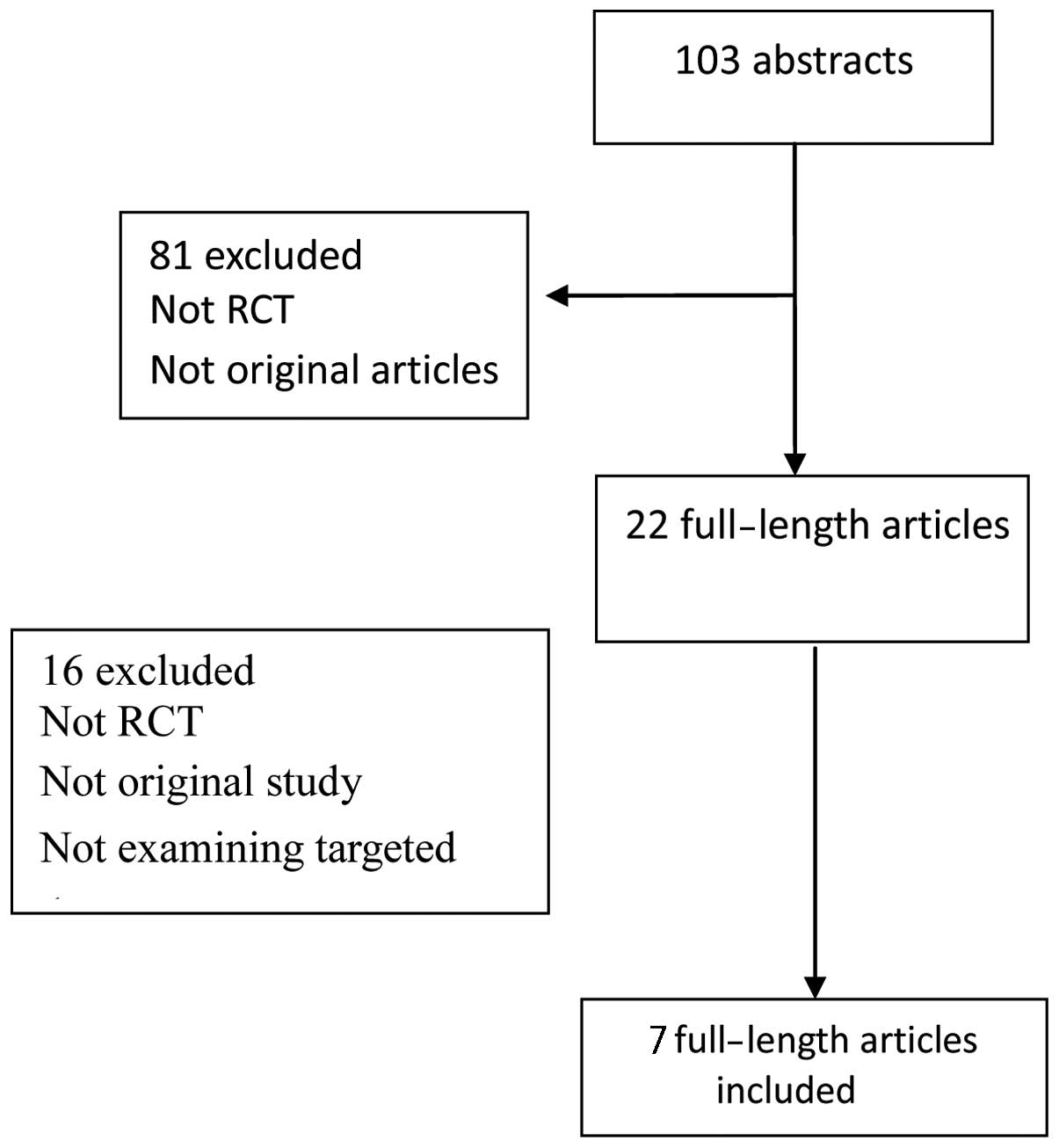
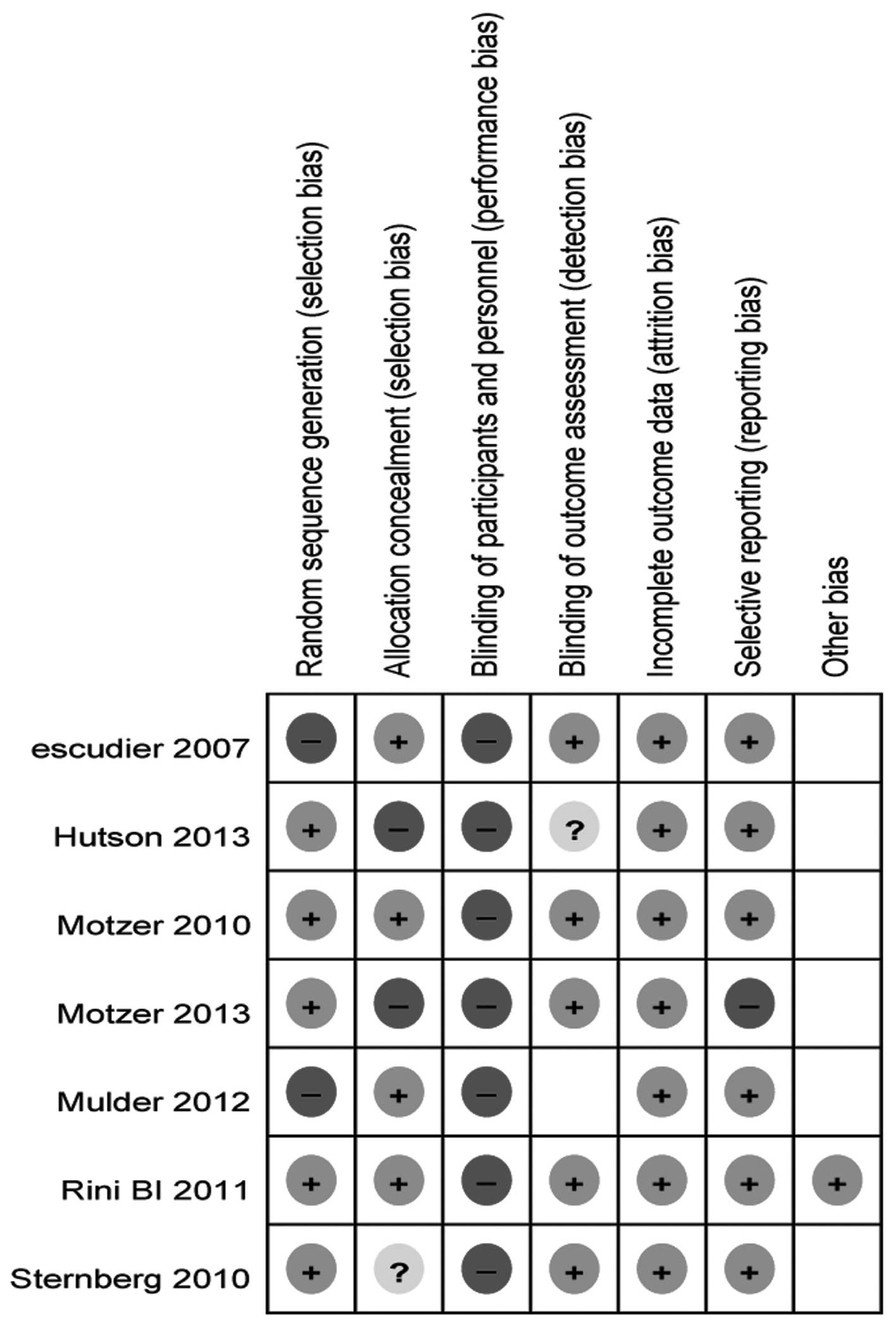
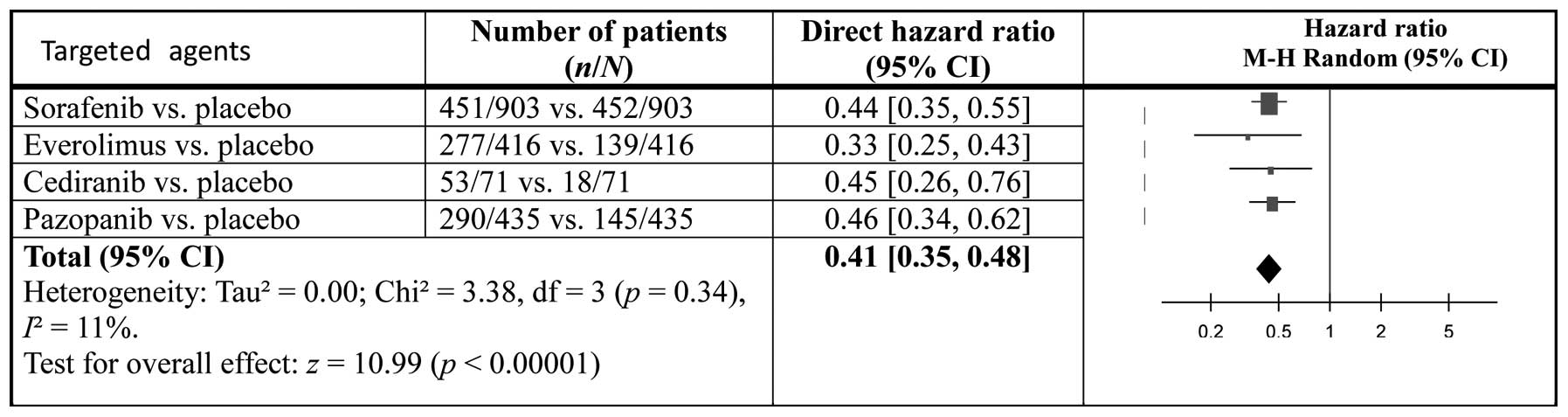
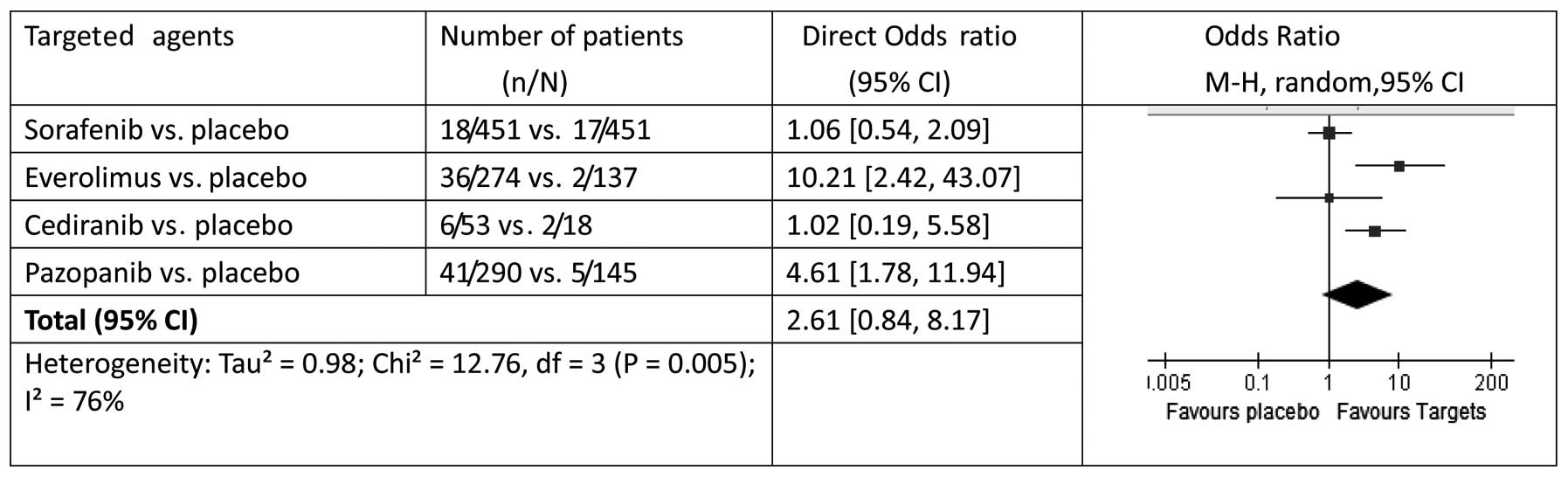
