Biliary tract cancer (BTC) includes a spectrum of
invasive malignancies that arise from the bile duct epithelium. It
is relatively uncommon but highly heterogeneous, comprising
gallbladder cancer (GBC; which arises from the gallbladder or
cystic duct) and cholangiocarcinoma (CCA; which arises from the
intrahepatic, perihilar or distal biliary tree). BTC accounts for
<1% of all human types of cancer and ~3% of digestive system
tumors globally (1,2). In recent years, the incidence of BTC
has been increasing globally, particularly in Asian countries,
where it represents an important health problem (3,4).
Globally, adenocarcinoma accounts for ~90% of all BTC cases
(5,6). BTC is usually diagnosed at advanced
stage. The majority of patients with symptomatic BTC, such as those
presenting with biliary obstruction, have incurable tumors with
invasion of the surrounding organs, lymph nodes and distant
metastases, which results in poor prognoses (2,7).
At present, surgery remains the gold standard for treating
early-stage tumors; however, only a limited number of patients have
this opportunity (8,9). The 5-year overall survival rate of
BTC is <20% for patients from the United States of America and
Europe (10). Furthermore,
despite undergoing curative resection with a negative margin (R0),
BTC still have a high recurrence rate of >50% (8,9,11).
Gemcitabine-platinum chemotherapy is a recommended first-line
treatment for advanced BTC; however, its efficacy is not
satisfactory (12). The emergence
of targeted therapies and immunotherapies, such as Durvalumab and
Pembrolizumab, has revolutionized the treatment paradigm of
malignant tumors. Immunotherapy plus chemotherapy has been verified
to be effective in treating BTC (13-15). Investigating the key molecular
mechanisms and identifying novel therapeutic targets for BTC is
imperative.
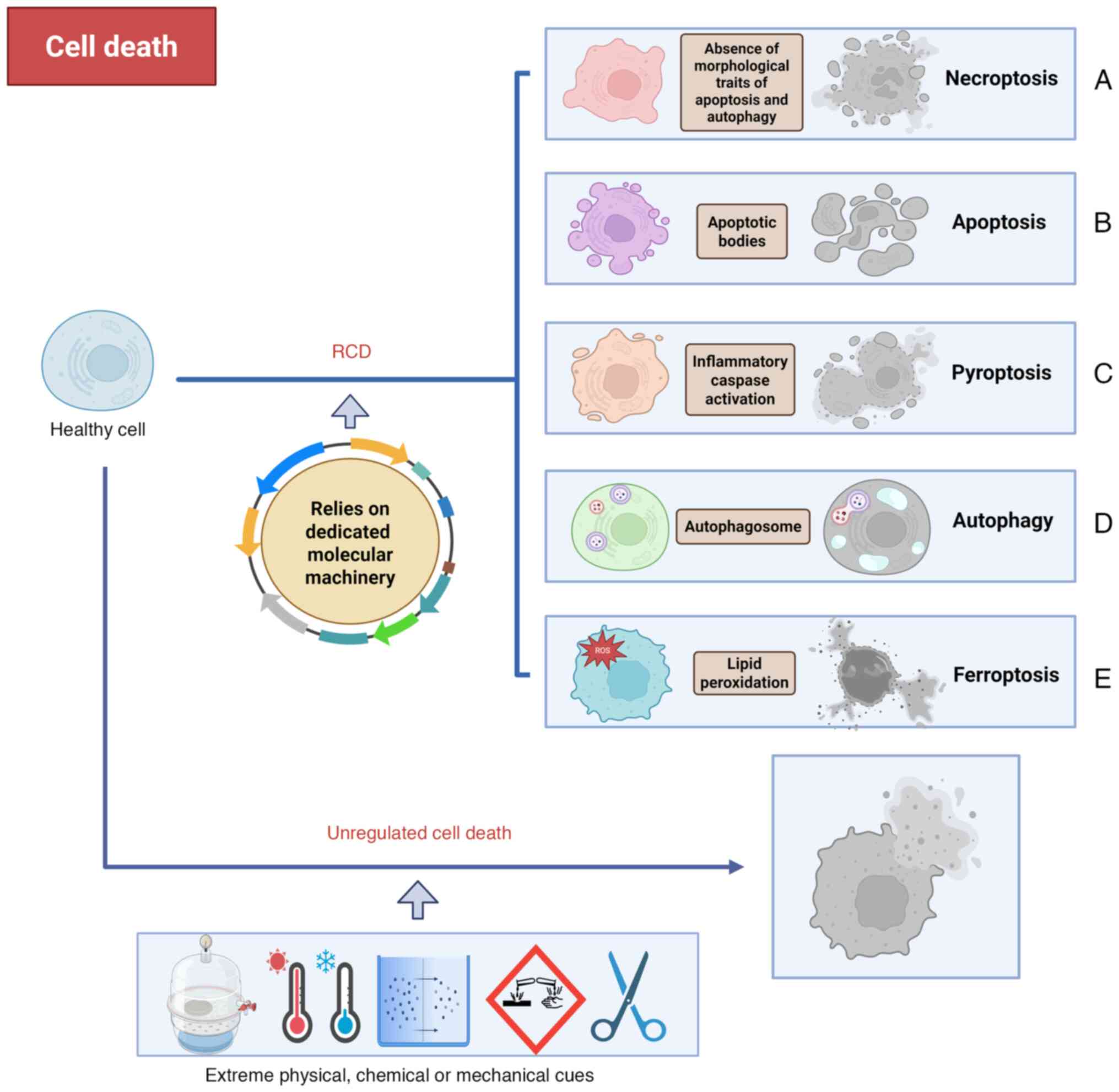
Cell death can occur either due to unregulated
causes or following a regulated process (Fig. 1) (16). Unregulated cell death is the
instantaneous demise of cells due to severe physical, chemical or
mechanical causes. Unlike unregulated cell death, regulated cell
death (RCD) is characterized by organized signaling cascades and
specific molecular mechanisms, suggesting that it can be controlled
(17). The process of RCD can be
influenced, either delayed or accelerated, through pharmacological
or genetic interventions. A study by Dixon et al (18) first proposed the notion of
'ferroptosis' in 2012 (19).
Ferroptosis is a new type of RCD, differing from necroptosis
(Fig. 1A) (20), apoptosis (Fig. 1B) (21), pyroptosis (Fig. 1C) (22) and autophagy (Fig. 1D) (23) in morphology, genetics and
biochemistry (24,25). As its name implies, ferroptosis is
an iron-dependent mechanism of cell death (26). Ferroptosis is triggered by lipid
peroxidation (LPO), which results from an imbalanced cellular
metabolism and redox homeostasis. The morphological evidence of
ferroptosis includes the presence of small mitochondria with
increased mitochondrial membrane densities, reduced or missing
mitochondrial crista, outer mitochondrial membrane rupture and a
normal nucleus (Fig. 1E)
(27,28). Ferroptosis is indicated to serve
an important pathophysiological role in the development and
occurrence of numerous diseases, such as ischemic heart disease and
acute kidney injury, but particularly in cancer (19,29). Non-small cell lung cancer,
hepatocellular carcinoma, pancreatic cancer, breast cancer and
renal cell carcinoma are reported to be sensitive to ferroptosis
(30-34). Additionally, various ferroptosis
inducers, such as sorafenib, sulfasalazine and artemisinin,
demonstrate a mitigation of tumor progression (35). Due to the issue of drug
resistance, there is a growing interest regarding the potential of
targeting ferroptosis as a therapeutic strategy for malignancies
(36-38).
The present review aimed to provide a comprehensive
overview of the signaling pathways and molecular mechanisms of
ferroptosis, the research progress on ferroptosis in the occurrence
and development of BTC, and the potential application of
ferroptosis as a targeted therapy for BTC.
Following the first description of ferroptosis, a
form of non-apoptosis and iron-dependent RCD, in 2012, there has
been an increasing interest to investigate the process and
regulation of ferroptosis (39).
Previously, several studies investigated the mechanisms involved in
ferroptosis (40-42). The present study aimed to review
the core mechanisms of ferroptosis based on three aspects, namely
iron metabolism, lipid metabolism and antioxidant defense
systems.
Iron is one of the crucial minor elements for human
health, and the correct intracellular level of iron is
indispensable for a well-functioning metabolic balance (43). Iron is mainly obtained from
dietary intake, existing in two distinct oxidation states. These
are the divalent (Fe2+) and trivalent (Fe3+)
ions (44). The continuous
interconversion of Fe2+ and Fe3+ allows
iron-dependent cofactors, such as cytochrome P450s, to effectively
carry out their catalytic functions in various biological
processes, including nucleic acid synthesis as well as repair,
epigenetic regulation and cellular respiration (45). While in the Fe2+ state
or combined with a transporter protein, iron is primarily absorbed
in the duodenum and upper jejunum epithelial cells. The less
soluble Fe3+ ion must first be reduced to absorbable
ferrous Fe2+ and is then transported via divalent metal
transporter 1 (DMT1) into epithelial cells (46). Absorbed iron then either enters
the blood circulation via ferroportin (FPN) or is stored as
ferritin. Fe3+ is the primary form of iron in blood
circulation. FPN, encoded by the transporter solute carrier family
40 member 1 gene, is the only known iron-exporting protein in
mammalian cells. It is responsible for exporting Fe3+ to
the extracellular space (47).
Subsequently, multi-copper ferroxidases on the epithelial cell
membrane re-oxidize Fe2+ to Fe3+, which is
then bound by transferrin (TF) and transported in the plasma
(44). Lactotransferrin also
contributes positively to the regulation of iron absorption by
enhancing iron uptake, similar to TF (48). The majority of cells mainly obtain
non-heme iron through two primary pathways that involve TF-bound
iron uptake and non-TF-bound iron (NTBI) uptake. TF binds to
transferrin receptor (TFR)1 and forms endosomes through
clathrin-dependent endocytosis. Within cells, TF-bound iron
separates, and six-transmembrane epithelial antigen of prostate 3
reduces Fe3+ to Fe2+, which then enters the
cytoplasm via DMT1 for subsequent use or storage as ferritin
(49,50). When there is an iron overload,
such as in hemochromatosis, excess iron surpasses the capacity of
TF, resulting in the increased circulation and uptake of NTBI
(forming an unstable iron pool) through transporters such as solute
carrier family 39 member 14 (51). The main repository for
intracellular iron is the ferritin dimer, consisting of ferritin
heavy and light chains. Nuclear receptor coactivator 4 (NCOA4)
increases iron levels by facilitating ferritin degradation
(52-54).
Ferroptosis relies on iron, as suggested by its
name. An elevated iron level in the body has a direct association
with the onset of ferroptosis (18). Excessive iron levels increase the
production of reactive oxygen species (ROS), leading to cell
dysfunction or death, tissue damage and diseases, such as human
leukocyte antigen-linked hemochromatosis and Friedreich ataxia
(44). An excessive accumulation
of iron can result in ferroptosis either through Fenton reactions
or by activating arachidonate lipoxygenases and cytochrome P450
oxidoreductase-mediated phospholipid peroxidation metabolism
(55,56). As an important step in
ferroptosis, the Fenton reaction involves the non-enzymatic
oxidation of organic compounds, such as polyunsaturated fatty acids
(PUFAs), into inorganic states by a mixture of hydrogen peroxide
(H2O2) and Fe2+ (37). Glutathione peroxidase 4 (GPX4) is
the major neutralizing enzyme of phospholipid (PL) hydroperoxides
(OOHs; PLOOHs). GPX4 knockdown results in an accumulation of
PLOOHs, which induces an increase in lipid peroxidation and
ferroptosis (57).
