The World Health Organization (WHO) currently
classifies gliomas into four grades, where a new classification was
proposed in 2016 (1). These grades
of interest are as follows: i) Diffuse low-grade gliomas (WHO I and
II), which include diffuse astrocytoma and oligodendroglioma; ii)
diffuse high-grade gliomas (WHO III), which include anaplastic
astrocytoma with various mutated genes; and iii) glioblastoma (GBM;
WHO IV), which is the most lethal variety of glioma (2). Glioblastoma multiforme (GBM) is a
common type of brain malignancy that remains largely incurable.
Despite the development of multi-modal therapeutic interventions,
such as radiotherapy combined with temozolomide (TMZ) (3) and electrofield therapy combined with
TMZ (4), the median survival rate
of patients with GBM remains at ≤15 months (5).
TMZ is an orally administered alkylating antitumor
drug that can cross the blood-brain barrier with a bioavailability
reaching ~100%. It can be used to effectively treat newly diagnosed
and relapsed GBM to prolong the survival rate of patients (6,7).
Thus, TMZ is the main adjuvant therapeutic agent used in the
treatment of glioma in clinical practice (8). However, the development of resistance
to TMZ in glioma impairs its therapeutic efficacy, resulting in
recurrence (9). A previous study
evaluated the responses of 1,035 GBM tumors to therapies using
multiple assaying technologies and discovered that gene mutations
are largely responsible for resistance to treatment. The mechanisms
of action of TMZ involve the alkylation of the O6 site
of guanine (O6MeG), resulting in a base mismatch
following subsequent DNA replication, causing DNA damage (10). TMZ resistance has become one of the
largest obstacles in prolonging the survival of patients with
glioma. Several mechanisms have been proposed to underlie the
resistance to TMZ. The most common mode lies in the DNA repair
system, including the O6MeG DNA methyltransferase (MGMT)
pathway (11), DNA mismatch repair
and base excision repair (12,13).
In addition to the DNA repair system, GBM stemness and autophagy
can play critical roles in mediating resistance to TMZ. Other
factors, such as the EGFR, PTEN, p53 and PI3K/AKT/mTOR pathways,
have all been reported to participate in the resistance process.
Due to the high occurrence of TMZ resistance, the development of
novel effective treatment methods for gliomas is of utmost
urgency.
According to The Encyclopedia of DNA Elements
statistics, RNAs account for 75% of the human genome, whilst the
ratio of RNA to protein-coding genes is only ~3% (14). Influenced by the central genetic
dogma, the untranslated region of genes was previously considered
by prejudice to be 'junk' (15).
However, at the end of the last century, the discovery of small
regulatory non-coding RNAs (ncRNAs) has completely changed the
understanding of the role of ncRNAs (16). Among these ncRNAs, there is a class
of RNAs with base-pair lengths of ≥200 nucleotides, named long
non-coding RNAs (lncRNAs). The function of lncRNAs in protein
expression is similar to that of conventional RNAs. Both are
transcribed from DNA by RNA polymerase II and are then modified,
namely being 5′-capped, polyadenylated and/or spliced, before
eventually becoming mature lncRNA (17,18).
Compared with mRNAs, lncRNAs contain fewer but longer exons, where
their expression levels in different tissues are typically lower
(19,20). lncRNAs have been reported to
perform a variety of molecular functions. They have been reported
to regulate gene expression directly and indirectly by targeting
specific proteins, including Polycomb group proteins and enhancer
of zeste homolog 2 (EZH2). In addition, lncRNAs can also function
as precursors of small RNAs and modulate RNA processing events.
lncRNAs can also directly bind to proteins and modulate their
function (21). It has been
reported that lncRNAs and microRNAs (miRNAs/miRs) typically perform
reciprocal functions. miRNAs can promote the degradation of
lncRNAs, thereby diminishing the molecular functions of lncRNAs,
whereas lncRNAs can also conversely target miRNAs directly to
regulate their expression. lncRNAs can compete with miRNAs for
binding to the same sites on mRNAs. Furthermore, lncRNAs can be
spliced to generate miRNAs (22).
Accumulating evidence has indicated that lncRNAs can function as
regulatory molecules in cancer development. Han et al
(23) previously reported key
lncRNAs that can influence glioma pathogenesis using microarrays.
However, the most recent network-based algorithms failed to
adequately reflect the regulatory role in human tumors. Zhang et
al (24) successfully
developed a novel global network-based framework, termed
'LncRDNetFlow', to verify that lncRNAs play a key role in
regulating cellular processes in different malignancies. Apart from
the aforementioned reports, lncRNAs that are associated with cancer
have been previously detected in the body fluids of patients with
cancer, which suggests the potential of using lncRNAs as biomarkers
and therapeutic targets for cancer applications (25). Of note, lncRNAs have been reported
to participate in the development of resistance to chemotherapy in
various human malignancies, such as head and neck squamous cell
carcinoma, hepatocellular carcinoma, gastric cancer, pancreatic
cancer, bladder cancer, including glioma (26-32).
In conclusion, lncRNAs are promising therapeutic
targets for the treatment of human diseases, particularly glioma.
Therefore, the present review article first summarizes research
findings from the past 5 years regarding the signaling pathways
underlying the resistance of glioma to TMZ, with a focus on
oncogenic or tumor suppressive lncRNAs. Moreover, the association
between lncRNAs and combination therapy with TMZ and
immunosuppressive therapy for glioma is discussed, in an aim to
provide further insight into the clinical treatment of glioma.
However, lncRNA taurine upregulated 1 (TUG1) was
considered to be a suppressor of glioma cell proliferation by
promoting apoptosis. The overexpression of TUG1 was found to
increase the expression of caspases-3 and -9, whilst decreasing the
expression of Bcl-1, suggesting that the suppressive effects of
TUG1 were due to the inhibition of the pathways associated with
cell apoptosis (49).
Gliomas have also been reported to regulate the
expression of certain lncRNAs. Glioma cells retain the ability to
secrete exosomes containing mRNAs, miRNAs, lncRNAs and angiogenic
proteins into the surrounding microenvironment to regulate the
function of target cells, by targeting downstream signaling
pathways (50,51). Using electron microscopy and
western blot analysis, exosomes secreted by glioma cells were
previously found to transport lncRNA-activated by TGF-β (ATB) into
astrocytes to activate them. Subsequently, lncRNA-ATB in glioma
cell-derived exosomes were demonstrated to promote the
proliferation of glioma cells by inhibiting miR-204-3p (52). Angiogenesis also plays a key role
in glioma growth. It was reported that glioma-derived exosomes
enriched in lncRNA-POU class 3 homeobox 3 promoted angiogenesis in
glioma (53). Therefore, these
aforementioned findings suggest that gliomas can export lncRNAs by
secreting exosomes.
c-Met is a receptor tyrosine kinase, the expression
of which has been found to be upregulated in TMZ-resistant GBM
(54). MGMT is an enzyme that
removes the methyl group at O6MeG, thereby affecting the
activity of TMZ (11). Wu et
al (55) reported that
lncRNA-TMZ-associated lncRNA (TALC), a novel lncRNA associated with
GBM recurrence, upregulated the expression of MGMT and promoted TMZ
resistance by activating c-Met signaling.
The knockdown of K-homology splicing regulatory
protein (KSRP) expression has been demonstrated to inhibit the
expression of miR-198 (61). In
addition, miR-198 may enhance sensitivity to TMZ by inhibiting the
expression of MGMT, which is abrogated by TGF-β1 through the
suppression of KSRP expression in GBM cells. TGF-β1 may also
upregulate the expression of lncRNAs H19 and HOXD cluster antisense
RNA 2 by activating Smad, which promotes GBM resistance to TMZ by
activating MGMT and inhibiting miR-198. However, KSRP knockdown was
shown to abolished the effects of TGF-β1 and lncRNA H19 on miR-198
and MGMT. In the clinic, TMZ tended to be more effective in
patients with lower expression levels of TGF-β1 or lncRNA H19.
Collectively, these observations suggest that TGF-β1 can confer
resistance to TMZ by regulating the processing of lncRNAs and
increasing the expression of MGMT (61).
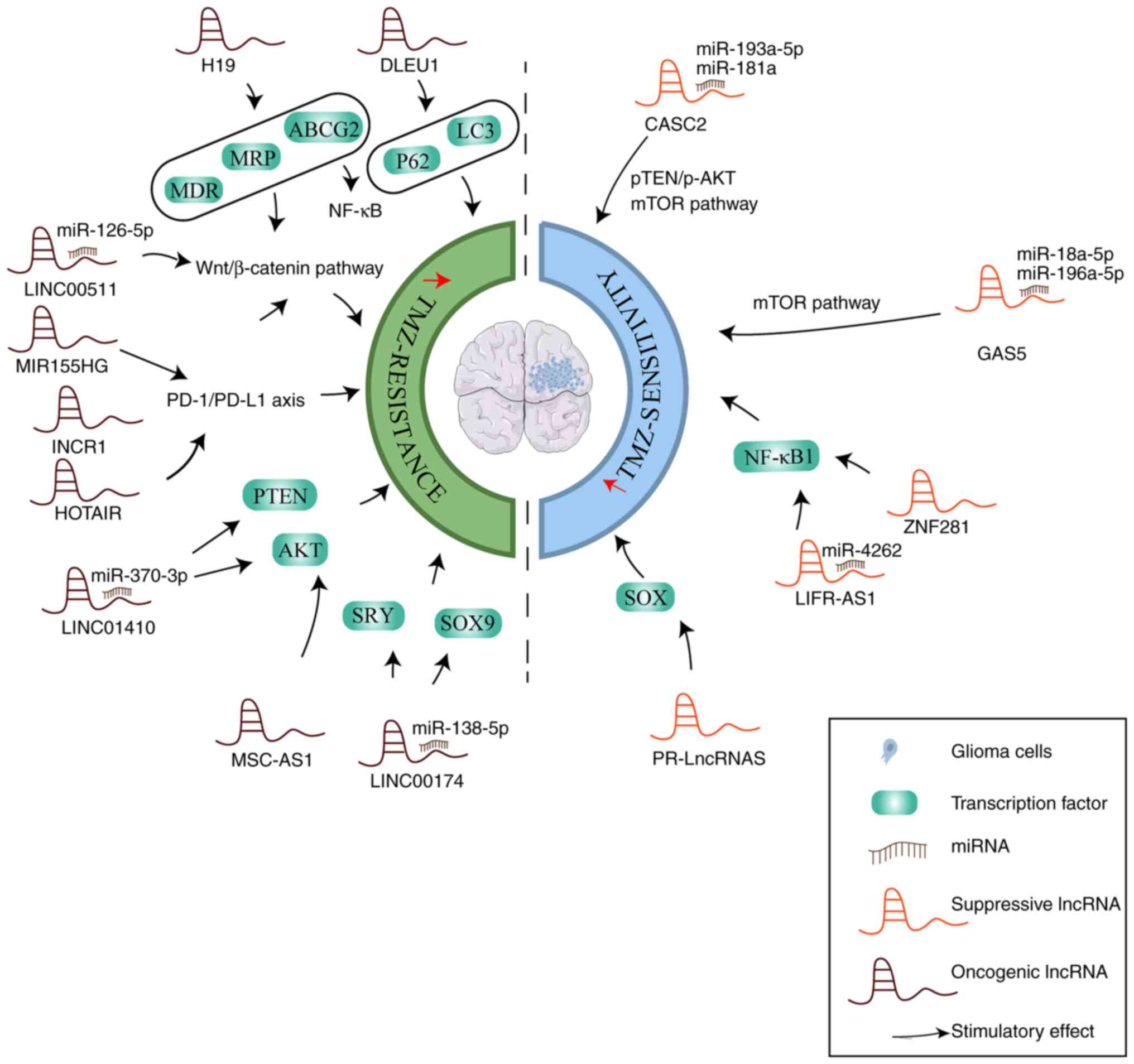
A literature search revealed various lncRNAs
affecting TMZ resistance in glioma, as well as their functions and
targets (62-82). This list of lncRNAs is presented in
Table I.
