Introduction
Primary liver cancer is the common cause of the
global cancer burden, and 90% of primary liver cancer cases are
hepatocellular carcinoma (HCC) (1). HCC ranks sixth in incidence among
malignant tumors and third among cancer-related deaths (2). According to the 2020 global cancer
statistics, the incidence and mortality rate associated with liver
cancer in China ranked first worldwide (2). In recent years, with advancements
being made in systemic therapy, non-surgical treatments for HCC
have gradually emerged. An objective response rate of ~30% can be
achieved using anti-angiogenic drugs combined with immunotherapy
for the treatment of advanced or unresectable liver cancer
(3). Although the efficacy of
advanced HCC treatments has notably improved, serious challenges
remain. It has been found that, during the clinical application of
targeted drugs, some patients are susceptible to drug resistance
and the median survival rate remains low (3). Therefore, the development of
effective therapeutic drugs is mandatory in order to provide
patients with HCC with more treatment opportunities and improved
survival benefits.
Ferroptosis is a newly discovered oxidative cell
death characterized by cystine depletion or glutathione peroxidase
4 (GPX4) inactivation and the iron-dependent accumulation of lethal
lipid peroxidation on the plasma membrane or elsewhere within the
cell (4). Studies have confirmed
that ferroptosis plays a critical regulatory role in the
pathogenesis and treatment of cancers, including HCC, and is mainly
suppressed in tumor cells to maintain survival (5). For example, ubiquitin-like
modification activator 1 triggers the inhibition of ferroptosis by
upregulating nuclear factor erythroid-2 related factor 2 (Nrf2)
signaling and downregulating Fe2+ levels, promoting the
development of HCC (6).
Furthermore, sorafenib and sulfasalazine synergistically inhibit
branched chain acid aminotransferase 2, which induces the
ferroptosis of HepG2 cells (7). In
addition, the inhibition of ADP-ribosylation factor 6 activates
long-chain acyl-CoA synthetase 4, inducing ferroptosis, to overcome
gemcitabine resistance in pancreatic cancer cells (8); this action also downregulates
microRNA-522, promotes arachidonic acid 5 lipid oxygenase
expression to induce ferroptosis, and enhances cisplatin/paclitaxel
sensitivity in gastric cancer cells (9). These findings suggest that the
induction of ferroptosis may present a novel therapeutic approach
for HCC treatment.
Traditional Chinese medicine (TCM) involves the
combination of the traditional culture and wisdom of the Chinese
nation (10). TCM is characterized
by less toxicity and fewer side-effects, more therapeutic targets
and an enhanced efficacy (10).
TCM contains numerous bioactive components, some of which exhibit
activity against HCC (11).
Therefore, identifying novel drugs for the treatment of HCC from
TCM monomer compounds may be a practical approach. The authors
screened a drug library of TCM monomers containing 1,444 compounds
and identified polyphyllin VI as a potential therapeutic drug for
HCC.
Polyphyllin VI (PPVI), an active saponin isolated
from Paris polyphylla, has been shown to exert potent
anticancer effects in a variety of malignant tumors. PPVI has been
shown to induce non-small cell lung cancer (NSCLC) cell death
through the induction of the NF-κB/NLR family pyrin domain
containing 3/gasdermin D signaling axis (12), to exert anticancer effects against
breast cancer cells by modulating RELT-like protein 2 (13), and to induce the apoptosis and
autophagy of osteosarcoma through c-Jun N-terminal kinase (14). However, the effects of PPVI on HCC
remain unclear. The present study investigated the therapeutic
effects of PPVI on HCC and assessed the possible mechanisms through
which these effects may be exerted.
Materials and methods
Reagents and antibodies
Traditional Chinese Medicine Library (cat. no.
L8300), PPVI (cat. no. S9302), β-lapachone (cat. no. S7261),
3-methyladenine (3-MA, cat. no. S2767) inhibitors and Ac-DEVD-CHO
(cat. no. S7901) were purchased from Selleck Chemicals; Stattic
(cat. no. HY-13818) and ferrostatin-1 (Fer-1, cat. no. HY-100579)
were purchased from MedChem Express. GPX4 (cat. no. ab125066),
signal transducer and activator of transcription 3 (STAT3; cat. no.
ab68153), phosphorylated (p-)STAT3 (pY705; cat. no. ab76315),
N-cadherin (cat. no. ab18203), Vimentin (cat. no. ab92547),
E-cadherin (cat. no. ab40772) and goat anti-rabbit IgG H&L
(HRP; cat. no. ab6721) antibodies were purchased from Abcam. The
GAPDH (2118S) antibody was purchased from Cell Signaling
Technology, Inc. The goat anti-rabbit IgG (H+L) cross-adsorbed
secondary antibody and cyanine3 (A-10520) were purchased from
Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture and treatment
The HCCLM3 cells were purchased from the China
Center for Type Culture Collection (CCTCC), and the Huh7 cells were
purchased from Suyan Biotechnology Co., Ltd. The THLE-2 cells were
purchased from Meisen Cell Technology Co., Ltd. The HCCLM3 and Huh7
Cells were cultured in DMEM/high-glucose medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2. The THLE-2 cells require a special coating medium
(CTCC-002-30, Meisen Cell Technology Co., Ltd.) and were incubated
overnight in an incubator at 37°C and 5% CO2 and
cultured in BEGM™ BulletKit™ medium (cat. no. CC-3170, Lonza Group,
Ltd.) with 10% FBS. The HCC cells were observed and representative
images were captured under an inverted microscope (Ts2R-FL, Nikon
Corporation; ×100 magnification) at 0 and 24 h following drug
intervention. The HCC cells were treated with PPVI (4 µM) at
37°C for 24 h, following which different assays were performed.
Inhibitors, such as 3-MA (1 mM), Ac-DEVD-CHO (50 µM) and
Fer-1 (1 µM) for 2 h prior to treatment with PPVI (4
µM) for 24 h.
Transfection with overexpression
plasmids
The plasmids that included PIRES2-EGFP
(hSTAT3-IRES2-EGFP, oeSTAT3 and BW2486) and PIRES2-EGFP
(No-Targeting Plasmid, the control plasmid and BVA15) were
purchased from Hangzhou Youming Biotechnology Co., Ltd. For
transfection, Lipofectamine 3000 transfection reagent (GlpBio)
diluted in Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) was
used (1:1 ratio; two tubes). After seeding, the cells were grown to
70-90% confluency and then transfected, with 5 µg/µl
of the transfected DNA concentration. The DNA master mix was
prepared by diluting DNA in Opti-MEM, then adding Lipofectamine
3000 reagent and mixing well. The diluted DNA was added to each
tube of diluted Lipofectamine 3000 reagent and incubated for 10-15
min at room temperature. The DNA-lipid complex was added to the
cells and incubated for 3 days at 37°C.
Subcutaneous tumor model and drug
administration
All animal experiments were approved (No. 20220070)
by the Animal Ethics Committee (AEC) of the China Science and
Technology Industry Holdings (Shenzhen) Co., Ltd. Male BALB/c nude
mice (n=9), 4 weeks old, were purchased from the Guangdong Medical
Laboratory Animal Center. All mice were housed under the following
conditions: A 12-h light/dark cycle (lights on, 07:00; lights off,
19:00), a temperature of 22±2°C and a humidity of 50±10%, and were
provided with free access to standard chow and water. The Huh7
cells (1×107 in 0.1 ml DMEM) were injected
subcutaneously into the right flanks of the nude mice. After 7
days, the mice were randomly divided into three groups (three mice
per group) as follows: The control group (0.9% NaCl/day),
PPVI-treated group (PPVI at 10 mg/kg/day) and the group treated
with PPVI (10 mg/kg/day) + Fer-1 (10 mg/kg/day). The weight of the
tumors and tumor size were measured daily. Following the
intraperitoneal administration of the treatments for 10 consecutive
days, the mice were sacrificed, and the serum was used to measure
alanine transaminase (ALT, cat. no. C009-2-1) and aspartate
aminotransferase (AST, cat. no. C010-2-1) (both from Jiancheng
Bioengineering Institute) levels. The mice were euthanized by an
intraperitoneal injection of pentobarbital sodium (200 mg/kg).
Blood was then obtained from the orbital sinus, and the livers and
subcutaneous tumors were removed. During the animal experiments,
the maximum diameter of the tumors was measured daily. When the
longest diameter of a single tumor was 20 mm, the experiment was
terminated and the animals were euthanized. Following euthanasia,
the death of the animal was confirmed by documenting the
disappearance of the pain response (i.e., unresponsiveness to toe
compressions with hands or forceps) and the observation of cardiac
arrest and apnea. Liver and tumor tissues were collected for
detection using hematoxylin and eosin (H&E) staining and
immunohistochemistry, as described below.
Cell counting kit-8 (CCK-8) assay
The HCC cells were treated with the indicated
concentrations of PPVI (from 1 to 10 µM) or Stattic (6
µM) for 24 h. The HCC cells were then treated with or
without 3-MA (1 mM), Ac-DEVD-CHO (50 µM) and Fer-1 (1
µM) inhibitors for 2 h prior to treatment with PPVI for 24
h. Cell viability was assessed using a CCK-8 assay (cat. no. C0043,
Beyotime Institute of Biotechnology). The optical density (OD)
value of the sample was obtained at 450 nm using a microplate
reader (BioTek Instruments, Inc.).
Cell cloning assay
After the HCCLM3 or Huh7 cells were seeded in
six-well plates at a density of 800 cells/well, they were subjected
to PPVI (2, 4, 6, 8 and 10 µM) or PPVI (4 µM) with
Fer-1 (1 µM). The medium was replaced for 3 days and the
cells were cultured until the number of monoclonal cells was
>50. The cells were fixed with 4% paraformaldehyde at 25°C for
30 min, stained with crystal violet (cat. no. BL802A, Biosharp Life
Sciences) for 30 min at 25°C, and photographed using a digital
camera (Ts2R-FL, Nikon Corporation).
Lactate dehydrogenase (LDH) cytotoxicity
assay
The HCCLM3 or Huh7 cells were treated with or
without 3-MA (1 mM), Ac-DEVD-CHO (50 µM) and Fer-1 (1
µM) inhibitors for 2 h prior to treatment with PPVI (4
µM) for 24 h. Following the instructions provided with the
LDH Cytotoxicity Detection Kit (Beyotime Institute of
Biotechnology), the plates were incubated at 37°C for 30 min in the
dark. The OD values at 490 nm were obtained using a microplate
reader (BioTek Instruments, Inc.).
