Cancer poses a significant global health challenge
as the second leading cause of death after cardiovascular disease.
Extensive studies have been carried out over the years on cancer
remedies, encompassing traditional interventions such as
chemotherapy, radiotherapy and surgical procedures (1). Considering the elevated rate of
occurrence and distribution, there is a pressing demand for
substitute treatment options with improved efficacy and safety
profiles compared with the aforementioned interventions (1). Hence, there is a requirement for
comprehensive research to improved understand the pathways involved
in cancer formation and growth. The tumor microenvironment (TME) is
integral to the development of cancer by enabling the spread of
information that supports the growth of tumor cells, formation of
new blood vessels (angiogenesis) and dissemination to distant
sites. Communication between cancer cells and surrounding tissues
results in changes to the local environment that support tumor
progression, immune evasion and invasion (2). Furthermore, interactions with
distally located non-cancerous tissues contribute to the
establishment of new habitats for cancer cells to thrive and aid in
the spread of the disease (2).
Endothelial cells (ECs) are specialized cells lining
both large and small blood vessels throughout the body. They serve
an important role in regulating coagulation, inflammation and blood
pressure, as well as the development of new blood vessels. This
role is indispensable for their function in the supply of oxygen
and nutrients, as well as the removal of metabolic wastes from the
internal organs. The endothelial lining primarily acts as a barrier
between the blood and the surrounding tissue, while permitting
limited exchange of cellular and molecular substances. Disruption
of this barrier function can lead to the promotion of blood clot
formation (3). Therefore, the
growth of new blood vessels is vital for the development of
embryonic organs, tissue repair and wound healing (3). Angiogenesis, which is the creation
of new capillaries, involves numerous physiological and
pathological processes. This intricate and well-organized process
culminates in the development of fully operational blood vessels
(4). Studies have examined the
significance of ECs in the process of angiogenesis, which plays a
major role in the advancement of tumors (5,6).
In addition to their involvement in angiogenesis through
proliferation, migration and adhesion, vascular endothelial cells
(VECs) can undergo a process similar to epithelial-to-mesenchymal
transition (EMT). VECs have the ability to transition their
phenotype to exhibit mesenchymal characteristics, which in turn
affects tumor advancement (7).
This mechanism is termed endothelial-to-mesenchymal transition
(EndoMT), affecting the advancement of tumors in various manners.
Moreover, exosomes have the capacity to affect tumor progression by
initiating both EMT and EndoMT. Along with the endothelial
compartment, cancer-associated fibroblasts (CAFs) are the most
prevalent stromal cells in the TME and serve a crucial role in
advancing tumors. Tumor cells engage with one another to create a
myofibroblastic microenvironment that enhances tumor development
and viability and sustains malignancy (8). Consequently, CAFs affect both the
arrangement and growth characteristics of tumors. CAFs can
originate from normal fibroblasts and epithelial cells (8); they can also originate from VECs
through the EndoMT process (9).
Exosomes (diameter: 40-160 nm) function as important
vehicles for intercellular communication within both nearby and
far-reaching cells. They act as valuable indicators for diagnosing
and predicting diseases (10).
Exosomes are notably involved in the spread of tumors, are present
in high quantities in bodily fluids and help maintain the stability
of the biomarkers they transport. As a result, they enhance the
accuracy of cancer detection, monitoring of treatments and
forecasting of cancer outcomes (10). They are secreted into the
extracellular space by ECs and various other cells, such as
platelets, immune cells, smooth muscle cells and cancer cells
(10-12). The continuous exchange of
bioactive molecules within exosomes takes place extracellularly,
enabling intercellular communication in both healthy and diseased
conditions (13). The interaction
between tumors and exosomes results in alteration of the TME,
thereby promoting tumor proliferation, resilience, evasion of the
immune system and infiltration (14). Exosomes, an important element of
the TME, have been extensively studied in recent decades and have
demonstrated distinct roles in tumor development, advancement,
spread, vascularization and resistance to drugs (15). Furthermore, distinctive genetic
characteristics passed down from the initial tumor cells provide
tumor-derived exosomes (TEXs) with the capacity to anticipate tumor
advancement and outlook. They can be modified as therapeutic
carriers to transport a range of small compounds; mRNAs, non-coding
RNAs (ncRNAs), DNAs, proteins, or anticancer drugs and convey them
to target cells. Exosomes exhibit considerable promise in cancer
treatment (16,17). They serve as potential preclinical
biomarkers for a variety of types of cancer (such as lung,
hepatocellular, pancreatic, colorectal, melanoma, breast, prostate,
ovarian, glioblastoma and nasopharyngeal) (18-24). Studies have demonstrated that
disrupting the production, release, or absorption of exosomes
originating from tumors shows great potential for advancing the
field of cancer treatment (25,26).
The aim of the present review was to elucidate the
crosstalk between vascular endothelium and exosomes in tumor
progression, focusing on the roles of EndoMT and exosomes in
tumorigenesis, metastasis, tumor drug resistance and the TME as
well as their applications in tumor therapy and diagnostic
prognosis. These advances will help to realize clinical
applications based on vascular endothelium and exosomes in
cancer.
The TME is a critical area of study in cancer
research. Cancer cells reside alongside stromal cells in a complex
environment, relying on interactions with various components of the
TME for their growth and spread. In tumor tissues, ECs are mainly
divided into lymphatic endothelial cells (LECs) and VECs, including
arterial endothelial cells (ArtECs), venous endothelial cells
(VenECs) and capillary-like endothelial cells (CapECs). LECs
exhibit two different differentiation lineages: One is responsible
for lymphangiogenesis and the other is involved in antigen
presentation (27). VECs serve an
essential role in tumor angiogenesis and the provision of blood
supply, which are vital processes for the progression of solid
tumors. As a result, targeting angiogenic factors and their
receptors through anti-angiogenic therapy has become a significant
approach in the treatment of tumors (27-30).
To support the rapid growth of tumors, cancer cells
induce the development of new lymphatic and blood vessels from
existing ones to satisfy their high oxygen and nutrient
requirements. Previous research has shown that blood and lymphatic
vessels associated with tumors serve a crucial role in aiding
immune evasion and disrupting immune surveillance by T cells. The
tumor vasculature contributes to the TME by promoting conditions
favorable to cancer cell survival, such as hypoxia, acidity and
high interstitial pressure, while also acting as a physical barrier
to T cell penetration (31). The
ECs that compose the tumor vasculature actively impede the
recruitment, attachment and function of T cells (31). Previous research has recognized
diverse elements of the TME that affect malignant behavior and
advancement (32). Alongside
malignant cells, the TME is comprised of adipocytes, fibroblasts,
immune cells, dendritic cells, CAFs and ECs of the blood and
lymphatic vessels. Each of these cellular categories possesses a
distinct immune capability, which can dictate the survival of the
tumor and its effect on surrounding cells (32,33).
In the TME, cancer cells engage in constant
interactions with various elements through the secretion of soluble
factors (such as cytokines, chemokines, growth factors and enzymes
for matrix remodeling) or by direct communication with stromal
cells. This leads to chronic inflammation, immune suppression and a
microenvironment that supports angiogenesis, facilitating cancer
cell metastasis and hindering the effectiveness of treatments, such
as immunotherapy. Of note, this interaction is bidirectional, with
TME matrix components effectively influencing cancer cell behavior
throughout all stages of cancer progression. For instance, research
indicates that the immune system can eliminate early-stage tumors
through immunosurveillance (34).
Nevertheless, cancer cells can later evade immune detection and
even exploit the immune system to support tumor growth (34). The development of an immune escape
cancer cell phenotype involves various mechanisms, which include
reduced immunogenicity from the loss of tumor-associated antigens
or major histocompatibility complex class I molecules, changes in
DNA copy numbers and oncogenic signaling, along with heightened
expression of immune checkpoint proteins, such as programmed
death-ligand 1 (PD-L1), indoleamine 2,3-dioxygenase and tryptophan
2,3-dioxygenase (TDO), as well as shifts in metabolism leading to
lowered pH levels and increased immunogenicity of TDO. This results
in alterations in the immune response, promoting immune evasion in
cancer cells (35). Furthermore,
cancer cells manipulate the polarization, activity and expansion of
different immune cell subpopulations by enhancing the production of
immunosuppressive and pro-tumorigenic cytokines (31). They also interact with ECs,
causing changes in their structural integrity and functional
properties, ultimately dampening antitumor immune responses
(31).
Small solid tumors trigger tumor angiogenesis to
meet the high demands for energy and essential nutrients (such as
oxygen and glucose) required for growth. This process involves the
creation of new blood vessels within an existing vascular network
(36). A recent study has shown
that angiogenesis can occur at any stage of tumor progression and
it involves the development of neovascularization from established
vascular beds, with tumor tissue infiltrating more CapECs than
VenECs and ArtECs compared with adjacent non-tumor tissue. The
tumor angiogenic trajectory is a differential trajectory from
VenECs to ArtECs passing through CapECs; tumor angiogenesis starts
from VenECs (27).
Pathological angiogenesis results mainly from an
imbalance in pro- and anti-angiogenic signaling within the TME.
Primary factors promoting angiogenesis include vascular endothelial
growth factor-A (VEGF-A), basic fibroblast growth factor and
interleukin-8 (IL-8), among others. These cytokines are prevalent
in the TME and counteract vaso-suppressive signals, promoting the
activation of pro-angiogenic pathways by inhibiting vasopressor and
endothelial suppressor effects (37). Furthermore, hypoxia plays a key
role in tumor angiogenesis (27).
Cancer cells are able to release significant quantities of VEGF,
leading to the stimulation of VEGF-independent blood vessel
formation. This is achieved through the secretion of various
pro-angiogenic molecules, such as placental growth factor (PGF),
VEGF-C, VEGF-D and platelet-derived growth factor-C (PDGF-C).
Additionally, cancer cells exhibit a response to survival-promoting
and metastasis-promoting VEGF cues in an autocrine or paracrine
manner. This response is triggered by pro-survival and
pro-metastatic VEGF signaling (36). Controversially, placental growth
factor (PIGF) is a member of the VEGF family and has been reported
to have both pro- and anti-angiogenic properties. In preclinical
tumor models, the efficacy of anti-PlGF therapy in inhibiting
angiogenesis and stopping tumor growth remains a conflicting result
(38).
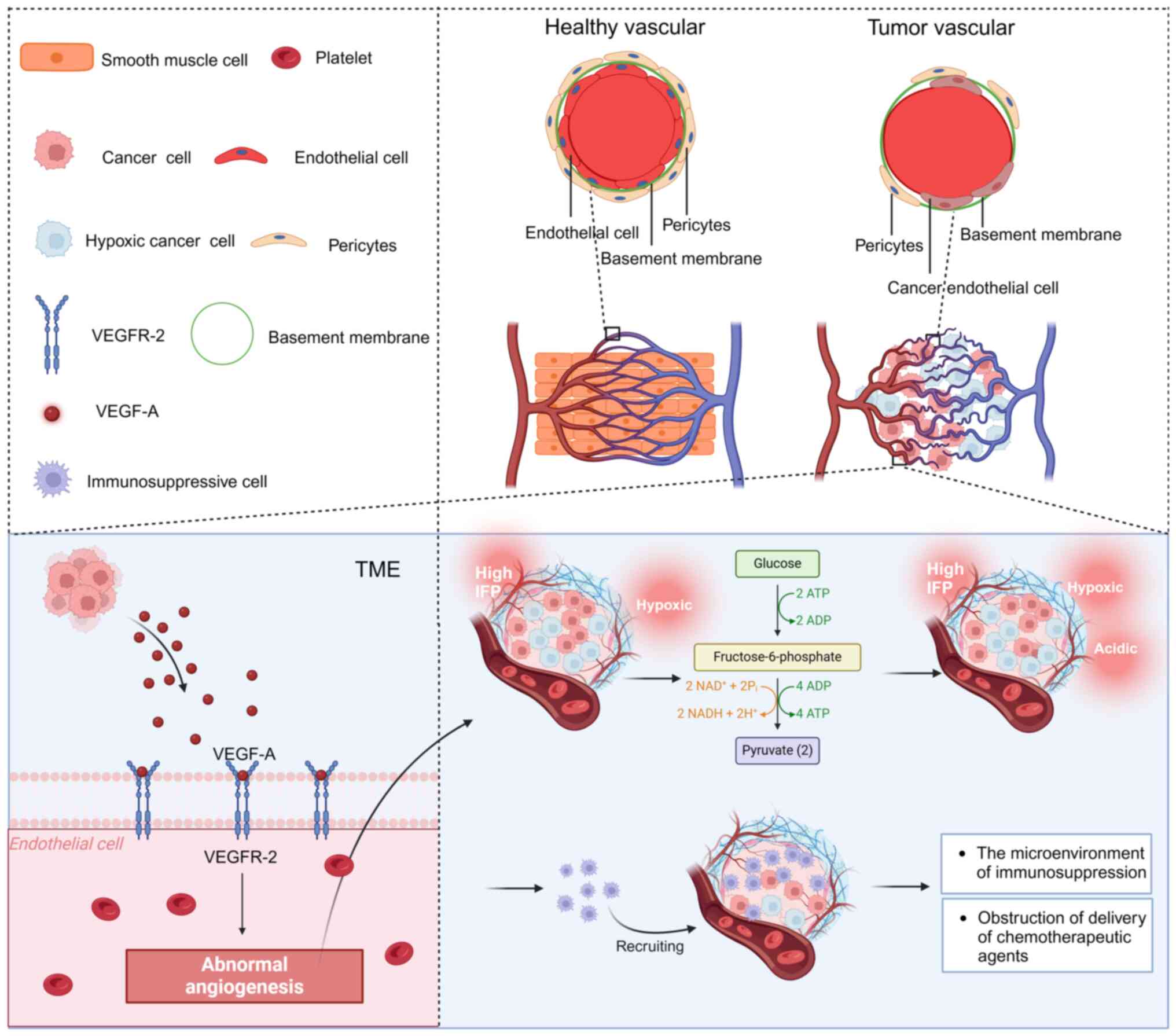
The present study presents the process of
angiogenesis stimulated by the critical pro-angiogenic factor VEGF.
Despite its involvement in sustaining the blood supply in tumors,
tumor angiogenesis results in an unstable, disordered and
underdeveloped vascular structure, characterized by poor
circulation. This significantly atypical angiogenesis plays a role
in upholding the tumor's tumorigenic and immune-suppressing TME and
has a profound effect on the cancer cells' evasion of immune
monitoring, metastasis and response to immunotherapy (39). The disorderly vascular arrangement
leads to inconsistent and reduced blood flow, linked to the
immature, unstable, leaking and curved nature of the blood vessels.
Moreover, the high interstitial fluid pressure (IFP) within the
tumor, caused by vascular leakage, renders these vessels prone to
collapse, thereby narrowing the perfused tumor region (Fig. 1) (39).
The hypoxic and acidic TME resulting from elevated
anaerobic glycolysis in cancer cells enhances the development of
genetically and epigenetically altered tumor cells, thus increasing
their invasive properties. Importantly, hypoxia and acidosis
facilitate the attraction and attachment of immune-suppressing
cells, diminish the antitumor effectiveness of infiltrating T cells
and hinder the distribution of therapeutic drugs and immune-based
treatments, thereby impeding the elimination of cancer cells
through radiotherapy, chemotherapy and immunotherapy (40).
Targeting the VEGF/vascular endothelial growth
factor receptor (VEGF/VEGFR) pathway has been the most commonly
used combination strategy in studies related to immunotherapy.
Initially, treatment with anti-angiogenic drugs, such as the
anti-VEGF antibody bevacizumab, was employed to prolong patient
survival by obstructing VEGF/VEGFR-driven blood vessel formation,
thereby halting tumor advancement (41). Despite encouraging preliminary
findings in preclinical trials, this vascular-directed therapy,
referred to as vascular obstruction, did not produce the expected
outcomes in patients with cancer. Subsequent preclinical
investigations indicated that blocking angiogenesis resulted in an
augmentation of hypoxic regions within tumors, ultimately promoting
tumor expansion and metastatic spread (42). Certainly, hypoxia induced by
anti-VEGF/VEGFR therapy could contribute to the angiogenic relapse
and drug resistance observed following vascular blockade
approaches. This could potentially involve various
immunosuppressive cell types, such as
Gr1+CD11b+ myeloid-derived suppressor cells
(MDSCs) and tumor-associated macrophages (43). The cancer cell-derived vasopressor
chemokine C-X-C motif chemokine ligand 14 recruits myeloid cells
and subsequently stimulates phosphoinositide-3-kinase (PI3K)
signaling in these cells. Therefore, blocking this pathway is
essential for the sustained benefits of anti-angiogenic treatment
(44).
Enhancing the function of blood vessels through
vascular normalization enhances tumor blood flow and optimizes the
delivery of medication (including small amounts of chemotherapy
drugs and monoclonal antibodies), ultimately boosting the
effectiveness of treatment (45,46). This increase in vascular transport
capability is heavily reliant on sufficient blood circulation
within the tumor (47). Other
approaches, aside from blocking the VEGF/VEGFR pathway, have
demonstrated the ability to promote vascular normalization
(48,49). For instance, chloroquine (CQ), a
first-generation autophagy inhibitor, induces vascular
normalization in vivo through activation of the Notch
signaling pathway in ECs within the blood (50). CQ decreases tumor growth and
enhances the TME (51). CQ
normalizes the vascular structure and function of tumors, leading
to improved blood flow. This normalization results in reduced
hypoxia, decreased cancer cell invasion and metastasis and enhanced
delivery and effectiveness of chemotherapy (51). Research conducted on animals
indicates that even with its minimal impact on the immune system,
CQ does not hinder the ability of the body to fight against tumors
and may enhance the effectiveness of immunotherapy (52).
