Introduction
Sentinel lymph node biopsy (SLNB) has become an
alternative to axillary lymph node dissection (ALND) for nodal
staging in breast cancer (1,
2). If the sentinel lymph node
(SLN) is free of metastasis, metastatic disease is not likely to be
present in the axillary lymph nodes; therefore, ALND may be avoided
(3). However, SLNB has been
reported to be associated with false-negative results and local
recurrence following SLNB (4).
In keeping with the characteristically tortuous and
aberrant pattern of tumor neovasculature, metastatic lymph nodes
also exhibit peripheral and mixed vascularity. Color Doppler
ultrasonography only provides information regarding macrovessel
flow and morphology; therefore, it is difficult to accurately
diagnose lymph node metastasis using this method (5). Sonazoid, a new generation contrast
agent for ultrasonography, allows for visualization of lymph node
microvessels. Compared to previously used imaging modalities,
contrast-enhanced ultrasonography (CEUS) with Sonazoid may enable a
more accurate evaluation of lymph node metastasis.
This is a case report of axillary lymph node
metastasis (ALNM) following SLNB, which was accurately diagnosed
with CEUS using Sonazoid.
Case report
A 40-year-old woman underwent total mastectomy and
SLNB for cancer of the left breast. The histopathological
examination revealed invasive ductal carcinoma, T1bN0M0, stage IA,
estrogen receptor-negative, progesterone receptor-negative, human
epidermal growth factor receptor-negative and MIB-1 index 6%. The
