Oncolytic viruses (OVs) represent an innovative
approach in cancer therapy, characterized by their ability to
selectively lyse tumor cells while sparing adjacent normal tissues.
They also potentiate the anti-tumor immune response through the
release of tumor-associated antigens and the activation of
inflammatory pathways within the tumor microenvironment (TME)
(1). Preclinical toxicology
studies have demonstrated that OVs exhibit low toxicity and high
tolerability (2). To date, five
OVs have successfully undergone clinical translation (3), including the oncolytic adenovirus
H101, talimogene laherparepvec (T-VEC), Rigvir, G47Δ and
nadofaragene firadenovec-vncg. They show significant potential in
clinical applications (4-8). However, in solid tumors, factors
such as abnormal lymphatic structures, high vascular permeability,
a dense extracellular matrix and the requirement for OVs to
traverse the endothelial layer to reach target cells collectively
reduce the penetration range of OVs. Additionally, interactions
between OVs and antigen-presenting cells can trigger innate immune
responses and antiviral immunity, increasing the likelihood of
clearance by the host's immune system (9). Another key obstacle is the
heterogeneity of tumors, which leads to incomplete responses to
specific monotherapies (10).
These limitations hinder the widespread clinical translation of OV
therapy.
Targeted therapy functions by selectively
interacting with specific sites to inhibit enzymes and growth
factor receptors essential for proliferation, inducing apoptosis in
cancer cells and modulating gene expression, thereby altering
protein functions in normal cells to disrupt pathways associated
with carcinogenesis and tumor progression (11). However, due to genetic
alterations, adaptive responses or bypass mechanisms that allow
cancer cells to endure the selective pressure exerted by the
treatment, resistance may be acquired, which limits the long-term
efficacy and developmental potential of targeted therapies
(12).
Previously research indicates that the integration
of OVs with targeted therapeutics can inhibit tumor initiation and
progression through various mechanisms, effectively overcoming the
limitations associated with OVs and targeted drug monotherapies,
thereby enhancing the overall efficacy of cancer treatment. As a
result, combination therapy is gradually becoming one of the
leading approaches in oncology. Earlier reviews mainly explained
the basic principles and potential strategies of combining OVs with
key cellular signaling pathway modulators in cancer, without
categorizing the specific mechanisms (13). Certain reviews have summarized the
progress of OVs in combination with various therapies, such as
chemotherapy, radiotherapy and mainly novel immunotherapies like
immune checkpoint inhibitors (10,14,15). This article primarily elaborates
on the mechanisms and progress of OVs combined with targeted drugs,
categorizing them according to different mechanisms, while further
exploring how to select more rational combination approaches based
on the characteristics of different tumors, such as OVs targeting
tumor surface nonspecific and specific receptors and monitoring of
mutated targets. By discussing the differences between human and
mouse immune systems, potential combination toxicities and their
solutions, as well as future strategies to address drug resistance,
the article highlights key points for translating preclinical
research into clinical applications. Based on the above
discussions, it connects preclinical evidence with trial design
elements such as patient selection criteria, recommended endpoints
and overlapping toxicity safety monitoring, providing a valuable
reference for the future clinical translation of OVs combined with
targeted drugs.
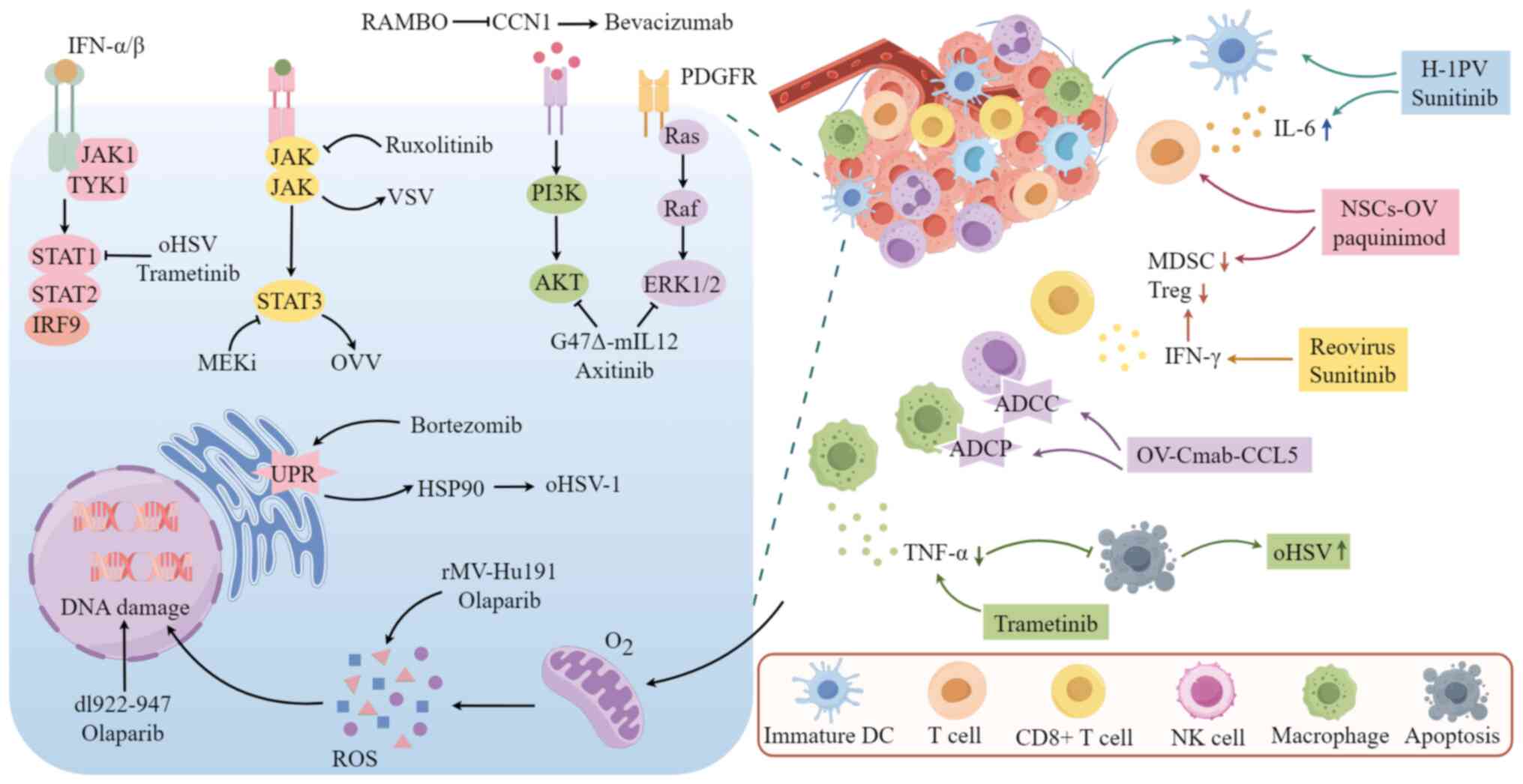
The mechanism of action of OVs combined with
molecularly targeted drugs is mainly related to signaling pathways,
immune responses, DNA damage, apoptosis and autophagic cell death.
In this chapter, a detailed explanation of the above will be
provided.
Inhibiting key targets in signaling pathways related
to cell proliferation can enhance the replication of OVs and their
oncolytic effects, thereby exerting antitumor activity. The
mitogen-activated protein kinase (MAPK), Janus kinase (JAK)/signal
transducer and activator of transcription (STAT) and
phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways are
jointly involved in regulating cell proliferation, differentiation,
survival and apoptosis, with complex crosstalk between them. MAPK
kinase (MEK) is one of the key targets in the MAPK signaling
pathway (16). Lee et al
(17) demonstrated that
inhibiting MEK phosphorylation can activate STAT3, thereby
promoting oncolytic vaccinia virus (OVV) replication in
doxorubicin-resistant ovarian cancer and enhancing the anti-tumor
effects. STAT3 activation also promotes the replication of
oncolytic herpes simplex virus (oHSV) in glioma cells (18). MEK inhibitors also suppress the
antiviral response facilitated by IFN signaling through
STAT1/STAT2-dependent pathways. IFNs orchestrate innate immune
responses, serving as a formidable first line of defense against
pathogen invasion (19). This
initiates a signaling cascade through the JAK-STAT pathway
(20). STAT1 and STAT2 play
pivotal roles in type I and type II IFN signaling, significantly
contributing to the cellular antiviral response and adaptive
immunity. The primary pathways through which IFNs exert their
antiviral effects include the oligoadenylate
synthetase-ribonuclease L system and the RNA-dependent protein
kinase (PKR) pathway, which degrade viral RNA and inhibit viral
protein synthesis, respectively. Zhou et al (21) found that trametinib boosts viral
replication in BRAF V600E and BRAF wild-type (wt)/KRAS mutated
tumor cells by reducing STAT1 and PKR phosphorylation.
Additionally, the JAK-1/2 inhibitor ruxolitinib increases vesicular
stomatitis virus (VSV) replication by inhibiting the JAK/STAT
signaling pathway, enhancing its sensitivity in melanoma and lung
cancer (22,23). Targeting the Cysteine-rich 61
(CCN1)-AKT pathway can enhance bevacizumab's effectiveness by
reducing glioma infiltration. Elevated CCN1 expression is
associated with increased AKT phosphorylation in tumor cells
(24). Studies have shown that
CCN1 stimulates the expression of PI3K/AKT in various types of
cancer cells, including glioma, breast cancer, gastric cancer and
renal cancer cells (25-27). Rapid antiangiogenesis mediated by
oncolytic virus (RAMBO) is an engineered oHSV designed to express
angiogenesis inhibitors and is capable of suppressing the
expression of CCN1 (28). A study
found that the angiostatin produced by RAMBO can inhibit
bevacizumab-induced CCN1 expression, reducing bevacizumab-induced
glioma infiltration by blocking the CCN1-AKT signaling pathway,
thereby enhancing the efficacy of bevacizumab against malignant
gliomas (28).
Tyrosine kinase inhibitor (TKI) synergistically
augments the antitumor efficacy of OVs by inhibiting the
phosphorylation of AKT, which are activated by OVs. G47Δ-mIL12, an
advanced version based on the third-generation oHSV-1 G47Δ,
enhancing tumor-specific replication and safety by deleting the α47
gene and US11 promoter to prevents class I major histocompatibility
complex (MHC) downregulation and inhibiting angiogenesis (4,29,30). Research conducted by indicates
that the selective TKI axitinib enhances the antitumor effect of
G47Δ-mIL12 in glioblastoma (GBM) by blocking the platelet-derived
growth factor receptor (PDGFR) pathway, inhibiting phosphorylated
ERK1/2 and reducing G47Δ-mIL12-induced AKT phosphorylation.
Furthermore, this combination also promotes apoptosis and affects
stem-like cell characteristics (31). TKI can also promote OV replication
by inhibiting the antiviral response mediated by IFN signaling.
Research by Jha et al (32) demonstrated that multi-target TKI
sunitinib weakens the antiviral response by inhibiting VSV-induced
activation of the IFN pathway, thereby enhancing VSV
replication.
IFN is also a key pathway in antiviral immunity, and
the transcription of IFN-regulated genes induced by the JAK/STAT
pathway inhibits OVs replication through multiple mechanisms
(19). By inhibiting the type I
IFN pathway to weaken the antiviral response of specific tumor
cells, thereby enhancing the spread and oncolytic activity of OVs
in tumor cells. Trastuzumab-DM1 (T-DM1), an antibody-drug
conjugate, consists of the targeted therapeutic agent trastuzumab
linked to the microtubule-disrupting agent and mertansine
derivative DM1. This conjugate primarily exerts its
microtubule-inhibiting effect in human EGFR2 (HER2)-overexpressing
tumor cells through the specific targeting of trastuzumab (33). Arulanandam et al (34) found that T-DM1 targets
HER2-overexpressing tumor cells to release DM1, which inhibits the
type I IFN pathway and enhances the cytotoxic effect of TNF-α on
neighboring cells, thereby increasing the spread and oncolytic
effect of the VSVΔ51 virus in VSV-resistant cancer cells. This
study did not emphasize the antitumor effect of trastuzumab itself
(34). In addition, IκB kinase
inhibitors BMS-345541 and TPCA-1 enhance VSV replication by
inhibiting type I IFN-mediated antiviral responses, thereby
improving the efficacy against glioma (35).
Targeted drugs combined with OVs enhance cytotoxic
effects on tumor cells in the TME by activating immune cells,
promoting cytokine release and reducing immunosuppression.
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells
that expand in inflammation and tumors and are effective inhibitors
of T cell-mediated immune responses (36). Lawson et al (37) found that sunitinib can alleviate
immunosuppression induced by coxsackievirus, such as the
accumulation of MDSCs, and stimulate the number of
immune-stimulating cells, enhancing the anti-tumor immune response
in mouse renal cell carcinoma and lung squamous cell carcinoma.
Similarly, Moehler et al (38) found that sunitinib with H-1
parvovirus significantly boosts immune stimulation in melanoma.
S100A8/9 are low-molecular-weight calcium-binding proteins, which
can regulate the accumulation of MDSCs (39). Chai et al (40) confirmed that neural stem
cell-delivered OV combined with S100A8/9 inhibitor paquinimod
enhanced anti-GBM efficacy by increasing T-cell infiltration,
shifting macrophages to a pro-inflammatory phenotype and reducing
MDSCs. A recent study reported that KRAS inhibitors themselves
promote innate and adaptive immunity, and when combined with OVV,
they reduce immunosuppressive cells such as MDSCs and T-regulatory
cells (Tregs) in the TME, synergistically enhancing antitumor
effects (41).
TANK-binding kinase 1 (TBK1) inhibitors promote OVs
replication and their sensitivity to resistant cells by enhancing
immune cell responses. Intercellular adhesion molecule 1 (ICAM-1)
is a cell surface receptor used by immune cells for adhesion and
migration, and it is often upregulated during viral infections
(42). TBK1 is a serine/threonine
kinase, and its increased expression or abnormal activity can
promote the survival and proliferation of cancer cells (43). Guo et al (44) reported that TBK1 inhibitors can
promote VSVΔ51 replication by enhancing ICAM-1-mediated natural
killer (NK) cell immunity and increase the sensitivity of VSVΔ51 to
chemoresistant colorectal cancer cells.
MEK inhibitors enhance viral replication by
decreasing TNF-α secretion and blocking its apoptotic effects. Due
to early cell death from TNF-α-induced apoptosis, the replication
and antitumor impact of oHSV-1 are limited (45). Yoo et al (46) discovered that the MEK inhibitor
trametinib inhibits apoptosis in GBM cells following oHSV infection
by reducing TNF-α levels, thereby promoting viral replication.
Furthermore, oHSV aids trametinib in crossing the blood-brain
barrier and prevents MAPK pathway reactivation, enhancing tumor
sensitivity to MEK inhibition.
