Introduction
Gastric carcinoma (GC) is the third commonest cause
of cancer death globally (1).
Multiple risk factors, including gene polymorphism, dietary habits
and smoking, have been reported as key factors in GC. However,
H. pylori infection is known as the main risk, accounting
for 90% of new cases of non-cardia gastric cancer (2). Growing evidence shows that H.
pylori virulence factors have important roles in the
carcinogenesis pattern 'chronic gastritis-gastric mucosa
atrophy-intestinal metaplasia-dysplasia-gastric cancer' (3-5).
Particularly, CagA is demonstrated to be the critical risk factor
for chronic infection (6,7). It can interact with a variety of
intracellular proteins, perturb multiple intracellular signaling
pathways and promote the malignant transformation of the host cells
(6). However, the molecular
mechanism of H. pylori (CagA+)-induced GC is still not fully
understood.
microRNAs (miRNAs/miRs) serve crucial roles in a
variety of biological processes, including tumorigenesis and
progression (8). Aberrant
regulation of miRNAs can function as oncogenes or anti-oncogenes by
binding with its specific target mRNAs, resulting in mRNA
degradation or/and translational repression (9). Studies have revealed the regulatory
roles of miRNAs in H. pylori-induced inflammation and
carcinogenesis (10-13).
As an ancient miRNA, miR-7 is involved in the
fine-tuning of several signaling pathways, serving primarily as a
tumor suppressor in various cancers (14). It can suppress tumor metastasis via
neuro-oncological ventral antigen 2 (NOVA2) in non-small cell lung
cancer (15), suppress the
progression of pancreatic cancer via mitogen-activated protein
kinase kinase 9 (MAP3K9) (16) and
reduce breast cancer stem cell metastasis by suppressing RELA
proto-oncogene, NF-κB subunit (RELA) and decreasing the expression
of endothelial cell-selective adhesion molecule (ESAM) (17). miR-7 is downregulated in GC
(18); it can inhibit
proliferation and angiogenesis and promotes apoptosis of GC cells
via Raf-1 (19), increase
cisplatin sensitivity of GC cells through suppressing mTOR
(20), inhibit the invasion and
metastasis of GC cells through inhibitions of EGFR (21) and insulin-like growth factor-1
receptor (IGF1R) (22).
Particularly, it can form a regulatory feedback circuit with NF-κB
(23) and act as a potential
therapeutic target for aberrant NF-κB-driven distant metastasis of
GC (24).
miR-153 exerts opposite functions as a tumor
promoter or suppressor in different types of cancer. It can improve
sensitivity to gefitinib by regulating the expression of ABCE1 in
lung cancer (25), inhibit
triple-negative breast cancer by targeting zine finger
E-box-binding homeobox2 (ZEB2)-induced epithelial-mesenchymal
transition (EMT) (26), regulate
the progression of ovarian carcinoma by targeting myeloid cell
leukemia 1 (MCL1) (27) and
decrease tryptophan catabolism and inhibit angiogenesis via the
repression of indoleamine 2,3-dioxygenase 1 in bladder cancer
(28). Meanwhile, high expression
of miR-153 is relevant for a poor prognosis for prostate cancer
patients (29). miR-153 also
increases invasion and therapeutic resistance by pleiotropic
effects in colorectal cancer progression (30). Nevertheless, relevant studies of
miR-153 in GC are rare. miR-153 acts as a tumor suppressor by
aiming at Krüppel-like factor 5 (KLF5) in GC (31).
To date, the synergistic roles of miRNAs have not
been well elucidated in GC. In the present study, an integrated
approach was adopted and identified that miR-7 and miR-153 were
involved in H. pylori-induced gastric carcinogenesis and
progression. The present study systematically validated that
simultaneous overexpression of miR-7 and miR-153 significantly
promoted apoptosis, inhibited proliferation and inflammatory
response by multiple targets. In addition, it was discovered that a
combination of miR-7 and miR-153 might serve as novel diagnostic
and therapeutic targets in H. pylori CagA (+)-associated
GC.
Materials and methods
Cell lines, bacterial strains and
culture
Human normal gastric epithelial cell line
GES-1(RRID:CVCL_EQ22) and human GC cell lines MKN-45
(RRID:CVCL_0434), MKN-28 (RRID:CVCL_1416), HGC-27 (RRID:CVCL_1279),
SNU-1 (RRID:CVCL_0099) and AGS (RRID:CVCL_0139) were purchased from
the BeNa Culture Collection. GC cell line NCL-87 was purchased from
the National Collection of Authenticated Cell Cultures. Cell lines
were cultured in RPMI-1640 medium (HyClone; Cytiva) and maintained
in a humidified atmosphere containing 5% CO2 at 37°C.
All cell cultures were supplemented with 10% fetal bovine serum
(FBS; Biological Industries), 100 IU/ml penicillin and 100
µg/ml streptomycin (Gibco, Thermo Fisher Scientific,
Inc.).
H. pylori clinical isolate SBK was isolated
from a gastric ulcer patient and preserved in Central Lab of Weihai
Municipal Hospital. CagA-knockout H. pylori SBK was
established according to the previously described method (32). H. pylori strain ATCC 26695
and its corresponding CagA-knockout H. pylori (named 0547)
were kindly provided by Professor Boqing Li of Binzhou Medical
University (Yantai, China). All strains were maintained in
Colombian agar plates supplemented with 8% sheep blood under a
microaerophilic environment.
Cell chronic infection model
GES-1 cells were co-cultured with H. pylori
strain ATCC 26695 or isolated SBK at a multiplicity of infection
(MOI) of 100:1 to construct the H. pylori chronic infection
model, as previously described (33). However, only the SBK-treated GES-1
survived and was termed GES-1/HP. GES-1cells without exposure to
H. pylori underwent the same procedure and were designated
as control cells.
Construction and sequencing of miRNA
libraries
High-throughput sequencing was performed to identify
miRNAs as follows: steps. Total RNA isolated from H.
pylori-infected GC tissues and paired adjacent normal tissues
were fractionated with a 15% native polyacrylamide gel (Invitrogen;
Thermo Fisher Scientific, Inc.) and small RNAs of 18-30 nt were
purified. Small RNAs were reverse transcribed into cDNA and further
amplified. The sequence of the amplified products was performed on
the Illumina HiSeq 2500 platform (Guangzhou RiboBio Co., Ltd.).
miRNA/RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total miRNAs or RNA were extracted from
1×107 cells with RNAiso for Small RNA or RNAiso Plus
(Takara Bio, Inc.) according to the manufacturer's protocol, and
the concentrations were measured with the NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc.). Then, 1-3
µg miRNA or RNA was reverse transcribed. RT-qPCR was
performed using TB Green Premix Ex Taq II (Takara Biotechnology
Co., Ltd.) on the 7500 Real-Time PCR instrument (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. U6
small nuclear RNA and β-actin were used as an internal control for
miRNA and mRNA assays, respectively. Specific primers used for SP1,
Bcl-xL, Bcl2, β-actin, miR-7, miR-153 and U6 were as follows:
5′-CCT CAG TGC ATT GGG TAC TTC (forward) and 5′-ACC AAG CTG AGC TCC
ATG AT (reverse) for SP1; 5′-GAG CTG GTG GTT GAC TTT CTC (forward)
and 5′-TCC ATC TCC GAT TCA GTC CCT (reverse) for Bcl-xL; 5′-GGT GGG
GTC ATG TGT GTG G (forward) and 5′-CGG TTC AGG TAC TCA GTC ATC C
(reverse) for Bcl2; 5′-CAT GTA CGT TGC TAT CCA GGC (forward) and
5′CTC CTT AAT GTC ACG CAC GAT-(reverse) for β-actin; 5′-CCA CGT TGG
AAG ACT AGT GAT TT (forward) and 5′TAT GGT TTT GAC GAC TGT GTG
AT-(reverse) for miR-7; 5′-AAC GAA CTT GCA TAG TCA CAA AAG
(forward) and 5′TAT GGT TTT GAC GAC TGT GTG AT-(reverse) for
miR-153; 5′-CAG CAC ATA TAC TAA AAT TGG AAC G (forward) and 5′ACG
AAT TTG CGT GTC ATC C-(reverse) for U6. Fold changes of target
miRNAs or genes were calculated by the 2−ΔΔCq method
from triplicate experiments (34).
Western blotting
Cells or tissue samples were collected and lysed
with T-PER (Thermo Fisher, Inc.) containing protease inhibitor and
phosphatase inhibitor cocktail (Biotool, LLC), then incubated 30
min on ice and centrifuged at 13,700 × g at 4°C for 15 min. The
supernatants were collected carefully and determined by BCA protein
concentration assay kit (Beyotime Institute of Biotechnology).
Total proteins (30 µg) were separated by 10% SDS-PAGE. Gels
were transferred onto PVDF membranes by wet transfer. Membranes
were blocked in 5% BSA in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.05%
Tween-20, pH 8.0) at 37°C for 2 h, incubated with primary
antibodies to Bax (1:1,000; cat. no. 5023), Bcl-xL (1:1,000; cat.
no. 2764), Bcl-2 (1:1,000; cat. no. 15071), Cleaved Caspase-3
(1:1,000; cat. no. 9661), phosphorylated (p)-AKT (cat. no. 4060),
IL-1β (1:1,000; cat. no. 12703S) and AKT (1:1,000; cat. no. 4685;
Cell Signaling Technology, Inc.); P53 (1:1,000; cat. no.
60283-2-Ig), P27 (1:1,000; cat. no. 25614-1-AP), SP1 (1:1,000; cat.
no. 21962-1-AP), Cyclin A2 (1:1,000; cat. no. 66391-1-Ig), Cyclin
E2 (1:1,000; cat. no. 11935-1-AP), Toll-like receptor 4 (TLR4;
1:1,000; cat. no. 19811-1-AP), Krüppel-like factor (KLF)5 (1:1,000;
cat. no. 21017-1-AP; Proteintech Group, Inc.) and Ki-67 (1:1,000;
cat. no. AF1738) and NF-κB p65 (1:1,000; cat. no. AF0246; Beyotime
Institute of Biotechnology) overnight at 4°C, and then incubated
with appropriate HRP-conjugated secondary antibody (1:5,000; cat.
nos. A0208 or A0216; Beyotime) at room temperature for 30 min.
Finally, the specific bands were visualized using super sensitive
ECL chemiluminescence detection reagent (Tiangen Biotech Co., Ltd.)
and imaged with Chemidoc XRS+ (Bio-Rad Laboratories, Inc.).
