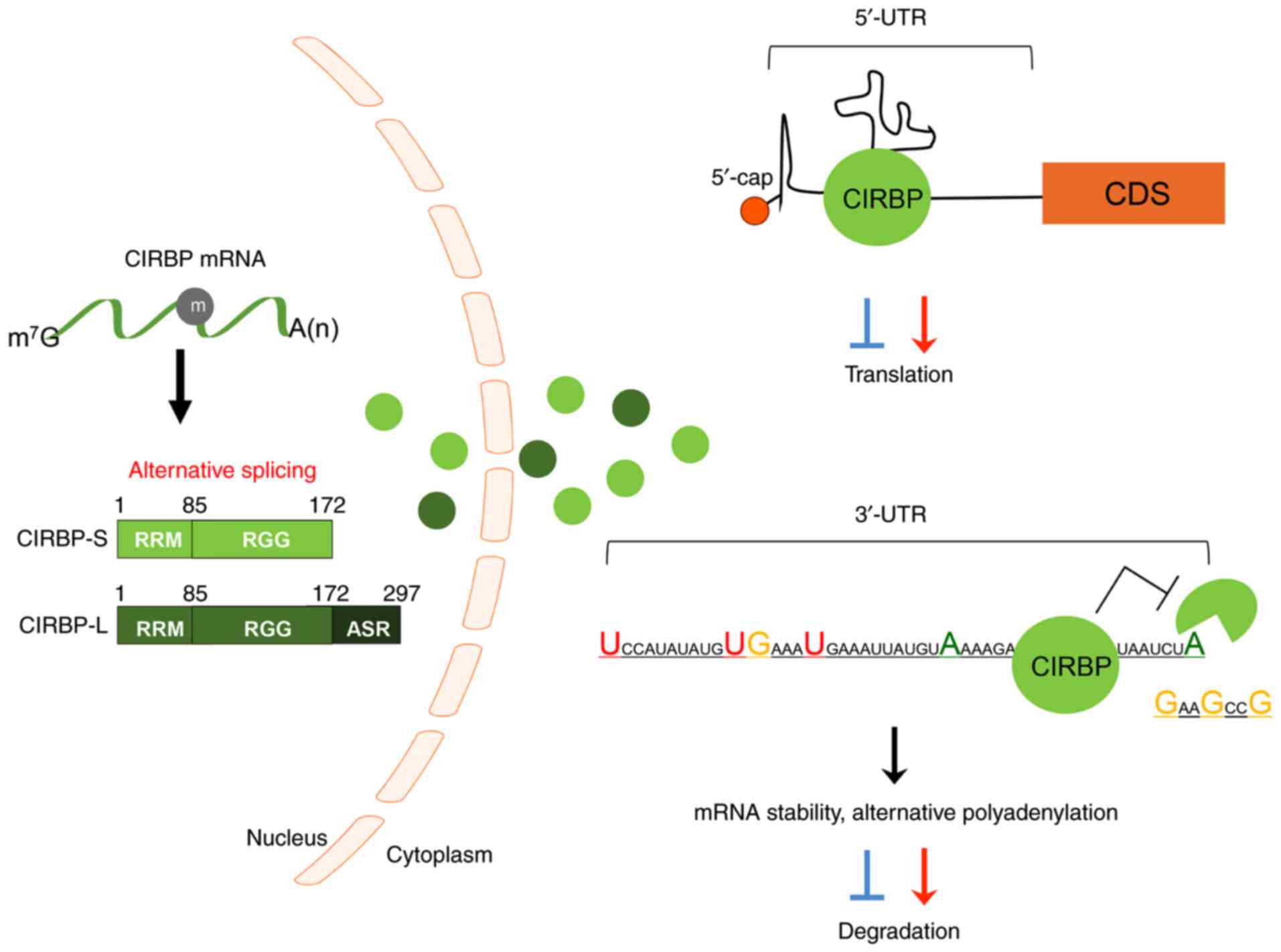
Cold-inducible RNA-binding protein (CIRBP; also
called CIRP and hnRNP A18) was identified as a cold-shock protein
and an RNA-binding protein (RBP) expressed following a variety of
stressors, such as hypoxia, cold shock and UV radiation (1-3).
In total, two major CRIBP transcripts are expressed in cells
through N6-methyladenosine modification-mediated
alternative splicing (2,4-6).
The large isoform of CIRBP (CIRBP-L) contains 297 amino acids and
another short one (CIRBP-S) encodes 172 amino acids (Fig. 1). CIRBP is translated in the
nucleus and migrates to the cytoplasm following stimulation
(1,7). CIRBP contains an RNA-recognition
motif (RRM) in the N-terminal domain and an arginine-rich motif
(RGG) in the C-terminal region (1); it interacts with the 5′ or 3′-UTR of
partner mRNAs through its RRM and regulates its expression
post-transcriptionally (1,8).
The RGG domain of CIRBP induces the protein-protein interaction,
thereby modulating the protein-RNA interaction. Therefore, it is
likely that CIRBP acts as a chaperone protein to interact and
support RNA structure, assembly and transport of various proteins
(9).
Moreover, CIRBP participates in multiple cellular
signaling pathways as a crucial regulator. In the apoptosis
pathway, mild hypothermia can protect cells from death in part
through CIRBP, which activates the MAPK and NF-κB pathways
(3). This indicates that CIRBP
functions as a regulator of cell viability by activating survival
signaling. Under mild hypothermia and UV radiation, CIRBP
upregulates the expression of thioredoxin (TRX), which protects
cells from oxidative damage by sequestering reactive oxygen species
(ROS) (10,11). These findings indicate that CIRBP
can induce anti-senescence signaling through TRX-mediated
antioxidant activity. In addition, CIRBP is involved in various
biological processes, including DNA repair, circadian clock
regulation, telomere integrity, nutrient deficiency, inflammatory
response signaling and cardiac electrophysiology (12-18). Furthermore, CIRBP is also involved
in various human diseases, including sepsis, Alzheimer's disease
and pancreatitis (19-24).
In recent years, numerous studies have suggested the
involvement of CIRBP in several forms of human cancer. In the
present review, the roles of CIRBP and its target mRNAs in cancer
are summarized, and its potential as a therapeutic target is
evaluated.
RBPs not only serve important roles in multiple
physiological signaling pathways, but also act as important
regulators of cancer genesis and progression. Several studies have
reported that RBPs influence cancer progression by acting as either
oncogenes or tumor suppressors (25,26). In order for normal cells to
develop into cancer cells, they must go through a multi-step
process to acquire the hallmarks of cancer. Hallmarks of cancer
have been previously described and updated with newly identified
characteristics of cancer (27).
In the present review, the role of CIRBP in human cancers was
summarized based on the hallmarks of cancer. Similar to other RBPs,
CIRBP has a promotive or inhibitory regulatory effect on
carcinogenesis, depending on the cancer subtype (Table I).
The most fundamental characteristic of cancer cells
is the capacity to maintain unlimited proliferation. Healthy
tissues maintain structure and function by carefully regulating
cell growth to ensure cell number homeostasis, whereas cancer cells
exhibit excessive proliferation (28). CIRBP significantly promotes the
proliferation of breast and bladder cancer cells (29,30). Recently, it has been reported that
CIRBP expression is elevated in luminal breast cancer, promoting
cell proliferation and clonogenicity (31). Notably, CIRBP levels are closely
associated with a less favorable survival rate in patients with the
luminal subtype (31). Moreover,
CIRBP enhances the proliferation of immature male germ cells
through its interaction with dual-specificity
tyrosine-phosphorylation-regulated kinase 1B (DYRK1B) in mice
(32).
