Introduction
Cervical carcinoma is the most malignant and
life-threatening type of cancer in women. Indeed, as the fourth
most common malignant tumor worldwide. According to the World
Health Organization (WHO), in 2020, there were a total of 604,000
new cases and 342,000 deaths. In 2015, it was estimated that there
were 111,000 new cases of cervical cancer in China, with an
estimated death toll of ~ 33,800 individuals (1), highlighting the treatment of cervical
cancer is of great importance (2,3), and
research focused on its pathogenesis and therapeutic targets has
generated interest in various fields (4).
Several programmed cell death mechanisms, such as
apoptosis, have been investigated as important mechanisms of
anticancer defense (4). However,
the association between pyroptosis and cancer remains to be further
clarified. Pyroptosis is a form of programmed cell death;
morphological changes involve cells continuously expanding until
the membrane ruptures, resulting in the release of cellular
contents, which triggers a strong inflammatory response (5). The specific process of pyroptosis
includes stimulation by pathogens, followed by recognition of these
signals by intracellular NOD-like receptor (NLR) and activation of
caspase-1 through the binding of the adaptor protein
apoptosis-associated speck-like protein containing a caspase
recruitment domain (ASC) to pro-caspase-1 (6). Subsequently, activated caspase-1
cleaves gasdermin (GSDM) D, thus forming holes in cell membrane and
releasing cellular contents, which eventually induces pyroptosis
(7,8). In addition, pyroptosis is
characterized by the production of activated IL-1β and IL-18, which
are released to the extracellular space, causing an inflammatory
response (9,10). The expression of NLR protein 3
(NLRP3)-related downstream molecules, including ASC, caspase-1,
IL-1β and IL-18, in atherosclerotic plaques is significantly higher
compared with that in non-atherosclerotic vessels; the upregulation
of their expression levels is closely associated with plaque
fragility, suggesting that pyroptosis activation mediates the
evolution of atherosclerotic lesions (11). It has also been reported that
pyroptosis may contribute to cancer prevention and treatment
(12). A study exploring the
mechanism of gastric cancer chemotherapy demonstrated that GSDME
can convert chemotherapy-induced apoptosis into pyroptosis to
achieve therapeutic purposes (13). Additionally,
α-naphthoyl-ethyltrimethylammonium represents a potential novel
antitumor molecule, as it has been reported to induce pyroptosis of
epithelial ovarian cancer cells (14). Based on these studies, it appears
that pyroptosis may be of value in the treatment of cancer.
Comprehensive treatment of cervical cancer is mainly
based on surgery and radiotherapy, supplemented by chemotherapy in
clinical practice (15).
Considering the fertility requirements, drug tolerance and side
effects of traditional treatments, novel strategies against
cervical cancer require further studies on potential molecular
targets. Long non-coding RNAs (lncRNAs) are a class of RNA
molecules with transcripts >200 nucleotide in length that are
usually involved in protein-coding gene regulation at the
epigenetic, transcriptional and post-transcriptional levels.
Numerous lncRNAs have been reported to be involved in several
physiological and pathological mechanisms, particularly malignant
tumorigenesis (16). For example,
lncRNA plasmacytoma variant translocation 1 is closely associated
with myelocytomatosis progression and may represent a therapeutic
target (17). lncRNA
metastasis-associated lung adenocarcinoma transcript 1
(MALAT1) was initially recognized in non-small cell lung
cancer (NSCLC) and is one of the most studied lncRNAs in cancer
owing to its high abundance and conservation. For example,
MALAT1 suppresses breast cancer metastasis by targeting TEA
domain family members (18). In
addition, lncRNA MALAT1 can promote tumorigenesis in
colorectal, ovarian and gallbladder cancer (19–21).
In addition, lncRNA MALAT1 was shown to regulate downstream
microRNAs (miRNAs/miRs) by binding complementary sequences,
including miR-129-5p, and the miR-129-5p/high mobility group box
protein 1 axis, during colon cancer development (22), miR-200a during lung cancer cell
proliferation (23) and miR-124,
and the miR-124/calpain small subunit 1 axis, in nasopharyngeal
carcinoma (24). Although lncRNA
MALAT1 serves a crucial role in regulating cancer
progression, including cell proliferation, invasion (19) and epithelial-to-mesenchymal
transition (25), limited research
has been conducted on the role of lncRNA MALAT1 in
pyroptosis (26), particularly on
cervical tumorigenesis. The present study hypothesized that lncRNA
MALAT1 is the key mediator on pyroptosis during cervical
cancer, regulating tumor progression. The present study aimed to
elucidate the potential mechanism underlying lncRNA MALAT1
during cervical cancer and to offer important insights into
pyroptosis.
Materials and methods
Human subjects
A total of 11 patients (age, 48–62 years) with newly
diagnosed cervical carcinoma, who received treatment at the First
Affiliated Hospital of Harbin Medical University (Harbin, China)
between January 2019 and August 2020, were enrolled in the present
study. Ethical approval was obtained from the Ethics Committee of
the First Affiliated Hospital of Harbin Medical University
(approval no. IRB-AF/SC-04/02.0; 11 November 2020); tumor samples
were collected from the patients upon written informed consent.
Human carcinoma and paracarcinoma tissues (1–5 cm away from
carcinoma tissue) were collected for the culture of isolated
primary cells.
Animals
The experimental protocols involving animals were
approved by the Animal Ethical Care Committee of Qiqihar Medical
University (Qiqihar, China; approval no. QMU-AECC-2020-43; 8 May
2020) and complied with the Guide for the Use and Care of
Laboratory Animals published by the USA National Institutes of
Health (NIH Publication No. 85–23, revised 1996) (27). BALB/c nude female mice (n=10; age,
6 weeks; weight, 18–22 g) were provided by the Animal Center of
Qiqihar Medical University and were housed in a dedicated room with
a 12-h dark/light cycle, controlled temperature (22±1°C) and
constant humidity (55±5%); mice had free access to a standard diet
and water. After 1 week of acclimatization, 2×106 U14
mouse cervical carcinoma cells (BeNa Culture Collection; Beijing
Beina Chunglian Institute of Biotechnology) were intraperitoneally
(i.p.) injected in the C57BL/6 mice to prepare the U14 ascites
tumor cells; cells were allowed to grow for 7 days, ensuring the
abdominal ascites did not exceed 20% body weight (endpoint weight,
20.3–24.2 g). The abdominal ascites were collected as follows: i)
Mice were anesthetized with 150 mg/kg tribromoethanol i.p. (cat.
no. M2910; Nanjing Aibei Biotechnology Co., Ltd.) until loss of
corneal reflex, muscle tightness and response to skin pinch; ii)
the abdomen was swabbed with 75% alcohol, a small incision was made
in the center of the skin overlying the peritoneal wall and the
skin was firmly pulled to expose the peritoneal wall; iii) i) a 25
G needle was inserted into the peritoneal membrane, avoiding
inserting needle into intestine or bladder, and 5 ml saline was
injected into the peritoneal cavity; iv) the abdomen was massaged
for approximately 10–15 sec, then the needle was withdrawn slowly;
v) using one hand, the fluid was pushed to one side of the
peritoneum and, with the other hand, the needle was inserted into
the side of cavity with plenty of fluid and as much of the fluid as
possible was withdrawn from the peritoneum; vi) the needle was
removed, the contents of the syringe were dispensed into a
centrifuge tube on ice, which was then centrifuged at 4°C, 183 × g
for 5 min to collect cell pellet; vii) the U14 ascites tumor cells
were resuspended and passaged for 10 days. The U14 cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fishers Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. Following collection of ascetic fluid
the mice were euthanized with pentobarbital sodium (100 mg/kg;
i.p.).
The U14 cervical cells were subsequently divided
into two groups and transfected as follows: i) Short hairpin
(sh)RNA negative control (sh-Control) and ii) shRNA against lncRNA
MALAT1 (sh-MALAT1). The effectiveness of transfection was
confirmed as shown in Fig. S1.
The tumor cell density was adjusted to 1×107 cells/ml
using normal saline. The right subaxillary skin of the mice was
disinfected and inoculated with 0.2 ml U14 cervical cancer cell
suspension for the establishment of 20 nude mice bearing U14
cervical tumor xenografts (n=10 mice/group). After 4 weeks, the
maximum tumor diameter was 14 mm; tumors were excised after
euthanasia using 100 mg/kg pentobarbital injection. The tumor
volume was calculated as the follows: Tumor volume=(length ×
width2)/2. The tumor index included tumor diameter
(range, 0.7–1.4 cm), volume (range. 0.1–0.726 cm3) and
weight (range, 0.12–0.62 g).
Isolated primary cell culture
Carcinoma and paracarcinoma tissues were isolated
from four patients with cervical cancer and prepared for culture
according to the following steps: i) Tissue block was placed into a
pre-cooled sterile culture medium on ice at room temperature for 5
min; ii) tissue was were washed at room temperature with
non-FBS-containing DMEM for 3 times under and cut; iii) tissue was
enzymatically dissociated by the addition of 0.05% trypsin-EDTA for
overnight at 4°C; iv) rinsed with 0.2% collagenase I for 10 min and
collected the cell supernatant at room temperature v) repeated the
step iv until none tissue coarse left; v) cell supernatant was
filtered with 100 µm mesh filter and centrifuged at 183 × g for 10
min at 4°C, and the supernatant was discarded; viii) the cell
pellet was resuspended in fresh culture medium; and ix) cells were
incubated overnight at 37°C in a humidified air atmosphere
containing 5% CO2; x) Upon attainment of dense culture
(after 2–3 weeks of primary culture), cells were passaged by
mechanical dissociation via trituration with a Pasteur pipet, and
transferred into separated culture medium containing plasks at room
temperature (28). The culture
medium used was DMEM with high glucose containing 5% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 1% antibiotics and HEPES (10 mM).
The viability of primary cervical cancer cells was evaluated by
trypan blue assay; the number of live cells was >70%.
HeLa cell culture
The HeLa cervical cancer cell line was obtained from
Shanghai Institutes for Biological Sciences and was cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplied with 10%
FBS and 1% penicillin/streptomycin (100 µg/ml) in 5% CO2
at 37°C.
