Introduction
Numerous transcription factors have been found to
mediate key roles in hematopoiesis (1). Runt-related transcription factor 1
(RUNX1), which is also known as acute myeloid leukemia (AML)1, is
one of the key transcription factors for hematopoiesis. RUNX1 was
first cloned from the breakpoint of the t(8;21) chromosome
translocation in AML cases of the AML-M2 subtype of the
French-American-British (FAB) classification (2). Chromosome aberrations and mutations
in RUNX1 has been frequently reported to associate with leukemia
pathogenesis (3,4). The t(8;12) translocation rearranges
the RUNX1 locus to generate a fusion protein RUNX1-ETO (5), which trans-dominantly
interferes with normal RUNX1 function to facilitate leukemogenesis
(6,7). Point mutations in this locus that
result in loss-of-function or dominant-negative mutations in this
gene were frequently found in cases of sporadic AML,
myelodysplastic syndrome/myeloproliferative neoplasms or pedigrees
of familial platelet disorder with predisposition to AML
development (8-12).
Conventional disruption experiments previously
revealed that RUNX1 is required for the initial mid-gestation
development of hematopoietic stem cells from the hemogenic
endothelium (13-15). In addition, conditional knock-out
and knock-in techniques applied in adult mouse models demonstrated
the possible function of RUNX1 in regulating the size of
circulating and bone marrow myeloid precursor cell populations,
platelet production and T-/B-lymphoid development and function
(16-19). RUNX1 encodes a DNA-binding subunit
of the hetero-dimeric transcriptional factor complex known as
core-binding factor (CBF) (20,21). The N-terminus of RUNX1, the Runt
domain, is responsible for both association with the β subunit CBFβ
and binding to DNA at the consensus sequence TGT/cGGT. (22). By contrast, domains at the
C-terminus serve as the recruiting sites for regulatory co-factors
(23,24).
RUNX1 performs its biological functions through the
transcriptional regulation of its target genes, including
colony-stimulating factor 1 (CSF1/M-CSF) receptor, hematopoietic
transcription factors, such as PU.1, CCAAT/enhancer binding protein
α, cell cycle-related genes, such as p14ARF and
p21WAF1, microRNA (miR)-24, miR-223, IL-3 and granzyme B
(25-28). RUNX1 expression was found to be
upregulated during the differentiation of K562 leukemia cells
following treatment with tetradecanoylphorbol 13-acetate (TPA)
(29). TPA activates protein
kinase C and promotes cancer, such as papilloma and squamous cell
carcinoma, alongside the tumor initiator 7,12-dimetylbenzanthracene
(DMBA) (30).
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a secretory and/or membrane-bound protein that
has been extensively studied (31). TRAIL induces apoptosis by binding
to its specific receptors, death receptor 5 (DR5) and DR4, on the
target cell surface, where the resultant signal mediates
TRAIL-induced apoptosis by interacting with Fas associated via
death domain and activating caspases (32). TRAIL is a member of the TNF family
of cytokines that induces apoptosis (32). Previously, TNF-α, a TNF family
member, was reported to alter the lactate dehydrogenase (LDH)
isotype in non-Hodgkin's lymphomas and cell surface CD marker
profiles upstream of apoptosis induction in K562 cells (33,34). A number of previous studies also
support the notion that TRAIL has the capability to preferentially
kill tumor cells instead of non-cancerous normal cells, such that
recombinant TRAIL is now under exploration in a number of on-going
clinical trials for the treatment of malignant neoplasms (35,36). TRAIL efficacy was found to be
increased by co-treatment with other therapeutic agents, such as
histone deacetylase inhibitors (HDACis) in Jurkat T-cell leukemia
cells (37). Histone deacetylases
(HDACs) reduce histone acetylation and serve as transcriptional
repressors (38). Oncoproteins,
such as EWS-Fli1, aberrantly disrupt physiological transcriptional
conditions by cooperating with HDACs (38). Therefore, HDACis can inhibit the
function and expression of oncogenes and have potential as
anti-tumor agents. To date, five HDACis, namely valproic acid
(VPA), vorinostat, panobinostat, belinostat, and romidepsin, have
been approved by the Food and Drug Administration in the United
States for the treatment of cutaneous T-cell lymphomas, multiple
myeloma or peripheral T-cell lymphomas (39). TRAIL is endogenously expressed by
numerous cell types in the immune system, including natural killer
(NK) cells, T lymphocytes, natural killer T cells, dendritic cells,
and macrophages (40). This
protein participates in the immune surveillance system against
malignant tumors (40), because
TRAIL- or TRAIL-R-deficient mice frequently develop lymphomas,
exhibit xenografted carcinogen-induced tumors that grow faster and
show increased lymph node metastases in xenograft models (41-43). Cloning of the promoter region of
the human TRAIL gene revealed its structure, which contains
putative binding sites for a number of transcription factors,
including nuclear factor of activated T-cells, activator protein 1
(AP-1) and specificity protein 1 (Sp1) (44). Specificity protein 1, NF-κB,
FOXO3a, c-Myc and p53 have all been reported to regulate the
transcription of the TRAIL gene (45-47). However, details of the regulatory
mechanisms underlying TRAIL expression remain to be fully
elucidated. In the RUNX1-ETO-inducible U937 pro-monocytic leukemia
cell line, RUNX1-ETO induction was reported to upregulate TRAIL
expression (48). However, the
effects of wild-type RUNX1 on TRAIL regulation under physiological
conditions remain unclear.
In the present study, the transcriptional
relationship between RUNX1 and TRAIL was investigated using RT-qPCR
and luciferase reporter assays in a panel of leukemia cell lines.
In addition, the biological function of TRAIL on leukemia cell
lines was assessed.
Materials and methods
DNA and plasmid constructs
Hemagglutinin (HA)-tagged mouse RUNX1b encoded in
the pRc/CMV plasmid (Invitrogen; Thermo Fisher Scientific, Inc.)
was previously described (49).
HA-tagged mouse RUNX1b was first digested by HindIII and
EcoRI before being filled-in, where the complementary
nucleotides were added to the 5′-overhang of the digest-end using
DNA polymerase I (Klenow; New England Biolabs). The filled-in DNA
fragment was then subjected to blunt-end ligation to the
NotI-digested/filled-in site of the pRc/CMV plasmid. The
sequence of the plasmid construct was analyzed and confirmed by
Sanger sequencing using the 3500 Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and BigDye Terminator
v1.1 cycle sequencing kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). FLAG-tagged mouse CBFβ (CBFβ-FLAG)
encoded in pRc/CMV was also produced as previously described
(10,50). The mouse CBFβ-FLAG was amplified
by PCR using AmpliTaq Gold™ DNA polymerase (Thermo Fisher
Scientific, Inc.) before being ligated into the TA-cloning site of
the pCR2.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.). The
CBFβ-FLAG DNA was then excised from the plasmid by XbaI and
SpeI and subjected to the cohesive-end ligation at the
XbaI site of the pRc/CMV plasmid. The sequence of the
construct was analyzed and confirmed by Sanger sequencing using the
3500 Genetic Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and BigDye Terminator v1.1 cycle sequencing kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Runx1 and
CBFβ are highly homologous between human and mouse (51). The amino acid sequence of human
RUNX1 holds 96.4% sequence identity to that of murine RUNX1
(accession no. NP_001001890.1 for human RUNX1; accession no.
NP_001104493.1 for murine RUNX1). Similarly, the amino acid
sequence of human CBFβ has 98.3% sequence identity to mouse CBFβ
(accession no. NP_001746.1 for human CBFβ; accession no.
NP_001154930.1 for mouse CBFβ). Mouse RUNX1 and CBFβ can both
regulate human transcription of RUNX1 target genes, since ectopic
human RUNX1 expression has been previously demonstrated to rescue
the hematopoietic function lost in RUNX1-deficient mouse cells
(23,52,53). Therefore, RUNX1 and CBFβ are
considered to be functionally interchangeable between humans and
mice, at least under these previous experimental conditions.
RUNX1-ETO cDNA kindly donated by Dr. Misao Ohki (Chromatin Function
in Leukemogenesis Project and Cancer Genomics Division, National
Cancer Center Research Institute, Tokyo, Japan) was amplified with
KpnI site-linked oligonucleotides (forward, 5′-GGG GTA CCG
TGA TGC CGT ATC CCC GTA GAT GCC-3′ and reverse, 5′-GGG GTA CCC TAG
CGA GGG GTT GTC TCT ATG GTG-3′) using PrimeSTAR GXL DNA polymerase
(cat. no. R050A; Takara Bio Inc.) by PCR (98°C for 10 sec, followed
by 30 cycles of 98°C for 10 sec, 60°C for 15 sec, 68°C for 60 sec,
followed by 68°C 5 min). It was then digested by KpnI and
subcloned into the KpnI site of the pcDNA3.1 plasmid
(Invitrogen; Thermo Fisher Scientific Inc.). The sequence of
construct was confirmed by Sanger sequencing using 3500 the Genetic
Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). pcDNA3.1 and pRc/CMV are both
mammalian expression vectors that express exogenous genes by using
the same CMV promoter and enhancer sequences. Therefore, expression
levels of the exogenous genes following transfection with these two
vectors are considered to be comparable. However, the pcDNA3.1
plasmid contains more restriction enzyme sites in its multiple
cloning site compared with pRc/CMV. To generate the pTRAIL PF
reporter construct, the human wild-type TRAIL promoter obtained
from the human genome library, a λPS library from human peripheral
blood leukocytes (MoBiTec GmbH) using lambda phage λPS vector
containing human genome DNA was inserted into the reporter plasmid
pGV-B2, which contains a firefly luciferase gene (Toyo B-Net Co.,
Ltd.; https://www.toyo-b-net.co.jp/bio/product-pgv2.html).
The human TRAIL promoter 5′-deletion mutants pTRAIL/-1523,
pTRAIL/-819 and pTRAIL/-165 (40)
were kindly donated by Dr B Mark Evers (Department of Surgery,
University of Texas Medical Branch, Galveston, USA), which were
inserted into the reporter plasmid pGL2m, derived from pGL2-basic
by removal of the cryptic AP-1 site. Site-directed mutagenesis of
the plasmids was performed using PCR-based methods. Mt1, where the
wild-type sequence of ACCACA was changed to ATAACG and Mt2, where
the wild-type sequences TGTGGT was changed to CGTTAT, were
generated by site-directed mutagenesis (54) as aforementioned. The plasmid was
amplified by inverse-PCR with oligonucleotide primers (Mt1 forward,
5′-GTA GCA TGA GAA AAA taA CgT ATG GAA GTT TCA G-3′ and reverse,
5′-CTG AAA CTT CCA TAc GTt aTT TTT CTC ATG CTA C-3′ and Mt2
forward, 5′-GAG GCT TAG AGC TCc GTt aTA GAA TGA GGA TAT G-3′ and
reverse, 5′-CAT ATC CTC ATT CTA taA CgG AGC TCT AAG CCT C-3′)
containing the planned mutation shown in small letters using
PrimeStar GXL DNA polymerase (cat. no. R050A; Takara Bio Inc.),
before the template plasmid was destroyed by digestion with
DpnI. These amplified PCR products underwent spontaneous
circularisation due to the 'sticky' 5′ ends in the primer
sequences. These circularised DNA were used to transform competent
DH5α E.coli cells to produce intact recombinant plasmids.
