Introduction
It is estimated that >90% of cancers of the head
and neck regions are squamous cell carcinomas (HNSCC) that arise
from the oral cavity, pharynx and larynx (1). Alcohol and tobacco use (2) and human papillomavirus exposure
(3) are important risk factors.
As of 2018, cancer of the pharynx accounts for 1.7% of all
cancer-related mortalities worldwide (4). While the underlying mechanisms for
the development of HNSCC are complex (5), the tumor suppressor p53 plays an
important role in antagonizing the initiation and progression of
numerous cancers. By contrast, its alterations can lead to the
impairment of the cell cycle checkpoint and represent the early
events in progression observed for HNSCC cancers (6). Mutations at the DNA-binding domain
are considered particularly disruptive and are associated with poor
prognosis (7). Indeed, TP53 is
the most frequently mutated gene observed in HNSCC-derived cells
(69.9%) (8).
Standard treatment modalities for HNSCC tumors
consist of surgery, radiotherapy and chemotherapy that are often
accompanied by considerable morbidity (9). Despite the improvement in curative
efforts, the 5-year survival rate remains relatively constant at
~66% for all cancer stages (10).
While surgery is the primary option, numerous patients present with
advanced diseases that preclude resection and are only accessible
to radiotherapy and chemotherapy. As such, an important and
commonly used chemotherapy is the cytotoxic platinum-based drug,
cisplatin (11). Cisplatin has
been used to treat various cancers, such as biliary tract and
breast cancer, carcinoma of salivary gland origin, cervical, colon,
gastric and lung cancer, melanoma, prostate and pancreatic cancer,
squamous cell carcinoma of male genital tract and urothelial
bladder cancer (12). In regard
to unresectable oral squamous cell carcinoma tumors, cisplatin is
recommended as a standard agent in combination with radiation
(13). However, drug-resistance
and significant toxicity pose concerns for the use of cisplatin and
provide the impetus to explore other agents.
Detroit 562 is a pharyngeal squamous cell carcinoma
with a gain-of-function mutant p53 that is due to a R175H amino
acid substitution. The mutation resides on the L2 loop of the
DNA-binding domain and is characterized by the overexpression of
mutant p53 and the promotion of metastasis and mortality in a
xenograft mouse model (14). In
our previous effort to discover antiviral agents against poxvirus
by disrupting the poxviral processivity factor, an indole-based
small molecule (24a) was realized (15). The present study describes the
discovery of 24a as a candidate anticancer agent for future
studies. To accomplish this, the present study aimed to demonstrate
the ability of 24a to promote antiproliferative and pro-apoptotic
properties against an aggressive HNSCC-derived cell line. This was
done using cell viability and cell cycle analysis studies and the
probing of the expression profiles of various putative protein
targets involved in cell proliferation and cell death. Of
particular interest was the potential contribution of p53.
Materials and methods
Compounds
Cisplatin and nocodazole were purchased from
Sigma-Aldrich; Merck KGaA. 24a was synthesized and confirmed by
elemental analysis, liquid chromatography-mass spectrometry and
nuclear magnetic resonance spectroscopy at >95% purity as
previously described (15).
Cisplatin was prepared in PBS. Nocodazole and 24a were prepared in
DMSO.
Cells
The Detroit 562 cell line (cat. no. CCL-138) was
obtained from the American Type Culture Collection (ATCC) and
maintained in DMEM growth medium (Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% FBS (Corning, Inc.) and 25
µg/ml gentamicin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were confirmed negative for mycoplasma species by the
MycoAlert detection kit (Cambrex Bio Science Rockland, Inc.)
performed at the Department of Genetics Core at University of
Pennsylvania. Genotyping was performed at the Core using standard
methodology for short tandem repeat (STR) profiling in accordance
with the American National Standards Institute's Authentication of
Human Cell Lines: Standardization of STR Profiling (ANSI/ATCC
ASN-0002-2011) at eight autosomal loci and amelogenin. Cells were
authenticated by 93% match to the reference STR database of ATCC.
Cells were cultured at 5% CO2 in a humidified incubator
at 37°C. The normal fibroblast cell line, GM04390, was obtained
from the Genetics Core and similarly maintained. B38 and BSC-1 cell
lines (ATCC) were similarly maintained in 10% FBS/DMEM growth
medium, HeLa cells (ATCC) were maintained in RPMI-1640 growth
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS and 25 µg/ml gentamicin, and MDA-MB-231 cells (ATCC)
were maintained in Leibovitz's L-15 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 25 µg/ml
gentamicin. For all experiments, cells were not subjected to
synchronization prior to compound treatments.
Cytotoxicity
Detroit 562 cells were seeded ~8×103
cells/well overnight in 96-well plates in 100 µl of growth
medium to obtain 15-20% confluence at the time of treatment. For
cisplatin treatment, PBS served as mock treatment. By comparison,
nocodazole and 24a treatments were maintained at 1% DMSO
throughout, with 1% DMSO serving as mock treatment. Compounds were
serially diluted two-fold in growth media and prepared in
triplicate. Fresh growth medium exchange occurred after 3 days. On
the 6th day, cells typically reached 80-90% confluence, indicating
at least two cell population doubling. Following treatment, 20
µl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
prepared in PBS) was added to each well, and the plate was
incubated another 2 h in a humidified incubator at 37°C. The growth
media were then removed by aspiration, and the solid formazan in
each well was dissolved in 150 µl of isopropanol containing
4 mM HCl and 0.1% P-20 by remaining for 1 h at room temperature.
Absorbance was measured at 590 nm by a microplate reader. Cell
viability was determined relative to the mock treatment and fitted
by non-linear regression. Cell viability studies for GM04390A, B38,
BSC-1, HeLa, and MDA-MB-231 were performed at the same plating
density and processing (Fig.
S1). Detection of cell viability was determined by staining
with the nucleic acid-binding fluorescent dye, CyQUANT GR,
according to the manufacture's recommendation (Invitrogen; Thermo
Fisher Scientific, Inc.) and measured on a fluorescence microplate
reader at 520 nm emission.
Cell cycle analysis
Cells were seeded at 1.3×106 cells/dish
overnight in six-cm dishes to yield ~40% confluence and then
treated with 24a or 1% DMSO for 24 h. Following treatment,
suspended cells were collected from growth media by centrifugation
at 600 × g for 5 min at 25°C. Adherent cells were washed with PBS,
trypsinized and similarly collected by centrifugation as the
suspended cells. Both cell collections were resuspended in PBS and
pooled. Ice-cold ethanol was added to yield 70% final concentration
and the cells were fixed by incubating at 4°C for 2 h. The fixed
cells were then collected by centrifugation at 600 × g for 5 min at
4°C and rehydrated in PBS. The cells were next similarly collected
by centrifugation, resuspended in 100 µl staining solution
consisting of 15 µg/ml propidium iodide (PI), 10
µg/ml RNAse, 5 mM EDTA and 5 mg/ml BSA (Thermo Fisher
Scientific, Inc.) in PBS and incubated overnight in the dark at
4°C. The cells were then diluted with an addition of 300 µl
PBS, filtered through a 100-µm mesh, and measured on a
NovoCyte flow cytometer (ACEA Bioscience, Inc.) at 488-nm
excitation and detection by the phycoerythrin channel, with no
further compensation of the fluorescent signals. The cell cycle
distribution was determined with the NovoExpress software version
1.3.0 (ACEA Bioscience, Inc.).