Cell apoptosis assay
This was performed according to the manufacturer's
protocol as described previously (33). Briefly, cells were harvested,
resuspended and labelled using an Annexin V-FITC apoptosis
detection kit (BD Biosciences). Then, cells were analyzed by flow
cytometer BD FACSaria II (BD Biosciences), and the total percentage
of early + late apoptotic cells were processed for the apoptotic
rate using the CellQuest Pro software v8.0.1 (BD Biosciences).
EdU assay
This was performed in accordance with the
manufacturer's protocol using the BeyoClick EdU Cell Proliferation
kit with Alexa Fluor 488 (Beyotime Institute of Biotechnology). In
brief, cells were incubated with 10 µM EdU reagent at 37°C
for 2 h, fixed with 4% paraformaldehyde for 15 min and permeated
with 0.3% Triton X-100 for another 15 min at room temperature. The
cells were incubated with the Click Reaction Mixture at room
temperature for 30 min and then incubated with Hoechst 33342 for 10
min in a dark place. The ratio of EdU-positive cells to the total
number of Hoechst 33342-positive cells was calculated as the cell
proliferation rate.
Cell transfection
GES-1/HP cells and HGC-27 cells under H.
pylori infection (5×105 cells/well) were transfected
with miR-7-5p mimics (50 nmol; cat. no. miR1181031074037-1-5),
miR-153-3p mimics (50 nmol; cat. no. miR10000439-1-5), combination
of miR-7-5p (25 nmol) and miR-153-3p mimics (25 nmol), mimics
negative control (NC, 50 nmol; cat. no. miR1N0000001-1-5; Guangzhou
RiboBio Co., Ltd.), SP1 short interfering (si)RNA (cat. no. A10001)
and negative control siRNA (cat. no. A06001; Shanghai GenePharma
Co., Ltd.) in 6-well plates using the Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol at 37°C for 6 h. The
sequences of validated siRNA for SP1 were: 5′-CCA GCA ACA UGG GAA
UUA UTT (forward) and 5′-AUA AUU CCC AUG UUG CUG GTT (reverse) for
si-1; 5′-GCC GUU GGC UAU AGC AAA UTT (forward) and 5′-AUU UGC UAU
AGC CTT AAC GGC TT (reverse) for si-2; 5′-GCC CUU AUU ACC ACC AAU
ATT (forward) and 5′-UAU UGGUGG UAA UAA GGG CTT (reverse) for si-3;
the sequences of negative control were 5′-UUC UCC GAA CGU GUC ACG
UTT (forward) and 5′-ACG UGA CAC GUU CGG AGA ATT (reverse). The
time interval between transfection and subsequent experimentation
was 48 h.
Dual-luciferase reporter assay
Bcl-xL-3′-UTR/wild-type (WT), Bcl-xL-3′-UTR/mutant
(MUT), Bcl2-3′-UTR/WT and Bcl2-3′-UTR/Mut were cloned and embedded
into the psiCHECK-2 plasmid. Cells were co-transfected with either
WT or MUT luciferase reporter vector and either mimic miRNAs or
negative control using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.), respectively. After 48 h
transfection, cells were treated using Dual-Luciferase Reporter
Gene Assay kit (Beyotime Institute of Biotechnology). Dual
fluorescent signals were detected with a luminometer (GloMax
Navigator; Promega Corporation) and each value for Renilla
luciferase activity was normalized by firefly luciferase
activity.
Cell cycle assay
The cell cycle assay was conducted using a cell
cycle and apoptosis kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Cells transfected with
miR-7, miR-153 or miR-7 and miR-153 together at 37°C for 48 h were
digested and stained with propidium iodide at 37°C for 30 min in
the dark. Then, the cell DNA content was determined by the flow
cytometer BD FACSaria II (BD Biosciences), and the cell cycle was
analyzed using the FlowJo software (v10.8.2; FlowJo LLC).
Fluorescence in situ hybridization (FISH)
analysis
A FAM-labeled Locked Nucleic Acid (LNA) probe for
detecting miR-7 and a Cy3-labeled LNA probe for detecting miR-153
were synthesized and performed on paraffin sections using a
fluorescence in situ hybridization kit (Shanghai GenePharma Co.,
Ltd.). In brief, firstly, the dissected tissues were fixed with 10%
formalin for 48 h at room temperature. After rinsing the tissues
with running tap water and dehydrating the tissues in EtOH baths at
70, 95 and 100% ethanol, successively, the tissues were incubated
in a 65°C paraffin bath twice, 30 min each. Then, the
paraffin-embedded tissues were sectioned at 4 µm.
Subsequently, the paraffin sections were dewaxed, rehydrated,
digested with proteinase K at 37°C for 20 min, and dehydrated
through a graded series of alcohol. Then, the sections were
hybridized with the probes directed against miR-7 and miR-153 at
37°C for overnight in wet box, and further counterstained with
4,6-diamidino-2-phenylindole (DAPI) at room temperature for 10 min.
Images were captured using fluorescence microscopy (Nikon
Corporation).
Immunohistochemical (IHC) analysis
Paraffin sections were made from H.
pylori-infected GC tissues, adjacent normal tissues and
xenograft tumor samples according the following steps: First, the
dissected tissues were fixed with 10% formalin for 48 h at room
temperature. After rinsing the tissues with running tap water and
dehydrating the tissues in EtOH baths in the 70, 95 and 100%
ethanol, successively, the tissues were incubated in a 65°C
paraffin bath twice, 30 min each. Then, the paraffin-embedded
tissues were sectioned at 4 µm. The paraffin sections were
dewaxed and underwent antigen retrieval with citrate buffer. The
slides were blocked with 0.1% BSA and incubated overnight with the
following antibodies: Bcl-xL (1:50; cat. no. 2764; Cell Signaling
Technology, Inc.), Bcl-2 (1:50; cat. no. 15071; Cell Signaling
Technology, Inc.), Ki-67 (1:100; cat. no. AF1738; Beyotime
Institute of Biotechnology), SP1 (1:50; cat. no. 21962-1-AP;
Proteintech Group, Inc.) or P53 (1:100; cat. no. 60283-2-Ig) at 4°C
overnight, and then incubated with HRP-conjugated secondary
antibodies (1:50; Beyotime Institute of Biotechnology) at 37°C for
1 h. Finally, the target proteins were visualized on-site with the
3,3′-diaminobenzidine substrate (Beyotime Institute of
Biotechnology) and images captured using fluorescence microscopy
(Nikon Corporation).
Chromatin immunoprecipitation (ChIP)
ChIP assay was conducted following the instructions
of the ChIP Assay kit (Beyotime Institute of Biotechnology).
Briefly, cultured cells (1×107 cells in 10 cm dish) were
fixed with 1% formaldehyde at 37°C for 10 min, and then neutralized
with 125 mM glycine solution (1.1 ml) at room temperature for 5
min. Chromatin solutions were sonicated and incubated with anti-SP1
antibody (1:100) or control IgG (1:100) overnight with rotation at
4°C. DNA-protein cross-links were disassembled and chromatin DNA
was purified and subjected to PCR analysis with the primers: 5′-CCG
CTC GAG CGG CCA GGT CGG CGA GAA TCC T-3′ and 5′-CCC AAG CTT GGG TTA
GCG CCA GTC TTG AGC ACA T-3′ for SP1, 5′-TAC TAG CGG TTT TAC GGG
CG-3′ and 5′-TCG AAC AGG AGG AGC AGA GAG CGA-3′ for GAPDH as
a negative control. DNA isolated from total nuclear extract was
used the PCR input control, and the PCR products were analyzed by
gel electrophoresis using a 2% agarose gel. PrimeSTAR HS DNA
Polymerase (Takara Bio Inc.) was used for the PCR process.
Thermocycling conditions was initial denaturation: 98°C for 5 min;
30 cycles of denaturation (98°C for 10 sec), annealing (60°C for 15
sec) and elongation (72°C for 15 sec).
Nude mouse tumor xenograft model
A total of 20 Balb/c male nude mice, 4-6 weeks old,
weighing 20-25 g, were obtained from Weihai Desheng Technology
Testing Co., Ltd. and housed in standard pathogen-free conditions
environment with a temperature of 22±1°C, relative humidity of
50±1% and a 12-h light/dark cycle. All animal studies (including
the mouse euthanasia procedure) were approved by the Ethics
Committee of Weihai Municipal Hospital (approval no. 20220103) and
conducted in compliance with the regulations and guidelines of
Weihai Municipal Hospital institutional animal care and conducted
according to the AAALAC and the IACUC guidelines.
To establish a xenograft model, 1×107
HGC-27 cells were suspended in 100 µl PBS and inoculated
subcutaneously into the right hind flank. After 2 weeks, the
transplanted nude mice were randomly divided into three groups
(n=5/group): i) MicrON hsa-miR-7 (cat. no. miR4180703093100-4-5;
Guangzhou RiboBio Co., Ltd.), ii) micrON hsa-miR-153 (cat. no.
miR40000439; Guangzhou RiboBio Co., Ltd.) and iii) MicrON hsa-miR-7
and micrON hsa-miR-153 together were injected into the unilateral
implanted tumor at the dose of 5 nmol (in 20 µl PBS) per
mouse under anesthesia with isoflurane inhalation (2-3% induction,
1-2% maintenance). The implanted tumor on the other side was
injected with micrON agomir NC#22 (cat. no. miR4N0000001-2-5;
Guangzhou RiboBio Co., Ltd.) as NC. Tumor volume were calculated as
π/6 × width2 × length every 4 days for 5 times. After 6
weeks, mice were euthanized by cervical dislocation under
anesthesia (1% sodium pentobarbital intraperitoneal injection, 30
mg/kg) and tumor tissues were excised, measured, weighed and used
for IHC and FISH. The mice were euthanized when reaching the
following humane endpoints: i) Tumor burden was >10% of body
weight and the diameter of tumor was >20 mm; ii) tumor
ulceration, infection or necrosis was observed; iii) tumor
interferes with eating or walking; iv) rapid weight loss (loss of
15-20% of original weight). Notably, none of these endpoints were
reached during the experiments. Animal health and behavior were
monitored twice a day (at the beginning and end of the day) by an
experienced individual. A comprehensive judgment on death was made
by observing cessation of respiration, heartbeat and pupil and
nerve reflexes.
IL-1β levels quantification (ELISA)
IL-1β levels in GES-1 cells treated with or without
H. pylori infection and biopsy specimens obtained from the
antrum of gastric patients with H. pylori infection and
matched normal tissues were determined in duplicated samples by an
ELISA (cat. no. PI305; Beyotime Institute of Biotechnology).
