As the global population ages and lifestyles change,
the burden of cancer continues to increase. According to global
cancer statistics, there were nearly 20 million new cancer cases
and ~9.7 million cancer-related deaths worldwide in 2022 (1). It is expected that by 2050, the
number of new cancer cases will exceed 35 million (2), and cancer will become a notable
challenge to global public health. Due to innovations in cancer
treatment methods and drugs, the overall mortality rate has
decreased. In the United States, recent statistics indicate that
approximately 4.5 million cancer-related deaths were averted
between 1991 and 2022 (3).
However, owing to the involvement of complex factors in tumor
development, effective curative methods are still lacking (4). Historically, treatment methods for
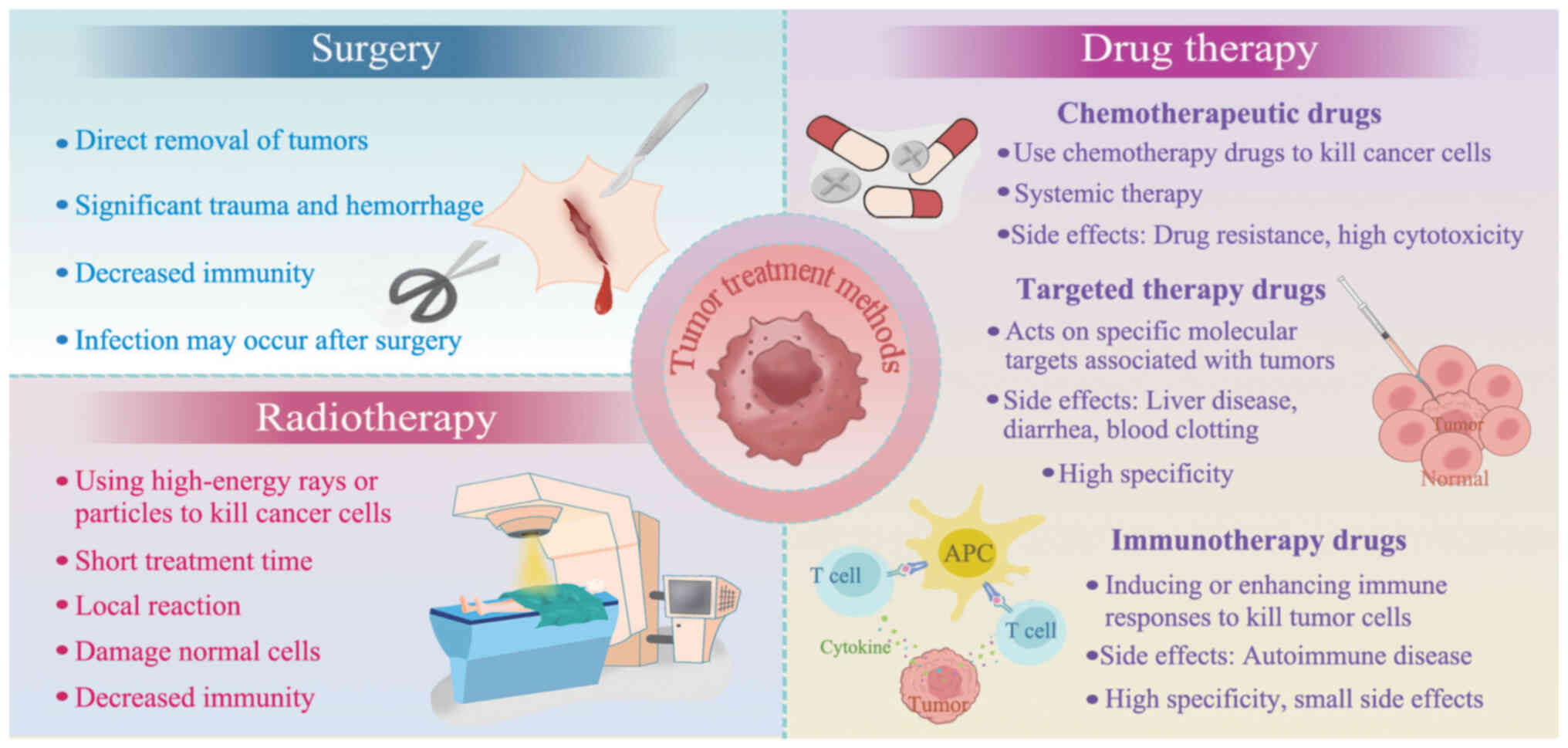
tumors have primarily included surgery and radiation and drug
therapy (Fig. 1). Although
surgical treatment can directly remove tumors, it often causes
notable trauma and bleeding, and postoperative immunity may decline
(5). Additionally, the healing
response of wounds can potentially lead to the growth of metastatic
tumors (6), resulting in
decreased safety. Radiation therapy uses high-energy rays or
particles to kill cancer cells with a short treatment duration and
generally localized reactions. Drug therapy relies mainly on
chemotherapeutic drugs to inhibit or kill cancer cells.
Chemotherapy is often used in combination with surgery or
radiotherapy to control tumor growth. However, both radiation and
chemotherapy can damage normal cells while killing tumor cells
(7) and may also lead to
complications (8). For example,
both treatment modalities induce mucositis (9) and bone marrow suppression (10,11). Radiotherapy can also cause skin
disorders (12) and carries the
risk of inducing secondary malignancies (13). Chemotherapy may result in
gastrointestinal complications (14), premature ovarian failure and
infertility (15). In addition to
chemotherapeutic drugs, targeted therapeutic and immunotherapeutic
drugs have become an important means of tumor treatment (16,17). Targeted therapy prevents the
proliferation of cancer cells by interfering with specific
molecules required for carcinogenesis or tumor proliferation
(18), whereas immunotherapeutic
drugs are safer because they kill tumor cells by inducing or
enhancing immune responses (19).
Hybrid cell vaccine immunization (20), heat shock proteins (HSP) (21), bacterial extracts (22) and T body therapies (23) are commonly used. Mycomedicine
(24) is an immunotherapeutic
strategy and their metabolites are regarded as key oncological
therapeutic drugs because they enhance immune responses by inducing
apoptosis or autophagy and decreasing tumor metastasis. In recent
years, with the rapid development of sequencing technology and
immunology, tumor immunotherapy has gradually become an emerging
tumor therapeutic approach (25,26).
Cancer originates from the abnormal proliferation of
normal cells. Cancer cells are similar to normal cells in terms of
morphology and biological characteristics, making it difficult for
the immune system to recognize and attack them (27,28). When tumor cells are mistakenly
identified by the immune system as self-components, they evade
immune surveillance, allowing them to grow and spread. To address,
researchers have explored various methods aimed at enhancing the
immune system antitumor response and improving its ability to
suppress tumors (29-31). Tumor immunotherapy actively or
passively activates the immune system to generate tumor-specific
immune responses, thereby inhibiting or killing tumor cells. Unlike
traditional treatment methods, immunotherapy does not directly kill
cancer cells; instead, it enhances the immune system and mobilizes
immune cells to recognize and kill tumor cells. This results in
higher specificity and efficiency and fewer side effects (32). Numerous types of tumor
immunotherapy have been developed, including monoclonal antibody
therapy (33), immune checkpoint
inhibitors (ICIs) (34), tumor
vaccines (such as Provenge and Cimavax) (35,36), adoptive cell therapy (37) [such as T cell receptor (TCR)
therapy (38,39), chimeric antigen receptor (CAR) T
cell therapy (CAR-T) (40), and
chimeric antigen receptor natural killer (CAR-NK) cell therapy
(41)] and non-specific immune
modulators. Among these, peptide-based neoantigen vaccines are
gradually becoming popular in the field of tumor immunotherapy
owing to their unique advantages (42,43): Neoantigen vaccines can accurately
identify tumor cells and enhance tumor-specific immune responses,
thereby achieving the targeted destruction of tumors (44). In addition, neoantigen vaccines
stimulate long-term immune memory, decreasing the risk of tumor
recurrence and metastasis (45),
making them a highly promising option for personalized
treatment.
Research on tumor immunology can be traced back to
the early 20th century. Paul Ehrlich proposed the concept of immune
surveillance', suggesting that the immune system is capable of
recognizing and suppressing spontaneously arising tumor cells,
thereby preventing their progression into detectable malignancies
(46). This hypothesis laid the
groundwork for future research in tumor immunology. In 1943, Gross
was the first to demonstrate that methylcholanthrene-induced
sarcomas could elicit an immune response in mice (47). In 1966, Baldwin (48) confirmed the recognition and
rejection response of the immune system to spontaneous tumors,
providing a theoretical foundation for the study of tumor
antigens.
The unique advantage of tumor immunotherapy stems
from its ability to fully utilize the selective recognition and
attack mechanisms of the human immune system, thereby specifically
killing tumor cells without damaging healthy cells (49). This mechanism relies on the
inherent antitumor characteristics of the human adaptive immune
system in which CD8+ and CD4+ T cells serve
crucial roles. After antigen recognition, T cells signal tumor
cells displaying the antigen to undergo cell cycle arrest and cell
death by recognizing peptide fragments on the cell surface via
major histocompatibility complex (MHC) class I and II molecules and
release paracrine signals to elicit an antitumor response (50). Currently, neoantigen vaccines used
in clinical applications primarily include peptide, dendritic cell
(DC) and RNA vaccines (51-53), which are directly injected into
patients subcutaneously or into the lymph nodes. Peptide vaccines
are made directly from neoantigen peptides; after vaccination, the
neoantigens can bind to the corresponding human leukocyte antigen
(HLA) on DC cells in the body, presenting them to T cells and
inducing an immune response (42). DC vaccines use DCs loaded with
neoantigen peptides to present neoantigens directly to T cells
(52). RNA vaccines involve the
injection of RNA fragments that encode neoantigen peptides, which
are translated into neoantigen peptides in the body and presented
by HLA (54).
Since the beginning of the 21st century, research on
tumor antigens has advanced rapidly, with breakthroughs in the
immunotherapy of melanoma (55-57). Ott et al (58) predicted individual tumor
neoantigen vaccines in tests with patients suffering from malignant
melanoma and found that these neoantigen vaccines induced responses
in 16% of CD8+ T cells and 60% of CD4+ T
cells. Among six vaccinated patients, four had no recurrence at 25
months post-vaccination, whereas two patients who experienced
disease progression underwent complete tumor regression after
receiving anti-PD-1 treatment, and their neoantigen-specific T cell
repertoire was expanded. Tumor neoantigen vaccine NeoVax
demonstrates long-term efficacy in eight patients with high-risk
melanoma (59). Personalized
neoantigen vaccination is effective for tumor types beyond melanoma
(60). In a previous study, a
personalized neoantigen-targeted vaccine was used to immunize
patients with newly diagnosed glioblastoma after surgical resection
and conventional radiotherapy. Patients who did not receive
dexamethasone developed multifunctional neoantigen-specific
CD4+ and CD8+ T cell responses that were rich
in memory phenotypes and showed an increase in the number of
tumor-infiltrating T cells. This demonstrates that
neoantigen-targeted vaccines favorably alter the immune environment
of glioblastoma. Furthermore, combination therapy with tumor
vaccines and immunosuppressive treatment is more effective than
single treatments (61,62) and has good prognostic outcomes in
the treatment of various types of cancer, including melanoma
(63), non-small cell lung cancer
(NSCLC) (64) and bladder cancer
(65). Notably, Sipuleucel-T
(66,67), a therapeutic cancer vaccine, has
been approved by the US. Food and Drug Administration for the
treatment of asymptomatic or minimally symptomatic metastatic
castration-resistant prostate cancer (68). These advancements clearly
demonstrate the clinical value and broad potential of cancer
vaccines in modern cancer treatment.
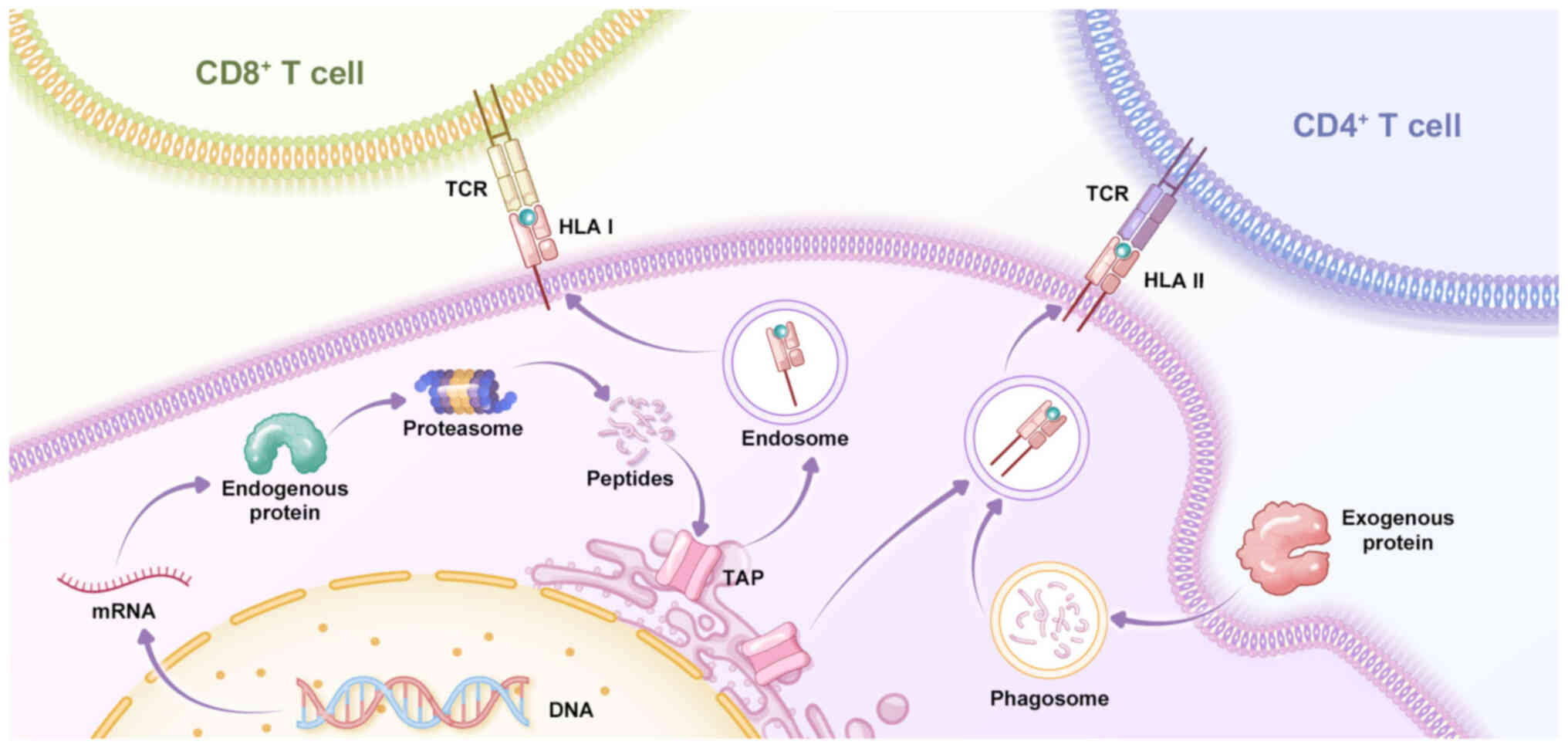
In tumor cells, mutated genes are transcribed into
mRNA and translated into corresponding mutated proteins. These
mutated proteins are degraded by the proteasome into many short
peptides (69), which are
recognized by antigen-presenting cells (APCs). After entering the
endoplasmic reticulum, short peptides are further processed by
N-terminal peptidases and bind HLA in tumor cells to form
peptide-HLA complexes (pHLA). These pHLA complexes leave the
endoplasmic reticulum and are transported to the cell membrane,
where they are recognized by TCR and trigger an antitumor immune
response (70) (Fig. 2). The mutated short peptides that
induce an immune response in tumor cells are referred to as
neoantigens.
