Introduction
Adrenocortical carcinoma (ACC) is a rare, yet highly
aggressive tumor type (1). In
addition to gross genomic alterations, mutational events in several
key genes involved in the β-catenin pathway (e.g., CTNNB1
and ZNRF3), the p53/Rb pathway (e.g., TP53,
CDKN2A and RB1) and chromatin remodeling (e.g.,
MEN1, TERT and DAXX) are associated with ACC
tumorigenesis (1-3). The overexpression of insulin-like
growth factor (IGF) II and the IGF2 gene is observed in the
majority of ACC; however, this does not occur in adrenocortical
adenoma (ACA) or normal adrenal glands (4-6).
The IGF2 gene is located within 90 kb from
the H19 gene in chromosomal region 11p15.5 (7). H19 produces an untranslated,
yet spliced and polyadenylated transcript with possible tumor
suppressor function (8), and
IGF2 encodes the growth factor, IGFII. The IGF2-H19
locus is subjected to parental imprinting, which is frequently lost
in cancer by the loss of imprinting, leading to the overexpression
of IGF2/IGFII (9-11). Furthermore, IGFII has been reported
to promote malignant transformation in the mammary gland in
vivo (12). However, it has
not been found to exert an effect on the tumor phenotype in
vitro (13), and only mildly
contributes to the development of ACC in vivo (14,15).
Given that IGFII acts on the IGF1 receptor (IGF1R), the application
of an IGF1R blocker has been attempted in anticancer therapy to
counteract IGFII signaling in ACC (16-21).
In ACC, H19 expression has been reported to be decreased as
compared to that in ACA (6), while
IGF2/IGFII expression is known to be markedly increased in
the vast majority of ACC cases (4,6,22-24).
MicroRNAs (miRNAs/miRs) are short non-coding RNAs
that generally suppress the expression of target genes by mRNA
degradation or translational repression (25). Three miRNAs are known to be
transcribed from the IGF2-H19 locus, i.e., miR-483-3p
and miR-483-5p from IGF2 intron 7, and miR-675
from H19. miR-483-3p is overexpressed in different
tumor types, including ACC (13,26-28).
Furthermore, the p53 upregulated modulator of apoptosis (PUMA) is a
target of miR-483-3p, and the inhibition of
miR-483-3p inhibits proliferation and increases apoptosis
in vitro, and tumorigenicity in vivo (13,27).
miR-483-5p is also overexpressed in ACC (26-29)
and their inhibition in vitro reduces cell growth, although
it does not affect the apoptosis of ACC cells (27). On the other hand, miR-483-5p
has been shown to regulate the N-Myc downstream-regulated gene 2
and promote the invasion of ACC cells (30). Notably, miR-483-5p is
detectable in serum samples of patients with ACC, and its high
expression level is associated with a degree of malignancy and a
poor survival (31-33). The overexpression of miR-675
has been reported in various types of cancer, e.g. gastric,
colorectal, esophageal and breast cancer (34-37);
however, the decreased expression has also been observed in
non-small cell lung cancer (38).
In adrenocortical tumors, miR-675 has only been analyzed in
a small cohort of 13 patients (4 patients with ACC and 9 patients
with ACA), while the significance of its deregulation in ACC
remains to be investigated (39).
The present study aimed to comprehensively
characterize the expression of RNAs and miRNAs generated from the
IGF2-H19 locus in adrenocortical tissues and associate their
expression levels with global protein expression profiles in ACA
and ACC. The analysis revealed the expression of several proteins
involved in mitochondrial respiratory complexes that inversely
correlated with the miR-483-5p level. Functionally,
miR-483-5p was demonstrated to regulate the expression of
the NADH:ubiquinone oxidoreductase subunit C1 (NDUFC1) and the
mitochondrial oxygen consumption rate.
Materials and methods
Tissue material
Fresh-frozen tumor tissues were obtained from the
Karolinska University Hospital Biobank for patients treated
surgically for ACC (n=35) or ACA (n=43) between 1986 and 2010.
Tumors were classified according to the WHO criteria (40). In addition, normal adrenal gland
samples were obtained from patients undergoing nephrectomy for
other reasons, or from patients with adrenocortical tumors, and
histologically verified as non-malignant. The samples were obtained
with informed consent and the study of the tissue material was
approved by the Ethical Committee of the Karolinska University
Hospital.
The clinical information for all cases is presented
in Table SI. The screening series
included 6 cases with ACA and 8 cases with ACC used for proteomics
analysis in a previous study by the authors (41). An extended series of samples from
nine normal adrenal glands, 43 ACA and 29 ACC (30 samples) was used
for the reverse transcription-quantitative PCR (RT-qPCR) analysis
of RNAs and miRNAs. The results from the quantification analyses of
miR-483-3p and miR-483-5p have been previously
published for most cases (27).
Additionally, samples from 13 normal adrenal glands, 25 ACA and 25
ACC (29 samples) were used for western blot analyses. The ACA and
ACC cases included in the different analyses are presented in
Table SI.
Data analysis from TCGA and
genotype-tissue expression (GTEx)
Comparisons of IGF2 or H19 RNA
expression levels between ACCs and normal adrenal glands, and
principal component analysis (PCA) were performed in Gene
Expression Profiling Interactive Analysis [GEPIA; (42)]. The RNA expression levels were
based on the RNA sequencing expression data of 77 ACC cases from
The Cancer Genome Atlas (TCGA) and 128 normal adrenal glands from
GTEx. PCA was performed using the 46 genes whose proteins inversely
correlated with miR-483-5p and significantly differentially
expressed between ACA and ACC (P<0.05, Table SII). Correlations between the
expression of IGF2 and H19, IGF2 and
miR-483-3p or miR-483-5p, H19 and
miR-675 were assessed in the StarBase Pan-Cancer Analysis
Platform (43). This platform
includes RNA and miRNA sequencing data of 79 ACC samples from
TCGA.
Cell line and cell culture
All in vitro cell assays were performed using
the NCI-H295R ACC cell line purchased from the American Type
Culture Collection (cat. no. CRL-2128; ATCC). The cells were
maintained in DMEM:F12 medium (cat. no. 31330038; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 2.5% NuSerum Growth
medium (Corning 355100; Thermo Fisher Scientific, Inc.) and 1%
insulin-transferrin-selenium basal medium supplement (cat. no.
41400045; Thermo Fisher Scientific, Inc.). The cells were incubated
at 37°C in a humidified CO2 incubator. The authenticity
of the cell line was re-verified prior to the experiments by
genotyping of short tandem repeats (STRs) performed by the National
Genomics Infrastructure-Uppsala (SciLifeLab, Uppsala University,
Uppsala, Sweden). The NCI-H295R cell line is the only human ACC
cell line that is capable of secreting major adrenocortical
steroids (44), representing the
cellular origin of ACC cells, and it is the most extensively used
cell line in in vitro models of ACC.
RT-qPCR analysis
The expression levels of mature miRNAs and RNAs were
evaluated using RT-qPCR with an Applied Biosystems 7900HT Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). cDNA for miRNA analysis was synthesized from 25 ng total RNA
using the TaqMan MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). miRNA assays for
miR-483-3p (ID 002339), miR-483-5p (ID 002338) and
miR-675 (ID 002005) were purchased from Applied Biosystems
(Thermo Fisher Scientific, Inc.) and normalization was performed
using RNU6B (ID 001093). The high Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was adopted to generate cDNA from 100 ng total RNA for RNA
expression analysis and specific TaqMan probes targeting
IGF2 (ID 00277496_s1) and H19 (ID 00399293_g1) were
used for RT-qPCR. Normalization was performed using 18S (ID
99999901). All TaqMan probes were labeled with FAM-MGB (Applied
Biosystems); the reverse transcription and qPCR conditions followed
the manufacturer's instructions. All reactions were performed in
triplicate and the relative expression levels were determined using
the ΔΔCq method and reported as 2−ΔΔCq
(45).
Proteomics data analysis
Proteomics profiling for 6 cases of ACA and 8 cases
of ACC in the screening series has been described in a previous
study by the authors (41). The
protein expression levels of the screening series were correlated
with their corresponding IGF2, H19,
miR-483-3p, miR-483-5p and miR-675 transcripts
using Pearson's correlation analysis. The mRNA targets of
miR-483-3p, miR-483-5p and miR-675 were
predicted using TargetScan 7.1 (https://www.targetscan.org/vert_71/). Ingenuity
pathway analysis of complex 'omics' data (IPA; IPA version
14400082; Ingenuity® Systems, www.
ingenuity.com) was employed to assess the theoretical
interactions and cellular networks of the proteins that correlated
with IGF2.