Briefly, biopsy samples were immediately placed in 2 ml of PBS (pH
7.4), frozen on dry ice, homogenized and centrifuged (10,000 × g,
4°C for 10 min). Then, total protein in supernatants or cell
culture supernatants was measured and IL-1β levels were analyzed
according to the manufacturer's instructions.
Patient samples
All blood and paired samples of H.
pylori-infected GC tissues and adjacent normal tissues were
obtained from Weihai Municipal Hospital. All samples were
clinically and pathologically confirmed.
Tissue microarray analysis
GC tissue microarrays (TMAs) consisting of 24 cases
of gastric disease (including six cases of GC tissue with matched
adjacent normal gastric tissue) were obtained from Shanghai Outdo
Biotech Co., Ltd. FISH probes and specific antibodies or were
applied to slides followed the protocol of IHC and FISH described
previously. Slides were scanned with a confocal scanner and images
captured under fluorescence microscopy (Nikon Corporation). The
staining intensity of TMAs was evaluated and scored using Image-Pro
Plus 6.0 (Media Cybernetics, Inc.).
Bioinformation analysis
TargetScan (https://www.targetscan.org/vert_80/), miRDB
(https://mirdb.org/) and picTar (https://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi)
are widely applicable to predict biological targets of miRNAs. In
this study, we used TargetScan, miRDB and picTar to synthetically
analyzed the targets of miR-7 or miR-153. Pathway Commons
(http://www.pathwaycommons.org/) is a web
resource for biological pathway data. The present study used
Pathway Commons to analyzed the interaction between Sp1 and
p53.
Statistical analysis
For the majority of experiments, the two-tailed,
unpaired, Student's test was applied to compare groups. For paired
GC and adjacent non-tumor tissues, the two-tailed, paired,
Student's test was used (GraphPad Prism V. 7.0 software;
Dotmatics). Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Dunnett's post hoc test. Pearson
correlation analysis was used to analyze correlation of two
indexes. Data are presented as mean ± SD. P-values <0.05 was
considered significant. receiver operating characteristic curve was
introduced for assessing the diagnostic potential of miR-7, miR-153
and their combine in GC via Statistical Product and Service
Solutions (SPSS) 22.0 (IBM, Armonk, NY, USA).
Results
miR-7 and miR-153 were downregulated in H.
pylori-associated GC. Expression profile changes of miRNAs were
analyzed between H. pylori-infected GC tissues and compared
adjacent normal tissues using the Illumina HisSeq 2500
high-throughput sequencing (miRNA-seq) technique. A total of 331
miRNAs (159 upregulated and 172 downregulated, Table SI) were identified after fold
change filtering and illustrated with hierarchical clustering
analysis, scatter plots and volcano plots (Fig. 1A-C), respectively. Partially
differently expressed miRNAs were further confirmed in our
established chronic H. pylori-infected model GES-1/HP cells
(Fig. 1D). Notably, both miR-153
and miR-7 were found to be significantly downregulated in H.
pylori infected GC tissues and GES-1/HP cells, revealing that
dysregulation of miR-7 and miR-153 is not only involved in H.
pylori-induced gastric malt lymphoma (13).
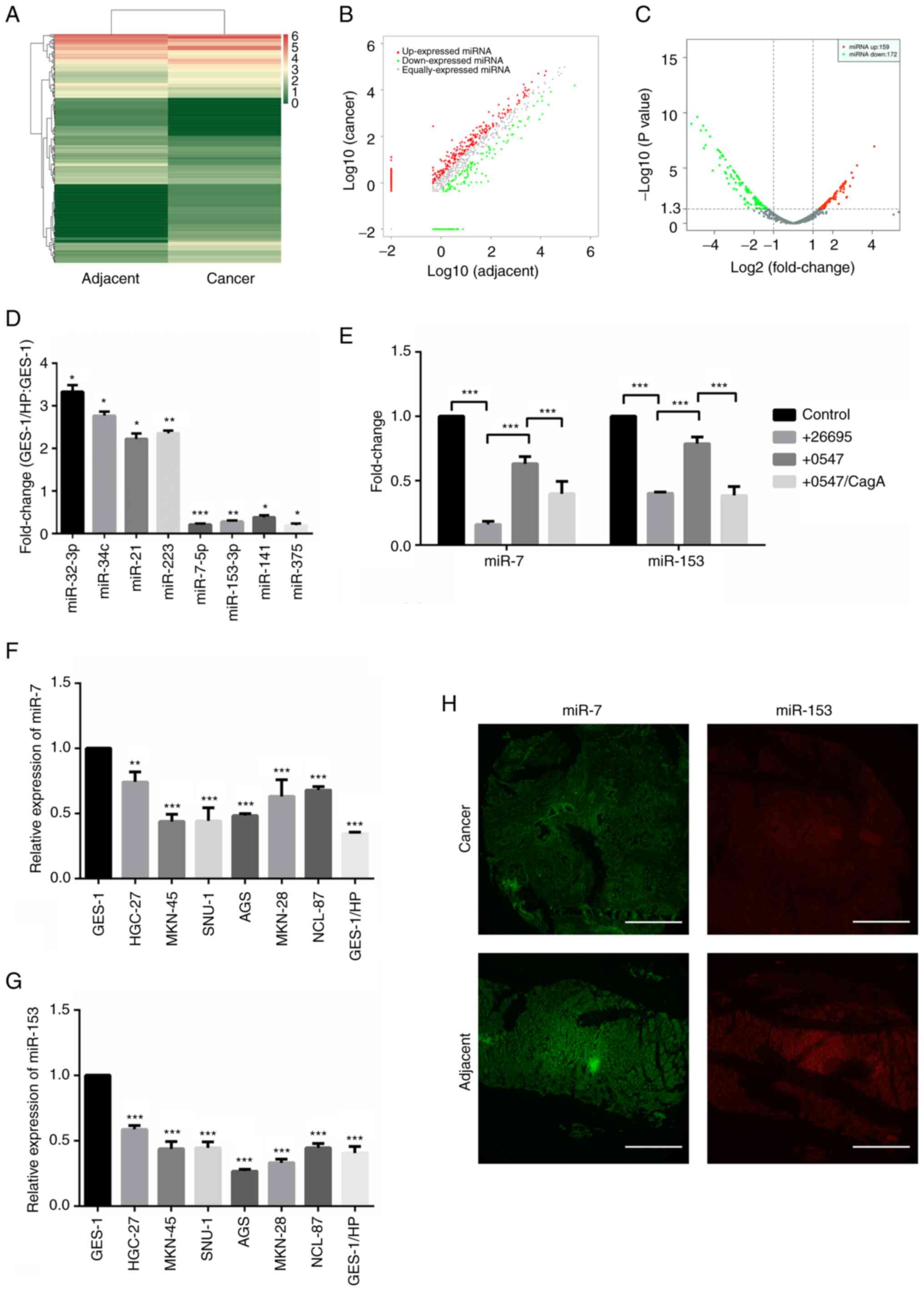 | Figure 1miR-7 and miR-153 are downregulated
in H. pylori-associated GC. (A) Representative heatmap of
differential expression levels of miRNAs in H. pylori (+)
gastric cancer tissues compared with adjacent normal tissues
obtained by sequencing on the Illumina HiSeq 2500 platform. (B)
Scatter plots used to evaluate differences in the expression of
miRNAs. (C) Volcano plot indicating differential expression. P≤0.05
and fold change ≥2 were considered statistically significant. (D)
Partial upregulated and downregulated miRNAs validated by RT-qPCR.
*P<0.05, **P<0.01,
***P<0.001. (E) Fold changes of miR-7 and miR-153
detected with RT-qPCR under treatment with H. pylori 26695
and 0547 with or without the addition of the recombinant protein
CagA. ***P<0.001. (F) Relative expression of miR-7
was detected in GES-1, GC cells and GES-1/HP cells by RT-qPCR.
***P<0.001. (G) Relative expression of miR-153
detected in GES-1, GC cells and GES-1/HP cells by RT-qPCR.
***P<0.001. (H) Differential expressions of miR-7 and
miR-153 were detected in H. pylori-infected GC tissues and
adjacent normal tissues by fluorescence in situ
hybridization. Scale bar, 500 µm. H. pylori,
Helicobacter pylori; GC, gastric cancer; miRNA/miR,
microRNA; RT-qPCR, reverse transcription-quantitative PCR; CagA,
cytotoxin-associated gene A. |
CagA plays an important role on miRNA expression
(35). To clarify the effect of
CagA on the downregulation of miR-7 and miR-153, GES-1 cells were
co-cultured with H. pylori strain 26695 and its
corresponding CagA knockout strain 0547 with or without the
addition of recombinant protein CagA, respectively. The results
showed that infection of H. pylori strain 26695
significantly decreased the expression of miR-7 and miR-153 in
GES-1 cells. Meanwhile, compared with infection of H. pylori
strain 26695, a clear elevation was found in expression of miR-7
and miR-153 accompanied by knockout of CagA. The expression of
miR-7 and miR-153 was further weakened with the addition of
recombinant protein CagA, indicating that CagA might account for
the downregulation of miR-7 and miR-153 (Fig. 1E). Subsequently, miR-7 and miR-153
were analyzed in GES-1 cells, GC cell lines and GES-1/HP cells and
the results showed that miR-7 and miR-153 were both downregulated
(Fig. 1F and G). The lower
expression of miR-7 and miR-153 was confirmed by FISH analysis in
H. pylori-infected GC tissues compared with adjacent normal
tissues (Fig. 1H).
miR-7 and miR-153 combination significantly
accelerates apoptosis and autophagy in H. pylori-associated GC
cells. Numerous miRNAs regulate programmed cell death including
apoptosis, autophagy and necroptosis. Particularly, some miRNAs
serve important roles in crosstalk between apoptosis and autophagy.
Our previous results demonstrated that apoptosis was inhibited in
GES-1/HP cells. Some critical apoptosis-related proteins
significantly changed, e.g., Bcl-xL, Bcl2 and P65 were
significantly increased, while Bax and cleaved caspase-3 were
decreased (33). Furthermore, as
shown in Fig. 2A, an obvious
increase in apoptosis was found when GES-1 cells were treated with
SBK/ΔCagA. Western blotting showed that Bax and cleaved caspase-3
were enhanced in these cells (Fig.
2B).
