The immune system serves as the primary defense
mechanism in the body against external pathogens and abnormal
cells; it plays a vital role in identifying and eliminating tumor
cells (1). The relationship
between tumors and immune function represents a complex and
intricate biological network that has emerged as the key focus in
research on cancer. Hypoxia is a common characteristic of solid
tumors (2). During tumor growth,
cancer cells progressively form an immunosuppressive hypoxic tumor
microenvironment (TME), which promotes the proliferation and
metastasis of tumors by influencing processes such as metabolism,
angiogenesis and immune evasion (3). In the TME, critical transcription
factors, particularly hypoxia-inducible factors (HIFs) and their
downstream signaling pathways, are activated. These signals
regulate the differentiation, function and metabolic reprogramming
of immune cells, thereby shaping the direction and strength of the
tumor immune response. Ultimately, HIFs influence angiogenesis,
cell proliferation and invasion in tumors, mediating the
progression and development of tumors (4).
Several epidemiological studies have identified a
significant correlation between HIFs and greater incidence and
mortality in various types of cancer. The two primary HIF isoforms,
HIF-1α and HIF-2α, serve as key regulators under hypoxic conditions
and play crucial roles in tumor immune evasion by modulating the
innate and adaptive immune systems (5). Hypoxia upregulates the transcription
and expression of HIF-1α, which subsequently activates the immune
checkpoint comprising programmed cell death protein 1 (PD-1) and
its ligand PD-L1 (6). This
pathway indirectly suppresses the activation and proliferation of
T-cells, leading to immune evasion by tumor cells. Similarly, the
interaction between CD137 expressed on T cells and its ligand
CD137L can activate dendritic cells and macrophages; this, in turn,
allows them to recognize and eliminate cancer cells. However,
HIF-1α present in tumor cells can prevent this process, causing the
tumor cells to escape from adaptive immunity (7,8).
Compared with HIF-1α, HIF-2α more prominently induces the
expression of genes associated with invasion and stem cell
properties, such as matrix metalloproteinases and stem cell factor
octamer-binding transcription factor 3/4 (OCT-3/4) (9,10).
When these genes are expressed, the invasive and metastatic
capabilities of tumor cells are enhanced (11). In clear cell renal cell carcinoma
(ccRCC), HIF-2α promotes the secretion of immunosuppressive
cytokines such as transforming growth factor-β (TGF-β) and
interleukin (IL) 10 by upregulating stem cell factors, thereby
fostering an immunosuppressive TME (12).
The induction of HIF-dependent genes not only
improves the ability of the tumor to adapt to more hypoxic
conditions but also promotes angiogenesis, cell survival, invasion
and metastasis in tumors (4).
Numerous preclinical and clinical studies have investigated
targeted cancer therapeutic strategies aimed at inhibiting or
activating HIFs. The methods available for inhibiting the response
of HIF-1α to hypoxia include, but are not limited to, small
interfering RNA (siRNA) therapy, blocking of the dimerization of
HIF-1α and β subunits and the use of anticancer agents that
directly inhibit HIF-1α by targeting the PI3K/AKT/HIF-1α pathway
(13). However, in clinical
trials, HIF-targeted therapeutic techniques have often failed to
achieve the desired outcomes and HIF inhibitors are associated with
significant dose-limiting side effects. As tumor immunity is
complex, deciding whether to target all HIF isoforms or selectively
target HIF-1α or HIF-2α requires careful consideration of their
distinct functions, as well as the different roles HIFs play across
various types of cells. Additionally, hypoxia-induced reduction in
pH leads to an acidic TME, which leads to a decrease in the
concentration of the drug due to ion trapping, a reduction in the
apoptosis of cancer cells and an increase in the activity of
multidrug transporter P-glycoprotein (P-gp) (13-15). These factors contribute to
HIF-mediated therapeutic failure and/or an increase in tumor drug
resistance.
The present review comprehensively examined the
mechanisms and effects of HIFs on both innate and adaptive immune
systems in the hypoxic TME. It evaluated the potential of HIFs as
critical therapeutic targets in cancer treatment and proposed
strategies for developing novel HIF inhibitors to systematically
combat cancer.
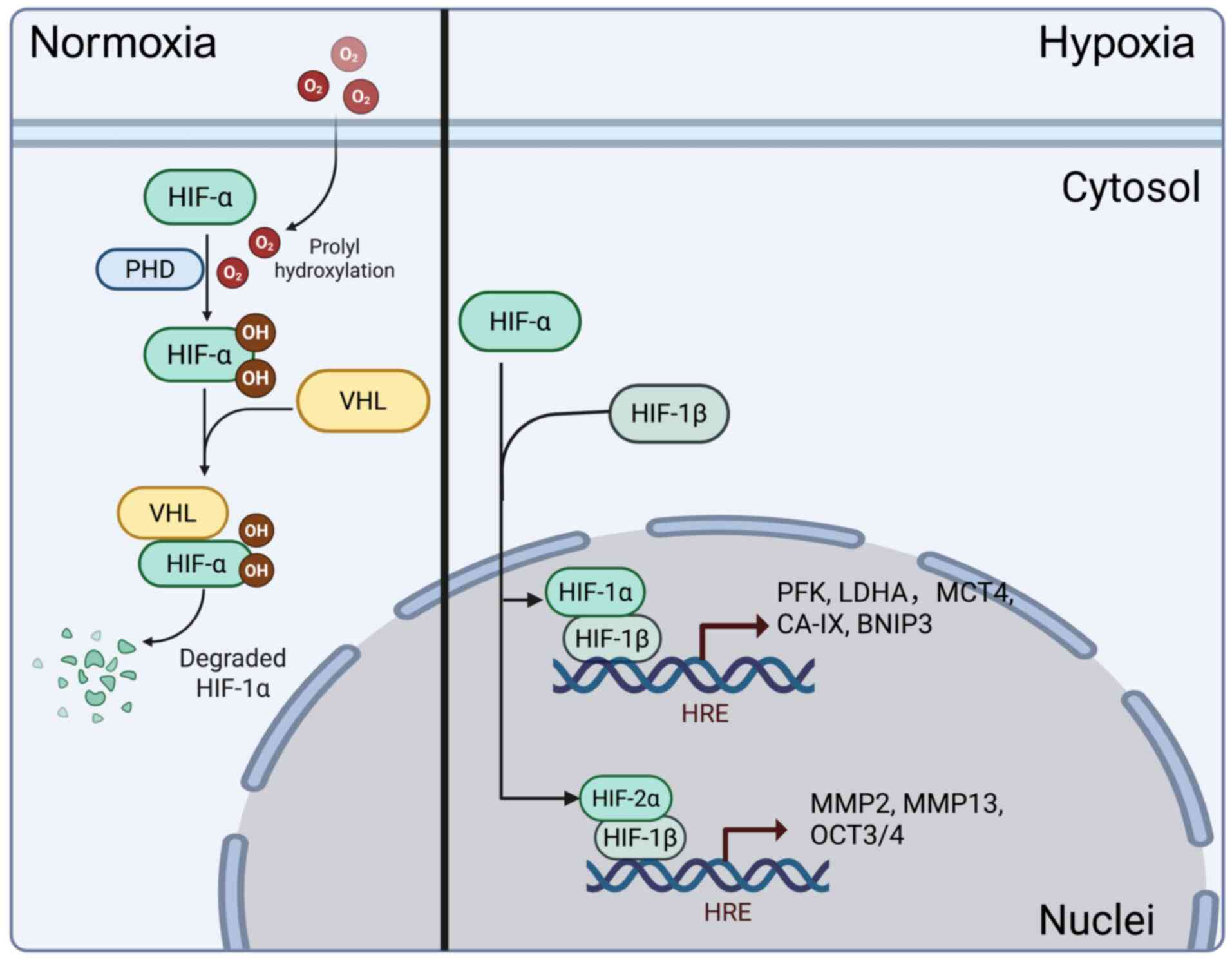
HIFs is a heterodimeric transcription factor
composed of α and β subunits. The α subunit exhibits
oxygen-dependent expression, while the β subunit exhibits
constitutive expression. A total of three α subunits (HIF-1α,
HIF-2α and HIF-3α) and three β subunits (HIF-1β, HIF-2β and HIF-3β,
also known as ARNT1, ARNT2 and ARNT3) are known. Although the HIF-β
subunit shows excellent stability, the stability of the HIF-α
subunit fluctuates in response to changes in oxygen tension,
regulated by prolyl hydroxylase (PHD)1, PHD2 and PHD3 (16-18). Under normoxic conditions, oxygen
is used by PHDs to hydroxylate two conserved proline residues on
the HIF-α subunit. These hydroxylated proline residues are
recognized by the Von Hippel-Lindau (VHL) E3 ubiquitin ligase
complex, leading to proteasomal degradation of the HIF-α subunit
(19). However, under hypoxic
conditions, PHDs lack sufficient oxygen as a substrate to perform
their dioxygenase function, thereby preventing VHL from recognizing
the HIF-α subunit, resulting in its stabilization. As a result, the
HIF-α subunit translocates to the nucleus, where it dimerizes with
the HIF-β subunit and binds to DNA at hypoxia response elements,
thereby driving HIF-dependent transcription (20,21).
Transcriptional targets dependent on HIFs spans
numerous biological processes, including angiogenesis, glycolysis,
chromatin remodeling, cell cycle regulation and even genes involved
in the oxygen-sensing pathway. Through genome-wide analyses of
hypoxic transcriptomic responses and HIF binding sites, researchers
have found that, in any specific cell type, at least 500-1,000
genes are directly or indirectly regulated by HIFs (22-24). Concerning target genes, HIF-1 and
HIF-2 exhibit a degree of specificity. HIF-1 generally induces
genes encoding glycolytic enzymes, such as phosphofructokinase and
lactate dehydrogenase A (LDHA); those involved in the regulation of
pH, such as monocarboxylate transporter 4 and carbonic anhydrase IX
and genes that promote apoptosis, such as Bcl2 interacting protein
3 (BNIP3) and Bcl2/Adenovirus interacting protein 3 (BNIP3L/NIX)
(11,24). By contrast, HIF-2 generally
induces genes associated with invasion processes, such as MMP2 and
MMP13 and the stem cell factor OCT-3/4 (11). These two heterodimeric
transcription factors may substitute for each other under certain
circumstances, besides specifically regulating downstream target
genes, HIF-1 and HIF-2 share some common targets, such as vascular
endothelial growth factor A (VEGF-A) and glucose transporter 1
(GLUT1) (25,26). In human tissues, HIF-1α is widely
expressed, while HIF-2α, initially identified as Endothelial PAS
domain protein 1, was previously considered an endothelial-specific
HIF-α isoform (27). However,
studies have found that under hypoxic conditions, the expression of
HIF-2α is not limited to the vasculature; its transcriptional
response is widely activated under prolonged hypoxia, complementing
rather than redundantly overlapping with the function of HIF-1α
(28,29) (Fig.
1).
The immune system is a network that operates due to
the interaction of lymphoid organs, cells, humoral factors and
cytokines. It is divided into nonspecific immunity (innate
immunity) and specific immunity (adaptive immunity); the two types
of immunity differ in their activation times, the types of immune
cells involved and their modes of action (30). HIF-1α and HIF-2α are broadly
expressed and detectable in nearly all innate and adaptive immune
cell populations. The unique expression patterns of HIF-1α and
HIF-2α in immune cells depend on intrinsic and extrinsic factors,
with the balance between them contributing to the regulation of
overlapping or distinct sets of target genes. Their expression and
stabilization in immune cells can be triggered not only by hypoxia
but also by other factors associated with pathological stress, such
as inflammation, infectious microorganisms and cancer (31-33). HIFs regulate various types of
immune processes, enhancing the bactericidal capacity of phagocytes
and driving the differentiation of T cells and cytotoxic activity.
Additionally, HIF-mediated cellular metabolism is a critical immuno
regulatory factor, influencing the development, fate and function
of myeloid cells and lymphocytes (31,34).
In the hypoxic TME of solid tumors, >7,000 types
of mRNAs that are regulated by the transcription of HIFs have been
identified in cancer cells. These mRNAs contribute to important
aspects of cancer progression, including tumor angiogenesis,
metabolic reprogramming, cell motility and invasion and resistance
to chemotherapy and radiotherapy (35-38). However, in the hypoxic TME of
solid tumors, besides cancer cells, there are also immune cells
that are either resident or recruited from oxygen-rich blood
circulation (39). Therefore,
compared with cancer cells, the expression of HIFs in immune cells
exerts a more complex influence on tumor immunity. Depending on the
type of immune cells, HIFs demonstrate varied roles and effects
across different tumor models. In the following sections, we
comprehensively reviewed the roles of HIFs in various cells of the
innate and adaptive immune systems within the TME.
