Gastric cancer (GC) continues to have a high global
burden, ranking third in terms of cancer-related mortality and
fifth in terms of incidence (1,2).
Annually, ~660,000 individuals die from GC, despite improvements in
early detection and treatment methods (3); by 2040, it is estimated to increase
to ~1.8 million new cases and 1.3 million fatalities (4). GC follows a multistage progression
pattern driven by chronic inflammation, including atrophy,
metaplasia, dysplasia and invasive carcinoma, termed the 'Correa
pathway' proposed by Correa et al (5) in 1975. Current research is improving
the understanding of the molecular mechanisms underlying the
progression of precancerous lesions through inflammation-mediated
epigenetic changes, such as microbiota dysbiosis, while therapeutic
studies are investigating novel approaches to target particular
pathways, such as glycosylation, in order to prevent metastasis
(6,7). When a terminally differentiated cell
type that is not typically found at a specific anatomical location
replaces pre-existing terminally differentiated cells, this is
termed metaplasia (8). In most
cases, the metaplasia lineage is characterized by mucus secretion.
A key characteristic of the metaplastic lineage is the remodeling
of the gastric mucosa by mucus-secreting cell cohorts in response
to damage (9), and mucus
production is typically the distinguishing characteristic of this
lineage. In the gastric mucosa, two main forms of metaplasia have
been identified: i) Intestinal metaplasia (IM); and ii) spasmolytic
polypeptide-expressing metaplasia (SPEM).
SPEM, also termed pseudopyloric metaplasia or mucous
metaplasia, is a regenerative lesion that presents histologically
as Brunner's glands or deep proventricular glands. It is
distinguished by the expression of Trefoil factor 2 (TFF2),
Griffonia simplicifolia lectin II (GS II) and mucin (MUC) 6
(10). SPEM formation is
frequently accompanied by abnormal gastric pit hyperplasia,
macrophage infiltration and glandular structure disarray. Chronic
damage (such as inflammation or NSAID use) causes the gastric
epithelium to ablate, which leads to the formation of SPEM during
regeneration. Similar to intestinal cup cells (MUC5AC, MUC6 and
MUC2), these metaplasia cells typically exhibit a distinct
mucus-secreting phenotype, altering the defensive and digestive
characteristics of the gastric mucosa (10,11). SPEM and IM are two distinct
lineages of metaplasia cells. However, SPEM may precede IM and
promote carcinogenesis via a variety of mechanisms (12). According to independent clinical
studies carried out in the US, Japan and Iceland, SPEM is found in
>90% of GC resection specimens, is linked to >50% of early
GCs and is frequently found in the paracarcinoma or dysplasia
region (13-15). Furthermore, animal models have
confirmed that SPEM is an important intermediate stage in gastric
precancerous lesions (16-18).
Interleukins (ILs) are potent secreted regulators of
various cell types and activities. By targeting and influencing
various cell signaling pathways, they aid in the development and
progression of inflammatory diseases and various types of cancer.
ILs also regulate processes such as cell death, proliferation,
differentiation and migration, and are involved in cellular
communication during homeostasis and disease (19-22). IL production and function are
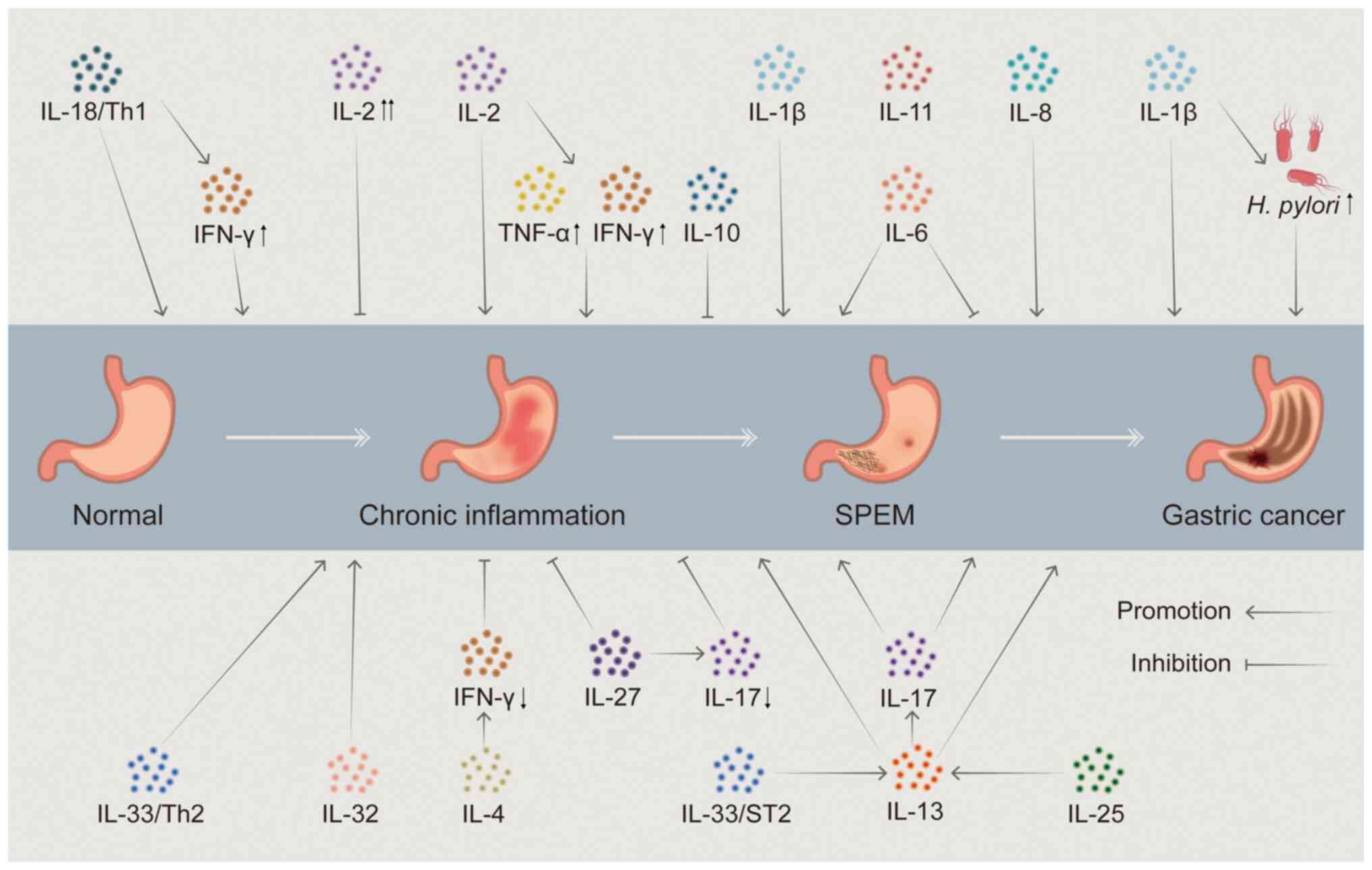
central regulators of SPEM development. Research has verified that
the development of SPEM is closely associated with chronic
inflammation and involves an imbalance in the network of
pro-inflammatory/anti-inflammatory ILs, such as IL-1β, IL-10,
IL-17A and IL-33. ILs bind to high-affinity receptors on the cell
membrane and activate intracellular signaling pathways, including
NF-κB, MAPK and JAK-STAT, resulting in gene transcription changes.
This in turn regulates local cell proliferation, differentiation
and reprogramming (23-26) and induces or alters specific
cellular functions (27-29). The IL network may disturb the
normal homeostasis of gastric mucosal cells by controlling local
inflammation, cellular infiltration and multi-pathway interactions.
This could lead to the development of SPEM or be a major factor in
the transformation of SPEM into GC (30).
The present review summarized recent findings on
IL-related mechanisms involved in SPEM formation and progression.
Furthermore, the roles of various ILs in SPEM and the relationship
between IL changes and SPEM after Helicobacter pylori (H.
pylori) infection are described. In addition, the present
review offered a comprehensive summary of the features of the
different SPEM experimental models that are employed in IL studies.
Finally, by describing the progress and limitations of ILs for
therapeutic use, novel directions for future investigation are
discussed.
The mucosal barrier, made up of closely knit
epithelial cells and a thick layer of mucus, protects the gastric
epithelium from harmful agents such as ingested food, bacteria,
gastric acid and digestive enzymes, stopping the stomach contents
from penetrating into the underlying tissues (31). Pepsinogen-producing chief cells,
mucus-producing surface foveolar cells, neck cells and
acid-producing parietal cells make up the majority of the gastric
mucosal barrier (32). The chief
cell is a differentiated cell lineage, and a subset of it serves as
'reserve' stem cells in the gastric mucosa. During gastric injury,
parietal cells become deficient due to a variety of pathogenic
factors, resulting in a compensatory proliferative response of
gastric stem and progenitor cells, as well as metaplasia with
zymogenic chief cells that can be recruited back into the cell
cycle. Metaplasia in the stomach, which results in the structural
change of its glands, is considered to be an 'adaptive' process
that reacts to a range of endogenous or exogenous aggressors,
including pH, hormones, chemicals and microbiota changes (33). SPEM is a type of metaplasia that
occurs during the healing of gastric mucosal damage. This is due to
the metaplasia cells expressing spasmolytic polypeptide, also
termed TFF2. Fully differentiated gastric chief cells can be
reprogrammed into mucin-secreting metaplasia cells, and the
presence of mature chief cells in the corpus is the source of SPEM
cells via transdifferentiation. These metaplasia changes take place
concurrently with or in response to parietal cell death and
inflammation (34). SPEM cells
are histologically localized at the base of metaplasia glands
following injury, and their specificity was determined by the
co-expression of molecular markers including GS II lectin, TFF2,
Muc6, CD44v9 and AQP5 (35). The
formation of metaplasia lineage cells, proliferation of neck cells
and amplification of the small foveolar/tuft cell lineage in the
gastric mucosa are characteristic pathological alterations
(36,37). Additionally, excessive immune
activation in the stomach can damage epithelial cells and induce
the development of SPEM (38).
Acute SPEM may be a healing mechanism, but when it persists in an
environment of chronic inflammation, chronic SPEM is strongly
associated with the development of gastric adenocarcinoma (34,38). It has been recorded that >95%
of GCs are adenocarcinomas, and according to Laurén's
classification, they can be divided into two types: i) Diffuse; and
ii) intestinal (39). SPEM is
present in almost all GC types, and research on animal models with
acute oxyntic atrophy, transgenic and knockout (KO) genes, chronic
infection and genetic modification indicates that SPEM is a key
precancerous intermediate in the malignant transformation of
gastric mucositis (9,40). Although SPEM is partially
reversible, intestinal SPEM and/or IM are considered precancerous
lesions that may be irreversible in the presence of chronic
inflammatory stimuli (41,42).
Dysplasia progression is an inevitable stage of cancer development
(39). The susceptibility of
gastric precancerous lesions to alterations in the expression of
numerous ILs suggests that immune cells and different ILs
implicated in the progression of metaplasia may be important in
predicting the risk of carcinogenesis (27,39,43,44). Thus, additional identification and
clarification of ILs associated with the SPEM response will greatly
enhance the present understanding of gastric carcinogenesis.
ILs are a subset of cytokines and a family of
signaling proteins that are essential for the regulation and
coordination of immune system responses. The family consists of
small proteins secreted by different cells to facilitate cellular
communication and interaction (45). At least 40 ILs have been
identified, a number of which can be classified into separate
families or superfamilies (46).
