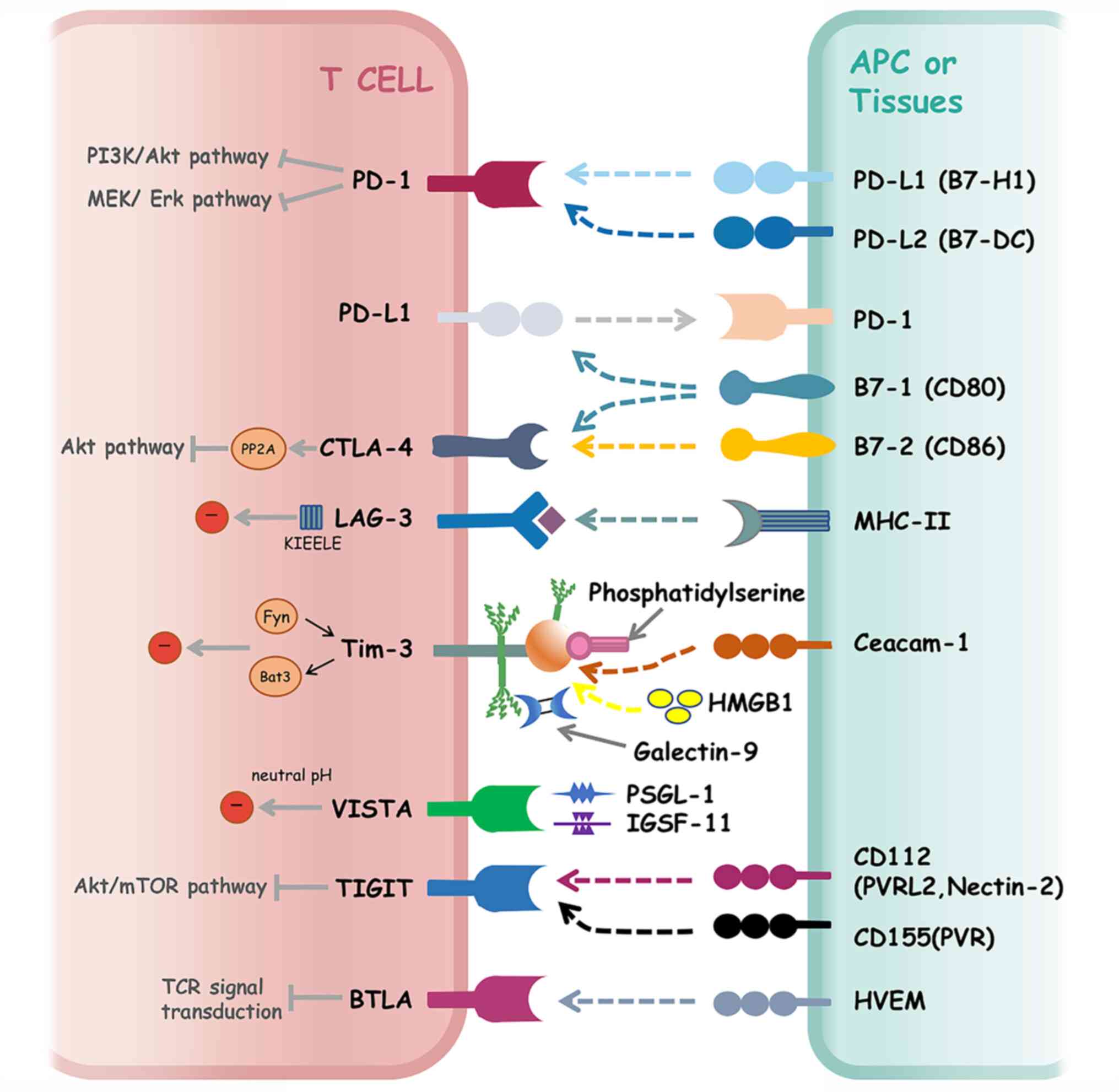
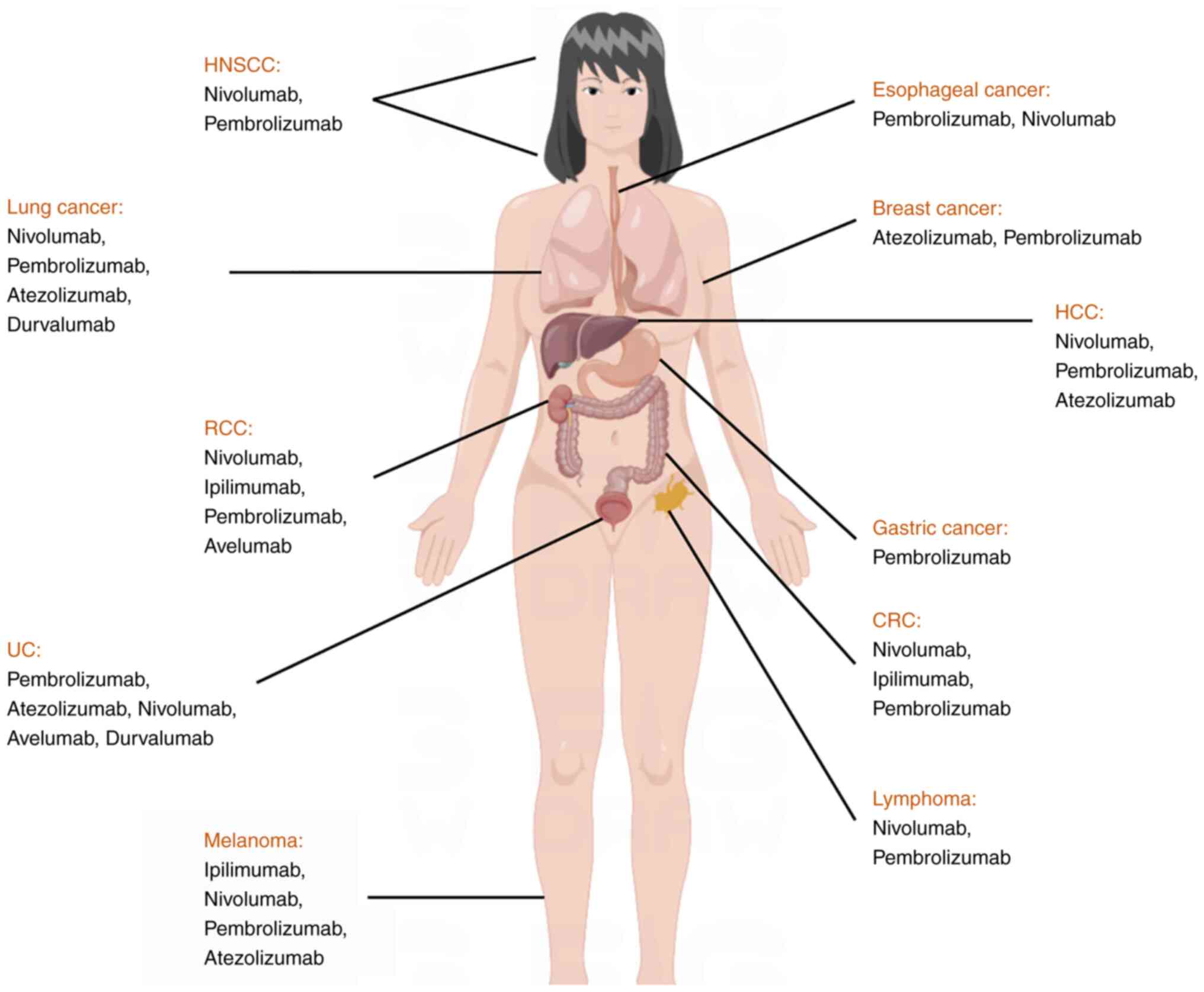
|
1
|
Li K and Tian H: Development of
small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new
therapeutic strategy for tumour immunotherapy. J Drug Target.
27:244–256. 2019. View Article : Google Scholar
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dillman RO: Cancer immunotherapy. Cancer
Biother Radiopharm. 26:1–64. 2011.PubMed/NCBI
|
|
5
|
Stewart TJ and Smyth MJ: Improving cancer
immunotherapy by targeting tumor-induced immune suppression. Cancer
Metastasis Rev. 30:125–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P, Wagner K, Wolchok JD and Allison
JP: Novel cancer immunotherapy agents with survival benefit: Recent
successes and next steps. Nat Rev Cancer. 11:805–812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoos A: Development of immuno-oncology
drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug
Discov. 15:235–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharpe AH: Mechanisms of costimulation.
Immunol Rev. 229:5–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudd CE, Taylor A and Schneider H: CD28
and CTLA-4 coreceptor expression and signal transduction. Immunol
Rev. 229:12–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunet JF, Denizot F, Luciani MF,
Roux-Dosseto M, Suzan M, Mattei MG and Golstein P: A new member of
the immunoglobulin superfamily-CTLA-4. Nature. 328:267–270. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krummel MF and Allison JP: CD28 and CTLA-4
have opposing effects on the response of T cells to stimulation. J
Exp Med. 182:459–465. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linsley PS, Brady W, Urnes M, Grosmaire
LS, Damle NK and Ledbetter JA: CTLA-4 is a second receptor for the
B cell activation antigen B7. J Exp Med. 174:561–569. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walunas TL, Lenschow DJ, Bakker CY,
Linsley PS, Freeman GJ, Green JM, Thompson CB and Bluestone JA:
Pillars article: CTLA-4 can function as a negative regulator of T
cell activation. Immunity. 1994. 1: 405-413. J Immunol.
187:3466–3474. 2011.PubMed/NCBI
|
|
20
|
Linsley PS, Greene JL, Brady W, Bajorath
