Introduction
Brain metastases (BM) can be detected in 10-20% of
patients with non-small cell lung cancer (NSCLC) at the initial
diagnosis and the percentage extends up to 50% during disease
progression (1-3). The development of BM leads to an
evident incidence of neurocognitive and functional deficits in
patients with NSCLC, which adversely affects the quality of life
(4). BM is closely related to a
poor prognosis, and the median overall survival (OS) of patients
with NSCLC with BM is limited only up to 4-6 months (5,6).
However, the molecular mechanisms underlying the high morbidity,
poor prognosis, and high mortality rate of patients with NSCLC with
BM remain largely unknown.
In terms of histological subtypes, BM occurs most
frequently in patients with lung adenocarcinoma (54 and 58.6%), as
compared to non-adenocarcinoma subtypes (large cell carcinoma,
17.7%; and squamous cell carcinoma, 9.9%) (7,8).
Moreover, patients with NSCLC harboring epidermal growth factor
receptor (EGFR) mutations or anaplastic lymphoma kinase
(ALK) rearrangements are at a greater risk of developing BM,
in the aspect of molecular subtypes (9,10).
Pathogenic driver mutations have been reported to be vital for
therapeutic decision-making, since targeted therapies significantly
improve survival outcomes for the majority of patients (11). Specifically, a subgroup of patients
with BM responds well to EGFR tyrosine kinase inhibitors
(EGFR-TKIs) (12). However,
actionable genomic alterations are not currently available for
recurrent or progressive diseases, due to the unsafety of the
invasive biopsy procedure and/or the inaccessibility of tumor
sites, particularly for patients with BM. Currently, knowledge
about pathogenic driver mutations is most frequently obtained from
primary or metastatic tumor tissue at easily accessible sites;
however, a divergent mutation landscape from these sites has been
observed (13,14). Thus, safe, convenient and
replaceable approaches are essential to acquiring genomic
characterization for patients with NSCLC with BM.
Liquid biopsy is regarded as a promising
minimally-invasive approach to obtaining tumor cells, as opposed to
invasive tissue biopsies in molecular diagnostics in recent years
(15). Cell-free deoxyribonucleic
acid (cfDNA), which has been previously detected in blood and body
fluids, is released from cells when they undergo necrosis,
apoptosis and lysis (16,17). Circulating tumor DNA (ctDNA), the
tumor-derived fraction of cfDNA, possesses the potential ability to
represent the whole tumor burden across different metastatic sites,
partly circumventing tumor spatial heterogeneity issues (18). Since the reliability of a
single-tumor tissue biopsy for the obtainment of the whole mutation
landscape, the limitations of personalized medicine approaches are
a dilemma. Multiregional biopsy analysis has already been proposed
by Gerlinger et al (19),
in order to profile a more complete mutation landscape and predict
the therapeutic outcome. Additionally, several studies have
suggested that a dynamic sampling of somatic mutations from ctDNA
analysis may represent a larger clonal hierarchy (20-23),
rendering the realization of therapeutic decisions and tracking
therapeutic outcomes safer and more convenient, even across
different metastatic sites.
In the present study, targeted next-generation
sequencing (NGS) of tumor tissues and peripheral blood samples from
patients with NSCLC was conducted, using a hybridization
capture-based panel consisting of 95 known cancer genes. The
present study aimed to identify the characteristic of genomic
alternations in patients with advanced NSCLC with or without BM.
The novelty of the present study was, to the best of our knowledge,
systematical comparisons of the differences in somatic mutations,
somatic interactions, key signaling pathways, functional biological
information and clinical actionability for the therapy of targeted
agents between the BM group and the non-BM group. The findings
presented herein may possibly aid towards the elucidation of the
underlying molecular mechanisms underlying the initiation or
progression of BM in NSCLC. Furthermore, it was also investigated
whether ctDNA analysis may serve as a more credible alternative for
genomic profiling in patients with advanced NSCLC with BM than in
patients without BM.
Patients and methods
Patients and sample collection
In total, 120 patients with NSCLC from the
Department of Pathology at the Affiliated 3201 Hospital of Xi'an
Jiaotong University were recruited between May, 2017 and December,
2020. The pathological diagnosis was verified by three pulmonary
pathologists, based on the 4th edition of the World Health
Organization Classification of Lung Tumors (24). Tumors with histological components
other than NSCLC were excluded. In total, one fresh tumor tissue,
68 formalin-fixed and paraffin-embedded (FFPE) tumor specimens and
51 peripheral blood specimens were collected for next-generation
sequencing (NGS) analysis. Written informed consent was acquired
from all participating individuals. The study was performed
according to the Code of Ethics of the World Medical Association
(Declaration of Helsinki) (25),
and it was approved by the Medical Ethics Committee of Affiliated
3201 Hospital of Xi'an Jiaotong University. The corresponding
Institutional Review Board (IRB) number was No.008(2017).
DNA extraction and quality control
Genomic DNA (gDNA) from fresh tumor tissues, FFPE
tumor specimens and plasma was extracted using the QIAamp DNA Mini
kit (Qiagen GmbH), GeneRead DNA FFPE kit (Qiagen GmbH) and the
HiPure Circulating DNA Midi kit C (Magen Biotechnology Co., Ltd.),
respectively. The Qubit® 3.0 Fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc.) and NanoDrop ND-1000 (Thermo
Scientific, Inc.) were used for the quantity and purity evaluation
of the gDNA. The fragmentation status was evaluated using the
Agilent 2100 Bioanalyzer instrument (Agilent Technologies, Inc.)
with the High Sensitivity DNA Reagent (Agilent Technologies, Inc.)
to produce a DNA integrity number. Additionally, the step of
quality control (QC) was also performed to evaluate FFPE DNA
integrity by multiplex Polymerase Chain Reaction (PCR). In brief,
gDNA (30 ng) was amplified using three different primers of the
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, sized
at 200-400 base pairs (Table SI).
The Agilent 2100 Bioanalyzer instrument (Agilent Technologies,
Inc.) was used to determine the concentration of multiplex PCR
products. The fragmentation of gDNA from FFPE was estimated by the
average yield ratio (AYR) value, which was calculated by dividing
the yield ratio of reference DNA (Promega Corporation) by each
amplicon's yield ratio.
Library preparation and hybridization
capture
In total, 300 ng gDNA from each sample was
mechanically fragmented using an E220 focused ultrasonicator
Covaris (Covaris, LLC.). The targeted size of the DNA fragment was
between 150 and 200 bp. Subsequently, 10-100 ng DNA was used for
library construction with the KAPA library preparation kit (Kapa
Biosystems Inc.; Roche Diagnostics), which was constructed with
end-repair, A-tailing and adapter ligation without additional
fragmentation, according to the manufacturer's instructions.
Finally, the NGS libraries were captured using the xGen Lockdown
Probe pool (Integrated DNA Technologies, Inc.), and the captured
DNA fragments were amplified for 13 cycles of PCR, using 1X KAPA
HiFi Hot Start Ready Mix (Kapa Biosystems Inc.; Roche Diagnostics).
The thermal cycling conditions used were as follows: Initial
denaturation for 45 sec at 98°C followed by 13 cycles of 98°C for
15 sec, 60°C for 30 sec, and 72°C for 30 sec. The final extension
was performed at 72°C for 60 sec.
Illumina sequencing
Following QC and quantification using the Agilent
2100 Bioanalyzer (Agilent Technologies, Inc.) and Qubit®
3.0 Fluorometer (Invitrogen; Thermo Fisher Scientific, Inc.), the
NGS libraries were sequenced on an Illumina NextSeq CN500 platform
with a medium flux chip (NextSeq CN500 Mid Output v2 kit; Illumina
Inc.).
Bioinformatics analysis
Clean data were obtained following filtering
low-quality reads, which includes reads with adapter sequences and
reads with length <36 bp. All filtered reads were aligned to the
human genome (University of California Santa Cruz ID: hg19) using
the Burrows-Wheeler-Alignment Tool (BWA v.0.7.12; Wellcome Trust
Sanger Institute) (26).
Subsequently, the Picard and Genome Analysis Toolkit (GATK v.3.2;
Broad Institute) method was used for duplicate removal, local
realignment, and base quality score recalibration, and it was also
adopted for generating quality statistics, including mapped reads,
mean mapping quality, and mean coverage. Finally, the VarDict
(v.1.6.0; GitHub, Inc.) was adopted for the identification of
single nucleotide variation (SNV) and Insertion/Deletion (InDel)
(27).
The ANNOVAR software tool (v. 20210202; https://annovar.openbioinformatics.org/en/latest/) was
used for annotating somatic variants (28). The candidates of somatic variants
were identified by the following filter conditions: i) Removal of
the variants coverage depth (VDP) <10; ii) removal of the
variant sites with mutant allele frequency (MAF) >0.001 in the
1,000 Genomes databases (1,000 Genomes Project Consortium;
https://www.internationalgenome.org/)
and Exome Aggregation Consortium (ExAC) (https://ncbiinsights.ncbi.nlm.nih.gov/tag/exac/);
iii) retainment of variant sites with MAF≥0.001 and <0.1 in the
1,000 Genomes databases with COSMIC evidence (http://cancer.sanger.ac.uk/cosmic); iv)
retainment of variations in the exon or splicing region (10 bp
upstream and downstream of splicing sites); v) remove synonymous
mutations; vi) remove unknown variant classification; and vii)
removal of the functional benign variant sites, predicted by
PolyPhen-2 (Polymorphism Phenotyping v2; http://genetics.bwh.harvard.edu/pph2/) (29) or MutationTaster
(MutationTaster2020; https://www.muta-tiontaster.org/) (30). Additionally, the association
between the identified somatic mutations and their clinical
significance was established by OncoKB Precision Oncology Database
(http://oncokb.org/). Kyoto Encyclopedia Of Genes and
Genomes (KEGG) and Gene Ontology (GO) enrichment analysis were used
to explore the biological consequences of mutant genes by using the
cluster Profiler package (http://bioconductor.org/packages/release/bioc/html/clusterProfiler.Html)
in R software (R 4.0.3, R Core Team; https://www.RProject.org) (31).
