According to the Global Cancer Statistics 2020
report, lung cancer ranks as the second most common type of cancer
in incidence, accounting for 11.4% of all diagnosed cancer cases
(1). Lung cancer is a prime
contributor to cancer-related deaths, with 1.8 million global
deaths from lung cancer each year (1). Non-small cell lung cancer (NSCLC) is
the predominant pathological type of lung cancer, accounting for
~85% of all reported cases (2).
Patients with advanced NSCLC who received combination chemotherapy
have a 5-year survival rate of only 2.8% (3).
Implementing immune checkpoint inhibitors (ICIs) can
improve survival in advanced NSCLC. Pembrolizumab showed notable
survival benefits in both previously untreated and treated advanced
NSCLC, in which the 5-year survival rate was 23.2 and 15.5%,
respectively (4). Programmed
death 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) blockers,
including nivolumab, pembrolizumab, cemiplimab, atezolizumab, and
durvalumab, have been authorized by the US Food and Drug
Administration for advanced and metastatic NSCLC (5-7).
Additionally, anti-PD-1/PD-L1 treatments combined
with anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)
treatments have also been used for the management of advanced and
recurrent NSCLC (8-11). The NEOSTER trial showed that the
treatment with nivolumab combined with ipilimumab in neoadjuvant
therapy for resectable NSCLC significantly increased the
pathological response rate and reduced tumor retention (12).
The emergence of ICIs has revolutionized the
therapeutic approaches for the management of NSCLC. Immune-related
adverse events (irAEs) have also attracted significant attention,
in particular, checkpoint inhibitor pneumonitis (CIP). CIP is more
prone to occur in ICI-treated NSCLC than in other cancer types
(13), with the rate of a grade
≥3 CIP being 2.3× higher than in different cancer types (14), which may be due to an increased
chance of having respiratory comorbidities such as chronic
obstructive pulmonary disease (COPD) and pre-existing interstitial
lung disease (ILD) and receiving chest irradiation in NSCLC
(13,15). Although CIP is rare, it has become
one of the primary causes of ICI-related treatment interruption and
death (16,17). The characteristics of occurrence,
pathogenesis, high-risk factors, clinical and radiological
manifestation, and management of CIP remain unclear; therefore,
herein, the above issues are explored and summarized.
Among patients with malignant tumors who were
administered PD-1/PD-L1 blockers, the global morbidity of irAEs was
26.82%, and the incidence of severe irAEs was 6.10% (6); common irAEs included pneumonia,
colitis, hepatitis, rash, endocrine diseases, and nephritis
(18). Following the Common
Terminology Criteria for Adverse Events [version 5.0], CIP can be
classified into 5 grades (19):
In neoadjuvant therapy, anti-PD-1 therapy and combined
immunotherapy had CIP rates of 1.1-5.0 and 5.0%, respectively; the
occurrence of grade ≥3 CIP was 0.0-5.0% and 0.0, respectively
(12,20,21). The incidence of CIP in first-line
treatment with anti-PD-1/PD-L1 therapy and combined immunotherapy
was 1.1-8.0 and 3.8-12.8%, respectively; the incidence of grade ≥3
CIP was 0-3.0 and 2.3-5.7%, respectively. In consolidation therapy,
anti-PD-L1 therapy had CIP rates of 10.7-19.0%, and the incidence
of grade ≥3 CIP was 1.7-3.0%. In second line and above treatment,
anti-PD-1/PD-L1 therapy had CIP rates of 1.0-4.5%, and the
incidence of grade ≥3 CIP was 0.0-2.1% (Table I). However, a meta-analysis
involving 1,885 patients with Stage III NSCLC showed that the
incidence of CIP and the incidence of grade ≥3 CIP were 35 and 6%,
respectively, when adopting durvalumab as a consolidation regimen
in the real world (22). In
retrospective studies, the incidence of CIP and the incidence of
grade ≥3 CIP was 4.7-18.0 and 2.5-6.5%, respectively (Table II) (23-31). Based on the above studies, CIP in
the real world is higher than that of prospective clinical
trials.
The typical clinical manifestations of CIP are
dyspnea (38.5-78.6%), cough (22.7-88.1%), fever (9.1-40.5%), and
chest pain (2.4-7.0%), although 8.8-33.0% of CIP patients are
asymptomatic (6,35-40). Compared to other respiratory
diseases, the clinical manifestations of CIP lack specificity.
Therefore, radiological characteristics are critical to the
diagnosis.
The prime radiological patterns of CIP are
organizing pneumonia (OP) (65-86%), nonspecific interstitial
pneumonia (NSIP) (15-31.3%), and hypersensitive pneumonia (HP)
(7-38.1%). In addition, the unique radiological patterns of CIP
include traction bronchiectasis, consolidation, reticular changes,
central lobular nodules, and honeycomb changes (6,26,28,35,38,41,42). Studies have shown that the
radiological characteristics of CIP are related to its severity. In
grade ≥3 CIP, acute interstitial pneumonia (AIP) and acute
respiratory distress syndrome (ARDS) are the primary
manifestations, followed by OP, while in grade 1-2 CIP, NSIP, and
HP are the most common manifestations (41). HP and cryptogenic organizing
pneumonia were associated with improved efficacy of ICIs, with a
median progression-free survival (PFS) of 44.29 weeks and 57 weeks,
respectively (28). In addition,
high-resolution computed tomography (HRCT) is promising for
diagnosing CIP, especially when interstitial pulmonary fibrosis is
considered (36). Clinical and
radiological characteristics can help to establish a preliminary
diagnosis of CIP, but tumor progression, infection, ILD, and
thromboembolism must first be excluded (43).
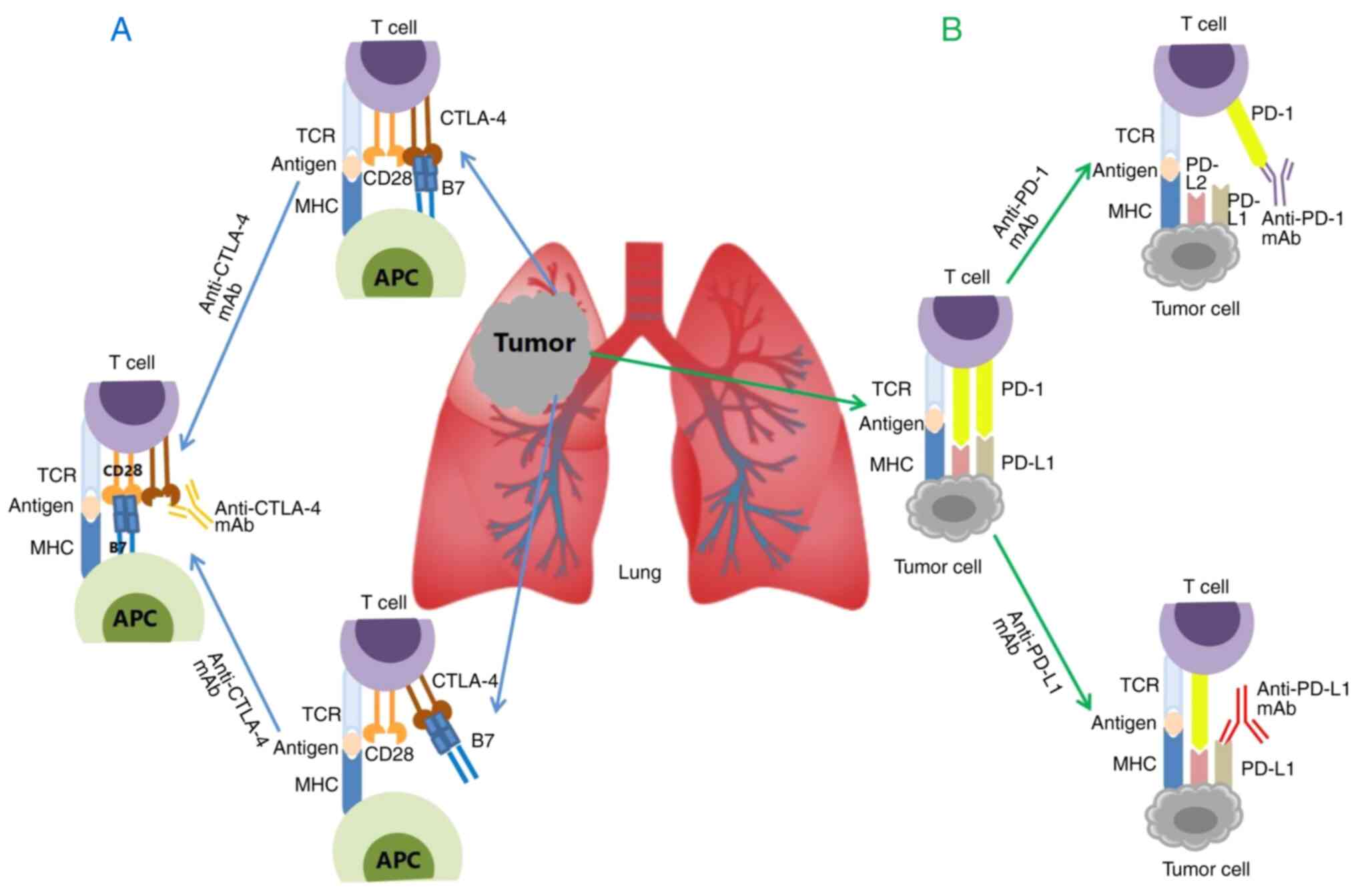
Tumor cells typically use immune suppression and
tolerance mechanisms to evade immune clearance, activating CTLA-4
and PD-1/PD-L1 signals to destroy or inhibit immune regulatory
pathways (44). CTLA-4 is
structurally similar and homologous to the T cell co-stimulatory
molecule Cluster of Differentiation 28 (CD28). CTLA-4 can compete
with CD28 to bind to the ligands B7-1 (CD80) and B7-2 (CD86).
