Introduction
Germ cell tumors (GCTs) are rare, heterogeneous
neoplasms derived from primordial germ cells (PGCs). While they
typically develop in the gonads, they can also arise in
extragonadal midline locations, particularly in children (1). Diagnosis often involves imaging
studies and evaluation of serum markers, including α-fetoprotein,
β-human chorionic gonadotropin and lactate dehydrogenase (LDH)
(2). GCTs account for ~3% of
childhood cancers (3) and
represent ~11% of adolescent cancer cases diagnosed post-puberty
(4). In adults, although GCTs
represent only ~1% of cancers, they are the most common testicular
cancer in young adults (5) and
account for 20-25% of all ovarian neoplasms (6).
The diverse histologies observed in GCTs arise from
the totipotent nature of PGCs and the specific differentiation
stage at which genetic alterations occur (7). According to Teilum's classification,
germinomas, known as seminomas (SEs) in the testes and
dysgerminomas in the ovaries, are undifferentiated cells with
pluripotent features that originate directly from PGCs (Fig. 1). Embryonal carcinomas (ECs) arise
after early embryonic differentiation and can develop into to a
variety of histological subtypes. When ECs follow an embryonic
developmental pathway, they may differentiate into teratomas (TEs),
which contain tissues derived from all three germ layers: Endoderm,
mesoderm and ectoderm. Conversely, if cells undergo extra-embryonic
differentiation, they may give rise to yolk sac tumors (YSTs),
characterized by extra-embryonic mesoblast overgrowth, or
choriocarcinomas (CC), which display trophoblastic differentiation
(7,8). Non-germinomatous GCTs are generally
more aggressive than germinomas, exhibiting increased proliferation
and a higher metastatic potential (9,10).
By contrast, germinomas are associated with a more favorable
prognosis due to their high sensitivity to chemotherapy and
radiotherapy (11).
Surgical resection is typically the first-line
treatment for GCTs (7); however,
systemic therapy is generally required for disease control.
Etoposide- and cisplatin-based chemotherapy has remained the
standard of care for >4 decades, despite the associated toxicity
(12). Cisplatin remains the
cornerstone of treatment, achieving cure rates of 80-90% (13,14). Nonetheless, ~30% of patients show
an incomplete response or develop resistance to cisplatin, leading
to poor clinical outcomes and reduced survival (14,15). Alternative treatments demonstrate
response rates of only 20-40%, with median survival times of ~6-8
months (16).
The biology of cisplatin resistance is
multifactorial (17) and has been
linked to several molecular alterations, including changes in tumor
protein p53, mouse double minute 2 homolog (12), DNA methylation patterns (18), dysregulation of the
platelet-derived growth factor receptor β/AKT signaling pathway
(19) and overexpression of ERBB4
oncogenic EGFR-like receptor (20). These resistance mechanisms are
often categorized into four types: Pre-target (before DNA-binding);
on-target (related to DNA-cisplatin adducts); post-target (cell
death signaling pathways induced by cisplatin-mediated DNA damage);
or off-target (involving pathways not directly linked to
cisplatin-induced signals) (20).
Therefore, comprehensive investigations into these resistance
mechanisms are essential for identifying novel treatment
strategies.
Epithelial-mesenchymal plasticity (EMP) has emerged
as a key hallmark of cancer, particularly in its role as a driver
of metastasis and chemoresistance (21). EMP is a process characterized by a
series of molecular and morphological alterations, accompanied by
the expression of specific markers, leading to the suppression of
epithelial cell characteristics. During EMP, cells acquire
mesenchymal properties, adopting a more malignant phenotype with
enhanced invasion, migration and dissemination capabilities
(22,23). However, given the paucity of
studies investigating the association between EMP and cisplatin
resistance in GCTs, the present review was conducted to compile and
discuss the available evidence.
Unraveling the mechanisms and implications
of EMP
EMP was initially described as
epithelial-mesenchymal transition (EMT), a process in which cells
were considered to exist in one of two distinct states, namely an
epithelial or mesenchymal phenotype (24). Subsequently, the concept of EMT
was redefined as a reversible and transient process, applicable to
diverse contexts, and thus renamed EMP (25,26). A further study revealed that the
cells can adopt intermediate or hybrid phenotypes, known as partial
EMP, displaying characteristics of both epithelial and mesenchymal
cells (27). At present, it is
widely accepted that these hybrid states are prevalent in the tumor
microenvironment (TM), where not all cells complete the full EMP
process, meaning they may never fully acquire a mesenchymal
phenotype (27).
Epithelial cells are tightly organized with minimal
surrounding extracellular matrix (ECM); their distinct
characteristics are defined by structural components such as tight
junctions, adherens junctions, desmosomes and gap junctions, which
maintain strong cell-to-cell adhesion and tissue integrity
(28). These junctions are
responsible for maintaining both structural and functional
integrity. Specifically, they firmly hold cells in place and
prevent individual cell displacement. These cell-to-cell
interactions involve E-cadherin (CDH1)-mediated junctions and
desmosomes, as well as cell-ECM interactions mediated by integrins
and other molecules. Together, these interactions confer polarity
to epithelial cells with distinct basal and apical functions
(29). The polarity of epithelial
cells is maintained by tight junctions, which create distinct
apical and basal regions and form an effective barrier. This
barrier regulates the passage of molecules and ions, thus
preserving cell polarity. Adherens junctions, which are mediated by
the transmembrane protein CDH1, serve to provide robust
cell-to-cell adhesion by connecting to actin filaments, thereby
ensuring structural stability (29). Desmosomes function as anchors for
cells by interacting with intermediate filaments (30). Additionally, gap junctions,
composed of connexins, facilitate direct intercellular
communication. Together, these structures promote the integrity and
cohesion of epithelial tissue, with key markers including CDH1,
desmoplakin, cytokeratins, claudins and occludins (31).
In contrast to epithelial cells, mesenchymal cells
display reduced intercellular adhesion and lack apical-basal
polarity. These cells interact with ECM components through
integrins at focal adhesion sites, enabling cell movement. The
cells also feature a cytoplasm rich in vimentin (VIM) filaments, a
mesodermal marker, and form irregular structures with enhanced
migratory capacity. Other expressed markers include N-cadherin
(CDH2), smooth muscle α-actin (α-SMA), fibroblast-specific protein
1 (FSP-1), fibronectin (FN1), type I collagen (COL1A1) and matrix
metalloproteinases (MMPs) (32).
During EMP, epithelial cells lose cellular polarity
by downregulating the expression of cytokeratins and adhesion
molecules, such as CDH1. Concurrently, the expression of
mesenchymal markers, such as α-SMA, FSP-1, VIM, CDH2, FN1, COL1A1
and MMPs, is upregulated. Cells with polyhedral morphologies begin
to adopt a fibroblast-like morphology (28). This transition is accompanied by
enhanced migratory and invasive capacities, resistance to apoptosis
and increased production of ECM components (32).
EMP can be triggered in various biological contexts
by diverse signaling molecules. These molecules trigger distinct
signaling pathways, ultimately activating a specific set of
transcription factors (TFs), often referred to as 'master
regulators' of EMP. These include members of the Snail family
(Snail1 and Snail2/Slug), the zinc finger E-box-binding homeobox
proteins (Zeb1 and Zeb2) and the Twist family (Twist1 and Twist2).
These TFs suppress the expression of epithelial markers and
activate mesenchymal markers (33).
EMP is categorized into three types. EMP type 1 has
been associated with a variety of physiological processes,
particularly during embryogenesis (Fig. 1), where it plays a key role in the
migration and differentiation of cells that form the germ layers
(34). These germ layers are the
origin of tissue and organ development. EMP type 1 has been
demonstrated to be non-aggressive and non-invasive; it has been
shown to be essential for the proper functioning of physiological
processes, including organ and tissue formation, placenta
development and embryo implantation (35). EMP type 1 is also involved in the
formation of melanocytes from the neural crest, which is essential
for pigmentation (22).
EMP type 2 plays an important role in tissue
regeneration and fibrosis, including conditions such as renal and
pulmonary fibrosis, and can be categorized into physiological
(tissue regeneration) and pathological (persistent fibrosis)
processes (36). In its
physiological form, cells mobilize into fibroblast-like phenotypes,
facilitating tissue repair after trauma (37). This regenerative phase is linked
to the inflammatory response, which subsides once inflammation is
resolved, as observed in wound healing and tissue regeneration
(37). However, when inflammation
persists, physiological EMP type 2 can progress to a pathological
state (31), contributing to
chronic fibrosis and organ damage, as observed in the kidney and
liver (38). This process is
driven by inflammatory cells and fibroblast-like cells, which
release various inflammatory signals (39) and contribute to a collagen-rich
ECM. Protein markers, such as VIM, serve as molecular targets to
identify signs of persistent inflammation that may progress to a
chronic state, thereby establishing a link with EMP type 2
(38).
EMP type 3 is closely linked to cancer progression,
underscoring the importance of studies exploring the connection
between EMP and tumor dissemination (Fig. 1). Such studies can provide an
understanding of the molecular mechanisms influencing signaling
pathways that regulate EMP activation or its inhibition (40,41), potentially informing novel
therapeutic strategies. Furthermore, identifying specific markers
associated with EMP is essential for understanding a tumor's
metastatic potential, improving patient survival predictions and
developing targeted therapies for treatment-resistant cancer
(42).
The acquired plasticity of cancer cells through EMP
has been widely documented across various cancer types and is
strongly associated with resistance to cisplatin (43,44). In the following sections, key
studies are reviewed that explore the role of EMP markers in GCTs,
focusing on their involvement in cisplatin resistance, drawing
insights from both in vitro and in vivo models.
EMP analysis in vitro models of
GCTs
Different cell lines, whose characteristics are
presented in Table I, have been
used to investigate the molecular mechanisms, genetic alterations
and therapeutic responses in GCTs. Most cell lines have been
isolated from the testes of adult individuals (aged 22 years or
older). Cell lines representing the various histological types of
GCTs from children and adolescents are scarce or unavailable,
except for choriocarcinoma cell lines from fetal placentas. This
lack of pediatric and adolescent cell lines significantly hinders
research and molecular studies. Next, the present study will review
key studies that have used GCT cell lines to research biomarkers
and therapeutic targets, offering insights into future therapeutic
strategies.
 | Table ICharacteristics of human germ cell
tumor cell lines. |
Table I
Characteristics of human germ cell
tumor cell lines.
| Cell lines | Primary tissue | Sex | Age, years | Histologies |
|---|
| NTERA-2 | Testis | Male | 22 | Embryonal
carcinoma |
| 2102EP | Testis | Male | 23 | Embryonal
carcinoma |
| 1777N Rpmet | Testisa | Male | 25 | Embryonal
carcinoma |
| NCCIT | Mediastinum | Male | 24 | Embryonal
carcinoma |
| 833KE | Abdomen | Male | 19 | Embryonal
carcinoma |
| BeWo | Placenta | Male | Fetus |
Choriocarcinoma |
| JAR | Placenta | Male | Fetus |
Choriocarcinoma |
| JEG3 | Placenta | Male | Fetus |
Choriocarcinoma |
| TCam-2 | Testis | Mal | 35 | Seminoma |
| SEM-1 | Mediastinum | Male | 58 | Seminoma |
| GCT27 | Testis | Male | NI | Teratoma |
| SuSa | Testis | Male | 46 | Teratoma |
| NOY-1 | Ovary | Female | 28 | Yolk sac tumor |
| H12.1 | Testis | Male | 19 | Mixed (embryonal
carcinoma and teratoma) |
| 1411HP | Testisb | Male | 17 | Mixed (embryonal
carcinoma and yolk sac tumor) |
ECM remodeling and its role in tumor
behavior
Both normal and transformed mesenchymal and
epithelial cells rely on ECM proteins for growth and
differentiation in vitro. These proteins play a key role in
processes such as motility, wound healing and tumor metastasis,
mechanisms that are closely linked to EMP. In teratoma cell
culture, cells secrete adhesion proteins such as vitronectin, FN1,
laminin and type IV collagen (45). In human GCTs, differentiated cells
in low-density cultures produce FN1 in a differentiation-dependent
manner, with less differentiated cells exhibiting decreased FN1
synthesis (46).
In this regard, some studies indicate that
modulation of those ECM proteins influences cell adhesion,
plasticity and invasion capacity in vitro (47,48). In BeWo cells, the heme oxygenase-1
(HMOX1) gene, which is upregulated in various tumor types,
has been shown to increase adhesion by modulating laminin and FN1.
This process, dependent on peroxidasin homolog, a cell surface
peroxidase, occurs at both the gene level and HMOX1 protein level
(Table II) (47). Therefore, the protein network
activated by HMOX1 influences key cellular functions, such as
adhesion, signaling and transport, supporting tumor growth and
dissemination (47).
 | Table IIAssociation between EMP and
resistance in different germ cell tumor cell lines. |
Table II
Association between EMP and
resistance in different germ cell tumor cell lines.
A, Embryonal
carcinoma
|
|---|
| First author,
year | Cell lines | Association with
EMP | (Refs.) |
|---|
| Pulzová et
al, 2021 | 1777N and
Rpmet | Cell adhesion
remodeling. | (48) |
| Liu et al,
2022 | NCCIT | Long non-coding RNA
LINC00313I is upregulated in TGCT tissues. Moreover,
LINC00313I-silencing is associated with decreased migration and
invasion in the NCCIT cell line. Potential inhibitor:
Panobinostat. | (66) |
| Abada et al,
2014 | NTERA-2 | Cisplatin triggers
differentiation which leads to resistance. This process is linked
with reduced expression of NANOG and POU5F1 and
increased NES, STMN2 and FN1. | (81) |
| Wu et al,
2012 | NCCIT and
NTERA-2 | Hypoxia reduces
levels of POU5F1 protein which triggers drug resistance.