J, Ledbetter JA and Peach R: Human B7-1 (CD80) and B7-2 (CD86) bind
with similar avidities but distinct kinetics to CD28 and CTLA-4
receptors. Immunity. 1:793–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibson HM, Hedgcock CJ, Aufiero BM, Wilson
AJ, Hafner MS, Tsokos GC and Wong HK: Induction of the CTLA-4 gene
in human lymphocytes is dependent on NFAT binding the proximal
promoter. J Immunol. 179:3831–3840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waterhouse P, Penninger JM, Timms E,
Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW:
Lymphoproliferative disorders with early lethality in mice
deficient in Ctla-4. Science. 270:985–988. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tivol EA, Borriello F, Schweitzer AN,
Lynch WP, Bluestone JA and Sharpe AH: Loss of CTLA-4 leads to
massive lymphoproliferation and fatal multiorgan tissue
destruction, revealing a critical negative regulatory role of
CTLA-4. Immunity. 3:541–547. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Read S, Greenwald R, Izcue A, Robinson N,
Mandelbrot D, Francisco L, Sharpe AH and Powrie F: Blockade of
CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in
vivo. J Immunol. 177:4376–4383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wing K, Onishi Y, Prieto-Martin P,
Yamaguchi T, Miyara M, Fehervari Z, Nomura T and Sakaguchi S:
CTLA-4 control over Foxp3+ regulatory T cell function. Science.
322:271–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider H, Smith X, Liu H, Bismuth G and
Rudd CE: CTLA-4 disrupts ZAP70 microcluster formation with reduced
T cell/APC dwell times and calcium mobilization. Eur J Immunol.
38:40–47. 2008. View Article : Google Scholar
|
|
27
|
Wang XB, Fan ZZ, Anton D, Vollenhoven AV,
Ni ZH, Chen XF and Lefvert AK: CTLA4 is expressed on mature
dendritic cells derived from human monocytes and influences their
maturation and antigen presentation. BMC Immunol. 12:212011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boasso A, Herbeuval JP, Hardy AW, Winkler
C and Shearer GM: Regulation of indoleamine 2,3-dioxygenase and
tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells.