Angiogenesis inhibitors are used in cancer therapy
to affect the formation of new blood vessels in tumors, which opens
up new frontiers for the treatment of a wide range of solid tumors.
However, due to the complexity of tumor angiogenesis and limited
research, anti-angiogenic therapy still has some limitations
(53). Examples include lack of
therapeutic efficacy, drug resistance and prevalence of treatment
modalities (53).
It is important to note that excessive restriction
of angiogenesis affects drug transport and exacerbates the
pathological manifestations of TME. This induces a stronger hypoxic
response and tumor aggressiveness, which ultimately leads to drug
resistance and even cancer metastasis (54).
Drug resistance has been limiting the clinical
outcomes of targeted antiangiogenic therapy (55). This can involve a high degree of
heterogeneity of the TME, endothelial heterogeneity, tumor cell
autophagy, cancer stem cell (CSC) differentiation, stromal cell
infiltration, tumor type, genetic mutations in the tumor or target,
stage of tumor progression, the patient's medication history and
other factors, all of which can affect a patient's response to, and
tolerance of, antitumor therapy (56-58).
In addition, there is a lack of effective biomarkers
characterizing changes in the vasculature and TME to prevent tumor
escape and monitor patient response to drugs and treatment
progression (59,60). Exploration of effective
cancer-specific biomarkers responsive to the angiogenic system to
enhance anti-angiogenic efficacy is a pressing concern. Indeed, the
development of biomarkers will be a great challenge due to a number
of factors such as the complexity of tumor angiogenesis, tumor
heterogeneity and variability and limitations of preclinical and
clinical trials.
Combination therapies based on antiangiogenic drugs
have been used in the antitumor field since the approval of the
first angiogenesis inhibitor, bevacizumab, for the treatment
(61). In recent years,
combination therapy with angiogenesis inhibitors and immune
checkpoint inhibitors has attracted considerable research
attention. Treatment of patients with hepatocellular carcinoma
(HCC) and patients with renal carcinoma using a combination of
programmed cell death 1 (PD1) and VEGFR2 inhibitors yielded
improved results compared with monotherapy (62-64). If immunotherapy is accompanied by
anti-angiogenic drugs targeting the VEGF pathway, it can neutralize
excess VEGF while reversing VEGF-induced immunosuppression and
enhancing the immune function of patients, rebuild the vascular
system of the tumor tissue, normalize the vascular network, inhibit
excessive angiogenesis and limit tumor growth, invasion and
metastasis (65). However, the
effectiveness and toxicity of this combination therapy need to be
optimized by further studies on the dose, duration and sequence of
treatment in different patients (66,67).
In tumor angiogenesis, various angiogenic tyrosine
kinases act synergistically to induce a series of intracellular
signaling cascades rather than working individually (68). The development of novel selective
multi-targeted kinase inhibitors can effectively avoid the adverse
events of broad-spectrum inhibitors, exert multiple anti-angiogenic
effects, avoid drug-drug interactions and develop a more stable
pharmacokinetic profile (69).
Hyperactivation of pro-angiogenic factors and
over-inhibition of anti-angiogenic mediators contribute to abnormal
angiogenesis in tumor tissues (53). Therefore, endogenous
anti-angiogenic components or their derivatives may be beneficial
for vascular normalization and therapeutic efficacy. For example,
endostatin can compete with fibroblast growth factor to limit VEC
proliferation and tumor development. Recombinant human endostatin
is a non-cytotoxic angiogenesis inhibitor approved by the Chinese
Food and Drug Administration for the treatment of various cancers,
including non-small cell lung cancer (70,71).
Moreover, studies have demonstrated the significant
effect of small ncRNAs termed micro-RNAs (miRNAs) on the process of
angiogenesis. These molecules serve a crucial role in modulating
gene expression via RNA interference mechanisms (72,73). For example, hypoxia induces
pro-angiogenic miRNAs (including miR-210 and miR-494) (74). Specific miRNAs such as miR-16,
which also disrupts the transforming growth factor-beta (TGF-β)
pathway, affect the pathway of VEGF and VEGFR and manage the
process of angiogenesis (75).
miR-494 enhances the migration of VECs and stimulates angiogenesis
by inhibiting phosphatase and tensin homolog (PTEN), leading to
activation of the protein kinase B (AKT)/endothelial nitric oxide
synthase pathway (76).
The present study also discussed the mechanisms
through which TEXs, including but not limited to miRNAs, can alter
the state of VECs and, thus, affect tumor growth. Since
tumor-associated conditions can promote miRNA expression to support
a highly angiogenic TME, miRNAs can be considered potential targets
for anti-angiogenic/vascular normalization approaches. However,
further studies are needed to improve understanding of the
mechanism by which specific targeting of miRNAs can enhance the
efficacy of immunotherapy. Further research is warranted to
investigate whether exosomes can be used as carriers of miRNAs and
good drug delivery vehicles for exosome-mediated targeting of
vascular endothelial-specific molecules to improve tumor
therapeutic responses. Moreover, it is important to determine
whether tumor vascular normalization strategies are the optimal
therapeutic options for improving T-cell function and
immunotherapy. Such research advances will broaden our knowledge on
the role of endothelial function and dysfunction, increase
understanding and control of the TME and facilitate the
optimization of anti-angiogenic and vascular modification therapies
for cancer and other diseases.
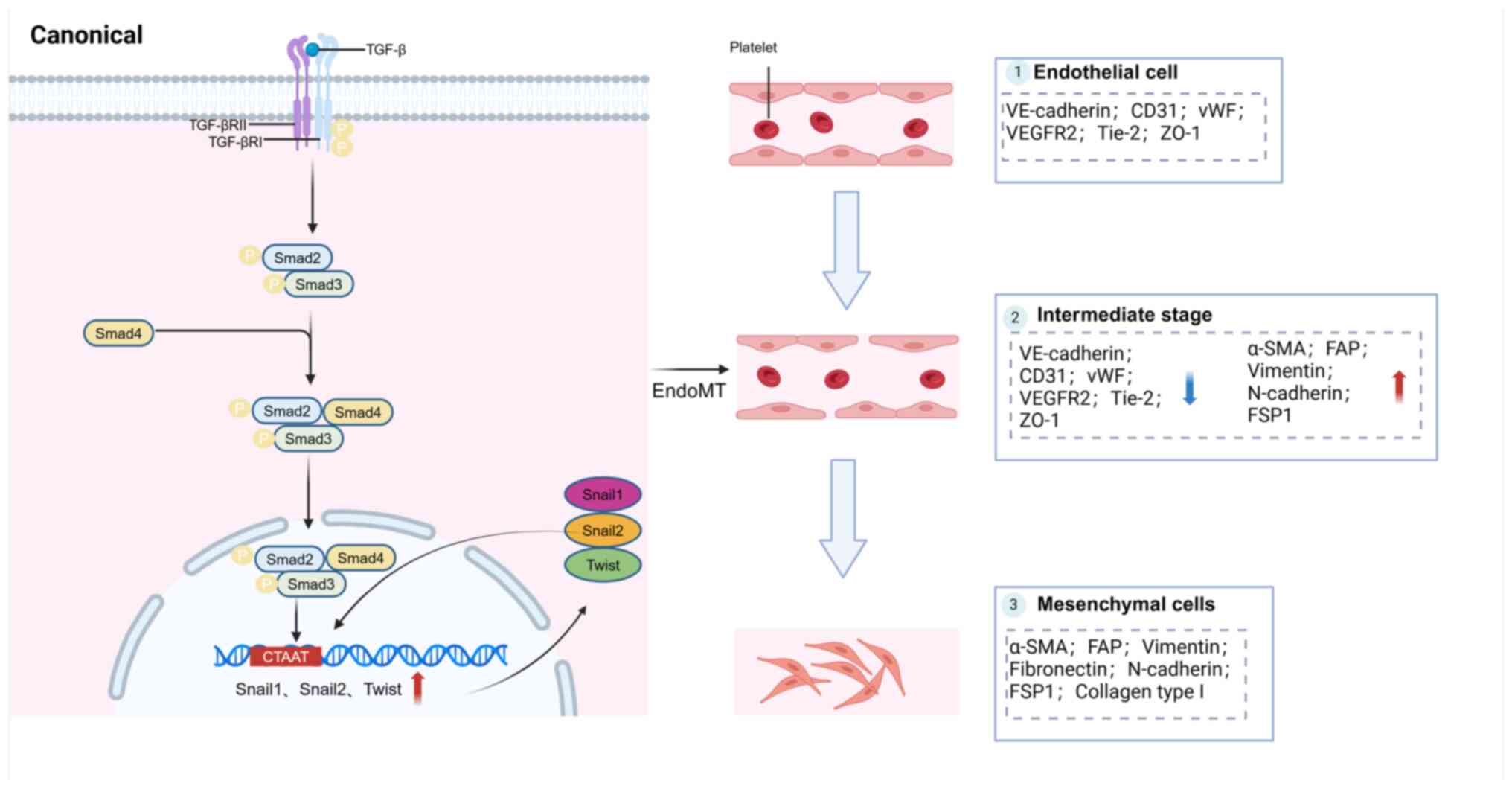
EndoMT is a dynamic process in which VECs
transition, thereby abolishing their distinctive characteristics
and gaining mesenchymal properties (77). Typical characteristics include
morphological transformation of VECs, altered gene expression and
functional changes. Morphological transformation refers to the
change from an ovoid cell shape to an elongated, spindle-shape.
Changes in gene expression involve a decrease in specific protein
markers, such as von Willebrand factor, platelet endothelial cell
adhesion molecule-1, vascular endothelial cadherin, VEGFR2, Tie2
and zonula occludens-1. There is also an increase in the expression
of mesenchymal proteins, such as alpha-smooth muscle actin,
fibroblast activation protein, vimentin, fibronectin, N-cadherin,
fibroblast-specific protein 1 and collagen type I. Functional
changes include the loss of intercellular connections, heightened
vascular permeability, decrease in vasculature formation and the
acquisition of migratory, invasive and contractile qualities
(78). Some of these protein
markers are consistent with EMT (Table I) (79).
Research studies show that TGF-β is significantly
involved in the development and advancement of EndoMT (80,81). The Smad2/3-mediated pathways are
considered classic and powerful pathways in cellular signaling. In
this process, homodimers of activated TGF-β bind to heterodimeric
TGF-β receptor complexes on the cell surface, initiating the
activation of the TGF-β pathway. Interaction between activated
TGF-β molecules and their respective cell surface receptors induces
a series of intricate molecular reactions. Smad proteins, which
belong to a diverse family of intracellular proteins, serve a
crucial role in mediating this cascade of events in conventional
TGF-β signaling (82). They
transduce signals triggered by TGF-β cell surface binding from the
cell membrane to the nucleus (Fig.
2) (83). The TGF-β receptor
initiates a signaling pathway involving two transmembrane serine
threonine kinases; TGF-β receptor types I and II. Upon binding of
TGF-β, the receptor complex undergoes autophosphorylation of TGF-β
receptor type II, followed by transphosphorylation of specific
residues in TGF-β receptor I to form an active complex. This leads
to phosphorylation of serine residues in Smad2 and Smad3 proteins,
resulting in their activation. Thereafter, the phosphorylated
Smad2/Smad3 complex interacts with Smad4 and translocates to the
nucleus. Within the nucleus, the Smad2/Smad3/Smad4 complex binds to
Smad binding elements of TGF-β response genes, thereby promoting
their transcription. This mechanism involves the binding of Smad
proteins to specific sites in the promoter regions of target genes,
facilitating gene expression in response to TGF-β signaling
(78).
Of note, tumor cells release extracellular vesicles
(EVs) that also serve a role in regulating EndoMT. Yeon et
al (84) reported that
exosomes derived from both breast cancer cells and mouse melanoma
cells triggered EndoMT in VECs. Moreover, Yamada et al
(85) demonstrate that colon
cancer cell-derived conditioned medium contains EVs capable of
inducing EndoMT in human umbilical vein endothelial cells (HUVECs).
This process may be facilitated by miR-92a-3p. In addition, EVs may
regulate the EndoMT process in pre-metastatic ecological niches
(86). Additional clarification
concerning the effector molecules found in EVs that trigger EndoMT
is imperative to enhance our comprehension of the biological
activities of EVs in the advancement of tumors. Moreover, this
knowledge will be useful in addressing illnesses facilitated by the
interaction between EVs and vascular VECs.
The high occurrence rate of EndoMT across various
types of cancer in humans and the wide array of tumor-related
factors that trigger EndoMT imply that EndoMT could be a major
event in the advancement of tumors and have a significant function
in disease progression. VECs that undergo EndoMT hasten tumor
growth through the enhancement of tumor cell growth, viability and
vascularization. Furthermore, this process may facilitate tumor
spread by affecting numerous essential processes, such as the
transition from epithelial to mesenchymal states, movement,
infiltration, internalization and externalization of tumor cells.
Moreover, EndoMT may facilitate tumor evasion from the immune
system and resistance to treatments (87).
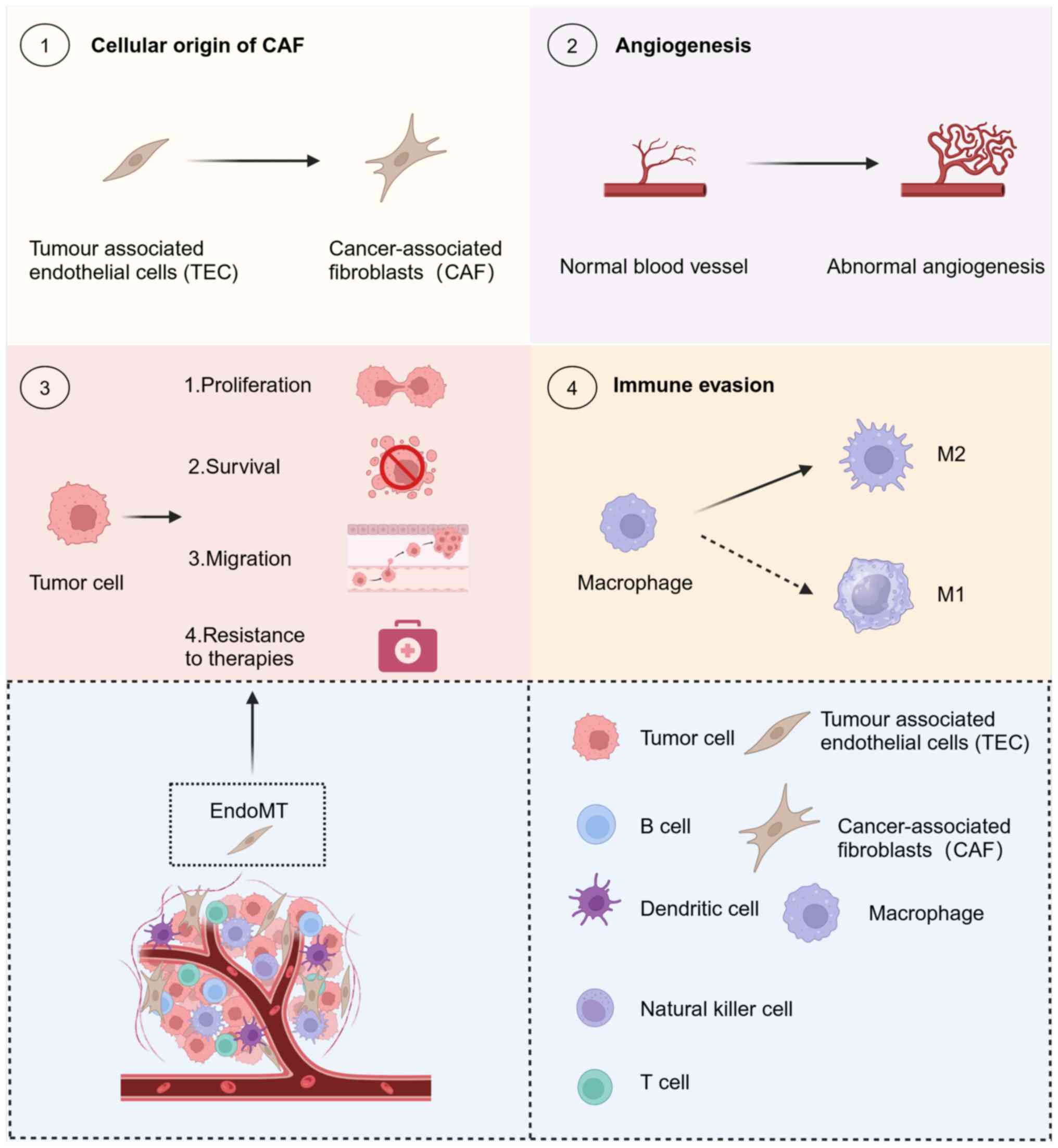
CAFs are important stromal cells in the TME,
controlling tumor development, spread, immune system suppression
and resistance to medication (88-90). They originate from a range of cell
types, primarily pericytes, cells of the blood vessel walls, VECs
undergoing EndoMT, cancer cells undergoing EMT, fibroblasts
remaining in tissues (such as hepatic stellate cells) and cells
derived from the bone marrow [such as mesenchymal stromal cells
(MSCs)] (91). EndoMT is involved
in various processes, making significant contributions. For
instance, >50 and 40% of CAFs stem from EndoMT in gliomas and
melanomas, respectively (9,92).
Improved insight into the heterogeneity of CAFs can be achieved by
investigating the involvement of EndoMT in promoting CAF
differentiation and its influence on tumor advancement.
Continuous angiogenesis is a common characteristic
of cancerous growth and a focal point of studies on therapies
targeting tumors. The mesenchymal transition of VECs can lead to
partial loss of their blood vessel-forming capabilities, while they
retain or acquire the capacity to produce and release VEGF,
stimulating angiogenesis through signaling pathways (93). This process affects the efficacy
of anti-angiogenic therapy (94).