In addition to its role in carcinoma, CIRBP
expression is also increased in pituitary corticotroph adenoma,
which promotes cell proliferation and tumor growth via Erk
signaling (33). However, certain
reports have revealed that CIRBP can suppress the tumorigenesis of
breast cancer cells (34,35). High expression of CIRBP in breast
tissue has been correlated with a more favorable prognosis in
postmenopausal women with breast cancer who have experienced
childbirth (34). Another study
also reported that CIRBP overexpression interferes with cell
proliferation during mammary gland development (35). In addition, CIRBP expression is
highest in normal endometrium, but significantly reduced in
endometrial carcinoma (36).
Recently, CIRBP was also reported to induce translation of p27, a
CDK inhibitor, thereby reducing cell proliferation (37).
Telomeres are essential for genome stability, as
they protect the fusion of linear chromosomes (38). Telomeres are extended and
maintained by telomerase, which is comprised of telomerase reverse
transcriptase (TERT) and telomerase RNA component (TERC). Although
it is virtually silent in somatic cells, TERT expression is
activated in numerous tumor types, giving cancer cells the hallmark
feature of replicative immortality (39). For the maintenance of telomere
length, CIRBP has been identified as a telomerase-associating
protein through its RRG domain (40). Upon direct interaction with TERC,
CIRBP promotes the formation of the telomerase complex. In
addition, CIRBP enhances the telomerase activity through
stabilization of TERT mRNA. As activated TERT is a common trait in
most cancer types, this may represent an important approach to
understanding the exact role of CIRBP in the regulation of
telomerase activity.
Apoptosis acts as a natural barrier to tumorigenesis
and is suppressed in tumors that have successfully progressed to a
treatment-resistant state (41).
Previous studies have reported an association between CIRBP and
apoptosis. For example, CIRBP-overexpressing cells have a reduced
rate of apoptosis owing to reduced DNA damage (42,43). A recent study reported that CIRBP
inhibits amyloid β-induced activation of apoptosis via
anti-oxidative pathways in cortical neurons (44). Notably, CIRBP stimulates NLRP3
inflammasome activation and simultaneously induces caspase-1
activation and IL-1β release, resulting in pyroptosis, a type of
inflammatory cell death (45).
Additionally, cancer cells must evade pathways involving tumor
suppressor genes, such as p53 and retinoblastoma protein, which
negatively regulate proliferation (46). It has been reported that CIRBP
inhibits p53, thereby reducing apoptosis (42) and suppressing the damage of
testicular tissue (47), but the
exact mechanism is still unknown.
Cancer cells use the inflammatory microenvironment
to promote tumor growth. Tumor-promoting inflammation is closely
associated with tumor progression and metastasis (48). Certain studies have reported that
CIRBP acts as a mediator of cancer-associated inflammation in
numerous cancer types. Chronic inflammation is known to increase
the risk of intestinal cancer in patients with inflammatory bowel
disease (IBD) (49). In patients
with IBD, CIRBP is positively correlated with IL-23A (50), a known oncogenic cytokine, and
IL-17, which is known to enhance cancer-induced inflammation
(51,52). Moreover, CIRBP expression is
higher in inflammatory cells compared with epithelial cells in
patients with IBD, and the same result is observed in patients with
colitis-associated colorectal cancer (CAC) (52). In another study, CIRBP deficiency
resulted in decreased expression of inflammatory cytokines in
liver-specific macrophages and attenuated tumorigenesis in mice
(53). Oral chronic inflammation
is a crucial part of oral squamous cell carcinoma (OSCC) promotion
(54). The expression of CIRBP
and toll-like receptor 4 (TLR4) is high, and a positive correlation
in their expression levels has been reported in patients with OSCC
(55). In a previous study, it
was reported that CIRBP induced an inflammatory response through
TLR4 (15). Overall, these
findings indicate that CIRBP can modulate the development of cancer
through the regulation of the inflammatory response.
A major characteristic that distinguishes cancer
cells from normal cells is their ability to spread through invasion
and metastasis. Metastasis is the major cause of cancer-related
mortality in patients. In addition to the previously mentioned role
of CIRBP in proliferative signaling, several studies have reported
that CIRBP is involved in the metastasis of multiple cancer types
(56,57). CIRBP is upregulated in 57% of
human bladder cancer tissues and cancer cell lines, and it is
reported to enhance migration and metastasis in vivo and
in vitro (29). Breast
cancer is one of the leading causes of cancer-associated mortality
in women (58). Notably,
progressive breast cancer is virtually incurable and the cause of a
high mortality rate in patients. CIRBP downregulation was shown to
reduce the invasion and migration capacity of breast cancer cells,
and CIRBP upregulation was observed in more aggressive breast
cancer subtypes compared with ductal carcinoma, in situ
(30). Moreover, CIRBP exhibited
strong metastasis-promoting activity in invasive ductal carcinoma
(59) and invasive brain
metastases (60). In addition,
epithelial-mesenchymal transition (EMT) is a crucial process for
cancers metastasizing from the original site to other organs
(61). During TGF-β-induced EMT,
CIRBP silencing was shown to inhibit the upregulation of the master
regulator, Snail, thereby suppressing the migration of
hepatocellular carcinoma cells (62). This indicates that CIRBP is
involved in metastasis of HCC and, therefore, the low survival rate
of patients with HCC. However, in contrast to its oncogenic role in
certain cancer types, several studies have shown that CIRBP can
suppress cancer metastasis (56,63). CIRBP is negatively correlated with
distant metastasis in nasopharyngeal cancer (56), and is downregulated in patients
with aggressive metastatic TNBC (63).
Angiogenesis is regulated by chemical signals such
as VEGF, which binds to endothelial cell receptors and initiates
intracellular signaling to promote the growth of new blood vessels
(64). Neoangiogenesis represents
an important step in cancer and is required to supply nutrients and
oxygen to the tumoral cells, and to remove the waste products
(65). Melanoma tumors with
decreased CIRBP expression exhibit specifically downregulated VEGF
expression compared with controls when using the angiogenesis
proteome profiler array (30).
Conversely, strong staining of CD31, an angiogenesis marker, was
observed in a skin wound-healing sample of CIRBP-knockout mice
compared with wild-type mice (66). Moreover, a recent study
demonstrated that knockdown of CIRBP enhances the regeneration of
ischemic muscle tissues, damaged by unilateral ligation of the
hindlimb femoral artery, through acceleration of angiogenesis and
M2-like macrophage polarization (67). These studies strongly indicate
that CIRBP serves a role in angiogenesis, which may modulate tumor
growth.
CIRBP is commonly overexpressed in a number of
cancer tissues and cell lines. It acts as an oncogene by increasing
the stability and translation of cancer-associated mRNA targets.
However, several studies have also suggested the potential of CIRBP
as a tumor suppressor by modulating the stability of target mRNAs
(Fig. 1; Table II). CIRBP can bind the 5′ and
3′-UTRs of mRNAs, as well as poly U sequences at the 3′-ends
(68). It has been suggested that
its binding is important for the translation of interacting mRNAs
by regulating polyadenylation and 3′-end cleavage (7,37).