Using transcriptome sequencing, a previous study
found that the Wnt/β-catenin pathway was significantly activated in
TMZ-treated glioma cells in a PI3K/Akt pathway-dependent manner.
Mechanistically, Akt activation promoted β-catenin translocation
into the nucleus, resulting in the transcriptional activation of
Wnt/β-catenin signaling to suppress apoptosis whilst promoting TMZ
resistance (83). Sex-determining
region Y-box9 protein (SOX9) has been found to be overexpressed in
GBM, where it can stimulate GBM growth by activating Wnt/β-catenin
signaling (84). In another study,
Li et al (71) explored the
effects of LINC00174 on glioma cells. They found that LINC00174
expression was increased in glioma tissues, and that LINC00174
knockdown significantly inhibited the proliferation of
TMZ-resistant glioma cells. Mechanistically, the knockdown of
LINC00174 expression suppressed the growth of TMZ-resistant glioma
by sponging miR-138-5p, which negatively targets SOX9. Therefore,
LINC00174 was proposed to activate Wnt/β-catenin signaling by
targeting the miR-138-5p/SOX9 axis. Furthermore, in vivo
experiments also revealed consistent findings (71).
It has been previously demonstrated that the
expression level of lncRNA H19 is significantly increased in
patients with glioma resistant to TMZ (73). An in vitro TMZ
drug-resistant cell model was established and the model was
verified using RT-qPCR. Silencing H19 expression enhanced the
sensitivity of TMZ-resistant cells to TMZ by downregulating the
expression of a number of genes, including multidrug resistance
mutation, multidrug resistance-associated protein and ATP binding
cassette subfamily G member 2. Therefore, H19 was proposed to be a
novel therapeutic target for TMZ-resistant gliomas (73). In addition, other studies have
previously investigated the specific mechanisms involved in lncRNA
H19-mediated regulation. H19 knockdown was found to inhibit EMT by
promoting the expression of the epithelial marker, E-cadherin,
whilst inhibiting that of the mesenchymal markers, vimentin and
zinc finger E-box binding homeobox (ZEB)1. Furthermore, H19
downregulation was found to decrease the expression of β-catenin
and its downstream targets, c-Myc and surviving, in TMZ-treated
glioma cells. However, H19 reversed the resistance of glioma cells
to TMZ by repressing EMT and targeting Wnt/β-Catenin pathway
(72). A recent study also
reported that lncRNA PSMG3-AS1 was significantly upregulated in
GBM. PSMG3-AS1 was observed to promote TMZ resistance in glioma
cells by binding to c-Myc in the nucleus (74).
The silencing of lncRNA miR-155 host gene (MIR155HG)
expression was previously reported to promote glioma sensitivity to
TMZ through the Wnt/β catenin pathway by inhibiting poly-pyrimidine
tract-binding protein 1 expression (64). Furthermore, the knockdown of
LINC00511 expression enhanced the sensitivity of U87-R glioma cells
to TMZ by targeting miR-126-5p and activating Wnt/β-catenin
signaling (82). It has also been
previously reported that EGFR signaling plays a key role in GBM
(85), by regulating lncRNA
nuclear enriched abundant transcript 1 (NEAT1) expression and in
turn promoting glioma cell growth by targeting the Wnt/β-catenin
pathway. Taken together, these results suggest that NEAT1 is a
potential therapeutic target in GBM (86).
TMZ has been shown to induce GBM cell senescence and
autophagy, which promote GBM cell survival without affecting
proliferation (87). TMZ-induced
senescence has been found to be dependent on p21 activity. GSCs are
a main cause of TMZ resistance. MMP14 is an enzyme that is
localized to the cell membrane (88). It was previously reported that TMZ
treatment increased MMP14 expression in GSCs, which activated Notch
signaling (89). In another study,
the expression of lncRNA LINC00021 was revealed to be markedly
upregulated in TMZ-resistant GBM cells, and the silencing LINC00021
expression promoted TMZ sensitivity, whilst suppressing the
apoptosis of TMZ-resistant cells (75). Additionally, the expression of p21
was found to be downregulated by LINC00021 by targeting EZH2.
Western blot analysis revealed that LINC00021 knockdown inhibited
Notch expression. Therefore, LINC00021 was concluded to promote the
malignant features of GSCs and the chemoresistant phenotype through
the p21/Notch axis (75).
EMT has been observed to not only promote cell
migration, but also chemoresistance in tumor cells. Wen et
al (90) reported that the
expression of EMT markers was upregulated in oxaliplatin-resistant
human gastric cancer cell lines. In addition, EMT has been found to
be associated with the resistance of pancreatic cancer to treatment
(91). In another study, Peng
et al (92) discussed that
EMT can be regulated through miRNAs and summarized a list of drug
candidates that may suppress EMT in cancer. EMT was also identified
to be part of the mechanism underlying cancer resistance to EGFR
tyrosine kinase inhibitors in both mouse models and in clinical
non-small cell lung cancer (NSCLC) specimens (92). However, the effects of EMT on TMZ
resistance in glioma cells remain unclear and require further
investigation. Previously, a novel lncRNA TCONA_00004099 was
identified through the analysis of sequencing data, the expression
levels of which were high in glioma tissues and cell lines
(79). In both in vitro and
in vivo models, TCONA_00004099 knockdown was found to
inhibit glioma progression and promote TMZ-induced U87 and U251
cell apoptosis. This mechanism of resistance associated with
TCONA_00004099 in glioma was then examined using miRNA mimics.
TCONA_00004099 was demonstrated to sponge the miRNA TCONA_00004099
and its target protein tyrosine phosphatase, receptor type F, which
is involved in EGF signaling (79). Therefore, these findings reveal
potentially novel therapeutic targets for TMZ-resistant glioma.
lncRNA EGFR-AS1 was previously observed to promote the
proliferation, migration and invasion, whilst inhibiting the
apoptosis of glioma cells by regulating miR-133b/ribosomal receptor
for activated C-kinase 1 (RACK1) (80). However, the effects of lnc-EGFR-AS1
on the sensitivity of GBM cells to TMZ requires further
investigation both in vivo and in vitro (80). lncRNA HOTTIP expression was
reported to be significantly elevated in SF268 glioma cells,
particularly resistant to TMZ (93). Moreover, the expression of miR-10b
and the mesenchymal markers, ZEB1/ZEB2, was increased, while the
expression of E-cadherin was decreased in SF268, indicating that
EMT plays a crucial role in TMZ resistance. Moreover, EMT was
reversed in HOTTIP overexpressing cell lines by silencing miR-10b.
Thus, HOTTIP regulated TMZ resistance in glioma cells via the EMT
signaling pathway by targeting miR-10b (93).
FGFR3, platelet-derived growth factor receptor
(PDGFR) and EGFR have all been found to be among the upregulated
receptor tyrosine kinases involved in GBM tumorigenesis (94,95),
which are also associated with the EMT process. The expression
profile of FGFR3 was consistent with that of EGFR, whereby FGFR
inhibition enhanced NSCLC sensitivity to EGFR inhibitors (96). Furthermore, PDGFR was found to
promote the EMT process in glioma by promoting ZEB1 expression, an
inducer of EMT (97). Cui et
al (76) previously reported
that lncRNA colon cancer-associated transcript 1 (CCAT1) expression
was increased in glioma tissues, whilst that of miR-181b was
downregulated. CCAT1 silencing was observed to significantly
suppress the malignant biological behavior of glioma cells, which
was reversed with miR-181b inhibitors. In addition, CCAT1 was found
to function as a ceRNA with miR-181b to promote the expression of
FGFR3 and PDGFR. Collectively, CCAT1 downregulation suppressed
glioma growth by targeting miR-181b, resulting in the
downregulation of the endogenous targets, FGFR3 and PDGFR (76). However, the effects of CCAT1 on the
resistance of gliomas to TMZ and EMT require further in vivo
and in vitro investigations.
The function of p38 MAPK signaling is to relay
extracellular stimuli through the cell (98). The activation of the p38 MAPK
pathway has been reported to promote glioma invasiveness (99). Nuclear transcription factor
erythroid 2p45 (NF-E2)-related factor 2 (Nrf2) has also been shown
to promote the resistance of glioma cells to the combined treatment
of TMZ and radiotherapy (100).
In addition, another previous study found that TMZ increased Nrf2
expression by activating the p38 MAPK signaling pathway, leading to
the development of TMZ resistance (101).
The role of lnc-TALC in promoting the resistance of
GBM to TMZ has been observed. Furthermore, lnc-TALC can be
incorporated into exosomes and exported to tumor-associated
macrophages to promote M2 polarization. M2 macrophage polarization
has been shown to be associated with the secretion of complement
components C5/C5a, which occurs after the binding of lnc-TALC to
enolase 1 to promote the phosphorylation of p38 MAPK. Furthermore,
C5/C5a can promote the resistance to chemotherapy of GBM. Taken
together, lnc-TALC can potentially regulate the microenvironment of
GBM to improve GBM resistance to TMZ chemotherapy through the p38
MAPK pathway (70).
PTEN is a protein phosphatase that targets the lipid
phosphatidylinositol-3,4,5-triphosphate (PIP3), which is the
product of PI3K. PTEN mutation and PI3K activation promote the
accumulation of PIP3, resulting in the activation of AKT (102). PI3K/AKT signaling activation is
associated with the expression of MGMT, the main factor of TMZ
resistance. Supporting this, the pan-PI3K inhibitor, PX-866, has
been found to block TMZ-induced autophagy, whilst promoting GBM
cell death (103). In addition,
the activation of PTEN/PI3K/AKT signaling has been reported to be
an essential step in the development of TMZ resistance in GBM cells
in a MGMT-dependent manner (104-106).