Lipopolysaccharide (LPS)
treatment
Before treatment, cells (1×106 cells/ml)
were seeded and serum-starved with FBS-free restriction treatment
medium overnight, according to the study by Qiu et al
(29). LPS (Beijing Solarbio
Science & Technology Co., Ltd.) dissolved in sterile deionized
water was used at a final cell culture concentration of 1 µg/ml and
cells were incubated for 24 h in 5% CO2 at 37°C
(28).
Cell transfection
Isolated primary cervical carcinoma cells from human
patients, U14 and HeLa cells were seeded into 6-well plates at a
density of 2×106 cells/well. When the confluence reached
80%, the cells were incubated in serum-free medium for 24 h before
transfection. Transfection was performed using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Lentiviral particles (Shanghai
GenePharma Co., Ltd.) containing the specific shRNAs (100 nM) for
sh-Control (5′-GGGUGAACUCACGUCAGAA-3′), sh-MALAT1-1
(5′-CACAGGGAAAGCGAGUGGUUGGU-3′) and sh-MALAT1-2)
(5′-GACAGGUAUCUCUUCGUUAUC-3′) were transduced into the cells for 12
h, and fresh medium was then changed for subsequent 48 h culture in
5% CO2 at 37°C. The interference efficiency was detected
by reverse transcription-quantitative PCR (RT-qPCR). Similarly,
sirtuin 1 (SIRT1) shRNA
(5′-TGCGGGAATCCAAAGGATAATTTTCAAGAGAAATTATCCTTTGGATTCCCGCTTTTTC-3′)
and sh-Control
(5′-TGCAACAAGATGAAGAGCACCAATTCAAGAGATGGTGCTCTTCATCTTGTTGTTTTTTC-3′)
were also purchased from Shanghai GenePharma Co., Ltd. and used to
transfect cells as aforementioned.
The MALAT1 overexpression plasmid (pcDNA3.1- MALAT1;
NCBI accession: NR_002819.4, nucleotides 3,207-8,411 bp) was
constructed as previously described (26,27).
The MALAT1-vector plasmid and the empty vector plasmid were
purchased from Shanghai GenePharma Co., Ltd. A total of 50 ng/ml
plasmid was transfected into cells using Lipofectamine®
2000 Transfection Reagent (Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's instructions.
miR-124 mimics (50 nM) and negative control (NC; 50 nM) were
transfected into cells using X-treme GENE reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C. The sequences of the
miR-124 mimics were as follows: Sense 5′-UAAGGCACGCGGUGAAUGCC-3′
and anti-sense 5′-CAUUCACCGCGUGCCUUAUU-3′. The sequences of the
negative controls were as follows: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′. After 6 h transfection, the medium was
replaced with normal growth medium, and total RNA and protein were
extracted at 24 h post-transfection.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
cDNA synthesis was performed using a High-Capacity cDNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The levels of
lncRNA MALAT1, miR-124, SIRT1, NLRP3, caspase-1 and
ASC were determined by the SYBR Green I (Roche Diagnostics)
incorporation method using an ABI 7500 Fast Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation for
30 sec at 95°C; followed by 40 cycles of 5 sec at 95°C and 30 sec
at 60°C. GAPDH was used as the internal control for lncRNA
MALAT1, SIRT1, NLRP3, caspase-1 and ASC; U6
was used as the internal control for miR-124. The primer sequences
are shown in Table I. The relative
expression levels were calculated using the 2−ΔΔCq
method (30).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| lncRNA
MALAT1 | F:
TGAGTCGAGCTCTGCCAAGTCCTGGAGAAATAGTAG |
|
| R:
AGTCATGGGCCCTGAAGACAGATTAGTAAAAGCA |
| NLRP3 | F: GAT
CTTCGCTGCGATCAACA |
|
| R:
GGGATTCGAAACACGTGCATTA |
|
Caspase-1 | F:
GCCTGTTCCTGTGATGT GGAG |
|
| R:
TGCCCACAGACATTCATACAGTTTC |
| ASC | F:
CTGACGGATGAGCAGTACCA |
|
| R:
CAGATGATTTGGTGGGATT |
| SIRT1 | F:
GUAUUGCUGAACAGAUGGAUU |
|
| R:
UCCAUCUGUUCAGCAAUACUU |
| GAPDH | F:
CCTGCACCACCAACTGCTTA |
|
| R:
-GGCCATCCACAGTCTT CTGAG |
| miRNA-124 | F:
UUAAGGCACGCGGUGAAUGC |
|
| R:
CAGTGCGTGTCGTGTCGTGGAT |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT |
|
| R:
5′-CGCTTCACGAATTTGCGTGTCAT |
Western blotting
Total protein for western blot analysis was
extracted and dissolved in RIPA buffer (Beijing Solarbio Science
& Technology Co., Ltd.) with protease inhibitors
(Sigma-Aldrich; Merck KGaA). The BCA method (Beyotime Institute of
Biotechnology) was used to quantify the protein concentration.
Proteins (80 µg/15 µl into each well) were separated by 10%
SDS-PAGE and then transferred to a nitrocellulose membrane. The
membranes were blocked with 5% skimmed milk for 2 h at room
temperature, then incubated with primary antibodies against SIRT1
(1:1,000, Abcam, Catalog No. ab110304), NLRP3 (dilution at 1:1,000,
Abcam, Catalog No. ab263899), caspase-1 (dilution at 1:1,000,
Abcam, Catalog No. ab207802), cleaved caspase-1 (dilution at
1:1,000, Cell Signaling Technology, Inc. Catalog No. 89332), ASC
(dilution at 1:1,000, Abcam, Catalog No. ab283684) and GAPDH
(dilution at 1:500, OriGene Technologies, Inc. Catalog No.
TA802519) at 4°C for 12 h. The membranes were then incubated with
FITC-conjugated goat anti-rabbit (1:10,000, Invitrogen; Thermo
Fisher Scientific, Inc. catalog No. A-11008) or goat anti-mouse
secondary antibody (dilution at 1:10,000, Invitrogen; Thermo Fisher
Scientific, Inc. Catalog No. A-11001) was incubated for 2 h at room
temperature in the dark. Odyssey Infrared Imaging System (LI-COR
Biosciences) and its analysis software (Odyssey version 3.0) were
used to calculate the relative expression level compared with the
internal control (GAPDH).
ELISA
IL-1β and IL-18 in cell culture medium were
determined using Human IL-1β (Interleukin 1 Beta) ELISA Kit (Cat.
No. E-EL-H0149) and Human IL-18 (Interleukin 18) ELISA Kit (Cat.
No. E-EL-H0253), respectively, from Elabscience Biotechnology,
Inc., following the manufacturer's instructions.
Immunofluorescence staining
Hela cells (seeding density of 70% were fixed with
4% paraformaldehyde solution (Beyotime Institute of Biotechnology)
for 30 min at room temperature and permeabilized with 0.4% Triton
X-100 (Beyotime Institute of Biotechnology; catalog No. P0096-100
ml) for 30 min at room temperature. After 2-h blocking with goat
serum (Beyotime Institute of Biotechnology, Catalog No. C0265) at
room temperature, cells were then incubated with anti-NLRP3 (1:100;
Abcam, Catalog No. ab263899) antibody at 4°C for 24 h followed by
incubation with FITC-labeled goat anti-rabbit immunoglobulin G
(1:500; Invitrogen; Thermo Fisher Scientific, Inc. Catalog No.
A16118) in the dark for 2 h at room temperature. DAPI (1 µg/ml;
Beyotime Institute of Biotechnology) was used for nuclear staining;
cells were incubated at room temperature in the dark. Conventional
fluorescence microscopy (Nikon Corporation) was used for image
capturing, and images were analyzed at ×200 magnification. Three
complete and non-overlapping views were randomly selected, and the
mean optical density under each view were measured as manufacture
of Image-pro plus (version 6.0).
TUNEL staining assay
Cell death was assessed using a TUNEL assay kit
(Beijing Solarbio Science & Technology Co., Ltd.) in accordance
with the manufacturer's instructions. Cells were stained with DAPI
for nuclear staining, and three complete and non-overlapping views
were randomly selected for examination under an Olympus IX51
fluorescence microscope (Olympus Corporation) at ×100
magnification.
Measurement of caspase activity
Cell lysate (200 ml) was used for caspase activity
detection using Caspase-1 Activity Assay kit (cat. no. C1101;
Beyotime Institute of Biotechnology), Caspase-4 Activity Assay kit
(cat. no. C1121; Beyotime Institute of Biotechnology) and Caspase-5
Colorimetric Analysis kit (cat. no. 55R-1283; AmyJet Scientific
Inc.). Briefly, cells were collected with 50 µl of ice-cold lysis
buffer for 1 h. Then, caspase activities were detected by
spectrophotometer at A405 nm according to the manufacturer's
instructions.
Bioinformatics analysis
Online bioinformatics tools for lncRNAs, including
LncACTdb (http://www.bio-bigdata.net/LncACTdb; version 3.0/Sep.
2021), NPInter (http://bigdata.ibp.ac.cn/npinter; version 4.0/Sep.
2019) and LncBase (http://www.microrna.gr/LncBase; version 3.0/2019) were
used to predict putative target miRNAs of lncRNA MALAT1. A
Venn diagram was used to search for targets withing the
intersections among these databases. Bioinformatic tools for miRNAs
and its potential mRNA targets were also used, including starBase
(https://starbase.sysu.edu.cn/starbase2/index.php;
version 2.0/Sep. 2013), TargetScan (http://www.targetscan.org; version 7.2/March 2018) and
miRDB (http://mirdb.org/miRDB; version 6.0/June
2019).
For predicting the downstream target mRNAs of
selected miRNAs, first a Venn diagram was used to identify possible
candidates among the aforementioned databases. Furthermore, a
literature search of the PubMed database (PMID: 33040786; PMID:
29844574; PMID: 33061806; PMID: 32232409; PMID: 34369271) with the
key words ‘pyroptosis and cervical cancer’ was used to review
possible regulators reported in previous studies. Finally, SIRT1
from bioinformatic databases and literature searches was identified
as a potential target of miR-124 and selected for further
examination in the present study.