Uncontrolled LPO represents a key hallmark of
ferroptosis. A key initiation step of ferroptosis involves an
excessive oxidation of PUFA-PLs (19). The susceptibility of PUFAs to
peroxidation is attributed to their diallyl moieties (55). The magnitude of LPO is influenced
by both the abundance and distribution of PUFAs within
phospholipids (28). Arachidonic
acid (AA) and adrenic acid (AdA) serve as the primary LPO
substrates in ferroptosis. Acyl-CoA synthetase long-chain family
member 4 (ACSL4) catalyzes the esterification of AA or AdA with
CoA, generating oxidizable AA- or AdA-CoA. Subsequently,
lysophosphatidylcholine acyltransferase 3 (LPCAT3) facilitates the
PL remodeling of AA- or AdA-CoA and membrane
phosphatidylethanolamine (PE) into AA- or AdA-PE, anchoring it to
the cell or mitochondrial membranes (58). Conversely, acyl-CoA synthetase
long-chain family member 3 (ACSL3) and stearoyl-CoA desaturase 1
(SCD1) confer cellular resistance to ferroptosis by catalyzing the
synthesis of monounsaturated fatty acid (MUFA) and the displacement
of PUFAs from plasma membrane phospholipids (55,59,60). Subsequently, 15-lipoxygenase (LOX)
then oxidizes AA-PE or AdA-PE to PL-PUFA-OOHs, which acts as a
ferroptosis signal (58).
However, 12-LOX can mediate the ACSL4-independent pathway of LPO
production via the p53/solute carrier family 7 member 11
(SLC7A11)/12-LOX axis (61).
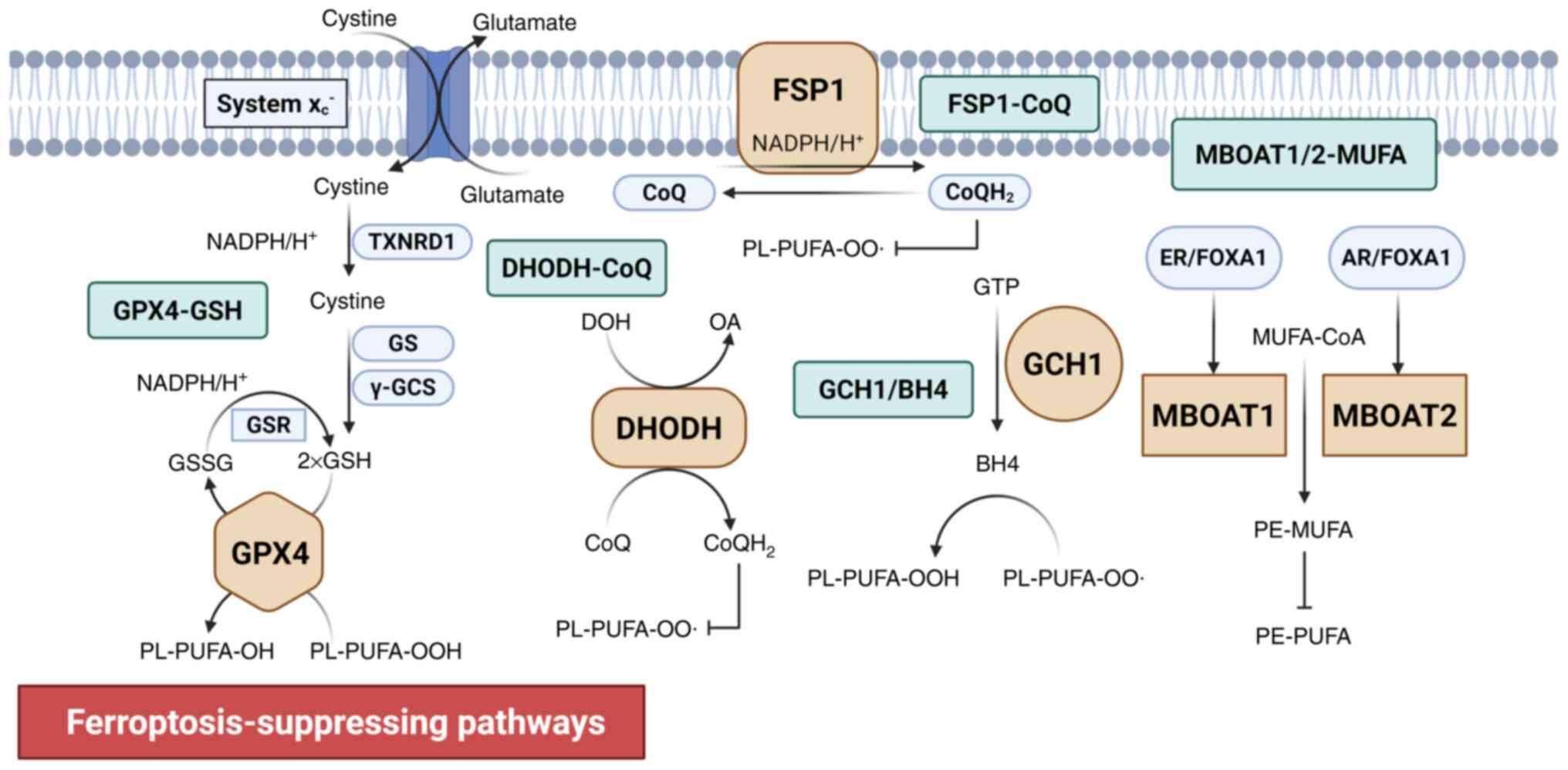
FSP1 is a GSH-independent ferroptosis suppressor,
acting in parallel to GPX4. When GPX4 is knocked out, FSP1 (also
known as apoptosis-inducing factor mitochondrial-associated protein
2) is activated as a compensatory antioxidant system. FSP1 is
located on the plasma membrane and uses NAD(P)H to reduce CoQ to
ubiquinol (CoQH2), which acts as an antioxidant by
binding to radicals. This neutralizes lipid peroxide free radicals
and blocks the propagation of LPO (66,67) (Fig.
2).
The GCH1-BH4 system is another important
GPX4-independent ferroptosis inhibitor. GCH1 prevents ferroptosis
via the synthesis of its metabolic derivatives, BH4 and
dihydrobiopterin (BH2), and lipid remodeling. GCH1 overexpression
selectively protects those PLs containing two PUFA chains from
degradation, which potentially has two simultaneous mechanisms: i)
Acting as an antioxidant that directly binds to radicals; and ii)
contributing to the synthesis of CoQ (68,69) (Fig.
2).
As PL-modifying enzymes, MBOAT1 and MBOAT2 function
as GPX4/FSP1-independent ferroptosis suppressors. MBOAT1 and 2
inhibit ferroptosis by remodeling PLs by selectively incorporating
MUFAs into lyso-PE. This increases the cellular MUFA-PE levels and
decreases the PUFA-PE levels (71). However, MBOAT1 and 2 function as
sex hormone-dependent regulators, and their transcription is
upregulated by androgen and estrogen receptors (71) (Fig.
2).
The TME serves pivotal roles in the initiation,
progression, metastasis and therapeutic resistance of tumors
(72-75). It is a dynamic interacting network
that is comprised of diverse cellular components, including
malignant cells, immune cells, cancer-associated fibroblasts and
endothelial cells, alongside acellular elements such as the
extracellular matrix, cytokines and metabolites (76). Ferroptosis engages in a complex
bidirectional crosstalk with the TME, particularly through
metabolic reprogramming and immunomodulation, which regulates BTC
malignancy and treatment responses (77,78).
Ferroptosis in malignant cells modulates antitumor
immunity within the TME through immunogenic signaling (79,80). Previous evidence indicates that
ferroptotic cancer cells release damage-associated molecular
patterns (DAMPs), a hallmark of immunogenic cell death (ICD), which
function as endogenous adjuvants to potentiate antitumor immunity
(81,82). As well as the classic DAMPs, such
as high mobility group protein 1 (83-85), adenosine triphosphate (86,87), calcium reticulum protein (84,86) and the recently identified
proteoglycan decorin (88),
ferroptotic cancer cells release immunomodulatory cytokines such as
C-X-C motif chemokine ligand 1 (CXCL1), tumor necrosis factor (TNF)
and interferon (IFN)-β, that contribute to the remodeling of the
TME (89). However, whether
ferroptosis is a classic form of ICD is still an ongoing scientific
debate (89). In addition, the
specific role of ferroptotic cancer cells in antitumor immunity is
also contradictory. A previous study demonstrates that tumor cell
competition for cystine compromises the function of CD8+ T cells
within the TME. Cystine deprivation triggers glutamate
accumulation, which potentiates CD36-mediated LPO (90). However, ferroptotic cells can
activate antitumor immunity by expressing
1-steaoryl-2-15-HpETE-sn-glycero-3-PE. This serves as a signal that
mediates phagocytosis by engaging Toll-like receptor 2 on
macrophages (91) and enhancing
the activities of natural killer cells (92).
Immune cells exhibit differential susceptibility to
ferroptosis within the TME, and their functional heterogeneity
enables distinct populations to either potentiate or suppress
ferroptosis in malignant cells (80). CD8+ T cells exhibit an increased
sensitivity to GPX4 inhibition compared with malignant cells,
resulting in a premature impairment of antitumor immunity prior to
notable tumor cell death (93).
However, these effector lymphocytes demonstrate relative resistance
to system Xc− inhibitors, such as sulfasalazine, as
inhibition of system Xc− did not affect T cell
proliferation and antitumor immune responses (94). Activated CD8+ T cells with high
expression levels of fatty acid transporter CD36 increases the risk
of LPO and ferroptosis (95).
Compared with tumor-derived CD8+ T cells, tumor-infiltrating
regulatory T cells (Tregs) typically have reduced levels of LPO,
suggesting that they are less prone to ferroptosis in the TME
(93). However, system
Xc− inhibitors rarely impair the viability of Tregs
(96). Tumor-associated
macrophages (TAMs) are one of the most abundant types of immune
cells in the TME and include the proinflammatory antitumor M1 and
anti-inflammatory protumor M2 types (97,98). The sensitivity of these two types
of cells to ferroptosis is different due to the high levels of
inducible nitric oxide synthase and nitric oxide-free radicals in
M1 type compared with M2 type TAMs (99). Another group of immune-suppressive
cells, namely myeloid-derived suppressor cells (MDSCs), exhibit
notable resistance to ferroptosis, which is associated with the
upregulation of system Xc− and neutral ceramidase
N-acylsphingosine amidohydrolase expression levels (100).
Metabolic reprogramming is a defining hallmark of
malignancy, triggering cancer metastasis, aggressiveness and
therapeutic resistance (101).
Remodeled metabolic environments and immunosuppressive environments
enable cancer cells to evade RCD (78). This metabolic reprogramming
extends beyond canonical adaptations such as the Warburg effect (in
which preferential glycolysis reduces mitochondrial ROS
generation), and includes the suppression of pyruvate
dehydrogenase, limiting the acetyl-CoA flux for PUFA biosynthesis,
which is critical for ferroptosis (102-104). Within the TME, MDSCs increase
immunosuppression through IL-6/JAK2/STAT3-mediated SLC7A11
upregulation, which depletes the extracellular cystine pools
(105). This metabolic
competition induces a GSH deprivation-induced ferroptosis in CD8+ T
cells, further inactivating antitumor immunity (90).
To resist ferroptosis, cancer cells use lipid
metabolic reprogramming as a defense strategy via two primary
mechanisms: i) PL membrane restructuring via the activation of the
sterol regulatory element-binding protein-1-SCD1 axis, replacing
oxidation-vulnerable PUFAs with MUFAs (106); and ii) mevalonate
pathway-derived antioxidant production, such as isopentenyl
pyrophosphate from cholesterol metabolism, which neutralizes lipid
peroxides (107,108). Iron homeostasis is also
inhibited through TFR1-mediated uptake and ferritin heavy chain
(FTH)-dependent storage, which reduces the levels of cytotoxic free
Fe2+ (109,110). Furthermore, gut
microbiota-derived metabolites, such as short-chain fatty acids
(such as butyrate) and tryptophan metabolites (such as
indole-acrylic acid), also affect the TME and influence the
resistance of cancer cells to ferroptosis (111,112).