In glioma tissues and cells resistant to TMZ, the
expression of the lncRNA musculin antisense RNA1 (MSC-AS1) has been
demonstrated to be increased. MSC-AS1 knockdown has been shown to
induce cell apoptosis and promote TMZ sensitivity by targeting the
PI3K/AKT pathway (65).
Cytoplasmic polyadenylation element binding protein (CPEB4) is an
RNA-binding protein that participates in autophagy. The increased
expression of CPEB4 has been found to be associated with migration,
tumor growth, angiogenesis, metastasis and invasion (66). Additionally, the suppression of
lncRNA-forkhead box D2-antisense RNA1 (FoxD2-AS1) expression has
been found to inhibit the invasion, proliferation, migration and of
TMZ-resistant glioma cells by promoting miR-98-5p expression, which
inhibits CPEB4 expression (67).
These data suggest that FoxD2-AS1 can inhibit glioma
chemoresistance to TMZ by modulating the miR-98-5p/CPEB4 axis
(58). Furthermore, the expression
of CPEB4, as a target of miR-373-3p, was also found to be regulated
by MSC-AS1. Specifically, MSC-AS1 knockdown inhibited the malignant
behavior of glioma cells and increased their sensitivity to TMZ by
sponging miR-373-3p, which activated the PI3K/Akt pathway both
in vitro or in vivo (65). Similarly, the expression of the
non-catalytic region of tyrosine kinase adaptor protein 1-antisense
RNA1 was reported to be increased in glioma tissues and cells
resistant to TMZ, which facilitated the resistance to TMZ by
activating the miR-137/tripartite motif containing 24 pathways
(81). In addition, in a previous
study, the expression of the lncRNA LINC01410 was found to be
upregulated in GBM and in GBM cells resistant to TMZ. LINC01410
silencing promoted the sensitivity of TMZ-resistant cells whilst
also upregulating the expression of PTEN but reducing the
phosphorylation of AKT. Inhibition of miR-370-3p reversed the
effects of LINC01410 silencing on the malignant biological behavior
of GBM cells. Based on these findings, LINC01410 was concluded to
promote glioma growth and TMZ resistance by sponging miR-370-3p to
inhibit the PTEN/AKT pathway (62).
p53 is a key transcription factor that can regulate
the transcription of target genes in response to DNA damage. p53
suppresses the gene expression of O6-alkylguanine DNA
alkyltransferase. In addition, Y-box binding protein 1 and mouse
double minute 2 homolog were found to be overexpressed in glioma
cells and were previously found to be associated with TMZ
resistance by directly targeting p53 (107,108). Therefore, p53 may be associated
with the resistance of glioma cells to TMZ.
The levels of lncRNA brain cytoplasmic RNA1 (BCYRN1)
were previously found to be increased in the blood and tumor
tissues from patients with GBM. Furthermore, the expression of
BCYRN1 differed significantly from that of isocitrate dehydrogenase
1 (IDH1) and the p53 status and was markedly higher compared with
both p53 and Ki-67 expression. The silencing of BCYRN1 expression
was previously reported to inhibit the proliferation, migration,
invasion and reverse resistance to TMZ in GB cells by sponging
miR-218-5p (68).
Compared with normal tissues, higher expression
levels of lncRNA CCAT2 were previously found in glioma tissues and
cells and were found to negatively correlate with those of miR-424
(69). In addition, different
glioma cell lines exhibited varying degrees of resistance to
chemotherapeutic agents. Specifically, SHG44 was the most resistant
cell line whereas U251 cells were more sensitive to agents, such as
TMZ. However, regardless of the cell line tested, the knockdown of
CCAT2 expression or the overexpression of miR-424 markedly
inhibited the proliferation, invasion and migration of glioma cells
(69). Furthermore, checkpoint
kinase 1 (CHK1), which is activated by DNA damage, was reported to
be a target of miR-424. The overexpression of CHK1 reversed the
effects of miR-424 overexpression in both p53 wild-type and p53
mutant glioma cells (109). Taken
together, these findings suggest that lncRNA CCAT2 expression is
upregulated in glioma tissues and cells, which in turn promotes
tumor growth and resistance to TMZ possibly through the
miR-424/CHK1 axis (69).
PIM1 is a serine/threonine kinase that plays a key
role in promoting cell proliferation and conferring chemoresistance
in various malignancies, such as prostate cancer, HER2-positive
breast cancer, high-risk neuroblastoma, triple negative breast
cancer and so on (110-113). The expression of survivin can be
decreased by the MGMT inhibitor (114). In addition, TMZ-induced
senescence results in tumor cell cycle arrest, promoting TMZ
resistance. Song et al (115) previously indicated that the
knockdown of survivin promoted glioma cell sensitivity to TMZ
treatment by inducing the apoptosis of senescent cells.
Furthermore, survivin nuclear trapping has been found to facilitate
GBM response to TMZ (116).
The lncRNA potassium voltage-gated channel subfamily
Q member 1 opposite strand/antisense transcript one gene (KCNQ1OT1)
was previously reported to promote the proliferation and colony
formation of glioma cells by negatively regulating miRNA-761
expression. It was also found to impair the effects of TMZ on the
promotion of the apoptosis of glioma cells in vitro.
Furthermore, the overexpression of KCNQ1OT1 was found to increase
survivin expression by promoting PIM1 expression in glioma cells.
In vivo, the experimental results were consistent with their
in vitro counterparts. Taken together, it was found that
lncRNA KCNQ1OT1 played a significant role in glioma sensitivity to
TMZ by sponging miR-761 to activate PIM-1/survivin signaling, which
may be exploited as a potential prognostic target (78).
Over the past decade, immune checkpoint inhibitors
have become a topic of extensive research for tumor treatment.
Among these, PD-1/PD-L1 have been found to exert promising
therapeutic effects against various malignancies. PD-L1 has also
been shown to be strongly expressed in a wide range of tumor cells,
including breast cancer, oral squamous cell carcinoma and
nasopharyngeal carcinoma (117-119). The PD-1/PD-L1 signaling pathway
plays a constructive role in tumor immunosuppression by inducing
T-cell death and activating tumor immune escape (120).
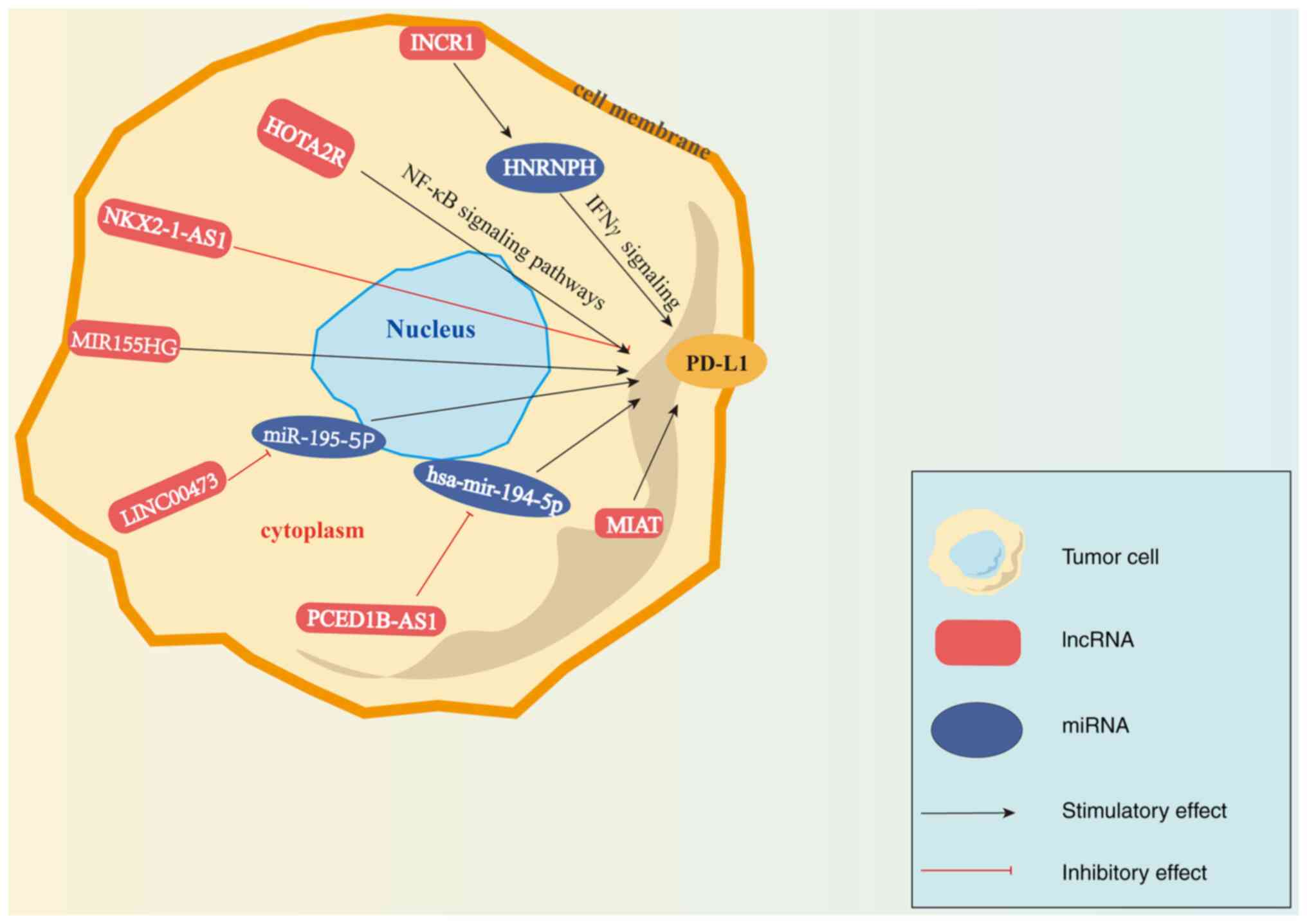
Furthermore, several studies have demonstrated that
a number of lncRNAs are associated with PD-1/PD-L1 in different
types of cancer (Fig. 1). A
previous study demonstrated that human antisense lncRNA-NK-2
homeobox 1 antisense RNA1 (NKX2-1-AS1) negatively regulated the
expression of endogenous CD274/PD-L1, and that NKX2-1-AS1 knockdown
was able to upregulate the expression of genes that are involved in
cell adhesion and checkpoints (121). In addition, PD-1/PD-L1 was found
to exert promoting effects on diffuse large-B-cell lymphoma
(DLBCL), and lncRNA SNHG14 expression negatively correlated with
miR-152-3p, the expression of which was decreased in DLBCL tissues
and cell lines. Furthermore, SNHG14/miR-152-3p inhibited apoptosis
and promoted cell proliferation in cytotoxic T-lymphocytes (CTLs)
in DLBCL by sponging the PD-1/PD-L1 checkpoint (122). Similarly, SNHG15 lncRNA, which is
highly expressed in gastric cancer cells, was reported to increase
the expression of PD-L1 (123).