Proteasome inhibition promotes OVs replication by
increasing the expression of heat shock proteins (HSPs). HSP90 has
previously been shown to be important for the nuclear localization
of HSV polymerase (47). In line
with this, Yoo et al (48)
found that in head and neck squamous cell carcinoma, ovarian
cancer, malignant peripheral nerve sheath tumor and glioma, the
proteasome inhibitor bortezomib increases the expression of HSP90
by inducing unfolded protein response accumulation, thereby
promoting oHSV-1 replication and enhancing antitumor efficacy.
Using OVs to infect tumor cells allows targeted
drugs to be released and connects their targets with immune cell
receptors to enhance cell-killing effects. Tian et al
(49) created OV-Cmab-CCL5, an
oHSV encoding a bispecific fusion protein that combines cetuximab's
IgG1 form with C-C motif chemokine ligand 5 (CCL5). This targets
EGFR and CCL5 receptors to increase CCL5 levels in tumors and boost
immune cell infiltration. It can also enhance the killing effect on
GBM cells by promoting antibody-dependent cellular phagocytosis and
antibody-dependent cellular cytotoxicity through linking Fcgamma
receptors on macrophages and NK cells with EGFR on tumor cells.
Poly ADP-ribose polymerase (PARP) inhibitors (PARPi)
enhance antitumor effects by blocking DNA repair induced by OVs,
causing persistent DNA damage and promoting cancer cell death. The
dl922-947, a second-generation oncolytic adenovirus with a 24-base
pair deletion in the E1A-conserved region 2, enhances viral genome
production in host cells (50).
Passaro et al (51) found
that DNA damage induced by dl922-947 activates the PARP repair
mechanism in the DNA damage response signaling pathway, while the
PARPi olaparib can restore PARP activation induced by it and
exacerbate DNA damage, thereby promoting apoptosis and enhancing
the anti-tumor efficacy against thyroid cancer. The same antitumor
mechanism was observed in the treatment of melanoma with reovirus
type 3 Dearing strain (ReoT3D) combined with talazoparib (52). Recent research reports that PARP1
has been identified as a replication restriction factor for HSV-1,
and olaparib promotes viral replication by inhibiting PARP1,
thereby enhancing antitumor efficacy in GBM and triple-negative
breast cancer (53).
Furthermore, the combination of OVs and PARPi can
induce excessive reactive oxygen species (ROS) production, leading
to increased DNA damage and S-phase arrest, thereby enhancing the
inhibition of tumor cell proliferation. While ROS are common in
cells, excessive ROS can cause cell death (54) and activate oncogenic pathways,
leading to DNA damage and genetic instability (55). PARPi can have antitumor effects by
increasing ROS and ROS-induced DNA damage (56,57). The measles virus (MV) is a
promising alternative virus (58), which has been proven to exert
oncolytic effects on ovarian cancer through ROS-induced apoptosis
(59). Zhang et al
(60) discovered that the
recombinant Chinese Hu191 MV (rMV-Hu191) modified by a viral
reverse genetic system, works with olaparib to enhance oxidative
DNA damage in pancreatic ductal adenocarcinoma cells by increasing
ROS levels. This combination also significantly halts the cell
cycle in the S phase and inhibits cell proliferation by blocking
DNA division (60).
In mammalian cells, p53 and cyclin-dependent kinases
jointly manage the G1/S transition, linking apoptosis to cell cycle
progression (61). Irinotecan is
a topoisomerase I inhibitor that can induce the expression and
phosphorylation of the tumor antigen p53, promoting the expression
of apoptosis-regulating factors (62). Napabucasin (BBI608) is a stem cell
inhibitor that can directly inhibit the transcription of
STAT3-derived genes and can also indirectly inhibit other related
genes and pathways (63). Babaei
et al (64) found that
combining ReoT3D, Irinotecan (CPT-11) and BBI608 significantly
reduced the S phase of CT26 cells, arrested them in G0/G1 and G2/M
phases, induced apoptosis by causing a sub-G1 fraction to form and
downregulated KRAS and STAT3 mRNA.
Autophagy is a fundamental biological process that
is crucial for preventing cellular damage, maintaining cell
survival during nutrient scarcity and responding to cytotoxic
stimuli. The regulation of autophagy by the PI3K/AKT/mTOR signaling
pathway allows tumors to exploit this process for survival benefits
through the dysregulation of the pathway (65-67). Everolimus (RAD001) is a widely
used mTOR inhibitor that targets mTOR complex 1 and inhibits
autophagy (68). Alonso et
al (69) demonstrated that
the combination of RAD001 with Delta-24-RGD, an oncolytic
adenovirus with a 24-base pair deletion in the E1A gene (70), induces autophagic cell death and
enhances anti-glioma efficacy, although the precise underlying
mechanism remains to be elucidated. The anti-angiogenic and
immunosuppressive properties of RAD001 may also contribute to these
outcomes. Furthermore, they revealed that Delta-24-RGD increases
glioma cell sensitivity to RAD001 by circumventing G1 phase arrest,
without impacting the replication of Delta-24-RGD, indicating an
additional potential anti-tumor mechanism.
The above content regarding composite molecular
machineries involves various targeted drugs that exhibit
significant differences in targets, mechanisms of action and tumor
sensitivity. MAPK inhibitors primarily act on kinases within the
MAPK pathway, such as BRAF, MEK and ERK. MEK inhibitors promote OVV
replication by activating STAT3 and can also increase viral
replication by reducing antiviral responses through the inhibition
of STAT activation. TKIs synergistically enhance antitumor effects
by reducing AKT phosphorylation activated by OVs, while OVs
engineered to specifically express endostatin can increase tumor
sensitivity to bevacizumab by inhibiting AKT. Pathway inhibitors
can also suppress targets related to the IFN antiviral pathway,
promoting OV replication. The presence of immunosuppressive cells
in the TME is also a key reason for poor efficacy. Targeted drugs
combined with OVs synergistically enhance antitumor effects by
boosting innate and adaptive immunity and reducing
immunosuppression. PARPi primarily target PARP enzymes responsible
for repairing double-strand DNA damage, exerting antitumor effects
by inducing sustained DNA damage through increased ROS and
inhibition of OV-induced DNA repair. Furthermore, OVs can synergize
with inhibitors related to apoptosis and autophagy pathways for
antitumor effects (Fig. 1). The
different characteristics of these inhibitors provide clearer
guidance for selecting targeted drugs in combination therapy.
Choosing the appropriate drugs based on tumors with different
target mutations can markedly enhance the precision and antitumor
efficacy of combination therapy. Due to the numerous molecular
target mutations in tumors, a wide variety of targeted drugs have
been developed and approved for use. However, the exploration of
combination studies remains limited, with the combined mechanisms
focusing only on major pathways such as MAPK, JAK/STAT and
PI3K/AKT. The Wnt, Notch and Hippo signaling pathways, which also
play important roles in multiple cancers (71-73), have not yet been reported, to the
best of our knowledge. The potential interactions between OVs and
the toxic side effects of the targeted drugs themselves could
reduce the therapeutic window and introduce unpredictable
pharmacokinetics/pharmacodynamics. Furthermore, this strategy also
has some inherent limitations. Tumor heterogeneity may lead to
certain tumors being insensitive to both treatments, and since OVs
are mainly administered via local injection, their efficacy against
distant metastatic lesions is limited. The use of certain potent
apoptosis-inducing targeted drugs may prematurely eliminate OVs,
which in turn could restrict viral replication.
Research indicates that combining OVs with targeted
drugs is safer and more effective for treating solid tumors than
using them separately. Some animal studies suggest that small
molecule targeted drugs can hinder tumor growth by reducing
angiogenesis, interstitial fluid pressure (IFP) and barriers to OVs
reaching target cells. HF10 is a spontaneously occurring HSV-1
(74). Yamamura et al
(75) found that erlotinib and
HF10 work synergistically in a pancreatic cancer model by reducing
angiogenesis and IFP, leading to a higher intratumoral virus
distribution and significant tumor growth inhibition. Tan et
al (76) found that combining
the OV HF10 with bevacizumab improved tumor suppression compared to
either treatment alone in breast cancer models. This synergy was
linked to increased vascular permeability, VEGF-induced viral
replication in endothelial cells, immune attacks on infected
vessels, enhanced tumor hypoxia boosting viral protein synthesis
and increased apoptosis. Another study reported that bevacizumab in
combination with dl922-947 significantly inhibited tumor growth in
tumor xenografts in thyroid anaplastic carcinoma tumors and
increased the distribution of the virus in tumors (77). However, Mahller et al
(78) found that erlotinib in
combination with oHSV did not show enhanced antitumor effects in a
xenograft mouse model of human malignant peripheral nerve sheath
tumor. The distribution of OVs is strongly influenced by the TME,
among which IFP is a great challenge to efficient virus
distribution, and tumor angiogenesis is one of the main causes of
high IFP.
The aforementioned preclinical studies have
demonstrated that the combination of OVs with small molecule
targeted therapies can partially overcome the barrier of the
endothelial layer, thereby facilitating the distribution and
replication of the virus within the tumor and enhancing its
anti-tumor efficacy. Animal models have further indicated that this
combination exhibits significant anti-tumor effects. However, the
precise molecular mechanisms underlying these effects remain
unidentified, and it is elusive whether they align with the
mechanisms discussed in this article or involve alternative
pathways. Given the discrepancies between the TMEs in vivo
and in vitro, in vitro findings alone are
insufficient to substantiate the translation of these therapies
into clinical practice. Consequently, extensive preclinical
research, including exploratory studies on the mechanisms involved,
is necessary to advance the development of this combination
therapy.
Although the immune organs of humans and mice are
highly conserved in their macroscopic anatomical structure, there
are significant differences in the composition, distribution and
expression of key immune molecules in their immune cells (Table I) (79). Due to significant immune
differences between the TME of mouse tumor models and human
spontaneous tumors, their clinical application value is limited.
The discovery and application of immunodeficient mice provide a
crucial breakthrough for reconstructing the human immune system
in vivo, thereby accurately simulating the human TME. Their
development has undergone continuous iterative upgrades, evolving
from simple T-cell deficiencies (such as nude mice) to severe
combined immunodeficiency models with multiple functional
deficiencies in immune cells such as T, B and NK cells (79). Most of the research data in this
study are derived from this type of model. However, immunodeficient
mice completely lose the ability to initiate adaptive immune
responses, displaying severe defects in the innate immune system.
They are not suitable for studying the human immune system itself
or the diseases it participates in, but this is the primary
requirement for constructing humanized mouse models from
immunodeficient mice (80).
Humanized mice refer to immunodeficient mice co-implanted with
human tumors and immune components, which can realistically assess
the mechanisms of immune system activation and efficacy in
preclinical studies of OVs in combination with targeted drugs,
providing more optimized combination treatment strategies for
clinical trial design.
Certain OVs have the ability to preferentially
infect certain types of tumor cells before any modifications. This
natural tropism primarily depends on the specific binding between
viral surface proteins and specific receptors on the host cell
surface. Many tumor cells overexpress certain receptors required
for viral invasion. For example, the coxsackievirus and adenovirus
receptor (CAR) is expressed at low levels in normal tissues but is
often overexpressed in various malignancies, allowing
coxsackievirus and adenovirus to preferentially target and infect
these tumors such as laryngeal cancer, thyroid cancer, lung cancer
and female reproductive system tumors (81,82). CD46 is mainly present on the
surface of prostate cancer and bladder cancer and can be recognized
by adenoviruses and measles viruses (83-86). The expression of integrins in
tumor cells is usually abnormal, allowing various viruses such as
adenovirus, coxsackievirus and reovirus to enter cells through
integrin-associated ligands (87-91). Table II shows the natural affinity of
many viruses for nonspecific receptors on the surface of certain
tumor cells (Table II). It can
serve directly as a therapeutic option or as a framework for viral
modification. In addition to common receptors, the surface
receptors of different types of tumors also have their specific
characteristics. OVs Ankara-5T4, constructed to target the specific
receptors 5T4 for colorectal cancer, have shown higher specificity
and efficacy (92,93). In addition, colorectal cancer is
associated with gut microbiota dysbiosis, but there are no reports
on the genetic modification of OVs in relation to this connection
(94,95). R-405, designed to target the
prostate cancer-specific receptor prostate-specific membrane
antigen, also demonstrates strong antitumor effects (96,97). The development and research of OVs
targeting different tumor-specific receptors are shown in the table
(Table III). Therefore, we can
enable OVs to acquire the ability to bind new receptors and further
enhance tumor targeting by inserting antibody fragments or peptides
that specifically bind to tumor-associated antigens.
Over the past two decades, clinical research has
progressively investigated the integration of OVs with targeted
therapies, yielding promising outcomes in certain instances. A
meta-analysis encompassing 12 studies evaluated the objective
response rate, survival rate and incidence of adverse reactions in
cancer patients receiving a combination of OVs and conventional
therapy compared to conventional therapy alone. The findings
revealed that the combination therapy was significantly associated
with an increased objective response rate (ORR) (P=0.04) and a
higher incidence of grade ≥3 adverse reactions (P=0.02) and grade
≥3 neutropenia (P=0.01), although no significant difference in
survival was observed (98).
Nonetheless, further investigation is warranted. In a phase I trial
conducted by Hirooka et al (99), patients with histologically
confirmed locally advanced pancreatic cancer without distant
metastasis and unresectable tumors received one cycle of erlotinib
and gemcitabine, followed by intratumoral injection of HF10 under
endoscopic ultrasound guidance. The primary endpoint was safety
assessment and the secondary endpoint was efficacy evaluation. The
study results showed that among the 10 subjects, 2 experienced
serious adverse events unrelated to HF10, indicating that the doses
used in this trial were safe and effective. Among the 9 subjects
who completed all four HF10 injections, the overall response
included 3 partial responses, 4 patients with stable disease and 2
cases with progressive disease, with a median progression-free
survival (PFS) of 6.3 months and a median overall survival (OS) of
15.5 months, and 2 subjects were able to undergo surgical
resection. The results suggest the safety and efficacy of this
therapeutic approach (100).
However, the study only compared the efficacy between different
HF10 titers and did not set up a control group or erlotinib
monotherapy group, gemcitabine monotherapy group or erlotinib plus
gemcitabine to further demonstrate the superiority of HF10
injection after treatment with erlotinib and gemcitabine.
Sorafenib in combination with JX-594 has been
studied in hepatocellular carcinoma (HCC). JX-594 (Pexa-Vec) was
constructed by inserting human granulocyte-monocyte
colony-stimulating factor and lacZ into the thymidine kinase gene
region of the Wyeth strain vaccinia virus (101). After infecting tumor cells, it
can stimulate toll like receptors, activate dendritic cells (DCs),
increase NK, cytotoxic T-lymphocyte and white blood cell
infiltration, and promote the secretion of a variety of cytokines,
such as IFN, IL-1, IL-6 and IL-12 (101). Heo et al (102) discovered that administering
JX-594 before sorafenib significantly inhibited tumor growth
compared to control and other treatment sequences in mouse models.
