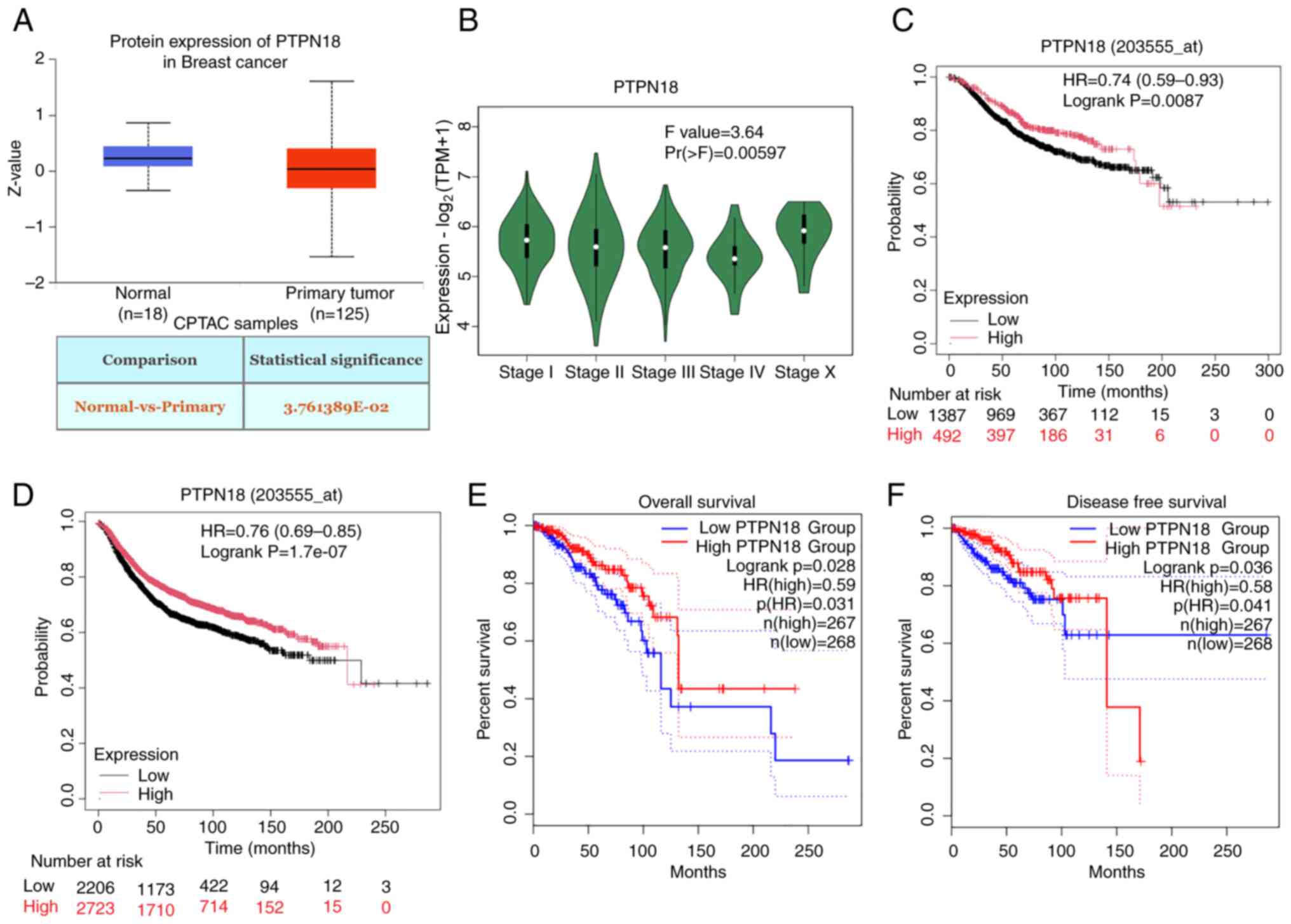
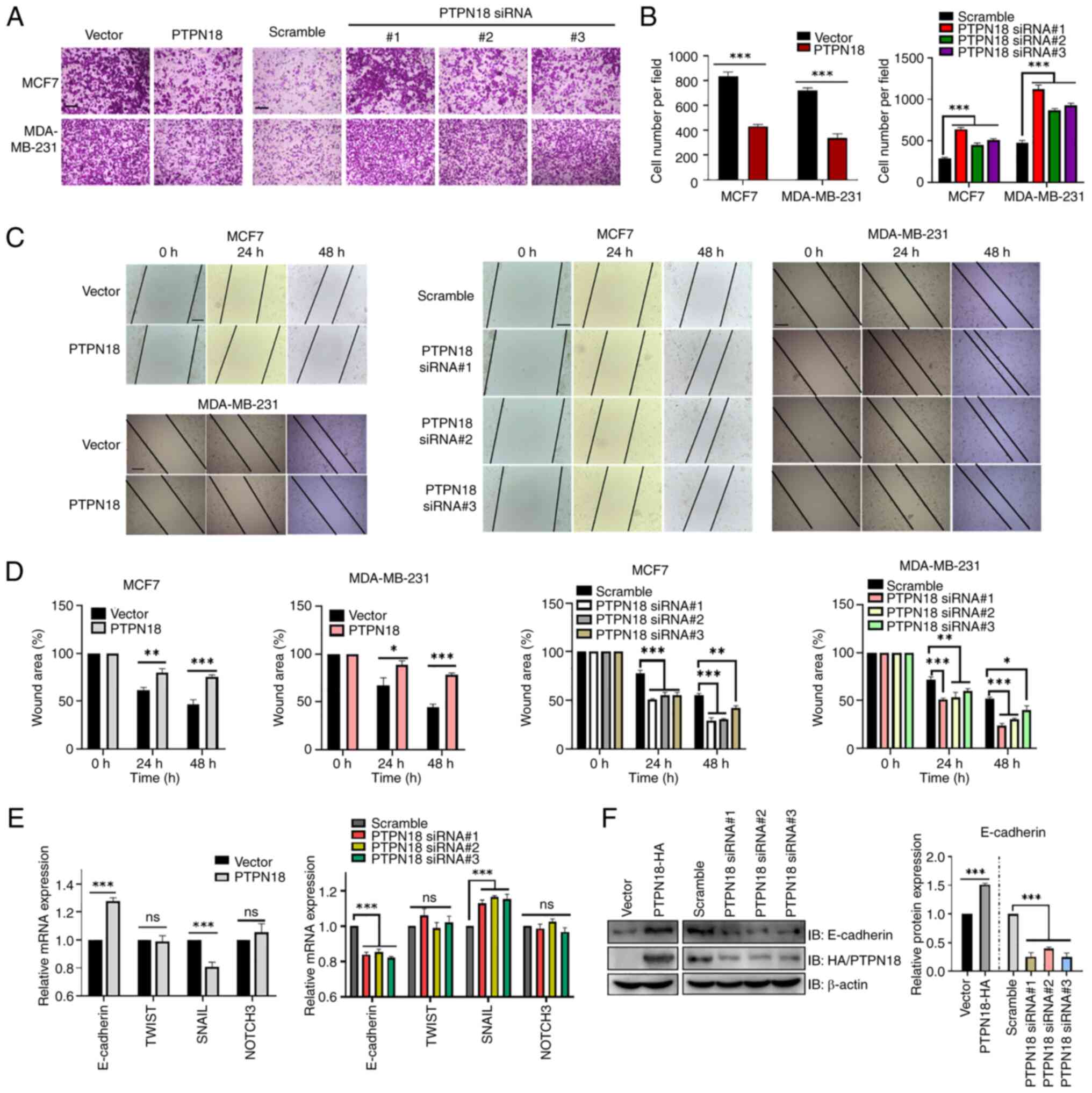
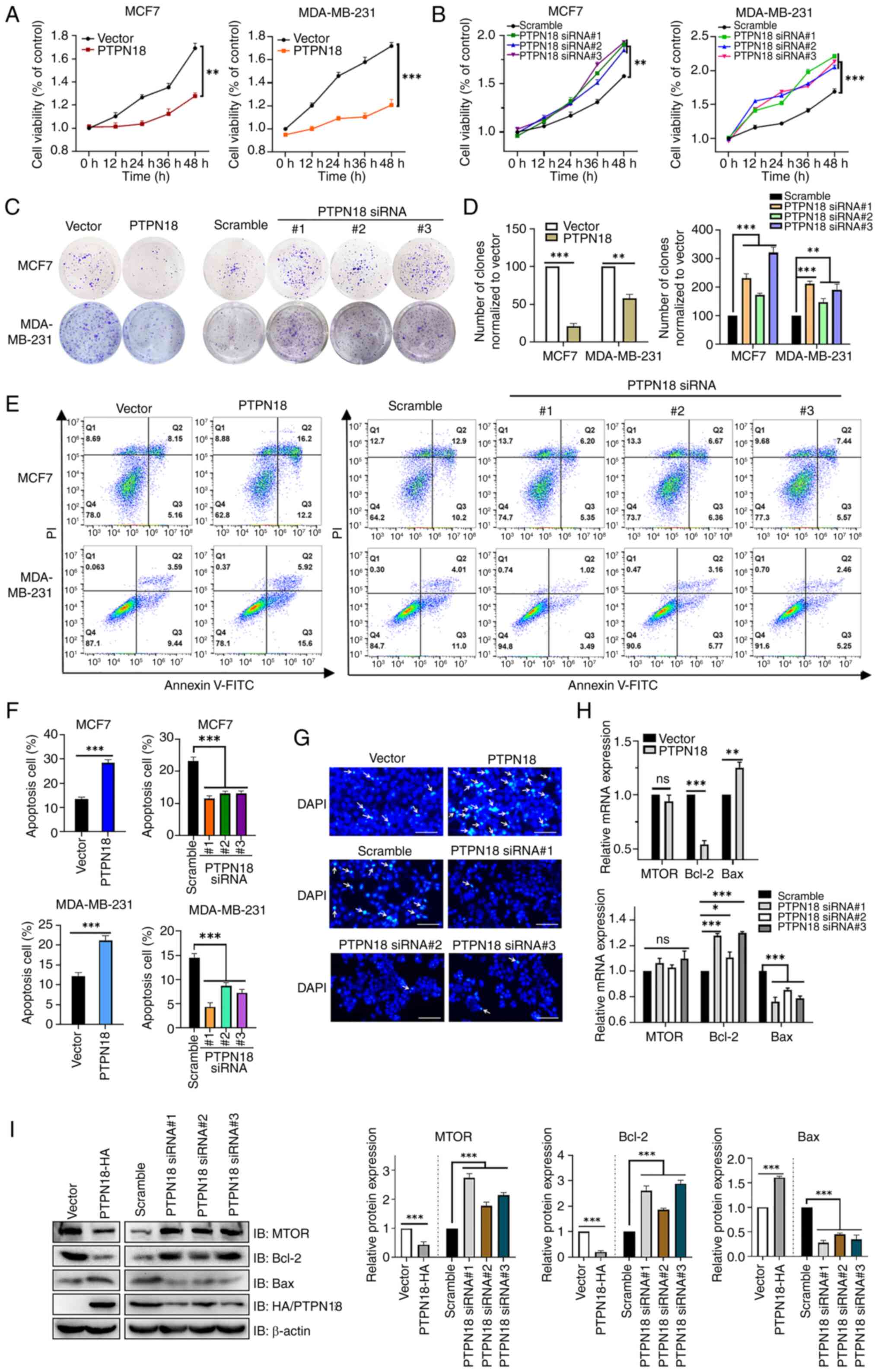
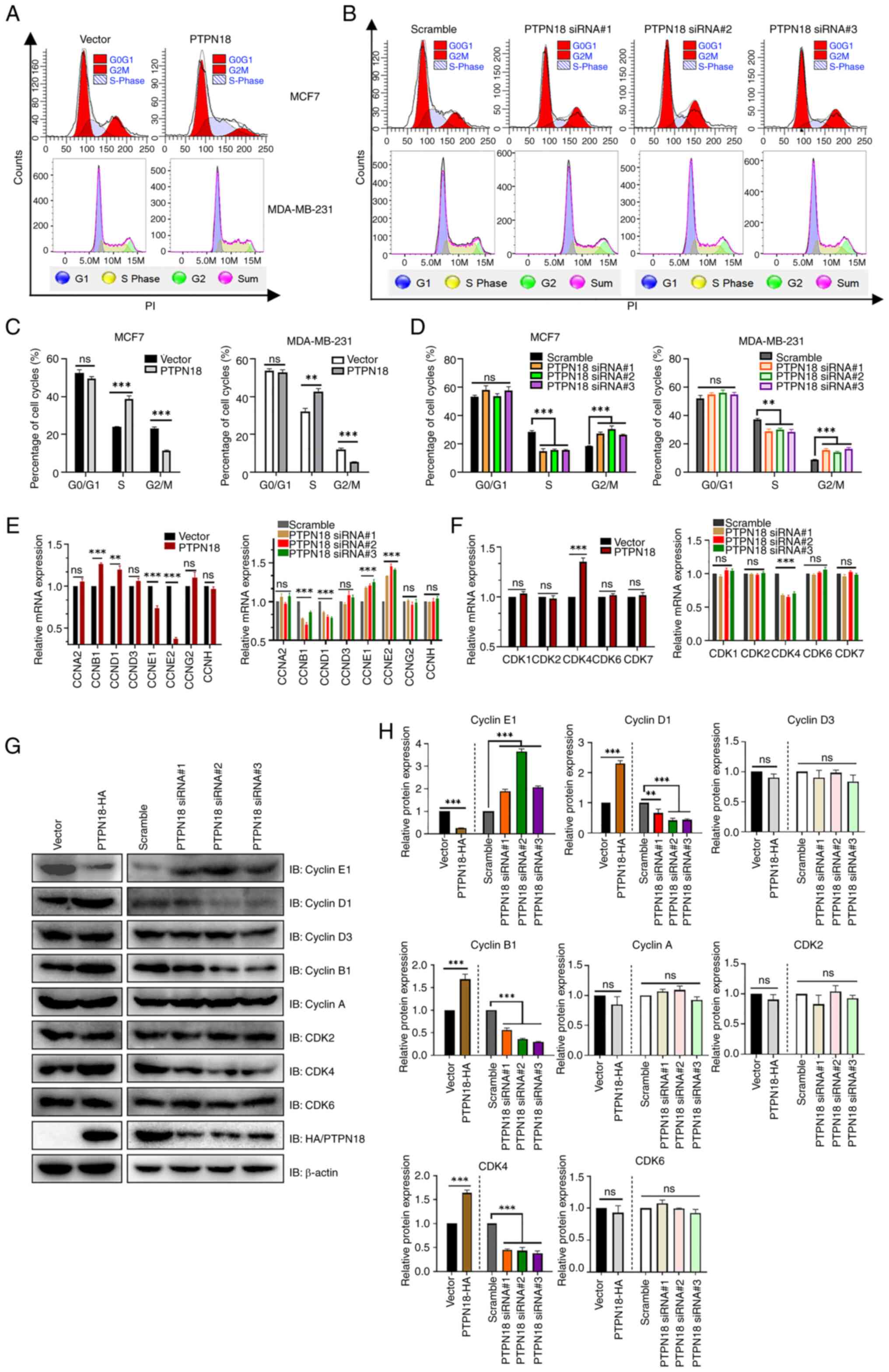
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Xiong X, Zheng LW, Ding Y, Chen YF, Cai
YW, Wang LP, Huang L, Liu CC, Shao ZM and Yu KD: Breast cancer:
Pathogenesis and treatments. Signal Transduct Target Ther.
10:492025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Radenkovic S, Konjevic G, Jurisic V,
Karadzic K, Nikitovic M and Gopcevic K: Values of MMP-2 and MMP-9
in tumor tissue of basal-like breast cancer patients. Cell Biochem
Biophys. 68:143–152. 2014. View Article : Google Scholar
|
|
4
|
Radenkovic S, Milosevic Z, Konjevic G,
Karadzic K, Rovcanin B, Buta M, Gopcevic K and Jurisic V: Lactate
dehydrogenase, catalase, and superoxide dismutase in tumor tissue
of breast cancer patients in respect to mammographic findings. Cell
Biochem Biophys. 66:287–295. 2013. View Article : Google Scholar
|
|
5
|
Konjević G, Jurisić V and Spuzić I:
Association of NK cell dysfunction with changes in LDH
characteristics of peripheral blood lymphocytes (PBL) in breast
cancer patients. Breast Cancer Res Treat. 66:255–263. 2001.
