Prostate cancer (PCa) is among the most prevalent
malignancies in males globally and has emerged as a notable public
health concern (1). The
pathophysiology of PCa is intricate and early-stage symptoms often
go undetected; however, as the condition progresses, it may
markedly affect the prognosis of patients and treatment efficacy
(2,3). Androgen pathway inhibitors and
chemotherapeutic medicines act as first-line therapeutic options
for PCa, while prostate-specific antigen testing, multi-parametric
magnetic resonance imaging and positron emission
tomography-computed tomography imaging function as first-line
diagnostic techniques, considerably enhancing the survival rates
and prognosis of patients. Despite advancements in medical
technology and the growing complexity of diagnostic and therapeutic
approaches for PCa, the treatment and prognosis remain poor due to
delayed diagnosis, postoperative recurrence, complications,
complexities in treatment and resistance (4-6).
For example, the effectiveness of non-selective immune checkpoint
inhibitor therapy in patients remains ambiguous and its application
in the early stages of treatment-resistant clones may promote the
emergence of highly cross-resistant clones, thereby diminishing the
clinical efficacy of subsequent androgen pathway inhibitor therapy
(7). Thus, further investigations
are required, to determine the specific pathophysiology of PCa and
identify novel treatment targets that may improve patient
prognoses.
In ~1980, researchers demonstrated that copper may
be associated with programmed cell death (8). In 2022, Tsvetkov et al
(9) highlighted a novel mechanism
of copper-induced cell death; namely, cuproptosis (9). The results of this previous study
provided novel insights into the role of copper in tumor growth.
Cuproptosis is an emerging form of cell death in PCa, with evidence
suggesting that it suppresses tumor progression. Inducing
cuproptosis or modulating cuproptosis-related genes (CRGs) may
exhibit potential as novel anti-cancer therapies. Thus, further
investigations are required to determine the specific processes
associated with cuproptosis and the potential role it may play in
PCa.
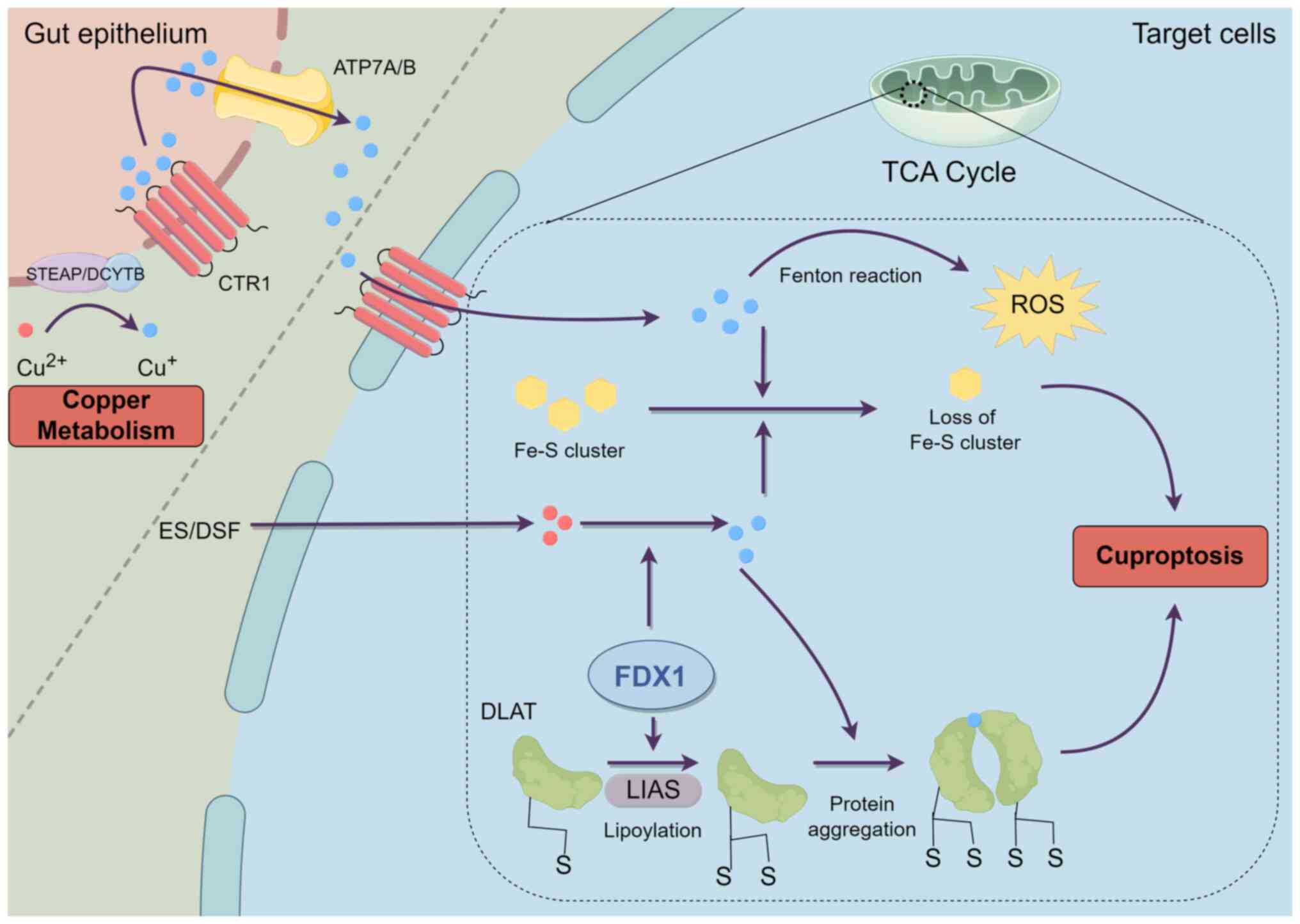
However, excessive accumulation of intracellular
copper ions may be harmful to cells (26). Results of a previous study
(27) indicated that excessively
high copper levels promote the generation of reactive oxygen
species (ROS), resulting in oxidative stress and DNA damage that
may lead to cell death. Alterations in the valence state of copper
ions result in harm to bio-organic molecules, including proteins,
nucleic acids and lipids. These alterations may disrupt the
production of iron-sulfur (Fe-S) clusters; thus, impacting enzyme
activity (28). Excess copper
ions associate with the lipoylated constituents of the
tricarboxylic acid cycle (TCA) within mitochondria. Ferredoxin 1
(FDX1), a member of the ferredoxin protein family, co-regulates the
proteolipoylation of proteins, such as dihydrolipoic acid
S-acetyltransferase (DLAT) and lipoic acid synthetase (LIAS). FDX1
reduces Cu2+ to Cu+, which exhibits higher
levels of toxicity. This ion subsequently binds to lipoylated
proteins, causing DLAT aggregation, resulting in proteotoxic stress
and cellular apoptosis. Moreover, Cu+ may destabilize
the architecture of Fe-S cluster proteins and impede the synthesis
of Fe-S proteins, which are integral to mitochondrial stability.
The depletion of Fe-S cluster proteins markedly compromises
cellular metabolic functions and energy maintenance, leading to an
escalation of oxidative free radicals and the production of ROS,
thereby increasing cytotoxicity and culminating in cell death
(Fig. 1). In addition, FDX1 and
LIAS may be affected by cuproptosis, resulting in reduced
expression (9,29,30). Thus, the homeostatic management of
copper is crucial to avert cytotoxicity and sustain overall health.
Notably, the processes and attributes of cuproptosis may exhibit
potential in the treatment of specific disorders.
The identification of cuproptosis has offered novel
insights into the initiation and advancement of tumor pathologies.
Copper participates in the biological mechanisms of tumor
proliferation and migration during the initial stages, activating
metastasis-associated enzymes and signaling pathways to facilitate
the spread of cancer (26).
Notably, it binds to and activates critical molecules within
various tumor signaling pathways (31,32), thereby influencing cancers
directly or indirectly, and is intricately associated with the
regulation of anti-tumor immunity (33). Moreover, copper directly
influences or indirectly modifies the activity of key components,
such as the Notch system; thus, affecting tumor angiogenesis,
metabolism and inflammatory response (34). Copper also plays a key role in
cancer cell proliferation (35)
and results of a previous study (36) revealed that metallothionein, which
is associated with copper storage, was markedly diminished in tumor
tissues. Collectively, these results suggest that the capacity of
cancer cells for regulating copper levels may be compromised.
Moreover, copper facilitates angiogenesis, which is intricately
associated with the advancement of malignant tumors (37,38). Results of previous studies
(39-41) demonstrate that copper may activate
numerous angiogenic factors and stabilize nuclear hypoxia-inducible
factor-1, thereby augmenting the expression of pro-angiogenic
factors. In addition, copper facilitated angiogenesis in numerous
in vitro and in vivo models, whereas copper chelators
inhibited tumor angiogenesis (42). Results of a further previous study
(43) revealed that increased
copper concentrations in tumor cells facilitated tumor
angiogenesis, resulting in tumor advancement and spread.