Epigenetic regulation is a heritable mechanism that
regulates the expression of genes through chemical modifications
without altering the DNA sequence. It primarily involves DNA
methylation, histone modifications, non-coding RNA (ncRNA)-mediated
regulation and RNA methylation (113).
Several key genes are dynamically controlled by DNA
methylation. For example, in normal tissues, the core enzyme GPX4,
which is essential for eliminating lipid peroxides, has notably
reduced expression levels due to transcriptional repression caused
by the hypermethylation of CpG islands within its promoter region
compared with cancer tissues. This suppression increases cellular
susceptibility to ferroptosis (114-117). By contrast, the promoter of the
system Xc− light chain subunit SLC7A11 is frequently
hypomethylated, leading to its elevated expression levels (118,119). This enhances cystine uptake and
GSH synthesis, which inhibits ferroptosis. Furthermore,
hypermethylation of the FSP1 promoter results in a reduction to its
expression levels, which sensitizes cells to ferroptosis (120,121).
RNA methylation, the most abundant
post-transcriptional modification in eukaryotic mRNA, also
contributes to ferroptosis regulation. AlkB homolog 5 regulates
glutamate-cysteine ligase modifier subunit (GCLM) mRNA levels
though N6-methyladenosine (m6A) modification
of GCLM and YTH m6A RNA binding protein-mediated decay
of GCLM, which regulates ferroptosis (122). Additionally,
methyltransferase-like protein 16 (METTL16) serves a role in
ferroptosis and tumorigenesis by catalyzing the m6A
modification of Sentrin/small ubiquitin-like modifier-specific
protease 3 mRNA and regulating the stability of lactotransferrin
(123).
Histone modifications serve an important role in
shaping chromatin structure. It exerts precise transcriptional
control over ferroptosis-associated genes, such as SLC7A11 and
ACSL3, and impacts gene expression and cancer development (124-126). p53 promotes the nuclear
translocation of a histone H2B monoubiquitylation (H2Bub1)
deubiquitinase, ubiquitin-specific protease 7 (USP7), which
negatively regulates H2Bub1 levels. The interaction between p53 and
USP7 reduces the occupancy of H2Bub1 at the regulatory region of
SLC7A11, leading to transcriptional repression of SLC7A11and
enhancing the ferroptosis induction sensitivity of cells (125). Histone acetylation and
methylation also demonstrate bidirectional regulatory effects on
ferroptosis (126-129).
ncRNAs regulate the expression of
ferroptosis-associated genes via post-transcriptional regulation,
forming protein-RNA interaction networks. Specifically, microRNA
(miR)-137/miR-9 and miR-15a-5p promote ferroptosis by targeting
SLC7A11 and GPX4 mRNA, respectively (130,131), whereas miR-27a and miR-4717
exert anti-ferroptosis effects by targeting ACSL4 and NCOA4
(132,133). Long ncRNAs (lncRNAs) also
participate in ferroptosis regulation by acting as protein decoys,
chromatin modifiers or miRNA sponges (134,135).
Ferroptosis induction has emerged as a novel
strategy to overcome tumor therapy resistance (35,36). However, tumor cells establish
complex ferroptosis defense networks by remodeling immune-metabolic
environments, notably elevating the threshold for ferroptosis
initiation (78). The development
of this resistance limits the clinical application of
ferroptosis-inducing therapies (78). However, a series of innovative
strategies have been developed, providing a theoretical foundation
for designing effective treatments to circumvent resistance.
Recently identified, reversible palmitoylation modification of GPX4
is a key regulator of ferroptosis. The palmitoylation inhibitor
2-bromopalmitate notably enhances the antitumor efficacy of
ferroptosis inducers by inhibiting GPX4 palmitoylation (136). Furthermore, targeted protein
degradation represents an emerging therapeutic paradigm. The
GPX4-targeted autophagy-targeting chimera (GPX4-AUTAC), based on
selective autophagic degradation, induces ferroptosis and
demonstrates antitumor activity in vitro, in vivo and
in patient-derived organoids (137). In addition,
proteolysis-targeting chimeras that target GPX4/DHODH have also
been designed (138,139). The use of combination strategies
that target immune checkpoints alongside ferroptosis inducers are
also gaining attention (140).
Furthermore, the development of novel nanodrug delivery systems
offers additional approaches to overcome chemoresistance, such as
high-density lipoprotein nanoparticles with dual metabolic
disruption (selenium deprivation and LPO) (141) and antibody-targeted
nanoplatforms (such as Fe-MOF@Erastin@ Herceptin), which deliver
ferroptosis inducers (such as erastin) specifically to tumor cells
(142).
Ferroptosis is demonstrated to have therapeutic
potential in various types of cancer, including hepatocellular
carcinoma, lung carcinoma, lymphoma, pancreatic ductal carcinoma
and renal cell carcinoma (30-34). BTC is an uncommon type of
gastrointestinal malignancy that has a poor prognosis. In the early
stages of disease, surgical resection with negative margins can
effectively treat BTC (8,9). For unresectable and metastatic
disease, gemcitabine and cisplatin chemotherapies plus
immunotherapy (such as with Durvalumab or Pembrolizumab) are the
only preferred regimens approved by the National Comprehensive
Cancer Network (143,144). With the increasing number of
patients with BTC, investigating novel targets and alternative
options is crucial. Ferroptosis, which is considered to be one of
the most promising potential antitumor methods, can affect the
occurrence and development of BTC by regulating intracellular iron
and ROS levels, providing new treatment options for patients with
BTC (70,124,145). The following section primarily
concentrates on the advancements in ferroptosis-associated research
in BTC, including potential therapeutic targets, treatment options
and associated mechanisms (Table
I; Fig. 3).
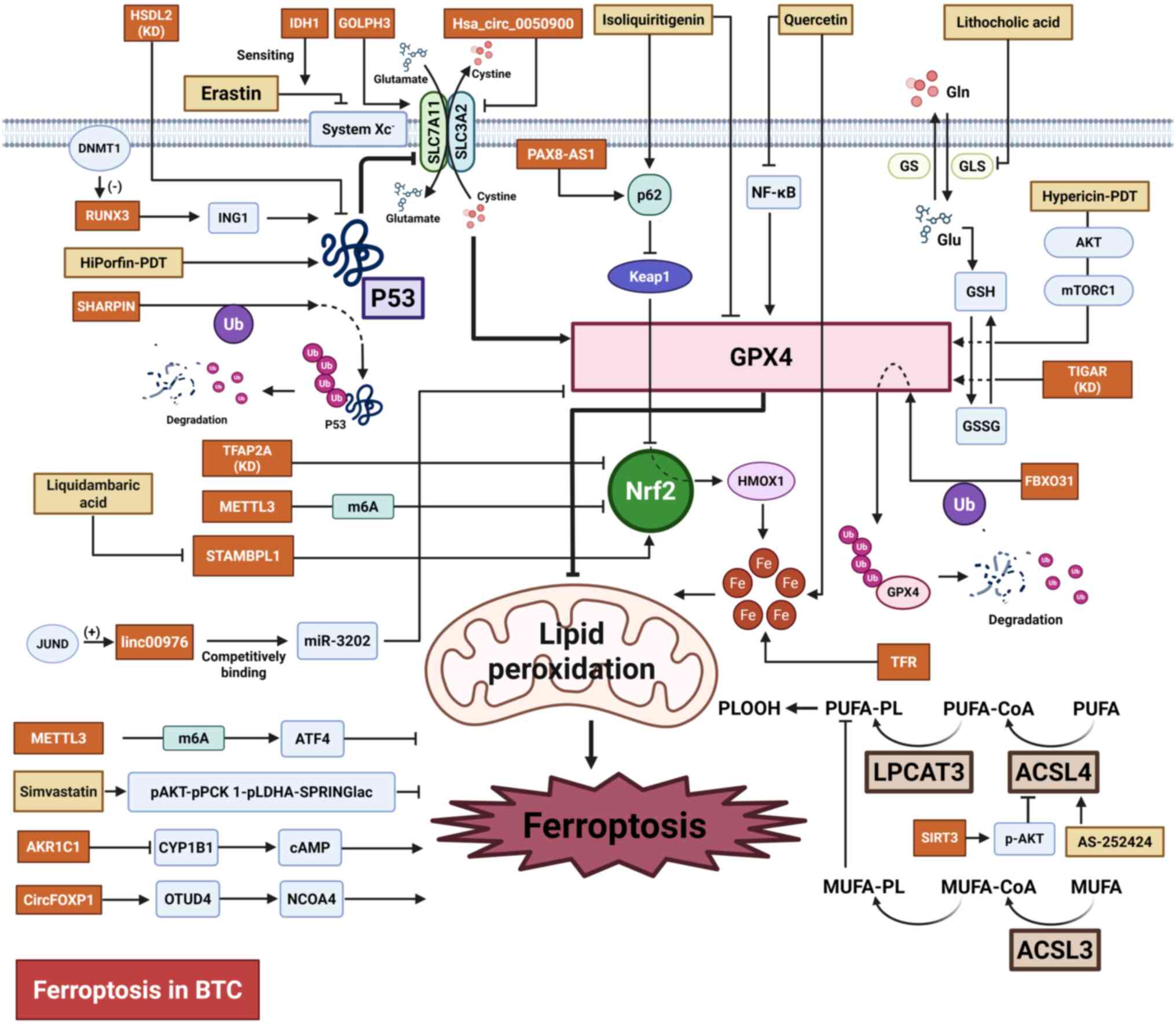
TP53 is acknowledged as a classic tumor suppressor
gene, which serves important roles in controlling the cell cycle,
cell proliferation and cell death (146,147). TP53 serves a role in controlling
the susceptibility to ferroptosis via both transcription-dependent
and transcription-independent mechanisms (148). Shank-associated RH domain
interacting protein (SHARPIN) is a crucial part of the complex
responsible for activating the linear ubiquitin chain, which
inhibits ferroptosis through the p53/SLC7A11/GPX4 signaling pathway
and promotes cell proliferation in CCA. Silencing the SHARPIN gene
results in the suppression of p53 ubiquitination and degradation,
as well as a decreased expression of SLC7A11, GPX4, superoxide
dismutase (SOD)-1 and SOD-2. Targeting SHARPIN may serve as a
potential strategy benefiting CCA treatment (149). Human hydroxysteroid
dehydrogenase-like 2 (HSDL2) is also a regulator of cancer
progression and lipid metabolism. Knocking down HSDL2 promotes CCA
progression by inhibiting ferroptosis through the p53/SLC7A11 axis
(150). As a member of
Runt-domain family, Runt-related transcription factor 3 (RUNX3) has
a reduced expression level in GBC. This downregulation, mediated by
promoter DNA hypermethylation, is notably associated with adverse
outcomes in patients with GBC. RUNX3 activates the transcription of
inhibitor of growth protein 1, which leads to the suppression of
SLC7A11 through a p53-dependent pathway and the induction of
ferroptosis (151). Golgi
phosphoprotein 3 also promotes CCA malignancy by inhibiting
ferroptosis through SLC7A11 upregulation (152). Additionally, TP53-induced
glycolysis and apoptosis regulator (TIGAR) is associated with
adverse outcomes and ferroptosis resistance in patients with
intrahepatic CCA (iCCA). TIGAR encodes an enzyme responsible
for regulating glycolysis and scavenging ROS. Knockdown of TIGAR
reduced the expression of GPX4, a key inhibitor of ferroptosis
(153). Furthermore, combining
TIGAR knockdown with cisplatin treatment synergistically induces a
notable increase in ferroptosis (153).
