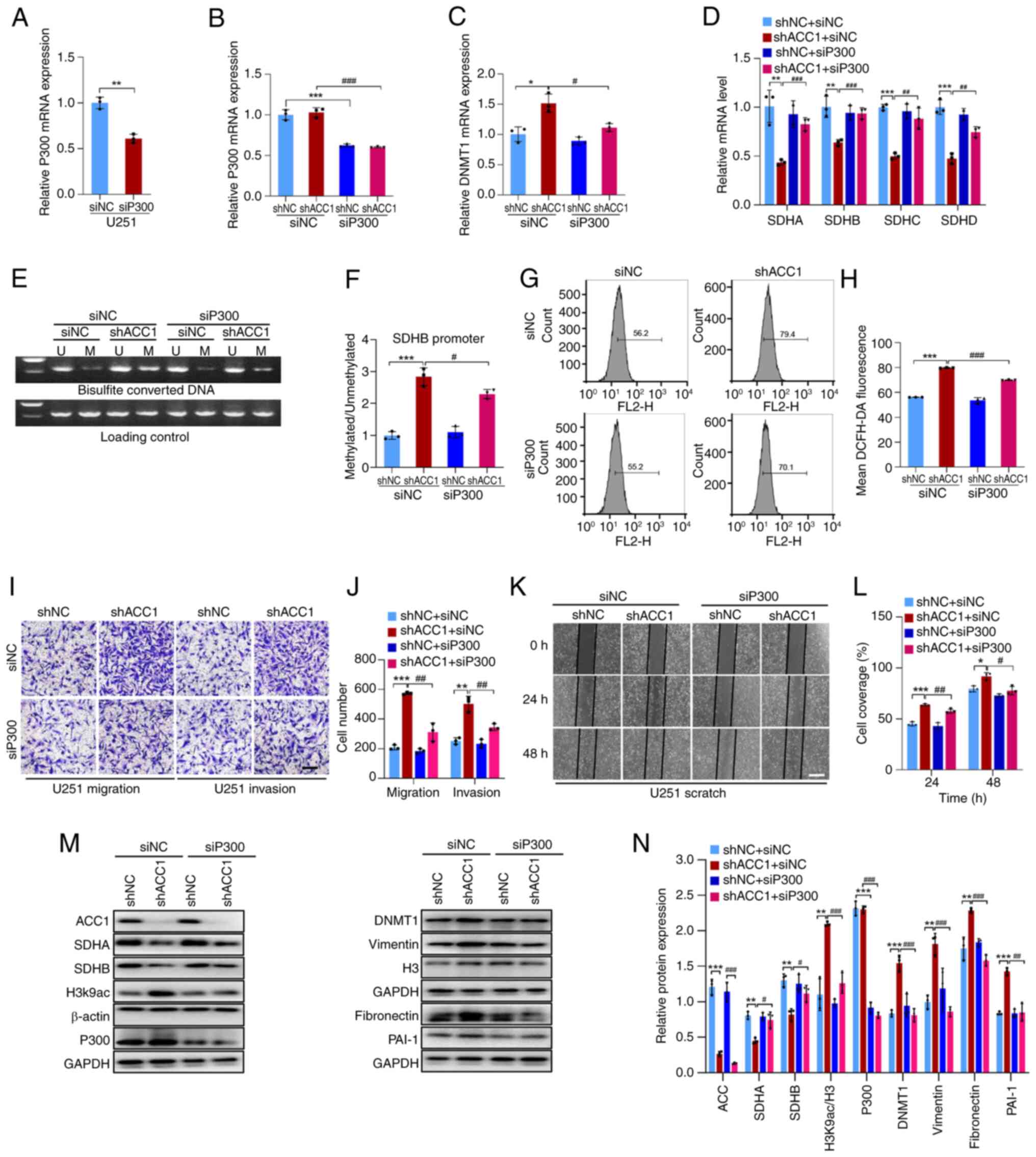
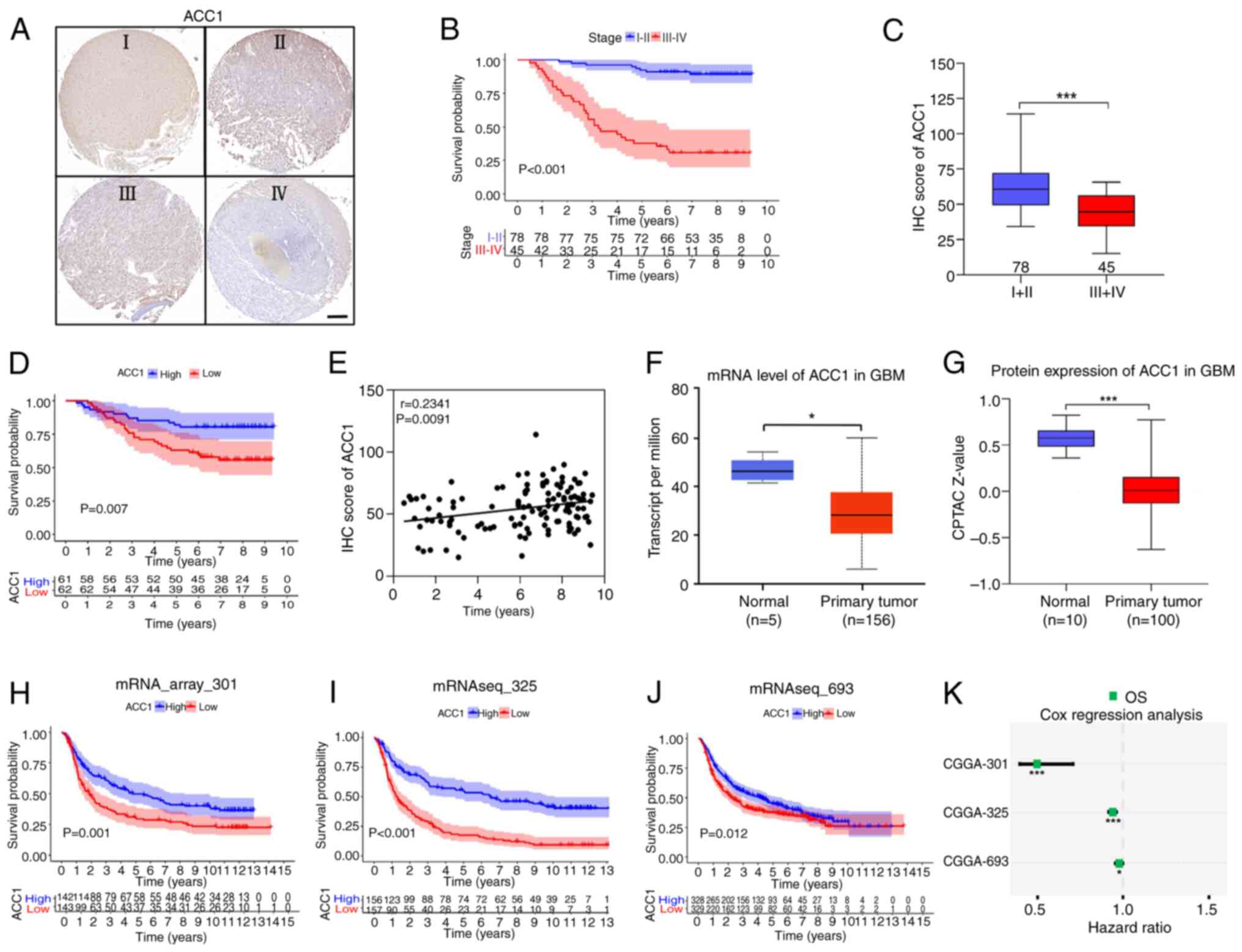
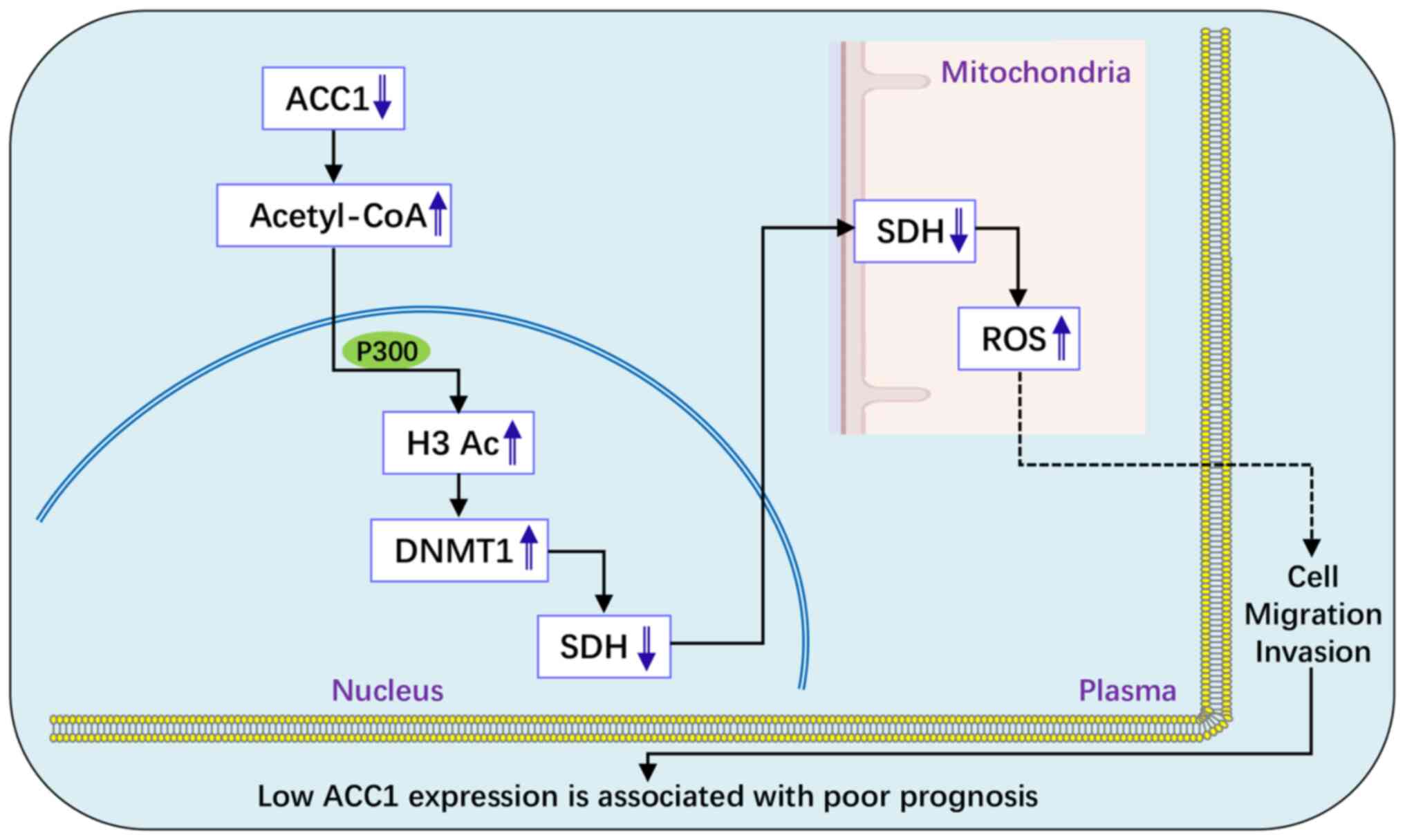
|
1
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumors in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delgado-López PD and Corrales-García EM:
Survival in glioblastoma: A review on the impact of treatment
modalities. Clin Transl Oncol. 18:1062–1071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khasraw M and Lassman AB: Advances in the
treatment of malignant gliomas. Curr Oncol Rep. 12:26–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucke-Wold B, Rangwala BS, Shafique MA,
Siddiq MA, Mustafa MS, Danish F, Nasrullah RMU, Zainab N and Haseeb
A: Focus on current and emerging treatment options for glioma: A
comprehensive review. World J Clin Oncol. 15:482–495. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duzan A, Reinken D, McGomery TL, Ferencz
NM, Plummer JM and Basti mM: Endocannabinoids are potential
inhibitors of glioblastoma multiforme proliferation. J Integr Med.
21:120–129. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunkeler M, Hagmann A, Stuttfeld E, Chami
M, Guri Y, Stahlberg H and Maier T: Structural basis for regulation
of human acetyl-CoA carboxylase. Nature. 558:470–474. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rios Garcia M, Steinbauer B, Srivastava K,
Singhal M, Mattijssen F, Maida A, Christian S, Hess-Stumpp H,
Augustin HG, Müller-Decker K, et al: Acetyl-CoA carboxylase
1-dependent protein acetylation controls breast cancer metastasis
and recurrence. Cell Metab. 26:842–855.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye B, Yin L, Wang Q and Xu C: ACC1 is
overexpressed in liver cancers and contributes to the proliferation
of human hepatoma Hep G2 cells and the rat liver cell line BRL 3A.