In the context of stress-induced regulation, abnormal upregulation
of CIRBP promotes hypoxia inducible factor (HIF)-1α expression
(29). Due to stabilization of
the HIF-1α mRNA transcript, increased HIF-1α can bind to the
promoter region of prostaglandin I2 synthase, a tumor suppressor,
resulting in its downregulation (29) and an increase in the growth and
invasion of cancer cells. An in vitro study demonstrated
that CIRBP can also increase the mRNA stability of cyclin E1 in
breast cancer (69). Responding
to DNA damage, CIRBP can bind to the 3′-UTRs of TRX, replication
protein A2 and ATR serine/threonine kinase mRNAs and increase their
translational efficiencies (7,10,70). A recent study reported that, in
luminal breast cancer, CIRBP is upregulated and enhances oncogenic
properties by downregulating the CST3 mRNA expression levels
(31). Notably, CIRBP can also
enhance telomere maintenance by upregulating TERT mRNA levels
(40). In most human cancer
cells, active telomerase is upregulated, highlighting the
importance of TERT expression and telomerase activity in promoting
cancer progression (71,72). Other CIRBP-mediated regulatory
effects have also been reported in human cancers. For example,
CIRBP can increase phosphorylation of ribosomal protein S6, and
eukaryotic translation initiation factor 4E-binding protein1, a
protein that regulates the elongation phases of translation
(73). In addition, CIRBP can
promote cell proliferation by upregulating cyclin D1 and
downregulating p27 via ERK signaling (33). Within the MAPK pathway, ERK
signaling is involved in various human diseases, including
inflammatory-related diseases and cancer (74,75). Additionally, CIRBP reduces
phosphorylation of p27 by interacting with DYRK1B and inhibiting
its binding to p27 in mouse germ cells (32). CIRBP also interferes with the
phosphorylation of cyclin D1 by DYRK1B, thereby stabilizing cyclin
D1 and ultimately increasing proliferation (32). Conversely, another study showed
that CIRBP had an anti-proliferative function by binding to the
5′-UTR of p27 and increasing p27 expression in mouse embryonic
fibroblasts (37).
The association between cancer and inflammation has
been reported in numerous studies. In chronic airway inflammation
disease, CIRBP upregulates mucin-5AC, which is associated with
pulmonary disease via NF-κB/TLR4 signaling (76). In a CAC mouse model, CIRBP
depletion reduced the level of inflammation markers, such as TNF-α
and IL-23, and consequently decreased the susceptibility to CAC
development (52). CIRBP can
induce ROS accumulation by increasing the expression of
inflammatory cytokines (IL-6 and IL-1β) in liver-specific
macrophages. Conversely, CIRBP-knockout mice exhibited a decreased
level of inflammatory cytokines with attenuated ROS accumulation
(53). Together, these studies
suggest that CIRBP may function as a tumor promoter or tumor
suppressor by modulating the expression of inflammatory
mediators.
Applicable prognostic cancer biomarkers in cancer
are crucial for better tumor prediction and treatment planning.
Several studies have shown the potential of RBPs as prognostic
markers for various types of cancer, such as gastric or breast
cancer (77,78). Consequently, databases such as
TCGA (https://portal.gdc.cancer.gov/) and
GEO (https://www.ncbi.nlm.nih.gov/geo/) containing the
expression level of CIRBP in samples from patients with cancer were
selected, and the potential of CIRBP as a prognostic marker in
human cancers was presented (Table
III).
A recent study indicated that CIRBP is methylated in
the plasma of non-small cell lung carcinoma (NSCLC) with occult
lymph node metastasis. RNA sequencing data obtained from The Cancer
Genome Atlas (TCGA) also revealed that the mRNA expression levels
of CIRBP are higher in metastatic tissues compared with primary
breast tumor samples (79).
Similar to previous RNA sequencing data, CIRBP is upregulated in
invasive ductal carcinoma (59)
and in patient with brain metastases with a high recurrence rate
(60). These studies suggest that
CIRBP can promote cancer metastasis. Conversely, CIRBP is inversely
correlated with lymph node invasion and distant metastasis in
nasopharyngeal carcinoma (56).
Additionally, CIRBP is differentially upregulated in non-triple
negative breast cancer (TNBC) compared with metastasis-related TNBC
(63). Although the evidence of
CIRBP involvement in metastasis is still incomplete, CIRBP may
potentially represent a crucial component of the metastatic
process.
To overcome low survival rate of patients with
metastatic cancer, it is necessary to identify the biomarkers for
early diagnosis before metastasis to distant organs. Recently,
genomic profiling analysis using Gene Expression Omnibus and TCGA
datasets revealed that high expression levels of CIRBP are
correlated with good prognosis in patients with early-stage NSCLC
with low metastasis (80).
Stratification according to TNM classification revealed that a
higher CIRBP expression level is frequently detected in T1-T2, M0
and I-II tumors compared with T3-T4, M1 and III-IV nasopharyngeal
carcinoma tissues, respectively (56). Likewise, gene expression profiles
based on microarrays have demonstrated that CIRBP is significantly
upregulated in benign tumors compared with malignant ovarian
cancers (81). Conversely, CIRBP
is significantly associated with histological classification,
clinical stages and lymph node metastasis in OSCC samples (55). Although it is important to
classify the subtypes of breast cancer, there is currently no good
parameter to distinguish invasive breast carcinoma (IBC) from
ductal carcinoma in situ (DCIS) (82). By screening autoantibodies using
protein microarrays with DCIS and IBC samples, CIRBP was identified
as an autoantibody signature that could discriminate DCIS from IBC.
This result indicates that CIRBP may represent a novel prognostic
marker in breast cancer (83).
CIRBP is also a splicing factor (SF), which are important factors
in cancer progression (84,85). By comparing RNA expression levels
of various SFs between primary cancer and their metastatic
counterparts from TCGA, it was found that CIRBP expression is
higher in metastatic tissues compared with original tumors
(79). Along with SF, alternative
splicing events (ASEs) are also responsible for cancer development
and progression (86,87). RNA sequencing and ASE-related
datasets of breast cancer samples obtained from TCGA revealed that
CIRBP, may serve as a predictor for survival in prognostic-related
ASE (59). Together, these
results suggest that CIRBP may function as a prognostic marker in a
number of cancer types.
The use of cytotoxic drugs is the main treatment
method for advanced and aggressive cancers, and cancers without
specific therapeutic targets. However, resistance to cytotoxic
chemotherapy and drug side effects are major barriers to attaining
a complete response (88).
Several studies have reported that resistance to chemotherapy is
enhanced by secretory molecules that can promote the repair
signaling coordinated by TLR4 (89,90). CIRBP can trigger the secretion of
TNF-α through the activation of TLR4 and NF-κB in macrophages.
Several studies have also reported that CIRBP can mediate
inflammatory signaling via regulation of TLR4 signaling (76,91). Based on these results,
CIRBP-derived oligopeptides or neutralizing antibodies were
demonstrated to ameliorate sepsis-mediated injury of the lung and
kidney (15,92,93). These CIRBP antagonists can block
the interaction of extracellular CIRBP with TLR4/myeloid
differentiation 2 receptor complex to inhibit the downstream
signaling (15).