SENP1 can normalize POU5F1 levels. Potential inhibitor:
SENP1. | (64) |
| Koch et al,
2003 | NTERA-2, 2102 EP
and NCCIT | Hypoxia reduces
drug cytotoxicity. Potential inhibitor: Erythropoietin. | (65) |
|
| B,
Choriocarcinoma |
|
| First author,
year | Cell lines | Association with
EMP | (Refs.) |
|
| Tauber et
al, 2010 | BeWo | High expression of
HMOX1 gene and ECM remodeling. | (47) |
| Butler et
al, 2009 | BeWo | Increased ILK,
along with the expression of p-AKT and SNAIL proteins. | (53) |
| Ng et al,
2011 | BeWo | Increased invasive
capacity with increased TWIST and decreased
CDH1. | (55) |
| Iwaki et al,
2004 | BeWo | Hypoxia increases
mRNA levels of fibronectin domains and integrin α-5,
but not integrin α-1. | (62) |
| DaSilva-Arnold
et al, 2019 | BeWo | Overexpression of
ZEB2 changes the morphology of BeWo and JEG-3 cells towards
a mesenchymal phenotype and promotes a gene expression profile
consistent with EMP. | (58) |
| Xu et al,
2022 | JEG3 | High expression of
HIF-1α is found. HIF-1α depends on DEC1 to trigger EMP by
β-catenin signaling. Potential inhibitor: DEC1 inhibitors. | (61) |
| Zhang et al,
2024 | BeWo and JAR | Transcription
factor TWIST1 is found to be upregulated compared with a
normal extravillous trophoblast cell line. | (56) |
| Tian et al,
2015 | JAR and JEG-3 | Overexpression of
HIF-1α depends on NOTCH signaling to promote EMP. Potential
inhibitor: DAPT. | (60) |
| Xue et al,
2020 | JAR JEG-3 | Forskolin promotes
invasion, migration, vasculogenic-like network formation and
NOTCH-1-mediated EMP. | (63) |
|
| C, Seminoma |
|
| First author,
year | Cell lines | Association with
EMP | (Refs.) |
|
| Teveroni et
al, 2022 | SEM-1 | PTTG1
depends on ZEB1 to repress CDH1. This cooperation
facilitates cell invasion and cell growth. | (59) |
|
| D, Various cell
lines |
|
| First author,
year | Cell lines | Association with
EMP | (Refs.) |
|
| Skowron et
al, 2022 | TCam-2, 2102EP, JAR
and GCT72 | ECM remodeling
promotes migration and invasion. Secreted collagen I/IV and
fibronectin causes decreased sensitivity to cisplatin. | (50) |
The EC 2102EP cell line, derived from a primary
tumor, and 1777NRpmet, a differentiated EC cell line with immature
teratoma features derived from a metastatic tumor, were analyzed in
a previous study, revealing increased expression of tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein β and
γ polypeptide, caldesmon 1, filamin A, VIM and vinculin
genes in 1777NRpmet compared with the primary tumor, while
PARK7 expression decreased (48). These findings suggest a role for
cell adhesion remodeling and ECM crosslinking in testes invasion,
EMP and metastasis (48).
ECM remodeling has also been observed in
cisplatin-resistant ovarian cancer cell lines, characterized by
elevated expression of the collagen type VI α 3 chain gene.
Furthermore, these cells demonstrated increased chemoresistance
when cultured on collagen IV-coated dishes (49). Similarly, co-culture of GCT cell
lines with TM cells has shown an interplay, where exposure to
TM-secreted components such as collagen I/IV and FN1 decreased
sensitivity to cisplatin (50).
These studies demonstrate that several factors contribute to the
critical role of ECM remodeling and adhesion molecules in
triggering cell plasticity, cisplatin resistance and tumor cell
survival.
Beyond ECM proteins, their interaction with other
cell components, such as integrins and their signaling pathways,
has also been implicated in the EMP process and treatment
resistance (51,52). Integrin-linked kinase (ILK) is
related to adhesion to the extracellular environment, transduction
signaling, and interaction with β-catenin (CTNNB1) and cytoskeletal
proteins. BeWo cells exhibit increased ILK activity during the
differentiation process, along with the expression of
phosphorylated AKT and SNAIL proteins (53).
During EMP, dynamic alterations occur not only in
ECM-cell interactions but also in cell-cell adhesion, modulating
adhesion and structural proteins. In this setting, CDH2 is
upregulated while CDH1 is downregulated, a mechanism that plays a
crucial role in malignancies, favoring tumor cell metastasis and
migration. Upregulation of CDH1 may be linked to decreased
migratory ability in cancer cells and increased sensitivity to cell
death, likely due to the inhibition of the EMP process (43). These changes are widely associated
with TFs such as TWIST, ZEB1/2 and SNAIL/SLUG (54).
EMP and transcriptional regulation
EMP is governed by specific EMP TFs that orchestrate
transcriptional reprogramming to promote the loss of epithelial
traits and acquisition of mesenchymal properties.
Expression of TWIST and CDH1 genes was
previously evaluated in BeWo cells during differentiation and
fusion (55). This
differentiation process was accompanied by an increase in
TWIST expression and a decrease in CDH1 expression,
even in the presence of exogenous TWIST expression. However,
differentiation failed when TWIST was knocked down.
Moreover, treatment with 8-Br-cAMP increased TWIST levels.
This demonstrates the role of these molecules in the
differentiation and invasive capacity promoted in the EMP process
(55). A study on gestational
trophoblastic disease evaluated the expression and influence of
TWIST1 gene silencing in the CC BeWo and JAR cell lines
(56). It was revealed that this
TF was upregulated, and its knockout significantly inhibited the
proliferation, migration and invasion of these cells. Additionally,
protein analysis revealed that silencing of the TWIST1 gene led to
increased expression of CDH1 and decreased expression of CDH2 and
VIM. Thus, TWIST1 silencing promotes an epithelial
phenotype, inhibiting EMP and malignant behavior (56). Furthermore, increased TWIST
protein expression was observed in cisplatin-resistant ovarian
cancer cell lines (57).
In the CC BeWo and JEG-3 cell lines, overexpression
of ZEB2 enhanced cell migration and invasion capabilities.
Alongside ZEB2 upregulation, changes were observed in gene
expression, cell morphology and protein levels (58). At the gene expression level,
alterations occurred in EMP markers, though some divergence was
noted among cell clones. Additionally, JEG-3 clones overexpressing
ZEB2 exhibited differential gene expression of other EMP
markers, such as decreased SNAIL and increased SLUG
and TWIST1, distinct from BeWo clones. This suggests that
ZEB2 overexpression activates cell-specific downstream
pathways to promote EMP. Lastly, protein expression of EMP markers
was more pronounced in BeWo cells than that in JEG-3 cells
(58).
Our group investigated the role of EMP TF
SLUG in GCTs (21). The
analysis revealed distinct expression profiles of EMP markers
across different histologies. SEs exhibited lower expression of EMP
markers, while ECs and mixed GCTs showed higher expression.
SNAIL and SLUG displayed varying expression levels in
each histology, and patients with lower SLUG expression had
a longer median progression-free survival time. Furthermore,
integrated analyses showed that patients expressing low levels of
both factors (SNAILlowSLUGlow) had a
higher progression-free survival rate compared to those with high
expression of both TFs
(SNAILhighSLUGhigh) (21).
The role of EMP mediators in enhancing invasiveness
is evident across various GCT types, potentially involving
different molecular pathways. In human SE, Securin (PTTG1)
has been identified as an EMP mediator. This gene promotes
invasiveness by expressing MMP2 protein, which facilitates
migration and invasion (59). In
the SE SEM-1 cell line, PTTG1 relies on ZEB1 to
exhibit invasion and cell growth features, and to form an axis that
represses CDH1 expression. Additionally, database analysis
shows that in seminoma tumors, where PTTG1 is more localized in the
nucleus compared with the non-seminoma subtype, CDH1
expression is significantly lower than that observed in
non-seminomas (59). Based on
these observations, the PTTG1-ZEB1-CDH1 axis appears
particularly relevant in SEs compared with NGGCTs (59). However, further research is needed
to elucidate the specific mechanisms through which these mediators
influence tumor progression and response to treatment.
Hypoxia, long non-coding RNAs and
emerging mechanisms
In addition to differentiation-related pathways,
external environmental factors, such as hypoxia, influence
resistance mechanisms and EMP in GCTs. The role of Notch receptor
(NOTCH) signaling was previously investigated in CC, focusing on
how it links hypoxia to EMP (60). Overexpression of hypoxia-inducible
factor 1-α (HIF-1α) protein in JAR and JEG-3 cell lines reduced the
expression of epithelial markers (CDH1, Cytokeratin 18 and
Cytokeratin 19) while enhancing cell migration and invasiveness.
HIF-1α overexpression was positively correlated with NOTCH1 and
Hairy and enhancer of split-1 proteins. Inhibiting NOTCH1 with
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester reversed the EMP changes, increasing CDH1, CK18 and CK19,
while reducing the activities of MMP2 and MMP9. Thus, HIF-1α
promotes CC metastasis through EMP via the NOTCH signaling pathway
(60).
Moreover, JEG-3 cells exhibit higher HIF-1α protein
expression compared with control chorionic trophoblast cells.
Knocking down HIF-1α decreases cell proliferation and
migration. Additionally, it is associated with reduced cell
invasion, increased CDH1, and decreased VIM and
α-SMA, thereby suppressing EMP. HIF-1α has been shown
to modulate deleted in esophageal cancer 1 (DEC1). However,
when DEC1 is overexpressed, it partially reverses the
effects of HIF-1α knockdown, indicating that both
HIF-1α and DEC1 regulate EMP mediators (VIM, α-SMA
and Wnt/CTNNB1 signaling) (61).
In one study using BeWo cells and the early placenta, hypoxic
conditions induced higher mRNA levels of genes encoding FN1 domains
and integrin α-5 compared with normoxic conditions, while the
levels of integrin α-1 mRNA decreased (62).
Hypoxia is also associated with vasculogenic mimicry
(VM), a process whereby cancer cells form tubular channels
mimicking blood vessels. These cells may undergo EMP to exhibit an
endothelial-like phenotype, enhancing tumor aggressiveness and
facilitating metastasis (63). In
this context, in a previous study, JAR and JEG-3 cells treated with
forskolin, a cAMP activator, exhibited increased VM, migration and
invasive capacity. These cells produced MMP2 and MMP9 at both the
gene and protein levels, increasing the expression of mesenchymal
markers via NOTCH1 signaling. NOTCH1 signaling can be triggered
under hypoxic conditions and is linked to EMP markers (63).
In GCTs, hypoxia reduces POU5F1 protein levels in EC
cells, contributing to cisplatin resistance. However,
overexpression of the sentrin-specific peptidase 1 (SENP1)
gene normalizes POU5F1 protein levels and restores drug sensitivity
(64). Similarly, in EC NTERA-2,
2102EP and NCCIT cell lines exposed to cisplatin under hypoxic and
normoxic conditions, cisplatin was less effective under hypoxia.
Hypoxic cells displayed a higher IC50
(NCCIT>2102EP>NTERA-2), indicating that hypoxia reduces
cytotoxicity not only for cisplatin but also for other drugs,
suggesting it is not exclusively linked to cisplatin resistance in
GCTs (65).
Another factor associated with EMP is long
non-coding RNAs, such as SPRY4, LINC00467 and
LINC00313, which regulate gene expression. LINC00313
was found to be upregulated in TGCT cells, serving as a good
biomarker for diagnosis and prognosis. In silico analyses
revealed that LINC00313 was associated with lower immune
cell infiltration (CD4+, CD8+ and dendritic
cells) and an altered immune microenvironment (66). In vitro studies
demonstrated that LINC00313 promotes migration and invasion,
and acts as an EMP mediator by upregulating VIM, ZEB1, SNAIL,
CTNNB1 and CDH2 proteins, potentially through microRNA
(miRNA/miR)-138-5p, miR-150-5p, miR-204-5p and miR-205-5p (66). Moreover, long non-coding RNAs are
reportedly involved in cisplatin resistance in other tumors
(43). Thus, targeting
LINC00313 could be a promising strategy to overcome
cisplatin resistance by inhibiting EMP, thereby reducing cancer
cell proliferation, migration and invasion.
Despite the scarcity of studies exploring these
associations, research on EMP markers in GCT cell lines, primarily
BeWo, JAR and JEG-3 (53-56,58,63), has focused on differentiation,
invasion and EMP in pregnancy-related phenomena rather than GCTs.
Even fewer studies have evaluated EMP in GCTs and its association
with cisplatin resistance. In summary, various GCT cell lines have
provided valuable insights into the molecular mechanisms underlying
EMP. ECM remodeling, integrin signaling and dynamic regulation of
key TFs, such as TWIST, ZEB1 and SLUG are
critical drivers of EMP and tumor progression. However, the limited
availability of pediatric and adolescent GCT cell lines poses a
significant challenge to understanding the EMP mechanisms in these
populations. Additionally, external factors, such as hypoxia and
long non-coding RNAs, add complexity to the regulation of EMP and
chemoresistance in GCTs.
