Introduction
Cancer is a paramount societal, public health and
economic challenge in the twenty-first century, accounting for
nearly 16.8% of global deaths (one in six deaths) and 22.8% of
deaths attributable to non-communicable diseases (1). The disease is responsible for 3 in
10 premature deaths from non-communicable diseases worldwide (30.3%
among individuals aged 30-69 years), ranking amongst the three
principal causes of mortality within this demographic across 177 of
183 nations (2). In the United
States in 2025, there will be ~2,041,910 new cancer diagnoses,
equating to ~5,600 cases daily (3). Oncological research has
predominantly focused on intrinsic characteristics of neoplastic
cells, with comparatively limited attention to extracellular
stromal components. The extracellular matrix (ECM) constitutes a
crucial element of tumor stroma. Alterations in ECM protein
expression represent significant mechanisms influencing tumor cell
proliferation, migration and invasion, with Fibulin2 serving as a
typical protein. Even intracellular modifications in Fibulin2
expression may have a substantial impact on the ECM due to the
secretory properties of Fibulin2. Furthermore, Fibulin2 is
considered to be a promising biomarker in multi-tumor research
contexts (4-7).
Fibulin2 was initially characterized by Pan et
al (8), who identified it in
a murine fibroblast cDNA library using the Fibulin1 cDNA probe.
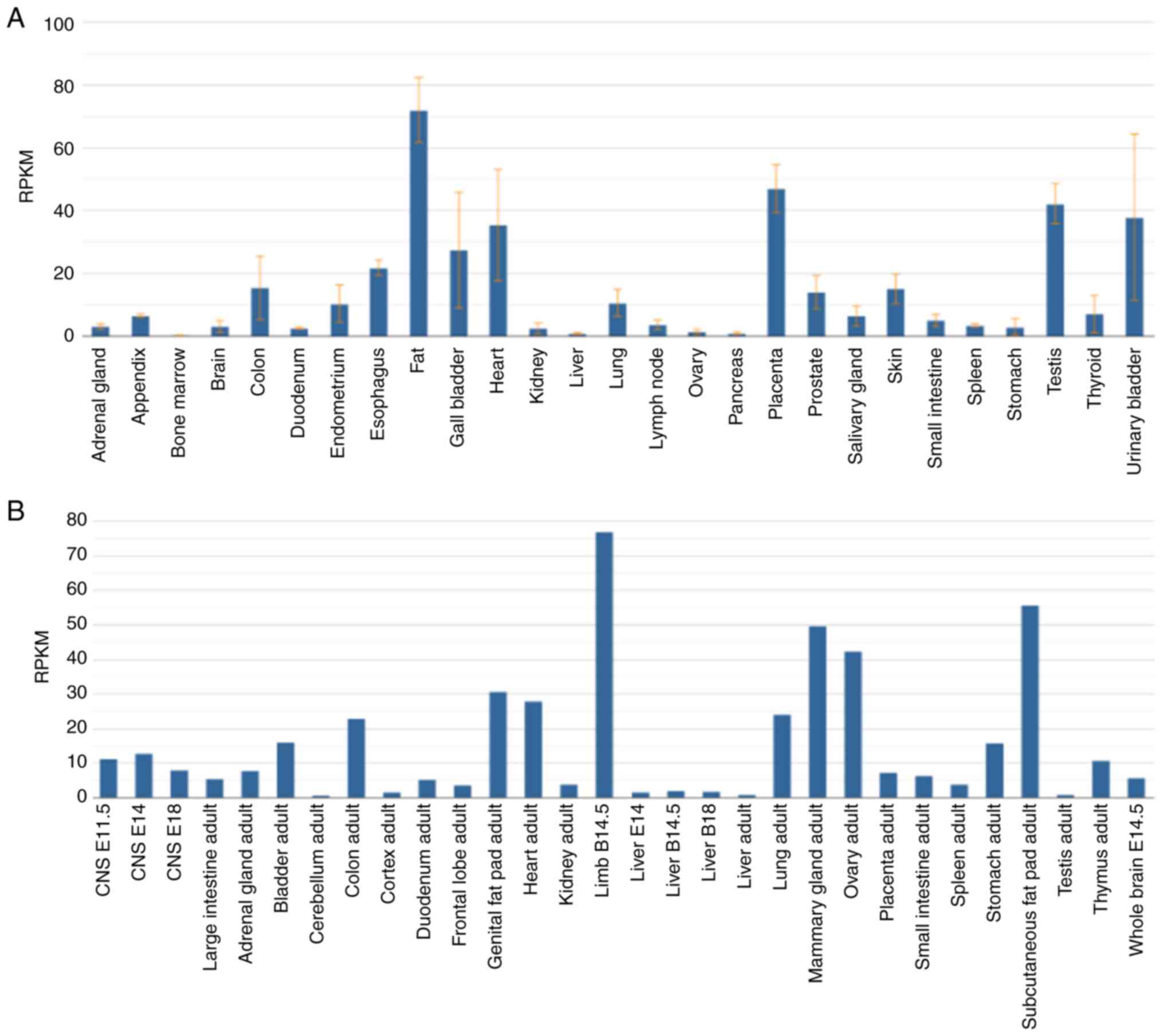
Fibulin2 is expressed in humans and mice (Fig. 1A and B). In human physiology,
Fibulin2 is highly expressed in cardiac tissue, placenta, testes
and the urinary bladder, but expressed at low levels in brain
tissue, splenic parenchyma and bone marrow (Fig. 1A). Fibulin2 exhibits aberrant
expression across malignancies of diverse organs (9), such as colorectal cancer (4) and lung cancer (10). The protein structures of human and
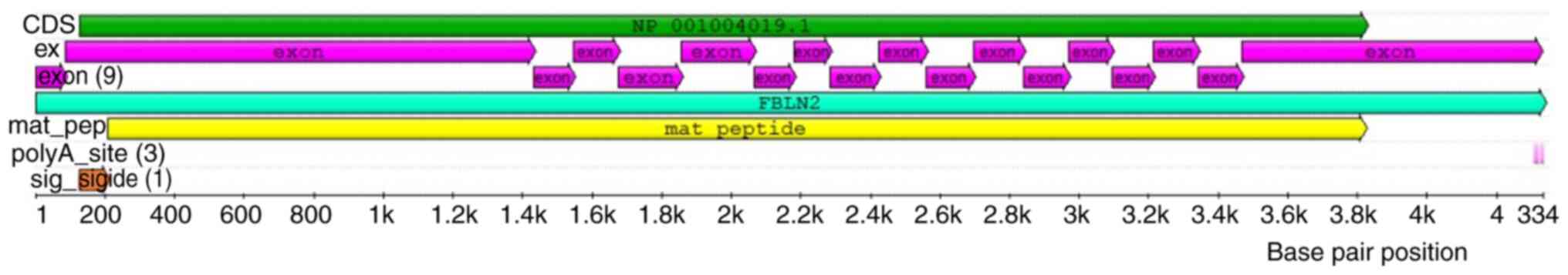
murine Fibulin2 exhibit remarkable homology (8,11)
(Fig. 2A and B), providing a
robust theoretical basis for using murine cellular systems and
animal models as surrogates for human studies in oncological
research. In the Na, I, II and III domains, the amino acid sequence
identity is ~90%, whereas the Nb domain shows only 62% sequence
identity (8,11). Human Fibulin2 contains 18 exons
(11) (Fig. 3), while mouse Fibulin2 contains 17
exons (8). Surface plasmon
resonance and/or solid-phase microplate analyses have shown that
Fibulin2 has high binding affinity for Perlecan (12,13), laminin5 (14,15), Fibronectin (16), collagen XVIII (17), Aggrecan, Versican, Brevican lectin
domains (18), the C-terminal
region V of Perlecan (19), and
the short arms of Laminin-5 and Laminin-1 (20), which helps maintain the ECM
(21). Consequently, ECM
stabilization mediated by Fibulin2 represents a pivotal mechanism
underlying tumor suppression.
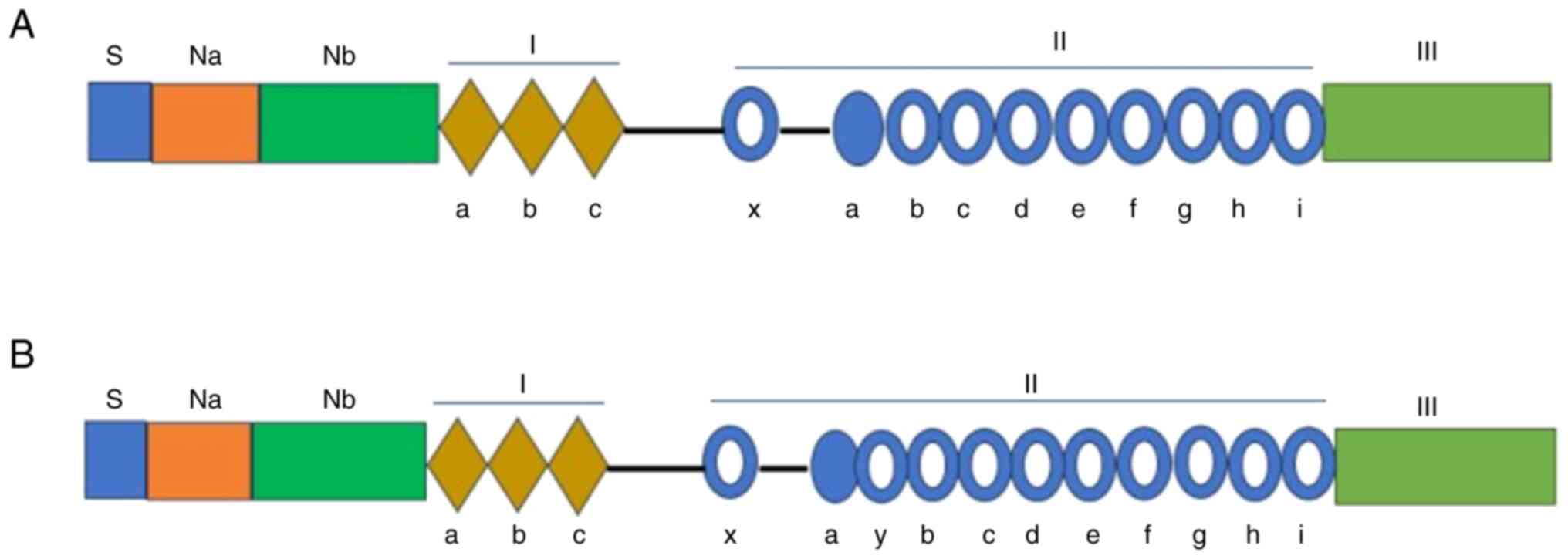 | Figure 2Protein structure of human Fibulin2
and mouse Fibulin2. (A) Diagram of the protein domains of human
Fibulin2 starting with the S, Na and Nb subdomains, followed by
domain I, II and III. Diamonds indicate anaphylatoxin-like motifs
in domain Ⅰ. Solid circles represent EGF-like repeats, while those
depicted as hollow rounds contain the consensus sequence for
calcium binding. (B) Diagram of the protein domains of mouse
Fibulin2 showing that the Fibulin2 protein comprises S, Na, Nb, I,
II and III domains. At the N-terminus are domains Na and Nb, which
span 408 amino acids, and include the 'Na' subdomain containing 22
cysteines and the 'Nb' subdomain devoid of cysteine. Domain I
consists of three anaphylatoxin-like motifs. Domain II encompasses
11 EGF-like repeats, all of which except IIa possess a consensus
sequence for calcium binding. Domain I is connected via a
50-amino-acid linker segment to the first EGF module (IIx) of
domain II, which is followed by another 34-amino-acid linker
segment and then 10 EGF modules. Domain III is depicted as a light
green box. Na, N-terminal cysteine-rich; Nb, N-terminal
cysteine-free; S, signal peptide. |
Nevertheless, Fibulin2 does not universally exert
inhibitory effects across all malignancies; rather, it acts as a
promoter in certain neoplastic contexts. For instance, increased
Fibulin2 expression inhibits cell proliferation in nasopharyngeal
carcinoma (22), Kaposi's sarcoma
(23) and breast cancer (24). Conversely, suppression of Fibulin2
expression effectively attenuates lung adenocarcinoma progression
(10). Therefore, the present
review summarizes the diverse roles of Fibulin2 across various
malignancies, while examining underlying mechanisms, alongside
comprehensive analysis of the potential of Fibulin2 as a biomarker.
Regarding the mechanistic influence of Fibulin2 on tumor
development and its utility as a tumor marker, several critical
issues persist. The mechanisms governing Fibulin2 function across
numerous malignancies require further elucidation. The role and
mechanistic basis of the effects of Fibulin2 on tumor stroma remain
poorly understood. Furthermore, its clinical application as a
biomarker is not yet mature. Therefore, the present overview
provides guidance for the clinical translation of Fibulin2 as both
a therapeutic target and predictive marker in oncological
diseases.
Summary of the mechanisms and roles of
Fibulin2
Fibulin2 acts as an upstream regulator of the
Ras-MEK-ERK1/2 pathway in hepatocellular carcinoma and the TGF-β/
Smad2/TGFB induced factor homeobox 2 (TGIF2) pathway or β-catenin
in gastric cancer (25-27). Differences in Fibulin2 expression
between tumor tissues and adjacent normal tissues lead to the
activation of these pathways (25,26). However, to the best of our
knowledge, the factors causing altered Fibulin2 expression in liver
and gastric cancer, as well as its upstream regulators, remain
unclear. In p53-null immortalized mouse keratinocytes and
RasV12-transformed mouse keratinocytes, which are skin cancer
models, Fibulin2 is regulated by the upstream molecule Integrin
α3β1 (28). Changes in
Fbln2 expression in lung adenocarcinoma cells alter the
expression of downstream Integrin genes, including Itga1,
Itga2, Itga10 and Itgb1 (10). A reciprocal regulatory
relationship may exist between Fibulin2 and certain Integrin
proteins, which warrants further investigation. In nasopharyngeal
carcinoma, Fbln2 acts as an upstream inhibitor of
VEGF-165, VEGF-189 and MMP-2. The upstream
factors regulating Fbln2 expression in nasopharyngeal
carcinoma remain to be elucidated (22). Given that Fibulin2 is a secreted
protein, it likely exerts autocrine or paracrine effects on its
producing cells or neighboring cells, potentially binding to
receptors such as Integrin proteins to establish feedback loops.
