Introduction
The ultraviolet spectrum of solar radiation (UVR)
that reaches surface of the earth includes both UVA and UVB
corresponding to the wavelengths of 320-400 and 290-320 nm,
respectively. Both induce various biological effects that are
wavelength dependent (1-4). UVR exposure can lead to a range of
skin pathologies including skin aging, solar keratosis and melanoma
or non-melanoma skin cancers (NMSC). Due to its pleiotropic effects
UVR is defined as a full cutaneous carcinogen with UVB being an
important etiological factor responsible for basal (BCC) and
squamous (SCC) cell carcinomas (4-7).
The two chromophore dependent (predominantly UVB) and chromophore
independent mechanisms, such as generation of reactive oxygen
species (ROS), are involved in epidermal cancerogenesis (2,3,5,8,9), a
process further amplified by modification of local homeostasis,
including immunosuppression (10). While the mortality for BCC and SCC
is low, they are the commonest human cancers with a significant
economic burden on the health care system in the USA and worldwide
(11). Their incidence increases
in immunocompromised patients, including those with organ
transplantation, where tumors behave more aggressively. In
addition, military personnel are unavoidably exposed in an
unprotected manner to high doses of UVR during training or when
deployed to locations with high solar radiation. Therefore,
occupational overexposure to UVB will be experienced by those who
work outdoors or who are exposed intermittently, potentially
leading to the development and progression of BCC and SCC. While a
number of therapeutic approaches are being tested against these
cancers, they are costly and not fully effective, which in part
could be secondary to the phenomenon of UVB-induced field of
cancerization (12). Therefore,
there is an urgent need to establish economical and non-toxic
chemopreventive and therapeutic measures against UVB-induced
cancer.
UVB also has a beneficial role in biology where
transformation of 7-dehydrocholesterol (7DHC) to vitamin D3 (D3) in
the skin supplies much of this hormonal precursor for systemic use
(13,14). Higher doses of UVB lead to
photoisomerization of the previtamin D3 intermediate to lumisterol
(L3) and tachysterol (T3) (13).
In the classical pathway, D3 is activated by hydroxylation at C25
producing 25(OH)D3 in the liver and subsequently at C1 producing
1,25(OH)2D3 (kidney) with local (skin) production also
occurring (13,15,16). 1,25(OH)2D3, in addition
to regulating calcium homeostasis, has pleiotropic activities that
include anti-cancer effects (13,15-18). In the skin, it regulates formation
of the epidermal barrier, hair cycling and attenuates
cancerogenesis and inflammatory processes (13,16,19-22). 1,25(OH)2D3 can also
protect against UVB-induced cell death and DNA damage (23-26) with topically applied D3 precursor
being able to inhibit UVB-induced cancerogenesis in mice (27).
We previously reported an alternative metabolic
pathway of D3 activation initiated by the action of CYP11A which
converted D3 to 20(OH)D3 and 22(OH)D3 with some further side-chain
hydroxylations producing di-hydroxy (such as
20,23(OH)2D3) and tri-hydroxy species. The major product
was 20(OH)D3 and this can be further modified by other CYP enzymes
including CYP2R1, CYP27A1 and CYP27B1 (28-34). Many of these pathway products and
intermediates have been detected in both humans and animals
(28,29,31,33,34). 20(OH)D3 has been shown to be
present in serum and to accumulate together with its downstream
derivatives in human epidermis (28,29,31). Thus, 20(OH)D3 and related
metabolites are physiologically relevant endogenous bioregulators
that can be defined as natural products (35). They show antiproliferative,
prodifferentiation, anti-inflammatory and anti-cancer effects that
are comparable to those of 1,25(OH)2D3 (30,36-41). They also protect human
keratinocytes against UVB damage (42-45) and topically applied 20(OH)D3
attenuates UVB-induced skin damage in Skh:hr1 mice (46).
While the phenotypic effects of canonical
1,25(OH)2D3 are predominantly mediated through
interaction with the vitamin D receptor (VDR) through genomic and
non-genomic mechanisms (13,47-50), CYP11A1-derived vitamin D
hydroxyderivatives, in addition to acting on VDR (51-53), can also act on retinoid-related
orphan receptors (RORs) α and γ (54-56) and the aryl hydrocarbon receptor
(AhR) (57). Furthermore,
clinico-pathological analyses of human melanoma samples have shown
that expression of VDR and RORα and γ changes during tumor
progression and correlates with disease outcomes (58-62).
Notably, 20(OH)D3 and 20,23(OH)2D3 are
noncalcemic and non-toxic at extremely high doses in rats and mice
(37,39,63,64) unlike the majority of low-calcemic
derivatives of D3 that have been chemically synthesized (18,65), identifying the former as potential
therapeutics.
Therefore, using a wide range of CYP11A1-derived
vitamin D hydroxyderivatives, some modified by 1α-hydroxylation by
CYP27B1, the present study examined their anticancer potential in
human SCC and murine BCC lines. In addition, it analyzed the
expression of the VDR, RORα and γ, and megalin/LRP2 which is a
transmembrane receptor for vitamin d-binding protein for cellular
import of 25(OH)D3 (66-68), in a panel of benign and malignant
epidermal lesions.
Materials and methods
Vitamin D derivatives
Vitamin D3 and D2, 1,25(OH)2D3 and
25(OH)D3 were purchased from MilliporeSigma. Hydroxyderivatives of
D3 and D2 were synthesized enzymatically or non-enzymatically as
described in Fig. S1 and
purified as previously described (30,32,63,69-76). The purity of the compounds was
>99%. The compounds were dissolved in ethanol and applied to
cell cultures as previously described (44,51,57). All vitamin D-derived compounds
were dissolved in ethanol. The active compounds were prepared as
stock solutions at 10−4 M and then diluted with medium
to final concentrations ranging from 10−7 to
10−12 M (as indicated in the figures) and added to cell
cultures. In each experiment, the control with vehicle (ethanol)
was prepared.
Cell culture
The murine basal cell carcinoma (BCC) cell line
ASZ001 and human skin squamous cell carcinoma (SCC) cell lines were
used for the experiments. ASZ001 was a gift from Dr Ervin Epstein
(University of California, San Francisco, CA) (77,78), A431 was purchased from ATCC (cat.
no. CRL-1555) and SCC13 was a gift from Dr. S. K. Katiyar
(University of Alabama at Birmingham, Birmingham, AL) (79). The BCC cell line was cultured in
154CF medium (Thermo Fisher Scientific, Inc.) (77,78) and SCC cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Corning Inc.),
supplemented with 10% fetal bovine serum (FBS; MilliporeSigma),
antibiotic-antimycotic solution (Corning Inc.) and maintained in 5%
CO2 at 37°C (79). For
experimental treatment, charcoal-treated FBS (ctFBS) was used as
previously described (44).
Proliferation assays
The MTS, sulforhodamine B (SRB) tests were used to
measure proliferation of BCC and SCC cells as described previously
(80,81). BCC and SCC cells were seeded on
96-well plates in complete culture medium for 24 h in 5%
CO2 at 37°C. The next day the medium was replaced with
serum-free medium and the cells starved for the subsequent 24 h.
Cells were treated with secosteroids dissolved in ethanol and added
to culture medium with 10% charcoal-treated FBS, for 24, 48 and 72
h. To assess the effects of secosteroids on BCC and SCC cells, the
metabolic activity was tested using a tetrazolium compound and an
electron coupling reagent (MTS; CellTiter 96 AQueous One Solution
Cell Proliferation Assay, Promega Corporation), as previously
described (80,81): 20 µl of MTS/PMS solution
was added to the cells and incubated for 2 h at 37°C then read the
absorbance at 490 nm using Cytation 5 (BioTek Instruments, Inc.).
SRB was also used to determine cell number and cell viability by
colorimetric evaluation of total protein content as the dye binds
to cellular proteins. The BCC and SCC cells were assayed as
described previously (82).
Briefly, after the treatment with secosteroids, BCC and SCC cells
were fixed for 1 h with 10% trichloroacetic acid at 4°C and
incubated with 0.4% sulforhodamine B in 1% acetic acid for 30 min
at room temperature. Next, the protein-bound dye was dissolved in
Tris base solution and the absorbance was measured at 565 nm using
Cytation 5 (BioTek Instruments, Inc.).
Spheroids and colony formation
To assess the ability of the secosteroids being
tested to inhibit anchorage-independent growth of BCC and SCC
cells, the spheroid formation test was performed as previously
described (81). Briefly, cells
were seeded into ultra-low attachment 96-well plates in culture
medium supplemented with epidermal growth factor (MilliporeSigma),
basic fibroblast growth factor (MilliporeSigma), insulin (Thermo
Fisher Scientific, Inc.), bovine serum albumin (MilliporeSigma) and
B27 supplement (Thermo Fisher Scientific, Inc.). After seven days
the plate was scanned using a Cytation 5 microplate reader and
Gene5 software (BioTek Instruments, Inc.).
For colony formation assay, cells were plated in
6-well plates (1,000 cells/well) and incubated with graded
concentrations of the secosteroids for 6 days. After methanol (70%)
fixation at 4°C and staining with 0.1% crystal violet, the colonies
were counted using Cytation 5 (BioTek Instruments, Inc.) and
analyzed by Gene 5 software. The large (>0.5 mm), medium
(>0.25 mm) and small colonies (>0.1 mm) colonies were
counted.
Western blot analysis
RIPA Lysis and Extraction Buffer (Thermo Scientific;
cat. no. 89901) was used for whole cell extract and Nuclear Extract
kit (Active Motif, Inc.; cat. no. 54001) for nuclear and
cytoplasmic fractions as described previously (43,44). Protein concentration was measured
with Pierce BCA Protein Assay kit (Thermo Scientific; cat. no.
23225). For protein separation 4-15% Mini-PROTEAN TGX Stain-Free
Protein Gels, 10 well, 30 µl (Bio-Rad Laboratories, Inc.;
cat. no. 4568083) were used with 20 µg of proteins loaded
per lane. The proteins were transferred to Immobilon-P PVDF
membranes (MilliporeSigma; cat. no. IPVH00010). Prior incubation
with primary antibodies the membranes were incubated with 5%
skimmed milk in TBS-T buffer (Tris-buffer saline containing 0.1%
(v/v) Tween 20 and 5% (w/v) skim milk) for 1 h at room
temperature.
Immunodetection of VDR protein expression was
performed using VDR antibody (D-6; cat. no. sc-13133; Santa Cruz
Biotechnology, Inc.) at a 1:200 dilution (1 h incubation at room
temperature) followed by treatment of secondary antibody, m-IgGκ
BP-HRP (cat. no. sc-516102; Santa Cruz Biotechnology, Inc.; 1 h
incubation at room temperature at 1:2,000 dilution). RORα
Polyclonal Antibody (cat. no. PA1-812; Invitrogen; Thermo Fisher
Scientific, Inc.; 1:1,000) and RORg(t) monoclonal Antibody (cat.
no. 14-6988-82; Invitrogen; Thermo Fisher Scientific, Inc.; 1:500)
were used for RORα and RORγ protein expression, respectively at 1 h
at room temperature. Goat Anti-Rabbit IgG (cat. no. ab6721; Abcam;
1:5,000) and Anti-rat IgG, HRP-linked Antibody (cat. no. 7077; Cell
Signaling Technology, Inc.; 1:2,000) were used as secondary
antibodies for RORα and RORg, respectively. The membranes were
reprobed with following antibodies to measure the protein loading.
Lamin C: lamin A/C antibody (N-18) (Santa Cruz; cat. no. sc-6215)
for primary antibody (1 h incubation at room temperature at 1:200
dilution) with donkey anti-goat IgG-HRP (Santa Cruz; cat. no.
sc-2020) for secondary antibody (1 h at room temperature at 1:5,000
dilution). α-Tubulin: alpha Tubulin Antibody (Thermo Fisher
Scientific, Inc.; cat. no. 62204) for primary antibody (1 h
incubation at room temperature at 1:5,000 dilution) and goat
anti-mouse IgG-HRP (Santa Cruz; cat. no. sc-2005) for secondary
antibody (1 h incubation at room temperature at 1:5,000 dilution).
β-Actin: anti-β-Actin-Peroxidase antibody, mouse monoclonal
(MilliporeSigma; cat. no. A3854) with 1hr incubation at room
temperature at 1:10,000 dilution. After each incubation with
primary antibody membranes were washed three times with TBS-T
(Tris-buffer saline containing 0.1% (v/v) Tween 20) for 10 min each
prior incubation with secondary antibody. Immuno-reactivity was
detected using the SuperSignal West Pico PLUS Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc., cat. no. 34580) or
Immobilon Crescendo Western HRP substrate (MilliporeSigma; cat. no.
WBLUF0500). The integrated optical density of the bands was
analyzed by ImageJ software, version 1.53a (National Institutes of
Health).
