Introduction
Endometrial cancer (EC), a common, malignant tumor
presenting in young individuals, has a high invasiveness and low
differentiation, and has become a major threat to the health of
women worldwide (1,2). Despite early diagnostic and
therapeutic developments in recent years, patients with late-stage
EC still have a poor prognosis and exhibit aggressive metastases
(3). Hence, in order to determine
effective intervention strategies for EC, the exploration of the
mechanisms underlying its occurrence and development is
critical.
Metabolic disorders, such as diabetes, are a major
risk factor for EC progression (4). Hyperglycemia leads to an increase in
the activity of the hexosamine biosynthesis pathway to promote
O-GlcNAcylation, which is a necessary post-translational
modification (PTM) (5,6). The entire process is both reversible
and dynamic, and is driven by O-GlcNAc transferase (OGT) and
inverted by O-GlcNAcase (OGA) (7). O-GlcNAcylation is involved in
numerous key cellular behaviors, such as translation, metabolism
and apoptosis, with the potential to alter protein function either
indirectly or directly in concert with phosphorylation (8,9). The
dysregulation of O-GlcNAc metabolism has been linked to a
variety of diseases, including diabetes, cancer and inflammation
(10-12). A wide variety of molecules, closely
related to tumorigenesis and cancer development, are modified by
O-GlcNAcylation, which affects their proliferative, invasive
and metabolic properties (13-15).
Although aberrant O-GlcNAcylation is associated with EC cell
invasion and metastasis (16,17),
the mechanisms through which O-GlcNAcylation influences the
development and progression of EC remain unclear.
The Hippo pathway is an evolutionarily conserved
tumor suppressor pathway that regulates organ development and
maintains internal environment homeostasis (18-20).
Yes-associated protein (YAP) is a transcriptional co-activator and
a key component of the Hippo pathway (21). Activated macrophage stimulating 1/2
phosphorylates and activates its substrate, large tumor suppressor
kinase 1/2 (LATS1/2), when the Hippo pathway is operating, which in
turn directly causes downstream YAP phosphorylation and inactivates
it by promoting its cytoplasmic retention. The inhibition of the
Hippo pathway causes YAP to enter the nucleus in a
non-phosphorylated state and bind to transcription factors, such as
the TEA domain protein family, to promote cell proliferation and
transformation (22,23). High glucose levels activate YAP
signaling (24-26), which is critical to the development
of cancers, including EC (27-29).
Moreover, YAP is a target protein for O-GlcNAc modification
(30); however, to date, at least
to the best of our knowledge, there are no relevant studies
available on EC that depict this mechanism. The present study thus
aimed to examine the effects of O-GlcNAcylation on the
malignancy of EC and its association with YAP.
Materials and methods
Cells and cell culture
The AN3CA (CL-0505) and HEC-1-B (CL-0100) cell
lines, provided by Procell Life Science & Technology Co., Ltd.,
were cultured at 37°C and 5% CO2 in MEM supplemented
with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific,
Inc.). OSMI-1 (HY-119738, MedChemExpress) and Thiamet-G (TMG;
HY-12588, MedChemExpress) were dissolved in dimethyl sulfoxide
(DMSO; PYG0040, Wuhan Boster Biological Technology, Ltd.).
Generation of stable cell lines
The AN3CA (50,000 cells/well) and HEC-1-B (50,000
cells/well) cells were cultured for 24 h in six-well plates and the
medium was discarded. Subsequently, 940 µl medium, 40
µl infection enhancing solution (HitransG P, 25X) and 20
µl virus (1×108 TU/ml) were added to the six-well
plates followed by incubation at 37°C for 12 h. After switching to
normal medium and continuing incubation for 48 h, the cells were
cultured in medium containing puromycin (2 µg/ml, HY-K1057,
MedChemExpress) for 2 weeks to establish a stable cell line. The
third generation was used. The MOI values of both cell lines were
10. Virus and infection enhancement solution were provided by
Shanghai Genechem Co., Ltd. The sequences of the shRNAs used are
presented in Table SI.
Western blot analysis
The cells were treated with RIPA lysis buffer (cat.
no. AR0102, Wuhan Boster Biological Technology, Ltd.) containing
phosphatase inhibitors (cat. no. AR1195, Wuhan Boster Biological
Technology, Ltd.) and broad-spectrum protease inhibitors (cat. no.
AR1193, Wuhan Boster Biological Technology, Ltd.), and whole
protein lysates were harvested. The protein concentration was
determined using the BCA protein concentration assay kit (cat. no.
P0012S, Beyotime Institute of Biotechnology). Equal amounts of 50
µg whole cell lysate were separated on 10% SDS-PAGE gel and
then electrophoretically transferred to PVDF membrane. After being
blocked with 10% skimmed milk in TBST at 27°C for 1 h, the PVDF
membranes were incubated overnight at 4°C with primary and
secondary antibodies, followed by ECL luminescence detection (cat.
no. AR1196, Wuhan Boster Biological Technology, Ltd.). Analysis of
the strips was performed using ImageJ software (V1.8.0.112,
National Institutes of Health). The antibodies and dilutions used
in the present study were as follows: Anti-OGT (1:1,000; cat. no.
ab96718, Abcam), anti-O-GlcNAcylation (1:1,000; cat. no.
ab2739, Abcam), anti-YAP (1:2,000; cat. no. 13584-1-AP, Proteintech
Group, Inc.), anti-phosphorylated (p-)YAP (1:1,000; cat. no.
13008S, Cell Signaling Technology, Inc.), anti-LATS1 (1:1,000; cat.
no. 3477S, Cell Signaling Technology, Inc.), anti-LATS2 (1:1,000;
cat. no. 20276-1-AP, Proteintech Group, Inc.), anti-connective
tissue growth factor (CTGF; 1:2,000; cat. no. 25474-1-AP,
Proteintech Group, Inc.), anti-Histone-H3 (1:2,000; cat. no.
17168-1-AP, Proteintech Group, Inc.), anti-β-actin (1:2,000; cat.
no. BM0005, Wuhan Boster Biological Technology, Ltd.) and
anti-tubulin (1:1,000; cat. no. 2148, Cell Signaling Technology,
Inc.).
Co-immunoprecipitation
Cell lysates were obtained according to the kit
instructions (AM001-01, ACE Biotechnology LLC) and incubated with
20 µl of prepared protein A/G immunoprecipitated magnetic
beads (IC-8110, InCellGene LLC) for 12 h at 4°C according to the
instructions of the manufacturer. Following magnetic separation,
the target antibody was added to the antigen-magnetic beads and
incubated at 4°C for 12 h. The antigen was then eluted and the
eluted antigen was examined using western blot analysis. The
antibodies used for immunoprecipitation were as follows: Anti-OGT
(1:200; cat. no. ab96718, Abcam), anti-IgG (1:200; cat. no.
IC-8109, InCellGene LLC), anti-O-GlcNAcylation (1:200; cat.
no. ab2739, Abcam), anti-YAP (1:200; cat. no. 13584-1-AP,
Proteintech Group, Inc.), anti-LATS1 (1:200; cat. no. 3477S, Cell
Signaling Technology, Inc.) and anti-LATS2 (1:200; cat. no.
20276-1-AP, Proteintech Group, Inc.).
Immunohistochemistry
Human endometrial cancer tissue microarray slides
were purchased from Shanghai Xinchao Biotechnology (cat. no.
HUteA060CS01). Ethics approval (no. KY20203047) was provided by the
Medical Ethics Committee of the First Hospital Affiliated to the
Air Force Medical University. The slides included 28 endometrial
cancer specimens (age range, 36-73 years) and 15 pairs of
carcinomas and para-cancerous tissue specimens (age range, 36-73
years). Dewaxing and rehydration were followed by antigen repair in
citrate buffer at pH 6.0. The sections were closed with endogenous
peroxidase blocking solution for 10 min, followed by immersion in
PBS solution for 5 min. Incubation with primary antibody was
performed overnight at 4°C after the sections are closed with goat
serum (cat. no. AR0009, Wuhan Boster Biological Technology, Ltd.).
The primary antibodies used were as follows: Anti-OGT (1:100; cat.
no. PB9767, Wuhan Boster Biological Technology, Ltd.) and
anti-O-GlcNAc (1:50; cat. no. PTM-952, Jingjie PTM BioLab
(Hangzhou) Co. Ltd.). The sections were then incubated with
secondary antibodies (1:1; cat. no. BA1056, Wuhan Boster Biological
Technology, Ltd.) at 37°C for 1 h following three washes with PBS.