However, such feedback mechanisms have not received sufficient
attention in current tumor-related studies, and further research is
needed to demonstrate their existence across various tumor types.
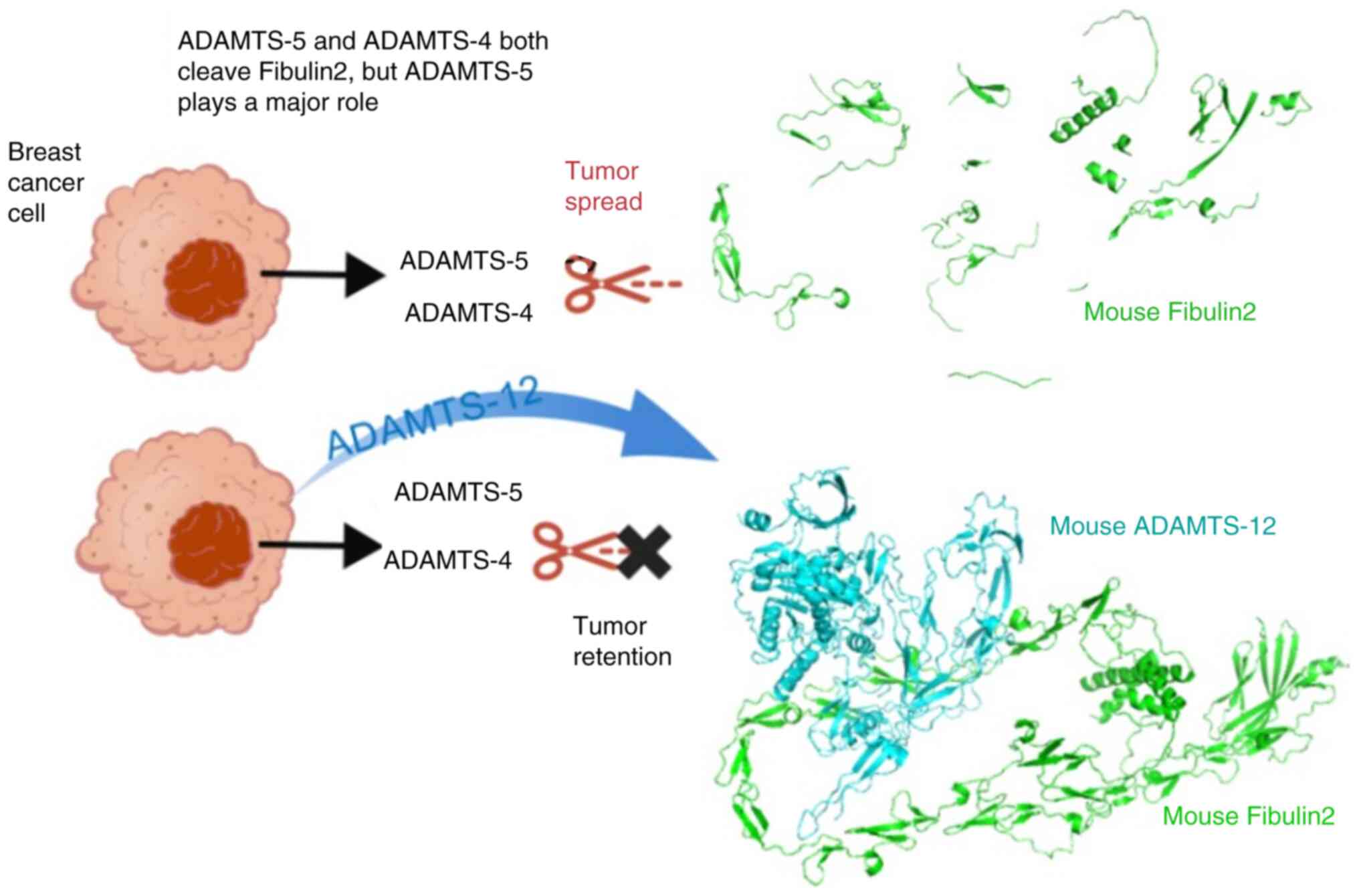
In the extracellular space, Fibulin2 interacts with multiple
proteins: Integrin β1 in non-small cell lung cancer (29), a disintegrin and metalloproteinase
with thrombospondin motifs (ADAMTS)-5 and ADAMTS-12 in breast
cancer (30), Nidogen1 in
colorectal cancer (CRC) (31),
and mucin 4 (MUC4) in pancreatic cancer (32). These are protein-protein
interactions rather than upstream-downstream regulatory
relationships (Fig. 4). In breast
cancer, ADAMTS-5 cleaves Fibulin2, and this role can be inhibited
by ADAMTS-12 (30). Differences
in mechanisms of Fibulin2 regulating tumor development across tumor
types are shown in Table I.
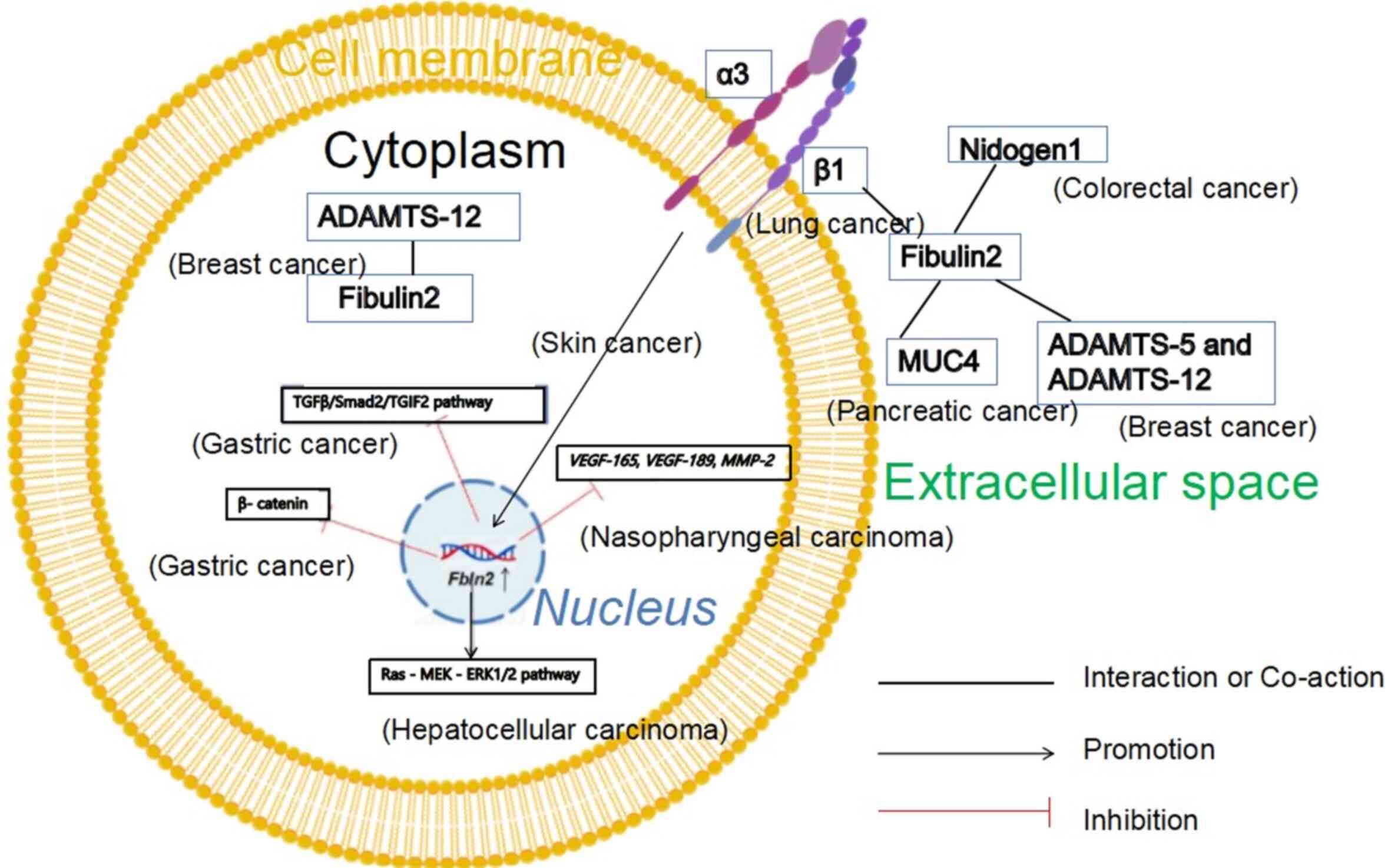 | Figure 4Mechanistic roles of Fibulin2 in
various tumors. The yellow circle denotes the phospholipid bilayer,
and the small blue circle inside represents the nucleus. Solid
lines indicate an interaction or co-action relationship between
molecules. Arrow-headed lines represent a positive promoting
relationship between molecules, and red lines denote an inhibitory
relationship between molecules. Overexpression of Fbln2 in
gastric cancer cells results in diminished expression of β-catenin,
phosphorylated-Smad2 and TGIF2 in the TGF-β/Smad2/TGIF2 pathway. In
liver cancer cells, overexpression of Fbln2 activates the
Ras-MEK-ERK1/2 pathway. In nasopharyngeal carcinoma cells,
overexpression of Fbln2 leads to decreased expression of
VEGF-165, VEGF-189 and MMP-2. Overexpression
of ADAMTS-12 and Fibulin2 in breast cancer cells can inhibit tumor
development. Fibulin2 and Integrin β1 exhibit partial
colocalization near the cell membrane in non-small cell lung cancer
tissue sections. As secreted proteins, Fibulin2 and Nidogen1
collectively promote the development of CRC. The interaction
between MUC4 and Fibulin2 promotes the development of pancreatic
cancer. ADAMTS-5, ADAMTS-12 and Fibulin2 are secreted proteins. In
breast cancer, ADAMTS-5 cleaves Fibulin2, and this role can be
inhibited by ADAMTS-12. BioGDP.com was
used to generate figures (https://biogdp.com/?tg=CFXL). ADAMTS, a disintegrin
and metalloproteinase with thrombospondin motifs; MUC4, mucin 4;
TGIF2, TGFB induced factor homeobox 2. |
 | Table IDifferences in mechanisms of Fibulin2
regulating tumor development across tumor types. |
Table I
Differences in mechanisms of Fibulin2
regulating tumor development across tumor types.
A, Pro-carcinogenic
|
|---|
| First author/s,
year | Tumor type | Mechanism | (Refs.) |
|---|
| Baird et al,
2013 | Lung
adenocarcinoma | Fbln2
promotes the adhesion of tumor cells to type I collagen and the
expression of Itga1, Itga2, Itga10 and
Itgb1 | (10) |
| Vaes et al,
2021 | CRC | Fibulin2 and
Nidogen1 jointly promote tumor development | (31) |
| Hu et al,
2023 | Hepatocellular
carcinoma | Fibulin2
facilitates the activation of the Ras-MEK-ERK1/2 signaling
pathway | (25) |
| Missan et
al, 2014 |
Immortalized/transformed
keratinocytes | Fibulin2 expression
is regulated by Integrin α3β1 | (28) |
|
| B,
Anti-carcinogenic |
|
| First author/s,
year | Tumor type | Mechanism | (Refs.) |
|
| Law et al,
2012 | Nasopharyngeal
carcinoma | Fbln2
inhibits the expression of VEGF-165, VEGF-189 and
MMP-2 | (22) |
| Ma et al,
2019 | Gastric cancer | Fibulin2
downregulates β-catenin and its downstream c-myc and cyclin D1 | (26) |
| Zhou et al,
2025 | Gastric cancer | Fibulin2 inhibits
the TGF-β/Smad2-TGIF2 pathway | (27) |
| Senapati et
al, 2012 | Pancreatic
cancer | MUC4 interacts with
Fibulin2, disrupting the intact BM and promoting tumor
development | (32) |
| Fontanil et
al, 2017; Fontanil et al, 2014 | Breast cancer | Fibulin2 and
ADAMTS-12 jointly inhibit tumor development; ADAMTS-5 cleaves
Fibulin2, promoting tumor development | (30,80) |
Roles and mechanisms of Fibulin2 in
promoting various tumor types
In the enteric nervous system of patients with
colorectal carcinoma, Ndrg4 expression is absent or markedly
reduced (33), while enteric
nerve cells concurrently secrete higher levels of Fibulin2.
Fibulin2 and Nidogen1 promote human colorectal carcinoma cell
proliferation and migration. A study substantiated its conclusions
by stimulating cells with Fibulin2 and Nidogen1 proteins, then
assessing cell proliferation and migration (31). However, the study failed to
demonstrate the isolated effects of Fibulin2 on CRC cells and
mechanisms (31). In an
experiment investigating subcutaneous tumor formation in lung
adenocarcinoma, tumors were generated in mice by injecting tumor
cells in PBS subcutaneously into the right flank. Tumors formed by
Fbln2-knockdown cells exhibited reduced size, weight and
tissue consistency compared with those formed by normal cells
(10). In vitro
experiments revealed that Fbln2-knockdown cells, compared
with normal cells, exhibited markedly attenuated type I collagen
adhesion capacity alongside markedly reduced tumor cellular
pseudopodia (10). Itga1,
Itga2, Itga10 and Itgb1 were notably reduced
following Fbln2 depletion in lung adenocarcinoma cells
(10). These genes encode
Integrin proteins α1β1, α2β1, α10β1 and α11β1, respectively, which
can bind to collagen (34). In
summary, Fibulin2 is closely associated with other ECM proteins in
lung adenocarcinoma. Aberrant Fibulin2 expression leads to abnormal
changes of other ECM proteins such as integrin proteins and
collagen (10).