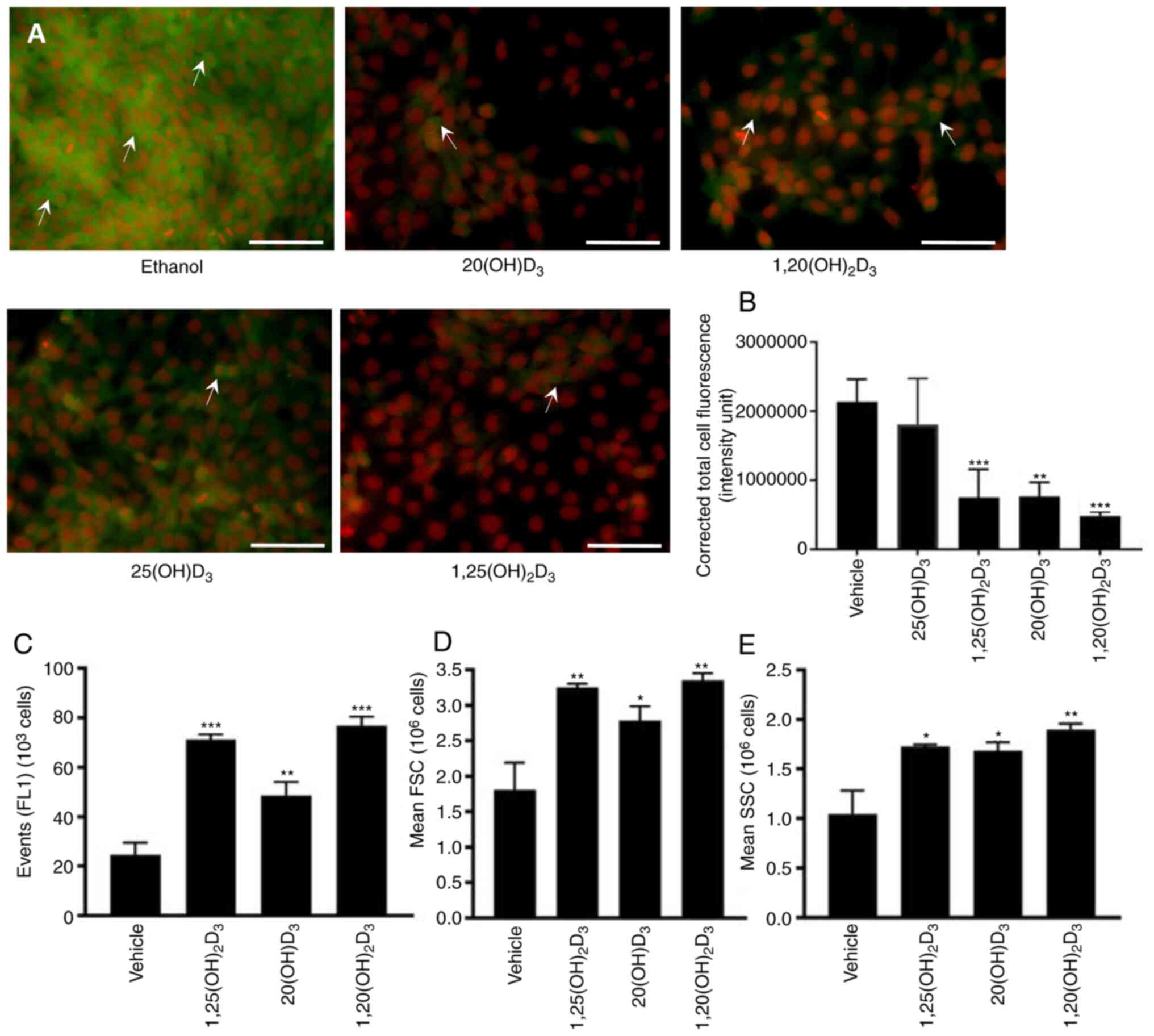
Flow cytometry
A431 cells were treated with 100 nM
1,20(OH)2D3,
1,25(OH)2D3, 20(OH)D3, or ethanol
(vehicle) for 24 h. To test the stimulation of differentiation by
vitamin D3 derivatives, after treatment the cells were fixed in 2%
paraformaldehyde (Thermo Fisher Scientific, Inc.) in PBS for 1 h at
room temperature. Following washing with PBS, pellets were
resuspended in 100 µl of permeabilizing solution containing
0.1% BSA and 0.1% NaN3 in PBS and 0.2 µg primary
antibody against involucrin (Novocastra Laboratories Ltd.). Cells
stained with isotype control antibody (IgG1; Caltag
Medsystems) were used as controls. After overnight incubation at
4°C, cells were washed with PBS and resuspended in 100 µl of
permeabilizing solution containing sheep anti-mouse FITC-conjugated
secondary antibody (1:50; cat. no. NCL-SAM-FITC; Novocastra
Laboratories Ltd.). After 3 h of incubation at 4°C, cells were
washed with PBS, centrifuged and resuspended in 500 µl of
PBS. Samples were read and analyzed with an Accuri C6 (BD
Bioscience) flow cytometer and BD Accuri C6 Plus software 8 (BD
Bioscience). The FL-1 signal (collected from 10,000 events inside
scatter/forward scatter window after debris exclusion) was
recorded. Forward (relative to cell size) and side (relative to
cell granularity) scatter were recorded. The number of events
(positive cells)-FL-1 signal values are presented as difference
between mean fluorescence intensity of sample stained with specific
and isotype control antibody (dMFI). Scatter signal values were
presented as MSI (mean signal intensity).
Immunofluorescence studies
VDR, RORα and RORγ in BCC and SCC cells were
immunostained as previously described (81) (for details see Fig. S2: Scheme for immunohistochemistry
and characteristic of reagents used). Briefly, the cells were
seeded in a 96-well plate and grown until reaching ~70% confluence
then fixed with 4% buffered formalin for 20 min at room
temperature. Following permeabilization, the cells were blocked
with 0.5% Triton X-100 and 2% bovine serum albumin (BSA) for 1 h at
room temperature, followed by overnight incubation with primary
antibodies (VDR; clone 9A7; Thermo Fisher Scientific, Inc., cat.
no. MA1-710, 1:50) and RORα (1:400) and RORγ (1:400) [both
antibodies developed at NIEHS and characterized as reported in
(54)] overnight at 4°C and
incubation with the secondary antibody (goat anti-rat IgG (H+L)
Cross-Adsorbed Secondary Antibody, cat. no. A-11006A, Alexa Fluor
488goat anti-rabbit immunoglobulin G (IgG; H + L) Cross-Adsorbed
Secondary Antibody, Alexa Fluor 488, cat. no. A-11008; both Thermo
Fisher Scientific, Inc.), diluted 1:1,000 for 1 h at room
temperature. After washing the cell nuclei were stained with
propidium iodide (PI; MilliporeSigma) and examined with a Cytation
5 microplate reader (BioTek Instruments, Inc.). For changes in GLI1
and β-catenin expression, ASZ001 and A431 lines were treated with
20(OH)D3, 1,20(OH)2D3, 25(OH)D3, 1,25(OH)2D3
or vehicle (ethanol) at 10−7 M at 37°C for 24 h. After
washing the cell nuclei were stained with propidium iodide (PI;
MilliporeSigma) and examined with a Cytation 5 microplate reader
(BioTek Instruments, Inc.).
Immunohistochemistry
For immunohistochemistry, the standard
formalin-fixed paraffin-embedded skin samples of normal skin BCC,
SCC in situ and invasive SCC were included in this study
(Table SI). The authors declare
that this investigation was carried out following the rules of the
Declaration of Helsinki of 1975 (revised in 2008) and this study
was approved by the institutional review board (IRB) of the
University of Alabama at Birmingham under IRB-940831016 (OCCC
Tissue Procurement CORE Facility) and IRB-00000726 (Title
E150427002, Dr. A. Slominski PI). The IRB of the University of
Alabama at Birmingham, which gave its permission for conducting the
present study, waived the requirement to obtain patients' informed
consent for this research. VDR, RORα, RORγ and megalin in tissues
samples of normal skin, BCC and SCC were labelled as previously
described (83,84) (for details see Fig. S2). Briefly, heat-induced antigen
retrieval (96°C, 20 min) in high pH unmasking solution (Vector
Laboratories, Inc.) was performed on formalin-fixed paraffin
embedded sections, followed by blocking of endogenous peroxidase
for 10 min. The sections were incubated 30 min at room temperature
with anti-Lrp2/Megalin antibody (cat. no. ab76969; Abcam) or
overnight with other antibodies (anti-VDR; clone 9A7; cat. no.
MA1-710) or anti-RORγ and anti-RORα [generated as previously
described (54)] with subsequent
incubation at room temperature with secondary antibodies conjugated
with HRP (ready-to-use anti-rabbit (cat. no. MP-7451) and
anti-mouse (cat. no. MP-7452) ImmPRESS antibodies, Vector
Laboratories, Inc. for RORs and megalin; anti-rat antibody, (1:200;
cat. no. ab97057; Abcam) for VDR. The immunolabelling was
visualized with peroxidase substrate ImmPACT NovaRED (Vector
Laboratories Inc.) and the sections were mounted with permanent
mounting (Thermo Fisher Scientific, Inc.) under coverslips.
Sections were analyzed under a light microscope (magnification,
×200). The mean staining intensity was assessed by two observers
(ATS and AAB).
The analysis of the expression of RORA and
RORC mRNA level followed public genomics data repository
Gene Expression Omnibus, accession number GSE7553 http://www.ncbi.nlm.nih.gov/geo). For details on
data collection, methods and patient's characteristic performed by
Riker et al (85) please
refer to their original paper.
Reverse transcription-quantitative (RT-q)
PCR
Cultures at 70% confluency were used for treatment.
After treatment for 24 h with secosteroids, cells were detached
using trypsin and centrifuged at 271 × g for 5 min at 4°C. Cell
pellets were then collected, and total RNA was isolated using an
Absolutely RNA Miniprep kit (1.2 ml per 4×106 cells)
(Agilent Technologies, Inc.). The RNA purity and quantification
were performed with Cytation 5 (BioTek Instruments, Inc.) and the
absorbance 260/280 ratio ~2 checked for the purity. Total RNA (0.5
µg) was used for cDNA synthesis with a High-Capacity cDNA
Reverse Transcription kit (Applied Biosystems, Waltham, MA, USA)
following the manufacturer's protocol. The cDNA (750 ng) was used
for qPCR. β-actin was used as an internal control. qPCR was
performed using Kapa SYBR Fast qPCR Master Mix (Kapa Biosystems;
Roche Diagnostics) with QuantStudio 6 Flex Real-Time PCR Systems
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in
quadruplicate, following the manufacturer's protocol and as
previously described (43,44).
Primer sequences are listed in Table
SII. Data were collected with QuantStudio Real-Time PCR
Software v1.2 (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the amount of mRNA was normalized by the comparative
2−ΔΔCq method followed by calculating the fold change,
using β-actin as a housekeeping gene as described previously
(43,44). The experiments were repeated three
times.
Statistical analysis
Data exported from Microsoft Excel were analyzed
using GraphPad Prism 7 (GraphPad Software, Inc.) statistical
software and are presented as means ± SD or SEM as indicated in the
figures legend. The data were analyzed by one way ANOVA with
Dunnett's multi comparison post-hoc test or the Student's t-test as
detailed in figure legends.
Results
The present study investigated the in vitro
antitumor effects of CYP11A1-derived D3-hydroxyderivatives using
established lines of human squamous (A431 and SCC-13) and mouse
basal cell (ASZ0001) carcinoma. The structures of the secosteroids
that were tested are presented in Table I, with stereochemistry shown where
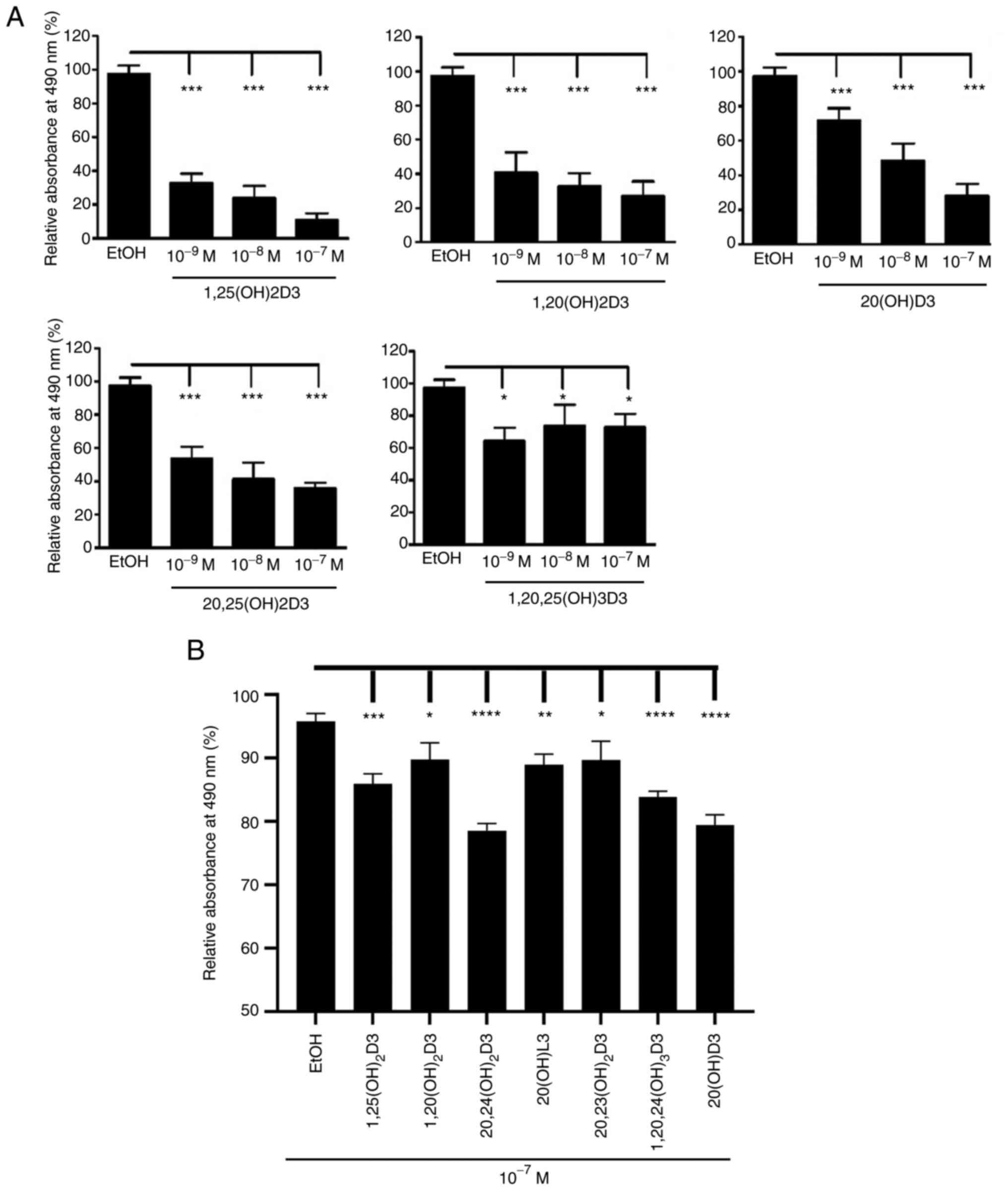
known. The MTS tests showed that a range of representative vitamin
D3-hydroxyderivatives inhibited proliferation of basal and squamous
cell carcinoma cells in a dose-dependent manner (Fig. 1). Similar inhibitory effects were
observed using the RBS assay (data not shown).