Blood. 105:1574–1581. 2005. View Article : Google Scholar
|
|
29
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hammers HJ, Plimack ER, Infante JR, Rini
BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA,
et al: Safety and efficacy of nivolumab in combination with
ipilimumab in metastatic renal cell carcinoma: The CheckMate 016
study. J Clin Oncol. 35:3851–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: Ipilimumab versus placebo after radiotherapy in
patients with metastatic castration-resistant prostate cancer that
had progressed after docetaxel chemotherapy (CA184-043): A
multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.
15:700–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le DT, Lutz E, Uram JN, Sugar EA, Onners
B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM and Laheru
DA: Evaluation of ipilimumab in combination with allogeneic
pancreatic tumor cells transfected with a GM-CSF gene in previously
treated pancreatic cancer. J Immunother. 36:382–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duffy AG, Ulahannan SV, Makorova-Rusher O,
Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T,
ElGindi M, et al: Tremelimumab in combination with ablation in
patients with advanced hepatocellular carcinoma. J Hepatol.
66:545–551. 2017. View Article : Google Scholar :
|
|
36
|
Selby MJ, Engelhardt JJ, Quigley M,
Henning KA, Chen T, Srinivasan M and Korman AJ: Anti-CTLA-4
antibodies of IgG2a isotype enhance antitumor activity through
reduction of intratumoral regulatory T cells. Cancer Immunol Res.
1:32–42. 2013. View Article : Google Scholar
|
|
37
|
Marangoni F, Zhakyp A, Corsini M, Geels
SN, Carrizosa E, Thelen M, Mani V, Prüßmann JN, Warner RD, Ozga AJ,
et al: Expansion of tumor-associated Treg cells upon disruption of
a CTLA-4-dependent feedback loop. Cell. 184:3998–4015.e19. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: Expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zamani MR, Aslani S, Salmaninejad A, Javan
MR and Rezaei N: PD-1/PD-L and autoimmunity: A growing
relationship. Cell Immunol. 310:27–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar
|
|
48
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ahmadzadeh M, Johnson LA, Heemskerk B,
Wunderlich JR, Dudley ME, White DE and Rosenberg SA: Tumor
antigen-specific CD8 T cells infiltrating the tumor express high
levels of PD-1 and are functionally impaired. Blood. 114:1537–1544.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yaghoubi N, Soltani A, Ghazvini K,
Hassanian SM and Hashemy SI: PD-1/PD-L1 blockade as a novel
treatment for colorectal cancer. Biomed Pharmacother. 110:312–318.
2019. View Article : Google Scholar
|
|
51
|
Patsoukis N, Wang Q, Strauss L and
Boussiotis VA: Revisiting the PD-1 pathway. Sci Adv.
6:eabd27122020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sharpe AH, Wherry EJ, Ahmed R and Freeman
GJ: The function of programmed cell death 1 and its ligands in
regulating autoimmunity and infection. Nat Immunol. 8:239–245.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong X, Tumang JR, Gao W, Bai C and
Rothstein TL: PD-L2 expression extends beyond dendritic
cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and
phosphatidylcholine binding. Eur J Immunol. 37:2405–2410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018. View Article : Google Scholar
|
|
55
|
Ribas A and Hu-Lieskovan S: What does
PD-L1 positive or negative mean? J Exp Med. 213:2835–2840. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brown JA, Dorfman DM, Ma FR, Sullivan EL,
Munoz O, Wood CR, Greenfield EA and Freeman GJ: Blockade of
programmed death-1 ligands on dendritic cells enhances T cell
activation and cytokine production. J Immunol. 170:1257–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brahmer JR, Drake CG, Wollner I, Powderly
JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller
TL, et al: Phase I study of single-agent anti-programmed death-1
(MDX-1106) in refractory solid tumors: Safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gettinger S, Rizvi NA, Chow LQ, Borghaei
H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman
JW, et al: Nivolumab monotherapy for first-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 34:2980–2987.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-p rogrammed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Patnaik A, Kang SP, Rasco D, Papadopoulos
KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L,
Espino G, et al: Phase I study of pembrolizumab (MK-3475; anti-PD-1
monoclonal antibody) in patients with advanced solid tumors. Clin
Cancer Res. 21:4286–4293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rihawi K, Gelsomino F, Sperandi F, Melotti
B, Fiorentino M, Casolari L and Ardizzoni A: Pembrolizumab in the
treatment of metastatic non-small cell lung cancer: A review of
current evidence. Ther Adv Respir Dis. 11:353–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Suzman DL, Agrawal S, Ning YM, Maher VE,
Fernandes LL, Karuri S, Tang S, Sridhara R, Schroeder J, Goldberg
KB, et al: FDA approval summary: Atezolizumab or pembrolizumab for
the treatment of patients with advanced urothelial carcinoma
ineligible for cisplatin-containing chemotherapy. Oncologist.
24:563–569. 2019. View Article : Google Scholar
|
|
70
|
U.S. Food and Drug: FDA approves first
treatment for advanced form of the second most common skin cancer.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-advanced-form-second-most-common-skin-cancer-0.
Accessed January 20, 2023
|
|
71
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Peters S, Gettinger S, Johnson ML, Jänne
PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE,
Carcereny Costa E, et al: Phase II trial of atezolizumab as
first-line or subsequent therapy for patients with programmed
death-ligand 1-selected advanced non-small-cell lung cancer
(BIRCH). J Clin Oncol. 35:2781–2789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: Atezolizumab as first-line treatment in
cisplatin-ineligible patients with locally advanced and metastatic
urothelial carcinoma: A single-arm, multicentre, phase 2 trial.
Lancet. 389:67–76. 2017. View Article : Google Scholar
|
|
74
|
Petrylak DP, Powles T, Bellmunt J, Braiteh
F, Loriot Y, Morales-Barrera R, Burris HA, Kim JW, Ding B, Kaiser
C, et al: Atezolizumab (MPDL3280A) monotherapy for patients with
metastatic urothelial cancer: Long-term outcomes from a phase 1
study. JAMA Oncol. 4:537–544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hamilton G and Rath B: Avelumab: Combining
immune checkpoint inhibition and antibody-dependent cytotoxicity.