Immunohistochemistry revealed Fibulin2 upregulation in lung cancer
tissues compared with adjacent normal tissues. In vitro
validation relied exclusively on Fbln2 knockdown.
Incorporating overexpression experiments would enhance
methodological rigor (10). In
p53-null immortalized mouse keratinocytes and RasV12-transformed
mouse keratinocytes, knockdown of Fbln2 diminished cell
invasion; however, subcutaneous injection of Fbln2-knockdown
cells failed to produce a notable reduction in tumor growth volume
compared with controls (28).
Fibulin2 expression was regulated by Integrin α3β1 and promoted the
invasion of immortalized/transformed keratinocytes (28). The research was limited by overly
simplistic mechanistic exploration, necessitating further
investigation of more specific mechanisms. In mouse non-small cell
lung cancer tissue sections, ECM-expressed Fibulin2 interacts with
cell membrane-expressed Integrin β1. The interaction between
Integrin β1 and Fibulin2 mediates tumor cellular adhesion to the
ECM while conferring chemotherapy drug resistance (29). The study employed
immunofluorescence double-labeling experiments to demonstrate
partial colocalization of Integrin β1 and Fibulin2, subsequently
inferring disease mechanisms (29). Additional experiments are
necessary, including experiments verifying whether Integrin β1 is a
receptor of Fibulin2 and assessing its impact on tumors. In human
hepatocellular carcinoma cells, Fibulin2 promotes carcinoma cell
proliferation while inhibiting apoptosis through Ras-MEK-ERK1/2
signaling pathway activation (25). In in vitro experiments on
liver cancer cells, Fbln2 knockdown and overexpression were
performed, and the expression changes of key proteins in the
Ras-MEK-ERK1/2 signaling pathway were detected by western blotting
(WB) (25). Fibulin2 exerts
tumor-promoting effects in both in vitro and in vivo
experiments (25). However, this
merely establishes an association between Fibulin2 and the
Ras-MEK-ERK1/2 signaling pathway rather than a direct regulatory
relationship, as indirect mechanisms may exist. Future research
could use glutathione S-transferase (GST) pull-down to further
verify the direct interactions between Fibulin2 and these pathway
proteins. Fibulin2 expression alterations within neoplastic tissues
and corresponding modifications in tumor cell proliferation,
migration and invasion (demonstrating the pro-carcinogenic
properties of Fibulin2) are shown in Table II.
 | Table IIChanges in Fibulin2 expression in
tumor tissue and changes in tumor cell proliferation, migration and
invasion (Fibulin2 exerts a pro-carcinogenic effect). |
Table II
Changes in Fibulin2 expression in
tumor tissue and changes in tumor cell proliferation, migration and
invasion (Fibulin2 exerts a pro-carcinogenic effect).
| First author/s,
year | Tumor | Tumor vs. non-tumor
tissue | Fibulin2
expression | Proliferation | Migration | Invasion | (Refs.) |
|---|
| Vaes et al,
2021 | CRC | Carcinoma vs.
paracarcinoma | Upregulated | Increased after
Nidogen1 and Fibulin2 stimulation | Increased after
Nidogen1 and Fibulin2 stimulation | | (31) |
| Baird et al,
2013 | Lung
adenocarcinoma | Carcinoma vs.
paracarcinoma | Upregulated | Decreased after
knockdown of Fbln2 | Decreased after
knockdown of Fbln2 | Decreased after
knockdown of Fbln2 | (10) |
| Hu et al,
2023 | Hepatocellular
carcinoma | Carcinoma vs.
paracarcinoma | Upregulated | Decreased after
knockdown of Fbln2; increased after overexpression of
Fbln2 | | | (25) |
Fibulin2 is associated with Integrin proteins in
mechanistic investigations of lung adenocarcinoma (10), non-small cell lung carcinoma
(29), p53-null immortalized
murine keratinocytes and RasV12-transformed murine keratinocytes
(28). In a hepatocellular
carcinoma study, the authors did not study Integrin proteins
(25), the Ras-MEK-ERK1/2
signaling pathway constitutes a crucial downstream pathway of
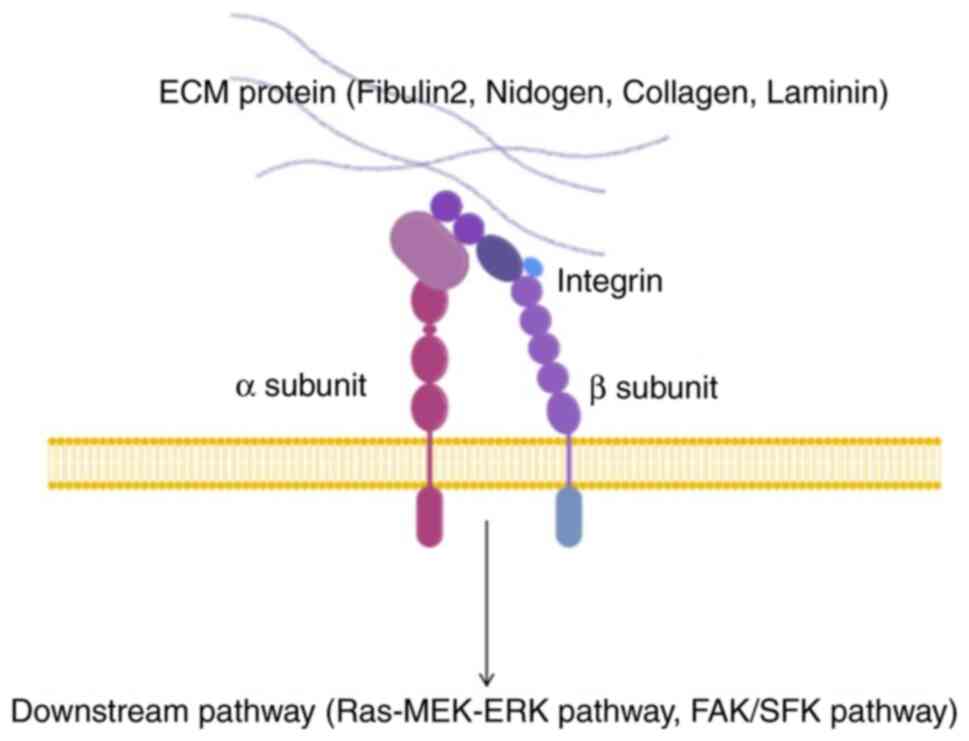
Integrin according to previous investigations (35-37). Integrins, as principal ECM
cell-surface receptors, represent essential mediators of cellular
communication with the tumor microenvironment (TME) (35,38,39). Integrins comprise a family of 24
heterodimeric receptors (40).
Integrins consist of two subunits: α and β subunits, which have
extensive extracellular domains and short cytoplasmic domains
(41,42). These interact with ECM proteins
outside the cell membrane while connecting to downstream pathways,
including the Ras-MEK-ERK1/2 and FAK/SFK pathways in the cytoplasm
(35). When ECM protein,
including Fibulin2, Nidogen, Collagen and Laminin, or Integrin
expression is altered, fibrosis or remodeling can change tissue
stiffness, and downstream Integrin pathways such as the
Ras-MEK-ERK1/2 and FAK/SFK pathways may be activated, affecting
tumor migration, invasion and proliferation (35,43,44) (Fig.
5).
Limitations
Most studies examining the tumor-promoting functions
of Fibulin2 are simplistic. Future research may include additional
experiments to enhance persuasiveness. Furthermore, other factors
impact future drug development. For example, studies have used
immortalized cell lines such as the HCT116 and Caco-2 human CRC
cell lines (31), and mouse
models such as nude mice subcutaneously injected with SNU398 cells
(25). These models differ
substantially from primary cells and the human body, potentially
leading to the failure of related drugs in human applications.
Inferring disease mechanisms solely from protein spatial
associations, exemplified by partial Integrin β1 and Fibulin2
colocalization in lung carcinoma tissue sections (29), may fail to establish causative
relationships, resulting in ineffective therapeutic targets.
Focusing on single signaling pathways, such as the Ras-MEK-ERK1/2
signaling cascade in hepatocellular carcinoma (25), while disregarding other potential
pathways, may lead to compensatory pathway activation and
therapeutic resistance after single-target inhibition, thereby
limiting the applicability of conclusions. To the best of our
knowledge, the impact of changes in Fibulin2 expression on tumor
stroma activity [which depends on cancer-associated fibroblasts
(CAFs)] has not been investigated. Tumor stromal activity and
changes in CAF properties substantially influence tumor
development. In advanced-stage malignancies, activated stroma
promotes further genetic and epigenetic alterations in carcinoma
cells (45-47) while supporting carcinoma
progression (48). CAFs are one
of the cell types in the TME. CAFs exhibit proliferative, migratory
and high secretory activities (49,50). Due to their altered morphology
[multispindled rather than single-spindled (51)] and Acta2 and prolyl
endopeptidase expression, CAFs are frequently referred to as
myofibroblasts (50). CAFs
secrete ECM factors, including tenascin, periostin, secreted
protein acidic and rich in cysteine, and collagens (51-58). The expression levels of MMPs and
other enzymes that degrade and metabolize the ECM are increased in
CAFs, enabling cell penetration through the ECM (59-61). CAFs also secrete high levels of
growth factors, cytokines and chemokines, promoting intrinsic
malignant hallmarks of carcinoma cells through autocrine and
paracrine mechanisms (62). In
these studies where Fibulin2 had a tumor-promoting effect (10,25,28,31), while Fibulin2 was secreted by
tumor cells and acted on the stroma, it remained unclear how the
stroma reciprocally affects tumor cells. Whether tumor cell
secretion of Fibulin2 impacts CAFs requires further
exploration.
Roles and mechanisms of Fibulin2 in
inhibiting various tumor types
Alterations in Fibulin2 expression in tumor tissues
and corresponding changes in tumor cell proliferation, migration
and invasion (demonstrating the anti-carcinogenic properties of
Fibulin2) are summarized in Table
III. In human gastric cancer, Fibulin2 expression in malignant
tissues is reduced compared with that in adjacent normal tissues,
and is negatively associated with β-catenin (26). β-catenin is a bifunctional protein
with both cell adhesion and signal transduction activities, and was
initially identified through a study of the cell adhesion molecule
E-cadherin (63). β-catenin is an
essential component of the Wnt/β-catenin signaling pathway, serving
a pivotal role in gastric carcinoma pathogenesis and progression
(64). A study has established
direct or indirect regulatory relationships between the Fibulin
family and β-catenin, with both substantially influencing gastric
carcinoma development (65).
β-catenin is typically localized in the cytoplasm, with its
expression suppressed through ubiquitin/proteasome-mediated protein
degradation (66). Following
construction of Fbln2 overexpression plasmids and
transfection of them into AGS and SGC-790 human gastric cancer cell
lines, WB analysis has confirmed that Fibulin2 overexpression
downregulated β-catenin and its downstream c-myc and cyclin D1,
thereby inhibiting the proliferation of gastric cancer cells
(26). Immunohistochemistry
demonstrated downregulation of Fibulin2 in gastric cancer tissues
compared with adjacent normal tissues (26). In vitro validation relied
exclusively on Fbln2 overexpression, and incorporation of
knockdown experiments would enhance methodological rigor (26). Another study has confirmed that
reduced Fibulin2 expression in gastric carcinoma tissues promotes
tumor cell proliferation and metastasis through activation of the
TGF-β/Smad2/TGIF2 pathway (27).
The study used normal and Fbln2-knockdown human gastric
cancer cell lines for RNA sequencing, demonstrating upregulation of
the TGF-β signaling pathway in Fbln2-knockdown gastric
cancer cells (27). Following
Fbln2 knockdown and overexpression in gastric carcinoma
cells, WB analysis revealed corresponding increases and decreases
in Smad2 and TGIF2 phosphorylation (27). Addition of TGF-β signaling pathway
inhibitor reversed these expression changes (27). The specific mechanism by which
decreased Fbln2 expression in cells alters the activity of
the TGF-β pathway requires further exploration. This establishes an
association rather than a direct regulatory relationship between
Fibulin2 and the TGF-β/Smad2/TGIF2 pathway, as indirect mechanisms
cannot be excluded. Future research could use GST pull-down to
demonstrate direct interactions between Fibulin2 and pathway
proteins.
 | Table IIIChanges in Fibulin2 expression in
tumor tissue and changes in tumor cell proliferation, migration and
invasion (Fibulin2 exerts an anti-carcinogenic effect). |
Table III
Changes in Fibulin2 expression in
tumor tissue and changes in tumor cell proliferation, migration and
invasion (Fibulin2 exerts an anti-carcinogenic effect).
| First author/s,
year | Tumor | Tumor vs. non-tumor
tissue | Fibulin2
expression | Proliferation | Migration | Invasion | (Refs.) |
|---|
| Law et al,
2012 | Nasopharyngeal
carcinoma | Nasopharyngeal
carcinoma biopsy tissue vs. non-tumor tissue | Downregulated | Decreased after
Fbln2s overexpression | Decreased after
Fbln2s overexpression | Decreased after
Fbln2s overexpression | (22) |
| Ren et al,
2016 | Glioma | | | Decreased after
Fibulin2 overexpression | Decreased after
Fibulin2 overexpression | Decreased after
Fibulin2 overexpression | (7) |
| Zhang et al,
2020 | Breast cancer | | | Decreased after
Fibulin2 overexpression | Decreased after
Fibulin2 overexpression | Decreased after
Fibulin2 overexpression | (24) |
| Alcendor et
al, 2011 | Kaposi's
sarcoma | Carcinoma tissue
vs. paracarcinoma tissue | Downregulated | | | | (23) |
| Ma et al,
2019 | Gastric cancer | Carcinoma tissue
vs. paracarcinoma tissue | Downregulated | Decreased after
Fibulin2 overexpression | | | (26) |
Kaposi's sarcoma, which originates from human
vascular endothelial cells, is characterized by vascular
proliferation caused by HIV infection of endothelial cells
(23). In cutaneous microvascular
endothelial cells after 10 days of infection, Fibulin2 protein and
mRNA expression are decreased by 50- and 26-fold, respectively.