Encouraged by these findings, they tested this sequence in patients
with advanced HCC and found it to be well-tolerated, leading to
rapid and significant tumor necrosis, with a 50-100% increase in
tumor necrosis for patients who did not achieve durable objective
tumor remission with JX-594 alone completed JX-594 treatment, and
JX-594 was injected directly into the tumors under imaging guidance
(102). However, only 3 patients
were evaluated in this study and are not representative of the
entire HCC population, and did not set evaluation endpoints or
provide statistical analysis results, resulting in poor
generalizability of the results and reduced reliability,
potentially leading to misleading conclusions. Rare adverse events
could also not be detected. In a randomized, multicenter phase IIb
trial, intravenous injection of Pexa-Vec in patients with advanced
HCC who have progressed after sorafenib treatment or are intolerant
to sorafenib, OS is primarily being evaluated, with secondary
endpoints including time to progression (TTP), ORR, disease control
rate (DCR), time to symptomatic progression, safety, tolerability
and quality of life. The study results showed that Pexa-Vec was
generally well-tolerated, but there were no significant differences
in the evaluated endpoints, which may be due to a high dropout rate
preventing accurate assessment of the outcomes (103). In a phase Ⅲ, randomized,
open-label study, for patients with advanced HCC who have not
received systematic treatment, Pexa-Vec was first administered via
intratumoral injection, followed by sorafenib, to evaluate OS
compared to sorafenib alone. Secondary objectives included TTP,
PFS, ORR and DCR, as well as assessing and comparing the safety of
the two treatment groups. The study results showed that compared to
sorafenib alone, treatment with sorafenib following Pexa-Vec did
not show a statistically significant benefit and was less
effective, with a higher incidence of serious adverse events
(104). The study indicates that
the dosing sequence of JX-594 and sorafenib may impact their
effectiveness. Investigating the mechanisms behind their
interaction could advance the use of OVs with targeted drugs in
clinical research. Table IV
summarizes the study subjects, treatment methods, endpoints,
results and limitations of the aforementioned clinical studies on
OVs combined with targeted drugs and/or other chemotherapeutic
agents, as well as those have not reported results. There are
currently relatively few clinical trials of OVs combined with
targeted drugs, suggesting that there may be obstacles to clinical
translation and highlighting the importance of preclinical research
in clinical trials.
In some rare case studies, OVs combined with
molecularly targeted drugs can alleviate the patient's disease and
improve the prognosis. CF33-hNIS-anti-PD-L1 (CHECKvacc) is a novel
chimeric orthopoxvirus encoding a single-stranded variable fragment
of human sodium iodide symporter and anti-programmed cell death
ligand 1, with potent anticancer activity in triple-negative breast
cancer xenografts (105). A
pathology report reported that a patient with metastatic
triple-negative breast cancer progressed after multiple lines of
therapy with extensive skin metastases, received intratumoral
injection of CHECKvacc followed by sequential treatment with
trastuzumab deruxtecan, with a clinical complete response lasting 7
months and a DFS of 10 months, The tumor marker CA15-3 was
significantly reduced (106).
The patient's decision to cease therapy after multiple treatments
makes it unclear whether remission is solely due to the combination
therapy, requiring more research. A pathology report highlighted a
patient with advanced poorly differentiated rectal adenocarcinoma
and liver metastases who achieved complete remission and stable
disease for 7 years following FOLFOX-4 chemotherapy, bevacizumab
and Rigvir (107). However,
since most pathological reports are retrospective, issues of data
completeness and consistency cannot be verified, and they also have
disadvantages such as high selectivity, poor population
representativeness and a high risk of bias, and the results do not
represent the safety and efficacy of this combination in the
overall population, but can be linked to the existing literature as
a way to share lessons learned to help identify new trends and
potential uses.
Malignant tumors constitute the primary cause of
mortality worldwide, with their incidence continuously rising
(108). Therapeutic strategies,
encompassing surgery, chemotherapy, radiotherapy, immunotherapy and
targeted therapy, have progressed from monotherapy to combination
therapy to address tumor drug resistance (109,110). OVs have emerged as an innovative
approach to cancer treatment, distinguished by their
tumor-targeting capabilities, which minimize harm to normal cells
and reduce the occurrence of side effects. Clinical trials have not
reported any fatalities or severe adverse events attributable to OV
therapy, highlighting its favorable safety profile. However, the
inherent limitations of OVs may affect their therapeutic efficacy.
Targeted drugs can activate the phosphorylation of relevant targets
to promote viral replication, and inhibit pathway activation and
antiviral responses caused by OVs, thereby increasing OV
replication, distribution and oncolytic effects. This enhancement
may extend to other targets involved in tumorigenesis or immune
microenvironment regulation. The relationship between MEK and STAT3
leads to the inhibition of MEK promoting STAT3 activation, thereby
enhancing viral replication. Existing studies have reported a
negative correlation between ERK and STAT3 (111-113). This negative correlation has
been confirmed as a potential mechanism underlying the development
of drug resistance, suggesting that a similar mechanism may also
exist. Targeted drugs can also synergize with OVs to enhance
immunity, exacerbate DNA damage and promote tumor cell apoptosis,
exerting a strong antitumor effect. The diversity in the types and
mechanisms of action of targeted drugs, coupled with the variety of
OVs and the potential for their genetic modification, provides
numerous opportunities. These opportunities include the elimination
of factors that adversely affect their anti-tumor effects, as well
as the potential for synergistic and cumulative effects. Such
interactions underscore the promising prospects for the combined
use of OVs and targeted drugs. In addition to the simultaneous
administration of these therapeutic agents, researchers have also
developed genetically modified OVs that incorporate molecularly
targeted drugs. This innovative strategy seeks to leverage the
anti-tumor properties of both the targeted drugs and the OVs.
However, extensive genetic modifications limit the replication and
efficacy of the virus. Therefore, revisiting the fundamental
principles of virology and basic immunology is crucial for
rationally designing more effective oncolytic virus constructs
(114). Multiple preclinical
studies have confirmed that OVs combined with targeted drugs can
enhance anti-tumor immune responses through multiple mechanisms,
and initial results have been achieved in clinical trial progress.
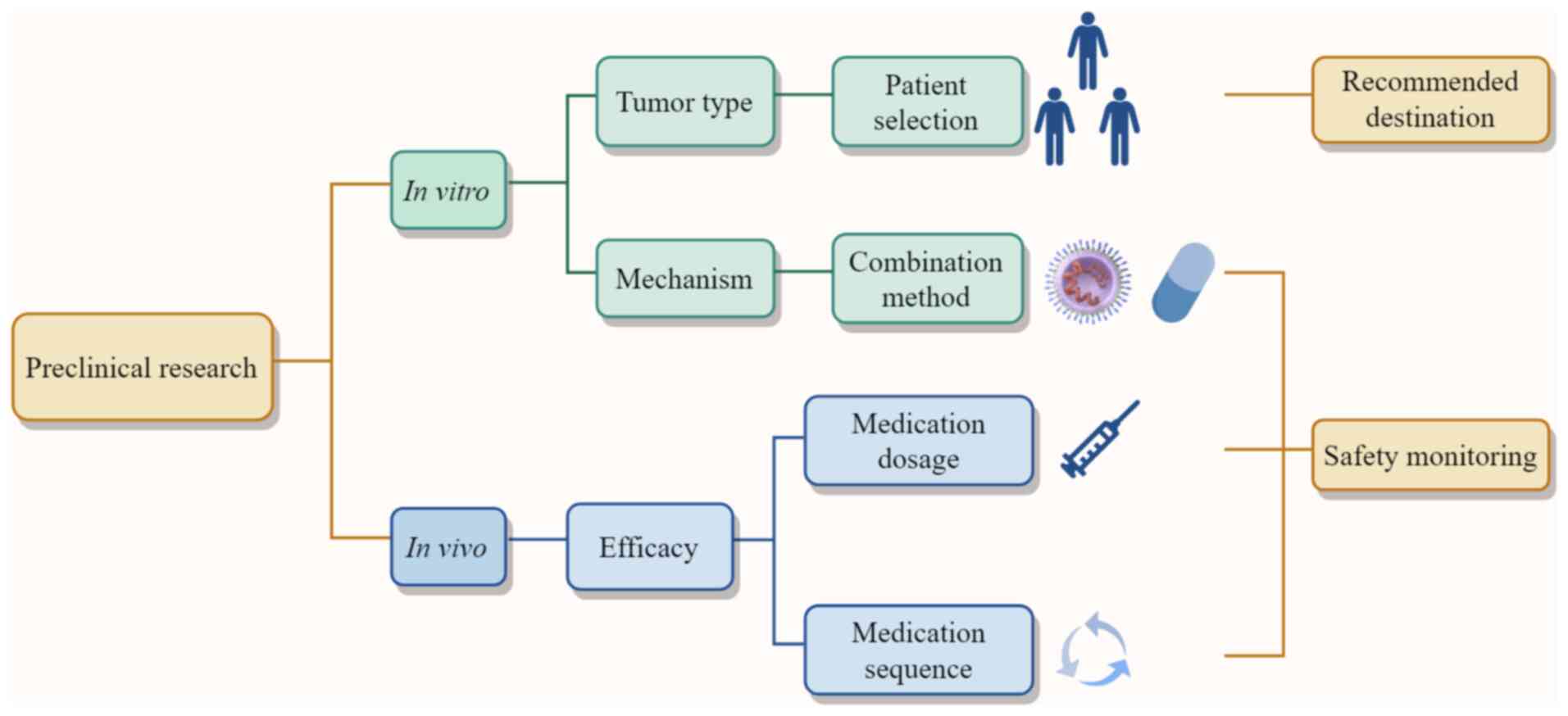
To this end, in the present study, preclinical evidence was
connected with trial design elements, including patient selection,
recommended endpoints, and overlapping toxicity safety monitoring,
to construct a clinical translation pathway (Fig. 2). Based on mechanisms of action,
potential combination strategies and priority tumor types can be
suggested, which in turn provides criteria for patient selection,
while the combined efficacy observed in animal models offers
guidance on drug dosage and administration sequence. Continuous
safety monitoring during these explorations may help prevent
serious adverse events caused by potential toxicities. How to
further optimize OVs and targeted drugs based on their own
characteristics and limitations to advance clinical trials more
effectively is a key focus for the future.
Overactivation of signaling pathways is a cause of
cancer pathogenesis, being involved in various tumors and playing a
critical role in guiding clinical treatment choices and
classification (115). Different
gene mutation statuses, such as EGFR and KRAS mutations in lung
adenocarcinoma, show significant differences in biological behavior
and clinicopathological features, leading to completely different
treatment options (116). In
breast cancer, classification is based on the expression of
estrogen receptor, progesterone receptor and HER2 (Luminal A,
Luminal B, HER2-positive, triple-negative), which directly
determines subsequent treatment strategies (117). Once the key signaling pathways
driving tumor growth are identified, targeted drugs can be
developed against critical nodes in these pathways. These pathways
can also serve as predictive markers for evaluating treatment
efficacy and prognosis. For instance, phosphatase and tensin
homolog protein loss leading to overactivation of the PI3K-AKT
pathway in prostate cancer generally indicates poor prognosis
(118). Single-cell RNA
sequencing suggests that the C2 insulin-like growth factor 2 tumor
subtype is associated with poor prognosis in high-grade serous
ovarian cancer, providing a promising target for future therapies
(119). Additionally, signaling
pathway targets can be used as monitoring indicators for studying
resistance mechanisms and adjusting treatment plans; analyzing
pathway changes through re-biopsy can reveal resistance mechanisms
and guide subsequent treatment. Approximately half of the
resistance to first-generation EGFR inhibitors is due to the
emergence of the secondary T790M mutation. To address this, the
third-generation EGFR inhibitor Osimertinib was developed, which
effectively overcomes T790M resistance and has become the standard
treatment option after resistance develops (120). Through time-of-flight cytometry,
it was found that AXL inhibition can induce JAK1-STAT3 signaling to
compensate for the loss of AXL, enhancing the potential for distant
metastasis in lung cancer (121). AXL is a member of the TAM
receptor family, typically overexpressed in cancers, and has become
a potential target for malignant tumors (122). The use of sequencing technology
to predict targeted drug targets has formed a set of multi-omics
integration strategies. By sequencing the genome, transcriptome and
by other omics, key genes related to diseases can be systematically
identified, and their potential as drug targets can be evaluated.
Advances in targeted drug development have led to new drugs
addressing various mutations, enhancing the effectiveness and
overcoming the limitations of older treatments. Consequently,
precise combination strategies based on molecular typing can
significantly enhance anti-tumor effects and reduce drug
resistance.
As the first line of defense against the invasion of
external pathogenic microorganisms, the antiviral response pathway,
in addition to the IFN pathway, The cyclic GMP-AMP synthase
(cGAS)-stimulator of interferon genes (STING) and
retinoic-acid-inducible gene-I (RIG-I)/mitochondrial antiviral
signaling protein (MAVS), also play key roles in antiviral innate
immunity, belonging to the cellular stress response pathway. The
release of viral DNA and tumor cell nuclear DNA can activate the
cGAS-STING pathway, subsequently triggering a series of signaling
cascades, thereby inducing the production of type I IFNs and
initiating adaptive immune responses (123). However, in various cancers,
interruption or loss of the cGAS-STING pathway has been observed,
which prevents OVs from effectively activating this pathway,
resulting in a failure to initiate immune responses (124-126). The development of various STING
agonists has shown enhanced stability and efficacy in anti-tumor
applications (127-129). Sibal et al (130) found that the STING activator
2'3'-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP)
combined with the attenuated HSV-1 strain C-REV could induce a more
sustained anti-tumor immune response, but they did not conduct an
in-depth analysis of how 2'3'-cGAMP affects the intracellular
process dynamics related to viral replication mechanisms (130). The RIG-I/MAVS pathway primarily
recognizes viral RNA in the cytoplasm. RIG-I has high affinity for
double-stranded (ds)RNA with three phosphates on the 5' end. After
binding, it also activates the transcription and expression of type
I IFNs and various pro-inflammatory cytokines (131). It was reported that HSV-1
infection induces mitochondrial damage and release of mitochondrial
DNA, which can trigger cGAS/STING/interferon regulatory factor 3
and RIG-I-MAVS signaling pathways, suggesting that the RIG-I-MAVS
pathway can recognize more than just viral RNA (132). Current research mainly focuses
on OVs transforming 'cold' tumors into 'hot' tumors and promoting
immune cell infiltration and tumor killing by activating the
cGAS-STING and RIG-I/MAVS pathway, generating a strong antitumor
immune response, and tumors lacking cGAS or STING expression are
more easily infected, thereby enhancing oncolytic activity
(133-137). However, if this pathway, which
is central to antiviral immunity, is excessively or prematurely
activated, it may lead to viral clearance, thereby weakening the
direct oncolytic effect of the virus. Precisely regulating the
balance between viral replication and immune activation is a major
challenge for studying the combined use of OVs and related pathway
activators. Furthermore, there are no reports in the literature on
whether specific inhibitors exist that can temporarily modulate
innate antiviral pathways to allow OV spread without causing
systemic immunosuppression, which may become a direction for future
research.