CTLA-4 has greater affinity and activity than CD28, reducing
CD28/B7 interactions, and may transmit intracellular inhibitory
signals after binding to B7 molecules (45). In addition, studies have confirmed
that CTLA-4 can remove CD80 and CD86 molecules on the surface of
antigen-presenting cells (APCs), which reduces the activation of
effector T cells (46).
PD-L1 is one of the ligands of PD-1 and is primarily
expressed on somatic cells exposed to anti-inflammatory cytokines.
The binding of PD-1 and PD-L1 inhibits the effects of T cells
(44,47). At the same time, chronic
inflammatory factor-mediated expression of PD-L1 in the tumor
microenvironment leads to PD-1-mediated depletion of T cells and
inhibits the anti-tumor cytotoxic T cell response (47-49). That is, the binding between CTLA-4
and B7 molecules, removing B7 molecules from APCs, and the
relationship between PD-1 and PD-L1 ultimately reduces the
activation of T cells, thus improving the survival of tumor cells.
This mechanism suggests that CTLA-4 mAbs can block CTLA-4
inhibitory signals, and PD-1 and PD-L1 mAbs can block PD-1/PD-L1
inhibitory signals, restoring T cells' tumor-killing effect and
achieving tumor growth inhibition (Fig. 1) (43).
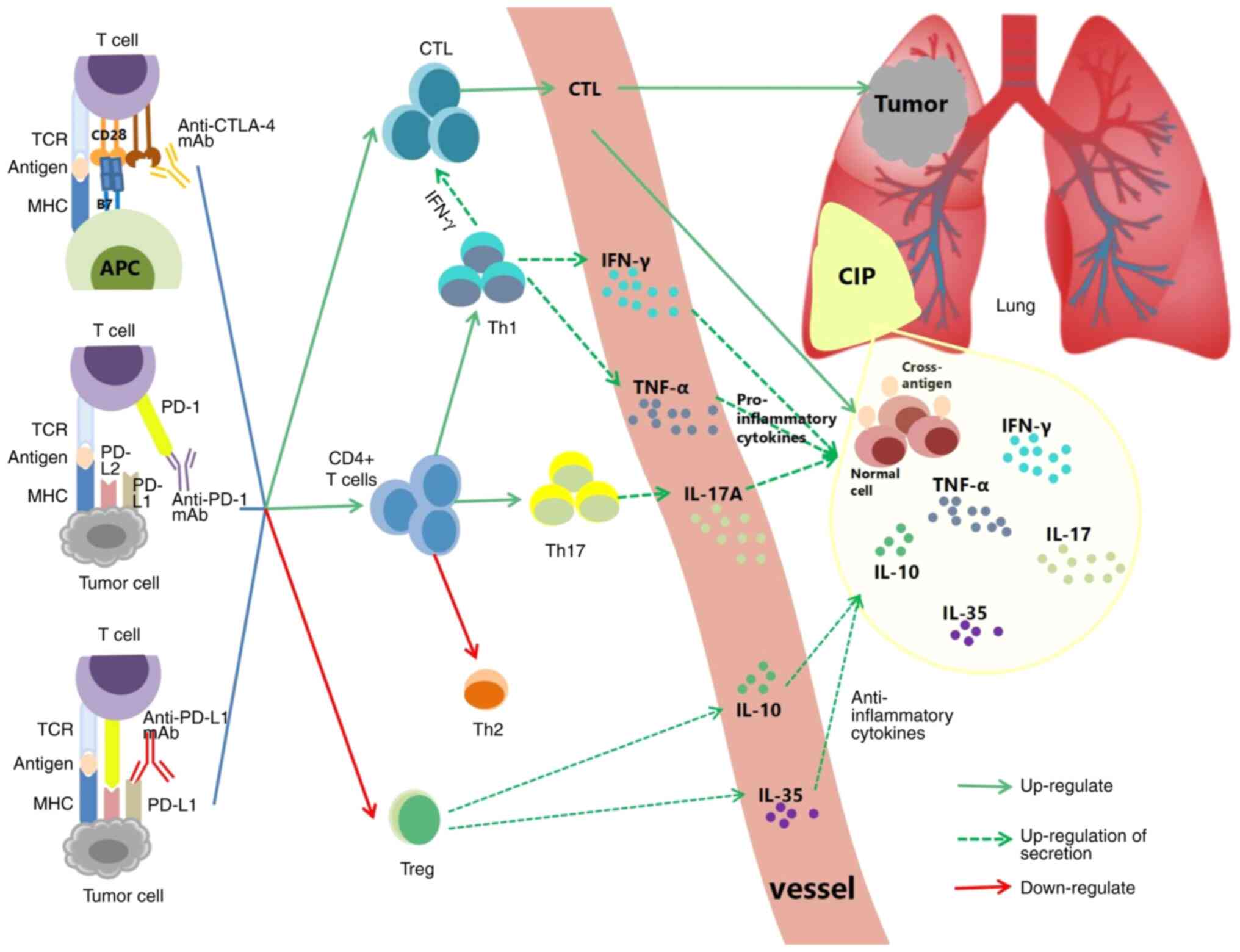
Anti-PD-1, anti-PD-L1, and anti-CTLA-4 mAb can block
CTLA-4 and PD-1/PD-L1 signaling, respectively. This process may
also lead to excessive activation and amplification of CTL, Helper
T (Th) cells, and downregulation of regulatory T cells (Tregs), and
ultimately lead to induction of CIP (Fig. 2) (50-52).
Prior to treatment with steroids, analysis of
bronchoalveolar lavage fluid (BALF) from 12 CIP patients and 6
patients without CIP showed that the number of lymphocytes
increased by >20% in the CIP group. Subsequent flow cytometry
analysis revealed that the CD3+CD8+T cells
and TNF-αhighIFN-γhigh CD8+T cells
increased (50). Histochemical
analysis of pneumonia tissues from CIP patients revealed that
CD8+T cells increased in pneumonia tissues (51). During steroid reduction, the
specific proliferation of PD-1+CD8+ T cells
was observed in the pulmonary pathology of relapsed CIP but not in
normal tissues (53).
Furthermore, comparison of the
complementarity-determining region 3 of the T cell receptor (TCR) β
chain in irAE-lesions and tumor-infiltrating lymphocytes (TILs) via
sequencing revealed that the T cell pools of two groups overlapped
significantly (51). Subudhi
et al (54) found that the
number of CD8+T cell clones in the peripheral blood was
closely correlated with irAEs (P=0.01), especially with grade 2-3
irAEs (P<0.0001) in patients who received ipilimumab, a CTLA-4
blocker. TIL-like T cells in inflammatory tissues and peripheral
blood suggest the existence of cross-antigens shared in tumor and
normal tissues. If the cross-antigens appeared in the lung tissue,
the specific CTL may damage the normal lung tissue and thereby
cause CIP.
Tregs belong to the inhibitory CD4+ T cell subgroup,
which is primarily involved in establishing peripheral tolerance by
inhibiting effector T cells and inhibiting immune-mediated tissue
destruction against autoantigens. PD-1+ and
CTLA-4+ Tregs negatively regulate the inflammatory
response induced by CD8+T cells (52,64). Since the PD-1/PD-L1 axis is
blocked, Treg differentiation is blocked, leading to a decrease in
Treg levels in the tumor microenvironment (38). The conjugation of anti-CTLA-4
antibodies to CTLA-4 can also lead to Treg depletion or functional
blockade, thereby enhancing T-cell activation (52). The proportion of immunosuppressive
CTLA-4highPD-1high alveolar Tregs is notably
decreased in the BALF following the development of CIP (50), which may promote Th1 cell
responses, as seen in NSCLC patients with CIP (60). Not only does Treg depletion
facilitate CIP, but alveolar Tregs participate in the regression of
lung injury (65). Thus, the
depletion and dysfunction of Tregs may accelerate CIP.
In addition to immune cells, cytokines are also
involved in the development of CIP. The dysregulation of cytokines
is associated with severe irAEs and may thus be used in determining
a prognosis (70).
