Introduction
Patients with recurrent or metastatic head and neck
squamous cell carcinoma (R/M HNSCC) have few treatment options with
little or no lasting response to therapy (1). With the introduction of checkpoint
inhibitors (CPI), a new choice for head and neck tumors arrived.
For R/M HNSCC, immunotherapy with the programmed cell death
ligand-1 (PD-L1) inhibitors pembrolizumab (alone or in combination
with chemotherapy) and nivolumab as a monotherapy are already
established as first- and second-line therapies (2,3).
A number of tumor entities, including HNSCC, express
PD-L1 and PD-L2, which interact with their programmed cell death-1
(PD-1) receptor to limit the function of activated T cells
(4). There is growing knowledge
about the molecular processes that induce the expression of PD-L1
and PD-L2 or modulate their protein stability (5,6). In
40% of HNSCC, there is an inflammatory phenotype with
tumor-infiltrating lymphocytes. This plus the known high mutation
rate resulting in the expression of possibly immunogenic
neo-antigens justifies the use of CPI (7).
PD-1 antibodies are the first immunotherapeutic
agents that were able to produce a stable response and a reduced
mortality rate in R/M HNSCC patients (8-11).
However, the number of patients who respond to CPI is low (8,11)
which requires an improved prediction of therapy success. PD-L1
expression is widely used as a biomarker to predict response to CPI
(12). However, especially in a
heterogeneous disease such as HNSCC, a multitude of
additional/other predictors are essential for diagnosis, monitoring
and prediction of disease progression and therapeutic outcome
(response/failure). It has been suggested that exosomes as a liquid
biomarker can indicate an active HNSCC disease (13). Exosomes are nanosized lipid
vesicles ranging from 30-150 nm in size and are among the smallest
of all extracellular vesicles, also called small extracellular
vesicles (sEVs). Exosomes re-assemble the cargo of their parent
cells reflecting the immunosuppressive molecular profiles (14-17).
Tumor cells are particularly efficient exosome producers and
utilize exosomes to reliably inhibit anti-tumor immune responses
and thus contribute to immune evasion (13). Data indicate that PD-1 and PD-L1
expression on exosomes from HNSCC patients can be used as surrogate
markers for disease progression and therapeutic response following
surgery and/or chemoradiotherapy (CRT) (18-20).
Data from melanoma and non-small cell lung cancer imply that
exosomal PD-L1 is an early indicator for therapeutic response to
anti-PD-1 CPI (21,22). However, studies on HNSCC are still
rare.
At present, CPI is approved in combination with
platinum-based chemotherapy or as monotherapy as it has been
demonstrated that radiotherapy (RT) exerts immunomodulatory effects
followed by additional immune activation (23). Currently, the effect of
combinatorial treatment consisting of CPI and (C) RT in HNSCC is
the subject of various clinical trials (ClinicalTrials.gov Identifier: NCT03480672,
NCT03532737, NCT03349710 and numerous others). These trials note
that patient sub-stratification prior to therapy selection is
clearly indicated (24) and
exosomal PD-L1 expression could be a valuable tool for
sub-stratification.
In light of these studies, it was considered crucial
to explore the effect of established therapies such as RT,
platinum-based chemotherapy (CT), combined CRT and cetuximab
(Cetux), an antibody against EGFR, on PD-L1 expression.
To the best of the authors' knowledge, this is the
first study to address the effect of combined CT with
normal-fractionated RT on checkpoint regulation, a combination that
is considered standard either in the definitive or adjuvant setting
for HNSCC treatment. It was hypothesized that checkpoint modulation
may affect treatment response to immunotherapy in the same or
subsequent therapy lines. Additionally, the present study
hypothesized interrelations between PD-L1 modulation in cell lines
and exosomes and signaling cascades in HNSCC known to mediate
survival and therapy resistance, such as MEK/ERK.
Materials and methods
Cell culture
The cell lines UM-SCC-11B, UM-SCC-14C and UM-SCC-22B
were obtained from Dr T.E. Carey (University of Michigan, Ann
Arbor, MI, USA). Origins of the cell lines were larynx, oral cavity
and hypopharynx, respectively (Table
I) (25). Original tumors were
not human papilloma virus-driven. The cells were cultivated in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific
Inc.) supplemented with 10% fetal calf serum (FCS)
(exosome-depleted for some experiments) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.).
 | Table ICharacteristics of the HNSCC cell
lines (25). |
Table I
Characteristics of the HNSCC cell
lines (25).
| Characteristic | UM-SC-11B | UM-SCC-14C | UM-SCC-22B |
|---|
| Specimen | Primary | Local
recurrence | Primary |
| Tumor site | Larynx | Floor of mouth | Hypopharynx |
| TNM status | T2N2a | T2N0 | T2N1 |
| Previous
therapy | CT | Su+CRT | None |
Reagents and antibodies
The EGFR inhibitor Cetux (Merck KGaA) was dissolved
in 0.9% sodium chloride and stored in aliquots, in accord with the
manufacturer's instructions. Cisplatin was dissolved in DMSO
according to the manufacturer's instructions (cat. no. NSC 119875;
Selleck Chemicals GmbH). Details and concentrations are summarized
in the specific experiments. Appropriate concentrations of the
respective compounds were determined by previous titration
experiments (data not shown).
Immunohistochemistry
Cells (20,000) were seeded in 8 well chamber slides
(Sarstedt, Inc.), cultured for 24 h at 37°C and fixed by ice-cold
ethanol/acetone (2:1) followed by endogenous peroxidase blockage
with DAKO Peroxidase blocking solution (Agilent Technologies
Deutschland GmbH). After preincubation with sheep normal serum 1:10
(BIOZOL Diagnostica Vertrieb GmbH) for 30 min at room temperature
to avoid unspecific binding, primary antibodies [phosphorylated
(p)44/42 MAPK (ERK1/2) (Thr202/Tyr204) cat. no. 9201, CST, Inc.,
1:200; ERK1/2, cat. no. 9102, CST, Inc., 1:100; PD-L1, cat. no.
13684, CST, Inc., 1:200] were incubated overnight at 4°C, followed
by biotinylated secondary anti-rabbit or anti-mouse antibodies
(Cytiva Europe GmbH, cat no. RPN 1004 and cat. no. RPN 1001)
diluted 1:200 in streptavidin biotinylated horseradish peroxidase
for 45 min at room temperature. Afterwards,
3-amino-9-ethylcarbazole (AEC; ScyTek Biotech Life Sciences) was
added. All washing procedures were performed in PBS. Slides were
counterstained by quickly dipping into hematoxylin at room
temperature. Sections incubated without the primary antibody served
as negative controls. Controls were also performed with the
secondary antibody only (data not shown).
Irradiation experiments
Cells (20,000) from each cell line were seeded per
well in six-well plates and irradiated day 2 post-seeding on five
consecutive days with a daily dose of 2 Gy by the use of a linear
accelerator with a photon energy of 6 MV (Synergy; Elekta AB) and
polymethylmethacrylate plates as water and tissue equivalents,
respectively, or kept untreated as controls. Separate lots were
additionally treated with Cetux (5 µg/ml) and cisplatin (1
and 5 µM), respectively, at 37°C on days 3, 5 and 7 in the
course of change of media. Cells were left to recover again on days
8 and 9 and were harvested on day 10. Controls were mock-treated.
Each experiment was performed three times.
Western blot analysis
Cells were washed with PBS and lysed with ice-cold
RIPA Buffer (MilliporeSigma). The protein concentration of RIPA
lysates was measured by DC Protein assay (Bio-Rad Laboratories
Inc.) according to the manufacturer's instructions. A volume of
homogenate containing 20 µg of total protein was separated
by SDS-PAGE. Gradient gels (4-12%) were transferred to
polyvinylidene fluoride membranes and blotted using primary
antibodies [Phospho-p44/42 MAPK (ERK 1/2) (Thr202/Tyr204); cat. no.
9101; CST, Inc.; 1:1,000; p44/42 MAPK (ERK1/2); cat. no. 9102; CST,
Inc.; 1:1,000; PD-L1; cat. no. 13684; CST, Inc.; 1:1,000; GAPDH;
cat. no. 5174; CST Inc.; 1:10,000] (overnight at 4°C, with shaking)
and HRP-conjugated secondary anti-rabbit or anti-mouse antibodies
(Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. 31460 and
cat. no. 31450; 1:10,000; 1 h at room temperature). Blocking
reagents were 5% BSA (MilliporeSigma) and 5% milk (MilliporeSigma),
respectively, according to the manufacturer's instructions, for 1 h
at room temperature. For luminescence detection, the membrane was
coated with 1 ml luminol and 1 ml peroxide solution and then
analyzed with an iBright FL 1000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Densitometric quantification was performed using
the gel analysis function of ImageJ (National Institutes of Health;
Version 2.9.0/1.53t).
Ex vivo treatment of HNSCC vital tissue
cultures
Fresh tissue HNSCC samples (n=9, five from the
oropharynx, two from the oral cavity, two from the larynx) were
procured immediately after surgical resection at the Department of
Otorhinolaryngology, Head and Neck Surgery, Mannheim University
Hospital, Germany. Informed written consent was obtained from all
patients after the review of the local ethics board (approval no.
2019-528N). Samples were processed as previously described
(26). For ex vivo analysis
of tumor response to cisplatin, tumor sections were maintained in
twelve-well plates with inserts (Thinsert; Greiner Bio One Ltd.) in
DMEM, supplemented with 10% fetal bovine serum and antibiotics
(penicillin 100 U/ml and streptomycin 100 µg/ml) at 37°C.
After 1 day in culture, samples were treated with cisplatin (80
mg/m2) on days 1, 3 and 7 after change of media at 37°C.
Non-treated controls were processed in parallel. The tissue slices
were harvested on day 10 to be evaluated for histopathological and
immunohistochemical analysis.
Morphological and immunohistochemical
evaluation of ex vivo models
Ex vivo cultivated tissue was formalin-fixed
and paraffin-embedded (FFPE) using an automatic embedding machine.
From these FFPE tissue blocks, 0.5 µm sections were cut and
deparaffinized. Hematoxylin (10 min) and eosin staining (10 min)
was performed at room temperature to visualize tissue morphology.
Anti-Ki-67 (cat. no. M7420; Proteintech Group, Inc.; 1:50) was used
to assess the proliferative activity of the tumor samples. PD-L1
expression was visualized by mAb PD-L1 (E1L3N®)
XP® (cat. no. 13684; CST, Inc.; 1:200). Incubation with
the primary antibodies was at 4°C overnight. A secondary
biotinylated multilink secondary anti-rabbit antibody (cat. no. RPN
1004V; Cytiva; 1:200) served for protein detection. This step was
followed by the application of H2O2 (7%) for
7 min, then Streptavidin-Biotinylated HRP Complex (Cytiva) was
added for 45 min. For staining AEC (ScyTek Biotech Life Sciences)
was used. Precipitate development of the substrate solution was
monitored under the microscope. The sections were counterstained
with hematoxylin by quickly dipping at room temperature. For PD-L1
expression, the TPS (tumor proportion score) was evaluated by two
independent observers. The number of positively membrane-bound
stained tumor cells was divided by the number of all tumor
cells.
