Introduction
Renal cell carcinoma (RCC) is one of the major
causes of cancer-related mortality. There were an estimated ~64,700
new cases of RCC and 13,570 deaths in 2012 in the United States
(1). Over the last few years, a
number of tyrosine kinase inhibitors (TKIs) have been proven to be
effective and are currently widely used for the treatment of
metastatic RCC. However, the effect of these TKIs appears to be
rather limited, with only 31% of naive cases exhibiting an
objective response [complete response (CR) or partial response
(PR)] to sunitinib treatment in the first-line setting (2) and only 10% of cases with previous
cytokine therapy exhibiting a PR to treatment with sorafenib
(3). However, thus far, only a
limited number of factors that predict the response of RCC to TKIs
have been reported. A significant decrease in serum vascular
endothelial growth factor (VEGF) receptor-2 levels and/or an
increase in serum VEGF levels were observed in patients exhibiting
an objective tumor response (4,5).
Hypothyroidism and hypertension associated with TKI treatment were
also reported to be correlated with a favorable response (6,7).
Although previous studies suggested that TKIs may
affect the immune system (8,9),
only a limited number of studies have investigated immunological
biomarkers for therapeutic prediction. Adotevi et al
(10) reported that a decrease in
regulatory T cells was correlated with a favorable overall survival
in cases with metastatic RCC who received sunitinib-based
antiangiogenic therapy. Thus, we conducted a prospective study to
invesigate predictive immunological biomarkers.
Patients and methods
Patients
Patients with histologically proven RCC with at
least one measurable metastatic lesion, who were diagnosed between
March, 2012 and June, 2013, were enrolled in this study. Sunitinib,
sorafenib or axitinib were administered orally as previously
described (2,3,11).
Tumor response was assessed 8–12 weeks after the initiation of TKI
treatment according to the response evaluation criteria in solid
tumors and was classified as CR, PR, stable disease (SD) or
progressive disease (PD) (12).
We collected blood samples from the 13 patients
prior to treatment. The plasma was deep frozen at −80°C and stored
before measuring the immune function.
Cytokines
A total of 27 cytokines including interleukin
(IL)-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10,
IL-12, IL-13, IL-15, IL-17, eotaxin, basic fibroblast growth
factor, granulocyte colony-stimulating factor, granulocyte
macrophage colony-stimulating factor (GM-CSF), interferon-γ
(IFN-γ), IFN-γ-induced protein 10, monocyte chemoattractant
protein-1, macrophage inflammatory protein (MIP)-1α,
platelet-derived growth factor (PDGF)-BB, MIP-1β, regulated on
activation, normal T-cell expressed and secreted, tumor necrosis
factor-α and VEGF were measured twice by BioPlex Pro Human Cytokine
27 Plex assay (M50-0KCAF0Y; Bio-Rad, Hercules, CA, USA). The assay
was performed according to the manufacturer’s instructions.
Briefly, plasma was centrifuged at 15,000 × g for 10 min at 4°C.
The samples were then incubated with microbeads labeled with
specific antibodies to one of the aforementioned cytokines for 60
min. Following a washing step, the beads were incubated with the
detection antibody cocktail, with each antibody specific to a
single cytokine, for 30 min. After another washing step, the beads
were incubated with streptavidin-phycoerythrin for 10 min, washed
again and the concentration of each cytokine was determined using
the array reader. The samples were tested in duplicate on a 96-well
plate alongside the standard curve used to generate the results.
Unknown concentrations were calculated from a standard curve
generated from Bio-Rad supplied standards.
Statistical analysis
The correlation between clinical and cytokine data
was analyzed by analysis of variance (ANOVA) and Tukey-Kramer’s
test using JMP software, version 10.0.0 (SAS, Institute, Cary, NC,
USA).
This study was approved by the Institutional Ethics
Committee of the Faculty of Medicine and Graduate School of
Medicine of the University of Tokyo (no. H22-23-400).
Results
Patient characteristics
A total of 13 patients (8 treated with sunitinib, 1
with sorafenib and 4 with axitinib), including 11 men and 2 women,
with a median age of 63 years (range, 50–77 years), were recruited
in this study (Table I). The
performance status was 0 in 8 and 1 in 5 cases. Eight tumors were
located in the right and 5 in the left kidney. Radical nephrectomy
was performed in 11 and partial nephrectomy in 2 patients.
