Introduction
Increasing evidence has supported the notion that
tumor-targeted local radiation therapy (RT) may induce an
in-situ tumor vaccine and potentiate immune responses to
immunotherapy (1). Thus, in
addition to its local antitumor effects, RT may induce systemic
immune activation via the release of tumor-associated antigens from
dying tumor cells, which are taken up by dendritic cells to
cross-present them to naïve CD8+ T cells in the lymph
nodes. Following activation, the tumor antigen-specific T cells can
recognize and destroy tumor cells in the body at distant,
unirradiated anatomical sites, thereby turning tumors into
endogenous 'vaccines' through abscopal effects (2-4).
Therefore, alterations in the T-cell receptor (TCR) repertoire
post-RT may be useful as biomarkers predicting RT-induced systemic
T-cell immune activation.
RT is an established treatment option for the
management of localized prostate cancer (LPCa) (5), which aims to directly kill tumor
cells in the prostate gland and occasion- ally at distant anatomic
sites (6). The immune link for
such an abscopal effect in patients with LPCa has been attributed
to the generation of autoantibodies (7). Notably, RT has been shown to promote
antibody production against tumor antigens in patients with
non-small cell lung cancer (NSCLC) (8). The link with cellular antitumor
immunity has been demonstrated with the emergence of tumor
peptide-specific CD8+ T cells in patients with cancer
treated with RT (9), whereas
CD8+ T-cell depletion in animals has been shown to
significantly attenuate radiation-induced therapeutic effects
against tumors (10). Although RT
may induce some immune responses, the details are still not clear.
High-throughput sequencing of the T-cell receptor variable β (TCR
Vβ) chain repertoire is a tool used to examine how therapy changes
antigen-specific T-cell immunity. Profiling the TCR Vβ repertoire
in serial samples from patients can reveal features such as clonal
expansion, persistence and turnover of T-cell clones (11). Previous studies have demonstrated
that TCRs clustered by sequence similarity potentially target
similar antigens (11,12). By characterizing the TCR Vβ
repertoire before and after RT in patients with LPCa, the present
study aimed to provide indications as to whether RT affects
systemic cellular immunity, resulting in TCR Vβ clonal frequency
changes. Evaluation of blood samples at baseline and 3 months
post-RT revealed a dynamic remodeling of the circulating T-cell
repertoire based on the expansion and contraction of TCR Vβ
clonotypes, as well as on the appearance of new ones. To the best
of our knowledge, these findings provide the first evidence that RT
in LPCa may induce systemic immune changes, which could presumably
modulate clinical outcomes. Furthermore, these findings support the
application of RT in LPCa in combination with other therapeutic
treatments.
Materials and methods
Patients and sample collection
A total of 10 patients (age range, 58-83 years;
median age, 74 years), treated with external beam RT, were
recruited in the present study. Patients received either primary RT
(n=8), adjuvant RT post-radical prostatectomy (n=1) or salvage RT
post-radical prostatectomy (n=1) at the Department of Radiation
Oncology, Saint Savas Cancer Hospital (Athens, Greece). Variable RT
regimes with a daily dose/fraction ranging from 1.8 to 2.2 Gy
(median, 2 Gy) and total radiation doses between 66 and 72 Gy
(median, 70 Gy) were applied for an overall period of 35-38 days
(median, 37 days). Detailed clinical and radiation characteristics
are presented in Table I.
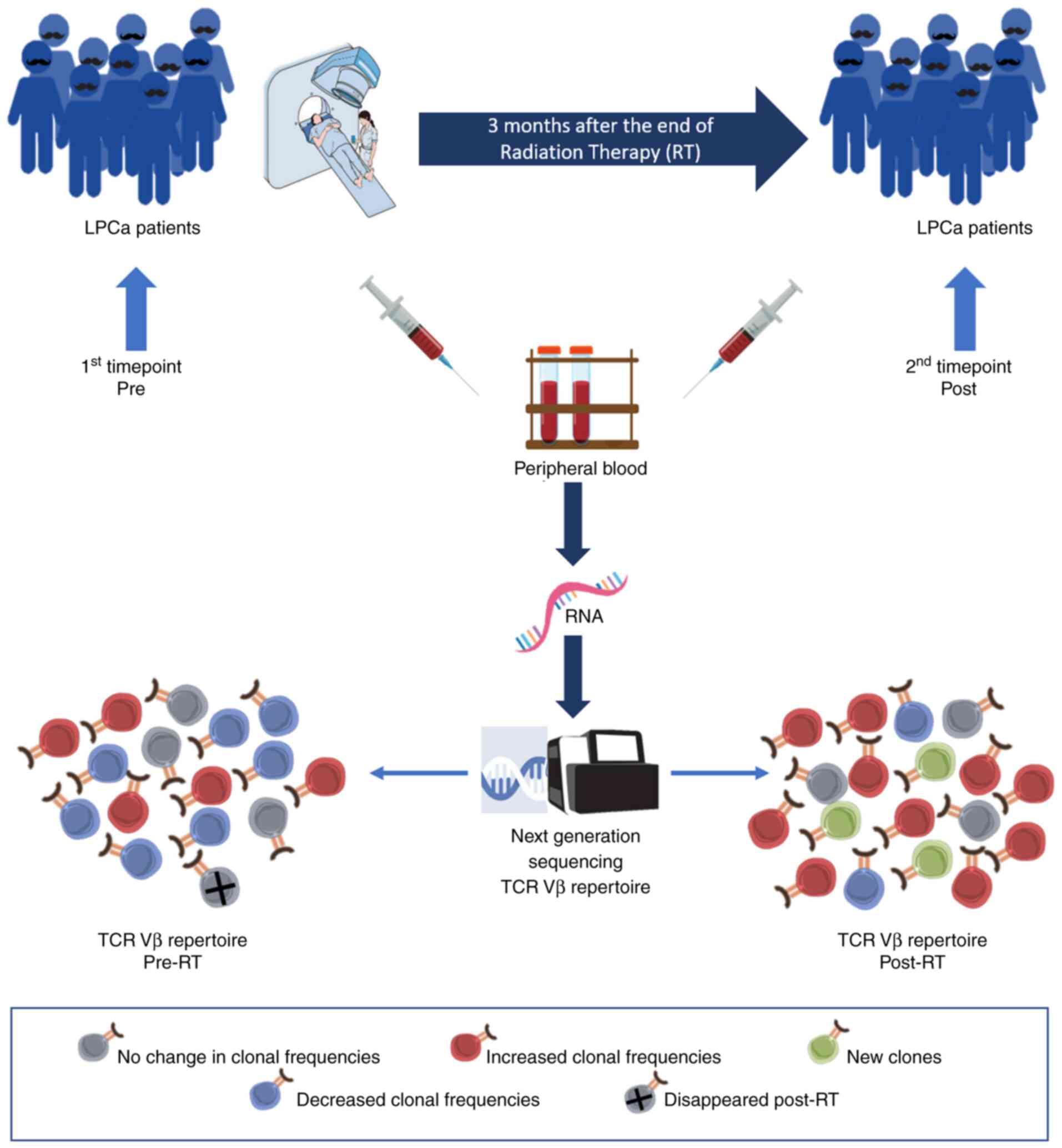
Peripheral blood samples were collected at two distinct
time-points: At diagnosis and 3 months after the completion of the
therapy. Fig. 1 shows the
experimental procedure of the present study.
 | Table IClinicopathological characteristics
of the patients with localized prostate cancer (n=10) enrolled in
the present study and details of RT. |
Table I
Clinicopathological characteristics
of the patients with localized prostate cancer (n=10) enrolled in
the present study and details of RT.
| Characteristic | Value |
|---|
| Median age at
diagnosis, years (range) | 74 (58-83) |
| PSA | |
| <10 ng/ml | 6 (60%) |
| 10-20 ng/ml | 1 (10%) |
| >20 ng/ml | 3 (30%) |
| Gleason score | |
| <6 | 3 (30%) |
| 7 (3+4) | 4 (40%) |
| 7 (4+3) | 2 (20%) |
| >8 | 1 (10%) |
| T stage | |
| T1c | 4 (40%) |
| T2a, T2b, T2c | 4 (40%) |
| T3a, T3b | 2 (20%) |
| Type of RT | |
| Primary | 8 (80%) |
| Adjuvant | 1 (10%) |
| Salvage | 1 (10%) |
| External beam 3D
conformal RT characteristics | |
| Median daily dose,
Gy (range) | 2 (1.8-2.2) |
| Median total dose,
Gy (range) | 70 (66-72) |
| Median radiation
treatment schedule, days (range) | 37 (35-38) |
Ethics approval
The present study was conducted in accordance with
the Declaration of Helsinki and all of the participants provided
written informed consent. The present study was approved by the
Saint Savas Cancer Hospital IRB (approval no.
IRB-ID6777/14-06-2017) and the Ethical Committee of the National
and Kapodistrian University of Athens as part of a larger project
(approval no. ID247/28-01-2020).