Cell wound healing assay
The HCCLM3 and Huh7 cells were seeded in a six-well
plate at a density of 4×105 cells/well. When the cell
density reached 90% confluency, the confluent cell monolayer was
scraped using a pipette tip. The cells were incubated in serum-free
medium as designed for the experiments. Images of the same
scratched area at 0 and 24 h were captured (Ts2R-FL, Nikon
Corporation).
Transwell invasion assay
The cells were starved and incubated for 12 h in
advance, and 2.5×105 HCC cells were seeded in the upper
chamber (cat. no. 3422, Corning, Inc.) of the Transwell, followed
by PPVI (4 µM) or PPVI (4 µM) + Fer-1 (1 µM)
for 24 h. The lower chamber contained DMEM with 20% FBS. Following
incubation of the Transwell plate at 37°C for 24 h, the invaded
cells were fixed with formaldehyde for 30 min at 25°C and stained
with crystal violet (cat. no. BL802A, Biosharp Life Sciences) for
30 min at 25°C. Finally, the invaded cells were observed and
photographed under an inverted microscope (Nikon, Ts2R-FL).
Transmission electron microscopy
(TEM)
After the HCCLM3 and Huh7 cells were treated with
PPVI (4 µM) and incubated for 24 h at 37°C, the cells were
collected by centrifugation (3,000 × g, 2 min, 37°C), supplemented
with 2.5% Gluta fixative (Beijing Solarbio Science & Technology
Co., Ltd.) at 4°C for 48 h. The cells were then dehydrated in a
graded ethanol series, infiltrated with propylene oxide, embedded
in an epoxy resin (cat. no. 10000418, SPI Supplies) at 37°C for 12
h and sectioned to a thickness of 70 nm. Subsequently, the
ultrathin sections were observed and photographed using an HT7800
transmission electron microscope (Hitachi, Ltd.).
Mitochondrial membrane potential
detection (JC-1)
The HCCLM3 or Huh7 cells were seeded in six-well
plates, and treated with PPVI (2, 4 and 6 µM) or PPVI (4
µM) with Fer-1 (1 µM) for 24 h. The JC-1 fluorescent
probe (Beyotime Institute of Biotechnology) was then added
according to the instructions of the manufacturer to observe the
mitochondrial membrane potential (MMP) changes under a fluorescence
microscope (DMIL4000, Leica Microsystems, Inc.).
Glutathione (GSH) detection
Following 24 h of treatment with PPVI (2, 4 and 6
µM) or PPVI (4 µM) with or without Fer-1 (1
µM), the working solution was added according to the
instructions provided with the GSH assay kit (cat. no. S0053,
Beyotime Institute of Biotechnology) using a single-point assay,
and absorbance was measured using a microplate reader (BioTek
Instruments, Inc.) after 25 min.
Malondialdehyde (MDA) detection
The HCCLM3 or Huh7 cells were homogenized in MDA
lysis buffer, and centrifuged at 13,000 × g for 10 min at 4°C to
obtain 200 µl supernatant. The MDA assay kit (cat. no.
MAK085, MilliporeSigma) was used to detect the MDA content.
Finally, the absorbance value was measured at 532 nm using a
microplate reader (BioTek Instruments, Inc.).
Iron content determination
The HCCLM3 or Huh7 cells were treated with PPVI (2,
4 and 6 µM) or PPVI (4 µM) with Fer-1 (1 µM)
for 24 h, and the Fe2+ levels were determined according
to the instructions provided with the iron assay kit (cat. no.
MAK025, MilliporeSigma).
Divalent iron on probe detection
FerroOrange fluorescent probes (Dojindo
Laboratories, Inc.) were loaded in the HCC cells following 24 h of
PPVI (2, 4 and 6 µM) or PPVI (4 µM) with or without
Fer-1 (1 µM). According to the instructions provided by the
manufacturer, following 30 min at 37°C of incubation, the changes
in the Fe2+ content were observed and photographed using
a fluorescence microscope (Ts2R-FL, Nikon Corporation).
Active oxygen determination
Intracellular superoxide anion levels were
determined using dihydroethidium (DHE; Beyotime Institute of
Biotechnology). Following the manufacturer's instructions, a DHE
solution was incubated with the cells at 37°C for 30 min and the
cells were then observed and photographed under a fluorescence
microscope (Ts2R-FL, Nikon Corporation).
Western blot analysis
The cells were lysed and homogenized using RIPA
lysis buffer (cat. no. 89900, Thermo Fisher Scientific, Inc.), and
the protein concentration was detected using a BCA assay kit (cat.
no. 23227, Thermo Fisher Scientific, Inc.). The proteins were
separated by SDS-PAGE (10% gels) and transferred onto PVDF
membranes. After the membranes were incubated with 5% skim milk at
25°C for 1 h, they were incubated with the corresponding primary
antibodies (1:1,000; all antibodies used are listed above in the
Reagents and antibodies section) at 4°C overnight, followed by
incubation with the secondary antibody (1:2,000) for 1 h at 25°C.
The bands were detected using a chemiluminescent HRP substrate
(WBKLS0500, MilliporeSigma) and visualized with the ChemiDoc XRS
system (Bio-Rad Laboratories, Inc.). Densitometric analysis of the
western blots was performed using ImageJ V1.8.0 (National
Institutes of Health, Inc.).
Immunofluorescence staining
The HCC cells were treated with PPVI (2, 4 and 6
µM) or PPVI (4 µM) with or without Fer-1 (1
µM) and incubated for 24 h at 37°C. The cells were fixed
with 4% polyformaldehyde, permeabilized with 0.5% Triton X-100
(T9284, MilliporeSigma), blocked with 5% goat serum (BL210A,
Biosharp Life Sciences) and incubated overnight with the required
amount of primary antibody such as GPX4, N-cadherin, Vimentin and
E-cadherin (1:200) in a humidified box at 4°C. The cells were
incubated with the goat anti-rabbit IgG (H+L) antibody, Cy3 (1:200)
at 25°C for 1 h in the dark. Nuclear DAPI (BL105A, Biosharp Life
Sciences) was used for counterstaining for 15 min at 25°C in the
dark with antifade mounting medium (P0126, Beyotime Institute of
Biotechnology) to mount the slides and the slides were then
photographed (Ts2R-FL, Nikon Corporation). The procedure was
repeated with the Huh7 cells.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the HCC cells using the
AG RNAex isolation system (Accurate Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Complementary DNA was
synthesized using reverse transcription kits (cat. no. AG11728,
Jiangsu Accuracy Biotechnology Co., Ltd.). qPCR was performed using
a QuantStudio5 system (Thermo Fisher Scientific, Inc.) with
SYBR-Green (cat. no. AG11718, Jiangsu Accuracy Biotechnology Co.,
Ltd.) and the thermo-cycling conditions were as follows: 95°C for
30 sec, 95°C for 5 sec and 60°C for 30 sec, for 40 cycles. The
2−ΔΔCq method was used to analyze the RT-qPCR data
(15). The sequences of the
primers used are as follows: GPX4 forward, 5′-CCC GAT ACG CTG AGT
GTG GTT TG-3′ and reverse, 5′-CCT TGC CCT TGG GTT GGA TCT TC-3′;
STAT3 forward, 5′-TGT GAT GCT TCCCTG ATT GTG ACT G-3′ and reverse,
5′-TCG CTT GGT GGT GGA GGA GAA C-3′; and GAPDH forward, 5′-CAA GGC
TGT GGG CAA GGT CAT C-3′ and reverse, 5′-GTG TCG CTG TTG AAG TCA
GAG GAG-3′.
Immunohistochemistry
The tissues were paraffin-embedded, sectioned,
dewaxed in xylene, soaked in 100 to 70% gradient ethanol and placed
in PBS. Citric acid retrieval solution (cat. no. P0086, Beyotime
Institute of Biotechnology) was then added, and the slices were
placed in the microwave for 20 min at 100°C and then rinsed with
PBS three times, for 5 min each. After washing, 5% blocking serum
was added in a dropwise manner for blocking for 30 min at 37°C, and
primary antibody such as p-STAT3, STAT3, GPX4 (1:200) with 5% BSA
was then added in a dropwise manner for overnight incubation at
4°C. The following day, MaxVision™ HRP-Polymer anti-mouse/rabbit
IHC kit (cat. no. KIT-5020, MXB Biotechnologies) was added 50
µl followed by incubation at 25°C for 15 min. Subsequently,
50 µl developer diaminobenzidine working solution (cat. no.
DAB-0031, MXB Biotechnology Co., Ltd.) was added followed by
incubation at room temperature for 3 min. The sections were
counterstained with hematoxylin for 40 sec at 25°C, dehydrated,
mounted, and observed under a microscope (Axio Imager M2, Zeiss
AG).
Network pharmacology-based and molecular
docking analyses
The human targets of PPVI were obtained from the
SwissTarget prediction database (http://swisstargetpre-diction.ch/), and the DrugBank
(https://www.drugbank.ca/), GeneCards (https://www.genecards.org/), DisGeNET (http://www.disgenet.org/home), Online Mendelian
Inheritance in Man (OMIM, https://www.omim.org/), TTD (http://db.idrblab.net/ttd) and PharmGKB databases
(https://www.pharmgkb.org/) were then
searched using the key words 'liver cancer' and 'liver carcinoma'.
All obtained targets were merged and duplicate targets were
removed. The targets of PPVI were used to intersect with the
targets of liver cancer to obtain the anti-liver cancer targets of
PPVI. The data were uploaded to the STRING 11.5 database to obtain
protein-protein interaction (PPI) information and analyzed using
Cytoscape 3.9.1 (https://cytoscape.org/). Cytoscape was used to
calculate network topology parameters, including degree centrality,
closeness centrality, betweenness centrality, and clustering
coefficient. Finally, AutoDock 4.2.6 (https://autodock.scripps.edu/) was used to perform
molecular docking between PPVI and key targets, and PyMOL
(https://pymol.org/2/) was used to obtain a visual
2D map.
