity of UHRF1
toward chromatin. Mol Cell. 72:739–752.e9. 2018. View Article : Google Scholar
|
|
21
|
Mishima Y, Brueckner L, Takahashi S,
Kawakami T, Otani J, Shinohara A, Takeshita K, Garvilles RG,
Watanabe M, Sakai N, et al: Enhanced processivity of Dnmt1 by
monoubiquitinated histone H3. Genes Cells. 25:22–32. 2020.
View Article : Google Scholar
|
|
22
|
Li T, Wang L, Du Y, Xie S, Yang X, Lian F,
Zhou Z and Qian C: Structural and mechanistic insights into
UHRF1-mediated DNMT1 activation in the maintenance DNA methylation.
Nuclic Acids Res. 46:3218–3231. 2018. View Article : Google Scholar
|
|
23
|
Alhosin M, Omran Z, Zamzami MA, Al-Malki
AL, Choudhry H, Mousli M and Bronner C: Signalling pathways in
UHRF1-dependent regulation of tumor suppressor genes in cancer. J
Exp Clin Cancer Res. 35:1742016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai C, Shi D and Gu W: Negative regulation
of the acetyltransferase TIP60-p53 interplay by UHRF1
(ubiquitin-like with PHD and RING finger domains 1). J Biol Chem.
288:19581–19592. 2013. View Article : Google Scholar :
|
|
26
|
Guan D, Factor D, Liu Y, Wang Z and Kao
HY: The epigenetic regulator UHRF1 promotes ubiquitination-mediated
degradation of the tumor-suppressor protein promyelocytic leukemia
protein. Oncogene. 32:3819–3828. 2013. View Article : Google Scholar :
|
|
27
|
Achour M, Fuhrmann G, Alhosin M, Rondé P,
Chataigneau T, Mousli M, Schini-Kerth VB and Bronner C: UHRF1
recruits the histone acetyltransferase Tip60 and controls its
expression and activity. Biochem Biophys Res Commun. 390:523–528.
2009. View Article : Google Scholar
|
|
28
|
Kamine J, Elangovan B, Subramanian T,
Coleman D and Chinnadurai G: Identification of a cellular protein
that specifically interacts with the essential cysteine region of
the HIV-1 Tat transactivator. Virology. 216:357–366. 1996.
View Article : Google Scholar
|
|
29
|
Yamamoto T and Horikoshi M: Novel
substrate specificity of the histone acetyltransferase activity of
HIV-1-Tat interactive protein Tip60. J Biol Chem. 272:30595–30598.
1997. View Article : Google Scholar
|
|
30
|
Hilfiker A, Hilfiker-Kleiner D, Pannuti A
and Lucchesi JC: mof, a putative acetyl transferase gene related to
the Tip60 and MOZ human genes and to the SAS genes of yeast, is
required for dosage compensation in Drosophila. EMBO J.
16:2054–2060. 1997. View Article : Google Scholar
|
|
31
|
Lee KK and Workman JL: Histone
acetyltransferase complexes: One size doesn't fit all. Nat Rev Mol
Cell Biol. 8:284–295. 2007. View Article : Google Scholar
|
|
32
|
Doyon Y, Selleck W, Lane WS, Tan S and
Côté J: Structural and functional conservation of the NuA4 histone
acetyltransferase complex from yeast to humans. Mol Cell Biol.
24:1884–1896. 2004. View Article : Google Scholar
|
|
33
|
Voss AK and Thomas T: MYST family histone
acetyltransferases take center stage in stem cells and development.
Bioessays. 31:1050–1061. 2009. View Article : Google Scholar
|
|
34
|
Sheikh BN and Akhtar A: The many lives of
KATs-detectors, integrators and modulators of the cellular
environment. Nat Rev Genet. 20:7–23. 2019. View Article : Google Scholar
|
|
35
|
Kim CH, Kim JW, Jang SM, An JH, Seo SB and
Choi KH: The chromodomain-containing histone acetyltransferase
TIP60 acts as a code reader, recognizing the epigenetic codes for
initiating transcription. Biosci Biotechnol Biochem. 79:532–538.