Construction of Detroit 562 cells
expressing fluorescence ubiquitination-based cell cycle indicator
(FUCCI)
VSV-G pseudotyped lentiviral particles were
generated from the transfection of 293T cells (ATCC) by the
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's recommendation
with the 3rd generation pBOB-EF1-FastFUCCI-Puro transfer vector
(plasmid no. 86849), the 2nd generation packaging plasmid
pCMV-dR8.2 dpr (plasmid no. 8455) and the 2nd generation envelope
plasmid pCMV-VSV-G (plasmid no. 8454) obtained from Addgene, Inc.
293T cells (at ~95% confluence in a 10-cm dish) were transfected
with 10 µg transfer vector, 5 µg packaging plasmid
and 5 µg envelope plasmid and the media harvested 48 and 72
h post-transfection. For the generation of the FastFUCCI-expressing
cells, Detroit 562 cells (~2×105 cells/well of a 24-well
plate) were transduced with virus at 10 multiplicity of infection
for 72 h and selected with 2 µg/ml puromycin over a 2-week
period. Following puromycin selection, cells were diluted to allow
the expansion of various clones and maintained at 1 µg/ml
puromycin. Clonal expansion proceeded for another 2 weeks so as to
generate cell stocks and enough cells for experimentation, totaling
31 days post-transduction. The expressed licensing factor Cdt1 was
fused to the fluorescent protein, monomeric Kusabira Orange 2, to
create the FUCCI reporter that allows visualization of cells in the
G1 phase. A fusion protein of geminin with fluorescent
monomeric Azami Green allows for the monitoring of cells in the
G2 phase.
Microscopy
FastFUCCI-expressing Detroit 562 cells were seeded
at 5×104 cells and cultured in 35-mm glass dishes and
the images were captured on a DMi8 fluorescence microscope (Leica
Microsystems GmbH). For the assessment of membrane integrity, the
cells were incubated at 37°C with 10 µg/ml PI one hour prior
to imaging.
Isothermal titration calorimetry
(ITC)
DNA-binding was measured by ITC on a MicroCal iTC200
instrument (Malvern Panalytical, Ltd.) as previously described
(16). Briefly, fragmented salmon
sperm DNA was dialyzed in 10 mM sodium phosphate, 0.15 M NaCl, pH
7.8 and prepared at 20 µg/ml concentration in the dialysate
supplemented with 1% DMSO and 0.005% P-20 to serve as the ligand.
The sample-representing buffer, 60 µM ethidium bromide
(EtBr), or 100 µM 24a-was similarly prepared in the
dialysate. Data are displayed as raw heat values without further
deconvolution.
Protein extraction, protein array and
western blot analysis
Detroit 562 cells were seeded at 5×106
cells in a 15-cm dish and cultured to ~80% confluence. Cells were
then trypsinized and seeded at ~3×106 cells/dish in nine
6-cm dishes, allowing for three dishes per treatment. After
overnight growth and upon reaching ~80% confluence, the dishes were
treated for 24 h with 0, 0.1 and 1 µM 24a at 37°C. DMSO was
maintained at 1% throughout. The suspended cells were then
collected from growth media by centrifugation at 600 × g for 5 min
at 25°C. The adherent cells were washed twice with PBS and the
cells from the washings were similarly collected. The non-ionic
buffer supplied by the Proteome Profiler Human Apoptosis Array kit
(cat. no. ARY009; lot no. 1487784; R&D Systems, Inc.) was
supplemented with complete protease inhibitor cocktail and 1.5%
phosphatase inhibitor cocktail 3 (both from Sigma-Aldrich; Merck
KGaA); a total of 600 µl buffer was used to lyse the three
plates representing each treatment. While keeping the cells on ice,
cell lysis was aided by scraping with a cell scraper and subsequent
sonication of the pooled fractions with six 5-sec pulses. After
centrifugation for 10 min at 18,407 × g at 4°C, the concentrations
of the clarified lysates were determined by BCA method using BSA as
standard. The lysates were aliquoted and stored at -80°C until use.
For protein profiling, the Apoptosis Array permitted the detection
of 35 apoptosis-related proteins by antibodies spotted on
nitrocellulose membranes. A total of 400 µg protein was used
per membrane according to the manufacturer's protocol and
visualized by chemiluminescent detection (using manufacturer's
supplied reagents) on the Amersham Imager 680 instrument (Cytiva).
Blot densitometry was analyzed using ImageJ software, version 1.53k
(National Institutes of Health).
For western blot analysis, typically 10 or 20
µg total proteins were loaded into each well of a 4-12%
Bis-Tris minigel and detected by standard western blot technique.
Briefly, membranes were blocked for 1 h in 5% milk in TBS/0.1%
Tween-20 at room temperature by rocking and incubated overnight
with primary antibodies (prepared in blocking buffer) at 4°C. The
membranes were then washed three times with TBS/0.1% Tween-20 at
room temperature and incubated with HRP-conjugated secondary
antibody (prepared in blocking buffer) for 1 h and similarly
washed. Antibodies against β-actin (cat. no. 4970), GAPDH (cat. no.
5174), death receptor (DR)4 (cat. no. 42533), DR5 (cat. no. 8074),
DR6 (cat. no. 93026), Fas (cat. no. 4233), phosphorylated
(p)-Atg1/ULK1 (cat. no. 6888), poly (ADP-ribose) polymerase 1
(PARP; cat. no. 9542) p53 (cat. no. 2527), p-p53 (cat. nos. 9288
for protein phosphorylation at Ser-9; 9284 at Ser-15; 2526 at
Ser-33; and 9281 at Ser-392; cat. no. 2525 for protein acetylation
at Lys-382), p63 (cat. no. 39692) p-p63 (cat. no. 4981,
phosphorylation at Ser-160/162), p73 (cat. no. 14620) p-p73 (cat.
no. 4665, phosphorylation at Tyr-99), Rip1 (cat. no. 3493), tumor
necrosis factor receptors (TNFR)1 (cat. no. 3736) and TNFR2 (cat.
no. 3727) were purchased from Cell Signaling Technology, Inc. Atg7
(cat. no. Ab133528) was purchased from Abcam and Beclin-1 (cat. no.
NB500-249) was purchased from Novus Biologicals LLC. The primary
antibodies were used at 1:1,000 dilution and the secondary antibody
(anti-rabbit IgG, HRP-linked antibody; cat. no. 7074; Cell
Signaling Technology, Inc.) at 1:4,000 dilution. Detection was
performed by chemiluminescence using the SuperSignal West Pico PLUS
reagent (Thermo Fisher Scientific, Inc.) and visualization on the
Amersham Imager 680 instrument (Cytiva), followed by densitometry
determination using ImageJ software.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
Apoptotic cells were detected colorimetrically using
the HT TiterTACS Apoptosis Detection kit (R&D Systems, Inc.).