Identification of the factors that support tumor
cell growth and targeted interventions could hinder tumor
development. EndoMT can enhance tumor cell proliferation, thus
hastening tumor growth in HCC and breast cancer (95,96). The exact molecular mechanism
underlying this facilitation process needs to be further
elucidated.
Tumor cells exhibit increased resistance to hypoxia,
starvation and other severe conditions caused by tumor expansion
and medical treatments. Importantly, EndoMT may be a critical
factor in this mechanism. Breast cancer cells cultured alongside
VECs undergoing EndoMT demonstrated improved resilience to
starvation and higher rates of survival (96). This suggests that inhibition of
EndoMT reduces tumor cell survival and may provide new avenues for
tumor therapy.
The process of tumor metastasis is complex and
consists of multiple steps, starting with the invasion and
migration of cancer cells from the original tumor location. This is
followed by infiltration in the living system, exit from the
circulation and vascular network and ultimately establishment and
expansion at the secondary site of metastasis (97). EndoMT affects tumor metastasis at
the original location and exerts a significant effect on the
secondary site. This enhances the EMT, as well as the migration and
infiltration of tumor cells at the primary tumor location. The
enhancement of vascular permeability may also benefit tumor cell
internalization. Similarly, EndoMT at the metastatic site aids in
the extrication of tumor cells and can potentially contribute to
the establishment of a pre-metastatic microenvironment.
Nevertheless, there has been limited research on
EndoMT occurring at the metastatic site. The presence of EndoMT has
not been identified in metastatic tumor tissues of human origin.
Using a live mouse model of breast cancer, Smeda et al
(100) demonstrate that EndoMT
within the lung endothelium coincides with a rise in vascular
permeability at the onset of metastasis. According to research
findings, human melanoma cells that have metastasized to the brain
triggered EndoMT in brain VECs. This process results in the
disruption of the monolayer's integrity, which is confirmed by a
decrease in transendothelial electrical resistance. Additionally,
there is an increase in metastatic cell adhesion to the endothelial
layer, along with a heightened melanoma cell transendothelial
migration (101). Notably, Kim
et al (86) reveal that
EVs discharged by breast carcinoma cells prompted EndoMT in VECs of
the liver sinusoids. The results indicate that cancer cells have a
tendency to trigger EndoMT at the location of metastasis. This may
contribute to the creation of pre-metastatic microenvironments
aiding in cancer cell extravasation and, therefore, supporting
tumor spread. Detecting EndoMT in metastatic human tumor samples
and investigating whether this process precedes or follows tumor
spread can enhance our comprehension of the involvement of EndoMT
in tumor metastasis.
Chemotherapy, radiotherapy and targeted therapy have
shown effectiveness in treating advanced tumors. Nonetheless, the
process of EndoMT can lead to tumor cell resistance to these
treatments. Recent research conducted by Huang et al
(92) demonstrated that
suppressing EndoMT markedly increases the responsiveness of glioma
cells to treatment with temozolomide. According to Choi et
al (102), the use of
radiotherapy triggers EndoMT in VECs, leading to a decrease in the
effectiveness of this treatment. Furthermore, the expression of
VEGFR2 was diminished in VECs that underwent EndoMT, rendering them
impervious to anti-VEGF treatment in cases of glioblastoma
(94). Focusing on tumor
treatment-related EndoMT and implementing targeted interventions
can improve treatment outcomes.
The immune surveillance function of the immune
system is critical for identifying and removing abnormal elements
in the body, such as tumor cells resulting from genetic mutations.
Failure of immune surveillance can result in the growth and
advancement of tumors. Previous studies have identified numerous
factors contributing to the induction of tumor immunosuppression
(103-107). Notably, EndoMT can promote tumor
immune escape by inducing macrophage M2 polarization (108). In the TME, tumor-infiltrating
lymphocytes (TILs) serve a vital role as stromal cells and are
significantly linked to tumor immunomodulation (109). EndoMT-induced irregularities in
the development, blood vessel formation, blood flow and openness of
CAFs can directly or indirectly impact the entry and performance of
TILs (110,111) (Fig. 3). Additional research focusing on
the interactions between EndoMT and TILs might broaden the
scientific knowledge in this area.
To summarize, the vascular endothelium plays a major
role within the TME. Throughout tumor growth and metastasis, VECs
are responsible for forming the structural framework of the tumor
vascular network and for regulating both the normal and abnormal
processes within the TME. Initially, VECs affect the blood flow to
tumors by controlling the permeability and hemodynamic
characteristics of blood vessels. Within tumor tissues, these cells
are able to control the permeability of blood vessel walls, thus
facilitating the transportation of necessary nutrients and oxygen
to fuel tumor growth. Additionally, VECs are involved in modulating
the immune response in the TME. By secreting cell adhesion
molecules, they attract immune and inflammation-related cells to
the tumor, influencing tumor growth and metastasis.
Furthermore, these cells are involved in managing
tumor neovascularization and angiogenesis. As tumors develop, they
release various factors that stimulate the proliferation and
movement of nearby VECs, leading to the creation of a new vascular
network to sustain tumor growth. The diverse population of VECs
that undergo EndoMT promotes tumor progression, metastasis, immune
suppression and treatment resistance through various mechanisms.
Understanding the precise molecular pathways involved in these
processes will guide targeted therapies for EndoMT (87).
In the TME, the vascular endothelium plays a complex
and diverse role. This role is intricately linked to tumor growth,
metastasis and drug resistance, as well as various aspects of tumor
blood supply and angiogenesis. Further investigation into the
mechanisms underlying the function of the vascular endothelium
within the TME may lead to the identification of novel targets for
tumor development. Additionally, it may offer novel insight and
strategies for combating tumors.
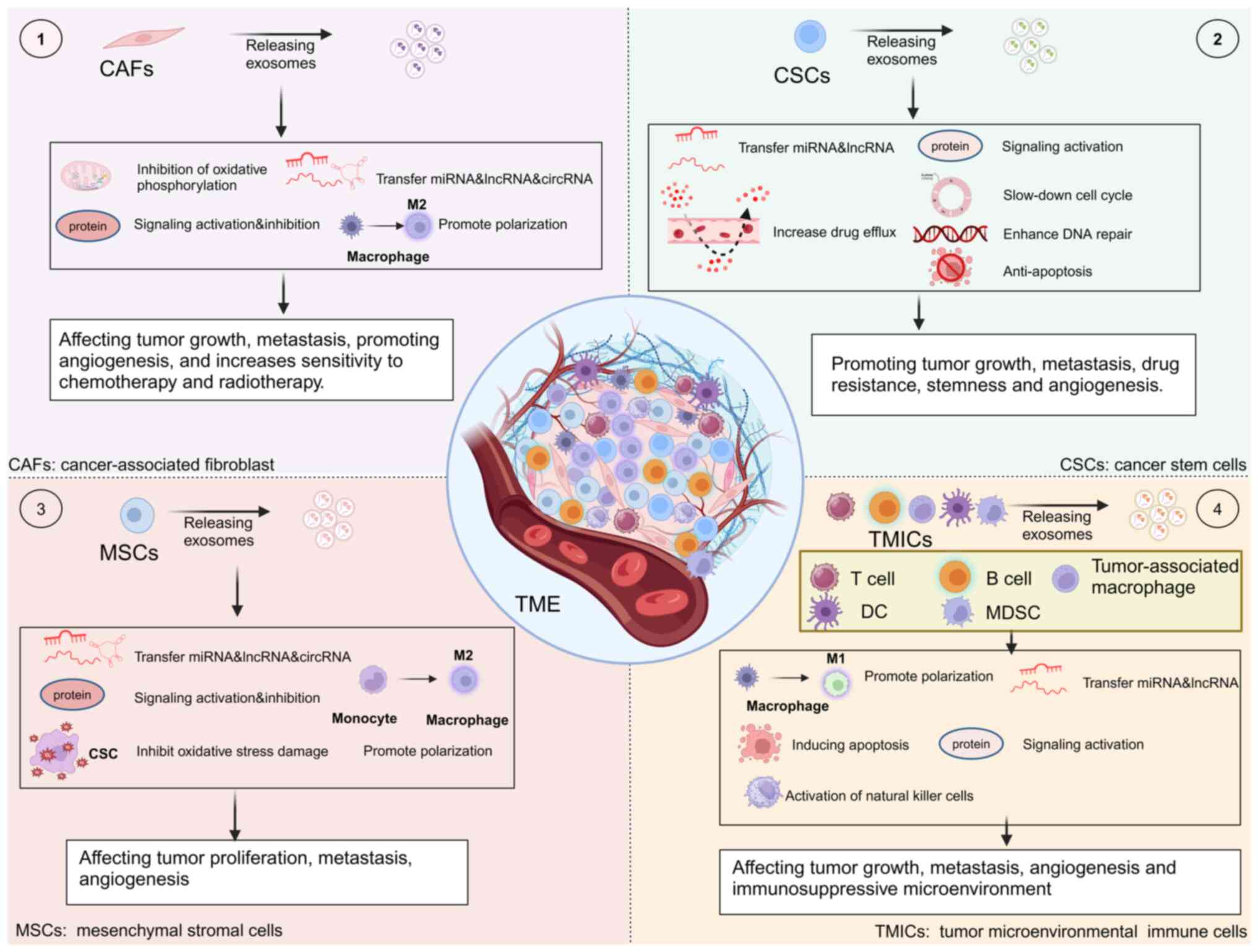
The TME is composed of a diverse array of elements,
such as cancerous cells, different types of stromal cells,
cytokines, growth factors, enzymes and the surrounding environment
where they are located. It plays a critical role in controlling the
development, advancement and spread of cancer, as shown by
extensive evidence available across various types of cancer. As
tumors advance and spread to other parts of the body, the cancer
cells do not function in isolation; instead, they engage in
interactions with the entirety of the TME (112,113). A growing body of research
suggests that exosomes serve an important role in the regulation of
the TME (114). CAFs, CSCs, MSCs
and tumor microenvironmental immune cells (TMICs) are the most
important types of cells in the TME. In the following sections, we
examine the key cells within the TME and their significant
associations with exosomes (Fig.
4).
In most types of solid cancer, CAFs are the major
cellular component of the TME (115). Cancer-associated
fibroblast-derived exosomes (CDEs) are one of the key factors in
oncogenic transformation (116).
In colorectal cancer (CRC), the role of CDEs in
enhancing resistance to cancer drugs and facilitating metastasis
cannot be overlooked. For instance, CDEs have the ability to
stimulate angiogenesis and the advancement of tumors in colorectal
cancer (CRC). Moreover, they can trigger the dedifferentiation of
cancer cells via the Wnt signaling pathway, thereby contributing to
the development of resistance to chemotherapy in CRC (117). CAF-induced activation of the Wnt
signaling pathway enhances cell stemness, promotes EMT, the
development of metastasis and resistance to chemotherapy in CRC by
upregulating miR-92a-3p expression (118). CDEs in CRC exhibit elevated
miR-93-5p levels compared with normal fibroblasts. This increased
miR-93-5p content promotes the proliferation of SW480 cells and
imparts radioresistance to these cells (119). Resistance to treatment with
5-fluorouracil is frequently observed in patients with CRC.
miR-181d-5p, an exosome originating from CAFs, directly inhibits
neurocalcin delta, thereby reducing sensitivity to 5-fluorouracil
in CRC cells (120). Exosome
miR-200b-3p deletion in hypoxic CAFs decreases sensitivity to
treatment with 5-fluorouracil in CRC through high mobility group
box 3 targeting (121). CDEs
promote CRC progression and metastasis through miR-181b-3p and
miR-345-5p (122,123). CDEs harbor the long non-coding
RNA (lncRNA) long intergenic non-protein coding RNA 659
(LINC00659), which enhances the proliferation, invasion, migration
and EMT advancement of CRC cells by modulating the
miR-342-3p/annexin A2 signaling pathway (124). CDE circular RNA-NEDD4 binding
protein 2 like 2 enhances the stemness of CRC cells and their
resistance to oxaliplatin by increasing the expression of
eukaryotic translation initiation factor 4A3 (125).
Elevated expression levels of miR-21, miR-378e and
miR-143 are observed in CDEs of patients with breast cancer. The
introduction of these three miRNAs into breast cancer cells induces
an increase in stemness and facilitated the EMT process (126). CDEs exhibit a more potent effect
on promoting breast cancer cell migration and invasion, compared
with exosomes derived from normal fibroblasts. Moreover, the
expression of miR-18b is increased in CDEs. The presence of miR-18b
in CDEs facilitates breast cancer cell invasion, migration and EMT
progression through its specific interaction with the
3′untranslated region of transcription elongation factor A-like 7
(127). Exosomes transfer
miR-20a-5p from CD63 CAFs to breast cancer cells, leading to the
development of resistance to cyclin dependent kinase 4/6 (CDK4/6)
inhibitors through the miR-20a-5p/retinoblastoma 1 (miR-20a-5p/RB1)
axis (128).
Cancer cell growth is promoted by CDEs through the
inhibition of mitochondrial oxidative phosphorylation.
Additionally, CDEs increase glycolysis and reduce
glutamine-dependent carboxylation in pancreatic cancer cells
(115). Furthermore, miR-106b
promotes gemcitabine resistance by directly targeting tumor protein
53-induced nuclear protein-1 in cancer cells (129). Exosomal miRNA-320a derived from
CAFs induces M2 polarization of macrophages to enhance cell
proliferation and invasion in pancreatic cancer (130).
In ESCC cells, the promotion of proliferation,
invasion and migration is facilitated by two proteins found in
CDEs, namely tumor necrosis factor receptor superfamily member 10b
and interleukin enhancer binding factor 3 (131). Additionally, experiments
conducted in vitro and in vivo demonstrate that CDEs
are able to enhance the proliferation and spread of esophageal
squamous cell carcinoma (ESCC) (132). LINC01410, a lncRNA secreted by
CDEs, enhances metastasis and EMT of ESCC both in vitro and
in vivo through the miR-122-5p/pyruvate kinase M1/2 axis
(133).
miR-210 is expressed at high levels in NSCLC and
acts through CDEs to enhance the migration and invasion of NSCLC
cells. This is achieved by directly targeting Up frameshift 1,
leading to activation of the PTEN/PI3K/AKT pathway involved in the
process of EMT (134). CDEs
confer resistance to cisplatin in NSCLC cells by transferring
miRNA-130a (135).
Emerging data indicate that taurine upregulated gene
1 (TUG1) plays a vital role in cancer advancement, with CDE TUG1
stimulating the movement, penetration and glucose metabolism of HCC
cells through the miR-524-5p/SIX homeobox 1 pathway (136). The circular RNA-zinc finger RNA
binding protein (circZFR) exhibits high expression levels in CAFs
and CDEs. Moreover, the addition of CAFs to the culture medium
enhances resistance to cisplatin in HCC cells. Xenograft studies
demonstrate that depletion of circZFR suppressed tumor progression
and reduced resistance to cisplatin. Conversely, exposure to CDEs
upregulated circZFR levels, suppressed the signal transducer and
activator of transcription 3/nuclear factor-κB (STAT3/NF-κB)
signaling pathway, facilitated tumor growth and heightened
resistance to cisplatin (137).
Bladder cancer CDEs increase HCC cell metastasis through lncRNA
zinc finger E-box binding homeobox 2-antisense RNA 1 by stimulating
invasion and EMT (138).
By contrast, certain CDEs may also possess
oncostatic properties. For instance, in HCC, a notable decrease in
miR-150-3p is observed in CDEs, resulting in the inhibition of
tumor cell migration and invasiveness (141). Additionally, diminished
miR-150-3p expression in HCC tissues poses a significant risk for
recurrence in patients with HCC. Importantly, individuals
displaying low levels of miR-150-3p in plasma exosomes exhibit
notably reduced survival rates compared with those with elevated
miR-150-3p levels (141). CDEs
transport circular RNA-interferon gamma receptor 2 (circIFNGR2) and
suppress the malignant advancement of ovarian cancer through the
circIFNGR2/miR-378/ST5 pathway (142).
Cancer-initiating cells, commonly referred to as
CSCs, constitute a minority of the diverse cell population found
within tumor tissues. Nevertheless, CSCs do not remain stagnant, as
they continuously shift between self-differentiation and
de-differentiation, leading to a constant state of transformation
alongside non-CSCs. These CSCs possess an infinite capacity for
self-renewal and variation, contributing significantly to the
development of tumors, their recurrent nature, metastatic spread
and resistance to treatment (143). Exosomes are carriers of
information in the transition from non-CSCs to CSCs and in the
regulation of CSC homeostasis through specific mechanisms (144). The dynamic interconversion state
of CSCs is maintained by exosomal lncRNA fragile X messenger
ribonucleoprotein 1-antisense RNA 1 via a mechanism that triggers
Toll like receptor 7-NF-κB signaling (145). Exosomes from CSCs deliver
miR-19b-3p into cells of clear cell renal carcinoma and trigger
EMT, leading to the enhancement of metastasis (146). Furthermore, exosomes originating
from HCC cells contain p120 catenin, which hinders the growth of
HCC cells, as well as the spread and growth of CSCs by targeting
the STAT3 pathway (147).
Exosomes serve a critical role in CSC drug
resistance due to various mechanisms. These include increased
efficiency of DNA repair, enhanced anti-apoptotic abilities,
delayed progression of the cell cycle, drug expulsion and the
activation of detoxification enzymes (148). EVs originating from pancreatic
CSCs resistant to gemcitabine facilitate the transfer of drug
resistance characteristics to gemcitabine-sensitive pancreatic
cancer cells via transfer of miR-210 (149). RAB27B in breast cancer
facilitated the transfer of exosomes from stromal cells to breast
cancer cells containing exosomal 5′-triphosphates. This transfer
led to the activation of the retinoic acid-inducible gene I (RIG-I)
signal in recipient cells, subsequently triggering the
interferon-related DNA damage resistant signature (IRDS) genes to
confer resistance to DNA damage. Additionally, the notch receptor 3
(NOTCH3) pathway was concurrently activated to modulate the
proliferation of CSCs (150). Of
note, aside from pro-angiogenic factors (such as IL-8 and VEGF),
CSCs serve a role in promoting angiogenesis through exosome
secretion. They exhibit resistance to intratumor hypoxia by
accessing blood supply via autophagy or directly forming tube-like
structures (150). Conversely,
the vascular niche within the TME also secretes growth factors
through autocrine and paracrine pathways to facilitate CSC growth
and preserve their stem-like properties. This mutual enhancement
mechanism between angiogenesis and CSCs is present in the liver TME
and contributes to the progression and unfavorable outcome of HCC
(151).
