Glioma is one of the most common types of brain
tumors, accounting for ~30% of all brain tumors and 80% of all
malignant brain tumors (1). There
are several types of gliomas, including astrocytomas,
oligodendrogliomas and ependymomas. Low-grade gliomas (LGGs) grow
slowly, whereas high-grade gliomas, such as glioblastoma multiforme
(GBM), are highly aggressive (2,3).
LGGs generally have a more favorable prognosis, whereas GBMs have a
5-year survival rate of <7% (4,5).
Common symptoms vary depending on the tumor location and may
include headaches, seizures, memory loss, personality changes,
nausea and disrupted vision (6).
Standard treatments for gliomas include surgery, radiation therapy
and chemotherapy (7).
Additionally, immunotherapy and targeted therapies are emerging as
approaches for glioma treatment (8-10).
Metabolic adaptations, particularly the Warburg
effect, facilitate rapid tumor growth by shifting cellular energy
production toward glycolysis, even in the presence of oxygen
(11). This metabolic shift leads
to the excessive accumulation of lactate, which has traditionally
been considered to be a metabolic waste product (12,13). However, recent studies have
highlighted the role of lactate as a crucial signaling molecule
that is capable of modifying proteins through lactylation, which is
an epigenetic and metabolic regulatory mechanism that impacts tumor
behavior (14,15). Lactylation, which is a type of
post-translational modification (PTM) that was first identified as
a histone modification, influences gene expression and immune cell
differentiation (16-19). In addition to histone lactylation,
non-histone protein lactylation has been implicated in various
cellular processes, including tumor cell proliferation, immune
suppression, angiogenesis and therapy resistance (20-22).
Lactylation has been demonstrated to serve critical
roles in glioma development and progression (23). Lactylation-related genes that were
identified using single-cell RNA-sequencing data revealed two
distinct molecular subtypes, forming a prognostic signature that
classified patients with glioma into high-score and low-score
groups (24). Notably, high-score
patients exhibited greater immune infiltration, elevated expression
of chemokines and immune checkpoints, and poorer overall survival
but an enhanced response to immunotherapy compared with low-score
patients. This classification provides a valuable tool to predict
survival outcomes and optimize treatment strategies (24). A pan-cancer multi-omics analysis
of lactylation-associated genes has revealed that the prognostic
significance of the lactylation score differs across tumor types.
The lactylation score is a protective factor in LGG, kidney renal
clear cell carcinoma, adrenocortical carcinoma, rectum
adenocarcinoma and uveal melanoma (25). Higher lactylation scores are
associated with a cold tumor microenvironment (TME), reduced immune
cell infiltration and poorer responses to immunotherapy (25). The present review investigates the
mechanisms and functional significance of lactate-induced protein
lactylation in gliomas, emphasizing its implications for tumor
progression and potential therapeutic interventions. The functions
of protein lactylation in non-glioma cancer types are also
described, indicating that protein lactylation occurs not only in
gliomas but also serves a role in the development and progression
of other tumors.
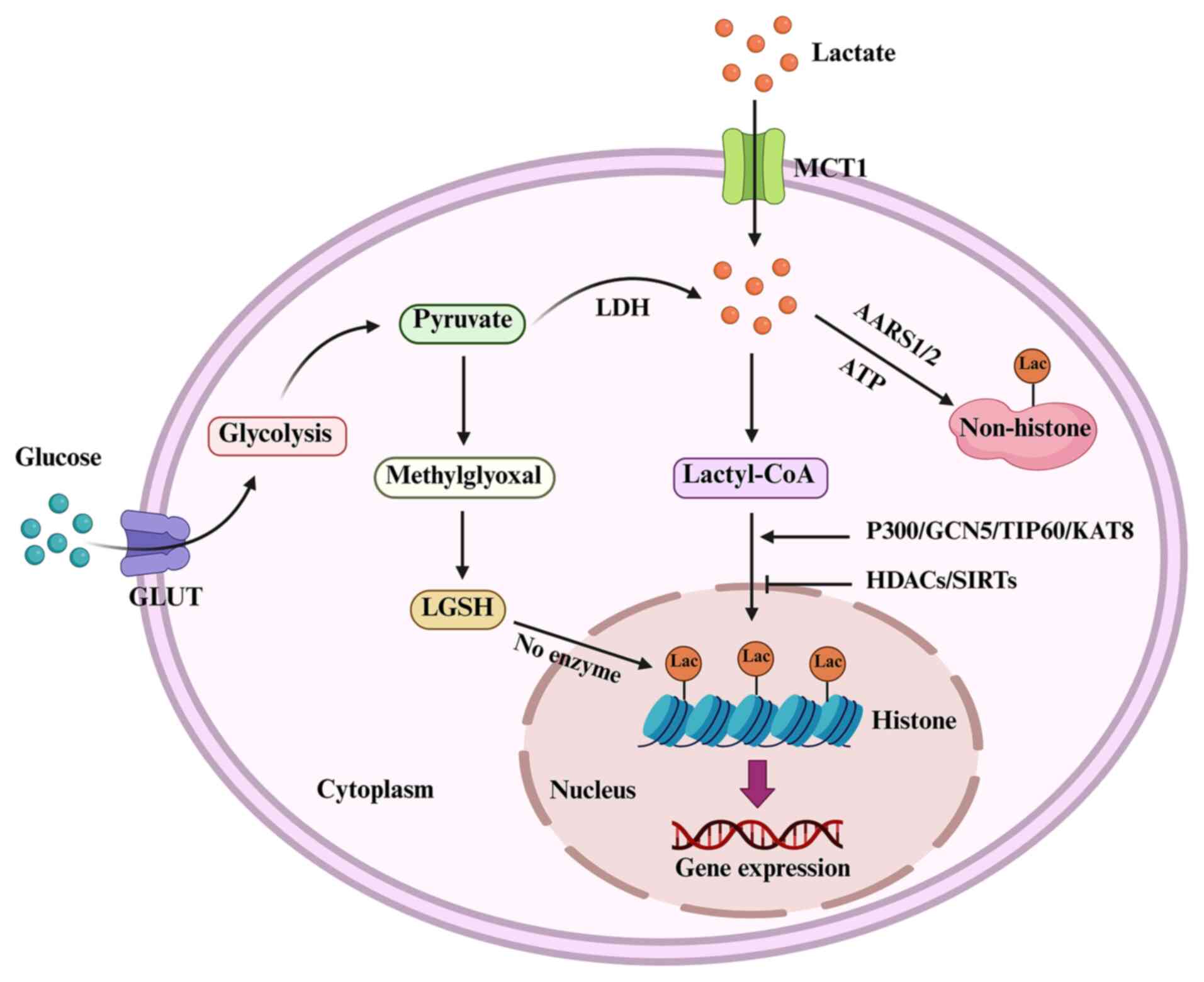
Under normal conditions, cells generate ATP through
glycolysis and mitochondrial oxidative phosphorylation (26,27). During these processes, glycolysis
converts glucose into pyruvate, which then enters the tricarboxylic
acid (TCA) cycle in mitochondria for energy production. However, a
number of cancer cells preferentially rely on aerobic glycolysis,
which is a phenomenon known as the Warburg effect (28,29). In cancer cells, most pyruvate is
converted into lactate rather than entering the TCA cycle. This
metabolic shift is influenced by multiple factors, including
hypoxia, hypoxia-inducible factor 1-α (HIF-1α) activation, oncogene
activation and mitochondrial dysfunction (30). HIF-1α is frequently upregulated in
tumor cells and enhances the expression of key glycolytic enzymes,
such as hexokinase, phosphofructokinase and pyruvate kinase M2
(PKM2), and it promotes the expression of lactate dehydrogenase A
(LDHA) (31,32).
Protein lactylation is a type of PTM that involves
the covalent attachment of lactate-derived acyl groups to lysine
residues on histone or other proteins (43). This process is medicated by
specific enzymes that regulate the addition and removal of lactyl
groups. Several key enzymes, including histone lactyltransferases
[p300 and lysine acetyltransferase 2A (KAT2A)], acyl-CoA synthetase
short chain family member 2 (ACSS2), LDHs and histone deacetylases
(HDACs), have been demonstrated to contribute to protein
lactylation (44-46). p300, which is primarily known as a
histone acetyltransferase, also mediates histone lactylation by
transferring the lactyl group from lactyl-CoA to lysine residues on
histones (47). KAT2A, which is
also known as general control non-depressible 5, has been reported
to exhibit lactyltransferase activity. ACSS2 converts lactate into
lactyl-CoA, which serves as a donor molecule for protein
lactylation (45). KAT2A often
works in conjunction with ACSS2, which produces lactyl-CoA from
lactate, enabling KAT2A to perform its lactyltransferase function
(45).
LDHA and LDHB catalyze the conversion of pyruvate to
lactate, increasing intracellular lactate levels, which can provide
the necessary substrate for lactyl-CoA synthesis (48). Sirtuin 1 (SIRT1) has been
identified as a delactylase that is responsible for removing
lactylation marks from histones (49). Additionally, HDAC2 and HDAC3 have
been implicated in the removal of lactylation, further regulating
this modification (50-52). Although chromobox protein homolog
3 (CBX3) is not a direct lactyltransferase, it binds to lactylated
histones and facilitates transcriptional regulation through
interactions with p300 (53).
MCT1 and MCT4 regulate lactate transport into and out of the cell,
which influences the availability of lactate for lactyl-CoA
synthesis (54,55). Therefore, protein lactylation is a
highly dynamic process that involves a complex interplay of
multiple enzymes that work together to maintain a balance between
lactylation and delactylation (56) (Fig.
1).
Both histone and nonhistone proteins are modified,
and these modifications serve important roles in gene expression,
metabolism and cell signaling (57). For example, tumor-derived lactate
induces bevacizumab resistance in colorectal cancer (CRC) by
enhancing rubicon-like autophagy enhancer expression through
histone H3 lysine 18 lactylation (H3K18la) (58). Similarly, H3K18la promotes
cisplatin resistance in bladder cancer by enhancing glycolytic
metabolism and activating the key transcription factors Y-box
binding protein 1 (YBX1) and YY1, while targeted inhibition of
H3K18la restores cisplatin sensitivity (59). Evodiamine suppresses semaphorin
3A-mediated angiogenesis and programmed death-ligand 1 (PD-L1)
expression in prostate cancer by impairing HIF-1α histone
lactylation and inducing ferroptosis (60). In CRC, G protein-coupled receptor
37 promotes glycolysis and histone lactylation through the Hippo
pathway, promoting liver metastasis (61). Furthermore, the m5C
methyltransferase NOP2/Sun RNA methyltransferase 2 (NSUN2) promotes
CRC progression by reprogramming glucose metabolism through the
NSUN2/YBX1/m5C-enolase 1 axis, whereas lactate accumulation
enhances NSUN2 expression and its lactylation via H3K18la (62). In pancreatic cancer, H3K18la is
highly expressed in tumor tissues, and is positively associated
with the serum lactate, CA19-9 and CEA levels, demonstrating its
potential for use as a novel biomarker for diagnosis and prognosis
(63). Elevated H3K18la levels in
pancreatic ductal adenocarcinoma (PDAC) are associated with poor
prognosis and promote the transcription of TTK and BUB1 mitotic
checkpoint serine/threonine kinase B, which enhance p300 expression
and glycolysis (64).