Statistical analysis
The maftools package in R software (R 4.0.3, R Core
Team;https://www.R-Project.org) was used to
create somatic mutation landscapes, co-Barplots, co-Oncoplots,
lollipop plots, and spectrums of co-occurring and mutually
exclusive genomic alterations. Fisher's exact test was used to
evaluate the statistical differences in categorical variables
between the BM group and the non-BM group using R software (R
4.0.3, R Core Team; https://www.RProject.org). Statistical analyses were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In the present retrospective study, the numbers of
patients with different stages of NSCLC were as follows: I, II, III
and IV were as follows: i) Stage I, 6 patients (aged 61-78 years;
median, 76 years); ii) stage II, 3 patients (aged 57-71 years;
median, 58 years); iii) stage III, 21 patients (aged 43-77 years;
median, 61 years); and iv) stage IV, 90 patients (aged 30-84 years;
median, 63 years). Patients with stage IV NSCLC (n=90) were divided
into the BM group (n=26, aged 43-84 years) and non-BM group (n=64,
aged 30-84 years) by clinically detectable metastatic lesions.
There were no significant differences in the demographic and
clinical characteristics between the BM and the non-BM group
(Table I).
 | Table ICharacteristics of patients with
advanced NSCLC according to brain metastatic progression. |
Table I
Characteristics of patients with
advanced NSCLC according to brain metastatic progression.
| Clinical
characteristics | No. of
patients | Brain metastases
| P-value |
|---|
| Yes | No |
|---|
| Total sample | 90 | 26 | 64 | 0.6378 |
| Tissue | 53 | 14 | 39 | |
| ctDNA | 37 | 12 | 25 | |
| Age, years | | | | 0.9074 |
| ≤50 | 18 | 5 | 13 | |
| >50 | 72 | 21 | 51 | |
| Sex | | | | 0.8135 |
| Male | 54 | 15 | 39 | |
| Female | 36 | 11 | 25 | |
| Histology | | | | 0.999 |
|
Adenocarcinoma | 80 | 24 | 56 | |
| Squamous
carcinoma | 9 | 2 | 7 | |
| Adenosquamous
carcinoma | 1 | 0 | 1 | |
| Family history of
lung cancer | | | | 0.999 |
| Yes | 1 | 0 | 1 | |
| No | 89 | 26 | 63 | |
| History of
pulmonary infection | | | | 0.8931 |
| Yes | 44 | 13 | 31 | |
| No | 46 | 13 | 33 | |
| History of
smoking | | | | 0.6771 |
| Once | 12 | 2 | 10 | |
| Now | 26 | 8 | 18 | |
| Never | 52 | 16 | 36 | |
| History of alcohol
consumption | | | | 0.9312 |
| Once | 8 | 2 | 6 | |
| Now | 19 | 6 | 13 | |
| Never | 63 | 18 | 45 | |
| Pre-existing
metabolic disease | | | | 0.1556 |
| Yes | 19 | 3 | 16 | |
| No | 58 | 20 | 38 | |
| Unknown | 13 | 3 | 10 | |
| Vascular
invasion | | | | 0.7495 |
| Yes | 76 | 23 | 53 | |
| No | 14 | 3 | 11 | |
| Nerve invasion | | | | 0.5002 |
| Yes | 11 | 2 | 9 | |
| No | 79 | 24 | 55 | |
| CYFRA21-1 at
baseline | | | | 0.5084 |
| Normal | 13 | 6 | 7 | |
| Elevated | 26 | 9 | 17 | |
| Unknown | 51 | 11 | 40 | |
| CEA at
baseline | | | | 0.603 |
| Normal | 24 | 6 | 18 | |
| Elevated | 60 | 20 | 40 | |
| Unknown | 6 | 0 | 6 | |
| NSE at
baseline | | | | 0.7294 |
| Normal | 28 | 12 | 16 | |
| Elevated | 12 | 4 | 8 | |
| Unknown | 50 | 10 | 40 | |
| SCC at
baseline | | | | 0.1427 |
| Normal | 24 | 11 | 13 | |
| Elevated | 12 | 2 | 10 | |
| Unknown | 54 | 13 | 41 | |
Landscape of somatic mutations
To delineate the somatic mutation landscape, the
somatic mutations from the tumor tissue DNA of 69 patients were
first analyzed by applying an NGS panel of 95 known cancer genes
(Table SII). The present study
mainly focused on protein-altering variants, based on the
annotation of somatic SNVs and InDels. A total of 157 somatic
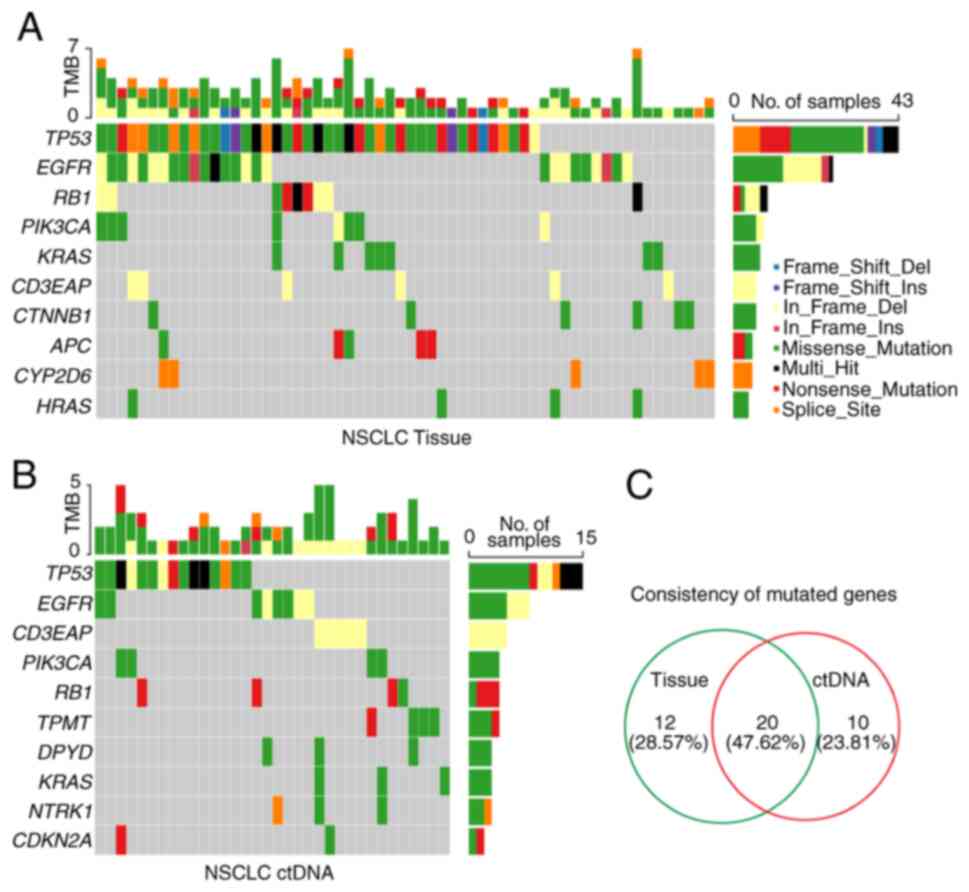
variants of 32 mutated genes were detected in 62 out of 69 (89.86%)
tumor tissues (Fig. 1A and
Table SIII). To explore the
feasibility of genomic profiling using peripheral blood samples,
the same NGS panel was used to detect 51 plasma-derived ctDNA. In
total, 77 somatic variants of 30 mutated genes were identified in
38 out of 51 (74.51%) peripheral blood samples, which was lower
than that from tissue DNA (Fig. 1B
and Table SIII). Furthermore, the
percentages of tissue DNA-specific mutated genes and ctDNA-specific
mutated genes were 28.57% (12/42) and 23.81% (10/42), respectively,
whereas the consistency rate of mutated genes was 47.62% (20/42) in
NSCLC (Fig. 1C and Table SIII).