RNA isolation
Total RNA was extracted from peripheral blood
samples collected in K2EDTA tubes (BD Vacutainer™; BD
Biosciences) using the PureLink™ Total RNA Blood Kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The RNA was then quantified using a Qubit Fluorometer
3.0 with the Qubit™ RNA HS Assay Kit (Invitrogen; Thermo Fisher
Scientific, Inc.).
TCR Vβ library preparation for next
generation sequencing
The Oncomine™ TCR Beta-LR Assay (cat. no. A35386;
Thermo Fisher Scientific, Inc.) was adopted for profile analysis of
the TCR Vβ repertoire in blood samples from patients with LPCa that
were subjected to RT. This is a highly sensitive, RNA-based next
generation sequencing assay suitable for the characterization of
the TCR Vβ sequences, including all complementarity-determining
regions (CDR1, CDR2, CDR3) of the variable domain. The assay
accurately determines TCR Vβ diversity and clonal expansion, and
allows for identification of allele-specific polymorphisms in
peripheral blood samples.
The extracted RNA from the peripheral blood of
patients before and after RT was used for TCR Vβ analysis using the
Oncomine TCR Beta-LR Assay, according to the manufacturer's
instructions. Briefly, following DNase treatment with ezDNase™
(Invitrogen; Thermo Fisher Scientific, Inc.), a minimum of 25 ng
RNA was reverse transcribed using the Superscript™ VILO™ cDNA
Synthesis Kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Target amplification was
performed using the Oncomine TCR Beta-LR Assay followed by library
preparation using the Ion AmpliSeq™ Library Kit Plus and the Ion
Select™ Barcode Adapters (Ion Torrent; Thermo Fisher Scientific,
Inc.). Following purification with the Agencourt™ AMPure™ XP
Reagent (Beckman Coulter, Inc.), all individual libraries were
quantified using the Ion Library TaqMan™ Quantitation Kit (Ion
Torrent; Thermo Fisher Scientific, Inc.), diluted to 25 pM and
pooled. Following template preparation and chip loading with the
Ion Chef™ System, libraries were sequenced with the Ion GeneStudio™
S5 System using the Ion 510™ & Ion 520™ & Ion 530™ Kit-Chef
(cat. no. A34461) and the Ion 530™ Chip Kit (cat. no. A27763) (all
from Ion Torrent; Thermo Fisher Scientific, Inc.) (single-end
sequencing, 400 bp nucleotide length). Subsequent immune repertoire
analysis was performed using the Ion Reporter™ Software 5.16 with
the Oncomine TCR Beta-LR Single Sample workflow (both from Ion
Torrent; Thermo Fisher Scientific, Inc.).
Graph preparation and statistical
analyses
TCR Vβ sequencing results were automatically
analyzed using the Ion Reporter Software. Additional data plotting
and statistical analyses were performed using GraphPad Prism 6.0
for Windows (GraphPad Software, Inc.). Data are presented as the
mean ± standard deviation. Non-parametric Wilcoxon's test was
performed for the identification of differences in TCR Vβ sequences
among patients before and after RT. For some of the analyses, the
patients were divided into two groups, based on the appearance of
new TCR Vβ clones (Group II) or not (Group I) following RT.
Individual paired t-tests were performed for each patient to
analyze V gene segments before and after RT, and unpaired t-tests
were performed for each V gene to analyze its frequency in Group I
vs. Group II. P<0.05 was considered to indicate a statistically
significant difference. ClustalX graphical alignment tool was used
for clonotype-sequence comparisons (13).
Results
Effect of RT on TCR clonal frequencies in
patients with LPCa
A total of 20 samples from 10 patients with LPCa
receiving RT, as aforementioned, were included in the present
analysis. The median age was 74 years (range, 58-83 years). Six
patients had a Gleason Score (GS) of 7 (GS 3+4, n=4; GS 4+3, n=2),
three patients had a GS <6 and one patient had a GS >8.
Clinical and external beam radiation characteristics are summarized
in Table I.
RT-induced changes in the diversity of the TCR Vβ
repertoire were determined by TCR Vβ sequencing of RNA isolated
from peripheral blood at baseline and 3 months post-RT. This time
point (i.e., 3 months) was chosen based on previous findings, which
have demonstrated significant RT-induced immune changes in patients
with cancer (14). On average,
534,677.95 sequence reads were obtained, which were mapped to the V
and joining segments, and could identify unique TCR Vβ clonotypes.
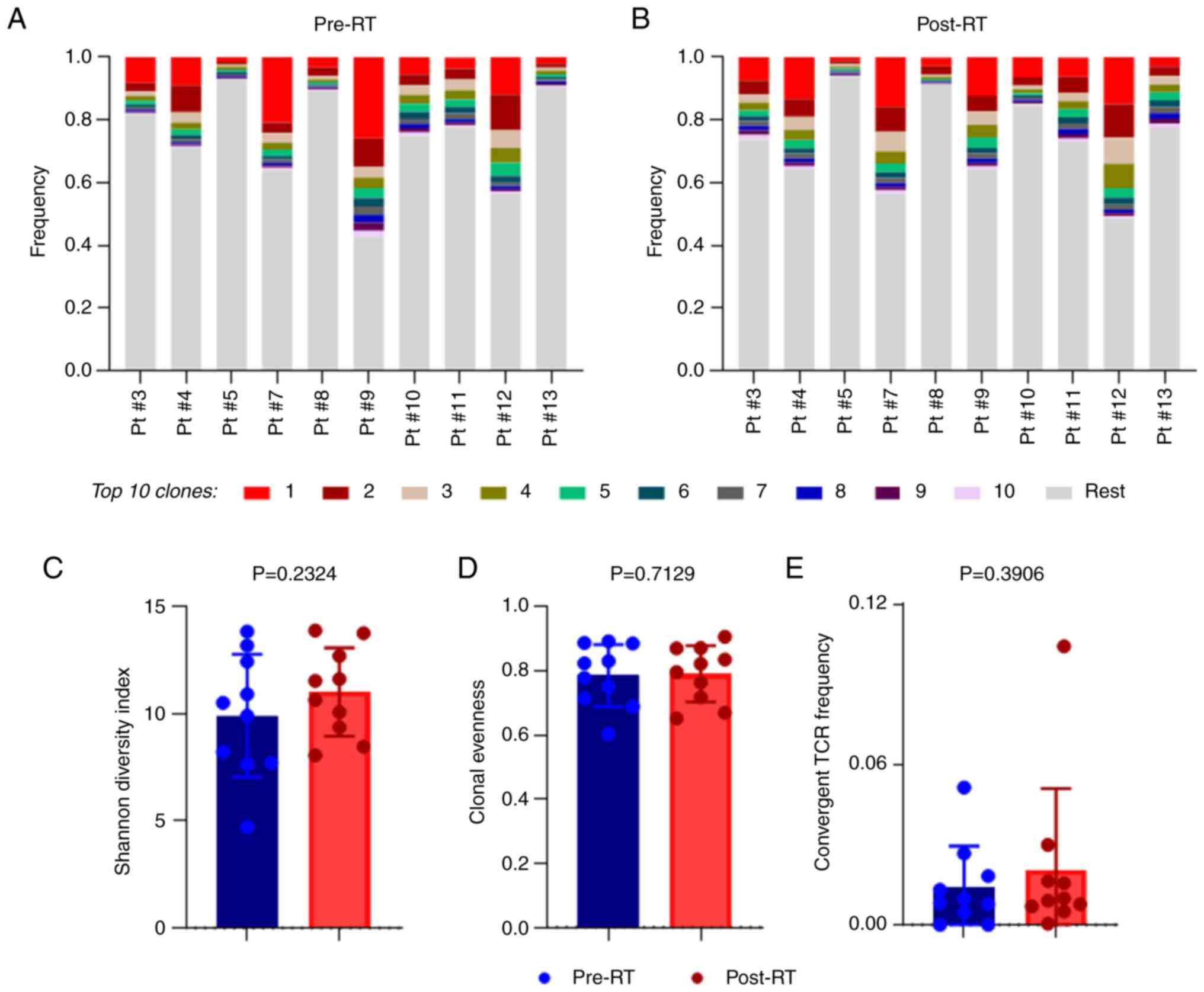
The frequencies of the top 10 clonotypes for all 10 patients
analyzed either pre-RT (Fig. 2A)
or post-RT (Fig. 2B) ranged from
0.0023 to 0.2591, which suggested a high variability in TCR Vβ
diversity between patients.
To explore the effect of RT on the TCR Vβ
repertoire, T-cell complexity was measured both at baseline and
post-RT. It was revealed that the diversity index was increased
post-RT compared with baseline; however, this was not significant
(Fig. 2C). The similarity in the
TCR Vβ repertoire before and after RT was also reflected by clonal
evenness, as shown in Fig. 2D. In
addition, the frequency of convergent TCR Vβs was also similar at
both time-points (Fig. 2E).
There were changes in clonal frequencies (CFs) among
the top 10 TCR Vβ clonotypes following RT. Table SI presents a representative
example of how the frequencies of the top 10 clonotypes changed
post-RT in one patient. Fig. 3
shows the alterations in the top 10 TCR Vβ clonotype frequencies
before and post-treatment in each patient. The CDR3 amino acid (AA)
sequences of the clonotypes, which entered the top 10 post-RT in
all patients, were mainly occupied by polar (S, T, Y, Q) and
negatively charged (D, E) residues (average frequency 58±11%).