IL-17A produced by Th17 cells, is a pro-inflammatory
cytokine involved in various inflammatory diseases. Overexpression
of IL-17A and Th17 cells leads to tissue damage, inflammation, and
autoimmune activation (76-78). Spleen cells of PD-1−/−
mice have been reported to produce more IL-17A than wild-type mice
post-stimulation (concanavalin A, PMA + lonomycin, or αCD3 + αCD28)
(79). High levels of IL-17 at
baseline were predictive of grade 3 diarrhea/enteritis in melanoma
treated with ipilimumab (P=0.02) (80). IL-17A levels in the serum and BALF
were elevated when CIP occurred in NSCLC patients. IL-17A in serum
significantly decreased when CIP was improved or restored (P=0.034)
and was positively correlated with the proportion of Th17 cells and
the Th17/Treg ratio (60).
Another study demonstrated that IL-17A in the BALF of CIP patients
was significantly higher than that of lung cancer and ILD patients
(68). In conclusion, elevated
IL-17A levels may promote CIP.
IL-1β is a critical pro-inflammatory factor,
primarily synthesized and secreted by monocytes and macrophages.
High levels of IL-1β in the serum can promote acute lung injury and
pulmonary fibrosis (67,81). Elevated IL-1β at baseline and
early in anti-PD-1 therapy (1-6 weeks after anti-PD-1 therapy) is
predictive of irAEs (70). A case
report demonstrated that the levels of IL-1β were significantly
elevated in the serum of CIP patients (21.9 pg/ml) (82). Suresh et al (50) observed that the number of
monocytes expressing IL-1β in the BALF increased noticeably, while
soluble IL-1β levels decreased during the development of CIP. This
may be due to the late time of BALF collection (at least 2-3 days
after the onset of CIP symptoms), whereas elevations in IL-1β
generally occur early in lung injury. According to the above
studies, IL-1β may be involved in the pathogenesis of CIP through
pro-inflammatory responses, although its secretion in CIP requires
further observation and analysis.
IL-10 and IL-35, which are produced by Tregs, are
important anti-inflammatory cytokines with anti-fibrotic effects
(83,84). IL-10 can inhibit the production of
TNF-α, IL-6, and IL-1β of monocytes (85). IL-35 can reduce the activation of
Th1 and Th17 cells and inhibit the secretion of cytokines such as
IL-17A, TNF-α, and IFN-γ (86,87). IL-35 can promote the production of
IL-10 (88). IL-10 is elevated
when CIP occurs, and high levels of IL-10 (≥3.79 pg/ml) are
positively correlated with severe CIP (P=0.057) (74). Wang et al (89) performed a subgroup analysis of 40
NSCLC patients with irAEs and found baseline IL-10 levels were an
independent prognostic risk factor for CIP (OR=9.969, 95% CI
1.144-86.843, P=0.037). CIP was prone to occur in the high IL-10
group (≥0.704 pg/ml) compared with the low IL-10 group (<0.704
pg/ml) (45.65 vs. 9.52%, P=0.004). The levels of IL-35 in the serum
and BALF were elevated when CIP occurred in NSCLC patients. IL-35
levels in the serum were significantly decreased when CIP improved
or resolved (P=0.044) and positively associated with the proportion
of Th1 cells and the Th1/Th2 ratio (60). In conclusion, IL-10 and IL-35 may
influence CIP, while an increase in the levels of IL-10 and IL-35
may be secondary to the pro-inflammatory response. However, the
specific mechanism warrants further exploration.
In addition to the aforementioned immune disorders
and abnormal cytokine secretion, autoantibodies, and microbial
flora may also affect the occurrence of CIP. In NSCLC patients who
were treated with PD-1 blockers, irAEs were found to be associated
with preexisting rheumatoid factor (68 vs. 40%, P=0.006) and
autoantibodies (60 vs. 32%, P=0.002), such as thyroid peroxidase
antibody, anti-thyroglobulin, and antinuclear antibody; however, no
statistical differences in the occurrence of CIP was found in the
subgroup analyses (90).
Tumor-associated autoantibodies can increase CIP,
such as antibodies against p53, NY-ESO-1, TRIM21, HUD, and BRCA2
(91). Furthermore, the levels of
anti-CD74 autoantibodies increased 1.34-fold in patients with CIP
compared with before treatment with ICIs, but the fold increase was
not observed in patients without CIP, which revealed that the
fold-change of anti-CD74 autoantibodies was related to the
development of CIP (92). The
relationship between microbial flora and irAEs has also attracted
attention. For example, the enrichment of Firmicutes is more
likely to lead to ICI-related diarrhea (93). However, the connection between CIP
and microbial flora is vague.
In real-world settings, several NSCLC patients have
pre-existing respiratory diseases, such as ILD, COPD, and asthma.
Pre-existing ILD may accelerate CIP in NSCLC (35,38,94-96), and CIP was found to occur earlier
in NSCLC patients with previous ILD during ICI treatment (1.3 vs.
2.3 months) (24). In another
study consisting of 461 NSCLC patients, the ILD group (n=49) more
frequently developed CIP (n=412) (30.6 vs. 9.5%, P<0.01) and
grade ≥3 CIP (16.3 vs. 3.6%, P<0.01) than the non-ILD group
(97). However, mild ILD may not
increase the incidence of CIP and grade ≥3 CIP. Fujimoto et
al (98) defined mild
interstitial pneumonia as a predicted vital capacity of ≥80% and
manifesting as usual interstitial pneumonia on HRCT. In their
study, CIP occurred in only 2 of 18 NSCLC patients after nivolumab
therapy, both grade 2. In another study involving 10 patients with
mild ILD, it was also found that there was no significant
difference in the incidence and severity of CIP between those with
and without prior ILD who received first-line pembrolizumab
monotherapy in NSCLC (20.0 vs. 22.6% and 10.0 vs. 11.3%,
respectively) (99).
In conclusion, the application of PD-1 blockers in
patients with mild ILD may be safe, but more severe ILD may be more
closely related to CIP in NSCLC, which requires further
confirmation. Interestingly, adopting the anti-PD-L1 mAb in a
bleomycin-induced pulmonary fibrosis mouse model can alleviate
pulmonary fibrosis (100). There
is substantial heterogeneity in the effects of PD-L1 blockers in
ILD. Further comparison and analysis of the immune background of
patients with ILD who develop CIP and the changes in the
microenvironment during the development of the two diseases are
necessary, which may offer a reliable basis for utilizing ICIs in
NSCLC with pre-existing ILD. In addition, COPD and asthma may also
contribute to CIP (101,102). In the KEYNOTE-001 trial, CIP was
more common in patients with prior COPD and asthma (5.4 vs. 3.1%)
(101). Grades 3-4 CIP was more
prone to occur in patients with concomitant asthma (100.0 vs.
28.6%) (103).
A meta-analysis found that compared with PD-L1
blockers, the use of PD-1 blockers was more likely to result in a
patient developing CIP, with Grade 3 or 4 pneumonitis also being
more commonly observed with PD-1 blockers (1.1 vs. 0.4%, P=0.02)
(108,113). A higher incidence of grade ≥3
CIP was also observed in patients treated with PD-1 blockers
compared with PD-L1 blockers in stage III NSCLC (8.6 vs. 4.4%,
P=0.01) (114). A review
involving 48 trials demonstrated that CIP was more likely to occur
with PD-1 blockers than CTLA-4 blockers (OR 6.4, 95% CI 3.2-12.7)
(115). CIP was more common when
treated with pembrolizumab than with nivolumab (63 vs. 37%,
P=0.004) (23). Additionally,
untreated NSCLC is more likely to result in CIP compared with NSLC
previously treated with PD-1/PD-L1 blockers (113). Compared with the use of ICIs as
a second-line treatment, CIP and grade ≥3 CIP were more likely to
occur if ICIs were used as a first-line treatment (14,108). The KEYNOTE-598 trial showed that
pembrolizumab plus ipilimumab was more likely to result in CIP and
grade ≥3 CIP than pembrolizumab alone (12.8 vs. 5.3% and 5.7 vs.
2.5%, respectively) (10). In the
Lung-MAP S1400I trial (8) and the
MYSTIC trial (11), the morbidity
of CIP and grade ≥3 CIP in the PD-1/PD-L1 plus CTLA-4 blocker group
tended to be higher compared with that in the PD-1/PD-L1 blocker
monotherapy group.
Excluding previous respiratory disease, the history
of radiotherapy, chemotherapy, and EGFR-TKI therapy, and types of
ICIs, age (35,116), smoking history (117,118), histological type (34,117), Eastern Cooperative Oncology
Group (ECOG) score (38,42), extra-thoracic metastasis (35,119), serum albumin (23), and lung function (120) may be related to the occurrence
of CIP.
However, several studies have shown that age, sex,
smoking history, ECOG score, extra-thoracic metastasis,
histological type, previous COPD, previous chemotherapy,
radiotherapy, and EGFR-TKI treatment history, and the type of ICIs
are not related to the occurrence of CIP (28,35,43,50,116).
According to the guidelines and consensus
recommendations for grade 1 CIP, monitoring symptoms and pulmonary
function, and performing a chest CT is recommended. If symptoms
improve, close follow-up and ICI treatment should be resumed.
However, if conditions worsen, ICI treatment should be suspended.
