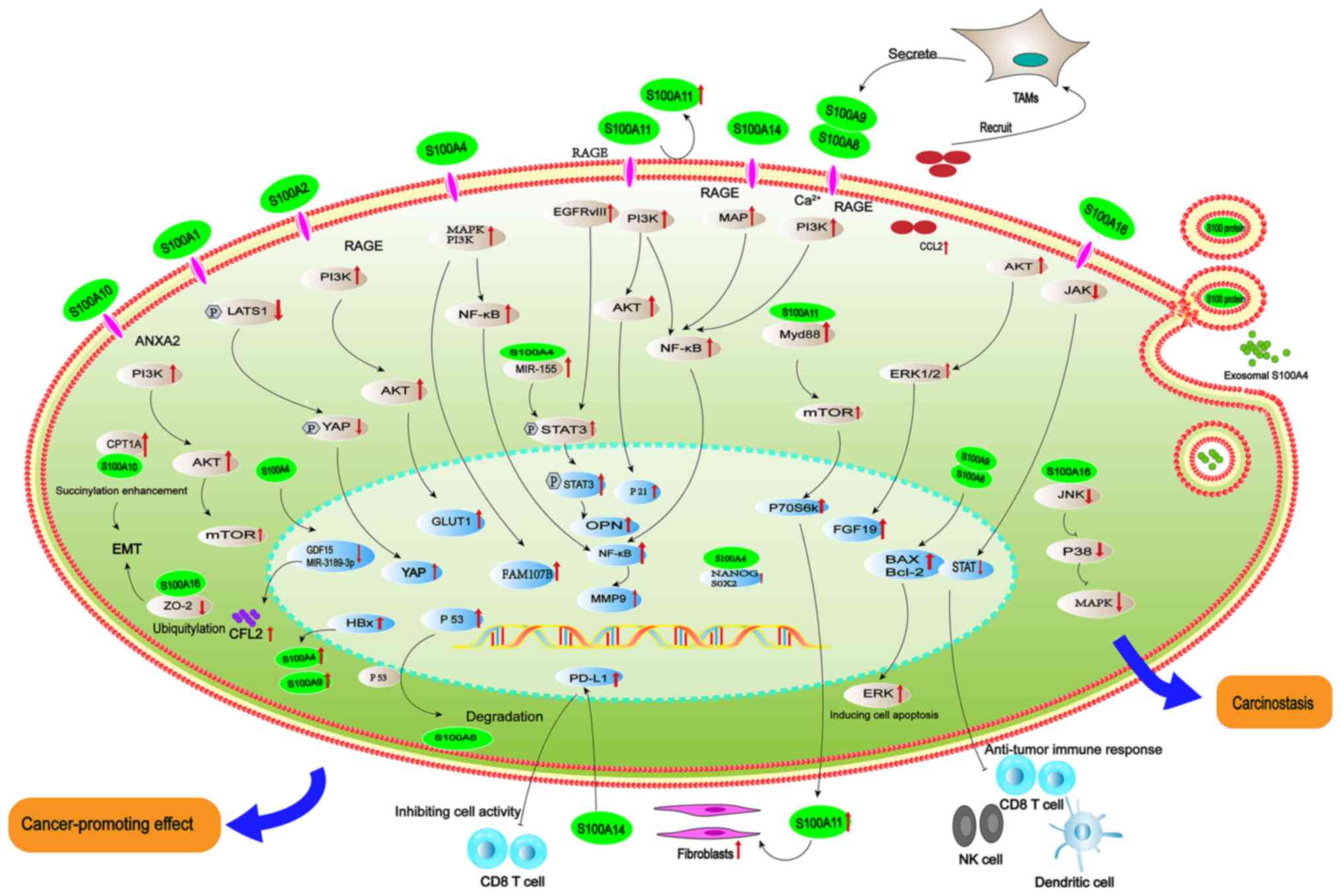
Digestive tract cancer is one of the most common
types of malignant tumors worldwide and primarily includes
esophageal cancer (EC), gastric cancer (GC), pancreatic cancer
(PC), liver cancer (LC) and colorectal cancer (CRC). According to
the World Health Organization, digestive tract cancer accounts for
more than a quarter (26%) of global cancer incidence, and more than
a third (35%) of all cancer-associated deaths across the world
(1). Therefore, it is urgent to
identify early screening methods for digestive tract cancer, as
well as improved approaches for prognosis of patients with advanced
disease. Improving the prognosis of patients with digestive tract
cancer, particularly those with advanced disease, is an urgent
issue. Accordingly, it is key to identify new targets for the early
diagnosis and treatment of digestive tract cancer to improve
disease survival and overall quality of life.
S100 proteins belong to a polygenic calcium-binding
family composed of small acidic proteins. S100 proteins are widely
expressed with high tissue- and cell-specificity and were firstly
extracted from bovine brain tissues by Moore in 1965 (2). S100 proteins dissolve in saturated
ammonium sulfate solution at neutral pH (3). In human, the S100 protein family
consists of 20 members (4), 16 of
which are located on chromosome 1q21 and known as group A S100
proteins (5) (Table I). The corresponding genes are
highly conserved and encode small proteins with ~100 amino acids in
size. S100 proteins have a highly similar calcium binding protein
sequence, known as the elongation factor (EF hand). When calcium
ion binds the EF hand, S100 proteins bind to the corresponding
receptors and participate in various cellular processes, including
proliferation, differentiation and apoptosis (6). Increasing evidence has demonstrated
that the S100 protein family is associated with pathogenesis of
digestive tract cancer, including LC (7–10).
The aberrant expression of specific S100 isoforms drives LC, such
as S100A4, S100A6, S100A8, S100A9 and S100A11 (7). The present review aimed to summarize
studies on the role of S100 protein family in digestive tract
cancer. Elucidating the effects and underlying mechanisms of S100
proteins may provide insight into the pathogenesis of digestive
tract cancer. In particular, S100 inhibitors for cancer treatment
may have significance, given the pivotal role of S100 signaling in
tumorigenesis and tumor biology (4).
S100 protein family consists of 25 members,
characterized by low molecular weight and a symmetrical dimeric
structure. S100 proteins exhibit notable homology with both
calmodulin and calcium-binding proteins (11). The conformation of S100 protein
changes when bound to Ca2+, exposing hydrophobic amino
acids in the first helix and hinge regions of the C-terminal EF
hand (12). S100 protein members
serve as intracellular Ca2+ sensors during
carcinogenesis via the regulation of
Ca2+/S100A4/myosin-IIA complex and other mechanisms
(13,14).