 | Table IChemical structures of vitamin D
hydroxyderivatives analyzed. |
Table I
Chemical structures of vitamin D
hydroxyderivatives analyzed.
| Chemical name | Chemical
structure | Chemical name | Chemical
structure |
|---|
1,25(OH)2D3
1α,25-dihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1R,7aR,E)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  |
20,23(OH)2D3
20S,23S-dihydroxyvitamin D3
(2S,4S)-2-((1S,7aS,E)-4-((Z)-2-((S)-5-hydroxy-2-methylenecyclohexylidene)
ethylidene)-7a-methyloctahydro-1H-inden-1-yl)-6-methylheptane-2,4-diol |  |
1,20(OH)2D3
1α,20S-dihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1S,7aS,E)-1-(2-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  |
20,24(OH)2D3
20S,24R-dihydroxyvitamin D3
(2S,5R)-2-((1S,7aS,E)-4-((Z)-2-((S)-5-hydroxy-2-methylenecyclohexylidene)
ethylidene)-7a-methyloctahydro-1H-inden-1-yl)-6-methylheptane-2,5-diol |  |
1,20,23(OH)3D3
1α,20S,23S-trihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1S,7aS,E)-1-((4S)-2,4-dihydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  |
20,25(OH)2D3
20S,25-dihydroxyvitamin D3
2-((1S,7aS,E)-4-((Z)-2-((S)-5-hydroxy-2-methylenecyclohexylidene)
ethylidene)-7a-methyloctahydro-1H-inden-1-yl)-6-methylheptane-2,6-diol |  |
1,20,24(OH)3D3
1α,20S,24R-trihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1S,7aS,E)-1-((5R)-2,5-dihydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  |
17,20(OH)2D2
17R,20S-dihydroxyvitamin D2
(1R,7aS,E)-4-((Z)-2-((S)-5-hydroxy-2-methylenecyclo-hexylidene)ethylidene)-1-((2S,5R,E)-2-hydroxy-5,6-dimethylhept-3-en-2-yl)-7a-methyloctahydro-1H-inden-1-ol |  |
1,20,25(OH)3D3
1α,20S,25-trihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1S,7aS,E)-1-(2,6-dihydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  | 20(OH)D2
20-hydroxyvitamin D2
(1S,Z)-3-(2-((1S,7aS,E)-1-((2S,5R,E)-2-hydroxy-5,6-dimethylhept-3-en-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol |  |
1,20,26(OH)3D3
1α,20S,26-trihydroxyvitamin D3
(1R,3S,Z)-5-(2-((1S,7aS,E)-1-((2S)-2,7-dihydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol |  | 22(OH)D3
22-hydroxyvitamin D3
(1S,Z)-3-(2-((1R,7aR,E)-1-((2S)-3-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol |  |
20(OH)D3
20S-hydroxyvitamin D3
(1S,Z)-3-(2-((1S,7aS,E)-1-((S)-2-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol |  | 25(OH)D3
25-dihydroxyvitamin D3
(1S,Z)-3-(2-((1R,7aR,E)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol |  |
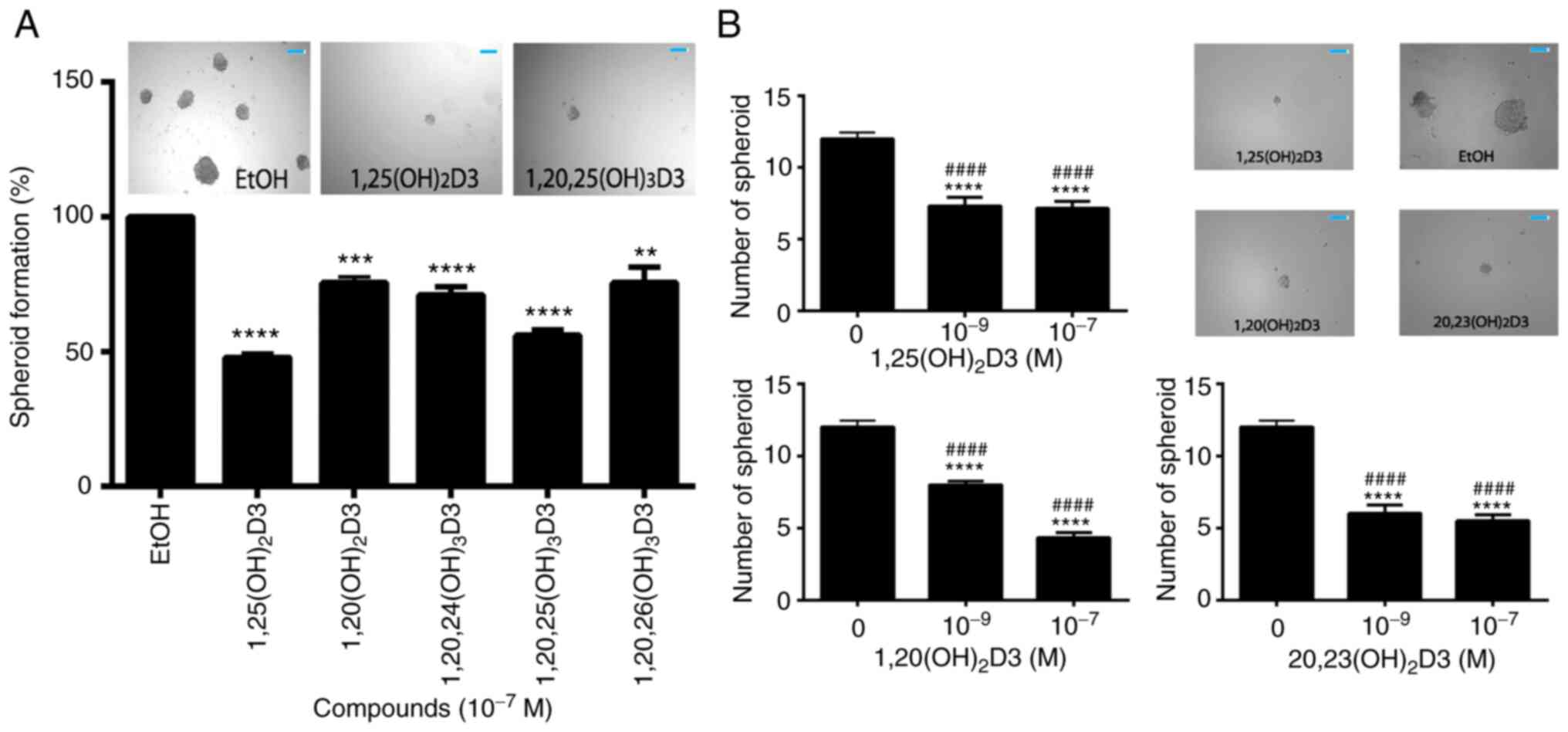
1,20,24(OH)3D3 and 1,25(OH)2D3
were selected to test the effect of secosteroids on colony
formation by the murine basal cell carcinoma line, ASZ001 (Fig. 2). The two secosteroids caused a
dose-dependent inhibition of the formation of large (>0.5 mm),
medium (>0.25 mm) and small colonies (>0.1 mm). Using
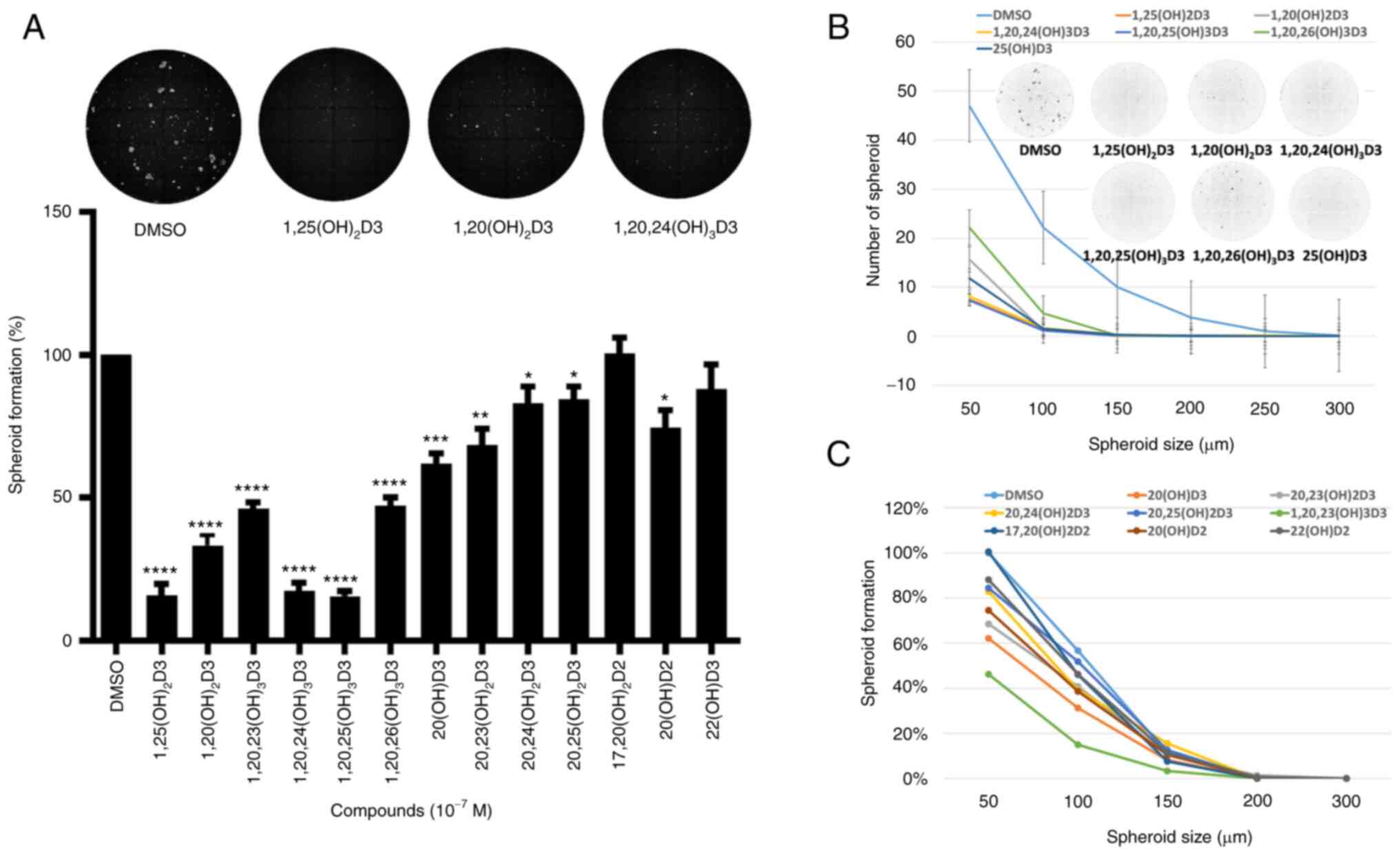
spheroid formation assays the antitumor activities of all the
CYP11A1-derived hydroxyderivatives of vitamin D3 under study
(Table I) were tested against the
ASZ001 cell line (Fig. 3). The
inhibition of spheroid formation by different secosteroids, with or
without hydroxyl group at C1α and in comparison with canonical
1,25(OH)2D3, is shown in Fig. 3A. Only 22(OH D3 and
17,20(OH)2D2 failed to significantly inhibit spheroid
formation at the dose (10−7 M) tested. Secosteroids
having a C1α hydroxyl group generally showed greater inhibition
than those lacking this functional group. Fig. 3B and C show the effect of
secosteroids on spheroid formation depending on their size. Similar
inhibitory effects on spheroid formation by representative
secosteroids containing a 1α-hydroxyl group, in comparison with
1,25(OH)2D3, (control) were observed in A431 cell
carcinoma cells (Fig. 4A). The
dose dependency of selected secosteroids on spheroid formation by
SCC13 squamous cells is shown in Fig.
4B with significant inhibition being observed already at
10−9 M.