Statistical analysis
All experiments were repeated independently at least
three times. GraphPad Prism 9.4 software (GraphPad Software, Inc.)
was used for data analysis using an unpaired t-test and one-way
ANOVA (with Tukey's multiple comparisons test). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
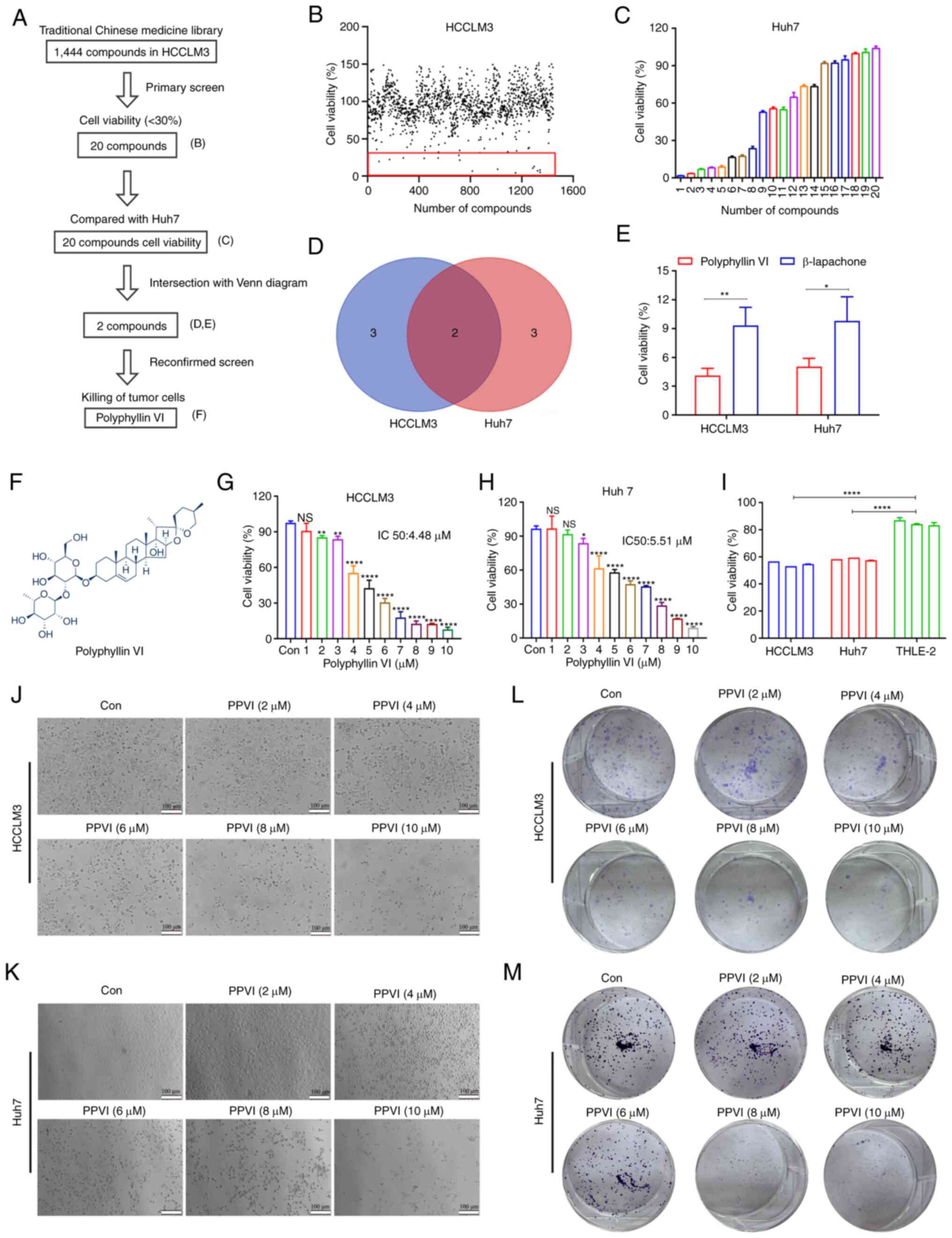
Identification of compounds that can
induce cell death in HCC cells using high-throughput screening
To identify compounds with therapeutic efficacy in
HCC, a pilot library was screened which comprised ~1,444 TCM
monomeric representative compounds with an ability to inhibit cell
activity in HCCLM3 cells at a concentration of 10 µM. The
screening process followed in the present study is illustrated in
Fig. 1A. The HCCLM3 cells were
treated with 1,444 monomers for 24 h and cell viability was then
examined. As shown in Fig. 1B, 20
compounds (Fig. S1A and B)
inhibited the growth of the HCCLM3 cells by <30%. Subsequently,
the Huh7 cells were treated with these monomers 10 µM for 24
h, and a second screening was performed using CCK-8 assay (Fig. 1C). The Venn diagram shown in
Fig. 1D illustrates the screening
process, demonstrating monomeric molecules with toxic effects in
both HCCLM3 and Huh7 cells. The intersection of the Venn diagram
indicates two compounds, PPVI and β-lapachone. The Huh7 cells were
then treated with PPVI and β-lapachone (10 µM) for 24 h,
which was found to exert potent cytotoxic effects on both cell
lines (Fig. 1E). The further
comparison of the inhibitory effects of PPVI and β-lapachone on HCC
cell (HCCLM3 and Huh7 cells) viability revealed that PPVI exerted
the most pronounced inhibitory effect on the viability of HCC cells
(Fig. 1E). The chemical structural
formula of PPVI is presented in Fig.
1F.
To further analyze the cytotoxic effects of PPVI on
HCC cells, the cells were treated with various concentrations of
PPVI. The results revealed that PPVI inhibited the viability of
HCCLM3 and Huh7 cells in a concentration-dependent manner (Fig. 1G and H). Compared with the normal
liver cells (THLE-2), PPVI (4 µM) also exerted a significant
inhibitory effect on HCC cell viability (Fig. 1I). The light microscopy images
demonstrated that cell contraction and cell death increased with
the increasing concentrations of PPVI (Fig. 1J and K). Cell cloning experiments
also revealed that the proliferative capacities of the HCCLM3 and
Huh7 cells were negatively associated with the concentration of
PPVI (Fig. 1L and M).
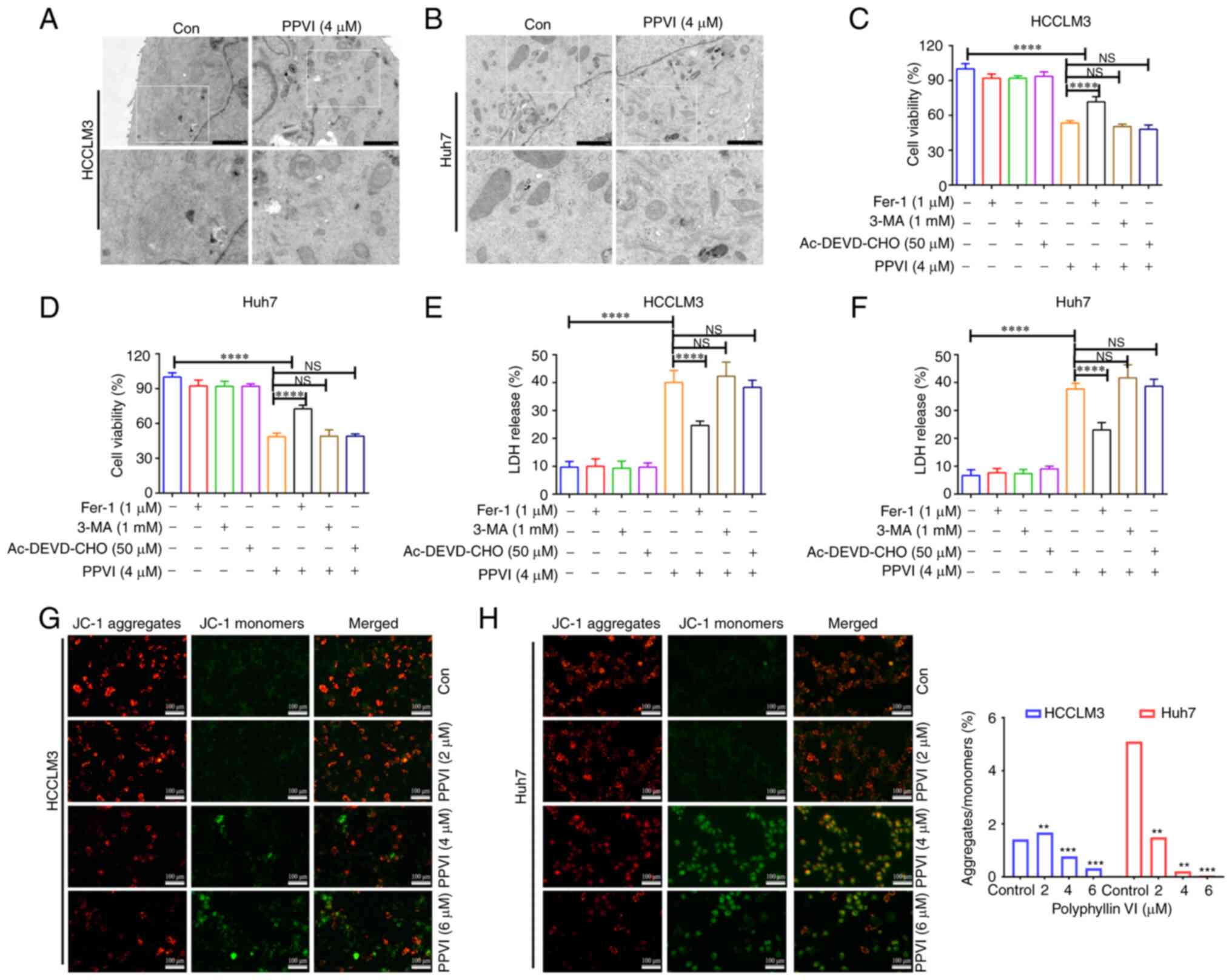
Ferroptosis contributes to the cytotoxic
effects of PPVI on HCC cells
The present study then examined the morphology of
the treated cells in order to explore the mechanisms underlying
PPVI-induced cell death. TEM analysis revealed that the HCCLM3 and
Huh7 cells treated with PPVI (4 µM) for 24 h exhibited a
decrease in mitochondrial volume, an increase in bilayer membrane
density, and a decrease or disappearance of mitochondrial cristae
(Fig. 2A and B), which are typical
morphological features of ferroptosis. To verify whether this form
of cell death involved ferroptosis, the HCCLM3 and Huh7 cells were
treated with PPVI in the absence or presence of several cell death
inhibitors. Combination treatment with the autophagy inhibitor,
3-MA, and the pyroptosis inhibitor, Ac-DEVD-CHO, did not prevent
the death of the cells treated with PPVI (Fig. 2C-F), and the ferroptosis inhibitor,
Fer-1, exerted the most pronounced reversal effect (Fig. 2C and D). Apoptosis or necrosis can
cause damage to the cell membrane structure, and the quantitative
analysis of cytotoxicity was achieved by detecting the activity of
LDH released from the cells with plasma membrane rupture (Fig. 2E and F). Furthermore, in the
fluorescence images of JC-1-stained mitochondria (Fig. 2G and H), red fluorescence
representing an intact MMP was observed in the control group and a
low MMP was observed in the PPVI-treated groups (2, 4 and 6
µM) with increasing concentrations.