S100 proteins serve a key role in regulating cell
proliferation, differentiation, and apoptosis by interacting with
enzymes, cytoskeletal subunits, receptors, transcriptional factors
and nucleic acids (15). S100
proteins exert biological effects in either an autocrine or
paracrine manner (16). S100
proteins are implicated in inflammation, tissue repair and
resistance to pathogens by binding to various receptors, including
G protein-coupled receptor, scavenger receptor and receptor for
advanced glycation end products (RAGE), and activating mTOR,
Src/annexin A2 (ANXA2)/AKT and PI3K signaling pathways (17,18).
Besides, S100 family members participate in regulating
neuroinflammation in astrocytes and microglia and may serve as
diagnostic and therapeutic targets (for instance, S100A8/A9)
(19). The expression of S100
protein members is specific in different types of cancer.
Dysregulation of S100 family proteins occurs in most types of
cancers, suggesting their key roles in tumorigenesis. S100P is
upregulated in multiple cancers, such as lung cancer, CRC, and PC
(20). S100A4 is an important
regulator of immunosuppressive T cells in human glioma, affecting
immune microenvironment balance and survival (21). S100A7 is significantly associated
with the prognosis of head and neck squamous carcinoma (22). S100A6 regulates cancer cell
proliferation, apoptosis, migration and invasion, which is also
associated with poor prognosis (4,23).
S100A10, also known as p11, is found to mediate the conversion of
plasminogen to plasmin primarily by binding to ANXA2 (24). This interaction results in
degradation of the extracellular matrix, facilitating the
dissemination of cancer cells through the bloodstream. In breast
cancer, S100A14 enhances the phosphorylation of HER2 and the
activation of AKT/ERK signaling pathway, thereby promoting the
development of breast cancer (25). Taken together, the aforementioned
studies have suggested key roles of S100 family members (including
S100P, S100A4, S100A6, S100A7, S100A10, and S100A14) in the
development and progression of various types of cancers. The
present study summarizes the role of S100 family in digestive tract
cancers to provide insights into cancer pathogenesis and novel
therapeutic strategies.
GC is one of the most common types of malignant
tumors across the world. The majority of patients with GC are
diagnosed at advanced stage with poor prognosis. Therefore, it is
necessary to explore more effective strategies for the early
diagnosis of GC. A previous study demonstrated that S100A4 promotes
proliferation and migration of GC cells by regulating a downstream
effector family with sequence similarity 107 member B via the PI3K
signaling (Fig. 1) (26). Another study found that S100A4
increases the stemness of cancer cells via upregulating NANOG and
SOX2, thereby promoting gastric carcinogenesis (27). Bian et al (28) reported that silencing S100A4 using
small interfering RNA inhibited the proliferation and migration of
GC cells. Furthermore, this effect is enhanced by microRNA (miRNA
or miR)-3189-3p mimics, which targeting cofilin-2 and further
inhibiting GC cell proliferation and migration (28). Accordingly, S100A4 may serve as a
promising biomarker and treatment target for GC due to its key
effects in regulating the biological behavior of cancer cells
(Table II).
Another well-established S100 family member is
S100A9, the expression and function of which exhibit variability
across types of cancer (29–31).
Enhanced expression of S100A9 has been observed in GC and promotes
the proliferation and migration of cancer cells (30). S100A9 plays dual roles, acting both
as a pro- and anti-tumor factor independently and forming
heterodimers with S100A8 (S100A8/A9) during gastric carcinogenesis
(31). It is involved in
inflammation in GC. At low concentrations, S100A8/A9 promotes
cancer cell proliferation and migration by activating NF-κB-, RAGE-
and MAP kinase-dependent signaling pathways (32). Conversely, at high concentrations,
S100A8/A9 exhibits cytotoxic (33)
and pro-apoptosis effects on GC cells by regulating Bax/Bcl-2
expression and activation of ERK (34). Positive association between S100A9
expression and the overall survival of patients with GC has been
demonstrated (35), suggesting the
protective role of S100A9 at high concentration against GC. It has
also been well documented that endogenous S100A8/A9 inhibits
migration and invasion of GC cells, whereas exogenous S100A8/A9
functions as a heterodimer to activate NF-κB signaling, thereby
promoting the development of GC (32,36).
This discrepancy may be attributed to differences in cancer
microenvironment (37). Given the
complicated effects of S100A9 under varying concentrations and
cellular locations, it is key to elucidate the precise molecular
mechanisms of S100A9 in regulating GC.
S100A10, a key member of the S100 family, is
upregulated in some malignant tumors, including lung cancer
(38) and LC (39). Similarly, increased expression of
S100A10 has been found in GC (40). S100A10 can promote gastric
carcinogenesis by enhancing GC cell proliferation and the
consumption of glucose via the Src/ANXA2/AKT/mTOR signaling pathway
(40). Besides, enhanced
succinylation of S100A10 promotes GC invasion and metastasis
depending on the activity of carnitine palmitoyltransferase 1A
(41). Taken together, S100A10
emerges as a promising therapeutic target due to its key role in
regulating the growth, invasion and metastasis of GC.
Liver is one of the most important digestive organs
responsible for the metabolisms of lipid, fatty acid and other
substances by regulating various active factors, such as growth
factors and cytokines (46,47).
Effective therapeutic strategies for HCC include surgery,
chemotherapy, targeted therapy and liver transplantation. However,
the prognosis of HCC remains poor (48). Therefore, it is necessary to
explore more effective strategies to achieve early intervention and
treatment of HCC. Increasing studies have demonstrated that S100
proteins play important roles in HCC and may serve as useful
diagnostic and prognostic markers (49–52).
Increased expression of S100A1 is observed in HCC tissue and is
positively related to tumor and tumor grade and survival rate
(53). Moreover, S100A1
contributes to HCC by inhibiting phosphorylation of LATS1 and
yes-associated protein via the Hippo pathway (53). S100A4 is a risk factor for GC
(26). Zhai et al (50) reported that S100A4 is involved in
HCC pathogenesis by promoting EMT and upregulating MMP-9 via the
NF-κB pathway (51,52). In HCC, Hepatitis B virus X (HBx)
protein can enhance the expression of S100A4, thus promoting the
proliferation of HCC cells (54).
