Introduction
Ferroptosis, first defined by Dixon et al
(1) in 2012, is a relatively
novel type of programmed cell death distinguished from apoptosis,
necroptosis and pyroptosis by the presence of smaller mitochondria
and a reduced number of mitochondrial cristae. Ferroptosis was
initially explored in mammalian systems and is now known as one of
the most prevalent forms of cell death (2). Ferroptosis is driven by lethal lipid
peroxidation resulting from unbalanced redox homeostasis and
cellular metabolism, which is inhibited by antioxidant systems,
mainly including the glutathione peroxidase 4 (GPX4)-glutathione
(3), ferroptosis suppressor
protein 1 (FSP1)-CoQH2 (4,5),
GTP cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (6) and dihydroorotate dehydrogenase
(DHODH)-CoQH2 systems (7).
RAS-selective lethal 3 (RSL3) and Erastin are two small-molecule
RSL compounds that induce ferroptosis, which is inhibited by
ferrostatin-1 (Fer-1). RSL3 directly inactivates GPX4, inhibiting
its ability to convert lipid hydroperoxides to lipid alcohols and
triggering ferroptosis, whereas Erastin suppresses system
Xc-, decreasing cystine uptake and enhancing the levels
of reactive oxygen species (ROS) (1,8,9).
Fer-1 is thought to have antioxidant abilities as it scavenges
alkoxyl radicals and other rearrangement products generated by
ferrous iron from lipid hydroperoxides (10). Dixon et al (1) found that Fer-1 specifically
inhibited ferroptosis induced by RSL but not the other known forms
of cell death. Various signalling pathways, such as the p53 and
PI3K/AKT/mTOR signalling pathways, linked to cancer regulate
ferroptosis in cancer cells, and accumulating evidence suggests
that ferroptosis has an essential role in cancer progression
(11,12).
Long non-coding RNAs (lncRNAs), defined as
non-coding transcripts with sequences >200 nucleotides, are
involved in gene expression regulation in diverse biological
processes, including as RNA decoys (13), microRNA (miRNA) sponges (14) and scaffolds (15). lncRNAs have important roles as
tumour suppressors or oncogenes, influencing the emergence of
cancer (16). Recently, RNA
sequencing (RNA-Seq) and bioinformatics analyses have demonstrated
the involvement of certain lncRNAs in ferroptosis. For instance,
H19 has an important role in ferroptosis caused by curcumenol by
regulating miR-19b-3p-mediated ferritin heavy chain 1 (FTH1)
downregulation (17). Moreover,
Qin et al (18)
constructed a 5-lncRNA (AC055720.2, LINC00900, DPP4-DT, LINC02454
and AC012038.2) signature related to ferroptosis to predict thyroid
cancer prognosis and immune responses. Nevertheless, the function
and regulatory mechanisms of lncRNAs associated with ferroptosis in
cancer have not yet been fully investigated.
Lung cancer-associated transcript 1 (LUCAT1) was
first identified in the airways of patients with a history of
smoking; hence, it is sometimes referred to as smoke and
cancer-associated lncRNA-1 (19).
LUCAT1 expression is essential for tumour-node-metastasis staging
and overall survival predictions for several malignancies,
including liver cancer (20),
gastric cancer (21), colorectal
cancer (22,23) and renal cell carcinoma (24). Previous bioinformatics data have
indicated that LUCAT1 is a ferroptosis-related lncRNA that can be
used in prognostic or diagnostic signatures in renal cell carcinoma
(25,26) and glioma (27). However, to the best of our
knowledge, the role of LUCAT1 in lung cancer ferroptosis and its
underlying mechanisms remain unknown.
Therefore, the aim of the present study was to
identify ferroptosis-related lncRNAs and investigate their roles
and regulatory mechanisms in lung cancer. Lung cancer cells were
treated with the ferroptosis inducer, RSL3, and inhibitor, Fer-1,
and ferroptosis-associated lncRNAs were identified by RNA-Seq.
Investigating the functions of these lncRNAs in ferroptosis offers
a novel theoretical foundation for therapeutic strategies in lung
cancer.
Materials and methods
Cell culture
The human normal lung epithelial cell line, BEAS-2B
(cat. no. HTX2075), was obtained from Otwo Biotech (Shenzhen
Haodihuatuo Biotechnology Co., Ltd.). The lung cancer cell line,
A549 (cat. no. CCL-185), and the 293T cell line (cat. no.
CRL-11268) were obtained from the American Type Culture Collection.
The lung cancer cell line, H460 (cat. no. CL-0299), was obtained
from Procell Life Science & Technology Co., Ltd. All cell lines
were authenticated by their suppliers through STR profiling. The
A549, BEAS-2B, and 293T cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with 10% foetal bovine serum (Gemini Bio
Products), 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C with 5% CO2. H460 cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) with the same
supplements and conditions.
RNA-Seq
A549 cells were treated in triplicate with 0.7%
dimethyl sulfoxide (DMSO; solvent used to dissolve RSL3 and Fer-1),
2 μM RSL3 (28) (cat. no.
HY-100218A; MedChemExpress), or 2 μM RSL3 and 5 μM
Fer-1 (29) (cat. no. S7243;
Selleck Chemicals) for 24 h. Total RNA was extracted using TRIzol
reagent (cat. no. T9424; Sigma-Aldrich; Merck KGaA), and the
quantity and purity of the extracted RNA were analysed using an RNA
6000 Nano LabChip Kit (cat. no. 5067-1511; Agilent Technologies,
Inc.) by Bioanalyzer 2100 (Agilent Technologies, Inc.). The 2x 150
bp paired-end sequencing was performed using an Illumina Novaseq™
6000 at LC-Bio Technology (Hangzhou) Co., Ltd.
Bioinformatics analysis
Using genes from the aforementioned 9 samples with a
mean fragments per kilobase of transcript per million mapped reads
value >1, differentially expressed genes (DEGs) between the
treatment and control samples were identified using DEseq2 software
(version 1.40.2; https://github.com/mikelove/DESeq2), based on fold
change >1.5 and P<0.05 as the cut-off. Biological Process
(BP) of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analyses of the DEGs were performed using
Metascape (https://metascape.org/gp) (30). Heatmaps, volcano plots and Venn
diagrams were created using OmicStudio (https://www.omicstudio.cn). Protein-protein
interaction (PPI) networks were examined using STRING (https://www.string-db.org/). The co-expression
analysis of mRNA-lncRNA was performed using the online resource,
weishengxin (https://www.bioinformatics.com.cn). GO analysis of
LUCAT1 and the prediction of target genes of DElncRNAs was
performed using LncACTdb 3.0 (http://bio-bigdata.hrbmu.edu.cn/LncACTdb) (31). Expression specificity analysis of
mRNA and lncRNA was conducted using index τ, following the method
described by Li et al (32). The location of LUCAT1 was
predicted using lncLocator (http://www.csbio.sjtu.edu.cn/bioinf/lncLocator2/)
(33). LUCAT1 data were obtained
from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) [accession nos.
GSE104462 (34) and GSE158458
(35)] and were associated with
liver cancer lines (including HepG2, Hep3B and Huh7). Potential
miRNAs targeting LUCAT1 or the 3'-untranslated region (3'-UTR) of
GCH1 were predicted using DIANA-LncBase v3 (https://diana.e-ce.uth.gr/lncbasev3/interactions)
(36) or TargetScan (http://www.targetscan.org). miR-34a-5p and LUCAT1 or
GCH1 binding sites were predicted using miRDB (http://www.mirdb.org) (37) or TargetScan. Pan-cancer data were
downloaded from the UCSC Xena Pan-Cancer Atlas Hub (https://xenabrowser.net/datapages/), and the
expression of lncRNAs were analysed using R software (version
4.2.2; https://cran.r-project.org/src/base/R-4/).
Plasmid, stable cell line generation,
small interfering RNA (siRNA), miRNA mimics and miRNA inhibitors
transfections
The LUCAT1-expression vector was constructed using
the PB-CMV-MCS-EF1α-RedPuro vector (cat. no. PB514B-2; System
Biosciences, LLC) by YouBio. A549 cells in 6-well plates were
co-transfected with 0.5 μg LUCAT1-expression vector (LUCAT1)
or its control (Vector) and 0.2 μg Super PiggyBac
Transposase Expression Vector (cat. no. PB210PA-1; System
Biosciences, LLC) using the jetPRIME transfection reagent (cat. no.
101000046; Polyplus-transfection SA) at room temperature, and cells
that stably expressed LUCAT1 were selected using 2 μg/ml
puromycin (cat. no. A1113803; Gibco; Thermo Fisher Scientific,
Inc.) and subsequently maintained with 1 μg/ml puromycin.
LUCAT1 siRNA (siLUCAT1) and its control (siNC), mimics of
miR-34a-5p (miR-34a-5p) and its control (miR-NC), and inhibitors of
miR-34a-5p (anti-miR-34a-5p) and its control (anti-NC), synthesised
by Genepharm, Inc., were transfected into H460 cells at a final
concentration of 50 nM under the same conditions as aforementioned.
The sequences used in these experiments are shown in Table SI. The gene RNA expression level
was detected 48 h after transfection, and the protein expression
level was detected 72 h after transfection.
Cell viability assay
The viability of cells was assessed using Cell
Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular
Technologies, Inc.). To measure cell proliferation, cells were
seeded at a density of 1×103 cells/well into 96-well
plates and cultured for 0, 24, 48 and 72 h. To measure
cytotoxicity, cells were seeded at a density of 5×103
cells/well into 96-well plates, cultured overnight and treated with
RSL3 and Erastin (A549 cells were treated with 2 μM RSL3 or
20 μM Erastin and H460 cells were treated with 8 μM
RSL3 or 20 μM Erastin) for 24 h. After adding the mixture
(CCK-8 reagent: medium, 1:10) into the wells and incubating at 37°C
for 1 h, a microplate reader (LabSystems Multiskan MS; Thermo
Fisher Scientific, Inc.) was used to measure the absorbance at 450
nm.