Gene ontology (GO) enrichment
analysis
The gene signature corresponding to the 46 proteins
that inversely correlated with miR-483-5p, and significantly
differentially expressed between ACA and ACC in the proteomics
cohort (Table SII) was annotated
based on biological processes using GO enrichment analysis
(http://geneontology.org/). The analysis was based
on the PANTHER overrepresentation test (released on August 3, 2019;
http://www.pantherdb.org/) and the GO database
(released on February 2, 2019). P-values <0.05 following the
Bonferroni correction were considered to indicate statistically
significant differences.
Transfections with miRNA inhibitors
Transfections were performed by suspending
3×106 NCI-H295R cells in 100 µl Ingenio solution
(Mirus Bio) together with 100 pmol mirVana miR-483-5p
inhibitor, miR-483-3p inhibitor or Negative Control #1
(MH12629, MH12478 and 4464076, respectively; Thermo Fisher
Scientific, Inc.) in Ingenio cuvettes (MIR50121; Mirus Bio). The
cells were electroporated using the program T-20 of the Amaxa
Nucleofector device (Lonza Group, Ltd.) and then transferred into a
culture plate containing pre-warmed complete DMEM:F12 medium. The
plate was incubated at 37°C in a humidified CO2
incubator. The cell culture medium was replaced at 24 h
post-transfection and harvested for metabolic profiling or western
blot analysis following 48 h of transfection. At least three
biological replicates were performed for each transfection. The
efficiency of miR-483-5p silencing was assessed using
RT-qPCR.
Real-time metabolic profiling
Mitochondrial and glycolytic functions were measured
using the Mito Stress Test and Glycolysis Stress Test, respectively
(Agilent Technologies, Inc.) using the Seahorse XFe24 Analyser
(Agilent Technologies, Inc.). The transfected cells were seeded at
1.5×105/well in 24-well plates (Seahorse XF24 V7 PS
Culture Microplates, Agilent Technologies, Inc.). The culture media
were replaced after 24 h and incubated for a further 24 h at 37°C.
Culture media were changed to the Seahorse XF Base Medium (Agilent
Technologies, Inc.) supplemented with 5.5 mM glucose, 1 mM
L-glutamine and 1 mM sodium pyruvate at pH 7.4 and incubated at
37°C with CO2 for 1 h. For the evaluation of
mitochondrial respiration, the oxygen consumption rate (OCR) was
measured at the basal level and following sequential loading with 1
µM oligomycin (ATP synthase inhibitor), 0.5 µM
carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP, an
uncoupler of oxidative phosphorylation), 1 µM rotenone and
antimycin A (complex I and III inhibitor, respectively). For the
assessment of glycolysis, the extracellular acidification rate
(ECAR) was analyzed at basal conditions and after sequential
loading with 1 µM oligomycin (inhibiting ATP synthase in the
mitochondria leading to enhanced glycolysis dependency), and 50 mM
2-deoxyglucose (2-DG, a competitive inhibitor of glucose). Both OCR
and ECAR were normalized to the total protein content in each well
as measured using the Bradford assay (Bio-Rad Laboratories,
Inc.).
Western blot analysis
Cell pellets or tissue samples were suspended in
RIPA lysis and extraction buffer (cat. no. 89901; Thermo Fisher
Scientific, Inc.) supplemented with 1 mM phenylmethanesulfonyl
fluoride (PMSF; Sigma-Aldrich; Merck KGaA), 1% cOmplete™ proteinase
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and 1% phosphatase
inhibitor cocktail 2 and 3 (Sigma-Aldrich; Merck KGaA). The lysates
were vortexed repeatedly and left on ice for 60 min or until
homogenization. The protein concentration was quantified using the
Bradford assay (Bio-Rad Laboratories). Denatured lysates (40
µg) were separated in 10% NuPAGE™ Bis-Tris denaturing
pre-cast gels (Invitrogen; Thermo Fisher Scientific, Inc.) and
transferred onto nitrocellulose membranes (cat. no. 88013, Thermo
Fisher Scientific, Inc.) at 4°C. The membranes were blocked in 5%
BSA or 1% non-fat dried milk (for total OXPHOS WB) in TBS
containing 0.1% Tween-20 (Sigma-Aldrich; Merck KGaA). The membranes
were then incubated overnight at 4°C with the primary antibodies,
anti-NDUFC1 (cat. no. PA5-68240; Thermo Fisher Scientific, Inc.) at
a 1:500 dilution and total OXPHOS WB Antibody Cocktail (ab110413,
Abcam) at a 1:250 dilution. Anti-β-actin (A1978, Sigma-Aldrich;
Merck KGaA) diluted to 1:2,500 was used for normalization purposes.
IRDye 800CW goat anti-mouse IgG (LI-COR Biosciences) at a 1:10,000
dilution or HRP-conjugated goat anti-rabbit IgG (cat. no. 31466,
Invitrogen; Thermo Fisher Scientific, Inc.) at a 1:2,000 dilution
or goat anti-mouse IgG (cat. no. 62-6520 Invitrogen; Thermo Fisher
Scientific, Inc.) at a 1:10,000 dilution were used as secondary
antibodies. Novex Sharp pre-stained protein standard (LC5800,
Thermo Fisher Scientific, Inc.) and Precision Plus Protein All Blue
protein standards were used as molecular weight markers.
Chemiluminescent signals were detected using the SuperSignal™ West
Femto Maximum Sensitivity Substrate (cat. no. 34096, Thermo Fisher
Scientific, Inc.) and the Odyssey Fc imaging system (LI-COR
Biosciences) and quantified using Image Studio Lite 5.2 software
(LI-COR Biosciences).
Statistical analyses
Statistica 10.0 (StatSoft, Inc., Tulsa, OK) or IBM
SPSS Statistics version 24.0 (IBM Corp., Armonk, NY) was used for
all statistical analyses, unless otherwise specified. Differences
in expression levels between two sample groups were calculated
using the Mann-Whitney U-test or the Student's t-test. One-way
ANOVA with post hoc Tukey's test was used to compare the three
transfection conditions for the metabolic assays. Correlations
between IGF2 and H19 and miRNA (miR-483-3p,
miR-483-5p and miR-675) expression levels were
assessed using Spearman's rank order correlation analysis.
Difference in NDUFC1 levels in transfection experiments were
evaluated using the Student's t-test. All analyses were two-tailed
and P-values <0.05 were considered to indicate statistically
significant differences.
