Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, with non-small-cell lung cancer (NSCLC)
accounting for 80–85% of lung cancer cases (1). Chemotherapy combined with
radiotherapy are traditionally used for the treatment of NSCLC.
Over the last few years, targeted therapy has been gradually
applied for the treatment of NSCLC and has been proven to be
effective to a certain extent (2).
Among the targeted drugs used for NSCLC, those acting on the
epidermal growth factor receptor (EGFR) are attracting increasing
attention, such as the tyrosine kinase inhibitor (TKI) gefitinib
(Iressa; AstraZeneca, London, UK) and the monoclonal antibody
cetuximab (Erbitux; Merck, Darmstadt, Germany). These two dtugs
have similar mechanisms of action against NSCLC. In clinical
practice, gefitinib may be administered orally alone, while
cetuximab is administered intravenously in combination with
chemotherapy. Gefitinib and cetuximab-based therapies have been
proven to be effective for advanced NSCLC to a certain extent
(3,4); however, there is currently no
systematic review directly based on these two therapies and the
differences between them in terms of efficacy and safety have not
been determined.
Indirect comparisons are undertaken to address such
issues. Using the same intervention as a bridge, the two therapies
are compared with the intervention through a direct meta-analysis
and, on the basis of the results, indirect comparisons are
subsequently conducted. With conventional chemotherapy as the
intervention, we performed a systematic evaluation for gefitinib
and cetuximab-based therapies based on the most updated results of
these studies and weighed the two therapies indirectly against the
clinical benefits and toxicities, with the aim of providing
references for clinical decisions for patients with advanced
NSCLC.
Materials and methods
Literature search
Several engines, including Medline, Embase,
Elsevier, the Cochrane Library Register of Controlled Trials and
the Science Citation Index, were searched for randomized controlled
trials (RCTs) using the keywords ‘random/trial’, ‘gefitinib’,
‘cetuximab’, ‘chemotherapy’ and ‘non-small-cell lung cancer/NSCLC’.
The deadline for trial publication eligible for the analysis was
April 30, 2013.
Study selection
The relevant studies were carefully selected using
the following criteria: i) RCTs published in English; ii) patients
with advanced (stage IIIB/IV) NSCLC and no obvious abnormalities of
other organs; iii) comparison of gefitinib therapy vs. conventional
chemotherapy (one or more combinations of cisplatin, carboplatin,
docetaxel, gemcitabine, pemetrexed and vinorelbine) and
cetuximab-based therapy vs. conventional chemotherapy; and iv) all
or part of the data on objective response rate (ORR), 1-year
survival, progression-free survival (PFS), overall survival (OS)
and adverse events (AEs) were provided. Studies were excluded by
any of the following criteria: i) objective unrelated to this
study; ii) phase I clinical trial; iii) no controlled clinical
trials; iv) no or insufficient mature data; and v) reviews,
comments and case reports.
Data extraction and conversion
According to the recommended guidelines of the
Cochrane Handbook for Systematic Reviews (5), the extraction form, created with
Microsoft Excel, included author, year of publication,
interventions, sample size, dose, clinical efficacy and AEs.
As for the data that could not be adopted directly,
appropriate transforming was required. For qualitative data, number
of events = effective sample size × event rate. For quantitative
data, the conversion methods were as follows: When the confidence
interval (CI) was provided within a group, i) if the sample size
was ≥100, under the 95% CI, standard deviation (SD) = √N ×
(upper limit of CI - lower limit of CI)/3.92); ii) if the sample
size was ≤60, 3.92 was replaced with 2 × t value; iii) if the
sample size was 60–100, either method was applicable. When CI was
provided between the groups, standard error (SE) was estimated
first with the method described above, where N = n1 +
n2, and then SD was calculated with the formula SD = SE/
√1/n1 + 1/n2 (6).
Quality assessment
An open assessment of the trials was performed with
the Jadad scale (7). The Jadad
score ranged between 0 and 5 points with the major indicators of
attrition and exclusions, randomization method and blinding.
Studies scoring ≥3 were considered to be of high quality (8).