The constructs were confirmed by Sanger sequencing using the 3500
Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and BigDye Terminator v1.1 cycle sequencing kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Cell Culture
HeLa cells (Riken BioResource Center) were cultured
in DMEM (FUJIFILM Wako Pure Chemical Corporation) with 10% FBS
(Sigma-Aldrich; Merck KGaA) and 2 mmol/l glutamine (Gibco; Thermo
Fisher Scientific, Inc.). The leukemia cell line K562 was cultured
in DMEM/F-12 (FUJIFILM Wako Pure Chemical Corporation) with 10% FBS
and 2 mmol/l glutamine. The Kasumi-1 leukemia cell line (55) carrying t(8;21), which expresses
the RUNX1-ETO fusion protein (kindly provided by Dr. Toshiya Inaba,
Department of Molecular Oncology and Leukemia Program Project,
Research Institute for Radiation Biology and Medicine, Hiroshima
University, Hiroshima, Japan) and KG-1, an AML cell line without
RUNX1-ETO (Riken BioResource Center), were cultured in RPMI-1640
with 10% FBS. SKNO-1 leukemia cells carrying the t(8;21)
translocation and express the RUNX1-ETO fusion protein, were
obtained from the Japanese Collection of Research Bioresources Cell
Bank and cultured in RPMI-1640 supplemented with 10% FBS and 10
ng/ml granulocyte macrophage (GM)-CSF (cat. no. 300-03; PeproTech,
Inc.). All cells were maintained at 37°C in humidified air with 5%
CO2.
Differentiation of K562 cells
K562 cells were treated with 25 nM TPA (cat. no.
P8139; Sigma-Aldrich; Merck KGaA) for 48 h at 37°C (29), subjected to the centrifugation by
cytospin-preparation at 78 × g for 6 min at room temperature
(Shandon Cytospin 3 Centrifuge; Thermo Fisher Scientific, Inc.) and
then processed with May-Gruenwald-Giemsa staining (Giemsa for 30
min at room temperature) followed by light microscopic examination
with an Eclipse E800 optical microscope (Nikon Corporation). The
magnification was ×400. The morphological changes of
differentiation, including increased cell size and
cytoplasm-to-nucleus ratio, formation of numerous vesicles and
lobulated nuclei, was observed. TPA was dissolved in DMSO, which
was added into control samples to a final concentration of 0.1%
volume of medium.
Luciferase assay
Expression plasmids [RUNX1 in pRc/CMV (0.75
µg) and CBFβ in pRc/CMV (0.25 µg)] and reporter
plasmids (1.25 µg; pTRAIL PF, pTRAIL/-1523, pTRAIL/-819,
pTRAIL/-165, Mt1, Mt2 and pGL2m) were transfected into
5×104 HeLa and K562 cells using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). phRL-TK plasmid
(0.0625 µg; cat. no. E6241; Promega Corporation) was
co-transfected as an internal control. After incubation at 37°C for
48 h, the cells were lysed using passive lysis buffer contained in
the Dual-Luciferase® Reporter Assay System (cat. no.
E1960; Promega Corporation). Luciferase activities were measured
using the Dual-Luciferase® Reporter Assay System with a
luminometer (Lumat LB 9507; Titertek-Berthold). The cell lysate (20
µl) were mixed with luciferase assay reagent II (50
µl) and Photinus pyralis luciferase activity was
measured with a luminometer. Stop & Glo reagent (50 µl)
was then added to the mixture and Renilla reniformis
luciferase activity driven by the TK promoter was measured with a
luminometer. The luciferase activities were normalized by the
activity of TK.
Transfections
For K562 cells (1×106), the expression
plasmids for RUNX1 in pRc/CMV (3 µg) and for CBFβ in pRc/CMV
(2 µg) or RUNX1 small interfering RNA (siRNA; 300 pmol) was
transfected by electroporation (program T-016 of Nucleofector™ 2b
device; Lonza Group, Ltd.) in 100 µl buffer contained within
the AMAXA Cell Line Nucleofector Kit V (cat. no. VCA-1003; Lonza
Group, Ltd.) in a cuvette equipped with aluminium electrodes of the
kit at room temperature. Nucleofector™ 2b device and AMAXA Cell
Line Nucleofector Kit V were used for the transfection of K562,
Kasumi-1 and KG-1 cells. For Kasumi-1 cells (1×106),
RUNX1-ETO siRNA (300 pmol) was transfected using program L-014 of
Nucleofector™ 2b in 100 µl RPMI-1640. For KG-1 cells
(1×106), RUNX1-ETO in pcDNA3.1 plasmid (2 µg) was
transfected using program V-001 of Nucleofector™ 2b (Lonza) in 100
µl RPMI-1640. After electroporation, all cells were
immediately collected with 500 µl RPMI1640 containing 10%
FBS as recovery medium at room temperature. K562 cells were
analyzed 48 h after RUNX1 and CBFβ transfection or 72 h after RUNX1
RNA siRNA transfection after electroporation. Kasumi-1 and KG-1
cells were analyzed 24 h after electroporation.
RUNX1 siRNA (assay ID, HSS141472; sense, 5′-UCU GGU
CAC UGU GAU GGC UGG CAA U-3′ and antisense, 5′-AUU GCC AGC CAU CAC
AGU GAC CAG A-3′) was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. Stealth RNAi™ siRNA Negative Control Hi GC duplex
was used as negative control (cat. no. 12935400; Invitrogen, Thermo
Fisher Scientific, Inc.). Whenever RUNX1 cDNA and CBFβ cDNA were
transfected, the empty pRc/CMV plasmid was used as a negative
control because RUNX1 cDNA and CBFβ cDNA were inserted into this
vector.
For luciferase reporter assays, RUNX1 cDNA and CBFβ
cDNA were expressed as effectors. Empty pRc/CMV was used as the
corresponding negative control. RUNX1-ETO siRNA was synthesized by
Eurofins Scientific (Sense, 5′-CCU CGA AAU CGU ACU GAG AAG-3′ and
antisense, 5′-UCU CAG UAC GAU UUC GAG GUU-3′) and transfected. As a
negative control, Stealth RNAi™ siRNA Negative Control Hi GC duplex
was also used. RUNX1-ETO cDNA, which was inserted into the pcDNA3.1
plasmids, had empty pcDNA3.1 as the control vector.
Measurement of mRNA expression
Expression plasmids were transfected into K562 cells
using Nucleofector™ 2b. After 48 h, total RNA was isolated from
cells using Isogen (cat. no. 311-02501) or Isogen II (cat. no.
311-07361; both Nippon Gene Co., Ltd.). cDNA was generated using
SuperScript III (cat. no. 18080044) or IV (cat. no. 18090010)
reverse transcriptases (Thermo Fisher Scientific, Inc.), oligo
(dT)15 primer (cat. no. 3805; Takara Bio, Inc.) and dNTP
mixture (cat. no. 4030; Takara Bio, Inc.) at 55°C for 10 min
followed by 80°C for 10 min. cDNA was quantified using Thunderbird™
SYBR™ qPCR mix (cat. no. QPS-201; Toyobo Life Science), specific
primers synthesized by Eurofins and StepOnePlus (Thermo Fisher
Scientific, Inc.). The thermocycling conditions were 95°C for 10
sec, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min,
then 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. Primer
sequences are listed as follows: TRAIL forward, 5′-GAG AGT AGC AGC
TCA CAT AAC T-3′ and reverse, 5′-TCA TGG ATG ACC AGT TCA CCA TT-3′;
RUNX1 forward, 5′-CTG AGC CCA GGC AAG ATG AG-3′ and reverse, 5′-GTC
TTG TTG CAG CGC CAG TG-3′; CBFβ forward, 5′-TCG AGA ACG AGG AGT TCT
TCA GGA-3′ and reverse, 5′-AGG CGT TCT GGA AGC GTG TCT-3′;
RUNX1/ETO forward, 5′-CAC TGT CTT CAC AAA CCC AC-3′ and reverse,
5′-ATG AAC TGG TTC TTG GAG CTC CTT-3′; DR4 forward, 5′-ACC TTC AAG
TTT GTC GTC GTC-3′ and reverse, 5′-CCA AAG GGC TAT GTT CCC ATT-3′;
DR5 forward, 5′-GCC CCA CAA CAA AAG AGG TC-3′ and reverse, 5′-AGG
TCA TTC CAG TGA GTG CTA-3′; β-actin forward, 5′-GCT GTG CTA CGT CGC
CCT G-3′ and reverse, 5′-GGA GGA GCT GGA AGC AGC C-3′ and GAPDH
forward, 5′-TGC ACC ACC AAC TGC TTA GC-3′ and reverse, 5′-GGC ATG
GAC TGT GGT CAT GAG-3′. β-actin and GAPDH were used as the
reference genes. The quantification method used was relative
standard curve (56).
Cell viability assay
The recombinant human soluble TRAIL protein was a
lyophilized powder purchased from PeproTech, Inc. The recombinant
TRAIL was dissolved in water, diluted in PBS and used for
experimentation. Equivalent volumes of PBS was added in the control
groups. Cells were treated with recombinant human soluble TRAIL for
3 days (at 10, 50, or 100 ng/ml) at 37°C, before cell numbers were
counted using a Bürker-Turk counting chamber (Erma, Inc.) under an
Olympus CK40 inverted light microscope (Olympus Corporation),
capable of phase contrast observation, using ×100 magnification.
Lactate dehydrogenase (LDH) assay was performed using the
Cytotoxicity LDH Assay Kit-WST (cat. no. CK12; Dojindo Molecular
Technologies, Inc.). Collected cells (2×105 cells) were
suspended in PBS with 0.1% Triton X-100 and 10 µg/ml of
propidium iodide (PI) at room temperature for 5 min, before DNA
content was analyzed by BD FACS Canto II and BD FACSDiva software
v6.1.3 (BD Biosciences) (57).
TRAIL was co-treated with 1 mM sodium butyrate (NaB; Sigma-Aldrich;
Merck KGaA), 2 mM VPA (cat. no. 197-09722; FUJIFILM Wako Pure
Chemical Corporation) and 20 µM caspase inhibitor z-VAD-fmk
(cat. no. 3175-v; Peptide Institute, Inc.) for 72 h at 37°C
(37). NaB and VPA were dissolved
in distilled water, whilst z-VAD-fmk was dissolved in DMSO. When
zVAD-fmk was used, solvent DMSO was added in control samples and
the final concentration of DMSO was 0.1% volume of medium.
Preparation of recombinant proteins
To produce hexa-histidine (His6)-tagged
RUNX1 proteins, partial cDNA encoding the Runt domain (amino acids
24-189) of mouse RUNX1b originally in the pRc/CMV plasmid was
amplified by PCR using PrimeSTAR GXL DNA polymerase (cat. no.
R050A; Takara Bio Inc.), digested by KpnI and inserted into
the pQE30 plasmid (Qiagen, Inc.). To generate
His6-tagged CBFβ, full length mouse CBFβ originally in
the pRc/CMV plasmid was amplified by PCR using PrimeSTAR GXL DNA
polymerase (cat. no. R050A; Takara Bio Inc.), digested by
KpnI and inserted into the pET3a vector (Qiagen, Inc.).