Consequently, increased copper concentrations may facilitate tumor
development and progression. Excess copper levels may lead to
cytotoxicity and trigger cuproptosis, which suppress tumor
progression. Notably, excess copper-induced cuproptosis may exert
inhibitory effects in numerous types of cancer (44). Copper exerts a dual influence on
tumors and their metabolism, facilitating tumor cell proliferation
and differentiation, while simultaneously suppressing proliferation
and triggering apoptosis. This phenomenon is intricately associated
with its biochemical characteristics and the complex tumor
microenvironment (45).
Elevated copper concentrations in PCa cell tissues
are a hallmark of patients with PCa (46), as demonstrated using in
vitro cell line studies (47-49) and a xenograft mouse model
(49,50). Notably, patients with PCa exhibit
higher than expected serum copper levels (51-53) and elevated serum copper levels may
be associated with a poor prognosis in patients with PCa (54). The RNA interference-mediated
knockdown of CTR1 decreased copper ion absorption in PCa cells,
resulting in the substantial inhibition of tumor growth (50). Collectively, these findings
highlighted the role that increased copper ion concentrations may
play in sustaining the biological processes of PCa cells, while
diminished copper ion levels may fail to support the physiological
functions of tumor cells.
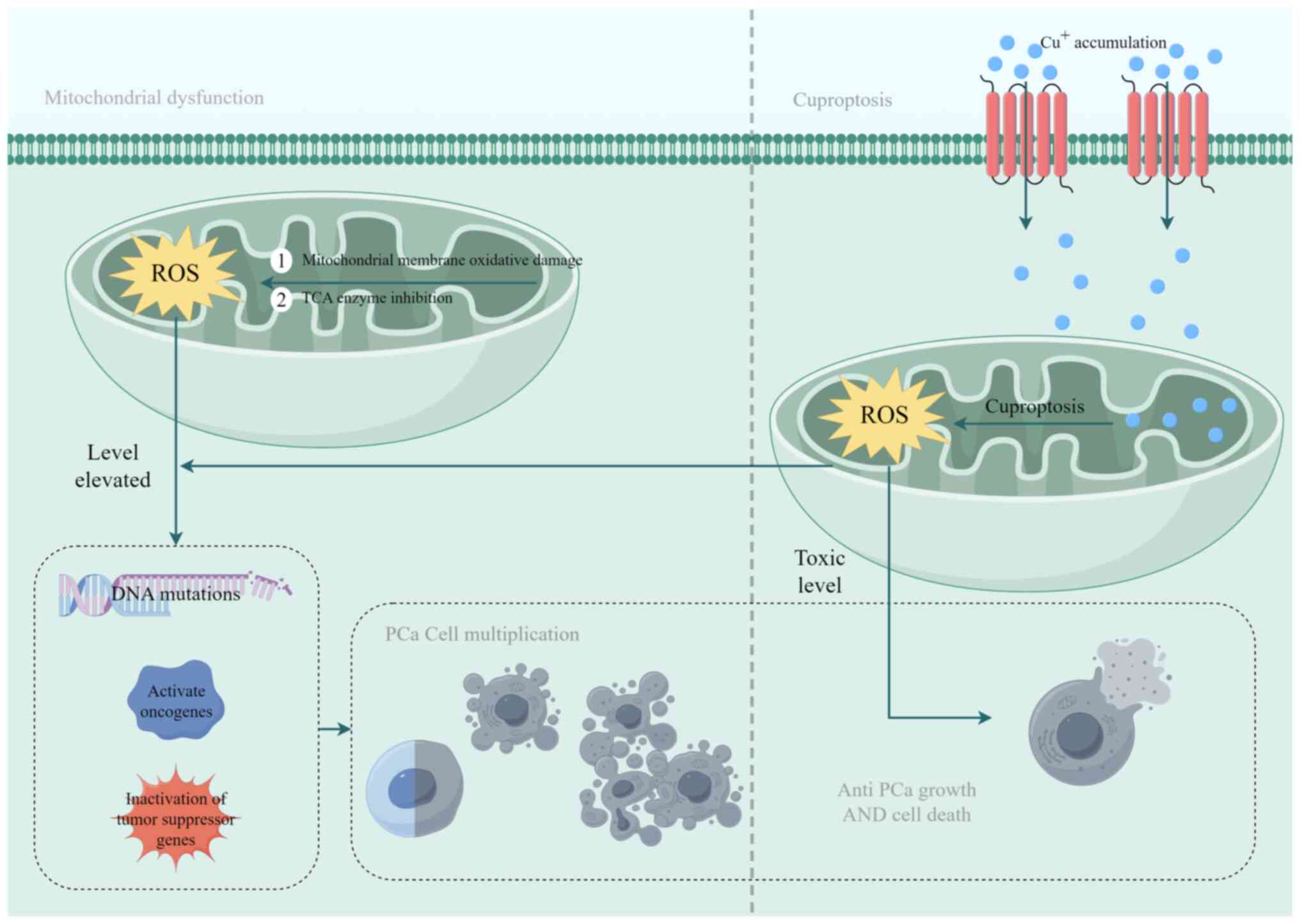
Mitochondrial dysfunction is pivotal for
cuproptosis, resulting in oxidative damage to the mitochondrial
membrane and compromised enzyme performance in the TCA cycle
(29). By contrast, excessive
copper accumulation in cellular mitochondria initiates cuproptosis
(68), producing substantial
amounts of ROS. During PCa progression, ROS levels are increased in
PCa cells (69), resulting in DNA
mutations, oncogene activation and tumor suppressor gene
inactivation. This cascade facilitates malignant cell
transformation and the activation of stress-responsive survival
pathways, thereby exacerbating the aberrant proliferation of PCa
cells (70). In addition, the
process of cuproptosis generates ROS, further advancing PCa
progression (71). Notably, a
requisite level of ROS is essential for tumor cell growth and DNA
mutation; however, severe oxidative stress may deplete the
capabilities of the antioxidant system, resulting in apoptosis
(72). In cuproptosis, ROS are
continuously generated, leading to toxic concentrations in PCa
cells and notable effects on tumor growth. Results of previous
studies (73-75) indicate that the excessive creation
and accumulation of ROS are employed to impede PCa progression.
Moreover, soy isoflavones, such as genistein and soy glycosides,
may elevate copper concentrations in PCa cells through mobilizing
endogenous copper and disrupting the expression of copper
transporter protein genes, CTR1 and ATP7A, in cancer cells. This
process subsequently induced high levels of ROS production,
ultimately resulting in apoptosis in PCa cells (76). Moreover, curcumin analogs
(77) and felicidin (78) exhibit analogous mechanisms of
action to impede PCa cell proliferation. Results of a previous
study (79) reveal that
alterations in the ATP7B gene inhibits the regulation of copper ion
distribution in vivo, resulting in increased copper levels
in PCa cells. Similarly, ATP7B protein levels were markedly
enhanced in docetaxel-resistant PCa cell lines, resulting in
diminished intracellular copper levels. Notably, these levels may
be promoted through silencing ATP7B expression or using a
combination of disulfiram and copper to enhance intracellular
copper concentrations (80). This
modified intra- and extracellular copper distribution results in a
substantial inhibitory effect on PCa growth, which may be
associated with the excessive production of ROS due to copper ion
carriers entering PCa cells, as well as antioxidant deficiencies
present in these cells (79).
Results of a previous study (81)
highlight a reciprocal interaction between cuproptosis and
copper-induced oxidative stress. The accumulation of ROS may
facilitate the progression of PCa and exert an inhibitory effect on
it. Cuproptosis may induce excessive ROS production to promote
tumor cell death; however, investigations surrounding this dual
mechanism are limited (Fig. 2).
The specific impact of cuproptosis on PCa requires further
validation through relevant experiments to elucidate the mechanism
underlying cuproptosis in the context of PCa development.
Copper participates in tumor metabolism and promotes
tumor progression. Notably, copper is associated with the
progression and spread of PCa (82,83) and it also facilitates tumor
angiogenesis (37,38). Angiogenesis markedly contributes
to the progression and metastasis of PCa (84), primarily driven by angiogenic
factors, including vascular endothelial growth factor, fibroblast
growth factor, hypoxia-inducible factor (HIF) and interleukin
(85). Results of a previous
study (79) reveal that a
reduction in copper levels diminishes HIF activity and obstructed
tumor angiogenesis. Moreover, restrictions in angiogenesis may
limit PCa progression (86).
Copper chelators may also diminish intracellular copper ion
concentrations and lower copper bioavailability, thereby reducing
copper-induced angiogenesis (42). Therefore, lowering copper
concentrations to impede tumor angiogenesis may mitigate tumor
advancement.
Complexities in lipid metabolism may contribute to
carcinogenesis and disease progression. Results of a previous study
(87) reveal that disturbances in
lipid metabolism are associated with disease advancement and the
development of castration-resistant prostate cancer (CRPC). In
addition, cholesteryl esters (CE) are excessively accumulated in
high-grade PCa and metastatic sites (88). The Wnt/β-catenin signaling pathway
plays an important role in the metastasis of PCa and the depletion
of CE markedly obstructs Wnt3a secretion through reducing
unsaturated fatty acid levels, which inhibit the activation of the
Wnt/β-catenin signaling pathway and restrict the acylation of
Wnt3a, thereby inhibiting the metastasis of PCa (89). Copper ions also activate the
Wnt/β-catenin signaling pathway, resulting in augmented cancer stem
cell characteristics and increased resistance to numerous therapies
(90). Elevated serum copper
levels may be associated with increased serum total cholesterol and
HDL cholesterol levels, as well as a heightened risk of
dyslipidemia characterized by high serum total cholesterol and LDL
cholesterol (91). Results of
previous studies (92,93) reveal that zebrafish and grass carp
exhibit elevated copper concentrations in their livers when
subjected to excess copper, resulting in increased lipid
accumulation and levels of triglycerides. These findings indicate
that alterations in copper levels, particularly in excess, may
influence the progression of PCa by affecting lipid metabolism and
signaling system activity; however, further molecular or animal
studies are required to validate these mechanisms.