Simultaneously, mRNA levels of the ECM-binding partners fibronectin
and tropoelastin are decreased 5- and 25-fold, respectively. This
weakens the binding of Fibulin2 to ECM proteins, including
fibronectin and tropoelastin, compromising basement membrane (BM)
stability and promoting tumor progression (23). Fibronectin is crucial for numerous
cell functions, including migration, proliferation and
differentiation (67).
Transcriptional downregulation of fibronectin has been associated
with highly metastatic breast carcinoma cells in murine models
(68). Tropoelastin is the
soluble precursor of elastin, inducible by ultraviolet irradiation
and degradable by MMP-12 (69).
Loss of tropoelastin causes developmental tissue disorders,
including aneurysms, atherosclerosis and reduced skin elasticity
(70). Tropoelastin and
fibronectin are essential ECM proteins critical for wound healing
(71). The study investigating
Kaposi's sarcoma failed to establish causative relationships
between Fibulin2 alterations and changes in fibronectin or
tropoelastin (23). Future
research should include cellular Fbln2 knockdown and
overexpression for validation.
In astrocytoma research, U251 cells (a human glioma
cell line) exhibited reduced migration and invasion following
Fibulin2 overexpression. The conclusion that Fibulin2 inhibits
astrocytoma was confirmed by comparing Fbln2-overexpressing
U251 cells with negative controls transfected with empty vectors
(7). A previous study revealed
that across different astrocytoma grades, Fibulin2 inhibits tumor
development (7). The authors
suggested an ECM-stabilizing function of Fibulin2; however,
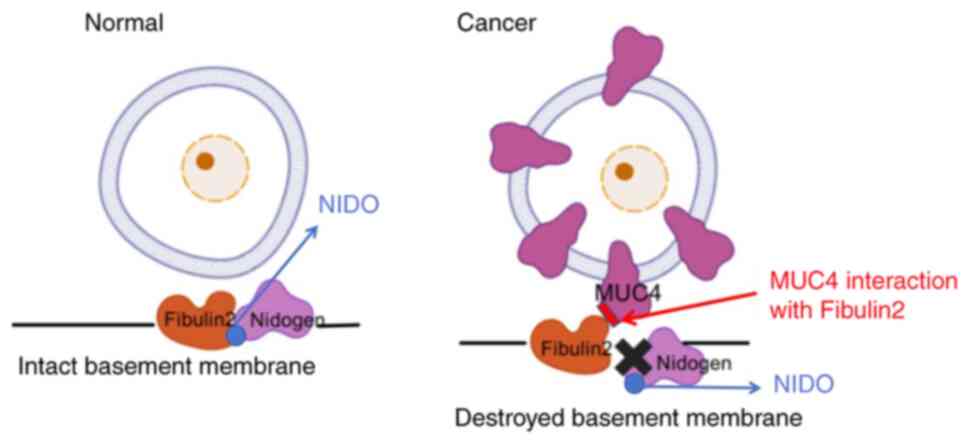
experimental verification is required (7). During pancreatic carcinoma
development, MUC4 protein expression is markedly upregulated on
pancreatic carcinoma cell surfaces and MUC4 binds to Fibulin2
(32). This binding interferes
with the normal interaction between Fibulin2 and the G1 domain of
Nidogen (NIDO), disrupting BM integrity (32) (Fig.
6). Tumor cells consequently breach the BM more readily,
facilitating invasion and metastasis. In pancreatic cancer tissue
sections, immunofluorescence double-labeling experiments have shown
colocalization of MUC4 and Fibulin2 (32). However, immunofluorescence
double-labeling experiments merely suggest potential connections
between these proteins and do not convincingly demonstrate the
roles of MUC4 and Fibulin2 in pancreatic carcinoma (32). Future studies may use conditional
knockout of MUC4 and Fibulin2 in murine models for further
validation. Additionally, numerous previous articles and reviews
have erroneously characterized Fibulin2 as an oncogenic protein in
pancreatic carcinoma when citing the conclusions of this study
about pancreatic carcinoma. The original study provides no evidence
to suggest that Fibulin2 promotes pancreatic carcinoma metastasis
and invasion (32).
Breast carcinoma is a prevalent malignancy in women
with a high incidence (72). In a
breast carcinoma study, transfection of Fbln2 plasmids into
human breast carcinoma cell lines reduced tumor cell migration and
invasion compared with those of negative control lentiviral vector
groups (24). Fibulin2 expression
is reduced in breast carcinoma tissues compared with adjacent
normal tissues (73). Knockdown
of Fbln2 is associated with disruption of the type IV
collagen sheath surrounding breast cells in vitro (74); Ibrahim et al (74) hypothesized that its downregulation
may be associated with BM disruption and early invasion. However,
the BM composition is complex, precluding exclusion of compensation
by other Fibulin family members (such as Fibulin1 and Fibulin5) or
ECM proteins, which requires demonstration (75). A study involving 272 patients with
breast carcinoma from a Norwegian hospital found a positive
association between elevated perivascular Fibulin2 expression in
breast carcinoma and survival rates (76). Elevated Fibulin2 expression was
associated with luminal breast carcinoma, pronounced elastic tissue
hyperplasia in the tumor stroma and favorable prognosis, while
reduced expression was associated with the basal-like phenotype,
triple-negative breast carcinoma, interval breast carcinoma,
vascular invasion and poor prognosis (76). The study lacked mechanistic
exploration and only reported observed associations.
The TME is currently being investigated as a novel
therapeutic target for cancer. ADAMTS is secreted by carcinoma and
stromal cells, potentially modifying the TME through various
mechanisms, thereby promoting or inhibiting tumors (77). ADAMTS-12 is a secreted
metalloproteinase that has functions in tissue remodeling and cell
migration or adhesion (78,79). In 293-EBNA cells, yeast two-hybrid
screening and co-immunoprecipitation experiments demonstrated that
the carboxyl-terminal region of Fibulin2 interacts with the spacer
region of ADAMTS-12 (80). When
Fibulin2 and ADAMTS-12 were concurrently overexpressed in breast
cancer cells, they reduced cell invasion and migration in
vitro, inhibited cell migration on relevant matrices, decreased
mammosphere unit formation, and suppressed tumor growth in
vivo (80). Fibulin2 and
ADAMTS-12 expression in breast cancer tissue was negatively
associated with histopathological tumor stage, with patients
exhibiting the best prognosis when both were highly expressed
(80). ADAMTS-4 and ADAMTS-5 are
members of the ADAMTS secreted metalloproteinase family (81). A previous study has indicated that
both ADAMTS-5 and Fibulin2 are expressed in the stromal and
epithelial components of breast cancer tissue, with
immunofluorescence double staining showing partial colocalization
(30). ADAMTS-4 and ADAMTS-5,
predominantly ADAMTS-5, can specifically cleave Fibulin2, enhancing
breast carcinoma cell migration and invasion, while increasing the
tumorigenic potential (30)
(Fig. 7). The study compared the
invasion of breast cancer cell lines with simultaneous
overexpression of ADAMTS-5 and Fibulin2, breast cancer cell lines
with overexpression of ADAMTS-5 or Fibulin2 alone and the control
group (30). The invasion
capacity of MCF-7 cells was increased by the simultaneous
overexpression of Fibulin-2 and ADAMTS-5 compared with the control
group and overexpression of Fibulin2 alone (30). However, the invasion capacity of
MCF-7 cells was decreased by the simultaneous overexpression of
Fibulin-2 and ADAMTS-5 compared with overexpression of ADAMTS-5
alone (30). ADAMTS-5-mediated
Fibulin2 degradation is inhibited by ADAMTS-12 (Fig. 7) (30). However, in vivo experiments
only showed partial colocalization of ADAMTS-5 or ADAMTS-12 with
Fibulin2 via immunofluorescence or immunohistochemistry, which is
insufficient to establish their relationship with breast cancer
(30). Future research using
conditional gene-knockout mice to substantiate the relationship
between these proteins and breast cancer would be more compelling.
When mammary fibroblasts are cultured in medium containing excess
Fibulin2 and ADAMTS-5, the expression levels of α-smooth muscle
actin (α-SMA) increase. Stimulation with excess Fibulin2 alone can
also increase α-SMA expression levels, as observed by WB (the
authors did not conduct statistical analysis), indicating that
Fibulin2 promotes breast fibroblast activation (30). Breast fibroblasts are a key
component of the tumor stroma (82). This indicates that changes in
Fibulin2 expression in breast cancer cells can activate stromal
fibroblasts and potentially drive their differentiation into CAFs,
a phenomenon closely related to the secretory properties of
Fibulin2 (30). Similar
mechanisms may occur in other tumor types; however, they have
received limited attention to date and warrant further
investigation.
Due to the opposing roles of different ADAMTS
subtypes in breast cancer, developing highly specific inhibitors
that selectively block the tumor-promoting activities of ADAMTS
subtypes without affecting their potential tumor-suppressive
effects remains challenging. Small-molecule inhibitors targeting
aggrecanases, including ADAMTS-4 and ADAMTS-5, have advanced to a
phase III clinical trial for orthopedic applications (83). This provides valuable insights for
the development of ADAMTS-targeted therapeutics for breast cancer
treatment.
Cancer progression is accompanied by uncontrolled
tumor growth, local invasion and metastasis; processes that depend
heavily on the proteolytic activities of multiple MMPs. These
enzymes affect tissue integrity by degrading ECM components such as
Fibulin2 (84). In a
nasopharyngeal carcinoma study involving samples from 30 patients,
Fbln2 levels in carcinoma tissues substantially exceeded
those in normal tissues, as detected by gene chip technology
(22). In nasopharyngeal cancer
cell lines, overexpression of Fbln2s, which encodes the
short isoform of Fibulin2, downregulated the expression levels of
VEGF-165, VEGF-189 and MMP-2 compared with
those in the control group (22).
Sustained angiogenesis is indispensable for both tumor growth and
metastasis (85). MMP-2 is an
effective gelatinase capable of cleaving protein components of the
ECM and is involved in the invasion and metastasis process of tumor
cells (86). Evidence has
indicated that Fibulin2 is cleaved by MMP-2 (87), and inhibits nasopharyngeal
carcinoma cell migration and invasion by strictly regulating
MMP-2 expression (22).
However, the study could not establish a direct regulatory
relationship between Fbln2 and VEGF-165,
VEGF-189 or MMP-2, because there may be unknown
molecules or signaling pathways mediating the interaction between
Fbln2 and VEGF-165, VEGF-189 or MMP-2.
WB analysis of osteosarcoma cell line lysates has indicated
multiple Fibulin2 fragments (87). Gelatinase MMP-2 facilitates
Fibulin2 degradation through MMP-dependent mechanisms in
osteosarcoma cell lines (87).
Following addition of an MMP-2 inhibitor to the cell culture
medium, WB indicated no change in the cleaved Fibulin2 fragments,
indicating that unidentified mechanisms require exploration
(87). This study using an
osteosarcoma cell line relied entirely on in vitro
experiments, and in vivo experiments are required for
verification, as the in vivo environment is more complex
than the in vitro cell culture environment (87).
Most documented MMP inhibitors exhibit non-specific
binding and have diminished efficacy, attributable to their
pronounced sequence homology with other MMPs (88). To date, no effective MMP inhibitor
has successfully completed clinical trials and secured regulatory
approval from the Food and Drug Administration for tumor treatment
(84,88).
Limitations
Studies on the tumor-inhibitory effect of Fibulin2
predominantly focus on preserving BM structural integrity (7,32,74). Few studies have investigated the
mechanisms of interaction between Fibulin2 and classical signaling
pathways. Only the association among Fibulin2, β-catenin and the
TGF-β/Smad2/TGIF2 pathways has been demonstrated in gastric cancer
studies (26,27). An intricate association exists
between the β-catenin and TGF-β signaling pathways and the
aforementioned Integrins (89,90). Whether Fibulin2 indirectly
regulates the β-catenin and TGF-β/Smad2/TGIF2 pathways through
Integrins warrants comprehensive investigation. Some methodological
considerations profoundly impact future pharmaceutical development
efforts. For example, the use of established cell lines, such as
the HGC27 and MKN28 cell lines, to study gastric cancer fails to
replicate the complexity of primary cells isolated from living
tissues (27). Inferring causal
relationships in disease mechanisms based solely on gene or protein
expression associations or simultaneous alterations, exemplified by
MUC4 and Fibulin2 in pancreatic cancer (32), and simultaneous changes in
Fibulin2, fibronectin and tropoelastin in Kaposi's sarcoma
(23), may result in ineffective
drug targets. Focusing on signaling pathways, such as the
TGF-β/TGIF2 pathway in gastric cancer (27), while disregarding other potential
pathways, may lead to therapeutic resistance due to the activation
of compensatory pathways following single-target inhibition. The
role of tumor stroma in inhibiting or promoting tumors is
associated not only with signaling pathway activation but is also
closely associated with stromal cells, such as the activity or
senescence state of fibroblasts (91). Secretion of growth factors with
inhibitory functions by non-activated fibroblasts (92) or upregulation of the tissue
inhibitor of metalloproteinases (TIMP) family are potential
mechanisms by which tumor stroma suppresses neoplastic progression
(93,94). Notably, both MMPs and the
previously discussed ADAMTS are strictly regulated by TIMPs in
normal tissues (95,96). Expression of TIMP family proteins
in fibroblasts governs ECM structural organization and stromal cell
architecture (97). These
proteins function as endogenous negative regulators of MMP
activity, and numerous malignancies exhibit aberrant TIMP and/or
MMP expression patterns (93,98-99). Loss or reduction of TIMP
expression leads to enhanced MMP functionality, facilitating
stromal activation and subsequent tumor progression (100,101). TIMP overexpression attenuates
tumorigenesis, growth, angiogenesis and metastasis in some cancer
types, such as pancreatic cancer (94). Whether altered Fibulin2 expression
in tumor cells influences fibroblast activity and stromal TIMP
family expression requires further elucidation.