Histologically, the tumors were diagnosed as 11 clear cell RCCs and
2 papillary RCCs. All the patients had developed metastasis, with
the most common metastatic site being the lung (10 cases), followed
by bone (5 cases).
 | Table ICorrelation between the clinical
effect of tyrosine kinase inhibitors (TKIs) and clinicopathological
characteristics among patients with metastatic renal cancer. |
Table I
Correlation between the clinical
effect of tyrosine kinase inhibitors (TKIs) and clinicopathological
characteristics among patients with metastatic renal cancer.
| | Clinical
effecta | |
|---|
| |
| |
|---|
| Clinical
characteristics | Total (n=13) | PR (n=5) | SD (n=4) | PD (n=4) | P-value |
|---|
| Gender |
| Male | 11 | 4 | 4 | 3 | 0.603 |
| Female | 2 | 1 | 0 | 1 | |
| Age (years) |
| ≥65 | 7 | 2 | 2 | 3 | 0.593 |
| <65 | 6 | 3 | 2 | 1 | |
| Performance
status |
| 0 | 8 | 3 | 2 | 3 | 0.780 |
| 1 | 5 | 2 | 2 | 1 | |
| Laterality |
| Right | 8 | 2 | 3 | 3 | 0.479 |
| Left | 5 | 3 | 1 | 1 | |
| Nephrectomy |
| Radical | 11 | 4 | 4 | 3 | 0.603 |
| Partial | 2 | 1 | 0 | 1 | |
| Histology |
| Clear cell RCC | 11 | 5 | 2 | 4 | 0.085 |
| Papillary RCC | 2 | 0 | 2 | 0 | |
| Nuclear grade |
| G1/G2 | 12 | 4 | 4 | 4 | 0.449 |
| G3 | 1 | 1 | 0 | 0 | |
| Stage |
| pT1 | 6 | 3 | 1 | 2 | 0.593 |
| pT2/pT3/pT4 | 7 | 2 | 3 | 2 | |
| Lymphovascular
invasion |
| 0 | 2 | 1 | 1 | 0 | 0.603 |
| 1 | 11 | 4 | 3 | 4 | |
| Lung metastasis |
| No | 3 | 1 | 2 | 0 | 0.267 |
| Yes | 10 | 4 | 2 | 4 | |
| Bone metastasis |
| No | 8 | 2 | 4 | 2 | 0.180 |
| Yes | 5 | 3 | 0 | 2 | |
| TKIs |
| Sunitinib | 8 | 4 | 3 | 1 | 0.219 |
| Others | 5 | 1 | 1 | 3 | |
| Dose intensity
(%) |
| 100 | 7 | 2 | 2 | 3 | 0.593 |
| <100 | 6 | 3 | 2 | 1 | |
| Previous
treatment |
| No | 2 | 1 | 1 | 0 | 0.603 |
| Yes | 11 | 4 | 3 | 4 | |
| Previous TKI
treatment |
| No | 8 | 4 | 3 | 1 | 0.219 |
| Yes | 5 | 1 | 1 | 3 | |
| Previous cytokine
treatment |
| No | 5 | 3 | 1 | 1 | 0.479 |
| Yes | 8 | 2 | 3 | 3 | |
| Previous mTOR
inhibitor treatment |
| No | 10 | 4 | 3 | 3 | 0.980 |
| Yes | 3 | 1 | 1 | 1 | |
Treatment
Two cases received TKI treatment as first-line
therapy. Previous systemic treatment included TKIs in 5, mammalian
target of rapamycin (mTOR) inhibitors in 3 and cytokines in 8
patients. PR was achieved in 5 cases (38%), SD in 4 (30%) and PD
developed in 4 cases (30%). The dose was reduced in 6 patients
(46%) due to adverse events.