2015. View Article : Google Scholar
|
|
36
|
Squatrito M, Gorrini C and Amati B: Tip60
in DNA damage response and growth control: Many tricks in one HAT.
Trends Cell Biol. 16:433–442. 2006. View Article : Google Scholar
|
|
37
|
Kimura A, Matsubara K and Horikoshi M: A
decade of histone acetylation: Marking eukaryotic chromosomes with
specific codes. J Biochem. 138:647–662. 2005. View Article : Google Scholar
|
|
38
|
Kim MY, Ann EJ, Kim JY, Mo JS, Park JH,
Kim SY, Seo MS and Park HS: Tip60 histone acetyltransferase acts as
a negative regulator of Notch1 signaling by means of acetylation.
Mol Cell Biol. 27:6506–6519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sapountzi V, Logan IR and Robson CN:
Cellular functions of TIP60. Int J Biochem Cell Biol. 38:1496–1509.
2006. View Article : Google Scholar
|
|
40
|
Putnik J, Zhang CD, Archangelo LF, Tizazu
B, Bartels S, Kickstein M, Greif PA and Bohlander SK: The
interaction of ETV6 (TEL) and TIP60 requires a functional histone
acetyltransferase domain in TIP60. Biochim Biophys Acta.
1772:1211–1224. 2007. View Article : Google Scholar
|
|
41
|
Ikura T, Ogryzko VV, Grigoriev M, Groisman
R, Wang J, Horikoshi M, Scully R, Qin J and Nakatani Y: Involvement
of the TIP60 histone acetylase complex in DNA repair and apoptosis.
Cell. 104:463–473. 2000. View Article : Google Scholar
|
|
42
|
Judes G, Rifaï K, Ngollo M, Daures M,
Bignon YJ, Penault-Llorca F and Bernard-Gallon D: A bivalent role
of TIP60 histone acetyl transferase in human cancer. Epigenomics.
7:1351–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Idrissou M, Rifaï K, Daures M,
Penault-Llorca F, Bignon YJ and Bernard-Gallon D: Exciting history
of Tip60 and its companions in carcinogenesis across the
heterochromatin landscapes. OMICS. 22:626–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Frank SR, Parisi T, Taubert S, Fernandez
P, Fuchs M, Chan HM, Livingston DM and Amati B: MYC recruits the
TIP60 histone acetyltransferase complex to chromatin. EMBO Rep.
4:575–580. 2003. View Article : Google Scholar
|
|
45
|
Berns K, Hijmans EM, Mullenders J,
Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M,
Nijkamp W, Weigelt B, et al: A large-scale RNAi screen in human
cells identifies new components of the p53 pathway. Nature.
428:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mo F, Zhuang X, Liu X, Yao PY, Qin B, Su
Z, Zang J, Wang Z, Zhang J, Dou Z, et al: Acetylation of Aurora B
by TIP60 ensures accurate chromosomal segregation. Nat Chem Biol.
12:226–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
DeRan M, Pulvino M, Greene E, Su C and
Zhao J: Transcriptional activation of histone genes requires
NPAT-dependent recruitment of TRRAP-Tip60 complex to histone
promoters during the G1/S phase transition. Mol Cell Biol.
28:435–447. 2008. View Article : Google Scholar
|
|
48
|
Niida H, Katsuno Y, Sengoku M, Shimada M,
Yukawa M, Ikura M, Ikura T, Kohno K, Shima H, Suzuki H, et al:
Essential role of Tip60-dependent recruitment of ribonucleotide
reductase at DNA damage sites in DNA repair during G1 phase. Genes
Dev. 24:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taubert S, Gorrini C, Frank SR, Parisi T,
Fuchs M, Chan HM, Livingston DM and Amati B: E2F-dependent histone
acetylation and recruitment of the Tip60 acetyltransferase complex
to chromatin in late G1. Mol Cell Biol. 24:4546–4556. 2004.