Results
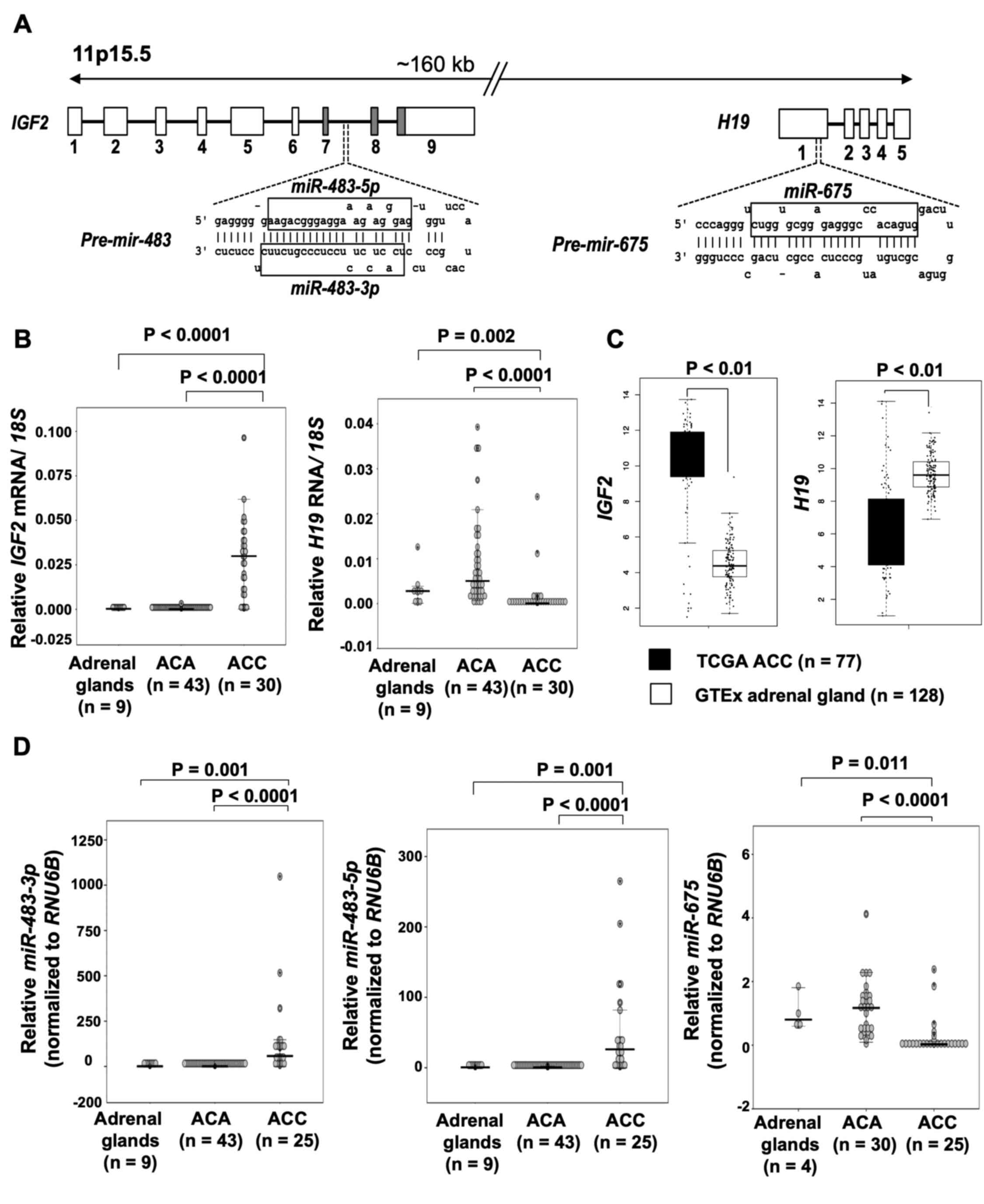
Expression of RNAs and miRNAs from the
IGF2-H19 locus
The RNA and miRNA transcripts generated from
IGF2 and H19 (Fig.
1A) were quantified using RT-qPCR in an extended series of
normal adrenal glands, ACAs and ACCs. The results are illustrated
in Fig. 1B-D. Elevated levels of
IGF2 and decreased levels of H19 were observed in the
majority of ACC tissues as compared to ACA tissues and normal
adrenals. Concordant with the findings in the present cohort, the
ACC samples (n=77) in TCGA dataset also exhibited higher levels of
IGF2 RNA and lower levels of H19, as compared to
normal adrenal glands from the GTEx (n=128) datasets.
miR-483-3p and miR-483-5p generated from IGF2
were both upregulated in ACC vs. ACA and normal adrenals, as
previously published for a subset of the cases (27). miR-675 transcribed from
H19 was underexpressed in ACC as compared to ACA and normal
adrenals.
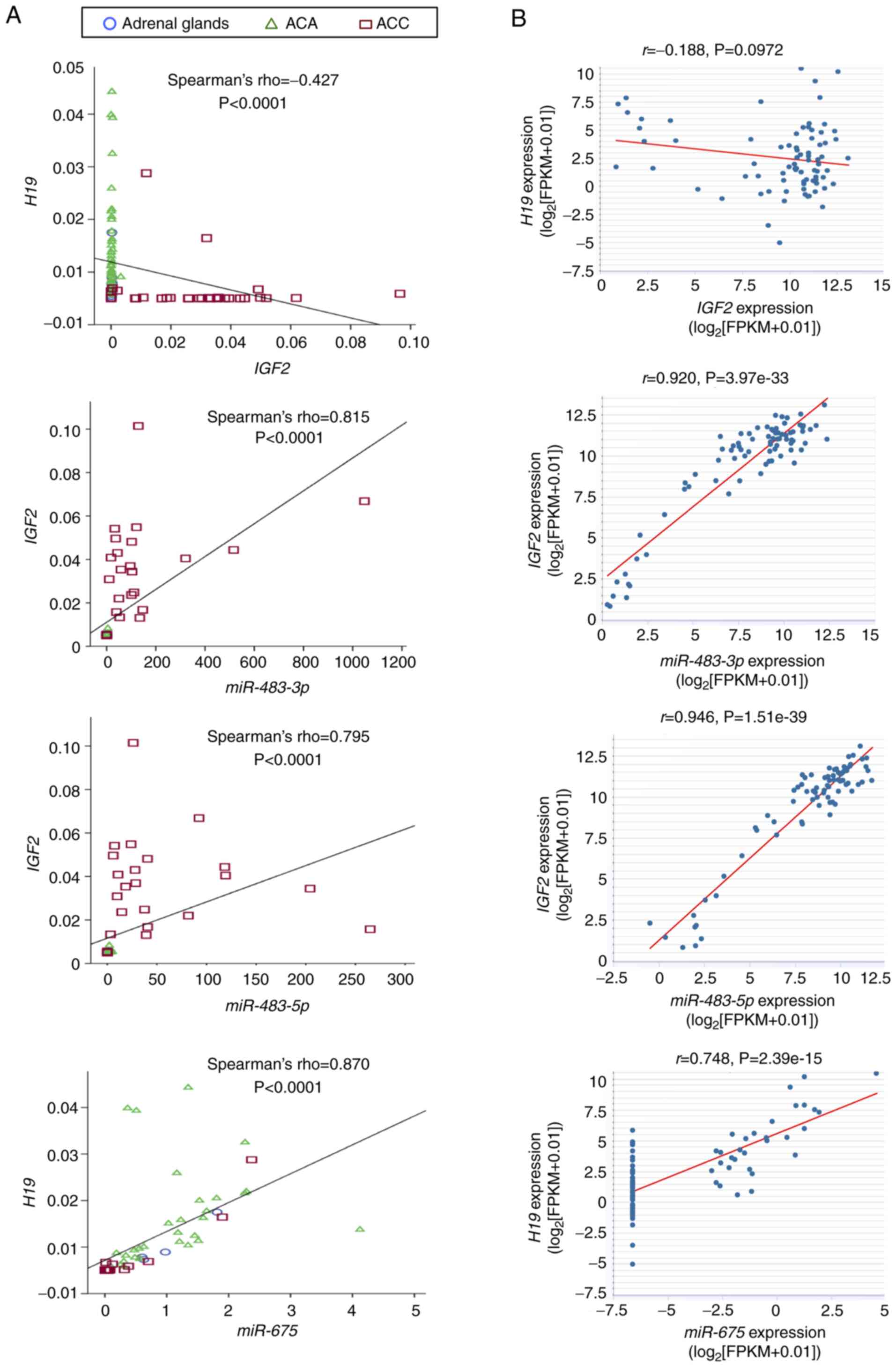
A comparison of the RNAs and miRNAs analyzed in the
extended series of normal adrenal glands, ACA and ACC revealed a
moderately inverse correlation between IGF2 and H19
(Fig. 2A). Strong positive
correlations were found between miR-483-3p and
miR-483-5p and their host IGF2, as well as between
miR-675 and its host H19 (Fig. 2A). Similar findings were
subsequently revealed in TCGA dataset with 79 ACC cases (Fig. 2B). A weak inverse correlation,
although not statistically significant, was observed between
H19 and IGF2. Strong positive correlations were
observed between IGF2 and miR-483-3p and
miR-483-5p, as well as between H19 and
miR-675.
