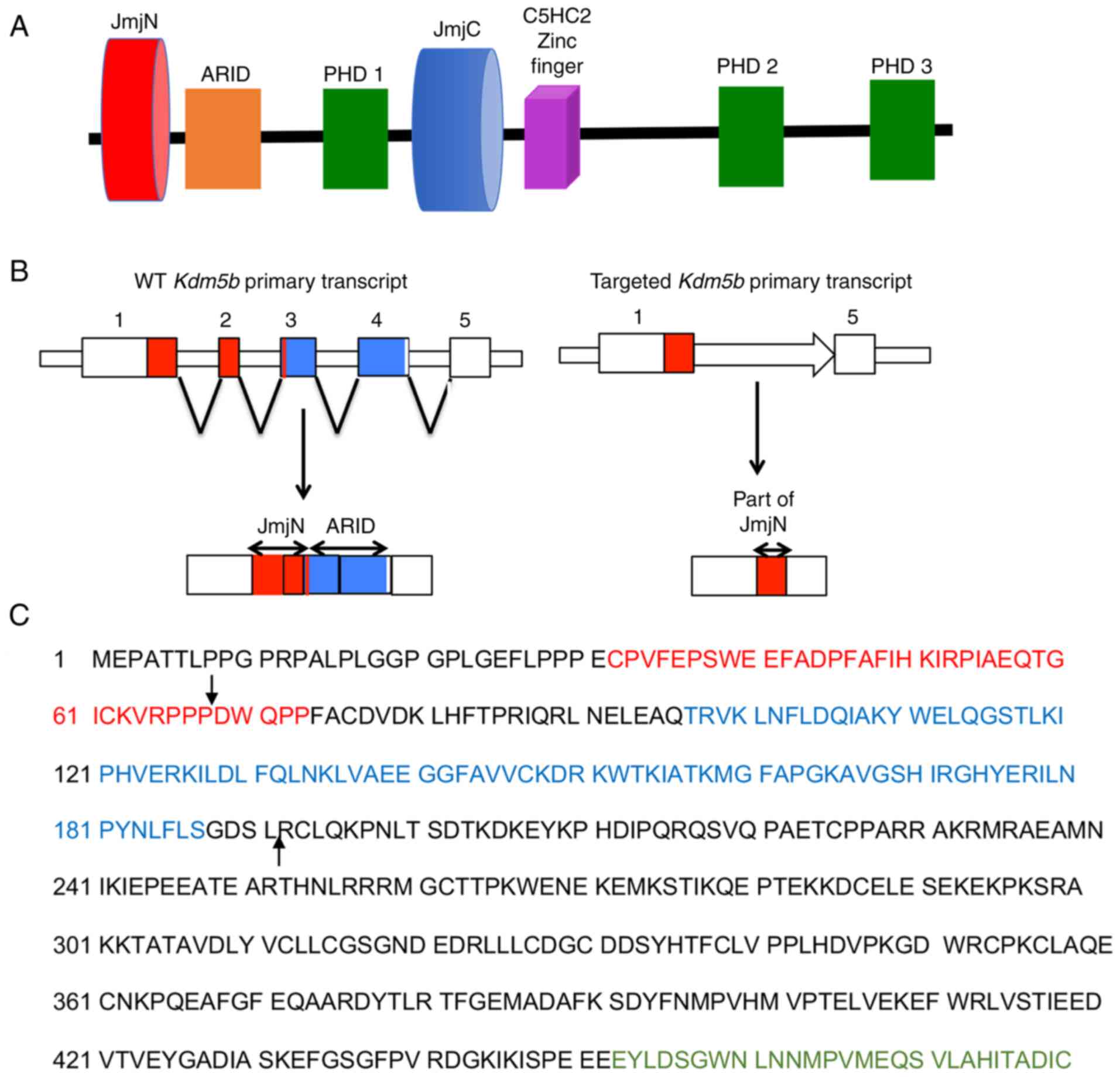
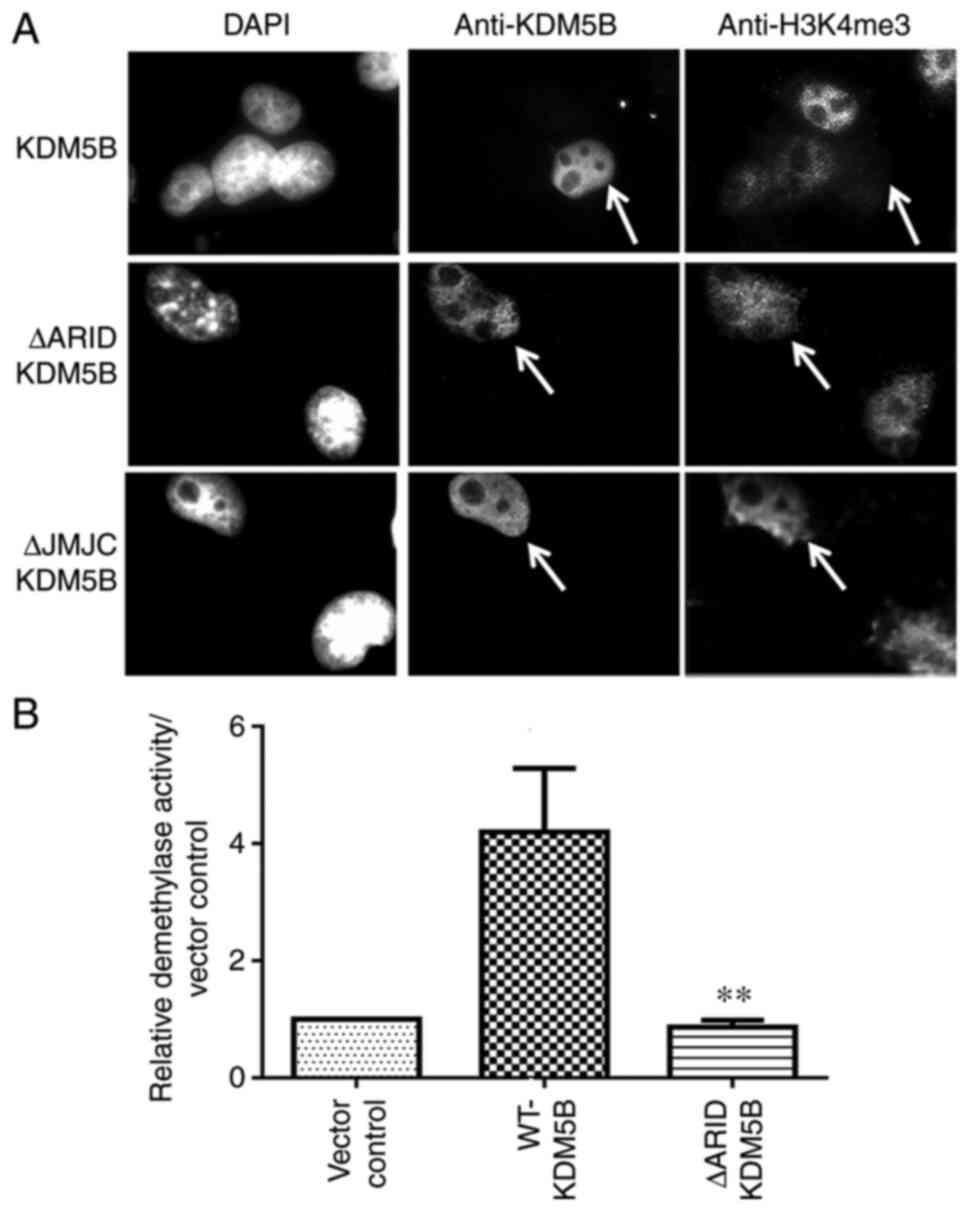
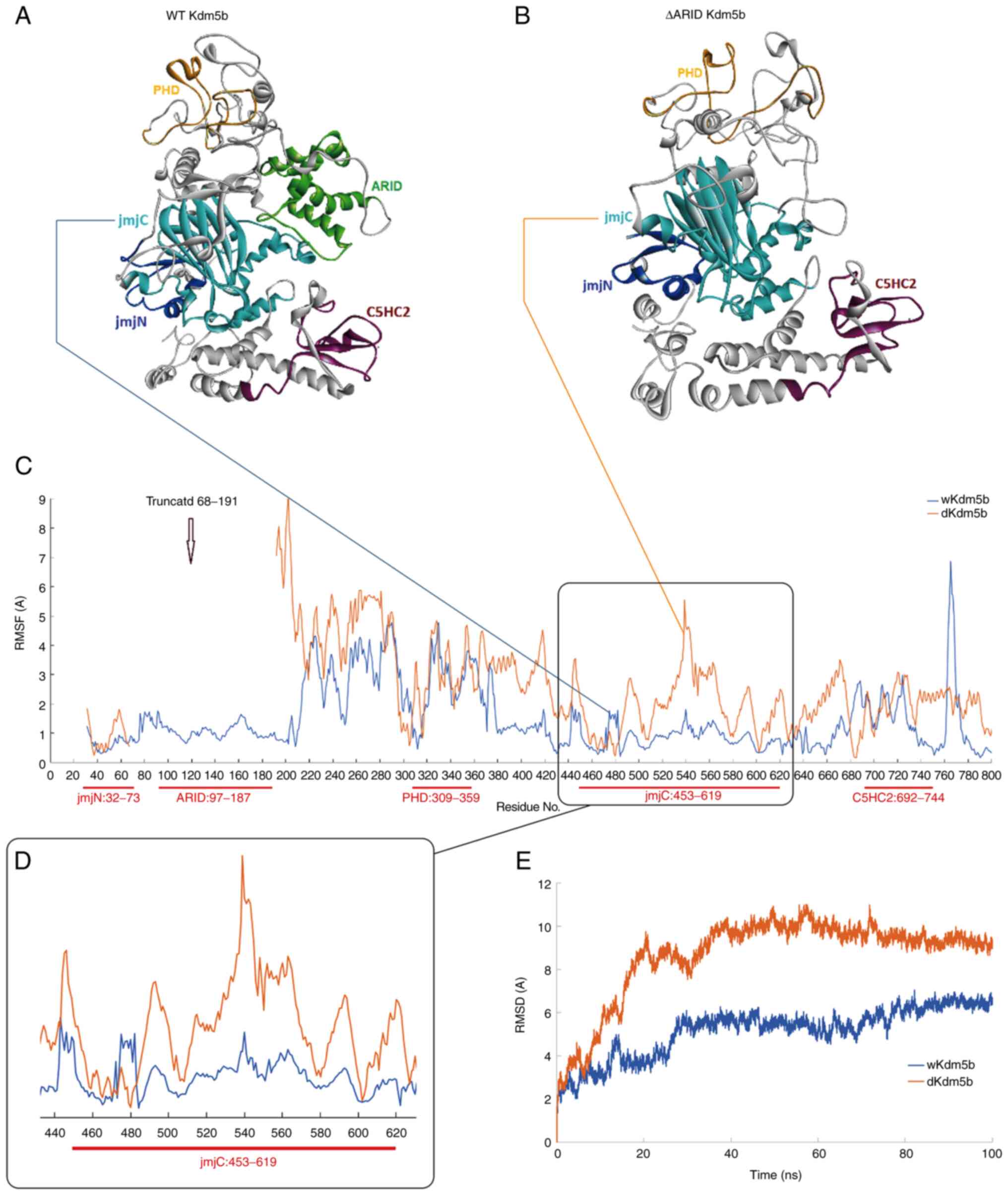
|
1
|
Bernstein BE, Kamal M, Lindblad-Toh K,
Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK,
Kulbokas EJ III, Gingeras TR, et al: Genomic maps and comparative
analysis of histone modifications in human and mouse. Cell.