EMP pathway intersections underlying
cisplatin resistance
As aforementioned, cisplatin resistance mechanisms
can be classified as pre-target, on-target, post-target and
off-target (20). A comparison
between NTERA-2 cells and their resistant counterparts, NTERA-2R
cells, reveals similar behavior in drug uptake, efflux and
DNA-binding. However, the resistant lineage exhibits alterations in
cell cycle regulation and cell death response. These findings
suggest that resistance mechanisms in NTERA-2R are less related to
DNA binding and damage induction, and more associated with cellular
responses to this damage, implicating post-target or off-target
mechanisms (13). Supporting this
hypothesis, our group demonstrated that NTERA-2R cells showed
upregulation of DNA repair-related genes (O-6-methylguanine-DNA
methyltransferase; complex subunit, DNA damage recognition and
repair factor; and DNA polymerase delta 4, accessory subunit)
compared with NTERA-2 cells (Table
III). This overexpression was accompanied by more aggressive
cellular behaviors, such as increased proliferation, colony
formation and migration. As expected, NTERA-2R cells exhibited a
lower rate of apoptosis following cisplatin treatment compared with
NTERA-2 cells. However, combining the proteasome inhibitor MG-132
with cisplatin made the apoptosis rate of the two cell lines
comparable, highlighting a potential combinatorial strategy to
overcome cisplatin resistance (67).
 | Table IIIAdditional molecular mechanisms of
resistance observed in germ cell tumors. |
Table III
Additional molecular mechanisms of
resistance observed in germ cell tumors.
| First author,
year | Cell lines | Association with
resistance | (Refs.) |
|---|
| Lengert et
al, 2022 | NTERA-2 and
NTERA-2R | DNA repair genes
increased: MGMT, XPC and POLD4. Potential
inhibitor: Proteasome inhibitor (MG-132). | (67) |
| Fichtner et
al, 2022 | NTERA-2R, NCCIT-R
and 2102EP-R | Upregulated
proteins ANXA1, TAGLN, COL1A1 and VIM. Upregulated protein ANXA1;
downregulated COL1A1. Upregulated protein ANXA1; downregulated
TAGLN and VIM. | (14) |
| Cardoso et
al, 2024 | NTERA-2R | Cisplatin treatment
increased FN1, VIM, ACTA2, COL1A1,
TGF-β and SLUG. | (21) |
| Schmidtova et
al, 2019 | NTERA-2 | Increase in
ALDH1A1, ALDH1A3 and NANOG. Decrease in
ALDH1A2 and ALDH1B1. Decreased proteins NANOG and
SOX2. | (73) |
| Schmidtova et
al, 2019 | NCCIT-R | Increase in
ALDH1A2, ALDH1A3, NANOG and CD133. No
protein expression changes. Potential inhibitor: ALDH inhibitor
(Disulfiram). | (73) |
| Miranda-Gonçalves
et al, 2021 | NCCIT-R | Increased
VIRMA. | (90) |
| Port et al,
2011 | NTERA-2R, NCCIT-R
and 2102EP-R | Upregulation of
hsa-miR-10b and hsa-miR-512-3p. | (91) |
| Lobo et al,
2020 | NTERA-2R | Increased
expression of genes HDAC8, 9 and 11. Only HDAC11
protein was significantly expressed. Potential inhibitor: HDAC
inhibitors (Belinostat and Panobinostat). | (17) |
| Schmidtova et
al, 2020 | NOY-1-R | Increase of CD133,
ABCG2 and ALDH3A1. Reduced gene and promoter methylation. Increased
expression of ALDH1A3. accompanied by higher ALDH enzymatic
activity. | (74) |
| Roška et al,
2020 | H12.1D | Upregulated
TRIB3; IGFBP2, NANOG, POU5F1 and SOX2 genes
downregulated. | (75) |
| Schmidtova et
al, 2021 | TCam-2R and
NCCIT-R | Increased gene and
protein expression of β-catenin. | (84) |
| Schmidtova et
al, 2021 | NTERA-2R | Decreased β-catenin
and cyclin D1 expression levels. Potential inhibitor: Wnt/β-catenin
inhibitor (PRI-724). | (84) |
| Funke et al,
2023 | JAR-R and
2102EP-R | Overexpression of
NAE1. Potential inhibitor: NAE1 inhibitor (MLN4924). | (88) |
| Skowron et
al, 2020 | TCam-2, 2102EP,
NCCIT and JAR | Increased gene and
protein CDK4/CDK4; CDK6/CDK6 expression weak or absent. Potential
inhibitor: CDK4/6 inhibitors (palbociclib and ribociclib). | (92) |
| Noel et al,
2010 | 833K-R, Susa-R and
GCT27-R | Upregulation of
CCND1. | (93) |
Regarding DNA repair-related protein expression, a
study involving NTERA-2, NCCIT and 2102EP cell lines, and their
resistant counterparts, revealed deregulated proteins in at least
two resistant cell lines. These included downregulation of
cystathionine β-synthase and cystathionine γ-lyase, alongside
upregulation of annexin A1 (ANXA1), L-LDH A chain and
NADPH-adrenodoxin oxidoreductase. Additionally, transgelin (TAGLN)
was upregulated in NTERA-2R cells but downregulated in 2102EP-R
cells, while COL1A1 and VIM showed contrasting expression patterns,
being upregulated in NTERA-2R cells but downregulated in NCCIT-R
and 2102EP-R cells, respectively (Table III). Among these proteins,
ANXA1, TAGLN, COL1A1 and VIM are associated with EMP in GCTs or
other cancer types (68-70). Gene enrichment analysis detected
DNA repair-associated proteins across all resistant lineages, but
an EMP gene set was detected in NTERA-2R cells. These results
further corroborate the association of cisplatin resistance and EMP
in GCTs (14).
Our group also evaluated EMP markers in NTERA-2 and
NTERA-2R cells through mRNA quantification, with some findings
confirmed at the protein level. After 72 h of cisplatin treatment,
mRNA levels of FN1, VIM, α smooth muscle actin,
COL1A1, TGF-β and SLUG were higher in
resistant cells compared with those in parental cells (Table III) (21). Additionally, CDH1 and its
corresponding protein exhibited an increasing trend in NTERA-2R,
while CDH2 and its protein displayed a decreasing trend
(21). No differences were
observed in SNAIL expression levels (21). Together, the findings suggest that
EMP may be involved in chemoresistance (21).
Upregulation of cancer stem cell (CSC) markers is a
well-documented phenomenon in chemoresistant solid tumors, with
aldehyde dehydrogenase (ALDH) as a key marker (71,72). However, in GCTs, this upregulation
is not always consistent. A comparative study of
cisplatin-sensitive NTERA-2 cells and their resistant counterpart
(NTERA-2R) revealed increased gene expression levels of aldehyde
dehydrogenase 1 family member A1 (ALDH1A1), ALDH1A3
and NANOG, alongside decreased levels of ALDH1A2 and
ALDH1B1 in the resistant NTERA-2 cells. However, NANOG and
SOX2 protein levels were decreased in the NTERA-2R cells. In
another EC cell line, NCCIT-R, ALDH1A2, ALDH1A3,
NANOG and prominin-1 (CD133) levels were
increased, but protein levels showed no significant changes
(Table III). Moreover,
treatment with the ALDH inhibitor disulfiram reduced cell
viability, which was potentiated by combined cisplatin treatment.
This combination also reduced the viability of resistant cells in a
3D spheroid model (73).
Similarly, CSC markers were evaluated in an ovarian
YST cell line (NOY-1) and its cisplatin-resistant variant
(NOY-1-R). The resistant cells exhibited increased protein
expression of CD133, ATP binding cassette subfamily G member 2 (JR
Blood Group) (ABCG2) and ALDH3A1. This upregulation was associated
with reduced gene promoter methylation, increased expression of
ALDH1A3 and higher ALDH enzymatic activity (Table III). These findings underscore
the potential role of the ALDH protein family in cisplatin
resistance in refractory YST and suggest cross-resistance to ALDH
inhibitors in cisplatin-resistant GCTs (74).
Pluripotency markers have also been examined in
cisplatin-sensitive cell lines (H12.1, 2102EP and NTERA-2) and
their resistant counterparts (H12.1D, 1411HP and 1777NRpmet).
Several genes related to pluripotency, cell metabolism,
proliferation and migration were identified. Upregulated genes
included PCP4, tribbles pseudokinase 3 (TRIB3),
ID2 and SLC40A1, while downregulated genes included
insulin like growth factor binding protein 2 (IGFBP2),
L1TD1, NANOG, POU5F1 and SOX2 (75) (Table III). Among these, TRIB3
(76), IGFBP2 (77), NANOG (78), POU5F1 (79) and SOX2 (80) are associated with EMP-related
processes.
Signaling pathways related to differentiation can
modulate FN1 expression in cisplatin-resistant NTERA-2 cells. In a
previous study, cisplatin treatment reduced the expression of the
TFs NANOG and POU5F1, which maintain the
undifferentiated state, while promoting expression of
differentiation markers, such as nestin (NES), Stathmin-2
(STMN2) and FN1. Cells that did not downregulate
NANOG and POU5F1 failed to develop resistance to
cisplatin. Overexpression of NANOG prevented
cisplatin-induced resistance, indicating a link between cellular
differentiation and drug resistance (81).
The Wnt/CTNNB1 signaling pathway is known to play a
role in GCTs (82,83). The effects of Wnt/CTNNB1 signaling
pathway inhibition using PRI-724 were evaluated in GCT cell lines
(NTERA-2, JEG-3, TCam-2 and NCCIT) and their resistant variants
(NTERA-2R, JEG-3-R, TCam-2-R and NCCIT-R). Gene and protein
expression levels of CTNNB1 and cyclin D1 were increased in
TCam-2-R and NCCIT-R cells but decreased in NTERA-2R cells.
Treatment with PRI-724 induced pro-apoptotic effects (activation of
caspases 3/7) in all cell lines, indicating that the Wnt/CTNNB1
pathway contributes to resistance (84).
EMP can be regulated by post-translational
modifications (85), which are
known to influence cancer aggressiveness and cisplatin resistance
in tumors (86,87). One such process is neddylation,
which involves conjugation of the ubiquitin-like molecule NEDD8 to
a target protein, altering its stability, function or subcellular
localization. Neddylated proteins may undergo degradation via the
ubiquitin-proteasome system, and aberrant degradation of tumor
suppressor proteins through this pathway can contribute to
carcinogenesis (88,89).
A genome-scale CRISPR/Cas9 screen showed
upregulation of NEDD8-activating enzyme E1 subunit 1 (NAE1)
gene in cisplatin-resistant colonies of JARMPHv2/SAMv2 and
2102EPMPHv2/SAMv2 cell lines. Inhibition of neddylation with
MLN4924 sensitized these cells to cisplatin, resulting in
apoptosis, G2/M cell cycle arrest, γH2AX/p27
accumulation and differentiation into mesoderm/endoderm lineages in
TGCT cells, while fibroblasts remained unaffected (88). NAE1 inhibition has also been
evaluated in ovarian cancer, in which, both in vivo and
in vitro, exposure to this inhibitor augmented cisplatin
activity, showing synergetic capacity, and even resensitized
cisplatin-resistant cells/tumors. The drug caused stabilization of
NEDD8, and it also promoted apoptosis induced by oxidative stress
(86).
Epigenetic alterations, particularly in RNA, have
been studied, with N6-methyladenosine (m6A) being the most common
mRNA modification. In GCTs, the protein levels of m6A writer
complex vir-like m6A methyltransferase associated (VIRMA) were
higher in cisplatin-resistant NCCIT-R cells compared with those in
NCCIT cells (88). Knockdown of
VIRMA reduced m6A abundance, increased cisplatin sensitivity, and
decreased cell viability, tumor cell proliferation, migration and
invasion in NCCIT cells (90).
miRNA expression was also evaluated in the NTERA-2,
NCCIT and 2102EP cell lines, and their cisplatin-resistant
counterparts (NTERA-2R, NCCIT-R and 2102EP-R). Differentially
expressed miRNAs included hsa-miR-10b and hsa-miR-512-3p, which
were both upregulated 2-3-fold in all resistant cell lines.
Notably, hsa-miR-10b was implicated in breast cancer invasion and
metastasis (91).
Histone deacetylases (HDACs), which regulate
chromatin accessibility, were investigated in GCT cell lines.
Differential expression of HDACs, including HDAC1, HDAC2 and HDAC7,
was observed. TCam-2 cells displayed lower histone expression. In
NTERA-2R cells, significant gene-level expression of HDAC8,
HDAC9 and HDAC11 was observed. However, only HDAC11 showed
significant protein expression. Treatment with HDAC inhibitors,
such as belinostat and panobinostat, reduced cell viability in a
time- and dose-dependent manner, inducing cell cycle arrest and
apoptosis. Pre-treatment with non-toxic doses of belinostat
enhanced cisplatin sensitivity (17).
Cell cycle dysregulation was also analyzed in
TGCTs. Gene and protein analysis revealed high expression of
CDK4/CDK4 in TCam-2, 2102EP, NCCIT and JAR cell lines,
whereas CDK6/CDK6 expression was weak or absent (Table III). Treatment with CDK4/6
inhibitors (palbociclib and ribociclib) reduced cell viability and
induced cell cycle arrest and apoptosis, suggesting these
inhibitors as potential therapeutic options for both
cisplatin-sensitive and -resistant GCTs (92). Cisplatin resistance was associated
with cyclin D1 (CCND1) upregulation in resistant cell lines
(833K-R, Susa-R and GCT27-R), encoding cyclin D1, a protein
involved in G1/S cell cycle transition. Knockdown of
CCND1 reduced cell viability and increased cell death
following cisplatin treatment (93).
Cisplatin-resistant GCT cell lines exhibit
alterations across multiple biological processes, including DNA
repair (94), CSC markers
(95), post-translational
modifications, epigenetic changes (85), epitranscriptomic alterations and
cell cycle dysregulation (96).
This current review focuses on how these processes converge on EMP,
either as drivers or consequences, providing potential therapeutic
targets for treatment.
Despite valuable insights from available studies,
critical limitations must be acknowledged. Much of the data on EMP
in GCTs are derived from choriocarcinoma-derived cell lines (BeWo,
JAR and JEG-3), which, although informative, primarily model
placental development and pregnancy-related pathologies rather than
non-gestational GCTs. This limits the translatability of findings
to the broader spectrum of GCTs, especially those in pediatric and
adolescent populations. Moreover, few studies have directly
investigated the association between EMP and cisplatin resistance
in GCTs, leaving gaps in understanding how these processes
intersect and contribute to treatment failure. The reliance on
limited in vitro models and the scarcity of cell lines
representing various histological subtypes and age groups further
constrain generalizability. Future research should focus on
developing and characterizing additional GCT models, particularly
from pediatric cases, and integrating EMP-related mechanisms with
chemoresistance pathways to uncover clinically relevant
targets.
EMP analysis in in vivo models of
GCTs
Although recent studies have focused on elucidating
key insights into plasticity, to the best of our knowledge, few
studies demonstrate a specific association between animal
experimentation, GCT cellular models and cisplatin resistance. It
is evident that numerous studies are limited to standardizing
cellular models for xenografts or developing animal models that
best represent neoplasia development. These efforts are intended to
facilitate future detection in humans through patient-derived
xenograft (PDX) models (Fig. 2).