Additionally, TTK phosphorylates LDHA, leading to increased lactate
production and H3K18la levels (64).
Downregulation of aldo-keto reductase family 1
member B10 reduces cell proliferation and alters the expression of
key target genes, including Bcl-2, Bax, Pan histone lysine
lactylation (Kla) and H3K18la, highlighting the role of histone
lactylation in the pathogenesis of hepatocellular carcinoma (HCC)
(65). In HCC, protein arginine
methyltransferase 3 (PRMT3) facilitates tumor immunosuppression by
upregulating PD-L1 expression through the PRMT3-pyruvate
dehydrogenase kinase 1-lactate-H3K18la axis (66). Targeting this pathway with JX06 or
anti-PD-L1 therapy effectively suppresses tumor growth and restores
CD8+ T cell infiltration, suggesting a promising
immunotherapeutic approach (66).
In ovarian cancer, high histone H3K18la levels are associated with
poor prognosis, advanced tumor stage, shorter platinum recurrence
time and increased metastasis (67). Similarly, elevated H3K18la levels
in gastric cancer are associated with poor prognosis (49). SIRT1, which acts as a histone
delactylase, disrupts a positive feedback loop involving H19,
glycolysis and H3K18la, suggesting a novel strategy for the
treatment of gastric cancer (49). In breast cancer, elevated H3K18la
in tumor tissues promotes peroxisome proliferator activated
receptor δ expression, which increases AKT transcription and
phosphorylation to support cell survival under anaerobic glycolytic
conditions (52).
Nonhistone protein lactylation has been reported in
various cancer types, including PDAC, breast cancer and HCC
(16). The p53 tumor suppressor
protein is a key regulator of cell cycle arrest, apoptosis, DNA
repair and senescence, and it functions as a critical guardian of
genomic stability to prevent tumorigenesis (68). Recently, p53 has been identified
to be lactylated. Specifically, alanyl-tRNA synthetase 1 acts as a
lactate sensor in tumor cells, mediating global lysine lactylation,
including the lactylation of p53, which impairs its function and
promotes tumorigenesis (69).
Notably, β-alanine disrupts this process and reduces cancer
progression (69). In pancreatic
cancer, RHOF promotes tumor progression by upregulating c-Myc,
enhancing PKM2-mediated glycolysis and inducing Snail1 lactylation,
which drives epithelial-mesenchymal transition and promotes tumor
growth (70). Elevated
lactylation in PDAC reshapes the TME and promotes immunotherapy
resistance by inducing K63 lactylation of endosulfine α (ENSA) and
activating STAT3-C-C motif chemokine ligand 2 (CCL2) signaling.
Targeting the ENSA-K63la or CCL2 pathway enhances the efficacy of
immune checkpoint blockade therapy (71).
In triple-negative breast cancer (TNBC), increased
levels of the m6A modification via the HDAC2-mediated delactylation
of METTL3 promote cisplatin resistance. Inhibition of HDAC2 by
tucidinostat enhances cisplatin sensitivity (51). In cervical cancer, lactate-induced
lactylation of DCBLD1 at K172 stabilizes its expression, promoting
glucose-6-phosphate dehydrogenase activity and the subsequent
pentose phosphate pathway activation, thereby facilitating cancer
cell proliferation and metastasis (72). In HCC, lactylation at K28 inhibits
adenylate kinase 2 function, thus promoting cell proliferation and
metastasis by regulating cellular metabolism (73). Additionally, a study has
identified lactylation of phosphofructokinase platelet at lysine
688 and aldolase A at lysine 147 in colon cancer cells (74). These studies highlight the
metabolic impact of protein lactylation on cancer progression and
treatment (70-73).
Protein lactylation has been identified to be a
pivotal regulator of gene expression by promoting the transcription
of genes that are involved in cell proliferation, angiogenesis,
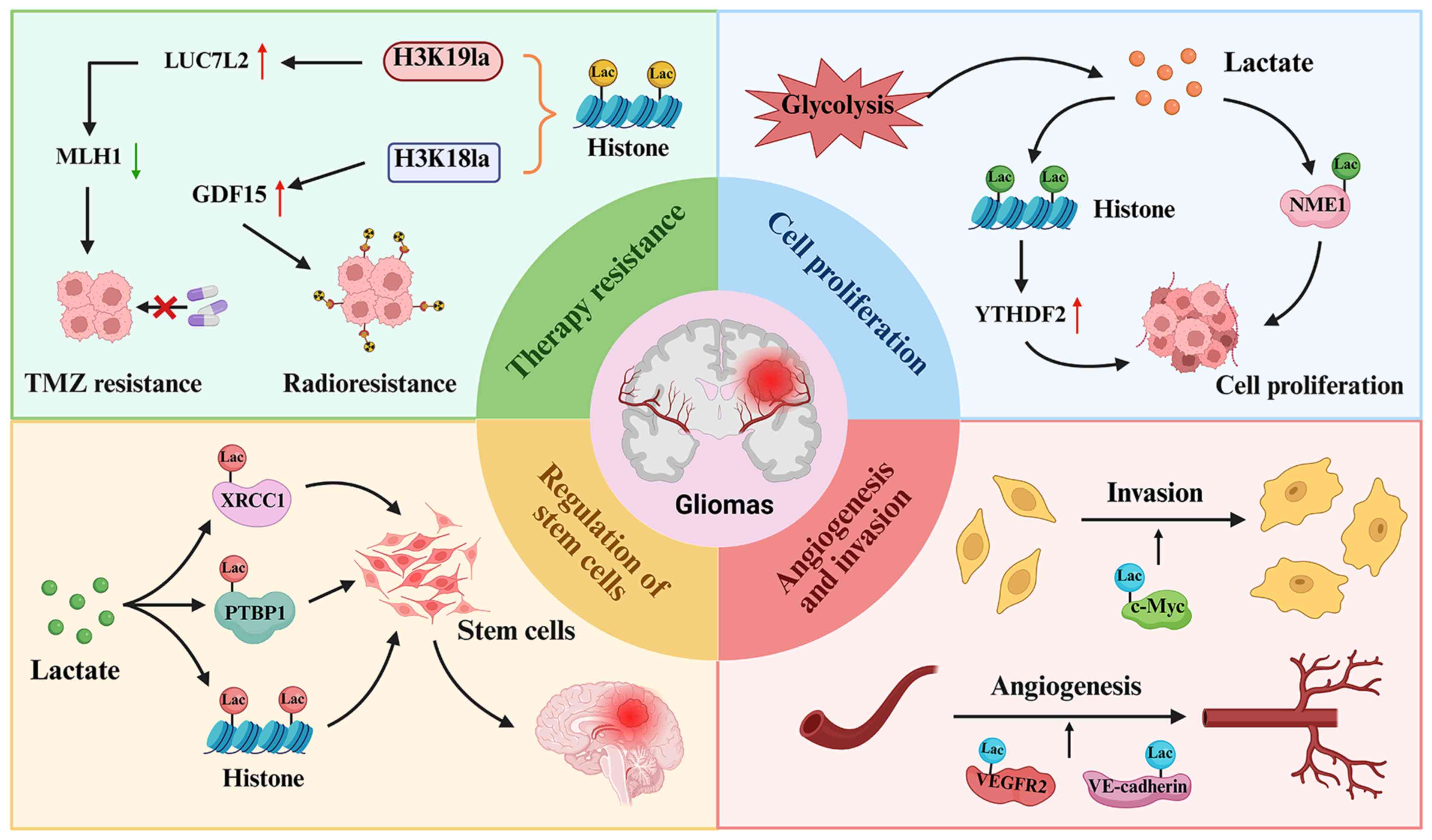
apoptosis, the cell cycle, invasion and drug resistance (20). This section discusses the
functional roles of protein lactylation in gliomas (Fig. 2).
Numerous studies have shown that protein lactylation
regulates cell proliferation in a wide range of human cancer types,
including gliomas (75,76). Batsios et al (75) revealed a crucial role of lactate
in nucleotide biosynthesis in pediatric diffuse midline gliomas
(DMGs). The oncogenic H3K27M mutation (a mutation in the histone H3
gene where lysine 27 is replaced with methionine) upregulated
phosphoglycerate kinase 1, leading to increased lactate production
from glucose in DMGs. Lactate subsequently activated the nucleoside
diphosphate kinase NME/NM23 nucleoside diphosphate kinase 1 (NME1)
through lactylation, promoting the synthesis of nucleoside
triphosphates, which are essential for tumor cell proliferation.
Their study established a novel H3K27M-lactate-NME1 axis,
suggesting potential clinical strategies for tumor monitoring and
therapy evaluation (75). Dong
et al (76) reported that
lactylation of transcription factors, such as HIF-1α, augmented
hypoxia-driven tumor progression. Hypoxia-induced HIF-1α
upregulation enhanced glycolysis and promoted glioma progression by
promoting BCL2 interacting protein 3 (BNIP3)-dependent mitophagy.
Notably, H3K18la upregulated YTH N6-methyladenosine RNA binding
protein F2 (YTHDF2) to sustain mitophagy. Conversely, YTHDF2
downregulation disrupted this process and inhibited tumor
progression. Therefore, HIF-1α orchestrates metabolic reprogramming
through BNIP3-dependent mitophagy, which is a process that is
modulated by H3K18la-induced YTHDF2 expression, ultimately
contributing to glioma cell proliferation and invasion (76).
Lactylation of proteins has been implicated in the
regulation of cellular invasion and angiogenesis in GBM. For
example, dexamethasone (Dex) inhibits GBM cell viability,
migration, invasion and glycolysis (77). The oncogene C-Myc is highly
expressed in GBM cells but its expression is reduced following Dex
treatment (78). Dex suppresses
c-Myc lactylation by inhibiting glycolysis, leading to decreased
stability of the c-Myc protein. Notably, the administration of
sodium lactate reverses the inhibitory effects of Dex, restoring
GBM cell proliferation and invasion (77). These findings suggest that Dex
modulates GBM progression by disrupting glycolysis-dependent c-Myc
stabilization via the regulation of c-Myc lactylation, highlighting
potential metabolic vulnerability that can be used in the treatment
of GBM (77). Additionally,
MAPK6P4 promotes vasculogenic mimicry (VM) in GBM by encoding the
peptide P4-135aa, which phosphorylates KLF transcription factor 15,
enhancing its stability and nuclear translocation to activate LDHA.