In addition, exosome miR-26a from glioma stem cells
stimulates angiogenesis in human brain microvascular VECs by
activating the PI3K/AKT signaling pathway (152). The piwi-like RNA-mediated gene
silencing 2-induced CSCs secrete exosomes with the ability to
transform normal fibroblasts into CAFs, ultimately facilitating
tumor progression (153). Lung
CSC-derived exosome miR-210-3p targets fibroblast growth factor
receptor like 1 to promote lung cancer metastasis (154). Exosomal lncRNA dedicator of
cytokinesis 9-antisense 2 derived from thyroid-like carcinoma
(papillary thyroid carcinoma) CSCs stimulates the Wnt/β-catenin
pathway, enhancing proliferation, migration, invasion, EMT and
stemness in this type of cancer (155). CSCs secrete exosomes in a
RAB27A-dependent manner to trigger the expression of Nanog and
resistance to regorafenib in differentiated cells. Exosomal lncRNA
cyclin dependent kinase inhibitor 2B-antisense 1 derived from CSCs
enhances the growth and spread of thyroid cancer through the
TGF-β1/Smad2/3 signaling pathway or the miR-122-5p/prolyl
4-hydroxylase subunit alpha 1 axis (156,157). MSCs, highly metastatic melanoma
CSCs, release exosomes that enhance the invasiveness of melanoma
parental cells, low metastatic melanoma cells and hasten metastatic
advancement through miR-1268a (158). In addition, circular RNA-zinc
finger E-box binding homeobox 1 and circular RNA-actin filament
associated protein 1 derived from exosomes of HCC CSCs were
significantly linked to the stemness of HCC and unfavorable
prognosis and were able to control the process of EMT (159).
MSCs are recognized as a highly promising cell
source for tissue engineering owing to their convenient
availability and pluripotent characteristics, which enable their
differentiation into adipocytes, osteoblasts, cardiomyocytes and
neurons. Exosomes are the primary paracrine mediators of MSCs
(160). Exosomes derived from
MSCs control cancer indicators and boost tumor advancement through
the transfer of particular miRNAs to adjacent cells. Specifically,
MSCs from bone marrow discharge exosomes containing miR-214; this
suppresses oxidative stress-induced harm in CSCs, thus supporting
tumor growth (161). Exosomes
from MSCs associated with tumors have elevated concentrations of
miR-155. Uptake of miR-155 by tumor cells resembling atypical
teratoma/rhabdomyosarcoma leads to suppression of the tumor
inhibitory gene SWI/SNF related, matrix associated, actin dependent
regulator of chromatin, subfamily a, member 4 (a target of miR-155)
and promotes the migration of these tumors (162). Exosomes derived from human MSCs
associated with glioma and containing miR-1587 serve a role in
enhancing the proliferation and formation of clones of glioma stem
cells. This is achieved by reducing the expression of the tumor
suppressor nuclear receptor corepressor 1 in glioma stem cell-like
cells (163). Exosomes derived
from MSCs suppress EndoMT, promote angiogenesis and uphold vascular
equilibrium. Of note, exosomes derived from MSCs harbor miRNAs with
anti-angiogenic properties, including miR-16 and miR-100, which
hinder angiogenesis in breast cancer cells within the TME by
suppressing VEGF (164-166). Biswas et al (167) showed that exosomes released by
tumor-educated MSCs from humans and mice promote the rapid
advancement of breast cancer. This is achieved through the
conversion of monocytic MDSCs into M2-polarized macrophages with
strong immunosuppressive abilities at the tumor sites. Exosomes
derived from MSCs containing miR-133b suppresses the proliferation,
invasion and migration of glioma cells by blocking the enhancer of
zeste 2 polycomb repressive complex 2 subunit and Wnt/β-catenin
signaling pathways (168). In
addition, exosomes derived from MSCs regulate osteosarcoma
progression through miR-150 as well as miR-21-5p (169,170). Exosome miR-3940-5p from MSCs
inhibits the invasion, EMT, metastasis and growth of CRC cells by
targeting integrin subunit α6 (171). Exosomes derived from MSCs
suppress the proliferation, migration, invasion and angiogenesis of
CSCs originating from HCC through the lncRNA chromosome 5 open
reading frame 66-antisense 1/miR-127-3p/dual specificity
phosphatase 1/extracellular signal-regulated kinase axis (172). In a study, MSCs exhibit anti-HCC
activity through the exosomal circ563/miR-148a-3p/metal regulatory
transcription factor 1 pathway (173).
TMICs predominantly consist of myeloid cells (such
as tumor-associated macrophages, dendritic cells, MDSCs) and
lymphocytes (T cells and B cells) (174). Macrophages are classified into
two major macrophage subsets. M1 macrophage-derived exosomes
(M1-Exos) can inhibit tumor progression by directly promoting
apoptosis or enhancing tumor immune response (175). In addition, M1-Exos could also
reduce the expression of PD-L1 in gastric cancer cells through
miR-16-5p, thereby promoting the activation and killing ability of
T cells (176). Notably, Jiang
et al (177) found that
M1-Exos could also serve a dual role as a tumor suppressor. M1-Exos
highly expressed the lncRNA HOXA distal transcript antisense RNA
(HOTTIP), which inhibited the proliferation, invasion and
metastasis of head and neck squamous cell carcinoma cells by
upregulating the TLR5/NF-κB signaling pathway. In addition, it
polarizes circulating monocytes into antitumor M1 macrophages
thereby inhibiting cancer progression. M1-Exos may serve a greater
role as a regulator of other immune cell functions in the TME.
Thus, further research on M1-Exos is warranted. M2
macrophage-derived exosomes (M2-Exos) miR-221-3p promotes tumor
cell proliferation and G1/S transformation by decreasing
the levels of CDKN1B in tumor cells (178). M2-Exos miR-501-3p downregulates
transforming growth factor beta receptor 3 expression and finally
promotes pancreatic ductal adenocarcinoma cell invasion and
migration through the TGF-β signaling pathway (179). M2-Exos also promote esophageal
cancer invasion and metastasis by downregulating miR-26a and
upregulating activating transcription factor 2 via lncRNA AFAP1-AS1
(180). Moreover, M2-Exos can
carry miR-155-5p and miR-221-5p to ECs and promote angiogenesis in
pancreatic ductal adenocarcinoma by targeting E2F2 (181).
Engineered exosomes have become a research hotspot
in recent years. However, exosomes extracted from different cells
of different populations are heterogeneous. How can we overcome the
heterogeneity and find the exosomes that can serve the most
important role? Tumor heterogeneity is also extensive. How can we
select the exosomes that serve the most effective role specifically
for different tumors? In addition, the tumor immune
microenvironment is complex. Can exosomes overcome the functions of
immunosuppressive factors and immunosuppressive cells, thus
allowing immune killer cells to serve a greater role? Further
research on exosome-based antitumor therapies and efficacy is
warranted.
Angiogenesis is a process that plays an important
role in the development of cancer, from the beginning of
carcinogenesis to the advanced stages of cancer (192). Tumor cells use different
strategies to communicate with neighboring tissues to promote tumor
progression; one such strategy is the release of exosomes (193,194). Exosomes contain various cargoes
that can accelerate angiogenesis and serve an important role in
cancer invasion (195). Evidence
suggests that ncRNAs, particularly lncRNAs and miRNAs, serve an
important role in the regulation of angiogenesis (196). Angiogenesis in tumor tissues is
a dynamic process with spatio-temporal specificity. Do exosomes
serve different roles at different stages of angiogenesis? Is the
relationship between exosomes and angiogenesis the same in tumors
of different tissue origins? These questions should be explored in
detail.
Cancer cells have specific mechanisms of vascular
development, including the unique ability of host blood vessels to
adulterate tumors or to express EC phenotypes and form structures
that resemble blood vessels (197). At the initial stage of tumor
progression, tumor tissues develop independently of the structure
of the vascular network, oxygen and nutrients and growth factors.
They reach the tumor cells mainly due to the diffusion of nearby
blood vessels. At later stages of development, tumor growth is
dependent on further blood supply (198). Tumor neovascularization promotes
tumor growth by providing oxygen, nutrients and metabolite
replacement. Adequate blood supply to the tumor promotes the entry
of tumor cells into the bloodstream, which initiates the metastatic
process (199).
The development of blood vessels involves two
primary processes, namely vasculogenesis and angiogenesis. A key
distinction between them lies in their mechanisms; angiogenesis
refers to the creation of new blood vessels from those that already
exist, whereas vasculogenesis pertains to the generation of new
blood vessels from precursors termed ECs or angioblasts originating
from the mesoderm. This process subsequently leads to the formation
of basic capillary networks that eventually mature into fully
developed blood vessels (200).
The generation of new blood vessels begins in the
early stages of cancer development and is related to the amount of
exosomes produced by the tumor. A proangiogenic effect is observed
using exosomes derived from cancer cell lines, as well as exosomes
isolated from blood samples of patients with cancer and urine of
patients with bladder cancer (201). Notably, gliomas have a more
abundant vascular system compared with other solid tumors. In
vitro and in vivo studies have shown that exosomes
secreted by gliomas are rich in angiogenic proteins (202,203). In addition, other solid tumors,
such as pancreatic and breast cancers, produce exosomes that
promote angiogenesis (195). In
addition to solid tumors, chronic granulocytic leukemia cells also
produce exosomes that interact directly with ECs and have an effect
on angiogenesis (204).
One of the factors determining the development and
progression of cancer is an adequate supply of oxygen and nutrients
(205). During the initial
stages of tumor development, vascular organization in the TME is
poor. Pro-angiogenic signaling predominates, which activates
clusters of angiographically inactive cancer cells. This mechanism
is termed the angiogenic switch. In the TME, this is achieved by
transferring angiogenic factors from cancer cells to VECs (206).
In TME remodeling, angiogenesis is induced,
involving a number of molecules transported by TEX. The angiogenic
potential of TEX depends on the conditions under which they are
secreted. It has been shown that HUVECs cultured with esophageal
squamous-cell carcinoma-secreted exosomes under hypoxic conditions
have improved angiogenic capacity compared with those cultured
under normal conditions (207).
Studies show that miR-23a, an exosome derived from hypoxic lung
cancer cells and HCC, stimulates angiogenesis (208,209). Hypoxia may be an influential
factor in the role of exosomes at different stages of tumor
vascularization.
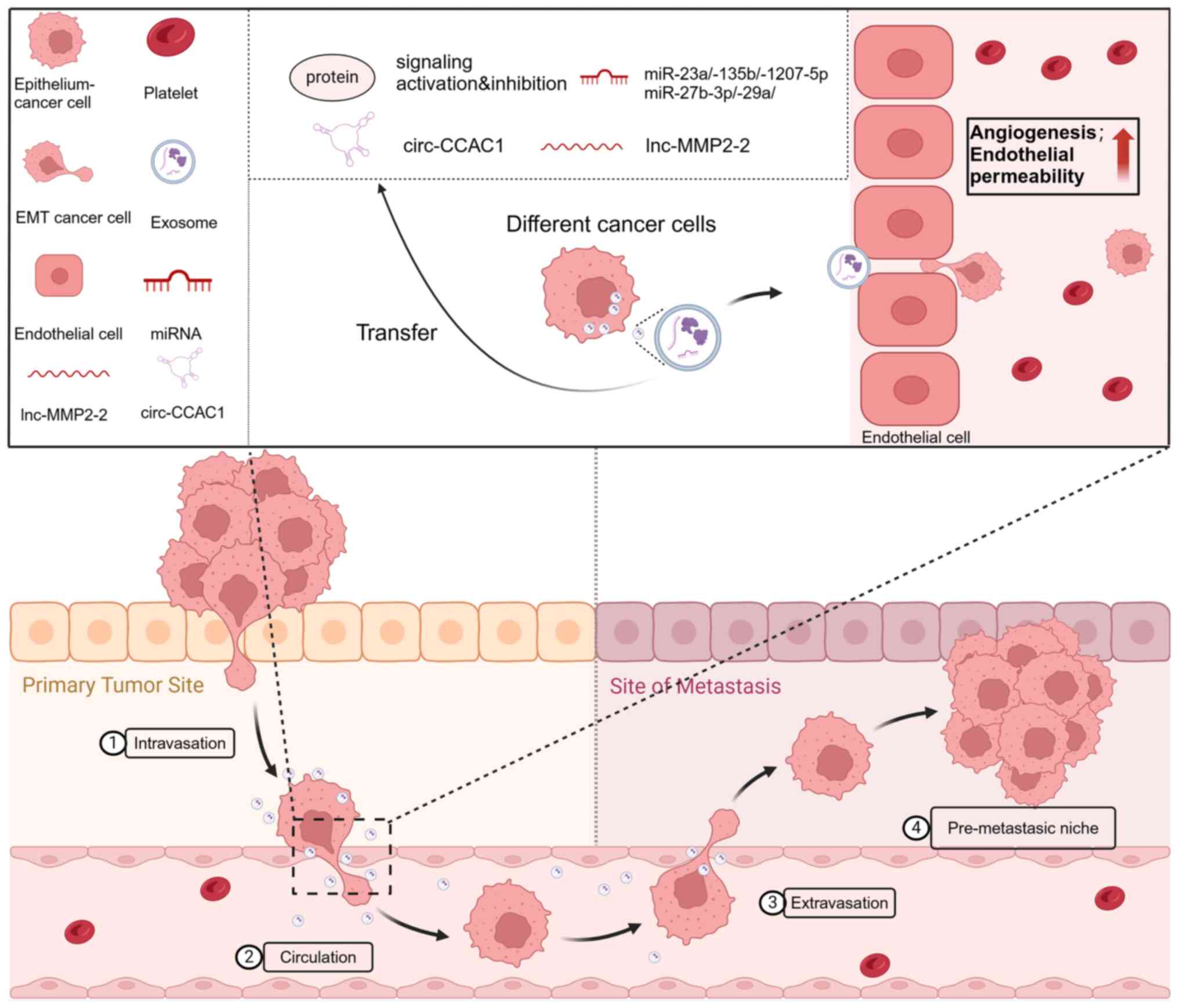
Tumor cell exosomes are able to influence EC
characteristics via their internal cargo. This can lead to
stimulation of angiogenesis or changes in vascular endothelial
permeability, ultimately affecting tumor spread (Fig. 5). Following exposure to low oxygen
levels, exosomes containing the tetraspanin protein CO-029 can
enhance tumor development by boosting angiogenesis (210). Relevant pathways and regulatory
mechanisms identified in studies are listed (Table II) (208,211-220).
Primary tumors release systemic signals, such as
exosomes, to promote the formation of a pre-metastatic
microenvironment. Studies have revealed the importance of exosomes
in the establishment of a pre-metastatic microenvironment by cancer
cells. For example, melanoma cell-derived exosomes promote
angiogenesis in lymph nodes or lung tissue, thus creating a
favorable microenvironment for melanoma cell metastatic
colonization and growth (221,222). Exosomes from melanoma cells
deliver MET (a tyrosine kinase receptor) to myeloid progenitor
cells. In addition, they induce vascular leakage at pre-metastatic
sites and reprogram myeloid progenitor cells to a pro-angiogenic
phenotype that promotes bone metastasis (223).
By contrast, exosomes from parental lung cancer
cells contain miR-192, which effectively blocks metastatic
angiogenesis and inhibits cancer cell colonization by suppressing
the expression of IL-8, intercellular adhesion molecules and CXCL1
in endothelial precursor cells of the bone microenvironment.
Moreover, exosomes from a highly metastatic subpopulation contained
less miR-192, which promoted the formation of the pre-metastatic
microenvironment of bone metastasis (224).
There are 10 major tumor angiogenesis process,
including hypoxia, extracellular matrix (ECM) organization,
vascular development, cell migration, cell proliferation, VEGF
response, wound healing, lipid response, hemostasis and platelet
aggregation (27). Exosomes
influence the progression of these processes through the cargoes
they carry. For example, hypoxia plays a key role in tumor
angiogenesis (27),
hypoxia-inducible factor 1 (HIF-1) plays an essential role in the
hypoxic tumor microenvironment (225). In breast cancer, MSC-derived
exosomal miR-100 induces VEGF expression by regulating HIF-1α
(166). In pancreatic cancer,
TEX-enriched protease regulates ECM as demonstrated for degradation
of collagens, laminin and fibronectin; this has important
implications for tumor adhesion, motility and invasiveness
(226). Exosomal regulation of
the ECM may contribute to physiologic and pathologic angiogenesis,
wound healing and coagulation after vessel rupture (226). Xie et al (227) demonstrate that exosomal
miR-582-3p promotes gastric cancer progression by regulating VEGF
expression. One study suggests that EVs, containing both
microvesicles and exosomes, derived from breast-cancer cell lines
induce tissue factor (TF)-independent platelet activation and
aggregation, as well as TF-dependent plasma clotting and platelet
aggregation by means of thrombin generation (228). These roles may vary depending on
the tumor type and microenvironment. Further studies will help to
reveal the specific mechanism underlying the role of exosomes in
tumor angiogenesis and provide new strategies for tumor
therapy.