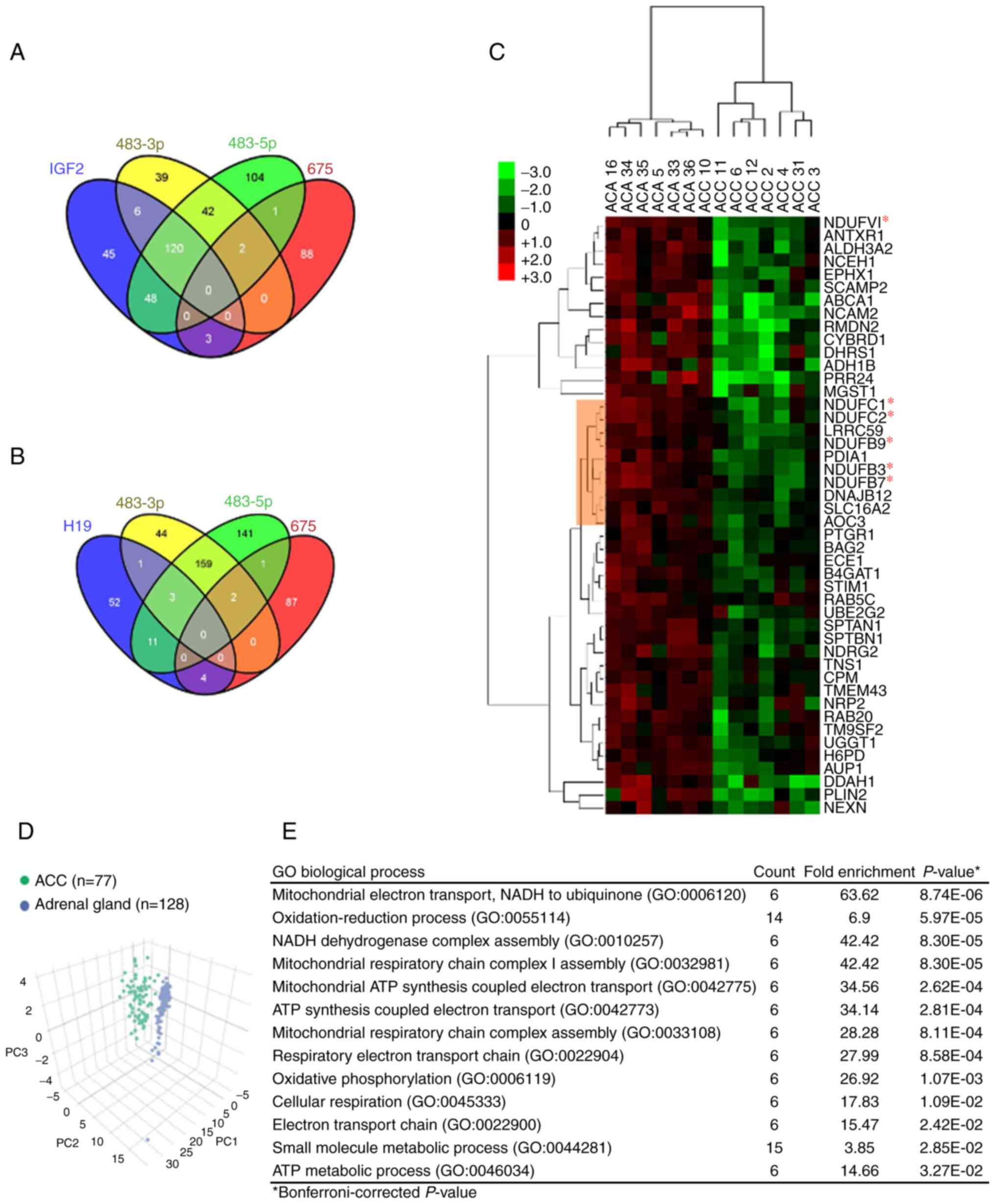
Comparative analysis of transcripts with
the proteome
Protein expression profiles determined using mass
spectrometry for the 6 cases of ACA and 8 cases of ACC in the
screening series (41) were
compared with the expression levels of the RNAs (IGF2 and
H19) and miRNAs (miR-483-3p, miR-483-5p and
miR-675). The numbers of proteins overlapping with the
various transcripts are illustrated in Venn diagrams in Fig. 3A and B. In total, six proteins were
associated with both IGF2 and miR-483-3p, 48 proteins
were associated with IGF2 and miR-483-5p, and 120
proteins were associated with IGF2 and both
miR-483-3p and miR-483-5p. Only four proteins were
associated with H19 and miR-675.
A comparison of the proteomics data with the
expression of IGF2 or H19 identified 222 proteins
that correlated with IGF2 (Table SIII) and 71 that correlated with
H19 (Table SIV). The
proteomics data were also investigated to identify proteins
exhibiting an inverse expression to the three miRNAs, since such
proteins would represent potential targets. This identified seven
proteins whose expression inversely correlated with that of
miR-483-3p, including one predicted target using TargetScan
7.1 (Table SV). For
miR-483-5p, 101 inversely correlated proteins were revealed
including nine predicted targets (Table SII). For miR-675, 11
inversely expressed proteins were identified, including one
predicted target (Table SVI).
Among the 101 proteins that were inversely
correlated with miR-483-5p, 46 of them were differentially
expressed between ACA and ACC based on the proteomics data
(Table SII). Based on these 46
protein expression patterns, all ACC cases (apart from ACC case no.
10) were clustered together and separated from the ACA cluster
(Fig. 3C). Using TCGA and GTEx
datasets, it was also observed that this 46-gene signature could
distinguish ACC from normal adrenal glands (Fig. 3D). GO analysis of these 46 genes
revealed a significant enrichment of biological processes related
to mitochondrial metabolism (Fig.
3E). Notably, six proteins (NDUFV1, NDUFC1, NDUFC2, NDUFB9,
NDUFB3 and NDUFB7) are subunits of the mitochondrial respiratory
chain complex I. Among these six subunits, only NDUFC1 is a
predicted target of miR-483-5p according to TargetScan
7.1.
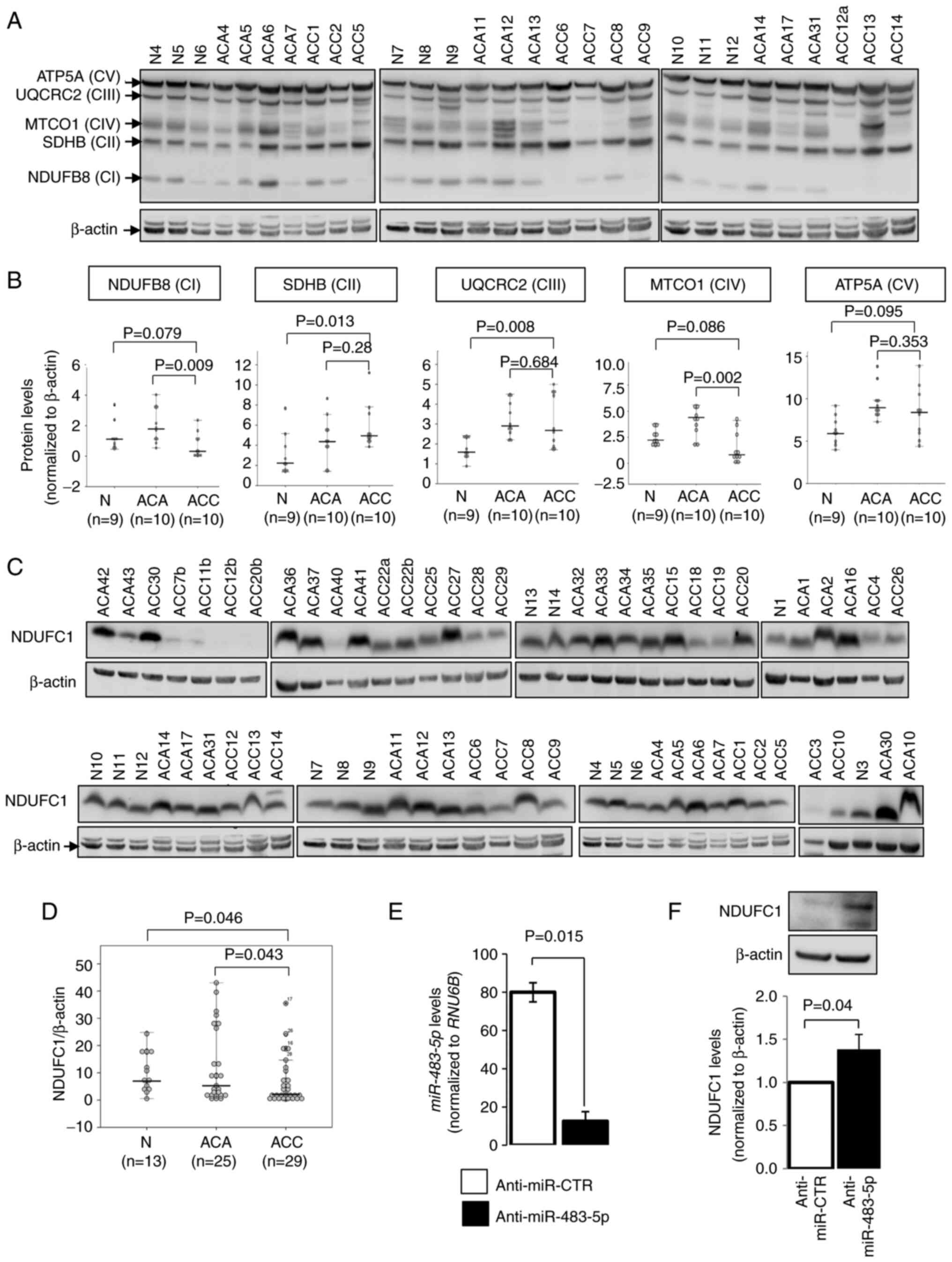
Protein expression of mitochondrial
respiratory complexes in adrenal cortical tissues
To further assess the association between
mitochondrial respiratory complexes and ACC, the expression levels
of mitochondrial respiratory complex I-V were examined in nine
adrenal glands, 10 ACA tissues and 10 ACC tissues using western
blot analysis, and the total OXPHOS antibody cocktail representing
each of the complexes (Fig. 4A).