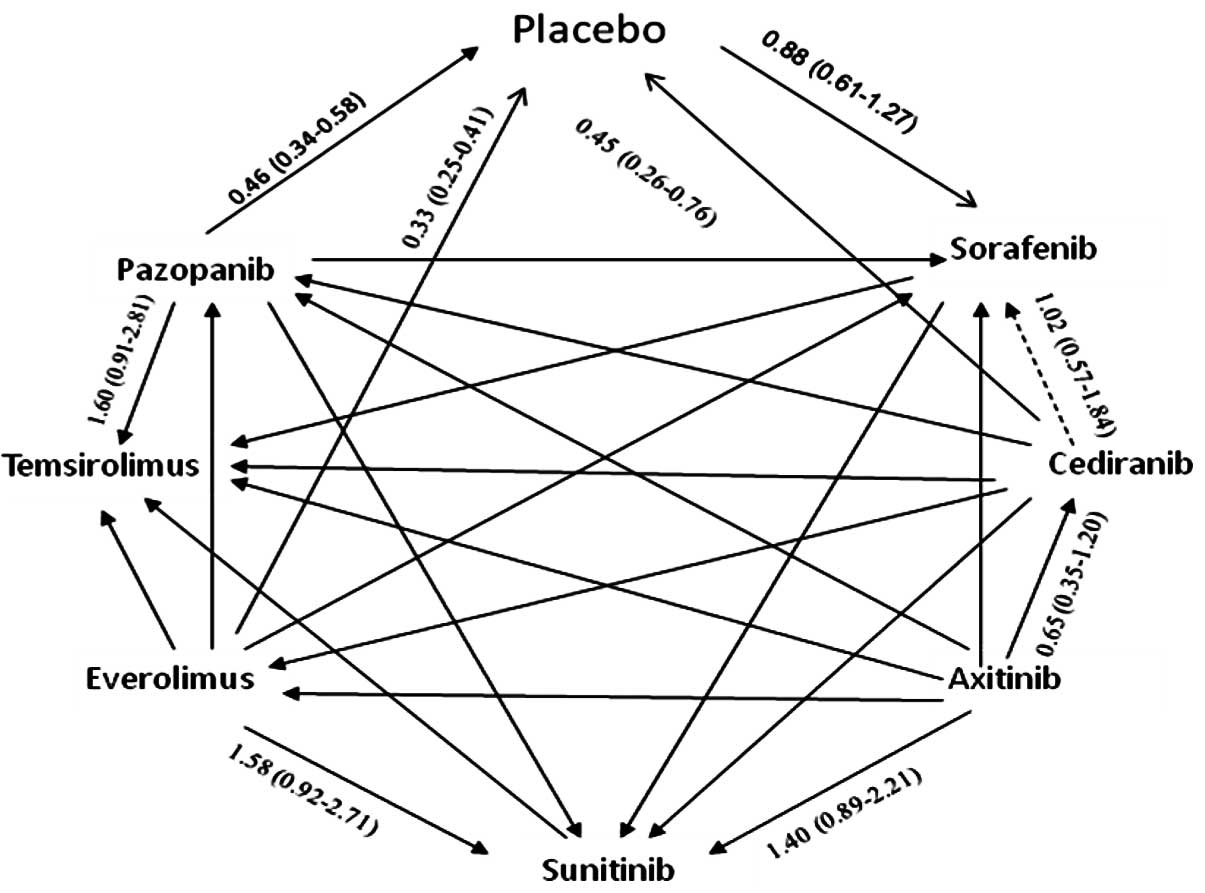
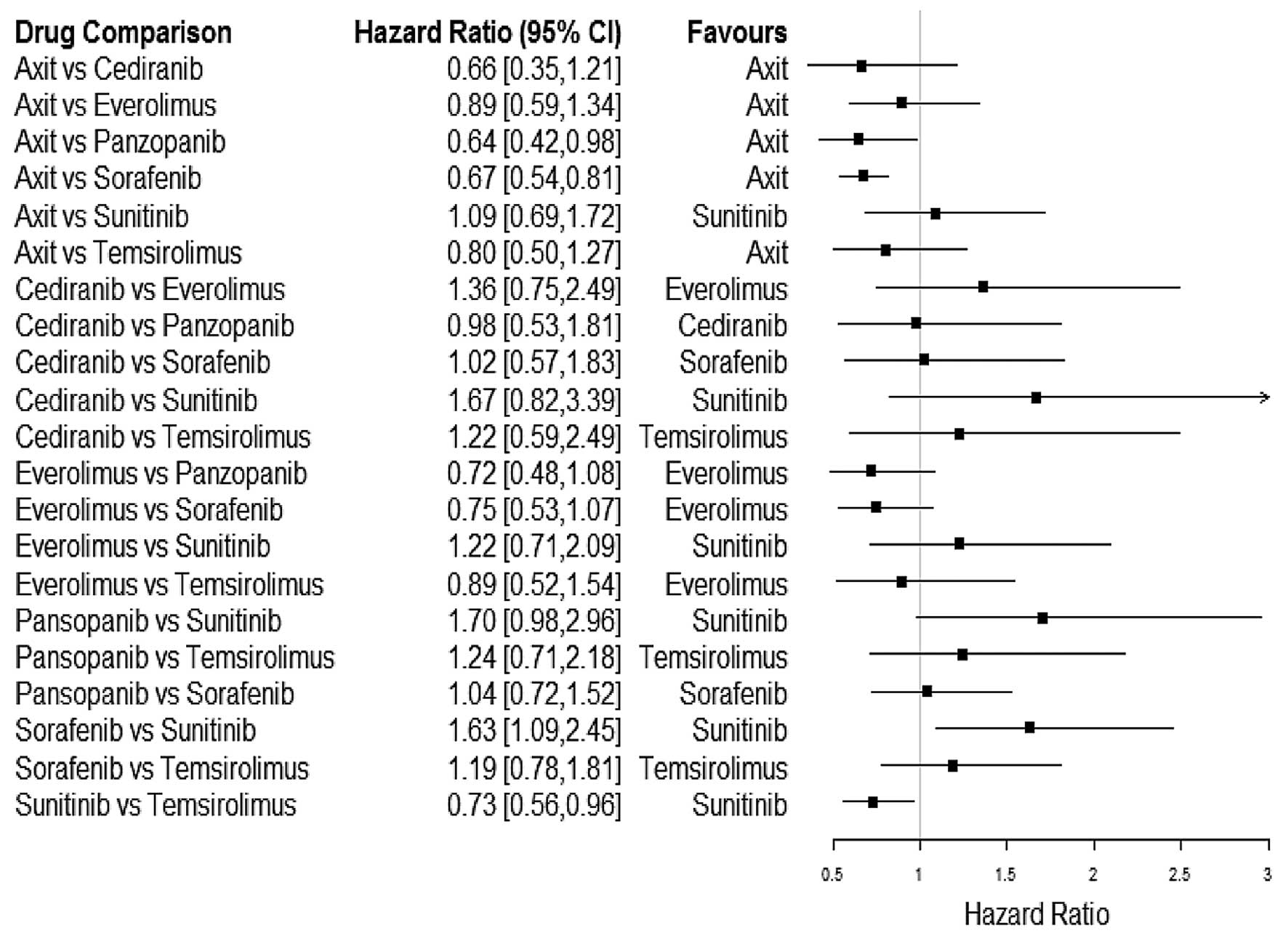
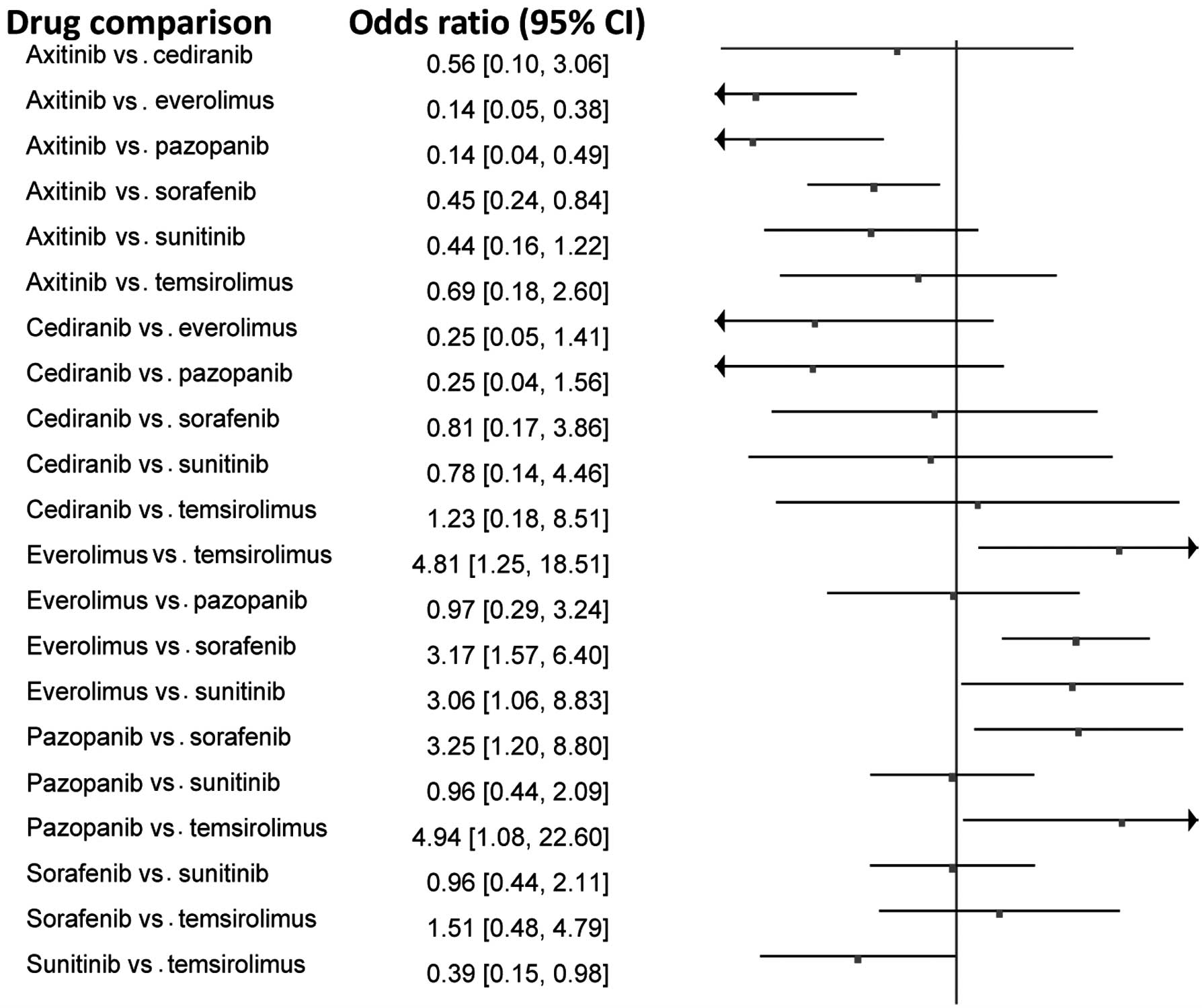