The core of cancer immunotherapy is the presence of
tumor antigens in tumor cells, which are recognized by the immune
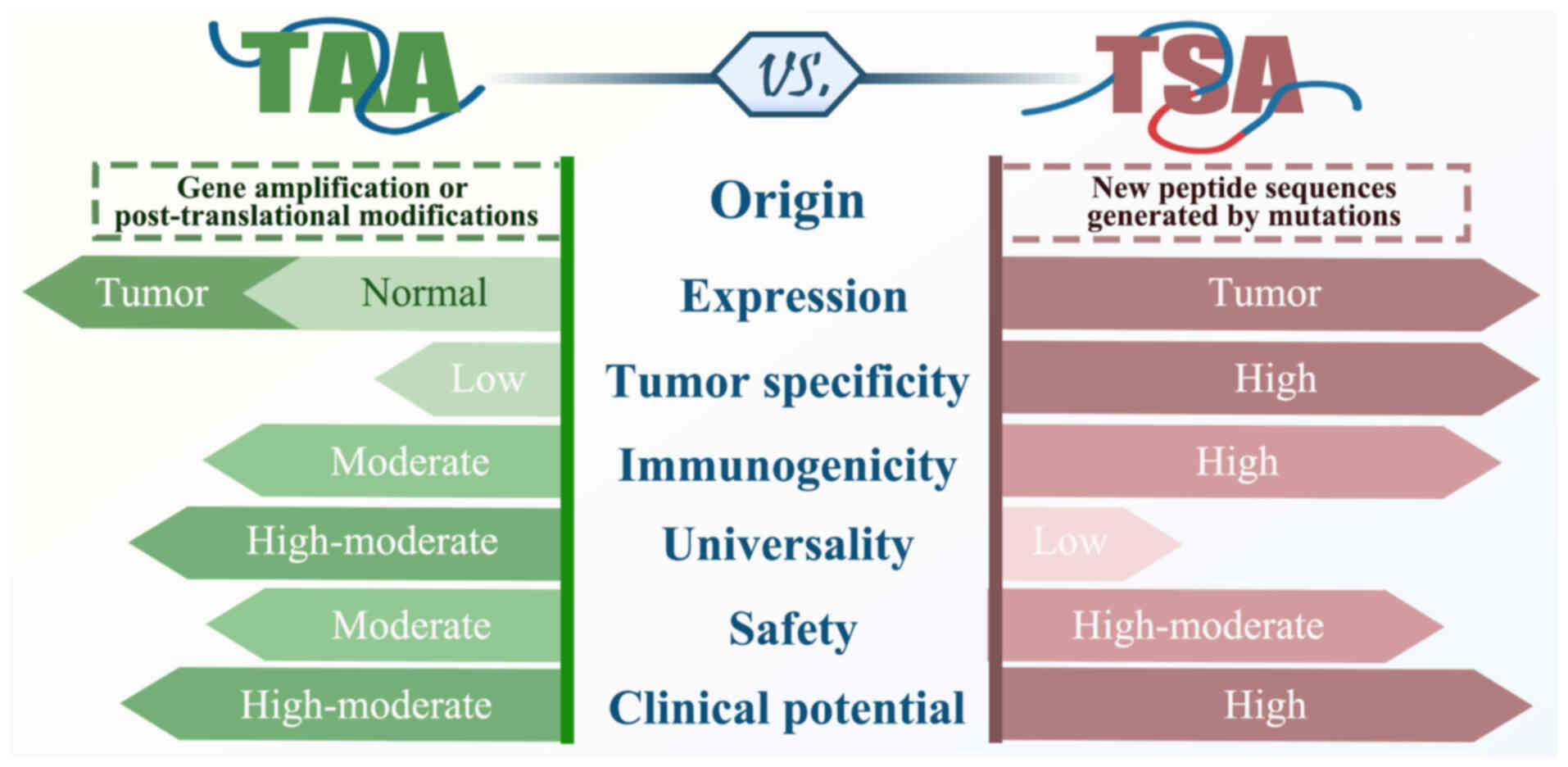
system. Based on their specificity, tumor antigens are divided into
tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs)
(Fig. 3).
By contrast, TSA originates from mutations in the
cancer genome, is present only in tumor cells and is virtually
absent in normal tissues, making it highly tumor-specific. TSA also
has greater immunogenicity and better MHC affinity and is not
affected by central immune tolerance (81). Because normal tissue cells do not
express TSA, immunotherapy targeting TSA does not cause off-target
damage to non-tumor tissue and has a better safety profile
(82). Therefore, TSA, as a tumor
neoantigen, shows potential for clinical applications.
The generation of neoantigens is typically
associated with genomic mutation and the number and types of
mutation vary according to the cancer type (83,84), including single nucleotide
variants (SNVs), insertions/deletions (INDELs) (85) and gene fusion (86,87). Point mutations account for 95% of
tumor mutations, whereas INDELs and frameshift mutations constitute
the remaining portion (88).
Changes in amino acid sequences and spatial structures caused by
INDELs, or frameshift mutations are more pronounced, and mutated
peptides have a stronger affinity for MHC, making them more likely
to be recognized by T cells as neoantigens (89). Therefore, research has mainly
focused on SNVs and INDELs (90),
which frequently occur in tumor cells and trigger abnormal protein
translation under certain conditions (such as altered RNA splicing
(91-93) or imbalances in translational
regulation (94)), leading to the
formation of neoantigens. Trials of neoantigen vaccines for
melanoma indicate that neoantigens derived from SNVs expand T cell
populations (95) and induce
disease regression (58,96). Although neoantigens generated from
SNVs and INDELs have been widely studied (90,97,98), their clinical applications remain
limited. These neoantigens often have high patient specificity
(99-101), meaning that the mutation may not
be the same across patients, resulting in poor universality of
neoantigen vaccines and limited clinical efficacy. Fusion
gene-derived neoantigens have higher immunogenicity (102). Wei et al (103) collected 6,552 tumor samples from
The Cancer Genome Atlas (TCGA), including colon adenocarcinoma,
stomach adenocarcinoma, uterine corpus endometrial carcinoma,
melanoma and predicted a total of 67,502 fusion neoantigens.
Compared with SNVs and INDELs, fusion genes can generate up to
6-fold more candidate neoantigens and up to 11-fold more specific
candidate neoantigens. Moreover, 5.8% of the fusion neoantigens in
TCGA are shared between patients. However, such fusion genes are
relatively rare events (104).
Splice variants, endogenous retroelements (105) and other tumor-specific processes
may also generate neoantigens. In addition to neoantigens derived
from mutations in protein-coding genes, peptides produced from
non-coding regions have immunogenic potential. Non-coding
transcripts can be created from non-coding exons, introns and
untranslated regions, as well as non-canonical reading frames
within coding regions (106).
Laumont et al (107)
discovered numerous antigens from non-coding regions, including
both mutated and unmutated antigens, by studying traditional
non-coding sequences in patients with leukemia and lung cancer.
Huang et al (108) found
that peptides encoded by circular RNA (circRNA) not only exhibit
immunogenicity but can also effectively induce specific T cell
responses, thereby triggering a strong antitumor immune response.
These findings provide novel ideas for tumor immunotherapy,
suggesting the development of vaccines or immunotherapy strategies
using circRNAs and peptides encoded within tumor cells.
Neoantigen screening is a key step in tumor
immunotherapy to identify antigens that can effectively activate
immune responses and target tumor cell-specific mutations. In 2012,
a study of the exome of mouse tumors demonstrated that whole-exome
sequencing (WES) could be used to identify neoantigens (36), marking a development in the
screening of tumor-specific neoantigens based on genomic analysis.
In 2013, Robbins (109) used
exome sequencing to identify mutated proteins expressed in
patients. The aforementioned study used an MHC molecule-antigen
epitope affinity algorithm for simulated predictive assessment and
synthesized candidate antigen epitopes to validate the immune
response. This method can quickly identify mutated antigens
expressed in tumor cells that can be recognized by
tumor-infiltrating lymphocytes. In 2017, Sahin et al
(96) identified individual
mutations using exome sequencing technology and designed and
synthesized personalized tumor vaccines for patients with melanoma.
The results showed that all subjects who received the vaccine
developed a strong and efficient specific antitumor T cell
response, with two of five patients with metastatic tumors
exhibiting objective responses following vaccination. To the best
of our knowledge, the aforementioned study was the first time a
personalized mutant vaccine has been applied in humans, providing a
new path for neoantigen immunotherapy. Exome sequencing technology
is widely used in cancer immunology research and clinical practice
because of its advantages such as large sequencing data volume,
high sequencing depth, mature sequencing technology and reliable
detection of mutated genes (110-112).
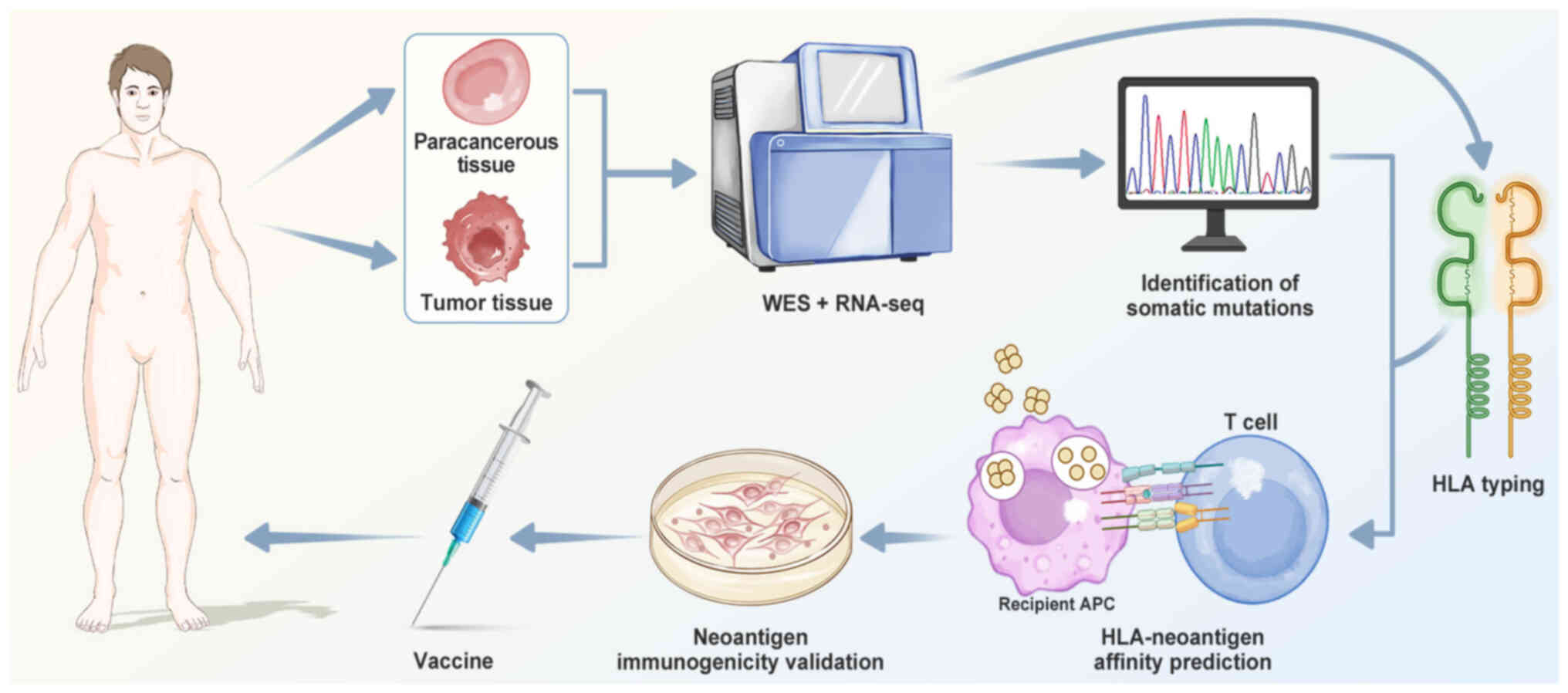
The existing methods for screening neoantigens
involve identification of somatic mutations, HLA typing,
HLA-neoantigen affinity prediction and neoantigen immunogenicity
validation (Fig. 4).
Tumor mutation sites are filtered by performing
exome sequencing of tumors and adjacent normal tissue (110,113), aligning the sequence reads with
the reference genome and using mutation identification tools
(Table I) (114-132) to detect somatic mutations (such
as SNVs and INDELs). RNA-seq can identify neoantigens generated
from gene fusions, along with other biological information, such as
gene expression levels and copy number variations (133), which can refine the selection of
tumor mutation sites. Combining WES data with RNA-seq can confirm
whether mutated genes are expressed in tumor cells, thereby
filtering more reliable candidate neoantigens.
HLA is a key molecule in the immune system that is
involved in antigen recognition. It is encoded by the HLA gene
complex located on chromosome 6p21.3 and is primarily involved in
functions such as antigen recognition and presentation and immune
response regulation, exhibiting a high degree of genetic
polymorphism (134). Based on
structural and functional differences, HLA is divided into three
classes: HLA I, HLA II and HLA III (135). HLA I and II encode molecules
that bind and present antigens, allowing cytotoxic T lymphocytes to
interact with mature HLA cell surface proteins through
antigen-binding grooves and enabling the immune system to recognize
and respond to antigens. HLA I genes present endogenous antigens
(such as tumor antigens) to CD8+ T cells (136), activating cytotoxic immune
responses, and thereby helping the immune system identify and
eliminate tumor cells. By contrast, HLA II molecules are primarily
responsible for presenting exogenous antigens, serving a key role
in initiating immune responses in CD4+ T cells (136), and participating in immune
regulation. HLA III genes are located between HLA I and II
(135), and functions of these
genes remain unclear. In the application of tumor vaccines, HLA
typing affects the prediction of neoantigens (137), thereby affecting vaccine
preparation. Therefore, accurate HLA typing is key for personalized
immunotherapy.
In clinical practice, HLA typing commonly employs
three methods: PCR sequence-specific primers and oligonucleotides
and sequence-based typing (SBT) (138). Among these, the SBT method has
become the gold standard for HLA typing, as recommended by the
World Health Organization, because of its high efficiency and
accuracy. However, these typing methods are labor-intensive and
expensive. Therefore, with the widespread adoption of
next-generation sequencing (NGS) technology, second-generation
sequencing is gradually being used for HLA gene typing (139). Compared with other methods, NGS
technology offers advantages such as high throughput and speed, and
the ability to simultaneously detect classical HLA I and II genes,
as well as non-classical HLA I genes (140,141). Compared with sequence-specific
oligonucleotide probe (SSOP) methods, NGS-based genotyping reduces
reagent consumption, minimizes technical variability between
experiments, and significantly improves the resolution of HLA
allele typing (142).
Furthermore, current HLA typing prediction software can achieve up
to 99% accuracy in predicting HLA class I alleles (143). Several tools are available for
predicting HLA molecular types (Table II) (144-160), among which, OptiType, Polysolver
and PHLAT are considered the best performing HLA I typing
prediction tools (161,162). HLA typing obtained from WES
shows better predictive results than RNA-seq data (149).
HLA molecules exhibit high numbers of polymorphisms,
with each HLA molecule recognizing a range of peptides and
presenting antigenic peptides to T lymphocytes, thereby triggering
an immune response. Although the number of short peptides generated
by mutations is high, tumor antigens are often masked by
polysaccharides or other molecules, making it difficult to process
and effectively present these antigenic peptides. Ultimately, the
number of peptide segments that can bind HLA and be recognized by T
cells to trigger an immune response is limited (163). Of 100-200 peptides generated by
mutations, only one may bind to a specific HLA molecule (164). Therefore, a large number of
candidate false-positive neoantigen peptides exist between the
identification of tumor somatic mutations through sequencing and
the recognition of neoantigens by TCRs that lead to an immune
response.
Traditional screening of candidate positive peptides
relies on cytological experiments such as competitive binding
(165) and enzyme-linked
immunospot (ELISPOT) assays (166). However, depending solely on
these experimental methods is often time-consuming and
labor-intensive. Therefore, techniques such as genomics and
bioinformatics to predict the affinity between HLA and antigen
peptides and select suitable tumor antigens are key in
immunotherapy (167).
Currently, a number of peptide-HLA binding affinity
datasets are available for the training and validation of
prediction software, including the Immune Epitope Database (IEDB)
(168), SYFPEITHI (169), and MHCBN (170). The development of online
prediction algorithms for peptide-MHC affinity is progressing, with
the accuracy of predictions improving (171,172). The discovery of allele-specific
motifs (173) has enabled
algorithms to provide more precise predictions (174). Bioinformatics software tools
have emerged to predict the affinity between HLA and antigen
peptides; commonly used tools include NetMHC (175), NetMHCpan (176), SMM (177) and SMMPMBEC (178). These prediction tools primarily
rely on three types of algorithms: Machine learning (ML),
structure-based approaches, and linear regression (LR). ML-based
methods predict peptide-binding affinity by learning a function
that maps a peptide to regions of binding affinity based on
available known binding peptides (179). Representative software includes
the artificial neural network-based method NetMHC (175) and the pan-specific method
NetMHCpan (176).