His6-tagged proteins of RUNX1 containing the Runt domain
(amino acids 24-189) and CBFβ were expressed in the XL-1
Blue strain of E.coli and resuspended in a buffer (4 ml)
containing 0.1 M sodium phosphate, 10 mM Tris, 6 M guanidine-HCl,
0.1% NP-40, 1 mM PMSF and 10 mM 2-mercaptoethanol and adjusted to
Ph 8.0 with 6 N NaOH. The total volume of the lysate was mixed with
300 µl Ni-NTA agarose beads (>15 µmol
Ni2+/ml gel; 50% slurry; cat. no. 145-09681; FUJIFILM
Wako Pure Chemical Corporation) for 1 h at 4°C and purified using a
100-ml syringe (cat. no. 08-649; Nipro Medical Corporation) with
0.34 mm chromatography paper (cat. no. 3030917; Cytiva) as a
column. The column was washed with buffer A plus 0.8 mM imidazole
and His-tagged proteins were eluted at 4°C in buffer B consisting
of buffer A with 250 mM imidazole and 20% glycerol. These
procedures were performed as described previously (58). The eluted proteins (10 µl)
were resolved on 12.5% SDS-PAGE and stained with Coomassie
brilliant blue staining. The His-tagged protein (His-tagged RUNX1,
18 kDa; His-tagged CBFβ, 21 kDa) amounts were estimated by
comparing with the BSA (66 kDa; Sigma-Aldrich; Merck KGaA) band
signal intensity visually on the SDS-PAGE gel.
Electrophoresis mobility shift assay
(EMSA)
EMSA was performed using the LightShift
Chemiluminescent EMSA kit (cat. no. 20148 and 89880; Thermo Fisher
Scientific, Inc.). DNA probes (Eurofins Scientific) were prepared
by annealing the following oligonucleotides: Control (CT),
biotin-5′-GTC TGT GGT TTC TGT GGT TTC TGT GGT TT-3′ and 5′-AAA CCA
CAG AAA CCA CAG AAA CCA CAG AC-3′; Wild-type (WT)1, biotin-5′-GAA
AAA CCA CAT ATG
G-3′ and 5′-CCA TAT GTG
GTT TTT C-3′; mutant (MT)1, biotin-5′-GAA AAA TAA CGT ATG G-3′ and 5′-CCA
TAC GTT ATT TTT
C-3′; WT2, biotin-5′-AGC TCT GTG GTA GAA T-3′ and 5′-ATT
CTA CCA CAG AGC
T-3′ and MT2, biotin-5′-AGC TCC GTT ATA GAA T-3′ and
5′-ATTCTATAACGGAGCT-3′. The DNA probes
(0.8 pmol) and recombinant His6-tagged RUNX1 (15 ng) and
His6-tagged CBFβ (60 ng) were reacted in 1X binding
buffer (cat. no. 20148A) and 50 ng/ml poly (dI-dC; cat. no. 20148E)
supplied by the manufacturer in a 10-µl reaction volume at
15°C for 30 min. The reaction mixture was resolved on a 10%
polyacrylamide gel in 0.5X TBE buffer at 4°C and transferred onto a
nylon membrane (cat. no. 10416196; Cytiva), fixed using a UV
cross-linker (UV Stratalinker 2400 (Stratagene; Agilent
Technologies, Inc.) under the auto cross-link condition of 1.2 mJ
(×100), 25-50 sec at room temperature and then reacted with
streptavidin-HRP (cat. no. 89880) for 15 min at room temperature.
The signal was detected using the Chemiluminescent substrate
solution (cat. no. 89880) using an ImageQuant LAS 500 (Cytiva).
Bioinformatics analysis
TRAIL expression in bone marrow samples from
patients with AML was analyzed by surveying the BloodPool: AML
samples with normal cells data set of the BloodSpot (https://servers.binf.ku.dk/bloodspot/)
database (59) using data of
GSE42519 (60), GSE13159
(61,62), GSE15434 (63), GSE61804 (64), GS E14468 (65-67) and The Cancer Genome Atlas (TCGA)
database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga).
Statistical analysis
Experiments were repeated three times and
statistically analyzed using a two-tailed, unpaired Student's
t-test by Microsoft Excel 2016 or 2019 (Microsoft Corporation) or
one-way ANOVA followed by Tukey's post hoc test by RStudio Desktop
2021.09.1+372 (RStudio; https://www.rstudio.com). Samples were adjudged to be
significantly different at P<0.05. The results were presented as
the mean ± standard deviation.
Results
TRAIL expression is increased after K562
differentiation induced by TPA stimulation in a RUNX1-dependent
manner
The human leukemia cell line K562 can be induced to
differentiate into cells of a megakaryocytic lineage, where RUNX1
expression was found to be upregulated during this process
(29). To investigate if TRAIL
expression was also affected by this differentiation process, K562
cells was stimulated with 25 nM tetradecanoylphorbol 13-acetate
(TPA) for 48 h, following which morphological changes, including
increased cell size and cytoplasm-to-nucleus ratio, many vesicles
and lobulated nuclei, were observed (Fig. 1A). TRAIL expression was next
measured in these cells. TRAIL expression was significantly
increased after TPA stimulation, which appeared to be at least
partially dependent on RUNX1 (Fig.
1B), since RUNX1 knockdown significantly reversed the
TPA-induced increase in TRAIL. In the presence of TPA, RUNX1
expression was also significantly reduced by transfection with
siRNA targeting RUNX1 compared with that in cells transfected with
negative siRNA (Fig. 1C). The
effects of RUNX1 siRNA transfection on K562 cell viability and cell
cycle progression without TPA treatment was subsequently examined.
RUNX1 siRNA did not alter cell viability or cell cycle progression
compared with those in cells after control siRNA transfection in
this experimental condition (Fig.
S1A and B). In addition, TRAIL transcriptional induction in
K562 cells after differentiation may be dependent on increased
RUNX1 expression. However, the effects of RUNX1 siRNA were
negligible without TPA treatment, which could be explained by the
low basal endogenous RUNX1 expression levels in K562 cells.
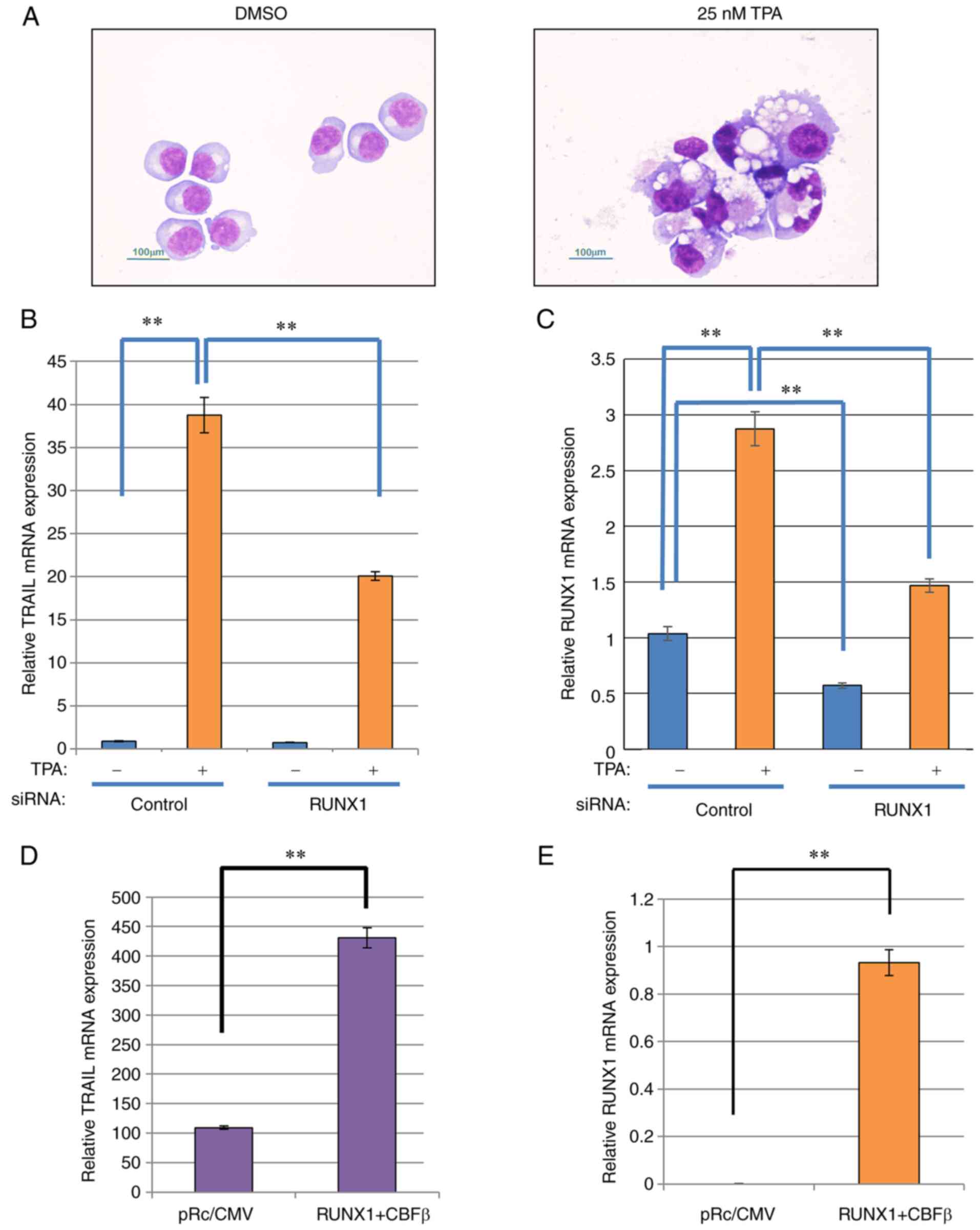 | Figure 1TRAIL expression is increased after
K562 differentiation model in a RUNX1-dependent manner. (A)
May-Gruenwald-Giemsa staining of the TPA-treated K562 cells. The
cells were treated with TPA, attached to glass slides using
Cytospin 3 and examined using May-Gruenwald-Giemsa staining. Scale
bars, 100 µm. (B and C) RUNX1 siRNA was transfected into
K562 cells by electroporation. After 24 h, the cells were treated
with TPA for 48 h for differentiation after which the relative
expression of (B) TRAIL and (C) RUNX1 was measured by RT-qPCR using
actin for normalization. (D and E) RUNX1 and CBFβ expression
plasmids were co-transfected into K562 cells by electroporation.
After 48 h, total RNA was prepared and the relative expression of
(D) TRAIL and (E) RUNX1 was measured by RT-qPCR using actin for
normalization. **P<0.01. TPA, tetradecanoylphorbol
13-acetate; siRNA, small interfering RNA; RUNX1, Runt-related
transcription factor 1; CBFβ, core-binding factor β; TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; RT-qPCR, reverse
transcription-quantitative PCR. |
To verify the dependency of this observed TRAIL
transcriptional induction on RUNX1 expression, mouse RUNX1 was
exogenously expressed in K562 cells. The transfection efficiency of
the individual RUNX1 and CBFβ plasmids into K562 cells was first
confirmed by the observed increased RUNX1 and CBFβ expression
compared with that in their corresponding control
plasmid-transfected cells (Fig.
S1C). The transfection efficiency in K562 cells was ~80%. As
shown in Fig. 1D and E, ectopic
expression of mouse RUNX1 along-side its dimerization partner,
CBFβ, into K562 cells resulted in a significant increase in
endogenous TRAIL and RUNX1 mRNA expression. However, the expression
of the TRAIL receptors DR4 and DR5 was not affected by ectopic
RUNX1 and CBFβ expression (Fig.
S1D). Therefore, these data suggest that TRAIL expression
induction is dependent on RUNX1 expression.
RUNX1 activates the TRAIL promoter
To determine if RUNX1 can activate TRAIL
transcription through the TRAIL promoter sequences, promoter
constructs of human TRAIL was subjected to a series of luciferase
reporter assays. Human TRAIL promoter sequences containing 1523
bases upstream of the transcriptional start site of the TRAIL gene
(Fig. 2A) were cloned as
previously described (44), where
it was established that this fragment can function as a regulatory
cis-element (44).