The transcription factor Nrf2 controls the
expression of genes involved in counteracting oxidative and
electrophilic stresses, which serves a role in regulating the
cellular antioxidant response (154,155). A study by Huang et al
(156) reports that
transcription factor activating enhancer-binding protein 2 α
(TFAP2A) may serve a role as a regulator of ferroptosis in GBC via
the Nrf2 signaling pathway. GBC cells exhibit elevated levels of
TFAP2A, compared with non-tumorigenic human intrahepatic bile duct
cells (H69), and knocking down TFAP2A lead to a decrease in GBC
cell proliferation, migration and invasion, as well as a decreased
expression of oxidative stress-associated genes, such as heme
oxygenase 1 and Nrf2. A study by Zheng et al (157) demonstrates high
methyltransferase-like 3 expression levels in cisplatin-resistant
iCCA cells compared with parental cells. This upregulation enhances
m6A modifications, leading to the inhibition of
ferroptosis and a resistance to cisplatin through the stabilization
of Nrf2 mRNA and an increase in the Nrf2 protein expression levels.
Additionally, overexpression of METTL16 in patients with CCA is
associated with poor prognosis. Mechanistically, METTL16 increases
the m6A modifications of activating transcription factor
4 (ATF4) mRNA, which increases the expression of ATF4 and
subsequently results in the suppression of ferroptosis (158).
At present, GPX4 is recognized as the only enzyme
with the ability to directly reduce complex phospholipid
hydroperoxide (159). Therefore,
GPX4, which is responsible for the efficient removal of
phospholipid hydroperoxides, is critical for cell survival
(160). When GPX4 fails to
effectively remove PLOOHs, there is an increase in LPO and
ferroptosis (57,161). GPX4 is also one of the important
adverse prognostic indicators in iCCA (162). A study by Lei et al
(163) demonstrates that JUND
enhances the transcription of linc00976, which promotes the
development of CCA and prevents ferroptosis by regulating the
miR-3202/GPX4 axis. F-box protein 31 (FBXO31) stimulates the
ubiquitination process of GPX4 and consequently promotes the
degradation of the GPX4 proteasome, which enhances the occurrence
of ferroptosis. Additionally, FBXO31-upregulated cancer stem
cell-like cells present enhanced sensitivity to cisplatin compared
with control cells (164). In
addition, lncRNA paired box 8-antisense RNA 1 binds to p62 and
activates Nrf2, which promotes GPX4 transcription and stabilizes
GPX4 mRNA by interacting with insulin-like growth factor 2 mRNA
binding protein 3. This inhibits ferroptosis and promotes
resistance to gemcitabine and cisplatin. Treatment with JKE-1674 (1
μM), a GPX4 inhibitor, combined with gemcitabine (5
μM) and cisplatin (10 μM) exhibits an improved
antitumor potential in preclinical models of both subcutaneous
tumor and orthotopic mice models, compared with gemcitabine and
cisplatin therapy (165).
Previous studies reveal ACSL4 promotes ferroptosis
by catalyzing the esterification of long-chain PUFAs into PUFA-CoA,
which serve as substrates for LPO (166,167). Data from the Gene Expression
Omnibus and The Cancer Genome Atlas databases demonstrates that
there is a higher level of ACSL4 in CCA compared with normal
adjacent tissues. Additionally, ACSL4 levels are associated with
the prognosis of patients with CCA as well as the immune
infiltration in CCA (168).
Targeting the ACSL4 pathway may be an anti-cancer approach. A study
by Liao et al (169)
reveals that, via ACSL4-dependent lipid reprogramming, IFNγ from
cytotoxic T lymphocytes and AA from the TME induce tumor cell
ferroptosis. Furthermore, cancer cells that derive from migration
exhibit a higher ferroptosis sensitivity and PUFA lipid contents
compared with primary-tumor-derived cells. This supports the
possibility of targeting ferroptosis in the treatment of cancer
(170). Specific and targeted
inhibitors of ACSL4 with anti-ferroptosis function such as
AS-252424 have been screened and identified (171). In GBC, sirtuin 3 has tumor
suppressive effects through the induction of the expression of
ACSL4 and AKT-dependent ferroptosis, and inhibition of the
epithelial-mesenchymal transition (172). Another member of the acyl-CoA
synthetase long chain family, ACSL3, synthesizes MUFAs that
suppress PUFAs peroxidation, which confers ferroptosis protection.
Furthermore, ACSL3 is an unfavorable prognostic biomarker in
patients with CCA and a mediator of ferroptosis resistance in CCA
cells. Therefore, ACSL3 may be a potential therapeutic target
(173).
Furthermore, several other factors potentially
regulate ferroptosis sensitivity. For example, circular
(circ)forkhead box protein P1 and Homo sapiens_circ_0050900
affect ferroptosis by interacting with OTU domain-containing
protein 4 and regulating SLC3A2, respectively (174,175). TFR modulates the ferroptosis
sensitivity of cells by regulating intracellular iron levels.
Additionally, TFR is also an adverse prognostic factor in patients
with iCCA (176). The regulatory
importance of the E26 transformation-specific variant 4-ALY/REF
export factor-pyruvate kinase M2 and Aldo-keto reductase family 1
member C1-cytochrome P450 family 1 subfamily B member 1-cAMP axes
also warrant attention (177,178). Additionally, gut microbiotas
inhibit ferroptosis in iCCA by altering glutamine metabolism
through the regulation of the activin receptor-like kinase 5/NADPH
oxidase 1 axis (179).
Ferroptosis serves a role in the pathogenesis,
progression and treatment resistance of BTC. Furthermore,
accumulating evidence demonstrates that ferroptosis-associated
indicators are notably associated with patient prognosis and
represent potential prognostic biomarkers (Table II). Techniques such as RNA
sequencing and immunohistochemistry reveal elevated expression of
ferroptosis-associated markers, including ACSL3/4, SLC7A11 and
GPX4, in BTC tumor tissues compared with normal adjacent tissues,
which is notably associated with poorer clinical outcomes (168,173,176,180-183). By contrast, isocitrate
dehydrogenase 1 (IDH1) mutations are associated with a more
favorable prognosis in BTC (183,184). In addition, other key
ferroptosis-associated markers, such as LPCAT3 and FSP1, are also
associated with prognosis in various solid tumors including ovarian
cancer and lung adenocarcinoma; however, their clinical validation
in BTC requires further investigation (185-192). Taken together, these
ferroptosis-associated indicators may serve as novel prognostic
markers for BTC, potentially offering a new perspective for precise
prognosis assessment and individualized treatment strategies.
Furthermore, integrating these indicators to establish
multi-parameter prognostic models may enhance the accuracy of
existing BTC prognostic assessment systems.
Traditional Chinese medicine (TCM) is currently
recognized as a viable complementary treatment in malignancies
(196). Accumulating evidence
demonstrates that TCM can notably enhance the chemosensitivity of a
patient, potentiate the antitumor efficacy of therapeutic agents
and mitigate treatment-associated adverse effects in cancer
management (197,198). Quercetin (QE), a flavonoid in
flowers, stems and leaves of various plants, possesses the ability
to impede the progression of breast and colon cancer by
interrupting the cell cycle (199,200). Previous studies demonstrate that
QE induces ferroptosis in iCCA cells by inhibiting the NF-κB
signaling pathway, which inhibits iCCA cell invasion (201,202). Isoliquiritigenin (ISL), also
known as 2',4',4-trihydroxychalcone, is also a type of flavonoid
and is extracted from the root of the liquorice plant. ISL induces
apoptosis and inhibits proliferation in tumors (203,204). A study by Wang et al
(205) reveals that ISL triggers
ferroptosis in GBC by activating the p62-Kelch-like ECH-associated
protein 1-Nrf2-haem oxygenase-1 (HMOX1) signaling pathway and
reducing the expression of GPX4. Silencing HMOX1 or increasing GPX4
expression levels decreases the susceptibility of GBC cells to
ISL-induced ferroptosis and enhances the survival of GBC cells
(205). Another naturally
occurring triterpenoid compound named liquidambaric acid (LCD) also
exhibits notable therapeutic potential. LCD can bind to and inhibit
signal transducing adaptor molecule binding protein like 1, which
stabilizes Nrf2 through de-ubiquitination, and notably enhances CCA
cell proliferation and migration while impeding ferroptosis
(206). Wu-Mei-Wan, a
long-utilized TCM formula, demonstrates potential efficacy as a
second-line GBC therapy. It enhances gemcitabine chemosensitivity
and induces ferroptosis through phosphorylated (p)-STAT3-mediated
transcriptional regulation of ferroptosis-associated targets,
downregulating GPX4, HIF-1α and FTH1, while upregulating ACSL4
(207).
At present, chemoimmunotherapy is the first-line
treatment option for BTC due to the durvalumab plus gemcitabine and
cisplatin in advanced BTC and KEYNOTE-966 studies (143,144). A study by Zhu et al
(208) reveals that, in
AKT-hyperactivated iCCA, the p-AKT-p-phosphoenolpyruvate
carboxykinase 1 (PCK1)-p-lactate dehydrogenase A-SPRINGlac axis is
a driver of ferroptosis resistance. This combines p-PCK1-mediated
glycolytic activation and mevalonate flux reprogramming. However,
simvastatin treatment effectively reverses this resistance,
underscoring its therapeutic potential for improving the
chemoimmunotherapy efficacy through ferroptosis sensitivity
(208).
In addition, metabolic products such as lithocholic
acid (LCA) are reagents involved in inhibiting tumorigenesis. A
study by Li et al (209)
reveals that LCA induces ferroptosis in GBC by suppressing
glutaminase-mediated glutamine metabolism, which may be a
tumor-suppressive mechanism with therapeutic potential.
PDT is a treatment modality that selectively
destroys tumor tissue through the light activation of
photosensitizers and release of ROS (210). The tumor-selective destruction
capacity of PDT minimizes damage to healthy tissues, which suggests
it may be a potential antitumor therapeutic strategy (211,212). The first-generation
photosensitizer-HiPorfin-mediated PDT promotes the apoptosis and
ferroptosis of CCA cells through the activation of the
p53/SLC7A11/GPX4 signaling pathway (213). Furthermore, the
second-generation photosensitizer-Hypericin-mediated PDT induces
ferroptosis in CCA by inhibiting the AKT/mTORC1/GPX4 signaling
pathway (214). These findings
provide possible insights into individualized precision therapy for
CCA. Furthermore, combination strategies represent novel
therapeutic paradigms for BTC. Surufatinib (SUR), a multi-targeted
kinase inhibitor, has comparable clinical efficacy in patients with
advanced CCA compared with regorafenib, which is one of the
recommend regimens used for subsequent-line therapy of BTC
(12,215). Mechanistically, both SUR and PDT
induce ferroptosis through the upregulation of ACSL4 and
suppression of GPX4, and their effect is synergistically enhanced
during combination treatment (215). In addition, an integrated
nanotherapeutic platform known as CMArg@ Lip has been successfully
developed for combined PDT and gas therapy. This platform
encapsulates the photosensitizer, NO gas-generating agent and Nrf2
inhibitor with ROS-responsive liposomes, which enables it to
effectively address the challenges of tumor hypoxia and the
antioxidant microenvironment (216).
Ferroptosis, an iron-dependent, LPO-driven form of
RCD, is a research focus in BTC therapeutics. Accumulating evidence
demonstrates marked vulnerability of BTC cells to ferroptosis,
unveiling novel avenues for targeted treatment. Despite promising
advances, challenges persist. While core ferroptosis pathways, such
as the GPX4-GSH axis and system Xc− regulation, are
delineated, the integrated regulatory network, particularly
crosstalk with metabolic reprogramming and TME interactions, is yet
to be fully elucidated. At present, clinically applicable
biomarkers for predicting ferroptosis sensitivity are lacking,
which hampers patient stratification. Furthermore, current
ferroptosis inducers, such as erastin analogs and RSL3 derivatives,
have limitations in tumor specificity, systemic toxicity and
pharmacokinetic profiles.