Fibulin2 as a biomarker for tumors
Studies on Fibulin2 as a biomarker are summarized
in Table IV. For case-control
studies involving human populations in Table IV, according to the 2011 Evidence
Hierarchy of the Oxford Centre for Evidence-based Medicine, the
evidence level of most studies was 4 (102).
 | Table IVFibulin2: A potential biomarker in
tumors and other diseases. |
Table IV
Fibulin2: A potential biomarker in
tumors and other diseases.
| First author/s,
year | Disease | Source | Expression | Comparison/
evidence level | (Refs.) |
|---|
| Ren et al,
2016 | Astrocytoma | Human
astrocytomas | Downregulation | Grades II/III/IV
vs. grade I/4 | (7) |
| Whiteaker et
al, 2007 | Breast cancer | Mouse plasma | Upregulation | Breast cancer mouse
vs. normal mice | (103) |
| Ibrahim et
al, 2020 | HCM | Human serum | Upregulation | Patients with HCM
vs. healthy individuals/4 | (107) |
| Sofela et
al, 2021; Sofela et al, 2021 | Meningioma | Human plasma | Upregulation | Grade II vs. grade
I/4 | (5,6) |
| Li et al,
2022 | Infection | Human plasma | Upregulation | Patients with
infection vs. healthy individuals/3 | (108) |
| Knittel et
al, 1999 | Liver fibrosis | Rat liver
myofibroblasts | Upregulation | Liver stellate
cells vs. liver myofibroblasts | (109) |
| Setiawati et
al, 2024 | Meningioma | Human tumor
tissue | Fibulin2 expression
was elevated in patients <50 years old or exhibiting high
histopathological grading | High vs. low
Fibulin2 expression/4 | (106) |
| Klingen et
al, 2021 | Breast cancer | Human breast cancer
tissue | Diminished Fibulin2
expression in perivascular regions was associated with attenuated
survival outcomes | High vs. low
Fibulin2 expression/4 | (76) |
| WalyEldeen et
al, 2024 | Breast cancer | Human breast cancer
mRNA data | High Fbln2
mRNA expression was associated with favorable prognosis in
relatively early breast cancer, with contrary associations observed
in relatively late lesions | High vs. low
Fbln2 expression/4 | (105) |
| Takakura et
al, 2023 | Advanced CRC | Human serum | Fibulin2 expression
was increased in recurrent and advanced CRC | Patients with
advanced CRC vs. healthy individuals/4 | (4) |
| Ibrahim et
al, 2018 | Breast cancer | Human breast cancer
tissue | Higher Fbln2
mRNA expression was associated with improved survival in low and
intermediate grade breast cancer, exhibiting contrary associations
in high-grade breast cancer | High vs. low
Fbln2 expression/4 | (74) |
| Avsar et al,
2019 | Lung cancer | Human blood | Downregulation | Patients with lung
cancer vs. healthy individuals/4 | (104) |
Fibulin2 as a marker to distinguish
patients with tumors from normal populations
In breast cancer mouse models, Fibulin2 expression
in plasma was substantially higher than that in normal mice,
indicating its potential as a plasma biomarker (103). The study used mice rather than
humans as research subjects. Due to species differences, the
effectiveness of the marker in humans requires verification,
limiting the applicability of the conclusions. Using liquid
chromatography-tandem mass spectrometry (LC-MS/MS) and label-free
quantitative methods, differences in Fibulin2 expression have been
detected in the sera of patients with advanced colon cancer and
healthy individuals. Fibulin2 is a diagnostic marker for advanced
colon cancer (4). The sample
sizes for the patient and normal control groups were only 8 and 10,
respectively, in the study investigating advanced colon cancer,
representing inadequate sample sizes (4). Consequently, the cohort lacks
representativeness, predisposing it to false positives or false
negatives. Future research should expand the sample size to enhance
the robustness of the conclusions. In a study on patients with lung
cancer, Fbln2 gene expression levels of the enriched
epithelial cells of peripheral blood lymphocytes were found to be
decreased ~2-fold in patients with metastatic and non-metastatic
lung cancer compared with healthy controls (104). Fbln2 is a potential
biomarker to distinguish patients with lung cancer from healthy
controls (104).
Fibulin2 as a marker to distinguish
different grades of tumors
Surgical specimens of astrocytomas have been
analyzed using LC-MS/MS, WB and reverse transcription-quantitative
PCR, revealing that Fibulin2 was downregulated in grade II/III/IV
astrocytomas compared with grade I astrocytomas, with negative
expression being associated with advanced clinical stages (7). The sample sizes of patients with
different grades of glioma were only 5 each, representing small
sample sizes that may reduce diagnostic accuracy. Fibulin2
expression in the plasma of patients with grade II meningioma is
higher than that in grade I patients, indicating that Fibulin2 is a
potential marker to distinguish between patients with grade II and
I meningioma (5,6).
Fibulin2 as a marker to predict the
prognosis of patients
In studies of Fibulin2 as a marker to predict the
prognosis of patients (74,105), comprehensive analysis of human
sample data from multiple databases yielded information on patients
with breast cancer, including their stages, molecular subtypes and
treatment conditions. Based on the Kaplan-Meier plotter dataset,
after chemotherapy, high Fbln2 expression was associated
with improved overall survival in Her2− patients. In
grade 2 patients (including those after chemotherapy/hormonal
therapy), unstratified patients with breast cancer,
Her2− patients, Luminal− patients and
estrogen receptor+ patients after chemotherapy, high
Fbln2 expression was associated with improved
recurrence-free survival. Conversely, in grade 3 patients, low
Fbln2 expression was associated with improved overall
survival. In Her2+ patients, estrogen
receptor− patients and grade 3 patients, low
Fbln2 expression was associated with improved
recurrence-free survival. Analysis of sample data from patients
with breast cancer with different molecular subtypes in The Cancer
Genome Atlas dataset revealed that in Luminal B patients, low
Fbln2 expression was associated with improved overall
survival. In Her2+ patients, high Fbln2
expression was associated with improved overall survival (105). Another study used the
Kaplan-Meier plotter dataset to collect the mRNA expression and
survival data of patients with breast cancer, analyzing the
association between Fbln2 expression and prognosis in
different subgroups (74). In
patients with lymph node-negative and intermediate-grade breast
cancer, high Fbln2 mRNA expression was associated with
improved distant metastasis-free survival compared with that of
patients with low expression. By contrast, in patients with
high-grade breast cancer, the opposite was true: Elevated
Fbln2 mRNA expression was associated with poorer prognosis
than low expression (74). The
conclusions of both studies were derived from database analyses
(74,105). However, samples from public
databases are prone to heterogeneity, so clinical validation of
their findings is necessary. In a study analyzing human meningioma
tissue samples, high-grade histopathology was associated with
elevated Ki-67 and Fibulin2 expression. The higher the histological
grade of meningioma was based on histopathology, the poorer the
prognosis of patients. Higher grades were associated with increased
risks of recurrence, progression and mortality. The younger age
group (<50 years) exhibited higher Fibulin2 expression than the
older age group (>50 years). Fibulin2 expression exhibited an
association with age and histopathological grading (106). The sample sizes for low-grade
and high-grade gliomas were both 25, representing relatively small
cohorts. Furthermore, as the study was a cross-sectional study, it
could not determine whether elevated Fibulin2 expression was the
cause or consequence of increased meningioma grading.
Fibulin2 as a marker in non-tumor
diseases
In a study examining patients with hypertrophic
cardiomyopathy (HCM), serum Fibulin2 expression in patients with
HCM markedly exceeded that of normal individuals, suggesting that
Fibulin2 may be beneficial for HCM diagnosis (107). This retrospective study included
95 consecutive patients with obstructive HCM eligible for septal
myectomy surgery. However, HCM has numerous subtypes, with sampling
limited to specific subtypes, introducing bias that affects the
generalizability of the conclusions to HCM overall. In a study
examining patients with infections, plasma Fibulin2 expression in
patients with infections was higher than that in normal individuals
(108). All patients in the
study were from the emergency department, and the disease types
included were limited, leading to selection bias in the study
population. The applicability of the conclusions supporting
Fibulin2 as a clinical diagnostic marker for infection remains
limited. Immunohistochemical expression of Fibulin2 distinguishes
liver stellate cells from liver myofibroblasts after liver injury,
suggesting that liver myofibroblasts are a unique cell population
involved in matrix production during liver fibrosis caused by
chronic liver injury (109).
Differences in Fibulin2 expression have been observed between liver
stellate cells and liver myofibroblasts in normal livers, livers
with acute damage and livers with chronic damage (109). This study provides a novel
marker for researchers to label liver myofibroblasts in related
research, although its application remains limited to basic
research. Future research should explore whether Fibulin2 can be
translated into a clinical diagnostic marker for liver-related
diseases based on this finding.
Challenges and limitations of clinical
translation
Although Fibulin2 shows considerable potential as a
therapeutic target and biomarker, several challenges and
limitations remain. Addressing these impediments is crucial for
successful clinical translation.
Mechanisms of Fibulin2 in tumors are not
fully understood
Currently, studies on Fibulin2 receptors remain
limited. In infected bone marrow mesenchymal stem cells, Fibulin2
binds to the transmembrane receptor Notch2 (110). In a rat model of neuropathic
pain, Fibulin2 expression in the spinal dorsal horn was upregulated
(111). Fibulin2 specifically
binds to the B1a subunit of γ-aminobutyric acid B receptor,
inhibiting its activity and thereby exacerbating pain (111). Following multiple sclerosis,
Fibulin2 suppresses oligodendrocyte generation through Notch
pathway activation (112). In
tumor research, Integrin proteins have been demonstrated to be
receptors of Fibulin2 (29). The
mechanistic evidence in numerous studies exhibits
oversimplification (10,22,28,29). These studies predominantly involve
Fbln2 knockdown or overexpression, followed by detection of
expression changes in specific key proteins in vitro. Other
studies primarily encompass influencing the alteration of the BM
(7,32,74). The impact of Fibulin2 on
neoplastic processes is complex, requiring further investigation
into more comprehensive mechanistic pathways. This would facilitate
the design of precision-targeted pharmaceuticals against
disease-associated molecular targets.
Side effects and toxicities
Due to the complexity of the function of Fibulin2,
targeting Fibulin2 may induce various adverse effects and
toxicities. Beyond its role in tumors, Fibulin2 serves a pivotal
role in maintaining tissue integrity (74,75,113), regulating fibrosis and immunity
(114-120), and supporting tissue development
and repair (121-126). For example, Fibulin2-deficient
mouse pups developed blisters, demonstrating that Fibulin2
deficiency during development could cause BM rupture. However,
adult Fibulin2-deficient mice exhibited no blisters, likely due to
compensatory mechanisms mediated by other ECM components (75,113). Reduced Fbln2 expression
disrupts sheath formation in the mammary epithelium and
downregulates Integrin β1 expression, compromising BM integrity.
Fibulin2 serves a crucial role in stabilizing the BM structure of
the mammary epithelium (74). The
Fibulin2 protein exhibits close associations with fibrosis. By
establishing cardiac hypertrophy models in Fibulin2 homozygous and
wild-type mice via angiotensin II infusion, Fibulin2 knockout has
been shown to inhibit myocardial fibrosis via H&E staining,
Masson staining and immunohistochemistry analysis (114). This finding was corroborated by
observations that Fbln2 reduced fibrosis markers in primary
cardiomyocyte fibroblasts from Fibulin2 homozygous and wild-type
mice stimulated with TGF-β1 (114). Consistent results were obtained
when establishing myocardial infarction models in Fibulin2
homozygous and wild-type mice: The survival rate of Fibulin2
homozygous mice was higher than that of wild-type mice (115). A 5-day mouse model of skin
injury revealed markedly elevated Fibulin2 expression in mice with
skin injuries compared with normal mice, as detected by nucleic
acid analysis and immunofluorescence (116). Unilateral ureteral obstruction
leads to renal fibrosis. Immunofluorescence staining in mice after
7 days of unilateral ureteral obstruction showed higher Fibulin2
expression compared with that in normal mice (117). After establishing a liver
fibrosis model in rats, immunofluorescence revealed higher Fibulin2
expression in rats with liver fibrosis compared with normal rats
(118). Fibulin2 is upregulated
in patients with idiopathic pulmonary fibrosis (119). Fibulin2 inhibition suppresses
α-SMA, collagen type Iα1 and fibronectin expression in human lung
fibroblast-derived MRC-5 cells (119). Fibulin2 influences immune
function, with reduced levels being associated with immune
impairment after bone trauma (120). In terms of tissue development
and repair, Fibulin2 is a key mediator of the neurogenic effect of
TGF-β1 on adult neural stem cells (121). Fibulin2-mediated TGF-β signaling
in astrocyte extracellular vesicles promotes synapse formation
(122). During myoblast
differentiation, Fbln2 expression is upregulated, and
Fbln2 is indispensable for myoblast differentiation
(123-125). Fibulin2 regulates smooth muscle
cell migration during blood vessel wall repair (126).
Consequently, the functions of Fibulin2 exhibit
extraordinary complexity. Although targeting of Fibulin2 has
therapeutic potential in tumor treatment, it may cause
immunosuppression (120),
disrupt the balance between ECM production and degradation, and
induce severe complications such as excessive collagen deposition
and fibrosis (114-119).
