Introduction
Malignant ascites is common in gastrointestinal
cancer patients with peritoneal metastasis (1). Treatment of malignant ascites
includes anticancer drugs, diuretics and paracentesis. Although
paracentesis is effective in improving symptoms such as abdominal
fullness and appetite loss, it may lead to loss of protein, since
malignant ascites contains considerable amounts of protein.
Furthermore, the decrease in circulating plasma volume due to
drainage of large amounts of ascitic fluid may cause renal
dysfunction.
Cell-free and concentrated ascites reinfusion
therapy (CART) is reported to be a useful treatment option for
improving the symptoms of massive ascites (2–5).
CART completely removes cells from the ascitic fluid and returns
the protein from the drained ascites into the body of the patient.
However, the safety of CART for malignant ascites of
gastrointestinal cancer patients has not been fully elucidated.
In the present study, we analyzed laboratory data,
including serum albumin and creatinine, prior to and after CART and
measured the amount of total protein and albumin in drained and
concentrated ascites to evaluate the safety of CART.
Materials and methods
Patients
A total of 51 sessions of CART were performed in 5
patients (4 with gastric cancer and 1 with appendiceal cancer) at
Nagoya University Hospital between September, 2011 and February,
2013. All the patients underwent systemic chemotherapy prior to and
after the first session of CART. This study was approved by the
Ethics Committee of the Nagoya University Graduate School of
Medicine.
CART procedures
We determined the presence of ascites with
ultrasonography and puncture was performed with an 18 gauge needle
under local anesthesia. Drainage was continued until the flow
stopped spontaneously or was interrupted at the physicians’
discretion. We confirmed that endotoxins were not detected in the
collected ascites, as it is known that CART is unable to eliminate
endotoxins with filtration (6).
The collected ascites was filtered through the columns of the
AHF-MO model (Asahi Kasei Medical, Tokyo, Japan) and filtered
ascites was then concentrated using the columns of the AHF-UP model
(Asahi Kasei Medical). The designed optimal concentration ratio was
1/10. The procedure was continued until the whole amount of ascites
was processed or until the filters were clogged.
Statistical analysis
An analysis of variance with repeated measures was
used to compare the laboratory data between time points.
Results
Patient characteristics and CART
We performed a total of 51 sessions of CART in 5
patients. The patient characteristics are summarized in Table I. The interval between CART
sessions was 16.5±12.7 days (range, 2–66 days; median 14 days).
Diuretics were administered to all 5 patients. Four of the 5
patients developed fever (>38°C) immediately after initiating
the reinfusion of concentrated ascites and a corticosteroid was
administered to 3 patients as an antipyretic. No other side effects
were observed. The amount of collected ascites per session was
1,180–7,210 ml (mean, 4,007 ml). The amount of protein in collected
and concentrated ascites is presented in Table II. The recovery rate of total
protein and albumin was ~63%. All the patients eventually succumbed
to cancer progression.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Case no. | Age, years | Gender | Origin of al
cancer | Pathologic
diagnosis | Diuretics | CART sessions | Fever | Corticosteroids |
|---|
| 1 | 63 | F | Stomach | Tub2-poor | + | 13 | + | − |
| 2 | 69 | F | Stomach | Tub2-poor | + | 10 | + | + |
| 3 | 68 | F | Stomach | Poor | + | 2 | − | − |
| 4 | 74 | M | Stomach | Poor | + | 8 | + | + |
| 5 | 44 | M | Appendix | Muc | + | 18 | + | + |
 | Table IIAmount of ascites and protein in
collected and concentrated ascites in one concentrated ascites
reinfusion therapy session. |
Table II
Amount of ascites and protein in
collected and concentrated ascites in one concentrated ascites
reinfusion therapy session.
