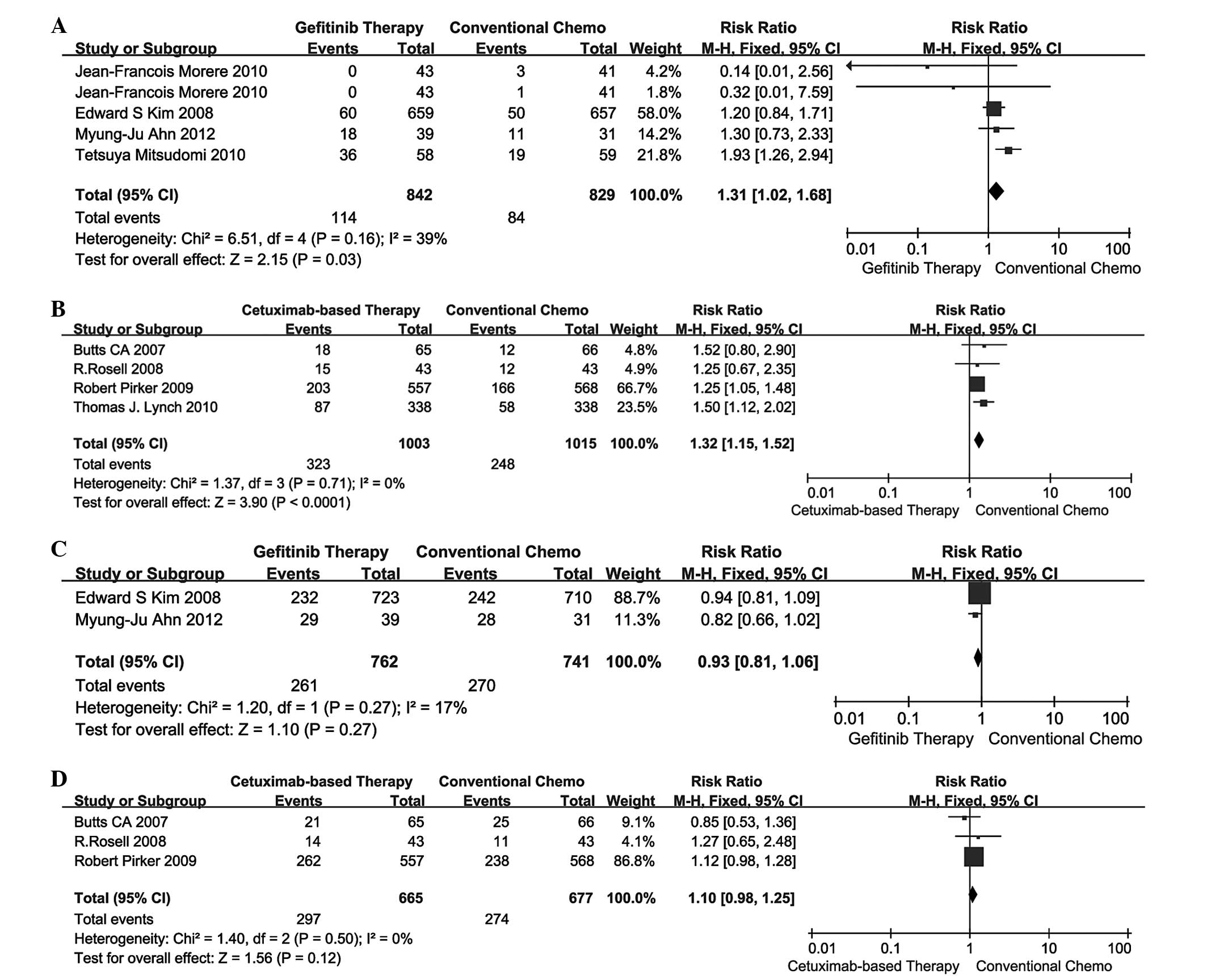
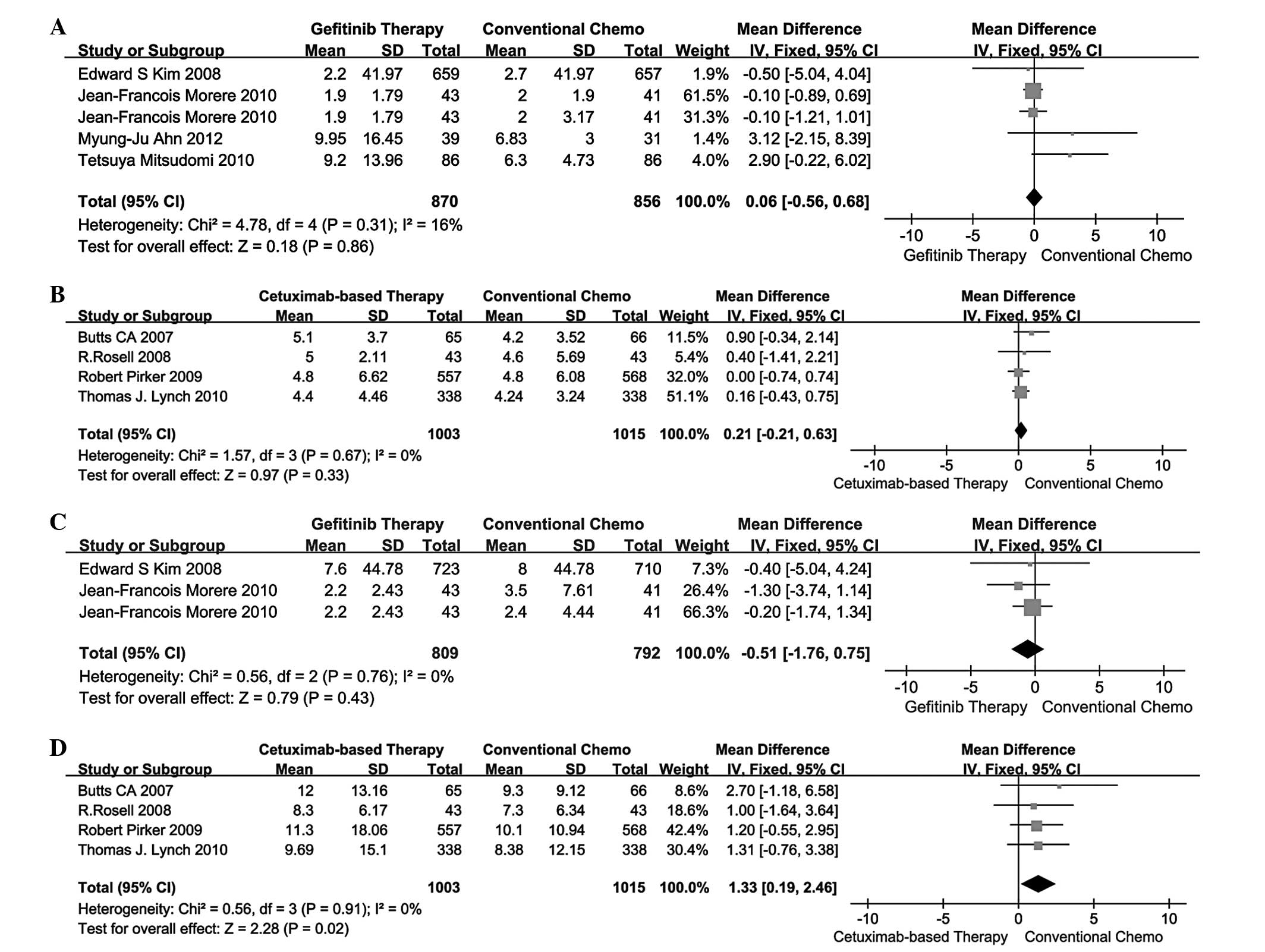
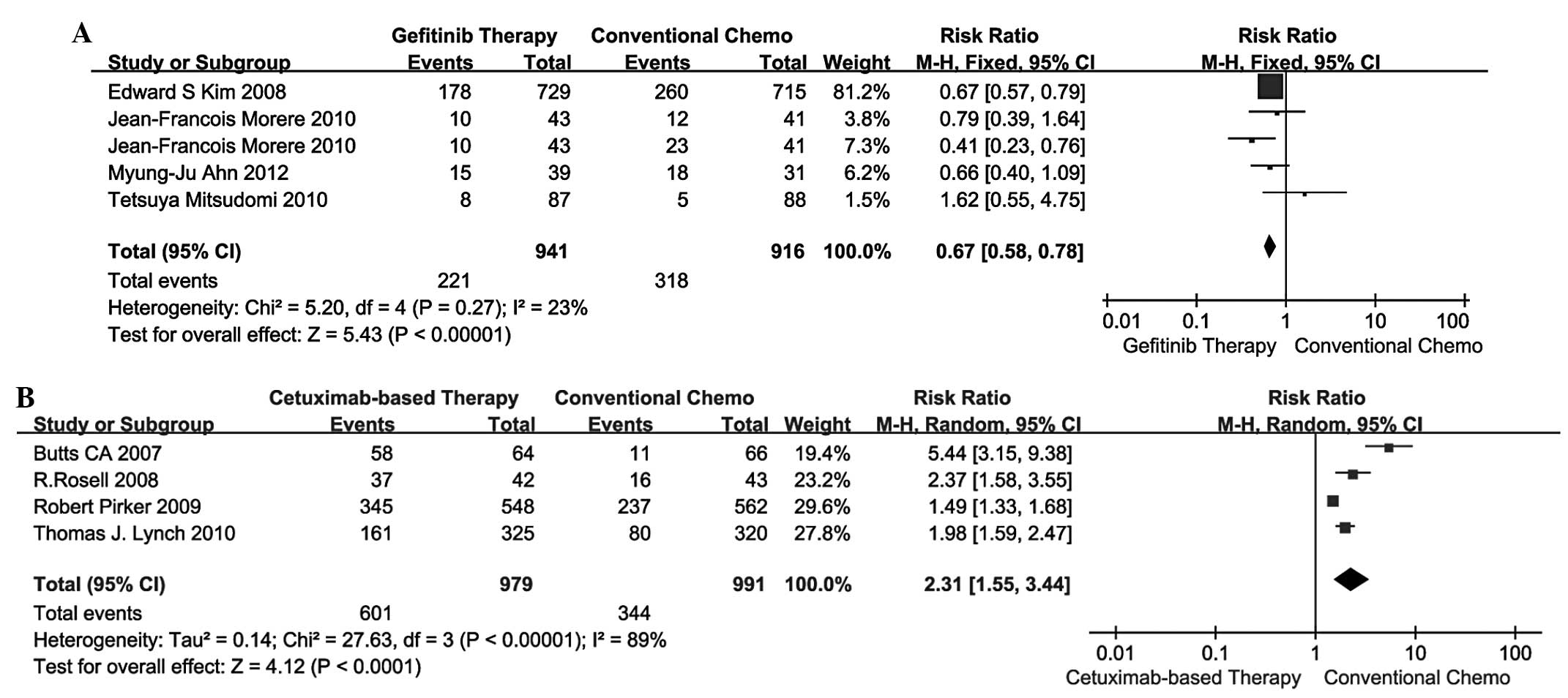
|
1
|
Ozkaya S, Findik S, Dirican A and Atici
AG: Long-term survival rates of patients with stage IIIB and IV
non-small cell lung cancer treated with cisplatin plus vinorelbine
or gemcitabine. Exp Ther Med. 4:1035–1038. 2012.PubMed/NCBI
|
|
2
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ku GY, Haaland BA and de Lima Lopes G Jr:
Gefitinib vs. chemotherapy as first-line therapy in advanced
non-small cell lung cancer: meta-analysis of phase III trials. Lung
Cancer. 74:469–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin H, Jiang J, Liang X, Zhou X and Huang
R: Chemotherapy with cetuximab or chemotherapy alone for untreated
advanced non-small-cell lung cancer: a systematic review and
meta-analysis. Lung Cancer. 70:57–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higgins JPT and Green S: Cochran. Handbook
for Systematic Reviews of Interventions, version 5.1.0. The
Cochrane Collaboration, . 2011, https://www.cochrane-handbook.orgAccessed. June
3–2013
|
|
6
|
Liu M: Design and Implementation Methods
of Systematic Reviews and Meta-analysis. People's Medical
Publishing House; Beijing: pp. 86–89. 2011, (In Chinese).