Expert Opin Biol Ther. 17:515–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga
E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, et al:
Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1
infected participants on suppressive antiretroviral therapy. J
Infect Dis. 215:1725–1733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Workman CJ and Vignali DAA: Negative
regulation of T cell homeostasis by lymphocyte activation gene-3
(CD223). J Immunol. 174:688–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Goldberg MV and Drake CG: LAG-3 in cancer
immunotherapy. Curr Top Microbiol Immunol. 344:269–278. 2011.
|
|
84
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Demeure CE, Wolfers J, Martin-Garcia N,
Gaulard P and Triebel F: T Lymphocytes infiltrating various tumour
types express the MHC class II ligand lymphocyte activation gene-3
(LAG-3): Role of LAG-3/MHC class II interactions in cell-cell
contacts. Eur J Cancer. 37:1709–1718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gandhi MK, Lambley E, Duraiswamy J, Dua U,
Smith C, Elliott S, Gill D, Marlton P, Seymour J and Khanna R:
Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident
with the suppression of latent membrane antigen-specific CD8+
T-cell function in Hodgkin lymphoma patients. Blood. 108:2280–2289.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, et al: Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are
negatively regulated by LAG-3 and PD-1 in human ovarian cancer.
Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li FJ, Zhang Y, Jin GX, Yao L and Wu DQ:
Expression of LAG-3 is coincident with the impaired effector
function of HBV-specific CD8(+) T cell in HCC patients. Immunol
Lett. 150:116–122. 2013. View Article : Google Scholar
|
|
89
|
Andrews LP, Marciscano AE, Drake CG and
Vignali DA: LAG3 (CD223) as a cancer immunotherapy target. Immunol
Rev. 276:80–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ruffo E, Wu RC, Bruno TC, Workman CJ and
Vignali DAA: Lymphocyte-activation gene 3 (LAG3): The next immune
checkpoint receptor. Semin Immunol. 42:1013052019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gleason MK, Lenvik TR, McCullar V, Felices
M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ,
Panoskaltsis-Mortari A, et al: Tim-3 is an inducible human natural
killer cell receptor that enhances interferon gamma production in
response to galectin-9. Blood. 119:3064–3072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Anderson AC: Tim-3, a negative regulator
of anti-tumor immunity. Curr Opin Immunol. 24:213–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nakayama M, Akiba H, Takeda K, Kojima Y,
Hashiguchi M, Azuma M, Yagita H and Okumura K: Tim-3 mediates
phagocytosis of apoptotic cells and cross-presentation. Blood.
113:3821–3830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang D and Lotze MT: Tumor immunity times
out: TIM-3 and HMGB1. Nat Immunol. 13:808–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang YH, Zhu C, Kondo Y, Anderson AC,
Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et
al: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion.
Nature. 517:386–390. 2015. View Article : Google Scholar
|
|
96
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma patients. J
Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang ZZ, Grote DM, Ziesmer SC, Niki T,
Hirashima M, Novak AJ, Witzig TE and Ansell SM: IL-12 upregulates
TIM-3 expression and induces T cell exhaustion in patients with
follicular B cell non-Hodgkin lymphoma. J Clin Invest.
122:1271–1282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang
F, Sun J, Yang Q, Zhang X and Lu B: TIM-3 expression characterizes
regulatory T cells in tumor tissues and is associated with lung
cancer progression. PLoS One. 7:e306762012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Markwick LJ, Riva A, Ryan JM, Cooksley H,
Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A,
et al: Blockade of PD1 and TIM3 restores innate and adaptive
immunity in patients with acute alcoholic hepatitis.
Gastroenterology. 148:590–602 e10. 2015. View Article : Google Scholar
|
|
101
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Levin SD, Taft DW, Brandt CS, Bucher C,
Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K,
Ardourel D, et al: Vstm3 is a member of the CD28 family and an
important modulator of T-cell function. Eur J Immunol. 41:902–915.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Boles KS, Vermi W, Facchetti F, Fuchs A,
Wilson TJ, Diacovo TG, Cella M and Colonna M: A novel molecular
interaction for the adhesion of follicular CD4 T cells to
follicular DC. Eur J Immunol. 39:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Manieri NA, Chiang EY and Grogan JL:
TIGIT: A Key inhibitor of the cancer immunity cycle. Trends
Immunol. 38:20–28. 2017. View Article : Google Scholar
|
|
106
|
U.S. National Library of Medicine:
Zimberelimab (AB122) With TIGIT Inhibitor Domvanalimab (AB154) in
PD-1 Relapsed/Refractory Melanoma. https://clinicaltrials.gov/ct2/show/NCT05130177?term=NCT05130177&draw=2&rank=1.
Accessed January 20, 2023
|
|
107
|
U.S. National Library of Medicine: COM902
(A TIGIT Inhibitor) in Subjects With Advanced Malignancies.
https://clinicaltrials.gov/ct2/show/NCT04354246?term=NCT04354246&draw=2&rank=1.
Accessed January 20, 2023
|
|
108
|
U.S. National Library of Medicine: Study
to Assess the Safety and Efficacy of AZD2936 in Participants With
Advanced or Metastatic Non-small Cell Lung Cancer (ARTEMIDE-01).
https://clinicaltrials.gov/ct2/show/NCT04995523?term=NCT04995523&draw=2&rank=1.
Accessed January 20, 2023
|
|
109
|
Watanabe N, Gavrieli M, Sedy JR, Yang J,
Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et
al: BTLA is a lymphocyte inhibitory receptor with similarities to
CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Karakatsanis S, Bertsias G, Roussou P and
Boumpas D: Programmed death 1 and B and T lymphocyte attenuator
immunoreceptors and their association with malignant
T-lymphoproliferative disorders: Brief review. Hematol Oncol.