120:169–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cruz C, Della Rosa M, Krueger C, Gao Q,
Horkai D, King M, Field L and Houseley J: Tri-methylation of
histone H3 lysine 4 facilitates gene expression in ageing cells.
Elife. 7:e340812018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roa CR and Dou Y: Hijacked in cancer: The
KMT2 (MLL) family of methyltransferases. Nat Rev Cancer.
15:334–346. 2015. View
Article : Google Scholar
|
|
4
|
Maiques-Diaz A and Somervaille TC: LSD1:
Biologic roles and therapeutic targeting. Epigenomics. 8:1103–1016.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harmeyer KM, Facompre ND, Herlyn M and
Basu D: JARID1 histone demethylases: Emerging targets in cancer.
Trends Cancer. 3:713–725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu PJ, Sundquist K, Baeckstrom D, Poulsom
R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P,
Freemont P and Taylor-Papadimitriou J: A novel gene (PLU-1)
containing highly conserved putative DNA/chromatin binding motifs
is specifically up-regulated in breast cancer. J Biol Chem.
274:15633–15645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamane K, Tateishi K, Klose RJ, Fang J,
Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P
and Zhang Y: PLU-1 is an H3K4 demethylase involved in
transcriptional repression and breast cancer cell proliferation.
Mol Cell. 25:801–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang Y, Zhu Z, Han G, Ye X, Xu B, Peng Z,
Ma Y, Yu Y, Lin H, Chen AP and Chen CD: JARID1B is a histone H3
lysine 4 demethylase up-regulated in prostate cancer. Proc Natl
Acad Sci USA. 104:19226–19231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xhabija B and Kidder BL: KDM5B is a master
regulator of the H3K4-methylome in stem cells, development and
cancer. Semin Cancer Biol. 57:79–85. 2019. View Article : Google Scholar :
|
|
10
|
Jose A, Shenoy GG, Sunil Rodrigues G,
Kumar NAN, Munisamy M, Thomas L, Kolesar J, Rai G, Rao PPN and Rao
M: Histone demethylase KDM5B as a therapeutic target for cancer
therapy. Cancers (Basel). 12:21212020. View Article : Google Scholar
|
|
11
|
Scibetta AG, Santangelo S, Coleman J, Hall
D, Chaplin T, Copier J, Catchpole S, Burchell J and
Taylor-Papadimitriou J: Functional analysis of the transcription
repressor PLU-1/JARID1B. Mol Cell Biol. 27:7220–7235. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu S, Teng YC, Yuan C, Wu YT, Chan MY,
Cheng AN, Lin PH, Juan LJ and Tsai MD: The ARID domain of H3K4
demethylase RBP2 binds to a DNA CCGCCC motif. Nat Struct Mol Bio.
15:419–421. 2008. View Article : Google Scholar
|
|
13
|
Klein BJ, Piao L, Xi Y, Rincon-Arano H,
Rothbart SB, Peng D, Wen H, Larson C, Zhang X, Zheng X, et al: The
histone-H3K4-specific demethylase KDM5B binds to its substrate and
product through distinct PHD fingers. Cell Rep. 6:315–335. 2014.
View Article : Google Scholar
|
|
14
|
Johansson C, Velupillai S, Tumber A,
Szykowska A, Hookway ES, Nowak RP, Strain-Damerell C, Gileadi C,
Philpott M, Burgess-Brown N, et al: Structural analysis of human
KDM5B guides histone demethylase inhibitor development. Nat Chem
Biol. 12:539–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Longbotham JE, Chio CM, Dharmarajan V,
Trnka MJ, Torres IO, Goswami D, Ruiz K, Burlingame AL, Griffin PR
and Fujimori DG: Histone H3 binding to the PHD1 domain of histone
demethylase KDM5A enables active site remodeling. Nat Commun.