Of note, complex I and IV were lower in ACC compared to ACA
(P=0.009 and P=0.002, respectively; Fig. 4B). On the other hand, complex II
and III were higher in ACC compared to adrenal glands (P=0.013 and
P=0.008, respectively), whereas they did not differ significantly
compared to ACA (Fig. 4B).
To validate the involvement of complex I in ACCs,
NDUFC1 protein expression was also evaluated in an extended series
of clinical samples, consisting of 13 normal adrenal glands, 25 ACA
and 29 ACC samples (from 25 patients with ACC), using western blot
analysis (Fig. 4C). Concordantly,
NDUFC1 expression was lower in ACC compared to ACA and normal
adrenals (P=0.043 and P=0.046, respectively; Fig. 4D). The inhibition of
miR-483-5p, using anti-miR-483-5p, in the NCI-H295R ACC cell
line increased NDUFC1 protein expression (Fig. 4E and F), suggesting a putative role
of miR-483-5p in mitochondrial respiratory activities.
Role of miR-483 in the cellular
metabolism of ACC cells
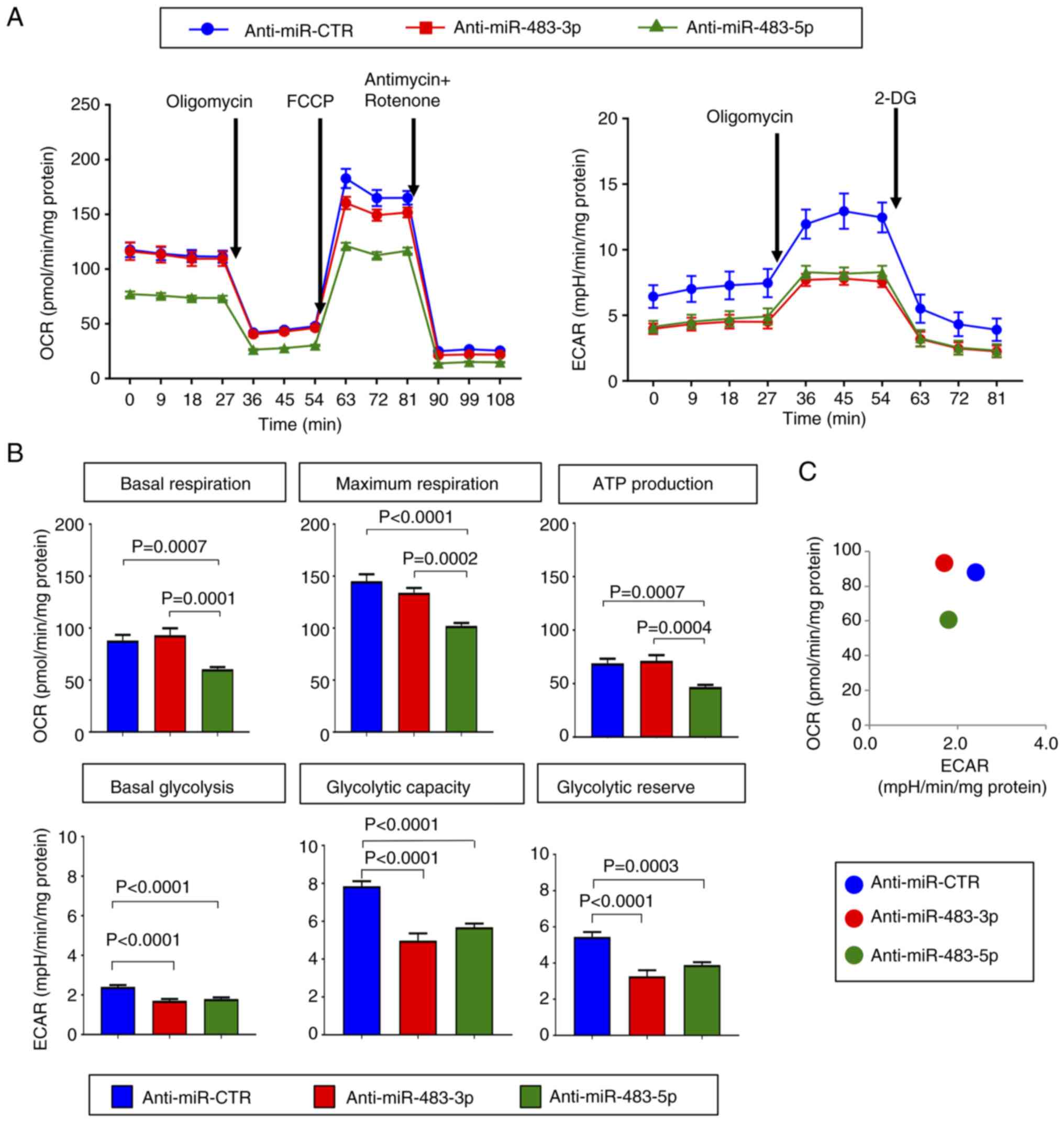
To further validate the effects of
miR-483-5p on metabolic activities, the mitochondrial OCR
and ECAR were measured using a Seahorse system. The OCR profile
exhibited a significant decrease in the basal and maximum
respiration upon the inhibition of miR-483-5p, but not that
of miR-483-3p, using anti-miR-483-5p and anti-miR-483-3p,
respectively (Fig. 5A and B).
Additionally, ATP production generated from the mitochondrial
respiration was also lower in the cells transfected with
anti-miR-483-5p, compared to those transfected with anti-miR-483-3p
or negative control-transfected cells (Fig. 5B).
The measurement of the ECAR, an indicator of
glycolysis, revealed a decrease in basal glycolysis, glycolytic
capacity and reserve in the anti-miR-483-5p-transfected cells.
Similar effects were also observed upon the inhibition of
miR-483-3p (Fig. 5A and B).
To obtain an overview of the bioenergetics profile, the OCR vs.
ECAR was plotted at basal conditions. As illustrated in Fig. 5C, the cells in which
miR-483-5p was inhibited were least energetic, as
demonstrated by lower respiration and glycolysis rates, whereas the
cells in which miR-483-3p was inhibited were less
glycolytic, but were similarly oxidative as the
anti-miR-CTR-transfected cells.
Discussion
Several studies have demonstrated that the
overexpression of IGF2 is a very frequent alteration in ACC,
which suggests a role for this growth factor in ACC tumor
development (4-6). The present study further analyzed
this alteration by measuring RNA and miRNA transcripts from the
IGF2 and H19 loci and associated these results with
global protein levels determined using proteomics. The present
study confirmed previous findings (4,5) that
IGF2 expression is elevated in ACC compared to normal
adrenal and to ACA. The higher transcription of IGF2 was, as
expected, paralleled by an increased expression of
miR-483-3p and miR-483-5p (hosted by IGF2) in
ACC. It was also demonstrated that H19 expression was
generally higher in ACA compared to normal adrenal, and lower in
ACC compared to ACA and normal adrenals. This observation
corroborates previous findings of low H19 levels in some
hormonally active ACC (24).