32:113–119. 2014. View Article : Google Scholar
|
|
111
|
Pasero C and Olive D: Interfering with
coinhibitory molecules: BTLA/HVEM as new targets to enhance
anti-tumor immunity. Immunol Lett. 151:71–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
M'Hidi H, Thibult ML, Chetaille B, Rey F,
Bouadallah R, Nicollas R, Olive D and Xerri L: High expression of
the inhibitory receptor BTLA in T-follicular helper cells and in
B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am
J Clin Pathol. 132:589–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hosseinkhani N, Derakhshani A, Shadbad MA,
Argentiero A, Racanelli V, Kazemi T, Mokhtarzadeh A, Brunetti O,
Silvestris N and Baradaran B: The role of V-domain Ig suppressor of
T cell activation (VISTA) in cancer therapy: Lessons learned and
the road ahead. Front Immunol. 12:6761812021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
U.S. National Library of Medicine: A Study
of CA-170 (Oral PD-L1 PD-L2 and VISTA Checkpoint Antagonist) in
Patients With Advanced Tumors and Lymphomas. https://clinicaltrials.gov/ct2/show/NCT02812875?term=NCT02812875&draw=2&rank=1.
Accessed January 20, 2023
|
|
115
|
U.S. National Library of Medicine: A Study
of Safety Pharmacokinetics, Pharmacodynamics of JNJ-61610588 in
Participants With Advanced Cancer. https://clinicaltrials.gov/ct2/show/NCT02671955?term=NCT02671955&draw=2&rank=1.
Accessed January 20, 2023
|
|
116
|
U.S. National Library of Medicine: Phase 1
Study of CI-8993 Anti-VISTA Antibody in Patients With Advanced
Solid Tumor Malignancies. https://clinicaltrials.gov/ct2/show/NCT04475523?term=NCT04475523&draw=2&rank=1.
Accessed January 20, 2023
|
|
117
|
Motzer RJ, Rini BI, McDermott DF, Redman
BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S,
Logan TF, et al: Nivolumab for metastatic renal cell carcinoma:
Results of a randomized phase II trial. J Clin Oncol. 33:1430–1437.
2015. View Article : Google Scholar
|
|
118
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
McDermott DF, Sosman JA, Sznol M, Massard
C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV,
et al: Atezolizumab, an anti-programmed death-ligand 1 antibody, in
metastatic renal cell carcinoma: Long-term safety, clinical
activity, and immune correlates from a phase Ia study. J Clin
Oncol. 34:833–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Augustin RC, Delgoffe GM and Najjar YG:
Characteristics of the tumor microenvironment that influence immune
cell functions: Hypoxia, oxidative stress, metabolic alterations.
Cancers (Basel). 12:38022020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang DR, Wu XL and Sun YL: Therapeutic
targets and biomarkers of tumor immunotherapy: Response versus
non-response. Signal Transduct Target Ther. 7:3312022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar
|
|
126
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Mahoney KM and Atkins MB: Prognostic and
predictive markers for the new immunotherapies. Oncology (Williston
Park). 28(Suppl 3): S39–S48. 2014.
|
|
129
|
Kim J, Myers AC, Chen L, Pardoll DM,
Truong-Tran QA, Lane AP, McDyer JF, Fortuno L and Schleimer RP:
Constitutive and inducible expression of b7 family of ligands by
human airway epithelial cells. Am J Respir Cell Mol Biol.
33:280–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar
|
|
131
|
Meng X, Huang Z, Teng F, Xing L and Yu J:
Predictive biomarkers in PD-1/PD-L1 checkpoint blockade
immunotherapy. Cancer Treat Rev. 41:868–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mansfield AS, Murphy SJ, Peikert T, Yi ES,
Vasmatzis G, Wigle DA and Aubry MC: Heterogeneity of programmed
cell death ligand 1 expression in multifocal lung cancer. Clin
Cancer Res. 22:2177–2182. 2016. View Article : Google Scholar
|
|
133
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lee V, Murphy A, Le DT and Diaz LA Jr:
Mismatch repair deficiency and response to immune checkpoint
blockade. Oncologist. 21:1200–1211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lynch HT, Jascur T, Lanspa S and Boland
CR: Making sense of missense in Lynch syndrome: The clinical
perspective. Cancer Prev Res (Phila). 3:1371–1374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ratner D and Lennerz JK: Implementing
keytruda/pembrolizumab testing in clinical practice. Oncologist.
23:647–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schwitalle Y, Kloor M, Eiermann S,
Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A and
von Knebel Doeberitz M: Immune response against frameshift-induced
neopeptides in HNPCC patients and healthy HNPCC mutation carriers.
Gastroenterology. 134:988–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Drescher KM, Sharma P, Watson P, Gatalica
Z, Thibodeau SN and Lynch HT: Lymphocyte recruitment into the tumor
site is altered in patients with MSI-H colon cancer. Fam Cancer.
8:231–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar :
|
|
141
|
Goel G and Sun W: Advances in the
management of gastrointestinal cancers-an upcoming role of immune
checkpoint blockade. J Hematol Oncol. 8:862015. View Article : Google Scholar
|
|
142
|
Mouw KW, Goldberg MS, Konstantinopoulos PA
and D'Andrea AD: DNA damage and repair biomarkers of immunotherapy
response. Cancer Discov. 7:675–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gubin MM, Zhang X, Schuster H, Caron E,
Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et
al: Checkpoint blockade cancer immunotherapy targets
tumour-specific mutant antigens. Nature. 515:577–581. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Van Allen EM, Miao D, Schilling B, Shukla
SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger
SM, et al: Genomic correlates of response to CTLA-4 blockade in
metastatic melanoma. Science. 350:207–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hellmann MD, Callahan MK, Awad MM, Calvo
E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G,
Szustakowski JD, et al: Tumor mutational burden and efficacy of
nivolumab monotherapy and in combination with ipilimumab in
small-cell lung cancer. Cancer Cell. 33:853–861.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human c'ancer genomes reveals the
landscape of tumor mutational burden. Genome Med. 9:342017.
View Article : Google Scholar
|
|
152
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lawrence MS, Stojanov P, Polak P, Kryukov
GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH,
Roberts SA, et al: Mutational heterogeneity in cancer and the
search for new cancer-associated genes. Nature. 499:214–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Pardoll D: Cancer and the immune system:
Basic concepts and targets for intervention. Semin Oncol.