Dropwise additions of HRP-streptavidin (cat. no. BA1088, Wuhan
Boster Biological Technology, Ltd.) were added, followed by DAB
color development. The tissue microarray was scanned using a
microscope (Pannoramic MIDI, 3DHISTECH) and the histochemistry
score (H-SCORE) was calculated by analyzing the histochemical
results using Aperio ImageScope software (Version 12, Leica
Biosystems).
Cell viability assays
The AN3CA and HEC-1-B cells were seeded in 96-well
plates (5,000 cells/well for AN3CA cells and 8,000 cells/well for
HEC-1-B cells). Measurements were performed at pre-determined time
points. Cell Counting Kit-8 (CCK-8; BB-4202, Bestbio) solution was
added to the 96-well plates, and following incubation for 2 h with
protection from light, spectrophotometric measurements were carried
out at 450 nm using an Infinite M200PRO multimode plate reader
(Tecan Group, Ltd.).
The cells were seeded in a 96-well plate and
incubated at 37°C for 24 h prior to the addition of 100 µl
EdU (50 µM, cat. no. C10310, Guangzhou RiboBio Co., Ltd.).
After 2 h, the 96-well plate was washed with PBS, and the cells
were fixed with 4% paraformaldehyde at 27°C for 30 min, and glycine
(2 mg/ml, AR1200, Boster Biological Technology, Ltd.) was then
added to the cells and incubated at 27°C for 5 min. After washing
with PBS, cells were incubated at 27°C for 10 min following the
adding 0.5% TritonX-100. Subsequently, Apollo staining and DNA
staining were performed according to the instructions of the
manufacturer (C0085, Beyotime Institute of Biotechnology). Finally,
observation was performed using a fluorescence microscope (Nikon
Eclipse Ni-U).
Wound healing assay
The cells were inoculated in six-well plates
(1.5×106 cells/well), cultured for 12 h and then
serum-starved for 24 h. A 50 µl pipette tip was passed
through the cell monolayer to create a 'wound'. To control for the
possible effects of proliferation, serum-free medium was used for
cell culture. Images of the wound area were obtained in a fixed
location once a day using an inverted microscope (Nikon Eclipse
Ti2; Nikon Corporation), and wound closure rates were calculated
using ImageJ software (V1.8.0.112, National Institutes of
Health).
Transwell assay
Transwell assays were employed to evaluate the
invasive and migratory potential of the cells. For the detection of
the cell invasive ability, 100,000 cells were added to the upper
chamber of the Transwell (354480, Matrigel Biocoat Invasion
Chambers, Corning, Inc.). For the detection of the migratory
ability, 30,000 cells were added to the upper chamber (3422,
Corning Transwell, Corning, Inc.). It is important to note that the
lower chamber contained 10% FBS, while the upper chamber did not.
The upper chamber was removed 48 h following incubation at 37°C,
and the Transwell membrane fixed with 4% paraformaldehyde at 27°C
for 30 min and stained with crystal violet (C0121, Beyotime
Institute of Biotechnology) at 27°C for 5 min. After dissolving the
crystal purple dye in 33% acetic acid, the absorbance was measured
at 570 nm using TECAN Infinite M200 Pro (Tecan Group, Ltd.).
Mass spectrometric analysis
The cells were treated with RIPA lysis buffer
containing phosphatase inhibitors and broad-spectrum protease
inhibitors, and whole protein lysates were harvested. The extracted
protein was added to dithiothreitol (10 mM, M109-5G, Amresco, LLC).
This was followed by incubation at 37°C for 1 h and iodoacetamide
was then added (40 mM, M216-30G, Amresco, LLC) followed by
incubation at 37°C for 45 min. The samples were then diluted using
ammonium bicarbonate (A6141, MilliporeSigma) to the pH of the
sample of 8. Trypsin (V5280, Promega Biotech Co., Ltd.) was added
at a 50:1 ratio of protein to trypsin overnight at 37°C. The
samples were passed through a desalting column and eluted with 70%
acetonitrile. The enrichment of the glycosylated peptides was then
performed by adding the samples to the activated hydrophilic
interaction liquid chromatography enrichment column (Atlantis,
Waters) and incubating at 27°C for 2 h; the flow-through solution
was discarded. For one more elution, 0.5% formic acid was added to
5% acetonitrile +0.5% formic acid. The flow-through solution was
collected and lyophilized. The samples were analyzed using
high-performance liquid chromatography coupled with a Q Exactive HF
mass spectrometer (Thermo Fisher Scientific, Inc.). The Homo
sapiens SP database was searched using Byonic software (Protein
Metrics Inc). The search parameters were set as follows: 15 ppm for
precursor ion mass tolerance, 0.02 Da for fragment ion mass
tolerance, 2 for the maximum number of missed cleavages, and the
Dynamic Modification was set to M Oxidation (15.995 Da) and Acetyl
(Protein N-terminal).
GeneCards
The GeneCards database (www.genecards.org) provides a comprehensive and
authoritative summary of human gene annotation data (31). The GeneCards database was searched
using 'endometrial cancer' to obtain genes associated with
endometrial cancer.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The RNAsimple Total RNA kit (Tiangen Biotech Co.,
Ltd.) was used to isolate total RNA from the EC cells, and the
PrimerScript RT Reagent kit (Takara Bio, Inc.) was used for reverse
transcription. qPCR was performed using ChamQ Universal SYBR qPCR
Master Mix (Vazyme Biotech Co., Ltd.) and the CFX Connect system
(Bio-Rad Laboratories, Inc.). The PCR thermocycling conditions were
set according to the kit instructions (Vazyme Biotech Co., Ltd.).
The 2−ΔΔCq method (32)
was used to calculate the relative transcript levels of target
genes, and ACTIN was used as an internal reference. The genes
examined were: ankyrin repeat domain 1 (ANKRD1), connective tissue
growth factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61)
and glucose transporter 3 (GLUT3). The primers used for RT-qPCR in
the present study are presented in Table SII.
Cycloheximide (CHX) chase
experiments
The medium was changed to medium containing 50
µM CHX (HY-12320, MedChemExpress) when the cell density in
the culture dish reached 70%. The time of medium change was
considered as the beginning of receiving CHX treatment, and
different treatment times (0, 4, 8, 12, 16 and 24 h) were selected
for protein extraction and western blot analysis.
Immunofluorescence staining
The cells were seeded in confocal Petri dishes one
day in advance. At 37°C, following fixation with 4%
paraformaldehyde for 15 min, the cells were washed with PBS and
incubated with 0.5% Triton X-100 for 5 min. The cells were washed
with PBS and blocked with 5% goat serum at 37°C for 30 min. The
cells were incubated overnight at 4°C with the primary antibody
(YAP; 1:100; cat. no. 13584-1-AP, Proteintech Group, Inc.). PBST
was used to wash the cells thrice, and the secondary antibody
(1:200; cat. no. ab150075, Abcam) was then added followed by
incubation at 37°C for 2 h. Nuclei were counterstained with DAPI
(G1012, Wuhan Servicebio Technology Co., Ltd.) for 5 min at 37°C.
An anti-fading solution (P0126, Beyotime Institute of
Biotechnology) was added in a dropwise manner, and observation was
performed with a Nikon A1+ laser confocal microscope (Nikon
Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 23.0
software (IBM Corp.). The statistical significance of differences
between two datasets was calculated using the two-tailed paired or
unpaired Student's t-test; for comparison of multiple groups, one-
or two-way ANOVA was performed followed using Dunnett's for
multiple post hoc comparisons. P-values <0.05 were considered to
indicate statistically significant differences.