Therefore, in this section, the present study will discuss markers
of cellular plasticity and approaches in cisplatin resistance
models in GCTs.
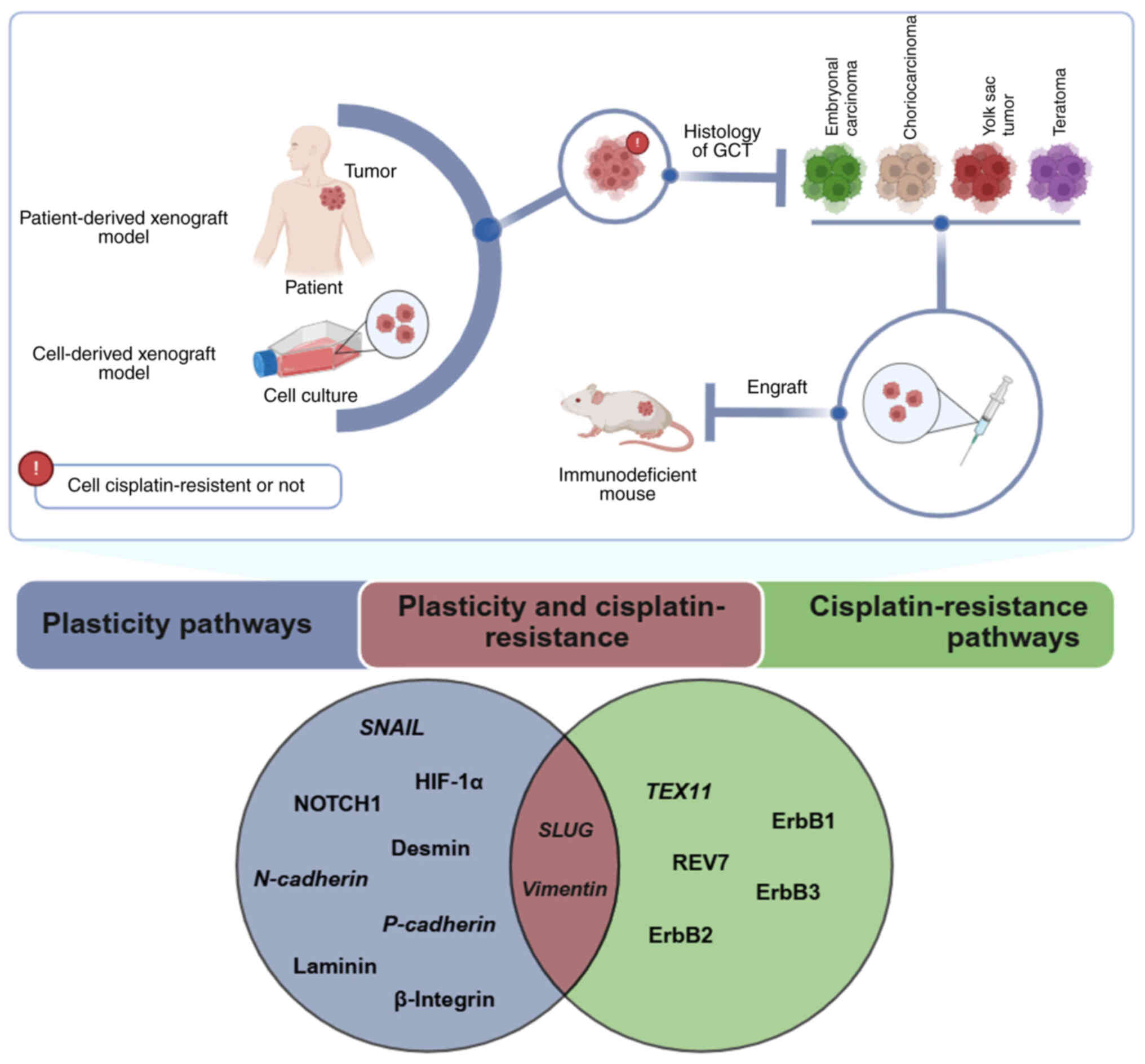 | Figure 2Overview of in vivo GCT models
and key findings on plasticity and cisplatin resistance. Cellular
plasticity pathways, shown in blue, include the genes SNAIL,
SLUG, HIF-1α, N-cadherin and P-cadherin and the
proteins NOTCH1, desmin, laminin and β-integrin. Cisplatin
resistance pathways, in green, involve the TEX11 gene and
the proteins REV7, ErbB1, ErbB2 and ErbB3. SLUG and
vimentin genes are the only plasticity markers linked to
cisplatin resistance in GCTs (in brown). Created in BioRender.
Pinto, M. (2025) https://BioRender.com/z54o788. GCT, germ cell tumor;
HIF-1α, hypoxia-inducible factor 1-α; TEX11,
testis-expressed 11; REV7, reversionless 7. |
Markers of cellular plasticity in
GCTs
Most studies on cellular plasticity in GCTs focus
on key TFs, proteins expressed during this phenotypic transition in
differentiating cells, and splice variants linked to tumor
angiogenesis directly associated with plasticity proteins. The lack
of data connecting animal experimentation, GCT cellular models and
cisplatin resistance can be attributed primarily to experimental
limitations and notable differences between human and animal tumors
(97).
In murine EC stem cells, commonly used in
vivo experiments, the cytoskeleton initially expresses VIM,
followed by keratin polypeptides after differentiation (97). By contrast, human EC cells exhibit
a divergent pattern of gene expression, initially producing keratin
polypeptides and subsequently undergoing spontaneous or induced
differentiation, resulting in the expression of VIM and other
intermediate filaments, including neurofilaments (97). For this reason, studies have used
xenograft models to address these differences, as discussed
below.
Species differences were evident in a study of
xenografts of human teratocarcinoma NTERA-2 and 2102EP cells in
nude mice, which produced solid tumors (97). Using a polyclonal antibody for
human epidermal keratin raised in rabbits and three monoclonal
antibodies for specific keratin polypeptides (AE-1, AE-3 and
RGE53), researchers analyzed intermediate filament protein
expression (97). The results
showed that tumors derived from NTERA-2 reacted with all keratin
antibodies and exhibited positive cells for neurofilaments and
mesenchymal areas containing VIM and desmin. By contrast,
2102EP-derived tumors expressed only keratin polypeptides. These
findings demonstrated differences in intermediate filament
expression between human and murine teratocarcinomas (97).
An important aspect of plasticity is the role of
TFs such as HIF-1α and SNAIL (98). HIF-1α has therapeutic potential in
cases where chemotherapy has been unsuccessful, as elevated HIF-1α
levels have been observed in CC. Positive HIF-1α expression is
correlated with NOTCH1 in CC cell lines, suggesting that
HIF-1α-induced plasticity depends on NOTCH1 signaling (98). Reduction of endogenous NOTCH1
signaling was associated with disruption of plasticity, while its
activation was linked to increased invasion and metastasis in cells
overexpressing HIF-1α in in vivo models of CC. Thus, NOTCH1
is directly related to invasion and metastasis in CC, and its
inhibition may be a promising therapeutic target by limiting
invasion and metastasis through suppression of plasticity (98).
Another TF, SNAIL, has been found to be strongly
associated with plasticity. Evidence shows that both SNAIL and SLUG
are present in the germ cells of normal human testes, similar to
observations in mice (99,100).
Positive regulation of SNAIL has been associated with the induction
of metastasis and poor prognosis, while its silencing suppresses
tumor growth and invasiveness in breast cancer (101). Although negative regulation of
SNAIL has been observed in CC cells treated with a NOTCH1
inhibitor, this evaluation was conducted only in vitro
(98).
Studies have analyzed the direct or indirect
presence of proteins characteristic of the mesenchymal phenotype in
in vivo models, such as CDH2, laminin and integrin β-1
(102,103). Through the mating of mice with
recessive and null N- or P-cadherin mutations, pluripotent
embryonic stem cells generated in vitro underwent
differentiation in vivo into TEs (102). The results showed that the
differentiation and histogenesis occurred within the TEs, as cells
lacking N- and P-cadherin exhibited predominantly adherent
structures and significant qualitative and quantitative
differentiation. Although the cells were inoculated near the
animals' lymph nodes, none metastasized or caused mortality in the
host, with the studies noting that some cells likely still
expressed CDH1 (102).
Furthermore, a study using syngeneic 129/Sv male
mice examined the impact of the absence of integrin β-1, a protein
involved in recognizing various laminins and collagen IV, on
teratoma development. Absence of integrin β-1 was found to be
efficient for analysis compared with TEs derived from wild-type
cells (103). Two stem cell
lines, D3 (wild-type) and G201 (integrin β-1-deficient via double
knockout of the integrin gene), were inoculated into the mice.
After 21 days, tumors were surgically collected. The results showed
that animals inoculated with the deficient cell line produced 90%
fewer TEs compared with the wild-type, with abundant epithelial
cells but losses in cuboidal shape, irregular layer arrangement and
reduced fluorescence in laminin α-1 staining (103). The mutant epithelial cells
exhibited a partially widened basal membrane, loop formation and
multilayered cells. Additionally, selective negative regulation of
laminin-1 was observed, indicating a loss of molecular contacts
with cellular receptors and aberrant structural characteristics
(103).
It was demonstrated that F9 mouse EC cells have
higher affinity for FN1 than for laminin in terms of attachment and
dissemination in the animal organism. Laminin is predominantly
found in the pulmonary matrix, while FN1 is found in the liver.
Thus, the low affinity of these cells for laminin in the lung
caused their rapid elimination from that organ. These results were
obtained from a study evaluating cell migration after injection
into the tail vein of mice, showing that the tumor cell adhesion to
organs is necessary but not sufficient for metastasis (104).
A key driver of tumor growth and metastasis is high
vascularization due to angiogenesis (105). The splice variant FN1B
stands out as a specific biomarker of angiogenesis expressed around
new blood vessels in various human cancer types, such as
glioblastoma and small cell lung cancer. Through culturing mouse
embryonal teratocarcinoma cells and inoculating them into
4-week-old female mice, a study observed via microPET imaging, that
FN1B serves as a promising biomarker for microPET imaging
targeting (105).
Taken together, these studies provide significant
insights into the molecular and cellular mechanisms underlying
plasticity in GCTs, particularly through in vivo models and
characterization of key proteins, TFs and angiogenesis-related
biomarkers. However, distinct differences in intermediate filament
expression between human and murine tumors, as well as limited
exploration of species-specific pathways, highlight the complexity
of translating findings across models. Moreover, while substantial
progress has been made in linking plasticity to invasion and
metastasis, the role of plasticity in treatment resistance,
particularly to cisplatin, remains insufficiently understood.
Bridging this knowledge gap will require integrating advanced
experimental approaches focusing on the interplay between
plasticity mechanisms and therapeutic responses, ultimately paving
the way for more precise and effective treatments for GCTs.
Approaches in cisplatin resistance
models
Several studies on resistance have focused on
demonstrating cisplatin resistance in in vivo models,
particularly in PDX models. However, some of these studies
investigated this phenomenon in tumor types other than GCTs, such
as ovarian (106,107), lung (108,109), liver (110,111), colorectal (112), gastric (113,114) and brain (115) cancer. Thus, the primary
objective of these studies was to characterize cisplatin
resistance, analyze genes, proteins and signaling pathways involved
in chemoresistance, compare different drugs associated with
chemoresistance and establish new targeted drugs for treatment.
Nevertheless, few studies have explored the interaction between
cisplatin resistance and plasticity, as highlighted below.
A notable study examined the association between
plasticity and treatment resistance, using a novel cyclic peptide,
MTI-101, in synergy with cisplatin in lung cancer (109). In vivo data indicated
that the treatment increased CDH1 and decreased VIM expression,
suggesting that chronic treatment with MTI-101 could reduce
metastatic disease (109).
Despite these notable results, a direct association between
cisplatin resistance, plasticity and GCT models was not observed. A
similar observation was reported using zebrafish xenografts to
evaluate the therapeutic benefits of cisplatin and valproic acid in
patient-derived laryngeal cancer cell lines (116). Evidence highlighted that the
RK45 cell line activated genes associated with the epithelial
phenotype. Following treatment with both chemotherapeutics,
upregulation of CDH1, which encodes CDH1, and its placental
form, CDH3, was observed (116). Additionally, negative regulation
of the TFs ZEB1 and FGFR1, genes that induce
plasticity progression and may be associated with the weak response
of RK33 cells to the combination of cisplatin and valproic acid
drugs, was noted. Meanwhile, the RK33 cell line exhibited
upregulation of mesenchymal phenotype genes, such as VIM (116).
Some studies have explored cisplatin resistance in
xenograft models using GCT cell lines. An in vivo model
utilizing the GCT NTERA-2 cell line, including both the parental
and cisplatin-resistant strains, was employed to evaluate drug
sensitivity. It was reported that both cell lines were sensitive to
the DNA methyltransferase inhibitor guadecitabine, suggesting that
this agent may offer a potential therapeutic alternative for
patients with cisplatin-resistant GCTs. This finding highlights the
potential of epigenetic therapies in overcoming resistance and
improving treatment outcomes for these patients (117). The same cell line, NTERA-2, was
used to investigate the effects of cholecalciferol (inactive form
of vitamin D) and 1,25(OH)2D3 on cisplatin
treatment in an in vivo xenotransplantation mouse model,
using tumor growth and tumor size as endpoints. In contrast to the
findings with guadecitabine, treatment with the active form of
vitamin D showed no antitumor activity in vivo (118).
Beyond the use of xenograft models derived from
established cell lines, research has focused on developing PDX
models. These PDX models preserve essential characteristics of
original patient tumor cells, including maintaining high genetic
and transcriptional stability. Importantly, key mutations present
in primary tumors have been shown to remain consistent across
successive PDX passages (119,120). PDX models show greater
similarity to human tumors compared with cell lines. Analyses of
cisplatin resistance in PDX have shown results resembling those
observed in the corresponding patient, highlighting the superiority
of PDX models in predicting drug response (119).
