Introduction
Ovarian cancer (OC) ranked as the third most common type of cancer of the female reproductive system worldwide in 2020 (1), accounting for more mortalities than any other gynecological cancer type (2). Advanced disease typically presents with local abdominal metastases in the peritoneal cavity, whereas distant metastases are relatively rare (3). In general, cancer cell metastasis is a highly coordinated complex multistep process that requires the increased motility of the neoplastic cells, in order for them to approach surrounding host tissue and detach from the primary tumor (4). In OC, the flow of peritoneal fluid or blood generates physical forces that cause neoplastic cells to separate from the primary tumor and to enter either the abdominal cavity or the capillary bed. In 80% of patients with serous cancer, OC cells disseminate via either route, ultimately homing to the omentum, a known predilection site for OC metastasis, containing mainly adipose tissue (5-7). A previous study suggested that the omental fat pad plays a crucial role during the homing, nidation, invasion and subsequent growth and progression of the metastatic cells. In particular, it has been demonstrated that metastatic OC cells make contact to host omental adipocytes and obtain growth-promoting cytokines and energy-dense nutrients originating from those cells (8). Stromal fat cells interacting in this manner with the malignant cells have been termed as 'cancer-associated adipocytes' to account for the fact that they have been conditioned by the invading cancer cells to secrete factors promoting cancer cell survival and growth. These aggressive cancer cells stimulate the catabolism of stored triglycerides in the adipocytes to provide free fatty acids (FAs), which are then shifted from the adipocytes to the cancer cells and degraded by beta-oxidation for energy supply. This parasitic association feeds the OC cells at the expense of the cancer-associated adipocytes. The stromal cells, which have been derived from mesenchymal stem cells in the omentum, become the main energy source for OC cells at the metastatic site (5).
In general, FAs and lipids fulfill many vital functions in OC cells, building blocks for new cell membranes, raw material for essential biomolecules, providing bioenergy during β-oxidation, acting as second messengers in growth signaling, and as functional groups during post-translational modification of proteins (9-11). In addition to the omentum, the ascitic fluid in the peritoneal cavity, which often harbors substantial amounts of floating OC cells, is also rich in lipids. Thus, it has been recognized that OC belongs to a group of malignancies exquisitely dependent on FAs and lipids, more than on glucose, for energy supply and growth (12-15). Accordingly, the authors and another research group have previously demonstrated that FA synthase (FASN), the rate-limiting enzyme in endogenous de novo synthesis of saturated long chain-free FAs serving as raw material for lipid production, is routinely overexpressed in OC, causing hyperactivation of FA synthesis and accumulation of lipids (9,16). Moreover, it has been demonstrated that the inhibition of this pathway by drugs interfering with the enzyme function of FASN causes cell growth arrest and apoptosis, reduces chemoresistance and decelerates the clinical progression of OC (17,18). Therefore, it has been recognized that FASN represents a potentially powerful molecular target for novel anticancer drugs. The present study was less focused on the endogenous regulation of lipid biosynthesis in OC, since this has already been previously elucidated in great detail (13,14,16-24). For example, we and others have shown that activation of oncogenic signaling correlates with upregulation of endogenous FA production during development and progression of OC (14,16,17,20,22). Moreover, a direct molecular crosstalk between ErbB/AKT/PI3K signaling and FASN pathways has been described in OC (17,18,19,22). By contrast, the roles of stromal cells in the modulation of the effects of anti-lipogenic drugs have not yet been well documented. Consequently, in the present study, the interaction between cancer cells and associated stromal cells when de novo FA synthesis has been blocked was investigated. Notably, while ample evidence proves the anti-proliferative and pro-apoptotic efficacy of this category of therapeutics (17-20), their influence on the adipotaxis homing during OC cell metastasis described above remains largely unknown (25). Furthermore, the entire variety of responses available to cancer cells to alleviate or fully compensate for the drug-enforced blockade of endogenous FA supply has not yet been established. Such alternative lipid supply pathways will have significant bearing on possible resistance phenomena to FASN targeting therapeutics and on their effects on OC cell metastasis. In particular, the ability of cancer cells to counteract the depletion of FA and lipids due to the drug-mediated blockade of de novo FA synthesis and to activate bypass routes appears to be dependent on a number of factors determined by the cancer cells and the stromal cells. For example, a previous study by the authors demonstrated that OC cells in monoculture are highly sensitive to FASN blockers. Accordingly, they become eradicated before they can activate salvage pathways to import exogenous FA and lipids from the microenvironment (21). By contrast, the data reported in a study by Drury et al (26) suggested that colon cancer cells can counteract the effects of FASN inhibitors by upregulating the import of exogenous lipids from the environment. Apart from the difference in histological cancer type, another notable difference between these two studies was the detection status of stromal cells. In OC, experiments have been performed in monocultures of cancer cells without adipocytes (21), in colorectal cancer, experiments have been performed in the presence of adipocytes (26). Considering these differences in experimental design, the effects of co-cultured stromal adipocytes on the sensitivity of OC cells against FASN inhibitory drugs were examined in the present study. Multiple functional interdependences between fibroblasts and OC cells (27,28), and between adipocytes and breast cancer cells have been documented already in much detail (29). For example, cancer associated fibroblasts have been found to promote cell migration, metastasis and chemoresistance in OC cells through paracrine loops involving stromal derived factor-1α/CXCR4 and/or EGF/integrin α5 signaling (27,28). In addition, cancer-associated adipocytes have been shown to provide FA to breast cancer cells for proliferation and migration (29). By contrast, the interaction between OC cells and adipocytes is still an active area of research. Thus, the present study focused on the role of lipid metabolism during this crucial tumor-stroma crosstalk. Accordingly, the main aim of the present study was to assess the extent of OC cell exploitation of nearby adipocytes to counteract FA deficiency due to blocked FASN. In addition, the authors aimed to reconcile the inconsistencies between previously published colorectal cancer data and recent findings on OC through a more in-depth analysis. As a consequence, a number of different OC-adipocyte co-culture systems were applied and the effects of serum and of adipocytes of different origin on OC cell Transwell motility and intercellular transfer of FA from stroma adipocytes to the OC cells in the presence or absence of FASN inhibitory drugs were determined. The outcome may be useful to assess the probability of promoting secondary resistance to FASN inhibitors and metastasis.
Materials and methods
Maintenance of OC cell cultures and generation of stable mCherry-containing transgenic OC cell lines
A2780 (donated by Professor Michael Krainer, Department of Medicine I, Medical University Vienna, Austria) and OVCAR3 (ATCC, cat. no. HTB-161) OC cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% v/v fetal calf serum (FCS), 100 μg/ml penicillin/streptomycin (PS) (Gibco; Thermo Fisher Scientific, Inc.) and 2 mM glutamine (Gibco; Thermo Fisher Scientific, Inc.) at 37°C, 5% CO2 and 95% humidity (30,31), and absence of viral, bacterial, fungal and mycoplasmal infection was routinely monitored using a commercially available test system (Venor GeM; Merck KGaA, Darmstadt, Germany). Species origin was examined using species-specific PCR, and cell line authenticity was proven using fluorescent nonaplex-PCR of short tandem repeat markers by the Leibniz-Institut DSMZ GmbH.
High-level transgene expression of fluorescent proteins was achieved by the retroviral transduction of a cDNA for red mCherry, as previously described (32,33). Briefly, 2×106 293T cells (Takara Bio USA, Inc.) maintained in DMEM plus 10% FCS were transfected in T25 cell culture flasks (Thermo Fisher Scientific, Inc.) by calcium phosphate co-precipitation with 5 μg of the Moloney murine leukemia virus (MMLV)-derived retroviral expression plasmid pQCXIP (Takara Bio USA, Inc.) containing sequences for puromycin resistance and mCherry, and with 3.75 μg p-gag-pol-gpt (34) and 1.25 μg pVSV-G (Takara Bio USA, Inc.) as helper plasmids. Following the addition of the transfection mix, 293T cells were incubated at 37°C for 5 h. The transfection medium was then replaced with fresh medium. Supernatants of the resultant 293T transfectants were collected after 72 h, filtered through 0.22-μm cellulose acetate filters (Corning, Inc.) supplemented with 8 μg/ml polybrene (Merck KGaA) and added to A2780 or OVCAR3 OC cells for retrovirus transduction for 24 h without prior titering. The subsequent selection of mCherry-transgene expressing OC cells was performed with puromycin (0.5 μg/ml; Thermo Fisher Scientific, Inc.) for 2 weeks. These fluorescent mCherry-A2780 and mCherry-OVCAR3 OC cells were used for further experimentation.
Culture and adipogenic differentiation of mesenchymal cells 3T3L1 cells
Murine 3T3L1 fibroblasts (Zen-Bio Inc.) at 5×103/cm2 were cultured for 7 days in 'pre-adipocyte medium' with the addition of 1% penicillin/streptomycin/amphotericin (PSF; Zen-Bio Inc), changing the medium each day until the cells reached 100% confluency. After 2 days, the medium was changed to 'differentiation medium' (Zen-Bio Inc., cat. no. DM-2-L1) with the addition of PSF and the cells were allowed to differentiate into adipocytes for 72 h. Differentiated cultures were then maintained for 7-14 days in 'adipocyte medium' (Zen-Bio Inc.) with the addition of PSF; the medium was changed every other day and the cells were prepared for further experimentation as indicated by the accumulation of large lipid droplets in the cells using phase contrast microscopy with an Olympus IX73 microscope (Olympus Corporation) (Fig. S1A).
Human mesenchymal stem cells (hMSC) immortalized by stable transduction of the catalytic subunit of human telomerase (hTert; hMSC-Tert cells)
hMSC-Tert cells represent hMSC cells that have been stably transduced with the catalytic subunit of the human telomerase gene (hTert). They were kindly obtained from Professor Moustapha Kassem (Department of Endocrinology, University Hospital of Odense, Denmark) (35). Cells were seeded at a density of 3×104/cm2 in DMEM (Gibco; Thermo Fisher Scientific, Inc.), with the addition of also 10% FCS and 100 μg/ml PS and cultured for 2 days until reaching a 80-100% confluency. Cells were then washed with PBS (Gibco; Thermo Fisher Scientific, Inc.) and the medium was changed to DMEM containing 5% FCS, 100 μg/ml PS, 0.1 μM dexamethasone, 450 μM 3-isobutyl-1-methylxanthine, 2 μM insulin, 1 μM rosiglitazone and 1 μM UO126 (MilliporeSigma) to initiate adipogenic differentiation, which was continued for 18 days with the medium changed daily. The final medium included only DMEM, 5% FCS and 100 μg/ml PS without supplementing any additional additives. After 2 days, adipocytes were ready for experimental assays as demonstrated by the accumulation of large lipid droplets in the cells using phase contrast microscopy with an Olympus IX73 microscope (Olympus Corporation) (Fig. S1B).