The circadian clock is an important molecular
mechanism for the maintenance of homeostasis and its imbalance
facilitates tumor progression (94). In various cancer types, circadian
genes are associated with chemoresistance and cancer progression
(95,96). Thus, there is a novel approach
that indirectly or directly targets circadian clock genes to remove
cancer and improve survival rates (97,98). Several studies have suggested that
CIRBP can be used in cancer treatment by regulating circadian genes
(13,68). Chemotherapeutic drugs can induce
apoptosis, necrosis and autophagy in cancerous tissues (99,100). As CIRBP exerts a protective role
in apoptosis in neurons and cardiac cells, combined therapy of
cytotoxic drugs with anti-CIRBP therapeutics may improve the
response efficacy and survival rate in patients with neuronal and
cardiac abnormalities (101,102).
Small molecules that complement biologics, such as
antibodies, have advantages of cost effectiveness and cell
permeability for applications in cancer therapy. Several chemical
probes targeting specific RBPs have been shown to be able to
function as selective inhibitors by modulating RBP-target mRNA
interactions (103-107). Recently, it has been reported
that a probe can interfere with CIRBP-RNA associations, inhibit
cytotoxic T-lymphocyte protein-4 and TRX expression, and suppress
the progression of various cancer types without side effects
(108). Further studies are
needed to apply these CIRBP antagonists for cancer therapy in the
future.
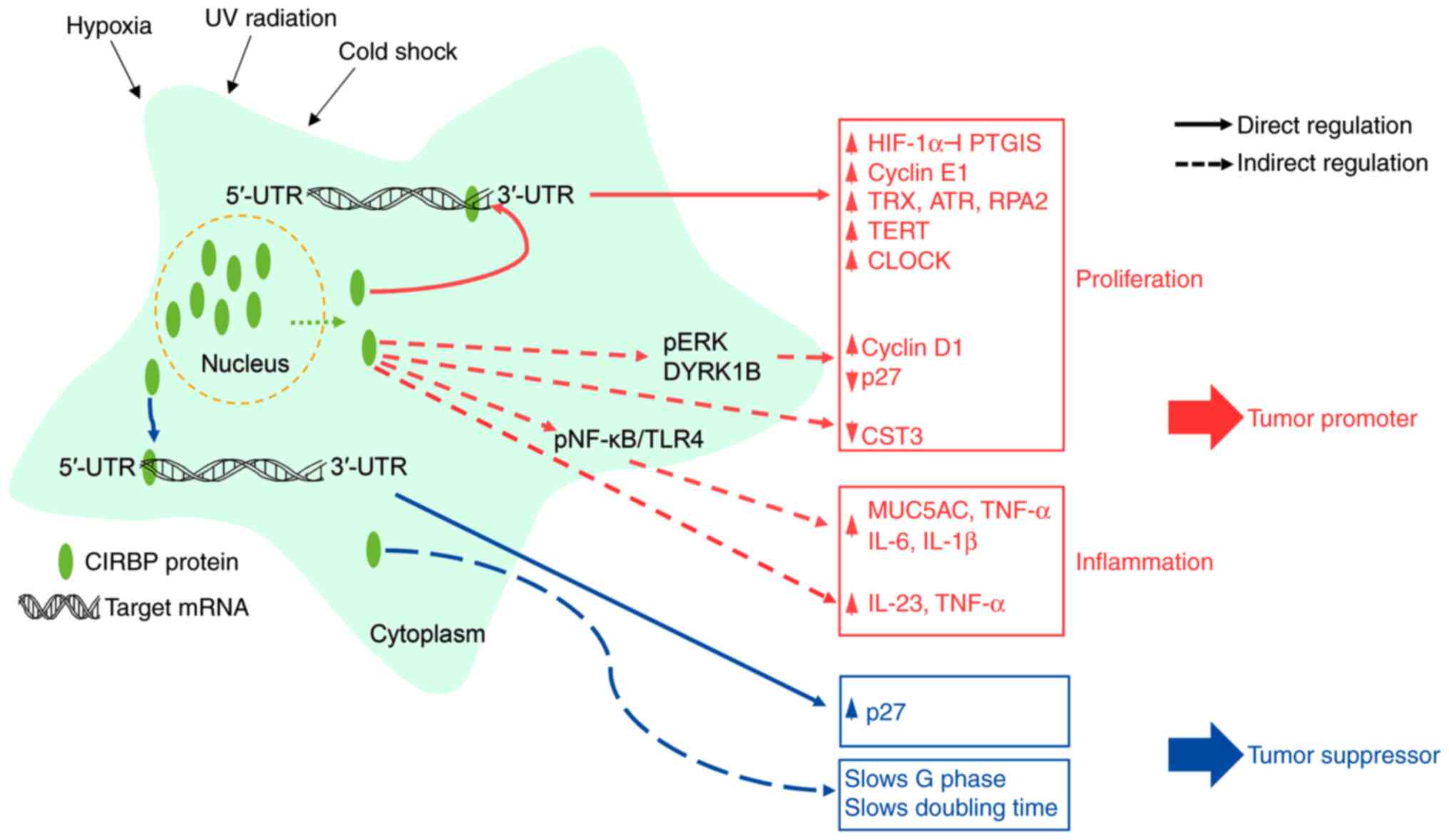
The present review summarized recent findings about
the roles of CIRBP in cancer development, metastasis and cancer
therapy (Fig. 2). During cancer
proliferation and metastasis, the function of CIRBP appears to be
driven primarily by promoting the stability and translation of
target mRNAs. Conversely, certain studies have demonstrated that
CIRBP serves as a tumor suppressor in cancer progression by
modulating the multiple steps of cell proliferation. These
controversial roles of CIRBP in human cancers may originate from
the alternative splicing of the CIRBP transcript (2,4-6).
Differentially expressed splicing variants may interact and
modulate the different target mRNAs, depending on cancer subtypes
or cell contexts. To understand the exact role of CIRBP in cancers,
target mRNAs of each splicing isoform should be identified and the
regulatory mechanism analyzed in human cancers. Clinical studies
have shown that CIRBP may represent a prognostic marker of cancer
progression. Although numerous studies have reported roles of CIRBP
in cancer biology, further detailed studies are required to
elucidate the exact role of CIRBP in human cancers and to evaluate
the potential of the application of CIRBP-targeted cancer
therapy.
Not applicable.
Y-MK and SH designed and discussed the contents and
wrote the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
De Leeuw F, Zhang T, Wauquier C, Huez G,
Kruys V and Gueydan C: The cold-inducible RNA-binding protein
migrates from the nucleus to cytoplasmic stress granules by a
methylation-dependent mechanism and acts as a translational
repressor. Exp Cell Res. 313:4130–4144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Fageeh MB and Smales CM: Cold-inducible
RNA binding protein (CIRP) expression is modulated by alternative
mRNAs. RNA. 15:1164–1176. 2009. View Article : Google Scholar :
|
|
3
|
Kaneko T and Kibayashi K: Mild hypothermia
facilitates the expression of cold-inducible RNA-binding protein
and heat shock protein 70.1 in mouse brain. Brain Res.
1466:128–136. 2012. View Article : Google Scholar
|
|
4
|
Horii Y, Shimaoka H, Horii K, Shiina T and
Shimizu Y: Mild hypothermia causes a shift in the alternative
splicing of cold-inducible RNA-binding protein transcripts in
Syrian hamsters. Am J Physiol Regul Integr Comp Physiol.