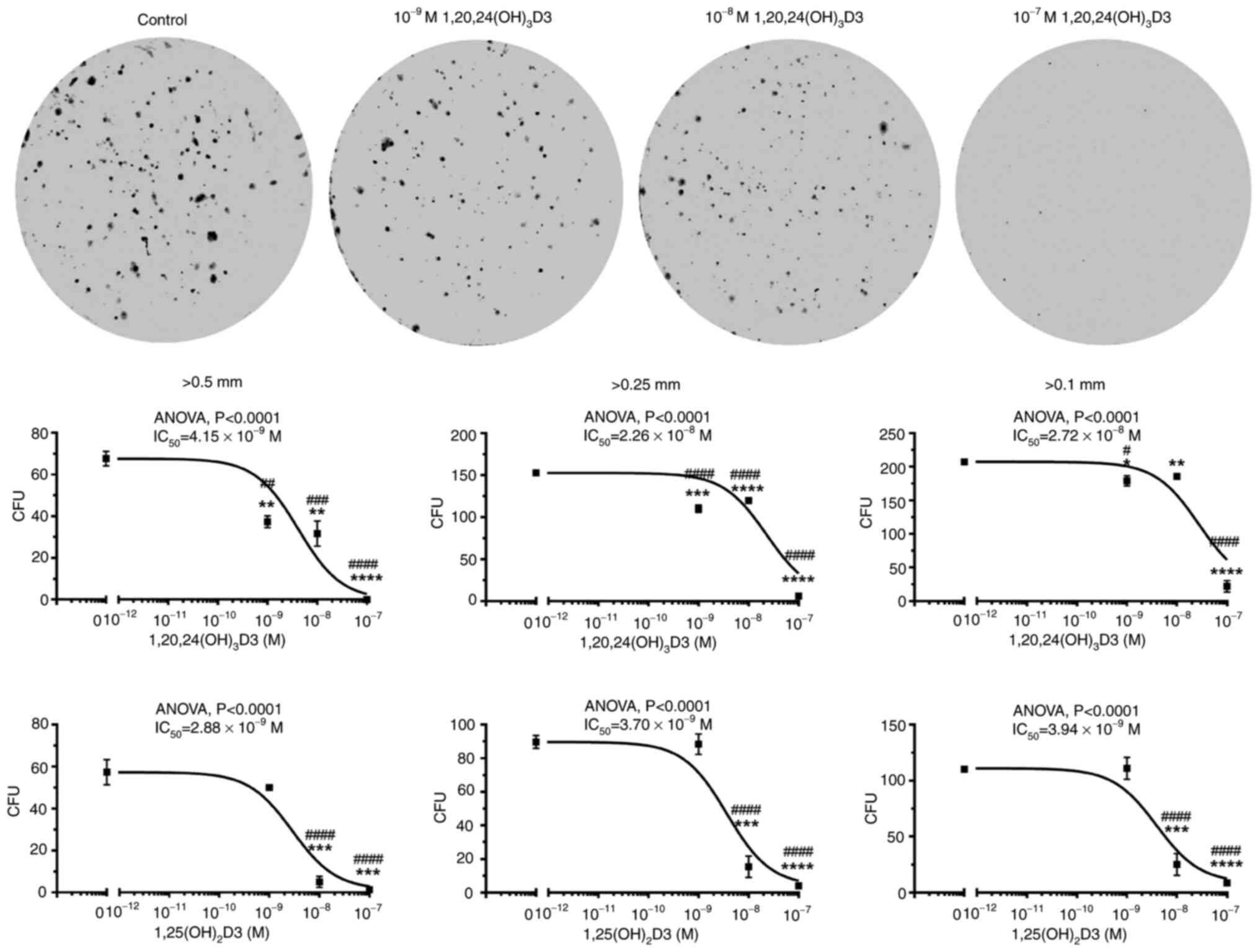 | Figure 21,20,24(OH)3D3 and
1,25(OH)2D3 inhibit colony formation by murine ASZ001
cells. Cells were plated in 6 well-plates (1,000 cells/well) and
incubated with graded concentrations of the secosteroids for six
days. After methanol fixation and staining with 0.1% crystal
violet, the colonies sized >0.5, >0.25, >0.1 mm were
counted using Cytation 5 and analyzed by Gene 5 software (BioTek
Instruments, Inc.). Representative images for
1,20,24(OH)3D3 treatment are shown at the top of the
Figure. Data points for the dose responses (lower panels) are shown
as means ± SEM (n=3) *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 by Student's
t-test; #P<0.05, ##P<0.01,
###P<0.001, ####P<0.0001 at one-way
AVOVA with Dunnett's multi comparison post-hoc test.
IC50 values were calculated from the non-fitted
dose-response curves. |
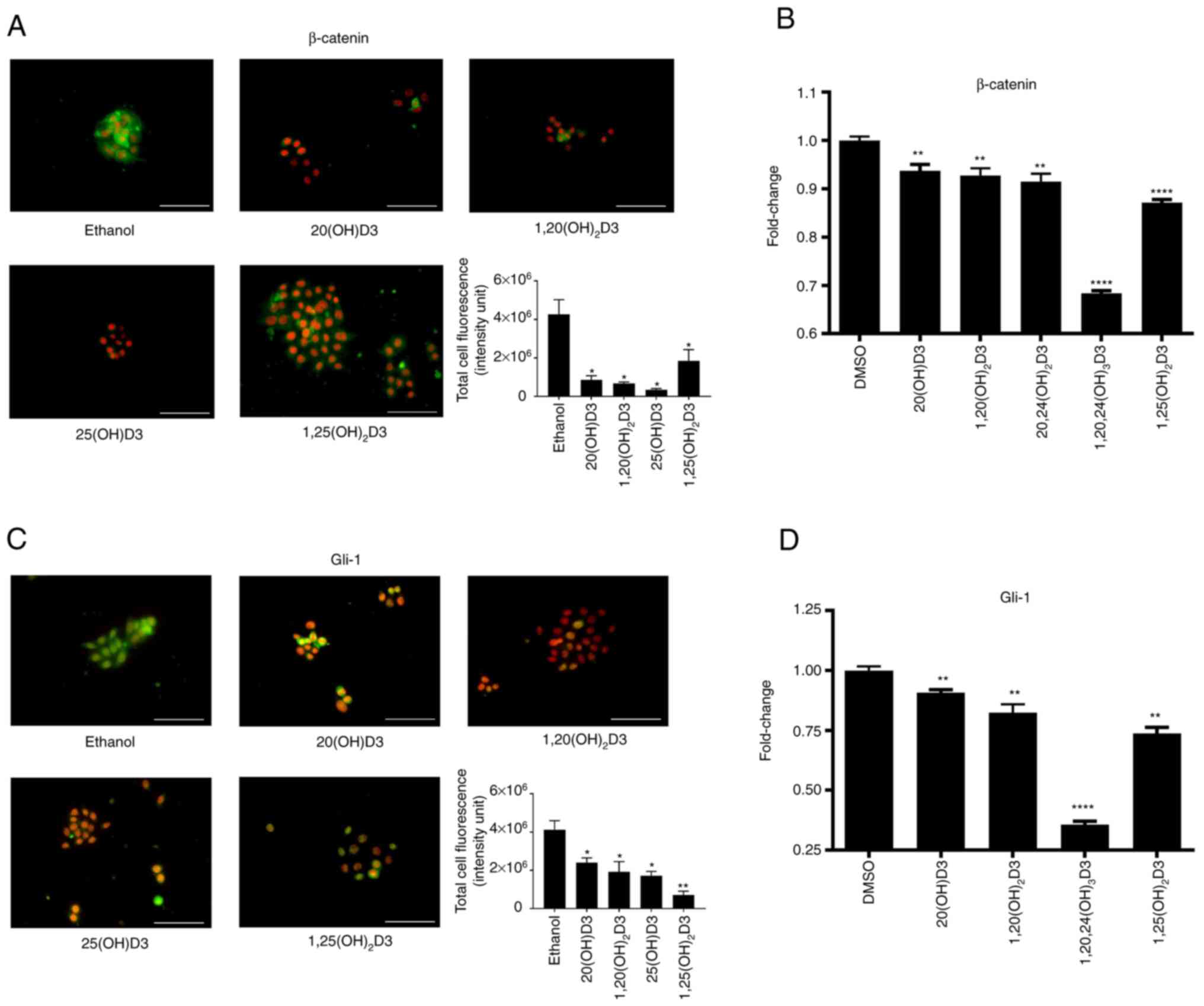
As the authors have previously shown that the
antitumor effects against oral squamous cell carcinoma are
associated with inhibition of β-catenin and glioma-associated
oncogene 1 (Gli1) (80), the
effect of vitamin D-hydroxyderivatives on the expression of
β-catenin and Gli1 were tested in the murine ASZ001 carcinoma line.
The selected secosteroids, including 25(OH)D3 and
1,25(OH)2D3 as well as CYP11A1-derived 20(OH)D3 and
1,20(OH)2D3, significantly inhibited intracellular
expression of these proteins (Fig.
5A-D). These effects were further substantiated by inhibition
of the expression of Gli1 and Ctnnb1 genes, by a
panel of vitamin D derivatives (Fig.
5A-D). A similar inhibitory effect on Gli1 expression by
20(OH)D3, 1,20(OH)2D3 and 1,25(OH)2D3, but
not by 25(OH D3 was observed in the A431 line (Fig. 6A and B). The expression of
β-catenin was not evaluated as it was below the level of detection
by the procedure employed. The effects of 20(OH)D3 and
1,20(OH)2D3 on involucrin expression was also tested in
comparison with 1,25(OH)2D3 in the A431 cells.
Stimulation by all three secosteroids was observed (Fig. 6C-D).
SCC and BCC lines expressed VDR and RORα and γ,
which are receptors for vitamin D3 hydroxyderivatives, as well as
is the C1α hydroxylase CYP27B1 (Fig.
7A and B). The relative expression of VDR and RORγ in cell
extracts was the highest in the ASZ0001 line with RORα being
similar in the three lines. CYP27B1 expression was highest in the
A431 cells and lowest in the ASZ001 cell extracts (Fig. 7A). The nuclear receptors showed
both a cytoplasmic and nuclear staining pattern, while western
blotting showed predominant location of the VDR in the cytoplasm of
the ASZ001 line (Fig. 7A). The
effects of representative vitamin D3 hydroxyderivatives on the gene
expression of vitamin D inactivating (Cyp24A1) and
activating enzymes (Cyp11a1 and CYP27b1) and nuclear
receptors (Vdr, Rora and Rorc) were also
tested (Fig. 7B). As expected,
the secosteroids with a C1α(OH) showed the strongest stimulation of
the expression of Cyp24a1. A smaller stimulation of
Cyp27b1 expression (up to 1.5-fold) was seen with all the
secosteroids tested except 1,20,24(OH)3D3 which showed
no effect. Only a marginal effect was observed for Cyp11a1
expression with 1,20(OH)2D3 decreasing it and
1,25(OH)2D3 increasing it. The majority of the vitamin
D3 hydroxyderivatives tested stimulated Vdr gene expression
to some degree, with 1,20,24(OH)3D3 showing the
strongest effect (1.8 fold). All the secosteroids had an inhibitory
effect on Rora. The effects on Rorc were small and
variable, mainly inhibiting expression but stimulating it in the
case of 1,25(OH)2D3 (Fig.
7B).
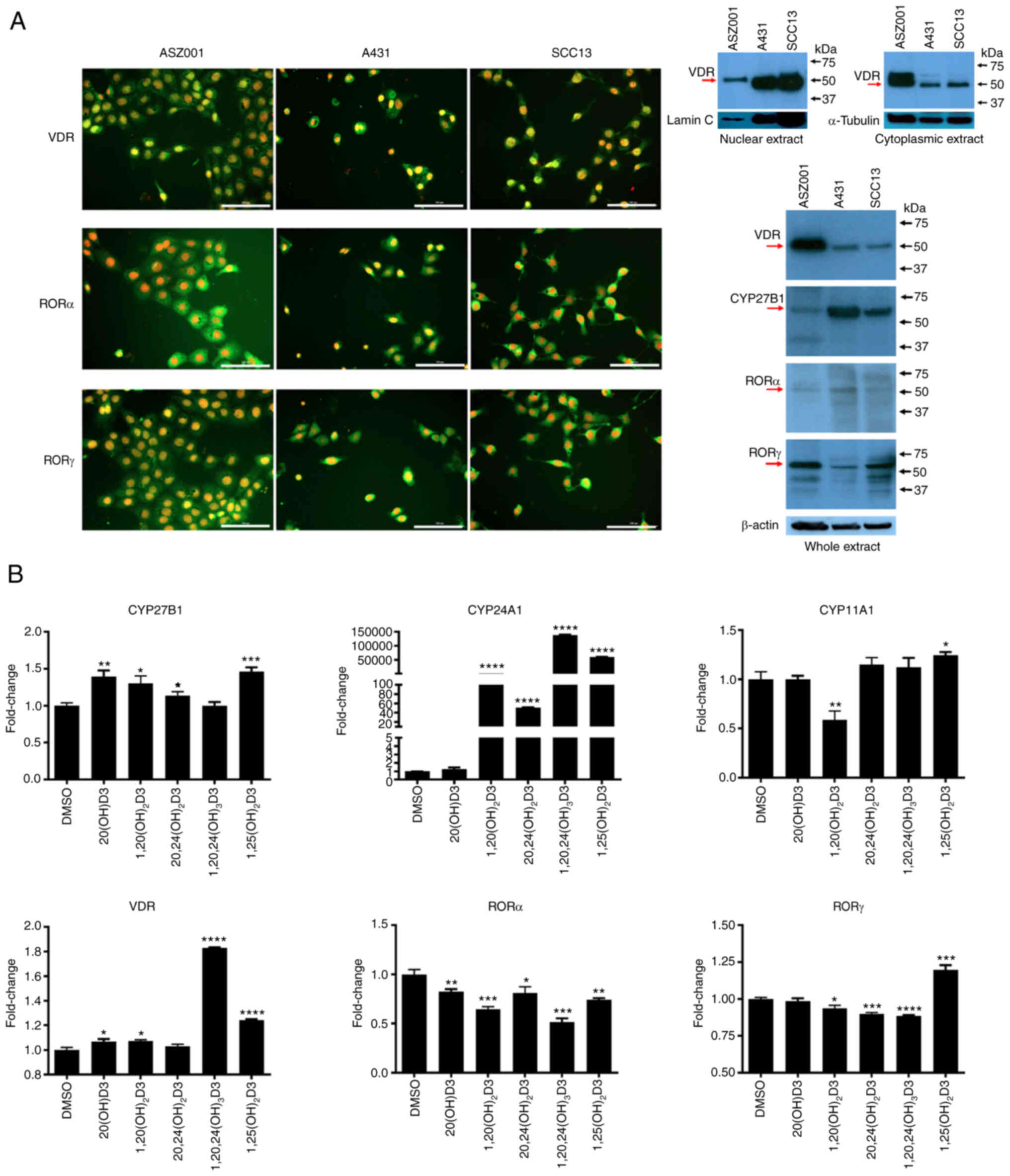 | Figure 7Expression of nuclear receptors for
vitamin D3 hydroxyderivatives and CYP enzymes involved in vitamin D
metabolism in murine ASZ001, human A431 and human SCC13 carcinoma
lines. (A) Immunofluorescence detection (left panel) was performed
using antibodies against VDR (MA1-710) and RORα and RORγ (54). The antigen is in green, while cell
nuclei are red-counterstained with propidium iodide; scale bar=100
µm. Right panel shows detection of VDR and RORα and RORγ by
western blotting (arrows) using specific anti-receptor antibodies
(see Materials and methods) for isolated cytoplasmic or nuclear
fractions (VDR) from the cells or whole extracts (CYP27B1, RORα and
RORγ). Human hepatoma and human melanoma SKMEL-188 cells were used
as positive controls for RORα and RORγ. Loading was evaluated using
antibodies against lamin C and α-tubulin for nuclear or cytoplasmic
fractions, respectively, while with anti-β-actin for whole
extracts. (B) shows expression at the mRNA level of Cyp27b1,
Cyp24a1, Cyp11a1, Vdr, Rora and Rorc, respectively in
ASZ001 murine carcinoma line treated with 10−7 M of the
secosteroids listed on the x-axis. Data represent fold change,
using β-actin as a housekeeping gene and are shown as means ± SEM
(n=4) with *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 by Student's
t-test. ROR, retinoid-related orphan receptor; VDR, vitamin D
receptor. |
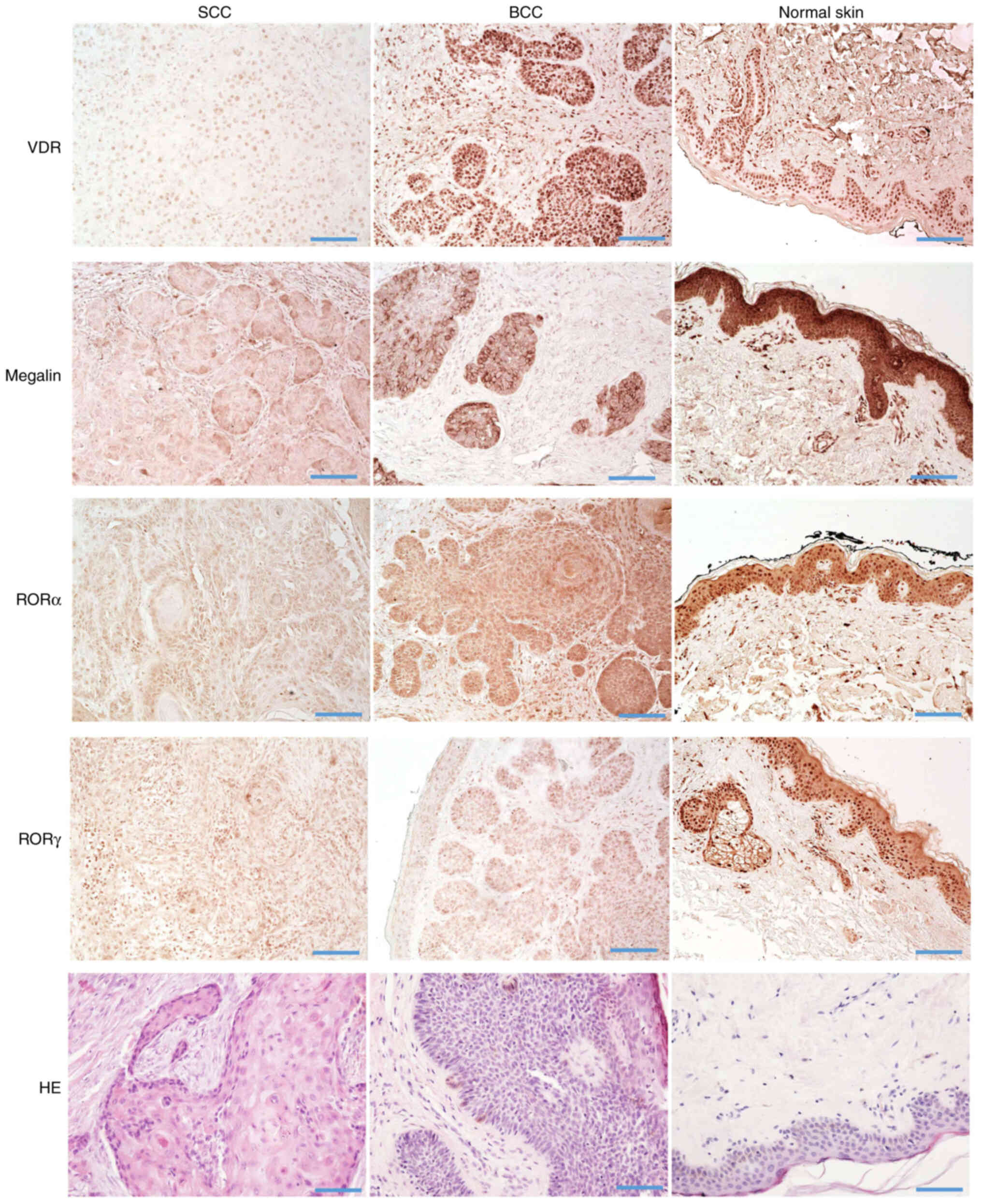
The relative expression of the above nuclear
receptors and megalin/LRP2 in normal skin (n=35), BCC (n=12), SSC
in situ (n=22) and SCC (n=14) were analyzed by