Zhou et al (124) found
that in pancreatic cancer tissues and cell lines, the expression of
LINC00473 was increased, whilst that of miR-195-5p was decreased,
and the expression of PD-L1 was increased. Mechanistically,
LINC00473 upregulated PD-L1 expression by targeting miR-195-5p.
Furthermore, CD8+ T-cells could be activated either by
the silencing of LINC00473 expression or increasing miR-195-5p
expression, which inhibited PC progression (124). In addition to these
aforementioned pre-clinical findings, a database analyses have also
revealed the potential of lncRNAs in regulating PD-1/PD-L1
signaling. According to The Cancer Genome Atlas-liver
hepatocellular carcinoma (HCC) dataset, PC-esterase
domain-containing 1B-antisense RNA1 promoted PD-L1 and PD-L2
expression by inhibiting hsa-miR-194-5p expression, resulting in
immunosuppression in HCC (125).
Similarly, the association between lncRNA-myocardial infarction
associated transcript (MIAT) and immunomodulatory regulation in HCC
was also investigated. The results revealed that the expression of
MIAT in HCC was positively associated with that of PD-1/PD-L1.
Furthermore, MIAT reduced the sensitivity of HCC to sorafenib, an
anticancer drug, mediating immune escape (126).
Previous studies have demonstrated that various
lncRNAs can regulate the PD-1/PD-L1 axis in gliomas. The lncRNA
interferon (IFN)-stimulated non-coding RNA 1 (INCR1) can be
transcribed from the PD-L1 locus following stimulation by tumor
IFNs and can promote checkpoint blockade resistance in GBM. INCR1
knockdown was found to inhibit PD-L1 expression and enhance GBM
cell sensitivity to cytotoxic T-cell-mediated cell death (127). Mechanistically, INCR1 was found
to promote the expression of PD-L1 genes by binding to
heterogeneous nuclear ribonucleoprotein H1 (HNRNPH1), a nuclear
ribonucleoprotein, to negatively regulate the transcription of
PD-L1. In summary, INCR1 lncRNA can regulate PD-L1 expression and
responses to immune checkpoint therapy by targeting HNRNPH1 after
IFNγ signaling (127). Similarly,
the lncRNA HOTAIR was also found to be highly expressed in glioma
cells (77). Bioinformatics
analysis revealed that HOTAIR activated the expression of proteins
in the NF-κB signaling pathway. Furthermore, the downregulation of
HOTAIR expression was found to inhibit PD-L1 expression in
vivo, suggesting that HOTAIR can improve the sensitivity of
glioma cells to immune checkpoint inhibitor therapy. In summary,
HOTAIR positively regulated PD-L1 expression by targeting the NF-κB
signaling pathway in gliomas (77). According to the existing public
bioinformatics database, higher levels of the lncRNA MIR155HG have
been shown to be associated with a poorer overall survival of
patients with GBM. In addition, the levels of MIR155HG expression
have been demonstrated to be significantly consistent with those of
PD-L1, suggesting that MIR155HG can be used predict the efficacy of
immune checkpoint inhibitor therapy (63). Taken together, lncRNAs may
represent a therapeutic target that can regulate the effects of
PD-1/PD-L1 treatment in various types of cancer, particularly
gliomas. However, T-cells in GBM are very unresponsive to
immunotherapy, and PD-1 checkpoint blockade cannot be overcome
through intracellular regulation. Further research on combination
therapy is still in progress (128). Due to chemo-immunotherapy
resistance, Miyazaki et al (129) used a TMZ-treated glioma model to
examine the effects of anti-PD-L1 antibody on the tumor
microenvironment. They also established TMZ-resistant TS (TMZRTS)
cells to examine the effects of anti-PD-L1 anti-body on glioma
growth. The results of their study revealed that anti-PD-L1
antibody significantly suppressed tumor growth and markedly
decreased CD163-positive Mϕ infiltration into tumors. However, the
safety and effectiveness of clinical therapy need to be further
studied (129). Nivolumab is a
type of PD-1 inhibitor. Recent research has reported that a patient
with GBM received nivolumab treatment for almost 2 years following
standard radiotherapy and TMZ therapy. Magnetic resonance imaging
revealed a continuous shrinking of the tumor (130), suggesting that the combined
application with PD-1 inhibitor may be effective for the treatment
of gliomas. Clinically, TMZ is used as an adjuvant therapy for
patients with glioma, while the prognosis is poor. Thus, exploring
the role of PD-1/PD-L1 combined with TMZ is of therapeutic
significance. Notably, it was demonstrated that stereotactic
radiation, which to date is a conventional treatment for glioma,
combined with anti-PD-1 blockade enhanced the long-term survival
rate of tumor-bearing mice (131), which provided further evidence of
the potential of combining TMZ with PD-1 inhibitors. In a previous
clinical study, 47 patients were divided into two subgroups
(132). One subgroup of 27
patients, which was named as ADCTA, was treated with concomitant
chemo-radiotherapy (CCRT). Moreover, the patients also received
post-surgical adjuvant immunotherapy with an autologous dendritic
cell/tumor antigen vaccine. The other subgroup was only treated
with CCRT and TMZ without immunotherapy as the control group. The
results of that study demonstrated that the ADCTA group exhibited a
markedly low TIL PD-1+/CD8+ ratio within the
GBM tumor compared with the control group, which prolonged the
overall survival rate of the patients. On the whole, that study
suggested that adjuvant immune therapy for the immune system
combined with conventional therapies, such as TMZ has great
potential for the treatment of GBM (132). In conclusion, the com-bination of
immune checkpoint inhibitors and anti-tumor chemical drugs, such as
TMZ, has a promising therapeutic effect on glioma, which further
provides new insight into the treatment of gliomas in clinical
practice.
lncRNA growth arrest specific 5 (GAS5) has been
reported to play a constructive role in various tumor types, such
as gastric cancer and NSCLC. It has been observed to inhibit the
proliferation, migration and invasion of human glioma cells by
targeting miR-18a-5p or miR-196a-5p (137,138). In addition, GAS5 can reverse
glioma cell resistance to cisplatin by preventing excessive
autophagy through activation of mTOR signaling (139). Similarly, in another previous
study (140), which investigated
the U251 and U87 cell lines, the expression of GAS5 was found to be
decreased, and the upregulation of GAS5 was able to inhibit tumor
growth by sponging glutathione-S-transferase M3, the overexpression
of which has been associated with chemoresistance. However, the
effects of GAS5 on the resistance of glioma cells to TMZ require
further examination in vitro and in vivo (140).
lncRNAs can also regulate resistance to TMZ by
targeting the PTEN, p53 and PI3K/AKT/mTOR pathways (141). In a previous study, the
overexpression of lncRNA CACS2 was found to reverse the resistance
of glioma cells to TMZ by suppressing miR-181a expression (142). In addition, CASC2 promoted PTEN
protein expression and inhibited AKT phosphorylation by targeting
miR-181a in a reversible manner by miR-181a overexpression.
Therefore, CASC2 may be a clinically relevant lncRNA that can
mediate TMZ resistance by regulating miR-181a expression (142).
NF-κB is a transcription factor and plays a key role
in cell survival, inflammation and immune responses. In particular,
NF-κB has been found to be associated with cellular senescence in
response to chemotherapy. Accordingly, senescence induced by
chemotherapy and mediated by NF-κB is a key characteristic of
superior therapeutic outcomes (143). Therefore, NF-κB will likely
affect TMZ resistance by modulating cellular senescence.
The p53-regulated tumor suppressor signature of
lncRNAs (PR-lncRNAs) was first identified in colorectal cancer
(144). In glioma, the expression
of PR-lncRNAs was found to be negatively associated with its grade
(145). A previous functional
study reported that PR-lncRNA knockdown aggravated glioma
malignancy by inhibiting SOX activity. This suggests that the
downregulation of PR-lncRNAs can abrogate the beneficial effects of
PR-lncRNAs on cell proliferation, migration, invasion and
resistance to TMZ in vitro (145). Similarly, the expression of
leukemia inhibitory factor receptor-antisense RNA1 (LIFR-AS1) has
also been found to be decreased, whereas that of miR-4262 was found
to be increased in glioma tissues and cell lines. The
overexpression of LIFR-AS1 inhibited glioma cell proliferation and
enhanced glioma cell sensitivity to TMZ by inactivating the
miR-4262/NF-κB pathway (146).
lncRNA-zinc finger protein 281 (ZNF281) was previously reported to
be expressed at lower levels in glioma stem-like cells (U251s)
compared with those in normal brain tissues. Furthermore,
lncRNA-ZNF281 was able to suppress the self-renewal capacity and
invasion of glioma stem cells by activating the NF-κB signaling
pathway in vitro and in vivo (147).
The various mechanisms of lncRNAs in the regulation
of TMZ resistance in glioma are illustrated in Fig. 2.
GBM, which is considered to originate from neural
stem cells or progenitors, remains to be one of the greatest
threats to human health to date. Regrettably, even with the
traditional treatment methods of gliomas, including surgery,
radiation therapy and alkylating agent chemotherapy, such as TMZ,
the overall survival of patients with gliomas has not improved
satisfactorily (148).
Additionally, >50% patients with glioma treated with TMZ develop
resistance to this drug, which has become a major obstacle in the
clinical treatment efficacy of glioma (9). Apart from DNA repair systems, the
mechanism underlying chemotherapy resistance also included
translesion synthesis (TLS). When DNA lesions occur due to
chemotherapy and/or radiation, such as following treatment with
cisplatin and TMZ, TLS can bypass these types of lesions at the
cost of increasing the risk of mutations (149), which in turn increases the risk
of developing chemotherapeutic resistance. The TLS process involves
several key regulators, including RAD18 (150), DNA polymerases (POL)κ, POLι, POLη
or REV1 (which introduce a nucleotide opposite the lesion) and TLS
DNA polymerase, such as POLζ (comprising of the
Rev3L/Rev7/Pold2d/Pold3 complex) (149,151). Peng et al (152) previously reported that the
overexpression of POLκ increased the TMZ resistance of GBM cells
which were previously TMZ-sensitive. The higher expression of POLκ
significantly promoted TMZ sensitivity in vitro and in
orthotopic xenograft mouse models. Furthermore, other TLS
polymerases, such as Rev1 and Rev7, have been proposed as targets
for developing agents for reversing chemoresistance (151,153). RAD18 was previously found to be
overexpressed in GBM cell lines, where it promoted resistance to
radiotherapy by inhibiting the expression of p53 (154).
lncRNAs have been demonstrated to regulate the
survival, proliferation, apoptosis and invasion of GBM cells
(155). Furthermore, lncRNAs play
a key role in regulating the resistance of gliomas to TMZ by
sponging miRNAs and various signaling pathways. However, TMZ can
conversely regulate the levels of lncRNA expression to enhance the
effectiveness of TMZ treatment. Recently, lncRNA ATXN8OS was found
to be mainly located in the cytoplasm and to be downregulated in
glioma cell lines (156).