Differences in somatic mutations between
the BM and non-BM group
Patients with NSCLC with BM have been shown to be
significantly associated with an increased mortality rate (4). However, the underlying molecular
mechanisms of the initiation and progression of BM have not yet
been fully elucidated in NSCLC. In the present study, to explore
this issue, the genomic characterizations of patients both from the
BM and the non-BM group were first depicted using an NGS panel. In
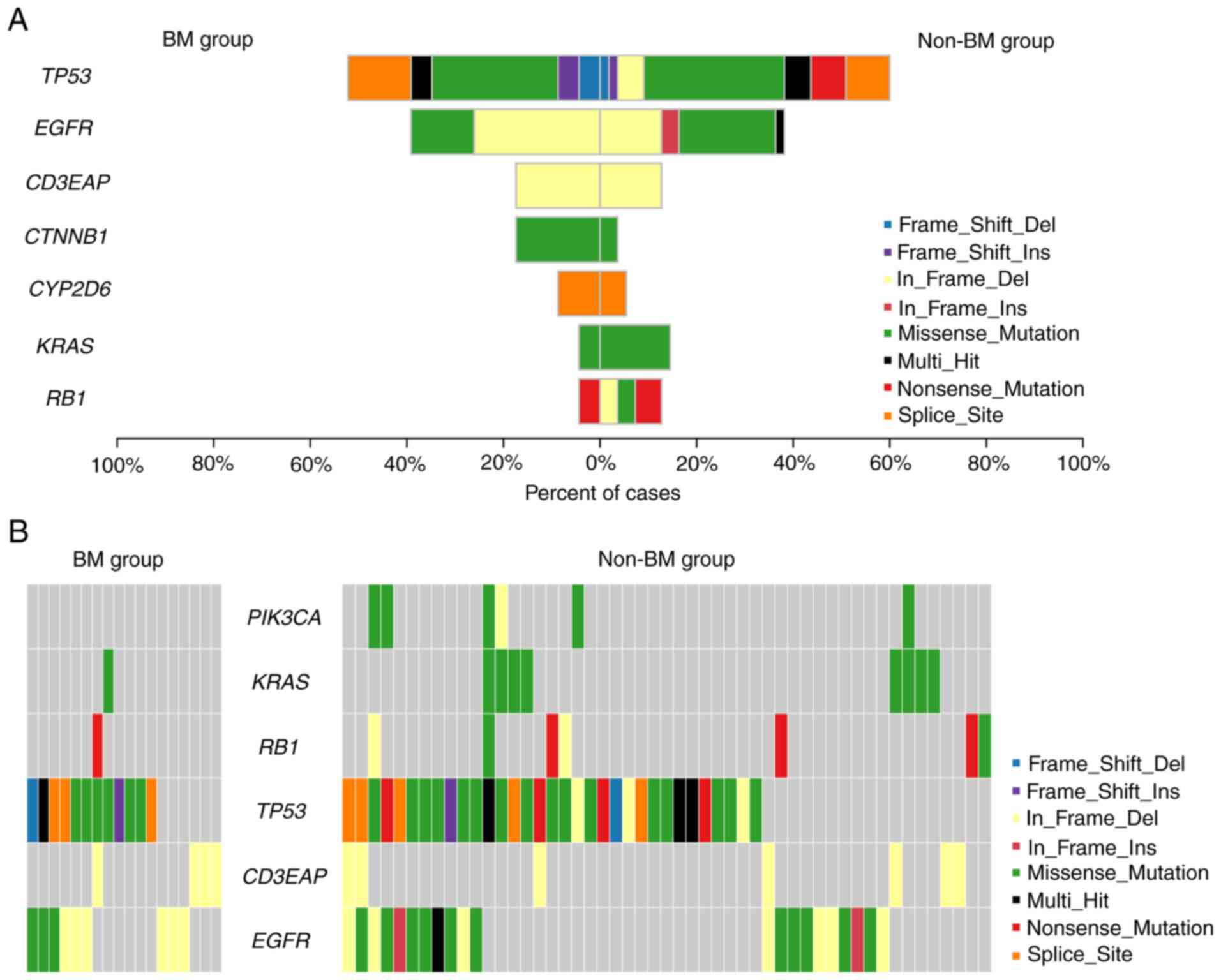
total, 47 somatic variants of 17 mutated genes were identified in
23 out of 26 (88.46%) patients with BM (Figs. 2 and S1A, and Table SIV), and 127 somatic variants of
30 mutated genes were observed in 55 of 64 (85.94%) non-BM patients
(Figs. 2 and S1C, and Table SIV). To further discover the
molecular differences between the BM and the non-BM group, a Venn
diagram was plotted (Fig. S1B).
Among the total number of 35 mutant genes, ALK, cytidine
deaminase (CDA), SMAD family member 4 (SMAD4),
superoxide dismutase 2 (SOD2), and Von Hippel-Lindau tumor
suppressor (VHL) were uniquely present in the BM group,
whereas 18 mutant genes [ATM serine/threonine kinase (ATM),
cyclin-dependent kinase inhibitor 2A (CDKN2A), Erb-B2
receptor tyrosine kinase 4 (ERBB4), HRAS,
PIK3CA, etc.] only emerged in the non-BM group. In total, 12
mutant genes [APC regulator of WNT signaling pathway (APC),
DNA-directed RNA polymerase I subunit RPA34 (CD3EAP),
catenin beta-1 (CTNNB1), cytochrome P450 2D6
(CYP2D6), dihydropyrimidine dehydrogenase (DPYD),
EGFR, Kirsten rat sarcoma (KRAS), NRAS,
TP53, telomerase reverse transcriptase (TERT),
thiopurine S-methyltransferase (TPMT) and RB transcriptional
co-repressor 1 (RB1)] were concurrently present in both
groups.
Moreover, the frequencies and sites of the above
concurrently mutated genes were also investigated. Of note, apart
from CD3EAP, DPYD and TPMT, the frequencies
and/or sites of the concurrently mutated genes were different
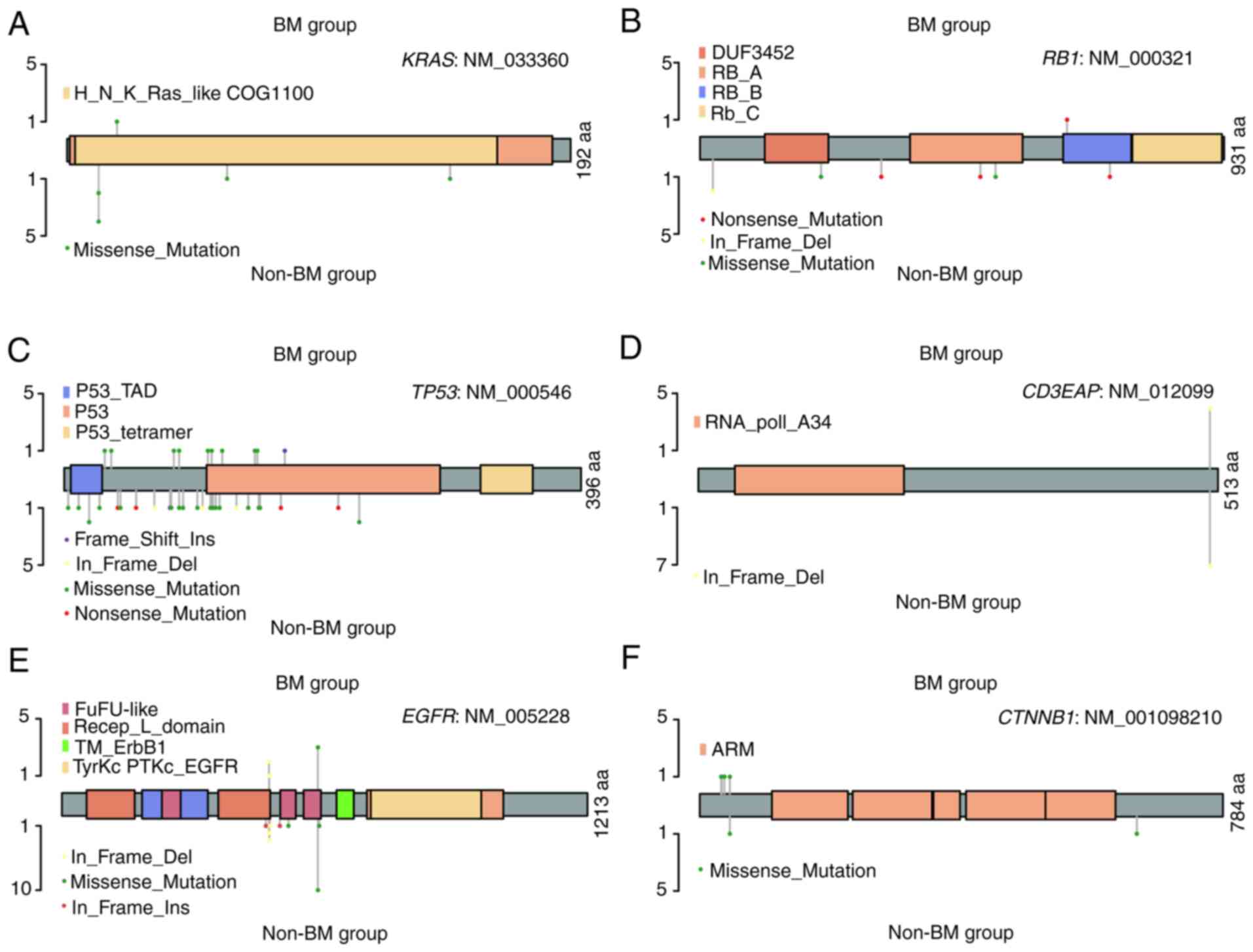
(Fig. 3 and Table SV). In relation to the KRAS
gene, marked differences were observed in the mutation sites and
frequencies (p.L19F vs. p.G12D, p.G12V, p.Q61H, and p.A146T, 3.85
vs. 12.5%) of these two groups (Fig.
3A and Table SV). Although
about half of the patients harbored TP53 mutations in the
two groups, the types and sites of amino acid alterations were
different (Fig. 3C and Table SV). The frameshift insertion of
TP53 was exclusively identified in the BM group, and
in-frame deletion and nonsense mutation were only detected in the
non-BM group.