RT-induced alterations in V gene segment
usage frequencies in patients with LPCa
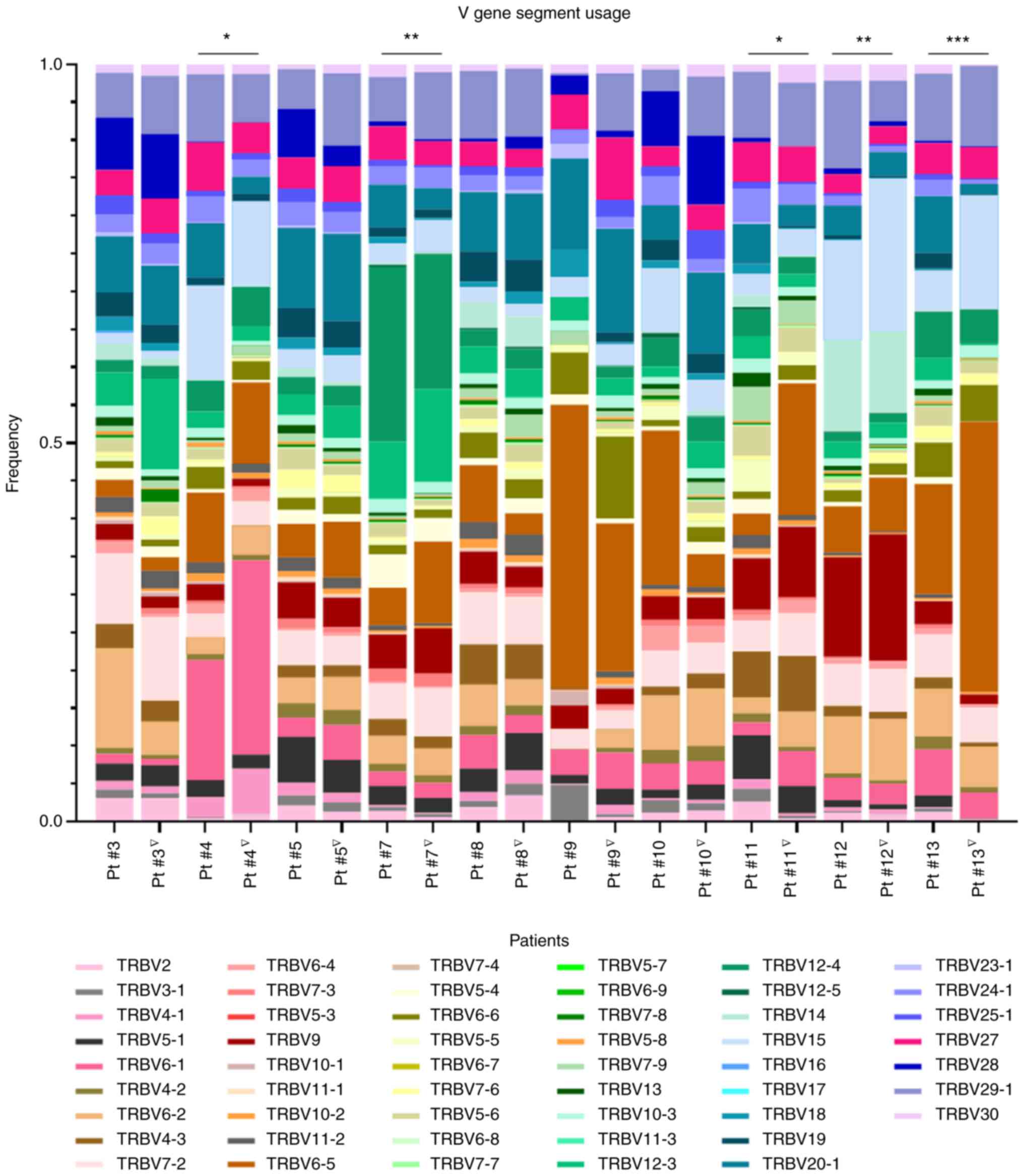
The highly expressed Vβ gene segments were also
analyzed pre-RT as well as post-RT for each of the 10 patients and
the results are shown in Fig. 4.
Unpaired grouped analysis of each one of the V gene segment usage
frequencies for all the patients pre- and post-RT did not give
statistically significant differences (data not shown). However, by
comparing V gene segment usage pre- and post-RT for each patient,
statistically significant differences were identified in the
frequencies of five patients (Pt #4, Pt #7, Pt #11, Pt #12 and Pt
#13). Notably, >2 fold-change increases (ranging from 2.03 to
26.31) and decreases (ranging from 2.01 to 157.59) among the 53
different TCR Vβ clonotypes tested pre- and post-RT, although not
statistically significant, were frequently observed for all
patients (at various numbers for every patient) and were suggestive
of RT-induced alterations in systemic T-cell immunity (Fig. S1). Subsequently, the patients
were divided into two groups, based on the appearance of new TCR Vβ
clones (Group II) or not (Group I) following RT. Notably, the three
patients (Pt #4, Pt #9 and Pt#13) who developed new clonotypes
(NCs) (Group II) all had a high GS [8 or 7 (4+3)], in contrast to
the other patients who had a GS of 6 or 7 (3+4) (Group I) and did
not develop NCs. Statistically significant differences in the usage
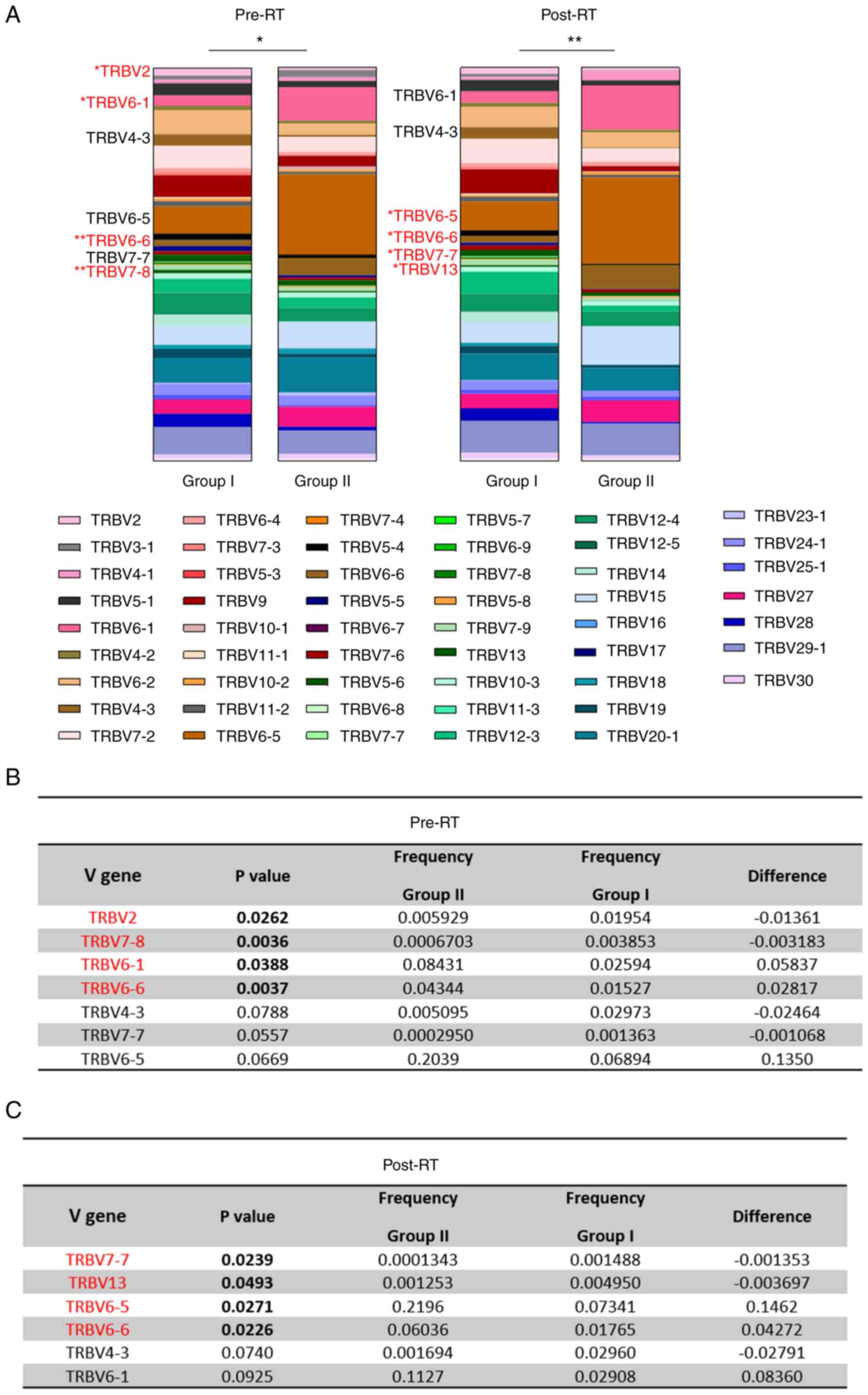
of specific V gene segments between the two groups of patients were
detected, both before and after RT (Fig. 5A). Individual t-tests were
performed for each V gene and revealed statistically significant
alterations in the usage of certain V gene segments between the two
patient groups. Specifically, before RT, there was a statistically
significant higher usage frequency of TRBV2 (P=0.0262) and TRBV7-8
(P=0.0036) and lower usage frequency of TRBV6-1 (P=0.0388) and
TRBV6-6 (P=0.0037) in the low-risk patient group (Group I) as
compared with the high-risk group (Group II) (Fig. 5B). Moreover, there were some
notable differences in the CFs between the two groups of patients
pre-RT that were not statistically significant. For example, the
frequency of TRBV4-3 and TRBV7-7 was lower (P=0.0788 and 0.0557,
respectively), and that of TRBV6-5 was higher (P=0.0669) in Group
II compared with Group I. Post-RT, there was a statistically
significant higher usage frequency of TRBV7-7 (P=0.0239) and TRBV13
(P=0.0493), and lower usage frequency of TRBV6-5 (P=0.0271) and
TRBV6-6 (P=0.0226) in the low-risk patient group (Group I) as
compared with the high-risk group (Group II) (Fig. 5C). In addition, post-RT there were
non-significant trends in CF differences between Groups I and II.