Human primary omental cells
Pooled human primary omental cells were collected at Zen Bio, Inc. after obtaining informed consent from the donors. In accordance with the Declaration of Helsinki, the use of this cell line did not require a formal vote of the Ethics Committee of the Medical University of Vienna, since it was a commercially available cell collection. Therefore, it was waived by the named ethics committee. After purchase from Zen-Bio, Inc. (cat. no. OP-F-3; https://www.zen-bio.com/products/cells/visceral_adipocytes.php) the cryopreserved cells were plated in three types of proprietary culture media ('omental pre-adipocyte medium', 'omental differentiation medium', 'omental adipocyte medium') obtained from Zen-Bio Inc at a density of 4×104/cm2 and grown for 1 day in 'omental pre-adipocyte medium' (Zen-Bio Inc, cat. no. OM-PM) to reach 100% confluency. The medium was then changed to 'omental differentiation medium' (Zen-Bio Inc, cat. no. OM-DM) and the cells were incubated for 7 days at 37°C before the 'omental differentiation medium' was replaced with 'omental adipocyte medium' (Zen-Bio Inc, cat. no. OM-AM), and cells were incubated for another 7 days. Mature adipocytes were ready for further experimentation after a total of 15-16 days, as shown by the accumulation of large lipid droplets in the cells using phase contrast microscopy with an Olympus IX73 microscope (Olympus Corporation) (Fig. S1C).
FASN inhibitors
FASN inhibitory drugs G28UCM (Professor Ramon Colomer, Universidad Autónoma de Madrid; Professor María. L. López Rodríguez, Universidad Complutense de Madrid, Spain) (36-38) and Fasnall (Professor Timothy A.J. Haystead, Duke University School of Medicine Durham, NC; Professor Jesse J. Kwiek, The Ohio State University, Columbus, OH) (39) were dissolved in 100% DMSO and further diluted 1:1,000 in RPMI-1640 medium.
Migration assay Serum-stimulated OC cell migration
In total, 5×104 mCherry-A2780 or 12.5×104 mCherry-OVCAR3 OC cells expressing the red fluorescent transgene were plated in RPMI-1640 medium containing 0.4% FCS in 24-well FluoroBlok cell culture inserts (apical chamber) with pores of 8 μm diameter (Corning, Inc.), which were inserted into the basal chambers of 24-well plates (Corning, Inc.) containing RPMI-1640 medium with 0, 5 or 30% FCS as chemoattractant. For the direct treatment of the OC cells, solvent (DMSO ≤0.1% v/v) without (control groups) or with 0.63-90 μM of the FASN inhibitors G28UCM or Fasnall (experimental groups) was added. The sustainability of the drug effects was examined by pretreatment of the OC cells for 24 h at 37°C with solvent (control groups) or 0.63-90 μM of the inhibitors (experimental groups) before drug washout with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) and transfer into the migration assembly. Constitutively fluorescent OC cells harboring the mCherry transgene were then allowed to migrate for 24 h through the pores from the apical chamber down to the basal chamber before determining fluorescence in the basal chamber of the migration assembly with a SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices, LLC) and/or with an Olympus IX73 fluorescence microscope (Olympus Corporation) followed by quantification using ImageJ 1.54G software (National Institutes of Health).
3T3L1 adipocyte-stimulated OC cell migration
The technical setup, loading of fluorescent OC cells, direct drug treatment and pretreatment followed by subsequent drug washout with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.), and data acquisition of the migration assay were performed as described above for serum-stimulated OC cell migration, with the exception that the basal chambers of the 24-well plates (Corning, Inc.) contained 'adipocyte media' (Zen-Bio Inc., cat. no. AM-1-L1) with or without differentiated murine 3T3L1 adipocytes as chemoattractants.
hMSC-Tert adipocyte-stimulated OC cell migration
The methodology of the migration assay was performed as described above, with the exception that the basal chambers of the 24-well plates contained adipogenic differentiated hMSC-Tert cells in DMEM medium which contained 5% FCS and 1% PS (35) as a chemoattractant.
Primary human omental adipocyte-stimulated OC cell migration
The methodology of the migration assay was as described above with the exception that that the basal chambers of the 24-well plates contained differentiated primary human omental adipocytes in 'omental adipocyte medium' (Zen-Bio Inc, cat. no. OM-AM) as chemoattractants.
Measuring FA transfer from adipocytes to OC cells
Sterile glass cloning cylinders (Merck KGaA) were placed in the center of the wells of black-walled, flat- and clear-bottomed 24-well plates (Ibidi GmbH). Subsequently, murine or human mesenchymal cells were transferred into these wells outside the cylinders at densities of 5×103/cm2 (3T3L1), 3×104/cm2 (hMSC-Tert) or 4×104/cm2 (human primary omental cells) and subjected to the adipogenic differentiation protocols as described. When adipocytes were fully differentiated, 3×104 fluorescent OC cells were plated into the cloning cylinders and allowed to attach overnight. Subsequently, the cells were exposed for 24 h to medium without (control groups) or with 0.63-90 μM of the FASN inhibitors G28UCM or Fasnall (experimental groups). Cells were subsequently washed with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) and the adipocytes were depleted from FCS for 1 h prior to the addition of medium containing 1.5 μM green fluorescent FA marker Bodipy FL C16 (Thermo Fisher Scientific, Inc.). Differentiated adipocytes were allowed to incorporate the fluorescent FAs for 4 h, followed by thorough washout with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) of excess non-incorporated marker and the removal of the cloning cylinders. Hereafter, the OC cells were in close contact with the adipocytes and were able to receive fluorescent FAs from them. Transcellular transfer was maintained for 24 h, with cells either continuing to be treated ('Direct Treatment' regimen) with media containing solvent alone (control groups) or solvent plus 0.63-90 μM of the inhibitors (experimental groups) or left untreated during that FA transfer period ('Pretreatment' regimen) before the experiment was terminated. Transfer of the green fluorescent FA marker Bodipy from adipocytes to OC cells was measured in the central OC monolayer using a SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices, LLC). The data obtained for FA import were correlated with the red fluorescence signals derived from the transgene label constitutively expressed in the OC, which were then used to estimate cell numbers for data normalization and to detect growth inhibitory effects of the FASN antagonists. Fluorescent labels were also evaluated with an Olympus IX73 fluorescence microscope (Olympus Corporation) followed by quantification using ImageJ 1.54G software (National Institutes of Health).
Statistical analysis
The results are presented as mean values ± standard deviation of at least triplicate experiments. Where appropriate, differences between control and experimental data were analyzed for statistical significance using one-tailed Student's t-test or two-way ANOVA followed by post hoc Tukey's or Scheffe tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of FASN promotes OC cell motility and migration
Using an in vitro fluorescent cell migration system, the migration of mCherry-A2780 and mCherry-OVCAR3 OC cell lines was determined at baseline culture conditions (0% FCS, control group) and in the presence of 5 or 30% FCS as a chemoattractant (experimental groups). It was observed that the chemotactic activity of mCherry-A2780 and mCherry-OVCAR3 OC cells was highly sensitive to serum. Baseline motility at 0% FCS was markedly lower in mCherry-OVCAR3 than in mCherry-A2780 OC cells; however, maximal positive chemotaxis was achieved at an FCS concentration of 5% in both cell lines (Fig. S2). These OC cells are thus chemotactically attracted along the serum concentration gradient.
Subsequently, the effects of the FASN inhibitors, G28UCM and Fasnall, on the migration of mCherry-A2780 cells were determined. This cell line represents the best characterized OC cell lipid metabolism model in our hands and was therefore used as the preferred system for subsequent assays. In Fig. 1A and S3A-C it is demonstrated that cell migration was slightly stimulated following 24 h of drug exposure in the presence of serum (black bars in Fig. 1A). Of note, when the cells were treated with G28UCM (Fig. 1A, left panel), a drug-mediated increase in cell migration was observed, even in the absence of serum (white bars in Fig. 1A). Thus, FASN inhibitors, previously shown to block de novo lipid synthesis (18-20,38) promote cell motility. Notably, when the cells were pretreated with FASN inhibitors for 24 h and the assay was then performed in drug-free medium, the increase in cell migration was much more pronounced and was evident even at lower drug concentrations, regardless of the presence or absence of serum (Fig. 1B). These results suggested that random motility (white bars in Fig. 1) and targeted chemo-taxis (black bars in Fig. 1) were stimulated by the drugs and increased even further as cells recovered from a transient drug exposure (Fig. 1B).
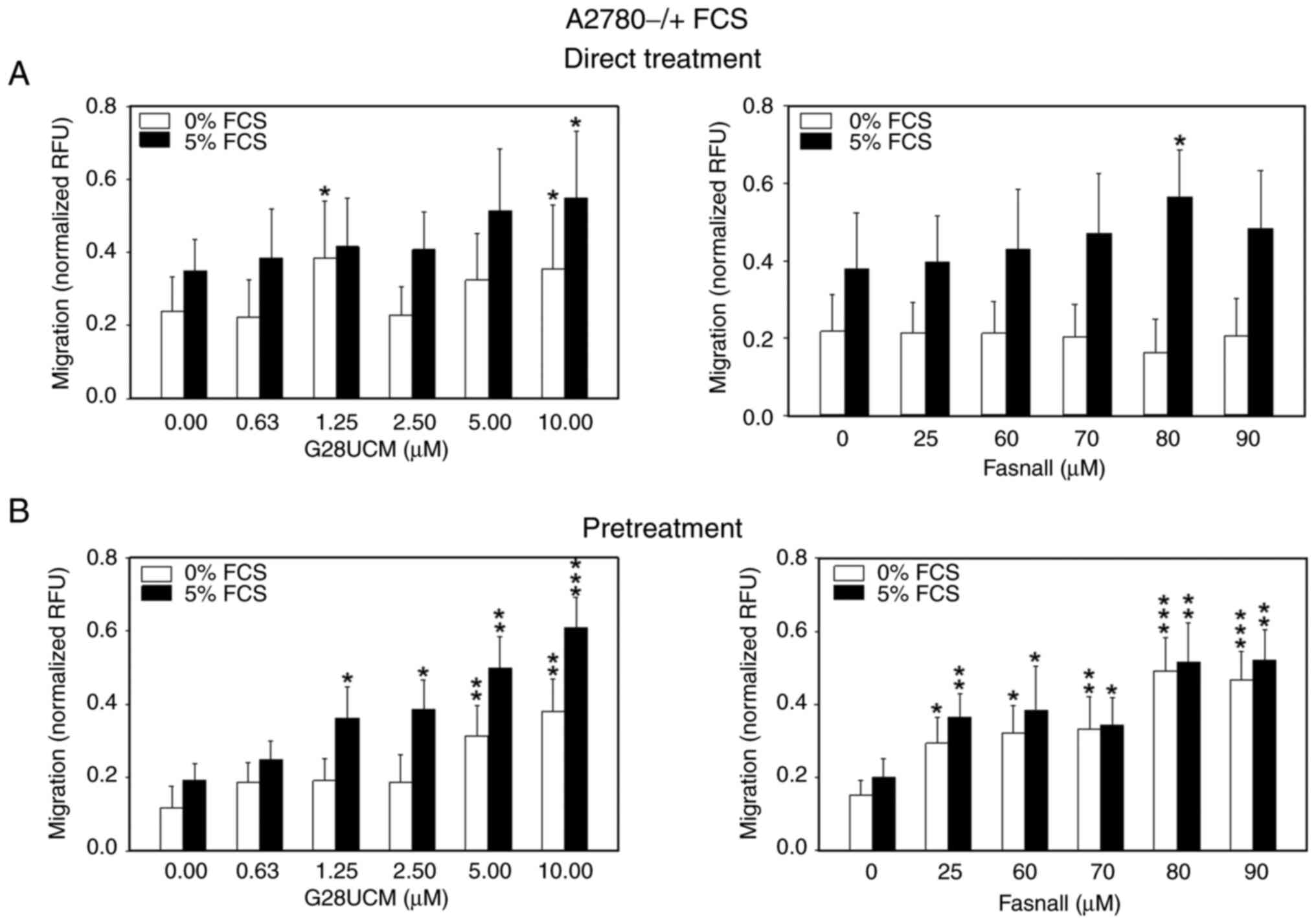 |
Figure 1.