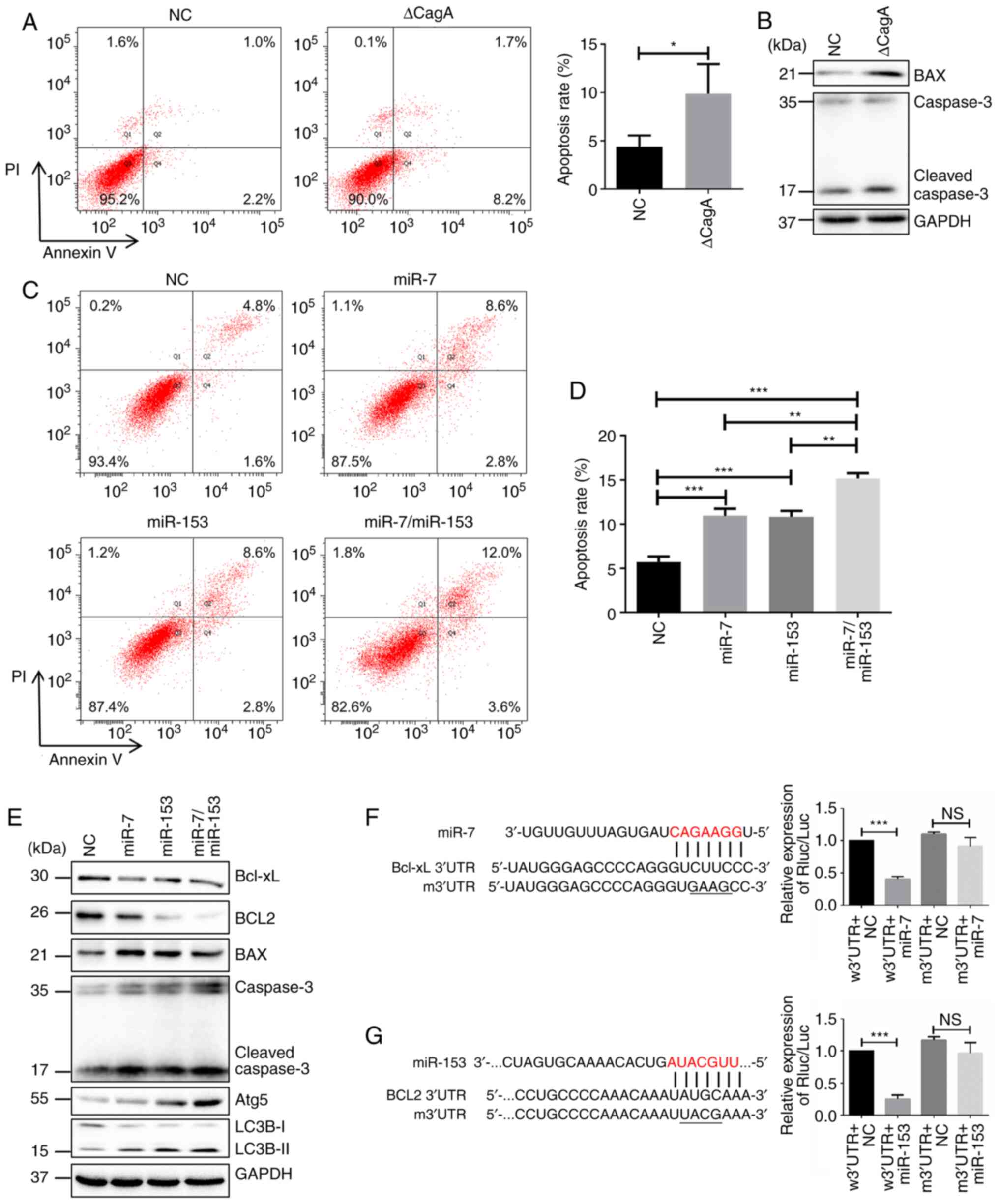 | Figure 2miR-7 and miR-153 significantly
accelerates apoptosis of H. pylori-associated GC cells. (A)
Apoptosis changes detected by flow cytometry in GES-1 cells
following CagA knockdown in H. pylori SBK strain.
*P<0.05. (B) Changes of apoptosis protein determined
by western blotting. (C and D) Apoptosis changes detected in flow
cytometry when miR-7, miR-153 or their combination overexpressed in
GES-1/HP cells compared with NC. *P<0.05,
**P<0.01, ***P<0.001. (E) Changes of
apoptosis and autophagy related protein determined by western
blotting. (F) Left: Putative miR-7 binding sites on 3′-UTR of
Bcl-xL and the site discrepancies between wild-type and mutant
type; right: GES-1/HP cells co-transfected with miR-7 mimic or
mimic-NC and constructed reporter plasmids and relative luciferase
activities were detected. ***P<0.001, NS, not
significant. (G) Left: Putative miR-153 binding sites on 3′-UTR of
Bcl2 and the site discrepancies between wild-type and mutant type;
right: GES-1/HP cells co-transfected with miR-153 mimic or mimic-NC
and constructed reporter plasmids and relative luciferase
activities of transfected cells were detected.
***P<0.001, NS, not significant. miRNA/miR, microRNA;
H. pylori, Helicobacter pylori; GC, gastric cancer;
NC, negative control; w3′UTR, wild-type; m3′UTR, mutant. |
To clarify the functional role of miR-7 and miR-153
on cell apoptosis, miR-7, miR-153, or their combination were
overexpressed in GES-1/HP and H. pylori-infected HGC-27
cells (Fig. S1), respectively. As
shown in Figs. 2C and D and
S2A, miR-7 or miR-153 enhanced
the apoptosis of the above cells. Western blotting showed that
Bcl-xL and Bcl2 were more repressed, while Bax and cleaved
caspase-3 were more upregulated in cells co-expressing both miR-7
and miR-153 compared to those overexpressing miR-7 or miR-153 alone
(Figs. 2E and S2E). Moreover, it was found that
autophagy marker Atg5 and LC3B increased with overexpression of
miR-7 and miR-153 (Figs. 2E and
S2E), consistent with previous
reports (36,37). Collectively, these results
demonstrated that miR-7 and miR-153 serve important roles in the
crosstalk between apoptosis and autophagy.
Subsequently, bioinformatics algorithms (including
TargetScan, miRDB and PicTar) indicated that there was a potential
binding site of miR-7 in 3′UTR of Bcl-xL and a potential binding
site of miR-153 in 3′UTR of Bcl2 (Fig.
2F and G, left). Dual-luciferase reporter assay showed that
overexpression of miR-7 or miR-153 inhibited luciferase activity in
Bcl-xL-w3′UTR reporter gene constructs or BCL-2-w3′UTR reporter
gene constructs, respectively, but did not affect mutation reporter
gene constructs (right of Fig. 2F and
G, right). These results proved that Bcl-xL was a direct target
of miR-7 and BCL-2 was a direct target of miR-153 in H.
pylori-associated GC cells.
Combination of miR-7 and miR-153 significantly
represses the proliferation of H. pylori-associated GC cells.
Furthermore, the effects of miR-7 and miR-153 on cell proliferation
were determined in GES-1/HP and H. pylori-infected HGC-27
cells, respectively. The results of EdU assay (Figs. 3A and S2B) and clone formation assay (Figs. 3B and S2C) confirmed that cell growth and
proliferation ability were decreased with overexpression of miR-7,
miR-153, or their combination. Western blotting results showed that
the expression of p-AKT was significantly downregulated. Meanwhile,
cell-cycle associated protein P53 and P27 were significantly
upregulated when GES-1/HP and H. pylori-infected HGC-27
cells were overexpressed with miR-7, miR-153, or their combination
(Figs. 3C and S2E). Cell cycle detection also showed
that overexpression of miR-7, miR-153, or their combination could
induce G1 phase arrest (Figs. 3D and S2D).
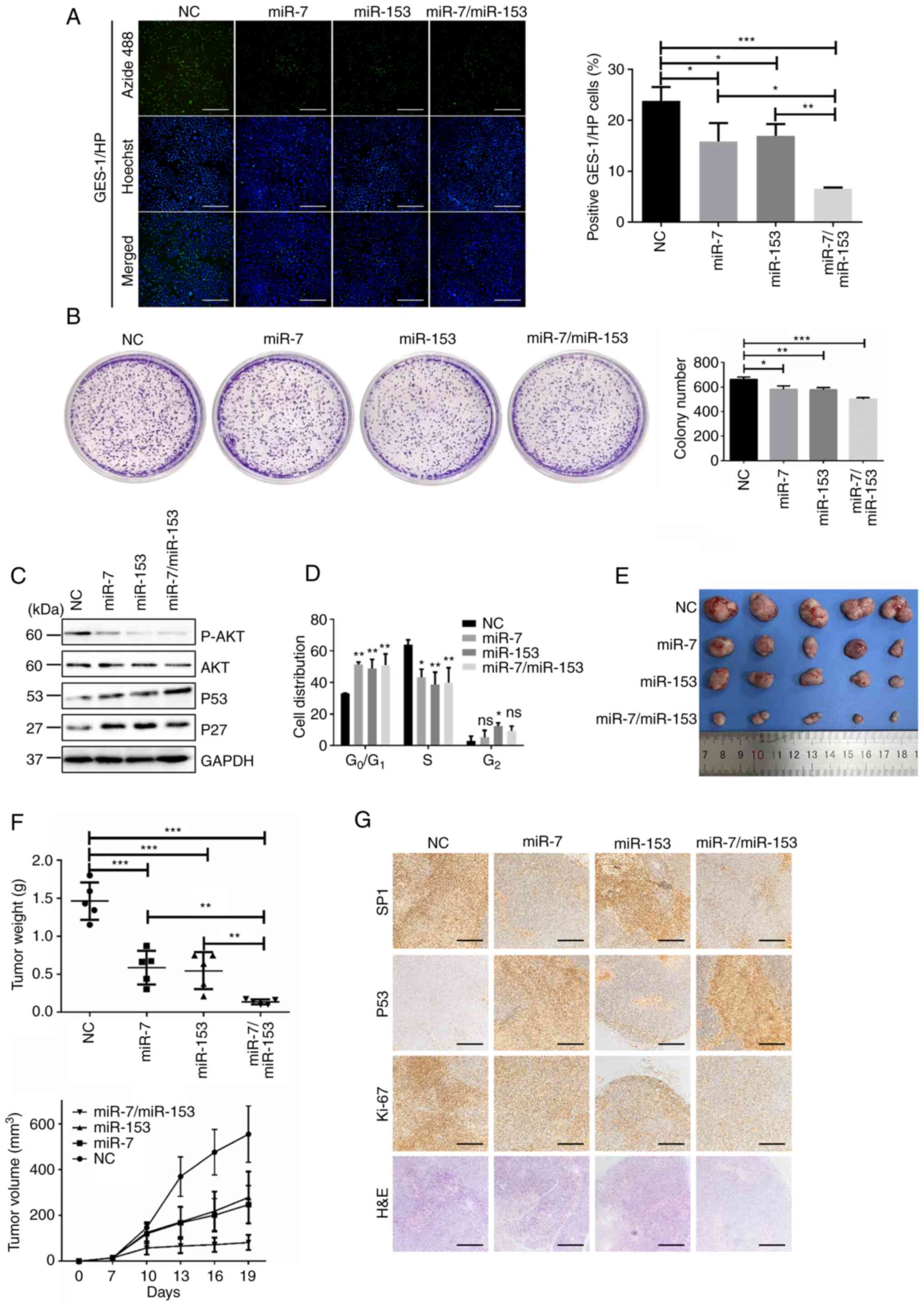 | Figure 3miR-7 and miR-153 combination
obviously represses the proliferation of H.
pylori-associated GC cells. (A) Representative images
illustrating the EdU and Hoechst staining of GES-1/HP cells
transfected with NC, miR-7 or/and miR-153 (left) and analysis of
EdU-positive GES-1/HP cells by and EdU incorporation assay (right).