For example, the frequency of TRBV4-3 was lower (P=0.0740) and that
of TRBV6-1 was higher (P=0.0925) in Group II compared to Group I.
Notably, the usage of the TRBV6-6 segment was found to be higher in
the high-risk group of patients compared with the low-risk group,
both pre- and post-RT. This particular V gene segment was also
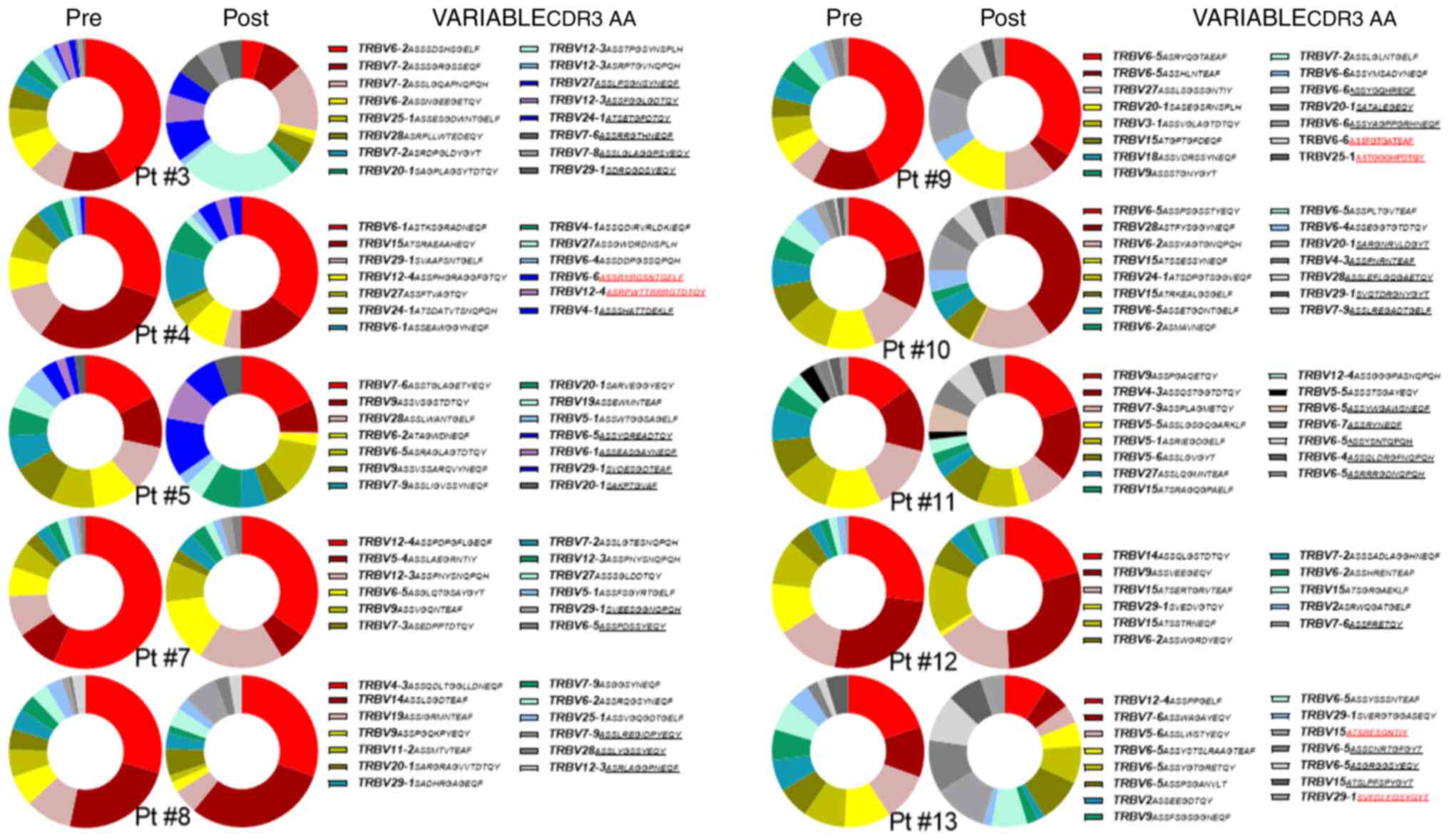
detected within the NCs of Pt #4 and Pt #9, post-RT (Fig. 3). Intragroup analyses revealed no
statistically significant differences between the frequencies of V
genes pre- and post-RT among patients belonging to Group I, whereas
in Group II, the frequency of TRBV9 was higher post-RT in
comparison with the frequency pre-RT (P=0.0336) (data not
shown).
RT-induced alterations in the top 10 TCR
clonal frequencies and emergence of new clonotypes
Subsequently, the present study aimed to identify
TCR Vβ CFs that were not detect- able among the top 10 clonotypes
before RT, but appeared among the top 10 clonotypes post-RT. By
contrast, the present study also searched for TCR Vβ CFs that were
present in the top 10 at baseline but were not detectable among the
top 10 clonotypes post-RT. Notably, some clonotypes that had a low
CF at baseline, with some being far below the top 10 TCR Vβ CFs,
were identified that had expanded post-RT and were in the top 10
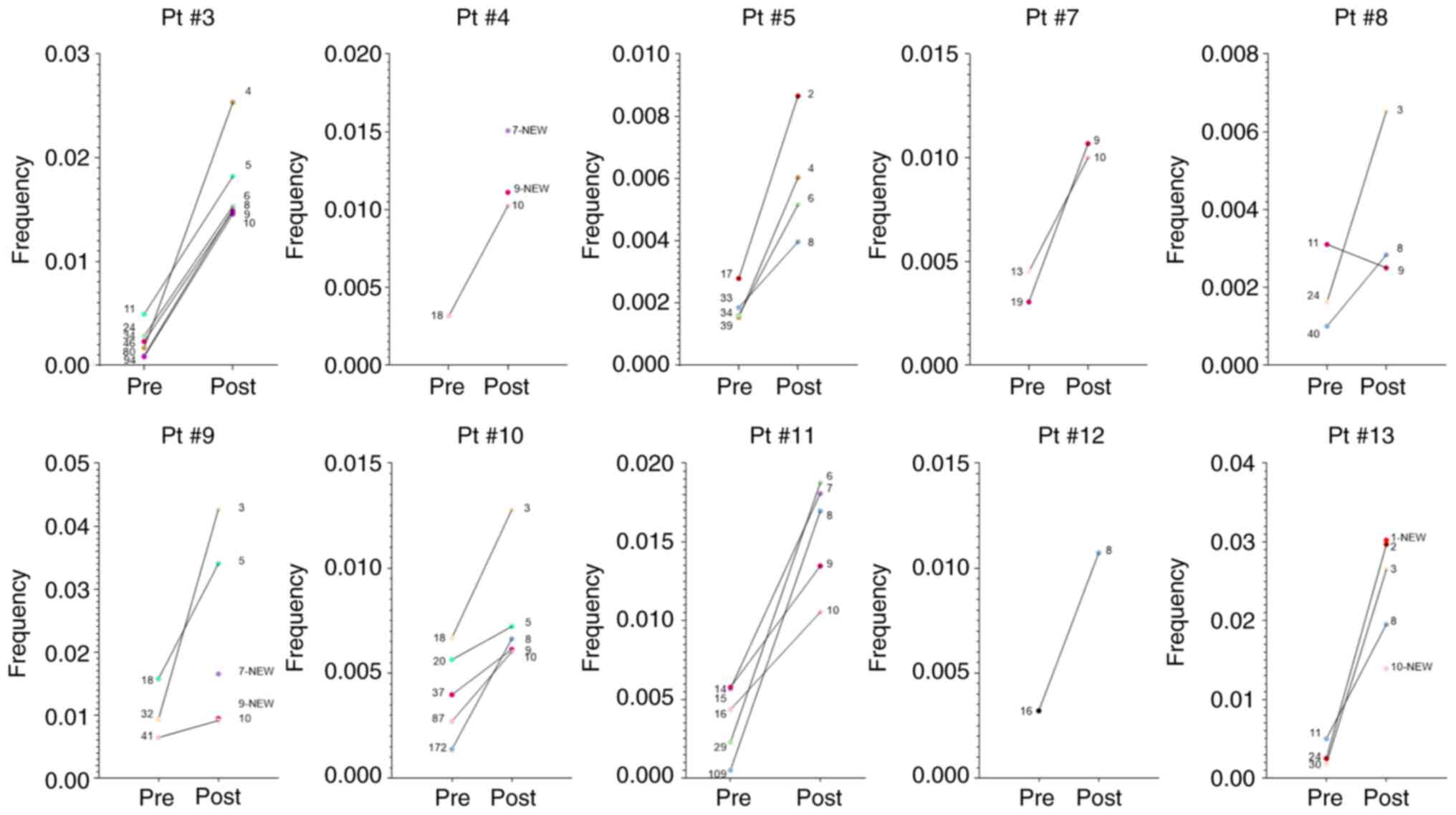
TCR Vβ CF. For example, as shown in Fig. 6, Pt #3 had six clonotypes that
were below the top 10 TCR Vβ CFs (ranked at positions 11, 24, 34,
46, 80 and 94), with frequencies ranging from 0.0008 to 0.0049,
which were increased post-RT, ranging from 0.014 to 0.025, thus
entering the top 10 CFs (ranked at positions 4, 5, 6, 8, 9 and 10).