Colony formation assay
Cells were seeded at a density of 1×103
cells/well into 6-well plates, cultured overnight and treated with
RSL3 or Erastin (A549 cells were treated with 0.1 μM RSL3 or
4 μM Erastin and H460 cells were treated with 2 μM
RSL3 or 4 μM Erastin) for 10 days. After 20 min of methanol
fixation at room temperature, 0.2% crystal violet was used to stain
the cells for 5 min at room temperature. ImageJ software (version
1.53a; National Institutes of Health) was used to count colonies
with >50 cells, and analysis was performed using GraphPad Prism
(version 9.0; Dotmatics).
Lipid ROS assay
Lipid ROS was detected using a BODIPY 581/591 C11
Kit (cat. no. D3861; Invitrogen; Thermo Fisher Scientific, Inc.).
Cells were seeded at a density of 1.5×105 cells/well
into 6-well plates, cultured overnight and treated with RSL3 or
Erastin (A549 cells were treated with 2 μM RSL3 or 20
μM Erastin and H460 cells were treated with 8 μM RSL3
or 20 μM Erastin) at 37°C for 24 h. Subsequently, the cells
were harvested and stained with 2 μM C11-BODIPY (581/591)
probe at 37°C for 30 min in the dark. At least 1×104
cells were collected to measure the fluorescence using the BD
FACSAria™ II flow cytometer (BD Biosciences). FlowJo V10 software
(FlowJo, LLC) was used to analyse the data.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol reagent was used to extract the total RNA,
and the extracted RNA was reverse transcribed into cDNA using an
ImProm-II™ Reverse Transcriptase Reagent Kit (cat. no.
A3803; Promega Corporation) according to the manufacturer's
instructions. qPCR was performed in a Mx3000P instrument (Agilent
Technologies, Inc.) using PowerUp™ SYBR™ Green Master Mix (cat. no.
A24742; Applied Biosystems; Thermo Fisher Scientific, Inc.). For
detecting miRNA expression, poly(A) tails were added before RT
using E. coli poly(A) polymerase (cat. no. M0276S; New
England BioLabs, Inc.). The qPCR conditions were as follows: i)
50°C for 2 min; ii) 95°C for 2 min; and iii) 40 cycles of 95°C for
5 sec and 60°C for 30 sec. To normalise the levels of miRNA
expression and other genes, U6 or glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) RNA were utilised, and the relative gene
expression levels were calculated using the 2−ΔΔCq
method (38). Table SII depicts the RT-qPCR primer
sequences used in this assay.
Western blotting
RIPA Lysis Buffer (cat. no. ZE122-101S; Genstar
Biosolutions Co., Ltd.) containing Protease Inhibitor Cocktail
(cat. no. B14001; Bimake) was used to extract the total proteins
from cells. The Pierce™ BCA Protein Assay Kit (cat. no. 23227;
Thermo Fisher Scientific, Inc.) was utilised to quantify protein
concentrations in the samples. Then, samples with 15 μg of
protein were separated via 12% SDS-PAGE gels and transferred to
nitrocellulose membranes (cat. no. 66485; Pall Life Sciences).
After blocking the membrane with 5% non-fat milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies overnight at 4°C. The following primary antibodies were
used: GAPDH (1:20,000; cat. no. 60004-1-Ig; ProteinTech Group,
Inc.); GCH1 (1:2,000; cat. no. 28501-1-AP; ProteinTech Group,
Inc.); GPX4 (1:5,000; cat. no. ab125066; Abcam) and FSP1 (1:2,000;
cat. no. 20886-1-AP; ProteinTech Group, Inc.). Then, the membranes
were incubated with secondary antibodies at room temperature for 1
h. The secondary antibodies used were as follows: Goat anti-mouse
(1:5,000; cat. no. SA00001-1; ProteinTech Group, Inc.) and goat
anti-rabbit (1:5,000; cat. no. SA00001-2; ProteinTech Group, Inc.).
The signals were visualised using HRP Substrate Luminol Reagent
(cat. no. WBKLS0500; MilliporeSigma) and imaged using a
chemiluminescence imaging system (Tanon-4600SF; Tanon Science and
Technology Co., Ltd.). ImageJ software was used for the
semi-quantitation of protein.
Dual-luciferase reporter assay
Full-length LUCAT1 was inserted into the pGL3-basic
vector (Promega Corporation) using XbaI and NdeI
(cat. nos. R0145S and R0111S; New England BioLabs, Inc.) to create
the LUCAT1 reporter vector. The GCH1 3'-UTR reporter vector was
also constructed by insertion into the pGL3-basic vector. Using the
QuickMutation™ Plus Kit (cat. no. D0208S; Beyotime Institute of
Biotechnology), the binding sites 'CACUGCC' (position 869-875 in
LUCAT1 or position 255-261 in the GCH1 3'-UTR) were changed to
'GCGACGG' to create mutated variants of the LUCAT1 and the GCH1
3'-UTR reporter vector. Dual-luciferase reporter assays were
conducted by co-transfecting 293T cells with the Renilla
Luciferase Expression Vector (Promega Corporation), the LUCAT1 or
GCH1 3'-UTR reporter vector, and miR-NC or miR-34a-5p mimics using
jetPRIME transfection reagent. After 48 h of transfection, the
cells were lysed by the Dual-Luciferase Reporter Assay System (cat.
no. E1910; Promega Corporation), and the luciferase activities were
measured by GloMax® 20/20 Luminometer (Promega
Corporation). Transfection efficiencies were standardised using
Renilla luciferase. Table
SIII shows the sequences of the primers used in this assay.
Statistical analysis
The data are presented as the mean ± SD. Unpaired
Student's t-test (two-tailed) and one-way analysis of variance
(ANOVA) or two-way ANOVA followed by Tukey's test were used to
analyse the differences between the different groups, using
GraphPad Prism software. P<0.05 was used to indicate a
statistically significant difference.
Results
Identification of DEmRNAs involved in
regulating ferroptosis
To identify genes involved in ferroptosis, A549
cells were treated with DMSO, RSL3 or RSL3 + Fer-1. RSL3 (2
μM) inhibited approximately half of the cell viability of
A549 cells, which was prevented by co-incubation with 5 μM
Fer-1 (Fig. S1). Subsequently,
RNA-Seq was performed. When comparing the RSL3 treatment group with
the DMSO group, 291 DEmRNAs were identified, including the
ferroptosis-related genes, solute carrier family 7 member 11,
prostaglandin-endoperoxide synthase 2, FTH1, heme oxygenase 1 and
TP53 (1,39-42) (Fig.
1A and B), of which 82 were rescued (expression level was
restored) by co-incubation with Fer-1, indicating that these 82
DEmRNAs may be important for ferroptosis (Fig. 1C). The DEmRNAs were enriched in
the BP terms, 'response to oxygen levels' and 'positive control of
programmed cell death' (Fig. 1D).
KEGG pathway analysis revealed that the ferroptosis-related, 'HIF-1
signaling pathway' (43), and
'TNF signaling pathway' (44)
were also enriched during ferroptosis (Fig. 1E). Among the top 10 hub mRNAs in
the PPI network displayed using STRING, jun proto-oncogene, C-X-C
motif chemokine ligand 8, epiregulin, cyclin-dependent kinase
inhibitor 1A, serpin family E member 1 and DNA damage-inducible
transcript 3 were previously reported to be involved in ferroptosis
(45-50), suggesting that the remaining four
genes, growth arrest and DNA damage inducible α (GADD45A),
epithelial mitogen (EPGN), heparin-binding EGF-like growth factor
(HBEGF) and transforming growth factor α (TGFA), may also have
roles in ferroptosis (Fig. 1F and
G). These results therefore identified novel genes that may be
involved in ferroptosis caused by alkoxyl radicals and other
rearrangement products generated by ferrous iron from lipid
hydroperoxides.
