Cancer stem cells (CSCs) are a specialized subset of
tumor cells with properties akin to those of normal stem cells,
such as self-renewal (the ability to generate identical copies of
themselves) and differentiation (the capacity to form diverse cell
types). Unlike normal stem cells, which maintain tissue balance,
CSCs drive uncontrolled tumor growth and are linked to treatment
resistance and relapse (1). These
cells are hypothesized to be responsible for tumor initiation,
progression and resistance to conventional therapies (2). Over the past decade, extensive
research has focused on understanding the biology of CSCs and their
role in cancer progression. CSCs are believed to maintain tumor
growth through their unique self-renewal capacity and resistance to
apoptosis, making them critical targets for therapeutic
intervention (1). Studies have
identified specific surface markers and signaling pathways that
regulate CSC behavior, such as the Wnt, Notch and Hedgehog pathways
(1,3). These findings provide insights into
the mechanisms that underlie CSC-driven tumorigenesis and highlight
potential therapeutic strategies to target these cells.
Digestive tract tumors, including esophageal,
gastric, colorectal and pancreatic cancer, are among the most
common and aggressive malignancies worldwide (4). According to the latest global cancer
statistics, gastric cancer is the fifth most common cancer and the
third leading cause of cancer-related death, whereas colorectal
cancer is the third most common cancer globally (4). Esophageal cancer and pancreatic
cancer are also associated with high mortality rates (4). These tumors often exhibit high rates
of recurrence and metastasis, which contribute to poor patient
outcomes (5). Previous studies
have implicated CSCs in the pathogenesis and progression of
digestive tract tumors. For example, CSCs have been shown to drive
tumor initiation, promote metastasis and contribute to therapeutic
resistance in gastric and colorectal cancer (6,7).
The identification of CSC-specific markers and pathways has
provided new avenues for developing targeted therapies aimed at
improving patient survival and quality of life.
Research on CSCs in digestive tract tumors has
advanced significantly in recent years. Studies have identified
specific CSC markers, such as CD44, CD133 and leucine rich repeat
containing G protein-coupled receptor 5 (LGR5), which are
associated with tumor aggressiveness and poor prognosis (8,9).
Furthermore, high expression of high mobility group box 2 (HMGB2),
a non-histone chromatin-binding protein, has been linked to poor
survival outcomes in patients with gastric cancer and colorectal
cancer. A meta-analysis of multiple studies revealed that elevated
HMGB2 is associated with a shorter overall survival (OS) time in
patients with digestive cancer, highlighting its potential as a
prognostic biomarker and therapeutic target (10). Additionally, research has explored
the role of CSCs in promoting epithelial-mesenchymal transition
(EMT) and metastasis in hepatocellular carcinoma and pancreatic
ductal adenocarcinoma. These findings underscore the importance of
CSCs in driving tumor progression and therapeutic resistance in
digestive tract cancer (11,12).
The present review aims to comprehensively explore
the biology of CSCs in digestive tract tumors, focusing on their
role in tumorigenesis, progression and therapeutic resistance. The
current understanding of CSC-specific markers and signaling
pathways are critically evaluated, highlighting their clinical
relevance in predicting patient outcomes and guiding treatment
strategies. Furthermore, the emerging therapeutic approaches that
target CSCs, including small molecule inhibitors, monoclonal
antibodies and combination therapies are discussed. By integrating
findings from preclinical studies and clinical trials, the present
review seeks to provide insights into the potential of CSC-targeted
therapies to improve patient outcomes in digestive cancer. Future
research directions and challenges in developing effective
CSC-targeted therapies will also be addressed, emphasizing the need
for innovative strategies to overcome treatment resistance and
increase therapeutic efficacy.
The biology of CSCs is complex and multifaceted,
involving intricate interactions between genetic and epigenetic
factors, signaling pathways and the tumor microenvironment (TME)
(13). Understanding these
mechanisms is crucial for developing effective therapeutic
strategies targeting CSCs and improving patient outcomes in
digestive cancer.
CSCs are a distinct subpopulation of cancer cells
characterized by their ability to self-renew, differentiate into
various cell types and drive tumorigenesis. CSCs are hypothesized
to be responsible for tumor initiation, progression and resistance
to conventional therapies (1).
Normal stem cells in tissues (such as the gut lining or bone
marrow) are strictly controlled to replace damaged cells and
maintain organ function. By contrast, CSCs acquire genetic and
epigenetic abnormalities that disrupt this regulation, allowing
them to proliferate uncontrollably and spread to other organs
(metastasis) (2). Studies have
shown that CSCs can repopulate tumors even after therapy, making
them a critical target for therapeutic intervention (14,15). For example, in gastric cancer,
CSCs have been implicated in therapeutic resistance and tumor
relapse, highlighting their role in maintaining tumor growth and
heterogeneity (16).
The identification and characterization of CSCs rely
on specific surface markers and functional assays. Surface markers
are proteins on cell membranes that can act as 'barcodes' to
identify specific cell types. In CSCs, markers such as CD44, CD133
and aldehyde dehydrogenase 1 (ALDH1; an enzyme involved in
metabolism) are often upregulated and are correlated with
aggressive tumor behavior. For example, CD44+ CSCs in
colorectal cancer show enhanced ability to initiate tumors and
resist drugs (17,18). CD44, a transmembrane glycoprotein,
is a defining CSCs marker in multiple cancer types, including
breast, colorectal and head and neck malignancies. CD44+
CSCs exhibit enhanced tumor-initiating capacity and resistance to
conventional therapies (19).
CD133 (prominin-1) is another widely studied marker, implicated in
CSC tumorigenicity in glioblastoma, liver and pancreatic cancer.
CD133+ cells exhibit increased sphere-forming ability
and heightened tumorigenicity in xenograft models (20). ALDH1 is an intracellular enzyme
frequently used to identify CSCs in breast, lung and prostate
cancer. High ALDH1 activity is linked to enhanced self-renewal,
chemoresistance and tumor initiation (20). However, the variability in marker
expression across different cancer types underscores the need for
context-dependent analysis of CSC markers.
While CD44 and CD133 remain widely used CSC markers,
their expression varies significantly across digestive tumor
subtypes. For instance, CD44+ cells dominate in
colorectal cancer, whereas CD133+ populations are more
prevalent in pancreatic CSCs (21). Notably, Tian et al
(22) demonstrated heterogeneous
CD44/CD133 expression in esophageal squamous cell carcinoma (ESCC),
with only 40% of tumors showing co-expression, questioning their
universal applicability. Single-cell sequencing further revealed
that ALDH1+ gastric CSCs (GCSCs) comprise distinct
subclones with divergent Wnt and Notch dependencies (23), underscoring the need for
context-specific marker validation.
The maintenance of CSCs is regulated by complex
molecular pathways and influenced by both genetic and epigenetic
factors. Key signaling pathways include Wnt/β-catenin (24), Notch (25) and Hedgehog (26), which are critical for CSC
self-renewal and differentiation. For instance, aberrant activation
of the Wnt/β-catenin pathway leads to β-catenin accumulation and
nuclear translocation, activating genes associated with
proliferation, invasion and survival (27). This dysregulation is observed in
colorectal and breast cancer, where nuclear β-catenin expression is
correlated with increased tumor aggressiveness and recurrence
(28,29). Epigenetic factors, such as DNA
methylation and histone modification, also serve significant roles
in maintaining CSCs properties (30). Studies have shown that epigenetic
modifications can silence tumor suppressor genes and activate
oncogenes, contributing to CSC maintenance and tumorigenesis
(31,32). Additionally, genetic mutations and
chromosomal instability can drive the acquisition of stem cell-like
properties in cancer cells, further promoting tumor progression and
therapeutic resistance (33).
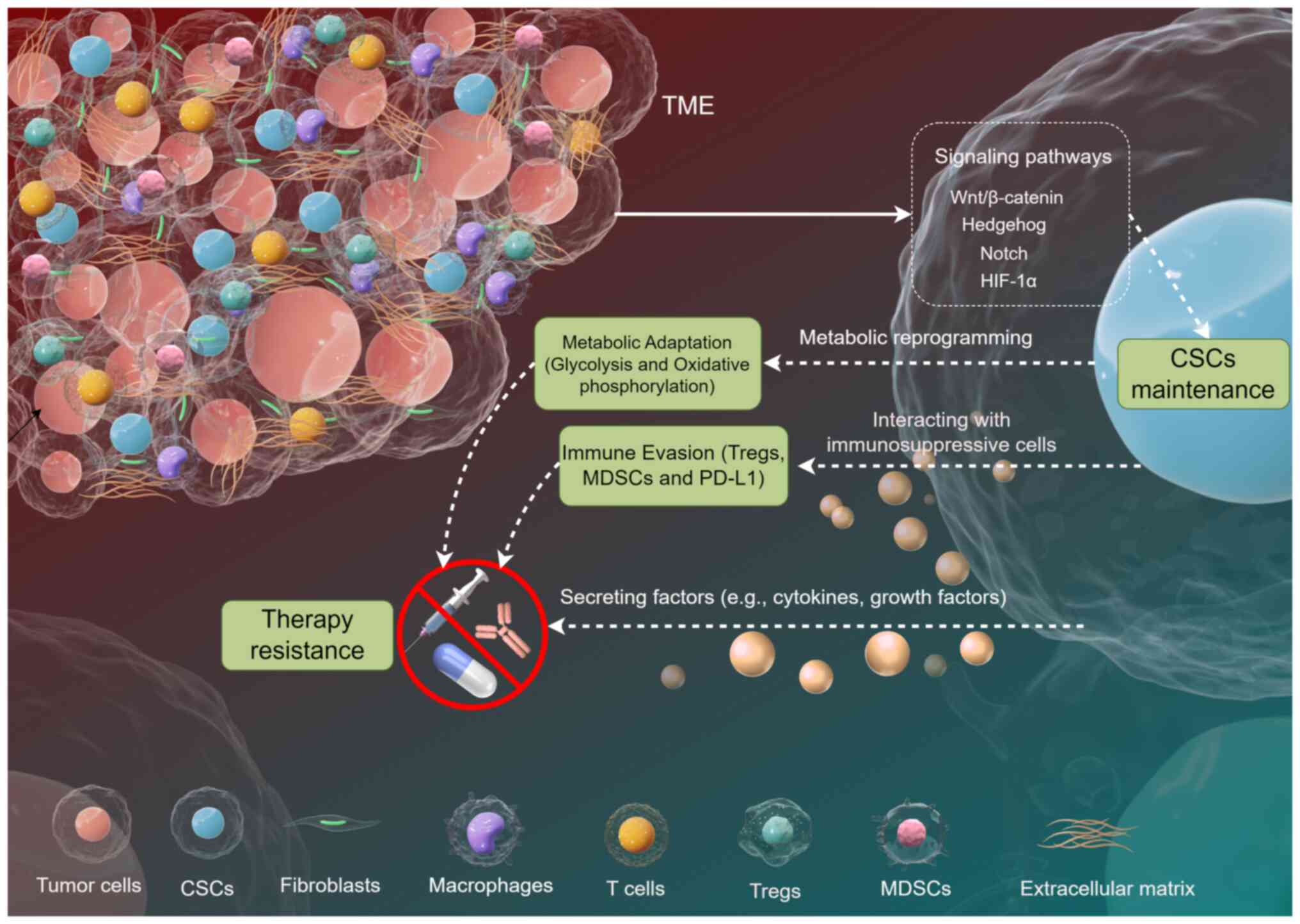
The interaction between CSCs and the TME is a
dynamic and complex process that significantly impacts tumor
progression, therapeutic resistance and patient outcomes (2). Understanding these interactions is
crucial for developing novel therapeutic strategies targeting both
CSCs and their microenvironment to improve treatment efficacy in
digestive cancer.