Migration of OC cells is stimulated by FASN inhibitors even in the absence of serum. (A) 5×104 red fluorescent mCherry-A2780 OC cells were plated in media containing 0.4% FCS in 24-well FluoroBlok cell culture inserts (apical chamber) with pores of 8 μm diameter, which were inserted into the basal chambers of 24-well plates containing media with 0% (white bars) or 5% FCS (black bars) as chemoattractant. Subsequently, solvent (DMSO, final concentration ≤0.1%) without (control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall (experimental groups), was added ('Direct Treatment'). (B) Fluorescent mCherry-A2780 cells were first pretreated for 24 h with plain solvent (control group) or with various concentrations of FASN inhibitors G28UCM or Fasnall in RPMI-1640 containing 5% FCS (experimental groups). Solvent- and FASN-inhibitor pretreated mCherry-A2780 OC cells were then transferred to drug-free RPMI-1640 with 0.4% FCS and plated in the apical chamber. Basal chambers contained media with 0% (white bars) or 5% FCS (black bars; 'Pretreatment'). In both experimental settings presented in A and B, fluorescent cells were then allowed to migrate for 24 h through the pores from the apical chamber down to the basal chamber before determining fluorescence in the basal chamber with a fluorometer. Data are provided in RFU normalized to seeded mCherry-A2780 cell number. All data are presented as the mean ± SD, n ≥3. Data were analyzed using two-way ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM drug) in 0% (white bars) or 5% FCS (black bars), respectively. OC, ovarian cancer; FASN, fatty acid synthase; FCS, fetal calf serum; RFU, relative fluorescence units; SD, standard deviation.
|
During progression, OC cells detach from the primary tumor and spread via the peritoneal fluid or the blood to colonize abdominal tissues surrounding the peritoneal cavity. Among these, the omental adipose tissue is a highly favored site for invasion and settlement. Moreover, omental adipocytes have been shown to directly feed metastatic OC cells with high energy nutrients, including FAs (8). A consequent aim in the present study was to investigate whether the presence of adipocytes in proximity to the cancer cells can alter OC cell motility. As expected, OC cell migration was stimulated when OC cells were co-cultured with 3T3L1 differentiated murine adipocytes, albeit mCherry-OVCAR3 were less responsive than mCherry-A2780 cells (Fig. S4). Evidence from previous studies suggests that cancer-associated fibroblasts can exert similar stimulating effects on OC cell migration and metastasis. Thus, it appears that the cancer-promoting activity of stromal cells in OC is not only limited to adipocytes, but may also occur in fibroblasts (27,28). Subsequently, it was examined whether the blockade of de novo fatty acid synthesis is able to alter the migratory ability of the OC cells. To this end, mCherry-A2780 OC cells were co-cultured with 3T3L1 adipocytes in the migration assembly. In Fig. 2 it is demonstrated that the FASN inhibitors, G28UCM (left panels) and Fasnall (right panels), stimulated mCherry-A2780 cell migration under baseline conditions in the absence (Fig. 2A, white bars) or presence of adipocytes in a concentration-dependent manner (Figs. 2A, black bars, and S3D-F). However, when the cancer cells were pretreated with the FASN inhibitors and the drugs had been washed out prior to the initiation of the co-culture, drug-mediated stimulation of cell migration was only observed in the absence (Fig. 2B, white bars), but not in the presence of adipocytes (Fig. 2B, black bars) relative to 0 μM inhibitor (control group), respectively. Two fully human co-culture systems were then employed. While data indicated that differentiated hMSC-Tert adipocytes attracted mCherry-A2780 OC cells less strongly than 3T3L1 adipocytes [as can be observed comparing normalized relative fluorescence units (RFU) values from untreated controls of Fig. 2, black bars (>1.5 RFU) with those presented in Fig. 3 (≤1.0 RFU)], direct treatment of the cells with FASN inhibitors markedly upregulated OC migration towards hMSC-Tert cells (Figs. 3A and S3G-I). However, when the FASN inhibitors were washed out prior to co-cultivation, stimulation of OC cell attraction to hMSC-Tert adipocytes was observed only at relatively high drug concentrations (Fig. 3B). By contrast, the drug-induced stimulation of OC cell attraction was less intensive when differentiated primary mortal human omental adipocytes were used instead of the immortalized hMSC-Tert cell line. Under these conditions, drug-induced mCherry-A2780 cell attraction to adipocytes was not robust at all. It only occurred after direct treatment with the highest concentration of G28UCM or after pretreatment with the highest dose of Fasnall (Figs. 4 and S3J-L).
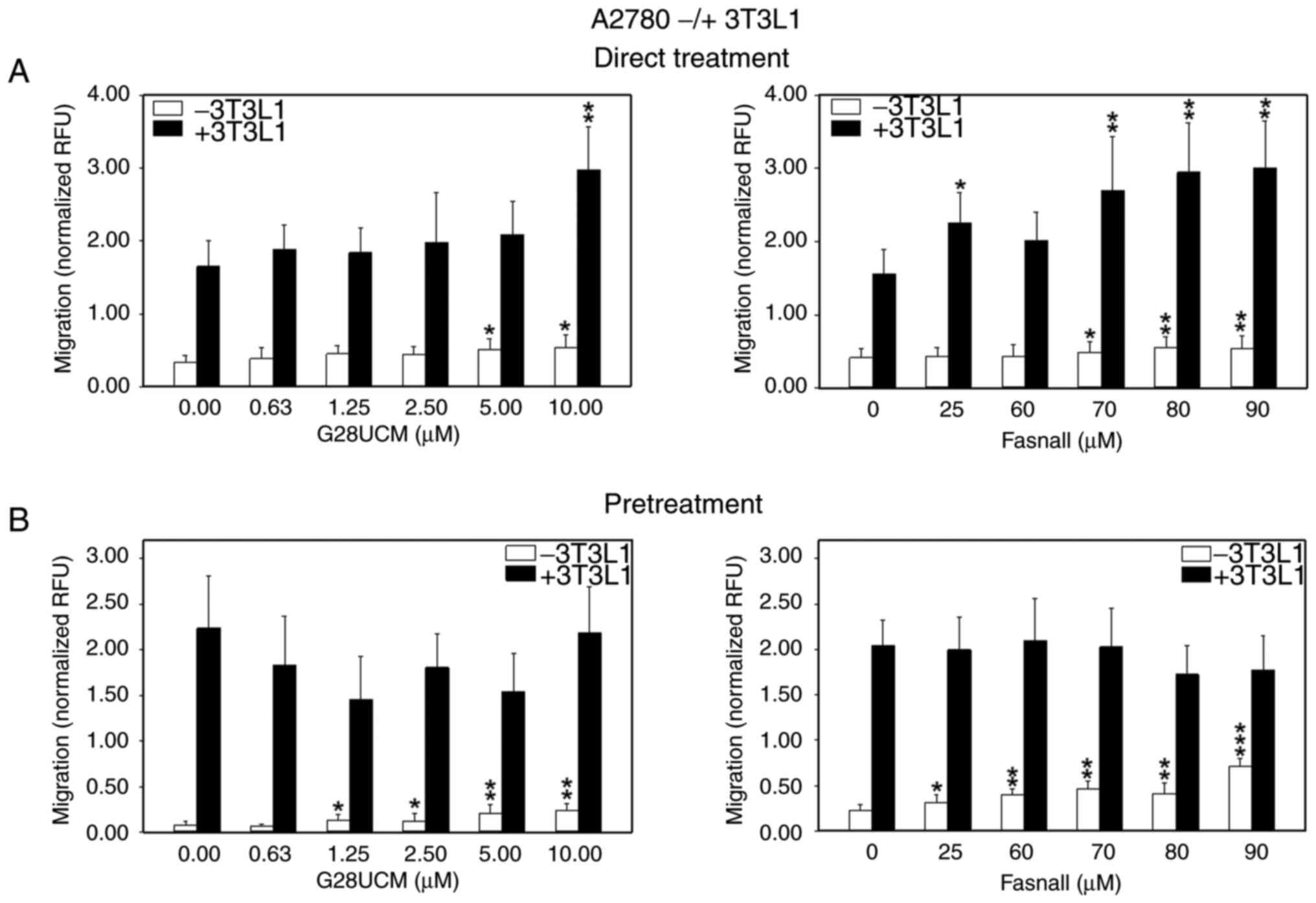 |
Figure 2.
Effects of FASN inhibitors on the migration of OC cells in the absence (white bars) or presence (black bars) of differentiated murine 3T3L1 adipocytes. (A) In total, 5×104 red fluorescent mCherry-A2780 OC cells per insert were plated in RPMI-1640 containing 5% FCS in 24-well FluoroBlok cell culture inserts (apical chamber) with pores of 8 μm diameter, which were inserted into the basal chambers of 24-well plates containing adipocyte media with (black bars) or without differentiated murine 3T3L1 adipocytes (white bars) as chemoattractant. Subsequently, solvent (DMSO, final concentration ≤0.1%) without (0 μM control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall, (experimental groups) was added ('Direct Treatment'). (B) FluorescentmCherry-A2780 cells were first pretreated for 24 h with plain solvent (0 μM control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall in RPMI-1640 containing 5% FCS (experimental groups). Drugs were then washed out and OC cells were transferred into the apical chambers. Basal chambers contained adipocyte media without (white bars) or with differentiated murine 3T3L1 adipocytes (black bars) ('Pretreatment'). In both experimental settings presented in (A and B), fluorescent cells were then allowed to migrate for 24 h through the pores from the apical chamber down to the basal chamber before determining fluorescence in the basal chamber with a fluorometer. Data are provided in RFU, normalized to seeded mCherry-A2780 cell number/insert. All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control group (0 μM). FASN, fatty acid synthase; OC, ovarian cancer; RFU, relative fluorescence units; SD, standard deviation.