LDHA, in turn, facilitates the lactylation of VEGFR2 and vascular
endothelial cadherin, leading to their increased expression.
Conversely, MAPK6P4 deficiency suppresses VM development, as
confirmed in xenograft mouse models (79). VEGFR2 has been shown to serve an
important role in tumor metastasis and angiogenesis through the
regulation of multiple signaling pathways, including the PI3K, AKT
and MAPK pathways (80,81). Therefore, VEGFR2 lactylation may
be linked to tumor angiogenesis in GBM.
CSCs exhibit stem-like properties, including
self-renewal, differentiation potential and tumorigenic capacity
(82). Extensive evidence
suggests that glioma stem cells (GSCs) are the primary contributors
to glioma progression, recurrence and drug resistance (83-85). The common biomarkers for GSCs
include CD133, nextin, CD44, integrin a6, CD15, SOX2, aldehyde
dehydrogenase 1 family member A3 (ALDH1A3), L1 cell adhesion
molecule and A2B5 (86,87). Protein lactylation has been
demonstrated to participate in the maintenance of CSCs in glioma.
For example, the ALDH1A3-PKM2 interaction enhances lactate
accumulation in glioblastoma stem cells, leading to X-ray repair
cross complementing 1 (XRCC1) lactylation. This modification
increases the affinity of XRCC1 for importin α, promoting its
nuclear translocation and affecting DNA repair, thereby influencing
tumor progression (88). Another
study revealed that global lactylation levels were elevated in
GSCs, with polypyrimidine tract binding protein 1 (PTBP1)
hyperlactylation maintaining GSC self-renewal and promoting glioma
progression. Specifically, lactylation of PTBP1 prevented its
degradation via tripartite motif containing 21, enhanced its
RNA-binding capacity, stabilized
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 mRNA and
increased glycolysis. Conversely, SIRT1 counteracted this process
by inducing PTBP1 delactylation, thereby suppressing tumor growth
(89). Additionally, Warburg
effect-induced H3 histone lactylation drives the expression of
NF-κB-related LINC01127 in GBM, promoting self-renewal by
regulating MAP4K4 expression and activating the JNK/NF-κB axis.
Targeting the JNK/NF-κB pathway hinders tumor growth (90). Overall, these findings underscore
the critical roles of protein lactylation in sustaining cancer
stemness and driving glioma development and progression.
Drug resistance, which includes intrinsic resistance
and acquired resistance, is a major obstacle in cancer therapy and
contributes to poor prognosis (91). Intrinsic resistance refers to a
pre-existing insensitivity of cancer cells to therapy, while
acquired resistance develops after initial responsiveness through
adaptive changes or mutations (92,93). Gliomas are resistant to standard
therapies, including radiotherapy, chemotherapy and immunotherapy
(94-96). Resistance to temozolomide (TMZ)
remains a major challenge in glioma treatment (97). The mechanisms of drug resistance
include efflux of drugs, drug inactivation, alteration of drug
targets and epigenetic alterations (98,99). For example, upregulation of efflux
transporters expels chemotherapeutic agents from GBM cells,
reducing intracellular drug concentrations (100). Intercellular adhesion molecule-1
reduces TMZ sensitivity in gliomas with acquired resistance by
promoting the membrane localization of ATP binding cassette
subfamily B member 1 transporters (101). O-6-methylguanine-DNA
methyltransferase (MGMT) repairs TMZ-induced O6-methylguanine DNA
lesions, with its promoter hypermethylation predicting TMZ
sensitivity, while MGMT genomic rearrangements drive chemotherapy
resistance in gliomas (102).
Mutations in tumor-derived protein tyrosine phosphatase receptor
type K impair its functional activity and modify cell
responsiveness to chemotherapeutic agents in glioma (103). USP6 N-terminal like (USP6NL), in
coordination with the EGFR signaling pathway, modulates
ubiquitin-dependent DNA repair mechanisms and contributes to TMZ
resistance in GBM (104). TNF
receptor associated factor 4 (TRAF4) sustains the deubiquitination
of Caveolin-1, thereby enhancing glioblastoma stem cell
characteristics and resistance to TMZ (105). Additionally, TMA exposure
upregulates high mobility group box 1, which facilitates GSC
formation through activation of the toll like receptor 2
(TLR2)/nuclear paraspeckle assembly transcript 1/Wnt signaling
cascade (106). Therefore,
various factors such as USP6NL, TRAF4 and TLR2 contribute to TMZ
resistance in GBM (107).
Lactylation has been revealed to participate in the
regulation of drug resistance in breast cancer, HCC and GBM
(108-110). For instance, cancer-associated
fibroblast (CAF)-derived lactate promotes doxorubicin (DOX)
resistance in TNBC by enhancing histone lactylation-mediated ZFP64
expression. ZFP64 inhibits ferroptosis through the upregulation of
GTP cyclohydrolase 1 and ferritin heavy chain 1, while targeting
this pathway can restore DOX sensitivity (110). Similarly, USP34 promotes HCC
progression and cisplatin resistance by regulating histone
lactylation levels, and USP34 knockdown enhances cisplatin
sensitivity (109). In GBM, the
interaction between ALDH1A3 and PKM2 promotes PKM2 tetramerization
and lactate accumulation. By profiling the lactylated proteome in
GSCs with elevated lactate levels, lysine 247 of XRCC1 has been
identified as a key lactylation site (88). This modification enhances the
binding affinity between XRCC1 and importin α, thereby facilitating
XRCC1 nuclear import and promoting more efficient DNA repair
(88). Using high-throughput
screening of a small-molecule compound library, D34-919 has been
found to effectively disrupt the interaction between ALDH1A3 and
PKM2, thereby inhibiting ALDH1A3-induced PKM2 tetramer formation.
Treatment with D34-919 increases the susceptibility of GBM cells to
apoptosis following chemoradiotherapy. Collectively, these results
highlight ALDH1A3-driven PKM2 tetramerization as a promising target
for enhancing chemoradiotherapy sensitivity in GBM (88).
Studies have shown that protein lactylation governs
immune evasion and immunotherapy in human cancer (113-116). For example, serine and arginine
rich splicing factor 10 (SRSF10) promotes immune evasion and
anti-programmed cell death protein 1 (PD-1) resistance in HCC by
driving a positive feedback loop that involves glycolysis, lactate
production, H3K18la and M2 macrophage polarization (113). Pharmacological inhibition of
SRSF10 with 1C8 enhances the efficacy of anti-PD-1 therapy,
indicating that SRSF10 is a potential therapeutic target for
overcoming immune resistance (113). Glycolysis-driven H3K18la
upregulates B7 homolog 3 protein (B7-H3) expression via the cAMP
responsive element binding protein 1/E1A binding protein P300 axis,
promoting tumor immune evasion by inhibiting CD8+ T cell
cytotoxicity. Notably, the inhibition of glycolysis and B7-H3
enhances antitumor immunity and improves the efficacy of anti-PD-1
therapy (116). In ovarian
cancer, knockdown of LDHB suppresses cancer cell proliferation,
reduces lactate production, enhances T cell-mediated immune
activation and inhibits PD-L1 expression by decreasing H3K18la at
the PD-L1 promoter, thereby reducing immune evasion (114). In cervical cancer, lactate that
is secreted by tumor cells promotes M2 macrophage polarization by
upregulating glycerol-3-phosphate dehydrogenase 2 expression
through H3K18la-mediated histone lactylation, contributing to tumor
progression (115). In bladder
cancer, single-cell analysis has identified parkin RBR E3 ubiquitin
protein ligase (PRKN) as a key regulator of mitophagy in M2
macrophages, with H3K18la-enhanced PRKN expression promoting M2
polarization, immune suppression and tumor progression (117). In gastric cancer, CAFs reduce
the efficacy of PD-1/PD-L1 immunotherapy by secreting lysyl
oxidase, activating TGF-β signaling, promoting insulin like growth
factor 1 expression, enhancing glycolysis and inducing
H3K18la-mediated PD-L1 transcription, thereby reinforcing immune
evasion (118).
Lactate-induced protein lactylation has been
identified to regulate the tumor immune microenvironment and
influence immunotherapy strategies across various cancer types,
including gliomas (119,120). H3K18la and H3K9la function as
transcription initiators in CD8+ T cells, modulating
their activity and antitumor immunity through metabolic and
epigenetic modulation (121).
For example, H3K18la promotes immune evasion in non-small cell lung
cancer (NSCLC) by activating the POM121/MYC/PD-L1 pathway. However,
metabolic reprogramming and immunotherapy can restore
CD8+ T-cell cytotoxicity and enhance antitumor efficacy
(122). In glioma, high-score
cases based on lactylation-related genes exhibit greater immune
infiltration, elevated checkpoint expression and poorer survival
(123). In addition, lactate
production in GBM induces histone lactylation, leading to
CD47-dependent immune evasion by promoting an immunosuppressive
transcriptional program. Targeting lactate production or inhibiting
CBX3 enhances anti-CD47 therapeutic efficacy and tumor cell
phagocytosis, providing a promising strategy to overcome immune
resistance in GBM (53).
Hypoxia enhances the malignancy of glioma cells by
promoting glycolysis and lactic acid accumulation (124). This lactate is absorbed by
macrophages to induce M2 polarization through the MCT-1/H3K18la
signaling pathway, which subsequently upregulates tumor necrosis
factor superfamily member 9 expression and facilitates glioma
progression (124). Chimeric
antigen receptor (CAR) T-cell therapy is an advanced immunotherapy
that harnesses the power of the immune system in patients to fight
cancer (125,126). CAR-T cells recognize specific
antigens on cancer cells and destroy cancer cells with high
specificity, and this approach has shown success in the treatment
of certain hematologic malignancies, such as B-cell acute
lymphoblastic leukemia and diffuse large B-cell lymphoma, as well
as some solid tumors (127,128). One group has revealed that
oxamate enhanced the immune activation of tumor-infiltrating CAR-T
cells in a GBM model by altering immune molecule phenotypes,
increasing regulatory T cell infiltration, and reducing the histone
H3K18la-mediated expression of CD39, CD73 and C-C motif chemokine
receptor 8 (129). Another group
has revealed that EGFR activation triggered ERK-mediated
phosphorylation of ACSS2, leading to its nuclear translocation and
interaction with KAT2A, enabling histone H3 lactylation. This
process activated Wnt/β-catenin, NF-κB and PD-L1 expression,
promoting brain tumor growth and immune evasion (45).
