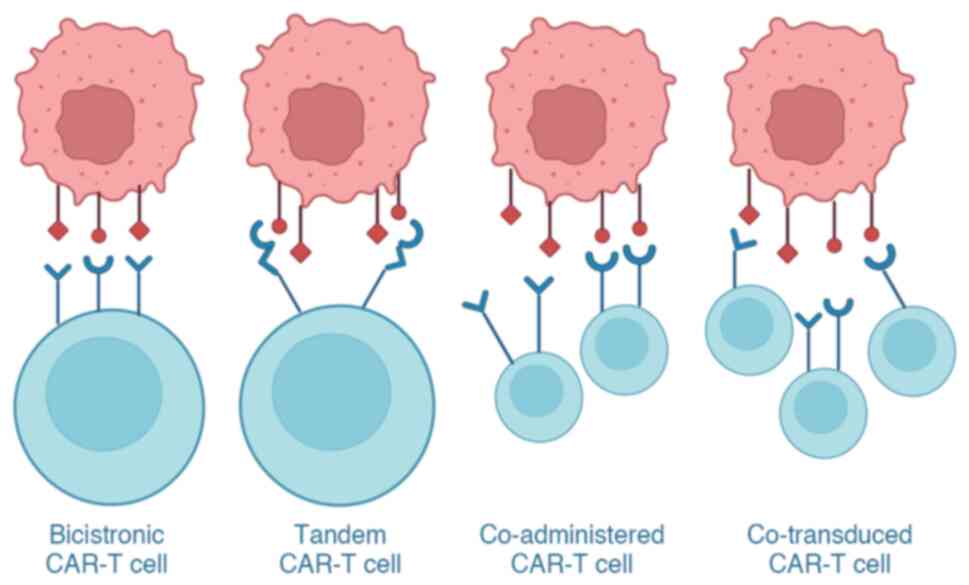
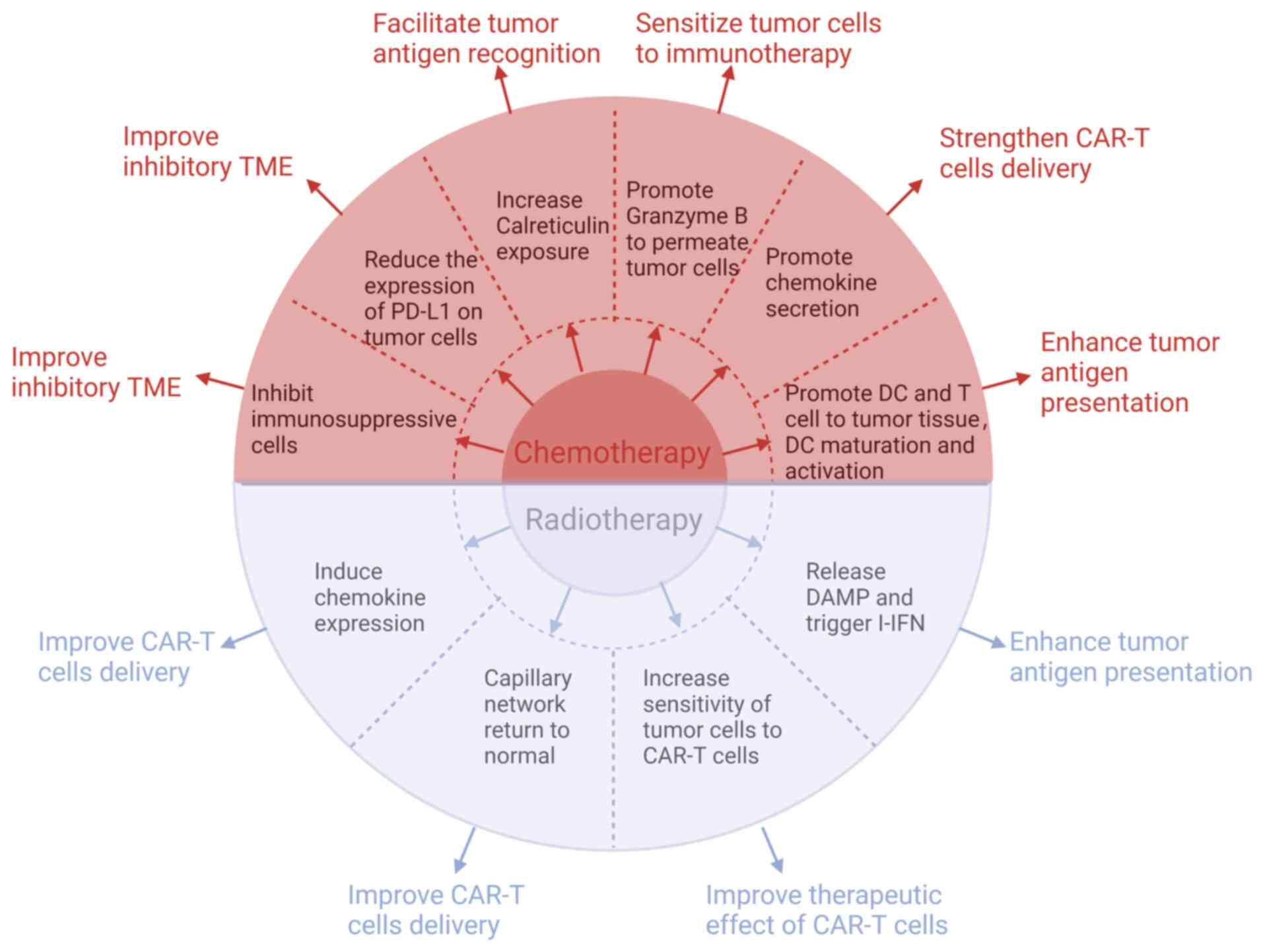
|
1
|
Misaghi A, Goldin A, Awad M and Kulidjian
AA: Osteosarcoma: A comprehensive review. SICOT J. 4:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer. 75(1 Suppl): S203–S210. 1995. View Article : Google Scholar
|
|
3
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
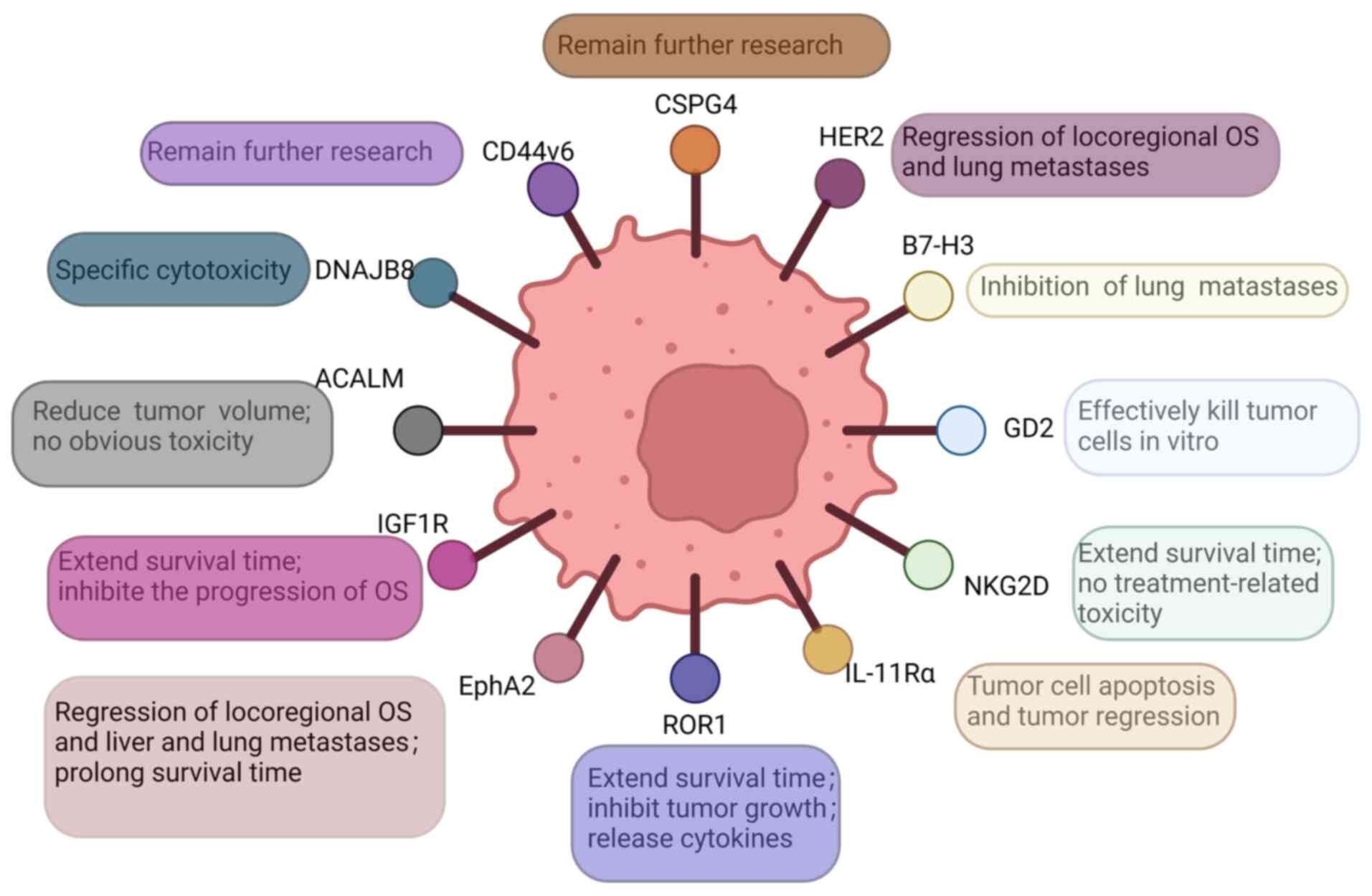
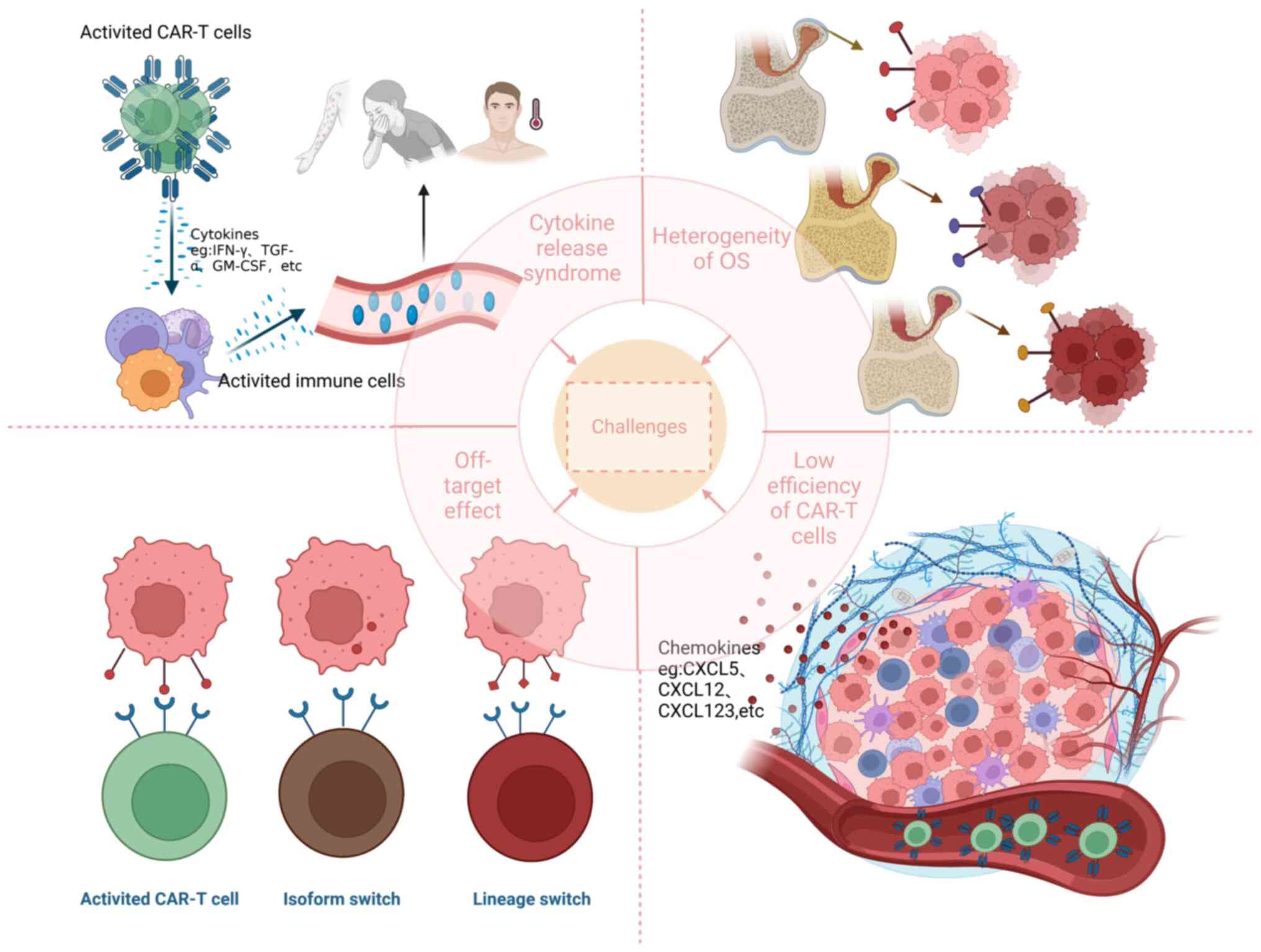
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizzo A, Nannini M, Astolfi A, Indio V, De
Iaco P, Perrone AM, De Leo A, Incorvaia L, Di Scioscio V and
Pantaleo MA: Impact of chemotherapy in the adjuvant setting of
early stage uterine leiomyosarcoma: A systematic review and updated
meta-analysis. Cancers (Basel). 12:18992020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Y, Yang D, Yang Q, Lv X, Huang W,
Zhou Z, Wang Y, Zhang Z, Yuan T, Ding X, et al: Single-cell RNA
landscape of intratumoral heterogeneity and immunosuppressive
microenvironment in advanced osteosarcoma. Nat Commun. 11:63222020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizzo A, Pantaleo MA, Saponara M and
Nannini M: Current status of the adjuvant therapy in uterine
sarcoma: A literature review. World J Clin Cases. 7:1753–1763.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santoni M, Rizzo A, Mollica V, Matrana MR,
Rosellini M, Faloppi L, Marchetti A, Battelli N and Massari F: The
impact of gender on the efficacy of immune checkpoint inhibitors in
cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol.
170:1035962022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stancovski I, Schindler DG, Waks T, Yarden
Y, Sela M and Eshhar Z: Targeting of T lymphocytes to
Neu/HER2-expressing cells using chimeric single chain Fv receptors.
J Immunol. 151:6577–6582. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Wu L, Huang C, Liu R, Li Z, Liu L
and Shan B: Challenges and strategies of clinical application of
CAR-T therapy in the treatment of tumors-a narrative review. Ann
Transl Med. 8:10932020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abbas MZ: Strategic use of patent
opposition safeguard to improve equitable access to innovative
