Introduction
Obesity prevalence continues to rise globally, with
>1.9 billion adults classified as overweight and >650 million
classified as obese, according to the latest World Health
Organization reports (1). Obesity
is a key risk factor for numerous chronic diseases, including type
2 diabetes mellitus (2),
cardiovascular diseases (3) and
osteoarthritis (4). Obesity leads
to insulin resistance, which can progress to type 2 diabetes
(2). The excess fat tissue alters
metabolic processes, increasing the production of pro-inflammatory
cytokines and free fatty acids, which in turn impair insulin
signaling (2). Obesity also
elevates the risk of cardiovascular diseases. The excess adipose
tissue increases the workload of the heart, raises the blood
pressure and contributes to the buildup of plaque in the arteries
(3). Furthermore, obesity is
linked to a higher incidence of osteoarthritis, as the excess
weight puts additional stress on joints, causing cartilage
degradation and inflammation (4).
The global economic burden of obesity is enormous, with healthcare
costs related to obesity-related diseases increasing in both
developed and developing nations (5).
BC stands out as a leading cause of cancer-related
mortality among women globally. In 2020 alone, there were ~2.3
million new cases diagnosed, accounting for a significant
proportion of all cancer cases (6). The impact of BC extends beyond
physical health, affecting psychological wellbeing and the quality
of life (6). The diagnosis of BC
often leads to heightened anxiety, depression and body image issues
(7). Conventional treatments,
including surgery, chemotherapy and radiation therapy, while
effective in numerous cases, are associated with a range of side
effects. These can include lymphedema, fatigue, nausea, hair loss
and, in some instances, long-term organ damage (8). The economic costs of BC treatment
are also substantial, including direct medical expenses as well as
indirect costs due to lost productivity (9). Despite advances in early detection
and treatment, certain subtypes of BC, such as triple-negative BC
(TNBC), remain particularly aggressive and challenging to treat,
with limited targeted therapies available (10).
Recent research has begun to unravel the complex
relationship among obesity, chronic breast inflammation and BC
development (11).
Epidemiological studies have established a clear association
between obesity and an increased risk of BC, particularly in
postmenopausal women (11,12).
The mechanisms underlying this link are multifaceted. Adipose
tissue in the breast is not inert; it actively secretes a variety
of inflammatory mediators. These include cytokines such as IL-6 and
TNF-α, which create a pro-inflammatory microenvironment (13). Chronic inflammation is now
recognized as a key driver of carcinogenesis. Persistent low-grade
inflammation leads to DNA damage through the production of reactive
oxygen species (ROS) and nitrogen species (13). Inflammatory cells also secrete
growth factors and angiogenic factors, promoting cell proliferation
and the formation of new blood vessels to supply growing tumors
(13). Emerging evidence suggests
that specific signaling pathways, such as the NF-κB pathway, are
activated in obese breast tissue (14). This pathway regulates the
expression of genes involved in cell survival, proliferation and
immune responses (14).
Cyclooxygenase-2 (COX-2) upregulation in obese breast tissue
results in increased prostaglandin E2 levels, which suppress
apoptosis and induce DNA damage (15). Furthermore, the PI3K/AKT/mTOR
pathway is often dysregulated in obesity-related BC (16). This pathway is critical for the
regulation of cell proliferation and metabolism, and its activation
can lead to uncontrolled cell proliferation and resistance to cell
death (16).
The present review aims to comprehensively explore
the molecular mechanisms that connect obesity, chronic breast
inflammation and breast carcinogenesis. By delving into the latest
research findings from PubMed-listed studies (https://pubmed.ncbi.nlm.nih.gov/), the present
review aims to provide a detailed understanding of how
obesity-induced inflammation contributes to the development and
progression of BC. The search strategy employed the following key
words and Boolean operators: ('obesity' OR 'adiposity') AND
('chronic inflammation' OR 'breast inflammation') AND ('breast
cancer' OR 'breast carcinogenesis') AND ('molecular mechanisms' OR
'signaling pathways'). Articles published in English between 2013
and 2025 were included, with priority given to meta-analyses,
systematic reviews and high-impact original research. The clinical
implications of these molecular insights are also discussed,
focusing on novel diagnostic approaches, biomarkers for early
detection and risk assessment, and innovative prevention and
treatment strategies tailored to the obese population. Furthermore,
the present review aims to identify gaps in the current knowledge
and propose future research directions, such as long-term
intervention studies and the exploration of personalized medicine
approaches based on the obese inflammatory phenotype in patients
with BC. The ultimate goal is to enhance the prevention, early
detection and treatment of BC in the context of the obesity
epidemic, improving outcomes for this vulnerable patient population
and reducing the global burden of BC.
Obesity and BC epidemiology
Obesity represents a significant global health
challenge and is a well-established modifiable risk factor for
postmenopausal BC, while its association with premenopausal BC risk
appears more complex (17-19).
Furthermore, a substantial body of evidence has demonstrated that
obesity adversely impacts prognosis and treatment outcomes across
various BC subtypes, contributing to cancer-related mortality
(20-22). This section synthesizes key
epidemiological findings on the relationships among obesity, BC
risk, prognosis and treatment response (Table SI).
Obesity as a risk factor for BC
development
Numerous large-scale epidemiological studies have
demonstrated that an elevated BMI increases the risk of developing
postmenopausal BC (19,23) (Table SI). A secondary analysis of the
Women's Health Initiative (WHI) observational study revealed a
clear positive association between higher BMI and increased risk of
invasive postmenopausal BC (18).
Crucially, Ladoire et al (21) demonstrated that even among
postmenopausal women with a normal BMI, higher levels of body fat
were independently associated with an increased BC risk, suggesting
that adiposity itself, beyond BMI classification, is a critical
factor. The risk appears to be further amplified by metabolic
dysfunction. Agnoli et al (12) found that metabolic syndrome, a
cluster of conditions often associated with obesity (including
hypertension, dyslipidemia, insulin resistance and central
adiposity), conferred an elevated risk of BC in postmenopausal
women compared with that of those without metabolic syndrome. The
underlying mechanisms linking obesity to carcinogenesis involve
chronic low-grade inflammation and oxidative stress (24). Dias et al (24), in a nested case-control study
within the Malmö Diet and Cancer Cohort, provided evidence that
systemic low-grade inflammation and oxidative stress biomarkers
were associated with an increased risk of invasive postmenopausal
BC, suggesting that these inflammatory and oxidative processes may
mechanistically link obesity to breast carcinogenesis by promoting
genomic instability and epithelial cell proliferation. Kabat et
al (19) further investigated
metabolic phenotypes, suggesting that specific obesity-related
metabolic disturbances contribute to BC risk beyond overall
adiposity. Recent data from the WHI reported by Chlebowski et
al (25) reinforced these
findings, revealing higher BC incidence and mortality rates among
postmenopausal women with obesity or metabolic syndrome compared
with those without these conditions. The relationship between
obesity and premenopausal BC risk appears inconsistent, with some
studies suggesting a lower risk in women with a higher BMI,
possibly due to hormonal modulation (19,23).
Impact of obesity on BC prognosis and
outcomes
Beyond increasing BC incidence, obesity is strongly
linked to poorer prognosis in women diagnosed with BC compared with
that of non-obese counterparts, affecting disease-free survival
(DFS) and overall survival (OS) (21,25-27) (Table SI). Ladoire et al
(21), in a pooled analysis of
two large French randomized trials, reported that obese women (BMI
≥30 kg/m2) with node-positive BC had worse DFS and OS
compared with non-obese patients. This adverse prognostic effect
was particularly pronounced in hormone receptor-positive
(HoR+) disease. Analysis of the CALGB 9741 trial by
Ligibel et al (22)
confirmed that a higher BMI was associated with increased
recurrence and mortality risks in women with node-positive BC,
especially within the luminal A subtype as defined by the PAM50
gene expression assay. Widschwendter et al (26), analyzing the SUCCESS A trial
focusing on high-risk early BC (EBC), also found that obesity was
an independent negative prognostic factor for DFS, OS and distant
DFS. Gennari et al (27)
reported that obese patients with high-risk EBC treated with
adjuvant chemotherapy had shorter DFS and OS times compared with
normal-weight patients. Biganzoli et al (28) observed distinct recurrence
dynamics based on baseline BMI, with obese patients showing a
persistently elevated recurrence risk over time compared with
non-obese patients.
However, the prognostic impact appears to vary by BC
subtype. While consistently negative in HoR+ disease,
some studies suggest a potentially less detrimental or even neutral
effect in HER2+ BC or TNBC (26,29). Widschwendter et al
(26) noted that the negative
impact of obesity was significant in HoR+ disease but
not in TNBC within their cohort. Studies continue to explore these
nuances. Lammers et al (29) specifically investigated
HoR+ BC, confirming that a higher BMI was associated
with worse prognosis in this HoR+ patient subgroup
(n=3,521; 78% of the study cohort). Furthermore, obesity may
influence tumor biology and dissemination; Tzschaschel et al
(30) found an association
between obesity and increased detection of circulating tumor cells
in patients with EBC, potentially indicating a mechanism for worse
outcomes. Tangalakis et al (31) suggested that obesity might not
significantly influence the management or outcomes of advanced BC
in elderly patients, highlighting the context-dependency of the
effects of obesity.
Obesity is generally linked to worse prognosis in
HoR+ BC; however, its impact on TNBC remains debated
(32-35). A secondary analysis of the
SUCCESS-A trial indicated that BMI ≥30 kg/m2 did not
significantly influence DFS or OS in the TNBC subgroup [n=769;
hazard ratio (HR), 1.12; 95% CI, 0.82-1.53] (32). Conversely, metabolomics studies
have identified obesity-associated lipid-peroxidation products,
particularly 4-hydroxy-2-nonenal (4-HNE), as activators of the
nuclear factor erythroid 2-related factor 2 (Nrf2) cytoprotective
axis in TNBC cells, promoting doxorubicin and carboplatin
resistance (33-35). Integration of these contradictory
data suggests that the prognostic neutrality of obesity in early
TNBC cohorts may not extend to tumors with high 4-HNE/Nrf2
signaling, a hypothesis requiring prospective validation.
Obesity, treatment response and
interventions
Obesity can modulate the efficacy of BC therapies
and influence treatment-related side effects (36). Studies in the neoadjuvant and
adjuvant settings suggest differential responses based on BMI
(35-37). Di Cosimo et al (36), in an exploratory analysis of the
NeoALTTO trial, observed that obese patients with HER2+
BC had lower rates of pathological complete response following dual
HER2-targeted therapy plus chemotherapy compared with normal-weight
patients. An analysis of the ALTTO trial by Martel et al
(37) also indicated that a
higher BMI was associated with worse outcomes in patients with
HER2+ EBC treated with adjuvant trastuzumab-based
therapy, particularly in terms of DFS. Similarly, sub-analysis of
the APHINITY trial (pertuzumab and trastuzumab) by Dauccia et
al (38) revealed that a
higher BMI was associated with an increased risk of invasive DFS
(IDFS) events in HER2+ EBC, specifically among
node-positive patients, consistent with the subgroup analysis of
patients with HER2− early BC with node-positive disease,
showing an increased risk of IDFS events with higher BMI.
Weight gain during treatment is another concern.
Sedjo et al (39)
documented significant weight gain among overweight and obese BC
survivors prior to enrolling in a weight-loss intervention study.
Mutschler et al (40)
demonstrated that weight gain during adjuvant chemotherapy was an
independent negative prognostic factor for DFS in patients with
high-risk EBC. Conversely, intentional weight loss may improve
outcomes (41,42). Goodwin et al (41) conducted the LISA trial, a
randomized study showing that a telephone-based weight loss
intervention was feasible and effective in reducing weight among
postmenopausal women receiving adjuvant letrozole for BC. While
LISA was not powered for survival endpoints, it established the
principle of intervention feasibility. Babatunde et al
(42) demonstrated that a dietary
and physical activity intervention successfully reduced chronic
inflammation markers in obese African-American women, a group at
heightened risk of obesity-related BC. The impact of obesity
extends to survivorship issues; Inglis et al (43) linked excess body weight in BC
survivors to increased cancer-related fatigue, systemic
inflammation and adverse serum lipid profiles. Furthermore,
Kiecolt-Glaser et al (44)
demonstrated that obesity, alongside chemotherapy, was associated
with lower primary vaccine responses (reduced anti-typhoid Vi IgG
titers) in BC survivors, underscoring obesity-related immune
suppression. Analyses have also explored how obesity might
influence specific treatments. Poggio et al (45), analyzing the GIM2 trial, reported
that the benefit of dose-dense adjuvant chemotherapy on
disease-free survival did not differ significantly between obese
(BMI ≥30 kg/m2) and non-obese patients with early BC
(P=0.17), indicating no evidence for schedule-specific
BMI-dependent efficacy in that cohort. Meanwhile, Biganzoli et
al (46), examining the SOLE
trial, observed that obese patients receiving extended letrozole
had a shorter disease-free survival than non-obese patients,
indicating BMI-related prognostic divergence during prolonged
endocrine therapy. Macis et al (47) explored adiponectin as a potential
mediator of the obesity-BC risk relationship.