View Article : Google Scholar :
|
|
50
|
Hu Y, Fisher JB, Koprowski S, McAllister
D, Kim MS and Lough J: Homozygous disruption of the Tip60 gene
causes early embryonic lethality. Dev Dyn. 238:2912–2921. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sakuraba K, Yasuda T, Sakata M, Kitamura
YH, Shirahata A, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa
G, et al: Down-regulation of Tip60 gene as a potential marker for
the malignancy of colorectal cancer. Anticancer Res. 29:3953–3955.
2009.
|
|
52
|
Sakuraba K, Yokomizo K, Shirahata A, Goto
T, Saito M, Ishibashi K, Kigawa G, Nemoto H and Hibi K: TIP60 as a
potential marker for the malignancy of gastric cancer. Anticancer
Res. 31:77–79. 2011.PubMed/NCBI
|
|
53
|
Gorrini C, Squatrito M, Luise C, Syed N,
Perna D, Wark L, Martinato F, Sardella D, Verrecchia A, Bennett S,
et al: Tip60 is a haplo-insufficient tumour suppressor required for
an oncogene-induced DNA damage response. Nature. 448:1063–1067.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA,
Tran C, Chen C, Chung CH, Huber O, Rose DW, et al: Transcriptional
regulation of a metastasis suppressor gene by Tip60 and
beta-catenin complexes. Nature. 434:921–926. 2005. View Article : Google Scholar
|
|
55
|
Jha S, Vande Pol S, Banerjee NS, Dutta AB,
Chow LT and Dutta A: Destabilization of TIP60 by human
papillomavirus E6 results in attenuation of TIP60-dependent
transcriptional regulation and apoptotic pathway. Mol Cell.
38:700–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brown JA, Bourke E, Eriksson LA and Kerin
MJ: Targeting cancer using KAT inhibitors to mimic lethal
knockouts. Biochem Soc Trans. 44:979–986. 2016. View Article : Google Scholar :
|
|
57
|
Ashraf W, Bronner C, Zaayter L, Ahmad T,
Richert L, Alhosin M, Ibrahim A, Hamiche A, Mely Y and Mousli M:
Interaction of the epigenetic integrator UHRF1 with the MYST domain
of TIP60 inside the cell. J Exp Clin Cancer Res. 36:1882017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang ZM, Rothbart SB, Allison DF, Cai Q,
Harrison JS, Li L, Wang Y, Strahl BD, Wang G and Song J: An
allosteric interaction links USP7 to deubiquitination and chromatin
targeting of UHRF1. Cell Re. 12:1400–1406. 2015. View Article : Google Scholar
|
|
59
|
Cheng J, Yang H, Fang J, Ma L, Gong R,
Wang P, Li Z and Xu Y: Molecular mechanism for USP7-mediated DNMT1
stabilization by acetylation. Nat Commun. 6:70232015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Clamme JP, Azoulay J and Mély Y:
Monitoring of the formation and dissociation of
polyethylenimine/DNA complexes by two photon fluorescence
correlation spectroscopy. Biophys J. 84:1960–1968. 2003. View Article : Google Scholar
|
|
61
|
El Meshri SE, Dujardin D, Godet J, Richert
L, Boudier C, Darlix JL, Didier P, Mély Y and de Rocquigny H: Role
of the nucleocapsid domain in HIV-1 Gag oligomerization and
trafficking to the plasma membrane: A fluorescence lifetime imaging
microscopy investigation. J Mol Biol. 427:1480–1494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M and Murty VV: Identification of copy
number gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar
|
|
63
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Becker W: Advanced time-correlated single
photon counting applications. Springer; Heidelberg: 2015,
View Article : Google Scholar
|
|
65
|
Voss TC, Demarco IA and Day RN:
Quantitaive imaging of protein interactions in the cell nucleus.
Biotechniques. 38:413–424. 2005. View Article : Google Scholar :
|
|
66
|
Ma H, Chen H, Guo X, Wang Z, Sowa ME,
Zheng L, Hu S, Zeng P, Guo R, Diao J, et al: M phase
phosphorylation of the epigenetic regulator UHRF1 regulates its
physical association with the deubiquitylase USP7 and stability.