Results
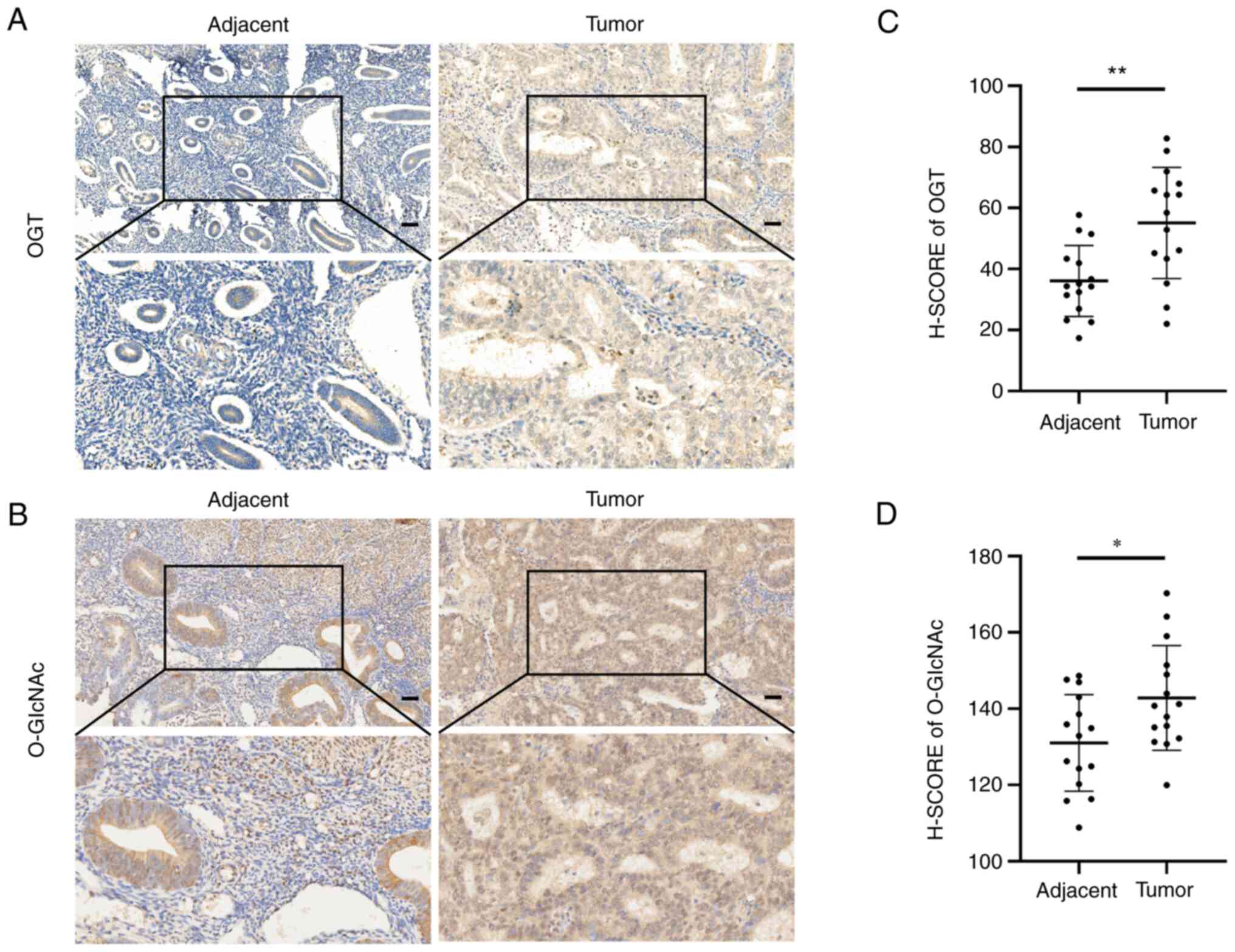
The level of O-GlcNAcylation is associated with the
malignancy of EC. To explore the effects of O-GlcNAcylation
on EC, OGT and O-GlcNAc expression was examined in cancer
tissues and paired tumor-adjacent tissues from 15 patients with EC.
The results of immunohistochemistry revealed that the EC tissues
expressed higher levels of OGT and O-GlcNAc than the paired
normal tumor-adjacent endometrial tissues (Fig. 1A and B). Likewise, the OGT and
O-GlcNAc H-SCOREs in the EC tissues were significantly
higher than those in the paired tumor-adjacent tissues (Fig. 1C and D).
In addition, the association between the H-SCORE of
O-GlcNAcylation and OGT, and the clinicopathological
features of 28 patients with EC was analyzed (Tables I and II, and Fig. S1). A high staining intensity of
O-GlcNAcylation was positive associated with lymph node
metastasis, histopathological grade and clinical stage. The
increased expression of OGT was positively associated with the
clinical stage and lymph node metastasis, although not with the
histopathological grade. Neither O-GlcNAcylation nor OGT
expression was associated with the age of the patients.
 | Table IEvaluation of the association between
O-GlcNAcylation and the clinical characteristics of 28
patients with endometrial cancer. |
Table I
Evaluation of the association between
O-GlcNAcylation and the clinical characteristics of 28
patients with endometrial cancer.
| Variable | No. of
patients | H-SCORE of
O-GlcNAc (± SE) | P-value |
|---|
| Age (years) | | | |
| <55 | 13 | 147.65±18.80 | 0.640505 |
| ≥55 | 15 | 150.72±15.59 | |
| Histopathological
grade | | | |
| Well
differentiated | 7 | 137.43±7.15 | 0.028965a |
| Moderately
differentiated + poorly differentiated | 21 | 153.25±17.45 | |
| Clinical stage | | | |
| I + II | 19 | 144.09±12.69 | 0.014766a |
| III + IV | 9 | 160.29±20.04 | |
| Lymph node
metastasis | | | |
| Yes | 8 | 163.81±16.87 | 0.002264b |
| No | 20 | 143.49±13.30 | |
 | Table IIEvaluation of the association between
OGT and the clinical characteristics of 28 patients with
endometrial cancer. |
Table II
Evaluation of the association between
OGT and the clinical characteristics of 28 patients with
endometrial cancer.
| Variable | No. of
patients | H-SCORE of OGT (±
SEM) | P-value |
|---|
| Age (years) | | | |
| <55 | 13 |
53.4715±24.44747 | 0.455603 |
| ≥55 | 15 |
47.3313±18.37585 | |
| Histopathological
grade | | | |
| Well
differentiated | 7 | 55.98±22.19 | 0.414238 |
| Moderately +
poorly differentiated | 21 | 48.25±21.09 | |
| Clinical stage | | | |
| I + II | 19 | 38.23±20.70 | 0.037619a |
| III + IV | 9 | 55.84±19.49 | |
| Lymph node
metastasis | | | |
| Yes | 8 | 56.42±19.72 | 0.010983a |
| No | 20 | 34.59±17.11 | |
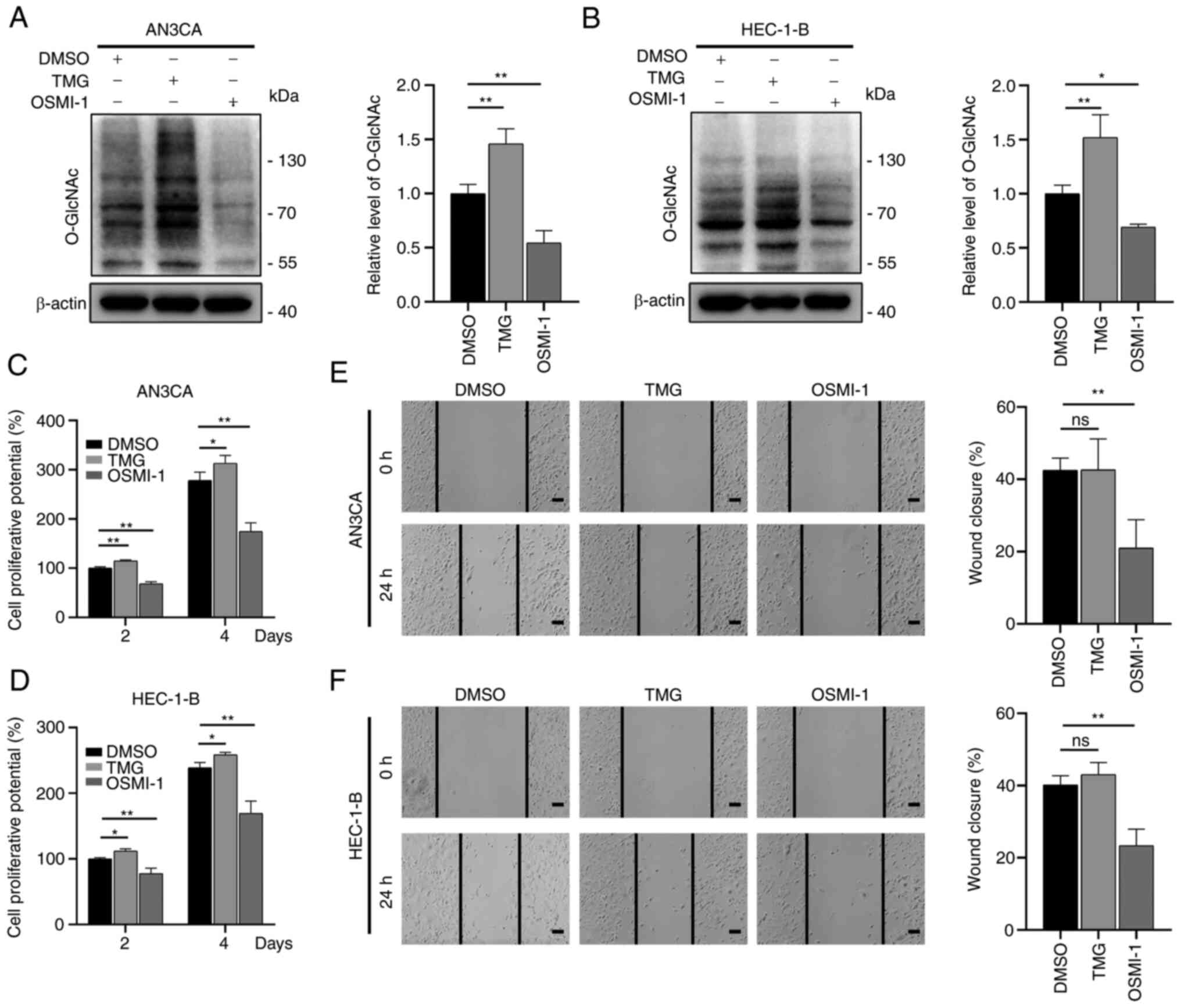
O-GlcNAcylation is essential for the
malignancy of EC cells
To examine the effects of O-GlcNAcylation on
EC, the EC cells were treated with TMG and OSMI-1, which are able
to regulate O-GlcNAcylation. The O-GlcNAcylation
levels increase upon treatment with TMG via the antagonization of
OGA activity, whereas these levels are reduced following treatment
with OSMI-1 through the inhibition of OGT activity (33,34).