Exosome isolation from cell
supernatants
For exosome enrichment, HNSCC cell lines were
cultured in two to three T75 culture flasks, treated and irradiated
according to the aforementioned protocol and the medium was
collected on days 5, 7 and 10. Medium with exosome-depleted FCS was
used for this experiment: Bovine exosomes were removed from the
serum by centrifugation at 120,000 × g and 4°C for 18 h.
Exosomes from cell culture supernatants were
prepared by size exclusion chromatography as previously described
by Hong et al (27):
Briefly, conditioned cell culture medium was differentially
centrifuged at 2,000 × g for 10 min at room temperature and at
14.000 × g for 30 min at 4°C, followed by ultrafiltration
(Millipore filter, 0.22 µm). The medium was concentrated 50
× using Vivaspin® 20 concentrators with an molecular
weight cutoff of 100,000 Da (Sartorius AG; cat. no. VS2041).
Self-made mini size exclusion chromatography columns were prepared.
The fourth fraction, verified to contain exosomes, was collected
and used for further studies.
Nanoparticle tracking analysis (NTA)
NTA was performed on ZetaView (Particle Metrix GmbH)
to determine the size distribution and concentration of the
isolated particles. Freshly prepared exosome samples were diluted
at 1:50-1:1,000 in PBS and measured at 11 test ranges with two
cycles. Concentration and size ranges were calculated by NTA 2.0
analytical software (Particle Metrix GmbH).
Transmission electron microscopy (TEM) of
exosomes
TEM was conducted as previously described at the
Electron Microscopy Core Facility of Heidelberg University
(20). Freshly prepared exosomes
were placed on carbon-coated formvar grids with 3% w/v uranyl
acetate at room temperature for ~3 sec. Exosomes were visualized by
a JEM1400 transmission electron microscope (JEOL Ltd.) with a
bottom-mounted 4K CMOS camera (TemCam F416; TVIPS). Micrographs
were analyzed using ImageJ (National Institutes of Health; Version
2.9.0/1.53t).
Measurement of protein content
The protein content of the isolated exosomes was
analyzed using Pierce BCA protein assay (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions.
Western blotting of exosomes
Exosomes (10 µg) were lysed in reducing
sample buffer (Thermo Fisher Scientific, Inc.; cat. no. 39000).
Samples were loaded onto 4-20% precast gels (Bio-Rad Laboratories,
Inc.; cat. no. 4561094) and SDS-PAGE was performed, followed by
protein transfer onto a PVDF membrane. The membrane was blocked
using 5% skimmed milk in TBST (0.1% Tween-20) for 1 h at room
temperature and incubated with primary antibodies overnight under
refrigeration (TSG101; cat. no. PA5-31260; Invitrogen; Thermo
Fisher Scientific, Inc.; 1:500; CD63; cat. no. 10628D; Invitrogen;
Thermo Fisher Scientific, Inc.; 1:250; CD9; cat. no. 10626D;
Invitrogen; Thermo Fisher Scientific, Inc.; 1:500; Grp94; cat. no.
2104; CST; 1:1,000; ApoA1; cat. no. 3350; CST; 1:1,000; TRAIL; cat.
no. ab2056; Abcam; 1:500; PD-L1; cat. no. 13684; CST; 1:1,000). The
membrane was exposed to the HRP-conjugated secondary anti-rabbit or
anti-mouse antibody (Invitrogen, cat. no. 31460 and cat. no. 31450,
1:10,000) for 1 h at room temperature. After additional three
washes, the chemiluminescent substrate (Thermo Fisher Scientific,
Inc.; cat. no. 34076) was added and subsequently the membrane was
imaged using an iBright FL 1000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Densitometric quantification was performed using
the gel analysis function of ImageJ (National Institutes of Health;
Version 2.9.0/1.53t).
Caspase-Glo® 3/7 assay
HNSCC cell lines were seeded in white-walled
96-well-plates at a density of 5,000 cells/well in 50 µl.
The cells were immediately treated with 50 µl exosomes (1
µg) in PBS and after 24 h an equal volume of luminogenic
caspase-3/7 substrate (Promega Corporation) was added and incubated
at room temperature for 1 h. Luminescence was read with a Tecan
Infinite® 200 PRO microplate reader (Tecan Group, Ltd.).
As negative control, cells were treated with PBS.
MTS proliferation assay
HNSCC cell lines were seeded in 96-well-plates and
treated with 1 µg exosomes, as described above. After 24 h
the MTS reagent (Abcam) was added 1:10, incubated 3 h at 37°C and
the absorbance was measured at 490 nm. As negative control, cells
were treated with PBS. In other studies, it has been shown that a
reliable proliferation can be detected by the MTS assay after 24 h
co-incubation (28-30).
Statistical analysis
Results were graphed and analyzed by the use of
GraphPad Prism software (version 9.4.1; Dotmatics). Data were
presented as means (bars) with standard error means (whiskers) of
three independent experiments. For western blotting results from
tumor cell lines Kruskal-Wallis tests (non-parametric, without
P-value correction) were performed. Uncorrected Dunn's test was
used as a post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
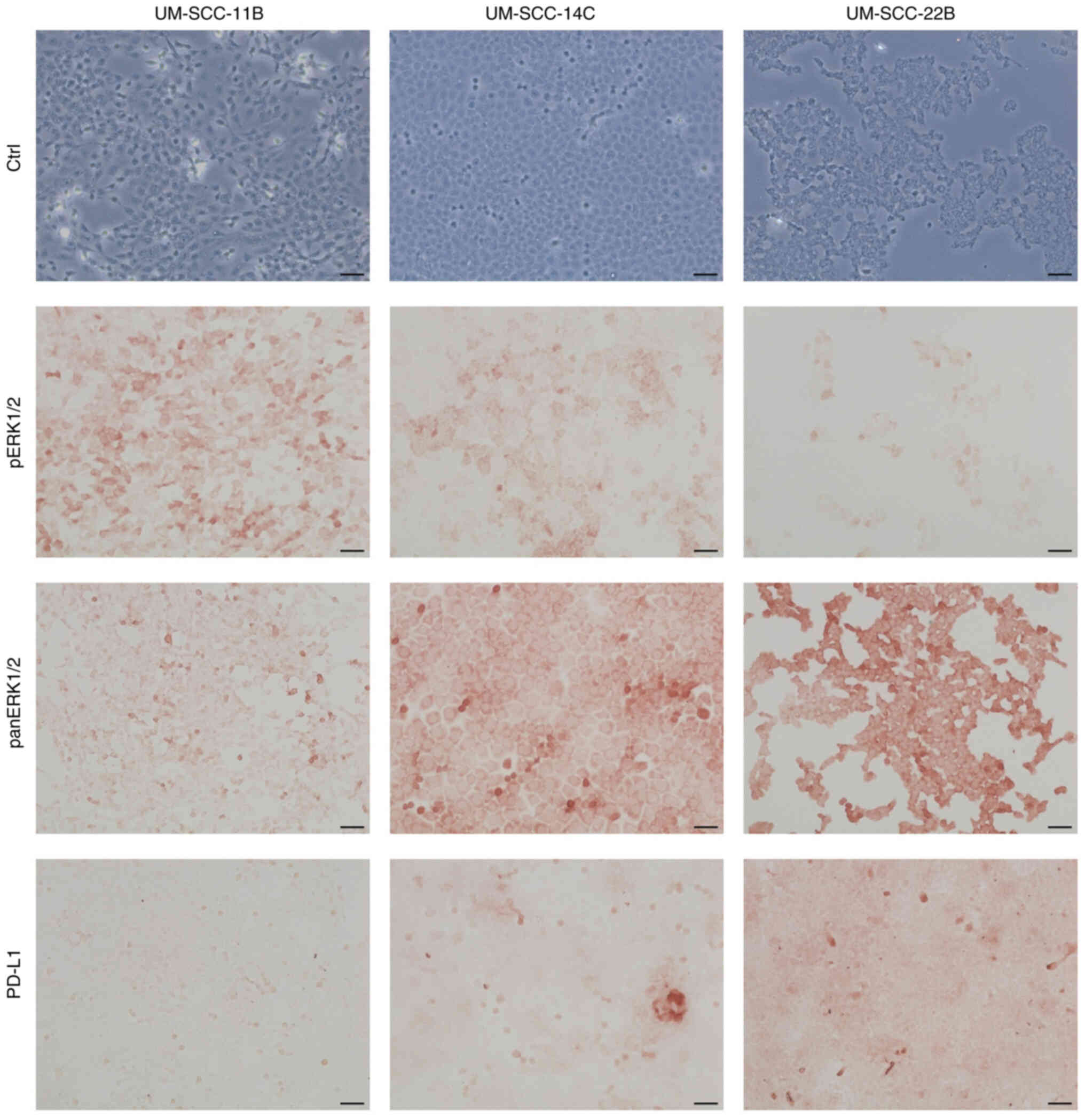
Heterogenic basal expression of target
proteins in immunohistochemistry of the HNSCC cell lines
Basal expression levels of pERK1/2, panERK1/2 and
PD-L1 were immunohistochemically assessed to illustrate the
heterogeneity of the in vitro cell culture model.
Differential expression of the markers was found in all three cell
lines. Expression levels of pERK1/2 were strongest in UM-SCC-11B
and weaker in UM-SCC-14C and UM-SCC22B. PD-L1 and panERK1/2
expression levels were stronger in UM-SCC-22B than in UM-SCC-11B
and UM-SCC-14C (Fig. 1).