Detroit 562 cells were seeded overnight at ~3×104 cells
per well in 96-well plates and treated at 37°C the next day (~50%
confluence at the time of treatment) with 2-fold serial dilutions
of 24a, with 1% DMSO maintained throughout. After 24 h, the cells
were processed and detected at 450 nm absorbance on a plate reader
according to the manufacturer's instructions.
Caspase activity
Detroit 562 cells were seeded overnight in 6-cm
dishes at ~2×104 cells per dish, resulting in ~40% cell
confluence at the time of treatment. The growth media were removed
and replaced with fresh growth media containing 0, 0.1 and 1.0
µM 24a, with DMSO maintained at 1% throughout. After 24 h,
suspended cells were collected from the corresponding growth media
by centrifugation at 25°C at 600 × g for 5 min. The adherent cells
were washed once with PBS, similarly collected and pooled with the
cells collected from suspension. Cells from each treatment (pooled
adherent and suspension fractions) were added with 500 µl of
the supplied lysis buffer and lysed by two freeze-thaw cycles. Cell
lysates were then clarified by centrifugation at 18,407 × g at 4°C
for 10 min and the protein concentrations were determined by BCA
method. A total of 20 µg protein was used for each reaction
according to the manufacturer's protocol. Caspase-3/7 activity was
assessed by measuring the ability of the cell lysates to cleave the
substrate DEDVpNA and produce the colorimetric pNA whose absorbance
is measured at 405 nm (CaspACE Assay; Promega Corporation).
Similarly, the substrate AcVEIDpNA (Cayman Chemical Company) was
used to measure caspase-6 activity.
Statistical analysis
Unless indicated, mean ± standard deviation values
were obtained from triplicate experiments. The comparison of 24a
treatments with corresponding mock treatments were analyzed using
Prism software (version 5; GraphPad Software, Inc.), using one-way
analysis of variance with post hoc Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
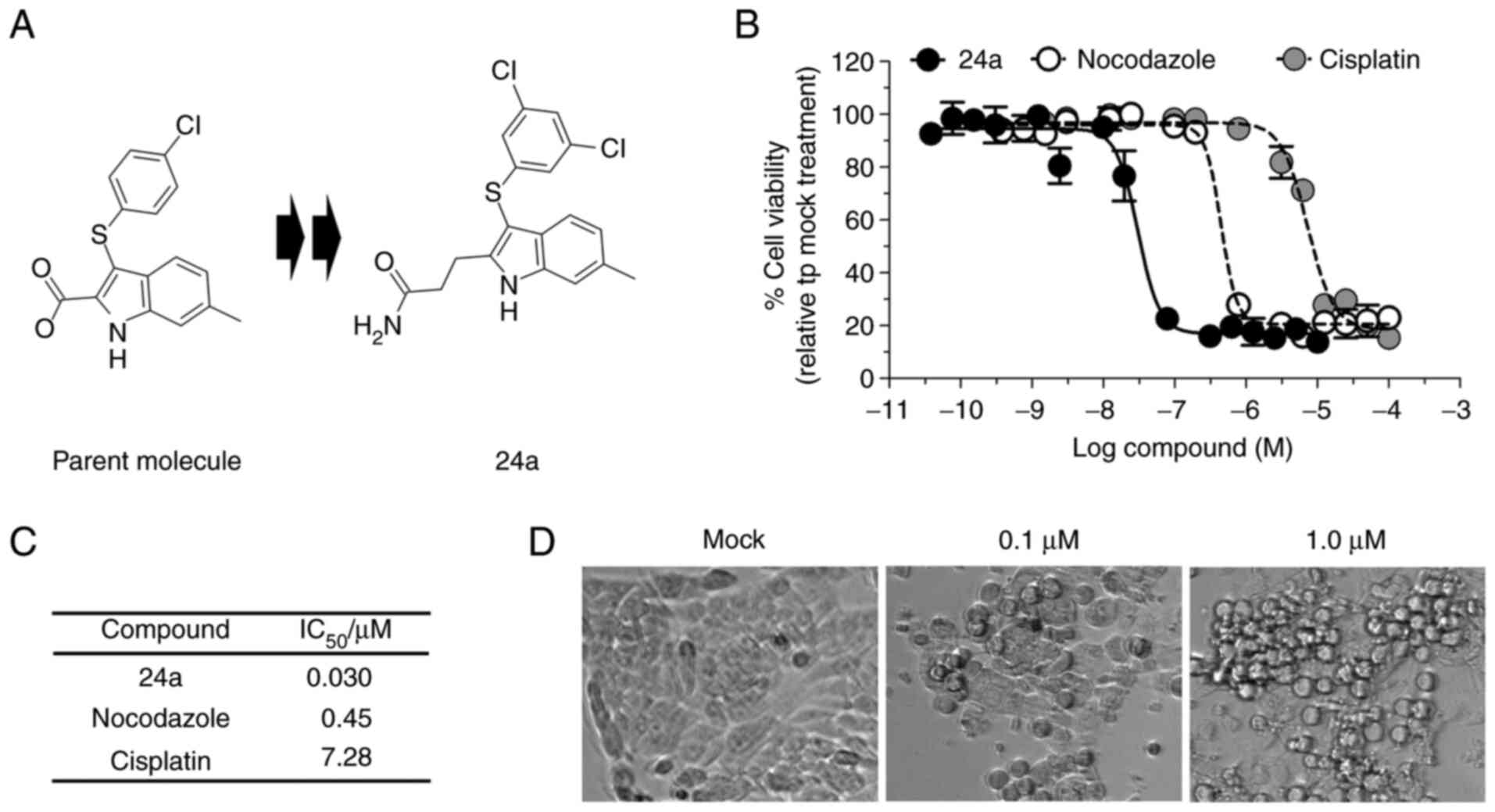
Identification of 24a as a potent
cytotoxic agent
A previous screen of small molecules against
vaccinia virus (VACV) has identified an indole-based scaffold from
which optimization steps are performed to produce antiviral pox
therapeutics (15). While the
optimized propanamide 24a (Fig.
1A) proves to have a potent antiviral activity against VACV
infection, it also exhibits appreciable cytotoxicity, effectively
eliminating the African green monkey kidney epithelial cells
(BSC-1) at a half-maximal inhibitory concentration
(IC50) of 16 µM following 24-h exposure (15). Investigation into longer exposure
(72 h) of 24a with various tumor cell lines unexpectedly revealed
the promotion of growth inhibition well into the nanomolar
concentrations (Fig. S1).
Therefore, it was sought to explore whether 24a was equally
effective at inhibiting cell growth of an HNSCC cell line.
The viability of Detroit 562 cells was measured
following 6 days of treatment, which equated to at least 2 doubling
of cell populations observed in the mock treatment. Indeed, 24a
showed potent growth inhibition, with an extracted
IC50=0.03 µM (Fig.
