Introduction
Colorectal cancer (CRC) represents one of the most
prevalent malignancies of the digestive tract. In 2020, CRC emerged
as the third most diagnosed cancer worldwide and ranked second
among global cancer mortality factors (1). Due to its insidious onset, most CRC
patients present with advanced-stage disease at initial diagnosis,
which limits opportunities for curative interventions such as
surgical resection and local ablation (2). Consequently, the development and
establishment of efficient multimodal therapeutic strategies have
emerged as a research hotspot globally.
Current therapeutic modalities for CRC primarily
encompass surgical resection, radiotherapy, chemotherapy and
targeted therapy, which have demonstrably provided symptomatic
relief, prolonged survival and improved the life quality of
patients. However, adjuvant pharmacological treatment regimens for
CRC, using chemotherapeutic agents such as irinotecan
hydrochloride, 5-fluorouracil (5-FU), oxaliplatin and cetuximab,
frequently induce irreversible damage to normal tissues and organs,
accompanied by severe adverse reactions and toxic side effects
(3). For instance, 5-FU
administration may precipitate severe gastrointestinal reactions
that exacerbate mucosal damage and related symptomatology (4). In addition, the emergence of drug
resistance to cetuximab has progressively become a major clinical
challenge requiring urgent attention (5). Therefore, developing effective yet
low-toxicity therapeutic agents for CRC may constitute a pivotal
strategy to enhance overall treatment efficacy.
Recent researches have elucidated that the
pathogenesis of CRC involves the dysregulation of a number of
cascading signaling pathways, including signal transducer and
activator of transcription 3 (STAT3), transforming growth factor-β
(TGF-β), phosphoinositide 3-kinase/protein kinase (PI3K/Akt) and
Wnt/β-catenin, characterized by aberrant silencing or activation of
specific targets (6). However,
current pharmacological interventions for CRC predominantly focus
on single-target therapies. While acknowledging the clinical
efficacy of specific single-target agents in CRC management (such
as immune checkpoint inhibitors), monotherapy targeting individual
signaling cascades or biological markers generally fail to achieve
optimal therapeutic outcomes. Naturally derived phytochemicals
exhibit marked advantages in modulating signal transduction
cascades through multi-target, multi-pathway and multi-effect
mechanisms, thereby enhancing treatment efficacy and quality of
life in CRC patients while effectively preventing recurrence and
potentiating targeted drug performance (7). Notable examples include paclitaxel,
the first plant-derived chemotherapeutic agent and camptothecin,
another plant-based antineoplastic alkaloid, have shown promising
clinical applications. These instances underscore the growing
significance of natural product-derived chemicals in anticancer
drug discovery and research.
Chronic inflammation and oxidative stress-induced
damage are closely implicated in the initiation and progression of
CRC. Under physiological conditions, the balanced production of
reactive oxygen species (ROS) and their elimination by endogenous
antioxidants maintains the body's oxidative-antioxidant
equilibrium. However, during pathological states, various
environmental and endogenous stressors stimulate excessive ROS
generation, disrupting this equilibrium and triggering oxidative
stress. The resultant oxidative stress induces macromolecular
oxidation of proteins, lipids and DNA/RNA, leading to lipid
peroxidation of biomembranes, denaturation of intracellular
proteins and enzymes and DNA damage. These alterations compromise
intestinal mucosal barrier integrity, ultimately promoting mucosal
injury, colitis and colorectal carcinogenesis (8,9).
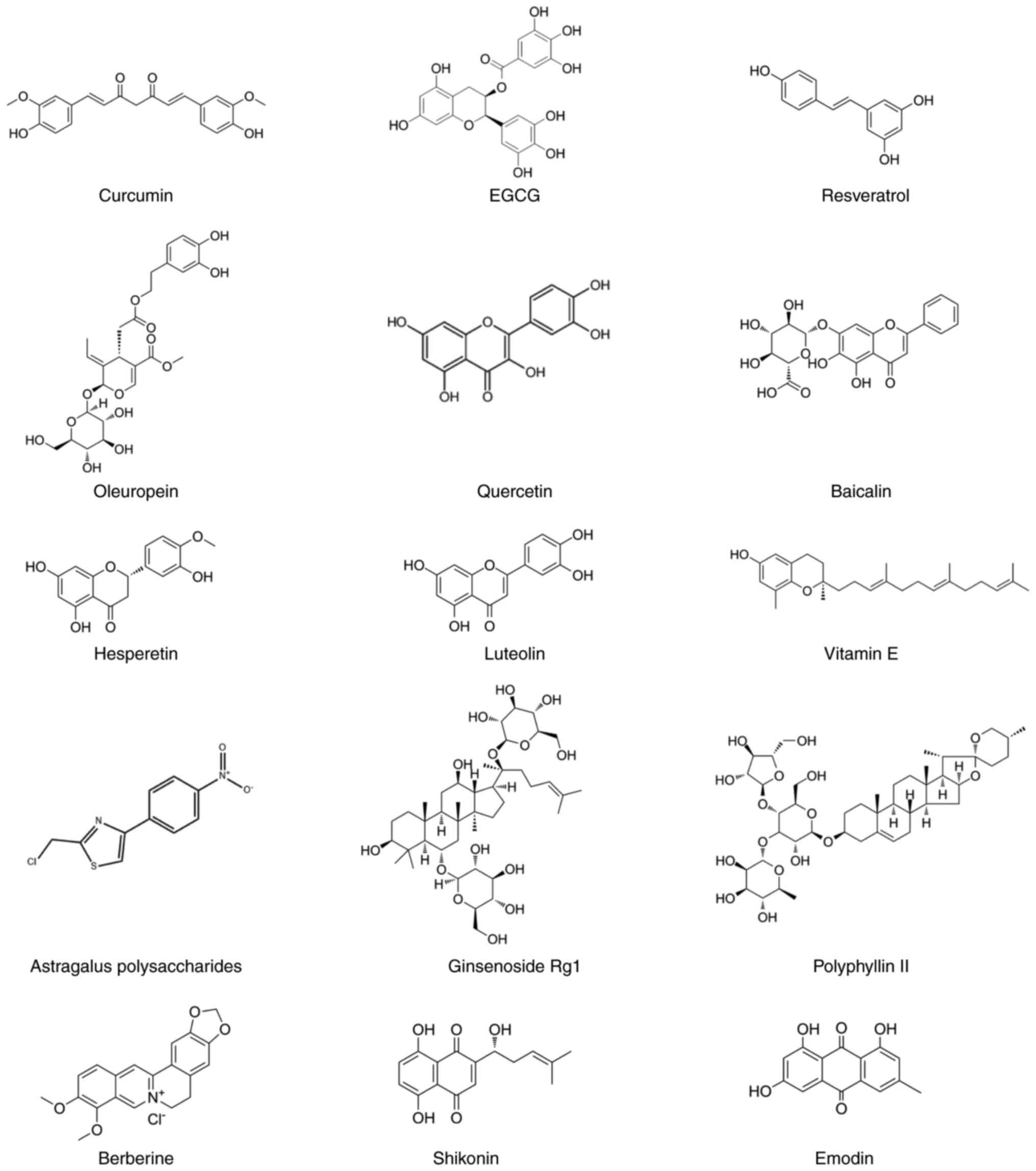
Over the past decade, numerous plant-based
antioxidants (PBAs) with anticancer properties have been
identified, such as curcumin and epigallocatechin gallate (EGCG)
(10). Chemically, PBAs under
investigation for CRC therapy comprise diverse structural classes
including phenolics, polysaccharides, alkaloids and terpenoids
(Fig. 1). While these monomer
compounds have demonstrated promising antitumor activities in
epidemiological, in vitro and preclinical studies across
various malignancies, their combinatorial application in CRC
chemotherapy remains underexplored and the precise synergistic
antitumor mechanisms remain incompletely elucidated (11). As oncology enters the era of
precision medicine, effective integration of research on PBAs
against CRC to maximize their therapeutic potential represents an
urgent priority in fundamental research.
The present review elucidated the preventive and
therapeutic effects of PBAs on the occurrence and progression of
CRC from the foundational cellular mechanisms and clinical
research. Various types of PBAs play roles by regulating the
signaling pathways related to CRC and modulating the expression of
target genes involved in these pathways. Rather than focusing
solely on a class of plant compounds or herbal medicines that
modulate the gut microbiota of CRC (12,13), the present review comprehensively
detailed the classification and therapeutic effects of various
plant-derived antioxidant monomer components with anti-CRC
properties. The present review also provided a more comprehensive
theoretical basis for further understanding of the molecular
mechanism of PBAs in the treatment of CRC. Additionally, there are
few clinical studies on the prevention and treatment of CRC using
PBAs and they are still facing bioavailability and safety problems.
Different drug delivery strategies and individualized treatment
concepts have been developed and the present review highlighted and
emphasized the limitations and challenges of current progress. It
requires further investigation to explore and promote the efficacy
of PBAs in CRC.
Foundational mechanisms of plant-based
antioxidants
Antioxidant and redox homeostasis
The mutation and transformation of normal tissue
cells into cancerous cells can be triggered by the accumulation of
free radicals during early stages, with oxygen-derived free
radicals subsequently participating in cancer progression. Free
radicals produced by Enterococcus bacteria in the colon may
directly induce colonic DNA mutations, thereby contributing to CRC
development (14). Oxidative
stress is widely thought to promote intestinal epithelial cell
damage by inducing genetic instability, specific gene alterations
and aberrant methylation, creating opportunities for colorectal
carcinogenesis. Excessive ROS generation triggers tissue or
intracellular oxidative stress, further inducing oxidative DNA
damage. Increased DNA damage stimulates the uptake of
ω-polyunsaturated fatty acids as a compensatory response (15). Concurrently, ROS activates both
intrinsic mitochondrial-mediated and extrinsic death
receptor-mediated apoptosis pathways, thereby promoting CRC
initiation and progression (16).
Gingerol (6-Shogaol, 20 mg/kg/day, orally), compared
with the control group [a mouse colorectal adenoma model induced by
Azoxymethane (AOM) and dextran sulfate sodium (DSS)], reportedly
demonstrates significant antioxidant stress effects by reducing
levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α),
lipid peroxidation, myeloperoxidase (MPO) and nitric oxide (NO),
while markedly enhancing activities of superoxide dismutase (SOD),
catalase (CAT) and glutathione (GSH) (17). These effects collectively promote
redox homeostasis restoration in colonic tissues. Curcumin
activates intracellular redox reactions to induce ROS generation,
which upregulates apoptotic receptors on tumor cell membranes. In
addition, curcumin enhances the expression and activity of p53, a
tumor suppressor that inhibits proliferation and promotes
apoptosis. This compound also potently inhibits NF-κB and COX-2
activities, both linked to overexpression of anti-apoptotic genes
such as Bcl-2. Curcumin further attenuates pro-survival PI3K
signaling while increasing mitogen-activated protein kinase (MAPK)
expression, stimulating endogenous ROS production, which may
activate a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress
colorectal cancer metastasis (18).
Anti-inflammatory and immunomodulatory
effects
Colitis-associated CRC (CAC) is now understood to
develop from chronic inflammatory bowel disease (IBD) (19). During early intestinal
inflammation and mucosal barrier disruption, luminal microbial
antigens trigger immune cell chemotaxis to the colonic mucosa.
These infiltrating cells secrete pro-inflammatory cytokines
including IL-6, IL-1β, TNF-α and interferon-γ (IFN-γ), establishing
a chronic inflammatory microenvironment. Concurrently,
immune-derived reactive oxygen/nitrogen species, such as inducible
nitric oxide synthase (iNOS), induce colonic epithelial DNA damage
(20). Nuclear factor-κB (NF-κB)
serves as a master regulator of inflammatory mediator production,
with its hyperactivation critical for maintaining colonic
inflammation and promoting epithelial dysplasia (21). Upon activation, inhibitors of
NF-κB (IκBs) undergo phosphorylation and degradation, releasing the
p65 subunit for nuclear translocation and pro-inflammatory gene
transcription (22). To
investigate the effect of Berberine (BBR) on intestinal
inflammatory response in AOM/DSS-induced CAC mice, BBR (daily
gavage of 100 mg/kg) markedly decreased the colonic levels of TNF
α, IL-6 and IL-1β compared with the CAC group (daily gavage of 100
μl PBS). Furthermore, compared with the CAC group, the
zonula occludens-1 (ZO-1), occludin and mucin-2 (MUC-2) mRNAs were
markedly upregulated in response to BBR (23). These results indicated that BBR
could improve colitis symptoms and epithelial injury in inflamed
mucosa, inhibiting CAC development. Toll-like receptor 4 (TLR4), a
pathogen recognition receptor expressed in colonic epithelia and
lamina propria immune cells, activates downstream inflammatory
pathways that exacerbate IBD-associated inflammation and
tumorigenesis (24). Compared
with the control group (constituting AOM/DSS-induced CAC mouse
model), Rosmarinic acid (30 mg/kg/day; orally) markedly decreased
83.3% of the polypoid tumor number and inhibited COX-2 and iNOS
protein levels in vivo. Furthermore, Rosmarinic acid (25
μM) competitively antagonizes TLR4, blocking NF-κB and STAT3
activation in HCT116 cells and HT29 cells exposed to the
inflammatory microenvironment (25). This suppression reduces
inflammatory mediator levels and confers chemo-preventive effects
against CAC.
