|
1
|
Ambrosio MR, Adriaens M, Derks K,
Migliaccio T, Costa V, Liguoro D, Cataldi S, D'Esposito V, Maneli
G, Bassolino R, et al: Glucose impacts onto the reciprocal
reprogramming between mammary adipocytes and cancer cells. Sci Rep.
14:246742024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dominiak A, Chelstowska B, Olejarz W and
Nowicka G: Communication in the cancer microenvironment as a target
for therapeutic interventions. Cancers (Basel). 12:12322020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou JX, Taramelli R, Pedrini E,
Knijnenburg T and Huang S: Extracting intercellular signaling
network of cancer tissues using ligand-receptor expression patterns
from whole-tumor and single-cell transcriptomes. Sci Rep.
7:88152017. View Article : Google Scholar : PubMed/NCBI
|
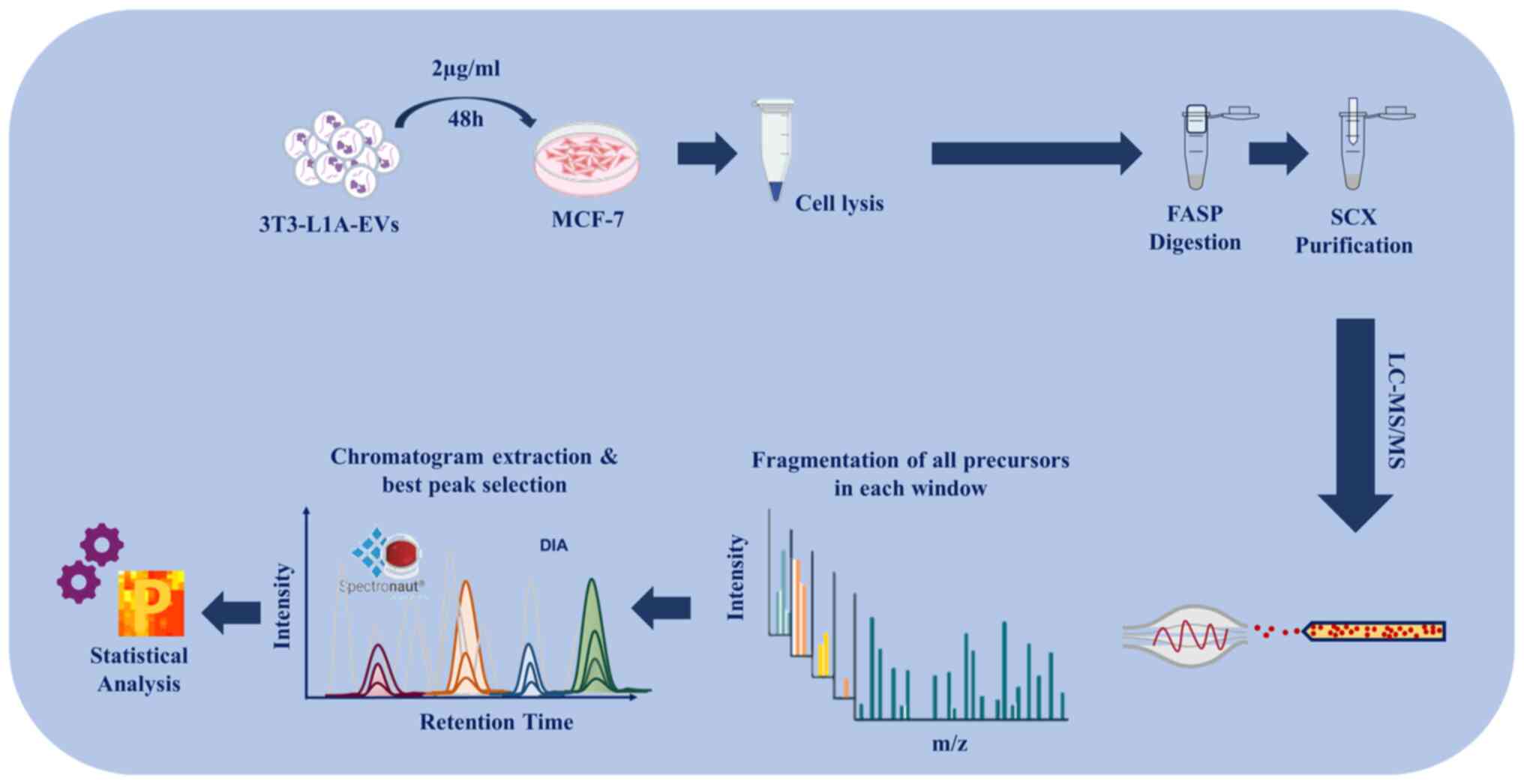
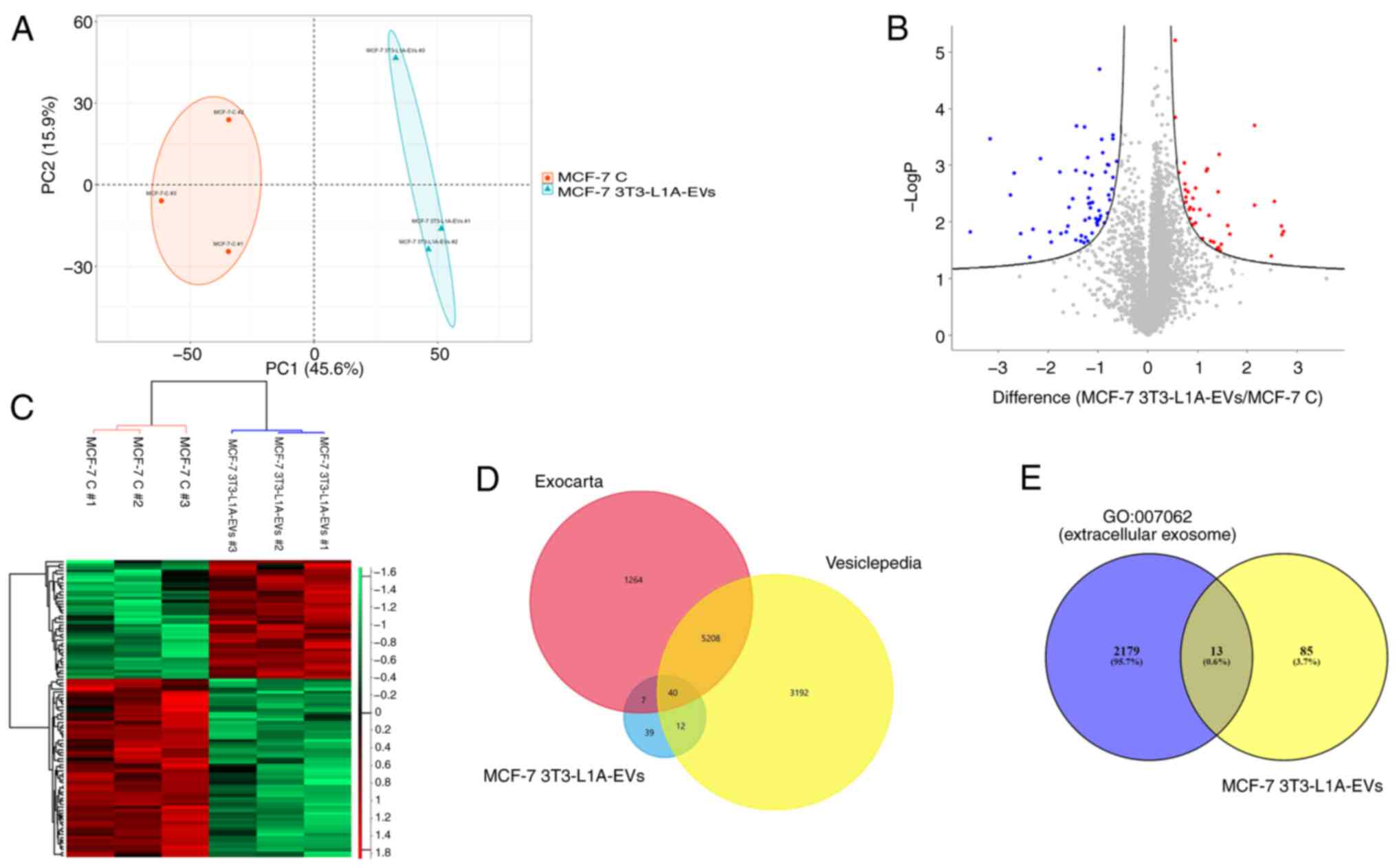
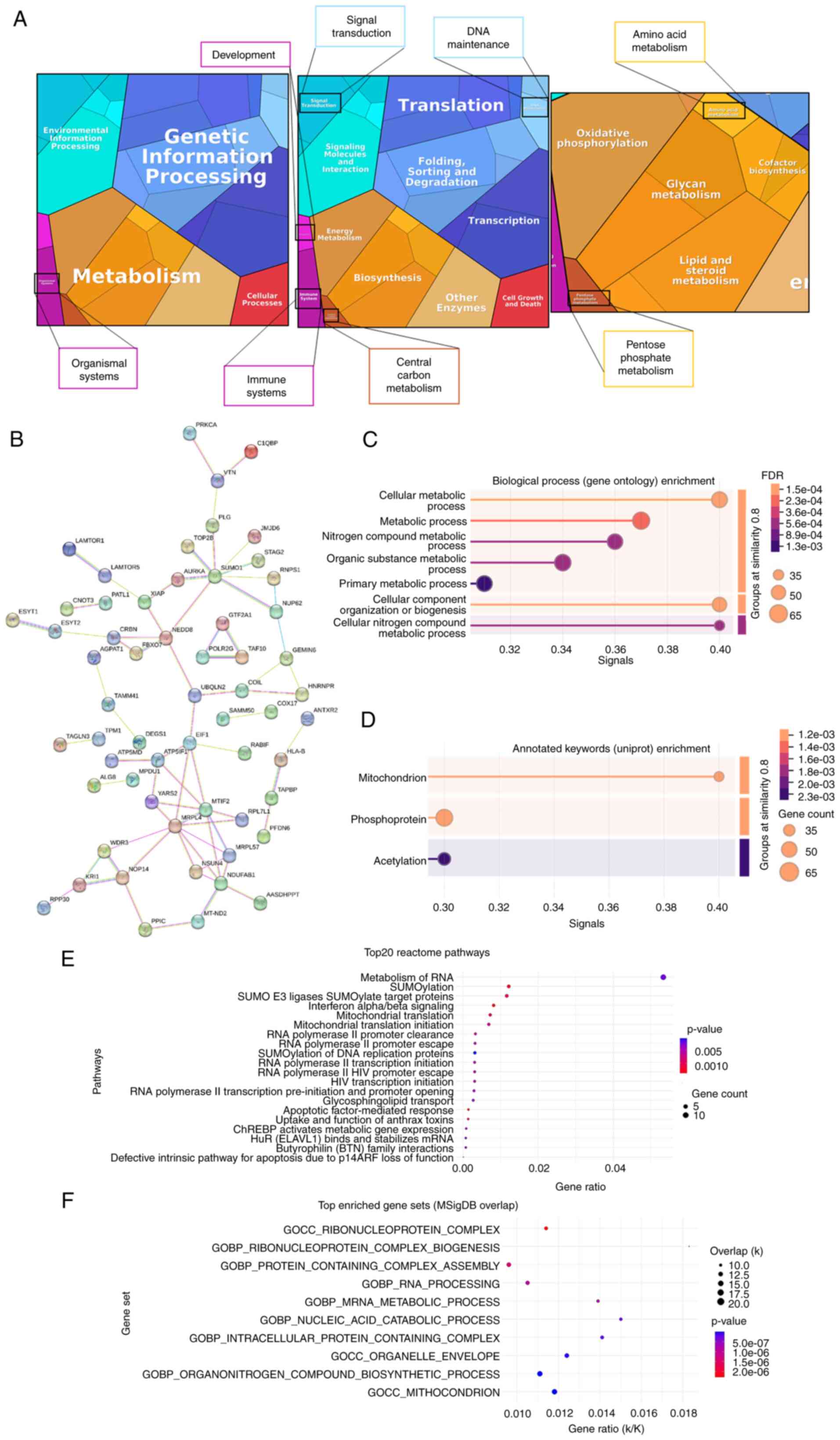
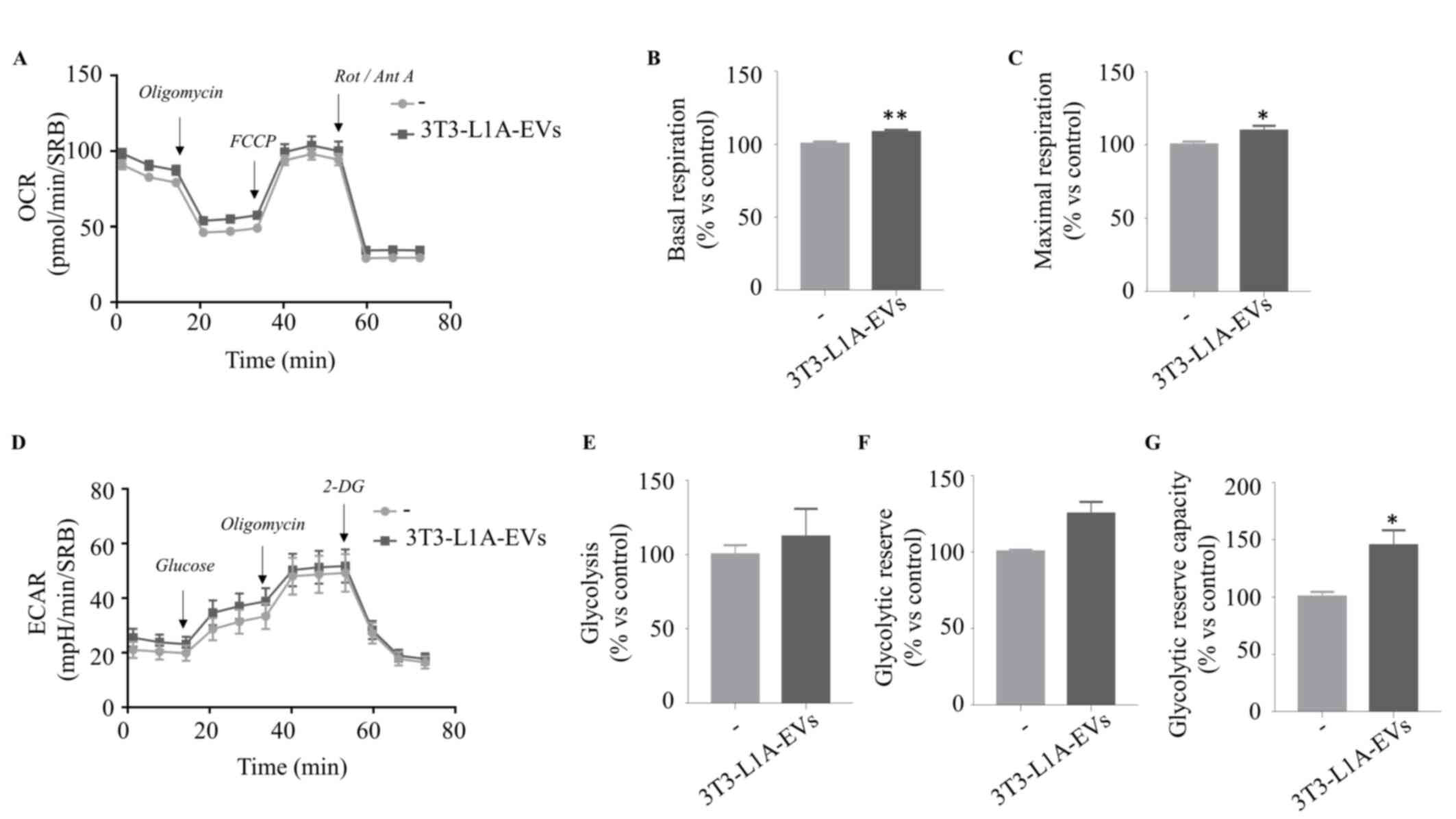
|
4
|
Andò S, Gelsomino L, Panza S, Giordano C,
Bonofiglio D, Barone I and Catalano S: Obesity, leptin and breast
cancer: epidemiological evidence and proposed mechanisms. Cancers
(Basel). 11:622019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelsomino L, Giordano C, Camera GL, Sisci
D, Marsico S, Campana A, Tarallo R, Rinaldi A, Fuqua S, Leggio A,
et al: Leptin signaling contributes to aromatase inhibitor
resistant breast cancer cell growth and activation of macrophages.