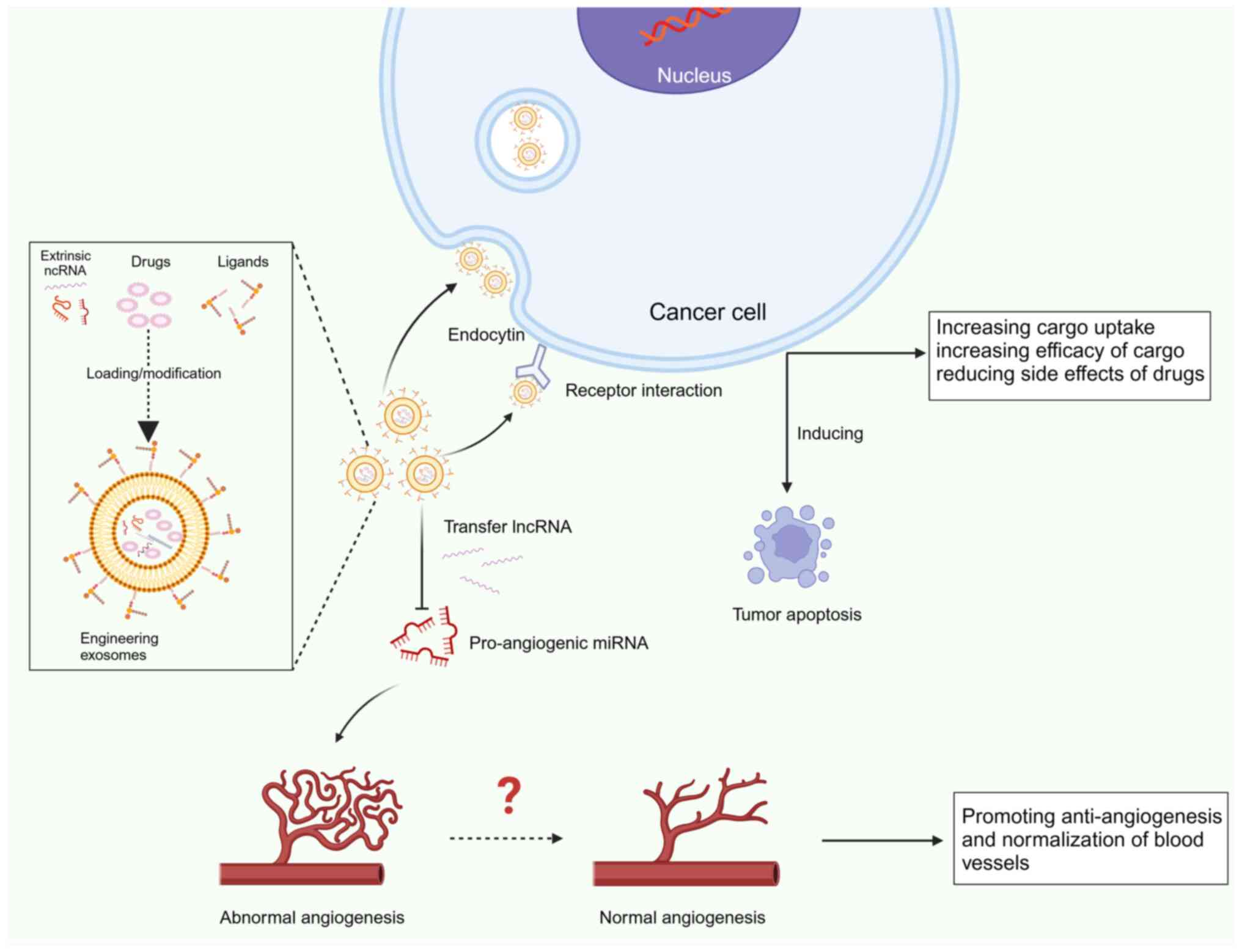
With the development of and advances in exosome
research, the use of engineered exosomes in cancer is gaining
attention. Although natural exosomes can deliver antitumor drugs,
they are mostly retained in the liver or spleen rather than in
tumor tissue. Consequently, the therapeutic effect is
unsatisfactory (229). Thus,
engineering technology provides exosomes with more specific
targeting and clinical application potential (Fig. 6).
The isolation of exosomes is important for the
study of their application in tumor therapy. Conventional methods
for exosome isolation include ultracentrifugation, size-based
filtration, size exclusion chromatography and polymer precipitation
(230). They are typically
subject to various constraints with regard to clinical
applications, such as cumbersome procedures, high costs and limited
throughput.
Microfluidic-based separation techniques have
recently attracted increasing attention for the microscale
separation, detection and analysis of exosomes. The technique uses
innovative sorting mechanisms such as acoustic, electrophoretic,
electromagnetic manipulation, nanowire-based traps, nanoscale
deterministic lateral displacement and viscoelastic flow (231). This results in a five-fold
increase in the number of key liposarcoma-associated EV cargoes in
30 min (232).
In short, isolation of high-purity exosomes is the
first step in studying the therapeutic function of engineered
exosomes. Conventional methods alone are often unable to meet
separation requirements, such as high purity, high yield and low
cost. Microfluidic-based isolation techniques are capable of
recovering, analyzing and quantifying exosomes in limited clinical
samples in a high-throughput manner with higher sensitivity and
multiplexed detection capabilities. This is the basis for the
development of efficiently engineered exosomes.
Biomodification is currently the most widely used
membrane modification strategy, including targeted peptide
modification and biomimetic exosome modification. For example,
cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide is a cyclic Arg-Gly-Asp
peptide, which endows the natural exosome with significant
tumor-targeting ability and high affinity for tumor vascular ECs in
osteosarcoma (233,234). Notably, this opens up the
possibility of exosome-based anti-angiogenic therapies.
Triple-negative breast cancer is a form of breast cancer
characterized by a high likelihood of metastasis and recurrence.
Raghav et al (235)
altered the surface of exosomes using a peptide that targets the
c-Met gene, a receptor for hepatocyte growth factor or scatter
factor. This modification causes an increase in c-Met on the
surface of triple-negative breast cancer cells. Studies conducted
in living organisms showed that the engineered nanoparticles
enclosed within exosomes markedly increases the efficiency of
cellular absorption and effectiveness of adriamycin against tumors.
This approach demonstrates remarkable effectiveness in targeting
tumors, resulting in improved inhibition of tumor growth and
triggering robust tumor cell death (236). Modification of exosomes with
peptides is a promising engineering approach.
Real-time imaging of engineered exosomes through
stochastic optical reconstruction microscopy, structured
illumination microscopy and confocal laser scanning microscopy is
also important in in vivo studies. Structured illumination
microscopy and confocal laser scanning microscopy can track the
dynamic intracellular distributions of exosomes within seconds.
This approach is suitable for studying the physiological and
tumor-killing effects of engineered exosomes on cancer cells
(241,242). It is important to choose a
particular combination of reporter and microscope based on the
biological question and the actual imaging equipment available at
this time.
Gene therapy is the transfer of genetic molecules
to a patient for the treatment of a disease (243). The ncRNAs can be used as gene
therapy vectors for a wide range of diseases, including cancer.
However, great challenges remain for the controlled expression of
ncRNA therapeutics in terms of the level, timing and location of
gene therapy. Engineered exosomes offer promise in this field; it
is realistic to deliver ncRNAs to the tumor site in a precise
temporal, spatial and dosage manner through engineered exosomes
(244-247). For instance, antimiRNA-21 and
antimiRNA-10b were encapsulated within polymeric nanocarriers and
subsequently coated with urokinase plasminogen activator-engineered
exosomes to improve the affinities for targeting tumors. As a
result, the systemic delivery of the urokinase plasminogen
activator-engineered exosomes polymeric nanocarriers nanococktail
in vivo demonstrated significant cancer inhibition, thereby
supporting the evidence for the combined antitumor impacts of
ncRNAs and engineered exosomes (248).
Exosomes serve a role in cancer progression by
promoting angiogenesis through the transportation of various
pro-angiogenic molecules, including VEGF and miRNAs (249). Numerous lncRNAs are dysregulated
in the context of angiogenesis within human malignant tumors.
Moreover, exosomes secreted by cancer cells harbor a multitude of
lncRNAs that promote angiogenesis. Consequently, exosomes derived
from tumors containing lncRNAs are perceived as potent modulators
of angiogenesis. Moreover, as aforementioned, exosomes encapsulate
miRNAs that serve a role in the regulation of angiogenesis. The
effect of these miRNAs on angiogenesis is manifested through the
direct modulation of VEGF or activation of signaling pathways (such
as NF-κB). Hence, targeting lncRNAs and miRNAs presents a promising
therapeutic approach to manipulating the angiogenic process in
diverse human ailments. The advances in high-throughput sequencing
techniques have simplified the recognition of ncRNA characteristics
involved in angiogenesis and the correlation between the expression
levels of various ncRNA subclasses, thereby delineating the
intricate network of interactions between miRNAs and lncRNAs. Such
strategies facilitate the development of novel therapeutic
interventions to modulate angiogenesis. The aforementioned ncRNAs
have emerged as valuable diagnostic and prognostic indicators for
human diseases, particularly types of cancer (250). Further research is warranted to
investigate the potential value of ncRNA-based anti-angiogenic
therapies by targeting engineered exosomes. Specifically,
determining whether the translocation of certain lnRNAs against
pro-angiogenic miRNAs to the endothelium of tumor blood vessels can
effectively inhibit aberrant angiogenesis in tumors is a major area
of study.
To create a conducive environment for cancer cell
survival and immune system suppression, cancer cells manipulate the
properties of stromal cells through various means, including the
release of exosomes. Research indicates that the impact of cancer
cells on the vascular endothelium plays an important role in
establishing and sustaining an immunosuppressive TME (31). Specifically, the vascular network
of the tumor hinders the infiltration of T cells, selectively
eliminates them and attracts immunosuppressive cells, thereby
hampering antitumor immune responses. Emerging evidence suggests
that strategies aimed at normalizing the tumor vasculature can
temporarily enhance its structural and functional abnormalities,
reduce hypoxia within the tumor, improve drug delivery, facilitate
immune cell penetration and synergize with immunotherapy for
prolonged efficacy. These discoveries hold significant implications
for the development of vascular normalization therapies;
nonetheless, numerous unanswered questions and obstacles persist.
Examples include lack of therapeutic efficacy, drug resistance and
prevalence of treatment modalities. These limitations encourage
researchers to develop novel angiogenesis inhibitors, explore the
druggability of additional targets, validate specific biomarkers
and offer more treatment options to patients with cancer. Whether
ncRNA-based anti-angiogenic therapy is feasible and has clinical
translational value through engineered exosomes should be further
investigated.
Novel approaches are required to enhance the
outcomes of anti-angiogenic treatment beyond VEGFR blockade.
Investigating the metabolic profiles and transport mechanisms of
VECs may unveil promising alternatives for therapy. Enhancing the
delivery of pharmacological inhibitors to these targets via
exosomes, natural drug carriers, could be a fruitful avenue.
Additionally, the role of stromal cells, such as CAFs, in promoting
angiogenesis should not be overlooked. Exploring potential targets
to hinder the recruitment of immunosuppressive cells that fuel
tumor growth, in light of the interactions between VECs and immune
cells, is of great importance.
In summary, directing efforts towards vascular
normalization in tumor vasculature could enhance the effectiveness
of current immunotherapies by reducing immunosuppression within the
TME. EndoMT encourages various malignant biological behaviors
within tumors. Potentially beneficial therapeutic effects could be
achieved by inhibiting or reversing EndoMT during tumor
advancement. For instance, it fosters tumor growth by stimulating
the proliferation, survival and angiogenesis of tumor cells,
promotes tumor metastasis by influencing critical processes (such
as EMT, cell migration, invasion, endocytosis and exocytosis) and
facilitates tumor immune evasion and resistance to therapy.
Nonetheless, limited research has been conducted thus far on EndoMT
in tumor therapy.
In theory, suppressing molecules and signaling
pathways initialized and sustained by EndoMT or effector molecules
enhancing tumor biological behavior could effectively impede tumor
progression. Nevertheless, only a small number of studies have
attempted to tackle these obstacles, primarily concentrating on
evaluating the efficacy of current medications. Markedly, EVs from
MSCs possess the ability to reverse EndoMT in VECs exposed to tumor
cells (84). More in-depth
understanding of the molecules and signaling pathways triggered and
sustained by EndoMT or effector molecules that enhance tumor
biological behaviors, coupled with the creation of specific
inhibitors, will enhance treatment plans and improve the outlook
for patients with cancer.
Cancer-related exosomes within the TME encompass a
variety of molecules, including proteins, DNA, mRNA, miRNA, lncRNA
and circular RNA. In cancer, some of these components function as
biomarkers, aiding in the early detection, diagnosis, prognosis and
evaluation of treatment effectiveness. Additionally, certain
molecules serve as messengers for intercellular communication
between cancer cells and surrounding cells or within the TME,
influencing processes such as tumor progression, invasion,
metastasis and resistance to therapies. Notably, exosomes derived
from tumors can enhance metastasis by modifying the behavior of
VECs. Notably, research has shown that exosomes serve a role in
connecting cardiovascular disease to cancer. For instance, exosomes
released by cardiomyocytes during heart failure facilitate
intercellular communication using miR-22-3p, which decreases the
vulnerability of cancer cells to iron-induced cell death, thereby
supporting tumor growth (251).
In a recent study, Caller et al (252) reported that hearts following
myocardial infarction, specifically cardiac MSCs (cMSCs), generate
an increased amount of cardiac small extracellular vesicles (sEVs)
containing tumorigenic material compared with healthy hearts.
Following a heart attack, cMSC-sEVs also transform inactive
macrophages into a state that promotes angiogenesis and
tumorigenesis in laboratory settings. Furthermore, the absorption
of cMSC-sEVs by cancer cells enhances the growth of tumors.
Engineered exosomes offer a number of advantages
over natural exosomes as drug delivery platforms. Nevertheless,
there are several challenges to the clinical application of
engineered exosomes. First, there is a lack of consensus on
standardized methods for isolating, quantifying and analyzing
engineered exosomes from complex tissues (such as blood, tissues
and urine) in the clinical stage. Second, the process through which
we can accurately quantify exosomes and their contents, including
the exosome number, protein content and ratio of these two remains
elusive. Finally, exosomes from different sources have different
functions and compositions. Hence, it is important to develop
methods for the selection of suitable and available exosomes. This
must be addressed before clinical development of exosome-based drug
delivery systems.
Numerous investigations have examined the molecular
mechanisms that drive the biological function of exosomes in
advancing tumors. Nevertheless, additional research is essential to
deepen our understanding and translate the diagnostic and
therapeutic potential of exosomes into practical use in clinical
settings. It is anticipated that through research, we can
effectively harness the benefits of exosomes as inherent
transporters and address their limitations in the immediate future.
The use of exosome-based strategies may lead to significant
advancements in the treatment of cancer.
In brief, both the endothelium and exosomes possess
the ability to affect tumor advancement in distinct manners and are
consistently associated with tumor progression, each fulfilling
crucial functions. The functions of the vascular endothelium and
exosomes in cancer are interconnected and reciprocally influential.
VECs aid in promoting tumor expansion and dissemination by
regulating the TME, whereas exosomes alter the activities of tumor
cells and adjacent stromal cells (including VECs) through the
transport of signaling molecules, collectively promoting tumor
progression. Consequently, a thorough examination of the mechanisms
involving vascular endothelium and exosomes in cancer could uncover
novel pathways of tumor formation and offer new perspectives and
strategies for cancer therapy and prevention.
Not applicable.
Writing of the original draft was by YD. Writing,
reviewing and editing was by YY. Supervision was by YH.
Supervision, writing, reviewing and editing was by XH. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Professor Youzhong
Wan of the China-Japan Union Hospital of Jilin University (Jilin,
China) for his support and guidance of this article.
The present study was supported by Jilin Scientific and
Technological Development Program (grant no. 20230508066RC),
Special Project for Health Research Talents of Jilin Province
(grant no. 2022SCZ04), Norman Bethune Program of Jilin University
(grant no. 2022B06) and Innovation and Entrepreneurship Talent
Funding Project of Jilin Province (grant no. 2023QN05).
|
1
|
Kamrani A, Hosseinzadeh R, Shomali N,
Heris JA, Shahabi P, Mohammadinasab R, Sadeghvand S, Ghahremanzadeh
K, Sadeghi M and Akbari M: New immunotherapeutic approaches for
cancer treatment. Pathol Res Pract. 248:1546322023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ragusa M, Barbagallo C, Cirnigliaro M,
Battaglia R, Brex D, Caponnetto A, Barbagallo D, Di Pietro C and
Purrello M: Asymmetric RNA distribution among cells and their
secreted exosomes: Biomedical meaning and considerations on
diagnostic applications. Front Mol Biosci. 4:662027. View Article : Google Scholar
|
|
3
|
Betz C, Lenard A, Belting HG and Affolter
M: Cell behaviors and dynamics during angiogenesis. Development.
143:2249–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szekanecz Z and Koch AE: Mechanisms of
Disease: Angiogenesis in inflammatory diseases. Nat Clin Pract
Rheumatol. 3:635–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nachmany I, Bogoch Y, Friedlander-Malik G,
Amar O, Bondar E, Zohar N, Hantisteanu S, Fainaru O, Lubezky N,
Klausner JM and Pencovich N: The transcriptional profile of
circulating myeloid derived suppressor cells correlates with tumor
development and progression in mouse. Genes Immun. 20:589–598.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang FT, Sun W, Zhang JT and Fan YZ:
Cancer-associated fibroblast regulation of tumor neo-angiogenesis
as a therapeutic target in cancer. Oncol Lett. 17:3055–3065.