Similar to these previous studies, the results of the present study
indicated that treatment with TMG increased the
O-GlcNAcylation levels, whereas treatment with OSMI-1
reduced the O-GlcNAcylation levels (Fig. 2A and B). The results of CCK-8 assay
revealed that the OSMI-1-treated cells (OSMI-1 group) had a
markedly impaired proliferative ability compared to the control
group (DMSO group), while the TMG-treated cells (TMG group) had a
significantly enhanced proliferative ability (Fig. 2C and D). In addition, the migratory
ability of the aforementioned cells was examined using wound
healing assays. The results revealed that the migratory ability of
the cells in the OSMI-1 group was markedly reduced in comparison
with the control group, while that of the TMG group was not
significantly altered (Fig. 2E and
F).
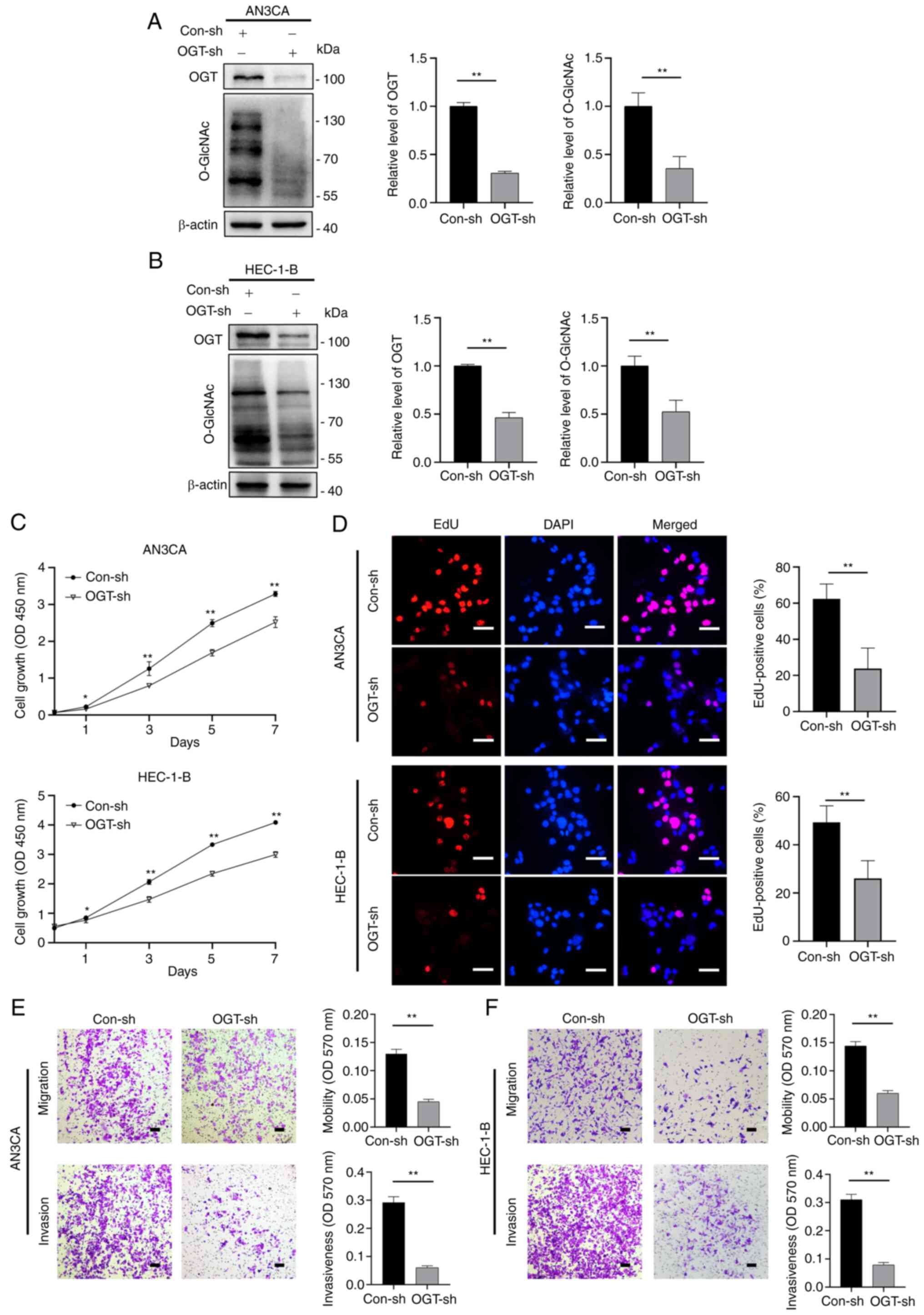
Subsequently, OGT-specific small hairpin RNA (shRNA)
were used to construct stable OGT-deficient AN3CA and HEC-1-B cell
lines. OGT expression was decreased in OGT-specific
shRNA-transfected cells compared to the control (Con-sh) cells, and
the O-GlcNAcylation levels were also found to be decreased
(Fig. 3A and B). When compared to
the control group, the OGT-deficient (OGT-sh) cells exhibited an
inhibited growth capacity in the CCK-8 and EdU cell proliferation
assays (Fig. 3C and D). The
results of wound healing assay also revealed an impaired wound
closure rate for the OGT-deficient cells (Fig. S2). Transwell assays were then used
to assess the migratory and invasive ability of the OGT-sh group
(in both AN3CA and HEC-1-B cells) and it was found that the
migratory and invasive ability of the cells decreased significantly
in the OGT-sh group (Fig. 3E and
F). These findings demonstrate that O-GlcNAcylation is
critical to EC proliferation and metastasis.