In total, for the 10 patients analyzed post-RT, 33 expanded TCR Vβ
clonotypes were identified, which entered the top 10 TCR Vβ CFs
(Fig. 6). Some exceptional cases
included two clonotypes that were ranked at positions 172 (Pt #10)
and 109 (Pt #11) at baseline, but post-RT were advanced to position
8 among the top 10 TCR Vβ CF (Fig.
6). Next, the present study searched for new TCR Vβ clonotypes
post-RT to suggest for a systemic T-cell immune activation more
convincingly in these patients. As shown in Fig. 3, the present study identified a
total of six NCs ranking in the top 10 TCR Vβ CF at positions 7 and
9 for Pt #4 (two clonotypes; Fig.
6), at positions 7 and 9 for Pt #9 (two clonotypes; Fig. 6) and at positions 1 and 10 for Pt#
13 (two clonotypes; Fig. 6).
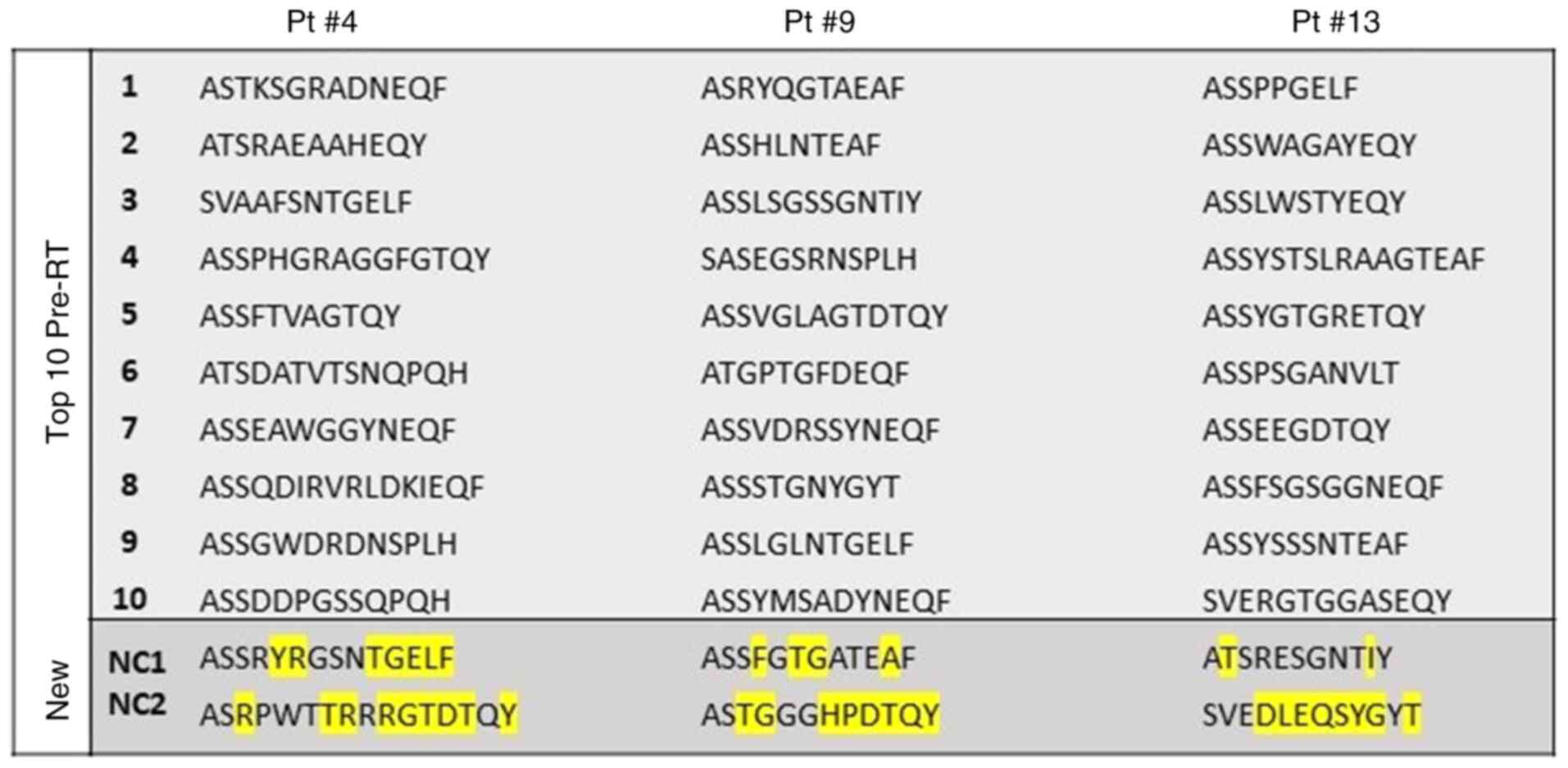
These identified NCs differed in their TCR Vβ CDR3 AA from the top
10 identified pre-RT. As shown in Fig. 7, NC1 of Pt #4 differed by seven
AAs (i.e., Y, R, T, G, E, L and F at positions 5, 6, 10, 11, 12, 13
and 14, respectively). The NC2 of this patient differed by nine AAs
(i.e., R, T, R, R, G, T, D, T and Y at positions 3, 7, 8, 10, 11,
12, 13, 14 and 16, respectively). Similarly, Pt #9 had a NC1, which
differed by four AAs (i.e. F, T, G and A at positions 4, 6, 7 and
11, respectively) and a NC2 that differed by eight AAs (i.e., T, G,
H, P, D, T, Q and Y at positions 3, 4, 7, 8, 9, 10, 11 and 12,
respectively), whereas the two NCs of Pt#13 differed by two AAs
(NC1; i.e., T and I at positions 2 and 10, respectively) and by
eight AAs (NC2; i.e., D, L, E, Q, S, Y, G and T at positions 4, 5,
6, 7, 8, 9, 10 and 12, respectively).