The expression of HIFs in T cells exhibits
stage-specific characteristics. While HIF-1α in cancer cells can
inhibit the activation of T cells via PD-L1 interaction (40), it primarily displays an
activation-promoting effect in naïve T cells (41). Studies have highlighted the
upregulation of aerobic glycolysis as a hallmark of T cell
activation (42,43). In response to T cell activation,
the transcriptional activity of HIF-1 increases, promoting the
upregulation of glycolytic enzymes, such as pyruvate kinase,
hexokinase 2 (HK2) and GLUT1; these enzymes and their associated
metabolic pathways are integral to the activation and function of T
cells (44-46). However, in tissues, hypoxia may
suppress T cell activation, with T cells exposed to higher oxygen
levels exhibiting a more robust activation profile than those in
hypoxic environments (47).
Glycoproteomic studies on the surfaces of primary human T cells
have shown that hypoxia substantially alters the CD8+ T
cell surface profile in a manner consistent with metabolic
reprogramming and an immunosuppressive state. CD4+ T
cells demonstrated similar responses, indicating a common
hypoxia-induced surface receptor program and suggesting that
hypoxic environments pose challenges to T cell activation (48). Therefore, the levels of HIF-1
during and after activation play a critical role in adapting to the
hypoxic environment and regulating the functions of T cells. The
evidence from experiments has shown that preconditioning human T
cells to hypoxia or activating HIF pathways before chimeric antigen
receptor T-cell therapy sustainably enhance T cell cytotoxic
functionality (49,50).
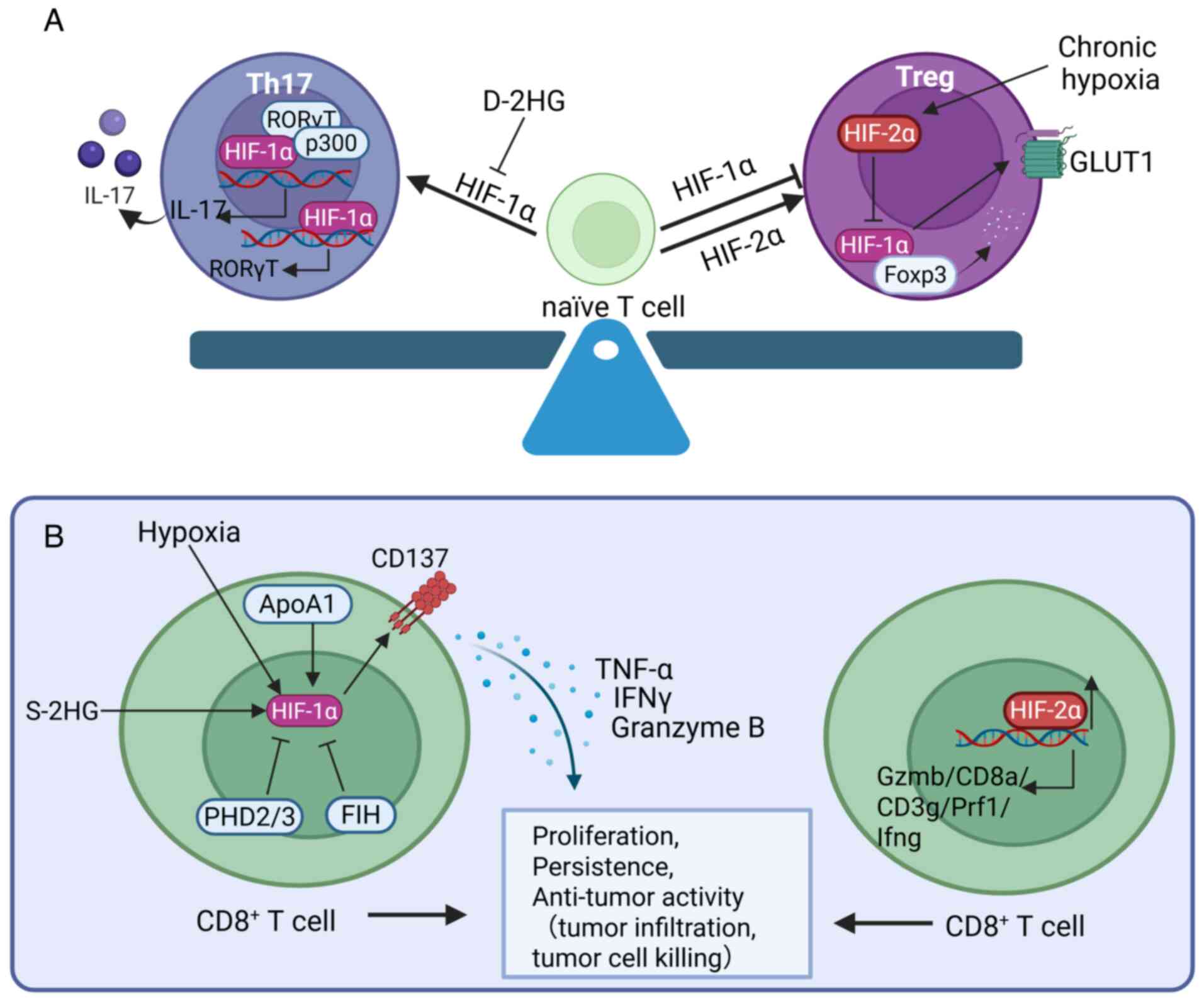
HIF-1α contributes to the polarization of naïve T
cells into Th17 cells. Naïve T cells differentiate into various
functional effector and regulatory subsets, the process being
partially regulated by the cytokine environment present during
antigen recognition. During the differentiation of naïve T cells,
HIF-1, as a key glycolysis-promoting factor, may be an important
driver of the differentiation of Th17 cells (51). HIF-1 controls cell fate decisions
through glycolysis, promoting the differentiation of naïve T cells
into Th17 cells rather than Treg cells. The absence of HIF-1α
results in a decrease in the differentiation of Th17 cells, while
Treg differentiation is enhanced (52). HIF-1 also promotes the development
of Th17 cells through direct transcriptional activation of
RAR-related orphan receptor C (RORC) and by forming a ternary
complex with RAR-related orphan receptor γt (RORγt) and p300 to
recruit the IL-17 promoter, thereby regulating the differentiation
program of Th17 signature genes (53,54). Additionally, HIF-1 suppresses Treg
development by binding to forkhead box P3 (Foxp3) and targeting it
for proteasomal degradation (53). Evidence from patients with
thymoma-associated myasthenia gravis suggests that a higher
RORγt/FOXP3 ratio may provide evidence for Th17/Treg imbalance,
potentially associated with an increase in HIF-1α levels (55). By contrast, in acute myeloid
leukemia patients, the metabolic product D-2-hydroxyglutarate
(D-2HG) affects the differentiation of T cells upon uptake. D-2HG
induces the instability of the HIF-1α protein, thereby increasing
the abundance of Treg subsets and reducing the differentiation of
Th17 cells (56). The intrinsic
expression of oxygen-sensing PHD proteins, which promote the
degradation of HIFs, in T cells is necessary for sustaining immune
evasion and tumor colonization in the lungs. PHD proteins limit the
differentiation of Th17 cells, promote the induction of Treg cells
and suppress CD8+ T cell effector functions,
contributing to IFN-γ-dependent tumor immune suppression (57) (Fig.
2A).
In Treg cells, HIF-1α promotes the instability and
degradation of Foxp3, induces IL-17 expression, stimulates IFN-γ
production and increases the fragility of Tregs (58). Through these activities, the
expression of HIF-1α in Tregs imparts antitumor immunity, thereby
protecting the host from tumor growth. Thus, selectively increasing
the expression of HIF-1α in Tregs can be considered a therapeutic
approach to cancer treatment. Despite some controversy (59), with reports suggesting that
hypoxia promotes the expression of Foxp3 (60,61), a more detailed examination
indicates that hypoxia inhibits the differentiation of Tregs, while
HIF-1α deletion rescues the differentiation of Tregs under hypoxic
conditions, providing increasing evidence of the intrinsic negative
effects of HIF-1α on FOXP3 and Tregs (52,53). Hsu et al (62) discovered that HIF-2α and HIF-1α
have opposite effects on the differentiation of Tregs. Although the
development of Tregs remains normal in mice with Foxp3-cre-specific
deletion of either HIF-1α or HIF-2α, Tregs lacking HIF-2α, but not
HIF-1α, display functional defects in suppressing effector T cells,
exhibit enhanced reprogramming toward IL-17-secreting cells and
confer resistance to the growth of MC38 colon adenocarcinoma and
the metastasis of B16F10 melanoma (62). This is attributed to the
inhibition of HIF-1α expression by HIF-2α, considering that HIF-2α
deficiency leads to upregulation of HIF-1α expression both
transcriptionally and post-transcriptionally, which increases Glut1
expression and a modest increase in Pdk1; these changes increase
glycolytic activity that is unfavorable for the development of
Tregs (62). The cross-talk
between these two isoforms may partially explain the controversy
surrounding hypoxia and the function of Tregs. For example,
Neildez-Nguyen et al (59)
observed that significant Treg proliferation Compared with normoxia
only occurs after prolonged culture (7 and 11 days) under hypoxic
conditions, as HIF-2α is expressed predominantly during long-term
hypoxia (63) (Fig. 2A).
In the blood, B cells are key cells responsible for
antibody production. Specific receptors on B cells recognize
harmful substances in the blood as antigens. After the antigens are
processed, with the help of T cells, B cells mature and
differentiate into plasma cells that secrete antibodies. B cells
also play roles in antigen presentation and the secretion of
cytokines (74,75). The development and selection of B
lymphocytes are core processes in adaptive immunity and
self-tolerance, relying on B cell receptor (BCR) signaling
(76). The activity of HIFs is
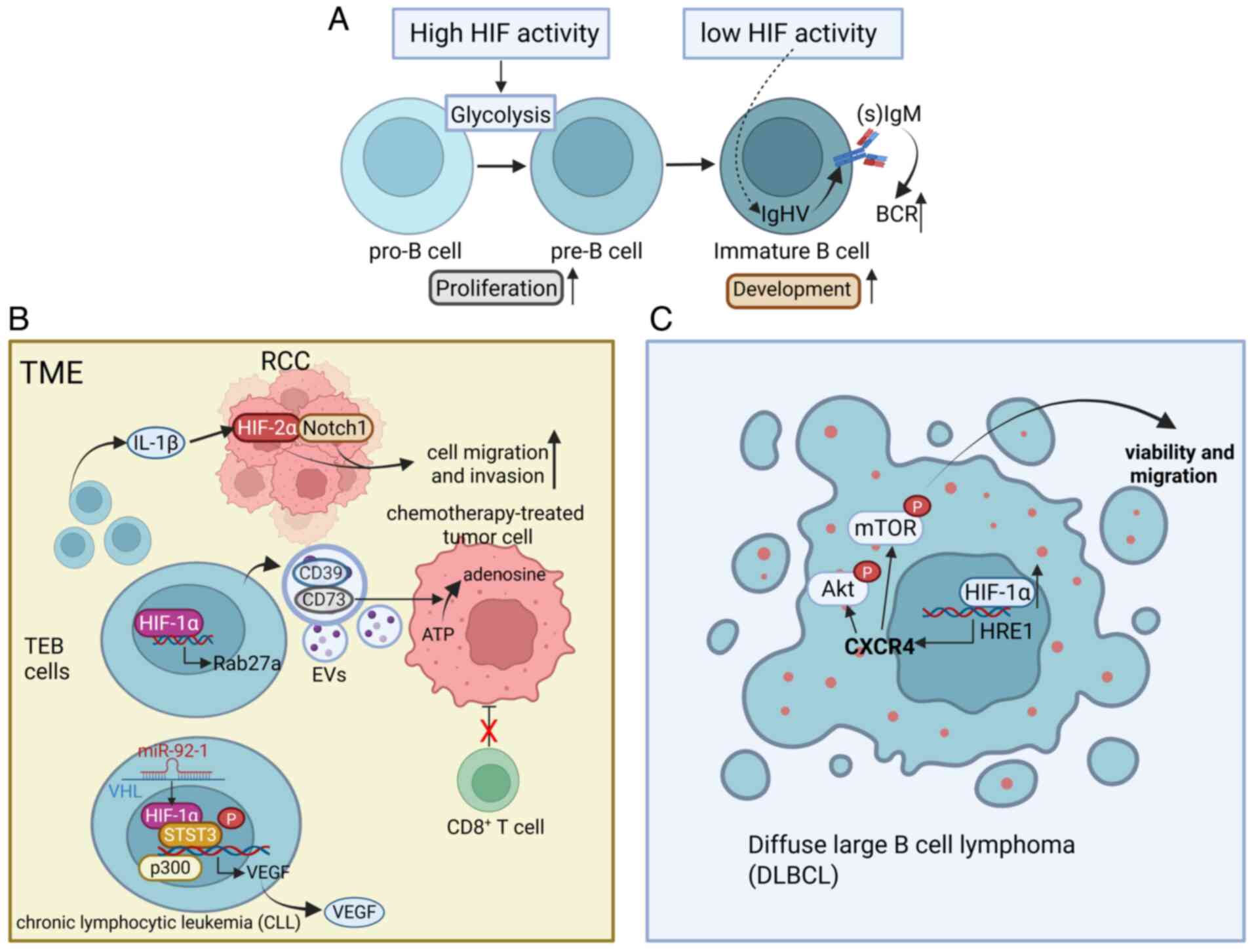
high in human and mouse bone marrow, particularly in pro-B and
pre-B cells and decreases at the immature B cell stage. This
stage-specific inhibition of HIFs is essential for the normal
development of B cells, as genetic activation of HIF-1α in mouse B
cells leads to reduced repertoire diversity, decreased BCR editing
and developmental arrest of immature B cells, resulting in a
reduction in the number of peripheral B cells (77) (Fig.