| Variables | Mean ± SD |
|---|
| Amount of collected
ascites (ml) | 4,007±1,304 |
| Total protein in
collected ascites (g) | 121.6±47.9 |
| Albumin in collected
ascites (g) | 63.8±30.7 |
| Amount of
concentrated ascites (ml) | 561.1±204.9 |
| Total protein in
concentrated ascites (g) | 75.0±29.8 |
| Albumin in
concentrated ascites (g) | 39.3±20.8 |
| Recovery rate of
total protein (%) | 63.1±14.9 |
| Recovery rate of
albumin (%) | 63.4±22.2 |
Comparison of laboratory data
We evaluated laboratory data prior to CART (on the
same or previous day), the following day, 1 week (5–10 days) and 2
weeks (11–17 days) later (Table
III). Case 2 received albumin transfusion 5 months prior to the
first CART session due to hypoalbuminemia and case 5 received an
albumin transfusion immediately prior to the last CART to treat
septic shock due to pneumonia. We excluded 6 sessions of case 2
from the analysis, as the patient received an erythrocyte
transfusion immediately after CART. Out of 51 sessions, 51, 40, 28
and 33 sessions were available for the evaluation of laboratory
data prior to CART, on the following day, 1 week later and 2 weeks
later, respectively. The serum levels of total protein and albumin
after CART were similar to those prior to CART. Hemoglobin and
serum creatinine concentration were decreased on the day following
CART and recovered to baseline levels in 1 week.
 | Table IIILaboratory data prior to and after
concentrated ascites reinfusion therapy (CART). |
Table III
Laboratory data prior to and after
concentrated ascites reinfusion therapy (CART).
| Variables | Prior to CART | Following day | One week later | Two weeks later |
|---|
| Total protein
(g/dl) | 5.65±0.57 | 5.50±0.61 | 5.79±0.58 | 5.68±0.58 |
| Albumin (g/dl) | 2.80±0.76 | 2.77±0.79 | 2.78±0.87 | 2.88±0.69 |
| Creatinine
(mg/dl) | 0.81±0.23 | 0.67±0.20 | 0.86±0.34 | 0.78±0.24 |
| Hemoglobin
(g/dl) | 10.88±3.01 | 9.36±2.93 | 10.91±2.78 | 10.59±3.19 |
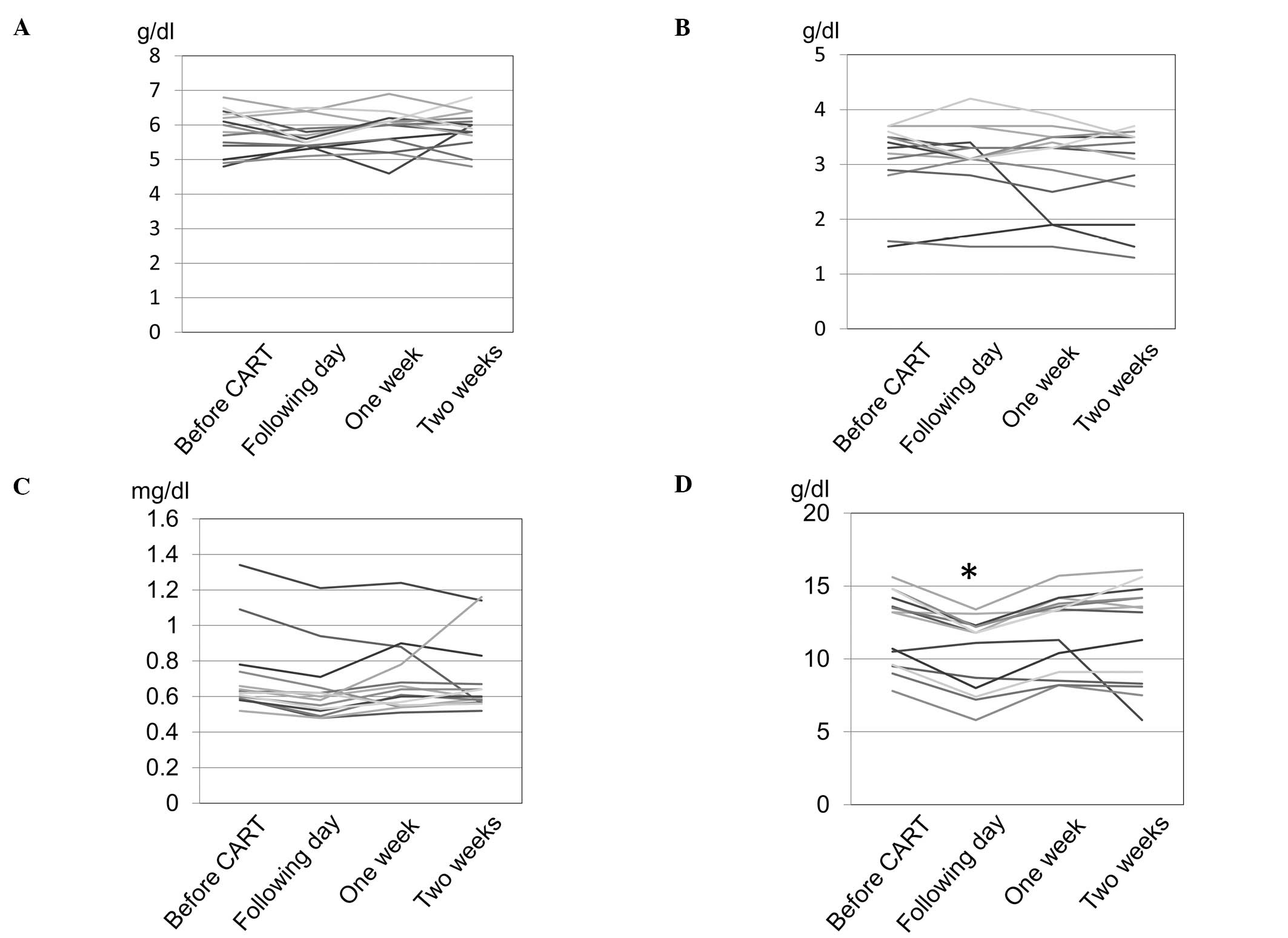
A total of 14 sessions were available for analysis
at all 4 time points (Table IV,
Fig. 1). Hemoglobin concentration
was significantly decreased on the day following CART compared to