Evidence suggests that targeting lactate metabolism
with inhibitors of LDHA
[7-benzyl-2,3-dihydroxy-6-methyl-4-propylnaphthalene-1-carboxylic
acid (FX11)] and MCT1 (AZD3965) reduces lactate accumulation,
thereby limiting lactylation-mediated tumor progression (130,131). For example, inhibition of LDHA
by FX11 has been shown to suppress tumor progression in various
cancer types, including lymphoma (132), pancreatic cancer (133,134), prostate cancer (135), osteosarcoma (136), neuroblastoma (137), gallbladder carcinoma (138), papillary thyroid carcinoma
(139) and breast cancer
(140). One study demonstrated
that inhibition of LDHA by FX11 suppressed proliferation, migration
and invasion, while promoting apoptosis in prostate cancer cells by
reducing the Warburg effect, glucose consumption, lactate
secretion, and MMP-9, PLAU and cathepsin B expression (131). AZD3965, which is a promising
MCT1 inhibitor, exerts antitumor effects by inducing metabolic
reprogramming (130); this
represents a novel therapeutic approach in various cancer types,
including small cell lung cancer (141,142), NSCLC (143), lymphoma (144,145), breast cancer (146) and bladder cancer (147). Additionally, AR-C155858
selectively inhibits MCT1 and MCT2, which are responsible for the
transport of monocarboxylates, such as lactate, pyruvate and ketone
bodies, across the cell membrane (148). One study has shown that
AR-C155858 inhibited the migration and invasion of pancreatic
cancer cells (149). Further
research is needed to explore the therapeutic potential of FX11,
AZD3965 and AR-C155858 in glioma.
Given that p300 is a key regulator of lactylation,
inhibitors of p300 (such as A-485) may suppress the lactylation of
both histone and nonhistone proteins, thereby altering glioma
epigenetics and metabolism. Several inhibitors of p300 have been
observed to have anticancer effects in various malignancies
(150-153). For example, in neuroblastoma,
the expression of cyclooxygenase-2, which is induced by enterovirus
71, is inhibited by pretreatment with the p300 inhibitor GR343
(150). In acute myeloid
leukemia, the p300 inhibitor C646 selectively enhances apoptosis
and triggers cell cycle arrest (154). Similarly, C646 suppresses human
papillomavirus E6-E7 transcription, leading to p53 accumulation,
inhibition of cell proliferation, disruption of glucose metabolism
and induction of apoptosis in cervical cancer cells (151). Additionally, C646 effectively
overcomes the resistance of resistant melanoma cells to BRAF
inhibitors (153). The p300
inhibitor A-485 has been demonstrated to suppress cell
proliferation, growth hormone secretion and tumor growth by
downregulating key oncogenes and pathways, including Pttg1, c-Myc,
cAMP and PI3K/AKT/mTOR, in growth hormone pituitary adenoma
(152). Furthermore, A-485
induces proteasome-mediated degradation of SOX10 in melanoma cells,
effectively inhibiting tumor cell proliferation (155).
In GBM, the CREB-binding protein/p300 inhibitor
CPI-1612 suppresses H3K27Ac, targets c-MYC, synergizes with TMZ and
penetrates into the brain, which makes it a promising therapeutic
candidate (156). Other
compounds have also been implicated in modulating
lactylation-driven tumor progression. Tanshinone I inhibits ovarian
cancer cell proliferation by blocking glycolysis, reducing histone
H3K18la, downregulating tumor-associated gene expression (such as
TTK, platelet-derived growth factor receptor β, YTHDF2 and
rubicon-like autophagy enhancer), and alleviating the
immunosuppressive TME (157).
Additionally, in lung cancer, collagen triple helix repeat
containing 1 (CTHRC1)+ CAFs promote EGFR-tyrosine kinase
inhibitor resistance by enhancing glycolysis and creating a histone
lactylation-driven positive feedback loop. Notably, gambogenic acid
disrupts this loop to improve therapeutic efficacy, suggesting that
CTHRC1+ CAFs are a predictive biomarker and therapeutic
target in lung cancer (158).
A study has demonstrated that structural maintenance
of chromosomes 4 serves a crucial role in CRC cell dormancy, where
its attenuation enhances glycolysis, increases lactate production
and upregulates ABC transporters via histone lactylation,
ultimately leading to chemotherapy resistance (159). In HCC, histone lactylation has
been linked to tumor prognosis and may serve as an independent
biomarker (160). The genes
nuclear receptor subfamily 6 group A member 1, oxysterol binding
protein 2 and unc-119 lipid binding chaperone B are associated with
prognosis, immunotherapy response and chemotherapy resistance in
HCC (160). Similarly, high
nijmegen breakage syndrome protein 1 lactylation predicts
chemotherapy resistance, whereas reduction of lactate levels via
LDHA inhibition impairs DNA repair and sensitizes tumors to therapy
(161). In oral squamous cell
carcinoma (OSCC), Kla-specific genes serve key roles in tumor
progression, with BCAM, cingulin like 1, diacylglycerol kinase γ
and oxidized low density lipoprotein receptor 1 serving as
prognostic markers (162).
Notably, BCAM promotes invasion, angiogenesis and chemotherapy
resistance in OSCC (162). In
ovarian cancer, malate enzyme 2 (ME2) promotes lactate production
and chemotherapy resistance by converting glutamine-derived malate
to pyruvate (163). In addition,
chemotherapy-induced glucose reduction triggers ME2 acetylation by
acetyl-CoA acetyltransferase 1, enhancing lactate production and
the lactylation of homologous recombination repair proteins,
leading to increased DNA repair capacity and drug resistance
(163).
Given the critical role of lactylation in therapy
resistance, strategies that target lactylation pathways may
sensitize gliomas to radiation and TMZ by impairing DNA repair
mechanisms and drug efflux pathways. Lactylation-related gene
expression has been used to classify patients with GBM into
high-score and low-score groups, with high-score patients
exhibiting an improved response to immunotherapy (123). Notably, the antiepileptic drug
stiripentol, which crosses the blood-brain barrier and inhibits
LDHA/B activity, inhibits lactylation, sensitizing GBM cells to TMZ
in both in vitro and in vivo models (111). Furthermore, blocking the
ACSS2-KAT2A interaction combined with anti-PD-1 therapy enhances
tumor inhibition in brain tumors (45). Furthermore, a lactate generation
inhibitor has been revealed to reprogram CSC metabolism, reduce
tumor immunosuppression and decrease CAR-regulatory T cell
infiltration, potentially enhancing the efficacy of CAR-T-cell
therapy in GBM (129).
Lactate-induced protein lactylation represents a
novel regulatory mechanism with profound implications for glioma
progression, immune suppression and therapy resistance (Fig. 2). Understanding the molecular
landscape of lactylation in gliomas provides novel avenues for the
development of therapeutic interventions. Several reviews have
addressed lactylation in the context of glioma progression
(164,165). Pienkowski et al (164) primarily focused on PTMs such as
phosphorylation, methylation, acetylation, ubiquitination and
glycosylation, but did not discuss lactylation in detail. Han et
al (165) explored the role
of protein lactylation in various brain diseases, including
cerebrovascular disorders, neurodegenerative diseases,
neuroinflammation, mental disorders and brain tumors; however, the
role of lactylation in GBM could not be discussed in detail due to
space limitations. Qiu et al (23) highlighted lactylation in GBM,
emphasizing its role as a link between epigenetic plasticity and
metabolic reprogramming. By contrast, the present review provides a
comprehensive discussion on the functional roles of protein
lactylation in GBM, including its involvement in proliferation,
angiogenesis, invasion, therapeutic resistance, regulation of CSCs
and immune evasion. Furthermore, we hypothesized that targeting
lactate metabolism, lactylation-modifying enzymes and immune
evasion mechanisms holds promise for improving glioma treatment
outcomes. It is important to mention that several critical
challenges must be addressed to fully elucidate the molecular
mechanisms underlying the role of lactylation in glioma development
and treatment. First, although p300 is known to catalyze
lactylation, to the best of our knowledge, the enzymatic regulation
of lactylation removal remains unclear. Identifying delactylases is
critical for better understanding the dynamic lactylation in
glioma. Characterizing lactyltransferase and delactylases will be
pivotal for developing targeted pharmacological modulators of
lactylation. Furthermore, the regulation of histone vs. non-histone
protein lactylation in different glioma subtypes and stages
requires further investigation. Second, the integration of
lactylation with glioma metabolism and the TME requires
comprehensive analysis. Given the hypoxic and lactate-rich nature
of the TME in glioma, lactylation could act as a critical mediator
of immunosuppression. Single-cell epigenomics ad spatial
transcriptomics could elucidate how lactylation shapes cellular
interactions in the glioma niche. Third, current inhibitors that
target p300 or lactate metabolism lack specificity for lactylation,
necessitating the development of small molecules that selectively
inhibit protein lactylation to design more precise therapeutic
strategies. Inhibitors of lactate production (LDHA inhibitors),
lactylation writers (p300) or delactylase activators could
synergize with chemotherapy or immune checkpoint blockade. Using
patient-derived glioma models and organoids could be essential for
preclinical validation of drugs targeting lactylation.
Additionally, p300 has been identified as an acetyltransferase
capable of catalyzing multiple types of protein modifications,
including acetylation (166,167), propionylation (168), butyrylation (169,170) and lactylation (171-173) in oncogenesis. Targeting of
lactylation by p300 inhibitors could also affect other PTMs in
tumorigenesis. Fourth, identifying lactylation-specific biomarkers
in gliomas could aid in early diagnosis, patient stratification and
treatment response monitoring. In parallel, lactylation-associated
gene signatures could be integrated into existing molecular
classification frameworks to enhance predictive accuracy for
therapy response. To the best of our knowledge, to date, no
clinical trials specifically targeting lactylation have been
reported. Therefore, clinical trials are essential to determine the
feasibility and efficacy of targeting lactylation in patients with
glioma in the future. Continued exploration of lactylation and its
role in gliomas may pave the way for the development of innovative
treatment strategies, ultimately improving patient outcomes and
advancing the field of glioma therapy.
Not applicable.