3A).
The B cells in the TME and the intracellular HIF
signals may play a significant role in the progression of cancer.
Renal cell carcinoma (RCC) tissues contain a higher number of B
cells than the surrounding normal renal tissues and these recruited
B cells are referred to as tumor-educated B cells (TEBs) (75). The IL-1β released by TEBs
activates the HIF-2α/Notch1 signaling pathway in RCC, promoting the
migration and invasion of RCC cells (78). The upregulation of HIF-1α in TEBs
induced by the TME further promotes the release of CD19-containing
extracellular vesicles (EVs) from B cells through the transcription
of Rab27a mRNA. The CD39 and CD73 proteins in these EVs hydrolyze
ATP in chemotherapy-treated tumor cells to adenosine, thereby
impairing the responses of CD8 T cells and reducing the efficacy of
tumor chemotherapy in humans and mice (79). In chronic lymphocytic leukemia
(CLL), miR-92-1 expressed in TEBs targets the VHL transcript to
inhibit its expression and the stabilized HIF-1α forms an active
complex with the coactivator p300 and phosphorylated signal
transducer and activator of transcription 3 at the promoter of
VEGF, which recruits RNA polymerase II, explaining the abnormal
autocrine VEGF in CLL (80)
(Fig. 3B).
Additionally, HIF-1α is associated with the
self-transformation of B cells, thereby contributing to the
formation of diffuse large B cell lymphoma (DLBCL), which is the
most common form of lymphoid malignancy. Under hypoxic conditions
or upon overexpression of HIF-1α under normoxic conditions, the
viability and migration of DLBCL cells increase markedly, while
downregulation of HIF-1α has the opposite effect. This process
involves the binding of HIF-1α to the functional site HRE1 on the
CXCR4 promoter, thereby activating its transcription. The
activation of CXCR4 mediated by HIF-1α further increases the
phosphorylation of AKT/mTOR under hypoxic conditions (81) (Fig.
3C).
Macrophages exhibit high plasticity and, in response
to different stimuli, unpolarized macrophages (M0) can
differentiate into cells with unique phenotypes that perform
different functions. Based on phenotypic and functional
characteristics, polarized macrophages are generally categorized
into classically activated M1 macrophages and alternatively
activated M2 macrophages. M1 macrophages are induced by Th1-type
cytokines and bacterial lipopolysaccharide (LPS); they are
typically pro-inflammatory and anti-tumoral, characterized by the
secretion of inflammatory cytokines such as IL-1β, IL-6 and tumor
necrosis factor (TNF)-α. By contrast, M2 macrophages are stimulated
by Th2-type cytokines; they play key roles in tumor initiation,
proliferation, metastasis and immune evasion and can secrete
anti-inflammatory cytokines such as IL-10 and transforming growth
factor (TGF)-β (82,83). Mantovani et al (84) further subdivided M2 macrophages
into four subsets: M2a, M2b, M2c and M2d. Among these macrophages,
M2d macrophages, activated through Toll-like receptors and
characterized by the expression of VEGF and IL-10, contribute to
angiogenesis and tumor progression and are thus also referred to as
tumor-associated macrophages (TAMs) (85). Macrophage polarization is a
dynamic process. The M1 and M2 phenotypes are not strictly
antagonistic; rather, they frequently coexist and can interconvert
under specific conditions. This plasticity allows macrophages to
adapt to microenvironmental changes and maintain tissue homeostasis
and systemic balance.
Macrophages are an important component of the immune
system. As they are frequently found in hypoxic tissues (86), their functions and polarization
states are strongly influenced by HIFs, as most transcriptional
responses to hypoxia are mediated by HIFs. In primary macrophages
from normal mice, the expression levels of HIF-1α and HIF-2α mRNAs
differ between M1-polarized and M2-polarized macrophages. HIF-1α is
induced by Th1-type cytokines during the polarization of
macrophages to the M1 phenotype, whereas HIF-2α is induced by
Th2-type cytokines during the M2 response. This differential
expression becomes more prominent in macrophages after
polarization. In mice specifically overexpressing HIF-1α in bone
marrow cells, macrophages exhibit an exaggerated inflammatory state
characterized by the upregulation of M1 markers (87,88).
ccRCC exhibits unique features in the context of HIF
signaling-mediated regulation of tumor immunity. Moreover, ccRCC is
commonly associated with the inactivation of the VHL tumor
suppressor gene, leading to the constitutive stabilization and
activation of both HIF-1α and HIF-2α (96). In ccRCC, HIF-2α acts as a tumor
promoter, whereas HIF-1α has tumor-suppressive effects. HIF-2α and
other hypoxia-associated factors are predominantly expressed in
tumor cells and HIF-2α is widely recognized as a key oncogenic
driver and a promising therapeutic target in ccRCC (97-99). By contrast, HIF-1α is primarily
expressed in TAMs. Cell-based studies have shown that the
overexpression of HIF-1α in TAMs suppresses the growth of ccRCC
xenografts (29). Consistent with
this notion, homozygous deletion of HIF1A is found in ~50%
of high-grade ccRCC cases, suggesting that HIF1A functions
as a tumor suppressor gene in ccRCC (100). However, clinical studies have
further revealed that HIF-1α is primarily expressed in TAMs and is
associated with higher tumor grades, an increase in metastatic
risk, resistance to anti-angiogenic therapy and a significant
reduction in overall survival. No differences were observed between
HIF-1α protein levels within TAMs compared with macrophages derived
from uninvolved kidneys, suggesting that increased HIF-1α levels in
high-grade ccRCC may be due to an increase in the number of immune
cells rather than an increase in HIF-1α expression (101). To summarize, these studies
illustrate the distinct functional roles of HIF-1α and HIF-2α in
tumor immunity. The effects of these HIFs may differ based on
upstream regulatory signals, providing important insights into the
functions and regulatory mechanisms of TAMs in the TME.
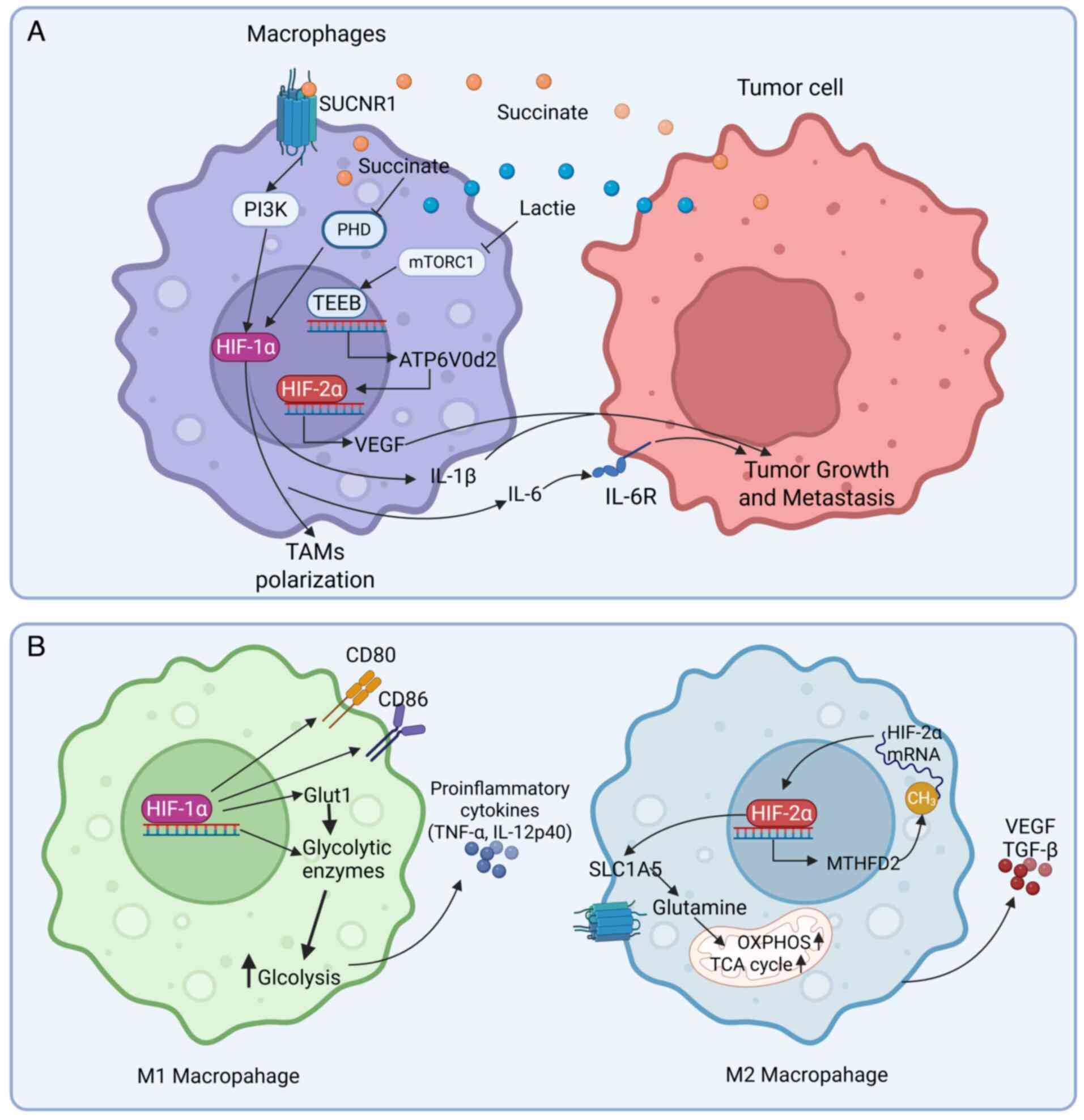
Under hypoxic conditions, the metabolic
reprogramming mediated by HIF may strongly influence the immune
function and polarization of macrophages. Hypoxic stimulation
induces HIF-1α, which upregulates key enzymes in the glycolytic
pathway, such as Glut1. This promotes rapid conversion of glucose
to pyruvate even under normoxic conditions and is known as the
Warburg effect (102,103). The resultant glycolytic
intermediates further drive the polarization of M1 macrophages.
Concurrently, M1 macrophages exhibit greater secretion of
pro-inflammatory cytokines, such as TNF-α and IL-12, along with an
increase in the levels of co-stimulatory molecules CD80 and CD86
(102). This metabolic
reprogramming supports the high energy demands of M1 macrophages
during immune responses against tumors while establishing a
positive feedback loop to reinforce their pro-inflammatory
phenotype (Fig. 4B). By contrast,
HIF-2α supports tumor progression through metabolic reprogramming.
Under hypoxic conditions, HIF-2α is stabilized and activated in
macrophages, triggering several metabolic adaptations, such as
upregulation of genes and expressions related to glycolysis
[methylenetetrahydrofolate dehydrogenase (NADP+ Dependent) 2,
MTHFD2] and glutamine metabolism (solute carrier family 1 member 5,
SLC1A5). The upregulation of SLC1A5 mediated by HIF-2α increases
glutamine transport into mitochondria, which fuels the
tricarboxylic acid cycle and oxidative phosphorylation (104). The upregulation of MTHFD2 by
HIF-2α promotes one-carbon metabolism, which can alter RNA
methylation patterns, particularly through an increase in the m6A
methylation of HIF-2α mRNA. This improves the translation
efficiency of HIF-2α, forming a positive feedback loop between the
expression of HIF-2α and metabolic reprogramming (105). These metabolic shifts allow
macrophages to produce more energy and biosynthetic precursors,
such as α-ketoglutarate, thereby supporting the rapid growth
demands of tumors (Fig. 4B).