In addition, cases that were characterized by
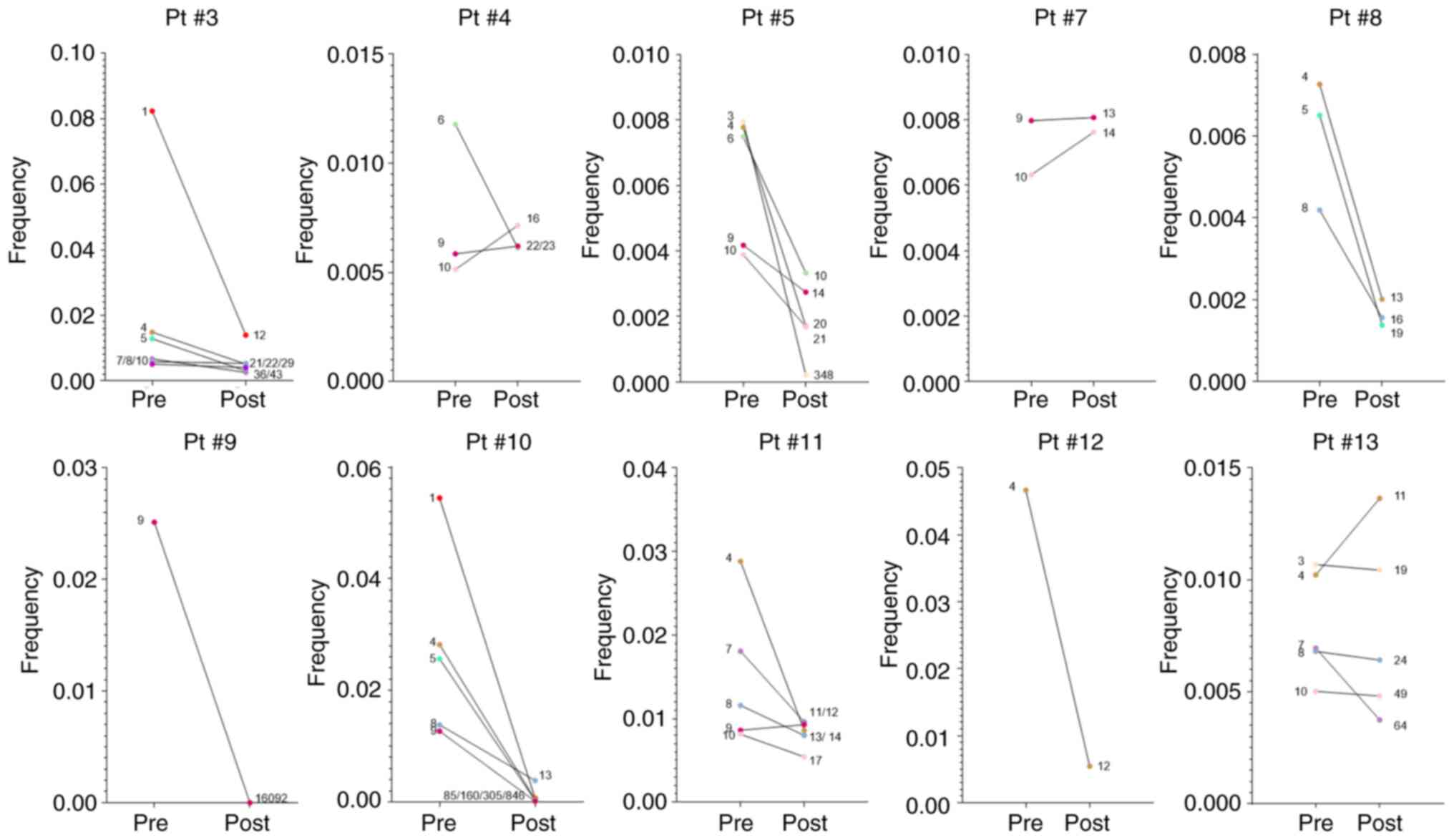
decreased TCR Vβ CF post-RT were identified (Fig. 8). For example, Pt #3 had six TCR
Vβ clonotypes, which at baseline were among the top 10 (ranking
positions 1, 4, 5, 7, 8 and 10) but post-RT ranked among positions
12-43 (Fig. 8). In total, for the
10 patients analyzed, 35 decreased TCR Vβ clonotypes were
identified post-RT, which were relegated from the top 10 TCR Vβ
CFs. Some exceptional cases included four clonotypes that were
lowered to positions 85, 160, 305 and 846 from positions 1, 4, 5
and 9, respectively (Pt #10) and one clonotype that was lowered
from position 9 to position 16,092 (Pt #9) (Fig. 8). Notably, six clonotypes were
detected, which although had increased CFs post-RT, they were still
relegated from the top 10 TCR Vβ CF; for Pt #4, one clonotype with
0.0058 CF pre-RT and 0.0062 CF post-RT ranked from position 9 to
22, and another one with 0.0051 CF pre-RT and 0.0071 CF post-RT
fell from position 10 to 16 (Fig.
8). Furthermore, for Pt #7, there were two clonotypes at
positions 10 and 9 with CFs 0.0063 and 0.0079 pre-RT, respectively,
which post-RT moved to positions 14 and 13, respectively, despite
increased CFs (0.0076 and 0.0080, respectively; Fig. 8). There were also another two
cases with clonotypes that were degraded post-RT compared with
pre-RT; however, they had higher CFs post-RT compared with pre-RT.
Specifically, in Pt #11 the clonotype at position 9 degraded to
position 12 (0.0086 to 0.0092) and in Pt #13 the clonotype at
position 4 degraded to position 11 (0.010 to 0.014) (Fig. 8). This could be due to the fact
that in these patients the frequencies of their TCR Vβ clonotypes
were relatively high at the lowest ranking of top 10 post-RT (i.e.,
position 10). These findings are presented in Table II, where the CFs of the TCR Vβ
clonotypes ranking at position 10 (of the top 10 TCR Vβ CFs)
post-RT for Pt #3, Pt #4, #7, #11 and #13 ranged from 0.010009 to
0.013911, thus being higher compared with the respective CFs for Pt
#5 (0.003327; 3.0 fold-4.2 fold), Pt #8 (0.002364; 4.3 fold-6.0
fold), Pt #9 (0.00917; 11.1 fold-15.4 fold), Pt #10 (0.00632; 1.58
fold-2.2 fold) and Pt #12 (0.008005; 1.25 fold-1.73 fold).
 | Table IICFs of the T-cell receptor variable β
clonotypes ranking in the last position (position 10) of the top 10
clonotypes in each patient post-radiation therapy. |
Table II
CFs of the T-cell receptor variable β
clonotypes ranking in the last position (position 10) of the top 10
clonotypes in each patient post-radiation therapy.
| Patient no. | CF of clone at
position 10 |
|---|
| Pt #3 | 0.014594 |
| Pt #4 | 0.010252 |
| Pt #5 | 0.003327 |
| Pt #7 | 0.010009 |
| Pt #8 | 0.002364 |
| Pt #9 | 0.00917 |
| Pt #10 | 0.006032 |
| Pt #11 | 0.010548 |
| Pt #12 | 0.008005 |
| Pt #13 | 0.013911 |
Discussion
RT is a standard treatment for PCa. Clinically,
although RT directly induces cancer cell death, an abscopal effect
expressed by the regression of distant tumors via systemic immune
activation is occasionally also observed (15). To the best of our knowledge,
details on TCR CF alterations post-RT linking an abscopal effect
with antitumor T-cell immunity in patients with LPCa have not yet
been described. In the present study, the dynamics of systemic
changes in frequencies among the top 10 TCR Vβ clonotypes before
and after RT were investigated, and it was revealed that among the
patients analyzed, a total of 33 TCR Vβ clonotypes were expanded in
frequencies that ranged from 0.81-fold to 33.18-fold. Taking into
consideration the fact that different clonotypes are characterized
by marked differences in their CDR3 AA sequence and length,
alterations in TCR CFs could indicate alterations in their
antigen-targeting and recognition properties. Consequently, the
detection of expanded TCR Vβ clonotypes post-RT was of particular
interest since these may signify disease prognosis and/or response
to therapy. To this end, significant differences in the usage of
specific V gene segments in the two groups of patients stratified
by high or low GS were detected, by comparing their CFs before and
after RT. Notably, TRBV6-6 segment usage was more frequent in the
high-risk group (Group II) compared with the low-risk group, both
pre- and post-RT. Similarly, the frequency of TRBV6-5 usage was
higher in the high-risk group compared with the low-risk group
post-RT. In a recent study, high usage of TRBV6-5 in patients with
advanced NSCLC was shown to be associated with disease progression
and a poor prognosis, and it was also revealed to be a predictive
marker of non-durable clinical benefit of anti-PD-1 treatment
(16). Moreover, deep TCR-β
sequencing in tissue samples from prostate tumors revealed an
abundance of both TRBV6-5 and TRBV6-6 in paracancerous tissue, but
not within the tumor (17). By
contrast, high usage of certain V segments has been associated with
a favorable prognosis in several tumor types. Notably, high
TRBV20-1 usage in patients with NSCLC has been reported to be
associated with improve- ments in both progression-free and overall
survival, as well as with an increased response to anti-PD-1
treatment (16). Taken together,
these data may suggest that the preferential usage of specific V
gene segments could have an important role in determining the
levels and duration of T-cell-mediated antitumor immunity. Of
particular interest was the detection of new TCR Vβ clonotypes
post-RT in patients with high GS, presumably recognizing new tumor
peptides released by RT-induced tumor cell death. Although PCa is
characterized by the expression of unique tumor antigens, which
could act as an excellent tool for triggering robust antitumor
immune responses (18), its
immunogenicity is still hampered by the immunosuppressive tumor
microenvironment and the low tumor mutation burden (TMB) (19). However, it has been reported that
TMB increases with certain tumor characteristics, such as a higher
GS (20,21). Moreover, TMB may be associated
with infiltrating immune cells in PCa (22).