RNA immunoprecipitation (RIP)
The binding of lncRNA MALAT1 to NLRP3 was
detected with a Magna RIP™ RNA-Binding Protein Immunoprecipitation
kit (EMD Millipore corp., 17–700). Hela cells (1–3×107
cells from manufacture, were washed with pre-cooled PBS and lysed
with RIPA lysis buffer (Beyotime Institute of Biotechnology) in an
ice bath for 5 min. The supernatant was collected after
centrifugation at 20,000 × g for 10 min at 4°C. An aliquot of the
cell extract was used as the input. Cell extract was incubated with
rabbit antibody against NLRP3 (1:100; Abcam) at room temperature
for 30 min for co-precipitation. A rabbit anti-human IgG (cat. no.
ab109489; 1:100; Abcam) was used as the negative control with a
volume of 1 ml at room temperature for 30 min. Samples were shaken
to the bottom and placed on a magnetic seat in an ice bath, and the
supernatant was discarded after 1 min. Then, 1 ml NT-2 was added
and the beads were shaken vigorously to mix. After 5-time
centrifugate (5,000 × g, 15 sec at 4°C) and washing with RIP Wash
Buffer, the precipitate was collected and used as the sample. RNA
was extracted after digestion by proteinase K at 55°C for
subsequent analysis. lncRNA MALAT1 content in
immunoprecipitated RNA was quantified by RT-qPCR and present as
relative RNA level compared with IgG IP.
Cell morphology
After transfection and/or LPS treatment, HeLa cells
were incubated for 24 h in a standard CO2 incubator, and
images were obtained with an inverted phase contrast microscope
(Olympus Corporation) to observe and record the morphology of the
cells.
Cell viability assay
Primary isolated cervical carcinoma cells from human
patients (104 cells/well) and HeLa cells (104
cells/well) were seeded in a 96-well plate. After 24 h transfection
and/or LPS treatment, Cell Counting Kit-8 reagent (Nanjing
Jiancheng Bioengineering Institute) was added each well (10
µl/well) and incubated for 4 h at 37°C. The optical density value
at 450 nm was determined with a Bio-Rad 680 microplate reader
(Bio-Rad Laboratories, Inc.).
Cell counting assay
Trypan blue staining (Beijing Solarbio Science &
Technology Co., Ltd.) was used for cell counting. Primary isolated
cervical carcinoma cells (and Hela cells (both 1×106
cells/ml) were resuspended and mixed with 0.1% trypan blue at room
temperature for 5 min. Cells were counted using a Countless II FL
automated cell counter (Invitrogen; Thermo Fisher Scientific, Inc.)
and the ratio of living cells vs. dead cells was analyzed.
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 software
(GraphPad Software, Inc.). Data are presented as the mean ±
standard error of the mean. All experiments were repeated three
times. Statistical comparisons between two groups were performed by
Student's t-test (paired t-test for the matched sample data and
unpaired t-test for comparisons of two groups). One- or two-way
ANOVA followed by Tukey's post hoc test was used for multiple
comparisons. Association analyses were assessed by the linear
regression index (r2) (31). P<0.05 was considered to indicate
a statistically significant difference.
Results
lncRNA MALAT1 is positively related
with cervical tumor growth and negatively associated with cell
death
To clarify the function of lncRNA MALAT1 in
cervical carcinoma, insight into its biological process from Gene
Ontology (GO) analysis was gained by LncACTdb database. The top two
GO term enrichment for lncRNA MALAT1 are ‘regulation of cell
proliferation’ and ‘regulation of cell cycle’ (Fig. 1A), which may be associated with
cervical tumor progression. Thus, the present study aimed to
determine the effect of lncRNA MALAT1 on cervical cancer
cell viability. Initially, primary cells were isolated from
paracarcinoma and carcinoma tissues of patients with cervical
cancer and cultures were established to compare the differential
expression of lncRNA MALAT1 in vitro. The expression level
of lncRNA MALAT1 was significantly increased in cervical
carcinoma cells compared with the paracarcinoma cells (Fig. 1B). Additionally, a positive
association was found between lncRNA MALAT1 levels and cell
viability, as well as with live cell number, whereas the opposite
was observed with the number of dead cells (Fig. 1C and D). These results suggested
that lncRNA MALAT1 is positively associated with cervical
cancer cell viability, and the loss of lncRNA MALAT1 may
exert an inhibitory effect on the development of cervical cancer.
To demonstrate this hypothesis, lncRNA MALAT1 was knocked
down with sh-MALAT1 in isolated primary cervical carcinoma
cells and in U14 cervical cancer cells (Fig. S1A and B, respectively). Knockdown
of lncRNA MALAT1 induced a marked increase in primary
cervical carcinoma cell death in vitro (Fig. 1E) and also led to a decrease in
in vivo tumor diameter, volume, and weight (Fig. 1F-H). These results suggested that
specific knockdown of lncRNA MALAT1 may function as a
suppressor of cervical cancer cells through mechanisms that remain
to be identified.
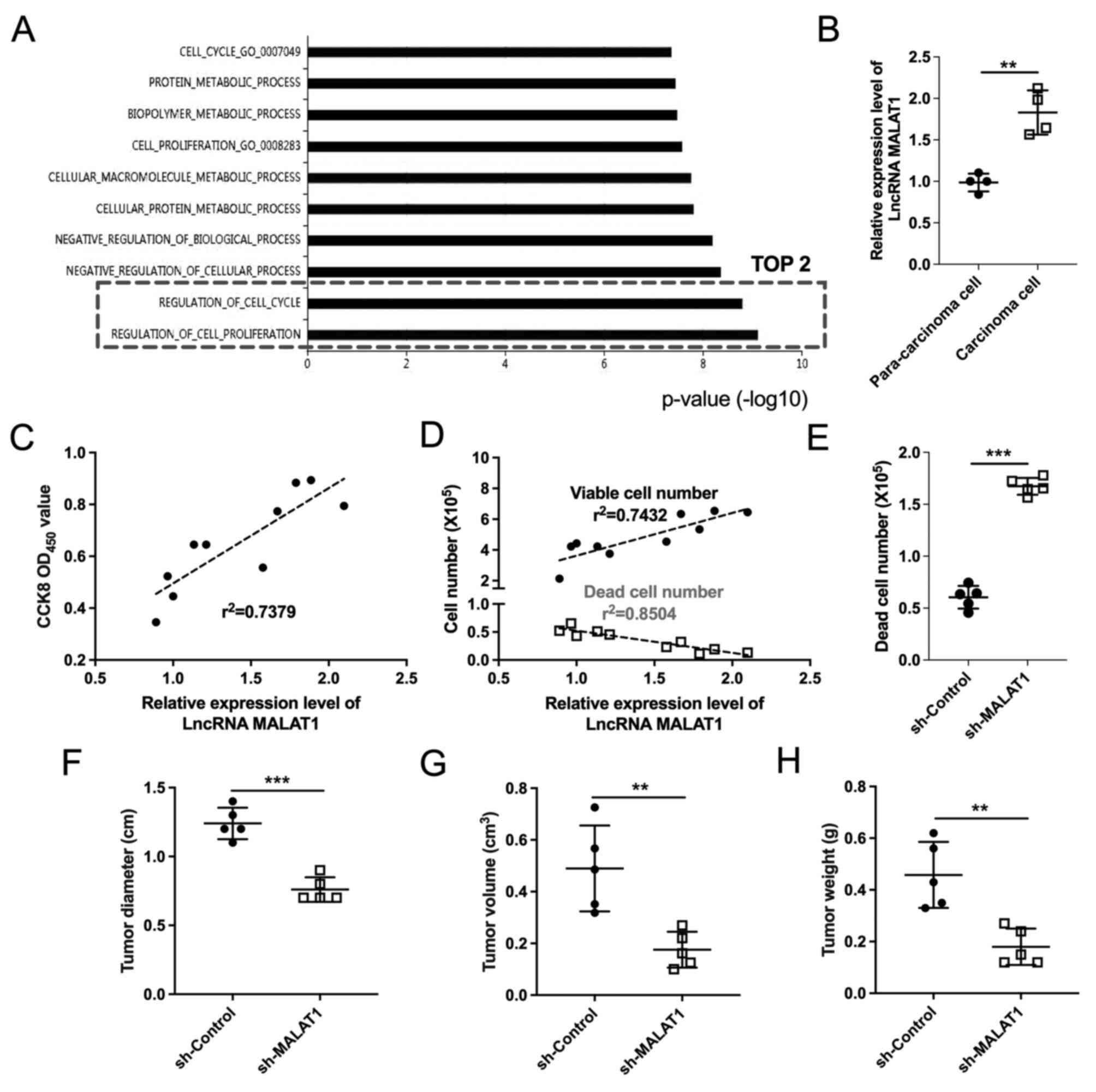 | Figure 1.LncRNA MALAT1 expression is
associated with cervical cancer progression. (A) Gene Ontology
analysis identified the top 10 biological processes associated with
lncRNA MALAT1. (B) lncRNA MALAT1 expression levels in
primary cells isolated from paracarcinoma and carcinoma tissues
were determined by reverse transcription-quantitative PCR. n=4
tissue samples/group; ***P<0.001. (C-E) Primary cells were
isolated from human cervical carcinoma tissues and examined for
viability and cell death. (C) Cell viability was determined by
CCK-8 assay (n=10). (D) Viable and dead cell numbers were
determined by trypan blue staining assay (n=10). (E) Number of dead
cells was determined in the sh-Control and sh-MALAT1 transfection
groups. n=5; ***P<0.001. (F) Tumor diameter (range, 0.7–1.4 cm),
(G) volume (range, 0.1–0.726 cm3) and (H) weight (range,
0.12–0.62 g) were determined in each group. n=5 mice/group;
**P<0.01, ***P<0.001. CCK-8, Cell Counting Kit-8; lncRNA,
long non-coding RNA; MALAT1, metastasis-associated lung
adenocarcinoma transcript 1; OD, optical density; sh, short
hairpin. |
In addition, lncRNA MALAT1 was overexpressed
in isolated primary cervical carcinoma cells for in-depth
exploration in vitro (Fig.
S1C). Increased lncRNA MALAT1 expression promoted cell
viability of cervical cancer cells with no significant change in
cell deaths (Fig. S2), which
suggested the potential of lncRNA MALAT1 overexpression on
promoting cervical cancer growth.
Subsequent in vitro experiments were
performed to further investigate the aforementioned effect of
MALAT1 knockdown on cell death in HeLa cells (Fig. S1D). After transfection with
sh-MALAT1, HeLa cervical cancer cells exhibited an increase in the
number of TUNEL-positive cells (Fig.