When OVs enter the body, their ability to induce a
strong anti-tumor immune response is also influenced by another key
factor. Previous research has indicated that OVs are swiftly
neutralized by antibodies upon entering the body, thereby
significantly diminishing their therapeutic efficacy (138,139). To overcome this challenge,
researchers have proposed employing vector delivery systems to
shield OVs from complement and neutralizing antibodies, thereby
enhancing tumor targeting. Polymer coatings (140) and cell-derived nanovesicles
(141) has been identified as
potential vectors for delivering OVs. Another promising approach
involves cell-based drug delivery systems, which provide advantages
such as extended drug circulation, improved efficacy, controlled
drug release and reduced immunogenicity and cytotoxicity (142). Furthermore, chimeric antigen
receptor T-cells have been reported as effective carriers for OVs,
offering significant advantages in OV therapy against distant
tumors (143). However, the
immunosuppressive mechanisms present within the TME may restrict
the effectiveness of T cells as OV vectors to tumors that are not
classified as immune deserts (144). By contrast, mesenchymal stem
cells have been extensively investigated as vectors for various
OVs, including oncolytic adenovirus, oncolytic HSV, oncolytic MV
and oncolytic reovirus (145).
This is attributed to their advantageous properties, such as tumor
homing, intrinsic anti-cancer capabilities, protection of OVs from
neutralizing antibodies and the ability to deliver viruses to tumor
sites via trojan horse strategies (146-148). The development and refinement of
targeted delivery systems for OVs substantially mitigate the risk
of clearance by the host immune system, thereby addressing some of
the challenges associated with combining OVs with molecular
targeted therapies and enhancing their anti-tumor efficacy.
However, in the development of targeted delivery systems, we must
consider not only the immunogenicity of the OVs themselves, but
also evaluate whether the delivery system could synergize with the
OVs to trigger potential risks such as cytokine storms. Therefore,
it is necessary to make a rational selection and modification of
the carrier. In addition, efficiently and stably assembling OVs
with the delivery system while maintaining the activity of both,
and achieving large-scale production at a relatively low cost,
remains a significant challenge.
The integration of OVs with molecularly targeted
therapies constitutes a promising domain for further exploration.
Current research predominantly focuses on preclinical trials, with
relatively few studies advancing to clinical trials. This limited
progression is likely due to the fact that many viruses do not
successfully advance beyond phase I trials (149), which are primarily designed to
assess safety and determine appropriate dosing parameters. To date,
only 13 viruses have exhibited adequate safety profiles and
preliminary clinical success to warrant progression to phase II
trials, which are intended to assess clinical efficacy (149). Current methodologies for drug
combination exhibit inherent limitations, such as the issue of
cumulative toxicity, necessitating further clinical trials to
ascertain their safety and efficacy. It is crucial to evaluate the
rationale behind these combination strategies and to optimize the
use of both the shared and unique properties of the drugs,
especially in cases where combinations are ineffective (114). Given that tumor cells frequently
develop resistance to monotherapies, employing a combination of
diverse therapeutic approaches may partially alleviate this
resistance (150-152). However, the potential
development of new drug resistance following combination therapy,
and the subsequent challenges it may pose to tumor eradication,
warrant further rigorous investigation and validation.
The efficacy of JX-594 in combination with sorafenib
in HCC mentioned in the aforementioned clinical study above is
affected by the order of administration, and priority
administration of JX-594 may be superior to sorafenib. The
underlying mechanisms may be related to the following factors.
Immune 'cold tumors' may be more dependent on OV to preferentially
initiate immunity (133-135). As a result, preferential
injection of OVs can induce immunogenic death of tumor cells,
release tumor-associated antigens, promote DC maturation, T-cell
infiltration and cytokine secretion, transform 'cold tumors' into
'hot tumors' and provide a more favorable immune environment for
subsequent targeted drugs to exert anti-tumor effects (136,153). Some targeted drugs may inhibit
OV replication, so prioritizing OV treatment ensures sufficient
viral spread and prevents targeted drugs from weakening its
oncolytic effect. When OVs are combined with certain antiangiogenic
drugs, which induced reduction in blood supply to tumors may limit
delivery of OV (154,155). Conversely, targeted drug
pretreatment can promote OV replication by downregulating the IFN
signaling pathway, a tumor cell defense mechanism (21,34). The use of immunomodulatory drugs
to remove immunosuppressive cells such as Tregs and relieve
immunosuppression may improve the efficacy of immunotherapy for OV
(37,40,41). IFP is one of the key factors
hindering the intratumoral distribution of OVs, and the prior use
of chemotherapy drugs such as cyclophosphamide can reduce tumor
IFP, further improve the intratumoral distribution of OV, and
enhance its oncolytic effect (156). Considering the above factors,
based on the dynamic changes of tumor characteristics, viral
characteristics, drug mechanism and TME, adjusting the order and
dose of drugs can enhance the anti-tumor effect.
OVs and targeted drugs each have their own toxic
effects, and when used in combination, careful consideration should
be given to whether potential additive toxicity could cause serious
adverse events. To date, the combination of therapies mentioned in
this study has not yet undergone clinical translation, and the
unique toxicities that may arise warrant attention. Protein kinase
inhibitors (PKIs) inhibit downstream signaling by targeting
specific oncogenic kinases and are one of the research focuses in
cancer therapy. However, most analyzed PKI product characteristic
summaries and European public assessment reports indicated severe
hepatotoxicity (157).
Furthermore, the hepatotoxicity caused by OVs themselves also needs
to be taken seriously when combined with targeted therapy.
Therefore, before treatment, patients at high risk of
hepatotoxicity should be identified through relevant biomarkers. In
addition, the nonspecific receptor CAR targeted by OVs is also
present in normal liver cells (158), and the virus can be genetically
engineered to avoid being taken up by the liver or infecting liver
cells.
Sunitinib, which is the most involved targeted drug
in mechanistic studies, shows a higher prevalence of reduced left
ventricular ejection fraction and bone marrow suppression (159). OVs therapy is generally not
considered to have significant bone marrow suppression
characteristics, and the incidence and severity of hematologic
toxicity are usually low. However, certain wild-type OVs, such as
oHSV-1, when administered locally to lesions, do not cause viremia,
but in rare cases, intravenous administration can lead to a strong
systemic immune response due to large-scale viral replication,
which may indirectly affect bone marrow function and result in
transient cytopenia (160). In
addition, bone marrow suppression is also the most common side
effect of PARPi (161).
Therefore, when considering the combination of sunitinib or PARPi
with OVs, the hematological toxicity caused by both should be of
concern. The complete blood count should be monitored regularly,
and when grade ≥3 toxicity occurs, it is usually necessary to
suspend treatment. Once recovery to grade ≤1 is achieved, the dose
needs to be reduced upon resuming to avoid irreversible
consequences.
Delta-24-RGD is commonly used for intratumoral
injection in the treatment of recurrent gliomas, with neurological
symptoms being the most common adverse events, such as headaches,
speech disorders and cerebral edema (70). ONC201 has shown significant
efficacy against certain high-grade gliomas in clinical trials, but
it can also lead to adverse events related to the nervous system
(162). The therapeutic effect
of their combined treatment on tumors is undeniable, but the
potential overlapping side effects on the central nervous system
should not be overlooked either. Furthermore, distinguishing
whether these neurological symptoms are caused by treatment-related
inflammation, which may indicate the effectiveness of the
treatment, or by the progression of the tumor itself, is crucial in
clinical practice and is also a challenge in management. Certain
wild-type viruses naturally have neurotropism, and by genetically
modifying these viruses, their ability to infect and damage normal
nerve cells can be significantly weakened (163-165).
At the same time, the size and location of the
tumor, previous treatment history such as radiotherapy that can
damage the blood-brain barrier, and pre-existing neurological
deficits may all amplify the neurotoxicity of combined therapy
(166). Bevacizumab combined
with RAMBO can enhance the efficacy against malignant glioma
through the CCN1-AKT pathway, but its dual anti-angiogenic effect
could significantly increase its impact on the vascular system,
excessively suppressing angiogenesis in both the tumor and
surrounding normal tissues, leading to tissue ischemia and necrosis
(167,168). Therefore, when conducting
further clinical research, the optimal order, dosage and interval
for administering the two drugs should be determined to balance
efficacy and toxicity. In addition, the new genes carried by the
genetically engineered recombinant oncolytic viruses may lead to
additional side effects. Based on studies of relevant OVs and the
toxicity of targeted drugs, future clinical development requires
cautious dose exploration and safety management.
In conclusion, the present study collected
literature on the mechanisms and clinical research of OVs combined
with targeted drugs over the past two decades. There may be
potential selection bias, while literature on this research area is
relatively sparse and outdated. Additionally, the weighting between
preclinical and clinical evidence is uneven and quantitative
synthesis of efficacy across studies is limited.
Not applicable.
SB and TS conceived the study and wrote the
manuscript, and conducted the literature search/selection and data
extraction. YT and QW revised and edited the manuscript. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This research was funded by the Guangxi Science and Technology
Program (grant nos. 2025GXNSFDA069034 and AD25069077), the National
Natural Science Foundation of China (grant nos. 82260484, 81860459,
82360587 and 82560548), the Key Laboratory of Early Prevention and
Treatment for Regional High-Frequency Tumors at Guangxi Medical
University, Ministry of Education (grant nos. GKE-ZZ2023021 and
GKE-ZZ202407) and the Key Projects of Guangxi Natural Science
Foundation (grant no. 2024GXNSFDA010022). Additionally, support was
provided by the '139' Plan for Cultivating High-Level and Key
Talents in Guangxi Medicine, China (grant no. 201903036).
|
1
|
Ma R, Li Z, Chiocca EA, Caligiuri MA and
Yu J: The emerging field of oncolytic virus-based cancer
immunotherapy. Trends Cancer. 9:122–139. 2023. View Article : Google Scholar :
|
|
2
|
Rasa A and Alberts P: Oncolytic virus
preclinical toxicology studies. J Appl Toxicol. 43:620–648. 2023.
View Article : Google Scholar
|
|
3
|
Shalhout SZ, Miller DM, Emerick KS and
Kaufman HL: Therapy with oncolytic viruses: Progress and
challenges. Nat Rev Clin Oncol. 20:160–177. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Todo T, Ito H, Ino Y, Ohtsu H, Ota Y,
Shibahara J and Tanaka M: Intratumoral oncolytic herpes virus G47
for residual or recurrent glioblastoma: A phase 2 trial. Nat Med.
28:1630–1639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gazal S, Gazal S, Kaur P, Bhan A and
Olagnier D: Breaking barriers: Animal viruses as oncolytic and
immunotherapeutic agents for human cancers. Virology.
600:1102382024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufman HL, Shalhout SZ and Iodice G:
Talimogene laherparepvec: Moving from first-in-class to
best-in-class. Front Mol Biosci. 9:8348412022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xi P, Zeng D, Chen M, Jiang L, Zhang Y,
Qin D, Yao Z and He C: Enhancing pancreatic cancer treatment: The
role of H101 oncolytic virus in irreversible electroporation. Front
Immunol. 16:15462422025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee T, Gianchandani A, Boorjian SA, Shore
ND, Narayan VM, Dinney CPN and Kamat AM: Intravesical
interferon-α2b gene therapy with nadofaragene firadenovec-vncg: A
contemporary review. Future Oncol. 21:2429–2438. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Zuo M, Zhou Q and Wang Y:
Oncolytic virotherapy in cancer treatment: Challenges and
optimization prospects. Front Immunol. 14:13088902023. View Article : Google Scholar
|
|
10
|
Lin D, Shen Y and Liang T: Oncolytic
virotherapy: Basic principles, recent advances and future
directions. Signal Transduct Target Ther. 8:1562023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YT, Tan YJ and Oon CE: Molecular
targeted therapy: Treating cancer with specificity. Eur J
Pharmacol. 834:188–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aldea M, Andre F, Marabelle A, Dogan S,
Barlesi F and Soria JC: Overcoming resistance to tumor-targeted and
immune-targeted therapies. Cancer Discov. 11:874–899. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Z, McGray AJR, Jiang W, Lu B, Kalinski
P and Guo ZS: Improving cancer immunotherapy by rationally
combining oncolytic virus with modulators targeting key signaling
pathways. Mol Cancer. 21:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Hu S and Wang X: Recent advances
in oncolytic virus combined immunotherapy in tumor treatment. Genes
Dis. 12:1015992025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C, Cillis J, Deshpande S, Park AK,
Valencia H, Kim SI, Lu J, Vashi Y, Yang A, Zhang Z, et al:
Oncolytic virotherapy in solid tumors: A current review. BioDrugs.
39:857–876. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar
|
|
17
|
Lee S, Yang W, Kim DK, Kim H, Shin M, Choi
KU, Suh DS, Kim YH, Hwang TH and Kim JH: Inhibition of MEK-ERK
pathway enhances oncolytic vaccinia virus replication in
doxorubicin-resistant ovarian cancer. Mol Ther Oncolytics.
25:211–224. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okemoto K, Wagner B, Meisen H, Haseley A,
Kaur B and Chiocca EA: STAT3 activation promotes oncolytic HSV1
replication in glioma cells. PLoS One. 8:e719322013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Q, Zhang R, Qiao C, Miao Y, Yuan Y
and Zheng H: Ubiquitination network in the type I IFN-induced
antiviral signaling pathway. Eur J Immunol. 53:e23503842023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schneider W M, Chevillotte M D and Rice
CM: Interferon-stimulated genes: A complex web of host defenses.
Annu Rev Immunol. 32:513–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Zhao J, Zhang JV, Wu Y, Wang L,
Chen X, Ji D and Zhou GG: Enhancing therapeutic efficacy of
oncolytic herpes simplex virus with MEK inhibitor trametinib in
some BRAF or KRAS-Mutated colorectal or lung carcinoma models.