Functional experiments proved that ATXN8OS enhanced TMZ sensitivity
of glioma in vivo and in vitro. Mechanistically,
ATXN8OS stabilized glutaminase 2 mRNA to promote the ferroptosis of
glioma cell lines, which may become a new target of TMZ resistance
(154). High mobility group box 1
protein (HMGB1), which is a highly conserved protein, has been
reported to be expressed in a variety of cell types and can be
secreted into the tumor micro-environment to function as a
tumor-derived signal. HMGB1 expression has been found to be
upregulated in GBM and to influence GBM growth. GSCs play a crucial
role in patients with TMZ-resistant glioma, and HMGB1 expression
has been found to be upregulated in GSCs (157). HMGB1 can then alter the
expression profile of mRNAs, lncRNAs and miRNAs in GSCs. Since
TMZ-induced HMGB1 expression can promote the formation of GSCs,
this process is inhibited when the secretion of HMGB1 is decreased.
In terms of the mechanism, TMZ-induced upregulation of HMGB1 has
been found to promote the formation of GSCs through the Toll-like
receptor 2/NEAT1/Wnt pathway. Taken together, TMZ-induced HMGB1 may
serve as a potential therapeutic target for the treatment of
patients with TMZ-resistant GBM (157). In addition, a number of
traditional Chinese medicine ingredients have also been reported to
affect TMZ resistance by regulating the expression of lncRNAs.
Isoliquiritigenin has been found to inhibit the expression of
metabolic enzymes of arachidonic acid (AA), such as
cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase-1
(mPGES-1) and cytochrome P450 (CYP) 4A11, all of which serve
significant roles in glioma angiogenesis. Furthermore,
isoliquiritigenin can enhance the therapeutic effects of TMZ in a
rat C6 glioma model. In terms of the mechanism, isoliquiritigenin
can reprogram COX-2-, mPGES-1- and CYP4A-mediated AA metabolism in
glioma by suppressing angiogenic Akt/fibroblast growth factor
2/TGFβ/VEGF signaling, which was achieved by sponging miR-194-5p
and lncRNA NEAT1 (158). To
conclude, lncRNAs may serve as potential therapeutic and prognostic
targets to improve the sensitivity of glioma to TMZ. However,
enormous research efforts remain necessary to transform this basic
concept into a feasible clinical strategy.
Not applicable.
The present review article was conceptualized by
all the authors (SL, XX, FP, JD and CP). SL was involved in the
study design and in the preparation of the original draft. FP, XX
and JD edited the manuscript. FP, JD and CP were involved in
funding-related administration. SL and FP were involved in
manuscript revision. All authors have read and agreed to the
published version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82003879 and U19A2010) the Key
Project of Science and Technology Department of Sichuan Province
(grant nos. 2020YFS0053 and 2021YFS0044), and the Youth Talent
Promotion Project of China Association for Science and Technology
(grant no. CACM-2020-QNRC1-01) and the Open Research Fund of
Chengdu University of Traditional Chinese Medicine Key Laboratory
of Systematic Research of Distinctive Chinese Medicine Resources in
Southwest China (grant no. 2021LF1026).
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rynkeviciene R, Simiene J, Strainiene E,
Stankevicius V, Usinskiene J, Miseikyte Kaubriene E, Meskinyte I,
Cicenas J and Suziedelis K: Non-coding RNAs in glioma. Cancers
(Basel). 11:172018. View Article : Google Scholar
|
|
3
|
De Sanctis V, Mazzarella G, Osti MF,
Valeriani M, Alfó M, Salvati M, Banelli E, Tombolini V and Enrici
RM: Radiotherapy and sequential temozolomide compared with
radiotherapy with concomitant and sequential temozolomide in the
treatment of newly diagnosed glioblastoma multiforme. Anticancer
Drugs. 17:969–975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strobel H, Baisch T, Fitzel R, Schilberg
K, Siegelin MD, Karpel-Massler G, Debatin KM and Westhoff MA:
Temozolomide and other alkylating agents in glioblastoma therapy.
Biomedicines. 7:692019. View Article : Google Scholar :
|
|
7
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: Mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar
|
|
8
|
Stupp R, Brada M, van den Bent MJ and Tonn
JC: High-grade glioma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 25(Suppl 3):
iii93–iii101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanzawa T, Bedwell J, Kondo Y, Kondo S and
Germano IM: Inhibition of DNA repair for sensitizing resistant
glioma cells to temozolomide. J Neurosurg. 99:1047–1052. 2003.
View Article : Google Scholar
|
|
11
|
Jiang G, Li LT, Xin Y, Zhang L, Liu YQ and
Zheng JN: Strategies to improve the killing of tumors using
temozolomide: Targeting the DNA repair protein MGMT. Curr Med Chem.
19:3886–3892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perazzoli G, Prados J, Ortiz R, Caba O,
Cabeza L, Berdasco M, Gónzalez B and Melguizo C: Temozolomide
resistance in glioblastoma cell lines: Implication of MGMT, MMR,
P-glycoprotein and CD133 expression. PLoS One. 10:e01401312015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang JB, Svilar D, Trivedi RN, Wang XH,
Goellner EM, Moore B, Hamilton RL, Banze LA, Brown AR and Sobol RW:
N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER
inhibitor potentiation of glioma cells to temozolomide. Neuro
Oncol. 13:471–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Comings DE: The structure and function of
chromatin. Adv Hum Genet. 3:237–431. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Non-coding RNAs in
Colorectal Cancer. Slaby O and Calin GA: Springer International
Publishing; Cham: pp. 3–17. 2016, View Article : Google Scholar
|
|
17
|
Ling H, Vincent K, Pichler M, Fodde R,
Berindan-Neagoe I, Slack FJ and Calin GA: Junk DNA and the long
non-coding RNA twist in cancer genetics. Oncogene. 34:5003–5011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmitz SU, Grote P and Herrmann BG:
Mechanisms of long noncoding RNA function in development and
disease. Cell Mol Life Sci. 73:2491–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu B and Wang S: Angio-LncRs: LncRNAs that
regulate angiogenesis and vascular disease. Theranostics.
8:3654–3675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon JH, Abdelmohsen K and Gorospe M:
Functional interactions among microRNAs and long noncoding RNAs.
Semin Cell Dev Biol. 34:9–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han L, Zhang K, Shi Z, Zhang J, Zhu J, Zhu
S, Zhang A, Jia Z, Wang G, Yu S, et al: LncRNA profile of
glioblastoma reveals the potential role of lncRNAs in contributing
to glioblastoma pathogenesis. Int J Oncol. 40:2004–2012.
2012.PubMed/NCBI
|
|
24
|
Zhang J, Zhang Z, Chen Z and Deng L:
Integrating multiple heterogeneous networks for novel
LncRNA-disease association inference. IEEE/ACM Trans Comput Biol
Bioinform. 16:396–406. 2019. View Article : Google Scholar
|
|
25
|
Bolha L, Ravnik-Glavač M and Glavač D:
Long noncoding RNAs as biomarkers in cancer. Dis Markers.
2017:72439682017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahinfar P, Baradaran B, Davoudian S,
Vahidian F, Cho WC and Mansoori B: Long Non-coding RNAs in
multidrug resistance of glioblastoma. Genes (Basel). 12:4552021.
View Article : Google Scholar
|
|
27
|
Jiang Y, Guo H, Tong T, Xie F, Qin X, Wang
X, Chen W and Zhang J: lncRNA lnc-POP11 upregulated by VN1R5
promotes cisplatin resistance in head and neck squamous cell
carcinoma through interaction with MCM5. Mol Ther. 30:448–467.
2022. View Article : Google Scholar
|
|
28
|
Chen KY, Zhu SG, He JW and Duan XP: LncRNA
CRNDE is involved in radiation resistance in hepatocellular
carcinoma via modulating the SP1/PDK1 axis. Neoplasma.
211230N18532022.Epub ahead of print. PubMed/NCBI
|
|
29
|
Wu J, Xu S, Li W, Lu Y, Zhou Y, Xie M, Luo
Y, Cao Y, He Y, Zeng T and Ling H: lncRNAs as hallmarks for
individualized treatment of gastric cancer. Anticancer Agents Med
Chem. 22:1440–1457. 2022. View Article : Google Scholar
|
|
30
|
Ye X, Wang LP, Han C, Hu H, Ni CM, Qiao
GL, Ouyang L and Ni JS: Increased m6A modification of
lncRNA DBH-AS1 suppresses pancreatic cancer growth and gemcitabine
resistance via the miR-3163/USP44 axis. Ann Transl Med. 10:3042022.
View Article : Google Scholar
|
|
31
|
Jiang X, Li H, Fang Y and Xu C: LncRNA
PVT1 contributes to invasion and doxorubicin resistance of bladder
cancer cells through promoting MDM2 expression and AURKB-mediated
p53 ubiquitination. Environ Toxicol. 37:1495–1508. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng M, Wang Q, Chen L, Zhao D, Tang J,
Xu J and He Z: LncRNA UCA1/miR-182-5p/MGMT axis modulates glioma
cell sensitivity to temozolomide through MGMT-related DNA damage
pathways. Hum Pathol. 123:59–73. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Sun S, Pu JK, Tsang AC, Lee D,
Man VO, Lui WM, Wong ST and Leung GK: Long non-coding RNA
expression profiles predict clinical phenotypes in glioma.
Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin X, Zhuang S, Chen X, Du J, Zhong L,
Ding J, Wang L, Yi J, Hu G, Tang G, et al: lncRNA ITGB8-AS1
functions as a ceRNA to promote colorectal cancer growth and
migration through integrin-mediated focal adhesion signaling. Mol
Ther. 30:688–702. 2022. View Article : Google Scholar
|
|
35
|
Li DQ, Ding YR, Che JH, Su Z, Yang WZ, Xu
L, Li YJ, Wang HH and Zhou WY: Tumor suppressive lncRNA MEG3 binds
to EZH2 and enhances CXCL3 methylation in gallbladder cancer.