immunohistochemical staining. The characteristics of samples
analyzed in this study are presented in Table SI. VDR, RORα and RORγ showed
mostly nuclear staining, with very faint cytoplasmic staining in
normal, peritumoral and cancerous tissues (Fig. 8). Megalin/LRP2 was localized
mostly in the cytoplasm with less expression on cell membranes.
Megalin/LRP2 was also found in normal, peritumoral and cancerous
tissues (Fig. 8).
Semiquantitative analysis of the VDR and
megalin/LRP2 expression showed similar levels of immunostaining in
SCC in situ and invasive and unaffected peritumoral skin;
however, their expression was significantly lower in comparison
with normal control skin samples (Fig. 9A). Compared to BCC, the VDR
expression level was similar in normal and peritumoral skin and BCC
samples (Fig. 9). On the other
hand, megalin/LRP2 showed significantly lower expression levels in
BCC in comparison with normal and peritumoral skin (Fig. 9A). RORα and RORγ expression in
peritumoral skin was similar to normal skin, while their expression
was significantly reduced in SCC (in situ and invasive) and
BCC (Fig. 9A). This trend was
also found for the mRNA expression of RORA and RORC
(Fig. 9B) using the genomic data
from the public repository (Gene Expression Omnibus, accession
number GSE7553).
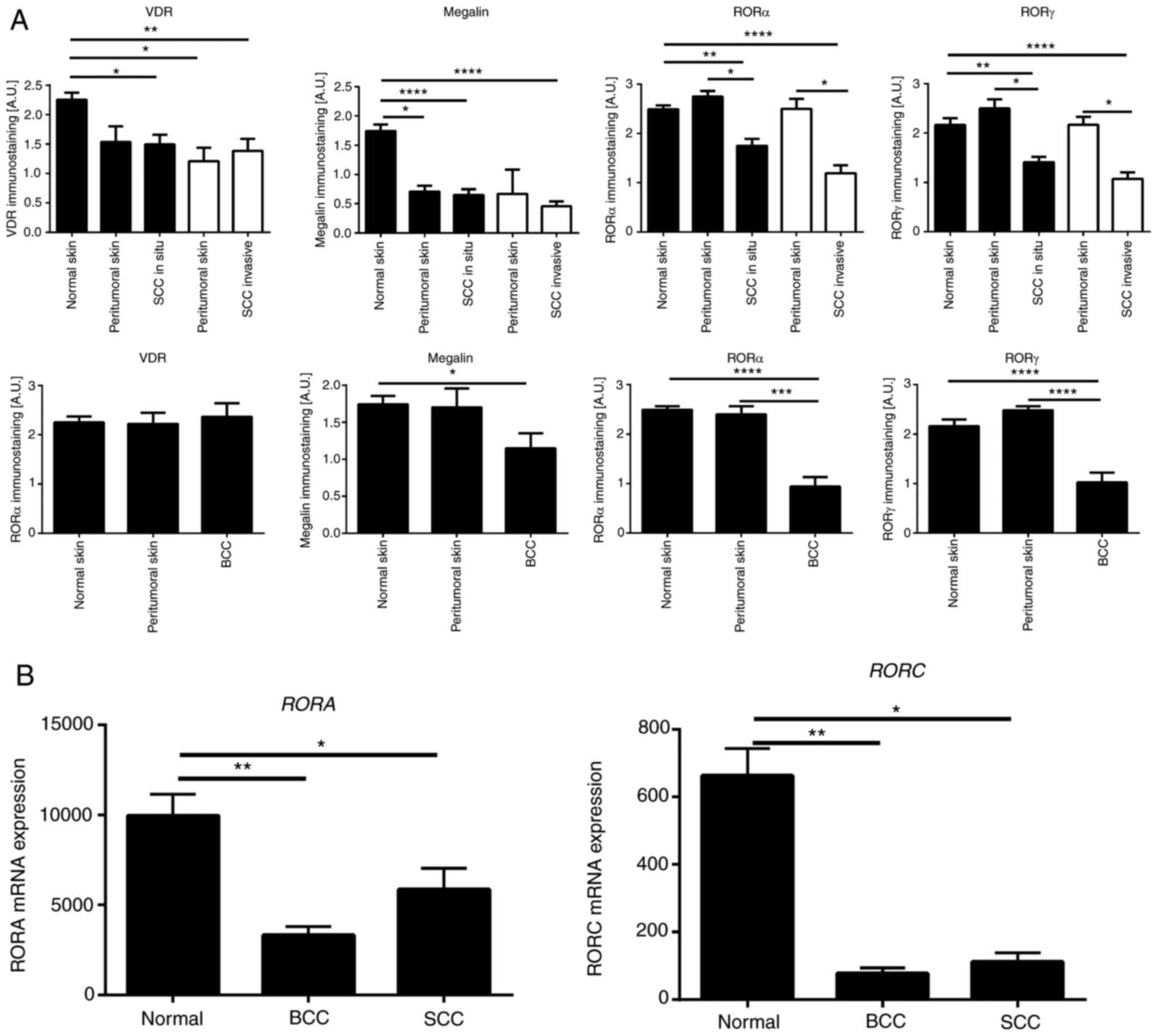 | Figure 9Quantitative analysis of nuclear
receptor expression in SCC and BCC. (A) Quantitative comparison of
immunostaining of VDR, RORα, RORγ and megalin in SCC (n=14), SCC
in situ (n=22), BCC (n=12), normal (n=35) and peritumoral
skin samples. Data are presented as mean values ± SD. Statistically
significant differences were determined with ANOVA followed by
Dunn's multiple comparisons test with *P<0.05,
**P<0.01, with ***P<0.001,
****P<0.0001. The immunohistochemical stained
archival formalin-fixed paraffin-embedded sections were used for
quantifications as described in the Materials and methods. (B)
Quantitative comparison of mRNA expression of RORA (probe
226682_at) and RORC (probe 228806_at) in SCC (n=11), BCC
(n=15) and normal skin (n=4) was performed using data from the
genomic repository (Gene Expression Omnibus, accession number
GSE7553 http://www.ncbi.nlm.nih.gov/geo). Data are shown as
means ± SD. Statistically significant differences were determined
with the ANOVA followed by Dunn's multiple comparisons test with
*P<0.05, **P<0.01. SCC, squamous cell
carcinomas; BCC, basal cell carcinomas; ROR, retinoid-related
orphan receptor; VDR, vitamin D receptor. |
Discussion
The present study demonstrated that active forms of
vitamin D3, including classical 1,25(OH)2D3 and novel
CYP11A1-derived hydroxyderivatives, show in vitro anticancer
activities against human A431- and SCC13- and murine
ASZ001-carcinoma cell lines as measured by cell proliferation
assays and colony- and spheroid-formation assays. While the
anti-tumor effects against NMSC of 1,25(OH)2D3 were
expected (27,77,86-92), the above data are the first
reports on the inhibitory effects of CYP11A1-derived
D3-hydroxyderivates on cutaneous lines of SCC and BCC. These data
are also consistent with the ability of CYP11A1-derived vitamin D
hydroxyderivatives to inhibit proliferation of primary and
immortalized non-tumorigenic epidermal keratinocytes (51,55,93) and their anti-cancer properties in
oral squamous cell carcinoma (80) and melanoma (94-96). The significance of these findings
is emphasized by the lack or low calcemic activities of the best
characterized of CYP11A1-derived hydroxyderivatives, namely
20(OH)D3, 20,23(OH)2D3 and 1,20(OH)2D3, and
their lack of measurable toxicity at pharmacological doses
(39). Furthermore, they have
been identified in the human body (29,31,34,45,97,98) and are considered as natural
products (35). Thus,
CYP11A1-derived hydroxyderivatives of vitamin D are excellent
candidates for principal or adjuvant therapeutics in the treatment
of cutaneous SCC and BCC.
Some differences in the efficacies of the vitamin
D3 hydroxyderivatives were observed depending on the cell line and
the positions of the hydroxyl groups on the secosteroids,
particularly the presence of the C1α-hydroxyl group. While the
anti-proliferative and anti-tumor effects of D3 hydroxyderivatives
were similar in both SCC lines, compounds with a C1α(OH) showed
higher efficacy in the BCC line for spheroid formation. These
differences can be rationalized by significantly higher expression
of the VDR and lower expression of CYP27B1 in the ASZ001 line in
comparison with A431 and SCC13 lines. Specifically, the VDR shows
high selectivity for 1,25(OH)2D3 (48,99) and other C1α-hydroxyderivatives of
D3 (52,53,55). Thus, higher levels of VDR and its
greater level in the cytoplasmic fraction of ASZ001 cells indicated
its relatively high availability in this BCC line for binding of
C1α-hydroxyderivatives, necessary for its translocation to the
nucleus and induction of transcriptional activity. Furthermore, the
relatively low expression of CYP27B1 in these cells pointed to less
efficient hydroxylation of CYP11-derived hydroxyderivatives that
lack a C1α(OH), thus potentiating the difference between
secosteroids with and without the C1α (OH).