|
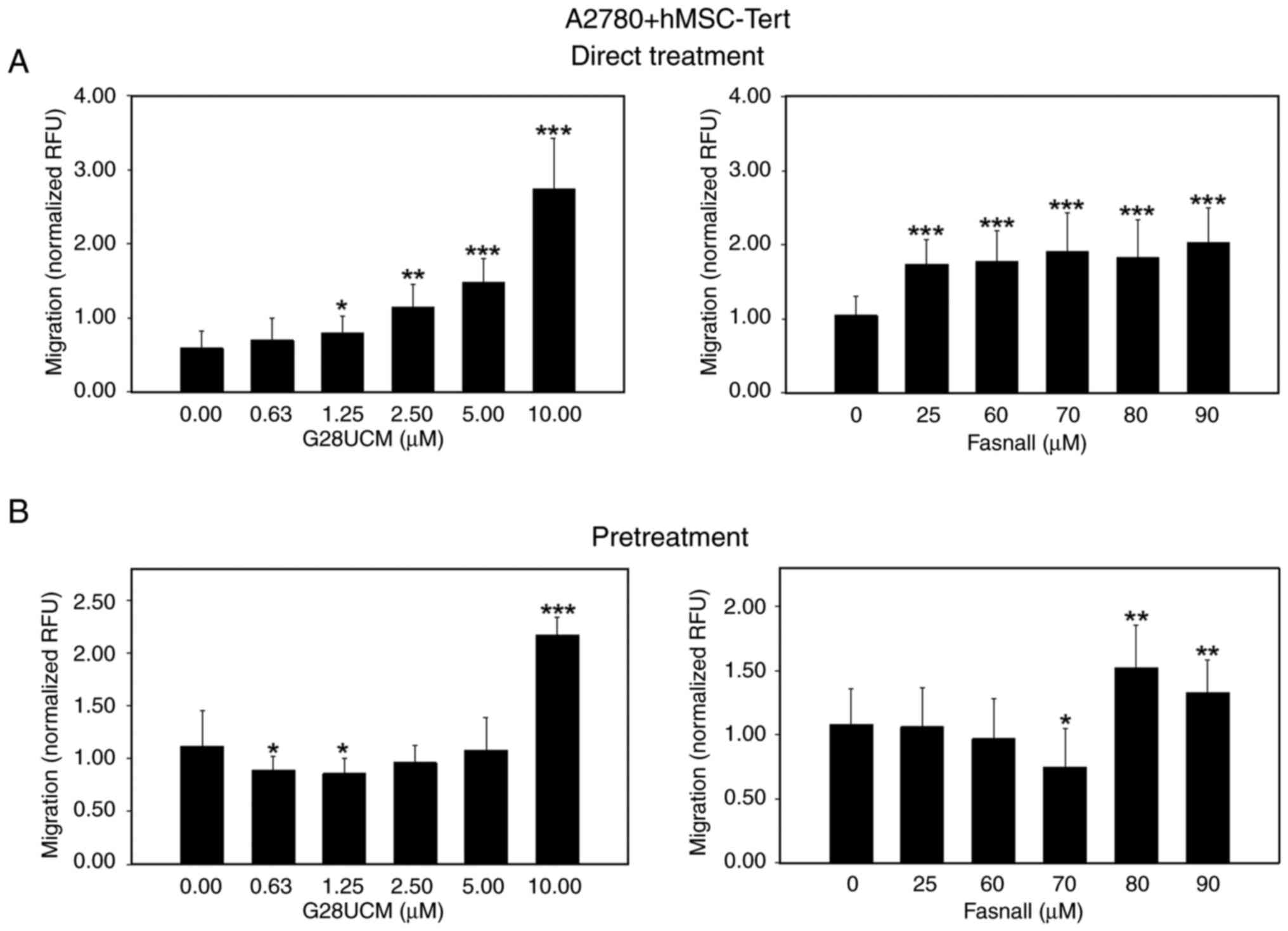 |
Figure 3.
Effects of FASN inhibitors on the migration of OC cells in the presence of an adipogenic differentiated telomerase-immortalized human mesenchymal stem cell line (hMSC-Tert). (A) In total, 5×104 red fluorescent mCherry-A2780 OC cells per insert were plated in RPMI-1640 containing 5% FCS in 24-well FluoroBlok cell culture inserts (apical chamber) with pores of 8 μm diameter, which were inserted into the basal chambers of 24-well plates containing differentiated human hMSC-Tert adipocytes in DMEM, 1% PS and 5% FCS as chemoattractant. Subsequently, solvent (DMSO, final concentration ≤0.1%) without (control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall (experimental groups), was added ('Direct Treatment'). (B) Fluorescent mCherry-A2780 cells were first pretreated for 24 h with plain solvent (control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall (experimental groups), in RPMI-1640 medium containing 5% FCS. Drugs were then washed out and OC cells were transferred into the apical chambers. Basal chambers contained differentiated human hMSC-Tert adipocytes in DMEM, 1% PS and 5% FCS ('Pretreatment'). In both experimental settings presented in (A and B), fluorescent mCherry-A2780 OC cells were then allowed to migrate for 24 h through the pores from the apical chamber down to the basal chamber before determining fluorescence in the basal chamber with a fluorometer. Data are provided in RFU, normalized to seeded mCherry-A2780 cell number/insert. All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM). FASN, fatty acid synthase; OC, ovarian cancer; FCS, fetal calf serum; hMSC-Tert, human mesenchymal stem cells immortalized by stable transduction of the catalytic subunit of human telomerase; RFU, relative fluorescence units; SD, standard deviation.
|
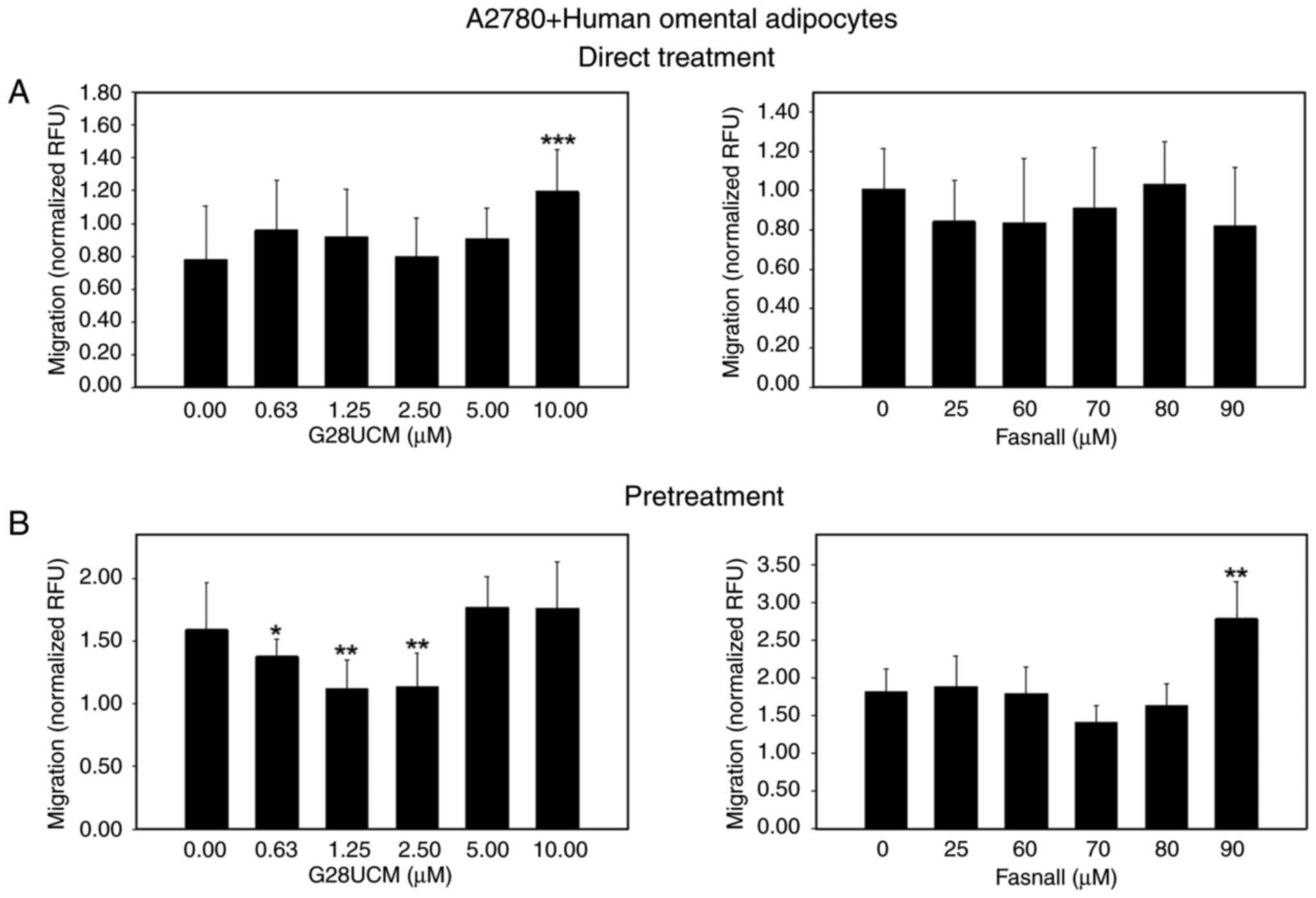 |
Figure 4.
Effects of FASN inhibitors on the migration of OC cells in the presence of differentiated primary human omental adipocytes. (A) In total, 5×104 red fluorescent mCherry-A2780 OC cells per insert were plated in RPMI-1640 medium containing 5% FCS in 24-well FluoroBlok cell culture inserts (apical chamber) with pores of 8 μm in diameter, which were inserted into the basal chambers of 24-well plates containing differentiated primary human omental adipocytes in omental adipocyte medium as chemoattractant. Subsequently, solvent (DMSO, final concentration ≤0.1%) without (control groups) or with various concentrations of the FASN inhibitors G28UCM or Fasnall (experimental groups), was added ('Direct Treatment'). (B) Fluorescent mCherry-A2780 cells were first pre-treated for 24 h with plain solvent (control groups) or with various concentrations of the FASN inhibitors, G28UCM or Fasnall (experimental groups) in RPMI-1640 containing 5% FCS. Drugs were then washed out and OC cells were transferred into the apical chambers. Basal chambers contained differentiated human omental adipocytes in omental adipocyte medium ('Pretreatment'). In both experimental settings A and B, fluorescent mCherry-A2780 OC cells were then allowed to migrate for 24 h through the pores from the apical chamber down to the basal chamber before determining fluorescence in the basal chamber with a fluorometer. Data are provided in RFU normalized to seeded mCherry-A2780 cell number/insert. All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM). FASN, fatty acid synthase; OC, ovarian cancer; FCS, fetal calf serum; RFU, relative fluorescence units; SD, standard deviation.
|
FASN inhibition promotes the transfer of FAs from adipocytes to OC cells
Following the investigation of whether FASN inhibitors are able to stimulate migration of OC cells towards stromal fat cells, the possible functional significance of this behavior was then analyzed. Specifically, it was investigated whether the inhibitors could facilitate the transfer of FAs from the fat cells to the OC cells, thereby promoting a parasitic nutritive loop in order to compensate for the lack of endogenous FA supply in the drug-treated cancer cells. To this end, a dedicated co-culture system was established, as specified in the Materials and methods section. The results presented in Figs. 5 and S5 revealed that the FASN inhibitors, G28UCM or Fasnall, stimulated the transfer of FAs from 3T3L1 adipocytes to mCherry-A2780 OC cells in a concentration-dependent manner to reach maximum extent, after which a plateau or a decline was observed, regardless of whether the cells were continuously (Figs. 5A, black bars, and S5A-C) or transiently (Fig. 5B, black bars) exposed to the drugs. The only difference observed was that the level of transfer was markedly lower following a temporary drug exposure (compare the y-axis scaling of Fig. 5A, black bars, and B, black bars). When differentiated hMSC-Tert or primary human omental adipocytes were used instead of murine 3T3L1, the effects of continuous drug exposure were relatively weakened when the OC cells were co-cultured with hMSC-Tert (Fig. 6A, black bars), but very prominent when cultured with human primary omental cells (Fig. 7A, black bars; Fig. S5D-F). The FA transfer was generally less strongly stimulated when the OC cells were only transiently exposed to the FASN inhibitors (Figs. 6B, and 7B, black bars). FA transport from adipocytes to OC cells certainly depends on the loading capacity of the co-cultivated adipocytes. To properly assess the data of FA transport into the OC cells, it was therefore essential to investigate to what extent the different types of adipocytes can import and buffer the fluorescent fatty acid marker first and then provide it to the OC cells, and whether this uptake may be influenced by the FASN drug treatment. As shown in Fig. S6A-C and J, it was demonstrated that 3T3L1 adipocytes accumulate the fluorescent label maximally followed by hMSC-Tert and primary human omental adipocytes, and it was observed that FA uptake into adipocytes was essentially resistant to FASN inhibitor treatment (Fig. S6D-I).