In conclusion, epidemiological evidence robustly
establishes obesity as a risk factor for postmenopausal BC
development and a negative prognostic factor impacting survival
outcomes across various BC subtypes, particularly HoR+
disease (Table SI). Obesity also
influences treatment efficacy, potentially contributing to reduced
response rates in neoadjuvant settings and worse survival outcomes
in adjuvant settings, while also exacerbating treatment-related
toxicities and survivorship issues (41,42). These findings underscore the
critical importance of addressing obesity within comprehensive BC
prevention and management strategies. Further research is required
to fully elucidate subtype-specific effects and optimize weight
management interventions to improve BC outcomes (47).
Molecular mechanisms linking obesity to
BC
Adipose expansion in obesity orchestrates a
protumorigenic milieu: Hypertrophic adipocytes release excess
leptin and reduced adiponectin, skewing signaling toward Janus
kinase 2 (JAK2)/STAT3 and PI3K/AKT/mTOR activation, while insulin
resistance-driven hyperinsulinemia amplifies insulin-like growth
factor-1 (IGF-1)-mediated mitogenesis (48-50). Concurrently, dying adipocytes
recruit M1 macrophages that form crown-like structures (CLS) and
secrete IL-6 and TNF-α, sustaining NF-κB and MAPK loops, which
promote DNA damage, epithelial-mesenchymal transition (EMT) and
angiogenesis (51,52). These intertwined adipokine,
metabolic and inflammatory circuits, including leptin-STAT3,
insulin-PI3K/AKT/mTOR and TNF-α/IL-6-driven NF-κB activation, are
schematically shown in Fig. 1
(48-52), which illustrates obesity-induced
breast tissue inflammation, CLS formation and downstream
protumorigenic signaling cascades.
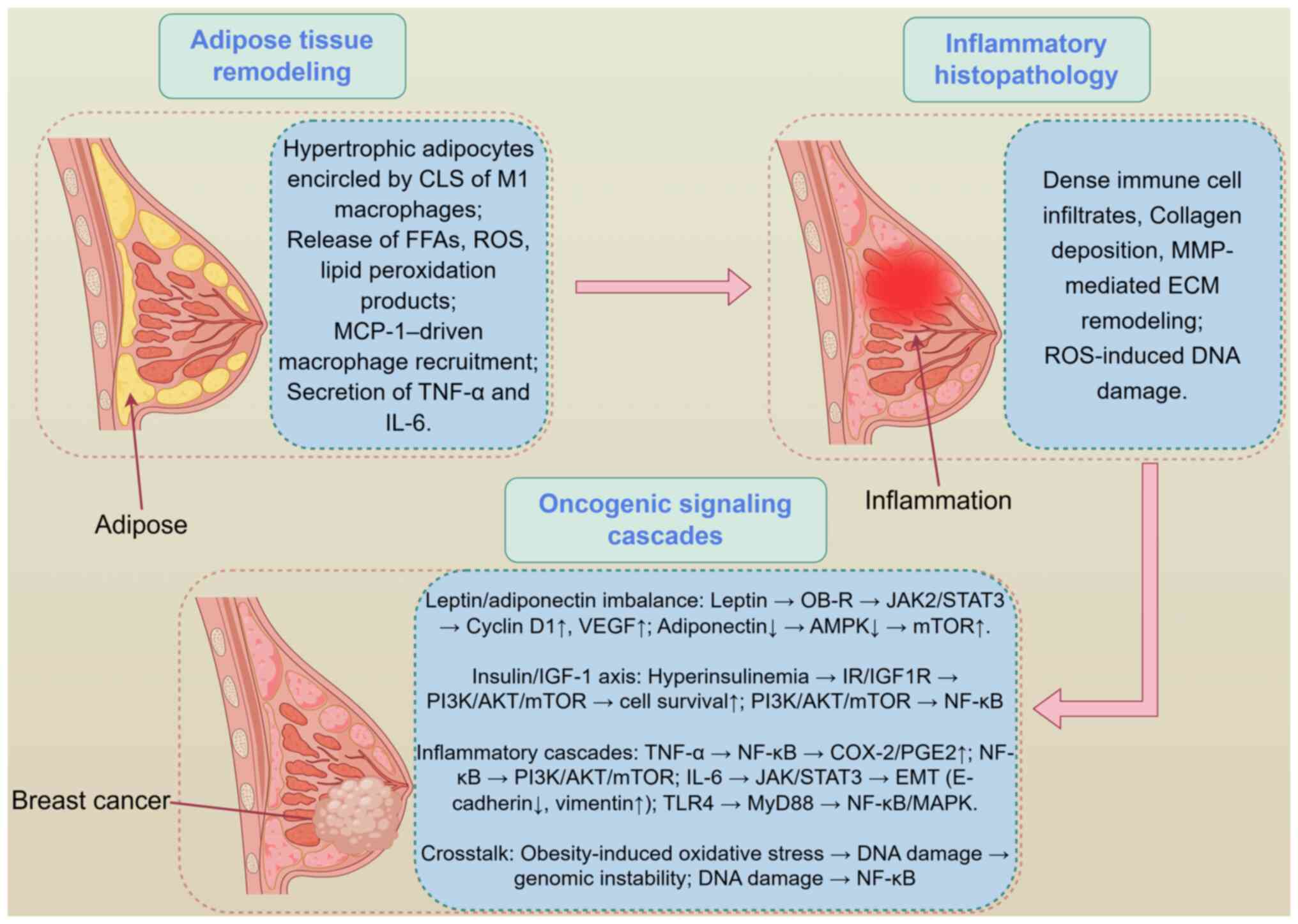 | Figure 1Obesity-induced molecular events and
chronic breast inflammation. By Figdraw (https://www.figdraw.com/static/index.html#/; 2.0
version; ID: RSAYO3b3b5). AMPK, AMP-activated protein kinase; CLS,
crown-like structures; COX-2, cyclooxygenase-2; ECM, extracellular
matrix; EMT, epithelial-mesenchymal transition; FFA, free fatty
acids; IGF-1, insulin-like growth factor-1; IGF1R, IGF-1 receptor;
IR, insulin receptor; JAK2, Janus kinase 2; MCP-1, monocyte
chemoattractant protein-1; MyD88, myeloid differentiation primary
response 88; PGE2, prostaglandin E2; ROS, reactive oxygen species;
TLR4, toll-like receptor 4. |
Adipokines and their roles
Leptin
The levels of leptin, predominantly produced by
adipose tissue, are elevated in obese individuals (48). Leptin binds to its receptor OB-R,
which is expressed in various tissues, including breast tissue
(48). The activation of leptin
signaling pathways promotes BC development and progression through
the JAK-STAT and PI3K-Akt pathways. Specifically, leptin activates
JAK2, leading to STAT3 phosphorylation and nuclear translocation,
which promotes cell proliferation and angiogenesis (48,49). Leptin also activates the PI3K-Akt
pathway, reducing apoptosis and stimulating mTOR-dependent cell
proliferation (50).
In breast epithelial cells, insulin receptor
substrate 1/2 (IRS-1/2) phosphorylation constitutes a shared
regulatory node that integrates leptin-activated JAK2/STAT3
signaling with the insulin resistance-driven PI3K/AKT/mTOR axis
(53). Leptin induces
JAK2-dependent phosphorylation of IRS-1 on Tyr608 and Ser307, which
impairs insulin-receptor signaling and promotes compensatory
hyperinsulinemia (53).
Conversely, chronic hyperinsulinemia leads to phosphorylation of
IRS-1/2 on additional serine residues (Ser612 and Ser636) via
mTOR/ribosomal protein S6 kinase B1 feedback loops, further
amplifying PI3K/AKT/mTOR activity (54). This bidirectional crosstalk
creates a feed-forward circuit in which leptin-driven JAK2/STAT3
signaling exacerbates insulin resistance, and hyperinsulinemia in
turn heightens leptin sensitivity, jointly accelerating breast
epithelial proliferation (55).
Adiponectin
Adiponectin, another important adipokine secreted by
adipose tissue, has antitumor properties (56). Adiponectin binds to its receptors
adiponectin receptor (AdipoR)1 and AdipoR2 on the surface of cells,
including breast cells, and activates the AMP-activated protein
kinase (AMPK) pathway (56). AMPK
is an energy-sensing enzyme that regulates cellular metabolism.
Activation of AMPK by adiponectin leads to a series of downstream
effects that inhibit cell proliferation and promote apoptosis
(57). Conversely,
gene-expression profiling has revealed increased adiponectin
signaling in aggressive Claudin-low and mesenchymal-type BCs,
indicating that adiponectin function may be context-dependent
rather than adiponectin being universally protective (58). However, adiponectin levels are
decreased in obesity, impairing these protective effects and
potentially contributing to BC development (59).
Insulin resistance and
hyperinsulinemia
Obesity is closely associated with insulin
resistance, characterized by reduced cellular responsiveness to
insulin (60). This condition is
driven by hypertrophic adipose tissue that secretes
pro-inflammatory cytokines such as TNF-α and IL-6, thereby
promoting chronic inflammation (60). These cytokines interfere with
insulin signaling by activating the NF-κB pathway, leading to the
expression of suppressor of cytokine signaling proteins that
inhibit IRS signaling (61).
Additionally, lipotoxicity from excess free fatty acids generates
diacylglycerols and ceramides, which activate protein kinase C
isoforms and impair insulin receptor (IR) and IRS function through
serine phosphorylation (62).
Oxidative stress further exacerbates insulin resistance by damaging
insulin signaling components (62).
Insulin resistance leads to hyperinsulinemia, which
can affect breast cells (63).
Insulin binds to IRs on breast cells, activating the PI3K-Akt-mTOR
pathway (63). This promotes cell
proliferation and survival by stimulating protein and lipid
synthesis and inhibiting apoptosis (63). Hyperinsulinemia also enhances the
activity of the IGF pathway. Insulin can bind to IGF-1 receptors
with lower affinity, but high insulin levels can amplify
growth-promoting signals through crosstalk between insulin and IGF
pathways (64). These activated
pathways synergistically promote cell proliferation, survival and
transformation, increasing the BC risk (64).
Inflammatory mediators from adipose
tissue
In summary, obesity drives BC development and
progression through molecular mechanisms such as adipokine
dysregulation (for example, elevated leptin levels and reduced
adiponectin levels), insulin resistance and compensatory
hyperinsulinemia, and chronic inflammation mediated by cytokines
such as IL-6 and TNF-α (19,63). Understanding these mechanisms is
crucial for developing targeted prevention and treatment strategies
for BC in obese individuals.
TNF-α and IL-6 levels are markedly elevated in obese
mammary fat (65,66). TNF-α promotes cell survival via
PI3K/Akt-dependent upregulation of Bcl-2 (65) and induces COX-2 transcription
(66). IL-6 triggers JAK/STAT3
phosphorylation, driving VEGF secretion and EMT (51). Conversely, local delivery or
adipocyte-derived IL-4/IL-13 can polarize macrophages toward an
anti-inflammatory M2 phenotype, which downregulates TNF-α and IL-6,
and attenuates NF-κB signaling, and thereby limits obesity-driven
epithelial proliferation (51,52). The canonical NF-κB and MAPK
cascades activated by these cytokines are described in the section
on molecular pathways in chronic breast inflammation, where they
are examined specifically within the chronically inflamed breast
microenvironment.