2A and B) and increased levels of IL-18 and IL-1β in the
culture medium (Fig. 2C). Thus, it
was hypothesized that pyroptosis may have occurred in HeLa cells
following knockdown of lncRNA MALAT1 expression. Thus, the
present study examined the activity of pyroptosis-related caspases,
the expression levels of NLRP3, caspase-1 and ASC, and examined
changes in cell morphology following sh-MALAT1 transfection. It was
observed that downregulated lncRNA MALAT1 markedly increased
caspase-1 activity (Fig. 2D),
increased protein (Fig. 2E and F)
and mRNA (Fig. 2G) expression
levels of NLRP3, cleaved caspase-1 and ASC (Fig. 2E-G), and increased the number of
cells exhibiting morphological evidence of membrane rupture
(Fig. 2H). These changes may
eventually lead cervical cancer cells to undergo pyroptosis.
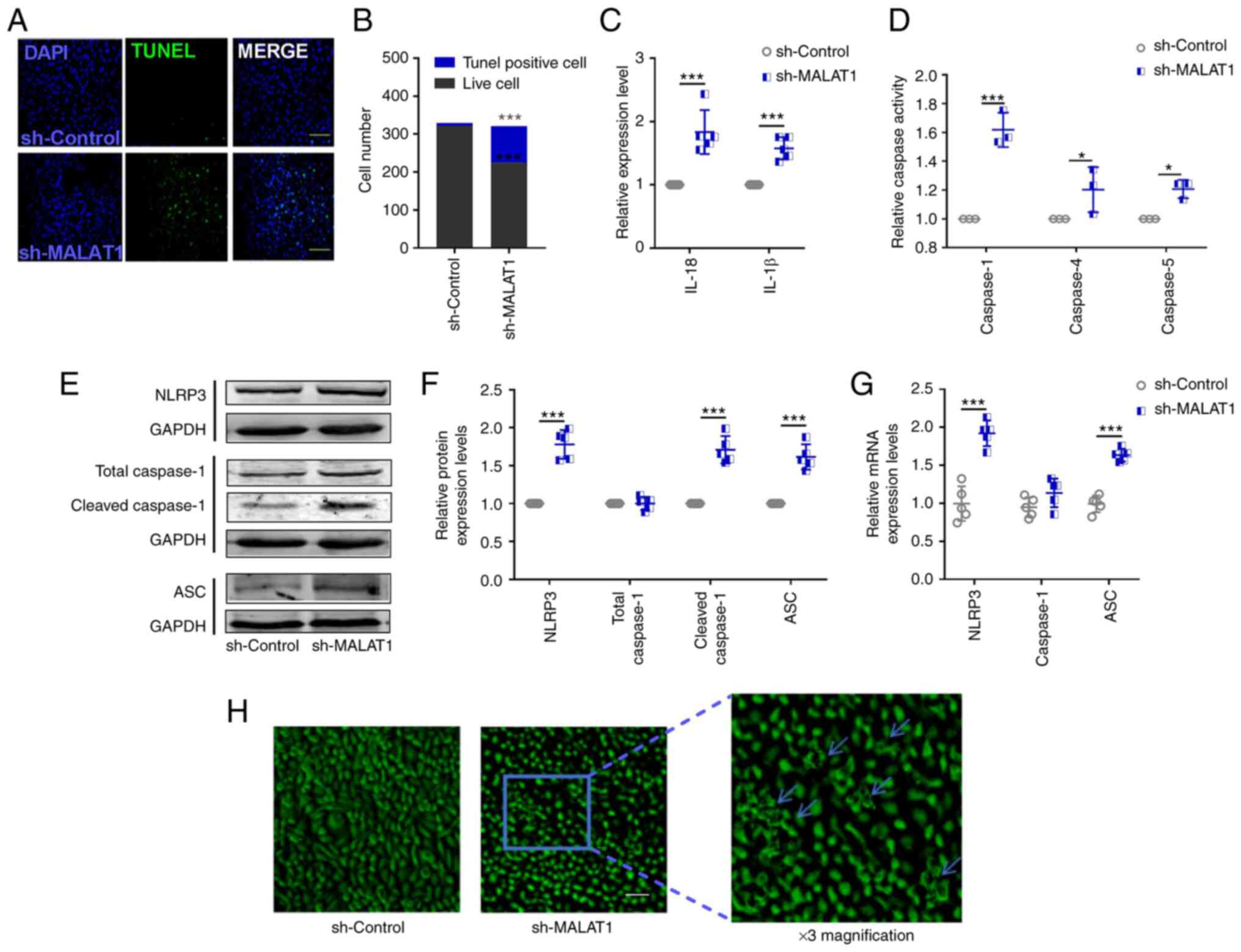 | Figure 2.Downregulation of lncRNA
MALAT1 induces the pyroptosis of HeLa cervical cancer cells.
(A) Representative images of the TUNEL-staining assay (scale bar,
50 µm) and (B) statistical analysis was performed to detect the
number of living and dead cells. n=3; ***P<0.001. (C) ELISA kits
were used to determine the levels of IL-18 and IL-1β in the culture
medium of transfected HeLa cells. n=5; ***P<0.001. (D) The
activities of caspase-1, caspase-4 and caspase-5 were determined
with a caspase assay kit. n=3; *P<0.05, ***P<0.001. (E and F)
Protein and (G) mRNA expression levels of pyroptosis-related
factors, NLRP3, caspase 1 and ASC, were determined by western
blotting and reverse transcription-quantitative PCR, respectively.
n=5; ***P<0.001. (H) Cell morphology was observed by inverted
phase contrast microscopy; blue arrows show the morphological
changes. Scale bar, 50 µm; three-fold magnification is shown on the
right. ASC, apoptosis-associated speck-like protein containing a
caspase recruitment domain; MALAT1, metastasis-associated
lung adenocarcinoma transcript 1; NLRP3, NOD-like receptor protein
3. |
Taken together, these results indicated that the
reduction of lncRNA MALAT1 expression may inhibit cervical
cancer cell viability by inducing pyroptosis.
Overexpression of lncRNA MALAT1
protects HeLa cells from LPS-induced pyroptosis
To explore the effects of lncRNA MALAT1 on
cervical cancer cells against pyroptosis, lncRNA MALAT1 was
overexpressed in LPS-treated HeLa cells; confirmation of successful
overexpression vector transfection was first confirmed in untreated
HeLa cells (Fig. S1E). LPS
treatment is generally used as a pyroptosis stimulus, which induces
typical pyroptotic characteristics, including cell morphology
(inflammasome-mediated membrane holes and cell swelling), decreased
cell viability and increased levels of inflammatory factors (IL-1β
and IL-18), as well as increased expression levels of NLRP3,
cleaved caspase-1 and ASC upregulation according to previous
studies (29,30). In the current study, lncRNA
MALAT1 was overexpressed in LPS-treated cells, which led to
a noticeable decrease in the number of swollen cells (Fig. 3A), an increase in HeLa cell
viability (Fig. 3B) and
downregulated levels of IL-1β and IL-18 (Fig. 3C), in addition to decreased protein
expression levels of NLRP3, cleaved caspase-1 and ASC (Fig. 3D-F). The results suggested that
lncRNA MALAT1 may block the development of pyroptosis in
LPS-treated HeLa cells.
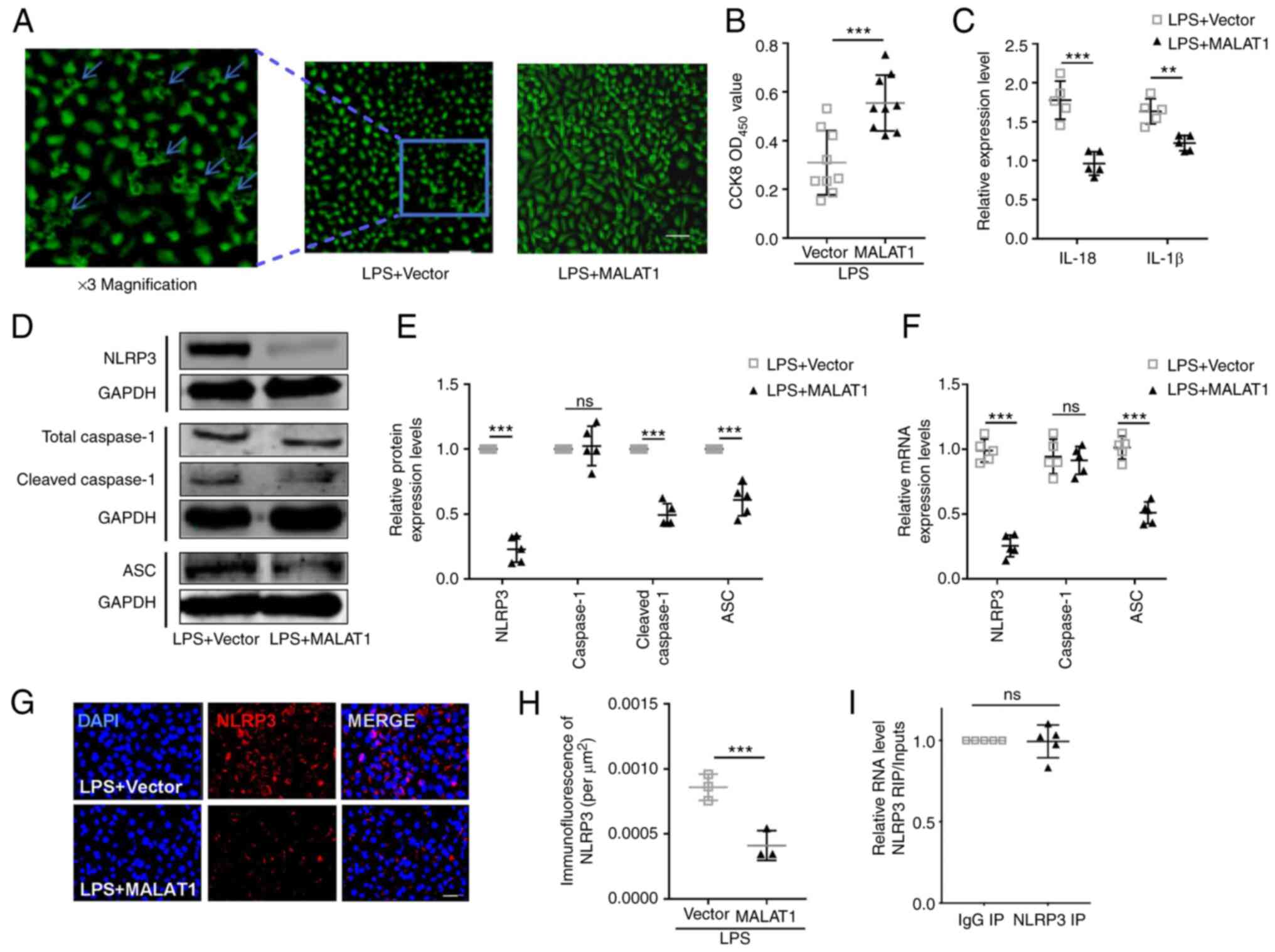 | Figure 3.Upregulation of lncRNA MALAT1
blocks the effect of LPS on HeLa cervical cancer cell pyroptosis.
