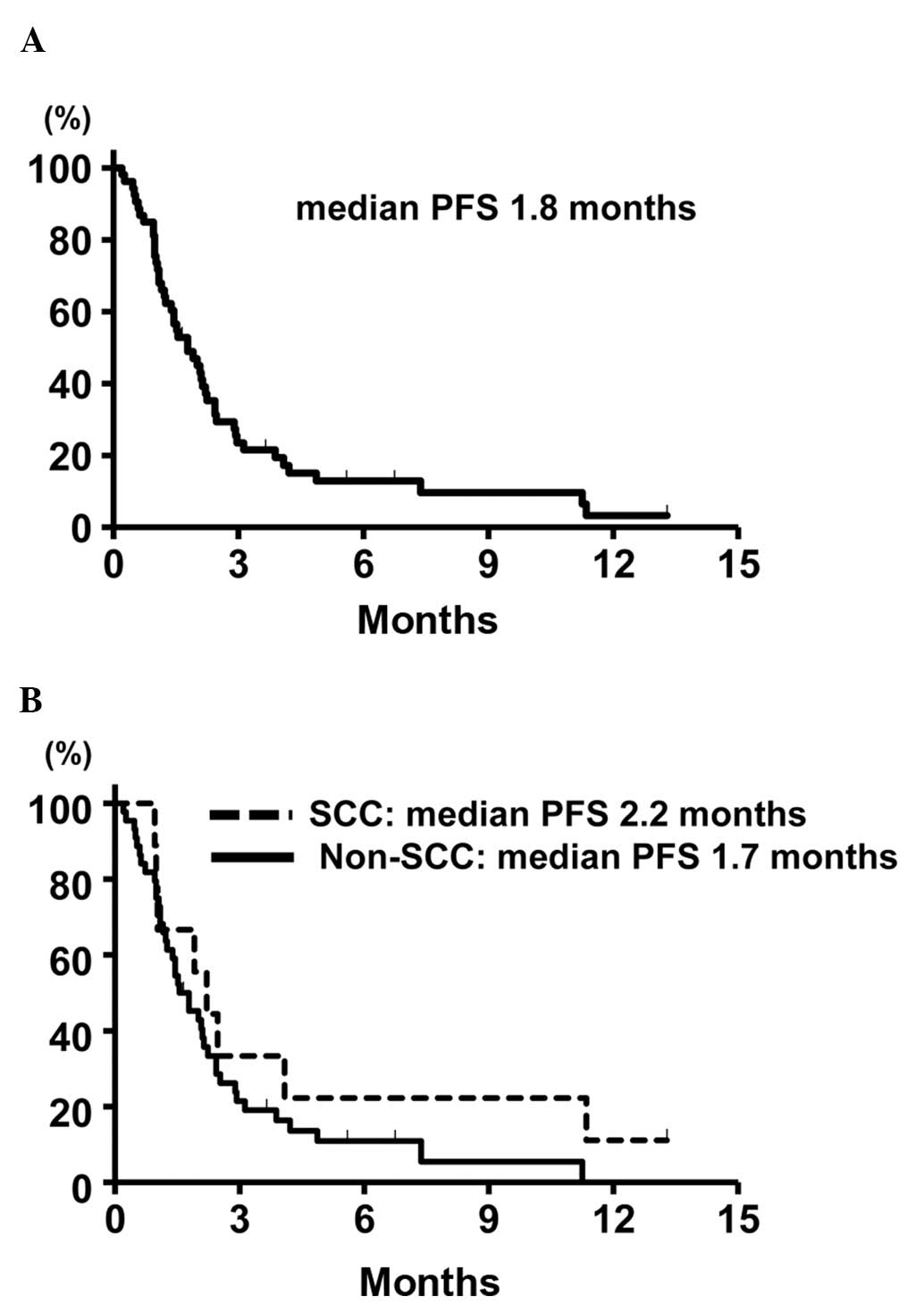
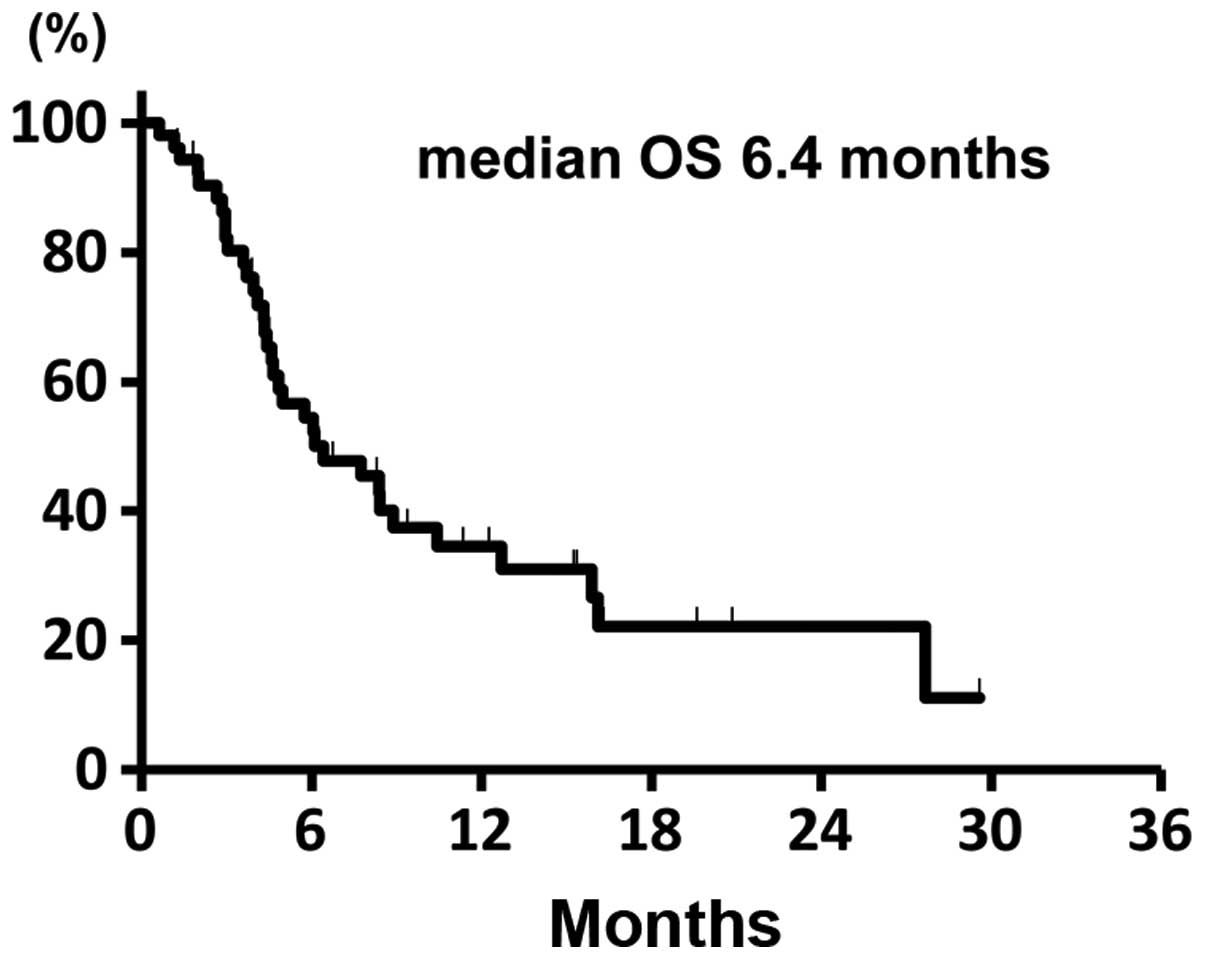
|
1
|
Shepherd FA, Rodrigues Pereira J, et al;
National Cancer Institute of Canada Clinical Trials Group.
Erlotinib in previously treated non-small-cell lung cancer. N Engl
J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
5
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosell R, Carcereny E, Gervais R, et al:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label,
randomised phase 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
Erlotinib as maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled phase 3
study. Lancet Oncol. 11:521–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura H, Fujiwara Y, Sone T, et al: High
sensitivity detection of epidermal growth factor receptor mutations
in the pleural effusion of non-small cell lung cancer patients.
Cancer Sci. 97:642–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Lee KY, Kim YC, et al: Detection
and comparison of peptide nucleic acid-mediated real-time
polymerase chain reaction clamping and direct gene sequencing for
epidermal growth factor receptor mutations in patients with
non-small cell lung cancer. Lung Cancer. 75:321–325. 2012.
View Article : Google Scholar
|
|
11
|
Linardou H, Dahabreh IJ, Kanaloupiti D, et
al: Assessment of somatic k-RAS mutations as a mechanism associated
with resistance to EGFR-targeted agents: a systematic review and
meta-analysis of studies in advanced non-small-cell lung cancer and
metastatic colorectal cancer. Lancet Oncol. 9:962–972. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
13
|
Goto K, Satouchi M, Ishii G, et al: An
evaluation study of EGFR mutation tests utilized for non-small-cell
lung cancer in the diagnostic setting. Ann Oncol. 23:2914–2919.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fossella FV, DeVore R, Kerr RN, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small-cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000.