Scale bar, 500 µm, *P<0.05,
**P<0.01. (B) Clone formation assay.
*P<0.05, **P<0.01,
***P<0.001. (C) Western blot analysis of cell
proliferation. (D) Analysis of cell cycle in GES-1/HP cell
overexpressed with miR-7 or/and miR-153. *P<0.05,
***P<0.001. (E) Isolated tumors of xenograft models.
(F) Tumor weights for various groups are shown on upper panel.
**P<0.01, ***P<0.001. Tumor growth
curve are displayed on lower panel. (G) Representative
immunohistochemical images of subcutaneous tumors of SP1, P53 and
Ki-67. Scale bar, 500 µm. miR, microRNA; H. pylori,
Helicobacter pylori; GC, gastric cancer; NC, negative
control; H&E, hematoxylin and eosin. |
In the xenograft model, miR-7 or miR-153
significantly reduced tumor volumes and weights and the miR-7 and
miR-153 combination group had a much greater inhibitory effect than
miR-7 or miR-153 alone (Fig. 3E and
F). IHC analysis revealed that miR-7, or miR-153, or miR-7 and
miR-153 treatment reduced proliferation via P53 and P27 (Fig. 3G).
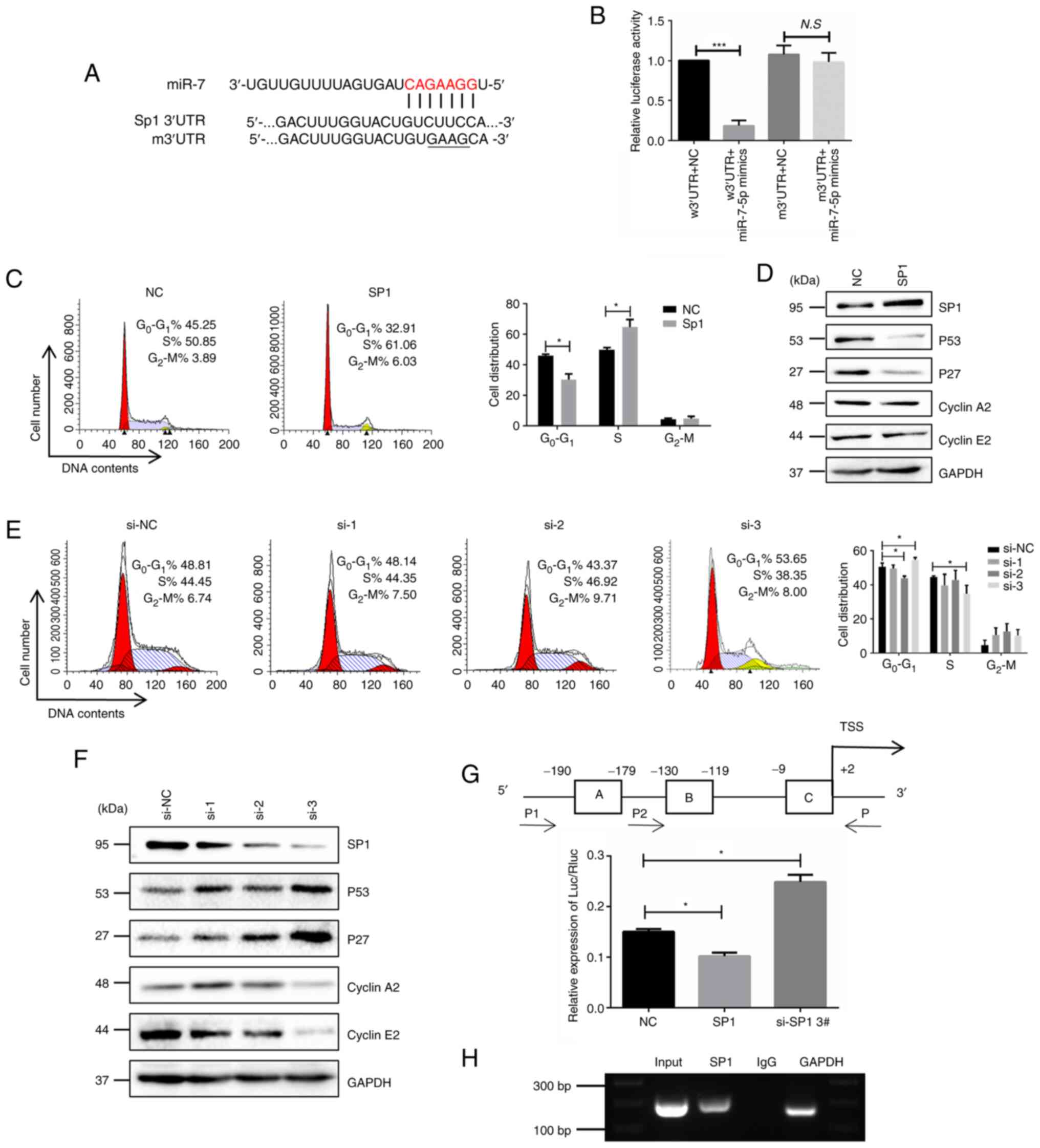
miR-7 induces cell cycle arrest in H.
pylori-associated GC cells via the Sp1/p53 axis. Bioinformatics
algorithms and Pathway Commons (http://www.pathwaycommons.org/) implied that miR-7
might induce cell cycle arrest via the Sp1/p53 axis. Bioinformatics
algorithms (including TargetScan, miRDB and PicTar) indicated a
potential binding site in 3′UTR of Sp1 (Fig. 4A). Dual-luciferase reporter assay
was performed in GES-1/HP cells and the result showed that miR-7
overexpression inhibited luciferase activity in Sp1-wild-type
(w)3′UTR reporter gene constructs, but did not affect Sp1-mutant
(m)3′UTR reporter gene constructs (Fig. 4B). These results proved that sp1
was a direct target of miR-7 in H. pylori-associated GC
cells.
Considering the puzzling interplay between p53 and
Sp1, Sp1 was overexpressed in GES-1 cell and Sp1-siRNA was adopted
in GES-1/HP cells. Cell cycle analysis showed that SP1 is mainly
involved in the G1 phase of the cell cycle (Fig. 4C and D). Western blotting showed
that overexpression of Sp1 downregulated the expression of P53 and
P27. Nevertheless, P53 and P27 were increased with Sp1 knockdown
(Fig. 4D-F). Further promoter
analysis showed three putative binding sites of SP1 in the
approximate transcription start site of P53 (Fig. 4H). Dual luciferase reporter gene
assay showed that SP1 overexpression significantly reduced the
relative luciferase activity, while the relative luciferase
activity was enhanced with SP1 knockdown (Fig. 4G). In addition, the ChIP assay
showed that SP1 could directly bind to the P53 promoter region. All
the findings suggested that cell cycle arrest was enhanced via the
Sp1/p53 axis when miR-7 was overexpressed in H.
pylori-associated GC cells.
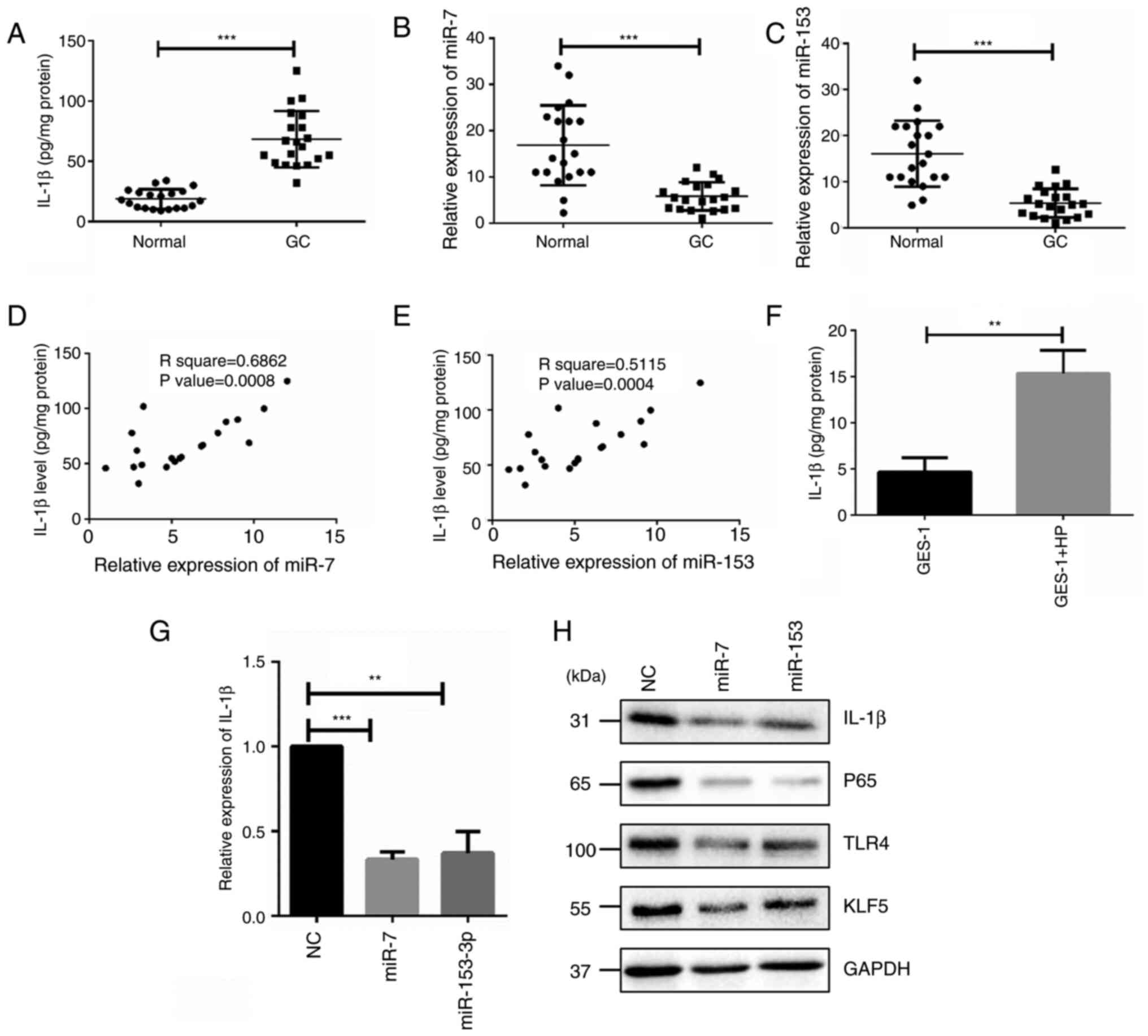
miR-7 and miR-153 regulates NF-kB-induced
inflammation via TLR4 and KLF5/IL-1β. Some evidence shows an
association between IL-1β gene polymorphisms and H. pylori
infection (38). Meanwhile, the
results of the present study also showed that expression of miR-7
and miR-153 were decreased with H. pylori infection. To
further uncover whether miR-7 and miR-153 control the activity of
IL-1β-mediated NF-kB signaling pathway, the present study assessed
the IL-1β levels in biopsy specimens obtained from the antrum of
gastric patients with H. pylori infection and matched normal
tissues. ELISA results showed that IL-1β was significantly enhanced
in patients with H. pylori infection (Fig. 5A). Same samples were used to
analyze the expression of miR-7 and miR-153. The RT-qPCR results
showed that miR-7 and miR-153 were weakened with H. pylori
infection (Fig. 5B and C).