The intestine constitutes the most significant
component of the human immune system, making the modulation of
intestinal immune status to restore homeostasis a promising
therapeutic approach for treating IBD and preventing CAC
development. During intestinal inflammation, myeloid-derived
suppressor cells (MDSCs) are recruited and activated in gut
tissues, where they suppress dendritic cell antigen
uptake/processing and subsequent CD4+ T cell
proliferation/activation. This impairment compromises pathogen
clearance at bacterial penetration sites, perpetuating chronic
inflammatory stimuli (26). The
resultant shift from acute to chronic inflammation creates a
permissive microenvironment for tumor initiation.
Madecassic acid (MA), a triterpenoid compound
isolated from Centella asiatica, blocks MDSC migration by
inhibiting γδT17 cell activation and related chemokine expression.
Compared with the control group (AOM/DSS mouse model) and the
positive control group (5-amino-o-hydroxybenzoic acid, 5-ASA; 75
mg/kg/day, orally), orally administrated of MA (25 mg/kg/day) can
markedly weaken the severity of CAC and reduce the incidence of
tumors and 5-ASA only delays the progression of tumors (27). This intervention enhances
antitumor immunity and suppresses CAC progression. Curcumin (20
μM) exerts dual actions by inducing CT26 tumor cell
apoptosis and heat shock protein 70 expression while recruiting
CD3+ T cells and F4/80+ macrophages to
inhibit CRC growth (28). In
addition, tumors from curcumin-treated rats (750 μg/kg, i.p.
on days 21 and 26) were infiltrated with numerous activated
lymphocytes, compared with the control group (untreated). The
proteome alterations showed that curcumin suppresses Foxp3
expression while enhancing type II interferon production, skewing T
cell differentiation toward Th1 phenotype and counteracting tumor
immune evasion (29).
Cell cycle and apoptosis regulation
Dysregulation of cell cycle progression represents a
critical mechanism underlying uncontrolled proliferation and
malignant transformation of tumor cells, with aberrant activation
of cell cycle checkpoints playing a pivotal role in this process.
Overexpression of Cyclin D1 and Cyclin B1, key rate-limiting
regulators for G1/S and G2/M phase
transitions, respectively, disrupts cellular proliferation control,
impairs differentiation and facilitates oncogenic progression
(30). Consequently, targeted
cell cycle arrest has emerged as a promising therapeutic avenue for
cancer cell elimination. EGCG has been shown to downregulate mRNA
expression of several cell cycle-related genes while enhancing
expression of the cyclin-dependent kinase inhibitor p21 and
apoptosis-associated death receptor 5 (31). Disrupted apoptotic processes
compromise the equilibrium between apoptosis and proliferation in
colonic epithelial cells, ultimately contributing to CRC
development. It demonstrates that various PBAs exert anti-apoptotic
effects via mitochondria-dependent apoptotic pathways, primarily by
modulating the expression of cysteine-aspartic acid protease
(caspase) family members and Bcl-2 family proteins (Fig. 2).
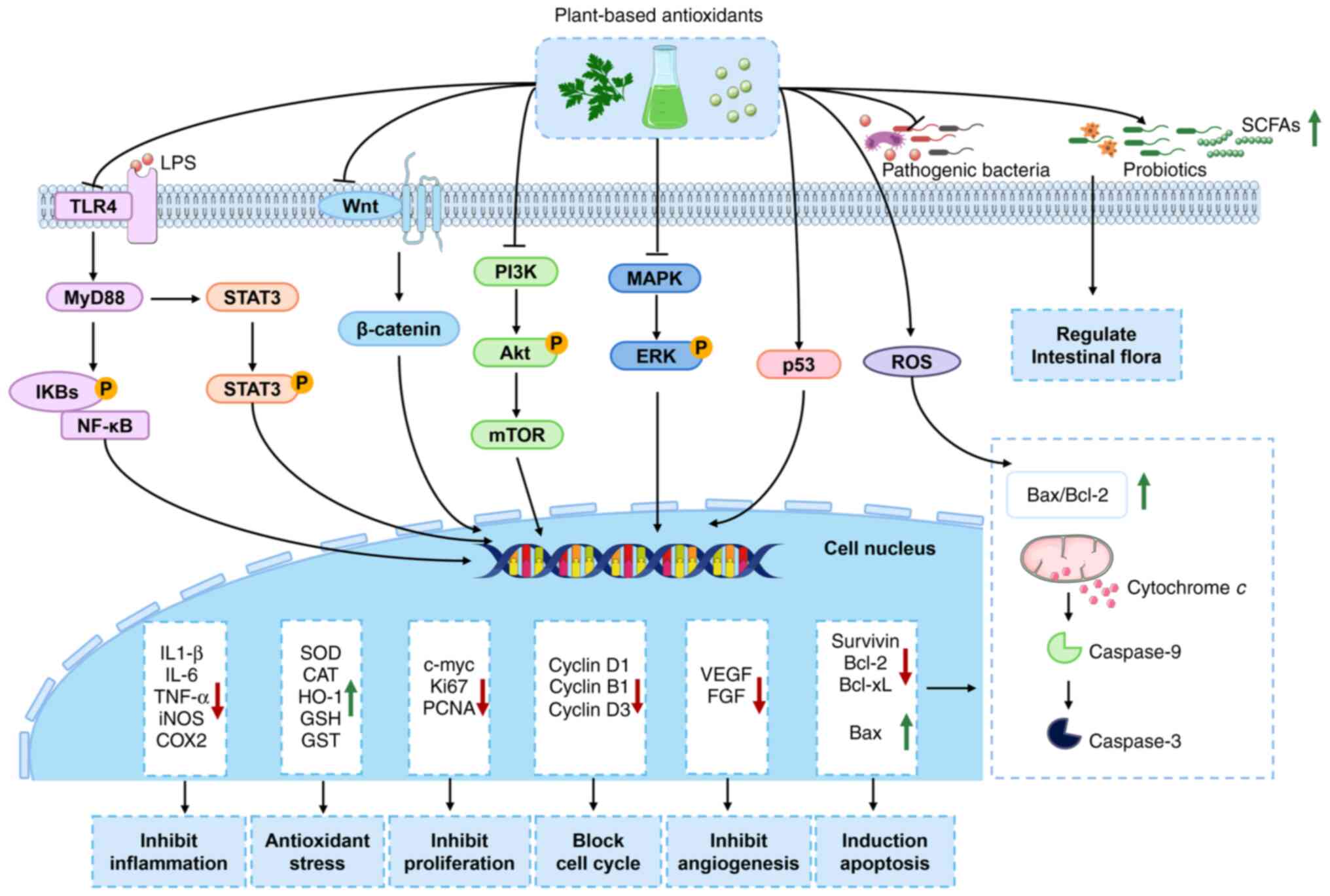 | Figure 2Signaling pathway diagram
illustrating the mechanisms of plant-based antioxidants against
CRC. Plant-based antioxidants exhibit significant efficacy in the
prevention and treatment of CRC. Its molecular mechanisms of action
encompass multi-targets, including NF-κB, TLR4, Wnt/β-catenin,
PI3K/Akt and MAPK/ERK signaling pathways, which suppress chronic
inflammation, alleviate oxidative stress, inhibit cell
proliferation and angiogenesis, induce cell apoptosis and cell
cycle arrest and regulate gut microbiota composition. CRC,
colorectal cancer; NF-κB, nuclear factor kappa-B; TLR4, toll-like
receptor 4; PI3K/Akt, phosphoinositide 3-kinase/protein kinase;
MAPK/ERK, mitogen-activated protein kinase/extracellular
signal-regulated kinase; LPS, lipopolysaccharide; IKBs, inhibitors
of NF-κB; STAT3, signal transducer and activator of transcription
3; mTOR, mammalian target of rapamycin; ROS, reactive oxygen
species; SCFAs, short chain fatty acids; iNOS, inducible nitric
oxide synthase; COX2, cyclooxygenase-2; SOD, superoxide dismutase;
CAT, catalase; HO-1, heme oxygenase 1; GSH, glutathione; GST,
glutathione S-transferase; VEGF, vascular endothelial growth
factor; FGF, fibroblast growth factor. |
The cell viability assay showed that curcumin
analogue, MS13 (1,5-Bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien
e-3-one) has a greater cytotoxicity effect on SW480
(EC50: 7.5±2.8 μM) and SW620 (EC50:
5.7±2.4 μM), compared with curcumin (SW480, EC50:
30.6±1.4 μM) and SW620, EC50: 26.8±2.1
μM). Subsequent analysis indicated that MS13 induced
apoptosis by enhancing caspase-3 activity while reducing Bcl-2
protein levels, thereby suppressing CRC cell growth (32). Furthermore, a significant
proportion of these phytochemicals activate mitochondrial apoptosis
by targeting aberrant signaling cascades within cancer cells,
including the PI3K/Akt, MAPK/extracellular signal-regulated kinase
(ERK) and p38 MAPK pathways (Fig.
2). In addition, it has been established that EGCG inhibits
tumor cell growth and apoptosis through the PI3K-Akt-Cyclin D1 and
p53 signaling axes. Its pro-apoptotic mechanism also involves
suppression of fasciclin-like arabinogalactan protein-mediated Jun
N-terminal kinase (JNK) signaling, leading to increased BAX/Bcl-2
ratio (31). These findings
collectively underscore the multi-targeted therapeutic potential of
PBAs in CRC management through coordinated regulation of
apoptosis-related proteins and oncogenic signaling networks.
Epigenetic modifications and miRNA
regulation
Recent investigations have elucidated the biological
activities of EGCG in inhibiting DNA methyltransferase (DNMT) and
microRNA expression, which play pivotal roles in cancer
therapeutics (31). EGCG
reportedly suppresses DNMT activity via radical scavenging and
antioxidant mechanisms, leading to demethylation and upregulated
expression of tumor suppressor genes P16 and P21,
ultimately inducing apoptosis and inhibiting tumorigenesis.
Notably, treatment of EGCG (20 μg/ml) to head and neck
cancer cell lines markedly reduced DNMT activity to 60% in SCC-1
and 80% in FaDu cells. An in vivo study demonstrated that
administration of EGCG (0.5%, w/w) inhibited tumor growth in
xenografts in nude mice (80%) compared with non-EGCG-treated
controls (33).
During the progression of IBD, numerous miRNAs
exhibit altered expression patterns and regulate the expression of
related tumor suppressor genes and proto-oncogenes, exerting
pro-carcinogenic effects by disrupting intestinal homeostasis.
Substantial evidence supports the association between CAC and
inflammation-induced aberrant miRNA expression. Pristimerin, a
natural triterpenoid compound isolated from Celastraceae
plants, alleviates intestinal inflammation symptoms in DSS-induced
colitis models through intraperitoneal injection of 0.4 mg/kg daily
for 5 days compared with the untreated model mice (34). This effect is mediated by
inhibiting intestinal miRNA-155 expression and suppressing the
NF-κB signaling pathway. Resveratrol, a naturally occurring
stilbene compound, has been reported to markedly inhibit LoVo and
SW480 cell viability by 50% at the concentration of 50 μM.
Compared with control groups, Resveratrol treatment led to
increased miR-769-5p expression and decreased MSI1 expression in
CRC cells (35). This indicates
that Resveratrol inhibits CRC cell proliferation and migration by
activating the miR-769-5p/MSI1 pathway. The aforementioned studies
overlap in their assertion that PBAs can attenuate intestinal
inflammation and suppress CRC by targeting miRNAs, highlighting the
need for developing more miRNA-targeted small molecule therapeutics
to expand clinical treatment options.