Role of non-coding RNAs in
obesity-related BC
Emerging evidence has highlighted the critical role
of non-coding RNAs, particularly long non-coding RNAs (lncRNAs), in
mediating obesity-driven breast inflammation and carcinogenesis
(67,68). The lncRNA HOX transcript antisense
RNA (HOTAIR) is upregulated in breast adipose tissue of obese
individuals and promotes tumor progression by recruiting
chromatin-modifying complexes that silence tumor suppressor genes
(67). In obese mouse models,
HOTAIR overexpression is associated with enhanced NF-κB and STAT3
signaling, driving pro-inflammatory cytokine production and
macrophage polarization towards an M2 tumor-promoting phenotype
(68).
Pre-clinical proof-of-concept studies have indicate
that antisense oligonucleotides (ASOs) targeting the
obesity-upregulated lncRNA HOTAIR can blunt tumor growth in murine
BC models (58,69). Gupta et al (69) first demonstrated that HOTAIR
recruits polycomb repressive complex 2 to silence
metastasis-suppressor loci; when HOTAIR was knocked down with
2'-O-methyl gapmer ASOs (25 mg kg−1; intraperitoneal;
twice weekly for 3 weeks), orthotopic 4T1 mammary tumors grew 45%
more slowly and exhibited a 60% reduction in pulmonary metastatic
foci (P<0.01) compared with those of control mice treated with
scrambled antisense oligonucleotides. In high-fat diet-induced
obese MMTV-PyMT mice, the same regimen decreased mammary tumor
multiplicity and lowered IL-6 and TNF-α protein levels in
peritumoral adipose tissue, indicating that HOTAIR blockade can
simultaneously restrain tumor progression and dampen obesity-driven
inflammation (58). RNA
sequencing (RNA-seq) of liver and kidney samples collected at
necropsy revealed no significant off-target transcriptomic
perturbations, and serum chemistry profiles remained within normal
limits, suggesting an acceptable acute-toxicity window (32). While these data are confined to
rodent systems and lack chronic-dosing or formal immunogenicity
analyses, they provide the first direct evidence that
pharmacological inhibition of HOTAIR can reverse obesity-promoted
mammary tumorigenesis and adipose inflammation, providing a
mechanistic rationale for future Good Laboratory Practice
toxicology and dose-finding studies (32).
RNA-seq analysis of mammary tissue from ASO-treated
obese mice revealed minimal off-target transcriptomic perturbations
beyond the intended HOTAIR network, with no significant toxicity in
liver or kidney function tests (70). To further assess systemic safety,
RNA-seq was also performed on liver and kidney tissues collected at
necropsy; these datasets showed no significant off-target gene
expression changes compared with scrambled-ASO controls, supporting
the specificity of the HOTAIR-targeting approach (70). However, comprehensive
organ-specific toxicity studies and assessments of immune
activation (for example, complement activation) remain pending,
highlighting the need for rigorous safety evaluations before
clinical translation (71).
Obesity-mediated tumor immune
microenvironment (TIME) remodeling
In addition to myeloid-derived suppressor cell
(MDSC) expansion via leptin/IL-6-JAK/STAT3 signaling, obesity
directly enforces T-cell exhaustion in breast tissue (72). Single-cell RNA-seq of mammary
tumors from high-fat diet-fed MMTV-PyMT mice revealed a 2.3-fold
increase in programmed cell death protein 1 (PD-1)+
T-cell immunoglobulin and mucin-domain containing-3+
CD8+ T cells vs. lean controls, accompanied by reduced
granzyme-B secretion (P<0.01) (72). Analogously, peripheral blood of
obese post-menopausal women (BMI ≥35 kg m−2) harbored
significantly higher exhausted T cell frequencies (PD-1+
cytotoxic T-lymphocyte associated protein 4+) that were
correlated with elevated serum leptin levels (r=0.62; P=0.003)
(73). These findings demonstrate
that obesity promotes the expansion of immunosuppressive myeloid
cells and concurrently impairs cytotoxic T-cell function, thereby
facilitating immune escape during early breast carcinogenesis.
Chronic breast inflammation and BC
Obesity-driven CLS formation, macrophage
infiltration and sustained TNF-α/IL release establish a
protumorigenic breast microenvironment (66,74,75). Histological immune clustering,
oxidative DNA damage and extracellular matrix remodeling converge
on NF-κB and MAPK activation, accelerating carcinogenesis (as
schematically outlined in Fig. 1,
which integrates these obesity-driven inflammatory and molecular
events into a unified protumorigenic framework).
Definition and characteristics of chronic
breast inflammation Histological features
Chronic breast inflammation is characterized by
immune cell infiltrates, including macrophages and lymphocytes,
often forming clusters around adipocytes or within the stromal
compartments (74). This
condition is associated with fibrosis and tissue remodeling,
leading to the deposition of extracellular matrix components such
as collagen and the activation of fibroblasts. These changes alter
breast tissue architecture, with activated fibroblasts depositing
extracellular matrix proteins such as collagen, thereby forming
fibrotic bands and replacing normal glandular tissue with dense
fibrous tissue (76). Proteolytic
enzymes such as MMPs are also activated, facilitating cell
migration and potentially promoting cancer cell dissemination
(77).
Causes of chronic breast inflammation in
the context of obesity
Obesity contributes to chronic breast inflammation
through several mechanisms. Adipocyte death is a key factor. In
obese individuals, the increased size of adipocytes can lead to
hypoxia and cell death. The dead adipocytes trigger an inflammatory
response, with infiltration of macrophages and the formation of CLS
around dead adipocytes (78).
These CLS are composed of macrophages encircling a necrotic core of
dead adipocytes and are associated with the secretion of
pro-inflammatory cytokines (78).
Additionally, lipid peroxidation and oxidative stress serve
significant roles in amplifying local inflammation and promoting
DNA damage within the obese breast tissue microenvironment
(66). The excessive accumulation
of lipids in obese breast tissue can lead to the generation of ROS
and lipid peroxidation products. These oxidative stress-related
molecules can damage cellular components, including DNA, proteins
and lipids, and activate inflammatory signaling pathways, further
exacerbating the inflammatory response in the breast tissue
(79).
Molecular pathways in chronic breast
inflammation-induced carcinogenesis
NF-κB pathway activation
The NF-κB pathway is a central player in the
inflammatory response and is frequently activated in chronic breast
inflammation (75). Inflammatory
mediators, such as TNF-α and IL-1β, which are produced by immune
cells in the inflamed breast tissue, can activate the NF-κB pathway
(75). Upon activation, NF-κB
translocates to the nucleus and regulates the transcription of
numerous genes involved in cell survival, proliferation and
inflammation. For example, NF-κB activation leads to the increased
expression of anti-apoptotic proteins such as Bcl-2 and Bcl-XL,
promoting cell survival. NF-κB activation also upregulates cyclin
D1 and other cell cycle-promoting genes, driving cell proliferation
(75). Furthermore, NF-κB induces
the production of pro-inflammatory cytokines and chemokines,
creating a self-sustaining inflammatory loop that contributes to
the development of a tumor-promoting microenvironment (75).
MAPK pathway activation
The MAPK pathway is another critical signaling
pathway involved in chronic breast inflammation-induced
carcinogenesis (80). There are
several types of MAPK pathways, including the ERK, JNK and p38
kinase pathways. In chronic breast inflammation, these pathways can
be activated by various stimuli, such as growth factors, cytokines
and oxidative stress (80). For
instance, ERK activation can be triggered by growth factors
released from inflammatory cells, leading to the phosphorylation
and activation of transcription factors such as Elk-1 and c-Fos,
which promote cell proliferation and differentiation (81). JNK and p38 pathways are often
activated in response to stress signals, including oxidative stress
and inflammatory cytokines. Their activation results in the
phosphorylation of transcription factors such as c-Jun and
activating transcription factor-2, which regulate the expression of
genes involved in cell survival, apoptosis and inflammation
(82). The activation of MAPK
pathways can lead to changes in breast cell behavior, such as
increased proliferation, migration and invasion, which are
hallmarks of cancer development (80-82).
Toll-like receptor (TLR)
signaling
TLRs are pattern recognition receptors that serve a
crucial role in the innate immune response (83). During chronic breast inflammation,
TLRs in breast tissue can be activated by various endogenous
ligands released from damaged cells or by pathogens (83). For example, TLR4 can be activated
by saturated fatty acids, the levels of which are elevated in the
breast tissue of obese individuals (83). Once activated, TLRs initiate
downstream signaling cascades, such as the MyD88-dependent pathway,
leading to the activation of NF-κB and MAPK pathways. This results
in the production of pro-inflammatory cytokines and chemokines,
amplifying the inflammatory response in the breast tissue (83). The chronic activation of TLR
signaling can create a prolonged inflammatory state, which
contributes to DNA damage, genomic instability and the promotion of
carcinogenesis (84).
Additionally, TLR signaling can also influence the TIME by
modulating the function and recruitment of immune cells,
potentially facilitating tumor immune evasion and progression
(85).
In summary, chronic breast inflammation,
particularly in the context of obesity, contributes to breast
carcinogenesis through various molecular pathways, including the
NF-κB, MAPK and TLR signaling pathways. These pathways drive the
development of a tumor-promoting microenvironment, characterized by
increased cell survival, proliferation and inflammation, thereby
increasing the risk of BC. Understanding these mechanisms provides
insights into potential therapeutic targets for the prevention and
treatment of BC in individuals with chronic breast
inflammation.
Interplay between obesity-related factors
and chronic breast inflammation in carcinogenesis
Dynamic, time-resolved crosstalk among adipocytes,
immune cells and epithelia converts obesity-associated inflammation
from a reversible stress into an irreversible oncogenic driver
(86). This section delineates
the sequential ignition, amplification and commitment phases that
precede malignant transformation, emphasizing dynamic feed-forward
loops, circadian regulation and epigenetic memory. Rather than
merely outlining isolated signaling events, it integrates temporal
and contextual dynamics to provide a more mechanistic understanding
of obesity-driven breast carcinogenesis (Fig. 2). Notably, the time-resolved
sequence summarized in Fig. 2 is
derived exclusively from ovariectomized (postmenopausal) MMTV-PyMT
mice and short-term explants of breast adipose tissue from obese
postmenopausal women (23,87,88).