A xenograft model of TGCT derived from a patient
with cisplatin resistance was used to evaluate potential
therapeutic strategies. The findings revealed the efficacy of short
interfering RNA targeting testis-expressed 11 (TEX11), a
gene significantly upregulated in the tumor. TEX11, known
for its role in meiosis, was implicated in promoting resistance to
cisplatin by inhibiting cisplatin-induced double-strand DNA
breakage. These results highlight TEX11 as a promising
therapeutic target for addressing cisplatin-resistant TGCTs
(121). Additionally,
reversionless 7 gene (REV7) deficiency is involved in DNA
damage repair, cell cycle regulation and gene expression, and it
sensitized cisplatin-resistant tumors in vivo. This was
observed in a tumor xenograft of a GCT (testicular EC) in SCID mice
(122). Thus, these studies
underscore the potential of targeting key molecular players, such
as TEX11 and REV7, in overcoming cisplatin resistance
in TGCTs.
A xenograft model was established in
immunodeficient mice using cisplatin-resistant cells derived from a
previously cisplatin-sensitive YST of a patient with ovarian
cancer. This model provided insights into the association between
CSC markers and cisplatin resistance (increased expression of
CD133, ALDH3A1 and ABCG2), underscoring potential targets to
address chemoresistance (74).
In the context of PDX models for GCTs, primary
choriocarcinoma tumors were implanted in mice to evaluate the
expression of the ErbB family of receptor tyrosine kinases and
assess the effects of their inhibitors (123). The ErbB family is associated
with tumor progression, correlating with worse prognosis and
resistance to conventional therapies (123). ErbB receptors are naturally
expressed in tissues of epithelial, mesenchymal and neuronal origin
(123). However, members of the
ErbB family (ErbB1, ErbB2 and ErbB3) exhibit distinct expression
patterns in tumors, such as CC, EC and YST (120). Specifically, ErbB1 is
upregulated in CC, ErbB2 is highly expressed in EC, and elevated
levels of ErbB3 are observed in CC, EC and YST (120). Therapeutic inhibition of these
markers has shown that targeting a single ErbB family member is
insufficient to disrupt tumor growth, as compensatory pathway
reactivation contributes to resistance against ErbB-targeted
therapies (120,124).
Despite studies identifying important pathways
associated with cisplatin resistance or sensitivity in GCTs, little
information addresses plasticity. To address this gap, our research
group conducted a study analyzing the expression of SLUG, a key
plasticity TF, in both parental and cisplatin-resistant xenograft
tumors (21). Parental and
cisplatin-resistant NTERA-2 cells were inoculated into athymic
mice, and significant upregulation of VIM and SLUG
expression was observed. This suggests that SLUG may serve
as an important TF for linking plasticity and cisplatin resistance
in GCTs (21). While this study
offers insights into the mechanisms of plasticity and cisplatin
resistance, murine models do not fully reflect the complexity of
human tumors. This is particularly due to species-specific
differences in gene expression and treatment response. However, a
deeper understanding of the molecular mechanisms underlying EMP and
drug resistance presents a promising avenue for the development of
innovative therapeutic strategies tailored to target TGCTs, thereby
opening new avenues for more effective and personalized
treatments.
Potential inhibitors targeting EMP
Inhibitors targeting EMP have become a key strategy
in combating cancer progression, metastasis and treatment
resistance. By modulating key signaling pathways and molecular
markers associated with plasticity, these inhibitors offer a
promising approach to mitigating the aggressive behavior of tumors
(125).
In JEG-3 cells, cyclosporin-A (CsA) was shown to
promote invasion through reduced CDH1 expression via the EGFR/ERK
signaling pathway. This effect was counteracted by U0126, an ERK
pathway inhibitor, demonstrating its ability to suppress
EMP-related invasive behavior (126). Similarly, the
phosphodiesterase-4 (PDE4) inhibitor rolipram has been highlighted
as a potential therapeutic agent. Rolipram effectively reduced
migration and invasion in JEG-3 and JAR cells by modulating EMP
markers at the mRNA and protein levels, including decreased
expression levels of MMP9 and TIMP1, and increased expression of
CDH1, positioning PDE4 inhibitors as promising candidates for
modulating EMP in cancer therapy (127,128). Thus, CsA and PDE4 inhibitors
demonstrate significant impacts on EMP and invasive behavior in
cancer cells.
Further advancements in overcoming cisplatin
resistance in GCTs include the use of monanchocidin A (MonA), an
alkaloid compound with antitumor properties. MonA demonstrated the
ability to downregulate VIM isoforms, suppress migration and alter
cell morphology in cisplatin-resistant NCCIT-R cells, suggesting
its potential role in addressing EMP-related resistance mechanisms
(129). Additionally,
panobinostat, an HDAC inhibitor, has shown potential as an
inhibitor of LINC00313, a long non-coding RNA linked to EMP
mediation. Although its effects on specific EMP markers require
further study, panobinostat represents a promising avenue for
therapeutic exploration (65).
These inhibitors hold considerable promise for further
investigation as potential therapeutic agents for GCT treatment,
particularly for addressing EMP-driven cisplatin resistance, a
critical unmet clinical need.
Insights from clinical trials in non-GCT
malignancies
To date, to the best of our knowledge, clinical
trials targeting EMP pathways in GCTs remain unavailable. However,
investigations into EMP-related targets in other malignancies
provide valuable insights. For instance, andecaliximab, a
monoclonal antibody inhibiting MMP9, a key EMP player, has
demonstrated manageable safety profiles and varying efficacy in
advanced cancer types, such as gastric and pancreatic
adenocarcinoma (130,131). In pancreatic adenocarcinoma,
combinations with chemotherapy regimens, such as gemcitabine and
nab-paclitaxel, achieved a progression-free survival time of 7.8
months and an objective response rate of 44.4%. In advanced gastric
cancer, one study evaluated andecaliximab as monotherapy and in
combination with nivolumab, showing a manageable safety profile and
clinical activity, with a median progression-free survival time of
1.4 and 4.6 months for monotherapy and combination therapy,
respectively (132). Another
study assessing andecaliximab combined with nivolumab in pretreated
metastatic gastric cancer reported a favorable safety profile but
no significant improvement in survival outcomes compared with
nivolumab alone (133). A phase
I study combining sapanisertib with ziv-aflibercept, a VEGF
inhibitor, in patients with advanced solid tumors, demonstrated a
disease control rate of 78%, with 74% achieving stable disease and
4% achieving a confirmed partial response (134).
Additionally, PRT543, an inhibitor of protein
arginine methyltransferase 5, which downregulates NOTCH1 and MYB
signaling, achieved a clinical benefit rate of 57% and a median
progression-free survival time of 5.9 months in adenoid cystic
carcinoma (135). Furthermore,
chidamide, an HDAC inhibitor, demonstrated anti-NOTCH1 activity and
clinical efficacy in T-cell acute lymphoblastic leukemia,
suggesting its utility in EMP-related pathways (136). Moreover, the combination of
bevacizumab, an antiangiogenic agent targeting VEGF, and
bortezomib, a proteasome inhibitor that suppresses HIF-1α
transcriptional activity, demonstrated clinical activity in a phase
I trial involving patients with advanced malignancies. Among the 91
patients treated, 12% achieved either a partial response or stable
disease lasting ≥6 months, highlighting the potential of dual
targeting of angiogenesis and HIF-1α in overcoming resistance to
antiangiogenic therapy (137).
These findings highlight the potential of EMP-targeting strategies
in broader oncology contexts and underscore the need to explore
their therapeutic value in GCTs, where emerging evidence suggests a
role for EMP in treatment resistance.
To offer a comprehensive summary and highlight
connections between all topics discussed in this review, Fig. 3 provides a graphical
representation synthesizing the key aspects of the article.
![Graphical representation of EMP
markers, cisplatin resistance and potential inhibitors across GCT
histologies. This figure illustrates the histologies studied both
in vivo and in vitro, including those related to
cisplatin resistance and the status of potential inhibitors for
targeted therapy. Red circles indicate association with cisplatin
resistance. Created in BioRender. Pinto, M. (2025) https://BioRender.com/x70i558.EMP,
epithelial-mesenchymal plasticity; GCT, germ cell tumor; CCND1,
cyclin D1; ErbB, ErbB family; CD133, prominin-1; ALDH3A1, aldehyde
dehydrogenase 3 family member A1; ABCG2, ATP binding cassette
subfamily G member 2; TRIB3, Tribbles pseudokinase 3; IGFBP2,
insulin-like growth factor binding protein 2; NANOG, homeobox
protein NANOG; POU5F1, POU class 5 homeobox 1; SOX2, SRY-related
HMG-box 2; CDK, cyclin-dependent kinase; VIRMA, Vir-like M6A
methyltransferase associated; ACTA2, actin alpha 2; COL1A1, type I
collagen; FN1, fibronectin; HDAC, histone deacetylase; POLD4, DNA
Polymerase Delta 4, Accessory Subunit; TGF-β, transforming growth
factor beta; VIM, vimentin; XPC, XPC complex subunit, DNA damage
recognition and repair factor; NES, nestin; STMN2, stathmin-2;
REV7; reversionless 7; has-miR, Homo sapiens microRNA; ANXA1,
annexin A1; TAGLN, Transgelin; TEXT11, testis-expressed 11;
LINC003131, long intergenic non-coding RNA; PTTG1, pituitary
tumor-transforming gene 1; DAPT,
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester; MLN4924, pevonedistat; MG-132, proteasome inhibitor; PDE4,
phosphodiesterase 4; SENP1, SUMO specific peptidase 1; ILK,
integrin linked kinase; SNAIL, snail family transcriptional
repressor 1; TWIST1, Twist-related protein 1; NAE1, NEDD8
activating enzyme E1 subunit 1; HIF-1α, hypoxia inducible factor 1
Subunit Alpha ; ZEB2, zinc finger E-box binding homeobox 2; HMOX1,
heme oxygenase 1; NOTCH1, notch receptor 1.](/article_images/ijo/67/6/ijo-67-06-05811-g02.jpg) | Figure 3Graphical representation of EMP
markers, cisplatin resistance and potential inhibitors across GCT
histologies. This figure illustrates the histologies studied both
in vivo and in vitro, including those related to
cisplatin resistance and the status of potential inhibitors for
targeted therapy. Red circles indicate association with cisplatin
resistance. Created in BioRender. Pinto, M. (2025) https://BioRender.com/x70i558.EMP,
epithelial-mesenchymal plasticity; GCT, germ cell tumor; CCND1,
cyclin D1; ErbB, ErbB family; CD133, prominin-1; ALDH3A1, aldehyde
dehydrogenase 3 family member A1; ABCG2, ATP binding cassette
subfamily G member 2; TRIB3, Tribbles pseudokinase 3; IGFBP2,
insulin-like growth factor binding protein 2; NANOG, homeobox
protein NANOG; POU5F1, POU class 5 homeobox 1; SOX2, SRY-related
HMG-box 2; CDK, cyclin-dependent kinase; VIRMA, Vir-like M6A
methyltransferase associated; ACTA2, actin alpha 2; COL1A1, type I
collagen; FN1, fibronectin; HDAC, histone deacetylase; POLD4, DNA
Polymerase Delta 4, Accessory Subunit; TGF-β, transforming growth
factor beta; VIM, vimentin; XPC, XPC complex subunit, DNA damage
recognition and repair factor; NES, nestin; STMN2, stathmin-2;
REV7; reversionless 7; has-miR, Homo sapiens microRNA; ANXA1,
annexin A1; TAGLN, Transgelin; TEXT11, testis-expressed 11;
LINC003131, long intergenic non-coding RNA; PTTG1, pituitary
tumor-transforming gene 1; DAPT,
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester; MLN4924, pevonedistat; MG-132, proteasome inhibitor; PDE4,
phosphodiesterase 4; SENP1, SUMO specific peptidase 1; ILK,
integrin linked kinase; SNAIL, snail family transcriptional
repressor 1; TWIST1, Twist-related protein 1; NAE1, NEDD8
activating enzyme E1 subunit 1; HIF-1α, hypoxia inducible factor 1
Subunit Alpha ; ZEB2, zinc finger E-box binding homeobox 2; HMOX1,
heme oxygenase 1; NOTCH1, notch receptor 1. |
Conclusion
The present review highlights several critical gaps
in the current understanding of EMP and its association with
cisplatin resistance in GCTs. A major limitation is the scarcity of
studies directly investigating EMP-associated cisplatin resistance
in GCTs, with most research focusing separately on EMP or
resistance mechanisms rather than their interplay. Additionally,
while in vitro studies using GCT cell lines have provided
some insights, there is a notable lack of in vivo research
exploring the connection between plasticity and resistance in GCTs.
These findings underscore the necessity for integrated studies that
combine both EMP and resistance mechanisms to enhance our
comprehension of the molecular mechanisms involved. Advancing
research in this area could lead to the identification of novel
therapeutic targets, ultimately improving outcomes for the 30% of
patients with GCT who have a poor prognosis or experience treatment
failure. To address the current knowledge gap, future research
should focus on longitudinal analysis of EMP markers in patient
cohorts, exploring the intricate association between plasticity
mechanisms and therapeutic responses. This will help validate their
prognostic utility and pave the way for developing more effective
and personalized treatments for GCTs.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ESDBG, TMDS, ALPO, AFSPB, IIVC, LS, LFL, MNR and
MTP contributed to the conception, design, literature search and
analysis of the study. ESDBG, TMDS, ALPO, AFSPB, IIVC, LS and MNR
drafted and wrote the manuscript. ESDBG, TMDS and AFSPB prepared
the figures. MTP supervised the review, critically evaluated and
revised the manuscript. Data authentication is not applicable. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work artificial
intelligence tools were used to translate the language of the
manuscript and, subsequently, the authors revised and edited the
content translated by the artificial intelligence tools as
necessary, taking full responsibility for the ultimate content of
the present manuscript.
Acknowledgements
Not applicable.
Funding
This study was supported by the São Paulo State Research Support
Foundation, Brazil (grant no. 2019/07502-8) and the Researcher
Support Program from Barretos Cancer Hospital.