TL, LL, HW and SW were involved in writing the
manuscript draft. Data authentication is not applicable. All
authors have read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Hunan Provincial Health
and Family Planning Commission Project (grant nos. 202204043722 and
20201100), the Hunan University of Chinese Medicine-Hospital
Collaborative Research Grant Program (grant nos. 2024XYLH248 and
2024XYLH252), and the Natural Science Foundation of Changsha (grant
no. kq2502355).
|
1
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao L, Hatami M, Ma W and Skutella T:
Vaccine-based immunotherapy and related preclinical models for
glioma. Trends Mol Med. 30:965–981. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tariq R, Hussain N, Bajwa MH, Aziz HF,
Shamim MS and Enam SA: Multicentric low-grade glioma: A systematic
review of a rare neuro-oncological disease. Clin Neurol Neurosurg.
251:1088212025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weller M, Wen PY, Chang SM, Dirven L, Lim
M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers.
10:332024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Awuah WA, Ben-Jaafar A, Roy S,
Nkrumah-Boateng PA, Tan JK, Abdul-Rahman T and Atallah O:
Predicting survival in malignant glioma using artificial
intelligence. Eur J Med Res. 30:612025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–87. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kampers LFC, Metselaar DS, Vinci M,
Scirocchi F, Veldhuijzen van Zanten S, Eyrich M, Biassoni V,
Hulleman E, Karremann M, Stucker W and Van Gool SW: The complexity
of malignant glioma treatment. Cancers (Basel). 17:8792025.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poorva P, Mast J, Cao B, Shah MV, Pollok
KE and Shen J: Killing the killers: Natural killer cell therapy
targeting glioma stem cells in high-grade glioma. Mol Ther.
33:2462–2478. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma X, Sun C, Ding X, Xu J, Zhang Y, Deng
T, Wang Y, Yang H, Ding R, Li H, et al: Mechanism analysis and
targeted therapy of IDH gene mutation in glioma. Am J Cancer Res.
15:248–270. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong G, Jiang L, Zhou J and Su Y:
Advancements in targeted and immunotherapy strategies for glioma:
Toward precision treatment. Front Immunol. 15:15370132024.
View Article : Google Scholar
|
|
11
|
Llibre A, Kucuk S, Gope A, Certo M and
Mauro C: Lactate: A key regulator of the immune response. Immunity.
58:535–554. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papaneophytou C: The warburg effect: Is it
always an enemy? Front Biosci (Landmark Ed). 29:4022024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong T, Wang QD, Loughran PA, Li YH, Scott
MJ, Billiar TR, Liu YT and Fan J: Mechanism of lactic
acidemia-promoted pulmonary endothelial cells death in sepsis: Role
for CIRP-ZBP1-PANoptosis pathway. Mil Med Res. 11:712024.PubMed/NCBI
|
|
14
|
Iozzo M, Pardella E, Giannoni E and
Chiarugi P: The role of protein lactylation: A kaleidoscopic
post-translational modification in cancer. Mol Cell. 85:1263–1279.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv M, Huang Y, Chen Y and Ding K:
Lactylation modification in cancer: Mechanisms, functions, and
therapeutic strategies. Exp Hematol Oncol. 14:322025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Huang Z, Chen Y, Tian H, Chai P,
Shen Y, Yao Y, Xu S, Ge S and Jia R: Lactate and lactylation in
cancer. Signal Transduct Target Ther. 10:382025. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao L, Qi H, Lv H, Liu W, Zhang R and
Yang A: Lactylation in health and disease: Physiological or
pathological? Theranostics. 15:1787–821. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Dong L and Wang K: Current and
future perspectives of lysine lactylation in cancer. Trends Cell
Biol. 35:190–193. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Zhang N, Ye J, Wang Z, Zhou X, Liu
J, Cai J, Li C and Chen L: Unveiling lactylation modification: A
new hope for cancer treatment. Biomed Pharmacother. 184:1179342025.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sui Y, Shen Z, Wang Z, Feng J and Zhou G:
Lactylation in cancer: Metabolic mechanism and therapeutic
strategies. Cell Death Discov. 11:682025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Malsi K, Xie S, Cai Y, Mohammed N, Xie
K, Lan T and Wu H: The role of lactylation in tumor growth and
cancer progression. Front Oncol. 15:15167852025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu Q, Deng H, Song P, Liu Y and Zhang M:
Lactylation in glioblastoma: A novel epigenetic modifier bridging
epigenetic plasticity and metabolic reprogramming. Int J Mol Sci.
26:33682025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu X, Zhou Z, Qiu P and Xin T: Integrated
single-cell and bulk RNA-sequencing data reveal molecular subtypes
based on lactylation-related genes and prognosis and therapeutic
response in glioma. Heliyon. 10:e307262024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Z, Wu H, Dai Y, Wang Z, Han H, Shen Y,
Zhang R and Wang X: A pan-cancer multi-omics analysis of
lactylation genes associated with tumor microenvironment and cancer
development. Heliyon. 10:e274652024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan T, Nagarajan M, Kang I, Wu C and
Wangpaichitr M: Targeting metabolic vulnerabilities to combat drug
resistance in cancer therapy. J Pers Med. 15:502025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clay R, Li K and Jin L: Metabolic
signaling in the tumor microenvironment. Cancers (Basel).
17:1552025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barba I, Carrillo-Bosch L and Seoane J:
Targeting the warburg effect in cancer: Where do we stand? Int J
Mol Sci. 25:31422024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang
J and Liu B: Targeting the Warburg effect: A revisited perspective
from molecular mechanisms to traditional and innovative therapeutic
strategies in cancer. Acta Pharm Sin B. 14:953–1008. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Xia M, Li J, Liu G, Sun Y, Chen X
and Zhong J: Warburg effect and lactylation in cancer: Mechanisms
for chemoresistance. Mol Med. 31:1462025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Upadhyay S, Khan S and Hassan MI:
Exploring the diverse role of pyruvate kinase M2 in cancer:
Navigating beyond glycolysis and the Warburg effect. Biochim
Biophys Acta Rev Cancer. 1879:1890892024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Basheeruddin M and Qausain S:
Hypoxia-Inducible Factor 1-Alpha (HIF-1alpha): An essential
regulator in cellular metabolic control. Cureus. 16:e638522024.
|
|
33
|
Sharma D, Singh M and Rani R: Role of LDH
in tumor glycolysis: Regulation of LDHA by small molecules for
cancer therapeutics. Semin Cancer Biol. 87:184–95. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Han S, Yao Y and Zhang G: Role of
human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4) in tumor
cells and the tumor microenvironment. Cancer Manag Res. 15:957–975.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh M, Afonso J, Sharma D, Gupta R and
Kumar V, Rani R, Baltazar F and Kumar V: Targeting monocarboxylate
transporters (MCTs) in cancer: How close are we to the clinics?
Semin Cancer Biol. 90:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Li S, Yu L, Leng J and Li N:
Targeting glycolysis: Exploring a new frontier in glioblastoma
therapy. Front Immunol. 15:15223922025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paul S, Ghosh S and Kumar S: Tumor
glycolysis, an essential sweet tooth of tumor cells. Semin Cancer
Biol. 86:1216–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun F, Li W, Du R, Liu M, Cheng Y, Ma J
and Yan S: Impact of glycolysis enzymes and metabolites in
regulating DNA damage repair in tumorigenesis and therapy. Cell
Commun Signal. 23:442025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kooshan Z, Cardenas-Piedra L, Clements J
and Batra J: Glycolysis, the sweet appetite of the tumor
microenvironment. Cancer Lett. 600:2171562024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wan L, Zhang H, Liu J, He Q, Zhao J, Pan
C, Zheng K and Tang Y: Lactylation and human disease. Expert Rev
Mol Med. 27:e102025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Luo N, Gong Z, Zhou W, Ku Y and
Chen Y: Lactate and lysine lactylation of histone regulate
transcription in cancer. Heliyon. 10:e384262024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu Y, Liu W, Luo Z, Xiao F and Sun B: New
insights into the roles of lactylation in cancer. Front Pharmacol.
15:14126722024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang W, Shan G, Bi G, Hu Z, Yi Y, Zeng D,
Lin Z and Zhan C: Lactylation and regulated cell death. Biochim
Biophys Acta Mol Cell Res. 1872:1199272025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li H, Liu C, Li R, Zhou L, Ran Y, Yang Q,
Huang H, Lu H, Song H, Yang B, et al: AARS1 and AARS2 sense
L-lactate to regulate cGAS as global lysine lactyltransferases.
Nature. 634:1229–1237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu R, Ye X, Lu X, Xiao L, Yuan M, Zhao H,
Guo D, Meng Y, Han H, Luo S, et al: ACSS2 acts as a lactyl-CoA
synthetase and couples KAT2A to function as a lactyltransferase for
histone lactylation and tumor immune evasion. Cell Metab.
37:361–376 e7. 2025. View Article : Google Scholar
|
|
46
|
Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z,
Geng D, Yue J, Tang Y, Tian L, et al: The alanyl-tRNA synthetase
AARS1 moonlights as a lactyltransferase to promote YAP signaling in
gastric cancer. J Clin Invest. 134:e1745872024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tsusaka T, Najar MA, Sharma I,
Marcinkiewicz MM, Crispim CVDS, Snyder NW, Burslem GM and Goldberg
EL: Class I histone deacetylases catalyze lysine lactylation.
bioRxiv [Preprint] 2025.02.25.640220. 2025.
|
|
48
|
Urbanska K and Orzechowski A:
Unappreciated Role of LDHA and LDHB to control apoptosis and
autophagy in tumor cells. Int J Mol Sci. 20:20852019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsukihara S, Akiyama Y, Shimada S, Hatano
M, Igarashi Y, Taniai T, Tanji Y, Kodera K, Yasukawa K, Umeura K,
et al: Delactylase effects of SIRT1 on a positive feedback loop
involving the H19-glycolysis-histone lactylation in gastric cancer.
Oncogene. 44:724–738. 2025. View Article : Google Scholar
|
|
50
|
Zou Y, Cao M, Tai M, Zhou H, Tao L, Wu S,
Yang K, Zhang Y, Ge Y, Wang H, et al: A feedback loop driven by
H4K12 lactylation and HDAC3 in macrophages regulates
lactate-induced collagen synthesis in fibroblasts via the TGF-beta
signaling. Adv Sci (Weinh). 12:e24114082025. View Article : Google Scholar
|
|
51
|
He X, Li Y, Li J, Li Y, Chen S, Yan X, Xie
Z, Du J, Chen G, Song J and Mei Q: HDAC2-Mediated METTL3
delactylation promotes DNA damage repair and chemotherapy
resistance in triple-negative breast cancer. Adv Sci (Weinh).