Viruses. 13:17582021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen TT, Ramsay L, Ahanfeshar-Adams M,
Lajoie M, Schadendorf D, Alain T and Watson IR: Mutations in the
IFNγ-JAK-STAT pathway causing resistance to immune checkpoint
inhibitors in melanoma increase sensitivity to oncolytic virus
treatment. Clin Cancer Res. 27:3432–3442. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel MR, Dash A, Jacobson BA, Ji Y,
Baumann D, Ismail K and Kratzke RA: JAK/STAT inhibition with
ruxolitinib enhances oncolytic virotherapy in non-small cell lung
cancer models. Cancer Gene Ther. 26:411–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otani Y, Ishida J, Kurozumi K, Oka T,
Shimizu T, Tomita Y, Hattori Y, Uneda A, Matsumoto Y, Michiue H, et
al: PIK3R1Met326Ile germline mutation correlates with cysteine-rich
protein 61 expression and poor prognosis in glioblastoma. Sci Rep.
7:73912017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long QZ, Zhou M, Liu XG, Du YF, Fan JH, Li
X and He DL: Interaction of CCN1 with αvβ3 integrin induces
P-glycoprotein and confers vinblastine resistance in renal cell
carcinoma cells. Anticancer Drugs. 24:810–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin BR, Chang CC, Chen LR, Wu MH, Wang MY,
Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, et al: Cysteine-rich 61
(CCN1) enhances chemotactic migration, transendothelial cell
migration, and intravasation by concomitantly up-regulating
chemokine receptor 1 and 2. Mol Cancer Res. 5:1111–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Y, Zhang Y, Nie Q and Chen X:
CCN1/Cyr61-PI3K/AKT signaling promotes retinal neovascularization
in oxygen-induced retinopathy. Int J Mol Med. 36:1507–1518. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomita Y, Kurozumi K, Yoo JY, Fujii K,
Ichikawa T, Matsumoto Y, Uneda A, Hattori Y, Shimizu T, Otani Y, et
al: Oncolytic herpes virus armed with vasculostatin in combination
with bevacizumab abrogates glioma invasion via the CCN1 and AKT
signaling pathways. Mol Cancer Ther. 18:1418–1429. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugawara K, Iwai M, Ito H, Tanaka M, Seto
Y and Todo T: Oncolytic herpes virus G47Δ works synergistically
with CTLA-4 inhibition via dynamic intratumoral immune modulation.
Mol Ther Oncolytics. 22:129–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma W, He H and Wang H: Oncolytic herpes
simplex virus and immunotherapy. BMC Immunol. 19:402018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saha D, Wakimoto H, Peters CW, Antoszczyk
SJ, Rabkin SD and Martuza RL: Combinatorial effects of VEGFR kinase
inhibitor axitinib and oncolytic virotherapy in mouse and human
glioblastoma stem-like cell models. Clin Cancer Res. 24:3409–3422.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jha BK, Dong B, Nguyen CT, Polyakova I and
Silverman RH: Suppression of antiviral innate immunity by sunitinib
enhances oncolytic virotherapy. Mol Ther. 21:1749–1757. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khoury R, Saleh K, Khalife N, Saleh M,
Chahine C, Ibrahim R and Lecesne A: Mechanisms of resistance to
antibody-drug conjugates. Int J Mol Sci. 24:96742023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arulanandam R, Taha Z, Garcia V, Selman M,
Chen A, Varette O, Jirovec A, Sutherland K, Macdonald E, Tzelepis
F, et al: The strategic combination of trastuzumab emtansine with
oncolytic rhabdoviruses leads to therapeutic synergy. Commun Biol.
3:2542020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du Z, Whitt MA, Baumann J, Garner JM,
Morton CL, Davidoff AM and Pfeffer LM: Inhibition of type I
interferon-mediated antiviral action in human glioma cells by the
IKK inhibitors BMS-345541 and TPCA-1. J Interferon Cytokine Res.
32:368–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dolcetti L, Marigo I, Mantelli B,
Peranzoni E, Zanovello P and Bronte V: Myeloid-derived suppressor
cell role in tumor-related inflammation. Cancer Lett. 267:216–225.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lawson KA, Mostafa AA, Shi ZQ, Spurrell J,
Chen W, Kawakami J, Gratton K, Thakur S and Morris DG: Repurposing
sunitinib with oncolytic reovirus as a novel immunotherapeutic
strategy for renal cell carcinoma. Clin Cancer Res. 22:5839–5850.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moehler M, Sieben M, Roth S, Springsguth
F, Leuchs B, Zeidler M, Dinsart C, Rommelaere J and Galle PR:
Activation of the human immune system by chemotherapeutic or
targeted agents combined with the oncolytic parvovirus H-1. BMC
Cancer. 11:4642011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ichikawa M, Williams R, Wang L, Vogl T and
Srikrishna G: S100A8/A9 activate key genes and pathways in colon
tumor progression. Mol Cancer Res. 9:133–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chai H, Xu H, Jiang S, Zhang T, Chen J,
Zhu R, Wang Y, Sun M, Liu B, Wang X, et al: Neural stem
cell-delivered oncolytic virus via intracerebroventricular
administration enhances glioblastoma therapy and immune modulation.
J Immunother Cancer. 13:e0129342025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu Z, Chen H, Feng C, Chen L, Ma C, Liu
Z, Qu Z, Bartlett DL, Lu B, Li K and Guo ZS: Specific inhibitor to
KRASG12C induces tumor-specific immunity and synergizes
with oncolytic virus for enhanced cancer immunotherapy. J
Immunother Cancer. 13:e0105142025. View Article : Google Scholar
|
|
42
|
Herman ML, Geanes ES, McLennan R, Greening
GJ, Mwitanti H and Bradley T: ICAM-1 autoantibodies detected in
healthy individuals and cross-react with functional epitopes.
Immunohorizons. 9:vlaf0252025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shin J, Lim J, Han D, Lee S, Sung NS, Kim
JS, Kim DK, Lee HY, Lee SK, Shin J, et al: TBK1 inhibitor amlexanox
exerts anti-cancer effects against endometrial cancer by regulating
AKT/NF-κB signaling. Int J Biol Sci. 21:143–159. 2025. View Article : Google Scholar :
|
|
44
|
Guo X, Feng H, Xi Z, Zhou J, Huang Z, Guo
J, Zheng J, Lyu Z, Liu Y, Zhou J, et al: Targeting TBK1 potentiates
oncolytic virotherapy via amplifying ICAM1-mediated NK cell
immunity in chemo-resistant colorectal cancer. J Immunother Cancer.
13:e0114552025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meisen WH, Wohleb ES, Jaime-Ramirez AC,
Bolyard C, Yoo JY, Russell L, Hardcastle J, Dubin S, Muili K, Yu J,
et al: The impact of macrophage- and microglia-secreted TNFα on
Oncolytic HSV-1 therapy in the glioblastoma tumor microenvironment.
Clin Cancer Res. 21:3274–3285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoo JY, Swanner J, Otani Y, Nair M, Park
F, Banasavadi-Siddegowda Y, Liu J, Jaime-Ramirez AC, Hong B, Geng
F, et al: Oncolytic HSV therapy increases trametinib access to
brain tumors and sensitizes them in vivo. Neuro Oncol.
21:1131–1140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Burch AD and Weller SK: Herpes simplex
virus type 1 DNA polymerase requires the mammalian chaperone hsp90
for proper localization to the nucleus. J Virol. 79:10740–10749.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yoo JY, Hurwitz BS, Bolyard C, Yu JG,
Zhang J, Selvendiran K, Rath KS, He S, Bailey Z, Eaves D, et al:
Bortezomib-induced unfolded protein response increases oncolytic
HSV-1 replication resulting in synergistic antitumor effects. Clin
Cancer Res. 20:3787–3798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tian L, Xu B, Chen Y, Li Z, Wang J, Zhang
J, Ma R, Cao S, Hu W, Chiocca EA, et al: Specific targeting of
glioblastoma with an oncolytic virus expressing a cetuximab-CCL5
fusion protein via innate and adaptive immunity. Nat Cancer.
3:1318–1335. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Napolitano F, Di Somma S, Castellano G,
Amato J, Pagano B, Randazzo A, Portella G and Malfitano AM:
Combination of dl922-947 oncolytic adenovirus and G-quadruplex
binders uncovers improved antitumor activity in breast cancer.
Cells. 11:24822022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Passaro C, Volpe M, Botta G, Scamardella
E, Perruolo G, Gillespie D, Libertini S and Portella G: PARP
inhibitor olaparib increases the oncolytic activity of dl922-947 in
in vitro and in vivo model of anaplastic thyroid carcinoma. Mol
Oncol. 9:78–92. 2015. View Article : Google Scholar
|
|
52
|
Kyula-Currie J, Roulstone V, Wright J,
Butera F, Legrand A, Elliott R, McLaughlin M, Bozhanova G, Krastev
D, Pettitt S, et al: The PARP inhibitor talazoparib synergizes with
reovirus to induce cancer killing and tumour control in vivo in
mouse models. Nat Commun. 16:62992025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong Y, Le H, Zhang X, Dai Y, Guo F, Ran
X, Hu G, Xie Q, Wang D and Cai Y: Identification of restrictive
molecules involved in oncolytic virotherapy using genome-wide
CRISPR screening. J Hematol Oncol. 17:362024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tapeinos C and Pandit A: Physical,
chemical, and biological structures based on ROS-Sensitive moieties
that are able to respond to oxidative microenvironments. Adv Mater.
28:5553–5585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang L, Wang D, Sonzogni O, Ke S, Wang Q,
Thavamani A, Batalini F, Stopka SA, Regan MS, Vandal S, et al:
PARP-inhibition reprograms macrophages toward an anti-tumor
phenotype. Cell Rep. 41:1114622022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tubbs A and Nussenzweig A: Endogenous DNA
damage as a source of genomic instability in cancer. Cell.
168:644–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Packiriswamy N, Upreti D, Zhou Y, Khan R,
Miller A, Diaz RM, Rooney CM, Dispenzieri A, Peng KW and Russell
SJ: Oncolytic measles virus therapy enhances tumor antigen-specific
T-cell responses in patients with multiple myeloma. Leukemia.
34:3310–3322. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou S, Li Y, Huang F, Zhang B, Yi T, Li
Z, Luo H, He X, Zhong Q, Bian C, et al: Live-attenuated measles
virus vaccine confers cell contact loss and apoptosis of ovarian
cancer cells via ROS-induced silencing of E-cadherin by
methylation. Cancer Lett. 318:14–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang CD, Jiang LH, Zhou X, He YP, Liu Y,
Zhou DM, Lv Y, Wu BQ and Zhao ZY: Synergistic antitumor efficacy of
rMV-Hu191 and Olaparib in pancreatic cancer by generating oxidative
DNA damage and ROS-dependent apoptosis. Transl Oncol.
39:1018122024. View Article : Google Scholar
|
|
61
|
Meikrantz W and Schlegel R: Apoptosis and
the cell cycle. J Cell Biochem. 58:160–174. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee B, Min JA, Nashed A, Lee SO, Yoo JC,
Chi SW and Yi GS: A novel mechanism of irinotecan targeting MDM2
and Bcl-xL. Biochem Biophys Res Commun. 514:518–523. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hubbard JM and Grothey A: Napabucasin: An
update on the first-in-class cancer stemness inhibitor. Drugs.
77:1091–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Babaei A, Soleimanjahi H, Soleimani M and
Arefian E: The synergistic anticancer effects of ReoT3D, CPT-11,
and BBI608 on murine colorectal cancer cells. Daru. 28:555–565.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen Y and Zhou X: Research progress of
mTOR inhibitors. Eur J Med Chem. 208:1128202020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Y and Zhang H: Regulation of
autophagy by mTOR signaling pathway. Adv Exp Med Biol. 1206:67–83.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoshida GJ: Therapeutic strategies of drug
repositioning targeting autophagy to induce cancer cell death: From
pathophysiology to treatment. J Hematol Oncol. 10:672017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Saran U, Foti M and Dufour JF: Cellular
and molecular effects of the mTOR inhibitor everolimus. Clin Sci
(Lond). 129:895–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Alonso MM, Jiang H, Yokoyama T, Xu J,
Bekele NB, Lang FF, Kondo S, Gomez-Manzano C and Fueyo J:
Delta-24-RGD in combination with RAD001 induces enhanced
anti-glioma effect via autophagic cell death. Mol Ther. 16:487–493.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lang FF, Conrad C, Gomez-Manzano C, Yung
WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD,
et al: Phase I study of DNX-2401 (Delta-24-RGD) oncolytic
adenovirus: Replication and immunotherapeutic effects in recurrent
malignant glioma. J Clin Oncol. 36:1419–1427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xue C, Chu Q, Shi Q, Zeng Y, Lu J and Li
L: Wnt signaling pathways in biology and disease: Mechanisms and
therapeutic advances. Signal Transduct Target Ther. 10:1062025.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang H, Hang W, Jing Z, Liu B, Wang X, Li
Y, Luo H, Lv H, Tao X, Timashev P, et al: The role of notch
signaling pathway in cancer: Mechanistic insights, therapeutic
potential, and clinical progress. Front Immunol. 16:15675242025.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang H, Yang J, Zheng X, Chen T, Zhang R,
Chen R, Cao T, Zeng F and Liu Q: The hippo pathway in breast
cancer: The extracellular matrix and hypoxia. Int J Mol Sci.
25:128682024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ushijima Y, Luo C, Goshima F, Yamauchi Y,
Kimura H and Nishiyama Y: Determination and analysis of the DNA
sequence of highly attenuated herpes simplex virus type 1 mutant
HF10, a potential oncolytic virus. Microbes Infect. 9:142–149.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yamamura K, Kasuya H, Sahin TT, Tan G,
Hotta Y, Tsurumaru N, Fukuda S, Kanda M, Kobayashi D, Tanaka C, et
al: Combination treatment of human pancreatic cancer xenograft
models with the epidermal growth factor receptor tyrosine kinase
inhibitor erlotinib and oncolytic herpes simplex virus HF10. Ann
Surg Oncol. 21:691–698. 2014. View Article : Google Scholar
|
|
76
|
Tan G, Kasuya H, Sahin TT, Yamamura K, Wu
Z, Koide Y, Hotta Y, Shikano T, Yamada S, Kanzaki A, et al:
Combination therapy of oncolytic herpes simplex virus HF10 and
bevacizumab against experimental model of human breast carcinoma
xenograft. Int J Cancer. 136:1718–1730. 2015. View Article : Google Scholar
|
|
77
|
Libertini S, Iacuzzo I, Perruolo G, Scala
S, Ieranò C, Franco R, Hallden G and Portella G: Bevacizumab
increases viral distribution in human anaplastic thyroid carcinoma
xenografts and enhances the effects of E1A-defective adenovirus
dl922-947. Clin Cancer Res. 14:6505–6514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mahller YY, Vaikunth SS, Currier MA,
Miller SJ, Ripberger MC, Hsu YH, Mehrian-Shai R, Collins MH,
Crombleholme TM, Ratner N and Cripe TP: Oncolytic HSV and erlotinib
inhibit tumor growth and angiogenesis in a novel malignant
peripheral nerve sheath tumor xenograft model. Mol Ther.