Gut microbiota regulation
CRC patients exhibit marked alterations in gut
microbiota composition and distribution, characterized by reduced
beneficial bacteria and increased pathogenic species. Pathogens
such as Escherichia coli, Fusobacterium,
Streptococcus and Enterococcus promote colorectal
carcinogenesis by secretion of oncogenic metabolites that induce
DNA damage, amplify inflammation and activate proliferative
signaling pathways (36).
Conversely, probiotics and their metabolites (such as short-chain
fatty acids, SCFAs) exert chemopreventive effects via
anti-mutagenesis, proliferation suppression, apoptosis induction,
mucosal barrier enhancement, inflammatory microenvironment
modulation and immune activation (37,38). Gut microbiota can produce ROS or
stimulate intestinal epithelial cells to produce ROS, thereby
promoting tumorigenesis and development (39).
PBAs have shown promise in alleviating gut dysbiosis
in CRC animal models. Compared with the NC group, an AOM/DSS
induced CRC model group (PBS, 150 mg/kg/day) exhibited a marked
reduction in colon length and an obvious increase in tumor count,
while the curcumin treatment groups (CRC-Cur, 150 mg/kg/day)
increased the length of the colon and markedly reduced the number
of tumors. The intestinal flora and intestinal metabolites analysis
showed that curcumin educed harmful bacteria (such as
Ileibacterium, Monoglobus and Desulfovibrio)
and increased the abundance of Clostridia_UCG-014,
Bifidobacterium and Lactobacillus in AOM/DSS-induced
CRC model mice (40). Patchouli
essential oil (40 mg/kg/day) markedly reduced the number of polyps
(47.14±15.66) compared with the control group (vehicle 0.5%
carboxymethyl cellulose + 1% DMSO, 81.86±12.36) in
ApcMin/+ mice while enhancing intestinal barrier
integrity and alleviating the inflammatory microenvironment. This
effect was associated with decreased abundance of pathogenic
bacteria (Desulfovibrio, Mycoplasma genitalium and
Clostridium difficile) and elevated fecal SCFAs, which
upregulated SCFA receptors (GPR41, GPR43 and GPR109a) and
peroxisome proliferator-activated receptor γ expression (41). Compared with a dextran sulfate
sodium (DSS)-induced colitis group (daily gavage of PBS for 3 weeks
with 2.5% DSS in drinking water for the last 6 days), an EGCG group
(50 mg/kg) altered the gut microbiome of DSS-treated mice by
increasing Akkermansia abundance and butyrate production.
The alteration of gut microbiome further promotes anti-inflammatory
effects and colonic barrier integrity (42). Current research on plant-based CRC
prevention has mainly focused on microbiota modulation and
metabolite production, with little emphasis on host-microbiota
crosstalk mechanisms involving antitumor immunity and epithelial
cell signaling. Accordingly, further investigations are warranted
to elucidate these interactive pathways.
Unique anti-tumor effects of key plant-based
antioxidants in CRC
Polyphenols
Curcumin
Curcumin, derived from the rhizome of Curcuma
longa (turmeric), exhibits diverse biological activities
including anti-cancer, antimicrobial, anti-inflammatory, antiviral,
antioxidant, anti-aging, anti-diabetic and cardiovascular
protective properties (43).
Systematic meta-analyses show that curcumin has therapeutic
potential for CRC, improves survival rates and enhances quality of
life (44-46). Curcumin targets proliferating
cancer stem-like cells (CSCs) within CRC premalignant adenoma and
early-stage cancer tissues. Curcumin decreases the proportion of
proliferating CSCs by direct binding to NANOG, thereby inhibiting
tumor development (47).
Moreover, Curcumin can inhibit CRC growth by inducing ferroptosis
via regulation of p53 and SLC7A11/glutathione/GPX4 axis (48). Combining curcumin (50 μM)
and metformin (40 μM) markedly suppresses the migration
ability of HCT116 cells and promote ROS-induced cell death
(49), which provides a potential
option for CRC treatment. Overall, these findings indicate that
curcumin plays an effective adjunct therapy for CRC on a number of
molecular targets. However, its low bioavailability and rapid
metabolism limit its clinical translation (46). Addressing these challenges through
more studies, determining effective doses and improving
formulations to enhance absorption is essential.
EGCG
The anti-cancer mechanisms of EGCG encompass
angiogenesis inhibition, induction of tumor cell death and
suppression of tumor growth.
EGCG downregulates the expression of HIF-1α, HIF-1β
and VEGF in CRC cells, reducing tumor vascular density and
effectively controlling tumor cell metastasis while inhibiting the
PI3K/Akt signaling pathway (31).
Furthermore, EGCG inhibits CRC cell proliferation and induces
apoptosis by blocking the activation of the receptor tyrosine
kinases family members EGFR, IGF-1R and VEGFR2 (50). In the Caco-2 cell line, EGCG (15
μM) downregulates the expression of MMP-2 and MMP-9 through
a NOX1/EGFR signaling pathway-dependent mechanism, while directly
inhibiting the enzymatic activity of MMPs, thereby effectively
suppressing tumor cell invasion and metastasis (51). A fibril composed of EGCG and
lysozyme (EGCG-LYS) demonstrated excellent siRNA delivery
efficiency in in vitro experiments. It could effectively
silence the expression of circMAP2K2 (hsa_circRNA_102415), thereby
inhibiting the epithelial-mesenchymal transition (EMT)-like
phenotype generated by circMAP2K2 through the protease-mediated
PCBP1/GPX1 axis, inhibiting the activated AKT/GSK3β signaling
pathway and achieving the goal of inhibiting the proliferation and
metastasis of gastric cancer cells, which provides a new tool for
the treatment of gastric cancer (52). Collectively, these findings
demonstrate that EGCG effectively alleviates tumor angiogenesis and
metastasis.
Apoptosis, a programmed cellular protective
mechanism, represents the primary pathway for eliminating tumor
cells through the induction of their programmed death. EGCG induces
apoptosis in gastrointestinal tumor cells in a dose-dependent
manner without affecting normal cell growth and it triggers cancer
cell death through a number of pathways (31). Specifically, EGCG enhances the
translocation of cytochrome c from the mitochondrial inner
membrane to the cytoplasm, inhibits ATP synthesis, disrupts
mitochondrial membrane potential, activates caspase cascades and
promotes tumor cell apoptosis (53). Moreover, treatment with 10
μg/ml EGCG for 24 h induced apoptosis and markedly
suppressed the proliferation in CACO-2 and CW-2 cells. The miRNA
analysis showed that the expression of hsa-miR-187-5p in CW-2 cells
was markedly downregulated following EGCG treatment (54). An in vitro dosage of 6
μM EGCG has an initial antagonistic effect on oxaliplatin
cytotoxicity and increases the sensitivity of HCT116 and HT29 cells
to subsequent oxaliplatin administration, which provides an
adjunctive treatment for CRC with lower and safer doses of EGCG
(55). EGCG also inhibits gastric
cancer cell proliferation by targeting STAT3 to inhibit M2
macrophages polarization induced by PLXNC1-mediated exosomes
(56). In an in vivo
study, mice received intraperitoneal injections of EGCG at a dose
of 50 μg/kg (0.1 ml) developed markedly smaller tumors than
the control group treated with 0.1 ml PBS alone (54). While EGCG primarily antagonizes
gastrointestinal tumors by inducing tumor cell apoptosis, the
methods of triggering such apoptosis are complex and diverse, with
a number of underlying mechanisms remaining unclear. Elucidating
the molecular mechanisms of tumor apoptosis represents one of the
significant future directions in cancer research to more
effectively induce this process. Furthermore, EGCG can be
administered orally or injected with an acceptable safety profile
and its safe dose of antitumor effects still needs more
reports.
Resveratrol
Resveratrol exerts multi-faceted effects on CRC
through various mechanisms, including suppression of cell
proliferation, inhibition of metastasis, induction of apoptosis,
stimulation of autophagy, modulation of immune responses,
alleviation of inflammation, regulation of gut microbiota and
enhancement of other anticancer drug efficacy (57). The expression of pro-inflammatory
cytokine tumor necrosis factor-β and its receptor activates nuclear
transcription factor NF-κB, which participates in CRC cell growth
and proliferation. Compared with HCT116 and SW480 cells in the
control groups, resveratrol (5 μM) suppressed the cancer
cell proliferation with the regulation of β1-integrin/FAK/p65-NF-kB
pathway and cyclin D1-signaling. CRC cells treated with resveratrol
markedly inhibited β1-integrin expression and reduced the
distribution of β1-integrin receptors on the cell surface (58). In addition, high concentration
(>10 μM) of resveratrol disrupted interactions between
CRC cells and stromal cells within the multicellular tumor
microenvironment, triggering apoptosis through promoting
hyperacetylation of p53 and FOXO3a as post-translational substrates
of Sirt-1 in the CRC tumor microenvironment (59). Furthermore, resveratrol markedly
reduced serine/threonine kinase 1/2/3 (Akt1/2/3) phosphorylation,
downregulated bone morphogenetic protein expression via PI3K/Akt
signaling inhibition, upregulated STATB2 to promote Bax-Caspase 3/9
apoptotic pathway protein expression in HCT116 cells and induced
CRC cell apoptosis (60).
Oxidative stress has been established as a
pro-carcinogenic factor and resveratrol alleviates oxidative stress
by up-regulating antioxidant enzymes. Reports suggest that
resveratrol achieves therapeutic efficacy by inhibiting SOD
activity or activating Nrf2-mediated antioxidant signaling
pathways, thereby suppressing oxidative stress in CRC. Resveratrol
(10 μmol/l) was found to activate Nrf2 and Sirt-1 to
regulate cellular oxidation, reducing 5-FU-induced oxidative stress
damage in normal cells at a 5 μg/ml concentration,
demonstrating a dose-response relationship (61). Furthermore, in a murine
cardiotoxicity model established via intraperitoneal injection of
5-FU at 15, 30 and 60 mg/kg, resveratrol was found to attenuate
myocardial cell toxicity induced by 5-FU, showing potential in
mitigating cardiac damage associated with long-term high-dose 5-FU
treatment for CRC (62).
Sprague-Dawley rats that received oxaliplatin (2 mg/kg/day,
cumulative dose: 6 mg/kg, i.p.) and oral resveratrol (7.14
mg/kg/day) was found not to develop mechanical allodynia or
hypersensitivity, which inhibited upregulation of NF-κB, TNF-α,
AIF3 and excitatory neuronal promoter c-fos, while increasing
expression of Nrf2, NQO-1, HO-1 and Sirt1. This combination
restored the GSH/GSSG ratio, preventing and antagonizing
chemotherapy-induced peripheral neuropathic pain (63).
Oleuropein
Oleuropein represents one of the primary phenolic
compounds in olive leaf extracts. It has demonstrated that
oleuropein exerts protective effects in acetic acid-induced
ulcerative colitis in rats by inhibiting the production of
intestinal inflammatory factors (64). There were three groups: Normal
control, positive control (ulcerative colitis and untreated) and
oleuropein group (treated with intrarectal oleuropein at a dose of
350 mg/kg). Compared with the positive control group, oleuropein
resulted in a significant reduction of MPO and NO levels and
increased SOD, CAT and GPX levels in colon tissues. Moreover, the
expression levels of pro-inflammatory cytokines (IL-1β, TNF-α,
IL-10, COX-2, iNOS and NF-κB) were also decreased in the oleuropein
group. In the intestinal tissues of rats treated with oleuropein,
expression of the pro-apoptotic gene Bcl-2-associated X protein was
reduced, while the anti-apoptotic gene Bcl-2 was
upregulated. These findings indicate that oleuropein alleviates
inflammation by suppressing aberrant apoptosis of intestinal
cells.
Flavonoids
Flavonoids represent a class of plant secondary
metabolites characterized by a basic structure comprising two
benzene rings interconnected via a three-carbon bridge,
establishing a C6-C3-C6 configuration (65). Baicalin, a natural flavonoid, has
been found to possess remarkable anti-CRC properties. Studies have
revealed its capacity (at 100 μg/ml) to induce cell cycle
G1 phase arrest in CRC cells, promote p53-independent
apoptosis and suppress both endogenous and exogenous TGF-β1-induced
EMT via inhibition of the TGF-β/Smad pathway (66). In an in vivo test,
orthotopic transplanted colon tumor model mice were randomly
divided into negative control, positive control (25 mg/kg of 5-FU),
low dose group (100 mg/kg of baicalin) and high dose group (200
mg/kg baicalin), respectively. The low-dose baicalin group
exhibited markedly tumor inhibition rates (ratios of average tumor
size of treated groups and negative control group) compared with
those in both positive control and high-dose baicalin groups.