In conclusion, the mechanisms underlying cuproptosis
in PCa primarily encompass oxidative stress, intracellular copper
distribution, angiogenesis and lipid metabolism dysregulation.
Further investigations into these mechanisms may facilitate a
deeper comprehension of cuproptosis in PCa and aid in identifying
potential therapeutic targets.
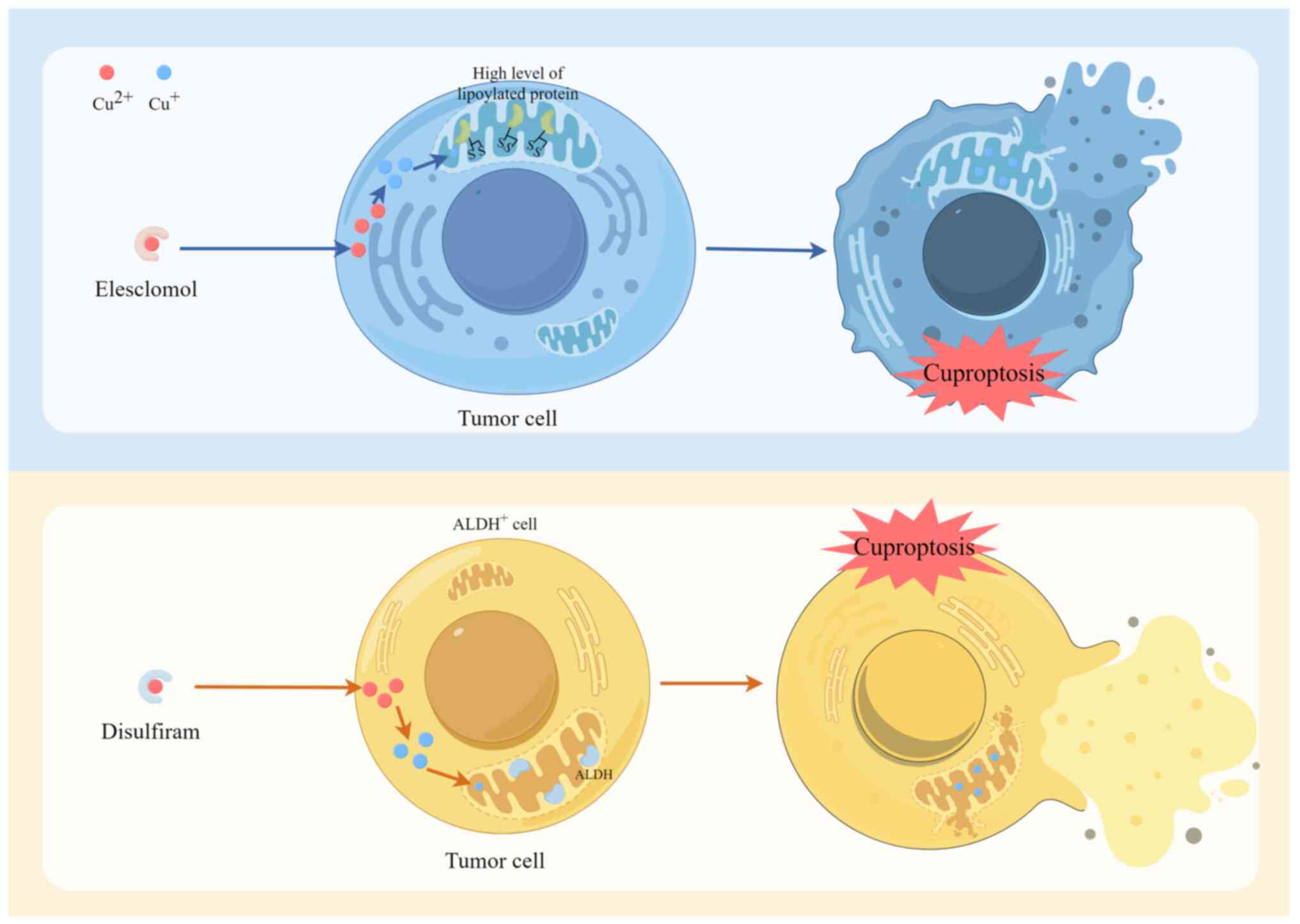
Elesclomol (ES) is a copper ion transporter that
facilitates the entry of copper ions into cells, often targeting
mitochondria. The entry of copper into cells leads to the
accumulation of ROS, consequently impeding tumor growth. In cancer
therapy, ES may leverage its capacity to produce cuproptosis to
eliminate malignant cells. Disulfiram (DSF) functions as a copper
ion transporter through binding to copper ions and facilitating
entry into the cell. Notably, DSF has previously been investigated
for the treatment of specific types of cancer, particularly those
with elevated levels of lipoylated proteins (94). The two aforementioned copper ion
carriers have been extensively researched for their ability to
carry Cu2+ into cells or mitochondria to facilitate
copper-dependent cell death (Fig.
3). Copper exerts a multifaceted impact on PCa growth and
proliferation; however, results of in vitro studies
(61,62) consistently demonstrate that ES
diminishes PCa cell viability via a reduction in FDX1 expression
levels. Moreover, ES promotes keratin formation in tumor cells,
inhibits autophagy in PCa via the DLAT/mammalian target of
rapamycin pathway and enhances sensitivity to docetaxel and
paclitaxel chemotherapy (95). In
addition, DSF compounds combined with copper have demonstrated
efficacy in breast cancer treatment through downregulating the
expression of the phosphatase and tensin homolog (PTEN) associated
with human chromosome 10 deletion and activating protein kinase B
(AKT) signaling. This indicates a potential for combination therapy
utilizing phosphatidylinositol 3-kinase (PI3K)/AKT inhibitors
(96). Results of further
previous studies (97,98) reveal that PCa may involve the same
PTEN and PI3K/AKT signaling pathways for targeted therapy,
highlighting that these pathways may inhibit PCa progression.
However, previous investigations focused on the role of PTEN and
PI3K/AKT signaling pathways in PCa progression remain limited.
Moreover, ES may exhibit potential in the treatment
of PCa. Results of a previous study (99) revealed that DSF may inhibit the
proliferation of PCa cell lines and this mechanism may be mediated
by other pharmacological agents or specific sensitizers, such as
copper (100). Thus, copper may
be used to suppress the growth of PCa cells (49,101), ultimately improving the survival
rate of patients (80).
Collectively, these results revealed that although
copper ion carrier-induced cell death is a key form of cell death,
few studies have confirmed the efficacy in PCa using in
vitro investigations. Results of previous research demonstrated
that several substances may elevate intracellular copper ion
concentrations, including the aforementioned copper ion carriers,
copper ion compounds and several copper chelators that inhibit the
increase in copper ion levels. Triethylenetetramine dihydrochloride
(102), 8-hydroxyquinoline
(103), penicillamine (104), methanobactin (105), Bathocuproine disulphonate
(106) and choline
tetrathiomolybdate (107) are
key compounds that regulate intracellular copper ion
concentrations, thereby facilitating or obstructing copper
ion-associated biological functions. The aforementioned drugs are
theoretically capable of stimulating cell proliferation; however,
no previous investigations have been undertaken regarding
cuproptosis in PCa. Preliminary experiments should be performed to
substantiate this hypothesis and to aid in the development of novel
cuproptosis-associated therapies.
A reduction in glutathione (GSH), a natural chelator
of intracellular copper ions, results in an elevation of
intracellular copper concentration, ultimately culminating in cell
death (9). In healthy prostate
cells, GSH neutralizes ROS produced by increased copper ion levels
from copper ion carriers; thus, providing an antioxidant effect and
reducing sensitivity to ROS. Conversely, PCa cells possess
diminished levels of GSH and are deficient in antioxidants,
rendering them susceptible to ROS generated by copper ion carriers.