12:e24131212025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Y, Meng W, Dai Y, Xu L, Ding N, Zhang J
and Zhuang X: Anaerobic metabolism promotes breast cancer survival
via Histone-3 Lysine-18 lactylation mediating PPARD axis. Cell
Death Discov. 11:542025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang S, Huang T, Wu Q, Yuan H, Wu X, Yuan
F, Duan T, Taori S, Zhao Y, Snyder NW, et al: Lactate reprograms
glioblastoma immunity through CBX3-regulated histone lactylation. J
Clin Invest. 134:e1768512024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fan M, Yang K, Wang X, Chen L, Gill PS, Ha
T, Liu L, Lewis NH, Williams DL and Li C: Lactate promotes
endothelial-to-mesenchymal transition via Snail1 lactylation after
myocardial infarction. Sci Adv. 9:eadc94652023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang T, Chen L, Kueth G, Shao E, Wang X,
Ha T, Williams DL, Li C, Fan M and Yang K: Lactate's impact on
immune cells in sepsis: Unraveling the complex interplay. Front
Immunol. 15:14834002024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Liang C, Wu C, Wan S and Xu L,
Wang S, Wang J, Huang X and Xu L: A rising star involved in tumour
immunity: Lactylation. J Cell Mol Med. 28:e701462024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi P, Ma Y and Zhang S: Non-histone
lactylation: Unveiling its functional significance. Front Cell Dev
Biol. 13:15356112025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar :
|
|
59
|
Li F, Zhang H, Huang Y, Li D, Zheng Z, Xie
K, Cao C, Wang Q, Zhao X, Huang Z, et al: Single-cell transcriptome
analysis reveals the association between histone lactylation and
cisplatin resistance in bladder cancer. Drug Resist Updat.
73:1010592024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu Y, Huang X, Liang C and Zhang P:
Evodiamine impairs HIF1A histone lactylation to inhibit
Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in
prostate cancer. Eur J Pharmacol. 957:1760072023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou J, Xu W, Wu Y, Wang M, Zhang N, Wang
L, Feng Y, Zhang T, Wang L and Mao A: GPR37 promotes colorectal
cancer liver metastases by enhancing the glycolysis and histone
lactylation via Hippo pathway. Oncogene. 42:3319–30. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen B, Deng Y, Hong Y, Fan L, Zhai X, Hu
H, Yin S, Chen Q, Xie X, Ren X, et al: Metabolic recoding of
NSUN2-mediated m(5)C modification promotes the progression of
colorectal cancer via the NSUN2/YBX1/m(5)C-ENO1 positive feedback
loop. Adv Sci (Weinh). 11:e23098402024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hou J, Guo M, Li Y and Liao Y: Lactylated
histone H3K18 as a potential biomarker for the diagnosis and
prediction of the severity of pancreatic cancer. Clinics (Sao
Paulo). 80:1005442024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li F, Si W, Xia L, Yin D, Wei T, Tao M,
Cui X, Yang J, Hong T and Wei R: Positive feedback regulation
between glycolysis and histone lactylation drives oncogenesis in
pancreatic ductal adenocarcinoma. Mol Cancer. 23:902024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu Y, Li Y, Zhou L, Cheng X and Gong Z:
Hepatic stellate cells promote hepatocellular carcinoma development
by regulating histone lactylation: Novel insights from single-cell
RNA sequencing and spatial transcriptomics analyses. Cancer Lett.
604:2172432024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ding CH, Yan FZ, Xu BN, Qian H, Hong XL,
Liu SQ, Luo YY, Wu SH, Cai LY, Zhang X, et al: PRMT3 drives
PD-L1-mediated immune escape through activating PDHK1-regulated
glycolysis in hepatocellular carcinoma. Cell Death Dis. 16:1582025.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chao J, Chen GD, Huang ST, Gu H, Liu YY,
Luo Y, Lin Z, Chen ZZ, Li X, Zhang B, et al: High histone H3K18
lactylation level is correlated with poor prognosis in epithelial
ovarian cancer. Neoplasma. 71:319–332. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang Y, Che X, Wang PW and Qu X: p53/MDM2
signaling pathway in aging, senescence and tumorigenesis. Semin
Cancer Biol. 101:44–57. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang
B and Zhou F: Alanyl-tRNA synthetase, AARS1, is a lactate sensor
and lactyltransferase that lactylates p53 and contributes to
tumorigenesis. Cell. 187:2375–2392 e33. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao R, Yi Y, Liu H, Xu J, Chen S, Wu D,
Wang L and Li F: RHOF promotes Snail1 lactylation by enhancing
PKM2-mediated glycolysis to induce pancreatic cancer cell
endothelial-mesenchymal transition. Cancer Metab. 12:322024.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun K, Zhang X, Shi J, Huang J, Wang S, Li
X, Lin H, Zhao D, Ye M, Zhang S, et al: Elevated protein
lactylation promotes immunosuppressive microenvironment and
therapeutic resistance in pancreatic ductal adenocarcinoma. J Clin
Invest. 135:e1870242025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Meng Q, Sun H, Zhang Y, Yang X, Hao S, Liu
B, Zhou H, Xu ZX and Wang Y: Lactylation stabilizes DCBLD1
activating the pentose phosphate pathway to promote cervical cancer
progression. J Exp Clin Cancer Res. 43:362024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S,
Shen X, Wu Y, Zhang S, Wang X, et al: Lactylome analysis suggests
lactylation-dependent mechanisms of metabolic adaptation in
hepatocellular carcinoma. Nat Metab. 5:61–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cheng Z, Huang H, Li M and Chen Y:
Proteomic analysis identifies PFKP lactylation in SW480 colon
cancer cells. iScience. 27:1086452023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Batsios G, Taglang C, Udutha S, Gillespie
AM, Robinson SP, Phoenix T, Mueller S, Venneti S, Koschmann C and
Viswanath P: Lactylation fuels nucleotide biosynthesis and
facilitates deuterium metabolic imaging of tumor proliferation in
H3K27M-mutant gliomas. bioRxiv. Jan 3–2025.Epub ahead of print.
|
|
76
|
Dong F, Yin H and Zheng Z:
Hypoxia-inducible factor-1alpha regulates BNIP3-dependent mitophagy
and mediates metabolic reprogramming through histone lysine
lactylation modification to affect glioma proliferation and
invasion. J Biochem Mol Toxicol. 39:e700692025. View Article : Google Scholar
|
|
77
|
Zhu J and Zhang Y: Dexmedetomidine
inhibits the migration, invasion, and glycolysis of glioblastoma
cells by lactylation of c-myc. Neurol Res. 46:1105–1112. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Trejo-Solis C, Castillo-Rodriguez RA,
Serrano-Garcia N, Silva-Adaya D, Vargas-Cruz S, Chavez-Cortez EG,
Gallardo-Perez JC, Zavala-Vega S, Cruz-Salgado A and
Magana-Maldonado R: Metabolic roles of HIF1, c-Myc, and p53 in
glioma cells. Metabolites. 14:2492024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang M, Zhao Y, Liu X, Ruan X, Wang P,
Liu L, Wang D, Dong W, Yang C and Xue Y: Pseudogene MAPK6P4-encoded
functional peptide promotes glioblastoma vasculogenic mimicry
development. Commun Biol. 6:10592023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Uba AI: Computer-aided design of VEGFR-2
inhibitors as anticancer agents: A review. J Mol Recognit.
38:e31042025. View Article : Google Scholar
|
|
81
|
Mehta K, Hegde M, Girisa S, Vishwa R,
Alqahtani MS, Abbas M, Shakibaei M, Sethi G and Kunnumakkara AB:
Targeting RTKs/nRTKs as promising therapeutic strategies for the
treatment of triple-negative breast cancer: Evidence from clinical
trials. Mil Med Res. 11:762024.PubMed/NCBI
|
|
82
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Agosti E, Zeppieri M, Ghidoni M, Ius T,
Tel A, Fontanella MM and Panciani PP: Role of glioma stem cells in
promoting tumor chemo- and radioresistance: A systematic review of
potential targeted treatments. World J Stem Cells. 16:604–614.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Friess D, Brauer S, Poysti A, Choudhury C
and Harris L: Tools to study neural and glioma stem cell
quiescence. Trends Neurosci. 47:736–748. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ramar V, Guo S, Hudson B and Liu M:
Progress in glioma stem cell research. Cancers (Basel). 16:1022023.
View Article : Google Scholar
|
|
86
|
Agosti E, Antonietti S, Ius T, Fontanella
MM, Zeppieri M and Panciani PP: Glioma stem cells as promoter of
glioma progression: A systematic review of molecular pathways and
targeted therapies. Int J Mol Sci. 25:79792024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tang J, Amin MA and Campian JL:
Glioblastoma stem cells at the nexus of tumor heterogeneity, immune
evasion, and therapeutic resistance. Cells. 14:5622025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li G, Wang D, Zhai Y, Pan C, Zhang J, Wang
C, Huang R, Yu M, Li Y, Liu X, et al: Glycometabolic
reprogramming-induced XRCC1 lactylation confers therapeutic
resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab.
36:1696–1710 e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou Z, Yin X, Sun H, Lu J, Li Y, Fan Y,
Lv P, Han M, Wu J, Li S, et al: PTBP1 lactylation promotes glioma
stem cell maintenance through PFKFB4-Driven glycolysis. Cancer Res.
85:739–757. 2025. View Article : Google Scholar
|
|
90
|
Li L, Li Z, Meng X, Wang X, Song D, Liu Y,
Xu T, Qin J, Sun N, Tian K, et al: Histone lactylation-derived
LINC01127 promotes the self-renewal of glioblastoma stem cells via
the cis-regulating the MAP4K4 to activate JNK pathway. Cancer Lett.
579:2164672023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Russo M, Chen M, Mariella E, Peng H,
Rehman SK, Sancho E, Sogari A, Toh TS, Balaban NQ, Batlle E, et al:
Cancer drug-tolerant persister cells: From biological questions to
clinical opportunities. Nat Rev Cancer. 24:694–717. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kuczynski EA, Sargent DJ, Grothey A and
Kerbel RS: Drug rechallenge and treatment beyond
progression-implications for drug resistance. Nat Rev Clin Oncol.