Exosome-derived S100A4 can induce the metastasis of HCC by
regulating cell adhesion and remodeling of extracellular matrix
(55,56). Furthermore, S100A4 is highly
expressed in hypermetastatic HCC cells, which can promote invasion
and metastasis by upregulating miR-155 and activating STAT3
(57,58). In addition, high expression of
S100A4 predicts poor prognosis of HCC (49). Accordingly, S100A4 is a key
regulator in HCC. Exosomal S100A4 may serve as a promising marker
for HCC progression and prognosis. Other S100 proteins are involved
in the pathogenesis of HCC, such as S100A6 (59), S100A9 (60), S100A10 (61), S100A11 (62) and S10013 (63). Among them, increased expression of
S100A6 leads to HCC cell proliferation and invasion by enhancing
degradation of p53 (64). In
addition to cancer cell proliferation and invasion, high expression
of S100A9 is associated with poor differentiation and increased
malignancy of HCC (65). S100A9
secreted by tumor-associated macrophages functions as a cancer
promoter by recruiting more macrophages and other inflammatory
cells via chemokine ligand 2, thereby establishing a positive
feedback loop that leads to increased production of S100A9 within
the tumor microenvironment (66).
Similar to S100A4, HBx protein can elevate the expression of S100A9
via the NF-κB pathway, which thus promotes hepatitis B
virus-associated HCC occurrence and metastasis (67). In addition, increased expression of
S100A11 occurs in HCC, contributing to the invasion and migration
of HCC cells (68). It also
enhances inflammation and fibrosis via epidermal growth factor
receptor variant III/STAT3 signaling (68), ultimately leading to poor prognosis
of HCC (62). A recent study
confirmed that S100A11 is superior to alpha fetoprotein antibody in
predicting haematogenous metastasis in patients with HCC (69). Certain S100 proteins and the key
signaling molecules can serve as promising targets for the
treatment of HCC in future, although more high-quality studies are
warranted to determine the precise molecular mechanisms.
There are increasing studies supporting the key role
of S100 protein members in PC (9,71,72).
Bachet et al (71)
demonstrated that S100A2 predicts longer disease-free and overall
survival in patients with pancreatic adenocarcinoma, suggesting a
predictive benefit of S100A2 in the adjuvant therapy of pancreatic
adenocarcinoma. By contrast, another study (9) has implicated that elevated expression
of S100A2 is related to progression and poor prognosis of patients
with PC. Moreover, a recent study has shown that S100A2 serves as a
predictive biomarker of CD8+ T and activated natural
killer (NK) cell infiltration in PC, suggesting a prognostic factor
for predicting the response to immunotherapy response in patients
with PC (72). The expression of
S100A2 is positively associated with PD-L1 and infiltration of M0
macrophages in the immune microenvironment (72). Accordingly, more studies are
warranted to elucidate the effect and mechanism of S100A2 in
regulating PC. Che et al (73) demonstrated that the expression of
S100A4 is positively associated with the differentiation grade and
metastasis of PC (71) and
promoted the PC cell proliferation, angiogenesis, invasion, and
progression. High expression of S100A4 leads to poor
differentiation of PC by inducing hypomethylation of the
corresponding intron (74). In
addition, expression of S100A4 is positively associated with the
serum levels of CA19.9, an important prognostic factor for PC
(75). Accordingly, S100A4 is a
tumor promoter in PC but its precise molecular mechanism remains
unclear. It has been well documented that the expression of S100A11
is increased in multiple types of cancers, including lung (76) and thyroid cancer (77). A previous study demonstrated
elevated expression of S100A11 in PC, which also predicts poor
cancer prognosis (78). Similar
findings have also been demonstrated in subsequent studies
(79–81). S100A11 promotes pancreatic
carcinogenesis by enhancing expression, phosphorylation and
activation of AKT, upregulating P21 and facilitating the transition
of G0/G1 cell cycle through the PI3K/AKT signaling pathway in
cancer cells (80). Moreover,
S100A11 can also function as a PC promoter by facilitating the
spread of fibroblast population and promoting cancer progression
via the RAGE/MyD88/mTOR/p70 signaling pathway (81). As a result, S100A11 may serve as a
promising therapeutic target due to the key modifying effects in
regulating PC. Increasing evidence has demonstrated the altered
expression of S100A14 in multiple malignant tumors, such as LC,
breast cancer and CRC (82–85).
Elevated expression of S100A14 has also been reported in several
publications (83–85). Zhuang et al (84) demonstrated that the overexpression
of S100A14 promotes the proliferation, migration and invasion of PC
cells by adhering to Ras and inhibiting CD8+ T cell
infiltration and cytolytic activity. S100A14 is found to inhibit
the activation of CD8+T cells by enhancing the
expression of PD-L1, which affects the immunotherapy response of
patients with PC (85).
Accordingly, S100A14 is also a PC promoter and prognostic
predictor. Fang et al (86)
demonstrated that S100A16 promotes PC progression by enhancing the
expression of FGF19 via the AKT/ERK1/2 signaling pathway. Besides,
S100A16 plays a critical role in regulating cancer immunity in PC,
affecting the function of CD8+ T, dendritic and NK cells
by inhibiting the activation of JAK/STAT signaling (87). S100A16 induces EMT by enhancing
TWIST expression and activating the STAT3 signaling pathway
(87). Moreover, the anti-tumor
effect of gemcitabine is augmented following inhibition of S100A16
(88). Therefore, elevated
expression of S100A16 predicts poor overall survival of patients
with PC (89,90). Taken together, S100 family members
are potential biomarkers for PC diagnosis, treatment and prognosis,
particularly S100A4, S100A11, S100A14 and S100A16. Nonetheless, the
underlying mechanisms of S100 family in PC warrant more studies
with high quality.
CRC is one of the most common types of
gastrointestinal cancer and has unclear etiology and pathogenesis
(91). It is the fourth most
common cause of cancer-related death worldwide after lung cancer,
LC and GC (92). It is urgent to
identify novel strategies for the early diagnosis and treatment of
CRC.
Taken together, S100 family members are involved in
the pathogenesis of CRC, particularly S100A2, S100A4, S100A8,
S100A14 and S100A16. They may serve as useful therapeutic targets
for CRC but the underlying mechanisms need to be elucidated in
future.
