Lung cancer is a prevalent malignancy worldwide;
according to the latest GLOBOCAN report, ~2.48 million new lung
cancer cases are diagnosed each year and it accounts for 12.4% of
global cancer incidence, thus ranking as the most common cancer
globally. In addition, lung cancer is the leading cause of
cancer-related deaths, with an estimated 1.8 million fatalities
representing 18.7% of total cancer mortality. Notably, China bears
the highest lung cancer incidence and mortality rates worldwide
(1).
Radiotherapy serves as a primary treatment for lung
cancer across different stages and pathological types, markedly
improving local control rates and survival (2,3).
However, intrinsic or acquired radioresistance remains a clinical
challenge to treatment outcomes and patient prognosis (4,5).
The present review focuses on the role of mTOR in
lung cancer radioresistance, explores associated molecular
mechanisms and evaluates the clinical prospects of mTOR inhibitors
for enhancing radiotherapy efficacy. To achieve this, a systematic
literature search was conducted. PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofscience.com/) and
Scopus (https://www.scopus.com/) were searched
up to May 2024 using key words such as 'mTOR', 'lung cancer',
'radiotherapy resistance', 'mTOR inhibitors', 'PI3K/AKT', 'SCLC'
and 'immunotherapy'. Findings from original research, including
in vitro cellular experiments and in vivo animal
models, were subsequently assessed, as well as relevant clinical
trials, meta-analyses and other review articles. Notably,
non-peer-reviewed materials were excluded unless critical original
data were required. By synthesizing these findings, the present
review aims to elucidate how mTOR signaling mediates
radioresistance and to propose optimized therapeutic strategies to
improve outcomes for patients with lung cancer.
mTOR is a highly conserved serine/threonine kinase
belonging to the phosphatidylinositol kinase-related kinase family.
The soil bacterium Streptomyces hygroscopicus, which
produces rapamycin, was initially identified in 1931. Vézina et
al (15) then discovered the
molecular target of rapamycin, mTOR, in 1975 on Easter Island (Rapa
Nui, Chile). The mature mTOR protein comprises 2,549 amino acid
residues (molecular weight: ~289 kDa) and is encoded by the mTOR
gene situated on chromosome 1p36.2. Following transcription,
splicing, translation and post-translational modifications, the
functional mTOR protein localizes to multiple intracellular
compartments, including the endoplasmic reticulum, Golgi apparatus,
mitochondrial outer membrane, lysosomes, cytoplasm and nucleus
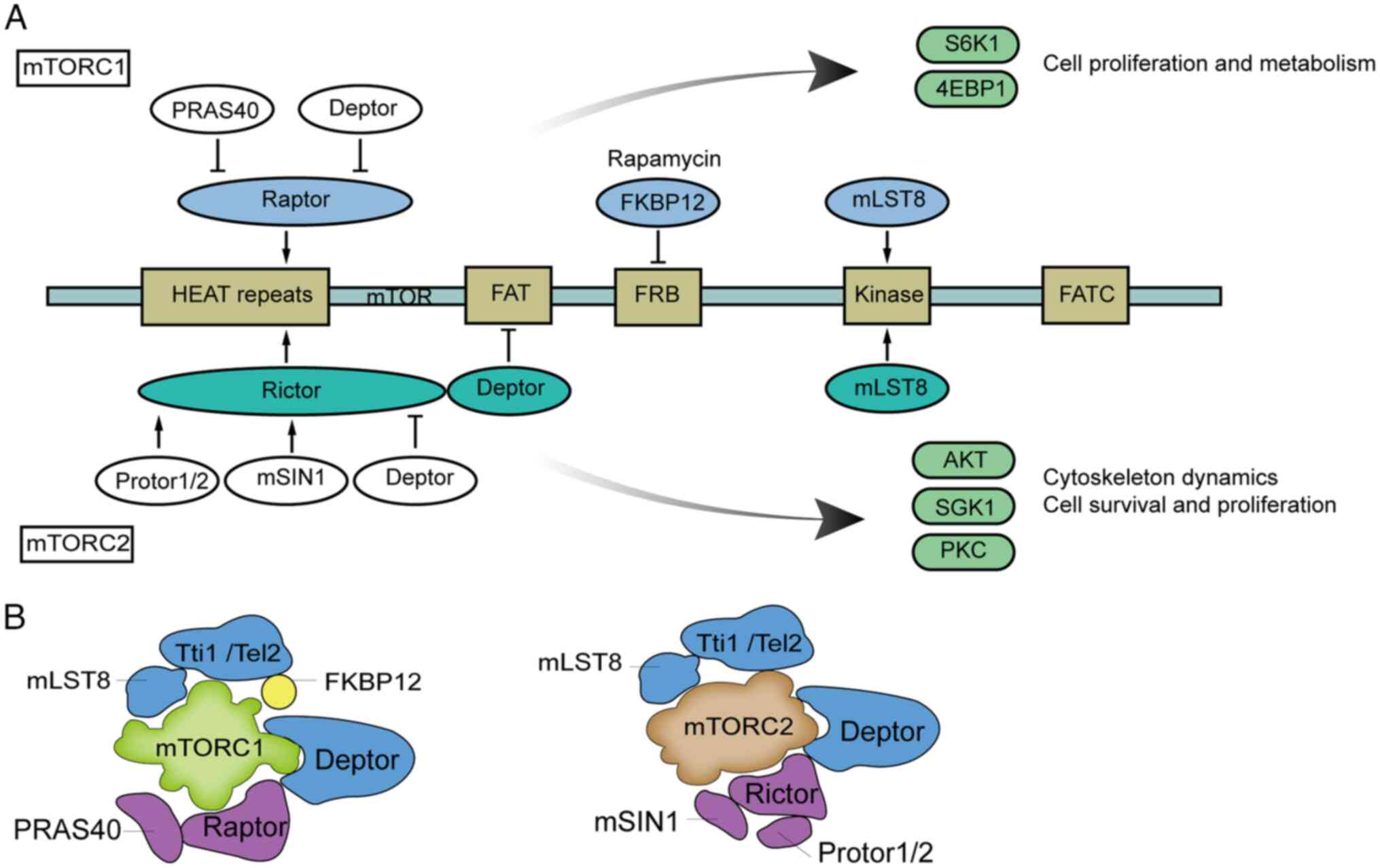
(16-18). Structurally, mTOR has two clusters
of repeats at its N-terminus. Each cluster has 20 tandem HEAT
repeats; HEAT stands for Huntingtin, EF3, the A subunit of PP2A,
and TOR1, and these repeats form hydrophobic surfaces, which are
critical for protein-protein interactions, membrane anchoring and
cytoplasmic trafficking. Adjacent to these, the focal adhesion
targeting (FAT) domain stabilizes protein complexes through
scaffold-like interactions, while the FKBP12-rapamycin binding
domain serves as the binding site for the FKBP12-rapamycin complex.
The kinase domain, functioning as the catalytic core,
phosphorylates serine/threonine residues in substrate proteins to
regulate downstream signaling. Finally, the C-terminal FATC domain
interacts spatially with the FAT domain to expose the catalytic
site, a configuration essential for the enzymatic activity of mTOR
(19) (Fig. 1A).
mTOR exerts its biological functions through two
distinct complexes: mTOR complex (mTORC)1 and mTORC2 (Fig. 1B). mTORC1 primarily regulates cell
proliferation and metabolism, with its core components including
mTOR, the scaffold protein regulatory-associated protein of mTOR
(Raptor), and mLST8/GβL (homologous to yeast TOR1, Kog1 and Lst8).
Additional regulatory proteins such as proline-rich AKT substrate
of 40 kDa and DEP domain-containing mTOR-interacting protein
modulate mTORC1 activity, while Tel2 and Tti1 are involved in its
assembly and stability. By sensing nutrient, energy and oxygen
availability, mTORC1 controls protein synthesis, autophagy and
metabolic pathways (20-22), and is potently inhibited by
rapamycin, from which its name originates. By contrast, mTORC2
governs cell survival and cytoskeletal reorganization by regulating
the AKT/PKB signaling pathway (23). Its core structure comprises mTOR,
the scaffold protein rapamycin-insensitive companion of mTOR, and
specific subunits including mammalian stress-activated protein
kinase-interacting protein 1 and Protor1/2 (homologous to yeast
TOR2, Avo3, Avo1 and Bit61/2). Unlike mTORC1, mTORC2 is not
directly sensitive to rapamycin inhibition. However, prolonged or
high-dose rapamycin treatment may indirectly suppress mTORC2
activity. The suppression of mTORC2 activity happens in
hepatocytes, adipocytes, T cells and certain cancer cells, and
impairs full AKT activation, subsequently reducing cell survival
and proliferation (24-26).
The mTOR signaling pathway forms a complex
regulatory network essential for eukaryotic cell proliferation,
metabolism and survival, integrating multiple upstream and
downstream effectors, as illustrated in Fig. 1A. mTORC1 activity is regulated by
upstream signals including the PI3K/AKT pathway (27,28), AMP-activated protein kinase (AMPK)
(29,30) and Rag GTPases (31). By sensing cellular nutrients,
energy and oxygen levels, mTORC1 coordinates protein synthesis,
autophagy, and metabolic processes. Its key downstream targets, S6
kinase 1 and 4EBP1, drive protein production and cell proliferation
while suppressing autophagy (20). By contrast, mTORC2 acts as a PI3K
signaling effector, enhancing cell survival and cytoskeletal
organization through insulin, insulin-like growth factor-1 and
leptin-mediated PI3K activation (32,33). It regulates the AKT/PKB pathway to
influence cell proliferation and survival (34) and mediates PKC phosphorylation to
support cytoskeletal remodeling and cell migration (35,36) (Fig.