Subsequently, this may result in PCa cell mortality (79). In addition, elevated copper
concentrations facilitate tumor angiogenesis and disrupt lipid
metabolism. Steroid-based compounds impede PCa progression through
the inhibition of CTR, thereby decreasing copper absorption by PCa
cells and mitigating the proliferative effects of copper. These
results indicate that the aforementioned compounds may represent
innovative anticancer agents targeting anti-copper therapy
(35). Results of a further study
(108) reveal that the pro-drug,
gamma-glutamyl transferase-activated prochelator releasing
dithiocarbamate (GGTDTC), is activated by gamma-glutamyl
transferase (GGT) in PCa cells. The release of dithiocarbamate
(DTC), which chelates with copper ions to form a Cu-DTC complex, is
highly toxic and promotes PCa cell death by triggering oxidative
stress to generate large amounts of ROS. Ultimately, this
interferes with protein ubiquitination. In PCa cells, GGT is often
overexpressed and the concentration of copper ions is high. In
addition, GGT exhibits a decreased antioxidant capacity, leading to
metabolic disorders. This compound is more selective to cancer
cells and therefore less toxic to non-tumor cells. In addition, GGT
exhibits improved selectivity (108). Thus, the use of copper
chelators, in addition to elevated copper ion concentrations that
mediate cuproptosis and impede tumor advancement, may diminish
intracellular copper levels and initiate cuproptosis. These
compounds may be used to leverage the highly toxic characteristics
of copper-chelator complexes, which may further inhibit tumor
progression to some degree. However, the specific mechanisms remain
to be fully elucidated.
Nanotechnology facilitates the targeted delivery of
copper ions to tumor sites via modifying responsive groups that
coordinate with copper compounds. This process enhances the
concentration of copper ions within tumor cells, leading to the
toxic oligomerization of lipoylated proteins and the degradation of
Fe-S cluster proteins. This ultimately induces cuproptosis in tumor
cells and produces a therapeutic effect against cancer (109). Researchers presented a novel
nanoparticle, DCM@GDY-CuMOF@DOX, capable of releasing
Cu+ in the presence of GSH, ultimately facilitating the
onset of cuproptosis (110).
Concurrently, doxorubicin (DOX) generates high levels of hydrogen
peroxide (H2O2), which, when catalyzed by
Cu+, is transformed into cytotoxic ROS via a Fenton-like
reaction. Notably, this process induces cancer cell death. ROS
produced via this method may trigger apoptosis, resulting in
oxidative DNA damage and mitochondrial impairment. By contrast to
the administration of DOX alone, nanoparticles enveloped within the
PCa cell membranes (DU145 cell membrane; DCM) exhibit isotypic
targeting capabilities, allowing them to specifically identify and
target identical PCa cells. This process may diminish the
likelihood of non-specific targeting during blood circulation. The
non-specific adsorption and elimination in the bloodstream extend
circulation time within the body, thereby minimizing toxicity to
healthy tissues and enhancing the precision of targeting PCa cells
for treatment. Haemolysis experiments, histopathological
assessments and serum biochemical analyses demonstrated that
nanoparticles did not induce notable damage to healthy cells and
tissues; however, they did exhibit targeting capabilities and
biocompatibility. Thus, these may exhibit potential in clinical
practice (110). Results of a
previous study (111)
demonstrate that cuprous oxide nanoparticles (CONPs) selectively
inhibited the proliferation of CRPC cells. Toxicological
investigations revealed that CONPs elevate copper demand and uptake
capacity and induce cell cycle arrest and apoptosis in cancer
cells. In addition, CONPs suppressed stem cell properties and the
Wnt signaling pathway and exhibited minimal effects on healthy
prostate epithelial cells. Moreover, Cu(DDC)2
nanoparticles facilitate the transport of Cu2+ into
cells, elevate intracellular copper levels and mediate the
apoptosis of paclitaxel-resistant PCa cells (112). These nanoparticles facilitated
tumor site-specific accumulation and induce cuproptosis through
loading copper ions and employing a responsive release mechanism,
offering a novel, targeted and biocompatible therapeutic
alternative for PCa treatment.
Moreover, liposomal copper (LpCu) effectively
transports copper ions into PCa cells via encapsulating copper
salts within liposomes. PCa cells treated with LpCu exhibit
metabolic toxicity comparable with that of free copper salts,
indicated by increased intracellular copper levels and increased
levels of ROS, apoptosis and necrosis (113). Specific steroid-based compounds
form complexes with copper ions, diminishing the concentration of
free copper ions within the cell. Thus, the influx of copper ions
into PCa cells via CTR1 is reduced, effectively impeding copper
absorption by PCa cells and disrupting their biosynthesis to
inhibit PCa cell proliferation (35).
Surgery remains the primary treatment option for
localized PCa and androgen deprivation therapy is effective for
patients with biochemically recurrent and metastatic
hormone-sensitive PCa. However, resistance and progression to CRPC
may still occur, along with suboptimal therapeutic outcomes
following chemotherapy and immunotherapy (54). At present, research is focused on
the role of cuproptosis in malignancies, to determine the effects
on cell growth and the specific underlying mechanisms.
Cuproptosis affects treatment resistance and
contributes to the management of PCa. Notably, enzalutamide
augments the susceptibility of CRPC cells to copper ion carriers
and improves treatment efficacy through facilitating
copper-dependent cell death. This combination therapy efficiently
suppresses the proliferation of CRPC cells and reverses the
resistance of enzalutamide-resistant cells both in vitro and
in vivo (43). By
contrast, enzalutamide-resistant PCa cells demonstrate high levels
of resistance to cuproptosis and the alteration of NUDT21 activity
or the addition of PDHA improve the effectiveness of the copper
ionophore, potentially leading to treatment resistance in PCa
(67). Results of a further
previous study revealed that a combination of disulfiram and copper
led to a notable reduction in the expression of ATP7B and the
elevation of intracellular copper levels in PCa cell lines,
rendering resistant cells sensitive to the growth-inhibitory and
apoptotic effects of docetaxel (80). Results of both in vivo and
in vitro studies demonstrate that a combination of DSF and
copper markedly reduces cell proliferation and the induction of
apoptosis, indicative of the increased antitumor efficacy of
docetaxel (80). Moreover, DSF
and copper directly interact with ATP7B, leading to the
downregulation of its downstream proteins, including copper
metabolism MURR1 Domain containing 1, α-clusterin and S-phase
kinase associated protein 2. Simultaneously, DSF and copper promote
upregulation of cyclin-dependent kinase inhibitor 1 thus inhibiting
tumor growth (80). Results of a
previous study (95) reveal that
a combination of ES and CuCl2 (ES-Cu) effectively
induces copper-mediated cytotoxicity in PCa cells, inhibits
autophagy via DLAT upregulation and subsequently promotes cell
retention in the G2/M phase, thereby augmenting the
chemotherapeutic efficacy of docetaxel. In vivo experiments
further demonstrate that the combination of ES-Cu and docetaxel
markedly suppresses tumor growth in a PCa transplantation model
using nude mice, where tumor proliferation was markedly inhibited
(95). Collectively, these data
indicated that copper-induced cell death may impact the sensitivity
of PCa to pharmacological agents.
Complanatoside A (CA) is a flavonoid derived from
the Traditional Chinese Medicine, Semen Astragali
Complanati. Results of a previous study (114) reveal that CA downregulates the
expression of the copper efflux-associated protein, ATOX1, promotes
the accumulation of intracellular copper ions, inhibits
mitochondrial activity and induces copper-mediated cell death.
Notably, this agent markedly suppresses the proliferation, invasion
and growth of PCa cells both in vivo and ex vivo,
with no notable damage to major organs in experimental animal
models (114). Nuclear
factor-activated T cell 5 (NFAT5) is a transcription factor that
activates T cells and plays a key role in cellular stress response,
immunological response and cell proliferation. Results of previous
study revealed that NFAT5 acts as a target gene of microRNA
(miR)-206 through bioinformatics analysis and database predictions.
In addition, results of a previous study (115) reveal that the long-chain
non-coding RNA (lncRNA), AP000842.3, acts as a target gene of
miR-206, functioning as a competitive endogenous RNA (ceRNA) that
modulates the expression levels of NFAT5. Thus, this lncRNA affects
the sensitivity of PCa cells to copper death inducers. In cellular
experiments, NFAT5 knockdown markedly increases the sensitivity of
PCa cells to copper-induced cytotoxicity and this was highlighted
by elevated intracellular copper ion concentrations and reduced ROS
levels (115). Therefore, the
interaction of lncRNA AP000842.3 with miR-206 may play a key role
in regulating NFAT5 expression. Notably, this regulatory mechanism
may be partly dependent on miR-206, providing a novel potential
target for PCa diagnosis and treatment (115).
Resveratrol, a polyphenol compound mainly located in
red grapes and pomegranates, is an antioxidant that inhibits PCa
cells through interacting with copper ions. This process
contributes to the redox of copper ions and the generation of ROS,
leading to an increase in intracellular oxidative stress and
interference with intracellular signaling pathways, such as MAPK,
PI3K/Akt and NF-κB pathways. Moreover, results of a this study
(116) reveal that resveratrol
inhibits cell cycle progression, thus inhibiting PCa cell
proliferation. This compound also activates key intracellular
apoptotic signaling pathways, such as the mitochondrial pathway and
death receptor pathway, to promote the apoptosis of PCa cells. By
contrast, copper ion chelation reverses the anticancer effects of
resveratrol, further confirming the key role of copper ions in the
action of resveratrol. In addition, the observed elevation of
copper ions may facilitate their absorption and storage, promoting
the harmful effects of resveratrol through the upregulation of the
copper transporter protein, CTR (116). Collectively, results of these
investigations indicated that copper and copper-induced
cytotoxicity may serve as viable therapeutic approaches for the
treatment of PCa.