S100A7 is an ideal diagnostic marker for oral
potentially malignant disorders (OPMDs) and oral squamous cell
carcinoma (OSCC) (114). The
disease-free survival of patients with esophageal squamous cell
carcinoma (ESCC) with higher expression of S100A8/A9 is shorter
(115). Besides, S100A8/A9
promotes migration and invasion of ESCC cells via Akt/p38 signaling
(115), suggesting a pivotal role
of S100A8/A9 in ESCC progression and prognosis. Moreover,
extracellular S100A14 enhances the proliferation and survival of
ESCC cells via interacting with RAGE and activating MAP and NF-κB
pathways (25). Accordingly, S100
family members S100A7, S100A8/A9 and S100A14 may serve as useful
markers for the diagnosis and treatment of oral cancer and EC.
To date, there is no effective strategy for the
early diagnosis of most types of cancer. It is urgent to identify
novel makers for early screening and effective treatment of
digestive tract cancers due to the rising incidence and
cancer-associated mortality. Tumor-targeted therapy, which
selectively kills cancer cells while sparing healthy cells, has
garnered significant attention in recent years (103,116). Molecular targeted therapy, also
known as a ‘biological missile’, has emerged as a key focus in
cancer research. The S100 protein has been identified as a highly
promising biological target in recent studies (88,117). S100 protein family has been
demonstrated to participate in regulating inflammation, immunity,
tissue repair and tumorigenesis. Most importantly, certain S100
family members (such as S100A4, S100A8/9, S100A11, S100A14, and
S100A16) have shown potential for the molecular diagnosis,
progression monitoring and prognostic prediction of digestive tract
cancer. These proteins are involved in regulating tumor
proliferation, metastasis, angiogenesis and immune evasion,
although the precise mechanisms are elusive (118–121). Thus, elucidating the effects and
potential mechanisms of S100 proteins in digestive tract cancer may
facilitate the identification of novel targets for targeted
therapy. Future studies should focus on validation of
cancer-specific S100 proteins as biomarkers for early detection,
anti-tumor targets and prognostic prediction. S100 family members
hold promise as potential targets for the diagnosis and targeted
therapy of digestive tract cancer.
Not applicable.
The present study was supported by National Natural Science
Foundation (grant nos. 82003042 and 82171790) and Natural Science
Foundation of Shandong Province, China (grant no. ZR2020KC001).
Not applicable.
ML, DX and SY wrote and revised the manuscript. SJ,
BC, PC, WD, HZ, SY, DX and YS collected the data and constructed
tables and figures. ML and SY confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore BW: A soluble protein characteristic
of the nervous system. Biochem Biophys Res Commun. 19:739–744.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zimmer DB, Cornwall EH, Landar A and Song
W: The S100 protein family: History, function, and expression.
Brain Res Bull. 37:417–429. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalez LL, Garrie K and Turner MD: Role
of S100 proteins in health and disease. Biochim Biophys Acta Mol
Cell Res. 1867:1186772020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marenholz I, Heizmann CW and Fritz G: S100
proteins in mouse and man: From evolution to function and pathology
(including an update of the nomenclature). Biochem Biophys Res
Commun. 322:1111–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donato R: Functional roles of S100
proteins, calcium-binding proteins of the EF-hand type. Biochim
Biophys Acta. 1450:191–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Delangre E, Oppliger E, Berkcan S,
Gjorgjieva M, Correia de Sousa M and Foti M: S100 proteins in fatty
liver disease and hepatocellular carcinoma. Int J Mol Sci.
23:110302022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You X, Li M, Cai H, Zhang W, Hong Y, Gao
W, Liu Y, Liang X, Wu T, Chen F and Su D: Calcium binding protein
S100A16 expedites proliferation, invasion and
epithelial-mesenchymal transition process in gastric cancer. Front
Cell Dev Biol. 9:7369292021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohuchida K, Mizumoto K, Miyasaka Y, Yu J,
Cui L, Yamaguchi H, Toma H, Takahata S, Sato N, Nagai E, et al:
Over-expression of S100A2 in pancreatic cancer correlates with
progression and poor prognosis. J Pathol. 213:275–282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng ML, Zhu XJ, Liu J, Shi PC, Kang YL,
Lin Z and Cao YP: An integrated bioinformatic analysis of the S100
gene family for the prognosis of colorectal cancer. Biomed Res Int.
2020:47469292020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heizmann CW: Intracellular calcium-binding
proteins: Structure and possible functions. J Cardiovasc Pharmacol.
8 (Suppl 8):S7–S12. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schäfer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: Functions and
pathology. Trends Biochem Sci. 21:134–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wright NT, Cannon BR, Wilder PT, Morgan
MT, Varney KM, Zimmer DB and Weber DJ: Solution structure of S100A1
bound to the CapZ peptide (TRTK12). J Mol Biol. 386:1265–1277.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malashkevich VN, Varney KM, Garrett SC,
Wilder PT, Knight D, Charpentier TH, Ramagopal UA, Almo SC, Weber
DJ and Bresnick AR: Structure of Ca2+-bound S100A4 and its
interaction with peptides derived from nonmuscle myosin-IIA.
Biochemistry. 47:5111–5126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia C, Braunstein Z, Toomey AC, Zhong J
and Rao X: S100 proteins as an important regulator of macrophage
inflammation. Front Immunol. 8:19082018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilston BA, Skaar EP and Chazin WJ:
Binding of transition metals to S100 proteins. Sci China Life Sci.
59:792–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuberappa PH, Bagalad BS, Ananthaneni A,
Kiresur MA and Srinivas GV: Certainty of S100 from physiology to
pathology. J Clin Diagn Res. 10:ZE10–ZE15. 2016.PubMed/NCBI
|
|
19
|
Wang S, Song R, Wang Z, Jing Z, Wang S and
Ma J: S100A8/A9 in inflammation. Front Immunol. 9:12982018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelfattah N, Kumar P, Wang C, Leu JS,
Flynn WF, Gao R, Baskin DS, Pichumani K, Ijare OB, Wood SL, et al:
Single-cell analysis of human glioma and immune cells identifies
S100A4 as an immunotherapy target. Nat Commun. 13:7672022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng G, Tsukamoto S, Okumura K, Ogawa H,
Ikeda S and Niyonsaba F: A pancancer analysis of the oncogenic role
of S100 calcium binding protein A7 (S100A7) in human tumors.
