Traditionally, cancer is considered to originate
from genomic instability, whereas recent research has indicated
that epigenetic changes alone are sufficient to cause cancer
(1,2). The main forms of cancer therapy
available at present are surgery, radiation therapy, chemotherapy,
targeted therapy and immunotherapy. Tumor immunotherapy, which
targets tumor escape mechanisms, has both targetability and
long-lasting therapeutic effects. The immunotherapeutic modalities
that are mainly used at present are lysosomal virus therapy, cancer
vaccines, cytokine therapy, chimeric antigen receptor T-cell
therapy and immune checkpoint inhibition therapy (3). However, immunotherapy remains
expensive, lengthy and associated with off-target effects, posing
challenges in improving treatment efficiency (4).
Nanomaterials have emerged as promising therapeutic
agents for cancer because of their ability to improve efficacy,
high stability and specificity, capacity to control drug release
more precisely and the inherent therapeutic properties of certain
nanomaterials in response to stimuli (5-7).
Transition-metal NPs, which are derived from transition metals
(d-group elements), are particularly notable for their high
stability, modifiable properties and ability to participate in
multiple therapeutic modalities (8). In comparison with conventional tumor
immunotherapy, transition-metal NPs are highly targeted; moreover,
they are capable of combining multiple effects of chemistry, optics
and thermodynamics for treatment and can improve the
bioavailability and in vivo residence time of drugs
(9,10). Studies have suggested that
transition-metal NPs hold great potential in tumor immunotherapy;
however, their safety concerns and potential adverse effects
require cautious consideration. The present review focused on the
properties of transition-metal NPs, their advantages and
limitations in immunotherapy and their potential clinical
applications. Furthermore, it explored the feasibility of
categorizing such materials for future clinical practice.
Gold NPs (AuNPs). AuNPs are the most stable
transitionmetal NPs and show excellent stability, oxidation
resistance, biocompatibility and low toxicity, rendering them
suitable for biomedical applications (11). Notably, their tunable size and
shape allow for diverse functionalization, which is facilitated by
negatively charged surfaces that enable conjugation with
biologically active groups, such as amines and sulfhydryl groups
(11,12).
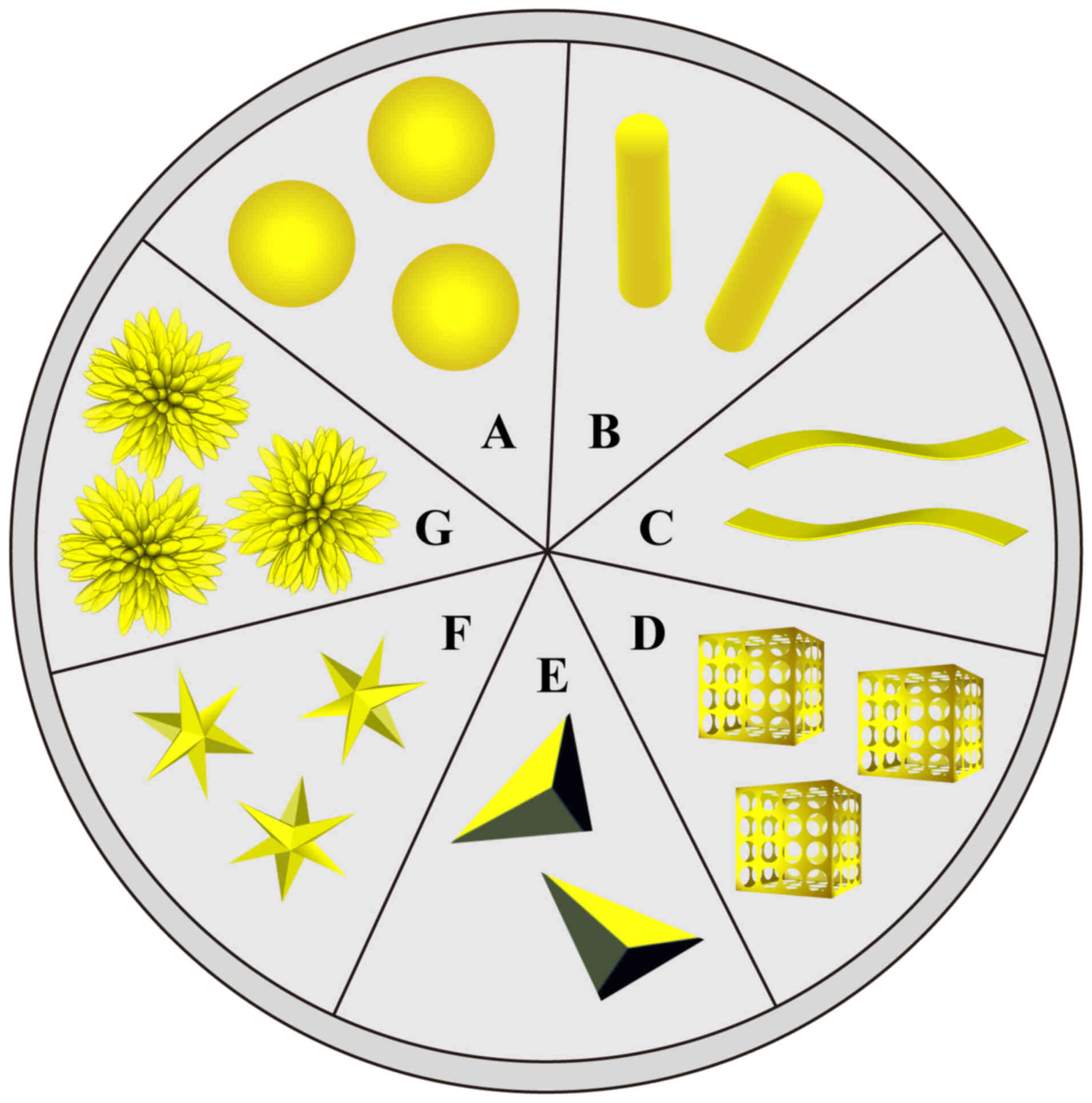
AuNPs can be classified on the basis of their
shapes, such as nanospheres, nanorods, nanoplates, nanocages,
nanotriangles, nanostars, nanoflowers and network dendrites, with
each shape showing a specific biomedical application (Fig. 1). AuNPs, particularly in nanoplate
or nanorod forms, have shown applications in imaging techniques
such as magnetic resonance imaging (MRI) and computed tomography
(CT) (13). Gold nanorods,
nanocages and nanoshells are considered excellent imaging NPs for
cancer therapy (14); for
example, an AuNP for CT imaging cancer therapy was studied by Wang
et al (15). AuNPs also
exhibit antimicrobial effects, possibly due to the surface charge,
with the surface modification between bacteria and AuNPs generating
reactive oxygen species (ROS). NPs also affect the cell membrane
permeability by disrupting intercellular communication (16). Bankar et al (17) have described the antibacterial
activity of network-dendritic AuNPs.
AuNPs are excellent drug carriers that can not only
carry small molecules but also transport large biomolecules through
different interactions, enabling drug release at the target
location (18). Additionally,
drug-loaded AuNPs can enhance drug accumulation and retention
(19). Gold nanospheres have a
uniform size, good tissue permeability and relatively simple
surface modifications, which are conducive to drug delivery
(20,21). Gold nanocages have hollow
interiors and porous structures, such that small molecules enter
the nanocages through the pores on the surfaces and are
encapsulated therein, which can be applied to the targeting area
(20).
In photothermal therapy (PTT), AuNPs convert light
into heat, thereby inducing high temperatures that cause
irreversible damage. Moreover, in vitro studies employing
tumor cell lines have confirmed that AuNPs can penetrate various
tumor cells, including lung, gastric and colorectal cancers, for
targeted therapy (22-24).
Despite these advantages, AuNPs should be used with
caution. The size, shape and surface properties of AuNPs are also
associated with their cytotoxicity. Additionally, modifications
such as PEGylation may induce toxicity (14). Exposure time and concentration
affect the cellular uptake of NPs and thus their cytotoxicity
(25).
AgNPs have attracted substantial attention because
of their nano-sized effects, surface properties and localized
surface plasmon resonance, making them valuable in antibiotics,
sensors and biomedical applications. Therefore, they have been
synthesized in various shapes to meet specific requirements
(26).
AgNPs have attracted growing interest in cancer
research because of their potential for next-generation diagnosis
and treatment, since they show broad anticancer effects by
inhibiting cancer cell growth and viability. AgNPs can induce
apoptosis and necrosis by generating ROS and damaging DNA (27). In vitro studies have shown
that AgNPs induce apoptosis by upregulating or downregulating gene
expression (28,29). They can also induce apoptosis by
altering key signaling pathways (30). Additionally, cancer cells treated
with AgNPs may experience cell cycle arrest (31). AgNPs inhibit tumor cell migration
and angiogenesis in vitro, indicating their possible role in
reducing distant metastasis (32). AgNPs can also be used to treat
cancer by coating them with polymers or by conjugating them with
drugs. Furthermore, because of their targeting ability and
biocompatibility, AgNPs show excellent anti-tumor efficacy and few
adverse reactions.
AgNPs inhibit tumor cell migration and invasion in a
concentration- and dose-dependent manner, which are critical
features for cancer development and progression (33,34). Although AgNPs have been shown to
inhibit tumor invasion, the underlying mechanism remains unknown.
One hypothesis suggests that AgNPs may reduce the production of
cytokines and growth factor proteins and inhibit the enzymatic
activity of matrix metalloproteinases in cancer cells (35).
Cu-based NPs mainly include CuNPs, CuONPs and CuS
NPs. CuNPs are of interest owing to their unique physical and
chemical properties, high surface-to-volume ratio, low preparation
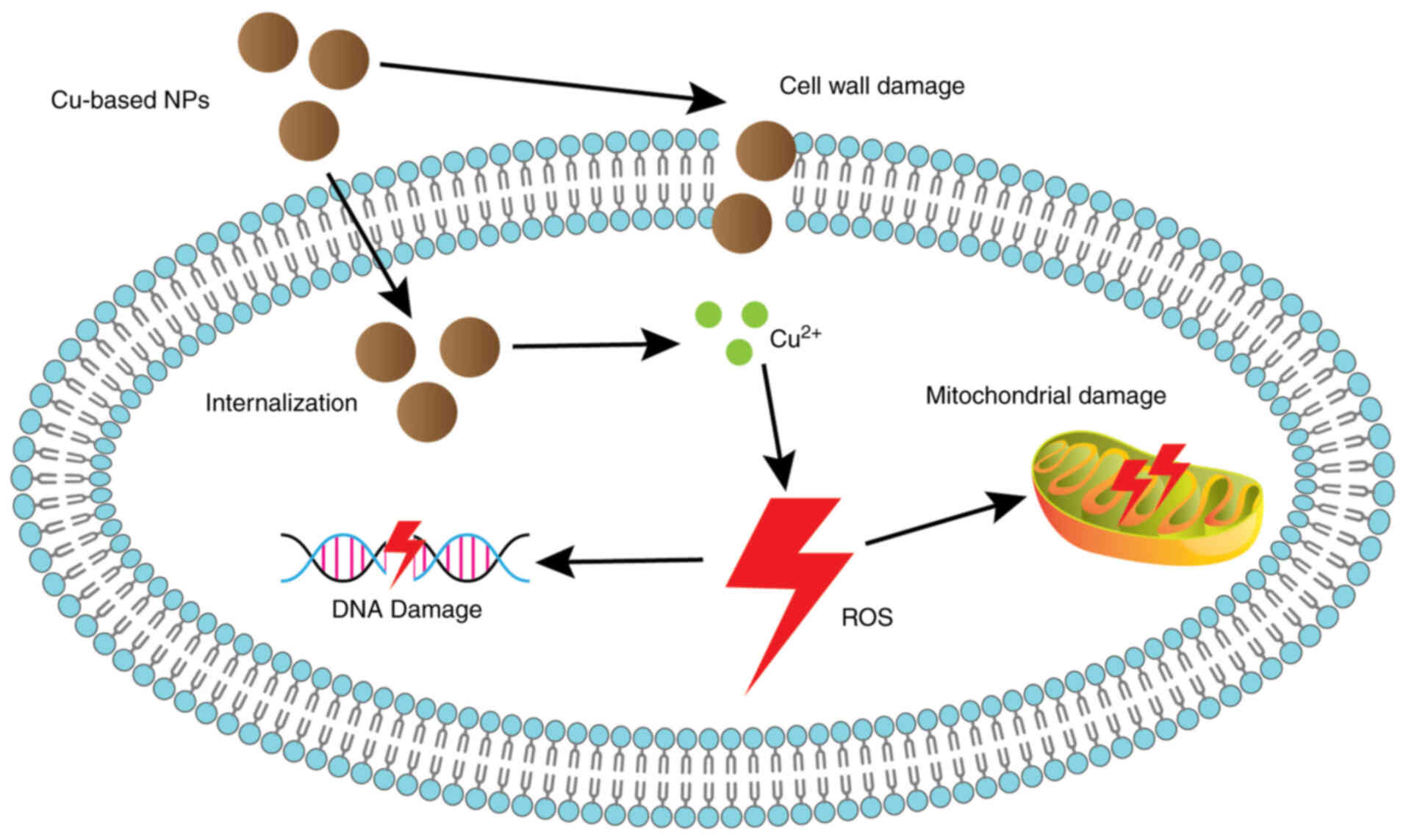
cost, low toxicity and good biocompatibility (36-38). Cu-based NPs exhibit antimicrobial
activity against Staphylococcus aureus, Escherichia
coli, Bacillus subtilis and Proteus vulgaris.