Differences in somatic interactions
between the BM and non-BM group
In NSCLC, patients presenting with EGFR
mutations have a much higher incidence of BM compared to those
without EGFR mutation. EGFR mutations and KRAS
mutations are usually mutually exclusive, and KRAS mutations
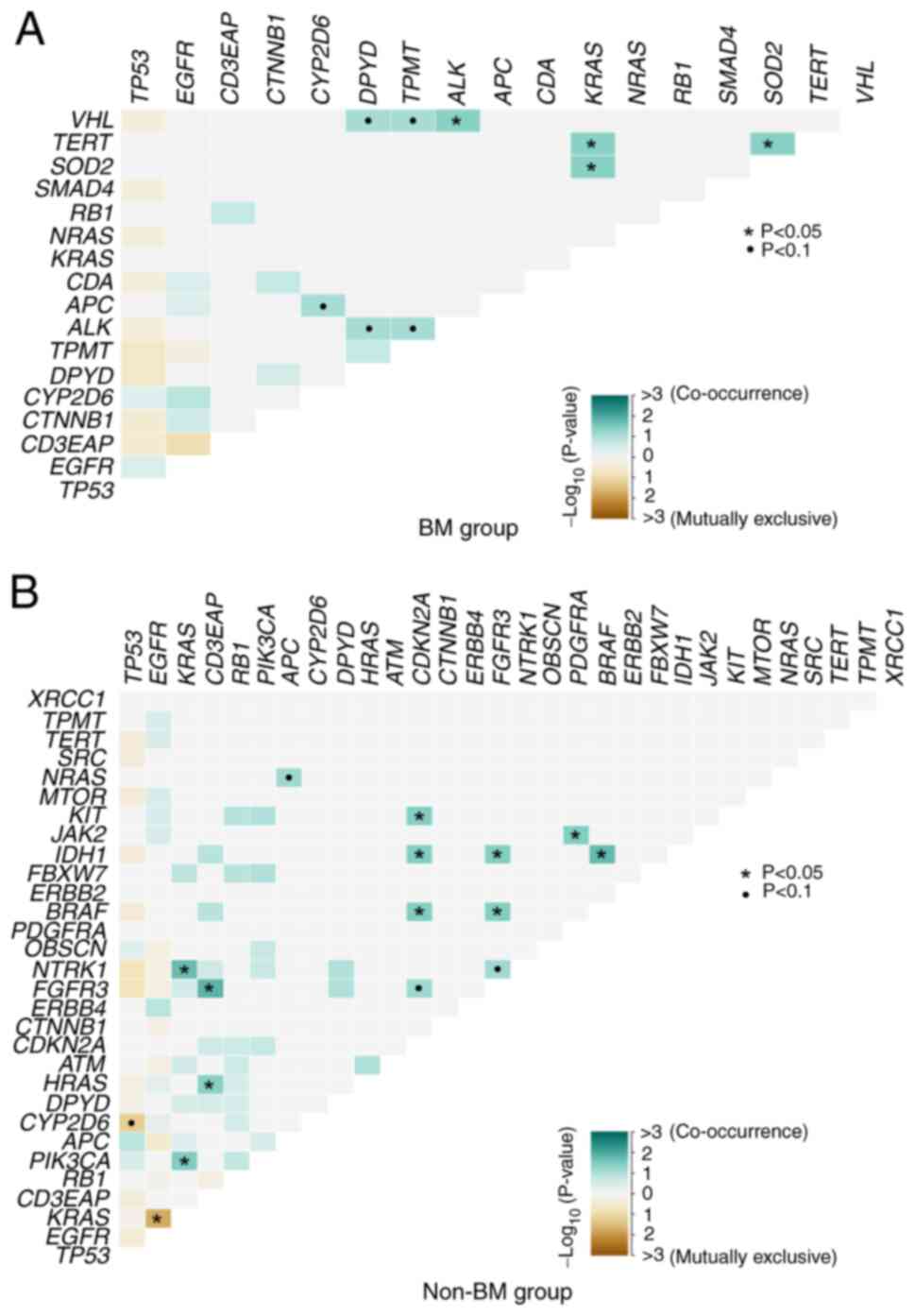
could confer resistance to EGFR-TKIs when they co-exist (32). In the present study, the somatic
interactions in the BM group were different from the interactions
of the non-BM group. EGFR and KRAS were a mutually exclusive
set of genes in the non-BM group (P=0.0183) (Fig. 4B and Table SV), whereas limited mutually
exclusive interactions were observed in the BM group (P=0.999)
(Fig. 4A and Table SVI). Additionally, obvious
differences were also detected in the co-occurring set of genes
between the two groups. As demonstrated in Fig. 4, VHL and ALK
(P=0.0435), SOD2 and KRAS (P=0.0435), TERT and
KRAS (P=0.0435), TERT and SOD2 (P=0.0435) were
co-occurring pair of genes in the BM group (Fig. 4A and Table SV), while FGFR3 and
CD3EAP (P=0.0141), isocitrate dehydrogenase [NADP(+)] 1
(IDH1) and BRAF (P=0.0182), neurotrophic receptor
tyrosine kinase 1 (NTRK1) and KRAS (P=0.0189),
PIK3CA and KRAS (P=0.0340), CDKN2A and
BRAF (P=0.0364), and interactions between other six
co-occurring pair of genes were detected in the BM group (Fig. 4B and Table SVI).
Key signaling pathways and biological
functions analysis of somatic mutations in the BM and the non-BM
group
In order to acquire a more incisive understanding of
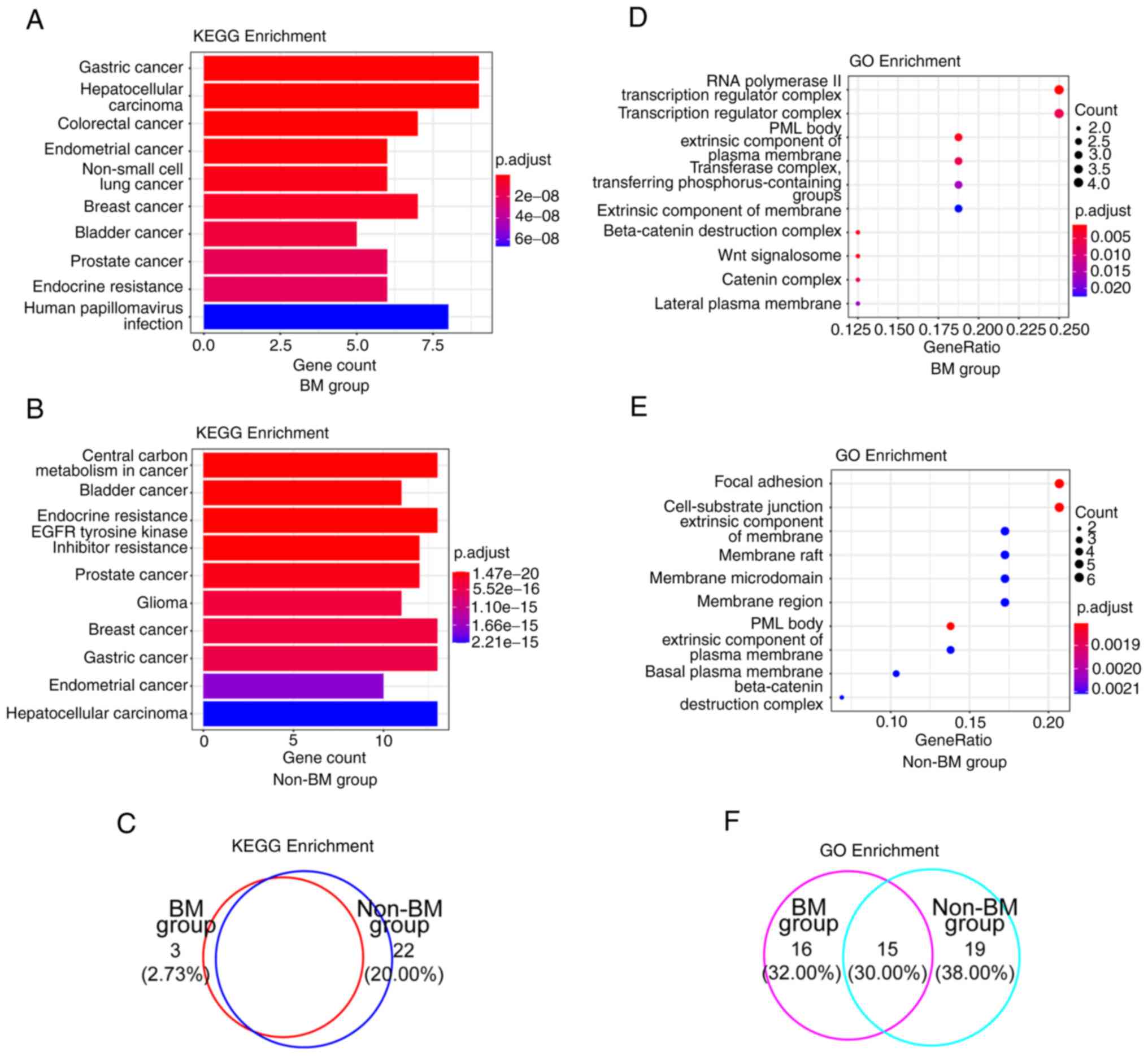
the biological consequences in these two groups, KEGG and GO
enrichment analyses were performed. The top 10 KEGG pathways
enriched by mutated genes were depicted according to gene count and
P-value, and most signaling pathways were cancer-related (Fig. 5A and B, and Table II). Since patients with BM respond
well to EGFR-TKIs, the P-value of EGFR-TKI resistance was
markedly higher in the BM group, than in the non-BM group (4.50E-04
vs. 7.84E-18) (Tables II and
SVII). In GO enrichment analysis,
functional categories were most involved in RNA polymerase II
transcription regulator complex in the BM group (Fig. 5D and Table III) and promyelocytic leukemia
nuclear body in the non-BM group (Fig.
5E and Table III).
Furthermore, five mutated genes uniquely present in the BM group
were prevailingly distributed in transcription regulator complex,
RNA polymerase complex, bicellular tight junction, tight junction,
apical junction complex, and so forth (Tables III and SVII). Collectively, a high consistency
of altered signaling pathways between these two groups according to
KEGG analysis was observed (Fig.