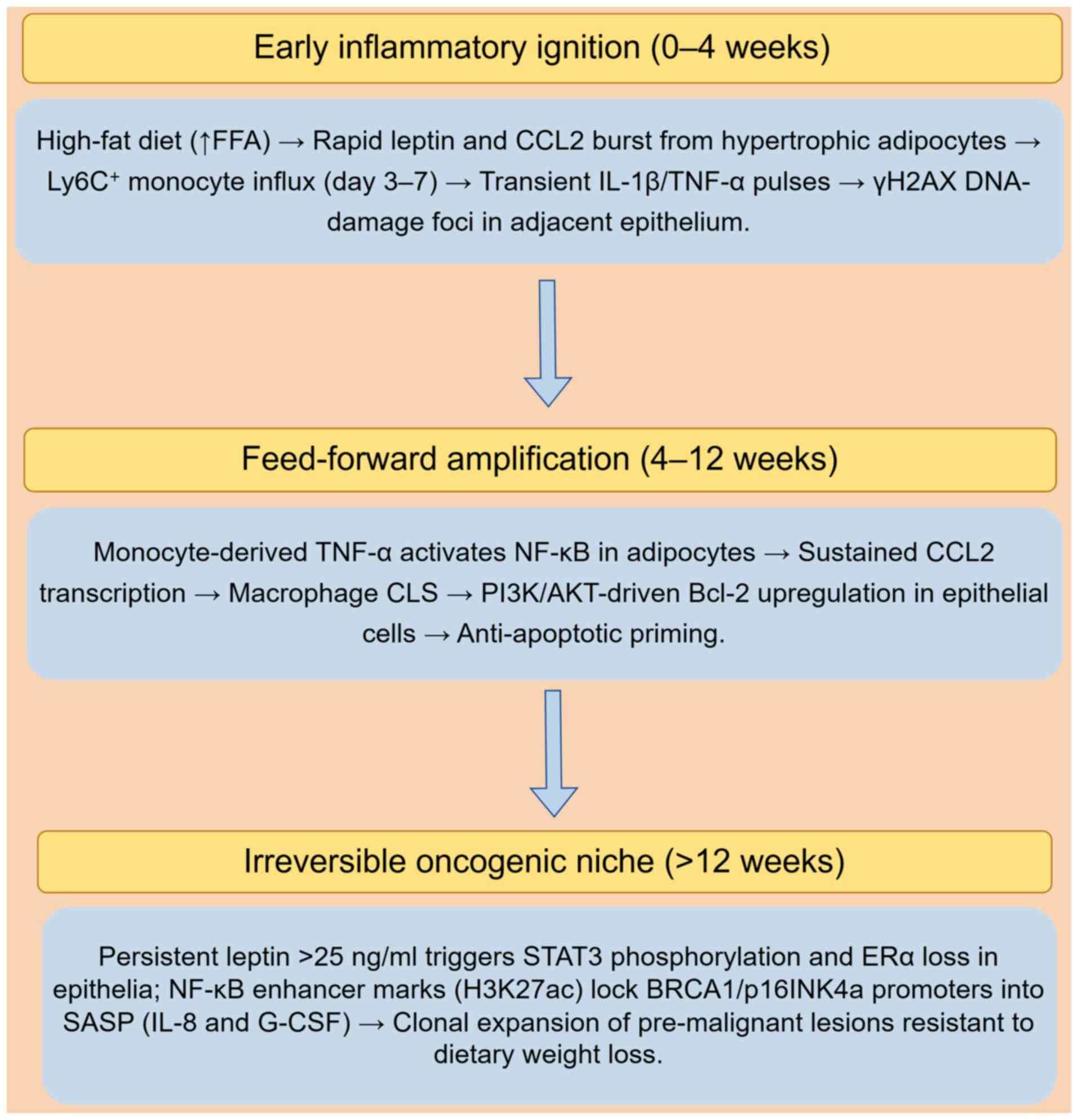 | Figure 2Time-resolved crosstalk in the obese
breast microenvironment during early carcinogenesis. By Figdraw
(https://www.figdraw.com/static/index.html#/; 2.0
version; ID: UAAWW758ae). The depicted adipocyte-immune
interactions and epigenetic reprogramming events are primarily
derived from high-fat diet-fed, ovariectomized (postmenopausal)
murine mammary tissue models and ex vivo explants from obese
postmenopausal women. γH2AX, phosphorylated histone H2AX; CCL2, C-C
motif chemokine ligand 2; CLS, crown-like structures; ERα, estrogen
receptor α; FFA, free fatty acids; G-CSF, granulocyte-colony
stimulating factor; H3K27ac, histone H3 lysine 27 acetylation;
Ly6C, lymphocyte antigen 6C; SASP, senescence-associated secretory
phenotype. |
Early inflammatory ignition (0-4
weeks)
High-fat feeding in MMTV-PyMT mice rapidly (within 7
days) elevates mammary C-C motif chemokine ligand 2 (CCL2) and
leptin secretion, leading to lymphocyte antigen 6C-positive
monocyte influx that precedes CLS formation (87). Single-cell RNA-seq time-courses
reveal that these monocytes transiently express IL-1β and TNF-α at
day 7, coinciding with phosphorylated histone H2AX DNA-damage foci
in adjacent epithelial cells (86). In human breast tissue explants
from obese women (BMI ≥30 kg/m2), a similar early
cytokine switch (IL-1β to IL-6) has been observed within 6 days
ex vivo, supporting the translational relevance (23).
Feed-forward amplification (4-12
weeks)
During this amplification phase, NF-κB activation in
adipocytes further amplifies CCL2 transcription, establishing a
self-sustaining macrophage retention loop (88). Simultaneously, monocyte-derived
TNF-α activates NF-κB in adipocytes, further reinforcing this
feed-forward inflammatory cycle (88). Concomitant PI3K/AKT signaling in
epithelial cells upregulates anti-apoptotic Bcl-2, rendering cells
resistant to physiological clearance (65). This feed-forward circuit is unique
to the obese state; in lean controls, macrophage numbers plateau
after day 7 and do not sustain NF-κB activity (86).
Irreversible oncogenic niche transition
(>12 weeks)
After chronicity, transient NF-κB pulses are
replaced by stable enhancer histone marks (histone H3 lysine 27
acetylation) at BRCA1 and p16INK4a promoters, locking
cells into a senescence-associated secretory phenotype that
continuously secretes IL-8 and granulocyte-colony stimulating
factor (88). Obesity-specific
miR-155 elevation persists even upon in vitro adipocyte
dedifferentiation, indicating a cell-autonomous memory transferable
to epithelial cells via exosomal cargo (89). Multiphoton intravital microscopy
demonstrates that CD68+ macrophages form periductal
niches that expand clonally once circulating leptin exceeds 25 ng
ml−1; epithelial cells within these niches exhibit STAT3
phosphorylation and loss of estrogen receptor α expression, changes
that do not regress after 8 weeks of dietary weight loss (90), signifying an irreversible
commitment to oncogenesis.
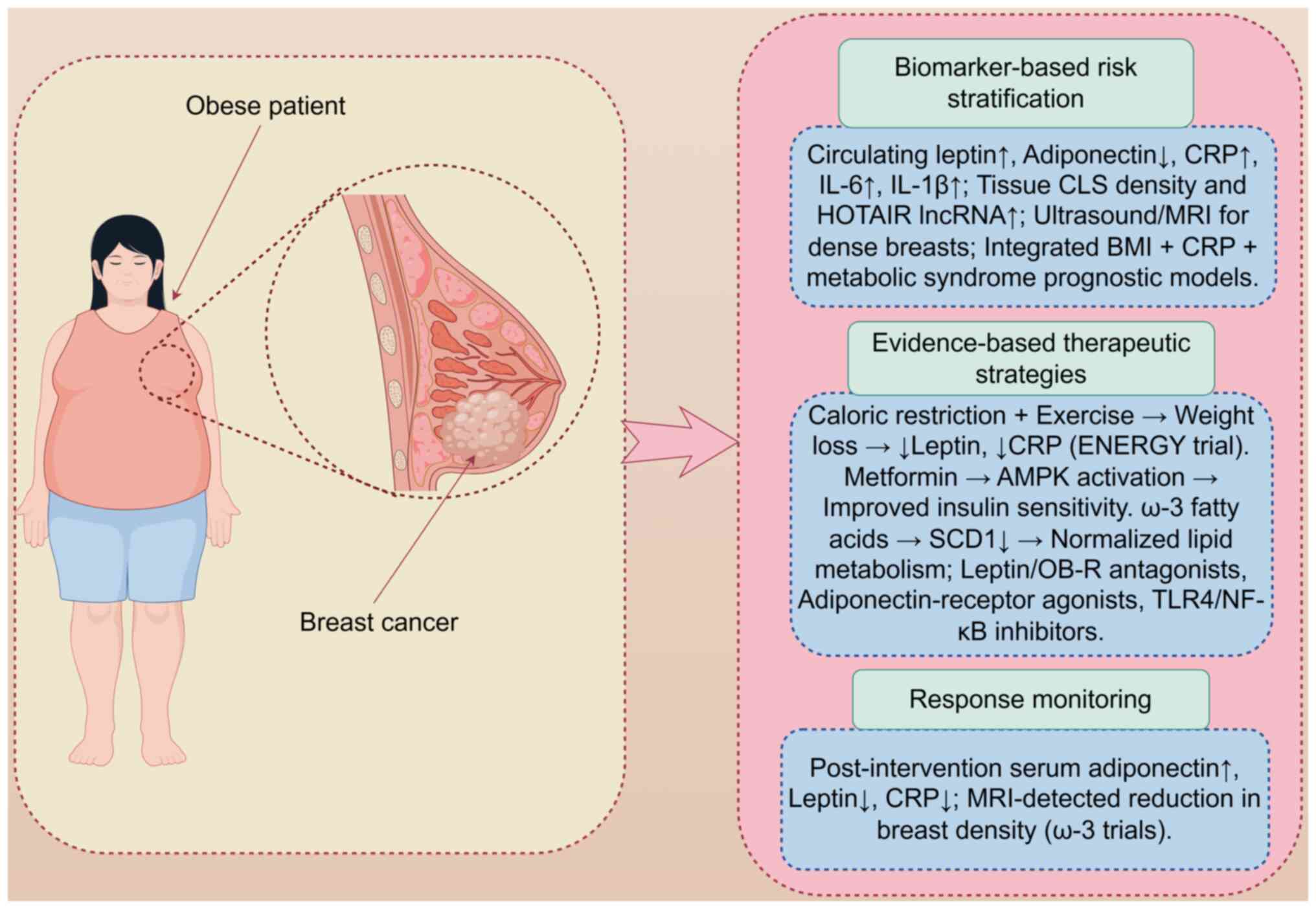
Clinical implications
Composite models combining elevated leptin, CRP and
IL-6 levels, and MRI-detected breast density outperform
single-parameter risk prediction (91-93). Lifestyle caloric restriction,
metformin-mediated AMPK activation and high-dose ω-3 fatty acids
reduce systemic inflammation and tumor-promoting signaling;
therapeutic monitoring via serial assessment of these biomarker
trajectories can track intervention efficacy and disease
progression (Fig. 3). Fig. 3 illustrates an integrated clinical
approach for obese patients with BC, encompassing risk
stratification using composite biomarkers and imaging, targeted
interventions including lifestyle modifications and
pharmacotherapy, and continuous monitoring of biomarker dynamics to
optimize outcomes (91,94,95).
Diagnosis
Biomarkers for the prediction of BC
risk in obese patients with chronic breast inflammation
In obese patients with chronic breast inflammation,
several biomarkers show promise in predicting BC risk. Adipokines
are a key focus. Leptin, the levels of which are elevated in
obesity, is linked to BC risk, and promotes cell proliferation and
inhibits apoptosis via pathways such as the JAK-STAT and PI3K-Akt
pathways (96). Conversely,
adiponectin levels are reduced in obesity, and adiponectin has
anti-inflammatory and anti-proliferative effects. Lower adiponectin
levels are associated with an increased risk of BC (97).
Inflammatory markers are also crucial. CRP, a
marker of systemic inflammation, is positively associated with BC
risk in obese postmenopausal women (91). Elevated CRP levels are linked to
shorter DFS (91). Similarly,
IL-6, IL-1β and TNF-α are upregulated in obese breast tissue. These
cytokines induce DNA damage through ROS production and promote a
tumor-supportive microenvironment (92).
Imaging techniques for the detection
of BC in obese individuals
Obesity poses challenges for traditional
mammography. The increased breast density and volume can obscure
tumors, reducing the sensitivity of mammography (98). Alternative imaging modalities are
being explored (98). Ultrasound
is useful for distinguishing between cystic and solid masses.
Ultrasound offers real-time imaging and avoids radiation exposure.
However, its sensitivity decreases for smaller tumors (99). MRI shows higher sensitivity than
traditional mammography in detecting BC in obese patients. MRI
provides detailed soft tissue contrast and can identify multiple
tumors. Despite its advantages, MRI is costly and may yield
false-positive results, requiring further confirmation (93).
Prognosis
Impact of obesity and chronic breast
inflammation on BC prognosis
Obesity impacts BC prognosis. Obese patients with
BC often have poorer OS rates compared with normal-weight patients.
They tend to present with higher tumor grades, larger tumor sizes
and increased lymph node involvement (100). Obesity-related inflammation
promotes tumor progression and metastasis (101). Additionally, compared with their
non-obese counterparts, obese patients are more likely to develop
resistance to endocrine therapy and chemotherapy (102).
Molecular mechanisms related to obesity and
inflammation influence prognosis. The chronic inflammatory state in
obese patients leads to elevated levels of pro-inflammatory
cytokines such as IL-6 and TNF-α. These cytokines activate
signaling pathways that promote cell proliferation, inhibit
apoptosis and enhance angiogenesis (52). The dysregulated adipokines leptin
and adiponectin also contribute to a poorer prognosis in obese
patients compared with their non-obese counterparts by creating a
tumor-supportive microenvironment (52). Furthermore, obesity-associated
insulin resistance and elevated IGF-1 levels are linked to
increased tumor recurrence and mortality (103).
Prognostic models incorporating
obesity-related and inflammatory factors
Prognostic models that integrate obesity-related
and inflammatory factors are being developed to better predict BC
outcomes. These models incorporate clinical data such as BMI, waist
circumference and metabolic markers (94). They also include levels of
inflammatory cytokines such as CRP, IL-6 and TNF-α. Laforest et
al (95) have shown that
combination of these factors improves the accuracy of prognosis
prediction. For instance, a model incorporating BMI and CRP levels,
along with components of metabolic syndrome such as waist
circumference, hypertension, dyslipidemia and insulin resistance,
demonstrated better predictive performance for disease recurrence
and survival compared with models using single factors (93). However, validation across diverse
patient populations is needed to ensure reliability and
applicability. Further research is ongoing to refine these models
and enhance their clinical utility (91).