317:R240–R247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gokhale NS, McIntyre AB, Mattocks MD,
Holley CL, Lazear HM, Mason CE and Horner SM: Altered
m6A modification of specific cellular transcripts
affects flaviviridae infection. Mol Cell. 77:542–555.e8. 2020.
View Article : Google Scholar
|
|
6
|
Liao Y, Tong L, Tang L and Wu S: The role
of cold-inducible RNA binding protein in cell stress response. Int
J Cancer. 141:2164–2173. 2017. View Article : Google Scholar
|
|
7
|
Yang C and Carrier F: The UV-inducible
RNA-binding protein A18 (A18 hnRNP) plays a protective role in the
genotoxic stress response. J Biol Chem. 276:47277–47284. 2001.
View Article : Google Scholar
|
|
8
|
Sheikh MS, Carrier F, Papathanasiou MA,
Hollander MC, Zhan Q, Yu K and Fornace AJ Jr: Identification of
several human homologs of hamster DNA damage-inducible transcripts.
Cloning and characterization of a novel UV-inducible cDNA that
codes for a putative RNA-binding protein. J Biol Chem.
272:26720–26726. 1997. View Article : Google Scholar
|
|
9
|
Fujita J: Cold shock response in mammalian
cells. J Mol Microbiol Biotechnol. 1:243–255. 1999.
|
|
10
|
Yang R, Weber DJ and Carrier F:
Post-transcriptional regulation of thioredoxin by the stress
inducible heterogenous ribonucleoprotein A18. Nucleic Acids Res.
34:1224–1236. 2006. View Article : Google Scholar
|
|
11
|
Lu J, Shen Y, Qian HY, Liu LJ, Zhou BC,
Xiao Y, Mao JN, An GY, Rui MZ, Wang T and Zhu CL: Effects of mild
hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of
hippocampus nerve cells in rats after cardiopulmonary
resuscitation. World J Emerg Med. 5:298–305. 2014. View Article : Google Scholar :
|
|
12
|
Haley B, Paunesku T, Protić M and
Woloschak GE: Response of heterogeneous ribonuclear proteins
(hnRNP) to ionising radiation and their involvement in DNA damage
repair. Int J Radiat Biol. 85:643–655. 2009. View Article : Google Scholar
|
|
13
|
Morf J, Rey G, Schneider K, Stratmann M,
Fujita J, Naef F and Schibler U: Cold-inducible RNA-binding protein
modulates circadian gene expression posttranscriptionally. Science.
338:379–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Denning NL, Aziz M, Murao A, Gurien SD,
Ochani M, Prince JM and Wang P: Extracellular CIRP as an endogenous
TREM-1 ligand to fuel inflammation in sepsis. JCI Insight.
5:e1341722020. View Article : Google Scholar :
|
|
15
|
Qiang X, Yang WL, Wu R, Zhou M, Jacob A,
Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al: Cold-inducible
RNA-binding protein (CIRP) triggers inflammatory responses in
hemorrhagic shock and sepsis. Nat Med. 19:1489–1495. 2013.
View Article : Google Scholar
|
|
16
|
Zhong P, Peng J, Yuan M, Kong B and Huang
H: Cold-inducible RNA-binding protein (CIRP) in inflammatory
diseases: Molecular insights of its associated signalling pathways.
Scand J Immunol. 93:e129492021. View Article : Google Scholar
|
|
17
|
Xie D, Geng L, Wang S, Xiong K, Zhao T,
Wang G, Feng Z, Lv F, Wang C, Liang D, et al: Cold-inducible
RNA-binding protein modulates atrial fibrillation onset by
targeting multiple ion channels. Heart Rhythm. 17:998–1008. 2020.
View Article : Google Scholar
|
|
18
|
Xie D, Geng L, Xiong K, Zhao T, Wang S,
Xue J, Wang C, Wang G, Feng Z, Zhou H, et al: Cold-Inducible
RNA-binding protein prevents an excessive heart rate response to
stress by targeting phosphodiesterase. Circ Res. 126:1706–1720.
2020. View Article : Google Scholar
|
|
19
|
Aziz M, Brenner M and Wang P:
Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol.
106:133–146. 2019. View Article : Google Scholar
|
|
20
|
Zhou Y, Dong H, Zhong Y, Huang J, Lv J and
Li J: The cold-inducible RNA-binding protein (CIRP) level in
peripheral blood predicts sepsis outcome. PLoS One.
10:e01377212015. View Article : Google Scholar :
|
|
21
|
Gong JD, Qi XF, Zhang Y and Li HL:
Increased admission serum cold-inducible RNA-binding protein
concentration is associated with prognosis of severe acute
pancreatitis. Clin Chim Acta. 471:135–142. 2017. View Article : Google Scholar
|
|
22
|
Sharma A, Brenner M and Wang P: Potential
role of extracellular CIRP in alcohol-induced Alzheimer's disease.
Mol Neurobiol. 57:5000–5010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo IS, Lee SY, Park CK, Lee JC, Kim Y,
Yoo SJ, Shim SC, Choi YS, Lee Y and Kang SW: Serum and synovial
fluid concentrations of cold-inducible RNA-binding protein in
patients with rheumatoid arthritis. Int J Rheum Dis. 21:148–154.
2018. View Article : Google Scholar
|
|
24
|
Lujan DA, Ochoa JL and Hartley RS:
Cold-inducible RNA binding protein in cancer and inflammation.
Wiley Interdiscip Rev RNA. Jan 11–2018.Epub ahead of print.
View Article : Google Scholar
|
|
25
|
Kanemura Y, Mori K, Sakakibara S, Fujikawa
H, Hayashi H, Nakano A, Matsumoto T, Tamura K, Imai T, Ohnishi T,
et al: Musashi1, an evolutionarily conserved neural RNA-binding
protein, is a versatile marker of human glioma cells in determining
their cellular origin, malignancy, and proliferative activity.
Differentiation. 68:141–152. 2001. View Article : Google Scholar
|
|
26
|
Wang Q, Wang F, Zhong W, Ling H, Wang J,
Cui J, Xie T, Wen S and Chen J: RNA-binding protein RBM6 as a tumor
suppressor gene represses the growth and progression in
laryngocarcinoma. Gene. 697:26–34. 2019. View Article : Google Scholar
|
|
27
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
28
|
Kong N, Zhang H, Feng C, Liu C, Xiao Y,
Zhang X, Mei L, Kim JS, Tao W and Ji X: Arsenene-mediated multiple
independently targeted reactive oxygen species burst for cancer
therapy. Nat Commun. 12:47772021. View Article : Google Scholar :
|
|
29
|
Lu M, Ge Q, Wang G, Luo Y and Wang X,
Jiang W, Liu X, Wu CL, Xiao Y and Wang X: CIRBP is a novel oncogene
in human bladder cancer inducing expression of HIF-1α. Cell Death
Dis. 9:10462018. View Article : Google Scholar
|
|
30
|
Chang ET, Parekh PR, Yang Q, Nguyen DM and
Carrier F: Heterogenous ribonucleoprotein A18 (hnRNP A18) promotes
tumor growth by increasing protein translation of selected
transcripts in cancer cells. Oncotarget. 7:10578–10593. 2016.