PPVI-induced ferroptosis in HCC
cells
To explore whether ferroptosis is a key determinant
of HCC cell death induced by PPVI, several ferroptosis events were
examined in HCC cells, including ROS accumulation, GSH, MDA, the
Fe2+ content and GPX4 expression. GSH levels in the
HCCLM3 and Huh7 cells were found to gradually decreased with the
increasing concentrations of PPVI (2, 4 and 6 µM) (Fig. 3A and B). However, PPVI upregulated
the MDA levels in a concentration-dependent manner (Fig. 3C and D). In addition, the
intracellular Fe2+ levels significantly increased upon
stimulation with 4 and 6 µM PPVI (Fig. 3E and F). Consistently, when the
Fe2+ content was detected using a FerroOrange
fluorescent probe, the accumulation of Fe2+ in the cells
was promoted by the increase in the PPVI concentration (Fig. 3G). The level of cellular ROS was
then detected using DHE. As the concentration of PPVI increased,
the red fluorescence in the HCC cells was enhanced, indicating that
the intracellular ROS level was positively associated with the
concentration of PPVI, which suggested that PPVI induced oxidative
stress in the HCC cells (Fig. 3H).
The results also revealed that the GPX4 protein expression levels
in the HCCLM3 and Huh7 cells were negatively associated with the
concentration of PPVI (Fig. 3I and
J).
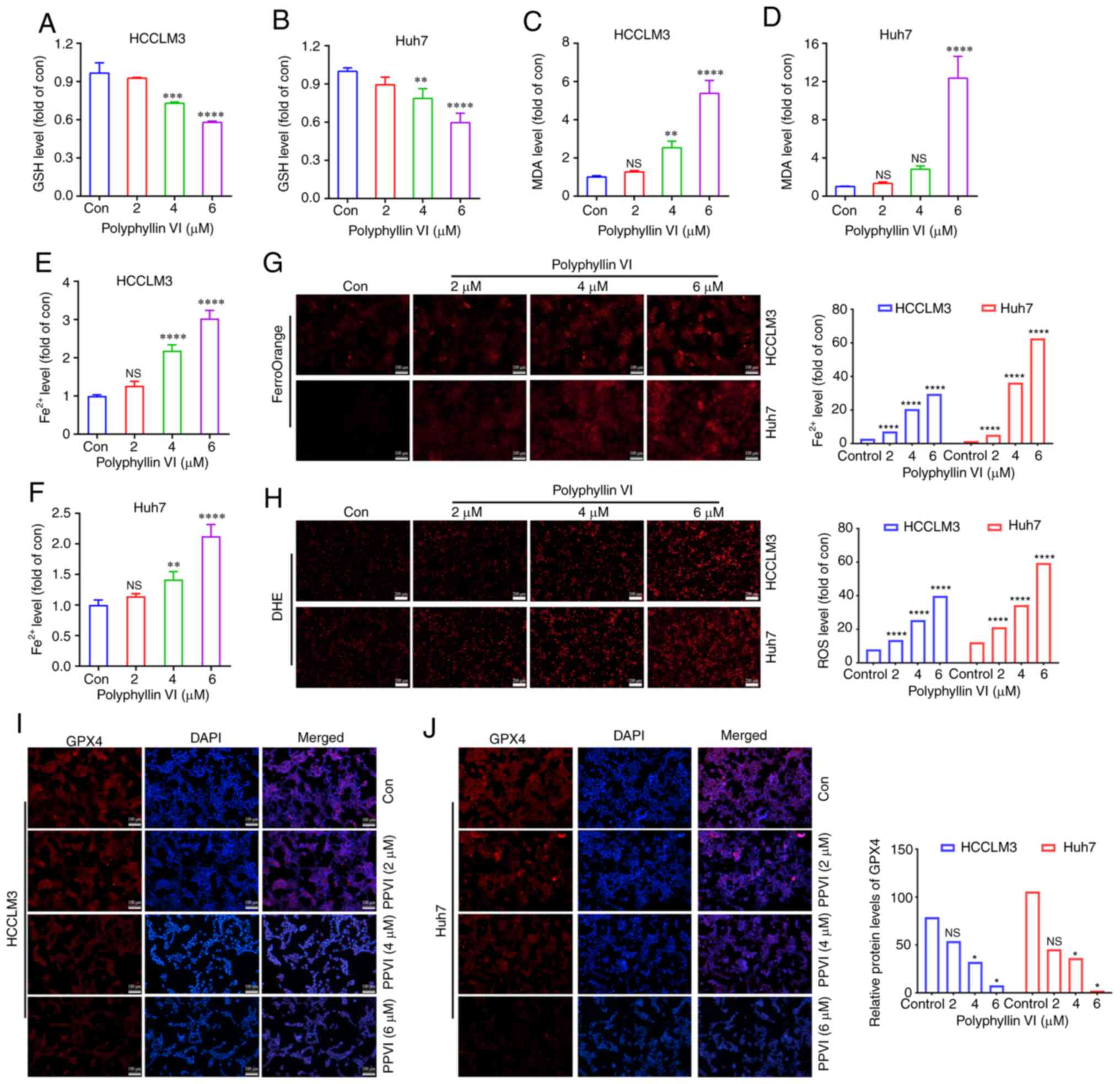 | Figure 3PPVI induces the ferroptosis of
hepatocellular carcinoma cells. The HCCLM3 and Huh7 cells were
treated with PPVI (2, 4 and 6 µM) for 24 h. (A and B) GSH
levels in HCCLM3 and Huh7 cells. (C and D) MDA level detection. (E
and F) Fe2+ level detection. (G) The Fe2+
content in HCCLM3 and Huh7 cells was detected using the FerroOrange
fluorescent probe. (H) ROS levels in HCCLM3 and Huh7 cells were
observed using DHE. (I and J) The protein expression levels of GPX4
in HCCLM3 and Huh7 cells were detected using immunofluorescence.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs.
control. NS, not significant; Con, control; PPVI, polyphyllin VI;
GSH, glutathione; MDA, malondialdehyde; DHE, dihydroethidium; ROS,
reactive oxygen species; GPX4, glutathione peroxidase 4. |
The ferroptosis inhibitor, Fer-1,
attenuates PPVI-induced cell death by inhibiting the ferroptosis of
HCC cells
To further investigate the association between
mitochondrial damage and ferroptosis induced by PPVI, the
ferroptosis inhibitor, Fer-1, was used. Mitochondrial fluorescence
staining was performed using HCC cells treated with PPVI + Fer-1,
and an increase in aggregate JC-1 and a decrease in monomeric JC-1
were observed compared to the PPVI group (Fig. 4A and B), suggesting that Fer-1 can
alleviated mitochondrial damage induced by PPVI. Changes in the
intracellular levels of GSH, MDA and Fe2+ were also
examined following co-treatment with PPVI and Fer-1. Consistently,
GSH expression increased in the PPVI + Fer-1 group compared with
that in the PPVI group (Fig. 4C and
D), whereas the contents of MDA and Fe2+ decreased
in the PPVI + Fer-1 group (Fig.
4E-H). Furthermore, the levels of ROS and ferrous iron were
decreased in the PPVI + Fer-1 group compared with the PPVI group
(Fig. 4I and J). Moreover, the
protein expression levels of GPX4 were measured following
co-treatment with PPVI and Fer-1 using western blot analysis.
Compared with the PPVI group, the protein expression level of GPX4
in the PPVI + Fer-1 group was higher (Fig. 4K and L). An analysis of the GPX4
mRNA levels also revealed that its expression was reduced by PPVI,
whereas it was increased in the PPVI + Fer-1 group (Fig. 4M). These results suggest that Fer-1
reverses PPVI-induced mitochondrial damage and the ferroptosis of
HCC cells.
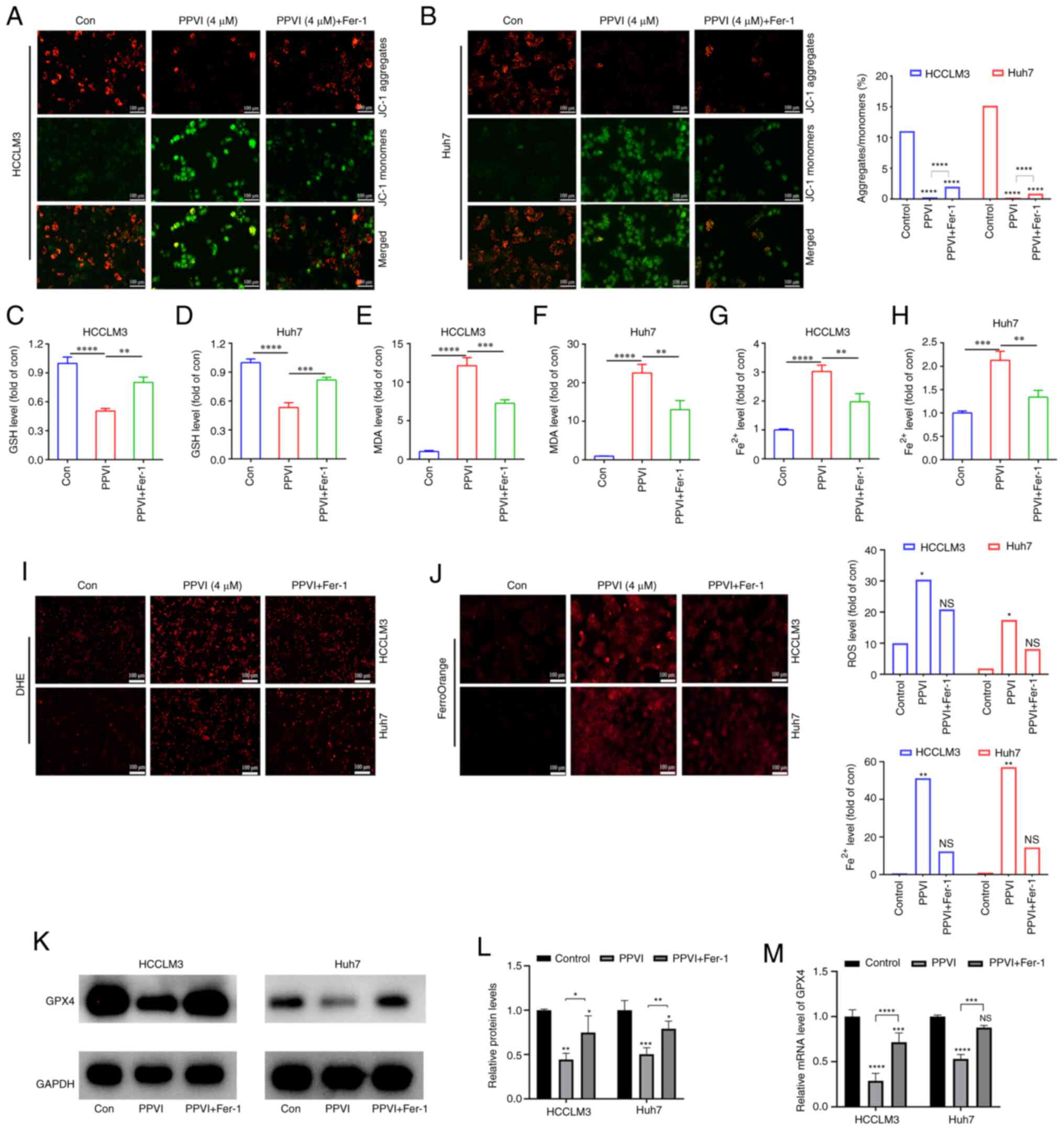 | Figure 4The ferroptosis inhibitor, Fer-1,
attenuates polyphyllin VI-induced cell death by inhibiting the
ferroptosis of HCC cells. The HCCLM3 and Huh7 cells were treated
with or without Fer-1 for 2 h prior to treatment with PPVI for 24
h, after which different assays were performed. (A and B) Changes
in the MMP of HCCLM3 and Huh7 cells were observed by MMP detection
with JC-1. (C and D) GSH level detection in HCCLM3 and Huh7 cells.