Biology (Basel). 11:2842022.PubMed/NCBI
|
|
23
|
Chen B, Zheng D, Liu C, Bhandari A,
Hirachan S, Shen C, Mainali S, Li H, Jiang W, Xu J, et al: S100A6
promotes the development of thyroid cancer and inhibits apoptosis
of thyroid cancer cells through the PI3K/AKT/mTOR pathway. Pathol
Res Pract. 242:1543252023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christensen MV, Høgdall CK, Jochumsen KM
and Høgdall EVS: Annexin A2 and cancer: A systematic review. Int J
Oncol. 52:5–18. 2018.PubMed/NCBI
|
|
25
|
Basnet S, Sharma S, Costea DE and Sapkota
D: Expression profile and functional role of S100A14 in human
cancer. Oncotarget. 10:2996–3012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo J, Bian Y, Wang Y, Chen L, Yu A and
Sun X: FAM107B is regulated by S100A4 and mediates the effect of
S100A4 on the proliferation and migration of MGC803 gastric cancer
cells. Cell Biol Int. 41:1103–1109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu A, Wang Y, Bian Y, Chen L, Guo J, Shen
W, Chen D, Liu S and Sun X: IL-1β promotes the nuclear
translocaiton of S100A4 protein in gastric cancer cells MGC803 and
the cell's stem-like properties through PI3K pathway. J Cell
Biochem. 119:8163–8173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bian Y, Guo J, Qiao L and Sun X:
miR-3189-3p mimics enhance the effects of S100A4 siRNA on the
inhibition of proliferation and migration of gastric cancer cells
by targeting CFL2. Int J Mol Sci. 19:2362018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan B, Zhang LH, Jia YN, Zhong XY, Liu YQ,
Cheng XJ, Wang XH, Xing XF, Hu Y, Li YA, et al: Presence of
S100A9-positive inflammatory cells in cancer tissues correlates
with an early stage cancer and a better prognosis in patients with
gastric cancer. BMC Cancer. 12:3162012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Z, Zhang C and Zhao Q: S100A9 as a
novel diagnostic and prognostic biomarker in human gastric cancer.
Scand J Gastroenterol. 55:338–346. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghavami S, Chitayat S, Hashemi M, Eshraghi
M, Chazin WJ, Halayko AJ and Kerkhoff C: S100A8/A9: A Janus-faced
molecule in cancer therapy and tumorgenesis. Eur J Pharmacol.
625:73–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shabani F, Farasat A, Mahdavi M and Gheibi
N: Calprotectin (S100A8/S100A9): A key protein between inflammation
and cancer. Inflamm Res. 67:801–812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghavami S, Rashedi I, Dattilo BM, Eshraghi
M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C and Los M:
S100A8/A9 at low concentration promotes tumor cell growth via RAGE
ligation and MAP kinase-dependent pathway. J Leukoc Biol.
83:1484–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shabani F, Mahdavi M, Imani M,
Hosseinpour-Feizi MA and Gheibi N: Calprotectin
(S100A8/S100A9)-induced cytotoxicity and apoptosis in human gastric
cancer AGS cells: Alteration in expression levels of Bax, Bcl-2,
and ERK2. Hum Exp Toxicol. 39:1031–1045. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Luo J, Rong J, He S, Zhang L and
Zheng F: Distinct prognostic roles of S100 mRNA expression in
gastric cancer. Pathol Res Pract. 215:127–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon CH, Moon HJ, Park HJ, Choi JH and
Park DY: S100A8 and S100A9 promotes invasion and migration through
p38 mitogen-activated protein kinase-dependent NF-κB activation in
gastric cancer cells. Mol Cells. 35:226–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Swann JB, Vesely MD, Silva A, Sharkey J,
Akira S, Schreiber RD and Smyth MJ: Demonstration of
inflammation-induced cancer and cancer immunoediting during primary
tumorigenesis. Proc Natl Acad Sci USA. 105:652–656. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katono K, Sato Y, Jiang SX, Kobayashi M,
Saito K, Nagashio R, Ryuge S, Satoh Y, Saegusa M and Masuda N:
Clinicopathological significance of S100A10 expression in lung
adenocarcinomas. Asian Pac J Cancer Prev. 17:289–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao JT, Chi BJ, Sun Y, Chi NN, Zhang XM,
Sun JB, Chen Y and Xia Y: LINC00174 is an oncogenic lncRNA of
hepatocellular carcinoma and regulates miR-320/S100A10 axis. Cell
Biochem Funct. 38:859–869. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Li XY, Li LX, Zhou RC, Sikong Y, Gu
X, Jin BY, Li B, Li YQ and Zuo XL: S100A10 accelerates aerobic
glycolysis and malignant growth by activating mTOR-signaling
pathway in gastric cancer. Front Cell Dev Biol. 8:5594862020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang C, Zhang C, Li X, Shen J, Xu Y, Shi
H, Mu X, Pan J, Zhao T, Li M, et al: CPT1A-mediated succinylation
of S100A10 increases human gastric cancer invasion. J Cell Mol Med.
23:293–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koh SA and Lee KH: HGF-mediated S100A11
overexpression enhances proliferation and invasion of gastric
cancer. Am J Transl Res. 10:3385–3394. 2018.PubMed/NCBI
|
|
43
|
Cui Y, Li L, Li Z, Yin J, Lane J, Ji J and
Jiang WG: Dual effects of targeting S100A11 on suppressing cellular
metastatic properties and sensitizing drug response in gastric
cancer. Cancer Cell Int. 21:2432021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang Y, Yu X, Zhao Y, Huang J, Li T, Chen
H, Zhou J, Huang Z and Yang Z: ADAMTS19 suppresses cell migration
and invasion by targeting S100A16 via the NF-κB pathway in human
gastric cancer. Biomolecules. 11:5612021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv H, Hou H, Lei H, Nie C, Chen B, Bie L,
Han L and Chen X: MicroRNA-6884-5p regulates the proliferation,
invasion, and EMT of gastric cancer cells by directly targeting
S100A16. Oncol Res. 28:225–236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu
W, Li R, Qie J, Zhen B, Wang Y, et al: A proteomics landscape of
circadian clock in mouse liver. Nat Commun. 9:15532018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kang JH, Toita R and Murata M: Liver
cell-targeted delivery of therapeutic molecules. Crit Rev
Biotechnol. 36:132–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N
and Zhao Y: Recent progress in treatment of hepatocellular
carcinoma. Am J Cancer Res. 10:2993–3036. 2020.PubMed/NCBI
|
|
49
|
Liu Z, Liu H, Pan H, Du Q and Liang J:
Clinicopathological significance of S100A4 expression in human
hepatocellular carcinoma. J Int Med Res. 41:457–462. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang J, Zhang DL, Jiao XL and Dong Q:
S100A4 regulates migration and invasion in hepatocellular carcinoma
HepG2 cells via NF-κB-dependent MMP-9 signal. Eur Rev Med Pharmacol
Sci. 17:2372–2382. 2013.PubMed/NCBI
|
|
52
|
Grotterød I, Maelandsmo GM and Boye K:
Signal transduction mechanisms involved in S100A4-induced
activation of the transcription factor NF-kappaB. BMC Cancer.