View Article : Google Scholar
|
|
31
|
Indacochea A, Guerrero S, Ureña M, Araujo
F, Coll O, LLeonart ME and Gebauer F: Cold-inducible RNA binding
protein promotes breast cancer cell malignancy by regulating
Cystatin C levels. RNA. 27:190–201. 2021. View Article : Google Scholar
|
|
32
|
Masuda T, Itoh K, Higashitsuji H,
Higashitsuji H, Nakazawa N, Sakurai T, Liu Y, Tokuchi H, Fujita T,
Zhao Y, et al: Cold-inducible RNA-binding protein (Cirp) interacts
with Dyrk1b/Mirk and promotes proliferation of immature male germ
cells in mice. Proc Natl Acad Sci USA. 109:10885–10890. 2012.
View Article : Google Scholar
|
|
33
|
Jian F, Chen Y, Ning G, Fu W, Tang H, Chen
X, Zhao Y, Zheng L, Pan S, Wang W, et al: Cold inducible RNA
binding protein upregulation in pituitary corticotroph adenoma
induces corticotroph cell proliferation via Erk signaling pathway.
Oncotarget. 7:9175–9187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peri S, de Cicco RL, Santucci-Pereira J,
Slifker M, Ross EA, Russo IH, Russo PA, Arslan AA, Belitskaya-Lévy
I, Zeleniuch-Jacquotte A, et al: Defining the genomic signature of
the parous breast. BMC Med Genomics. 5:462012. View Article : Google Scholar
|
|
35
|
Lujan DA, Garcia S, Vanderhoof J,
Sifuentes J, Brandt Y, Wu Y, Guo X, Mitchell T, Howard T, Hathaway
HJ and Hartley RS: Cold-inducible RNA binding protein in mouse
mammary gland development. Tissue Cell. 48:577–587. 2016.
View Article : Google Scholar
|
|
36
|
Hamid AA, Mandai M, Fujita J, Nanbu K,
Kariya M, Kusakari T, Fukuhara K and Fujii S: Expression of
cold-inducible RNA-binding protein in the normal endometrium,
endometrial hyperplasia, and endometrial carcinoma. Int J Gynecol
Pathol. 22:240–247. 2003. View Article : Google Scholar
|
|
37
|
Roilo M, Kullmann MK and Hengst L:
Cold-inducible RNA-binding protein (CIRP) induces translation of
the cell-cycle inhibitor p27Kip1. Nucleic Acids Res. 46:3198–3210.
2018. View Article : Google Scholar
|
|
38
|
Roake CM and Artandi SE: Regulation of
human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol.
21:384–397. 2020. View Article : Google Scholar
|
|
39
|
Yuan X, Larsson C and Xu D: Mechanisms
underlying the activation of TERT transcription and telomerase
activity in human cancer: Old actors and new players. Oncogene.
38:6172–6183. 2019. View Article : Google Scholar
|
|
40
|
Zhang Y, Wu Y, Mao P, Li F, Han X, Zhang
Y, Jiang S, Chen Y, Huang J, Liu D, et al: Cold-inducible
RNA-binding protein CIRP/hnRNP A18 regulates telomerase activity in
a temperature-dependent manner. Nucleic Acids Res. 44:761–775.
2016. View Article : Google Scholar
|
|
41
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar
|
|
42
|
Lee HN, Ahn SM and Jang HH: Cold-inducible
RNA-binding protein, CIRP, inhibits DNA damage-induced apoptosis by
regulating p53. Biochem Biophys Res Commun. 464:916–921. 2015.
View Article : Google Scholar
|
|
43
|
Sun W, Bergmeier AP, Liao Y, Wu S and Tong
L: CIRP sensitizes cancer cell responses to ionizing radiation.
Radiat Res. 195:93–100. 2021.
|
|
44
|
Su F, Yang S, Wang H, Qiao Z, Zhao H and
Qu Z: CIRBP ameliorates neuronal amyloid toxicity via antioxidative
and antiapoptotic pathways in primary cortical neurons. Oxid Med
Cell Longev. 2020:27861392020. View Article : Google Scholar
|
|
45
|
Yang WL, Sharma A, Wang Z, Li Z, Fan J and
Wang P: Cold-inducible RNA-binding protein causes endothelial
dysfunction via activation of Nlrp3 inflammasome. Sci Rep.
6:265712016. View Article : Google Scholar
|
|
46
|
Li H, Han X, Yang S, Wang Y, Dong Y and
Tang T: FOXP1 drives osteosarcoma development by repressing P21 and
RB transcription downstream of P53. Oncogene. 40:2785–2802. 2021.
View Article : Google Scholar
|
|
47
|
Zhou KW, Zheng XM, Yang ZW, Zhang L and
Chen HD: Overexpression of CIRP may reduce testicular damage
induced by cryptorchidism. Clin Invest Med. 32:E103–E111. 2009.
View Article : Google Scholar
|
|
48
|
Cho SY, Oh Y, Jeong EM, Park S, Lee D,
Wang X, Zeng Q, Qin H, Hu F, Gong H, et al: Amplification of
transglutaminase 2 enhances tumor-promoting inflammation in gastric
cancers. Exp Mol Med. 52:854–864. 2020. View Article : Google Scholar
|
|
49
|
Means AL, Freeman TJ, Zhu J, Woodbury LG,
Marincola-Smith P, Wu C, Meyer AR, Weaver CJ, Padmanabhan C, An H,
et al: Epithelial Smad4 Deletion Up-regulates inflammation and
promotes inflammation-associated cancer. Cell Mol Gastroenterol
Hepatol. 6:257–276. 2018. View Article : Google Scholar :
|
|
50
|
Kortylewski M, Xin H, Kujawski M, Lee H,
Liu Y, Harris T, Drake C, Pardoll D and Yu H: Regulation of the
IL-23 and IL-12 balance by Stat3 signaling in the tumor
microenvironment. Cancer Cell. 15:114–123. 2009. View Article : Google Scholar
|
|
51
|
Wu D, Wu P, Huang Q, Liu Y, Ye J and Huang
J: Interleukin-17: A promoter in colorectal cancer progression.
Clin Dev Immunol. 2013:4363072013. View Article : Google Scholar
|
|
52
|
Sakurai T, Kashida H, Watanabe T, Hagiwara
S, Mizushima T, Iijima H, Nishida N, Higashitsuji H, Fujita J and
Kudo M: Stress response protein cirp links inflammation and
tumorigenesis in colitis-associated cancer. Cancer Res.
74:6119–6128. 2014. View Article : Google Scholar
|
|
53
|
Sakurai T, Yada N, Watanabe T, Arizumi T,
Hagiwara S, Ueshima K, Nishida N, Fujita J and Kudo M:
Cold-inducible RNA-binding protein promotes the development of
liver cancer. Cancer Sci. 106:352–358. 2015. View Article : Google Scholar
|
|
54
|
Feller L, Altini M and Lemmer J:
Inflammation in the context of oral cancer. Oral Oncol. 49:887–892.
2013. View Article : Google Scholar
|
|
55
|
Ren WH, Zhang LM, Liu HQ, Gao L, Chen C,
Qiang C, Wang XL, Liu CY, Li SM, Huang C, et al: Protein
overexpression of CIRP and TLR4 in oral squamous cell carcinoma: An
immunohistochemical and clinical correlation analysis. Med Oncol.
31:1202014. View Article : Google Scholar
|
|
56
|
Lin TY, Chen Y, Jia JS, Zhou C, Lian M,
Wen YT, Li XY, Chen HW, Lin XL, Zhang XL, et al: Loss of Cirbp
expression is correlated with the malignant progression and poor
prognosis in nasopharyngeal carcinoma. Cancer Manag Res.