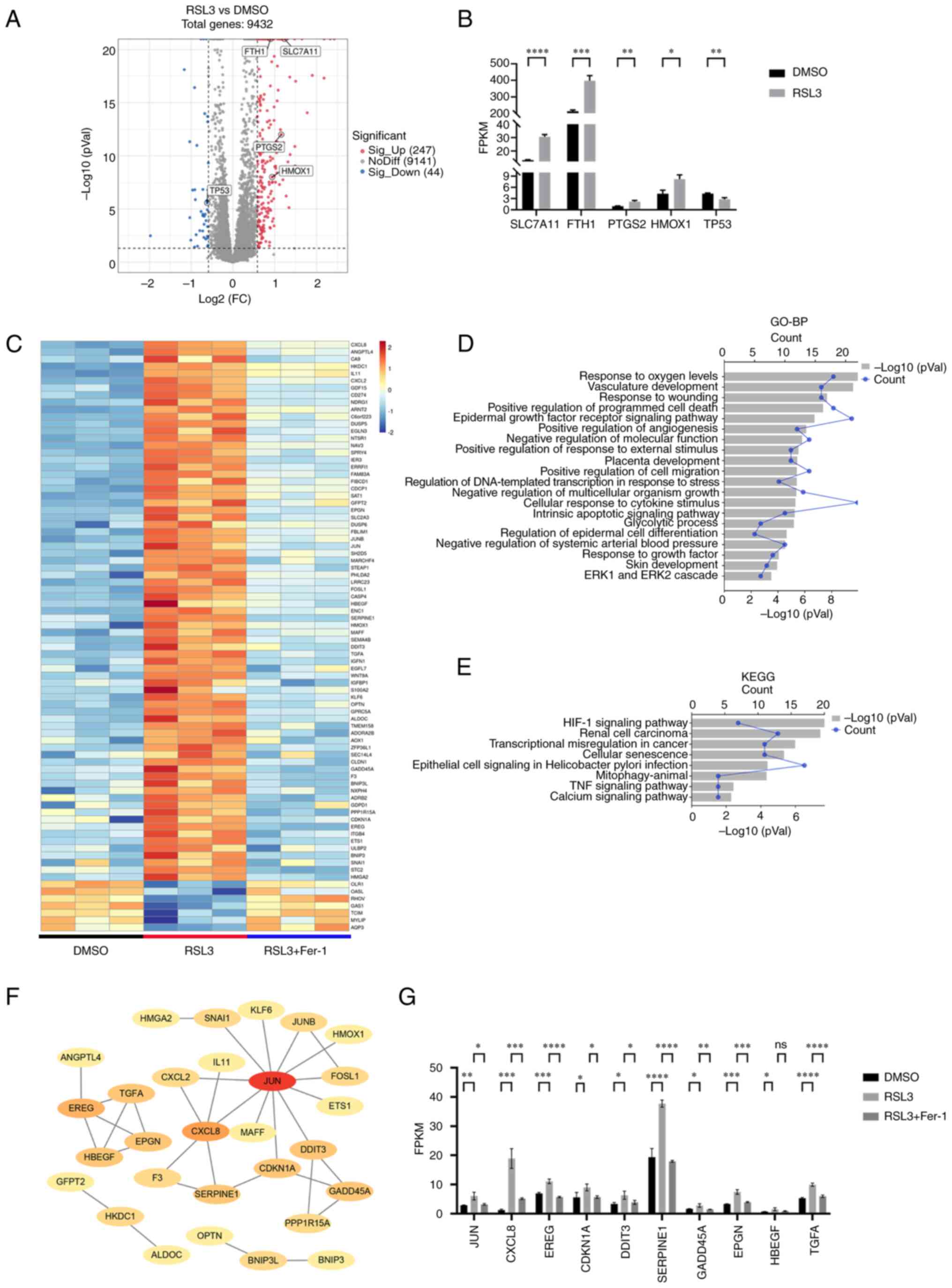 | Figure 1Expression profiles of mRNAs in
ferroptosis. (A) Volcano plot for the RSL3 group vs. the DMSO
group. (B) The FPKM value of ferroptosis-associated genes in the
DMSO and RSL3 groups. (C) Heatmap showing the overlap of 82 DEmRNAs
between the RSL3 vs. DMSO groups and the RSL3 vs. RSL3 + Fer-1
groups. (D) Top 20 GO-BP terms for the DEmRNAs. (E) KEGG terms for
the DEmRNAs. (F) PPI network of DEmRNAs. (G) The FPKM value of the
top 10 hub genes in the PPI network. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. RSL3, RAS-selective lethal 3; DEmRNAs,
differentially expressed mRNAs; Fer-1, ferrostatin-1; GO, Gene
Ontology; BP, Biological Process; KEGG, Kyoto Encyclopedia of Genes
and Genomes; PPI, protein-protein interaction; FPKM, fragments per
kilobase of transcript per million mapped reads; FC, fold change;
ns, not significant; SLC7A11, solute carrier family 7 member 11;
FTH1, ferritin heavy chain 1; PTGS2, prostaglandin-endoperoxide
synthase 2; HMOX1, heme oxygenase 1; JUN, jun proto-oncogene;
CXCL8, C-X-C motif chemokine ligand 8; EREG, epiregulin; CDKN1A,
cyclin-dependent kinase inhibitor 1A; DDIT3, DNA damage-inducible
transcript 3; GADD45A, growth arrest and DNA damage inducible α;
HBEGF, heparin-binding EGF-like growth factor. |
LUCAT1 is upregulated in RSL3-induced
ferroptosis, which is prevented by co-incubation with Fer-1
In addition to the 82 DEmRNAs, 18 DElncRNAs were
either upregulated or downregulated in the RSL3 group compared with
the DMSO group, which was prevented by co-incubation with Fer-1
(Fig. 2A-D). The genes targeted
by DElncRNAs were enriched in the BP terms 'regulation of growth'
and 'response to oxygen levels' (Fig.
2E). Additionally, KEGG pathway analysis revealed that the
'MAPK signaling pathway' (51,52), 'Hippo signaling pathway' (53) and 'Wnt signaling pathway'
(54) were enriched (Fig. 2F), suggesting that DElncRNAs are
involved in ferroptosis.
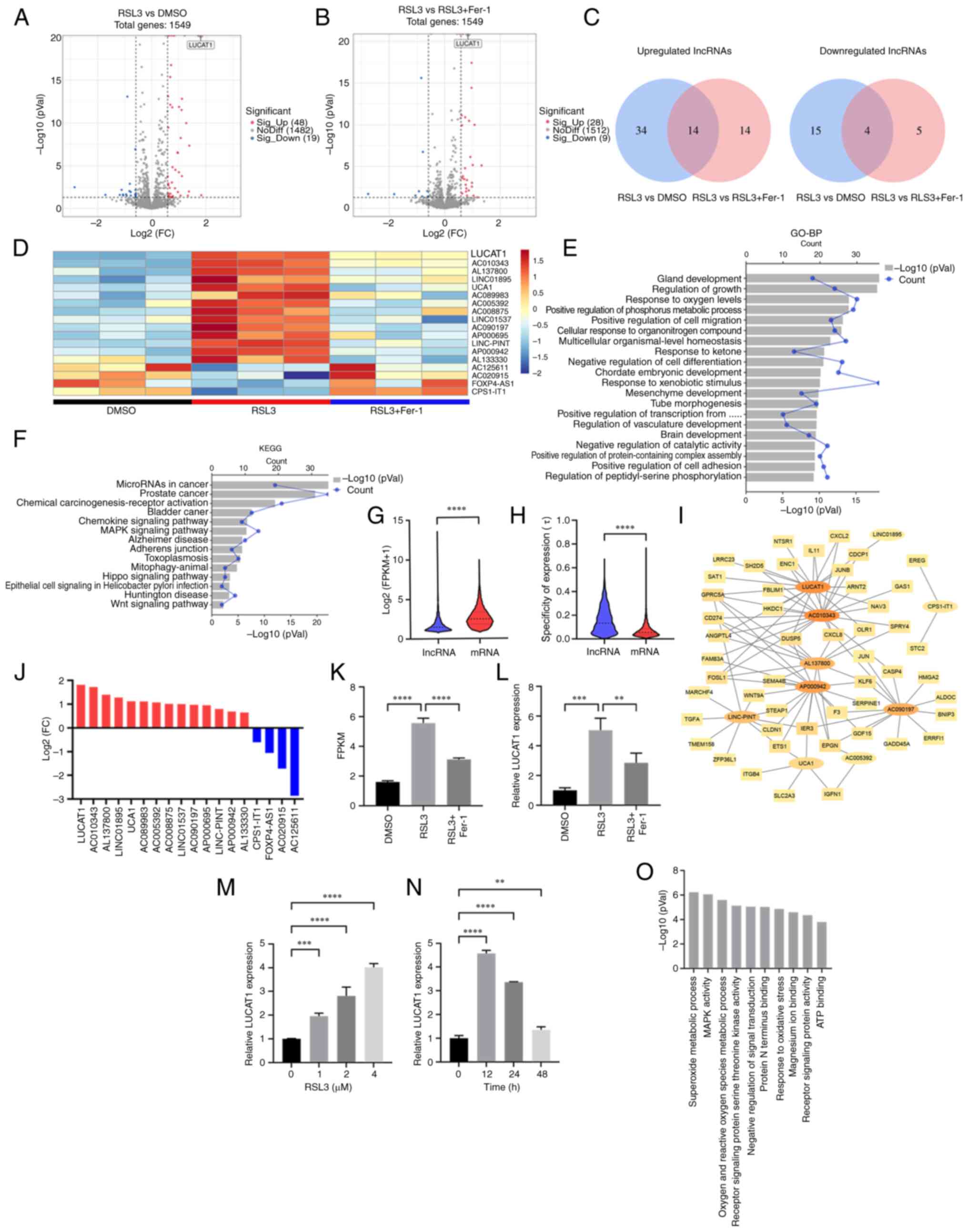 | Figure 2LUCAT1 is upregulated in RSL3-induced
ferroptosis, which is inhibited by Fer-1. (A) Volcano map for the
RSL3 vs. DMSO group. (B) Volcano map for the RSL3 vs. RSL3 + Fer-1
group. (C) The overlap of the DElncRNAs between the RSL3 vs. DMSO
group and the RSL3 vs. RSL3 + Fer-1 group. (D) Heatmap of the 18 DE
lncRNAs. (E) Top 20 GO-BP terms enriched for the target genes of
DElncRNAs. (F) KEGG terms enriched for the target genes of the
DElncRNAs. (G) Log2 (FPKM+1) of lncRNAs and mRNAs. (H)
Specific expression analysis of lncRNAs and mRNAs. (I)
Co-expression networks between DElncRNAs and DEmRNAs. (J) DElncRNAs
ranked in terms of their log2 FC values. (K) The FPKM
value for LUCAT1 was determined using RNA sequencing. (L) LUCAT1
expression in the DMSO, RSL3 and RSL3 + Fer-1 groups was detected
using RT-qPCR. (M) LUCAT1 expression in A549 cells treated with 0,
1, 2 or 4 μM RSL3 for 24 h was detected using RT-qPCR. (N)
LUCAT1 expression in A549 cells treated with 4 μM RSL3 for
0, 12, 24 or 48 h was detected using RT-qPCR. (O) GO analysis of
LUCAT1 was predicted using LncACTdb. **P<0.01,
***P<0.001, ****P<0.0001. All
experiments were repeated three times. LUCAT1, lung
cancer-associated transcript 1; RSL3, RAS-selective lethal 3;
Fer-1, ferrostatin-1; DE, differentially expressed; lncRNA, long
non-coding RNA; GO, Gene Ontology; BP, Biological Process; KEGG,
Kyoto Encyclopedia of Genes and Genomes; FC, fold change; FPKM,
fragments per kilobase of transcript per million mapped reads;
RT-qPCR, reverse transcription-quantitative PCR. |
Compared with mRNA expression levels, the global
expression level of lncRNA was much lower (Fig. 2G). However, the lncRNAs displayed
a greater degree of expression specificity compared with that of
the mRNAs (Fig. 2H). To identify
lncRNAs that were more important in ferroptosis, their correlations
with DEmRNAs were examined and mRNA-lncRNA co-expression networks
were constructed. Using a robust correlation (|r|>0.9,
P<0.05) as the cut-off for mRNA-lncRNA pairs, 93 nodes (16
DElncRNAs and 77 DEmRNAs) and 589 mRNA-lncRNA pairs (544 with a
positive correlation and 45 with a negative correlation) were
identified. The mRNA-lncRNA pairs with the top 100 correlation
coefficients (|r|) were used for network construction (Fig. 2I).