health technologies: A case study of CAR T-cell therapy Kymriah.
Glob Public Health. 17:3255–3265. 2022. View Article : Google Scholar
|
|
12
|
Astolfi A, Nannini M, Indio V, Schipani A,
Rizzo A, Perrone AM, De Iaco P, Pirini MG, De Leo A, Urbini M, et
al: Genomic database analysis of uterine leiomyosarcoma mutational
profile. Cancers (Basel). 12:21262020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berdeja JG, Madduri D, Usmani SZ,
Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M,
Lesokhin A, et al: Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma
(CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 398:314–324.
2021. View Article : Google Scholar : PubMed/NCBI
|
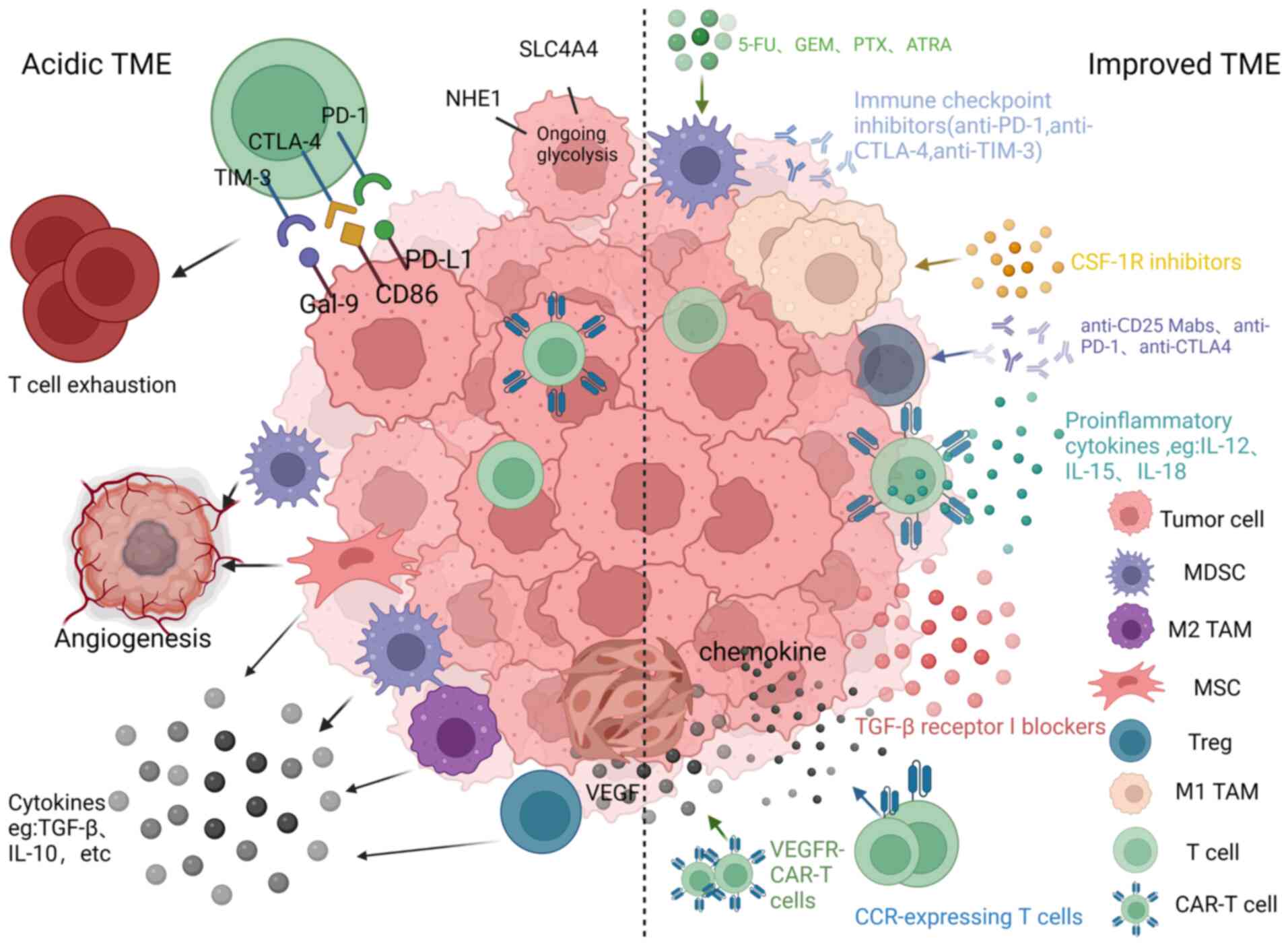
|
14
|
Van Oekelen O, Aleman A, Upadhyaya B,
Schnakenberg S, Madduri D, Gavane S, Teruya-Feldstein J, Crary JF,
Fowkes ME, Stacy CB, et al: Neurocognitive and hypokinetic movement
disorder with features of parkinsonism after BCMA-targeting CAR-T
cell therapy. Nat Med. 27:2099–2103. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-cell therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garfall AL, Stadtmauer EA, Hwang WT, Lacey
SF, Melenhorst JJ, Krevvata M, Carroll MP, Matsui WH, Wang Q,
Dhodapkar MV, et al: Anti-CD19 CAR T cells with high-dose melphalan
and autologous stem cell transplantation for refractory multiple
myeloma. JCI Insight. 4:e1276842018. View Article : Google Scholar
|
|
17
|
Guo B, Chen M, Han Q, Hui F, Dai H, Zhang
W, Zhang Y, Wang Y, Zhu H and Han W: CD138-directed adoptive
immunotherapy of chimeric antigen receptor (CAR)-modified T cells
for multiple myeloma. J Cell Immunother. 2:28–35. 2016. View Article : Google Scholar
|
|
18
|
Zhu J, Simayi N, Wan R and Huang W: CAR T
targets and microenvironmental barriers of osteosarcoma.
Cytotherapy. 24:567–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boettcher M, Joechner A, Li Z, Yang SF and
Schlegel P: Development of CAR T cell therapy in children-A
comprehensive overview. J Clin Med. 11:21582022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadelain M, Brentjens R and Rivière I: The
basic principles of chimeric antigen receptor design. Cancer Discv.