This antimicrobial property may be related to cell wall damage,
internalization of NPs into bacterial cells, Cu ion release, excess
ROS, oxidative stress, mitochondrial damage and DNA damage
(Fig. 2) (39). CuNPs act as anti-tumor agents
through various cytotoxic mechanisms, including ROS production,
cell cycle blockade, DNA damage, apoptosis and autophagy and are
effective against a wide range of cancer cell lines (39-41). Woźniak-Budych et al
(42) demonstrated that
sulfobetaine-stabilized Cu(I) oxide NPs promoted the suppression of
cancer cell growth in a concentration-dependent manner in tumor
cell line models. Due to their large surface area, CuNPs facilitate
high-density surface ligand attachment, making them suitable drug
carriers for the controlled release of anticancer drugs during
tumor treatment (43,44). In tumor therapy, CuS is a
potential photothermal agent owing to its good photothermal effect.
Yuan et al (45) used CuS
NPs as photothermal agents and found that encapsulated CuS NPs can
be used for PTT combined with chemotherapy, achieving improved
results in late-stage combined anticancer therapy in
vivo.
However, the stable synthesis of CuNPs still remains
challenging at present because Cu is easily oxidized. The rapid
dissolution of nanodrugs may also involve the oxidation of CuO,
which increases the toxicity of CuONPs.
Owing to their magnetic properties, iron-based NPs
have been broadly used in MRI, photodynamic therapy (PDT)/PTT
combination therapy and immunotherapy (46). Iron-based NPs include FeNPs, iron
oxide nanoparticles (IONPs) and iron-based bimetallic NPs, of which
IONPs are the most widely used (47). IONPs are cheap to produce and are
biocompatible (47). Their large
surface area reduces the amount of drugs required and their toxic
effects on cells (48). They are
degraded into ferric ions in vivo and participate in
physiological iron homeostasis. In addition, an in vitro
study demonstrated the antagonistic effect of
Fe3O4 NPs on ROS accumulation, indicating
their ability to regulate oxidative stress and apoptosis (49,50). As ferromagnetic materials, IONPs
can be surface-modified to obtain superparamagnetic iron oxide
nanoparticles (SPIONs), ensuring uniform size and stability at the
desired pH. In addition, elevated iron levels induce the Fenton
reaction, which leads to an increase in ROS (51). When ROS production exceeds the
cellular clearance capacity, it leads to lipid peroxidation and DNA
damage, resulting in iron death, a mechanism that provides ideas
for disease treatment (51).
Since the upregulation of the dehydrogenase redox system in tumor
cells affects lipid peroxidation, Chen et al (52) designed a layered double hydroxide
nanoplatform co-loaded with the ferroptosis agent IONPs and the
dehydrogenase inhibitor small interfering RNA (siR). Evidence from
both in vitro and in vivo studies has confirmed that
this platform synergistically induces cancer cell death by
releasing IONPs and siR to enable the mass production of ROS while
accelerating the accumulation of lipid peroxidation (52).
IONPs can generate excess ROS and disrupt biofilms,
which opens up the possibility of antimicrobial therapy (53). In addition, IONPs can be used for
tissue repair (54). SPIONs are
capable of targeted drug delivery by binding to drugs and
biomolecules, localizing them to target sites and releasing them
through the action of a magnetic field.
IONPs can be used in PTT, PDT, hyperthermia therapy
and tumor immunotherapy of tumors owing to their magnetic and
superparamagnetic properties (55). In PTT, IONPs can act as
photo-absorbents (56). IONPs may
also be suitable as intensifiers for radiotherapy (57,58). In magnetic hyperthermia therapy,
IONPs are exposed to an alternating magnetic field and produce a
local heating effect, causing protein denaturation, cancer cell
apoptosis and tissue damage through high temperatures >42°C.
IONPs used in hyperthermia therapy must be defined in terms of size
and shape to obtain improved therapeutic effects (59). Magnetic hyperthermia therapy can
also be used as a stimulating agent in tumor immunotherapy
(60,61). IONPs are not only competent in
transporting tumor-specific antigens to dendritic cells (DCs) and T
cells, but also in treating tumors by inducing a shift in
macrophage polarization toward the M1 type and increasing ROS
production (62,63).
Nanoscale iron-based metal-organic frameworks (MOFs)
play an important role in biomedical engineering because of the
extensive porosity, surface area and chemical and thermal
stability, as well as their good biocompatibility and
biodegradability (64-66). Nanoscale iron-based MOFs are ideal
for drug delivery systems because of their unique properties and
their catalytic and redox activities make them suitable for use in
biosensors and electrochemical sensors. This catalytic activity has
been used in nano-catalytic medicine to generate ROS for cancer
treatment. Using in vitro and in vivo studies, Yang
et al (67) demonstrated
that an iron-containing metal-organic framework [MOF(Fe)]
nano-catalyst, acting as a peroxidase mimic, catalyzes the
generation of highly oxidizing•OH radicals in cancer cells and
amplifies oxidative damage in synergy with autophagy-inhibiting
drugs. Iron metal-phenolic networks have a specific range of
absorption peaks in the ultraviolet range and pH-responsiveness and
can be efficiently dissociated to release drugs under acidic
conditions (68). They are
capable of functional modification and can undergo the Fenton
reaction to induce ferroptosis. Luo et al (69) designed an integrated nanoplatform
called FCS/GCS that takes advantage of the properties of
metal-phenolic networks to enhance the anti-tumor effect; thus
nanoconjugates are degraded and released in the acidic tumor
microenvironment (TME) and the Fenton reaction can occur, which
induces ferroptosis in vitro and in vivo.
Cancer vaccines. Cancer vaccines act against cancer
by activating the innate cellular or humoral immune systems, with
most vaccines triggering the proliferation of CD8+ T
cells that specifically identify and eliminate cancer cells
(70,71). To date, three cancer vaccines have
been approved by the United States Food and Drug Administration
(FDA) (72). Cancer nanovaccines,
whose antigens can be peptides, RNA, or DNA, are more flexible and
may elicit more robust immune responses. The simplest cancer
nanovaccine could be an antigen, an adjuvant, or even a
heterogeneous antigen in NPs with adjuvant properties (73). Currently, both therapeutic and
prophylactic vaccines against tumors are under investigation.
Metal ions are involved in several critical immune
processes in organisms; therefore, the application of
transition-metal NPs to tumor vaccines may promote effective
immunomodulation (74,75). NPs are modified to possess surface
properties similar to those of pathogens, thereby modulating the
interaction of the vaccine with antigen-presenting cells (APCs) and
enhancing antigen immunogenicity (76). In addition, NPs can increase the
specificity of tumor vaccines and minimize harm to normal cells in
comparison with conventional tumor vaccines. Transition-metal-based
nanomaterials are often used as adjuvants and drug carriers in
tumor nanovaccines.
A lack of adjuvants in vaccinations often results in
weaker immune responses. Currently, Toll-like receptor (TLR)
agonists and stimulator of interferon genes (STING) agonists are
being studied as vaccine adjuvants (77,78). The human body has 10 different
TLRs, which are distributed on the extracellular and endosomal
membranes of immune cells and can induce the secretion of
cytokines, chemokines and type I interferons, thereby activating
the innate immune system and mediating the activation of acquired
immune responses (79,80). Shinchi et al (80) took into account the high affinity
of AuNPs for thiol-functionalized molecules, such as the TLR7
ligand and antigens and combined the small-molecule synthetic TLR7
ligand 2-methoxyethoxy-8-oxo-9-(4-carboxy benzyl)adenine (1V209)
and α-mannose co-immobilized on the surface of AuNPs, which served
as a potent adjuvant to enhance the activation of TLR7 on endosomal
membranes. STING is a transmembrane protein integrated within the
endoplasmic reticulum that activates type I interferons (81). The cyclic GMP-AMP synthase-STING
pathway may act as an anti-tumor agent by inducing a
proinflammatory response dominated by type I interferons, which may
be a potential target for immunotherapy (82). Ding et al (83) designed a TME-responsive adjuvant
called MnOx nanospikes and prepared a vaccine using
ovalbumin (OVA) proteins as antigens to suppress primary and distal
tumor growth as well as tumor metastasis in vitro and in
vivo. In addition, a number of other cytokines with
immunostimulatory effects, such as IL-2, IL-1 and interferons, can
be used as adjuvants. Granulocyte-macrophage colony-stimulating
factor facilitates the attraction of DCs to the target site,
promotes DC maturation and facilitates antigen presentation
(84,85). Inorganic nanoadjuvants, which have
been found to enable the sustained release of antigens and
specifically increase the immune response, have also gained
widespread interest (86). Wang
et al (87) used human
tubercle bacillus-loaded Zn- and Mg-tricalcium phosphates as
adjuvants to boost the immune response and stimulate the secretion
of granulocyte-macrophage colony-stimulating factor, which markedly
inhibited the development of Lewis lung cancer cells in
vivo.
Transition-metal NPs can modulate their size and
shape to deliver antigens and adjuvants to specific tissues and
have been used as delivery carriers for cancer nanovaccines
(88,89). Vaccines that use AuNPs as carriers
exhibit high immunostimulatory activity (90). Zhang et al (91) studied multilayer polyelectrolytic
AuNPs with anionic poly I:C and antigenic peptides as nanocarriers,
which could induce more antigen-specific CD8+ T cells.