Furthermore, the expression of miR-675 (hosted by
H19) correlated with H19 levels in the present cohort
and in TCGA dataset.
In the ACC group, the concerted upregulation of
IGF2 together with miR-483-3p and miR-483-5p,
as well as the downregulation of H19 together with
miR-675 was observed. Each of these molecules could
potentially contribute to ACC development alone or cooperatively.
The present study then investigated protein expression profiles
[obtained using mass spectrometry (41)] of proteins from ACC and ACA, in
order to identify proteins that correlate with the transcription
from the H19-IGF2 locus. The correlation analysis of
IGF2 and H19 transcripts with the proteome took into
account both up- and downregulated proteins. Mass spectrometry
revealed a correlation between IGF2 mRNA and IGFII protein
levels, as well as an increased IGFII protein content in ACC
(Table SIII), demonstrating that
translation of increased IGF2 mRNAs indeed occur in ACC.
Among the 222 proteins overlapping with IGF2 mRNA
expression, 15 proteins (NDUFV1, ANTXR1, RAB20, PTGR1, ECE1, TNS1,
MGST1, UGGT1, H6PD, AOC3, TM9SF2, MGST2, SCAMP2, AUP1 and DNAJC16)
were significantly downregulated in ACC compared to ACA (Table SIII). One of these, NDUFV1, is
associated with mitochondrial oxidative phosphorylation. This
indicates that energy metabolism is an important marker for the ACC
phenotype.
The present study also applied IPA to the
IGF2 mRNA correlated proteins, to elucidate their
associations and ontology. The top two molecular and cellular
functions were energy metabolism and lipid metabolism. Further IPA
identified hepatocyte nuclear factor 4A and TP53 as the top two
upstream regulators, both of which are central to cancer (Table SVII). In addition, IPA suggested
seven of the molecules (COX17, NDUFV1, OGDH, PRDX3, TXN2, TXNRD2
and UQCRB) to be associated with mitochondrial dysfunction
(Table SVII). Notably, one of the
Weiss criteria for the classification of ACC is the presence of
<25% clear cells (46). Such
tumors instead have a majority of oxyphilic cells, which are
characterized by increased amounts of mitochondria (giving the
positive eosin staining). It is not known whether these
mitochondria are functional or whether they represent a
compensatory increase due to a defective mitochondrial function.
The present study also found 71 proteins that correlated with
H19 expression, and almost all exhibited a lower prevalence
in ACC vs. ACA (Table SIV). Of
the 71 identified proteins, 23 displayed significant differences
between ACC and ACA. Of note, the protein with the greatest
difference between ACC and ACA in association with H19
transcription is encoded by the potassium channel gene
KCNQ1, which is imprinted and associated with for example
Beckwith-Wiedemann syndrome (47).
Other notable proteins are two mitochondrial NDUF (NADH
dehydrogenase sub-complex) proteins, supporting the importance of
energy metabolism.
As it is assumed that the role of miRNAs is the
negative regulation of target genes, the present study analyzed the
inverse correlation of these with the proteome. In total, 11
proteins correlated inversely with miR-675, one of which was
predicted as a target. This apoptosis-related protein, tumor
necrosis factor alpha-induced protein 2 (TNFAIP2), is induced by
tumor necrosis factor-α and associated with various cancer types
(48-50). miR-483-5p exhibited an
inverse correlation with 101 proteins. By contrast, its companion
miR-483-3p only negatively correlated with seven proteins.
Of note, all except two of these were found in the list of proteins
inversely correlating with miR-483-5p. However, these two
proteins (H6PD and UBE2G2) were not predicted as targets using
TargetScan 7.1. The PUMA, a potential target of miR-483-3p
in ACC (27), was not detected
using mass spectrometry. Among the proteins inversely correlating
with miR-483-5p, a group of mitochondrial proteins/enzymes
may be noted, such as members of the NADH dehydrogenase complex, as
well as other mitochondrial molecules. In total, 46 of the proteins
with an inverse correlation with miR-483-5p were
differentially expressed between ACC and ACA. This signature was
also validated as a classifier for ACC in independent cohorts using
TCGA and GTEx datasets. GO analysis revealed the enrichment of
biological processes related to mitochondrial respiration. In
particular, six subunits of mitochondrial respiratory complex I
were downregulated in ACC. The decreased expression of
mitochondrial complex I was also validated in an extended series of
clinical samples using western blot analysis, suggesting the
deficiency of complex I in ACC. In line with these findings, Kimmel
et al (51) previously
demonstrated a poor oxygen uptake by the tumor mitochondria of the
rat ACC 494 model using the tricarboxylic acid cycle substrates
(α-ketoglutarate, malate and isocitrate), which led them to propose
that the tumor mitochondria could be deficiency in the flavoprotein
dehydrogeneases for NADH and NADPH oxidation. Notably, the effect
of several anti-ACC drugs (mitotane, niclosamide and ATR-101) is
also tightly linked to the disruption of mitochondrial function
(52-54). In addition, a deficiency in
mitochondrial respiratory complex I has also been demonstrated to
promote tumor progression in other tumor types, such as breast
cancer and hepatoma (55,56).
One of the mitochondrial respiratory complex I
subunits, NDUFC1, is also a predicted target of miR-483-5p.
The expression of this target was upregulated upon
miR-483-5p inhibition. The involvement of miR-483-5p
in mitochondrial respiration was also supported by the Mito Stress
Test using the Seahorse technology. Additionally, the inhibition of
miR-483-5p also reduced glycolysis, which led to a low
metabolic state resembling cellular quiescence. These results are
in line with the observations that the inhibition of
miR-483-5p had no effect on cell proliferation or apoptosis
in ACC (27,30). Although the target(s) of
miR-483-5p involved in glycolysis remains unknown, the
present study noted a common protein,
hexose-6-phosphate-dehydrogenase (H6PD), which inversely correlated
with both miR-483-3p and miR-483-5p. H6PD is an
enzyme that produces NAPDH in the endoplasmic reticulum for glucose
metabolism and glycolysis (57,58).
Even though this gene was not predicted as a target of
miR-483-3p and miR-483-5p by TargetScan 7.1, the
possibility of H6PD as a direct or indirect target of these miRNAs
has not yet been excluded.
The present data may be utilized to identify
differentially expressed molecules that may be developed into
diagnostic markers for the identification of ACC cases in the
absence of metastasis at the time of diagnosis. Furthermore, it may
potentially aid in the identification of those patients who could
benefit from adjuvant mitotane therapy (59,60).
The main findings of the present study were that correlations exist
between specific miRNA(s) and RNA(s) in the IGF2-H19
locus and the proteomes of ACC and ACA. The patterns of RNA
transcription from this locus form specific networks of protein
expression that appear to be associated with ACC. The present study
also reveals a link of the IGF2-H19 locus to energy
metabolism and deficiency of mitochondrial respiratory complex I in
ACC, suggesting the importance of mitochondrial dysfunction and
IGF2-H19 regulatory network in ACC development.
Supplementary Data
Availability of data and materials
The analyzed datasets presented in the present
study are available in the Gene Expression Profiling Interactive
Analysis (http://gepia.cancer-pku.cn/),
StarBase Pan-Cancer Analysis Platform (https://starbase.sysu.edu.cn/panCancer.php) and
ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org;
PXD000604).
Authors' contributions
PS, TJE, RB, WOL and CL conceived and designed the
study. PS, SC, HK, CX, RF, MoA, JG, HS and MaA performed the
experiments and analyzed the data. MK, AH, JZ and RB contributed to
the clinical materials and data. WOL and CL supervised the study,
and reviewed and approved the authenticity of all the raw data. All
authors critically reviewed, and have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The use of the tumor samples (Dnr 01-136; Dnr
2020-04226) and normal adrenals (Dnr 01-353; Dnr 2020-04226) in the
present study were approved by the Ethics Committee of Karolinska
Institutet (Dnr 01-136; Dnr 01-353) and by the Swedish Ethical
Review Authority (Dnr 2020-04226). Tissue samples were collected
with informed consent and was obtained from the patients prior to
surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Lisa Ånfalk
(Karolinska University Hospital) for assisting with the collection
of all tissue samples, and the Strategic Research Programme
Diabetes Facility for the Seahorse system.