View Article : Google Scholar
|
|
6
|
Radenkovic S, Konjevic G, Gavrilovic D,
Stojanovic-Rundic S, Plesinac-Karapandzic V, Stevanovic P and
Jurisic V: pSTAT3 expression associated with survival and
mammographic density of breast cancer patients. Pathol Res Pract.
215:366–372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veillette A, Rhee I, Souza CM and Davidson
D: PEST family phosphatases in immunity, autoimmunity, and
autoinflammatory disorders. Immunol Rev. 228:312–324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai B, Wang T, Zhang X, Ba X, Zhang N,
Zhao Y, Wang X, Yu Y and Wang B: PTPN22 activates the PI3K pathway
via 14-3-3τ in T cells. FEBS J. 290:4562–4576. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Yu Y, Bai B, Wang T, Zhao J,
Zhang N, Zhao Y, Wang X and Wang B: PTPN22 interacts with EB1 to
regulate T-cell receptor signaling. FASEB J. 34:8959–8974. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang B, Lemay S, Tsai S and Veillette A:
SH2 domain-mediated interaction of inhibitory protein tyrosine
kinase Csk with protein tyrosine phosphatase-HSCF. Mol Cell Biol.
21:1077–1088. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cong F, Spencer S, Côté JF, Wu Y, Tremblay
ML, Lasky LA and Goff SP: Cytoskeletal protein PSTPIP1 directs the
PEST-type protein tyrosine phosphatase to the c-Abl kinase to
mediate Abl dephosphorylation. Mol Cell. 6:1413–1423. 2000.
View Article : Google Scholar
|
|
12
|
Dowbenko D, Spencer S, Quan C and Lasky
LA: Identification of a novel polyproline recognition site in the
cytoskeletal associated protein, proline serine threonine
phosphatase interacting protein. J Biol Chem. 273:989–996. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Dowbenko D and Lasky LA: PSTPIP 2, a
second tyrosine phosphorylated, cytoskeletal-associated protein
that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem.
273:30487–30496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiota M, Tanihiro T, Nakagawa Y, Aoki N,
Ishida N, Miyazaki K, Ullrich A and Miyazaki H: Protein tyrosine
phosphatase PTP20 induces actin cytoskeleton reorganization by
dephosphorylating p190 RhoGAP in rat ovarian granulosa cells
stimulated with follicle-stimulating hormone. Mol Endocrinol.
17:534–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HM, Xu YF, Ning SL, Yang DX, Li Y, Du
YJ, Yang F, Zhang Y, Liang N, Yao W, et al: The catalytic region
and PEST domain of PTPN18 distinctly regulate the HER2
phosphorylation and ubiquitination barcodes. Cell Res.
24:1067–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gensler M, Buschbeck M and Ullrich A:
Negative regulation of HER2 signaling by the PEST-type
protein-tyrosine phosphatase BDP1. J Biol Chem. 279:12110–12116.
2004. View Article : Google Scholar
|
|
17
|
Wang T, Ba X, Zhang X, Zhang N, Wang G,
Bai B, Li T, Zhao J, Zhao Y, Yu Y and Wang B: Nuclear import of
PTPN18 inhibits breast cancer metastasis mediated by MVP and
importin β2. Cell Death Dis. 13:7202022. View Article : Google Scholar
|
|
18
|
Chu C, Geng Y, Zhou Y and Sicinski P:
Cyclin E in normal physiology and disease states. Trends Cell Biol.
31:732–746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siu KT, Rosner MR and Minella AC: An
integrated view of cyclin E function and regulation. Cell Cycle.