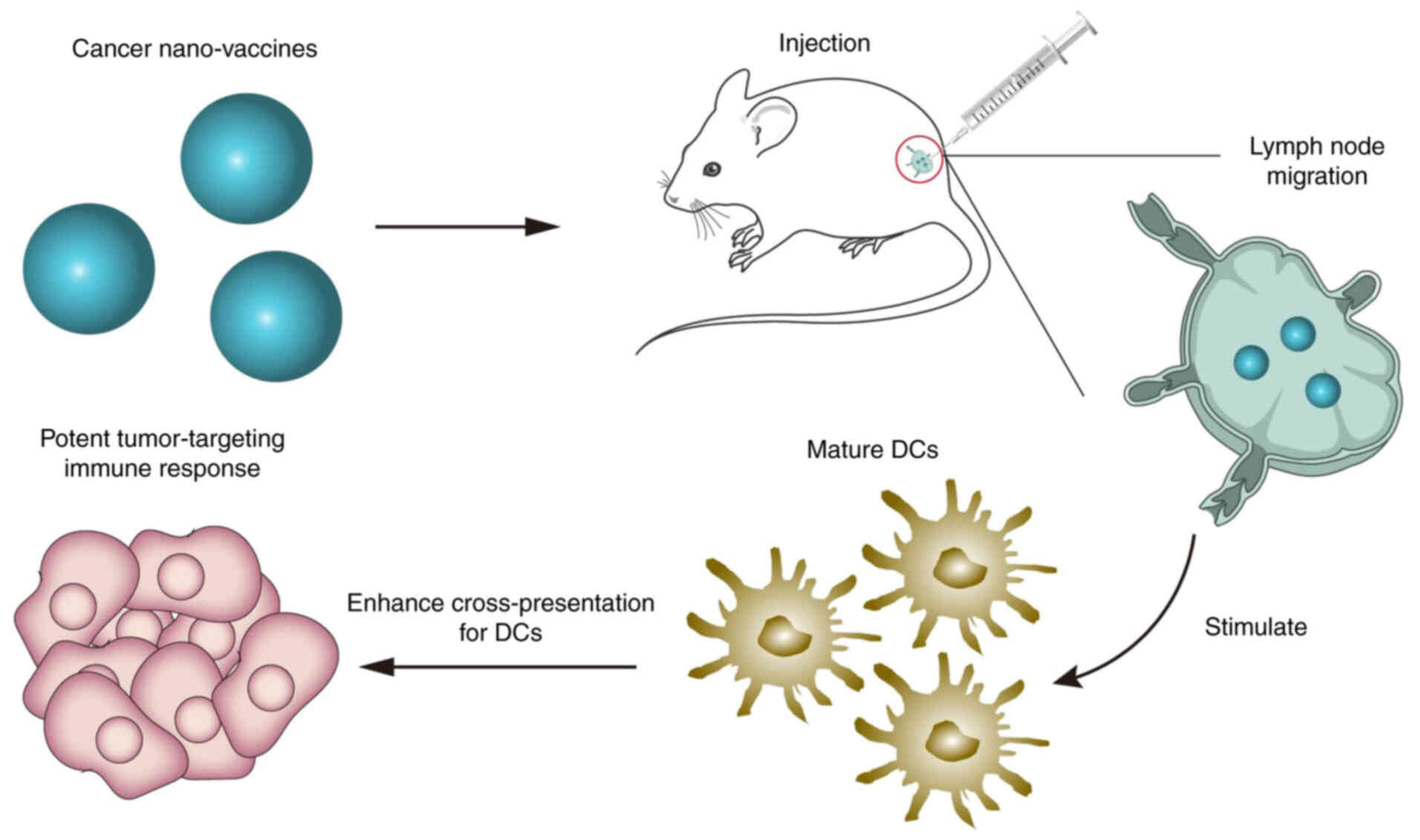
Zhao et al (92) prepared
a vaccine carrier containing Mn2+ ions and
meso-2,6-diaminopimelic acid (DAP) to encapsulate the OVA
antigen to form a cancer vaccine (Fig. 3). This vaccine could effectively
co-deliver OVA and DAP to the lymph nodes, stimulate the maturation
of DCs, enhance OVA cross-presentation to DCs and serve as a
prophylactic vaccine for B16-OVA melanoma tumors in
vivo.
Nanovaccines can also be integrated with other
immunotherapies to enhance their efficacy (73). Jin et al (93) developed polyethyleneimine modified
gold nanorods called GNRs-PEI. Subsequently, GNR-PEI/cyclic dimeric
guanosine monophosphate-adenosine monophosphate-laden macrophages
(GPc-RAWs) were injected into the tumor with simultaneous
introduction of a programmed cell death ligand 1 (PD-L1) antibody.
This strategy inhibits tumor growth and metastasis through a
combination of tumor vaccines and checkpoint inhibitors in
vivo. Chen et al (94)
assembled an iron nano-adjuvant containing IONPs and STING agonists
that activated STING while forming a vaccine with OVA antigens to
induce long-lasting anti-tumor immunity and prevent postoperative
recurrence and distant metastasis in vivo by synergistic
treatment with checkpoint inhibitors.
Immune checkpoint inhibitors. Cancer immunotherapies
promote immune system specificity against cancer. The blockade of
immune checkpoint programmed death receptor-1 (PD-1) and cytotoxic
T lymphocyte antigen-4 (CTLA-4) removes immune suppression,
enabling tumor clearance (95).
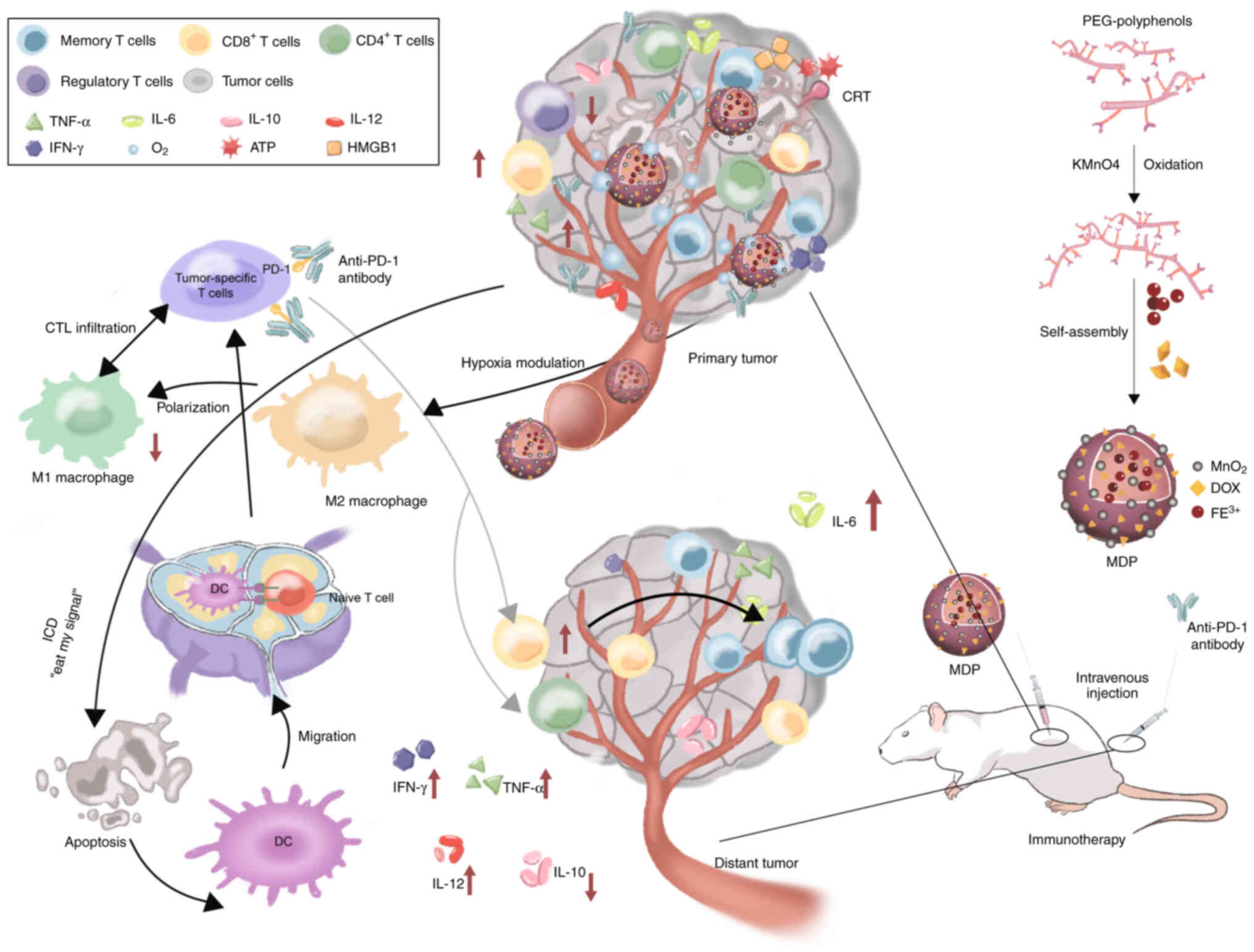
Nanometallic materials have become a hotspot in
research on checkpoint blockade immunotherapy. Hybrid nanometallic
frameworks function as immunogenic cell death (ICD) inducers,
enhancing tumor sensitivity to immunotherapy. Researchers have
developed a phenolic ICD inducer to improve antigen generation,
tumor-specific T-cell infiltration and PD-1 checkpoint blockade,
stimulating anti-tumor immunity and generating significant abscopal
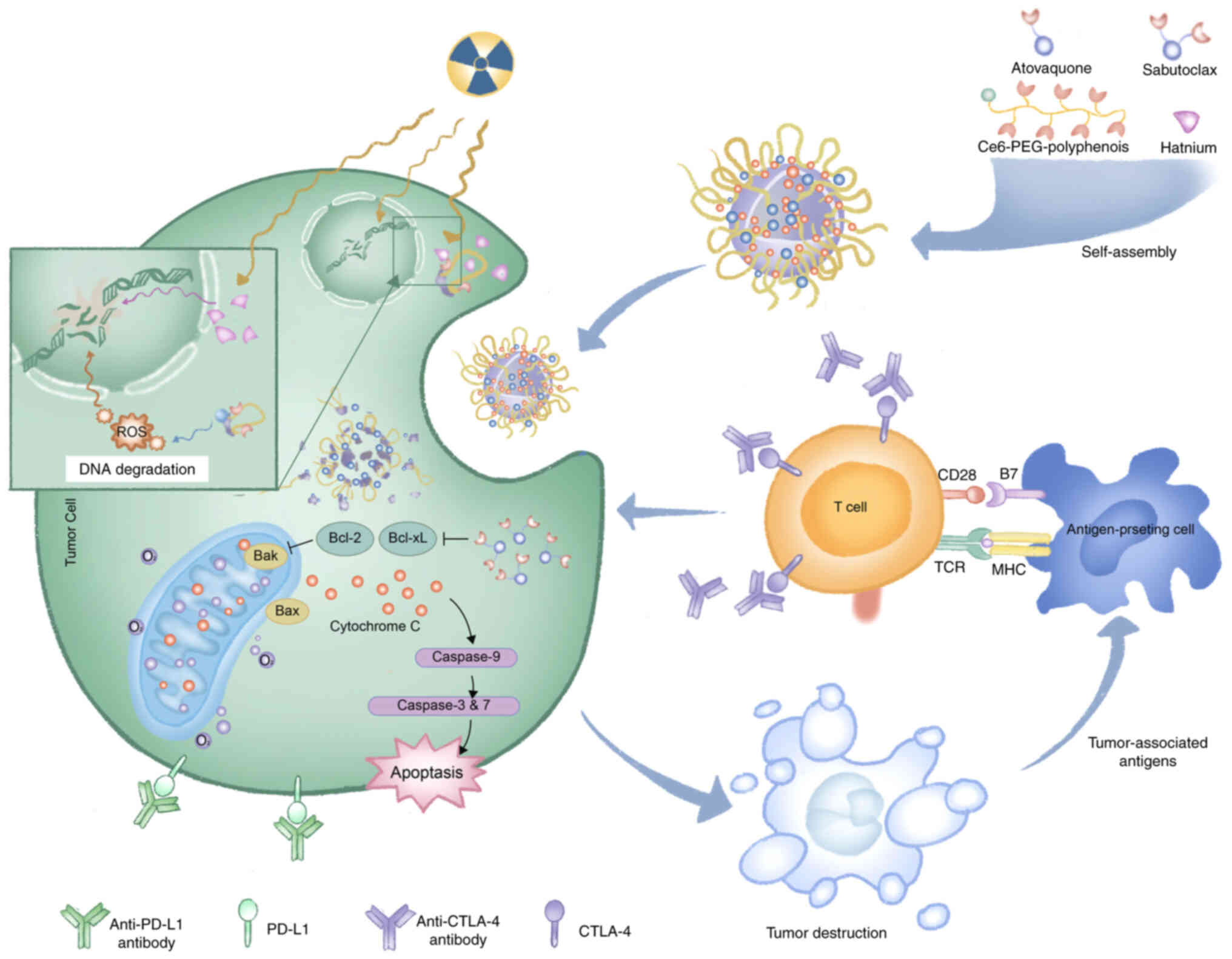
effects on distant tumors in vivo (Fig. 4) (96). Moreover, Sang et al
(97) developed metal-phenolic
network nanopumps to overcome resistance to combined
radiotherapy/anti-CTLA-4 immunotherapy. Evidence from both in
vitro and in vivo studies confirmed that these NPs
enhanced RT efficacy, alleviated hypoxia/induced apoptosis,
suppressed therapy-induced PD-L1 upregulation, effectively reversed
T-cell exhaustion and enabled immunotherapy for immunologically
non-responsive tumors (Fig.