3:388–398. 2013. View Article : Google Scholar
|
|
21
|
Morita R, Nishizawa S, Torigoe T,
Takahashi A, Tamura Y, Tsukahara T, Kanaseki T, Sokolovskaya A,
Kochin V, Kondo T, et al: Heat shock protein DNAJB8 is a novel
target for immunotherapy of colon cancer-initiating cells. Cancer
Sci. 105:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed N, Brawley VS, Hegde M, Robertson C,
Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al:
Human epidermal growth factor receptor 2 (HER2)-specific chimeric
antigen receptor-modified T cells for the immunotherapy of
HER2-positive sarcoma. J Clin Oncol. 33:1688–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Picarda E, Ohaegbulam KC and Zang X:
Molecular pathways: Targeting B7-H3 (CD276) for human cancer
immunotherapy. Clin Cancer Res. 22:3425–3431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Yu W, Zhu J, Wang J, Xia K, Liang
C and Tao H: Anti-CD166/4-1BB chimeric antigen receptor T cell
therapy for the treatment of osteosarcoma. J Exp Clin Cancer Res.
38:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu K, Middlemiss S, Saletta F, Gottschalk
S, McCowage GB and Kramer B: Chimeric antigen receptor-modified T
cells targeting EphA2 for the immunotherapy of paediatric bone
tumours. Cancer Gene Ther. 28:321–334. 2021. View Article : Google Scholar :
|
|
26
|
Reppel L, Tsahouridis O, Akulian J, Davis
IJ, Lee H, Fucà G, Weiss J, Dotti G, Pecot CV and Savoldo B:
Targeting disialoganglioside GD2 with chimeric antigen
receptor-redirected T cells in lung cancer. J Immunother Cancer.
10:e0038972022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang G, Yu L, Cooper LJN, Hollomon M,
Huls H and Kleinerman ES: Genetically modified T cells targeting
interleukin-11 receptor α-chain kill human osteosarcoma cells and
induce the regression of established osteosarcoma lung metastases.
Cancer Res. 72:271–281. 2012. View Article : Google Scholar
|
|
28
|
Huang X, Park H, Greene J, Pao J, Mulvey
E, Zhou SX, Albert CM, Moy F, Sachdev D, Yee D, et al: IGF1R- and
ROR1-specific CAR T cells as a potential therapy for high risk
sarcomas. PLoS One. 10:e01331522015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernández L, Metais JY, Escudero A, Vela
M, Valentín J, Vallcorba I, Leivas A, Torres J, Valeri A,
Patiño-García A, et al: Memory T cells expressing an NKG2D-CAR
efficiently target osteosarcoma cells. Clin Cancer Res.
23:5824–5835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riccardo F, Tarone L, Iussich S, Giacobino
D, Arigoni M, Sammartano F, Morello E, Martano M, Gattino F, Maria
R, et al: Identification of CSPG4 as a promising target for
translational combinatorial approaches in osteosarcoma. Ther Adv
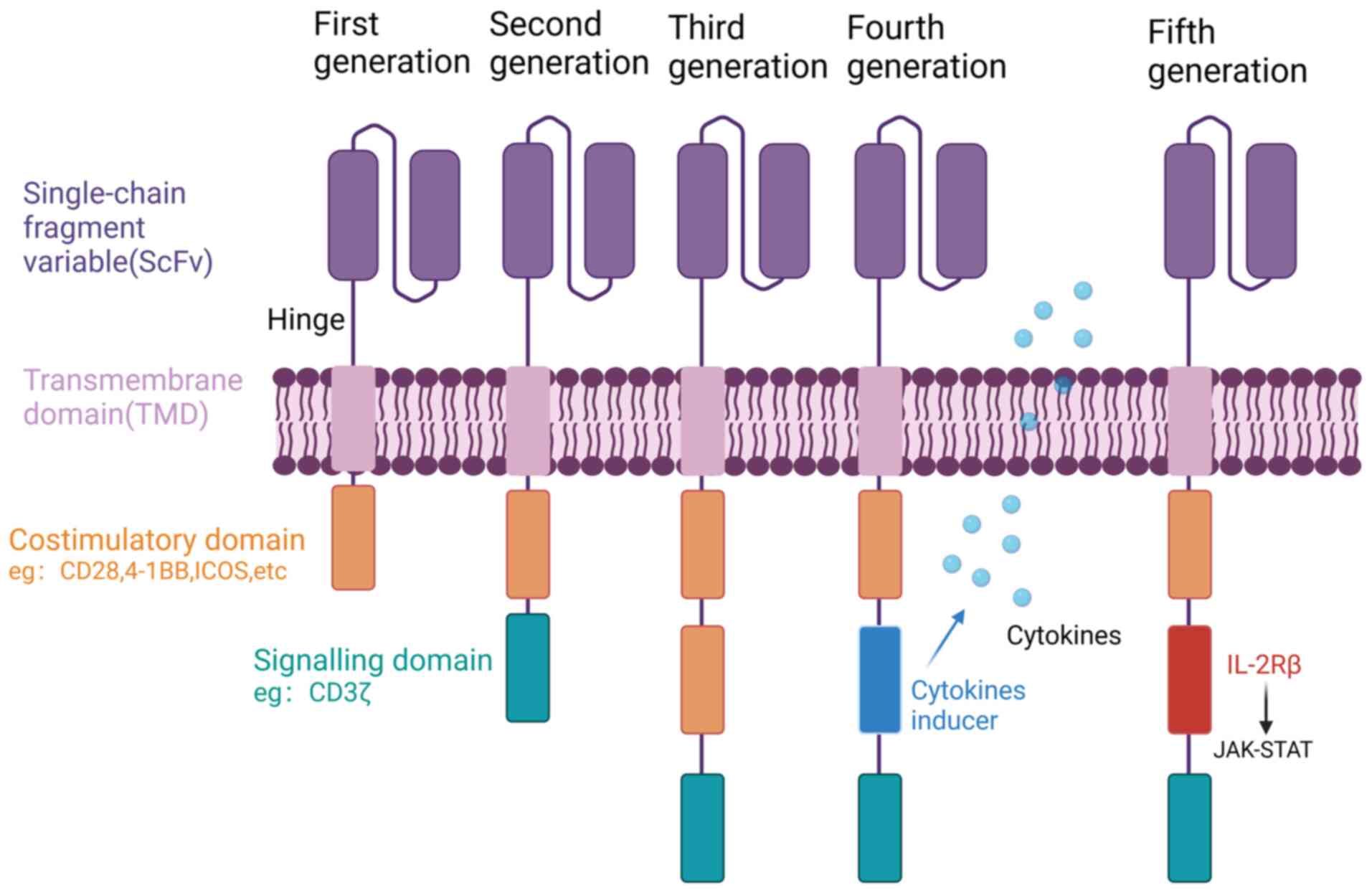
Med Oncol. 11:17588359198554912019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Ding C, Wang J, Sun G, Cao Y, Xu
L, Zhou L and Chen X: Prognostic significance of CD44V6 expression
in osteosarcoma: A meta-analysis. J Orthop Surg Res. 10:1872015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Z, Wu Z and Luo W: Chimeric antigen
receptor T-cell therapy: The light of day for osteosarcoma. Cancers
(Basel). 13:44692021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah NN and Fry TJ: Mechanisms of
resistance to CAR T cell therapy. Nat Rev Clin Oncol. 16:372–385.
2019.PubMed/NCBI
|
|
34
|
Guan Y, Zhang R, Peng Z, Dong D, Wei G and
Wang Y: Inhibition of IL-18-mediated myeloid derived suppressor
cell accumulation enhances anti-PD1 efficacy against osteosarcoma
cancer. J Bone Oncol. 9:59–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng B, Ren T, Huang Y, Sun K, Wang S,
Bao X, Liu K and Guo W: PD-1 axis expression in musculoskeletal
tumors and antitumor effect of nivolumab in osteosarcoma model of
humanized mouse. J Hematol Oncol. 11:162018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wallace A, Kapoor V, Sun J, Mrass P,
Weninger W, Heitjan DF, June C, Kaiser LR, Ling LE and Albelda SM:
Transforming growth factor-beta receptor blockade augments the
effectiveness of adoptive T-cell therapy of established solid
cancers. Clin Cancer Res. 14:3966–3974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fedorov VD, Themeli M and Sadelain M:
PD-1- and CTLA-4-based inhibitory chimeric antigen receptors
(iCARs) divert off-target immunotherapy responses. Sci Transl Med.
5:215ra1722013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JC, Chang YW, Hong CC, Yu YH and Su
JL: The role of the VEGF-C/VEGFRs axis in tumor progression and
therapy. Int J Mol Sci. 14:88–107. 2012. View Article : Google Scholar
|
|
39
|
Avanzi MP, Yeku O, Li X, Wijewarnasuriya
DP, van Leeuwen DG, Cheung K, Park H, Purdon TJ, Daniyan AF,
Spitzer MH and Brentjens RJ: Engineered tumor-targeted T cells
mediate enhanced anti-tumor efficacy both directly and through
activation of the endogenous immune system. Cell Rep. 23:2130–2141.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith TT, Moffett HF, Stephan SB, Opel CF,
Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD and
Stephan MT: Biopolymers codelivering engineered T cells and STING
agonists can eliminate heterogeneous tumors. J Clin Invest.
127:2176–2191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia T, Konno H and Barber GN: Recurrent
loss of STING signaling in melanoma correlates with susceptibility
to viral oncolysis. Cancer Res. 76:6747–6759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
DeSelm C, Palomba ML, Yahalom J, Hamieh M,
Eyquem J, Rajasekhar VK and Sadelain M: Low-dose radiation
conditioning enables CAR T cells to mitigate antigen escape. Mol
Ther. 26:2542–2552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chulanetra M, Morchang A, Sayour E,
Eldjerou L, Milner R, Lagmay J, Cascio M, Stover B, Slayton W,
Chaicumpa W, et al: GD2 chimeric antigen receptor modified T cells
in synergy with sub-toxic level of doxorubicin targeting
osteosarcomas. Am J Cancer Res. 10:674–687. 2020.PubMed/NCBI
|
|
44
|
Buka D, Dvořák J, Sitorová V, Hátlová J,
Richter I and Sirák I: Changes in the CD8+ density of tumor
infiltrating lymphocytes after neoadjuvant radiochemotherapy in
patients with rectal adenocarcinom. Klin Onkol. 29:204–209. 2016.In
Czech. View Article : Google Scholar
|
|
45
|
Makita S, Imaizumi K, Kurosawa S and
Tobinai K: Chimeric antigen receptor T-cell therapy for B-cell
non-Hodgkin lymphoma: Opportunities and challenges. Drugs Context.