10:2412010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo Q, Wang J, Cao Z, Tang Y, Feng C and
Huang F: Interaction of S100A1 with LATS1 promotes cell growth
through regulation of the Hippo pathway in hepatocellular
carcinoma. Int J Oncol. 53:592–602. 2018.PubMed/NCBI
|
|
54
|
Zhu K, Huang W, Wang W, Liao L, Li S, Yang
S, Xu J, Li L, Meng M, Xie Y, et al: Up-regulation of S100A4
expression by HBx protein promotes proliferation of hepatocellular
carcinoma cells and its correlation with clinical survival. Gene.
749:1446792020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui JF, Liu YK, Pan BS, Song HY, Zhang Y,
Sun RX, Chen J, Feng JT, Tang ZY, Yu YL, et al: Differential
proteomic analysis of human hepatocellular carcinoma cell line
metastasis-associated proteins. J Cancer Res Clin Oncol.
130:615–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schmidt-Hansen B, Ornås D, Grigorian M,
Klingelhöfer J, Tulchinsky E, Lukanidin E and Ambartsumian N:
Extracellular S100A4(mts1) stimulates invasive growth of mouse
endothelial cells and modulates MMP-13 matrix metalloproteinase
activity. Oncogene. 23:5487–5495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN,
Fu CJ, Chen HX, Yuan HF, Li ZW, Shi L, et al: Hepatocellular
carcinoma-associated mesenchymal stem cells promote hepatocarcinoma
progression: Role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology.
57:2274–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun H, Wang C, Hu B, Gao X, Zou T, Luo Q,
Chen M, Fu Y, Sheng Y, Zhang K, et al: Exosomal S100A4 derived from
highly metastatic hepatocellular carcinoma cells promotes
metastasis by activating STAT3. Signal Transduct Target Ther.
6:1872021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hua Z, Chen J, Sun B, Zhao G, Zhang Y,
Fong Y, Jia Z and Yao L: Specific expression of osteopontin and
S100A6 in hepatocellular carcinoma. Surgery. 149:783–791. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Arai K, Yamada T and Nozawa R:
Immunohistochemical investigation of migration inhibitory
factor-related protein (MRP)-14 expression in hepatocellular
carcinoma. Med Oncol. 17:183–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kittaka N, Takemasa I, Takeda Y, Marubashi
S, Nagano H, Umeshita K, Dono K, Matsubara K, Matsuura N and Monden
M: Molecular mapping of human hepatocellular carcinoma provides
deeper biological insight from genomic data. Eur J Cancer.
44:885–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sobolewski C, Abegg D, Berthou F, Dolicka
D, Calo N, Sempoux C, Fournier M, Maeder C, Ay AS, Clavien PA, et
al: S100A11/ANXA2 belongs to a tumour suppressor/oncogene network
deregulated early with steatosis and involved in inflammation and
hepatocellular carcinoma development. Gut. 69:1841–1854. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang C, Yao R, Chen J, Zou Q and Zeng L:
S100 family members: Potential therapeutic target in patients with
hepatocellular carcinoma: A STROBE study. Medicine (Baltimore).
100:e241352021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song D, Xu B, Shi D, Li S and Cai Y:
S100A6 promotes proliferation and migration of HepG2 cells via
increased ubiquitin-dependent degradation of p53. Open Med (Wars).
15:317–326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Németh J, Stein I, Haag D, Riehl A,
Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn
M, et al: S100A8 and S100A9 are novel nuclear factor kappa B target
genes during malignant progression of murine and human liver
carcinogenesis. Hepatology. 50:1251–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liao J, Li JZ, Xu J, Xu Y, Wen WP, Zheng L
and Li L: High S100A9+ cell density predicts a poor
prognosis in hepatocellular carcinoma patients after curative
resection. Aging (Albany NY). 13:16367–16380. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duan L, Wu R, Zhang X, Wang D, You Y,
Zhang Y, Zhou L and Chen W: HBx-induced S100A9 in NF-κB dependent
manner promotes growth and metastasis of hepatocellular carcinoma
cells. Cell Death Dis. 9:6292018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luo X, Xie H, Long X, Zhou M, Xu Z, Shi B,
Jiang H and Li Z: EGFRvIII mediates hepatocellular carcinoma cell
invasion by promoting S100 calcium binding protein A11 expression.
PLoS One. 8:e833322013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zheng M, Meng H, Li Y, Shi J, Han Y, Zhao
C, Chen J, Han J, Liang J, Chen Y, et al: S100A11 promotes
metastasis via AKT and ERK signaling pathways and has a diagnostic
role in hepatocellular carcinoma. Int J Med Sci. 20:318–328. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bachet JB, Maréchal R, Demetter P,
Bonnetain F, Cros J, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet
A, Louvet C, et al: S100A2 is a predictive biomarker of adjuvant
therapy benefit in pancreatic adenocarcinoma. Eur J Cancer.
49:2643–2653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Y, Wang C, Song J, Xu R, Ruze R and
Zhao Y: S100A2 is a prognostic biomarker involved in immune
infiltration and predict immunotherapy response in pancreatic
cancer. Front Immunol. 12:7580042021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Che P, Yang Y, Han X, Hu M, Sellers JC,
Londono-Joshi AI, Cai GQ, Buchsbaum DJ, Christein JD, Tang Q, et
al: S100A4 promotes pancreatic cancer progression through a dual
signaling pathway mediated by Src and focal adhesion kinase. Sci
Rep. 5:84532015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tsukamoto N, Egawa S, Akada M, Abe K,
Saiki Y, Kaneko N, Yokoyama S, Shima K, Yamamura A, Motoi F, et al:
The expression of S100A4 in human pancreatic cancer is associated
with invasion. Pancreas. 42:1027–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jia F, Liu M, Li X, Zhang F, Yue S and Liu
J: Relationship between S100A4 protein expression and pre-operative
serum CA19.9 levels in pancreatic carcinoma and its prognostic
significance. World J Surg Oncol. 17:1632019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Woo T, Okudela K, Mitsui H, Tajiri M, Rino
Y, Ohashi K and Masuda M: Up-regulation of S100A11 in lung
adenocarcinoma-its potential relationship with cancer progression.