Additionally, macrophage-derived HIF extends beyond
macrophages, influencing the TME by modulating other cell types,
such as fibroblasts and endothelial cells. HIF-1α and HIF-2α in
macrophages also exhibit antagonistic effects in regulating tumor
angiogenesis. Macrophage-specific HIF-1α deletion markedly reduces
the proportion of proangiogenic TME in mice, promoting tumor
oxygenation and the response to chemotherapy. By contrast, in
macrophage HIF-2α-deficient tumors, proangiogenic TEMs exhibit
increased CD31+ microvascular density. However, these
tumors experience exacerbated hypoxia and necrosis, probably due to
impaired adaptation to hypoxia. These findings suggest that in
wild-type macrophages, HIF-2α may suppress HIF-1α-dependent TEM
differentiation, thereby limiting excessive and dysfunctional
angiogenesis during tumor progression (106). Eubank et al (107) found that HIF-2α promotes
macrophage production of sVEGFR-1, a soluble decoy receptor that
inhibits VEGF signaling, whereas HIF-1α induces the expression of
VEGF. A lower sVEGFR-1/VEGF ratio, often seen in HIF-2α-deficient
settings, favors tumor angiogenesis and is associated with poor
prognosis (107). Despite its
usual pro-tumorigenic characteristics, HIF-2α can inhibit the
differentiation of macrophages into proangiogenic and M2-like TAMs
under certain conditions (Fig.
4B).
Recent studies suggest that HIF-3α may contribute to
the alternative M2-like polarization of TAMs, particularly in
response to anti-inflammatory signals. Transcriptomic analyses have
revealed that HIF-3α levels are higher in macrophages polarized by
glucocorticoids (such as dexamethasone), a model for M2-like TAMs
(108). This increase is
associated with genes involved in the regulation of transcription
of pluripotent stem cells, such as Klf4, implying that HIF-3α may
help establish a sustained anti-inflammatory or homeostatic state
in TAMs (89,108). HIF-3α is often described as a
negative regulator of HIF-1α signaling, suggesting that it may
inhibit the pro-inflammatory response of macrophages (109).
To summarize, during different stages of tumor
progression, HIFs are regulated by complex signaling networks,
which influence the functions and polarization states of
macrophages. While regulating the functionality of TAMs, tumor
growth and metastasis and antitumor immune responses, HIFs exhibit
overlapping yet distinct roles, reflecting their nuanced
contributions to the TME.
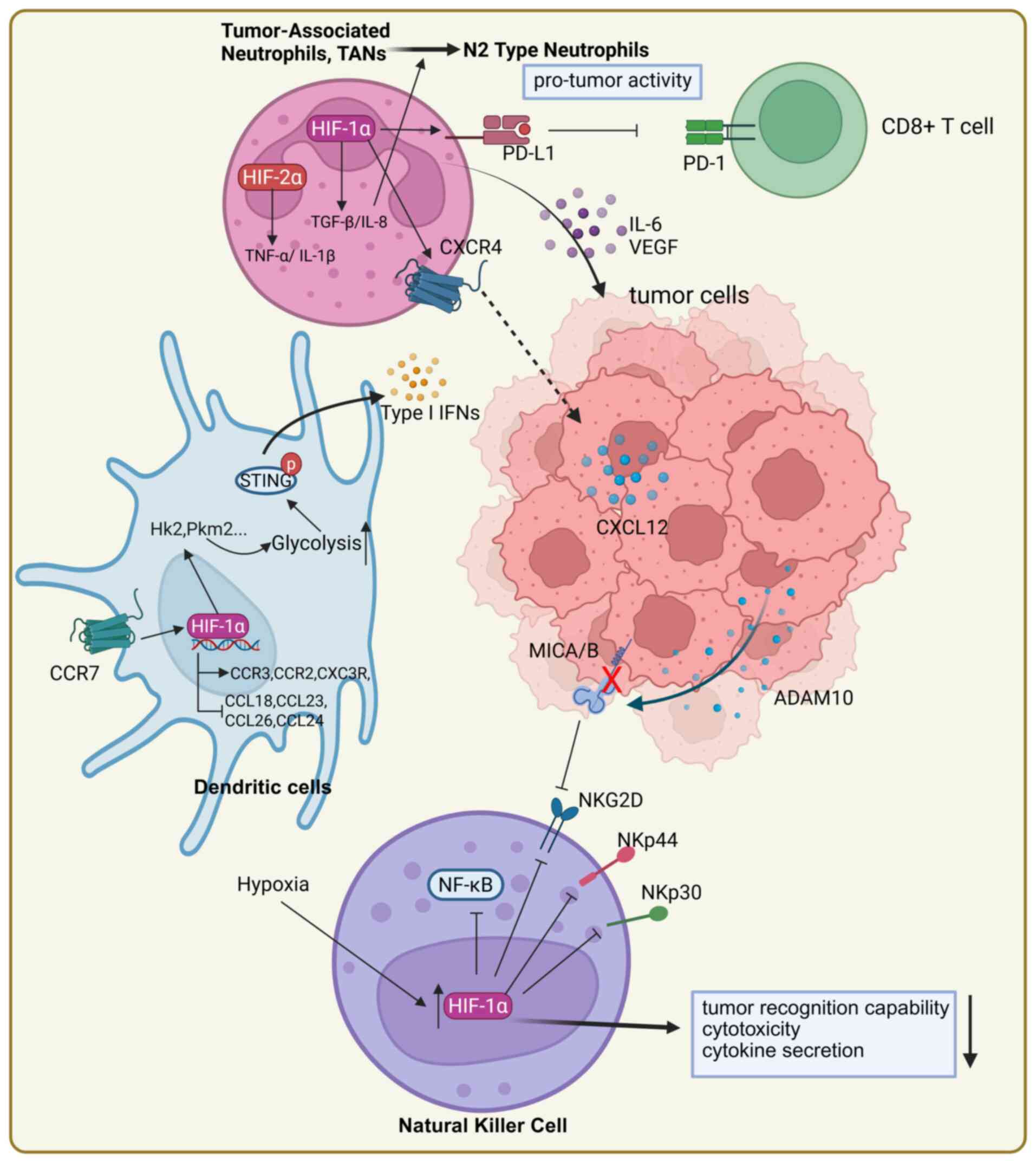
Neutrophils are key effector cells of the innate
immune system and play a complex dual role in tumor immunity. They
exert antitumor effects by directly killing tumor cells. On the
other hand, particularly under hypoxic conditions, neutrophils may
facilitate the initiation and progression of tumors. The
polarization, transcriptional regulation of tumor-associated
neutrophils (TANs) and their interactions with tumors and other
immune cells are closely associated with HIFs (110).
When the microenvironment changes, neutrophils
respond by exhibiting functional plasticity. In tumors, they can be
classified into antitumor N1 and pro-tumor N2 phenotypes. HIF-1α
promotes the formation of N2-type neutrophils by influencing
signaling in the TME. For example, HIF-1α regulates downstream
effectors, such as TGF-β and IL-8, which drive the polarization of
neutrophils toward the N2 phenotype and enhance their pro-tumor
activity (111,112). Additionally, N2-type TANs
promote the growth and invasion of tumors by secreting
pro-inflammatory cytokines, such as IL-6 and angiogenic factors,
such as VEGF (113). HIF-2α can
also enhance the immunosuppressive properties of TANs by inhibiting
their pro-inflammatory responses. Singhal et al (114) demonstrated that the deletion of
HIF-2α in neutrophils slows the growth of colorectal cancer by
reducing the secretion of pro-inflammatory cytokines (such as TNF-α
and IL-1β) and immunosuppressive cytokines.
Moreover, HIF-1α facilitates the migration of
neutrophils to tumor sites by regulating the expression of
chemokine receptors, with the CXCR4/CXCL12 axis serving as a key
pathway. By upregulating the expression of CXCR4, HIF-1α enhances
the responsiveness of neutrophils to the tumor-derived chemokine
CXCL12, thereby accelerating the directed migration of TANs to
tumor sites (115-117). Besides directly promoting the
migration of neutrophils toward tumors, HIF-1α may support tumor
immune evasion by modulating the immunosuppressive functions of
neutrophils. HIF-1α-activated neutrophils express PD-L1, which
inhibits the activity of tumor-infiltrating T cells, thereby
enhancing tumor immune escape (40,118,119). Moreover, when exposed to hypoxia
and signals from cancer and stromal cells, invasive neutrophils in
tumors are stimulated to form neutrophil extracellular traps
(NETs). In gastric cancer, hypoxic conditions induce neutrophil
infiltration and the formation of NETs via the activation of
HIF-1α. During this process, high-mobility group box 1 translocates
from the nucleus to the cytoplasm in gastric cancer cells and
mediates NET formation through the TLR4/p38 MAPK signaling pathway.
NETs exacerbate the progression of gastric cancer by promoting
angiogenesis rather than directly enhancing the proliferation of
cancer cells (120) (Fig. 5).
The role of HIF-3α in neutrophils is particularly
understudied, but recent studies have found its involvement in
stress-induced adaptation of neutrophils. In models of chronic
stress, glucocorticoid signaling upregulates the expression of the
HIF3A gene in neutrophils, linking HIF-3α to
neuroendocrine-immune cross-talk in the TME. This suggests that
HIF-3α may help fine-tune neutrophil responses to systemic cues,
such as stress hormones, rather than direct hypoxia. For example,
HIF-3α may modulate neutrophil migration (e.g., via CXCR4/CXCL12
axis), formation of NETs, or polarization toward a pro-tumor (N2)
phenotype (114). However, no
study has directly verified these hypotheses. As HIF-1α promotes
NETs and immunosuppressive functions in TANs, HIF-3α might act as a
counterbalance and is worthy of further study (120).
Dendritic cells (DCs) are complex antigen-presenting
cells, exhibiting phenotypic heterogeneity and functional
plasticity. They migrate from peripheral tissues to lymph nodes,
where they interact with T cells to trigger specific immune
responses. The normal chemotaxis and migratory capacity of DCs
greatly contribute to their ability to present tumor antigens
(121,122). DCs generated under hypoxia
display a distinguishable migratory phenotype. The migration of DCs
depends on the expression of chemokine receptors on their surface.
HIF-1α can regulate the expression of chemokine receptors (123). Under hypoxic conditions, the
mRNA expression of chemokine receptors CCR3, CX3CR1 and CCR2 is
enhanced in an HIF-1α-dependent manner; these genes are associated
with the functions of DCs; their upregulation increases ligand
sensitivity and migratory capacity of DCs. Concurrently, there is a
marked increase in the release of CCL17, CCL22 and IL-22. By
contrast, the mRNA expression of CCL18, CCL23, CCL26 and CCL24 is
decreased; these downregulated genes are involved in the
recruitment of other inflammatory leukocytes and a decrease in
their expression leads to impaired recruitment ability (124,125). Moreover, the most important
chemotactic response for the migration of DCs is mediated by C-C
motif chemokine receptor 7 (CCR7). Complex signaling pathways
involving PI3K/AKT, MAPK/NF-κB, HIF-1α and IRFs are activated by
CCR7 and play regulatory feedback roles in the migration of DCs in
a context-dependent manner (122). CCR7 stimulation activated the
HIF-1α transcription factor pathway in DCs, leading to metabolic
reprogramming toward glycolysis for the migration of DCs (126).
The metabolic state of DCs plays an important role
in maintaining their tumor immune function. Mature DCs primarily
rely on oxidative phosphorylation, while immature DCs primarily
rely on glycolysis (127). Under
hypoxic conditions, activation of HIF-1α leads to metabolic
reprogramming in DCs, altering their normal function. For example,
HIF-1α increases the glycolytic activity of DCs by upregulating the
expression of key glycolytic enzymes, such as HK2 (128,129). Hu et al (130) used LDHA/LDHB DC-conditional
knockout mice to demonstrate that the deficiency of LDHA/LDHB
inhibits glycolytic metabolism and ATP production, thereby
weakening the stimulator of interferon gene (STING)-dependent type
I interferon response and limiting the antitumor function of DCs.
Activation of the STING signaling pathway in DCs promotes
HIF-1α-mediated glycolysis, triggering a positive feedback loop
centered on PI3K, which drives effector T cell responses (130-132). Clinical trials have shown that
glycolysis also promotes the activity of STING-dependent DCs in
tissue samples from non-small cell lung cancer patients (130). This suggests that the cross-talk
between HIF-1α-mediated glycolytic metabolism and STING signaling
may enhance the antitumor activity of DCs.
However, an increase in glycolysis under hypoxic
conditions leads to the excessive accumulation of lactate. Lactate
interacts with HIF-1α to inhibit the interaction between DCs and T
cells, inducing the tumor immunosuppressive properties of DCs
(133). Under hypoxic
conditions, lactate produced by DCs promotes the expression of
NDUFA4L2 through HIF-1α. The increase in the expression of NDUFA4L2
suppresses the pro-inflammatory response driven by mitochondrial
reactive oxygen species and X-box binding protein in DCs (134). Additionally, the activation of
NF-κB under hypoxia further increases HIF-1α levels stabilized by
lactate (135). The positive
feedback loop formed through the HIF-1α/NDUFA4L2 signaling axis
limits the regulation of T cell immune responses by DCs, thereby
exacerbating the immunosuppressive effect of HIF-1α in the TME
(135).