Biomolecules. 10:5432020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catalano S, Leggio A, Barone I, De Marco
R, Gelsomino L, Campana A, Malivindi R, Panza S, Giordano C,
Liguori A, et al: A novel leptin antagonist peptide inhibits breast
cancer growth in vitro and in vivo. J Cell Mol Med. 19:1122–1132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barone I, Catalano S, Gelsomino L, Marsico
S, Giordano C, Panza S, Bonofiglio D, Bossi G, Covington KR, Fuqua
SA and Andò S: Leptin mediates tumor-stromal interactions that
promote the invasive growth of breast cancer cells. Cancer Res.
72:1416–1427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giordano C, Vizza D, Panza S, Barone I,
Bonofiglio D, Lanzino M, Sisci D, De Amicis F, Fuqua SA, Catalano S
and Andò S: Leptin increases HER2 protein levels through a
STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol
Oncol. 7:379–391. 2013. View Article : Google Scholar
|
|
9
|
Devericks EN, Carson MS, McCullough LE,
Coleman MF and Hursting SD: The obesity-breast cancer link: A
multidisciplinary perspective. Cancer Metastasis Rev. 41:607–625.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andò S, Naimo GD, Gelsomino L, Catalano S
and Mauro L: Novel insights into adiponectin action in breast
cancer: Evidence of its mechanistic effects mediated by ERα
expression. Obes Rev. 21:e130042020. View Article : Google Scholar
|
|
11
|
Mauro L, Naimo GD, Gelsomino L, Malivindi
R, Bruno L, Pellegrino M, Tarallo R, Memoli D, Weisz A, Panno ML
and Andò S: Uncoupling effects of estrogen receptor α on LKB1/AMPK
interaction upon adiponectin exposure in breast cancer. FASEB J.
32:4343–4355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naimo GD, Forestiero M, Paoli A, Malivindi
R, Gelsomino L, Győrffy B, Leonetti AE, Giordano F, Panza S,
Conforti FL, et al: ERα/LKB1 complex upregulates E-cadherin
expression and stimulates breast cancer growth and progression upon
adiponectin exposure. Int J Cancer. 153:1257–1272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naimo GD, Gelsomino L, Catalano S, Mauro L
and Andò S: Interfering role of ERα on adiponectin action in breast
cancer. Front Endocrinol (Lausanne). 11:662020. View Article : Google Scholar
|
|
14
|
Hartwig S, De Filippo E, Göddeke S, Knebel
B, Kotzka J, Al-Hasani H, Roden M, Lehr S and Sell H: Exosomal
proteins constitute an essential part of the human adipose tissue
secretome. Biochim Biophys Acta Proteins Proteom. 1867:1401722019.