D3-hydroxyderivatives can also activate other
nuclear and non-nuclear receptors besides the VDR (42,100). Specifically, CYP11A1-derived D
hydroxyderivatives act as inverse agonists on ROR α and γ (54,55), which are expressed in these lines,
providing additional regulatory targets. This is further
substantiated by our study in which knocking out RORγ in murine
fibroblasts reverses the antiproliferative and anti-fibrotic
effects of D3 hydroxyderivatives on these phenotypic traits
(56). In other tumor models, the
expression of these receptors decrease during melanoma progression
(59) and there is a positive
correlation between the expression of RORs and the expression of
HIF-1α (hypoxia-inducible factor 1α) (60). Notably, D3-hydroxyderivatives can
affect gene expression of enzymes involved in activation or
inactivation of active forms of D3, as well as their receptors
(Fig. 7B). Specifically, they
caused a small stimulation of CYP27B1 expression in a manner
unrelated to the absence or presence of a C1α(OH). They
significantly stimulated CYP24A1 [an inactivating enzyme
(101,102)] expression with selectivity for
compounds containing a C1α(OH). This further substantiated the
selectivity of the derivatives containing a C1α(OH) for the VDR,
since CYP24A1 is a well-established downstream target of the VDR
(16,103). There was little effect on
CYP11A1 expression and small but variable stimulation of
VDR expression by the various secosteroids. This is
consistent with previous reports showing that VDR expression
can be stimulated by active forms of vitamin D (30,104). All compounds inhibited
RORA expression and the majority also inhibited RORC
expression with the exception of 1,25(OH)2D3, which
stimulated the expression of this gene. In summary, the actions of
hydroxyderivatives of D3 are complex involving both VDR-dependent
and independent (RORs) mechanisms. Their actions may also be
influenced by their in vivo metabolism by vitamin
D-activating and inactivating enzymes and by their abilities to
regulate these enzymes, as well as their abilities to regulate
expression of their target receptors. This opens exciting
possibilities for future studies.
Application of vitamin D and of
1,25(OH)2D3 can inhibit BCC development and growth
through inhibition Hh signaling (27,77,87,88). Similarly, the authors have shown
that D3 hydroxyderivatives can inhibit Hh signaling and β-catenin
expression in oral SCC (80),
while another study has shown that 1,25(OH)2D3 inhibits
β-catenin signaling in epidermal keratinocytes (105). In agreement with these studies
the present study showed that classical and CYP11A1-derived D3
hydroxyderivatives can inhibit GLI1 and β-catenin expression in the
murine ASZ001 cell line and Gli1 in the human A431 line,
identifying these pathways as realistic targets for
bioregulation.
Immunocytochemistry performed on human biopsies of
SCC in situ, invasive SCC and BCC showed nuclear expression
of all three receptors. This finding is consistent with previous
reports on the VDR expression in NMSCs biopsies (90,106). However, some differences in the
level of immunoreactivity require explanation. Studies by Reichrath
et al (90,106) report an increase in VDR
immunoreactivity in SCC and BCC compared with normal skin. The
present study showed a slight but significant decrease in VDR
expression in SCC in comparison with normal skin and no difference
in VDR expression between BCC and control skin. These quantitative
differences could be secondary to differences in the patient
population, fixation protocols, tissue processing or different
antibodies used. However, in agreement with Reichrath et al
(90,106), the present study did not observe
any marked differences between different types of SCC and BCC and
observed an inhibition of SCC growth in vitro by
D3-hydroxyderivatives. With respect to RORα and γ, these receptors
have a similar expression in peritumoral and normal skin and show
comparatively lower expression in SCC and BCC. This pattern is
consistent with decreased expression of the receptors observed
during the progression of melanocytic lesions to a more malignant
phenotype (59) or with decreased
expression of corresponding genes in SCC and BCC as evaluated from
the public genomics data repository (GSE7553). RORs are targets for
CYP11A1-derived hydroxyderivatives (54,55). Therefore, a decrease in their
expression may reduce the anti-tumor effects of the
hydroxyderivatives via their inverse agonist activity on RORs.
Finally, the present study observed decreased expression in the SCC
and BCC samples of transmembrane glycoprotein-LRP2/megalin, which
is implicated in vitamin D3 transport into the cell (66-68). The decrease in expression of RORs,
VDR and LRP2/megalin was recently reported in
hyperproliferative/inflammatory diseases, such as psoriasis
(84). Thus, disturbed expression
of LRP2/megalin, VDR, RORα and RORγ in skin cancerous lesions
suggests defects in the vitamin D endocrine system in cancerous
tissue that could facilitate tumor growth. Their detection in all
SCC and BCC samples indicated that they can serve as realistic
targets for therapeutic activity of novel vitamin D
hydroxyderivatives. However, it should be noted that not only
cancerous keratinocytes express the receptors for vitamin D and are
targets for CYP11A1-derived hydroxymetabolites of vitamin D but
they also regulate a differentiation program in normal epidermal
keratinocytes. Specifically, hydroxymetabolites of vitamin can
induce differentiation, inhibit proliferation, act as
photoprotectants and as anti-aging and anti-inflammatory factors in
normal epidermal keratinocytes (51,53,93,107,108).
In conclusion, the present study identified several
CYP11A1-derived vitamin D analogs which are endogenously produced
(29,31,33,34,45,98), as potential cutaneous anti-cancer
therapeutics. Their anti-cancer activity can be mediated via
different mechanisms. It is well known that 1,25(OH)2D3
promotes the differentiation of keratinocytes (93,109). The present study demonstrated
that 20(OH D3, 1,20(OH)2D3 and 1,25(OH)2D3
also stimulated involucrin expression in skin cancer cells. The
other mechanisms relate to effects on Hh/GLI1 and β-catenin
signaling pathways uncontrolled activities of which promote cancer
development and progression (107,110). The present study showed that
GLI1 and β-catenin are downregulated following treatment with
CYP11A1-derived D3 hydroxyderivatives. Expression of nuclear
receptors for these hydroxyderivatives were further observed in all
samples of SCC and BCC, which potentially can be targeted in
vivo for anti-tumor activity. This is the first time, to the
best of the authors' knowledge, that a range of CYP11A1-derived
vitamin D hydroxyderivatives have been tested against cutaneous SCC
and BCC. The finding that RORs are expressed in skin cancer cells
is also new. It has previously been shown that
1,25(OH)2D3 inhibits the growth of BCC in mice through
the activation of the VDR and inhibition of Hh signaling, in a
VDR-independent manner, with the effects being stronger than those
of the cyclopamine (88). It was
also reported that D3 inhibits Hh signaling and growth of murine
BCC cells to a similar degree as cyclopamine with an effect
independent of the VDR (77).
Notably, topical application of the D3 prohormone can inhibit
UVB-induced BCC with orally delivered D3 having no effect (27). In addition, calcipotriol (analog
of 1,25(OH)2D3) inhibits cutaneous cancerogenesis in
mice (86) and in clinical
studies shows a synergistic effect with other therapeutic
modalities against actinic keratosis (86,111). While calcemic and other toxic
effects place limits on the therapeutic use of
1,25(OH)2D3 or its synthetic derivatives at
pharmacological doses, major CYP11A1-derived vitamin D derivatives
are noncalcemic and non-toxic or show low calcemic effects at
supra-pharmacological doses (37,39,63,64). They have also been detected in
natural products (35) and show
potent protective properties against UVB-induced damage (43,44). Besides the VDR they can act on
other nuclear receptors (42,57,112), including RORs (54), extending the therapeutic
possibilities for their use. The present study provided exciting
challenges for medicinal chemistry to develop more
receptor-specific ligands based on the lead structures of the
CYP11A1-derived hydroxyderivatives.
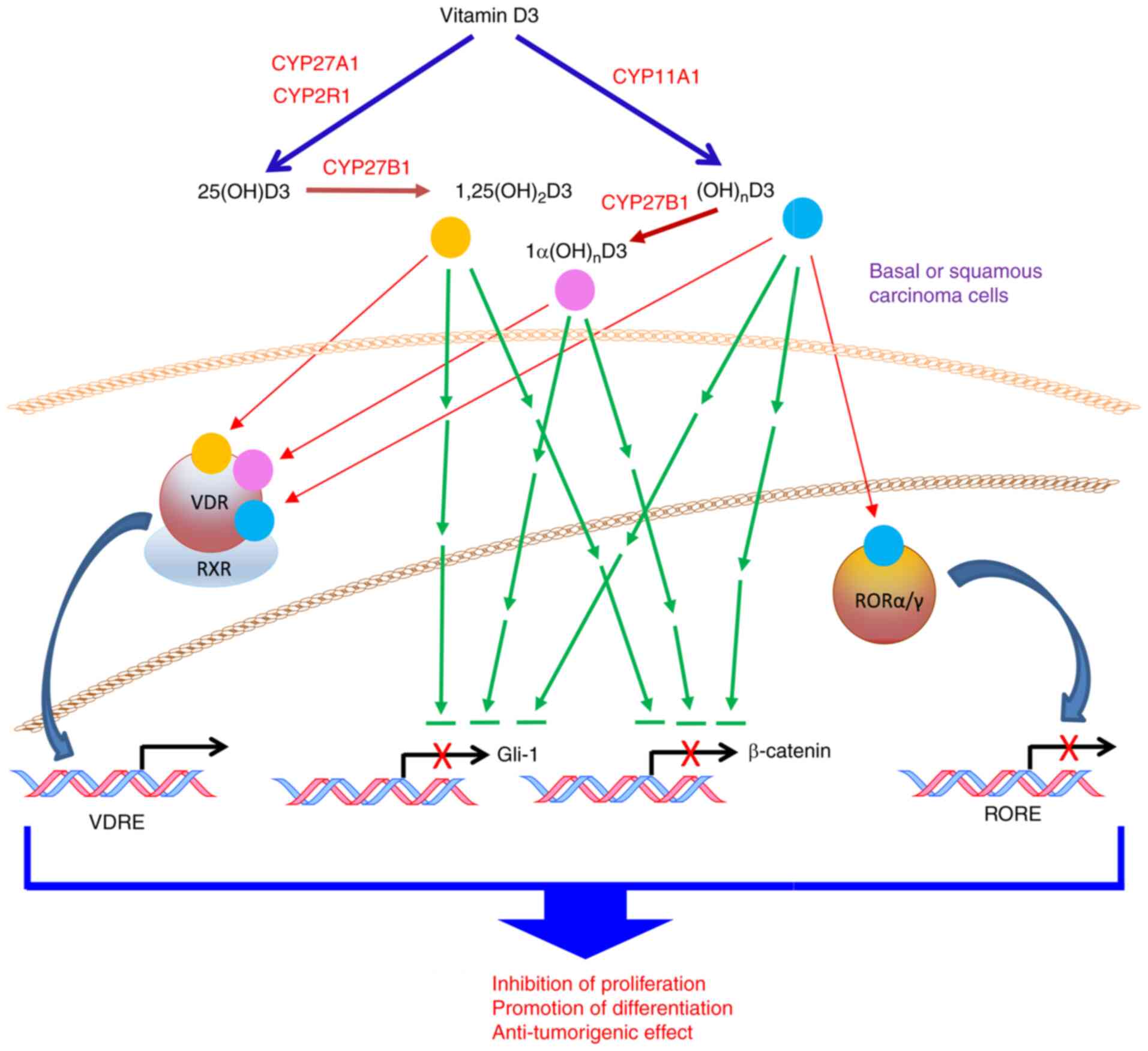
In summary, the present study identified
CYP11A1-derived vitamin D hydroxyderivatives as excellent
candidates for therapy or prevention of NMSCs, which can act on
distinct nuclear receptors and inhibit GLI1 and β-catenin signaling
pathways as a part of their anti-cancer activity with a proposed
mechanism of action shown in Fig.
10.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
ATS conceived and supervised the study, analyzed
the data and wrote, revised and edited the manuscript. AAB
performed the experiments, analyzed the data and wrote, revised and
edited the manuscript. TKK performed the experiments, analyzed the
data and drafted the manuscript. MME performed the experiments and
analyzed the data. ZJ performed the experiments, analyzed the data
and drafted the manuscript. SQ performed the experiments and
analyzed the data. RMS performed the experiments, analyzed the data
and drafted the manuscript. ASWO performed the experiments,
analyzed the data and drafted the manuscript. CL performed the
experiments, analyzed the data and drafted the manuscript. EP
performed the experiments and analyzed the data. WL synthesized
vitamin D derivatives, drafted the manuscript and revised and
edited the manuscript. AMJ provided anti-RORs antibodies, analyzed
the data and drafted, revised and edited the manuscript. RCT
synthesized vitamin D derivatives and drafted, revised and edited
the manuscript. EKYT synthesized vitamin D derivatives. CE analyzed
the data and drafted, revised and edited the manuscript. MA
analyzed data, provided cell lines and drafted, revised and edited
the manuscript. ATS and AAB confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The authors declare that this investigation was
carried out following the rules of the Declaration of Helsinki of
1975 (revised in 2008) and this study was approved by the
Institutional Review Board of the University of Alabama at
Birmingham under IRB-940831016 (OCCC Tissue Procurement CORE
Facility) and IRB-00000726 (Title E150427002; Dr. A. Slominski
PI).
Patient consent for publication
The IRB of the University of Alabama at Birmingham,
which gave its permission for conducting this study, waived the
requirement to obtain patients' informed consent for this
research.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was in part supported by the NIH (grant nos.
1R01AR073004, R01AR071189 and R21AI149267-01A1) and VA merit (grant
no. 1I01BX004293-01A1) to ATS, NIH/NCI grant nos. R21ES034595,
R01CA193885 and P01CA210946 to CAE, NIH/NCI grant nos. R01CA193609
and R01CA240447 to WL, the O'Neal Comprehensive Cancer Center Core
grant (grant no. P30CA13148) and by the Intramural Research Program
of the NIEHS, (grant no. NIH Z01-ES-101585) to AMJ). The contents
of the article are solely the responsibility of the authors and do
not necessarily represent the official views of the NIH.