Although the transcription of a number of TRAIL variants was
reported to start ~30 bp downstream of the start site, the previous
report (44) stated the
transcription start site to be at the +1 position of the TRAIL
promoter. Luciferase reporter assay using this DNA fragment of
pTRAIL PF revealed that co-expression with RUNX1 and its co-factor
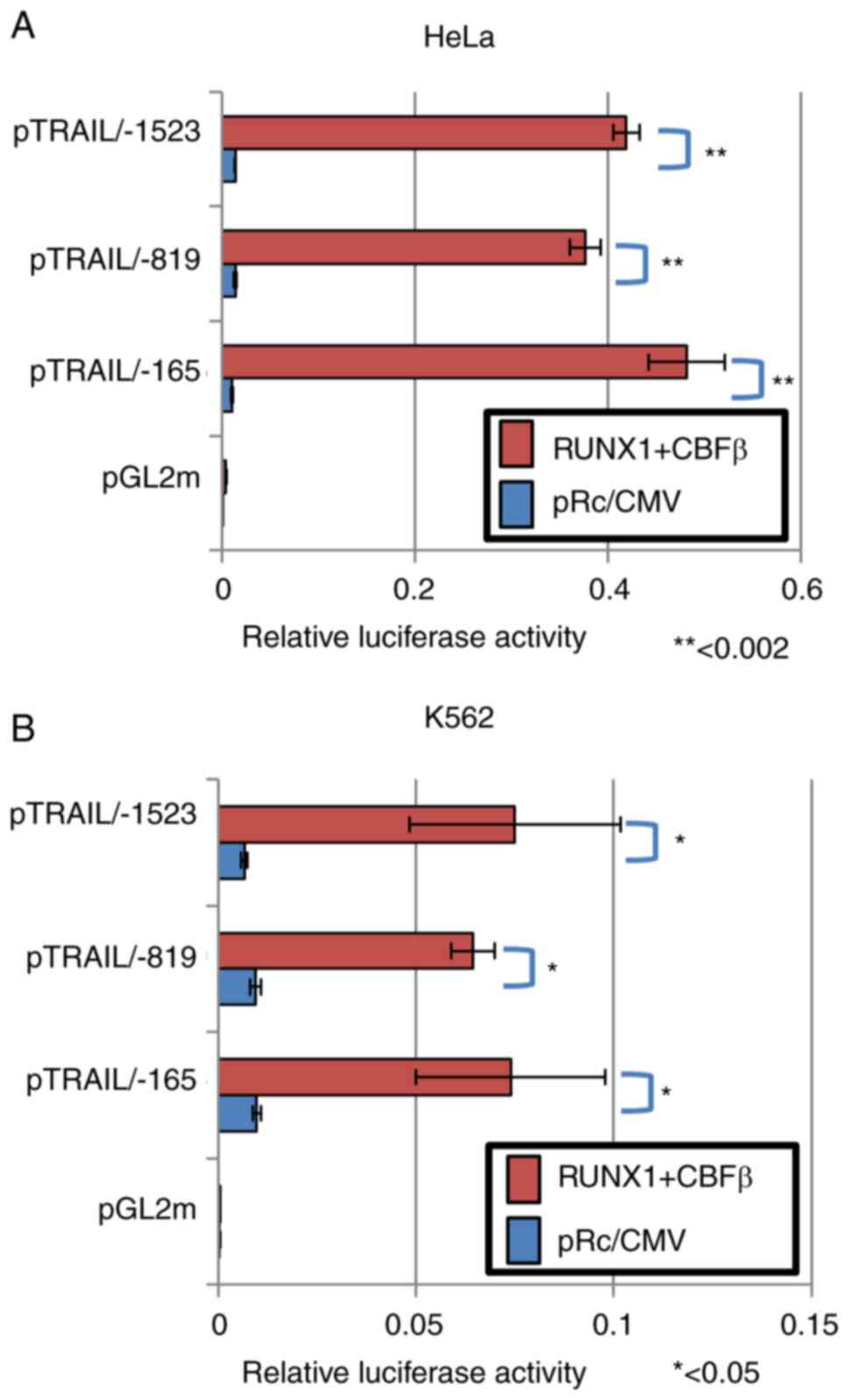
CBFβ significantly activated the human TRAIL promoter (Fig. 2B). The transfection efficiency in
HeLa cells was ~30%. Since the effector plasmids and reporter
plasmids were co-transfected into the cells, sufficient signal
values were detected even though the transfection efficiency was
low. This activation occurred in a CBFβ-dependent manner, because
ectopic transfection with either CBFβ or RUNX1 alone only weakly
and insignificantly activated TRAIL transcription, unlike when both
were transfected together (Fig.
2C). Therefore, the RUNX1/CBFβ combination may function as a
transcription factor complex of the human TRAIL gene. This is
consistent with a previous report, which stated that RUNX1 encodes
a DNA-binding subunit of the CBF complex (20,21), where the CBF complex requires both
RUNX1 and CBFβ to fully function as a transcription factor.
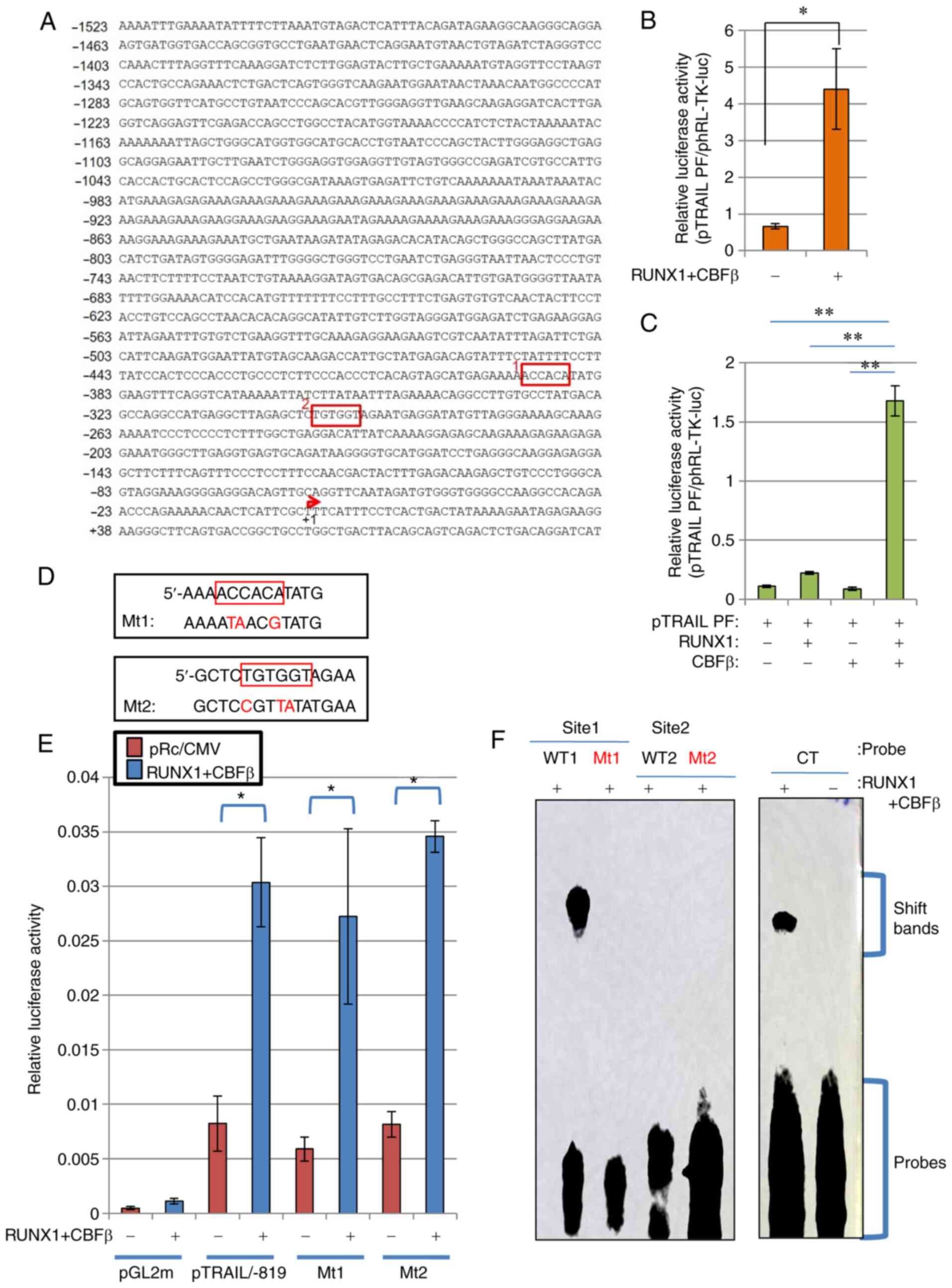 | Figure 2RUNX1 upregulates TRAIL transcription
independent of the consensus RUNX1 sequences on the TRAIL promoter.
(A) Nucleotide sequence of the human TRAIL promoter element are
depicted. RUNX1-binding consensus sequences, ACCACA and TGTGGT, are
shown in red boxes (Sites 1 and 2). The transcription start site is
indicated by the red arrow. (B and C) RUNX1 increased TRAIL
promoter activity. (B) RUNX1 and CBFβ expression plasmids were
transfected alongside the TRAIL promoter-luciferase reporter
plasmid (pTRAIL) into HeLa cells. At 48 h post-transfection, cell
lysates were prepared and luciferase assays were performed. (C) The
RUNX1 and/or CBFβ plasmids were transfected alongside the
pTRAIL-promoter construct in HeLa cells before luciferase
activities were measured. Results were normalized to Renilla
luciferase driven by the TK promoter. (D) Site-directed mutagenesis
of sites 1 and 2 was used to generate mutant 1 and mutant 2,
respectively. (E) pTRAIL/-819 luciferase constructs encoding the
TRAIL promoter with the Mt1 or Mt2 mutations were transfected into
HeLa cells alongside the RUNX1 and CBFβ expression plasmids, before
their activities were measured and compared with those of the
pTRAIL construct pTRAIL/-819. pGL2m, an empty reporter plasmid, was
used as the control. (F) Electrophoresis mobility shift assay was
performed to detect the formation RUNX1 protein-DNA complexes.
Recombinant His-tagged RUNX1 and His-tagged CBFβ produced in E.
coli were incubated with probes containing sequences shown in
(D). CT is a control probe with the RUNX1 consensus sequence. The
positions for of the protein-DNA complexes (shift bands) and free
probes are indicated on the right side of gel image.
*P<0.05 and **P<0.01. RUNX1,
Runt-related transcription factor 1; CBFβ, core-binding factor β;
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; WT,
wild-type; Mt, mutant. |
Consensus RUNX1 sequences on the TRAIL
promoter do not affect RUNX1-mediated TRAIL transcription
RUNX1 functions as a transcription factor by
specifically binding to DNA at the TGT/cGGT consensus sequences
(22). As shown in Fig. 2A, two consensus sites were found
in the complete human TRAIL promoter sequence (Fig. 2A). To analyze if these sites are
important for the regulatory effects of RUNX1 on TRAIL expression,
mutations were introduced into these sites by PCR-based
site-directed mutagenesis (Fig.