Proc Natl Acad Sci USA. 109:4828–4833. 2012. View Article : Google Scholar :
|
|
67
|
Alhosin M, Abusnina A, Achour M, Sharif T,
Muller C, Peluso J, Chataigneau T, Lugnier C, Schini-Kerth VB,
Bronner C and Fuhrmann G: Induction of apoptosis by thymoquinone in
lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent
pathway which targets the epigenetic integrator UHRF1. Biochem
Pharmacol. 79:1251–1260. 2010. View Article : Google Scholar
|
|
68
|
Achour M, Mousli M, Alhosin M, Ibrahim A,
Peluso J, Muller CD, Schini-Kerth VB, Hamiche A, Dhe-Peganon S and
Bronner C: Epigallocatechin-3-gallate up-regulates tumor suppressor
gene expression via a reactive oxygen species-dependent
down-regulation of UHRF1. Biochem Biophys Res Commun. 430:208–212.
2013. View Article : Google Scholar
|
|
69
|
León-González AJ, Jara-Palacios MJ, Abbas
M, Heredia FJ and Schini-Kerth VB: Role of epigenetic regulation on
the induction of apoptosis in Jurkat leukemia cells by white grape
pomace rich in phenolic compounds. Food Nut. 8:4062–4069. 2017.
|
|
70
|
Sharif T, Alhosin M, Auger C, Minker C,
Kim JH, Etienne-Selloum N, Bories P, Gronemeyer H, Lobstein A,
Bronner C, et al: Aronia melanocarpa juice induces a
redox-sensitive p73-related caspase-3-dependent apoptosis in human
leukemia cells. PLoS One. 7:e325262012. View Article : Google Scholar
|
|
71
|
Kim JK, Estève PO, Jacobsen SE and Pradhan
S: UHRF1 binds G9a and participates in p21 transcriptional
regulation in mammalian cells. Nucleic Acids Res. 37:493–505. 2009.
View Article : Google Scholar :
|
|
72
|
Polepalli S, George SM, Valli Sri Vidya R,
Rodrigues GS, Ramachandra L, Chandrashekar R, DN M, Rao PPN,
Pestell RG and Rao M: Role of UHRF1 in malignancy and its function
as a therapeutic target for molecular docking towards the SRA
domain. Int J Biochem Cell Biol. 114:1055582019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Boukhari A, Alhosin M, Bronner C, Sagini
K, Truchot C, Sick E, Schini-Kerth VB, André P, Mély Y, Mousli M
and Gies JP: CD47 activation-induced UHRF1 over-expression is
associated with silencing of tumor suppressor gene p16INK4A in
glioblastoma cells. Anticancer Res. 35:149–157. 2015.PubMed/NCBI
|
|
74
|
Jeanblanc M, Mousli M, Hopfner R, Bathami
K, Martinet N, Abbady AQ, Siffert JC, Mathieu E, Muller CD and
Bronner C: The retinoblastoma gene and its product are targeted by
ICBP90: A key mechanism in the G1/S transition during the cell
cycle. Oncogene. 24:7337–7345. 2005. View Article : Google Scholar
|
|
75
|
Unoki M, Brunet J and Mousli M: Drug
discovery targeting epigenetic codes: The great potential of UHRF1,
which links DNA methylation and histone modifications, as a drug
target in cancers and toxoplasmosis. Biochem Pharmacol.
78:1279–1288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang F, Yang YZ, Shi CZ, Zhang P, Moyer
MP, Zhang HZ, Zou Y and Qin HL: UHRF1 promotes cell growth and
metastasis through repression of p16(ink4a) in colorectal cancer.
Ann Surg Oncol. 19:2753–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xue B, Zhao J, Feng P, Xing J, Wu H and Li
Y: Epigenetic mechanism and target therapy of UHRF1 protein complex
in malignancies. Onco Targets Ther. 12:549–559. 2019. View Article : Google Scholar :
|
|
78
|
Subbaiah VK, Zhang Y, Rajagopalan D,
Abdullah AN, Yeo-Teh NS, Tomaić V, Banks L, Myers MP, Chow EK and
Jha S: E3 ligase EDD1/UBR5 is utilized by the HPV E6 oncogene to
destabilize tumor suppressor TIP60. Oncogene. 35:2062–2074. 2016.