Among the 18 DElncRNAs, LUCAT1 was the most highly
upregulated, ranking first among the candidates (Fig. 2J), and it had an important role in
the constructed mRNA-lncRNA co-expression network (Fig. 2I). Therefore, it was next
confirmed that LUCAT1 was upregulated following treatment with RSL3
and prevented by co-incubation with Fer-1 (Fig. 2K and L). After treating A549 cells
with RSL3, LUCAT1 expression increased in a dose-dependent manner
and maximum LUCAT1 expression occurred after 12 h of treatment with
4 μM RSL3 (Fig. 2M and N).
To obtain more evidence for the connection between LUCAT1 and
ferroptosis, the GEO database was utilised to explore the
relationship between LUCAT1 and other ferroptosis inducers in
cancer. It was found that LUCAT1 was elevated in HepG2 liver cancer
cells treated with Erastin and in two sorafenib-resistant liver
cancer lines (Hep3B and Huh7) by bioinformatic analysis (Fig. S2A-C), suggesting that ferroptosis
inducer-induced upregulation of LUCAT1 may be common in different
types of cancer. Furthermore, the top three GO terms for LUCAT1
predicted by the LncACTdb were 'superoxide metabolic process'
(55), 'MAPK activity' (51,52) and 'oxygen and reactive oxygen
species metabolic process' (56),
which all have important roles in ferroptosis (Fig. 2O). These results reflected a
possible close relationship between ferroptosis and LUCAT1.
Genes that are expressed at either high or low
levels in tumours influence the growth and treatment of the tumours
(57). LUCAT1 expression was
higher in 18 different types of cancer tissues than in the
corresponding normal tissues (Fig.
S3A). Compared with normal tissues, LUCAT1 expression was
higher in lung adenocarcinoma and lung squamous cell carcinoma
(Fig. S3B and C). Similarly, two
lung cancer cell lines, A549 and H460, expressed higher levels of
LUCAT1 compared with the BEAS-2B normal lung epithelial cell line
(Fig. S3D). These findings
suggested that LUCAT1 functions as an oncogene in lung cancer.
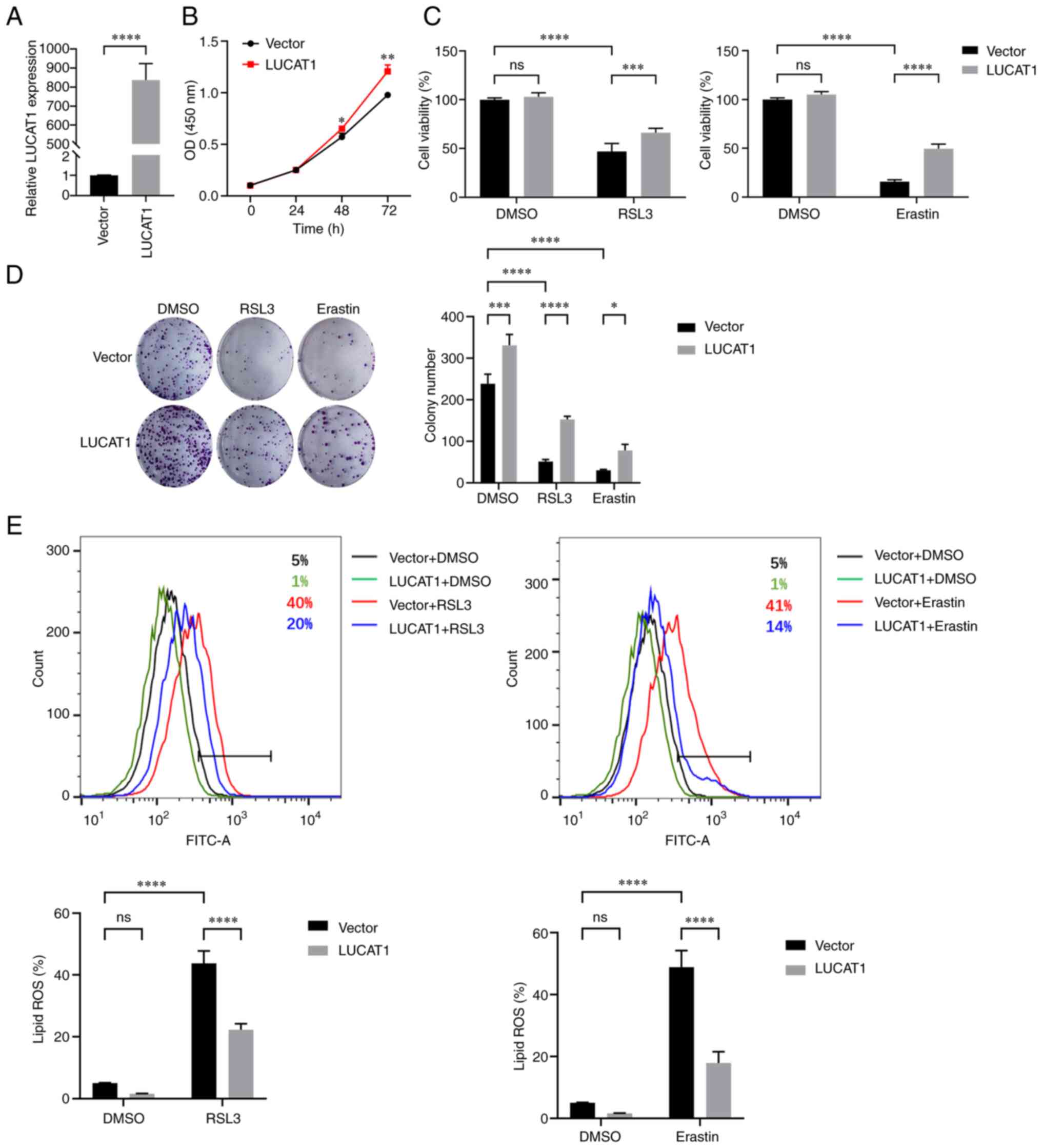
LUCAT1 overexpression inhibits RSL3- and
Erastin-induced ferroptosis
The expression of LUCAT1 was lower in A549 cells
than in H460 cells (Fig. S3D);
therefore, the effect of overexpressing LUCAT1 in A549 cells was
examined (Fig. 3A). As shown in
Fig. 3B, LUCAT1 overexpression
significantly enhanced A549 cell proliferation. When ferroptosis
was induced by RSL3 and Erastin, overexpression of LUCAT1 enhanced
the survival rate and colony formation of A549 cells (Fig. 3C and D). Similarly, lipid
peroxidation levels were elevated by RSL3 and Erastin treatment;
however, LUCAT1 overexpression reduced these levels (Fig. 3E). Collectively, these data
suggested that LUCAT1 was a ferroptosis suppressor in A549 cells
treated with RSL3 and Erastin.
Knocking down LUCAT1 expression promotes
RSL3- and Erastin-induced ferroptosis
As LUCAT1 was expressed at higher levels in H460
cells than in A549 cells (Fig.
S3D), the effect of knocking down LUCAT1 expression in H460
cells was next explored (Fig.
4A). Knocking down LUCAT1 expression significantly inhibited
the proliferation of H460 cells (Fig.
4B). Knocking down LUCAT1 expression also significantly reduced
the survival rate and colony formation capacity of H460 cells
following ferroptosis induction (Fig.
4C and D). Moreover, knocking down LUCAT1 expression increased
the level of lipid peroxidation upon ferroptosis induction
(Fig. 4E). These data suggested
that knocking down LUCAT1 expression promoted RSL3- and
Erastin-induced ferroptosis.