|
|
15
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
16
|
Garassino MC, Martelli O, Broggini M, et
al; TAILOR trialists. Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): a randomised controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi T, Ando M, Asami K, et al:
Randomized phase III trial of erlotinib versus docetaxel as second-
or third-line therapy in patients with advanced non-small cell lung
cancer : Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin
Oncol. May 19–2014.(Epub ahead of print).
|
|
18
|
Fukui T, Ohe Y, Tsuta K, et al:
Prospective study of the accuracy of EGFR mutational analysis by
high-resolution melting analysis in small samples obtained from
patients with non-small cell lung cancer. Clin Cancer Res.
14:4751–4757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karampeazis A, Voutsina A, Souglakos J, et
al: Pemetrexed versus erlotinib in pretreated patients with
advanced non-small cell lung cancer: a Hellenic Oncology Research
Group (HORG) randomized phase 3 study. Cancer. 119:2754–2764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hata A, Katakami N, Yoshioka H, et al: How
sensitive are epidermal growth factor receptor-tyrosine kinase
inhibitors for squamous cell carcinoma of the lung harboring EGFR
gene-sensitive mutations? J Thorac Oncol. 8:89–95. 2013. View Article : Google Scholar
|
|
21
|
Nakagawa K, Kudoh S, Ohe Y, et al:
Postmarketing surveillance study of erlotinib in Japanese patients
with non-small-cell lung cancer (NSCLC): an interim analysis of
3488 patients (POLARSTAR). J Thorac Oncol. 7:1296–1303. 2012.
View Article : Google Scholar
|
|
22
|
Yoshioka H, Hotta K, Kiura K, et al;
Okayama Lung Cancer Study Group. A phase II trial of erlotinib
monotherapy in pretreated patients with advanced non-small cell
lung cancer who do not possess active EGFR mutations: Okayama Lung
Cancer Study Group trial 0705. J Thorac Oncol. 5:99–104. 2010.
View Article : Google Scholar : PubMed/NCBI
|