PLoS One. 10:e01426422015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Anania MC, Miranda C, Vizioli MG, Mazzoni
M, Cleris L, Pagliardini S, Manenti G, Borrello MG, Pierotti MA and
Greco A: S100A11 overexpression contributes to the malignant
phenotype of papillary thyroid carcinoma. J Clin Endocrinol Metab.
98:E1591–E1600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xiao MB, Jiang F, Ni WK, Chen BY, Lu CH,
Li XY and Ni RZ: High expression of S100A11 in pancreatic
adenocarcinoma is an unfavorable prognostic marker. Med Oncol.
29:1886–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xiao M, Li T, Ji Y, Jiang F, Ni W, Zhu J,
Bao B, Lu C and Ni R: S100A11 promotes human pancreatic cancer
PANC-1 cell proliferation and is involved in the PI3K/AKT signaling
pathway. Oncol Lett. 15:175–182. 2018.PubMed/NCBI
|
|
80
|
Ji YF, Li T, Jiang F, Ni WK, Guan CQ, Liu
ZX, Lu CH, Ni RZ, Wu W and Xiao MB: Correlation between S100A11 and
the TGF-β1/SMAD4 pathway and its effects on the
proliferation and apoptosis of pancreatic cancer cell line PANC-1.
Mol Cell Biochem. 450:53–64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Takamatsu H, Yamamoto KI, Tomonobu N,
Murata H, Inoue Y, Yamauchi A, Sumardika IW, Chen Y, Kinoshita R,
Yamamura M, et al: Extracellular S100A11 plays a critical role in
spread of the fibroblast population in pancreatic cancers. Oncol
Res. 27:713–727. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pietas A, Schlüns K, Marenholz I, Schäfer
BW, Heizmann CW and Petersen I: Molecular cloning and
characterization of the human S100A14 gene encoding a novel member
of the S100 family. Genomics. 79:513–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu H, Gao W, Li X, Yu L, Luo D, Liu Y and
Yu X: S100A14 promotes progression and gemcitabine resistance in
pancreatic cancer. Pancreatology. 21:589–598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhuang H, Chen X, Dong F, Zhang Z, Zhou Z,
Ma Z, Huang S, Chen B, Zhang C and Hou B: Prognostic values and
immune suppression of the S100A family in pancreatic cancer. J Cell
Mol Med. 25:3006–3018. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang C, Chen Y, Xinpeng Y, Xu R, Song J,
Ruze R, Xu Q and Zhao Y: Construction of immune-related signature
and identification of S100A14 determining immune-suppressive
microenvironment in pancreatic cancer. BMC Cancer. 22:8792022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fang D, Zhang C, Xu P, Liu Y, Mo X, Sun Q,
Abdelatty A, Hu C, Xu H, Zhou G, et al: S100A16 promotes metastasis
and progression of pancreatic cancer through FGF19-mediated AKT and
ERK1/2 pathways. Cell Biol Toxicol. 37:555–571. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen T, Xia DM, Qian C and Liu SR:
Integrated analysis identifies S100A16 as a potential prognostic
marker for pancreatic cancer. Am J Transl Res. 13:5720–5730.
2021.PubMed/NCBI
|
|
88
|
Li T, Ren T, Huang C, Li Y, Yang P, Che G,
Luo L, Chen Y, Peng S, Lin Y and Zeng L: S100A16 induces
epithelial-mesenchymal transition in human PDAC cells and is a new
therapeutic target for pancreatic cancer treatment that synergizes
with gemcitabine. Biochem Pharmacol. 189:1143962021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Borroni EM, Savino B, Bonecchi R and
Locati M: Chemokines sound the alarmin: The role of atypical
chemokine in inflammation and cancer. Semin Immunol. 38:63–71.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tu G, Gao W, Li Y, Dian Y, Xue B, Niu L,
Yu X and Zhu H: Expressional and prognostic value of S100A16 in
pancreatic cancer via integrated bioinformatics analyses. Front
Cell Dev Biol. 9:6456412021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh
Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M and Saad A:
Colorectal cancer epidemiology: Recent trends and impact on
outcomes. Curr Drug Targets. 22:998–1009. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fukuda Y, Tanaka Y, Eto K, Ukai N, Sonobe
S, Takahashi H, Ikegami M and Shimoda M: S100-stained perineural
invasion is associated with worse prognosis in stage I/II
colorectal cancer: Its possible association with immunosuppression
in the tumor. Pathol Int. 72:117–127. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kaya T and Dursun A: Can lymphovascular
and perineural invasion be additional staging criteria in
colorectal cancer? J Coll Physicians Surg Pak. 31:657–662. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Alotaibi AM, Lee JL, Kim J, Lim SB, Yu CS,
Kim TW, Kim JH and Kim JC: Prognostic and oncologic significance of
perineural invasion in sporadic colorectal cancer. Ann Surg Oncol.
24:1626–1634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hatthakarnkul P, Ammar A, Pennel KAF,
Officer-Jones L, Cusumano S, Quinn JA, Matly AAM, Alexander PG, Hay
J, Andersen D, et al: Protein expression of S100A2 reveals it
association with patient prognosis and immune infiltration profile
in colorectal cancer. J Cancer. 14:1837–1847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang Y, Zeng Z, Li L, Lei S, Wu Y, Chen T
and Zhang J: Sinapine thiocyanate exhibited anti-colorectal cancer
effects by inhibiting KRT6A/S100A2 axis. Cancer Biol Ther.
24:22491702023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Schwartz L, Seyfried T, Alfarouk KO, Da
Veiga Moreira J and Fais S: Out of Warburg effect: An effective
cancer treatment targeting the tumor specific metabolism and
dysregulated pH. Semin Cancer Biol. 43:134–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li C, Chen Q, Zhou Y, Niu Y, Wang X, Li X,
Zheng H, Wei T, Zhao L and Gao H: S100A2 promotes glycolysis and
proliferation via GLUT1 regulation in colorectal cancer. FASEB J.
34:13333–13344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Destek S and Gul VO: S100A4 may be a good
prognostic marker and a therapeutic target for colon cancer. J
Oncol. 2018:18287912018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dahlmann M, Kobelt D, Walther W, Mudduluru
G and Stein U: S100A4 in cancer metastasis: Wnt signaling-driven
interventions for metastasis restriction. Cancers (Basel).
8:592016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: A small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hsieh YY, Cheng YW, Wei PL and Yang PM:
Repurposing of ingenol mebutate for treating human colorectal
cancer by targeting S100 calcium-binding protein A4 (S100A4).