patient received tamoxifen as adjuvant endocrine therapy. After 6
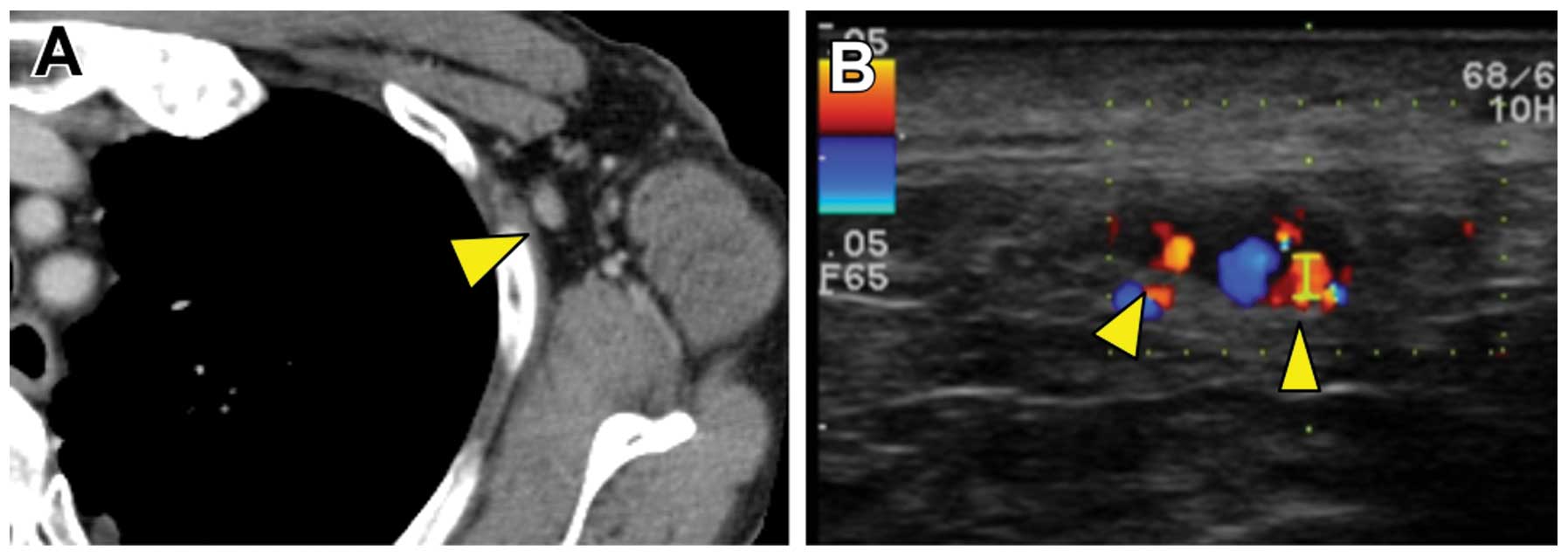
months, follow-up enhanced computed tomography (CT) revealed a left
axillary lymph node enlarged to 11 mm (Fig. 1A). Color Doppler ultrasonography
revealed pulsatile blood flow to the lymph node from several
directions (Fig. 1B). On the basis
of the CT and color Doppler results, lymph node metastasis was
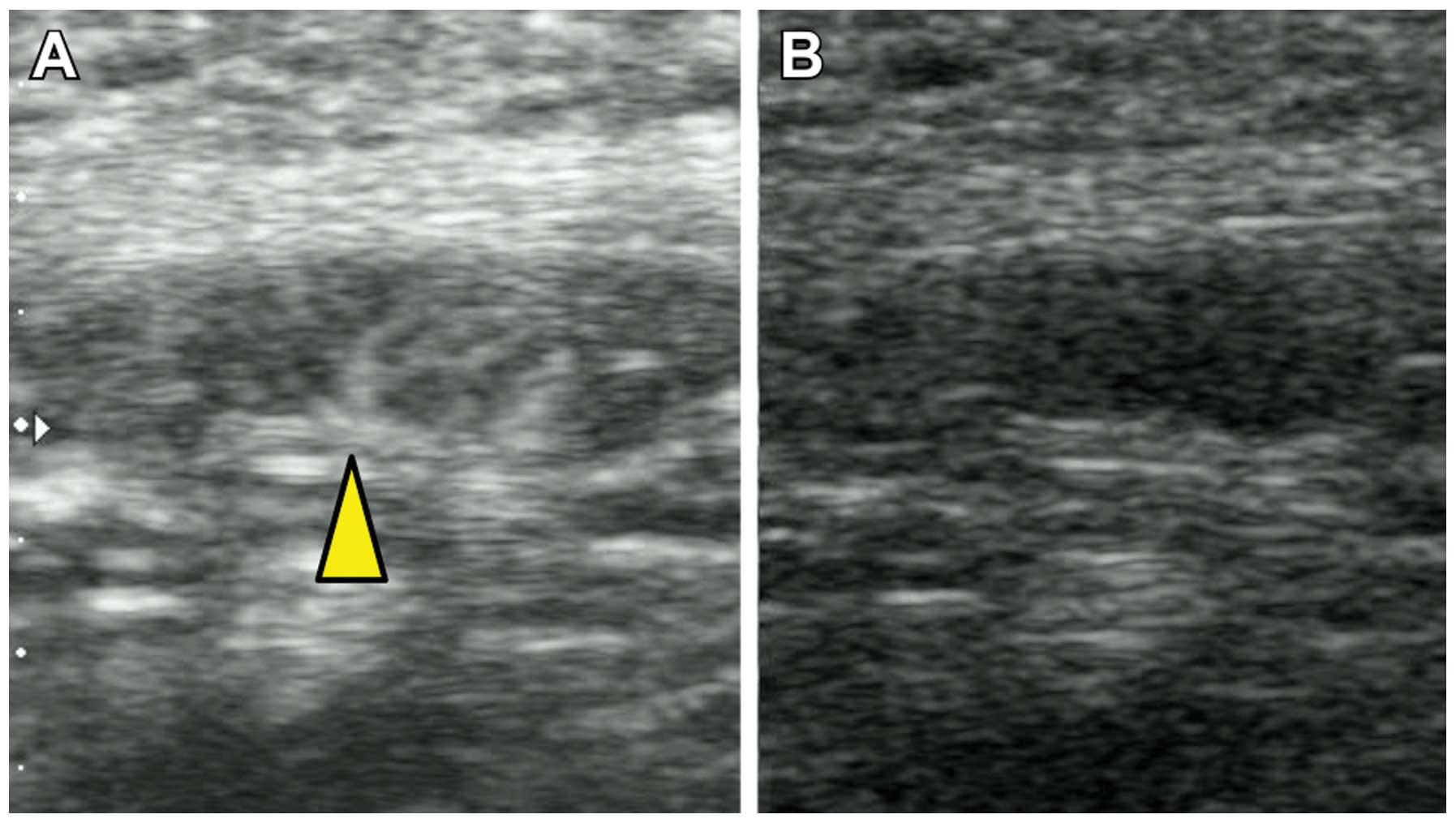
suspected. In contrast to the color Doppler, CEUS demonstrated
blood flow from only the hilum of the lymph node, suggesting that
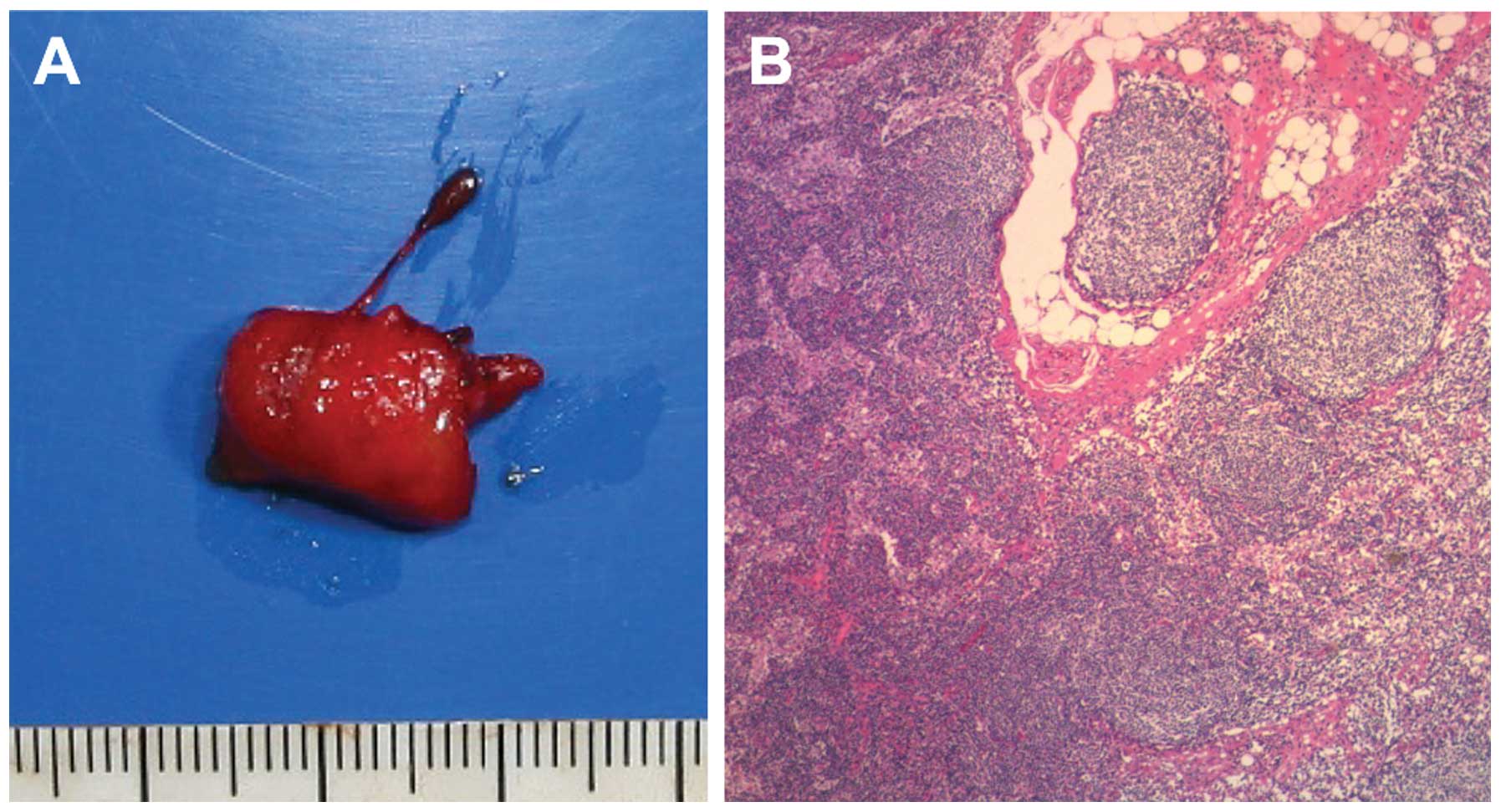
this lymph node may be clear of metastasis (Fig. 2). ALND was ultimately performed and
the histopathological findings confirmed that the axillary lymph
node was negative for metastasis (Fig.