5C), whereas the percentage was decreased in GO
analysis-related altered functional terms [Fig. 5F; KEGG analysis, 77.27% (85/110)
vs. GO analysis, 30.00% (15/50)].
 | Table IISignaling pathways enriched by KEGG
analysis in the BM group and the non-BM group. |
Table II
Signaling pathways enriched by KEGG
analysis in the BM group and the non-BM group.
| ID | Description | Adjusted
P-value | geneID |
|---|
| BM group |
| hsa05226 | Gastric cancer | 1.85E-12 |
TP53/TERT/KRAS/RB1/EGFR/SMAD4/CTNNB1/APC/NRAS |
| hsa05225 | Hepatocellular
carcinoma | 5.53E-12 |
TP53/TERT/KRAS/RB1/EGFR/SMAD4/CTNNB1/APC/NRAS |
| hsa05210 | Colorectal
cancer | 1.21E-10 |
TP53/KRAS/EGFR/SMAD4/CTNNB1/APC/NRAS |
| hsa05213 | Endometrial
cancer | 7.60E-10 |
TP53/KRAS/EGFR/CTNNB1/APC/NRAS |
| hsa05223 | Non-small cell lung
cancer | 2.89E-09 |
TP53/KRAS/RB1/EGFR/ALK/NRAS |
| hsa05224 | Breast cancer | 5.41E-09 |
TP53/KRAS/RB1/EGFR/CTNNB1/APC/NRAS |
| hsa05219 | Bladder cancer | 1.06E-08 |
TP53/KRAS/RB1/EGFR/NRAS |
| hsa05215 | Prostate
cancer | 1.78E-08 |
TP53/KRAS/RB1/EGFR/CTNNB1/NRAS |
| hsa01522 | Endocrine
resistance | 1.89E-08 |
TP53/KRAS/RB1/EGFR/CYP2D6/NRAS |
| hsa05165 | Human
papillomavirus infection | 6.64E-08 |
TP53/TERT/KRAS/RB1/EGFR/CTNNB1/APC/NRAS |
| Non-BM group |
| hsa05230 | Central carbon
metabolism in cancer | 1.47E-20 |
PDGFRA/NRAS/TP53/PIK3CA/KRAS/EGFR/HR
AS/MTOR/KIT/FGFR3/NTRK1/IDH1/ERBB2 |
| hsa05219 | Bladder cancer | 2.47E-19 |
NRAS/TP53/RB1/KRAS/EGFR/HRAS/CDKN2A/FGFR3/SRC/BRAF/ERBB2 |
| hsa01522 | Endocrine
resistance | 1.58E-18 |
CYP2D6/NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CDKN2A/MTOR/SRC/BRAF/ERBB2 |
| hsa01521 | EGFR tyrosine
kinase inhibitor resistance | 7.84E-18 |
PDGFRA/NRAS/PIK3CA/KRAS/EGFR/HRAS/JAK2/MTOR/FGFR3/SRC/BRAF/ERBB2 |
| hsa05215 | Prostate
cancer | 1.06E-16 |
PDGFRA/NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CTNNB1/MTOR/BRAF/ERBB2 |
| hsa05214 | Glioma | 3.59E-16 |
PDGFRA/NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CDKN2A/MTOR/BRAF |
| hsa05224 | Breast cancer | 3.76E-16 |
NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CTNNB1/APC/MTOR/KIT/BRAF/ERBB2 |
| hsa05226 | Gastric cancer | 4.50E-16 |
NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CTNNB1/APC/MTOR/BRAF/TERT/ERBB2 |
| hsa05213 | Endometrial
cancer | 1.77E-15 |
NRAS/TP53/PIK3CA/KRAS/EGFR/HRAS/CTNNB1/APC/BRAF/ERBB2 |
| hsa05225 | Hepatocellular
carcinoma | 2.21E-15 |
NRAS/TP53/PIK3CA/RB1/KRAS/EGFR/HRAS/CDKN2A/CTNNB1/APC/MTOR/BRAF/TERT |
 | Table IIIFunctional terms enriched by GO
enrichment analysis in the BM and the non-BM group. |
Table III
Functional terms enriched by GO
enrichment analysis in the BM and the non-BM group.
| ID | Description | Adjusted
P-value | geneID |
|---|
| BM group |
| GO:0090575 | RNA polymerase II
transcription regulator complex | 0.0010 |
TP53/RB1/SMAD4/CTNNB1 |
| GO:0030877 | Beta-catenin
destruction complex | 0.0017 |
CTNNB1/APC |
| GO:1990909 | Wnt
signalosome | 0.0017 |
CTNNB1/APC |
| GO:0016605 | PML body | 0.0023 |
TP53/TERT/RB1 |
| GO:0016342 | Catenin
complex | 0.0060 |
CTNNB1/APC |
| GO:0005667 | Transcription
regulator complex | 0.0060 |
TP53/RB1/SMAD4/CTNNB1 |
| GO:0019897 | Extrinsic component
of plasma membrane | 0.0060 |
KRAS/CTNNB1/APC |
| GO:0061695 | Transferase
complex, transferring phosphorus-containing groups | 0.0163 |
TP53/TERT/RB1 |
| GO:0016328 | Lateral plasma
membrane | 0.0173 |
CTNNB1/APC |
| GO:0019898 | Extrinsic component
of membrane | 0.0225 |
KRAS/CTNNB1/APC |
| Non-BM group |
| GO:0016605 | PML body | 0.0018 |
TP53/RB1/MTOR/TERT |
| GO:0005925 | Focal adhesion | 0.0018 |
KRAS/EGFR/JAK2/CTNNB1/FGFR3/SRC |
| GO:0030055 | Cell-substrate
junction | 0.0018 |
KRAS/EGFR/JAK2/CTNNB1/FGFR3/SRC |
| GO:0009925 | Basal plasma
membrane | 0.0021 |
ERBB4/EGFR/ERBB2 |
| GO:0019898 | Extrinsic component
of membrane | 0.0021 |
PIK3CA/KRAS/CTNNB1/APC/SRC |
| GO:0045121 | Membrane raft | 0.0021 |
KRAS/EGFR/JAK2/CTNNB1/SRC |
| GO:0098857 | Membrane
microdomain | 0.0021 |
KRAS/EGFR/JAK2/CTNNB1/SRC |
| GO:0019897 | Extrinsic component
of plasma membrane | 0.0021 |
KRAS/CTNNB1/APC/SRC |
| GO:0098589 | Membrane
region | 0.0021 |
KRAS/EGFR/JAK2/CTNNB1/SRC |
| GO:0030877 | Beta-catenin
destruction complex | 0.0021 |
CTNNB1/APC |
Clinical actionability for the therapy of
targeted agents
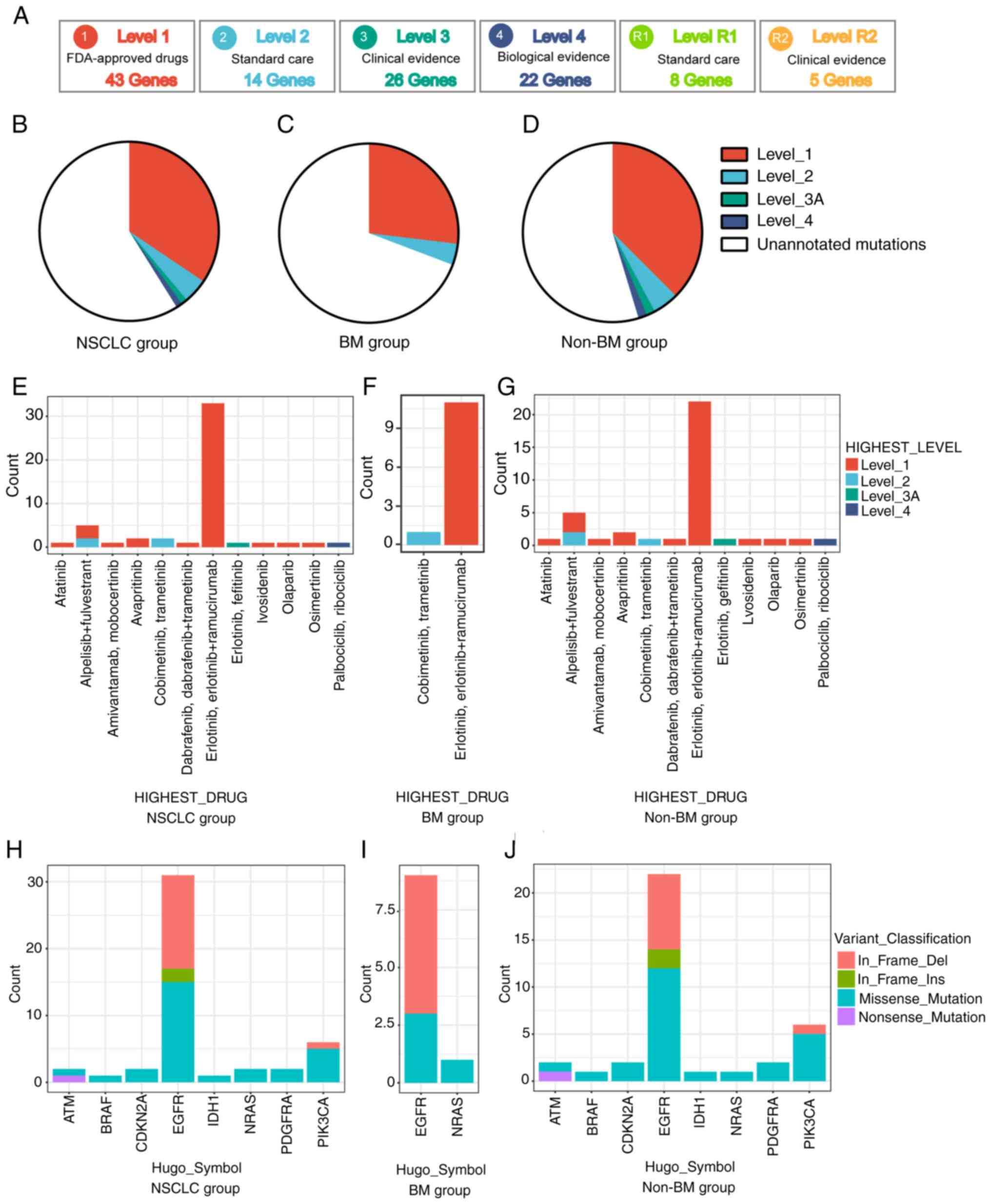
In order to evaluate the clinical utility of
anticipative molecular profiling, all mutations were divided into
different levels, according to the evidence of clinical
actionability in OncoKB (Fig. 6A).