For grade 2 CIP, ICI should be suspended, and methylprednisolone
1-2 mg/kg/d should be administered intravenously. After 48-72 h of
treatment, if the symptoms improve, the steroid dose should be
reduced by 5-10 mg per week for 4-6 weeks. If the disease worsens,
the treatment plan should be escalated. If there is the possibility
of a co-infection, empirical and spectral antibiotic therapy should
be considered. Chest CT and pulmonary function should be reviewed
every 3-4 days. When the patient recovers to grade ≤1 CIP, the
resumption of immunotherapy should be considered. For grade 3-4
CIP, ICIs should be discontinued permanently, the patient should be
hospitalized, and methylprednisolone 2-4 mg/kg/d should be
administered intravenously after 48 h of treatment. If the symptoms
improve, the dose of the steroid should be reduced after 8 weeks of
treatment. If the symptoms worsen, other immunosuppressants should
be considered (121-123).
There are currently four RCTs exploring CIP
treatment on the National Institutes of Health ongoing Trial
Registry, of which NCT04438382, NCT05899725, and NCT05280873 are
recruiting patients, and NCT04036721 was suspended due to
SARS-CoV-2 cases. Therefore, the outcomes of treatment for CIP in
randomized clinical trials are currently unknown.
Following the guidelines and consensus
recommendations, grade ≥2 CIP requires pharmacological
interventions (121-123). A large proportion of the data on
pharmacological interventions of grade ≥2 CIP originate from
retrospective studies. Among CIP patients receiving first-line
steroid therapy, the efficiency is 56-100% (Table III), Stroud et al
(124) attempted to treat grade
3-4 CIP with an IL-6R inhibitor (tocilizumab) on the basis of
corticosteroid therapy, and 11 of the 12 patients with grade 3-4
CIP exhibited improvements. Commonly used second-line drugs include
TNFα inhibitors (Infliximab), mycophenolate mofetil,
cyclophosphamide, and intravenous immunoglobulins (Table III) (124-129). Nintedanib has also shown promise
in improving CIP (130). IL-1
inhibitors (anakinra and canakinumab), IL-17 inhibitors
(ixekizumab, brodalumab, and secukinumab), integrin-4 inhibitors
(natalizumab), IL-23 and IL-12 inhibitors (ustekinumab), and anti-B
cell antibodies (rituximab and obinutuzumab) have been used to
improve irAEs (71,121-123), but their efficacy in CIP remains
unknown.
In addition to the applications of steroid
hormones, immunosuppressants, and cytokine antagonists to treat
irAEs, other strategies to reduce irAEs have also attracted
attention. A meta-analysis assessing 14 RCTs suggested that
atezolizumab may reduce the incidence of grade ≥3 CIP compared with
other immune-based schedules (131). IL-6 blockade combined with ICIs
can alleviate ICI-induced experimental autoimmune encephalomyelitis
(132). Thymosin α1 combined
with anti-CTLA-4 antibodies can significantly reduce the
gastrointestinal toxicity induced by anti-CTLA-4 antibodies
(133). However, the results of
the above two studies are based on animal experiments and have not
been confirmed in RCTs.
Following steroid treatment, most patients exhibit
improvement. However, ~14% (6/44) of CIP patients still have
persistent or worsening pneumonia during steroid reduction, and
chronic CIP requires ≥12 weeks of immunosuppressive therapy
(53). Lung cancer patients with
CIP have a better maximal tumor shrinkage rate (25.5 vs. 0.0%,
P=0.014) (38), better objective
response rate (61.90 vs. 29.91%), and better PFS (45.80 vs. 21.15
weeks) compared with those without CIP (28). Ono et al (26) found that patients with CIP had a
longer OS compared with patients without CIP (27.4 vs.14.8 months).
However, the common feature of these studies was the predominance
of grade 1-2 CIP and the use of close monitoring. Lung cancer
patients with grade ≥3 CIP have a markedly shorter OS (3.7 vs. 22.1
months, P<0.001) (74). The
grade ≥3 CIP-related mortality was 22.7-28.1% in NSCLC (29,42), and patients with grade ≥3 CIP had
a significantly shorter PFS (1.0 vs. 3.5 months) and OS (3.0 vs.
12.7 months) (42). In
conclusion, grade 1-2 CIP may be sued to predict the effectiveness
of an ICI treatment. In contrast, patients with grade ≥3 CIP may
exhibit a reduced response to ICI and shortened survival; thus,
assisting in the evaluation of the predictive prognosis of NSCLC
patients receiving ICI treatment. However, these findings require
further confirmation via randomized and prospective trials.
ICIs serve as a better treatment option for NSCLC;
however, additional attention should be focused on the resulting
irAEs, especially CIP. The real-world incidence of CIP is higher
than in randomized clinical trials. CIP is commonly seen early in
ICI treatment, especially within the first 6 months of initiation
of ICIs. The clinical and imaging manifestations of CIP lack
specificity, complicating the diagnosis. HRCT may be a promising
method in the imaging diagnosis, evaluation, and follow-up of CIP
since it can better reflect pulmonary interstitial changes.
Excessive activation and amplification of CTL, Th
cells, downregulation of Tregs, and over-secretion of
pro-inflammatory cytokines remain the dominant mechanisms
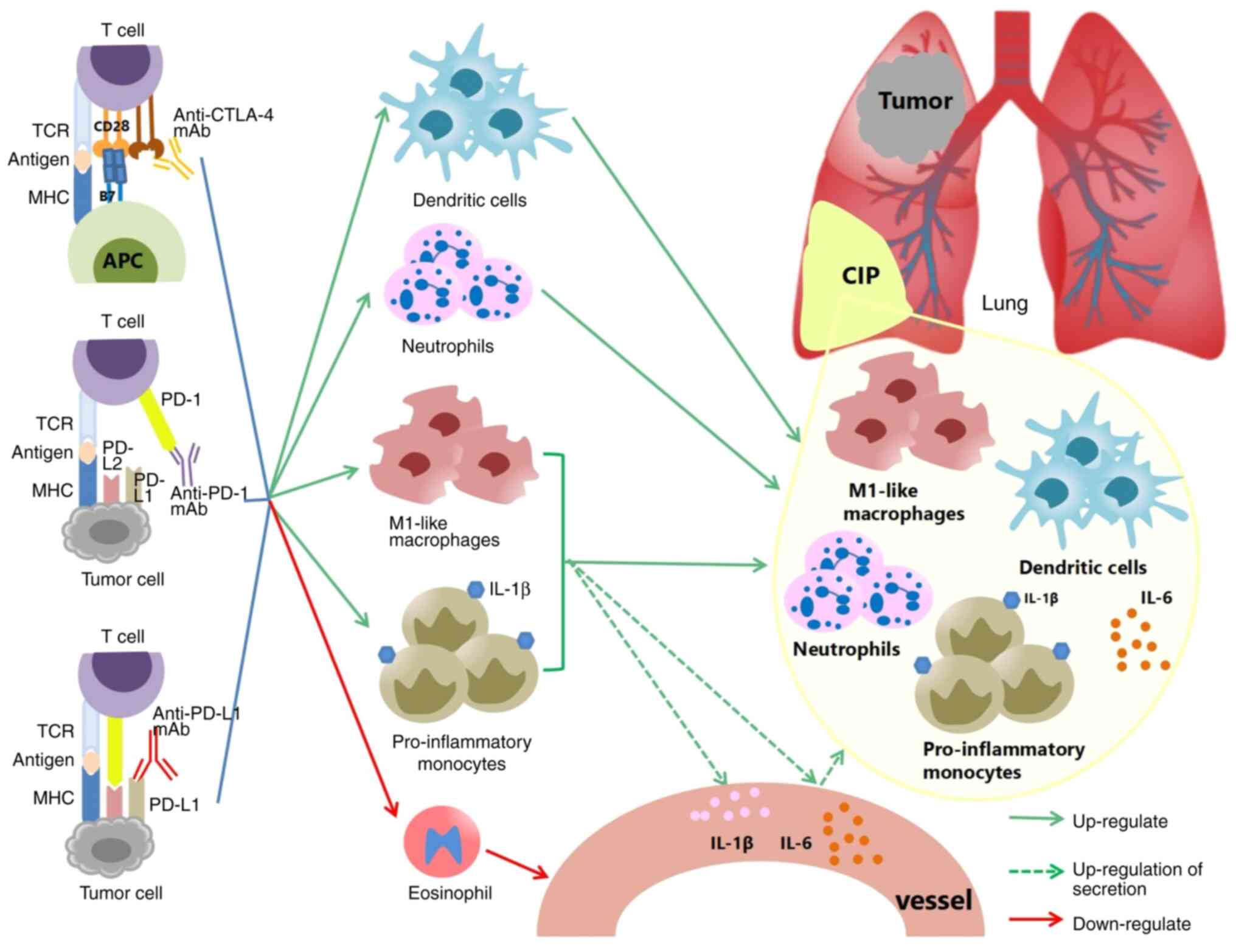
underlying the pathophysiology of CIP. The dysregulation of innate
immune cells, such as increased levels of inflammatory monocytes,
DCs, neutrophils and M1 polarization of macrophages, increased
IL-10 and IL-35, and a decrease in the eosinophil levels may
underlie the onset and progression of CIP. Nevertheless, several of
the above mechanistic findings are based on retrospective studies.