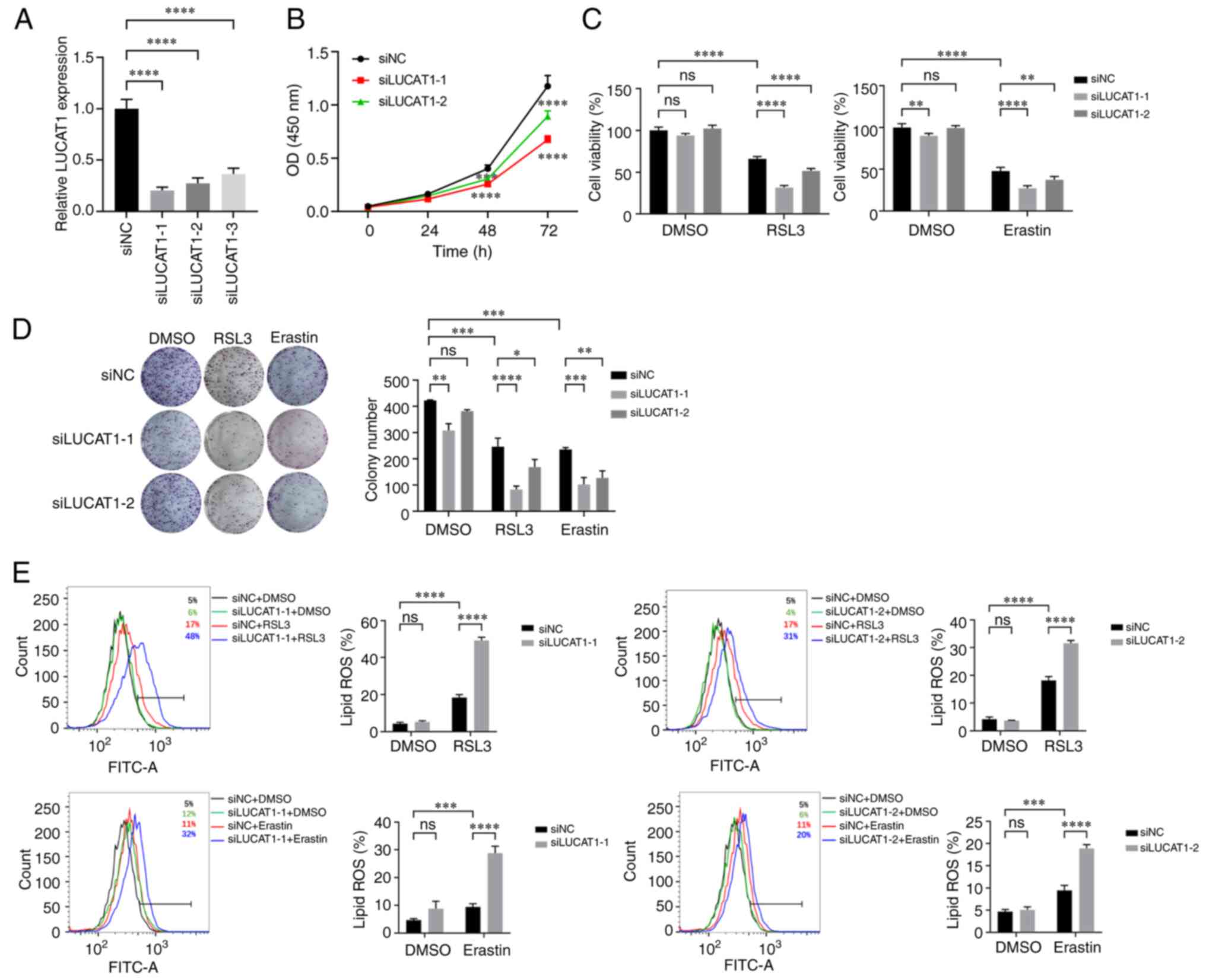 | Figure 4LUCAT1 knockdown promotes RSL3- and
Erastin-induced ferroptosis. (A) LUCAT1 expression in H460 cells
was detected using reverse transcription-quantitative PCR. (B) The
effect of LUCAT1 knockdown on cell proliferation was detected by
CCK-8. (C) H460 cells were treated with 8 μM RSL3 or 20
μM Erastin for 24 h and the cell viability was determined by
CCK-8. (D) Colony formation assay was performed following treatment
with 2 μM RSL3 or 4 μM Erastin. (E) The histograms of
lipid ROS for H460 cells treated with 8 μM RSL3 or 20
μM Erastin for 24 h. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. All experiments were repeated three
times. LUCAT1, lung cancer-associated transcript 1; RSL3,
RAS-selective lethal 3; CCK-8, Cell Counting Kit-8; ns, not
significant; ROS, reactive oxygen species; si(RNA), small
interfering RNA; NC, negative control. |
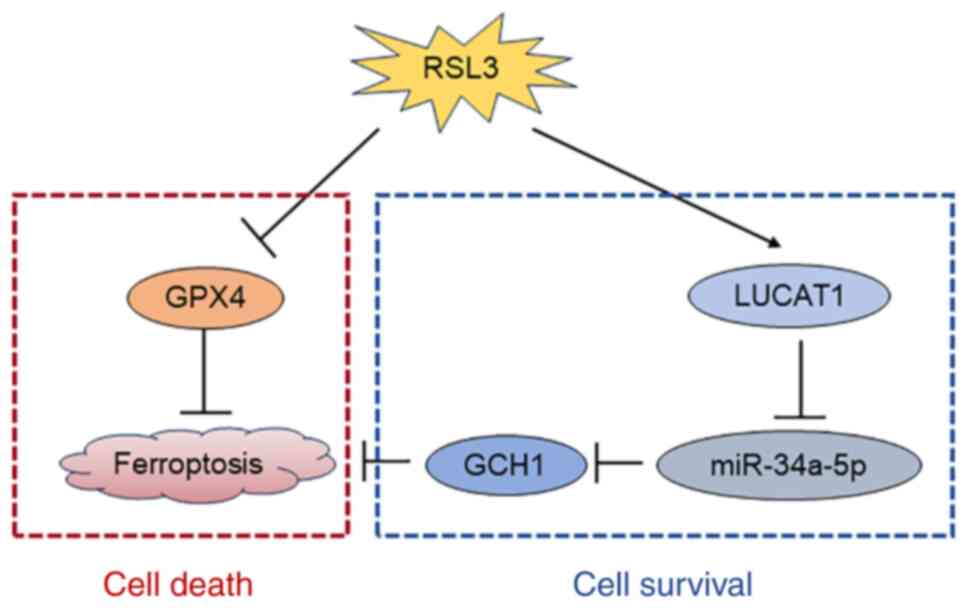
Knocking down LUCAT1 expression promotes
ferroptosis by regulating the miR-34a-5p/GCH1 axis
To investigate the mechanism by which LUCAT1
influences ferroptosis, the expression of the main ferroptosis
resistance genes were detected. FSP1 and GCH1 were downregulated in
LUCAT1-knock down cells, while GPX4 expression was not affected
(Fig. 5A). Owing to the greater
downregulation of GCH1, we considered that LUCAT1 primarily exerted
its effects through GCH1. LncLocator analysis indicated that LUCAT1
is predominantly localised in the cytoplasm (Fig. 5B); thus, it may act as a miRNA
sponge in regulating GCH1 expression. Next, the shared miRNAs
targeting the 3'-UTR of GCH1 (predicted by TargetScan) and LUCAT1
(predicted by DIANA-LncBase v3) were analysed and miR-34a-5p was
identified as a potential mediator between the two (Fig. 5C). As illustrated in Fig. 5D, the expression level of
miR-34a-5p was elevated upon LUCAT1 knockdown. Moreover,
transfection with miR-34a-5p mimics downregulated GCH1 expression
(Fig. 5E and F). Additionally,
miR-34a-5p mimics enhanced the RSL3-induced ferroptosis sensitivity
as determined via cell viability and lipid ROS assays (Fig. 5G and H). Conversely, inhibition of
miR-34a-5p with anti-miR-34a-5p increased the resistance of
ferroptosis mediated by LUCAT1 inhibition (Fig. 5I and J).