In a recent study, the β-chain CDR3 AA sequences of
various TCRs were grouped with the aim to sub-group those that
recognize the same antigenic epitope (12). By clustering epitope-specific TCR
AA sequences it was revealed that differences of at most one AA led
to the recognition of the same antigenic peptide. The present study
identified marked differences in the AA sequences for the CDR3 of
the TCR Vβ chain in the NCs post-RT as compared with the top 10
clonotypes pre-RT (ranging from 2–9 AA for all 10 patients tested),
which suggested that these recognize new antigens. The amino acid
distribution within the CDR3 has been shown to serve a critical
role in TCR assembly and function, and consequently, in the
degeneracy of TCR recognition (23). Although characteristics of paired
TCR α- and β-chains are more widely used for the determination of
T-cell specificity, some deductions can be made by studying
alterations in CDR3 AA sequences. It is widely noted that CDR3
mainly consists of 15 AAs (positions 104–118), from which the
flanking positions (104–107 and 113–118) are almost exclusively
expressed by germline-encoded V or J genes; consequently, these are
almost universally conserved. However, AAs in the central region
(positions 107–116) of CDR3 are those that directly contact
antigens (11). For example,
glycine has been found to enhance the flexibility of the CDR3 loop,
which in turn serves a role in TCR polyspecificity (24). Notably, in all NCs, except for NC1
of Pt #13, at least one amino acid substitution (or insertion) by
glycine was noted. Among the AAs with a higher frequency in all NCs
were threonine and tyrosine, which are both polar hydrophilic
residues. Apart from NCs, a general trend that was recorded
regarding the most frequent clonotypes post-RT was the enrichment
of CDR3 with polar and/or acidic AAs, which has been found to
contribute to the TCR bonding process and may be related to the
restricted localization of TCR on HLA-A2 (25). Notably, it has previously been
shown that the nature of AA residues (especially at position 109)
within the CDR3 has a crucial role for T-cell autoreactivity, which
increases significantly in the presence of hydrophobic residues
(26).
TCR sequencing has been used to examine intratumoral
T-cell responses in solid types of cancer (27,28); in a previous study, it was shown
that changes in pre- and post-treatment TCR repertoires were
associated with better outcomes in patients with lung cancer
(28). Furthermore, TCR diversity
has been shown to be prognostic for overall survival in the absence
of any treatment in patients with solid tumors, whereas
pre-treatment TCR clonality was revealed to be predictive of
response to anti-PD-1 treatment (29). Changes in TCR clonality following
stereotactic body RT in patients with NSCLC have also been
correlated with disease progression (30), further suggesting that radiation
effects on TCR clonality may serve as predictive biomarkers for
clinical outcomes.
The present study also detected a number of TCR Vβ
clonotypes whose frequencies were either increased or decreased
post-RT. Among all 10 patients examined, 33 TCR Vβ clonotypes were
identified that at baseline had frequencies not high enough to rank
among the top 10 TCR Vβ clono-types; however, post-RT these were
expanded and could be detected at high abundance. Inversely, 35
clonotypes at high baseline frequencies ranking among the top 10
TCR Vβ CFs were found at much lower frequencies post-RT. These
findings clearly suggested that RT may induce immune changes, which
differentially influence the expansion of various T-cell
clonotypes, either locally or in the blood stream, as a result of
systemic immune activation. Notably, reports have indicated that,
after irradiation, dying tumor cells can release damage-associated
molecular patterns that lead to a variety of immune pathways
affecting production of pro-inflammatory cytokines, including IL-1,
IL-18 (31,32) and type I and type II interferons
(33–37), which in turn activate pathways
involved in the antigen-processing machinery and presentation of
tumor peptides resulting in the induction of adaptive antitumor
immunity (12,15,31,38). Moreover, irradiation-induced IFN-γ
has been reported to upregulate the pro-inflammatory chemo-kines
CXCL10 and CXCL16, which in turn activate antitumor CD8+
T-cells (15,39-41). Thus, the present hypothesis to
build upon is that the RT-induced release of tumor peptides from
the damaged tumor cells in the presence of pro-inflammatory
mediators will favor the clonal expansion of T cells specifically
recognizing these peptides via their cognate TCRs, which will be
then presented at abundant frequencies post-RT. From this
perspective, it was proposed that these expanded T-cell clonotypes
will be further stimulated to extensive proliferative responses
during the continuous release of and stimulation by tumor peptides
in the periphery; therefore, their immunodominant outgrowth will
suppress the expansion of other T-cell clones having a low average
avidity of their involved TCRs for the presented peptide-MHC/HLA
class-I complexes. The present results are in line with those
recently reported by Chow et al (42), which showed that RT in patients
with renal cell cancer could induce immune changes in the
periphery, which were reflected as dynamic changes in their TCR
repertoire. Notably, radiation-induced immunogenicity has been
linked to increased type I and type II IFN responses leading to
upregulation of the cGAS-STING pathway and enhanced intratumoral
infiltration of CD8+ T-cells (40,43).
The present study has some limitations. Firstly, the
number of patients examined was low, and therefore a future study
with a larger sample size and clinical follow-up is required to
confirm the impact of changes in the TCR Vβ repertoires before and
after RT on prognostication. Secondly, the present study lacked
data regarding the impact of RT on TCR Vβ CF according to T-cell
subsets; for example, on CD8+ or CD4+ T
cells, or even CD4+ CD25+ FoxP3+
regulatory T cells, which are important for negatively regulating
antitumor immunity. Taken together, the results of the present
study identified clonal expansion and decreases in response to RT,
providing justification for RT as an immune-activating tool in
LPCa. The specific observations of T-cell expansion and decrease
within a period of 3 months post-RT offer novel therapeutic
combination strategies that may leverage RT-activated endogenous
systemic T-cell immunity for improved clinical outcomes to future
androgen deprivation therapies.
Supplementary Data
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Sequence Read Archive under
BioProject no. PRJNA818160 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA818160).
Authors' contributions
MG, ND, PK and SPF performed the experiments. MG,
ND, PB and SPF analyzed the data and performed statistical
analysis. MA, ADG and VZ also contributed to data analysis. SS, EM,
EV and CZ recruited the patients included in the study, performed
the blood sampling and recorded all relevant clinicopathological
data. MG, ND, CNB and SPF contributed to the interpretation of the
data. MG, ND, PB, VZ, CNB and SPF prepared the original manuscript.
CNB and SPF reviewed and edited the final version of the
manuscript. CNB conceptualized the study. MG and ND confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Saint Savas Cancer
Hospital IRB (approval no. IRB-ID6777/14-06-2017) and the Ethical
Committee of the National and Kapodistrian University of Athens as
part of a larger study (approval no. ID247/28-01-2020). Written
informed consent was obtained from all subjects involved in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This research has been co-financed by the European Regional
Development Fund of the European Union and Greek national funds
through the Operational Program Competitiveness, Entrepreneurship
and Innovation under the call RESEARCH-CREATE-INNOVATE (grant no.
T1EDK-01404).
References
|
1
|
Vanpouille-Box C, Pilones KA, Wennerberg
E, Formenti SC and Demaria S: In situ vaccination by radiotherapy
to improve responses to anti-CTLA-4 treatment. Vaccine.
33:7415–7422. 2015. View Article : Google Scholar
|
|
2
|
Song CW, Glatstein E, Marks LB, Emami B,
Grimm J, Sperduto PW, Kim MS, Hui S, Dusenbery KE and Cho LC:
Biological principles of stereotactic body radiation therapy (SBRT)
and stereotactic radiation surgery (SRS): Indirect cell death. Int
J Radiat Oncol Biol Phys. 110:21–34. 2021. View Article : Google Scholar
|
|
3
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar
|
|
4
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018. View Article : Google Scholar
|
|
5
|
Podder TK, Fredman ET and Ellis RJ:
Advances in radiotherapy for prostate cancer treatment. Adv Exp Med
Biol. 1096:31–47. 2018. View Article : Google Scholar
|
|
6
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar
|
|
7
|
Nesslinger NJ, Sahota RA, Stone B, Johnson
K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, et
al: Standard treatments induce antigen-specific immune responses in
prostate cancer. Clin Cancer Res. 13:1493–1502. 2007. View Article : Google Scholar
|
|
8
|
Lockney NA, Zhang M, Morris CG, Nichols
RC, Okunieff P, Swarts S, Zhang Z, Zhang B, Zhang A and Hoppe BS:
Radiation-induced tumor immunity in patients with non-small cell
lung cancer. Thorac Cancer. 10:1605–1611. 2019. View Article : Google Scholar
|
|
9
|
Schaue D, Comin-Anduix B, Ribas A, Zhang
L, Goodglick L, Sayre JW, Debucquoy A, Haustermans K and McBride
WH: T-cell responses to survivin in cancer patients undergoing
radiation therapy. Clin Cancer Res. 14:4883–4890. 2008. View Article : Google Scholar
|
|
10
|
Takeshima T, Chamoto K, Wakita D, Ohkuri
T, Togashi Y, Shirato H, Kitamura H and Nishimura T: Local
radiation therapy inhibits tumor growth through the generation of
tumor-specific CTL: Its potentiation by combination with Th1 cell
therapy. Cancer Res. 70:2697–2706. 2010. View Article : Google Scholar
|
|
11
|
Glanville J, Huang H, Nau A, Hatton O,
Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, et al:
Identifying specificity groups in the T cell receptor repertoire.
Nature. 547:94–98. 2017. View Article : Google Scholar
|
|
12
|
Meysman P, De Neuter N, Gielis S, Bui Thi
D, Ogunjimi B and Laukens K: On the viability of unsupervised
T-cell receptor sequence clustering for epitope preference.