Neoplasma. 69:538–549. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan D, Guo T, Zhu D, Ge H, Zhao Y, Huang
A, Wang X, Cao X, He C, Qian H and Yu H: Exosomal lncRNA ATB
derived from ovarian cancer cells promotes angiogenesis via
regulating miR-204-3p/TGFβR2 axis. Cancer Manag Res. 14:327–337.
2022. View Article : Google Scholar :
|
|
37
|
Yan Y, Xu Z, Li Z, Sun L and Gong Z: An
insight into the increasing role of LncRNAs in the pathogenesis of
gliomas. Front Mol Neurosci. 10:532017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng Z, Liu C and Wu M: New insights into
long noncoding RNAs and their roles in glioma. Mol Cancer.
17:612018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Bian EB, He XJ, Ma CC, Zong G, Wang
HL and Zhao B: Epigenetic repression of long non-coding RNA MEG3
mediated by DNMT1 represses the p53 pathway in gliomas. Int J
Oncol. 48:723–733. 2016. View Article : Google Scholar
|
|
40
|
Zeng H, Xu N, Liu Y, Liu B, Yang Z, Fu Z,
Lian C and Guo H: Genomic profiling of long non-coding RNA and mRNA
expression associated with acquired temozolomide resistance in
glioblastoma cells. Int J Oncol. 51:445–455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang X, Li XD, Fu Z, Zhou Y, Huang X and
Jiang X: Long non-coding RNA LINC00473/miR-195-5p promotes glioma
progression via YAP1-TEAD1-Hippo signaling. Int J Oncol.
56:508–521. 2020.PubMed/NCBI
|
|
42
|
Lei W, Wang ZL, Feng HJ, Lin XD, Li CZ and
Fan D: Long non-coding RNA SNHG12promotes the proliferation and
migration of glioma cells by binding to HuR. Int J Oncol.
53:1374–1384. 2018.PubMed/NCBI
|
|
43
|
Fang K, Liu P, Dong S, Guo Y, Cui X, Zhu
X, Li X, Jiang L, Liu T and Wu Y: Magnetofection based on
superparamagnetic iron oxide nanoparticle-mediated low lncRNA
HOTAIR expression decreases the proliferation and invasion of
glioma stem cells. Int J Oncol. 49:509–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu ZZ, Tian YF, Wu H, Ouyang SY and Kuang
WL: LncRNA H19 promotes glioma angiogenesis through
miR-138/HIF-1α/VEGF axis. Neoplasma. 67:111–118. 2020. View Article : Google Scholar
|
|
45
|
Jia P, Cai H, Liu X, Chen J, Ma J, Wang P,
Liu Y, Zheng J and Xue Y: Long non-coding RNA H19 regulates glioma
angiogenesis and the biological behavior of glioma-associated
endothelial cells by inhibiting microRNA-29a. Cancer Lett.
381:359–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Wang Y, Li J, Zhang Y, Yin H and
Han B: CRNDE, a long-noncoding RNA, promotes glioma cell growth and
invasion through mTOR signaling. Cancer Lett. 367:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng J, Liu X, Wang P, Xue Y, Ma J, Qu C
and Liu Y: CRNDE promotes malignant progression of glioma by
attenuating miR-384/PIWIL4/STAT3 axis. Mol Ther. 24:1199–1215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shree B, Tripathi S and Sharma V:
Transforming growth factor-beta-regulated LncRNA-MUF promotes
invasion by modulating the miR-34a snail1 axis in glioblastoma
multiforme. Front Oncol. 11:7887552022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. Exp Biol Med (Maywood). 241:644–649. 2016. View Article : Google Scholar
|
|
50
|
Arscott WT, Tandle AT, Zhao S, Shabason
JE, Gordon IK, Schlaff CD, Zhang G, Tofilon PJ and Camphausen KA:
Ionizing radiation and glioblastoma exosomes: Implications in tumor
biology and cell migration. Transl Oncol. 6:638–648. 2013.
View Article : Google Scholar
|
|
51
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bian EB, Chen EF, Xu YD, Yang ZH, Tang F,
Ma CC, Wang HL and Zhao B: Exosomal lncRNA-ATB activates astrocytes
that promote glioma cell invasion. Int J Oncol. 54:713–721.
2019.
|
|
53
|
Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu
YM, Wu L and Xu GH: Glioma cells promote angiogenesis through the
release of exosomes containing long non-coding RNA POU3F3. Eur Rev
Med Pharmacol Sci. 21:959–972. 2017.PubMed/NCBI
|
|
54
|
Li MY, Yang P, Liu YW, Zhang CB, Wang KY,
Wang YY, Yao K, Zhang W, Qiu XG, Li WB, et al: Low c-Met expression
levels are prognostic for and predict the benefits of temozolomide
chemotherapy in malignant gliomas. Sci Rep. 6:211412016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu P, Cai J, Chen Q, Han B, Meng X, Li Y,
Li Z, Wang R, Lin L, Duan C, et al: Lnc-TALC promotes
O6-methylguanine-DNA methyltransferase expression via regulating
the c-Met pathway by competitively binding with miR-20b-3p. Nat
Commun. 10:20452019. View Article : Google Scholar :
|
|
56
|
Wesolowska A, Kwiatkowska A, Slomnicki L,
Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S and
Kaminska B: Microglia-derived TGF-beta as an important regulator of
glioblastoma invasion-an inhibition of TGF-beta-dependent effects
by shRNA against human TGF-beta type II receptor. Oncogene.
27:918–930. 2008. View Article : Google Scholar
|
|
57
|
Han J, Alvarez-Breckenridge CA, Wang QE
and Yu J: TGF-β signaling and its targeting for glioma treatment.
Am J Cancer Res. 5:945–955. 2015.
|
|
58
|
Miyazawa K and Miyazono K: Regulation of
TGF-β family signaling by inhibitory smads. Cold Spring Harb
Perspect Biol. 9:a0220952017. View Article : Google Scholar
|
|
59
|
Brunen D, Willems SM, Kellner U, Midgley
R, Simon I and Bernards R: TGF-β: An emerging player in drug
resistance. Cell Cycle. 12:2960–2968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oshimori N, Oristian D and Fuchs E: TGF-β
promotes heterogeneity and drug resistance in squamous cell
carcinoma. Cell. 160:963–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nie E, Jin X, Miao F, Yu T, Zhi T, Shi Z,
Wang Y, Zhang J, Xie M and You Y: TGF-β1 modulates temozolomide
resistance in glioblastoma via altered microRNA processing and
elevated MGMT. Neuro Oncol. 23:435–446. 2021. View Article : Google Scholar
|
|
62
|
Fu T, Yang Y, Mu Z, Sun R, Li X and Dong
J: Silencing lncRNA LINC01410 suppresses cell viability yet
promotes apoptosis and sensitivity to temozolomide in glioblastoma
cells by inactivating PTEN/AKT pathway via targeting miR-370-3p.
Immunopharmacol Immunotoxicol. 43:680–692. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peng L, Chen Z, Chen Y, Wang X and Tang N:
MIR155HG is a prognostic biomarker and associated with immune
infiltration and immune checkpoint molecules expression in multiple
cancers. Cancer Med. 8:7161–7173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He X, Sheng J, Yu W, Wang K, Zhu S and Liu
Q: LncRNA MIR155HG promotes temozolomide resistance by activating
the Wnt/β-catenin pathway via binding to PTBP1 in glioma. Cell Mol
Neurobiol. 41:1271–1284. 2021. View Article : Google Scholar
|
|
65
|
Li C, Feng S and Chen L: MSC-AS1 knockdown
inhibits cell growth and temozolomide resistance by regulating
miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway. Mol Cell
Biochem. 476:699–713. 2021. View Article : Google Scholar :
|
|
66
|
Boustani MR, Mehrabi F, Yahaghi E,
Khoshnood RJ, Shahmohammadi M, Darian EK and Goudarzi PK: Somatic
CPEB4 and CPEB1 genes mutations spectrum on the prognostic
predictive accuracy in patients with high-grade glioma and their
clinical significance. J Neurol Sci. 363:80–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gu N, Wang X, Di Z, Xiong J, Ma Y, Yan Y,
Qian Y, Zhang Q and Yu J: Silencing lncRNA FOXD2-AS1 inhibits
proliferation, migration, invasion and drug resistance of
drug-resistant glioma cells and promotes their apoptosis via
microRNA-98-5p/CPEB4 axis. Aging (Albany NY). 11:10266–10283. 2019.
View Article : Google Scholar
|
|
68
|
Su YK, Lin JW, Shih JW, Chuang HY, Fong
IH, Yeh CT and Lin CM: Targeting BC200/miR218-5p signaling axis for
overcoming temozolomide resistance and suppressing glioma stemness.
Cells. 9:18592020. View Article : Google Scholar :
|
|
69
|
Ding J, Zhang L, Chen S, Cao H, Xu C and
Wang X: lncRNA CCAT2 enhanced resistance of glioma cells against
chemodrugs by disturbing the normal function of miR-424. Onco
Targets Ther. 13:1431–1445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Z, Meng X, Wu P, Zha C, Han B, Li L,
Sun N, Qi T, Qin J, Zhang Y, et al: Glioblastoma cell-derived
lncRNA-containing exosomes induce microglia to produce complement
C5, promoting chemotherapy resistance. Cancer Immunol Res.
9:1383–1399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li B, Zhao H, Song J, Wang F and Chen M:
LINC00174 down-regulation decreases chemoresistance to temozolomide
in human glioma cells by regulating miR-138-5p/SOX9 axis. Hum Cell.
33:159–174. 2020. View Article : Google Scholar
|
|
72
|
Jia L, Tian Y, Chen Y and Zhang G: The
silencing of LncRNA-H19 decreases chemoresistance of human glioma
cells to temozolomide by suppressing epithelial-mesenchymal
transition via the Wnt/β-catenin pathway. Onco Targets Ther.
11:313–321. 2018. View Article : Google Scholar :
|
|
73
|
Jiang P, Wang P, Sun X, Yuan Z, Zhan R, Ma
X and Li W: Knockdown of long noncoding RNA H19 sensitizes human
glioma cells to temozolomide therapy. Onco Targets Ther.
9:3501–3509. 2016.PubMed/NCBI
|
|
74
|
Zhou L, Huang X, Zhang Y, Wang L, Li H and
Huang H: PSMG3-AS1 enhances glioma resistance to temozolomide via
stabilizing c-Myc in the nucleus. Brain Behav. 12:e25312022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang S, Guo S, Liang C and Lian M: Long
intergenic noncoding RNA 00021 promotes glioblastoma temozolomide
resistance by epigenetically silencing p21 through Notch pathway.