Structure-based methods predict peptide-binding affinity by
calculating the minimum free energy of the pHLA (180) using residue-based statistical
energy functions, quantitative structure-activity relationship
analysis and quantitative sequence-activity models (181,182). However, owing to the limited
number of available crystal structures, the prediction speed and
accuracy of these methods are relatively low (183). The LR-based method, a
matrix-based approach, predicts peptide-binding affinity by
constructing a matrix from the alignment of peptides that represent
the motif information (184). As
the linear computational complexity is lower than the non-linear
computational complexity of other methods, these methods can
quickly predict the binding affinity. In addition to these
mainstream prediction algorithms, certain software programs
integrate multiple methods or use their own methods. For example,
TripHLApan (185) combines
triple-encoding matrices and recurrent neural networks, ForestMHC
(186) uses a random forest
classifier and Anthem (187)
develops an aggregating one-dependence estimators model based on
Bayesian ensemble methods to predict the binding affinity between
peptides and HLA (Table III)
(169,175-178,184-226).
Prediction algorithms for HLA I molecules are
mature, but there are relatively few algorithms for HLA II. Unlike
HLA I molecules, the peptide-binding groove of HLA II molecules is
open, leading to variations in both the length of peptides that
bind to HLA-II and the position of the binding core (227). The most common peptide lengths
that bind to HLA II range from 13 to 25 amino acids (228), while class I peptides typically
range from 8 to 15 amino acids (195). To the best of our knowledge,
there is no database regarding the interactions between HLA II and
neoantigens. Therefore, compared with the prediction methods for
HLA I, the binding predictions for HLA II are in a developmental
stage and face more challenges in practical applications (229).
Despite the progress in predictive algorithms,
currently available tools cannot reliably predict the
immunogenicity of peptides. Several comparative studies have been
conducted to evaluate the performance of software packages in
predicting affinity (230-234). The results show that artificial
neural network algorithms exhibit better predictive performance,
with no notable differences between the methods. Concurrently,
there are considerable differences in the results predicted by
these bioinformatics software programs, depending on the algorithm,
MHC type and peptide length (233). Regarding decision thresholds,
certain commonly recommended thresholds [half maximal inhibitory
concentration (IC50)≤500, percentile rank ≤2] can lead to low
sensitivity, potentially overlooking many MHC ligands (235,236). Additionally, applying a lower
binding affinity threshold (IC50≤5,000) generally does not improve
prediction performance. The strong binding affinity threshold
resulted in the most stable accuracy across different predictors,
but also resulted in the lowest accuracy values. Using strong
(IC50≤50) and intermediate (IC50≤500) binding affinity thresholds,
provides high predictive specificity (233).
To predict pHLA binding affinity more accurately,
integrated platforms for prediction methods have emerged (237), such as MHCcombine (233) and IEDB (168). Compared with individual methods,
using multiple approaches is more helpful in screening for
neoantigens, as algorithms vary in modeling strategies, training
datasets, and predictive performance. By combining multiple
approaches, the limitations of individual methods can be
compensated for, improving both the reliability and accuracy of
predictions. This enables a more comprehensive and effective
identification of potential neoantigen candidates. However, their
predictive accuracy remains unsatisfactory, especially in clinical
application (238). Other
factors affect the final immunogenicity of the predicted epitopes.
These include gene expression, RNA splicing, protein processing and
overall patterns of peptide loading and presentation by MHC
(239,240). Therefore, the prediction of
HLA-peptide binding needs to be improved based on these
factors.
Immunogenicity refers to the ability of peptides to
bind MHC molecules and induce adaptive immune responses. The key
factors in this process are MHC molecule presentation and TCR
recognition (241). Not every
expressed mutant peptide will be processed by MHC molecules and
presented on the cell surface, nor do all pHLAs induce T cell
activation and trigger an immune response (242,243). A review of 13 published studies
predicting candidate neoantigens as MHC binders demonstrated that
out of 1,948 new peptide-MHC combinations, only 53 elicited T cell
responses (244). Therefore, it
is important to validate the immunogenicity of neoantigens.
Full activation of T cells requires two signals. The
first signal originates from the specific binding of the TCR to
pHLA. The second signal originates from costimulatory molecules
expressed by APCs that interact with and activate T cells (245). Numerous tools are available for
predicting the recognition of neoantigen-specific T cells, which
assists in the preliminary screening of the immunogenicity of
neoantigens. Commonly used tools such as NetCTL (246) and NetCTLpan (247) generate a comprehensive score by
analyzing MHC presentation, proteasomal C terminal cleavage, and
transporter associated with antigen processing (TAP) transport
efficiency (rather than directly predicting T cell binding) to
identify potential cytotoxic T lymphocyte (CTL) epitopes within
protein sequences. There are also machine and deep learning
techniques to predict TCR and pHLA binding (248). These prediction tools screen
neoantigens further, reducing the experimental workload and the
consumption of scarce experimental materials.
Traditional antigen screening methods rely primarily
on immunochemical luminescence to detect the strength of T cell
effector functions in response to antigen stimulation. When TCRs
recognize their target antigens, T cells are activated and
subsequently release cytokines such as IL-2 and IFN-γ, as well as
granzyme and perforin, leading to the lysis and death of target
cells. Numerous detection methods assess the immunogenicity of
antigens based on the levels of cytokine release, such as ELISA
(249) and ELISPOT (250,251). The most common method is the
ELISPOT technique, which evaluates TCR antigen reactivity by
detecting changes in the cytokines secreted by T cells in response
to antigen stimulation through antibodies (252). This technique is widely used to
assess the responses of CD4+ and CD8+ T cells
to antigens or mitogenic stimulation (253,254). Owing to its simplicity and high
sensitivity, the ELISPOT assay is currently the primary method used
for immunological validation.
Chromium release assay is used to measure the
cytotoxic activity of effector cells in vitro (255). This method quantifies the
specific killing of target cells by T cells by co-culturing T cells
with target cells labeled with the radioactive isotope
51Cr, then detecting the number of radioactive pulses of
51Cr released into the supernatant following lysis of
the target cells (256,257). The Cr release assay has the
advantages of accurate results and high reproducibility; however,
owing to the short half-life of 51Cr, it is difficult to
perform multiple measurements. In addition, 51Cr is
radioactive, posing health risks to researchers and requiring
training in the handling of radioactive materials. These factors
limit the applicability of this method.
Organoids refer to a technology involving the
culture of pluripotent stem cells derived from human tissue in a
semi-solid matrix in the presence of mitogens and pathway
regulators (258). Compared with
traditional cancer cell lines, in vitro tumor organoids have
greater clinical relevance in tumor immunology: Their heterogeneous
three-dimensional structure and spatial arrangement reflect the
genomic, morphological and physiological characteristics of the
original tumor, making them more likely to accurately predict
patient response to drugs (259,260). This provides more precise
efficacy and effectiveness data for drug screening, aiding
identification of resistance mechanisms or understanding the
reasons for treatment failure. Co-culturing tumor organoids with
autologous immune cells can demonstrate the cytotoxic ability of T
cells against tumor organoids and test the immunogenicity of tumor
vaccines (261). Organoids
effectively retain the characteristics of the tumors within the
patient (262), contributing to
the development of personalized treatment plans.
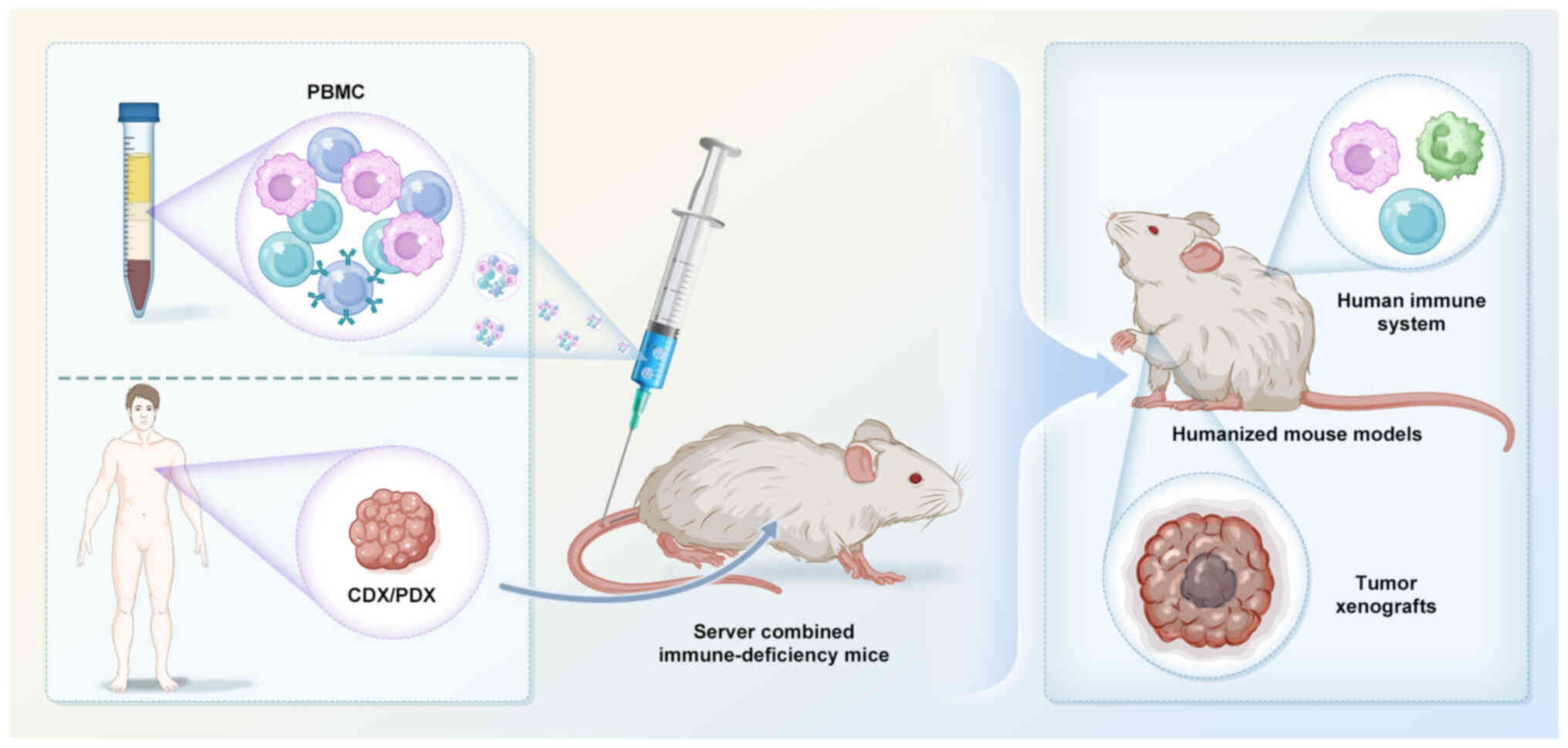
Humanized mouse models have been created by
transplanting human tissue (such as the fetal thymus and liver
tissue), peripheral blood mononuclear cells (PBMCs), or
CD34+ human hematopoietic stem cells and their
progenitors (263,264) into immunodeficient mice to
simulate the human immune system. Subsequently, human cancer cell
line- and patient-derived xenografts are implanted for immune
validation experiments (Fig. 5).
Among humanized mouse models, PBMC-based models are the most widely
used due to their relative ease of implementation and lower cost
(265,266). These humanized mouse models
effectively simulate human immune responses to determine tumor
immune evasion mechanisms, test novel immunotherapeutic strategies,
and validate the safety and efficacy of drugs (267). Humanized mouse models have
achieved notable results in the testing and validation of
immunotherapy and are widely used in preclinical research (268,269). Camacho et al (270) assessed a carcinoembryonic
antigen (CEA) vaccine using transgenic non-obese Diabetic/severe
combined immunodeficiency (NOD/scid)-DR1 mice and detected
CEA-specific cellular immune responses. In another study, Spranger
et al (271) developed
PBMC-NOD/scid IL2Rgnull (NSG) mouse models to evaluate
melanoma-associated antigen MART-1 vaccine, and the results showed
that the vaccine could induce antigen-specific CD8+ T
cell responses. These findings suggest that humanized mouse models
effectively recapitulate human immune cell responses to tumor
antigens and serve as valuable tools for studying anti-tumor immune
responses.
Melanoma is a highly aggressive and metastatic
malignant tumor that is often associated with ultraviolet radiation
exposure. Owing to its high tumor mutational burden (TMB) (272,273), melanoma is an ideal target for
neoantigen-based immunotherapy (274,275).
Lung cancer is the most common cancer worldwide,
accounting for 12.4% of newly diagnosed cases (1). The application of neoantigen
vaccines for the treatment of lung cancer has also progressed.
Phase I/II clinical trials are currently evaluating the safety and
immunogenicity of neoantigen vaccines (279-284) and their role as adjuvant
interventions for other immunotherapies.
Recent studies on tumor neoantigen vaccines have
demonstrated promising efficacy in the treatment of NSCLC (280,281). Li et al (282) reported the results of a phase I
clinical trial involving 24 patients with stage III/IV NSCLC who
received personalized neoantigen peptide vaccines. Following
vaccination, the median PFS was 6.0 and the median OS was 8.9
months. Additionally, all patients exhibited a significant increase
in the frequency of neoantigen-specific CD8+ T cells in
peripheral blood following the vaccination process. In the
KEYNOTE-603 study (280), 16
patients received treatment with the mRNA-4157 vaccine, including
11 patients with NSCLC; of these patients, 14 remained disease-free
throughout the study. This vaccine has progressed to a phase III
clinical trial (281).
The efficacy of neoantigen vaccines in combination
with other treatment modalities for lung cancer is widely
recognized (283,284). As demonstrated by Ott et
al (276), the combination
of neoantigen vaccines with ICIs can enhance the immune system
antitumor response. In a case report (283), a patient with advanced squamous
cell carcinoma who was resistant to PD-1 blockade was treated with
a neoantigen DC vaccine combined with ICIs. The results showed
significant tumor regression, with OS of 48 months and no severe
adverse reactions. Additionally, in a phase Ib clinical trial, Awad
et al (284) used the
personalized neoantigen vaccine NEO-PV-01 in combination with
pemetrexed, carboplatin and pembrolizumab as first-line treatment
for advanced NSCLC. Following vaccination, neoantigen-specific
CD4+ and CD8+ T cell responses were observed
along with increased infiltration of CD4+ T cells, which
exhibited a pronounced cytotoxic phenotype. These findings validate
the potential efficacy of combining neoantigen vaccines with
ICIs.
Gliomas are the most common primary intracranial
tumors of the brain, with a poor prognosis and limited curative
treatment options (286).
Gliomas are considered immune-privileged tumors (287), leading to limited effectiveness
of immunotherapy. However, an increasing number of
immunotherapeutic strategies have been developed (288).
Due to the notable intratumoral heterogeneity of
gliomas, research on glioma neoantigen vaccines has primarily
focused on developing personalized vaccines: For example, Hilf
et al (296) conducted a
phase I clinical trial of the Glioma Actively Personalized Vaccine
Consortium, in which researchers designed personalized vaccines
based on transcriptomic and immunopeptidomic mutation analyses of
tumors. The results showed that most patients responded to at least
one immunopeptide, with a significant increase in CD8+ T
cell numbers and a transition to a memory phenotype. Additionally,
patients exhibited new epitope-specific responses, primarily
TH1-type CD4+ T cell responses, while maintaining a good
safety profile. In a phase I/Ib study, Keskin et al
(60) adopted a similar strategy
using multi-epitope personalized neoantigen vaccines to treat
patients with glioblastoma: Following vaccination, patients
developed multifunctional CD4+ and CD8+
T-cell responses to neoantigens.
Compared with cancers such as melanoma and lung
cancer, the development of neoantigen-based therapies for other
tumor types is in its early stages. The personalized neoantigen
vaccine NEO-PV-01, developed by Ott et al (276), has also made breakthrough
progress in the treatment of bladder cancer. After synthesizing the
antigen peptides, they were mixed with the adjuvant
polyinosinic-polycytidylic acid stabilized with poly-L-lysine and
carboxymethylcellulose (poly-ICLC) (308) for bladder cancer therapy,
demonstrating the vaccine's safety and efficacy (276). Zeng et al (309) treated a patient with advanced
collecting duct carcinoma (CDC) using a personalized neoantigen
vaccine combined with NRTs. Following treatment, both the primary
CDC tumor and metastatic lesions remained in a stable disease state
for 9 months, suggesting that neoantigen-based immunotherapy may
improve the PFS of patients with advanced CDC.