(A) Morphological changes (blue arrows) in Hela cells were used to
assess cell pyroptosis by phase contrast microscopy. Scale bar, 50
µm Three-fold magnification is shown on the left for better
clarification. (B) Hela cell viability was determined by CCK-8
assay. n=5; ***P<0.001. (C) ELISA kits were used to determine
the levels of IL-18 and IL-1β in the culture medium. n=5;
**P<0.01, ***P<0.001. (D and E) Protein and (F) mRNA
expression levels of pyroptotic factors, including NLRP3, caspase-1
and ASC, were detected by western blotting and reverse
transcription-quantitative PCR, respectively. n=5; **P<0.01,
***P<0.001. (G) Representative images and (H) statistical
analysis of NLRP3 immunofluorescence; scale bar, 20 µm. n=5;
***P<0.001. (I) RNA immunoprecipitation analysis was used to
determine the regulatory association between lncRNA MALAT1
and NLRP3; n=3. ASC, apoptosis-associated speck-like protein
containing a caspase recruitment domain; CCK-8, Cell Counting
Kit-8; IP, immunoprecipitation; lncRNA, long non-coding RNA; LPS,
lipopolysaccharide; MALAT1, metastasis-associated lung
adenocarcinoma transcript 1; NLRP3, NOD-like receptor protein 3;
ns, not significant; OD, optical density. |
Based on these data, it was concluded that lncRNA
MALAT1 may affect the progression of cervical cancer, at
least in part through the regulation of pyroptosis. However, the
mechanism by which lncRNA MALAT1 initiates pyroptosis
remains unclear, including whether lncRNA MALAT1 directly
interacts with pyroptotic factors, such as NLRP3, or interferes
with them at the post-transcriptional level. NLRP3 is generally
recognized to initiate pyroptosis (31), thus, it was proposed that lncRNA
MALAT1 may directly affect the expression of NLRP3 as a
transcriptional factor. Immunofluorescent staining of NLRP3 showed
a downregulated expression in MALAT1 overexpressed cell
under the condition of LPS treatment (Fig. 3G and H). RIP assays were performed
in HeLa cells to verify whether there is a direct interaction
between lncRNA MALAT1 and NLRP3. Unexpectedly, no
significant difference was identified in the RIP analysis for an
lncRNA MALAT1/NLRP3 regulatory axis (Fig. 3I).
lncRNA MALAT1 affects the
miR-124/SIRT1 axis in LPS-treated cervical cancer cells
To explore the downstream target of lncRNA
MALAT1 in regulating the pyroptotic process in cervical
cancer cells, bioinformatics tools, Venn diagrams and literature
searches using the key words ‘pyroptosis and cervical cancer’ were
used. According to the results from three bioinformatics predictive
databases (LncACTdb, NPInter and LncBase) and a Venn diagram, which
was used to search for the intersection among the results of these
databases, five potential targets of lncRNA MALAT1 were
identified, including miR-25-3p, miR-92b-3p, miR96-5p, miR-124 and
miR-1 (Fig. 4A).
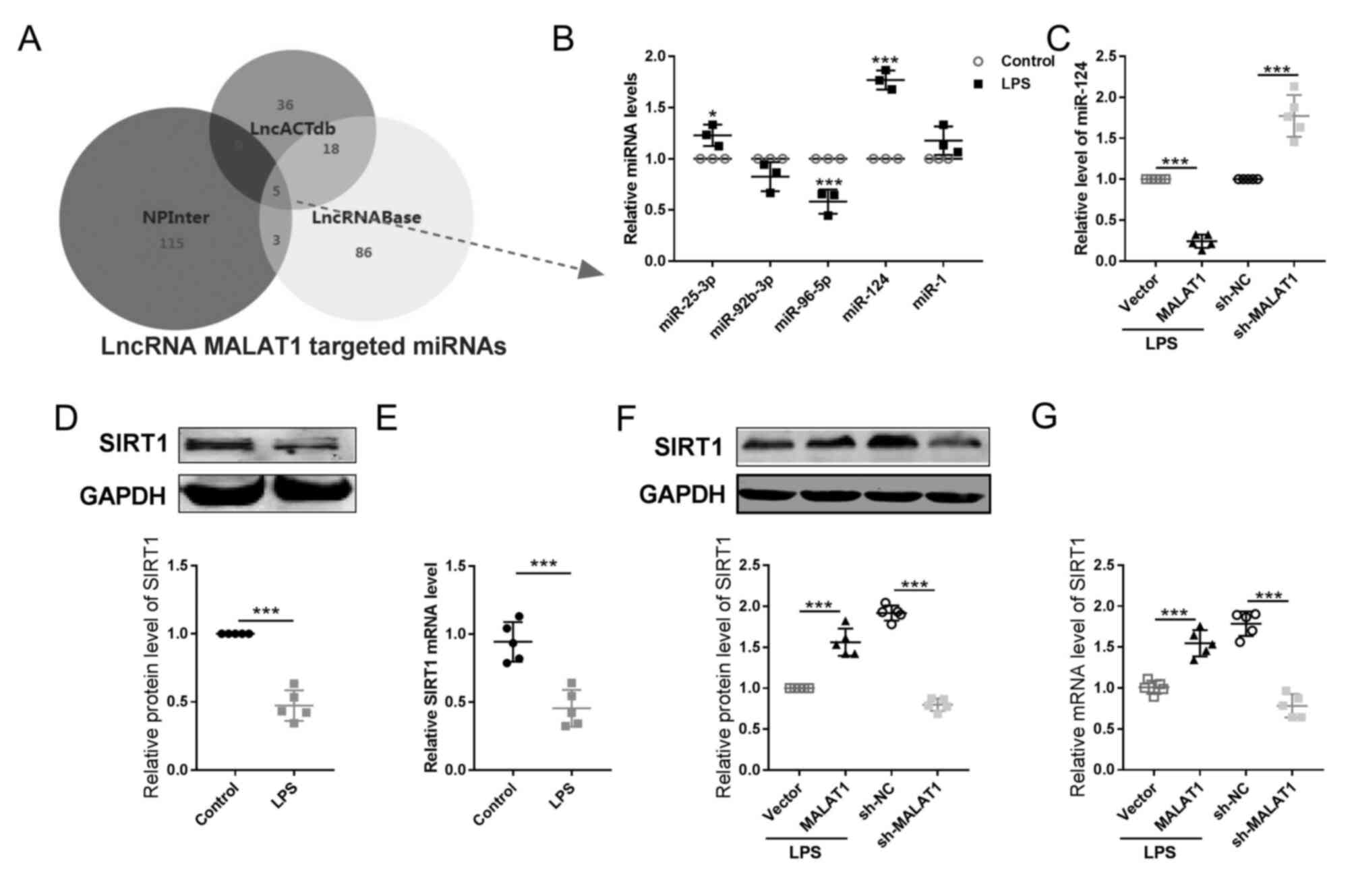 | Figure 4.lncRNA MALAT1 alters the
expression levels of miR-124/SIRT1 in cervical tumor cell during
pyroptosis. (A) Bioinformatics prediction analyses (LncACTdb,
NPInter and LncRNABase) of lncRNAs and a Venn diagram were used to
identify the possible downstream miRNA targets of lncRNA
MALATA1. (B) Expression levels of the five miRNA candidates
identified from the intersection of (A) were then detected by
RT-qPCR in HeLa cells treated with or without LPS. n=5; *P<0.05,
***P<0.001. (C) The levels of miR-124 was determined by RT-qPCR.
n=5; ***P<0.001. The expression levels of SIRT1 (D) protein and
(E) mRNA were detected by western blotting and RT-qPCR,
respectively. n=5; ***P<0.001. The expression levels of SIRT1
(F) protein and (G) mRNA were detected by western blotting and
RT-qPCR, respectively. n=5; ***P<0.001. lncRNA, long non-coding
RNA; LPS, lipopolysaccharide; MALAT1, metastasis-associated
lung adenocarcinoma transcript 1; miR/miRNA, microRNA; RT-qPCR,
reverse transcription-quantitative PCR; SIRT1, sirtuin 1. |
RT-qPCR was then performed to determine miRNA
expression levels and to investigate if these molecules serve a
role in lncRNA MALAT1-regulated pyroptosis in HeLa cells.
The level of miR-124 exhibited the most significant alteration
after LPS treatment (Fig. 4B); in
addition, knockdown and overexpression of lncRNA MALAT1
resulted in significant increase and decrease of miR-124 expression
levels, respectively (Fig. 4C).
These data suggested that miR-124 may be a downstream target of
lncRNA MALAT1, particularly in pyroptosis.
Apart from lncRNA MALAT1, other putative
targets of miR-124 were investigated; three bioinformatics
prediction tools (starBase, TargetScan and miRDB) were used
alongside a Venn diagram. A total of 82 potential targets of
miR-124 were identified with the result of PubMed database search
(using the key words ‘pyroptosis and cervical cancer’). A previous
study demonstrated the involvement of SIRT1 during pyroptosis,
suggesting SIRT1 could therefore be a target for the effective
treatment of cervical cancer (32)
(Fig. S3A). Therefore,
SIRT1 was selected as a potential target of miR-124
(Fig. S3).
miR-124/SIRT1 mediates the effect of
lncRNA MALAT1 on pyroptotic progression of HeLa cervical cancer
cells
To investigate the potential target of lncRNA
MALAT1 during pyroptosis, subsequent experiments were
divided into two parts: i) Verification the direct interaction
between miR-124 and SIRT1, and ii) SIRT1 mediating
the function of lncRNA MALAT1 in LPS-treated HeLa cells.