References
|
1
|
Oosterhuis JW and Looijenga LHJ: Human
germ cell tumours from a developmental perspective. Nat Rev Cancer.
19:522–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drozynska E, Bien E, Polczynska K,
Stefanowicz J, Zalewska-Szewczyk B, Izycka-Swieszewska E,
Ploszynska A, Krawczyk M and Karpinsky G: A need for cautious
interpretation of elevated serum germ cell tumor markers in
children. Review and own experiences. Biomark Med. 9:923–932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopes LF, Sonaglio V, Ribeiro KCB,
Schneider DT and de Camargo B: Improvement in the outcome of
children with germ cell tumors. Pediatr Blood Cancer. 50:250–253.
2008. View Article : Google Scholar
|
|
4
|
Veneris JT, Mahajan P and Frazier AL:
Contemporary management of ovarian germ cell tumors and remaining
controversies. Gynecol Oncol. 158:467–475. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trabert B, Chen J, Devesa SS, Bray F and
McGlynn KA: International patterns and trends in testicular cancer
incidence, overall and by histologic subtype, 1973-2007. Andrology.
3:4–12. 2015. View Article : Google Scholar :
|
|
6
|
Smith HO, Berwick M, Verschraegen CF,
Wiggins C, Lansing L, Muller CY and Qualls CR: Incidence and
survival rates for female malignant germ cell tumors. Obstet
Gynecol. 107:1075–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaikh F, Murray MJ, Amatruda JF, Coleman
N, Nicholson JC, Hale JP, Pashankar F, Stoneham SJ, Poynter JN,
Olson TA, et al: Paediatric extracranial germ-cell tumours. Lancet
Oncol. 17:e149–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teilum G: Ovarian Cancer. Gentil F and
Junqueira AC: Springer Berlin Heidelberg; Berlin, Heidelberg: 1968,
Available from: http://link.springer.com/10.1007/978-3-642-87755-1.
|
|
9
|
Yao X, Zhou H, Duan C, Wu X, Li B, Liu H
and Zhang Y: Comprehensive characteristics of pathological subtypes
in testicular germ cell tumor: Gene expression, mutation and
alternative splicing. Front Immunol. 13:10964942023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi K, Saito T, Kitamura Y,
Nobushita T, Kawasaki T, Hara N and Takahashi K: Oncological
outcomes in patients with stage I testicular seminoma and
nonseminoma: Pathological risk factors for relapse and feasibility
of surveillance after orchiectomy. Diagn Pathol. 8:572013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murray MJ and Nicholson JC: Germ cell
tumours in children and adolescents. Paediatr Child Health.
20:109–116. 2010. View Article : Google Scholar
|
|
12
|
Travis LB, Feldman DR, Fung C, Poynter JN,
Lockley M and Frazier AL: Adolescent and young adult germ cell
tumors: Epidemiology, genomics, treatment, and survivorship. J Clin
Oncol. 42:696–706. 2024. View Article : Google Scholar
|
|
13
|
Fenske AE, Glaesener S, Bokemeyer C,
Thomale J, Dahm-Daphi J, Honecker F and Dartsch DC: Cisplatin
resistance induced in germ cell tumour cells is due to reduced
susceptibility towards cell death but not to altered DNA damage
induction or repair. Cancer Lett. 324:171–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fichtner A, Bohnenberger H, Elakad O,
Richter A, Lenz C, Oing C, Ströbel P, Kueffer S, Nettersheim D and
Bremmer F: Proteomic profiling of cisplatin-resistant and
cisplatin-sensitive germ cell tumour cell lines using quantitative
mass spectrometry. World J Urol. 40:373–383. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selfe J, Goddard NC, McIntyre A, Taylor
KR, Renshaw J, Popov SD, Thway K, Summersgill B, Huddart RA,
Gilbert DC and Shipley JM: IGF1R signalling in testicular germ cell
tumour cells impacts on cell survival and acquired cisplatin
resistance. J Pathol. 244:242–253. 2018. View Article : Google Scholar :
|
|
16
|
Galvez-Carvajal L, Sanchez-Muñoz A,
Ribelles N, Saez M, Baena J, Ruiz S, Ithurbisquy C and Alba E:
Targeted treatment approaches in refractory germ cell tumors. Crit
Rev Oncol Hematol. 143:130–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lobo J, Guimarães-Teixeira C, Barros-Silva
D, Miranda-Gonçalves V, Camilo V, Guimarães R, Cantante M, Braga I,
Maurício J, Oing C, et al: Efficacy of HDAC inhibitors belinostat
and panobinostat against cisplatin-sensitive and
cisplatin-resistant testicular germ cell tumors. Cancers (Basel).
12:29032020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wermann H, Stoop H, Gillis AJ, Honecker F,
van Gurp RJ, Ammerpohl O, Richter J, Oosterhuis JW, Bokemeyer C and
Looijenga LH: Global DNA methylation in fetal human germ cells and
germ cell tumours: Association with differentiation and cisplatin
resistance. J Pathol. 221:433–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juliachs M, Muñoz C, Moutinho CA, Vidal A,
Condom E, Esteller M, Graupera M, Casanovas O, Germà JR, Villanueva
A and Viñals F: The PDGFRβ-AKT pathway contributes to CDDP-acquired
resistance in testicular germ cell tumors. Clin Cancer Res.
20:658–667. 2014. View Article : Google Scholar
|
|
20
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
21
|
Cardoso I, Rosa M, Moreno D, Tufi L, Ramos
L, Pereira L, Silva L, Galvão JMS, Tosi IC, Lengert AVH, et al:
Cisplatin-resistant germ cell tumor models: An exploration of the
epithelial-mesenchymal transition regulator SLUG. Mol Med Rep.
30:2282024. View Article : Google Scholar
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of β-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar
|
|
25
|
Nawshad A, LaGamba D, Polad A and Hay ED:
Transforming growth factor-β signaling during
epithelial-mesenchymal transformation: Implications for
embryogenesis and tumor metastasis. Cells Tissues Organs.
179:11–23. 2005. View Article : Google Scholar
|
|
26
|
Medici D, Hay ED and Olsen BR: Snail and
slug promote epithelial-Mesenchymal Transition through
β-catenin-T-cell Factor-4-dependent expression of transforming
growth factor-β3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grasset EM, Dunworth M, Sharma G, Loth M,
Tandurella J, Cimino-Mathews A, Gentz M, Bracht S, Haynes M, Fertig
EJ and Ewald AJ: Triple-negative breast cancer metastasis involves
complex epithelial-mesenchymal transition dynamics and requires
vimentin. Sci Transl Med. 14:eabn75712022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ugur D, Gungul TB, Yucel S, Ozcivici E,
Yalcin-Ozuysal O and Mese G: Connexin 32 overexpression increases
proliferation, reduces gap junctional intercellular communication,
motility and epithelial-to-mesenchymal transition in Hs578T breast
cancer cells. J Cell Commun Signal. 16:361–376. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zvrko E, Vuckovic L and Radunovic M:
Prognostic significance of a panel of two biomarkers (E-cadherin
and CD105) in laryngeal cancer. Polish J Pathol. 74:225–231. 2023.
View Article : Google Scholar
|
|
30
|
Jiang JX and Gu S: Gap junction- and
hemichannel-independent actions of connexins. Biochim Biophys Acta.
1711:208–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Acosta FM and Jiang JX: Gap
Junctions or Hemichannel-dependent and independent roles of
connexins in fibrosis, epithelial-mesenchymal transitions, and
wound healing. Biomolecules. 13:17962023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trelstad RL: The extracellular matrix in
development and regeneration. An interview with Elizabeth D. Hay.
Int J Dev Biol. 48:687–694. 2004. View Article : Google Scholar
|
|
33
|
Debnath P, Huirem RS, Dutta P and
Palchaudhuri S: Epithelial-mesenchymal transition and its
transcription factors. Biosci Rep. 42:BSR202117542022. View Article : Google Scholar
|
|
34
|
Piek E, Moustakas A, Kurisaki A, Heldin CH
and ten Dijke P: TGF-β type I receptor/ALK-5 and Smad proteins
mediate epithelial to mesenchymal transdifferentiation in NMuMG
breast epithelial cells. J Cell Sci. 112:4557–4568. 1999.
View Article : Google Scholar
|
|
35
|
Guo X, Yi H, Li TC, Wang Y, Wang H and
Chen X: Role of vascular endothelial growth factor (VEGF) in human
embryo implantation: Clinical implications. Biomolecules.
11:2532021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Batarfi WA, Mohd Yunus MH and Hamid AA:
The Effect of Hydroxytyrosol in Type II Epithelial-Mesenchymal
transition in human skin wound healing. Molecules. 28:26522023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marconi GD, Fonticoli L, Rajan TS,
Pierdomenico SD, Trubiani O, Pizzicannella J and Diomede F:
Epithelial-mesenchymal transition (EMT): The Type-2 EMT in wound
healing, tissue regeneration and organ fibrosis. Cells.
10:15872021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tennakoon A, Izawa T, Kuwamura M and
Yamate J: Pathogenesis of type 2 epithelial to mesenchymal
transition (EMT) in renal and hepatic fibrosis. J Clin Med.
5:42015. View Article : Google Scholar
|
|
39
|
Linsdell P: Cystic fibrosis transmembrane
conductance regulator (CFTR): Making an ion channel out of an
active transporter structure. Channels. 12:284–290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui H, Huang J, Lei Y, Chen Q, Hu Z, Niu
J, Wei R, Yang K, Li H, Lu T, et al: Design and synthesis of dual
inhibitors targeting snail and histone deacetylase for the
treatment of solid tumour cancer. Eur J Med Chem. 229:1140822022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zivotic M, Kovacevic S, Nikolic G,
Mioljevic A, Filipovic I, Djordjevic M, Jovicic V, Topalovic N,
Ilic K, Radojevic Skodric S, et al: SLUG and SNAIL as potential
immunohistochemical biomarkers for renal cancer staging and
survival. Int J Mol Sci. 24:122452023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ashrafizadeh M, Zarrabi A, Hushmandi K,
Kalantari M, Mohammadinejad R, Javaheri T and Sethi G: Association
of the epithelial-mesenchymal transition (EMT) with Cisplatin
resistance. Int J Mol Sci. 21:40022020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Chen J, Li J, Weberpals J, Davey S, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cooper S and Pera MF: Vitronectin
production by human yolk sac carcinoma cells resembling parietal
endoderm. Development. 104:565–574. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ruoslahti E, Jalanko H, Comings DE,
Neville AM and Raghavan D: Fibronectin from human germ-cell tumors
resembles amniotic fluid fibronectin. Int J Cancer. 27:763–767.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tauber S, Jais A, Jeitler M, Haider S,
Husa J, Lindroos J, Knöfler M, Mayerhofer M, Pehamberger H, Wagner
O and Bilban M: Transcriptome analysis of human cancer reveals a
functional role of Heme Oxygenase-1 in tumor cell adhesion. Mol
Cancer. 9:2002010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Borszéková Pulzová LB, Roška J, Kalman M,
Kliment J, Slávik P, Smolková B, Goffa E, Jurkovičová D, Kulcsár Ľ,
Lešková K, et al: Screening for the key proteins associated with
rete testis invasion in clinical stage I seminoma via label-free
quantitative mass spectrometry. Cancers (Basel). 13:55732021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sherman-Baust CA, Weeraratna AT, Rangel
LBA, Pizer ES, Cho KR, Schwartz DR, Shock T and Morin PJ:
Remodeling of the extracellular matrix through overexpression of
collagen VI contributes to cisplatin resistance in ovarian cancer
cells. Cancer Cell. 3:377–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Skowron MA, Eul K, Stephan A, Ludwig GF,
Wakileh GA, Bister A, Söhngen C, Raba K, Petzsch P, Poschmann G, et
al: Profiling the 3D interaction between germ cell tumors and
microenvironmental cells at the transcriptome and secretome level.
Mol Oncol. 16:3107–3127. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Henke E, Nandigama R and Ergün S:
Extracellular matrix in the tumor microenvironment and its impact
on cancer therapy. Front Mol Biosci. 6:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun L, Guo S, Xie Y and Yao Y: The
characteristics and the multiple functions of integrin β1 in human
cancers. J Transl Med. 21:7872023. View Article : Google Scholar
|
|
53
|
Butler TM, Elustondo PA, Hannigan GE and
MacPhee DJ: Integrin-linked kinase can facilitate syncytialization
and hormonal differentiation of the human trophoblast-derived BeWo
cell line. Reprod Biol Endocrinol. 7:512009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ng YH, Zhu H and Leung PCK: Twist
regulates cadherin-mediated differentiation and fusion of human
trophoblastic cells. J Clin Endocrinol Metab. 96:3881–3890. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang C, Song Y, Yin Y, Hou H and Ge Z:
Twist-1 stimulates the malignant behaviors of Hydatidiform mole via
the PI3K/AKT pathway. Discov Med. 36:2862024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bahar E, Kim JY, Kim HS and Yoon H:
Establishment of acquired cisplatin resistance in ovarian cancer
cell lines characterized by enriched metastatic properties with
increased twist expression. Int J Mol Sci. 21:76132020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
DaSilva-Arnold SC, Kuo CY, Davra V,
Remache Y, Kim PCW, Fisher JP, Zamudio S, Al-Khan A, Birge RB and
Illsley NP: ZEB2, a master regulator of the epithelial-mesenchymal
transition, mediates trophoblast differentiation. Mol Hum Reprod.
25:61–75. 2019. View Article : Google Scholar
|
|
59
|
Teveroni E, Di Nicuolo F, Vergani E,
Bianchetti G, Bruno C, Maulucci G, De Spirito M, Cenci T, Pierconti
F, Gulino G, et al: PTTG1/ZEB1 axis regulates E-cadherin expression
in human seminoma. Cancers (Basel). 14:48762022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu Y, Ren B and Wang M: HIF-1α contributes
to metastasis in choriocarcinoma by regulating DEC1 expression.