Recent studies have identified neoantigens derived
from RNA splicing aberrations in numerous types of cancer (93,311-313). These previously uncharacterized
shared neoantigens are widely expressed in tumors such as gliomas,
mesothelioma and prostate and liver cancer (314). It is hypothesized that these
neoantigens are endogenously generated and presented by tumor cells
under physiological conditions, effectively triggering
neoantigen-specific CD8+ T cell responses to eradicate
cancer cells (313,315). This provides a molecular
foundation for addressing tumor heterogeneity in immunotherapy.
Research on tumor immunotherapy has a history of
nearly 20 years, with thousands of related clinical research and
development projects registered on ClinicalTrials.gov in the United States. However, the
development of tumor vaccines is a long and challenging process.
Although the rapid development of bioinformatics and
high-throughput sequencing technologies has made the screening of
neoantigens more efficient and convenient, with the emergence of
more specialized algorithms and tools and both the accuracy and
speed of predictions have improved, tumor vaccines based on
neoantigens face issues that need to be addressed.
SNVs and INDELs are important neoantigen sources.
However, in addition to these, there are other types of neoantigen
sources, especially non-coding region neoantigens that have been
previously overlooked, which may have higher immunogenicity
(107,108,316). Research in this field is in its
early stages (317), and
relevant screening processes and standards require further
optimization and improvement (318).
The prediction and screening of neoantigens is
complex because of the variety of mutations involved, the diversity
of HLA molecules and the intricate mechanisms of immune
presentation (319).
Consequently, existing tools and algorithms struggle to simulate
and predict the binding and presentation processes of antigen
peptides to HLA molecules with 100% accuracy. Therefore, there is
room for improvement in the optimization of tools and processes,
especially for the prediction of HLA II molecule binding. Most
current prediction tools focus primarily on HLA I molecules,
whereas the support for HLA II molecules is lacking (229). Enhancing the accuracy of binding
predictions and expanding the support for different HLA types are
key issues to be addressed (320). Additionally, neoantigen data
remains relatively limited. Because neoantigen prediction relies on
a number of unknown factors, particularly individual differences
and the diversity of the tumor microenvironment (321), the uncertainty of the prediction
results is high, which impedes clinical application.
Current research mainly focuses on binding studies
between MHC and antigen peptide segments, but there is relatively
little research on the role of peptide segments in T cell
activation or their therapeutic potential (50,322,323). Although prediction models allow
the identification of a large number of neoantigens in clinical
trials of cancer vaccines, a small fraction of the predicted
antigens exhibit immunogenicity in clinical settings (43,296,324). Relying solely on computational
prediction of neoantigens may not accurately reflect their
therapeutic effectiveness in clinical application.
Based on the current clinical practice, numerous
neoantigen vaccine strategies have been developed using a single
biopsy sample (309,310). However, this approach has
several limitations. First, tumor heterogeneity means that a single
biopsy sample cannot fully represent variations within a tumor.
Candidate neoantigens may differ between tumor lesions, metastatic
sites and between primary and metastatic tumors (325). Neoantigen vaccines derived from
these samples may target a small subset of tumor antigens, thereby
limiting its widespread use.
Antigens that provoke a strong tumor rejection
response often exhibit individual specificity (326). Only a small number of
neoantigens are shared between patients, making it difficult to
develop neoantigen vaccines with broad applicability. Most studies
have concentrated on personalized tumor vaccines (52,54,133,276-285,297,303,307); however, the development of
personalized treatments is time-consuming and costly. In patients
with a short therapeutic window, the time is often insufficient to
complete the process. Therefore, although personalized tumor
vaccines have theoretical potential, developing broadly applicable
treatments within a short time remains a challenge.
Although tumor vaccines based on neoantigens have
made progress, their efficacy remains suboptimal because of tumor
heterogeneity and individual patient differences (325,326). To address these challenges,
several strategies have been proposed. Development of novel
multitarget antigen vaccines by targeting multiple neoantigens
simultaneously can activate a broader immune response, thereby
enhancing therapeutic efficacy (50,54,91). Compared with single-neoantigen
vaccines, multi-target vaccines may overcome tumor heterogeneity
and immune evasion. Current research primarily focuses on
tumor-specific neoantigens derived from mutations (97,319). However, recent studies have
identified non-traditional sources of neoantigens (98), such as gene fusion and RNA
splicing (311-313), which may exhibit high
immunogenicity. Additionally, neoantigens generated from aberrant
translation in non-coding regions warrant further investigation
(107,108,316). In-depth studies of these
neoantigens may reveal more effective immune epitopes. Compared
with personalized neoantigens, developing shared neoantigens is key
for large-scale applications (327). Shared neoantigens can be used
across a broader patient population, decreasing research and
development costs and shortening treatment timelines, thereby
creating favorable conditions for commercial applications. The
delivery method greatly influences the efficacy of neoantigen
vaccines. Each format (peptides, RNA, DNA, viral vectors, DCs) has
pros and cons, and should be chosen based on clinical context and
antigen features (171). To
enhance therapeutic outcomes, it's essential to establish a
comprehensive evaluation system, monitor immune responses, and
optimize formulations, adjuvants, and dosages using preclinical
models. Exploring optimal combinations of neoantigen vaccines with
adjuvants, such as poly-ICLC (328), has demonstrated potential in
clinical trials of personalized neoantigen vaccines (24,97,276). Additionally, studies on immune
induction mechanisms, including the molecular mimicry of tumor
antigens and the ability to initiate antigen epitope spreading,
should be conducted to develop more effective vaccine adjuvants and
enhance vaccine efficacy. Based on neoantigen vaccine therapy,
combining strategies that protect and activate T cells can decrease
T lymphocyte apoptosis in patients, thereby ensuring therapeutic
efficacy. Additionally, the integration of conventional treatments,
radiotherapy, targeted therapy and immunotherapy should be explored
to enhance T cell responses to vaccines and improve clinical
outcomes (78).
In summary, future research should focus on
screening and validation of neoantigens, as well as overcoming the
technical difficulties and costs encountered during the clinical
translation process (329).
Not applicable.
YTJ, BW and HF conceived and design of the study.
HF and BW prepared the original draft of the manuscript. HF drew
the figures. YTJ and BW revised the manuscript. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by Zhejiang Medical Science and
Technology Project (grant no. 2021KY092).
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization: Global cancer
burden growing, amidst mounting need for services. 2024.
|
|
3
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai Z, Yin Y, Shen C, Wang J, Yin X, Chen
Z, Zhou Y and Zhang B: Comparative effectiveness of preoperative,
postoperative and perioperative treatments for resectable gastric
cancer: A network meta-analysis of the literature from the past 20
years. Surg Oncol. 27:563–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krall JA, Reinhardt F, Mercury OA,
Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh
HL, Dougan SK and Weinberg RA: The systemic response to surgery
triggers the outgrowth of distant immune-controlled tumors in mouse
models of dormancy. Sci Transl Med. 10:eaan34642018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu WD, Sun G, Li J, Xu J and Wang X:
Mechanisms and therapeutic potentials of cancer immunotherapy in
combination with radiotherapy and/or chemotherapy. Cancer Lett.
452:66–70. 2019. View Article : Google Scholar
|
|
8
|
Pich O, Muiños F, Lolkema MP, Steeghs N,
Gonzalez-Perez A and Lopez-Bigas N: The mutational footprints of
cancer therapies. Nat Genet. 51:1732–1740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonis ST: Mucositis: The impact, biology
and therapeutic opportunities of oral mucositis. Oral Oncol.
45:1015–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao H, Xiong L, Song X, Jin P, Chen L,
Chen X, Yao H, Wang Y and Wang L: Angelica sinensis polysaccharides
ameliorate stress-induced premature senescence of hematopoietic
cell via protecting bone marrow stromal cells from oxidative
injuries caused by 5-fluorouracil. Int J Mol Sci. 18:22652017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Probin V and Zhou DH: Cancer
therapy-induced residual bone marrow injury-mechanisms of induction
and implication for therapy. Curr Cancer Ther Rev. 2:271–279. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryan JL: Ionizing radiation: The good, the
bad, and the ugly. J Invest Dermatol. 132:985–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dracham CB, Shankar A and Madan R:
Radiation induced secondary malignancies: A review article. Radiat
Oncol J. 36:85–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta K, Walton R and Kataria SP:
Chemotherapy-induced nausea and vomiting: Pathogenesis,
recommendations, and new trends. Cancer Treat Res Commun.
26:1002782021. View Article : Google Scholar
|
|
15
|
Spears N, Lopes F, Stefansdottir A, Rossi
V, De Felici M, Anderson RA and Klinger FG: Ovarian damage from
chemotherapy and current approaches to its protection. Hum Reprod
Update. 25:673–693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urruticoechea A, Alemany R, Balart J,
Villanueva A, Viñals F and Capellá G: Recent advances in cancer
therapy: An overview. Curr Pharm Des. 16:3–10. 2010. View Article : Google Scholar
|
|
17
|
Cavalcanti IDL and Soares JCS: Advances in
cancer treatment: From systemic chemotherapy to targeted therapy.
Springer Nature. 1–109. 2021.
|
|
18
|
Baudino TA: Targeted cancer therapy: The
next generation of cancer treatment. Curr Drug Discov Technol.
12:3–20. 2015. View Article : Google Scholar
|
|
19
|
Lollini PL, Cavallo F, Nanni P and Forni
G: Vaccines for tumour prevention. Nat Rev Cancer. 6:204–216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinho MP, Sundarasetty BS, Bergami-Santos
PC, Steponavicius-Cruz K, Ferreira AK, Stripecke R and Barbuto JAM:
Dendritic-tumor cell hybrids induce tumor-specific immune responses
more effectively than the simple mixture of dendritic and tumor
cells. Cytotherapy. 18:570–580. 2016. View Article : Google Scholar
|
|
21
|
Guzhova IV and Margulis BA: HSP70-based
anti-cancer immunotherapy. Hum Vaccin Immunother. 12:2529–2535.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Howell LM and Forbes NS: Bacteria-based
immune therapies for cancer treatment. Semin Cancer Biol.
86:1163–1178. 2022. View Article : Google Scholar
|
|
23
|
Eshhar Z: The T-body approach: Redirecting
T cells with antibody specificity. Handb Exp Pharmacol.
181:329–342. 2008. View Article : Google Scholar
|
|
24
|
Dai R, Liu M, Nik Nabil WN, Xi Z and Xu H:
Mycomedicine: A unique class of natural products with potent
anti-tumour bioactivities. Molecules. 26:11132021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lau TTY, Sefid Dashti ZJ, Titmuss E,
Pender A, Topham JT, Bridgers J, Loree JM, Feng X, Pleasance ED,
Renouf DJ, et al: The neoantigen landscape of the coding and
noncoding cancer genome space. J Mol Diagn. 24:609–618. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lybaert L, Lefever S, Fant B, Smits E, De
Geest B, Breckpot K, Dirix L, Feldman SA, van Criekinge W,
Thielemans K, et al: Challenges in neoantigen-directed
therapeutics. Cancer Cell. 41:15–40. 2023. View Article : Google Scholar
|
|
27
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar
|
|
28
|
Sánchez-Danés A and Blanpain C:
Deciphering the cells of origin of squamous cell carcinomas. Nat
Rev Cancer. 18:549–561. 2018. View Article : Google Scholar
|
|
29
|
Kim J, Lee BJ, Moon S, Lee H, Lee J, Kim
BS, Jung K, Seo H and Chung Y: Strategies to overcome hurdles in
cancer immunotherapy. Biomater Res. 28:00802024. View Article : Google Scholar
|
|
30
|
Farhadi Rad H, Tahmasebi H, Javani S,
Hemati M, Zakerhamidi D, Hosseini M, Alibabaei F, Banihashemian SZ,
Oksenych V and Eslami M: Microbiota and cytokine modulation:
Innovations in enhancing anticancer immunity and personalized
cancer therapies. Biomedicines. 12:27762024. View Article : Google Scholar
|
|
31
|
Ritu, Chandra P and Das A: Immune
checkpoint targeting antibodies hold promise for combinatorial
cancer therapeutics. Clin Exp Med. 23:4297–4322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Q, Zhang L, Luo C and Jiang M:
Emerging strategies in cancer therapy combining chemotherapy with
immunotherapy. Cancer Lett. 454:191–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weiner GJ: Building better monoclonal
antibody-based therapeutics. Nat Rev Cancer. 15:361–370. 2015.
View Article : Google Scholar :
|
|
34
|
Wei G, Zhang H, Zhao H, Wang J, Wu N, Li
L, Wu J and Zhang D: Emerging immune checkpoints in the tumor
microenvironment: Implications for cancer immunotherapy. Cancer
Lett. 511:68–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oka Y, Tsuboi A, Oji Y, Kawase I and
Sugiyama H: WT1 peptide vaccine for the treatment of cancer. Curr
Opin Immunol. 20:211–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castle JC, Kreiter S, Diekmann J, Löwer M,
van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C,
et al: Exploiting the mutanome for tumor vaccination. Cancer Res.
72:1081–1091. 2012. View Article : Google Scholar
|
|
37
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J and Wang L: The emerging world of
TCR-T cell trials against cancer: A systematic review. Technol
Cancer Res Treat. 18:15330338198310682019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao QJ, Jiang Y, Xiang SX, Kaboli PJ,
Shen J, Zhao YS, Wu X, Du FK, Li MX, Cho CH, et al: Engineered
TCR-T cell immunotherapy in anticancer precision medicine: Pros and
cons. Front Immunol. 12:6587532021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11:692021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daher M, Melo Garcia L, Li Y and Rezvani
K: CAR-NK cells: The next wave of cellular therapy for cancer. Clin
Transl Immunology. 10:e12742021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen XT, Yang J, Wang LF and Liu BR:
Personalized neoantigen vaccination with synthetic long peptides:
Recent advances and future perspectives. Theranostics.
10:6011–6023. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Goedegebuure SP and Gillanders WE:
Preclinical and clinical development of neoantigen vaccines. Ann
Oncol. 28(Suppl 12): xii11–xii17. 2017. View Article : Google Scholar
|
|
44
|
Lybaert L, Thielemans K, Feldman SA, van
der Burg SH, Bogaert C and Ott PA: Neoantigen-directed therapeutics
in the clinic: Where are we? Trends Cancer. 9:503–519. 2023.
View Article : Google Scholar
|
|
45
|
Luo Y, Zhou H, Mizutani M, Mizutani N, Liu
C, Xiang R and Reisfeld RA: A DNA vaccine targeting Fos-related
antigen 1 enhanced by IL-18 induces long-lived T-cell memory
against tumor recurrence. Cancer Res. 65:3419–3427. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ribatti D: The concept of immune
surveillance against tumors: The first theories. Oncotarget.
8:7175–7180. 2017. View Article : Google Scholar
|
|
47
|
Gross L: Intradermal immunization of C3H
mice against a sarcoma that originated in an animal of the same
line. Cancer Res. 3:326–333. 1943.
|
|
48
|
Baldwin RW: Tumour-specific immunity
against spontaneous rat tumours. Int J Cancer. 1:257–264. 1966.
View Article : Google Scholar
|
|
49
|
Dietrich CH, Allen JM, Lemmon AR, Lemmon
EM, Takiya DM, Evangelista O, Walden KKO, Grady PGS and Johnson KP:
Anchored hybrid enrichment-based phylogenomics of leafhoppers and
treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Syst
Divers. 1:57–72. 2017. View Article : Google Scholar
|
|
50
|
Richters MM, Xia H, Campbell KM,
Gillanders WE, Griffith OL and Griffith M: Best practices for
bioinformatic characterization of neoantigens for clinical utility.
Genome Med. 11:562019. View Article : Google Scholar
|
|
51
|
Aljabali AAA, Hamzat Y, Alqudah A and
Alzoubi L: Neoantigen vaccines: Advancing personalized cancer
immunotherapy. Explor Immunol. 5:10031902025. View Article : Google Scholar
|
|
52
|
Fennemann FL, de Vries IJM, Figdor CG and
Verdoes M: Attacking tumors from all sides: Personalized multiplex
vaccines to tackle intratumor heterogeneity. Front Immunol.