Despite the limited number of studies on cuproptosis
in PCa, previous studies revealed that CRGs may act as novel
therapeutic targets, playing a key role in the progression of PCa
(Table II). Moreover,
cuproptosis may be associated with key predictors of PCa,
exhibiting potential as a novel indicator of the prognosis of
patients with PCa and their responsiveness to treatment (Table III).
Irrespective of the trajectory of predictive
modeling, research is focused on the development of novel molecular
instruments for prognostic evaluation and clinical decision-making
in PCa. Cuproptosis-based prognostic models exhibit efficacy in
predicting outcomes of patients with PCa; however, the recurrence
prediction mediated by CDI surpasses that of the cuproptosis-based
prognostic models (123). CRLs
obtained from bioinformatics analysis not only aid in the
establishment of a prognostic prediction model for patients with
PCa, but also the results of cellular experiments reveal that the
knockdown of CRLs, particularly AC106820.5, may inhibit the
proliferation, migration and invasive capabilities of PCa cells
(127). Collectively, these data
illustrate that bioinformatics analyses may be used to identify
potential therapeutic targets in the treatment of PCa. Notably, the
aforementioned models may also be used to predict responsiveness to
immunotherapy, leading to the development of personalized treatment
options that improve patient outcomes and quality of life.
The redox activity of copper represents a dual
purpose in cellular viability; for example, it acts as a crucial
cofactor for enzymes that facilitate vital physiological processes
in cellular metabolism and may induce oxidative stress and cell
death when present in excess (131,132). The initial discovery of
cuproptosis elucidated the association between copper-induced cell
death and mitochondrial metabolism, thus furthering investigations
in copper biology and offering novel insights for future
investigations into cell death pathways. In PCa, increased copper
concentrations and a disruption of copper transporters result in
the accumulation of copper ions within tumor cells, facilitating
the initiation of cuproptosis. The activation of cuproptosis
facilitates the elimination of surplus copper ions, mitigates
cellular damage resulting from copper metabolism disorders and
suppresses the proliferation, invasion and metastasis of PCa cells,
thereby inhibiting tumor progression induced by copper metabolism
disorders. However, further investigations are required to
determine the specific underlying mechanisms.
In recent years, cuproptosis has become a novel
focus within PCa research, demonstrating potential in various
tumors to markedly impact the proliferation, invasion and
metastasis of tumor cells. Despite novel insights into the role of
cuproptosis in PCa, this field of study exhibits certain
limitations. Cuproptosis may inhibit the progression of PCa;
however, PCa requires elevated copper levels for its physiological
functions. In addition, FDX1 expression in PCa cells remains low to
prevent the initiation of cuproptosis. Androgens may enhance the
transcriptional levels of CTR1, ATP7B and STEAP in PCa cells via
androgen receptor activation, thereby increasing copper uptake and
facilitating PCa progression (49). Moreover, the hypothesis regarding
increased copper demand in PCa and the approaches to alleviate
levels of toxicity remain ambiguous and the specific molecular
mechanisms underlying cuproptosis have yet to be fully elucidated.
Thus, further investigations should focus on the specific signaling
pathways and regulatory mechanisms underlying this phenomenon.
Notably, these processes may include the accumulation of copper
ions in PCa cells, the functions of pivotal proteins and the
activation of signaling pathways associated with the onset of
cuproptosis. To date, previous research has focused on CRGs and
CRLs and the use of bioinformatics analyses, predictive model
development and in vitro experimentation. The aforementioned
studies have primarily utilized public databases for analysis,
which may result in limitations in the validity of studies, through
large-scale clinical samples and a lack of investigation into
clinical applications. Thus, further investigations should focus on
the use of genetic analysis of clinical samples to validate prior
findings and inform subsequent studies. Notably, the AlphaFold
(https://deepmind.google/science/alphafold/) suite of
tools constitutes a robust, integrated platform for structural
prediction. It is used to accurately predict the structures of
proteins interacting with various biomolecules (133). Thus, the development of novel
prediction models using AlphaFold 3 tools may further the
understanding of PCa at the molecular level and aid in the
identification of additional therapeutic targets. Further
investigations into drug sensitivity may lead to the development of
novel therapeutic targets, while analyses of immune cells inside
the tumor microenvironment may clarify the role of cuproptosis in
modulating their activity and the potential impact on tumor
proliferation and metastasis. Moreover, a multidisciplinary
research approach is required to elucidate the specific role of
cuproptosis in PCa, thereby establishing a foundation for the
advancement of novel therapies aimed at enhancing the prognosis and
quality of life of patients.
In conclusion, the present review article
demonstrated that cuproptosis may exhibit potential in the
treatment of PCa. Notably, copper-based nanomedicines, as well as
traditional copper ion carriers and chelators, may elicit more
potent anti-tumor effects. Additional investigations into the
transformation of conventional pharmaceuticals into nanoparticles,
alongside additional clinical trials, may aid in the development of
a novel therapeutic approach. However, these trials should be
preceded by a comprehensive understanding of the mechanisms
underlying copper metabolism and cuproptosis, aiming to determine
distinct interactions between copper and the tumor
microenvironment, as well as immune responses, which are essential
for drug design and efficacy prediction. Notably, the
identification of cuproptosis has prompted extensive investigations
into novel treatment options for PCa and this newly recognized
mechanism of regulatory cell death may contribute to theoretical
advancements and practical implementations in the field.
Not applicable.
ZL was responsible for writing the original draft,
reviewing and editing, project administration, supervision and
conceptualization. YC was responsible for writing the original
draft, reviewing and editing, supervision and visualization. KZ was
responsible for writing the original draft, supervision and
conceptualization. ZC was responsible for writing the original
draft and resources. YY was responsible for funding acquisition,
supervision, project administration, writing, reviewing and
editing. Data authentication is not applicable. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81503589, 81973866 and 82474520),
the Natural Science Foundation of Sichuan Province (grant no.
2022NSFSC0684) and the Sichuan Provincial Administration of
Traditional Chinese Medicine Science and Technology Research
Special Fund Project (grant no. 2021ZD016).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Siegel DA, O'Neil ME, Richards TB, Dowling
NF and Weir HK: Prostate cancer incidence and survival, by stage
and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal
Wkly Rep. 69:1473–1480. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z, Garzotto M, Davis EW II, Mori M,
Stoller WA, Farris PE, Wong CP, Beaver LM, Thomas GV, Williams DE,
et al: Sulforaphane bioavailability and chemopreventive activity in
men presenting for biopsy of the prostate gland: A Randomized
controlled trial. Nutr Cancer. 72:74–87. 2020. View Article : Google Scholar
|
|
4
|
Tilki D, van den Bergh RCN, Briers E, Van
den Broeck T, Brunckhorst O, Darraugh J, Eberli D, De Meerleer G,
De Santis M, Farolfi A, et al: EAU-EANM-ESTRO-ESUR-ISUP-SIOG
guidelines on prostate cancer. Part II-2024 update: Treatment of
relapsing and metastatic prostate cancer. Eur Urol. 86:164–182.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cornford P, van den Bergh RCN, Briers E,
Van den Broeck T, Brunckhorst O, Darraugh J, Eberli D, De Meerleer
G, De Santis M, Farolfi A, et al: EAU-EANM-ESTRO-ESUR-ISUP-SIOG
guidelines on prostate cancer-2024 update. Part I: Screening,
diagnosis, and local treatment with curative intent. Eur Urol.
86:148–163. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillessen S, Bossi A, Davis ID, de Bono J,
Fizazi K, James ND, Mottet N, Shore N, Small E, Smith M, et al:
Management of patients with advanced prostate cancer. Part I:
Intermediate-/high-risk and locally advanced disease, biochemical
relapse, and side effects of hormonal treatment: Report of the
advanced prostate cancer consensus conference 2022. Eur Urol.
83:267–293. 2023. View Article : Google Scholar :
|
|
7
|
Sandhu S, Moore CM, Chiong E, Beltran H,
Bristow RG and Williams SG: Prostate cancer. Lancet. 398:1075–1090.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halliwell B and Gutteridge JM: Oxygen
toxicity, oxygen radicals, transition metals and disease. Biochem
J. 219:1–14. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francque SM, Marchesini G, Kautz A,
Walmsley M, Dorner R, Lazarus JV, Zelber-Sagi S, Hallsworth K,
Busetto L, Frühbeck G, et al: Non-alcoholic fatty liver disease: A
patient guideline. JHEP Rep. 3:1003222021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mason KE: A conspectus of research on
copper metabolism and requirements of man. J Nutr. 109:1979–2066.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dancis A, Roman DG, Anderson GJ,
Hinnebusch AG and Klausner RD: Ferric reductase of Saccharomyces
cerevisiae: molecular characterization, role in iron uptake, and
transcriptional control by iron. Proc Natl Acad Sci USA.
89:3869–3873. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Georgatsou E, Mavrogiannis LA, Fragiadakis
GS and Alexandraki D: The yeast Fre1p/Fre2p cupric reductases
facilitate copper uptake and are regulated by the copper-modulated
Mac1p activator. J Biol Chem. 272:13786–13792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan D, Zhao L, Shi X, Ma X and Chen Z:
Copper in cancer: From pathogenesis to therapy. Biomed
Pharmacother. 163:1147912023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyd SD, Ullrich MS, Skopp A and Winkler
DD: Copper sources for Sod1 activation. Antioxidants (Basel).