IUBMB Life. 72:1747–1756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cui B, Li B, Liu Q and Cui Y: lncRNA CCAT1
promotes glioma tumorigenesis by sponging miR-181b. J Cell Biochem.
118:4548–4557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang Y, Yi K, Liu X, Tan Y, Jin W, Li Y,
Zhou J, Wang F and Kang C: HOTAIR up-regulation activates NF-κB to
induce immunoescape in gliomas. Front Immunol. 12:7854632021.
View Article : Google Scholar
|
|
78
|
Wang W, Han S, Gao W, Feng Y, Li K and Wu
D: Long noncoding RNA KCNQ1OT1 confers gliomas resistance to
temozolomide and enhances cell growth by retrieving PIM1 from
miR-761. Cell Mol Neurobiol. 42:695–708. 2022. View Article : Google Scholar
|
|
79
|
Wang Y, Shan A, Zhou Z, Li W, Xie L, Du B
and Lei B: LncRNA TCONS_00004099-derived microRNA regulates
oncogenesis through PTPRF in gliomas. Ann Transl Med. 9:10232021.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dong ZQ, Guo ZY and Xie J: The lncRNA
EGFR-AS1 is linked to migration, invasion and apoptosis in glioma
cells by targeting miR-133b/RACK1. Biomed Pharmacother.
118:1092922019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen M, Cheng Y, Yuan Z, Wang F, Yang L
and Zhao H: NCK1-AS1 increases drug resistance of glioma cells to
temozolomide by modulating miR-137/TRIM24. Cancer Biother
Radiopharm. 35:101–108. 2020. View Article : Google Scholar
|
|
82
|
Lu Y, Tian M, Liu J and Wang K: LINC00511
facilitates temozolomide resistance of glioblastoma cells via
sponging miR-126-5p and activating Wnt/β-catenin signaling. J
Biochem Mol Toxicol. 35:e228482021. View Article : Google Scholar
|
|
83
|
Tomar VS, Patil V and Somasundaram K:
Temozolomide induces activation of Wnt/β-catenin signaling in
glioma cells via PI3K/Akt pathway: Implications in glioma therapy.
Cell Biol Toxicol. 36:273–278. 2020. View Article : Google Scholar
|
|
84
|
Liu H, Liu Z, Jiang B, Peng R, Ma Z and Lu
J: SOX9 overexpression promotes glioma metastasis via Wnt/β-catenin
signaling. Cell Biochem Biophys. 73:205–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen Q, Cai J, Wang Q, Wang Y, Liu M, Yang
J, Zhou J, Kang C, Li M and Jiang C: Long noncoding RNA NEAT1,
regulated by the EGFR pathway, contributes to glioblastoma
progression through the WNT/β-catenin pathway by scaffolding EZH2.
Clin Cancer Res. 24:684–695. 2018. View Article : Google Scholar
|
|
87
|
Knizhnik AV, Roos WP, Nikolova T, Quiros
S, Tomaszowski KH, Christmann M and Kaina B: Survival and death
strategies in glioma cells: Autophagy, senescence and apoptosis
triggered by a single type of temozolomide-induced DNA damage. PLoS
One. 8:e556652013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Linder S, Wiesner C and Himmel M:
Degrading devices: Invadosomes in proteolytic cell invasion. Annu
Rev Cell Dev Biol. 27:185–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ulasov IV, Mijanovic O, Savchuk S,
Gonzalez-Buendia E, Sonabend A, Xiao T, Timashev P and Lesniak MS:
TMZ regulates GBM stemness via MMP14-DLL4-Notch3 pathway. Int J
Cancer. 146:2218–2228. 2020. View Article : Google Scholar
|
|
90
|
Wen Q, Chen Z, Chen Z, Chen J, Wang R,
Huang C and Yuan W: EphA2 affects the sensitivity of oxaliplatin by
inducing EMT in oxaliplatin-resistant gastric cancer cells.
Oncotarget. 8:47998–48011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gaianigo N, Melisi D and Carbone C: EMT
and treatment resistance in pancreatic cancer. Cancers (Basel).
9:1222017. View Article : Google Scholar
|
|
92
|
Peng F, Fan H, Li S, Peng C and Pan X:
MicroRNAs in epithelial-mesenchymal transition process of cancer:
potential targets for chemotherapy. Int J Mol Sci. 22:75262021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li Z, Li M, Xia P and Lu Z: HOTTIP
mediated therapy resistance in glioma cells involves regulation of
EMT-related miR-10b. Front Oncol. 12:8735612022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Loilome W, Joshi AD, ap Rhys CM,
Piccirillo S, Vescovi AL, Gallia GL and Riggins GJ: Glioblastoma
cell growth is suppressed by disruption of fibroblast growth factor
pathway signaling. J Neurooncol. 94:359–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Snuderl M, Fazlollahi L, Le LP, Nitta M,
Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD,
Betensky RA, et al: Mosaic amplification of multiple receptor
tyrosine kinase genes in glioblastoma. Cancer Cell. 20:810–817.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Raoof S, Ruddy D, Timonia D, Damon L,
Engelman J and Hata A: Abstract A142: Targeting FGFR to overcome
EMT-related resistance in EGFR-mutated non-small cell lung cancer.
Mol Cancer Ther. 17(1 Suppl): A1422018. View Article : Google Scholar
|
|
97
|
Zhang L, Zhang W, Li Y, Alvarez A, Li Z,
Wang Y, Song L, Lv D, Nakano I, Hu B, et al: SHP-2-upregulated ZEB1
is important for PDGFRα-driven glioma epithelial-mesenchymal
transition and invasion in mice and humans. Oncogene. 35:5641–5652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Brichkina A, Nguyen NT, Baskar R, Wee S,
Gunaratne J, Robinson RC and Bulavin DV: Proline isomerisation as a
novel regulatory mechanism for p38MAPK activation and functions.
Cell Death Differ. 23:1592–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metalloproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cong ZX, Wang HD, Zhou Y, Wang JW, Pan H,
Zhang DD, Zhang L and Zhu L: Temozolomide and irradiation combined
treatment-induced Nrf2 activation increases chemoradiation
sensitivity in human glioblastoma cells. J Neurooncol. 116:41–48.
2014. View Article : Google Scholar
|
|
101
|
Ma L, Liu J, Zhang X, Qi J, Yu W and Gu Y:
p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma
cells against TMZ. Med Oncol. 32:692015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Harder BG, Peng S, Sereduk CP, Sodoma AM,
Kitange GJ, Loftus JC, Sarkaria JN and Tran NL: Inhibition of
phosphatidylinositol 3-kinase by PX-866 suppresses
temozolomide-induced autophagy and promotes apoptosis in
glioblastoma cells. Mol Med. 25:492019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pridham KJ, Shah F, Hutchings KR, Sheng
KL, Guo S, Liu M, Kanabur P, Lamouille S, Lewis G, Morales M, et
al: Connexin 43 confers chemoresistance through activating PI3K.
Oncogenesis. 11:22022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zając A, Sumorek-Wiadro J, Langner E,
Wertel I, Maciejczyk A, Pawlikowska-Pawlęga B, Pawelec J, Wasiak M,
Hułas-Stasiak M, Bądziul D, et al: Involvement of PI3K pathway in
glioma cell resistance to temozolomide treatment. Int J Mol Sci.
22:51552021. View Article : Google Scholar
|
|
106
|
Zhang LH, Yin AA, Cheng JX, Huang HY, Li
XM, Zhang YQ, Han N and Zhang X: TRIM24 promotes glioma progression
and enhances chemoresistance through activation of the PI3K/Akt
signaling pathway. Oncogene. 34:600–610. 2015. View Article : Google Scholar
|
|
107
|
Cao X, Hou J, An Q, Assaraf YG and Wang X:
Towards the overcoming of anticancer drug resistance mediated by
p53 mutations. Drug Resist Updat. 49:1006712020. View Article : Google Scholar
|
|
108
|
Hientz K, Mohr A, Bhakta-Guha D and
Efferth T: The role of p53 in cancer drug resistance and targeted
chemotherapy. Oncotarget. 8:8921–8946. 2017. View Article : Google Scholar :
|
|
109
|
Hirose Y, Berger MS and Pieper RO:
Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates
temozolomide-induced toxicity in a p53-independent manner in human
glioblastoma cells. Cancer Res. 61:5843–5849. 2001.PubMed/NCBI
|
|
110
|
Holder SL and Abdulkadir SA: PIM1 kinase
as a target in prostate cancer: Roles in tumorigenesis, castration
resistance, and docetaxel resistance. Curr Cancer Drug Targets.
14:105–114. 2014. View Article : Google Scholar
|
|
111
|
Wang BW, Huang CH, Liu LC, Cheng FJ, Wei
YL, Lin YM, Wang YF, Wei CT, Chen Y, Chen YJ and Huang WC: Pim1
kinase inhibitors exert anti-cancer activity against HER2-positive
breast cancer cells through downregulation of HER2. Front
Pharmacol. 12:6146732021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Trigg RM, Lee LC, Prokoph N, Jahangiri L,
Reynolds CP, Amos Burke GA, Probst NA, Han M, Matthews JD, Lim HK,
et al: The targetable kinase PIM1 drives ALK inhibitor resistance
in high-risk neuroblastoma independent of MYCN status. Nat Commun.
10:54282019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wein L and Loi S: Mechanisms of resistance
of chemotherapy in early-stage triple negative breast cancer
(TNBC). Breast. 34(Suppl 1): S27–S30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bobustuc GC, Kassam AB, Rovin RA, Jeudy S,
Smith JS, Isley B, Singh M, Paranjpe A, Srivenugopal KS and Konduri
SD: MGMT inhibition in ER positive breast cancer leads to CDC2,
TOP2A, AURKB, CDC20, KIF20A, Cyclin A2, cyclin B2, cyclin D1, ERα
and survivin inhibition and enhances response to temozolomide.
Oncotarget. 9:29727–29742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Song Z, Pan Y, Ling G, Wang S, Huang M,
Jiang X and Ke Y: Escape of U251 glioma cells from
temozolomide-induced senescence was modulated by CDK1/survivin
signaling. Am J Transl Res. 9:2163–2180. 2017.PubMed/NCBI
|
|
116
|
Reich TR, Schwarzenbach C, Vilar JB, Unger
S, Mühlhäusler F, Nikolova T, Poplawski A, Baymaz HI, Beli P,
Christmann M and Tomicic MT: Localization matters: Nuclear-trapped
survivin sensitizes glioblastoma cells to temozolomide by elevating
cellular senescence and impairing homologous recombination. Cell
Mol Life Sci. 78:5587–5604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li Z, Wu X, Zhao Y, Xiao Y, Zhao Y, Zhang
T, Li H, Sha F, Wang Y, Deng L and Ma X: Clinical benefit of
neoadjuvant anti-PD-1/PD-L1 utilization among different tumors.