10:8242019. View Article : Google Scholar
|
|
53
|
Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong
F, Guo C, Wu X, Li Y, Li X, et al: Neoantigen vaccine: An emerging
tumor immunotherapy. Mol Cancer. 18:1282019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rojas LA, Sethna Z, Soares KC, Olcese C,
Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, et al:
Personalized RNA neoantigen vaccines stimulate T cells in
pancreatic cancer. Nature. 618:144–150. 2023. View Article : Google Scholar
|
|
55
|
Ehx G and Perreault C: Discovery and
characterization of actionable tumor antigens. Genome Med.
11:292019. View Article : Google Scholar
|
|
56
|
Finn OJ: Human tumor antigens yesterday,
today, and tomorrow. Cancer Immunol Res. 5:347–354. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sotirov S and Dimitrov I: Tumor-derived
antigenic peptides as potential cancer vaccines. Int J Mol Sci.
25:49342024. View Article : Google Scholar
|
|
58
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu Z, Leet DE, Allesøe RL, Oliveira G, Li
S, Luoma AM, Liu J, Forman J, Huang T, Iorgulescu JB, et al:
Personal neoantigen vaccines induce persistent memory T cell
responses and epitope spreading in patients with melanoma. Nat Med.
27:515–525. 2021. View Article : Google Scholar :
|
|
60
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239. 2019.
View Article : Google Scholar :
|
|
61
|
Ali OA, Lewin SA, Dranoff G and Mooney DJ:
Vaccines combined with immune checkpoint antibodies promote
cytotoxic T-cell activity and tumor eradication. Cancer Immunol
Res. 4:95–100. 2016. View Article : Google Scholar
|
|
62
|
Karyampudi L, Lamichhane P, Scheid AD,
Kalli KR, Shreeder B, Krempski JW, Behrens MD and Knutson KL:
Accumulation of memory precursor CD8 T cells in regressing tumors
following combination therapy with vaccine and anti-PD-1 antibody.
Cancer Res. 74:2974–2985. 2014. View Article : Google Scholar
|
|
63
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar
|
|
64
|
Qiu Z, Chen Z, Zhang C and Zhong W:
Achievements and futures of immune checkpoint inhibitors in
non-small cell lung cancer. Exp Hematol Oncol. 8:192019. View Article : Google Scholar :
|
|
65
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv42016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Higano CS, Armstrong AJ, Sartor AO,
Vogelzang NJ, Kantoff PW, McLeod DG, Pieczonka CM, Penson DF, Shore
ND, Vacirca J, et al: Real-world outcomes of sipuleucel-T treatment
in PROCEED, a prospective registry of men with metastatic
castration-resistant prostate cancer. Cancer. 125:4172–4180. 2019.
View Article : Google Scholar
|
|
68
|
Lin G, Elkashif A, Saha C, Coulter JA,
Dunne NJ and McCarthy HO: Key considerations for a prostate cancer
mRNA vaccine. Crit Rev Oncol Hematol. 208:1046432025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Blum JS, Wearsch PA and Cresswell P:
Pathways of antigen processing. Annu Rev Immunol. 31:443–473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Babbitt BP, Allen PM, Matsueda G, Haber E
and Unanue ER: Binding of immunogenic peptides to Ia
histocompatibility molecules. Nature. 317:359–361. 1985. View Article : Google Scholar
|
|
71
|
Abdel-Aal ABM, Lakshminarayanan V,
Thompson P, Supekar N, Bradley JM, Wolfert MA, Cohen PA, Gendler SJ
and Boons GJ: Immune and anticancer responses elicited by fully
synthetic aberrantly glycosylated MUC1 tripartite vaccines modified
by a TLR2 or TLR9 agonist. Chembiochem. 15:1508–1513. 2014.
View Article : Google Scholar
|
|
72
|
Vlad AM, Kettel JC, Alajez NM, Carlos CA
and Finn OJ: MUC1 immunobiology: From discovery to clinical
applications. Adv Immunol. 82:249–293. 2004. View Article : Google Scholar
|
|
73
|
Arab A, Yazdian-Robati R and Behravan J:
HER2-positive breast cancer immunotherapy: A focus on vaccine
development. Arch Immunol Ther Exp (Warsz). 68:22020. View Article : Google Scholar
|
|
74
|
Baxevanis CN, Sotiriadou NN, Gritzapis AD,
Sotiropoulou PA, Perez SA, Cacoullos NT and Papamichail M:
Immunogenic HER-2/neu peptides as tumor vaccines. Cancer Immunol
Immunother. 55:85–95. 2006. View Article : Google Scholar
|
|
75
|
Zanetti M: A second chance for telomerase
reverse transcriptase in anticancer immunotherapy. Nat Rev Clin
Oncol. 14:115–128. 2017. View Article : Google Scholar
|
|
76
|
Slingluff CL Jr, Chianese-Bullock KA,
Bullock TNJ, Grosh WW, Mullins DW, Nichols L, Olson W, Petroni G,
Smolkin M and Engelhard VH: Immunity to melanoma antigens: From
self-tolerance to immunotherapy. Adv Immunol. 90:243–295. 2006.
View Article : Google Scholar
|
|
77
|
Guo Q, Wang J, Xiao J, Wang L, Hu X, Yu W,
Song G, Lou J and Chen JF: Heterogeneous mutation pattern in tumor
tissue and circulating tumor DNA warrants parallel NGS panel
testing. Mol Cancer. 17:1312018. View Article : Google Scholar :
|
|
78
|
Hollingsworth RE and Jansen K: Turning the
corner on therapeutic cancer vaccines. NPJ Vaccines. 4:72019.
View Article : Google Scholar
|
|
79
|
Malacopol AT and Holst PJ: Cancer
vaccines: Recent insights and future directions. Int J Mol Sci.
25:112562024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Leisegang M, Engels B, Schreiber K, Yew
PY, Kiyotani K, Idel C, Arina A, Duraiswamy J, Weichselbaum RR,
Uckert W, et al: Eradication of large solid tumors by gene therapy
with a T-cell receptor targeting a single cancer-specific point
mutation. Clin Cancer Res. 22:2734–2743. 2016. View Article : Google Scholar
|
|
81
|
Chen P, Fang QX, Chen DB and Chen HS:
Neoantigen vaccine: An emerging immunotherapy for hepatocellular
carcinoma. World J Gastrointest Oncol. 13:673–683. 2021. View Article : Google Scholar
|
|
82
|
Pan RY, Chung WH, Chu MT, Chen SJ, Chen
HC, Zheng L and Hung SI: Recent development and clinical
application of cancer vaccine: Targeting neoantigens. J Immunol
Res. 2018:43258742018. View Article : Google Scholar
|
|
83
|
Vormehr M, Türeci Ö and Sahin U:
Harnessing tumor mutations for truly individualized cancer
vaccines. Annu Rev Med. 70:395–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Turajlic S, Litchfield K, Xu H, Rosenthal
R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M,
et al: Insertion-and-deletion-derived tumour-specific neoantigens
and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol.
18:1009–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang W, Lee KW, Srivastava RM, Kuo F,
Krishna C, Chowell D, Makarov V, Hoen D, Dalin MG, Wexler L, et al:
Immunogenic neoantigens derived from gene fusions stimulate T cell
responses. Nat Med. 25:767–775. 2019. View Article : Google Scholar :
|
|
87
|
Zhang J, White NM, Schmidt HK, Fulton RS,
Tomlinson C, Warren WC, Wilson RK and Maher CA: INTEGRATE: Gene
fusion discovery using whole genome and transcriptome data. Genome
Res. 26:108–118. 2016. View Article : Google Scholar :
|
|
88
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar
|
|
89
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar
|
|
90
|
Borden ES, Buetow KH, Wilson MA and
Hastings KT: Cancer neoantigens: Challenges and future directions
for prediction, prioritization, and validation. Front Oncol.
12:8368212022. View Article : Google Scholar
|
|
91
|
Anczuków O and Krainer AR: Splicing-factor
alterations in cancers. RNA. 22:1285–1301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kahles A, Lehmann KV, Toussaint NC, Hüser
M, Stark SG, Sachsenberg T, Stegle O, Kohlbacher O and Sander C;
Cancer Genome Atlas Research Network, Gunnar Rätsch: Comprehensive
analysis of alternative splicing across tumors from 8,705 patients.
Cancer Cell. 34:211–224 e6. 2018. View Article : Google Scholar
|
|
93
|
Cheng R, Xu Z, Luo M, Wang P, Cao H, Jin
X, Zhou W, Xiao L and Jiang Q: Identification of alternative
splicing-derived cancer neoantigens for mRNA vaccine development.
Brief Bioinform. 23:bbab5532022. View Article : Google Scholar
|
|
94
|
Weller C, Bartok O, McGinnis CS, Palashati
H, Chang TG, Malko D, Shmueli MD, Nagao A, Hayoun D, Murayama A, et
al: Translation dysregulation in cancer as a source for targetable
antigens. Cancer Cell. S1535-6108(25)00082-02025.Epub ahead of
print.
|
|
95
|
Carreno BM, Magrini V, Becker-Hapak M,
Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH,
Mardis ER and Linette GP: Cancer immunotherapy. A dendritic cell
vaccine increases the breadth and diversity of melanoma
neoantigen-specific T cells. Science. 348:803–808. 2015. View Article : Google Scholar :
|
|
96
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hacohen N, Fritsch EF, Carter TA, Lander
ES and Wu CJ: Getting personal with neoantigen-based therapeutic
cancer vaccines. Cancer Immunol Res. 1:11152013. View Article : Google Scholar
|
|
98
|
Capietto AH, Hoshyar R and Delamarre L:
Sources of cancer neoantigens beyond single-nucleotide variants.
Int J Mol Sci. 23:101312022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schumacher T, Bunse L, Pusch S, Sahm F,
Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, et al: A
vaccine targeting mutant IDH1 induces antitumour immunity. Nature.
512:324–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang QJ, Yu Z, Griffith K, Hanada KI,
Restifo NP and Yang JC: Identification of T-cell receptors
targeting KRAS-mutated human tumors. Cancer Immunol Res. 4:204–214.
2016. View Article : Google Scholar
|
|
101
|
Chheda ZS, Kohanbash G, Okada K, Jahan N,
Sidney J, Pecoraro M, Yang X, Carrera DA, Downey KM, Shrivastav S,
et al: Novel and shared neoantigen derived from histone 3 variant
H3.3K27M mutation for glioma T cell therapy. J Exp Med.
215:141–157. 2018. View Article : Google Scholar :
|
|
102
|
Wang Y, Shi T, Song X, Liu B and Wei J:
Gene fusion neoantigens: Emerging targets for cancer immunotherapy.
Cancer Lett. 506:45–54. 2021. View Article : Google Scholar
|
|
103
|
Wei Z, Zhou C, Zhang Z, Guan M, Zhang C,
Liu Z and Liu Q: The landscape of tumor fusion neoantigens: A
pan-cancer analysis. iScience. 21:249–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dai X, Theobard R, Cheng H, Xing M and
Zhang J: Fusion genes: A promising tool combating against cancer.
Biochim Biophys Acta Rev Cancer. 1869:149–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Smith CC, Selitsky SR, Chai S, Armistead
PM, Vincent BG and Serody JS: Alternative tumour-specific antigens.
Nat Rev Cancer. 19:465–478. 2019. View Article : Google Scholar :
|
|
106
|
Diederichs S, Bartsch L, Berkmann JC,
Fröse K, Heitmann J, Hoppe C, Iggena D, Jazmati D, Karschnia P,
Linsenmeier M, et al: The dark matter of the cancer genome:
Aberrations in regulatory elements, untranslated regions, splice
sites, non-coding RNA and synonymous mutations. EMBO Mol Med.
8:442–457. 2016. View Article : Google Scholar :
|
|
107
|
Laumont CM, Vincent K, Hesnard L, Audemard
É, Bonneil É, Laverdure JP, Gendron P, Courcelles M, Hardy MP, Côté
C, et al: Noncoding regions are the main source of targetable
tumor-specific antigens. Sci Transl Med. 10:eaau55162018.
View Article : Google Scholar
|
|
108
|
Huang D, Zhu X, Ye S, Zhang J, Liao J,
Zhang N, Zeng X, Wang J, Yang B, Zhang Y, et al: Tumour circular
RNAs elicit anti-tumour immunity by encoding cryptic peptides.
Nature. 625:593–602. 2024. View Article : Google Scholar
|
|
109
|
Robbins PF, Lu YC, El-Gamil M, Li YF,
Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al:
Mining exomic sequencing data to identify mutated antigens
recognized by adoptively transferred tumor-reactive T cells. Nat
Med. 19:747–752. 2013. View Article : Google Scholar
|
|
110
|
van Buuren MM, Calis JJ and Schumacher TN:
High sensitivity of cancer exome-based CD8 T cell neo-antigen
identification. Oncoimmunology. 3:e288362014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Biswas N, Chakrabarti S, Padul V, Jones LD
and Ashili S: Designing neoantigen cancer vaccines, trials, and
outcomes. Front Immunol. 14:11054202023. View Article : Google Scholar :
|
|
112
|
Roudko V, Greenbaum B and Bhardwaj N:
Computational prediction and validation of tumor-associated
neoantigens. Front Immunol. 11:272020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yadav M, Jhunjhunwala S, Phung QT,
Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J,
Weinschenk T, et al: Predicting immunogenic tumour mutations by
combining mass spectrometry and exome sequencing. Nature.
515:572–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar :
|
|
115
|
Chen S: Ultrafast one-pass FASTQ data
preprocessing, quality control, and deduplication using fastp.
Imeta. 2:e1072023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Langmead B and Salzberg SL: Fast
gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar
|
|
119
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar :
|
|
120
|
Pertea M, Kim D, Pertea GM, Leek JT and
Salzberg SL: Transcript-level expression analysis of RNA-seq
experiments with HISAT, StringTie and Ballgown. Nat Protoc.
11:1650–1667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kim D, Paggi JM, Park C, Bennett C and
Salzberg SL: Graph-based genome alignment and genotyping with
HISAT2 and HISAT-genotype. Nat Biotechnol. 37:907–915. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Genome
Project Data Processing Subgroup: The sequence alignment/map format
and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shumate A, Wong B, Pertea G and Pertea M:
Improved transcriptome assembly using a hybrid of long and short
reads with StringTie. PLoS Comput Biol. 18:e10097302022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
van der Auwera G and O'Connor BD: Genomics
in the cloud: Using docker, GATK, and WDL in Terra. O'Reilly Media,
Incorporated; 2020
|
|
126
|
Koboldt DC, Zhang Q, Larson DE, Shen D,
McLellan MD, Lin L, Miller CA, Mardis ER, Ding L and Wilson RK:
VarScan 2: Somatic mutation and copy number alteration discovery in
cancer by exome sequencing. Genome Res. 22:568–576. 2012.
View Article : Google Scholar
|
|
127
|
Lai Z, Markovets A, Ahdesmaki M, Chapman
B, Hofmann O, McEwen R, Johnson J, Dougherty B, Barrett JC and Dry
JR: VarDict: A novel and versatile variant caller for
next-generation sequencing in cancer research. Nucleic Acids Res.
44:e1082016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kim S, Scheffler K, Halpern AL, Bekritsky
MA, Noh E, Källberg M, Chen X, Kim Y, Beyter D, Krusche P and
Saunders CT: Strelka2: Fast and accurate calling of germline and
somatic variants. Nat Methods. 15:591–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Haas BJ, Dobin A, Stransky N, Li B, Yang
X, Tickle T, Bankapur A, Ganote C, Doak TG, Pochet N, et al:
STAR-fusion: Fast and accurate fusion transcript detection from
RNA-Seq. bioRxiv: 120295. 2017.
|
|
130
|
Yang H and Wang K: Genomic variant
annotation and prioritization with ANNOVAR and wANNOVAR. Nat
Protoc. 10:1556–1566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Mayakonda A, Lin DC, Assenov Y, Plass C
and Koeffler HP: Maftools: Efficient and comprehensive analysis of
somatic variants in cancer. Genome Res. 28:1747–1756. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Gubin MM, Artyomov MN, Mardis ER and
Schreiber RD: Tumor neoantigens: Building a framework for
personalized cancer immunotherapy. J Clin Invest. 125:3413–3421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shiina T, Hosomichi K, Inoko H and Kulski
JK: The HLA genomic loci map: expression, interaction, diversity
and disease. J Hum Genet. 54:15–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Klein J and Sato A: The HLA system. First
of two parts. N Engl J Med. 343:702–709. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Neefjes J, Jongsma M, Paul P and Bakke O:
Towards a systems understanding of MHC class I and MHC class II
antigen presentation. Nat Rev Immunol. 11:823–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Aurisicchio L, Pallocca M, Ciliberto G and
Palombo F: The perfect personalized cancer therapy: Cancer vaccines
against neoantigens. J Exp Clin Cancer Res. 37:862018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bontadini A: HLA techniques: Typing and
antibody detection in the laboratory of immunogenetics. Methods.