1A). Collectively, mTOR critically impacts physiological and
pathological processes such as apoptosis, metabolic regulation,
immune responses and carcinogenesis (37), with its aberrant activation
strongly associated with cancer progression (38), thus making it a prime therapeutic
target.
mTOR serves a critical role in the pathogenesis of
lung cancer. As a key effector of the PI3K/AKT signaling pathway,
mTOR regulates tumor growth and survival by modulating cellular
processes including proliferation, metabolism, apoptosis and
autophagy (7,39). Recent investigations have shown
that mTOR is ubiquitously activated in lung cancer, with aberrant
mTOR signaling observed in both non-small cell lung cancer (NSCLC)
and small cell lung cancer (SCLC) (40), closely associated with tumor
aggressiveness and therapy resistance (41). Table
I systematically outlines PI3K/AKT/mTOR-mediated resistance
mechanisms in lung cancer radiotherapy, encompassing target
mechanisms, interventions (such as pharmacological inhibitors,
genetic modifications and radiotherapy combinations), their
functions and corresponding references.
In NSCLC, mTOR overexpression (OE) drives tumor
initiation, progression and metastasis, serving as a potential
therapeutic target, and multiple mechanisms contribute to this
dysregulation. Granville et al (42) revealed that tobacco-mediated
carcinogenesis is dependent on mTOR activation. This previous study
also showed that rapamycin effectively reduces the size and
proliferation of tumors induced by NNK (a tobacco-specific
carcinogen). This finding implicates mTOR in tobacco-related lung
squamous carcinoma. Additionally, mTOR upregulation may arise from
genetic mutations (such as EGFR, KRAS and ALK) disrupting PI3K/AKT
signaling (43-47), epigenetic modifications (DNA
methylation and histone acetylation) (48), and tumor microenvironment factors.
Zhang et al (49)
demonstrated that mTOR signaling promotes angiogenesis and
metastasis in NSCLC. Inhibiting mTORC2, in turn, can suppress cell
migration, metastasis and epithelial-mesenchymal transition
(EMT).
Compared with NSCLC, SCLC is characterized by high
aggressiveness and rapid growth, and it is often diagnosed at
advanced stages. Studies have indicated that mTOR signaling is
similarly hyperactivated in SCLC, and is associated with its
aggressive phenotype and treatment resistance (50-52). mTOR governs SCLC growth and
survival by regulating proliferation, metabolism, apoptosis and
autophagy. In SCLC, MYC, a commonly amplified oncogene, activates
mTOR signaling through multiple pathways, such as the PI3K/AKT and
MAPK pathways, to accelerate tumor cell proliferation (53). Furthermore, eIF4E, a downstream
effector of mTORC1, supports tumor growth by regulating protein
synthesis. Matsumoto et al (54) identified aberrant activation of
the MYC-eIF4E axis as a primary driver of resistance to the mTOR
inhibitor everolimus in SCLC. This finding underscores the
interplay between mTOR signaling and MYC/eIF4E pathways. While both
NSCLC and SCLC may benefit from mTOR pathway modulation, their
therapeutic efficacy and resistance mechanisms differ. As noted,
mTOR activity in NSCLC is predominantly linked to EGFR signaling,
whereas resistance in SCLC involves MYC and eIF4E pathways.
mTOR serves a critical role in cancer
radioresistance. Radiotherapy eliminates cancer cells by inducing
DNA damage; however, some tumor cells evade this effect through
mTOR signaling activation, leading to radiation resistance
(11,55). By regulating biological processes,
such as cell proliferation, metabolism, autophagy and DNA repair,
mTOR promotes cancer cell survival during radiotherapy. In lung
cancer, abnormal mTOR pathway activation is strongly associated
with radioresistance (56).
Current evidence has demonstrated that mTOR promotes resistance to
radiotherapy in lung cancer through multiple mechanisms. To provide
a concise overview of these complex mechanisms, Table I, Table II, Table III and Table IV summarize key findings from
preclinical studies.
The PI3K/AKT/mTOR pathway is a critical
intracellular signaling cascade that regulates physiological
processes, including cell proliferation, apoptosis, angiogenesis
and energy metabolism in normal cells. Its aberrant activation
promotes tumor progression by suppressing apoptosis, accelerating
cell cycle progression, enhancing angiogenesis and promoting
metastasis (57-59). In lung cancer, the PI3K/AKT/mTOR
pathway is frequently activated via genetic mutations,
amplifications and receptor tyrosine kinase activation. This
activation is associated with intrinsic radiosensitivity, tumor
cell proliferation and hypoxia, all of which contribute to
radiotherapy resistance (12,60).
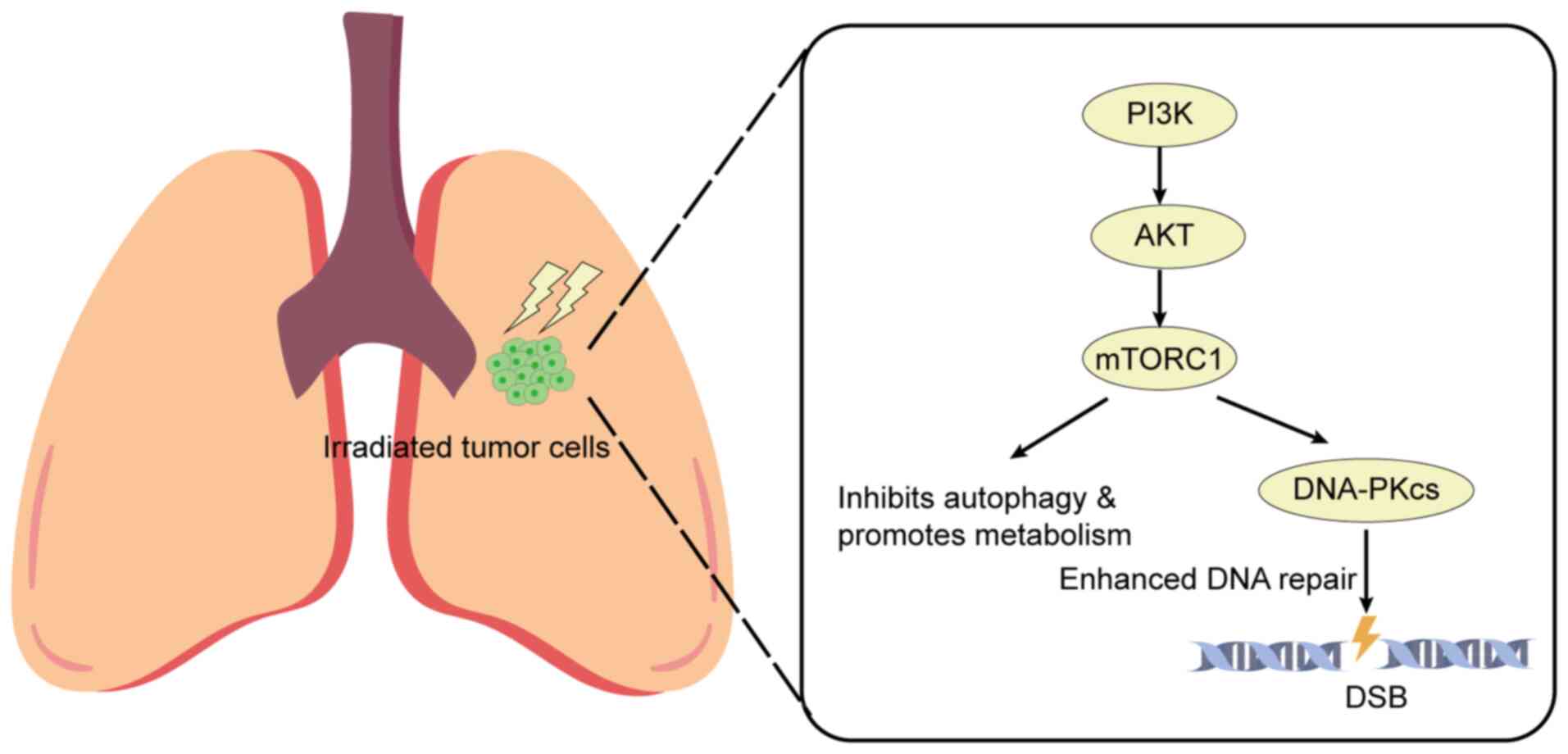
Mechanistically, activation of this pathway enhances
radioresistance by upregulating DNA repair proteins such as
DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which
accelerates the repair of radiation-induced DNA double-strand
breaks (61,62) (Fig.
2). This enhanced DNA repair is a primary escape mechanism for
irradiated tumor cells. However, Toulany et al (61) further revealed limitations in
radiosensitizing KRAS mutant NSCLC through PI3K inhibition alone.
These limitations are attributed to compensatory MEK-ERK pathway
activation. Tumor cells counteract PI3K suppression by enhancing
MEK-ERK signaling, which sustains survival via AKT-dependent
upregulation of DNA repair proteins (such as DNA-PKcs and Rad51),
ultimately impairing radiotherapy efficacy. Dual targeting of PI3K
and MEK has emerged as a promising strategy to overcome this
resistance (63). Kim et
al (64) demonstrated that
combining the MEK inhibitor trametinib with the mTOR inhibitor
temsirolimus may enhance radiosensitivity in NSCLC cells by
boosting radiation-induced apoptosis and inducing prolonged DNA
breaks.
Preclinical studies have established robust evidence
implicating PI3K/AKT/mTOR pathway activation in mediating tumor
cell radioresistance (Table I).