10:942019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vinogradova M, Gehling VS, Gustafson A,
Arora S, Tindell CA, Wilson C, Williamson KE, Guler GD, Gangurde P,
Manieri W, et al: An inhibitor of KDM5 demethylases reduces
survival of drug-tolerant cancer cells. Nat Chem Biol. 12:531–538.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horton JR, Engstrom A, Zoeller EL, Liu X,
Shanks JR, Zhang X, Johns MA, Vertino PM, Fu H and Cheng X:
Characterization of a linked jumonji domain of the KDM5/JARID1
family of histone H3 lysine 4 demethylases. J Biol Chem.
291:2631–2646. 2016. View Article : Google Scholar :
|
|
18
|
Torres IO, Kuchenbecker KM, Nnadi CI,
Fletterick RJ, Kelly MJ and Fujimori DG: Histone demethylase KDM5A
is regulated by its reader domain through a positive-feedback
mechanism. Nat Commun. 6:62042015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albert M, Schmitz SU, Kooistra SM,
Malatesta M, Morales Torres C, Rekling JC, Johansen JV, Abarrategui
I and Helin K: The histone demethylase Jarid1b ensures faithful
mouse development by protecting developmental genes from aberrant
H3K4me3. PLoS Genet. 9:e10034612013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catchpole S, Spencer-Dene B, Hall D,
Santangelo S, Rosewell I, Guenatri M, Beatson R, Scibetta AG,
Burchell JM and Taylor-Papadimitriou J: PLU-1/JARID1B/KDM5B is
required for embryonic survival and contributes to cell
proliferation in the mammary gland and in ER+ breast
cancer cells. Int J Oncol. 38:1267–1277. 2011.PubMed/NCBI
|
|
21
|
Zou MR, Cao J, Liu Z, Huh SJ, Polyak K and
Yan Q: Histone demethylase jumonji AT-rich interactive domain 1B
(JARID1B) controls mammary gland development by regulating key
developmental and lineage specification genes. J Biol Chem.
289:17620–17633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madsen B, Spencer-Dene B, Poulsom R, Hall
D, Lu PJ, Scott K, Shaw AT, Burchell JM, Freemont P and
Taylor-Papadimitriou J: Characterisation and developmental
expression of mouse Plu-1, a homologue of a human nuclear protein
(PLU-1) which is specifically up-regulated in breast cancer. Mech
Dev. 119(Suppl 1): S239–S246. 2002. View Article : Google Scholar
|
|
23
|
Barrett A, Madsen B, Copier J, Lu PJ,
Cooper L, Scibetta AG, Burchell J and Taylor-Papadimitriou J: PLU-1
nuclear protein, which is upregulated in breast cancer, shows
restricted expression in normal human adult tissues: A new
cancer/testis antigen? Int J Cancer. 101:581–586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnold K, Bordoli L, Kopp J and Schwede T:
The SWISS-MODEL workspace: A web-based environment for protein
structure homology modelling. Bioinformatics. 22:195–201. 2006.
View Article : Google Scholar
|
|
25
|
Schwede T, Kopp J, Guex N and Peitsch M:
Swiss-Model: An automated protein homology-modeling server. Nucleic
Acids Res. 31:3381–3385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Case DA, Cheatham TE III, Darden T, Gohlke
H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B and Woods
RJ: The Amber biomolecular simulation programs. J Comput Chem.
26:1668–1688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Darden T, York D and Pedersen L: Particle
mesh Ewald: An N log (N) method for Ewald sums in large systems. J
Chem Phys. 98:10089–10092. 1993. View Article : Google Scholar
|
|
28
|
Lebrun N, Mehler-Jacob C, Poirier K,
Zordan C, Lacombe D, Carion N, Billuart P and Bienvenu T: Novel
KDM5B splice variants identified in patients with developmental
disorders: Functional consequences. Gene. 679:305–313. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q, Obana EA, Radomski KL, Sukumar G,
Wynder C, Dalgard CL and Doughty ML: Inhibition of the histone
demethylase Kdm5b promotes neurogenesis and derepresses Reln
(reelin) in neural stem cells from the adult subventricular zone of
mice. Mol Biol Cell. 27:627–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zamurrad S, Hatch HAM, Drelon C,
Belalcazar HM and Secombe J: A Drosophila model of intellectual
disability caused by mutations in the histone demethylase KDM5.