(E and F) MDA level detection in HCCLM3 and Huh7 cells. (G and H)
Fe2+ levels in HCCLM3 and Huh7 cells. (I) ROS level
detection in HCC cells observed using DHE. (J) Fe2+
level detection in HCCLM3 and Huh7 cells detected using FerroOrange
fluorescent probe. (K and L) Western blot analysis of GPX4 in
HCCLM3 and Huh7 cells. (M) Relative mRNA expression of GPX4 in HCC
cells. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. control
or as indicated. NS, not significant; Con, control; HCC,
hepatocellular carcinoma; PPVI, polyphyllin VI; MMP, mitochondrial
membrane potential; DHE, dihydroethidium; ROS, reactive oxygen
species; GPX4, glutathione peroxidase 4; Fer-1, ferrostatin-1. |
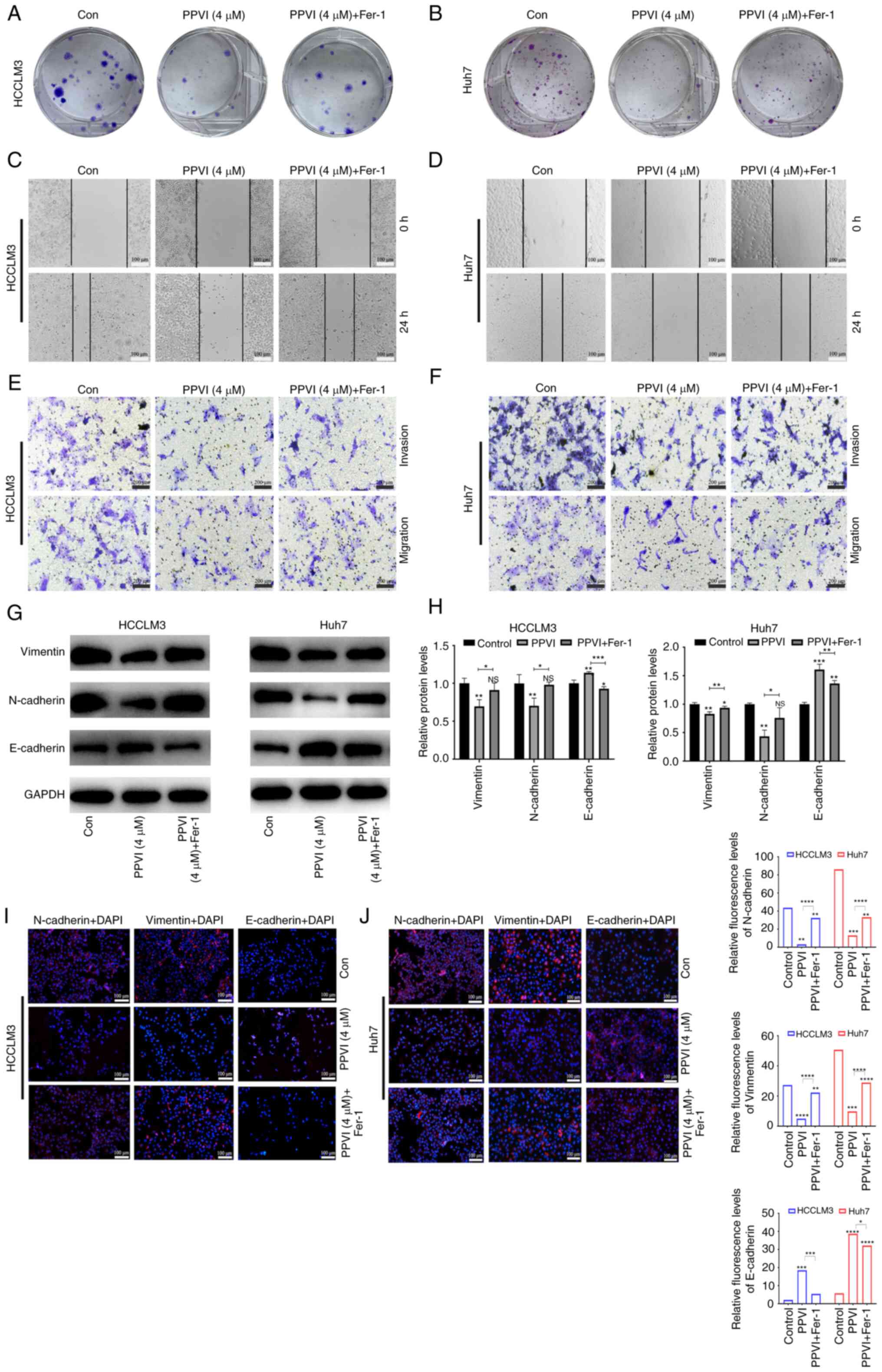
PPVI inhibits HCC cell invasion and
migration by inducing ferroptosis
The present study then examined the effects of PPVI
on the invasion and migration of HCC cells. Following treatment of
the HCCLM3 and Huh7 cells with PPVI or PPVI combined with Fer-1,
the PPVI + Fer-1 combination exhibited a lower ability to inhibit
colony formation than PPVI alone, indicating that Fer-1 reversed
cell death induced by PPVI (Fig. 5A
and B). The wound healing assay revealed that PPVI markedly
inhibited the wound-healing ability of the HCC cells compared with
the control group, whereas the PPVI-induced inhibition of the wound
healing ability was markedly reversed by Fer-1 (Fig. 5C and D). Transwell assays revealed
that PPVI markedly inhibited the number of invasive and migratory
cells, and Fer-1 reversed this motility of the cells (Fig. 5E and F). As epithelial-mesenchymal
transition (EMT) is one of the most critical mechanisms involved in
cell migration and invasion, the effects of PPVI on the progression
of EMT were investigated. The protein expression levels of
E-cadherin, N-cadherin and Vimentin were examined using western
blot analysis. The results revealed that PPVI increased the protein
expression levels of E-cadherin in the HCCLM3 and Huh7 cells,
whereas the levels of N-cadherin and Vimentin were decreased
(Fig. 5G and H). The protein
expression levels of E-cadherin were decreased in the PPVI + Fer-1
group, and the expression of N-cadherin and Vimentin was increased
compared with that in the PPVI group (Fig. 5G and H). The results of
immunofluorescence staining also revealed a similar result as those
obtained for protein expression (Fig.
5I and J). These results thus suggest that PPVI inhibits the
invasion and migration of HCC cells induced by the EMT
mechanism.
PPVI inhibits the STAT3/GPX4 pathway in
HCC cells
To examine the mechanisms by which PPVI induces the
ferroptosis of HCC cells, a network pharmacology-based strategy was
used to obtain PPVI targets in HCC by taking the intersection of
the utility targets of PPVI and the targets in liver cancer. These
target proteins were analyzed using a PPI network to obtain 42
nodes and 400 edges (Fig. 6A). The
target proteins in the PPI network were then selected according to
the criteria of degree ≥24, betweenness centrality ≥0.011, and
closeness centrality ≥0.707, and nine core target proteins were
finally identified, namely PIK3CA, JUN, FGF2, VEGFA, MTOR, CASP3,
SRC, TNF and STAT3 (Fig. 6A). PPVI
was molecularly docked with the aforementioned target proteins, and
the binding energy data indicated that PPVI had a strong binding
activity with STAT3 protein (Fig.
6B).