42:523–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Salgado R, Denkert C, Campbell C, Savas P,
Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J,
et al: Tumor-infiltrating lymphocytes and associations with
pathological complete response and event-free survival in
HER2-positive early-stage breast cancer treated with lapatinib and
trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA
Oncol. 1:448–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Basile D, Pelizzari G, Vitale MG, Lisanti
C, Cinausero M, Iacono D and Puglisi F: Atezolizumab for the
treatment of breast cancer. Expert Opin Biol Ther. 18:595–603.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018. View Article : Google Scholar
|
|
160
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Pagès F, Mlecnik B, Marliot F, Bindea G,
Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al:
International validation of the consensus immunoscore for the
classification of colon cancer: A prognostic and accuracy study.
Lancet. 391:2128–2139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Khalili JS, Liu S, Rodríguez-Cruz TG,
Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT,
Li Y, et al: Oncogenic BRAF(V600E) promotes stromal cell-mediated
immunosuppression via induction of interleukin-1 in melanoma. Clin
Cancer Res. 18:5329–5340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Frederick DT, Piris A, Cogdill AP, Cooper
ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et
al: BRAF inhibition is associated with enhanced melanoma antigen
expression and a more favorable tumor microenvironment in patients
with metastatic melanoma. Clin Cancer Res. 19:1225–1231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He
Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al: Loss of IFN-γ
pathway genes in tumor cells as a mechanism of resistance to
anti-CTLA-4 therapy. Cell. 167:397–404.e9. 2016. View Article : Google Scholar
|
|
166
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD-1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary resistance to PD-1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. 2017.
View Article : Google Scholar :
|
|
168
|
Riaz N, Havel JJ, Kendall SM, Makarov V,
Walsh LA, Desrichard A, Weinhold N and Chan TA: Recurrent SERPINB3
and SERPINB4 mutations in patients who respond to anti-CTLA4
immunotherapy. Nat Genet. 48:1327–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
170
|
George S, Miao D, Demetri GD, Adeegbe D,
Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter
SL, et al: Loss of PTEN is associated with resistance to Anti-PD-1
checkpoint blockade therapy in metastatic uterine leiomyosarcoma.
Immunity. 46:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z,
Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al: Potential predictive
value of TP53 and KRAS mutation status for response to PD-1
blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res.
23:3012–3024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lisberg A, Cummings A, Goldman JW,
Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M,
Hunt J, et al: A phase II study of pembrolizumab in EGFR-mutant,
PD-L1+, tyrosine kinase inhibitor Naïve patients with advanced
NSCLC. J Thorac Oncol. 13:1138–1145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kapur P, Peña-Llopis S, Christie A,
Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie XJ and Brugarolas J:
Effects on survival of BAP1 and PBRM1 mutations in sporadic
clear-cell renal-cell carcinoma: A retrospective analysis with
independent validation. Lancet Oncol. 14:159–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pawłowski R, Mühl SM, Sulser T, Krek W,
Moch H and Schraml P: Loss of PBRM1 expression is associated with
renal cell carcinoma progression. Int J Cancer. 132:E11–E17. 2013.
View Article : Google Scholar
|
|
177
|
Nam SJ, Lee C, Park JH and Moon KC:
Decreased PBRM1 expression predicts unfavorable prognosis in
patients with clear cell renal cell carcinoma. Urol Oncol.
33:340.e9–e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Miao D, Margolis CA, Gao W, Voss MH, Li W,
Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, et al:
Genomic correlates of response to immune checkpoint therapies in
clear cell renal cell carcinoma. Science. 359:801–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Manguso RT, Pope HW, Zimmer MD, Brown FD,
Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et
al: In vivo CRISPR screening identifies Ptpn2 as a cancer
immunotherapy target. Nature. 547:413–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Patel SJ, Sanjana NE, Kishton RJ,
Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM,
Yamamoto TN, et al: Identification of essential genes for cancer
immunotherapy. Nature. 548:537–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Pan D, Kobayashi A, Jiang P, Ferrari de
Andrade L, Tay RE, Luoma AM, Tsoucas D, Qiu X, Lim K, Rao P, et al:
A major chromatin regulator determines resistance of tumor cells to
T cell-mediated killing. Science. 359:770–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Shukla SA, Rooney MS, Rajasagi M, Tiao G,
Dixon PM, Lawrence MS, Stevens J, Lane WJ, Dellagatta JL, Steelman
S, et al: Comprehensive analysis of cancer-associated somatic
mutations in class I HLA genes. Nat Biotechnol. 33:1152–1158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
McGranahan N, Rosenthal R, Hiley CT, Rowan
AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P,
Herrero J, et al: Allele-specific HLA loss and immune escape in
lung cancer evolution. Cell. 171:1259–1271.e11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Rodig SJ, Gusenleitner D, Jackson DG,
Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C,
Weber JS, et al: MHC proteins confer differential sensitivity to
CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci
Transl Med. 10:eaar33422018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chowell D, Morris LGT, Grigg CM, Weber JK,
Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et
al: Patient HLA class I genotype influences cancer response to
checkpoint blockade immunotherapy. Science. 359:582–587. 2018.
View Article : Google Scholar :
|
|
186
|
Simeone E, Gentilcore G, Giannarelli D,
Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M,
Cavalcanti E, et al: Immunological and biological changes during
ipilimumab treatment and their potential correlation with clinical
response and survival in patients with advanced melanoma. Cancer
Immunol Immunother. 63:675–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Martens A, Wistuba-Hamprecht K, Geukes
Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B,
Capone M, et al: Baseline peripheral blood biomarkers associated
with clinical outcome of advanced melanoma patients treated with
ipilimumab. Clin Cancer Res. 22:2908–2918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Martens A, Wistuba-Hamprecht K, Yuan J,
Postow MA, Wong P, Capone M, Madonna G, Khammari A, Schilling B,
Sucker A, et al: Increases in absolute lymphocytes and circulating
CD4+ and CD8+ T cells are associated with positive clinical outcome
of melanoma patients treated with ipilimumab. Clin Cancer Res.