Future studies should prioritize the following: i)
Systematic investigations of ferroptosis synergism with immune
checkpoint inhibitors and molecular-targeted agents, using
multiomics approaches to decipher resistance mechanisms; ii)
development of integrated biomarker panels incorporating genetic,
epigenetic and microenvironmental features for precision patient
stratification; iii) therapeutic validation through physiologically
relevant platforms, including patient derived organoids, orthotopic
BTC models and humanized mouse systems simulating tumor-stroma
crosstalk; and iv) engineering of tumor-targeted nano-formulations,
such as the CMArg@Lip platform, to enhance the tumor-targeted
delivery of ferroptosis inducers while mitigating off-target
effects.
In summary, bridging mechanistic insights from
laboratory studies with rationally designed clinical trials
incorporating biomarker-driven enrollment may highlight ferroptosis
modulation as a potnetial therapeutic paradigm for BTC.
Not applicable.
RZ and YD contributed to literature searching and
screening, manuscript drafting and revision of the manuscript. SY
and HH contributed to the revision of the manuscript. FLi and FLiu
contributed to the study design and revision of the manuscript.
Data authentication is not applicable. All authors read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by 1.3.5 project for disciplines
of excellence (West China Hospital, Sichuan University; grant no.
ZYJC21046), 1.3.5 project for disciplines of excellence-Clinical
Research Incubation Project (West China Hospital, Sichuan
University; grant no. 2021HXFH001), China Telecom Sichuan Company
Biliary Tract Tumor Big Data Platform and Application Phase I
R&D Project (grant no. 312230752), National Natural Science
Foundation of China for Young Scientists Fund (grant no. 82303669),
Sichuan University-Sui Ning School-local Cooperation project (grant
no. 2022CDSN-18), and The Post-doctor Research Fund of West China
Hospital, Sichuan University (grant nos. ZYJC21046, 2021HXFH001 and
2024HXBH083).
|
1
|
Valle JW, Kelley RK, Nervi B, Oh DY and
Zhu AX: Biliary tract cancer. Lancet. 397:428–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brindley PJ, Bachini M, Ilyas SI, Khan SA,
Loukas A, Sirica AE, The BT, Wongkham S and Gores GJ:
Cholangiocarcinoma. Nat Rev Dis Primers. 7:652021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
4
|
Torre LA, Siegel RL, Islami F, Bray F and
Jemal A: Worldwide burden of and trends in mortality from
gallbladder and other biliary tract cancers. Clin Gastroenterol
Hepatol. 16:427–437. 2018. View Article : Google Scholar
|
|
5
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO classification of tumours editorial board: The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar :
|
|
6
|
Nakanuma Y and Kakuda Y: Pathologic
classification of cholangiocarcinoma: New concepts. Best Pract Res
Clin Gastroenterol. 29:277–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roa JC, García P, Kapoor VK, Maithel SK,
Javle M and Koshiol J: Gallbladder cancer. Nat Rev Dis Primers.
8:692022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vogel A, Bridgewater J, Edeline J, Kelley
RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A,
Valle JW, et al: Biliary tract cancer: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
34:127–140. 2023. View Article : Google Scholar
|
|
9
|
Ruff SM, Cloyd JM and Pawlik TM: Annals of
surgical oncology practice guidelines series: Management of primary
liver and biliary tract cancers. Ann Surg Oncol. 30:7935–7949.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baria K, De Toni EN, Yu B, Jiang Z, Kabadi
SM and Malvezzi M: Worldwide incidence and mortality of biliary
tract cancer. Gastro Hep Adv. 1:618–626. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamarca A, Edeline J and Goyal L: How I
treat biliary tract cancer. ESMO Open. 7:1003782022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benson AB, D'Angelica MI, Abrams T, Abbott
DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, et al:
NCCN Guidelines® insights: Biliary tract cancers,
version 2.2023. J Natl Compr Canc Netw. 21:694–704. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harding JJ, Khalil DN, Fabris L and
Abou-Alfa GK: Rational development of combination therapies for
biliary tract cancers. J Hepatol. 78:217–228. 2023. View Article : Google Scholar
|
|
14
|
Palmieri LJ, Lavolé J, Dermine S, Brezault
C, Dhooge M, Barré A, Chaussade S and Coriat R: The choice for the
optimal therapy in advanced biliary tract cancers: Chemotherapy,
targeted therapies or immunotherapy. Pharmacol Ther.
210:1075172020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamarca A, Barriuso J, McNamara MG and
Valle JW: Molecular targeted therapies: Ready for 'prime time' in
biliary tract cancer. J Hepatol. 73:170–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koren E and Fuchs Y: Modes of regulated
cell death in cancer. Cancer Discov. 11:245–265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar
|
|
21
|
Wyllie AH: Glucocorticoid-induced
thymocyte apoptosis is associated with endogenous endonuclease
activation. Nature. 284:555–556. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bergsbaken T and Cookson BT: Macrophage
activation redirects Yersinia-infected host cell death from
apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog.
3:e1612007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014. View Article : Google Scholar
|
|
27
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176. 2016.
View Article : Google Scholar :
|
|
28
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao GB, Chen L, Pan JF, Lei T, Cai X, Hao
Z, Wang Q, Shan G and Li J: LncRNA RGMB-AS1 inhibits HMOX1
ubiquitination and NAA10 activation to induce ferroptosis in
non-small cell lung cancer. Cancer Lett. 590:2168262024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar
|
|
32
|
Li J, Liu J, Zhou Z, Wu R, Chen X, Yu C,
Stockwell B, Kroemer G, Kang R and Tang D: Tumor-specific GPX4
degradation enhances ferroptosis-initiated antitumor immune
response in mouse models of pancreatic cancer. Sci Transl Med.
15:eadg30492023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao
X, Wang M, Chen Y and Zhang Q: Identification of a small molecule
as inducer of ferroptosis and apoptosis through ubiquitination of
GPX4 in triple negative breast cancer cells. J Hematol Oncol.
14:192021. View Article : Google Scholar
|
|
34
|
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK,
Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al: A
GPX4-dependent cancer cell state underlies the clear-cell
morphology and confers sensitivity to ferroptosis. Nat Commun.
10:16172019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen Z, Song J, Yung BC, Zhou Z, Wu A and
Chen X: Emerging strategies of cancer therapy based on ferroptosis.
Adv Mater. 30:e17040072018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedmann Angeli JP, Krysko DV and Conrad
M: Ferroptosis at the crossroads of cancer-acquired drug resistance
and immune evasion. Nat Rev Cancer. 19:405–414. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Angeli JPF, Shah R, Pratt DA and Conrad M:
Ferroptosis inhibition: Mechanisms and opportunities. Trends
Pharmacol Sci. 38:489–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar :
|
|
41
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar :
|
|
42
|
Zeng F, Nijiati S, Tang L, Ye J, Zhou Z
and Chen X: Ferroptosis detection: From approaches to applications.
Angew Chem Int Ed Engl. 62:e2023003792023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frey PA and Reed GH: The ubiquity of iron.
ACS Chem Biol. 7:1477–1481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hentze MW, Muckenthaler MU and Andrews NC:
Balancing acts: Molecular control of mammalian iron metabolism.
Cell. 117:285–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gonciarz RL, Collisson EA and Renslo AR:
Ferrous Iron-dependent pharmacology. Trends Pharmacol Sci. 42:7–18.
2021. View Article : Google Scholar
|
|
46
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Geng N, Shi BJ, Li SL, Zhong ZY, Li YC,
Xua WL, Zhou H and Cai JH: Knockdown of ferroportin accelerates
erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med
Pharmacol Sci. 22:3826–3836. 2018.PubMed/NCBI
|
|
48
|
Wang Y, Liu Y, Liu J, Kang R and Tang D:
NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem
Biophys Res Commun. 531:581–587. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alvarez SW, Sviderskiy VO, Terzi EM,
Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K
and Possemato R: NFS1 undergoes positive selection in lung tumours
and protects cells from ferroptosis. Nature. 551:639–643. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X,
Ren X, An Y, Wu Y, Sun W, et al: DHA inhibits proliferation and
induces ferroptosis of leukemia cells through autophagy dependent
degradation of ferritin. Free Radic Biol Med. 131:356–369. 2019.
View Article : Google Scholar
|
|
51
|
Knutson MD: Non-transferrin-bound iron
transporters. Free Radic Biol Med. 133:101–111. 2019. View Article : Google Scholar
|
|
52
|
Song N, Zhang J, Zhai J, Hong J, Yuan C
and Liang M: Ferritin: A multifunctional nanoplatform for
biological detection, imaging diagnosis, and drug delivery. Acc
Chem Res. 54:3313–3325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J,
Zhang Z and Wang X: Membrane Damage during ferroptosis is caused by
oxidation of phospholipids catalyzed by the oxidoreductases POR and
CYB5R1. Mol Cell. 81:355–369.e10. 2021. View Article : Google Scholar
|
|
57
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kagan VE, Mao G, Qu F, Angeli JP, Doll S,
Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017. View Article : Google Scholar
|
|
59
|
Magtanong L, Ko PJ, To M, Cao JY, Forcina
GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, et al:
Exogenous monounsaturated fatty acids promote a
Ferroptosis-resistant cell state. Cell Chem Biol. 26:420–432.e9.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tesfay L, Paul BT, Konstorum A, Deng Z,
Cox AO, Lee J, Furdui CM, Hegde P, Torti FM and Torti SV:
Stearoyl-CoA desaturase 1 protects ovarian cancer cells from
ferroptotic cell death. Cancer Res. 79:5355–5366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chu B, Kon N, Chen D, Li T, Liu T, Jiang
L, Song S, Tavana O and Gu W: ALOX12 is required for p53-mediated
tumour suppression through a distinct ferroptosis pathway. Nat Cell
Biol. 21:579–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T,
et al: Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422.e421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dai C, Chen X, Li J, Comish P, Kang R and
Tang D: Transcription factors in ferroptotic cell death. Cancer
Gene Ther. 27:645–656. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP Cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Soula M, Weber RA, Zilka O, Alwaseem H, La
K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K, et al:
Metabolic determinants of cancer cell sensitivity to canonical
ferroptosis inducers. Nat Chem Biol. 16:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang D, Feng Y, Zandkarimi F, Wang H,
Zhang Z, Kim J, Cai Y, Gu W, Stockwell BR and Jiang X: Ferroptosis
surveillance independent of GPX4 and differentially regulated by
sex hormones. Cell. 186:2748–2764.e2722. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lei G, Zhuang L and Gan B: The roles of
ferroptosis in cancer: Tumor suppression, tumor microenvironment,
and therapeutic interventions. Cancer Cell. 42:513–534. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Elhanani O, Ben-Uri R and Keren L: Spatial
profiling technologies illuminate the tumor microenvironment.
Cancer Cell. 41:404–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cui K, Wang K and Huang Z: Ferroptosis and
the tumor microenvironment. J Exp Clin Cancer Res. 43:3152024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bhowmick S, Banerjee S, Shridhar V and
Mondal S: Reprogrammed immuno-metabolic environment of cancer: The
driving force of ferroptosis resistance. Mol Cancer. 24:1612025.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim R, Taylor D, Vonderheide RH and
Gabrilovich DI: Ferroptosis of immune cells in the tumor
microenvironment. Trends Pharmacol Sci. 44:542–552. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zheng Y, Sun L, Guo J and Ma J: The
crosstalk between ferroptosis and anti-tumor immunity in the tumor
microenvironment: Molecular mechanisms and therapeutic controversy.