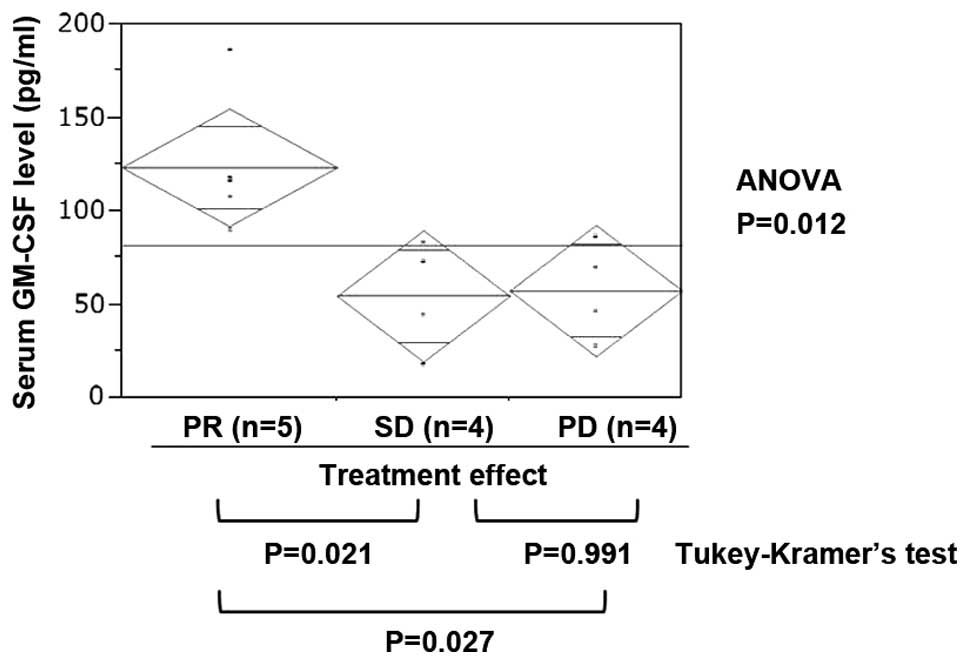
GM-CSF plasma levels by treatment
response
No clinical parameters exhibited a significant
correlation with treatment effect (Table I). Among the 27 investigated
cytokines, the plasma GM-CSF level in PR cases was significantly
higher compared to that in cases with SD or PD (Fig. 1, ANOVA, P=0.012; Tukey-Kramer’s
test: PR vs. SD, P=0.021; PR vs. PD, P=0.027; and SD vs. PD,
P=0.991). The IL-6 level was higher in PD cases, but the difference
was not statistically significant (Table II, P=0.141).
 | Table IICorrelation between the clinical
effect of tyrosine kinase inhibitors and cytokine levels in
patients with metastatic renal cancer. |
Table II
Correlation between the clinical
effect of tyrosine kinase inhibitors and cytokine levels in
patients with metastatic renal cancer.
| Clinical
effect | |
|---|
|
| |
|---|
| Cytokines | PR | SD | PD | P-value |
|---|
| GM-CSF | 123±36 | 54±29 | 57±25 | 0.012 |
| IL-1β | 3.8±4.6 | 1.9±1.2 | 1.6±0.2 | 0.494 |
| IL-1ra | 103±98 | 57±43 | 60±22 | 0.536 |
| IL-2 | 5.2±2 | 4.8±3.1 | 5±3 | 0.971 |
| IL-4 | 5.6±2.3 | 4.8±1.8 | 5.1±2.2 | 0.864 |
| IL-5 | 1.1±1.4 | 0.7±0.8 | 0.6±0.8 | 0.791 |
| IL-6 | 6±2.3 | 5±2.6 | 12±8.9 | 0.141 |
| IL-7 | 5.3±1.9 | 4.8±4.9 | 3.1±2.2 | 0.605 |
| IL-8 | 25±13 | 29±33 | 19±12 | 0.779 |
| IL-9 | 44±12 | 26±8.8 | 29±15 | 0.125 |
| IL-10 | 4.2±2.9 | 3.4±1.1 | 6.4±5.7 | 0.525 |
| IL-12 | 19±17 | 17±15 | 32±37 | 0.658 |
| IL-13 | 5.2±3.9 | 5.1±3.1 | 5.1±2.4 | 0.990 |
| IL-15 | 5±1.6 | 3.6±2.3 | 4.2±0.6 | 0.479 |
| IL-17 | 56±15 | 41±16 | 59±41 | 0.613 |
| Eotaxin | 183±152 | 128±126 | 112±61 | 0.667 |
| FGF-basic | 51±14 | 42±12 | 55±22 | 0.554 |
| G-CSF | 66±19 | 52±18 | 59±13 | 0.527 |
| IFN-γ | 610±893 | 207±94 | 178±21 | 0.462 |
| IP-10 | 2,381±1,857 | 1,386±749 | 1,906±1,432 | 0.616 |
| MCP-1 | 82±68 | 41±16 | 47±22 | 0.388 |
| MIP-1α | 2.9±1.1 | 7.7±12 | 2.9±1.7 | 0.561 |
| PDGF-BB | 309±306 | 862±146 | 213±128 | 0.508 |
| MIP-1β | 178±43 | 174±141 | 128±79 | 0.703 |
| RANTES | 3,364±138 | 2,630±763 | 2,679±771 | 0.523 |
| TNF-α | 88±92 | 62±47 | 43±6.9 | 0.580 |
| VEGF | 108±62 | 122±79 | 165±143 | 0.683 |
Discussion
We demonstrated that plasma GM-CSF may be a
predictive marker of the response to TKI treatment. Thus far, only
a few studies demonstrated the clinical utility of GM-CSF. The
plasma GM-CSF level was found to be higher in cervical cancer
patients compared to healthy controls (13), while in another study GM-CSF was
undetectable in non-cancer patients (14).