5).
Immune checkpoint agonist antibodies. Immune cells
are stimulated by exogenous antigens to avoid autoimmunity and
tissue damage through regulation of costimulatory and
co-suppressive receptors, that is, the regulation of immune
checkpoints (98). Evidence
support the important role of the costimulatory pathway in
mediating anti-cancer immunity. The targets of immune agonist
antibodies are mainly the B7-CD28 and tumor necrosis factor
receptor (TNFR) families (98,99).
Typical costimulatory receptors of the B7-CD28
family are CD28 and inducible T-cell costimulator (ICOS). CD28
activates downstream signaling after adhering to the ligands CD80
and CD86, driving T-cell function, proliferation and survival
(100). ICOS responds to ICOS
ligands to activate T cells and memory T cells. Thus, CD28 and ICOS
are potential targets for the development of therapeutic agonists
for tumor treatment (101,102). Chiang et al (103) reported an inherently therapeutic
fucoidan-dextran-based magnetic nanomedicine (IO@FuDex3)
combined with a checkpoint inhibitor and T-cell activators,
demonstrating in vivo that combining IONPs with anti-PD-L1
and agonist antibodies enhances the efficacy of immunotherapy.
The TNFR superfamily influences the maturation and
differentiation of B and T lymphocytes and is involved in
physiological processes such as apoptosis, inflammation and
autoimmunity (104). Six
receptors have been suggested to play major roles as immune
costimulators, namely, CD40, OX40, 4-1BB, CD27, GITR and CD30
(98). Among them, agonistic CD40
monoclonal antibody activates host APCs, especially DCs, thus
inducing anti-tumor T-cell responses, which are considered
promising for research (105).
Although nanomedicines targeting TNFR have been
investigated, nanomedicines based on transition-metal NPs targeting
TNFR are still lacking (106).
Overall, the use of immune checkpoint agonist antibodies
incorporating transition-metal NPs remains an area that requires
further exploration.
Adoptive cell therapy (ACT). ACT is a form of
treatment in which T cells are isolated from plasma or tumor
samples, expanded and activated in vitro, conditioned and
modified and injected back into the patient to activate the immune
system and suppress cancer (107-109). This method is based on the
principle of engineering T cells is to create T cells with
tumor-targeting structural domains, which enhances the specificity
of T-cell recognition in tumors (110,111). Due to the complexity and expense
of the natural activation process of T cells, the primary focus of
current research is the use of artificial antigen-presenting cells
(aAPCs), which mimic the function of APCs, to stimulate T cells to
initiate tumor-specific adaptive immunity (112). Nanoscale aAPCs have been
developed owing to their small size, low toxicity and low-dose
requirements (113,114).
PTT, which induces localized thermotherapy, is one
of the few NP-based therapies to enter clinical trials for cancer
and is expected to be applied synergistically with immunotherapy in
tumor treatment (120,121). Paholak et al (122) demonstrated that PTT mediated by
highly crystalline IONPs efficiently eliminated breast cancer stem
cells in a transformed model of triple-negative breast cancer,
thereby improving the long-term survival of patients with
metastatic breast cancer by inducing a systemic immune response
targeting distal cancer cells.
Nanoenzyme-based catalytic therapy is a novel
strategy for treating tumors; however, challenges such as hypoxia,
immunosuppression and insufficient endogenous
H2O2 levels in the TME limit the efficacy of
this strategy. Researchers have developed a trimetallic nanoenzyme
(Au@Pt@Rh) that exhibits endogenous peroxidase- and catalase-like
activities under acidic TME conditions. Au@Pt@Rh-activated
photothermal thermotherapy has also been shown to improve
peroxidase- and catalase-mimicking activity. By loading the
transforming growth factor-β inhibitor LY2157299, this nano-complex
successfully reprogrammed the immunosuppressive TME, alleviated
tumor hypoxia and generated highly toxic hydroxyl radicals (•OH)
(123).
Although PTT combined with immunotherapy shows
potential for tumor treatment, the toxicity of NPs and possible
damage to normal tissues from high temperatures and prolonged
irradiation remain to be addressed. Furthermore, considering the
effects of different temperatures on immune cells, the design of
specific strategies to maximize the effects of PTT and
immunotherapy requires further exploration.
PDT can induce an immune response and destroy tumor
tissues. PDT-mediated immunotherapy is a promising therapeutic
modality used to treat cancer. Duan et al developed
Zn-pyrophosphate NPs loaded with photosensitizer thermolipids
(ZnP@pyro) and found that ZnP@pyro PDT combined with anti-PD-L1
therapy eradicated primary breast tumors and generated systemic
tumor-specific cytotoxic T-cell responses to completely suppress
distant tumors. These results showed that NP-mediated PDT can
enhance immunotherapy by activating the innate and adaptive immune
systems in the TME (124).
Nevertheless, insufficient light-penetration depth remains an
unresolved limitation of PDT, posing a challenge to the clinical
translation of PDT and immune combination therapies.
Radiotherapy causes ionizing damage to tumor tissue
in an X-ray dose-dependent manner, with the maximum radiation dose
defined as the dose that causes no damage to adjacent normal tissue
(125). High-dose
hyperfractionated radiotherapy and radiotherapy sensitizers have
been investigated to enhance checkpoint blockade immunotherapy
(126). Ni et al
(127) reported two porous
Hf-based nMOFs that act as effective radioenhancers. More
importantly, in vitro and in vivo studies showed that
these nMOFs are capable of mediating low-dose radiotherapy in
combination with anti-PD-L1 treatment, which simultaneously treats
both local and distant tumors through a distant effect.
Nevertheless, further studies should explore these nMOFs in
relation to their therapeutic efficacy, toxic response and drug
design in terms of clinical transformation.
Conventional chemotherapy combined with metallic
nanomaterials is expected to synergistically inhibit tumor
progression, metastasis and recurrence. Xu et al (128) reported synergistic immunotherapy
for triple-negative breast cancer on the basis of the premise of
stimulating and promoting an ICD response using a transformable NP
that simultaneously delivered cisplatin, adjudin and WKYMVm. The
in vitro and in vivo results showed that the NP could
markedly inhibit primary tumor growth and lung metastasis and
enhance innate and adaptive anti-tumor immunity, resulting in
significant survival benefits.
To date, among transition-metal NPs, drugs that have
received FDA approval are mainly iron-based NPs, including INFed,
Dexferrum, Venofer, Feraheme, Injectafer, Monoferric, Feridex and
Ferrlecit (130,131). The main applications of these
iron-based NPs are in the treatment of iron-deficient anemia and
imaging (132).
Nanotherm® is an FDA-approved device with a SPION
formulation that causes apoptosis in cancer cells heated in a
high-frequency magnetic field (133). However, most transition-metal
NPs are not used clinically.
Clinical studies employing transition-metal NPs as
a therapeutic option for oncology patients have focused on
modalities such as chemotherapy and hyperthermia. Khoobchandani
et al (134) applied
AuNP-based Nano Swarna Bhasma to patients with stage IIIA or IIIB
breast cancer and found that the treatment group that received the
nanomedicine showed 100% clinical benefit in comparison with the
group that received standard of care treatment. Kumthekar et
al (135) employed
brain-penetrant RNA interference-based spherical nucleic
acid-containing AuNPs; they observed no treatment-related grade 4
or 5 toxicity after administration, while the presence of gold
accumulation in tumor-associated endothelial, macrophage and cancer
cells represented a potential strategy for the systemic treatment
of glioblastoma. In addition, drugs such as AuroLase and Magnablate
have been used in clinical trials for thermal ablation of cancer
(132).
Cancer immunotherapy has a promising future, in
which NPs have been tested in clinical trials related to cancer
vaccines and immune checkpoint inhibitors (ICIs). Rojas et
al (136) developed mRNA
neoantigen vaccines in real time from surgically removed pancreatic
ductal adenocarcinoma tumors using uridine mRNA-lipoplex NPs. After
an 18-month follow-up period, patients who had T cells expanded by
the vaccine exhibited a longer median relapse-free survival than
those who did not have vaccine-expanded T cells. Alonso et
al (137) selected
non-muscle invasive bladder cancer patients who were unresponsive
to Bacillus Calmette-Guérin and applied
OncoTherad® nanomedicine for treatment and found that it
works by binding the TLR4 to activate the innate immune system and
reduce immune checkpoint molecules. In addition, Zhang et al
(138) found that ICIs combined
with nanomedicine chemotherapy inhibited the progression of
esophageal squamous cell carcinoma.
Overall, most studies on cancer immunotherapy using
transition-metal NPs are in the preclinical stage and lack data
from clinical trials. The reasons for this worrisome state of
clinical transformation warrant further investigation.
Immunotherapy is affected by multiple factors such as off-target
toxicity, tissue heterogeneity, poor immune response durability and
adverse effects; often, only a limited number of patients respond
satisfactorily to immunotherapy (74). In addition, transition-metal NPs
face a number of challenges in drug delivery, release and
biodistribution and these factors directly determine their efficacy
in cancer treatment. Different routes of drug delivery are
associated with various biological barriers (139). Local delivery is more direct;
however, its invasiveness may pose other problems and local
delivery is limited by the site and type of cancer (140). Although systemic drug delivery
is more commonly used, it is influenced by a more complex set of
factors. During circulation, the NPs may be destabilized by shear
stress (141). Another challenge
is the absorption of NPs by the phagocytic cells in the
reticuloendothelial system (142). Different portals of entry often
allow NPs to target different cell populations and factors such as
the size, shape, charge and surface coating of transition-metal NPs
affect their biodistribution in vivo (89). The molecular mechanisms and
interactions between transition-metal NPs and biological systems
require further study. The heterogeneity of the TME and enhanced
permeability and retention in the human body lead to reduced
penetration of NPs and inhomogeneous tumor extravasation of NPs,
thus reducing their accumulation in the tumor.