2D), with the aim of interfering with RUNX1 binding to these
mutated sequences. Luciferase reporter assays using these mutant
constructs of the 819-bp TRAIL promoter sequences showed comparable
transcriptional activation by RUNX1 between wild type pTRAIL/-819
and mutant types Mt1 and Mt2, indicating that neither site were
important for TRAIL transcriptional regulation by RUNX1 (Fig. 2E).
Subsequently, EMSA was performed to directly assess
if RUNX1 can bind to the promoter sequences used for Fig. 2E. RUNX1 was unable to bind to the
mutant sequences, including MT1, MT2 and WT2 sequences (Fig. 2F). By contrast, RUNX1 protein was
found to bind the WT1 sequence (Fig.
2F). However, this binding was not responsible for the
transactivation of TRAIL promoter by RUNX1, since the luciferase
activities of the MT1 construct, which is the corresponding mutant
for WT1, was not affected (Fig.
2E).
To assess if these sequences are dispensable for the
transcription control of TRAIL, deletion mutants of the TRAIL
promoter sequences were subjected to luciferase reporter assay
following the ectopic expression of RUNX1 and CBFβ. The
transfection efficiency was ~30% in HeLa cells and ~10% in K562
cells. Luciferase in the deletion construct up to the -819 position
was activated by the presence of the RUNX1/CBFβ transcription
factor complex (Fig. 3A and B).
Luciferase in the longer construct containing sequences up to the
-1523 position was also activated (Fig. 3A and B). However, transcription of
the shorter deletion mutant up to the -165 position was also
activated by RUNX1 and CBFβ ectopic expression. These results were
reproducible in both HeLa and K562 cells (Fig. 3A and B). Therefore, these
consensus sites were concluded to be dispensable for the
transcriptional activation of the human TRAIL gene by RUNX1.
TRAIL expression is reduced in leukemia
cells with the RUNX1-ETO protein
The aforementioned findings gave rise to the
investigation into how TRAIL expression is regulated in cells where
RUNX1 function is impaired. TRAIL expression in patients with
t(8;21)AML was next measured. A public database of gene expression
profiles of healthy individuals and patients with malignant
hematopoiesis was analyzed through BloodSpot database (59) using data of GSE42519 (60), GSE13159 (61,62), GSE15434 (63), GSE61804 (64), GSE14468 (65-67) and TCGA database. TRAIL expression
was found to be significantly decreased in AML samples with t(8;21)
compared with that in other-type AML samples or normal cells
(Fig. 4A), which were obtained
from the bone marrow.
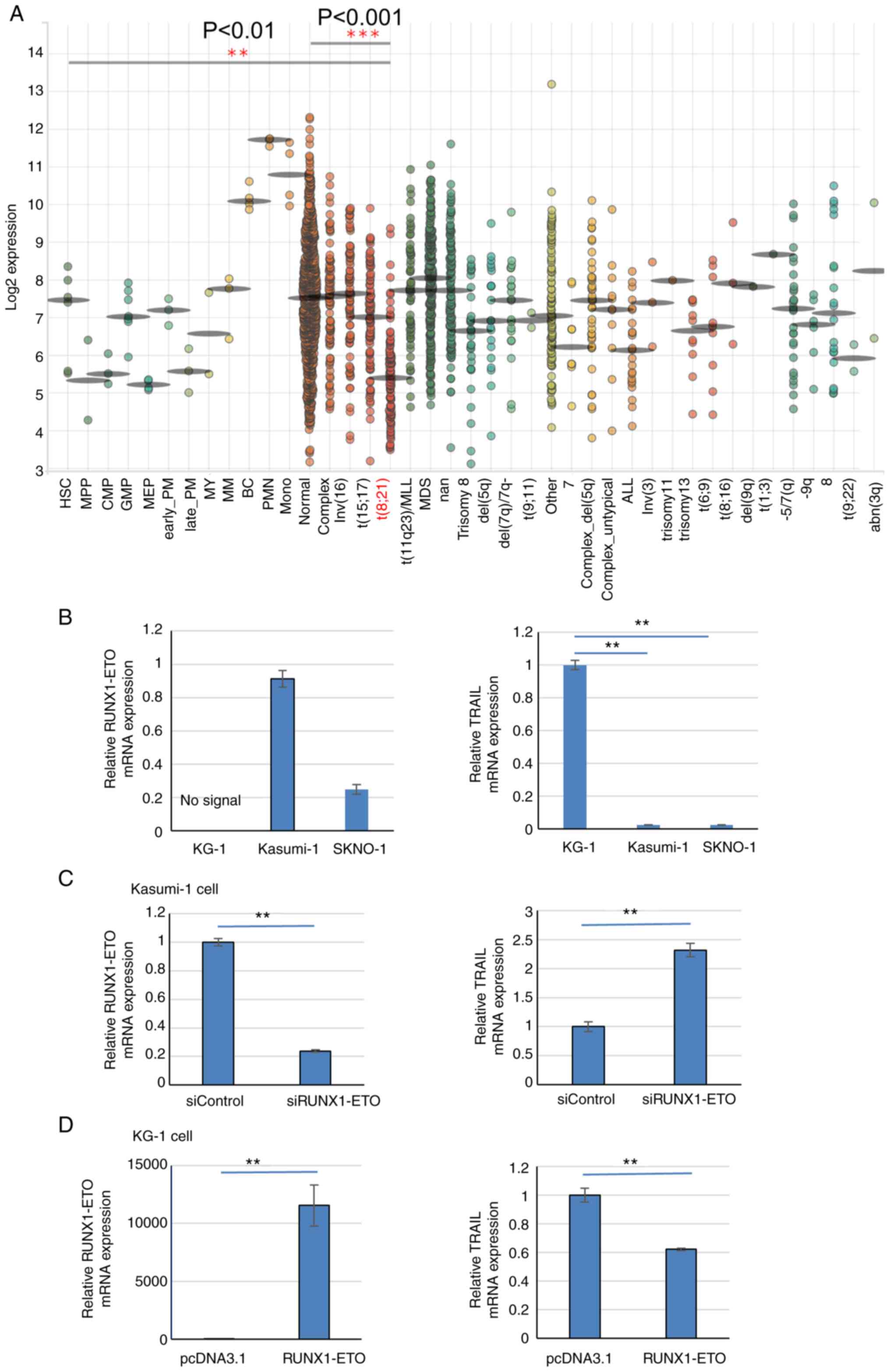 | Figure 4TRAIL expression is reduced in
t(8;21)AML patients and cell lines. (A) BloodSpot revealed that
TRAIL expression is lower in the t(8;21) subtype of AML among other
types of leukemia samples analyzed. The expression of TRAIL was
analyzed by the Microarray Innovations in Leukemia (MILE) study
GSE13159. (B) RT-qPCR results of RUNX1-ETO and TRAIL
expression in Kasumi-1 and SKNO-1 cells compared with KG-1 cells.
Data were standardized by GAPDH expression. (C) RUNX1-ETO
expression was knocked down by RUNX1-ETO siRNA in Kasumi-1
cells. In total, 24 h after siRNA transfection, RUNX1-ETO
and TRAIL mRNA levels were analyzed by RT-qPCR. Data were
standardized by GAPDH expression. (D) After 24 h of
RUNX1-ETO overexpression in KG-1 cells, RUNX1-ETO and
TRAIL mRNA levels were analyzed by RT-qPCR and normalized to
that of GAPDH expression. **P<0.01 and ***P<0.001.
AML, acute myeloid leukemia; Normal, AML with a normal karyotype;
Complex, AML with a complex karyotype; RT-qPCR, reverse
transcriptionquantitative PCR; si, small interfering; RUNX1,
Runt-related transcription factor 1; TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand. |
Next, TRAIL expression in the Kasumi-1 and SKNO-1
t(8;21) AML cell lines, which express the RUNX1-ETO chimeric
protein and trans-dominantly interfere with normal RUNX1
function (6). TRAIL mRNA
expression was markedly significantly reduced in Kasumi-1 and
SKNO-1 cells compared with that in the RUNX1-ETO-negative AML cell
line KG-1 (Fig. 4B). RUNX1-ETO
knockdown in Kasumi-1 cells significantly increased TRAIL
expression compared with that in cells transfected with the control
siRNA (Fig. 4C). The transfection
efficiency was ~65%. In addition, RUNX1-ETO overexpression in KG-1
cells, which do not contain the t(8;21) translocation,
significantly reduced TRAIL expression compared with that in cells
transfected with the empty plasmid (Fig. 4D). The transfection efficiency was
~60%. This suggest that RUNX1-ETO negatively regulates TRAIL
expression in AML cells with t(8;21).
Recombinant TRAIL exerts cytocidal
effects on t(8;21) AML cells
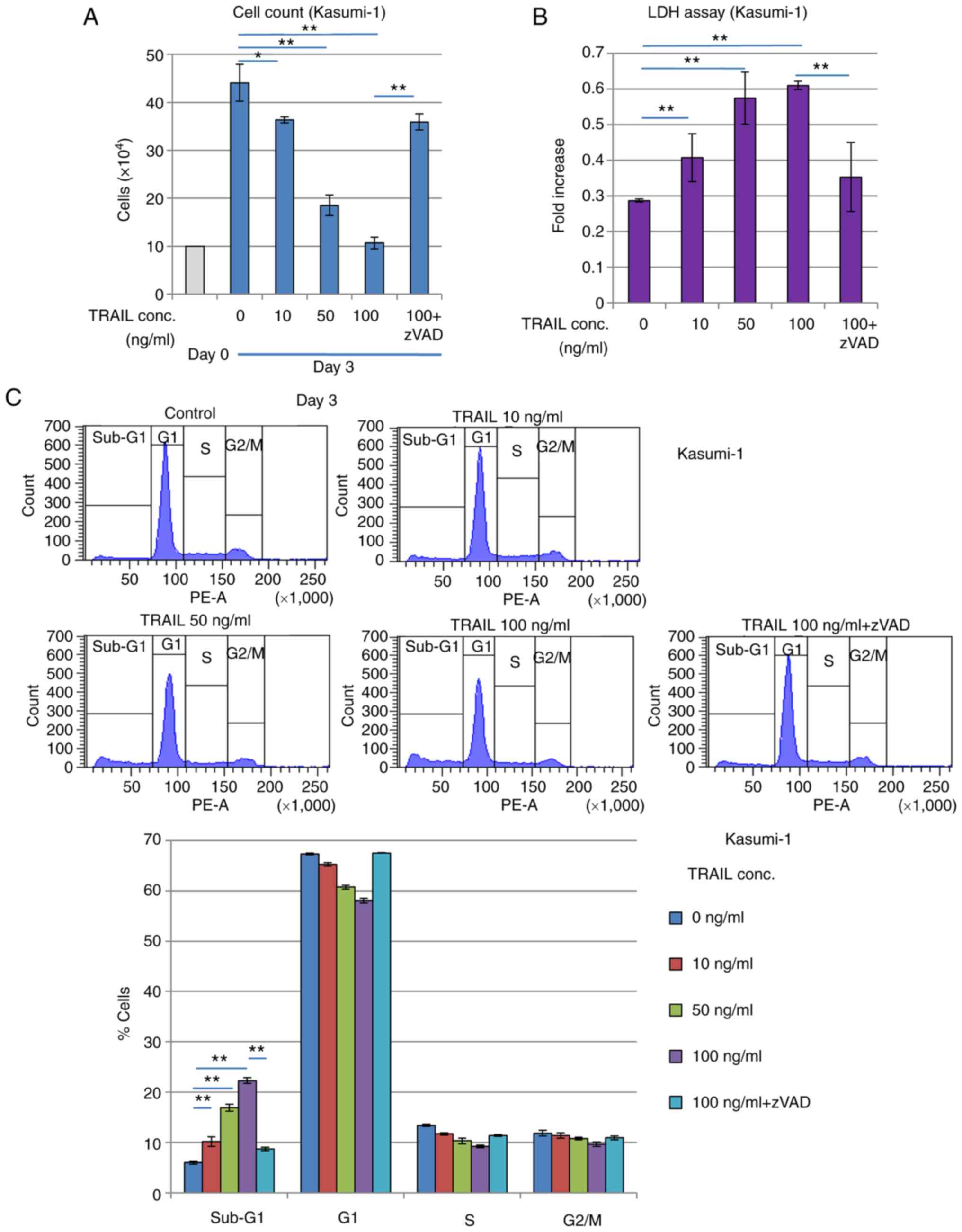
It was subsequently found that treatment with the
exogenous recombinant TRAIL protein suppressed the proliferation of
Kasumi-1 cells. Treatment with the recombinant TRAIL protein for 3
days reduced the number of viable Kasumi-1 cells whilst increasing
LDH release in a TRAIL concentration-dependent manner (Fig. 5A and B), suggesting that cell
death was induced. Cell cycle profiling revealed that the
sub-G1 phase fraction of Kasumi-1 cells was increased
whereas the G1 phase cell fraction was decreased in a
TRAIL concentration-dependent manner (Fig. 5C). These inhibitory effects
mediated by TRAIL treatment were significantly reversed by caspase
inhibitor zVAD-fmk treatment, suggesting that these effects were
caused by increasing apoptosis. By contrast, these inhibitory,
possibly apoptotic effects of TRAIL were significantly and weakly
observed in K562 cells, which have no RUNX1 mutations (Fig. S2), but was not observed in KG-1
cells, which are negative for RUNX1-ETO expression (Fig. S3). Furthermore, another AML cell
line, SKNO-1, which also expresses RUNX1-ETO, was found to have its
viability reduced and sub-G1 population increased by exogenous
TRAIL treatment, which was reversed by zVAD-fmk treatment (Fig. S4).