GM-CSF promotes the differentiation and expansion of
myeloid-derived suppressor cells (MDSCs) (15,16).
Antigen-specific CD8+ T-cell tolerance, induced by
MDSCs, is known to be one of the main mechanisms of tumor escape
(17). Knockdown of GM-CSF in
tumor cells may reverse the cytotoxicity to CD8 T lymphocytes:
Dolcetti et al (15) found
that lack of GM-CSF release from 4T1 mammary carcinoma cells
reduced the accumulation of Gr-1int/low MDSC subsets and
successfully inhibited tumor-induced tolerance in mice. Similarly,
Serafini et al (16)
demonstrated that inhibition of MDSC function abrogates the
proliferation of regulatory T cells and tumor-induced tolerance in
antigen-specific T cells, using the A20 B-cell lymphoma model in
vitro and in vivo. However, TKIs may reduce the number
of MDSCs in the tumor and normalize T-lymphocyte function: Xin
et al (18) demonstrated
that sunitinib directly induced RCC tumor cell apoptosis through
Stat3 inhibition, which was accompanied by a reduction in MDSCs and
tumor-infiltrating regulatory T cells.
These reports suggest that high levels of plasma
GM-CSF may promote the function of MDSCs and escape of tumor cells
from the host immune system. In patients with high GM-CSF levels,
TKIs may decrease the function of MDSCs that is upregulated by
GM-CSF and reverse the cytotoxicity of regulatory T lymphocytes
directly or indirectly, which may lower tumor-induced tolerance and
result in favorable treatment effects.
In our study, VEGF was not found to be significantly
associated with treatment effect, contrary to previous reports
(4,5). GM-CSF was reported to induce VEGF
release from the epithelium, resulting in the promotion of
carcinogenesis: Wang et al (19) demonstrated that, in a
colitis-associated cancer model, blocking GM-CSF activity in
vivo significantly decreased epithelial release of VEGF and
abrogated cancer formation. In the plasma, GM-CSF, which is
upstream of VEGF, may be a more sensitive biomarker for metastatic
RCC treatment compared to VEGF.
As regards other biomarkers, Tran et al
(20) screened pretreatment
cytokines and angiogenic factors in patients with metastatic RCC
who received pazopanib treatment and found that high IL-6 was
predictive for unfavorable progression-free survival. In our study,
IL-6 was also higher in PD cases, but the difference was not
statistically significant.
This study had certain limitations. First, this was
a single-institution study; and second, our sample size was
limited.