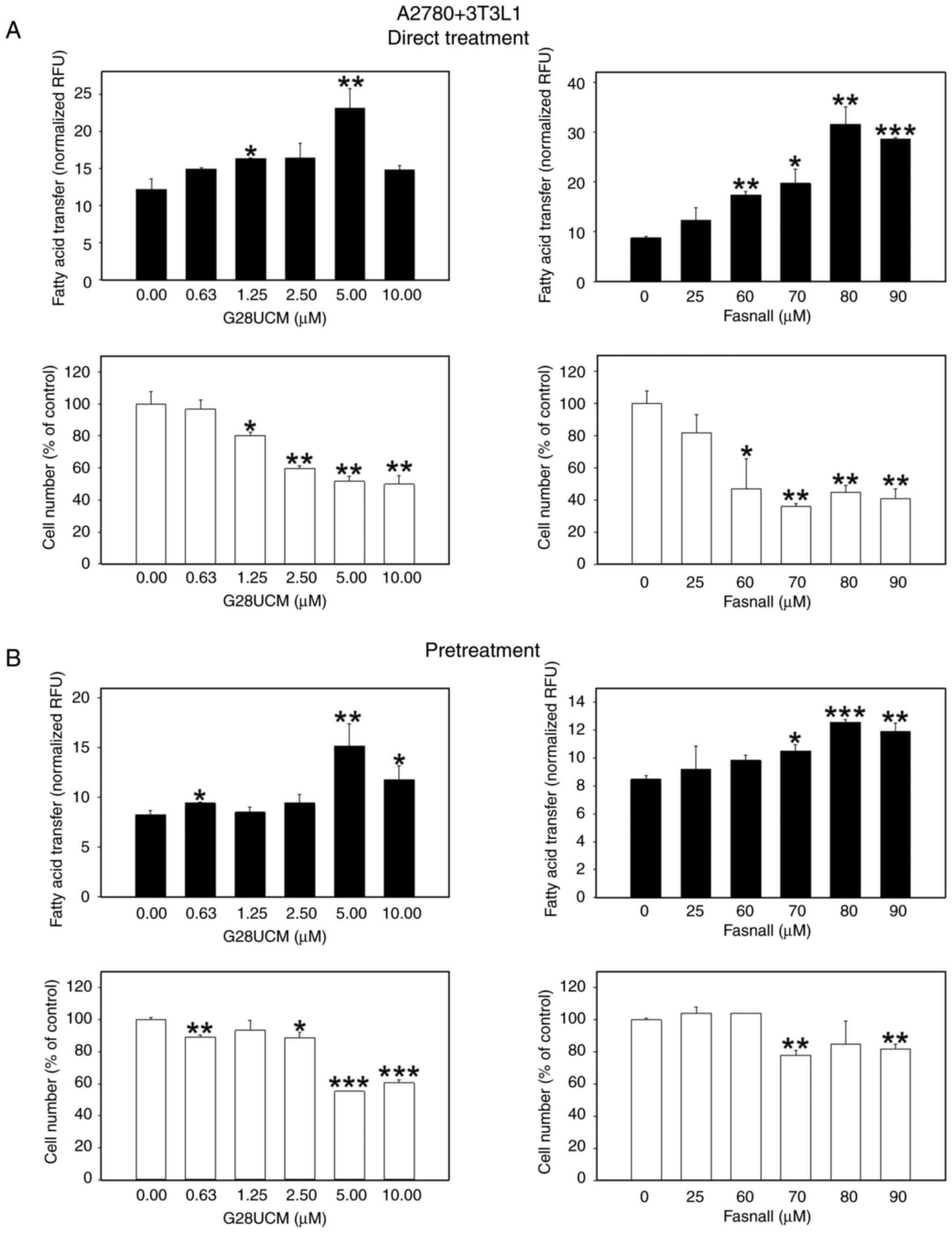 |
Figure 5.
Effects of the FASN inhibitors on the transcellular transfer of FA from differentiated murine 3T3L1 adipocytes into OC cells. In total, 3×104 red fluorescent mCherry-A2780 OC cells/cylinder suspended in RPMI-1640 with 5% FCS containing solvent (DMSO, final concentration ≤0.1%) without (0 μM, control groups) or with various concentrations of the FASN inhibitors G28UCM or Fasnall (experimental groups) were plated inside of glass cloning cylinders in the center of the wells of 24-well plates. On the outside of the cloning cylinders the wells contained monolayers of 7.5×103 differentiated murine 3T3L1 adipocytes in adipocyte media. Following a 24-h drug exposure of mCherry-A2780 OC cells, the 3T3L1 adipocyte monolayers were serum deprived for 1 h and then labeled with 1.5 μM of the green fluorescent FA marker Bodipy FL C16. After 4 h, adipocytes were washed once with HBSS with the addition of 0.2% FA-free BSA before the glass cloning cylinders separating OC cells from adipocytes were removed. All cells were then washed again five times with HBSS with the addition of 0.2 % FA-free BSA and incubated in RPMI-1640 containing 1% FCS with ('Direct Treatment') (A) or without ('Pretreatment') (B) various concentrations of G28UCM or Fasnall and co-cultured for another 24 h. The experiment was terminated by removing the media and washing the co-cultures once with 1% HBSS with 0.2% FA-free BSA before determining the import of the green fluorescent Bodipy FA marker from peripheral 3T3L1 adipocytes into the mCherry-A2780 OC cells with a fluorometer. The number of the central red fluorescent mCherry-A2780 OC cell was also determined by fluorometry. Data of green fluorescent Bodipy FA uptake into G28UCM-(left panels) or Fasnall-treated (right panels) mCherry-A2780 OC cells (experimental groups) and into untreated cells (0 μM, control groups) are provided in RFU, normalized to mCherry-A2780 cell number (black bars), and mCherry-A2780 OC cell numbers (white bars) were expressed as % of untreated solvent (≤0.1% DMSO) control cells (0 μM, control groups). All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM, control groups). FASN, fatty acid synthase; FA, fatty acids; OC, ovarian cancer; HBSS, Hank's balanced solution; BSA, bovine serum albumin; RFU, relative fluorescence units; SD, standard deviation.
|
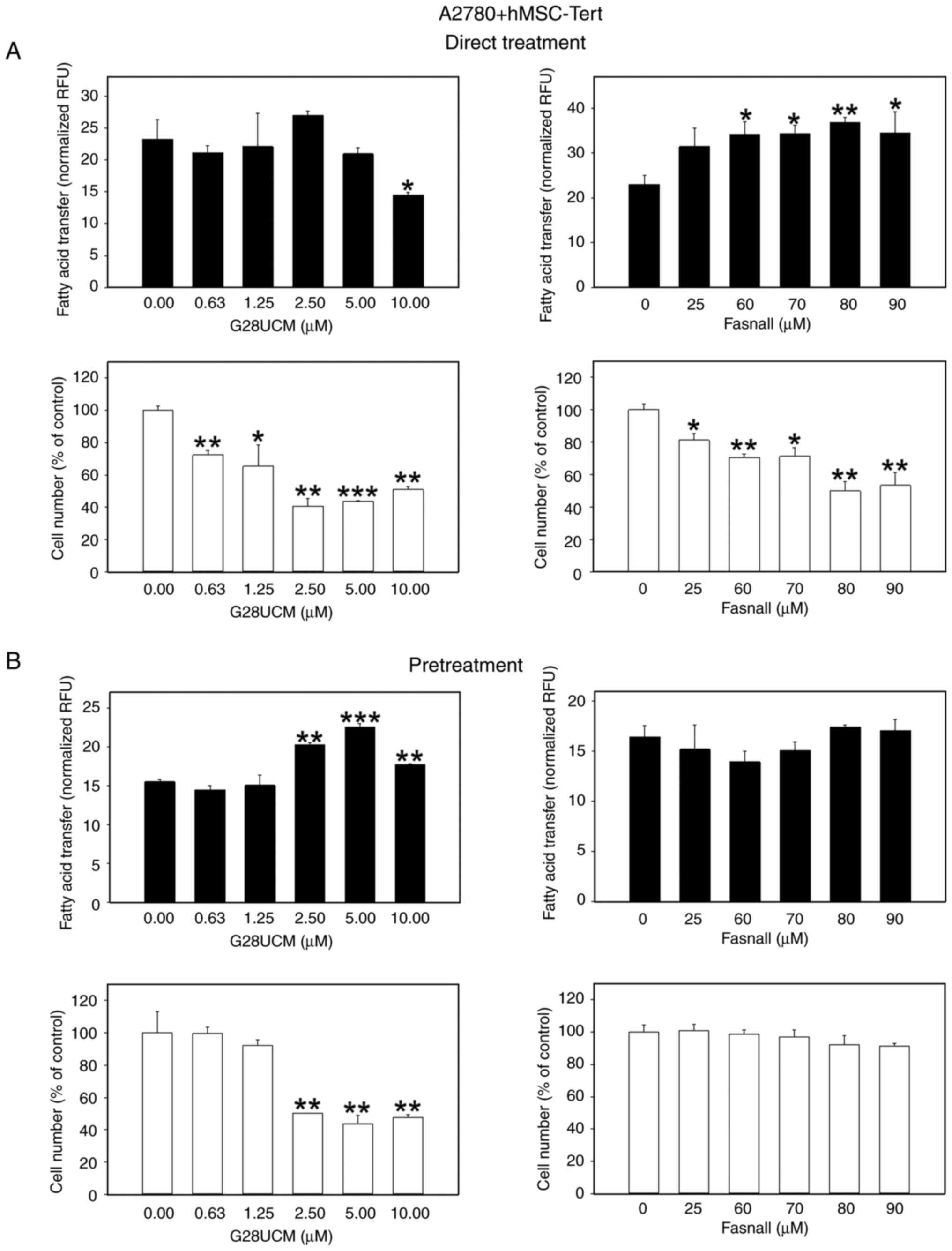 |
Figure 6.