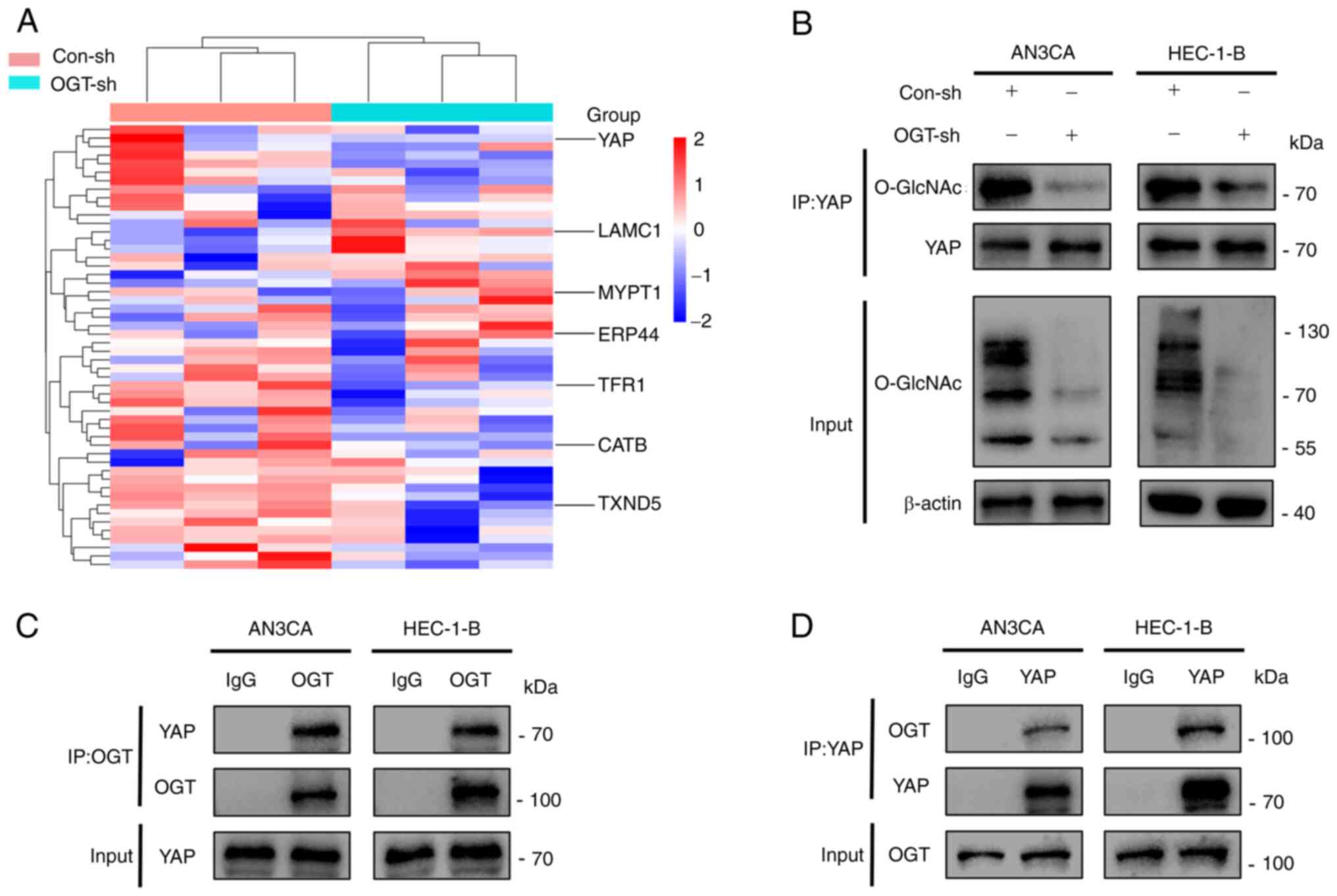
YAP combines with OGT and undergoes
O-GlcNAcylation
In order to elucidate the mechanisms through which
O-GlcNAcylation promotes the development of EC, the Con-sh
and OGT-sh HEC-1-B cells were analyzed using high-performance
liquid chromatography to identify differential proteins that could
be O-GlcNAcylated (Fig.
4A). It was found that YAP was O-GlcNAcylated, and the
knockdown of OGT resulted in a reduced YAP O-GlcNAcylation
(Fig. 4A). Combined with the
related genes of EC in the GeneCards database (https://www.genecards.org/) and the crucial role of
YAP in EC, it was hypothesized that O-GlcNAcylation may
affect tumor malignancy by regulating YAP. It was verified that YAP
was the target protein for O-GlcNAcylation through
co-immunoprecipitation experiments: Its O-GlcNAcylation
level was reduced as the global O-GlcNAcylation decreased
(Fig. 4B). Furthermore, it was
demonstrated that the knockdown of YAP significantly reduced the
proliferative and migratory abilities of the cells; however,
altering the O-GlcNAcylation of cells following the knockdown of
YAP had no significant effect on the proliferative and migratory
abilities of the cells (Fig. S3).
In addition, owing to the fact that OGT is the only enzyme known to
promote protein O-GlcNAcylation, the present study examined
whether YAP interacts with OGT. The result of
co-immunoprecipitation assays indicated that YAP interacted with
OGT (Fig. 4C and D). In summary,
it was demonstrated that YAP interacts with OGT, leading to its
O-GlcNAcylation.
YAP O-GlcNAcylation inhibits
phosphorylation by suppressing YAP/LATS1/2 expression to improve
the stability of YAP protein
In light of the fact that O-GlcNAcylation
affects the expression and function of multiple proteins, the
effects of O-GlcNAcylation on YAP expression were examined.
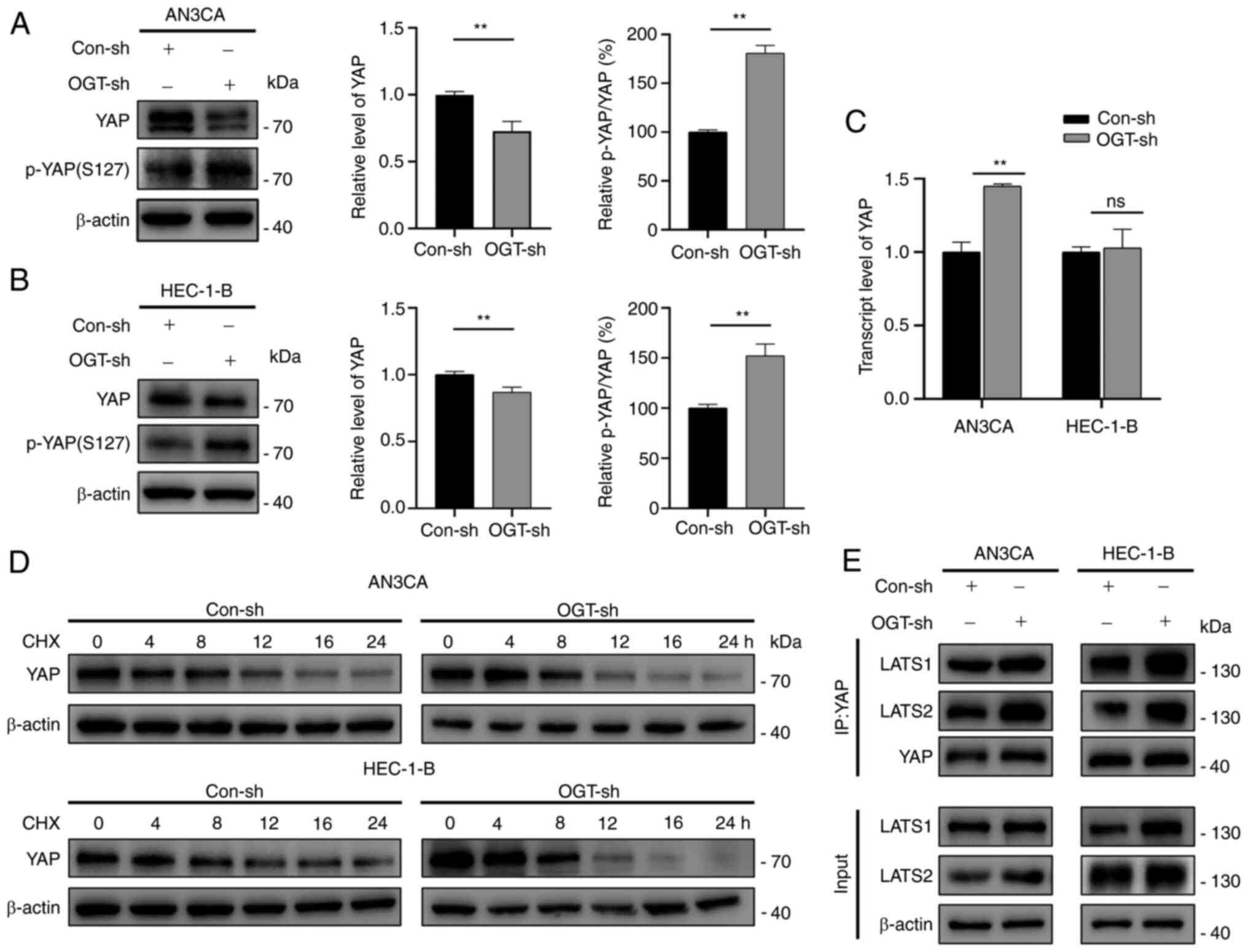
It was observed that YAP expression was decreased in the OGT-sh
group (Fig. 5A and B). YAP
transcriptional levels were measured in the Con-sh and OGT-sh
groups to investigate the mechanisms driving the changes in YAP
expression (Fig. 5C). The
transcriptional levels of YAP in the AN3CA OGT-sh group increased
significantly, whereas no significant difference was found between
the HEC-1-B Con-sh and OGT-sh groups (Fig. 5C). As protein concentration is
regulated by synthesis and degradation, it was hypothesized that
the changes in YAP expression were related to YAP degradation. As
demonstrated by CHX chase experiments, OGT knockdown reduced the
half-life of YAP in EC cells (Figs.
5D and S4), indicating that
O-GlcNAcylation modulates YAP expression by enhancing its
stability.
Since YAP phosphorylation at Ser127 (p-YAP)
inactivates YAP through cytosolic sequestration and subsequent
degradation (22), it was
hypothesized that the changes in YAP expression due to
O-GlcNAcylation were associated with its phosphorylation. It
was found that a decreased O-GlcNAcylation resulted in a
significantly increased p-YAP(S127)/YAP ratio (decreased YAP
expression with increased p-YAP expression; Fig. 5A and B). Through the kinase cascade
reaction, YAP, a Hippo pathway effector, is phosphorylated by
upstream kinases (LATS1/2) (22).