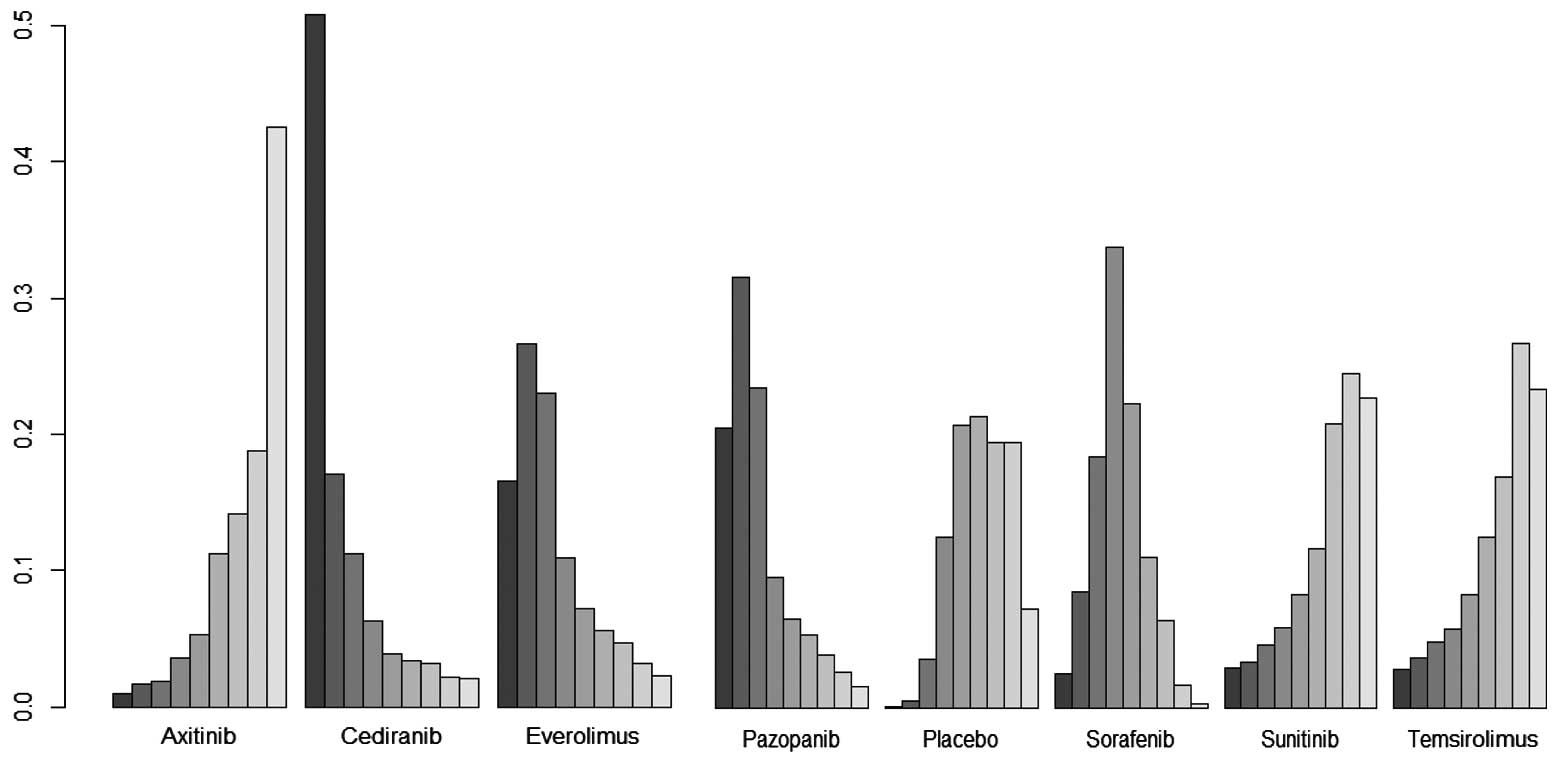
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Thun MJ, Ries LA, et al: Annual
report to the nation on the status of cancer, 1975–2005, featuring
trends in lung cancer, tobacco use, and tobacco control. J Natl
Cancer Inst. 100:1672–1694. 2008.
|
|
3
|
Mathew A, Devesa SS, Fraumeni JF Jr and
Chow WH: Global increases in kidney cancer incidence, 1973–1992.
Eur J Cancer Prev. 11:171–178. 2002.PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
5
|
Taiwan Cancer Registry. http://tcr.cph.ntu.edu.tw/main.php?Page=N1.
Accessed June 21, 2012
|
|
6
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Athar U and Gentile TC: Treatment options
for metastatic renal cell carcinoma: a review. Can J Urol.