1B and C). By comparison, the antimitotic agent nocodazole and
the chemotherapeutic agent cisplatin inhibited the proliferation of
Detroit 562 cells at IC50 values of 0.45 and 7.28
µM, respectively (Fig. 1B and
C). When the cells were visualized following a shorter
treatment time (24 h), morphological changes were evident in a
dose-dependent manner (Fig. 1D),
with the round and shrunken cells suggestive of apoptotic events
(17).
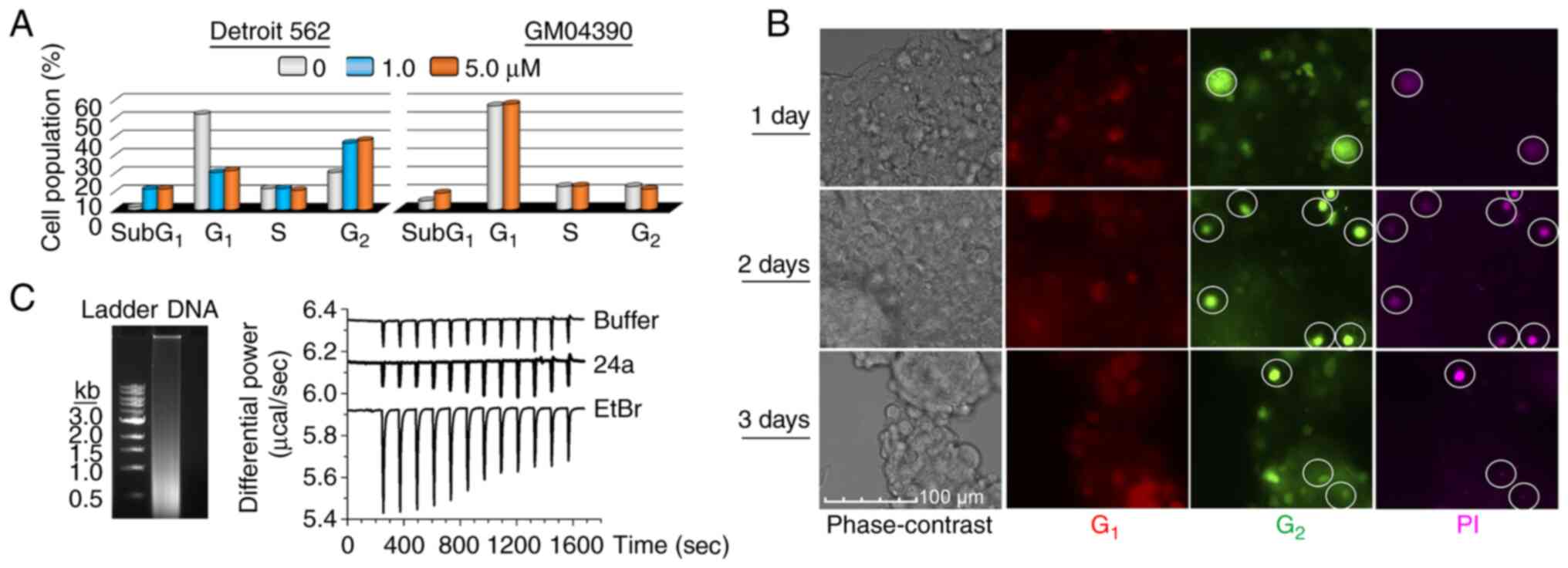
24a promotes G2/M
blockade
Since the potent growth inhibitory effect of 24a was
only observed after an extended treatment time, the effect on the
cell cycle was suspected and therefore examined. To accomplish
this, Detroit 562 cells were treated with 1 and 5 µM 24a for
24 h and characterized by flow cytometry after nuclear staining
with propidium iodide (PI). In comparison with the mock treatment,
the observed accumulation of cell populations in the G2
phase was 1.8- and 1.9-fold for 1 and 5 µM 24a treatments,
respectively (Figs. 2A and
S2). In addition, the
accumulation of subG1 cell populations further hinted at
the promotion of apoptosis. By comparison, 5 µM 24a failed
to accumulate normal fibroblasts (GM04390) in either the
G1 or G2 phase (Figs. 2A and S2), suggesting the sparing of cells
that were not as proliferative.
Next, it was sought to examine whether the
G2/M blockade could play a role in cell death. To
investigate real-time cell cycle progression, Detroit 562 cells
expressing the FUCCI were prepared from VSV-G pseudotyped
lentiviral particles produced by the all-in-one expression
cassette, FastFUCCI (18). By
monitoring the protein levels of the licensing factor Cdt1 (which
peaks during G1 and decreases entering S phase) and its
inhibitor geminin (which peaks during S, G2, and M
phases, but decreases during late mitosis and G1), DNA
replication could be monitored in live cells (19). The FastFUCCI-expressing Detroit
562 cells were treated with 0.03 µM 24a (at a low
concentration value that permitted the following of cell
progression without markedly causing cell death) and monitored 1-,
2- and 3-days post-treatments. At 1 h prior to microscopy, PI was
directly added to the growth medium to assess membrane integrity.
Compared with the undetectable PI-staining of the mock-treated
cells (Fig. S3), the
cell-impermeant PI specifically stained 24a-treated cells in the
G2 phase (Fig. 2B).
The absence of PI-staining of cells in the G1 phase
provided support that only cells that progressed to the
G2 phase lost membrane integrity and were susceptible to
cell death.
Finally, the lack of G1/S blockade by 24a
treatment would presumably argue against promiscuous DNA-binding as
a possible mode-of-action. To ensure that this was indeed the case,
direct DNA-binding was investigated by ITC. As expected, the
DNA-intercalating EtBr was able to generate a binding thermogram
when titrated with random, fragmented DNA, while 24a only generated
a thermogram similar to that of buffer alone, thus indicating the
lack of associated binding events (Fig. 2C). Taken together, 24a effectively
promoted growth arrest by G2/M blockade and cells
experiencing this blockade were susceptible to cell death.
The promotion of apoptosis as the key
underlying mode of cell death
To gain insight into the mode of cell death, protein
markers of necrosis (Rip1) and autophagy (p-Atg1, Atg7 and
Beclin-1) were initially examined by western blot analysis. As
revealed in Fig. 3A, the levels
of all investigated proteins either remained unchanged or decreased
when treated with 0.1 (low) and 1.0 µM (excess) 24a. Next,
TUNEL assay was performed following 24 h treatment to assess the
endpoint of apoptosis. Overall, the promotion of apoptosis was
observed over the mock treatment in all test concentrations; a
non-monotonic trend was exhibited by the increase of TUNEL response
for up to 0.25 µM 24a followed by decreasing response from
0.5-2 µM 24a (Fig. 3D). In
accordance with the TUNEL results, both caspase-3/7 and caspase-6
activities were significantly increased with increasing 24a
concentrations compared with mock treatments (Fig. 3B and C), demonstrating these
effector caspases as important contributors of apoptosis. Notably,
excess 24a consistently produced decreasing caspase activities
compared with the low dose, an observation consistent with the
decreasing TUNEL response at the same treatment concentration,
together implicating apoptosis as an important event at the low
dose. Given that poly (ADP-ribose) polymerase 1 (PARP) is a target
of caspases-3, -6 and -7 (20,21), PARP protein levels were further
assessed. Indeed, dose-dependent cleavage of PARP was observed
following 24a treatment (Figs. 3E
and 4C).