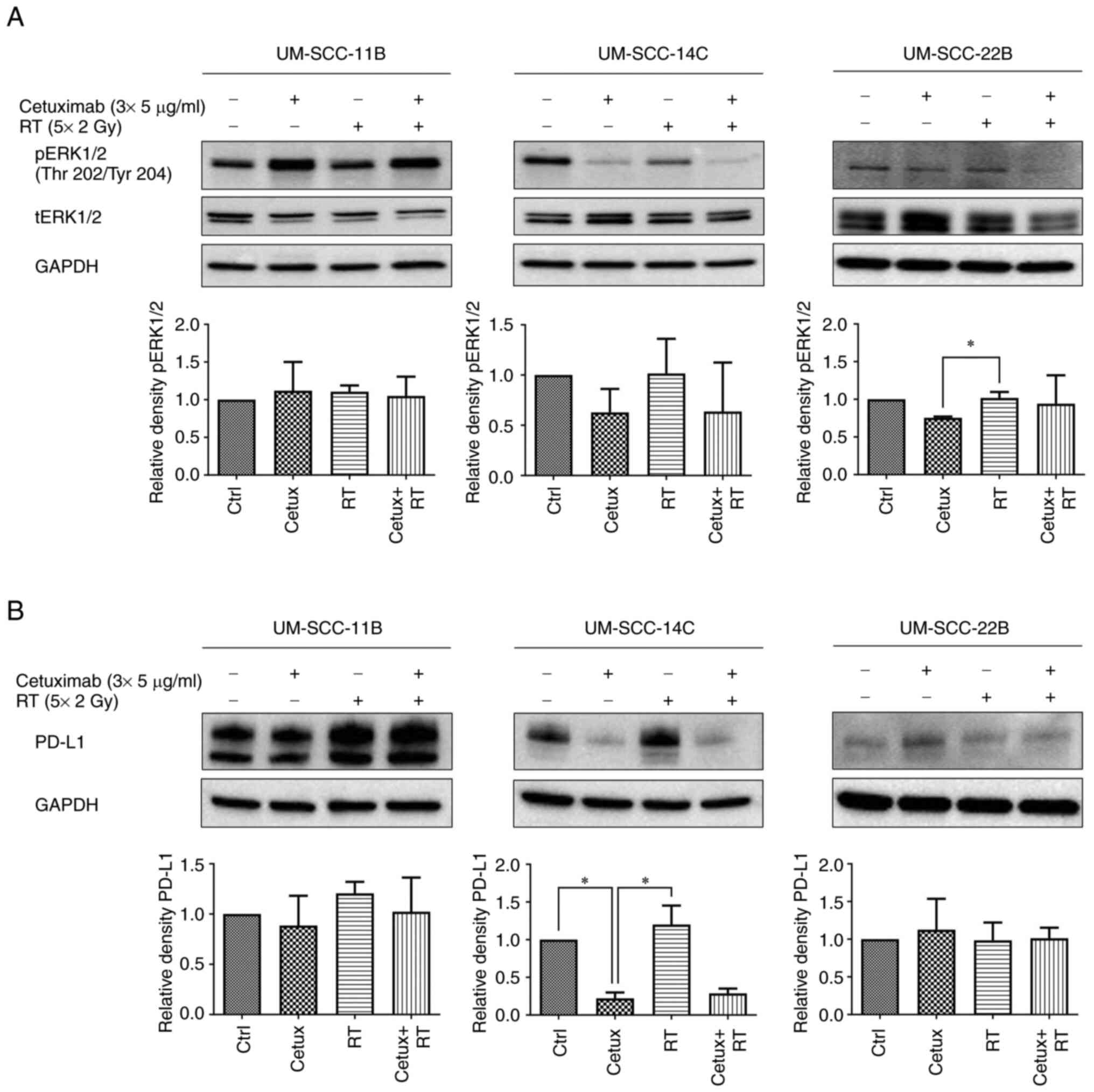
Basal and postradiogenic ERK1/2
phosphorylation is suppressed by EGFR blockade
Previously, the authors reported post-radiogenic
activation of the MAPK pathway in HNSCC by a single dose
irradiation (26). The present
study aimed for a fractionated RT scheme adapted to the
normofractionation with 5×2 Gy per week comparable to the
conditions applied to HNSCC patients in the clinic (with a break at
the weekend), combined with Cetux. Cetux/EGFR blockade similarly
suppressed basal pERK1/2 expression most clearly in UM-SCC-14C
cells. The effect was less pronounced in UM-SCC-11B and UM-SCC-22B
cells. However, EGFR blockade failed to inhibit postradiogenic
ERK1/2 activation in all three cell lines although EGFR is a direct
upstream activator of the Ras-RAF-MEK-ERK pathway (Fig. 2; pERK1/2/tERK1/2 ratios are given
in Table SIII).
Association of PD-L1 expression with
ERK1/2 phosphorylation post-treatment
PD-L1 expression revealed a similar pattern in
UM-SCC-14C cells with basal and postradiogenic inhibition of PD-L1
expression by EGFR blockade while UM-SCC-22B and UM-SCC-11B cell
lines did not display a strong response to treatment (Fig. 2; Table SI). An association of PD-L1
checkpoint regulation and MAPK signaling which are probably
co-regulated upon treatment, at least in UM-SCC-14C cells, was
hypothesized.
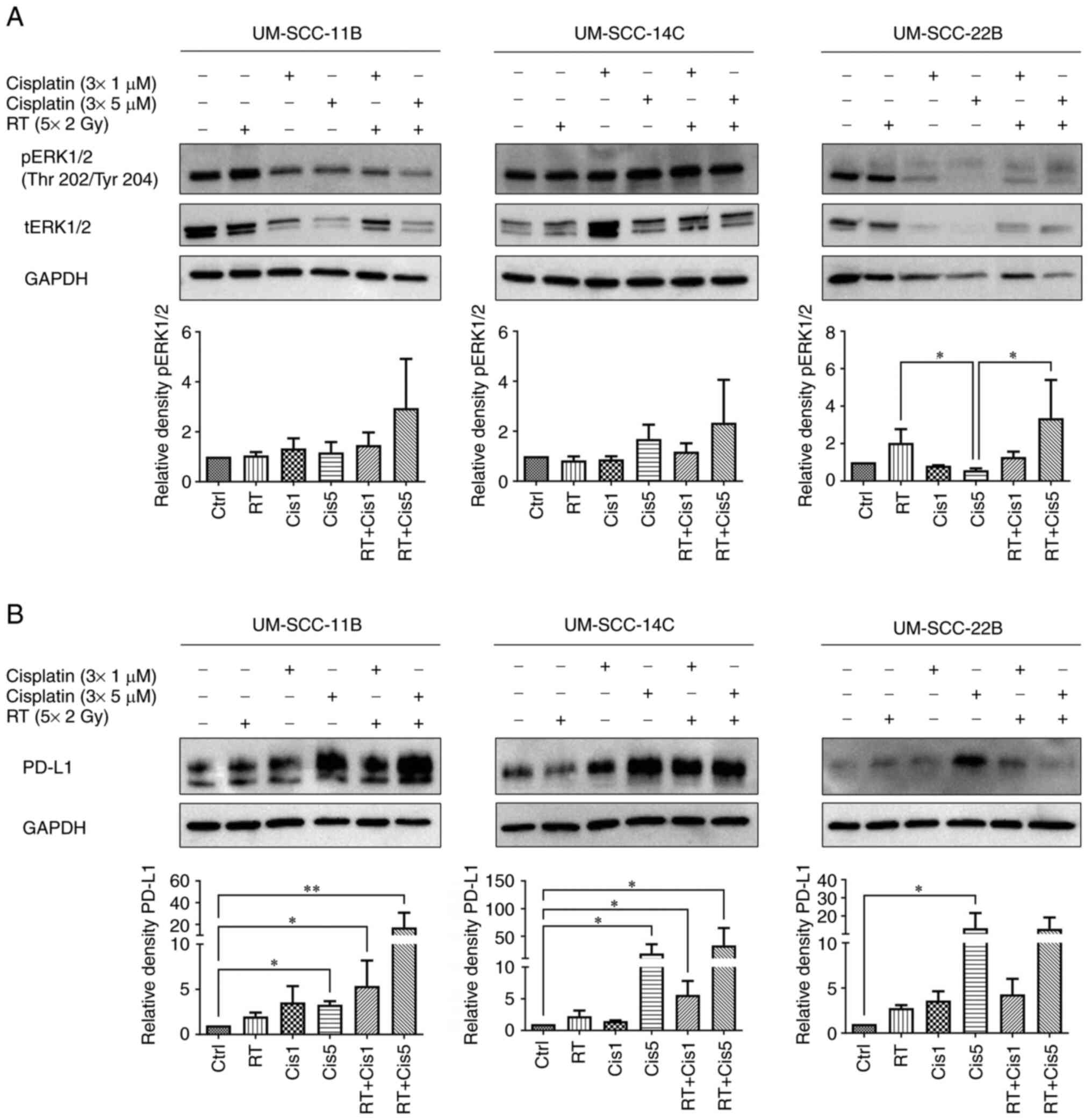
ERK1/2 is activated by RT and
cisplatin
Following combined treatment with RT and cisplatin
on days 3, 5 and 7 at two different concentrations (1 and 5
µM), ERK1/2 activation was assessed. A distinct upregulation
after combined RT and the high-dose cisplatin (5 µM)
concentration was observed in all three cell lines indicating a
potential mechanism of cellular resistance as an undesired
treatment effect (Fig. 3; Table SII; Table SIV). If ERK1/2 is activated upon
cancer treatment, a mechanism of cellular resistance is most likely
which has been previously described (31-35).
Postradiogenic cellular responses could be specified by
administering inhibitors of the MAPK ERK pathway (33-35).
Cisplatin and irradiation-induced
upregulation of PD-L1
After application of standard mainstays irradiation
and cisplatin, all three cell lines showed a distinct and
dose-dependent increase of PD-L1 expression levels after cisplatin
alone. UM-SCC-14C cells displayed the strongest rise. This
modulation of PD-L1 was enhanced after combined CRT. Again, the
effect was most marked in UM-SCC-14C cells. Effects of single RT
were negligible. Additionally, a nearly identical expression
pattern for was observed all treatment combinations in these lines
UM-SCC-11B and UM-SCC-14C when comparing PD-L1 with pERK1/2 levels.
Taken together, these findings indicated that cisplatin acts
synergistically with irradiation treatment to modulate the
PD-1/PD-L1 axis. As after RT/Cetux application, PD-L1 appeared
co-regulated with pERK1/2, again in UM-SCC-14C but additionally in
UM-SCC-11B cell lines. Notably, the effect of combined CRT was
supra-additive in these two cell lines while in 22B it was not
(Fig. 3; Table SII).
Cisplatin-induced PD-L1 modulation is
validated ex vivo
To refine these results and to adapt to a clinical
setting, the present study investigated the effect of cisplatin (80
µM) on PD-L1 regulation in eight independent ex vivo
tumor cultures; two samples displayed an elevated PD-L1 expression
following treatment compared with controls. The other samples
showed basal PD-L1 expression and cisplatin caused no induction,
reflecting the distinct heterogeneity in HNSCC (Fig. 4).