15:3954–3966. 2008.PubMed/NCBI
|
|
8
|
Shih YC, Chien CR, Xu Y, Pan IW, Smith GL
and Buchholz TA: Economic burden of renal cell carcinoma: Part I -
an updated review. Pharmacoeconomics. 29:315–329. 2011.PubMed/NCBI
|
|
9
|
Finley DS, Pantuck AJ and Belldegrun AS:
Tumor biology and prognostic factors in renal cell carcinoma.
Oncologist. 16(Suppl 2): 4–13. 2011. View Article : Google Scholar
|
|
10
|
Schünemann H, Brozek J and Oxman A: GRADE
handbook for grading quality of evidence and strength of
recommendation, version 3.2 (updated March 2009). The GRADE Working
Group. http://ims.cochrane.org/revman/gradepro.
Accessed June 21, 2012
|
|
11
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions, version 5.0.0. The
Cochrane Collaboration; 2008, View Article : Google Scholar
|
|
12
|
Lumley T: Network meta-analysis for
indirect treatment comparisons. Stat Med. 21:2313–2324. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu G and Ades AE: Combination of direct
and indirect evidence in mixed treatment comparisons. Stat Med.
23:3105–3124. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Review Manager (RevMan) computer program,
version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane
Collaboration; 2013
|
|
15
|
Bucher HC, Guyatt GH, Griffith LE and
Walter SD: The results of direct and indirect treatment comparisons
in meta-analysis of randomized controlled trials. J Clin Epidemiol.
50:683–691. 1997. View Article : Google Scholar
|
|
16
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: combining direct
and indirect evidence. BMJ. 331:897–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wells GA, Sultan SA, Chen L, Khan M and
Coyle D: Indirect Treatment Comparisons (ITC) computer program,
version 1.0. Ottawa: Canadian Agency for Drugs and Technologies in
Health; 2009
|
|
18
|
R Development Core Team. R: A Language and
Environment for Statistical Computing. R Foundation for Statistical
Computing; Vienna, Austria: http://www.R-project.org/.
Accessed August 14, 2013
|
|
19
|
Escudier B, Eisen T, Stadler WM, et al:
TARGET Study Group: Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motzer RJ, Escudier B, Oudard S, et al:
RECORD-1 Study Group: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sternberg CN, Davis ID, Mardiak J, et al:
Pazopanib in locally advanced or metastatic renal cell carcinoma:
results of a randomized phase III trial. J Clin Oncol.
28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mulders P, Hawkins R, Nathan P, et al:
Cediranib monotherapy in patients with advanced renal cell
carcinoma: results of a randomised phase II study. Eur J Cancer.
48:527–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, et al: Comparative effectiveness of axitinib
versus sorafenib in advanced renal cell carcinoma (AXIS): a
randomised phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hutson TE, Escudier B, Esteban E, et al:
Randomized phase III trial of temsirolimus versus sorafenib as
second-line therapy after sunitinib in patients with metastatic
renal cell carcinoma. J Clin Oncol. 32:760–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motzer RJ, Hutson TE, Cella D, et al:
Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N
Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mills EJ, Rachlis B, O’Regan C, et al:
Metastatic renal cell cancer treatments: an indirect comparison
meta-analysis. BMC Cancer. 9:342009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung HW and Chan AL: Multikinase
inhibitors in metastatic renal cell carcinoma: indirect comparison
meta-analysis. Clin Ther. 33:708–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Larkin J, Paine A, Tumur I, Cappelleri JC,
Healey PJ Sr, Foley G, Mitchell S, Kroes M and Chen C: Second-line
treatments for the management of advanced renal cell carcinoma:
systematic review and meta-analysis. Expert Opin Pharmacother.
14:27–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoyle M, Green C, Thompson-Coon J, Liu Z,
Welch K, Moxham T and Stein K: Cost-effectiveness of sorafenib for
second-line treatment of advanced renal cell carcinoma. Value
Health. 13:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoyle M, Green C, Thompson-Coon J, Liu Z,
Welch K, Moxham T and Stein K: Cost-effectiveness of temsirolimus
for first-line treatment of advanced renal cell carcinoma. Value
Health. 13:61–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions, version 5.1.0. The
Cochrane Collaboration; 2011
|