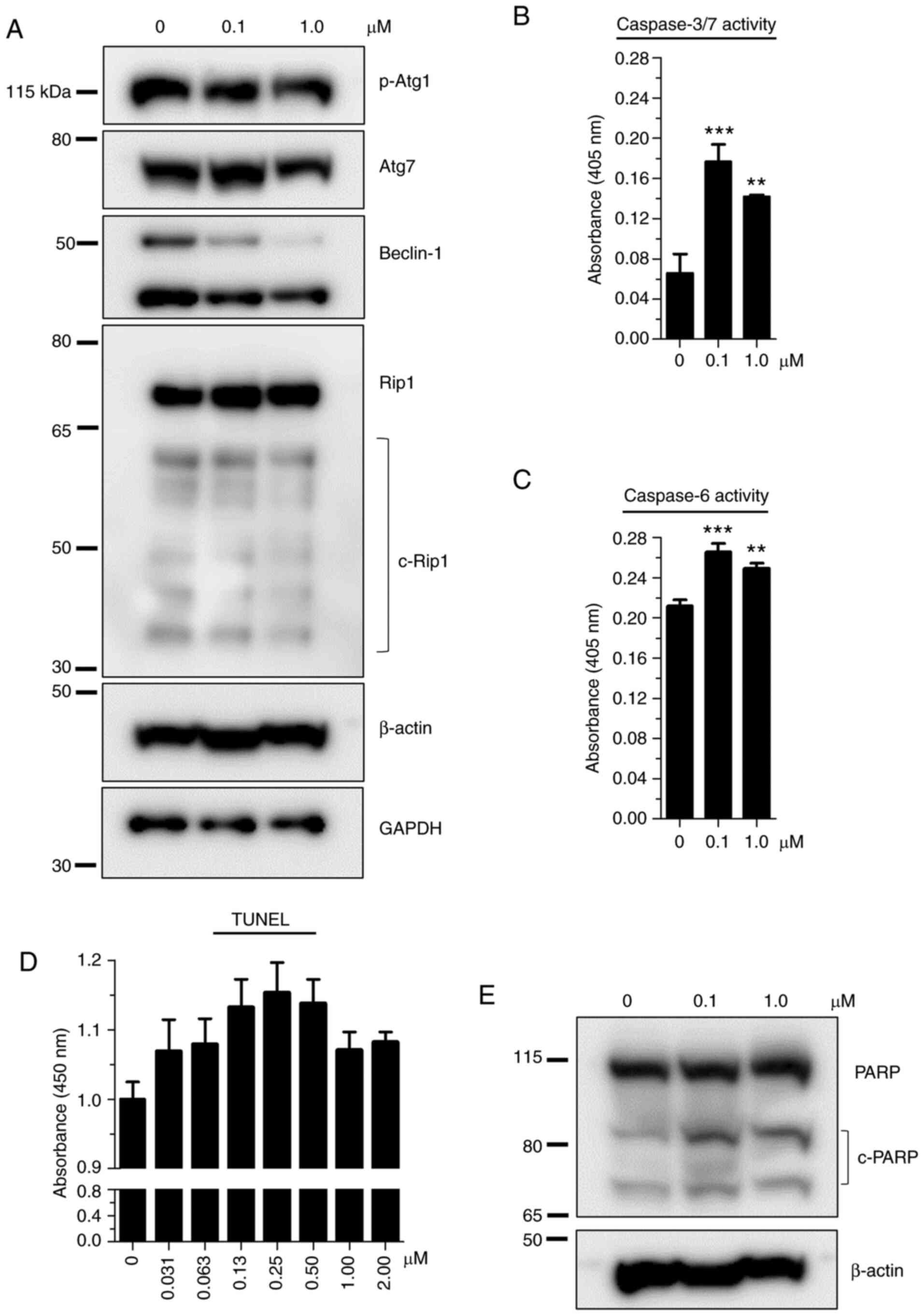 | Figure 3Examination of the effect of 24a on
the markers of autophagy, necrosis and apoptosis following 24 h
treatment. (A) Demonstration of the lack of (or negligible)
response from markers of necrosis and autophagy. Cell lysates were
prepared from Detroit 562 cells treated with 24a at the indicated
doses. As determined by western blot analysis, p-Atg1, Atg7 and
Beclin-1 represent protein markers of autophagy and Rip1 is a
marker of necrosis. Representative blots of duplicate experiments
are shown, with the typical β-actin as loading control for p-Atg1
and Atg7 and GAPDH as loading control for Beclin-1 and Rip1.