Clinical translation challenges
To the best of our knowledge, currently, there are
no clinical studies on Fibulin2 as a therapeutic target. Existing
clinical studies related to Fibulin2 only focus on its use as a
marker (5,6,7,107,108). The clinical translation of
Fibulin2-targeted drugs for cancer treatment faces numerous
obstacles, although current research has not yet reached this
stage. Beyond the aforementioned incomplete understanding of
mechanisms, there are substantial challenges in clinical drug
trials themselves. The following issues are similarly prevalent in
the research and development of oncology drugs. For example,
determining the optimal dosing and regimens for targeted drugs is
challenging due to their narrow therapeutic window and potential
toxicity (127). Careful dose
escalation studies and monitoring of adverse reactions are
necessary. Additionally, for combination therapies involving
targeted drugs and other anticancer drugs, synergistic dose
regimens need to be determined. Establishing appropriate clinical
trial endpoints and efficacy measures is quite complex, as
traditional endpoints such as overall survival and progression-free
survival may inadequately reflect the therapeutic effects and
potential long-term implications of targeted drugs (128). Determining the optimal
combination, sequence and potential interactions between treatments
is challenging. Extensive preclinical and clinical studies are
necessary to evaluate synergistic effects, additional benefits or
potential antagonistic interactions of treatments to maximize
therapeutic efficacy.
Challenges in the clinical application of
markers
Biomarkers should meet strict criteria to ensure
concordance between measured and actual physiological values,
including accuracy, precision, sensitivity, reproducibility,
stability (129,130), specificity, dynamics,
detectability and minimal invasiveness (131). Fibulin2 exhibits significant
expression differences across distinct stages of tumor development
and between normal and diseased conditions (4,5,6,7,103). Fibulin2 is also a potentially
effective biomarker in studies of infection and fibrosis (108,109). However, numerous studies are
limited by inadequate sample sizes. Furthermore, Fibulin2 can serve
as a marker in studies on different diseases, and thus, does not
meet the specificity criteria for a marker. Its low specificity
fundamentally hinders the clinical utility of Fibulin2 as a single
biomarker. In breast cancer, meningioma, infection and HCM,
Fibulin2 in human serum samples is detected using ELISA (5,103,107,108). ELISA has considerable
advantages, including high sensitivity, high specificity (132) and the ability to analyze
multiple samples simultaneously within short timeframes (133). This ensures practical
feasibility and convenience for large-scale screening programs and
facilitates high-throughput sample processing (133). However, its inherent limitations
include lengthy sample pretreatment and purification procedures,
unsuitability for rapid detection, high cost, lack of real-time
detection capabilities (134),
and the need for a relatively large sample volume (100-200
µl) (135). As a
biomarker, Fibulin2 should not merely aid in staging disease
progression or pathological exclusion but also provide therapeutic
guidance. This requires that Fibulin2 demonstrates systematic
changes corresponding to dynamic disease progression. Most studies
so far have not conducted comprehensive dynamic monitoring.
Future perspectives of clinical
translation
Emerging Fibulin2-targeted therapies
At present, there is a lack of pharmaceutical
investigations targeting Fibulin2 for tumor treatment. A key
contributing factor to this scarcity is the multifaceted complexity
of its biological functions as aforementioned. Fibulin2-directed
therapeutic interventions may induce considerable toxicity and
adverse sequelae (74,75,113-126). Next-generation small-molecule
inhibitors, novel monoclonal antibodies, ligand traps, gene
editing, RNA interference technologies, nanomaterials and
peptide-based therapies are emerging targeted treatments in cancer.
Beyond the need to further elucidate the mechanistic basis of the
role of Fibulin2 in tumors, the strategic application of these
emerging technologies in prospective Fibulin2-targed drugs will
prove instrumental in enhancing therapeutic specificity and potency
while reducing adverse effects.
Combining other therapies with
Fibulin2-targeted therapies
Integrating Fibulin2-targeted treatment with other
modalities is a strategic approach to enhance antitumor efficacy
and circumvent resistance mechanisms. For example, synergistic
combinations with immunotherapeutic agents, particularly immune
checkpoint inhibitors (such as anti-programmed cell death protein 1
and anti-cytotoxic T-lymphocyte-associated protein 4), show promise
by reactivating antitumor immune responses by reducing
immunosuppression (136,137). Combining Fibulin2-targeted
treatment with adoptive cell therapy, such as chimeric antigen
receptor-T cells, may enhance cellular persistence and
effectiveness (138).
Co-administering signaling pathway inhibitors with
Fibulin2-targeted treatment helps attenuate parallel signaling
pathways, thereby blocking the compensatory mechanisms exploited by
malignant cells. This strategy aims to enhance comprehensive
antitumor activity and delay drug resistance. Integrating
Fibulin2-targeted treatment with traditional chemotherapy and
radiotherapy increases tumor susceptibility to these treatments.
Concomitant use of epigenetic modulators can enhance therapeutic
responsiveness by systematically reprogramming the TME, which is
effective in addressing epigenetic changes that promote cancer
progression (139).
Combining Fibulin2 with other
markers
Given the limited specificity of Fibulin2 as a
single biomarker, integrating multiple biomarkers is advantageous
to address this limitation. While there are currently no reports on
the use of Fibulin2 in combination with other markers for tumor
detection, the use of microRNAs (miRNAs) and other proteins as
combined markers for early-stage cancer diagnosis has demonstrated
the considerable potential of combined marker detection, offering
valuable insights for combining Fibulin2 with other markers
(140,141). For example, Yu et al
(140) described a multi-marker
diagnostic method for early-stage hepatocellular carcinoma, using α
fetoprotein and miRNA-125b as combined markers to simultaneously
improve diagnostic sensitivity and specificity. Yuan et al
(141) proposed a new
combination of circulating miRNAs and plasma protein biomarkers for
pancreatic cancer diagnosis. These combined indicators exhibited
higher specificity in distinguishing pancreatic cancer from other
gastrointestinal cancers than CA19-9 and individual indicators
(141). Combining Fibulin2 with
other markers such as miRNAs for early cancer screening, diagnosis
and prognosis may enhance specificity compared with using Fibulin2
alone. The introduction of artificial intelligence (AI) and machine
learning can facilitate the identification of novel potential
combined biomarkers. AI algorithms excel in analyzing and
identifying unique combinations of gene mutations and using
large-scale genomic databases to identify cancer-specific markers
(142).
Improvements in traditional ELISA
detection methods
The quantification of Fibulin2 in serum and plasma
is performed using traditional ELISA. ELISA detection requires
air-conditioned laboratories, refrigeration facilities for
chemicals and reagents, a reliable power supply,
precision-calibrated equipment, and highly trained personnel
(135). Some laboratories or
hospitals still lack access to affordable infrastructure for these
complex diagnostic tests. To address these deficiencies,
laboratories worldwide have evaluated and implemented various
modified forms of ELISA. These innovative ELISA formats have
greater potential for clinical translation due to their low cost,
short detection time, portability and reduced reagent needs
(143-146). For example, microfluidic-based
ELISA, paper-ELISA and aptamer-ELISA can mitigate the limitations
of traditional ELISA to some extent (143-146).
Conclusion
The oncogenic or suppressive effects of altered
Fibulin2 expression in malignant cells vary markedly across diverse
tumors. The contributing factors include distinct mechanisms,
including the activation of different signaling pathways and the
complex relationships with BM or other ECM proteins, tumor
development stage, species and tumor origin. Although current
mechanistic studies help clarify aspects of pathogenic processes,
several outstanding issues remain to be resolved. These include
validating the upstream and downstream molecules of Fibulin2,
confirming the existence of feedback loops, exploring alternative
pathways, and clarifying the relationship between Fibulin2 and
stromal fibroblasts. These considerations are crucial to improve
the safety and effectiveness of future relevant drug research. The
use of Fibulin2 alone as a marker for tumor diagnosis or staging
remains not well-established. Future research should further
explore methods to enhance specificity, such as identifying novel
and specific markers to be used in combination with Fibulin2 as a
dual-marker system.
Availability of data and materials
Not applicable.
Authors' contributions
YY completed most of the work, wrote the original
draft and created illustrations. ZW wrote and revised the article,
and collected the literature. LW revised the article and collected
the literature. JF reviewed and edited the manuscript, provided
resources, and acquired funding. ZL supervised the study, edited
and reviewed the manuscript, was involved in project
administration, and acquired funding. Data authentication is not
applicable. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
TME
|
tumor microenvironment
|
|
CAF
|
cancer-associated fibroblast
|
|
ECM
|
extracellular matrix
|
|
BM
|
basement membrane
|
|
TIMP
|
tissue inhibitor of
metalloproteinases
|
|
WB
|
western blotting
|
|
ADAMTS
|
a disintegrin and metalloproteinase
with thrombospondin motifs
|
|
AI
|
artificial intelligence
|
|
HCM
|
hypertrophic cardiomyopathy
|
|
CRC
|
colorectal cancer
|
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the Key Research
and Development Program of Sichuan Province (grant no.
2023YFQ0009), the Chongqing Municipality Science-Health Joint
Traditional Chinese Medicine Research Project (grant no.
2023DBXM010), and the Sichuan Provincial Central Government-Guided
Local Science and Technology Development Special Project (grant no.
2023ZYD0288).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025.PubMed/NCBI
|
|
4
|
Takakura D, Ohashi S, Kobayashi N,
Tokuhisa M, Ichikawa Y and Kawasaki N: Targeted O-glycoproteomics
for the development of diagnostic markers for advanced colorectal
cancer. Front Oncol. 13:11049362023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sofela AA, Hilton DA, Ammoun S, Baiz D,
Adams CL, Ercolano E, Jenkinson MD, Kurian KM, Teo M, Whitfield PC,
et al: Fibulin-2: A novel biomarker for differentiating grade II
from grade I meningiomas. Int J Mol Sci. 22:5602021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sofela AA, McGavin L, Whitfield PC and
Hanemann CO: Biomarkers for differentiating grade II meningiomas
from grade I: A systematic review. Br J Neurosurg. 35:696–702.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren T, Lin S, Wang Z and Shang A:
Differential proteomics analysis of low- and high-grade of
astrocytoma using iTRAQ quantification. Onco Targets Ther.
9:5883–5895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan TC, Sasaki T, Zhang RZ, Fässler R,
Timpl R and Chu ML: Structure and expression of fibulin-2, a novel
extracellular matrix protein with multiple EGF-like repeats and
consensus motifs for calcium binding. J Cell Biol. 123:1269–1277.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Hui D and Fu X: Roles of
Fibulin-2 in carcinogenesis. Med Sci Monit.
26:e9180992020.PubMed/NCBI
|
|
10
|
Baird BN, Schliekelman MJ, Ahn YH, Chen Y,
Roybal JD, Gill BJ, Mishra DK, Erez B, O'Reilly M, Yang Y, et al:
Fibulin-2 is a driver of malignant progression in lung
adenocarcinoma. PLoS One. 8:e670542013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang RZ, Pan TC, Zhang ZY, Mattei MG,
Timpl R and Chu ML: Fibulin-2 (FBLN2): Human cDNA sequence, mRNA
expression, and mapping of the gene on human and mouse chromosomes.
Genomics. 22:425–430. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown JC, Sasaki T, Göhring W, Yamada Y
and Timpl R: The C-terminal domain V of perlecan promotes beta1
integrin-mediated cell adhesion, binds heparin, nidogen and
fibulin-2 and can be modified by glycosaminoglycans. Eur J Biochem.
250:39–46. 1997. View Article : Google Scholar
|
|
13
|
Hopf M, Göhring W, Kohfeldt E, Yamada Y
and Timpl R: Recombinant domain IV of perlecan binds to nidogens,
laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J
Biochem. 259:917–925. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki T, Göhring W, Mann K, Brakebusch C,
Yamada Y, Fässler R and Timpl R: Short arm region of laminin-5
gamma2 chain: Structure, mechanism of processing and binding to
heparin and proteins. J Mol Biol. 314:751–763. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talts JF, Andac Z, Göhring W, Brancaccio A
and Timpl R: Binding of the G domains of laminin alpha1 and alpha2
chains and perlecan to heparin, sulfatides, alpha-dystroglycan and
several extracellular matrix proteins. EMBO J. 18:863–870. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki T, Wiedemann H, Matzner M, Chu ML
and Timpl R: Expression of fibulin-2 by fibroblasts and deposition
with Fibronectin into a fibrillar matrix. J Cell Sci. 109(Pt 12):
2895–2904. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki T, Fukai N, Mann K, Göhring W,
Olsen BR and Timpl R: Structure, function and tissue forms of the
C-terminal globular domain of collagen XVIII containing the
angiogenesis inhibitor endostatin. EMBO J. 17:4249–4256. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olin AI, Mörgelin M, Sasaki T, Timpl R,
Heinegård D and Aspberg A: The proteoglycans aggrecan and Versican
form networks with fibulin-2 through their lectin domain binding. J
Biol Chem. 276:1253–1261. 2001. View Article : Google Scholar
|
|
19
|
Friedrich MV, Göhring W, Mörgelin M,
Brancaccio A, David G and Timpl R: Structural basis of
glycosaminoglycan modification and of heterotypic interactions of
perlecan domain V. J Mol Biol. 294:259–270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Utani A, Nomizu M and Yamada Y: Fibulin-2
binds to the short arms of laminin-5 and laminin-1 via conserved
amino acid sequences. J Biol Chem. 272:2814–2820. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Vega S, Iwamoto T and Yamada Y:
Fibulins: Multiple roles in matrix structures and tissue functions.
Cell Mol Life Sci. 66:1890–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Law EW, Cheung AK, Kashuba VI, Pavlova TV,
Zabarovsky ER, Lung HL, Cheng Y, Chua D, Kwong DLK, Tsao SW, et al:
Anti-angiogenic and tumor-suppressive roles of candidate
tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma.