Statistical methods
Treatment A and C were compared with the
intervention B and the direct evidence of AB and CB were obtained
to conduct the indirect comparisons of AC (9). A meta-analysis was used to obtain the
pooled AB and pooled CB using RevMan 5.0 software (The Cochrane
Collaboration, Oxford, UK). The statistics were risk ratio (RR) for
dichotomous variables and mean difference (MD) for numerical
variables, together with the 95% CI. If the test for heterogeneity
indicated good homogeneity (P>0.1 or I2≤50%) between
trials, the fixed-effects model was applied with the Mantel-Haeszel
method (10); in the opposite case
(P≤0.1 or I2>50%), the random-effects model
[DerSimonian and Laird method (11)] was used.
The Bucher approach was applied for indirect
comparisons. A comparison of A and C was conducted through the
difference between pooled AB and pooled CB, namely dAC =
dAB - dCB. The pooled effect size was
measured by lnRR for dichotomous variables and MD for numerical
variables. The variance of dAC equaled the sum of the
variance of AB and CB, namely Var(dAC) =
Var(dAB) + Var(dCB). For Var(dAB)
and Var(dCB), the computational formula was Var(d) =
[(upper limit of CI - lower limit of CI)/3.92]2. For
dichotomous variables, 95% CI of dAC = exp
[dAC ± 1.96 √Var(dAC)]
and for numerical variables, 95% CI of dAC =
dAC ± 1.96 √Var(dAC).
The hypothesis test was set for the results, as follows:
H0, dAC=0; H1, dAC≠0;
and
ZAC=|dAC|/√Var(dAC),
where ZAC exhibited a standard normal distribution as a
test statistic. The null hypothesis was rejected if P<0.05
(ZAC>1.96), i.e., if the effects between A and C
exhibited a statistically significant difference (12).
Results
Description of selected studies
A total of 104 articles were retrieved during the
primary search, of which 8 studies met the predetermined inclusion
criteria. A total of 4 studies (13–16),
including 935 patients who were randomized to receive either
gefitinib therapy or conventional chemotherapy, and another 4
studies (17–20), including 1,015 patients who
received either cetuximab-based therapy or conventional
chemotherapy, were included in the study. The main characteristics
of the 8 studies are summarized in Table I.
 | Table ICharacteristics of the 8 studies
included in the meta-analysis. |
Table I
Characteristics of the 8 studies
included in the meta-analysis.
| Study (year) | Group | Intervention | Treatment
schedule | Phase | Cases | End point | (Refs.) |
|---|
| Kim et al
(2008) | Treatment | Gefitinib | 250 mg/day p.o. | III | 733 | a–e | (13) |
| Control | Doc | 75 mg/m2
i.v. | III | 729 | a–e | |
| Mitsudomi et
al (2010) | Treatment | Gefitinib | 250 mg/day p.o. | III | 88 | a,c,e | (14) |
| Control | Cis + Doc | 80+60
mg/m2 i.v. | III | 89 | a,c,e | |
| Morère et al
(2010) | Treatment | Gefitinib | 250 mg/day
p.o. | II | 43 | a,c–e | (15) |
| Control | Gem | 1,250
mg/m2 i.v. | II | 42 | a,c–e | |
| Control | Doc | 75 mg/m2
i.v. | II | 42 | a,c–e | |
| Ahn et al
(2012) | Treatment | Gefitinib | 250 mg/day
p.o. | II | 40 | a–c,e | (16) |
| Control | Pem + Cis | 500+75
mg/m2 i.v. | II | 33 | a–c,e | |
| Rosell et al
(2008) | Treatment | Cetuximab + Cis +
Vin | 400+80+25
mg/m2 i.v. | II | 43 | a–e | (17) |
| Control | Cis + Vin | 80+25
mg/m2 i.v. | II | 43 | a–e | |
| Butts et al
(2007) | Treatment | Cetuximab + Gem +
Cis | 400+1,250+75
mg/m2 i.v. | II | 65 | a–e | (18) |
| Control | Gem + Cis | 1,250+75
mg/m2 i.v. | II | 66 | a–e | |
| Pirker et al
(2009) | Treatment | Cetuximab + Cis +
Vin | 400+80+25
mg/m2 i.v. | III | 557 | a–e | (19) |
| Control | Cis + Vin | 80+25
mg/m2 i.v. | III | 568 | a–e | |
| Lynch et al
(2010) | Treatment | Cetuximab + Doc +
Carbo | 400+75
mg/m2 + curve ≤6 i.v. | III | 338 | a,c–e | (20) |
| Control | Doc + Carbo | 75 mg/m2
+ curve ≤6 i.v. | III | 338 | a,c–e | |
All 8 studies were RCTs, of which 7 studies applied
the proper methods of randomization. Attrition and exclusions were
illustrated in detail, while double-blind methods were not
mentioned. The included studies were considered to be of high
quality, scoring 3 on the Jadad scale, except one study (13). The quality assessment of the
studies is presented in Table
II.