2019.PubMed/NCBI
|
|
7
|
Gasparics A, Kokeny G, Fintha A, Bencs R,
Mozes MM, Agoston EI, Buday A, Ivics Z, Hamar P, Gyorffy B, et al:
Alterations in SCAI expression during cell plasticity, fibrosis and
cancer. Pathol Oncol Res. 24:641–651. 2018. View Article : Google Scholar
|
|
8
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar
|
|
9
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhang L, Montgomery KC, Jiang L,
Lyon CJ and Hu TY: Advanced technologies for molecular diagnosis of
cancer: State of pre-clinical tumor-derived exosome liquid
biopsies. Mater Today Bio. 18:1005382022. View Article : Google Scholar
|
|
11
|
Liao J, Liu R, Yin L and Pu Y: Expression
profiling of exosomal miRNAs derived from human esophageal cancer
cells by solexa High-Throughput sequencing. Int J Mol Sci.
15:15530–15551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saunderson SC, Dunn AC, Crocker PR and
McLellan AD: CD169 mediates the capture of exosomes in spleen and
lymph node. Blood. 123:208–216. 2014. View Article : Google Scholar :
|
|
13
|
Andersen JS and Mann M: Organellar
proteomics: Turning inventories into insights. EMBO Rep. 7:874–879.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darband SG, Mirza-Aghazadeh-Attari M,
Kaviani M, Mihanfar A, Sadighparvar S, Yousefi B and Majidinia M:
Exosomes: Natural nanoparticles as bio shuttles for RNAi delivery.
J Control Rel. 289:158–170. 2018. View Article : Google Scholar
|
|
15
|
Wang S, Wang J, Wei W and Ma G: Exosomes:
The indispensable messenger in tumor pathogenesis and the rising
star in antitumor applications. Adv Biosyst. 3:e19000082019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Wu D, Ma X, Wang J, Hou W and
Zhang W: Exosomes as drug carriers for cancer therapy and
challenges regarding exosome uptake. Biomed Pharmacother.
128:1102372020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashed M, Bayraktar EK, Helal G, Abd-Ellah
M, Amero P, Chavez-Reyes A and Rodriguez-Aguayo C: Exosomes: From
garbage bins to promising therapeutic targets. Int J Mol Sci.
18:5382017. View Article : Google Scholar
|
|
18
|
Mannavola F, D'Oronzo S, Cives M, Stucci
LS, Ranieri G, Silvestris F and Tucci M: Extracellular vesicles and
epigenetic modifications are hallmarks of melanoma progression. Int
J Mol Sci. 21:522019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He C, Zheng S, Luo Y and Wang B: Exosome
theranostics: Biology and translational medicine. Theranostics.
8:237–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Kim SY, Tu W, Kim J, Xu A, Yang
YM, Matsuda M, Reolizo L, Tsuchiya T, Billet S, et al:
Extracellular vesicles in fatty liver promote a metastatic tumor
microenvironment. Cell Metab. 35:1209–1226.e13. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu H, Sun T, An J, Wen L, Liu F, Bu Z, Cui
Y and Feng J: Potential roles of exosomes in Parkinson's Disease:
From pathogenesis, diagnosis, and treatment to prognosis. Front
Cell Dev Biol. 8:862020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farooqi AA, Desai NN, Qureshi MZ,
Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V and
Daglia M: Exosome biogenesis, bioactivities and functions as new
delivery systems of natural compounds. Biotechnol Adv. 36:328–334.
2018. View Article : Google Scholar
|
|
23
|
Doyle L and Wang M: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das CK, Jena BC, Banerjee I, Das S, Parekh
A, Bhutia SK and Mandal M: Exosome as a novel shuttle for delivery
of therapeutics across biological barriers. Mol Pharm. 16:24–40.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang M and Zhou F: Biological
functions and clinical applications of exosomal long non-coding
RNAs in cancer. J Cell Mol Med. 24:11656–11666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tai Y, Chen KC, Hsieh JT and Shen TL:
Exosomes in cancer development and clinical applications. Cancer
Sci. 109:2364–2374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan X, Li X, Dong L, Liu T, Zhang M, Zhang
L, Zhang X, Huang L, Shi W, Sun H, et al: Tumour vasculature at
single-cell resolution. Nature. 632:429–436. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shashni B, Nishikawa Y and Nagasaki Y:
Management of tumor growth and angiogenesis in triple-negative
breast cancer by using redox nanoparticles. Biomaterials.
269:1206452021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Qu X, Cao B, Yang T, Bao Q, Yue H,
Zhang L, Zhang G, Wang L, Qiu P, et al: Selectively suppressing
tumor angiogenesis for targeted breast cancer therapy by
genetically engineered phage. Adv Mater. 32:e20012602020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schaaf MB, Garg AD and Agostinis P:
Defining the role of the tumor vasculature in antitumor immunity
and immunotherapy. Cell Death Dis. 9:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arneth B: Tumor microenvironment. Medicina
(Kaunas). 56:152019. View Article : Google Scholar
|
|
33
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blankenstein T, Coulie PG, Gilboa E and
Jaffee EM: The determinants of tumour immunogenicity. Nat Rev
Cancer. 12:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ntellas P, Mavroeidis L, Gkoura S, Gazouli
I, Amylidi AL, Papadaki A, Zarkavelis G, Mauri D, Karpathiou G,
Kolettas E, et al: Old Player-new tricks: Non angiogenic effects of
the VEGF/VEGFR pathway in cancer. Cancers (Basel). 12:31452020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chouaib S, Noman MZ, Kosmatopoulos K and
Curran MA: Hypoxic stress: Obstacles and opportunities for
innovative immunotherapy of cancer. Oncogene. 36:439–445. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reardon DA: Update on the use of
angiogenesis inhibitors in adult patients with brain tumors. Clin
Adv Hematol Oncol. 12:293–303. 2014.PubMed/NCBI
|
|
42
|
Winkler F, Kozin SV, Tong RT, Chae SS,
Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E,
et al: Kinetics of vascular normalization by VEGFR2 blockade
governs brain tumor response to radiation. Cancer Cell. 6:553–563.
2004.PubMed/NCBI
|
|
43
|
Casazza A, Laoui D, Wenes M, Rizzolio S,
Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JoA,
Tamagnone L and Mazzone M: Impeding macrophage entry into hypoxic
tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis
and restores antitumor immunity. Cancer Cell. 24:695–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rivera Lee B, Meyronet D, Hervieu V,
Frederick, Mitchell J, Bergsland E and Bergers G: Intratumoral
myeloid cells regulate responsiveness and resistance to
antiangiogenic therapy. Cell Reports. 11:577–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chauhan VP, Stylianopoulos T, Martin JD,
Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D and Jain RK:
Normalization of tumour blood vessels improves the delivery of
nanomedicines in a size-dependent manner. Nat Nanotechnol.
7:383–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stylianopoulos T and Jain RK: Combining
two strategies to improve perfusion and drug delivery in solid
tumors. Proc Natl Acad Sci USA. 110:18632–18637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weiss SA, Han SW, Lui K, Tchack J, Shapiro
R, Berman R, Zhong J, Krogsgaard M, Osman I and Darvishian F:
Immunologic heterogeneity of tumor-infiltrating lymphocyte
composition in primary melanoma. Hum Pathol. 57:16–125. 2016.
View Article : Google Scholar
|
|
48
|
Park JS, Kim IK, Han S, Park I, Kim C, Bae
J, Oh SJ, Lee S, Kim JH, Woo DC, et al: Normalization of tumor
vessels by Tie2 activation and Ang2 inhibition enhances drug
delivery and produces a favorable tumor microenvironment. Cancer
Cell. 30:953–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maes H, Olmeda D, Soengas MS and Agostinis
P: Vesicular trafficking mechanisms in endothelial cells as
modulators of the tumor vasculature and targets of antiangiogenic
therapies. FEBS J. 283:25–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kofler NM, Shawber CJ, Kangsamaksin T,
Reed HO, Galatioto J and Kitajewski J: Notch signaling in
developmental and tumor angiogenesis. Genes Cancer. 2:1106–1116.
2011. View Article : Google Scholar
|
|
51
|
Maes H, Kuchnio A, Peric A, Moens S, Nys
K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, et
al: Tumor vessel normalization by chloroquine independent of
autophagy. Cancer Cell. 26:190–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang X, De Vera ME, Buchser WJ, de Vivar
Chavez AR, Loughran P, Stolz DB, Basse P, Wang T, Van Houten B, Zeh
HJ III and Lotze MT: Inhibiting systemic autophagy during
interleukin 2 immunotherapy promotes long-term tumor regression.
Cancer Res. 72:2791–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Goel S, Wong AH and Jain RK: Vascular
normalization as a therapeutic strategy for malignant and
nonmalignant disease. Cold Spring Harb Perspect Med. 2:a0064862012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guerrouahen BS, Pasquier J, Kaoud NA,
Maleki M, Beauchamp MC, Yasmeen A, Ghiabi P, Lis R, Vidal F, Saleh
A, et al: Akt-activated endothelium constitutes the niche for
residual disease and resistance to bevacizumab in ovarian cancer.
Mol Cancer Ther. 13:3123–3136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McMillin DW, Negri JM and Mitsiades CS:
The role of tumour-stromal interactions in modifying drug response:
Challenges and opportunities. Nat Rev Drug Discov. 12:217–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jayson GC, Hicklin DJ and Ellis LM:
Antiangiogenic therapy-evolving view based on clinical trial
results. Nat Rev Clin Oncol. 9:297–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao Y, Arbiser J, D'Amato RJ, D'Amore PA,
Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, et al:
Forty-year journey of angiogenesis translational research. Sci
Transl Med. 3:114rv32011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tang T, Huang X, Zhang G, Hong Z, Bai X
and Liang T: Advantages of targeting the tumor immune
microenvironment over blocking immune checkpoint in cancer
immunotherapy. Signal Transduct Target Ther. 6:722021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and
Zhang B: Anti-angiogenic agents in combination with immune
checkpoint inhibitors: A promising strategy for cancer treatment.
Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ciciola P, Cascetta P, Bianco C, Formisano
L and Bianco R: Combining immune checkpoint inhibitors with
anti-angiogenic agents. J Clin Med. 9:6752020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer. 18:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Neves KB, Montezano AC, Lang NN and Touyz
RM: Vascular toxicity associated with anti-angiogenic drugs. Clin
Sci (Lond). 134:2503–2520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hilmi M, Neuzillet C, Calderaro J, Lafdil
F, Pawlotsky JM and Rousseau B: Angiogenesis and immune checkpoint
inhibitors as therapies for hepatocellular carcinoma: Current
knowledge and future research directions. J Immunother Cancer.
7:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiao L, Yan K, Yang Y, Chen N, Li Y, Deng
X, Wang L, Liu Y, Mu L, Li R, et al: Anti-vascular endothelial
growth factor treatment induces blood flow recovery through
vascular remodeling in high-fat diet induced diabetic mice.
Microvasc Re. 105:70–76. 2016. View Article : Google Scholar
|
|
69
|
Broekman F, Giovannetti E and Peters GJ:
Tyrosine kinase inhibitors: Multi-targeted or single-targeted? Mol
Cancer Ther. 2:80–93. 2011.
|
|
70
|
Hutzen B, Bid HK, Houghton PJ, Pierson CR,
Powell K, Bratasz A, Raffel C and Studebaker AW: Treatment of
medulloblastoma with oncolytic measles viruses expressing the
angiogenesis inhibitors endostatin and angiostatin. BMC Cancer.
14:2062014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mohajeri A, Pilehvar-Soltanahmadi Y,
Pourhassan-Moghaddam M, Abdolalizadeh J, Karimi P and Zarghami N:
Cloning and expression of recombinant human endostatin in periplasm
of escherichia coli expression system. Adv Pharm Bull. 6:187–194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Matejuk A, Collet G, Nadim M, Grillon C
and Kieda C: MicroRNAs and tumor vasculature normalization: Impact
on Anti-Tumor immune response. Arch Immunol Ther Exp (Warsz).
61:285–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yin R, Guo L, Zhang W and Zheng J: The
pleiotropic effects of miRNAs on tumor angiogenesis. J Cell
Biochem. 116:1807–1815. 2015. View Article : Google Scholar
|
|
74
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Karaa ZS, Iacovoni JS, Bastide A,
Lacazette E, Touriol C and Prats H: The VEGF IRESes are
differentially susceptible to translation inhibition by miR-16.
RNA. 15:249–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Azhar M, Runyan RB, Gard C, Sanford LP,
Miller ML, Andringa A, Pawlowski S, Rajan S and Doetschman T:
Ligand-specific function of transforming growth factor beta in
epithelial-mesenchymal transition in heart development. Dev Dyn.
238:431–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Piera-Velazquez S and Jimenez SA:
Endothelial to mesenchymal transition: Role in physiology and in
the pathogenesis of human diseases. Physiol Rev. 99:1281–1324.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Piera-Velazquez S, Li Z and Jimenez SA:
Role of endothelial-mesenchymal transition (EndoMT) in the
pathogenesis of fibrotic disorders. Am J Pathol. 179:1074–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Medici D and Olsen BR: The role of
endothelial-mesenchymal transition in heterotopic ossification. J
Bone Miner Res. 27:1619–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
van Meeteren LA and ten Dijke P:
Regulation of endothelial cell plasticity by TGF-β. Cell Tissue
Res. 347:177–186. 2011. View Article : Google Scholar
|
|
82
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2011. View Article : Google Scholar
|
|
84
|
Yeon JH, Jeong HE, Seo H, Cho S, Kim K, Na
D Chung S, Park J, Choi N and Kang JY: Cancer-derived exosomes
trigger endothelial to mesenchymal transition followed by the
induction of cancer-associated fibroblasts. Acta Biomater.
76:146–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yamada NO, Heishima K, Akao Y and Senda T:
Extracellular vesicles containing MicroRNA-92a-3p facilitate
partial Endothelial-mesenchymal transition and angiogenesis in
endothelial cells. Int J Mol Sci. 20:44062019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kim J, Lee C, Kim I, Ro J, Kim J, Min Y,
Park J, Sunkara V, Park YS, Michael I, et al: Three-dimensional
human liver-chip emulating premetastatic niche formation by breast
cancer-derived extracellular vesicles. ACS Nano. 14:14971–14988.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yin Z and Wang L:
Endothelial-to-mesenchymal transition in tumour progression and its
potential roles in tumour therapy. Ann Med. 55:1058–1069. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yin Z, Dong C, Jiang K, Xu Z, Li R, Guo K,
Shao S and Wang L: Heterogeneity of cancer-associated fibroblasts
and roles in the progression, prognosis, and therapy of
hepatocellular carcinoma. J Hematol Oncol. 12:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kobayashi H, Enomoto A, Woods SL, Burt AD,
Takahashi M and Worthley DL: Cancer-associated fibroblasts in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 16:282–295.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar
|
|
92
|
Huang M, Liu T, Ma P, Mitteer RA, Zhang Z,
Kim HJ, Yeo E, Zhang D, Cai P, Li C, et al: c-Met-mediated
endothelial plasticity drives aberrant vascularization and
chemoresistance in glioblastoma. J Clin Invest. 126:1801–1814.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nie L, Lyros O, Medda R, Jovanovic N,
Schmidt JL, Otterson MF, Johnson CP, Behmaram B, Shaker R and
Rafiee P: Endothelial-mesenchymal transition in normal human
esophageal endothelial cells cocultured with esophageal
adenocarcinoma cells: Role of IL-1β and TGF-β2. Am J Physiol Cell
Physiol. 307:C859–C877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu T, Ma W, Xu H, Huang M, Zhang D, He Z,
Zhang L, Brem S, O'Rourke DM, Gong Y, et al: PDGF-mediated
mesenchymal transformation renders endothelial resistance to
anti-VEGF treatment in glioblastoma. Nat Commun. 9:34392018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu K, Pan Q, Jia LQ, Dai Z, Ke AW, Zeng
HY, Tang ZY, Fan J and Zhou J: MiR-302c inhibits tumor growth of
hepatocellular carcinoma by suppressing the endothelial-mesenchymal
transition of endothelial cells. Sci Rep. 4:55242014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ghiabi P, Jiang J, Pasquier J, Maleki M,
Abu-Kaoud N, Halabi N, Guerrouahen BS, Rafii S and Rafii A: Breast
cancer cells promote a notch-dependent mesenchymal phenotype in
endothelial cells participating to a pro-tumoral niche. J Transl
Med. 13:272015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yoshimatsu Y, Wakabayashi I, Kimuro S,
Takahashi N, Takahashi K, Kobayashi M, Maishi N, Podyma-Inoue KA,
Hida K, Miyazono K and Watabe T: TNF-α enhances TGF-β-induced
endothelial-to-mesenchymal transition via TGF-β signal
augmentation. Cancer Sci. 111:2385–2399. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Smeda M, Kieronska A, Adamski MG,
Proniewski B, Sternak M, Mohaissen T, Przyborowski K, Derszniak K,
Kaczor D, Stojak M, et al: Nitric oxide deficiency and
endothelial-mesenchymal transition of pulmonary endothelium in the
progression of 4T1 metastatic breast cancer in mice. Breast Cancer
Res. 20:862018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Krizbai IA, Gasparics A, Nagyoszi P,
Fazakas C, Molnar J, Wilhelm I, Bencs R, Rosivall L and Sebe A:
Endothelial-mesenchymal transition of brain endothelial cells:
Possible role during metastatic extravasation. PLoS One.