Bioinformatics. 35:1461–1468. 2019. View Article : Google Scholar
|
|
13
|
Larkin MA, Blackshields G, Brown NP,
Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm
A, Lopez R, et al: Clustal W and Clustal X version 2.0.
Bioinformatics. 23:2947–2948. 2007. View Article : Google Scholar
|
|
14
|
Cavanaugh SX, Fuller CD, Kupelian PA,
Reddy C, Bradshaw P, Pollock BH and Fuss M: Time and PSA threshold
model prognosticates long-term overall and disease-specific
survival in prostate cancer patients as early as 3 months after
external beam radiation therapy. Prostate Cancer Prostatic Dis.
8:353–358. 2005. View Article : Google Scholar
|
|
15
|
Link B, Torres Crigna A, Holzel M,
Giordano FA and Golubnitschaja O: Abscopal effects in metastatic
cancer: Is a predictive approach possible to improve individual
outcomes? J Clin Med. 10:51242021. View Article : Google Scholar
|
|
16
|
Dong N, Moreno-Manuel A, Calabuig-Farinas
S, Gallach S, Zhang F, Blasco A, Aparisi F, Meri-Abad M, Guijarro
R, Sirera R, et al: Characterization of circulating T cell receptor
repertoire provides information about clinical outcome after PD-1
blockade in advanced non-small cell lung cancer patients. Cancers
(Basel). 13:29502021. View Article : Google Scholar
|
|
17
|
Liu S, Pan W, Cheng Z, Sun G, Zhu P, Chan
F, Hu Y, Zhang X and Dai Y: Characterization of the T-cell receptor
repertoire by deep T cell receptor sequencing in tissues from
patients with prostate cancer. Oncol Lett. 15:1744–1752. 2018.
|
|
18
|
Baxevanis CN, Fortis SP and Perez SA:
Prostate cancer: Any room left for immunotherapies? Immunotherapy.
11:69–74. 2019. View Article : Google Scholar
|
|
19
|
De Velasco MA and Uemura H: Prostate
cancer immunotherapy: Where are we and where are we going? Curr
Opin Urol. 28:15–24. 2018. View Article : Google Scholar
|
|
20
|
Ryan MJ and Bose R: Genomic alteration
burden in advanced prostate cancer and therapeutic implications.
Front Oncol. 9:12872019. View Article : Google Scholar
|
|
21
|
Fraser M, Sabelnykova VY, Yamaguchi TN,
Heisler LE, Livingstone J, Huang V, Shiah YJ, Yousif F, Lin X,
Masella AP, et al: Genomic hallmarks of localized, non-indolent
prostate cancer. Nature. 541:359–364. 2017. View Article : Google Scholar
|
|
22
|
Wang L, Pan S, Zhu B, Yu Z and Wang W:
Comprehensive analysis of tumour mutational burden and its clinical
significance in prostate cancer. BMC Urol. 21:292021. View Article : Google Scholar
|
|
23
|
Reiser JB, Darnault C, Gregoire C, Mosser
T, Mazza G, Kearney A, van der Merwe PA, Fontecilla-Camps JC,
Housset D and Malissen B: CDR3 loop flexibility contributes to the
degeneracy of TCR recognition. Nat Immunol. 4:241–247. 2003.
View Article : Google Scholar
|
|
24
|
Birnbaum ME, Mendoza JL, Sethi DK, Dong S,
Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW and
Garcia KC: Deconstructing the peptide-MHC specificity of T cell
recognition. Cell. 157:1073–1087. 2014. View Article : Google Scholar
|
|
25
|
Yu K, Shi J, Lu D and Yang Q: Comparative
analysis of CDR3 regions in paired human αβ CD8 T cells. FEBS Open
Bio. 9:1450–1459. 2019. View Article : Google Scholar
|
|
26
|
Stadinski BD, Shekhar K, Gomez-Tourino I,
Jung J, Sasaki K, Sewell AK, Peakman M, Chakraborty AK and Huseby
ES: Hydrophobic CDR3 residues promote the development of
self-reactive T cells. Nat Immunol. 17:946–955. 2016. View Article : Google Scholar
|
|
27
|
Reuben A, Gittelman R, Gao J, Zhang J,
Yusko EC, Wu CJ, Emerson R, Zhang J, Tipton C, Li J, et al: TCR
repertoire intra- tumor heterogeneity in localized lung
adenocarcinomas: An association with predicted neoantigen
heterogeneity and postsurgical recurrence. Cancer Discov.
7:1088–1097. 2017. View Article : Google Scholar
|
|
28
|
Dovedi SJ, Cheadle EJ, Popple AL, Poon E,
Morrow M, Stewar t R, Yusko EC, Sanders CM, Vignali M, Emerson RO,
et al: Fractionated radiation therapy stimulates antitumor immunity
mediated by both resident and infiltrating polyclonal T-cell
populations when combined with PD-1 blockade. Clin Cancer Res.
23:5514–5526. 2017. View Article : Google Scholar
|
|
29
|
Valpione S, Mundra PA, Galvani E, Campana
LG, Lorigan P, De Rosa F, Gupta A, Weightman J, Mills S, Dhomen N
and Marais R: The T cell receptor repertoire of tumor infiltrating
T cells is predictive and prognostic for cancer survival. Nat
Commun. 12:40982021. View Article : Google Scholar
|
|
30
|
Wu L, Zhu J, Rudqvist NP, Welsh J, Lee P,
Liao Z, Xu T, Jiang M, Zhu X, Pan X, et al: T-cell receptor
profiling and prognosis after stereotactic body radiation therapy
for stage I non-small-cell lung cancer. Front Immunol.
12:7192852021. View Article : Google Scholar
|
|
31
|
Di Virgilio F: Liaisons dangereuses:
P2X(7) and the inflammasome. Trends Pharmacol Sci. 28:465–472.
2007. View Article : Google Scholar
|
|
32
|
Martinon F, Mayor A and Tschopp J: The
inflammasomes: Guardians of the body. Annu Rev Immunol. 27:229–265.
2009. View Article : Google Scholar
|
|
33
|
Sun L, Wu J, Du F, Chen X and Chen ZJ:
Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates
the type I interferon pathway. Science. 339:786–791. 2013.
View Article : Google Scholar
|
|
34
|
Ablasser A, Goldeck M, Cavlar T, Deimling
T, Witte G, Röhl I, Hopfner KP, Ludwig J and Hornung V: cGAS
produces a 2′-5′-linked cyclic dinucleotide second messenger that
activates STING. Nature. 498:380–384. 2013. View Article : Google Scholar
|
|
35
|
Ishikawa H, Ma Z and Barber GN: STING
regulates intracellular DNA-mediated, type I interferon-dependent
innate immunity. Nature. 461:788–792. 2009. View Article : Google Scholar
|
|
36
|
Deng L, Liang H, Xu M, Yang X, Burnette B,
Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al:
STING-dependent cytosolic DNA sensing promotes radiation-induced
type I inter- feron-dependent antitumor immunity in immunogenic
tumors. Immunity. 41:843–852. 2014. View Article : Google Scholar
|
|
37
|
Burnette BC, Liang H, Lee Y, Chlewicki L,
Khodarev NN, Weichselbaum RR, Fu YX and Auh SL: The efficacy of
radio- therapy relies upon induction of type i interferon-dependent
innate and adaptive immunity. Cancer Res. 71:2488–2496. 2011.
View Article : Google Scholar
|
|
38
|
Rodriguez-Ruiz ME, Vanpouille-Box C,
Melero I, Formenti SC and Demaria S: Immunological mechanisms
responsible for radiation-induced abscopal effect. Trends Immunol.
39:644–655. 2018. View Article : Google Scholar
|
|
39
|
Matsumura S, Wang B, Kawashima N,
Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti
SC, Dustin ML and Demaria S: Radiation-induced CXCL16 release by
breast cancer cells attracts effector T cells. J Immunol.
181:3099–3107. 2008. View Article : Google Scholar
|
|
40
|
Lim JY, Gerber SA, Murphy SP and Lord EM:
Type I interferons induced by radiation therapy mediate recruitment
and effector function of CD8(+) T cells. Cancer Immunol Immunother.
63:259–271. 2014. View Article : Google Scholar
|
|
41
|
Matsumura S and Demaria S: Up-regulation
of the pro-inflammatory chemokine CXCL16 is a common response of
tumor cells to ionizing radiation. Radiat Res. 173:418–425. 2010.
View Article : Google Scholar
|
|
42
|
Chow J, Hoffend NC, Abrams SI, Schwaab T,
Singh AK and Muhitch JB: Radiation induces dynamic changes to the T
cell repertoire in renal cell carcinoma patients. Proc Natl Acad
Sci USA. 117:23721–23729. 2020. View Article : Google Scholar
|
|
43
|
Vanpouille-Box C, Alard A, Aryankalayil
MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN,
Formenti SC and Demaria S: DNA exonuclease Trex1 regulates
radiotherapy-induced tumour immunogenicity. Nat Commun.
8:156182017. View Article : Google Scholar
|