As standard therapeutic biomarkers, a cluster of gene mutations was
approved by the FDA. In the present cohort, 47 out of 120 (39.17%)
patients possessed at least one actionable alteration. Among the
patients with stage IV disease, level_1 accounted for 34.44%
(31/90), including missense mutations of BRAF, EGFR,
IDH1, PDGFRA and PIK3CA, a nonsense mutation
of ATM and an in-frame insertion of EGFR; level_2
accounted for 4.44% (4/90), including missense mutations of
NRAS and PIK3CA and an in-frame deletion of
PIK3CA; level_3 accounted for 1.11% (1/90), including an
in-frame insertion of EGFR; level_4 accounted for 1.11%
(1/90), including missense mutations of CDKN2A (Fig. 6B, E and H, and Table SVIII). Additionally, it was also
observed that non-BM patients had a slightly higher percentage of
actionable alterations than patients with BM, namely 45.31% (29/64)
vs. 30.77% (8/26) (Fig. 6D, F, G, I,
J, and Table SVIII).
ctDNA analysis has a higher consistency
of genomic profiling in the non-BM group as compared with that in
the BM group
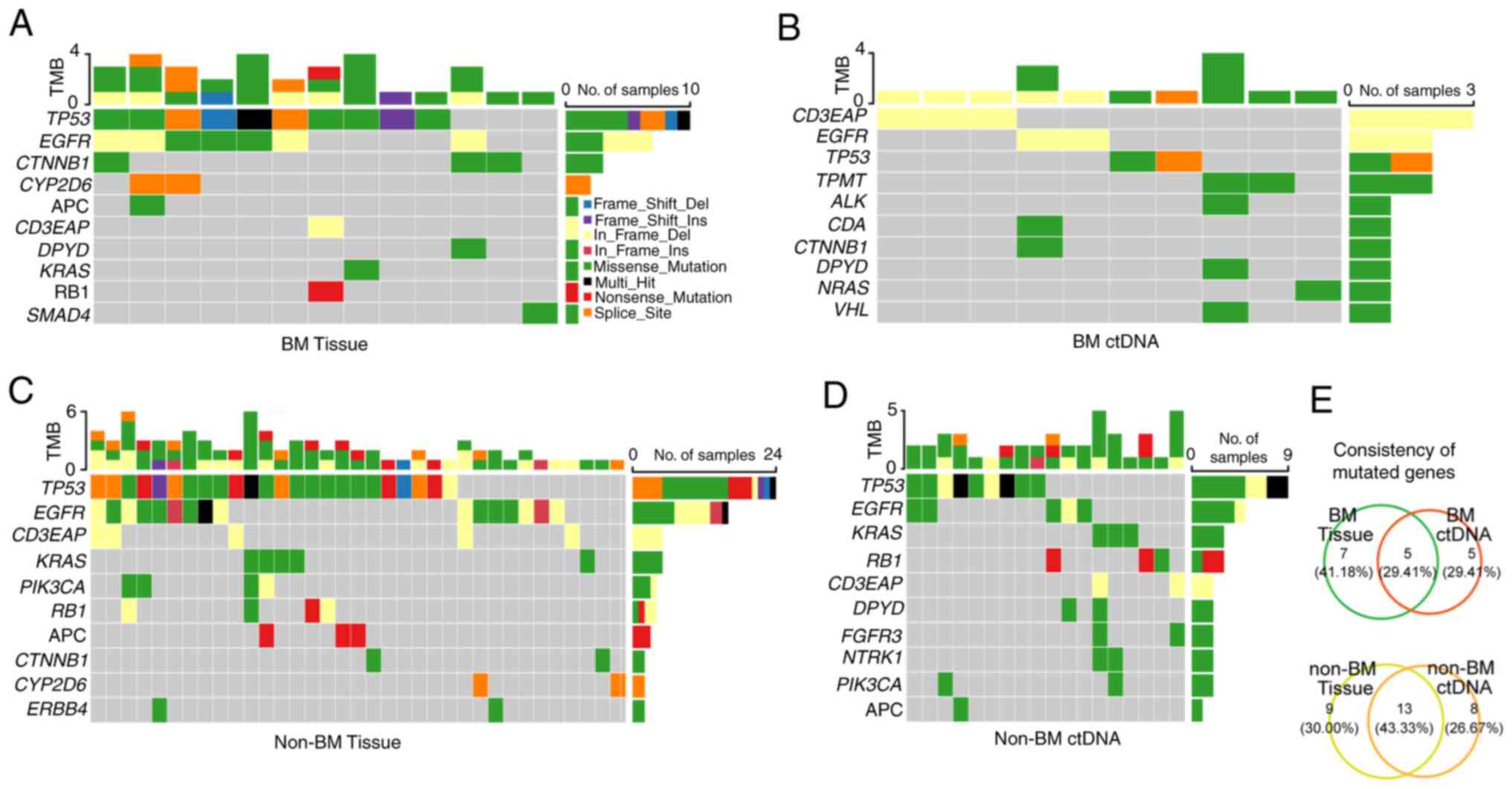
To compare the feasibility of genomic profiling of
advanced patients with or without BM using plasma-derived ctDNA,
somatic mutations from 14 tumor tissues and 12 peripheral blood
samples were analyzed in the BM group by the above NGS panel. A
total of 32 somatic variants of 12 mutated genes were identified in
13 out of 14 (92.86%) tumor tissue DNA samples (Fig. 7A and Table SIX), and 15 somatic variants of 10
mutant genes were detected in 10 of 12 (83.33%) plasma-derived
ctDNA (Fig. 7B and Table SIX). Meanwhile, eighty-three
somatic variants of 22 mutated genes in 36 of 39 (92.31%) tumor
tissue DNA were also detected (Fig.
7C and Table SX), as well as
44 somatic variants of 21 mutated genes in 19 out of 25 (76.00%)
plasma-derived ctDNA (Fig. 7D and
Table SX) in the non-BM group. In
summary, 43.33% (13/30) of the mutated genes were detected by both
tumor tissue DNA analysis and ctDNA analysis in the non-BM group
(Fig. 7E), whereas the percentage
was only 29.41% (5/17) in the BM group (Fig. 7E).
Discussion
Exploring the genomic alterations is crucial for
clinical management in NSCLC patients with BM. Although dynamic
mutation landscapes have been reported, systematic comparisons of
genomic characteristics between the BM and the non-BM groups remain
limited. In the present study, 174 somatic mutations of 35 mutated
genes were identified in 90 patients with stage IV NSCLC using an
NGS panel of 95 known cancer genes. Significant differences between
the BM and the non-BM group were detected in somatic mutations,
somatic interactions, key signaling pathways, functional biological
information and clinical actionability for the therapy of targeted
agents. Finally, it was also observed that ctDNA analysis presented
with a higher consistency for genomic profiling of the non-BM than
that of the BM group, indicating that ctDNA analysis may serve as a
more credible alternative for genomic profiling in advanced NSCLC
patients without BM.
In the present study, 17 mutated genes and 30
mutated genes were identified in the BM and the non-BM group,
respectively. Among these genes, five genes, including ALK,
CDA, SMAD4, SOD2 and VHL were uniquely
present in patients with BM. ALK is a tyrosine kinase and
its constitutively activated mutation renders ALK a
formidable cancer driver gene (33,34).
BM occurs frequently in tumors harboring ALK rearrangements
(10), and its clinical
significance has been considered to be critical. Several ALK
inhibitors have been reported to demonstrate conspicuous activity
in brain metastatic patients with crizotinib-resistant
ALK-positive NSCLC, including second-generation (brigatinib
and alectinib) (35-37), and third-generation therapeutics
(lorlatinib) (38,39). Additionally, ALK and
VHL (P=0.0435) are exclusively co-occurring genes in the BM
group, which was reported in Chinese patients with NSCLC for the
first time, to the best of our knowledge. More notably, as the
first generation of the blood-brain barrier (BBB)-penetrating TKIs,
AZD3759 can activate a p53-SMAD4 positive feedback loop and
lead to apoptosis in hepatoma cells (40), offering a promising future approach
for the treatment of brain metastatic NSCLC patients by AZD3759
(41). Collectively, the data of
the present study may contribute to an improved comprehension of
the underlying molecular mechanisms of patients with NSCLC with BM
and may provide prospective therapeutic targets for this specific
subgroup.
Additionally, five genes exclusively identified in
the BM group were distributed in the Hippo signaling pathway,
pyrimidine metabolism (PyM), and pantothenate and CoA biosynthesis,
according to KEGG enrichment analyses. As a key mediator in the
Hippo signaling pathway, Yes-associated protein (YAP) has been
founded to facilitate drug resistance and tumorigenesis in NSCLC
(42-45). Furthermore, YAP has been reported
to play a crucial role for the promotion of BM in lung
adenocarcinoma patients, and the inhibition of YAP by shRNA may
significantly suppress migration and invasion abilities of
metastatic NSCLC cell lines H2030-BrM3 in a murine model (46). Combined with the current results,
these findings may provide prospective therapeutic approaches by
modulating the members or mediators of the Hippo signaling pathway
in brain metastatic patients with NSCLC. As a complex enzymatic
network, the main function of PyM is to integrate de novo
nucleotide synthesis, nucleoside salvage, and catalytic degradation
of pyrimidines. In cancer cells, the de novo nucleotide
synthesis pathway continuously provides deoxyribonucleoside
triphosphates (dNTPs) to sustain uncontrolled proliferation, being
different from normal cell de novo nucleotide synthesis
(47,48). Until recently, PyM has been mainly
implicated in the differentiation of leukemic cells; however,
little is known about its roles in the differentiation of solid
tumors (49). To the best of our
knowledge, the present finding is the first report on PyM as an
exclusive signaling pathway in Chinese patients with NSCLC with BM.