22:4848–4858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wistuba-Hamprecht K, Martens A, Heubach F,
Romano E, Geukes Foppen M, Yuan J, Postow M, Wong P, Mallardo D,
Schilling B, et al: Peripheral CD8 effector-memory type 1 T-cells
correlate with outcome in ipilimumab-treated stage IV melanoma
patients. Eur J Cancer. 73:61–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kuzman JA, Stenehjem DD, Merriman J,
Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM and
Agarwal N: Neutrophil-lymphocyte ratio as a predictive biomarker
for response to high dose interleukin-2 in patients with renal cell
carcinoma. BMC Urol. 17:12017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Weide B, Martens A, Hassel JC, Berking C,
Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di
Giacomo AM, et al: Baseline biomarkers for outcome of melanoma
patients treated with pembrolizumab. Clin Cancer Res. 22:5487–5496.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Dall'Olio FG, Gelsomino F, Conci N,
Marcolin L, De Giglio A, Grilli G, Sperandi F, Fontana F,
Terracciano M, Fragomeno B, et al: PD-L1 expression in circulating
tumor cells as a promising prognostic biomarker in advanced
non-small-cell lung cancer treated with immune checkpoint
inhibitors. Clin Lung Cancer. 22:423–431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Krieg C, Nowicka M, Guglietta S, Schindler
S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP and
Becher B: High-dimensional single-cell analysis predicts response
to anti-PD-1 immunotherapy. Nat Med. 24:144–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yuan J, Zhou J, Dong Z, Tandon S, Kuk D,
Panageas KS, Wong P, Wu X, Naidoo J, Page DB, et al: Pretreatment
serum VEGF is associated with clinical response and overall
survival in advanced melanoma patients treated with ipilimumab.
Cancer Immunol Res. 2:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zang J, Hu Y, Xu X, Ni J, Yan D, Liu S, He
J, Xue J, Wu J and Feng J: Elevated serum levels of vascular
endothelial growth factor predict a poor prognosis of
platinum-based chemotherapy in non-small cell lung cancer. Onco
Targets Ther. 10:409–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Schalper KA, Carleton M, Zhou M, Chen T,
Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al:
Elevated serum interleukin-8 is associated with enhanced intratumor
neutrophils and reduced clinical benefit of immune-checkpoint
inhibitors. Nat Med. 26:688–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia
W, Cha JH, Hou J, Hsu JL, Sun L and Hung MC: Exosomal PD-L1 harbors
active defense function to suppress T cell killing of breast cancer
cells and promote tumor growth. Cell Res. 28:862–864. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tucci M, Passarelli A, Mannavola F, Stucci
LS, Ascierto PA, Capone M, Madonna G, Lopalco P and Silvestris F:
Serum exosomes as predictors of clinical response to ipilimumab in
metastatic melanoma. Oncoimmunology. 7:e13877062017. View Article : Google Scholar
|
|
201
|
Garrett WS: Cancer and the microbiota.
Science. 348:80–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zitvogel L, Ayyoub M, Routy B and Kroemer
G: Microbiome and anticancer immunosurveillance. Cell. 165:276–287.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar
|
|
206
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
207
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Arora S, Velichinskii R, Lesh RW, Ali U,
Kubiak M, Bansal P, Borghaei H, Edelman MJ and Boumber Y: Existing
and emerging biomarkers for immune checkpoint immunotherapy in
solid tumors. Adv Ther. 36:2638–2678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sucker A, Zhao F, Real B, Heeke C,
Bielefeld N, Maβen S, Horn S, Moll I, Maltaner R, Horn PA, et al:
Genetic evolution of T-cell resistance in the course of melanoma
progression. Clin Cancer Res. 20:6593–6604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sade-Feldman M, Jiao YJ, Chen JH, Rooney
MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum
H, Blackmon SM, et al: Resistance to checkpoint blockade therapy
through inactivation of antigen presentation. Nat Commun.
8:11362017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gettinger S, Choi J, Hastings K, Truini A,
Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al:
Impaired HLA class I antigen processing and presentation as a
mechanism of acquired resistance to immune checkpoint inhibitors in
lung cancer. Cancer Discov. 7:1420–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Benci JL, Xu B, Qiu Y, Wu TJ, Dada H,
Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et
al: Tumor interferon signaling regulates a multigenic resistance
program to immune checkpoint blockade. Cell. 167:1540–1554.e12.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wang B, Tian T, Kalland KH, Ke X and Qu Y:
Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends
Pharmacol Sci. 39:648–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Cancer Genome Atlas Network: Genomic
classification of cutaneous melanoma. Cell. 161:1681–1696. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Sumimoto H, Imabayashi F, Iwata T and
Kawakami Y: The BRAF-MAPK signaling pathway is essential for
cancer-immune evasion in human melanoma cells. J Exp Med.
203:1651–1656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Gao J, Ward JF, Pettaway CA, Shi LZ,
Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al:
VISTA is an inhibitory immune checkpoint that is increased after
ipilimumab therapy in patients with prostate cancer. Nat Med.
23:551–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Saleh R and Elkord E: Acquired resistance
to cancer immunotherapy: Role of tumor-mediated immunosuppression.
Semin Cancer Biol. 65:13–27. 2020. View Article : Google Scholar
|
|
221
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Wang L, Saci A, Szabo PM, Chasalow SD,
Castillo-Martin M, Domingo-Domenech J, Siefker-Radtke A, Sharma P,
Sfakianos JP, Gong Y, et al: EMT- and stroma-related gene
expression and resistance to PD-1 blockade in urothelial cancer.