Herein, to explore whether O-GlcNAcylation affects the
phosphorylation of YAP by blocking LATS1/2 binding to YAP,
co-immunoprecipitation assays were performed. The results suggested
that the reduced O-GlcNAcylation promoted the accessibility
of YAP to LATS1/2 (Fig. 5E). On
the whole, the results confirmed that O-GlcNAcylation
enhanced the stability of YAP by inhibiting its phosphorylation via
the suppression of the interaction of YAP and LATS1/2.
O-GlcNAcylation of YAP regulates its
activation and nuclear translocation
It has been shown that unphosphorylated YAP enters
the nucleus and actives transcription factors (21). However, whether YAP
O-GlcNAcylation leads to induces YAP nuclear entry remains
unclear. Therefore, the present study examined the distribution of
YAP by separating nuclear and cytoplasmic proteins. It was observed
that YAP expression was markedly reduced in the nucleus and
slightly decreased in the cytoplasm of the OGT-sh group (Fig. 6A). These results were in accordance
with those obtained by immunofluorescence staining, which confirmed
that YAP in the OGT-sh group was predominantly located in the
cytoplasm and less in the nucleus compared to the control group
(Fig. 6B and C).
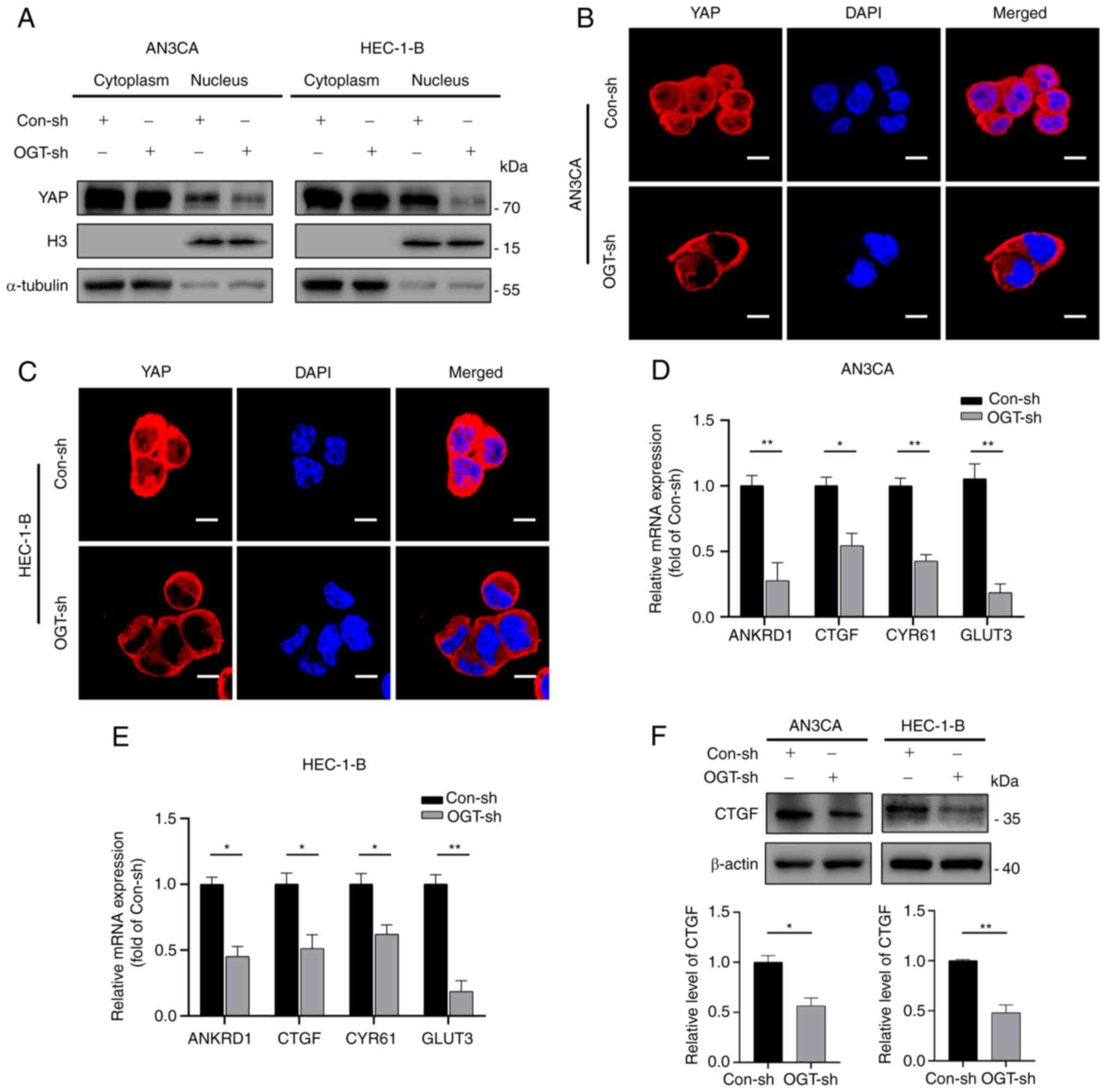 | Figure 6YAP O-GlcNAcylation promotes
its activation and nuclear translocation. (A) Nuclear-cytoplasmic
separation assays revealed the cellular sub-localization of YAP in
the different groups. (B and C) Immunofluorescence staining
revealed the cellular sub-localization of YAP in the different
groups. Scale bars, 10 µm. (D and E) mRNA expression of
ANKRD1, CTGF, CYR61 and GLUT3 was detected in endometrial cancer
using reverse transcription-quantitative PCR in EC cells. (F)
Western blot analysis was used to measure CTGF expression in
endometrial cancer cells. Data represent the mean ± SD (n=3).
*P<0.05 and **P<0.01. ns, not
significant; YAP, Yes-associated protein; OGT, O-GlcNAc
transferase; H3, Histone-H3; ANKRD1, ankyrin repeat domain 1; CTGF,
connective tissue growth factor; CYR61, cysteine-rich angiogenic
inducer 61; GLUT3, glucose transporter 3. |
Further research was conducted on the mRNA
expression of typical YAP target genes (ANKRD1, CTGF, CYR61 and
GLUT3) to examine the effects of O-GlcNAcylation on YAP
activity (Fig. 6D and E). The
results revealed that a decrease in O-GlcNAcylation reduced
the transcriptional levels of YAP downstream target genes (Fig. 6D and E). Subsequently, the protein
expression of CTGF was verified using western blot analysis.
Consistent with the results obtained for mRNA expression, reduced
O-GlcNAcylation resulted in a decreased expression of CTGF
(Fig. 6F). Thus, these results
demonstrate that YAP nuclear distribution and activation are
influenced by its O-GlcNAcylation level.
Discussion
By comparing EC tissues with normal tumor-adjacent
endometrial tissues, the present study found that OGT and
O-GlcNAcylation were significantly upregulated in EC
tissues. O-GlcNAcylation was positively associated with
lymph node metastasis, histopathological grade and clinical stage,
while OGT expression was positively associated with clinical stage
and lymph node metastasis, although not with histopathological
grade. O-GlcNAcylation in tissues may not only be regulated
via OGT, but also by OGA and the nutritional status in vivo;
however, the sample size in the present study was not sufficient to
cover all these aspects. In subsequent experiments, it was
confirmed that the reduction of O-GlcNAcylation affected EC
cell proliferation, migration and invasion.
In EC cells and tissues, YAP is upregulated and its
expression is significantly associated with tumor grade, stage,
post-operative recurrence/metastasis, and overall survival
(35-37). As YAP expression and function are
controlled by its phosphorylation (38), altering its phosphorylation affects
the malignancy of a variety of tumors by influencing YAP activation
and nucleation (39,40). YAP O-GlcNAcylation hinders
its phosphorylation, thus facilitating YAP entry into the nucleus
to exert pro-cancer effects (41,42).
Based on these findings, the present study identified that YAP was
O-GlcNAcylated in EC cells and that this modification
affected its expression. Further analyses revealed that a reduction
in the global O-GlcNAcylation levels in EC cells resulted in
the decreased O-GlcNAcylation of YAP and promoted YAP
phosphorylation by facilitating the binding of YAP to LATS1/2,
leading to its cytoplasmic retention and functional
inactivation.