 | Table IIQuality assessment of the 8 included
studies by the Jadad scale. |
Table II
Quality assessment of the 8 included
studies by the Jadad scale.
| Author (year) | Randomization | Blinding | Attrition and
exclusions | Jadad score | (Refs.) |
|---|
| Kim et al
(2008) | 1 | 0 | 1 | 2 | (13) |
| Mitsudomi et
al (2010) | 2 | 0 | 1 | 3 | (14) |
| Morère et al
(2010) | 2 | 0 | 1 | 3 | (15) |
| Ahn et al
(2012) | 2 | 0 | 1 | 3 | (16) |
| Rosell et al
(2008) | 2 | 0 | 1 | 3 | (17) |
| Butts et al
(2007) | 2 | 0 | 1 | 3 | (18) |
| Pirker et al
(2009) | 2 | 0 | 1 | 3 | (19) |
| Lynch et al
(2010) | 2 | 0 | 1 | 3 | (20) |
Statistical analysis of efficacy and
safety
ORR
A total of 4 studies (13–16)
compared gefitinib therapy vs. conventional chemotherapy and the
remaining 4 studies (17–20) compared cetuximab-based therapy vs.
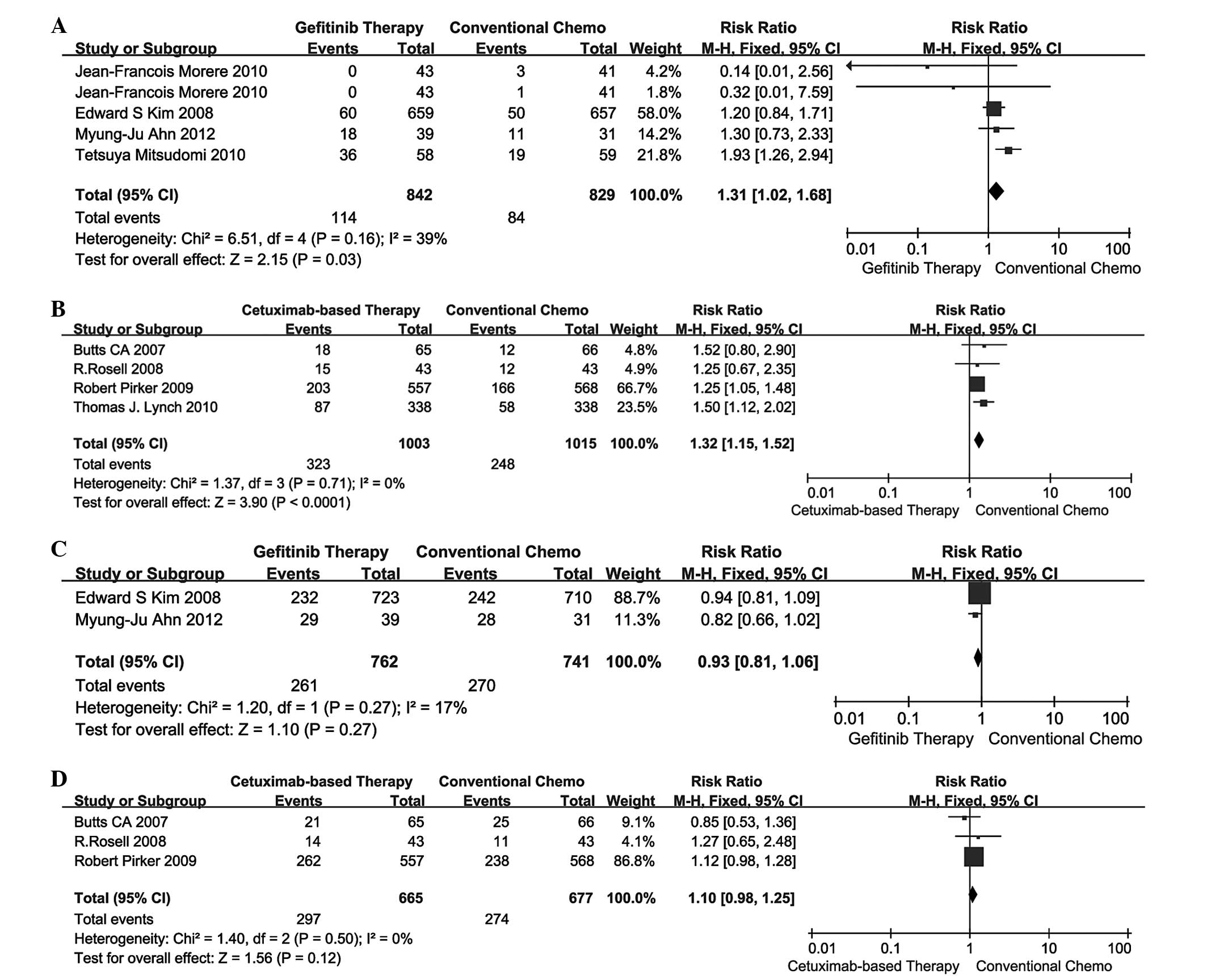
conventional chemotherapy in terms of ORR. The pooled analysis of
ORR using the fixed-effects model is presented in Fig. 1A and B. The RR for gefitinib
therapy vs. conventional chemotherapy and cetuximab-based therapy
vs. conventional chemotherapy was 1.31 (95% CI: 1.02–1.68) and 1.32
(95% CI: 1.15–1.52), respectively. Indirect comparisons between
gefitinib and cetuximab-based therapies revealed no significant