The TME is the 'ecosystem' surrounding cancer cells,
including both living cells (such as immune cells and fibroblasts)
and non-living elements [such as proteins and nutrients in the
extracellular matrix (ECM)]. This environment supports tumor growth
by providing nutrients, promoting blood vessel formation and
shielding cancer cells from the immune system (34). Cellular components include
cancer-associated fibroblasts (CAFs), immune cells [such as
macrophages, T cells and natural killer (NK) cells] and endothelial
cells. Non-cellular components consist of the ECM, growth factors,
cytokines and metabolites (35).
These components collectively influence tumor progression,
metastasis and therapeutic resistance (Fig. 1). For example, CAFs promote tumor
growth by secreting growth factors and remodeling the ECM, while
immune cells can either suppress or enhance tumor growth depending
on their polarization state (36). The ECM provides structural support
and facilitates cell migration and invasion, contributing to
metastasis (37).
Among the TME components, cytokines play a pivotal
role in shaping the behavior of CSCs. For instance, interleukin-6
(IL-6) and IL-8 have been shown to activate critical signaling
pathways such as STAT3 in CSCs, thereby promoting their
self-renewal and survival (38).
Matrix metalloproteinases (MMPs), which are enzymes that degrade
the ECM, also contribute to CSC maintenance by facilitating tumor
invasion and creating niches conducive to CSC proliferation
(39). The intricate interplay
between these TME components and CSCs highlights the importance of
considering the microenvironment in therapeutic strategies.
The TME plays a critical role in supporting the
survival and proliferation of CSCs. Hypoxia, a common feature of
the TME, enhances the properties of CSCs by activating
hypoxia-inducible factors (HIFs), which promote self-renewal and
resistance to apoptosis (25)
(Fig. 1). Studies have shown that
hypoxia increases the expression of CSCs markers such as CD44 and
ALDH1, driving tumor progression and therapeutic resistance
(25,40). Additionally, angiogenesis within
the TME provides nutrients and oxygen to CSCs, further promoting
their survival and proliferation. For instance, HMGB2, a
non-histone chromatin-binding protein, has been implicated in
promoting angiogenesis and enhancing the properties of CSCs in
digestive tract tumors. High HMGB2 expression is correlated with
shorter OS and disease-free survival (DFS), highlighting its role
in supporting CSC behavior (10).
CSCs employ various strategies to evade anti-tumor
immunity, often interacting with immunosuppressive cells such as
regulatory T cells (Tregs) and myeloid-derived suppressor cells
(MDSCs) (Fig. 1). Tregs suppress
anti-tumor immune responses by inhibiting cytotoxic T cells, while
MDSCs promote immune tolerance through the production of
immunosuppressive cytokines (42,43). CSCs can secrete factors that
attract and activate these immunosuppressive cells, creating a
favorable microenvironment for tumor growth (44). Furthermore, CSCs express immune
checkpoint molecules such as programmed death-ligand 1 (PD-L1),
which inhibit T cell activity and enhance immune evasion (45). For example, HMGB2 has been shown
to promote immune escape in non-small cell lung cancer by
upregulating PD-L1 expression, highlighting its role in immune
evasion mechanisms (46).
Moreover, CSCs can modulate the TME to reduce the
infiltration of cytotoxic T cells and NK cells. For instance, in
colorectal cancer, the fatty acid desaturase 1
(FADS1)/dihydroxydodecanoic acid (DDA) axis is activated under
hypoxic conditions in CSCs, impairing NK cell cytotoxicity
(47). This mechanism not only
facilitates immune evasion but also enhances the metastatic
potential of CSCs. Additionally, CSCs can exploit metabolic
pathways to alter the TME. Lactate, a byproduct of glycolysis, can
drive metastasis of normoxic colorectal CSCs (CCSCs) via peroxisome
proliferator-activated receptor γ coactivator 1-α
(PGC-1α)-dependent oxidative phosphorylation (48). This metabolic adaptation not only
supports the survival of CSCs but also creates a hostile
environment for immune cells.
CSCs exhibit notable metabolic flexibility, enabling
them to survive and thrive in the harsh TME. CSCs can switch
between glycolysis and oxidative phosphorylation depending on the
availability of nutrients and oxygen. For example, in hypoxic
conditions, CSCs upregulate glycolysis-related enzymes and
transporters to maintain energy production (25). This metabolic reprogramming is
often accompanied by the activation of specific signaling pathways,
such as the HIF-1α pathway, which further enhances the stemness and
survival of CSCs.
Furthermore, CSCs can utilize alternative metabolic
substrates to adapt to nutrient limitations. In colorectal cancer,
vitamin D has been shown to promote ferroptosis in CCSCs by
downregulating SLC7A11, a cystine transporter critical for redox
balance (49). This suggests that
CSCs can alter their metabolic pathways to resist oxidative stress
and maintain cellular homeostasis. Additionally, CSCs can exploit
the metabolic products of surrounding cells. For instance, in
gastric cancer, prostaglandin D2 (PGD2)/prostaglandin D2 receptor 2
(PTGDR2) signaling inhibits autophagy via autophagy-related protein
4B (ATG4B) ubiquitination, reducing CSC stemness (50). This highlights the intricate
interplay between metabolic adaptation and signaling pathways in
CSCs.
CSCs play a pivotal role in the pathogenesis and
progression of digestive tract tumors, driving tumorigenesis,
metastasis and therapeutic resistance. Understanding the biology of
CSCs and their molecular mechanisms is crucial for developing
effective therapeutic strategies targeting these cells, which could
significantly improve patient outcomes in digestive system
cancer.
Esophageal CSCs drive tumor initiation, progression
and therapeutic resistance through diverse molecular mechanisms
(Table I). This subsection
synthesizes key findings on CSC-related pathways in esophageal
cancer, critically evaluates their implications and highlights
unresolved controversies.
CSCs in ESCC exhibit a quiescent state, enabling
evasion of conventional therapies targeting rapidly dividing cells.
Chen et al (51)
demonstrated that ESCC CSCs downregulate DDR pathways, reducing
apoptosis under genotoxic stress. This quiescence is mediated by
suppressed checkpoint kinase 1/2 activation and impaired p53
signaling, allowing CSCs to accumulate mutations and survive
chemotherapy. However, this study relied on in vitro
sphere-forming assays and xenograft models, which may not fully
recapitulate the TME in human patients. Subsequent studies, such as
that by Zhao et al (52),
corroborated these findings but emphasized heterogeneity in DDR
pathways across CSC subpopulations, suggesting context-dependent
survival mechanisms.
Multiple signaling cascades converge to sustain CSC
self-renewal and chemoresistance. The Hippo/Yes-associated protein
1 (YAP1) pathway is a critical regulator in esophageal cancer. Song
et al (53) revealed that
YAP1 upregulates SRY-box transcription factor 9, enhancing CSC
properties such as tumorigenicity and spheroid formation.
Similarly, Xu et al (54)
identified a STAT3/miRNA (miR)-181b/cylindromatosis axis that
promotes CSC proliferation by modulating IL-6/STAT3 signaling.
While these studies highlight pathway-specific roles, conflicting
evidence exists regarding cross-talk between pathways. For
instance, Liu et al (55)
linked the HSP27/AKT/hexokinase 2 (HK2) axis to CSC metabolic
reprogramming, showing that AKT activation sustains stemness via
HK2-dependent glycolysis. This contrasts with the study by Kai
et al (56), in which
myosin heavy chain 9 was implicated in activating PI3K/AKT/mTOR to
drive CSC oncogenesis, suggesting overlapping yet distinct
metabolic dependencies.
Autocrine signaling mechanisms facilitate CSC
invasion and metastasis in esophageal cancer. Wang et al
(57) demonstrated that C-X-C
motif chemokine ligand 12/C-X-C motif chemokine receptor 4 (CXCR4)
axis activation in CSCs enhances MMP secretion, promoting ECM
degradation and metastatic spread. Conversely, Yue et al
(58) linked TGF-β1 to CSC
migration via Smad-dependent EMT activation. Despite mechanistic
clarity, these studies predominantly utilized monolayer cell
cultures, neglecting the contribution of stromal cells in the TME.
Recent work by Wei et al (59) addressed this gap, showing that
quiescin sulfhydryl oxidase 1 (QSOX1) in CSCs upregulates PD-L1 to
exclude CD8+ T cells, illustrating how cytokine networks
synergize with immune evasion.
Dysregulation of non-coding RNAs and epigenetic
modifiers underpins CSC plasticity in esophageal cancer. Guo et
al (60) reported that
miR-637 loss activates the Wiskott-Aldrich syndrome protein and
SCAR homolog/IL-8 pathway, augmenting CSC stemness and metastasis.
Similarly, Xun et al (61)
identified miR-191-3p as a suppressor of regulator of G-protein
signaling 1, which inhibits CXCR4/PI3K/AKT signaling. These
findings align with genome-wide methylation analyses by Yu et
al (62), which revealed
hypermethylation of tumor suppressor genes (such as Cadherin 1) in
CSCs. However, inconsistencies arise in biomarker specificity; for
example, Gupta et al (63)
found variable CD44/CD133 expression across ESCC subtypes,
questioning the universality of these markers.