Funding
The present study was supported by grants from the Swedish
Research Council (2021-03006), the Swedish Cancer Society (20 0843
and 20 0859), the Cancer Society in Stockholm (201223), the Gustav
V Jubilee Foundation (204103), the Stockholm County Council (RS
2019-1054) and Funds from Karolinska Institutet (2020-01851 and
2020-01768).
References
|
1
|
Assié G, Letouzé E, Fassnacht M, Jouinot
A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K,
René-Corail F, et al: Integrated genomic characterization of
adrenocortical carcinoma. Nat Genet. 46:607–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Juhlin CC, Goh G, Healy JM, Fonseca AL,
Scholl UI, Stenman A, Kunstman JW, Brown TC, Overton JD, Mane SM,
et al: Whole-exome sequencing characterizes the landscape of
somatic mutations and copy number alterations in adrenocortical
carcinoma. J Clin Endocrinol Metab. 100:E493–E502. 2015. View Article : Google Scholar
|
|
3
|
Zheng S, Cherniack AD, Dewal N, Moffitt
RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA,
Ciriello G, et al: Comprehensive pan-genomic characterization of
adrenocortical carcinoma. Cancer Cell. 29:723–736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boulle N, Logié A, Gicquel C, Perin L and
Le Bouc Y: Increased levels of insulin-like growth factor II
(IGF-II) and IGF-binding protein-2 are associated with malignancy
in sporadic adrenocortical tumors. J Clin Endocrinol Metab.
83:1713–1720. 1998.PubMed/NCBI
|
|
5
|
Gicquel C, Bertagna X, Schneid H,
Francillard-Leblond M, Luton JP, Girard F and Le Bouc Y:
Rearrangements at the 11p15 locus and overexpression of
insulin-like growth factor-II gene in sporadic adrenocortical
tumors. J Clin Endocrinol Metab. 78:1444–1453. 1994.PubMed/NCBI
|
|
6
|
Gicquel C, Raffin-Sanson ML, Gaston V,
Bertagna X, Plouin PF, Schlumberger M, Louvel A, Luton JP and Le
Bouc Y: Structural and functional abnormalities at 11p15 are
associated with the malignant phenotype in sporadic adrenocortical
tumors: Study on a series of 82 tumors. J Clin Endocrinol Metab.
82:2559–2565. 1997.PubMed/NCBI
|
|
7
|
Larsson C: Epigenetic aspects on therapy
development for gastroenteropancreatic neuroendocrine tumors.
Neuroendocrinology. 97:19–25. 2013. View Article : Google Scholar
|
|
8
|
Brannan CI, Dees EC, Ingram RS and
Tilghman SM: The product of the H19 gene may function as an RNA.
Mol Cell Biol. 10:28–36. 1990.PubMed/NCBI
|
|
9
|
Ogawa O, Eccles MR, Szeto J, McNoe LA, Yun
K, Maw MA, Smith PJ and Reeve AE: Relaxation of insulin-like growth
factor II gene imprinting implicated in Wilms' tumour. Nature.
362:749–751. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rainier S, Johnson LA, Dobry CJ, Ping AJ,
Grundy PE and Feinberg AP: Relaxation of imprinted genes in human
cancer. Nature. 362:747–749. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weksberg R, Shen DR, Fei YL, Song QL and
Squire J: Disruption of insulin-like growth factor 2 imprinting in
Beckwith-Wiedemann syndrome. Nat Genet. 5:143–150. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pravtcheva DD and Wise TL: Metastasizing
mammary carcinomas in H19 enhancers-Igf2 transgenic mice. J Exp
Zool. 281:43–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Veronese A, Lupini L, Consiglio J, Visone
R, Ferracin M, Fornari F, Zanesi N, Alder H, D'Elia G, Gramantieri
L, et al: Oncogenic role of miR-483-3p at the IGF2/483 locus.
Cancer Res. 70:3140–3149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drelon C, Berthon A, Ragazzon B, Tissier
F, Bandiera R, Sahut-Barnola I, de Joussineau C, Batisse-Lignier M,
Lefrancois-Martinez AM, Bertherat J, et al: Analysis of the role of
Igf2 in adrenal tumour development in transgenic mouse models. PLoS
One. 7:e441712012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heaton JH, Wood MA, Kim AC, Lima LO,
Barlaskar FM, Almeida MQ, Fragoso MC, Kuick R, Lerario AM, Simon
DP, et al: Progression to adrenocortical tumorigenesis in mice and
humans through insulin-like growth factor 2 and β-catenin. Am J
Pathol. 181:1017–1033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barlaskar FM, Spalding AC, Heaton JH,
Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E and Hammer GD:
Preclinical targeting of the type I insulin-like growth factor
receptor in adrenocortical carcinoma. J Clin Endocrinol Metab.
94:204–212. 2009. View Article : Google Scholar :
|
|
17
|
Fassnacht M, Berruti A, Baudin E, Demeure
MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL,
et al: Linsitinib (OSI-906) versus placebo for patients with
locally advanced or metastatic adrenocortical carcinoma: A
double-blind, randomised, phase 3 study. Lancet Oncol. 16:426–435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haluska P, Worden F, Olmos D, Yin D,
Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A
and Hammer GD: Safety, tolerability, and pharmacokinetics of the
anti-IGF-1R monoclonal antibody figitumumab in patients with
refractory adrenocortical carcinoma. Cancer Chemother Pharmacol.
65:765–773. 2010. View Article : Google Scholar :
|
|
19
|
Jones RL, Kim ES, Nava-Parada P, Alam S,
Johnson FM, Stephens AW, Simantov R, Poondru S, Gedrich R, Lippman
SM, et al: Phase I study of intermittent oral dosing of the
insulin-like growth factor-1 and insulin receptors inhibitor
OSI-906 in patients with advanced solid tumors. Clin Cancer Res.
21:693–700. 2015. View Article : Google Scholar
|
|
20
|
Lerario AM, Worden FP, Ramm CA, Hesseltine
EA, Stadler WM, Else T, Shah MH, Agamah E, Rao K and Hammer GD: The
combination of insulin-like growth factor receptor 1 (IGF1R)
antibody cixutumumab and mitotane as a first-line therapy for
patients with recurrent/metastatic adrenocortical carcinoma: A
multi-institutional NCI-sponsored trial. Horm Cancer. 5:232–239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naing A, Kurzrock R, Burger A, Gupta S,
Lei X, Busaidy N, Hong D, Chen HX, Doyle LA, Heilbrun LK, et al:
Phase I trial of cixutumumab combined with temsirolimus in patients
with advanced cancer. Clin Cancer Res. 17:6052–6060. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ilvesmäki V, Kahri AI, Miettinen PJ and
Voutilainen R: Insulin-like growth factors (IGFs) and their
receptors in adrenal tumors: High IGF-II expression in functional
adrenocortical carcinomas. J Clin Endocrinol Metab. 77:852–858.
1993.PubMed/NCBI
|
|
23
|
Laurell C, Velázquez-Fernández D, Lindsten
K, Juhlin C, Enberg U, Geli J, Höög A, Kjellman M, Lundeberg J,
Hamberger B, et al: Transcriptional profiling enables molecular
classification of adrenocortical tumours. Eur J Endocrinol.
161:141–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Kahri AI, Heikkilä P, Ilvesmäki V
and Voutilainen R: H19 and insulin-like growth factor-II gene
expression in adrenal tumors and cultured adrenal cells. J Clin
Endocrinol Metab. 80:492–496. 1995.PubMed/NCBI
|
|
25
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doghman M, El Wakil A, Cardinaud B, Thomas
E, Wang J, Zhao W, Peralta-Del Valle MH, Figueiredo BC, Zambetti GP
and Lalli E: Regulation of insulin-like growth factor-mammalian
target of rapamycin signaling by microRNA in childhood
adrenocortical tumors. Cancer Res. 70:4666–4675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Özata DM, Caramuta S, Velázquez-Fernández
D, Akçakaya P, Xie H, Höög A, Zedenius J, Bäckdahl M, Larsson C and
Lui WO: The role of microRNA deregulation in the pathogenesis of
adrenocortical carcinoma. Endocr Relat Cancer. 18:643–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soon PS, Tacon LJ, Gill AJ, Bambach CP,
Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson
BG and Sidhu SB: miR-195 and miR-483-5p identified as predictors of
poor prognosis in adrenocortical cancer. Clin Cancer Res.