8:2125672019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Zhu L, Zhang H, Chen S and Xiao
Y: CAR-T cell therapy in hematological malignancies: Current
opportunities and challenges. Front Immunol. 13:9271532022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kong Y, Tang L, You Y, Li Q and Zhu X:
Analysis of causes for poor persistence of CAR-T cell therapy in
vivo. Front Immunol. 14:10634542023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Asmamaw Dejenie T, Tiruneh G/Medhin M,
Dessie Terefe G, Tadele Admasu F, Wale Tesega W and Chekol Abebe E:
Current updates on generations, approvals, and clinical trials of
CAR T-cell therapy. Hum Vaccin Immunother. 18:21142542022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer? BMC Med.
14:732016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
García Merino A: Anticuerpos monoclonales.
Aspectos básicos. Neurología. 26:301–306. 2011. View Article : Google Scholar
|
|
51
|
Lu J, Ding J, Liu Z and Chen T:
Retrospective analysis of the preparation and application of
immunotherapy in cancer treatment (review). Int J Oncol. 60:122022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahmed N, Salsman VS, Yvon E, Louis CU,
Perlaky L, Wels WS, Dishop MK, Kleinerman EE, Pule M, Rooney CM, et
al: Immunotherapy for osteosarcoma: Genetic modification of T cells
overcomes low levels of tumor antigen expression. Mol Ther.
17:1779–1787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pulè MA, Straathof KC, Dotti G, Heslop HE,
Rooney CM and Brenner MK: A chimeric T cell antigen receptor that
augments cytokine release and supports clonal expansion of primary
human T cells. Mol Ther. 12:933–941. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamada R, Takahashi A, Torigoe T, Morita
R, Tamura Y, Tsukahara T, Kanaseki T, Kubo T, Watarai K, Kondo T,
et al: Preferential expression of cancer/testis genes in cancer
stem-like cells: Proposal of a novel sub-category,
cancer/testis/stem gene. Tissue Antigens. 81:428–434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nishizawa S, Hirohashi Y, Torigoe T,
Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H,
Morita R, et al: HSP DNAJB8 controls tumor-initiating ability in
renal cancer stem-like cells. Cancer Res. 72:2844–2854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Watanabe Y, Tsukahara T, Murata K, Hamada
S, Kubo T, Kanaseki T, Hirohashi Y, Emori M, Teramoto A,
Nakatsugawa M, et al: Development of CAR-T cells specifically
targeting cancer stem cell antigen DNAJB8 against solid tumours. Br
J Cancer. 128:886–895. 2023. View Article : Google Scholar :
|
|
57
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abdou AG, Kandil M, Asaad NY, Dawoud MM,
Shahin AA and Abd Eldayem AF: The prognostic role of Ezrin and
HER2/neu expression in osteosarcoma. Appl Immunohistochem Mol
Morphol. 24:355–363. 2016. View Article : Google Scholar
|
|
59
|
Xuan Y, Sheng Y, Zhang D, Zhang K, Zhang
Z, Ping Y, Wang S, Shi X, Lian J, Liu K, et al: Targeting CD276 by
CAR-T cells induces regression of esophagus squamous cell carcinoma
in xenograft mouse models. Transl Oncol. 14:1011382021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, He L, Sadagopan A, Ma T, Dotti G,
Wang Y, Zheng H, Gao X, Wang D, DeLeo AB, et al: Targeting
radiation-resistant prostate cancer stem cells by B7-H3 CAR T
cells. Mol Cancer Ther. 20:577–588. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Du H, Hirabayashi K, Ahn S, Kren NP,
Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene
K, et al: Antitumor responses in the absence of toxicity in solid
tumors by targeting B7-H3 via chimeric antigen receptor T cells.
Cancer Cell. 35:221–237.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Theruvath J, Sotillo E, Mount CW, Graef
CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A,
Majzner RG, et al: Locoregionally administered B7-H3-targeted CAR T
cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med.
26:712–719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Talbot LJ, Chabot A, Funk A, Nguyen P,
Wagner J, Ross A, Tillman H, Davidoff A, Gottschalk S and DeRenzo
C: A novel orthotopic implantation technique for osteosarcoma
produces spontaneous metastases and illustrates dose-dependent
efficacy of B7-H3-CAR T cells. Front Immunol. 12:6917412021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Majzner RG, Theruvath JL, Nellan A,
Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota
C, et al: CAR T cells targeting B7-H3, a pan-cancer antigen,
demonstrate potent preclinical activity against pediatric solid
tumors and brain tumors. Clin Cancer Res. 25:2560–2574. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Swart GWM: Activated leukocyte cell
adhesion molecule (CD166/ALCAM): Developmental and mechanistic
aspects of cell clustering and cell migration. Eur J Cell Biol.
81:313–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Federman N, Chan J, Nagy JO, Landaw EM,
McCabe K, Wu AM, Triche T, Kang H, Liu B, Marks JD and Denny CT:
Enhanced growth inhibition of osteosarcoma by cytotoxic polymerized
liposomal nanoparticles targeting the alcam cell surface receptor.
Sarcoma. 2012:1269062012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
He S, Li S, Guo J, Zeng X, Liang D, Zhu Y,
Li Y, Yang D and Zhao X: CD166-specific CAR-T cells potently target
colorectal cancer cells. Transl Oncol. 27:1015752023. View Article : Google Scholar
|
|
68
|
Kang BH, Jensen KJ, Hatch JA and Janes KA:
Simultaneous profiling of 194 distinct receptor transcripts in
human cells. Sci Signal. 6:rs132013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wykosky J and Debinski W: The EphA2
receptor and ephrinA1 ligand in solid tumors: Function and
therapeutic targeting. Mol Cancer Res. 6:1795–1806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fritsche-Guenther R, Noske A, Ungethüm U,
Kuban RJ, Schlag PM, Tunn PU, Karle J, Krenn V, Dietel M and Sers
C: De novo expression of EphA2 in osteosarcoma modulates activation
of the mitogenic signalling pathway. Histopathology. 57:836–850.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Doronin II, Vishnyakova PA, Kholodenko IV,
Ponomarev ED, Ryazantsev DY, Molotkovskaya IM and Kholodenko RV:
Ganglioside GD2 in reception and transduction of cell death signal
in tumor cells. BMC Cancer. 14:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Roth M, Linkowski M, Tarim J, Piperdi S,
Sowers R, Geller D, Gill J and Gorlick R: Ganglioside GD2 as a
therapeutic target for antibody-mediated therapy in patients with
osteosarcoma. Cancer. 120:548–554. 2014. View Article : Google Scholar
|
|
74
|
Long AH, Highfill SL, Cui Y, Smith JP,
Walker AJ, Ramakrishna S, El-Etriby R, Galli S, Tsokos MG, Orentas
RJ and Mackall CL: Reduction of MDSCs with all-trans retinoic acid
improves CAR therapy efficacy for sarcomas. Cancer Immunol Res.
4:869–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Suri M, Soni N, Okpaleke N, Yadav S, Shah
S, Iqbal Z, Alharbi MG, Kalra HS and Hamid P: A deep dive into the
newest avenues of immunotherapy for pediatric osteosarcoma: A
systematic review. Cureus. 13:e183492021.PubMed/NCBI
|
|
76
|
Park JA and Cheung NKV: GD2 or HER2
targeting T cell engaging bispecific antibodies to treat
osteosarcoma. J Hematol Oncol. 13:1722020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jiang J, Wang R, Yang L, Sha Y, Zhao S,
Guo J, Chen D, Zhong Z and Meng F: IL-11Rα-targeted nanostrategy
empowers chemotherapy of relapsed and patient-derived osteosarcoma.
J Control Release. 350:460–470. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lokau J, Schoeder V and Garbers C: The
role of interleukin-11 in osteosarcoma. Der Pathologe. 41:163–167.
2020.In German. View Article : Google Scholar
|
|
79
|
Li YS, Liu Q, He HB and Luo W: The
possible role of insulin-like growth factor-1 in osteosarcoma. Curr
Probl Cancer. 43:228–235. 2019. View Article : Google Scholar
|
|
80
|
Duan Z, Choy E, Harmon D, Yang C, Ryu K,
Schwab J, Mankin H and Hornicek FJ: Insulin-like growth factor-I
receptor tyrosine kinase inhibitor cyclolignan picropodophyllin
inhibits proliferation and induces apoptosis in multidrug resistant
osteosarcoma cell lines. Mol Cancer Ther. 8:2122–2130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tan X, Fan S, Wu W and Zhang Y:
MicroRNA-26a inhibits osteosarcoma cell proliferation by targeting
IGF-1. Bone Res. 3:150332015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu Y, Zhu ST, Wang X, Deng J, Li WH,
Zhang P and Liu BS: MiR-100 inhibits osteosarcoma cell
proliferation, migration, and invasion and enhances
chemosensitivity by targeting IGFIR. Technol Cancer Res Treat.
15:NP40–NP48. 2016. View Article : Google Scholar
|
|
83
|
Chen G, Fang T, Huang Z, Qi Y, Du S, Di T,
Lei Z, Zhang X and Yan W: MicroRNA-133a inhibits osteosarcoma cells
proliferation and invasion via targeting IGF-1R. Cell Physiol
Biochem. 38:598–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hojjat-Farsangi M, Moshfegh A,
Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A and Mellstedt
H: The receptor tyrosine kinase ROR1-an oncofetal antigen for
targeted cancer therapy. Semin Cancer Biol. 29:21–31. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dai B, Shen Y, Yan T and Zhang A:
Wnt5a/ROR1 activates DAAM1 and promotes the migration in
osteosarcoma cells. Oncol Rep. 43:601–608. 2020.PubMed/NCBI
|
|
86
|
Zhang T, Barber A and Sentman CL:
Generation of antitumor responses by genetic modification of
primary human T cells with a chimeric NKG2D receptor. Cancer Res.
66:5927–5933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ding H, Yang X and Wei Y: Fusion proteins
of NKG2D/NKG2DL in cancer immunotherapy. Int J Mol Sci. 19:1772018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Baumeister SH, Murad J, Werner L, Daley H,
Trebeden-Negre H, Gicobi JK, Schmucker A, Reder J, Sentman CL,
Gilham DE, et al: Phase I trial of autologous CAR T cells targeting
NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer
Immunol Res. 7:100–112. 2019. View Article : Google Scholar
|
|
89
|
Tao K, He M, Tao F, Xu G, Ye M, Zheng Y
and Li Y: Development of NKG2D-based chimeric antigen receptor-T
cells for gastric cancer treatment. Cancer Chemother Pharmacol.
82:815–827. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Li X, Zhang J and Mao L: Novel
cellular immunotherapy using NKG2D CAR-T for the treatment of
cervical cancer. Biomed Pharmacother. 131:1105622020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun B, Yang D, Dai H, Liu X, Jia R, Cui X,
Li W, Cai C, Xu J and Zhao X: Eradication of hepatocellular
carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res.