CSCs employ multifaceted strategies to resist
treatment in esophageal cancer. Xu et al (64) showed that STAT3 silencing
sensitizes CSCs to the HSP90 inhibitor, SNX-2112, while Liu et
al (65) linked ferroptosis
resistance to HSP27-glutathione peroxidase 4 (GPX4) upregulation.
Notably, Wei et al (59)
uncovered a novel immune evasion mechanism where QSOX1 elevates
PD-L1, enabling CSCs to bypass cytotoxic T cell surveillance. These
studies underscore the need for combinatorial therapies targeting
both CSCs and immune cells, although clinical validation remains
limited.
GCSCs are central to tumor initiation, metastasis
and therapeutic resistance. The functional regulation of GCSCs
involves intricate signaling networks, epigenetic reprogramming and
interactions with the TME (Table
II). In this subsection, key mechanisms governing GCSC biology
are dissected, representative studies are critically evaluated and
therapeutic implications are discussed.
GCSCs exploit microenvironmental cues to evade
immune surveillance and foster metastasis. Yang et al
(74) revealed that HIF-1α
induces EMT in GCSCs via Snail upregulation, facilitating
dissemination under hypoxia. Sun et al (75) implicated HER2 in GCSC invasion,
where HER2 inhibition reduces tumorigenicity in patient-derived
xenografts. Additionally, Seeneevassen et al (76) identified leukemia inhibitory
factor as a Hippo pathway activator that suppresses GCSC
tumorigenicity, suggesting a dual role for cytokines in niche
regulation. Recent studies also highlight the influence of the
microbiome. PGD2/PTGDR2 signaling, downregulated in gastric cancer
tissues, restricts GCSC self-renewal by inhibiting STAT3
phosphorylation (50,77), aligning with findings from GCSC
models.
CCSCs are pivotal drivers of tumor initiation,
progression, chemoresistance and metastasis. The unique biological
properties and regulatory mechanisms of CCSCs have been extensively
studied, revealing complex interactions between intrinsic signaling
pathways, epigenetic modifications and the TME (Table III). In this subsection, the key
mechanisms underlying CCSC functionality, supported by
representative studies, are summarized and their implications for
therapeutic targeting are discussed.
The Wnt/β-catenin pathway is a central regulator of
CCSC self-renewal and chemoresistance. Chen et al (82) demonstrated that miR-199a/b
upregulation in ALDH1+ CCSCs activates Wnt/β-catenin
signaling, enhancing ATP-binding cassette subfamily G member 2
(ABCG2)-mediated drug efflux and cisplatin resistance. Similarly,
Li et al (83) identified
that lysine-specific demethylase 3 epigenetically activates
Wnt/β-catenin by removing repressive H3K9 methylation marks,
thereby promoting stemness and tumorigenicity. These findings align
with Hua et al (84), who
showed that Tribbles pseudokinase 3 stabilizes β-catenin/T-cell
factor 4 complexes, amplifying stemness and EMT in CCSCs. However,
discrepancies arise in the role of downstream effectors. While Yu
et al (85) implicated
Special AT-rich sequence-binding protein 2 as a Wnt-driven
transcriptional coactivator, Zhu et al (86) highlighted SOX2-mediated
β-catenin/Beclin1 crosstalk in chemoresistance, suggesting pathway
plasticity across CCSC subpopulations.
CCSCs evade chemotherapy through dynamic
post-translational modifications. Izumi et al (87) revealed that F-box and WD repeat
domain-containing 7 (FBXW7), an E3 ubiquitin ligase, is
downregulated in CCSCs, leading to c-Myc stabilization and enhanced
survival under 5-FU treatment. Conversely, Honma et al
(88) reported that FBXW7
upregulation degrades pro-survival proteins, sensitizing CCSCs to
oxaliplatin. This paradox may reflect context-dependent roles of
FBXW7 in different chemotherapeutic regimens. Epigenetically,
Mukohyama et al (89)
identified miR-221 as a driver of chemoresistance by targeting
Quaking homolog, KH domain RNA-binding protein (QKI), a tumor
suppressor that restrains CCSC proliferation. These studies
underscore the need for personalized strategies targeting
ubiquitination or miRNA networks.
CSCs significantly influence clinical outcomes in
digestive tract tumors by driving therapeutic resistance,
recurrence and metastasis. This section evaluates their prognostic
value, mechanisms of drug resistance and emerging therapeutic
strategies in clinical trials, integrating findings from key
studies to highlight translational implications.
CSC-associated markers serve as robust predictors of
patient survival and treatment response. In esophageal cancer, CD44
and CD133 expression is associated with resistance to neoadjuvant
chemotherapy and poor survival. Specifically, Agawa et al
(96) demonstrated that
CD44+/CD133+ CSCs in ESCC predict poor
pathological response to cisplatin/5-FU regimens, with a 3-year
survival rate of 28 vs. 72% for marker-negative patients.
Similarly, Claudin 4 (CLDN4)-high ESCC cells exhibit stem-like
properties and resistance to concurrent chemoradiotherapy, as shown
by Lin et al (97),
linking CLDN4 to reduced OS [hazard ratio (HR), 2.1; P=0.003].
Meta-analyses by Trevellin et al (98) further confirmed that CSC markers
(such as ALDH1 and CD44) are consistently associated with shorter
DFS across esophageal and gastric cancer.
CSCs evade therapy through intrinsic and extrinsic
mechanisms. In esophageal cancer, ABT-263 (a BCL-2 inhibitor)
synergizes with chemotherapy by depleting CSCs via apoptosis
induction, achieving a 60% reduction in tumor volume in xenografts
(104). Nanog, a stemness
marker, mediates resistance to cisplatin; iron chelators targeting
Nanog reduce chemoresistance by 50% in vitro (105).
In colorectal cancer, dual PI3K/mTOR inhibitors
induce the differentiation of CD133+ CSCs, reducing
tumorigenicity by 70% (109).
CD44+/CD133+ CSCs are paradoxically sensitive
to trifluridine (110),
suggesting metabolic vulnerabilities. However, sphingosine kinase
1/HIF-1 axis inhibition (111)
and aurora kinase A/YAP1) targeting (112) overcome microenvironment-driven
resistance, suppressing metastasis by 45-60%.
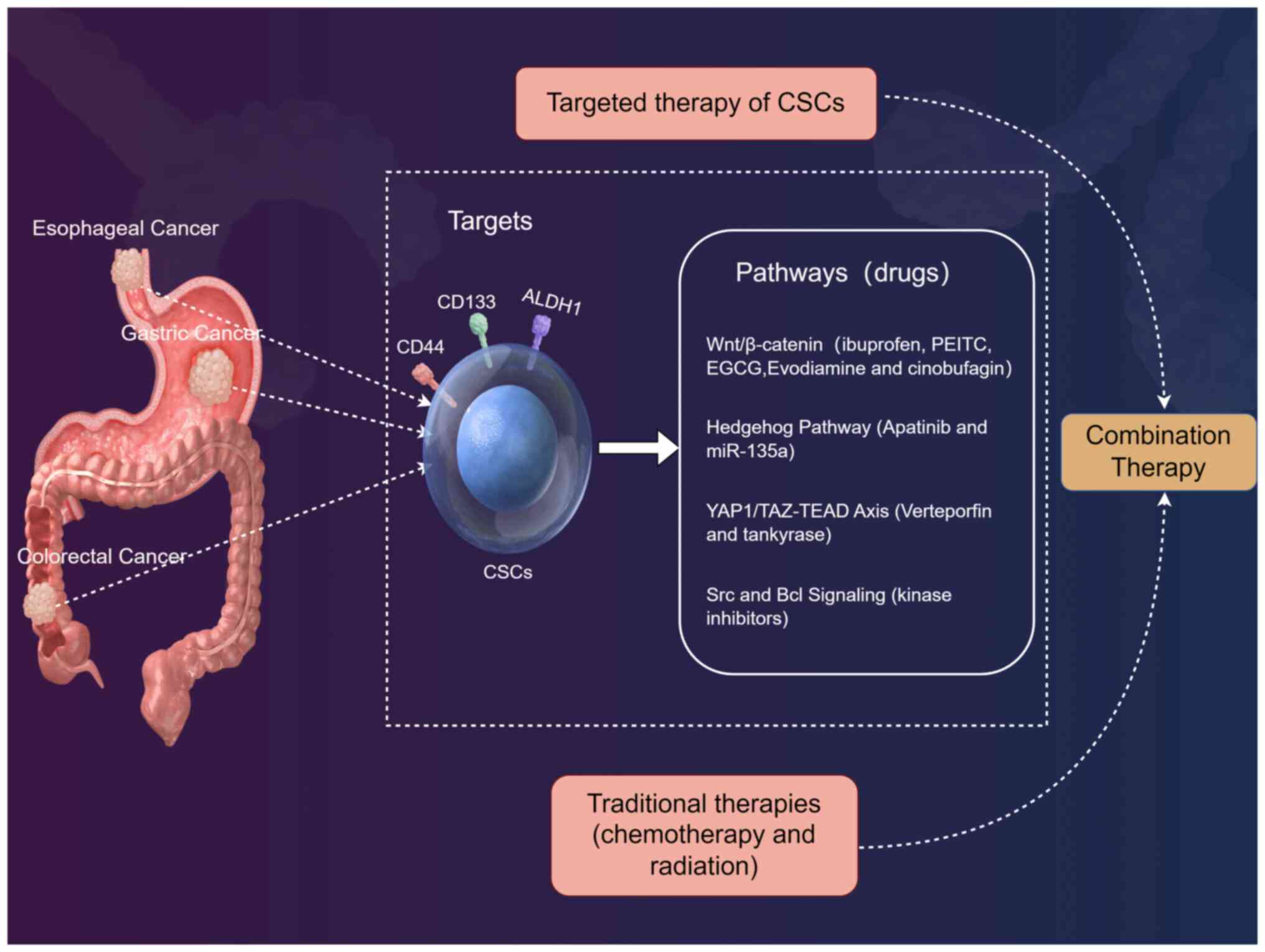
The development of therapies targeting CSCs in
digestive tract tumors has gained momentum, with strategies
focusing on disrupting CSC-specific pathways, enhancing drug
delivery and overcoming therapeutic resistance (Table IV). This section critically
evaluates current approaches, integrating findings from preclinical
and clinical studies, and discusses challenges and future
directions.