Supporting evidence for apoptosis following 24a treatment by (B)
caspase-3/7 and (C) caspase-6 activities, (D) TUNEL assay, and (E)
the cleavage of PARP protein. Values represent the mean ± SD of
n=3, with the treatment values compared with that of the
corresponding mock values. **P<0.01 and
***P<0.001 vs. 0 µM control. p-,
phosphorylated; c-, cleaved; PARP, poly (ADP-ribose) polymerase
1. |
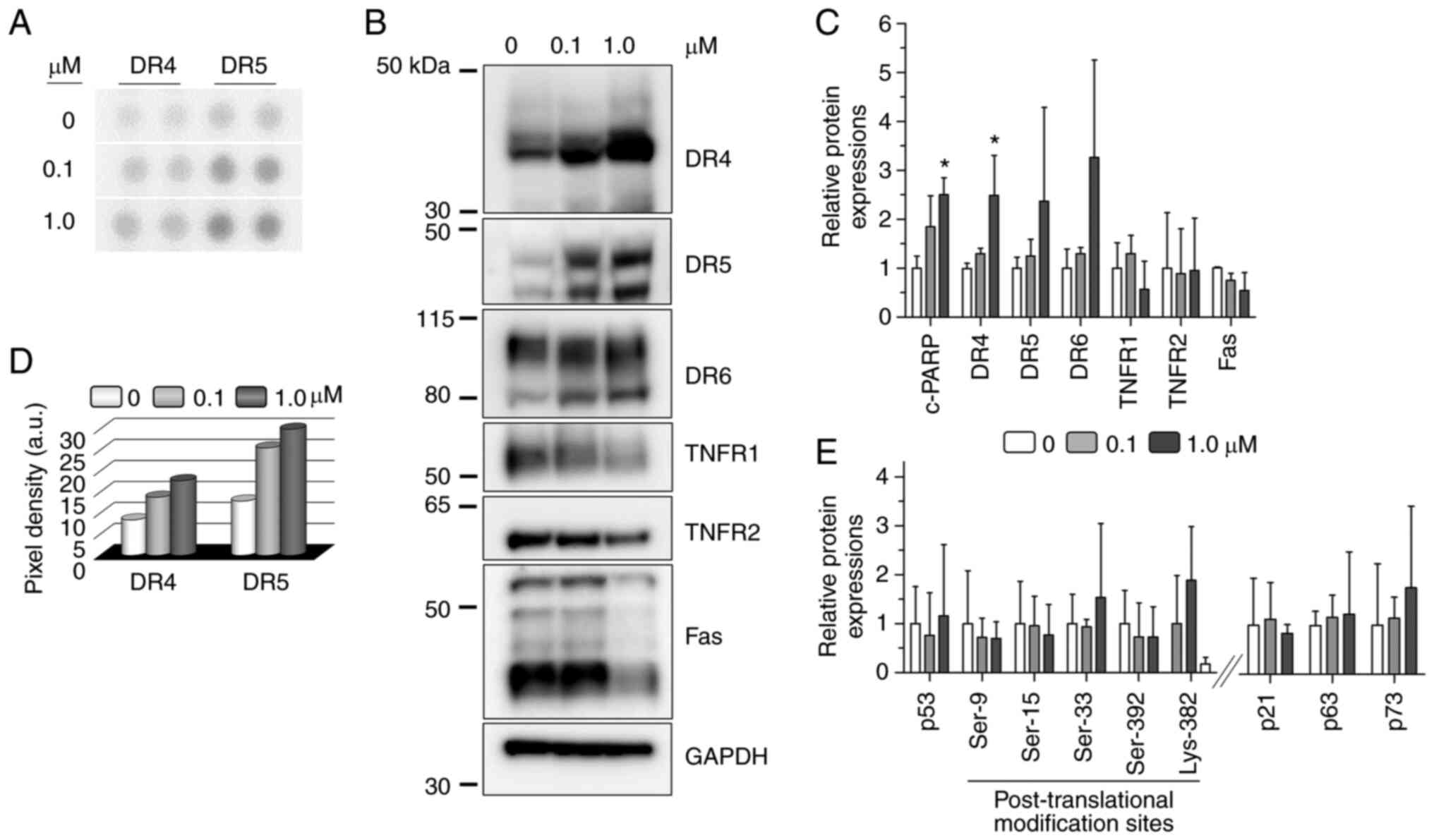
24a promotes the increase in expression
of DRs
Given that apoptosis plays an important role in the
observed cytotoxicity, it was next sought to gain insight into the
involvement of possible signaling proteins. To accomplish this,
Detroit 562 cells were treated with low and excess doses of 24a for
24 h and the lysates were specifically probed for apoptosis-related
proteins using the Human Apoptosis Array (Fig. S4). It is worth noting that a
longer incubation time was not investigated due to the depletion of
cell populations and the likely introduction of confounding cell
death events (data not shown). Out of the 35 apoptosis-related
proteins simultaneously probed in the array, only the response from
the DRs was appreciable, with quantifiable increase in the
expression levels of DR4 and DR5 compared with the mock treatment
(Fig. 4A and D). Western blot
analysis was further used to confirm the DR4 and DR5 results and to
sample other possible DRs that either gave weak responses between
the mock and treatments or were not included in the array.
Accordingly, both DR4 and DR5 showed a dose-dependent increase in
protein levels following 24a treatment (Fig. 4B and C). Another DR that was not
included among the array probes, DR6, also showed dose-dependent
increase in expression when exposed to 24a (Fig. 4B and C). In agreement with the
array, TNFR1 and Fas showed negligible to decreasing change in
protein levels following treatments (Fig. 4B and C). Similarly, a random
examination of TNFR2 (which is not included among the array probes)
showed negligible change in protein levels in response to 24a
(Fig. 4B and C). Taken together,
the results from the Apoptosis Array and independent probing by
western blot consistently showed 24a to specifically promote the
expression of DR4, DR5 and (to an extent) DR6 in response to 24a
treatment for both low and excess doses, while the other tumor
necrosis factor (TNF) superfamily members examined (TNFR1, TNFR2
and Fas) were not responsive.
24a does not promote p53, p63 and p73
activities
When cells are stressed or damaged, the tumor
suppressor p53 is an important transcriptional activator of target
genes that invoke apoptosis, senescence and cell cycle arrest.
However, the majority of human cancers contain incorrectly
functioning p53, with ~1/2 of these cancers owing to the
inactivation of the p53 protein as the result of mutations to the
TP53 gene (22). In particular,
68.2% of cell lines derived from an oral and pharyngeal cancer
panel have been demonstrated to exhibit TP53 mutations (8). Among the squamous cell carcinoma
cell lines, G>T (or an alternate G>A) transversion is
commonly observed concomitantly with the overexpression of p53
(23). Indeed, a c.524G>A
transversion results in the amino acid R175H substitution that
endows Detroit 562 with a gain-of-function p53 that promotes tumor
growth and metastasis (14). In
the present study, it was sought to determine whether p53 was
activated in response to 24a. Since the effect of p53 on cellular
functions largely requires post-translational modifications at more
than a single site (24,25), the status of various putative
phosphorylation and acetylation sites was investigated. As
summarized in Fig. 4E and shown
in Fig. S5, western blot results
showed the total protein levels of p53 and its post-translationally
modified forms remained constant following 24a treatment.
Next, the protein levels of the p53 paralogs (p63
and p73) were examined to assess their potential contributions.
While the wild-type proteins can serve redundant functions to p53,
differential promoter activation and splicing can generate dominant
negative (∆N) isoforms that lack the transactivation function,
thereby counteracting the tumor suppressive function of p53. Given
that p63 and p73 are rarely mutated, tumorigenesis is then
reflected by the expression levels of these ∆N isoforms relative to
the wild-type proteins (26). For
example, the alpha isoform of ∆Np63 is observed predominantly
overexpressed in HNSCC (27) and
likely contributes to oncogenesis (28). Similarly, the expression of the
∆Np73 isoforms also endows antiapoptotic and proliferative
properties (29). As summarized
in Fig. 4E and shown in Figs. S5 and S6 for Detroit 562 cell
lysates, the observed faster-migrating bands on the gels are more
in line with the expression of the ∆N isoforms of both p63 and p73.
As such, the negligible change in the expression levels following
24a treatment indicated the lack of contribution by both proteins.
In addition, 24a treatment produced no detectable protein
phosphorylation for either p63 at Ser-160/162 or p73 at Tyr-99
(Fig. S6), further indicating
the lack of contribution from either p63 or p73 on the basis of
phosphorylation status.
As a target of p53, p21WAF1/CIP1 can
suppress the growth of various tumor cell lines (30). Therefore, its presence is
considered indicative of wild-type p53 function (31). Accordingly, it was sought to
examine corresponding p21 protein levels in response to 24a
treatment. As summarized in Fig.
4E and shown in Fig. S5, p21
protein levels did not increase in a dose-dependent manner
following 24a treatment, further ruling out the involvement of
p53.