9:5002020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luza SC and Speisky HC: Liver copper
storage and transport during development: Implications for
cytotoxicity. Am J Clin Nutr. 63:812S–820S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pufahl RA, Singer CP, Peariso KL, Lin SJ,
Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE and O'Halloran
TV: Metal ion chaperone function of the soluble Cu(I) receptor
Atx1. Science. 278:853–856. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heaton DN, George GN, Garrison G and Winge
DR: The mitochondrial copper metallochaperone Cox17 exists as an
oligomeric, polycopper complex. Biochemistry. 40:743–751. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gromadzka G, Tarnacka B, Flaga A and
Adamczyk A: Copper dyshomeostasis in neurodegenerative
diseases-therapeutic implications. Int J Mol Sci. 21:92592020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
La Fontaine S and Mercer JFB: Trafficking
of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis.
Arch Biochem Biophys. 463:149–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lutsenko S, LeShane ES and Shinde U:
Biochemical basis of regulation of human copper-transporting
ATPases. Arch Biochem Biophys. 463:134–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lutsenko S, Bhattacharjee A and Hubbard
AL: Copper handling machinery of the brain. Metallomics. 2:596–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lutsenko S: Copper trafficking to the
secretory pathway. Metallomics. 8:84–852. 2016. View Article : Google Scholar
|
|
24
|
Csiszar K: Lysyl oxidases: A novel
multifunctional amine oxidase family. Prog Nucleic Acid Res Mol
Biol. 70:1–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pierson H, Yang H and Lutsenko S: Copper
transport and disease: What can we learn from organoids? Annu Rev
Nutr. 39:75–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Tar. 7:3782022. View Article : Google Scholar
|
|
27
|
Gaetke LM and Chow CK: Copper toxicity,
oxidative stress, and antioxidant nutrients. Toxicology.
189:147–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Jin D, Zhou S, Dong N, Ji Y, An P,
Wang J, Luo Y and Luo J: Regulatory roles of copper metabolism and
cuproptosis in human cancers. Front Oncol. 13:11234202023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang D, Chen X and Kroemer G: Cuproptosis:
A copper-triggered modality of mitochondrial cell death. Cell Res.
32:417–418. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric
cancer. Nat Commun. 14:65232023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie J, Yang Y, Gao Y and He J:
Cuproptosis: Mechanisms and links with cancers. Mol Cancer.
22:462023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Q, Huo C, Wang M, Huang H, Zheng X
and Xie M: Research progress on cuproptosis in cancer. Front
Pharmacol. 15:12905922024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu WQ, Lin WR, Yan L, Xu WH and Yang J:
Copper homeostasis and cuproptosis in cancer immunity and therapy.
Immunol Rev. 321:211–227. 2024. View Article : Google Scholar
|
|
34
|
Nowell CS and Radtke F: Notch as a tumour
suppressor. Nat Rev Cancer. 17:145–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie F and Peng F: Reduction in copper
uptake and inhibition of prostate cancer cell proliferation by
novel steroid-based compounds. Anticancer Res. 41:5953–5958. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Si M and Lang J: The roles of
metallothioneins in carcinogenesis. J Hematol Oncol. 11:1072018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baldari S, Di Rocco G and Toietta G:
Current biomedical use of copper chelation therapy. Int J Mol Sci.
21:10692020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ilyechova EY, Bonaldi E, Orlov IA,
Skomorokhova EA, Puchkova LV and Broggini M: CRISP-R/Cas9 mediated
deletion of copper transport genes CTR1 and DMT1 in NSCLC cell line
H1299. Biological and pharmacological consequences. Cells.
8:3222019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shao S, Si J and Shen Y: Copper as the
target for anticancer nanomedicine. Adv Ther. 2:2019, https://doi.org/10.1002/adtp.201800147.
|
|
40
|
Li Y: Copper homeostasis: Emerging target
for cancer treatment. IUBMB Life. 72:1900–1908. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lelièvre P, Sancey L, Coll JL, Deniaud A
and Busser B: The multifaceted roles of copper in cancer: A trace
metal element with dysregulated metabolism, but also a target or a
bullet for therapy. Cancers (Basel). 12:35942020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Zhang J, Yang H, Wu C, Dang X and
Liu Y: Copper depletion inhibits CoCl2-induced aggressive phenotype
of MCF-7 cells via downregulation of HIF-1 and inhibition of
Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep.
5:124102015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao X, Zhao H, Liu J, Wang M, Dai Z, Hao
W, Wang Y, Wang X, Zhang M, Liu P, et al: Enzalutamide sensitizes
castration-resistant prostate cancer to copper-mediated cell death.
Adv Sci (Weinh). 11:e24013962024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Chen Y, Zhang J, Yang Y, Fleishman
JS, Wang Y, Wang J, Chen J, Li Y and Wang H: Cuproptosis: A novel
therapeutic target for overcoming cancer drug resistance. Drug
Resist Update. 72:1010182024. View Article : Google Scholar
|
|
45
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross
JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, et al:
Connecting copper and cancer: From transition metal signalling to
metalloplasia. Nat Rev Cancer. 22:102–113. 2022. View Article : Google Scholar :
|
|
46
|
Stanislawska IJ, Figat R, Kiss AK and
Bobrowska-Korczak B: Essential elements and isoflavonoids in the
prevention of prostate cancer. Nutrients. 14:12252022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cater MA and Haupt Y: Clioquinol induces
cytoplasmic clearance of the X-linked inhibitor of apoptosis
protein (XIAP): Therapeutic indication for prostate cancer. Biochem
J. 436:481–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen D, Cui QC, Yang H, Barrea RA, Sarkar
FH, Sheng S, Yan B, Reddy GP and Dou QP: Clioquinol, a therapeutic
agent for Alzheimer's disease, has proteasome-inhibitory, androgen
receptor-suppressing, apoptosis-inducing, and antitumor activities
in human prostate cancer cells and xenografts. Cancer Res.
67:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Safi R, Nelson ER, Chitneni SK, Franz KJ,
George DJ, Zalutsky MR and McDonnell DP: Copper signaling axis as a
target for prostate cancer therapeutics. Cancer Res. 74:5819–5831.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cai H, Wu JS, Muzik O, Hsieh JT, Lee RJ
and Peng F: Reduced 64Cu uptake and tumor growth inhibition by
knockdown of human copper transporter 1 in xenograft mouse model of
prostate cancer. J Nucl Med. 55:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saleh SAK, Adly HM, Abdelkhaliq AA and
Nassir AM: Serum levels of selenium, zinc, copper, manganese, and
iron in prostate cancer patients. Curr Urol. 14:44–49. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaba M, Pirincci N, Yuksel MB, Gecit I,
Gunes M, Ozveren H, Eren H and Demir H: Serum levels of trace
elements in patients with prostate cancer. Asian Pac J Cancer Prev.
15:2625–2629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ozmen H, Erulas FA, Karatas F, Cukurovali
A and Yalcin O: Comparison of the concentration of trace metals
(Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid
peroxidation in patients with prostate cancer. Clin Chem Lab Med.
44:175–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu J, He J, Liu Z, Zhu X, Li Z, Chen A and
Lu J: Cuproptosis: Mechanism, role, and advances in urological
malignancies. Med Res Rev. 44:1662–1682. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baszuk P, Marciniak W, Derkacz R,
Jakubowska A, Cybulski C, Gronwald J, Dębniak T, Huzarski T,
Białkowska K, Pietrzak S, et al: Blood copper levels and the
occurrence of colorectal cancer in Poland. Biomedicines.
9:16282021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baltaci AK, Dundar TK, Aksoy F and
Mogulkoc R: Changes in the serum levels of trace elements before
and after the operation in thyroid cancer patients. Biol Trace Elem
Res. 175:57–64. 2017. View Article : Google Scholar
|
|
57
|
Jin Y, Zhang C, Xu H, Xue S, Wang Y, Hou
Y, Kong Y and Xu Y: Combined effects of serum trace metals and
polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: A
hospital based case-control study in China. Cancer Epidemiol.
35:182–187. 2011. View Article : Google Scholar
|
|
58
|
Aubert L, Nandagopal N, Steinhart Z,
Lavoie G, Nourreddine S, Berman J, Saba-El-Leil MK, Papadopoli D,
Lin S, Hart T, et al: Copper bioavailability is a KRAS-specific
vulnerability in colorectal cancer. Nat Commun. 11:37012020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lener MR, Scott RJ, Wiechowska-Kozlowska
A, Serrano-Fernández P, Baszuk P, Jaworska-Bieniek K, Sukiennicki
G, Marciniak W, Muszyńska M, Kładny J, et al: Serum concentrations
of selenium and copper in patients diagnosed with pancreatic
cancer. Cancer Res Treat. 48:1056–1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pavithra V, Sathisha TG, Kasturi K,
Mallika DS, Amos SJ and Ragunatha S: Serum levels of metal ions in
female patients with breast cancer. J Clin Diagn Res. 9:BC25–BC27.