10:e01196552015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi SH, Kim AR, Nam JK, Kim JM, Kim JY,
Seo HR, Lee HJ, Cho J and Lee YJ: Tumour-vasculature development
via endothelial-to-mesenchymal transition after radiotherapy
controls CD44v6+ cancer cell and macrophage polarization. Nat
Commun. 9:51082018. View Article : Google Scholar :
|
|
103
|
Ribas A: Adaptive immune resistance: How
cancer protects from immune attack. Cancer Discovery. 5:915–919.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Landsberg J, Kohlmeyer J, Renn M, Bald T,
Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M and Tüting
T: Melanomas resist T-cell therapy through inflammation-induced
reversible dedifferentiation. Nature. 490:412–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Knutson KL, Lu H, Stone B, Reiman JM,
Behrens MD, Prosperi CM, Gad EA, Smorlesi A and Disis ML:
Immunoediting of cancers may lead to epithelial to mesenchymal
transition. J Immunol. 177:1526–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fan C, Chen LL, Hsu TA, Chen CC, Chua KV,
LiC P and Huang TS: Endothelial-mesenchymal transition harnesses
HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal
adenocarcinoma. J Hematol Oncol. 12:1382019. View Article : Google Scholar
|
|
109
|
Liu X, Hoft DF and Peng G: Tumor
microenvironment metabolites directing T cell differentiation and
function. Trends Immunol. 43:132–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Riegler J, Gill H, Ogasawara A, Hedehus M,
Javinal V, Oeh J, Ferl GZ, Marik J, Williams S, Sampath D, et al:
VCAM-1 density and tumor perfusion predict T-cell infiltration and
treatment response in preclinical models. Neoplasia. 21:1036–1050.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Borriello L, Seeger RC, Asgharzadeh S and
DeClerck YA: More than the genes, the tumor microenvironment in
neuroblastoma. Cancer Lett. 380:304–314. 2016. View Article : Google Scholar
|
|
113
|
Marques P, Grossman AB and Korbonits M:
The tumour microenvironment of pituitary neuroendocrine tumours.
Front Neuroendocrinol. 58:1008522020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhao H, Yang L, Baddour J, Achreja A,
Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA,
et al: Tumor microenvironment derived exosomes pleiotropically
modulate cancer cell metabolism. ELife. 5:e102502016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Whiteside TL: Tumor-derived exosomes and
their role in cancer progression. Adv Clin Chem. 174:103–141. 2016.
View Article : Google Scholar
|
|
117
|
Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H,
Li XL, Tao DD, Wu YQ, Gong JP and Qin JC: Exosomal Wnt-induced
dedifferentiation of colorectal cancer cells contributes to
chemotherapy resistance. Oncogene. 38:1951–1965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen X, Liu J, Zhang Q, Liu B, Cheng Y,
Zhang Y, Sun Y, Ge H and Liu Y: Exosome-mediated transfer of
miR-93-5p from cancer-associated fibroblasts confer radioresistance
in colorectal cancer cells by downregulating FOXA1 and upregulating
TGFB3. J Exp Clin Cancer Res. 39:652020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pan S, Deng Y, Fu J, Zhang Y, Zhang Z and
Qin X: N6-methyladenosine upregulates miR-181d-5p in exosomes
derived from cancer-associated fibroblasts to inhibit 5-FU
sensitivity by targeting NCALD in colorectal cancer. Int J Oncol.
60:142022. View Article : Google Scholar :
|
|
121
|
Yuan H, Chen B, Chai R, Gong W, Wan Z,
Zheng B, Hu X, Guo Y, Gao S, Dai Q, et al: Loss of exosomal
micro-RNA-200b-3p from hypoxia cancer-associated fibroblasts
reduces sensitivity to 5-flourouracil in colorectal cancer through
targeting high-mobility group box 3. Front Oncol. 12:9201312022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jiang Y, Qiu Q, Jing X, Song Z, Zhang Y,
Wang C, Liu K, Ye F, Ji X, Luo F and Zhao R: Cancer-associated
fibroblast-derived exosome miR-181b-3p promotes the occurrence and
development of colorectal cancer by regulating SNX2 expression.
Biochem Biophys Res Commun. 641:177–185. 2023. View Article : Google Scholar
|
|
123
|
Shi W, Liu Y, Qiu X, Yang L and Lin G:
Cancer-associated fibroblasts-derived exosome-mediated transfer of
miR-345-5p promotes the progression of colorectal cancer by
targeting CDKN1A. Carcinogenesis. 44:317–327. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhou L, Li J, Tang Y and Yang M: Exosomal
LncRNA LINC00659 transferred from cancer-associated fibroblasts
promotes colorectal cancer cell progression via miR-342-3p/ANXA2
axis. J Transl Med. 19:82021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Qu Z, Yang KD, Luo BH and Zhang F:
CAFs-secreted exosomal cricN4BP2L2 promoted colorectal cancer
stemness and chemoresistance by interacting with EIF4A3. Exp Cell
Res. 418:1132662022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang X, Li Y, Zou L and Zhu Z: Role of
exosomes in crosstalk between Cancer-associated fibroblasts and
cancer cells. Front Oncol. 9:3561029. View Article : Google Scholar
|
|
127
|
Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q,
Shi L, Li H, Yin C, Luo H, Hao C, et al: Cancer-associated
fibroblast-derived exosomal miR-18b promotes breast cancer invasion
and metastasis by regulating TCEAL7. Cell Death Dis. 12:11202021.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sun J, Du R, Li X, Liu C, Wang D, He X, Li
G, Zhang K, Wang S, Hao Q, et al: CD63+ cancer-associated
fibroblasts confer CDK4/6 inhibitor resistance to breast cancer
cells by exosomal miR-20. Cancer Lett. 588:2167472024. View Article : Google Scholar
|
|
129
|
Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu
W, Wang D and Lou W: Exosomal miRNA-106b from cancer-associated
fibroblast promotes gemcitabine resistance in pancreatic cancer.
Exp Cell Res. 383:1115432019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhao M, Zhuang A, Fang Y and Chatterjee S:
Cancer-Associated fibroblast-derived exosomal miRNA-320a promotes
macrophage M2 polarization in vitro by regulating PTEN/PI3Kγ
signaling in pancreatic cancer. J Oncol. 2022:95146972022.
View Article : Google Scholar
|
|
131
|
Wang Z, Zhang M, Liu L, Yang Y, Qiu J, Yu
Y and Li J: Prognostic and immunological role of cancer-associated
fibroblasts-derived exosomal protein in esophageal squamous cell
carcinoma. Int Immunopharmacol. 124:1108372023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P
and Zhang S: Exosomal Sonic Hedgehog derived from cancer-associated
fibroblasts promotes proliferation and migration of esophageal
squamous cell carcinoma. Cancer Med. 9:2500–2513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shi Z, Jiang T, Cao B, Sun X and Liu J:
CAF-derived exosomes deliver LINC01410 to promote
epithelial-mesenchymal transition of esophageal squamous cell
carcinoma. Exp Cell Res. 412:1130332022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang F, Yan Y, Yang Y, Hong X, Wang M,
Yang Z, Liu B and Ye L: MiR-210 in exosomes derived from CAFs
promotes non-small cell lung cancer migration and invasion through
PTEN/PI3K/AKT pathway. Cell Signal. 73:1096752020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang T, Zhang P and Li HX: CAFs-Derived
Exosomal miRNA-130a confers Cisplatin resistance of NSCLC cells
through PUM2-dependent packaging. Int J Nanomedicine. 16:561–577.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lu L, Huang J, Mo J, Da X, Li Q, Fan M and
Lu H: Exosomal lncRNA TUG1 from cancer-associated fibroblasts
promotes liver cancer cell migration, invasion, and glycolysis by
regulating the miR-524-5p/SIX1 axis. Cell Mol Biol Lett. 27:172022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhou Y, Tang W, Zhuo H, Zhu D, Rong D, Sun
J and Song J: Cancer-associated fibroblast exosomes promote
chemoresistance to cisplatin in hepatocellular carcinoma through
circZFR targeting signal transducers and activators of
transcription (STAT3)/nuclear factor-kappa B (NF-κB) pathway.
Bioengineered. 13:4786–4797. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhuang J, Lu Q, Shen B, Huang X, Shen L,
Zheng X, Huang R, Yan J and Guo H: TGFβ1 secreted by
cancer-associated fibroblasts induces epithelial-mesenchymal
transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep.
5:119242015. View Article : Google Scholar
|
|
139
|
Wang Y, Li T, Yang L, Zhang X, Wang X, Su
X, Ji C and Wang Z: Cancer-associated fibroblast-released
extracellular vesicles carrying miR-199a-5p induces the progression
of gastric cancer through regulation of FKBP5-mediated AKT1/mTORC1
signaling pathway. Cell Cycle. 21:2590–2601. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Qu X, Liu B, Wang L, Liu L, Zhao W, Liu C,
Ding J, Zhao S, Xu B, Yu H, et al: Loss of cancer-associated
fibroblast-derived exosomal DACT3-AS1 promotes malignant
transformation and ferroptosis-mediated oxaliplatin resistance in
gastric cancer. Drug Resist Updat. 68:1009362023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yugawa K, Yoshizumi T, Mano Y, Itoh S,
Harada N, Ikegami T, Kohashi K, Oda Y and Mori M: Cancer-associated
fibroblasts promote hepatocellular carcinoma progression through
downregulation of exosomal miR-150-3p. Eur J Surg Oncol.
47:384–393. 2021. View Article : Google Scholar
|
|
142
|
Chen X, Ren X, E J, Zhou Y and Bian R:
Exosome-transmitted circ IFNGR2 modulates ovarian cancer metastasis
via miR-378/ST5 Axis. Mol Cell Biol. 43:22–42. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sun Z, Wang L, Dong L and Wang X: Emerging
role of exosome signalling in maintaining cancer stem cell dynamic
equilibrium. J Cell Mol Med. 22:3719–3728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Xu J, Liao K and Zhou W: Exosomes regulate
the transformation of cancer cells in cancer stem cell homeostasis.
Stem Cells Int. 2018:48373702018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Li W, Zhang L, Guo B, Deng J, Wu S, Li F,
Wang Y, Lu J and Zhou Y: Exosomal FMR1-AS1 facilitates maintaining
cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc
signaling in female esophageal carcinoma. Mol Cancer. 18:222019.
View Article : Google Scholar
|
|
146
|
Wang L, Yang G, Zhao D, Wang J, Bai Y,
Peng Q, Wang H, Fang R, Chen G, Wang Z, et al: CD103-positive CSC
exosome promotes EMT of clear cell renal cell carcinoma: Role of
remote MiR-19b-3p. Mol Cancer. 18:862019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Cheng Z, Lei Z, Yang P, Si A, Xiang D,
Tang X, Guo G, Zhou J and Hüser N: Exosome-transmitted p120-catenin
suppresses hepatocellular carcinoma progression via STAT3 pathways.
Mol Carcinog. 58:1389–1399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang J, Zheng Y and Zhao M: Exosome-Based
cancer therapy: Implication for targeting cancer stem cells. Front
Pharmacol. 7:5332017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yang Z, Zhao N, Cui J, Wu H, Xiong J and
Peng T: Exosomes derived from cancer stem cells of
gemcitabine-resistant pancreatic cancer cells enhance drug
resistance by delivering miR-210. Cell Oncol. 43:123–136. 2019.
View Article : Google Scholar
|
|
150
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yao H, Liu N, Lin MC and Zheng J: Positive
feedback loop between cancer stem cells and angiogenesis in
hepatocellular carcinoma. Cancer Lett. 379:213–219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang ZF, Liao F, Wu H and Dai J: Glioma
stem cells-derived exosomal miR-26a promotes angiogenesis of
microvessel endothelial cells in glioma. J Exp Clin Cancer Res.
38:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhang D, Li D, Shen L, Hu D, Tang B, Guo
W, Wang Z, Zhang Z, Wei G and He D: Exosomes derived from
Piwil2-induced cancer stem cells transform fibroblasts into
cancer-associated fibroblasts. Oncol Rep. 43:1125–1132.
2020.PubMed/NCBI
|
|
154
|
Wang L, He J, Hu H, Tu L, Sun Z, Liu Y and
Luo F: Lung CSC-derived exosomal miR-210-3p contributes to a
pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell
Mol Med. 24:6324–6339. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dai W, Jin X, Han L, Huang H, Ji Z, Xu X,
Tang M, Jiang B and Chen W: Exosomal lncRNA DOCK9-AS2 derived from
cancer stem cell-like cells activated Wnt/β-catenin pathway to
aggravate stemness, proliferation, migration, and invasion in
papillary thyroid carcinoma. Cell Death Dis. 11:7432020. View Article : Google Scholar
|
|
156
|
Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M
and Zhao R: Cancer stem cell-like cells-derived exosomal CDKN2B-AS1
stabilizes CDKN2B to promote the growth and metastasis of thyroid
cancer via TGF-β1/Smad2/3 signaling. Exp Cell Res. 419:1132682022.
View Article : Google Scholar
|
|
157
|
Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M
and Zhao R: Cancer stem cell-like cells-derived exosomal lncRNA
CDKN2B-AS1 promotes biological characteristics in thyroid cancer
via miR-122-5p/P4HA1 axis. Exp Cell Res. 22:19–29. 2023.
|
|
158
|
Li X, Liu D, Chen H, Zeng B, Zhao Q, Zhang
Y, Chen Y, Wang J and Xing HR: Melanoma stem cells promote
metastasis via exosomal miR-1268a inactivation of autophagy. Biol
Res. 55:292022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Han T, Chen L and Li K, Hu Q, Zhang Y, You
X, Han L, Chen T and Li K: Significant CircRNAs in liver cancer
stem cell exosomes: Mediator of malignant propagation in liver
cancer? Mol Cancer. 22:1972023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Deng H, Sun C, Sun Y, Li H, Yang L, Wu D,
Gao Q and Jiang X: Lipid, Protein, and MicroRNA composition within
mesenchymal stem Cell-derived exosomes. Cell Cell Reprogram.
20:178–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Sharma A: Role of stem cell derived
exosomes in tumor biology. Int J Cancer. 142:1086–1092. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yang YP, Nguyen PNN, Ma HI, Ho WJ, Chen
YW, Chien Y, Yarmishyn AA, Huang PI, Lo WL, Wang CY, et al: Tumor
mesenchymal stromal cells regulate cell migration of atypical
teratoid rhabdoid tumor through Exosome-mediated miR155/SMARCA4
pathway. Cancers (Basel). 11:7202019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Figueroa J, Phillips LM, Shahar T, Hossain
A, Gumin J, Kim H, Bean AJ, Calin GA, Fueyo J, Walters ET, et al:
Exosomes from glioma-associated mesenchymal stem cells increase the
tumorigenicity of Glioma Stem-like cells via transfer of miR-1587.
Cancer Res. 77:5808–5819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Toh WS, Lai RC, Zhang B and Lim SK: MSC
exosome works through a protein-based mechanism of action. Biochem
Soc Trans. 46:843–853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Lee C, Mitsialis SA, Aslam M, Vitali SH,
Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A and
Kourembanas S: Exosomes mediate the cytoprotective action of
mesenchymal stromal cells on hypoxia-induced pulmonary
hypertension. Circulation. 126:2601–2611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol.
40:457–470. 2017. View Article : Google Scholar
|
|
167
|
Biswas S, Mandal G, Roy Chowdhury S,
Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X,
Conejo-Garcia JR and Bhattacharyya A: Exosomes produced by
mesenchymal stem cells drive differentiation of myeloid cells into
immunosuppressive M2-Polarized macrophages in breast cancer. J
Immunol. 203:3447–3460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Xu H, Zhao G, Zhang Y, Jiang H, Wang W,
Zhao D, Hong J, Yu H and Qi L: Mesenchymal stem cell-derived
exosomal microRNA-133b suppresses glioma progression via
Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res
Ther. 10:3812019. View Article : Google Scholar
|
|
169
|
Xu Z, Zhou X, Wu J, Cui X, Wang M, Wang X
and Gao Z: Mesenchymal stem cell-derived exosomes carrying
microRNA-150 suppresses the proliferation and migration of
osteosarcoma cells via targeting IGF2BP1. Transl Cancer Res.
9:5323–5335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Qi J, Zhang R and Wang Y: Exosomal
miR-21-5p derived from bone marrow mesenchymal stem cells promote
osteosarcoma cell proliferation and invasion by targeting PIK3R1. J
Cell Mol Med. 25:11016–11030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li T, Wan Y, Su Z, Li J, Han M and Zhou C:
Mesenchymal stem Cell-derived exosomal microRNA-3940-5p inhibits
colorectal cancer metastasis by targeting integrin α6. Dig Dis Sci.
66:1916–1927. 2020. View Article : Google Scholar
|
|
172
|
Gu H, Yan C, Wan H, Wu L, Liu J, Zhu Z and
Gao D: Mesenchymal stem cell-derived exosomes block malignant
behaviors of hepatocellular carcinoma stem cells through a lncRNA
C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Human Cell.
34:1812–1829. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lyu ZZ, Li M, Yang MY, Han M and Yang Z:
Exosome-mediated transfer of circRNA563 promoting hepatocellular
carcinoma by targeting the microRNA148a-3p/metal-regulatory
transcription factor-1 pathway. World J Gastroenterol.
29:6060–6075. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yong SB, Chung JY, Song Y, Kim J, Ra S and
Kim YH: Non-viral nano-immunotherapeutics targeting tumor
microenvironmental immune cells. Biomaterials. 219:1194012019.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang Q, Fan Z, Zhang L, You Q and Wang L:
Strategies for targeting Serine/Threonine protein phosphatases with
small molecules in cancer. J Med Chem. 64:8916–8938. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li Z, Suo B, Long G, Gao Y, Song J, Zhang
M, Feng B, Shang C and Wang D: Exosomal miRNA-16-5p derived from M1
macrophages enhances T cell-dependent immune response by regulating
PD-L1 in gastric cancer. Front Cell Dev Biol. 8:5726892020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Jiang H, Zhou L, Shen N, Ning X, Wu D,
Jiang K and Huang X: M1 macrophage-derived exosomes and their key
molecule lncRNA HOTTIP suppress head and neck squamous cell
carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell
Death Dis. 13:1832022. View Article : Google Scholar
|
|
178
|
Li X and Tang M: Exosomes released from M2
macrophages transfer miR-221-3p contributed to EOC progression
through targeting CDKN1B. Cancer Med. 9:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar
|
|
180
|
Mi X, Xu R, Hong S, Xu T, Zhang W and Liu
M: M2 Macrophage-Derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a
affect cell migration and metastasis in esophageal cancer. Mol Ther
Nucl Acids. 22:779–790. 2020. View Article : Google Scholar
|
|
181
|
Yang Y, Guo Z, Chen W, Wang X, Cao M, Han
X, Zhang K, Teng B, Cao J, Wu W, et al: M2 Macrophage-Derived
exosomes promote angiogenesis and growth of pancreatic ductal
adenocarcinoma by Targeting E2F2. Mol Ther. 29:1226–1238. 2021.