Clin Transl Oncol. 25:1641–1649. 2022. View Article : Google Scholar
|
|
62
|
Iwaki T, Yamamoto K, Matsuura T, Sugimura
M, Kobayashi T and Kanayama N: Alteration of Integrins under
hypoxic stress in early placenta and choriocarcinoma cell line
BeWo. Gynecol Obstet Invest. 57:196–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xue Y, Sun R, Zheng W, Yang L and An R:
Forskolin promotes vasculogenic mimicry and invasion via
Notch-1-activated epithelial-to-mesenchymal transition in
syncytiolization of trophoblast cells in choriocarcinoma. Int J
Oncol. 56:1129–1139. 2020.PubMed/NCBI
|
|
64
|
Wu YC, Ling TY, Lu SH, Kuo HC, Ho HN, Yeh
SD, Shen CN and Huang YH: Chemotherapeutic sensitivity of
testicular germ cell tumors under hypoxic conditions is negatively
regulated by SENP1-controlled Sumoylation of OCT4. Cancer Res.
72:4963–4973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Koch S, Mayer F, Honecker F, Schittenhelm
M and Bokemeyer C: Efficacy of cytotoxic agents used in the
treatment of testicular germ cell tumours under normoxic and
hypoxic conditions in vitro. Br J Cancer. 89:2133–2139. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Z, Fang B, Cao J, Zhou Q, Zhu F, Fan
L, Xue L, Huang C and Bo H: LINC00313 regulates the metastasis of
testicular germ cell tumors through epithelial-mesenchyme
transition and immune pathways. Bioengineered. 13:12141–12155.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lengert AVH, Pereira LDNB, Cabral ERM,
Gomes INF, Jesus LM, Gonçalves MFS, Rocha AOD, Tassinari TA, Silva
LSD, Laus AC, et al: Potential new therapeutic approaches for
cisplatin-resistant testicular germ cell tumors. Front Biosci
(Landmark Ed). 27:2452022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun C, Zhang K, Ni C, Wan J, Duan X, Lou
X, Yao X, Li X, Wang M, Gu Z, et al: Transgelin promotes lung
cancer progression via activation of cancer-associated fibroblasts
with enhanced IL-6 release. Oncogenesis. 12:182023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhong W, Hou H, Liu T, Su S, Xi X, Liao Y,
Xie R, Jin G, Liu X, Zhu L, et al: Cartilage Oligomeric Matrix
Protein promotes epithelial-mesenchymal transition by interacting
with Transgelin in colorectal cancer. Theranostics. 10:8790–8806.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Oshi M, Tokumaru Y, Mukhopadhyay S, Yan L,
Matsuyama R, Endo I and Takabe K: Annexin A1 expression is
associated with Epithelial-Mesenchymal transition (EMT), cell
proliferation, prognosis, and drug response in pancreatic cancer.
Cells. 10:6532021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cortes-Dericks L, Froment L, Boesch R,
Schmid RA and Karoubi G: Cisplatin-resistant cells in malignant
pleural mesothelioma cell lines show ALD(Hhigh)CD44(+) phenotype
and sphere-forming capacity. BMC Cancer. 14:3042014. View Article : Google Scholar
|
|
72
|
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu ATH,
Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, et al: Cisplatin selects
for multidrug-resistant CD133+ cells in lung adenocarcinoma by
activating notch signaling. Cancer Res. 73:406–416. 2013.
View Article : Google Scholar
|
|
73
|
Schmidtova S, Kalavska K, Gercakova K,
Cierna Z, Miklikova S, Smolkova B, Buocikova V, Miskovska V,
Durinikova E, Burikova M, et al: Disulfiram overcomes cisplatin
resistance in human embryonal carcinoma cells. Cancers (Basel).
11:12242019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schmidtova S, Dorssers LCJ, Kalavska K,
Gillis AJM, Oosterhuis JW, Stoop H, Miklikova S, Kozovska Z,
Burikova M, Gercakova K, et al: Napabucasin overcomes cisplatin
resistance in ovarian germ cell tumor-derived cell line by
inhibiting cancer stemness. Cancer Cell Int. 20:3642020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Roška J, Wachsmannová L, Hurbanová L,
Šestáková Z, Mueller T, Jurkovičová D and Chovanec M: Differential
gene expression in cisplatin-resistant and -sensitive testicular
germ cell tumor cell lines. Oncotarget. 11:4735–4753. 2020.
View Article : Google Scholar
|
|
76
|
Makino S, Takahashi H, Okuzaki D, Miyoshi
N, Haraguchi N, Hata T, Matsuda C, Yamamoto H, Mizushima T, Mori M
and Doki Y: DCLK1 integrates induction of TRIB3, EMT, drug
resistance and poor prognosis in colorectal cancer. Carcinogenesis.
41:303–312. 2020. View Article : Google Scholar
|
|
77
|
Chen X, Zhang Y, Zhang P, Wei M, Tian T,
Guan Y, Han C, Wei W and Ma Y: IGFBP2 drives epithelial-mesenchymal
transition in hepatocellular carcinoma via activating the
Wnt/β-catenin pathway. Infect Agent Cancer. 18:732023. View Article : Google Scholar
|
|
78
|
Liu S, Sun J, Cai B, Xi X, Yang L, Zhang
Z, Feng Y and Sun Y: NANOG regulates epithelial-mesenchymal
transition and chemoresistance through activation of the STAT3
pathway in epithelial ovarian cancer. Tumor Biol. 37:9671–9680.
2016. View Article : Google Scholar
|
|
79
|
Bu X, Liu Y, Wang L, Yan Z, Xin G and Su
W: Oct4 promoted proliferation, migration, invasion, and
epithelial-mesenchymal transition (EMT) in colon cancer cells by
activating the SCF/c-Kit signaling pathway. Cell Cycle. 22:291–302.
2023. View Article : Google Scholar :
|
|
80
|
Fukusumi T, Guo T, Ren S, Haft S, Liu C,
Sakai A, Ando M, Saito Y, Sadat S and Califano JA: Reciprocal
activation of HEY1 and NOTCH4 under SOX2 control promotes EMT in
head and neck squamous cell carcinoma. Int J Oncol. 58:226–237.
2020. View Article : Google Scholar
|
|
81
|
Abada PB and Howell SB: Cisplatin induces
resistance by triggering differentiation of testicular embryonal
carcinoma cells. PLoS One. 9:e874442014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Honecker F, Kersemaekers AF, Molier M, van
Weeren PC, Stoop H, de Krijger RR, Wolffenbuttel KP, Oosterhuis W,
Bokemeyer C and Looijenga LH: Involvement of E-cadherin and
β-catenin in germ cell tumours and in normal male fetal germ cell
development. J Pathol. 204:167–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Young JC, Kerr G, Micati D, Nielsen JE,
Rajpert-De Meyts E, Abud HE and Loveland KL: WNT signalling in the
normal human adult testis and in male germ cell neoplasms. Hum
Reprod. 35:1991–2003. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Schmidtova S, Kalavska K, Liskova V, Plava
J, Miklikova S, Kucerova L, Matuskova M, Rojikova L, Cierna Z,
Rogozea A, et al: Targeting of deregulated Wnt/β-catenin signaling
by PRI-724 and LGK974 inhibitors in germ cell tumor cell lines. Int
J Mol Sci. 22:42632021. View Article : Google Scholar
|
|
85
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Olaizola P, Lee-Law PY, Fernandez-Barrena
MG, Alvarez L, Cadamuro M, Azkargorta M, O'Rourke CJ,
Caballero-Camino FJ, Olaizola I, Macias RIR, et al: Targeting
NAE1-mediated protein hyper-NEDDylation halts
cholangiocarcinogenesis and impacts on tumor-stroma crosstalk in
experimental models. J Hepatol. 77:177–190. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nawrocki ST, Kelly KR, Smith PG, Espitia
CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M,
Berger A and Carew JS: Disrupting protein NEDDylation with MLN4924
is a novel strategy to target cisplatin resistance in ovarian
cancer. Clin Cancer Res. 19:3577–3590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Funke K, Einsfelder U, Hansen A, Arévalo
L, Schneider S, Nettersheim D, Stein V and Schorle H: Genome-scale
CRISPR screen reveals neddylation to contribute to cisplatin
resistance of testicular germ cell tumours. Br J Cancer.
128:2270–2282. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhao Y, Morgan MA and Sun Y: Targeting
Neddylation pathways to inactivate Cullin-RING ligases for
anticancer therapy. Antioxid Redox Signal. 21:2383–2400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Miranda-Gonçalves V, Lobo J,
Guimarães-Teixeira C, Barros-Silva D, Guimarães R, Cantante M,
Braga I, Maurício J, Oing C, Honecker F, et al: The component of
the m6A writer complex VIRMA is implicated in aggressive
tumor phenotype, DNA damage response and cisplatin resistance in
germ cell tumors. J Exp Clin Cancer Res. 40:2682021. View Article : Google Scholar
|
|
91
|
Port M, Glaesener S, Ruf C, Riecke A,
Bokemeyer C, Meineke V, Honecker F and Abend M: Micro-RNA
expression in cisplatin resistant germ cell tumor cell lines. Mol
Cancer. 10:522011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Skowron MA, Vermeulen M, Winkelhausen A,
Becker TK, Bremmer F, Petzsch P, Schönberger S, Calaminus G, Köhrer
K, Albers P and Nettersheim D: CDK4/6 inhibition presents as a
therapeutic option for paediatric and adult germ cell tumours and
induces cell cycle arrest and apoptosis via canonical and
non-canonical mechanisms. Br J Cancer. 123:378–391. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Noel EE, Yeste-Velasco M, Mao X, Perry J,
Kudahetti SC, Li NF, Sharp S, Chaplin T, Xue L, McIntyre A, et al:
The association of CCND1 overexpression and cisplatin resistance in
testicular germ cell tumors and other cancers. Am J Pathol.
176:2607–2615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Moyret-Lalle C, Prodhomme MK, Burlet D,
Kashiwagi A, Petrilli V, Puisieux A, Seimiya H and Tissier A: Role
of EMT in the DNA damage response, double-strand break repair
pathway choice and its implications in cancer treatment. Cancer
Sci. 113:2214–2223. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Assani G and Zhou Y: Effect of modulation
of epithelial-mesenchymal transition regulators Snail1 and Snail2
on cancer cell radiosensitivity by targeting of the cell cycle,
cell apoptosis and cell migration/invasion. Oncol Lett. 17:23–30.
2019.PubMed/NCBI
|
|
97
|
Damjanov I, Clark RK and Andrews PW:
Cytoskeleton of human embryonal carcinoma cells. Cell Differ.
15:133–139. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Micati DJ, Hime GR, McLaughlin EA, Abud HE
and Loveland KL: Differential expression profiles of conserved
Snail transcription factors in the mouse testis. Andrology.
6:362–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Micati DJ, Radhakrishnan K, Young JC,
Rajpert-De Meyts E, Hime GR, Abud HE and Loveland KL: Snail factors
in testicular germ cell tumours and their regulation by the BMP4
signalling pathway. Andrology. 8:1456–14570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dominis M: The generation and in vivo
differentiation of murine embryonal stem cells genetically null for
either N-cadherin or N-and P-cadherin [Internet]. Article in the
International Journal of Developmental Biology. 1999, Available
from: https://www.researchgate.net/publication/12607650.
|
|
103
|
Sasaki T, Forsberg E, Bloch W, Addicks K,
Fässler R and Timpl R: Deficiency of β1 Integrins in teratoma
interferes with basement membrane assembly and Laminin-1
expression. Exp Cell Res. 238:70–81. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rusciano D, Lorenzoni P and Burger MM: The
role of both specific cellular adhesion and growth promotion in
liver colonization by F9 embryonal carcinoma cells. Int J Cancer.
48:450–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rossin R, Berndorff D, Friebe M,
Dinkelborg LM and Welch MJ: Small-animal PET of tumor angiogenesis
using a 76Br-labeled human recombinant antibody fragment to the
ED-B domain of Fibronectin. J Nuclear Med. 48:1172–1179. 2007.
View Article : Google Scholar
|
|
106
|
Andrews PA, Jones JA, Varki NM and Howell
SB: Rapid emergence of acquired cis-Diamminedichloroplatinum(II)
resistance in an in vivo model of human ovarian carcinoma. Cancer
Commun. 2:93–100. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Caffrey PB and Frenkel GD: Prevention of
carboplatin-induced resistance in human ovarian tumor xenografts by
selenite. Anticancer Res. 33:4249–4254. 2013.PubMed/NCBI
|
|
108
|
Caffrey PB, Frenkel GD, Mcandrew KL and
Marks K: A model of the development of cisplatin resistance in
human small cell lung cancer Xenografts. In Vivo. 30:745–750. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jones C, Dziadowicz S, Suite S, Eby A,
Chen WC, Hu G and Hazlehurst LA: Emergence of resistance to MTI-101
selects for a MET genotype and phenotype in EGFR driven PC-9 and
PTEN deleted H446 lung cancer cell lines. Cancers (Basel).
14:30622022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fuchs J, Wenderoth M, von Schweinitz D,
Haindl J and Leuschner I: Comparative activity of cisplatin,
ifosfamide, doxorubicin, carboplatin, and etoposide in
heterotransplanted hepatoblastoma. Cancer. 83:2400–2407. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Warmann S, Hunger M, Teichmann B, Flemming
P, Gratz KF and Fuchs J: The role of the MDR1 gene in the
development of multidrug resistance in human hepatoblastoma.
Cancer. 95:1795–1801. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rendón-Barrón MJ, Pérez-Arteaga E,
Delgado-Waldo I, Coronel-Hernández J, Pérez-Plasencia C,
Rodríguez-Izquierdo F, Linares R, González-Esquinca AR,
Álvarez-González I, Madrigal-Bujaidar E and Jacobo-Herrera NJ:
Laherradurin inhibits tumor growth in an Azoxymethane/dextran
sulfate sodium colorectal cancer model in vivo. Cancers (Basel).