Hesperetin, a flavonoid primarily derived from citrus fruits, has
been reported to prevent DSS-induced colitis. The mice were
randomly divided into four groups: control group, DSS group,
hesperetin treated group (20 mg/kg, injected daily
intraperitoneally) and DSS with hesperetin treated group (20 mg/kg,
injected daily intraperitoneally). The DSS group showed a lower
weight and colon length compared with the control group, while
these changes were rescued by hesperetin treatment. Hesperetin
enhances intestinal expression of ZO-1, occludin and MUC-2, while
reducing TNF-α, IL-6, IL-18, HMGB1 and IL-1β levels to exert
intestinal protective effects (67). Further investigation revealed
hesperetin's ability to decrease expression of receptor-interacting
protein kinase 3 (RIPK3) and mixed lineage kinase domain-like
protein (MLKL), two critical mediators of necroptosis pathways,
suggesting its capacity to ameliorate DSS-induced intestinal
inflammation through suppression of the RIPK3/MLKL necroptosis
signaling cascade (67). This
anti-inflammatory action preserves intestinal barrier homeostasis
and inhibits intestinal tissue carcinogenesis driven by persistent
inflammatory stimuli. Moreover, 50 or 100 mg/kg of luteolin has
been found to markedly attenuate DSS-induced murine colitis
symptoms compared with a DSS group, primarily by inhibiting JNK1/2,
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)
signaling, NF-κB and STAT3 pathways to exert anti-inflammatory,
anti-apoptotic and anti-autophagic effects for colonic homeostasis
restoration (68).
Vitamins
Defects in intestinal mucosal antioxidant defense
constitute the initiating factor in the pathogenesis of IBD, with
oxidative imbalance in the intestinal mucosa further associated
with disease activity and progression. Reduced plasma levels of
vitamins A, E and β-carotene, coupled with decreased antioxidant
enzyme activity in the intestinal mucosa, correlate with IBD
severity and may serve as indicators of disease activity (69). Notably, vitamin E (δ-tocotrienol),
a natural antioxidant, protects phagocytes and surrounding tissues
from oxidative assault by free radicals generated from neutrophils
and macrophages (70). This
compound inhibits the elevation of free radicals produced during
lipid and lipoprotein oxidative damage in IBD.
Polysaccharides
Polysaccharides, complex carbohydrate macromolecules
formed through condensation and dehydration of a number of
monosaccharide units, have recently attracted significant interest
in their anti-CRC effects. Tao et al (71) investigated the anti-tumor
activities of Dendrobium officinale polysaccharides,
Astragalus polysaccharides and Lentinus edodes
polysaccharides with varying molecular weights using a zebrafish
xenograft model. Compared with the model group (inhibition
0±5.09%), the results indicated that all three polysaccharides
inhibited the growth of HT29 cells in the xenograft model, with
Dendrobium officinale polysaccharides (250 μg/ml)
exhibiting the most significant inhibitory effect on CRC
(67.91±1.69%). Kyoto Encyclopedia of Genes and Genomes pathway
enrichment analysis suggested that their primary mechanisms may
involve immunomodulation and induction of apoptosis.
Astragalus polysaccharides, a major bioactive component in
Astragalus membranaceus, primarily consist of arabinose,
galactose, glucose, xylose and mannose (72). Compared with the AOM/DSS control
group, in vivo studies revealed that middle doses (200
mg/kg) of Astragalus polysaccharides effectively improved
CD8+ T cell function through modulation of the
STAT3/Gal-3/LAG3 pathway to inhibit CRC development (73). These findings suggest that
Astragalus polysaccharides may exert anti-CRC effects as a
novel strategy for future clinical development of natural
anti-tumor drugs.
Saponins
Ginsenoside Rg1, a bioactive component derived from
the traditional Chinese medicine Panax ginseng, exerts
therapeutic effects via modulation of various metabolic pathways of
the gut microbiota, primarily by enhancing tryptophan metabolite
levels to influence microbial tryptophan metabolism, thereby
alleviating intestinal inflammation (74). Polyphyllin, a natural steroidal
saponin derived from the traditional Chinese medicine Paris
polyphylla, encompasses compounds such as Polyphyllin I, II, VI
and VII, demonstrating significant anti-tumor, antimicrobial,
antioxidant, sedative, analgesic, hemostatic, immunomodulatory and
organ-protective effects, with particularly notable anti-tumor
activities (75). Li et al
(76) found that the percentages
of the apoptotic cells for HCT116 cells increased from 4.7-35.2% in
the control group and the Polyphyllin II treated group (4
μM) and for SW620 cells from 4.6-27.3% in the control group
and the Polyphyllin II treated group. In vivo study showed
Polyphyllin II (0.5 or 1 mg/kg, i.p. once every 3 days) suppressed
HCT116 tumor growth in nude mice. Further mechanism study revealed
that Polyphyllin II markedly induced G2/M-phase cell
cycle arrest and apoptosis and reduced the expression levels of
phosphorylated (p-)PI3K, p-Akt and p-mTOR in HCT116 and SW620
cells, promoting the expression of autophagy-related protein
LC3B-II. Further increases in LC3B-II expression were observed upon
treatment with the mTOR inhibitor rapamycin, indicating that
Polyphyllin II induces tumor cell autophagy by inhibiting the
PI3K/Akt/mTOR signaling pathway. Moreover, Polyphyllin II was found
to induce tumor cell apoptosis by suppressing the Janus kinase
2/signal transducer and activator of STAT3 signaling pathway,
exerting anti-CRC effects (76).
Alkaloids
Alkaloids are organic compounds containing one or
more basic nitrogen atoms arranged in cyclic structures. Berberine,
also known as berberrubine, is a quaternary ammonium isoquinoline
alkaloid isolated from the traditional Chinese medicine
Coptischinensis, demonstrating diverse biological activities
including anti-tumor, anti-oxidant, anti-inflammatory,
cholesterol-lowering, anti-diabetic, anti-obesity and
anti-microbial properties (77).
Its anti-cancer effects and mechanisms have been extensively
studied, establishing it as a potential anti-cancer drug candidate.
It has been reported that berberine alleviates AOM/DSS-induced
intestinal barrier damage in mice, thereby reducing microbial
invasion (78). In the AOM/DSS
mice model, berberine was daily administered with a dose of 50 and
100 mg/kg and aspirin was the positive control. Compared with the
AOM/DSS model group, berberine markedly reduced the number and load
of tumors in mice. Furthermore, berberine suppressed inflammation
and CRC development by increasing the abundance of short-chain
fatty acid-producing bacteria and decreasing pathogenic bacterial
populations (78). By reshaping
the gut microbiota composition, berberine increased the expression
of occludin and ZO-1, inhibited the activation of the
p-NF-κB/p-STAT3 pathway, consequently impeding colorectal
adenocarcinoma progression. Berberine's restorative mechanisms
against oxidative stress-induced DNA damage have been demonstrated
across murine models. In adenovirus-infected AOM/DSS mice
administered with 28 mg/kg berberine for 5 weeks, berberine
enhanced Dicer expression and reduced IL-6 expression, mitigating
intestinal injury (79).
Collectively, these findings indicate that berberine suppresses CRC
progression by reducing inflammation-related chemotactic factors,
enhancing antioxidant radical scavenging capacity and repairing DNA
damage.
Terpenoids
Terpenoids represent a class of compounds composed
of five-carbon isoprene units (C5H8),
categorized based on the number of isoprene units into
monoterpenes, sesquiterpenes, diterpenes, sesterterpenes,
triterpenes, tetraterpenes and polyterpenes (80). Zingiberene, a sesquiterpene
compound and the primary constituent of ginger essential oil,
exhibits anti-cancer, antioxidant, antibacterial, anti-inflammatory
and anti-angiogenic activities. In the aberrant crypt focus (ACF)
rat model induced with 1.2 Dimethylhydrazine (DMH) at a 20 mg/kg
dose, the experimental treatment group (DMH + Zingiberene at a 300
mg/kg concentration) showed decreased amount of AC in the distal
region compared with the positive induction control group (81). It also has shown that zingiberene
demonstrates specific activity against HT-29 cells with minimal
effects on non-cancerous cells. It promotes the formation of
autophagosomes in tumor cells, increasing LC3-II expression and
decreasing p62 expression to induce autophagy (82).
Quinones
There has been burgeoning research interest in the
potential of various plant-based quinone bioactive compounds for
CRC drug development due to their promising therapeutic efficacy.
Shikonin, a naphthoquinone component primarily sourced from the
traditional Chinese medicinal herb
Lithospermumerythrorhizon, activates ROS-mediated
endoplasmic reticulum stress to notably inhibit HCT116 cell
proliferation. In addition, 1.5 μM shikonin markedly
inhibited the HCT116 cell colonies compared with the control group.
In the HCT116 xenograft mouse model, a dose of 3 mg/kg (i.p.)
shikonin effectively inhibited tumor growth by 52.3% in
vivo. Moreover, shikonin downregulates Bcl-2 expression and
activates cleavage of caspase3/9 and PARP to induce apoptosis
(83). Emodin is a natural
anthraquinone compound with antioxidant, anti-inflammatory and
anti-tumor properties. In AOM/DSS-induced models, emodin (50 mg/kg)
reduced recruitment of inflammatory cells, expression of cytokines
and pro-inflammatory enzymes in the tumor microenvironment while
enhancing CD3+ T lymphocyte levels. In addition, emodin
decreased viability, migration and fibroblast-induced invasion
capacity of SW-620 and HCT116 cells in vitro (84).
Current challenges and progress in clinical
research
PBAs have shown promising therapeutic effects in CRC
cell lines and animal models. Several clinical trials investigating
the prevention and treatment of CRC with PBAs have been conducted
(Table I). Carroll et al
(85) assessed the effects of
oral curcumin (2 g/day or 4 g/day for 30 days) on PGE2 within ACF
in a nonrandomized, open-label clinical trial. Colonoscopy revealed
no significant ACF reduction in the 2 g/day group, whereas the 4
g/day group showed a 40% reduction. Cruz-Correa et al
(86) evaluated the regress
adenomas effects of curcumin (480 mg/day) and quercetin (20 mg/day)
in 5 post-colectomy familial adenomatous polyposis (FAP) patients.
Colonoscopy demonstrated a 60.4% reduction in polyp number and a
50.9% decrease in size compared with baseline. While this study
showed prominent therapeutic effects, a subsequent larger trial
involving 44 FAP patients found no significant differences in polyp
number or size (87). Panahi
et al (88) assessed the
effects of curcumin in 67 stage III CRC patients with chemotherapy
after the surgery. The results demonstrated that 8-week
curcuminoids capsules (500 mg daily) improved erythrocyte
sedimentation rate (ESR) and serum C-reactive protein (CRP) levels,
while also enhancing the quality of life in stage III CRC patients
Compared with the control group taking placebo capsules. The effect
of curcumin on the prognosis of CRC patients remains unclear.