The Wnt/β-catenin pathway is normally active during
embryonic development to guide cell fate decisions. Multiple
Wnt/β-catenin pathway inhibitors have shown efficacy in gastric and
colorectal cancer. For instance, ibuprofen suppresses GCSCs by
inhibiting Wnt/β-catenin signaling, reducing proliferation and
tumorigenicity in vitro and in vivo (113) (Fig. 2). Similarly, phenethyl
isothiocyanate and epigallocatechin-3-gallate downregulate
Wnt/β-catenin in CCSCs, impairing sphere formation and
chemoresistance (114,115). However, variability exists in
downstream effects; for example, TET1/FOXO4-mediated Wnt inhibition
suppresses metastasis but fails to eliminate quiescent GCSCs
(116), highlighting the need
for combinatorial approaches. Furthermore, evodiamine and
cinobufagin inhibit GCSC self-renewal via the Wnt/β-catenin and
AKT/GSK-3β pathways, respectively (117,118). Notably, cinobufagin enhances
5-FU sensitivity by suppressing β-catenin nuclear translocation
(118), illustrating the promise
of phytochemicals in overcoming chemoresistance.
Apatinib, a tyrosine kinase inhibitor, targets
Sonic Hedgehog in GCSCs, reducing stemness markers (such as CD44
and ALDH1) and tumor growth (119) (Fig. 2). Conversely, miR-135a inhibits
Hedgehog signaling in esophageal CSCs by targeting Smoothened, but
its clinical application is limited by delivery challenges
(120). These studies underscore
the context-dependent role of this pathway, necessitating
biomarker-guided therapies.
Verteporfin, a YAP1/TAZ-TEAD inhibitor, suppresses
GCSC tumorigenicity by disrupting transcriptional activity, leading
to reduced spheroid formation and metastasis (121) (Fig. 2). This aligns with findings in
colorectal cancer, where tankyrase inhibitors downregulate
YAP1-associated c-KIT, impairing CCSC viability (122). However, YAP1 crosstalk with
other pathways (such as PI3K/AKT) may necessitate dual targeting to
prevent resistance.
Src kinase inhibition blocks GCSC proliferation and
EMT by suppressing STAT3 and AKT phosphorylation (123) (Fig. 2). Similarly, in CRC, MEK
inhibitors combined with CSC-targeting agents overcome resistance
by depleting ALDH1+ CCSCs (124). These findings emphasize the
potential of kinase inhibitors in disrupting CSC survival networks.
The pan-BCL-2 inhibitor, navitoclax (ABT-263), synergizes with
chemotherapy in gastroesophageal carcinoma, inducing apoptosis in
CD44+ CSCs and reducing tumor recurrence (125). However, heterogeneity in BCL-2
family expression across CSC subpopulations may limit efficacy, as
observed in colorectal cancer models (126).
Combining CSC-targeted agents with conventional
therapies improves outcomes by addressing bulk tumors and residual
CSCs (Fig. 2). For example,
salinomycin-loaded carbon nanotubes selectively kill GCSCs while
sparing normal cells, enhancing the efficacy of 5-FU (127). In colorectal cancer, polymeric
micelles targeting CD44v6 deliver niclosamide, synergizing with
oxaliplatin to reduce CCSC-driven metastasis (128). Similarly, mithramycin A
represses ABCG2 in CRCSCs, sensitizing them to chemotherapy
(129). In addition,
aptamer-mediated survivin knockdown in CCSCs enhances 5-FU-induced
apoptosis and reduces immune evasion (130). Furthermore, inhibition of
cholesterol synthesis in GCSCs attenuates NK cell evasion,
suggesting a role for metabolic-immune crosstalk in combinatorial
regimens (131).
CSCs serve pivotal roles in the initiation,
progression and therapeutic resistance of digestive tract tumors.
The heterogeneity and plasticity of these cells pose significant
challenges for effective treatment (132). Emerging therapeutic strategies
targeting CSC-specific pathways, immune modulation and combination
therapies offer promising avenues to overcome resistance and
enhance patient outcomes (133).
Future research should focus on integrating biomarker-driven
approaches and innovative technologies to advance precision
medicine in the field of digestive oncology.
Despite promising preclinical data, the clinical
translation of CSC-targeted therapies remains challenging. For
example, vismodegib, a Hedgehog inhibitor, failed to improve
survival in gastric cancer trials due to compensatory YAP1
activation in residual CSCs (134,135). Similarly, Wnt inhibitors such as
PRI-724 have shown limited efficacy against colorectal cancer, as
CAF-derived IL-6 reactivates β-catenin via STAT3 (136). These failures highlight the need
to target CSC-stroma crosstalk. Emerging strategies such as
microbiota modulation (including F. nucleatum eradication)
and metabolic-immune combinations (including vitamin D + anti-PD-1)
may overcome these hurdles by simultaneously disrupting CSC niche
support and immune evasion (137,138).
Identifying and targeting CSCs is technically
challenging due to their heterogeneity and plasticity. Universal
markers such as CD44 and CD133 show variable expression across
tumor subtypes, necessitating context-specific validation (139). Furthermore, CSC subpopulations
exhibit divergent responses to therapies. For instance, delta-like
protein 1 inhibition suppresses Wnt-driven CCSCs but spares
LGR5+ subsets (140),
underscoring the need for multitargeted strategies. Single-cell
sequencing and spatial transcriptomics may elucidate clonal
dynamics, guiding personalized therapies. Furthermore, the TME
shields CSCs via cytokine loops and stromal support. Blocking
proprotein convertases disrupts GCSC-TME crosstalk, reducing
invasiveness (141). Similarly,
ruthenium-xanthoxylin complexes target HSP90 in CRCSCs, thereby
overcoming stroma-mediated resistance (142). Advances in genomic and proteomic
technologies have enabled tailored interventions against CSC
heterogeneity in digestive tract tumors.
However, preclinical models have inherent
limitations. Although animal models provide mechanistic insights,
they often fail to recapitulate human tumor complexity and
microenvironmental interactions, leading to clinical discrepancies
(143). Additionally, tumor
heterogeneity and CSC plasticity complicate therapy development, as
CSCs can adapt to microenvironmental changes and therapeutic
pressures. The TME, which is crucial for CSC support, is difficult
to target without affecting normal tissue homeostasis (144). Furthermore, a lack of robust
biomarkers for patient stratification and treatment response
assessment hinders the development of effective CSC-targeted
therapies.
Nanomaterials and CRISPR-based approaches offer
precision in future treatment. For example, SPION-driven atranorin
induces ferroptosis in GCSCs by modulating Xc-/GPX4 (6,145). Additionally, low-dose vitamin C
promotes CCSC differentiation via β-catenin membrane retention, a
strategy that is compatible with immune checkpoint inhibitors
(146). Although preclinical
data are promising, clinical trials remain limited. The active
hexose correlated compound/epigallocatechin gallate combination
reduces LGR5+ CCSCs in early-phase studies (147); however, its scalability and
toxicity require further evaluation. Future research should
prioritize biomarkers (such as ALDH1 and CD44) to stratify patients
and optimize trial designs.
In addition, the application of artificial
intelligence (AI) in predicting the vulnerabilities of CSCs has
recently been demonstrated, providing new ideas and methods for the
development of targeted therapies against CSCs (148). For example, deep learning models
have been employed to analyze large-scale CSC genomic and
transcriptomic data, aiming to identify potential therapeutic
targets and drug candidates. These models can predict the drug
sensitivity and resistance of CSCs, thereby offering a theoretical
basis for drug repurposing (149). For example, studies have
utilized deep learning algorithms to analyze the gene expression
profiles of CSCs from various digestive tract tumors and to predict
their responses to different drugs (150,151). The results have shown that some
conventional drugs may have potential inhibitory effects on CSCs.
Further experimental verification is ongoing to explore the
feasibility of these drugs in clinical applications. This
integration of AI with CSC research not only increases the
efficiency of target discovery but also optimizes therapeutic
strategies, offering new avenues for the individualized treatment
of digestive tract tumors.
Finally, patient-derived organoids (PDOs) have
garnered significant attention as promising tools for personalized
therapy screening. PDOs are three-dimensional cellular models
cultured from patient tumor tissues (152). PDOs retain the histological
structure and genetic characteristics of the original tumor to some
extent, making them powerful tools for evaluating drug efficacy and
guiding clinical treatment. A previous study has shown that PDOs
can effectively mimic the TME and CSC niche of digestive tract
tumors (153). By screening
various drugs using PDOs, researchers can identify therapeutic
regimens that are effective against CSCs and the bulk tumor cells,
providing a basis for personalized treatment. For example,
researchers successfully established PDO models from patients with
gastric or pancreatic ductal adenocarcinoma, and drug screening
experiments revealed that certain drugs could specifically targeted
CSCs in the PDOs, inhibiting tumor growth and metastasis (154,155). These findings demonstrate the
potential of PDOs to help predict therapeutic responses and guide
clinical treatment. The application of PDOs in personalized therapy
screening offers a new approach for improving the treatment
outcomes of digestive tract tumors.
In conclusion, although significant progress has
been made in understanding the biology of CSCs and their role in
digestive tract tumors, several challenges remain in translating
this knowledge into clinical practice. Future research should focus
on addressing the limitations of current CSC markers, reconciling
discrepancies between preclinical and clinical findings and
developing strategies to overcome CSC plasticity and
microenvironment-driven resistance. By integrating innovative
technologies and biomarker-driven approaches, the field of
CSC-targeted therapies can be advanced and precision medicine in
the field of digestive oncology can be improved.
CSCs play a pivotal role in the initiation,
progression and therapeutic resistance of digestive tract tumors.
The heterogeneity and plasticity of CSCs pose significant
challenges to effective treatment. Emerging therapeutic strategies
targeting CSC-specific pathways, immune modulation and combination
therapies offer promising avenues to overcome resistance and
enhance patient outcomes. Future research should focus on
integrating biomarker-driven approaches and innovative technologies
to advance precision medicine in digestive oncology.
Not applicable.
XC and XG equally contributed to the study by
conducting in-depth literature reviews on CSCs in digestive tract
tumors from PubMed, analyzing data related to their biological
characteristics and functions in esophageal and gastric cancer as
well as drafting relevant sections. CZ focused on CSCs in
colorectal cancer, collecting and analyzing data from PubMed and
writing the corresponding content. LL supervised the entire
project, critically evaluated the data and drafts based on the
PubMed literature and refined the manuscript to meet the
requirements of the journal. Data authentication is not applicable.