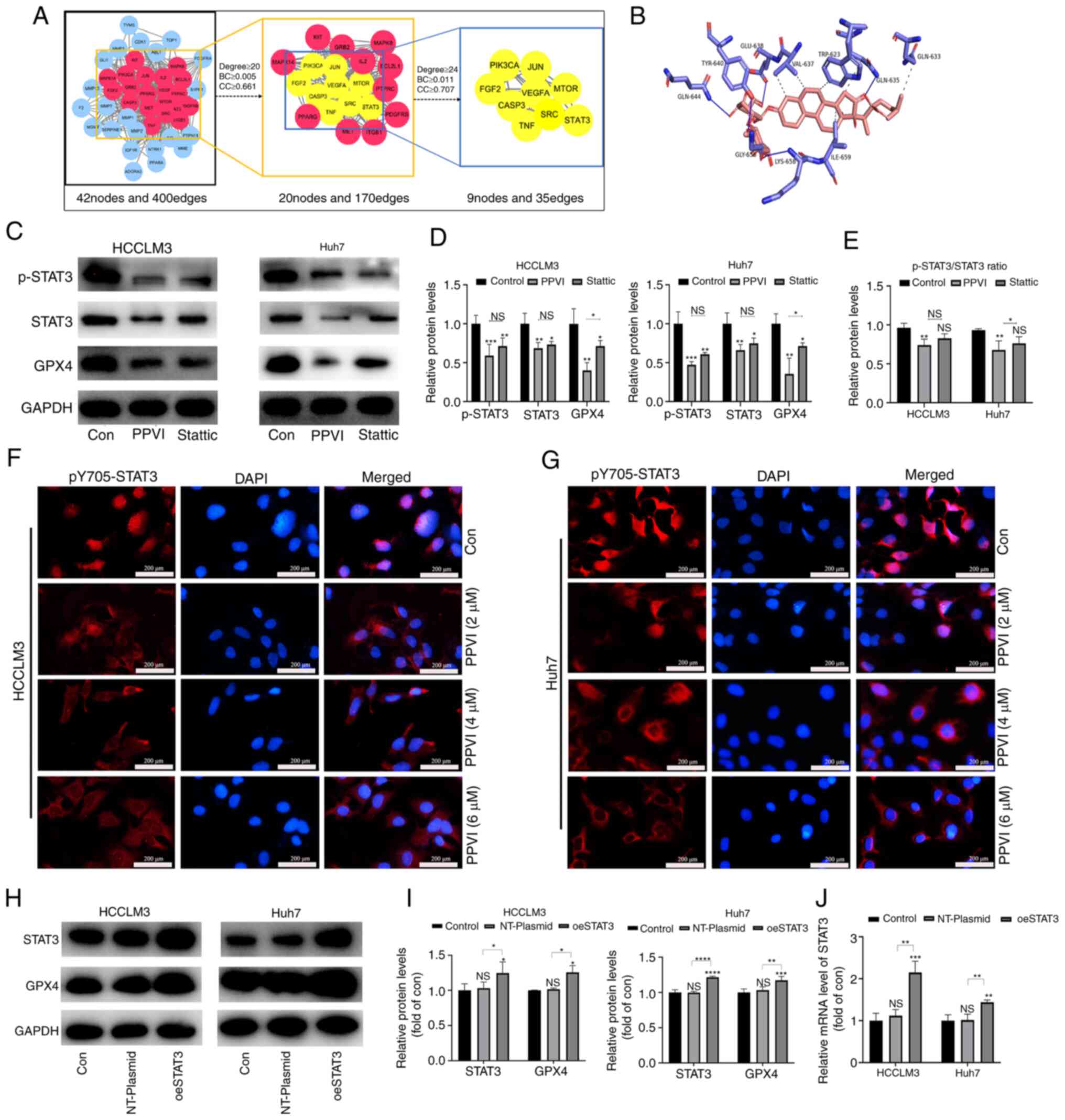 | Figure 6PPVI inhibits STAT3/GPX4 in
hepatocellular carcinoma cells. (A) The binding activity between
PPVI and STAT3 was examined using network pharmacology-based
analysis and molecular docking technology. (B) Simulation of the
combination of PPVI and STAT3. (C-E) HCCLM3 and Huh7 cells were
treated with PPVI or Stattic for 24 h. Western blot analysis of
GPX4, STAT3 and p-STAT3 protein expression in HCCLM3 and Huh7
cells. (F and G) Detection of pY705-STAT3 expression in HCCLM3 and
Huh7 cells treated with PPVI (2, 4 and 6 µM) using
immunofluorescence staining. (H and I) Western blot analysis of
GPX4, STAT3 and p-STAT3 levels in HCC cells with or without the
overexpression of STAT3. (J) RT-qPCR analysis of STAT3 mRNA
expression. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. control
or as indicated. NS, not significant (vs. control); Con, control;
PPVI, polyphyllin VI; STAT3, signal transducer and activator of
transcription 3; GPX4, glutathione peroxidase 4; Fer-1,
ferrostatin-1; oeSTAT3, STAT3 overexpression plasmid. |
The present study then examined whether STAT3
inhibition promoted the ferroptosis of HCC cells. The HCCLM3 and
Huh7 cells were treated with 4 µM PPVI and STAT3 inhibitor
(6 µM Stattic), respectively. The results of western blot
analysis revealed that PPVI significantly inhibited STAT3 and GPX4
protein expression, and the phosphorylation also decreased
accordingly, which followed the downward trend induced by Stattic
(Fig. 6C-E). Notably, the levels
of ROS, ferrous iron, MDA and GSH, and GPX4 protein expression
(based on western blot analysis) also exhibited similar changes in
the cells treated with PPVI or Stattic compared with the control
group (Fig. S2). Moreover, the
wound healing assay revealed that PPVI or Stattic inhibited the
wound healing ability of the HCC cells (Fig. S1C). Furthermore, the results of
immunofluorescence staining indicated that the nuclear
translocation of p-STAT3 in the HCC cells was suppressed with the
increasing PPVI concentrations (Fig.
6F and G). These results suggest that PPVI may regulate the
protein expression of the GPX4 through STAT3. Subsequently, to
investigate the mechanisms through which STAT3 regulates GPX4,
STAT3 was overexpressed in the HCCLM3 and Huh7 cells, and it was
confirmed that STAT3 increased the GPX4 protein and mRNA levels
(Fig. 6H-J).
PPVI induces ferroptosis by inhibiting
the STAT3/GPX4 axis in HCC cells
The present study then examined whether PPVI induces
ferroptosis by regulating the STAT3/GPX4 axis in HCC cells. For
this purpose, five groups were created, including one control group
with nothing added. To reduce the influence of plasmid vectors on
the experiment, an unloaded plasmid group and an unloaded plasmid +
PPVI group were utilized. The remaining two groups were the
overexpression STAT3 group and the overexpression STAT3 + PPVI
group. After the HCC cells that overexpressed STAT3 were treated
with 4 µM PPVI for 24 h, ROS and ferrous iron in the
NT-Plasmid + PPVI group increased significantly; however, this
effect was inhibited by STAT3 overexpression (Fig. 7A and B). Furthermore, the increase
in MDA and the decrease in GSH levels induced by PPVI were also
reversed by STAT3 overexpression (Fig.
7C and D). In addition, the PPVI-induced decrease in GPX4
expression was partially reversed by STAT3 overexpression (Fig. 7E and F). Morevoer, the
phosphorylation ratio of STAT3 is presented in Fig. 7G. Similarly, the PPVI-induced
decrease in the GPX4 mRNA levels was also reversed by STAT3
overexpression (Fig. 7H). Thus,
PPVI induces ferroptosis by inhibiting the STAT3/GPX4 axis in HCC
cells.
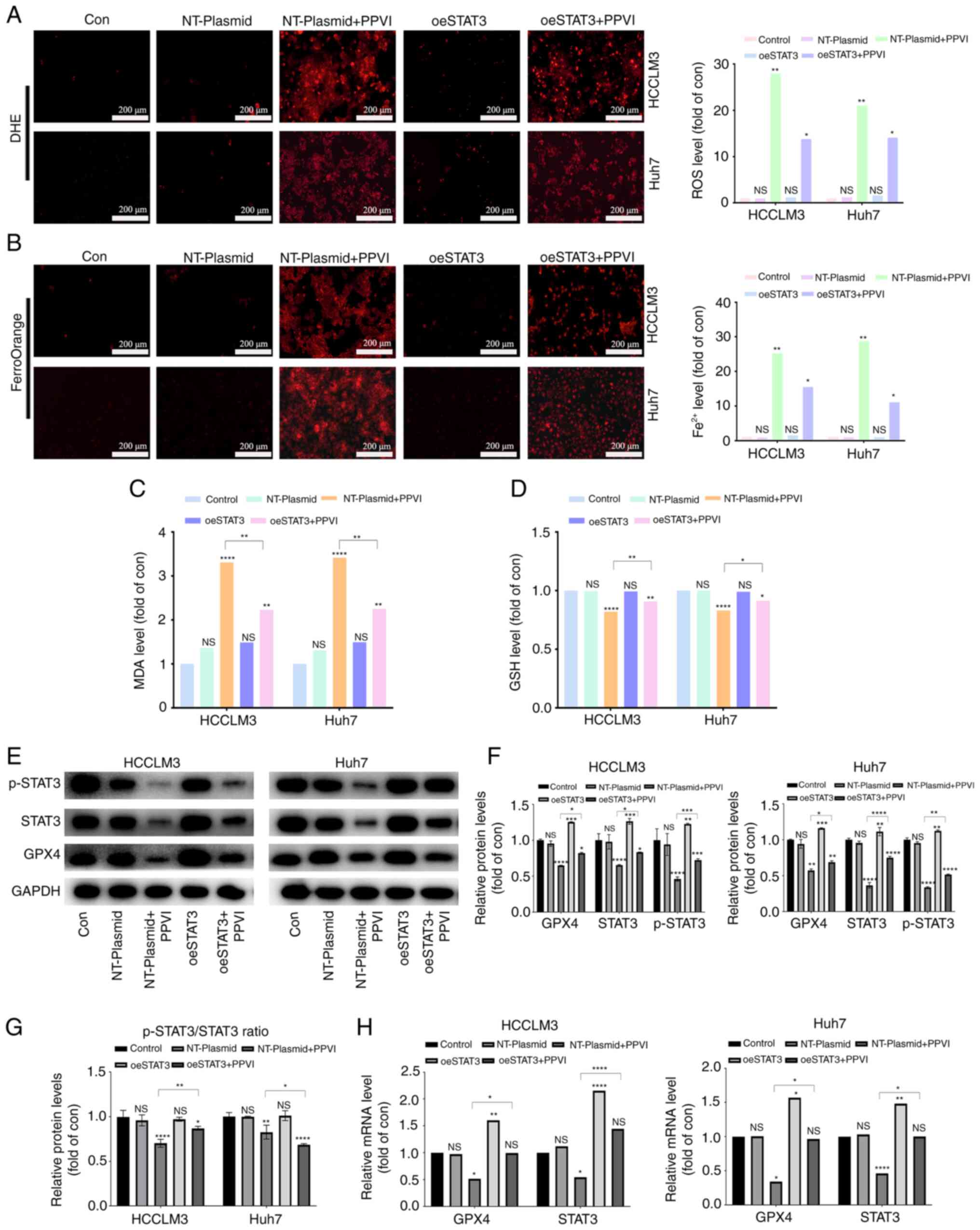 | Figure 7PPVI induces ferroptosis by
inhibiting the STAT3/GPX4 axis in hepatocellular carcinoma cells.
HCC cells with or without overexpression of STAT3 were treated with
PPVI. (A) ROS levels in HCCLM3 and Huh7 cells were observed using
DHE. (B) Fe2+ content in HCCLM3 and Huh7 cells was
detected using the FerroOrange fluorescent probe. (C and D) MDA and
GSH levels. (E-G) Protein expression of GPX4, p-STAT3 and STAT3 in
HCCLM3 and Huh7 cells was detected using western blot analysis. (H)
RT-qPCR analysis of STAT3 and GPX4 mRNA expression.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 vs. control
or as indicated. NS, not significant (vs. control); Con, control;
PPVI, polyphyllin VI; STAT3, signal transducer and activator of
transcription 3; GPX4, glutathione peroxidase 4; oeSTAT3, STAT3
overexpression plasmid; DHE, dihydroethidium. |
PPVI suppresses tumor growth in vivo via
the STAT3/GPX4 axis
Subsequently, the present study investigated the
mechanisms through which PPVI induces ferroptosis via regulation of
the STAT3/GPX4 signaling pathway in vivo. Huh7 cells were
injected subcutaneously into nude mice. Once the mice developed
palpable tumors, the mice were treated for 10 consecutive days with
intraperitoneal injections of PPVI, PPVI + Fer-1, or the same
volume of saline (Fig. 8A and B).