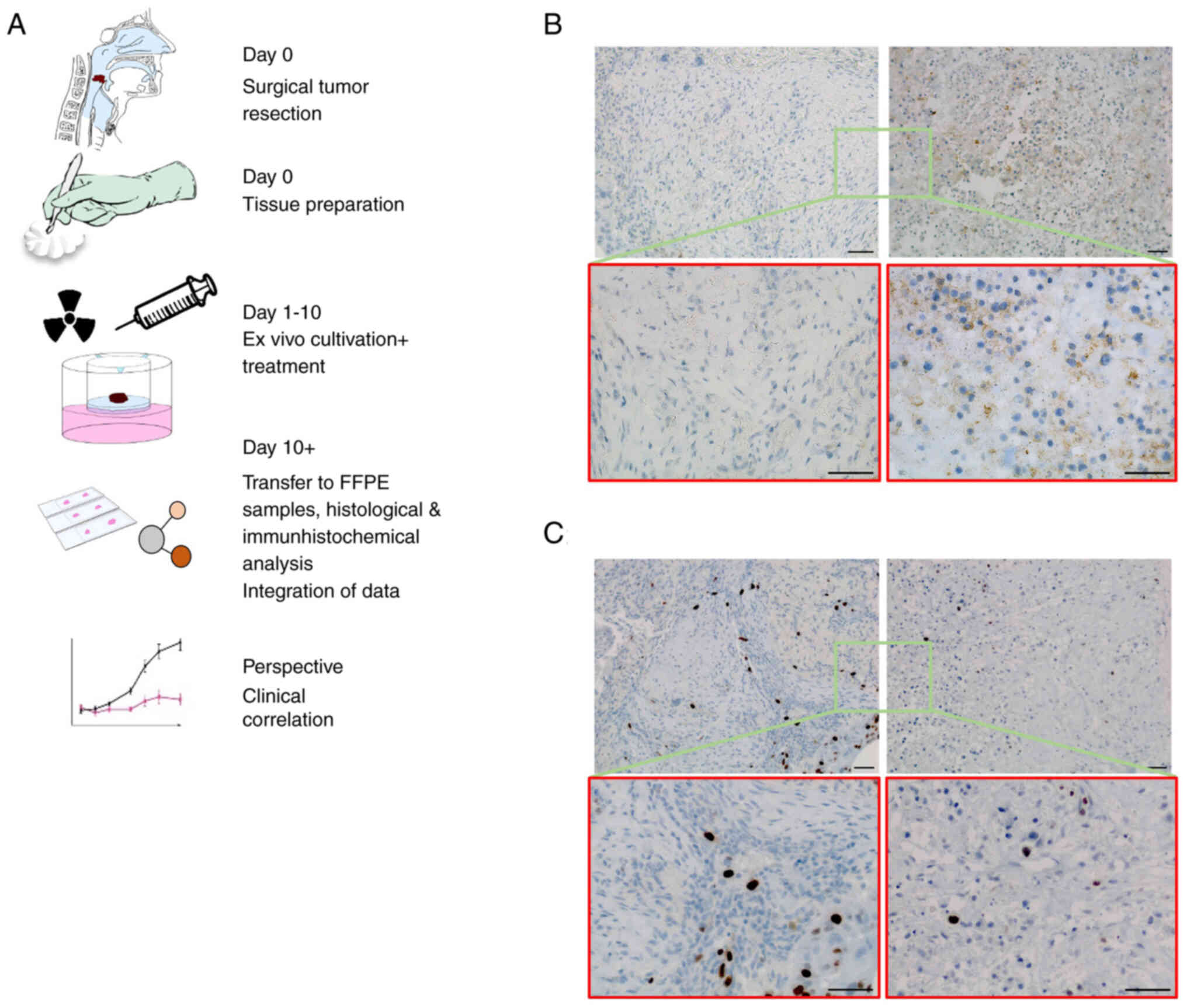 | Figure 4Immunohistochemical staining of ex
vivo HNSCC tissue cultures. (A) Workflow of the experimental
setting. After surgical resection, vital tumor tissues were cut
into 2-3-mm-thick slides and kept in culture for up to 10 days.
After experimental treatment samples were formalin-fixed and
paraffin-embedded. Tissue sections were analyzed by hematoxylin and
eosin staining and immunohistochemical staining. The correlation of
experimental data with clinical outcome most likely offers the
perspective of personalized therapy approaches. (B) Representative
immunohistochemistry staining of PD-L1 in ex vivo tumor
tissues with or without cisplatin treatment (3×80 µM). Left
panel: Moderate immunostaining of PD-L1 in an untreated
oropharyngeal SCC sample, scored with a TPS of 5%. Right panel:
Distinct induction of PD-L1 after cisplatin treatment in
corresponding samples from the same tumor, scored with a TPS of
25%. (C) Left panel: Low basal expression levels of the
proliferation marker Ki-67 in untreated controls of the same OPSCC.
Right panel: Further reduction of Ki-67 positive cells after
cisplatin treatment. Scale bar, 50 µm. Parts of the figure
were drawn by using pictures from Servier Medical Art (http://smart.servier.com/), licensed under a Creative
Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
HNSCC, head and neck squamous cell carcinoma; PD-L1, programmed
cell death ligand-1; SCC, squamous cell carcinoma; TPS, tumor
proportion score; OPSCC, oropharyngeal squamous cell carcinoma. |
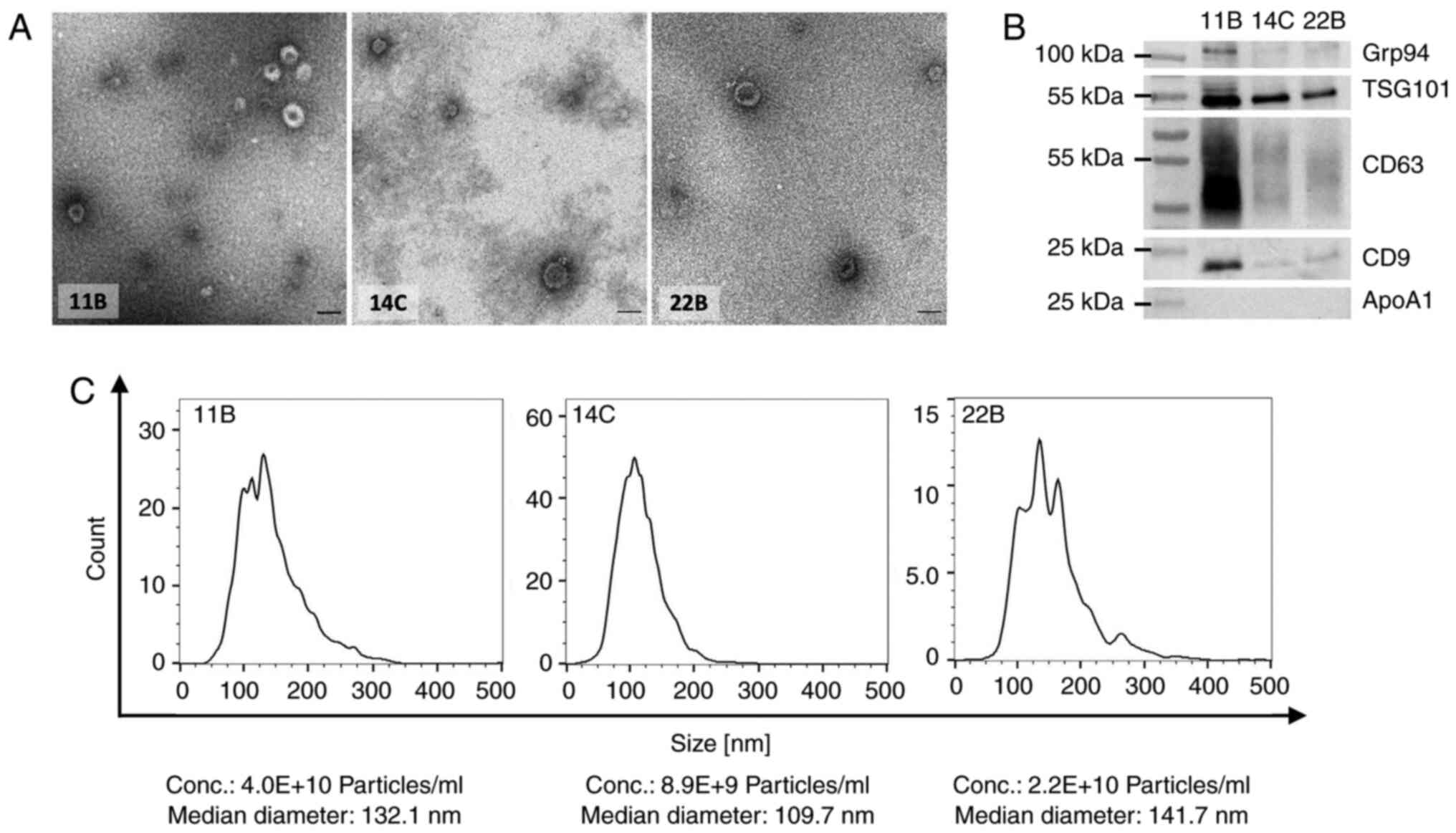
Characterization of exosomes
To validate the biomarker potential and the
functional role of exosomes in HNSCC cell lines treated with
irradiation and/or chemo- or targeted therapy, exosomes were
isolated from all three cell lines and their molecular cargo and
functional impact on untreated HNSCC cell lines analyzed.
The representative TEM images show typical size
ranges from 30-120 nm and typical vesicular shapes of the isolated
exosomes from all three cell lines (Fig. 5A). Western blots of exosomes
confirm the expression of the endosomal protein (TSG101),
tetraspanins (CD63 and CD9) and low expression/lack of isolation
byproducts (Grp94, ApoA1) as suggested by MISEV 2018 (Fig. 5B) (36). Nanoparticle tracking analysis
reveals comparable concentrations and median size ranges of the
particles in the preparations from all cell lines (Fig. 5C).