Nat Commun. 9:35032018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thiery JP and Chouaib S: New insights into
the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Arce Vargas F, Furness AJS, Solomon I,
Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A,
Sledzinska A, et al: Fc-optimized anti-CD25 depletes
tumor-infiltrating regulatory T cells and synergizes with PD-1
blockade to eradicate established tumors. Immunity. 46:577–586.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Shitara K and Nishikawa H: Regulatory T
cells: A potential target in cancer immunotherapy. Ann N Y Acad
Sci. 1417:104–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Gebhardt C, Sevko A, Jiang H,
Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L,
Beckhove P, Sucker A, et al: Myeloid cells and related chronic
inflammatory factors as novel predictive markers in melanoma
treatment with ipilimumab. Clin Cancer Res. 21:5453–5459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Arlauckas SP, Garris CS, Kohler RH,
Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman
GJ, Anthony RM, et al: In vivo imaging reveals a tumor-associated
macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci
Transl Med. 9:eaal36042017. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Mariathasan S, Turley SJ, Nickles D,
Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita
JL, Cubas R, et al: TGFβ attenuates tumour response to PD-L1
blockade by contributing to exclusion of T cells. Nature.
554:544–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Highfill SL, Cui Y, Giles AJ, Smith JP,
Zhang H, Morse E, Kaplan RN and Mackall CL: Disruption of
CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy.
Sci Transl Med. 6:237ra672014. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Holmgaard RB, Zamarin D, Munn DH, Wolchok
JD and Allison JP: Indoleamine 2,3-dioxygenase is a critical
resistance mechanism in antitumor T cell immunotherapy targeting
CTLA-4. J Exp Med. 210:1389–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Holmgaard RB, Zamarin D, Li Y, Gasmi B,
Munn DH, Allison JP, Merghoub T and Wolchok JD: Tumor-expressed IDO
recruits and activates MDSCs in a treg-dependent manner. Cell Rep.
13:412–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank
CU and Teng MWL: The promise of neoadjuvant immunotherapy and
surgery for cancer treatment. Clin Cancer Res. 25:5743–5751. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Liu J, Blake SJ, Yong MC, Harjunpää H,
Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ and
Teng MW: Improved efficacy of neoadjuvant compared to adjuvant
immunotherapy to eradicate metastatic disease. Cancer Discov.
6:1382–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Amaria RN, Reddy SM, Tawbi HA, Davies MA,
Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, et al:
Neoadjuvant immune checkpoint blockade in high-risk resectable
melanoma. Nat Med. 24:1649–1654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Liu SY, Dong S, Liao RQ, Jiang B, Zhang
JT, Lin JT, Zhang S, Yang J, Nie Q, Yang X, et al: LBA2 phase II
study of PD-L1 expression guidance on neoadjuvant (NA) Nivolumab
(Nivo) monotherapy with or without platinum-doublet chemotherapy in
resectable NSCLC. ESMO. 16(Suppl 1): S103632022.
|
|
238
|
Hannani D, Sistigu A, Kepp O, Galluzzi L,
Kroemer G and Zitvogel L: Prerequisites for the antitumor
vaccine-like effect of chemotherapy and radiotherapy. Cancer J.
17:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Teng F, Kong L, Meng X, Yang J and Yu J:
Radiotherapy combined with immune checkpoint blockade
immunotherapy: Achievements and challenges. Cancer Lett. 365:23–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Ngiow SF, McArthur GA and Smyth MJ:
Radiotherapy complements immune checkpoint blockade. Cancer Cell.
27:437–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wang C, Wang J, Zhang X, Yu S, Wen D, Hu
Q, Ye Y, Bomba H, Hu X, Liu Z, et al: In situ formed reactive
oxygen species-responsive scaffold with gemcitabine and checkpoint
inhibitor for combination therapy. Sci Transl Med. 10:eaan36822018.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Redmond WL, Linch SN and Kasiewicz MJ:
Combined targeting of costimulatory (OX40) and coinhibitory
(CTLA-4) pathways elicits potent effector T cells capable of
driving robust antitumor immunity. Cancer Immunol Res. 2:142–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Mayes PA, Hance KW and Hoos A: The promise
and challenges of immune agonist antibody development in cancer.
Nat Rev Drug Discov. 17:509–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Popovic A, Jaffee EM and Zaidi N: Emerging
strategies for combination checkpoint modulators in cancer
immunotherapy. J Clin Invest. 128:3209–3218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A
and Moon JJ: Designer vaccine nanodiscs for personalized cancer
immunotherapy. Nat Mater. 16:489–496. 2017. View Article : Google Scholar :
|
|
249
|
Madan RA, Mohebtash M, Arlen PM, Vergati
M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL,
Wright JJ, et al: Ipilimumab and a poxviral vaccine targeting
prostate-specific antigen in metastatic castration-resistant
prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol.
13:501–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar :
|
|
251
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Gay F, D'Agostino M, Giaccone L, Genuardi
M, Festuccia M, Boccadoro M and Bruno B: Immuno-oncologic
approaches: CAR-T cells and checkpoint inhibitors. Clin Lymphoma
Myeloma Leuk. 17:471–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
De Henau O, Rausch M, Winkler D, Campesato
LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et
al: Overcoming resistance to checkpoint blockade therapy by
targeting PI3Kγ in myeloid cells. Nature. 539:443–447. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Abdel-Wahab N, Shah M and Suarez-Almazor
ME: Adverse events associated with immune checkpoint blockade in
patients with cancer: A systematic review of case reports. PLoS
One. 11:e01602212016. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Palmieri DJ and Carlino MS: Immune
checkpoint inhibitor toxicity. Curr Oncol Rep. 20:722018.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Cousin S, Seneschal J and Italiano A:
Toxicity profiles of immunotherapy. Pharmacol Ther. 181:91–100.
2018. View Article : Google Scholar
|
|
258
|
Sznol M, Ferrucci PF, Hogg D, Atkins MB,
Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels
GA, et al: Pooled analysis safety profile of nivolumab and
ipilimumab combination therapy in patients with advanced melanoma.
J Clin Oncol. 35:3815–3822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Kottschade LA: Incidence and management of
immune-related adverse events in patients undergoing treatment with
immune checkpoint inhibitors. Curr Oncol Rep. 20:242018. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K; ESMO Guidelines Committee:
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
28(Suppl 4): iv119–iv142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Sullivan RJ and Weber JS: Immune-related
toxicities of checkpoint inhibitors: Mechanisms and mitigation
strategies. Nat Rev Drug Discov. 21:495–508. 2022. View Article : Google Scholar
|