Oncogene. 31:728–738. 2012. View Article : Google Scholar
|
|
23
|
Alcendor DJ, Knobel S, Desai P, Zhu WQ and
Hayward GS: KSHV regulation of fibulin-2 in Kaposi's sarcoma:
Implications for tumorigenesis. Am J Pathol. 179:1443–1454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Duan L, Zhang Y, Zhao H, Yang X
and Zhang C: Correlation of Fibulin-2 expression with
proliferation, migration and invasion of breast cancer cells. Oncol
Lett. 20:1945–1951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu X, Liu T, Li L, Gan H, Wang T, Pang P
and Mao J: Fibulin-2 facilitates malignant progression of
hepatocellular carcinoma. Turk J Gastroenterol. 34:635–644. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Lian C and Song Y: Fibulin-2
inhibits development of gastric cancer by downregulating β-catenin.
Oncol Lett. 18:2799–2804. 2019.PubMed/NCBI
|
|
27
|
Zhou M, Mao X, Shen K, Zhan Q, Ni H, Liu
C, Huang Z and Li R: FBLN2 inhibits gastric cancer proliferation
and metastasis via the TGFβ/TGIF2 pathway. Pathol Res Pract.
269:1558992025. View Article : Google Scholar
|
|
28
|
Missan DS, Chittur SV and DiPersio CM:
Regulation of fibulin-2 gene expression by integrin α3β1
contributes to the invasive phenotype of transformed keratinocytes.
J Invest Dermatol. 134:2418–2427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang MR, Chen RJ, Zhao F, Zhang HH, Bi QY,
Zhang YN, Zhang YQ, Wu ZC and Ji XM: Effect of Wenxia Changfu
formula combined with cisplatin reversing non-small cell lung
cancer cell adhesion-mediated drug resistance. Front Pharmacol.
11:5001372020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fontanil T, Álvarez-Teijeiro S, Villaronga
MÁ, Mohamedi Y, Solares L, Moncada-Pazos A, Vega JA, García-Suárez
O, Pérez-Basterrechea M, García-Pedrero JM, et al: Cleavage of
Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to
the tumorigenic potential of breast cancer cells. Oncotarget.
8:13716–13729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vaes N, Schonkeren SL, Rademakers G,
Holland AM, Koch A, Gijbels MJ, Keulers TG, de Wit M, Moonen L, Van
der Meer JRM, et al: Loss of enteric neuronal Ndrg4 promotes
colorectal cancer via increased release of Nid1 and Fbln2. EMBO
Rep. 22:e519132021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Senapati S, Gnanapragassam VS, Moniaux N,
Momi N and Batra SK: Role of MUC4-NIDO domain in the MUC4-mediated
metastasis of pancreatic cancer cells. Oncogene. 31:3346–3356.
2012. View Article : Google Scholar :
|
|
33
|
Melotte V, Lentjes MH, van den Bosch SM,
Hellebrekers DM, de Hoon JP, Wouters KA, Daenen KL,
Partouns-Hendriks IE, Stessels F, Louwagie J, et al: N-Myc
downstream-regulated gene 4 (NDRG4): A candidate tumor suppressor
gene and potential biomarker for colorectal cancer. J Natl Cancer
Inst. 101:916–927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barczyk M, Carracedo S and Gullberg D:
Integrins. Cell Tissue Res. 339:269–280. 2010. View Article : Google Scholar
|
|
35
|
Cooper J and Giancotti FG: Integrin
signaling in cancer: Mechanotransduction, stemness, epithelial
plasticity, and therapeutic resistance. Cancer Cell. 35:347–367.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nobre LT, Vidal AA, Almeida-Lima J,
Oliveira RM, Paredes-Gamero EJ, Medeiros VP, Trindade ES, Franco
CR, Nader HB and Rocha HA: Fucan effect on CHO cell proliferation
and migration. Carbohydr Polym. 98:224–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fischer C, Sanchez-Ruderisch H, Welzel M,
Wiedenmann B, Sakai T, André S, Gabius HJ, Khachigian L, Detjen KM
and Rosewicz S: Galectin-1 interacts with the {alpha}5{beta}1
fibronectin receptor to restrict carcinoma cell growth via
induction of p21 and p27. J Biol Chem. 280:37266–37277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamidi H and Ivaska J: Every step of the
way: Integrins in cancer progression and metastasis. Nat Rev
Cancer. 18:533–548. 2019. View Article : Google Scholar :
|
|
40
|
Humphries JD, Byron A and Humphries MJ:
Integrin ligands at a glance. J Cell Sci. 119:3901–3903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Springer TA and Wang JH: The
three-dimensional structure of integrins and their ligands, and
sconformational regulation of cell adhesion. Adv Protein Chem.
68:29–63. 2004. View Article : Google Scholar
|
|
42
|
Arnaout MA, Mahalingam B and Xiong JP:
Integrin structure, allostery, and bidirectional signaling. Annu
Rev Cell Dev Biol. 21:381–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erler JT, Bennewith KL, Nicolau M,
Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Couillard J, Demers M, Lavoie G and
St-Pierre Y: The role of DNA hypomethylation in the control of
stromelysin gene expression. Biochem Biophys Res Commun.
342:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hanson JA, Gillespie JW, Grover A, Tangrea
MA, Chuaqui RF, Emmert-Buck MR, Tangrea JA, Libutti SK, Linehan WM
and Woodson KG: Gene promoter methylation in prostate
tumor-associated stromal cells. J Natl Cancer Inst. 98:255–261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin HJ, Zuo T, Lin CH, Kuo CT,
Liyanarachchi S, Sun S, Shen R, Deatherage DE, Potter D, Asamoto L,
et al: Breast cancer-associated fibroblasts confer AKT1-mediated
epigenetic silencing of Cystatin M in epithelial cells. Cancer Res.
68:10257–10266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth-bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Micallef L, Vedrenne N, Billet F, Coulomb
B, Darby IA and Desmoulière A: The myofibroblast, multiple origins
for major roles in normal and pathological tissue repair.
Fibrogenesis Tissue Repair. 5(Suppl 1): S52012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Neuzillet C, Tijeras-Raballand A, Cros J,
Faivre S, Hammel P and Raymond E: Stromal expression of SPARC in
pancreatic adenocarcinoma. Cancer Metastasis Rev. 32:585–602. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rønnov-Jessen L, Petersen OW and Bissell
MJ: Cellular changes involved in conversion of normal to malignant
breast: Importance of the stromal reaction. Physiol Rev. 76:69–125.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chiquet-Ehrismann R, Mackie EJ, Pearson CA
and Sakakura T: Tenascin: An extracellular matrix protein involved
in tissue interactions during fetal development and oncogenesis.
Cell. 47:131–139. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine model. Int J Mol Med.
28:181–186. 2011.PubMed/NCBI
|
|
56
|
Mackie EJ, Chiquet-Ehrismann R, Pearson
CA, Inaguma Y, Taya K, Kawarada Y and Sakakura T: Tenascin is a
stromal marker for epithelial malignancy in the mammary gland. Proc
Natl Acad Sci USA. 84:4621–4625. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ouyang G, Liu M, Ruan K, Song G, Mao Y and
Bao S: Upregulated expression of periostin by hypoxia in
non-small-cell lung cancer cells promotes cell survival via the
Akt/PKB pathway. Cancer Lett. 281:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sternlicht MD, Lochter A, Sympson CJ, Huey
B, Rougier JP, Gray JW, Pinkel D, Bissell MJ and Werb Z: The
stromal proteinase MMP3/stromelysin-1 promotes mammary
carcinogenesis. Cell. 98:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hotary KB, Allen ED, Brooks PC, Datta NS,
Long MW and Weiss SJ: Membrane type I matrix metalloproteinase
usurps tumor growth control imposed by the three-dimensional
extracellular matrix. Cell. 114:33–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Valkenburg KC, de Groot AE and Pienta KJ:
Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin
Oncol. 15:366–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Melnik S, Dvornikov D, Müller-Decker K,
Depner S, Stannek P, Meister M, Warth A, Thomas M, Muley T, Risch
A, et al: Cancer cell specific inhibition of Wnt/β-catenin
signaling by forced intracellular acidification. Cell Discov.
4:372018. View Article : Google Scholar
|
|
65
|
Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long
LY, Li G, Ji XD, Shi S, Guan DX, et al: RACK1 suppresses gastric
tumorigenesis by stabilizing the β-catenin destruction complex.
Gastroenterology. 142:812–823.e15. 2012. View Article : Google Scholar
|
|
66
|
Austinat M, Dunsch R, Wittekind C,
Tannapfel A, Gebhardt R and Gaunitz F: Correlation between
beta-catenin mutations and expression of Wnt-signaling target genes
in hepatocellular carcinoma. Mol Cancer. 7:212008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Brenner KA, Corbett SA and Schwarzbauer
JE: Regulation of fibronectin matrix assembly by activated Ras in
transformed cells. Oncogene. 19:3156–3163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Werbajh SE, Urtreger AJ, Puricelli LI, de
Lustig ES, de Kier Joffé E and Kornblihtt AR: Downregulation of
fibronectin transcription in highly metastatic adenocarcinoma
cells. FEBS Lett. 440:277–281. 1998. View Article : Google Scholar
|
|
69
|
Seo JY, Lee SH, Youn CS, Choi HR, Rhie GE,
Cho KH, Kim KH, Park KC, Eun HC and Chung JH: Ultraviolet radiation
increases Tropoelastin mRNA expression in the epidermis of human
skin in vivo. J Invest Dermatol. 116:915–919. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Taddese S, Weiss AS, Jahreis G, Neubert RH
and Schmelzer CE: In vitro degradation of human tropoelastin by
MMP-12 and the generation of matrikines from domain 24. Matrix
Biol. 28:84–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yan L, Wang Y, Feng J, Ni Y, Zhang T, Cao
Y, Zhou M and Zhao C: Mechanism and application of fibrous proteins
in diabetic wound healing: A literature review. Front Endocrinol
(Lausanne). 15:14305432024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Giaquinto AN, Sung H, Newman LA, Freedman
RA, Smith RA, Star J, Jemal A and Siegel RL: Breast cancer
statistics 2024. CA Cancer J Clin. 74:477–495. 2024.PubMed/NCBI
|
|
73
|
Yi CH, Smith DJ, West WW and Hollingsworth
MA: Loss of fibulin-2 expression is associated with breast cancer
progression. Am J Pathol. 170:1535–1545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ibrahim AM, Sabet S, El-Ghor AA, Kamel N,
Anis SE, Morris JS and Stein T: Fibulin-2 is required for basement
membrane integrity of mammary epithelium. Sci Rep. 8:141392018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Longmate WM, Monichan R, Chu ML, Tsuda T,
Mahoney MG and DiPersio CM: Reduced fibulin-2 contributes to loss
of basement membrane integrity and skin blistering in mice lacking
Integrin α3β1 in the epidermis. J Invest Dermatol. 134:1609–1617.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Klingen TA, Chen Y, Aas H, Wik E and
Akslen LA: Fibulin-2 expression associates with vascular invasion
and patient survival in breast cancer. PLoS One. 16:e02497672021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cal S and López-Otín C: ADAMTS proteases
and cancer. Matrix Biol. 44-46:77–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Noël A, Gutiérrez-Fernández A, Sounni NE,
Behrendt N, Maquoi E, Lund IK, Cal S, Hoyer-Hansen G and López-Otín
C: New and paradoxical roles of matrix metalloproteinases in the
tumor microenvironment. Front Pharmacol. 3:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Luan Y, Kong L, Howell DR, Ilalov K,
Fajardo M, Bai XH, Di Cesare PE, Goldring MB, Abramson SB and Liu
CJ: Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage
oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis
Cartilage. 16:1413–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fontanil T, Rúa S, Llamazares M,
Moncada-Pazos A, Quirós PM, García-Suárez O, Vega JA, Sasaki T,
Mohamedi Y, Esteban MM, et al: Interaction between the ADAMTS-12
metalloprotease and fibulin-2 induces tumor-suppressive effects in
breast cancer cells. Oncotarget. 5:1253–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kelwick R, Desanlis I, Wheeler GN and
Edwards DR: The ADAMTS (A DisIntegrin and Metalloproteinase with
Thrombospondin motifs) family. Genome Biol. 16:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ and
Feng YM: Cancer-associated fibroblasts induce
epithelial-mesenchymal transition of breast cancer cells through
paracrine TGF-β signaling. Br J Cancer. 110:724–732. 2014.
View Article : Google Scholar
|
|
83
|
Cuffaro D, Ciccone L, Rossello A, Nuti E
and Santamaria S: Targeting Aggrecanases for osteoarthritis
therapy: From zinc chelation to exosite inhibition. J Med Chem.
65:13505–13532. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Niland S, Riscanevo AX and Eble JA: Matrix
metalloproteinases shape the tumor microenvironment in cancer
progression. Int J Mol Sci. 23:1462021. View Article : Google Scholar
|
|
85
|
Ghalehbandi S, Yuzugulen J, Pranjol MZI
and Pourgholami MH: The role of VEGF in cancer-induced angiogenesis
and research progress of drugs targeting VEGF. Eur J Pharmacol.
949:1755862023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Reddy RA, Varshini MS and Kumar RS: Matrix
metalloproteinase-2 (MMP-2): As an essential factor in cancer
progression. Recent Pat Anticancer Drug Discov. 20:26–44. 2025.
View Article : Google Scholar
|
|
87
|
Hergeth SP, Aicher WK, Essl M, Schreiber
TD, Sasaki T and Klein G: Characterization and functional analysis
of osteoblast-derived fibulins in the human hematopoietic stem cell
niche. Exp Hematol. 36:1022–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rashid ZA and Bardaweel SK: Novel matrix
metalloproteinase-9 (MMP-9) inhibitors in cancer treatment. Int J
Mol Sci. 24:121332023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Astudillo P: Extracellular matrix
stiffness and Wnt/β-catenin signaling in physiology and disease.