Expression levels of exosomal PD-L1 and
TRAIL
To assess the role of exosomes in inducing
apoptosis, the apoptosis markers PD-L1 and TRAIL were detected and
their relative density was compared with TSG101 in western blots
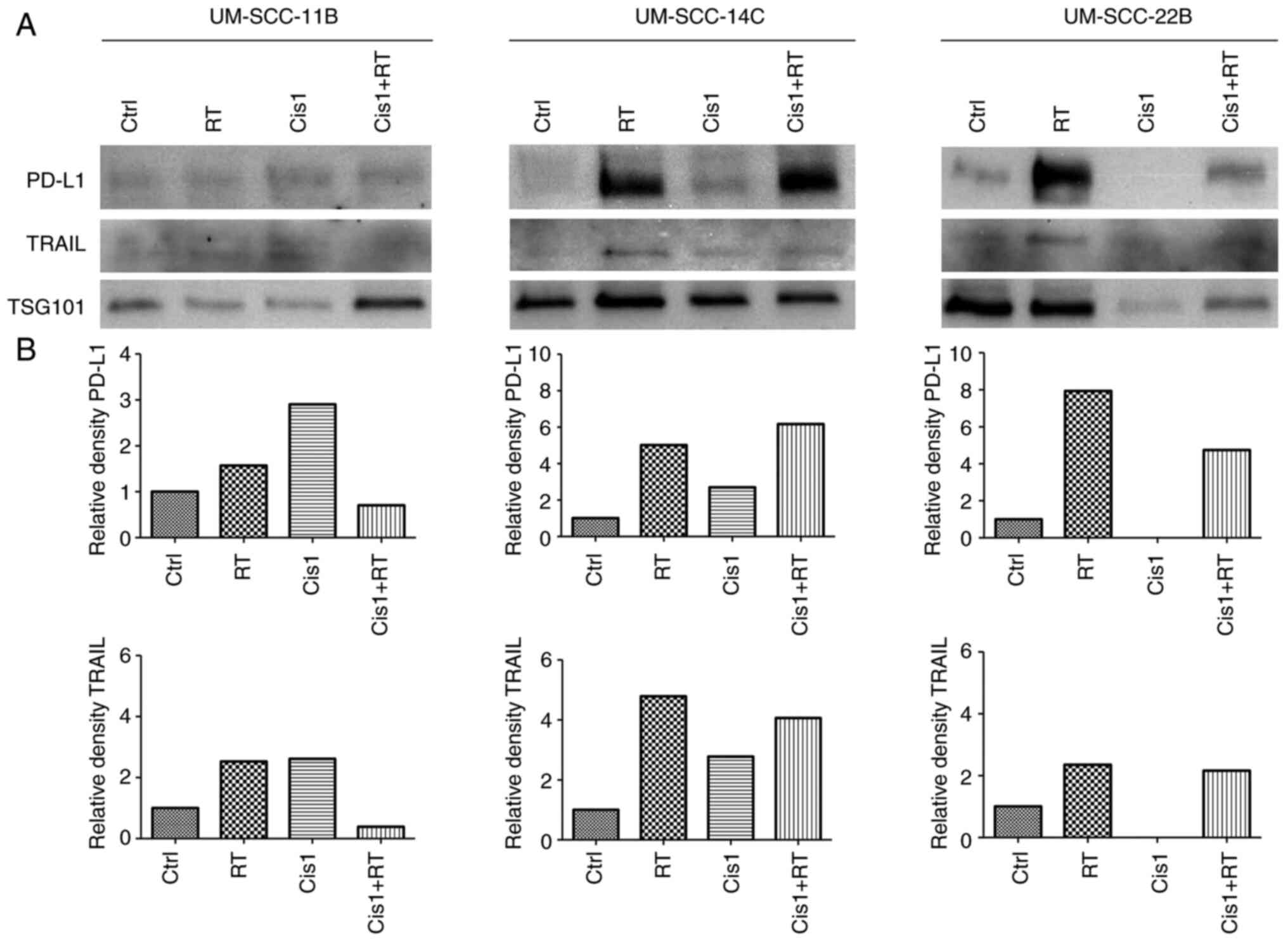
for the different treatment conditions (Fig. 6; Fig.
SI).
Relative PD-L1 and TRAIL expression in exosomes was
induced by monotherapy with RT in all three cell lines. Notably,
treatment with low-dose cisplatin (Cis1, 1 µM) resulted in
higher relative PD-L1 and TRAIL expression levels in exosomes from
UM-SCC-11B and UM-SCC-14C than in exosomes from UM-SCC-22B with no
expression.
Markedly, exosomes from HNSCC cell lines treated
with combined CRT (Cis1+RT) revealed controversial results: While
PD-L1 and TRAIL showed low expression levels in exosomes from
UM-SCC-11B, elevated expression levels were present in exosomes
from UM-SCC-14C and UM-SCC-22B cell lines.
Exosomes from HNSCC cell lines modulate
the proliferation and apoptosis of HNSCC cell lines
Exosomes from HNSCC cell lines treated with the
various therapeutic conditions were co-incubated with the untreated
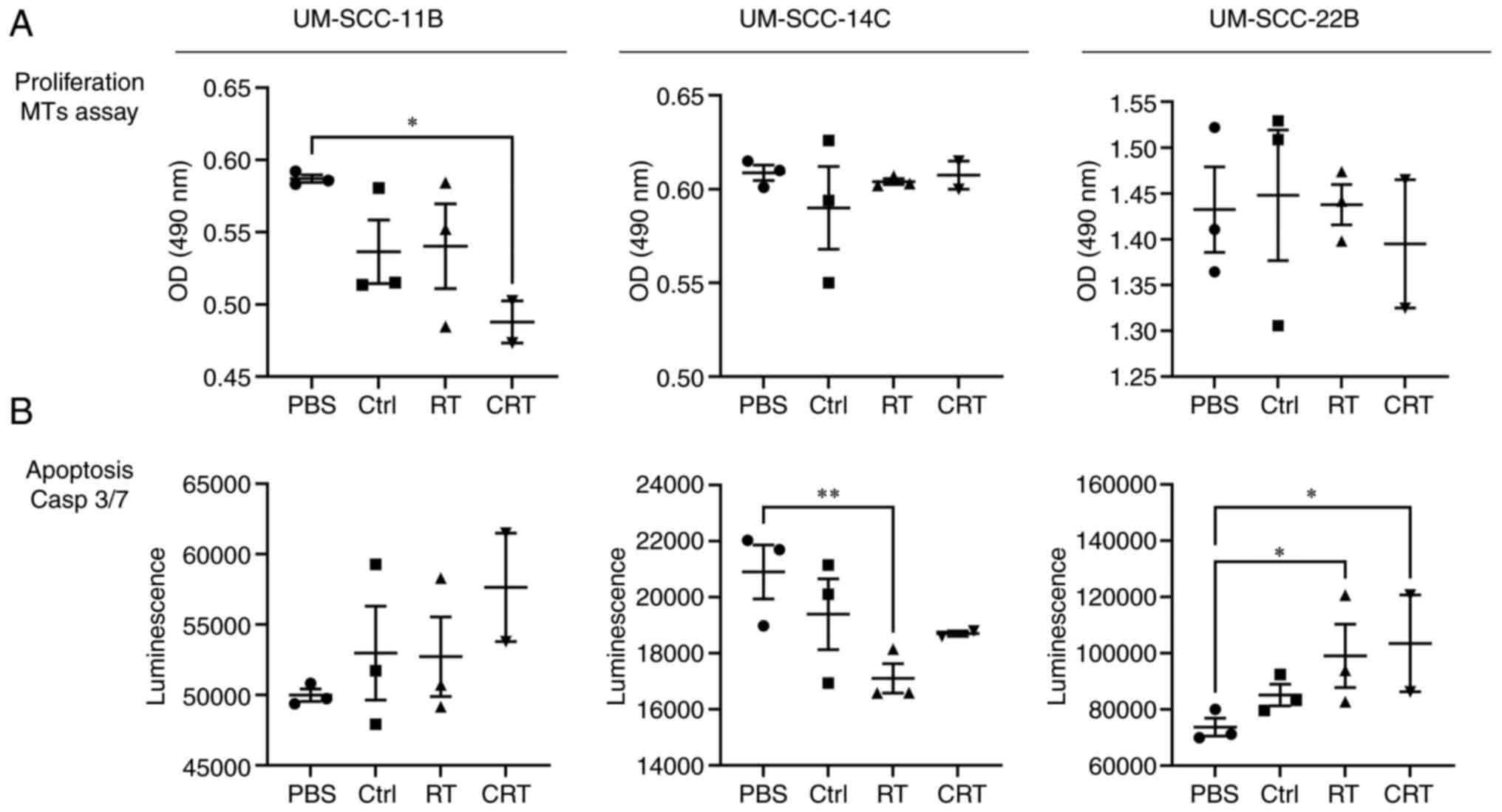
HNSCC cell lines for 24 h to monitor the proliferation (Fig. 7A) and apoptosis (Fig. 7B). Untreated HNSCC cell lines that
were co-incubated with exosomes from UM-SCC-11B post
chemoradiotherapy (Cis1+RT) revealed reduced proliferation.
Exosomes from other cell lines or treatment conditions did not
modulate the proliferation of the cell lines.
Exosomes from HNSCC cells treated with RT, cisplatin
and CRT induced apoptosis of UM-SCC-11B and UM-SCC-22B. Strikingly,
apoptosis was reduced in UM-SCC-14C co-incubated with exosomes from
the irradiated (RT) cell line (P<0.05) and combined therapy
(Cis1+RT or Cetux+RT), whereas monotherapy with chemotherapy or
targeted therapy alone did not modulate apoptosis.
Discussion
Although the PD-L1 expression status is
well-accepted for the medical justification of anti-PD-1 agents,
the clinical success is not as high as expected and requires
improved stratification. The expression of PD-L1 is undergoing
changes during the clinical course and treatment reflected by
altered expression levels after chemotherapeutic drugs or RT or as
a response to exogenous signals (IF-y) (37). Although CRT is considered a
standard therapy in HNSCC, there is only limited knowledge about
direct tumor cell-dependent upregulation of PD-L1 expression upon
exposure.
As taken into account in the present study and in
other studies, irradiation is known to exert immunomodulatory
effects and appears to activate immune responses by inducing DNA
damage and cell death as inflammatory signals occur through the
activation of cell survival pathways (38). Dovedi et al (39) postulate that acquired resistance to
radiotherapy can be overcome by concomitant but not sequential
administration of anti-PD-L1 mAb with fractionated irradiation.
Therefore it is mandatory to gain new insights into
the regulation of the PD-1/PD-L1 axis upon exposure to standard
head and neck cancer therapy, in particular fractionated RT
combined with cytotoxic agents. As the response rate in HNSCC
towards CPI is still low with <20% (40), the present study investigated the
impact of fractionated RT with platinum-based chemotherapy and
Cetux on PD-L1 expression in an in vitro HNSCC model
including established tumor cell lines and their exosomes and
primary patient explants.
A total of three HNSCC cell lines were used as an
in vitro model to mimic heterogeneity in HNSCC and to
improved understand patient-specific response patterns. For
validation, primary tumor material derived from HNSCC patients was
analyzed.
Supporting the hypothesis, a moderate increase of
PD-L1 was noticed in all three cell lines and their exosomes when
treated with RT alone.
Other groups have likewise observed an upregulation
of PD-L1 following RT in HNSCC and other entities (41-45).
For HNSCC, it has been described that an increase in PD-L1
expression in radioresistant cell lines after RT affects cell
proliferation due to the inactivation of GSK-3β (46). The observed induction of PD-L1 in
the HNSCC cell lines and their exosomes underlines the biomarker
potential of exosomes. Lately, it was reported that
PD-L1+ exosomes are a reliable biomarker for an active
HNSCC disease and effectively inhibit CD8+ -T cell
activity, which can be attenuated by anti-PD-1 therapy (18).
The present study discovered that exosomes from
irradiated HNSCC cells reduced apoptosis in untreated UM-SCC-14C
cells. Notably, Mutschelknaus et al (47) made similar observations; that
exosomes from irradiated HNSCC cell lines promote prolonged
survival of untreated HNSCC cell lines by the increase of DNA
double-stranded repair. Thus, exosomes from irradiated HNSCC cell
lines could be utilized as a survival mechanism by the tumor
protecting the non-irradiated cells from apoptosis and thus
supporting immune evasion.