Therapeutic strategies for obesity
management in BC
The established link between obesity and adverse BC
outcomes necessitates evidence-based therapeutic strategies
targeting weight management and metabolic dysregulation (104). This section synthesizes clinical
evidence on interventions spanning lifestyle modifications,
pharmacotherapy and molecularly targeted approaches, emphasizing
their mechanistic foundations and clinical applicability (Table SII).
Lifestyle interventions: Cornerstone of
weight management
Lifestyle interventions combining dietary
modification and physical activity constitute the most extensively
studied therapeutic approach. The landmark ENERGY trial
demonstrated that a structured 12-month behavioral intervention
(caloric restriction + 225 min/week moderate exercise) achieved
marked weight reduction (mean loss, 6.0% body weight) in
overweight/obese BC survivors, translating to clinically relevant
improvements in physical function and quality of life (105,106). Furthermore, this trial
established the feasibility of sustained weight management in
cancer survivors, a population often challenged by
treatment-related fatigue and metabolic alterations. Subsequent
research refined these approaches for specific subgroups:
Culturally adapted interventions for African American survivors
yielded a weight loss of 6.1% vs. 1.8% in controls (P<0.001)
(107), while telemedicine-based
programs effectively addressed barriers for rural populations,
maintaining a weight loss of 7.3% at 18 months (108). Exercise regimens require
optimization; Courneya et al (109) established a dose-response
relationship, showing that 300 min/week of aerobic exercise
significantly improved quality of life vs. 150 min/week in
postmenopausal survivors (P<0.05).
Pharmacological and metabolic inter
ventions
Pharmacological strategies target
obesity-associated metabolic pathways to enhance treatment
efficacy. Yam et al (110) conducted a phase II trial
combining metformin (500 mg twice daily), everolimus (5 mg/day) and
exemestane in obese metastatic HoR+ patients,
demonstrating a 48% clinical benefit rate and median
progression-free survival of 5.6 months. This synergistic approach
concurrently inhibited mTOR signaling and estrogen synthesis,
counteracting obesity-induced pathway activation. Similarly,
metformin monotherapy shows promise; Patterson et al
(111) designed the first
randomized controlled trial (RCT) examining metformin + weight loss
in survivors, with preliminary data suggesting enhanced insulin
sensitivity. Complementarily, nutritional pharmacology leverages
specific nutrients to modulate molecular pathways: High-dose ω-3
fatty acids (3.6 g/day) reduced breast density by 6.2% [Sandhu
et al (112); P=0.03],
and improved leptin/adiponectin ratios in high-risk women (113). Mechanistically, Manni et
al (114) identified
ω-3-mediated suppression of stearoyl-CoA desaturase-1 (reduction of
38%; P=0.008), a key enzyme in lipid metabolism promoting
tumorigenesis in obesity.
Targeting molecular mediators and
biomarkers
Emerging strategies focus on obesity-related
molecular mediators as therapeutic targets and response biomarkers.
Bowers et al (88)
demonstrated that caloric restriction reversed obesity-induced
pro-tumorigenic genomic signatures in triple-negative models,
suppressing tumor growth by 67% (P<0.001) through epigenetic
modulation. Clinically, molecular biomarkers facilitate
intervention personalization: Macis et al (47) established adiponectin as a
mediator of 19% of the obesity-BC risk association (P=0.02),
suggesting its utility for monitoring intervention efficacy.
Concurrently, interventions modulate inflammatory cascades;
combined exercise and weight loss in the WISER Survivor trial
significantly reduced CRP (-12.3%; P=0.03) and insulin resistance
(homeostatic model assessment of insulin resistance reduced by
18.5%; P=0.01) (90,115). Furthermore, recent translational
work has revealed that weight loss alters cancer-related proteins:
Bull et al (116)
identified 14 serum proteins (including leptin and IL-6) modulated
by weight loss, providing mechanistic insights for BC
applications.
Therapeutic strategies for obesity in BC span
lifestyle, pharmacological and molecular interventions,
collectively targeting metabolic dysregulation, inflammation and
tumor-promoting pathways. While lifestyle modifications remain
foundational, emerging biomarker-driven approaches enable
personalization. Crucially, overcoming implementation barriers
requires culturally adapted, accessible programs integrated into
standard oncology care. As evidenced by the reversal of
obesity-induced genomic alterations reported by Bowers et al
(88), these strategies hold
promise not only for improving outcomes but potentially disrupting
the obesity-BC pathogenetic axis.
Emerging evidence suggests that obesity modulates
the TIME in TNBC, influencing the response to immune checkpoint
inhibitors (ICIs) (72). In obese
TNBC models, leptin and IL-6 promote the expansion and recruitment
of MDSCs via JAK/STAT3 signaling, which dampens cytotoxic T-cell
activity and contributes to immunotherapy resistance (72). For instance, Pingili et al
(72) demonstrated that
diet-induced obesity increased MDSC infiltration and reduced PD-1
inhibitor efficacy in TNBC murine models, an effect reversible upon
MDSC depletion or leptin signaling blockade. Clinically, a
retrospective analysis has indicated that obese patients with TNBC
may exhibit lower objective response rates to anti-PD-1/programmed
death-ligand 1 (PD-L1) therapies compared with non-obese
counterparts (117), although
some studies have reported a paradoxical 'obesity benefit' in other
cancer types, underscoring context-dependent effects (72,73). These findings highlight the need
to consider obesity-associated immune dysregulation, particularly
MDSC-mediated suppression, when designing immunotherapy trials for
TNBC.
Limitations of current obesity-targeted
therapies in BC management
Despite mechanistic evidence supporting
obesity-targeted interventions (88), clinical translation faces
significant limitations. First, most lifestyle trials (such as the
ENERGY and LISA trials) are underpowered for survival endpoints,
with follow-up ≤5 years, precluding definitive conclusions on
long-term oncologic benefits (88,105,106). Second, pharmacologic approaches
exhibit constrained efficacy: Metformin monotherapy failed to
improve IDFS in the National Cancer Institute of Canada (NCIC)
MA.32 trial (HR, 1.01; 95% CI, 0.84-1.21; n=3,649), despite
preclinical AMPK activation (118). Everolimus-based regimens suffer
from dose-limiting toxicities (mucositis, 63% grade ≥3),
restricting applicability in obese populations with pre-existing
metabolic syndrome (110).
Third, biomarker-driven stratification remains rudimentary: While
adiponectin mediates 19% of the obesity-BC risk association
(47), to the best of our
knowledge, no trial has prospectively selected or stratified
patient cohorts based on adipokine signatures, leading to
heterogeneous responses. Fourth, trials disproportionately enroll
postmenopausal HoR+ patients [≥70% in the ENERGY trial
(105)], leaving TNBC and
premenopausal obesity-associated BC understudied. Finally,
weight-loss interventions exhibit poor durability: 40% of
participants in the WISER Survivor trial regained ≥5% body weight
within 18 months post-intervention (90), undermining sustained
anti-inflammatory effects. These gaps necessitate adaptive trial
designs integrating real-time metabolomic profiling and minimal
residual disease monitoring to circumvent empirical therapy
limitations.
Although lifestyle modification remains the
cornerstone, the pooled adherence across four RCTs in BC survivors
was only 54% at 12 months and fell to 34% by 36 months (105-108). Pharmacologic approaches face
uncertainty regarding long-term safety: The NCIC MA.32 trial showed
no excess adverse events with metformin after a median follow-up of
6.3 years (118); however, to
the best of our knowledge, liraglutide or tirzepatide have not been
prospectively evaluated in BC survivors for durations exceeding 2
years, and their chronic gastrointestinal and pancreatic safety
profiles remain undefined. Likewise, dose-limiting mucositis (grade
≥3 rate, 63%) restricted everolimus exposure in the
metformin-everolimus-exemestane phase-II study (110), underscoring the need for
rigorous toxicity surveillance.
The impact of obesity on immunotherapy response
also remains underexplored in clinical trials. While preclinical
data implicate MDSCs and adipokine signaling in ICI resistance
(117), to the best of our
knowledge, no prospective studies have stratified patients with
TNBC by obesity status or MDSC levels when evaluating PD-1/PD-L1
inhibitors at present. This represents a significant translational
gap, as combinatorial strategies targeting MDSCs or leptin may
enhance immunotherapy efficacy in obese patients with TNBC
(117).
Bariatric surgery and BC risk
Cohort data provide robust evidence for a
protective effect of bariatric surgery against postmenopausal BC,
with studies showing a 32-42% reduction in long-term incidence
among obese women following surgical weight loss (119,120). In the Swedish Obese Subjects
study, 1,116 women who underwent bariatric surgery (mainly
vertical-banded gastroplasty or gastric bypass) exhibited a 32%
reduction in post-menopausal BC incidence compared with matched
non-surgical controls (adjusted HR, 0.68; 95% CI, 0.48-0.96; median
follow-up, 21.3 years) (119).
Similarly, analysis of the US Kaiser Permanente database (n=22,198)
demonstrated a 42% lower BC risk after Roux-en-Y gastric bypass
(HR, 0.58; 95% CI, 0.42-0.79) that became apparent 5 years
post-procedure and persisted beyond 15 years post-procedure
(120). Mechanistically,
surgery-induced weight loss rapidly decreases circulating leptin,
CRP and IL-6 levels, while restoring adiponectin levels,
collectively reversing the obesity-driven chronic inflammatory
milieu implicated in mammary carcinogenesis (121).
Future directions
Despite significant progress, the molecular
mechanisms linking obesity, chronic breast inflammation and
carcinogenesis remain unclear. The interplay among signaling
pathways, including the NF-κB, MAPK and TLR signaling pathways, is
complex and context-dependent (92). For example, further research is
needed to elucidate how these pathways synergistically or
antagonistically drive cancer initiation and progression in
different stages of obesity and inflammation (52,94). Specifically, studies should focus
on the temporal dynamics of these pathways and their interactions
with adipokines and inflammatory mediators.
The role of novel factors such as exosomes and
extracellular vesicles in mediating crosstalk among adipose tissue,
immune cells and breast epithelial cells has only recently gained
attention (122). Future
research should explore the detailed mechanisms by which these
vesicles transfer oncogenic signals and modulate the tumor
microenvironment (122). For
instance, exosomes derived from obese adipose tissue have been
shown to promote BC progression by transferring miRNAs and proteins
that enhance cell proliferation and migration (122).
Long-term follow-up studies on obese patients with
chronic breast inflammation are lacking. Large-scale, longitudinal
studies are required to better understand the long-term impact of
obesity and chronic inflammation on BC risk and progression
(122). These studies should
account for ethnic and sex differences, as preliminary evidence
suggests variations in obesity-related cancer risk across different
populations (123).
Personalized medicine offers a promising avenue for
improving outcomes in obese patients at risk of BC. Advances in
genomic and proteomic technologies enable the identification of
individual molecular profiles related to obesity and chronic
inflammation (124). Future
research could focus on developing biomarker panels that predict
cancer risk and treatment response. By integrating genetic,
epigenetic and transcriptomic data, therapies can be tailored to
the unique profile of an individual, potentially improving efficacy
and reducing adverse effects (91).
The identification of novel therapeutic targets
through advanced omics technologies is another frontier.
Metabolomics can reveal altered metabolic pathways in obesity and
cancer, pointing to potential targets for metabolic modulation
(125). Integration of
untargeted plasma metabolomics with single-cell RNA-seq now enables
obesity subtyping: Simultaneous profiling of serum kynurenine/4-HNE
levels and mammary-tissue scRNA-seq delineated an
'inflammatory-proliferative' obese subtype (IL-6high
macrophages + leptinhigh adipocytes), which exhibited a
2.1-fold higher BC risk compared with a 'metabolically quiescent'
subtype (AdipoQhigh; AMPK-activated) in postmenopausal
women (n=34; metabolome-transcriptome correlation r=0.62;
P<0.01) (125). Such
metabolomics-plus-single-cell strategies refine risk prediction
beyond BMI and nominate subtype-specific molecular targets (for
example, enzymes in the kynurenine pathway such as indoleamine
2,3-dioxygenase 1) for precision prevention trials in obese cohorts
using pharmacological inhibitors (125). Transcriptomics and proteomics
can uncover previously unknown signaling nodes or protein
interactions that are critical in the inflammatory-carcinogenic
process. For example, lncRNAs and circular RNAs, which have been
implicated in regulating gene expression in cancer, may serve as
diagnostic biomarkers or therapeutic targets (71).