Discussion
Squamous cell carcinoma is the major form of
malignancy that arises in head and neck cancer and is challenging
to treat largely due to the detection of locally advanced diseases
(32). While surgery is
recommended for early-stage diseases, there is the need to avoid or
minimize undue morbidity and promote organ preservation (13). Such that unresectable tumors are
largely accessible by radiotherapy and chemotherapy, it follows
that the curative effort therefore must adopt multipronged
strategies whereby various drugs, in combination, attempt to
disrupt the various signaling events required by the cancer.
Indeed, cytotoxic agents remain the inevitable treatment options
for advanced cancers and metastases (33).
Cisplatin is a commonly used and important cytotoxic
agent that, through the aquation reaction (34), produces a highly potent
electrophile that can react to nucleophiles such as protein thiols
and nitrogen donor ligands of nucleic acid. Specifically, the
crosslinking of cisplatin to purines blocks cell division,
interferes with DNA repair and results in apoptosis, making it
effective against various cancers, including bladder, head and
neck, lung, ovarian and testicular cancers (12). Given the ability of cisplatin to
block non-homologous end joining in DNA repair (35), the concurrent treatment with
DNA-damaging radiation demonstrates cisplatin to be an effective
radiosensitizer for improving survival and reducing recurrence in
the management of unresectable HNSCC tumors (13). In another example, the combination
of cisplatin with 5-fluoruracil (another cytotoxic agent that
disrupts DNA synthesis) (36) and
cetuximab (a monoclonal antibody against epidermal growth factor
receptor, which is a marker of HNSCC) (37) represents an important first-line
treatment of recurrent and metastatic HNSCC tumors (38). However, with the improvement of
overall survival at only 10.1 months after this advanced treatment
strategy, it argues for the need to explore other options (39).
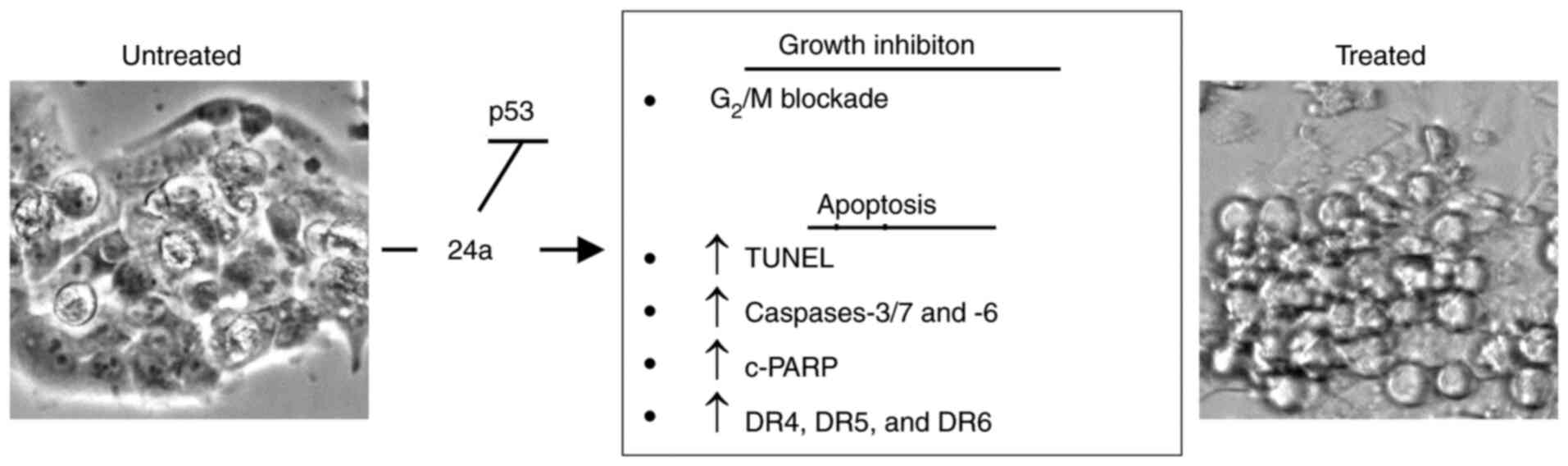
In the present study, 24a was identified as a potent
growth inhibitor against various tumor cell lines and specifically
described its cytotoxic action against a human pharyngeal carcinoma
cell line as owing to its antiproliferative and pro-apoptotic
properties (Fig. 5). Its
effectiveness appears to be largely due to the promotion of
apoptosis in cell populations arrested in the G2 phase.
Furthermore, the promotion of the expression of DR4 and DR5 could
prove interesting. Given that TRAIL promotes apoptosis in various
tumor cell lines and is viewed as a promising anticancer avenue
(40), the potential utility of
24a to promote synergy with TRAIL in a combination treatment could
represent one intriguing aspect for future studies.
To shed light on the underlying mechanism-of-action
of 24a, the tumor suppressor p53 was investigated due to its
contribution to the control of both the cell cycle and apoptosis,
as well as its role in the upregulation of DRs (41,42). Its importance is exemplified by
the fact that TP53 mutations are associated with poor prognosis for
patients with HNSCC (7).
Therefore, it was sought to explore whether 24a could prove
effective against an HNSCC cell model with a known TP53 mutation.
To this end, Detroit 562 serves such a purpose, as the R175H
mutation endows the cell line with a gain-of-function p53 that is
responsible for aggressive tumor growth and metastasis (14). Accordingly, the R175H mutation
represents a key hot spot found in numerous human cancers (43) and has been revealed to promote
genomic instability in a mouse model (44). As such, it was asked whether the
effectiveness of 24a could be due to the promotion of p53 activity.
This could be possible either by the restoration of wild-type
function through the reactivation of the R175H mutant or the
favoring of the residual wild-type p53 over the mutant protein.
Indeed, the suppression of tumorigenesis by wild-type activities
was observed for a heterozygous R175H mutant (44), and Detroit 562 is of unknown
zygosity according to the Catalogue of Somatic Mutations
(https://cancer.sanger.ac.uk/cosmic).
However, the present findings do not support the involvement of
p53, as evidenced by the lack of increase in the levels of
post-translationally modified p53 proteins or its downstream
effector p21. Additionally, no contribution was observed from its
paralogs p63 and p73.
In summary, the present study revealed that 24a is a
potent cytotoxic agent against malignant human pharyngeal squamous
carcinoma cells in vitro. Accordingly, the exhibited growth
inhibitory and pro-apoptotic properties appear to be due to
signaling pathway(s) independent of p53. As such, future studies
will be required to precisely identify the primary cellular target
of 24a. Additionally, eventual testing in an animal tumor model
will be required to demonstrate efficacy. In conclusion, 24a was
identified as a promising anticancer candidate that warrants future
studies.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
MN conceived and designed the study. MN, MRB and RPR
analyzed and interpreted the data. MN and MRB performed all the
experiments. MN, MRB and RPR confirm the authenticity of all the
raw data. MN wrote the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by The National Institute of
Allergy and Infectious Diseases (grant no. U01AI082211) and The
Joseph and Josephine Rabinowitz Award (School of Dental Medicine
Institutional Grant).