For example, scutellarin combined with iodine-125 seeds targets the
AKT/mTOR pathway to enhance apoptosis and inhibit proliferation in
NSCLC, offering a potential therapeutic approach (65). To validate the clinical relevance
of these findings, Sebastian et al (66) conducted a clinical study analyzing
tumor samples from 92 patients with T1-3N0 NSCLC treated with
stereotactic body radiotherapy (SBRT). This previous study revealed
that elevated PI3K pathway activity was significantly associated
with increased local recurrence risk (HR=1.72, 95% CI=1.40-98.0,
P=0.023) and shorter disease-free survival (DFS) (HR=3.98, 95%
CI=1.57-10.09, P=0.0035) in patients with early-stage NSCLC
receiving SBRT. Mechanistically, AKT/mTOR signaling deregulation
has been directly linked to chemoradiation resistance in lung
squamous cell carcinoma. Proteomics analysis of
chemoradiation-resistant patient-derived xenograft models and cell
lines has identified upregulated phosphorylated (p)-AKT and p-mTOR,
and mTOR kinase inhibitors have been shown to sensitize these
resistant cells to radiation (67). For KRAS-mutated NSCLC, single
targeting of PI3K often fails due to MEK/ERK-dependent Akt
reactivation. Dual inhibition of PI3K and MEK, however, can block
this compensatory signaling, impair DNA double-strand break repair
via non-homologous end joining, and significantly enhance
radiosensitivity (68).
Additionally, downstream effectors of PI3K/AKT/mTOR
signaling contribute to radioresistance through multiple routes.
For example, Akt/mTOR signaling-driven COX-2 overexpression fosters
radioresistance, and combining celecoxib (a COX-2 inhibitor) with
radiotherapy enhances apoptosis and prevents radioresistance
(69). Furthermore, SIRT6, an
epigenetic regulator, suppresses PI3K/Akt/mTOR signaling, and SIRT6
overexpression enhances radiosensitivity and inhibits tumor
progression in lung cancer (70).
Targeting these downstream nodes has shown promise. For example,
dysregulated PI3K/mTOR/EGFR crosstalk drives resistance, whereas
dual inhibitors targeting EGFR and PI3K/mTOR show selective
therapeutic benefit in lung cancer models, such as A549 cells
(71).
However, PI3K activity showed no significant
association with overall survival (P=0.49), regional recurrence
(P=0.15) or distant metastasis (P=0.85) according to a study by
Sebastian et al (66).
These results position PI3K activity as a potential biomarker for
predicting local recurrence and DFS in SBRT-treated NSCLC; however,
validation in larger multicenter cohorts remains essential to
confirm clinical applicability.
Autophagy is a key metabolic and homeostatic
mechanism that allows cells to adapt to environmental stress and
damage by degrading damaged organelles, misfolded proteins and
cellular debris, thereby preserving normal cellular function
(72). Under physiological
conditions, autophagy serves as a protective process essential for
clearing dysfunctional or senescent cellular components (73). In cancer cells, however, aberrant
activation of the mTOR signaling pathway suppresses autophagy,
allowing tumor cells to escape radiotherapy-induced damage
(74). Studies have indicated
that mTOR acts as a primary negative regulator of autophagy. It not
only inhibits autophagic flux but also enhances cancer cell
survival by promoting growth and metabolic reprogramming, thereby
fostering resistance to antitumor therapies such as radiotherapy
(75,76).
Collectively, these studies underline the dual role
of autophagy in lung cancer therapy and highlight the central
regulatory role of mTOR in autophagic processes. Co-targeting
autophagy and mTOR-associated signaling pathways may offer a more
effective strategy to enhance the therapeutic efficacy of
radiotherapy in lung cancer.
miRNAs are a class of non-coding RNAs that serve
pivotal roles in regulating gene expression and are frequently
dysregulated in diverse types of cancer (86). Studies have revealed that miRNAs
markedly contribute to tumorigenesis, progression and
radioresistance by modulating cancer cell proliferation, migration,
invasion and apoptosis, highlighting their potential value in early
diagnosis, prognosis prediction and therapeutic intervention
(87-89). In NSCLC, aberrant miRNA expression
profoundly impacts mTOR signaling pathway activity, thereby
influencing radiotherapy outcomes (Table III).
miRNAs frequently promote radioresistance by
suppressing tumor suppressor genes that regulate the mTOR pathway.
For example, miR-410, which is commonly upregulated in cancer,
promotes EMT and radioresistance by targeting PTEN to activate the
PI3K/mTOR axis (90-92). OE of miR-410 has been shown to be
positively associated with EMT-related markers (such as E-cadherin
downregulation and vimentin upregulation) and negatively associated
with PTEN expression. Genetic knockdown of miR-410 can suppress EMT
and enhance radiosensitivity, suggesting it may serve as a
potential biomarker or therapeutic target in NSCLC radiotherapy.
Restoring PTEN expression or administering mTOR inhibitors could
reverse miR-410-induced EMT and radioresistance, offering novel
strategies for NSCLC-targeted therapy. Similarly, miR-181a reduces
NSCLC radiosensitivity by suppressing the expression of PTEN, a
negative regulator of the PI3K/AKT/mTOR pathway (93). miR-21 also reduces NSCLC
radiosensitivity by targeting programmed cell death 4 to activate
PI3K/AKT/mTOR signaling (94).
Furthermore, Huang et al (95) revealed that circular PVT1 promotes
radioresistance by acting as a competing endogenous RNA to
sequester miR-1208, subsequently reactivating the PI3K/AKT/mTOR
pathway.
Conversely, miRNAs downregulated in lung cancer
enhance NSCLC radiosensitivity by directly targeting mTOR. For
example, miR-99a acts via mTOR as it is highly expressed in
radiation-sensitive A549 cells compared with resistant
counterparts. Its OE enhances radiosensitivity, whereas its
inhibition induces resistance in vitro and in vivo
(96). Similarly, miR-101-3p is
commonly downregulated in NSCLC tissues and cell lines (97), and modulates radiosensitivity via
mTOR. Li et al (98)
showed that in radiation-resistant A549R cells, miR-101-3p
upregulation can increase radiosensitivity; however, this effect is
attenuated by high mTOR activity, whereas its inhibition induces
resistance that can be reversed by rapamycin. This confirms that
miR-101-3p downregulation drives resistance by activating mTOR,
consistent with the mechanism of miR-99a. Another example is
miR-208a, which promotes cell proliferation and radioresistance in
NSCLC by targeting the AKT/mTOR pathway (99). Collectively, these studies
highlight mTOR as a core mediator of miRNA-regulated NSCLC
radiosensitivity, supporting co-targeting mTOR and these miRNAs as
a promising strategy to optimize lung cancer radiotherapy.
Although most research on mTOR and radioresistance
focuses on NSCLC, this pathway is also pivotal in highly aggressive
and resistant SCLC. The mechanisms underlying radioresistance in
SCLC often involve unique metabolic and epigenetic vulnerabilities,
as well as oncogene-driven signaling crosstalk (Table IV).
Metabolically, the PI3K/AKT/mTOR pathway actively
influences glucose metabolism to drive SCLC radioresistance
(100,101). In SCLC models, dual PI3K/mTOR
inhibitors (such as, BEZ235 and GSK2126458) promote autophagic
degradation of glucose-6-phosphate dehydrogenase (G6PD). G6PD is
the rate-limiting enzyme in the pentose phosphate pathway (PPP),
which is a key survival mechanism for SCLC cells. Disrupting the
PPP via G6PD degradation increases reactive oxygen species
accumulation and oxidative stress damage, ultimately enhancing IR
cytotoxicity and overcoming radioresistance (102). Additionally, SCLC frequently
harbors the MYC oncogene amplification, which drives aggressive
proliferation and mediates resistance to mTOR inhibitors such as
everolimus through the eIF4E axis (54).
Beyond metabolic and MYC-driven resistance, adaptive
survival pathways are another critical mTOR-related resistance
mechanism in SCLC. Therapy-induced DNA stress activates
compensatory pathways, such as PARP inhibition and SCLC DNA repair
strategies, which upregulate PI3K/mTOR. This feedback loop,
possibly via reduced LKB1 signaling, limits PARP inhibitor efficacy
alone (103,104). This highlights the use of mTOR
targeting to overcome SCLC therapy resistance. Additionally, in
lung cancer research, targeting hypoxia/HIF-1α via PI3K/Akt/mTOR
with Hsp90 inhibitors combined with radiotherapy blocks
radiation-induced HIF-1α stabilization and enhances antitumor
effects (105). Carbon ion
radiotherapy also attenuates HIF-1 signaling and mTOR induction
(106). In drug-resistant NSCLC,
CXCR4 inhibition eliminates cancer stem-like cells via
STAT3/Akt/mTOR signaling (107).
Furthermore, disrupting the NRF2-CHML-mTOR axis by CHML knockdown
inhibits NSCLC progression and overcomes chemo/radioresistance
(108). Moreover, rapamycin
combined with irradiation enhances lung cancer radiosensitivity and
protects normal lung cells (109). The activation of mTOR to counter
DNA damage in SCLC supports the combination of mTOR inhibitors with
DNA-damaging agents, such as radiation, highlighting the use of
mTOR targeting to overcome SCLC.
The mTOR pathway acts as a key regulator of T-cell
differentiation and function; therefore, its activity within tumor
cells and the tumor immune microenvironment is critical for
therapeutic responses.
Oncogenic activation of the PI3K/AKT/mTOR pathway is
strongly associated with the transcriptional and translational
regulation of programmed death-ligand 1 (PD-L1) expression on the
membranes of lung cancer cells (110). This drives immune evasion, as
PD-L1 binds to PD-1 on T cells to suppress antitumor immunity.
Common NSCLC mutations, such as EGFR and KRAS, activate this
pathway and subsequently increase PD-L1 expression, while
inhibiting mTOR can downregulate PD-L1, theoretically
're-sensitizing' tumors to immune attack (111).
Although mTOR inhibitors (rapalogs) are known as
immunosuppressants, they also function as immunomodulators that
promote antitumor responses, notably by expanding memory
CD8+ T cells. This dual role provides a strong rationale
for combination therapy as preclinical lung cancer models confirm
that pairing an mTOR inhibitor with a PD-1 antibody can markedly
reduce tumor growth and increase tumor-infiltrating T cells
(112). Targeting mTOR signaling
alongside immune checkpoints may therefore offer a potent
synergistic strategy for overcoming radioresistance and achieving
systemic antitumor effects (113).