Cell Rep. 22:2359–2369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuźbicki Ł, Lange D, Stanek-Widera A and
Chwirot BW: Prognostic significance of RBP2-H1 variant of JARID1B
in melanoma. BMC Cancer. 17:8542017. View Article : Google Scholar
|
|
32
|
Yamamoto S, Wu Z, Russnes HG, Takagi S,
Peluffo G, Vaske C, Zhao X, Moen Vollan HK, Maruyama R, Ekram MB,
et al: JARID1B is a luminal lineage-driving oncogene in breast
cancer. Cancer Cell. 25:762–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayami S, Yoshimatsu M, Veerakumarasivam
A, Unoki M, Iwai Y, Tsunoda T, Field HI, Kelly JD, Neal DE, Yamaue
H, et al: Overexpression of the JmjC histone demethylase KDM5B in
human carcinogenesis: Involvement in the proliferation of cancer
cells through the E2F/RB pathway. Mol Cancer. 9:592010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen X, Zhuang Z, Zhang Y, Chen Z, Shen L,
Pu W, Chen L and Xu Z: JARID1B modulates lung cancer cell
proliferation and invasion by regulating p53 expression. Tumour
Biol. 36:7133–7142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang B, Qi G, Tang F, Yuan S, Wang Z,
Liang X, Li B, Yu S, Liu J, Huang Q, et al: JARID1B promotes
metastasis and epithelial-mesenchymal transition via PTEN/AKT
signaling in hepatocellular carcinoma cells. Oncotarget.
6:12723–12739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan G, Li S, Yue M, Li C and Kang Z:
Lysine demethylase 5B suppresses CC chemokine ligand 14 to promote
progression of colorectal cancer through the Wnt/β-catenin pathway.
Life Sci. 264:1187262021. View Article : Google Scholar
|
|
37
|
Shigekawa Y, Hayami S, Ueno M, Miyamoto A,
Suzaki N, Kawai M, Hirono S, Okada KI, Hamamoto R and Yamaue H:
Overexpression of KDM5B/JARID1B is associated with poor prognosis
in hepatocellular carcinoma. Oncotarget. 9:34320–34335. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Montano MM, Yeh IJ, Chen Y, Hernandez C,
Kiselar JG, de la Fuente M, Lawes AM, Nieman MT, Kiser PD,
Jacobberger J, et al: Inhibition of the histone demethylase, KDM5B,
directly induces re-expression of tumor suppressor protein HEXIM1
in cancer cells. Breast Cancer Res. 21:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sayegh J, Cao J, Zou MR, Morales A, Blair
LP, Norcia M, Hoyer D, Tackett AJ, Merkel JS and Yan Q:
Identification of small molecule inhibitors of Jumonji AT-rich
interactive domain 1B (JARID1B) histone demethylase by a sensitive
high throughput screen. J Biol Chem. 288:9408–9417. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tumber A, Nuzzi A, Hookway ES, Hatch SB,
Velupillai S, Johansson C, Kawamura A, Savitsky P, Yapp C,
Szykowska A, et al: Potent and selective KDM5 inhibitor stops
cellular demethylation of H3K4me3 at transcription start sites and
proliferation of MM1S myeloma cells. Cell Chem Biol. 24:371–380.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Højfeldt JW, Agger K and Helin K: Histone
lysine demethylases as targets for anticancer therapy. Nat Rev Drug
Discov. 12:917–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kristensen LH, Nielsen AL, Helgstrand C,
Lees M, Cloos P, Kastrup JS, Helin K, Olsen L and Gajhede M:
Studies of H3K4me3 demethylation by KDM5B/Jarid1B/PLU1 reveals
strong substrate recognition in vitro and identifies
2,4-pyridine-dicarboxylic acid as an in vitro and in cell
inhibitor. FEBS J. 279:1905–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|