For the first part, the aim was to verify whether
miR-124 mediated the effects of lncRNA MALAT1 on pyroptosis.
Thus, cells were co-transfected with lncRNA MALAT1
overexpression vector with miR-124 mimics, attempting to disturb
the function of lncRNA MALAT1. After the determination of
transfection efficiency of miR-124 mimics in non-treated HeLa cells
(Fig. S4) and lncRNA
MALAT1 co-transfected HeLa cells (Fig. 5A), overexpression of miR-124,
compared to its NC group, led to a decrease in the level of lncRNA
MALAT1 expression (Fig.
5B). Additionally, the overexpression of miR-124 also
concurrently decreased the activity of HeLa cells (Fig. 5C). Our previous findings suggested
that lncRNA MALAT1 could ‘protect’ HeLa cells against
pyroptotic damage induced by LPS. However, in this context, we
discovered that miR-124 counteracted this ability of lncRNA
MALAT1, evidenced by a subsequent increase in the expression
of inflammatory factors IL-1β and IL-18 (Fig. 5D), promoted death of HeLa cells,
and reduced number of viable HeLa cells (Fig. 5E and F). Further examination of
pyroptotic factors NLRP3, ASC, and cleaved caspase 1 confirmed that
miR-124 inhibited lncRNA MALAT1's ability to protect HeLa
cells against pyroptosis induced by LPS stimulation (Fig. 5G-I).
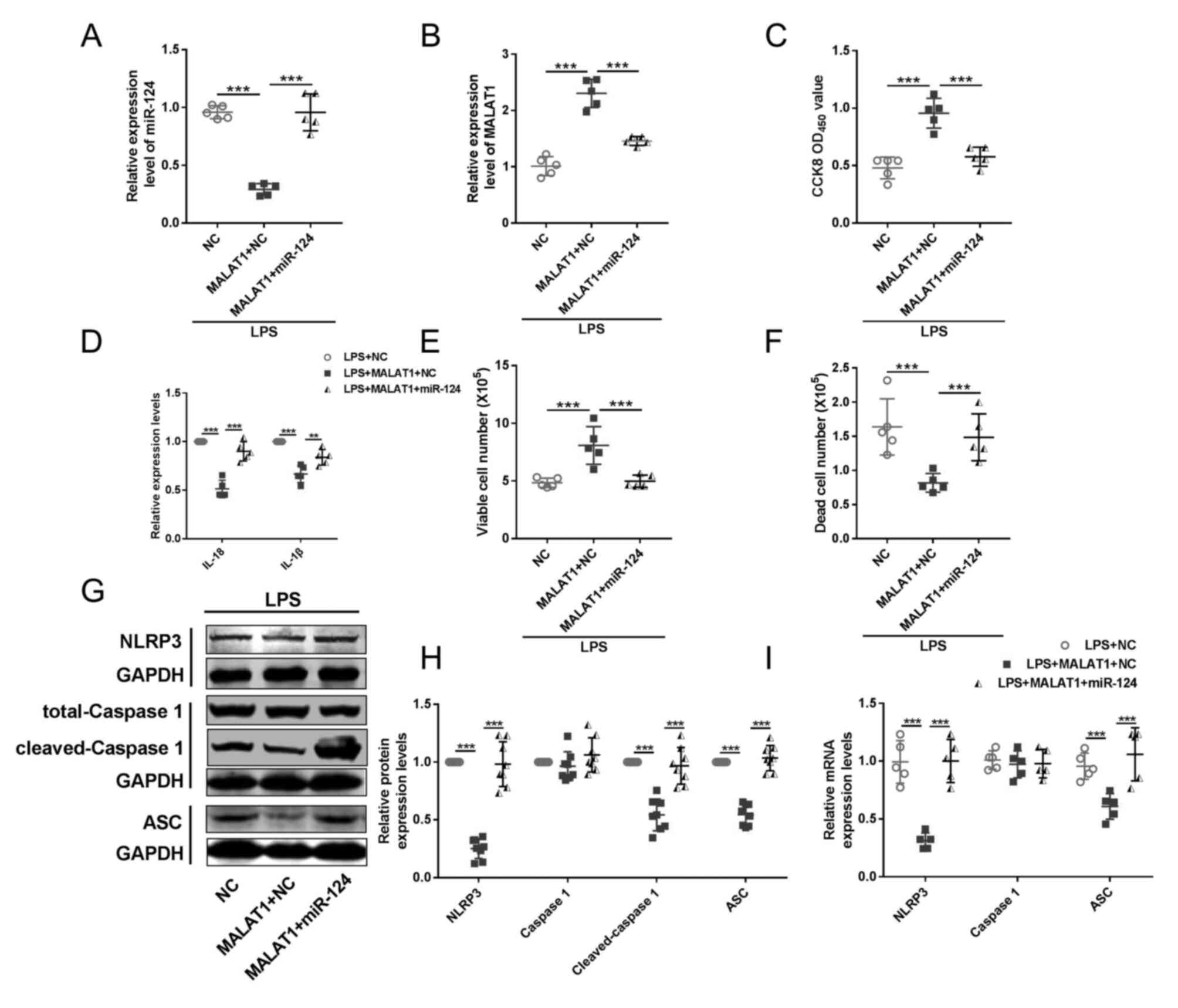 | Figure 5.miR-124 mediates the regulation of
lncRNA MALAT1 on HeLa cervical cancer cell pyroptosis.
RT-qPCR was performed to determine the expression levels of (A)
miR-124 and (B) lncRNA MALAT1 in LPS-treated HeLa cells.
n=5; ***P<0.001. (C) Cell viability was detected by CCK-8 assay.
n=5; ***P<0.001. (D) ELISA kits were used to determine the
levels of IL-18 and IL-1β in the culture medium. n=5; **P<0.01,
***P<0.001. Trypan blue analysis was used to facilitate the
detection of (E) viable and (F) dead cells. n=5; ***P<0.001. (G
and H) Western blotting and (I) RT-qPCR were performed to detect
the protein and mRNA expression levels, respectively, of pyroptotic
factors, including NLRP3, caspase-1, cleaved caspase-1 and ASC.
n=5; ***P<0.001. ASC, apoptosis-associated speck-like protein
containing a caspase recruitment domain; CCK-8, Cell Counting
Kit-8; lncRNA, long non-coding RNA; LPS, lipopolysaccharide;
MALAT1, metastasis associated lung adenocarcinoma transcript
1; miR, microRNA; NLRP3, NOD-like receptor protein 3; OD, optical
density; RT-qPCR, reverse transcription-quantitative PCR. |
For the second part, experiments were conducted to
verify whether SIRT1 underlies the function of lncRNA MALAT1
on pyroptosis. HeLa cells were co-transfected with lncRNA
MALAT1 overexpression vector and sh-SIRT1 or its NC
(Figs. S5A and 5B). Transfection of sh-SIRT1 functioned
as an antagonist against lncRNA MALAT1, abolishing the
protective effects of lncRNA MALAT1 in HeLa cells from LPS,
including reducing viable HeLa cells (Fig. 6C and D), increasing death number of
HeLa cells (Fig. 6E). Transfection
of sh-SIRT1 elevated inflammatory factors IL-1β and IL-18 (Fig. 6F) and pyroptotic factors NLRP3,
ASC, and cleaved caspase 1 (Fig.
6G-I). Taken together, these results demonstrated the
regulatory effect of the lncRNA MALAT1/miR-124/SIRT1 axis on
cervical cancer cell pyroptosis.
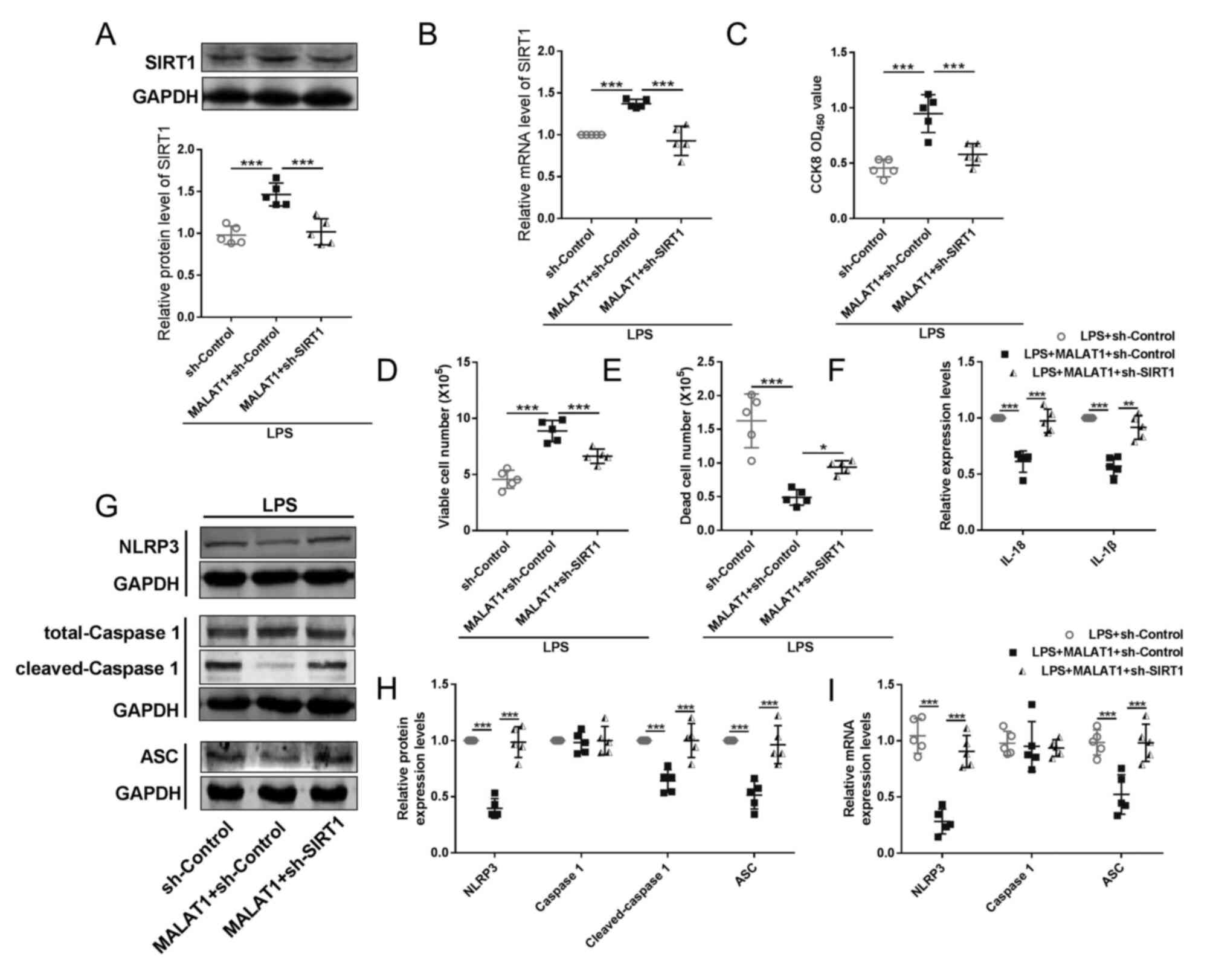 | Figure 6.SIRT1 is a downstream effector of
MALAT1 in HeLa cervical cancer cell pyroptosis. (A) Western
blotting and (B) RT-qPCR were performed to determine the SIRT1
protein and mRNA expression levels, respectively, in LPS-treated
HeLa cells. n=5; ***P<0.001. (C) Cell viability was detected by
CCK-8 assay (n=5 in each group). ***P<0.001. (D) ELISA kits were
used to determine the levels of IL-18 and IL-1β in the culture
medium (n=5 in each group). ***P<0.001. (E and F) Cell numbers
were detected by Trypan Blue analysis (n=5 in each group).