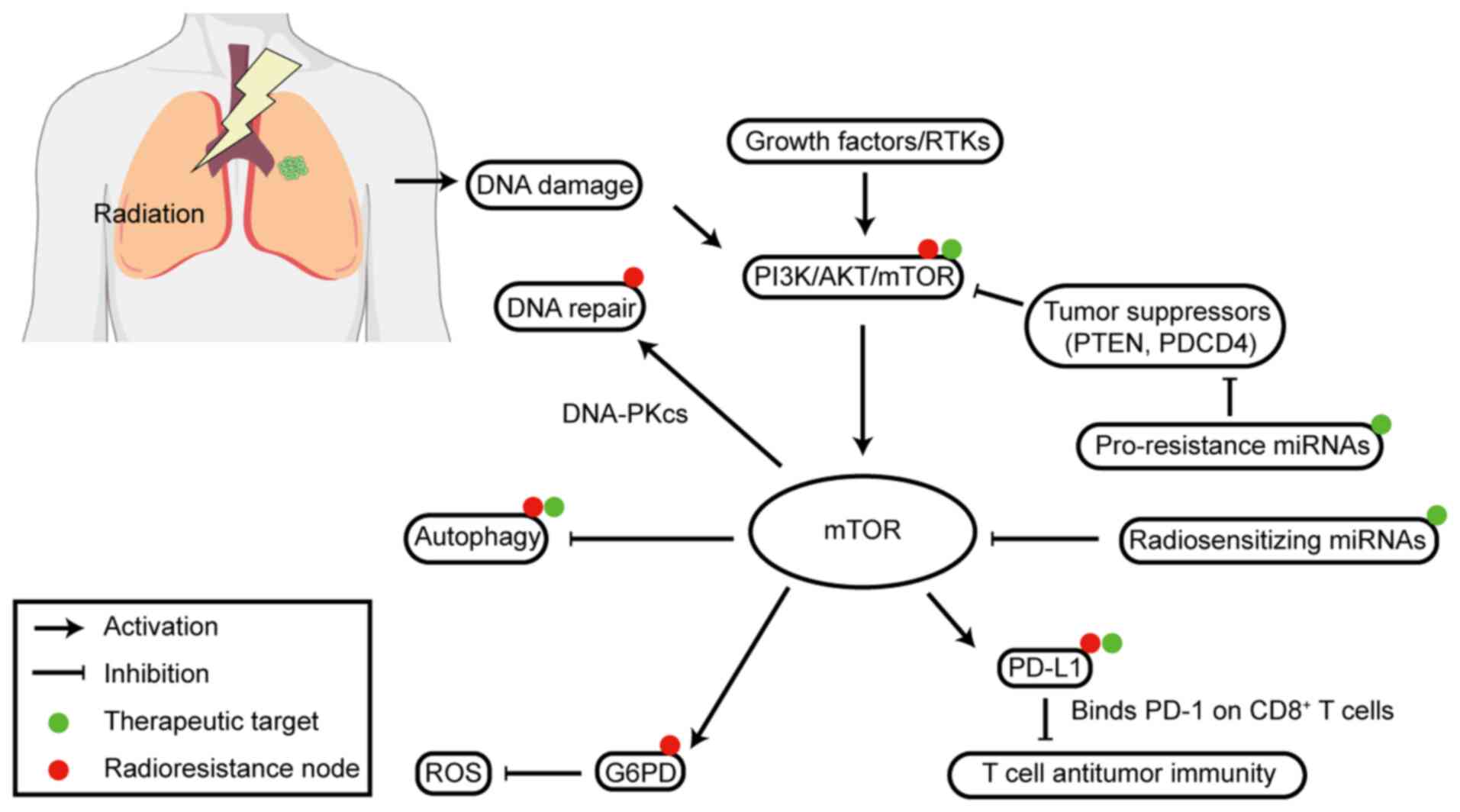
mTOR drives lung cancer radioresistance via the
PI3K/AKT axis. It regulates key processes such as DNA repair, miRNA
activity, autophagy and PD-L1-mediated immune evasion. Clear
radiosensitizing targets have been identified to counter these
resistance mechanisms (Fig. 3).
Notably, single mTOR inhibition shows limited clinical efficacy and
requires combination with other therapies (such as anti-PD-1/PD-L1
and autophagy modulators) (79,110). Future efforts should focus on
validating biomarkers, which may aid the development of
personalized regimens and improve radiotherapy benefits for
patients with lung cancer.
mTOR inhibitors target the mTOR pathway, which is
critical for regulating cell proliferation, metabolism and
survival. These inhibitors hold broad therapeutic potential across
multiple diseases, including autoimmune disorders, endocrine
conditions and cancer (114,115). Aberrant activation of the mTOR
signaling pathway serves a notable role in radioresistance in
NSCLC. By targeting this pathway, mTOR inhibitors demonstrate
marked antitumor potential. Specifically, they enhance tumor cell
radiosensitivity and counteract resistance caused by dysregulated
mTOR signaling. Furthermore, combining mTOR inhibitors with
radiotherapy generates synergistic antitumor effects, amplifying
therapeutic efficacy.
Preclinical studies have consistently demonstrated
the radiosensitizing potential of mTOR inhibitors. For example,
Ushijima et al (116)
assessed the impact of the mTOR inhibitor temsirolimus on
radioresistance under hypoxic conditions, using the A549 human lung
adenocarcinoma cell line. This previous study revealed that while
the D (10) value (dose required
to kill 90% of cells) for A549 cells was 14.2 Gy under hypoxia, the
combination with temsirolimus reduced this to 5.4 Gy (oxygen
enhancement ratio=1.1), demonstrating its ability to suppress
hypoxia-inducible factor-1α expression and overcome hypoxia-induced
radioresistance. These findings position temsirolimus as a
radiosensitizer for hypoxic tumors. Another study (117) reported that the mTOR inhibitor
everolimus exerts radiosensitizing effects by inducing
G2/M phase arrest in A549 cells. However, rapamycin
pretreatment was shown to abolish this arrest 8 h
post-radiotherapy. Early clinical trials also support this
strategy. In a phase I clinical trial (118) assessing temsirolimus combined
with thoracic radiotherapy (35 Gy/14f) in patients with NSCLC,
among the eight evaluable patients, three achieved partial
responses and two exhibited stable disease. These results
underscore the preclinical and clinical synergy between mTOR
inhibitors and radiotherapy, highlighting their potential to
improve treatment outcomes.
mTOR inhibitors are classified into three
generations based on their mechanisms of action. First-generation
inhibitors consist of antibiotic-derived allosteric inhibitors,
including rapamycin and its derivatives such as temsirolimus,
everolimus and ridaforolimus. Second-generation inhibitors are
ATP-competitive inhibitors that selectively target the active
kinase site of mTOR. These molecules, termed selective mTOR kinase
inhibitors, achieve complete blockade of both mTORC1 and mTORC2,
thereby preventing phosphorylation of PKB. Third-generation
inhibitors, known as RapaLink, are hybrid molecules formed by
conjugating the ATP-competitive inhibitor sapanisertib to rapalog
macrocycles via diverse linker chains. Structurally, this design
mimics a dual-inhibitor strategy, and such hybrid agents overcome
resistance arising from monotherapy with rapalogs or
ATP-competitive mTOR inhibitors. Furthermore, their multitarget
activity enhances drug selectivity and therapeutic efficacy
(119,120). By integrating rapamycin with
mTOR kinase inhibitors, third-generation compounds exhibit superior
antitumor potency and reduced off-target toxicity (Table V) (121).
Clinical investigations have explored these
different generations of inhibitors. First-generation rapalogs have
been evaluated extensively in combination strategies for NSCLC.
Early phase trials assessed rapamycin with sunitinib (122) and temsirolimus with pemetrexed
(123). Other studies examined
ridaforolimus in patients with NSCLC harboring KRAS mutations
(124) or evaluated everolimus
in a translational study for resectable NSCLC (125). This combination approach
continues to evolve with more rationale-driven pairings, such as
the investigation of nab-sirolimus with the KRAS G12C inhibitor
adagrasib (126). In parallel,
second-generation dual mTORC1/mTORC2 kinase inhibitors have
advanced into clinical studies. Initial first-in-human and
dose-escalation studies have established the safety and
tolerability of monotherapies such as AZD2014 (127) and CC-223 (128) in patients with advanced solid
tumors. This development subsequently informed strategies to
combine these potent dual inhibitors with other targeted agents,
such as pairing the PI3K/mTOR inhibitor SAR245409 with the MEK
inhibitor pimasertib (129) or
combining the PI3K/mTOR inhibitor gedatolisib (PF-05212384) with
the CDK4/6 inhibitor palbociclib (PD-0332991) (130). In summary, mTOR inhibitors
demonstrate substantial promise in enhancing the efficacy of
radiotherapy for lung cancer. By advancing the understanding of the
molecular mechanisms underlying the mTOR signaling pathway and
refining therapeutic strategies, more effective treatment regimens
can be developed for patients with NSCLC, ultimately improving
clinical outcomes and prognosis.
Despite promising preclinical evidence for mTOR
inhibition, several critical limitations and controversies must be
addressed to ensure successful clinical translation. The key
challenge lies in the notable disparity between the robust body of
preclinical mechanistic evidence and the limited clinical outcomes
achieved to date, which necessitates a realistic perspective on the
field. Most current research comes from in vitro studies and
xenograft models. This raises concerns about whether conclusions
can be reliably generalized to heterogeneous human tumors.
Furthermore, although a number of clinical trials investigating
mTOR inhibitors have been completed (Table V), the results often represent
preliminary phase I studies focused predominantly on determining
safety and maximum tolerated dose, rather than definitive efficacy.