The expression of PD-L1 on tumor cells, the presence
of tumor-infiltrating lymphocytes and high mutational burden
(48-50) indicate sensitivity towards CPI. RT
inducing these features is thus very likely to increase the
response. First, the observation that fractionated RT alone induces
PD-L1 is a well-known and undesired side-effect causing
immunosuppression (51). Oweida
et al (52) investigated
whether local irradiation can change the immunogenicity of the
tumor by sensitizing a poorly immunogenic HNSCC towards PD-L1
inhibition. They observed treatment resulting in a highly inflamed
tumoral phenotype and proposed an enhanced tumor cell killing by
increased T cell infiltration. Likewise, Deng et al
(53) proposed a cytotoxic T
cell-dependent mechanism after combined RT and PD-L1 blockade that
boosted the irradiation efficacy.
In addition, the killing of cancer and stroma cells
by antigen-specific cytotoxic T lymphocytes is strengthened if the
tumor antigen (Ag) is released by the application of local RT or
chemotherapeutic agents Such findings indicate that there is a
rationale for developing combination treatments of irradiation or
chemotherapy with CPI. (54).
Similarly, when treating with cisplatin the present
study observed a distinct induction of PD-L1 expression in all
three cell lines with the greatest increase in UM-SCC-14C and
UM-SCC-22B cells and in exosomes from UM-SCC-11B and UM-SCC-14C,
however, not from UM-SCC-22B.
In UM-SCC-14C and UM-SCC-11B, this induction was
even enhanced by combining cisplatin with fractionated RT, while in
exosomes an elevated induction in UM-SCC-14C and UM-SCC-22B was
observed. The data of the present study are in line with previous
publications: An upregulation of PD-L1 expression in HNSCC has been
observed after a number of cytotoxic treatments including
cisplatin, carboplatin, docetaxel, platinum and fluorouracil as
summarized by Lin et al (55). As a mechanism, the tumor immune
escape is likely to be promoted by an increase in PD-1/PD-L1
expression through the MAPK/ERK kinase pathways in HNSCC patients
(37).
While these results refer to a single treatment with
irradiation and cytotoxic agents, for the first time, to the best
of the authors' knowledge, the present study presented an induction
of PD-L1 surface expression in HNSCC upon cisplatin teamed with
fractionated RT (5×2 Gy) with supra-additive effects in two cell
lines compared with the application of monotherapy with cisplatin
or irradiation. So far, the combined and synergistic effect of CRT
on PD-L1 expression has not been described for HNSCC but has been
shown in other entities backing the observations the present study
(51). In melanoma, IL-6 acts as a
possible further intrinsic tumor cell trigger for regulating the
expression of PD-L1 after RT and CRT. Furthermore, PD-L1
upregulation occurs especially on vital tumor cells dependent on
the entity (51), which could be
an explanation for the heterogeneous expression depending on the
location of the tumor of origin.
In any case, these data clearly indicated that
standard therapy such as CRT is capable of affecting checkpoint
regulation in HNSCC. The role of PD-L1 as a prognosticator has been
recently addressed by various research groups for different tumor
entities. PD-L1 appears to be a negative prognostic factor in
NSCLC, yet remains controversially discussed (56). For HNSCC, a study indicates that
tumors with high PD-L1 expression potentially follow an unfavorable
clinical course (57), which is in
line with other studies (58,59).
In summary, patients whose tumors strongly express PD-L1 might
benefit from the (early) application of CPI and it is likely to be
advantageous to combine anti-PD (L)-1 agents with standard
pillars.
The effect of exosomal PD-L1 as an indicator for
therapeutic response has been controversially discussed in the
literature: In the follow-up of HNSCC therapy after surgery and/or
(C)RT high exosomal PD-L1 has been either linked to therapeutic
response and improved disease-free survival (19) or active disease and worse overall
survival (20). In metastatic
melanoma treated with anti-PD-1 CPI, an increase of the exosomal
load of PD-L1 was either correlated with therapeutic response
(60) or indicated a persistent
disease (61). It has been
hypothesized that high exosomal PD-L1 levels following therapy are
caused by immunomodulatory tumor responses hinting towards
therapeutic response (62).
However, another study emphasizes that the exosomal PD-L1 reflects
PD-L1 expression in tumor cells and thus the tumor burden (61). These controversies underline the
urgent need for larger biomarker studies since the observed
obstacles might be due to different immune escape mechanisms at
various time points.
By combining the EGFR blockade Cetux with
fractionated RT the present study found a suppression of basal and
postradiogenic pERK1/2 expression in UM-SCC-14C and, less
pronounced, in UM-SCC-11B cells. However, the blockade of the EGFR
failed to inhibit the Ras-RAF-MEK-ERK pathway directly downstream
in all three cell lines. Notably, this basal and postradiogenic
decrease after combined Cetux and RT in UM-SCC-14C cells could be
similarly observed for PD-L1 expression indicating that MAPK ERK
signaling and checkpoint regulation are associated in some but not
all HNSCC. In exosomes, however, a slight increase in PD-L1 and
almost no change in TRAIL expression was observed (Fig. S1). The functional assays revealed
a decreased apoptosis in untreated UM-SCC-11B and UM-SCC-14C cells
after treatment with exosomes from Cetux and irradiated cells
(Fig. 7). The results of the
present study may be limited by the fact that it did not measure
apoptosis directly but in an indirect way by Caspase 3/7
activity.
Theodoraki et al (63) observed that HNSCC patients,
enrolled in a phase I trial receiving a treatment combination of
irradiation, Cetux and imiplimab, increased exosomal PD-L1
expression during follow-up when the disease recurred. In
conclusion, the functional impact of exosomes secreted by
irradiated tumor cells that protect unirradiated HNSCC cells from
apoptosis might serve as one mechanism of tumor resistance and the
exosomal PD-L1 expression might reflect the failed therapeutic
response in HNSCC.
Notably, in contrast to Ock et al (37), in the present model cisplatin alone
did not or only moderately increase pERK1/2, although the
pERK1/2/total-ERK1/2 ratio was not determined, while Ock et
al (37) describe an enhanced
dose-dependent ratio of p-MEK/total-MEK following cisplatin
treatment in HNSCC cells. Ock et al (37) state that MEK regulation is a
crucial step in modulating PD-L1 expression in cancer. If tumor
cells are resistant to anti-EGFR treatment the MEK pathway is
activated and inhibition of the MEK pathway attenuates PD-L1
upregulation. An association between PD-L1 regulation and EGFR
downstream signaling, especially in the case of EGFR mutations, has
also been described for NSCLC (64,65).
The present study discovered a potential co-regulation of PD-L1 and
activated ERK1/2, most evident in UM-SCC-14C. This is in line with
Ota et al (64), who found
both EML4-ALK and mutant EGFR upregulating PD-L1 by activating
PI3K-AKT and MEK-ERK signaling pathways in NSCLC which reveals a
direct link between oncogenic drivers and PD-L1 expression.
Accordingly, Ebert et al (66) hypothesized that a dual blockade of
MEK and PD-L1 might cause synergistic effects. Jiang et al
concluded from their data that the potential therapeutic benefits
of combining targeted inhibitors and immune modulation to improve
patient outcomes should be investigated (67), a hypothesis which is supported by
the results of the present study. Furthermore, the results from the
EGFR blockade experiments point towards a similar direction,
indicating that post-radiogenic activation of MAPK signaling most
likely contributes to cellular defense in response to treatment and
can be tackled by EGFR inhibition in a context- and cell dependent
manner.
The results of the present study are relevant as the
efficacy of combined CRT and CPI is currently under investigation
in clinical trials in HNSCC. However, expression patterns varied
within the cell lines and exosomes examined. HNSCC are markedly
heterogeneous. They include several subcategories related to
anatomical location, etiology and molecular findings. The
heterogeneity of HNSCC at the molecular level has hampered both the
identification of specific targets and the development of targeted
therapeutics for this entity. The results of the present study
mirror the heterogeneity of HNSCC tumor cells and illustrate why
the individualized characterization of checkpoint regulation is
mandatory.
It is known that RT in combination with anti-PD-L1
agents stimulates CD8+ T cell-mediated anti-tumor immunity
(68). If single dosages are
applied, low dosages affect the vascularization of the tumor while
higher doses are linked with innate and adaptive immune mechanisms
via the mediation of type I interferon (IFN) (69). Hence, it is crucial to consider the
sequence of application of treatment modules as well as the
fractionation protocols which exert different effects on anti-tumor
immune responses (70-72). In the current study and previously,
the authors have assessed a combination scheme according to the
clinically applied regimen for HNSCC patients to take the clonal
selection of radioresistant tumor cells under fractionated
irradiation into account (73).
The results of the present study are in part
confirmed by results from other groups, yet, the current data
situation is inconclusive. Fournel et al (74) have already described upregulation
of PD-L1 by cisplatin in NSCLC. Park et al (75) stated that anti-PD-1 therapy may
enhance several features of anti-tumor immunity that have been
induced by platinum-based CT.
By contrast, Tran et al (76) demonstrated in a syngeneic oral
cancer model that cisplatin could have immune-enhancing as well as
immunosuppressive effects, which may be dose-dependent and
partially reversible by combining PD-1/PD-L1 blockade. They
discovered a robust increase in the CD8+ T cell number
of animals treated with anti-PD-1 alone which was not seen in
animals treated with cisplatin and anti-PD-1. At present, it is
unclear whether cisplatin can enhance or reduce anticancer
immunity. However, this is an essential question as chemotherapy is
frequently applied before or concurrent with CPI. One of the
standard therapies in HNSCC is CRT, either in a definitive or an
adjuvant approach. In this context, it seems important to assess a
potential synergistic effect of this regimen on PD-L1 expression.
In line with the results from Derer et al (68), the present study observed an
upregulation at lower levels after single treatment with cisplatin
compared with a combination exposure with fractionated RT.
Derer et al (51) suggested a tumor cell-mediated
upregulation of PD-L1 expression following, in particular, CRT that
is not only dependent on the somatic mutation prevalence of the
tumor entity after discovering an upregulation of PD-L1 surface
expression following fractionated irradiation in combination with
dacarbazine. It is generally accepted that the prevalence of
somatic mutations is associated with the immunogenicity of the
tumor cells and tumors with a high mutational burden display a
favorable response to immunotherapy.
The induction of PD-L1 by cisplatin has been
validated by our previously established ex vivo 3D HNSCC
model. The results support our observations in vitro. 3D
validation is of importance to take tumor stroma interactions into
account. Notably, similar effects of cisplatin on PD-L1 in HNSCC 2D
and 3D cultures were found. These findings of the present study
indicated that CRT has an effect on the increase of PD-L1 surface
expression on tumor cells in the absence of immune cells. In this
context, exosomes could serve as valuable biomarkers that reflect
the therapeutic response and could be targeted directly to reduce
exosome-mediated immunosuppression.