Cancer Commun (Lond). 43:1071–1096. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Demuynck R, Efimova I, Naessens F and
Krysko DV: Immunogenic ferroptosis and where to find it? J
Immunother Cancer. 9:e0034302021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fucikova J, Kepp O, Kasikova L, Petroni G,
Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G and Galluzzi L:
Detection of immunogenic cell death and its relevance for cancer
therapy. Cell Death Dis. 11:10132020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wen Q, Liu J, Kang R, Zhou B and Tang D:
The release and activity of HMGB1 in ferroptosis. Biochem Biophys
Res Commun. 510:278–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao YY, Lian JX, Lan Z, Zou KL, Wang WM
and Yu GT: Ferroptosis promotes anti-tumor immune response by
inducing immunogenic exposure in HNSCC. Oral Dis. 29:933–941. 2023.
View Article : Google Scholar
|
|
85
|
Han W, Duan X, Ni K, Li Y, Chan C and Lin
W: Co-delivery of dihydroartemisinin and pyropheophorbide-iron
elicits ferroptosis to potentiate cancer immunotherapy.
Biomaterials. 280:1213152022. View Article : Google Scholar :
|
|
86
|
Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J,
Huang J, He Q, Wu B, Zhang Z, et al: Irradiated tumor cell-derived
microparticles mediate tumor eradication via cell killing and
immune reprogramming. Sci Adv. 6:eaay97892020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Efimova I, Catanzaro E, Van der Meeren L,
Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C,
Bachert C, Coppieters F, et al: Vaccination with early ferroptotic
cancer cells induces efficient antitumor immunity. J Immunother
Cancer. 8:e0013692020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu J, Zhu S, Zeng L, Li J, Klionsky DJ,
Kroemer G, Jiang J, Tang D and Kang R: DCN released from
ferroptotic cells ignites AGER-dependent immune responses.
Autophagy. 18:2036–2049. 2022. View Article : Google Scholar :
|
|
89
|
Wiernicki B, Maschalidi S, Pinney J,
Adjemian S, Vanden Berghe T, Ravichandran KS and Vandenabeele P:
Cancer cells dying from ferroptosis impede dendritic Cell-mediated
Anti-tumor immunity. Nat Commun. 13:36762022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Han C, Ge M, Xing P, Xia T, Zhang C, Ma K,
Ma Y, Li S, Li W, Liu X, et al: Cystine deprivation triggers
CD36-mediated ferroptosis and dysfunction of tumor infiltrating
CD8+ T cells. Cell Death Dis. 15:1452024. View Article : Google Scholar :
|
|
91
|
Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li
ZQ, Wang G, Liu B, Liang L, Kurihara H, et al: Oxygenated
phosphatidylethanolamine navigates phagocytosis of ferroptotic
cells by interacting with TLR2. Cell Death Differ. 28:1971–1989.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kim KS, Choi B, Choi H, Ko MJ and Kim DH
and Kim DH: Enhanced natural killer cell anti-tumor activity with
nanoparticles mediated ferroptosis and potential therapeutic
application in prostate cancer. J Nanobiotechnology. 20:4282022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Drijvers JM, Gillis JE, Muijlwijk T,
Nguyen TH, Gaudiano EF, Harris IS, LaFleur MW, Ringel AE, Yao CH,
Kurmi K, et al: Pharmacologic screening identifies metabolic
vulnerabilities of CD8+ T cells. Cancer Immunol Res. 9:184–199.
2021. View Article : Google Scholar :
|
|
94
|
Arensman MD, Yang XS, Leahy DM,
Toral-Barza L, Mileski M, Rosfjord EC, Wang F, Deng S, Myers JS,
Abraham RT and Eng CH: Cystine-glutamate antiporter xCT deficiency
suppresses tumor growth while preserving antitumor immunity. Proc
Natl Acad Sci USA. 116:9533–9542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu S, Chaudhary O, Rodríguez-Morales P,
Sun X, Chen D, Zappasodi R, Xu Z, Pinto AFM, Williams A, Schulze I,
et al: Uptake of oxidized lipids by the scavenger receptor CD36
promotes lipid peroxidation and dysfunction in CD8+ T cells in
tumors. Immunity. 54:1561–1577.e7. 2021. View Article : Google Scholar
|
|
96
|
Xu C, Sun S, Johnson T, Qi R, Zhang S,
Zhang J and Yang K: The glutathione peroxidase Gpx4 prevents lipid
peroxidation and ferroptosis to sustain Treg cell activation and
suppression of antitumor immunity. Cell Rep. 35:1092352021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kapralov AA, Yang Q, Dar HH, Tyurina YY,
Anthonymuthu TS, Kim R, St Croix CM, Mikulska-Ruminska K, Liu B,
Shrivastava IH, et al: Redox lipid reprogramming commands
susceptibility of macrophages and microglia to ferroptotic death.
Nat Chem Biol. 16:278–290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhu H, Klement JD, Lu C, Redd PS, Yang D,
Smith AD, Poschel DB, Zou J, Liu D, Wang PG, et al: Asah2 represses
the p53-Hmox1 axis to protect Myeloid-derived suppressor cells from
ferroptosis. J Immunol. 206:1395–1404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ždralević M, Vučetić M, Daher B, Marchiq
I, Parks SK and Pouysségur J: Disrupting the 'Warburg effect'
re-routes cancer cells to OXPHOS offering a vulnerability point via
'ferroptosis'-induced cell death. Adv Biol Regul. 68:55–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Amos A, Amos A, Wu L and Xia H: The
Warburg effect modulates DHODH role in ferroptosis: A review. Cell
Commun Signal. 21:1002023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Song X, Liu J, Kuang F, Chen X, Zeh HJ
III, Kang R, Kroemer G, Xie Y and Tang D: PDK4 dictates metabolic
resistance to ferroptosis by suppressing pyruvate oxidation and
fatty acid synthesis. Cell Rep. 34:1087672021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dai Y, Cui C, Jiao D and Zhu X: JAK/STAT
signaling as a key regulator of ferroptosis: Mechanisms and
therapeutic potentials in cancer and diseases. Cancer Cell Int.
25:832025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Garcia-Bermudez J, Baudrier L, Bayraktar
EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B,
Freinkman E, et al: Squalene accumulation in cholesterol
auxotrophic lymphomas prevents oxidative cell death. Nature.
567:118–122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Warner GJ, Berry MJ, Moustafa ME, Carlson
BA, Hatfield DL and Faust JR: Inhibition of selenoprotein synthesis
by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol
Chem. 275:28110–28119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Qu L, He X, Tang Q, Fan X, Liu J and Lin
A: Iron metabolism, ferroptosis, and lncRNA in cancer: Knowns and
unknowns. J Zhejiang Univ Sci B. 23:844–862. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang S, Xin W, Anderson GJ, Li R, Gao L,
Chen S, Zhao J and Liu S: Double-edge sword roles of iron in
driving energy production versus instigating ferroptosis. Cell
Death Dis. 13:402022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
He Y, Ling Y, Zhang Z, Mertens RT, Cao Q,
Xu X, Guo K, Shi Q, Zhang X, Huo L, et al: Butyrate reverses
ferroptosis resistance in colorectal cancer by inducing
c-Fos-dependent xCT suppression. Redox Biol. 65:1028222023.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cui W, Guo M, Liu D, Xiao P, Yang C, Huang
H, Liang C, Yang Y, Fu X, Zhang Y, et al: Gut microbial metabolite
facilitates colorectal cancer development via ferroptosis
inhibition. Nat Cell Biol. 26:124–137. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cavalli G and Heard E: Advances in
epigenetics link genetics to the environment and disease. Nature.
571:489–499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang X, Sui S, Wang L, Li H, Zhang L, Xu
S and Zheng X: Inhibition of tumor propellant glutathione
peroxidase 4 induces ferroptosis in cancer cells and enhances
anticancer effect of cisplatin. J Cell Physiol. 235:3425–3437.
2020. View Article : Google Scholar
|
|
115
|
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z,
Wei X, Huang B, Shan Z, Liu J, Fan S, et al: Homocysteine induces
oxidative stress and ferroptosis of nucleus pulposus via enhancing
methylation of GPX4. Free Radic Biol Med. 160:552–565. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kim JW, Min DW, Kim D, Kim J, Kim MJ, Lim
H and Lee JY: GPX4 overexpressed non-small cell lung cancer cells
are sensitive to RSL3-induced ferroptosis. Sci Rep. 13:88722023.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lee J, You JH, Kim MS and Roh JL:
Epigenetic reprogramming of epithelial-mesenchymal transition
promotes ferroptosis of head and neck cancer. Redox Biol.
37:1016972020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar :
|
|
119
|
Liu J, Xia X and Huang P: xCT: A critical
molecule that links cancer metabolism to redox signaling. Mol Ther.
28:2358–2366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pontel LB, Bueno-Costa A, Morellato AE,
Carvalho Santos J, Roué G and Esteller M: Acute lymphoblastic
leukemia necessitates GSH-dependent ferroptosis defenses to
overcome FSP1-epigenetic silencing. Redox Biol. 55:1024082022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang X, Kong X, Feng X and Jiang DS:
Effects of DNA, RNA, and protein methylation on the regulation of
ferroptosis. Int J Biol Sci. 19:3558–3575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lv D, Zhong C, Dixit D, Yang K, Wu Q,
Godugu B, Prager BC, Zhao G, Wang X, Xie Q, et al: EGFR promotes
ALKBH5 nuclear retention to attenuate N6-methyladenosine and
protect against ferroptosis in glioblastoma. Mol Cell.
83:4334–4351.e4337. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang J, Xiu M, Wang J, Gao Y and Li Y:
METTL16-SENP3-LTF axis confers ferroptosis resistance and
facilitates tumorigenesis in hepatocellular carcinoma. J Hematol
Oncol. 17:782024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Y, Hu J, Wu S, Fleishman JS, Li Y, Xu
Y, Zou W, Wang J, Feng Y, Chen J and Wang H: Targeting epigenetic
and posttranslational modifications regulating ferroptosis for the
treatment of diseases. Signal Transduct Target Ther. 8:4492023.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang Y, Yang L, Zhang X, Cui W, Liu Y, Sun
QR, He Q, Zhao S, Zhang GA, Wang Y and Chen S: Epigenetic
regulation of ferroptosis by H2B monoubiquitination and p53. EMBO
Rep. 20:e475632019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ma M, Kong P, Huang Y, Wang J, Liu X, Hu
Y, Chen X, Du C and Yang H: Activation of MAT2A-ACSL3 pathway
protects cells from ferroptosis in gastric cancer. Free Radic Biol
Med. 181:288–299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li H, Liu W, Zhang X, Wu F, Sun D and Wang
Z: Ketamine suppresses proliferation and induces ferroptosis and
apoptosis of breast cancer cells by targeting KAT5/GPX4 axis.
Biochem Biophys Res Commun. 585:111–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang X, Du L, Qiao Y, Zhang X, Zheng W,
Wu Q, Chen Y, Zhu G, Liu Y, Bian Z, et al: Ferroptosis is governed
by differential regulation of transcription in liver cancer. Redox
Biol. 24:1012112019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zille M, Kumar A, Kundu N, Bourassa MW,
Wong VSC, Willis D, Karuppagounder SS and Ratan RR: Ferroptosis in
neurons and cancer cells is similar but differentially regulated by
histone deacetylase inhibitors. eNeuro. 6:ENEURO.0263-18.20192019.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yadav P, Sharma P, Sundaram S, Venkatraman
G, Bera AK and Karunagaran D: SLC7A11/xCT is a target of miR-5096
and its restoration partially rescues miR-5096-mediated ferroptosis
and anti-tumor effects in human breast cancer cells. Cancer Lett.
522:211–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Deng SH, Wu DM, Li L, Liu T, Zhang T, Li
J, Yu Y, He M, Zhao YY, Han R and Xu Y: miR-324-3p reverses
cisplatin resistance by inducing GPX4-mediated ferroptosis in lung
adenocarcinoma cell line A549. Biochem Biophys Res Commun.