that prior to CART and recovered to baseline levels in 1 week.
There were no significant differences in total protein, albumin and
creatinine levels prior to and after CART.
 | Table IVLaboratory data of 14 concentrated
ascites reinfusion therapy (CART) sessions for which laboratory
data were available at all four time points. |
Table IV
Laboratory data of 14 concentrated
ascites reinfusion therapy (CART) sessions for which laboratory
data were available at all four time points.
| Variables | Prior to CART | Following day | One week later | Two weeks later |
|---|
| Total protein
(g/dl) | 5.81±0.63 | 5.71±0.44 | 5.86±0.58 | 5.89±0.53 |
| Albumin (g/dl) | 3.11±0.72 | 3.08±0.72 | 3.01±0.75 | 2.94±0.81 |
| Creatinine
(mg/dl) | 0.71±0.23 | 0.64±0.20 | 0.69±0.20 | 0.69±0.21 |
| Hemoglobin
(g/dl) | 12.14±2.53 |
10.49±2.51a | 11.95±2.59 | 11.81±3.39 |
Discussion
Britton (7) first
investigated the use of CART for patients with liver cirrhosis.
CART as a treatment for malignant ascites has also been reported
for gastrointestinal (3) and
gynecological cancers (3,8,9). A
comparison between paracentesis with albumin transfusion and CART
for liver cirrhosis patients was reported and the two approaches
appeared to exert similar effects (10). However, albumin transfusion may
cause infection and entails problems associated with the overuse of
blood derivatives.
Drainage of massive amounts of ascitic fluid may
cause loss of proteins, including albumin, and may lead to renal
dysfunction due to the decrease in the circulating fluid volume. We
evaluated the changes in laboratory data and found that total
protein and albumin after CART remained at similar levels as those
prior to CART and that creatinine did not increase after CART. A
maximum of 18 sessions of CART were safely performed in 1 patient.
The decrease in serum creatinine and hemoglobin on the day
following CART is attributed to the dilution due to the increase of
circulating blood plasma, as both creatinine and hemoglobin
decreased to a similar degree (83 and 86%, respectively) and
returned to baseline levels in 1 week. It is considered that this
supplementation of plasma volume may contribute to maintaining
renal function.