In general, establishing optimized and multimodal
immunotherapy approaches could pave the way to a sensitivity
towards CPI that is more robust and occurs in a greater proportion
of patients. Combinations of ICI with anti-cancer and
non-anticancer drugs are currently examined for beneficial effects
in preclinical and early phase clinical trials (77-79).
Currently, the results of the present study are
reviewed and validated in HNSCC 3D tumor models and it is strongly
anticipated by preliminary data that the knowledge derived from the
present study will be applicable to established cancers in patients
exhibiting sensitivity to standard treatment. Designing and
establishing novel multimodal schemes consisting of CRT with CPI
will presumably help to overcome treatment resistance in HNSCC
patients. Results from ongoing clinical trials in which CPI enter
Standard of Care schemes are ardently awaited, however, as
previously described, unselected patient collectives might not
routinely experience benefits. For the guidance of novel drug
combinations such as anti-TIM-3 (80) or vaccines along with (C)RT and
CPIs, robust biomarker data from (pre-)clinical studies will be
paramount.
In light of the data from the present study, taking
a combined inhibition of the PD-1/PD-L1 axis and MAPK ERK signaling
into account is suggested, which will be evaluated using 2D/3D
HNSCC models in future studies. It is hypothesized that a complex
context-dependent PD-L1 regulation exists in HNSCC undergoing
chemoradio-/antibody therapy. Improved understanding of underlying
mechanisms and overcoming the immunosuppressive effects of exosomes
may provide the basis for the development of combinational
therapies with higher efficacy and response rates for the treatment
of HNSCC and other tumor types.
Supplementary Data
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
AA, KL, LT, MT, ES, AAz and SL were responsible for
data curation. KL and LT were responsible for formal analysis. AA,
NR and SL were responsible for funding acquisition. JS, MNT, AMR,
JF, CAW, BK, AL and CS made substantial contributions to conception
and design of the present study, the acquisition of data and
analysis and interpretation of data. AA, JK, KB, NR and SL
supervised the study. AA, LT, ES and SL were responsible for the
diagrams. AA and SL wrote the original draft of the manuscript. AA,
LT, MNT, JK, KB, NR and SL were responsible for writing, reviewing
and editing the manuscript. AA and SL confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients
after review of the local ethics board (approval nos. 2019-528N,
2021-552 and 2019-697N).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors gratefully acknowledge the excellent
technical support of Ms. Petra Prohaska (Department of
Otorhinolaryngology, Head and Neck Surgery, Medical Faculty
Mannheim of the University of Heidelberg). They are indebted to Dr
Stefan Hillmer (EMCF, University of Heidelberg) for assistance with
the TEM micrographs and Dr Katja Nitschke (Department of Urology
and Urosurgery, Medical Faculty Mannheim of the University of
Heidelberg) for supporting the nanoparticle tracking analyses.
Funding
The work on 3D HNSCC models is substantially supported by the
research funding program Development of Replacement and
Complementary Methods to Reduce Animal Testing provided by the
Ministry of Rural Affairs, Food and Consumer Protection
Baden-Wuerttemberg, Germany, to AA. (Staatshaushaltsplan 2020/2021
Kap. 0802, Tit. Gr. 74) and by the 3R network Baden-Wuerttemberg,
Ministry of Science Baden-Wuerttemberg, Germany, to NR and KB. This
research was funded by German Research Foundation (DFG) to SL (LU
2270/1-1).
References
|
1
|
Sacco AG and Cohen EE: Current treatment
options for recurrent or metastatic head and neck squamous cell
carcinoma. J Clin Oncol. 33:3305–3313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gillison ML, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: CheckMate 141: 1-Year update and subgroup analysis
of nivolumab as first-line therapy in patients with
recurrent/metastatic head and neck cancer. Oncologist.
23:1079–1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pai SI, Zandberg DP and Strome SE: The
role of antagonists of the PD-1:PD-L1/PD-L2 axis in head and neck
cancer treatment. Oral Oncol. 61:152–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keck MK, Zuo Z, Khattri A, Stricker TP,
Brown CD, Imanguli M, Rieke D, Endhardt K, Fang P, Brägelmann J, et
al: Integrative analysis of head and neck cancer identifies two
biologically distinct HPV and three non-HPV subtypes. Clin Cancer
Res. 21:870–881. 2015. View Article : Google Scholar
|
|
8
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chow LQM, Haddad R, Gupta S, Mahipal A,
Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, et al:
Antitumor activity of pembrolizumab in biomarker-unselected
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion
cohort. J Clin Oncol. 34:3838–3845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soulieres D, Cohen E, Le Tourneau C, Dinis
J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al:
Abstract CT115: Updated survival results of the KEYNOTE-040 study
of pembrolizumab vs standard-of-care chemotherapy for recurrent or
metastatic head and neck squamous cell carcinoma. Cancer Res. 78(13
Suppl): CT1152018. View Article : Google Scholar
|
|
11
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar
|
|
12
|
Gavrielatou N, Doumas S, Economopoulou P,
Foukas PG and Psyrri A: Biomarkers for immunotherapy response in
head and neck cancer. Cancer Treat Rev. 84:1019772020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ludwig S, Floros T, Theodoraki MN, Hong
CS, Jackson EK, Lang S and Whiteside TL: Suppression of lymphocyte
functions by plasma exosomes correlates with disease activity in
patients with head and neck cancer. Clin Cancer Res. 23:4843–4854.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cocucci E and Meldolesi J: Ectosomes and
exosomes: Shedding the confusion between extracellular vesicles.
Trends Cell Biol. 25:364–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Pol E, Böing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ludwig S, Sharma P, Wise P, Sposto R,
Hollingshead D, Lamb J, Lang S, Fabbri M and Whiteside TL: mRNA and
miRNA profiles of exosomes from cultured tumor cells reveal
biomarkers specific for HPV16-positive and HPV16-negative head and
neck cancer. Int J Mol Sci. 21:85702020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ludwig S, Marczak L, Sharma P, Abramowicz
A, Gawin M, Widlak P, Whiteside TL and Pietrowska M: Proteomes of
exosomes from HPV(+) or HPV(-) head and neck cancer cells:
Differential enrichment in immunoregulatory proteins.
Oncoimmunology. 8:15938082019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theodoraki MN, Yerneni SS, Hoffmann TK,
Gooding WE and Whiteside TL: Clinical significance of
PD-L1+ exosomes in plasma of head and neck cancer
patients. Clin Cancer Res. 24:896–905. 2018. View Article : Google Scholar
|
|
19
|
Theodoraki MN, Laban S, Jackson EK, Lotfi
R, Schuler PJ, Brunner C, Hoffmann TK, Whiteside TL and Hofmann L:
Changes in circulating exosome molecular profiles following
surgery/(chemo)radiotherapy: Early detection of response in head
and neck cancer patients. Br J Cancer. 125:1677–1686. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jablonska J, Rist M, Spyra I, Tengler L,
Domnich M, Kansy B, Giebel B, Thakur BK, Rotter N, Lang S and
Ludwig S: Evaluation of immunoregulatory biomarkers on plasma small
extracellular vesicles for disease progression and early
therapeutic response in head and neck cancer. Cells. 11:9022022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daassi D, Mahoney KM and Freeman GJ: The
importance of exosomal PDL1 in tumour immune evasion. Nat Rev
Immunol. 20:209–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rückert M, Flohr AS, Hecht M and Gaipl US:
Radiotherapy and the immune system: More than just immune
suppression. Stem Cells. 39:1155–1165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hecht M, Fietkau R and Gaipl US:
Definitive chemoradiotherapy of locally advanced head and neck
cancer in combination with immune checkpoint inhibition-new
concepts required. Strahlenther Onkol. 198:83–85. 2022.In German.
View Article : Google Scholar
|
|
25
|
Welters MJ, Fichtinger-Schepman AM, Baan
RA, Hermsen MA, van der Vijgh WJ, Cloos J and Braakhuis BJ:
Relationship between the parameters cellular differentiation,
doubling time and platinum accumulation and cisplatin sensitivity
in a panel of head and neck cancer cell lines. Int J Cancer.
71:410–415. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Affolter A, Muller MF, Sommer K,
Stenzinger A, Zaoui K, Lorenz K, Wolf T, Sharma S, Wolf J, Perner
S, et al: Targeting irradiation-induced mitogen-activated protein
kinase activation in vitro and in an ex vivo model for human head
and neck cancer. Head Neck. 38(Suppl 1): E2049–E2061. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong CS, Funk S, Muller L, Boyiadzis M and
Whiteside TL: Isolation of biologically active and morphologically
intact exosomes from plasma of patients with cancer. J Extracell
Vesicles. 5:292892016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rajamoorthi A, Shrivastava S, Steele R,
Nerurkar P, Gonzalez JG, Crawford S, Varvares M and Ray RB: Bitter
melon reduces head and neck squamous cell carcinoma growth by
targeting c-Met signaling. PLoS One. 8:e780062013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu SC, Hsieh YS, Yu CC, Lai YY and Chen
PN: Thymoquinone induces cell death in human squamous carcinoma
cells via caspase activation-dependent apoptosis and LC3-II
activation-dependent autophagy. PLoS One. 9:e1015792014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chien MH, Yang WE, Yang YC, Ku CC, Lee WJ,
Tsai MY, Lin CW and Yang SF: Dual targeting of the p38 MAPK-HO-1
axis and cIAP1/XIAP by demethoxycurcumin triggers caspase-mediated
apoptotic cell death in oral squamous cell carcinoma cells. Cancers
(Basel). 12:7032020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung EJ, Brown AP, Asano H, Mandler M,
Burgan WE, Carter D, Camphausen K and Citrin D: In vitro and in
vivo radio-sensitization with AZD6244 (ARRY-142886), an inhibitor
of mitogen-activated protein kinase/extracellular signal-regulated
kinase 1/2 kinase. Clin Cancer Res. 15:3050–3057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leiker AJ, DeGraff W, Choudhuri R, Sowers
AL, Thetford A, Cook JA, Van Waes C and Mitchell JB: Radiation
enhancement of head and neck squamous cell carcinoma by the dual
PI3K/mTOR inhibitor PF-05212384. Clin Cancer Res. 21:2792–2801.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Affolter A, Drigotas M, Fruth K,
Schmidtmann I, Brochhausen C, Mann WJ and Brieger J: Increased
radioresistance via G12S K-Ras by compensatory upregulation of MAPK
and PI3K pathways in epithelial cancer. Head Neck. 35:220–228.