7:1813–1823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang X, Wang Y, Yu L, Sakakura K, Visus C,
Schwab JH, Ferrone CR, Favoino E, Koya Y, Campoli MR, et al: CSPG4
in cancer: multiple roles. Curr Mol Med. 10:419–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rolih V, Barutello G, Iussich S, De Maria
R, Quaglino E, Buracco P, Cavallo F and Riccardo F: CSPG4: A
prototype oncoantigen for translational immunotherapy studies. J
Transl Med. 15:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Casanova JM, Almeida JS, Reith JD, Sousa
LM, Fonseca R, Freitas-Tavares P, Santos-Rosa M and
Rodrigues-Santos P: Tumor-infiltrating lymphocytes and cancer
markers in osteosarcoma: Influence on patient survival. Cancers
(Basel). 13:60752021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Deng Z, Niu G, Cai L, Wei R and Zhao X:
The prognostic significance of CD44V6, CDH11, and β-catenin
expression in patients with osteosarcoma. Biomed Res Int.
2013:4961932013. View Article : Google Scholar
|
|
96
|
Qiao GL, Song LN, Deng ZF, Chen Y and Ma
LJ: Prognostic value of CD44v6 expression in breast cancer: A
meta-analysis. Onco Targets Ther. 11:5451–5457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Saito S, Okabe H, Watanabe M, Ishimoto T,
Iwatsuki M, Baba Y, Tanaka Y, Kurashige J, Miyamoto Y and Baba H:
CD44v6 expression is related to mesenchymal phenotype and poor
prognosis in patients with colorectal cancer. Oncol Rep.
29:1570–1578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nakajima K, Taniguchi K and Mutoh KI:
Expression of CD44v6 as matrix-associated ectodomain in the bone
development. J Vet Med Sci. 72:1017–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang
C, Ye Z and Qian Q: Current progress in CAR-T cell therapy for
solid tumors. Int J Biol Sci. 15:2548–2560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu B, Yan L and Zhou M: Target selection
of CAR T cell therapy in accordance with the TME for solid tumors.
Am J Cancer Res. 9:228–241. 2019.PubMed/NCBI
|
|
101
|
Saifullah MK, Fox DA, Sarkar S, Abidi SM,
Endres J, Piktel J, Haqqi TM and Singer NG: Expression and
characterization of a novel CD6 ligand in cells derived from joint
and epithelial tissues. J Immunol. 173:6125–6133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ikeda K and Quertermous T: Molecular
isolation and characterization of a soluble isoform of activated
leukocyte cell adhesion molecule that modulates endothelial cell
function. J Biol Chem. 279:55315–55323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang Q, Zhang Z, Liu G, Li D, Gu Z, Zhang
L, Pan Y, Cui X, Wang L, Liu G, et al: B7-H3 targeted CAR-T cells
show highly efficient anti-tumor function against osteosarcoma both
in vitro and in vivo. BMC Cancer. 22:11242022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Majzner RG, Heitzeneder S and Mackall CL:
Harnessing the immunotherapy revolution for the treatment of
childhood cancers. Cancer Cell. 31:476–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mohammed S, Sukumaran S, Bajgain P,
Watanabe N, Heslop HE, Rooney CM, Brenner MK, Fisher WE, Leen AM
and Vera JF: Improving chimeric antigen receptor-modified T cell
function by reversing the immunosuppressive tumor microenvironment
of pancreatic cancer. Mol Ther. 25:249–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Anderson KG, Stromnes IM and Greenberg PD:
Obstacles posed by the tumor microenvironment to T cell activity: A
case for synergistic therapies. Cancer Cell. 31:311–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wagner LM and Adams VR: Targeting the PD-1
pathway in pediatric solid tumors and brain tumors. Onco Targets
Ther. 10:2097–2106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hashimoto K, Nishimura S and Akagi M:
Characterization of PD-1/PD-L1 immune checkpoint expression in
osteosarcoma. Diagnostics (Basel). 10:5282020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kawano M, Itonaga I, Iwasaki T and Tsumura
H: Enhancement of antitumor immunity by combining anti-cytotoxic T
lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed
dendritic cells in murine osteosarcoma. Oncol Rep. 29:1001–1006.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lussier DM, Johnson JL, Hingorani P and
Blattman JN: Combination immunotherapy with α-CTLA-4 and α-PD-L1
antibody blockade prevents immune escape and leads to complete
control of metastatic osteosarcoma. J Immunother Cancer. 3:212015.
View Article : Google Scholar
|
|
112
|
Sun CY, Zhang Z, Tao L, Xu FF, Li HY,
Zhang HY and Liu W: T cell exhaustion drives osteosarcoma
pathogenesis. Ann Transl Med. 9:14472021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu Y, Luo J, Li Y, Cao J and Wang X: IFNγ
and TNFα synergistically promote galectin 9 secretion by human
osteosarcoma cells MG-63 to prevent T cell killing. Int J Clin Exp
Pathol. 13:2009–2017. 2020.
|
|
114
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11:692021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hattinger CM, Salaroglio IC, Fantoni L,
Godel M, Casotti C, Kopecka J, Scotlandi K, Ibrahim T, Riganti C
and Serra M: Strategies to overcome resistance to immune-based
therapies in osteosarcoma. Int J Mol Sci. 24:7992023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Huang Q, Liang X, Ren T, Huang Y, Zhang H,
Yu Y, Chen C, Wang W, Niu J, Lou J and Guo W: The role of
tumor-associated macrophages in osteosarcoma
progression-therapeutic implications. Cell Oncol (Dordr).
44:525–539. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dumars C, Ngyuen JM, Gaultier A, Lanel R,
Corradini N, Gouin F, Heymann D and Heymann MF: Dysregulation of
macrophage polarization is associated with the metastatic process
in osteosarcoma. Oncotarget. 7:78343–78354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sugiyama D, Hinohara K and Nishikawa H:
Significance of regulatory T cells in cancer immunology and
immunotherapy. Exp Dermatol. 32:256–263. 2023. View Article : Google Scholar
|
|
120
|
Taylor A, Verhagen J, Blaser K, Akdis M
and Akdis CA: Mechanisms of immune suppression by interleukin-10
and transforming growth factor-beta: The role of T regulatory
cells. Immunology. 117:433–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Xiao H, Chen L, Luo G, Son H, Prectoni JH
and Zheng W: Effect of the cytokine levels in serum on
osteosarcoma. Tumor Biol. 35:1023–1028. 2014. View Article : Google Scholar
|
|
123
|
Tian B, Du X, Zheng S and Zhang Y: The
role of tumor microenvironment in regulating the plasticity of
osteosarcoma cells. Int J Mol Sci. 23:161552022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shen L, Li J, Liu Q, Song W, Zhang X,
Tiruthani K, Hu H, Das M, Goodwin TJ, Liu R and Huang L: Local
blockade of interleukin 10 and C-X-C motif chemokine ligand 12 with
nano-delivery promotes antitumor response in murine cancers. ACS
Nano. 12:9830–9841. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Rossowska J, Anger N, Szczygieł A,
Mierzejewska J and Pajtasz-Piasecka E: Reprogramming the murine
colon cancer microenvironment using lentivectors encoding shRNA
against IL-10 as a component of a potent DC-based
chemoimmunotherapy. J Exp Clin Cancer Res. 37:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zeng J, Chen S, Li C, Ye Z, Lin B, Liang
Y, Wang B, Ma Y, Chai X, Zhang X, et al: Mesenchymal stem/stromal
cells-derived IL-6 promotes nasopharyngeal carcinoma growth and
resistance to cisplatin via upregulating CD73 expression. J Cancer.
11:2068–2079. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y
and Qian A: Bone microenvironment and osteosarcoma metastasis. Int
J Mol Sci. 21:69852020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chang AI, Schwertschkow AH, Nolta JA and
Wu J: Involvement of mesenchymal stem cells in cancer progression
and metastases. Curr Cancer Drug Targets. 15:88–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pietrovito L, Leo A, Gori V, Lulli M,
Parri M, Becherucci V, Piccini L, Bambi F, Taddei ML and Chiarugi
P: Bone marrow-derived mesenchymal stem cells promote invasiveness
and transendothelial migration of osteosarcoma cells via a
mesenchymal to amoeboid transition. Mol Oncol. 12:659–676. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar :
|
|
132
|
Tsukamoto S, Honoki K, Fujii H, Tohma Y,
Kido A, Mori T, Tsujiuchi T and Tanaka Y: Mesenchymal stem cells
promote tumor engraftment and metastatic colonization in rat
osteosarcoma model. Int J Oncol. 40:163–169. 2012.
|
|
133
|
Zhang R, Liu Q, Zhou S, He H, Zhao M and
Ma W: Mesenchymal stem cell suppresses the efficacy of CAR-T toward
killing lymphoma cells by modulating the microenvironment through
stanniocalcin-1. Elife. 12:e829342023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang L, Song J, Xin X, Sun D, Huang H,
Chen Y, Zhang T and Zhang Y: Hypoxia stimulates the migration and
invasion of osteosarcoma via up-regulating the NUSAP1 expression.
Open Med (Wars). 16:1083–1089. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lv X, Li J, Zhang C, Hu T, Li S, He S, Yan
H, Tan Y, Lei M, Wen M and Zuo J: The role of hypoxia-inducible
factors in tumor angiogenesis and cell metabolism. Genes Dis.
4:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Guan G, Zhang Y, Lu Y, Liu L, Shi D, Wen
Y, Yang L, Ma Q, Liu T, Zhu X, et al: The HIF-1α/CXCR4 pathway
supports hypoxia-induced metastasis of human osteosarcoma cells.
Cancer Lett. 357:254–264. 2015. View Article : Google Scholar
|
|
137
|
Liu M, Wang D and Li N: MicroRNA-20b
downregulates HIF-1α and inhibits the proliferation and invasion of
osteosarcoma cells. Oncol Res. 23:257–266. 2016. View Article : Google Scholar
|
|
138
|
Galon J and Bruni D: Approaches to treat
immune hot, altered and cold tumours with combination
immunotherapies. Nat Rev Drug Discov. 18:197–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
de la Cruz-López KG, Castro-Muñoz LJ,
Reyes-Hernández DO, García-Carrancá A and Manzo-Merino J: Lactate
in the regulation of tumor microenvironment and therapeutic
approaches. Front Oncol. 9:11432019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kopecka J, Salaroglio IC, Perez-Ruiz E,
Sarmento-Ribeiro AB, Saponara S, De Las Rivas J and Riganti C:
Hypoxia as a driver of resistance to immunotherapy. Drug Resist
Updat. 59:1007872021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Avnet S, Di Pompo G, Chano T, Errani C,
Ibrahim-Hashim A, Gillies RJ, Donati DM and Baldini N:
Cancer-associated mesenchymal stroma fosters the stemness of
osteosarcoma cells in response to intratumoral acidosis via NF-κB
activation. Int J Cancer. 140:1331–1345. 2017. View Article : Google Scholar :
|
|
142
|
Bobulescu IA, Di Sole F and Moe OW: Na+/H+
exchangers: Physiology and link to hypertension and organ ischemia.