Typically, each IL interacts with a different receptor or group of
closely related receptors expressed on the target cell, and when
bound, the intracellular structural domain of the receptor is
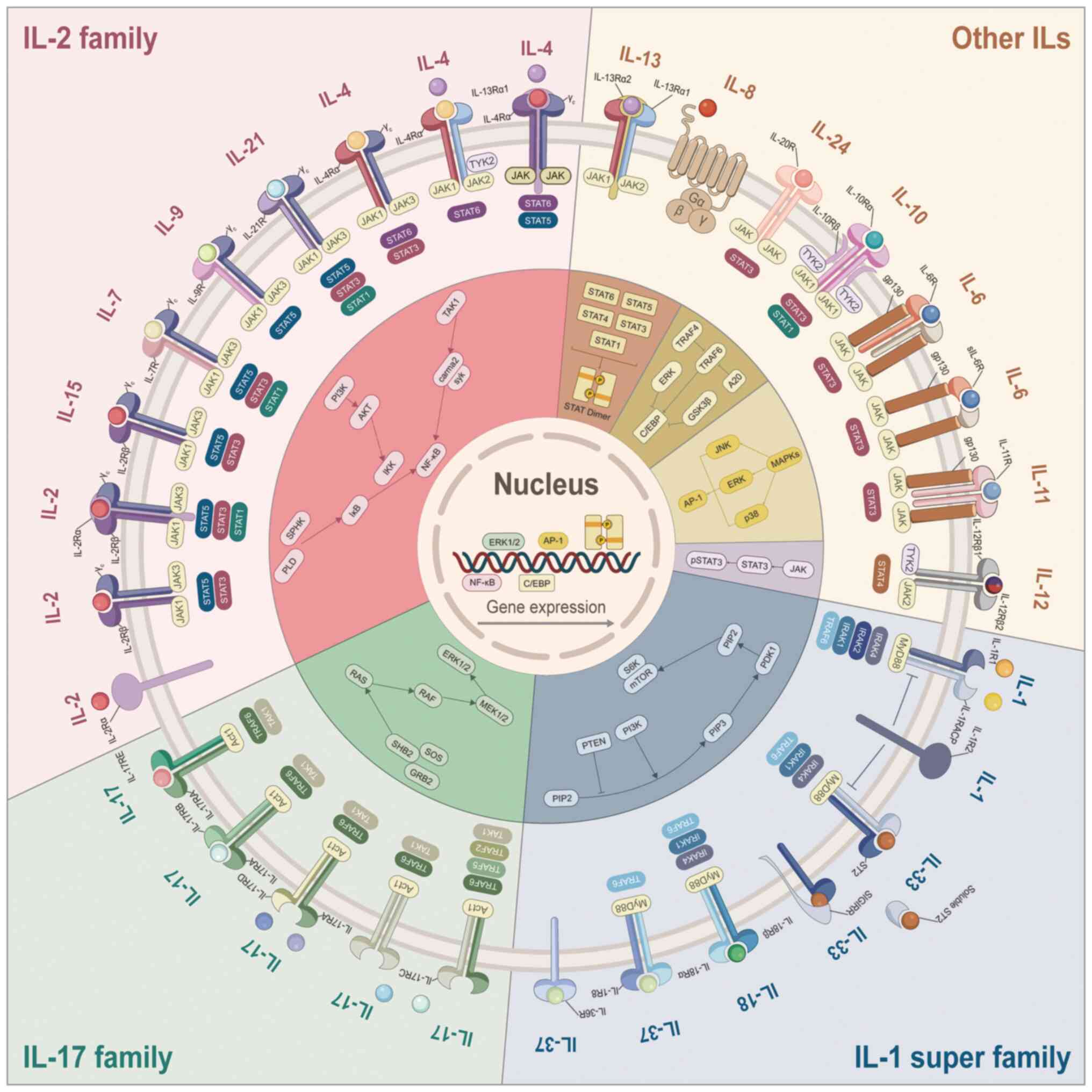
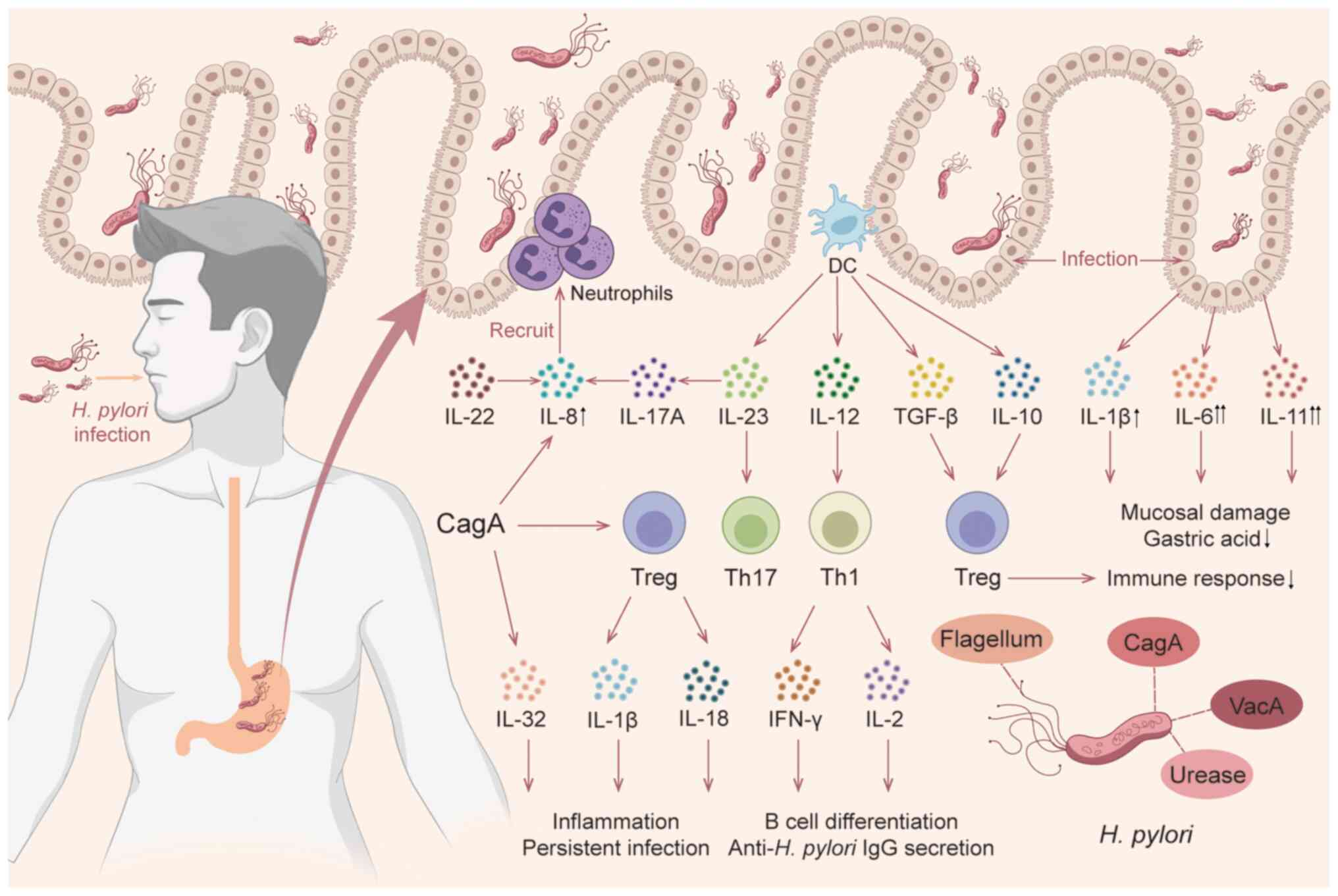
activated, triggering an intracellular signaling cascade (Fig. 1). Numerous ILs are especially
important for the onset and course of SPEM, and their pleiotropic
effects depend on a number of factors, including dose dependency,
receptor heterogeneity, cellular source diversity and signaling
pathway complexity (21). In the
present review, the cytokine signals associated with SPEM were
systematically categorized (Fig.
2), including the IL-1 superfamily, IL-2 family, IL-6 family,
IL-10 family, IL-12 family, IL-17 family and other ILs, as well as
cytokines closely related to ILs (Table I).
IL-1 is a polypeptide synthesized and secreted by
lymphocytes and monocyte macrophages and has various functions,
such as participation in immune response, thermogenesis and
mediation of inflammation (47).
Within a 430 kilobase region, the IL-1 gene cluster on chromosome
2q comprises three related genes, IL-1A, IL-IB and IL-1RN, which
encode IL-1α, IL-1β and IL-1 receptor antagonist (IL-1Ra) (27). IL-1α and IL-1β are alarm cytokines
(also termed alarm proteins), both of which initiate and amplify
local inflammation via the IL-1 receptor (IL-1R) (48). Additionally, IL-1β directly
stimulates the development of SPEM by strongly suppressing the
secretion of gastric acid and causing the atrophy of the gastric
mucosa. When pathogen-associated molecular patterns (PAMPs) or
damage-associated molecular patterns activate the pattern
recognition receptor, IL-1β is produced as an inactive precursor
(pro-IL-1β). The cleavage of pro-IL-1β into its active form by
caspase-1 proteolysis requires the activation of inflammasomes,
which are then released extracellularly (47). Inflammasome-mediated caspase-1
activation triggers both IL-1β release and pyroptosis, affecting
inflammation progression. In addition, IL-1β stimulates the
proliferation of gastric epithelial cells, which may further
contribute to gastric carcinogenesis (49). Prolonged and persistent acid
inhibition by IL-1β may facilitate H. pylori transfer from
the gastric antrum to the corpus, endangering acid-producing
parietal cells and promoting SPEM (50). IL-1Ra can also combine with the
IL-1β receptor, thereby affecting the action of IL-1β.
IL-18, a member of the IL-1 superfamily, is a
pleiotropic pro-inflammatory cytokine that is important in gastric
mucosal inflammation and immunomodulation (27,51). IL-18 signaling begins with a
ligand binding to the receptor IL-18Rα (also termed IL-1R-related
protein). When IL-18 binds to IL-18Rα, IL-18Rβ/IL-18RAcP is
recruited into the complex and activates downstream signaling
pathways such as NF-κB. IL-18 can dominate the gastric mucosal
response to H. pylori infection by promoting T helper (Th) 1
responses and inducing chronic persistent inflammatory changes
(52). Initially, it was referred
to as an IFN-inducing factor that could stimulate the production of
IFN-γ by splenocytes, hepatic lymphocytes and Th1 cells, as well as
increase the cytotoxicity of natural killer (NK) cells and
stimulate the production of IFN-γ in conjunction with IL-12
(53). The production of IFN-γ
causes the death of gastric epithelial cells that express the IFN-γ
receptor and is directly linked to the pathological process of SPEM
(54). Although the link between
IFN-γ and subsequent neoplasia remains uncertain, studies have
shown that IFN-γ is crucial during the SPEM stage of precancerous
lesions (55). Current studies
have typically focused on IL-18-related gene polymorphisms.
Research has demonstrated that IL-18RAP gene polymorphisms serve a
notable role in the development of gastric precancerous lesions by
controlling the IFN-γ production pathway, and may be linked to
precancerous lesions or susceptibility to GC in GC high-risk
populations (51,53). However, according to a different
study, there was no meaningful association between the progression
of gastric precancerous lesions and IL-18 promoter polymorphisms
(56). Therefore, there is a lack
of direct evidence that IL-18 serves a key role in gastric
precancerous lesions and SPEM progression.
IL-33, a member of the IL-1 superfamily, is a
cytokine involved in tissue homeostasis, pathogenic infections,
inflammation, allergies and the type 2 immune response. Through its
receptor ST2 (ST2L; also termed T1/ST2, IL1RL1, T1, Der4, Fit1 or
IL-33R), IL-33 forms a heterodimeric complex with IL-1 receptor
accessory protein (IL-1R3). IL-33 is extensively found on the
surfaces of Th2 cells, group 2 innate lymphocytes (ILC2s), mast
cells and macrophages (57,58). IL-33 serves as an intranuclear
transcriptional regulator, an extracellular alarmin and an immune
regulation mediator. Under healthy conditions, IL-33 translocates
to the nucleus and is involved in regulating gene expression
(59). When necrosis or cell
damage compromises the epithelial cell barrier, cells release
IL-33, which also acts as an alarm protein (60,61). The immune defense and repair
systems are coordinated by extracellular IL-33, which also
initiates adaptive immune responses. Increased extracellular IL-33
activates expanded tissue ILC2 and Treg and promotes the
recruitment and survival of immune cells (such as eosinophils, AAM,
Th2 cells and basophils). These cells and signals feed back to the
tissue to promote remodeling and limit inflammation. In the gastric
mucosa, IL-33 is mainly secreted by damaged epithelial cells, a
subset of mucous foveolar epithelial cells and M2-type macrophages
(60,62,63). When there is acute inflammation
and a loss of gastric mucosal parietal cells, macrophages
infiltrate and gastric pit epithelial cells release IL-33 to
synchronize immune defense and repair processes. IL-33 also
promotes epithelial proliferation through activation of the MAPK
pathway (ERK1/2, JNK and p38) (64). The IL-33/IL-13 axis is critical
for gastric metaplasia and gastric epithelial repair, and several
studies have linked reduced IL-33 in TFF2-deficient mice to
defective metaplasia development and delayed downstream gastric
epithelial repair (60,65,66). Following parietal cell loss, IL-33
stimulates the ST2 signaling axis, triggers a Th2-type immune
response, promotes the release of cytokines such as IL-13 and
causes the transdifferentiation of gastric chief cells into SPEM
cells (63). IL-33- or ST2-KO
mice are unable to form SPEM after L635-induced acute injury,
suggesting that appropriate ILs are required to promote
transdifferentiation as part of the gastric epithelial repair
process (63,67). IL-33 also enhances ILC2 activity
by upregulating GATA3 signaling, drives the gastric mucosal Th2
response and synergistically promotes goblet cell proliferation
with IL-25 (68). Furthermore,
IL-33 activates ILC2 in combination with IL-25 released by Tuft
cells, increasing type II cytokines such as IL-4, IL-5, IL-9 and
IL-13 (57,63,69). Among these cytokines, IL-13 and
IL-4 mediate M2 macrophage activation through the co-receptor
IL-4Rα (70). M2 macrophages and
eosinophils in turn produce IL-13 and IL-33, self-sustaining the
feed-forward cycle and stimulating mast cell activity (71,72). The resulting downstream release of
IL-13 facilitates the progression of SPEM. A series of
IL-33-mediated signaling events that promote SPEM progression are
centrally involved, and persistent inflammation of the gastric
mucosa can further lead to additional IM and carcinogenesis
(68). Long-term and high levels
of IL-33 cause M2 macrophage polarization, eosinophil proliferation
and severe infiltration into the stomach mucosa, resulting in the
maintenance of Th2-driven chronic inflammation (73). This increases the risk of cancer
and promotes metaplasia to transform into GC (60,62,64,68). Given that IL-33 stimulates
epithelial proliferation and metaplasia whilst also inducing and
maintaining chronic Th2-driven inflammation, it is a key mediator
of SPEM/intestinal SPEM manifestations.