The safety of transition-metal NPs in cancer
diagnosis and therapy is an important issue. Although previous
studies have demonstrated that transition-metal NPs can promote
anti-tumor immune responses in vivo (63), these materials have potential
safety risks. Immune responses to transition-metal NPs can also
cause inflammation and tissue damage (143,144). NPs can elicit allergic
reactions, limiting the feasibility and safety of repeated
administration (145). Oxidative
stress due to ROS imbalance is also a problem owing to the unique
ability of Cu-based NPs to generate ROS. In addition, long-term
accumulation of metal ions may lead to organ damage. Therefore,
future research should systematically evaluate both the short-term
immune responses and long-term toxicity of these nanomaterials.
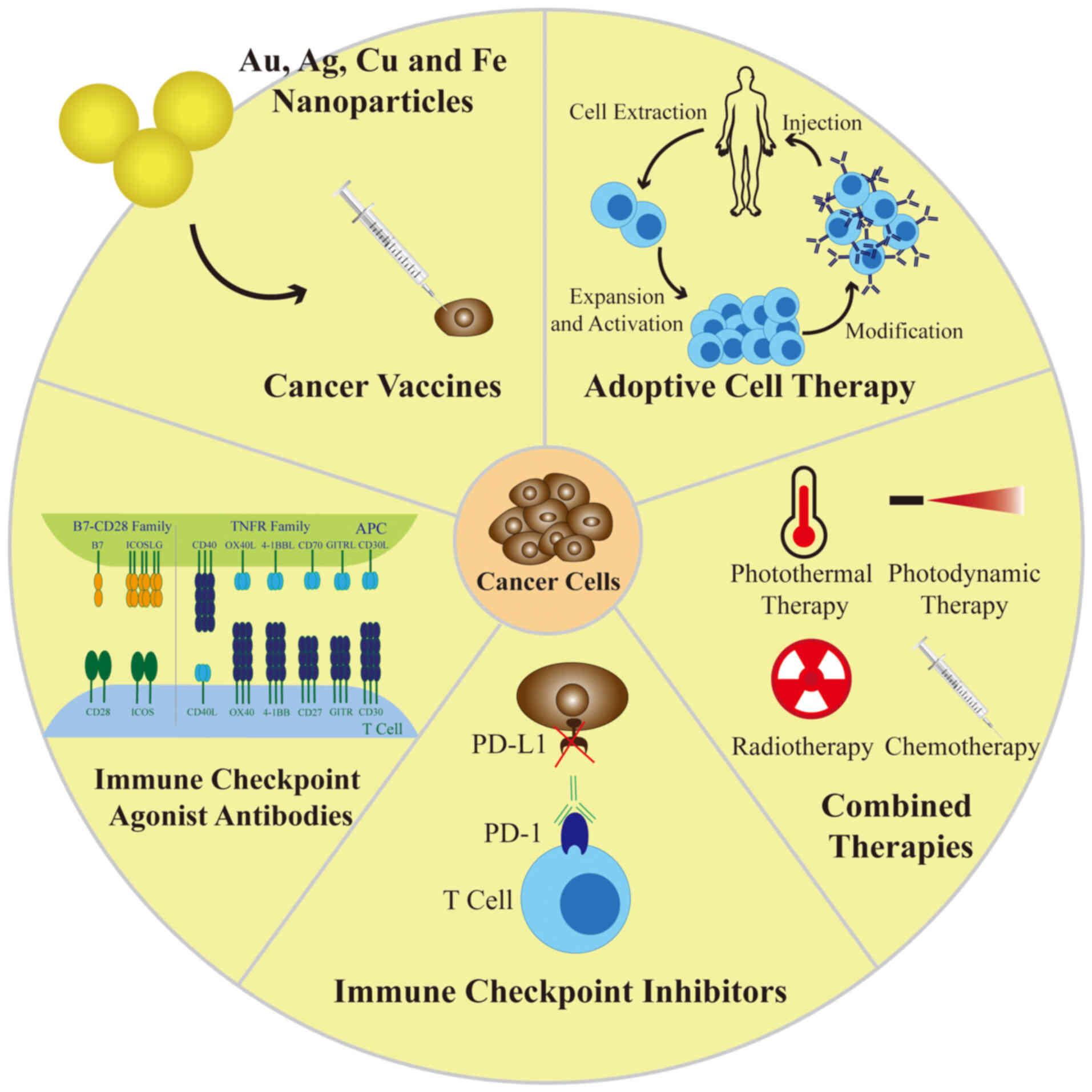
The present review critically analyzed the existing
applications and future potential of major transition-metal NPs in
cancer immunotherapy and a brief graphical summary of the review is
provided in Fig. 6. It began with
a detailed overview of the classification, properties, biomedical
roles and toxicity of major transition-metal NPs. The applications
of transition-metal NPs in tumor immunotherapies, including cancer
vaccines, ICIs, immune checkpoint agonist antibodies, ACT and
combination therapies, were then introduced. Finally, the current
status and challenges associated with clinical transformation were
discussed to provide ideas for subsequent clinical studies. To
provide a consolidated overview of biomedical applications, current
cancer research status and clinical transformation progress,
Table I presents a comparative
summary of transition metal NPs.
In conclusion, the efficacy and safety of
transition-metal NPs remain obstacles to clinical research
(153). Further research
regarding the design of NPs and their interactions in vivo
is required to improve specificity. Personalization of therapy for
different patient groups remains a challenge for clinical
transformation. In the future, systematic immunogenicity testing
combined with long-term clinical follow-up is recommended to
comprehensively evaluate the safety of metal-based materials.
Emerging technologies, such as single-cell transcriptomics and
spatial omics, may provide critical insights outlining how
nanoparticles reshape immune cell lineages and functions within the
TME, yielding a deeper mechanistic understanding. In addition, the
development of scalable manufacturing processes, demonstration of
batch-to-batch consistency and establishment of standardized
clinical-grade production workflows are essential steps toward
successful clinical translation. Transition-metal immunotherapy is
still in its infancy and much work remains to be conducted to make
the application of transition NPs in tumor immunotherapy a
reality.
Not applicable.
CS and YH were responsible for conceptualization.
ZD, ZC and CF were responsible for investigation. CF and YH were
responsible for project administration. CS and YH were responsible
for supervision. ZD and ZC were responsible for visualization. ZD
and ZC were responsible for writing the original draft. ZD, ZC, DX,
LX, CF, CS and YH were responsible for writing, reviewing and
editing. CS made a significant contribution to the manuscript
revision. Data authentication is not applicable. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was funded by National Key R&D Program of
China (grant nos. 2023YFC2508601, 2023YFC2508604 and
2023YFC2508605), Tongji University Medicine-X Interdisciplinary
Research Initiative (grant no. 2025-0554-ZD-08), Shanghai Hospital
Development Center Foundation (grant nos. SHDC22025208 and
SHDC12024125), Clinical Research Foundation of Shanghai Pulmonary
Hospital (grant no. LYRC202401), The Innovation Team Project of the
Faculty of Chinese Medicine Science, Guangxi University of Chinese
Medicine (grant nos. 2023CX001 and 2024ZZA004) and Project for
Enhancing Young and Middle-aged Teacher's Research Basis Ability in
Colleges of Guangxi (grant no. 2025KY1124).
|
1
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parreno V, Loubiere V, Schuettengruber B,
Fritsch L, Rawal CC, Erokhin M, Győrffy B, Normanno D, Di Stefano
M, Moreaux J, et al: Transient loss of Polycomb components induces
an epigenetic cancer fate. Nature. 629:688–696. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chong G, Zang J, Han Y, Su R,
Weeranoppanant N, Dong H and Li Y: Bioengineering of nano
metal-organic frameworks for cancer immunotherapy. Nano Res.
14:1244–1259. 2021. View Article : Google Scholar
|
|
4
|
Surendran SP, Moon MJ, Park R and Jeong
YY: Bioactive Nanoparticles for cancer immunotherapy. Int J Mol
Sci. 19:38772018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertrand N, Wu J, Xu X, Kamaly N and
Farokhzad OC: Cancer nanotechnology: The impact of passive and
active targeting in the era of modern cancer biology. Adv Drug
Deliv Rev. 66:2–25. 2014. View Article : Google Scholar :
|
|
6
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar :
|
|
7
|
Zheng X, Wu Y, Zuo H, Chen W and Wang K:
Metal nanoparticles as novel agents for lung cancer diagnosis and
therapy. Small. 19:e22066242023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao W, Li A, Zhang A, Zheng Y and Liu J:
Recent advances in functional-polymer-decorated transition-metal
nanomaterials for bioimaging and cancer therapy. ChemMedChem.
13:2134–2149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J and Zhang C: Regulation of
cancer-immunity cycle and tumor microenvironment by
nanobiomaterials to enhance tumor immunotherapy. Wiley Interdiscip
Rev Nanomed Nanobiotechnol. 12:e16122020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu W, Song X, Jiang Q, Guo W, Liu J, Chu
X and Lei Z: Transition metal oxide nanomaterials: New weapons to
boost anti-tumor immunity cycle. Nanomaterials (Basel).
14:10642024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daniel MC and Astruc D: Gold
nanoparticles: Assembly, supramolecular chemistry,
quantum-size-related properties, and applications toward biology,
catalysis, and nanotechnology. Chem Rev. 104:293–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KX, Shameli K, Yew YP, Teow SY,
Jahangirian H, Rafiee-Moghaddam R and Webster TJ: Recent
developments in the facile bio-synthesis of gold nanoparticles
(AuNPs) and their biomedical applications. Int J Nanomed.
15:275–300. 2020. View Article : Google Scholar
|
|
13
|
Hemalatha T, Prabu P, Gunadharini DN and
Gowthaman MK: Fabrication and characterization of dual acting oleyl
chitosan functionalised iron oxide/gold hybrid nanoparticles for
MRI and CT imaging. Int J Biol Macromol. 112:250–257. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh P, Pandit S, Mokkapati VRSS, Garg A,
Ravikumar V and Mijakovic I: Gold nanoparticles in diagnostics and
therapeutics for human cancer. Int J Mol Sci. 19:19792018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Deng J, He D, Yang E, Yang W, Shi
D, Jiang Y, Qiu Z, Webster TJ and Shen Y: PEGylated hollow gold
nanoparticles for combined X-ray radiation and photothermal therapy
in vitro and enhanced CT imaging in vivo. Nanomedicine. 16:195–205.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karthika V, Arumugam A, Gopinath K,
Kaleeswarran P, Govindarajan M, Alharbi NS, Kadaikunnan S, Khaled
JM and Benelli G: Guazuma ulmifolia bark-synthesized Ag, Au and
Ag/Au alloy nanoparticles: Photocatalytic potential, DNA/protein
interactions, anticancer activity and toxicity against 14 species
of microbial pathogens. J Photochem Photobiol, B. 167:189–199.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bankar A, Joshi B, Kumar AR and Zinjarde
S: Banana peel extract mediated synthesis of gold nanoparticles.
Colloids Surf, B Biointerfaces. 80:45–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar CG, Poornachandra Y and Mamidyala
SK: Green synthesis of bacterial gold nanoparticles conjugated to
resveratrol as delivery vehicles. Colloids Surf, B Biointerfaces.
123:311–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng J, Gu YJ, Cheng SH and Wong WT:
Surface functionalized gold nanoparticles for drug delivery. J
Biomed Nanotechnol. 9:1362–1369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Cao Z, Liu R, Liu L, Li H, Li X,
Chen Y, Lu C and Liu Y: AuNPs as an important inorganic
nanoparticle applied in drug carrier systems. Artif Cells Nanomed
Biotechnol. 47:4222–4233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DY, Kim M, Shinde S, Sung JS and
Ghodake G: Cytotoxicity and antibacterial assessment of gallic acid
capped gold nanoparticles. Colloids Surf, B Biointerfaces.