11:6959–6969. 2019. View Article : Google Scholar
|
|
57
|
Chen Z, Xiong S, Li J, Ou L, Li C, Tao J,
Jiang Z, Fan J, He J and Liang W: DNA methylation markers that
correlate with occult lymph node metastases of non-small cell lung
cancer and a preliminary prediction model. Transl Lung Cancer Res.
9:280–287. 2020. View Article : Google Scholar
|
|
58
|
Fahad Ullah M: Breast cancer: Current
perspectives on the disease status. Adv Exp Med Biol. 1152:51–64.
2019. View Article : Google Scholar
|
|
59
|
Huang R, Guo J, Yan P, Zhai S, Hu P, Zhu
X, Zhang J, Qiao Y, Zhang Y, Liu H, et al: The construction of bone
metastasis-specific prognostic model and co-expressed network of
alternative splicing in breast cancer. Front Cell Dev Biol.
8:7902020. View Article : Google Scholar
|
|
60
|
Dankner M, Caron M, Al-Saadi T, Yu W,
Ouellet V, Ezzeddine R, Maritan SM, Annis MG, Le PU, Nadaf J, et
al: Invasive growth associated with Cold-Inducible RNA-Binding
Protein expression drives recurrence of surgically resected brain
metastases. Neuro Oncol. 23:1470–1480. 2021. View Article : Google Scholar
|
|
61
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar
|
|
62
|
Lee HN, Ahn SM and Jang HH: Cold-inducible
RNA-binding protein promotes epithelial-mesenchymal transition by
activating ERK and p38 pathways. Biochem Biophys Res Commun.
477:1038–1044. 2016. View Article : Google Scholar
|
|
63
|
Joe S and Nam H: Prognostic factor
analysis for breast cancer using gene expression profiles. BMC Med
Inform Decis Mak. 16(Suppl 1): 562016. View Article : Google Scholar
|
|
64
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69(Suppl 3): S4–S10. 2005.
View Article : Google Scholar
|
|
65
|
Hashemi Goradel N, Ghiyami-Hour F,
Jahangiri S, Negahdari B, Sahebkar A, Masoudifar A and Mirzaei H:
Nanoparticles as new tools for inhibition of cancer angiogenesis. J
Cell Physiol. 233:2902–2910. 2018. View Article : Google Scholar
|
|
66
|
Idrovo JP, Jacob A, Yang WL, Wang Z, Yen
HT, Nicastro J, Coppa GF and Wang P: A deficiency in cold-inducible
RNA-binding protein accelerates the inflammation phase and improves
wound healing. Int J Mol Med. 37:423–428. 2016. View Article : Google Scholar
|
|
67
|
Kübler M, Beck S, Fischer S, Götz P,
Kumaraswami K, Ishikawa-Ankerhold H, Lasch M and Deindl E: Absence
of cold-inducible RNA-binding protein (CIRP) promotes angiogenesis
and regeneration of ischemic tissue by inducing M2-Like macrophage
polarization. Biomedicines. 9:3952021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu Y, Hu W, Murakawa Y, Yin J, Wang G,
Landthaler M and Yan J: Cold-induced RNA-binding proteins regulate
circadian gene expression by controlling alternative
polyadenylation. Sci Rep. 3:20542013. View Article : Google Scholar :
|
|
69
|
Guo X, Wu Y and Hartley RS: Cold-inducible
RNA-binding protein contributes to human antigen R and cyclin E1
deregulation in breast cancer. Mol Carcinog. 49:130–140. 2010.
View Article : Google Scholar
|
|
70
|
Yang R, Zhan M, Nalabothula NR, Yang Q,
Indig FE and Carrier F: Functional significance for a heterogenous
ribonucleo-protein A18 signature RNA motif in the 3′-untranslated
region of ataxia telangiectasia mutated and Rad3-related (ATR)
transcript. J Biol Chem. 285:8887–8893. 2010. View Article : Google Scholar :
|
|
71
|
Ouellette MM, Liao M, Herbert BS, Johnson
M, Holt SE, Liss HS, Shay JW and Wright WE: Subsenescent telomere
lengths in fibroblasts immortalized by limiting amounts of
telomerase. J Biol Chem. 275:10072–10076. 2000. View Article : Google Scholar
|
|
72
|
Nakayama J, Tahara H, Tahara E, Saito M,
Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T and Ishikawa F:
Telomerase activation by hTRT in human normal fibroblasts and
hepatocellular carcinomas. Nat Genet. 18:65–68. 1998. View Article : Google Scholar
|
|
73
|
Artero-Castro A, Callejas FB, Castellvi J,
Kondoh H, Carnero A, Fernández-Marcos PJ, Serrano M, Ramón y Cajal
S and Lleonart ME: Cold-inducible RNA-binding protein bypasses
replicative senescence in primary cells through extracellular
signal-regulated kinase 1 and 2 activation. Mol Cell Biol.
29:1855–1868. 2009. View Article : Google Scholar
|
|
74
|
García-Gómez R, Bustelo XR and Crespo P:
Protein-protein interactions: Emerging oncotargets in the RAS-ERK
pathway. Trends Cancer. 4:616–633. 2018. View Article : Google Scholar
|
|
75
|
Kim JY, Lee SG, Chung JY, Kim YJ, Park JE,
Koh H, Han MS, Park YC, Yoo YH and Kim JM: Ellipticine induces
apoptosis in human endometrial cancer cells: the potential
involvement of reactive oxygen species and mitogen-activated
protein kinases. Toxicology. 289:91–102. 2011. View Article : Google Scholar
|
|
76
|
Chen L, Ran D, Xie W, Xu Q and Zhou X:
Cold-inducible RNA-binding protein mediates cold air inducible
airway mucin production through TLR4/NF-κB signaling pathway. Int
Immunopharmacol. 39:48–56. 2016. View Article : Google Scholar
|
|
77
|
Li J, Zhou W, Wei J, Xiao X, An T, Wu W
and He Y: Prognostic value and biological functions of RNA binding
proteins in stomach adenocarcinoma. Onco Targets Ther.
14:1689–1705. 2021. View Article : Google Scholar
|
|
78
|
Zhang J, Xu A, Miao C, Yang J, Gu M and
Song N: Prognostic value of Lin28A and Lin28B in various human
malignancies: A systematic review and meta-analysis. Cancer Cell
Int. 19:792019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Koedoot E, Smid M, Foekens JA, Martens
JWM, Le Dévédec SE and van de Water B: Co-regulated gene expression
of splicing factors as drivers of cancer progression. Sci Rep.
9:54842019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
He R and Zuo S: A robust 8-gene prognostic
signature for early-stage non-small cell lung cancer. Front Oncol.
9:6932019. View Article : Google Scholar :
|
|
81
|
Biade S, Marinucci M, Schick J, Roberts D,
Workman G, Sage EH, O'Dwyer PJ, Livolsi VA and Johnson SW: Gene
expression profiling of human ovarian tumours. Br J Cancer.
95:1092–1100. 2006. View Article : Google Scholar
|
|
82
|
Virnig BA, Tuttle TM, Shamliyan T and Kane
RL: Ductal carcinoma in situ of the breast: A systematic review of
incidence, treatment, and outcomes. J Natl Cancer Inst.