10:571–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu J, Yang F, Hu J and Zhang X:
Nanoparticles for efficient drug delivery and drug resistance in
glioma: New perspectives. CNS Neurosci Ther. 30:e147152024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huo X, Li H, Xing Y, Liu W, Chen P, Du F,
Song L, Yu Z, Cao X and Tian J: Two decades of progress in glioma
methylation research: The rise of temozolomide resistance and
immunotherapy insights. Front Neurosci. 18:14407562024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen T, Ma W, Wang X, Ye Q, Hou X, Wang Y,
Jiang C, Meng X, Sun Y and Cai J: Insights of immune cell
heterogeneity, tumor-initiated subtype transformation, drug
resistance, treatment and detecting technologies in glioma
microenvironment. J Adv Res. 72:527–554. 2025. View Article : Google Scholar :
|
|
97
|
Davidson CL, Vengoji R, Jain M, Batra SK
and Shonka N: Biological, diagnostic and therapeutic implications
of exosomes in glioma. Cancer Lett. 582:2165922024. View Article : Google Scholar :
|
|
98
|
Brown R, Curry E, Magnani L,
Wilhelm-Benartzi CS and Borley J: Poised epigenetic states and
acquired drug resistance in cancer. Nat Rev Cancer. 14:747–753.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Munoz JL, Walker ND, Scotto KW and
Rameshwar P: Temozolomide competes for P-glycoprotein and
contributes to chemoresistance in glioblastoma cells. Cancer Lett.
367:69–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang X, Tan Y, Li T, Tan D, Fu B, Yang M,
Chen Y, Cao M, Xuan C, Du Q, et al: Intercellular adhesion
molecule-1 suppresses TMZ chemosensitivity in acquired
TMZ-resistant gliomas by increasing assembly of ABCB1 on the
membrane. Drug Resist Updat. 76:1011122024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Oldrini B, Vaquero-Siguero N, Mu Q, Kroon
P, Zhang Y, Galan-Ganga M, Bao Z, Wang Z, Liu H, Sa JK, et al: MGMT
genomic rearrangements contribute to chemotherapy resistance in
gliomas. Nat Commun. 11:38832020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Agarwal S, Al-Keilani MS, Alqudah MA,
Sibenaller ZA, Ryken TC and Assem M: Tumor derived mutations of
protein tyrosine phosphatase receptor type K affect its function
and alter sensitivity to chemotherapeutics in glioma. PLoS One.
8:e628522013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Su IC, Su YK, Chuang HY, Yadav VK,
Setiawan SA, Fong IH, Yeh CT, Huang HC and Lin CM:
Ubiquitin-specific protease 6 n-terminal-like protein (USP6NL) and
the epidermal growth factor receptor (EGFR) signaling axis
regulates ubiquitin-mediated DNA repair and temozolomide-resistance
in glioblastoma. Biomedicines. 10:15312022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li Y, Wang T, Wan Q, Wang Q, Chen Z, Gao
Y, Ye Y, Lin J, Zhao B, Wang H, et al: TRAF4 maintains
deubiquitination of caveolin-1 to drive glioblastoma stemness and
temozolomide resistance. Cancer Res. 82:3573–3587. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao XY, Zang J, Zheng MH, Zhang YF, Yue
KY, Cao XL, Cao Y, Li XX, Han H, Jiang XF and Liang L: Temozolomide
treatment induces HMGB1 to promote the formation of glioma stem
cells via the TLR2/NEAT1/Wnt pathway in glioblastoma. Front Cell
Dev Biol. 9:6208832021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pu J, Yuan K, Tao J, Qin Y, Li Y, Fu J, Li
Z, Zhou H, Tang Z, Li L, et al: Glioblastoma multiforme: An updated
overview of temozolomide resistance mechanisms and strategies to
overcome resistance. Discov Oncol. 16:7312025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yu D, Zhong Q, Wang Y, Yin C, Bai M, Zhu
J, Chen J, Li H and Hong W: Lactylation: The metabolic accomplice
shaping cancer's response to radiotherapy and immunotherapy. Ageing
Res Rev. 104:1026702025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fan M, Liu JS, Wei XL, Nie Y and Liu HL:
Histone lactylation-driven ubiquitin-specific protease 34 promotes
cisplatin resistance in hepatocellular carcinoma. Gastroenterology
Res. 18:23–30. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang K, Guo L, Li X, Hu Y and Luo N:
Cancer-associated fibroblasts promote doxorubicin resistance in
triple-negative breast cancer through enhancing ZFP64 histone
lactylation to regulate ferroptosis. J Transl Med. 23:2472025.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yue Q, Wang Z, Shen Y, Lan Y, Zhong X, Luo
X, Yang T, Zhang M, Zuo B, Zeng T, et al: Histone H3K9 lactylation
confers temozolomide resistance in glioblastoma via LUC7L2-Mediated
MLH1 intron retention. Adv Sci (Weinh). 11:e23092902024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu R, Ren X, Park YE, Feng H, Sheng X,
Song X, AminiTabrizi R, Shah H, Li L, Zhang Y, et al: Nuclear
GTPSCS functions as a lactyl-CoA synthetase to promote histone
lactylation and gliomagenesis. Cell Metab. 37:377–394 e9. 2025.
View Article : Google Scholar
|
|
113
|
Cai J, Song L, Zhang F, Wu S, Zhu G, Zhang
P, Chen S, Du J, Wang B, Cai Y, et al: Targeting SRSF10 might
inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy
in hepatocellular carcinoma. Cancer Commun (Lond). 44:1231–1260.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hu X, Huang Z and Li L: LDHB mediates
histone lactylation to activate PD-L1 and promote ovarian cancer
immune escape. Cancer Invest. 43:70–79. 2025. View Article : Google Scholar
|
|
115
|
Huang C, Xue L, Lin X, Shen Y and Wang X:
Histone lactylation-driven GPD2 mediates M2 macrophage polarization
to promote malignant transformation of cervical cancer progression.
DNA Cell Biol. 43:605–618. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ma Z, Yang J, Jia W, Li L, Li Y, Hu J, Luo
W, Li R, Ye D and Lan P: Histone lactylation-driven B7-H3
expression promotes tumor immune evasion. Theranostics.
15:2338–2359. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Deng X, Huang Y, Zhang J, Chen Y, Jiang F,
Zhang Z, Li T, Hou L, Tan W and Li F: Histone lactylation regulates
PRKN-Mediated mitophagy to promote M2 macrophage polarization in
bladder cancer. Int Immunopharmacol. 148:1141192025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li Z, Liang P, Chen Z, Chen Z, Jin T, He
F, Chen X and Yang K: CAF-secreted LOX promotes PD-L1 expression
via histone lactylation and regulates tumor EMT through TGFβ/IGF1
signaling in gastric cancer. Cell Signal. 124:1114622024.
View Article : Google Scholar
|
|
119
|
Wang W, Wang H, Wang Q, Yu X and Ouyang L:
Lactate-induced protein lactylation in cancer: Functions,
biomarkers and immunotherapy strategies. Front Immunol.
15:15130472025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hao ZN, Tan XP, Zhang Q, Li J, Xia R and
Ma Z: Lactate and lactylation: Dual regulators of T-cell-mediated
tumor immunity and immunotherapy. Biomolecules. 14:16462024.
View Article : Google Scholar :
|
|
121
|
Raychaudhuri D, Singh P, Chakraborty B,
Hennessey M, Tannir AJ, Byregowda S, Natarajan SM, Trujillo-Ocampo
A, Im JS and Goswami S: Histone lactylation drives CD8(+) T cell
metabolism and function. Nat Immunol. 25:2140–2151. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang C, Zhou L, Zhang M, Du Y, Li C, Ren
H and Zheng L: H3K18 lactylation potentiates immune escape of
non-small cell lung cancer. Cancer Res. 84:3589–3601. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
De Leo A, Ugolini A, Yu X, Scirocchi F,
Scocozza D, Peixoto B, Pace A, D'Angelo L, Liu JKC, Etame AB, et
al: Glucose-driven histone lactylation promotes the
immunosuppressive activity of monocyte-derived macrophages in
glioblastoma. Immunity. 57:1105–1123 e8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li M, Sun P, Tu B, Deng G, Li D and He W:
Hypoxia conduces the glioma progression by inducing M2 macrophage
polarization via elevating TNFSF9 level in a
histone-lactylation-dependent manner. Am J Physiol Cell Physiol.
327:C487–C504. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mulvey A, Trueb L, Coukos G and Arber C:
Novel strategies to manage CAR-T cell toxicity. Nat Rev Drug
Discov. 24:379–397. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Diorio C, Teachey DT and Grupp SA:
Allogeneic chimeric antigen receptor cell therapies for cancer:
Progress made and remaining roadblocks. Nat Rev Clin Oncol.
22:10–27. 2025. View Article : Google Scholar
|
|
127
|
Tang L, Huang ZP, Mei H and Hu Y: Insights
gained from single-cell analysis of chimeric antigen receptor
T-cell immunotherapy in cancer. Mil Med Res. 10:522023.PubMed/NCBI
|
|
128
|
Uslu U and June CH: Beyond the blood:
Expanding CAR T cell therapy to solid tumors. Nat Biotechnol.
43:506–515. 2025. View Article : Google Scholar
|
|
129
|
Sun T, Liu B, Li Y, Wu J, Cao Y, Yang S,
Tan H, Cai L, Zhang S, Qi X, et al: Oxamate enhances the efficacy
of CAR-T therapy against glioblastoma via suppressing
ectonucleotidases and CCR8 lactylation. J Exp Clin Cancer Res.
42:2532023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Silva A, Antunes B, Batista A,
Pinto-Ribeiro F, Baltazar F and Afonso J: In vivo anticancer
activity of AZD3965: A systematic review. Molecules. 27:1812021.
View Article : Google Scholar
|
|
131
|
Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ,
Xue J, Yang HQ, Li JL, Liu XF and Kuang SJ: Inhibition of LDHA
suppresses tumor progression in prostate cancer. Tumour Biol.
36:8093–8100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rajeshkumar NV, Dutta P, Yabuuchi S, de
Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK,
Hidalgo M, et al: Therapeutic targeting of the warburg effect in
pancreatic cancer relies on an absence of p53 function. Cancer Res.
75:3355–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Mohammad GH, Vassileva V, Acedo P, Olde
Damink SWM, Malago M, Dhar DK and Pereira SP: Targeting pyruvate
kinase M2 and lactate dehydrogenase a is an effective combination
strategy for the treatment of pancreatic cancer. Cancers (Basel).
11:13722019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hao J, Graham P, Chang L, Ni J, Wasinger
V, Beretov J, Deng J, Duan W, Bucci J, Malouf D, et al: Proteomic
identification of the lactate dehydrogenase A in a radioresistant
prostate cancer xenograft mouse model for improving radiotherapy.