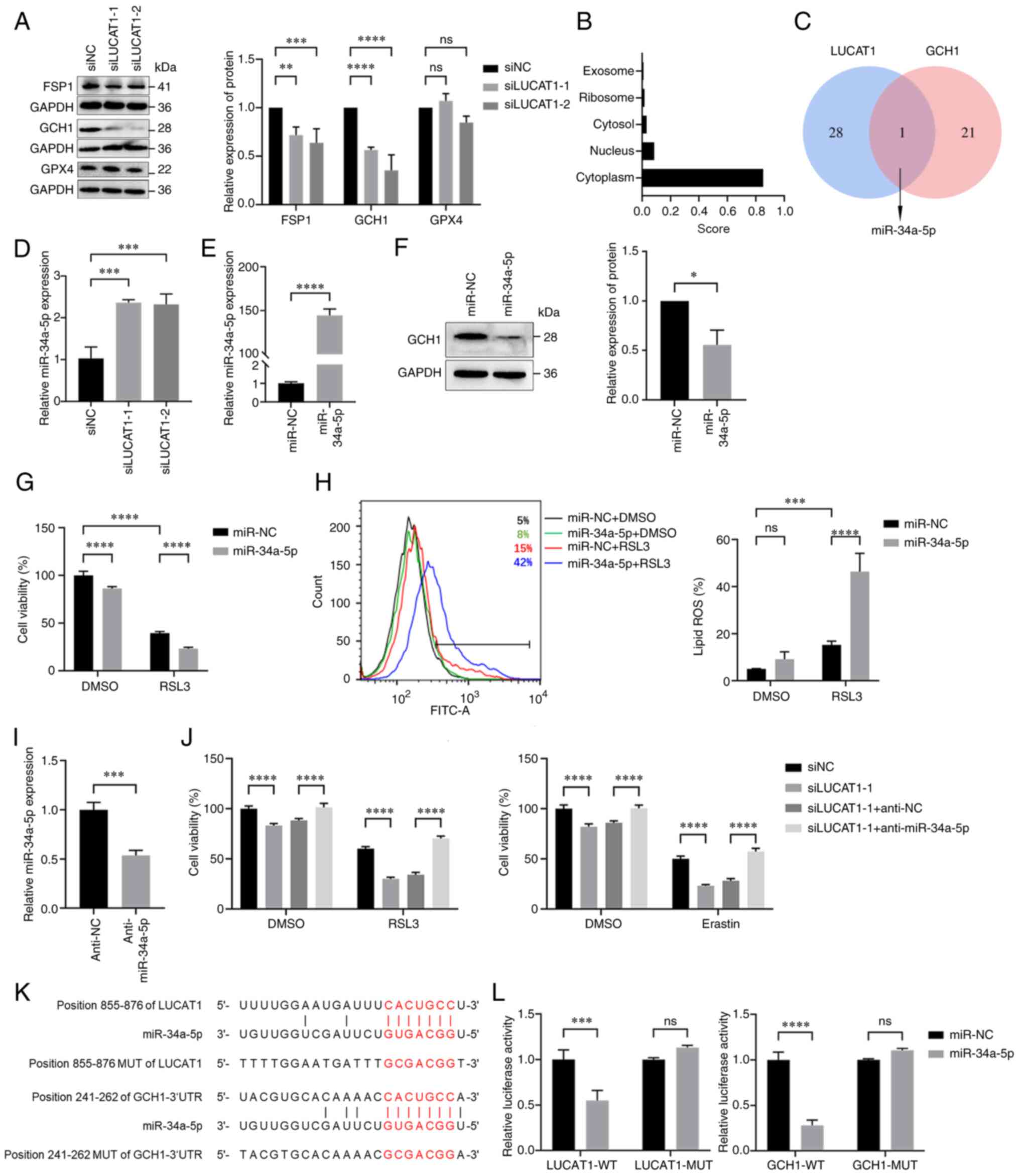 | Figure 5Knocking down LUCAT1 expression
promotes ferroptosis by regulating the miR-34a-5p/GCH1 axis. (A)
Expression of ferroptosis-associated proteins in H460 cells after
knocking down LUCAT1 expression was detected using western
blotting. (B) The subcellular localisation of LUCAT1 was predicted
using lncLocator. (C) Venn diagram of overlapping miRNAs related to
LUCAT1 and the 3'-UTR of GCH1. (D) miR-34a-5p expression in H460
cells after knocking down LUCAT1 expression was detected using
RT-qPCR. (E) miR-34a-5p expression in H460 cells transfected with
miR-34a-5p mimics was detected using RT-qPCR. (F) GCH1 protein
expression in H460 cells transfected with miR-34a-5p mimics was
detected using western blotting. (G) H460 cells were treated with 8
μM RSL3 for 24 h and the cell viability was determined by
using CCK-8. (H) The lipid ROS histograms for H460 cells treated
with 8 μM RSL3 for 24 h. (I) miR-34a-5p expression in H460
cells transfected with miR-34a-5p inhibitors was detected using
RT-qPCR. (J) The viability of H460 cells treated with siNC,
siLUCAT1-1, siLUCAT1-1 + anti-NC or siLUCAT1-1 + anti-miR-34a-5p
groups was assessed following treatment with 8 μM RSL3 or 20
μM Erastin for 24 h using CCK-8. (K) The miR-34a-5p binding
sites of LUCAT1 and 3'-UTR of GCH1 were predicted using miRDB and
TargetScan. (L) Dual-luciferase reporter assays were performed to
determine the associations between miR-34a-5p and LUCAT1 or GCH1.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. All
experiments were repeated three times. LUCAT1, lung
cancer-associated transcript 1; GCH1, GTP cyclohydrolase 1; 3'-UTR,
3'-untranslated region; FSP1, ferroptosis suppressor protein 1;
GPX4, glutathione peroxidase 4; CCK-8, Cell Counting Kit-8;
RT-qPCR, reverse transcription-quantitative PCR; ns, not
significant; ROS, reactive oxygen species; si(RNA), small
interfering RNA; NC, negative control; miR, microRNA; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; WT, wild-type; MUT,
mutated. |
Using miRDB and TargetScan, the potential miR-34a-5p
binding sites with LUCAT1 and the 3'-UTR of GCH1 were identified
(Fig. 5K). The potential binding
sites between miR-34a-5p and LUCAT1 or the 3'-UTR of GCH1 were then
validated using dual-luciferase reporter assays. The luciferase
activity was markedly lower in 293T cells transfected with
miR-34a-5p mimics than with miR-NC; however, no changes in
luciferase activity were observed in the LUCAT1-mutated (MUT) or
GCH1-MUT groups (Fig. 5L). In
conclusion, these findings suggested that LUCAT1 may promote
ferroptosis via the miR-34a-5p/GCH1 axis.
Discussion
As ferroptosis is a targetable cancer vulnerability,
more attention has been paid to the impact of
ferroptosis-associated genes in recent years (12). Therefore, lncRNA expression
profiles were analysed in the present study and it was discovered
that LUCAT1 expression was elevated following treatment with RSL3,
which was prevented by Fer-1. The results of the present study also
suggested that LUCAT1 acted as a miR-34a-5p sponge and upregulated
GCH1 expression, enhancing the resistance of lung cancer cells to
ferroptosis and thereby promoting cell survival (Fig. 6).
Ferroptosis-associated proteins have been
extensively reported, some of which have been targeted to increase
the sensitivity of cancer to certain drugs (58). For instance, Mao et al
(7) reported that DHODH is a
ferroptosis suppressor, and the DHODH inhibitor, brequinar,
selectively inhibited the growth of tumours with low GPX4
expression by inducing ferroptosis. In addition, the growth of the
tumours with high GPX4 expression was suppressed by inducing
ferroptosis following combined treatment with brequinar and the
FDA-approved drug, sulfasalazine. In the present study, GADD45A,
EPGN, HBEGF and TGFA were identified as potentially novel
ferroptosis-related genes by analysing their mRNA expression
profiles under ferroptosis conditions. Qi et al (59) also reported that sodium selenite
inhibited cervical cancer by activating GADD45A in a ROS-dependent
manner (56), which is essential
for ferroptosis. Although lncRNAs are vital in biological
processes, data on ferroptosis-related lncRNAs remains limited. In
the present study, the analysis of mRNA and lncRNA expression
profiles suggested the involvement of LUCAT1 in ferroptosis. The
findings indicated that LUCAT1 was significantly upregulated in
A549 cells treated with RSL3, which was prevented by Fer-1.
Previous studies have reported LUCAT1 upregulation in Erastinor
sorafenib-treated liver cancer cells and in pancreatic
adenocarcinoma cells subjected to cystine depletion (a
ferroptosis-inducing condition) (34,35,60,61). Moreover, nuclear factor erythroid
2-related factor 2 (62),
specificity protein 1 (63),
signal transducer and activator of transcription 3 (64) and E74-like ETS transcription
factor 1 (65) regulate LUCAT1
transcription; however, whether RSL3 controls these transcription
factors to regulate LUCAT1 expression requires further
exploration.
Previous reports have shown that LUCAT1 promotes
tumour cell migration, invasion (63) and metastasis (66), is linked to worse outcomes
(67) and has been described as a
key regulator of stemness in cancer (68). Vierbuchen et al (69) found that LUCAT1 levels are
elevated in a large cohort of patients with inflammatory disorders,
underlining its key role in immune regulation. Tao et al
(70) demonstrated that LUCAT1
prevents stem cell apoptosis and improves the efficacy of stem cell
therapy for ischaemic cardiovascular disease. Reduced LUCAT1
expression promotes G0/G1 phase block of the cell cycle in several
tumour cells (71-75), while high expression leads to the
development of cellular resistance, for instance cisplatin
resistance in non-small cell lung cancer (NSCLC) (76), methotrexate resistance in
osteosarcoma (77) and
camptothecin, 5-fluorouracil, adriamycin and oxaliplatin resistance
in colorectal cancer (22). In
the present study, LUCAT1 knockdown increased the sensitivity of
lung cancer cells to ferroptosis, indicating that this method could
be a novel approach to treating lung cancer.
In addition, in the present study, knocking down
LUCAT1 expression inhibited the expression of GCH1, which promotes
tumorigenesis in KRAS-driven lung cancer (78) and was identified as a ferroptosis
suppressor in a genome-wide activation screen (6). The localisations of lncRNAs are
closely associated with their functions (79). In the present study, LUCAT1 was
predicted to be localised in the cytoplasm, where it may act as a
miR-34a-5p sponge. miR-34a-5p has been shown to inhibit cell
proliferation in lung adenocarcinoma (80) and is a driver in
CdCl2-induced ferroptosis (81). The findings of the present study
demonstrated that miR-34a-5p inhibited GCH1 expression, which in
turn promoted ferroptosis in the presence of RSL3 in lung
cancer.
Although this study provides a new theoretical basis
for the treatment of lung cancer, there are still some limitations.
Specifically, the findings of the present study suggested that the
ferroptosis inducer, RSL3, regulated LUCAT1, yet the precise
regulatory mechanism remains unclear. The expression levels of
LUCAT1 in lung cancer and normal tissues were determined through
bioinformatics analysis; however, the lack of available clinical
samples hinders further validation. Furthermore, in vivo
experiments are necessary to substantiate the suppressive function
of LUCAT1 in ferroptosis. Consequently, additional research is
warranted to address these unresolved questions.
In summary, the results of the present study
suggested that LUCAT1 may have a critical role in ferroptosis.