Fig. 8C shows that the weight of
the mice in the saline group gradually decreased due to tumor
consumption, while the body weight of the nude mice in the PPVI
group decreased less than that in the PPVI + Fer-1 group. In the
later period, the weight of the mice in the saline group exceeded
that of the PPVI + Fer-1 group, which was deemed to be due to an
increase in tumor net weight. PPVI suppressed tumor growth and the
ferroptosis inhibitor, Fer-1, reversed this effect (Fig. 8D and E). Subsequently, liver
function was examined by measuring the ALT and AST levels in mouse
serum; the levels of PPVI and PPVI + Fer-1 were not significantly
altered compared to the control group (Fig. 8F and G). Furthermore, H&E
staining of liver and tumor tissues revealed that PPVI exerted no
evident hepatic damage and was thus a safe therapeutic agent
(Fig. 8H). The results of
immunohistochemistry revealed that Fer-1 reversed the decrease in
the expression of p-STAT3, STAT3 and GPX4 in the tumor mouse
tissues (Fig. 8I). These data
demonstrate the efficacy of PPVI in the treatment of liver cancer
in vivo and indicate that PPVI exerts its effects via the
STAT3/GPX4 axis.
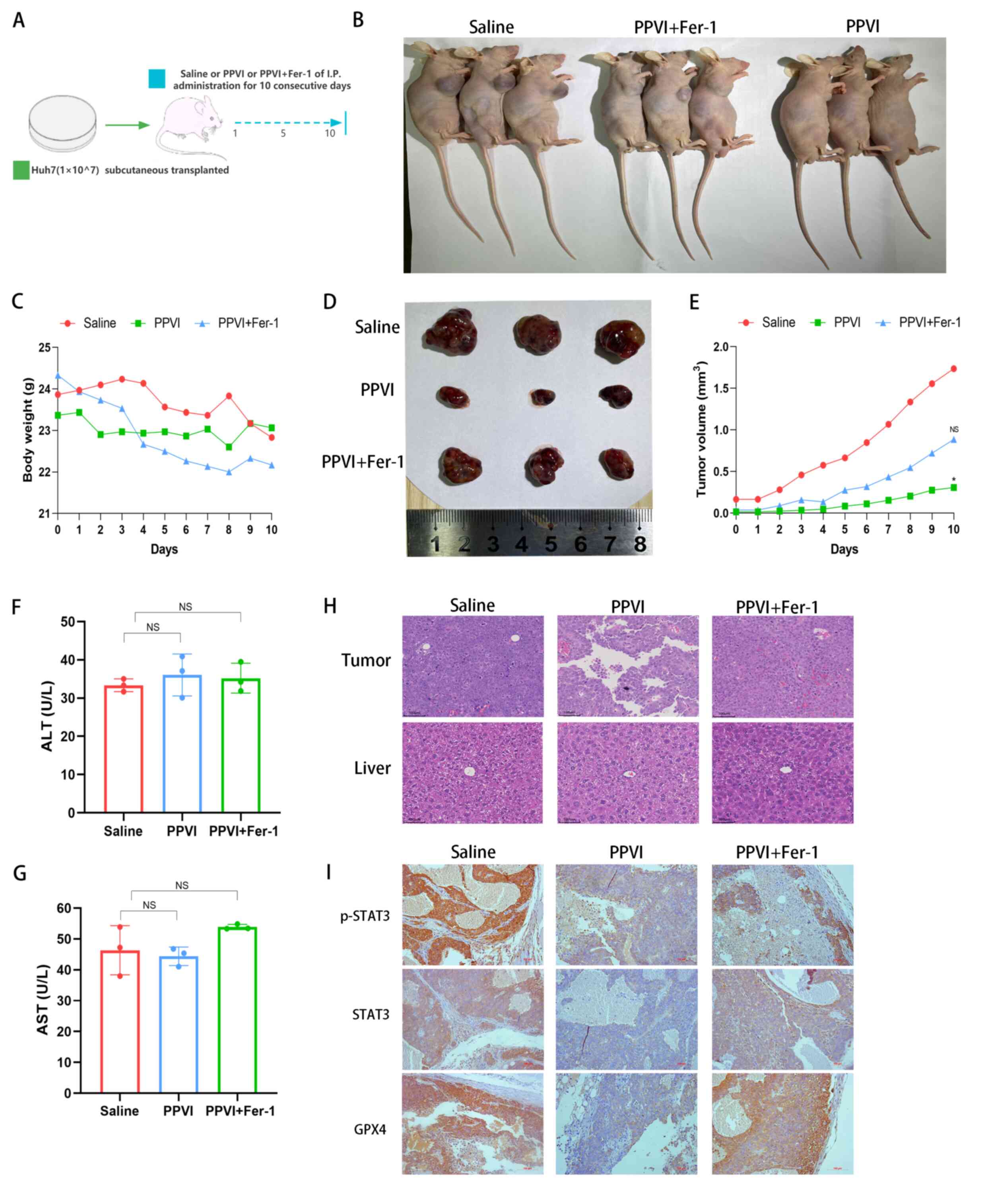 | Figure 8PPVI suppresses tumor growth in
vivo through the STAT3/GPX4 axis. (A) Schematic diagram of the
in vivo experiment. (B) Image of mice with subcutaneous
tumor xenografts. (C) Muse weight. (D) Images of subcutaneous
xenograft mouse tumors. (E) Changes in tumor volume in mice during
drug injection. (F and G) The levels of ALT and AST, which are
markers of liver function, were measured. (H) Hematoxylin and eosin
staining illustrating the morphology of mouse liver and tumor
tissue. (I) The level of p-STAT3, STAT3 and GPX4 proteins in the
indicated tumor tissue was using immunohistochemistry.
*P<0.05 vs. saline group. NS, not significant; PPVI,
polyphyllin VI; STAT3, signal transducer and activator of
transcription 3; GPX4, glutathione peroxidase 4; Fer-1,
ferrostatin-1; AST, aspartate aminotransferase; ALT, alanine
transaminase. |
Discussion
HCC presents a global health challenge with its
increasing morbidity and mortality (2). Exhaustive research on HCC has led to
considerable improvements being made the available monitoring
methods and treatment strategies for this disease (3). However, the 5-year survival rate of
patients remains poor (20%); chemotherapy remains the mainstay of
treatment for patients with advanced-stage HCC, and sorafenib is
the standard of care for these patients (3); however, it only provides patients
with a limited survival benefit (3). Therefore, the development of more
promising and therapeutically valuable HCC drugs, and the
identification of innovative therapeutic targets, remain
priorities. Current cancer therapies exert their anticancer effects
by triggering cancer cell death, and small-molecule compounds
targeting cancer cell death pathways are important and promising
cancer treatment agents (16,17).
The present study screened a library of small-molecule compounds of
TCM monomers and identified a popular drug (PPVI), which was
effective in inhibiting the proliferation, migration and invasion
of HCC cells, potentially targeting the STAT3/GPX4 axis to induce
ferroptosis.
The anticancer properties of PPVI have been
demonstrated in various cancer models; for example, PPVI has been
shown to induce the apoptosis of human osteosarcoma cells (14) and to induce NSCLC cell autophagic
death via the ROS-mediated mTOR signaling pathway (18). However, the form of death induced
by PPVI in HCC cells has not yet been reported, at least to the
best of our knowledge. In the present study, PPVI treatment
inhibited the viability of HCC cells. PPVI significantly reduced
the number of viable HCC cells and their colony formation capacity.
Furthermore, TEM revealed the typical morphological features of
ferroptosis in HCC cells treated with PPVI, including a reduced
mitochondrial volume and decreased or absent mitochondrial cristae.
To further determine whether this form of cell death involved
ferroptosis, the HCCLM3 and Huh7 cells were treated with PPVI in
the absence or presence of several inhibitors of cell death. Fer-1
combination treatment prevented PPVI-induced cell death, suggesting
that ferroptosis may be a key determinant of cell death in HCCLM3
and Huh7 cells treated with PPVI.
Ferroptosis is a new form of cell death discovered
by Dixon et al (4) in 2012.
It differs from necrosis, apoptosis and autophagy in genetics,
biochemical characteristics and morphology (4). Chen et al (5) found that ferroptosis played a key
role in tumor cytotoxicity and tumor progression. Several small
molecules induce ferroptosis and exert tumor-suppressive effects in
various experimental cancer models (19,20),
involving mechanisms primarily related to iron overload, lipid
peroxidation imbalance and cysteine transport (21). The accumulation of excess
Fe2+ exerts potent oxidative effects through the Fenton
reaction, which generates ROS and induces lipid peroxidation by
reacting with polyunsaturated fatty acids on the lipid membrane
(21). The present study found
that the GSH levels in HCCLM3 and Huh7 cells gradually decreased,
whereas the MDA, ROS and Fe2+ levels were significantly
increased in a concentration-dependent manner following treatment
with PPVI. Notably, Fer-1 not only alleviated the cellular
mitochondrial damage induced by PPVI, but also reduced the
biochemical characteristics of ferroptosis in HCC cells, including
ROS accumulation and lipid peroxidation. These findings strongly
suggest that ferroptosis contributes to the inhibitory effects of
PPVI on the growth of HCC cells. The key to ferroptosis is lipid
peroxidation, and GPX4 has been confirmed to play a crucial role in
inhibiting iron-catalyzed lipid peroxidation in microsomes; this
can be eliminated by reducing lipid hydroperoxides (22). For example, bufotalin has been
shown to induce ferroptosis in NSCLC cells by accelerating the
degradation of GPX4 and boosting intracellular Fe2+
(23). Furthermore, the
phyto-sesquiterpene lactones, DET and DETD-35, have been found to
induce ferroptosis in mutant melanoma through the inhibition of
GPX4 (24). The findings of the
present study are consistent with those of other research (25), demonstrating that GPX4 expression
in HCC cells is negatively associated with the PPVI concentration.
These findings provide new evidence of the anticancer effects of
PPVI on HCC, particularly by the induction of ferroptosis.