MedComm (2020). 2:60–68. 2021.
|
|
118
|
Zhou Y, Miao J, Wu H, Tang H, Kuang J,
Zhou X, Peng Y, Hu D, Shi D, Deng W, et al: PD-1 and PD-L1
expression in 132 recurrent nasopharyngeal carcinoma: The
correlation with anemia and outcomes. Oncotarget. 8:51210–51223.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Qin T, Zeng YD, Qin G, Xu F, Lu JB, Fang
WF, Xue C, Zhan JH, Zhang XK, Zheng QF, et al: High PD-L1
expression was associated with poor prognosis in 870 Chinese
patients with breast cancer. Oncotarget. 6:33972–33981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kathuria H, Millien G, McNally L, Gower
AC, Tagne JB, Cao Y and Ramirez MI: NKX21-AS1 negatively regulates
CD274/PD-L1, cell-cell interaction genes, and limits human lung
carcinoma cell migration. Sci Rep. 8:144182018. View Article : Google Scholar
|
|
122
|
Tian Y, Li L, Lin G, Wang Y, Wang L, Zhao
Q, Hu Y, Yong H, Wan Y and Zhang Y: lncRNA SNHG14 promotes
oncogenesis and immune evasion in diffuse large-B-cell lymphoma by
sequestering miR-152-3p. Leuk Lymphoma. 62:1574–1584. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Dang S, Malik A, Chen J, Qu J, Yin K, Cui
L and Gu M: LncRNA SNHG15 contributes to immuno-escape of gastric
cancer through targeting miR141/PD-L1. Onco Targets Ther.
13:8547–8556. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhou WY, Zhang MM, Liu C, Kang Y, Wang JO
and Yang XH: Long noncoding RNA LINC00473 drives the progression of
pancreatic cancer via upregulating programmed death-ligand 1 by
sponging microRNA-195-5p. J Cell Physiol. 234:23176–23189. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Fan F, Chen K, Lu X, Li A, Liu C and Wu B:
Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging
hsa-miR-194-5p induces immunosuppression in hepatocellular
carcinoma. Hepatol Int. 15:444–458. 2021. View Article : Google Scholar
|
|
126
|
Peng L, Chen Y, Ou Q, Wang X and Tang N:
LncRNA MIAT correlates with immune infiltrates and drug reactions
in hepatocellular carcinoma. Int Immunopharmacol. 89:1070712020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Mineo M, Lyons SM, Zdioruk M, von
Spreckelsen N, Ferrer-Luna R, Ito H, Alayo QA, Kharel P, Giantini
Larsen A, Fan WY, et al: Tumor interferon signaling is regulated by
a lncRNA INCR1 transcribed from the PD-L1 locus. Mol Cell.
78:1207–1223.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wagle N, Nguyen M, Carrillo J, Truong J,
Dobrawa L and Kesari S: Characterization of molecular pathways for
targeting therapy in glioblastoma. Chin Clin Oncol. 9:772020.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Miyazaki T, Ishikawa E, Matsuda M, Sugii
N, Kohzuki H, Akutsu H, Sakamoto N, Takano S and Matsumura A:
Infiltration of CD163-positive macrophages in glioma tissues after
treatment with anti-PD-L1 antibody and role of PI3Kγ inhibitor as a
combination therapy with anti-PD-L1 antibody in in vivo model using
temozolomide-resistant murine glioma-initiating cells. Brain Tumor
Pathol. 37:41–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Roth P, Valavanis A and Weller M:
Long-term control and partial remission after initial
pseudoprogression of glioblastoma by anti-PD-1 treatment with
nivolumab. Neuro Oncol. 19:454–456. 2017.PubMed/NCBI
|
|
131
|
Zeng J, See AP, Phallen J, Jackson CM,
Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E,
et al: Anti-PD-1 blockade and stereotactic radiation produce
long-term survival in mice with intracranial gliomas. Int J Radiat
Oncol Biol Phys. 86:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Jan CI, Tsai WC, Harn HJ, Shyu WC, Liu MC,
Lu HM, Chiu SC and Cho DY: Predictors of response to autologous
dendritic cell therapy in glioblastoma multiforme. Front Immunol.
9:7272018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Vargas-Toscano A, Nickel AC, Li G, Kamp
MA, Muhammad S, Leprivier G, Fritsche E, Barker RA, Sabel M,
Steiger HJ, et al: Rapalink-1 targets glioblastoma stem cells and
acts synergistically with tumor treating fields to reduce
resistance against temozolomide. Cancers (Basel). 12:38592020.
View Article : Google Scholar
|
|
135
|
Zou Y, Chen M, Zhang S, Miao Z, Wang J, Lu
X and Zhao X: TRPC5-induced autophagy promotes the TMZ-resistance
of glioma cells via the CAMMKβ/AMPKα/mTOR pathway. Oncol Rep.
41:3413–3423. 2019.PubMed/NCBI
|
|
136
|
Jiang C, Shen F, Du J, Fang X, Li X, Su J,
Wang X, Huang X and Liu Z: Upregulation of CASC2 sensitized glioma
to temozolomide cytotoxicity through autophagy inhibition by
sponging miR-193a-5p and regulating mTOR expression. Biomed
Pharmacother. 97:844–850. 2018. View Article : Google Scholar
|
|
137
|
Liu Q, Yu W, Zhu S, Cheng K, Xu H, Lv Y,
Long X, Ma L, Huang J, Sun S and Wang K: Long noncoding RNA GAS5
regulates the proliferation, migration, and invasion of glioma
cells by negatively regulating miR-18a-5p. J Cell Physiol.
234:757–768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhao X, Liu Y, Zheng J, Liu X, Chen J, Liu
L, Wang P and Xue Y: GAS5 suppresses malignancy of human glioma
stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim Biophys
Acta Mol Cell Res. 1864:1605–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huo JF and Chen XB: Long noncoding RNA
growth arrest-specific 5 facilitates glioma cell sensitivity to
cisplatin by suppressing excessive autophagy in an mTOR-dependent
manner. J Cell Biochem. 120:6127–6136. 2019. View Article : Google Scholar
|
|
140
|
Li G, Cai Y, Wang C, Huang M and Chen J:
LncRNA GAS5 regulates the proliferation, migration, invasion and
apoptosis of brain glioma cells through targeting GSTM3 expression.
The effect of LncRNA GAS5 on glioma cells. J Neurooncol.
143:525–536. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yan Y, Xu Z, Dai S, Qian L, Sun L and Gong
Z: Targeting autophagy to sensitive glioma to temozolomide
treatment. J Exp Clin Cancer Res. 35:232016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: LncRNA CASC2 interacts with miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Jing H and Lee S: NF-κB in cellular
senescence and cancer treatment. Mol Cells. 37:189–195. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sánchez Y, Segura V, Marín-Béjar O, Athie
A, Marchese FP, González J, Bujanda L, Guo S, Matheu A and Huarte
M: Genome-wide analysis of the human p53 transcriptional network
unveils a lncRNA tumour suppressor signature. Nat Commun.
5:58122014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Torres-Bayona S, Aldaz P, Auzmendi-Iriarte
J, Saenz-Antoñanzas A, Garcia I, Arrazola M, Gerovska D, Undabeitia
J, Querejeta A, Egaña L, et al: PR-LncRNA signature regulates
glioma cell activity through expression of SOX factors. Sci Rep.
8:127462018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ding H, Cui L and Wang C: Long noncoding
RNA LIFR-AS1 suppresses proliferation, migration and invasion and
promotes apoptosis through modulating miR-4262/NF-κB pathway in
glioma. Neurol Res. 43:210–219. 2021. View Article : Google Scholar
|
|
147
|
Li XT, Li JC, Feng M, Zhou YX and Du ZW:
Novel lncRNA-ZNF281 regulates cell growth, stemness and invasion of
glioma stem-like U251s cells. Neoplasma. 66:118–127. 2019.
View Article : Google Scholar
|
|
148
|
Weller M, Wick W, Aldape K, Brada M,
Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and
Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yang W and Gao Y: Translesion and repair
DNA polymerases: Diverse structure and mechanism. Annu Rev Biochem.
87:239–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bailly V, Lamb J, Sung P, Prakash S and
Prakash L: Specific complex formation between yeast RAD6 and RAD18
proteins: A potential mechanism for targeting RAD6
ubiquitin-conjugating activity to DNA damage sites. Genes Dev.
8:811–820. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wojtaszek JL, Chatterjee N, Najeeb J,
Ramos A, Lee M, Bian K, Xue JY, Fenton BA, Park H, Li D, et al: A
small molecule targeting mutagenic translesion synthesis improves
chemotherapy. Cell. 178:152–159.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Peng C, Chen Z, Wang S, Wang HW, Qiu W,
Zhao L, Xu R, Luo H, Chen Y, Chen D, et al: The error-prone DNA
polymerase κ promotes temozolomide resistance in glioblastoma
through Rad17-dependent activation of ATR-Chk1 signaling. Cancer
Res. 76:2340–2353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Vassel FM, Bian K, Walker GC and Hemann
MT: Rev7 loss alters cisplatin response and increases drug efficacy
in chemotherapy-resistant lung cancer. Proc Natl Acad Sci USA.
117:28922–28924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wu B, Wang H, Zhang L, Sun C, Li H, Jiang
C and Liu X: High expression of RAD18 in glioma induces
radiotherapy resistance via down-regulating P53 expression. Biomed
Pharmacother. 112:1085552019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Rezaei O, Tamizkar KH, Sharifi G, Taheri M
and Ghafouri-Fard S: Emerging role of long non-coding RNAs in the
pathobiology of glioblastoma. Front Oncol. 10:6258842021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Luo J, Bai R, Liu Y, Bi H, Shi X and Qu C:
Long non-coding RNA ATXN8OS promotes ferroptosis and inhibits the
temozolomide-resistance of gliomas through the ADAR/GLS2 pathway.
Brain Res Bull. 186:27–37. 2022.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gao XY, Zang J, Zheng MH, Zhang YF, Yue
KY, Cao XL, Cao Y, Li XX, Han H, Jiang XF and Liang L: Temozolomide
treatment induces HMGB1 to promote the formation of glioma stem
cells via the TLR2/NEAT1/Wnt pathway in glioblastoma. Front Cell
Dev Biol. 9:6208832021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang C, Chen Y, Wang Y, Liu X, Liu Y, Li
Y, Chen H, Fan C, Wu D and Yang J: Inhibition of COX-2, mPGES-1 and
CYP4A by isoliquiritigenin blocks the angiogenic Akt signaling in
glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J Exp
Clin Cancer Res. 38:3712019. View Article : Google Scholar : PubMed/NCBI
|