IL-2 is a pro- and anti-inflammatory cytokine
produced primarily by activated T cells that regulates immune
response and anti-tumor immunity. The IL-2 receptor consists of
three subunits: i) IL-2Rα (CD25); ii) IL-2Rβ (CD122); and iii)
common γ-chain (γc; CD132). The IL-2Rαβγc heterotrimer is
constitutively expressed on Tregs and ILC2s and has the highest
affinity for IL-2. In the context of chronic or acute inflammation,
mucosal cells proliferate at an accelerated rate to promote the
repair of damaged epithelium (74). H. pylori-associated
gastritis is closely related to IL-2, and the DMP-777 model shows
that parietal cell deficiency is sufficient to trigger SPEM, but
inflammation is critical for further differentiation and the
development of SPEM (75,76). Studies into the inflammatory
component in mice have shown that the progression of SPEM to
developmental abnormalities requires a Th1-dominated inflammatory
response (77,78). Th1 cells characteristically
secrete IFN-γ and IL-2 (79). Th1
cells in H. pylori-infected individuals produce large
amounts of IL-2, which induces T cell differentiation and immune
memory (80,81). IL-2 is considered to provide a
pathological basis for gastric carcinogenesis and SPEM by
suppressing the secretion of gastric acid and modifying Th1 immune
responses. A previous study found that the Th1-specific cytokines
IL-2 and IFN-γ directly inhibit acid secretion in mice, which can
lead to gastric atrophy and promote the development of precursor
lesions to GC (82). However,
there is no direct experimental evidence that indicates that IL-2
is directly related to SPEM differentiation and maturation.
Instead, IL-2 primarily mitigates tissue damage by reducing gastric
acid secretion and thus acid-related chronic inflammation (83). In summary, IL-2 serves an
important role in SPEM progression by regulating the intensity of
inflammation and the immune microenvironment, but its specific
signaling pathways and interactions with other cytokines still need
further exploration.
IL-4 is an important immunomodulatory cytokine
involved in the cascade transduction of several cytokines. Its
signaling is initiated through two heterodimeric receptor
complexes: i) Type I (IL-4Rα/γc) specifically bound to IL-4; and
ii) type II (IL-4Rα/IL-13Rα1) bound to both IL-4 and IL-13,
activating STAT6, JNK or IRS2 signaling pathways that drive
macrophage polarization towards the alternative activation
phenotype (M2a) (70,84). Activated JAK phosphorylates IL-4Rα
tyrosine residues, creating binding sites for STAT6, which
dimerizes and translocates to the nucleus to activate gene
transcription (85). IL-4 also
maintains immune homeostasis by balancing M1/M2 macrophage
polarization via STAT1/3 and STAT6 synergistic regulation (84,86). In gastric mucosal inflammation,
IL-4 acts as an anti-inflammatory factor to inhibit inflammation
and atrophy by reducing IFN-γ. It inhibits the secretion of
pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, and
regulates lymphocyte differentiation and the tumor microenvironment
(84). Due to the structural
similarity of the surface receptors, in a variety of diseases IL-4
acts predominantly in conjunction with another type II cytokine,
IL-13 (84). IL-4 and IL-13
activate macrophages by alternating, a process that is also an
important step in the progression of SPEM (70,86-88). IL-4 also serves a central role in
the Th2 cell phenotype, with studies revealing that sequential
activation of STAT5 and STAT6 enhances the subsequent response to
IL-4, ultimately leading to Th2 T cell differentiation (89,90). Studies have shown that IL-4 also
inhibits the growth of GC cells, and a notable increase in serum
IL-4 levels has also been reported in patients with GC (85,91,92). IL-4 is considered to be associated
with the repair of epithelial damage in the early stages, and the
mechanism of its association with cancer progression in the late
stages has not yet been demonstrated. Furthermore, a study
demonstrated that IL-4 notably increased M2 macrophage
differentiation in a diabetic mouse model, indicating that it may
serve a similar role in gastric mucosal injury repair (57).
IL-6, a pleiotropic cytokine, is secreted by immune
cells (such as monocytes, macrophages and lymphocytes) and
non-immune cells (such as endothelial cells, intestinal epithelial
cells, tumor-associated fibroblasts and tumor cells) (93-95). IL-6 regulates inflammatory
mediators and endocrine responses, and serves as a link between
innate and adaptive systems in host defense mechanisms (96). IL-6 signaling necessitates binding
to the specific receptor IL-6R or IL-11R, which then binds to the
common signal transduction receptor subunit glycoprotein 130
(gp130), causing its recruitment and homodimerization, eventually
leading to the activation of the JAK/STAT and
SHP2/Ras/MAPK/ERK1-2/activator protein 1 (AP-1) signaling cascades
(97-99). In gastric mucosal lesions, IL-6
regulates gastric mucosal homeostasis by regulating TFF1 and
mucosal proliferation, inflammation, angiogenesis and
apoptosis-related mediators, and its signaling regulates gastric
mucosal homeostasis by balancing SHP2/Ras/ERK and STAT3. Elevated
IL-6 levels were noted in chronic inflammatory conditions, and the
downregulation of TFF1 tumor suppressor activity was caused by the
absence of ERK/AP-1 signaling downstream of gp130. This, in turn,
results in the activation of constitutive and oncogenic STAT3,
which inhibits apoptotic processes and promotes cellular
proliferation and inflammatory responses. These factors ultimately
lead to the development of mucosal atrophy, metaplasia,
TFF2-associated changes in the SPEM lineage, aberrant cell
proliferation and the progression to dysplasia and submucosal
invasion (98). When IL-6 family
signaling pathways are interfered with, increased STAT3 signaling
may promote the growth of gastric adenomas, while increased
SHP2/ERK2 signaling may result in chronic mucosal inflammation
(100). Therapeutic strategies
targeting the IL-6/JAK/STAT3 axis, such as the JAK inhibitor
WP1066, have been shown to inhibit STAT3 phosphorylation and reduce
pro-inflammatory factors (IL-6, IL-11 and IL-1β) and growth factor
expression, thereby blocking tumor proliferation and inducing
apoptosis (101). Nonetheless,
there is insufficient evidence to support a causal role for IL-6 in
cancer progression, implying that this IL may serve a protective
rather than pathogenic role in the stomach (102).
IL-11 belongs to the IL-6 family and is a
pleiotropic cytokine. It is a key mediator of inflammation in the
gastrointestinal tract, influencing the activity of numerous immune
cell populations such as macrophages, mast cells, dendritic cells
(DCs) and lymphocytes (103).
IL-11 initiates signal transduction by binding to IL-11 receptor α,
which recruits the signal transduction receptor gp130. By extending
stem cell survival, decreasing apoptosis and promoting mitosis,
IL-11 can impact epithelial homeostasis and is crucial for gastric
injury, mucosal repair and cancer progression (104). Chronic IL-11 elevation causes
severe fundus damage, including inflammation, metaplasia, loss of
chief and parietal cells and increased proliferation, all of which
are closely linked to chronic atrophic gastritis in humans
(103). IL-11 has been shown to
mediate the hyperactivation of STAT3 by gp130 in a
gp130F/F mouse model of gastric tumorigenesis (105,106). Overexpression of IL-11 in the
stomach caused by mutations in the gp130-JAK-STAT3 pathway can
cause spontaneous atrophic gastritis to progress to locally
advanced epithelial hyperplasia, but not dysplasia or cancer
(104). Furthermore, alongside
IL-11-induced gastric atrophy, there is a modest influx of
polymorphonuclear cells, accompanied by elevated expression of
IL-1β and IL-33 (103). IL-33
modulates Th1/Th2 cytokine balance in epithelial cells (107), whereas IL-1β regulates the
expression of HK-ATPaseα subunit 64 and suppresses
gastrin-dependent acid secretion (108,109). Notably, IL-11 can directly
activate GC cells, thereby driving the development of an invasive
phenotype (110), and is
upregulated in both mouse and human GC as well as preneoplastic
mucosa (111).
IL-10 is an important anti-inflammatory cytokine and
serves a key role in immunomodulation by inhibiting the synthesis
of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12 and TNF-α
(112-114). IL-10 activity is mediated by
heterodimeric IL-10 receptors (IL-10R1 and IL-10R2) (112). By preventing macrophage-mediated
inflammatory responses and limiting pro-inflammatory signaling
pathways such as NF-κB, IL-10 facilitates tissue repair (112,113). In a tamoxifen-induced SPEM model
of gastric mucosa, IL-10 expression was notably reduced 3 days
after administration and coincided with a histological loss of ~90%
of parietal cells in the gastric mucosa. IL-10 levels were restored
after 10 and 21 days, accompanied by histological normalization,
suggesting that it may be involved in mucosal repair by modulating
parietal cell function (25).
Deficits in IL-10 can also result in the development of
precancerous microenvironments and chronic inflammation (42). In addition, intracellular IL-10
levels are significantly elevated in patients with advanced GC
(115). IL-10 present in
tumor-associated macrophages creates an immune-evasive
microenvironment (116), and
elevated IL-10 levels are associated with GC metastasis and poor
prognosis (117). The regulatory
mechanism of IL-10 on SPEM-related carcinogenesis is still being
investigated (42); however,
IL-10 is regarded as a key target for intervention in gastric
metaplasia and carcinogenesis (25,118).
IL-27, a heterodimeric cytokine composed of p28 and
EBI3, has a receptor consisting of IL-27Rα and gp130 and is widely
expressed in immune cells such as T cells (43). Studies have shown that IL-27
serves a key regulatory role in chronic gastritis and gastric
mucosal metaplasia. IL-27-deficient mice progress more rapidly
during gastric carcinogenesis, as evidenced by earlier and faster
development of severe gastric atrophy, metaplasia and inflammatory
infiltrates, whereas exogenous IL-27 treatment notably reduces the
severity of gastritis, atrophy and SPEM (43,119). IL-27 protective effects are
primarily twofold: i) Reduces immune cell secretion of IL-17A; and
ii) Transmits signals directly to gastric epithelial cells, slowing
the progression of gastric metaplasia and possibly promoting
reparative metaplasia (120).
Rapid disease progression and notable variations in the degree of
STAT signaling pathway activation in gastric epithelial cells are
linked to increased Th17 cell proportion and pro-inflammatory
cytokine levels (IL-6, G-CSF, MIP-2 and IL-5) in IL-27-deficient
models (119,121,122). Notably, IL-27 largely controls
metaplasia linked to chronic inflammation and has no discernible
impact on metaplasia brought on by acute injury (122). Based on this evidence, IL-27 may
serve a crucial protective role in preventing gastric
carcinogenesis and SPEM by regulating immune cell activity and
epithelial cell response.
The IL-17 cytokine family consists of six members
[IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25) and IL-17F]
(123-125). IL-17A (also commonly referred to
as IL-17) has been extensively studied and serves a crucial role in
host defense against microbes and in the development of
inflammatory diseases (126-130). IL-17 is derived from a wide
range of sources, including adaptive immune cells (such as
CD8+ T cells and γδ T cells) and innate immune cells or
myeloid cells (NK cells, macrophages, DCs, neutrophils and
eosinophils) (126,131-135). The receptor for IL-17A consists
of two protein monomers: i) IL-17 receptor A; and ii) IL-17
receptor C. The IL-17 receptor complex is expressed on numerous
cell types, including various types of epithelial cells (34). IL-17A promotes inflammation by
upregulating cellular adhesion molecules such as vascular cell
adhesion molecule-1 and intercellular adhesion molecule-1,
activating downstream signaling pathways (including NF-κB, MAPKs
and JAK2/STAT3), and stimulating the production of cytokines and
chemokines (including IL-1, IL-6 and monocyte chemotactic
protein-1) (123,136). IL-17A is a primary cause of
mural cell atrophy and metaplasia in chronic atrophic gastritis.
Overproduction of IL-17A in the setting of chronic inflammation
directly causes parietal cell apoptosis and accelerates the process
of gastric mucosal atrophy and metaplasia, whilst neutralization of
IL-17A successfully inhibits the aforementioned pathological
alterations (34,137). Patients with gastro-IM have
increased IL-17 levels, indicating that a prolonged Th17 response
may occur prior to cancer formation. The gastric epithelium may
undergo physiological and morphological changes as a result of a
persistent inflammatory state or immune response in the gastric
region. This increases the risk of tumor transformation due to
atrophy and decreased gastric acid levels (72). By upregulating the matrix
metalloproteinases MMP2/MMP9, disrupting the extracellular matrix
and activating the JAK2/STAT3 signaling pathway, IL-17A promotes
the formation of the tumor microenvironment by increasing the
migration and invasion of cancer cells (132). According to clinical evidence,
patients with GC had significantly higher serum IL-17A levels,
which may indicate that the protein could be used as a diagnostic
marker (133). In summary,
cytokine networks control the proinflammatory and procarcinogenic
effects of IL-17A, which is pleiotropic in gastric mucosal
inflammation, metaplasia and carcinogenesis.