Effects of FASN inhibitors on the transcellular transfer of FA from differentiated human hMSC-Tert adipocytes into OC cells. The experimental procedure was as described in Fig. 5 and in the Material and methods section except that on the outside of the cloning cylinders the wells contained monolayers of 4.5×104 differentiated hMSC-Tert adipocytes in DMEM with 5% FCS and 1% PS. After removing the cloning cylinders, all cells were washed 5X with HBSS with 0.2 % FA-free BSA and incubated in RPMI-1640 containing 1% FCS with ('Direct Treatment') (A) or without ('Pretreatment') (B) various concentrations of G28UCM or Fasnall and co-cultured for another 24 h. Data of green fluorescent Bodipy FA uptake into G28UCM-(left panels) or Fasnall-treated (right panels) mCherry-A2780 OC cells (experimental groups) and into untreated cells (0 μM, control groups) are provied in RFU normalized to mCherry-A2780 cell number (black bars), and mCherry-A2780 OC cell numbers (white bars) were expressed as % of untreated solvent (≤0.1% DMSO) control cells (0 μM, control groups). All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM, control groups). FASN, fatty acid synthase; FA, fatty acids; hMSC-Tert, human mesenchymal stem cells immortalized by stable transduction of the catalytic subunit of human telomerase; OC, ovarian cancer; FCS, fetal calf serum; PS, penicillin/streptomycin; RFU, relative fluorescence units; SD, standard deviation.
|
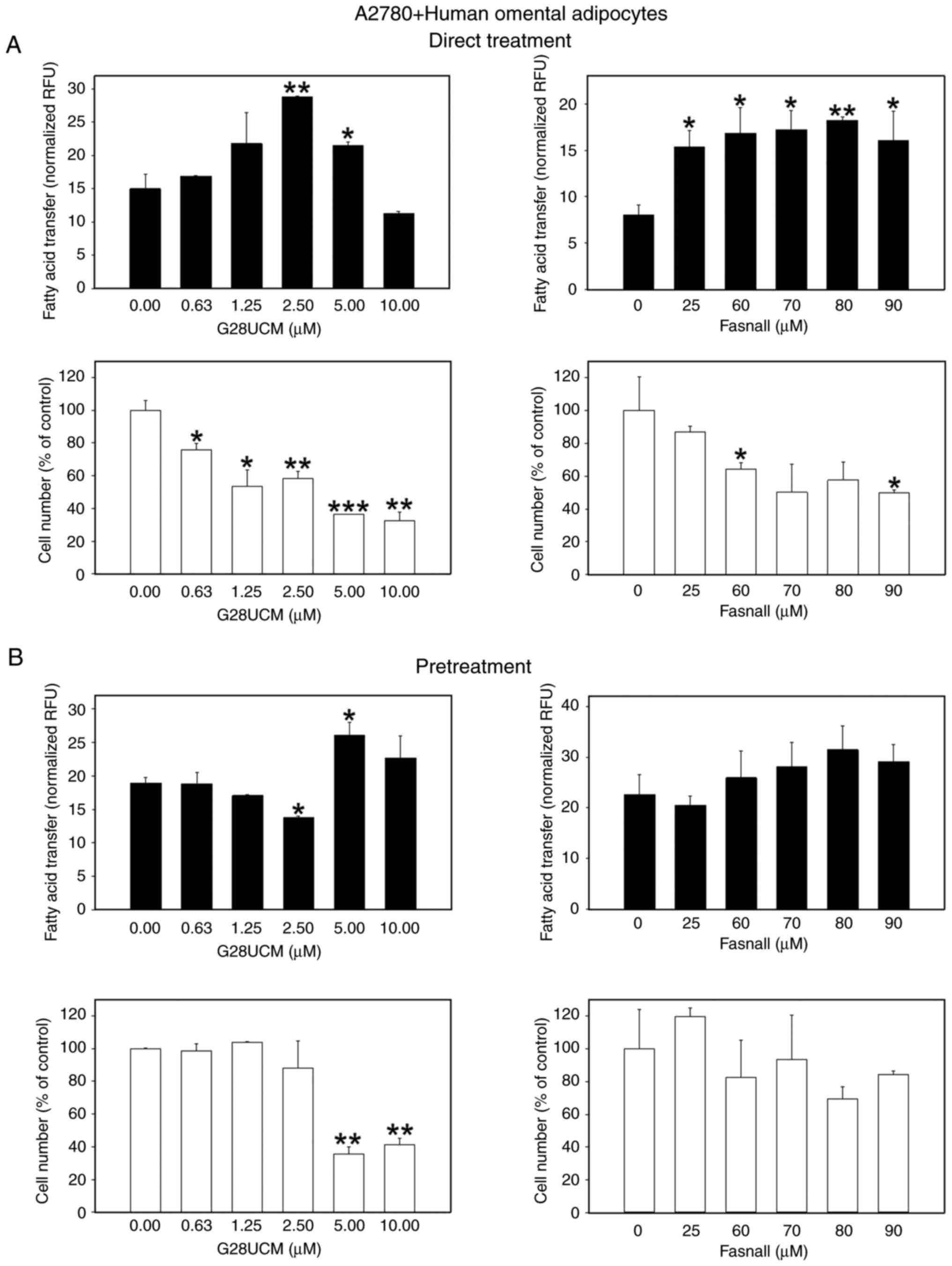 |
Figure 7.
Effects of FASN inhibitors on the transcellular transfer of FA from differentiated primary human omental adipocytes into OC cells. The experimental procedure was as described in Fig. 5 and in the Material and methods section except that on the outside of the cloning cylinders the wells contained monolayers of 6×104 differentiated human omental adipocytes in omental adipocyte media. Following the removal of the cloning cylinders, all cells were washed five times with HBSS with 0.2 % FA-free BSA and incubated in RPMI-1640 containing 1% FCS with ('Direct Treatment') (A) or without ('Pretreatment') (B) various concentrations of G28UCM or Fasnall and co-cultured for another 24 h. Data of green fluorescent Bodipy FA uptake into G28UCM-(left panels) or Fasnall-treated (right panels) mCherry-A2780 OC cells (experimental groups) and into untreated cells (0 μM, control groups) are provided in RFU) normalized to mCherry-A2780 cell number (black bars), and mCherry-A2780 OC cell numbers (white bars) were expressed as % of untreated solvent (≤0.1% DMSO) control cells (0 μM, control groups). All data are presented as the mean ± SD, n ≥3. Data were analyzed using ANOVA, followed by a Tukey's post hoc test. *P<0.05, **P<0.01 and ***P<0.001, relative to the solvent treated control (0 μM, control groups). FASN, fatty acid synthase; FA, fatty acids; OC, ovarian cancer; HBSS, Hank's balanced solution; BSA, bovine serum albumin; RFU, relative fluorescence units; SD, standard deviation.
|
Stromal adipocytes are unable to abolish the growth-inhibiting effects of FASN inhibitors on OC cells
Subsequently, it was investigated whether the drug-induced migration and supply of exogenous FAs may affect the anticancer activity of the drugs. To this end, the anti-proliferative effects of the FASN inhibitors on the OC cells in co-culture with 3T3L1 (Figs. 5, white bars, and S7), hMSC-Tert (Fig. 6, white bars), or primary human omental adipocytes (Fig. 7, white bars) were determined. Regardless of the origin of the co-cultured adipocytes, a significant concentration-dependent reduction in the OC cell count was observed following direct treatment with FASN inhibitors (Figs. 5A, 6A and 7A, white bars). This effect was less pronounced when the cells were only transiently exposed to the drugs, particularly when Fasnall was used (Figs. 5B, 6B and 7B, white bars).
Discussion
Metabolic pathways in cancer cells reveal numerous striking differences compared to their non-malignant counterparts. Accordingly, metabolic reprogramming in cancer cells has now been classified as a cancer hallmark (40,41). Nevertheless, the functional architecture and regulation of metabolic pathways varies a lot between individual types of cancer. For example, in OC, a close dependence on fatty acid- and lipid-metabolism rather than on aerobic glycolysis, the latter also known as the 'Warburg effect', has been demonstrated (13-15). OC cells typically grow in a low-oxygen environment in pelvic compartments (13), producing malignant ascites and spreading to the omentum mainly via the peritoneal cavity. Ascites fluid and omentum are poor in glucose but rich in lipids, therefore establishing lipids as the main fuel for OC (12,13). Accordingly, in OC cells the authors and other research groups observed not only elevated FASN expression and FA synthesis, but also intense FA import from ascites and omental compartments. This uptake is mediated by a variety of FA receptor and transport proteins. Together, these processes efficiently replenish the intracellular lipid stores in OC cells (8,14,21,22,42-46). Due to the crucial dependence on FA synthesis, the inhibition of FASN has been considered a promising therapeutic strategy against OC. While the direct anticancer effects of FASN inhibitory drugs have been well documented, particularly for OC among other tumors (18-20,23), the direct impact of these drugs on cancer cell motility and cell interactions at metastatic sites has not yet been established. However, this issue has significant relevance for the development of secondary resistance against FASN inhibitory drugs and increased metastasis. Therefore, the aim of the present study was to determine the effects of FASN enzyme inhibitors on cancer cell motility and on transcellular nutrition from cancer-associated adipocytes. The present study, to the best of our knowledge, is the first to demonstrate that FASN inhibitors stimulate random motility and targeted movement of OC cells toward adipocytes (Figs. 2-4 and S3), and facilitate the transfer of FA from adipocytes to OC cells (Figs. 5-7 and S5).
The drug-induced concentration-dependent transcellular passage of FA from the fat cells into the cancer cells was of moderate magnitude, and increased to a maximum at a medium drug concentration, subsequently decreasing again. In general, untreated OC cells responded differently to adipocytes of different provenience. OC cells were attracted to 3T3L1 adipocytes about twice as much, as compared to hMSC-Tert or primary omental adipocytes. Moreover, 3T3L1 cells accumulated lipids more prominently in comparison with hMSC-Tert and omental adipocytes. The lipid stores of 3T3L1 adipocytes were thus fully charged, while hMSC-Tert and omental adipocytes absorbed significantly less fluorescent label (Fig. S6A-C and J). This issue must definitely be considered when interpreting the data on FA uptake in cancer cells. Overall, this is provocative novel data requiring thoughtful interpretation.