View Article : Google Scholar
|
|
15
|
Durcin M, Fleury A, Taillebois E, Hilairet
G, Krupova Z, Henry C, Truchet S, Trötzmüller M, Köfeler H,
Mabilleau G, et al: Characterisation of adipocyte-derived
extracellular vesicle subtypes identifies distinct protein and
lipid signatures for large and small extracellular vesicles. J
Extracell Vesicles. 6:13056772017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Wu Y, Guo J, Fei X, Yu L and Ma S:
Adipocyte-derived exosomes promote lung cancer metastasis by
increasing MMP9 activity via transferring MMP3 to lung cancer
cells. Oncotarget. 8:81880–81891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fontana F, Anselmi M, Carollo E, Sartori
P, Procacci P, Carter D and Limonta P: Adipocyte-derived
extracellular vesicles promote prostate cancer cell aggressiveness
by enabling multiple phenotypic and metabolic changes. Cells.
11:23882022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lazar I, Clement E, Dauvillier S, Milhas
D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S,
et al: Adipocyte exosomes promote melanoma aggressiveness through
fatty acid oxidation: A novel mechanism linking obesity and cancer.
Cancer Res. 76:4051–4057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Tan J, Ou S, Chen J and Chen L:
Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth
in hepatocellular cancer by targeting the VHL/HIF axis. J Physiol
Biochem. 75:391–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
La Camera G, Gelsomino L, Malivindi R,
Barone I, Panza S, De Rose D, Giordano F, D'Esposito V, Formisano
P, Bonofiglio D, et al: Adipocyte-derived extracellular vesicles
promote breast cancer cell malignancy through HIF-1α activity.
Cancer Lett. 521:155–168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jafari N, Kolla M, Meshulam T, Shafran JS,
Qiu Y, Casey AN, Pompa IR, Ennis CS, Mazzeo CS, Rabhi N, et al:
Adipocyte-derived exosomes may promote breast cancer progression in
type 2 diabetes. Sci Signal. 14:eabj28072021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barone I, Gelsomino L, Accattatis FM,
Giordano F, Gyorffy B, Panza S, Giuliano M, Veneziani BM, Arpino G,
De Angelis C, et al: Analysis of circulating extracellular vesicle
derived microRNAs in breast cancer patients with obesity: A
potential role for Let-7a. J Transl Med. 21:2322023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aliakbari F, Stocek NB, Cole-André M,
Gomes J, Fanchini G, Pasternak SH, Christiansen G, Morshedi D,
Volkening K and Strong MJ: A methodological primer of extracellular
vesicles isolation and characterization via different techniques.
Biol Methods Protoc. 9:bpae0092024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JY, Li YJ, Hu XB, Huang S and Xiang DX:
Preservation of small extracellular vesicles for functional
analysis and therapeutic applications: A comparative evaluation of
storage conditions. Drug Deliv. 28:162–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gelsomino L, Caruso A, Tasan E, Leonetti
AE, Malivindi R, Naimo GD, Giordano F, Panza S, Gu G, Perrone B, et
al: Evidence that CRISPR-Cas9 Y537S-mutant expressing breast cancer
cells activate Yes-associated protein 1 to driving the conversion
of normal fibroblasts into cancer-associated fibroblasts. Cell
Commun Signal. 22:5452024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murfuni MS, Prestagiacomo LE, Giuliano A,
Gabriele C, Signoretti S, Cuda G and Gaspari M: Evaluation of PAC
and FASP performance: DIA-Based quantitative proteomic Analysis.
Int J Mol Sci. 25:51412024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rappsilber J, Mann M and Ishihama Y:
Protocol for micro-purification, enrichment, pre-fractionation and
storage of peptides for proteomics using StageTips. Nat Protoc.
2:1896–1906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tyanova S, Temu T, Sinitcyn P, Carlson A,
Hein MY, Geiger T, Mann M and Cox J: The Perseus computational
platform for comprehensive analysis of (prote)omics data. Nat
Methods. 13:731–740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liebermeister W, Noor E, Flamholz A,
Davidi D, Bernhardt J and Milo R: Visual account of protein
investment in cellular functions. Proc Natl Acad Sci USA.