3).
This study was approved by the Research Ethics
Committee of our hospital and the patient provided consent for the
findings of her case to be published.
Discussion
Breast cancer is the most common type of cancer in
women, exhibiting considerably varying incidence and mortality
rates worldwide. The 5-year survival rate in patients with breast
cancer reportedly ranges between 74 and 82% (6).
Since standard radical mastectomy for the treatment
of breast cancer was first established by Halsted, the surgical
procedures for breast cancer have continued to improve on the basis
of the results of randomized clinical trials (2, 7–11).
Breast-conserving surgery is now considered to be the standard
local treatment for early breast cancer. In addition, SLNB has
recently become an alternative to ALND for nodal staging (1, 2).
The SLN hypothesis states that tumor cells that are
shed from a primary carcinoma migrate through a lymphatic channel
to a single lymph node prior to involvement of the remaining lymph
nodes within that basin. The SLN is the first lymph node that
receives lymphatic drainage from a tumor and its identification and
analysis for tumor involvement may predict the status of the
remaining lymph nodes (12).
However, several issues have been reported when
using SLNB for nodal staging. First, it was reported that the
proportion of patients with successfully mapped SLNs ranged between
41 and 100%, with >50% of the studies reporting a rate <90%.
The false-negative rate ranged between 0 and 29%, with an average
rate of 7.3% (13). Second,
patients may experience local disease recurrence following SLNB.
Axillary local recurrence rates in patients with a negative SLNB
and no ALND were reported to range between 0 and 1.4% at 14–46
months of follow-up (4).
ALNM is a key factor for the prognosis of breast
cancer and significantly affects the decisions regarding the
selection of treatment modalities; thus, diagnostically accurate
methods for determining ALNM are crucial. Axillary ultrasonography
(AUS) is widely used for the detection of ALNM, as it is relatively
accurate and non-invasive. AUS is simple, easy and more
cost-effective compared to other modalities. Therefore, it is an
elemental test in breast cancer evaluation. The sensitivity and
specificity of AUS for the detection of ALNM were reported to be 61
and 82%, respectively (14).
Contrast-enhanced magnetic resonance imaging (cMRI) is generally
used to evaluate the regional extent of breast cancer prior to
breast-conserving surgery; it enables the assessment of the changes
in the extent of tumor growth pre- and post-chemotherapy and may be
used for screening of high-risk patients and of those with large
breasts, evaluating isolated ALNMs of unknown origin and evaluating
ALNMs in breast cancer (15,
16). The sensitivity and
specificity of cMRI for the prediction of ALNM range between
36–100% and 54–100%, respectively. These ranges are fairly wide, as
they are dependent on the definition of ALNM, the type of contrast
agent used, the size of the breast tumor and the number of
metastatic ALNs (17–21). Hwang et al (22) reported that the actual accuracy of
cMRI was similar to that of AUS. Imaging with
fluoro-2-deoxy-D-glucose-positron emission tomography (PET) may
also be used to evaluate ALNM. The fundamental strength of PET
imaging over conventional imaging is its ability to convey
functional information that even the most exquisitely detailed
anatomical image cannot provide. However, when surveyed across the
multitude of prior reports, PET has an overall sensitivity of 88%,
a specificity of 92% and an accuracy of 89%, although several of
the studies achieved a higher sensitivity at the expense of lower
specificity or vice versa. This may explain the wide variation in
the results (23).
Sonazoid, a new generation contrast agent for
ultrasonography, was first introduced on January 10, 2007 and is
approved for use only in Japan. The active ingredient of Sonazoid
is a perflubutane microbubble that is stabilized using hydrogenated
egg phosphatidyl serine sodium, which is a phospholipid.
Perflubutane is chemically stable and insoluble in water.
Therefore, it has a long lifespan in the body, as it hardly
dissolves in the blood. CEUS with Sonazoid for liver tumors is
currently frequently performed in Japan. Omoto et al
(24) reported an SNL detection
method using CEUS with Sonazoid in a human breast cancer patient.