Biochem Soc Trans. 48:1187–1198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Henderson NC, Rieder F and Wynn TA:
Fibrosis: From mechanisms to medicines. Nature. 587:555–566. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Caligiuri G and Tuveson DA: Activated
fibroblasts in cancer: Perspectives and challenges. Cancer Cell.
41:434–449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bhowmick NA, Chytil A, Plieth D, Gorska
AE, Dumont N, Shappell S, Washington MK, Neilson EG and Moses HL:
TGF-beta signaling in fibroblasts modulates the oncogenic potential
of adjacent epithelia. Science. 303:848–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cruz-Munoz W and Khokha R: The role of
tissue inhibitors of metalloproteinases in tumorigenesis and
metastasis. Crit Rev Clin Lab Sci. 45:291–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bloomston M, Shafii A, Zervos EE and
Rosemurgy AS: TIMP-1 overexpression in pancreatic cancer attenuates
tumor growth, decreases implantation and metastasis, and inhibits
angiogenesis. J Surg Res. 102:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen J and Khalil RA: Matrix
metalloproteinases in normal pregnancy and preeclampsia. Prog Mol
Biol Transl Sci. 148:87–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Murphy G: Tissue inhibitors of
metalloproteinases. Genome Biol. 12:2332011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shimoda M, Jackson HW and Khokha R: Tumor
suppression by stromal TIMPs. Mol Cell Oncol. 3:e9750822016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhai LL, Cai CY, Wu Y and Tang ZG:
Correlation and prognostic significance of MMP-2 and TFPI-2
differential expression in pancreatic carcinoma. Int J Clin Exp
Pathol. 8:682–691. 2015.PubMed/NCBI
|
|
99
|
Song G, Xu S, Zhang H, Wang Y, Xiao C,
Jiang T, Wu L, Zhang T, Sun X, Zhong L, et al: TIMP1 is a
prognostic marker for the progression and metastasis of colon
cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer
Res. 35:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shimoda M, Principe S, Jackson HW, Luga V,
Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas
C, et al: Loss of the Timp gene family is sufficient for the
acquisition of the CAF-like cell state. Nat Cell Biol. 16:889–901.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tjomsland V, Pomianowska E, Aasrum M,
Sandnes D, Verbeke CS and Gladhaug IP: Profile of MMP and TIMP
expression in human pancreatic stellate cells: Regulation by IL-1α
and TGFβ and implications for migration of pancreatic cancer cells.
Neoplasia. 18:447–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
https://gdt.gradepro.org/app/handbook/handbook.html#h.9rdbelsnu4iy.
Accessed 22 Jul 2023
|
|
103
|
Whiteaker JR, Zhang H, Zhao L, Wang P,
Kelly-Spratt KS, Ivey RG, Piening BD, Feng LC, Kasarda E, Gurley
KE, et al: Integrated pipeline for mass spectrometry-based
discovery and confirmation of biomarkers demonstrated in a mouse
model of breast cancer. J Proteome Res. 6:3962–3975. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Avsar M, Tambas M, Yalniz Z, Akdeniz D,
Tuncer SB, Kilic S, Erdogan OS, Ciftci R, Dagoglu N, Vatansever S
and Yazici H: The expression level of fibulin-2 in the circulating
RNA (ctRNA) of epithelial tumor cells of peripheral blood and tumor
tissue of patients with metastatic lung cancer. Mol Biol Rep.
46:4001–4008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
WalyEldeen AA, Sabet S, Anis SE, Stein T
and Ibrahim AM: FBLN2 is associated with basal cell markers Krt14
and ITGB1 in mouse mammary epithelial cells and has a preferential
expression in molecular subtypes of human breast cancer. Breast
Cancer Res Treat. 208:6872024. View Article : Google Scholar
|
|
106
|
Setiawati Y, Alimuddin T, Mulyani H and
Kamelia M: The prognostic role of fibulin-2 and Ki-67 index in
patients with meningioma: A study among minangkabau ethnicity.
Asian Pac J Cancer Prev. 25:2735–2742. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ibrahim AM, Roshdy M, Elshorbagy S, Hosny
M, Halawa S, Yehia D, Elfawy HA, Eldessouki A, Mohamed F, Ellithy
A, et al: An investigation of fibulin-2 in hypertrophic
cardiomyopathy. Int J Mol Sci. 21:71762020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li S, Jiang H, Xing W, Wang S, Zhang Y, Li
Y, Mao C, Zeng D, Lan P, Tang D, et al: A clinical diagnostic
study: Fibulin-2 is a novel promising biomarker for predicting
infection. Infect Dis Ther. 11:1057–1073. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Knittel T, Kobold D, Piscaglia F, Saile B,
Neubauer K, Mehde M, Timpl R and Ramadori G: Localization of liver
myofibroblasts and hepatic stellate cells in normal and diseased
rat livers: Distinct roles of (myo-)fibroblast subpopulations in
hepatic tissue repair. Histochem Cell Biol. 112:387–401. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li SD, Xing W, Wang SC, Li YB, Jiang H,
Zheng HX, Li XM, Yang J, Guo DB, Xie XY, et al: Fibulin2: A
negative regulator of BMSC osteogenic differentiation in infected
bone fracture healing. Exp Mol Med. 55:443–456. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Papon MA, Le Feuvre Y, Barreda-Gómez G,
Favereaux A, Farrugia F, Bouali-Benazzouz R, Nagy F,
Rodríguez-Puertas R and Landry M: Spinal inhibition of GABAB
receptors by the extracellular matrix protein fibulin-2 in
neuropathic rats. Front Cell Neurosci. 14:2142020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ghorbani S, Li C, Lozinski BM, Moezzi D,
D'Mello C, Dong Y, Visser F, Li H, Silva C, Khakpour M, et al:
Fibulin-2 is an extracellular matrix inhibitor of oligodendrocytes
relevant to multiple sclerosis. J Clin Invest. 134:e1769102024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sicot FX, Tsuda T, Markova D, Klement JF,
Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE and Chu ML: Fibulin-2
is dispensable for mouse development and elastic fiber formation.
Mol Cell Biol. 28:1061–1067. 2008. View Article : Google Scholar :
|
|
114
|
Zhang H, Wu J, Dong H, Khan SA, Chu ML and
Tsuda T: Fibulin-2 deficiency attenuates angiotensin II-induced
cardiac hypertrophy by reducing transforming growth factor-β
signaling. Clin Sci (Lond). 126:275–288. 2014. View Article : Google Scholar
|
|
115
|
Tsuda T, Wu J, Gao E, Joyce J, Markova D,
Dong H, Liu Y, Zhang H, Zou Y, Gao F, et al: Loss of fibulin-2
protects against progressive ventricular dysfunction after
myocardial infarction. J Mol Cell Cardiol. 52:273–282. 2012.
View Article : Google Scholar :
|
|
116
|
Fässler R, Sasaki T, Timpl R, Chu ML and
Werner S: Differential regulation of fibulin, tenascin-C, and
nidogen expression during wound healing of normal and
glucocorticoid-treated mice. Exp Cell Res. 222:111–116. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kida Y, Asahina K, Inoue K, Kawada N,
Yoshizato K, Wake K and Sato T: Characterization of vitamin
A-storing cells in mouse fibrous kidneys using Cygb/STAP as a
marker of activated stellate cells. Arch Histol Cytol. 70:95–106.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Piscaglia F, Dudás J, Knittel T, Di Rocco
P, Kobold D, Saile B, Zocco MA, Timpl R and Ramadori G: Expression
of ECM proteins fibulin-1 and -2 in acute and chronic liver disease
and in cultured rat liver cells. Cell Tissue Res. 337:449–462.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang Y, Zhang W, Zhang R and Xia Y:
Knockdown of FBLN2 suppresses TGF-β1-induced MRC-5 cell migration
and fibrosis by downregulating VTN. Tissue Cell. 81:1020052023.
View Article : Google Scholar
|
|
120
|
Li S, Jiang H, Wang S, Li Y, Guo D, Zhan
J, Li Q, Meng H, Chen A, Chen L, et al: Fibulin-2: A potential
regulator of immune dysfunction after bone trauma. Immun Inflamm
Dis. 11:e8462023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Radice PD, Mathieu P, Leal MC, Farías MI,
Ferrari C, Puntel M, Salibe M, Chernomoretz A and Pitossi FJ:
Fibulin-2 is a key mediator of the pro-neurogenic effect of
TGF-beta1 on adult neural stem cells. Mol Cell Neurosci. 67:75–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Patel MR and Weaver AM: Astrocyte-derived
small extracellular vesicles promote synapse formation via
fibulin-2-mediated TGF-β signaling. Cell Rep. 34:1088292021.
View Article : Google Scholar
|
|
123
|
Bois PR and Grosveld GC: FKHR (FOXO1a) is
required for myotube fusion of primary mouse myoblasts. EMBO J.
22:1147–1157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lehka L and Rędowicz MJ: Mechanisms
regulating myoblast fusion: A multilevel interplay. Semin Cell Dev
Biol. 104:81–92. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chan CY, Masui O, Krakovska O, Belozerov
VE, Voisin S, Ghanny S, Chen J, Moyez D, Zhu P, Evans KR, et al:
Identification of differentially regulated secretome components
during skeletal myogenesis. Mol Cell Proteomics.
10:M110.0048042011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ström A, Olin AI, Aspberg A and
Hultgårdh-Nilsson A: Fibulin-2 is present in murine vascular
lesions and is important for smooth muscle cell migration.
Cardiovasc Res. 69:755–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ball K, Dovedi SJ, Vajjah P and Phipps A:
Strategies for clinical dose optimization of T cell-engaging
therapies in oncology. MAbs. 15:21810162023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gueorguieva I, Cleverly AL, Stauber A,
Pillay NS, Rodon JA, Miles CP, Yingling JM and Lahn MM: Defining a
therapeutic window for the novel TGF-β inhibitor LY2157299
monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J
Clin Pharmacol. 77:796–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ou FS, Michiels S, Shyr Y, Adjei AA and
Oberg AL: Biomarker discovery and validation: Statistical
considerations. J Thorac Oncol. 16:537–545. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kraus VB: Biomarkers as drug development
tools: Discovery, validation, qualification and use. Nat Rev
Rheumatol. 14:354–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang Z, Wu H, Chong W, Shang L, Jing C
and Li L: Liquid biopsy in gastric cancer: Predictive and
prognostic biomarkers. Cell Death Dis. 13:9032022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Galarini R, Diana F, Moretti S, Puppini B,
Saluti G and Persic L: Development and validation of a new
qualitative ELISA screening for multiresidue detection of
sulfonamides in food and feed. Food Control. 35:300–310. 2014.
View Article : Google Scholar
|
|
133
|
Wang S, Xu B, Zhang Y and He JX:
Development of enzyme-linked immunosorbent assay (ELISA) for the
detection of neomycin residues in pig muscle, chicken muscle, egg,
fish, milk and kidney. Meat Sci. 82:53–58. 2009. View Article : Google Scholar
|
|
134
|
Petz M: Recent applications of surface
plasmon resonance biosensors for analyzing residues and
contaminants in food. Monatshefte für Chemie-Chemical Monthly.
140:953–964. 2009. View Article : Google Scholar
|
|
135
|
Ahirwar R, Bhattacharya A and Kumar S:
Unveiling the underpinnings of various non-conventional ELISA
variants: A review article. Expert Rev Mol Diagn. 22:761–774. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Moreno BH and Ribas A: Anti-programmed
cell death protein-1/ligand-1 therapy in different cancers. Br J
Cancer. 112:1421–1427. 2015. View Article : Google Scholar
|
|
137
|
Boutros C, Tarhini A, Routier E, Lambotte
O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S,
Berdelou A, et al: Safety profiles of anti-CTLA-4 and anti-PD-1
antibodies alone and in combination. Nat Rev Clin Oncol.
13:473–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Moreno V, Hernandez T, de Miguel M, Doger
B and Calvo E: Adoptive cell therapy for solid tumors: Chimeric
antigen receptor T cells and beyond. Curr Opin Pharmacol. 59:70–84.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang J, Xu J, Wang W, Zhang B, Yu X and
Shi S: Epigenetic regulation in the tumor microenvironment:
Molecular mechanisms and therapeutic targets. Signal Transduct
Target Ther. 8:2102023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yu H, Han R, Su J, Chen H and Li D:
Multi-marker diagnosis method for early hepatocellular carcinoma
based on surface plasmon resonance. Clin Chim Acta. 502:9–14. 2020.
View Article : Google Scholar
|
|
141
|
Yuan W, Tang W, Xie Y, Wang S, Chen Y, Qi
J, Qiao Y and Ma J: New combined microRNA and protein plasmatic
biomarker panel for pancreatic cancer. Oncotarget. 7:80033–80045.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ginghina O, Hudita A, Zamfir M, Spanu A,
Mardare M, Bondoc I, Buburuzan L, Georgescu SE, Costache M, Negrei
C, et al: Liquid biopsy and artificial intelligence as tools to
detect signatures of colorectal malignancies: A modern approach in
patient's stratification. Front Oncol. 12:8565752022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Weng X, Gaur G and Neethirajan S: Rapid
detection of food allergens by microfluidics ELISA-based optical
sensor. Biosensors (Basel). 6:242016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cheng CM, Martinez AW, Gong J, Mace CR,
Phillips ST, Carrilho E, Mirica KA and Whitesides GM: Paper-based
ELISA. Angew Chem Int Ed Engl. 49:4771–4774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kudłak B and Wieczerzak M: Aptamer based
tools for environmental and therapeutic monitoring: A review of
developments, applications, future perspectives. Crit Rev Environ
Sci Technol. 50:816–867. 2020. View Article : Google Scholar
|
|
146
|
Bauer M, Strom M, Hammond DS and Shigdar
S: Anything you can do, I can do better: Can aptamers replace
antibodies in clinical diagnostic applications? Molecules.
24:43772019. View Article : Google Scholar : PubMed/NCBI
|