The gut microbiota has emerged as a critical player
in systemic inflammation and metabolism (126). In obesity, dysbiosis of the gut
microbiota contributes to chronic inflammation and metabolic
dysfunction (126). Previous
studies have suggested that gut microbial metabolites, including
short-chain fatty acids and secondary bile acids, could influence
BC risk and progression (127,128). Future research could explore how
modulating the gut microbiota through prebiotics, probiotics or
fecal microbiota transplantation affects BC outcomes in obese
individuals. Specifically, obesity-associated enrichment of
deoxycholic acid (DCA), a secondary bile acid generated by
bacterial 7α-dehydroxylation, activates the farnesoid X receptor
(FXR)/NF-κB axis in breast adipose tissue and increases oxidative
DNA damage, thereby accelerating tumor initiation (127). In high-fat diet-fed mice,
elevated fecal DCA levels are associated with larger mammary tumor
volume and higher incidence of pulmonary metastases, an effect that
can be reversed by oral administration of a bile-acid sequestrant
or by genetic ablation of the FXR receptor (128). Understanding the molecular
mechanisms underlying gut microbiota-breast crosstalk, such as
through the production of inflammatory mediators or the modulation
of host metabolism, may open up novel avenues for prevention and
treatment of obesity-related BC (126,127). To accelerate mechanistic
discovery and therapeutic testing, obesity-driven breast
carcinogenesis research should incorporate next-generation animal
and culture models that faithfully replicate the inflamed obese
microenvironment.
Despite robust and accumulating preclinical
evidence demonstrating that obesity fuels breast carcinogenesis
through chronic inflammation, adipokine dysregulation and immune
microenvironment remodeling (13,51,65,72,88), several translational gaps persist
that hinder clinical implementation: i) At present, no phase I
trial has been registered that specifically depletes or reprograms
adipose-tissue macrophages (for example, via colony-stimulating
factor 1 receptor or C-C motif chemokine receptor 2 inhibitors) in
obese patients with BC, although mouse data show marked tumor
growth delay when CLS macrophages are eliminated (79,88); ii) biomarker-adaptive trials that
incorporate circulating leptin, adiponectin and MRI-determined
breast density as eligibility criteria have not yet been conducted,
limiting the ability to achieve precision enrollment in
obesity-related BC studies (105,106); iii) long-term oncological
endpoints (>5 years) are rarely captured in existing lifestyle
or metformin trials, leaving the magnitude of obesity-modifiable
recurrence risk uncertain (91);
and iv) pharmacokinetic studies evaluating chemotherapy or
endocrine-agent dosing in mammary fat volumes >35% of breast
mass have not been performed, potentially contributing to
under-dosing in obese women (45). Addressing these deficiencies
through dedicated early-phase studies will be critical to disrupt
the obesity-inflammation-carcinogenesis axis in clinical
settings.
Conclusion
Obesity fuels breast carcinogenesis via chronic
inflammation, metabolic dysfunction and adipokine dysregulation,
worsening BC prognosis and treatment response. Metformin, ω-3 and
biomarker-driven approaches show promise in mitigating these risks.
Future efforts must prioritize large-scale studies, personalized
medicine and novel targets (such as the gut microbiota) to disrupt
the obesity-BC axis and reduce the global disease burden.
Supplementary Data
Availability of data and materials
Not applicable.
Authors' contributions
FL and ZG conceived the study and designed its
methodology. FL curated the data and prepared the initial
manuscript, while both authors jointly investigated and visualized
the findings and supervised the project. The manuscript was
reviewed and edited collaboratively by FL and ZG, who also serves
as the guarantor of the work. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technology Project of
Baiyin First People's Hospital (grant no. 2024YK-08), and Baiyin
Municipal Science and Technology Bureau 2025 Municipal Science and
Technology Plan-Social Development Category (grant no.
2025-2-9S).
References
|
1
|
Lingvay I, Cohen RV, Roux CWL and
Sumithran P: Obesity in adults. Lancet. 404:972–987. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chandrasekaran P and Weiskirchen R: The
role of obesity in type 2 diabetes mellitus-an overview. Int J Mol
Sci. 25:18822024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koskinas KC, Van Craenenbroeck EM,
Antoniades C, Blüher M, Gorter TM, Hanssen H, Marx N, McDonagh TA,
Mingrone G, Rosengren A, et al: Obesity and cardiovascular disease:
An ESC clinical consensus statement. Eur Heart J. 45:4063–4098.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
King LK, March L and Anandacoomarasamy A:
Obesity & osteoarthritis. Indian J Med Res. 138:185–193.
2013.
|
|
5
|
GBD 2021 Adult BMI Collaborators: Global,
regional, and national prevalence of adult overweight and obesity,
1990-2021, with forecasts to 2050: A forecasting study for the
Global burden of disease study 2021. Lancet. 405:813–838. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
7
|
Wu Y, Zhou L, Zhang X, Yang X, Niedermann
G and Xue J: Psychological distress and eustress in cancer and
cancer treatment: Advances and perspectives. Sci Adv.
8:eabq79822022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teli BD and Behzadifar M, Beiranvand M,
Rezapour A, Ehsanzadeh SJ, Azari S, Bakhtiari A, Haghighatfard P,
Martini M, Saran M and Behzadifar M: The economic burden of breast
cancer in western Iran: A cross-sectional cost-of-illness study. J
Health Popul Nutr. 44:162025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong N, Wu H and Yu Z: Advancements and
challenges in triple-negative breast cancer: A comprehensive review
of therapeutic and diagnostic strategies. Front Oncol.
14:14054912024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu JJ, Zhang QY and Yang ZC: The
correlation between obesity and the occurrence and development of
breast cancer. Eur J Med Res. 30:4192025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agnoli C, Grioni S, Sieri S, Sacerdote C,
Ricceri F, Tumino R, Frasca G, Pala V, Mattiello A, Chiodini P, et
al: Metabolic syndrome and breast cancer risk: A case-cohort study
nested in a multicentre italian cohort. PLoS One. 10:e01288912015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cozzo AJ, Fuller AM and Makowski L:
Contribution of adipose tissue to development of cancer. Compr
Physiol. 8:237–282. 2017. View Article : Google Scholar
|
|
14
|
Xu Q, Yu J, Jia G, Li Z and Xiong H:
Crocin attenuates NF-κB-mediated inflammation and proliferation in
breast cancer cells by down-regulating PRKCQ. Cytokine.
154:1558882022. View Article : Google Scholar
|
|
15
|
Chen EP and Smyth EM: COX-2 and
PGE2-dependent immunomodulation in breast cancer. Prostaglandins
Other Lipid Mediat. 96:14–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma VR, Gupta GK and Sharma AK, Batra
N, Sharma DK, Joshi A and Sharma AK: PI3K/Akt/mTOR intracellular
pathway and breast cancer: Factors, mechanism and regulation. Curr
Pharm Des. 23:1633–1638. 2017. View Article : Google Scholar
|
|
17
|
Mohanty SS and Mohanty PK: Obesity as
potential breast cancer risk factor for postmenopausal women. Genes
Dis. 8:117–123. 2021. View Article : Google Scholar :
|
|
18
|
Neuhouser ML, Aragaki AK, Prentice RL,
Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan
BJ, Tinker LF, et al: Overweight, obesity, and postmenopausal
invasive breast cancer risk: A secondary analysis of the women's
health initiative randomized clinical trials. JAMA Oncol.
1:611–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabat GC, Kim MY, Lee JS, Ho GY, Going SB,
Beebe-Dimmer J, Manson JE, Chlebowski RT and Rohan TE: Metabolic
obesity phenotypes and risk of breast cancer in postmenopausal
women. Cancer Epidemiol Biomarkers Prev. 26:1730–1735. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Picon-Ruiz M, Morata-Tarifa C,
Valle-Goffin JJ, Friedman ER and Slingerland JM: Obesity and
adverse breast cancer risk and outcome: Mechanistic insights and
strategies for intervention. CA Cancer J Clin. 67:378–397.
2017.PubMed/NCBI
|
|
21
|
Ladoire S, Dalban C, Roché H, Spielmann M,
Fumoleau P, Levy C, Martin AL, Ecarnot F, Bonnetain F and
Ghiringhelli F: Effect of obesity on disease-free and overall
survival in node-positive breast cancer patients in a large French
population: a pooled analysis of two randomised trials. Eur J
Cancer. 50:506–516. 2014. View Article : Google Scholar
|
|
22
|
Ligibel JA, Cirrincione CT, Liu M, Citron
M, Ingle JN, Gradishar W, Martino S, Sikov W, Michaelson R, Mardis
E, et al: Body mass index, PAM50 subtype, and outcomes in
node-positive breast cancer: CALGB 9741 (Alliance). J Natl Cancer
Inst. 107:djv1792015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iyengar NM, Arthur R, Manson JE,
Chlebowski RT, Kroenke CH, Peterson L, Cheng TD, Feliciano EC, Lane
D, Luo J, et al: Association of body fat and risk of breast cancer
in postmenopausal women with normal body mass index: A secondary
analysis of a randomized clinical trial and observational study.
JAMA Oncol. 5:155–163. 2019. View Article : Google Scholar :
|
|
24
|
Dias JA, Fredrikson GN, Ericson U,
Gullberg B, Hedblad B, Engström G, Borgquist S, Nilsson J and
Wirfält E: Low-grade inflammation, oxidative stress and risk of
invasive post-menopausal breast cancer-A nested case-control study
from the malmö diet and cancer cohort. PLoS One. 11:e01589592016.
View Article : Google Scholar
|
|
25
|
Chlebowski RT, Aragaki AK, Pan K, Simon
MS, Neuhouser ML, Haque R, Rohan TE, Wactawski-Wende J, Orchard TS,
Mortimer JE, et al: Breast cancer incidence and mortality by
metabolic syndrome and obesity: The women's health initiative.
Cancer. 130:3147–3156. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Widschwendter P, Friedl TW, Schwentner L,
DeGregorio N, Jaeger B, Schramm A, Bekes I, Deniz M, Lato K,
Weissenbacher T, et al: The influence of obesity on survival in
early, high-risk breast cancer: Results from the randomized SUCCESS
A trial. Breast Cancer Res. 17:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gennari A, Amadori D, Scarpi E, Farolfi A,
Paradiso A, Mangia A, Biglia N, Gianni L, Tienghi A, Rocca A, et
al: Impact of body mass index (BMI) on the prognosis of high-risk
early breast cancer (EBC) patients treated with adjuvant
chemotherapy. Breast Cancer Res Treat. 159:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biganzoli E, Desmedt C, Fornili M, de
Azambuja E, Cornez N, Ries F, Closon-Dejardin MT, Kerger J, Focan
C, Di Leo L, et al: Recurrence dynamics of breast cancer according
to baseline body mass index. Eur J Cancer. 87:10–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lammers SWM, Geurts SME, van Hellemond
IEG, Swinkels ACP, Smorenburg CH, van der Sangen MJC, Kroep JR, de
Graaf H, Honkoop AH, Erdkamp FLG, et al: The prognostic and
predictive effect of body mass index in hormone receptor-positive
breast cancer. JNCI Cancer Spectr. 7:pkad0922023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tzschaschel M, Friedl TWP, Schochter F,
Schütze S, Polasik A, Fehm T, Pantel K, Schindlbeck C, Schneeweiss
A, Schreier J, et al: Association Between Obesity and Circulating
Tumor Cells in Early Breast Cancer Patients. Clin Breast Cancer.
23:e345–e353. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tangalakis LL, Cortina CS, Son JD, Poirier
J and Madrigrano A: Obesity does not influence management of
advanced breast cancer in the elderly. Clin Breast Cancer.