2015.PubMed/NCBI
|
|
61
|
Yang L, Zhang Y, Wang Y, Jiang P, Liu F
and Feng N: Ferredoxin 1 is a cuproptosis-key gene responsible for
tumor immunity and drug sensitivity: A pan-cancer analysis. Front
Pharmacol. 13:9381342022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang L, Tang Y, Zhang Y, Wang Y, Jiang P,
Liu F and Feng N: Comprehensiveness cuproptosis related genes study
for prognosis and medication sensitiveness across cancers, and
validation in prostate cancer. Sci Rep. 14:95702024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang Q, Zeng S and Liu W: Roles of
cuproptosis-related gene DLAT in various cancers: A bioinformatic
analysis and preliminary verification on pro-survival autophagy.
PeerJ. 11:e150192023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li C, Xiao Y, Cao H, Chen Y, Li S and Yin
F: Cuproptosis regulates microenvironment and affects prognosis in
prostate cancer. BIOL Trace Elem Res. 202:99–110. 2024. View Article : Google Scholar
|
|
65
|
Xiao S and Lou W: Integrated analysis
reveals a potential cuproptosis-related ceRNA axis
SNHG17/miR-29a-3p/GCSH in prostate adenocarcinoma. Heliyon.
9:e215062023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang D, Wang T, Zhou Y and Zhang X:
Comprehensive analyses of cuproptosis-related gene CDKN2A on
prognosis and immunologic therapy in human tumors. Medicine
(Baltimore). 102:e334682023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lin SC, Tsai YC, Chen YL, Lin HK, Huang
YC, Lin YS, Cheng YS, Chen HY, Li CJ, Lin TY and Lin SC:
Un-methylation of NUDT21 represses docosahexaenoic acid
biosynthesis contributing to enzalutamide resistance in prostate
cancer. Drug Resist Update. 77:1011442024. View Article : Google Scholar
|
|
68
|
Tang D, Kroemer G and Kang R: Targeting
cuproplasia and cuproptosis in cancer. Nat Rev Clin Oncol.
21:370–388. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Role of ROS and RNS sources in physiological and pathological
conditions. Oxid Med Cell Longev. 2016:12450492016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Han C, Wang Z, Xu Y, Chen S, Han Y, Li L,
Wang M and Jin X: Roles of reactive oxygen species in biological
behaviors of prostate cancer. Biomed Res Int. 2020:12696242020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu Y, Chen A, Wu Y, Ni J, Wang R, Mao Y,
Sun N and Mi Y: Identification of mitochondrial carrier homolog 2
as an important therapeutic target of castration-resistant prostate
cancer. Cell Death Dis. 16:702025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Baohai X, Shi F and Yongqi F: Inhibition
of ubiquitin specific protease 17 restrains prostate cancer
proliferation by regulation of epithelial-to-mesenchymal transition
(EMT) via ROS production. Biomed Pharmacother. 118:1089462019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lee W, Kim KY, Yu SN, Kim SH, Chun SS, Ji
JH, Yu HS and Ahn SC: Pipernonaline from Piper longum Linn. induces
ROS-mediated apoptosis in human prostate cancer PC-3 cells. Biochem
Biophys Res Commun. 430:406–412. 2013. View Article : Google Scholar
|
|
75
|
Kim SH, Kim KY, Yu SN, Park SG, Yu HS, Seo
YK and Ahn SC: Monensin induces PC-3 prostate cancer cell apoptosis
via ROS production and Ca2+ homeostasis disruption. Anticancer Res.
36:5835–5843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Farhan M, El Oirdi M, Aatif M, Nahvi I,
Muteeb G and Alam MW: Soy isoflavones induce cell death by
copper-mediated mechanism: Understanding its anticancer properties.
Molecules. 28:29252023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alhasawi M, Aatif M, Muteeb G, Alam MW,
Oirdi ME and Farhan M: Curcumin and its derivatives induce
apoptosis in human cancer cells by mobilizing and redox cycling
genomic copper ions. Molecules. 27:74102022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Farhan M, Rizvi A, Ali F, Ahmad A, Aatif
M, Malik A, Alam MW, Muteeb G, Ahmad S, Noor A and Siddiqui FA:
Pomegranate juice anthocyanidins induce cell death in human cancer
cells by mobilizing intracellular copper ions and producing
reactive oxygen species. Front Oncol. 12:9983462022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Denoyer D, Pearson HB, Clatworthy SA,
Smith ZM, Francis PS, Llanos RM, Volitakis I, Phillips WA, Meggyesy
PM, Masaldan S and Cater MA: Copper as a target for prostate cancer
therapeutics: copper-ionophore pharmacology and altering systemic
copper distribution. Oncotarget. 7:37064–37080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Song L, Nguyen V, Xie J, Jia S, Chang CJ,
Uchio E and Zi X: ATPase copper transporting beta (ATP7B) is a
novel target for improving the therapeutic efficacy of docetaxel by
disulfiram/copper in human prostate cancer. Mol Cancer Ther.
23:854–863. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vo T, Peng TY, Nguyen TH, Bui TNH, Wang
CS, Lee WJ, Chen YL, Wu YC and Lee IT: The crosstalk between
copper-induced oxidative stress and cuproptosis: A novel potential
anticancer paradigm. Cell Commun Signal. 22:3532024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Onuma T, Mizutani T, Fujita Y, Yamada S
and Yoshida Y: Copper content in ascitic fluid is associated with
angiogenesis and progression in ovarian cancer. J Trace Elem Med
Biol. 68:1268652021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Oliveri V: Selective targeting of cancer
cells by copper ionophores: An overview. Front Mol Biosci.
9:8418142022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Melegh Z and Oltean S: Targeting
angiogenesis in prostate cancer. Int J Mol Sci. 20:26762019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ioannidou E, Moschetta M, Shah S, Parker
JS, Ozturk MA, Pappas-Gogos G, Sheriff M, Rassy E and Boussios S:
Angiogenesis and anti-angiogenic treatment in prostate cancer:
mechanisms of action and molecular targets. Int J Mol Sci.
22:99262021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Marin-Aguilera M, Pereira MV, Jimenez N,
Reig Ò, Cuartero A, Victoria I, Aversa C, Ferrer-Mileo L, Prat A
and Mellado B: Glutamine and cholesterol plasma levels and clinical
outcomes of patients with metastatic castration-resistant prostate
cancer treated with taxanes. Cancers (Basel). 13:49602021.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee HJ, Li J, Vickman RE, Li J, Liu R,
Durkes AC, Elzey BD, Yue S, Liu X, Ratliff TL and Cheng JX:
Cholesterol esterification inhibition suppresses prostate cancer
metastasis by impairing the wnt/β-catenin pathway. Mol Cancer Res.
16:974–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu YT, Chen L, Li SJ, Wang WY, Wang YY,
Yang QC, Song A, Zhang MJ, Mo WT, Li H, et al: Dysregulated
Wnt/β-catenin signaling confers resistance to cuproptosis in cancer
cells. Cell Death Differ. 31:1452–1466. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Song X, Wang W, Li Z and Zhang D:
Association between serum copper and serum lipids in adults. Ann
Nutr Metab. 73:282–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pan YX, Zhuo MQ, Li DD, Xu YH, Wu K and
Luo Z: SREBP-1 and LXRα pathways mediated Cu-induced hepatic lipid
metabolism in zebrafish Danio rerio. Chemosphere. 215:370–379.
2019. View Article : Google Scholar
|
|
93
|
Xu YC, Xu YH, Zhao T, Wu LX, Yang SB and
Luo Z: Waterborne Cu exposure increased lipid deposition and
lipogenesis by affecting Wnt/β-catenin pathway and the beta-catenin
acetylation levels of grass carp Ctenopharyngodon idella. Environ
Pollut. 263(Pt B): 1144202020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang L, Yang P, Lip GYH and Ren J: Copper
homeostasis and cuproptosis in cardiovascular disease therapeutics.
Trends Pharmacol Sci. 44:573–585. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wen H, Qu C, Wang Z, Gao H, Liu W, Wang H,
Sun H, Gu J, Yang Z and Wang X: Cuproptosis enhances docetaxel
chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway
in prostate cancer. FASEB J. 37:e231452023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang H, Chen D, Ringler J, Chen W, Cui
QC, Ethier SP, Dou QP and Wu G: Disulfiram treatment facilitates
phosphoinositide 3-kinase inhibition in human breast cancer cells
in vitro and in vivo. Cancer Res. 70:3996–4004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bergez-Hernandez F, Irigoyen-Arredondo M
and Martinez-Camberos A: A systematic review of mechanisms of PTEN
gene down-regulation mediated by miRNA in prostate cancer. Heliyon.