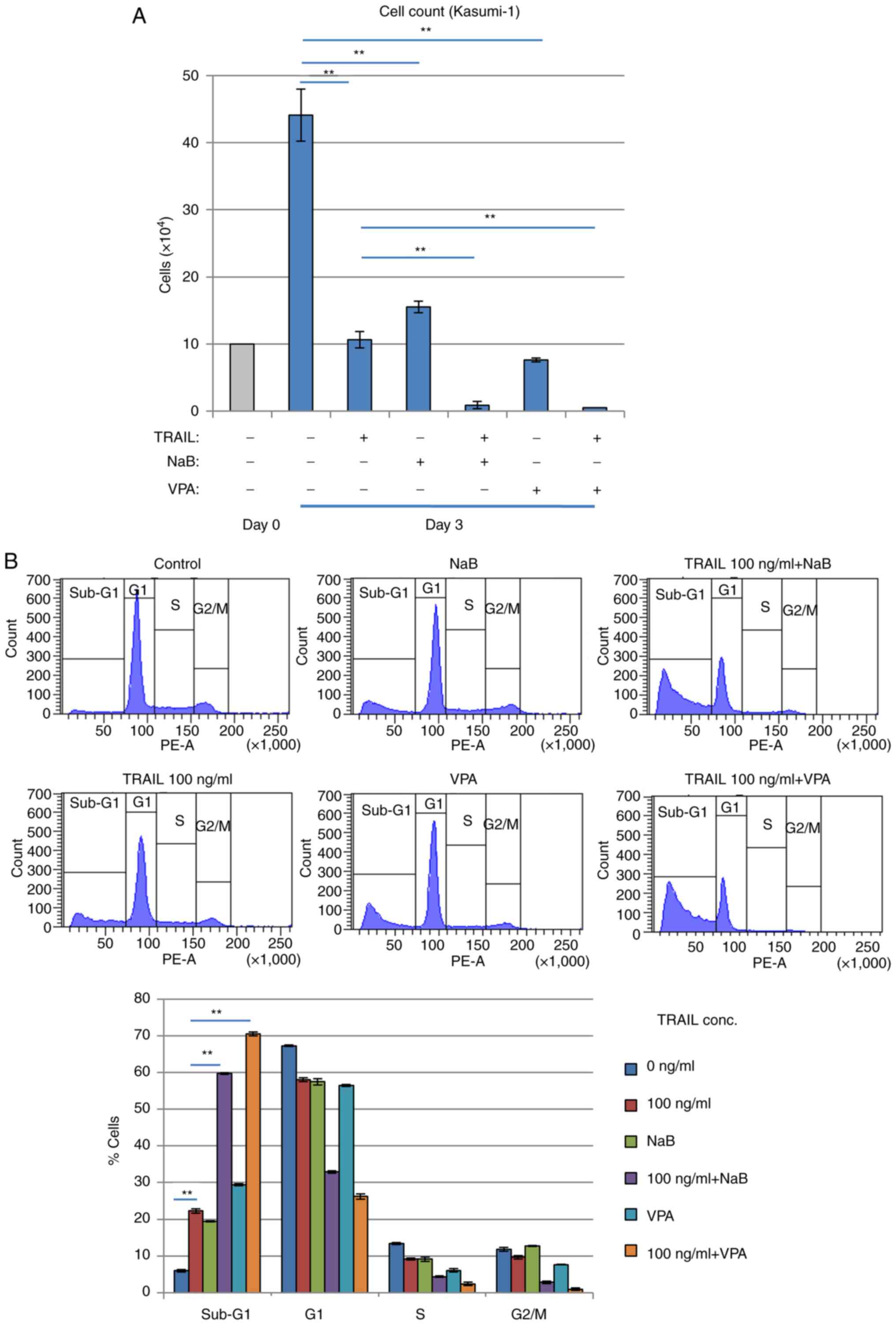
Combination of TRAIL and HDACi
efficiently kills Kasumi-1 cells
Inhibition of cell proliferation and increased
sub-G1 Kasumi-1 cell populations were potentiated
further after HDACis NaB or VPA, were combined with TRAIL (Fig. 6A and B). Since HDACis, including
NaB and VPA, can upregulate DR5 expression and sensitize malignant
human tumor cells such as Jurkat T-cell leukemia, to TRAIL-induced
apoptosis (37), TRAIL signaling
appeared to activate Kasumi-1 cell death. The combination of TRAIL
and HDACis was found to exert superior effects on cell viability
reduction and cell death induction compared with those mediated by
either alone in a dose-dependent manner in Kasumi-1 cells (Fig. S5).
Therefore, the effects of TRAIL on cell survival
likely depend on the presence of the RUNX1-fusion protein.
Discussion
The present study demonstrated that TRAIL is a novel
target gene of RUNX1, as supported by the observation that TRAIL
expression was increased following TPA-induced K562 megakaryocytic
differentiation, which is dependent on RUNX1. Previous reports
stated that TRAIL is expressed in megakaryocytes and platelets,
where it regulates megakaryocytic development (68-70). However, the upstream regulation
mechanism of TRAIL expression remains unclear. The present study
revealed that RUNX1 serves as a transcriptional regulator of TRAIL
during leukemia cell differentiation. Increasing RUNX1-ETO
expression was previously found to increased TRAIL expression in
the RUNX1-ETO-inducible U937 cell line (48). However, the present study
demonstrated that wild-type RUNX1 physiologically functioned as a
positive regulator of TRAIL expression whereas RUNX1-ETO suppressed
TRAIL expression in AML cells harboring t(8;21). This discrepancy
in results may be caused by differences in the cellular background
or experimental system. RUNX1 may indirectly upregulate TRAIL
transcription without dependence on consensus RUNX1 binding sites
in its promoter region. RUNX1 was previously found to regulate high
mobility group AT-hook 2 promoter activity without the need for
consensus sequences, likely by interacting with other intermediary
factors (71), which supports the
findings from the present study. The RNA sequencing approach should
be applied in future studies to clarify this hypothesis, which can
be used to visualize the network of transcription factors and
signal transduction components within complex cellular system. This
may shed light on the underlying mechanism of the RUNX1-TRAIL
axis.
A number of transcription factors that can regulate
TRAIL expression have been identified (45-47). The majority of these transcription
factors regulate TRAIL expression by binding to their DNA sequence
in the promoter region (45-47). By contrast, the present study
suggests that RUNX1 can be categorized as a transcription factor
that regulates TRAIL expression in an indirect manner. It will
therefore be important to examine if this indirect mechanism also
exists in the expression of other genes associated with
hematopoietic malignancy.
RUNX1 was initially recognized as a transcription
factor that mediates myeloid functions such as
myeloperoxidase-induction and macrophage-colony stimulating factor
1-receptor upregulation (5,6).
Further analyzes suggested that RUNX1 is also a regulator of T-cell
activation (16,17). Previous studies using RUNX1
gene-modified mice demonstrated that RUNX1 serves an essential role
in lymphoid function (16,17).
One such finding was that granzyme B (28), which is a secreted protease that
functions as an important effector molecule of activated cytotoxic
T-cells, was identified as a RUNX1 target gene. In the present
study, TRAIL was identified to be another RUNX1 target that
functions as an important effector molecule for lymphoid function.
The molecular mechanism of the immune system related to TRAIL
should be investigated further with focus on the involvement of
other members of the RUNX family.
Elucidation of the molecular mechanisms through
which RUNX1 expression is induced by TPA stimulation in K562 cells
remains a question that needs to be addressed. It has been
extensively reported that RUNX1 has two promoters, proximal and
distal, where this two-promoter organization is strongly conserved
into target genes of the RUNX2 and RUNX3 families through evolution
(50,72). Findings from the present study
therefore serves as an initial starting point for further
investigation into the mechanisms involved.
Since TRAIL preferentially kills cancer cells such
as colon, lung, breast, central nervous system, skin and kidney
cancers, recombinant TRAIL has been considered to be a promising
anti-cancer agent (73). Although
several types of malignancies remain resistant to TRAIL, induction
of its receptor DR5, may also be a viable option for sensitizing
these tumor cells to TRAIL (37,74,75). The present study in conjunction
with BloodSpot data revealed that TRAIL expression is reduced in
t(8;21) AML samples, suggesting that RUNX1 mutations caused the
TRAIL downregulation. Furthermore, compensation of TRAIL expression
reduced cell viability in Kasumi-1 and SKNO-1 cells, both of which
carry the t(8;21) translocation. By contrast, the WT
RUNX1-expressing AML cell line KG-1 and the chronic myelogenous
leukemia cell line K562 were resistant to TRAIL treatment. K562 was
previously reported to be resistant to TNF-α whereas NK cells
caused K562 cell death (76).
This suggest that K562 cells possess a common mechanism of death
ligand resistance. Death ligands, such as TNF-α, can not only
induce apoptosis, but can also induce cell proliferation depending
on the receptor profile (77).
Although TRAIL may also confer such a property, in the present
study TRAIL only reduced cell viability and cell cycle progression.
The possible proliferative effect of TRAIL warrants further
investigation, especially in a clinical setting. In the present
study, sub-G1 analysis was used to evaluate cell death,
which was also previously used to analyze TRAIL function (37,55,64,65). Annexin V/PI staining is an
alternative method that can be used to assess cell death, which
distinguishes between apoptosis and necrosis. To confirm apoptosis
induction in detail, Annexin V/PI assay accompanied by
sub-G1 analysis should be performed and is a limitation
of the present study.