Curr Opin Nephrol Hypertens. 14:485–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumor hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar
|
|
145
|
Yang Q, Liu J, Wu B, Wang X, Jiang Y and
Zhu D: Role of extracellular vesicles in osteosarcoma. Int J Med
Sci. 19:1216–1226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen C, Xie L, Ren T, Huang Y, Xu J and
Guo W: Immunotherapy for osteosarcoma: Fundamental mechanism,
rationale, and recent breakthroughs. Cancer Lett. 500:1–10. 2021.
View Article : Google Scholar
|
|
147
|
Prudowsky ZD and Yustein JT: Recent
insights into therapy resistance in osteosarcoma. Cancers (Basel).
13:832020. View Article : Google Scholar
|
|
148
|
Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D,
Ji B, Luo Y and Hu X: Beyond Warburg effect-dual metabolic nature
of cancer cells. Sci Rep. 4:49272014. View Article : Google Scholar
|
|
149
|
Tang HY, Guo JQ, Sang BT, Cheng JN and Wu
XM: PDGFRβ modulates aerobic glycolysis in osteosarcoma HOS cells
via the PI3K/AKT/mTOR/c-Myc pathway. Biochem Cell Biol. 100:75–84.
2022. View Article : Google Scholar
|
|
150
|
Zea AH, Rodriguez PC, Atkins MB, Hernandez
C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A,
O'Neill A, et al: Arginase-producing myeloid suppressor cells in
renal cell carcinoma patients: A mechanism of tumor evasion. Cancer
Res. 65:3044–3048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Rodríguez PC and Ochoa AC: Arginine
regulation by myeloid derived suppressor cells and tolerance in
cancer: Mechanisms and therapeutic perspectives. Immunol Rev.
222:180–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Pietrobon V and Marincola FM: Hypoxia and
the phenomenon of immune exclusion. J Transl Med. 19:92021.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Patel CH and Powell JD: Targeting T cell
metabolism to regulate T cell activation, differentiation and
function in disease. Curr Opin Immunol. 46:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Schubert ML, Schmitt M, Wang L, Ramos CA,
Jordan K, Muller-Tidow C and Dreger P: Side-effect management of
chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol.
32:34–48. 2021. View Article : Google Scholar
|
|
155
|
Almåsbak H, Aarvak T and Vemuri MC: CAR T
cell therapy: A game changer in cancer treatment. J Immunol Res.
2016:54746022016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Maus MV, Grupp SA, Porter DL and June CH:
Antibody-modified T cells: CARs take the front seat for hematologic
malignancies. Blood. 123:2625–2635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chen J, López-Moyado IF, Seo H, Lio CJ,
Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP and Rao A:
NR4A transcription factors limit CAR T cell function in solid
tumours. Nature. 567:530–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Klebanoff CA, Gattinoni L and Restifo NP:
Sorting through subsets: Which T-cell populations mediate highly
effective adoptive immunotherapy? J Immunother. 35:651–660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang BL, Qin DY, Mo ZM, Li Y, Wei W, Wang
YS, Wang W and Wei YQ: Hurdles of CAR-T cell-based cancer
immunotherapy directed against solid tumors. Sci China Life Sci.
59:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kershaw MH, Wang G, Westwood JA, Pachynski
RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM and Hwu
P: Redirecting migration of T cells to chemokine secreted from
tumors by genetic modification with CXCR2. Hum Gene Ther.
13:1971–1980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang
S, Fang Z, Zhao K, Konaparthi R, Hua S, et al: Targeting
YAP-dependent MDSC infiltration impairs tumor progression. Cancer
Discov. 6:80–95. 2016. View Article : Google Scholar :
|
|
162
|
Feig C, Jones JO, Kraman M, Wells RJ,
Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL,
et al: Targeting CXCL12 from FAP-expressing carcinoma-associated
fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic
cancer. Proc Natl Acad Sci USA. 110:20212–20217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Xia AL, Wang XC, Lu YJ, Lu XJ and Sun B:
Chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors:
Challenges and opportunities. Oncotarget. 8:90521–90531. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tian M, Cheuk AT, Wei JS, Abdelmaksoud A,
Chou HC, Milewski D, Kelly MC, Song YK, Dower CM, Li N, et al: An
optimized bicistronic chimeric antigen receptor against GPC2 or
CD276 overcomes heterogeneous expression in neuroblastoma. J Clin
Invest. 132:e1556212022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Muhammad N, Wang R, Li W, Zhang Z, Chang
Y, Hu Y, Zhao J, Zheng X, Mao Q and Xia H: A novel TanCAR targeting
IL13Rα2 and EphA2 for enhanced glioblastoma therapy. Mol Ther
Oncolytics. 24:729–741. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Han X, Wang Y, Wei J and Han W:
Multi-antigen-targeted chimeric antigen receptor T cells for cancer
therapy. J Hematol Oncol. 12:1282019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Hudecek M, Sommermeyer D, Kosasih PL,
Silva-Benedict A, Liu L, Rader C, Jensen MC and Riddell SR: The
nonsignaling extracellular spacer domain of chimeric antigen
receptors is decisive for in vivo antitumor activity. Cancer
Immunol Res. 3:125–135. 2015. View Article : Google Scholar
|
|
168
|
Srivastava S and Riddell SR: Engineering
CAR-T cells: Design concepts. Trends Immunol. 36:494–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Künkele A, Johnson AJ, Rolczynski LS,
Chang CA, Hoglund V, Kelly-Spratt KS and Jensen MC: Functional
tuning of CARs reveals signaling threshold above which CD8+ CTL
antitumor potency is attenuated due to cell fas-FasL-dependent
AICD. Cancer Immunol Res. 3:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
James SE, Greenberg PD, Jensen MC, Lin Y,
Wang J, Till BG, Raubitschek AA, Forman SJ and Press OW: Antigen
sensitivity of CD22-specific chimeric TCR is modulated by target
epitope distance from the cell membrane. J Immunol. 180:7028–7038.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wilkie S, Picco G, Foster J, Davies DM,
Julien S, Cooper L, Arif S, Mather SJ, Taylor-Papadimitriou J,
Burchell JM and Maher J: Retargeting of human T cells to
tumor-associated MUC1: The evolution of a chimeric antigen
receptor. J Immunol. 180:4901–4909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Hudecek M, Lupo-Stanghellini MT, Kosasih
PL, Sommermeyer D, Jensen MC, Rader C and Riddell SR: Receptor
affinity and extracellular domain modifications affect tumor
recognition by ROR1-specific chimeric antigen receptor T cells.
Clin Cancer Res. 19:3153–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Guest RD, Hawkins RE, Kirillova N, Cheadle
EJ, Arnold J, O'Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM,
et al: The role of extracellular spacer regions in the optimal
design of chimeric immune receptors: Evaluation of four different
scFvs and antigens. J Immunother. 28:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Brudno JN, Lam N, Vanasse D, Shen YW, Rose
JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, et al: Safety
and feasibility of anti-CD19 CAR T cells with fully human binding
domains in patients with B-cell lymphoma. Nat Med. 26:270–280.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Alabanza L, Pegues M, Geldres C, Shi V,
Wiltzius JJW, Sievers SA, Yang S and Kochenderfer JN: Function of
novel Anti-CD19 chimeric antigen receptors with human variable
regions is affected by hinge and transmembrane domains. Mol Ther.
25:2452–2465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Hombach A, Hombach AA and Abken H:
Adoptive immunotherapy with genetically engineered T cells:
Modification of the IgG1 Fc 'spacer' domain in the extracellular
moiety of chimeric antigen receptors avoids 'off-target' activation
and unintended initiation of an innate immune response. Gene Ther.
17:1206–1213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Almåsbak H, Walseng E, Kristian A, Myhre
MR, Suso EM, Munthe LA, Andersen JT, Wang MY, Kvalheim G,
Gaudernack G and Kyte JA: Inclusion of an IgG1-Fc spacer abrogates
efficacy of CD19 CAR T cells in a xenograft mouse model. Gene Ther.
22:391–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Watanabe N, Bajgain P, Sukumaran S, Ansari
S, Heslop HE, Rooney CM, Brenner MK, Leen AM and Vera JF:
Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology.
5:e12536562016. View Article : Google Scholar
|
|
179
|
Stoiber S, Cadilha BL, Benmebarek MR,
Lesch S, Endres S and Kobold S: Limitations in the design of
chimeric antigen receptors for cancer therapy. Cells. 8:4722019.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bridgeman JS, Hawkins RE, Bagley S,
Blaylock M, Holland M and Gilham DE: The optimal antigen response
of chimeric antigen receptors harboring the CD3zeta transmembrane
domain is dependent upon incorporation of the receptor into the
endogenous TCR/CD3 complex. J Immunol. 184:6938–6949. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Guedan S, Posey AD Jr, Shaw C, Wing A, Da
T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU,
Uribe-Herranz M, et al: Enhancing CAR T cell persistence through
ICOS and 4-1BB costimulation. JCI Insight. 3:e969762018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wan Z, Shao X, Ji X, Dong L, Wei J, Xiong
Z, Liu W and Qi H: Transmembrane domain-mediated Lck association
underlies bystander and costimulatory ICOS signaling. Cell Mol
Immunol. 17:143–152. 2020. View Article : Google Scholar :
|
|
183
|
Majzner RG, Rietberg SP, Sotillo E, Dong
R, Vachharajani VT, Labanieh L, Myklebust JH, Kadapakkam M, Weber
EW, Tousley AM, et al: Tuning the antigen density requirement for
CAR T-cell activity. Cancer Discov. 10:702–723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Fujiwara K, Tsunei A, Kusabuka H, Ogaki E,
Tachibana M and Okada N: Hinge and transmembrane domains of
chimeric antigen receptor regulate receptor expression and
signaling threshold. Cells. 9:11822020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Yan Z, Cao J, Cheng H, Qiao J, Zhang H,
Wang Y, Shi M, Lan J, Fei X, Jin L, et al: A combination of
humanised anti-CD19 and anti-BCMA CAR T cells in patients with
relapsed or refractory multiple myeloma: A single-arm, phase 2
trial. Lancet Haematol. 6:e521–e529. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Huang R, Li X, He Y, Zhu W, Gao L, Liu Y,
Gao L, Wen Q, Zhong JF, Zhang C and Zhang X: Recent advances in
CAR-T cell engineering. J Hematol Oncol. 13:862020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Imai C, Mihara K, Andreansky M, Nicholson
IC, Pui CH, Geiger TL and Campana D: Chimeric receptors with 4-1BB
signaling capacity provoke potent cytotoxicity against acute
lymphoblastic leukemia. Leukemia. 18:676–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Song DG, Ye Q, Poussin M, Harms GM, Figini
M and Powell DJ Jr: CD27 costimulation augments the survival and
antitumor activity of redirected human T cells in vivo. Blood.