Furthermore, Pearson correlation analysis showed that the
expression of miR-7 or miR-153 was negatively associated with the
expression of IL-1β in H. pylori-infected GC specimens
(Fig. 5D and E). Upregulation of
IL-1β level was also detected when GES-1 cells were treated with
H. pylori infection (Fig.
5F). Moreover, IL-1β was significantly reduced at both mRNA and
protein levels when miR-7 and miR-153 were overexpressed in
GES-1/HP cells (Fig. 5E and F).
Meanwhile, TLR4 and KLF5 were decreased (Fig. 5H), which is consistent with
previous reports, implying that miR-7 and miR-153 regulate
NF-kB-induced inflammation via TLR4 and KLF5.
Potentially diagnostic value of miR-7 and miR-153
in H. pylori-associated gastric GC. To investigate the
correlation between the expression of miR-7 and miR-153 levels and
clinicopathological features of H. pylori-associated GC
patients, 60 tissue specimens were adopted to analyze the clinical
correlation. It was found that low expression of miR-7 and miR-153
was significantly associated with the TNM stage and lymph node
metastasis (Table SII). Moreover,
the expression changes of miR-7, miR-153, Bcl-xL, SP1 and Bcl2 were
analyzed in 20 cases of GC tissues and paired adjacent normal
tissues. Downregulation of miR-7 and miR-153 and upregulation of
Bcl-xL, Sp1 and Bcl2 were found in GC tissues (Fig. 6A and B). Associations between
miR-7, miR-153 and their target Bcl-xL, SP1 and Bcl2 were further
confirmed by FISH and ISH analysis on tissue microarray (Fig. 6C).
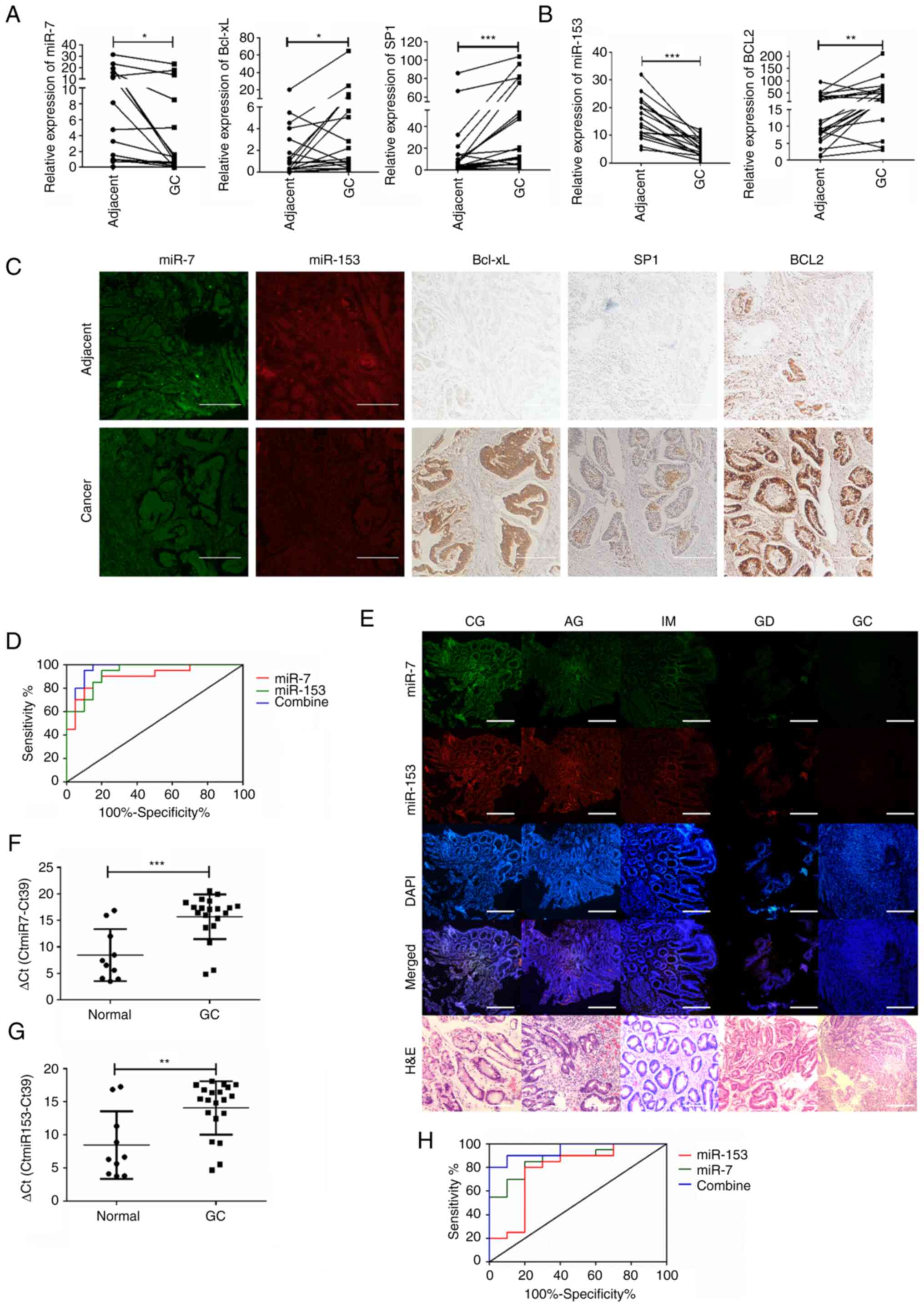 | Figure 6Potentially diagnostic value of miR-7
and miR-153 in H. pylori-associated gastric GC. (A)
Detection of miR-7 and its targets between GC and adjacent normal
tissues. *P<0.05. (B) Detection of miR-153 and its
targets between GC and adjacent normal tissues.
*P<0.05. (C) Differences of miR-7, miR-153 and their
targets were confirmed with FISH and IHC between GC and adjacent
normal tissues. Scale bar, 200 µm. (D) Diagnostic efficacy
of miR-7, miR-153 and their combine in GC tissues. ROC was
introduced for assessing the diagnostic efficacy of miR-7, miR-153
and their combine in GC tissues. GC tissues vs. normal tissues. (E)
Differential expressions of miR-7 and miR-153 were detected in GC
progression by FISH. Scale bar, 500 µm. (F and G) Detection
of miR-7 and miR-153 in serum samples between GC patients and
health control. n=10 in normal group and n=20 in GC group;
**P<0.05, ***P<0.001. (H) Diagnostic
efficacy of miR-7, miR-153 and their combine in GC patient serum.
ROC is introduced for assessing the diagnostic efficacy of miR-7,
miR-153 and their combination in GC patient serum. GC patient serum
vs. normal serum. miR, microRNA; H. pylori, Helicobacter
pylori; GC, gastric cancer; miR, microRNA; TLR4, Toll-like
receptor 4; KLF, Krüppel-like factor; FISH, fluorescence in
situ hybridization; IHC immunohistochemical; ROC, receiver
operating characteristic curve; CG, chronic gastritis; AG, atrophic
gastritis; IM, intestinal metaplasia; GD, gastric dysplasia. |
As shown in Fig.
6D, the area under the curve (AUC) value for combined detection
of miR-7 and miR-153 (AUC=0.9675, 95% Confidence Interval (CI):
0.9192-1.106) indicated a higher sensitivity and specificity than
miR-7 (AUC=0.9675, 95% CI: 0.8019-0.9981) or miR-153 (AUC=0.9325,
95% CI: 0.8598-1.005) alone in GC tissues. Furthermore, based on
the critical role of H. pylori in the carcinogenesis pattern
'chronic gastritis-gastric mucosa atrophy-intestinal
metaplasia-dysplasia-gastric cancer', GC tissues from different
phases of patients and paired adjacent normal tissues were obtained
by endoscopic biopsy or surgical samples and the expression of
miR-7 and miR-153 was detected. Results showed that miR-7 and
miR-153 were gradually decreased in the process of carcinogenesis
(Fig. 6E).
Finally, both miR-7 and miR-153 were detected in
serum between healthy individuals and GC patients with H.
pylori infection. The results showed that miR-7 and miR-153
were significantly downregulated (Fig.
6F and G) and the AUC value for combined detection of miR-7 and
miR-153 (AUC=0.9500, 95% CI: 0.8788-1.021) also indicated a higher
sensitivity and specificity than miR-7 (AUC=0.8750, 95% CI:
0.7493-1.001) or miR-153 (AUC=0.7800, 95% CI: 0.5823-0.9777) alone
in GC serum (Fig. 6H).
Collectively, the results showed the potential diagnostic value of
miR-7 and miR-153 in H. pylori-associated gastric GC.