Aoki et al (25) suggested
that CEUS may be useful in distinguishing tumor-induced from
inflammation-induced lymph node enlargement.
CEUS with Sonazoid may also allow for the
visualization of microvessels. Color Doppler ultrasonography
provides information on macrovessel flow and morphology and may
evaluate palpable lymph nodes more accurately compared to AUS;
however, it cannot be used to evaluate microvessels and is
therefore not applicable in the evaluation of non-palpable nodes
(5). We suggest that CEUS with
Sonazoid may enable an accurate diagnosis of ALNM.
In our department, we have performed preoperative
evaluation using CEUS for lymph node metastasis in a total of 14
cases (Table I). In addition, we
also performed plane ultrasonography, color Doppler
ultrasonography, enhanced CT and enhanced MRI during the
preoperative examination. An accurate diagnosis using CEUS was
possible in all the cases. In cases 6 and 13, as well as in the
present case, the results differed between CEUS and the other
imaging modalities. Considering these results, CEUS is more likely
to result in an accurate diagnosis of lymph node metastasis
compared to other modalities used for evaluation.
 | Table I.Comparison of results of axillary
lymph node metastasis detection by CT, Doppler US and CEUS. |
Table I.
Comparison of results of axillary
lymph node metastasis detection by CT, Doppler US and CEUS.
| Case no. | CT | US (with
Doppler) | CEUS | Pathological
findings |
|---|
| 1 | Negative | Negative | Negative | Negative |
| 2 | Negative | Negative | Negative | Negative |
| 3 | Negative | Positive | Positive | Positive |
| 4 | Negative | Negative | Negative | Negative |
| 5 | Negative | Negative | Negative | Negative |
| 6 | Negative | Negative | Positive | Positive |
| 7 | Positive | Negative | Negative | Negative |
| 8 | Negative | Negative | Negative | Negative |
| 9 | Negative | Negative | Negative | Negative |
| 10 | Negative | Negative | Negative | Negative |
| 11 | Negative | Negative | Negative | Negative |
| 12 | Negative | Negative | Negative | Negative |
| 13 | Negative | Negative | Positive | Positive |
| Present case | Positive | Positive | Negative | Negative |
| Total, (%) | 10/14 (71) | 11/14 (79) | 14/14 (100) | − |
In conclusion, we presented a case with axillary
lymph node enlargement following SLNB, in which CEUS with Sonazoid
resulted in an accurate diagnosis. On the basis of our experience
with this case, a clinical trial evaluating the detection of lymph
node metastasis by CEUS in breast cancer patients is currently
ongoing at our institution.
Acknowledgements
The authors would like to thank E. Tamura, M. Satoh,
T. Kaga, M. Ohmura, Y. Takada, C. Takeda and A. Fujibe for making
this study possible.
References
|
1
|
Lyman GH, Giuliano AE, Somerfield MR, et
al American Society of Clinical Oncology: American Society of
Clinical Oncology guideline recommendations for sentinel lymph node
biopsy in early-stage breast cancer. J Clin Oncol. 23:7703–7720.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCready D, Holloway C, Shelley W, et al:
Breast Cancer Disease Site Group of Cancer Care; Ontario's Program
in Evidence-Based Care: Surgical management of early stage invasive
breast cancer: a practice guideline. Can J Surg. 48:185–194.
2005.PubMed/NCBI
|
|
3
|
Dabakuyo TS, Fraisse J, Causeret S, et al:
A multicenter cohort study to compare quality of life in breast
cancer patients according to sentinel lymph node biopsy or axillary
lymph node dissection. Ann Oncol. 20:1352–1361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naik AM, Fey J, Gemignani M, et al: The
risk of axillary relapse after sentinel lymph node biopsy for
breast cancer is comparable with that of axillary lymph node
dissection: a follow-up study of 4008 procedures. Ann Surg.