119:696–706. 2012. View Article : Google Scholar
|
|
189
|
Maher J, Brentjens RJ, Gunset G, Riviere I
and Sadelain M: Human T-lymphocyte cytotoxicity and proliferation
directed by a single chimeric TCRzeta/CD28 receptor. Nat
Biotechnol. 20:70–75. 2002. View Article : Google Scholar
|
|
190
|
Guedan S, Chen X, Madar A, Carpenito C,
McGettigan SE, Frigault MJ, Lee J, Posey AD Jr, Scholler J,
Scholler N, et al: ICOS-based chimeric antigen receptors program
bipolar TH17/TH1 cells. Blood. 124:1070–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Mullard A: FDA approves first CAR T
therapy. Nat Rev Drug Discov. 16:6692017.
|
|
192
|
Carpenito C, Milone MC, Hassan R, Simonet
JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF,
Albelda SM, et al: Control of large, established tumor xenografts
with genetically retargeted human T cells containing CD28 and CD137
domains. Proc Natl Acad Sci USA. 106:3360–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Zhao Z, Condomines M, van der Stegen SJC,
Perna F, Kloss CC, Gunset G, Plotkin J and Sadelain M: Structural
design of engineered costimulation determines tumor rejection
kinetics and persistence of CAR T cells. Cancer Cell. 28:415–428.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Guedan S, Madar A, Casado-Medrano V, Shaw
C, Wing A, Liu F, Young RM, June CH and Posey AD Jr: Single residue
in CD28-costimulated CAR-T cells limits long-term persistence and
antitumor durability. J Clin Invest. 130:3087–3097. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
van der Merwe PA and Dushek O: Mechanisms
for T cell receptor triggering. Nat Rev Immunol. 11:47–55. 2011.
View Article : Google Scholar
|
|
196
|
Gaud G, Lesourne R and Love PE: Regulatory
mechanisms in T cell receptor signalling. Nat Rev Immunol.
18:485–497. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z,
Leibold J, Dobrin A, Cabriolu A, Hamieh M and Sadelain M:
Calibration of CAR activation potential directs alternative T cell
fates and therapeutic potency. Nat Med. 25:82–88. 2019. View Article : Google Scholar :
|
|
198
|
James JR: Tuning ITAM multiplicity on T
cell receptors can control potency and selectivity to ligand
density. Sci Signal. 11:eaan10882018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Bachiller M, Dobaño-López C,
Rodríguez-García A, Castellsagué J, Gimenez-Alejandre M,
Antoñana-Vildosola A, Martin-Antonio B, Delgado J, Pérez-Galán P,
Juan M, et al: Co-Transduced CD19/BCMA dual-targeting CAR-T cells
for the treatment of non-hodgkin lymphoma. Blood. 140(Suppl 1):
S7386–S7387. 2022. View Article : Google Scholar
|
|
200
|
Ghorashian S, Lucchini G, Richardson R,
Nguyen K, Terris C, Oporto-Espuelas M, Yeung J, Pinner D, Chu J,
Williams L, et al: Dual antigen targeting with co-transduced
CD19/22 CAR T cells may prevent antigen-negative relapse after CAR
T cell therapy for relapsed/refractory ALL. Blood. 140(Suppl 1):
S10352–S10354. 2022. View Article : Google Scholar
|
|
201
|
Wang L, Tan Su Yin E, Zhao H, Ni F, Hu Y
and Huang H: CAR-T cells: The Chinese experience. Expert Opin Biol
Ther. 20:1293–1308. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Sun C, Dotti G and Savoldo B: Utilizing
cell-based therapeutics to overcome immune evasion in hematologic
malignancies. Blood. 127:3350–3359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Dhupkar P, Gordon N, Stewart J and
Kleinerman ES: Anti-PD-1 therapy redirects macrophages from an M2
to an M1 phenotype inducing regression of OS lung metastases.
Cancer Med. 7:2654–2664. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ,
van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al:
Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances
anti-tumor efficacy in vivo. Nat Biotechnol. 36:847–856. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Kenderian SS, Ruella M, Shestova O,
Klichinsky M, Kim M, Porter DL, June CH and Gill S: Identification
of PD1 and TIM3 As checkpoints that limit chimeric antigen receptor
T cell efficacy in leukemia. Biol Blood Marrow Transplant. 22(3
Suppl): S19–S21. 2016. View Article : Google Scholar
|
|
206
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and
enhances antitumor immune activity. Clin Cancer Res. 11:6713–6721.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Sevko A, Michels T, Vrohlings M, Umansky
L, Beckhove P, Kato M, Shurin GV, Shurin MR and Umansky V:
Antitumor effect of paclitaxel is mediated by inhibition of
myeloid-derived suppressor cells and chronic inflammation in the
spontaneous melanoma mode. J Immunol. 190:2464–2471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Eriksson E, Wenthe J, Irenaeus S, Loskog A
and Ullenhag G: Gemcitabine reduces MDSCs, tregs and TGFβ-1 while
restoring the teff/treg ratio in patients with pancreatic cancer. J
Transl Med. 14:2822016. View Article : Google Scholar
|
|
209
|
Vincent J, Mignot G, Chalmin F, Ladoire S,
Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and
Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated
myeloid-derived suppressor cells resulting in enhanced T
cell-dependent antitumor immunity. Cancer Res. 70:3052–3061. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kusmartsev S, Su Z, Heiser A, Dannull J,
Eruslanov E, Kübler H, Yancey D, Dahm P and Vieweg J: Reversal of
myeloid cell-mediated immunosuppression in patients with metastatic
renal cell carcinoma. Clin Cancer Res. 14:8270–8278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Yoshida K, Okamoto M, Sasaki J, Kuroda C,
Ishida H, Ueda K, Ideta H, Kamanaka T, Sobajima A, Takizawa T, et
al: Anti-PD-1 antibody decreases tumour-infiltrating regulatory T
cells. BMC Cancer. 20:252020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Pyonteck SM, Akkari L, Schuhmacher AJ,
Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT,
Teijeiro V, et al: CSF-1R inhibition alters macrophage polarization
and blocks glioma progression. Nat Med. 19:1264–1272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Klug F, Prakash H, Huber PE, Seibel T,
Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et
al: Low-dose irradiation programs macrophage differentiation to an
iNOS+/M1 phenotype that orchestrates effective T cell
immunotherapy. Cancer Cell. 24:589–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Nadella V, Singh S, Jain A, Jain M,
Vasquez KM, Sharma A, Tanwar P, Rath GK and Prakash H: Low dose
radiation primed iNOS + M1macrophages modulate angiogenic
programming of tumor derived endothelium. Mol Carcinog.
57:1664–1671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Ruella M, Klichinsky M, Kenderian SS,
Shestova O, Ziober A, Kraft DO, Feldman M, Wasik MA, June CH and
Gill S: Overcoming the immunosuppressive tumor microenvironment of
Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer
Discov. 7:1154–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Choi SH, Myers J, Tomchuck S, Bonner M,
Eid S, Kingsley D, VanHeyst K, Kim SJ, Kim BG and Huang AY: Oral
TGF-βR1 inhibitor vactosertib promotes osteosarcoma regression by
targeting tumor proliferation and enhancing anti-tumor immunity.
Res Sq. rs.3.rs-27092822023.
|
|
217
|
Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu
W, Wei XF, Han W and Wang H: TGF-β inhibition via CRISPR promotes
the long-term efficacy of CAR T cells against solid tumors. JCI
Insight. 5:e1339772020. View Article : Google Scholar
|
|
218
|
Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu
H, Dotti G, Balyasnikova IV and Gottschalk S: Transgenic expression
of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but
results in antigen loss variants. Cancer Immunol Res. 5:571–581.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Chmielewski M, Kopecky C, Hombach AA and
Abken H: IL-12 release by engineered T cells expressing chimeric
antigen receptors can effectively Muster an antigen-independent
macrophage response on tumor cells that have shut down tumor
antigen expression. Cancer Res. 71:5697–5706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Loschinski R, Böttcher M, Stoll A, Bruns
H, Mackensen A and Mougiakakos D: IL-21 modulates memory and
exhaustion phenotype of T-cells in a fatty acid oxidation-dependent
manner. Oncotarget. 9:13125–13138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Huang Y, Si X, Shao M, Teng X, Xiao G and
Huang H: Rewiring mitochondrial metabolism to counteract exhaustion
of CAR-T cells. J Hematol Oncol. 15:382022. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Sukumar M, Liu J, Ji Y, Subramanian M,
Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly
ED, et al: Inhibiting glycolytic metabolism enhances CD8+ T cell
memory and antitumor function. J Clin Invest. 123:4479–4488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Geiger R, Rieckmann JC, Wolf T, Basso C,
Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et
al: L-arginine modulates T cell metabolism and enhances survival
and anti-tumor activity. Cell. 167:829–842.e13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Ghassemi S, Martinez-Becerra FJ, Master
AM, Richman SA, Heo D, Leferovich J, Tu Y, García-Cañaveras JC,
Ayari A, Lu Y, et al: Enhancing chimeric antigen receptor T cell
anti-tumor function through advanced media design. Mol Ther Methods
Clin Dev. 18:595–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar
|
|
226
|
Turtle CJ, Hanafi LA, Berger C, Hudecek M,
Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et
al: Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of
CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T
cells. Sci Transl Med. 8:355ra1162016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Mestermann K, Giavridis T, Weber J, Rydzek
J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H and Hudecek
M: The tyrosine kinase inhibitor dasatinib acts as a pharmacologic
on/off switch for CAR T cells. Sci Transl Med. 11:eaau59072019.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Varadarajan I and Lee DW: Management of
T-cell engaging immunotherapy complications. Cancer J. 25:223–230.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Fukumura D, Xavier R, Sugiura T, Chen Y,
Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK and Seed B:
Tumor induction of VEGF promoter activity in stromal cells. Cell.
94:715–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Chinnasamy D, Yu Z, Theoret MR, Zhao Y,
Shrimali RK, Morgan RA, Feldman SA, Restifo NP and Rosenberg SA:
Gene therapy using genetically modified lymphocytes targeting
VEGFR-2 inhibits the growth of vascularized syngenic tumors in
mice. J Clin Invest. 120:3953–3968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo
FC, Zhang J, Li D, Mo BH, Wen F, et al: Specificity redirection by
CAR with human VEGFR-1 affinity endows T lymphocytes with
tumor-killing ability and anti-angiogenic potency. Gene Ther.