111:8488–8493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Binder MJ and Pedley AM: The roles of
molecular chaperones in regulating cell metabolism. FEBS Lett.
597:1681–1701. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caruso A, Gelsomino L, Panza S, Accattatis
FM, Naimo GD, Barone I, Giordano C, Catalano S and Andò S: Leptin:
A heavyweight player in obesity-related cancers. Biomolecules.
13:10842023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gelsomino L, Naimo GD, Malivindi R,
Augimeri G, Panza S, Giordano C, Barone I, Bonofiglio D, Mauro L,
Catalano S and Andò S: Knockdown of leptin receptor affects
macrophage phenotype in the tumor microenvironment inhibiting
breast cancer growth and progression. Cancers (Basel). 12:20782020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gelsomino L, Naimo GD, Catalano S, Mauro L
and Andò S: The emerging role of adiponectin in female
malignancies. Int J Mol Sci. 20:21272019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elia I and Haigis MC: Metabolites and the
tumour microenvironment: From cellular mechanisms to systemic
metabolism. Nat Metab. 3:21–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chae HS and Hong ST: Overview of cancer
metabolism and signaling transduction. Int J Mol Sci. 24:122022.
View Article : Google Scholar
|
|
36
|
Cantor JR and Sabatini DM: Cancer cell
metabolism: One hallmark, many faces. Cancer Discov. 2:881–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Shay C, Saba NF and Teng Y: Cancer
metabolism and carcinogenesis. Exp Hematol Oncol. 13:102024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pham DV and Park PH: Tumor metabolic
reprogramming by adipokines as a critical driver of
obesity-associated cancer progression. Int J Mol Sci. 22:14442021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balaban S, Shearer RF, Lee LS, van
Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC,
Fazakerley DJ, Grewal T, et al: Adipocyte lipolysis links obesity
to breast cancer growth: Adipocyte-derived fatty acids drive breast
cancer cell proliferation and migration. Cancer Metab. 5:12017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown KA: Metabolic pathways in
obesity-related breast cancer. Nat Rev Endocrinol. 17:350–363.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Müller G, Schneider M, Biemer-Daub G and
Wied S: Microvesicles released from rat adipocytes and harboring
glycosylphosphatidylinositol-anchored proteins transfer RNA
stimulating lipid synthesis. Cell Signal. 23:1207–1223. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clement E, Lazar I, Attané C, Carrié L,
Dauvillier S, Ducoux-Petit M, Esteve D, Menneteau T, Moutahir M, Le
Gonidec S, et al: Adipocyte extracellular vesicles carry enzymes
and fatty acids that stimulate mitochondrial metabolism and
remodeling in tumor cells. EMBO J. 39:e1025252020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu S, Benito-Martin A, Pelissier Vatter
FA, Hanif SZ, Liu C, Bhardwaj P, Sethupathy P, Farghli AR, Piloco
P, Paik P, et al: Breast adipose tissue-derived extracellular
vesicles from obese women alter tumor cell metabolism. EMBO Rep.
24:e573392023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q,
Bai M, Liu R, Ning T, Zhang L, Yu Z, et al: Adipocyte-derived
exosomal MTTP suppresses ferroptosis and promotes chemoresistance
in colorectal cancer. Adv Sci (Weinh). 9:e22033572022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Galluzzi L, Kepp O, Vander Heiden MG and
Kroemer G: Metabolic targets for cancer therapy. Nat Rev Drug
Discov. 12:829–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martinez-Outschoorn UE, Peiris-Pagés M,
Pestell RG, Sotgia F and Lisanti MP: Cancer metabolism: A
therapeutic perspective. Nat Rev Clin Oncol. 14:11–31. 2017.