It has been demonstrated that downstream target
genes of YAP play a crucial role in tumorigenesis. CTGF is a
multifunctional signaling regulator that promotes cancer
development, progression and metastasis by regulating cell
proliferation (43). CTGF has been
found to be an independent prognostic factor in EC; it is closely
associated with the development of EC and can be used as a
prognostic biomarker in EC (44).
GLUT3 is a member of the GLUT family and is involved in the first
step of cellular glucose utilization and glycolysis. It has been
found that GLUT3 is involved in glucose uptake by EC cells and is
associated with the degree of differentiation of EC (45). CYR61 is a dynamically expressed
multifunctional matricellular protein that is associated with the
proliferative capacity of EC cells (46). A key mechanism through which YAP
exerts its influence on tumor function is by regulating the
expression of its downstream target proteins (47). YAP promotes the proliferation and
migration of colorectal cancer cells through the GLUT3/AMPK
signaling pathway (48); the
Hippo-YAP pathway upregulates CYR61/CTGF to promote the aggressive
phenotype of thyroid cancer (49).
The authors aim to focus on exploring the function of downstream
target genes of YAP protein in EC and to further explore the
localization of YAP and its downstream target genes in EC tissue in
future studies.
There is a frequent and dynamic crosstalk between
O-GlcNAcylation and phosphorylation. These PTMs can compete
for the same sites/residues (50,51)
and occur on Ser/Thr residues both at close distances from one
another (52,53) and relatively far from one another
(54). This crosstalk is reflected
in YAP by the fact that O-GlcNAcylation and phosphorylation
occur at residues in close proximity and that the PTMs have
specific effects on the function of the protein. When YAP is
O-GlcNAcylated at threonine 241, its serine 127
phosphorylation is inhibited, which promotes YAP nuclear
translocation in hepatocellular carcinoma cells (41). YAP undergoes O-GlcNAcylation
at serine 109, competing with serine 127 phosphorylation (42,55).
The present study identified the O-GlcNAcylation site of YAP
in EC cells by mass spectrometry (Fig. S5); however, the present
study only demonstrated that the O-GlcNAcylation of YAP
hinders the phosphorylation of serine 127. The specific PTM site
that affects YAP phosphorylation needs further investigation.
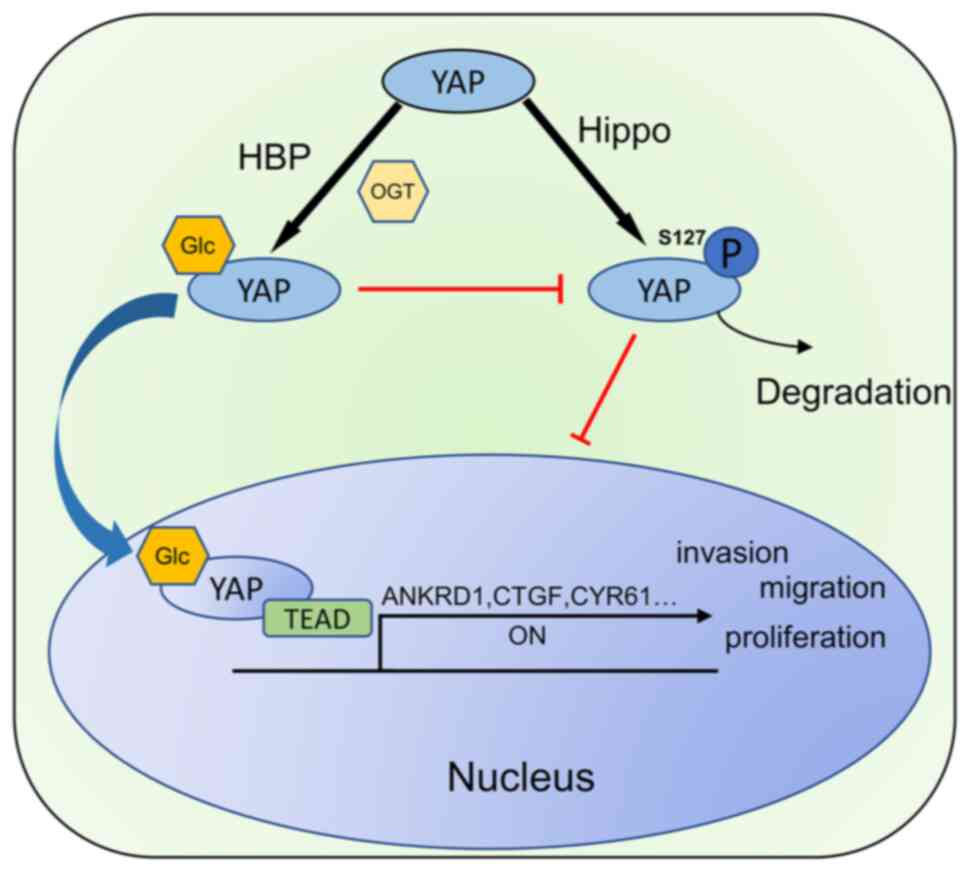
In conclusion, the present study demonstrated
elevated O-GlcNAcylation levels in EC and explored the
mechanisms of the tumorigenic role of O-GlcNAcylation. It
was demonstrated for the first time, to the best of our knowledge,
that the O-GlcNAcylation of YAP affects the phosphorylation
of its serine 127 site, which in turn regulates the malignancy of
EC (Fig. 7). The results obtained
herein suggest that targeting YAP O-GlcNAcylation may
represent a promising therapeutic strategy for EC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request. The data obtained from mass spectrometric analysis are
available at: https://www.iprox.cn/page/PSV023.html;?url=1685362579736KfYS
(password: HJVv).
Authors' contributions
FZ and BC designed the study. LZ and XY were
involved in the conceptualization of the study. LZ, JD and LQ
performed the experiments. YG, YL and LC were involved in data
processing. LZ, FZ and XY confirm the authenticity of all the raw
data. LZ, XY and FZ were involved in the writing of the manuscript.
FZ and BC supervised the study. All the authors were involved in
the discussions related to the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval (no. KY20203047) was provided by the
Medical Ethics Committee of the First Hospital Affiliated to the
Air Force Medical University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the Shaanxi Key
Laboratory of Free Radical Biology and Medicine (Xi'an, China) for
providing the experimental facilities and conditions.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81972440 and 82002735).
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Urick ME and Bell DW: Clinical
actionability of molecular targets in endometrial cancer. Nat Rev
Cancer. 19:510–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brooks RA, Fleming GF, Lastra RR, Lee NK,
Moroney JW, Son CH, Tatebe K and Veneris JL: Current
recommendations and recent progress in endometrial cancer. CA
Cancer J Clin. 69:258–279. 2019.PubMed/NCBI
|
|
4
|
Shahid RK, Ahmed S, Le D and Yadav S:
Diabetes and cancer: Risk, challenges, management and outcomes.
Cancers (Basel). 13:57352021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lam C, Low JY, Tran PT and Wang H: The
hexosamine biosynthetic pathway and cancer: Current knowledge and
future therapeutic strategies. Cancer Lett. 503:11–18. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma J, Wu C and Hart GW: Analytical and
biochemical perspectives of protein O-GlcNAcylation. Chem Rev.
121:1513–1581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X and Qian K: Protein
O-GlcNAcylation: Emerging mechanisms and functions. Nat Rev Mol
Cell Biol. 18:452–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang YH, Weng CL and Lin KI:
O-GlcNAcylation and its role in the immune system. J Biomed Sci.
27:572020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akimoto Y, Yan K, Miura Y, Tsumoto H, Toda
T, Fukutomi T, Sugahara D, Kudo A, Arai T, Chiba Y, et al:
O-GlcNAcylation and phosphorylation of β-actin Ser199 in
diabetic nephropathy. Am J Physiol Renal Physiol. 317:F1359–F1374.
2019. View Article : Google Scholar
|
|
10
|
Nie H and Yi W: O-GlcNAcylation, a sweet
link to the pathology of diseases. J Zhejiang Univ Sci B.
20:437–448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee BE, Suh PG and Kim JI: O-GlcNAcylation
in health and neurodegenerative diseases. Exp Mol Med.
53:1674–1682. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quik M, Hokke CH and Everts B: The role of
O-GlcNAcylation in immunity against infections. Immunology.
161:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JB, Pyo KH and Kim HR: Role and
function of O-GlcNAcylation in cancer. Cancers (Basel).
13:53652021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrer CM, Lynch TP, Sodi VL, Falcone JN,
Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN and Reginato MJ:
O-GlcNAcylation regulates cancer metabolism and survival stress
signaling via regulation of the HIF-1 pathway. Mol Cell.
54:820–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun L, Lv S and Song T: O-GlcNAcylation
links oncogenic signals and cancer epigenetics. Discov Oncol.