It is, therefore, necessary to obtain lung biopsies from CIP
patients, especially patients with grade ≥3 CIP for assessment.
Before ICI administration and during the process of CIP, analyzing
the components and changes of BALF may provide more evidence of the
molecular mechanisms underlying the development of CIP and other
pulmonary toxicities. Furthermore, autoantibodies and
microorganisms offer novel research avenues.
Although contested, several factors may facilitate
the onset of CIP, such as previous ILD, COPD, asthma, radiotherapy,
chemotherapy, EGFR-TKI therapy, PD-1 blockers, first-line
application of ICIs, and combined immunotherapy. First-line ICIs
plus chemotherapy may reduce the occurrence of CIP. Additional
trials are required to further assess the risk factors associated
with CIP. With a deeper understanding of CIP, a predictive model
may be established to promote the early detection, diagnosis, and
treatment of CIP and screen the optimal population for ICI
treatment.
Currently, the treatment of grade ≥2 CIP remains
steroid hormone therapy. Despite concerns regarding the toxic
effects and the potential to promote tumor progression, cytokine
blockers are promising therapeutic agents. The control rate of CIP
may be further upgraded by enhancing the targeting of cytokine
blockers, reducing their toxicity, and optimizing their combination
with steroid hormones. Multi-center, large samples, and
interdisciplinary research are imperative to achieve this goal.
Not applicable.
All authors contributed to the study conception and
design. XH, JR and QX prepared the manuscript, and collected and
assembled the data. XH, RL, DD, JR, JT, XS and JY performed the
data analysis and interpretation. XH drafted the manuscript. All
authors revised the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toi Y, Sugawara S, Kawashima Y, Aiba T,
Kawana S, Saito R, Tsurumi K, Suzuki K, Shimizu H, Sugisaka J, et
al: Association of immune-related adverse events with clinical
benefit in patients with advanced non-small-cell lung cancer
treated with nivolumab. Oncologist. 23:1358–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozkaya S, Findik S, Dirican A and Atici
AG: Long-term survival rates of patients with stage IIIB and IV
non-small cell lung cancer treated with cisplatin plus vinorelbine
or gemcitabine. Exp Ther Med. 4:1035–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naidoo J, Wang X, Woo KM, Iyriboz T,
Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin
AM, et al: Pneumonitis in patients treated with anti-programmed
death-1/programmed death ligand 1 therapy. J Clin Oncol.
35:709–717. 2017. View Article : Google Scholar
|
|
7
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar
|
|
8
|
Gettinger SN, Redman MW, Bazhenova L,
Hirsch FR, Mack PC, Schwartz LH, Bradley JD, Stinchcombe TE, Leighl
NB, Ramalingam SS, et al: Nivolumab plus ipilimumab vs nivolumab
for previously treated patients with stage IV squamous cell lung
cancer: The lung-MAP S1400I phase 3 randomized clinical trial. JAMA
Oncol. 7:1368–1377. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyer M, Şendur MAN, Rodríguez-Abreu D,
Park K, Lee DH, Çiçin I, Yumuk PF, Orlandi FJ, Leal TA, Molinier O,
et al: Pembrolizumab plus ipilimumab or placebo for metastatic
non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%:
Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol.
39:2327–2338. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft
A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al:
Durvalumab with or without tremelimumab vs standard chemotherapy in
first-line treatment of metastatic non-small cell lung cancer: The
MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6:661–674.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khoja L, Day D, Wei-Wu Chen T, Siu LL and
Hansen AR: Tumour- and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen K and Sun B: Incidence and risk of
PD-1/PD-L1 inhibitor-associated pneumonia in advance cancer
patients: A meta-analysis. Zhongguo Fei Ai Za Zhi. 23:927–940.
2020.In Chinese. PubMed/NCBI
|
|
15
|
Yamaguchi T, Shimizu J, Hasegawa T, Horio
Y, Inaba Y, Hanai N, Muro K and Hida T: Pre-existing interstitial
lung disease is associated with onset of nivolumab-induced
pneumonitis in patients with solid tumors: A retrospective
analysis. BMC Cancer. 21:9242021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi FS: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
systematic review and meta-analysis. JAMA Oncol. 2:1607–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hahn AW, Gill DM, Agarwal N and Maughan
BL: PD-1 checkpoint inhibition: Toxicities and management. Urol
Oncol. 35:701–707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE) v 5.0. Available
from: https://ctep.cancer.gov/protocoldevelopment/electronic_applica-tions/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
|
|
20
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zhang T, Huang Y, Li W, Zhao J,
Yang Y, Li C, Wang L and Bi N: Real-world safety and efficacy of
consolidation durvalumab after chemoradiation therapy for stage III
non-small cell lung cancer: A systematic review and meta-analysis.
Int J Radiat Oncol Biol Phys. 112:1154–1164. 2022. View Article : Google Scholar
|
|
23
|
Fukihara J, Sakamoto K, Koyama J, Ito T,
Iwano S, Morise M, Ogawa M, Kondoh Y, Kimura T, Hashimoto N and
Hasegawa Y: Prognostic impact and risk factors of immune-related
pneumonitis in patients with non-small-cell lung cancer who
received programmed death 1 inhibitors. Clin Lung Cancer.
20:442–450.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibaki R, Murakami S, Matsumoto Y,
Yoshida T, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N,
Kusumoto M, et al: Association of immune-related pneumonitis with
the presence of preexisting interstitial lung disease in patients
with non-small lung cancer receiving anti-programmed cell death 1
antibody. Cancer Immunol Immunother. 69:15–22. 2020. View Article : Google Scholar
|
|
25
|
Fujimoto D, Miura S, Yoshimura K, Wakuda
K, Oya Y, Yokoyama T, Yokoi T, Asao T, Tamiya M, Nakamura A, et al:
Pembrolizumab plus chemotherapy-induced pneumonitis in chemo-naïve
patients with non-squamous non-small cell lung cancer: A
multicentre, retrospective cohort study. Eur J Cancer. 150:63–72.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ono K, Ono H, Toi Y, Sugisaka J, Aso M,
Saito R, Kawana S, Aiba T, Odaka T, Matsuda S, et al: Association
of immune-related pneumonitis with clinical benefit of
anti-programmed cell death-1 monotherapy in advanced non-small cell
lung cancer. Cancer Med. 10:4796–4804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu X, Zhao J, Zhou J, Zhou F, Jiang T,
Jiang S, Sun X, You X, Wu F, Ren S, et al: Association of baseline
peripheral-blood eosinophil count with immune checkpoint
inhibitor-related pneumonitis and clinical outcomes in patients
with non-small cell lung cancer receiving immune checkpoint
inhibitors. Lung Cancer. 150:76–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui P, Huang D, Wu Z, Tao H, Zhang S, Ma
J, Liu Z, Wang J, Huang Z, Chen S, et al: Association of
immune-related pneumonitis with the efficacy of PD-1/PD-L1
inhibitors in non-small cell lung cancer. Ther Adv Med Oncol.
12:17588359209220332020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang A, Xu Y, Zang X, Wu C, Gao J, Sun X,
Xie M, Ma X, Deng H, Song J, et al: Radiographic features and
prognosis of early- and late-onset non-small cell lung cancer
immune checkpoint inhibitor-related pneumonitis. BMC Cancer.
21:6342021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamagata A, Yokoyama T, Fukuda Y and
Ishida T: Impact of interstitial lung disease associated with
immune checkpoint inhibitors on prognosis in patients with
non-small-cell lung cancer. Cancer Chemother Pharmacol. 87:251–258.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao Y, Zhou J, Hsu S, Ding N, Li J, Zhang
Y, Xu X, Tang X, Wei T, Zhu Z, et al: Risk factors for immune
checkpoint inhibitor-related pneumonitis in non-small cell lung
cancer. Transl Lung Cancer Res. 11:295–306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suresh K, Voong KR, Shankar B, Forde PM,
Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL,
et al: Pneumonitis in non-small cell lung cancer patients receiving
immune checkpoint immunotherapy: incidence and risk factors. J
Thorac Oncol. 13:1930–1939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee
YJ, Cho YJ, Yoon HI, Lee JH, Lee CT and Park JS: Characteristics,
incidence, and risk factors of immune checkpoint inhibitor-related
pneumonitis in patients with non-small cell lung cancer. Lung
Cancer. 125:150–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Shao C, Li S, Xu Y, Xu K, Zhang Y,
Huang H, Wang M and Xu Z: Programmed cell death 1 (PD-1)/PD-ligand
1(PD-L1) inhibitors-related pneumonitis in patients with advanced
non-small cell lung cancer. Asia Pac J Clin Oncol. 16:299–304.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Tang L, Zhou Y, He W and Li W:
Immune checkpoint inhibitor-associated pneumonitis in non-small
cell lung cancer: Current understanding in characteristics,
diagnosis, and management. Front Immunol. 12:6639862021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Gao F, Jin S, Gao W, Chen S and
Guo R: Checkpoint inhibitor pneumonitis in Chinese lung cancer
patients: Clinical characteristics and risk factors. Ann Palliat
Med. 9:3957–3965. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim ST, Sheshadri A, Shannon V,
Kontoyiannis DP, Kantarjian H, Garcia-Manero G, Ravandi F, Im JS,
Boddu P, Bashoura L, et al: Distinct immunophenotypes of T cells in
bronchoalveolar lavage fluid from leukemia patients with immune
checkpoint inhibitors-related pulmonary complications. Front
Immunol. 11:5904942021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Zhao Y, Zhang X, Si X, Song P,
Xiao Y, Yang X, Song L, Shi J, Zhao H and Zhang L: Clinical
characteristics and management of immune checkpoint
inhibitor-related pneumonitis: A single-institution retrospective
study. Cancer Med. 10:188–198. 2021. View Article : Google Scholar
|
|
41
|
Nishino M, Ramaiya NH, Awad MM, Sholl LM,
Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF and Hodi FS: PD-1
inhibitor-related pneumonitis in advanced cancer patients:
Radiographic patterns and clinical course. Clin Cancer Res.