549:54–60. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Bao C, Zhang J, Xian SY and Chen F:
MicroRNA-670-3p suppresses ferroptosis of human glioblastoma cells
through targeting ACSL4. Free Radic Res. 55:853–864. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ma LL, Liang L, Zhou D and Wang SW: Tumor
suppressor miR-424-5p abrogates ferroptosis in ovarian cancer
through targeting ACSL4. Neoplasma. 68:165–173. 2021. View Article : Google Scholar
|
|
134
|
Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C
and Xu S: LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress
during erastin-induced ferroptosis in HepG2 hepatocellular
carcinoma cells. Sci Rep. 9:161852019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang Y, Guo S, Wang S, Li X, Hou D, Li H,
Wang L, Xu Y, Ma B, Wang H and Jiang X: LncRNA OIP5-AS1 inhibits
ferroptosis in prostate cancer with long-term cadmium exposure
through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf.
220:1123762021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Huang B, Wang H, Liu S, Hao M, Luo D, Zhou
Y, Huang Y, Nian Y, Zhang L, Chu B and Yin C:
Palmitoylation-dependent regulation of GPX4 suppresses ferroptosis.
Nat Commun. 16:8672025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Gong R, Wan X, Jiang S, Guan Y, Li Y,
Jiang T, Chen Z, Zhong C, He L, Xiang Z, et al: GPX4-AUTAC induces
ferroptosis in breast cancer by promoting the selective autophagic
degradation of GPX4 mediated by TRAF6-p62. Cell Death Differ. May
20–2025. View Article : Google Scholar : Epub ahead of
print.
|
|
138
|
Yao L, Yang N, Zhou W, Akhtar MH, Zhou W,
Liu C, Song S, Li Y, Han W and Yu C: Exploiting cancer
vulnerabilities by blocking of the DHODH and GPX4 pathways: A
multifunctional Bodipy/PROTAC nanoplatform for the efficient
synergistic ferroptosis therapy. Adv Healthc Mater.
12:e23008712023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Dong J, Ma F, Cai M, Cao F, Li H, Liang H,
Li Y, Ding G, Li J, Cheng X and Qin JJ: Heat shock protein 90
interactome-mediated proteolysis targeting chimera (HIM-PROTAC)
degrading glutathione peroxidase 4 to trigger ferroptosis. J Med
Chem. 67:16712–16736. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang S, Zhu L, Li T, Lin X, Zheng Y, Xu D,
Guo Y, Zhang Z, Fu Y, Wang H, et al: Disruption of MerTK increases
the efficacy of checkpoint inhibitor by enhancing ferroptosis and
immune response in hepatocellular carcinoma. Cell Rep Med.
5:1014152024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lamperis SM, McMahon KM, Calvert AE, Rink
JS, Vasan K, Pandkar MR, Crentsil EU, Chalmers ZR, McDonald NR,
Kosmala CJ, et al: CRISPR screen reveals a simultaneous targeted
mechanism to reduce cancer cell selenium and increase lipid
oxidation to induce ferroptosis. Proc Natl Acad Sci USA.
122:e25028761222025. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gao J, Ye T, Miao H, Liu M, Wen L, Tian Y,
Fu Z, Sun L, Wang L and Wang Y: Antibody-functionalized iron-based
nanoplatform for ferroptosis-augmented targeted therapy of
HER2-positive breast cancer. Bioact Mater. 52:702–718.
2025.PubMed/NCBI
|
|
143
|
Burris HA III, Okusaka T, Vogel A, Lee MA,
Takahashi H, Breder V, Blanc JF, Li J, Bachini M, Żotkiewicz M, et
al: Durvalumab plus gemcitabine and cisplatin in advanced biliary
tract cancer (TOPAZ-1): Patient-reported outcomes from a
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 25:626–635. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Cheok CF, Verma CS, Baselga J and Lane DP:
Translating p53 into the clinic. Nat Rev Clin Oncol. 8:25–37. 2011.
View Article : Google Scholar
|
|
147
|
Bykov VJN, Eriksson SE, Bianchi J and
Wiman KG: Targeting mutant p53 for efficient cancer therapy. Nat
Rev Cancer. 18:89–102. 2018. View Article : Google Scholar
|
|
148
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zeng C, Lin J, Zhang K, Ou H, Shen K, Liu
Q, Wei Z, Dong X, Zeng X, Zeng L, et al: SHARPIN promotes cell
proliferation of cholangiocarcinoma and inhibits ferroptosis via
p53/SLC7A11/GPX4 signaling. Cancer Sci. 113:3766–3775. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ma S, Ma Y, Qi F, Lei J, Chen F, Sun W,
Wang D, Zhou S, Liu Z, Lu Z, et al: HSDL2 knockdown promotes the
progression of cholangiocarcinoma by inhibiting ferroptosis through
the P53/SLC7A11 axis. World J Surg Oncol. 21:2932023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Cai C, Zhu Y, Mu J, Liu S, Yang Z, Wu Z,
Zhao C, Song X, Ye Y, Gu J, et al: DNA methylation of RUNX3
promotes the progression of gallbladder cancer through repressing
SLC7A11-mediated ferroptosis. Cell Signal. 108:1107102023.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yin Z, Liu Q, Gao Y, Wang R, Qi Y, Wang D,
Chen L, Yin X, He M and Li W: GOLPH3 promotes tumor malignancy via
inhibition of ferroptosis by upregulating SLC7A11 in
cholangiocarcinoma. Mol Carcinog. 63:912–925. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Toshida K, Itoh S, Iseda N, Izumi T,
Yoshiya S, Toshima T, Ninomiya M, Iwasaki T, Oda Y and Yoshizumi T:
Impact of TP53-induced glycolysis and apoptosis regulator on
malignant activity and resistance to ferroptosis in intrahepatic
cholangiocarcinoma. Cancer science. 115:170–183. 2024. View Article : Google Scholar
|
|
154
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar :
|
|
156
|
Huang HX, Yang G, Yang Y, Yan J, Tang XY
and Pan Q: TFAP2A is a novel regulator that modulates ferroptosis
in gallbladder carcinoma cells via the Nrf2 signalling axis. Eur
Rev Med Pharmacol Sci. 24:4745–4755. 2020.PubMed/NCBI
|
|
157
|
Zheng X, Li H, Lin J, Li P, Yang X, Luo Z
and Jin L: METTL3-mediated m6A modification promotes
chemoresistance of intrahepatic cholangiocarcinoma by up-regulating
NRF2 to inhibit ferroptosis in cisplatin-resistant cells. J
Chemother. 37:596–606. 2025. View Article : Google Scholar
|
|
158
|
Zhao S, Cao J, Liang R, Peng T, Wu S, Liu
Z, Wu Y, Song L, Sun C, Liu Y, et al: METTL16 suppresses
ferroptosis in cholangiocarcinoma by promoting ATF4 via
m6A modification. Int J Biol Sci. 21:189–203. 2025.
View Article : Google Scholar :
|
|
159
|
Ursini F, Maiorino M, Valente M, Ferri L
and Gregolin C: Purification from pig liver of a protein which
protects liposomes and biomembranes from peroxidative degradation
and exhibits glutathione peroxidase activity on phosphatidylcholine
hydroperoxides. Biochim Biophys Acta. 710:197–211. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Yant LJ, Ran Q, Rao L, Van Remmen H,
Shibatani T, Belter JG, Motta L, Richardson A and Prolla TA: The
selenoprotein GPX4 is essential for mouse development and protects
from radiation and oxidative damage insults. Free Radic Biol Med.
34:496–502. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shah R, Shchepinov MS and Pratt DA:
Resolving the role of lipoxygenases in the initiation and execution
of ferroptosis. ACS Cent Sci. 4:387–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Hori Y, Yoh T, Nishino H, Okura K,
Kurimoto M, Takamatsu Y, Satoh M, Nishio T, Koyama Y, Ishii T, et
al: Ferroptosis-related gene glutathione peroxidase 4 promotes
reprogramming of glucose metabolism via Akt-mTOR axis in
intrahepatic cholangiocarcinoma. Carcinogenesis. 45:119–130. 2024.
View Article : Google Scholar
|
|
163
|
Lei S, Cao W, Zeng Z, Zhang Z, Jin B, Tian
Q, Wu Y, Zhang T, Li D, Hu C, et al: JUND/linc00976 promotes
cholangiocarcinoma progression and metastasis, inhibits ferroptosis
by regulating the miR-3202/GPX4 axis. Cell Death Dis. 13:9672022.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhu Z, Zheng Y, He H, Yang L, Yang J, Li
M, Dai W and Huang H: FBXO31 sensitizes cancer stem cells-like
cells to cisplatin by promoting ferroptosis and facilitating
proteasomal degradation of GPX4 in cholangiocarcinoma. Liver Int.
42:2871–2888. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Chen ZW, Shan JJ, Chen M, Wu Z, Zhao YM,
Zhu HX, Jin X, Wang YX, Wu YB, Xiang Z, et al: Targeting GPX4 to
induce ferroptosis overcomes chemoresistance mediated by the
PAX8-AS1/GPX4 axis in intrahepatic cholangiocarcinoma. Adv Sci
(Weinh). 12:e010422025. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
167
|
Gan B: ACSL4, PUFA, and ferroptosis: New
arsenal in anti-tumor immunity. Signal Transduct Target Ther.
7:1282022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liu S, Fan S, Wang Y, Chen R, Wang Z,
Zhang Y, Jiang W, Chen Y, Xu X, Yu Y, et al: ACSL4 serves as a
novel prognostic biomarker correlated with immune infiltration in
Cholangiocarcinoma. BMC Cancer. 23:4442023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liao P, Wang W, Wang W, Kryczek I, Li X,
Bian Y, Sell A, Wei S, Grove S, Johnson JK, et al: CD8+ T cells and
fatty acids orchestrate tumor ferroptosis and immunity via ACSL4.
Cancer Cell. 40:365–378.e6. 2022. View Article : Google Scholar :
|
|
170
|
Wang Y, Hu M, Cao J, Wang F, Han JR, Wu
TW, Li L, Yu J, Fan Y, Xie G, et al: ACSL4 and polyunsaturated
lipids support metastatic extravasation and colonization. Cell.
188:412–429.e27. 2025. View Article : Google Scholar
|
|
171
|
Huang Q, Ru Y, Luo Y, Luo X, Liu D, Ma Y,
Zhou X, Linghu M, Xu W, Gao F and Huang Y: Identification of a
targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci
Adv. 10:eadk12002024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Liu L, Li Y, Cao D, Qiu S, Li Y, Jiang C,
Bian R, Yang Y, Li L, Li X, et al: SIRT3 inhibits gallbladder
cancer by induction of AKT-dependent ferroptosis and blockade of
epithelial-mesenchymal transition. Cancer Lett. 510:93–104. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sae-Fung A, Vinayavekhin N, Fadeel B and
Jitkaew S: ACSL3 is an unfavorable prognostic marker in
cholangiocarcinoma patients and confers ferroptosis resistance in
cholangiocarcinoma cells. NPJ Precis Oncol. 8:2842024. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wang P, Hu Z, Yu S, Su S, Wu R, Chen C, Ye
Y, Wang H, Ye X, Zhou Z, et al: A novel protein encoded by
circFOXP1 enhances ferroptosis and inhibits tumor recurrence in
intrahepatic cholangiocarcinoma. Cancer Lett. 598:2170922024.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Shi X, Yang J, Wang M, Xia L, Zhang L and
Qiao S: Hsa_circ_0050900 affects ferroptosis in intrahepatic
cholangiocarcinoma cells by targeting hsa-miR-605-3p to regulate
SLC3A2. Oncol Lett. 27:22024. View Article : Google Scholar
|
|
176
|
Toshida K, Itoh S, Iseda N, Izumi T, Bekki
Y, Yoshiya S, Toshima T, Iwasaki T, Oda Y and Yoshizumi T: The
association of transferrin receptor with prognosis and biologic
role in intrahepatic cholangiocarcinoma. Ann Surg Oncol.