|
|
7
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
8
|
Schulz KF, Altman DG, Moher D, et al:
CONSORT 2010 statement: updated guidelines for reporting parallel
group randomised trials. BMC Med. 8(18)2010. View Article : Google Scholar
|
|
9
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Controlled clinical trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HN, Yan JZ, Tang JF and Shao R:
Indirect comparisons for efficacy of gefitinib and erlotinib in
patients with non-small cell lung cancer. Chin J Pharm Econ.
1:15–19. 2013.(In Chinese).
|
|
11
|
Silagy C, Lancaster T, Stead L, et al:
Nicotin. replacement therapy for smoking cessation. The Cochrane
Library, John Wiley & Sons; Oxford: 2004
|
|
12
|
Liao WQ: Simulation Study of Multivariate
Meta-analysis and Indirect Comparisons. Southern Medical
University; Guangzhou: pp. 23–26. 2011, (In Chinese).
|
|
13
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsudomi T, Morita S, Yatabe Y, et al
West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): an
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar
|
|
15
|
Morère JF, Bréchot JM, Westeel V, et al:
Randomized phase II trial of gefitinib or gemcitabine or docetaxel
chemotherapy in patients with advanced non-small-cell lung cancer
and a performance status of 2 or 3 (IFCT-0301 study). Lung Cancer.
70:301–307. 2010.PubMed/NCBI
|
|
16
|
Ahn MJ, Yang JC, Liang J, et al:
Randomized phase II trial of first-line treatment with
pemetrexed-cisplatin, followed sequentially by gefitinib or
pemetrexed, in East Asian, never-smoker patients with advanced
non-small cell lung cancer. Lung Cancer. 77:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosell R, Robinet G, Szczesna A, et al:
Randomized phase II study of cetuximab plus cisplatin/vinorelbine
compared with cisplatin/vinorelbine alone as first-line therapy in
EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol.
19:362–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butts CA, Bodkin D, Middleman EL, et al:
Randomized phase II study of gemcitabine plus cisplatin or
carboplatin, with or without cetuximab, as first-line therapy for
patients with advanced or metastatic non-small-cell lung cancer. J
Clin Oncol. 25:5777–5784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pirker R, Pereira JR, Szczesna A, et al
FLEX Study Team: Cetuximab plus chemotherapy in patients with
advanced non-small-cell lung cancer (FLEX): an open-label
randomised phase III trial. Lancet. 373:1525–1531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch TJ, Patel T, Dreisbach L, et al:
Cetuximab and first-line taxane/carboplatin chemotherapy in
advanced non-small-cell lung cancer: results of the randomized
multicenter phase III trial BMS099. J Clin Oncol. 28:911–917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim EM: Frontline gefitinib in
advanced non-small cell lung cancer: meta-analysis of published
randomized trials. Ann Thorac Med. 5:153–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsao MS, Sakurada A, Cutz JC, et al:
Erlotinib in lung cancer - molecular and clinical predictors of
outcome. New Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pirker R, Pereira JR, von Pawel J, et al:
EGFR expression as a predictor of survival for first-line
chemotherapy plus cetuximab in patients with advanced
non-small-cell lung cancer: analysis of data from the phase 3 FLEX
study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: combining direct
and indirect evidence. BMJ. 331:897–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bucher HC, Guyatt GH, Griffith LE and
Walter SD: The results of direct and indirect treatment comparisons
in meta-analysis of randomized controlled trials. J Clin Epidemiol.
50:683–691. 1997. View Article : Google Scholar
|
|
27
|
Song F, Harvey I and Lilford R: Adjusted
indirect comparison may be less biased than direct comparison for
evaluating new pharmaceutical interventions. J Clin Epidemiol.
61:455–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song F, Altman DG, Glenny AM and Deeks JJ:
Validity of indirect comparison for estimating efficacy of
competing interventions: empirical evidence from published
meta-analyses. BMJ. 326(472)2003. View Article : Google Scholar : PubMed/NCBI
|