149:162–167. 2017. View Article : Google Scholar
|
|
22
|
Lee KD, Nagajyothi PC, Sreekanth TVM and
Park S: Eco-friendly synthesis of gold nanoparticles (AuNPs) using
Inonotus obliquus and their antibacterial, antioxidant and
cytotoxic activities. J Ind Eng Chem. 26:67–72. 2015. View Article : Google Scholar
|
|
23
|
Naraginti S and Li Y: Preliminary
investigation of catalytic, antioxidant, anticancer and
bactericidal activity of green synthesized silver and gold
nanoparticles using Actinidia deliciosa. J Photochem Photobiol, B.
170:225–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vijayakumar S, Vaseeharan B,
Malaikozhundan B, Gopi N, Ekambaram P, Pachaiappan R, Velusamy P,
Murugan K, Benelli G, Suresh Kumar R and Suriyanarayanamoorthy M:
Therapeutic effects of gold nanoparticles synthesized using Musa
paradisiaca peel extract against multiple antibiotic resistant
Enterococcus faecalis biofilms and human lung cancer cells (A549).
Microb Pathogen. 102:173–183. 2017. View Article : Google Scholar
|
|
25
|
Mironava T, Hadjiargyrou M, Simon M,
Jurukovski V and Rafailovich MH: Gold nanoparticles cellular
toxicity and recovery: Effect of size, concentration and exposure
time. Nanotoxicology. 4:120–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ziyu P and Haodong J: Controlled synthesis
of silver nanomaterials and their environmental applications. Prog
Chem. 35:1229–1257. 2023.
|
|
27
|
Huy TQ, Huyen PTM, Le AT and Tonezzer M:
Recent advances of silver nanoparticles in cancer diagnosis and
treatment. Anticancer Agents Med Chem. 20:1276–1287. 2020.
View Article : Google Scholar
|
|
28
|
Mohamed AF, Nasr M, Amer ME, Abuamara TMM,
Abd-Elhay WM, Kaabo HF, Matar EER, El Moselhy LE, Gomah TA, Deban
MAE and Shebl RI: Anticancer and antibacterial potentials induced
post short-term exposure to electromagnetic field and silver
nanoparticles and related pathological and genetic alterations: In
vitro study. Infect Agent Cancer. 17:42022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan YG, Zhang S, Hwang JY and Kong IK:
Silver Nanoparticles potentiates cytotoxicity and apoptotic
potential of camptothecin in human cervical cancer cells. Oxid Med
Cell Longev. 2018:61213282018. View Article : Google Scholar
|
|
30
|
Jeong JK, Gurunathan S, Kang MH, Han JW,
Das J, Choi YJ, Kwon DN, Cho SG, Park C, Seo HG, et al:
Hypoxia-mediated autophagic flux inhibits silver
nanoparticle-triggered apoptosis in human lung cancer cells. Sci
Rep. 6:216882016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin M, Xu X, Han H, Dai J, Sun R, Yang L,
Xie J and Wang Y: Preparation of triangular silver nanoparticles
and their biological effects in the treatment of ovarian cancer. J
Ovarian Res. 15:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noorbazargan H, Amintehrani S, Dolatabadi
A, Mashayekhi A, Khayam N, Moulavi P, Naghizadeh M, Mirzaie A,
Mirzaei Rad F and Kavousi M: Anti-cancer & anti-metastasis
properties of bioorganic-capped silver nanoparticles fabricated
from Juniperus chinensis extract against lung cancer cells. AMB
Express. 11:612021. View Article : Google Scholar
|
|
33
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meenakshisundaram S, Krishnamoorthy V,
Jagadeesan Y, Vilwanathan R and Balaiah A: Annona muricata assisted
biogenic synthesis of silver nanoparticles regulates cell cycle
arrest in NSCLC cell lines. Bioorg Chem. 95:1034512020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miranda RR, Sampaio I and Zucolotto V:
Exploring silver nanoparticles for cancer therapy and diagnosis.
Colloids Surf B Biointerfaces. 210:1122542022. View Article : Google Scholar
|
|
36
|
Jia B, Mei Y, Cheng L, Zhou J and Zhang L:
Preparation of copper nanoparticles coated cellulose films with
antibacterial properties through one-step reduction. ACS Appl Mater
Interfaces. 4:2897–2902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luque-Jacobo CM, Cespedes-Loayza AL,
Echegaray-Ugarte TS, Cruz-Loayza JL, Cruz I, de Carvalho JC and
Goyzueta-Mamani LD: Biogenic synthesis of copper nanoparticles: A
systematic review of their features and main applications.
Molecules. 28:48382023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith AM, Duan H, Rhyner MN, Ruan G and
Nie S: A systematic examination of surface coatings on the optical
and chemical properties of semiconductor quantum dots. Phys Chem
Chem Phys. 8:3895–3903. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Letchumanan D, Sok SPM, Ibrahim S, Nagoor
NH and Arshad NM: Plant-based biosynthesis of copper/copper oxide
nanoparticles: An update on their applications in biomedicine,
mechanisms, and toxicity. Biomolecules. 11:5642021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rehana D, Mahendiran D, Kumar RS and
Rahiman AK: Evaluation of antioxidant and anticancer activity of
copper oxide nanoparticles synthesized using medicinally important
plant extracts. Biomed Pharmacother. 89:1067–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahmood RI, Kadhim AA, Ibraheem S,
Albukhaty S, Mohammed-Salih HS, Abbas RH, Jabir MS, Mohammed MKA,
Nayef UM, AlMalki FA, et al: Biosynthesis of copper oxide
nanoparticles mediated Annona muricata as cytotoxic and apoptosis
inducer factor in breast cancer cell lines. Sci Rep. 12:161652022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Woźniak-Budych MJ, Przysiecka Ł,
Maciejewska BM, Wieczorek D, Staszak K, Jarek M, Jesionowski T and
Jurga S: Facile synthesis of sulfobetaine-stabilized Cu2O
nanoparticles and their biomedical potential. ACS Biomater Sci Eng.
3:3183–3194. 2017. View Article : Google Scholar
|
|
43
|
Wozniak-Budych MJ, Langer K, Peplinska B,
Przysiecka L, Jarek M, Jarzebski M and Jurga S: Copper-gold
nanoparticles: Fabrication, characteristic and application as drug
carriers. Mater Chem Phys. 179:242–253. 2016. View Article : Google Scholar
|
|
44
|
Tortella GR, Pieretti JC, Rubilar O,
Fernández-Baldo M, Benavides-Mendoza A, Diez MC and Seabra AB:
Silver, copper and copper oxide nanoparticles in the fight against
human viruses: progress and perspectives. Crit Rev Biotechnol.
42:431–449. 2022. View Article : Google Scholar
|
|
45
|
Yuan Z, Qu S, He Y, Xu Y, Liang L, Zhou X,
Gui L, Gu Y and Chen H: Thermosensitive drug-loading system based
on copper sulfide nanoparticles for combined photothermal therapy
and chemotherapy in vivo. Biomater Sci. 6:3219–3230. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang M, Wang W, Cui Y, Chu X, Sun B, Zhou
N and Shen J: Magnetofluorescent Fe3O4/carbon quantum dots coated
single-walled carbon nanotubes as dual-modal targeted imaging and
chemo/photodynamic/photothermal triple-modal therapeutic agents.
Chem Eng J. 338:526–538. 2018. View Article : Google Scholar
|
|
47
|
Frtús A, Smolková B, Uzhytchak M, Lunova
M, Jirsa M, Kubinová Š, Dejneka A and Lunov O: Analyzing the
mechanisms of iron oxide nanoparticles interactions with cells: A
road from failure to success in clinical applications. J Control
Release. 328:59–77. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xuan S, Wang F, Lai JM, Sham KW, Wang YX,
Lee SF, Yu JC, Cheng CH and Leung KC: Synthesis of biocompatible,
mesoporous Fe(3)O(4) nano/microspheres with large surface area for
magnetic resonance imaging and therapeutic applications. ACS Appl
Mater Interfaces. 3:237–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Wang Z, Li X, Zhang Y, Yin M, Li
J, Song H, Shi J, Ling D, Wang L, et al: Deciphering active
biocompatibility of iron oxide nanoparticles from their intrinsic
antagonism. Nano Res. 11:2746–2755. 2018. View Article : Google Scholar
|
|
50
|
Jin R, Lin B, Li D and Ai H:
Superparamagnetic iron oxide nanoparticles for MR imaging and
therapy: Design considerations and clinical applications. Curr Opin
Pharmacol. 18:18–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochim Biophys
Acta-Gen Subj. 1861:1893–1900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen S, Yang J, Liang Z, Li Z, Xiong W,
Fan Q, Shen Z, Liu J and Xu Y: Synergistic functional nanomedicine
enhances ferroptosis therapy for breast tumors by a blocking
defensive redox system. ACS Appl Mater Interfaces. 15:2705–2713.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cormode DP, Gao L and Koo H: Emerging
biomedical applications of enzyme-like catalytic nanomaterials.
Trends Biotechnol. 36:15–29. 2018. View Article : Google Scholar
|
|
54
|
Zhou C, Wang C, Xu K, Niu Z, Zou S, Zhang
D, Qian Z, Liao J and Xie J: Hydrogel platform with tunable
stiffness based on magnetic nanoparticles cross-linked GelMA for
cartilage regeneration and its intrinsic biomechanism. Bioact
Mater. 25:615–628. 2023.PubMed/NCBI
|
|
55
|
Ashraf N, Ahmad F, Da-Wei L, Zhou RB,
Feng-Li H and Yin DC: Iron/iron oxide nanoparticles: Advances in
microbial fabrication, mechanism study, biomedical, and
environmental applications. Crit Rev Microbiol. 45:278–300. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Z, Sun Y, Shen J, Wei J, Yu C, Kong
B, Liu W, Yang H, Yang S and Wang W: Iron/iron oxide core/shell
nanoparticles for magnetic targeting MRI and near-infrared
photothermal therapy. Biomaterials. 35:7470–7478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang X, Liu Z, Lou Z, Chen F, Chang S,
Miao Y, Zhou Z, Hu X, Feng J, Ding Q, et al: Radiosensitivity
enhancement of Fe3O4@Ag nanoparticles on human glioblastoma cells.
Artif Cell Nanomed Biotechnol. 46(Supp1): 975–984. 2018. View Article : Google Scholar
|
|
58
|
Zhong D, Zhao J, Li Y, Qiao Y, Wei Q, He
J, Xie T, Li W and Zhou M: Laser-triggered aggregated cubic
α-Fe2O3@Au nanocomposites for magnetic resonance imaging and
photothermal/enhanced radiation synergistic therapy. Biomaterials.
219:1193692019. View Article : Google Scholar
|
|
59
|
Dennis CL and Ivkov R: Physics of heat
generation using magnetic nanoparticles for hyperthermia. Int J
Hyperthermia. 29:715–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Grauer O, Jaber M, Hess K, Weckesser M,
Schwindt W, Maring S, Wölfer J and Stummer W: Combined
intracavitary thermotherapy with iron oxide nanoparticles and
radiotherapy as local treatment modality in recurrent glioblastoma
patients. J Neurooncol. 141:83–94. 2019. View Article : Google Scholar :
|
|
61
|
Toraya-Brown S and Fiering S: Local tumour
hyperthermia as immunotherapy for metastatic cancer. Int J
Hyperthermia. 30:531–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Goldberg MS: Immunoengineering: How
nanotechnology can enhance cancer immunotherapy. Cell. 161:201–204.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zanganeh S, Hutter G, Spitler R, Lenkov O,
Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M,
et al: Iron oxide nanoparticles inhibit tumour growth by inducing
pro-inflammatory macrophage polarization in tumour tissues. Nat
Nanotechnol. 11:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ge X, Wong R, Anisa A and Ma S: Recent
development of metal-organic framework nanocomposites for
biomedical applications. Biomaterials. 281:1213222022. View Article : Google Scholar
|
|
65
|
Auer B, Telfer SG and Gross AJ: Metal
organic frameworks for bioelectrochemical applications.