15:7684–7692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patterson EE, Holloway AK, Weng J, Fojo T
and Kebebew E: MicroRNA profiling of adrenocortical tumors reveals
miR-483 as a marker of malignancy. Cancer. 117:1630–1639. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agosta C, Laugier J, Guyon L, Denis J,
Bertherat J, Libé R, Boisson B, Sturm N, Feige JJ, Chabre O and
Cherradi N: MiR-483-5p and miR-139-5p promote aggressiveness by
targeting N-myc downstream-regulated gene family members in
adrenocortical cancer. Int J Cancer. 143:944–957. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chabre O, Libé R, Assie G, Barreau O,
Bertherat J, Bertagna X, Feige JJ and Cherradi N: Serum miR-483-5p
and miR-195 are predictive of recurrence risk in adrenocortical
cancer patients. Endocr Relat Cancer. 20:579–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Decmann A, Bancos I, Khanna A, Thomas MA,
Turai P, Perge P, Pintér JZ, Tóth M, Patócs A and Igaz P:
Comparison of plasma and urinary microRNA-483-5p for the diagnosis
of adrenocortical malignancy. J Biotechnol. 297:49–53. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patel D, Boufraqech M, Jain M, Zhang L, He
M, Gesuwan K, Gulati N, Nilubol N, Fojo T and Kebebew E: MiR-34a
and miR-483-5p are candidate serum biomarkers for adrenocortical
tumors. Surgery. 154:1224–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung
JJ and Kwok TT: Oncofetal H19-derived miR-675 regulates tumor
suppressor RB in human colorectal cancer. Carcinogenesis.
31:350–358. 2010. View Article : Google Scholar
|
|
36
|
Zhai LL, Wang P, Zhou LY, Yin JY, Tang Q,
Zhang TJ, Wang YX, Yang DQ, Lin J and Deng ZQ: Over-expression of
miR-675 in formalin-fixed paraffin-embedded (FFPE) tissues of
breast cancer patients. Int J Clin Exp Med. 8:11195–11201.
2015.PubMed/NCBI
|
|
37
|
Zhou YW, Zhang H, Duan CJ, Gao Y, Cheng
YD, He D, Li R and Zhang CF: miR-675-5p enhances tumorigenesis and
metastasis of esophageal squamous cell carcinoma by targeting
REPS2. Oncotarget. 7:30730–30747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He D, Wang J, Zhang C, Shan B, Deng X, Li
B, Zhou Y, Chen W, Hong J, Gao Y, et al: Down-regulation of
miR-675-5p contributes to tumor progression and development by
targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol
Cancer. 14:732015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schmitz KJ, Helwig J, Bertram S, Sheu SY,
Suttorp AC, Seggewiss J, Willscher E, Walz MK, Worm K and Schmid
KW: Differential expression of microRNA-675, microRNA-139-3p and
microRNA-335 in benign and malignant adrenocortical tumours. J Clin
Pathol. 64:529–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DeLellis RA, Lloyd RV, Heitz PU and Eng C:
Pathology and genetics of tumours of endocrine organs. World Health
Organization Classification of Tumours. 3rd edition. 8. IARC Press;
Lyon, France: 2004
|
|
41
|
Kjellin H, Johansson H, Höög A, Lehtiö J,
Jakobsson PJ and Kjellman M: Differentially expressed proteins in
malignant and benign adrenocortical tumors. PLoS One. 9:e879512014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45(W1):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42(Database Issue): D92–D97. 2014. View Article : Google Scholar
|
|
44
|
Gazdar AF, Oie HK, Shackleton CH, Chen TR,
Triche TJ, Myers CE, Chrousos GP, Brennan MF, Stein CA and La Rocca
RV: Establishment and characterization of a human adrenocortical
carcinoma cell line that expresses multiple pathways of steroid
biosynthesis. Cancer Res. 50:5488–5496. 1990.PubMed/NCBI
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
46
|
Weiss LM: Comparative histologic study of
43 metastasizing and nonmetastasizing adrenocortical tumors. Am J
Surg Pathol. 8:163–169. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Valente FM, Sparago A, Freschi A,
Hill-Harfe K, Maas SM, Frints SGM, Alders M, Pignata L, Franzese M,
Angelini C, et al: Transcription alterations of KCNQ1 associated
with imprinted methylation defects in the Beckwith-Wiedemann locus.
Genet Med. 21:1808–1820. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Corrêa GT, Bandeira GA, Cavalcanti BG, de
Carvalho Fraga CA, dos Santos EP, Silva TF, Gomez RS, Guimarães AL
and De Paula AM: Association of-308 TNF-α promoter polymorphism
with clinical aggressiveness in patients with head and neck
squamous cell carcinoma. Oral Oncol. 47:888–894. 2011. View Article : Google Scholar
|
|
49
|
Schteingart DE, Giordano TJ, Benitez RS,
Burdick MD, Starkman MN, Arenberg DA and Strieter RM:
Overexpression of CXC chemokines by an adrenocortical carcinoma: A
novel clinical syndrome. J Clin Endocrinol Metab. 86:3968–3974.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shih CM, Lee YL, Chiou HL, Chen W, Chang
GC, Chou MC and Lin LY: Association of TNF-alpha polymorphism with
susceptibility to and severity of non-small cell lung cancer. Lung
Cancer. 52:15–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kimmel GL, Péron FG, Haksar A, Bedigian E,
Robidoux WF Jr and Lin MT: Ultrastructure, steroidogenic potential,
and energy metabolism of the Snell adrenocortical carcinoma 494. A
comparison with normal adrenocortical tissue. J Cell Biol.
62:152–163. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng Y, Kerppola RE and Kerppola TK:
ATR-101 disrupts mitochondrial functions in adrenocortical
carcinoma cells and in vivo. Endocr Relat Cancer. 23:1–19. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Poli G, Guasti D, Rapizzi E, Fucci R, Canu
L, Bandini A, Cini N, Bani D, Mannelli M and Luconi M:
Morphofunctional effects of mitotane on mitochondria in human
adrenocortical cancer cells. Endocr Relat Cancer. 20:537–550. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Satoh K, Zhang L, Zhang Y, Chelluri R,
Boufraqech M, Nilubol N, Patel D, Shen M and Kebebew E:
Identification of niclosamide as a novel anticancer agent for
adrenocortical carcinoma. Clin Cancer Res. 22:3458–3466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee JH, Lee YK, Lim JJ, Byun HO, Park I,
Kim GH, Xu WG, Wang HJ and Yoon G: Mitochondrial respiratory
dysfunction induces claudin-1 expression via reactive oxygen
species-mediated heat shock factor 1 activation, leading to
hepatoma cell invasiveness. J Biol Chem. 290:21421–21431. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santidrian AF, Matsuno-Yagi A, Ritland M,
Seo BB, LeBoeuf SE, Gay LJ, Yagi T and Felding-Habermann B:
Mitochondrial complex I activity and NAD+/NADH balance regulate
breast cancer progression. J Clin Invest. 123:1068–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Marbet P, Klusonova P, Birk J, Kratschmar
DV and Odermatt A: Absence of hexose-6-phosphate dehydrogenase
results in reduced overall glucose consumption but does not prevent
11β-hydroxysteroid dehydrogenase-1-dependent glucocorticoid
activation. FEBS J. 285:3993–4004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marini C, Ravera S, Buschiazzo A, Bianchi
G, Orengo AM, Bruno S, Bottoni G, Emionite L, Pastorino F,
Monteverde E, et al: Discovery of a novel glucose metabolism in
cancer: The role of endoplasmic reticulum beyond glycolysis and
pentose phosphate shunt. Sci Rep. 6:250922016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Berruti A, Grisanti S, Pulzer A, Claps M,
Daffara F, Loli P, Mannelli M, Boscaro M, Arvat E, Tiberio G, et
al: Long-term outcomes of adjuvant mitotane therapy in patients
with radically resected adrenocortical carcinoma. J Clin Endocrinol
Metab. 102:1358–1365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Calabrese A, Basile V, Puglisi S, Perotti
P, Pia A, Saba L, Berchialla P, Porpiglia F, Veltri A, Volante M,
et al: Adjuvant mitotane therapy is beneficial in non-metastatic
adrenocortical carcinoma at high risk of recurrence. Eur J
Endocrinol. 180:387–396. 2019. View Article : Google Scholar : PubMed/NCBI
|