View Article : Google Scholar :
|
|
182
|
Chen S, Lv M, Fang S, Ye W, Gao Y and Xu
Y: Poly(I:C) enhanced anti-cervical cancer immunities induced by
dendritic cells-derived exosomes. Int J Biol Macromol.
113:1182–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Viaud S, Terme M, Flament C, Taieb J,
André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz
T, et al: Dendritic cell-derived exosomes promote natural killer
cell activation and proliferation: A role for NKG2D ligands and
IL-15Ralpha. PLoS One. 4:e49422009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Wang Y, Yin K, Tian J, Xia X, Ma J, Tang
X, Xu H and Wang S: Granulocytic Myeloid-Derived suppressor cells
promote the stemness of colorectal cancer cells through exosomal
S100A9. Adv Sci (Weinh). 6:19012782019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhou JH, Yao ZX, Zheng Z, Yang J, Wang R,
Fu SJ, Pan XF, Liu ZH and Wu K: G-MDSCs-derived Exosomal
miRNA-143-3p promotes proliferation via targeting of ITM2B in lung
cancer. Onco Targets Ther. 13:9701–9719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhou WJ, Zhang J, Xie F, Wu JN, Ye JF,
Wang J, Wu K and Li MQ: CD45RO-CD8+ T cell-derived exosomes
restrict estrogen-driven endometrial cancer development via the
ERβ/miR-765/PLP2/Notch axis. Theranostics. 11:5330–5345. 2021.
View Article : Google Scholar :
|
|
187
|
Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang
Q, Yang Y, Wang L, Cao X and Wang J: Activated T cell exosomes
promote tumor invasion via Fas signaling pathway. J Immunol.
188:5954–5961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Xie Y, Zhang X, Zhao T, Li W and Xiang J:
Natural CD8+25+ regulatory T cell-secreted
exosomes capable of suppressing cytotoxic T lymphocyte-mediated
immunity against B16 melanoma. Biochem Biophys Res Commun.
438:152–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Guyon N, Garnier D, Briand J, Nadaradjane
A, Bougras-Cartron G, Raimbourg J, Campone M, Heymann D, Vallette
FM, Frenel JS and Cartron PF: Anti-PD1 therapy induces
lymphocyte-derived exosomal miRNA-4315 release inhibiting
Bim-mediated apoptosis of tumor cells. Cell Death Dis. 11:10482020.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu
C, Chen Y, Zhou W, Lin A, Yu L, et al: Specific decrease in
B-cell-derived extracellular vesicles enhances
post-chemotherapeutic CD8+ T cell responses. Immunity.
50:738–750.e7. 2019. View Article : Google Scholar
|
|
191
|
Yang Z, Wang W, Zhao L, Wang X, Gimple RC,
Xu L, Wang Y, Rich JN and Zhou S: Plasma cells shape the
mesenchymal identity of ovarian cancers through transfer of
exosome-derived microRNAs. Sci Adv. 7:eabb07372021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Aguilar-Cazares D, Chavez-Dominguez R,
Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON and
Lopez-Gonzalez JS: Contribution of angiogenesis to inflammation and
cancer. Front Oncol. 9:13992019. View Article : Google Scholar
|
|
193
|
Dominiak A, Chełstowska B, Olejarz W and
Nowicka G: Communication in the cancer microenvironment as a target
for therapeutic interventions. Cancers (Basel). 12:12322020.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Stec M, Baj-Krzyworzeka M, Baran J,
Węglarczyk K and Zembala M, Barbasz J, Szczepanik A and Zembala M:
Isolation and characterization of circulating micro(nano)vesicles
in the plasma of colorectal cancer patients and their interactions
with tumor cells. Oncol Rep. 34:2768–2775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Aslan C, Maralbashi S, Salari F, Kahroba
H, Sigaroodi F, Kazemi T and Kharaziha P: Tumor-derived exosomes:
Implication in angiogenesis and antiangiogenesis cancer therapy. J
Cell Physiol. 234:16885–16903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zhao Z, Sun W, Guo Z, Zhang J, Yu H and
Liu B: Mechanisms of lncRNA/microRNA interactions in angiogenesis.
Life Sci. 254:1169002020. View Article : Google Scholar
|
|
197
|
Folkman J, Merler E, Abernathy C and
Williams G: Isolation of a tumor factor responsible for
angiogenesis. J Exp Med. 133:275–288. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Weinstein N, Mendoza L, Gitler I and Klapp
J: A Network model to explore the effect of the Micro-environment
on endothelial cell behavior during angiogenesis. Front Physiol.
8:9602017. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Vavourakis V, Wijeratne PA, Shipley R,
Loizidou M, Stylianopoulos T and Hawkes DJ: A validated multiscale
In-silico model for mechano-sensitive tumour angiogenesis and
growth. PLoS Comput Biol. 13:e10052592017. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Varberg KM, Winfree S, Dunn KW and
Haneline LS: Kinetic analysis of vasculogenesis quantifies dynamics
of vasculogenesis and angiogenesis in vitro. J Vis Exp. 57044:2018.
View Article : Google Scholar
|
|
201
|
Ludwig N and Whiteside TL: Potential roles
of tumor-derived exosomes in angiogenesis. Expert Opin Ther
Targets. 22:409–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Kaur B, Cork SM, Sandberg EM, Devi NS,
Zhang Z, Klenotic PA, Febbraio M, Shim H, Mao H, Tucker-Burden C,
et al: Vasculostatin inhibits intracranial glioma growth and
negatively regulates in vivo angiogenesis through a CD36-dependent
mechanism. Cancer Res. 69:1212–1220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Taverna S, Flugy A, Saieva L, Kohn EC,
Santoro A, Meraviglia S, De Leo G and Alessandro R: Role of
exosomes released by chronic myelogenous leukemia cells in
angiogenesis. Int J Cancer. 130:2033–2043. 2012. View Article : Google Scholar
|
|
205
|
Siemann DW and Horsman MR: Modulation of
the tumor vasculature and oxygenation to improve therapy. Pharmacol
Ther. 153:107–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hida K, Maishi N, Annan DA and Hida Y:
Contribution of tumor endothelial cells in cancer progression. Int
J Mol Sci. 19:12722018. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y,
Wang C, Zhang Q, Yang S, Cao L, Zhang X, et al: Hypoxic exosomes
facilitate angiogenesis and metastasis in esophageal squamous cell
carcinoma through altering the phenotype and transcriptome of
endothelial cells. Int J Mol Sci. 38:3892019.
|
|
208
|
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC,
Tsai PH, Wu CY and Kuo PL: Hypoxic lung cancer-secreted exosomal
miR-23a increased angiogenesis and vascular permeability by
targeting prolyl hydroxylase and tight junction protein ZO-1.
Oncogene. 36:4929–4942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sruthi TV, Edatt L, Raji GR, Kunhiraman H,
Shankar SS, Shankar V, Ramachandran V, Poyyakkara A and Kumar SVB:
Horizontal transfer of miR-23a from hypoxic tumor cell colonies can
induce angiogenesis. J Cell Physiol. 233:3498–3514. 2018.
View Article : Google Scholar
|
|
210
|
Gesierich S, Berezovskiy I, Ryschich E and
Zöller M: Systemic induction of the angiogenesis switch by the
tetraspanin D6.1A/CO-029. Cancer Res. 66:7083–7094. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sheldon H, Heikamp E, Turley H, Dragovic
R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RCA, et
al: New mechanism for Notch signaling to endothelium at a distance
by Delta-like 4 incorporation into exosomes. Blood. 116:2385–2394.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Tang MKS, Yue PYK, Ip PP, Huang RL, Lai
HC, Cheung ANY, Tse KY, Ngan HYS and Wong AST: Soluble E-cadherin
promotes tumor angiogenesis and localizes to exosome surface.
Nature Commun. 9:22702018. View Article : Google Scholar
|
|
213
|
Svensson KJ, Kucharzewska P, Christianson
HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W
and Belting M: Hypoxia triggers a proangiogenic pathway involving
cancer cell microvesicles and PAR-2-mediated heparin-binding EGF
signaling in endothelial cells. Proc Natl Acad Sci USA.
108:13147–13152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wu D, Deng S, Li L, Liu T, Zhang T, Li J,
Yu Y and Xu Y: TGF-β1-mediated exosomal lnc-MMP2-2 increases
blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis
to promote non-small cell lung cancer brain metastasis. Cell Death
Dis. 12:7212021. View Article : Google Scholar
|
|
216
|
Dou R, Liu K, Yang C, Zheng J, Shi D, Lin
X, Wei C, Zhang C, Fang Y, Huang S, et al: EMT-cancer cells-derived
exosomal miR-27b-3p promotes circulating tumour cells-mediated
metastasis by modulating vascular permeability in colorectal
cancer. Cell Death Dis. 11:e5952021.
|
|
217
|
Liu K, Dou R, Yang C, Di Z, Shi D, Zhang
C, Song J, Fang Y, Huang S, Xiang Z, et al: Exosome-transmitted
miR-29a induces colorectal cancer metastasis by destroying the
vascular endothelial barrier. Carcinogenesis. 44:356–367. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Xu Y, Leng K, Yao Y, Kang P, Liao G, Han
Y, Shi G, Ji D, Huang P, Zheng W, et al: Circular RNA,
Cholangiocarcinoma-associated circular RNA 1, contributes to
Cholangiocarcinoma progression, induces angiogenesis, and disrupts
vascular endothelial barriers. Hepatology. 73:1419–1435. 2021.
View Article : Google Scholar
|
|
219
|
Li K, Xue W, Lu Z, Wang S, Zheng J, Lu K,
Li M, Zong Y, Xu F, Dai J, et al: Tumor-derived exosomal ADAM17
promotes pre-metastatic niche formation by enhancing vascular
permeability in colorectal cancer. J Exp Clin Cancer Res.
43:592024. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Nazarenko I, Rana S, Baumann A, McAlear J,
Hellwig A, Trendelenburg M, Lochnit G, Preissner KT and Zöller M:
Cell surface tetraspanin Tspan8 contributes to molecular pathways
of exosome-induced endothelial cell activation. Cancer Res.
70:1668–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Akoto T and Saini S: Role of exosomes in
prostate cancer metastasis. Int J Mol Sci. 22:35282021. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Valencia K, Luis-Ravelo D, Bovy N, Antón
I, Martínez-Canarias S, Zandueta C, Ormazábal C, Struman I, Tabruyn
S, Rebmann V, et al: miRNA cargo within exosome-like vesicle
transfer influences metastatic bone colonization. Mol Oncol.
8:689–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
You L, Wu W, Wang X, Fang L, Adam V,
Nepovimova E, Wu Q and Kuca K: The role of hypoxia-inducible factor
1 in tumor immune evasion. Med Res Rev. 41:1622–1643. 2021.
View Article : Google Scholar
|
|
226
|
Mu W, Rana S and Zöller M: Host matrix
modulation by tumor exosomes promotes motility and invasiveness.
Neoplasia. 15:875–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F,
Ma P, Jiang H, Wu X, Shu Y and Xu T: Exosomal circSHKBP1 promotes
gastric cancer progression via regulating the miR-582-3p/HUR/VEGF
axis and suppressing HSP90 degradation. Mol Cancer. 19:1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Gomes FG, Sandim V, Almeida VH, Rondon
AMR, Succar BB, Hottz ED, Leal AC, Verçoza BRF, Rodrigues JCF,
Bozza PT, et al: Breast-cancer extracellular vesicles induce
platelet activation and aggregation by tissue factor-independent
and -dependent mechanisms. Thromb Res. 159:24–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Zhang X, Zhang H, Gu J, Zhang J, Shi H,
Qian H, Wang D, Xu W, Pan J and Santos HA: Engineered extracellular
vesicles for cancer therapy. Adv Mater. 33:e20057092021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Peterson MF, Otoc N, Sethi JK, Gupta A and
Antes TJ: Integrated systems for exosome investigation. Methods.
87:31–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Contreras-Naranjo JC, Wu HJ and Ugaz VM:
Microfluidics for exosome isolation and analysis: Enabling liquid
biopsy for personalized medicine. Lab Chip. 17:3558–3577. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Casadei L, Choudhury A, Sarchet P, Mohana
Sundaram P, Lopez G, Braggio D, Balakirsky G, Pollock R and Prakash
S: Cross-flow microfiltration for isolation selective capture and
release of liposarcoma extracellular vesicles. J Extracell
Vesicles. 10:e120622021. View Article : Google Scholar
|
|
233
|
Huang X, Wu W, Jing D, Yang L, Guo H, Wang
L, Zhang W, Pu F and Shao Z: Engineered exosome as targeted lncRNA
MEG3 delivery vehicles for osteosarcoma therapy. J Control Release.
343:107–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Lu Y, Li L, Lin Z, Li M, Hu X, Zhang Y,
Peng M, Xia H and Han G: Enhancing osteosarcoma killing and CT
imaging using ultrahigh drug loading and NIR-responsive bismuth
Sulfide@ Mesoporous silica nanoparticles. Adv Healthc Mater.
7:e18006022018. View Article : Google Scholar
|
|
235
|
Raghav KP, Wang W, Liu S, Chavez-MacGregor
M, Meng X, Hortobagyi GN, Mills GB, Meric-Bernstam F, Blumenschein
GR and Gonzalez-Angulo AM: cMET and Phospho-cMET protein levels in
breast cancers and survival outcomes. Clin Cancer Res.
18:2269–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Li S, Wu Y, Ding F, Yang J, Li J, Gao X,
Zhang C and Feng J: Engineering macrophage-derived exosomes for
targeted chemotherapy of triple-negative breast cancer. Nanoscale.
12:10854–10862. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Gonçalves MS: Fluorescent labeling of
biomolecules with organic probes. Clin Cancer Res. 109:190–212.
2009.
|
|
238
|
Gray WD, Mitchell AJ and Searles CD: An
accurate, precise method for general labeling of extracellular
vesicles. MethodsX. 2:360–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Takahashi Y, Nishikawa M, Shinotsuka H,
Matsui Y, Ohara S, Imai T and Takakura Y: Visualization and in vivo
tracking of the exosomes of murine melanoma B16-BL6 cells in mice
after intravenous injection. J Biotechnol. 165:77–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Lai CP, Mardini O, Ericsson M, Prabhakar
S, Maguire C, Chen JW, Tannous BA and Breakefield XO: Dynamic
biodistribution of extracellular vesicles in vivo using a
multimodal imaging reporter. ACS Nano. 8:483–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Bose RJC, Uday Kumar S, Zeng Y, Afjei R,
Robinson E, Lau K, Bermudez A, Habte F, Pitteri SJ, Sinclair R, et
al: Tumor cell-derived extracellular vesicle-coated nanocarriers:
An efficient theranostic platform for the cancer-specific delivery
of anti-miR-21 and imaging agents. ACS Nano. 12:10817–10832. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang
D, Dong H and Zhang X: Engineered exosome-mediated near-infrared-II
region V(2)C quantum dot delivery for nucleus-target
low-temperature photothermal therapy. ACS Nano. 13:1499–1510.
2019.PubMed/NCBI
|
|
243
|
Anguela XM and High KA: Entering the
modern era of gene therapy. Annu Rev Med. 70:273–288. 2019.
View Article : Google Scholar
|
|
244
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar :
|
|
245
|
Paunovska K, Loughrey D and Dahlman JE:
Drug delivery systems for RNA therapeutics. Natu Rev Genet.
23:265–280. 2022. View Article : Google Scholar
|
|
246
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Winkle M, El-Daly SM, Fabbri M and Calin
GA: Noncoding RNA therapeutics-challenges and potential solutions.
Nat Rev Drug Discov. 20:629–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Bose RJ, Kumar US, Garcia-Marques F, Zeng
Y, Habte F, McCarthy JR, Pitteri S, Massoud TF and Paulmurugan R:
Engineered cell-derived vesicles displaying targeting peptide and
functionalized with nanocarriers for therapeutic microRNA delivery
to triple-negative breast cancer in mice. Adv Healthc Mater.
11:e21013872022. View Article : Google Scholar :
|
|
249
|
Olejarz W, Kubiak-Tomaszewska G,
Chrzanowska A and Lorenc T: Exosomes in Angiogenesis and
Anti-angiogenic therapy in cancers. Int J Mol Sci. 21:58402020.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Ghafouri-Fard S, Shoorei H, Mohaqiq M and
Taheri M: Non-coding RNAs regulate angiogenic processes. Vascular
Pharmacol. 133-134:1067782020. View Article : Google Scholar
|
|
251
|
Yuan Y, Mei Z, Qu Z, Li G, Yu S, Liu Y,
Liu K, Shen Z, Pu J, Wang Y, et al: Exosomes secreted from
cardiomyocytes suppress the sensitivity of tumor ferroptosis in
ischemic heart failure. Signal Transduct Target Ther. 8:1212023.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Caller T, Rotem I, Shaihov-Teper O,
Lendengolts D, Schary Y, Shai R, Glick-Saar E, Dominissini D,
Motiei M, Katzir I, et al: Small extracellular vesicles from
infarcted and failing heart accelerate tumor growth. Circulation.
149:1729–1748. 2024. View Article : Google Scholar : PubMed/NCBI
|