In conclusion, high pre-treatment plasma levels of
GM-CSF, which is an inducer of immune tolerance, were significantly
associated with a favorable response of metastatic RCC to TKI
treatment. The result suggests the potential of GM-CSF as a
predictive biomarker of the response to TKI treatment. However,
further investigation is required to determine the effects of TKIs
on abrogating cancer immune tolerance.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudier B, Eisen T, Stadler WM, et al;
TARGET Study Group. Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji S, Nakano M, Sato H, Tang XY,
Osamura YR, Terachi T, Uchida T and Takeya K: The current status of
tailor-made medicine with molecular biomarkers for patients with
clear cell renal cell carcinoma. Clin Exp Metastasis. 31:111–134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deprimo SE, Bello CL, Smeraglia J, Baum
CM, Spinella D, Rini BI, Michaelson MD and Motzer RJ: Circulating
protein biomarkers of pharmacodynamic activity of sunitinib in
patients with metastatic renal cell carcinoma: modulation of VEGF
and VEGF-related proteins. J Transl Med. 5:322007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Cohen DP, Lu DR, Chen I,
Hariharan S, Gore ME, Figlin RA, Baum MS and Motzer RJ:
Hypertension as a biomarker of efficacy in patients with metastatic
renal cell carcinoma treated with sunitinib. J Natl Cancer Inst.
103:763–773. 2011. View Article : Google Scholar
|
|
7
|
Clemons J, Gao D, Naam M, Breaker K,
Garfield D and Flaig TW: Thyroid dysfunction in patients treated
with sunitinib or sorafenib. Clin Genitourin Cancer. 10:225–231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wongkajornsilp A, Wamanuttajinda V,
Kasetsinsombat K, Duangsa-ard S, Sa-ngiamsuntorn K, Hongeng S and
Maneechotesuwan K: Sunitinib indirectly enhanced anti-tumor
cytotoxicity of cytokine-induced killer cells and
CD3+CD56+ subset through the co-culturing
dendritic cells. PLoS One. 8:e789802013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu Y, Zhao W, Meng F, Qu B, Zhu X, Sun Y,
Shu Y and Xu Q: Sunitinib impairs the proliferation and function of
human peripheral T cell and prevents T-cell-mediated immune
response in mice. Clin Immunol. 135:55–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adotevi O, Pere H, Ravel P, et al: A
decrease of regulatory T cells correlates with overall survival
after sunitinib-based antiangiogenic therapy in metastatic renal
cancer patients. J Immunother. 33:991–998. 2010. View Article : Google Scholar
|
|
11
|
Rini BI, Escudier B, Tomczak P, et al:
Comparative effectiveness of axitinib versus sorafenib in advanced
renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet.
378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
13
|
Lawicki S, Bedkowska GE, Gacuta-Szumarska
E, Knapp P and Szmitkowski M: Pretreatment plasma levels and
diagnostic utility of hematopoietic cytokines in cervical cancer or
cervical intraepithelial neoplasia patients. Folia Histochem
Cytobiol. 50:213–219. 2012. View Article : Google Scholar
|
|
14
|
Biancotto A, Wank A, Perl S, Cook W, Olnes
MJ, Dagur PK, Fuchs JC, Langweiler M, Wang E and McCoy JP: Baseline
levels and temporal stability of 27 multiplexed serum cytokine
concentrations in healthy subjects. PLoS One. 8:e760912013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dolcetti L, Peranzoni E, Ugel S, et al:
Hierarchy of immunosuppressive strength among myeloid-derived
suppressor cell subsets is determined by GM-CSF. Eur J Immunol.
40:22–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serafini P, Mgebroff S, Noonan K and
Borrello I: Myeloid-derived suppressor cells promote
cross-tolerance in B-cell lymphoma by expanding regulatory T cells.
Cancer Res. 68:5439–5449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaraj S, Gupta K, Pisarev V, Kinarsky L,
Sherman S, Kang L, Herber DL, Schneck J and Gabrilovich DI: Altered
recognition of antigen is a mechanism of CD8+ T cell
tolerance in cancer. Nat Med. 13:828–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin H, Zhang C, Herrmann A, Du Y, Figlin R
and Yu H: Sunitinib inhibition of Stat3 induces renal cell
carcinoma tumor cell apoptosis and reduces immunosuppressive cells.
Cancer Res. 69:2506–2513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Han G, Wang K, et al:
Tumor-derived GM-CSF promotes inflammatory colon carcinogenesis via
stimulating epithelial release of VEGF. Cancer Res. 74:716–726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tran HT, Liu Y, Zurita AJ, et al:
Prognostic or predictive plasma cytokines and angiogenic factors
for patients treated with pazopanib for metastatic renal-cell
cancer: a retrospective analysis of phase 2 and phase 3 trials.
Lancet Oncol. 13:827–837. 2012. View Article : Google Scholar
|