Several completed clinical trials (NCT02439385, NCT01490996 and
NCT01948661) are expected to provide further elucidation upon
publication of their results.
 | Table IClinical researches of PBAs. |
Table I
Clinical researches of PBAs.
| First author/s,
year | Type of PBA | Type of study | Patients | Group | Outcomes | (Refs.) |
|---|
| Carroll et
al, 2011 | Curcumin | Open-label
trial | 44 eligible smokers
with 8 or more ACF on screening colonoscopy | 2 g/day or 4 g/day
for 30 days | No significant ACF
reduction in the 2 g/day group, 40% reduction in ACF number in the
4 g/day group | (85) |
| Cruz-Correa et
al, 2006 | Curcumin | Single-arm
trial | 5 post-colectomy
FAP patients | Curcumin (480
mg/day) and quercetin (20 mg/day) | 60.4% reduction in
polyp number and 50.9% decrease in size compared with baseline | (86) |
| Cruz-Correa et
al, 2018 | Curcumin | Randomized
controlled trial | 44 FAP patients who
had not undergone colectomy | Curcumin (1,500 mg
orally, twice per day) or identical-appearing placebo capsules for
12 months | No significant
difference in mean polyp size between the curcumin group and the
placebo group | (87) |
| Panahi et
al, 2021 | Curcumin | Randomized
controlled trial | 67 stage III CRC
patients with chemotherapy after the surgery | Treatment group
receiving curcuminoids capsules (500 mg/day) (n=36), or the control
group taking placebo capsules (n=36) for 8 weeks | A significant
change in CRP and ESR in treatment group. A significant improvement
in functional and global quality of life in treatment group | (88) |
| Patel et al,
2010 | Resveratrol | Single-arm
trial | 20 CRC patients
before surgical resection | 0.5 or 1g daily for
8 days | 5% reduction in
tumor cell proliferation | (89) |
| Seufferlein et
al, 2022 | EGCG | Randomized
controlled trial | 1,001 patients with
colon adenomas | EGCG (150 mg/day)
or placebo groups over 3 years | No significant
difference in adenoma rate between the EGCG group and the placebo
group | (90) |
| Sinicrope et
al, 2021 | EGCG | Randomized
controlled trial | 39 patients with at
least 5 rectal ACF | EGCG (780 mg/day)
or placebo groups over 6 months | No significant
differences in ACF number, total ACF burden and adenoma
recurrence | (91) |
| Bonelli et
al, 2013 | Vitamin | Randomized
controlled trial | 411
post-polypectomy patients | Active compound
(200 μg selenium, 30 mg zinc, 2 mg vitamin A, 180 mg vitamin
C, 30 mg vitamin E) or a placebo daily for 5 years | 39% reduction of
the risk of recurrence in the intervention group Compared with the
placebo group | (92) |
| Oliai Araghi et
al, 2019 | Vitamin | Randomized
controlled trial | 2,524 Participants
aged 65 years and over with an elevated homocysteine Level | Folic acid (400
μg/day) and vitamin B12 (500 μg/day) vs. placebo over
2 to 3 years | Vitamin B12 were
markedly associated with a higher risk of CRC | (93) |
| Greenberg et
al, 1994 | Vitamin | Randomized
controlled trial | 864 patients who
had at least one histologically confirmed adenoma removed from the
large bowel | Beta carotene (25
mg daily); vitamin C (1 g daily) and vitamin E (400 mg daily); or
the beta carotene plus vitamins C and E; placebo | There was no
evidence that either beta carotene or vitamins C and E reduced the
incidence of adenomas | (94) |
| Gaziano et
al, 2009 | Vitamin | Randomized
controlled trial | 14,641 male
physicians aged 50 years or older | 400 IU of vitamin E
every other day and 500 mg of vitamin C daily; placebo | Neither vitamin E
nor vitamin C had a significant effect on CRC incidence | (95) |
| Wang et al,
2012 | Vitamin | Randomized
controlled trial | 816 CRC patients
and 815 controls | Dietary intakes of
vitamin C and vitamin E were assessed by a PC-assisted interview
regarding 148 food items | Intake of vitamin C
and vitamin E were not related to CRC risk in either men or
women | (96) |
| Ng et al,
2019 | Vitamin | Randomized
controlled trial | 139 patients with
advanced or metastatic CRC | mFOLFOX6 plus
bevacizumab chemotherapy every 2 weeks and either high-dose vitamin
D3 (n=69) or standard-dose vitamin D3 (n=70) daily | No significant
improvement in progression-free survival between high-dose and
standard-dose groups | (97) |
| Chen et al,
2020 | Berberine | Randomized
controlled trial | 553 participants
who had colorectal adenomas that had undergone complete
polypectomy | Berberine (0.3 g
twice daily) or placebo | Lower adenoma
recurrence in the berberine group vs. the placebo group | (98) |
Patel et al (89) enrolled 20 CRC patients who
received 0.5 g or 1.0 g/day resveratrol for 8 days before surgery
intervention. Post-intervention tissue analysis revealed a 5%
reduction in tumor cell proliferation. Further clinical trials
(NCT00256334 and NCT00433576) have been conducted, with the results
eagerly expected.
Seufferlein et al (90) investigated EGCG's preventive
effects on CRC, with 1,001 colon adenoma patients randomized to
EGCG (150 mg/day) or placebo groups. At 3 years, colonoscopy showed
adenoma recurrence rates of 55.7% (placebo) vs. 51.1% (EGCG), with
no statistical significance. Sinicrope et al (91) also studied EGCG's effects on 39
patients with 35 rectal ACFs, with no significant
differences in ACF number, total ACF burden and adenoma recurrence
observed. Several clinical trials (NCT02891538 and NCT01360320)
investigating the preventive effects of EGCG have been completed,
though their results remain unpublished.
The clinical evidence regarding vitamin
supplementation for CRC prevention remains contradictory. Bonelli
et al (92) demonstrated
in a double-blind randomized trial that vitamins A, C and E could
markedly reduce intestinal adenoma recurrence in patients with
prior polypectomy. However, Oliai et al (93) reported that vitamin B12 could
potentially increase risk of CRC. A number of relevant clinical
trials have demonstrated no significant effects of vitamins on CRC
outcomes. In Greenberg et al's study (94) of 864 adenoma patients, vitamin C
and E supplementation showed no difference in adenoma incidence
compared with placebo groups. Gaziano et al's large-scale
trial (95) involving 14,641 male
physicians found no effect of vitamin E and C on CRC incidence.
Similarly, Wang et al (96) analyzed antioxidant vitamin C and E
intake in 816 CRC patients vs. 815 controls, showing no association
with cancer risk. Ng et al (97) evaluated vitamin D3 supplementation
in advanced/metastatic CRC, revealing no significant improvement in
progression-free survival (PFS) between high-dose and standard-dose
groups. Ongoing clinical trials (NCT02969681, NCT01574027,
NCT00905918 and NCT02603757) are currently underway, whose findings
are expected to further clarify the role of vitamins in CRC.
A multicenter, double-blind, randomized controlled
trial has demonstrated berberine's efficacy in CRC prevention. In
this study, Chen et al (98) enrolled 1,108 patients with
colorectal adenomas, which were randomly allocated to the berberine
group (n=553, 0.3 g twice daily) and placebo group (n=555).
Colonoscopy evaluation after a 2-year's follow-up revealed lower
adenoma recurrence in the berberine group (n=155, 36%) compared
with the placebo group (n=216, 47%). These results warrant
validation through ongoing clinical trials (NCT03281096,
NCT02226185 and NCT03333265).
Despite the potential anticancer effects
demonstrated in preclinical studies, PBAs have not shown clear
clinical benefits in CRC and their clinical translation faces a
number of challenges. Indeed, low bioavailability is a critical
limiting factor. For example, bioavailability of oral curcumin is
<1% (99), with resulting
concentrations in plasma much lower than the doses in vitro.
Second, the core roles of PBAs in CRC remain unclear. While current
research reveals a number of anti-tumor mechanisms (gene
expression, signaling pathways and epigenetics) of PBAs in CRC, the
heterogeneity across models (cell lines, animal species) and
differences between models and human obscure the core mechanisms.
Third, clinical trial design requires optimization. Dose selection
lacks standardization: Carroll et al (85) found efficacy with 4 g/day
curcumin, whereas a larger trial by Cruz-Correa et al
(87) showed no benefit at 480
mg/day, highlighting the need for pharmacokinetic-guided
individualized dosing. Moreover, the influence of patient
heterogeneity on the anticancer effects of PBAs warrants further
confirmation. For example, genetic backgrounds of FAP patients may
affect curcumin response (86,87).
Directions for future research
Improving bioavailability represents the central
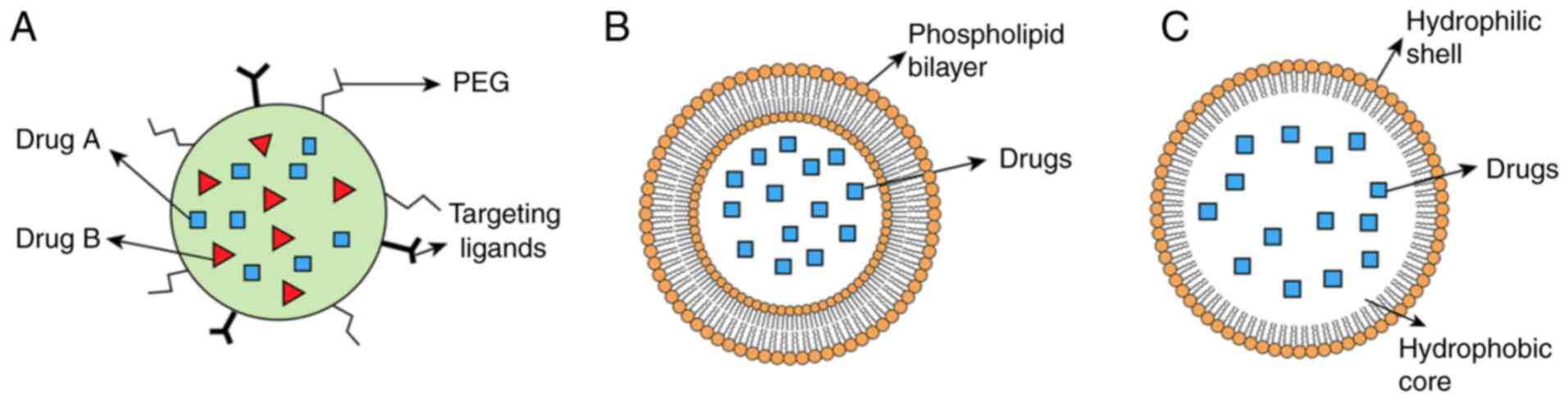
goal for clinical translation of PBAs. Nano-drug delivery system
has become a research focus in the context of PBAs given its
ability to improve drug solubility, prolong circulation time and
enhance tumor targeting (100,101). Currently, nanoparticles, nano
micelles and liposomes are relatively reliable nano-drug delivery
systems to improve the bioavailability of PBAs (Fig. 3).
Nanoparticles are generally classified into three
classes: Inorganic, organic and carbon-based. Various strategies
such as surface modification of nanoparticles with synthetic
polymer polyethylene glycol (PEG) can improve the water solubility
of nanoparticles (101). Zhang
et al (102) developed
erythrocyte membrane-coated resveratrol nanoparticles modified with
PCL-PEG, markedly prolonging half-life of resveratrol. Sun et
al (103) designed
PEG-modified amphiphilic cyclodextrin nanoparticles for co-delivery
of ginsenoside and quercetin, enhancing duration of drug in CRC
models. In addition, designing nanoparticles according to the
characteristics of PBAs represents an effective way to improve
bioavailability.
Nanomicelles, self-assembled amphiphilic polymer
structures, feature a hydrophilic shell to enhance pharmacokinetics
and a hydrophobic core to encapsulate the drug and control release
(104). Ran et al
(105) encapsulated ginsenoside
compound K in nanomicelles fabricated via ultrasonic self-assembly
of O-carboxymethyl chitosan, N-isopropylacrylamide and
thermoresponsive IR820. The nanomicelles markedly improved water
solubility and bioavailability of ginsenoside. Zhu et al
(106) incorporated silybin into
Soluplus-PVPVA nanomicelles, enhancing the pharmacokinetics of
silybin.
Liposomes are biocompatible and biodegradable
spherical vesicles with hydrophilic cores and lipid bilayers
(107). These structures serve
as effective carriers for the delivery of PBAs. Notably, curcumin
liposomes exhibit improved solubility and anticancer activity
against CRC cell lines (82,108), with clinical studies confirming
safety and tolerability in CRC patients (109).
Developing nano-drug delivery systems combining
PBAs with chemotherapeutics is another direction of further
research. Curcumin can reportedly reverse the resistance of CRC
cell lines to 5-FU, irinotecan and oxaliplatin through a number of
mechanisms (110,111). Quercetin can synergistically
enhance the killing effect of doxorubicin, 5-FU and oxaliplatin on
CRC cells (112-114). Rutin can alleviate
ensartinib-induced hepatotoxicity (115). Howells et al (116) conducted a study of 28 patients
with metastatic CRC receiving either FOLFOX alone or FOLFOX
combined with curcumin. The results showed that the curcumin-FOLFOX
combination exhibited favorable safety and superior clinical
outcomes compared with FOLFOX. Therefore, the combination of PBAs
and chemotherapeutic drugs may enhance the therapeutic effect of
chemotherapeutic drugs and appropriate nano-drug delivery systems
can further improve the utilization and targeting of drugs. Sen
et al (117) developed a
liposome containing both apigenin and 5-FU, which demonstrated
improved anti-tumor effects than 5-FU. Liu et al (118) designed a nanoparticle loaded
with irinotecan and quercetin with Conatumumab modified to target
CRC cells, with the nanoparticle yielding improved anti-tumor
effects without systemic toxicity.