The sensitivity of Kasumi-1 to TRAIL was found to
be increased by the combined treatment with HDACi. Cell viability
assay was performed to assess the effects of TRAIL alongside
different concentrations of HDACis, which found that cell viability
was reduced by combined treatment. HDACis was previously reported
to upregulate TRAIL receptor expression and sensitize target cells
to TRAIL-induced apoptosis in Jurkat cells. In addition, HDACis may
regulate the oncogenic pathway to induce AML cell death because
RUNX1-ETO can bind to HDACs to abrogate the normal transcriptional
function of RUNX1 (38). It is
therefore important to analyze TRAIL function in normal
myeloid/lymphoid development and to evaluate the possibility of
TRAIL as a novel treatment method for this type of leukemia.
However, the lack of in vivo validation is a limitation of
the present study. To elucidate the mechanism of malignant
transformation and therapy of AML, further in vivo analysis
is required. In addition, results from clinical trials testing the
effects of TRAIL on AML are expected to yield promising
results.
Taken together, the present study demonstrated
TRAIL to be a transcriptional target of RUNX1, where the effects
are likely to be indirect. Additionally, TRAIL treatment may be
useful for inducing apoptosis on leukemia cells that expresses the
RUNX1-ETO mutant. The present study therefore provides novel
insights into the regulation of TRAIL expression and roles of the
transcription factor RUNX1.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TY designed the study and performed the biological
analyzes. KY performed the luciferase assays. and KT, YK, AES, NK,
AM and TS analyzed experimental data. TO designed the study. TY and
TO can confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Toshiya Inaba,
Department of Molecular Oncology and Leukemia Program Project,
Research Institute for Radiation Biology and Medicine, Hiroshima
University, Hiroshima, Japan for valuable advice on Kasumi-1 and
SKNO-1 culture conditions and distribution of Kasumi-1 cells. The
authors are grateful to Dr Misao Ohki, Chromatin Function in
Leukemogenesis Project and Cancer Genomics Division, National
Cancer Center Research Institute, Tokyo, Japan, for supplying
RUNX1-ETO cDNA. The authors would like to thank Dr B Mark Evers,
Department of Surgery, University of Texas Medical Branch,
Galveston, USA, for the supply of human TRAIL promoter 5′-deletion
mutants pTRAIL/-1523, pTRAIL/-819 and pTRAIL/-165.
Funding
The present study was supported by JSPS KAKENHI (grant nos.
JP15K09487, JP16K09857, JP16K10038, JP17K09936 and JP19K08846). It
was also supported in part by Shimizu Foundation for Immunology and
Neuroscience Grant for 2016, Children's Cancer Association of Japan
Financial Support for 2016 and Research Institute for Production
Development Grant for 2017.
Abbreviations:
|
RUNX1
|
Runt-related transcription factor
1
|
|
AML1
|
acute myeloid leukemia 1
|
|
TRAIL
|
tumor-necrosis factor-related
apoptosis inducing ligand
|
References
|
1
|
Orkin SH and Zon LI: Hematopoiesis: An
evolving paradigm for stem cell biology. Cell. 132:631–644. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyoshi H, Shimizu K, Kozu T, Maseki N,
Kaneko Y and Ohki M: t(8;21) breakpoints on chromosome 21 in acute
myeloid leukemia are clustered within a limited region of a single
gene, AML1. Proc Natl Acad Sci USA. 88:10431–10434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Look AT: Oncogenic transcription factors
in the human acute leukemias. Science. 278:1059–1064. 1997.
View Article : Google Scholar
|
|
4
|
Cancer Genome Atlas Research Network; Ley
TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley
K, Triche TJ Jr, Laird PW, et al: Genomic and epigenomic landscapes
of adult de novo acute myeloid leukemia. N Engl J Med.
368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyoshi H, Kozu T, Shimizu K, Enomoto K,
Maseki N, Kaneko Y, Kamada N and Ohki M: The t(8;21) translocation
in acute myeloid leukemia results in production of an AML1-MTG8
fusion transcript. EMBO J. 12:2715–2721. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frank R, Zhang J, Uchida H, Meyers S,
Hiebert SW and Nimer SD: The AML1/ETO fusion protein blocks
transactivation of the GM-CSF promoter by AML1B. Oncogene.
11:2667–2674. 1995.PubMed/NCBI
|
|
7
|
Meyers S, Lenny N and Hiebert SW: The
t(8;21) fusion protein interferes with AML-1B-dependent
transcriptional activation. Mol Cell Biol. 15:1974–1982. 1995.
View Article : Google Scholar
|
|
8
|
Osato M, Asou N, Abdalla E, Hoshino K,
Yamasaki H, Okubo T, Suzushima H, Takatsuki K, Kanno T, Shigesada K
and Ito Y: Biallelic and heterozygous point mutations in the runt
domain of the AML1/PEBP2alphaB gene associated with myeloblastic
leukemias. Blood. 93:1817–1824. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song WJ, Sullivan MG, Legare RD, Hutchings
S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, et
al: Haploinsufficiency of CBFA2 causes familial thrombocytopenia
with propensity to develop acute myelogenous leukaemia. Nat Genet.
23:166–175. 1999. View
Article : Google Scholar
|
|
10
|
Mizutani S, Yoshida T, Zhao X, Nimer SD,
Taniwaki M and Okuda T: Loss of RUNX1/AML1 arginine-methylation
impairs peripheral T cell homeostasis. Br J Haematol. 170:859–873.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumura T, Nakamura-Ishizu A, Muddineni
SSNA, Tan DQ, Wang CQ, Tokunaga K, Tirado-Magallanes R, Sian S,
Benoukraf T, Okuda T, et al: Hematopoietic stem cells acquire
survival advantage by loss of RUNX1 methylation identified in
familial leukemia. Blood. 136:1919–1932. 2020. View Article : Google Scholar
|
|
12
|
DiFilippo EC, Coltro G, Carr RM,
Mangaonkar AA, Binder M, Khan SP, Rodriguez V, Gangat N, Wolanskyj
A, Pruthi RK, et al: Spectrum of abnormalities and clonal
transformation in germline RUNX1 familial platelet disorder and a
genomic comparative analysis with somatic RUNX1 mutations in
MDS/MPN overlap neoplasms. Leukemia. 34:2519–2524. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuda T, van Deursen J, Hiebert SW,
Grosveld G and Downing JR: AML1, the target of multiple chromosomal
translocations in human leukemia, is essential for normal fetal
liver hematopoiesis. Cell. 84:321–330. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Stacy T, Binder M, Marin-Padilla
M, Sharpe AH and Speck NA: Disruption of the Cbfa2 gene causes
necrosis and hemorrhaging in the central nervous system and blocks
definitive hematopoiesis. Proc Natl Acad Sci USA. 93:3444–3449.
1996. View Article : Google Scholar
|
|
15
|
North T, Gu TL, Stacy T, Wang Q, Howard L,
Binder M, Marín-Padilla M and Speck NA: Cbfa2 is required for the
formation of intra-aortic hematopoietic clusters. Development.
126:2563–2575. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniuchi I, Osato M, Egawa T, Sunshine MJ,
Bae SC, Komori T, Ito Y and Littman DR: Differential requirements
for Runx proteins in CD4 repression and epigenetic silencing during
T lymphocyte development. Cell. 111:621–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ichikawa M, Asai T, Saito T, Seo S,
Yamazaki I, Yamagata T, Mitani K, Chiba S, Ogawa S, Kurokawa M and
Hirai H: AML-1 is required for megakaryocytic maturation and
lymphocytic differentiation, but not for maintenance of
hematopoietic stem cells in adult hematopoiesis. Nat Med.
10:299–304. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Growney JD, Shigematsu H, Li Z, Lee BH,
Adelsperger J, Rowan R, Curley DP, Kutok JL, Akashi K, Williams IR,
et al: Loss of Runx1 perturbs adult hematopoiesis and is associated
with a myeloproliferative phenotype. Blood. 106:494–504. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Bruijn M and Dzierzak E: Runx
transcription factors in the development and function of the
definitive hematopoietic system. Blood. 129:2061–2069. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Wang Q, Crute BE, Melnikova IN,
Keller SR and Speck NA: Cloning and characterization of subunits of
the T-cell receptor and murine leukemia virus enhancer core-binding
factor. Mol Cell Biol. 13:3324–3339. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogawa E, Maruyama M, Kagoshima H, Inuzuka
M, Lu J, Satake M, Shigesada K and Ito Y: PEBP2/PEA2 represents a
family of transcription factors homologous to the products of the
Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci
USA. 90:6859–6863. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyers S, Downing JR and Hiebert SW:
Identification of AML-1 and the (8;21) translocation protein
(AML-1/ETO) as sequence-specific DNA-binding proteins: The runt
homology domain is required for DNA binding and protein-protein
interactions. Mol Cell Biol. 13:6336–6345. 1993.PubMed/NCBI
|
|
23
|
Kanno T, Kanno Y, Chen LF, Ogawa E, Kim WY
and Ito Y: Intrinsic transcriptional activation-inhibition domains
of the polyomavirus enhancer binding protein 2/core binding factor
alpha subunit revealed in the presence of the beta subunit. Mol
Cell Biol. 18:2444–2454. 1998. View Article : Google Scholar
|
|
24
|
Kitabayashi I, Yokoyama A, Shimizu K and
Ohki M: Interaction and functional cooperation of the
leukemia-associated factors AML1 and p300 in myeloid cell
differentiation. EMBO J. 17:2994–3004. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otto F, Lübbert M and Stock M: Upstream
and downstream targets of RUNX proteins. J Cell Biochem. 89:9–18.
2003. View Article : Google Scholar
|
|
26
|
Rossetti S and Sacchi N: RUNX1: A microRNA
hub in normal and malignant hematopoiesis. Int J Mol Sci.
14:1566–1588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imperato MR, Cauchy P, Obier N and Bonifer
C: The RUNX1-PU.1 axis in the control of hematopoiesis. Int J
Hematol. 101:319–329. 2015. View Article : Google Scholar
|
|
28
|
Wargnier A, Legros-Maida S, Bosselut R,
Bourge JF, Lafaurie C, Ghysdael CJ, Sasportes M and Paul P:
Identification of human granzyme B promoter regulatory elements
interacting with activated T-cell-specific proteins: implication of
Ikaros and CBF binding sites in promoter activation. Proc Natl Acad
Sci USA. 92:6930–6934. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elagib KE, Racke FK, Mogass M, Khetawat R,
Delehanty LL and Goldfarb AN: RUNX1 and GATA-1 coexpression and
cooperation in megakaryocytic differentiation. Blood.
101:4333–4341. 2003. View Article : Google Scholar
|
|
30
|
Verma AK, Wheeler DL, Aziz MH and
Manoharan H: Protein kinase cepsilon and development of squamous
cell carcinoma, the nonmelanoma human skin cancer. Mol Carcinog.
45:381–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C and Smith
CA: Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar
|
|
32
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jurisic V, Bumbasirevic V, Konjevic G,
Djuricic B and Spuzic I: TNF-alpha induces changes in LDH isotype
profile following triggering of apoptosis in PBL of non-Hodgkin's
lymphomas. Ann Hematol. 83:84–91. 2004. View Article : Google Scholar
|
|
34
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stuckey DW and Shah K: TRAIL on trial:
Preclinical advances in cancer therapy. Trends Mol Med. 19:685–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Twomey JD, Kim SR, Zhao L, Bozza WP and
Zhang B: Spatial dynamics of TRAIL death receptors in cancer cells.