Discussion
H. pylori is considered a class I carcinogen
and an inducer of the long-term evolution of gastric tissue from
malignant precancerous lesions to GC (39). Growing evidence has shown the
interaction between H. pylori infection and dysregulation of
miRNA expression (40-42). In the present study, a total of 331
miRNAs were identified as abnormal expression between H.
pylori-infected GC tissues and compared adjacent normal
tissues. Among them, a number of miRNAs have been identified as
possessing an association with H. pylori-induced gastric
carcinogenesis. For example, miR-375 is downregulated in H.
pylori-induced GC (43), it
can inhibit H. pylori-induced gastric carcinogenesis by
blocking JAK2-STAT3 signaling (44) and reduce the stemness of GC cells
through triggering ferroptosis (45); miR-196a-5p is increased in H.
pylori-positive GC tissue (13,46).
In the present study, miR-153 and miR-7 were both downregulated
with infection of H. pylori and demonstrated to be
associated with the virulence factor CagA. Further analysis showed
that downregulation of miR-7 and miR-153 can be detected in both
serums and clinical tissues of H. pylori-associated patients
with GC and has been associated with the TNM stage and lymph node
metastasis; therefore, conjoint analysis of miR-7 and miR-153 might
serve as potential candidate marker in early diagnosis of H.
pylori-associated GC.
Bcl-2 families have essential roles in apoptotic
regulation and have garnered interest as therapeutic targets. The
present study identified that Bcl-xL is the target gene of miR-7
and Bcl2 is the target gene of miR-153. Particularly, the combined
application of miR-7 and miR-153 can significantly enhance the
apoptosis of GES-1/HP cells. Growing evidence has shown that Bcl2
is target of miR-7 in different cancer cells (47,48).
However, there was no obvious change on the expression of Bcl2 when
miR-7 was overexpressed in GES-1/HP cells, consistent with the
previous result of genome-wide screenings (23). It was hypothesized that there is
cell specificity. In addition, Bcl2 has been reported to be the
target of miR-153 in human glioma (49), colorectal cancer (50) and chronic myeloid leukemia cells
(51). The results of the present
study confirmed that Bcl2 also can be negative-regulated by miR-153
in GES-1/HP cells.
Previous studies have shown that there is negative
association between miR-7 and SP1 and that the miR-7/SP1 axis
exerts pivotal roles via different downstream pathways. The
miR-7/SP1/TP53BP1 axis may serve a key role in the radiosensitivity
of non-small cell lung cancer (52) and miR-7/SP1 axis acts as an
effector of MTA2 to affect KLK10 levels and mobility of cervical
cancer cells (53). The present
study showed that SP1 also can be repressed in miR-7-overexpressed
GES-1/HP cells and cell proliferation was significantly attenuated
via P53-mediated cell cycle arrest. Previous studies show that
there is a puzzling interplay between p53 and Sp1. As transcription
factors, both p53 and Sp1 regulate critical cellular life and death
decisions. Intriguingly, p53 and Sp1 have similar consensus
sequences on GC-boxes and may compete in binding to specific
promoters and act in opposite directions (54,55).
Li et al (56) report that
Sp1 is a crucial factor for p53-mediated apoptosis but not cell
cycle arrest, which conflicts with the presumed function of Sp1 as
an oncogene. The present study showed that SP1 can repress the
expression of P53 by directly binding to the promoter of p53.
Studies have shown the connection between
inflammation and cancer (57,58).
IL-1β, one of the inflammatory factors, is a potent
pro-inflammatory cytokine and has been associated with an increased
risk of gastritis and non-cardia GC (59). The present study confirmed its
obvious elevation in GC with H. pylori infection (Fig. 5A). TLR4 has reported to possess a
role in the host response to H. pylori infection (60,61),
KLF5 expression is also significantly upregulated following
co-culture of H. pylori (62). TLR4 and KLF5 are both involved in
IL-1β activated NF-κB cascade (63,64).
In the present study, overexpression of miR-7 and miR-153 inhibited
the expression of NF-κB, miR-7 downregulated TLR4 and miR-153
decreased the expression of KLF5 in accordance with previous
studies (65,66).
In conclusion, the present study uncovered a more
comprehensive understanding of miR-7 and miR-153 in apoptosis,
proliferation and inflammation of H. pylori-associated GC
cells and provided insights into miR-7 and miR-153 as potential
diagnostic marker of GC. Furthermore, its findings suggested that
miR-7 and miR-153-targeted therapeutics may represent a potential
strategy for H. pylori CagA-associated gastric cancer.
Supplementary Data
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable request.
The raw data sequencing data from this study have been deposited in
the National Genomics Data Center, China National Center for
Bioinformation/Beijing Institute of Genomics Chinese Academy of
Sciences repository (https://ngdc.cncb.ac.cn/gas-human/; accession no.
HRA003675).
Authors' contributions
YS, DG, JFL, LNG, PL, YMQ, HYC, TL, XC, LYS and YRW
designed and performed experiments, analyzed data, interpreted
results and prepared the manuscript. ZJD helped with designing
experiments and evaluating the manuscript. MYW designed
experiments, analyzed data and wrote the manuscript. DG, LYS and XC
revised the manuscript. MYW and PL confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by the Ethics Committee of Weihai Municipal
Hospital (approval no. 2021021). Informed consent to be included in
the study was obtained from all patients.
Patient consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to Professor Boqing Li of
Binzhou Medical University (Yantai, China) for H. pylori
strain ATCC 26695 and its corresponding CagA-knockout strain.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 82102398), Shandong Provincial
Natural Science Foundation (grant nos. ZR2020QC073, ZR2021MH409 and
ZR2020MH296), Shandong Province Medical and Health Science and
Technology Development Plan (grant nos. 2018WS106 and 202211000314)
and the Incubation Project of Weihai Municipal Hospital (grant nos.
FH-2021-XZ02 and FH-2021-JY16).
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
Helicobacter pylori. Int J Cancer. 136:487–490. 2015. View Article : Google Scholar
|
|
3
|
Wen S and Moss SF: Helicobacter pylori
virulence factors in gastric carcinogenesis. Cancer Lett. 282:1–8.
2009. View Article : Google Scholar :
|
|
4
|
Nejati S, Karkhah A, Darvish H, Validi M,
Ebrahimpour S and Nouri HR: Influence of Helicobacter pylori
virulence factors CagA and VacA on pathogenesis of gastrointestinal
disorders. Microb Pathog. 117:43–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Javed S, Skoog EC and Solnick JV: Impact
of Helicobacter pylori virulence factors on the host immune
response and gastric pathology. Curr Top Microbiol Immunol.
421:21–52. 2019.PubMed/NCBI
|
|
6
|
Takahashi-Kanemitsu A, Knight CT and
Hatakeyama M: Molecular anatomy and pathogenic actions of
Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell
Mol Immunol. 17:50–63. 2020. View Article : Google Scholar :
|
|
7
|
Gao S, Song D, Liu Y, Yan H and Chen X:
Helicobacter pylori CagA PROTEIN ATTENUates 5-Fu sensitivity of
gastric cancer cells through upregulating cellular glucose
metabolism. Onco Targets Ther. 13:6339–6349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dastmalchi N, Safaralizadeh R and Banan
Khojasteh SM: The correlation between microRNAs and Helicobacter
pylori in gastric cancer. Pathog Dis. 77:ftz0392019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parizadeh SM, Jafarzadeh-Esfehani R, Avan
A, Ghandehari M, Goldani F and Parizadeh SM: The prognostic and
predictive value of microRNAs in patients with H. pylori-positive
gastric cancer. Curr Pharm Des. 24:4639–4645. 2018. View Article : Google Scholar
|
|
12
|
Zou D, Xu L, Li H, Ma Y, Gong Y, Guo T,
Jing Z, Xu X and Zhang Y: Role of abnormal microRNA expression in
Helicobacter pylori associated gastric cancer. Crit Rev Microbiol.
45:239–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blosse A, Levy M, Robe C, Staedel C,
Copie-Bergman C and Lehours P: Deregulation of miRNA in
Helicobacter pylori-induced gastric MALT lymphoma: From mice to
human. J Clin Med. 8:8452019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korać P, Antica M and Matulić M: MiR-7 in
cancer development. Biomedicines. 9:3252021. View Article : Google Scholar
|
|
15
|
Xiao H: MiR-7-5p suppresses tumor
metastasis of non-small cell lung cancer by targeting NOVA2. Cell
Mol Biol Lett. 24:602019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia J, Cao T, Ma C, Shi Y, Sun Y, Wang ZP
and Ma J: miR-7 suppresses tumor progression by directly targeting
MAP3K9 in pancreatic cancer. Mol Ther Nucleic Acids. 13:121–132.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Pan M, Wang J, You C, Zhao F, Zheng
D, Guo M, Xu H, Wu D, Wang L and Dou J: miR-7 reduces breast cancer
stem cell metastasis via inhibiting RELA to decrease ESAM
expression. Mol Ther Oncolytics. 18:70–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong D, Piao YS, Yamashita S, Oshima H,
Oguma K, Fushida S, Fujimura T, Minamoto T, Seno H, Yamada Y, et
al: Inflammation-induced repression of tumor suppressor miR-7 in
gastric tumor cells. Oncogene. 31:3949–3960. 2012. View Article : Google Scholar
|
|
19
|
Lin J, Liu Z, Liao S, Li E, Wu X and Zeng
W: Elevated microRNA-7 inhibits proliferation and tumor
angiogenesis and promotes apoptosis of gastric cancer cells via
repression of Raf-1. Cell Cycle. 19:2496–2508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu N, Lian YJ, Dai X and Wang YJ: miR-7
increases cisplatin sensitivity of gastric cancer cells through
suppressing mTOR. Technol Cancer Res Treat. 16:1022–1030. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie J, Chen M, Zhou J, Mo MS, Zhu LH, Liu
YP, Gui QJ, Zhang L and Li GQ: miR-7 inhibits the invasion and
metastasis of gastric cancer cells by suppressing epidermal growth
factor receptor expression. Oncol Rep. 31:1715–1722. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Dou W, He L, Liang S, Tie J, Liu
C, Li T, Lu Y, Mo P, Shi Y, et al: MicroRNA-7 functions as an
anti-metastatic microRNA in gastric cancer by targeting
insulin-like growth factor-1 receptor. Oncogene. 32:1363–1372.