22:6051–6060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tone M, Izumo T, Awano N, Kuse N, Inomata
M, Jo T, Yoshimura H, Minami J, Takada K, Miyamoto S and Kunitoh H:
High mortality and poor treatment efficacy of immune checkpoint
inhibitors in patients with severe grade checkpoint inhibitor
pneumonitis in non-small cell lung cancer. Thorac Cancer.
10:2006–2012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suresh K, Naidoo J, Lin CT and Danoff S:
Immune checkpoint immunotherapy for non-small cell lung cancer:
Benefits and pulmonary toxicities. Chest. 154:1416–1423. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schildberg FA, Klein SR, Freeman GJ and
Sharpe AH: Coinhibitory pathways in the B7‑CD28 ligand‑receptor
family. Immunity. 44:955–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qureshi OS, Zheng Y, Nakamura K, Attridge
K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z,
et al: Trans-endocytosis of CD80 and CD86: A molecular basis for
the cell-extrinsic function of CTLA-4. Science. 332:600–603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baumeister SH, Freeman GJ, Dranoff G and
Sharpe AH: Coinhibitory pathways in immunotherapy for cancer. Annu
Rev Immunol. 34:539–573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang SP, Gergich K, Lubiniecki GM, de
Alwis DP, Chen C, Tice MAB and Rubin EH: Pembrolizumab KEYNOTE-001:
An adaptive study leading to accelerated approval for two
indications and a companion diagnostic. Ann Oncol. 28:1388–1398.
2017. View Article : Google Scholar :
|
|
50
|
Suresh K, Naidoo J, Zhong Q, Xiong Y,
Mammen J, de Flores MV, Cappelli L, Balaji A, Palmer T, Forde PM,
et al: The alveolar immune cell landscape is dysregulated in
checkpoint inhibitor pneumonitis. J Clin Invest. 129:4305–4315.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Läubli H, Koelzer VH, Matter MS, Herzig P,
Dolder Schlienger B, Wiese MN, Lardinois D, Mertz KD and Zippelius
A: The T cell repertoire in tumors overlaps with pulmonary
inflammatory lesions in patients treated with checkpoint
inhibitors. Oncoimmunology. 7:e13863622017. View Article : Google Scholar
|
|
52
|
Rowshanravan B, Halliday N and Sansom DM:
CTLA-4: A moving target in immunotherapy. Blood. 131:58–67. 2018.
View Article : Google Scholar
|
|
53
|
Naidoo J, Cottrell TR, Lipson EJ, Forde
PM, Illei PB, Yarmus LB, Voong KR, Feller-Kopman D, Lee H, Riemer
J, et al: Chronic immune checkpoint inhibitor pneumonitis. J
Immunother Cancer. 8:e0008402020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Subudhi SK, Aparicio A, Gao J, Zurita AJ,
Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L,
et al: Clonal expansion of CD8 T cells in the systemic circulation
precedes development of ipilimumab-induced toxicities. Proc Natl
Acad Sci USA. 113:11919–11924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ivanova EA and Orekhov AN: T helper
lymphocyte subsets and plasticity in autoimmunity and cancer: An
overview. Biomed Res Int. 2015:3274702015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee J, Lozano-Ruiz B, Yang FM, Fan DD,
Shen L and González-Navajas JM: The multifaceted role of Th1, Th9,
and Th17 cells in immune checkpoint inhibition therapy. Front
Immunol. 12:6256672021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dejima H, Hu X, Chen R, Zhang J, Fujimoto
J, Parra ER, Haymaker C, Hubert SM, Duose D, Solis LM, et al:
Immune evolution from preneoplasia to invasive lung adenocarcinomas
and underlying molecular features. Nat Commun. 12:27222021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dulos J, Carven GJ, van Boxtel SJ, Evers
S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P,
Punt CJ, et al: PD-1 blockade augments Th1 and Th17 and suppresses
Th2 responses in peripheral blood from patients with prostate and
advanced melanoma cancer. J Immunother. 35:169–178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yoshino K, Nakayama T, Ito A, Sato E and
Kitano S: Severe colitis after PD-1 blockade with nivolumab in
advanced melanoma patients: potential role of Th1-dominant immune
response in immune-related adverse events: Two case reports. BMC
Cancer. 19:10192019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang YN, Lou DF, Li DY, Jiang W, Dong JY,
Gao W and Chen HC: Elevated levels of IL-17A and IL-35 in plasma
and bronchoalveolar lavage fluid are associated with checkpoint
inhibitor pneumonitis in patients with non-small cell lung cancer.
Oncol Lett. 20:611–622. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Passat T, Touchefeu Y, Gervois N, Jarry A,
Bossard C and Bennouna J: Physiopathological mechanisms of
immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and
anti-PD-L1 antibodies in cancer treatment. Bull Cancer.
105:1033–1041. 2018.In French. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Franken A, Van Mol P, Vanmassenhove S,
Donders E, Schepers R, Van Brussel T, Dooms C, Yserbyt J, De Crem
N, Testelmans D, et al: Single-cell transcriptomics identifies
pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in
immune checkpoint inhibitor-related pneumonitis. J Immunother
Cancer. 10:e0053232022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gianchecchi E and Fierabracci A:
Inhibitory receptors and pathways of lymphocytes: The role of PD-1
in treg development and their involvement in autoimmunity onset and
cancer progression. Front Immunol. 9:23742018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
D'Alessio FR, Tsushima K, Aggarwal NR,
West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM,
McDyer JF and King LS: CD4+CD25+Foxp3+ Tregs resolve experimental
lung injury in mice and are present in humans with acute lung
injury. J Clin Invest. 119:2898–2913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin X, Deng J, Deng H, Yang Y, Sun N, Zhou
M, Qin Y, Xie X, Li S, Zhong N, et al: Comprehensive analysis of
the immune microenvironment in checkpoint inhibitor pneumonitis.
Front Immunol. 12:8184922022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Jia X, Du Y, Mao Z, Zhang Y, Shen Y,
Sun H, Liu M, Niu G, Wang J, et al: Eosinophil as a biomarker for
diagnosis, prediction, and prognosis evaluation of severe
checkpoint inhibitor pneumonitis. Front Oncol. 12:8271992022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kowalski B, Valaperti A, Bezel P, Steiner
UC, Scholtze D, Wieser S, Vonow-Eisenring M, Widmer A, Kohler M and
Franzen D: Analysis of cytokines in serum and bronchoalveolar
lavage fluid in patients with immune-checkpoint
inhibitor-associated pneumonitis: A cross-sectional case-control
study. J Cancer Res Clin Oncol. 148:1711–1720. 2022. View Article : Google Scholar
|
|
69
|
Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin
Q, Wu G, Cui J, Chang J, Cheng Y, et al: Sugemalimab versus placebo
after concurrent or sequential chemoradiotherapy in patients with
locally advanced, unresectable, stage III non-small-cell lung
cancer in China (GEMSTONE-301): Interim results of a randomised,
double-blind, multicentre, phase 3 trial. Lancet Oncol. 23:209–219.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lim SY, Lee JH, Gide TN, Menzies AM,
Guminski A, Carlino MS, Breen EJ, Yang JYH, Ghazanfar S, Kefford
RF, et al: Circulating cytokines predict immune-related toxicity in
melanoma patients receiving anti-PD-1-based immunotherapy. Clin
Cancer Res. 25:1557–1563. 2019. View Article : Google Scholar
|
|
71
|
Martins F, Sykiotis GP, Maillard M, Fraga
M, Ribi C, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F,
et al: New therapeutic perspectives to manage refractory immune
checkpoint-related toxicities. Lancet Oncol. 20:e54–e64. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S,
Xie X, Liu M, Xie Z, Qin Y, et al: Peripheral blood biomarkers for
early diagnosis, severity, and prognosis of checkpoint
inhibitor-related pneumonitis in patients with lung cancer. Front
Oncol. 11:6988322021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou C, Yang Y, Lin X, Fang N, Chen L,
Jiang J, Deng H, Deng Y, Wan M, Qiu G, et al: Proposed clinical
phases for the improvement of personalized treatment of checkpoint
inhibitor-related pneumonitis. Front Immunol. 13:9357792022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Iwanaga N and Kolls JK: Updates on T
helper type 17 immunity in respiratory disease. Immunology.