Overexpression of LUCAT1 induced ferroptosis resistance to rescue
cell survival in the presence of RSL3. By contrast, knocking down
LUCAT1 expression enhanced susceptibility to RSL3- and
Erastin-induced ferroptosis via miR-34a-5p-mediated GCH1
downregulation in NSCLC cells. Therefore, the results of the
present study identified a novel pathway, the
LUCAT1/miR-34a-5p/GCH1 axis, which regulated ferroptosis
sensitivity and may be targeted for the treatment of lung
cancer.
Supplementary Data
Availability of data and materials
The RNA sequencing data generated in the present
study may be found in the GEO database under accession number
GSE247883 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247883.
All other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
XZ, FX, HF and FT conceived and designed the
experiments. FT, RZ, KD, YZ, HY, HL, QW and ZC performed the
experiments. FT and CG analysed the data. FT wrote the manuscript.
XZ, FX, HF and CG revised the manuscript. XZ and FT confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BP
|
Biological Process
|
|
DHODH
|
dihydroorotate dehydrogenase
|
|
Fer-1
|
ferrostatin-1
|
|
FSP1
|
ferroptosis suppressor protein 1
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
GCH1
|
GTP cyclohydrolase 1
|
|
GO
|
Gene Ontology
|
|
GPX4
|
glutathione peroxidase 4
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
lncRNA
|
long non-coding RNA
|
|
LUCAT1
|
lung cancer-associated transcript
1
|
|
miRNA
|
microRNA
|
|
NSCLC
|
non-small cell lung cancer
|
|
PPI
|
protein-protein interaction
|
|
RSL3
|
RAS-selective lethal 3
|
Acknowledgments
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant nos. 82073489 and 32071290).
References
|
1
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.
|
|
2
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285.
2017.
|
|
3
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019.
|
|
4
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019.
|
|
5
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019.
|
|
6
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020.
|
|
7
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021.
|
|
8
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003.
|
|
9
|
Yang WS and Stockwell BR: Synthetic lethal
screening identifies compounds activating iron-dependent,
nonapoptotic cell death in oncogenic-RAS-harboring cancer cells.
Chem Biol. 15:234–245. 2008.
|
|
10
|
Miotto G, Rossetto M, Di Paolo ML, Orian
L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin
M, Zennaro L, et al: Insight into the mechanism of ferroptosis
inhibition by ferrostatin-1. Redox Biol. 28:1013282020.
|
|
11
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019.
|
|
12
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022.
|
|
13
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010.
|
|
14
|
Karreth FA, Tay Y, Perna D, Ala U, Tan SM,
Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al:
In vivo identification of tumor-suppressive PTEN ceRNAs in an
oncogenic BRAF-induced mouse model of melanoma. Cell. 147:382–395.
2011.
|
|
15
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010.
|
|
16
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015.
|
|
17
|
Zhang R, Pan T, Xiang Y, Zhang M, Xie H,
Liang Z, Chen B, Xu C, Wang J, Huang X, et al: Curcumenol triggered
ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1
axis. Bioact Mater. 13:23–36. 2021.
|
|
18
|
Qin Y, Zhang D, Zhang H, Hou L, Wang Z,
Yang L, Zhang M, Zhao G, Yao Q, Ling R and Zhang J: Construction of
a ferroptosis-related five-lncRNA signature for predicting
prognosis and immune response in thyroid carcinoma. Cancer Cell
Int. 22:2962022.
|
|
19
|
Thai P, Statt S, Chen CH, Liang E,
Campbell C and Wu R: Characterization of a novel long noncoding
RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer
cell lines. Am J Respir Cell Mol Biol. 49:204–211. 2013.
|
|
20
|
Jiao Y, Li Y, Ji B, Cai H and Liu Y:
Clinical value of lncRNA LUCAT1 expression in liver cancer and its
potential pathways. J Gastrointestin Liver Dis. 28:439–447.
2019.
|
|
21
|
Chi J, Liu T, Shi C, Luo H, Wu Z, Xiong B,
Liu S and Zeng Y: Long non-coding RNA LUCAT1 promotes proliferation
and invasion in gastric cancer by regulating miR-134-5p/YWHAZ axis.
Biomed Pharmacother. 118:1092012019.
|
|
22
|
Huan L, Guo T, Wu Y, Xu L, Huang S, Xu Y,
Liang L and He X: Hypoxia induced LUCAT1/PTBP1 axis modulates
cancer cell viability and chemotherapy response. Mol Cancer.
19:112020.
|
|
23
|
Zhou Q, Hou Z, Zuo S, Zhou X, Feng Y, Sun
Y and Yuan X: LUCAT1 promotes colorectal cancer tumorigenesis by
targeting the ribosomal protein L40-MDM2-p53 pathway through
binding with UBA52. Cancer Sci. 110:1194–1207. 2019.
|
|
24
|
Wang Y, Li Z, Li W, Zhou L and Jiang Y:
Prognostic significance of long non-coding RNAs in clear cell renal
cell carcinoma: A meta-analysis. Medicine (Baltimore).
98:e172762019.
|
|
25
|
Shu X, Zhang Z, Yao ZY and Xing XL:
Identification of five ferroptosis-related lncRNAs as novel
prognosis and diagnosis signatures for renal cancer. Front Mol
Biosci. 8:7636972022.
|
|
26
|
Xing XL, Yao ZY, Ou J, Xing C and Li F:
Development and validation of ferroptosis-related lncRNAs prognosis
signatures in kidney renal clear cell carcinoma. Cancer Cell Int.
21:5912021.
|
|
27
|
He Y, Ye Y, Tian W and Qiu H: A novel
lncRNA panel related to ferroptosis, tumor progression, and
microenvironment is a robust prognostic indicator for glioma
patients. Front Cell Dev Biol. 9:7884512021.
|
|
28
|
Deng SH, Wu DM, Li L, Liu T, Zhang T, Li
J, Yu Y, He M, Zhao YY, Han R and Xu Y: miR-324-3p reverses
cisplatin resistance by inducing GPX4-mediated ferroptosis in lung
adenocarcinoma cell line A549. Biochem Biophys Res Commun.
549:54–60. 2021.
|
|
29
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020.
|
|
30
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019.
|
|
31
|
Wang P, Guo Q, Qi Y, Hao Y, Gao Y, Zhi H,
Zhang Y, Sun Y, Zhang Y, Xin M, et al: LncACTdb 3.0: An updated
database of experimentally supported ceRNA interactions and
personalized networks contributing to precision medicine. Nucleic
Acids Res. 50(D1): D183–D189. 2022.
|
|
32
|
Li T, Chen B, Yang P, Wang D, Du B and
Kang L: Long non-coding RNA derived from lncRNA-mRNA co-expression
networks modulates the locust phase change. Genomics Proteomics
Bioinformatics. 18:664–678. 2020.
|
|
33
|
Lin Y, Pan X and Shen HB: lncLocator 2.0:
A cell-line-specific subcellular localization predictor for long
non-coding RNAs with interpretable deep learning. Bioinformatics.
37:2308–2316. 2021.
|
|
34
|
Zhang X, Du L, Qiao Y, Zhang X, Zheng W,
Wu Q, Chen Y, Zhu G, Liu Y, Bian Z, et al: Ferroptosis is governed
by differential regulation of transcription in liver cancer. Redox
Biol. 24:1012112019.
|
|
35
|
Gao R, Buechel D, Kalathur RKR, Morini MF,
Coto-Llerena M, Ercan C, Piscuoglio S, Chen Q, Blumer T, Wang X, et
al: USP29-mediated HIF1α stabilization is associated with sorafenib
resistance of hepatocellular carcinoma cells by upregulating
glycolysis. Oncogenesis. 10:522021.
|
|
36
|
Karagkouni D, Paraskevopoulou MD,
Tastsoglou S, Skoufos G, Karavangeli A, Pierros V, Zacharopoulou E
and Hatzigeorgiou AG: DIANA-LncBase v3: Indexing experimentally
supported miRNA targets on non-coding transcripts. Nucleic Acids
Res. 48(D1): D101–D110. 2020.
|
|
37
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48(D1): D127–D131. 2020.
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
39
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014.
|
|
40
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
|
|
41
|
Kwon MY, Park E, Lee SJ and Chung SW: Heme
oxygenase-1 accelerates erastin-induced ferroptotic cell death.
Oncotarget. 6:24393–24403. 2015.
|
|
42
|
Tian Y, Lu J, Hao X, Li H, Zhang G, Liu X,
Li X, Zhao C, Kuang W, Chen D and Zhu M: FTH1 inhibits ferroptosis
through ferritinophagy in the 6-OHDA model of Parkinson's disease.
Neurotherapeutics. 17:1796–1812. 2020.
|
|
43
|
Dong H, Zhang C, Shi D, Xiao X, Chen X,
Zeng Y, Li X and Xie R: Ferroptosis related genes participate in
the pathogenesis of spinal cord injury via HIF-1 signaling pathway.
Brain Res Bull. 192:192–202. 2023.
|
|
44
|
Cao Y, Luo F, Peng J, Fang Z, Liu Q and
Zhou S: KMT2B-dependent RFK transcription activates the TNF-α/NOX2
pathway and enhances ferroptosis caused by myocardial
ischemia-reperfusion. J Mol Cell Cardiol. 173:75–91. 2022.
|
|
45
|
Liu H, Zhang B, Chen S, Zhang Y, Ye X, Wei
Y, Zhong G and Zhang L: Identification of ferroptosis-associated
genes exhibiting altered expression in response to cardiopulmonary
bypass during corrective surgery for pediatric tetralogy of fallot.