2013. View Article : Google Scholar
|
|
34
|
Affolter A, Fruth K, Brochhausen C,
Schmidtmann I, Mann WJ and Brieger J: Activation of
mitogen-activated protein kinase extracellular signal-related
kinase in head and neck squamous cell carcinomas after irradiation
as part of a rescue mechanism. Head Neck. 33:1448–1457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Affolter A, Samosny G, Heimes AS,
Schneider J, Weichert W, Stenzinger A, Sommer K, Jensen A, Mayer A,
Brenner W, et al: Multikinase inhibitors sorafenib and sunitinib as
radiosensitizers in head and neck cancer cell lines. Head Neck.
39:623–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
37
|
Ock CY, Kim S, Keam B, Kim S, Ahn YO,
Chung EJ, Kim JH, Kim TM, Kwon SK, Jeon YK, et al: Changes in
programmed death-ligand 1 expression during cisplatin treatment in
patients with head and neck squamous cell carcinoma. Oncotarget.
8:97920–97927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barker HE, Paget JTE, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirsch L, Zitvogel L, Eggermont A and
Marabelle A: PD-Loma: A cancer entity with a shared sensitivity to
the PD-1/PD-L1 pathway blockade. Br J Cancer. 120:3–5. 2019.
View Article : Google Scholar :
|
|
41
|
Kong Y, Ma Y, Zhao X, Pan J, Xu Z and
Zhang L: Optimizing the treatment schedule of radiotherapy combined
with anti-PD-1/PD-L1 immunotherapy in metastatic cancers. Front
Oncol. 11:6388732021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Narits J, Tamm H and Jaal J: PD-L1
induction in tumor tissue after hypofractionated thoracic
radiotherapy for non-small cell lung cancer. Clin Transl Radiat
Oncol. 22:83–87. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kordbacheh T, Honeychurch J, Blackhall F,
Faivre-Finn C and Illidge T: Radiotherapy and anti-PD-1/PD-L1
combinations in lung cancer: Building better translational research
platforms. Ann Oncol. 29:301–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wimmer S, Deloch L, Hader M, Derer A,
Grottker F, Weissmann T, Hecht M, Gostian AO, Fietkau R, Frey B and
Gaipl US: Hypofractionated radiotherapy upregulates several immune
checkpoint molecules in head and neck squamous cell carcinoma cells
independently of the HPV status while ICOS-L is upregulated only on
HPV-positive cells. Int J Mol Sci. 22:91142021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kikuchi M, Clump DA, Srivastava RM, Sun L,
Zeng D, Diaz-Perez JA, Anderson CJ, Edwards WB and Ferris RL:
Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1
monoclonal antibody for assessing radiation-induced PD-L1
upregulation in head and neck cancer and melanoma. Oncoimmunology.
6:e13290712017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schulz D, Stancev I, Sorrentino A, Menevse
AN, Beckhove P, Brockhoff G, Hautmann MG, Reichert TE, Bauer RJ and
Ettl T: Increased PD-L1 expression in radioresistant HNSCC cell
lines after irradiation affects cell proliferation due to
inactivation of GSK-3beta. Oncotarget. 10:573–583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mutschelknaus L, Peters C, Winkler K,
Yentrapalli R, Heider T, Atkinson MJ and Moertl S: Exosomes derived
from squamous head and neck cancer promote cell survival after
ionizing radiation. PLoS One. 11:e01522132016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ribas A and Hu-Lieskovan S: What does
PD-L1 positive or negative mean? J Exp Med. 213:2835–2840. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Derer A, Spiljar M, Bäumler M, Hecht M,
Fietkau R, Frey B and Gaipl US: Chemoradiation increases PD-L1
expression in certain melanoma and glioblastoma cells. Front
Immunol. 7:6102016. View Article : Google Scholar
|
|
52
|
Oweida A, Lennon S, Calame D, Korpela S,
Bhatia S, Sharma J, Graham C, Binder D, Serkova N, Raben D, et al:
Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint
blockade in orthotopic murine head and neck squamous cell
carcinoma. Oncoimmunology. 6:e13561532017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang B, Bowerman NA, Salama JK, Schmidt
H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley
DA, et al: Induced sensitization of tumor stroma leads to
eradication of established cancer by T cells. J Exp Med. 204:49–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin W, Chen M, Hong L, Zhao H and Chen Q:
Crosstalk between PD-1/PD-L1 blockade and its combinatorial
therapies in tumor immune microenvironment: A focus on HNSCC. Front
Oncol. 8:5322018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rossi G, Russo A, Tagliamento M, Tuzi A,
Nigro O, Vollome G Sini C, Grassi M, Bello MGD, Coco S, et al:
Präzisionsmedizin bei NSCLC im zeitalter der immuntherapie: Neue
biomarker zur selektion der am besten geeigneten therapie oder des
am besten geeigneten patienten. Kompass Pneumol. 8:300–317. 2020.
View Article : Google Scholar
|
|
57
|
Müller T, Braun M, Dietrich D, Aktekin S,
Höft S, Kristiansen G, Göke F, Schröck A, Brägelmann J, Held SAE,
et al: PD-L1: A novel prognostic biomarker in head and neck
squamous cell carcinoma. Oncotarget. 8:52889–52900. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Balermpas P, Rödel F, Krause M, Linge A,
Lohaus F, Baumann M, Tinhofer I, Budach V, Sak A, Stuschke M, et
al: The PD-1/PD-L1 axis and human papilloma virus in patients with
head and neck cancer after adjuvant chemoradiotherapy: A
multicentre study of the German cancer consortium radiation
oncology group (DKTK-ROG). Int J Cancer. 141:594–603. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hong AM, Ferguson P, Dodds T, Jones D, Li
M, Yang J and Scolyer RA: Significant association of PD-L1
expression with human papillomavirus positivity and its prognostic
impact in oropharyngeal cancer. Oral Oncol. 92:33–39. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cordonnier M, Nardin C, Chanteloup G,
Derangere V, Algros MP, Arnould L, Garrido C, Aubin F and Gobbo J:
Tracking the evolution of circulating exosomal-PD-L1 to monitor
melanoma patients. J Extracell Vesicles. 9:17108992020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
da Silva JL, Dos Santos ALS, Nunes NCC, de
Moraes Lino da Silva F, Ferreira CGM and de Melo AC: Cancer
immunotherapy: The art of targeting the tumor immune
microenvironment. Cancer Chemother Pharmacol. 84:227–240. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Theodoraki MN, Yerneni S, Gooding WE, Ohr
J, Clump DA, Bauman JE, Ferris RL and Whiteside TL: Circulating
exosomes measure responses to therapy in head and neck cancer
patients treated with cetuximab, ipilimumab, and IMRT.
Oncoimmunology. 8:15938052019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ota K, Azuma K, Kawahara A, Hattori S,
Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S,
et al: Induction of PD-L1 expression by the EML4-ALK oncoprotein
and downstream signaling pathways in non-small cell lung cancer.
Clin Cancer Res. 21:4014–4021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Han JJ, Kim DW, Koh J, Keam B, Kim TM,
Jeon YK, Lee SH, Chung DH and Heo DS: Change in PD-L1 expression
after acquiring resistance to gefitinib in EGFR-mutant
non-small-cell lung cancer. Clin Lung Cancer. 17:263–270.e2. 2016.
View Article : Google Scholar
|
|
66
|
Ebert PJR, Cheung J, Yang Y, McNamara E,
Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, et
al: MAP kinase inhibition promotes T cell and anti-tumor activity
in combination with PD-L1 checkpoint blockade. Immunity.
44:609–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013. View Article : Google Scholar
|
|
68
|
Derer A, Frey B, Fietkau R and Gaipl US:
Immune-modulating properties of ionizing radiation: Rationale for
the treatment of cancer by combination radiotherapy and immune
checkpoint inhibitors. Cancer Immunol Immunother. 65:779–786. 2016.
View Article : Google Scholar
|
|
69
|
Burnette BC, Liang H, Lee Y, Chlewicki L,
Khodarev NN, Weichselbaum RR, Fu YX and Auh SL: The efficacy of
radiotherapy relies upon induction of type I interferon-dependent
innate and adaptive immunity. Cancer Res. 71:2488–2496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y,
Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al: Therapeutic
effects of ablative radiation on local tumor require
CD8+ T cells: changing strategies for cancer treatment.
Blood. 114:589–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lugade AA, Moran JP, Gerber SA, Rose RC,
Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma
tumors increases the generation of tumor antigen-specific effector
cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dewan MZ, Galloway AE, Kawashima N,
Dewyngaert JK, Babb JS, Formenti SC and Demaria S: Fractionated but
not single-dose radiotherapy induces an immune-mediated abscopal
effect when combined with anti-CTLA-4 antibody. Clin Cancer Res.
15:5379–5388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rong C, Muller MF, Xiang F, Jensen A,
Weichert W, Major G, Plinkert PK, Hess J and Affolter A: Adaptive
ERK signalling activation in response to therapy and in silico
prognostic evaluation of EGFR-MAPK in HNSCC. Br J Cancer.
123:288–297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fournel L, Wu Z, Stadler N, Damotte D,
Lococo F, Boulle G, Ségal-Bendirdjian E, Bobbio A, Icard P,
Trédaniel J, et al: Cisplatin increases PD-L1 expression and
optimizes immune check-point blockade in non-small cell lung
cancer. Cancer Lett. 464:5–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Park SJ, Ye W, Xiao R, Silvin C, Padget M,
Hodge JW, Van Waes C and Schmitt NC: Cisplatin and oxaliplatin
induce similar immunogenic changes in preclinical models of head
and neck cancer. Oral Oncol. 95:127–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tran L, Allen CT, Xiao R, Moore E, Davis
R, Park SJ, Spielbauer K, Van Waes C and Schmitt NC: Cisplatin
alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition
in head and neck squamous cell carcinoma. Cancer Immunol Res.
5:1141–1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Varayathu H, Sarathy V, Thomas BE, Mufti
SS and Naik R: Combination strategies to augment immune check point
inhibitors efficacy-implications for translational research. Front
Oncol. 11:5591612021. View Article : Google Scholar
|
|
78
|
Califano JA, Khan Z, Noonan KA, Rudraraju
L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C, et al:
Tadalafil augments tumor specific immunity in patients with head
and neck squamous cell carcinoma. Clin Cancer Res. 21:30–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Merlano MC, Merlotti AM, Licitra L, Denaro
N, Fea E, Galizia D, Di Maio M, Fruttero C, Curcio P, Vecchio S, et
al: Activation of immune responses in patients with
relapsed-metastatic head and neck cancer (CONFRONT phase I-II
trial): Multimodality immunotherapy with avelumab, short-course
radiotherapy, and cyclophosphamide. Clin Transl Radiat Oncol.
12:47–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim JE, Patel MA, Mangraviti A, Kim ES,
Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H, et al:
Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation
results in regression of murine gliomas. Clin Cancer Res.
23:124–136. 2017. View Article : Google Scholar :
|