156:3–8. 2019. View Article : Google Scholar
|
|
77
|
Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma
YG, Wang XX, Liu HZ, Sun W and Hu ZW: Blocking IL-17A promotes the
resolution of pulmonary inflammation and fibrosis via
TGF-beta1-dependent and -independent mechanisms. J Immunol.
187:3003–3014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
McAlees JW, Lajoie S, Dienger K, Sproles
AA, Richgels PK, Yang Y, Khodoun M, Azuma M, Yagita H, Fulkerson
PC, et al: Differential control of CD4(+) T-cell subsets by the
PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur J Immunol.
45:1019–1029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tarhini AA, Zahoor H, Lin Y, Malhotra U,
Sander C, Butterfield LH and Kirkwood JM: Baseline circulating
IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of
relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother
Cancer. 3:392015. View Article : Google Scholar
|
|
81
|
Kolb M, Margetts PJ, Anthony DC, Pitossi F
and Gauldie J: Transient expression of IL-1beta induces acute lung
injury and chronic repair leading to pulmonary fibrosis. J Clin
Invest. 107:1529–1536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen Z and He J: Infliximab in the
treatment of tislelizumab-induced steroid-refractory immune
checkpoint inhibitor-related pneumonia: Case report and literature
review. Transl Cancer Res. 11:3309–3314. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shamskhou EA, Kratochvil MJ, Orcholski ME,
Nagy N, Kaber G, Steen E, Balaji S, Yuan K, Keswani S, Danielson B,
et al: Hydrogel-based delivery of Il-10 improves treatment of
bleomycin-induced lung fibrosis in mice. Biomaterials. 203:52–62.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Osuna-Gómez R, Barril S, Mulet M, Zamora
Atenza C, Millan-Billi P, Pardessus A, Brough DE, Sabzevari H,
Semnani RT, Castillo D and Vidal S: The immunoregulatory role of
IL-35 in patients with interstitial lung disease. Immunology.
168:610–621. 2023. View Article : Google Scholar
|
|
85
|
de Waal Malefyt R, Haanen J, Spits H,
Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H
and de Vries JE: Interleukin 10 (IL-10) and viral IL-10 strongly
reduce antigen-specific human T cell proliferation by diminishing
the antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Okada K, Fujimura T, Kikuchi T, Aino M,
Kamiya Y, Izawa A, Iwamura Y, Goto H, Okabe I, Miyake E, et al:
Effect of interleukin (IL)-35 on IL-17 expression and production by
human CD4+ T cells. PeerJ. 5:e29992017. View Article : Google Scholar
|
|
87
|
Wang HM, Zhang XH, Feng MM, Qiao YJ, Ye
LQ, Chen J, Fan FF and Guo LL: Interleukin-35 suppresses the
antitumor activity of T cells in patients with non-small cell lung
cancer. Cell Physiol Biochem. 47:2407–2419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Castellani ML, Anogeianaki A, Felaco P,
Toniato E, De Lutiis MA, Shaik B, Fulcheri M, Vecchiet J, Tetè S,
Salini V, et al: IL-35, an anti-inflammatory cytokine which expands
CD4+CD25+ Treg cells. J Biol Regul Homeost Agents. 24:131–135.
2010.PubMed/NCBI
|
|
89
|
Wang H, Zhou F, Zhao C, Cheng L, Zhou C,
Qiao M, Li X and Chen X: Interleukin-10 is a promising marker for
immune-related adverse events in patients with non-small cell lung
cancer receiving immunotherapy. Front Immunol. 13:8403132022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Toi Y, Sugawara S, Sugisaka J, Ono H,
Kawashima Y, Aiba T, Kawana S, Saito R, Aso M, Tsurumi K, et al:
Profiling preexisting antibodies in patients treated with anti-PD-1
therapy for advanced non-small cell lung cancer. JAMA Oncol.
5:376–383. 2019. View Article : Google Scholar
|
|
91
|
Zhou J, Zhao J, Jia Q, Chu Q, Zhou F, Chu
X, Zhao W, Ren S, Zhou C and Su C: Peripheral blood autoantibodies
against to tumor-associated antigen predict clinical outcome to
immune checkpoint inhibitor-based treatment in advanced non-small
cell lung cancer. Front Oncol. 11:6255782021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tahir SA, Gao J, Miura Y, Blando J,
Tidwell RSS, Zhao H, Subudhi SK, Tawbi H, Keung E, Wargo J, et al:
Autoimmune antibodies correlate with immune checkpoint
therapy-induced toxicities. Proc Natl Acad Sci USA.
116:22246–22251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chaput N, Lepage P, Coutzac C, Soularue E,
Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et
al: Baseline gut microbiota predicts clinical response and colitis
in metastatic melanoma patients treated with ipilimumab. Ann Oncol.
28:1368–1379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yamaguchi T, Shimizu J, Hasegawa T, Horio
Y, Inaba Y, Yatabe Y and Hida T: Pre-existing pulmonary fibrosis is
a risk factor for anti-PD-1-related pneumonitis in patients with
non-small cell lung cancer: A retrospective analysis. Lung Cancer.
125:212–217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kanai O, Kim YH, Demura Y, Kanai M, Ito T,
Fujita K, Yoshida H, Akai M, Mio T and Hirai T: Efficacy and safety
of nivolumab in non-small cell lung cancer with preexisting
interstitial lung disease. Thorac Cancer. 9:847–855. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang M, Fan Y, Nie L, Wang G, Sun K and
Cheng Y: Clinical outcomes of immune checkpoint inhibitor therapy
in patients with advanced non-small cell lung cancer and
preexisting interstitial lung diseases: A systematic review and
meta-analysis. Chest. 161:1675–1686. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tasaka Y, Honda T, Nishiyama N, Tsutsui T,
Saito H, Watabe H, Shimaya K, Mochizuki A, Tsuyuki S, Kawahara T,
et al: Non-inferior clinical outcomes of immune checkpoint
inhibitors in non-small cell lung cancer patients with interstitial
lung disease. Lung Cancer. 155:120–126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fujimoto D, Yomota M, Sekine A, Morita M,
Morimoto T, Hosomi Y, Ogura T, Tomioka H and Tomii K: Nivolumab for
advanced non-small cell lung cancer patients with mild idiopathic
interstitial pneumonia: A multicenter, open-label single-arm phase
II trial. Lung Cancer. 134:274–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yamaguchi O, Kaira K, Shinomiya S, Mouri
A, Hashimoto K, Shiono A, Miura Y, Akagami T, Imai H, Kobayashi K
and Kagamu H: Pre-existing interstitial lung disease does not
affect prognosis in non-small cell lung cancer patients with PD-L1
expression ≥50% on first-line pembrolizumab. Thorac Cancer.
12:304–313. 2021. View Article : Google Scholar
|
|
100
|
Lu Y, Zhong W, Liu Y, Chen W, Zhang J,
Zeng Z, Huang H, Qiao Y, Wan X, Meng X, et al: Anti-PD-L1 antibody
alleviates pulmonary fibrosis by inducing autophagy via inhibition
of the PI3K/Akt/mTOR pathway. Int Immunopharmacol. 104:1085042022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sul J, Blumenthal GM, Jiang X, He K,
Keegan P and Pazdur R: FDA approval summary: Pembrolizumab for the
treatment of patients with metastatic non-small cell lung cancer
whose tumors express programmed death-ligand 1. Oncologist.
21:643–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo
H and Zhu H: The mechanism and risk factors for immune checkpoint
inhibitor pneumonitis in non-small cell lung cancer patients.
Cancer Biol Med. 17:599–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Galant-Swafford J, Troesch A, Tran L,
Weaver A, Doherty TA and Patel SP: Landscape of immune-related
pneumonitis in cancer patients with asthma being treated with
immune checkpoint blockade. Oncology. 98:123–130. 2020. View Article : Google Scholar
|
|
104
|
Barrón F, Sánchez R, Arroyo-Hernández M,
Blanco C, Zatarain-Barrón ZL, Catalán R, Ramos-Ramírez M, Cardona
AF, Flores-Estrada D and Arrieta O: Risk of developing checkpoint
immune pneumonitis and its effect on overall survival in non-small
cell lung cancer patients previously treated with radiotherapy.
Front Oncol. 10:5702332020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang
F, Han C, Long Y, Li Y, Zheng X, et al: Risk factors for
pneumonitis in patients treated with anti-programmed death-1
therapy: A case-control study. Cancer Med. 7:4115–4120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Voong KR, Hazell SZ, Fu W, Hu C, Lin CT,
Ding K, Suresh K, Hayman J, Hales RK, Alfaifi S, et al:
Relationship between prior radiotherapy and checkpoint-inhibitor
pneumonitis in patients with advanced non-small-cell lung cancer.