Personalized treatment involving PBAs in CRC will
contribute to its clinical translation. First, the mechanism
underlying the efficacy of different PBAs in treating CRC needs to
be elucidated. Integrating single-cell sequencing or spatial
transcriptome technology will help to identify the core targets of
specific cell subsets (119).
The core targets can subsequently be harnessed to identify
populations that benefit from PBAs or those for whom they are
unsuitable. For instance, microsatellite-stable CRC cell lines are
more sensitive to curcumin (120), while EGCG may restore
TCF4-chromatin interactions and activate the Wnt pathway in
p53-mutant models, paradoxically promoting tumorigenesis (121). Thus, microsatellite-stable CRC
patients could be potential beneficiaries of curcumin, while EGCG
should be avoided in patients with p53 mutations. Future efforts
should focus on integrating molecular subtyping and biomarkers to
identify the potential population, thereby advancing the clinical
application of PBAs in CRC treatment.
Conclusion
CRC remains a formidable global health challenge,
with conventional therapies often limited by toxicity, drug
resistance and poor patient compliance. PBAs, characterized by
their multi-target, multi-pathway mechanisms, have emerged as
promising candidates for CRC prevention and treatment. Preclinical
studies highlight their ability to modulate oxidative stress,
inflammation, apoptosis, epigenetic dysregulation and gut
microbiota imbalance; key drivers of CRC pathogenesis. Compounds
such as curcumin, EGCG, resveratrol and berberine demonstrate
pleiotropic effects, including chemo-sensitization, immune
modulation and synergy with conventional therapies. Notably, these
natural agents mitigate chemotherapy-induced toxicity while
enhancing therapeutic efficacy, underscoring their potential as
adjunctive or alternative treatments.
However, clinical translation faces significant
hurdles and clinical efficacy from limited phase I/II trials. Low
bioavailability, inconsistent clinical outcomes and heterogeneous
patient responses remain critical barriers. In this regard, while
curcumin exhibits dose-dependent adenoma reduction in trials, its
poor absorption limits clinical utility. Similarly, EGCG and
vitamin supplementation trials revealed mixed results, emphasizing
the need for optimized trial designs, standardized dosing and
biomarker-driven patient stratification. Advances in nano-drug
delivery systems, such as nanoparticles, liposomes and
nanomicelles, offer promising solutions to enhance solubility,
stability and tumor targeting. Combinatorial strategies integrating
PBAs with chemotherapeutics (such as FOLFOX-curcumin) further
demonstrate improved safety and efficacy, warranting expanded
clinical exploration.
Future research should prioritize elucidating core
mechanisms through advanced technologies such as single-cell
sequencing and spatial transcriptomics, enabling precise
identification of molecular targets and responsive patient
subgroups. Personalized approaches, informed by CRC molecular
subtypes (such as microsatellite stability, p53 status), will
refine therapeutic applications. In addition, deeper investigations
into gut microbiota-PBAs crosstalk and host-microbe interactions
may unlock novel preventive and therapeutic avenues.
In conclusion, PBAs represent a versatile and
sustainable frontier in CRC management. While challenges persist,
interdisciplinary innovations in drug delivery, mechanism
elucidation and precision medicine are key to unlocking their full
clinical potential, ultimately bridging the gap between traditional
phytotherapy and modern oncology.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization, investigation and writing the
original draft was by DF, HF, KY, YW, BN and XL. DF, HF, KY, YW, BN
and XL were responsible for writing, review and editing. DF, HF,
KY, YW, BN and XL were responsible for visualization. DF, HF and XL
were responsible for supervision. HF, XL and DF were responsible
for project administration. Data authentication is not applicable.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82303765) and the Beien funding from
the Bethune Charitable Foundation (grant no. bnmr-2023-004).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Patel SG, Karlitz JJ, Yen T, Lieu CH and
Boland CR: The rising tide of early-onset colorectal cancer: A
comprehensive review of epidemiology, clinical features, biology,
risk factors, prevention, and early detection. Lancet Gastroenterol
Hepatol. 7:262–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen EX, Jonker DJ, Loree JM, Kennecke HF,
Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, et al:
Effect of combined immune checkpoint inhibition vs best supportive
care alone in patients with advanced colorectal cancer: The
Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 6:831–838.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hudita A, Radu IC, Galateanu B, Ginghina
O, Herman H, Balta C, Rosu M, Zaharia C, Costache M, Tanasa E, et
al: Bioinspired silk fibroin nano-delivery systems protect against
5-FU induced gastrointestinal mucositis in a mouse model and
display antitumor effects on HT-29 colorectal cancer cells in
vitro. Nanotoxicology. 15:973–994. 2021.PubMed/NCBI
|
|
5
|
Georgiou A, Stewart A, Vlachogiannis G,
Pickard L, Valeri N, Cunningham D, Whittaker SR and Banerji U: A
phospho-proteomic study of cetuximab resistance in
KRAS/NRAS/BRAF(V600) wild-type colorectal cancer. Cell Oncol
(Dordr). 44:1197–1206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Q, Geng S, Luo H, Wang W, Mo YQ, Luo Q,
Wang L, Song GB, Sheng JP and Xu B: Signaling pathways involved in
colorectal cancer: Pathogenesis and targeted therapy. Signal
Transduct Target Ther. 9:2662024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Liu M, Jafari M and Tang J: A
critical assessment of Traditional Chinese Medicine databases as a
source for drug discovery. Front Pharmacol. 15:13036932024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sies H, Mailloux RJ and Jakob U:
Fundamentals of redox regulation in biology. Nat Rev Mol Cell Biol.
25:701–719. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porter RJ, Arends MJ, Churchhouse AMD and
Din S: Inflammatory bowel disease-associated colorectal cancer:
Translational risks from mechanisms to medicines. J Crohns Colitis.
15:2131–2141. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu
W and Zheng Q: Natural products as anticancer agents: Current
status and future perspectives. Molecules. 27:83672022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Yue GGL, Leung PC, Wong CK and Lau
CBS: A review on the molecular mechanisms, the therapeutic
treatment including the potential of herbs and natural products,
and target prediction of obesity-associated colorectal cancer.
Pharmacol Res. 175:1060312022. View Article : Google Scholar
|
|
12
|
Bu F, Tu Y, Wan Z and Tu S: Herbal
medicine and its impact on the gut microbiota in colorectal cancer.
Front Cell Infect Microbiol. 13:10960082023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding Y and Yu Y: Therapeutic potential of
flavonoids in gastrointestinal cancer: Focus on signaling pathways
and improvement strategies (Review). Mol Med Rep. 31:1092025.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janney A, Powrie F and Mann EH:
Host-microbiota maladaptation in colorectal cancer. Nature.
585:509–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zińczuk J, Maciejczyk M, Zaręba K,
Pryczynicz A, Dymicka-Piekarska V, Kamińska J, Koper-Lenkiewicz O,
Matowicka-Karna J, Kędra B, Zalewska A and Guzińska-Ustymowicz K:
Pro-Oxidant enzymes, redox balance and oxidative damage to
proteins, lipids and DNA in colorectal cancer tissue. is oxidative
stress dependent on tumour budding and inflammatory infiltration?
Cancers (Basel). 12:16362020. View Article : Google Scholar
|
|
16
|
Monticelli S and Cejka P: DNA sensing and
repair systems unexpectedly team up against cancer. Nature.
625:457–458. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajeigbe OF, Maruf OR, Anyebe DA, Opafunso
IT, Ajayi BO and Farombi EO: 6-shogaol suppresses AOM/DSS-mediated
colorectal adenoma through its antioxidant and anti-inflammatory
effects in mice. J Food Biochem. 46:e144222022. View Article : Google Scholar
|
|
18
|
Liu C, Rokavec M, Huang Z and Hermeking H:
Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress
colorectal cancer metastasis. Cell Death Differ. 30:1771–1785.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun R, Zhang Y, Zhao X, Tang T, Cao Y,
Yang L, Tian Y, Zhang Z, Zhang P and Xu F: Temporal and spatial
metabolic shifts revealing the transition from ulcerative colitis
to colitis-associated colorectal cancer. Adv Sci (Weinh).
12:e24125512025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bardelčíková A, Šoltys J and Mojžiš J:
Oxidative stress, inflammation and colorectal cancer: An overview.
Antioxidants (Basel). 12:9012023. View Article : Google Scholar
|
|
21
|
Mandal M, Mamun MAA, Rakib A, Kumar S,
Park F, Hwang DJ, Li W, Miller DD and Singh UP: Modulation of
occludin, NF-κB, p-STAT3, and Th17 response by DJ-X-025 decreases
inflammation and ameliorates experimental colitis. Biomed
Pharmacother. 185:1179392025. View Article : Google Scholar
|
|
22
|
Li Q, Chen Y, Zhang D, Grossman J, Li L,
Khurana N, Jiang H, Grierson PM, Herndon J, DeNardo DG, et al:
IRAK4 mediates colitis-induced tumorigenesis and chemoresistance in
colorectal cancer. JCI Insight. 4:e1308672019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M, Ma Y, Yu G, Zeng B, Yang W, Huang
C, Dong Y, Tang B and Wu Z: Integration of microbiome, metabolomics
and transcriptome for in-depth understanding of berberine
attenuates AOM/DSS-induced colitis-associated colorectal cancer.
Biomed Pharmacother. 179:1172922024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burgueño JF, Fritsch J, González EE,
Landau KS, Santander AM, Fernández I, Hazime H, Davies JM,
Santaolalla R, Phillips MC, et al: Epithelial TLR4 Signaling
Activates DUOX2 to induce microbiota-driven tumorigenesis.
Gastroenterology. 160:797–808.e6. 2021. View Article : Google Scholar
|
|
25
|
Jin BR, Chung KS, Hwang S, Hwang SN, Rhee
KJ, Lee M and An HJ: Rosmarinic acid represses colitis-associated
colon cancer: A pivotal involvement of the TLR4-mediated
NF-κB-STAT3 axis. Neoplasia. 23:561–573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernández-Rocha C, Turpin W, Borowski K,
Stempak JM, Sabic K, Gettler K, Tastad C, Chasteau C, Korie U,
Hanna M, et al: After surgically induced remission, Ileal and
colonic mucosa-associated microbiota predicts crohn's disease
recurrence. Clin Gastroenterol Hepatol. 23:612–620.e10. 2025.
View Article : Google Scholar
|
|
27
|
Yun X, Zhang Q, Fang Y, Lv C, Chen Q, Chu
Y, Zhu Y, Wei Z, Xia Y and Dai Y: Madecassic acid alleviates
colitis-associated colorectal cancer by blocking the recruitment of
myeloid-derived suppressor cells via the inhibition of IL-17
expression in γδT17 cells. Biochem Pharmacol. 202:1151382022.
View Article : Google Scholar
|
|
28
|
Kuo IM, Lee JJ, Wang YS, Chiang HC, Huang
CC, Hsieh PJ, Han W, Ke CH, Liao ATC and Lin CS: Potential
enhancement of host immunity and anti-tumor efficacy of nanoscale
curcumin and resveratrol in colorectal cancers by modulated
electro-hyperthermia. BMC Cancer. 20:6032020. View Article : Google Scholar
|
|
29
|
Pouliquen DL, Malloci M, Boissard A, Henry
C and Guette C: Proteomes of residual tumors in curcumin-treated
rats reveal changes in microenvironment/malignant cell crosstalk in
a highly invasive model of mesothelioma. Int J Mol Sci.
23:137322022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montalto FI and De Amicis F: Cyclin D1 in
cancer: A molecular connection for cell cycle control, adhesion and
invasion in tumor and stroma. Cells. 9:26482020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alam M, Gulzar M, Akhtar MS, Rashid S,
Zulfareen Tanuja, Shamsi A and Hassan MI:
Epigallocatechin-3-gallate therapeutic potential in human diseases:
Molecular mechanisms and clinical studies. Mol Biomed. 5:732024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ismail NI, Othman I, Abas F, H Lajis N and
Naidu R: The curcumin analogue, MS13
(1,5-Bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadiene-3-one), inhibits
cell proliferation and induces apoptosis in primary and metastatic
human colon cancer cells. Molecules. 25:37982020. View Article : Google Scholar
|
|
33
|
Agarwal A, Kansal V, Farooqi H, Prasad R
and Singh VK: Epigallocatechin gallate (EGCG), an active phenolic
compound of green tea, inhibits tumor growth of head and neck
cancer cells by targeting DNA hypermethylation. Biomedicines.