However, further studies are required in order to discern whether
PyM signaling pathway plays a critical role in the initiation
and/or progression of BM in NSCLC.
More importantly, it was also demonstrated that
ctDNA analysis is more feasible as an alternative for somatic
mutation landscapes of non-BM patients than that of BM patients by
the higher consistency between ctDNA analysis and tumor DNA
analysis (43.33 vs. 29.41%). A possible explanation for the
discrepancies between ctDNA analysis and tumor DNA analysis may be
the inhibition of tumor cell release into the bloodstream by the
BBB in patients with BM (50,51).
Thus, the basic detection rate of genomic alterations derived from
peripheral blood ctDNA in the BM group has been reported to be
lower than that in the non-BM group (52,53).
In a similar study, Aldea et al (53) demonstrated that ctDNA was positive
in 52% of isolated central nervous system progression (iCNS) vs.
84% in extra-CNS only (noCNS), which was accompanied by a lower
detection rate of pathogenic driver mutations (37 vs. 77%) and
resistance alterations (6 vs. 45%). However, it cannot be
overlooked that liquid biopsy is a potent method with which to
improve the identification of actionable biomarkers when tumor
tissue is unavailable (52,54,55).
In the present study, plasma-derived ctDNA analysis improved the
detection rate of EGFR actionable mutations by a 15.39%
(4/26) increase in the BM group, and four patients had the
opportunity to receive targeted therapies (erlotinib/erlotinib +
ramucirumab/afatinib/gefitinib/osimertinib/dacomitinib) and/or
participate in clinical trials. Consequently, the data of the
present study are consistent with those of previous studies in
which the identification of actionable mutations is growing in
advanced NSCLC patients with the aid of plasma-derived ctDNA
(56,57).
In the present study, one of the main limitations
is that NGS data were obtained from tumor tissue DNA or
plasma-derived ctDNA without the simultaneous analysis of matched
normal tissue to delete the germline mutations. Thus, for a single
sample the analysis was not complete; however, it may be considered
adequate for the acquisition of actionable genomic alterations for
the application of guided clinical treatment based on the suitable
filter conditions (please see the 'Materials and methods' section,
'Bioinformatics analysis') (30,58-65).
However, it cannot be disregarded that either multiregional
biopsies or more than one type of biopsies may be costlier than the
analysis of a single tumor sample without the inclusion of matched
normal tissue. Another main limitation is the absence of a genomic
profile, derived from brain tumor tissue DNA due to difficulties in
the acquisition of brain tissue samples from NSCLC patients with
BM. Additionally, although all patients were recruited for a
prospective study, the collection of NGS data was retrospective.
Lastly, further multiple-institution research with larger sample
sizes is required to validate the present conclusions.
In conclusion, the somatic mutation landscapes of
NSCLC with and without BM were compared, and significant
differences in somatic mutations, somatic interactions, key
signaling pathways, functional biological information, and clinical
actionability for the therapy of targeted agents were observed
between the BM group and the non-BM group. Moreover, plasma-derived
ctDNA analysis may be a more reliable alternative for genomic
profiling of advanced patients without BM, based on the higher
consistency between ctDNA analysis and tumor DNA analysis in
NSCLC.
Supplementary Data
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request. The next generation sequencing data are
available at the NCBI BioProject database (Reference no.
PRJNA759391; https://www.ncbi.nlm.nih.gov/bioproject/PRJNA759391).
Authors' contributions
RN, JZ, JL and WL conceptualized and designed the
present study. RN, HZ, JM, PL, SW and JZ were involved in the
acquisition of samples. YW and SW performed the high-throughput
sequencing experiments. YW, HJ, WH and LJ performed the
bioinformatics analysis. HJ, WH, YX and LJ were involved in the
statistical analysis. RN, HJ, LJ, YW, JZ and WL were responsible
for administrative/technical/material support and study
supervision. HJ and JL wrote the manuscript. RN, HJ, WH, HZ, JM,
PL, LJ, YX, SW, and JL critically revised the article. All authors
have read and approved the final manuscript. LJ and WL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The part of this study involving human participants
was reviewed and approved by the Medical Ethics Committee of
Affiliated 3201 Hospital of Xi'an Jiaotong University
[No.008(2017)]. Written informed consent was obtained from all
participants involved in the present study, according to national
legislation and institutional requirements.
Patient consent for publication
The publication of data was approved by all
patients.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Schuette W: Treatment of brain metastases
from lung cancer: Chemotherapy. Lung Cancer. 45(Suppl 2):
S253–S257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khalifa J, Amini A, Popat S, Gaspar LE and
Faivre-Finn C: International Association for the Study of Lung
Cancer Advanced Radiation Technology C: Brain metastases from
NSCLC: Radiation therapy in the Era of targeted therapies. J Thorac
Oncol. 11:1627–1643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langer CJ and Mehta MP: Current management
of brain metastases, with a focus on systemic options. J Clin
Oncol. 23:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Owen S and Souhami L: The management of
brain metastases in non-small cell lung cancer. Front Oncol.
4:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lagerwaard FJ, Levendag PC, Nowak PJ,
Eijkenboom WM, Hanssens PE and Schmitz PI: Identification of
prognostic factors in patients with brain metastases: A review of
1292 patients. Int J Radiat Oncol Biol Phys. 43:795–803. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi AA, Digumarthy SR, Temel JS, Halpern
EF, Kuester LB and Aquino SL: Does initial staging or tumor
histology better identify asymptomatic brain metastases in patients
with non-small cell lung cancer? J Thorac Oncol. 1:205–210. 2006.
View Article : Google Scholar
|
|
8
|
Mujoomdar A, Austin JH, Malhotra R, Powell
CA, Pearson GD, Shiau MC and Raftopoulos H: Clinical predictors of
metastatic disease to the brain from non-small cell lung carcinoma:
Primary tumor size, cell type, and lymph node metastases.
Radiology. 242:882–888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thorac Oncol. 9:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fallet V, Cadranel J, Doubre H, Toper C,
Monnet I, Chinet T, Oliviero G, Foulon G, De Cremoux H, Vieira T,
et al: Prospective screening for ALK: Clinical features and outcome
according to ALK status. Eur J Cancer. 50:1239–1246. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heigener DF, Kerr KM, Laing GM, Mok TSK,
Moiseyenko FV and Reck M: Redefining treatment paradigms in
first-line advanced non-small-cell lung cancer. Clin Cancer Res.
25:4881–4887. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brastianos PK, Carter SL, Santagata S,
Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS,
Horowitz PM, Cibulskis K, et al: Genomic characterization of brain
metastases reveals branched evolution and potential therapeutic
targets. Cancer Discov. 5:1164–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih DJH, Nayyar N, Bihun I, Dagogo-Jack
I, Gill CM, Aquilanti E, Bertalan M, Kaplan A, D'Andrea MR,
Chukwueke U, et al: Genomic characterization of human brain
metastases identifies drivers of metastatic lung adenocarcinoma.
Nat Genet. 52:371–377. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandes Marques J, Pereira Reis J,
Fernandes G, Hespanhol V, Machado JC and Costa JL: Circulating
tumor DNA: A step into the future of cancer management. Acta Cytol.
63:456–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahr S, Hentze H, Englisch S, Hardt D,
Fackelmayer FO, Hesch RD and Knippers R: DNA fragments in the blood
plasma of cancer patients: Quantitations and evidence for their
origin from apoptotic and necrotic cells. Cancer Res. 61:1659–1665.
2001.PubMed/NCBI
|
|
17
|
Wan JCM, Massie C, Garcia-Corbacho J,
Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R and Rosenfeld N:
Liquid biopsies come of age: Towards implementation of circulating
tumour DNA. Nat Rev Cancer. 17:223–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keller L and Pantel K: Unravelling tumour
heterogeneity by single-cell profiling of circulating tumour cells.
Nat Rev Cancer. 19:553–567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murtaza M, Dawson SJ, Pogrebniak K, Rueda
OM, Provenzano E, Grant J, Chin SF, Tsui DWY, Marass F, Gale D, et
al: Multifocal clonal evolution characterized using circulating
tumour DNA in a case of metastatic breast cancer. Nat Commun.
6:87602015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marusyk A, Janiszewska M and Polyak K:
Intratumor heterogeneity: The Rosetta stone of therapy resistance.
Cancer Cell. 37:471–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rolfo C, Mack PC, Scagliotti GV, Baas P,
Barlesi F, Bivona TG, Herbst RS, Mok TS, Peled N, Pirker R, et al:
Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A
statement paper from the IASLC. J Thorac Oncol. 13:1248–1268. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen G, Cai Z, Li Z, Dong X, Xu H, Lin J,
Chen L, Zhang H, Liu X and Liu J: Clonal evolution in long-term
follow-up patients with hepatocellular carcinoma. Int J Cancer.
143:2862–2870. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gandevia B and Tovell A: Declaration of
Helsinki. Med J Aust. 2:320–321. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H and Durbin R: Fast and accurate
long-read alignment with Burrows-Wheeler transform. Bioinformatics.
26:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai Z, Markovets A, Ahdesmaki M, Chapman
B, Hofmann O, McEwen R, Johnson J, Dougherty B, Barrett JC and Dry
JR: VarDict: A novel and versatile variant caller for
next-generation sequencing in cancer research. Nucleic Acids Res.