References
|
1
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV light touches the brain and
endocrine system through skin, and why. Endocrinology.
159:1992–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brash DE: UV signature mutations.
Photochem Photobiol. 91:15–26. 2015. View Article : Google Scholar :
|
|
3
|
Wondrak GT: Let the sun shine in:
Mechanisms and potential for therapeutics in skin photodamage. Curr
Opin Investig Drugs. 8:390–400. 2007.PubMed/NCBI
|
|
4
|
D'Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slominski AT, Zmijewski MA, Semak I,
Zbytek B, Pisarchik A, Li W, Zjawiony J and Tuckey RC: Cytochromes
p450 and skin cancer: Role of local endocrine pathways. Anticancer
Agents Med Chem. 14:77–96. 2014. View Article : Google Scholar
|
|
7
|
Athar M, Walsh SB, Kopelovich L and Elmets
CA: Pathogenesis of nonmelanoma skin cancers in organ transplant
recipients. Arch Biochem Biophys. 508:159–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo HL, Nakajima S, Ma L, Walter B, Yasui
A, Ethell DW and Owen LB: Differential biologic effects of CPD and
6-4PP UV-induced DNA damage on the induction of apoptosis and
cell-cycle arrest. BMC Cancer. 5:1352005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wondrak GT, Roberts MJ, Jacobson MK and
Jacobson EL: 3-hydroxypyridine chromophores are endogenous
sensitizers of photooxidative stress in human skin cells. J Biol
Chem. 279:30009–30020. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slominski AT, Zmijewski MA, Skobowiat C,
Zbytek B, Slominski RM and Steketee JD: Sensing the environment:
Regulation of local and global homeostasis by the skin's
neuroendocrine system. Adv Anat Embryol Cell Biol. 212:v–vii.
1–115. 2012.PubMed/NCBI
|
|
11
|
Lim HW, Collins SAB, Resneck JS Jr,
Bolognia JL, Hodge JA, Rohrer TA, Van Beek MJ, Margolis DJ, Sober
AJ, Weinstock MA, et al: The burden of skin disease in the United
States. J Am Acad Dermatol. 76:958–972.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlson JA, Slominski A, Murphy MJ and
Wilson V: Evidence of skin field cancerization. Field
Cancerization: Basic Science and Clical Applications. Dakubo G:
Nova Science Publishers Inc; pp. 317–369. 2011
|
|
13
|
Holick MF: Vitamin D: A millenium
perspective. J Cell Biochem. 88:296–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holick MF, Tian XQ and Allen M:
Evolutionary importance for the membrane enhancement of the
production of vitamin D3 in the skin of poikilothermic animals.
Proc Natl Acad Sci USA. 92:3124–3126. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bikle DD: Vitamin D and the skin. J Bone
Miner Metab. 28:117–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bikle DD: Vitamin D metabolism and
function in the skin. Mol Cell Endocrinol. 347:80–89. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plum LA and DeLuca HF: Vitamin D, disease
and therapeutic opportunities. Nat Rev Drug Discov. 9:941–955.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bikle DD: Vitamin D receptor, UVR, and
skin cancer: A potential protective mechanism. J Invest Dermatol.
128:2357–2361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elias PM: Structure and function of the
stratum corneum extracellular matrix. J Invest Dermatol.
132:2131–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bikle DD: Vitamin D: Newly discovered
actions require reconsideration of physiologic requirements. Trends
Endocrinol Metab. 21:375–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Indra AK, Castaneda E, Antal MC, Jiang M,
Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, et al:
Malignant transformation of DMBA/TPA-induced papillomas and nevi in
the skin of mice selectively lacking retinoid-X-receptor alpha in
epidermal keratinocytes. J Invest Dermatol. 127:1250–1260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dixon KM, Tongkao-On W, Sequeira VB,
Carter SE, Song EJ, Rybchyn MS, Gordon-Thomson C and Mason RS:
Vitamin D and death by sunshine. Int J Mol Sci. 14:1964–1977. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song EJ, Gordon-Thomson C, Cole L, Stern
H, Halliday GM, Damian DL, Reeve VE and Mason RS:
1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA
damage and contributes to photoprotection. J Steroid Biochem Mol
Biol. 136:131–138. 2013. View Article : Google Scholar
|
|
25
|
Bikle DD, Elalieh H, Welsh J, Oh D,
Cleaver J and Teichert A: Protective role of vitamin D signaling in
skin cancer formation. J Steroid Biochem Mol Biol. 136:271–279.
2013. View Article : Google Scholar :
|
|
26
|
Demetriou SK, Ona-Vu K, Teichert AE,
Cleaver JE, Bikle DD and Oh DH: Vitamin D receptor mediates DNA
repair and is UV inducible in intact epidermis but not in cultured
keratinocytes. J Invest Dermatol. 132:2097–2100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Makarova A, Wang G, Dolorito JA, Kc S,
Libove E and Epstein EH Jr: Vitamin D3 produced by skin
exposure to UVR inhibits murine basal cell carcinoma
carcinogenesis. J Invest Dermatol. 137:2613–2619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slominski AT, Li W, Kim TK, Semak I, Wang
J, Zjawiony JK and Tuckey RC: Novel activities of CYP11A1 and their
potential physiological significance. J Steroid Biochem Mol Biol.
151:25–37. 2015. View Article : Google Scholar
|
|
29
|
Slominski AT, Kim TK, Li W, Postlethwaite
A, Tieu EW, Tang EKY and Tuckey RC: Detection of novel
CYP11A1-derived secosteroids in the human epidermis and serum and
pig adrenal gland. Sci Rep. 5:148752015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuckey RC, Li W, Shehabi HZ, Janjetovic Z,
Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, et al:
Production of 22-hydroxy metabolites of vitamin d3 by cytochrome
p450scc (CYP11A1) and analysis of their biological activities on
skin cells. Drug Metab Dispos. 39:1577–1588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slominski AT, Kim TK, Shehabi HZ, Semak I,
Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, et
al: In vivo evidence for a novel pathway of vitamin D3
metabolism initiated by P450scc and modified by CYP27B1. FASEB J.
26:3901–3915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang EK, Chen J, Janjetovic Z, Tieu EW,
Slominski AT, Li W and Tuckey RC: Hydroxylation of CYP11A1-derived
products of vitamin D3 metabolism by human and mouse CYP27B1. Drug
Metab Dispos. 41:1112–1124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slominski AT, Kim TK, Shehabi HZ, Tang EK,
Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W and Tuckey RC: In
vivo production of novel vitamin D2 hydroxy-derivatives by human
placentas, epidermal keratinocytes, Caco-2 colon cells and the
adrenal gland. Mol Cell Endocrinol. 383:181–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slominski AT, Kim TK, Li W and Tuckey RC:
Classical and non-classical metabolic transformation of vitamin D
in dermal fibroblasts. Exp Dermatol. 25:231–232. 2016. View Article : Google Scholar :
|
|
35
|
Kim TK, Atigadda V, Brzeminski P, Fabisiak
A, Tang EKY, Tuckey RC and Slominski AT: Detection of
7-dehydrocholesterol and vitamin D3 derivatives in honey.
Molecules. 25:25832020. View Article : Google Scholar :
|
|
36
|
Slominski AT, Janjetovic Z, Kim TK, Wright
AC, Grese LN, Riney SJ, Nguyen MN and Tuckey RC: Novel vitamin D
hydroxyderivatives inhibit melanoma growth and show differential
effects on normal melanocytes. Anticancer Res. 32:3733–3742.
2012.PubMed/NCBI
|
|
37
|
Wang J, Slominski A, Tuckey RC, Janjetovic
Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D and Li W:
20-Hydroxyvitamin D3 inhibits proliferation of cancer
cells with high efficacy while being non-toxic. Anticancer Res.
32:739–746. 2012.PubMed/NCBI
|
|
38
|
Janjetovic Z, Brozyna AA, Tuckey RC, Kim
TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM and Slominski AT:
High basal NF-κB activity in nonpigmented melanoma cells is
associated with an enhanced sensitivity to vitamin D3 derivatives.
Br J Cancer. 105:1874–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slominski AT, Janjetovic Z, Fuller BE,
Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J,
Miller D, et al: Products of vitamin D3 or 7-dehydrocholesterol
metabolism by cytochrome P450scc show anti-leukemia effects, having
low or absent calcemic activity. PLoS One. 5:e99072010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Slominski A, Kim TK, Zmijewski MA,
Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite
A, Miller DD, et al: Novel vitamin D photoproducts and their
precursors in the skin. Dermatoendocrinol. 5:7–19. 2013. View Article : Google Scholar
|
|
41
|
Wasiewicz T, Szyszka P, Cichorek M,
Janjetovic Z, Tuckey RC, Slominski AT and Zmijewski MA: Antitumor
effects of vitamin D analogs on hamster and mouse melanoma cell
lines in relation to melanin pigmentation. Int J Mol Sci.
16:6645–6667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slominski AT, Chaiprasongsuk A, Janjetovic
Z, Kim TK, Stefan J, Slominski RM, Hanumanthu VS, Raman C, Qayyum
S, Song Y, et al: Photoprotective properties of vitamin D and
lumisterol hydroxyderivatives. Cell Biochem Biophys. 78:165–180.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaiprasongsuk A, Janjetovic Z, Kim TK,
Tuckey RC, Li W, Raman C, Panich U and Slominski AT:
CYP11A1-derived vitamin D3 products protect against
UVB-induced inflammation and promote keratinocytes differentiation.
Free Radic Biol Med. 155:87–98. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chaiprasongsuk A, Janjetovic Z, Kim TK,
Jarrett SG, D'Orazio JA, Holick MF, Tang EKY, Tuckey RC, Panich U,
Li W and Slominski AT: Protective effects of novel derivatives of
vitamin D3 and lumisterol against UVB-induced damage in
human keratinocytes involve activation of Nrf2 and p53 defense
mechanisms. Redox Biol. 24:1012062019. View Article : Google Scholar
|
|
45
|
Slominski AT, Janjetovic Z, Kim TK,
Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W and Tuckey
RC: Novel non-calcemic secosteroids that are produced by human
epidermal keratinocytes protect against solar radiation. J Steroid
Biochem Mol Biol. 148:52–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tongkao-on W, Carter S, Reeve VE, Dixon
KM, Gordon-Thomson C, Halliday GM, Tuckey RC and Mason RS: CYP11A1
in skin: An alternative route to photoprotection by vitamin D
compounds. J Steroid Biochem Mol Biol. 148:72–78. 2015. View Article : Google Scholar
|
|
47
|
Bikle D and Christakos S: New aspects of
vitamin D metabolism and action-addressing the skin as source and
target. Nat Rev Endocrinol. 16:234–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bikle DD: Vitamin D: Newer concepts of its
metabolism and function at the basic and clinical level. J Endocr
Soc. 4:bvz0382020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Reichrath J, Zouboulis CC, Vogt T and
Holick MF: Targeting the vitamin D endocrine system (VDES) for the
management of inflammatory and malignant skin diseases: An
historical view and outlook. Rev Endocr Metab Disord. 17:405–417.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carlberg C: Vitamin D genomics: From in
vitro to in vivo. Front Endocrinol (Lausanne). 9:2502018.
View Article : Google Scholar
|
|
51
|
Slominski AT, Kim TK, Janjetovic Z, Tuckey
RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, et al:
20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with
potent antiproliferative and prodifferentiation activities in
normal and malignant cells. Am J Physiol Cell Physiol.
300:C526–C541. 2011. View Article : Google Scholar :
|
|
52
|
Kim TK, Wang J, Janjetovic Z, Chen J,
Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W and Slominski AT:
Correlation between secosteroid-induced vitamin D receptor activity
in melanoma cells and computer-modeled receptor binding strength.
Mol Cell Endocrinol. 361:143–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin Z, Marepally SR, Goh ESY, Cheng CYS,
Janjetovic Z, Kim TK, Miller DD, Postlethwaite AE, Slominski AT,
Tuckey RC, et al: Investigation of 20S-hydroxyvitamin D analogs and
their 1α-OH derivatives as potent vitamin D3 receptor
agonists with anti-inflammatory activities. Sci Rep. 8:14782018.
View Article : Google Scholar
|
|
54
|
Slominski AT, Kim TK, Takeda Y, Janjetovic
Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey
RC and Jetten AM: RORα and ROR γ are expressed in human skin and
serve as receptors for endogenously produced noncalcemic
20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 28:2775–2789.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Slominski AT, Kim TK, Hobrath JV, Oak ASW,
Tang EKY, Tieu EW, Li W, Tuckey RC and Jetten AM: Endogenously
produced nonclassical vitamin D hydroxy-metabolites act as 'biased'
agonists on VDR and inverse agonists on RORα and RORγ. J Steroid
Biochem Mol Biol. 173:42–56. 2017. View Article : Google Scholar
|
|
56
|
Janjetovic Z, Postlethwaite A, Kang HS,
Kim TK, Tuckey RC, Crossman DK, Qayyum S, Jetten AM and Slominski
AT: Antifibrogenic activities of CYP11A1-derived vitamin
D3-hydroxyderivatives are dependent on RORγ. Endocrinology.
162:bqaa1982021. View Article : Google Scholar
|
|
57
|
Slominski AT, Kim TK, Janjetovic Z,
Brożyna AA, Żmijewski MA, Xu H, Sutter TR, Tuckey RC, Jetten AM and
Crossman DK: Differential and overlapping effects of
20,23(OH)2 D3 and 1,25(OH)2 D3 on gene
expression in human epidermal keratinocytes: Identification of AhR
as an alternative receptor for 20,23(OH)2 D3. Int J Mol
Sci. 19:30722018. View Article : Google Scholar
|
|
58
|
Markiewicz A, Brożyna AA, Podgórska E,
Elas M, Urbańska K, Jetten AM, Slominski AT, Jóźwicki W,
Orłowska-Heitzman J, Dyduch G and Romanowska-Dixon B: Vitamin D
receptors (VDR), hydroxylases CYP27B1 and CYP24A1 and
retinoid-related orphan receptors (ROR) level in human uveal tract
and ocular melanoma with different melanization levels. Sci Rep.