56:471–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
De Mattos-Arruda L, Vazquez M, Finotello
F, Lepore R, Porta E, Hundal J, Amengual-Rigo P, Ng CKY, Valencia
A, Carrillo J, et al: Neoantigen prediction and computational
perspectives towards clinical benefit: Recommendations from the
ESMO precision medicine working group. Ann Oncol. 31:978–990. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hosomichi K, Shiina T, Tajima A and Inoue
I: The impact of next-generation sequencing technologies on HLA
research. J Hum Genet. 60:665–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Danzer M, Niklas N, Stabentheiner S, Hofer
K, Pröll J, Stückler C, Raml E, Polin H and Gabriel C: Rapid,
scalable and highly automated HLA genotyping using next-generation
sequencing: A transition from research to diagnostics. BMC
Genomics. 14:2212013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Smith AG, Pereira S, Jaramillo A, Stoll
ST, Khan FM, Berka N, Mostafa AA, Pando MJ, Usenko CY, Bettinotti
MP, et al: Comparison of sequence-specific oligonucleotide probe vs
next generation sequencing for HLA-A, B, C, DRB1, DRB3/B4/B5, DQA1,
DQB1, DPA1, and DPB1 typing: Toward single-pass high-resolution HLA
typing in support of solid organ and hematopoietic cell transplant
programs: Toward single-pass high-resolution HLA typing in support
of solid organ and hematopoietic cell transplant programs. HLA.
94:296–306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wittig M, Anmarkrud JA, Kässens JC, Koch
S, Forster M, Ellinghaus E, Hov JR, Sauer S, Schimmler M, Ziemann
M, et al: Development of a high-resolution NGS-based HLA-typing and
analysis pipeline. Nucleic Acids Res. 43:e702015. View Article : Google Scholar :
|
|
144
|
Boegel S, Löwer M, Schäfer M, Bukur T, de
Graaf J, Boisguérin V, Türeci O, Diken M, Castle JC and Sahin U:
HLA typing from RNA-seq sequence reads. Genome Med. 4:1022012.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Warren RL, Choe G, Freeman DJ, Castellarin
M, Munro S, Moore R and Holt RA: Derivation of HLA types from
shotgun sequence datasets. Genome Med. 4:952012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Warren RL: HLA predictions from long
sequence read alignments, streamed directly into HLAminer. arXiv:
2209.09155. 2022.
|
|
147
|
Kim HJ and Pourmand N: HLA typing from
RNA-seq data using hierarchical read weighting [corrected]. PLoS
One. 8:e678852013. View Article : Google Scholar
|
|
148
|
Liu C, Yang X, Duffy B, Mohanakumar T,
Mitra RD, Zody MC and Pfeifer JD: ATHLATES: Accurate typing of
human leukocyte antigen through exome sequencing. Nucleic Acids
Res. 41:e1422013. View Article : Google Scholar :
|
|
149
|
Szolek A, Schubert B, Mohr C, Sturm M,
Feldhahn M and Kohlbacher O: OptiType: Precision HLA typing from
next-generation sequencing data. Bioinformatics. 30:3310–3316.
2014. View Article : Google Scholar
|
|
150
|
Shukla SA, Rooney MS, Rajasagi M, Tiao G,
Dixon PM, Lawrence MS, Stevens J, Lane WJ, Dellagatta JL, Steelman
S, et al: Comprehensive analysis of cancer-associated somatic
mutations in class I HLA genes. Nat Biotechnol. 33:1152–1158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Huang Y, Yang J, Ying D, Zhang Y,
Shotelersuk V, Hirankarn N, Sham PC, Lau YL and Yang W:
HLAreporter: A tool for HLA typing from next generation sequencing
data. Genome Med. 7:252015. View Article : Google Scholar :
|
|
152
|
Nariai N, Kojima K, Saito S, Mimori T,
Sato Y, Kawai Y, Yamaguchi-Kabata Y, Yasuda J and Nagasaki M:
HLA-VBSeq: Accurate HLA typing at full resolution from whole-genome
sequencing data. BMC Genomics. 16(Suppl 2): S72015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Xie C, Yeo ZX, Wong M, Piper J, Long T,
Kirkness EF, Biggs WH, Bloom K, Spellman S, Vierra-Green C, et al:
Fast and accurate HLA typing from short-read next-generation
sequence data with xHLA. Proc Natl Acad Sci USA. 114:8059–8064.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kawaguchi S, Higasa K, Shimizu M, Yamada R
and Matsuda F: HLA-HD: An accurate HLA typing algorithm for
nextgeneration sequencing data. Hum Mutat. 38:788–797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kawaguchi S, Higasa K, Yamada R and
Matsuda F: Comprehensive HLA typing from a current allele database
using next-generation sequencing data. Methods Mol Biol.
1802:225–233. 2018. View Article : Google Scholar
|
|
156
|
Kawaguchi S and Matsuda F: High-definition
genomic analysis of HLA genes via comprehensive HLA allele
genotyping. Methods Mol Biol. 2131:31–38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ka S, Lee S, Hong J, Cho Y, Sung J, Kim
HN, Kim HL and Jung J: HLAscan: Genotyping of the HLA region using
next-generation sequencing data. BMC Bioinformatics. 18:2582017.
View Article : Google Scholar :
|
|
158
|
Hayashi S, Yamaguchi R, Mizuno S, Komura
M, Miyano S, Nakagawa H and Imoto S: ALPHLARD: A Bayesian method
for analyzing HLA genes from whole genome sequence data. BMC
Genomics. 19:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Bai Y, Wang D and Fury W: PHLAT: Inference
of high-resolution HLA types from RNA and whole exome sequencing.
Methods Mol Biol. 1802:193–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Orenbuch R, Filip I, Comito D, Shaman J,
Pe'er I and Rabadan R: arcasHLA: High-resolution HLA typing from
RNAseq. Bioinformatics. 36:33–40. 2020. View Article : Google Scholar :
|
|
161
|
Bauer DC, Zadoorian A, Wilson LOW;
Melbourne Genomics Health Alliance; Thorne NP: Evaluation of
computational programs to predict HLA genotypes from genomic
sequencing data. Brief Bioinform. 19:179–187. 2018.
|
|
162
|
Kiyotani K, Mai TH and Nakamura Y:
Comparison of exome-based HLA class I genotyping tools:
Identification of platform-specific genotyping errors. J Hum Genet.
62:397–405. 2017. View Article : Google Scholar
|
|
163
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ferrari G, Kostyu DD, Cox J, Dawson DV,
Flores J, Weinhold KJ and Osmanov S: Identification of highly
conserved and broadly cross-reactive HIV type 1 cytotoxic T
lymphocyte epitopes as candidate immunogens for inclusion in
Mycobacterium bovis BCG-vectored HIV vaccines. AIDS Res Hum
Retroviruses. 16:1433–1443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kessler JH, Benckhuijsen WE, Mutis T,
Melief CJM, van der Burg SH and Drijfhout JW: Competition-based
cellular peptide binding assay for HLA class I. Curr Protoc
Immunol. Chapter 18: Unit 18.12. 2004. View Article : Google Scholar
|
|
166
|
Wulf M, Hoehn P and Trinder P:
Identification and validation of T-cell epitopes using the
IFN-gamma ELISPOT assay. Methods Mol Biol. 524:439–446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yang B, Jeang J, Yang A, Wu TC and Hung
CF: DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother.
10:3153–3164. 2014. View Article : Google Scholar
|
|
168
|
Vita R, Mahajan S, Overton JA, Dhanda SK,
Martini S, Cantrell JR, Wheeler DK, Sette A and Peters B: The
immune epitope database (IEDB): 2018 Update. Nucleic Acids Res.
47(D1): D339–D343. 2019. View Article : Google Scholar :
|
|
169
|
Rammensee H, Bachmann J, Emmerich NP,
Bachor OA and Stevanović S: SYFPEITHI: Database for MHC ligands and
peptide motifs. Immunogenetics. 50:213–219. 1999. View Article : Google Scholar
|
|
170
|
Bhasin M, Singh H and Raghava GPS: MHCBN:
A comprehensive database of MHC binding and non-binding peptides.
Bioinformatics. 19:665–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Blass E and Ott PA: Advances in the
development of personalized neoantigen-based therapeutic cancer
vaccines. Nat Rev Clin Oncol. 18:215–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Garcia-Garijo A, Fajardo CA and Gros A:
Determinants for neoantigen identification. Front Immunol.
10:13922019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Falk K, Rötzschke O, Stevanović S, Jung G
and Rammensee HG: Allele-specific motifs revealed by sequencing of
self-peptides eluted from MHC molecules. Nature. 351:290–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Rötzschke O, Falk K, Stevanović S, Jung G,
Walden P and Rammensee HG: Exact prediction of a natural T cell
epitope. Eur J Immunol. 21:2891–2894. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Nielsen M, Lundegaard C, Worning P,
Lauemøller SL, Lamberth K, Buus S, Brunak S and Lund O: Reliable
prediction of T-cell epitopes using neural networks with novel
sequence representations. Protein Sci. 12:1007–1017. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Nielsen M and Andreatta M: NetMHCpan-3.0;
Improved prediction of binding to MHC class I molecules integrating
information from multiple receptor and peptide length datasets.
Genome Med. 8:332016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Peters B and Sette A: Generating
quantitative models describing the sequence specificity of
biological processes with the stabilized matrix method. BMC
Bioinformatics. 6:1322005. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kim Y, Sidney J, Pinilla C, Sette A and
Peters B: Derivation of an amino acid similarity matrix for
peptide: MHC binding and its application as a Bayesian prior. BMC
Bioinformatics. 10:3942009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lundegaard C, Lund O and Nielsen M:
Prediction of epitopes using neural network based methods. J
Immunol Methods. 374:26–34. 2011. View Article : Google Scholar :
|
|
180
|
Schueler-Furman O, Altuvia Y, Sette A and
Margalit H: Structure-based prediction of binding peptides to MHC
class I molecules: Application to a broad range of MHC alleles.
Protein Sci. 9:1838–1846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Kumar N and Mohanty D: MODPROPEP: A
program for knowledge-based modeling of protein-peptide complexes.
Nucleic Acids Res. 35(Web Server Issue): W549–W555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Hattotuwagama CK, Doytchinova IA and
Flower DR: Toward the prediction of class I and II mouse major
histocompatibility complex-peptide-binding affinity: In silico
bioinformatic step-by-step guide using quantitative
structure-activity relationships. Methods Mol Biol. 409:227–245.
2007. View Article : Google Scholar
|
|
183
|
Luo H, Ye H, Ng HW, Shi L, Tong W,
Mendrick DL and Hong H: Machine learning methods for predicting
HLA-peptide binding activity. Bioinform Biol Insights. 9(Suppl 3):
S21–S29. 2015.
|
|
184
|
Zhang H, Lund O and Nielsen M: The
PickPocket method for predicting binding specificities for
receptors based on receptor pocket similarities: Application to
MHC-peptide binding. Bioinformatics. 25:1293–1299. 2009. View Article : Google Scholar :
|
|
185
|
Wang M, Lei C, Wang J, Li Y and Li M:
TripHLApan: Predicting HLA molecules binding peptides based on
triple coding matrix and transfer learning. Brief Bioinform.
25:bbae1542024. View Article : Google Scholar
|
|
186
|
Boehm KM, Bhinder B, Raja VJ, Dephoure N
and Elemento O: Predicting peptide presentation by major
histocompatibility complex class I: An improved machine learning
approach to the immunopeptidome. BMC Bioinformatics. 20:72019.
View Article : Google Scholar
|
|
187
|
Mei S, Li F, Xiang D, Ayala R, Faridi P,
Webb GI, Illing PT, Rossjohn J, Akutsu T, Croft NP, et al: Anthem:
A user customised tool for fast and accurate prediction of binding
between peptides and HLA class I molecules. Brief Bioinform.
22:bbaa4152021. View Article : Google Scholar
|
|
188
|
Reche PA, Glutting JP and Reinherz EL:
Prediction of MHC class I binding peptides using profile motifs.
Hum Immunol. 63:701–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Reche PA, Glutting JP, Zhang H and
Reinherz EL: Enhancement to the RANKPEP resource for the prediction
of peptide binding to MHC molecules using profiles. Immunogenetics.
56:405–419. 2004. View Article : Google Scholar
|
|
190
|
Reche PA and Reinherz EL: Prediction of
peptide-MHC binding using profiles. Methods Mol Biol. 409:185–200.
2007. View Article : Google Scholar
|
|
191
|
Buus S, Lauemøller SL, Worning P, Kesmir
C, Frimurer T, Corbet S, Fomsgaard A, Hilden J, Holm A and Brunak
S: Sensitive quantitative predictions of peptide-MHC binding by a
'Query by Committee' artificial neural network approach. Tissue
Antigens. 62:378–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Lundegaard C, Lamberth K, Harndahl M, Buus
S, Lund O and Nielsen M: NetMHC-3.0: Accurate web accessible
predictions of human, mouse and monkey MHC class I affinities for
peptides of length 8-11. Nucleic Acids Res. 36(Web Server Issue):
W509–W512. 2008. View Article : Google Scholar
|
|
193
|
Andreatta M and Nielsen M: Gapped sequence
alignment using artificial neural networks: Application to the MHC
class I system. Bioinformatics. 32:511–517. 2016. View Article : Google Scholar
|
|
194
|
Sidney J, Assarsson E, Moore C, Ngo S,
Pinilla C, Sette A and Peters B: Quantitative peptide binding
motifs for 19 human and mouse MHC class I molecules derived using
positional scanning combinatorial peptide libraries. Immunome Res.
4:22008. View Article : Google Scholar :
|
|
195
|
Jurtz V, Paul S, Andreatta M, Marcatili P,
Peters B and Nielsen M: NetMHCpan-4.0: Improved peptide-MHC class I
interaction predictions integrating eluted ligand and peptide
binding affinity data. J Immunol. 199:3360–3368. 2017. View Article : Google Scholar
|
|
196
|
Hoof I, Peters B, Sidney J, Pedersen LE,
Sette A, Lund O, Buus S and Nielsen M: NetMHCpan, a method for MHC
class I binding prediction beyond humans. Immunogenetics. 61:1–13.
2009. View Article : Google Scholar
|
|
197
|
Reynisson B, Alvarez B, Paul S, Peters B
and Nielsen M: NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved
predictions of MHC antigen presentation by concurrent motif
deconvolution and integration of MS MHC eluted ligand data. Nucleic
Acids Res. 48(W1): W449–W454. 2020. View Article : Google Scholar
|
|
198
|
Bassani-Sternberg M, Chong C, Guillaume P,
Solleder M, Pak H, Gannon PO, Kandalaft LE, Coukos G and Gfeller D:
Deciphering HLA-I motifs across HLA peptidomes improves neo-antigen
predictions and identifies allostery regulating HLA specificity.
PLoS Comput Biol. 13:e10057252017. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Gfeller D, Schmidt J, Croce G, Guillaume
P, Bobisse S, Genolet R, Queiroz L, Cesbron J, Racle J and Harari
A: Improved predictions of antigen presentation and TCR recognition
with MixMHCpred2.2 and PRIME2.0 reveal potent SARS-CoV-2
CD8+ T-cell epitopes. Cell Syst. 14:72–83.e5. 2023.
View Article : Google Scholar
|
|
200
|
Liu G, Li D, Li Z, Qiu S, Li W, Chao CC,
Yang N, Li H, Cheng Z, Song X, et al: PSSMHCpan: A novel PSSM-based
software for predicting class I peptide-HLA binding affinity.
Gigascience. 6:1–11. 2017. View Article : Google Scholar
|
|
201
|
O'Donnell TJ, Rubinsteyn A and Laserson U:
MHCflurry 2.0: Improved pan-allele prediction of MHC class
I-presented peptides by incorporating antigen processing. Cell
Syst. 11:42–48.e7. 2020. View Article : Google Scholar
|
|
202
|
O'Donnell TJ, Rubinsteyn A, Bonsack M,
Riemer AB, Laserson U and Hammerbacher J: MHCflurry: Open-source
class I MHC binding affinity prediction. Cell Syst. 7:129–132.e4.