*P<0.05, **P<0.01, ***P<0.001. (G-I) RT-qPCR and western
blotting were performed to detect the mRNA and protein levels of
pyroptotic factors, including NOD-like receptor protein 3,
caspase-1, cleaved caspase-1 and apoptosis-associated speck-like
protein containing a caspase recruitment domain (n=5).
***P<0.001. CCK-8, Cell Counting Kit-8; LPS, lipopolysaccharide;
MALAT1, metastasis-associated lung adenocarcinoma transcript 1; OD,
optical density; SIRT1, sirtuin 1; RT-qPCR, reverse
transcription-quantitative PCR. |
Discussion
Although improvements have been made in terms of
cervical cancer treatment (33),
cervical cancer treatments face several challenges that limit their
efficiency. Cervical cancer is primarily caused by infection with
high-risk subtypes of the human papillomavirus (HPV). Although it
is a preventable disease, limited resources, especially in low- and
middle-income countries (LMICs) often lead to advanced and
untreatable disease at the time of diagnosis. Early-stage detection
is associated with significantly improved survival rates. However,
screening programs may not be well-established, leading to delayed
diagnosis and treatment initiation (34). The cost of cervical cancer
treatment can be a significant burden for patients, even for those
with insurance (35); 4) Recurrent
cervical cancer remains challenging to treat, with poor prognosis
and low overall survival rates (36). Despite these challenges, ongoing
research is focused on developing promising treatments such as
immunotherapies, targeted therapies, combination treatments, and
genetic treatment approaches.
Gene targeted therapies have notable treatment
effects, although limited effective targets and the difficulty in
discovering new targets remain problematic. The basic requirements
of therapeutic targets include: i) Significantly aberrant
expression in carcinoma tissues compared with that in normal
tissues; ii) association with cell viability, particularly
proliferation and metastasis; and iii) their specific
knockdown/knockout or overexpression must lead to a marked
inhibition of cancer cell viability. Only factors exhibiting such
characteristics should be considered as potential targets against
cancer.
The present study focused on lncRNA MALAT1
owing to its biological functions; according to GO analysis, the
top two biological processes were ‘regulation of cell
proliferation’ and ‘regulation of cell cycle’. The positive
relationship between lncRNA MALAT1 and cell proliferation
has been demonstrated in a number of cancer, fibroblast and smooth
muscle cells with different downstream molecules (37–40).
For example, lncRNA MALAT1 promotes lung cancer cell
proliferation as a miR-200a-specific sponge (18). Overexpression of lncRNA
MALAT1 enhances human periodontal ligament stem cell
proliferation by regulating fibroblast growth factor 2 (41). lncRNA MALAT1 impedes the
proliferation of synoviocytes through β-catenin promoter
methylation in rheumatoid arthritis (42). Based on these results, it was
hypothesized that specific knockdown of lncRNA MALAT1 could
be a potential treatment against cell overproliferation-induced
diseases. Previous studies indicated that knockdown of lncRNA
MALAT1 ameliorated osteoarthritis by promoting the apoptosis
of chondrocytes (43).
Furthermore, particularly in the progression of carcinoma,
inhibition of lncRNA MALAT1 showed markedly beneficial
properties. For example, lncRNA MALAT1 knockdown hinders
prostate cancer progression by regulating the miR-140/baculoviral
IAP repeat containing 6 axis (44)
and inhibits cell proliferation by promoting the apoptosis of acute
myeloid leukemia cells through the regulation of miR-96 (45). Previous studies also have reported
that inhibiting lncRNA MALAT1 protects
low-MALAT1-expressing normal cells, including cardiomyocytes
(46), podocytes (47), endothelial cells (48) and renal tubular epithelial cells
(27), which is the opposite
function to that observed in pathological tissues (such as
carcinoma and atherosclerotic lesions), suggesting that lncRNA
MALAT1 may be a suitable therapeutic target. Therefore, the
present study aimed to investigate the effects of lncRNA
MALAT1 on cervical cancer. Primary cervical cancer cells
were isolated from carcinoma tissues, and a negative relationship
between cell death and the expression of lncRNA MALAT1 was
observed; thus, the process of cell death was investigated in
detail.
There has been an increased interest in pyroptosis
over the past decade; however, studies focusing on pyroptosis still
faces numerous limitations, particularly in the context of cancer
development, which is complex and varies among species and organs
(48). However, a previous study
identified small molecule-mediated pyroptosis as an effective
inhibitor of various tumor cells (49). Precise modulation of pyroptosis
against intestinal tumors, inflammation or infectious disorders in
humans has been previously reported (50). lncRNA growth arrest specific 5 has
been reported to suppress ovarian cancer by inducing pyroptosis
(51). Knockdown of lncRNA
X-inactive specific transcript inhibits the progression of NSCLC by
targeting pyroptosis (52). lncRNA
ADAM metallopeptidase with thrombospondin type 1 motif 9-antisense
RNA 2 inhibits gastric cancer development by regulating the
miR-223-3p/NLRP3 axis (53).
Pyroptosis-targeted treatments may lead to a promising, novel
approach to the treatment of patients with cancer. In the present
study, upregulation of IL-18 and IL-1β was observed in the culture
medium of cervical cancer cells, which suggested the involvement of
pyroptosis of cervical cancer cells in lncRNA MALAT1-related
cell death.
The present results revealed a negative association
between lncRNA MALAT1 and pyroptosis in cervical tumor
cells, and overexpression of MALAT1 blocked LPS-induced
pyroptosis. In addition, it was observed that lncRNA MALAT1
altered the levels of miR-124/SIRT1 in cervical cancer cells, and
that the lncRNA MALAT1/miR-124/SIRT1 axis may regulate
cervical cell pyroptosis, which, at least in part, was associated
with tumor growth. Our findings provide a potential therapeutic
strategy against cervical cancer by promoting pyroptosis.
The mechanism by which this regulatory axis is
connected with pyroptotic factors was investigated in the present
study. A previous study demonstrated the direct interaction between
NLRP3 and p53 by chromatin immunoprecipitation analysis (21), and SIRT1 was reported to regulate
the deacetylation of p53 (54).
Whether specifically inactivating lncRNA MALAT1 expression
in cervical tumors using gene engineering tools could ameliorate
the mortality and morbidity of cervical carcinoma, or other cancer
types, thus providing more beneficial effects to improve the
outcomes, requires further investigation.
In conclusion, lncRNA MALAT1 may regulate
pyroptosis through the miR-124/SIRT1 axis in cervical cancer cells.
The present findings suggested that lncRNA MALAT1 may be a
potential target for improving the clinical effects of treatment in
the progression of cervical carcinoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (82104173/82104169).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TL, DX and GZ contributed to the conception of the
study. MJ, YW, YJ, SC, LD and XJ performed the experiment. TL and
DX contributed significantly to analysis and manuscript
preparation. GZ and DX performed the data analyses and wrote the
manuscript. TL helped perform the analysis with constructive
discussions. MJ, DX, GZ and YZ confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of The First Affiliated Hospital of Harbin Medical
University (approval no. IRB-AF/SC-04/02.0; 11 November 2020).
Tumor samples were collected from the patients upon written
informed consent. The experimental protocols involving animals were
approved by the Animal Ethical Care Committee of Qiqihar Medical
University (approval no. QMU-AECC-2020-43; 8 May 2020) and complied
with the Guide for the Use and Care of Laboratory Animals published
by the USA National Institutes of Health (NIH Publication No.
85-23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu X, Sun G, Zheng R, Zhang S, Zeng H, Sun
K, Wang S, Chen R and Wei W: Incidence and mortality of cervical
cancer in China in 2015. J Natl Cancer Cent. 2:70–77. 2022.
View Article : Google Scholar
|
|
2
|
Balasubramaniam SD, Balakrishnan V, Oon CE
and Kaur G: Key molecular events in cervical cancer development.
Medicina (Kaunas). 55:3842019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barquet-Muñoz SA, Rendón-Pereira GJ,
Acuña-González D, Peñate MV, Herrera-Montalvo LA, Gallardo-Alvarado
LN, Cantú-de León DF and Pareja R: Role of pelvic and para-aortic
lymphadenectomy in abandoned radical hysterectomy in cervical
cancer. World J Surg Oncol. 15:232017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bansal S, Lewin SN, Burke WM, Deutsch I,
Sun X, Herzog TJ and Wright JD: Sarcoma of the cervix: Natural
history and outcomes. Gynecol Oncol. 118:134–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang L, Liu S, Li S, Chen Y, Xie B and
Zhou J: Induction mechanism of ferroptosis, necroptosis, and
pyroptosis: A novel therapeutic target in nervous system diseases.