Clin Lung Cancer. 20:e470–e479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lin GF, Xu Y, Lin H, Yang DY, Chen L,
Huang LL, Su XS, Xu YX and Zeng YM: The association between the
incidence risk of pneumonitis and PD-1/PD-L1 inhibitors in advanced
NSCLC: A meta-analysis of randomized controlled trials. Int
Immunopharmacol. 99:1080112021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang M, Liang H, Wang W, Zhao S, Cai X,
Zhao Y, Li C, Cheng B, Xiong S, Li J, et al: Immune-related adverse
events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1
inhibitor alone in first-line treatment for advanced non-small cell
lung cancer: A meta-analysis of randomized control trials. Cancer.
127:777–786. 2021. View Article : Google Scholar
|
|
110
|
Matsuo N, Azuma K, Kojima T, Ishii H,
Tokito T, Yamada K and Hoshino T: Comparative incidence of
immune-related adverse events and hyperprogressive disease in
patients with non-small cell lung cancer receiving immune
checkpoint inhibitors with and without chemotherapy. Invest New
Drugs. 39:1150–1158. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Oshima Y, Tanimoto T, Yuji K and Tojo A:
EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated
patients with non-small cell lung cancer. JAMA Oncol. 4:1112–1115.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oxnard GR, Yang JCH, Yu H, Kim SW, Saka H,
Horn L, Goto K, Ohe Y, Mann H, Thress KS, et al: TATTON: A
multi-arm, phase Ib trial of osimertinib combined with selumetinib,
savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol.
31:507–516. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Khunger M, Rakshit S, Pasupuleti V,
Hernandez AV, Mazzone P, Stevenson J, Pennell NA and Velcheti V:
Incidence of pneumonitis with use of programmed death 1 and
programmed death-ligand 1 inhibitors in non-small cell lung cancer:
A systematic review and meta-analysis of trials. Chest.
152:271–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Balasubramanian A, Onggo J, Gunjur A, John
T and Parakh S: Immune checkpoint inhibition with chemoradiotherapy
in stage III non-small-cell lung cancer: A systematic review and
meta-analysis of safety results. Clin Lung Cancer. 22:74–82. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gomatou G, Tzilas V, Kotteas E, Syrigos K
and Bouros D: Immune checkpoint inhibitor-related pneumonitis.
Respiration. 99:932–942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li M, Spakowicz D, Zhao S, Patel SH, Johns
A, Grogan M, Miah A, Husain M, He K, Bertino EM, et al: Brief
report: inhaled corticosteroid use and the risk of checkpoint
inhibitor pneumonitis in patients with advanced cancer. Cancer
Immunol Immunother. 69:2403–2408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhou P, Zhao X and Wang G: Risk factors
for immune checkpoint inhibitor-related pneumonitis in cancer
patients: A systemic review and meta-analysis. Respiration.
101:1035–1050. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sawa K, Sato I, Takeuchi M and Kawakami K:
Risk of pneumonitis in non-small cell lung cancer patients with
preexisting interstitial lung diseases treated with immune
checkpoint inhibitors: A nationwide retrospective cohort study.
Cancer Immunol Immunother. 72:591–598. 2023. View Article : Google Scholar
|
|
119
|
Suresh K, Psoter KJ, Voong KR, Shankar B,
Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, et
al: Impact of checkpoint inhibitor pneumonitis on survival in NSCLC
patients receiving immune checkpoint immunotherapy. J Thorac Oncol.
14:494–502. 2019. View Article : Google Scholar
|
|
120
|
Reuss JE, Brigham E, Psoter KJ, Voong KR,
Shankar B, Ettinger DS, Marrone KA, Hann CL, Levy B, Feliciano JL,
et al: Pretreatment lung function and checkpoint inhibitor
pneumonitis in NSCLC. JTO Clin Res Rep. 2:1002202021.PubMed/NCBI
|
|
121
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S and Larkin J: Management of toxicities from
immunotherapy: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28(Suppl 4): iv119–iv142. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the Society for
Immunotherapy of Cancer (SITC) toxicity management working group. J
Immunother Cancer. 5:952017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Stroud CR, Hegde A, Cherry C, Naqash AR,
Sharma N, Addepalli S, Cherukuri S, Parent T, Hardin J and Walker
P: Tocilizumab for the management of immune mediated adverse events
secondary to PD-1 blockade. J Oncol Pharm Pract. 25:551–557. 2019.
View Article : Google Scholar
|
|
125
|
Karayama M, Inui N, Inoue Y, Yasui H,
Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Asada K, et
al: Six-week oral prednisolone therapy for immune-related
pneumonitis: A single-arm phase II study. J Immunother Cancer.
11:e0070562023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Luo J, Beattie JA, Fuentes P, Rizvi H,
Egger JV, Kern JA, Leung DYM, Lacouture ME, Kris MG, Gambarin M, et
al: Beyond steroids: Immunosuppressants in steroid-refractory or
resistant immune-related adverse events. J Thorac Oncol.
16:1759–1764. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Balaji A, Hsu M, Lin CT, Feliciano J,
Marrone K, Brahmer JR, Forde PM, Hann C, Zheng L, Lee V, et al:
Steroid-refractory PD-(L)1 pneumonitis: Incidence, clinical
features, treatment, and outcomes. J Immunother Cancer.
9:e0017312021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Beattie J, Rizvi H, Fuentes P, Luo J,
Schoenfeld A, Lin IH, Postow M, Callahan M, Voss MH, Shah NJ, et
al: Success and failure of additional immune modulators in
steroid-refractory/resistant pneumonitis related to immune
checkpoint blockade. J Immunother Cancer. 9:e0018842021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Camard M, Besse B, Cariou PL, Massayke S,
Laparra A, Noel N, Michot JM, Ammari S, Pavec JL and Lambotte O:
Prevalence and outcome of steroid-resistant/refractory pneumonitis
induced by immune checkpoint inhibitors. Respir Med Res.
82:1009692022.PubMed/NCBI
|
|
130
|
Xie XH, Deng HY, Lin XQ, Wu JH, Liu M, Xie
ZH, Qin YY and Zhou CZ: Case report: Nintedanib for
pembrolizumab-related pneumonitis in a patient with non-small cell
lung cancer. Front Oncol. 11:6738772021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gu J, Shi L, Jiang X, Wen J, Zheng X, Cai
H and Zhang W: Severe immune-related adverse events of immune
checkpoint inhibitors for advanced non-small cell lung cancer: A
network meta-analysis of randomized clinical trials. Cancer Immunol
Immunother. 71:2239–2254. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hailemichael Y, Johnson DH, Abdel-Wahab N,
Foo WC, Bentebibel SE, Daher M, Haymaker C, Wani K, Saberian C,
Ogata D, et al: Interleukin-6 blockade abrogates immunotherapy
toxicity and promotes tumor immunity. Cancer Cell. 40:509–523.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Renga G, Bellet MM, Pariano M, Gargaro M,
Stincardini C, D'Onofrio F, Mosci P, Brancorsini S, Bartoli A,
Goldstein AL, et al: Thymosin α1 protects from CTLA-4 intestinal
immunopathology. Life Sci Alliance. 3:e2020006622020. View Article : Google Scholar
|
|
134
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as first-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen
G, Zhang L, Huang D, Cang S, Yang Z, et al: Sintilimab plus
platinum and gemcitabine as first-line treatment for advanced or
metastatic squamous NSCLC: Results from a randomized, double-blind,
phase 3 trial (ORIENT-12). J Thorac Oncol. 16:1501–1511. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar
|
|
142
|
Sezer A, Kilickap S, Gümüş M, Bondarenko
I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov
O, et al: Cemiplimab monotherapy for first-line treatment of
advanced non-small-cell lung cancer with PD-L1 of at least 50%: A
multicentre, open-label, global, phase 3, randomised, controlled
trial. Lancet. 397:592–604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Herbst RS, Giaccone G, de Marinis F,
Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z,
Geater S, et al: Atezolizumab for first-line treatment of
PD-L1-selected patients with NSCLC. N Engl J Med. 383:1328–1339.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Nishio M, Barlesi F, West H, Ball S,
Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S,
Orlandi F, et al: Atezolizumab plus chemotherapy for first-line
treatment of nonsquamous NSCLC: Results From the randomized phase 3
IMpower132 trial. J Thorac Oncol. 16:653–664. 2021. View Article : Google Scholar
|
|
146
|
Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R,
Yu Y, Yao W, Chang J, Chen J, et al: Sugemalimab versus placebo, in
combination with platinum-based chemotherapy, as first-line
treatment of metastatic non-small-cell lung cancer (GEMSTONE-302):
Interim and final analyses of a double-blind, randomised, phase 3
clinical trial. Lancet Oncol. 23:220–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar
|
|
148
|
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok
T, Zhang L, Tu HY, Wu L, Feng J, et al: Nivolumab versus docetaxel
in a predominantly Chinese patient population with previously
treated advanced NSCLC: CheckMate 078 randomized phase III clinical
trial. J Thorac Oncol. 14:867–875. 2019. View Article : Google Scholar : PubMed/NCBI
|