Of the 5 patients, 4 developed fever after CART,
which was considered to be caused by substances in ascites. Katoh
et al (11) reported that
multiple substances, including fibrin, were the cause of fever
after the reinfusion of ascites. The inflammatory cytokine
interleukin-6 has also been considered to be a candidate substance
causing high fever after CART (12). In our study, corticosteroid was
administered at the onset of fever and also administered as a
prophylaxis just prior to reinfusion of ascites in patients who had
developed fever during prior CART sessions.
In the present study, we analyzed the laboratory
data prior to and after CART and concluded that CART was not
associated with renal dysfunction and protein loss. Therefore, CART
is effective and safe for improving the symptoms of malignant
ascites from gastrointestinal cancer.
References
|
1
|
Chung M and Kozuch P: Treatment of
malignant ascites. Curr Treat Options Oncol. 9:215–233. 2008.
View Article : Google Scholar
|
|
2
|
Inoue N, Yamazaki Z, Oda T, Sugiura M and
Wada T: Treatment of intractable ascites by continuous reinfusion
of the sterilized, cell-free and concentrated ascitic fluid. Trans
Am Soc Artif Intern Organs. 23:699–702. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japanese CART Study Group. Matsusaki K,
Ohta K, Yoshizawa A and Gyoda Y: Novel cell-free and concentrated
ascites reinfusion therapy (KM-CART) for refractory ascites
associated with cancerous peritonitis: its effect and future
perspectives. Int J Clin Oncol. 16:395–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamoto S, Britton RC and Kolff WJ:
Ultrafiltration of ascites. Trans Am Soc Artif Intern Organs.
6:15–21. 1960.
|
|
5
|
Takahashi K, Shibukawa T, Moriyama M and
Kitao M: Changes in biochemical data in ascites and serum before
and after the treatment of intractable ascites for patients with
terminal gynecologic tumor: re-infusion of cell-free and
concentrated ascitic fluid. Nihon Gan Chiryo Gakkai Shi.
20:2097–2103. 1985.
|
|
6
|
Maeda A, Takeda K, Tsuruya K, et al: A
case of cell-free and concentrated ascites reinfusion therapy
effective for refractory ascites in spontaneous bacterial
peritonitis in a renal transplant patient. Case Rep Nephrol Urol.
2:138–144. 2012. View Article : Google Scholar
|
|
7
|
Britton RC: A new technique for rapid
control of cirrhotic ascites. Arch Surg. 83:364–369. 1961.
View Article : Google Scholar
|
|
8
|
Ueda T, Maehara M, Takahashi Y, et al:
Clinical significance of cell-free and concentrated ascites
re-infusion therapy for advanced and recurrent gynecological
cancer. Anticancer Res. 32:2353–2357. 2012.PubMed/NCBI
|
|
9
|
Kitao M, Takahashi K and Iwaka O:
Treatment of intractable ascites at the terminal stage of ovarian
carcinoma - reinfusion of the cell-free, concentrated ascitic
fluid. Nihon Sanka Fujinka Gakkai Zasshi. 35:1668–1671. 1983.(In
Japanese).
|
|
10
|
Graziotto A, Rossaro L, Inturri P and
Salvagnini M: Reinfusion of concentrated ascitic fluid versus total
paracentesis. A randomized prospective trial. Dig Dis Sci.
42:1708–1714. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh S, Tatsukawa H, Kondoh M, Inoue M,
Ida K and Miyagawa F: Prevention of the febrile reaction occurring
on reinfusion of cell-free and concentrated autogenous ascites. Jpn
J Med. 30:311–317. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orimi S, Mizuno K, Narahara M, Umakosi H,
Kaihara M and Hashimoto M: A study of appropriate flow rate
settings for cell-free and concentrated ascites reinfusion therapy
and change of cytokine concentrations in ascites. Ther Apher Dial.
15:411–414. 2011.PubMed/NCBI
|