9:91422019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brożyna AA, Jóźwicki W, Skobowiat C,
Jetten A and Slominski AT: RORα and RORγ expression inversely
correlates with human melanoma progression. Oncotarget.
7:63261–63282. 2016. View Article : Google Scholar
|
|
60
|
Brożyna AA, Jóźwicki W, Jetten AM and
Slominski AT: On the relationship between VDR, RORα and RORγ
receptors expression and HIF1-α levels in human melanomas. Exp
Dermatol. 28:1036–1043. 2019. View Article : Google Scholar
|
|
61
|
Brożyna AA, Hoffman RM and Slominski AT:
Relevance of vitamin D in melanoma development, progression and
therapy. Anticancer Res. 40:473–489. 2020. View Article : Google Scholar
|
|
62
|
Slominski AT, Brożyna AA, Zmijewski MA,
Jóźwicki W, Jetten AM, Mason RS, Tuckey RC and Elmets CA: Vitamin D
signaling and melanoma: Role of vitamin D and its receptors in
melanoma progression and management. Lab Invest. 97:706–724. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen J, Wang J, Kim TK, Tieu EW, Tang EK,
Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, et al:
Novel vitamin D analogs as potential therapeutics: Metabolism,
toxicity profiling, and antiproliferative activity. Anticancer Res.
34:2153–2163. 2014.PubMed/NCBI
|
|
64
|
Slominski A, Janjetovic Z, Tuckey RC,
Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M and
Postlethwaite AE: 20S-Hydroxyvitamin D3, noncalcemic product of
CYP11A1 action on vitamin D3, exhibits potent antifibrogenic
activity in vivo. J Clin Endocrinol Metab. 98:E298–E303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Carlberg C and Molnar F: Current status of
vitamin D signaling and its therapeutic applications. Curr Top Med
Chem. 12:528–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao Y, Zhou S, Luu S and Glowacki J:
Megalin mediates 25-hydroxyvitamin D3 actions in human
mesenchymal stem cells. FASEB J. 33:7684–7693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nykjaer A, Dragun D, Walther D, Vorum H,
Jacobsen C, Herz J, Melsen F, Christensen EI and Willnow TE: An
endocytic pathway essential for renal uptake and activation of the
steroid 25-(OH) vitamin D3. Cell. 96:507–515. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaseda R, Hosojima M, Sato H and Saito A:
Role of megalin and cubilin in the metabolism of vitamin D(3). Ther
Apher Dial. 15(Suppl 1): S14–S17. 2011. View Article : Google Scholar
|
|
69
|
Slominski A, Semak I, Zjawiony J, Wortsman
J, Li W, Szczesniewski A and Tuckey RC: The cytochrome P450scc
system opens an alternate pathway of vitamin D3 metabolism. FEBS J.
272:4080–4090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tieu EW, Tang EK, Chen J, Li W, Nguyen MN,
Janjetovic Z, Slominski A and Tuckey RC: Rat CYP24A1 acts on
20-hydroxyvitamin D(3) producing hydroxylated products with
increased biological activity. Biochem Pharmacol. 84:1696–1704.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tang EK, Li W, Janjetovic Z, Nguyen MN,
Wang Z, Slominski A and Tuckey RC: Purified mouse CYP27B1 can
hydroxylate 20,23-dihydroxyvitamin D3, producing
1alpha,20,23-trihydroxyvitamin D3, which has altered biological
activity. Drug Metab Dispos. 38:1553–1559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tuckey RC, Li W, Zjawiony JK, Zmijewski
MA, Nguyen MN, Sweatman T, Miller D and Slominski A: Pathways and
products for the metabolism of vitamin D3 by cytochrome P450scc.
FEBS J. 275:2585–2596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tieu EW, Li W, Chen J, Baldisseri DM,
Slominski AT and Tuckey RC: Metabolism of cholesterol, vitamin D3
and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by
human CYP27A1. J Steroid Biochem Mol Biol. 129:163–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li W, Chen J, Janjetovic Z, Kim TK,
Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D and Slominski A:
Chemical synthesis of 20S-hydroxyvitamin D3, which shows
antiproliferative activity. Steroids. 75:926–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lin Z, Marepally SR, Ma D, Kim TK, Oak AS,
Myers LK, Tuckey RC, Slominski AT, Miller DD and Li W: Synthesis
and biological evaluation of vitamin D3 metabolite
20S,23S-dihydroxyvitamin D3 and Its 23R epimer. J Med Chem.
59:5102–5108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Slominski A, Semak I, Wortsman J, Zjawiony
J, Li W, Zbytek B and Tuckey RC: An alternative pathway of vitamin
D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to
20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J.
273:2891–2901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall
E, Wu A, So PL, Hebert J, Bikle D and Epstein EH Jr: Vitamin D3
inhibits hedgehog signaling and proliferation in murine basal cell
carcinomas. Cancer Prev Res (Phila). 4:744–751. 2011. View Article : Google Scholar
|
|
78
|
So PL, Langston AW, Daniallinia N, Hebert
JL, Fujimoto MA, Khaimskiy Y, Aszterbaum M and Epstein EH Jr:
Long-term establishment, characterization and manipulation of cell
lines from mouse basal cell carcinoma tumors. Exp Dermatol.
15:742–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chaudhary SC, Singh T, Talwelkar SS,
Srivastava RK, Arumugam A, Weng Z, Elmets CA, Afaq F, Kopelovich L
and Athar M: Erb-041, an estrogen receptor-β agonist, inhibits skin
photocarcinogenesis in SKH-1 hairless mice by downregulating the
WNT signaling pathway. Cancer Prev Res (Phila). 7:186–198. 2014.
View Article : Google Scholar
|
|
80
|
Oak ASW, Bocheva G, Kim TK, Brożyna AA,
Janjetovic Z, Athar M, Tuckey RC and Slominski AT: Noncalcemic
vitamin D hydroxyderivatives inhibit human oral squamous cell
carcinoma and down-regulate hedgehog and WNT/β-catenin pathways.
Anticancer Res. 40:2467–2474. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Brożyna AA, Kim TK, Zabłocka M, Jóźwicki
W, Yue J, Tuckey RC, Jetten AM and Slominski AT: Association among
vitamin D, retinoic acid-related orphan receptors, and vitamin D
hydroxyderivatives in ovarian cancer. Nutrients. 12:35412020.
View Article : Google Scholar
|
|
82
|
Brozyna AA, VanMiddlesworth L and
Slominski AT: Inhibition of melanogenesis as a radiation sensitizer
for melanoma therapy. Int J Cancer. 123:1448–1456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Slominski AT, Brożyna AA, Zmijewski MA,
Janjetovic Z, Kim TK, Slominski RM, Tuckey RC, Mason RS, Jetten AM,
Guroji P, et al: The role of classical and novel forms of vitamin D
in the pathogenesis and progression of nonmelanoma skin cancers.
Adv Exp Med Biol. 1268:257–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Brożyna AA, Żmijewski MA, Linowiecka K,
Kim TK, Slominski RM and Slominski AT: Disturbed expression of
vitamin D and retinoic acid-related orphan receptors α and γ and of
megalin in inflammatory skin diseases. Exp Dermatol. 31:781–788.
2022. View Article : Google Scholar
|
|
85
|
Riker AI, Enkemann SA, Fodstad O, Liu S,
Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, et al: The
gene expression profiles of primary and metastatic melanoma yields
a transition point of tumor progression and metastasis. BMC Med
Genomics. 1:132008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cunningham TJ, Tabacchi M, Eliane JP,
Tuchayi SM, Manivasagam S, Mirzaalian H, Turkoz A, Kopan R,
Schaffer A, Saavedra AP, et al: Randomized trial of calcipotriol
combined with 5-fluorouracil for skin cancer precursor
immunotherapy. J Clin Invest. 127:106–116. 2017. View Article : Google Scholar :
|
|
87
|
Hadden MK: Hedgehog and vitamin D
signaling pathways in development and disease. Vitam Horm.
100:231–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Uhmann A, Niemann H, Lammering B, Henkel
C, Hess I, Nitzki F, Fritsch A, Prüfer N, Rosenberger A, Dullin C,
et al: Antitumoral effects of calcitriol in basal cell carcinomas
involve inhibition of hedgehog signaling and induction of vitamin D
receptor signaling and differentiation. Mol Cancer Ther.
10:2179–2188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dixon KM, Norman AW, Sequeira VB, Mohan R,
Rybchyn MS, Reeve VE, Halliday GM and Mason RS:
1α,25(OH)2-vitamin D and a nongenomic vitamin D analogue
inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer
Prev Res (Phila). 4:1485–1494. 2011. View Article : Google Scholar
|
|
90
|
Reichrath J, Rafi L, Rech M, Mitschele T,
Meineke V, Gartner BC, Tilgen W and Holick MF: Analysis of the
vitamin D system in cutaneous squamous cell carcinomas. J Cutan
Pathol. 31:224–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bikle DD: Vitamin D and the skin:
Physiology and pathophysiology. Rev Endocr Metab Disord. 13:3–19.
2012. View Article : Google Scholar
|
|
92
|
Dixon KM, Sequeira VB, Deo SS, Mohan R,
Posner GH and Mason RS: Differential photoprotective effects of
1,25-dihydroxyvitamin D3 and a low calcaemic deltanoid. Photochem
Photobiol Sci. 11:1825–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zbytek B, Janjetovic Z, Tuckey RC,
Zmijewski MA, Sweatman TW, Jones E, Nguyen MN and Slominski AT:
20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by
cytochrome P450scc, stimulates keratinocyte differentiation. J
Invest Dermatol. 128:2271–2280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Podgorska E, Kim TK, Janjetovic Z,
Urbanska K, Tuckey RC, Bae S and Slominski AT: Knocking out the
vitamin D receptor enhances malignancy and decreases responsiveness
to vitamin D3 hydroxyderivatives in human melanoma cells. Cancers
(Basel). 13:31112021. View Article : Google Scholar
|
|
95
|
Slominski AT, Brożyna AA, Skobowiat C,
Zmijewski MA, Kim TK, Janjetovic Z, Oak AS, Jozwicki W, Jetten AM,
Mason RS, et al: On the role of classical and novel forms of
vitamin D in melanoma progression and management. J Steroid Biochem
Mol Biol. 177:159–170. 2018. View Article : Google Scholar :
|
|
96
|
Skobowiat C, Oak AS, Kim TK, Yang CH,
Pfeffer LM, Tuckey RC and Slominski AT: Noncalcemic
20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and
in vivo models. Oncotarget. 8:9823–9834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jenkinson C: The vitamin D metabolome: An
update on analysis and function. Cell Biochem Funct. 37:408–423.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jenkinson C, Desai R, Slominski AT, Tuckey
RC, Hewison M and Handelsman DJ: Simultaneous measurement of 13
circulating vitamin D3 and D2 mono and dihydroxy metabolites using
liquid chromatography mass spectrometry. Clin Chem Lab Med.
59:1642–1652. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Carlberg C and Muñoz A: An update on
vitamin D signaling and cancer. Semin Cancer Biol. 79:217–230.
2022. View Article : Google Scholar
|
|
100
|
Zmijewski MA and Carlberg C: Vitamin D
receptor(s): In the nucleus but also at membranes? Exp Dermatol.
29:876–884. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tuckey RC, Cheng CYS and Slominski AT: The
serum vitamin D metabolome: What we know and what is still to
discover. J Steroid Biochem Mol Biol. 186:4–21. 2019. View Article : Google Scholar :
|
|
102
|
Christakos S, Li S, De La Cruz J and Bikle
DD: New developments in our understanding of vitamin metabolism,
action and treatment. Metabolism. 98:112–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016. View Article : Google Scholar :
|
|
104
|
Medeiros JFP, de Oliveira Borges MV,
Soares AA, dos Santos JC, de Oliveira ABB, da Costa CHB, Cruz MS,
Bortolin RH, de Freitas RCC, Dantas PMS, et al: The impact of
vitamin D supplementation on VDR gene expression and body
composition in monozygotic twins: Randomized controlled trial. Sci
Rep. 10:119432020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hu L, Bikle DD and Oda Y: Reciprocal role
of vitamin D receptor on β-catenin regulated keratinocyte
proliferation and differentiation. J Steroid Biochem Mol Biol.
144(Pt A): 237–241. 2014. View Article : Google Scholar
|
|
106
|
Reichrath J, Kamradt J, Zhu XH, Kong XF,
Tilgen W and Holick MF: Analysis of 1,25-dihydroxyvitamin D(3)
receptors (VDR) in basal cell carcinomas. Am J Pathol. 155:583–589.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bikle DD, Ng D, Oda Y, Hanley K, Feingold
K and Xie Z: The vitamin D response element of the involucrin gene
mediates its regulation by 1,25-dihydroxyvitamin D3. J Invest
Dermatol. 119:1109–1113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe
EM Jr and Slominski AT: 20,23-dihydroxyvitamin D3, novel P450scc
product, stimulates differentiation and inhibits proliferation and
NF-kappaB activity in human keratinocytes. J Cell Physiol.
223:36–48. 2010.
|
|
109
|
Grund-Gröschke S, Stockmaier G and Aberger
F: Hedgehog/GLI signaling in tumor immunity-new therapeutic
opportunities and clinical implications. Cell Commun Signal.
17:1722019. View Article : Google Scholar
|
|
110
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Torezan L, Grinblat B, Haedersdal M,
Valente N, Festa-Neto C and Szeimies RM: A randomized split-scalp
study comparing calcipotriol-assisted methyl aminolaevulinate
photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the
treatment of actinic keratosis. Br J Dermatol. 179:829–835. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Slominski AT, Kim TK, Qayyum S, Song Y,
Janjetovic Z, Oak ASW, Slominski R M, Raman C, Stefan J,
Mier-Aguilar CA, et al: Vitamin D and lumisterol derivatives can
act on liver X receptors (LXRs). Sci Rep. 11:80022021. View Article : Google Scholar : PubMed/NCBI
|