All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Haddadin L and Sun X: Stem cells in
cancer: From mechanisms to therapeutic strategies. Cells.
14:5382025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Tanani M, Rabbani SA, Satyam SM,
Rangraze IR, Wali AF, El-Tanani Y and Aljabali AAA: Deciphering the
role of cancer stem cells: Drivers of tumor evolution, therapeutic
resistance, and precision medicine strategies. Cancers (Basel).
17:3822025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez RS, Raza A, Propst R, Adeyi O,
Bateman J, Sopha SC, Shaw J and Auerbach A: Recent advances in
digestive tract tumors: Updates from the 5th edition of the world
health organization 'blue book'. Arch Pathol Lab Med. 145:607–626.
2021. View Article : Google Scholar
|
|
6
|
Li K, Dan Z and Nie YQ: Gastric cancer
stem cells in gastric carcinogenesis, progression, prevention and
treatment. World J Gastroenterol. 20:5420–5426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ayob AZ and Ramasamy TS: Cancer stem cells
as key drivers of tumour progression. J Biomed Sci. 25:202018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jahangiri L: Cancer stem cell markers and
properties across gastrointestinal cancers. Curr. Tissue
Microenviron. Rep. 4:77–89. 2023. View Article : Google Scholar
|
|
9
|
Sarabia-Sánchez MA, Tinajero-Rodríguez JM,
Ortiz-Sánchez E and Alvarado-Ortiz E: Cancer stem cell markers:
Symphonic masters of chemoresistance and immune evasion. Life Sci.
355:1230152024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, He S, Wang Z, Xi H, Lu W and Lin X:
Predictive and clinicopathological importance of HMGB2 in various
carcinomas: A meta and bioinformatic approach. Sci Rep.
15:110032025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Meng WJ and Wang ZQ: Cancer stem
cells and the tumor microenvironment in gastric cancer. Front
Oncol. 11:8039742022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Steed A, Co M and Chen X: Cancer
stem cells, epithelial-mesenchymal transition, ATP and their roles
in drug resistance in cancer. Cancer Drug Resist. 4:684–709.
2021.PubMed/NCBI
|
|
13
|
Sinha S, Hembram KC and Chatterjee S:
Targeting signaling pathways in cancer stem cells: A potential
approach for developing novel anti-cancer therapeutics. Int Rev
Cell Mol Biol. 385:157–209. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Becerril-Rico J, Alvarado-Ortiz E,
Toledo-Guzmán ME, Pelayo R and Ortiz-Sánchez E: The cross talk
between gastric cancer stem cells and the immune microenvironment:
a tumor-promoting factor. Stem Cell Res Ther. 12:4982021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kapoor-Narula U and Lenka N: Cancer stem
cells and tumor heterogeneity: Deciphering the role in tumor
progression and metastasis. Cytokine. 157:1559682022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Otaegi-Ugartemendia M, Matheu A and
Carrasco-Garcia E: Impact of cancer stem cells on therapy
resistance in gastric cancer. Cancers (Basel). 14:14572022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhumal SN, Choudhari SK, Patankar S, Ghule
SS, Jadhav YB and Masne S: Cancer stem cell markers, CD44 and
ALDH1, for assessment of cancer risk in OPMDs and lymph node
metastasis in oral squamous cell carcinoma. Head Neck Pathol.
16:453–465. 2022. View Article : Google Scholar :
|
|
19
|
Hassn Mesrati M, Syafruddin SE, Mohtar MA
and Syahir A: CD44: A multifunctional mediator of cancer
progression. Biomolecules. 11:18502021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gopalan V Islam F and Lam AK: Surface
markers for the identification of cancer stem cells. Methods Mol
Biol. 1692:17–29. 2018. View Article : Google Scholar
|
|
21
|
Makohon-Moore A and Iacobuzio-Donahue CA:
Pancreatic cancer biology and genetics from an evolutionary
perspective. Nat Rev Cancer. 16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian S, Ma R, Liu Y, Chen F, Huang X, Yang
Q, Nian W and Fan Z: Clinicopathological significance of cancer
stem cell marker CD44/SOX2 in esophageal squamous cell carcinoma
(ESCC) patients and construction of a nomogram to predict overall
survival. Transl Cancer Res. 13:2971–2984. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang G, Yuan C, Zhang C, Yang F, Tan Y,
Chen D, Li H and Qian K: Single-cell sequencing reveals the immune
microenvironment associated with gastric cancer. Genes Dis.
12:1012182025. View Article : Google Scholar
|
|
24
|
Xue C, Chu Q, Shi Q, Zeng Y, Lu J and Li
L: Wnt signaling pathways in biology and disease: Mechanisms and
therapeutic advances. Signal Transduct Target Ther. 10:1062025.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang T, Jia Z, Li J, Cao H, Xu H, Cong L,
Ma D, Wang X and Liu J: Unraveling the triad of hypoxia, cancer
cell stemness, and drug resistance. J Hematol Oncol. 18:322025.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Wang Y, Xun X, Zhang C, Xiang X,
Cheng Q, Hu S, Li Z and Zhu J: Hedgehog signaling promotes
sorafenib resistance in hepatocellular carcinoma patient-derived
organoids. J Exp Clin Cancer Res. 39:222020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tufail M, Jiang CH and Li N: Wnt signaling
in cancer: From biomarkers to targeted therapies and clinical
translation. Mol Cancer. 24:1072025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lanauze CB, Sehgal P, Hayer K, Torres-Diz
M, Pippin JA, Grant SFA and Thomas-Tikhonenko A: Colorectal
Cancer-Associated Smad4 R361 Hotspot Mutations Boost Wnt/β-Catenin
Signaling through Enhanced Smad4-LEF1 Binding. Mol Cancer Res.
19:823–833. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Knowles E, Zardawi SJ, McNeil CM,
Millar EK, Crea P, Musgrove EA, Sutherland RL and O'Toole SA:
Cytoplasmic localization of beta-catenin is a marker of poor
outcome in breast cancer patients. Cancer Epidemiol Biomarkers
Prev. 19:301–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aine M, Nacer DF, Arbajian E, Veerla S,
Karlsson A, Häkkinen J, Johansson HJ, Rosengren F,
Vallon-Christersson J, Borg A and Staaf J: The DNA methylation
landscape of primary triple-negative breast cancer. Nat Commun.
16:30412025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z and Zhang Y: Transcriptional
regulation of cancer stem cell: regulatory factors elucidation and
cancer treatment strategies. J Exp Clin Cancer Res. 43:992024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Darwiche N: Epigenetic mechanisms and the
hallmarks of cancer: An intimate affair. Am J Cancer Res.
10:1954–1978. 2020.PubMed/NCBI
|
|
33
|
Liu B, Peng Z, Zhang H, Zhang N, Liu Z,
Xia Z, Huang S, Luo P and Cheng Q: Regulation of cellular
senescence in tumor progression and therapeutic targeting:
mechanisms and pathways. Mol Cancer. 24:1062025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia H, Chen X, Zhang L and Chen M: Cancer
associated fibroblasts in cancer development and therapy. J Hematol
Oncol. 18:362025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi Z, Hu C, Li Q and Sun C:
Cancer-associated fibroblasts as the 'Architect' of the lung cancer
immune microenvironment: Multidimensional roles and synergistic
regulation with radiotherapy. Int J Mol Sci. 26:32342025.
View Article : Google Scholar
|
|
37
|
Yu S, Wang S, Wang X and Xu X: The axis of
tumor-associated macrophages, extracellular matrix proteins, and
cancer-associated fibroblasts in oncogenesis. Cancer Cell Int.
24:3352024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang B, Lang X and Li X: The role of
IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol.
12:10231772022. View Article : Google Scholar :
|
|
39
|
Li YR, Fang Y, Lyu Z, Zhu Y and Yang L:
Exploring the dynamic interplay between cancer stem cells and the
tumor microenvironment: Implications for novel therapeutic
strategies. J Transl Med. 21:6862023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rabinovich I, Sebastião APM, Lima RS,
Urban CA, Junior ES, Anselmi KF, Elifio-Esposito S, De Noronha L
and Moreno-Amaral AN: Cancer stem cell markers ALDH1 and
CD44+/CD24− phenotype and their prognosis impact in invasive ductal
carcinoma. Eur J Histochem. 62:29432018.
|
|
41
|
Wang D, Li Y, Ge H, Ghadban T, Reeh M and
Güngör C: The extracellular matrix: A key accomplice of cancer stem
cell migration, metastasis formation, and drug resistance in PDAC.
Cancers (Basel). 14:39982022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeutic targets. J Hematol Oncol. 15:612022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin B, Cai Y, Chen L, Li Z and Li X:
Immunosuppressive MDSC and Treg signatures predict prognosis and
therapeutic response in glioma. Int Immunopharmacol.
141:1129222024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin Y, Song Y, Zhang Y, Li X, Kan L and
Han S: New insights on anti-tumor immunity of CD8+ T cells: Cancer
stem cells, tumor immune microenvironment and immunotherapy. J
Transl Med. 23:3412025. View Article : Google Scholar :
|
|
45
|
Galassi C, Musella M, Manduca N, Maccafeo
E and Sistigu A: The immune privilege of cancer stem cells: A key
to understanding tumor immune escape and therapy failure. Cells.
10:23612021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo H, Hu B, Gu XR, Chen J, Fan XQ, Zhang
W, Wang RT, He XD, Guo W, Dai N, et al: The miR-23a/27a/24 − 2
cluster drives immune evasion and resistance to PD-1/PD-L1 blockade
in non-small cell lung cancer. Mol Cancer. 23:2852024. View Article : Google Scholar
|
|
47
|
Geng S, Zhu L, Wang Y, Liu Q, Yu C, Shi S
and Yu S: Co-Colorectal cancer stem cells employ the FADS1/DDA axis
to evade NK cell-mediated immunosuppression after co-cultured with
NK cells under hypoxia. Int Immunopharmacol. 143(Pt 3): 1135352024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu S, Zhao H, Hu Y, Yan C, Mi Y, Li X,
Tao D and Qin J: Lactate promotes metastasis of normoxic colorectal
cancer stem cells through PGC-1α-mediated oxidative
phosphorylation. Cell Death Dis. 13:6512022. View Article : Google Scholar
|
|
49
|
Guo S, Zhao W, Zhang W, Li S, Teng G and
Liu L: Vitamin D promotes ferroptosis in colorectal cancer stem
cells via SLC7A11 downregulation. Oxid Med Cell Longev.