difference in ORR (RR=0.99; 95% CI: 0.75–1.32; P=0.9584; Table III).
 | Table IIIResults of indirect comparisons
between gefitinib and cetuximab-based therapies. |
Table III
Results of indirect comparisons
between gefitinib and cetuximab-based therapies.
| Indicator | RR/MD | 95% CI | P-value |
|---|
| Objective response
rate |
0.99 | 0.75 to 1.32 |
0.9584 |
| One-year survival
rate |
0.85 | 0.71 to 1.01 |
0.0696 |
| Progression-free
survival |
−0.15 | −0.90 to 0.60 |
0.6946 |
| Overall
survival |
−1.84 | −3.53 to −0.15 |
0.0331 |
| Grade 3/4 adverse
events |
0.29 | 0.19 to 0.44 |
0.0001 |
Survival rate
A total of 2 studies (13,16)
compared gefitinib therapy vs. conventional chemotherapy and 3
studies (17–19) compared cetuximab-based therapy vs.
conventional chemotherapy in terms of 1-year survival rate. The
pooled analysis of 1-year survival rate using the fixed-effects
model is presented in Fig. 1C and
D. The RR for gefitinib therapy vs. conventional chemotherapy
and cetuximab-based therapy vs. conventional chemotherapy was 0.93
(95% CI: 0.81–1.06) and 1.10 (95% CI: 0.98–1.25), respectively.
Indirect comparisons between gefitinib and cetuximab-based
therapies revealed no significant difference in 1-year survival
rate (RR=0.85; 95% CI: 0.71–1.01; P=0.0696; Table III).