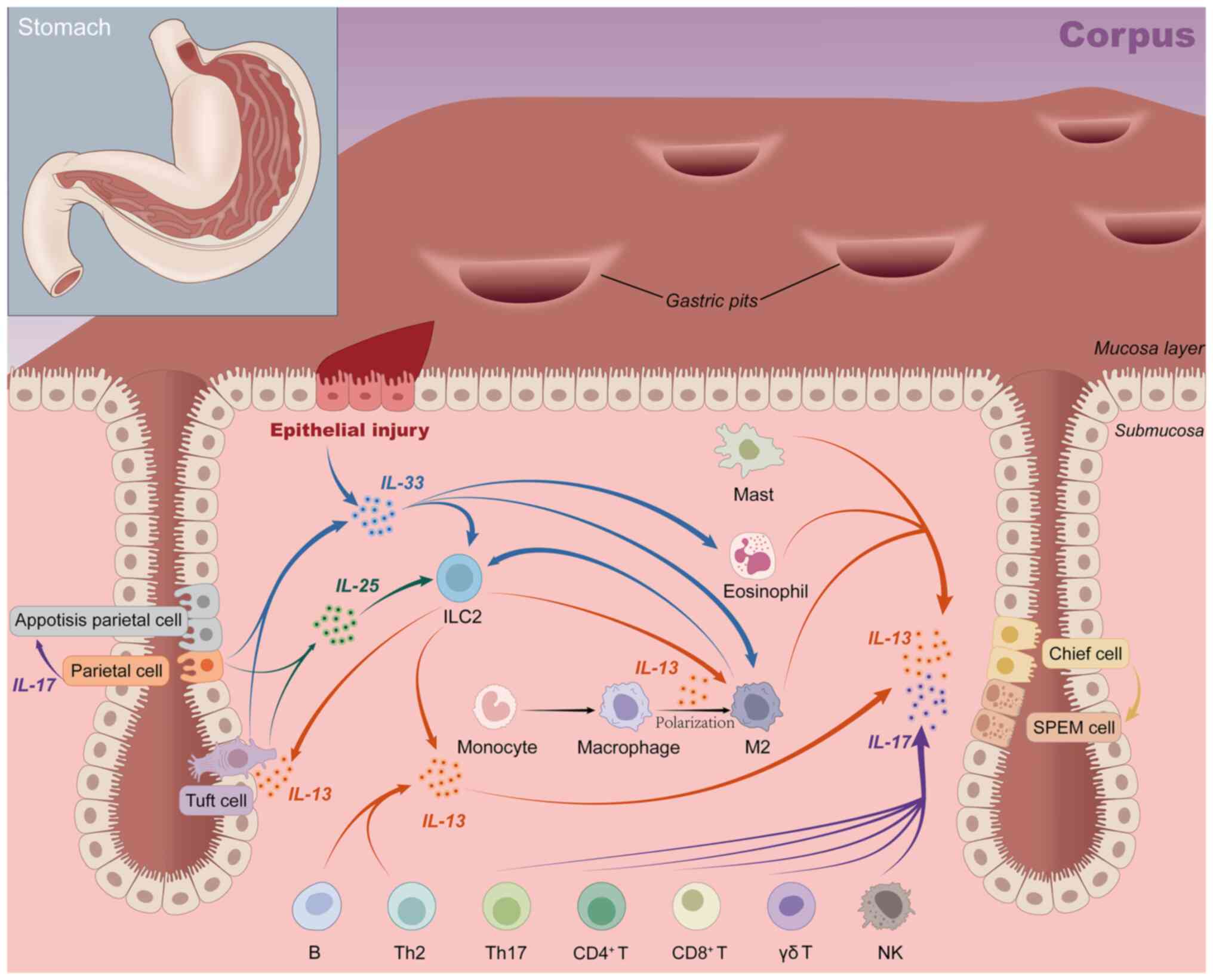
IL-25, also termed IL-17E, is a member of the IL-17
cytokine family. IL-25 is an important regulator of the type 2
immune response, and its increased expression is closely related to
the metaplasia microenvironment after parietal cell loss (138). Its receptor is expressed on the
surface of ILC2 cells, and when the gastric mucosa is severely
injured, surface mucus cells produce IL-33 and IL-25, which
stimulate ILC2 to secrete IL-13 together (Fig. 3). IL-13 is an important driver of
the reprogramming of chief cells into SPEM cells and the
development of pyloric metaplasia. Cluster cells further activate
ILC2 by secreting IL-25, forming a signaling loop (138,139). Notably, IL-25 also indirectly
triggers epithelial cell proliferation by inducing IL-13 production
in ILC2 (139). The IL-25/ILC2
axis is implicated in the development of metaplasia, but it also
facilitates the malignant progression of metaplasia to tumor cells.
According to the aforementioned findings, inhibiting IL-25
signaling or ablation of tuft cells or ILC2 has notable therapeutic
potential in both the early (gastric metaplasia) and advanced
stages of the tumor trajectory.
IL-8, also termed CXCL8, belongs to the CXC
chemokine family. By binding to the G protein-coupled receptors
CXCR1 and CXCR2, it activates downstream signaling pathways that
contribute to angiogenesis, the inflammatory response and the
growth of tumors (140). Its
expression is influenced by inflammatory factors (such as TNF-α and
IL-1β), environmental stress (including hypoxia and chemotherapy)
and hormones (including androgens and dexamethasone), and is
associated with a poor cancer prognosis (140-142). IL-8 was first identified for its
neutrophil chemotactic and degranulation effects, but its
pro-inflammatory properties and link to tumor metastasis are
especially important in GC (143). The chronic inflammatory state
caused by IL-8 may accelerate the progression of early
pre-cancerous lesions in GC, but no study has directly demonstrated
a link between IL-8 and SPEM.
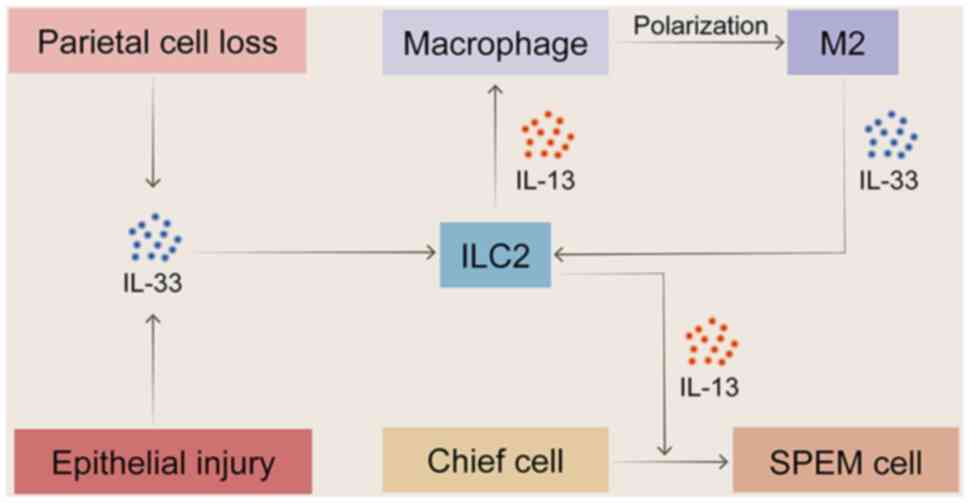
IL-13 is the central cytokine of the Th2 immune
response, mediating local tissue effects in gastritis such as
chemokine secretion, goblet cell proliferation, mucus production
and smooth muscle changes (84).
There are six immune cell types that secrete IL-13, and in
autoimmune gastritis models, mast cells produce the majority of
them. They also penetrate the gastric mucosa during chronic
inflammation (138,144,145). Another key source of IL-13 is
ILC2s, which are also found in inflammatory stomach tissue. IL4Rα,
IL13Rα1 and IL13Rα2 are three heterodimeric receptors that deliver
IL-13 signaling (146,147). The process involves JAK
signaling transducers (including JAK1 and JAK3) and the activator
of transcription (STAT) pathway (63,144). The most important of these is
STAT6, where IL-13 induces STAT6 phosphorylation and gene
transcriptional regulation to propel chief cell
transdifferentiation toward the SPEM phenotype (39,144,147). The gastric epithelium expresses
IL-4Rα, and IL-13 affects epithelial cells directly, which leads to
the expansion of mucus neck cells and the development of SPEM.
Furthermore, IL-13 can directly control chief cells through
IL-13Rα. In gastric metaplasia, its importance is mainly associated
with mucus hypersecretion, a specific feature of SPEM. IL-13 is a
core effector molecule of the IL-33/ILC2s/IL-13 axis (Fig. 4), and after chemically-induced
acute parietal cell loss, ILC2 is the major source of IL-13. In
conjunction with amphiphysin and IL-4, IL-13 promotes the
recruitment of eosinophils and the activation of M2-type
macrophages, as well as the induction and growth of SPEM (36,39). By contrast, ILC2 depletion or
ablation notably inhibits metaplasia induction, cell proliferation
and macrophage infiltration (36,38). Mice lacking the IL-33 receptor
(ST2) are unable to develop SPEM following L635 induction. However,
exogenous recombinant IL-13 aids in the redevelopment of SPEM, and
neutralizing IL-13 notably reverses the metaplasia in gastritis
mice (62,144). This suggests that IL-13 serves
an important role in the pathogenesis of SPEM, with IL-33 acting
only as its upstream inducer (63,148).
Existing research on the role of IL-13 in the
progression of SPEM to malignancy takes two opposing perspectives.
A previous study suggested that IL-13 mainly promotes the
maturation and stabilization of SPEM. By activating the IL-13/STAT6
axis, it causes STAT6 phosphorylation in SPEM cells and boosts
mucin production, resulting in a mature and proliferative SPEM cell
phenotype (39). Another study
reported the potential pro-cancer mechanism of IL-13; ILC2-derived
IL-13 promotes the growth and secretion of IL-25 by gastric mucosal
tufted cells, which in turn triggers ILC2 activity. This creates an
IL-13/IL-25 feedback loop and speeds up the development of cancer
from SPEM (138).
Simultaneously, TNF+ Tregs release IL-13, which
activates STAT3, directly promoting the malignant biologicals of
cancer cells (149). In
addition, the increase of the Th2 response and IL-4/IL-13 in
chronic inflammatory environments can trigger Tet3-mediated
epigenetic disorders. This process causes changes in gene
expression programs, which initiate epithelial transformation and
differentiation of precancerous cells (149). In conclusion, interventions
targeting IL-13 may be expected to alleviate gastrointestinal tumor
precursors and possibly reverse the development of gastric
metaplasia.
Animal models and gastric organoids are the two
most common types of SPEM models used in IL research. There are
three types of animal models: i) Drug-induced models; ii)
transgenic mouse models; and iii) H. pylori infection models
(Table II). Specific drugs cause
SPEM by directly inducing parietal cell death, making it an ideal
model for studying specific stages of the metaplasia process due to
its rapid and precise action. The drugs commonly used to induce
SPEM are DMP-777, L635 and tamoxifen. As a leukocyte elastase
protease inhibitor, DMP-777 was administered for 10-14 days to
induce gastric oxidative atrophy and metaplasia, accompanied by
mild inflammation (165). Its
analog L635 rapidly induces parietal cell loss in an inflammatory
environment, and treatment for 3 days can trigger inflammatory
infiltration and proliferative SPEM (advanced SPEM). Alterations in
its lineage are strikingly similar to those seen following 6-12
months of H. pylori infection (166-168). Tamoxifen, as a chemotherapeutic
drug, can rapidly induce SPEM formation through a selective
estrogen receptor-independent pathway within 3 days following oral
or intraperitoneal injection (169). The drug causes proton leakage by
damaging cells rich in proton pumps and mitochondria (such as
parietal cells), reducing mitochondrial phosphorylation efficiency
and interfering with intracellular pH homeostasis. Its resulting
loss of parietal cells can be reversed by the proton pump inhibitor
omeprazole, confirming that its action is dependent on active acid
secretion (170). Therefore,
tamoxifen-induced SPEM is considered a reversible precursor of
precancerous lesions (42,165,169,170).
In the autoimmune transgenic TxA23 mouse model, CD4+ T
cells cause chronic gastritis by targeting
H+/K+ ATPase and inducing apoptosis of
gastric parietal cells. This mimics a number of aspects of human
atrophic gastritis and metaplasia, including chronic inflammation
and parietal cell atrophy, mucus neck cell proliferation, SPEM and,
eventually, gastric intraepithelial tumors (34). Focal SPEM lesions were observed in
2-month-old mice, while extensive and severe SPEM lesions were
observed in 12-month-old mice. SPEM was also highly proliferated in
H. felis-infected mice. A total of 6 months after H.
pylori infection, mice showed acid secretion atrophy and
TFF2-expressing metaplasia (9).