15:279–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bin Y, Ren J and Zhang H, Zhang T, Liu P,
Xin Z, Yang H, Feng Z, Chen Z and Zhang H: Against all odds: The
road to success in the development of human immune reconstitution
mice. Animal Model Exp Med. 7:460–470. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen J, Liao S, Xiao Z and Pan Q, Wang X,
Shen K, Wang S, Yang L, Guo F, Liu HF and Pan Q: The development
and improvement of immunodeficient mice and humanized immune system
mouse models. Front Immunol. 13:10075792022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Reeh M, Bockhorn M, Görgens D, Vieth M,
Hoffmann T, Simon R, Izbicki JR, Sauter G, Schumacher U and Anders
M: Presence of the coxsackievirus and adenovirus receptor (CAR) in
human neoplasms: A multitumour array analysis. Br J Cancer.
109:1848–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cohen CJ, Shieh JT, Pickles RJ, Okegawa T,
Hsieh JT and Bergelson JM: The coxsackievirus and adenovirus
receptor is a transmembrane component of the tight junction. Proc
Natl Acad Sci USA. 98:15191–15196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Su Y, Liu Y, Behrens CR, Bidlingmaier S,
Lee NK, Aggarwal R, Sherbenou DW, Burlingame AL, Hann BC, Simko JP,
et al: Targeting CD46 for both adenocarcinoma and neuroendocrine
prostate cancer. JCI Insight. 3:e1214972018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Do MH, To PK, Cho YS, Kwon SY, Hwang EC,
Choi C, Cho SH, Lee SJ, Hemmi S and Jung C: Targeting CD46 enhances
anti-tumoral activity of adenovirus type 5 for bladder cancer. Int
J Mol Sci. 19:26942018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Trinh HV, Lesage G, Chennamparampil V,
Vollenweider B, Burckhardt CJ, Schauer S, Havenga M, Greber UF and
Hemmi S: Avidity binding of human adenovirus serotypes 3 and 7 to
the membrane cofactor CD46 triggers infection. J Virol.
86:1623–1637. 2012. View Article : Google Scholar :
|
|
86
|
Dhiman N, Jacobson RM and Poland GA:
Measles virus receptors: SLAM and CD46. Rev Med Virol. 14:217–229.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nestić D, Uil TG, Ma J, Roy S, Vellinga J,
Baker AH, Custers J and Majhen D: αvβ3 integrin is required for
efficient infection of epithelial cells with human adenovirus type
26. J Virol. 93:e01474–18. 2018.
|
|
88
|
Weis SM and Cheresh DA: αV integrins in
angiogenesis and cancer. Cold Spring Harb Perspect Med.
1:a0064782011. View Article : Google Scholar
|
|
89
|
Lyle C and McCormick F: Integrin
alphavbeta5 is a primary receptor for adenovirus in CAR-negative
cells. Virol J. 7:1482010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Koehler M, Petitjean SJL, Yang J,
Aravamudhan P, Somoulay X, Lo Giudice C, Poncin MA, Dumitru AC,
Dermody TS and Alsteens D: Reovirus directly engages integrin to
recruit clathrin for entry into host cells. Nat Commun.
12:21492021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ioannou M and Stanway G: Tropism of
Coxsackie virus A9 depends on the +1 position of the RGD
(arginine-glycine-aspartic acid) motif found at the C' terminus of
its VP1 capsid protein. Virus Res. 294:1982922021. View Article : Google Scholar
|
|
92
|
Stern PL and Harrop R: 5T4 oncofoetal
antigen: An attractive target for immune intervention in cancer.
Cancer Immunol Immunother. 66:415–426. 2017. View Article : Google Scholar
|
|
93
|
Scurr M, Pembroke T, Bloom A, Roberts D,
Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et
al: Effect of modified vaccinia ankara-5T4 and low-dose
cyclophosphamide on antitumor immunity in metastatic colorectal
cancer: A Randomized clinical trial. JAMA Oncol. 3:e1725792017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jian C, Jing Z, Yinhang W, Jinlong D,
Yuefen P, Quan Q and Shuwen H: Colorectal cancer and gut viruses: A
visualized analysis based on CiteSpace knowledge graph. Front
Microbiol. 14:12398182023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yi J, Quji S, Guo L, Chai Z, Kong X and
Meng J: Exploring novel strategies of oncolytic viruses and gut
microbiota to enhance CAR-T cell therapy for colorectal cancer.
Cell Immunol. 417:1050262025. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Boixareu C, Taha T, Venkadakrishnan VB, de
Bono J and Beltran H: Targeting the tumour cell surface in advanced
prostate cancer. Nat Rev Urol. 22:569–589. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Vannini A, Parenti F, Bressanin D, Barboni
C, Zaghini A, Campadelli-Fiume G and Gianni T: Towards a precision
medicine approach and in situ vaccination against prostate cancer
by PSMA-Retargeted oHSV. Viruses. 13:20852021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li Y, Shen Y, Tang T, Tang Z, Song W, Yang
Z, Zhang X, Wang M, Bai X and Liang T: Oncolytic virus combined
with traditional treatment versus traditional treatment alone in
patients with cancer: A meta-analysis. Int J Clin Oncol.
25:1901–1913. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hirooka Y, Kasuya H, Ishikawa T, Kawashima
H, Ohno E, Villalobos IB, Naoe Y, Ichinose T, Koyama N, Tanaka M,
et al: A Phase I clinical trial of EUS-guided intratumoral
injection of the oncolytic virus, HF10 for unresectable locally
advanced pancreatic cancer. BMC Cancer. 18:5962018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kim JH, Oh JY, Park BH, Lee DE, Kim JS,
Park HE, Roh MS, Je JE, Yoon JH, Thorne SH, et al: Systemic armed
oncolytic and immunologic therapy for cancer with JX-594, a
targeted poxvirus expressing GM-CSF. Mol Ther. 14:361–370. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Breitbach CJ, Parato K, Burke J, Hwang TH,
Bell JC and Kirn DH: Pexa-Vec double agent engineered vaccinia:
Oncolytic and active immunotherapeutic. Curr Opin Virol. 13:49–54.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Heo J, Breitbach CJ, Moon A, Kim CW, Patt
R, Kim MK, Lee YK, Oh SY, Woo HY, Parato K, et al: Sequential
therapy with JX-594, a targeted oncolytic poxvirus, followed by
sorafenib in hepatocellular carcinoma: Preclinical and clinical
demonstration of combination efficacy. Mol Ther. 19:1170–1179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Moehler M, Heo J, Lee HC, Tak WY, Chao Y,
Paik SW, Yim HJ, Byun KS, Baron A, Ungerechts G, et al:
Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec
in patients with advanced hepatocellular carcinoma after sorafenib
failure: A randomized multicenter Phase IIb trial (TRAVERSE).
Oncoimmunology. 8:16158172019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Abou-Alfa GK, Galle PR, Chao Y, Erinjeri
J, Heo J, Borad MJ, Luca A, Burke J, Pelusio A, Agathon D, et al:
PHOCUS: A phase 3, Randomized, open-label study of sequential
treatment with pexa-vec (JX-594) and sorafenib in patients with
advanced hepatocellular carcinoma. Liver Cancer. 13:248–264. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chaurasiya S, Yang A, Zhang Z, Lu J,
Valencia H, Kim SI, Woo Y, Warner SG, Olafsen T, Zhao Y, et al: A
comprehensive preclinical study supporting clinical trial of
oncolytic chimeric poxvirus CF33-hNIS-anti-PD-L1 to treat breast
cancer. Mol Ther Methods Clin Dev. 24:102–116. 2021. View Article : Google Scholar
|
|
106
|
Yuan Y, Egelston C, Colunga Flores O,
Chaurasiya S, Lin D, Chang H, Chong LMO, Seiz A, Shah M, Meisen WH,
et al: CF33-hNIS-anti-PD-L1 oncolytic virus followed by
trastuzumab-deruxtecan in a patient with metastatic triple negative
breast cancer: A case study. Ther Adv Med Oncol.
15:175883592312106752023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tilgase A, Olmane E, Nazarovs J, Brokāne
L, Erdmanis R, Rasa A and Alberts P: Multimodality treatment of a
colorectal cancer stage IV patient with FOLFOX-4, bevacizumab,
rigvir oncolytic virus, and surgery. Case Rep Gastroenterol.
12:457–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Filho AM, Laversanne M, Ferlay J, Colombet
M, Piñeros M, Znaor A, Parkin DM, Soerjomataram I and Bray F: The
GLOBOCAN 2022 cancer estimates: Data sources, methods, and a
snapshot of the cancer burden worldwide. Int J Cancer.
156:1336–1346. 2025. View Article : Google Scholar
|
|
109
|
Liu S, Jiang W, Sheng J, Wang L and Cui M:
Adoptive cell therapy for cancer: Combination strategies and
biomarkers. Front Immunol. 16:16037922025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Goswami S, Pauken KE, Wang L and Sharma P:
Next-generation combination approaches for immune checkpoint
therapy. Nat Immunol. 25:2186–2199. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xie B, Zhang L, Hu W, Fan M, Jiang N, Duan
Y, Jing D, Xiao W, Fragoso RC, Lam KS, et al: Dual blockage of
STAT3 and ERK1/2 eliminates radioresistant GBM cells. Redox Biol.
24:1011892019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nagathihalli NS, Castellanos JA,
Lamichhane P, Messaggio F, Shi C, Dai X, Rai P, Chen X, VanSaun MN
and Merchant NB: Inverse correlation of STAT3 and MEK signaling
mediates resistance to RAS pathway inhibition in pancreatic cancer.
Cancer Res. 78:6235–6246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Vultur A, Villanueva J, Krepler C, Rajan
G, Chen Q, Xiao M, Li L, Gimotty PA, Wilson M, Hayden J, et al: MEK
inhibition affects STAT3 signaling and invasion in human melanoma
cell lines. Oncogene. 33:1850–1861. 2014. View Article : Google Scholar
|
|
114
|
Appleton E, Chiocca EA, Ungerechts G,
Melcher A and Vile R: Oncolytic viruses as anticancer agents:
clinical progress and remaining challenges. Lancet. 406:1295–1312.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hossain MA: Targeting the RAS upstream and
downstream signaling pathway for cancer treatment. Eur J Pharmacol.
979:1767272024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zou J, Han W, Hu Y, Zeng C, Li J, Lei W,
Cao J, Fei Q, Shao M, Yi J, et al: Gene mutation, clinical
characteristics and pathology in resectable lung adenocarcinoma.
World J Surg Oncol. 23:162025. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yang T, Li W, Huang T and Zhou J: Genetic
testing enhances the precision diagnosis and treatment of breast
cancer. Int J Mol Sci. 24:166072023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chaudagar K, Hieromnimon HM, Khurana R,
Labadie B, Hirz T, Mei S, Hasan R, Shafran J, Kelley A, Apostolov
E, et al: Reversal of lactate and PD-1-mediated macrophage
immunosuppression controls growth of PTEN/p53-deficient prostate
cancer. Clin Cancer Res. 29:1952–1968. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhao F, Jiang X, Li Y, Huang T, Xiahou Z,
Nie W and Li Q: Characterizing tumor biology and immune
microenvironment in high-grade serous ovarian cancer via
single-cell RNA sequencing: Insights for targeted and personalized
immunotherapy strategies. Front Immunol. 15:15001532025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cooper AJ, Sequist LV and Lin JJ:
Third-generation EGFR and ALK inhibitors: Mechanisms of resistance
and management. Nat Rev Clin Oncol. 19:499–514. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Taverna JA, Hung CN, DeArmond DT, Chen M,
Lin CL, Osmulski PA, Gaczynska ME, Wang CM, Lucio ND, Chou CW, et
al: Single-cell proteomic profiling identifies combined AXL and
JAK1 inhibition as a novel therapeutic strategy for lung cancer.
Cancer Res. 80:1551–1563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yadav M, Sharma A, Patne K, Tabasum S,
Suryavanshi J, Rawat L, Machaalani M, Eid M, Singh RP, Choueiri TK,
et al: AXL signaling in cancer: From molecular insights to targeted
therapies. Signal Transduct Target Ther. 10:372025. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shen M, Jiang X, Peng Q, Oyang L, Ren Z,
Wang J, Peng M, Zhou Y, Deng X and Liao Q: The cGAS-STING pathway
in cancer immunity: mechanisms, challenges, and therapeutic
implications. J Hematol Oncol. 18:402025. View Article : Google Scholar
|
|
124
|
Xia T, Konno H, Ahn J and Barber GN:
Deregulation of STING signaling in colorectal carcinoma constrains
DNA damage responses and correlates with tumorigenesis. Cell Rep.
14:282–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xia T, Konno H and Barber GN: Recurrent
loss of STING signaling in melanoma correlates with susceptibility
to viral oncolysis. Cancer Res. 76:6747–6759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
de Queiroz N, Xia T, Konno H and Barber
GN: Ovarian cancer cells commonly exhibit defective STING signaling
which affects sensitivity to viral oncolysis. Mol Cancer Res.
17:974–986. 2019. View Article : Google Scholar :
|
|
127
|
Meric-Bernstam F, Sweis RF, Hodi FS,
Messersmith WA, Andtbacka RHI, Ingham M, Lewis N, Chen X, Pelletier
M, Chen X, et al: Phase I dose-escalation trial of MIW815
(ADU-S100), an intratumoral STING agonist, in patients with
advanced/metastatic solid tumors or lymphomas. Clin Cancer Res.
28:677–688. 2022. View Article : Google Scholar
|
|
128
|
Chang W, Altman MD, Lesburg CA, Perera SA,
Piesvaux JA, Schroeder GK, Wyss DF, Cemerski S, Chen Y, DiNunzio E,
et al: Discovery of MK-1454: A potent cyclic dinucleotide
stimulator of interferon genes agonist for the treatment of cancer.
J Med Chem. 65:5675–5689. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Song Z, Wang X, Zhang Y, Gu W, Shen A,
Ding C, Li H, Xiao R, Geng M, Xie Z and Zhang A: Structure-activity
relationship study of amidobenzimidazole analogues leading to
potent and systemically administrable stimulator of interferon gene
(STING) agonists. J Med Chem. 64:1649–1669. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sibal PA, Matsumura S, Ichinose T,
Bustos-Villalobos I, Morimoto D, Eissa IR, Abdelmoneim M, Aboalela
MAM, Mukoyama N, Tanaka M, et al: STING activator 2'3'-cGAMP
enhanced HSV-1-based oncolytic viral therapy. Mol Oncol.
18:1259–1277. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Thoresen D, Wang W, Galls D, Guo R, Xu L
and Pyle AM: The molecular mechanism of RIG-I activation and
signaling. Immunol Rev. 304:154–168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Berry N, Suspène R, Caval V, Khalfi P,
Beauclair G, Rigaud S, Blanc H, Vignuzzi M, Wain-Hobson S and
Vartanian JP: Herpes simplex virus type 1 infection disturbs the
mitochondrial network, leading to type I interferon production
through the RNA polymerase III/RIG-I pathway. mBio.