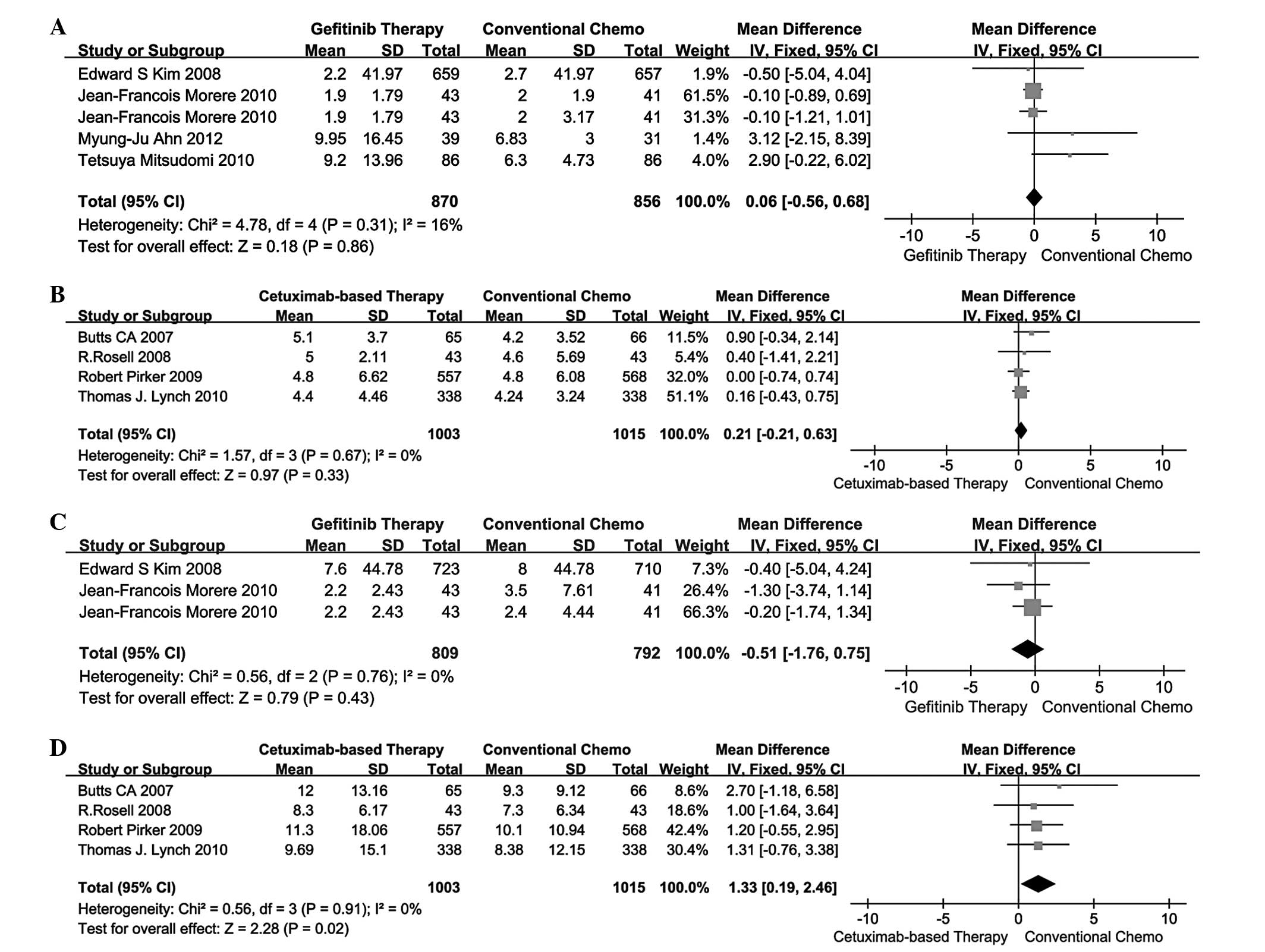
PFS
A total of 4 studies (13–16)
compared gefitinib therapy vs. conventional chemotherapy and the
remaining 4 studies (17–20) compared cetuximab-based therapy vs.
conventional chemotherapy in terms of PFS. The pooled analysis of
PFS using the fixed-effects model is presented in Fig. 2A and B. The MD for gefitinib
therapy vs. conventional chemotherapy and cetuximab-based therapy
vs. conventional chemotherapy was 0.06 (95% CI: −0.56 to 0.68) and
0.21 (95% CI: −0.21 to 0.63). Indirect comparisons between
gefitinib and cetuximab-based therapies revealed no significant
difference in PFS (MD=-0.15; 95% CI: −0.90 to 0.60; P=0.6946;
Table III).
OS
A total of 2 studies (13,15)
compared gefitinib therapy vs. conventional chemotherapy and 4
studies (17–20) compared cetuximab-based therapy vs.
conventional chemotherapy in terms of OS. The pooled analysis of OS
using the fixed-effects model is presented in Fig. 2C and D. The MD for gefitinib
therapy vs. conventional chemotherapy and cetuximab-based therapy
vs. conventional chemotherapy was −0.51 (95% CI: −1.76 to 0.75) and
1.33 (95% CI: 0.19–2.46). Indirect comparisons between gefitinib
and cetuximab-based therapies revealed that the latter exhibited a
significant advantage over the former in terms of OS (MD=-1.84; 95%
CI: −3.53 to −0.15; P=0.0331; Table
III).