The detection methods for mouse model establishment include
histological analysis and marker detection. Histological analysis
identified the morphological features (such as focal lesions and
glandular structural abnormalities) and inflammation of the
metaplastic glands by H&E staining. When inflammation occurs,
different types of metaplastic glands can form in nearby focal
lesions, creating a complex glandular assembly made up of a variety
of intestinal or gastric cell lineages that co-positively test for
different cell lineage markers. Cell lineage markers, such as HE4,
TFF2, MUC6, CD44v9 of SPEM and TFF3, MUC2, tail-type homeobox 1/2
or α-defense 5 of IM, were used to identify the lineage. Together
with the identification of associated ILs, Ki67, CD44, MUC1,
MUC5AC, p53, VEGF and other biomarkers, it can be used to assess
the malignant potential of SPEM (74).
Gastric organoids, also referred to as gastroids,
are 3-dimensional spheroids made by researchers from glands that
have separated from the stomach corpus mucosa. These gastroids
allowed the examination of the direct effects of ILs on gastric
epithelial cells (144). Gastric
glands were isolated from the target mice, and Matrigel was used to
cultivate whole gastric glands. They were placed in 24- or 48-well
plates, and cultured gastric organoids were treated with
recombinant IL (Table SI).
Typical images were captured of organoids aged 10 days following a
7-day IL treatment (34,39,138), and microphotographs were used to
examine changes in gastroids. Organoid diameter, relative organoid
diameter, organoid size, survival rate, Muc6 expression level,
AQP5/MUC6 double positive rate, structural departure from normal
tissue, height deviation from normal tissue, growth arrest,
mortality and structural collapse were among the statistical
indicators. This model is a crucial tool for studying the onset and
progression of SPEM as it closely resembles the cellular makeup and
behavior of the normal organism, making it easier to add ILs from
exogenous sources.
At present, SPEM detection in clinical practice has
not yet been popularized, but there is a growing interest in the
importance of metaplasia in precancerous lesions. Endoscopy, tissue
biopsy and biomarker detection are the most effective diagnostic
methods for gastric precancerous lesions (74). To lower the risk of inflammatory
cancer transformation and enhance the quality of life of patients,
the recommended management strategies include routine gastroscopy
monitoring, H. pylori eradication, lifestyle modifications
and medication interventions. The therapeutic potential of ILs has
gained interest from basic and translational cancer researchers. A
growing number of ongoing clinical trials have highlighted their
value as therapeutic agents and targets (171-175). Among these, IL-13 and its
receptor were found to be promising targets for treatment in order
to prevent and/or reverse the development of metaplasia in atrophic
gastritis (144). IL-13 and
IL-4Ra neutralizing antibodies (lebrikizumab, dupilumab) have been
used in clinical settings, and antagonizing the IL-33/IL-13
signaling pathway has become a prospective treatment paradigm to
reduce the progression of metaplasia and tumor transformation
(144,176). By controlling the cytokine
environment and CD4+ T cell infiltration, systemic IL-27
administration prevents gastric injury and aids in the recovery of
acid glands (43). Non-small cell
lung cancer, myelodysplastic syndrome and chronic myeloid leukemia
have been treated with IL-1 neutralization strategies (such as
canakinumab and anakinra) and the IL-6 antibody siltuximab
(177,178). Furthermore, IL-2 monotherapy has
shown early success in treating melanoma, renal cell carcinoma and
gastrointestinal tract cancer, and may be useful in reducing the
risk of tumor transformation and metaplasia (179). Innovative IL-12 therapies (such
as oncolytic viruses, nanoparticles and CAR-T cells) can enhance
the therapeutic potential of gastrointestinal cancer through
precise delivery (180,181).
Current clinical trials related to GC focus on
innovative applications of engineered ILs (Table SII). The safety of
Bempegaldesleukin, a polyethylene glycolized non-α IL-2 variant,
which extends half-life and reduces toxicity by blocking the IL-2Rα
subunit binding site via a PEG residue, has been validated
(ClinicalTrials.gov Identifier:
NCT02983045, https://clinicaltrials.gov/ct2/show/NCT02983045).
The comparable drug THOR707 (SAR444245) enhances anti-tumor
activity through selective activation of intermediate affinity
IL-2R (ClinicalTrials.gov Identifier:
NCT04009681, https://clinicaltrials.gov/ct2/show/NCT04009681).
In addition, ALKS 4230, which uses an IL-2/IL-2Rα circular
replacement structure to optimize receptor targeting, is in phase
I/II studies (ClinicalTrials.gov Identifier: NCT03861793,
https://clinicaltrials.gov/ct2/show/NCT03861793).
VG161 is a recombinant human IL-12/15/PD-L1B triple factor tumor
lysing HSV-1 injection that induces systemic anti-tumor immunity
via local cytokine release (ClinicalTrials.gov Identifier: NCT06008925,
https://clinicaltrials.gov/ct2/show/NCT06008925).
Recombinant human nsIL12 tumor solubilizing adenovirus injection
(BioTTT001) in combination with SOX chemotherapy and teraplizumab
is being evaluated for synergistic efficacy against peritoneal
metastasis of GC (ClinicalTrials.gov Identifier: NCT06283121,
https://clinicaltrials.gov/ct2/show/NCT06283121).
The engineered modification breaks through the limitations of
traditional IL therapy and provides a novel direction for GC
immunotherapy.
However, IL therapy is challenged by its short
half-life and narrow therapeutic window, leading to suboptimal
efficacy and unpredictable side effects (21). Common adverse reactions such as
influenza-like symptoms (including fever, chills and fatigue) are
short but notably affect the quality of life of patients. Severe
side effects included capillary leak syndrome, hypotension, fluid
retention, hepatotoxicity (liver function damage and elevated
enzyme levels), bone marrow suppression (increased risk of anemia,
infection and bleeding) and cardiovascular complications
(arrhythmia and fluid excess) (45). IL therapy is difficult to develop
and is not considered suitable as a standalone treatment for
clinical conditions. The pleiotropic nature of ILs contributes to
the aforementioned issues by causing dose-limiting toxicity or
inadequate efficacy (46,182). Future research must elucidate
the mechanism of IL-mediated SPEM in order to develop novel
therapies and investigate IL modification strategies, such as
targeted delivery mechanisms, PEGylation, fusion construction,
affinity regulation and synergy with other immunotumor drugs
(177,183-185). The emergence of more effective
immunotherapy and improved understanding of the tumor
microenvironment provide novel methods for the treatment of cancer
using the IL network (74,137).
Furthermore, patients with advanced disease are currently included
in the majority of trials, which may not be the optimal setting for
IL-based therapies. Future research should focus on identifying and
treating gastric metaplasia with a tendency to become cancerous
(28,41,186). Specific directions may include:
i) Developing more personalized IL modification strategies for
patients with early-stage SPEM, ii) designing combination trials of
IL therapy in patients with high-risk gastric metaplasia to halt
malignant progression, and iii) exploring the efficacy of
IL-targeted therapies in well-defined SPEM cohorts with targeted
design. Further research to target early precancerous lesions,
combined with tumor microenvironment regulation, deepen the
understanding of the pathogenesis of SPEM and provide direction for
targeted therapy to alleviate the progression of GC is
warranted.
The present review described the role of various
ILs and their expression products in the formation and progression
of SPEM. IL effects are complex and multifactorial. It was
summarized that certain ILs, such as IL-2, IL-4, IL-6, IL-8, IL-18
and IL-32, have not yet been studied to confirm a direct
relationship with SPEM but are closely related to IM and GC
development, which require further investigation. M2 macrophages
promote SPEM progression in the context of inflammation and
parietal cell atrophy, and the establishment of a chronic
inflammatory environment through pro-inflammatory IL recruitment is
also a potential direction for research. Studies have focused on
the role that these ILs may serve in GC-associated with
angiogenesis, metastasis and chemotherapy resistance, which are key
factors influencing carcinogenesis and tumor progression, quality
and patient survival. Although there are still a number of
challenges to overcome in the development of IL- or IL-targeted
therapies, the development of biologics and small-molecule
inhibitors that selectively target pathogenesis could improve
therapeutic efficacy and minimize side effects. With the discovery
of specific markers, the understanding of SPEM origin and cancer
progression will be improved, which may contribute to the simple
and accurate diagnosis of early developmental abnormalities. These
results provide a research direction for the pathogenesis of SPEM
and open up new avenues for future diagnosis and treatment.
Not applicable.
Conceptualization, literature investigation,
writing the original draft, writing, review and editing, and
visualization were performed by TZ, MJ and XZ. WG, SZ and HL made
substantial contributions to writing, reviewing and editing. HL and
MJ were responsible for conceptualization (substantial contribution
to the design of the work), supervision and project administration.
Data authentication is not applicable. All authors read and
approved the final manuscript, and agree to be accountable for all
aspects of the work.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present work was supported by grants from the National
Natural Science Foundation of China (grant no. 82274442) and the
Tianjin Municipal Education Commission (grant no. 2024KJ043).
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI
|
|
2
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
4
|
Morgan E, Arnold M, Camargo MC, Gini A,
Kunzmann AT, Matsuda T, Meheus F, Verhoeven RHA, Vignat J,
Laversanne M, et al: The current and future incidence and mortality
of gastric cancer in 185 countries, 2020-40: A population-based
modelling study. EClinicalMedicine. 47:1014042022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correa P, Haenszel W, Cuello C, Tannenbaum
S and Archer M: A model for gastric cancer epidemiology. Lancet.
2:58–60. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan L, Li W, Chen F, Wang J, Chen J, Chen
Y and Ye W: Inflammation as a mediator of microbiome
Dysbiosis-associated DNA methylation changes in gastric
premalignant lesions. Phenomics. 3:496–501. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang N, He HW, He YY, Gu W, Xu MJ and Liu
L: Xiaotan Sanjie recipe, a compound Chinese herbal medicine,
inhibits gastric cancer metastasis by regulating GnT-V-mediated
E-cadherin glycosylation. J Integr Med. 21:561–574. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giroux V and Rustgi AK: Metaplasia: Tissue
injury adaptation and a precursor to the dysplasia-cancer sequence.
Nat Rev Cancer. 17:594–604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen CP, Mills JC and Goldenring JR:
Murine models of gastric corpus preneoplasia. Cell Mol
Gastroenterol Hepatol. 3:11–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang M, Hu S, Min M, Ni Y, Lu Z, Sun X,
Wu J, Liu B, Ying X and Liu Y: Dissecting transcriptional
heterogeneity in primary gastric adenocarcinoma by single cell RNA
sequencing. Gut. 70:464–475. 2021. View Article : Google Scholar
|
|
11
|
Muthupalani S, Ge Z, Joy J, Feng Y, Dobey
C, Cho HY, Langenbach R, Wang TC, Hagen SJ and Fox JG: Muc5ac null
mice are predisposed to spontaneous gastric antro-pyloric
hyperplasia and adenomas coupled with attenuated H. pylori-induced
corpus mucous metaplasia. Lab Invest. 99:1887–1905. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldenring JR: Spasmolytic
polypeptide-expressing metaplasia (SPEM) cell lineages can be an
origin of gastric cancer. J Pathol. 260:109–111. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt PH, Lee JR, Joshi V, Playford RJ,
Poulsom R, Wright NA and Goldenring JR: Identification of a
metaplastic cell lineage associated with human gastric
adenocarcinoma. Lab Invest. 79:639–646. 1999.PubMed/NCBI
|
|
14
|
Halldórsdóttir AM, Sigurdardóttrir M,
Jónasson JG, Oddsdóttir M, Magnússon J, Lee JR and Goldenring JR:
Spasmolytic polypeptide-expressing metaplasia (SPEM) associated
with gastric cancer in Iceland. Dig Dis Sci. 48:431–441. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi H, Goldenring JR, Kaminishi M
and Lee JR: Identification of spasmolytic polypeptide expressing
metaplasia (SPEM) in remnant gastric cancer and surveillance
postgastrectomy biopsies. Dig Dis Sci. 47:573–578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katz JP, Perreault N, Goldstein BG, Actman
L, McNally SR, Silberg DG, Furth EE and Kaestner KH: Loss of Klf4
in mice causes altered proliferation and differentiation and
precancerous changes in the adult stomach. Gastroenterology.
128:935–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oshima H, Matsunaga A, Fujimura T,
Tsukamoto T, Taketo MM and Oshima M: Carcinogenesis in mouse
stomach by simultaneous activation of the Wnt signaling and
prostaglandin E2 pathway. Gastroenterology. 131:1086–1095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldenring JR, Nam KT, Wang TC, Mills JC
and Wright NA: Spasmolytic polypeptide-expressing metaplasia and
intestinal metaplasia: Time for reevaluation of metaplasias and the
origins of gastric cancer. Gastroenterology. 138:2207–2210.e1.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reyes ME, Pulgar V, Vivallo C, Ili CG,
Mora-Lagos B and Brebi P: Epigenetic modulation of cytokine
expression in gastric cancer: Influence on angiogenesis, metastasis
and chemoresistance. Front Immunol. 15:13475302024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bockerstett KA and DiPaolo RJ: Regulation
of gastric carcinogenesis by inflammatory cytokines. Cell Mol
Gastroenterol Hepatol. 4:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Briukhovetska D, Dörr J, Endres S, Libby
P, Dinarello CA and Kobold S: Interleukins in cancer: From biology
to therapy. Nat Rev Cancer. 21:481–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasmin R, Siraj S, Hassan A, Khan AR,
Abbasi R and Ahmad N: Epigenetic regulation of inflammatory
cytokines and associated genes in human malignancies. Mediators
Inflamm. 2015:2017032015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oshima M, Oshima H, Matsunaga A and Taketo
MM: Hyperplastic gastric tumors with spasmolytic
polypeptide-expressing metaplasia caused by tumor necrosis
factor-alpha-dependent inflammation in cyclooxygenase-2/microsomal
prostaglandin E synthase-1 transgenic mice. Cancer Res.