102:170–178. 2010. View Article : Google Scholar
|
|
83
|
Mangé A, Lacombe J, Bascoul-Mollevi C,
Jarlier M, Lamy PJ, Rouanet P, Maudelonde T and Solassol J: Serum
autoantibody signature of ductal carcinoma in situ progression to
invasive breast cancer. Clin Cancer Res. 18:1992–2000. 2012.
View Article : Google Scholar
|
|
84
|
Sveen A, Kilpinen S, Ruusulehto A, Lothe
RA and Skotheim RI: Aberrant RNA splicing in cancer; expression
changes and driver mutations of splicing factor genes. Oncogene.
35:2413–2427. 2016. View Article : Google Scholar
|
|
85
|
Anczuków O and Krainer AR: Splicing-factor
alterations in cancers. RNA. 22:1285–1301. 2016. View Article : Google Scholar
|
|
86
|
Zhao D, Zhang C, Jiang M, Wang Y, Liang Y,
Wang L, Qin K, Rehman FU and Zhang X: Survival-associated
alternative splicing signatures in non-small cell lung cancer.
Aging (Albany NY). 12:5878–5893. 2020. View Article : Google Scholar
|
|
87
|
Anczuków O, Akerman M, Cléry A, Wu J, Shen
C, Shirole NH, Raimer A, Sun S, Jensen MA, Hua Y, et al:
SRSF1-regulated alternative splicing in breast cancer. Mol Cell.
60:105–117. 2015. View Article : Google Scholar
|
|
88
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar
|
|
89
|
Rajput S, Volk-Draper LD and Ran S: TLR4
is a novel determinant of the response to paclitaxel in breast
cancer. Mol Cancer Ther. 12:1676–1687. 2013. View Article : Google Scholar
|
|
90
|
Perera PY, Mayadas TN, Takeuchi O, Akira
S, Zaks-Zilberman M, Goyert SM and Vogel SN: CD11b/CD18 acts in
concert with CD14 and Toll-like receptor (TLR) 4 to elicit full
lipopolysaccharide and taxol-inducible gene expression. J Immunol.
166:574–581. 2001. View Article : Google Scholar
|
|
91
|
Bolognese AC, Sharma A, Yang WL, Nicastro
J, Coppa GF and Wang P: Cold-inducible RNA-binding protein
activates splenic T cells during sepsis in a TLR4-dependent manner.
Cell Mol Immunol. 15:38–47. 2018. View Article : Google Scholar
|
|
92
|
Khan MM, Yang WL, Brenner M, Bolognese AC
and Wang P: Cold-inducible RNA-binding protein (CIRP) causes
sepsis-associated acute lung injury via induction of endoplasmic
reticulum stress. Sci Rep. 7:413632017. View Article : Google Scholar
|
|
93
|
Zhang F, Brenner M, Yang WL and Wang P: A
cold-inducible RNA-binding protein (CIRP)-derived peptide
attenuates inflammation and organ injury in septic mice. Sci Rep.
8:30522018. View Article : Google Scholar
|
|
94
|
Sulli G, Lam MTY and Panda S: Interplay
between circadian clock and cancer: New frontiers for cancer
treatment. Trends Cancer. 5:475–494. 2019. View Article : Google Scholar
|
|
95
|
Hadadi E, Taylor W, Li XM, Aslan Y,
Villote M, Rivière J, Duvallet G, Auriau C, Dulong S,
Raymond-Letron I, et al: Chronic circadian disruption modulates
breast cancer stemness and immune microenvironment to drive
metastasis in mice. Nat Commun. 11:31932020. View Article : Google Scholar
|
|
96
|
Fang L, Yang Z, Zhou J, Tung JY, Hsiao CD,
Wang L, Deng Y, Wang P, Wang J and Lee MH: Circadian clock gene
CRY2 degradation is involved in chemoresistance of colorectal
cancer. Mol Cancer Ther. 14:1476–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rosenberg LH, Lafitte M, Quereda V, Grant
W, Chen W, Bibian M, Noguchi Y, Fallahi M, Yang C, Chang JC, et al:
Therapeutic targeting of casein kinase 1δ in breast cancer. Sci
Transl Med. 7:318ra2022015. View Article : Google Scholar
|
|
98
|
Oshima T, Niwa Y, Kuwata K, Srivastava A,
Hyoda T, Tsuchiya Y, Kumagai M, Tsuyuguchi M, Tamaru T, Sugiyama A,
et al: Cell-based screen identifies a new potent and highly
selective CK2 inhibitor for modulation of circadian rhythms and
cancer cell growth. Sci Adv. 5:eaau90602019. View Article : Google Scholar
|
|
99
|
Ricci MS and Zong WX: Chemotherapeutic
approaches for targeting cell death pathways. Oncologist.
11:342–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar
|
|
101
|
Chen M, Fu H, Zhang J, Huang H and Zhong
P: CIRP downregulation renders cardiac cells prone to apoptosis in
heart failure. Biochem Biophys Res Commun. 517:545–550. 2019.
View Article : Google Scholar
|
|
102
|
Li S, Zhang Z, Xue J, Liu A and Zhang H:
Cold-inducible RNA binding protein inhibits
H2O2-induced apoptosis in rat cortical
neurons. Brain Res. 1441:47–52. 2012. View Article : Google Scholar
|
|
103
|
Wang L, Rowe RG, Jaimes A, Yu C, Nam Y,
Pearson DS, Zhang J, Xie X, Marion W, Heffron GJ, et al:
Small-molecule inhibitors disrupt let-7 oligouridylation and
release the selective blockade of let-7 processing by LIN28. Cell
Rep. 23:3091–3101. 2018. View Article : Google Scholar
|
|
104
|
Minuesa G, Albanese SK, Xie W, Kazansky Y,
Worroll D, Chow A, Schurer A, Park SM, Rotsides CZ, Taggart J, et
al: Small-molecule targeting of MUSASHI RNA-binding activity in
acute myeloid leukemia. Nat Commun. 10:26912019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu X, Gardashova G, Lan L, Han S, Zhong C,
Marquez RT, Wei L, Wood S, Roy S, Gowthaman R, et al: Targeting the
interaction between RNA-binding protein HuR and FOXQ1 suppresses
breast cancer invasion and metastasis. Commun Biol. 3:1932020.
View Article : Google Scholar
|
|
106
|
François-Moutal L, Felemban R, Scott DD,
Sayegh MR, Miranda VG, Perez-Miller S, Khanna R, Gokhale V,
Zarnescu DC and Khanna M: Small molecule targeting TDP-43's RNA
recognition motifs reduces locomotor defects in a drosophila model
of amyotrophic lateral sclerosis (ALS). ACS Chem Biol.
14:2006–2013. 2019. View Article : Google Scholar
|
|
107
|
Baker JD, Uhrich RL, Strovas TJ, Saxton AD
and Kraemer BC: Targeting pathological tau by small molecule
inhibition of the poly(A):MSUT2 RNA-protein interaction. ACS Chem
Neurosci. 11:2277–2285. 2020. View Article : Google Scholar
|
|
108
|
Solano-Gonzalez E, Coburn KM, Yu W, Wilson
GM, Nurmemmedov E, Kesari S, Chang ET, MacKerell AD, Weber DJ and
Carrier F: Small molecules inhibitors of the heterogeneous
ribonuclear protein A18 (hnRNP A18): A regulator of protein
translation and an immune checkpoint. Nucleic Acids Res.
49:1235–1246. 2021. View Article : Google Scholar
|