2018. View Article : Google Scholar
|
|
203
|
Phloyphisut P, Pornputtapong N, Sriswasdi
S and Chuangsuwanich E: MHCSeqNet: A deep neural network model for
universal MHC binding prediction. BMC Bioinformatics. 20:2702019.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Hu Y, Wang Z, Hu H, Wan F, Chen L, Xiong
Y, Wang X, Zhao D, Huang W and Zeng J: ACME: Pan-specific
peptide-MHC class I binding prediction through attention-based deep
neural networks. Bioinformatics. 35:4946–4954. 2019. View Article : Google Scholar
|
|
205
|
Wu J, Wang W, Zhang J, Zhou B, Zhao W, Su
Z, Gu X, Wu J, Zhou Z and Chen S: DeepHLApan: A deep learning
approach for neoantigen prediction considering both HLA-peptide
binding and immunogenicity. Front Immunol. 10:25592019. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Sarkizova S, Klaeger S, Le PM, Li LW,
Oliveira G, Keshishian H, Hartigan CR, Zhang W, Braun DA, Ligon KL,
et al: A large peptidome dataset improves HLA class I epitope
prediction across most of the human population. Nat Biotechnol.
38:199–209. 2020. View Article : Google Scholar
|
|
207
|
Shao XM, Bhattacharya R, Huang J,
Sivakumar IKA, Tokheim C, Zheng L, Hirsch D, Kaminow B, Omdahl A,
Bonsack M, et al: High-throughput prediction of MHC class I and II
neoantigens with MHCnuggets. Cancer Immunol Res. 8:396–408. 2020.
View Article : Google Scholar
|
|
208
|
Yang X, Zhao L, Wei F and Li J:
DeepNetBim: Deep learning model for predicting HLA-epitope
interactions based on network analysis by harnessing binding and
immunogenicity information. BMC Bioinformatics. 22:2312021.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Albert BA, Yang Y, Shao XM, Singh D, Smit
KN, Anagnostou V and Karchin R: Deep neural networks predict class
I major histocompatibility complex epitope presentation and
transfer learn neoepitope immunogenicity. Nat Mach Intell.
5:861–872. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Sturniolo T, Bono E, Ding J, Raddrizzani
L, Tuereci O, Sahin U, Braxenthaler M, Gallazzi F, Protti MP,
Sinigaglia F and Hammer J: Generation of tissue-specific and
promiscuous HLA ligand databases using DNA microarrays and virtual
HLA class II matrices. Nat Biotechnol. 17:555–561. 1999. View Article : Google Scholar
|
|
211
|
Singh H and Raghava GP: ProPred:
Prediction of HLA-DR binding sites. Bioinformatics. 17:1236–1237.
2001. View Article : Google Scholar
|
|
212
|
Wan J, Liu W, Xu Q, Ren Y, Flower DR and
Li T: SVRMHC prediction server for MHC-binding peptides. BMC
Bioinformatics. 7:4632006. View Article : Google Scholar
|
|
213
|
Nielsen M, Lundegaard C and Lund O:
Prediction of MHC class II binding affinity using SMM-align, a
novel stabilization matrix alignment method. BMC Bioinformatics.
8:2382007. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Nielsen M and Lund O: NN-align. An
artificial neural network-based alignment algorithm for MHC class
II peptide binding prediction. BMC Bioinformatics. 10:2962009.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Jensen KK, Andreatta M, Marcatili P, Buus
S, Greenbaum JA, Yan Z, Sette A, Peters B and Nielsen M: Improved
methods for predicting peptide binding affinity to MHC class II
molecules. Immunology. 154:394–406. 2018. View Article : Google Scholar
|
|
216
|
Nielsen M, Lundegaard C, Blicher T, Peters
B, Sette A, Justesen S, Buus S and Lund O: Quantitative predictions
of peptide binding to any HLA-DR molecule of known sequence:
NetMHCIIpan. PLoS Comput Biol. 4:e10001072008. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Andreatta M, Karosiene E, Rasmussen M,
Stryhn A, Buus S and Nielsen M: Accurate pan-specific prediction of
peptide-MHC class II binding affinity with improved binding core
identification. Immunogenetics. 67:641–650. 2015. View Article : Google Scholar :
|
|
218
|
Kaabinejadian S, Barra C, Alvarez B, Yari
H, Hildebrand WH and Nielsen M: Accurate MHC motif deconvolution of
immunopeptidomics data reveals a significant contribution of DRB3,
4 and 5 to the total DR immunopeptidome. Front Immunol.
13:8354542022. View Article : Google Scholar :
|
|
219
|
Pfeifer N and Kohlbacher O: Multiple
instance learning allows MHC class II epitope predictions across
alleles. Lect Notes Comput Sci. 5251:210–221. 2008. View Article : Google Scholar
|
|
220
|
Bordner AJ and Mittelmann HD: MultiRTA: A
simple yet reliable method for predicting peptide binding
affinities for multiple class II MHC allotypes. BMC Bioinformatics.
11:4822010. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Zhang L, Chen Y, Wong HS, Zhou S,
Mamitsuka H and Zhu S: TEPITOPEpan: Extending TEPITOPE for peptide
binding prediction covering over 700 HLA-DR molecules. PLoS One.
7:e304832012. View Article : Google Scholar
|
|
222
|
Chen B, Khodadoust MS, Olsson N, Wagar LE,
Fast E, Liu CL, Muftuoglu Y, Sworder BJ, Diehn M, Levy R, et al:
Predicting HLA class II antigen presentation through integrated
deep learning. Nat Biotechnol. 37:1332–1343. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Racle J, Michaux J, Rockinger GA, Arnaud
M, Bobisse S, Chong C, Guillaume P, Coukos G, Harari A, Jandus C,
et al: Robust prediction of HLA class II epitopes by deep motif
deconvolution of immunopeptidomes. Nat Biotechnol. 37:1283–1286.
2019. View Article : Google Scholar
|
|
224
|
Racle J, Guillaume P, Schmidt J, Michaux
J, Larabi A, Lau K, Perez MAS, Croce G, Genolet R, Coukos G, et al:
Machine learning predictions of MHC-II specificities reveal
alternative binding mode of class II epitopes. Immunity.
56:1359–1375.e13. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Abelin JG, Harjanto D, Malloy M, Suri P,
Colson T, Goulding SP, Creech AL, Serrano LR, Nasir G, Nasrullah Y,
et al: Defining HLA-II ligand processing and binding rules with
mass spectrometry enhances cancer epitope prediction. Immunity.
51:766–779.e17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Liu Z, Jin J, Cui Y, Xiong Z, Nasiri A,
Zhao Y and Hu J: DeepSeqPanII: An interpretable recurrent neural
network model with attention mechanism for peptide-HLA class II
binding prediction. IEEE/ACM Trans Comput Biol Bioinform.
19:2188–2196. 2022. View Article : Google Scholar
|
|
227
|
Rammensee HG, Friede T and Stevanoviíc S:
MHC ligands and peptide motifs: First listing. Immunogenetics.
41:178–228. 1995. View Article : Google Scholar
|
|
228
|
Chicz RM, Urban RG, Lane WS, Gorga JC,
Stern LJ, Vignali DA and Strominger JL: Predominant naturally
processed peptides bound to HLA-DR1 are derived from MHC-related
molecules and are heterogeneous in size. Nature. 358:764–768. 1992.
View Article : Google Scholar
|
|
229
|
Wang P, Sidney J, Dow C, Mothé B, Sette A
and Peters B: A systematic assessment of MHC class II peptide
binding predictions and evaluation of a consensus approach. PLoS
Comput Biol. 4:e10000482008. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Yu K, Petrovsky N, Schönbach C, Koh JYL
and Brusic V: Methods for prediction of peptide binding to MHC
molecules: A comparative study. Mol Med. 8:137–148. 2002.
View Article : Google Scholar
|
|
231
|
Peters B, Bui HH, Frankild S, Nielson M,
Lundegaard C, Kostem E, Basch D, Lamberth K, Harndahl M, Fleri W,
et al: A community resource benchmarking predictions of peptide
binding to MHC-I molecules. PLoS Comput Biol. 2:e652006. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Gowthaman U, Chodisetti SB, Parihar P and
Agrewala JN: Evaluation of different generic in silico methods for
predicting HLA class I binding peptide vaccine candidates using a
reverse approach. Amino Acids. 39:1333–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Bonsack M, Hoppe S, Winter J, Tichy D,
Zeller C, Küpper MD, Schitter EC, Blatnik R and Riemer AB:
Performance evaluation of MHC class-I binding prediction tools
based on an experimentally validated MHC-peptide binding data set.
Cancer Immunol Res. 7:719–736. 2019. View Article : Google Scholar
|
|
234
|
Trolle T, Metushi IG, Greenbaum JA, Kim Y,
Sidney J, Lund O, Sette A, Peters B and Nielsen M: Automated
benchmarking of peptide-MHC class I binding predictions.
Bioinformatics. 31:2174–2181. 2015. View Article : Google Scholar :
|
|
235
|
Engels B, Engelhard VH, Sidney J, Sette A,
Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA and Schreiber
H: Relapse or eradication of cancer is predicted by peptide-major
histocompatibility complex affinity. Cancer Cell. 23:516–526. 2013.
View Article : Google Scholar :
|
|
236
|
Nogueira C, Kaufmann JK, Lam H and
Flechtner JB: Improving cancer immunotherapies through empirical
neoantigen selection. Trends Cancer. 4:97–100. 2018. View Article : Google Scholar
|
|
237
|
Liao WWP and Arthur JW: Predicting peptide
binding to major histocompatibility complex molecules. Autoimmun
Rev. 10:469–473. 2011. View Article : Google Scholar
|
|
238
|
Backert L and Kohlbacher O:
Immunoinformatics and epitope prediction in the age of genomic
medicine. Genome Med. 7:1192015. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Türeci Ö, Löwer M, Schrörs B, Lang M,
Tadmor A and Sahin U: Challenges towards the realization of
individualized cancer vaccines. Nat Biomed Eng. 2:566–569. 2018.
View Article : Google Scholar
|
|
240
|
Schumacher TN, Scheper W and Kvistborg P:
Cancer neoantigens. Annu Rev Immunol. 37:173–200. 2019. View Article : Google Scholar
|
|
241
|
Xie N, Shen G, Gao W, Huang Z, Huang C and
Fu L: Neoantigens: Promising targets for cancer therapy. Signal
Transduct Target Ther. 8:92023. View Article : Google Scholar :
|
|
242
|
Bassani-Sternberg M, Bräunlein E, Klar R,
Engleitner T, Sinitcyn P, Audehm S, Straub M, Weber J,
Slotta-Huspenina J, Specht K, et al: Direct identification of
clinically relevant neoepitopes presented on native human melanoma
tissue by mass spectrometry. Nat Commun. 7:134042016. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Sette A, Vitiello A, Reherman B, Fowler P,
Nayersina R, Kast WM, Melief CJ, Oseroff C, Yuan L, Ruppert J, et
al: The relationship between class I binding affinity and
immunogenicity of potential cytotoxic T cell epitopes. J Immunol.
153:5586–5592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Bjerregaard AM, Nielsen M, Jurtz V, Barra
CM, Hadrup SR, Szallasi Z and Eklund AC: An analysis of natural T
cell responses to predicted tumor neoepitopes. Front Immunol.
8:15662017. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Shah K, Al-Haidari A, Sun J and Kazi JU: T
cell receptor (TCR) signaling in health and disease. Signal
Transduct Target Ther. 6:4122021. View Article : Google Scholar
|
|
246
|
Larsen MV, Lundegaard C, Lamberth K, Buus
S, Brunak S, Lund O and Nielsen M: An integrative approach to CTL
epitope prediction: A combined algorithm integrating MHC class I
binding, TAP transport efficiency, and proteasomal cleavage
predictions. Eur J Immunol. 35:2295–2303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Stranzl T, Larsen MV, Lundegaard C and
Nielsen M: NetCTLpan: Pan-specific MHC class I pathway epitope
predictions. Immunogenetics. 62:357–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Zhou C, Zhu C and Liu Q: Toward in silico
identification of tumor neoantigens in immunotherapy. Trends Mol
Med. 25:980–992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Chen P, Chen D, Bu D, Gao J, Qin W, Deng
K, Ren L, She S, Xu W, Yang Y, et al: Dominant neoantigen
verification in hepatocellular carcinoma by a single-plasmid system
coexpressing patient HLA and antigen. J Immunother Cancer.
11:e0063342023. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Chudley L, McCann KJ, Coleman A, Cazaly
AM, Bidmon N, Britten CM, van der Burg SH, Gouttefangeas C, Jandus
C, Laske K, et al: Harmonisation of short-term in vitro culture for
the expansion of antigen-specific CD8(+) T cells with detection by
ELISPOT and HLA-multimer staining. Cancer Immunol Immunother.
63:1199–1211. 2014. View Article : Google Scholar :
|
|
251
|
Slota M, Lim JB, Dang Y and Disis ML:
ELISpot for measuring human immune responses to vaccines. Expert
Rev Vaccines. 10:299–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Czerkinsky CC, Nilsson LA, Nygren H,
Ouchterlony O and Tarkowski A: A solid-phase enzyme-linked
immunospot (ELISPOT) assay for enumeration of specific
antibody-secreting cells. J Immunol Methods. 65:109–121. 1983.
View Article : Google Scholar
|
|
253
|
Taguchi T, McGhee JR, Coffman RL, Beagley
KW, Eldridge JH, Takatsu K and Kiyono H: Detection of individual
mouse splenic T cells producing IFN-gamma and IL-5 using the
enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods.
128:65–73. 1990. View Article : Google Scholar
|
|
254
|
Miyahira Y, Murata K, Rodriguez D,
Rodriguez JR, Esteban M, Rodrigues MM and Zavala F: Quantification
of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol
Methods. 181:45–54. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Verneris MR, Karimi M, Baker J, Jayaswal A
and Negrin RS: Role of NKG2D signaling in the cytotoxicity of
activated and expanded CD8+ T cells. Blood. 103:3065–3072. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Wonderlich J, Shearer G, Livingstone A,
Brooks A, Soloski MJ and Presby MM: Induction and measurement of
cytotoxic T lymphocyte activity. Curr Protoc Immunol.
120:3.11.1–3.11.29. 2018.
|
|
257
|
Chan JK, Hamilton CA, Cheung MK, Karimi M,
Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, et al:
Enhanced killing of primary ovarian cancer by retargeting
autologous cytokine-induced killer cells with bispecific
antibodies: A preclinical study. Clin Cancer Res. 12:1859–1867.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Clevers H and Tuveson DA: Organoid models
for cancer research. Annu Rev Cancer Biol. 3:223–234. 2019.
View Article : Google Scholar
|
|
259
|
Jacob F, Salinas RD, Zhang DY, Nguyen PTT,
Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, et
al: A patient-derived glioblastoma organoid model and biobank
recapitulates inter- and intra-tumoral heterogeneity. Cell.
180:188–204.e22. 2020. View Article : Google Scholar
|
|
260
|
Lee SH, Hu W, Matulay JT, Silva MV,
Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et
al: Tumor evolution and drug response in patient-derived organoid
models of bladder cancer. Cell. 173:515–528.e17. 2018. View Article : Google Scholar
|
|
261
|
Cattaneo CM, Dijkstra KK, Fanchi LF,
Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN
and Voest EE: Tumor organoid-T-cell coculture systems. Nat Protoc.
15:15–39. 2020. View Article : Google Scholar
|
|
262
|
Weng G, Tao J, Liu Y, Qiu J, Su D, Wang R,
Luo W and Zhang T: Organoid: Bridging the gap between basic
research and clinical practice. Cancer Lett. 572:2163532023.
View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Shultz LD, Ishikawa F and Greiner DL:
Humanized mice in translational biomedical research. Nat Rev
Immunol. 7:118–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Rongvaux A, Takizawa H, Strowig T,
Willinger T, Eynon EE, Flavell RA and Manz MG: Human
hemato-lymphoid system mice: Current use and future potential for
medicine. Annu Rev Immunol. 31:635–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Morillon YM II, Sabzevari A, Schlom J and
Greiner JW: The development of next-generation PBMC humanized mice
for preclinical investigation of cancer immunotherapeutic agents.
Anticancer Res. 40:5329–5341. 2020. View Article : Google Scholar
|
|
266
|
Ehx G, Ritacco C and Baron F:
Pathophysiology and preclinical relevance of experimental
graft-versus-host disease in humanized mice. Biomark Res.
12:1392024. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Chuprin J, Buettner H, Seedhom MO, Greiner
DL, Keck JG, Ishikawa F, Shultz LD and Brehm MA: Humanized mouse
models for immuno-oncology research. Nat Rev Clin Oncol.