Mol Med Rep. 19:3431–3440. 2019.PubMed/NCBI
|
|
9
|
Garn H, Krause H, Enzmann V and Drössler
K: An improved MTT assay using the electron-coupling agent
menadione. J Immunol Methods. 168:253–256. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Owens KM, Aykin-Burns N, Dayal D, Coleman
MC, Domann FE and Spitz DR: Genomic instability induced by mutant
succinate dehydrogenase subunit D (SDHD) is mediated by O2(-•) and
H2O2. Free Radic Biol Med. 52:160–166. 2012. View Article : Google Scholar
|
|
11
|
Turchini J and Gill AJ: Morphologic clues
to succinate dehydrogenase (SDH) deficiency in pheochromocytomas
and paragangliomas. Am J Surg Pathol. 44:422–424. 2020. View Article : Google Scholar
|
|
12
|
Neppala P, Banerjee S, Fanta PT, Yerba M,
Porras KA, Burgoyne AM and Sicklick JK: Current management of
succinate dehydrogenase-deficient gastrointestinal stromal tumors.
Cancer Metastasis Rev. 38:525–535. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rani V, Deep G, Singh RK, Palle K and
Yadav UC: Oxidative stress and metabolic disorders: Pathogenesis
and therapeutic strategies. Life Sci. 148:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang H, Li J, Qu K, Wan Y, Liu S, Zheng
W, Zhang Z and Liu C: CRIF1 overexpression facilitates tumor growth
and metastasis through inducing ROS/NFκB pathway in hepatocellular
carcinoma. Cell Death Dis. 11:3322020. View Article : Google Scholar
|
|
15
|
Checa J and Aran JM: Reactive oxygen
species: Drivers of physiological and pathological processes. J
Inflamm Res. 13:1057–1073. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarmiento-Salinas FL, Perez-Gonzalez A,
Acosta-Casique A, Ix-Ballote A, Diaz A, Treviño S, Rosas-Murrieta
NH, Millán-Perez-Peña L and Maycotte P: Reactive oxygen species:
Role in carcinogenesis, cancer cell signaling and tumor
progression. Life Sci. 284:1199422021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Wang L, Wang C, Wu Y, Chen D and
Lee TH: Potential implications of hydrogen peroxide in the
pathogenesis and therapeutic strategies of gliomas. Arch Pharm Res.
43:187–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleinman HK and Martin GR: Matrigel
basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Sharpe MA, Ismail N and Baskin DS:
Metabolic sculpting of the mitochondria, cell signaling and the
cancer phenotype. Transl Cancer Res. 6:S182–S188. 2017. View Article : Google Scholar
|
|
21
|
Zhang W and Lang R: Succinate metabolism:
A promising therapeutic target for inflammation,
ischemia/reperfusion injury and cancer. Front Cell Dev Biol.
11:12669732023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki mM and Bird A: DNA methylation
landscapes: Provocative insights from epigenomics. Nat Rev Genet.
9:465–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bestor T, Laudano A, Mattaliano R and
Ingram V: Cloning and sequencing of a cDNA encoding DNA
methyltransferase of mouse cells. The carboxyl-terminal domain of
the mammalian enzymes is related to bacterial restriction
methyltransferases. J Mol Biol. 203:971–983. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashapkin VV, Kutueva LI and Vanyushin BF:
Dnmt2 is the most evolutionary conserved and enigmatic cytosine DNA
methyltransferase in eukaryotes. Genetika. 52:269–282. 2016.In
Russian. PubMed/NCBI
|
|
26
|
Liao HF, Tai KY, Chen WS, Cheng LC, Ho HN
and Lin SP: Functions of DNA methyltransferase 3-like in germ cells
and beyond. Biol Cell. 104:571–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robertson KD, Uzvolgyi E, Liang G,
Talmadge C, Sumegi J, Gonzales FA and Jones PA: The human DNA
methyltransferases (DNMTs) 1, 3a and 3b: Coordinate mRNA expression
in normal tissues and overexpression in tumors. Nucleic Acids Res.