12:e02557212021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Farhangnia P, Khorramdelazad H, Nickho H
and Delbandi AA: Current and future immunotherapeutic approaches in
pancreatic cancer treatment. J Hematol Oncol. 17:402024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Dan J, Cai J, Zhong Y, Wang C, Huang S,
Zeng Y, Fan Z, Xu C, Hu L, Zhang J, et al: Oncolytic virus M1
functions as a bifunctional checkpoint inhibitor to enhance the
antitumor activity of DC vaccine. Cell Rep Med. 4:1012292023.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shen KY, Zhu Y, Xie SZ and Qin LX:
Immunosuppressive tumor microenvironment and immunotherapy of
hepatocellular carcinoma: Current status and prospectives. J
Hematol Oncol. 17:252024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang JW, Feng Q, Liu JH and Xun JJ:
Opportunities, challenges, and future perspectives of oncolytic
virus therapy for malignant melanoma. Front Immunol.
16:16536832025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang JW, Liu JH, Liu YL, Xu WZ and Zhang
ZB: Oncolytic virus therapy in the elderly: Immune frailty,
challenges, and perspectives. Front Immunol. 16:16866592025.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lang SI, Giese NA, Rommelaere J, Dinsart C
and Cornelis JJ: Humoral immune responses against minute virus of
mice vectors. J Gene Med. 8:1141–1150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hirasawa K, Nishikawa SG, Norman KL,
Coffey MC, Thompson BG, Yoon CS, Waisman DM and Lee PW: Systemic
reovirus therapy of metastatic cancer in immune-competent mice.
Cancer Res. 63:348–353. 2003.PubMed/NCBI
|
|
140
|
Chen G, Yuan Y, Li Y, He Q, Qin Z, Hu H,
Gao C, Xu Z, Xu Q, Gao Q and Li F: Enhancing oncolytic virus
efficiency with methionine and N-(3-aminoprolil)methacrylamide
modified acrylamide cationic block polymer. J Mater Chem B.
12:3741–3750. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Perera AS and Coppens MO: Re-designing
materials for biomedical applications: From biomimicry to
nature-inspired chemical engineering. Philos Trans A Math Phys Eng
Sci. 377:201802682019.PubMed/NCBI
|
|
142
|
Pang L, Zhang C, Qin J, Han L, Li R, Hong
C, He H and Wang J: A novel strategy to achieve effective drug
delivery: Exploit cells as carrier combined with nanoparticles.
Drug Deliv. 24:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhang Z, Yang N, Xu L, Lu H, Chen Y, Wang
Z, Lu Q, Zhong K, Zhu Z, Wang G, et al: Systemic delivery of
oncolytic herpes virus using CAR-T cells enhances targeting of
antitumor immuno-virotherapy. Cancer Immunol Immunother.
73:1732024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Reale A, Calistri A and Altomonte J:
Giving oncolytic viruses a free ride: Carrier cells for oncolytic
virotherapy. Pharmaceutics. 13:21922021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ghasemi Darestani N, Gilmanova AI,
Al-Gazally ME, Zekiy AO, Ansari MJ, Zabibah RS, Jawad MA, Al-Shalah
SAJ, Rizaev JA, Alnassar YS, et al: Mesenchymal stem cell-released
oncolytic virus: An innovative strategy for cancer treatment. Cell
Commun Signal. 21:432023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Collet G, Grillon C, Nadim M and Kieda C:
Trojan horse at cellular level for tumor gene therapies. Gene.
525:208–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mader EK, Maeyama Y, Lin Y, Butler GW,
Russell HM, Galanis E, Russell SJ, Dietz AB and Peng KW:
Mesenchymal stem cell carriers protect oncolytic measles viruses
from antibody neutralization in an orthotopic ovarian cancer
therapy model. Clin Cancer Res. 15:7246–7255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hakkarainen T, Särkioja M, Lehenkari P,
Miettinen S, Ylikomi T, Suuronen R, Desmond RA, Kanerva A and
Hemminki A: Human mesenchymal stem cells lack tumor tropism but
enhance the antitumor activity of oncolytic adenoviruses in
orthotopic lung and breast tumors. Hum Gene Ther. 18:627–641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Cook M and Chauhan A: Clinical application
of oncolytic viruses: A systematic review. Int J Mol Sci.
21:75052020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Rathod LS, Sakle NS and Mokale SN: KRAS
inhibitors in drug resistance and potential for combination
therapy. Tumori. 111:20–40. 2025. View Article : Google Scholar
|
|
151
|
Rahimi A, Baghernejadan Z, Hazrati A,
Malekpour K, Samimi LN, Najafi A, Falak R and Khorramdelazad H:
Combination therapy with immune checkpoint inhibitors in colorectal
cancer: Challenges, resistance mechanisms, and the role of
microbiota. Biomed Pharmacother. 186:1180142025. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
He X, Deng H, Liu W, Hu L and Tan X:
Advances in understanding drug resistance mechanisms and innovative
clinical treatments for melanoma. Curr Treat Options Oncol.
25:1615–1633. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Xuan Y, Yan W, Wang R, Wang X, Guo Y, Dun
H, Huan Z, Xu L, Han R, Sun X, et al: GM-CSF and IL-21-armed
oncolytic vaccinia virus significantly enhances anti-tumor activity
and synergizes with anti-PD1 immunotherapy in pancreatic cancer.
Front Immunol. 15:15066322025. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ottolino-Perry K, Tang N, Head R, Ng C,
Arulanandam R, Angarita FA, Acuna SA, Chen Y, Bell J, Dacosta RS
and McCart JA: Tumor vascularization is critical for oncolytic
vaccinia virus treatment of peritoneal carcinomatosis. Int J
Cancer. 134:717–730. 2014. View Article : Google Scholar
|
|
155
|
Arulanandam R, Batenchuk C, Angarita FA,
Ottolino-Perry K, Cousineau S, Mottashed A, Burgess E, Falls TJ, De
Silva N, Tsang J, et al: VEGF-mediated induction of PRD1-BF1/Blimp1
expression sensitizes tumor vasculature to oncolytic virus
infection. Cancer Cell. 28:210–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kurozumi K, Hardcastle J, Thakur R, Yang
M, Christoforidis G, Fulci G, Hochberg FH, Weissleder R, Carson W,
Chiocca EA and Kaur B: Effect of tumor microenvironment modulation
on the efficacy of oncolytic virus therapy. J Natl Cancer Inst.
99:1768–1781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Maliepaard M, Faber YS and van Bussel MTJ:
Reported hepatotoxicity and hepatotoxicity guidance in the product
information of protein kinase inhibitors in oncology registered at
the European medicines agency. Pharmacol Res Perspect.
11:e010672023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Godoy P, Hewitt NJ, Albrecht U, Andersen
ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger
J, et al: Recent advances in 2D and 3D in vitro systems using
primary hepatocytes, alternative hepatocyte sources and
non-parenchymal liver cells and their use in investigating
mechanisms of hepatotoxicity, cell signaling and ADME. Arch
Toxicol. 87:1315–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Rock EP, Goodman V, Jiang JX, Mahjoob K,
Verbois SL, Morse D, Dagher R, Justice R and Pazdur R: Food and
drug administration drug approval summary: Sunitinib malate for the
treatment of gastrointestinal stromal tumor and advanced renal cell
carcinoma. Oncologist. 12:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fukuhara H, Ino Y and Todo T: Oncolytic
virus therapy: A new era of cancer treatment at dawn. Cancer Sci.
107:1373–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
LaFargue CJ, Dal Molin GZ, Sood AK and
Coleman RL: Exploring and comparing adverse events between PARP
inhibitors. Lancet Oncol. 20:e15–e28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Arrillaga-Romany I, Gardner SL, Odia Y,
Aguilera D, Allen JE, Batchelor T, Butowski N, Chen C, Cloughesy T,
Cluster A, et al: ONC201 (Dordaviprone) in recurrent H3 K27M-Mutant
diffuse midline glioma. J Clin Oncol. 42:1542–1552. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Peters C, Paget M, Tshilenge KT, Saha D,
Antoszczyk S, Baars A, Frost T, Martuza RL, Wakimoto H and Rabkin
SD: Restriction of replication of oncolytic herpes simplex virus
with a deletion of γ34.5 in glioblastoma stem-like cells. J Virol.
92:e00246–18. 2018. View Article : Google Scholar :
|
|
164
|
Nakashima H, Nguyen T, Kasai K, Passaro C,
Ito H, Goins WF, Shaikh I, Erdelyi R, Nishihara R, Nakano I, et al:
Toxicity and efficacy of a novel GADD34-expressing oncolytic HSV-1
for the treatment of experimental glioblastoma. Clin Cancer Res.
24:2574–2584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Balathasan L, Tang VA, Yadollahi B, Brun
J, Labelle M, Lefebvre C, Swift SL and Stojdl DF: Activating
peripheral innate immunity enables safe and effective oncolytic
virotherapy in the brain. Mol Ther Oncolytics. 7:45–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Takata F, Nakagawa S, Matsumoto J and
Dohgu S: Blood-Brain barrier dysfunction amplifies the development
of neuroinflammation: Understanding of cellular events in brain
microvascular endothelial cells for prevention and treatment of BBB
Dysfunction. Front Cell Neurosci. 15:6618382021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhuang H, Shi S, Yuan Z and Chang JY:
Bevacizumab treatment for radiation brain necrosis: mechanism,
efficacy and issues. Mol Cancer. 18:212019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Seet RC, Rabinstein AA, Lindell PE, Uhm JH
and Wijdicks EF: Cerebrovascular events after bevacizumab
treatment: an early and severe complication. Neurocrit Care.
15:421–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Zhou BX and Li Y: Significance of
desmoglein-2 on cell malignant behaviors via mediating MAPK
signaling in cervical cancer. Kaohsiung J Med Sci. 36:336–343.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Sun R, Ma C, Wang W and Yang S:
Upregulation of desmoglein 2 and its clinical value in lung
adenocarcinoma: A comprehensive analysis by multiple bioinformatics
methods. PeerJ. 8:e84202020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Han CP, Yu YH, Wang AG, Tian Y, Zhang HT,
Zheng ZM and Liu YS: Desmoglein-2 overexpression predicts poor
prognosis in hepatocellular carcinoma patients. Eur Rev Med
Pharmacol Sci. 22:5481–5489. 2018.PubMed/NCBI
|
|
172
|
Cai F, Zhu Q, Miao Y, Shen S, Su X and Shi
Y: Desmoglein-2 is overexpressed in non-small cell lung cancer
tissues and its knockdown suppresses NSCLC growth by regulation of
p27 and CDK2. J Cancer Res Clin Oncol. 143:59–69. 2017. View Article : Google Scholar
|
|
173
|
Tan LY, Mintoff C, Johan MZ, Ebert BW,
Fedele C, Zhang YF, Szeto P, Sheppard KE, McArthur GA, Foster-Smith
E, et al: Desmoglein 2 promotes vasculogenic mimicry in melanoma
and is associated with poor clinical outcome. Oncotarget.
7:46492–46508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kamekura R, Kolegraff KN, Nava P, Hilgarth
RS, Feng M, Parkos CA and Nusrat A: Loss of the desmosomal cadherin
desmoglein-2 suppresses colon cancer cell proliferation through
EGFR signaling. Oncogene. 33:4531–4536. 2014. View Article : Google Scholar :
|
|
175
|
Brennan D and Mahoney MG: Increased
expression of Dsg2 in malignant skin carcinomas: A
tissue-microarray based study. Cell Adh Migr. 3:148–154. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen L, Liu X, Zhang J and Liu Y, Gao A,
Xu Y, Lin Y, Du Q, Zhu Z, Hu Y and Liu Y: Characterization of
desmoglein 2 expression in ovarian serous tumors and its prognostic
significance in high-grade serous carcinoma. Int J Clin Exp Pathol.
11:4977–4986. 2018.PubMed/NCBI
|
|
177
|
Ramani VC, Hennings L and Haun RS:
Desmoglein 2 is a substrate of kallikrein 7 in pancreatic cancer.
BMC Cancer. 8:3732008. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Biedermann K, Vogelsang H, Becker I,
Plaschke S, Siewert JR, Höfler H and Keller G: Desmoglein 2 is
expressed abnormally rather than mutated in familial and sporadic
gastric cancer. J Pathol. 207:199–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Mundy RM, Baker AT, Bates EA, Cunliffe TG,
Teijeira-Crespo A, Moses E, Rizkallah PJ and Parker AL: Broad
sialic acid usage amongst species D human adenovirus. Npj Viruses.
1:12023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Rodrigues E and Macauley MS:
Hypersialylation in cancer: Modulation of inflammation and
therapeutic opportunities. Cancers (Basel). 10:2072018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hu K, He S, Xiao J, Li M, Luo S, Zhang M
and Hu Q: Interaction between herpesvirus entry mediator and HSV-2
glycoproteins mediates HIV-1 entry of HSV-2-infected epithelial
cells. J Gen Virol. 98:2351–2361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Montgomery RI, Warner MS, Lum BJ and Spear
PG: Herpes simplex virus-1 entry into cells mediated by a novel
member of the TNF/NGF receptor family. Cell. 87:427–436. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ren S, Tian Q, Amar N, Yu H, Rivard CJ,
Caldwell C, Ng TL, Tu M, Liu Y, Gao D, et al: The immune
checkpoint, HVEM may contribute to immune escape in non-small cell
lung cancer lacking PD-L1 expression. Lung Cancer. 125:115–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Tsang JYS, Chan KW, Ni YB, Hlaing T, Hu J,
Chan SK, Cheung SY and Tse GM: Expression and clinical significance
of herpes virus entry mediator (HVEM) in breast cancer. Ann Surg
Oncol. 24:4042–4050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Lan X, Li S, Gao H, Nanding A, Quan L,
Yang C, Ding S and Xue Y: Increased BTLA and HVEM in gastric cancer
are associated with progression and poor prognosis. Onco Targets
Ther. 10:919–926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Migita K, Sho M, Shimada K, Yasuda S,
Yamato I, Takayama T, Matsumoto S, Wakatsuki K, Hotta K, Tanaka T,
et al: Significant involvement of herpesvirus entry mediator in
human esophageal squamous cell carcinoma. Cancer. 120:808–817.