References
|
1
|
Vigneswaran N and Williams MD:
Epidemiologic trends in head and neck cancer and aids in diagnosis.
Oral Maxillofac Surg Clin North Am. 26:123–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brennan JA, Boyle JO, Koch WM, Goodman SN,
Hruban RH, Eby YJ, Couch MJ, Forastiere AA and Sidransky D:
Association between cigarette smoking and mutation of the p53 gene
in squamous-cell carcinoma of the head and neck. N Engl J Med.
332:712–717. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Souza G, Kreimer AR, Viscidi R, Pawlita
M, Fakhry C, Koch WM, Westra WH and Gillison ML: Case-control study
of human papillomavirus and oropharyngeal cancer. N Engl J Med.
356:1944–1956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poeta ML, Manola J, Goldwasser MA,
Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D,
Saunders J, et al: TP53 mutations and survival in squamous-cell
carcinoma of the head and neck. N Engl J Med. 357:2552–2561. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin D, Abba MC, Molinolo AA,
Vitale-Cross L, Wang Z, Zaida M, Delic NC, Samuels Y, Lyons JG and
Gutkind JS: The head and neck cancer cell oncogenome: A platform
for the development of precision molecular therapies. Oncotarget.
5:8906–8923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Algazi AP and Grandis JR: Head and neck
cancer in 2016: A watershed year for improvements in treatment? Nat
Rev Clin Oncol. 14:76–78. 2017. View Article : Google Scholar
|
|
10
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bar-Ad V, Palmer J, Yang H, Cognetti D,
Curry J, Luginbuhl A, Tuluc M, Campling B and Axelrod R: Current
management of locally advanced head and neck cancer: The
combination of chemotherapy with locoregional treatments. Semin
Oncol. 41:798–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartner L: Chemotherapy for oral cancer.
Dent Clin North Am. 62:87–97. 2018. View Article : Google Scholar
|
|
14
|
Sano D, Xie TX, Ow TJ, Zhao M, Pickering
CR, Zhou G, Sandulache VC, Wheeler DA, Gibbs RA, Caulin C and Myers
JN: Disruptive TP53 mutation is associated with aggressive disease
characteristics in an orthotopic murine model of oral tongue
cancer. Clin Cancer Res. 17:6658–6670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nuth M, Guan H, Zhukovskaya N, Saw YL and
Ricciardi RP: Design of potent poxvirus inhibitors of the
heterodimeric processivity factor required for viral replication. J
Med Chem. 56:3235–3246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nuth M, Guan H, Xiao Y, Kulp JL III,
Parker MH, Strobel ED, Isaacs SN, Scott RW, Reitz AB and Ricciardi
RP: Mutation and structure guided discovery of an antiviral small
molecule that mimics an essential C-Terminal tripeptide of the
vaccinia D4 processivity factor. Antiviral Res. 162:178–185. 2019.
View Article : Google Scholar
|
|
17
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh SB, Mascalchi P, Rodriguez E, Lin Y,
Jodrell DI, Richards FM and Lyons SK: A quantitative FastFUCCI
assay defines cell cycle dynamics at a single-cell level. J Cell
Sci. 130:512–520. 2017.
|
|
19
|
Sakaue-Sawano A, Kurokawa H, Morimura T,
Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi
H, et al: Visualizing spatiotemporal dynamics of multicellular
cell-cycle progression. Cell. 132:487–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufmann SH, Desnoyers S, Ottaviano Y,
Davidson NE and Poirier GG: Specific proteolytic cleavage of
poly(ADP-ribose) polymerase: An early marker of
chemotherapy-induced apoptosis. Cancer Res. 53:3976–3985.
1993.PubMed/NCBI
|
|
21
|
Chaitanya GV, Alexander JS and Babu PP:
PARP-1 cleavage fragments: Signatures of cell-death proteases in
neurodegeneration. Cell Commun Signal. 8:312010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Somers KD, Merrick MA, Lopez ME, Incognito
LS, Schechter GL and Casey G: Frequent p53 mutations in head and
neck cancer. Cancer Res. 52:5997–6000. 1992.PubMed/NCBI
|
|
24
|
Giaccia AJ and Kastan MB: The complexity
of p53 modulation: Emerging patterns from divergent signals. Genes
Dev. 12:2973–2983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bode AM and Dong Z: Post-translational
modification of p53 in tumorigenesis. Nat Rev Cancer. 4:793–805.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deyoung MP and Ellisen LW: p63 and p73 in
human cancer: Defining the network. Oncogene. 26:5169–5183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sniezek JC, Matheny KE, Westfall MD and
Pietenpol JA: Dominant negative p63 isoform expression in head and
neck squamous cell carcinoma. Laryngoscope. 114:2063–2072. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nylander K, Coates PJ and Hall PA:
Characterization of the expression pattern of p63α and δnp63α in
benign and malignant oral epithelial lesions. Int J Cancer.
87:368–372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bisso A, Collavin L and Del Sal G: p73 as
a pharmaceutical target for cancer therapy. Curr Pharm Des.
17:578–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leemans CR, Braakhuis BJM and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View Article : Google Scholar
|
|
33
|
Bailly C, Thuru X and Quesnel B: Combined
cytotoxic chemotherapy and immunotherapy of cancer: Modern times.
NAR Cancer. 2:zcaa0022020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knox RJ, Friedlos F, Lydall DA and Roberts
JJ: Mechanism of cytotoxicity of anticancer platinum drugs:
Evidence that cis-di amminedichloroplatinum(II) and
cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(ii) differ only
in the kinetics of their interaction with dna. Cancer Res.
46:1972–1979. 1986.PubMed/NCBI
|
|
35
|
Boeckman HJ, Trego KS and Turchi JJ:
Cisplatin sensitizes cancer cells to ionizing radiation via
inhibition of nonhomologous end joining. Mol Cancer Res. 3:277–285.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grem JL: 5-Fluorouracil: Forty-plus and
still ticking. A review of its preclinical and clinical
development. Invest New Drugs. 18:299–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pendleton KP and Grandis JR:
Cisplatin-based chemotherapy options for recurrent and/or
metastatic squamous cell cancer of the head and neck. Clin Med
Insights Ther. 2013:10.4137/CMT.S10409. 2013.PubMed/NCBI
|
|
40
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu GS, Burns TF, McDonald ER III, Jiang W,
Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sheikh MS and Fornace AJ Jr: Death and
decoy receptors and p53-mediated apoptosis. Leukemia. 14:1509–1513.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hainaut P and Hollstein M: p53 and human
cancer: The first ten thousand mutations. Adv Cancer Res.
77:81–137. 2000. View Article : Google Scholar
|
|
44
|
Liu DP, Song H and Xu Y: A common gain of
function of p53 cancer mutants in inducing genetic instability.
Oncogene. 29:949–956. 2010. View Article : Google Scholar :
|