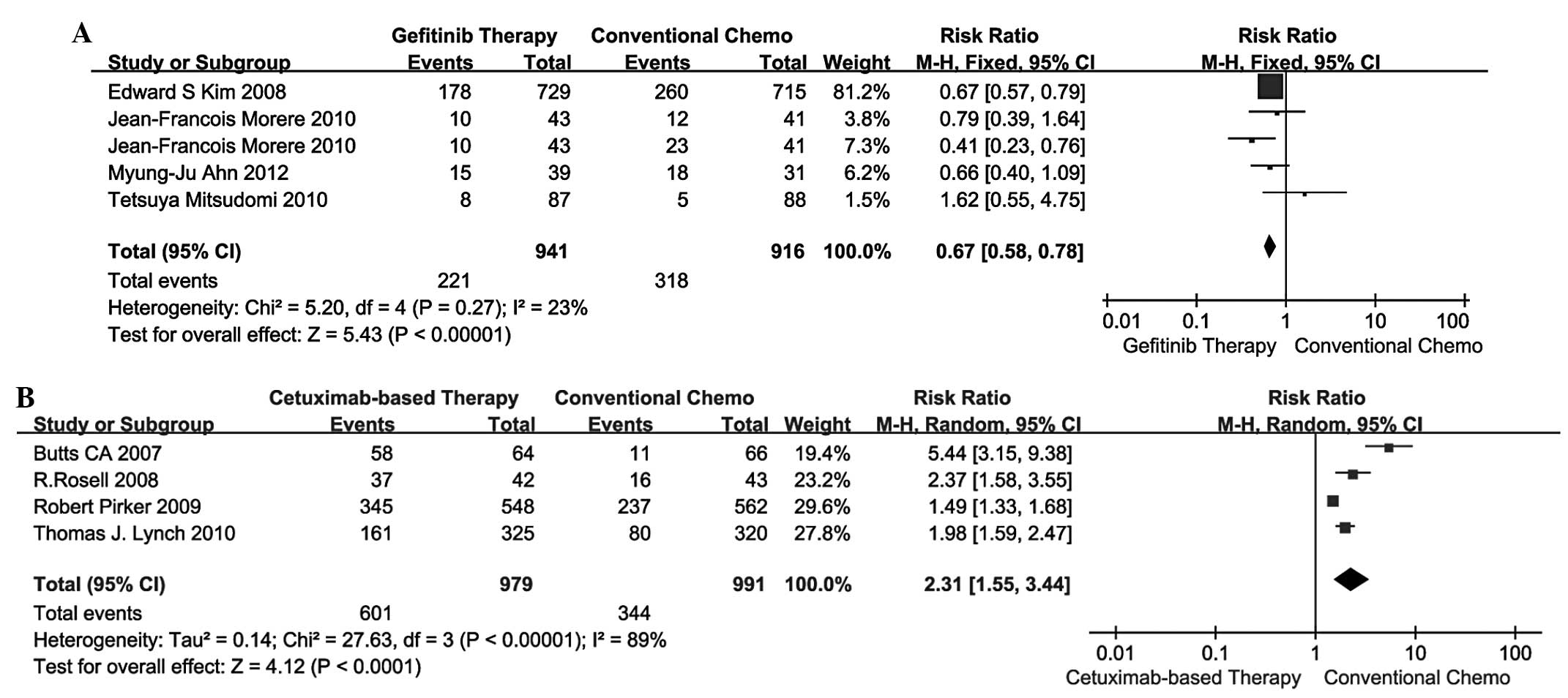
Grade 3/4 AEs
A total of 4 studies (13–16)
compared gefitinib therapy vs. conventional chemotherapy and the
remaining 4 studies (17–20) compared cetuximab-based therapy vs.
conventional chemotherapy in terms of 3/4 AEs. The pooled analysis
of 3/4 AEs using the fixed-effects model is shown in Fig. 3A, while the results using the
random-effects model are shown in Fig.
3B. The RR for gefitinib therapy vs. conventional chemotherapy
and cetuximab-based therapy vs. conventional chemotherapy was 0.67
(95% CI: 0.58–0.78) and 2.31 (95% CI: 1.55–3.44). Indirect
comparisons between gefitinib and cetuximab-based therapies
revealed that the former exhibited a significant advantage over the
latter in terms of 3/4 AEs (RR=0.29; 95% CI: 0.19–0.44; P=0.0001;
Table III).