View Article : Google Scholar
|
|
49
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou C, Huang YQ, Da MX, Jin WL and Zhou
FH: Adipocyte-derived extracellular vesicles: Bridging the
communications between obesity and tumor microenvironment. Discov
Oncol. 14:922023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blandin A, Dugail I, Hilairet G, Ponnaiah
M, Ghesquière V, Froger J, Ducheix S, Fizanne L, Boursier J, Cariou
B, et al: Lipidomic analysis of adipose-derived extracellular
vesicles reveals specific EV lipid sorting informative of the
obesity metabolic state. Cell Rep. 42:1121692023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gelsomino L, Barone I, Caruso A, Giordano
F, Brindisi M, Morello G, Accattatis FM, Panza S, Cappello AR,
Bonofiglio D, et al: Proteomic profiling of extracellular vesicles
released by leptin-treated breast cancer cells: A potential role in
cancer metabolism. Int J Mol Sci. 23:129412022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sakaue T, Dorayappan KDP, Zingarelli R,
Khadraoui W, Anbalagan M, Wallbillich J, Bognar B, Wanner R,
Cosgrove C, Suarez A, et al: Obesity-induced extracellular vesicles
proteins drive the endometrial cancer pathogenesis: Therapeutic
potential of HO-3867 and Metformin. Oncogene. 43:3586–3597. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mathiesen A, Haynes B, Huyck R, Brown M
and Dobrian A: Adipose tissue-derived extracellular vesicles
contribute to phenotypic plasticity of prostate cancer cells. Int J
Mol Sci. 24:12292023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Giordano C, Gelsomino L, Barone I, Panza
S, Augimeri G, Bonofiglio D, Rovito D, Naimo GD, Leggio A, Catalano
S and Andò S: Leptin modulates exosome biogenesis in breast cancer
cells: An additional mechanism in cell-to-cell communication. J
Clin Med. 8:10272019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Q, Guan C, Liu C, Li H, Wu J and Sun
C: Targeting hypoxia-inducible factor-1alpha: A new strategy for
triple-negative breast cancer therapy. Biomed Pharmacother.
156:1138612022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhi S, Chen C, Huang H, Zhang Z, Zeng F
and Zhang S: Hypoxia-inducible factor in breast cancer: Role and
target for breast cancer treatment. Front Immunol. 15:13708002024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luo S, Jiang Y, Zheng A, Zhao Y, Wu X, Li
M, Du F, Chen Y, Deng S, Chen M, et al: Targeting hypoxia-inducible
factors for breast cancer therapy: A narrative review. Front
Pharmacol. 13:10646612022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu ZJ, Semenza GL and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Infantino V, Santarsiero A, Convertini P,
Todisco S and Iacobazzi V: Cancer cell metabolism in hypoxia: Role
of HIF-1 as Key regulator and therapeutic target. Int J Mol Sci.
22:57032021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yun Z, Maecker HL, Johnson RS and Giaccia
AJ: Inhibition of PPAR gamma 2 gene expression by the
HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of
adipogenesis by hypoxia. Dev Cell. 2:331–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu X, Zhang T, Cheng X and Ma L: Breast
cancer cells and adipocytes in hypoxia: Metabolism regulation.
Discov Oncol. 15:112024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aird R, Wills J, Roby KF, Bénézech C,
Stimson RH, Wabitsch M, Pollard JW, Finch A and Michailidou Z:
Hypoxia-driven metabolic reprogramming of adipocytes fuels cancer
cell proliferation. Front Endocrinol (Lausanne). 13:9895232022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mirabelli M, Misiti R, Sicilia L, Brunetti
FS, Chiefari E, Brunetti A and Foti DP: Hypoxia in human obesity:
New insights from inflammation towards insulin resistance-a
narrative review. Int J Mol Sci. 25:98022024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gaspar JM and Velloso LA: Hypoxia
inducible factor as a central regulator of metabolism-implications
for the development of obesity. Front Neurosci. 12:8132018.
View Article : Google Scholar
|
|
66
|
He Q, Gao Z, Yin J, Zhang J, Yun Z and Ye
J: Regulation of HIF-1{alpha} activity in adipose tissue by
obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am
J Physiol Endocrinol Metab. 300:E877–E885. 2011. View Article : Google Scholar : PubMed/NCBI
|