2023:47721342023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Q, Tian H, Ge K and Wang F, Gao P,
Chen A, Wang L, Zhao Y, Lian C and Wang F: PGD2/PTGDR2 signaling
pathway affects the self-renewal capacity of gastric cancer stem
cells by regulating ATG4B ubiquitination. Front Oncol.
14:14960502024. View Article : Google Scholar
|
|
51
|
Chen Y, Li D, Wang D, Liu X, Yin N, Song
Y, Lu SH, Ju Z and Zhan Q: Quiescence and attenuated DNA damage
response promote survival of esophageal cancer stem cells. J Cell
Biochem. 113:3643–3652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Y, Bao Q, Schwarz B, Zhao L,
Mysliwietz J, Ellwart J, Renner A, Hirner H, Niess H, Camaj P, et
al: Stem cell-like side populations in esophageal cancer: A source
of chemotherapy resistance and metastases. Stem Cells Dev.
23:180–192. 2014. View Article : Google Scholar
|
|
53
|
Song S, Ajani JA, Honjo S, Maru DM, Chen
Q, Scott AW, Heallen TR, Xiao L, Hofstetter WL, Weston B, et al:
Hippo coactivator YAP1 upregulates SOX9 and endows esophageal
cancer cells with stem-like properties. Cancer Res. 74:4170–4182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu DD, Zhou PJ, Wang Y, Zhang L, Fu WY,
Ruan BB, Xu HP, Hu CZ, Tian L, Qin JH, et al: Reciprocal activation
between STAT3 and miR-181b regulates the proliferation of
esophageal cancer stem-like cells via the CYLD pathway. Mol Cancer.
15:402016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu CC, Chou KT, Hsu JW, Lin JH, Hsu TW,
Yen DH, Hung SC and Hsu HS: High metabolic rate and stem cell
characteristics of esophageal cancer stem-like cells depend on the
Hsp27-AKT-HK2 pathway. Int J Cancer. 145:2144–2156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kai JD, Cheng LH, Li BF, Kang K, Xiong F,
Fu JC and Wang S: MYH9 is a novel cancer stem cell marker and
prognostic indicator in esophageal cancer that promotes oncogenesis
through the PI3K/AKT/mTOR axis. Cell Biol Int. 46:2085–2094. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang X, Cao Y, Zhang S, Chen Z, Fan L,
Shen X, Zhou S and Chen D: Stem cell autocrine CXCL12/CXCR4
stimulates invasion and metastasis of esophageal cancer.
Oncotarget. 8:36149–36160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yue D, Zhang Z, Li J, Chen X, Ping Y, Liu
S, Shi X, Li L, Wang L, Huang L, et al: Transforming growth
factor-beta1 promotes the migration and invasion of sphere-forming
stem-like cell subpopulations in esophageal cancer. Exp Cell Res.
336:141–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wei JR, Zhang B, Zhang Y, Chen WM, Zhang
XP, Zeng TT, Li Y, Zhu YH, Guan XY and Li L: QSOX1 facilitates
dormant esophageal cancer stem cells to evade immune elimination
via PD-L1 upregulation and CD8 T cell exclusion. Proc Natl Acad Sci
USA. 121:e24075061212024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo M, Lian J, Liu Y, Dong B, He Q, Zhao
Q, Zhang H, Qi Y, Zhang Y and Huang L: Loss of miR-637 promotes
cancer cell stemness via WASH/IL-8 pathway and serves as a novel
prognostic marker in esophageal squamous cell carcinoma. Biomark
Res. 10:772022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xun J, Ma Y, Wang B, Jiang X, Liu B, Gao
R, Zhai Q, Cheng R, Wu X, Wu Y and Zhang Q: RGS1 targeted by
miR-191-3p inhibited the stemness properties of esophageal cancer
cells by suppressing CXCR4/PI3K/AKT signaling. Acta Histochem.
126:1521902024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu X, Teng Y, Jiang X, Yuan H and Jiang W:
Genome-Wide DNA methylation pattern of cancer stem cells in
esophageal cancer. Technol Cancer Res Treat.
19:15330338209837932020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gupta P, Rizvi SZ, Lal N, Gupta V,
Srivastav AN and Musa O: Expression of CD44 and CD133 stem cell
markers in squamous cell carcinoma of esophagus. Indian J Pathol
Microbiol. 64:472–478. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu DD, Chen SH, Zhou PJ, Wang Y, Zhao ZD,
Wang X, Huang HQ, Xue X, Liu QY, Wang YF and Zhang R: Suppression
of Esophageal Cancer Stem-like Cells by SNX-2112 Is Enhanced by
STAT3 Silencing. Front Pharmacol. 11:5323952020. View Article : Google Scholar :
|
|
65
|
Liu CC, Li HH, Lin JH, Chiang MC, Hsu TW,
Li AF, Yen DH, Hsu HS and Hung SC: Esophageal Cancer Stem-like
Cells Resist Ferroptosis-Induced Cell Death by Active Hsp27-GPX4
Pathway. Biomolecules. 12:482021. View Article : Google Scholar
|
|
66
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar
|
|
67
|
Xu XF, Gao F, Wang JJ, Long C, Chen X, Tao
L, Yang L, Ding L and Ji Y: BMX-ARHGAP fusion protein maintains the
tumorigenicity of gastric cancer stem cells by activating the
JAK/STAT3 signaling pathway. Cancer Cell Int. 19:1332019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y
and Fan D: MiR-19b/20a/92a regulates the self-renewal and
proliferation of gastric cancer stem cells. J Cell Sci. 126(Pt 18):
4220–4229. 2013.PubMed/NCBI
|
|
69
|
Han ME, Baek SJ, Kim SY, Kang CD and Oh
SO: ATOH1 can regulate the tumorigenicity of gastric cancer cells
by inducing the differentiation of cancer stem cells. PLoS One.
10:e01260852015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shen C, Wang J, Xu Z, Zhang L, Gu W and
Zhou X: ONECUT2 which is targeted by hsa-miR-15a-5p enhances
stemness maintenance of gastric cancer stem cells. Exp Biol Med
(Maywood). 246:2645–2659. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li LQ, Pan D, Zhang SWY, Xie D, Zheng XL
and Chen H: Autophagy regulates chemoresistance of gastric cancer
stem cells via the Notch signaling pathway. Eur Rev Med Pharmacol
Sci. 22:3402–3407. 2018.PubMed/NCBI
|
|
72
|
Xin L, Li SH, Liu C, Zeng F, Cao JQ, Zhou
LQ, Zhou Q and Yuan YW: Methionine represses the autophagy of
gastric cancer stem cells via promoting the methylation and
phosphorylation of RAB37. Cell Cycle. 19:2644–2652. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Togano S, Yashiro M, Masuda G, Sugimoto A,
Miki Y, Yamamoto Y, Sera T, Kushiyama S, Nishimura S, Kuroda K, et
al: Gastric cancer stem cells survive in stress environments via
their autophagy system. Sci Rep. 11:206642021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian
F, Shi Y, Li PA, Liu CY and Yu PW: HIF-1α induces the
epithelial-mesenchymal transition in gastric cancer stem cells
through the Snail pathway. Oncotarget. 8:9535–9545. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun LF, Yang K, Wang YG, Liu YX, Hou PX,
Lu ZH, Chen XL, Zhang WH, Zhou ZG, Mo XM and Hu JK: The Role of
HER2 in self-renewal, invasion, and tumorigenicity of gastric
cancer stem cells. Front Oncol. 10:16082020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Seeneevassen L, Giraud J, Molina-Castro S,
Sifré E, Tiffon C, Beauvoit C, Staedel C, Mégraud F, Lehours P,
Martin OCB, et al: Leukaemia inhibitory factor (LIF) inhibits
cancer stem cells tumorigenic properties through hippo kinases
activation in gastric cancer. Cancers (Basel). 12:20112020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Q and Wang F, Huang Y, Gao P, Wang
N, Tian H, Chen A, Li Y and Wang F: PGD2/PTGDR2 signal affects the
viability, invasion, apoptosis, and stemness of gastric cancer stem
cells and prevents the progression of gastric cancer. Comb Chem
High Throughput Screen. 27:933–946. 2024. View Article : Google Scholar
|
|
78
|
Wang X, Zhang F, Yang J, Huang X, Chao X,
Ayidu A and Abudureyimu A: The chemotherapeutic effect of
docetaxel, cisplatin and fluorouracil regimen on gastric cancer
stem cells. J Nanosci Nanotechnol. 17:983–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang H, Wang M, He Y, Deng T, Liu R, Wang
W, Zhu K, Bai M, Ning T, Yang H, et al: Chemotoxicity-induced
exosomal lncFERO regulates ferroptosis and stemness in gastric
cancer stem cells. Cell Death Dis. 12:11162021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mao X, Wang L, Chen Z, Huang H, Chen J, Su
J, Li Z, Shen G, Ren Y, Li Z, et al: SCD1 promotes the stemness of
gastric cancer stem cells by inhibiting ferroptosis through the
SQLE/cholesterol/mTOR signalling pathway. Int J Biol Macromol.
275(Pt 2): 1336982024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ni T, Chu Z, Tao L, Zhao Y, Lv M, Zhu M,
Luo Y, Sunagawa M, Wang H and Liu Y: Celastrus orbiculatus extract
suppresses gastric cancer stem cells through the TGF-β/Smad
signaling pathway. J Nat Med. 78:100–113. 2024. View Article : Google Scholar
|
|
82
|
Chen B, Zhang D, Kuai J, Cheng M, Fang X
and Li G: Upregulation of miR-199a/b contributes to cisplatin
resistance via Wnt/β-catenin-ABCG2 signaling pathway in ALDHA1(+)
colorectal cancer stem cells. Tumour Biol. 39:10104283177151552017.
View Article : Google Scholar
|
|
83
|
Li J, Yu B, Deng P, Cheng Y, Yu Y, Kevork
K, Ramadoss S, Ding X, Li X and Wang CY: KDM3 epigenetically
controls tumorigenic potentials of human colorectal cancer stem
cells through Wnt/β-catenin signalling. Nat Commun. 8:151462017.
View Article : Google Scholar
|
|
84
|
Hua F, Shang S, Yang YM, Zhang HZ, Xu TL,
Yu JJ, Zhou DD, Cui B, Li K, Lv XX, et al: TRIB3 interacts With
β-Catenin and TCF4 to increase stem cell features of colorectal
cancer stem cells and tumorigenesis. Gastroenterology.