31:8627–8637. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Wang X, Duan W, Ma Z, Wen H, Mao X and Liu
C: ETV4/ALYREF-mediated glycolytic metabolism through PKM2 enhances
resistance to ferroptosis and promotes the development of
intrahepatic cholangiocarcinoma. Cancer Metab. 13:192025.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Liu C, Zhang C, Wu H, Zhao Z, Wang Z,
Zhang X, Yang J, Yu W, Lian Z, Gao M and Zhou L: The
AKR1C1-CYP1B1-cAMP signaling axis controls tumorigenicity and
ferroptosis susceptibility of extrahepatic cholangiocarcinoma. Cell
Death Differ. 32:506–520. 2025. View Article : Google Scholar
|
|
179
|
Zhang Q, Zhou J, Zhai D, Jiang Q, Yang M
and Zhou M: Gut microbiota regulates the ALK5/NOX1 axis by altering
glutamine metabolism to inhibit ferroptosis of intrahepatic
cholangiocarcinoma cells. Biochim Biophys Acta Mol Basis Dis.
1870:1671522024. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Amontailak S, Titapun A, Jusakul A, Thanan
R, Kimawaha P, Jamnongkan W, Thanee M, Sirithawat P and Techasen A:
Prognostic values of Ferroptosis-related proteins ACSL4, SLC7A11,
and CHAC1 in cholangiocarcinoma. Biomedicines. 12:20912024.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Saisomboon S, Kariya R, Boonnate P,
Sawanyawisuth K, Cha'on U, Luvira V, Chamgramol Y, Pairojkul C,
Seubwai W, Silsirivanit A, et al: Diminishing acetyl-CoA
carboxylase 1 attenuates CCA migration via AMPK-NF-κB-snail axis.
Biochim Biophys Acta Mol Basis Dis. 1869:1666942023. View Article : Google Scholar
|
|
182
|
Wang J, Zhang M, Zhang L, Cai H, Zhou S,
Zhang J and Wang Y: Correlation of Nrf2, HO-1, and MRP3 in
gallbladder cancer and their relationships to clinicopathologic
features and survival. J Surg Res. 164:e99–e105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sarcognato S, Sacchi D, Fabris L, Zanus G,
Gringeri E, Niero M, Gallina G and Guido M: Ferroptosis in
intrahepatic cholangiocarcinoma: IDH1(105GGT) single nucleotide
polymorphism is associated with its activation and better
prognosis. Front Med (Lausanne). 9:8862292022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Rizzato M, Brignola S, Munari G, Gatti M,
Dadduzio V, Borga C, Bergamo F, Pellino A, Angerilli V, Mescoli C,
et al: Prognostic impact of FGFR2/3 alterations in patients with
biliary tract cancers receiving systemic chemotherapy: The BITCOIN
study. Eur J Cancer. 166:165–175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Qian Y, Liang X, Kong P, Cheng Y, Cui H,
Yan T, Wang J, Zhang L, Liu Y, Guo S, et al: Elevated DHODH
expression promotes cell proliferation via stabilizing β-catenin in
esophageal squamous cell carcinoma. Cell Death Dise. 11:8622020.
View Article : Google Scholar
|
|
186
|
Hu J, Jiang Q, Mao W, Zhong S, Sun H and
Mao K: STARD7 could be an immunological and prognostic biomarker:
From pan-cancer analysis to hepatocellular carcinoma validation.
Discov Oncol. 15:5432024. View Article : Google Scholar
|
|
187
|
Sun J, Zhou C, Ma Q, Chen W, Atyah M, Yin
Y, Fu P, Liu S, Hu B, Ren N and Zhou H: High GCLC level in tumor
tissues is associated with poor prognosis of hepatocellular
carcinoma after curative resection. J Cancer. 10:3333–3343. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Wang M, Lu Y, Wang H, Wu Y, Xu X and Li Y:
High ATF4 expression is associated with poor prognosis, amino acid
metabolism, and autophagy in gastric cancer. Front Oncol.
11:7401202021. View Article : Google Scholar
|
|
189
|
Luis G, Godfroid A, Nishiumi S, Cimino J,
Blacher S, Maquoi E, Wery C, Collignon A, Longuespée R,
Montero-Ruiz L, et al: Tumor resistance to ferroptosis driven by
Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid
Biding Protein-4 (FABP4) in tumor microenvironment promote tumor
recurrence. Redox Biol. 43:1020062021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wei JL, Wu SY, Yang YS, Xiao Y, Jin X, Xu
XE, Hu X, Li DQ, Jiang YZ and Shao ZM: GCH1 induces
immunosuppression through metabolic reprogramming and IDO1
upregulation in triple-negative breast cancer. J Immunother Cancer.
9:e0023832021. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Wen F, Ling H, Ran R, Li X, Wang H, Liu Q,
Li M and Yu T: LPCAT3 regulates the proliferation and metastasis of
serous ovarian cancer by modulating arachidonic acid. Transl Oncol.
52:1022562025. View Article : Google Scholar :
|
|
192
|
Takahara H, Kanazawa T, Oshita H, Tomita
Y, Hananoi Y, Ishibashi S, Ikeda M, Furukawa A, Kinoshita M,
Yamamoto K, et al: GPX4 and FSP1 expression in lung adenocarcinoma:
Prognostic implications and Ferroptosis-based therapeutic
strategies. Cancers (Basel). 16:38882024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Su L, Huang Y, Zheng L, Zhu Z, Wu Y and Li
P: Isocitrate dehydrogenase 1 mutation in cholangiocarcinoma
impairs tumor progression by sensitizing cells to ferroptosis. Open
Med (Wars). 17:863–870. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Dong S, An S, Liu Q, Wang X, Hu Y and
Jiang A: Study on the synergistic mechanism of photodynamic therapy
combined with ferroptosis inducer to induce ferroptosis in
cholangiocarcinoma. Lancet Oncol. 56:845–853. 2024.
|
|
196
|
Li S, Chen X, Shi H, Yi M, Xiong B and Li
T: Tailoring traditional Chinese medicine in cancer therapy. Mol
Cancer. 24:272025. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Xi Z, Dai R, Ze Y, Jiang X, Liu M and Xu
H: Traditional Chinese medicine in lung cancer treatment. Mol
Cancer. 24:572025. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Wu J, Tang G, Cheng CS, Yeerken R, Chan
YT, Fu Z, Zheng YC, Feng Y and Wang N: Traditional Chinese medicine
for the treatment of cancers of hepatobiliary system: From clinical
evidence to drug discovery. Mol Cancer. 23:2182024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Kim HI, Lee SJ, Choi YJ, Kim MJ, Kim TY
and Ko SG: Quercetin induces apoptosis in glioblastoma cells by
suppressing Axl/IL-6/STAT3 signaling pathway. Am J Chin Med.
49:767–784. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Mohammed HA, Sulaiman GM, Anwar SS,
Tawfeeq AT, Khan RA, Mohammed SAA, Al-Omar MS, Alsharidah M, Rugaie
OA and Al-Amiery AA: Quercetin against MCF7 and CAL51 breast cancer
cell lines: Apoptosis, gene expression and cytotoxicity of
nano-quercetin. Nanomedicine (Lond). 16:1937–1961. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Song Y, Zhang Z, Chai Q, Zheng H, Qi Y,
Xia G, Yu Z, Yang R, Huang J, Li Y, et al: Quercetin inhibits
intrahepatic cholangiocarcinoma by inducing ferroptosis and
inhibiting invasion via the NF-[Formula: See text]B pathway. Am J
Chin Med. 51:701–721. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Song Y, Zhang Z, Chai Q, Zheng H, Qi Y,
Xia G, Yu Z, Yang R, Huang J, Li Y, et al: ERRATUM: Quercetin
inhibits intrahepatic cholangiocarcinoma by inducing ferroptosis
and inhibiting invasion via the NF-[Formula: See text]B pathway. Am
J Chin Med. 51:1613–1614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Chen C, Shenoy AK, Padia R, Fang D, Jing
Q, Yang P, Su SB and Huang S: Suppression of lung cancer
progression by isoliquiritigenin through its metabolite 2, 4, 2',
4'-Tetrahydroxychalcone. J Exp Clin Cancer Res. 37:2432018.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Chen C, Huang S, Chen CL, Su SB and Fang
DD: Isoliquiritigenin inhibits ovarian cancer metastasis by
reversing Epithelial-to-mesenchymal transition. Molecules.
24:37252019. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Wang Z, Li W, Wang X, Zhu Q, Liu L, Qiu S,
Zou L, Liu K, Li G, Miao H, et al: Isoliquiritigenin induces HMOX1
and GPX4-mediated ferroptosis in gallbladder cancer cells. Chin Med
J (Engl). 136:2210–2220. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Wang Z, Zhang Y, Shen Y, Zhu C, Qin X and
Gao Y: Liquidambaric acid inhibits cholangiocarcinoma progression
by disrupting the STAMBPL1/NRF2 positive feedback loop.
Phytomedicine. 136:1563032025. View Article : Google Scholar
|
|
207
|
Zhao J, Shi L, Yang Y, Zhu J, Zhou Z, Dong
P, Liu S, Yang Z and Gong W: Wu-Mei-Wan promotes ferroptosis in
gallbladder cancer through STAT3 negative regulation: An integrated
HPLC, proteomics, network pharmacology, and experimental validation
study. J Ethnopharmacol. 347:1196712025. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhu J, Xiong Y, Zhang Y, Liang H, Cheng K,
Lu Y, Cai G, Wu Y, Fan Y, Chen X, et al: Simvastatin overcomes the
pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and
chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic
cholangiocarcinoma. Cancer Commun (Lond). 45:1038–1071. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Li W, Wang Z, Lin R, Huang S, Miao H, Zou
L, Liu K, Cui X, Wang Z, Zhang Y, et al: Lithocholic acid inhibits
gallbladder cancer proliferation through interfering
glutaminase-mediated glutamine metabolism. Biochem Pharmacol.
205:1152532022. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Pham TC, Nguyen VN, Choi Y, Lee S and Yoon
J: Recent strategies to develop innovative photosensitizers for
enhanced photodynamic therapy. Chem Rev. 121:13454–13619. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Brown SB, Brown EA and Walker I: The
present and future role of photodynamic therapy in cancer
treatment. Lancet Oncol. 5:497–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Alzeibak R, Mishchenko TA, Shilyagina NY,
Balalaeva IV, Vedunova MV and Krysko DV: Targeting immunogenic
cancer cell death by photodynamic therapy: Past, present and
future. J Immunother Cancer. 9:e0019262021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Yan X, Li Z, Chen H, Yang F, Tian Q and
Zhang Y: Photodynamic therapy inhibits cancer progression and
induces ferroptosis and apoptosis by targeting P53/GPX4/SLC7A11
signaling pathways in cholangiocarcinoma. Photodiagnosis Photodyn
Ther. 47:1041042024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
An W, Zhang K, Li G, Zheng S, Cao Y and
Liu J: Hypericin mediated photodynamic therapy induces ferroptosis
via inhibiting the AKT/mTORC1/GPX4 axis in cholangiocarcinoma.
Transl Oncol. 52:1022342025. View Article : Google Scholar :
|
|
215
|
Huang YP, Wang YX, Zhou H, Liu ZT, Zhang
ZJ, Xiong L, Zou H and Wen Y: Surufatinib combined with
photodynamic therapy induces ferroptosis to inhibit
cholangiocarcinoma in vitro and in tumor models. Front Pharmacol.
15:12882552024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Wang W, Gao Y, Xu J, Zou T, Yang B, Hu S,
Cheng X, Xia Y and Zheng Q: A NRF2 regulated and the
immunosuppressive microenvironment reversed nanoplatform for
cholangiocarcinoma Photodynamic-Gas therapy. Adv Sci (Weinh).
11:e23071432024. View Article : Google Scholar : PubMed/NCBI
|