EMT, the process by which epithelial cancer cells
acquire a mesenchymal phenotype with metastatic and invasive
properties, is one of the most critical events in cancer
progression (26). It has been
confirmed that drug-resistant cancer cells that normally undergo
EMT are more likely to be killed by ferroptosis inducers. For
example, β-elemene or cetuximab enhances the cytotoxic effects of
RSL3 in KRAS-mutant colorectal cancer cells by improving
RSL3-induced ferroptosis (27,28).
To investigate whether PPVI can inhibit EMT in HCC cells by
inducing ferroptosis, the present study detected several
EMT-related markers using immunofluorescence staining and western
blot analysis. The results revealed that PPVI increased the
expression of the epithelial marker, E-cadherin, enhanced cell-cell
adhesion junctions, and decreased the protein expression levels of
N-cadherin and Vimentin in HCCLM3 and Huh7 cells, whereas the
ferroptosis inhibitor, Fer-1, reversed these effects. These results
suggest that PPVI inhibits EMT in HCC cells by inducing
ferroptosis.
To clarify the mechanisms through which PPVI
induces ferroptosis, nine core target proteins were identified by
intersection screening based on the compound target database and
the liver cancer target database. Using AutoDock molecular docking
software, PPVI was found to target and regulate STAT3, an
oxidation-responsive transcription factor that plays an active role
in ferroptosis (29). In a
previous study, treatment with a STAT3 inhibitor was found to
enhance sensitivity to cisplatin by reactivating ferroptosis in
cells (30). In another study, the
inhibition of STAT3 downregulated SLC7A11, GPX4 and FTH1 expression
to trigger ferroptosis, and inhibit tumor growth and reduce
chemical resistance in gastric cancer (31). In addition, it was also previously
demonstrated that impairing STAT3/Nrf2/GPx4 signaling induced
ferroptosis and enhanced the sensitivity of osteosarcoma cells to
cisplatin (32). In the present
study, it was found that PPVI, similar to the STAT3 inhibitor,
Stattic, inhibited the activation of STAT3, induced ferroptosis and
reduced the levels of GPX4 in HCC cells, exerting a significant
antitumor effect. This is consistent with the conclusion of other
researchers, in that Stattic can exert antitumor effects by
inhibiting STAT3 phosphorylation and pancreatic cancer cell
proliferation (33). In the
present study, STAT3 was then overexpressed and it was confirmed
that PPVI blocked GPX4 expression by inhibiting the phosphorylation
of STAT3 through the accumulation of GSH, MDA and FE2+,
consistent with the findings of previous others (34). Finally, in vivo experiments
revealed PPVI exerted anticancer effects with relative
biosafety.
In conclusion, the present study demonstrates that
the natural product, PPVI, is a novel ferroptosis inducer with the
potential to induce ferroptosis and inhibit HCC cell metastasis and
invasion. The present study discovered a novel mechanism of the
anticancer effects of PPVI in HCC, which inhibits tumor growth by
inhibiting the STAT3/GPX4 axis, inducing ferroptosis, and
inhibiting the metastasis and invasion of HCC cells by regulating
EMT. These data suggest PPVI may serve as a potential new drug for
the treatment of HCC, and these data may thus provide a new
direction for the treatment of HCC. However, the present study had
certain limitations. First, it was not determined whether other
molecular targets were involved and whether PPVI can bind to
multi-target proteins involved in anticancer effects or
ferroptosis. Second, other important molecular mechanisms through
which STAT3 regulates the ferroptosis of HCC cells have not yet
been identified. Thus, further studies are warranted to explore the
multi-target proteins bound by PPVI, as well as the molecular
mechanisms by which STAT3 regulates ferroptosis in HCC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request. The raw data and original images from the
present study are included in the Figshare database (https://figshare.com/s/de9b1ad82b37321478c2). Further
inquiries can be directed to the corresponding authors.
Authors' contributions
XZhou and ZH conceived the study and revised the
manuscript. QH, JL and MM performed the experiments and drafted the
original manuscript. ML, RH and XZhong participated in conducting
the study and in data analysis. XS, JS and WF edited the manuscript
and were involved in data curation. WM, WZ and BZ were responsible
for the statistical analysis. QH and JL confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved (No. 20220070)
by the Animal Ethics Committee (AEC) of the China Science and
Technology Industry Holdings (Shenzhen) Co., Ltd.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Shenzhen
Science and Technology Project (Nos. JCYJ20210324120405015 and
JCYJ20180302173542393).
References
|
1
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo
M: Challenges of combination therapy with immune checkpoint
inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shan Y, Yang G, Huang H, Zhou Y, Hu X, Lu
Q, Guo P, Hou J, Cao L, Tian F and Pan Q: Ubiquitin-like modifier
activating Enzyme 1 as a novel diagnostic and prognostic indicator
that correlates with ferroptosis and the malignant phenotypes of
liver cancer cells. Front Oncol. 10:5924132020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang K, Zhang Z, Tsai HI, Liu Y, Gao J,
Wang M, Song L, Cao X, Xu Z, Chen H, et al: Branched-chain amino
acid aminotransferase 2 regulates ferroptotic cell death in cancer
cells. Cell Death Differ. 28:1222–1236. 2021. View Article : Google Scholar :
|
|
8
|
Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M,
Sun Q, Zhang Z, Liu W, Xu W, et al: Abrogation of ARF6 promotes
RSL3-induced ferroptosis and mitigates gemcitabine resistance in
pancreatic cancer cells. Am J Cancer Res. 10:1182–1193.
2020.PubMed/NCBI
|
|
9
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Yang S, Wang K, Bao X, Liu Y, Zhou
S, Liu H, Qiu Y, Wang T and Yu H: Alkaloids from traditional
Chinese medicine against hepatocellular carcinoma. Biomed
Pharmacother. 120:1095432019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R,
Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al: PPVI induces
caspase-1-mediated pyroptosis via the induction of
ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer.
Cancers (Basel). 12:1932020. View Article : Google Scholar
|
|
13
|
Wang P, Yang Q, Du X, Chen Y and Zhang T:
Targeted regulation of Rell2 by microRNA-18a is implicated in the
anti-metastatic effect of polyphyllin VI in breast cancer cells.
Eur J Pharmacol. 851:161–173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan YL, Jiang N, Li ZY, Song ZZ, Yang ZH,
Xue WH, Zhang XJ and Du Y: Polyphyllin VI induces apoptosis and
autophagy in human osteosarcoma cells by modulation of ROS/JNK
activation. Drug Des Devel Ther. 13:3091–3103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Ke B, Tian M, Li J, Liu B and He G:
Targeting programmed cell death using small-molecule compounds to
improve potential cancer therapy. Med Res Rev. 36:983–1035. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strasser A and Vaux DL: Cell death in the
origin and treatment of cancer. Mol Cell. 78:1045–1054. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng JF, Qin DL, Mei QB, Qiu WQ, Pan R,
Xiong R, Zhao Y, Law BY, Wong VK, Tang Y, et al: Polyphyllin VI, a
saponin from Trillium tschonoskii Maxim. induces apoptotic and
autophagic cell death via the ROS triggered mTOR signaling pathway
in non-small cell lung cancer. Pharmacol Res. 147:1043962019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Ye J, Xie Q, Liu B and Liu M:
Targeting regulated cell death with pharmacological small
molecules: An update on autophagy-dependent cell death,
ferroptosis, and necroptosis in cancer. J Med Chem. 65:2989–3001.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv
H, AlQudsy LHH and Shang P: Ferroptosis, a novel pharmacological
mechanism of anti-cancer drugs. Cancer Lett. 483:127–136. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar
|
|
23
|
Zhang W, Jiang B, Liu Y, Xu L and Wan M:
Bufotalin induces ferroptosis in non-small cell lung cancer cells
by facilitating the ubiquitination and degradation of GPX4. Free
Radic Biol Med. 180:75–84. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang MT, Tsai LC, Nakagawa-Goto K, Lee KH
and Shyur LF: Phyto-sesquiterpene lactones DET and DETD-35 induce
ferroptosis in vemurafenib sensitive and resistant melanoma via
GPX4 inhibition and metabolic reprogramming. Pharmacol Res.
178:1061482022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Gong M, Zhang W, Mo J, Zhang S,
Zhu Z, Wang X, Zhang B, Qian W, Wu Z, et al: Thiostrepton induces
ferroptosis in pancreatic cancer cells through STAT3/GPX4
signalling. Cell Death Dis. 13:6302022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bakir B, Chiarella AM, Pitarresi JR and
Rustgi AK: EMT, MET, plasticity, and tumor metastasis. Trends Cell
Biol. 30:764–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen P, Li X, Zhang R, Liu S, Xiang Y,
Zhang M, Chen X, Pan T, Yan L, Feng J, et al: Combinative treatment
of β-elemene and cetuximab is sensitive to KRAS mutant colorectal
cancer cells by inducing ferroptosis and inhibiting
epithelial-mesenchymal transformation. Theranostics. 10:5107–5119.
2020. View Article : Google Scholar :
|
|
28
|
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X,
Jiang L and Ye L: Cetuximab promotes RSL3-induced ferroptosis by
suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant
colorectal cancer. Cell Death Dis. 12:10792021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Wang C, Yang T, Wang H, Zhao S,
Sun N, Chen Y, Zhang H and Fan H: Chlorogenic acid alleviates
chronic stress-induced duodenal ferroptosis via the inhibition of
the IL-6/JAK2/STAT3 signaling pathway in rats. J Agric Food Chem.
70:4353–4361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Q and Wang K: The induction of
ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the
sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int.
43:1245–1256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ouyang S, Li H, Lou L, Huang Q, Zhang Z,
Mo J, Li M, Lu J, Zhu K, Chu Y, et al: Inhibition of
STAT3-ferroptosis negative regulatory axis suppresses tumor growth
and alleviates chemoresistance in gastric cancer. Redox Biol.
52:1023172022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Y, Gao X, Zou L, Lei M, Feng J and Hu
Z: Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis
in osteosarcoma cells. Oxid Med Cell Longev. 2021:17834852021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo H, Xiao Y, Yuan Z, Yang X, Chen J,
Chen C, Wang M, Xie L, Chen Q, Tong Y, et al: Inhibition of
STAT3Y705 phosphorylation by Stattic suppresses
proliferation and induces mitochondrial-dependent apoptosis in
pancreatic cancer cells. Cell Death Discov. 8:1162022. View Article : Google Scholar
|
|
34
|
Gaschler MM, Andia AA, Liu H, Csuka JM,
Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E,
et al: FINO2 initiates ferroptosis through GPX4
inactivation and iron oxidation. Nat Chem Biol. 14:507–515. 2018.
View Article : Google Scholar : PubMed/NCBI
|