A major therapeutic challenge stems from the complexity of the
PI3K/AKT/mTOR network (131):
First-generation inhibitors (rapalogs) are mostly cytostatic with
modest single-agent efficacy. Their inhibition of mTORC1 fails to
suppress a negative feedback loop, leading to paradoxical AKT
phosphorylation and activation. This AKT activation sustains cell
survival and proliferation, limiting the clinical use of rapalogs
as monotherapy and requiring combination or dual-targeting agents
(45,115,132-134). Compounding this, although the
PI3K/AKT/mTOR pathway is frequently dysregulated in lung cancer,
the utility of biomarkers for patient selection remains
context-dependent; while PIK3CA mutations have been shown to
predict rapalog sensitivity in breast cancer models, PTEN loss of
function is not consistently associated with sensitivity, and
co-occurring oncogenic drivers (such as HER2 mutations) can further
obscure predictive value (135,136). The lack of standardized, broadly
validated biomarkers that account for such context-specificity
still presents a notable barrier to robust patient stratification
and therapeutic guidance in clinical trials.
Additionally, mTOR inhibitors are associated with a
unique spectrum of adverse events, including metabolic
disturbances, mucositis, fatigue, and pulmonary complications such
as pneumonitis and dyspnea (137-140). Critically, the long-standing use
of rapalogs as immunosuppressants in transplant patients raises
concerns that their immunosuppressive properties could counteract
desired anticancer effects, particularly when combining mTOR
inhibition with immunotherapies designed to enhance antitumor
immunity (141,142). Collectively, these limitations,
from pathway redundancy and biomarker gaps to toxicity and
immunological conflicts, highlight the need for further research to
optimize mTOR-targeted strategies and overcome barriers to
effective clinical translation.
The role of mTOR in lung cancer radioresistance has
been extensively studied and well-established. The present review
summarizes the mechanisms driving aberrant activation of the mTOR
signaling pathway in lung cancer and its influence on radiotherapy
resistance. Specifically, mTOR markedly enhances tumor cell
radioresistance by regulating multiple biological processes,
including cellular proliferation, autophagy, DNA repair, miRNA
regulation and EMT. Furthermore, mTOR drives adaptive resistance by
regulating metabolic resilience and promoting immune evasion
through PD-L1 expression.
Although mTOR inhibitors hold considerable promise
in lung cancer radiotherapy, several challenges remain. For
example, drug resistance may arise as tumor cells evade mTOR
inhibitor activity through activation of alternative signaling
pathways or compensatory mechanisms. Consequently, combination
therapies, such as co-administration with PI3K or MEK inhibitors,
may represent effective strategies to overcome resistance.
Additionally, mTOR inhibitors are associated with toxicity and side
effects, including metabolic disturbances and immunosuppression,
necessitating further optimization of dosing regimens and
therapeutic protocols.
Not applicable.
XP, HL, YL, and HW conceptualized the study. XP
wrote the manuscript. LY and HW reviewed the manuscript. XP, HL, YL
and HW contributed to manuscript editing. Data authentication is
not applicable. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82272758 and 82273466), the Hunan
Cancer Hospital Climb Plan (grant nos. ZX2020001 and ZX2020005),
the Hunan Provincial Science and Technology Department (grant no.
2023ZJ1125), the Hunan Provincial Health High-Level Talent
Scientific Research Project (grant no. R2023057) and the National
Key Clinical Specialty Scientific Research Project (grant no.
Z2023025).
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Lehrer EJ, Singh R, Wang M, Chinchilli VM,
Trifiletti DM, Ost P, Siva S, Meng MB, Tchelebi L and Zaorsky NG:
Safety and survival rates associated with ablative stereotactic
radiotherapy for patients with oligometastatic cancer: A systematic
review and meta-analysis. JAMA Oncol. 7:92–106. 2021. View Article : Google Scholar
|
|
3
|
Gonsalves D, Ocanto A, Martín M and
Couñago F: Radiotherapy in early stages of lung cancer. Revisiones
en Cancer. 37:133–147. 2023.
|
|
4
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bache M, Kadler F, Struck O, Medenwald D,
Ostheimer C, Güttler A, Keßler J, Kappler M, Riemann A, Thews O, et
al: Correlation between Circulating miR-16, miR-29a, miR-144 and
miR-150, and the Radiotherapy response and survival of
non-small-cell lung cancer patients. Int J Mol Sci. 24:128352023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panwar V, Singh A, Bhatt M, Tonk RK,
Azizov S, Raza AS, Sengupta S, Kumar D and Garg M: Multifaceted
role of mTOR (mammalian target of rapamycin) signaling pathway in
human health and disease. Signal Transduct Target Ther. 8:3752023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei F, Liu Y, Guo Y, Xiang A, Wang G, Xue
X and Lu Z: MiR-99b-targeted mTOR induction contributes to
irradiation resistance in pancreatic cancer. Mol Cancer. 12:812013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woo Y, Lee HJ, Jung YM and Jung YJ:
MTOR-mediated antioxidant activation in solid tumor
radioresistance. J Oncol. 2019:59568672019. View Article : Google Scholar
|
|
10
|
Yu CC, Hung SK, Lin HY, Chiou WY, Lee MS,
Liao HF, Huang HB, Ho HC and Su YC: Targeting the PI3K/AKT/mTOR
signaling pathway as an effectively radiosensitizing strategy for
treating human oral squamous cell carcinoma in vitro and in vivo.
Oncotarget. 8:68641–68653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wanigasooriya K, Tyler R, Barros-Silva JD,
Sinha Y, Ismail T and Beggs AD: Radiosensitising cancer using
phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT) or
mammalian target of rapamycin (mTOR) inhibitors. Cancers (Basel).
12:12782020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mardanshahi A, Gharibkandi NA, Vaseghi S,
Abedi SM and Molavipordanjani S: The PI3K/AKT/mTOR signaling
pathway inhibitors enhance radiosensitivity in cancer cell lines.
Mol Biol Rep. 48:1–14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng YQ, Gu SX, Chen YS, Gao XD, Ren YX,
Chen JC, Lu YY, Zhang H and Cao S: Virtual screening and
optimization of novel mTOR inhibitors for radiosensitization of
hepatocellular carcinoma. Drug Des Devel Ther. 14:1779–1798. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ihlamur M, Akgül B, Zengin Y, Korkut ŞV,
Kelleci K and Abamor EŞ: The mTOR signaling pathway and mTOR
inhibitors in cancer: Next-generation inhibitors and approaches.
Curr Mol Med. 24:478–494. 2024. View Article : Google Scholar
|
|
15
|
Vézina C, Kudelski A and Sehgal SN:
Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of
the producing streptomycete and isolation of the active principle.
J Antibiot (Tokyo). 28:721–726. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pignataro G, Capone D, Polichetti G,
Vinciguerra A, Gentile A, Di Renzo G and Annunziato L:
Neuroprotective, immunosuppressant and antineoplastic properties of
mTOR inhibitors: Current and emerging therapeutic options. Curr
Opin Pharmacol. 11:378–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drenan RM, Liu X, Bertram PG and Zheng XF:
FKBP12-rapamycin-associated protein or mammalian target of
rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and
the Golgi apparatus. J Biol Chem. 279:772–778. 2004. View Article : Google Scholar
|
|
18
|
Helfenberger KE, Argentino GF, Benzo Y,
Herrera LM, Finocchietto P and Poderoso C: Angiotensin II regulates
mitochondrial mTOR pathway activity dependent on Acyl-CoA
synthetase 4 in adrenocortical cells. Endocrinology.
163:bqac1702022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y and Zhou X: Research progress of
mTOR inhibitors. Eur J Med Chem. 208:1128202020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali M, Bukhari SA, Ali M and Lee HW:
Upstream signalling of mTORC1 and its hyperactivation in type 2
diabetes (T2D). BMB Rep. 50:601–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang G, Chen L, Qin S, Zhang T, Yao J, Yi
Y and Deng L: Mechanistic target of rapamycin complex 1: From a
nutrient sensor to a key regulator of metabolism and health. Adv
Nutr. 13:1882–1900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Workman JJ, Chen H and Laribee RN:
Environmental signaling through the mechanistic target of rapamycin
complex 1: mTORC1 goes nuclear. Cell Cycle. 13:714–725. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon MS: The role of mammalian target of
rapamycin (mTOR) in insulin signaling. Nutrients. 9:11762017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamming DW, Ye L, Katajisto P, Goncalves
MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima
RS, et al: Rapamycin-induced insulin resistance is mediated by
mTORC2 loss and uncoupled from longevity. Science. 335:1638–1643.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarbassov DD, Ali SM, Sengupta S, Sheen
JH, Hsu PP, Bagley AF, Markhard AL and Sabatini DM: Prolonged
rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell.
22:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Tan J, Liu X, Guo W, Li M, Liu X,
Liu Y, Dai W, Hu L, Wang Y, et al: Cytoplasmic endonuclease G
promotes nonalcoholic fatty liver disease via mTORC2-AKT-ACLY and
endoplasmic reticulum stress. Nat Commun. 14:62012023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng H, Kasada A, Ueno M, Hoshii T,
Tadokoro Y, Nomura N, Ito C, Takase Y, Vu HT, Kobayashi M, et al:
Distinct roles of Rheb and Raptor in activating mTOR complex 1 for
the self-renewal of hematopoietic stem cells. Biochem Biophys Res
Commun. 495:1129–1135. 2018. View Article : Google Scholar
|
|
28
|
Miricescu D, Totan A, Stanescu-Spinu II,
Badoiu SC, Stefani C and Greabu M: PI3K/AKT/mTOR signaling pathway
in breast cancer: From molecular landscape to clinical aspects. Int
J Mol Sci. 22:1732020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoki K, Kim J and Guan KL: AMPK and mTOR
in cellular energy homeostasis and drug targets. Annu Rev Pharmacol
Toxicol. 52:381–400. 2012. View Article : Google Scholar
|
|
30
|
Chun Y and Kim J: AMPK-mTOR signaling and
cellular adaptations in hypoxia. Int J Mol Sci. 22:97652021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lama-Sherpa TD, Jeong MH and Jewell JL:
Regulation of mTORC1 by the Rag GTPases. Biochem Soc Trans.