2014. View Article : Google Scholar
|
|
187
|
Ahn AR, Noh SJ, Hussein UK, Park HS, Chung
MJ, Lee H, Moon WS, Kang MJ, Kim HJ, Lee NR, et al: FAM83H and
Nectin1 expression are related with survival and relapse of bladder
urothelial carcinoma patients. BMC Urol. 21:1432021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tampakis A, Tampaki EC, Nonni A, Droeser
R, Posabella A, Tsourouflis G, Kontzoglou K, Patsouris E, von Flüe
M and Kouraklis G: Nectin-1 expression in colorectal cancer: Is
there a group of patients with high risk for early disease
recurrence? Oncology. 96:318–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Cocchi F, Menotti L, Dubreuil P, Lopez M
and Campadelli-Fiume G: Cell-to-cell spread of wild-type herpes
simplex virus type 1, but not of syncytial strains, is mediated by
the immunoglobulin-like receptors that mediate virion entry,
nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB). J Virol.
74:3909–3917. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Girgis NM, Dehaven BC, Fan X, Viner KM,
Shamim M and Isaacs SN: Cell surface expression of the vaccinia
virus complement control protein is mediated by interaction with
the viral A56 protein and protects infected cells from complement
attack. J Virol. 82:4205–4214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Marques C, Reis CA, Vivès RR and Magalhães
A: Heparan sulfate biosynthesis and sulfation profiles as
modulators of cancer signalling and progression. Front Oncol.
11:7787522021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Li C, Luo P, Guo F, Jia X, Shen M, Zhang
T, Wang S and Du T: Identification of HSPG2 as a bladder pro-tumor
protein through NID1/AKT signaling. Cancer Cell Int. 24:3452024.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Lambrecht V, Le Bourhis X, Toillon RA,
Boilly B and Hondermarck H: Alterations in both heparan sulfate
proteoglycans and mitogenic activity of fibroblast growth factor-2
are triggered by inhibitors of proliferation in normal and breast
cancer epithelial cells. Exp Cell Res. 245:239–244. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yi B, Qiu Y, Ji W, Wei M, Liu C, Peng Z,
Zhang Y, Quan Z, Tang Z and Su C: Desulfation of cell surface HSPG
is an effective strategy for the treatment of gallbladder
carcinoma. Cancer Lett. 381:349–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Xiong R, Long Q, Zhang X, Xu J, Liu Y,
Xiong L, Yang S, Feng G, Song G and Liu K: HOXD11 upregulates JAM-A
and exerts oncogenic properties via NF-κB signaling pathway in
esophageal squamous cell carcinoma. Hum Cell. 36:244–257. 2023.
View Article : Google Scholar
|
|
196
|
Aravamudhan P, Guzman-Cardozo C, Urbanek
K, Welsh OL, Konopka-Anstadt JL, Sutherland DM and Dermody TS: The
murine neuronal receptor NgR1 is dispensable for reovirus
pathogenesis. J Virol. 96:e00055222022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Rosager AM, Sørensen MD, Dahlrot RH, Boldt
HB, Hansen S, Lathia JD and Kristensen BW: Expression and
prognostic value of JAM-A in gliomas. J Neurooncol. 135:107–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ikeo K, Oshima T, Shan J, Matsui H, Tomita
T, Fukui H, Watari J and Miwa H: Junctional adhesion molecule-A
promotes proliferation and inhibits apoptosis of gastric cancer.
Hepatogastroenterology. 62:540–545. 2015.PubMed/NCBI
|
|
199
|
McSherry EA, McGee SF, Jirstrom K, Doyle
EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM and Gallagher WM:
JAM-A expression positively correlates with poor prognosis in
breast cancer patients. Int J Cancer. 125:1343–1351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Marcq I, Nyga R, Cartier F, Amrathlal RS,
Ossart C, Ouled-Haddou H, Ghamlouch H, Galmiche A, Chatelain D,
Lamotte L, et al: Identification of SLAMF3 (CD229) as an inhibitor
of hepatocellular carcinoma cell proliferation and tumour
progression. PLoS One. 8:e829182013. View Article : Google Scholar :
|
|
201
|
Agresta L, Lehn M, Lampe K, Cantrell R,
Hennies C, Szabo S, Wise-Draper T, Conforti L, Hoebe K and Janssen
EM: CD244 represents a new therapeutic target in head and neck
squamous cell carcinoma. J Immunother Cancer. 8:e0002452020.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhang J, Zhu Y, Wang Q, Kong Y, Sheng H,
Guo J, Xu J and Dai B: Poliovirus receptor CD155 is up-regulated in
muscle-invasive bladder cancer and predicts poor prognosis. Urol
Oncol. 38:41.e11–41.e18. 2020. View Article : Google Scholar
|
|
203
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Clinical significance of CD155 expression in human pancreatic
cancer. Anticancer Res. 35:2287–2297. 2015.PubMed/NCBI
|
|
204
|
Li YC, Zhou Q, Song QK, Wang RB, Lyu S,
Guan X, Zhao YJ and Wu JP: Overexpression of an immune checkpoint
(CD155) in breast cancer associated with prognostic significance
and exhausted tumor-infiltrating lymphocytes: A cohort study. J
Immunol Res. 2020:39489282020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Zhuo B, Li Y, Gu F, Li Z, Sun Q, Shi Y,
Shen Y, Zhang F, Wang R and Wang X: Overexpression of CD155 relates
to metastasis and invasion in osteosarcoma. Oncol Lett.
15:7312–7318. 2018.PubMed/NCBI
|
|
206
|
Murakami D, Matsuda K, Iwamoto H, Mitani
Y, Mizumoto Y, Nakamura Y, Matsuzaki I, Iwamoto R, Takahashi Y,
Kojima F, et al: Prognostic value of CD155/TIGIT expression in
patients with colorectal cancer. PLoS One. 17:e02659082022.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Finkelshtein D, Werman A, Novick D, Barak
S and Rubinstein M: LDL receptor and its family members serve as
the cellular receptors for vesicular stomatitis virus. Proc Natl
Acad Sci USA. 110:7306–7311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Behrouj H, Erfani M and Mokarram P:
Examining the expression of low-density lipoprotein receptor (LDLR)
and low-density lipoprotein receptor-related protein 6 (LRP6) genes
in breast cancer cell lines. Mol Biol Res Commun. 13:85–88.
2024.PubMed/NCBI
|
|
209
|
Zhang GM, Chen W, Yao Y, Luo L and Sun LJ:
LDLR promotes growth and invasion in renal cell carcinoma and
activates the EGFR pathway. Neoplasma. 69:113–122. 2022. View Article : Google Scholar
|
|
210
|
Tang S, Chen K, Zheng F, Fu Z, Niu Y, Liu
X, Ni H, Yuan X, Cui Z, Lu W, et al: High serum LDL promotes EMT
and stemness through LDLR/FOXQ1/NF-κB1 pathway in epithelial
ovarian cancer. Oncogene. 44:4587–4600. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Örbom A, Evans-Axelsson S, Jansson B,
Vilhelmsson Timmermand O, Tran TA, Bjartell A and Strand SE:
Intratumoral distribution and pharmacokinetics of the radiolabeled
ICAM-1 targeting monoclonal antibody R6.5 in a prostate cancer
mouse model. Nuklearmedizin. 64:163–169. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gultekin O, Gonzalez-Molina J, Sarhan D,
Groes-Kofoed N, Hassan MU, Lehti K and Salehi S: Systemic and
tumor-specific inflammatory markers VCAM-1 and ICAM-1 as indicators
of extent of surgery and oncologic outcome in advanced ovarian
cancer. Transl Oncol. 59:1024622025. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Chen YH, Chu CC, Liu JF, Lai HS and Chen
YT: C-X-C motif ligand 1 induces cell migration by upregulating
ICAM-1 expression by activating PI3K/Akt and NF-κB signaling
pathway in liver cancer. Adv Biol (Weinh). 9:e24002952025.
View Article : Google Scholar
|
|
214
|
Annels NE, Mansfield D, Arif M,
Ballesteros-Merino C, Simpson GR, Denyer M, Sandhu SS, Melcher AA,
Harrington KJ, Davies B, et al: Phase I trial of an ICAM-1-targeted
immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent
against non muscle-invasive bladder cancer. Clin Cancer Res.
25:5818–5831. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Tempia-Caliera AA, Horvath LZ, Zimmermann
A, Tihanyi TT, Korc M, Friess H and Büchler MW: Adhesion molecules
in human pancreatic cancer. J Surg Oncol. 79:93–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Wang Q, Yang Q, Liu C, Wang G, Song H,
Shang G, Peng R, Qu X, Liu S, Cui Y, et al: Molecular basis of
differential receptor usage for naturally occurring CD55-binding
and -nonbinding coxsackievirus B3 strains. Proc Natl Acad Sci USA.
119:e21185901192022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Koretz K, Brüderlein S, Henne C and Möller
P: Decay-accelerating factor (DAF, CD55) in normal colorectal
mucosa, adenomas and carcinomas. Br J Cancer. 66:810–814. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Madjd Z, Durrant LG, Bradley R, Spendlove
I, Ellis IO and Pinder SE: Loss of CD55 is associated with
aggressive breast tumors. Clin Cancer Res. 10:2797–2803. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Mäenpää A, Junnikkala S, Hakulinen J,
Timonen T and Meri S: Expression of complement membrane regulators
membrane cofactor protein (CD46), decay accelerating factor (CD55),
and protectin (CD59) in human malignant gliomas. Am J Pathol.
148:1139–1152. 1996.PubMed/NCBI
|
|
220
|
Mustafa T, Klonisch T, Hombach-Klonisch S,
Kehlen A, Schmutzler C, Koehrle J, Gimm O, Dralle H and Hoang-Vu C:
Expression of CD97 and CD55 in human medullary thyroid carcinomas.
Int J Oncol. 24:285–294. 2004.PubMed/NCBI
|
|
221
|
Yamayoshi S, Iizuka S, Yamashita T,
Minagawa H, Mizuta K, Okamoto M, Nishimura H, Sanjoh K, Katsushima
N, Itagaki T, et al: Human SCARB2-dependent infection by
coxsackievirus A7, A14, and A16 and enterovirus 71. J Virol.
86:5686–5696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Zhang D, Fang J, Shan J, Xu L, Wu Y, Lu B,
Zhang X, Wang C, Sun P and Wang Q: SCARB2 associates with
tumor-infiltrating neutrophils and predicts poor prognosis in
breast cancer. Breast Cancer Res Treat. 207:15–24. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Wang F, Gao Y, Xue S, Zhao L, Jiang H,
Zhang T, Li Y, Zhao C, Wu F, Siqin T, et al: SCARB2 drives
hepatocellular carcinoma tumor initiating cells via enhanced MYC
transcriptional activity. Nat Commun. 14:59172023. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Hance KW, Rogers CJ, Zaharoff DA, Canter
D, Schlom J and Greiner JW: The antitumor and immunoadjuvant
effects of IFN-alpha in combination with recombinant poxvirus
vaccines. Clin Cancer Res. 15:2387–2396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Duggan MC, Jochems C, Donahue RN, Richards
J, Karpa V, Foust E, Paul B, Brooks T, Tridandapani S, Olencki T,
et al: A phase I study of recombinant (r) vaccinia-CEA(6D)-TRICOM
and rFowlpox-CEA(6D)-TRICOM vaccines with GM-CSF and IFN-α-2b in
patients with CEA-expressing carcinomas. Cancer Immunol Immunother.
65:1353–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Qi C, Liu C, Peng Z, Zhang Y, Wei J, Qiu
W, Zhang X, Pan H, Niu Z, Qiu M, et al: Claudin-18 isoform
2-specific CAR T-cell therapy (satri-cel) versus treatment of
physician's choice for previously treated advanced gastric or
gastro-oesophageal junction cancer (CT041-ST-01): A randomised,
open-label, phase 2 trial. Lancet. 405:2049–2060. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Liu S, Li F, Deng L, Ma Q, Lu W, Zhao Z,
Liu H, Zhou Y, Hu M, Wang H, et al: Claudin18.2 bispecific T cell
engager armed oncolytic virus enhances antitumor effects against
pancreatic cancer. Mol Ther Oncolytics. 30:275–285. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Yoon AR, Hong J, Kim M and Yun CO:
Hepatocellular carcinoma-targeting oncolytic adenovirus overcomes
hypoxic tumor microenvironment and effectively disperses through
both central and peripheral tumor regions. Sci Rep. 8:22332018.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Cordeiro R, Oliveira D, Santo D, Coelho J
and Faneca H: Mesoporous silica-glycopolymer hybrid nanoparticles
for dual targeted chemotherapy and gene therapy to liver cancer
cells. Int J Pharm. 675:1255532025. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Deng Y, Yang B, Yang Z, Xiao H, Zou Y, Zou
C, Yang S, Sun X, Wang Y, Bai J, et al: Engineered E. coli OMVs
carrying the membrane-binding hGC33 fragment precisely target liver
cancer and effectively treat tumor. Int J Nanomedicine.
20:6573–6590. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
West CE, Mirshahi UL, Ruth KS, Sharp LN,
Arni AM, Turnbull C, Wright CF, Vaidya B, Owens MM, Carey DJ and
Patel KA: Medullary thyroid cancer risk and mortality in carriers
of incidentally identified MEN2A RET variants. JAMA Netw Open.
8:e25179372025. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Cañizo CG, Guerrero-Ramos F, Perez Escavy
M, Lodewijk I, Suárez-Cabrera C, Morales L, Nunes SP,
Munera-Maravilla E, Rubio C, Sánchez R, et al: Characterisation of
the tumour microenvironment and PD-L1 granularity reveals the
prognostic value of cancer-associated myofibroblasts in
non-invasive bladder cancer. Oncoimmunology. 14:24382912025.
View Article : Google Scholar
|
|
233
|
Okato A, Utsumi T, Ranieri M, Zheng X,
Zhou M, Pereira LD, Chen T, Kita Y, Wu D, Hyun H, et al: FGFR
inhibition augments anti-PD-1 efficacy in murine FGFR3-mutant
bladder cancer by abrogating immunosuppression. J Clin Invest.
134:e1692412024. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Cheng L, Lopez-Beltran A, Massari F,
MacLennan GT and Montironi R: Molecular testing for BRAF mutations
to inform melanoma treatment decisions: A move toward precision
medicine. Mod Pathol. 31:24–38. 2018. View Article : Google Scholar :
|
|
235
|
Rosenkrans ZT, Erbe AK, Clemons NB, Feils
AS, Medina-Guevara Y, Jeffery JJ, Barnhart TE, Engle JW, Sondel PM
and Hernandez R: ImmunoPET demonstrates that Co-Targeting GD2 and
B7-H3 with bispecific antibodies enhances tumor selectivity in
preclinical melanoma models. Bioconjug Chem. 36:1595–1603. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Krug C, Birkholz K, Paulus A, Schwenkert
M, Schmidt P, Hoffmann N, Hombach A, Fey G, Abken H, Schuler G, et
al: Stability and activity of MCSP-specific chimeric antigen
receptors (CARs) depend on the scFv antigen-binding domain and the
protein backbone. Cancer Immunol Immunother. 64:1623–1635. 2015.
View Article : Google Scholar : PubMed/NCBI
|