156:708–721.e15. 2019. View Article : Google Scholar
|
|
85
|
Yu W, Ma Y, Shankar S and Srivastava RK:
SATB2/β-catenin/TCF-LEF pathway induces cellular transformation by
generating cancer stem cells in colorectal cancer. Sci Rep.
7:109392017. View Article : Google Scholar
|
|
86
|
Zhu Y, Huang S, Chen S, Chen J, Wang Z,
Wang Y and Zheng H: SOX2 promotes chemoresistance, cancer stem
cells properties, and epithelial-mesenchymal transition by
β-catenin and Beclin1/autophagy signaling in colorectal cancer.
Cell Death Dis. 12:4492021. View Article : Google Scholar
|
|
87
|
Izumi D, Ishimoto T, Miyake K, Eto T,
Arima K, Kiyozumi Y, Uchihara T, Kurashige J, Iwatsuki M, Baba Y,
et al: Colorectal cancer stem cells acquire chemoresistance through
the upregulation of F-Box/WD repeat-containing protein 7 and the
consequent degradation of c-Myc. Stem Cells. 35:2027–2036. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Honma S, Hisamori S, Nishiuchi A, Itatani
Y, Obama K, Shimono Y and Sakai Y: F-Box/WD repeat
domain-containing 7 induces chemotherapy resistance in colorectal
cancer stem cells. Cancers (Basel). 11:6352019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mukohyama J, Isobe T, Hu Q, Hayashi T,
Watanabe T, Maeda M, Yanagi H, Qian X, Yamashita K, Minami H, et
al: miR-221 Targets QKI to Enhance the tumorigenic capacity of
human colorectal cancer stem cells. Cancer Res. 79:5151–5158. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing
D, Huang S, Cai X and Chen Y: Cancer associated fibroblasts-derived
exosomes contribute to radioresistance through promoting colorectal
cancer stem cells phenotype. Exp Cell Res. 391:1119562020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Montalbán-Hernández K, Cantero-Cid R,
Casalvilla-Dueñas JC, Avendaño-Ortiz J, Marín E, Lozano-Rodríguez
R, Ter rón-A rcos V, Vica r io-Bravo M, Ma rcano C,
Saavedra-Ambrosy J, et al: Colorectal cancer stem cells fuse with
monocytes to form tumour hybrid cells with the ability to migrate
and evade the immune system. Cancers (Basel). 14:34452022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cavallucci V, Palucci I, Fidaleo M,
Mercuri A, Masi L, Emoli V, Bianchetti G, Fiori ME, Bachrach G,
Scaldaferri F, et al: Proinflammatory and cancer-promoting
pathobiont fusobacterium nucleatum directly targets colorectal
cancer stem cells. Biomolecules. 12:12562022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tamura S, Isobe T, Ariyama H, Nakano M,
Kikushige Y, Takaishi S, Kusaba H, Takenaka K, Ueki T, Nakamura M,
et al: E-cadherin regulates proliferation of colorectal cancer stem
cells through NANOG. Oncol Rep. 40:693–703. 2018.PubMed/NCBI
|
|
94
|
Zou W, Zhang Y, Bai G, Zhuang J, Wei L,
Wang Z, Sun M and Wang J: siRNA-induced CD44 knockdown suppresses
the proliferation and invasion of colorectal cancer stem cells
through inhibiting epithelial-mesenchymal transition. J Cell Mol
Med. 26:1969–1978. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vishnubalaji R, Manikandan M, Fahad M,
Hamam R, Alfayez M, Kassem M, Aldahmash A and Alajez NM: Molecular
profiling of ALDH1(+) colorectal cancer stem cells reveals
preferential activation of MAPK, FAK, and oxidative stress
pro-survival signalling pathways. Oncotarget. 9:13551–13564. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Agawa K, Yamashita K, Nakagawa A, Yamada
K, Watanabe A, Mukohyama J, Saito M, Fujita M, Takiguchi G, Urakawa
N, et al: Simple cancer stem cell markers predict neoadjuvant
chemotherapy resistance of esophageal squamous cell carcinoma.
Anticancer Res. 41:4117–4126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lin CH, Li HY, Liu YP, Kuo PF, Wang WC,
Lin FC, Chang WL, Sheu BS, Wang YC, Hung WC, et al: High-CLDN4 ESCC
cells harbor stem-like properties and indicate for poor concurrent
chemoradiation therapy response in esophageal squamous cell
carcinoma. Ther Adv Med Oncol. 11:17588359198753242019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Trevellin E, Pirozzolo G, Fassan M and
Vettor R: Prognostic value of stem cell markers in esophageal and
esophagogastric junction cancer: A meta-analysis. J Cancer.
11:4240–4249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nishikawa S, Konno M, Hamabe A, Hasegawa
S, Kano Y, Ohta K, Fukusumi T, Sakai D, Kudo T, Haraguchi N, et al:
Aldehyde dehydrogenase high gastric cancer stem cells are resistant
to chemotherapy. Int J Oncol. 42:1437–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gong DY, Chen X, Yang TL, Wang Y, Guo Y,
Zeng JH and Chen SZ: Upregulation of ECT2 is associated with
transcriptional program of cancer stem cells and predicts poor
clinical outcome in gastric cancer. Oncol Lett.
20:542020.PubMed/NCBI
|
|
101
|
Becerril-Rico J, Grandvallet-Contreras J,
Ruíz-León MP, Dorantes-Cano S, Ramírez-Vidal L, Tinajero-Rodríguez
JM and Ortiz-Sánchez E: Circulating gastric cancer stem cells as
blood screening and prognosis factor in gastric cancer. Stem Cells
Int. 2024:99991552024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Catalano V, Dentice M, Ambrosio R, Luongo
C, Carollo R, Benfante A, Todaro M, Stassi G and Salvatore D:
Activated thyroid hormone promotes differentiation and
chemotherapeutic sensitization of colorectal cancer stem cells by
regulating Wnt and BMP4 signaling. Cancer Res. 76:1237–1244. 2016.
View Article : Google Scholar
|
|
103
|
Prieur A, Cappellini M, Habif G, Lefranc
MP, Mazard T, Morency E, Pascussi JM, Flacelière M, Cahuzac N, Vire
B, et al: Targeting the Wnt pathway and cancer stem cells with
anti-progastrin humanized antibodies as a potential treatment for
K-RAS-mutated colorectal cancer. Clin Cancer Res. 23:5267–5280.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen Q, Song S, Wei S, Liu B, Honjo S,
Scott A, Jin J, Ma L, Zhu H, Skinner HD, et al: ABT-263 induces
apoptosis and synergizes with chemotherapy by targeting stemness
pathways in esophageal cancer. Oncotarget. 6:25883–25896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Narusaka T, Ohara T, Noma K, Nishiwaki N,
Katsura Y, Kato T, Sato H, Tomono Y, Kikuchi S, Tazawa H, et al:
Nanog is a promising chemoresistant stemness marker and therapeutic
target by iron chelators for esophageal cancer. Int J Cancer.
149:347–357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu
PF and Cheng XD: 5-Fluorouracil chemotherapy of gastric cancer
generates residual cells with properties of cancer stem cells. Int
J Biol Sci. 11:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu C, Wang JL, Wu DZ, Yuan YW and Xin L:
Methionine restriction enhances the chemotherapeutic sensitivity of
colorectal cancer stem cells by miR-320d/c-Myc axis. Mol Cell
Biochem. 477:2001–2013. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yu M, Fei B and Chu S: Targeting HNRNPA2B1
to overcome chemotherapy resistance in gastric cancer stem cells:
Mechanisms and therapeutic potential. J Biol Chem. 301:1082342025.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim MJ, Koo JE, Han GY, Kim B, Lee YS, Ahn
C and Kim CW: Dual-Blocking of PI3K and mTOR improves
chemotherapeutic effects on SW620 human colorectal cancer stem
cells by inducing differentiation. J Korean Med Sci. 31:360–370.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tsunekuni K, Konno M, Haraguchi N, Koseki
J, Asai A, Matsuoka K, Kobunai T, Takechi T, Doki Y, Mori M and
Ishii H: CD44/CD133-positive colorectal cancer stem cells are
sensitive to trifluridine exposure. Sci Rep. 9:148612019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Khoei SG, Sadeghi H and Dermani FK:
Targeting the SPHK1/HIF1 PATHWAY TO INHIBIT colorectal cancer stem
cells niche. J Gastrointest Cancer. 51:716–717. 2020. View Article : Google Scholar
|
|
112
|
Rio-Vilariño A, Cenigaonandia-Campillo A,
García-Bautista A, Mateos-Gómez PA, Schlaepfer MI, Del
Puerto-Nevado L, Aguilera O, García-García L, Galeano C, de Miguel
I, et al: Inhibition of the AURKA/YAP1 axis is a promising
therapeutic option for overcoming cetuximab resistance in
colorectal cancer stem cells. Br J Cancer. 130:1402–1413. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Akrami H, Moradi B, Borzabadi Farahani D
and Mehdizadeh K: Ibuprofen reduces cell proliferation through
inhibiting Wnt/β catenin signaling pathway in gastric cancer stem
cells. Cell Biol Int. 42:949–958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen Y, Li Y, Wang XQ, Meng Y, Zhang Q,
Zhu JY, Chen JQ, Cao WS, Wang XQ, Xie CF, et al: Phenethyl
isothiocyanate inhibits colorectal cancer stem cells by suppressing
Wnt/β-catenin pathway. Phytother Res. 32:2447–2455. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(-)-epigallocatechin-3-gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9:5722017.
View Article : Google Scholar
|
|
116
|
Qi J, Cui D, Wu QN, Zhao Q, Chen ZH, Li L,
Birchmeier W, Yu Y and Tao R: Targeting Wnt/β-catenin signaling by
TET1/FOXO4 inhibits metastatic spreading and self-renewal of cancer
stem cells in gastric cancer. Cancers (Basel). 14:32322022.
View Article : Google Scholar
|
|
117
|
Wen Z, Feng S, Wei L, Wang Z, Hong D and
Wang Q: Evodiamine, a novel inhibitor of the Wnt pathway, inhibits
the self-renewal of gastric cancer stem cells. Int J Mol Med.