51:655–664. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SJ, DeStefano MA, Oh WJ, Wu CC,
Vega-Cotto NM, Finlan M, Liu D, Su B and Jacinto E: mTOR complex 2
regulates proper turnover of insulin receptor substrate-1 via the
ubiquitin ligase subunit Fbw8. Mol Cell. 48:875–887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sural-Fehr T, Singh H, Cantuti-Catelvetri
L, Zhu H, Marshall MS, Rebiai R, Jastrzebski MJ, Givogri MI,
Rasenick MM and Bongarzone ER: Inhibition of the
IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal
vulnerability in a genetic lysosomal glycosphingolipidosis. Dis
Model Mech. 12:dmm0365902019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lazorchak AS and Su B: Perspectives on the
role of mTORC2 in B lymphocyte development, immunity and
tumorigenesis. Protein Cell. 2:523–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X and Gao T: mTORC2 phosphorylates
protein kinase Cζ to regulate its stability and activity. EMBO Rep.
15:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baffi TR, Lordén G, Wozniak JM, Feichtner
A, Yeung W, Kornev AP, King CC, Del Rio JC, Limaye AJ, Bogomolovas
J, et al: mTORC2 controls the activity of PKC and Akt by
phosphorylating a conserved TOR interaction motif. Sci Signal.
14:eabe45092021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carter CC, Mast FD, Olivier JP, Bourgeois
NM, Kaushansky A and Aitchison JD: Dengue activates mTORC2
signaling to counteract apoptosis and maximize viral replication.
Front Cell Infect Microbiol. 12:9799962022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katholnig K, Schütz B, Fritsch SD,
Schörghofer D, Linke M, Sukhbaatar N, Matschinger JM, Unterleuthner
D, Hirtl M, Lang M, et al: Inactivation of mTORC2 in macrophages is
a signature of colorectal cancer that promotes tumorigenesis. JCI
Insight. 4:e1241642019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mehta D, Rajput K, Jain D, Bajaj A and
Dasgupta U: Unveiling the role of mechanistic target of rapamycin
kinase (MTOR) signaling in cancer progression and the emergence of
MTOR inhibitors as therapeutic strategies. ACS Pharmacol Transl
Sci. 7:3758–3779. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qiu W, Ren M, Wang C, Fu Y and Liu Y: The
clinicopathological and prognostic significance of mTOR and p-mTOR
expression in patients with non-small cell lung cancer: A
meta-analysis. Medicine (Baltimore). 101:e323402022. View Article : Google Scholar
|
|
41
|
Zeng AQ, Chen X, Dai Y and Zhao JN:
Betulinic acid inhibits non-small cell lung cancer by repolarizing
tumor-associated macrophages via mTOR signaling pathway. Zhongguo
Zhong Yao Za Zhi. 49:2376–2384. 2024.In Chinese. PubMed/NCBI
|
|
42
|
Granville CA, Warfel N, Tsurutani J,
Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila
RI and Dennis PA: Identification of a highly effective rapamycin
schedule that markedly reduces the size, multiplicity, and
phenotypic progression of tobacco carcinogen-induced murine lung
tumors. Clin Cancer Res. 13:2281–2289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raskova Kafkova L, Mierzwicka JM,
Chakraborty P, Jakubec P, Fischer O, Skarda J, Maly P and Raska M:
NSCLC: From tumorigenesis, immune checkpoint misuse to current and
future targeted therapy. Front Immunol. 15:13420862024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bang J, Jun M, Lee S, Moon H and Ro SW:
Targeting EGFR/PI3K/AKT/mTOR signaling in hepatocellular carcinoma.
Pharmaceutics. 15:21302023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu YY, Wu HC, Wu JE, Huang KY, Yang SC,
Chen SX, Tsao CJ, Hsu KF, Chen YL and Hong TM: The dual PI3K/mTOR
inhibitor BEZ235 restricts the growth of lung cancer tumors
regardless of EGFR status, as a potent accompanist in combined
therapeutic regimens. J Exp Clin Cancer Res. 38:2822019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu L, Ding R, Song S, Liu J, Li J, Ju X
and Ju B: Single-cell RNA sequencing reveals the mechanism of
PI3K/AKT/mTOR signaling pathway activation in lung adenocarcinoma
by KRAS mutation. J Gene Med. 26:e36582024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ducray SP, Natarajan K, Garland GD, Turner
SD and Egger G: The transcriptional roles of ALK fusion proteins in
tumorigenesis. Cancers (Basel). 11:10742019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao T, Fan J, Abu-Zaid A, Burley SK and
Zheng XFS: Nuclear mTOR signaling orchestrates transcriptional
programs underlying cellular growth and metabolism. Cells.
13:7812024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Q, Zhang Y, Chen Y, Qian J, Zhang X
and Yu K: A Novel mTORC1/2 Inhibitor (MTI-31) inhibits tumor
growth, epithelial-mesenchymal transition, metastases, and improves
antitumor immunity in preclinical models of lung cancer. Clin
Cancer Res. 25:3630–3642. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Marinov M, Ziogas A, Pardo OE, Tan LT,
Dhillon T, Mauri FA, Lane HA, Lemoine NR, Zangemeister-Wittke U,
Seckl MJ and Arcaro A: AKT/mTOR pathway activation and BCL-2 family
proteins modulate the sensitivity of human small cell lung cancer
cells to RAD001. Clin Cancer Res. 15:1277–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Li C, Guo C, Zhao Q, Cao J, Huang
HY, Yue M, Xue Y, Jin Y, Hu L and Ji H: PI3K/Akt/mTOR signaling
orchestrates the phenotypic transition and chemo-resistance of
small cell lung cancer. J Genet Genomics. 48:640–651. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He C: Activating invasion and metastasis
in small cell lung cancer: Role of the tumour immune
microenvironment and mechanisms of vasculogenesis,
epithelial-mesenchymal transition, cell migration, and organ
tropism. Cancer Rep (Hoboken). 7:e700182024.
|
|
53
|
Fiorentino FP, Tokgün E, Solé-Sánchez S,
Giampaolo S, Tokgün O, Jauset T, Kohno T, Perucho M, Soucek L and
Yokota J: Growth suppression by MYC inhibition in small cell lung
cancer cells with TP53 and RB1 inactivation. Oncotarget.
7:31014–31028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsumoto M, Seike M, Noro R, Soeno C,
Sugano T, Takeuchi S, Miyanaga A, Kitamura K, Kubota K and Gemma A:
Control of the MYC-eIF4E axis plus mTOR inhibitor treatment in
small cell lung cancer. BMC Cancer. 15:2412015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chang L, Graham PH, Ni J, Hao J, Bucci J,
Cozzi PJ and Li Y: Targeting PI3K/Akt/mTOR signaling pathway in the
treatment of prostate cancer radioresistance. Crit Rev Oncol
Hematol. 96:507–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu N and Wang P: Development of
PI3K/AKT/mTOR signaling pathway and hypofractionated radiotherapy
in non-small cell lung cancer. Chin J Clin Oncol. 40:1196–1198.
2013.In Chinese.
|
|
57
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang J, Chen L, Wu J, Ai D, Zhang JQ,
Chen TG and Wang L: Targeting the PI3K/AKT/mTOR signaling pathway
in the treatment of human diseases: Current status, trends, and
solutions. J Med Chem. 65:16033–16061. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schuurbiers OC, Kaanders JH, van der
Heijden HF, Dekhuijzen RP, Oyen WJ and Bussink J: The
PI3-K/AKT-pathway and radiation resistance mechanisms in non-small
cell lung cancer. J Thorac Oncol. 4:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Toulany M, Iida M, Keinath S, Iyi FF,
Mueck K, Fehrenbacher B, Mansour WY, Schaller M, Wheeler DL and
Rodemann HP: Dual targeting of PI3K and MEK enhances the radiation
response of K-RAS mutated non-small cell lung cancer. Oncotarget.
7:43746–43761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang T, Cui GB, Zhang J, Zhang F, Zhou
YA, Jiang T and Li XF: Inhibition of PI3 kinases enhances the
sensitivity of non-small cell lung cancer cells to ionizing
radiation. Oncol Rep. 24:1683–1689. 2010.PubMed/NCBI
|
|
63
|
Chen K, Shang Z, Dai AL and Dai PL: Novel
PI3K/Akt/mTOR pathway inhibitors plus radiotherapy: Strategy for
non-small cell lung cancer with mutant RAS gene. Life Sci.
255:1178162020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim SY, Jeong EH, Lee TG, Kim HR and Kim
CH: The combination of trametinib, a MEK inhibitor, and
temsirolimus, an mTOR Inhibitor, radiosensitizes lung cancer cells.
Anticancer Res. 41:2885–2894. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He GH, Xing DJ, Jin D, Lu Y, Guo L, Li YL
and Li D: Scutellarin improves the radiosensitivity of non-small
cell lung cancer cells to iodine-125 seeds via downregulating the
AKT/mTOR pathway. Thorac Cancer. 12:2352–2359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sebastian NT, Webb A, Shilo K, Robb R,
Xu-Welliver M, Haglund K, Brownstein J, DeNicola GM, Shen C and
Williams TM: A PI3K gene expression signature predicts for
recurrence in early-stage non-small cell lung cancer treated with
stereotactic body radiation therapy. Cancer. 129:3971–3977. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Choi EJ, Ryu YK, Kim SY, Wu HG, Kim JS,
Kim IH and Kim IA: Targeting epidermal growth factor
receptor-associated signaling pathways in non-small cell lung
cancer cells: Implication in radiation response. Mol Cancer Res.
8:1027–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Holler M, Grottke A, Mueck K, Manes J,
Jücker M, Rodemann HP and Toulany M: Dual Targeting of Akt and
mTORC1 impairs repair of DNA double-strand breaks and increases
radiation sensitivity of human tumor cells. PLoS One.