65:9147–9151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee C, Lee H, Hwang SY, Moon CM and Hong
SN: IL-10 plays a pivotal role in Tamoxifen-induced spasmolytic
Polypeptide-expressing metaplasia in gastric mucosa. Gut Liver.
11:789–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demitrack ES, Gifford GB, Keeley TM,
Horita N, Todisco A, Turgeon DK, Siebel CW and Samuelson LC: NOTCH1
and NOTCH2 regulate epithelial cell proliferation in mouse and
human gastric corpus. Am J Physiol Gastrointest Liver Physiol.
312:G133–G144. 2017. View Article : Google Scholar :
|
|
27
|
Negovan A, Iancu M, Fülöp E and Bănescu C:
Helicobacter pylori and cytokine gene variants as predictors of
premalignant gastric lesions. World J Gastroenterol. 25:4105–4124.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng X, Yang M, Ye T, Feng J, Xu X, Yang
H, Wang X, Bao L, Li R, Xue B, et al: Mitochondrial GRIM-19 loss in
parietal cells promotes spasmolytic polypeptide-expressing
metaplasia through NLR family pyrin domain-containing 3
(NLRP3)-mediated IL-33 activation via a reactive oxygen species
(ROS)-NRF2-Heme oxygenase-1(HO-1)-NF-кB axis. Free Radic Biol Med.
202:46–61. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berraondo P, Sanmamed MF, Ochoa MC,
Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME,
Ponz-Sarvise M, Castañón E and Melero I: Cytokines in clinical
cancer immunotherapy. Br J Cancer. 120:6–15. 2019. View Article : Google Scholar :
|
|
30
|
Kureshi CT and Dougan SK: Cytokines in
cancer. Cancer Cell. 43:15–35. 2025. View Article : Google Scholar :
|
|
31
|
El-Zaatari M, Kao JY, Tessier A, Bai L,
Hayes MM, Fontaine C, Eaton KA and Merchant JL: Gli1 deletion
prevents Helicobacter-induced gastric metaplasia and expansion of
myeloid cell subsets. PLoS One. 8:e589352013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goldenring JR, Nam KT and Mills JC: The
origin of pre-neoplastic metaplasia in the stomach: Chief cells
emerge from the Mist. Exp Cell Res. 317:2759–2764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang WQ, Rathinavelu S, Samuelson LC and
Merchant JL: Interferon gamma induction of gastric mucous neck cell
hypertrophy. Lab Invest. 85:702–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bockerstett KA, Osaki LH, Petersen CP, Cai
CW, Wong CF, Nguyen TM, Ford EL, Hoft DF, Mills JC, Goldenring JR
and DiPaolo RJ: Interleukin-17A promotes parietal cell atrophy by
inducing apoptosis. Cell Mol Gastroenterol Hepatol. 5:678–690.e1.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caldwell B, Meyer AR, Weis JA, Engevik AC
and Choi E: Chief cell plasticity is the origin of metaplasia
following acute injury in the stomach mucosa. Gut. 71:1068–1077.
2022. View Article : Google Scholar
|
|
36
|
Meyer AR, Engevik AC, Madorsky T, Belmont
E, Stier MT, Norlander AE, Pilkinton MA, McDonnell WJ, Weis JA,
Jang B, et al: Group 2 innate lymphoid cells coordinate damage
response in the stomach. Gastroenterology. 159:2077–2091.e8. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He L, Zhang X, Zhang S, Wang Y, Hu W, Li
J, Liu Y, Liao Y, Peng X, Li J, et al: H. pylori-facilitated
TERT/Wnt/β-Catenin triggers spasmolytic Polypeptide-expressing
metaplasia and oxyntic atrophy. Adv Sci (Weinh). 12:e24012272025.
View Article : Google Scholar
|
|
38
|
Busada JT, Peterson KN, Khadka S, Xu X,
Oakley RH, Cook DN and Cidlowski JA: Glucocorticoids and androgens
protect from gastric metaplasia by suppressing group 2 innate
lymphoid cell activation. Gastroenterology. 161:637–652.e4. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Contreras-Panta EW, Lee SH, Won Y,
Norlander AE, Simmons AJ, Peebles RS Jr, Lau KS, Choi E and
Goldenring JR: Interleukin 13 promotes maturation and proliferation
in metaplastic gastroids. Cell Mol Gastroenterol Hepatol.
18:1013662024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldenring JR and Nomura S:
Differentiation of the gastric mucosa III. Animal models of oxyntic
atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol.
291:G999–G1004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee SH, Jang B, Min J, Contreras-Panta EW,
Presentation KS, Delgado AG, Piazuelo MB, Choi E and Goldenring JR:
Up-regulation of aquaporin 5 defines spasmolytic
Polypeptide-expressing metaplasia and progression to incomplete
intestinal metaplasia. Cell Mol Gastroenterol Hepatol. 13:199–217.
2022. View Article : Google Scholar
|
|
42
|
Park DJ and Kim SE: The Role of IL-10 in
gastric spasmolytic Polypeptide-expressing Metaplasia-related
carcinogenesis. Gut Liver. 11:741–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bockerstett KA, Petersen CP, Noto CN,
Kuehm LM, Wong CF, Ford EL, Teague RM, Mills JC, Goldenring JR and
DiPaolo RJ: Interleukin 27 protects from gastric atrophy and
metaplasia during chronic autoimmune gastritis. Cell Mol
Gastroenterol Hepatol. 10:561–579. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Brito BB, da Silva FAF and de Melo FF:
Role of polymorphisms in genes that encode cytokines and
Helicobacter pylori virulence factors in gastric carcinogenesis.
World J Clin Oncol. 9:83–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saleh RO, Jasim SA, Kadhum WR, Hjazi A,
Faraz A, Abid MK, Yumashev A, Alawadi A, Aiad IAZ and Alsalamy A:
Exploring the detailed role of interleukins in cancer: A
comprehensive review of literature. Pathol Res Pract.
257:1552842024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Propper DJ and Balkwill FR: Harnessing
cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol.
19:237–253. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Santos JC, Ladeira MS, Pedrazzoli J Jr and
Ribeiro ML: Relationship of IL-1 and TNF-α polymorphisms with
Helicobacter pylori in gastric diseases in a Brazilian population.
Braz J Med Biol Res. 45:811–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Starzyńska T, Ferenc K, Wex T, Kähne T,
Lubiński J, Lawniczak M, Marlicz K and Malfertheiner P: The
association between the interleukin-1 polymorphisms and gastric
cancer risk depends on the family history of gastric carcinoma in
the study population. Am J Gastroenterol. 101:248–254. 2006.
View Article : Google Scholar
|
|
50
|
Xue H, Lin B, Ni P, Xu H and Huang G:
Interleukin-1B and interleukin-1 RN polymorphisms and gastric
carcinoma risk: A meta-analysis. J Gastroenterol Hepatol.
25:1604–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang YM, Li ZX, Tang FB, Zhang Y, Zhou T,
Zhang L, Ma JL, You WC and Pan KF: Association of genetic
polymorphisms of interleukins with gastric cancer and precancerous
gastric lesions in a high-risk Chinese population. Tumour Biol.
37:2233–2242. 2016. View Article : Google Scholar
|
|
52
|
Myung DS, Lee WS, Park YL, Kim N, Oh HH,
Kim MY, Oak CY, Chung CY, Park HC, Kim JS, et al: Association
between interleukin-18 gene polymorphism and Helicobacter pylori
infection in the Korean population. Sci Rep. 5:115352015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheung H, Chen NJ, Cao Z, Ono N, Ohashi PS
and Yeh WC: Accessory protein-like is essential for IL-18-mediated
signaling. J Immunol. 174:5351–5357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Osaki LH, Bockerstett KA, Wong CF, Ford
EL, Madison BB, DiPaolo RJ and Mills JC: Interferon-γ directly
induces gastric epithelial cell death and is required for
progression to metaplasia. J Pathol. 247:513–523. 2019. View Article : Google Scholar :
|
|
55
|
Syu LJ, El-Zaatari M, Eaton KA, Liu Z,
Tetarbe M, Keeley TM, Pero J, Ferris J, Wilbert D, Kaatz A, et al:
Transgenic expression of interferon-γ in mouse stomach leads to
inflammation, metaplasia, and dysplasia. Am J Pathol.
181:2114–2125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Leung WK, Chan MC, To KF, Man EP, Ng EK,
Chu ES, Lau JY, Lin SR and Sung JJ: H. pylori genotypes and
cytokine gene polymorphisms influence the development of gastric
intestinal metaplasia in a Chinese population. Am J Gastroenterol.
101:714–720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He R, Yin H, Yuan B, Liu T, Luo L, Huang
P, Dai L and Zeng K: IL-33 improves wound healing through enhanced
M2 macrophage polarization in diabetic mice. Mol Immunol. 90:42–49.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Carriere V, Roussel L, Ortega N, Lacorre
DA, Americh L, Aguilar L, Bouche G and Girard JP: IL-33, the
IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
USA. 104:282–287. 2007. View Article : Google Scholar :
|
|
60
|
Buzzelli JN, Chalinor HV, Pavlic DI,
Sutton P, Menheniott TR, Giraud AS and Judd LM: IL33 is a stomach
alarmin that initiates a skewed Th2 response to injury and
infection. Cell Mol Gastroenterol Hepatol. 1:203–221.e3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schwartz C, O'Grady K, Lavelle EC and
Fallon PG: Interleukin 33: An innate alarm for adaptive responses
beyond Th2 immunity-emerging roles in obesity, intestinal
inflammation, and cancer. Eur J Immunol. 46:1091–1100. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
De Salvo C, Pastorelli L, Petersen CP,
Buttò LF, Buela KA, Omenetti S, Locovei SA, Ray S, Friedman HR,
Duijser J, et al: Interleukin 33 triggers early
Eosinophil-dependent events leading to metaplasia in a chronic
model of Gastritis-prone mice. Gastroenterology. 160:302–316.e7.
2021. View Article : Google Scholar
|
|
63
|
Petersen CP, Meyer AR, De Salvo C, Choi E,
Schlegel C, Petersen A, Engevik AC, Prasad N, Levy SE, Peebles RS,
et al: A signalling cascade of IL-33 to IL-13 regulates metaplasia
in the mouse stomach. Gut. 67:805–817. 2018. View Article : Google Scholar
|
|
64
|
Huang N, Cui X, Li W, Zhang C, Liu L and
Li J: IL-33/ST2 promotes the malignant progression of gastric
cancer via the MAPK pathway. Mol Med Rep. 23:3612021. View Article : Google Scholar :
|
|
65
|
Engevik AC, Feng R, Choi E, White S,
Bertaux-Skeirik N, Li J, Mahe MM, Aihara E, Yang L, DiPasquale B,
et al: The development of spasmolytic polypeptide/TFF2-expressing
metaplasia (SPEM) during gastric repair is absent in the aged
stomach. Cell Mol Gastroenterol Hepatol. 2:605–624. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao
CM, Podolsky DK and Wang TC: TFF2/SP-deficient mice show decreased
gastric proliferation, increased acid secretion, and increased
susceptibility to NSAID injury. J Clin Invest. 109:193–204. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nozaki K, Ogawa M, Williams JA, Lafleur
BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S and
Goldenring JR: A molecular signature of gastric metaplasia arising
in response to acute parietal cell loss. Gastroenterology.
134:511–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Privitera G, Williams JJ and De Salvo C:
The importance of Th2 immune responses in mediating the progression
of Gastritis-associated metaplasia to gastric cancer. Cancers
(Basel). 16:5222024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Meyer AR and Goldenring JR: Injury,
repair, inflammation and metaplasia in the stomach. J Physiol.
596:3861–3867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bernink JH, Germar K and Spits H: The role
of ILC2 in pathology of type 2 inflammatory diseases. Curr Opin
Immunol. 31:115–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li W, Huang X, Han X, Zhang J, Gao L and
Chen H: IL-17A in gastric carcinogenesis: Good or bad? Front
Immunol. 15:15012932024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pisani LF, Teani I, Vecchi M and
Pastorelli L: Interleukin-33: Friend or foe in gastrointestinal
tract cancers? Cells. 12:14812023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ye Q, Zhu Y, Ma Y, Wang Z and Xu G:
Emerging role of spasmolytic polypeptide-expressing metaplasia in
gastric cancer. J Gastrointest Oncol. 15:2673–2683. 2024.