Int J Mol Sci. 24:101272023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng X, Chen W, Gong F, Chen Y and Chen
E: The role and mechanism of pyroptosis and potential therapeutic
targets in sepsis: A review. Front Immunol. 12:7119392021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaczanowski S: Apoptosis: Its origin,
history, maintenance and the medical implications for cancer and
aging. Phys Biol. 13:0310012016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z,
Zhang Z and Dai Z: Propofol directly induces caspase-1-dependent
macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell
Death Dis. 10:5422019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen HH, Yang YX, Meng X, Luo XY, Li XM,
Shuai ZW, Ye DQ and Pan HF: NLRP3: A promising therapeutic target
for autoimmune diseases. Autoimmun Rev. 17:694–702. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue Y, Enosi Tuipulotu D, Tan WH, Kay C
and Man SM: Emerging activators and regulators of inflammasomes and
pyroptosis. Trends Immunol. 40:1035–1052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Varghese GP, Folkersen L, Strawbridge RJ,
Halvorsen B, Yndestad A, Ranheim T, Krohg-Sørensen K, Skjelland M,
Espevik T, Aukrust P, et al: NLRP3 inflammasome expression and
activation in human atherosclerosis. J Am Heart Assoc.
5:e0030312016. View Article : Google Scholar
|
|
13
|
Wang Y, Yin B, Li D, Wang G, Han X and Sun
X: GSDME mediates caspase-3-dependent pyroptosis in gastric cancer.
Biochem Biophys Res Commun. 495:1418–1425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang
Y, Yu T, Wu X, Shi Y, Ma P and Shu Y: Pyroptosis: A new frontier in
cancer. Biomed Pharmacother. 121:1095952020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao L, Wu X, Zhang J, Liu L, Sui X, Zhang
R, Liu W, Shen F, Sun Y and Xi X: α-NETA induces pyroptosis of
epithelial ovarian cancer cells through the GSDMD/caspase-4
pathway. FASEB J. 33:12760–12767. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Webb K, Prakash V, Kirresh O and Stewart
A: A case of aortitis during cisplatin-based chemotherapy for
cervical cancer. BJR Case Rep. 5:201800542018.PubMed/NCBI
|
|
17
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tseng YY, Moriarity BS, Gong W, Akiyama R,
Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell
TC, et al: PVT1 dependence in cancer with MYC copy-number increase.
Nature. 512:82–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Zhang X, Hu X, Zhou W, Zhang P,
Zhang J, Yang S and Liu Y: The effects of lncRNA MALAT1 on
proliferation, invasion and migration in colorectal cancer through
regulating SOX9. Mol Med. 24:522018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Y, Feng SJ, Qiu S, Shao N and Zheng
JH: LncRNA MALAT1 promotes proliferation and metastasis in
epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med
Pharmacol Sci. 21:3176–3184. 2017.PubMed/NCBI
|
|
22
|
Lin N, Yao Z, Xu M, Chen J, Lu Y, Yuan L,
Zhou S, Zou X and Xu R: Long noncoding RNA MALAT1 potentiates
growth and inhibits senescence by antagonizing ABI3BP in
gallbladder cancer cells. J Exp Clin Cancer Res. 38:2442019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Q, Meng WY, Jie Y and Zhao H: LncRNA
MALAT1 induces colon cancer development by regulating
miR-129-5p/HMGB1 axis. J Cell Physiol. 233:6750–6757. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng C, Zhao Y, Li Y, Zhang T, Ma Y and
Liu Y: LncRNA MALAT1 promotes lung cancer proliferation and
gefitinib resistance by acting as a miR-200a sponge. Arch
Bronconeumol (Engl Ed). 55:627–633. 2019.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi B, Wang Y and Yin F:
MALAT1/miR-124/Capn4 axis regulates proliferation, invasion and EMT
in nasopharyngeal carcinoma cells. Cancer Biol Ther. 18:792–800.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Jiang T, Liang X, Shu S, Xiang X,
Zhang W, Guo T, Xie W, Deng W and Tang X: lncRNA MALAT1 mediated
high glucose-induced HK-2 cell epithelial-to-mesenchymal transition
and injury. J Physiol Biochem. 75:443–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Zhuo H, Ye MY, Huang GX, Fan M and
Huang XZ: LncRNA MALAT1 promoted high glucose-induced pyroptosis of
renal tubular epithelial cell by sponging miR-30c targeting for
NLRP3. Kaohsiung J Med Sci. 36:682–691. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
23:1424–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019:81518362019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong M, Yao Y and Zhang H: Antitumor
activity of enzymatically hydrolyzed Ganoderma lucidum
polysaccharide on U14 cervical carcinoma-bearing mice. Int J
Immunopathol Pharmacol. 33:20587384198694892019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang T, Li Y, Zhu R, Song P, Wei Y, Liang
T and Xu G: Transcription factor p53 suppresses tumor growth by
prompting pyroptosis in non-small-cell lung cancer. Oxid Med Cell
Longev. 2019:87468952019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
So D, Shin HW, Kim J, Lee M, Myeong J,
Chun YS and Park JW: Cervical cancer is addicted to SIRT1 disarming
the AIM2 antiviral defense. Oncogene. 37:5191–5204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rerucha CM, Caro RJ and Wheeler VL:
Cervical cancer screening. Am Fam Physician. 97:441–448.
2018.PubMed/NCBI
|
|
34
|
Prince S: Cervical cancer treatments:
Current challenges and future points of view. J Mol Oncol Res.
6:1252022.
|
|
35
|
PDQ Adult Treatment Editorial Board, .
Financial toxicity and cancer treatment (PDQ®): Health
professional version. 2022 Sep 20. PDQ Cancer Information Summaries
[Internet]. National Cancer Institute; Bethesda, MD: 2002
|
|
36
|
Chao X, Song X, Wu H, You Y, Wu M and Li
L: Selection of treatment regimens for recurrent cervical cancer.
Front Oncol. 11:6184852021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin L, Li Q, Hao W, Zhang Y, Zhao L and
Han W: Upregulation of LncRNA Malat1 induced proliferation and
migration of airway smooth muscle cells via miR-150-eIF4E/Akt
signaling. Front Physiol. 10:13372019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cooper DR, Wang C, Patel R, Trujillo A,
Patel NA, Prather J, Gould LJ and Wu MH: Human adipose-derived stem
cell conditioned media and exosomes containing MALAT1 promote human
dermal fibroblast migration and ischemic wound healing. Adv Wound
Care (New Rochelle). 7:299–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao K, Lin Y, Gao W, Xiao Z, Medina R,
Dmitriev P, Cui J, Zhuang Z, Zhao X, Qiu Y, et al: Blocking lncRNA
MALAT1/miR-199a/ZHX1 axis inhibits glioblastoma proliferation and
progression. Mol Ther Nucleic Acids. 18:388–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Xu L and Zhan X: LncRNA MALAT1
regulates diabetic cardiac fibroblasts through the Hippo-YAP
signaling pathway. Biochem Cell Biol. 98:537–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen P, Huang Y, Wang Y, Li S, Chu H and
Rong M: MALAT1 overexpression promotes the proliferation of human
periodontal ligament stem cells by upregulating fibroblast growth
factor 2. Exp Ther Med. 18:1627–1632. 2019.PubMed/NCBI
|
|
42
|
Li GQ, Fang YX, Liu Y, Meng FR, Wu X,
Zhang CW, Zhang Y, Liu D and Gao B: MALAT1-driven inhibition of wnt
signal impedes proliferation and inflammation in fibroblast-like
synoviocytes through CTNNB1 promoter methylation in rheumatoid
arthritis. Hum Gene Ther. 30:1008–1022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Wang F, Chen G, He R and Yang L:
LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3
axis. Cell Biosci. 9:542019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hao T, Wang Z, Yang J, Zhang Y, Shang Y
and Sun J: MALAT1 knockdown inhibits prostate cancer progression by
regulating miR-140/BIRC6 axis. Biomed Pharmacother. 123:1096662020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu N, Chen L, Wang C and Zhao H: MALAT1
knockdown inhibits proliferation and enhances cytarabine
chemosensitivity by upregulating miR-96 in acute myeloid leukemia
cells. Biomed Pharmacother. 112:1087202019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu A, Sun W and Mou F: lncRNA-MALAT1
promotes high glucose-induced H9C2 cardiomyocyte pyroptosis by
downregulating miR-141-3p expression. Mol Med Rep. 23:2592021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zuo Y, Chen L, He X, Ye Z, Li L, Liu Z and
Zhou S: Atorvastatin regulates MALAT1/miR-200c/NRF2 activity to
protect against podocyte pyroptosis induced by high glucose.
Diabetes Metab Syndr Obes. 14:1631–1645. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song Y, Yang L, Guo R, Lu N, Shi Y and
Wang X: Long noncoding RNA MALAT1 promotes high glucose-induced
human endothelial cells pyroptosis by affecting NLRP3 expression
through competitively binding miR-22. Biochem Biophys Res Commun.
509:359–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ruan J, Wang S and Wang J: Mechanism and
regulation of pyroptosis-mediated in cancer cell death. Chem Biol
Interact. 323:1090522020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou CB and Fang JY: The role of
pyroptosis in gastrointestinal cancer and immune responses to
intestinal microbial infection. Biochim Biophys Acta Rev Cancer.
1872:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Yang C, Li Y, Chen A, Li L and You
Z: LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome
formation. Biosci Rep. 38:BSR201711502018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu J, Yao L, Zhang M, Jiang J, Yang M and
Wang Y: Downregulation of LncRNA-XIST inhibited development of
non-small cell lung cancer by activating miR-335/SOD2/ROS signal
pathway mediated pyroptotic cell death. Aging (Albany NY).
11:7830–7846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren N, Jiang T, Wang C, Xie S, Xing Y,
Piao D, Zhang T and Zhu Y: LncRNA ADAMTS9-AS2 inhibits gastric
cancer (GC) development and sensitizes chemoresistant GC cells to
cisplatin by regulating miR-223-3p/NLRP3 axis. Aging (Albany NY).
12:11025–11041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen H, Lin X, Yi X, Liu X, Yu R, Fan W,
Ling Y, Liu Y and Xie W: SIRT1-mediated p53 deacetylation inhibits
ferroptosis and alleviates heat stress-induced lung epithelial
cells injury. Int J Hyperthermia. 39:977–986. 2022. View Article : Google Scholar : PubMed/NCBI
|




