Drug Resist Updat. 19:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakata S, Yoshida T, Horinaka M, Shiraishi
T, Wakada M and Sakai T: Histone deacetylase inhibitors upregulate
death receptor 5/TRAIL-R2 and sensitize apoptosis induced by
TRAIL/APO2-L in human malignant tumor cells. Oncogene.
23:6261–6271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farooqi AA, Naqvi SK, Perk AA, Yanar O,
Tabassum S, Ahmad MS, Mansoor Q, Ashry MS, Ismail M, Naoum GE and
Arafat WO: Natural agents-mediated targeting of histone
deacetylases. Arch Immunol Ther Exp (Warsz). 66:31–44. 2018.
View Article : Google Scholar
|
|
39
|
Zhou M, Yuan M, Zhang M, Lei C, Aras O,
Zhang X and An F: Combining histone deacetylase inhibitors (HDACis)
with other therapies for cancer therapy. Eur J Med Chem.
226:1138252021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Falschlehner C, Schaefer U and Walczak H:
Following TRAIL's path in the immune system. Immunology.
127:145–154. 2009. View Article : Google Scholar :
|
|
41
|
Zerafa N, Westwood JA, Cretney E, Mitchell
S, Waring P, Iezzi M and Smyth MJ: TRAIL deficiency accelerates
hematological malignancies. J Immunol. 175:5586–5590. 2005.
View Article : Google Scholar
|
|
42
|
Cretney E, Takeda K, Yagita H, Glaccum M,
Peschon JJ and Smyth MJ: Increased susceptibility to tumor
initiation and metastasis in TNF-related apoptosis-inducing
ligand-deficient mice. J Immunol. 168:1356–1361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Finnberg N, Klein-Szanto AJ and El-Deiry
WS: TRAIL-R deficiency in mice promotes susceptibility to chronic
inflammation and tumorigenesis. J Clin Invest. 118:111–123. 2008.
View Article : Google Scholar
|
|
44
|
Wang Q, Ji Y, Wang X and Evers BM:
Isolation and molecular characterization of the 5′-upstream region
of the human TRAIL gene. Biochem Biophys Res Commun. 276:466–471.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Allen JE and El-Deiry WS: Regulation of
the human TRAIL gene. Cancer Biol Ther. 13:1143–1151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Azahri NS and Kavurma MM: Transcriptional
regulation of tumour necrosis factor-related apoptosis-inducing
ligand. Cell Mol Life Sci. 70:3617–3629. 2013. View Article : Google Scholar
|
|
47
|
Nebbioso A, Carafa V, Conte M, Tambaro FP,
Abbondanza C, Martens J, Nees M, Benedetti R, Pallavicini I,
Minucci S, et al: c-Myc modulation and acetylation is a key HDAC
inhibitor target in cancer. Clin Cancer Res. 23:2542–2555. 2017.
View Article : Google Scholar
|
|
48
|
Barbetti V, Tusa I, Cipolleschi MG, Rovida
E and Sbarba PD: AML1/ETO sensitizes via TRAIL acute myeloid
leukemia cells to the pro-apoptotic effects of hypoxia. Cell Death
Dis. 4:e5362013. View Article : Google Scholar :
|
|
49
|
Okuda T, Takeda K, Fujita Y, Nishimura M,
Yagyu S, Yoshida M, Akira S, Downing JR and Abe T: Biological
characteristics of the leukemia-associated transcriptional factor
AML1 disclosed by hematopoietic rescue of AML1-deficient embryonic
stem cells by using a knock-in strategy. Mol Cell Biol. 20:319–328.
2000. View Article : Google Scholar
|
|
50
|
Fukushima-Nakase Y, Naoe Y, Taniuchi I,
Hosoi H, Sugimoto T and Okuda T: Shared and distinct roles mediated
through C-terminal subdomains of acute myeloid
leukemia/Runt-related transcription factor molecules in murine
development. Blood. 105:4298–4307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ito Y: Oncogenic potential of the RUNX
gene family: 'Overview'. Oncogene. 23:4198–4208. 2004. View Article : Google Scholar
|
|
52
|
Kanno Y, Kanno T, Sakakura C, Bae SC and
Ito Y: Cytoplasmic sequestration of the polyomavirus enhancer
binding protein 2 (PEBP2)/core binding factor alpha (CBFalpha)
subunit by the leukemia-related PEBP2/CBFbeta-SMMHC fusion protein
inhibits PEBP2/CBF-mediated transactivation. Mol Cell Biol.
18:4252–4261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goyama S, Yamaguchi Y, Imai Y, Kawazu M,
Nakagawa M, Asai T, Kumano K, Mitani K, Ogawa S, Chiba S, et al:
The transcriptionally active form of AML1 is required for
hematopoietic rescue of the AML1-deficient embryonic para-aortic
splanchno-pleural (P-Sp) region. Blood. 104:3558–3564. 2004.
View Article : Google Scholar
|
|
54
|
Liu H and Naismith JH: An efficient
one-step site-directed deletion, insertion, single and
multiple-site plasmid mutagenesis protocol. BMC Biotechnol.
8:912008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Asou H, Tashiro S, Hamamoto K, Otsuji A,
Kita K and Kamada N: Establishment of a human acute myeloid
leukemia cell line (Kasumi-1) with 8;21 chromosome translocation.
Blood. 77:2031–2036. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
57
|
Horinaka M, Yoshida T, Tomosugi M, Yasuda
S, Sowa Y and Sakai T: Myeloid zinc finger 1 mediates sulindac
sulfide-induced upregulation of death receptor 5 of human colon
cancer cells. Sci Rep. 4:60002014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kagoshima H, Akamatsu Y, Ito Y and
Shigesada K: Functional dissection of the alpha and beta subunits
of transcription factor PEBP2 and the redox susceptibility of its
DNA binding activity. J Biol Chem. 271:33074–33082. 1996.
View Article : Google Scholar
|
|
59
|
Bagger FO, Sasivarevic D, Sohi SH, Laursen
LG, Pundhir S, Sønderby CK, Winther O, Rapin N and Porse BT:
BloodSpot: A database of gene expression profiles and
transcriptional programs for healthy and malignant haematopoiesis.
Nucleic Acids Res. 44(D1): D917–D924. 2016. View Article : Google Scholar :
|
|
60
|
Rapin N, Bagger FO, Jendholm J,
Mora-Jensen H, Krogh A, Kohlmann A, Thiede C, Borregaard N,
Bullinger L, Winther O, et al: Comparing cancer vs normal gene
expression profiles identifies new disease entities and common
transcriptional programs in AML patients. Blood. 123:894–904. 2014.
View Article : Google Scholar
|
|
61
|
Kohlmann A, Kipps TJ, Rassenti LZ, Downing
JR, Shurtleff SA, Mills KI, Gilkes AF, Hofmann WK, Basso G,
Dell'orto MC, et al: An international standardization programme
towards the application of gene expression profiling in routine
leukaemia diagnostics: The microarray innovations in LEukemia study
prephase. Br J Haematol. 142:802–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Haferlach T, Kohlmann A, Wieczorek L,
Basso G, Kronnie GT, Béné MC, De Vos J, Hernández JM, Hofmann WK,
Mills KI, et al: Clinical utility of microarray-based gene
expression profiling in the diagnosis and subclassification of
leukemia: Report from the international microarray innovations in
leukemia study group. J Clin Oncol. 28:2529–2537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Klein HU, Ruckert C, Kohlmann A, Bullinger
L, Thiede C, Haferlach T and Dugas M: Quantitative comparison of
micro-array experiments with published leukemia related gene
expression signatures. BMC Bioinformatics. 10:4222009. View Article : Google Scholar
|
|
64
|
Warnat-Herresthal S, Perrakis K, Taschler
B, Becker M, Baßler K, Beyer M, Günther P, Schulte-Schrepping J,
Seep L, Klee K, et al: scalable prediction of acute myeloid
leukemia using high-dimensional machine learning and blood
transcriptomics. iScience. 23:1007802020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wouters BJ, Löwenberg B,
Erpelinck-Verschueren CA, van Putten WL, Valk PJ and Delwel R:
Double CEBPA mutations, but not single CEBPA mutations, define a
subgroup of acute myeloid leukemia with a distinctive gene
expression profile that is uniquely associated with a favorable
outcome. Blood. 113:3088–3091. 2009. View Article : Google Scholar :
|
|
66
|
Taskesen E, Bullinger L, Corbacioglu A,
Sanders MA, Erpelinck CA, Wouters BJ, van der Poel-van de
Luytgaarde SC, Damm F, Krauter J, Ganser A, et al: Prognostic
impact, concurrent genetic mutations, and gene expression features
of AML with CEBPA mutations in a cohort of 1182 cytogenetically
normal AML patients: Further evidence for CEBPA double mutant AML
as a distinctive disease entity. Blood. 117:2469–2475. 2011.
View Article : Google Scholar
|
|
67
|
Taskesen E, Babaei S, Reinders MM and de
Ridder J: Integration of gene expression and DNA-methylation
profiles improves molecular subtype classification in acute myeloid
leukemia. BMC Bioinformatics. 16(Suppl 4): S52015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang L, Wang L, Zhao CH, Zhu XJ, Hou Y,
Jun P and Hou M: Contributions of TRAIL-mediated megakaryocyte
apoptosis to impaired megakaryocyte and platelet production in
immune thrombocytopenia. Blood. 116:4307–4316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Melloni E, Secchiero P, Celeghini C,
Campioni D, Grill V, Guidotti L and Zauli G: Functional expression
of TRAIL and TRAIL-R2 during human megakaryocytic development. J
Cell Physiol. 204:975–982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Crist SA, Elzey BD, Ludwig AT, Griffith
TS, Staack JB, Lentz SR and Ratliff TL: Expression of TNF-related
apoptosis-inducing ligand (TRAIL) in megakaryocytes and platelets.
Exp Hematol. 32:1073–1081. 2004. View Article : Google Scholar
|
|
71
|
Lam K, Muselman A, Du R, Harada Y, Scholl
AG, Yan M, Matsuura S, Weng S, Harada H and Zhang DE: Hmga2 is a
direct target gene of RUNX1 and regulates expansion of myeloid
progenitors in mice. Blood. 124:2203–2212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rini D and Calabi F: Identification and
comparative analysis of a second runx3 promoter. Gene. 273:13–22.
2001. View Article : Google Scholar
|
|
73
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yoshida T, Shiraishi T, Nakata S, Horinaka
M, Wakada M, Mizutani Y, Miki T and Sakai T: Proteasome inhibitor
MG132 induces death receptor 5 through CCAAT/enhancer-binding
protein homologous protein. Cancer Res. 65:5662–5667. 2005.
View Article : Google Scholar
|
|
75
|
Yoshida T, Maoka T, Das SK, Kanazawa K,
Horinaka M, Wakada M, Satomi Y, Nishino H and Sakai T:
Halocynthiaxanthin and peridinin sensitize colon cancer cell lines
to tumor necrosis factor-related apoptosis-inducing ligand. Mol
Cancer Res. 5:615–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jurisić V, Spuzić I and Konjević G: A
comparison of the NK cell cytotoxicity with effects of TNF-alpha
against K-562 cells, determined by LDH release assay. Cancer Lett.
138:67–72. 1999. View Article : Google Scholar
|
|
77
|
Jurisic V, Bogdanovic G, Kojic V, Jakimov
D and Srdic T: Effect of TNF-alpha on Raji cells at different
cellular levels estimated by various methods. Ann Hematol.
85:86–94. 2006. View Article : Google Scholar
|