12:542021. View Article : Google Scholar
|
|
16
|
Jaskiewicz NM and Townson DH:
Hyper-O-GlcNAcylation promotes epithelial-mesenchymal transition in
endometrial cancer cells. Oncotarget. 10:2899–2910. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ciesielski P, Jóźwiak P, Forma E and
Krześlak A: TET3- and OGT-dependent expression of genes involved in
epithelial-mesenchymal transition in endometrial cancer. Int J Mol
Sci. 22:132392021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma S, Meng Z, Chen R and Guan KL: The
Hippo pathway: Biology and pathophysiology. Annu Rev Biochem.
88:577–604. 2019. View Article : Google Scholar
|
|
19
|
Wang S, Zhou L, Ling L, Meng X, Chu F,
Zhang S and Zhou F: The crosstalk between Hippo-YAP pathway and
innate immunity. Front Immunol. 11:3232020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ibar C and Irvine KD: Integration of
Hippo-YAP signaling with metabolism. Dev Cell. 54:256–267. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misra JR and Irvine KD: The Hippo
signaling network and its biological functions. Annu Rev Genet.
52:65–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dey A, Varelas X and Guan KL: Targeting
the Hippo pathway in cancer, fibrosis, wound healing and
regenerative medicine. Nat Rev Drug Discov. 19:480–494. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chao ML, Luo S, Zhang C, Zhou X, Zhou M,
Wang J, Kong C, Chen J, Lin Z, Tang X, et al:
S-nitrosylation-mediated coupling of G-protein alpha-2 with CXCR5
induces Hippo/YAP-dependent diabetes-accelerated atherosclerosis.
Nat Commun. 12:44522021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei F, Wang A, Wang Q, Han W, Rong R, Wang
L, Liu S, Zhang Y, Dong C and Li Y: Plasma endothelial
cells-derived extracellular vesicles promote wound healing in
diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging (Albany
NY). 12:12002–12018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortillon J, Le Bail JC, Villard E, Léger
B, Poirier B, Girardot C, Beeske S, Ledein L, Blanchard V, Brieu P,
et al: High glucose activates YAP signaling to promote vascular
inflammation. Front Physiol. 12:6659942021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nguyen CDK and Yi C: YAP/TAZ signaling and
resistance to cancer therapy. Trends Cancer. 5:283–296. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konno T, Kohno T, Okada T, Shimada H,
Satohisa S, Kikuchi S, Saito T and Kojima T: ASPP2 suppression
promotes malignancy via LSR and YAP in human endometrial cancer.
Histochem Cell Biol. 154:197–213. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu G, Murshed A, Li H, Ma J, Zhen N, Ding
M, Zhu J, Mao S, Tang X, Liu L, et al: O-GlcNAcylation enhances
sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in
liver cancer. Cell Death Discov. 7:832021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Safran M, Dalah I, Alexander J, Rosen N,
Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, et al:
GeneCards version 3: The human gene integrator. Database (Oxford).
2010:baq0202010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Wu J, Tan Z, Li H, Lin M, Jiang Y, Liang
L, Ma Q, Gou J, Ning L, Li X and Guan F: Melatonin reduces
proliferation and promotes apoptosis of bladder cancer cells by
suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J
Pineal Res. 71:e127652021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi T, Horimoto Y, Oyama M, Nakatani
S, Kobata K, Tamura M, Arata Y and Hatanaka T: Osteoclast
differentiation is suppressed by increased O-GlcNAcylation due to
thiamet G treatment. Biol Pharm Bull. 43:1501–1505. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Song T, Zhou S and Kong X: YAP
promotes the malignancy of endometrial cancer cells via regulation
of IL-6 and IL-11. Mol Med. 25:322019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsujiura M, Mazack V, Sudol M, Kaspar HG,
Nash J, Carey DJ and Gogoi R: Yes-associated protein (YAP)
modulates oncogenic features and radiation sensitivity in
endometrial cancer. PLoS One. 9:e1009742014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng Y, Huang H, Han Y and Zhu Y:
Expression of YAP in endometrial carcinoma tissues and its effect
on epithelial to mesenchymal transition. Transl Cancer Res.
9:7248–7258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan F, Qian M, He Q, Zhu H and Yang B: The
posttranslational modifications of Hippo-YAP pathway in cancer.
Biochim Biophys Acta Gen Subj. 1864:1293972020. View Article : Google Scholar
|
|
39
|
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou
A, Liu J, Che L and Li J: Long noncoding RNA GAS5 inhibits
progression of colorectal cancer by interacting with and triggering
YAP phosphorylation and degradation and is negatively regulated by
the m6A reader YTHDF3. Mol Cancer. 18:1432019.
View Article : Google Scholar
|
|
40
|
Wang R, Du Y, Shang J, Dang X and Niu G:
PTPN14 acts as a candidate tumor suppressor in prostate cancer and
inhibits cell proliferation and invasion through modulating
LATS1/YAP signaling. Mol Cell Probes. 53:1016422020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu
X, Zhu G, Zhao Y, Chen Y, Yu Y, et al: The essential role of YAP
O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat
Commun. 8:152802017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng C, Zhu Y, Zhang W, Liao Q, Chen Y,
Zhao X, Guo Q, Shen P, Zhen B, Qian X, et al: Regulation of the
Hippo-YAP pathway by glucose sensor O-GlcNAcylation. Mol Cell.
68:591–604 e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen YW, Zhou YD, Chen HZ, Luan X and
Zhang WD: Targeting CTGF in cancer: An emerging therapeutic
opportunity. Trends Cancer. 7:511–524. 2021. View Article : Google Scholar
|
|
44
|
Li XT, Li JY, Zeng GC, Lu L, Jarrett MJ,
Zhao Y, Yao QZ, Chen X and Yu KJ: Overexpression of connective
tissue growth factor is associated with tumor progression and
unfavorable prognosis in endometrial cancer. Cancer Biomark.
25:295–302. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Krzeslak A, Wojcik-Krowiranda K, Forma E,
Jozwiak P, Romanowicz H, Bienkiewicz A and Brys M: Expression of
GLUT1 and GLUT3 glucose transporters in endometrial and breast
cancers. Pathol Oncol Res. 18:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
MacLaughlan SD, Palomino WA, Mo B, Lewis
TD, Lininger RA and Lessey BA: Endometrial expression of Cyr61: A
marker of estrogenic activity in normal and abnormal endometrium.
Obstet Gynecol. 110:146–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim H, Son S, Ko Y, Lee JE, Kim S and Shin
I: YAP, CTGF and Cyr61 are overexpressed in tamoxifen-resistant
breast cancer and induce transcriptional repression of ERα. J Cell
Sci. 134:jcs2565032021. View Article : Google Scholar
|
|
48
|
Kuo CC, Ling HH, Chiang MC, Chung CH, Lee
WY, Chu CY, Wu YC, Chen CH, Lai YW, Tsai IL, et al: Metastatic
colorectal cancer rewrites metabolic program through a
Glut3-YAP-dependent signaling circuit. Theranostics. 9:2526–2540.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuo CY, Chang YC, Chien MN, Jhuang JY, Hsu
YC, Huang SY and Cheng SP: SREBP1 promotes invasive phenotypes by
upregulating CYR61/CTGF via the Hippo-YAP pathway. Endocr Relat
Cancer. 29:47–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheng X and Hart GW: Alternative
O-glycosylation/O-phosphorylation of serine-16 in murine estrogen
receptor beta: Post-translational regulation of turnover and
transactivation activity. J Biol Chem. 276:10570–10575. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hardivillé S, Hoedt E, Mariller C,
Benaïssa M and Pierce A: O-GlcNAcylation/phosphorylation cycling at
Ser10 controls both transcriptional activity and stability of
delta-lactoferrin. J Biol Chem. 285:19205–19218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rani L, Mittal J and Mallajosyula SS:
Effect of phosphorylation and O-GlcNAcylation on proline-rich
domains of tau. J Phys Chem B. 124:1909–1918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fu Y, Ning L, Feng J, Yu X, Guan F and Li
X: Dynamic regulation of O-GlcNAcylation and phosphorylation on
STAT3 under hypoxia-induced EMT. Cell Signal. 93:1102772022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Whelan SA, Lane MD and Hart GW: Regulation
of the O-linked beta-N-acetylglucosamine transferase by insulin
signaling. J Biol Chem. 283:21411–21417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Wu Z, He J, Jin Y, Chu C, Cao Y, Gu
F, Wang H, Hou C, Liu X and Zou Q: OGT regulated O-GlcNAcylation
promotes papillary thyroid cancer malignancy via activating YAP.
Oncogene. 40:4859–4871. 2021. View Article : Google Scholar : PubMed/NCBI
|