44:e1082016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang K, Li M and Hakonarson H: ANNOVAR:
functional annotation of genetic variants from high-throughput
sequencing data. Nucleic Acids Res. 38:e1642010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adzhubei IA, Schmidt S, Peshkin L,
Ramensky VE, Gerasimova A, Bork P, Kondrashov AS and Sunyaev SR: A
method and server for predicting damaging missense mutations. Nat
Methods. 7:248–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwarz JM, Rodelsperger C, Schuelke M and
Seelow D: MutationTaster evaluates disease-causing potential of
sequence alterations. Nat Methods. 7:575–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JJ, Riely GJ and Shaw AT: Targeting
ALK: Precision medicine takes on drug resistance. Cancer Discov.
7:137–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holla VR, Elamin YY, Bailey AM, Johnson
AM, Litzenburger BC, Khotskaya YB, Sanchez NS, Zeng J, Shufean MA,
Shaw KR, et al: ALK: A tyrosine kinase target for cancer therapy.
Cold Spring Harb Mol Case Stud. 3:a0011152017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Camidge DR, Kim DW, Tiseo M, Langer CJ,
Ahn MJ, Shaw AT, Huber RM, Hochmair MJ, Lee DH, Bazhenova LA, et
al: Exploratory analysis of brigatinib activity in patients with
anaplastic lymphoma kinase-positive non-small-cell lung cancer and
brain metastases in two clinical trials. J Clin Oncol.
36:2693–2701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gadgeel SM, Gandhi L, Riely GJ, Chiappori
AA, West HL, Azada MC, Morcos PN, Lee RM, Garcia L, Yu L, et al:
Safety and activity of alectinib against systemic disease and brain
metastases in patients with crizotinib-resistant ALK-rearranged
non-small-cell lung cancer (AF-002JG): Results from the
dose-finding portion of a phase 1/2 study. Lancet Oncol.
15:1119–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tomasini P, Egea J, Souquet-Bressand M,
Greillier L and Barlesi F: Alectinib in the treatment of
ALK-positive metastatic non-small cell lung cancer: Clinical trial
evidence and experience with a focus on brain metastases. Ther Adv
Respir Dis. Feb 21–2019.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Solomon BJ, Besse B, Bauer TM, Felip E,
Soo RA, Camidge DR, Chiari R, Bearz A, Lin CC, Gadgeel SM, et al:
Lorlatinib in patients with ALK-positive non-small-cell lung
cancer: Results from a global phase 2 study. Lancet Oncol.
19:1654–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naito T, Shiraishi H and Fujiwara Y:
Brigatinib and lorlatinib: Their effect on ALK inhibitors in NSCLC
focusing on resistant mutations and central nervous system
metastases. Jpn J Clin Oncol. 51:37–44. 2021. View Article : Google Scholar
|
|
40
|
Chao D, Pang L, Shi Y, Wang W and Liu K:
AZD3759 induces apoptosis in hepatoma cells by activating a
p53-SMAD4 positive feedback loop. Biochem Biophys Res Commun.
509:535–540. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hochmair M: Medical treatment options for
patients with epidermal growth factor receptor mutation-positive
non-small cell lung cancer suffering from brain metastases and/or
leptomeningeal disease. Target Oncol. 13:269–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lorenzetto E, Brenca M, Boeri M, Verri C,
Piccinin E, Gasparini P, Facchinetti F, Rossi S, Salvatore G,
Massimino M, et al: YAP1 acts as oncogenic target of 11q22
amplification in multiple cancer subtypes. Oncotarget. 5:2608–2621.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
You B, Yang YL, Xu Z, Dai Y, Liu S, Mao
JH, Tetsu O, Li H, Jablons DM and You L: Inhibition of ERK1/2
down-regulates the Hippo/YAP signaling pathway in human NSCLC
cells. Oncotarget. 6:4357–4368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng H, Zhang Z, Rodriguez-Barrueco R,
Borczuk A, Liu H, Yu J, Silva JM, Cheng SK, Perez-Soler R and
Halmos B: Functional genomics screen identifies YAP1 as a key
determinant to enhance treatment sensitivity in lung cancer cells.
Oncotarget. 7:28976–28988. 2016. View Article : Google Scholar :
|
|
45
|
Hsu PC, You B, Yang YL, Zhang WQ, Wang YC,
Xu Z, Dai Y, Liu S, Yang CT, Li H, et al: YAP promotes erlotinib
resistance in human non-small cell lung cancer cells. Oncotarget.
7:51922–51933. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hsu PC, Miao J, Huang Z, Yang YL, Xu Z,
You J, Dai Y, Yeh CC, Chan G, Liu S, et al: Inhibition of
yes-associated protein suppresses brain metastasis of human lung
adenocarcinoma in a murine model. J Cell Mol Med. 22:3073–3085.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Villa E, Ali ES, Sahu U and Ben-Sahra I:
Cancer cells tune the signaling pathways to empower de novo
synthesis of nucleotides. Cancers(Basel). 11:6882019.
|
|
48
|
Buj R and Aird KM: Deoxyribonucleotide
triphosphate metabolism in cancer and metabolic disease. Front
Endocrinol (Lausanne). 9:1772018. View Article : Google Scholar
|
|
49
|
Shiotani T, Hashimoto Y, Fujita J,
Yamauchi N, Yamaji Y, Futami H, Bungo M, Nakamura H, Tanaka T and
Irino S: Reversal of enzymic phenotype of thymidine metabolism in
induced differentiation of U-937 cells. Cancer Res. 49:6758–6763.
1989.PubMed/NCBI
|
|
50
|
Hanssen A, Riebensahm C, Mohme M, Joosse
SA, Velthaus JL, Berger LA, Bernreuther C, Glatzel M, Loges S,
Lamszus K, et al: Frequency of circulating tumor cells (CTC) in
patients with brain metastases: Implications as a risk assessment
marker in oligo-metastatic disease. Cancers (Basel). 10:5272018.
View Article : Google Scholar
|
|
51
|
Riebensahm C, Joosse SA, Mohme M, Hanssen
A, Matschke J, Goy Y, Witzel I, Lamszus K, Kropidlowski J, Petersen
C, et al: Clonality of circulating tumor cells in breast cancer
brain metastasis patients. Breast Cancer Res. 21:1012019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye Y, Luo Z and Shi D: Use of cell free
DNA as a prognostic biomarker in non-small cell lung cancer
patients with bone metastasis. Int J Biol Markers. 34:381–388.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aldea M, Hendriks L, Mezquita L, Jovelet
C, Planchard D, Auclin E, Remon J, Howarth K, Benitez JC, Gazzah A,
et al: Circulating tumor DNA analysis for patients with
oncogene-addicted NSCLC with isolated central nervous system
progression. J Thorac Oncol. 15:383–391. 2020. View Article : Google Scholar
|
|
54
|
Gedvilaite V, Schveigert D and Cicenas S:
Cell-free DNA in non-small cell lung cancer. Acta Med Litu.
24:138–144. 2017.PubMed/NCBI
|
|
55
|
Nygaard AD, Garm Spindler KL, Pallisgaard
N, Andersen RF and Jakobsen A: The prognostic value of KRAS mutated
plasma DNA in advanced non-small cell lung cancer. Lung Cancer.
79:312–317. 2013. View Article : Google Scholar
|
|
56
|
Aggarwal C, Thompson JC, Black TA, Katz
SI, Fan R, Yee SS, Chien AL, Evans TL, Bauml JM, Alley EW, et al:
Clinical implications of plasma-based genotyping with the delivery
of personalized therapy in metastatic non-small cell lung cancer.
JAMA Oncol. 5:173–180. 2019. View Article : Google Scholar
|
|
57
|
Mack PC, Banks KC, Espenschied CR, Burich
RA, Zill OA, Lee CE, Riess JW, Mortimer SA, Talasaz A, Lanman RB
and Gandara DR: Spectrum of driver mutations and clinical impact of
circulating tumor DNA analysis in non-small cell lung cancer:
Analysis of over 8000 cases. Cancer. 126:3219–3228. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Adzhubei I, Jordan DM and Sunyaev SR:
Predicting functional effect of human missense mutations using
PolyPhen-2. Curr Protoc Hum Genet. Chapter 7: Unit7 20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
1000 Genomes Project Consortium; Auton A,
Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL,
McCarthy S, McVean GA and Abecasis GR: A global reference for human
genetic variation. Nature. 526:68–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kircher M, Witten DM, Jain P, O'Roak BJ,
Cooper GM and Shendure J: A general framework for estimating the
relative pathogenicity of human genetic variants. Nat Genet.
46:310–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ng PC and Henikoff S: SIFT: Predicting
amino acid changes that affect protein function. Nucleic Acids Res.
31:3812–3814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
McNulty SN, Parikh BA, Duncavage EJ,
Heusel JW and Pfeifer JD: Optimization of population frequency
cutoffs for filtering common germline polymorphisms from tumor-only
next-generation sequencing data. J Mol Diagn. 21:903–912. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sukhai MA, Misyura M, Thomas M, Garg S,
Zhang T, Stickle N, Virtanen C, Bedard PL, Siu LL, Smets T, et al:
Somatic tumor variant filtration strategies to optimize tumor-only
molecular profiling using targeted next-generation sequencing
panels. J Mol Diagn. 21:261–273. 2019. View Article : Google Scholar
|
|
64
|
Hiltemann S, Jenster G, Trapman J, van der
Spek P and Stubbs A: Discriminating somatic and germline mutations
in tumor DNA samples without matching normals. Genome Res.
25:1382–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Teer JK, Zhang Y, Chen L, Welsh EA, Cress
WD, Eschrich SA and Berglund AE: Evaluating somatic tumor mutation
detection without matched normal samples. Hum Genomics. 11:222017.
View Article : Google Scholar : PubMed/NCBI
|