11:57–64. 2012. View Article : Google Scholar :
|
|
20
|
Caldon CE and Musgrove EA: Distinct and
redundant functions of cyclin E1 and cyclin E2 in development and
cancer. Cell Div. 5:22010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang HC and Clurman BE: Cyclin E in
normal and neoplastic cell cycles. Oncogene. 24:2776–2786. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fagundes R and Teixeira LK: Cyclin E/CDK2:
DNA replication, replication stress and genomic instability. Front
Cell Dev Biol. 9:7748452021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scaltriti M, Eichhorn PJ, Cortés J,
Prudkin L, Aura C, Jiménez J, Chandarlapaty S, Serra V, Prat A,
Ibrahim YH, et al: Cyclin E amplification/overexpression is a
mechanism of trastuzumab resistance in HER2+ breast cancer
patients. Proc Natl Acad Sci USA. 108:3761–3766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radenković N, Milutinović M, Nikodijević
D, Jovankić J and Jurišić V: Sample preparation of adherent cell
lines for flow cytometry: protocol optimization-our experience with
SW-480 colorectal cancer cell line. Indian J Clin Biochem.
40:74–79. 2025. View Article : Google Scholar
|
|
25
|
Vuletic A, Konjevic G, Milanovic D,
Ruzdijic S and Jurisic V: Antiproliferative effect of
13-cis-retinoic acid is associated with granulocyte differentiation
and decrease in cyclin B1 and Bcl-2 protein levels in G0/G1
arrested HL-60 cells. Pathol Oncol Res. 16:393–401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scherbakov AM, Vorontsova SK, Khamidullina
AI, Mrdjanovic J, Andreeva OE, Bogdanov FB, Salnikova DI, Jurisic
V, Zavarzin IV and Shirinian VZ: Novel pentacyclic derivatives and
benzylidenes of the progesterone series cause anti-estrogenic and
antiproliferative effects and induce apoptosis in breast cancer
cells. Invest New Drugs. 41:142–152. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47(W1): W556–W560.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Posta M and Győrffy B: Pathway-level
mutational signatures predict breast cancer outcomes and reveal
therapeutic targets. Br J Pharmacol. 182:5734–5747. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vasaikar S, Straub P, Wang J and Zhang B:
LinkedOmics: analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46(D1): D956–D963. 2018. View Article : Google Scholar :
|
|
32
|
Szklarczyk D, Kirsch R, Koutrouli M,
Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT,
Pyysalo S, et al: The STRING database in 2023: Protein-protein
association networks and functional enrichment analyses for any
sequenced genome of interest. Nucleic Acids Res. 51(D1): D638–D646.
2023. View Article : Google Scholar
|
|
33
|
Kim YW, Wang H, Sures I, Lammers R,
Martell KJ and Ullrich A: Characterization of the PEST family
protein tyrosine phosphatase BDP1. Oncogene. 13:2275–2279.
1996.PubMed/NCBI
|
|
34
|
Cheng J, Daimaru L, Fennie C and Lasky LA:
A novel protein tyrosine phosphatase expressed in
lin(lo)CD34(hi)Sca(hi) hematopoietic progenitor cells. Blood.
88:1156–1167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Zhong Q, Deng N, Zhou X, Wang H,
Ouyang J, Guan Z, Cheng B, Xiang L, Huang Y, et al: Sphingolipid
metabolism-related genes for the diagnosis of metabolic syndrome by
integrated bioinformatics analysis and Mendelian randomization
identification. Diabetol Metab Syndr. 17:2342025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cuvillier O, Nava VE, Murthy SK, Edsall
LC, Levade T, Milstien S and Spiegel S: Sphingosine generation,
cytochrome c release, and activation of caspase-7 in
doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells.
Cell Death Differ. 8:162–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang S, Huang J, Liu P, Li J and Zhao S:
Apoptosis-inducing factor (AIF) nuclear translocation mediated
caspase-independent mechanism involves in X-ray-induced MCF-7 cell
death. Int J Radiat Biol. 93:270–278. 2017. View Article : Google Scholar
|
|
38
|
Pozo-Guisado E, Merino JM, Mulero-Navarro
S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A and
Fernandez-Salguero PM: Resveratrol-induced apoptosis in MCF-7 human
breast cancer cells involves a caspase-independent mechanism with
downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 115:74–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Peng Y and Wei W: Cell cycle on the
crossroad of tumorigenesis and cancer therapy. Trends Cell Biol.