Discussion
As demonstrated by the indirect comparisons,
cetuximab-based therapy was found to be superior to TKIs, such as
gefitinib, regarding efficacy. A recently published meta-analysis
recommended that gefitinib therapy not be used for the management
of patients with advanced NSCLC in the first-line setting (21). Other studies also reported that the
activity of EGFR-TKIs may be restricted to a subset of tumors with
specific molecular characteristics, highlighting the need for
appropriate patient selection (22,23).
Furthermore, certain studies proved the OS benefit of
cetuximab-based therapy and suggested that advanced NSCLC patients
with high EGFR gene expression may benefit more from
cetuximab-based therapy (24).
As regards safety, gefitinib appears to be superior
to cetuximab-based therapy in terms of 3/4 AEs. Despite the
limitations of the safety indicator itself, one plausible
explanation for this discrepancy is the difference in the
administration methods, i.e., the oral administration of gefitinib
is considered to be safer compared to the intravenous
administration of cetuximab. Another possible reason is that
gefitinib is more uncomplicated and controllable compared to
cetuximab-based therapy containing several chemotherapeutic drugs,
such as cisplatin, docetaxel and gemcitabine, which is associated
with more risks.
The indirect comparison adopted in our study is
controversial. Certain investigators have suggested that indirect
comparison compromises the randomness of original RCTs and
inevitably induces bias (25). In
the study of Bucher et al (26), indirect comparison was associated
with significantly more bias compared to direct comparisons, while
Song et al (27) reached
the opposite conclusion with 3 case studies. Another study by Song
et al (28) further
confirmed the reliability of the results of indirect comparison.
Indirect comparison remains a reasonable option in the absence of
direct comparison of two drugs and a number of medical journals,
such as JAMA, Lancet and BMJ have accepted the findings of indirect
comparison (12).
However, our results must be interpreted with
caution, as there were certain limitations to our study. Although
each of the 8 included studies was considered to be of high
quality, the total number of articles was insufficient to draw a
credible conclusion. The sample size of included trials was also
insufficient for a funnel plot to detect publication bias. Due to
the lack or inconformity of patient selection regarding details
such as gender, age, smoking history and race, subgroup analyses
were not feasible. The analyses also revealed some heterogeneity
within the study results, such as safety data of cetuximab-based
therapy. One must also consider the limitation on methodology of
indirect comparisons and the lack of unpublished or ongoing
RCTs.
Despite all the limitations, our results may
contribute to a better understanding of gefitinib and
cetuximab-based therapies in patients with advanced NSCLC. Based on
the present meta-analysis and indirect comparisons, we concluded
that cetuximab-based therapy may be associated with a more
significant improvement in OS compared to gefitinib therapy, while
gefitinib was superior in terms of safety, with a lower incidence
of grade 3/4 AEs. There were no significant differences between
gefitinib and cetuximab-based therapies in terms of ORR, 1-year
survival rate and PFS in patients with advanced NSCLC. Further
studies are required to confirm our findings and evaluate the
cost-effectiveness of the two therapies, in order to provide a
better reference for clinical practice.
Acknowledgements
We would like to thank Professor Feng Yu and Dr
Hongchao Li of the China Pharmaceutical University for their
assistance with the writing of this manuscript.
References
|
1
|
Ozkaya S, Findik S, Dirican A and Atici
AG: Long-term survival rates of patients with stage IIIB and IV
non-small cell lung cancer treated with cisplatin plus vinorelbine
or gemcitabine. Exp Ther Med. 4:1035–1038. 2012.PubMed/NCBI
|
|
2
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ku GY, Haaland BA and de Lima Lopes G Jr:
Gefitinib vs. chemotherapy as first-line therapy in advanced
non-small cell lung cancer: meta-analysis of phase III trials. Lung
Cancer. 74:469–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin H, Jiang J, Liang X, Zhou X and Huang
R: Chemotherapy with cetuximab or chemotherapy alone for untreated
advanced non-small-cell lung cancer: a systematic review and
meta-analysis. Lung Cancer. 70:57–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higgins JPT and Green S: Cochran. Handbook
for Systematic Reviews of Interventions, version 5.1.0. The
Cochrane Collaboration, . 2011, https://www.cochrane-handbook.orgAccessed. June
3–2013
|
|
6
|
Liu M: Design and Implementation Methods
of Systematic Reviews and Meta-analysis. People's Medical
Publishing House; Beijing: pp. 86–89. 2011, (In Chinese).