19:197–199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
33
|
Zhang Y, Sano M, Shinmura K, Tamaki K,
Katsumata Y, Matsuhashi T, Morizane S, Ito H, Hishiki T, Endo J, et
al: 4-hydroxy-2-nonenal protects against cardiac
ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol
Cell Cardiol. 49:576–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pizzimenti S, Toaldo C, Pettazzoni P,
Dianzani MU and Barrera G: The 'two-faced' effects of reactive
oxygen species and the lipid peroxidation product 4-hydroxynonenal
in the hallmarks of cancer. Cancers (Basel). 2:338–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong H and Yin H: Role of lipid
peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing
on mitochondria. Redox Biol. 4:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Cosimo S, Porcu L, Agbor-Tarh D,
Cinieri S, Franzoi MA, De Santis MC, Saura C, Huober J, Fumagalli
D, Izquierdo M, et al: Effect of body mass index on response to
neo-adjuvant therapy in HER2-positive breast cancer: An exploratory
analysis of the NeoALTTO trial. Breast Cancer Res. 22:1152020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martel S, Lambertini M, Agbor-Tarh D,
Ponde NF, Gombos A, Paterson V, Hilbers F, Korde L, Manukyants A,
Dueck A, et al: Body mass index and weight change in patients with
HER2-positive early breast cancer: Exploratory analysis of the
ALTTO BIG 2-06 trial. J Natl Compr Canc Netw. 19:181–189. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dauccia C, Alice Franzoi M, Martel S,
Agbor-Tarh D, Fielding S, Piccart M, Bines J, Loibl S, Di Cosimo S,
Vaz-Luis I, et al: Body mass index and weight changes in patients
with HER2-positive early breast cancer: A sub-analysis of the
APHINITY trial. Eur J Cancer. 223:1154892025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sedjo RL, Byers T, Ganz PA, Colditz GA,
Demark-Wahnefried W, Wolin KY, Azrad M and Rock CL: Weight gain
prior to entry into a weight-loss intervention study among
overweight and obese breast cancer survivors. J Cancer Surviv.
8:410–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mutschler NS, Scholz C, Friedl TWP,
Zwingers T, Fasching PA, Beckmann MW, Fehm T, Mohrmann S, Salmen J,
Ziegler C, et al: Prognostic impact of weight change during
adjuvant chemotherapy in patients with high-risk early breast
cancer: Results from the ADEBAR study. Clin Breast Cancer.
18:175–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goodwin PJ, Segal RJ, Vallis M, Ligibel
JA, Pond GR, Robidoux A, Blackburn GL, Findlay B, Gralow JR,
Mukherjee S, et al: Randomized trial of a telephone-based weight
loss intervention in postmenopausal women with breast cancer
receiving letrozole: The LISA trial. J Clin Oncol. 32:2231–2239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Babatunde OA, Arp Adams S, Truman S, Sercy
EMSPH, Murphy AE, Khan SMSW, Hurley TGMS, Wirth MD, Choi SK,
Johnson HBA and Hebert ScD JR: The impact of a randomized dietary
and physical activity intervention on chronic inflammation among
obese African-American women. Women Health. 60:792–805. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inglis JE, Kleckner AS, Lin PJ, Gilmore
NJ, Culakova E, VanderWoude AC, Mustian KM, Fernandez ID, Dunne RF,
Deutsch J and Peppone LJ: Excess body weight and cancer-related
fatigue, systemic inflammation, and serum lipids in breast cancer
survivors. Nutr Cancer. 73:1676–1686. 2021. View Article : Google Scholar
|
|
44
|
Kiecolt-Glaser JK, Renna M, Peng J,
Sheridan J, Lustberg M, Ramaswamy B, Wesolowski R, VanDeusen JB,
Williams NO, Sardesai SD, et al: Breast cancer survivors' typhoid
vaccine responses: Chemotherapy, obesity, and fitness make a
difference. Brain Behav Immun. 103:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Poggio F, Blondeaux E, Tagliamento M,
Perachino M, Nardin S, Conte B, Giuliano M, Arpino G, De Laurentiis
M, Gravina A, et al: Efficacy of adjuvant chemotherapy schedules
for breast cancer according to body mass index: results from the
phase III GIM2 trial. ESMO Open. 9:1036502024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Biganzoli G, Isnaldi E, Richard F, Marano
G, Boracchi P, Maetens M, Floris G, Neven P, Jerusalem G, Munzone
E, et al: Prognostic relation of body mass index on extended
aromatase inhibition treatment in postmenopausal patients with
estrogen receptor positive breast cancer: A retrospective analysis
of the SOLE trial. Eur J Cancer. 222:1154382025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Macis D, Bellerba F, Aristarco V,
Johansson H, Guerrieri-Gonzaga A, Lazzeroni M, Sestak I, Cuzick J,
DeCensi A, Bonanni B and Gandini S: A mediation analysis of obesity
and adiponectin association with postmenopausal breast cancer risk:
A nested cohort study in the International breast cancer
intervention study II (IBIS-II) prevention trial. Nutrients.
16:20982024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Surmacz E: Obesity hormone leptin: A new
target in breast cancer? Breast Cancer Res. 9:3012007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou W, Guo S and Gonzalez-Perez RR:
Leptin pro-angiogenic signature in breast cancer is linked to IL-1
signalling. Br J Cancer. 104:128–137. 2011. View Article : Google Scholar :
|
|
50
|
Wang L, Tang C, Cao H, Li K, Pang X, Zhong
L, Dang W, Tang H, Huang Y, Wei L, et al: Activation of IL-8 via
PI3K/Akt-dependent pathway is involved in leptin-mediated
epithelial-mesenchymal transition in human breast cancer cells.
Cancer Biol Ther. 16:1220–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qi Y, Li R and Han M: Tumor-associated
macrophages induce epithelial-mesenchymal transition and promote
lung metastasis in breast cancer by activating the IL-6/STAT3/TGM2
axis. Int Immunopharmacol. 143(Pt 2): 1133872024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luan D, Dadpey B, Zaid J, Bridge-Comer PE,
DeLuca JH, Xia W, Castle J and Reilly SM: Adipocyte-Secreted IL-6
sensitizes macrophages to IL-4 signaling. Diabetes. 72:367–374.
2023. View Article : Google Scholar :
|
|
53
|
Zhou Y and Rui L: Leptin signaling and
leptin resistance. Front Med. 7:207–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang M, Zhang K, Zhang Z, Zeng X, Huang
Z, Qin P, Xie Z, Cai X, Ashrafizadeh M, Tian Y and Wei R:
PI3K/AKT/mTOR axis in cancer: From pathogenesis to treatment.
MedComm (2020). 6:e702952025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kwon O, Kim KW and Kim MS: Leptin
signalling pathways in hypothalamic neurons. Cell Mol Life Sci.
73:1457–1477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Wen K, Han X, Liu R and Qu Q:
Adiponectin mediates antiproliferative and apoptotic responses in
endometrial carcinoma by the AdipoRs/AMPK pathway. Gynecol Oncol.
137:311–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar :
|
|
58
|
Fosam A and Perry RJ: Current mechanisms
in obesity and tumor progression. Curr Opin Clin Nutr Metab Care.
23:395–403. 2020.PubMed/NCBI
|
|
59
|
Wang Y, Luo M, Wang F, Tong Y, Li L, Shu
Y, Qiao K, Zhang L, Yan G, Liu J, et al: AMPK induces degradation
of the transcriptional repressor PROX1 impairing branched amino
acid metabolism and tumourigenesis. Nat Commun. 13:72152022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tong Y, Xu S, Huang L and Chen C: Obesity
and insulin resistance: Pathophysiology and treatment. Drug Discov
Today. 27:822–830. 2022. View Article : Google Scholar
|
|
61
|
Hotamisligil GS, Peraldi P, Budavari A,
Ellis R, White MF and Spiegelman BM: IRS-1-mediated inhibition of
insulin receptor tyrosine kinase activity in TNF-alpha- and
obesity-induced insulin resistance. Science. 271:665–668. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Piro S, Anello M, Di Pietro C, Lizzio MN,
Patanè G, Rabuazzo AM, Vigneri R, Purrello M and Purrello F:
Chronic exposure to free fatty acids or high glucose induces
apoptosis in rat pancreatic islets: possible role of oxidative
stress. Metabolism. 51:1340–1347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lopez R, Arumugam A, Joseph R, Monga K,
Boopalan T, Agullo P, Gutierrez C, Nandy S, Subramani R, de la Rosa
JM and Lakshmanaswamy R: Hyperglycemia enhances the proliferation
of non-tumorigenic and malignant mammary epithelial cells through
increased leptin/IGF1R signaling and activation of AKT/mTOR. PLoS
One. 8:e797082013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bartella V, Cascio S, Fiorio E, Auriemma
A, Russo A and Surmacz E: Insulin-dependent leptin expression in
breast cancer cells. Cancer Res. 68:4919–4927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tiwari P, Blank A, Cui C, Schoenfelt KQ,
Zhou G, Xu Y, Khramtsova G, Olopade F, Shah AM, Khan SA, et al:
Metabolically activated adipose tissue macrophages link obesity to
triple-negative breast cancer. J Exp Med. 216:1345–1358. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Morris PG, Hudis CA, Giri D, Morrow M,
Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, et
al: Inflammation and increased aromatase expression occur in the
breast tissue of obese women with breast cancer. Cancer Prev Res
(Phila). 4:1021–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Potolitsyna E, Hazell Pickering S, Germier
T, Collas P and Briand N: Long non-coding RNA HOTAIR regulates
cytoskeleton remodeling and lipid storage capacity during
adipogenesis. Sci Rep. 12:101572022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang J, Chen M, Zhai Y and Fu Y: HOTAIR
regulates lipopolysaccharide-induced inflammatory response in
hepatocytes. J Cell Physiol. 235:4247–4255. 2020. View Article : Google Scholar
|
|
69
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kado T, Nawaz A, Takikawa A, Usui I and
Tobe K: Linkage of CD8(+) T cell exhaustion with high-fat
diet-induced tumourigenesis. Sci Rep. 9:122842019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ivanisevic T and Sewduth RN: Multi-Omics
integration for the design of novel therapies and the
identification of novel biomarkers. Proteomes. 11:342023.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pingili AK, Chaib M, Sipe LM, Miller EJ,
Teng B, Sharma R, Yarbro JR, Asemota S, Al Abdallah Q, Mims TS, et
al: Immune checkpoint blockade reprograms systemic immune landscape
and tumor microenvironment in obesity-associated breast cancer.
Cell Rep. 35:1092852021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Z, Aguilar EG, Luna JI, Dunai C,
Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et
al: Paradoxical effects of obesity on T cell function during tumor
progression and PD-1 checkpoint blockade. Nat Med. 25:141–151.
2019. View Article : Google Scholar :
|
|
74
|
Danforth DN: The role of chronic
inflammation in the development of breast cancer. Cancers (Basel).
13:39182021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mao H, Zhao X and Sun SC: NF-κB in
inflammation and cancer. Cell Mol Immunol. 22:811–839. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Andrijauskaite K and Wargovich MJ: Role of
natural products in breast cancer related symptomology: Targeting
chronic inflammation. Semin Cancer Biol. 80:370–378. 2022.
View Article : Google Scholar
|
|
77
|
Suman S, Sharma PK, Rai G, Mishra S, Arora
D, Gupta P and Shukla Y: Current perspectives of molecular pathways
involved in chronic inflammation-mediated breast cancer. Biochem
Biophys Res Commun. 472:401–409. 2016. View Article : Google Scholar
|
|
78
|
Greenlee H, Shi Z, Hibshoosh H, Giri DD,
Ahmed A, Williams S, Falcone DJ, Winston LA, Zhou XK, Hudis CA, et
al: Obesity-associated breast inflammation among hispanic/latina
breast cancer patients. Cancer Prev Res (Phila). 12:21–30. 2019.
View Article : Google Scholar
|
|
79
|
Xiao L, Xian M, Zhang C, Guo Q and Yi Q:
Lipid peroxidation of immune cells in cancer. Front Immunol.
14:13227462024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kubatka P, Bojkova B, Nosalova N, Huniadi
M, Samuel SM, Sreenesh B, Hrklova G, Kajo K, Hornak S, Cizkova D,
et al: Targeting the MAPK signaling pathway: Implications and
prospects of flavonoids in 3P medicine as modulators of cancer cell
plasticity and therapeutic resistance in breast cancer patients.