Electroanalysis. 35:e2022001452023. View Article : Google Scholar
|
|
66
|
Kim SN, Park CG, Huh BK, Lee SH, Min CH,
Lee YY, Kim YK, Park KH and Choy YB: Metal-organic frameworks,
NH(2)-MIL-88(Fe), as carriers for ophthalmic delivery of
brimonidine. Acta Biomater. 79:344–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang B, Ding L, Yao H, Chen Y and Shi J: A
metal-organic framework (MOF) fenton nanoagent-enabled
nanocatalytic cancer therapy in synergy with autophagy inhibition.
Adv Mater. 32:e19071522020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu P, Shi X, Zhong S, Peng Y, Qi Y, Ding
J and Zhou W: Metal-phenolic networks for cancer theranostics.
Biomater Sci. 9:2825–2849. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Luo S, Ma D, Wei R, Yao W, Pang X, Wang Y,
Xu X, Wei X, Guo Y, Jiang X, et al: A tumor microenvironment
responsive nanoplatform with oxidative stress amplification for
effective MRI-based visual tumor ferroptosis. Acta Biomater.
138:518–527. 2022. View Article : Google Scholar
|
|
70
|
Farhood B, Najafi M and Mortezaee K: CD8+
cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell
Physiol. 234:8509–8521. 2019. View Article : Google Scholar
|
|
71
|
Lin MJ, Svensson-Arvelund J, Lubitz GS,
Marabelle A, Melero I, Brown BD and Brody JD: Cancer vaccines: the
next immunotherapy frontier. Nat Cancer. 3:911–926. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gupta M, Wahi A, Sharma P, Nagpal R, Raina
N, Kaurav M, Bhattacharya J, Rodrigues Oliveira SM, Dolma KG, Paul
AK, et al: Recent advances in cancer vaccines: Challenges,
achievements, and futuristic prospects. Vaccines (Basel).
10:20112022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Musetti S and Huang L:
Nanoparticle-mediated remodeling of the tumor microenvironment to
enhance immunotherapy. ACS Nano. 12:11740–11755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li J, Ren H and Zhang Y: Metal-based
nano-vaccines for cancer immunotherapy. Coord Chem Rev.
454:2143452022. View Article : Google Scholar
|
|
75
|
Pradeu T and Vivier E: The discontinuity
theory of immunity. Sci Immunol. 1:AAG04792016. View Article : Google Scholar
|
|
76
|
Duan X, Chan C and Lin W:
Nanoparticle-mediated immunogenic cell death enables and
potentiates cancer immunotherapy. Angew Chem Int Ed Engl.
58:670–680. 2019. View Article : Google Scholar
|
|
77
|
Yang Y, Huang CT, Huang X and Pardoll DM:
Persistent Toll-like receptor signals are required for reversal of
regulatory T cell-mediated CD8 tolerance. Nat Immunol. 5:508–515.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hanson MC, Crespo MP, Abraham W, Moynihan
KD, Szeto GL, Chen SH, Melo MB, Mueller S and Irvine DJ:
Nanoparticulate STING agonists are potent lymph node-targeted
vaccine adjuvants. J Clin Invest. 125:2532–2546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kaur A, Baldwin J, Brar D, Salunke DB and
Petrovsky N: Toll-like receptor (TLR) agonists as a driving force
behind next-generation vaccine adjuvants and cancer therapeutics.
Curr Opin Chem Biol. 70:1021722022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shinchi H, Yamaguchi T, Moroishi T, Yuki
M, Wakao M, Cottam HB, Hayashi T, Carson DA and Suda Y: Gold
nanoparticles coimmobilized with small molecule toll-like receptor
7 ligand and α-mannose as adjuvants. Bioconjugate Chem.
30:2811–2821. 2019. View Article : Google Scholar
|
|
81
|
Ishikawa H and Barber GN: STING is an
endoplasmic reticulum adaptor that facilitates innate immune
signalling. Nature. 455:674–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Motedayen Aval L, Pease JE, Sharma R and
Pinato DJ: Challenges and opportunities in the clinical development
of STING agonists for cancer immunotherapy. J Clin Med. 9:33232020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ding B, Zheng P, Jiang F, Zhao Y, Wang M,
Chang M, Ma P and Lin J: MnOx nanospikes as nanoadjuvants and
immunogenic cell death drugs with enhanced antitumor immunity and
antimetastatic effect. Angew Chem Int Ed Engl. 59:16381–16384.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Slingluff CL Jr, Petroni GR, Olson WC,
Smolkin ME, Ross MI, Haas NB, Grosh WW, Boisvert ME, Kirkwood JM
and Chianese-Bullock KA: Effect of granulocyte/macrophage
colony-stimulating factor on circulating CD8+ and CD4+ T-cell
responses to a multipeptide melanoma vaccine: Outcome of a
multicenter randomized trial. Clin Cancer Res. 15:7036–7044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Serafini P, Carbley R, Noonan KA, Tan G,
Bronte V and Borrello I: High-dose granulocyte-macrophage
colony-stimulating factor-producing vaccines impair the immune
response through the recruitment of myeloid suppressor cells.
Cancer Res. 64:6337–6343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Behzadi M, Vakili B, Ebrahiminezhad A and
Nezafat N: Iron nanoparticles as novel vaccine adjuvants. Eur J
Pharm Sci. 159:1057182021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang X, Li X, Onuma K, Sogo Y, Ohno T and
Ito A: Zn- and Mg- containing tricalcium phosphates-based adjuvants
for cancer immunotherapy. Sci Rep. 3:22032013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shukla R, Bansal V, Chaudhary M, Basu A,
Bhonde RR and Sastry M: Biocompatibility of gold nanoparticles and
their endocytotic fate inside the cellular compartment: A
microscopic overview. Langmuir. 21:10644–10654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dobrovolskaia MA and McNeil SE:
Immunological properties of engineered nanomaterials. Nat
Nanotechnol. 2:469–478. 2007. View Article : Google Scholar
|
|
90
|
Tao Y, Ju E, Li Z, Ren J and Qu X:
Engineered CpG- antigen conjugates protected gold nanoclusters as
smart self-vaccines for enhanced immune response and cell imaging.
Adv Funct Mater. 24:1004–1010. 2014. View Article : Google Scholar
|
|
91
|
Zhang P, Chiu YC, Tostanoski LH and Jewell
CM: Polyelectrolyte multilayers assembled entirely from immune
signals on gold nanoparticle templates promote antigen-specific T
cell response. ACS Nano. 9:6465–6477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y,
Zhou H, Peng R, Wang J and Liu Z: Nanoscale coordination polymer
based nanovaccine for tumor immunotherapy. ACS Nano.
13:13127–13135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jin C, Zhang Y, Zhang G, Wang B and Hua P:
Combination of GNRs-PEI/cGAMP-laden macrophages-based photothermal
induced in situ tumor vaccines and immune checkpoint blockade for
synergistic anti-tumor immunotherapy. Biomater Adv. 133:1126032022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen F, Li T, Zhang H, Saeed M, Liu X,
Huang L, Wang X, Gao J, Hou B, Lai Y, et al: Acid-ionizable iron
nanoadjuvant augments STING activation for personalized vaccination
immunotherapy of cancer. Adv Mater. 35:e22099102023. View Article : Google Scholar
|
|
95
|
Ljunggren HG, Jonsson R and Höglund P:
Seminal immunologic discoveries with direct clinical implications:
The 2018 Nobel Prize in Physiology or Medicine honours discoveries
in cancer immunotherapy. Scand J Immunol. 88:e127312018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xie L, Wang G, Sang W, Li J, Zhang Z, Li
W, Yan J, Zhao Q and Dai Y: Phenolic immunogenic cell death
nanoinducer for sensitizing tumor to PD-1 checkpoint blockade
immunotherapy. Biomaterials. 269:1206382021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sang W, Zhang Z, Wang G, Xie L, Li J, Li
W, Tian H and Dai L: A triple-kill strategy for tumor eradication
reinforced by metal-phenolic network nanopumps. Adv Funct Mater.
32:21131682022. View Article : Google Scholar
|
|
98
|
Mayes PA, Hance KW and Hoos A: The promise
and challenges of immune agonist antibody development in cancer.
Nat Rev Drug Discov. 17:509–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lim SH, Beers SA, Al-Shamkhani A and Cragg
MS: Agonist antibodies for cancer immunotherapy: History, hopes and
challenges. Clin Cancer Res. 30:1712–1723. 2023. View Article : Google Scholar
|
|
100
|
Boomer JS and Green JM: An enigmatic tail
of CD28 signaling. Cold Spring Harb Perspect Biol. 2:a0024362010.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Q and Vignali DA: Co-stimulatory and
co-inhibitory pathways in autoimmunity. Immunity. 44:1034–1051.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yoshinaga SK, Whoriskey JS, Khare SD,
Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T,
et al: T-cell co-stimulation through B7RP-1 and ICOS. Nature.
402:827–832. 1999. View
Article : Google Scholar
|
|
103
|
Chiang CS, Lin YJ, Lee R, Lai YH, Cheng
HW, Hsieh CH, Shyu WC and Chen SY: Combination of fucoidan-based
magnetic nanoparticles and immunomodulators enhances
tumour-localized immunotherapy. Nat Nanotechnol. 13:746–754. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vonderheide RH and Glennie MJ: Agonistic
CD40 antibodies and cancer therapy. Clin Cancer Res. 19:1035–1043.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang Y, Zhao G, Chen YF, Zhou SK, Wang Y,
Sun YQ, Shen S, Xu CF and Wang J: Engineering nano-clustered
multivalent agonists to cross-link TNF receptors for cancer
therapy. Aggregate. 4:e3932023. View Article : Google Scholar
|
|
107
|
Gupta J, Safdari HA and Hoque M:
Nanoparticle mediated cancer immunotherapy. Semin Cancer Biol.
69:307–324. 2021. View Article : Google Scholar
|
|
108
|
June CH, Riddell SR and Schumacher TN:
Adoptive cellular therapy: A race to the finish line. Sci Transl
Med. 7:280ps72015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Krishna S, Lowery FJ, Copeland AR,
Bahadiroglu E, Mukherjee R, Jia L, Anibal JT, Sachs A, Adebola SO,
Gurusamy D, et al: Stem-like CD8 T cells mediate response of
adoptive cell immunotherapy against human cancer. Science.
370:1328–1334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
June CH, O'Connor RS, Kawalekar OU,
Ghassemi S and Milone MC: CAR T cell immunotherapy for human
cancer. Science. 359:1361–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Met Ö, Jensen KM, Chamberlain CA, Donia M
and Svane IM: Principles of adoptive T cell therapy in cancer.