16:5732024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Venkatasamy A, Guerin E, Blanchet A,
Orvain C, Devignot V, Jung M, Jung AC, Chenard MP, Romain B,
Gaiddon C and Mellitzer G: Ultrasound and transcriptomics identify
a differential impact of cisplatin and histone deacetylation on
tumor structure and microenvironment in a Patient-derived in vivo
model of gastric cancer. Pharmaceutics. 13:14852021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Satta T, Isobe K, Yamauchi M, Nakashima I,
Akiyama S, Itou K, Watanabe T and Takagi H: Establishment of drug
resistance in human gastric and colon carcinoma xenograft lines.
Jpn J Cancer Res. 82:593–598. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rocha CRR, Garcia CCM, Vieira DB, Quinet
A, de Andrade-Lima LC, Munford V, Belizário JE and Menck CF:
Glutathione depletion sensitizes cisplatin- and
temozolomide-resistant glioma cells in vitro and in vivo. Cell
Death Dis. 5:e1505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gumbarewicz E, Tylżanowski P, Łuszczki J,
Kałafut J, Czerwonka A, Szumiło J, Wawruszak A, Kupisz K, Polberg
K, Smok-Kalwat J and Stepulak A: Differential molecular response of
larynx cancer cell lines to combined VPA/CDDP treatment. Am J
Cancer Res. 11:2821–2837. 2021.PubMed/NCBI
|
|
117
|
Albany C, Hever-Jardine MP, von Herrmann
KM, Yim CY, Tam J, Warzecha JM, Shin L, Bock SE, Curran BS,
Chaudhry AS, et al: Refractory testicular germ cell tumors are
highly sensitive to the second generation DNA methylation inhibitor
guadecitabine. Oncotarget. 8:2949–2959. 2017. View Article : Google Scholar :
|
|
118
|
Jørgensen A, Blomberg Jensen M, Nielsen
JE, Juul A and Rajpert-De Meyts E: Influence of vitamin D on
cisplatin sensitivity in testicular germ cell cancer-derived cell
lines and in a NTera2 xenograft model. J Steroid Biochem Mol Biol.
136:238–246. 2013. View Article : Google Scholar
|
|
119
|
de Vries G, Rosas-Plaza X, Meersma GJ,
Leeuwenburgh VC, Kok K, Suurmeijer AJH, van Vugt MATM, Gietema JA
and de Jong S: Establishment and characterisation of testicular
cancer patient-derived xenograft models for preclinical evaluation
of novel therapeutic strategies. Sci Rep. 10:189382020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Juliachs M, Castillo-Ávila W, Vidal A,
Piulats JM, Garcia del Muro X, Condom E, Hernández-Losa J, Teixidó
C, Pandiella A, Graupera M, et al: ErbBs inhibition by lapatinib
blocks tumor growth in an orthotopic model of human testicular germ
cell tumor. Int J Cancer. 133:235–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kitayama S, Ikeda K, Sato W, Takeshita H,
Kawakami S, Inoue S and Horie K: Testis-expressed gene 11 inhibits
cisplatin-induced DNA damage and contributes to chemoresistance in
testicular germ cell tumor. Sci Rep. 12:184232022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sakurai Y, Ichinoe M, Yoshida K, Nakazato
Y, Saito S, Satoh M, Nakada N, Sanoyama I, Umezawa A, Numata Y, et
al: Inactivation of REV7 enhances chemosensitivity and overcomes
acquired chemoresistance in testicular germ cell tumors. Cancer
Lett. 489:100–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, et al: Activation of ERBB2 signaling causes resistance to the
EGFR-directed therapeutic antibody cetuximab. Sci Transl Med.
3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jonckheere S, Adams J, De Groote D,
Campbell K, Berx G and Goossens S: Epithelial-mesenchymal
transition (EMT) as a therapeutic target. Cells Tissues Organs.
211:157–182. 2022. View Article : Google Scholar
|
|
126
|
Zhao HB, Wang C, Li RX, Tang CL, Li MQ, Du
MR, Hou XF and Li DJ: E-Cadherin, as a negative regulator of
invasive behavior of human trophoblast cells, is Down-regulated by
cyclosporin A via epidermal growth Factor/extracellular
Signal-regulated protein kinase signaling pathway1. Biol Reprod.
83:370–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hsien Lai S, Zervoudakis G, Chou J, Gurney
ME and Quesnelle KM: PDE4 subtypes in cancer. Oncogene.
39:3791–3802. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Huang Y, Zheng Y, Wang Q and Qi C:
Rolipram suppresses migration and invasion of human choriocarcinoma
cells by inhibiting phosphodiesterase 4-mediated
epithelial-mesenchymal transition. J Biochem Mol Toxicol.
37:e233632023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dyshlovoy SA, Venz S, Hauschild J,
Tabakmakher KM, Otte K, Madanchi R, Walther R, Guzii AG, Makarieva
TN, Shubina LK, et al: Anti-migratory activity of marine alkaloid
monanchocidin A-proteomics-based discovery and confirmation.
Proteomics. 16:1590–1603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ooki A, Satoh T, Muro K, Takashima A,
Kadowaki S, Sakai D, Ichimura T, Mitani S, Kudo T, Chin K, et al: A
phase 1b study of andecaliximab in combination with S-1 plus
platinum in Japanese patients with gastric adenocarcinoma. Sci Rep.
12:11002022. View Article : Google Scholar
|
|
131
|
Bendell J, Sharma S, Patel MR, Windsor KS,
Wainberg ZA, Gordon M, Chaves J, Berlin J, Brachmann CB,
Zavodovskaya M, et al: Safety and efficacy of andecaliximab
(GS-5745) plus gemcitabine and nab-paclitaxel in patients with
advanced pancreatic adenocarcinoma: Results from a phase I study.
Oncologist. 25:954–962. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yoshikawa AK, Yamaguchi K, Muro K,
Takashima A, Ichimura T, Sakai D, Kadowaki S, Chin K, Kudo T,
Mitani S, et al: Safety and tolerability of andecaliximab as
monotherapy and in combination with an anti-PD-1 antibody in
Japanese patients with gastric or gastroesophageal junction
adenocarcinoma: A phase 1b study. J Immunother Cancer.
10:e0035182022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shah MA, Cunningham D, Metges JP, Van
Cutsem E, Wainberg Z, Elboudwarej E, Lin KW, Turner S, Zavodovskaya
M, Inzunza D, et al: Randomized, open-label, phase 2 study of
andecaliximab plus nivolumab versus nivolumab alone in advanced
gastric cancer identifies biomarkers associated with survival. J
Immunother Cancer. 9:e0035802021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Coleman N, Stephen B, Fu S, Karp D,
Subbiah V, Ahnert JR, Piha-Paul SA, Wright J, Fessahaye SN, Ouyang
F, et al: Phase I study of sapanisertib (CB-228/TAK-228/MLN0128) in
combination with Ziv-aflibercept in patients with advanced solid
tumors. Cancer Med. 13:e68772024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ferrarotto R, Swiecicki PL, Zandberg DP,
Baiocchi RA, Wesolowski R, Rodriguez CP, McKean M, Kang H, Monga V,
Nath R, et al: PRT543, a protein arginine methyltransferase 5
inhibitor, in patients with advanced adenoid cystic carcinoma: An
open-label, phase I dose-expansion study. Oral Oncol.
149:1066342024. View Article : Google Scholar
|
|
136
|
Xi M, Guo S, Bayin C, Peng L, Chuffart F,
Bourova-Flin E, Rousseaux S, Khochbin S, Mi JQ and Wang J:
Chidamide inhibits the NOTCH1-MYC signaling axis in T-cell acute
lymphoblastic leukemia. Front Med. 16:442–458. 2022. View Article : Google Scholar
|
|
137
|
Falchook GS, Wheler JJ, Naing A, Jackson
EF, Janku F, Hong D, Ng CS, Tannir NM, Lawhorn KN, Huang M, et al:
Targeting hypoxia-inducible factor-1α (HIF-1α) in combination with
antiangiogenic therapy: A phase I trial of bortezomib plus
bevacizumab. Oncotarget. 5:10280–10292. 2014. View Article : Google Scholar : PubMed/NCBI
|















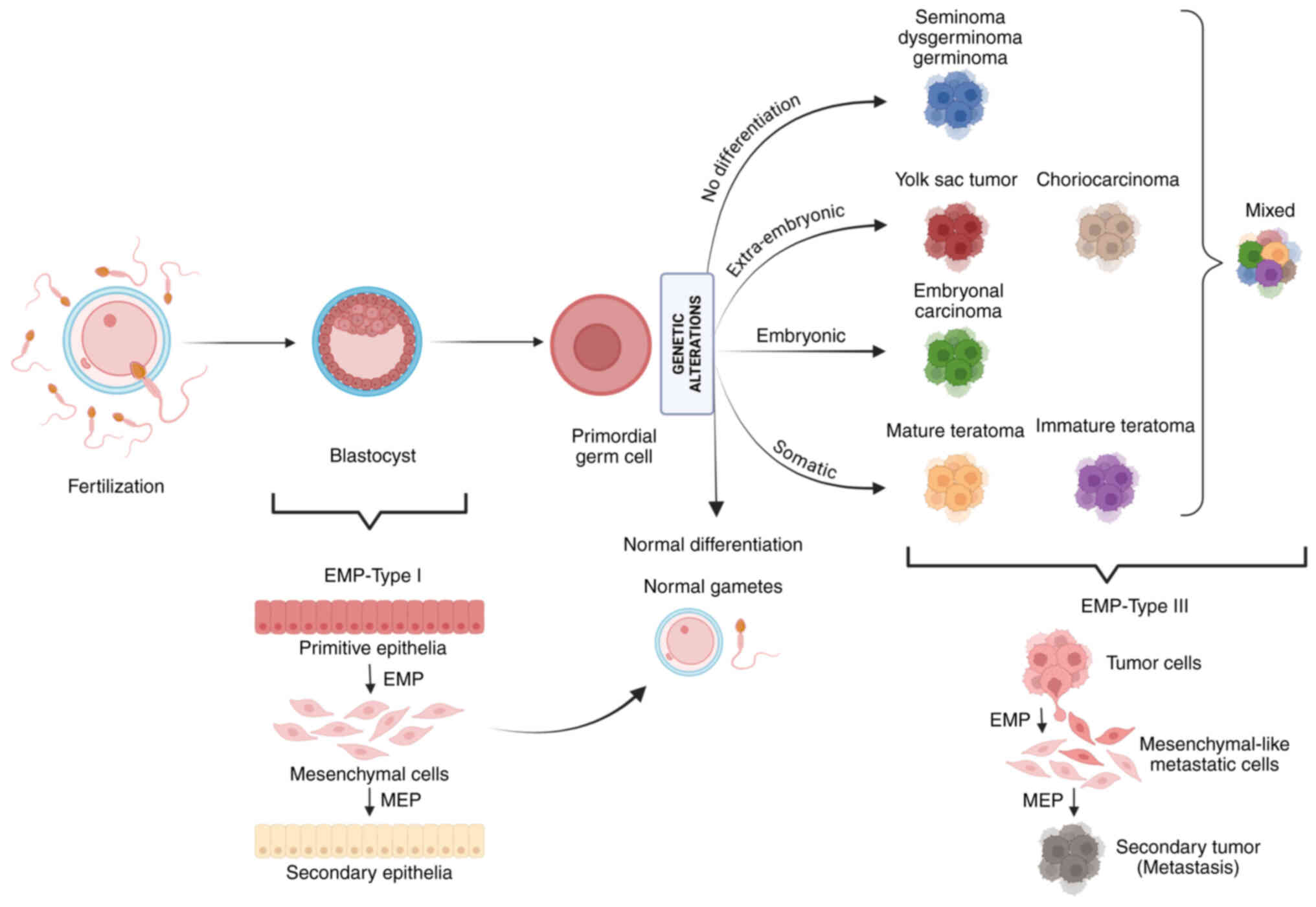

![Graphical representation of EMP
markers, cisplatin resistance and potential inhibitors across GCT
histologies. This figure illustrates the histologies studied both
in vivo and in vitro, including those related to
cisplatin resistance and the status of potential inhibitors for
targeted therapy. Red circles indicate association with cisplatin
resistance. Created in BioRender. Pinto, M. (2025) https://BioRender.com/x70i558.EMP,
epithelial-mesenchymal plasticity; GCT, germ cell tumor; CCND1,
cyclin D1; ErbB, ErbB family; CD133, prominin-1; ALDH3A1, aldehyde
dehydrogenase 3 family member A1; ABCG2, ATP binding cassette
subfamily G member 2; TRIB3, Tribbles pseudokinase 3; IGFBP2,
insulin-like growth factor binding protein 2; NANOG, homeobox
protein NANOG; POU5F1, POU class 5 homeobox 1; SOX2, SRY-related
HMG-box 2; CDK, cyclin-dependent kinase; VIRMA, Vir-like M6A
methyltransferase associated; ACTA2, actin alpha 2; COL1A1, type I
collagen; FN1, fibronectin; HDAC, histone deacetylase; POLD4, DNA
Polymerase Delta 4, Accessory Subunit; TGF-β, transforming growth
factor beta; VIM, vimentin; XPC, XPC complex subunit, DNA damage
recognition and repair factor; NES, nestin; STMN2, stathmin-2;
REV7; reversionless 7; has-miR, Homo sapiens microRNA; ANXA1,
annexin A1; TAGLN, Transgelin; TEXT11, testis-expressed 11;
LINC003131, long intergenic non-coding RNA; PTTG1, pituitary
tumor-transforming gene 1; DAPT,
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester; MLN4924, pevonedistat; MG-132, proteasome inhibitor; PDE4,
phosphodiesterase 4; SENP1, SUMO specific peptidase 1; ILK,
integrin linked kinase; SNAIL, snail family transcriptional
repressor 1; TWIST1, Twist-related protein 1; NAE1, NEDD8
activating enzyme E1 subunit 1; HIF-1α, hypoxia inducible factor 1
Subunit Alpha ; ZEB2, zinc finger E-box binding homeobox 2; HMOX1,
heme oxygenase 1; NOTCH1, notch receptor 1.](/article_images/ijo/67/6/ijo-67-06-05811-g02.jpg)