View Article : Google Scholar
|
|
75
|
Melchiades JL, Zabaglia LM, Sallas ML,
Orcini WA, Chen E, Smith MAC, Payão SLM and Rasmussen LT:
Polymorphisms and haplotypes of the interleukin 2 gene are
associated with an increased risk of gastric cancer. The possible
involvement of Helicobacter pylori. Cytokine. 96:203–207. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shin WG, Jang JS, Kim HS, Kim SJ, Kim KH,
Jang MK, Lee JH, Kim HJ and Kim HY: Polymorphisms of interleukin-1
and interleukin-2 genes in patients with gastric cancer in Korea. J
Gastroenterol Hepatol. 23:1567–1573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mohammadi M, Czinn S, Redline R and Nedrud
J: Helicobacter-specific cell-mediated immune responses display a
predominant Th1 phenotype and promote a delayed-type
hypersensitivity response in the stomachs of mice. J Immunol.
156:4729–4738. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Roth KA, Kapadia SB, Martin SM and Lorenz
RG: Cellular immune responses are essential for the development of
Helicobacter felis-associated gastric pathology. J Immunol.
163:1490–1497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang E, Chua W, Ng W and Roberts TL:
Peripheral cytokine levels as a prognostic indicator in gastric
cancer: A review of existing literature. Biomedicines. 9:19162021.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bevington SL, Keane P, Soley JK, Tauch S,
Gajdasik DW, Fiancette R, Matei-Rascu V, Willis CM, Withers DR and
Cockerill PN: IL-2/IL-7-inducible factors pioneer the path to T
cell differentiation in advance of lineage-defining factors. EMBO
J. 39:e1052202020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bamford KB, Fan X, Crowe SE, Leary JF,
Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE and Ernst PB:
Lymphocytes in the human gastric mucosa during Helicobacter pylori
have a T helper cell 1 phenotype. Gastroenterology. 114:482–492.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Padol IT and Hunt RH: Effect of Th1
cytokines on acid secretion in pharmacologically characterised
mouse gastric glands. Gut. 53:1075–1081. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Togawa S, Joh T, Itoh M, Katsuda N, Ito H,
Matsuo K, Tajima K and Hamajima N: Interleukin-2 gene polymorphisms
associated with increased risk of gastric atrophy from Helicobacter
pylori infection. Helicobacter. 10:172–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Van Dyken SJ and Locksley RM:
Interleukin-4- and interleukin-13-mediated alternatively activated
macrophages: Roles in homeostasis and disease. Annu Rev Immunol.
31:317–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pan K, Li Q, Guo Z and Li Z: Healing
action of Interleukin-4 (IL-4) in acute and chronic inflammatory
conditions: Mechanisms and therapeutic strategies. Pharmacol Ther.
265:1087602025. View Article : Google Scholar
|
|
86
|
Labonte AC, Tosello-Trampont AC and Hahn
YS: The role of macrophage polarization in infectious and
inflammatory diseases. Mol Cells. 37:275–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu YC, Zou XB, Chai YF and Yao YM:
Macrophage polarization in inflammatory diseases. Int J Biol Sci.
10:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Petersen CP, Weis VG, Nam KT, Sousa JF,
Fingleton B and Goldenring JR: Macrophages promote progression of
spasmolytic polypeptide-expressing metaplasia after acute loss of
parietal cells. Gastroenterology. 146:1727–1738.e8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yoshimoto T: The hunt for the source of
primary Interleukin-4: How we discovered that natural killer T
cells and basophils determine T helper type 2 cell differentiation
in vivo. Front Immunol. 9:7162018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhou JY, Alvarez CA and Cobb BA:
Integration of IL-2 and IL-4 signals coordinates divergent
regulatory T cell responses and drives therapeutic efficacy. Elife.
10:e574172021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang Z, Chen Z, Que Z, Fang Z, Zhu H and
Tian J: Chinese medicines and natural medicine as immunotherapeutic
agents for gastric cancer: Recent advances. Cancer Rep (Hoboken).
7:e21342024.
|
|
92
|
Yang F, Shaibu Z, Liu Q and Zhu W:
Cytokine profiles as predictive biomarkers for treatment outcomes
in advanced gastric cancer patients undergoing PD-1 blockade
immunochemotherapy: A meta-analysis. Clin Exp Med. 25:1362025.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM,
Choi KS, Son SY, Han SU, Brekken RA, Lee D and Hur H: Targeting
interleukin-6 as a strategy to overcome Stroma-induced resistance
to chemotherapy in gastric cancer. Mol Cancer. 18:682019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X,
Li J, Li C, Yan M, Zhu Z, et al: IL-6 secreted by cancer-associated
fibroblasts promotes epithelial-mesenchymal transition and
metastasis of gastric cancer via JAK2/STAT3 signaling pathway.
Oncotarget. 8:20741–20750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Matsuo K, Oka M, Murase K, Soda H, Isomoto
H, Takeshima F, Mizuta Y, Murata I and Kohno S: Expression of
interleukin 6 and its receptor in human gastric and colorectal
cancers. J Int Med Res. 31:69–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Leja M, Wex T and Malfertheiner P: Markers
for gastric cancer premalignant lesions: Where do we go? Dig Dis.
30:268–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Giraud AS, Jackson C, Menheniott TR and
Judd LM: Differentiation of the gastric mucosa IV. Role of trefoil
peptides and IL-6 cytokine family signaling in gastric homeostasis.
Am J Physiol Gastrointest Liver Physiol. 292:G1–G5. 2007.
View Article : Google Scholar
|
|
99
|
Kamimura D, Ishihara K and Hirano T: IL-6
signal transduction and its physiological roles: The signal
orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38.
2003.PubMed/NCBI
|
|
100
|
Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim
SY, Blaser MJ and Lee YC: Helicobacter pylori CagA phosphorylation
status determines the gp130-activated SHP2/ERK and JAK/STAT signal
transduction pathways in gastric epithelial cells. J Biol Chem.
285:16042–16050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Judd LM, Menheniott TR, Ling H, Jackson
CB, Howlett M, Kalantzis A, Priebe W and Giraud AS: Inhibition of
the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and
in vivo. PLoS One. 9:e959932014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hwang IR, Hsu PI, Peterson LE, Gutierrez
O, Kim JG, Graham DY and Yamaoka Y: Interleukin-6 genetic
polymorphisms are not related to Helicobacter pylori-associated
gastroduodenal diseases. Helicobacter. 8:142–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Howlett M, Chalinor HV, Buzzelli JN,
Nguyen N, van Driel IR, Bell KM, Fox JG, Dimitriadis E, Menheniott
TR, Giraud AS and Judd LM: IL-11 is a parietal cell cytokine that
induces atrophic gastritis. Gut. 61:1398–1409. 2012. View Article : Google Scholar
|
|
104
|
Buzzelli JN, O'Connor L, Scurr M, Chung
Nien Chin S, Catubig A, Ng GZ, Oshima M, Oshima H, Giraud AS,
Sutton P, et al: Overexpression of IL-11 promotes premalignant
gastric epithelial hyperplasia in isolation from germline
gp130-JAK-STAT driver mutations. Am J Physiol Gastrointest Liver
Physiol. 316:G251–G262. 2019. View Article : Google Scholar
|
|
105
|
Howlett M, Giraud AS, Lescesen H, Jackson
CB, Kalantzis A, Van Driel IR, Robb L, Van der Hoek M, Ernst M,
Minamoto T, et al: The interleukin-6 family cytokine interleukin-11
regulates homeostatic epithelial cell turnover and promotes gastric
tumor development. Gastroenterology. 136:967–977. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tebbutt NC, Giraud AS, Inglese M, Jenkins
B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et
al: Reciprocal regulation of gastrointestinal homeostasis by SHP2
and STAT-mediated trefoil gene activation in gp130 mutant mice.
Nature medicine. 8:1089–1097. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pastorelli L, Garg RR, Hoang SB, Spina L,
Mattioli B, Scarpa M, Fiocchi C, Vecchi M and Pizarro TT:
Epithelial-derived IL-33 and its receptor ST2 are dysregulated in
ulcerative colitis and in experimental Th1/Th2 driven enteritis.
Proc Natl Acad Sci USA. 107:8017–8022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Saha A, Hammond CE, Gooz M and Smolka AJ:
The role of Sp1 in IL-1beta and H. pylori-mediated regulation of
H,K-ATPase gene transcription. Am J Physiol Gastrointest Liver
Physiol. 295:G977–G986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wallace JL, Cucala M, Mugridge K and
Parente L: Secretagogue-specific effects of interleukin-1 on
gastric acid secretion. Am J Physiol. 261:G559–G564.
1991.PubMed/NCBI
|
|
110
|
Ham IH, Lee D and Hur H: Role of
Cancer-associated fibroblast in gastric cancer progression and
resistance to treatments. J Oncol. 2019:62707842019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Howlett M, Menheniott TR, Judd LM and
Giraud AS: Cytokine signalling via gp130 in gastric cancer. Biochim
Biophys Acta. 1793:1623–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Qu X, Tang Y and Hua S: Immunological
approaches towards cancer and inflammation: A cross talk. Front
Immunol. 9:5632018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gao L, Weck MN, Michel A, Pawlita M and
Brenner H: Association between chronic atrophic gastritis and serum
antibodies to 15 Helicobacter pylori proteins measured by multiplex
serology. Cancer Res. 69:2973–2980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sugai H, Kono K, Takahashi A, Ichihara F,
Kawaida H, Fujii H and Matsumoto Y: Characteristic alteration of
monocytes with increased intracellular IL-10 and IL-12 in patients
with advanced-stage gastric cancer. J Surg Res. 116:277–287. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X,
Lv K, He X, Fang H, Jin K, et al: Poor clinical outcomes and
immunoevasive contexture in intratumoral IL-10-Producing
macrophages enriched gastric cancer patients. Ann Surg.
275:e626–e635. 2022. View Article : Google Scholar
|
|
117
|
Park HS, Kwon WS, Park S, Jo E, Lim SJ,
Lee CK, Lee JB, Jung M, Kim HS, Beom SH, et al: Comprehensive
immune profiling and immune-monitoring using body fluid of patients
with metastatic gastric cancer. J Immunother Cancer. 7:2682019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yarmohammadi R, Najafi K, Noroozbeygi M,
Didehvar K, Rastin A, Ataei F, Atashzar MR and Shushtari SS: The
Role of IL-6, IL-10 and CRP in gastrointestinal cancers. Cell Biol
Int. 49:1061–1078. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nguyen TLM, Khurana SS, Sagartz JE, Mills
JC and DiPaolo R: EBI3 (IL-27/IL-35) regulates the progression from
atrophic gastritis to gastric cancer by Regulating IL-17 production
in the gastric mucosa. Gastroenterology. 146(Suppl 1): S64–S65.
2014. View Article : Google Scholar
|
|
120
|
Bockerstett KA, Nguyen TLM, Wong CF, Ford
EL and DiPaolo RJ: IL-27 Regulates gastric metaplasia through
effects on both immune and epithelial cells. Gastroenterology.
152(Suppl 1): S55–S56. 2017. View Article : Google Scholar
|
|
121
|
Bockerstett K, Petersen C, Ford E,
Goldenring JR and DiPaolo R: IL-17A promotes the progression from
gastritis to gastric cancer. Gastroenterology. 150(Suppl 1): S110.
2016. View Article : Google Scholar
|
|
122
|
Bockerstett KA, Petersen C, Noto CN, Kuehm
L, Wong CF, Ford EL, Mills J, Goldenring JR and DiPaolo RJ:
Interleukin-27-producing immune cells in the gastric mucosa protect
the stomach from inflammation-induced atrophy and metaplasia.
Gastroenterology. 156(Suppl 1): S186. 2019. View Article : Google Scholar
|
|
123
|
Gu C, Wu L and Li X: IL-17 family:
Cytokines, receptors and signaling. Cytokine. 64:477–485. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhao J, Chen X, Herjan T and Li X: The
role of interleukin-17 in tumor development and progression. J Exp
Med. 217:e201902972020. View Article : Google Scholar :
|
|
125
|
Kabir S: The role of interleukin-17 in the
Helicobacter pylori induced infection and immunity. Helicobacter.
16:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Meng XY, Zhou CH, Ma J, Jiang C and Ji P:
Expression of interleukin-17 and its clinical significance in
gastric cancer patients. Med Oncol. 29:3024–3028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Brackman LC, Jung MS, Green EH, Joshi N,
Revetta FL, McClain MS, Markham NO, Piazuelo MB and Scott Algood
HM: IL-17 signaling protects against Helicobacter pylori-induced
gastric cancer. Gut microbes. 16:24304212024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tangye SG and Puel A: The Th17/IL-17 Axis
and host defense against fungal infections. J Allergy Clin Immunol
Pract. 11:1624–1634. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kabil AK, Liu LT, Xu C, Nayyar N, González
L, Chopra S, Brassard J, Beaulieu MJ, Li Y, Damji A, et al:
Microbial dysbiosis sculpts a systemic ILC3/IL-17 axis governing
lung inflammatory responses and central hematopoiesis. Mucosal
Immunol. 18:1139–1158. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang J and Shen M: The Role of IL-17 in
systemic autoinflammatory diseases: Mechanisms and therapeutic
perspectives. Clin Rev Allergy Immunol. 68:272025. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhong F, Cui D, Tao H, Du H and Xing C:
IL-17A-producing T cells and associated cytokines are involved in
the progression of gastric cancer. Oncol Rep. 34:2365–2374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang J, Li S, Zhao Y, Ma P, Cao Y, Liu C,
Zhang X, Wang W, Chen L and Li Y: Cancer-associated fibroblasts
promote the migration and invasion of gastric cancer cells via
activating IL-17a/JAK2/STAT3 signaling. Ann Transl Med. 8:8772020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Karabulut M, Usul Afsar C, Serimez M and
Karabulut S: Serum IL-17 levels can be diagnostic for gastric
cancer. J BUON. 24:1601–1609. 2019.PubMed/NCBI
|
|
134
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Onishi RM and Gaffen SL: Interleukin-17
and its target genes: Mechanisms of interleukin-17 function in
disease. Immunology. 129:311–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Otani K, Watanabe T, Tanigawa T, Okazaki
H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N and
Arakawa T: Anti-inflammatory effects of IL-17A on Helicobacter
pylori-induced gastritis. Biochem Biophys Res Commun. 382:252–258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chong Y, Yu D, Lu Z and Nie F: Role and
research progress of spasmolytic polypeptide-expressing metaplasia
in gastric cancer (Review). Int J Oncol. 64:332024. View Article : Google Scholar :
|
|
138
|
O'Keefe RN, Carli ALE, Baloyan D, Chisanga
D, Shi W, Afshar-Sterle S, Eissmann MF, Poh AR, Pal B, Seillet C,
et al: A tuft cell-ILC2 signaling circuit provides therapeutic
targets to inhibit gastric metaplasia and tumor development. Nat
Commun. 14:68722023. View Article : Google Scholar
|
|
139
|
von Moltke J, Ji M, Liang HE and Locksley
RM: Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial
response circuit. Nature. 529:221–225. 2016. View Article : Google Scholar
|
|
140
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
He R, Li X, Zhang S, Liu Y, Xue Q, Luo Y,
Yu B, Li X and Liu Z: Dexamethasone inhibits IL-8 via glycolysis
and mitochondria-related pathway to regulate inflammatory pain. BMC
Anesthesiol. 23:3172023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and interleukin-8
in gastric cancer. World J Gastroenterol. 19:8192–8202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Noto CN, Hoft SG, Bockerstett KA, Jackson
NM, Ford EL, Vest LS and DiPaolo RJ: IL13 acts directly on gastric
epithelial cells to promote metaplasia development during chronic
gastritis. Cell Mol Gastroenterol Hepatol. 13:623–642. 2022.
View Article : Google Scholar :
|
|
145
|
Zhao R, Cao G, Zhang B, Wei L, Zhang X,
Jin M, He B, Zhang B, He Z and Bie Q: TNF+ regulatory T cells
regulate the stemness of gastric cancer cells through the
IL13/STAT3 pathway. Front Oncol. 13:11629382023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Grünig G, Warnock M, Wakil AE, Venkayya R,
Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD,
Locksley RM and Corry DB: Requirement for IL-13 independently of
IL-4 in experimental asthma. Science. 282:2261–2263. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Seyfizadeh N, Seyfizadeh N, Gharibi T and
Babaloo Z: Interleukin-13 as an important cytokine: A review on its
roles in some human diseases. Acta Microbiol Immunol Hung.
62:341–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bockerstett K, Lewis SA, Wolf KJ, Noto CN,
Jackson NM, Ford EL, Ahn TH and DiPaolo RJ: Single-cell
transcriptional analyses of spasmolytic polypeptide-expressing
metaplasia arising from acute drug injury and chronic inflammation
in the stomach. Gut. 69:1027–1038. 2020. View Article : Google Scholar
|
|
149
|
Miska J, Lui JB, Toomer KH, Devarajan P,
Cai X, Houghton J, Lopez DM, Abreu MT, Wang G and Chen Z:
Initiation of inflammatory tumorigenesis by CTLA4 insufficiency due
to type 2 cytokines. J Exp Med. 215:841–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dahl CA, Schall RP, He HL and Cairns JS:
Identification of a novel gene expressed in activated natural
killer cells and T cells. J Immunol. 148:597–603. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Meng D, Dong H, Wang C, Zang R and Wang J:
Role of interleukin-32 in cancer progression (review). Oncol Lett.
27:542024. View Article : Google Scholar
|
|
152
|
Han S and Yang Y: Interleukin-32: Frenemy
in cancer? BMB Rep. 52:165–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Fung KY, Nguyen PM and Putoczki TL:
Emerging roles for Interleukin-18 in the gastrointestinal tumor
microenvironment. Adv Exp Med Biol. 1240:59–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Khawar MB, Abbasi MH and Sheikh N: IL-32:
A novel pluripotent inflammatory interleukin, towards gastric
inflammation, gastric cancer, and chronic rhino sinusitis.
Mediators Inflamm. 2016:84137682016. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bagheri V, Memar B, Momtazi AA, Sahebkar
A, Gholamin M and Abbaszadegan MR: Cytokine networks and their
association with Helicobacter pylori infection in gastric
carcinoma. J Cell Physiol. 233:2791–2803. 2018. View Article : Google Scholar
|
|
156
|
Li ML, Hong XX, Zhang WJ, Liang YZ, Cai
TT, Xu YF, Pan HF, Kang JY, Guo SJ and Li HW: Helicobacter pylori
plays a key role in gastric adenocarcinoma induced by spasmolytic
polypeptide-expressing metaplasia. World J Clin Cases.
11:3714–3724. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kuo HY, Chang WL, Yeh YC, Cheng HC, Tsai
YC, Wu CT, Lin SH, Yang HB, Lu CC and Sheu BS: Spasmolytic
polypeptide-expressing metaplasia associated with higher
expressions of miR-21, 155, and 223 can be regressed by
Helicobacter pylori eradication in the gastric cancer familial
relatives. Helicobacter. 24:e125782019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yamaoka Y, Kodama T, Kita M, Imanishi J,
Kashima K and Graham DY: Relation between clinical presentation,
Helicobacter pylori density, interleukin 1beta and 8 production,
and cagA status. Gut. 45:804–811. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
El-Omar EM, Carrington M, Chow WH, McColl
KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N,
et al: Interleukin-1 polymorphisms associated with increased risk
of gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Jackson CB, Judd LM, Menheniott TR,
Kronborg I, Dow C, Yeomans ND, Boussioutas A, Robb L and Giraud AS:
Augmented gp130-mediated cytokine signalling accompanies human
gastric cancer progression. J Pathol. 213:140–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yamaoka Y, Kita M, Kodama T, Sawai N and
Imanishi J: Helicobacter pylori cagA gene and expression of
cytokine messenger RNA in gastric mucosa. Gastroenterology.
110:1744–1752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Adamsson J, Ottsjö LS, Lundin SB,
Svennerholm AM and Raghavan S: Gastric expression of IL-17A and
IFNγ in Helicobacter pylori infected individuals is related to
symptoms. Cytokine. 99:30–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar
|
|
165
|
Goldenring JR, Ray GS, Coffey RJ, Meunier
PC, Haley PJ, Barnes TB and Car BD: Reversible drug-induced oxyntic
atrophy in rats. Gastroenterology. 118:1080–1093. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal
RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, et al:
Mature chief cells are cryptic progenitors for metaplasia in the
stomach. Gastroenterology. 139:2028–2037.e9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Weis VG, Sousa JF, LaFleur BJ, Nam KT,
Weis JA, Finke PE, Ameen NA, Fox JG and Goldenring JR:
Heterogeneity in mouse spasmolytic polypeptide-expressing
metaplasia lineages identifies markers of metaplastic progression.
Gut. 62:1270–1279. 2013. View Article : Google Scholar
|
|
168
|
Nomura S, Yamaguchi H, Ogawa M, Wang TC,
Lee JR and Goldenring JR: Alterations in gastric mucosal lineages
induced by acute oxyntic atrophy in wild-type and gastrin-deficient
mice. Am J Physiol Gastrointest Liver Physiol. 288:G362–G375. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Cardoso CM, Custódio JB, Almeida LM and
Moreno AJ: Mechanisms of the deleterious effects of tamoxifen on
mitochondrial respiration rate and phosphorylation efficiency.
Toxicol Appl Pharmacol. 176:145–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Huh WJ, Khurana SS, Geahlen JH, Kohli K,
Waller RA and Mills JC: Tamoxifen induces rapid, reversible
atrophy, and metaplasia in mouse stomach. Gastroenterology.
142:21–24.e27. 2012. View Article : Google Scholar
|
|
171
|
Kudo-Saito C, Imazeki H, Nagashima K,
Shoji H, Tsugaru K, Takahashi N, Kawakami T, Amanuma Y, Wakatsuki
T, Okano N, et al: IL33-ST2 axis is a predictive biomarker for
anti-PD1 therapeutic efficacy in advanced gastric cancer. J Transl
Med. 23:11252025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Leidner R, Conlon K, McNeel DG,
Wang-Gillam A, Gupta S, Wesolowski R, Chaudhari M, Hassounah N, Lee
JB, Ho Lee L, et al: First-in-human phase I/Ib study of NIZ985, a
recombinant heterodimer of IL-15 and IL-15Rα, as a single agent and
in combination with spartalizumab in patients with advanced and
metastatic solid tumors. J Immunother Cancer. 11:e0077252023.
View Article : Google Scholar
|
|
173
|
Lordick F, Thuss-Patience P, Bitzer M,
Maurus D, Sahin U and Türeci Ö: Immunological effects and activity
of multiple doses of zolbetuximab in combination with zoledronic
acid and interleukin-2 in a phase 1 study in patients with advanced
gastric and gastroesophageal junction cancer. J Cancer Res Clin
Oncol. 149:5937–5950. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Gooderham M, Ameen M, Hong HC, Katoh N,
Langley RG, Peris K, Reich K, Silvestre JF, Simpson E, Werfel T, et
al: Efficacy of up to 4 years of tralokinumab in adults with
moderate-to-severe atopic dermatitis. J Eur Acad Dermatol Venereol.
Nov 11–2025. View Article : Google Scholar : Epub ahead of
print.
|
|
175
|
Song EJ, Ehst B, Glick B, Lewitt GM, Rich
P, Ezra N, Bagel J, Anschutz T, Bialik B, Duan C, et al: Efficacy
and safety of risankizumab in genital or scalp psoriasis in the
UnlIMMited phase 4 randomized clinical trial at week 16. Dermatol
Ther (Heidelb). Oct 25–2025. View Article : Google Scholar : Epub ahead of
print.
|
|
176
|
Yu S, Yang M, Lim KM, Cho Y, Kim H, Lee K,
Jeong SH, Coffey RJ, Goldenring JR and Nam KT: Expression of LRIG1,
a negative regulator of EGFR, is dynamically altered during
different stages of gastric carcinogenesis. Am J Pathol.
188:2912–2923. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Dickerson LK, Carter JA, Kohli K and
Pillarisetty VG: Emerging interleukin targets in the tumour
microenvironment: Implications for the treatment of
gastrointestinal tumours. Gut. 72:1592–1606. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Mohamed AH, Ahmed AT, Al Abdulmonem W,
Bokov DO, Shafie A, Al-Hetty HRAK, Hsu CY, Alissa M, Nazir S,
Jamali MC and Mudhafar M: Interleukin-6 serves as a critical factor
in various cancer progression and therapy. Med Oncol. 41:1822024.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Tanigawa K and Redmond WL: Current
landscape and future prospects of interleukin-2 receptor (IL-2R)
agonists in cancer immunotherapy. Oncoimmunology. 14:24526542025.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Dong C, Tan D, Sun H, Li Z, Zhang L, Zheng
Y, Liu S, Zhang Y and He Q: Interleukin-12 delivery strategies and
advances in tumor immunotherapy. Curr Issues Mol Biol.
46:11548–11579. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Xu Y, Sun X and Tong Y: Interleukin-12 in
multimodal tumor therapies for induction of anti-tumor immunity.
Discov Oncol. 15:1702024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lee HM, Lee HJ and Chang JE: Inflammatory
cytokine: An attractive target for cancer treatment. Biomedicines.
10:21162022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K and
Dai Z: Targeting cytokine and chemokine signaling pathways for
cancer therapy. Signal Transduct Target Ther. 9:1762024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Holder PG, Lim SA, Huang CS, Sharma P,
Dagdas YS, Bulutoglu B and Sockolosky JT: Engineering interferons
and interleukins for cancer immunotherapy. Adv Drug Deliv Rev.
182:1141122022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Tomasovic LM, Liu K, VanDyke D, Fabilane
CS and Spangler JB: Molecular engineering of interleukin-2 for
enhanced therapeutic activity in autoimmune diseases. BioDrugs.
38:227–248. 2024. View Article : Google Scholar :
|
|
186
|
Jeong H, Lee B, Kim KH, Cho SY, Cho Y,
Park J, Lee Y, Oh Y, Hwang BR, Jang AR, et al: WFDC2 Promotes
spasmolytic Polypeptide-Expressing metaplasia through the
Up-Regulation of IL33 in response to injury. Gastroenterology.
161:953–967.e15. 2021. View Article : Google Scholar
|