240:462–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang WT, Chang J and Metreweli C: Patients
with breast cancer: differences in color Doppler flow and
gray-scale US features of benign and malignant axillary lymph
nodes. Radiology. 215:568–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fisher B, Anderson S, Bryant J, et al:
Twenty-year follow-up of a randomized trial comparing total
mastectomy, lumpectomy, and lumpectomy plus irradiation for the
treatment of invasive breast cancer. N Engl J Med. 347:1233–1241.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobson JA, Danforth DN, Cowan KH, et al:
Ten-year results of a comparison of conservation with mastectomy in
the treatment of stage I and II breast cancer. N Engl J Med.
332:907–911. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lacour J, Le M, Caceres E, Koszarowski T,
Veronesi U and Hill C: Radical mastectomy versus radical mastectomy
plus internal mammary dissection. Ten year results of an
international cooperative trial in breast cancer. Cancer.
51:1941–1943. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maddox WA, Carpenter JT, Laws HL, et al: A
randomized prospective trial of radical (Halsted) mastectomy versus
modified radical mastectomy in 311 breast cancer patients. Ann
Surg. 198:207–212. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner L, Swindell R, Bell WG, et al:
Radical versus modified radical mastectomy for breast cancer. Ann R
Coll Surg Engl. 63:239–243. 1981.PubMed/NCBI
|
|
12
|
Cabanas RM: An approach for the treatment
of penile carcinoma. Cancer. 39:456–466. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim T, Giuliano AE and Lyman GH: Lymphatic
mapping and sentinel lymph node biopsy in early-stage breast
carcinoma: a meta-analysis. Cancer. 106:4–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houssami N, Ciatto S, Turner RM, Cody HS
and Macaskill P: Preoperative ultrasound-guided needle biopsy of
axillary nodes in invasive breast cancer: meta-analysis of its
accuracy and utility in staging the axilla. Ann Surg. 254:243–251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko EY, Han BK, Shin JH and Kang SS: Breast
MRI for evaluating patients with metastatic axillary lymph node and
initially negative mammography and sonography. Korean J Radiol.
8:382–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mameri CS, Kemp C, Goldman SM, Sobral LA
and Ajzen S: Impact of breast MRI on surgical treatment, axillary
approach, and systemic therapy for breast cancer. Breast J.
14:236–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcia Fernández A, Fraile M, Giménez N,
et al: Use of axillary ultrasound, ultrasound-fine needle
aspiration biopsy and magnetic resonance imaging in the
preoperative triage of breast cancer patients considered for
sentinel node biopsy. Ultrasound Med Biol. 37:16–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harnan SE, Cooper KL, Meng Y, et al:
Magnetic resonance for assessment of axillary lymph node status in
early breast cancer: a systematic review and meta-analysis. Eur J
Surg Oncol. 37:928–936. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kvistad KA, Rydland J, Smethurst HB,
Lundgren S, Fjosne HE and Haraldseth O: Axillary lymph node
metastases in breast cancer: preoperative detection with dynamic
contrast-enhanced MRI. Eur Radiol. 10:1464–1471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peare R, Staff RT and Heys SD: The use of
FDG-PET in assessing axillary lymph node status in breast cancer: a
systematic review and meta-analysis of the literature. Breast
Cancer Res Treat. 123:281–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valente SA, Levine GM, Silverstein MJ, et
al: Accuracy of predicting axillary lymph node positivity by
physical examination, mammography, ultrasonography, and magnetic
resonance imaging. Ann Surg Oncol. 19:1825–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang SO, Lee SW, Kim HJ, Kim WW, Park HY
and Jung JH: The comparative study of ultrasonography,
contrast-enhanced MRI, and18F-FDG PET/CT for detecting axillary
lymph node metastasis in T1 breast cancer. J Breast Cancer.
16:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quon A and Gambhir SS: FDG-PET and beyond:
molecular breast cancer imaging. J Clin Oncol. 23:1664–1673. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omoto K, Matsunaga H, Take N, et al:
Sentinel node detection method using contrast-enhanced
ultrasonography with Sonazoid in breast cancer: preliminary
clinical study. Ultrasound Med Biol. 35:1249–1256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aoki T, Moriyasu F, Yamamoto K, Shimizu M,
Yamada M and Imai Y: Image of tumor metastasis and inflammatory
lymph node enlargement by contrast-enhanced ultrasonography. World
J Radiol. 3:298–305. 2011. View Article : Google Scholar : PubMed/NCBI
|