Sci Prog. 104:3685042110502752021.
|
|
46
|
Koyanagi A, Kotani H, Iida Y, Tanino R,
Kartika ID, Kishimoto K and Harada M: Protective roles of
cytoplasmic p21Cip1/Waf1 in senolysis and ferroptosis of
lung cancer cells. Cell Prolif. 55:e133262022.
|
|
47
|
Deng H, Lin Y, Gan F, Li B, Mou Z, Qin X,
He X and Meng Y: Prognostic model and immune infiltration of
ferroptosis subcluster-related modular genes in gastric cancer. J
Oncol. 2022:58135222022.
|
|
48
|
Jehl A, Conrad O, Burgy M, Foppolo S,
Vauchelles R, Ronzani C, Etienne-Selloum N, Chenard MP, Danic A,
Dourlhes T, et al: Blocking EREG/GPX4 sensitizes head and neck
cancer to cetuximab through ferroptosis induction. Cells.
12:7332023.
|
|
49
|
Ji HZ, Chen L, Ren M, Li S, Liu TY, Chen
HJ, Yu HH and Sun Y: CXCL8 promotes endothelial-to-mesenchymal
transition of endothelial cells and protects cells from
Erastin-induced ferroptosis via CXCR2-mediated activation of the
NF-κB signaling pathway. Pharmaceuticals (Basel). 16:12102023.
|
|
50
|
Sun K, Hou L, Guo Z, Wang G, Guo J, Xu J,
Zhang X and Guo F: JNK-JUN-NCOA4 axis contributes to chondrocyte
ferroptosis and aggravates osteoarthritis via ferritinophagy. Free
Radic Biol Med. 200:87–101. 2023.
|
|
51
|
Ko J, Jang S, Kwon W, Kim SY, Jang S, Kim
E, Ji YR, Park S, Kim MO, Choi SK, et al: Protective effect of GIP
against monosodium glutamate-induced ferroptosis in mouse
hippocampal HT-22 cells through the MAPK signaling pathway.
Antioxidants (Basel). 11:1892022.
|
|
52
|
Zhu L, Cao P, Yang S, Lin F and Wang J:
Prolonged exposure to environmental levels of microcystin-LR
triggers ferroptosis in brain via the activation of Erk/MAPK
signaling pathway. Ecotoxicol Environ Saf. 267:1156512023.
|
|
53
|
Wang W, Zhu L, Li H, Ren W, Zhuo R, Feng
C, He Y, Hu Y and Ye C: Alveolar macrophage-derived exosomal
tRF-22-8BWS7K092 activates Hippo signaling pathway to induce
ferroptosis in acute lung injury. Int Immunopharmacol.
107:1086902022.
|
|
54
|
Chen J, Chen Z, Yu D, Yan Y, Hao X, Zhang
M and Zhu T: Neuroprotective effect of hydrogen sulfide subchronic
treatment against TBI-induced ferroptosis and cognitive deficits
mediated through Wnt signaling pathway. Cell Mol Neurobiol.
43:4117–4140. 2023.
|
|
55
|
Liu L, Wang M, Gong N, Tian P and Deng H:
Se improves GPX4 expression and SOD activity to alleviate
heat-stress-induced ferroptosis-like death in goat mammary
epithelial cells. Anim Cells Syst (Seoul). 25:283–295. 2021.
|
|
56
|
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan
X and Wu C: Application of glutathione depletion in cancer therapy:
Enhanced ROS-based therapy, ferroptosis, and chemotherapy.
Biomaterials. 277:1211102021.
|
|
57
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017.
|
|
58
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021.
|
|
59
|
Qi L, Wang Y, Su S, Wang M, Jablonska E,
Jia Y, Wang R, Hao S, Feng C, Li G, et al: Sodium selenite inhibits
cervical cancer growth via ROS mediated AMPK/FOXO3a/GADD45a axis.
Chem Biol Interact. 367:1101712022.
|
|
60
|
Zhang Y, Luo M, Cui X, O'Connell D and
Yang Y: Long noncoding RNA NEAT1 promotes ferroptosis by modulating
the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ.
29:1850–1863. 2022.
|
|
61
|
Wang S, Chen J, Li P and Chen Y: LINC01133
can induce acquired ferroptosis resistance by enhancing the FSP1
mRNA stability through forming the LINC01133-FUS-FSP1 complex. Cell
Death Dis. 14:7672023.
|
|
62
|
Bhattacharjee S, Li J and Dashwood RH:
Emerging crosstalk between long non-coding RNAs and Nrf2 signaling.
Cancer Lett. 490:154–164. 2020.
|
|
63
|
Zhang L, Liu SK, Song L and Yao HR:
SP1-induced up-regulation of lncRNA LUCAT1 promotes proliferation,
migration and invasion of cervical cancer by sponging miR-181a.
Artif Cells Nanomed Biotechnol. 47:556–564. 2019.
|
|
64
|
Wang X, Guo S, Zhao R, Liu Y and Yang G:
STAT3-activated long non-coding RNA lung cancer associated
transcript 1 drives cell proliferation, migration, and invasion in
hepatoblastoma through regulation of the miR-301b/STAT3 axis. Hum
Gene Ther. 30:702–713. 2019.
|
|
65
|
Wang L, Tang D, Wu T and Sun F:
ELF1-mediated LUCAT1 promotes choroidal melanoma by modulating RBX1
expression. Cancer Med. 9:2160–2170. 2020.
|
|
66
|
Wang L, Xie Y, Wang J, Zhang Y, Liu S,
Zhan Y, Zhao Y, Li J, Li P and Wang C: Characterization of a novel
LUCAT1/miR-4316/VEGF-A axis in metastasis and glycolysis of lung
adenocarcinoma. Front Cell Dev Biol. 10:8335792022.
|
|
67
|
Sun Y, Jin SD, Zhu Q, Han L, Feng J, Lu
XY, Wang W, Wang F and Guo RH: Long non-coding RNA LUCAT1 is
associated with poor prognosis in human non-small lung cancer and
regulates cell proliferation via epigenetically repressing p21 and
p57 expression. Oncotarget. 8:28297–28311. 2017.
|
|
68
|
Gutierrez-Cruz JA, Maldonado V and
Melendez-Zajgla J: Regulation of the cancer stem phenotype by long
non-coding RNAs. Cells. 11:23522022.
|
|
69
|
Vierbuchen T, Agarwal S, Johnson JL, Galia
L, Lei X, Stein K, Olagnier D, Gaede KI, Herzmann C, Holm CK, et
al: The lncRNA LUCAT1 is elevated in inflammatory disease and
restrains inflammation by regulating the splicing and stability of
NR4A2. Proc Natl Acad Sci USA. 120:e22137151202023.
|
|
70
|
Tao Y, Liu Q, Wu R, Xiao C, Ni C, Wang K,
Hu W, Zhong Z, Zhao J, Li Q, et al: Long noncoding RNA LUCAT1
enhances the survival and therapeutic effects of mesenchymal
stromal cells post-myocardial infarction. Mol Ther Nucleic Acids.
27:412–426. 2021.
|
|
71
|
Zhang K, Wang Q, Zhong B and Gong Z:
LUCAT1 as an oncogene in tongue squamous cell carcinoma by
targeting miR-375 expression. J Cell Mol Med. 25:4543–4550.
2021.
|
|
72
|
Nai Y, Pan C, Hu X and Ma Y: LncRNA LUCAT1
contributes to cell proliferation and migration in human pancreatic
ductal adenocarcinoma via sponging miR-539. Cancer Med. 9:757–767.
2020.
|
|
73
|
Liu Z, Gao H, Peng Q and Yang Y: Long
noncoding RNA LUCAT1 promotes multiple myeloma cell growth by
regulating the TGF-β signaling pathway. Technol Cancer Res Treat.
19:15330338209457702020.
|
|
74
|
Mou E and Wang H: LncRNA LUCAT1
facilitates tumorigenesis and metastasis of triple-negative breast
cancer through modulating miR-5702. Biosci Rep.
39:BSR201904892019.
|
|
75
|
Luzón-Toro B, Fernández RM,
Martos-Martínez JM, Rubio-Manzanares-Dorado M, Antiñolo G and
Borrego S: LncRNA LUCAT1 as a novel prognostic biomarker for
patients with papillary thyroid cancer. Sci Rep. 9:143742019.
|
|
76
|
Shen Q, Xu Z and Xu S: Long non-coding RNA
LUCAT1 contributes to cisplatin resistance by regulating the
miR-514a-3p/ULK1 axis in human non-small cell lung cancer. Int J
Oncol. 57:967–979. 2020.
|
|
77
|
Han Z and Shi L: Long non-coding RNA
LUCAT1 modulates methotrexate resistance in osteosarcoma via
miR-200c/ABCB1 axis. Biochem Biophys Res Commun. 495:947–953.
2018.
|
|
78
|
Cronin SJF, Rao S, Tejada MA, Turnes BL,
Licht-Mayer S, Omura T, Brenneis C, Jacobs E, Barrett L,
Latremoliere A, et al: Phenotypic drug screen uncovers the
metabolic GCH1/BH4 pathway as key regulator of EGFR/KRAS-mediated
neuropathic pain and lung cancer. Sci Transl Med.
14:eabj15312022.
|
|
79
|
Sang L, Yang L, Ge Q, Xie S, Zhou T and
Lin A: Subcellular distribution, localization, and function of
noncoding RNAs. Wiley Interdiscip Rev RNA. 13:e17292022.
|
|
80
|
Li W, Pan T, Jiang W and Zhao H:
HCG18/miR-34a-5p/HMMR axis accelerates the progression of lung
adenocarcinoma. Biomed Pharmacother. 129:1102172020.
|
|
81
|
Hao R, Ge J, Song X, Li F, Sun-Waterhouse
D and Li D: Cadmium induces ferroptosis and apoptosis by modulating
miR-34a-5p/Sirt1axis in PC12 cells. Environ Toxicol. 37:41–51.
2022.
|