Several studies have reported that the maturation
and function of DCs are influenced by several HIF-1α-modulated
factors, such as VEGF and IL-10, in the TME. The production of VEGF
by human tumors can inhibit the functional maturation of DCs and
accumulation of MDSCs that inhibit the functions of T cells
(136,137). HIF-1α also induces
tumor-associated DCs to express PD-L1 and other T-cell inhibitory
molecules, weakening the function of CD8+ T-cells and
ultimately leading to tumor immune evasion (138,139).
To summarize, HIF-1α regulates the function of DCs
in the TME through multiple mechanisms, markedly influencing DC
maturation, metabolism, migration and the formation of
immunosuppressive phenotypes. Activation of HIF-1α not only affects
the antigen-presenting capability of DCs but also plays a key role
in tumor initiation and progression by driving metabolic
reprogramming and immune evasion pathways (Fig. 5).
NK cells are crucial effector cells in innate
immunity, capable of directly killing tumor cells. The activity of
NK cells is regulated by their surface receptors, which include
both activating and inhibitory receptors. The hypoxic conditions in
the TME and the activation of HIF negatively affect the activity of
NK cells. In vitro studies have shown that hypoxic
stimulation and activation of HIF-1α markedly reduce the expression
of activating receptors on the surface of NK cells, such as NKp30,
NKp44 and NKG2D. This weakens the ability of NK cells to recognize
tumor cells, which decreases their cytotoxicity and ability to
secrete cytokines (140).
Moreover, HIF-1α induces tumor cells to release A disintegrin and
metalloproteinase domain 10 (ADAM10), which cleaves surface MICA/B
ligands. This shedding decreases the level of NKG2D on the surface
of NK cells, ultimately facilitating immune evasion (141). Some researchers found that
conditional knockout of HIF-1α in NK cells of mice inhibited the
growth of tumor cells and upregulated activation markers such as
CD69, while promoting the activation of the NF-κB pathway, thus
enhancing antitumor responses (142). Clinically, low HIF-1α expression
in infiltrating NK cells is markedly associated with high
NF-κB/IFN-γ expression profiles and this characteristic is
associated with markedly higher survival rates in cancer patients
(142). A similar result was
found by Nakazawa et al (143), who observed that in glioblastoma
(GBM), overexpression of HIF-1α impaired the function of NK cells,
reducing their cytotoxicity against tumor cells. Knocking out
HIF-1α in NK cells markedly enhanced the cytotoxicity of NK cells
under hypoxic conditions and induced apoptosis in GBM cells
(143). These studies suggest
that HIF-1α directly participates in the negative regulation of the
tumor-killing functions of NK cells.
The role of HIF-1α in regulating NK cells is complex
and context-dependent. Under hypoxic conditions, human NK cell
lines stimulated with IL-2 exhibit enhanced expression of HIF-1α,
which in turn improves the antitumor cytotoxicity and IFN-γ
secretion capacity of NK cells (144). In this process, the stability of
HIF-1α is important for maximizing the effector function of NK
cells.
Moreover, based on its behavior in other cell types,
HIF-3α may inhibit HIF-1α-driven pathways in NK cells, thereby
attenuating their antitumor activity. Alternatively, HIF-3α may
support the metabolic adaptation of NK cells under prolonged
hypoxia, similar to its role in some epithelial cells (145). Future studies need to verify
whether HIF-3α is expressed in NK cells, how it is regulated and
whether it affects key functions such as target cell killing and
IFN-γ secretion.
To summarize, the effects of HIF-1α on NK cells in
tumors or cancer are multifaceted, complex and highly dynamic.
HIF-1α impairs the function of NK cells in the TME through various
mechanisms, including its effect on the activity of NK cells,
receptor expression and secretion functions. These combined effects
contribute to tumor immune evasion (Fig. 5).
Several studies have reported that HIFs not only
regulate tumor cell proliferation, metabolism and angiogenesis but
also play a complex role in immune regulation within the TME.
Numerous natural and synthetic compounds have been identified as
potential inhibitors of HIFs (Table
I), which can specifically regulate the function of HIFs and be
used in cancer treatment. The available therapeutic strategies
targeting HIFs primarily include siRNA therapy, blocking the
upstream regulators of HIFs, or directly inhibiting HIF-1α with
anticancer drugs (13). For
example, a phase I clinical study demonstrated that the
topoisomerase inhibitor topotecan could inhibit the translation of
the HIF-1α protein, thereby reducing tumor angiogenesis and growth
(146). The selective HIF-2α
inhibitor belzutifan has demonstrated significant therapeutic
advancement in clinical trials (147). Belzutifan binds to the PAS-B
domain of HIF-2α, blocking its dimerization with ARNT, thus
inhibiting the transcriptional activation of downstream genes
(147). In patients with RCC,
belzutifan showed significant antitumor activity, with an objective
response rate of 49% (147). On
the other hand, its clinical application has health hazards, such
as anemia, which is a targeted adverse effect (147). The existing HIF inhibitors still
face significant challenges in clinical application. The primary
concern is dose-limiting adverse effects due to different
pharmacokinetic characteristics (148). This limits the long-term use of
these drugs in cancer treatment. Additionally, the acidic TME may
reduce drug concentrations, impair the tissue-specific delivery of
HIF inhibitors and increase the activity of the multidrug
transporter P-gp, leading to enhanced tumor resistance, all of
which interfere with the therapeutic effectiveness of HIF
inhibitors (14,15).
Precision-targeted drug delivery systems can
overcome these challenges and can be used to develop effective
therapeutic strategies: i) HIF-activating immunotherapeutic agents
can be coupled with molecules that specifically recognize tumor
cells or stromal components to increase drug concentration at the
targeted site. ii) Stimuli-responsive lipid-based microparticles or
nanovesicle carriers can be used to allow AI-optimized drug release
kinetics and spatiotemporally controlled tissue-specific
distribution; iii) Prodrugs that can be selectively activated under
specific conditions in the TME (such as low pH, high ATP
concentration, or overexpression of certain proteases) need to be
developed; iv) By fusing mitochondrial targeting sequences with
functional domains (such as HIF-inhibitory domains and succinate
dehydrogenase-activating domains), chimeric peptides with specific
mitochondrial-tumor targeting properties can be developed to
selectively inhibit the cross-talk between HIF and mitochondrial
metabolism in cancer cells, ultimately reversing the Warburg effect
and the immunosuppressive TME (149-151). Preliminary investigation into
these strategies has been conducted. For example, a recent study
demonstrated that PX478 (an HIF inhibitor) conjugated with silk
fibroin nanoparticles reverses multidrug resistance by enhancing
the efficacy of doxorubicin in MCF-7/ADR cells (152,153). Wu et al (149) found that the chimeric peptide
Mito-HIF-1α effectively decreased lactate production in cancer
cells by 70% in a pancreatic cancer model, while restoring
mitochondrial respiratory function in CD8+ T cells.
Designing and developing emerging therapeutic strategies, including
active targeted delivery (immunoconjugate), intelligent material
design (lipid carriers), microenvironment-responsive mechanisms
(prodrug activation) and precise organelle intervention
(mitochondrial chimeric peptides), may increase therapeutic
efficacy while minimizing systemic adverse effects. These
approaches can overcome the limitations of current drug development
strategies, providing more effective options for treating cancer
patients. However, further interdisciplinary research and clinical
validation are needed to develop and optimize these therapeutic
strategies.
Over the past three decades, research on HIFs has
considerably advanced our understanding of cellular adaptation to
hypoxia, particularly in the field of cancer biology. These studies
have revealed the central regulatory functions of the HIF pathway
in the proliferation of tumor cells, metabolic reprogramming and
angiogenesis, while also highlighting its complex role in immune
regulation. The distinct and often opposing functions of HIF-1α and
HIF-2α in the TME highlight the context-dependent nature of this
signaling pathway. This is especially evident in immune cells,
where HIFs orchestrate a delicate balance between metabolic
adaptation and functional responses across diverse populations,
including T cells, B cells, macrophages, neutrophils, DCs and NK
cells, directly influencing their antitumor efficacy and
persistence (Table II).
Although HIF-targeted therapeutic techniques have
achieved preliminary clinical success, their broader application
faces significant challenges. Dose-limiting toxicities, drug
resistance and the complicating effects of the acidic TME on drug
delivery remain major obstacles. The available strategies to
overcome these limitations include the development of
precision-targeted drug delivery systems, such as
nanoparticle-based platforms and the optimization of combination
therapy. Future studies should further assess the multifaceted
roles of HIF signaling in complex immune environments, with an
emphasis on understanding the cell-type-specific functions of
different HIF isoforms and their interaction networks. By
integrating HIF biology with emerging technologies in drug
delivery, single-cell multi-omics and spatial transcriptomics,
researchers can advance the rational design of next-generation
immunotherapeutic strategies. Finally, targeted manipulation of the
HIF pathway is highly promising for developing more precise and
effective cancer treatments that modulate both metabolism and
immunity while preserving immune function.
The main strength of the present review lies in its
systematic synthesis of recent discoveries concerning HIF-driven
regulation of the immune system, integrating mechanistic insights
with translational relevance. Unlike previous reviews that have
focused on tumor cell metabolism or angiogenesis, the present
review emphasized the cell-type-specific roles of HIFs across major
immune populations. By highlighting the metabolic and immunologic
duality of HIF signaling, it provided a nuanced perspective on how
hypoxia influences tumor-immune dynamics and identifies potential
targets for combined HIF and immunotherapy.
However, the present review had several
limitations. i) Most of the mechanistic insights summarized here
are derived from preclinical or in vitro models and their
physiological relevance in human tumors requires further
validation. ii) The spatial and temporal heterogeneity of oxygen
gradients, metabolic states and immune cell composition in the TME
makes it difficult to extrapolate experimental findings across
different types of cancer. iii) Unlike HIF-1α and HIF-2α, HIF-3α
has important functions in macrophage polarization, neutrophil
activation and NK cytotoxicity; moreover, HIF-3α plays a more
subtle and environment-dependent role. However, research on the
regulatory effect of HIF-3α on tumor-infiltrating immune cells is
still lacking and further studies are needed. Moreover, existing
HIF-targeted therapeutic interventions are hampered by contextual
efficacy and off-target toxicity. Future studies should use
single-cell multi-omics, metabolic imaging and spatial
transcriptomic technologies to elucidate cell-specific regulatory
networks and prioritize well-designed clinical trials to confirm
the immunomodulatory effect of HIF inhibition.
To summarize, while the current understanding of
HIFs in tumor-infiltrating immune cells provides a solid conceptual
foundation, translating these insights into clinically actionable
strategies will require continued interdisciplinary effort.
Not applicable.
QS, CL and YL conceived and designed the research.
CL and CQ drafted the manuscript. YL revised the manuscript. JW and
XL collated the literature. Data authentication is not applicable.
All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Shandong Province Natural
Science Foundation (grant no. ZR2025QC326), Taishan Scholars (grant
no. TSQN 202312181) and Youth Innovation Team Project of Shandong
province (grant no. 2023RW102).
|
1
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15:772022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao C, Liu X, Zhang C and Zhang Q: Tumor
hypoxia: From basic knowledge to therapeutic implications. Semin
Cancer Biol. 88:172–186. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhuang Y, Liu K, He Q, Gu X, Jiang C and
Wu J: Hypoxia signaling in cancer: Implications for therapeutic
interventions. MedComm (2020). 4:e2032023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wicks EE and Semenza GL: Hypoxia-inducible
factors: Cancer progression and clinical translation. J Clin
Invest. 132:e1598392022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loboda A, Jozkowicz A and Dulak J: HIF-1
and HIF-2 transcription factors-similar but not identical. Mol
Cells. 29:435–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You L, Wu W, Wang X, Fang L, Adam V,
Nepovimova E, Wu Q and Kuca K: The role of hypoxia-inducible factor
1 in tumor immune evasion. Med Res Rev. 41:1622–1643. 2021.
View Article : Google Scholar
|
|
7
|
Palazón A, Martínez-Forero I, Teijeira A,
Morales-Kastresana A, Alfaro C, Sanmamed MF, Perez-Gracia JL,
Peñuelas I, Hervás-Stubbs S, Rouzaut A, et al: The HIF-1α hypoxia
response in tumor-infiltrating T lymphocytes induces functional
CD137 (4-1BB) for immunotherapy. Cancer Discov. 2:608–623. 2012.
View Article : Google Scholar
|
|
8
|
Labiano S, Palazón A, Bolaños E,
Azpilikueta A, Sánchez-Paulete AR, Morales-Kastresana A, Quetglas
JI, Perez-Gracia JL, Gúrpide A, Rodriguez-Ruiz M, et al:
Hypoxia-induced soluble CD137 in malignant cells blocks
CD137L-costimulation as an immune escape mechanism. Oncoimmunology.
5:e10629672016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li NA, Wang H, Zhang J and Zhao E:
Knockdown of hypoxia inducible factor-2α inhibits cell invasion via
the downregulation of MMP-2 expression in breast cancer cells.