32:30–44. 2022. View Article : Google Scholar
|
|
40
|
Ekholm-Reed S, Mendez J, Tedesco D,
Zetterberg A, Stillman B and Reed SI: Deregulation of cyclin E in
human cells interferes with prereplication complex assembly. J Cell
Biol. 165:789–800. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Caldon CE, Sergio CM, Sutherland RL and
Musgrove EA: Differences in degradation lead to asynchronous
expression of cyclin E1 and cyclin E2 in cancer cells. Cell Cycle.
12:596–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Crncec A, Lau HW, Ng LY, Ma HT, Mak JPY,
Choi HF, Yeung TK and Poon RYC: Plasticity of mitotic cyclins in
promoting the G2-M transition. J Cell Biol. 224:e2024092192025.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Qian WP and Sun QY: Cyclins
regulating oocyte meiotic cell cycle progression†. Biol Reprod.
101:878–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao SC, Dong MZ, Zhao BW, Liu SL, Guo JN,
Sun SM, Li YY, Xu YH and Wang ZB: Fangchinoline inhibits mouse
oocyte meiosis by disturbing MPF activity. Toxicol In Vitro.
99:1058762024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
VanArsdale T, Boshoff C, ArndtK T and
Abraham RT: Molecular pathways: Targeting the cyclin D-CDK4/6 axis
for cancer treatment. Clin Cancer Res. 21:2905–2910. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kloet DEA, Polderman PE, Eijkelenboom A,
Smits LM, van Triest MH, van den Berg MCW, Groot Koerkamp MJ, van
Leenen D, Lijnzaad P, Holstege FC and Burgering BMT: FOXO target
gene CTDSP2 regulates cell cycle progression through Ras and
p21(Cip1/Waf1). Biochem J. 469:289–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amente S, Zhang J, Lavadera ML, Lania L,
Avvedimento EV and Majello B: Myc and PI3K/AKT signaling
cooperatively repress FOXO3a-dependent PUMA and GADD45a gene
expression. Nucleic Acids Res. 39:9498–9507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Halappanavar SS, St-Germain JR,
Tsang BK and Li Q: Role of Akt/protein kinase B in the activity of
transcriptional coactivator p300. Cell Mol Life Sci. 61:1675–1683.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sunayama J, Tsuruta F, Masuyama N and
Gotoh Y: JNK antagonizes Akt-mediated survival signals by
phosphorylating 14-3-3. J Cell Biol. 170:295–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Foley TM, Payne SN, Pasch CA, Yueh AE, Van
De Hey DR, Korkos DP, Clipson L, Maher ME, Matkowskyj KA, Newton MA
and Deming DA: Dual PI3K/mTOR inhibition in colorectal cancers with
APC and PIK3CA mutations. Mol Cancer Res. 15:317–327. February
9–2017.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dbouk HA and Backer JM: A beta version of
life: p110β takes center stage. Oncotarget. 1:729–733. 2010.
View Article : Google Scholar
|
|
52
|
Geering B, Cutillas PR, Nock G, Gharbi SI
and Vanhaesebroeck B: Class IA phosphoinositide 3-kinases are
obligate p85-p110 heterodimers. Proc Natl Acad Sci USA.
104:7809–7814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luo J and Cantley LC: The negative
regulation of phosphoinositide 3-kinase signaling by p85 and its
implication in cancer. Cell Cycle. 4:1309–1312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zheng Y, Peng M, Wang Z, Asara JM and
Tyner AL: Protein tyrosine kinase 6 directly phosphorylates AKT and
promotes AKT activation in response to epidermal growth factor. Mol
Cell Biol. 30:4280–4292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen R, Kim O, Yang J, Sato K, Eisenmann
KM, McCarthy J, Chen H and Qiu Y: Regulation of Akt/PKB activation
by tyrosine phosphorylation. J Biol Chem. 276:31858–31862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J and Wang G: Cyclin E expression and
chemotherapeutic sensitivity in breast cancer cells. J Huazhong
Univ Sci Technolog Med Sci. 26:565–566. 2006. View Article : Google Scholar
|
|
57
|
Waltersson MA, Askmalm MS, Nordenskjöld B,
Fornander T, Skoog L and Stål O: Altered expression of cyclin E and
the retinoblastoma protein influences the effect of adjuvant
therapy in breast cancer. Int J Oncol. 34:441–448. 2009.PubMed/NCBI
|