10:e349502024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Marques RB, Aghai A, de Ridder CMA,
Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR,
Trapman J and van Weerden WM: High efficacy of combination therapy
using PI3K/AKT inhibitors with androgen deprivation in prostate
cancer preclinical models. Eur Urol. 67:1177–1185. 2015. View Article : Google Scholar
|
|
99
|
Lin J, Haffner MC, Zhang Y, Lee BH,
Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, et
al: Disulfiram is a DNA demethylating agent and inhibits prostate
cancer cell growth. Prostate. 71:333–343. 2011. View Article : Google Scholar :
|
|
100
|
Iljin K, Ketola K, Vainio P, Halonen P,
Kohonen P, Fey V, Grafström RC, Perälä M and Kallioniemi O:
High-throughput cell-based screening of 4910 known drugs and
drug-like small molecules identifies disulfiram as an inhibitor of
prostate cancer cell growth. Clin Cancer Res. 15:6070–6078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tesson M, Rae C, Nixon C, Babich JW and
Mairs RJ: Preliminary evaluation of prostate-targeted radiotherapy
using 131I-MIP-1095 in combination with radiosensitising
chemotherapeutic drugs. J Pharm Pharmacol. 68:912–921. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Castoldi F, Hyvonen MT, Durand S,
Aprahamian F, Sauvat A, Malik SA, Baracco EE, Vacchelli E, Opolon
P, Signolle N, et al: Chemical activation of SAT1 corrects
diet-induced metabolic syndrome. Cell Death Differ. 27:2904–2920.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vetrik M, Mattova J, Mackova H, Kucka J,
Pouckova P, Kukackova O, Brus J, Eigner-Henke S, Sedlacek O, Sefc
L, et al: Biopolymer strategy for the treatment of Wilson's
disease. J Control Release. 273:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mao L, Huang CH, Shao J, Qin L, Xu D, Shao
B and Zhu BZ: An unexpected antioxidant and redox activity for the
classic copper-chelating drug penicillamine. Free Radic Biol Med.
147:150–158. 2020. View Article : Google Scholar
|
|
105
|
Kenney GE and Rosenzweig AC: Chemistry and
biology of the copper chelator methanobactin. ACS Chem Biol.
7:260–268. 2012. View Article : Google Scholar :
|
|
106
|
Lenartowicz M, Moos T, Ogorek M, Jensen TG
and Moller LB: Metal-dependent regulation of ATP7A and ATP7B in
fibroblast cultures. Front Mol Neurosci. 9:682016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lee K, Briehl MM, Mazar AP,
Batinic-Haberle I, Reboucas JS, Glinsmann-Gibson B, Rimsza LM and
Tome ME: The copper chelator ATN-224 induces
peroxynitrite-dependent cell death in hematological malignancies.
Free Radic Biol Med. 60:157–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bakthavatsalam S, Sleeper ML, Dharani A,
George DJ, Zhang T and Franz KJ: Leveraging γ-Glutamyl transferase
to direct cytotoxicity of copper dithiocarbamates against prostate
cancer cells. Angew Chem Int Ed Engl. 57:12780–12784. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wei C and Fu Q: Cell death mediated by
nanotechnology via the cuproptosis pathway: A novel horizon for
cancer therapy. VIEW-CHINA. 4:202300012023. View Article : Google Scholar
|
|
110
|
Xie W, Zhang Y, Xu Q, Zhong G, Lin J, He
H, Du Q, Tan H, Chen M, Wu Z, et al: A Unique approach: biomimetic
graphdiyne-based nanoplatform to treat prostate cancer by combining
cuproptosis and enhanced chemodynamic therapy. Int J Nanomedicine.
19:3957–3972. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang Y, Yang QW, Yang Q, Zhou T, Shi MF,
Sun CX, Gao XX, Cheng YQ, Cui XG and Sun YH: Cuprous oxide
nanoparticles inhibit prostate cancer by attenuating the stemness
of cancer cells via inhibition of the Wnt signaling pathway. Int J
Nanomedicine. 12:2569–2579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen W, Yang W, Chen P, Huang Y and Li F:
Disulfiram copper nanoparticles prepared with a stabilized metal
ion ligand complex method for treating drug-resistant prostate
cancers. ACS Appl Mater Interfaces. 10:41118–41128. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang Y, Zeng S, Lin TM, Krugner-Higby L,
Lyman D, Steffen D and Xiong MP: Evaluating the anticancer
properties of liposomal copper in a nude xenograft mouse model of
human prostate cancer: Formulation, in vitro, in vivo, histology
and tissue distribution studies. Pharm Res. 31:3106–3119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhao Y, Wang R, Hu C, Wang Y, Li Z, Yin D
and Tan S: Complanatoside A disrupts copper homeostasis and induces
cuproptosis via directly targeting ATOX1 in prostate cancer.
Toxicol Appl Pharmacol. 496:1172572025. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhou G, Chen C, Wu H, Lin J, Liu H, Tao Y
and Huang B: LncRNA AP000842.3 triggers the malignant progression
of prostate cancer by regulating cuproptosis related gene NFAT5.
Technol Cancer Res Treat. 23:153303382412555852024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Farhan M: Cytotoxic activity of the red
grape polyphenol resveratrol against human prostate cancer cells: A
molecular mechanism mediated by mobilization of nuclear copper and
generation of reactive oxygen species. Life (Basel).
14:6112024.PubMed/NCBI
|
|
117
|
Wang X, Chen X, Xu C, Zhou W and Wu D:
Identification of cuproptosis-related genes for predicting the
development of prostate cancer. Open Med (Wars). 18:202307172023.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cheng B, Tang C, Xie J, Zhou Q, Luo T,
Wang Q and Huang H: Cuproptosis illustrates tumor micro-environment
features and predicts prostate cancer therapeutic sensitivity and
prognosis. Life Sci. 325:1216592023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Jin L, Mei W, Liu X, Sun X, Xin S, Zhou Z,
Zhang J, Zhang B, Chen P, Cai M and Ye L: Identification of
cuproptosis-related subtypes, the development of a prognosis model,
and characterization of tumor microenvironment infiltration in
prostate cancer. Front Immunol. 13:9740342022. View Article : Google Scholar
|
|
120
|
Wang H, Xie M, Zhao Y and Zhang Y:
Establishment of a prognostic risk model for prostate cancer based
on Gleason grading and cuprotosis related genes. J Cancer Res Clin
Oncol. 150:3762024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yao K, Zhang R, Li L, Liu M, Feng S, Yan
H, Zhang Z and Xie D: The signature of cuproptosis-related immune
genes predicts the tumor microenvironment and prognosis of prostate
adenocarcinoma. Front Immunol. 14:11813702023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang J, Jiang S, Gu D, Zhang W, Shen X,
Qu M, Yang C, Wang Y and Gao X: Identification of novel molecular
subtypes and a signature to predict prognosis and therapeutic
response based on cuproptosis-related genes in prostate cancer.
Front Oncol. 13:11626532023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ma S, Xu M, Zhang J, Li T, Zhou Q, Xi Z,
Wang Z, Wang J and Ge Y: Analysis and functional validations of
multiple cell death patterns for prognosis in prostate cancer. Int
Immunopharmacol. 143(Pt 1): 1132162024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cheng X, Zeng Z, Yang H, Chen Y, Liu Y,
Zhou X, Zhang C and Wang G: Novel cuproptosis-related long
non-coding RNA signature to predict prognosis in prostate
carcinoma. BMC Cancer. 23:1052023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ma Z, Liang H, Cui R, Ji J, Liu H, Liu X,
Shen P, Wang H, Wang X, Song Z and Jiang Y: Construction of a risk
model and prediction of prognosis and immunotherapy based on
cuproptosis-related LncRNAs in the urinary system pan-cancer. Eur J
Med Res. 28:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jiang S, Li Z, Dou R, Lin Z, Zhang J,
Zhang W, Chen Z, Shen X, Ji J, Qu M, et al: Construction and
validation of a novel cuproptosis-related long noncoding RNA
signature for predicting the outcome of prostate cancer. Front
Genet. 13:9768502022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhong H, Lai Y, Ouyang W, Yu Y, Wu Y, He
X, Zeng L, Qiu X, Chen P, Li L, et al: Integrative analysis of
cuproptosis-related lncRNAs: Unveiling prognostic significance,
immune microenvironment, and copper-induced mechanisms in prostate
cancer. Cancer Pathog Ther. 3:48–59. 2024. View Article : Google Scholar
|
|
128
|
Ren L, Yang X, Wang W, Lin H, Huang G, Liu
Z, Pan J and Mao X: A cuproptosis-related LncRNA signature:
Integrated analysis associated with biochemical recurrence and
immune landscape in prostate cancer. Front Genet. 14:10967832023.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yu Z, Deng H, Chao H, Song Z and Zeng T:
Construction of a cuproptosis-related lncRNA signature to predict
biochemical recurrence of prostate cancer. Oncol Lett. 28:5262024.
View Article : Google Scholar
|
|
130
|
Lu Y, Wu J, Li X, Leng Q, Tan J, Huang H,
Zhong R, Chen Z and Zhang Y: Cuproptosis-related lncRNAs emerge as
a novel signature for predicting prognosis in prostate carcinoma
and functional experimental validation. Front Immunol.
15:14711982024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nyvltova E, Dietz JV, Seravalli J,
Khalimonchuk O and Barrientos A: Coordination of metal center
biogenesis in human cytochrome c oxidase. Nat Commun. 13:36152022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J
and Chen X: Copper metabolism in cell death and autophagy.
Autophagy. 19:2175–2195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Abramson J, Adler J, Dunger J, Evans R,
Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick
J, et al: Accurate structure prediction of biomolecular
interactions with AlphaFold 3. Nature. 630:493–500. 2024.
View Article : Google Scholar : PubMed/NCBI
|