Oncol Lett. 11:3743–3748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79. 2012.
|
|
11
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong Y, Liu L, Xia Y, Qi Y, Chen Y, Chen
L, Zhang P, Kong Y, Qu Y, Wang Z, et al: Tumor infiltrating mast
cells determine oncogenic HIF-2α-conferred immune evasion in clear
cell renal cell carcinoma. Cancer Immunol Immunother. 68:731–741.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jing X, Yang F, Shao C, Wei K, Xie M, Shen
H and Shu Y: Role of hypoxia in cancer therapy by regulating the
tumor microenvironment. Mol Cancer. 18:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wohlkoenig C, Leithner K, Deutsch A,
Hrzenjak A, Olschewski A and Olschewski H: Hypoxia-induced
cisplatin resistance is reversible and growth rate independent in
lung cancer cells. Cancer Lett. 308:134–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zub KA, Sousa MM, Sarno A, Sharma A,
Demirovic A, Rao S, Young C, Aas PA, Ericsson I, Sundan A, et al:
Modulation of cell metabolic pathways and oxidative stress
signaling contribute to acquired melphalan resistance in multiple
myeloma cells. PLoS One. 10:e01198572015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl hydroxylation.
Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia X, Lemieux ME, Li W, Carroll JS, Brown
M, Liu XS and Kung AL: Integrative analysis of HIF binding and
transactivation reveals its role in maintaining histone methylation
homeostasis. Proc Natl Acad Sci USA. 106:4260–4265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schödel J, Oikonomopoulos S, Ragoussis J,
Pugh CW, Ratcliffe PJ and Mole DR: High-resolution genome-wide
mapping of HIF-binding sites by ChIP-seq. Blood. 117:e207–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mole DR, Blancher C, Copley RR, Pollard
PJ, Gleadle JM, Ragoussis J and Ratcliffe PJ: Genome-wide
association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha
DNA binding with expression profiling of hypoxia-inducible
transcripts. J Biol Chem. 284:16767–16775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warnecke C, Zaborowska Z, Kurreck J,
Erdmann VA, Frei U, Wiesener M and Eckardt KU: Differentiating the
functional role of hypoxia-inducible factor (HIF)-1alpha and
HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin
is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J.
18:1462–1464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujiwara S, Nakagawa K, Harada H, Nagato
S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S and Ohnishi T:
Silencing hypoxia-inducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.PubMed/NCBI
|
|
27
|
Tian H, McKnight SL and Russell DW:
Endothelial PAS domain protein 1 (EPAS1), a transcription factor
selectively expressed in endothelial cells. Genes Dev. 11:72–82.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiesener MS, Jürgensen JS, Rosenberger C,
Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei
UA, Pugh CW, et al: Widespread hypoxia-inducible expression of
HIF-2alpha in distinct cell populations of different organs. FASEB
J. 17:271–273. 2003. View Article : Google Scholar
|
|
29
|
Raval RR, Lau KW, Tran MG, Sowter HM,
Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL and Ratcliffe
PJ: Contrasting properties of hypoxia-inducible factor 1 (HIF-1)
and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol
Cell Biol. 25:5675–5686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parkin J and Cohen B: An overview of the
immune system. Lancet. 357:1777–1789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palazon A, Goldrath AW, Nizet V and
Johnson RS: HIF transcription factors, inflammation, and immunity.
Immunity. 41:518–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peyssonnaux C, Datta V, Cramer T, Doedens
A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V and Johnson
RS: HIF-1alpha expression regulates the bactericidal capacity of
phagocytes. J Clin Invest. 115:1806–1815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cowman SJ and Koh MY: Revisiting the HIF
switch in the tumor and its immune microenvironment. Trends Cancer.
8:28–42. 2022. View Article : Google Scholar
|
|
34
|
Taylor CT and Scholz CC: The effect of HIF
on metabolism and immunity. Nat Rev Nephrol. 18:573–587. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vito A, El-Sayes N and Mossman K:
Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells.
9:9922020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liikanen I, Lauhan C, Quon S, Omilusik K,
Phan AT, Bartrolí LB, Ferry A, Goulding J, Chen J, Scott-Browne JP,
et al: Hypoxia-inducible factor activity promotes antitumor
effector function and tissue residency by CD8+ T cells. J Clin
Invest. 131:e1437292021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dhalla NS, Camargo RO, Elimban V, Dhadial
RS and Xu YJ: Role of Skeletal Muscle Angiogenesis in Peripheral
Artery Disease. Biochemical Basis and Therapeutic Implications of
Angiogenesis. 517–532. 2017. View Article : Google Scholar
|
|
38
|
de Heer EC, Jalving M and Harris AL: HIFs,
angiogenesis, and metabolism: Elusive enemies in breast cancer. J
Clin Invest. 130:5074–5087. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ozga AJ, Chow MT and Luster AD: Chemokines
and the immune response to cancer. Immunity. 54:859–874. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palmer CS, Ostrowski M, Balderson B,
Christian N and Crowe SM: Glucose metabolism regulates T cell
activation, differentiation, and functions. Front Immunol. 6:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Menk AV, Scharping NE, Moreci RS, Zeng X,
Guy C, Salvatore S, Bae H, Xie J, Young HA, Wendell SG, et al:
Early TCR signaling induces rapid aerobic glycolysis enabling
distinct acute T cell effector functions. Cell Rep. 22:1509–1521.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang CH, Curtis JD, Maggi LB Jr, Faubert
B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih
J, Qiu J, et al: Posttranscriptional control of T cell effector
function by aerobic glycolysis. Cell. 153:1239–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leone RD and Powell JD: Metabolism of
immune cells in cancer. Nat Rev Cancer. 20:516–531. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J and Green DR: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Finlay DK, Rosenzweig E, Sinclair LV,
Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K
and Cantrell DA: PDK1 regulation of mTOR and hypoxia-inducible
factor 1 integrate metabolism and migration of CD8+ T cells. J Exp
Med. 209:2441–2453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohta A, Diwanji R, Kini R, Subramanian M,
Ohta A and Sitkovsky M: In vivo T cell activation in lymphoid
tissues is inhibited in the oxygen-poor microenvironment. Front
Immunol. 2:272011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Byrnes JR, Weeks AM, Shifrut E, Carnevale
J, Kirkemo L, Ashworth A, Marson A and Wells JA: Hypoxia is a
dominant remodeler of the effector T cell surface proteome relative
to activation and regulatory T cell suppression. Mol Cell
Proteomics. 21:1002172022. View Article : Google Scholar
|
|
49
|
Cunha PP, Minogue E, Krause LCM, Hess RM,
Bargiela D, Wadsworth BJ, Barbieri L, Brombach C, Foskolou IP,
Bogeski I, et al: Oxygen levels at the time of activation determine
T cell persistence and immunotherapeutic efficacy. Elife.
12:e842802023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu X, Chen J, Li W, Xu Y, Shan J, Hong J,
Zhao Y, Xu H, Ma J, Shen J and Qian C: Hypoxia-responsive CAR-T
cells exhibit reduced exhaustion and enhanced efficacy in solid
tumors. Cancer Res. 84:84–100. 2024. View Article : Google Scholar
|
|
51
|
Wang R and Solt LA: Metabolism of murine
TH 17 cells: Impact on cell fate and function. Eur J Immunol.
46:807–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi LZ, Wang R, Huang G, Vogel P, Neale G,
Green DR and Chi H: HIF1alpha-dependent glycolytic pathway
orchestrates a metabolic checkpoint for the differentiation of TH17
and Treg cells. J Exp Med. 208:1367–1376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al: Control of
T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell.
146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Altınönder İ, Kaya M, Yentür SP, Çakar A,
Durmuş H, Yegen G, Özkan B, Parman Y, Sawalha AH and
Saruhan-Direskeneli G: Thymic gene expression analysis reveals a
potential link between HIF-1A and Th17/Treg imbalance in thymoma
associated myasthenia gravis. J Neuroinflammation. 21:1262024.
View Article : Google Scholar
|
|
56
|
Böttcher M, Renner K, Berger R, Mentz K,
Thomas S, Cardenas-Conejo ZE, Dettmer K, Oefner PJ, Mackensen A,
Kreutz M and Mougiakakos D: D-2-hydroxyglutarate interferes with
HIF-1α stability skewing T-cell metabolism towards oxidative
phosphorylation and impairing Th17 polarization. Oncoimmunology.
7:e14454542018. View Article : Google Scholar
|
|
57
|
Clever D, Roychoudhuri R, Constantinides
MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z,
Pan JH, et al: Oxygen sensing by T cells establishes an
immunologically tolerant metastatic niche. Cell. 166:1117–1131.e14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Overacre-Delgoffe AE, Chikina M, Dadey RE,
Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK,
Sander C, et al: Interferon-γ drives Treg fragility to
promote anti-tumor immunity. Cell. 169:1130–1141.e11. 2017.
View Article : Google Scholar
|
|
59
|
Neildez-Nguyen TMA, Bigot J, Da Rocha S,
Corre G, Boisgerault F, Paldi A and Galy A: Hypoxic culture
conditions enhance the generation of regulatory T cells.
Immunology. 144:431–443. 2015. View Article : Google Scholar :
|
|
60
|
Clambey ET, McNamee EN, Westrich JA,
Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC,
Stenmark KR, Colgan SP and Eltzschig HK: Hypoxia-inducible factor-1
alpha-dependent induction of FoxP3 drives regulatory T-cell
abundance and function during inflammatory hypoxia of the mucosa.
Proc Natl Acad Sci USA. 109:E2784–E2793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu J, Cui H, Zhu Z, Wang L, Li H and Wang
D: Effect of HIF1α on Foxp3 expression in CD4+ CD25-T lymphocytes.
Microbiol Immunol. 58:409–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsu TS, Lin YL, Wang YA, Mo ST, Chi PY,
Lai AC, Pan HY, Chang YJ and Lai MZ: HIF-2α is indispensable for
regulatory T cell function. Nat Commun. 11:50052020. View Article : Google Scholar
|
|
63
|
Koh MY and Powis G: Passing the baton: The
HIF switch. Trends Biochem Sci. 37:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Palazon A, Tyrakis PA, Macias D, Veliça P,
Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk
I, et al: An HIF-1α/VEGF-A axis in cytotoxic T cells regulates
tumor progression. Cancer Cell. 32:669–683.e5. 2017. View Article : Google Scholar
|
|
65
|
Bisilliat Donnet C, Acolty V, Azouz A,
Taquin A, Henin C, Trusso Cafarello S, Denanglaire S, Mazzone M,
Oldenhove G, Leo O, et al: PHD2 constrains antitumor CD8+ T-cell
activity. Cancer Immunol Res. 11:339–350. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dvorakova T, Finisguerra V, Formenti M,
Loriot A, Boudhan L, Zhu J and Van den Eynde BJ: Enhanced tumor
response to adoptive T cell therapy with PHD2/3-deficient CD8 T
cells. Nat Commun. 15:77892024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bargiela D, Cunha PP, Veliça P, Krause
LCM, Brice M, Barbieri L, Gojkovic M, Foskolou IP, Rundqvist H and
Johnson RS: The factor inhibiting HIF regulates T cell
differentiation and anti-tumour efficacy. Front Immunol.
15:12937232024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Walter Jackson I, Yang Y, Salman S, Dordai
D, Lyu Y, Datan E, Drehmer D, Huang TY, Hwang Y and Semenza GL:
Pharmacologic HIF stabilization activates costimulatory receptor
expression to increase antitumor efficacy of adoptive T cell
therapy. Sci Adv. 10:eadq23662024. View Article : Google Scholar
|
|
69
|
Tyrakis PA, Palazon A, Macias D, Lee KL,
Phan AT, Veliça P, You J, Chia GS, Sim J, Doedens A, et al:
S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate.
Nature. 540:236–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lv Q, Su T, Liu W, Wang L, Hu J, Cheng Y,
Ning C, Shan W, Luo X and Chen X: Low serum apolipoprotein A1
levels impair antitumor immunity of CD8+ T cells via the
HIF-1α-glycolysis pathway. Cancer Immunol Res. 12:1058–1073. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Veliça P, Cunha PP, Vojnovic N, Foskolou
IP, Bargiela D, Gojkovic M, Rundqvist H and Johnson RS: Modified
Hypoxia-inducible factor expression in CD8+ T cells
increases antitumor efficacy. Cancer Immunol Res. 9:401–414. 2021.
View Article : Google Scholar
|
|
72
|
Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang
G, Hudaihed A, Filisio F, Giles-Davis W, Xu X, Karakousis GC, et
al: Enhancing CD8+ T cell fatty acid catabolism within a
metabolically challenging tumor microenvironment increases the
efficacy of melanoma immunotherapy. Cancer Cell. 32:377–391.e9.