20:970–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Slaney CY, Kershaw MH and Darcy PK:
Trafficking of T cells into tumors. Cancer Res. 74:7168–7174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
van Schalkwyk MC, Papa SE, Jeannon JP,
Guerrero Urbano T, Spicer JF and Maher J: Design of a phase I
clinical trial to evaluate intratumoral delivery of ErbB-targeted
chimeric antigen receptor T-cells in locally advanced or recurre
head and neck cancer. Hum Gene Ther Clin Dev. 24:134–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Sridhar P and Petrocca F: Regional
delivery of chimeric antigen receptor (CAR) T-cells for cancer
therapy. Cancers (Basel). 9:922017. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Milner JJ, Toma C, Yu B, Zhang K, Omilusik
K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al: Runx3
programs CD8(+) T cell residency in non-lymphoid tissues and
tumors. Nature. 552:253–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Caruana I, Savoldo B, Hoyos V, Weber G,
Liu H, Kim ES, Ittmann MM, Marchetti D and Dotti G: Heparanase
promotes tumor infiltration and antitumor activity of
CAR-redirected T lymphocytes. Nat Med. 21:524–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Craddock JA, Lu A, Bear A, Pule M, Brenner
MK, Rooney CM and Foster AE: Enhanced tumor trafficking of GD2
chimeric antigen receptor T cells by expression of the chemokine
receptor CCR2b. J Immunother. 33:780–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Maus MV, Haas AR, Beatty GL, Albelda SM,
Levine BL, Liu X, Zhao Y, Kalos M and June CH: T cells expressing
chimeric antigen receptors can cause anaphylaxis in humans. Cancer
Immunol Res. 1:26–31. 2013. View Article : Google Scholar
|
|
240
|
Till BG, Jensen MC, Wang J, Chen EY, Wood
BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al:
Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle
cell lymphoma using genetically modified autologous CD20-specific T
cells. Blood. 112:2261–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Ajina A and Maher J: Prospects for
combined use of oncolytic viruses and CAR T-cells. Review. J
Immunother Cancer. 5:902017. View Article : Google Scholar
|
|
242
|
Kim DS, Dastidar H, Zhang C, Zemp FJ, Lau
K, Ernst M, Rakic A, Sikdar S, Rajwani J, Naumenko V, et al: Smac
mimetics and oncolytic viruses synergize in driving anticancer
T-cell responses through complementary mechanisms. Nat Commun.
8:3442017. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Scott EM, Duffy MR, Freedman JD, Fisher KD
and Seymour LW: Solid tumor immunotherapy with T cell engager-armed
oncolytic viruses. Macromol Biosci. 18:17001872018. View Article : Google Scholar
|
|
244
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Chmielewski M and Abken H: TRUCKS, the
fourth-generation CAR T cells: Current developments and clinical
translation. Adv Cell Gene Ther. 3:e842020. View Article : Google Scholar
|
|
246
|
Zhang H, Zhao P and Huang H: Engineering
better chimeric antigen receptor T cells. Exp Hematol Oncol.
9:342020. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Köksal H, Müller E, Inderberg EM, Bruland
Ø and Wälchli S: Treating osteosarcoma with CAR T cells. Scand J
Immunol. 89:e127412019. View Article : Google Scholar
|
|
248
|
Noordam L, Kaijen MEH, Bezemer K,
Cornelissen R, Maat LAPWM, Hoogsteden HC, Aerts JGJV, Hendriks RW,
Hegmans JPJJ and Vroman H: Low-dose cyclophosphamide depletes
circulating naïve and activated regulatory T cells in malignant
pleural mesothelioma patients synergistically treated with
dendritic cell-based immunotherapy. Oncoimmunology. 7:e14743182018.
View Article : Google Scholar
|
|
249
|
Ge Y, Domschke C, Stoiber N, Schott S,
Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, et al:
Metronomic cyclophosphamide treatment in metastasized breast cancer
patients: Immunological effects and clinical outcome. Cancer
Immunol Immunother. 61:353–362. 2012. View Article : Google Scholar
|
|
250
|
Hu J, Sun C, Bernatchez C, Xia X, Hwu P,
Dotti G and Li S: T-cell homing therapy for reducing regulatory T
cells and preserving effector T-cell function in large solid
tumors. Clin Cancer Res. 24:2920–2934. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Alizadeh D, Trad M, Hanke NT, Larmonier
CB, Janikashvili N, Bonnotte B, Katsanis E and Larmonier N:
Doxorubicin eliminates myeloid-derived suppressor cells and
enhances the efficacy of adoptive T-cell transfer in breast cancer.
Cancer Res. 74:104–118. 2014. View Article : Google Scholar
|
|
252
|
Seliger B and Quandt D: The expression,
function, and clinical relevance of B7 family members in cancer.
Cancer Immunol Immunother. 61:1327–1341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Murad JP, Tilakawardane D, Park AK, Lopez
LS, Young CA, Gibson J, Yamaguchi Y, Lee HJ, Kennewick KT, Gittins
BJ, et al: Pre-conditioning modifies the TME to enhance solid tumor
CAR T cell efficacy and endogenous protective immunity. Mol Ther.
29:2335–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Kohli K, Pillarisetty VG and Kim TS: Key
chemokines direct migration of immune cells in solid tumors. Cancer
Gene Ther. 29:10–21. 2022. View Article : Google Scholar :
|
|
255
|
Srivastava S, Furlan SN, Jaeger-Ruckstuhl
CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee
SM, Amezquita RA, et al: Immunogenic chemotherapy enhances
recruitment of CAR-T cells to lung tumors and improves antitumor
efficacy when combined with checkpoint blockade. Cancer Cell.
39:193–208.e10. 2021. View Article : Google Scholar
|
|
256
|
Motyka B, Korbutt G, Pinkoski MJ, Heibein
JA, Caputo A, Hobman M, Barry M, Shostak I, Sawchuk T, Holmes CF,
et al: Mannose 6-phosphate/insulin-like growth factor II receptor
is a death receptor for granzyme B during cytotoxic T cell-induced
apoptosis. Cell. 103:491–500. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Ramakrishnan R, Huang C, Cho HI, Lloyd M,
Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E and
Gabrilovich DI: Autophagy induced by conventional chemotherapy
mediates tumor cell sensitivity to immunotherapy. Cancer Res.
72:5483–5493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Trapani JA, Sutton VR, Thia KYT, Li YQ,
Froelich CJ, Jans DA, Sandrin MS and Browne KA: A
clathrin/dynaminand mannose-6-phosphate receptor-independent
pathway for granzyme B-induced cell death. J Cell Biol.
160:223–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Parente-Pereira AC, Whilding LM, Brewig N,
van der Stegen SJ, Davies DM, Wilkie S, van Schalkwyk MC,
Ghaem-Maghami S and Maher J: Synergistic chemoimmunotherapy of
epithelial ovarian cancer using ErbB-Retargeted T cells combined
with carboplatin. J Immunol. 191:2437–2445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Proietti E, Moschella F, Capone I and
Belardelli F: Exploitation of the propulsive force of chemotherapy
for improving the response to cancer immunotherapy. Mol Oncol.
6:1–14. 2012. View Article : Google Scholar
|
|
261
|
Senovilla L, Vitale I, Martins I, Tailler
M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O,
Niso-Santano M, et al: An immunosurveillance mechanism controls
cancer cell ploidy. Science. 337:1678–1684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Martins I, Tesniere A, Kepp O, Michaud M,
Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL,
Zitvogel L and Kroemer G: Chemotherapy induces ATP release from
tumor cells. Cell Cycle. 8:3723–3728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Garg AD, Galluzzi L, Apetoh L, Baert T,
Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R,
Cirone M, et al: Molecular and translational classifications of
DAMPs in immunogenic cell death. Front Immunol. 6:5882015.
View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Sistigu A, Yamazaki T, Vacchelli E, Chaba
K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et
al: Cancer cell-autonomous contribution of type I interferon
signaling to the efficacy of chemotherapy. Nat Med. 20:1301–1309.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Vierboom MP, Bos GM, Ooms M, Offringa R
and Melief CJ: Cyclophosphamide enhances anti-tumor effect of
wild-type p53-specific CTL. Int J Cancer. 87:253–260. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Higgins JP, Bernstein MB and Hodge JW:
Enhancing immune responses to tumor-associated antigens. Cancer
Biol Ther. 8:1440–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10:17588340177425752018. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
269
|
Crouse J, Kalinke U and Oxenius A:
Regulation of antiviral T cell responses by type I interferons. Nat
Rev Immunol. 15:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Burnette BC, Liang H, Lee Y, Chlewicki L,
Khodarev NN, Weichselbaum RR, Fu YX and Auh SL: The efficacy of
radiotherapy relies upon induction of type I interferon-dependent
innate and adaptive immunity. Cancer Res. 71:2488–2496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Diamond MS, Kinder M, Matsushita H,
Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM,
Kalinke U, et al: Type I interferon is selectively required by
dendritic cells for immune rejection of tumors. J Exp Med.
208:1989–2003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Fuertes MB, Kacha AK, Kline J, Woo SR,
Kranz DM, Murphy KM and Gajewski TF: Host type I IFN signals are
required for antitumor CD8+ T cell responses through CD8{alpha}+
dendritic cells. J Exp Med. 208:2005–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Weiss T, Weller M, Guckenberger M, Sentman
CL and Roth P: NKG2D-based CAR T cells and radiotherapy exert
synergistic efficacy in glioblastoma. Cancer Res. 78:1031–1043.
2018. View Article : Google Scholar
|
|
274
|
Matsumura S, Wang B, Kawashima N,
Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti
SC, Dustin ML and Demaria S: Radiation-induced CXCL16 release by
breast cancer cells attracts effector T cells. J Immunol.
181:3099–3107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Zhang B, Bowerman NA, Salama JK, Schmidt
H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley
DA, et al: Induced sensitization of tumor stroma leads to
eradication of established cancer by T cells. J Exp Med. 204:49–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Ganss R, Ryschich E, Klar E, Arnold B and
Hämmerling GJ: Combination of T-cell therapy and trigger of
inflammation induces remodeling of the vasculature and tumor
eradication. Cancer Res. 62:1462–1470. 2002.PubMed/NCBI
|
|
277
|
Yovino S and Grossman SA: Severity,
etiology and possible consequences of treatment-related lymphopenia
in patients with newly diagnosed high-grade gliomas. CNS Oncol.
1:149–154. 2012. View Article : Google Scholar
|