11:e01547452016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang P, He D, Song E, Jiang M and Song Y:
Celecoxib enhances the sensitivity of non-small-cell lung cancer
cells to radiation-induced apoptosis through downregulation of the
Akt/mTOR signaling pathway and COX-2 expression. PLoS One.
14:e02237602019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xiong L, Tan B, Lei X, Zhang B, Li W, Liu
D and Xia T: SIRT6 through PI3K/Akt/mTOR signaling pathway to
enhance radiosensitivity of non-Small cell lung cancer and inhibit
tumor progression. IUBMB Life. 73:1092–1102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hamid MB, Serafin AM and Akudugu JM:
Selective therapeutic benefit of X-rays and inhibitors of EGFR,
PI3K/mTOR, and Bcl-2 in breast, lung, and cervical cancer cells.
Eur J Pharmacol. 912:1746122021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Biswas U, Roy R, Ghosh S and Chakrabarti
G: The interplay between autophagy and apoptosis: Its implication
in lung cancer and therapeutics. Cancer Lett. 585:2166622024.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gargalionis AN, Papavassiliou KA and
Papavassiliou AG: Implication of mTOR Signaling in NSCLC:
Mechanisms and therapeutic perspectives. Cells. 12:20142023.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Loizzo D, Pandolfo SD, Rogers D, Cerrato
C, di Meo NA, Autorino R, Mirone V, Ferro M, Porta C, Stella A, et
al: Novel insights into autophagy and prostate cancer: A
comprehensive review. Int J Mol Sci. 23:38262022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang J, Gong M, Fan X, Huang D, Zhang J
and Huang C: Autophagy-related signaling pathways in non-small cell
lung cancer. Mol Cell Biochem. 477:385–393. 2022. View Article : Google Scholar
|
|
77
|
Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha
YI and Lu B: Autophagy upregulation by inhibitors of caspase-3 and
mTOR enhances radiotherapy in a mouse model of lung cancer.
Autophagy. 4:659–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fei HR, Tian H, Zhou XL, Yang MF, Sun BL,
Yang XY, Jiao P and Wang FZ: Inhibition of autophagy enhances
effects of PF-04691502 on apoptosis and DNA damage of lung cancer
cells. Int J Biochem Cell Biol. 78:52–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yan J, Xie Y, Wang F, Chen Y, Zhang J, Dou
Z, Gan L, Li H, Si J, Sun C, et al: Carbon ion combined with
tigecycline inhibits lung cancer cell proliferation by inducing
mitochondrial dysfunction. Life Sci. 263:1185862020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim KW, Moretti L, Mitchell LR, Jung DK
and Lu B: Combined Bcl-2/mammalian target of rapamycin inhibition
leads to enhanced radiosensitization via induction of apoptosis and
autophagy in non-small cell lung tumor xenograft model. Clin Cancer
Res. 15:6096–6105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim EJ, Jeong JH, Bae S, Kang S, Kim CH
and Lim YB: mTOR inhibitors radiosensitize PTEN-deficient
non-small-cell lung cancer cells harboring an EGFR activating
mutation by inducing autophagy. J Cell Biochem. 114:1248–1256.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kim KW, Myers CJ, Jung DK and Lu B:
NVP-BEZ-235 enhances radiosensitization via blockade of the
PI3K/mTOR pathway in cisplatin-resistant non-small cell lung
carcinoma. Genes Cancer. 5:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liang N, Zhong R, Hou X, Zhao G, Ma S,
Cheng G and Liu X: Ataxia-telangiectasia mutated (ATM) participates
in the regulation of ionizing radiation-induced cell death via
MAPK14 in lung cancer H1299 cells. Cell Prolif. 48:561–572. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang X, Ji J, Yang Y, Zhang J and Shen L:
Stathmin1 increases radioresistance by enhancing autophagy in
non-small-cell lung cancer cells. Onco Targets Ther. 9:2565–2574.
2016.PubMed/NCBI
|
|
85
|
Lai C, Zhang J, Tan Z, Shen LF, Zhou RR
and Zhang YY: Maf1 suppression of ATF5-dependent mitochondrial
unfolded protein response contributes to rapamycin-induced
radio-sensitivity in lung cancer cell line A549. Aging (Albany NY).
13:7300–7313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: Mirna-based biomarkers,
therapies, and resistance in cancer. Int J Biol Sci. 6:2628–2647.
2020. View Article : Google Scholar
|
|
87
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Avvari S, Prasad DKV and Khan IA: Role of
MicroRNAs in cell growth proliferation and tumorigenesis. Role of
MicroRNAs in Cancers. Prasad D and Santosh Sushma P: Springer;
Singapore: pp. 37–51. 2022, View Article : Google Scholar
|
|
90
|
Chen Y, Li WW, Peng P, Zhao WH, Tian YJ,
Huang Y, Xia S and Chen Y: mTORC1 inhibitor RAD001 (everolimus)
enhances non-small cell lung cancer cell radiosensitivity in vitro
via suppressing epithelial-mesenchymal transition. Acta Pharmacol
Sin. 40:1085–1094. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yuan Y, Liao H, Pu Q, Ke X, Hu X, Ma Y,
Luo X, Jiang Q, Gong Y, Wu M, et al: miR-410 induces both
epithelial-mesenchymal transition and radioresistance through
activation of the PI3K/mTOR pathway in non-small cell lung cancer.
Signal Transduct Target Ther. 5:852020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li T, Wei L, Zhang X, Fu B, Zhou Y, Yang
M, Cao M, Chen Y, Tan Y, Shi Y, et al: Serotonin Receptor HTR2B
facilitates colorectal cancer metastasis via CREB1-ZEB1
axis-mediated epithelial-mesenchymal transition. Mol Cancer Res.
22:538–554. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen Y, Liao W, Yuan A, Xu H, Yuan R and
Cao J: MiR-181a reduces radiosensitivity of non-small-cell lung
cancer via inhibiting PTEN. Panminerva Med. 64:374–383. 2022.
View Article : Google Scholar
|
|
94
|
Jiang LP, He CY and Zhu ZT: Role of
microRNA-21 in radiosensitivity in non-small cell lung cancer cells
by targeting PDCD4 gene. Oncotarget. 8:23675–23689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang M, Li T, Wang Q, Li C, Zhou H, Deng
S, Lv Z, He Y, Hou B and Zhu G: Silencing circPVT1 enhances
radiosensitivity in non-small cell lung cancer by sponging
microRNA-1208. Cancer Biomark. 31:263–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yin H, Ma J, Chen L, Piao S, Zhang Y,
Zhang S, Ma H, Li Y, Qu Y, Wang X and Xu Q: MiR-99a enhances the
radiation sensitivity of non-small cell lung cancer by targeting
mTOR. Cell Physiol Biochem. 46:471–481. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Meng X, Sun Y, Liu S and Mu Y: miR-101-3p
sensitizes lung adenocarcinoma cells to irradiation via targeting
BIRC5. Oncol Lett. 21:2822021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li Z, Qu Z, Wang Y, Qin M and Zhang H:
miR-101-3p sensitizes non-small cell lung cancer cells to
irradiation. Open Med (Wars). 15:413–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He
Y, Chen G, Zhou Q, Wang W, Zhou X, et al: Radiation-induced
miR-208a increases the proliferation and radioresistance by
targeting p21 in human lung cancer cells. J Exp Clin Cancer Res.
35:72016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Deng H, Chen Y, Li P, Hang Q, Zhang P, Jin
Y and Chen M: PI3K/AKT/mTOR pathway, hypoxia, and glucose
metabolism: Potential targets to overcome radioresistance in small
cell lung cancer. Cancer Pathog Ther. 1:56–66. 2023. View Article : Google Scholar
|
|
101
|
Liu B, Huang ZB, Chen X, See YX, Chen ZK
and Yao HK: Mammalian target of rapamycin 2 (MTOR2) and C-MYC
modulate glucosamine-6-phosphate synthesis in glioblastoma (GBM)
cells through glutamine: fructose-6-phosphate aminotransferase 1
(GFAT1). Cell Mol Neurobiol. 39:415–434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Deng H, Chen Y, Wang L, Zhang Y, Hang Q,
Li P, Zhang P, Ji J, Song H, Chen M and Jin Y: PI3K/mTOR inhibitors
promote G6PD autophagic degradation and exacerbate oxidative stress
damage to radiosensitize small cell lung cancer. Cell Death Dis.
14:6522023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cardnell RJ, Feng Y, Mukherjee S, Diao L,
Tong P, Stewart CA, Masrorpour F, Fan Y, Nilsson M, Shen Y, et al:
Activation of the PI3K/mTOR pathway following PARP Inhibition in
small cell lung cancer. PLoS One. 11:e01525842016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Knelson EH, Patel SA and Sands JM: PARP
inhibitors in small-cell lung cancer: Rational combinations to
improve responses. Cancers (Basel). 13:7272021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim WY, Oh SH, Woo JK, Hong WK and Lee HY:
Targeting heat shock protein 90 overrides the resistance of lung
cancer cells by blocking radiation-induced stabilization of
hypoxia-inducible factor-1alpha. Cancer Res. 69:1624–1632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Subtil FS, Wilhelm J, Bill V, Westholt N,
Rudolph S, Fischer J, Scheel S, Seay U, Fournier C, Taucher-Scholz
G, et al: Carbon ion radiotherapy of human lung cancer attenuates
HIF-1 signaling and acts with considerably enhanced therapeutic
efficiency. FASEB J. 28:1412–1421. 2014. View Article : Google Scholar
|
|
107
|
Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB,
Ko YG, Lee JS, Lee SJ, Lee JC and Park MJ: Upregulation of CXCR4 is
functionally crucial for maintenance of stemness in drug-resistant
non-small cell lung cancer cells. Oncogene. 32:209–221. 2013.