2017. View Article : Google Scholar
|
|
73
|
Loisel-Meyer S, Swainson L, Craveiro M,
Oburoglu L, Mongellaz C, Costa C, Martinez M, Cosset FL, Battini
JL, Herzenberg LA, et al: Glut1-mediated glucose transport
regulates HIV infection. Proc Natl Acad Sci USA. 109:2549–2554.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rawlings DJ, Metzler G, Wray-Dutra M and
Jackson SW: Altered B cell signalling in autoimmunity. Nat Rev
Immunol. 17:421–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Franchina DG, Grusdat M and Brenner D:
B-cell metabolic remodeling and cancer. Trends Cancer. 4:138–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Herzog S, Reth M and Jumaa H: Regulation
of B-cell proliferation and differentiation by pre-B-cell receptor
signalling. Nat Rev Immunol. 9:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burrows N, Bashford-Rogers RJM, Bhute VJ,
Peñalver A, Ferdinand JR, Stewart BJ, Smith JEG, Deobagkar-Lele M,
Giudice G, Connor TM, et al: Dynamic regulation of
hypoxia-inducible factor-1α activity is essential for normal B cell
development. Nat Immunol. 21:1408–1420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li S, Huang C, Hu G, Ma J, Chen Y, Zhang
J, Huang Y, Zheng J, Xue W, Xu Y and Zhai W: Tumor-educated B cells
promote renal cancer metastasis via inducing the
IL-1β/HIF-2α/Notch1 signals. Cell Death Dis. 11:1632020. View Article : Google Scholar
|
|
79
|
Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu
C, Chen Y, Zhou W, Lin A, Yu L, et al: Specific decrease in
B-cell-derived extracellular vesicles enhances
post-chemotherapeutic CD8+ T cell responses. Immunity.
50:738–750.e7. 2019. View Article : Google Scholar
|
|
80
|
Ghosh AK, Shanafelt TD, Cimmino A,
Taccioli C, Volinia S, Liu CG, Calin GA, Croce CM, Chan DA, Giaccia
AJ, et al: Aberrant regulation of pVHL levels by microRNA promotes
the HIF/VEGF axis in CLL B cells. Blood. 113:5568–5574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jin Z, Xiang R, Dai J, Wang Y and Xu Z:
HIF-1α mediates CXCR4 transcription to activate the AKT/mTOR
signaling pathway and augment the viability and migration of
activated B cell-like diffuse large B-cell lymphoma cells. Mol
Carcinog. 62:676–684. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gao J, Liang Y and Wang L: Shaping
polarization of tumor-associated macrophages in cancer
immunotherapy. Front Immunol. 13:8887132022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Anderson NR, Minutolo NG, Gill S and
Klichinsky M: Macrophage-based approaches for cancer immunotherapy.
Cancer Res. 81:1201–1208. 2021. View Article : Google Scholar
|
|
86
|
Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H,
Xiao GG, Rao L and Duo Y: Macrophages in immunoregulation and
therapeutics. Signal Transduct Target Ther. 8:2072023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Takeda N, O'Dea EL, Doedens A, Kim JW,
Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A and
Johnson RS: Differential activation and antagonistic function of
HIF-{alpha} isoforms in macrophages are essential for NO
homeostasis. Genes Dev. 24:491–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang T, Liu H, Lian G, Zhang SY, Wang X
and Jiang C: HIF1α-induced glycolysis metabolism is essential to
the activation of inflammatory macrophages. Mediators Inflamm.
2017:90293272017. View Article : Google Scholar
|
|
89
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Leek RD, Talks KL, Pezzella F, Turley H,
Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC and Harris AL:
Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha)
expression in tumor-infiltrative macrophages to tumor angiogenesis
and the oxidative thymidine phosphorylase pathway in Human breast
cancer. Cancer Res. 62:1326–1329. 2002.PubMed/NCBI
|
|
91
|
Kawanaka T, Kubo A, Ikushima H, Sano T,
Takegawa Y and Nishitani H: Prognostic significance of HIF-2alpha
expression on tumor infiltrating macrophages in patients with
uterine cervical cancer undergoing radiotherapy. J Med Invest.
55:78–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Imtiyaz HZ, Williams EP, Hickey MM, Patel
SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B and Simon
MC: Hypoxia-inducible factor 2alpha regulates macrophage function
in mouse models of acute and tumor inflammation. J Clin Invest.
120:2699–2714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia
Y, Wei Z, Xie X, Yin B, Chen F, et al: Lactate inhibits ATP6V0d2
expression in tumor-associated macrophages to promote
HIF-2α-mediated tumor progression. J Clin Invest. 129:631–646.
2019. View Article : Google Scholar :
|
|
94
|
Wu JY, Huang TW, Hsieh YT, Wang YF, Yen
CC, Lee GL, Yeh CC, Peng YJ, Kuo YY, Wen HT, et al: Cancer-derived
succinate promotes macrophage polarization and cancer metastasis
via succinate receptor. Mol Cell. 77:213–227.e5. 2020. View Article : Google Scholar
|
|
95
|
Tannahill GM, Curtis AM, Adamik J,
Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ,
Kelly B, Foley NH, et al: Succinate is an inflammatory signal that
induces IL-1β through HIF-1α. Nature. 496:238–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei
MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al: Mutations of the
VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7:85–90.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cho H, Du X, Rizzi JP, Liberzon E,
Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA,
et al: On-target efficacy of a HIF-2α antagonist in preclinical
kidney cancer models. Nature. 539:107–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ricketts CJ, Crooks DR and Linehan WM:
Targeting HIF2α in Clear-cell renal cell carcinoma. Cancer Cell.
30:515–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen W, Hill H, Christie A, Kim MS,
Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, et
al: Targeting renal cell carcinoma with a HIF-2 antagonist. Nature.
539:112–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shen C, Beroukhim R, Schumacher SE, Zhou
J, Chang M, Signoretti S and Kaelin WG Jr: Genetic and functional
studies implicate HIF1α as a 14q kidney cancer suppressor gene.
Cancer Discov. 1:222–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cowman SJ, Fuja DG, Liu XD, Tidwell RSS,
Kandula N, Sirohi D, Agarwal AM, Emerson LL, Tripp SR, Mohlman JS,
et al: Macrophage HIF-1α is an independent prognostic indicator in
kidney cancer. Clin Cancer Res. 26:4970–4982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu Q, Wang Y, Dong L, He Y, Liu R, Yang Q,
Cao Y, Wang Y, Jia A, Bi Y and Liu G: Regulations of glycolytic
activities on macrophages functions in tumor and infectious
inflammation. Front Cell Infect Microbiol. 10:2872020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pouysségur J, Marchiq I, Parks SK,
Durivault J, Ždralević M and Vucetic M: 'Warburg effect' controls
tumor growth, bacterial, viral infections and immunity-Genetic
deconstruction and therapeutic perspectives. Semin Cancer Biol.
86:334–346. 2022. View Article : Google Scholar
|
|
104
|
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo
JH, Kim JK, Heo Y, Lee HS, Lee MY, et al: A variant of SLC1A5 is a
mitochondrial glutamine transporter for metabolic reprogramming in
cancer cells. Cell Metab. 31:267–283.e12. 2020. View Article : Google Scholar
|
|
105
|
Green NH, Galvan DL, Badal SS, Chang BH,
LeBleu VS, Long J, Jonasch E and Danesh FR: MTHFD2 links RNA
methylation to metabolic reprogramming in renal cell carcinoma.
Oncogene. 38:6211–6225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Steinberger KJ, Forget MA, Bobko AA,
Mihalik NE, Gencheva M, Roda JM, Cole SL, Mo X, Hoblitzell EH,
Evans R, et al: Hypoxia-inducible factor α subunits regulate
Tie2-expressing macrophages that influence tumor oxygen and
perfusion in murine breast cancer. J Immunol. 205:2301–2311. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Eubank TD, Roda JM, Liu H, O'Neil T and
Marsh CB: Opposing roles for HIF-1α and HIF-2α in the regulation of
angiogenesis by mononuclear phagocytes. Blood. 117:323–332. 2011.
View Article : Google Scholar :
|
|
108
|
Deochand DK, Dacic M, Bale MJ, Daman AW,
Chaudhary V, Josefowicz SZ, Oliver D, Chinenov Y and Rogatsky I:
Mechanisms of epigenomic and functional convergence between
glucocorticoid- and IL4-driven macrophage programming. Nat Commun.
15:90002024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hara S, Hamada J, Kobayashi C, Kondo Y and
Imura N: Expression and characterization of hypoxia-inducible
factor (HIF)-3alpha in human kidney: Suppression of HIF-mediated
gene expression by HIF-3alpha. Biochem Biophys Res Commun.
287:808–813. 2001. View Article : Google Scholar
|
|
110
|
Wu G, Pan B, Shi H, Yi Y, Zheng X, Ma H,
Zhao M, Zhang Z, Cheng L, Huang Y and Guo W: Neutrophils' dual role
in cancer: From tumor progression to immunotherapeutic potential.
Int Immunopharmacol. 140:1127882024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Qiu B, Yuan P, Du X, Jin H, Du J and Huang
Y: Hypoxia inducible factor-1α is an important regulator of
macrophage biology. Heliyon. 9:e171672023. View Article : Google Scholar
|
|
112
|
Masucci MT, Minopoli M and Carriero MV:
Tumor associated neutrophils. their role in tumorigenesis,
metastasis, prognosis and therapy. Front Oncol. 9:11462019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: 'N1' versus 'N2'
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Singhal R, Kotla NK, Solanki S, Huang W,
Bell HN, El-Derany MO, Castillo C and Shah YM: Disruption of
hypoxia-inducible factor-2α in neutrophils decreases
colitis-associated colon cancer. Am J Physiol Gastrointest Liver
Physiol. 326:G53–G66. 2024. View Article : Google Scholar
|
|
115
|
LaGory EL and Giaccia AJ: The
ever-expanding role of HIF in tumour and stromal biology. Nat Cell
Biol. 18:356–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Santagata S, Ieranò C, Trotta AM,
Capiluongo A, Auletta F, Guardascione G and Scala S: CXCR4 and
CXCR7 signaling pathways: A focus on the Cross-talk between cancer
cells and tumor microenvironment. Front Oncol. 11:5913862021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lin N and Simon MC: Hypoxia-inducible
factors: Key regulators of myeloid cells during inflammation. J
Clin Invest. 126:3661–3671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Du J, Wang C, Chen Y, Zhong L, Liu X, Xue
L, Zhang Y, Li Y, Li X, Tang C, et al: Targeted downregulation of
HIF-1α for restraining circulating tumor microemboli mediated
metastasis. J Control Release. 343:457–468. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ding XC, Wang LL, Zhang XD, Xu JL, Li PF,
Liang H, Zhang XB, Xie L, Zhou ZH, Yang J, et al: The relationship
between expression of PD-L1 and HIF-1α in glioma cells under
hypoxia. J Hematol Oncol. 14:922021. View Article : Google Scholar
|
|
120
|
Li J, Xia Y, Sun B, Zheng N, Li Y, Pang X,
Yang F, Zhao X, Ji Z, Yu H, et al: Neutrophil extracellular traps
induced by the hypoxic microenvironment in gastric cancer augment
tumour growth. Cell Commun Signal. 21:862023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar
|
|
122
|
Liu J, Zhang X, Cheng Y and Cao X:
Dendritic cell migration in inflammation and immunity. Cell Mol
Immunol. 18:2461–2471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
McGettrick AF and O'Neill LAJ: The role of
HIF in immunity and inflammation. Cell Metab. 32:524–536. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ricciardi A, Elia AR, Cappello P, Puppo M,
Vanni C, Fardin P, Eva A, Munroe D, Wu X, Giovarelli M and Varesio
L: Transcriptome of hypoxic immature dendritic cells: Modulation of
chemokine/receptor expression. Mol Cancer Res. 6:175–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Köhler T, Reizis B, Johnson RS, Weighardt
H and Förster I: Influence of hypoxia-inducible factor 1α on
dendritic cell differentiation and migration. Eur J Immunol.
42:1226–1236. 2012. View Article : Google Scholar
|
|
126
|
Liu J, Zhang X, Chen K, Cheng Y, Liu S,
Xia M, Chen Y, Zhu H, Li Z and Cao X: CCR7 chemokine
receptor-inducible lnc-Dpf3 restrains dendritic cell migration by
inhibiting HIF-1α-mediated glycolysis. Immunity. 50:600–615.e15.
2019. View Article : Google Scholar
|
|
127
|
Kim MK and Kim J: Properties of immature
and mature dendritic cells: Phenotype, morphology, phagocytosis,
and migration. RSC Adv. 9:11230–11238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kelly B and O'Neill LA: Metabolic
reprogramming in macrophages and dendritic cells in innate
immunity. Cell Res. 25:771–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cao L, Wang M, Dong Y, Xu B, Chen J, Ding
Y, Qiu S, Li L, Karamfilova Zaharieva E, Zhou X and Xu Y: Circular
RNA circRNF20 promotes breast cancer tumorigenesis and Warburg
effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 11:1452020.