11:7892023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian M, Peng S, Wang S, Li X, Li H and
Shen L: Pristimerin reduces dextran sulfate sodium-induced colitis
in mice by inhibiting microRNA-155. Int Immunopharmacol.
94:1074912021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu H, Zhang L, Hao L and Fan D:
Resveratrol inhibits colorectal cancer cell tumor property by
activating the miR-769-5p/MSI1 pathway. Mol Biotechnol.
67:1893–1907. 2025. View Article : Google Scholar
|
|
36
|
Wang Z, Dan W, Zhang N, Fang J and Yang Y:
Colorectal cancer and gut microbiota studies in China. Gut
Microbes. 15:22363642023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chattopadhyay I, Dhar R, Pethusamy K,
Seethy A, Srivastava T, Sah R, Sharma J and Karmakar S: Exploring
the role of gut microbiome in colon cancer. Appl Biochem
Biotechnol. 193:1780–1799. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu P, Wang Y, Yang G, Zhang Q, Meng L,
Xin Y and Jiang X: The role of short-chain fatty acids in
intestinal barrier function, inflammation, oxidative stress, and
colonic carcinogenesis. Pharmacol Res. 165:1054202021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su ACY, Ding X, Lau HCH, Kang X, Li Q,
Wang X, Liu Y, Jiang L, Lu Y, Liu W, et al: Lactococcus lactis
HkyuLL 10 suppresses colorectal tumourigenesis and restores gut
microbiota through its generated alpha-mannosidase. Gut.
73:1478–1488. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng W, Xiong X, Lu M, Huang S, Luo Y,
Wang Y and Ying Y: Curcumin suppresses colorectal tumorigenesis
through restoring the gut microbiota and metabolites. BMC Cancer.
24:11412024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leong W, Huang G, Liao W, Xia W, Li X, Su
Z, Liu L, Wu Q, Wong VKW, Law BYK, et al: Traditional Patchouli
essential oil modulates the host's immune responses and gut
microbiota and exhibits potent anti-cancer effects in Apc(Min/+)
mice. Pharmacol Res. 176:1060822022. View Article : Google Scholar
|
|
42
|
Wu Z, Huang S, Li T, Li N, Han D, Zhang B,
Xu ZZ, Zhang S, Pang J, Wang S, et al: Gut microbiota from green
tea polyphenol-dosed mice improves intestinal epithelial
homeostasis and ameliorates experimental colitis. Microbiome.
9:1842021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Urošević M, Nikolić L, Gajić I, Nikolić V,
Dinić A and Miljković V: Curcumin: biological activities and modern
pharmaceutical forms. Antibiotics (Basel). 11:1352022. View Article : Google Scholar
|
|
44
|
Weng W and Goel A: Curcumin and colorectal
cancer: An update and current perspective on this natural medicine.
Semin Cancer Biol. 80:73–86. 2022. View Article : Google Scholar
|
|
45
|
López-Gómez L and Uranga JA: Polyphenols
in the prevention and treatment of colorectal cancer: A systematic
review of clinical evidence. Nutrients. 16:27352024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Neira M, Mena C, Torres K and Simón L: The
potential benefits of curcumin-enriched diets for adults with
colorectal cancer: A systematic review. Antioxidants (Basel).
14:3882025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khan S, Karmokar A, Howells L, Britton RG,
Parrott E, Palacios-Gallego R, Tufarelli C, Cai H, Higgins J,
Sylvius N, et al: An old spice with new tricks: Curcumin targets
adenoma and colorectal cancer stem-like cells associated with poor
survival outcomes. Cancer Lett. 629:2178852025. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ming T, Lei J, Peng Y, Wang M, Liang Y,
Tang S, Tao Q, Wang M, Tang X, He Z, et al: Curcumin suppresses
colorectal cancer by induction of ferroptosis via regulation of p53
and solute carrier family 7 member 11/glutathione/glutathione
peroxidase 4 signaling axis. Phytother Res. 38:3954–3972. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chuang HY, Chan HW and Shih KC:
Suppression of colorectal cancer growth: Interplay between curcumin
and metformin through DMT1 downregulation and ROS-mediated
pathways. Biofactors. 51:e21372025. View Article : Google Scholar
|
|
50
|
Li D, Cao D, Cui Y, Sun Y, Jiang J and Cao
X: The potential of epigallocatechin gallate in the chemoprevention
and therapy of hepatocellular carcinoma. Front Pharmacol.
14:12010852023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu W and Oteiza PI: NADPH oxidase 1: A
target in the capacity of dimeric ECG and EGCG procyanidins to
inhibit colorectal cancer cell invasion. Redox Biol. 65:1028272023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong J, Zheng Z, Zhou M, Wang Y, Chen J,
Cen J, Cao T, Yang T, Xu Y, Shu G, et al: EGCG-LYS Fibrils-Mediated
CircMAP2K2 silencing decreases the proliferation and metastasis
ability of gastric cancer cells in vitro and in vivo. Adv Sci
(Weinh). 10:e23040752023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guan Y, Wu Q, Li M, Chen D, Su J, Zuo L,
Zhu B and Li Y: Epigallocatechin-3-gallate Induced HepG2 Cells
Apoptosis through ROSmediated AKT/JNK and p53 signaling pathway.
Curr Cancer Drug Targets. 23:447–460. 2023. View Article : Google Scholar
|
|
54
|
Suetsugu F, Tadokoro T, Fujita K, Fujihara
S, Sasaki K, Omayu E, Nakatani K, Koyama Y, Kozuka K, Matsui T, et
al: Antitumor effects of epigallocatechin-3-gallate on colorectal
cancer: An in vitro and in vivo study. Anticancer Res.
45:2937–2947. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Z, Wang M, Huang J, Lin M and Wei P:
Dichotomic role of low-concentration EGCG in the oxaliplatin
sensitivity of colorectal cancer cells. Dokl Biochem Biophys.
515:29–35. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi J, Ye Z, Xu H, Zhang H, Cao H, Li X,
Wang T, Dong C, Du Y, Dong S and Zhou W: EGCG targeting STAT3
transcriptionally represses PLXNC1 to inhibit M2 polarization
mediated by gastric cancer cell-derived exosomal miR-92b-5p.
Phytomedicine. 135:1561372024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu SX, Xiong RG, Huang SY, Zhou DD,
Saimaiti A, Zhao CN, Shang A, Zhang YJ, Gan RY and Li HB: Effects
and mechanisms of resveratrol for prevention and management of
cancers: An updated review. Crit Rev Food Sci Nutr. 63:12422–12440.
2023. View Article : Google Scholar
|
|
58
|
Brockmueller A, Shayan P and Shakibaei M:
Evidence that β1-integrin is required for the anti-viability and
anti-proliferative effect of resveratrol in CRC cells. Int J Mol
Sci. 23:47142022. View Article : Google Scholar
|
|
59
|
Brockmueller A, Buhrmann C, Shayan P and
Shakibaei M: Resveratrol induces apoptosis by modulating the
reciprocal crosstalk between p53 and Sirt-1 in the CRC tumor
microenvironment. Front Immunol. 14:12255302023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dariya B, Girish BP, Merchant N, Srilatha
M and Nagaraju GP: Resveratrol: Biology, metabolism, and
detrimental role on the tumor microenvironment of colorectal
cancer. Nutr Rev. 82:1420–1436. 2024. View Article : Google Scholar
|
|
61
|
Chen S, Tamaki N, Kudo Y, Tsunematsu T,
Miki K, Ishimaru N and Ito HO: Protective effects of resveratrol
against 5-fluorouracil-induced oxidative stress and inflammatory
responses in human keratinocytes. J Clin Biochem Nutr. 69:238–246.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Choi CY, Lim SC, Lee TB and Han SI:
Molecular basis of resveratrol-induced resensitization of acquired
drug-resistant cancer cells. Nutrients. 14:6992022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Recalde MD, Miguel CA, Noya-Riobó MV,
González SL, Villar MJ and Coronel MF: Resveratrol exerts
anti-oxidant and anti-inflammatory actions and prevents
oxaliplatin-induced mechanical and thermal allodynia. Brain Res.
1748:1470792020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Motawea MH, Abd Elmaksoud HA, Elharrif MG,
Desoky AAE and Ibrahimi A: Evaluation of anti-inflammatory and
antioxidant profile of oleuropein in experimentally induced
ulcerative colitis. Int J Mol Cell Med. 9:224–233. 2020.PubMed/NCBI
|
|
65
|
Sokal-Dembowska A, Jarmakiewicz-Czaja S
and Filip R: Flavonoids and their role in preventing the
development and progression of MAFLD by modifying the microbiota.
Int J Mol Sci. 25:111872024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang B, Bai H, Sa Y, Zhu P and Liu P:
Inhibiting EMT, stemness and cell cycle involved in
baicalin-induced growth inhibition and apoptosis in colorectal
cancer cells. J Cancer. 11:2303–2317. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang J, Lei H, Hu X and Dong W:
Hesperetin ameliorates DSS-induced colitis by maintaining the
epithelial barrier via blocking RIPK3/MLKL necroptosis signaling.
Eur J Pharmacol. 873:1729922020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vukelić I, Detel D, Batičić L, Potočnjak I
and Domitrović R: Luteolin ameliorates experimental colitis in mice
through ERK-mediated suppression of inflammation, apoptosis and
autophagy. Food Chem Toxicol. 145:1116802020. View Article : Google Scholar
|
|
69
|
Panda SK, Peng V, Sudan R, Ulezko Antonova
A, Di Luccia B, Ohara TE, Fachi JL, Grajales-Reyes GE, Jaeger N,
Trsan T, et al: Repression of the aryl-hydrocarbon receptor
prevents oxidative stress and ferroptosis of intestinal
intraepithelial lymphocytes. Immunity. 56:797–812.e4. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu Q, Luo Y, Lu H, Xie T, Hu Z, Chu Z and
Luo F: The potential role of vitamin E and the mechanism in the
prevention and treatment of inflammatory bowel disease. Foods.
13:8982024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tao S, Ren Z, Yang Z, Duan S, Wan Z, Huang
J, Liu C and Wei G: Effects of different molecular weight
polysaccharides from dendrobium officinale kimura & migo on
human colorectal cancer and transcriptome analysis of
differentially expressed genes. Front Pharmacol. 12:7044862021.
View Article : Google Scholar
|
|
72
|
Cao Q, Zhou R, Guo S, Meng K, Yang X, Liu
M, Ma B, Su C and Duan X: PLGA-Astragalus polysaccharide
nanovaccines exert therapeutic effect in colorectal cancer. Int J
Nanomedicine. 19:9437–9458. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li Q, Zhang C, Xu G, Shang X, Nan X, Li Y,
Liu J, Hong Y, Wang Q and Peng G: Astragalus polysaccharide
ameliorates CD8(+) T cell dysfunction through STAT3/Gal-3/LAG3
pathway in inflammation-induced colorectal cancer. Biomed
Pharmacother. 171:1161722024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cheng H, Liu J, Zhang D, Wang J, Tan Y,
Feng W and Peng C: Ginsenoside Rg1 alleviates acute ulcerative
colitis by modulating gut microbiota and microbial tryptophan
metabolism. Front Immunol. 13:8176002022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang R, Gao W, Wang Z, Jian H, Peng L, Yu
X, Xue P, Peng W, Li K and Zeng P: Polyphyllin I induced
ferroptosis to suppress the progression of hepatocellular carcinoma
through activation of the mitochondrial dysfunction via
Nrf2/HO-1/GPX4 axis. Phytomedicine. 122:1551352024. View Article : Google Scholar
|
|
76
|
Li JK, Sun HT, Jiang XL, Chen YF, Zhang Z,
Wang Y, Chen WQ, Zhang Z, Sze SCW, Zhu PL and Yung KKL: Polyphyllin
II induces protective autophagy and apoptosis via Inhibiting
PI3K/AKT/mTOR and STAT3 signaling in colorectal cancer cells. Int J
Mol Sci. 23:118902022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Z, Wang Y, Xu Q, Ma J, Li X, Yan J,
Tian Y, Wen Y and Chen T: Berberine and health outcomes: An
umbrella review. Phytother Res. 37:2051–2066. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yan S, Chang J, Hao X, Liu J, Tan X, Geng
Z and Wang Z: Berberine regulates short-chain fatty acid metabolism
and alleviates the colitis-associated colorectal tumorigenesis
through remodeling intestinal flora. Phytomedicine. 102:1542172022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu X, Chen X, Liu H, He ZW, Wang Z, Wei
LJ, Wang WY, Zhong S, He Q, Zhang Z, et al: Rescuing Dicer
expression in inflamed colon tissues alleviates colitis and
prevents colitis-associated tumorigenesis. Theranostics.