20:192–206. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Guil-Luna S, Sedlik C and Piaggio E:
Humanized mouse models to evaluate cancer immunotherapeutics. Annu
Rev Cancer Biol. 5:119–136. 2021. View Article : Google Scholar
|
|
269
|
De La Rochere P, Guil-Luna S, Decaudin D,
Azar G, Sidhu SS and Piaggio E: Humanized mice for the study of
immuno-oncology. Trends Immunol. 39:748–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Camacho RE, Wnek R, Fischer P, Shah K,
Zaller DM, Woods A, La Monica N, Aurisicchio L, Fitzgerald-Bocarsly
P and Koo GC: Characterization of the NOD/scid-[Tg]DR1 mouse
expressing HLA-DRB1*01 transgene: A model of SCID-hu mouse for
vaccine development. Exp Hematol. 35:1219–1230. 2007. View Article : Google Scholar
|
|
271
|
Spranger S, Frankenberger B and Schendel
DJ: NOD/scid IL-2Rg(null) mice: A preclinical model system to
evaluate human dendritic cell-based vaccine strategies in vivo. J
Transl Med. 10:302012. View Article : Google Scholar
|
|
272
|
Li R, Han DS, Shi JP, Han YX, Tan P, Zhang
R and Li JM: Choosing tumor mutational burden wisely for
immunotherapy: A hard road to explore. Biochim Biophys Acta Rev
Cancer. 1874:1884202020. View Article : Google Scholar
|
|
273
|
Ahmed J, Das B, Shin S and Chen A:
Challenges and future directions in the management of tumor
mutational burden-high (TMB-H) advanced solid malignancies. Cancers
(Basel). 15:58412023. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Teixido C, Castillo P, Martinez-Vila C,
Arance A and Alos L: Molecular markers and targets in melanoma.
Cells. 10:23202021. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Ott PA, Hu-Lieskovan S, Chmielowski B,
Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD,
Lin JJ, et al: A phase Ib trial of personalized neoantigen therapy
plus anti-PD-1 in patients with advanced melanoma, non-small cell
lung cancer, or bladder cancer. Cell. 183:347–362.e24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Mørk SK, Kadivar M, Bol KF, Draghi A,
Wulff Westergaard MC, Skadborg SK, Overgaard N, Sørensen AB,
Rasmussen IS, Andreasen LV, et al: Personalized therapy with
peptide-based neoantigen vaccine (EVX-01) including a novel
adjuvant, CAF®09b, in patients with metastatic melanoma.
Oncoimmunology. 11:20232552022. View Article : Google Scholar
|
|
278
|
Weber JS, Carlino MS, Khattak A, Meniawy
T, Ansstas G, Taylor MH, Kim KB, McKean M, Long GV, Sullivan RJ, et
al: Individualised neoantigen therapy mRNA-4157 (V940) plus
pembrolizumab versus pembrolizumab monotherapy in resected melanoma
(KEYNOTE-942): A randomised, phase 2b study. Lancet. 403:632–644.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao
S, Wang P, Su X, Qin Y, Wang Y, et al: Personalized neoantigen
pulsed dendritic cell vaccine for advanced lung cancer. Signal
Transduct Target Ther. 6:262021. View Article : Google Scholar :
|
|
280
|
Gainor JF, Patel MR, Weber JS, Gutierrez
M, Bauman JE, Clarke JM, Julian R, Scott AJ, Geiger JL, Kirtane K,
et al: T-cell responses to individualized neoantigen therapy
mRNA-4157 (V940) alone or in combination with pembrolizumab in the
phase 1 KEYNOTE-603 study. Cancer Discov. 14:2209–2223. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Lee JM, Spicer J, Nair S, Khattak A, Brown
M, Meehan RS, Shariati NM, Deng X, Samkari A and Chaft JE: The
phase 3 INTerpath-002 study design: Individualized neoantigen
therapy (INT) V940 (mRNA-4157) plus pembrolizumab vs placebo plus
pembrolizumab for resected early-stage non-small-cell lung cancer
(NSCLC). J Clin Oncol. 42(16 Suppl): TPS81162024. View Article : Google Scholar
|
|
282
|
Li F, Deng L, Jackson KR, Talukder AH,
Katailiha AS, Bradley SD, Zou Q, Chen C, Huo C, Chiu Y, et al:
Neoantigen vaccination induces clinical and immunologic responses
in non-small cell lung cancer patients harboring EGFR mutations. J
Immunother Cancer. 9:e0025312021. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Gao S, Wang J, Zhu Z, Fang J, Zhao Y, Liu
Z, Qin H, Wei Y, Xu H, Dan X, et al: Effective personalized
neoantigen vaccine plus anti-PD-1 in a PD-1 blockade-resistant lung
cancer patient. Immunotherapy. 15:57–69. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Awad MM, Govindan R, Balogh KN, Spigel DR,
Garon EB, Bushway ME, Poran A, Sheen JH, Kohler V, Esaulova E, et
al: Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and
anti-PD-1 as first-line treatment for non-squamous non-small cell
lung cancer. Cancer Cell. 40:1010–1026.e11. 2022. View Article : Google Scholar
|
|
285
|
McCann K, von Witzleben A, Thomas J, Wang
C, Wood O, Singh D, Boukas K, Bendjama K, Silvestre N, Nielsen FC,
et al: Targeting the tumor mutanome for personalized vaccination in
a TMB low non-small cell lung cancer. J Immunother Cancer.
10:e0038212022. View Article : Google Scholar :
|
|
286
|
Moen MD: Bevacizumab: In previously
treated glioblastoma. Drugs. 70:181–189. 2010. View Article : Google Scholar
|
|
287
|
Engelhardt B: The blood-central nervous
system barriers actively control immune cell entry into the central
nervous system. Curr Pharm Des. 14:1555–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Johanns TM, Bowman-Kirigin JA, Liu C and
Dunn GP: Targeting neoantigens in glioblastoma: An overview of
cancer immunogenomics and translational implications. Neurosurgery.
64(CN Suppl 1): S165–S176. 2017. View Article : Google Scholar
|
|
289
|
Dang L, Yen K and Attar EC: IDH mutations
in cancer and progress toward development of targeted therapeutics.
Ann Oncol. 27:599–608. 2016. View Article : Google Scholar
|
|
290
|
Ni YQ, Shen PB, Wang XC, Liu HY, Luo HY
and Han XZ: The roles of IDH1 in tumor metabolism and immunity.
Future Oncol. 18:3941–3953. 2022. View Article : Google Scholar
|
|
291
|
de la Fuente MI: Targeting IDH1/IDH2
mutations in gliomas. Curr Opin Neurol. 35:787–793. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Pellegatta S, Valletta L, Corbetta C,
Patanè M, Zucca I, Riccardi Sirtori F, Bruzzone MG, Fogliatto G,
Isacchi A, Pollo B and Finocchiaro G: Effective immuno-targeting of
the IDH1 mutation R132H in a murine model of intracranial glioma.
Acta Neuropathol Commun. 3:42015. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Platten M, Bunse L, Wick A, Bunse T, Le
Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, et al: A
vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
592:463–468. 2021. View Article : Google Scholar :
|
|
294
|
Platten M, Schilling D, Bunse L, Wick A,
Bunse T, Riehl D, Green E, Sanghvi K, Karapanagiotou-Schenkel I,
Harting I, et al: Atim-33. Noa-16: A first-in-man multicenter phase
I clinical trial of the German neurooncology working group
evaluating a mutation-specific peptide vaccine targeting idh1r132h
in patients with newly diagnosed malignant astrocytomas. Neuro
Oncol. 20(Suppl 6): vi8–vi9. 2018. View Article : Google Scholar
|
|
295
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar
|
|
296
|
Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur
V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V,
van der Burg SH, et al: Actively personalized vaccination trial for
newly diagnosed glioblastoma. Nature. 565:240–245. 2019. View Article : Google Scholar
|
|
297
|
Narita Y, Arakawa Y, Yamasaki F, Nishikawa
R, Aoki T, Kanamori M, Nagane M, Kumabe T, Hirose Y, Ichikawa T, et
al: A randomized, double-blind, phase III trial of personalized
peptide vaccination for recurrent glioblastoma. Neuro Oncol.
21:348–359. 2019. View Article : Google Scholar :
|
|
298
|
Bruix J and Sherman M; Practice Guidelines
Committee American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
299
|
Tampaki M, Papatheodoridis GV and
Cholongitas E: Intrahepatic recurrence of hepatocellular carcinoma
after resection: An update. Clin J Gastroenterol. 14:699–713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
300
|
European Association for the Study of the
Liver: EASL clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar
|
|
301
|
Charneau J, Suzuki T, Shimomura M,
Fujinami N and Nakatsura T: Peptide-based vaccines for
hepatocellular carcinoma: A review of recent advances. J Hepatocell
Carcinoma. 8:1035–1054. 2021. View Article : Google Scholar
|
|
302
|
Liu C, Shao J, Dong Y, Xu Q, Zou Z, Chen
F, Yan J, Liu J, Li S, Liu B and Shen J: Advanced HCC patient
benefit from neoantigen reactive T cells based immunotherapy: A
case report. Front Immunol. 12:6851262021. View Article : Google Scholar :
|
|
303
|
Cai Z, Su X, Qiu L, Li Z, Li X, Dong X,
Wei F, Zhou Y, Luo L, Chen G, et al: Personalized neoantigen
vaccine prevents postoperative recurrence in hepatocellular
carcinoma patients with vascular invasion. Mol Cancer. 20:1642021.
View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Peng S, Chen S, Hu W, Mei J, Zeng X, Su T,
Wang W, Chen Z, Xiao H, Zhou Q, et al: Combination neoantigen-based
dendritic cell vaccination and adoptive T-cell transfer induces
antitumor responses against recurrence of hepatocellular carcinoma.
Cancer Immunol Res. 10:728–744. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Shen J, Wang LF, Zou ZY, Kong WW, Yan J,
Meng FY, Chen FJ, Du J, Shao J, Xu QP, et al: Phase I clinical
study of personalized peptide vaccination combined with
radiotherapy for advanced hepatocellular carcinoma. World J
Gastroenterol. 23:5395–5404. 2017. View Article : Google Scholar
|
|
306
|
Guo Z, Yuan Y, Chen C, Lin J, Ma Q, Liu G,
Gao Y, Huang Y, Chen L, Chen LZ, et al: Durable complete response
to neoantigen-loaded dendritic-cell vaccine following anti-PD-1
therapy in metastatic gastric cancer. NPJ Precis Oncol. 6:342022.
View Article : Google Scholar :
|
|
307
|
Yu YJ, Shan N, Li LY, Zhu YS, Lin LM, Mao
CC, Hu TT, Xue XY, Su XP, Shen X and Cai ZZ: Preliminary clinical
study of personalized neoantigen vaccine therapy for microsatellite
stability (MSS)-advanced colorectal cancer. Cancer Immunol
Immunother. 72:2045–2056. 2023. View Article : Google Scholar
|
|
308
|
Sultan H, Salazar AM and Celis E:
Poly-ICLC, a multi-functional immune modulator for treating cancer.
Semin Immunol. 49:1014142020. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Zeng Y, Zhang W, Li Z, Zheng Y, Wang Y,
Chen G, Qiu L, Ke K, Su X, Cai Z, et al: Personalized
neoantigen-based immunotherapy for advanced collecting duct
carcinoma: Case report. J Immunother Cancer. 8:e0002172020.
View Article : Google Scholar :
|
|
310
|
Chen Z, Zhang S, Han N, Jiang J, Xu Y, Ma
D, Lu L, Guo X, Qiu M, Huang Q, et al: A neoantigen-based peptide
vaccine for patients with advanced pancreatic cancer refractory to
standard treatment. Front Immunol. 12:6916052021. View Article : Google Scholar
|
|
311
|
Kwok DW, Stevers NO, Etxeberria I, Nejo T,
Colton Cove M, Chen LH, Jung J, Okada K, Lakshmanachetty S, Gallus
M, et al: Tumour-wide RNA splicing aberrations generate actionable
public neoantigens. Nature. 639:463–473. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Rosenberg-Mogilevsky A, Siegfried Z and
Karni R: Generation of tumor neoantigens by RNA splicing
perturbation. Trends Cancer. 11:12–24. 2025. View Article : Google Scholar
|
|
313
|
Huang P, Wen F, Tuerhong N, Yang Y and Li
Q: Neoantigens in cancer immunotherapy: Focusing on alternative
splicing. Front Immunol. 15:14377742024. View Article : Google Scholar :
|
|
314
|
Jayasinghe RG, Cao S, Gao Q, Wendl MC, Vo
NS, Reynolds SM, Zhao Y, Climente-González H, Chai S, Wang F, et
al: Systematic analysis of splice-site-creating mutations in
cancer. Cell Rep. 23:270–281.e3. 2018. View Article : Google Scholar
|
|
315
|
Smart AC, Margolis CA, Pimentel H, He MX,
Miao D, Adeegbe D, Fugmann T, Wong KK and Van Allen EM: Intron
retention is a source of neoepitopes in cancer. Nat Biotechnol.
36:1056–1058. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Wang F, Cai G, Wang Y, Zhuang Q, Cai Z, Li
Y, Gao S, Li F, Zhang C, Zhao B and Liu X: Circular RNA-based
neoantigen vaccine for hepatocellular carcinoma immunotherapy.
MedComm (2020). 5:e6672024. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Hu ZX, Guo XY, Li ZT, Meng ZQ and Huang
SL: The neoantigens derived from transposable elements-A hidden
treasure for cancer immunotherapy. Biochim Biophys Acta Rev Cancer.
1879:1891262024. View Article : Google Scholar
|
|
318
|
Li M, Wang Y, Wu P, Zhang S, Gong Z, Liao
Q, Guo C, Wang F, Li Y, Zeng Z, et al: Application prospect of
circular RNA-based neoantigen vaccine in tumor immunotherapy.
Cancer Lett. 563:2161902023. View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Lang F, Schrörs B, Löwer M, Türeci Ö and
Sahin U: Identification of neoantigens for individualized
therapeutic cancer vaccines. Nat Rev Drug Discov. 21:261–282. 2022.
View Article : Google Scholar
|
|
320
|
Gurung HR, Heidersbach AJ, Darwish M, Chan
PPF, Li J, Beresini M, Zill OA, Wallace A, Tong AJ, Hascall D, et
al: Systematic discovery of neoepitope-HLA pairs for neoantigens
shared among patients and tumor types. Nat Biotechnol.
42:1107–1117. 2024. View Article : Google Scholar
|
|
321
|
Shen KY, Zhu Y, Xie SZ and Qin LX:
Immunosuppressive tumor microenvironment and immunotherapy of
hepatocellular carcinoma: Current status and prospectives. J
Hematol Oncol. 17:252024. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Cherepnev G, Volk HD and Kern F: Use of
peptides and peptide libraries as T-cell stimulants in flow
cytometric studies. Methods Cell Biol. 75:453–479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Gascoigne NR: Do T cells need endogenous
peptides for activation? Nat Rev Immunol. 8:895–900. 2008.
View Article : Google Scholar
|
|
324
|
Shemesh CS, Hsu JC, Hosseini I, Shen BQ,
Rotte A, Twomey P, Girish S and Wu B: Personalized cancer vaccines:
Clinical landscape, challenges, and opportunities. Mol Ther.
29:555–570. 2021. View Article : Google Scholar
|
|
325
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar
|
|
326
|
Prehn RT and Main JM: Immunity to
methylcholanthrene-induced sarcomas. J Natl Cancer Inst.
18:769–778. 1957.PubMed/NCBI
|
|
327
|
Hao Q, Long YH, Yang Y, Deng YQ, Ding ZY,
Yang L, Shu Y and Xu H: Development and clinical applications of
therapeutic cancer vaccines with individualized and shared
neoantigens. Vaccines (Basel). 12:7172024. View Article : Google Scholar
|
|
328
|
D'Alise AM, Brasu N, De Intinis C, Leoni
G, Russo V, Langone F, Baev D, Micarelli E, Petiti L, Picelli S, et
al: Adenoviral-based vaccine promotes neoantigen-specific
CD8+ T cell stemness and tumor rejection. Sci Transl
Med. 14:eabo76042022. View Article : Google Scholar
|
|
329
|
Tsimberidou AM: Targeted therapy in
cancer. Cancer Chemother Pharmacol. 76:1113–1132. 2015. View Article : Google Scholar
|