Oncotarget. 7:74269–74285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gao S, Tu DN, Li H, Jiang JX, Cao X, You
JB and Zhou XQ: Pharmacological or genetic inhibition of LDHA
reverses tumor progression of pediatric osteosarcoma. Biomed
Pharmacother. 81:388–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rellinger EJ, Craig BT, Alvarez AL, Dusek
HL, Kim KW, Qiao J and Chung DH: FX11 inhibits aerobic glycolysis
and growth of neuroblastoma cells. Surgery. 161:747–752. 2017.
View Article : Google Scholar
|
|
138
|
He Y, Chen X, Yu Y, Li J, Hu Q, Xue C,
Chen J, Shen S, Luo Y, Ren F, et al: LDHA is a direct target of
miR-30d-5p and contributes to aggressive progression of gallbladder
carcinoma. Mol Carcinog. 57:772–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hou X, Shi X, Zhang W, Li D, Hu L, Yang J,
Zhao J, Wei S, Wei X, Ruan X, et al: LDHA induces EMT gene
transcription and regulates autophagy to promote the metastasis and
tumorigenesis of papillary thyroid carcinoma. Cell Death Dis.
12:3472021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Jiang J, Roman J, Xu HN and Li LZ: An
observation on enhanced extracellular acidification and lactate
production induced by inhibition of lactate dehydrogenase A. Adv
Exp Med Biol. 1269:163–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Polanski R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar :
|
|
142
|
Bola BM, Chadwick AL, Michopoulos F,
Blount KG, Telfer BA, Williams KJ, Smith PD, Critchlow SE and
Stratford IJ: Inhibition of monocarboxylate transporter-1 (MCT1) by
AZD3965 enhances radiosensitivity by reducing lactate transport.
Mol Cancer Ther. 13:2805–2816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Huang CY, Hsu LH, Chen CY, Chang GC, Chang
HW, Hung YM, Liu KJ and Kao SH: Inhibition of alternative cancer
cell metabolism of EGFR mutated non-small cell lung cancer serves
as a potential therapeutic strategy. Cancers (Basel). 12:1812020.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Beloueche-Babari M, Wantuch S, Casals
Galobart T, Koniordou M, Parkes HG, Arunan V, Chung YL, Eykyn TR,
Smith PD and Leach MO: MCT1 INHIBITOR AZD3965 increases
mitochondrial metabolism, facilitating combination therapy and
noninvasive magnetic resonance spectroscopy. Cancer Res.
77:5913–5924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Beloueche-Babari M, Casals Galobart T,
Delgado-Goni T, Wantuch S, Parkes HG, Tandy D, Harker JA and Leach
MO: Monocarboxylate transporter 1 blockade with AZD3965 inhibits
lipid biosynthesis and increases tumour immune cell infiltration.
Br J Cancer. 122:895–903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Benyahia Z, Blackman MCNM, Hamelin L,
Zampieri LX, Capeloa T, Bedin ML, Vazeille T, Schakman O and
Sonveaux P: In vitro and in vivo characterization of MCT1 inhibitor
AZD3965 confirms preclinical safety compatible with breast cancer
treatment. Cancers (Basel). 13:5692021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Silva A, Felix A, Cerqueira M, Goncalves
CS, Sampaio-Marques B, Longatto-Filho A, Baltazar F and Afonso J:
Effects of lactate transport inhibition by AZD3965 in
muscle-invasive urothelial bladder cancer. Pharmaceutics.
15:26882023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Puri S and Juvale K: Monocarboxylate
transporter 1 and 4 inhibitors as potential therapeutics for
treating solid tumours: A review with structure-activity
relationship insights. Eur J Med Chem. 199:1123932020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kong SC, Nohr-Nielsen A, Zeeberg K,
Reshkin SJ, Hoffmann EK, Novak I and Pedersen SF: Monocarboxylate
transporters MCT1 and MCT4 regulate migration and invasion of
pancreatic ductal adenocarcinoma cells. Pancreas. 45:1036–1047.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Tung WH, Hsieh HL, Lee IT and Yang CM:
Enterovirus 71 modulates a COX-2/PGE2/cAMP-dependent viral
replication in human neuroblastoma cells: Role of the
c-Src/EGFR/p42/p44 MAPK/CREB signaling pathway. J Cell Biochem.
112:559–570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
He H, Lai Y, Hao Y, Liu Y, Zhang Z, Liu X,
Guo C, Zhang M, Zhou H, Wang N, et al: Selective p300 inhibitor
C646 inhibited HPV E6-E7 genes, altered glucose metabolism and
induced apoptosis in cervical cancer cells. Eur J Pharmacol.
812:206–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ji C, Xu W, Ding H, Chen Z, Shi C, Han J,
Yu L, Qiao N, Zhang Y, Cao X, et al: The p300 inhibitor A-485
exerts antitumor activity in growth hormone pituitary adenoma. J
Clin Endocrinol Metab. 107:e2291–e2300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Zhang F, Tang X, Fan S, Liu X, Sun J, Ju
C, Liang Y, Liu R, Zhou R, Yu B, et al: Targeting the p300/NONO
axis sensitizes melanoma cells to BRAF inhibitors. Oncogene.
40:4137–4150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Gao XN, Lin J, Ning QY, Gao L, Yao YS,
Zhou JH, Li YH, Wang LL and Yu L: A histone acetyltransferase p300
inhibitor C646 induces cell cycle arrest and apoptosis selectively
in AML1-ETO-positive AML cells. PLoS One. 8:e554812013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Waddell A, Grbic N, Leibowitz K, Wyant WA,
Choudhury S, Park K, Collard M, Cole PA and Alani RM: p300 KAT
regulates SOX10 stability and function in human melanoma. Cancer
Res Commun. 4:1894–1907. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mladek AC, Yan H, Tian S, Decker PA,
Burgenske DM, Bakken K, Hu Z, He L, Connors MA, Carlson BL, et al:
RBBP4-p300 axis modulates expression of genes essential for cell
survival and is a potential target for therapy in glioblastoma.
Neuro Oncol. 24:1261–1272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Jin Z, Yun L and Cheng P: Tanshinone I
reprograms glycolysis metabolism to regulate histone H3 lysine 18
lactylation (H3K18la) and inhibits cancer cell growth in ovarian
cancer. Int J Biol Macromol. 291:1390722025. View Article : Google Scholar
|
|
158
|
Zhang C, Zhou W, Xu H, Xu J, Li J, Liu X,
Lu X, Dai J, Jiang Y, Wang W, et al: Cancer-associated fibroblasts
promote EGFR-TKI resistance via the CTHRC1/glycolysis/H3K18la
positive feedback loop. Oncogene. 44:1400–1414. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Sun X, He L, Liu H, Thorne RF, Zeng T, Liu
L, Zhang B, He M, Huang Y, Li M, et al: The diapause-like
colorectal cancer cells induced by SMC4 attenuation are
characterized by low proliferation and chemotherapy insensitivity.
Cell Metab. 35:1563–1579 e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wu Q, Li X, Long M, Xie X and Liu Q:
Integrated analysis of histone lysine lactylation (Kla)-specific
genes suggests that NR6A1, OSBP2 and UNC119B are novel therapeutic
targets for hepatocellular carcinoma. Sci Rep. 13:186422023.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen H, Li Y, Li H, Chen X, Fu H, Mao D,
Chen W, Lan L, Wang C, Hu K, et al: NBS1 lactylation is required
for efficient DNA repair and chemotherapy resistance. Nature.
631:663–669. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Huang G, Chen S, He J, Li H, Ma Z, Lubamba
GP, Wang L, Guo Z and Li C: Histone lysine lactylation
(Kla)-induced BCAM promotes OSCC progression and Cis-platinum
resistance. Oral Dis. 31:1116–1132. 2025. View Article : Google Scholar
|
|
163
|
Zheng C, Tan H, Niu G, Huang X, Lu J, Chen
S, Li H, Zhu J, Zhou Z, Xu M, et al: ACAT1-Mediated ME2 acetylation
drives chemoresistance in ovarian cancer by linking glutaminolysis
to lactate production. Adv Sci (Weinh). 12:e24164672025. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Pienkowski T, Kowalczyk T, Cysewski D,
Kretowski A and Ciborowski M: Glioma and post-translational
modifications: A complex relationship. Biochim Biophys Acta Rev
Cancer. 1878:1890092023. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Han M, He W, Zhu W and Guo L: The role of
protein lactylation in brain health and disease: Current advances
and future directions. Cell Death Discov. 11:2132025. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wu X, Zhang X, Tang S and Wang Y: The
important role of the histone acetyltransferases p300/CBP in cancer
and the promising anticancer effects of p300/CBP inhibitors. Cell
Biol Toxicol. 41:322025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chen Q, Yang B, Liu X, Zhang XD, Zhang L
and Liu T: Histone acetyltransferases CBP/p300 in tumorigenesis and
CBP/p300 inhibitors as promising novel anticancer agents.
Theranostics. 12:4935–4948. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liu B, Lin Y, Darwanto A, Song X, Xu G and
Zhang K: Identification and characterization of propionylation at
histone H3 lysine 23 in mammalian cells. J Biol Chem.
284:32288–32295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Chen Y, Sprung R, Tang Y, Ball H, Sangras
B, Kim SC, Falck JR, Peng J, Gu W and Zhao Y: Lysine propionylation
and butyrylation are novel post-translational modifications in
histones. Mol Cell Proteomics. 6:812–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Bhattacharya A, Chatterjee S, Bhaduri U,
Singh AK, Vasudevan M, Sashidhara KV, Guha R, Nazir A, Rath SK,
Natesh N and Kundu TK: Butyrylation meets adipogenesis-probed by a
p300-catalyzed acylation-specific small molecule inhibitor:
Implication in anti-obesity therapy. J Med Chem. 65:12273–12291.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Jiang C, He X, Chen X, Huang J, Liu Y,
Zhang J, Chen H, Sui X, Lv X, Zhao X, et al: Lactate accumulation
drives hepatocellular carcinoma metastasis through facilitating
tumor-derived exosome biogenesis by Rab7A lactylation. Cancer Lett.
627:2176362025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Deng J, Li Y, Yin L, Liu S, Li Y, Liao W,
Mu L, Luo X and Qin J: Histone lactylation enhances GCLC expression
and thus promotes chemoresistance of colorectal cancer stem cells
through inhibiting ferroptosis. Cell Death Dis. 16:1932025.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Dai J, Lu X, Zhang C, Qu T, Li W, Su J,
Guo R, Yin D, Wu P, Han L and Zhang E: NNMT promotes acquired
EGFR-TKI resistance by forming EGR1 and lactate-mediated double
positive feedback loops in non-small cell lung cancer. Mol Cancer.
24:792025. View Article : Google Scholar : PubMed/NCBI
|