Firstly, the main products of the enzyme reaction catalyzed by FASN are long-chain saturated FAs (47). Therefore, the lipid membranes of FASN-rich cancer cells typically contain more saturated and less unsaturated FA than those of normal cells. This imbalance affects the physical properties of the cancer cell membranes. Their rigidity increases with increasing proportion of saturated FA (48-50). FASN blocking, as for example by an inhibitor, reverts the lipid composition to non-malignant (14,39) and membrane fluidity gets restored, facilitating cell plasticity, flexibility and motility (48,50). Therefore, the observed FASN inhibitor-mediated increase of cell motility most likely is due to the diminished degree of saturation of the lipid membranes. Notably, the OC cells were very sensitive to the drugs, since the effect was observed even in unstimulated cells, in the absence of serum and/or adipocytes (Figs. 1 and 2), and even when the inhibitor has already been withdrawn following temporary drug exposure (Figs. 1B and 2B). In addition, long-term direct treatment with FASN inhibitors further enhanced adipocyte-stimulated migration of OC cells (Fig. 2A). According to Nieman et al (8), OC cell attraction to adipocytes is mediated by adipokines, including interleukin-8, which has been identified in the secretome of cancer-associated adipocytes. In the model applied in the present study, adipotaxis appeared to be a slow process requiring long-term exposure to the drugs and was hardly observed after drug washout (Figs. 2B and 4B).
FASN inhibitor-treated OC cells become more motile, and also appear to receive more FAs from the co-cultured adipocytes. Transcellular FA transfer exhibited a dose-dependent maximum. Beyond that, the number of labelled FAs in the OC cells decreased even below the baseline level of the untreated cells on some occasions. A potential limitation of the present study may be that not all micrographs used in the semi-quantification of fluorescence intensity, when confirming the fluorography data, are available. However, generally, fluorography is much more reliable and superior to microscopy when quantification is required, which was the preferred method for the present study. The observed moderate, yet statistically significant tendency of treated OC cells to import more exogenous FAs from nearby adipocytes is a challenging new finding of particular interest. Although the data presented in the current study are in line with evidence from colorectal cancer (26), they are contradictory to our previous report where we demonstrated that inhibition of FASN does not increase the uptake of exogenous lipids in OC cells (21). Thus, we are faced with the discerning task of reconciling these seemingly contradictory data and giving them a deeper meaning. Drury et al (26) reported that inhibition of FASN in colorectal cancer cells may cause increased import of exogenous FA, whereas the opposite has been previously observed in OC, where decreased FA uptake was observed when endogenous de novo synthesis of FA was inhibited (21). A number of biological differences between both systems may account for this discordance. Whereas colorectal cancer cell cultures could withstand long-term blockade or persistent knockdown of FASN without being eradicated (26), cultivated OC cells were clearly of increased sensitivity (21). This may explain the different outcomes of the previous studies in colorectal carcinoma vs. those in OC; however, it does not resolve the inconsistency arising between the novel data presented here and our previous results in OC (21). A comparison of the conditions of the current culture setup with those of our previous model may however clarify the issue. In the present model, instead of exploring uptake of freely dissolved FA from the extracellular space, the direct intercellular transfer of FA from co-cultured stroma cells into the OC cells after FASN inhibitor treatment was investigated. This process requires mobilization and discharge of FAs from fat cell stores to become available for cancer cells. Accordingly, tumor-associated adipocytes have been reported to exhibit increased lipolysis. Previously published evidence suggests that adipocyte-derived interleukins-6 and -8 attract OC cells toward the adipocytes. Once attached to the adipocytes the OC cells induce hormone-sensitive lipase/adipose triglyceride lipase-mediated lipolysis in fat cells (29,51) to mobilize FAs from triglycerides stored in the lipid droplets of the fat cells (29). Fatty acid binding protein 4 (FABP4) then carries the liberated FA to the adipocyte membrane and passes them on to a lipid binding protein known as fatty acid translocase/cluster of differentiation 36 (CD36), which is overexpressed on the plasma membrane of the OC cells enabling the cancer cells to accumulate exogenous FA (8,15,24,52) and use them for energy production through β-oxidation (8). Notably, data on colorectal cancer indicate that blockade of FASN further aggravates upregulation of CD36 and fuels FA uptake and β-oxidation (26). Thus, the cessation of endogenous FA biogenesis renders cancer cells fully dependent on exogenous supply of lipids. Importantly, whereas this condition enables survival of the bulk tumor cells, it does not support survival of the cancer stem cell population. These self-sufficient and self-renewing cells are essential for sustained tumor growth and progression (4). It has been demonstrated that OC stem cells contain high levels of FASN and fatty acid desaturases (53-57), designating them as independent from external lipid supply. Thus, targeting endogenous production of FA may represent an efficient strategy to specifically eradicate OC stem cells. Overall, multiple evidence points the way to some very promising antitumor therapeutic strategies. For instance, FA handling proteins, including FABP4 or CD36 can be targeted, or lipogenic enzymes, including FASN or FA desaturase, can be blocked. Both strategies can be used either as single drug treatments or in combination.
Therefore, different biological processes and culture conditions were examined (import from cell-free extracellular space versus direct transfer from one cell type to another). Co-culture models naturally reflect the in vivo situation better than monocultures. Accordingly, OC cells have been reported to be less sensitive to drug treatment when cultured in the presence of supportive stroma (13,48). For example, in OC monocultures maximal effects on FA uptake were obtained with concentrations as low as 1.25 μM G28UCM and 25 μM Fasnall (21), whereas in the present study, 3- to 4-fold higher concentrations of inhibitor were required for maximal changes in FA uptake. Furthermore, in the present study, the inhibition of FASN culminated in an increase instead of a decrease of FA uptake. A reduction was observed only when maximal concentrations of G28UCM or Fasnall were used, which probably was due to direct cancer cell toxicity of these high drug doses. The data of the present study corroborate previous reports demonstrating discrepancies between in vitro and in vivo efficacy of FASN inhibitory drugs in cancer (59).
The aim of the present study was to investigate the role of cancer-associated stromal fat cells in alleviating FA deficiency in OC cells exposed to FASN blockers. A second aim was to reconcile the inconsistencies between previous data in colorectal cancer and our recent findings in OC monocultures. FASN inhibition has been demonstrated in the former study to stimulate FA import into colorectal cancer cells (26), in our study however, the blockade of FASN reduced the uptake of FA into OC cells (21). Accordingly, it is now required to refine our earlier conclusion that FASN inhibitors do not stimulate the uptake of exogenous fatty acids, which applied for monoculture. In mixed cell culture, however, it appears to have stimulating effects on FA uptake. While stromal adipocytes may be unable to neutralize the anti-proliferative effects of FASN inhibitors, in the clinical setting the therapeutic efficacy of FASN inhibition may be attenuated by compensatory upregulation of dietary import of exogenous FAs. This issue has not yet been definitively resolved but establishes FA transport pathways as a major focus for molecular targeted treatment. Data presented here suggest that OC cells are rendered more tolerant to FASN drugs when co-cultured with adipocytes and activate salvage pathways, in order to obtain required FAs from the adipocytes. Consequently, surviving OC cells become dependent from adipocyte-derived lipids and change their phenotype towards higher migration, invasiveness and metastatic potential. Interestingly, 5-(tetradecyloxy)-2-furoic acid, which targets acetyl-CoA carboxylase, another lipogenic enzyme upstream of FASN, has previously been demonstrated to accelerate malignant progression, particularly cell migration and invasion, in chemoresistant cancer cells (59). This may suggest that stromal cells may reduce the therapeutic efficacy of FASN inhibitors under certain conditions. A combined inhibition of fatty acid synthesis and fatty acid transport could therefore be a powerful approach against OC. Fortunately, we have recently shown that OC cells are highly sensitive to inhibitors of FA transport pathways impeding FA import, blocking cell multiplication and inducing apoptosis (15). Consequently, studies combining interference with endogenous FA biosynthesis and exogenous FA import, are warranted and ongoing. Of note, recent data obtained in obese mice on a high-fat diet with elevated body weights and in cancer cells cultured in high-fat media revealed decreases in p53 levels and p53-dependent transcription, causing elevated colony formation and increased cell migration. In addition, tumor-bearing mice on a high-fat diet had an increased tumor burden, increased angiogenesis and decreased apoptosis (60). Similarly, two pancreatic cancer cell lines cultured in oleic acid demonstrated increased proliferation and cell migration (61). These types of studies establish a connection between molecular regulation of cancer metabolism and systemic nutrition. Moreover, experimental data indicate that pro-inflammatory cytokines, including interleukins-6 and -8, among other molecules, attract OC cells towards adipocytes. In addition, lipogenic gene expression is known to be controlled by epigenetic modulators, including coactivator-associated arginine methyltransferase 1 (CARM1) (62) and by lipogenic transcription factors including the family of sterol regulatory element-binding proteins (SREBP) (63). Moreover, SREBP1 has been reported to stimulate the migration and invasion of breast and OC cells (64). Thus, it is highly probable that CARM1 will contribute to stimulation of adipotaxis homing of OC cells. Although this issue was outside the scope of the present study, it may be quite a promising topic for follow-up studies.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TWG conceived and planned the experiments, supervised the study, and wrote the manuscript. RW carried out the experiments. TWG and RW analyzed the samples and the data. AR and MG planned and produced stable transfectants. ASB and MP contributed critical conception and design of the study. PV assisted in data interpretation and manuscript writing. TWG and RW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Pooled human primary omental cells were collected at Zen Bio, Inc. after obtaining informed consent from the donors. In accordance with the Declaration of Helsinki, the use of this cell line did not require a formal vote of the Ethics Committee of the Medical University of Vienna, since it was a commercially available cell collection. Therefore, it was waived by the named ethics committee.
Patient consent for publication
Not applicable.
Competing interests
MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Servier. All other authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Acknowledgments
The authors would like to thank Ramón Colomer (Hospital Universitario la Princesa, Madrid, Spain) and María Luz López-Rodríguez (Universidad Complutense de Madrid, Spain) for supply of G28UCM, and Timothy A.J. Haystead, Philip Hughes (Duke University School of Medicine, Durham, NC) and Jesse J. Kwiek (Ohio State University, Columbus, OH) for providing Fasnall. In addition, credit is given to Moustapha Kassem (University Hospital of Odense, Denmark) for providing hMSC-Tert cells.