10:5749–5762. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Q, Zhao X, Jiang Y, Jin B and Wang L:
Functions of representative terpenoids and their biosynthesis
mechanisms in medicinal plants. Biomolecules. 13:17252023.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lima DAN, Pelegrini BB, Uechi FAA, Varago
RC, Pimenta BB, Kaneshima AMS, Kaneshima EN, Souza PDC, Pedroso RB,
Silveira TGV and Becker TCA: Evaluation of antineoplasic activity
of zingiber officinale essential oil in the colorectal region of
wistar rats. Asian Pac J Cancer Prev. 21:2141–2147. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen H, Tang X, Liu T, Jing L and Wu J:
Zingiberene inhibits in vitro and in vivo human colon cancer cell
growth via autophagy induction, suppression of PI3K/AKT/mTOR
Pathway and caspase 2 deactivation. J Buon. 24:1470–1475.
2019.PubMed/NCBI
|
|
83
|
Qi H, Zhang X, Liu H, Han M, Tang X, Qu S,
Wang X and Yang Y: Shikonin induced apoptosis mediated by
endoplasmic reticulum stress in colorectal cancer cells. J Cancer.
13:243–252. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang Y, Pu W, Bousquenaud M, Cattin S,
Zaric J, Sun LK and Rüegg C: Emodin inhibits inflammation,
carcinogenesis, and cancer progression in the AOM/DSS model of
colitis-associated intestinal tumorigenesis. Front Oncol.
10:5646742021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Carroll RE, Benya RV, Turgeon DK, Vareed
S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C,
Meyskens FL Jr and Brenner DE: Phase IIa clinical trial of curcumin
for the prevention of colorectal neoplasia. Cancer Prev Res
(Phila). 4:354–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cruz-Correa M, Shoskes DA, Sanchez P, Zhao
R, Hylind LM, Wexner SD and Giardiello FM: Combination treatment
with curcumin and quercetin of adenomas in familial adenomatous
polyposis. Clin Gastroenterol Hepatol. 4:1035–1038. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cruz-Correa M, Hylind LM, Marrero JH,
Zahurak ML, Murray-Stewart T, Casero RA Jr, Montgomery EA,
Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, et al: Efficacy and
safety of curcumin in treatment of intestinal adenomas in patients
with familial adenomatous polyposis. Gastroenterology. 155:668–673.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Panahi Y, Saberi-Karimian M, Valizadeh O,
Behnam B, Saadat A, Jamialahmadi T, Majeed M and Sahebkar A:
Effects of curcuminoids on systemic inflammation and quality of
life in patients with colorectal cancer undergoing chemotherapy: A
randomized controlled trial. Adv Exp Med Biol. 1328:1–9. 2021.
View Article : Google Scholar
|
|
89
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Seufferlein T, Ettrich TJ, Menzler S,
Messmann H, Kleber G, Zipprich A, Frank-Gleich S, Algül H, Metter
K, Odemar F, et al: Green tea extract to prevent colorectal
adenomas, results of a randomized, placebo-controlled clinical
trial. Am J Gastroenterol. 117:884–894. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sinicrope FA, Viggiano TR, Buttar NS, Song
LMWK, Schroeder KW, Kraichely RE, Larson MV, Sedlack RE, Kisiel JB,
Gostout CJ, et al: Randomized phase II trial of polyphenon e versus
placebo in patients at high risk of recurrent colonic neoplasia.
Cancer Prev Res (Phila). 14:573–580. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bonelli L, Puntoni M, Gatteschi B, Massa
P, Missale G, Munizzi F, Turbino L, Villanacci V, De Censi A and
Bruzzi P: Antioxidant supplement and long-term reduction of
recurrent adenomas of the large bowel. A double-blind randomized
trial. J Gastroenterol. 48:698–705. 2013. View Article : Google Scholar
|
|
93
|
Oliai Araghi S, Kiefte-de Jong JC, van
Dijk SC, Swart KMA, van Laarhoven HW, van Schoor NM, de Groot
LCPGM, Lemmens V, Stricker BH, Uitterlinden AG and van der Velde N:
Folic acid and vitamin B12 supplementation and the risk of cancer:
Long-term Follow-up of the B vitamins for the prevention of
osteoporotic fractures (B-PROOF) trial. Cancer Epidemiol Biomarkers
Prev. 28:275–282. 2019. View Article : Google Scholar
|
|
94
|
Greenberg ER, Baron JA, Tosteson TD,
Freeman DH Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl
HD, Haile RW, et al: A clinical trial of antioxidant vitamins to
prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J
Med. 331:141–147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gaziano JM, Glynn RJ, Christen WG, Kurth
T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD and Buring
JE: Vitamins E and C in the prevention of prostate and total cancer
in men: The physicians' health study II randomized controlled
trial. JAMA. 301:52–62. 2009. View Article : Google Scholar
|
|
96
|
Wang Z, Joshi AM, Ohnaka K, Morita M,
Toyomura K, Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, et al:
Dietary intakes of retinol, carotenes, vitamin C, and vitamin E and
colorectal cancer risk: The Fukuoka colorectal cancer study. Nutr
Cancer. 64:798–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ng K, Nimeiri HS, McCleary NJ, Abrams TA,
Yurgelun MB, Cleary JM, Rubinson DA, Schrag D, Miksad R, Bullock
AJ, et al: Effect of high-dose vs standard-dose vitamin D3
supplementation on progression-free survival among patients with
advanced or metastatic colorectal cancer: The SUNSHINE randomized
clinical trial. JAMA. 321:1370–1379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen YX, Gao QY, Zou TH, Wang BM, Liu SD,
Sheng JQ, Ren JL, Zou XP, Liu ZJ, Song YY, et al: Berberine versus
placebo for the prevention of recurrence of colorectal adenoma: A
multicentre, double-blinded, randomised controlled study. Lancet
Gastroenterol Hepatol. 5:267–275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mao Q, Min J, Zeng R, Liu H, Li H, Zhang
C, Zheng A, Lin J, Liu X and Wu M: Self-assembled traditional
Chinese nanomedicine modulating tumor immunosuppressive
microenvironment for colorectal cancer immunotherapy. Theranostics.
12:6088–6105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ali ES, Sharker SM, Islam MT, Khan IN,
Shaw S, Rahman MA, Uddin SJ, Shill MC, Rehman S, Das N, et al:
Targeting cancer cells with nanotherapeutics and nanodiagnostics:
Current status and future perspectives. Semin Cancer Biol.
69:52–68. 2021. View Article : Google Scholar
|
|
101
|
Cheng Z, Li M, Dey R and Chen Y:
Nanomaterials for cancer therapy: Current progress and
perspectives. J Hematol Oncol. 14:852021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang Z, Ji Y, Hu N, Yu Q, Zhang X, Li J,
Wu F, Xu H, Tang Q and Li X: Ferroptosis-induced anticancer effect
of resveratrol with a biomimetic nano-delivery system in colorectal
cancer treatment. Asian J Pharm Sci. 17:751–766. 2022.PubMed/NCBI
|
|
103
|
Sun D, Zou Y, Song L, Han S, Yang H, Chu
D, Dai Y, Ma J, O'Driscoll CM, Yu Z and Guo J: A cyclodextrin-based
nanoformulation achieves co-delivery of ginsenoside Rg3 and
quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm
Sin B. 12:378–393. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tawfik SM, Azizov S, Elmasry MR, Sharipov
M and Lee YI: Recent advances in nanomicelles delivery systems.
Nanomaterials (Basel). 11:702020. View Article : Google Scholar
|
|
105
|
Ran P, Wang W, An Z, Gao N, He Y and Wu Z:
Preparation and in vitro biological studies of photothermal
response ginsenoside CK-carboxymethyl chitosan-based prodrug
nanomicelles for synergistic therapy. Int J Biol Macromol.
316:1447472025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhu C, Gong S, Ding J, Yu M, Ahmad E, Feng
Y and Gan Y: Supersaturated polymeric micelles for oral silybin
delivery: The role of the Soluplus-PVPVA complex. Acta Pharm Sin B.
9:107–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li M, Du C, Guo N, Teng Y, Meng X, Sun H,
Li S, Yu P and Galons H: Composition design and medical application
of liposomes. Eur J Med Chem. 164:640–653. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rahman S, Cao S, Steadman KJ, Wei M and
Parekh HS: Native and β-cyclodextrin-enclosed curcumin: Entrapment
within liposomes and their in vitro cytotoxicity in lung and colon
cancer. Drug Deliv. 19:346–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Y, Li Z, Huang Y, Xu Y and Zou B:
Nanotechnology and curcumin: A novel and promising approach in
digestive cancer therapy. Nanomedicine (Lond). 18:2081–2099. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bhattacharjya D and Sivalingam N:
Mechanism of 5-fluorouracil induced resistance and role of piperine
and curcumin as chemo-sensitizers in colon cancer. Naunyn
Schmiedebergs Arch Pharmacol. 397:8445–8475. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Brockmueller A, Samuel SM, Mazurakova A,
Busselberg D, Kubatka P and Shakibaei M: Curcumin, calebin A and
chemosensitization: How are they linked to colorectal cancer? Life
Sci. 318:1215042023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Suman I, Jezidzic A, Dobric D and
Domitrovic R: Differential effects of rutin and its aglycone
quercetin on cytotoxicity and chemosensitization of HCT 116 colon
cancer cells to anticancer drugs 5-fluorouracil and doxorubicin.
Biology (Basel). 14:5272025.PubMed/NCBI
|
|
113
|
Lee J, Jang CH, Kim Y, Oh J and Kim JS:
Quercetin-induced glutathione depletion sensitizes colorectal
cancer cells to oxaliplatin. Foods. 12:17332023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Deng H, Wei F, Han W, Li Y, Xu X, Zhang L
and Zhang Y: Synergistic chemotherapy and immunomodulatory effects
of Quercetin in cancer: A review. Front Immunol. 16:15479922025.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wu W, Li J, Yin Y, Zhou Y, Huang X, Cao Y,
Chen X, Zhou Y, Du J, Xu Z, et al: Rutin attenuates
ensartinib-induced hepatotoxicity by non-transcriptional regulation
of TXNIP. Cell Biol Toxicol. 40:382024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Howells LM, Iwuji COO, Irving GRB, Barber
S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel
SR, et al: Curcumin combined with FOLFOX chemotherapy is safe and
tolerable in patients with metastatic colorectal cancer in a
randomized phase IIa trial. J Nutr. 149:1133–1139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sen K, Banerjee S and Mandal M: Dual drug
loaded liposome bearing apigenin and 5-Fluorouracil for synergistic
therapeutic efficacy in colorectal cancer. Colloids Surf B
Biointerfaces. 180:9–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu Y, Zhang H, Cui H, Zhang F, Zhao L,
Liu Y and Meng Q: Combined and targeted drugs delivery system for
colorectal cancer treatment: Conatumumab decorated, reactive oxygen
species sensitive irinotecan prodrug and quercetin co-loaded
nanostructured lipid carriers. Drug Deliv. 29:342–350. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Vandereyken K, Sifrim A, Thienpont B and
Voet T: Methods and applications for single-cell and spatial
multi-omics. Nat Rev Genet. 24:494–515. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lu L, Przybylla R, Shang Y, Dai M, Krohn
M, Krämer OH, Mullins CS and Linnebacher M: Microsatellite Status
and IĸBα expression levels predict sensitivity to pharmaceutical
curcumin in colorectal cancer cells. Cancers (Basel). 14:10322022.
View Article : Google Scholar
|
|
121
|
Kadosh E, Snir-Alkalay I, Venkatachalam A,
May S, Lasry A, Elyada E, Zinger A, Shaham M, Vaalani G, Mernberger
M, et al: The gut microbiome switches mutant p53 from
tumour-suppressive to oncogenic. Nature. 586:133–138. 2020.
View Article : Google Scholar : PubMed/NCBI
|