2013. View Article : Google Scholar
|
|
23
|
Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen
GF, Zhou JF, Li T, Hu SJ, Zhou L, et al: MicroRNA-7/NF-κB signaling
regulatory feedback circuit regulates gastric carcinogenesis. J
Cell Biol. 210:613–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye T, Yang M, Huang D, Wang X, Xue B, Tian
N, Xu X, Bao L, Hu H, Lv T and Huang Y: MicroRNA-7 as a potential
therapeutic target for aberrant NF-κB-driven distant metastasis of
gastric cancer. J Exp Clin Cancer Res. 38:552019. View Article : Google Scholar
|
|
25
|
Wang L, Lv X, Fu X, Su L, Yang T and Xu P:
MiR-153 inhibits the resistance of lung cancer to gefitinib via
modulating expression of ABCE1. Cancer Biomark. 25:361–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi D, Li Y, Fan L, Zhao Q, Tan B and Cui
G: Upregulation of miR-153 inhibits triple-negative breast cancer
progression by targeting ZEB2-mediated EMT and contributes to
better prognosis. Onco Targets Ther. 12:9611–9625. 2019. View Article : Google Scholar
|
|
27
|
Li C, Zhang Y, Zhao W, Cui S and Song Y:
miR-153-3p regulates progression of ovarian carcinoma in vitro and
in vivo by targeting MCL1 gene. J Cell Biochem. 120:19147–19158.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Mao S, Shi D, Zhang J, Zhang Z,
Guo Y, Wu Y, Wang R, Wang L, Huang Y and Yao X: MicroRNA-153
decreases tryptophan catabolism and inhibits angiogenesis in
bladder cancer by targeting indoleamine 2,3-dioxygenase 1. Front
Oncol. 9:6192019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bi CW, Zhang GY, Bai Y, Zhao B and Yang H:
Increased expression of miR-153 predicts poor prognosis for
patients with prostate cancer. Medicine (Baltimore). 98:e167052019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Pickard K, Jenei V, Bullock MD,
Bruce A, Mitter R, Kelly G, Paraskeva C, Strefford J, Primrose J,
et al: miR-153 supports colorectal cancer progression via
pleiotropic effects that enhance invasion and chemotherapeutic
resistance. Cancer Res. 73:6435–6447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ouyang Y, Yuan W and Qiu S: MicroRNA-153
functions as a tumor suppressor in gastric cancer via targeting
Kruppel-like factor 5. Exp Ther Med. 16:473–482. 2018.PubMed/NCBI
|
|
32
|
Guo Y, Zhang T, Shi Y, Zhang J, Li M, Lu
F, Zhang J, Chen X and Ding S: Helicobacter pylori inhibits GKN1
expression via the CagA/p-ERK/AUF1 pathway. Helicobacter.
25:e126652020. View Article : Google Scholar
|
|
33
|
Liu JF, Guo D, Kang EM, Wang YS, Gao XZ,
Cong HY, Liu P, Zhang NQ and Wang MY: Acute and chronic infection
of H. pylori caused the difference in apoptosis of gastric
epithelial cells. Microb Pathog. 150:1047172021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Hayashi Y, Tsujii M, Wang J, Kondo J,
Akasaka T, Jin Y, Li W, Nakamura T, Nishida T, Iijima H, et al:
CagA mediates epigenetic regulation to attenuate let-7 expression
in Helicobacter pylori-related carcinogenesis. Gut. 62:1536–1546.
2013. View Article : Google Scholar
|
|
36
|
Yuan J, Li Y, Liao J, Liu M, Zhu L and
Liao K: MicroRNA-7 inhibits hepatocellular carcinoma cell invasion
and metastasis by regulating Atg5-mediated autophagy. Transl Cancer
Res. 9:3965–3972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou S, Guo M, Xi H, Zhang L, Zhao A, Hou H
and Fang W: MicroRNA-153-3p sensitizes melanoma cells to
dacarbazine by suppressing ATG5-mediated autophagy and apoptosis.
Transl Cancer Res. 9:5626–5636. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren HY, Wen LS, Geng YH, Huang JB, Liu JF,
Shen DY and Meng JR: Association between IL-1B gene polymorphisms
and Helicobacter pylori infection: A meta-analysis. Microb Pathog.
137:1037692019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar
|
|
40
|
Han T, Jing X, Bao J, Zhao L, Zhang A,
Miao R, Guo H, Zhou B, Zhang S, Sun J and Shi J: H. pylori
infection alters repair of DNA double-strand breaks via SNHG17. J
Clin Invest. 130:3901–3918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
So JBY, Kapoor R, Zhu F, Koh C, Zhou L,
Zou R, Tang YC, Goo PCK, Rha SY, Chung HC, et al: Development and
validation of a serum microRNA biomarker panel for detecting
gastric cancer in a high-risk population. Gut. 70:829–837. 2021.
View Article : Google Scholar
|
|
42
|
Liu Y, Zhu J, Ma X, Han S, Xiao D, Jia Y
and Wang Y: ceRNA network construction and comparison of gastric
cancer with or without Helicobacter pylori infection. J Cell
Physiol. 234:7128–7140. 2019. View Article : Google Scholar
|
|
43
|
Zhang Z, Chen S, Fan M, Ruan G, Xi T,
Zheng L, Guo L, Ye F and Xing Y: Helicobacter pylori induces
gastric cancer via down-regulating miR-375 to inhibit dendritic
cell maturation. Helicobacter. 26:e128132021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miao L, Liu K, Xie M, Xing Y and Xi T:
miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis
by blocking JAK2-STAT3 signaling. Cancer Immunol Immunother.
63:699–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ni H, Qin H, Sun C, Liu Y, Ruan G, Guo Q,
Xi T, Xing Y and Zheng L: MiR-375 reduces the stemness of gastric
cancer cells through triggering ferroptosis. Stem Cell Res Ther.
12:3252021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee JW, Kim N, Park JH, Kim HJ, Chang H,
Kim JM, Kim JW and Lee DH: Differential MicroRNA expression between
gastric cancer tissue and non-cancerous gastric mucosa according to
Helicobacter pylori status. J Cancer Prev. 22:33–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong T, Ding J and Li W: miR-7 reverses
breast cancer resistance to chemotherapy by targeting MRP1 And
BCL2. Onco Targets Ther. 12:11097–11105. 2019. View Article : Google Scholar
|
|
48
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun D, Mu Y and Piao H: MicroRNA-153-3p
enhances cell radiosensitivity by targeting BCL2 in human glioma.
Biol Res. 51:562018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He Y, Zhang L, Tan F, Wang LF, Liu DH,
Wang RJ and Yin XZ: MiR-153-5p promotes sensibility of colorectal
cancer cells to oxaliplatin via targeting Bcl-2-mediated autophagy
pathway. Biosci Biotechnol Biochem. 84:1645–1651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li YL, Tang JM, Chen XY, Luo B, Liang GH,
Qu Q and Lu ZY: MicroRNA-153-3p enhances the sensitivity of chronic
myeloid leukemia cells to imatinib by inhibiting B-cell
lymphoma-2-mediated autophagy. Hum Cell. 33:610–618. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo G, Li L, Song G, Wang J, Yan Y and
Zhao Y: miR-7/SP1/TP53BP1 axis may play a pivotal role in NSCLC
radiosensitivity. Oncol Rep. 44:2678–2690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin CL, Ying TH, Yang SF, Wang SW, Cheng
SP, Lee JJ and Hsieh YH: Transcriptional suppression of miR-7 by
MTA2 induces Sp1-mediated KLK10 expression and metastasis of
cervical cancer. Mol Ther Nucleic Acids. 20:699–710. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang MJ, Pei DS, Qian GW, Yin XX, Cheng Q,
Li LT, Li HZ and Zheng JN: p53 regulates Ki-67 promoter activity
through p53- and Sp1-dependent manner in HeLa cells. Tumour Biol.
32:905–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Drayman N, Ben-Nun-Shaul O, Butin-Israeli
V, Srivastava R, Rubinstein AM, Mock CS, Elyada E, Ben-Neriah Y,
Lahav G and Oppenheim A: p53 elevation in human cells halt SV40
infection by inhibiting T-ag expression. Oncotarget. 7:52643–52660.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Zhang Y, Ströse A, Tedesco D, Gurova
K and Selivanova G: Integrated high-throughput analysis identifies
Sp1 as a crucial determinant of p53-mediated apoptosis. Cell Death
Differ. 21:1493–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
El-Omar EM, Carrington M, Chow WH, McColl
KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N,
et al: Interleukin-1 polymorphisms associated with increased risk
of gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kawahara T, Kuwano Y, Teshima-Kondo S,
Kawai T, Nikawa T, Kishi K and Rokutan K: Toll-like receptor 4
regulates gastric pit cell responses to Helicobacter pylori
infection. J Med Invest. 48:190–197. 2001.PubMed/NCBI
|
|
61
|
Kawahara T, Teshima S, Oka A, Sugiyama T,
Kishi K and Rokutan K: Type I Helicobacter pylori
lipopolysaccharide stimulates toll-like receptor 4 and activates
mitogen oxidase 1 in gastric pit cells. Infect Immun. 69:4382–4389.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Noto JM, Khizanishvili T, Chaturvedi R,
Piazuelo MB, Romero-Gallo J, Delgado AG, Khurana SS, Sierra JC,
Krishna US, Suarez G, et al: Helicobacter pylori promotes the
expression of Krüppel-like factor 5, a mediator of carcinogenesis,
in vitro and in vivo. PLoS One. 8:e543442013. View Article : Google Scholar
|
|
63
|
Xie Z, Jie Z, Wang G, Sun X, Tang P, Chen
S, Qin A, Wang J and Fan S: TGF-β synergizes with ML264 to block
IL-1β-induced matrix degradation mediated by Krüppel-like factor 5
in the nucleus pulposus. Biochim Biophys Acta Mol Basis Dis.
1864:579–589. 2018. View Article : Google Scholar
|
|
64
|
Tang J, Xu L, Zeng Y and Gong F: Effect of
gut microbiota on LPS-induced acute lung injury by regulating the
TLR4/NF-kB signaling pathway. Int Immunopharmacol. 91:1072722021.
View Article : Google Scholar
|
|
65
|
Zhang XD, Fan QY, Qiu Z and Chen S: MiR-7
alleviates secondary inflammatory response of microglia caused by
cerebral hemorrhage through inhibiting TLR4 expression. Eur Rev Med
Pharmacol Sci. 22:5597–5604. 2018.PubMed/NCBI
|
|
66
|
Yu L, Xu Q, Yu W, Duan J and Dai G: LncRNA
cancer susceptibility candidate 15 accelerates the breast cancer
cells progression via miR-153-3p/KLF5 positive feedback loop.
Biochem Biophys Res Commun. 506:819–825. 2018. View Article : Google Scholar : PubMed/NCBI
|