36:1657–1663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sun J, Zhang S and Wang M, Cheng H, Wang
Y, He S, Zuo Q, Wang N, Li Q and Wang M: Cinobufacini enhances the
therapeutic response of 5-Fluorouracil against gastric cancer by
targeting cancer stem cells via AKT/GSK-3β/β-catenin signaling
axis. Transl Oncol. 47:1020542024. View Article : Google Scholar
|
|
119
|
Cao W, Li Y, Sun H, Yang C, Zhu J, Xie C,
Li X, Wu J, Geng S, Wang L, et al: Apatinib suppresses gastric
cancer stem cells properties by inhibiting the sonic hedgehog
pathway. Front Cell Dev Biol. 9:6798062021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang C, Zheng X, Ye K, Sun Y, Lu Y, Fan Q
and Ge H: miR-135a inhibits the invasion and migration of
esophageal cancer stem cells through the hedgehog signaling pathway
by targeting Smo. Mol Ther Nucleic Acids. 19:841–852. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Giraud J, Molina-Castro S, Seeneevassen L,
Sifré E, Izotte J, Tiffon C, Staedel C, Boeuf H, Fernandez S,
Barthelemy P, et al: Verteporfin targeting YAP1/TAZ-TEAD
transcriptional activity inhibits the tumorigenic properties of
gastric cancer stem cells. Int J Cancer. 146:2255–2267. 2020.
View Article : Google Scholar
|
|
122
|
Jang MK, Mashima T and Seimiya H:
Tankyrase inhibitors target colorectal cancer stem cells via
AXIN-dependent downregulation of c-KIT tyrosine kinase. Mol Cancer
Ther. 19:765–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hu CT, Lin CF, Shih HM, You RI, Wu WS and
Chen TC: Blockade of Src signaling prevented stemness gene
expression and proliferation of patient-derived gastric cancer stem
cells. Tzu Chi Med J. 37:65–71. 2024. View Article : Google Scholar
|
|
124
|
Lamichhane A, Shahi Thakuri P, Singh S,
Rafsanjani Nejad P, Heiss J, Luker GD and Tavana H: Therapeutic
targeting of cancer stem cells prevents resistance of colorectal
cancer cells to MEK inhibition. ACS Pharmacol Transl Sci.
5:724–734. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Song S, Chen Q, Li Y, Lei G, Scott A, Huo
L, Li CY, Estrella JS, Correa A, Pizzi MP, et al: Targeting cancer
stem cells with a pan-BCL-2 inhibitor in preclinical and clinical
settings in patients with gastroesophageal carcinoma. Gut.
70:2238–2248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Park SR, Kim SR, Hong IS and Lee HY: A
novel therapeutic approach for colorectal cancer stem cells:
Blocking the PI3K/Akt signaling axis with caffeic acid. Front Cell
Dev Biol. 8:5859872020. View Article : Google Scholar
|
|
127
|
Yao HJ, Zhang YG, Sun L and Liu Y: The
effect of hyaluronic acid functionalized carbon nanotubes loaded
with salinomycin on gastric cancer stem cells. Biomaterials.
35:9208–9223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Andrade F, Rafael D, Vilar-Hernández M,
Montero S, Martínez-Trucharte F, Seras-Franzoso J, Díaz-Riascos ZV,
Boullosa A, García-Aranda N, Cámara-Sánchez P, et al: Polymeric
micelles targeted against CD44v6 receptor increase niclosamide
efficacy against colorectal cancer stem cells and reduce
circulating tumor cells in vivo. J Control Release. 331:198–212.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Quarni W, Dutta R, Green R, Katiri S,
Patel B, Mohapatra SS and Mohapatra S: Mithramycin a inhibits
colorectal cancer growth by targeting cancer stem cells. Sci Rep.
9:152022019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
AlShamaileh H, Wang T, Xiang D, Yin W,
Tran PH, Barrero RA, Zhang PZ, Li Y, Kong L, Liu K, et al:
Aptamer-mediated survivin RNAi enables 5-fluorouracil to eliminate
colorectal cancer stem cells. Sci Rep. 7:58982017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhu L and Wang H: Cholesterol-regulated
cellular stiffness may enhance evasion of NK cell-mediated
cytotoxicity in gastric cancer stem cells. FEBS Open Bio.
14:855–866. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chen W, Dong J, Haiech J, Kilhoffer MC and
Zeniou M: Cancer stem cell quiescence and plasticity as major
challenges in cancer therapy. Stem Cells Int. 2016:17409362016.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Huang T, Song X, Xu D, Tiek D, Goenka A,
Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer
initiation, progression, and therapy resistance. Theranostics.
10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kim R, Ji JH, Kim JH, Hong JY, Lim HY,
Kang WK, Lee J and Kim ST: Safety and anti-tumor effects of
vismodegib in patients with refractory advanced gastric cancer: A
single-arm, phase-II trial. J Cancer. 13:1097–1102. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Rao X, Zhang C, Luo H, Zhang J, Zhuang Z,
Liang Z and Wu X: Targeting gastric cancer stem cells to enhance
treatment response. Cells. 11:28282022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhong B, Cheng B, Huang X, Xiao Q, Niu Z,
Chen YF, Yu Q, Wang W and Wu XJ: Colorectal cancer-associated
fibroblasts promote metastasis by up-regulating LRG1 through
stromal IL-6/STAT3 signaling. Cell Death Dis. 13:162021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Luo J, Chen H, Ma F, Xiao C, Sun B, Liu Y,
Tang H, Yang Y, Liu W and Luo Z: Vitamin D metabolism pathway
polymorphisms are associated with efficacy and safety in patients
under anti-PD-1 inhibitor therapy. Front Immunol. 13:9374762022.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar
|
|
139
|
Mohan A, Raj Rajan R, Mohan G, Kollenchery
Puthenveettil P and Maliekal TT: Markers and reporters to reveal
the hierarchy in heterogeneous cancer stem cells. Front Cell Dev
Biol. 9:6688512021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li Y, Lu Y, Wu M, Wang H, Gong Y and Gu Y:
Neogambogic acid suppresses characteristics and growth of
colorectal cancer stem cells by inhibition of DLK1 and
Wnt/β-catenin pathway. Eur J Pharmacol. 929:1751122022. View Article : Google Scholar
|
|
141
|
Zaafour A, Seeneevassen L, Nguyen TL,
Genevois C, Nicolas N, Sifré E, Giese A, Porcheron C, Descarpentrie
J, Dubus P, et al: Inhibition of proprotein convertases activity
results in repressed stemness and invasiveness of cancer stem cells
in gastric cancer. Gastric Cancer. 27:292–307. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Santos LS, Silva VR, de Castro MVL, Dias
RB, Valverde LF, Rocha CAG, Soares MBP, Quadros CA, Dos Santos ER,
Oliveira RMM, et al: New ruthenium-xanthoxylin complex eliminates
colorectal cancer stem cells by targeting the heat shock protein 90
chaperone. Cell Death Dis. 14:8322023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Soufizadeh P, Mansouri V and Ahmadbeigi N:
A review of animal models utilized in preclinical studies of
approved gene therapy products: trends and insights. Lab Anim Res.
40:172024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Johnson JI, Decker S, Zaharevitz D,
Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M,
Arbuck S, Hollingshead M and Sausville EA: Relationships between
drug activity in NCI preclinical in vitro and in vivo models and
early clinical trials. Br J Cancer. 84:1424–1431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ni Z, Nie X, Zhang H, Wang L, Geng Z, Du
X, Qian H, Liu W and Liu T: Atranorin driven by nano materials
SPION lead to ferroptosis of gastric cancer stem cells by weakening
the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis
and its expression. Int J Med Sci. 19:1680–1694. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Shanavas S, Sen U, Banerjee R, Shenoy PS
and Bose B: Effective targeting of colorectal cancer stem cells by
inducing differentiation mediated by low-dose vitamin C via
β-catenin retention in the cell membrane. J Cell Biochem.
126:e306862025. View Article : Google Scholar
|
|
147
|
Paganelli F, Chiarini F, Palmieri A,
Martinelli M, Sena P, Bertacchini J, Roncucci L, Cappellini A,
Martelli AM, Bonucci M, et al: The Combination of AHCC and ETAS
decreases migration of colorectal cancer cells, and reduces the
expression of LGR5 and Notch1 genes in cancer stem cells: A novel
potential approach for integrative medicine. Pharmaceuticals
(Basel). 14:13252021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mao Y, Shangguan D, Huang Q, Xiao L, Cao
D, Zhou H and Wang YK: Emerging artificial intelligence-driven
precision therapies in tumor drug resistance: Recent advances,
opportunities, and challenges. Mol Cancer. 24:1232025. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang Z, Wang ZX, Chen YX, Wu HX, Yin L,
Zhao Q, Luo HY, Zeng ZL, Qiu MZ and Xu RH: Integrated analysis of
single-cell and bulk RNA sequencing data reveals a pan-cancer
stemness signature predicting immunotherapy response. Genome Med.
14:452022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Xia X, Zhu C, Zhong F and Liu L: TransCDR:
A deep learning model for enhancing the generalizability of drug
activity prediction through transfer learning and multimodal data
fusion. BMC Biol. 22:2272024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhao X, Singhal A, Park S, Kong J,
Bachelder R and Ideker T: Cancer mutations converge on a collection
of protein assemblies to predict resistance to replication stress.
Cancer Discov. 14:508–523. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Varinelli L, Illescas O, Lorenc EJ,
Battistessa D, Di Bella M, Zanutto S and Gariboldi M: Organoids
technology in cancer research: from basic applications to advanced
ex vivo models. Front Cell Dev Biol. 13:15693372025. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Beshiri ML, Tice CM, Tran C, Nguyen HM,
Sowalsky AG, Agarwal S, Jansson KH, Yang Q, McGowen KM, Yin J, et
al: A PDX/Organoid biobank of advanced prostate cancers captures
genomic and phenotypic heterogeneity for disease modeling and
therapeutic screening. Clin Cancer Res. 24:4332–4345. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhu Z, Shen J, Ho PC, Hu Y, Ma Z and Wang
L: Transforming cancer treatment: Integrating patient-derived
organoids and CRISPR screening for precision medicine. Front
Pharmacol. 16:15631982025. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li P, Huang M, Li M, Li G, Ma Y, Zhao Y,
Wang X, Zhang Y and Shi C: Combining molecular characteristics and
therapeutic analysis of PDOs predict clinical responses and guide
PDAC personalized treatment. J Exp Clin Cancer Res. 44:722025.
View Article : Google Scholar : PubMed/NCBI
|