27:2291–2298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Singh K, Shing V, Gupta A, Arenberg
BC, Huffstutler RD, Lee DY and Sack MN: Mitochondrial fatty acid
oxidation regulates monocytic type I interferon signaling via
histone acetylation. Sci Adv. 11:eadq93012025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eberlé D, Hegarty B, Bossard P, Ferré P
and Foufelle F: SREBP transcription factors: Master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F,
Zhang Y, Wu F, Chai R, Wang Z, Zhang C, et al: Chinese glioma
genome atlas (CGGA): A comprehensive resource with functional
genomic data from Chinese glioma patients. Genomics Proteomics
Bioinformatics. 19:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghandi M, Huang FW, Jané-Valbuena J,
Kryukov GV, Lo CC, McDonald ER III, Barretina J, Gelfand ET,
Bielski CM, Li H, et al: Next-generation characterization of the
cancer cell line encyclopedia. Nature. 569:503–508. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vitale AM, D'Amico G, Santonocito R,
Spinnato G, Di Marco M, Scalia F, Campanella C, Tringali G, Giusti
I, Dolo V, et al: An overview of glioblastoma multiforme in vitro
experimental models. J Biol Res. 6:1982024.
|
|
34
|
Hong X, Chedid K and Kalkanis SN:
Glioblastoma cell line-derived spheres in serumcontaining medium
versus serum-free medium: A comparison of cancer stem cell
properties. Int J Oncol. 41:1693–1700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernández-Vega AM, Del Moral-Morales A,
Zamora-Sánchez CJ, Piña-Medina AG, González-Arenas A and
Camacho-Arroyo I: Estradiol induces epithelial to mesenchymal
transition of human glioblastoma cells. Cells. 9:19302020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan S, Lu Y, Yang J, Chen G, Kim S, Feng
L, Ogasawara M, Hammoudi N, Lu W, Zhang H, et al: Metabolic
activation of mitochondria in glioma stem cells promotes cancer
development through a reactive oxygen species-mediated mechanism.
Stem Cell Res Ther. 6:1982015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu L, Wang F, Xu J and Chen Z: PTPN2
induced by inflammatory response and oxidative stress contributed
to glioma progression. J Cell Biochem. 120:19044–19051. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT
and Chen YC: Contribution of reactive oxygen species to
migration/invasion of human glioblastoma cells U87 via
ERK-dependent COX-2/PGE2 activation. Neurobiol Dis.
37:118–129. 2010. View Article : Google Scholar
|
|
39
|
Recillas-Targa F: Cancer epigenetics: An
overview. Arch Med Res. 53:732–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan Y, Peng X, Wang Y, Li B and Zhao G:
Comprehensive analysis of HDAC family identifies HDAC1 as a
prognostic and immune infiltration indicator and HDAC1-related
signature for prognosis in glioma. Front Mol Biosci. 8:7200202021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan HH and Porter AG: p21(WAF1) negatively
regulates DNMT1 expression in mammalian cells. Biochem Biophys Res
Commun. 382:171–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng L, Yuan Z, Ling H, Fukasawa K,
Robertson K, Olashaw N, Koomen J, Chen J, Lane WS and Seto E: SIRT1
deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters
its activities. Mol Cell Biol. 31:4720–4734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harada T, Ohguchi H, Grondin Y, Kikuchi S,
Sagawa M, Tai YT, Mazitschek R, Hideshima T and Anderson KC: HDAC3
regulates DNMT1 expression in multiple myeloma: Therapeutic
implications. Leukemia. 31:2670–2677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang
S, Kao HY, Xu Y, Willis J, Markowitz SD, et al: DNMT1 stability is
regulated by proteins coordinating deubiquitination and
acetylation-driven ubiquitination. Sci Signal. 3:ra802010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kishikawa S, Ugai H, Murata T and Yokoyama
KK: Roles of histone acetylation in the Dnmt1 gene expression.
Nucleic Acids Res. Suppl:209–210. 2002. View Article : Google Scholar
|
|
46
|
Li Z, Wang P, Cui W, Yong H, Wang D, Zhao
T, Wang W, Shi M, Zheng J and Bai J: Tumour-associated macrophages
enhance breast cancer malignancy via inducing ZEB1-mediated DNMT1
transcriptional activation. Cell Biosci. 12:1762022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mateska I, Witt A, Hagag E, Sinha A,
Yilmaz C, Thanou E, Sun N, Kolliniati O, Patschin M, Abdelmegeed H,
et al: Succinate mediates inflammation-induced adrenocortical
dysfunction. Elife. 12:e830642023. View Article : Google Scholar : PubMed/NCBI
|