Toxicol Appl Pharmacol. 449:1161342022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ghoul A, Serova M, Astorgues-Xerri L,
Bieche I, Bousquet G, Varna M, Vidaud M, Phillips E, Weill S,
Benhadji KA, et al: Epithelial-to-mesenchymal transition and
resistance to ingenol 3-angelate, a novel protein kinase C
modulator, in colon cancer cells. Cancer Res. 69:4260–4269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sack U, Walther W, Scudiero D, Selby M,
Aumann J, Lemos C, Fichtner I, Schlag PM, Shoemaker RH and Stein U:
S100A4-induced cell motility and metastasis is restricted by the
Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells.
Mol Biol Cell. 22:3344–3354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hernández-Maqueda JG, Luna-Ulloa LB,
Santoyo-Ramos P, Castañeda-Patlán MC and Robles-Flores M: Protein
kinase C delta negatively modulates canonical Wnt pathway and cell
proliferation in colon tumor cell lines. PLoS One. 8:e585402013.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Schöpe PC, Zinnow V, Ishfaq MA, Smith J,
Herrmann P, Shoemaker RH, Walther W and Stein U: Cantharidin and
its analogue norcantharidin inhibit metastasis-inducing genes
S100A4 and MACC1. Int J Mol Sci. 24:11792023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Srikrishna G: S100A8 and S100A9: New
insights into their roles in malignancy. J Innate Immun. 4:31–40.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li S, Xu F, Li H, Zhang J, Zhong A, Huang
B and Lai M: S100A8+ stroma cells predict a good
prognosis and inhibit aggressiveness in colorectal carcinoma.
Oncoimmunology. 6:e12602132016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li S, Zhang J, Qian S, Wu X, Sun L, Ling
T, Jin Y, Li W, Sun L, Lai M and Xu F: S100A8 promotes
epithelial-mesenchymal transition and metastasis under TGF-β/USF2
axis in colorectal cancer. Cancer Commun (Lond). 41:154–170. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hashida H and Coffey RJ: Significance of a
calcium-binding protein S100A14 expression in colon cancer
progression. J Gastrointest Oncol. 13:149–162. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sun X, Wang T, Zhang C, Ning K, Guan ZR,
Chen SX, Hong TT and Hua D: S100A16 is a prognostic marker for
colorectal cancer. J Surg Oncol. 117:275–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ou S, Liao Y, Shi J, Tang J, Ye Y, Wu F,
Wang W, Fei J, Xie F and Bai L: S100A16 suppresses the
proliferation, migration and invasion of colorectal cancer cells in
part via the JNK/p38 MAPK pathway. Mol Med Rep. 23:1642021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sood A, Mishra D, Kharbanda OP, Chauhan
SS, Gupta SD, Deo SSV, Yadav R, Ralhan R, Kumawat R and Kaur H:
Role of S100 A7 as a diagnostic biomarker in oral potentially
malignant disorders and oral cancer. J Oral Maxillofac Pathol.
26:166–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tanigawa K, Tsukamoto S, Koma YI, Kitamura
Y, Urakami S, Shimizu M, Fujikawa M, Kodama T, Nishio M, Shigeoka
M, et al: S100A8/A9 induced by interaction with macrophages in
esophageal squamous cell carcinoma promotes the migration and
invasion of cancer cells via Akt and p38 MAPK pathways. Am J
Pathol. 192:536–552. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhong C, Niu Y, Liu W, Yuan Y, Li K, Shi
Y, Qiu Z, Li K, Lin Z, Huang Z, et al: S100A9 derived from
chemoembolization-induced hypoxia governs mitochondrial function in
hepatocellular carcinoma progression. Adv Sci (Weinh).
9:e22022062022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Low RRJ, Fung KY, Gao H, Preaudet A,
Dagley LF, Yousef J, Lee B, Emery-Corbin SJ, Nguyen PM, Larsen RH,
et al: S100 family proteins are linked to organoid morphology and
EMT in pancreatic cancer. Cell Death Differ. 30:1155–1165. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dong JX, Zhang LF, Liu DB, Li ZG, Gao F,
Wang LP and Dong JH: Circular ribonucleic acid circ-FADS2 promotes
colorectal cancer cell proliferation and invasion by regulating
miR-498/S100A16. J Physiol Pharmacol. 73:2022.
|
|
119
|
Treese C, Hartl K, Pötzsch M, Dahlmann M,
von Winterfeld M, Berg E, Hummel M, Timm L, Rau B, Walther W, et
al: S100A4 is a strong negative prognostic marker and potential
therapeutic target in adenocarcinoma of the stomach and esophagus.
Cells. 11:10562022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Liao WC, Chen CT, Tsai YS, Wang XY, Chang
YT, Wu MS and Chow LP: S100A8, S100A9 and S100A8/A9 heterodimer as
novel cachexigenic factors for pancreatic cancer-induced cachexia.
BMC Cancer. 23:5132023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zeng X, Guo H, Liu Z, Qin Z, Cong Y, Ren
N, Zhang Y and Zhang N: S100A11 activates the pentose phosphate
pathway to induce malignant biological behaviour of pancreatic
ductal adenocarcinoma. Cell Death Dis. 13:5682022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou X, Shi M, Cao J, Yuan T, Yu G, Chen
Y, Fang W and Li H: S100 calcium binding protein A10, a novel
oncogene, promotes the proliferation, invasion, and migration of
hepatocellular carcinoma. Front Genet. 12:6950362021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang X, Huang H, Sze KM, Wang J, Tian L,
Lu J, Tsui YM, Ma HT, Lee E, Chen A, et al: S100A10 promotes HCC
development and progression via transfer in extracellular vesicles
and regulating their protein cargos. Gut. 72:1370–1384. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sun Q, Fu C, Liu J, Li S and Zheng J:
Knockdown of LPCAT1 repressed hepatocellular carcinoma growth and
invasion by targeting S100A11. Ann Clin Lab Sci. 53:212–221.
2023.PubMed/NCBI
|
|
125
|
Lin H, Yang P, Li B, Chang Y, Chen Y, Li
Y, Liu K, Liang X, Chen T, Dai Y, et al: S100A10 promotes
pancreatic ductal adenocarcinoma cells proliferation, migration and
adhesion through JNK/LAMB3-LAMC2 axis. Cancers (Basel). 15:2022022.
View Article : Google Scholar : PubMed/NCBI
|