|
|
7
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
8
|
Schulz KF, Altman DG, Moher D, et al:
CONSORT 2010 statement: updated guidelines for reporting parallel
group randomised trials. BMC Med. 8(18)2010. View Article : Google Scholar
|
|
9
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Controlled clinical trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HN, Yan JZ, Tang JF and Shao R:
Indirect comparisons for efficacy of gefitinib and erlotinib in
patients with non-small cell lung cancer. Chin J Pharm Econ.
1:15–19. 2013.(In Chinese).
|
|
11
|
Silagy C, Lancaster T, Stead L, et al:
Nicotin. replacement therapy for smoking cessation. The Cochrane
Library, John Wiley & Sons; Oxford: 2004
|
|
12
|
Liao WQ: Simulation Study of Multivariate
Meta-analysis and Indirect Comparisons. Southern Medical
University; Guangzhou: pp. 23–26. 2011, (In Chinese).
|
|
13
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsudomi T, Morita S, Yatabe Y, et al
West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): an
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar
|
|
15
|
Morère JF, Bréchot JM, Westeel V, et al:
Randomized phase II trial of gefitinib or gemcitabine or docetaxel
chemotherapy in patients with advanced non-small-cell lung cancer
and a performance status of 2 or 3 (IFCT-0301 study). Lung Cancer.
70:301–307. 2010.PubMed/NCBI
|
|
16
|
Ahn MJ, Yang JC, Liang J, et al:
Randomized phase II trial of first-line treatment with
pemetrexed-cisplatin, followed sequentially by gefitinib or
pemetrexed, in East Asian, never-smoker patients with advanced
non-small cell lung cancer. Lung Cancer. 77:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosell R, Robinet G, Szczesna A, et al:
Randomized phase II study of cetuximab plus cisplatin/vinorelbine
compared with cisplatin/vinorelbine alone as first-line therapy in
EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol.
19:362–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butts CA, Bodkin D, Middleman EL, et al:
Randomized phase II study of gemcitabine plus cisplatin or
carboplatin, with or without cetuximab, as first-line therapy for
patients with advanced or metastatic non-small-cell lung cancer. J
Clin Oncol. 25:5777–5784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pirker R, Pereira JR, Szczesna A, et al
FLEX Study Team: Cetuximab plus chemotherapy in patients with
advanced non-small-cell lung cancer (FLEX): an open-label
randomised phase III trial. Lancet. 373:1525–1531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch TJ, Patel T, Dreisbach L, et al:
Cetuximab and first-line taxane/carboplatin chemotherapy in
advanced non-small-cell lung cancer: results of the randomized
multicenter phase III trial BMS099. J Clin Oncol. 28:911–917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim EM: Frontline gefitinib in
advanced non-small cell lung cancer: meta-analysis of published
randomized trials. Ann Thorac Med. 5:153–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsao MS, Sakurada A, Cutz JC, et al:
Erlotinib in lung cancer - molecular and clinical predictors of
outcome. New Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pirker R, Pereira JR, von Pawel J, et al:
EGFR expression as a predictor of survival for first-line
chemotherapy plus cetuximab in patients with advanced
non-small-cell lung cancer: analysis of data from the phase 3 FLEX
study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: combining direct
and indirect evidence. BMJ. 331:897–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bucher HC, Guyatt GH, Griffith LE and
Walter SD: The results of direct and indirect treatment comparisons
in meta-analysis of randomized controlled trials. J Clin Epidemiol.
50:683–691. 1997. View Article : Google Scholar
|
|
27
|
Song F, Harvey I and Lilford R: Adjusted
indirect comparison may be less biased than direct comparison for
evaluating new pharmaceutical interventions. J Clin Epidemiol.
61:455–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song F, Altman DG, Glenny AM and Deeks JJ:
Validity of indirect comparison for estimating efficacy of
competing interventions: empirical evidence from published
meta-analyses. BMJ. 326(472)2003. View Article : Google Scholar : PubMed/NCBI
|