EPMA J. 16:437–463. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
82
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pallathadka H, Khaleel AQ, Zwamel AH,
Malathi H, Sharma S, Rizaev JA, Mustafa YF, Pramanik A, Shuhata
Alubiady MH and Jawad MA: Multi-Drug resistance and breast cancer
progression via toll-like receptors (TLRs) signaling. Cell Biochem
Biophys. 82:3015–3030. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pálmai-Pallag T and Bachrati CZ:
Inflammation-induced DNA damage and damage-induced inflammation: A
vicious cycle. Microbes Infect. 16:822–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou J, Zhang L, Liu S, DeRubeis D and
Zhang D: Toll-like receptors in breast cancer immunity and
immunotherapy. Front Immunol. 15:14180252024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kanda H, Tateya S, Tamori Y, Kotani K,
Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K and
Kasuga M: MCP-1 contributes to macrophage infiltration into adipose
tissue, insulin resistance, and hepatic steatosis in obesity. J
Clin Invest. 116:1494–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang F, Yao T, Yang W, Wu P, Liu Y and
Yang B: Protocol to detect nucleotide-protein interaction in vitro
using a non-radio-active competitive electrophoretic mobility shift
assay. STAR Protoc. 3:1017302022. View Article : Google Scholar
|
|
88
|
Bowers LW, Doerstling SS, Shamsunder MG,
Lineberger CG, Rossi EL, Montgomery SA, Coleman MF, Gong W, Parker
JS, Howell A, et al: Reversing the genomic, epigenetic, and
triple-negative breast cancer-enhancing effects of obesity. Cancer
Prev Res (Phila). 15:581–594. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kong W, He L, Richards EJ, Challa S, Xu
CX, Permuth-Wey J, Lancaster JM, Coppola D, Sellers TA, Djeu JY and
Cheng JQ: Upregulation of miRNA-155 promotes tumour angiogenesis by
targeting VHL and is associated with poor prognosis and
triple-negative breast cancer. Oncogene. 33:679–689. 2014.
View Article : Google Scholar :
|
|
90
|
Sturgeon KM, Brown JC, Sears DD, Sarwer DB
and Schmitz KH: WISER survivor trial: combined effect of exercise
and weight loss interventions on inflammation in breast cancer
survivors. Med Sci Sports Exerc. 55:209–215. 2023. View Article : Google Scholar :
|
|
91
|
Holm JB, Baggesen E, Cronin-Fenton D,
Frystyk J, Bruun JM, Christiansen P and Borgquist S: Circulating
C-reactive protein levels as a prognostic biomarker in breast
cancer across body mass index groups. Sci Rep. 14:144862024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mallya S, Gangadhar V, Aldrin SE, Acharya
M, Kabekkodu SP, Kolathur KK and Chakrabarty S: Insights into the
molecular and genetic role of obesity in breast cancer
pathogenesis. Cancer Biol Ther. 26:25013452025. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Aristokli N, Polycarpou I, Themistocleous
SC, Sophocleous D and Mamais I: Comparison of the diagnostic
performance of Magnetic Resonance Imaging (MRI), ultrasound and
mammography for detection of breast cancer based on tumor type,
breast density and patient's history: A review. Radiography (Lond).
28:848–856. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Harborg S, Feldt M, Cronin-Fenton D,
Klintman M, Dalton SO, Rosendahl AH and Borgquist S: Obesity and
breast cancer prognosis: pre-diagnostic anthropometric measures in
relation to patient, tumor, and treatment characteristics. Cancer
Metab. 11:82023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Laforest S, Ennour-Idrissi K, Ouellette G,
Gauthier MF, Michaud A, Durocher F, Tchernof A and Diorio C:
Associations between markers of mammary adipose tissue dysfunction
and breast cancer prognostic factors. Int J Obes (Lond).
45:195–205. 2021. View Article : Google Scholar
|
|
96
|
Coradini D: Adipokines, cell polarity
disruption and breast cancer. Aging (Albany NY). 13:22625–22626.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zha JM, Zhang M, Wang T, Li HS, Ban QY,
Liu M, Jiang XX, Guo SY, Wang J, Zhou YR, et al: Association of
overweight and inflammatory indicators with breast cancer: A
cross-sectional study in Chinese women. Int J Womens Health.
16:783–795. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Houssami N: Breast cancer screening at a
crossroads: Supplemental imaging for dense breasts. Lancet.
405:1887–1889. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Thigpen D, Kappler A and Brem R: The role
of ultrasound in screening dense breasts-A review of the literature
and practical solutions for implementation. Diagnostics (Basel).
8:202018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Robinson PJ, Bell RJ and Davis SR: Obesity
is associated with a poorer prognosis in women with hormone
receptor positive breast cancer. Maturitas. 79:279–286. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Blair CK, Wiggins CL, Nibbe AM, Storlie
CB, Prossnitz ER, Royce M, Lomo LC and Hill DA: Obesity and
survival among a cohort of breast cancer patients is partially
mediated by tumor characteristics. NPJ Breast Cancer. 5:332019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Suzuki R, Saji S and Toi M: Impact of body
mass index on breast cancer in accordance with the life-stage of
women. Front Oncol. 2:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bocian-Jastrzębska A, Malczewska-Herman A
and Kos-Kudła B: Role of leptin and adiponectin in carcinogenesis.
Cancers (Basel). 15:42502023. View Article : Google Scholar
|
|
104
|
Hillers-Ziemer LE, Kuziel G, Williams AE,
Moore BN and Arendt LM: Breast cancer microenvironment and obesity:
challenges for therapy. Cancer Metastasis Rev. 41:627–647. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Demark-Wahnefried W, Colditz GA, Rock CL,
Sedjo RL, Liu J, Wolin KY, Krontiras H, Byers T, Pakiz B, Parker
BA, et al: Quality of life outcomes from the exercise and nutrition
enhance recovery and good health for you (ENERGY)-randomized weight
loss trial among breast cancer survivors. Breast Cancer Res Treat.
154:329–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rock CL, Flatt SW, Byers TE, Colditz GA,
Demark-Wahnefried W, Ganz PA, Wolin KY, Elias A, Krontiras H, Liu
J, et al: Results of the exercise and nutrition to enhance recovery
and good health for you (ENERGY) trial: A behavioral weight loss
intervention in overweight or obese breast cancer survivors. J Clin
Oncol. 33:3169–3176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Stolley M, Sheean P, Gerber B, Arroyo C,
Schiffer L, Banerjee A, Visotcky A, Fantuzzi G, Strahan D, Matthews
L, et al: Efficacy of a weight loss intervention for African
American breast cancer survivors. J Clin Oncol. 35:2820–2828. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Befort CA, Klemp JR, Sullivan DK, Shireman
T, Diaz FJ, Schmitz K, Perri MG and Fabian C: Weight loss
maintenance strategies among rural breast cancer survivors: The
rural women connecting for better health trial. Obesity (Silver
Spring). 24:2070–2077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Courneya KS, McNeil J, O'Reilly R,
Morielli AR and Friedenreich CM: Dose-Response effects of aerobic
exercise on quality of life in postmenopausal women: Results from
the breast cancer and exercise trial in Alberta (BETA). Ann Behav
Med. 51:356–364. 2017. View Article : Google Scholar
|
|
110
|
Yam C, Esteva FJ, Patel MM, Raghavendra
AS, Ueno NT, Moulder SL, Hess KR, Shroff GS, Hodge S, Koenig KH, et
al: Efficacy and safety of the combination of metformin, everolimus
and exemestane in overweight and obese postmenopausal patients with
metastatic, hormone receptor-positive, HER2-negative breast cancer:
A phase II study. Invest New Drugs. 37:345–351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Patterson RE, Marinac CR, Natarajan L,
Hartman SJ, Cadmus-Bertram L, Flatt SW, Li H, Parker B,
Oratowski-Coleman J, Villaseñor A, et al: Recruitment strategies,
design, and participant characteristics in a trial of weight-loss
and metformin in breast cancer survivors. Contemp Clin Trials.
47:64–71. 2016. View Article : Google Scholar :
|
|
112
|
Sandhu N, Schetter SE, Liao J, Hartman TJ,
Richie JP, McGinley J, Thompson HJ, Prokopczyk B, DuBrock C,
Signori C, et al: Influence of obesity on breast density reduction
by omega-3 fatty acids: Evidence from a Randomized clinical trial.
Cancer Prev Res (Phila). 9:275–282. 2016. View Article : Google Scholar
|
|
113
|
Fabian CJ, Befort CA, Phillips TA,
Nydegger JL, Kreutzjans AL, Powers KR, Metheny T, Klemp JR, Carlson
SE, Sullivan DK, et al: Change in blood and benign breast
biomarkers in women undergoing a weight-loss intervention
randomized to high-dose ω-3 fatty acids versus placebo. Cancer Prev
Res (Phila). 14:893–904. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Manni A, Richie JP, Schetter SE,
Calcagnotto A, Trushin N, Aliaga C and El-Bayoumy K: Stearoyl-CoA
desaturase-1, a novel target of omega-3 fatty acids for reducing
breast cancer risk in obese postmenopausal women. Eur J Clin Nutr.
71:762–765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
D'Alonzo NJ, Qiu L, Sears DD, Chinchilli
V, Brown JC, Sarwer DB, Schmitz KH and Sturgeon KM: WISER survivor
trial: Combined effect of exercise and weight loss interventions on
insulin and insulin resistance in breast cancer survivors.
Nutrients. 13:31082021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bull CJ, Hazelwood E, Legge DN, Corbin LJ,
Richardson TG, Lee M, Yarmolinsky J, Smith-Byrne K, Hughes DA,
Johansson M, et al: Impact of weight loss on cancer-related
proteins in serum: results from a cluster randomised controlled
trial of individuals with type 2 diabetes. EBioMedicine.
100:1049772024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mastrolonardo EV, Llerena P, De Ravin E,
Nunes K, Kaki PC, Bridgham KM, Amin DR, Campbell DJ, Philips R,
Koeneman SH, et al: Improved survival with elevated BMI following
immune checkpoint inhibition across various solid tumor cancer
types. Cancer. 131:e357992025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ,
Ennis M, Lemieux J, Ligibel JA, Hershman DL, Mayer IA, Hobday TJ,
et al: Effect of metformin vs placebo on invasive disease-free
survival in patients with breast cancer: The MA.32 Randomized
clinical trial. JAMA. 327:1963–1973. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Aminian A, Wilson R, Al-Kurd A, Tu C,
Milinovich A, Kroh M, Rosenthal RJ, Brethauer SA, Schauer PR,
Kattan MW, et al: Association of bariatric surgery with cancer risk
and mortality in adults with obesity. JAMA. 327:2423–2433. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wiggins T, Antonowicz SS and Markar SR:
Cancer risk following bariatric surgery-systematic review and
meta-analysis of National population-based cohort studies. Obes
Surg. 29:1031–1039. 2019. View Article : Google Scholar
|
|
121
|
Zhang K, Luo Y, Dai H and Deng Z: Effects
of bariatric surgery on cancer risk: Evidence from meta-analysis.
Obes Surg. 30:1265–1272. 2020. View Article : Google Scholar
|
|
122
|
Hao Y, Xiao J, Fu P, Yan L, Zhao X, Wu X,
Zhou M, Zhang X, Xu B, Li X, et al: Increases in BMI contribute to
worsening inflammatory biomarkers related to breast cancer risk in
women: a longitudinal study. Breast Cancer Res Treat. 202:117–127.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Motevalli M and Stanford FC: Personalized
lifestyle interventions for prevention and treatment of
obesity-related cancers: A call to action. Cancers (Basel).
17:12552025. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gkrinia EMM and Belančić A: The mechanisms
of chronic inflammation in obesity and potential therapeutic
strategies: A narrative review. Curr Issues Mol Biol. 47:3572025.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen Z, Li Z, Li H and Jiang Y:
Metabolomics: A promising diagnostic and therapeutic implement for
breast cancer. Onco Targets Ther. 12:6797–6811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Acevedo-Román A, Pagán-Zayas N,
Velázquez-Rivera LI, Torres-Ventura AC and Godoy-Vitorino F:
Insights into gut dysbiosis: Inflammatory diseases, obesity, and
restoration approaches. Int J Mol Sci. 25:97152024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jaye K, Chang D, Li CG and Bhuyan DJ: Gut
metabolites and breast cancer: The continuum of dysbiosis, breast
cancer risk, and potential breast cancer therapy. Int J Mol Sci.
23:94902022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Thirunavukkarasan M, Wang C, Rao A, Hind
T, Teo YR, Siddiquee AA, Goghari MAI, Kumar AP and Herr DR:
Short-chain fatty acid receptors inhibit invasive phenotypes in
breast cancer cells. PLoS One. 12:e01863342017. View Article : Google Scholar : PubMed/NCBI
|