Funding
The present study was financially supported by the 'Initiative Krebsforschung' of the Medical University of Vienna, and by the Herzfelder Familienstiftung, Vienna, Austria. Further financial support was acquired through funds originating from the Austrian Federal Ministry for Digital and Economic Affairs, the Austrian National Foundation for Research, Technology and Development and the Christian Doppler Research Association.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar
|
|
2
|
American Cancer Society: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html retrieved on 23.12.09.
|
|
3
|
Lee MI, Jung YJ, Kim DI, Paik HJ, Lee S, Jung CS, Kim JY and Kim HY: Metastasis to breast from ovarian cancer and primary ovarian cancer concurrently diagnosis. Gland Surg. 10:1806–1811. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schulenburg A, Brämswig K, Herrmann H, Karlic H, Mirkina I, Hubmann R, Laffer S, Marian B, Shehata M, Krepler C, et al: Neoplastic stem cells: Current concepts and clinical perspectives. Crit Rev Oncol Hematol. 76:79–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, Broaddus R, Kolonin M, Mok SC, Thompson MS, et al: Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 8:e818592013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST and Mok SC: Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 309:C444–C4456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rickard BP, Conrad C, Sorrin AJ, Ruhi MK, Reader JC, Huang SA, Franco W, Scarcelli G, Polacheck WJ, Roque DM, et al: Malignant ascites in ovarian cancer: Cellular, acellular, and biophysical determinants of molecular characteristics and therapy response. Cancers (Basel). 13:43182021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al: Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 17:1498–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grunt TW: Interacting Cancer Machineries: Cell signaling, lipid metabolism, and epigenetics. Trends Endocrinol Metab. 29:86–98. 2018. View Article : Google Scholar
|
|
10
|
Scaglia N, Frontini-López YR and Zadra G: Prostate cancer progression: As a matter of fats. Front Oncol. 11:7198652021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao Y, Yi Q, XiaoWu X, WeiBo C, GuangChen Z and XueMin C: Acetyl-CoA: An interplay between metabolism and epigenetics in cancer. Front Mol Med. 2:10445852022. View Article : Google Scholar
|
|
12
|
Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Ogishima J, et al: Detachment from the primary site and suspension in ascites as the initial step in metabolic reprogramming and metastasis to the omentum in ovarian cancer. Oncol Lett. 15:1357–1361. 2018.PubMed/NCBI
|
|
13
|
Chen RR, Yung MMH, Xuan Y, Zhan S, Leung LL, Liang RR, Leung THY, Yang H, Xu D, Sharma R, et al: Targeting of lipid metabolism with a metabolic inhibitor cocktail eradicates peritoneal metastases in ovarian cancer cells. Commun Biol. 2:2812019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grunt TW, Slany A, SemkovaM, Colomer R, López-Rodrı́guez ML, Wuczkowski M, Wagner R, Gerner C and Stübiger G: Membrane disruption, but not metabolic rewiring, is the key mechanism of anticancer-action of FASN-inhibitors: A multi-omics analysis in ovarian cancer. Sci Rep. 10:148772020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lemberger L, Wagner R, Heller G, Pils D and Grunt TW: Pharmacological inhibition of lipid import and transport proteins in ovarian Cancer. Cancers (Basel). 14:60042022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji Z, Shen Y, Feng X, Kong Y, Shao Y, Meng J, Zhang X and Yang G: Deregulation of lipid metabolism: The critical factors in ovarian cancer. Front Oncol. 10:5930172020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A and Testa JR: Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 24:3574–3582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner R, Stübiger G, Veigel D, Wuczkowski M, Lanzerstorfer P, Weghuber J, Karteris E, Nowikovsky K, Wilfinger-Lutz N, Singer CF, et al: Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells. Oncotarget. 8:11600–11613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomek K, Wagner R, Varga F, Singer CF, Karlic H and Grunt TW: Blockade of fatty acid synthase induces ubiquitination and degradation of phosphoinositide-3-kinase signaling proteins in ovarian cancer. Mol Cancer Res. 9:1767–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veigel D, Wagner R, Stübiger G, Wuczkowski M, Filipits M, Horvat R, Benhamú B, López-Rodríguez ML, Leisser A, Valent P, et al: Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. Int J Cancer. 136:2078–2090. 2015. View Article : Google Scholar
|
|
21
|
Grunt TW, Lemberger L, Colomer R, López Rodríguez ML and Wagner R: The pharmacological or genetic blockade of endogenous de novo fatty acid synthesis does not increase the uptake of exogenous lipids in ovarian cancer cells. Front Oncol. 11:6108852021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grunt TW, Wagner R, Grusch M, Berger W, Singer CF, Marian B, Zielinski CC and Lupu R: Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells. Biochem Biophys Res Commun. 385:454–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang CS, Matsuura K, Huang NJ, Robeson AC, Huang B, Zhang L and Kornbluth S: Fatty acid synthase inhibition engages a novel caspase-2 regulatory mechanism to induce ovarian cancer cell death. Oncogene. 34:3264–3272. 2015. View Article : Google Scholar :
|
|
24
|
Zhao G, Cardenas H and Matei D: Ovarian Cancer-Why Lipids Matter. Cancers (Basel). 26:18702019. View Article : Google Scholar
|
|
25
|
Gálvez BG, San Martín N and Rodríguez C: TNF-alpha is required for the attraction of mesenchymal precursors to white adipose tissue in Ob/ob mice. PLoS One. 4:e44442009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drury J, Rychahou PG, He D, Jafari N, Wang C, Lee EY, Weiss HL, Evers BM and Zaytseva YY: Inhibition of fatty acid synthase upregulates expression of CD36 to sustain proliferation of colorectal cancer cells. Front Oncol. 10:11852020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai JM, Sun K, Li C, Cheng M, Guan JH, Yang LN and Zhang LW: Cancer-associated fibroblasts contribute to cancer metastasis and apoptosis resistance in human ovarian cancer via paracrine SDF-1α. Clin Transl Oncol. 25:1606–1616. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Q, Yang Z, Xu S, Li X, Yang X, Jin P, Liu Y, Zhou X, Zhang T, Gong C, et al: Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J Exp Med. 216:688–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, et al: Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5:12017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grunt TW, Somay C, Oeller H, Dittrich E and Dittrich C: Comparative analysis of the effects of dimethyl sulfoxide and retinoic acid on the antigenic pattern of human ovarian adenocarcinoma cells. J Cell Sci. 103:501–509. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harant H, Korschineck I, Krupitza G, Fazeny B, Dittrich C and Grunt TW: Retinoic acid receptors in retinoid responsive ovarian cancer cell lines detected by polymerase chain reaction following reverse transcription. Br J Cancer. 68:530–536. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Inglés-Prieto Á and Janovjak H: Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 33:1713–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ries A, Flehberger D, Slany A, Pirker C, Mader JC, Mohr T, Schelch K, Sinn K, Mosleh B, Hoda MA, et al: Mesothelioma-associated fibroblasts enhance proliferation and migration of pleural mesothelioma cells via c-Met/PI3K and WNT signaling but do not protect against cisplatin. J Exp Clin Cancer Res. 42:272023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Markowitz D, Goff S and Bank A: A safe packaging line for gene transfer: Separating viral genes on two different plasmids. J Virol. 62:1120–1124. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG and Kassem M: Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 20:592–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blancafort A, Giró-Perafita A, Oliveras G, Palomeras S, Turrado C, Campuzano Ò, Carrión-Salip D, Massaguer A, Brugada R, Palafox M, et al: Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs. PLoS One. 10:e01312412015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Puig T, Turrado C, Benhamú B, Aguilar H, Relat J, Ortega-Gutiérrez S, Casals G, Marrero PF, Urruticoechea A, Haro D, et al: Novel inhibitors of fatty acid synthase with anticancer activity. Clin Cancer Res. 15:7608–1765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turrado C, Puig T, García-Cárceles J, Artola M, Benhamú B, Ortega-Gutiérrez S, Casals G, Marrero PF, Urruticoechea A, Haro D, et al: New synthetic inhibitors of fatty acid synthase with anticancer activity. J Med Chem. 55:5013–5023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alwarawrah Y, Hughes P, Loiselle D, Carlson DA, Darr DB, Jordan JL, Xiong J, Hunter LM, Dubois LG, Thompson JW, et al: Fasnall, a Selective FASN Inhibitor, Shows Potent Anti-tumor Activity in the MMTV-Neu Model of HER2(+) Breast Cancer. Cell Chem Biol. 23:678–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hanahan D and Weinberg RA: Hallmarks of cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hanahan D: Hallmarks of cancer: New dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hilvo M, de Santiago I, Gopalacharyulu P, Schmitt WD, Budczies J, Kuhberg M, Dietel M, Aittokallio T, Markowetz F, Denkert C, et al: Accumulated metabolites of hydroxybutyric acid serve as diagnostic and prognostic biomarkers of ovarian High-Grade serous carcinomas. Cancer Res. 76:796–804. 2016. View Article : Google Scholar :
|
|
43
|
Pua TL, Wang FQ and Fishman DA: Roles of LPA in ovarian cancer development and progression. Future Oncol. 5:1659–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leinster DA, Kulbe H, Everitt G, Thompson R, Perretti M, Gavins FN, Cooper D, Gould D, Ennis DP, Lockley M, et al: The peritoneal tumour microenvironment of high-grade serous ovarian cancer. J Pathol. 227:136–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim S, Kim B and Song YS: Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 107:1173–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghoneum A, Afify H, Salih Z, Kelly M and Said N: Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget. 9:22832–22849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Impheng H, Pongcharoen S, Richert L, Pekthong D and Srisawang P: The selective target of capsaicin on FASN expression and de novo fatty acid synthesis mediated through ROS generation triggers apoptosis in HepG2 cells. PLoS One. 9:e1078422014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lou E, Vogel RI, Hoostal S, Klein M, Linden MA, Teoh D and Geller MA: Tumor-Stroma proportion as a predictive biomarker of resistance to Platinum-Based chemotherapy in patients with ovarian cancer. JAMA Oncol. 5:1222–1224. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Romanauska A and Köhler A: Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat. Dev Cell. 56:2562–2578. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, et al: De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 70:8117–8126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nieman KM, Romero IL, Van Houten B and Lengyel E: Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, Nieman KM, Pascual G, Benitah SA, Montag A, et al: Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 37:2285–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mukherjee A, Chiang CY, Daifotis HA, Nieman KM, Fahrmann JF, Lastra RR, Romero IL, Fiehn O and Lengyel E: Adipocyte-Induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res. 80:1748–1761. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, et al: Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 130:387–398. 2011. View Article : Google Scholar
|
|
55
|
Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D and Cheng JX: Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 20:303–314.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grunt TW and Valent P: Increased lipid desaturation and ovarian cancer stem cells. Transl Cancer Res. 6(Suppl 3): S472–S475. 2017. View Article : Google Scholar
|
|
57
|
Cao Y: Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest. 129:3006–3017. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zaytseva YY, Rychahou PG, Le AT, Scott TL, Flight RM, Kim JT, Harris J, Liu J, Wang C, Morris AJ, et al: Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget. 9:24787–24800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wong A, Chen S, Yang LK, Kanagasundaram Y and Crasta K: Lipid accumulation facilitates mitotic slippage-induced adaptation to anti-mitotic drug treatment. Cell Death Discov. 4:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park R, Jang M, Park YI, Park Y, Namkoong S, Lee JI and Park J: Elevated levels of CTRP1 in obesity contribute to tumor progression in a p53-Dependent manner. Cancers (Basel). 13:36192021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Garcia DI, Hurst KE, Bradshaw A, Janakiraman H, Wang C and Camp ER: High-fat diet drives an aggressive pancreatic cancer phenotype. J Surg Res. 264:163–172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lombardi S, Goldman AR, Tang HY, Kossenkov AV, Liu H, Zhou W, Herlyn M, Lin J and Zhang R: Targeting fatty acid reprogramming suppresses CARM1-expressing ovarian cancer. Cancer Res Commun. 3:1067–1077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bao J, Zhu L, Zhu Q, Su J, Liu M and Huang W: SREBP-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol Lett. 12:2409–2416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nie LY, Lu QT, Li WH, Yang N, Dongol S, Zhang X and Jiang J: Sterol regulatory element-binding protein 1 is required for ovarian tumor growth. Oncol Rep. 30:1346–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|





