View Article : Google Scholar
|
|
79
|
Rajagopalan D, Pandey AK, Xiuzhen MC, Lee
KK, Hora S, Zhang Y, Chua BH, Kwok HS, Bhatia SS, Deng LW, et al:
TIP60 represses telomerase expression by inhibiting Sp1 binding to
the TERT promoter. PLoS Pathog. 13:e10066812017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Achour M, Jacq X, Rondé P, Alhosin M,
Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A,
Hughes AD, et al: The interaction of the SRA domain of ICBP90 with
a novel domain of DNMT1 is involved in the regulation of VEGF gene
expression. Oncogene. 27:2187–2197. 2008. View Article : Google Scholar
|
|
81
|
Gao Y, Wang Y, Zhou C, Kong S, Lu J, Wang
H and Yang J: Ubiquitin-specific protease 7 (USP7) is essential for
endometrial stromal cell decidualization in mice. Dev Growth
Differ. 61:176–185. 2019. View Article : Google Scholar
|
|
82
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Felle M, Joppien S, Németh A, Diermeier S,
Thalhammer V, Dobner T, Kremmer E, Kappler R and Längst G: The
USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1
and regulates the stability of UHRF1. Nucleic Acids Res.
39:8355–8365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mistry H, Gibson L, Yun JW, Sarras H,
Tamblyn L and McPherson JP: Interplay between Np95 and Eme1 in the
DNA damage response. Biochem Biophys Res Commun. 375:321–325. 2008.
View Article : Google Scholar
|
|
85
|
Chen H, Ma H, Inuzuka H, Diao J, Lan F,
Shi YG, Wei W and Shi Y: DNA damage regulates UHRF1 stability via
the SCF(β-TrCP) E3 ligase. Mol Cell Biol. 33:1139–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He J, Zhu Q, Wani G, Sharma N, Han C, Qian
J, Pentz K, Wang QE and Wani AA: Ubiquitin-specific protease 7
regulates nucleotide excision repair through deubiquitinating XPC
protein and preventing XPC protein from undergoing ultraviolet
light-induced and VCP/p97 protein-regulated proteolysis. J Biol
Chem. 289:27278–27289. 2014. View Article : Google Scholar :
|
|
87
|
He M, Zhu Z, Shah AA, Zou H, Tao J, Chen Q
and Wan Y: The emerging role of deubiquitinating enzymes in genomic
integrity, diseases, and therapeutics. Cell Biosci. 6:622016.
View Article : Google Scholar :
|
|
88
|
Kwon SK, Saindane M and Baek KH: p53
stability is regulated by diverse deubiquitinating enzymes. Biochim
Biophys Acta Rev Cancer. 1868:404–411. 2017. View Article : Google Scholar
|
|
89
|
Sheng Y, Saridakis V, Sarkari F, Duan S,
Wu T, Arrowsmith CH and Frappier L: Molecular recognition of p53
and MDM2 by USP7/HAUSP. Nat Struct Mol Biol. 13:285–291. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hou H, Sun D and Zhang X: The role of MDM2
amplification and overexpression in therapeutic resistance of
malignant tumors. Cancer Cell Int. 19:2162019. View Article : Google Scholar :
|
|
91
|
Nininahazwe L, Liu B, He C, Zhang H and
Chen ZS: The emerging nature of ubiquitin-specific protease 7
(USP7): A new target in cancer therapy. Drug Discov Today.
26:490–502. 2021. View Article : Google Scholar
|
|
92
|
Bronner C: Control of DNMT1 abundance in
epigenetic inheritance by acetylation, ubiquitylation, and the
histone code. Sci Sign. 4:pe32011.
|
|
93
|
Jang SY, Hong D, Jeong SY and Kim JH:
Shikonin causes apoptosis by up-regulating p73 and down-regulating
ICBP90 in human cancer cells. Biochem Biophys Res Commun.
465:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|