Semin Immunopathol. 41:49–58. 2019. View Article : Google Scholar
|
|
112
|
Wang C, Sun W, Ye Y, Bomba HN and Gu Z:
Bioengineering of artificial antigen presenting cells and lymphoid
organs. Theranostics. 7:3504–3516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ichikawa J, Yoshida T, Isser A, Laino AS,
Vassallo M, Woods D, Kim S, Oelke M, Jones K, Schneck JP and Weber
JS: Rapid expansion of highly functional antigen-specific T cells
from patients with melanoma by nanoscale artificial
antigen-presenting cells. Clin Cancer Res. 26:3384–3396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zheng C, Zhang J, Chan HF, Hu H, Lv S, Na
N, Tao Y and Li M: Engineering nano-therapeutics to boost adoptive
cell therapy for cancer treatment. Small Methods. 5:e20011912021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Youn W, Ko EH, Kim MH, Park M, Hong D,
Seisenbaeva GA, Kessler VG and Choi IS: Cytoprotective
encapsulation of individual jurkat T cells within durable TiO2
Shells for T-cell therapy. Angew Chem Int Ed Engl. 56:10702–10706.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nie W, Wei W, Zuo L, Lv C, Zhang F, Lu GH,
Li F, Wu G, Huang LL, Xi X and Xie HY: Magnetic nanoclusters armed
with responsive PD-1 antibody synergistically improved adoptive
T-cell therapy for solid tumors. ACS Nano. 13:1469–1478. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Parkhurst MR, Riley JP, Dudley ME and
Rosenberg SA: Adoptive transfer of autologous natural killer cells
leads to high levels of circulating natural killer cells but does
not mediate tumor regression. Clin Cancer Res. 17:6287–6297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Baruch EN, Berg AL, Besser MJ, Schachter J
and Markel G: Adoptive T cell therapy: An overview of obstacles and
opportunities. Cancer. 123:2154–2162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lin X, Li F, Gu Q, Wang X, Zheng Y, Li J,
Guan J, Yao C and Liu X: Gold-seaurchin based immunomodulator
enabling photothermal intervention and αCD16 transfection to boost
NK cell adoptive immunotherapy. Acta Biomater. 146:406–420. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kong C and Chen X: Combined photodynamic
and photothermal therapy and immunotherapy for cancer treatment: A
review. Int J Nanomedicine. 17:6427–6446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xiong Y, Rao Y, Hu J, Luo Z and Chen C:
Nanoparticle-based photothermal therapy for breast cancer
noninvasive treatment. Adv Mater. 37:e23051402025. View Article : Google Scholar
|
|
122
|
Paholak HJ, Stevers NO, Chen H, Burnett
JP, He M, Korkaya H, McDermott SP, Deol Y, Clouthier SG, Luther T,
et al: Elimination of epithelial-like and mesenchymal-like breast
cancer stem cells to inhibit metastasis following
nanoparticle-mediated photothermal therapy. Biomaterials.
104:145–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Song G, Shao X, Qu C, Shi D, Jia R, Chen
Y, Wang J and An H: A large-pore mesoporous Au@Pt@Rh trimetallic
nanostructure with hyperthermia-enhanced enzyme-mimic activities
for immunomodulation-improved tumor catalytic therapy. Chem Eng J.
477:1471612023. View Article : Google Scholar
|
|
124
|
Duan X, Chan C, Guo N, Han W, Weichselbaum
RR and Lin W: Photodynamic therapy mediated by nontoxic core-shell
nanoparticles synergizes with immune checkpoint blockade to elicit
antitumor immunity and antimetastatic effect on breast cancer. J Am
Chem Soc. 138:16686–16695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Thariat J, Hannoun-Levi JM, Sun Myint A,
Vuong T and Gérard JP: Past, present, and future of radiotherapy
for the benefit of patients. Nat Rev Clin Oncol. 10:52–60. 2013.
View Article : Google Scholar
|
|
126
|
Wardman P: Chemical radiosensitizers for
use in radiotherapy. Clin Oncol (R Coll Radiol). 19:397–417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ni K, Lan G, Chan C, Quigley B, Lu K, Aung
T, Guo N, La Riviere P, Weichselbaum RR and Lin W: Nanoscale
metal-organic frameworks enhance radiotherapy to potentiate
checkpoint blockade immunotherapy. Nat Commun. 9:23512018.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Xu CF, Yu YL, Sun Y, Kong L, Yang CL, Hu
M, Yang T, Zhang J, Hu Q and Zhang Z: Transformable
nanoparticle-enabled synergistic elicitation and promotion of
immunogenic cell death for triple-negative breast cancer
immunotherapy. Adv Funct Mater. 29:19052132019. View Article : Google Scholar
|
|
129
|
Liu Y, Qiao L, Zhang S, Wan G, Chen B,
Zhou P, Zhang N and Wang Y: Dual pH-responsive multifunctional
nanoparticles for targeted treatment of breast cancer by combining
immunotherapy and chemotherapy. Acta Biomater. 66:310–324. 2018.
View Article : Google Scholar
|
|
130
|
Bobo D, Robinson KJ, Islam J, Thurecht KJ
and Corrie SR: Nanoparticle-based medicines: A Review of
FDA-approved materials and clinical trials to date. Pharm Res.
33:2373–2387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fenton OS, Olafson KN, Pillai PS, Mitchell
MJ and Langer R: Advances in biomaterials for drug delivery. Adv
Mater. May 7–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Anselmo AC and Mitragotri S: Nanoparticles
in the clinic: An update. Bioeng Transl Med. 4:e101432019.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Huang Y, Hsu JC, Koo H and Cormode DP:
Repurposing ferumoxytol: Diagnostic and therapeutic applications of
an FDA-approved nanoparticle. Theranostics. 12:796–816. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Khoobchandani M, Katti KK, Karikachery AR,
Thipe VC, Srisrimal D, Dhurvas Mohandoss DK, Darshakumar RD, Joshi
CM and Katti KV: New approaches in breast cancer therapy through
green nanotechnology and nano-ayurvedic medicine - pre-clinical and
pilot human clinical investigations. Int J Nanomedicine.
15:181–197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kumthekar P, Ko CH, Paunesku T, Dixit K,
Sonabend AM, Bloch O, Tate M, Schwartz M, Zuckerman L, Lezon R, et
al: A first-in-human phase 0 clinical study of RNA
interference-based spherical nucleic acids in patients with
recurrent glioblastoma. Sci Transl Med. 13:eabb39452021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rojas LA, Sethna Z, Soares KC, Olcese C,
Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, et al:
Personalized RNA neoantigen vaccines stimulate T cells in
pancreatic cancer. Nature. 618:144–150. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Alonso JCC, de Souza BR, Reis IB, de
Arruda Camargo GC, de Oliveira G, de Barros Frazão Salmazo MI,
Gonçalves JM, de Castro Roston JR, Caria PHF, da Silva Santos A, et
al: OncoTherad() (MRB-CFI-1) Nanoimmunotherapy: A promising
strategy to treat bacillus calmette-guérin-unresponsive
non-muscle-invasive bladder cancer: Crosstalk among T-Cell CX3CR1,
immune checkpoints, and the toll-like receptor 4 signaling pathway.
Int J Mol Sci. 24:175352023. View Article : Google Scholar
|
|
138
|
Zhang G, Yuan J, Pan C, Xu Q, Cui X, Zhang
J, Liu M, Song Z, Wu L, Wu D, et al: Multi-omics analysis uncovers
tumor ecosystem dynamics during neoadjuvant toripalimab plus
nab-paclitaxel and S-1 for esophageal squamous cell carcinoma: A
single-center, open-label, single-arm phase 2 trial. EBioMedicine.
90:1045152023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Blanco E, Shen H and Ferrari M: Principles
of nanoparticle design for overcoming biological barriers to drug
delivery. Nat Biotechnol. 33:941–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Mitchell MJ, Billingsley MM, Haley RM,
Wechsler ME, Peppas NA and Langer R: Engineering precision
nanoparticles for drug delivery. Nat Rev Drug Discov. 20:101–124.
2021. View Article : Google Scholar
|
|
141
|
Hosta-Rigau L and Städler B: Shear stress
and its effect on the interaction of myoblast cells with nanosized
drug delivery vehicles. Mol Pharmaceut. 10:2707–2712. 2013.
View Article : Google Scholar
|
|
142
|
Moghimi SM and Szebeni J: Stealth
liposomes and long circulating nanoparticles: Critical issues in
pharmacokinetics, opsonization and protein-binding properties. Prog
Lipid Res. 42:463–478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Nizzero S, Ziemys A and Ferrari M:
Transport barriers and oncophysics in cancer treatment. Trends
Cancer. 4:277–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wagner AM, Gran MP and Peppas NA:
Designing the new generation of intelligent biocompatible carriers
for protein and peptide delivery. Acta Pharm Sin B. 8:147–164.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hirai T, Yoshioka Y, Izumi N, Ichihashi K,
Handa T, Nishijima N, Uemura E, Sagami K, Takahashi H, Yamaguchi M,
et al: Metal nanoparticles in the presence of lipopolysaccharides
trigger the onset of metal allergy in mice. Nat Nanotechnol.
11:808–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Radulescu DM, Surdu VA, Ficai A, Ficai D,
Grumezescu AM and Andronescu E: Green synthesis of metal and metal
oxide nanoparticles: A review of the principles and biomedical
applications. Int J Mol Sci. 24:153972023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chithrani BD, Ghazani AA and Chan WC:
Determining the size and shape dependence of gold nanoparticle
uptake into mammalian cells. Nano Lett. 6:662–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wills JW, Summers HD, Hondow N, Sooresh A,
Meissner KE, White PA, Rees P, Brown A and Doak SH: Characterizing
nanoparticles in biological matrices: Tipping points in
agglomeration state and cellular delivery in vitro. ACS Nano.
11:11986–12000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Agarwal R, Jurney P, Raythatha M, Singh V,
Sreenivasan SV, Shi L and Roy K: Effect of shape, size, and aspect
ratio on nanoparticle penetration and distribution inside solid
tissues using 3D spheroid models. Adv Healthc Mater. 4:2269–2280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kou L, Bhutia YD, Yao Q, He Z, Sun J and
Ganapathy V: Transporter-guided delivery of nanoparticles to
improve drug permeation across cellular barriers and drug exposure
to selective cell types. Front Pharmacol. 9:272018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li W, Jiang Y and Lu J:
Nanotechnology-enabled immunogenic cell death for improved cancer
immunotherapy. Int J Pharm. 634:1226552023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mulens-Arias V, Rojas JM and Barber DF:
The use of iron oxide nanoparticles to reprogram macrophage
responses and the immunological tumor microenvironment. Front
Immunol. 12:6937092021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Younis MA, Tawfeek HM, Abdellatif AAH,
Abdel-Aleem JA and Harashima H: Clinical translation of
nanomedicines: Challenges, opportunities, and keys. Adv Drug Deliv
Rev. 181:1140832022. View Article : Google Scholar
|
|
154
|
Almeida JPM, Lin AY, Figueroa ER, Foster
AE and Drezek RA: In vivo gold nanoparticle delivery of peptide
vaccine induces anti-tumor immune response in prophylactic and
therapeutic tumor models. Small. 11:1453–1459. 2015. View Article : Google Scholar :
|
|
155
|
Johannsen M, Gneveckow U, Thiesen B,
Taymoorian K, Cho CH, Waldöfner N, Scholz R, Jordan A, Loening SA
and Wust P: Thermotherapy of prostate cancer using magnetic
nanoparticles: Feasibility, imaging, and three-dimensional
temperature distribution. Eur Urol. 52:1653–1661. 2007. View Article : Google Scholar
|
|
156
|
Maier-Hauff K, Ulrich F, Nestler D,
Niehoff H, Wust P, Thiesen B, Orawa H, Budach V and Jordan A:
Efficacy and safety of intratumoral thermotherapy using magnetic
iron-oxide nanoparticles combined with external beam radiotherapy
on patients with recurrent glioblastoma multiforme. J Neurooncol.
103:317–324. 2011. View Article : Google Scholar :
|
|
157
|
Coyne DW: Ferumoxytol for treatment of
iron deficiency anemia in patients with chronic kidney disease.
Expert Opin Pharmacother. 10:2563–2568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shah A and Dobrovolskaia MA: Immunological
effects of iron oxide nanoparticles and iron-based complex drug
formulations: Therapeutic benefits, toxicity, mechanistic insights,
and translational considerations. Nanomedicine. 14:977–990. 2018.
View Article : Google Scholar : PubMed/NCBI
|