View Article : Google Scholar
|
|
130
|
Hu Z, Yu X, Ding R, Liu B, Gu C, Pan XW,
Han Q, Zhang Y, Wan J, Cui XG, et al: Glycolysis drives STING
signaling to facilitate dendritic cell antitumor function. J Clin
Invest. 133:e1660312023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xu K, Yin N, Peng M, Stamatiades EG,
Chhangawala S, Shyu A, Li P, Zhang X, Do MH, Capistrano KJ, et al:
Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support
effector T helper 17 cell responses. Immunity. 54:976–987.e7. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xu K, Yin N, Peng M, Stamatiades EG, Shyu
A, Li P, Zhang X, Do MH, Wang Z, Capistrano KJ, et al: Glycolysis
fuels phosphoinositide 3-kinase signaling to bolster T cell
immunity. Science. 371:405–410. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shi R, Tang YQ and Miao H: Metabolism in
tumor microenvironment: Implications for cancer immunotherapy.
MedComm (2020). 1:47–68. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
134
|
O'Neill LA, Kishton RJ and Rathmell J: A
guide to immunometabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sanmarco LM, Rone JM, Polonio CM,
Fernandez Lahore G, Giovannoni F, Ferrara K, Gutierrez-Vazquez C,
Li N, Sokolovska A, Plasencia A, et al: Lactate limits CNS
autoimmunity by stabilizing HIF-1α in dendritic cells. Nature.
620:881–889. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gabrilovich D, Ishida T, Oyama T, Ran S,
Kravtsov V, Nadaf S and Carbone DP: Vascular endothelial growth
factor inhibits the development of dendritic cells and dramatically
affects the differentiation of multiple hematopoietic lineages in
vivo. Blood. 92:4150–4166. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Gabrilovich DI, Chen HL, Girgis KR,
Cunningham HT, Meny GM, Nadaf S, Kavanaugh D and Carbone DP:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Noman MZ, Hasmim M, Messai Y, Terry S,
Kieda C, Janji B and Chouaib S: Hypoxia: A key player in antitumor
immune response. A review in the theme: Cellular responses to
hypoxia. Am J Physiol Cell Physiol. 309:C569–C579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Baginska J, Viry E, Paggetti J, Medves S,
Berchem G, Moussay E and Janji B: The critical role of the tumor
microenvironment in shaping natural killer cell-mediated anti-tumor
immunity. Front Immunol. 4:4902013. View Article : Google Scholar
|
|
141
|
Barsoum IB, Hamilton TK, Li X, Cotechini
T, Miles EA, Siemens DR and Graham CH: Hypoxia induces escape from
innate immunity in cancer cells via increased expression of ADAM10:
Role of nitric oxide. Cancer Res. 71:7433–7441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ni J, Wang X, Stojanovic A, Zhang Q,
Wincher M, Bühler L, Arnold A, Correia MP, Winkler M, Koch PS, et
al: Single-cell RNA sequencing of tumor-infiltrating NK cells
reveals that inhibition of transcription factor HIF-1α unleashes NK
cell activity. Immunity. 52:1075–1087.e8. 2020. View Article : Google Scholar
|
|
143
|
Nakazawa T, Morimoto T, Maeoka R, Yamada
K, Matsuda R, Nakamura M, Nishimura F, Yamada S, Park YS, Tsujimura
T and Nakagawa I: Characterization of HIF-1α knockout primary human
natural killer cells including populations in allogeneic
glioblastoma. Int J Mol Sci. 25:58962024. View Article : Google Scholar
|
|
144
|
Cluff E, Magdaleno CC, Fernandez E, House
T, Swaminathan S, Varadaraj A and Rajasekaran N: Hypoxia-inducible
factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR
pathway in hypoxic NK cells and supports effector functions in NKL
cells and ex vivo expanded NK cells. Cancer Immunol Immunother.
71:1989–2005. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Heikkilä M, Pasanen A, Kivirikko KI and
Myllyharju J: Roles of the human hypoxia-inducible factor (HIF)-3α
variants in the hypoxia response. Cell Mol Life Sci. 68:3885–3901.
2011. View Article : Google Scholar
|
|
146
|
Kummar S, Chen A, Ji J, Zhang Y, Reid JM,
Ames M, Jia L, Weil M, Speranza G, Murgo AJ, et al: Phase I study
of PARP inhibitor ABT-888 in combination with topotecan in adults
with refractory solid tumors and lymphomas. Cancer Res.
71:5626–5634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jonasch E, Donskov F, Iliopoulos O,
Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie
JK, Welsh SJ, et al: Belzutifan for renal cell carcinoma in von
Hippel-Lindau disease. N Engl J Med. 385:2036–2046. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Cheng B, Ma X, Zhou Y, Liu J, Fei X, Pan
W, Peng X, Wang W and Chen J: Recent progress in the development of
hypoxia-inducible factor 2α (HIF-2α) modulators: Inhibitors,
agonists, and degraders (2009-2024). Eur J Med Chem.
275:1166452024. View Article : Google Scholar
|
|
149
|
Wu H, Zhao X, Hochrein SM, Eckstein M,
Gubert GF, Knöpper K, Mansilla AM, Öner A, Doucet-Ladevèze R,
Schmitz W, et al: Mitochondrial dysfunction promotes the transition
of precursor to terminally exhausted T cells through
HIF-1α-mediated glycolytic reprogramming. Nat Commun. 14:68582023.
View Article : Google Scholar
|
|
150
|
Jiao X, Zhang Y, Li W, Zhou X, Chu W, Li
Y, Wang Z, Sun X, Xu C and Gan Y: HIF-1α inhibition attenuates
severity of Achilles tendinopathy by blocking NF-κB and MAPK
pathways. Int Immunopharmacol. 106:1085432022. View Article : Google Scholar
|
|
151
|
Taylor CT, Doherty G, Fallon PG and
Cummins EP: Hypoxia-dependent regulation of inflammatory pathways
in immune cells. J Clin Invest. 126:3716–3724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Li Z, Cheng G, Zhang Q, Wu W, Zhang Y, Wu
B, Liu Z, Tong X, Xiao B, Cheng L and Dai F: PX478-loaded silk
fibroin nanoparticles reverse multidrug resistance by inhibiting
the hypoxia-inducible factor. Int J Biol Macromol. 222:2309–2317.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Tiwari H, Rai N, Singh S, Gupta P, Verma
A, Singh AK, Kajal, Salvi P, Singh SK, Gautam V, et al: Recent
advances in Nanomaterials-based targeted drug delivery for
preclinical cancer diagnosis and therapeutics. Bioengineering
(Basel). 10:7602023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Park K, Lee HE, Lee SH, Lee D, Lee T and
Lee YM: Molecular and functional evaluation of a novel HIF
inhibitor, benzopyranyl 1,2,3-triazole compound. Oncotarget.
8:7801–7813. 2017. View Article : Google Scholar :
|
|
155
|
Wang Z, Wu J, Humphries B, Kondo K, Jiang
Y, Shi X and Yang C: Upregulation of Histone-lysine
methyltransferases plays a causal role in hexavalent
chromium-induced cancer stem Cell-like property and cell
transformation. Toxicol Appl Pharmacol. 342:22–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Spera D, Siciliano T, De Tommasi N, Braca
A and Vessières A: Antiproliferative cardenolides from Periploca
graeca. Planta Med. 73:384–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Sapra P, Kraft P, Mehlig M, Malaby J, Zhao
H, Greenberger LM and Horak ID: Marked therapeutic efficacy of a
novel polyethylene glycol-SN38 conjugate, EZN-2208, in xenograft
models of B-cell non-Hodgkin's lymphoma. Haematologica.
94:1456–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Sapra P, Zhao H, Mehlig M, Malaby J, Kraft
P, Longley C, Greenberger LM and Horak ID: Novel delivery of SN38
markedly inhibits tumor growth in xenografts, including a
camptothecin-11-refractory model. Clin Cancer Res. 14:1888–1896.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ban HS, Kim BK, Lee H, Kim HM, Harmalkar
D, Nam M, Park SK, Lee K, Park JT, Kim I, et al: The novel
hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer
metabolism, thereby suppressing tumor growth. Cell Death Dis.
8:e28432017. View Article : Google Scholar
|
|
160
|
Wang L, Syn NL, Subhash VV, Any Y, Thuya
WL, Cheow ESH, Kong L, Yu F, Peethala PC, Wong AL, et al: Pan-HDAC
inhibition by panobinostat mediates chemosensitization to
carboplatin in non-small cell lung cancer via attenuation of EGFR
signaling. Cancer Lett. 417:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lee WY, Chen PC, Wu WS, Wu HC, Lan CH,
Huang YH, Cheng CH, Chen KC and Lin CW: Panobinostat sensitizes
KRAS-mutant non-small-cell lung cancer to gefitinib by targeting
TAZ. Int J Cancer. 141:1921–1931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Bali P, Pranpat M, Swaby R, Fiskus W,
Yamaguchi H, Balasis M, Rocha K, Wang HG, Richon V and Bhalla K:
Activity of suberoylanilide hydroxamic Acid against human breast
cancer cells with amplification of her-2. Clin Cancer Res.
11:6382–6389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Kim KH, Kim D, Park JY, Jung HJ, Cho YH,
Kim HK, Han J, Choi KY and Kwon HJ: NNC 55-0396, a T-type Ca2+
channel inhibitor, inhibits angiogenesis via suppression of
hypoxia-inducible factor-1α signal transduction. J Mol Med (Berl).
93:499–509. 2015. View Article : Google Scholar
|
|
164
|
Courtney KD, Infante JR, Lam ET, Figlin
RA, Rini BI, Brugarolas J, Zojwalla NJ, Lowe AM, Wang K, Wallace
EM, et al: Phase I Dose-escalation trial of PT2385, a
first-in-class hypoxia-inducible factor-2α antagonist in patients
with previously treated advanced clear cell renal cell carcinoma. J
Clin Oncol. 36:867–874. 2018. View Article : Google Scholar
|
|
165
|
Courtney KD, Ma Y, Diaz de Leon A,
Christie A, Xie Z, Woolford L, Singla N, Joyce A, Hill H,
Madhuranthakam AJ, et al: HIF-2 complex dissociation, target
inhibition, and acquired resistance with PT2385, a First-in-class
HIF-2 inhibitor, in patients with clear cell renal cell carcinoma.
Clin Cancer Res. 26:793–803. 2020. View Article : Google Scholar
|
|
166
|
Ma Y, Joyce A, Brandenburg O, Saatchi F,
Stevens C, Toffessi Tcheuyap V, Christie A, Do QN, Fatunde O,
Macchiaroli A, et al: HIF2 inactivation and tumor suppression with
a Tumor-Directed RNA-silencing drug in mice and humans. Clin Cancer
Res. 28:5405–5418. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Keefe SM, Hoffman-Censits J, Cohen RB,
Mamtani R, Heitjan D, Eliasof S, Nixon A, Turnbull B, Garmey EG,
Gunnarsson O, et al: Efficacy of the nanoparticle-drug conjugate
CRLX101 in combination with bevacizumab in metastatic renal cell
carcinoma: Results of an investigator-initiated phase I-IIa
clinical trial. Ann Oncol. 27:1579–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Huang YC, Huang FI, Mehndiratta S, Lai SC,
Liou JP and Yang CR: Anticancer activity of MPT0G157, a derivative
of indolylbenzenesulfonamide, inhibits tumor growth and
angiogenesis. Oncotarget. 6:18590–18601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Lee S, Kwon OS, Lee CS, Won M, Ban HS and
Ra CS: Synthesis and biological evaluation of kresoxim-methyl
analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1
accumulation in cancer cells. Bioorg Med Chem Lett. 27:3026–3029.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lecomte S, Chalmel F, Ferriere F,
Percevault F, Plu N, Saligaut C, Surel C, Lelong M, Efstathiou T
and Pakdel F: Glyceollins trigger anti-proliferative effects
through estradiol-dependent and independent pathways in breast
cancer cells. Cell Commun Signal. 15:262017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Palayoor ST, Mitchell JB, Cerna D, Degraff
W, John-Aryankalayil M and Coleman CN: PX-478, an inhibitor of
hypoxia-inducible factor-1alpha, enhances radiosensitivity of
prostate carcinoma cells. Int J Cancer. 123:2430–2437. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Piorecka K, Kurjata J and Stanczyk WA:
Acriflavine, an acridine derivative for biomedical application:
Current state of the art. J Med Chem. 65:11415–11432. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Baker LC, Boult JK, Walker-Samuel S, Chung
YL, Jamin Y, Ashcroft M and Robinson SP: The HIF-pathway inhibitor
NSC-134754 induces metabolic changes and anti-tumour activity while
maintaining vascular function. Br J Cancer. 106:1638–1647. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Mabjeesh NJ, Escuin D, LaVallee TM,
Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW
and Giannakakou P: 2ME2 inhibits tumor growth and angiogenesis by
disrupting microtubules and dysregulating HIF. Cancer Cell.
3:363–375. 2003. View Article : Google Scholar : PubMed/NCBI
|