View Article : Google Scholar
|
|
108
|
Dodson M, Dai W, Anandhan A, Schmidlin CJ,
Liu P, Wilson NC, Wei Y, Kitamura N, Galligan JJ, Ooi A, et al:
CHML is an NRF2 target gene that regulates mTOR function. Mol
Oncol. 16:1714–1727. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zheng H, Wang M, Wu J, Wang ZM, Nan HJ and
Sun H: Inhibition of mTOR enhances radiosensitivity of lung cancer
cells and protects normal lung cells against radiation. Biochem
Cell Biol. 94:213–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lastwika KJ, Wilson W III, Li QK, Norris
J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et
al: Control of PD-L1 expression by oncogenic activation of the
AKT-mTOR pathway in non-small cell lung cancer. Cancer Res.
76:227–238. 2016. View Article : Google Scholar
|
|
111
|
Xiao P, Sun LL, Wang J, Han RL, Ma Q and
Zhong DS: LKB1 gene inactivation does not sensitize non-small cell
lung cancer cells to mTOR inhibitors in vitro. Acta Pharmacol Sin.
36:1107–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li H, Li X, Liu S, Guo L, Zhang B, Zhang J
and Ye Q: Programmed cell death-1 (PD-1) checkpoint blockade in
combination with a mammalian target of rapamycin inhibitor
restrains hepatocellular carcinoma growth induced by hepatoma
cell-intrinsic PD-1. Hepatology. 66:1920–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dong L, Lv H, Li W, Song Z, Li L, Zhou S,
Qiu L, Qian Z, Liu X, Feng L, et al: Co-expression of PD-L1 and
p-AKT is associated with poor prognosis in diffuse large B-cell
lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR
pathway in tumor cells. Oncotarget. 7:33350–33362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chiarini F, Evangelisti C, McCubrey JA and
Martelli AM: Current treatment strategies for inhibiting mTOR in
cancer. Trends Pharmacol Sci. 36:124–135. 2015. View Article : Google Scholar
|
|
115
|
Mohindra NA and Platanias LC: Catalytic
mammalian target of rapamycin inhibitors as antineoplastic agents.
Leuk Lymphoma. 56:2518–2523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ushijima H, Suzuki Y, Oike T, Komachi M,
Yoshimoto Y, Ando K, Okonogi N, Sato H, Noda SE, Saito J and Nakano
T: Radio-sensitization effect of an mTOR inhibitor, temsirolimus,
on lung adenocarcinoma A549 cells under normoxic and hypoxic
conditions. J Radiat Res. 56:663–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen H, Ma Z, Vanderwaal RP, Feng Z,
Gonzalez-Suarez I, Wang S and Zhang J, Roti Roti JL, Gonzalo S and
Zhang J: The mTOR inhibitor rapamycin suppresses DNA double-strand
break repair. Radiat Res. 175:214–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Waqar SN, Robinson C, Bradley J, Goodgame
B, Rooney M, Williams K, Gao F and Govindan R: A phase I study of
temsirolimus and thoracic radiation in non-small-cell lung cancer.
Clin Lung Cancer. 15:119–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Waldner M, Fantus D, Solari M and Thomson
AW: New perspectives on mTOR inhibitors (rapamycin, rapalogs and
TORKinibs) in transplantation. Br J Clin Pharmacol. 82:1158–1170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dancey J: MTOR signaling and drug
development in cancer. Nat Rev Clin Oncol. 7:209–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Occhiuzzi MA, Lico G, Ioele G, De Luca M,
Garofalo A and Grande F: Recent advances in PI3K/PKB/mTOR
inhibitors as new anticancer agents. Eur J Med Chem.
246:1149712023. View Article : Google Scholar
|
|
122
|
Waqar SN, Gopalan PK, Williams K,
Devarakonda S and Govindan R: A phase I trial of sunitinib and
rapamycin in patients with advanced non-small cell lung cancer.
Chemotherapy. 59:8–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Waqar SN, Baggstrom MQ, Morgensztern D,
Williams K, Rigden C and Govindan R: A Phase I Trial of
temsirolimus and pemetrexed in patients with advanced non-small
cell lung cancer. Chemotherapy. 61:144–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Riely GJ, Brahmer J, Planchard D, Crinò L,
Doebele RC, Lopez LAM, Gettinger SN, Schumann C, Li X, Atkins BM,
et al: A randomized discontinuation phase II trial of ridaforolimus
in non-small cell lung cancer (NSCLC) patients with KRAS mutations.
J Clin Oncol. 30(Suppl 15): 75322011.
|
|
125
|
National Library of Medicine: Adagrasib in
Combination With Nab-Sirolimus in Patients With Advanced Solid
Tumors. Non-Small Cell Lung Cancer With a KRAS G12C Mutation
(KRYSTAL-19). ClinicalTrials.gov ID NCT05840510. https://clinicaltrials.gov/study/NCT05840510.
|
|
126
|
Owonikoko TK, Ramalingam SS, Miller DL,
Force SD, Sica GL, Mendel J, Chen Z, Rogatko A, Tighiouart M,
Harvey RD, et al: A translational, pharmacodynamic, and
pharmacokinetic phase IB clinical study of everolimus in resectable
non-small cell lung cancer. Clin Cancer Res. 21:1859–1868. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bendell JC, Kelley RK, Shih KC, Grabowsky
JA, Bergsland E, Jones S, Martin T, Infante JR, Mischel PS,
Matsutani T, et al: A phase I dose-escalation study to assess
safety, tolerability, pharmacokinetics, and preliminary efficacy of
the dual mTORC1/mTORC2 kinase inhibitor CC-223 in patients with
advanced solid tumors or multiple myeloma. Cancer. 121:3481–3490.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Basu B, Dean E, Puglisi M, Greystoke A,
Ong M, Burke W, Cavallin M, Bigley G, Womack C, Harrington EA, et
al: First-in-human pharmacokinetic and pharmacodynamic study of the
dual m-TORC 1/2 inhibitor AZD2014. Clin Cancer Res. 21:3412–3419.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Heist RS, Infante JR, Campana F, Egile C,
Jego V, Damstrup L, Mita M, Grande E and Rizv N: 443O-Pimasertib
(Pim) and Sar245409 (Sar)-a Mek and Pi3K/Mtor inhibitor
combination: A Phase Ib trial with expansions in selected
genotype-defined solid tumors. Ann Oncol. 25(Suppl 4): iv1462014.
View Article : Google Scholar
|
|
130
|
National Library of Medicine: Study of the
CDK4/6 Inhibitor Palbociclib (PD-0332991) in Combination With the
PI3K/mTOR Inhibitor Gedatolisib (PF-05212384) for Patients With
Advanced Squamous Cell Lung Pancreatic, Head & Neck Other Solid
Tumors. ClinicalTrials.gov ID NCT03065062. https://clinicaltrials.gov/study/NCT03065062.
|
|
131
|
McCay J and Gribben JG: PI3 kinase, AKT,
and mTOR inhibitors. Precision Cancer Therapies. O'Connor OA,
Ansell SM and Seymour JF: 1. John Wiley & Sons, Inc.; pp.
113–129. 2023
|
|
132
|
Saran U, Foti M and Dufour JF: Cellular
and molecular effects of the mTOR inhibitor everolimus. Clin Sci
(Lond). 129:895–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rodrik-Outmezguine VS, Okaniwa M, Yao Z,
Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de
Stanchina E, et al: Overcoming mTOR resistance mutations with a
new-generation mTOR inhibitor. Nature. 534:272–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Porcelli L, Quatrale AE, Mantuano P,
Silvestris N, Rolland JF, Biancolillo L, Paradiso A and Azzariti A:
Synergistic antiproliferative and antiangiogenic effects of EGFR
and mTOR inhibitors. Curr Pharm Des. 19:918–926. 2013. View Article : Google Scholar
|
|
135
|
Weigelt B, Warne PH and Downward J: PIK3CA
mutation, but not PTEN loss of function, determines the sensitivity
of breast cancer cells to mTOR inhibitory drugs. Oncogene.
30:3222–3233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sanaei MJ, Razi S, Pourbagheri-Sigaroodi A
and Bashash D: The PI3K/Akt/mTOR pathway in lung cancer; oncogenic
alterations, therapeutic opportunities, challenges, and a glance at
the application of nanoparticles. Transl Oncol. 18:1013642022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Papadimitrakopoulou VA, Soria JC, Jappe A,
Jehl V, Klimovsky J and Johnson BE: Everolimus and erlotinib as
second- or third-line therapy in patients with advanced
non-small-cell lung cancer. J Thorac Oncol. 7:1594–1601. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ponticelli C: The pros and the cons of
mTOR inhibitors in kidney transplantation. Expert Rev Clin Immunol.
10:295–305. 2015. View Article : Google Scholar
|
|
139
|
Boers-Doets CB, Raber-Durlacher JE,
Treister NS, Epstein JB, Arends AB, Wiersma DR, Lalla RV, Logan RM,
van Erp NP and Gelderblom H: Mammalian target of rapamycin
inhibitor-associated stomatitis. Future Oncol. 9:1883–1892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Gartrell BA, Ying J, Sivendran S, Boucher
KM, Choueiri TK, Sonpavde G, Oh WK, Agarwal N and Galsky MD:
Pulmonary complications with the use of mTOR inhibitors in targeted
cancer therapy: A systematic review and meta-analysis. Target
Oncol. 9:195–204. 2014. View Article : Google Scholar
|
|
141
|
Gaumann A, Schlitt HJ and Geissler EK:
Immunosuppression and tumor development in organ transplant
recipients: The emerging dualistic role of rapamycin. Transpl Int.
21:207–217. 2008. View Article : Google Scholar
|
|
142
|
El Hage A and Dormond O: Combining mtor
inhibitors and T cell-based immunotherapies in cancer treatment.
Cancers (Basel). 13:13592021. View Article : Google Scholar : PubMed/NCBI
|